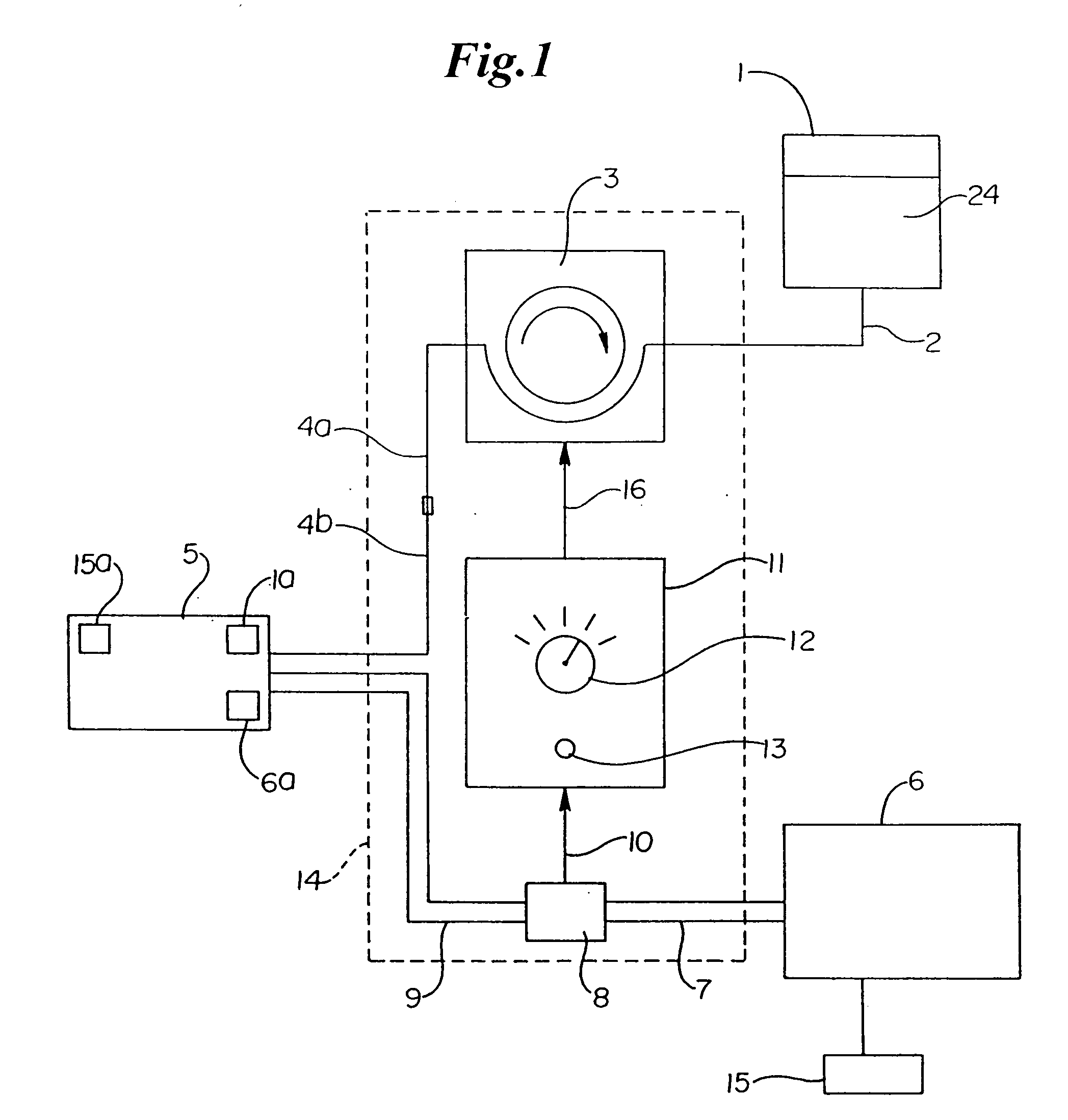
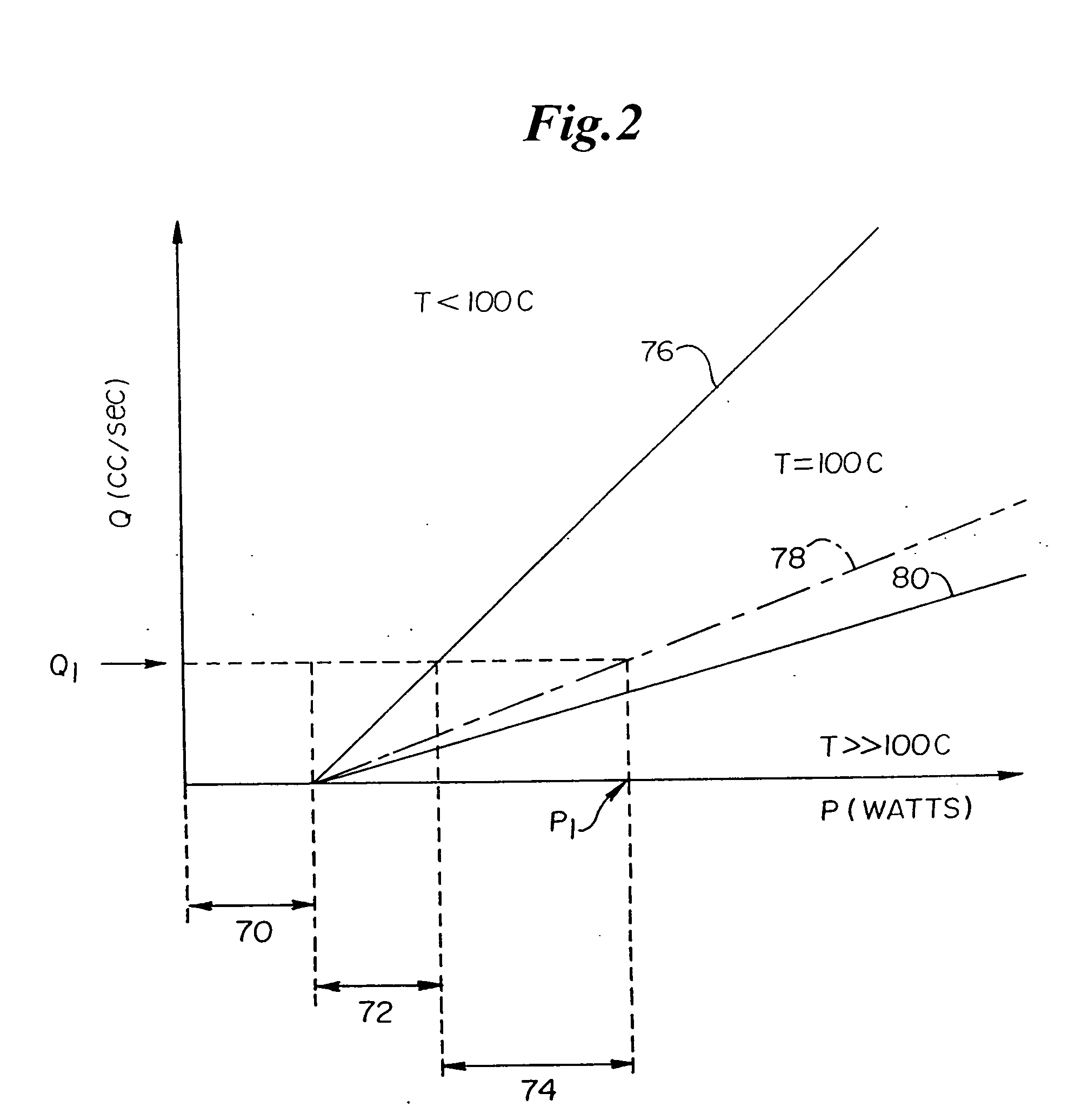
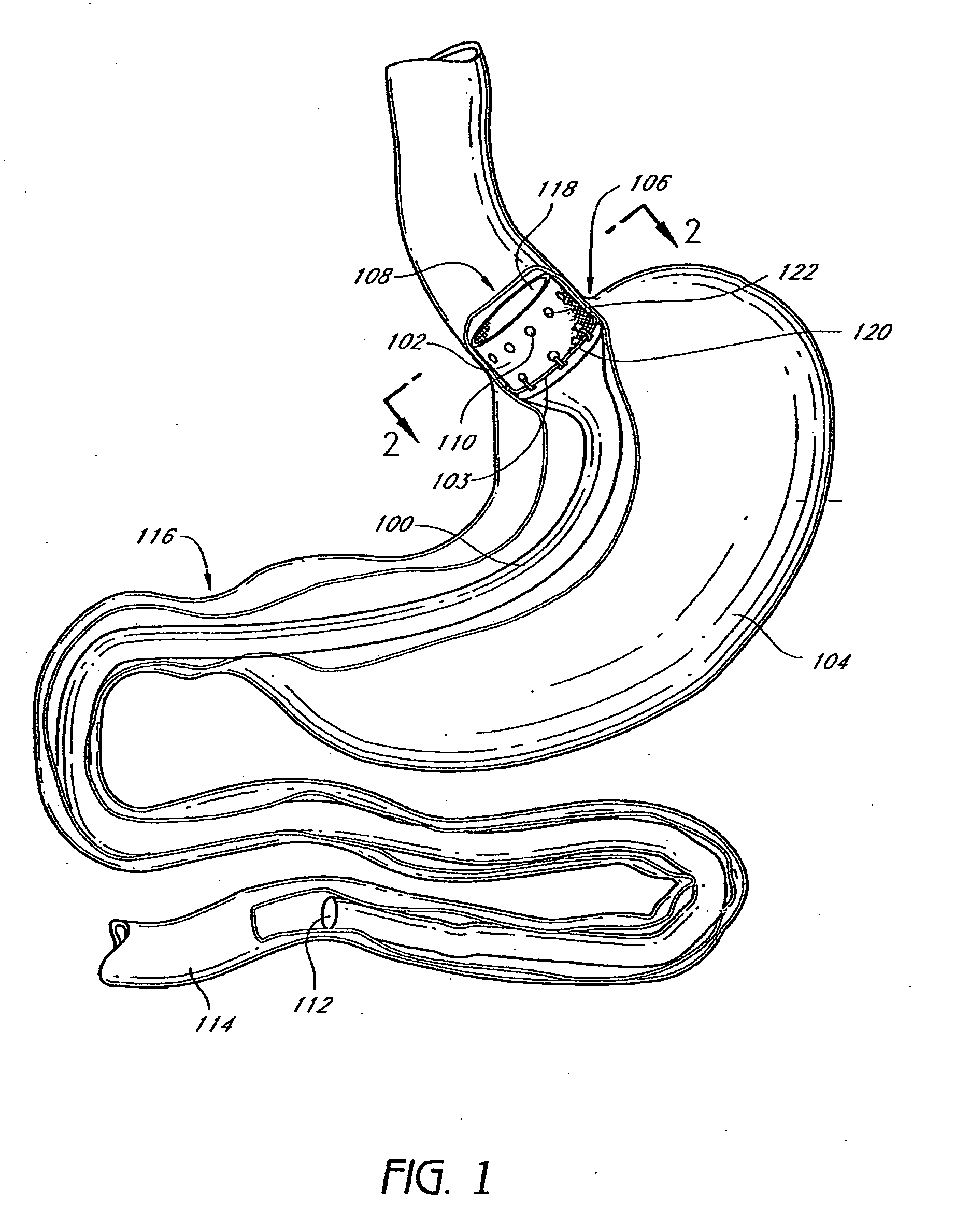
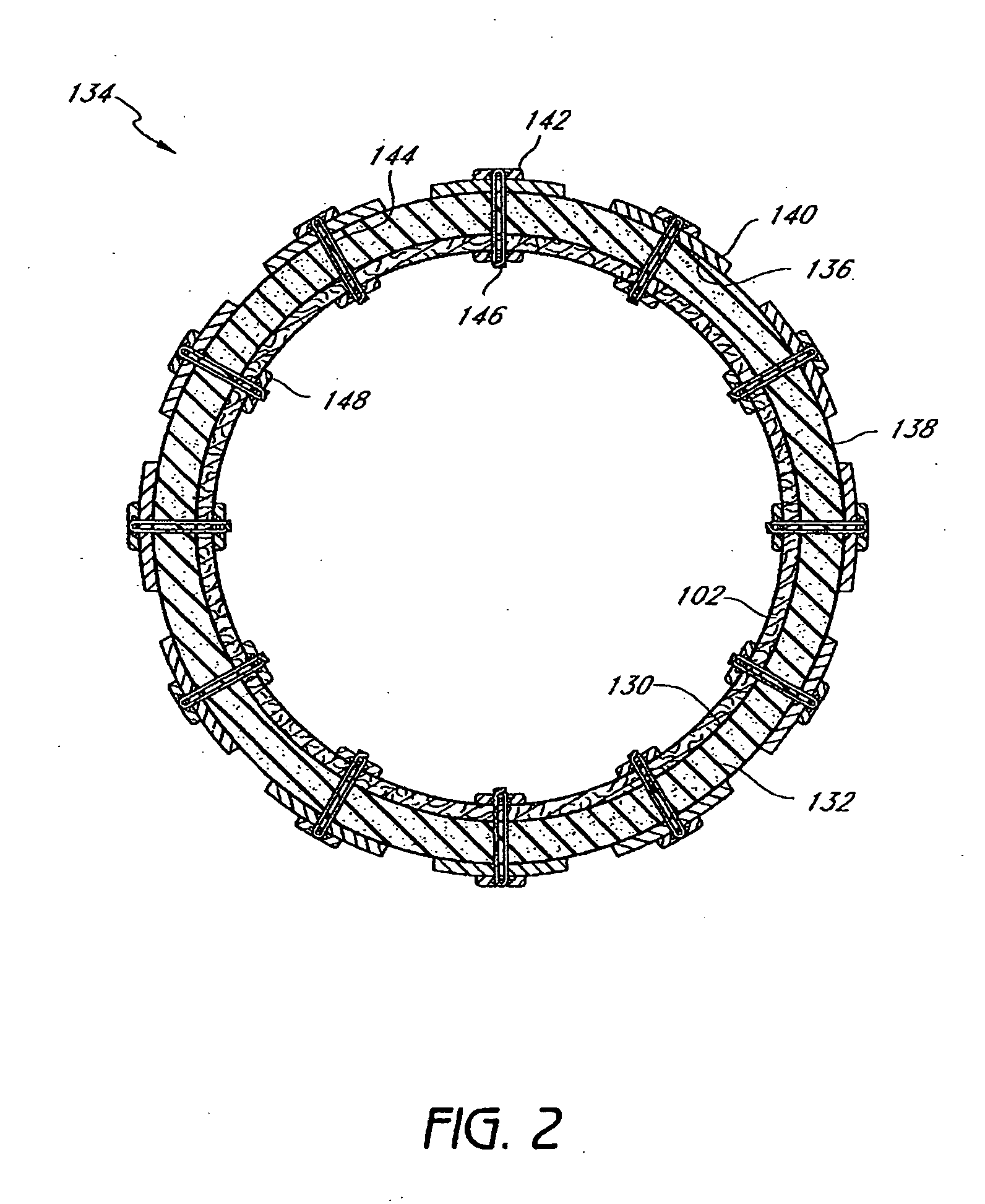
Patents
Literature
6841 results about "Gastrointestinal tract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
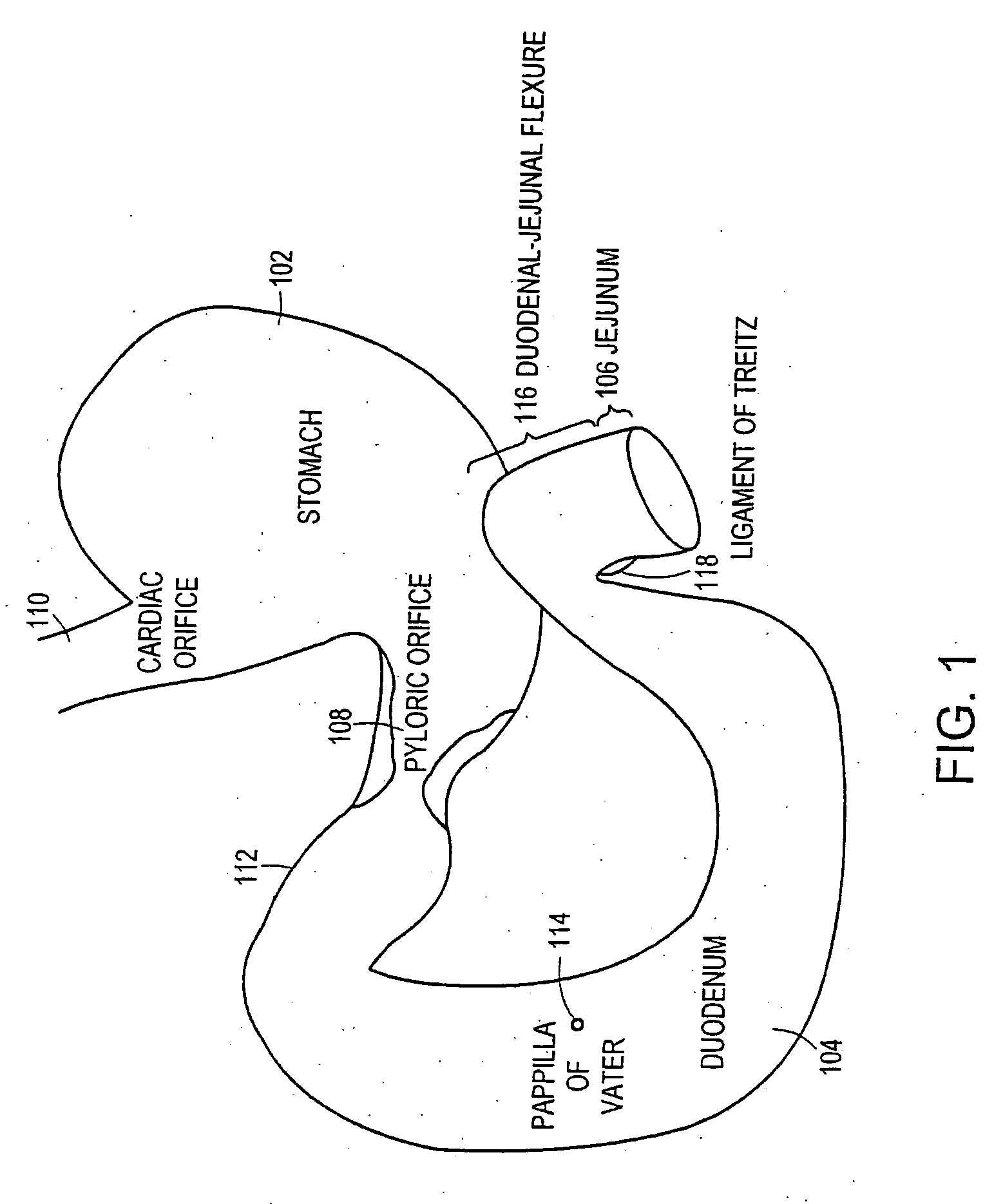
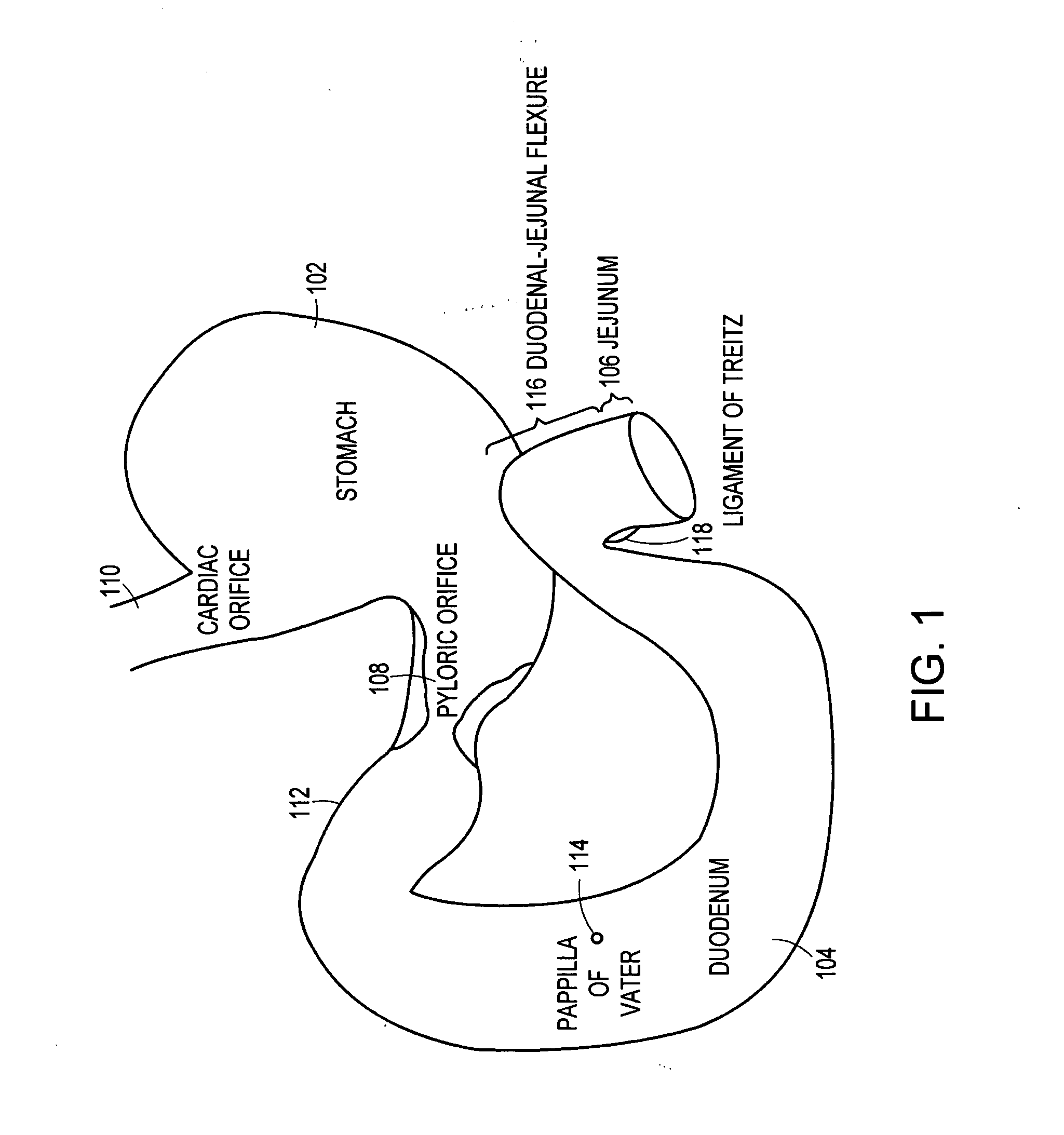
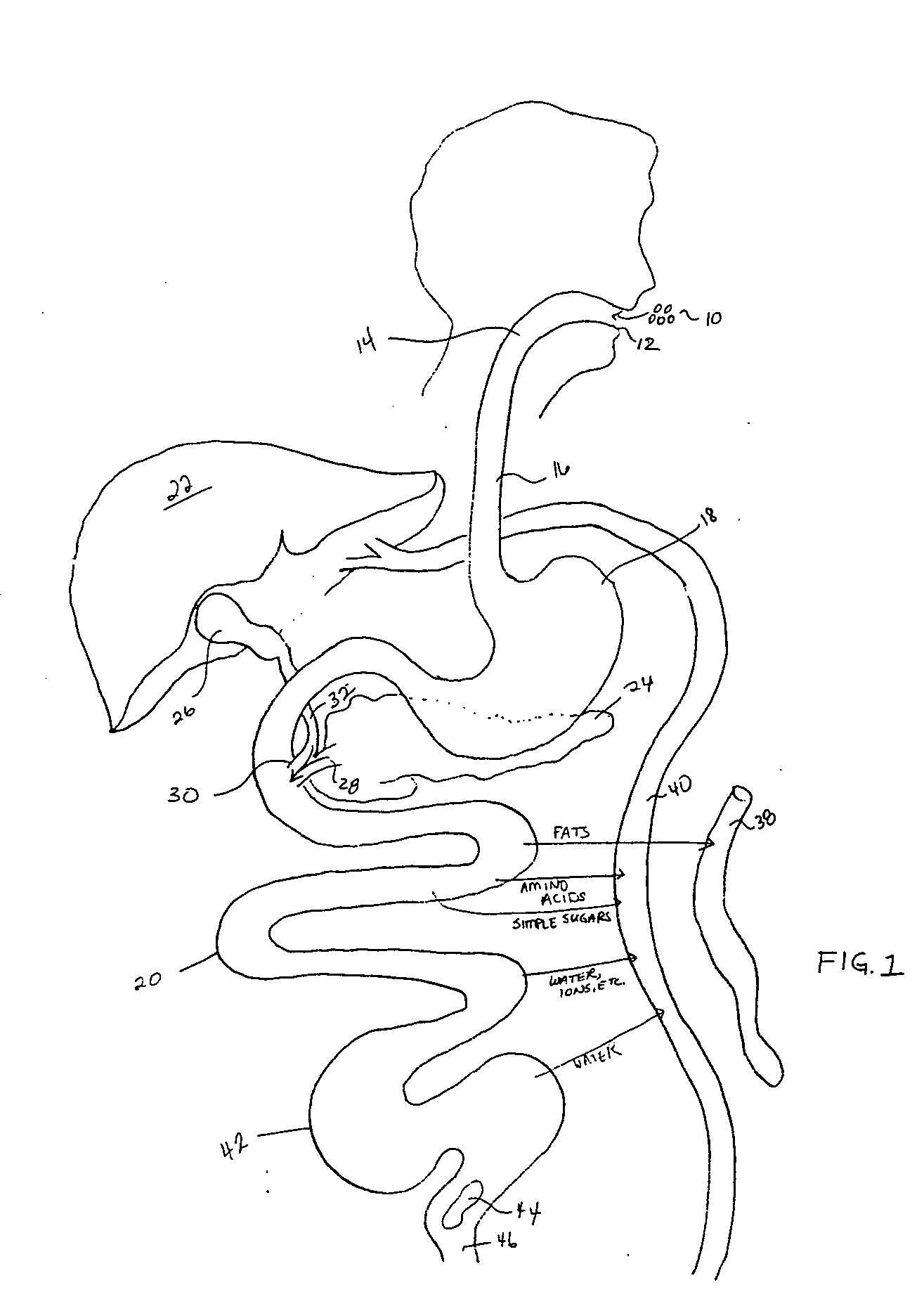
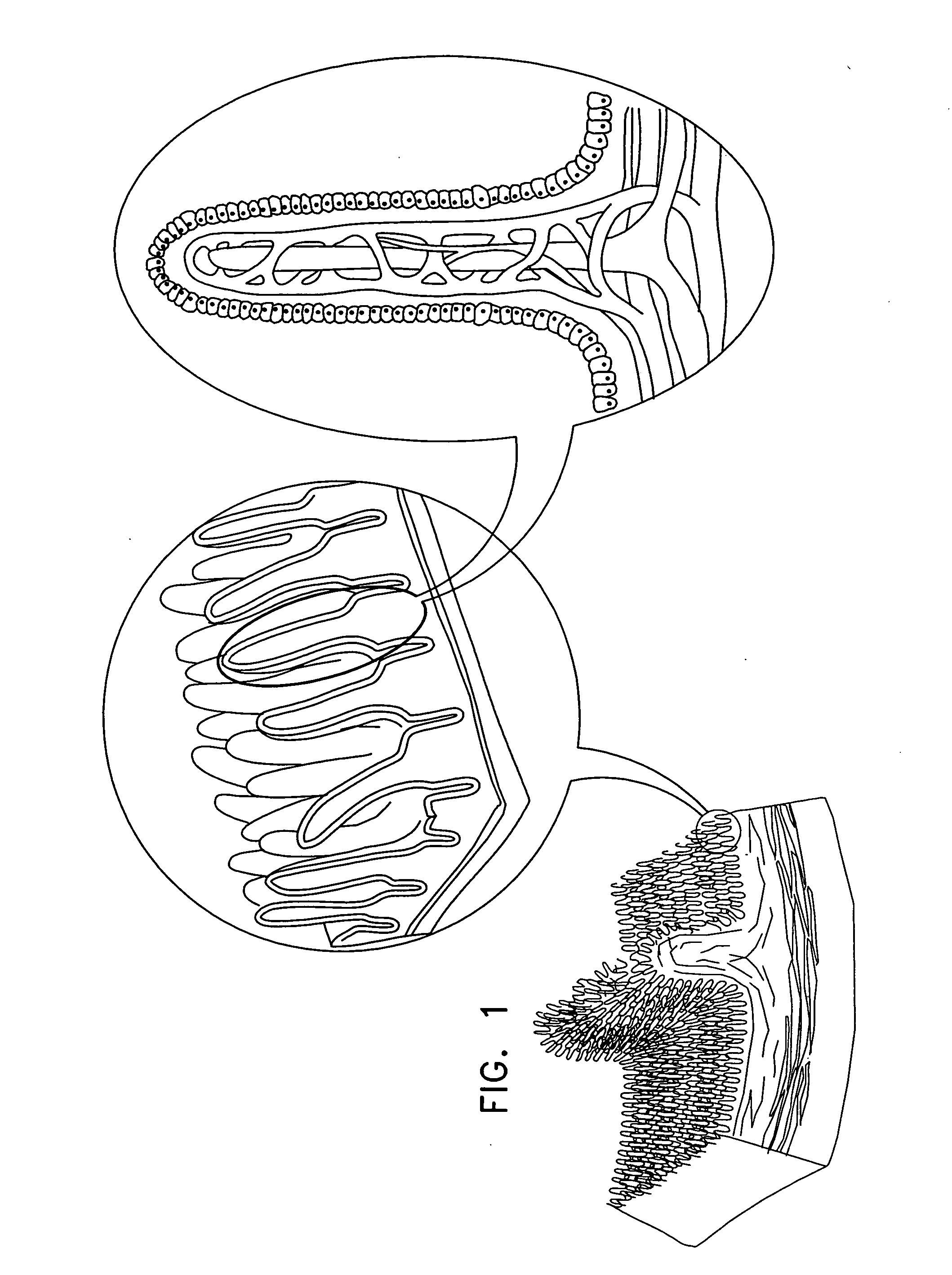
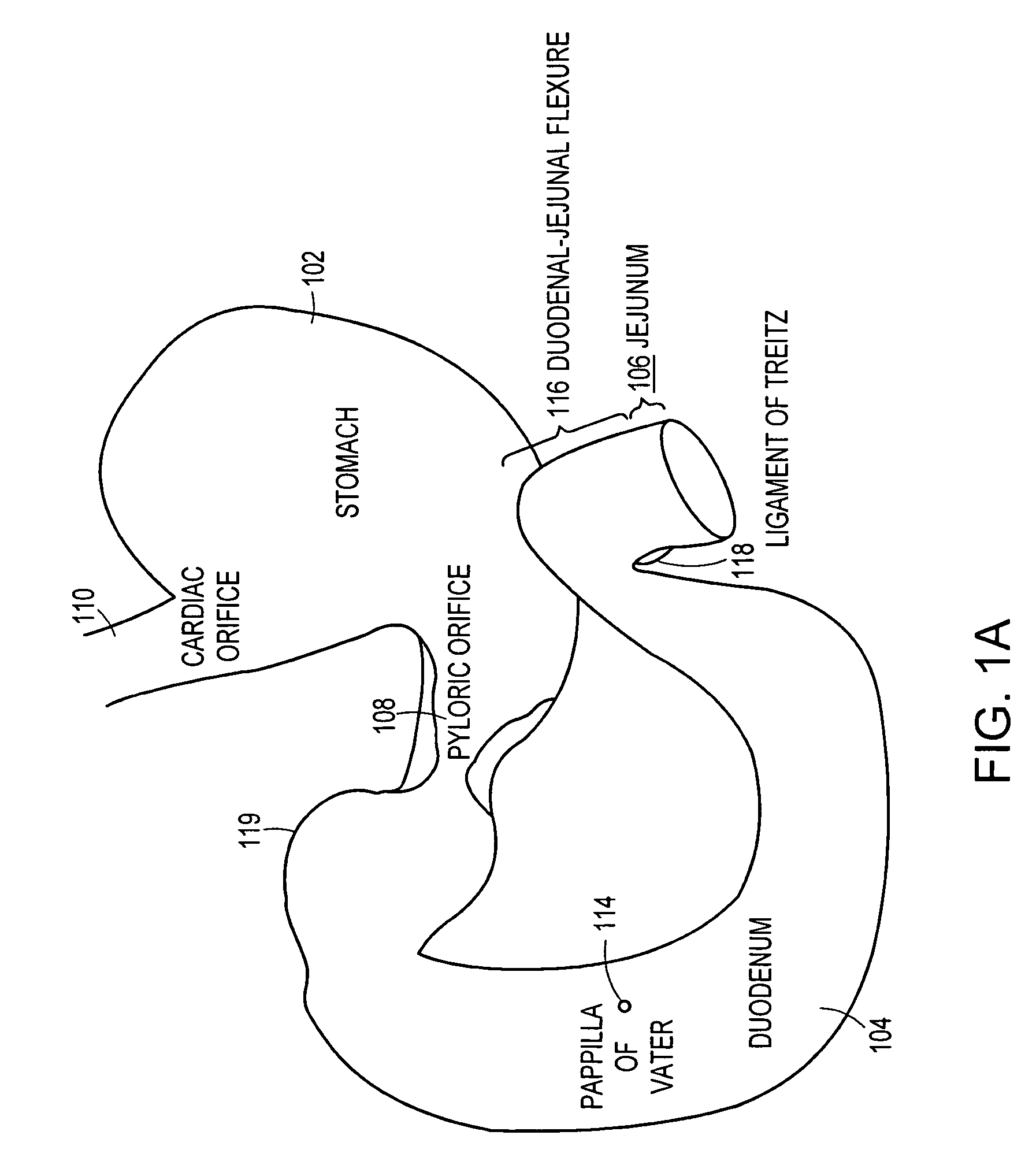
The gastrointestinal tract (digestive tract, digestional tract, GI tract, GIT, gut, or alimentary canal) is an organ system within humans and other animals which takes in food, digests it to extract and absorb energy and nutrients, and expels the remaining waste as feces. The mouth, esophagus, stomach and intestines are part of the gastrointestinal tract. Gastrointestinal is an adjective meaning of or pertaining to the stomach and intestines. A tract is a collection of related anatomic structures or a series of connected body organs.
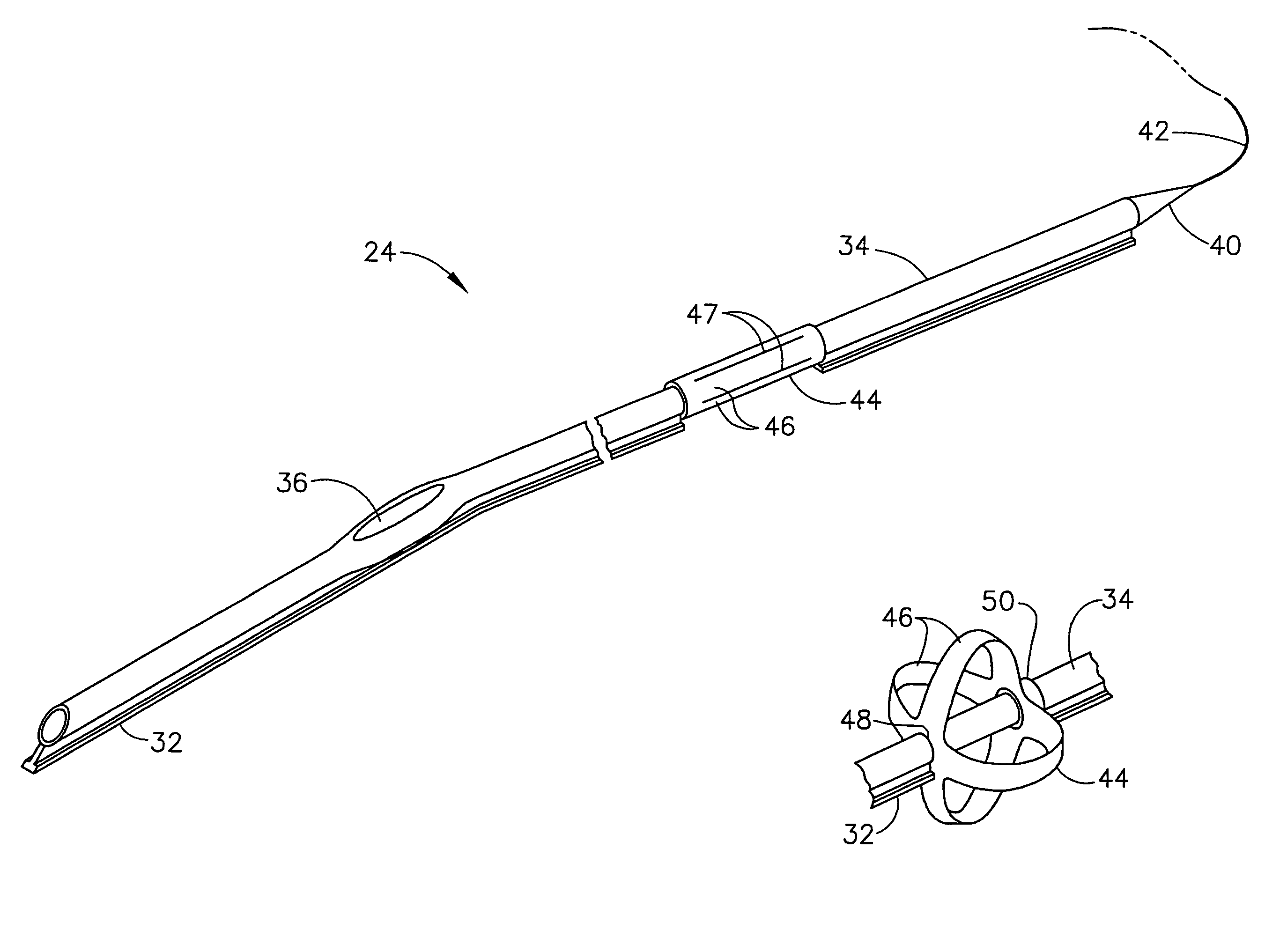
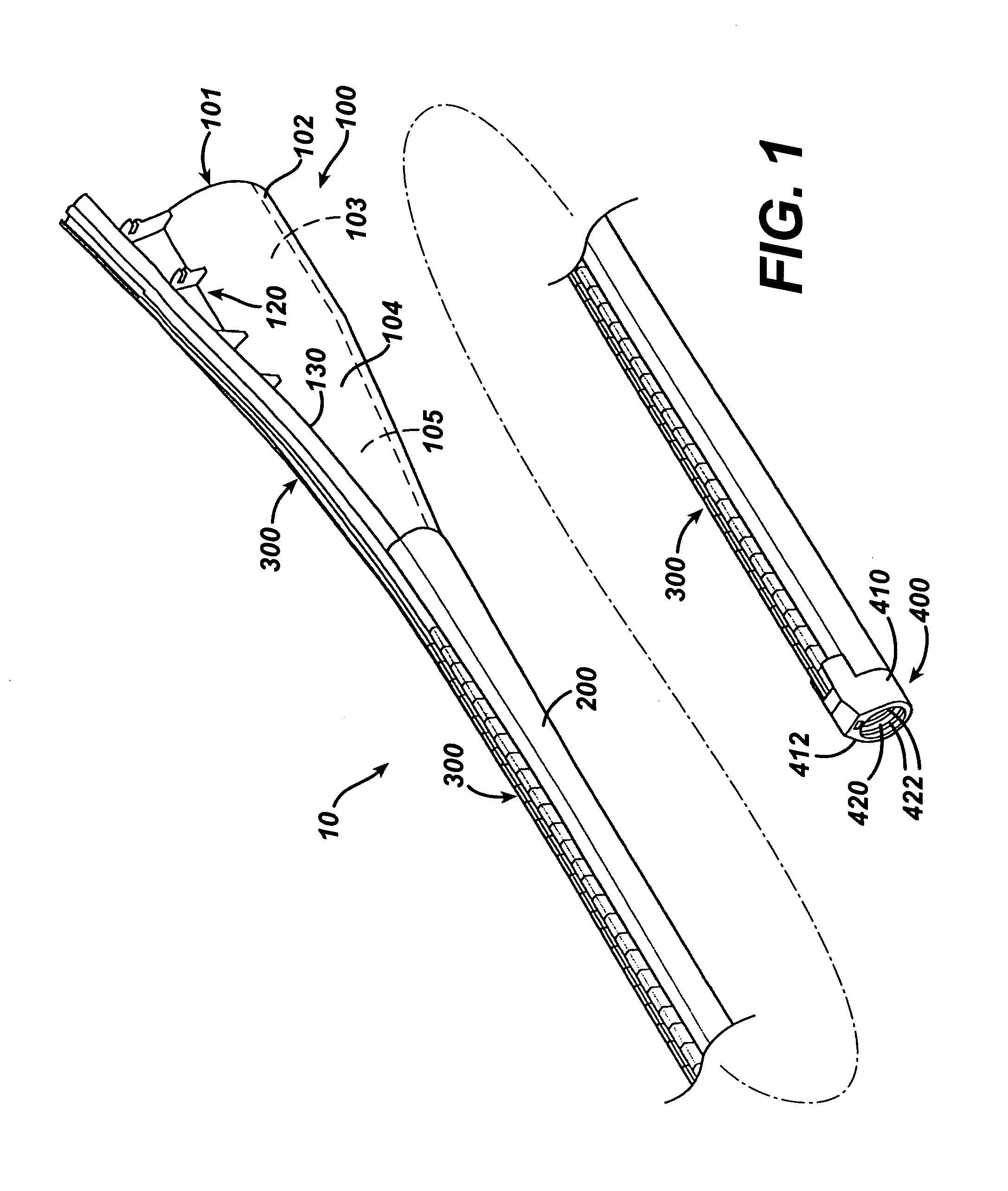
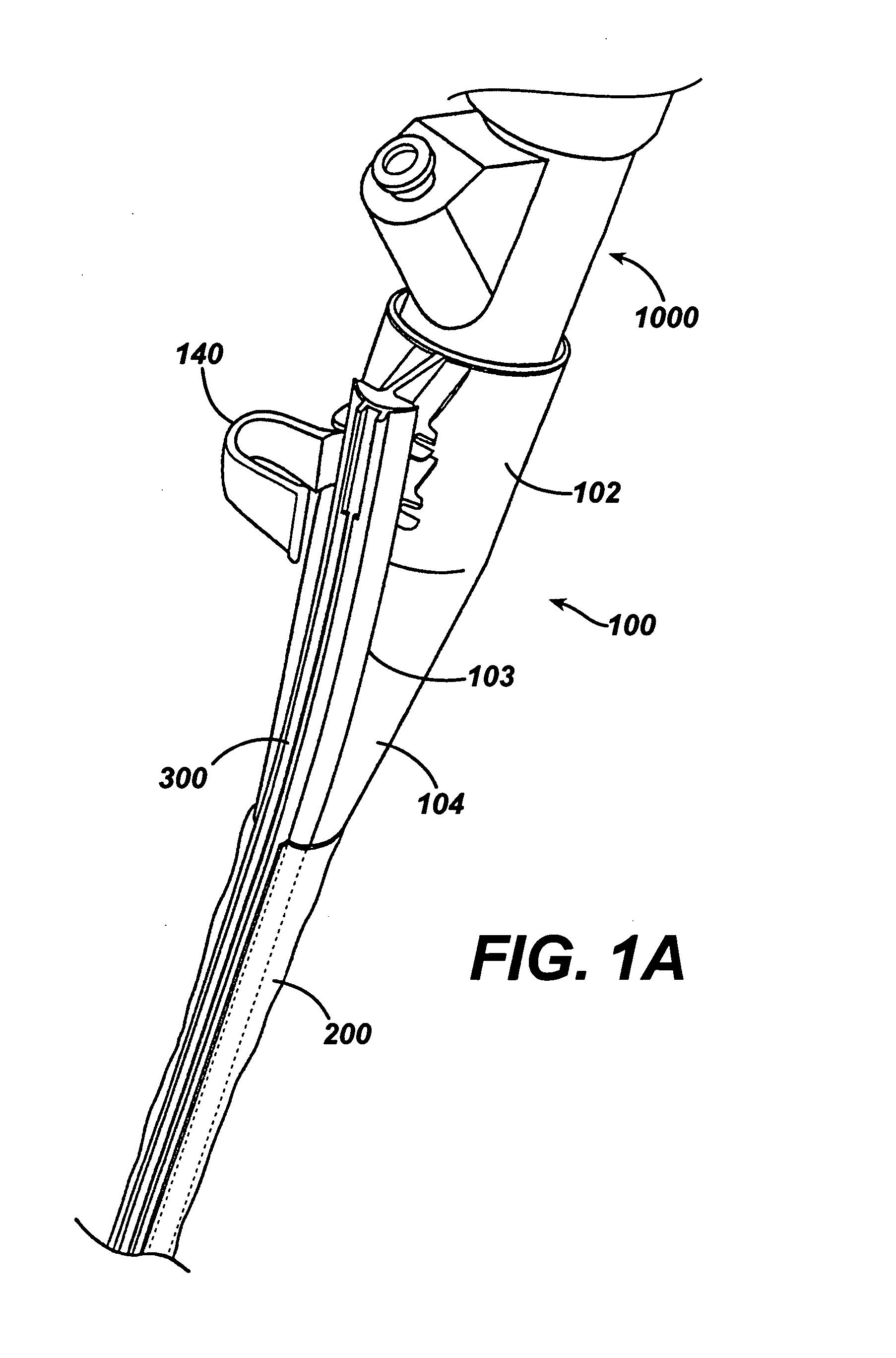
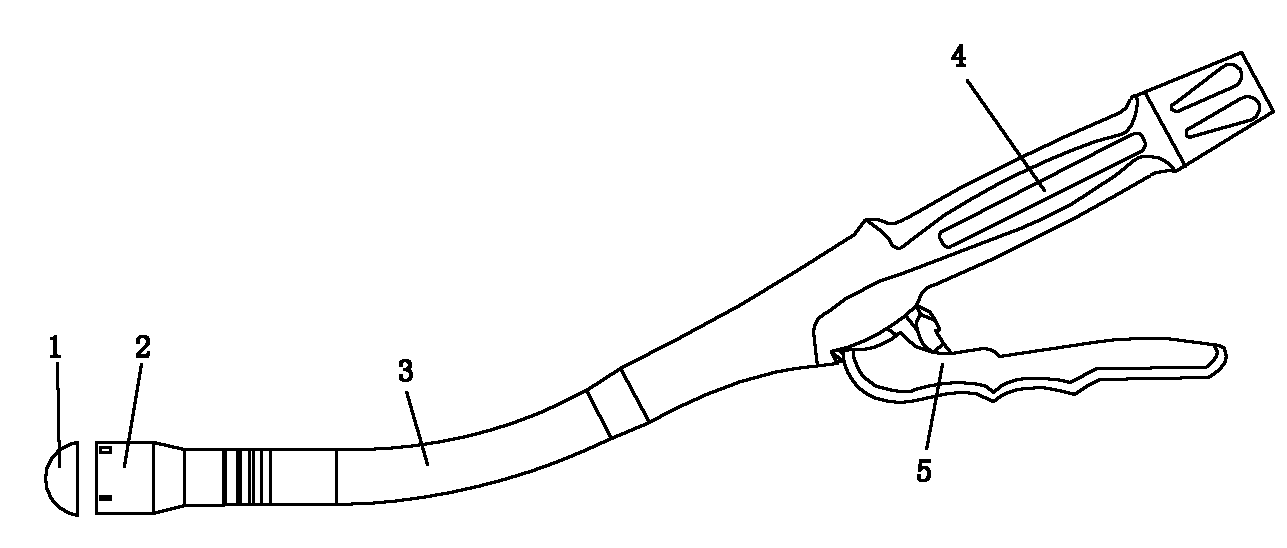
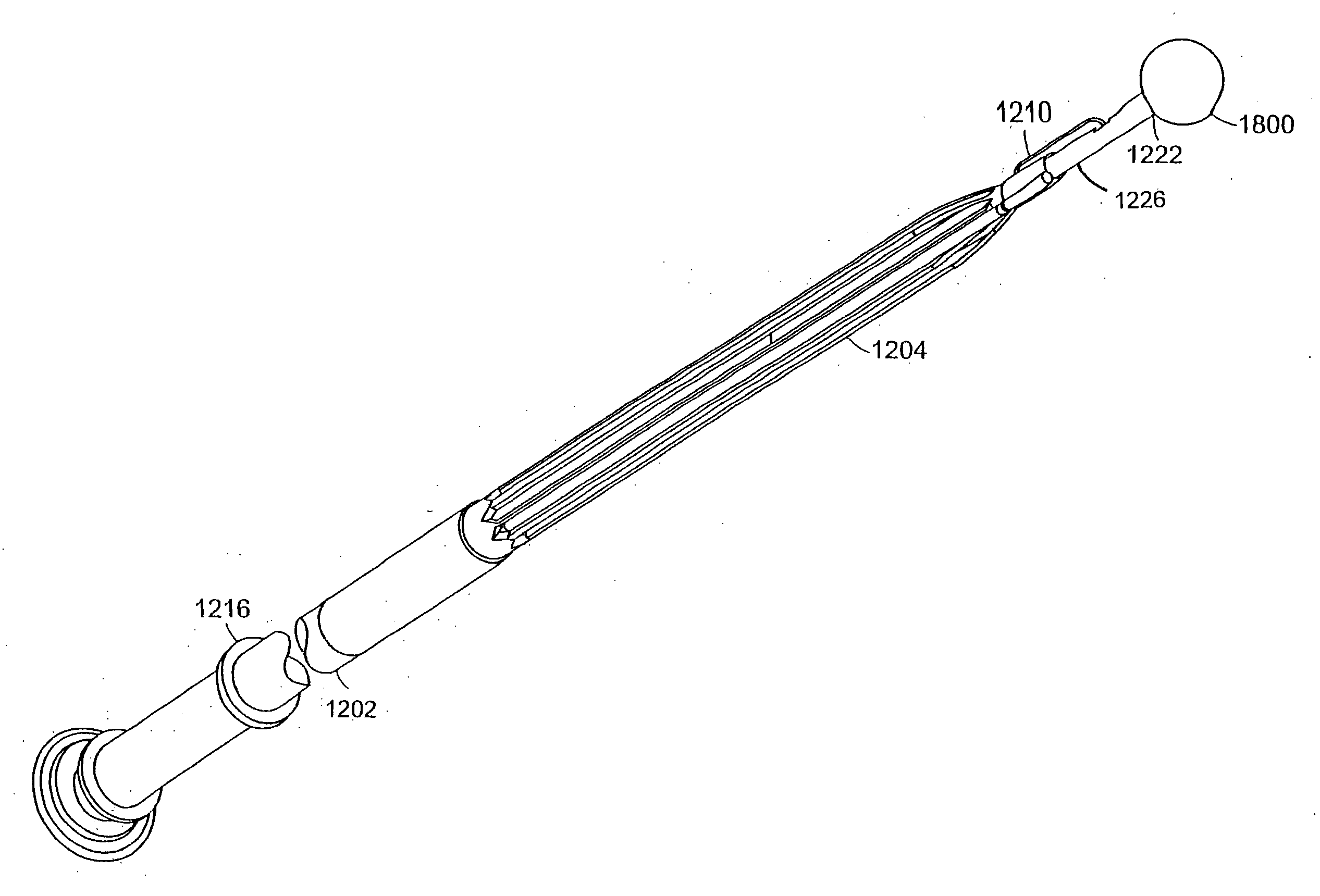
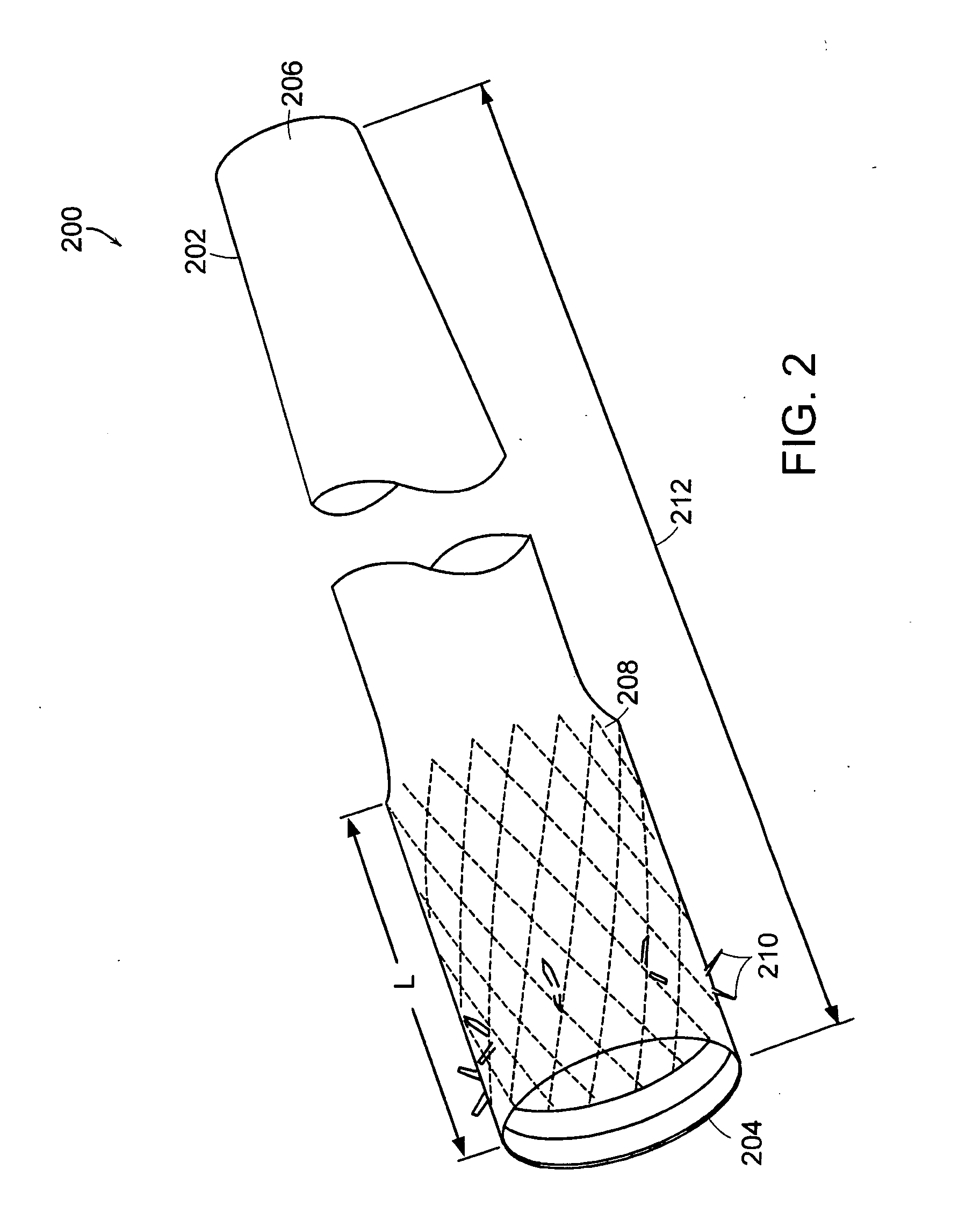
Intubation device for enteral feeding
An intubation device is provided for use with a guide apparatus having a track that is adapted to be associated with an endoscope such that bending of the track is substantially decoupled from bending of the endoscope. The intubation device includes an elongated, flexible tube and a mating member attached to the tube and adapted to slidingly engage the track external of the endoscope. The intubation device further includes a tissue bolster disposed on the proximal portion of the tube and changeable between a collapsed and an expanded configuration. The tube is positionable inside the upper gastrointestinal tract of a patient such that the proximal end of the tube is externalized through the gastric and abdominal walls of the patient, and wherein the tissue bolster is securable against the inner gastric wall when the tissue bolster is in the expanded configuration.
Owner:ETHICON ENDO SURGERY INC
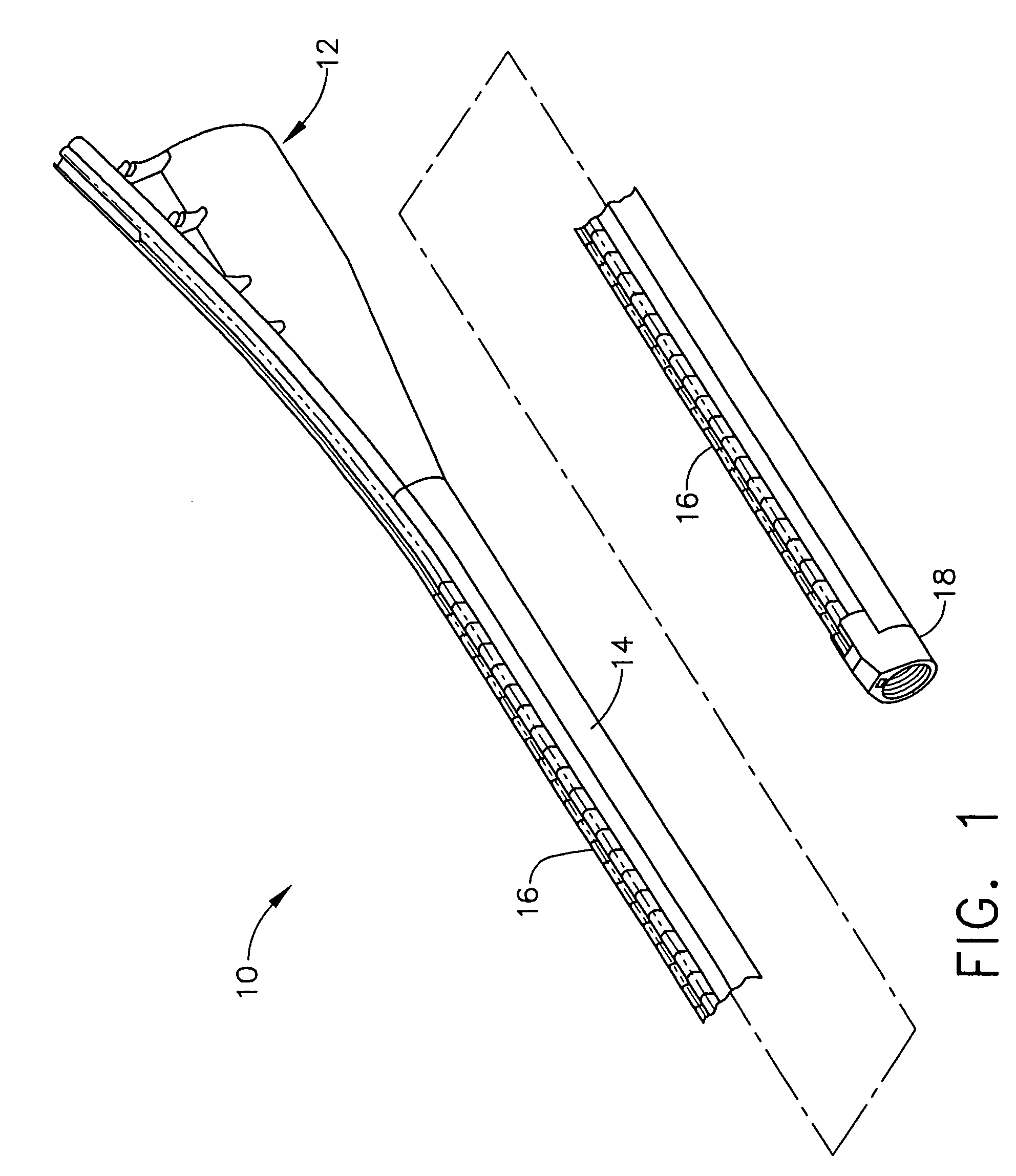
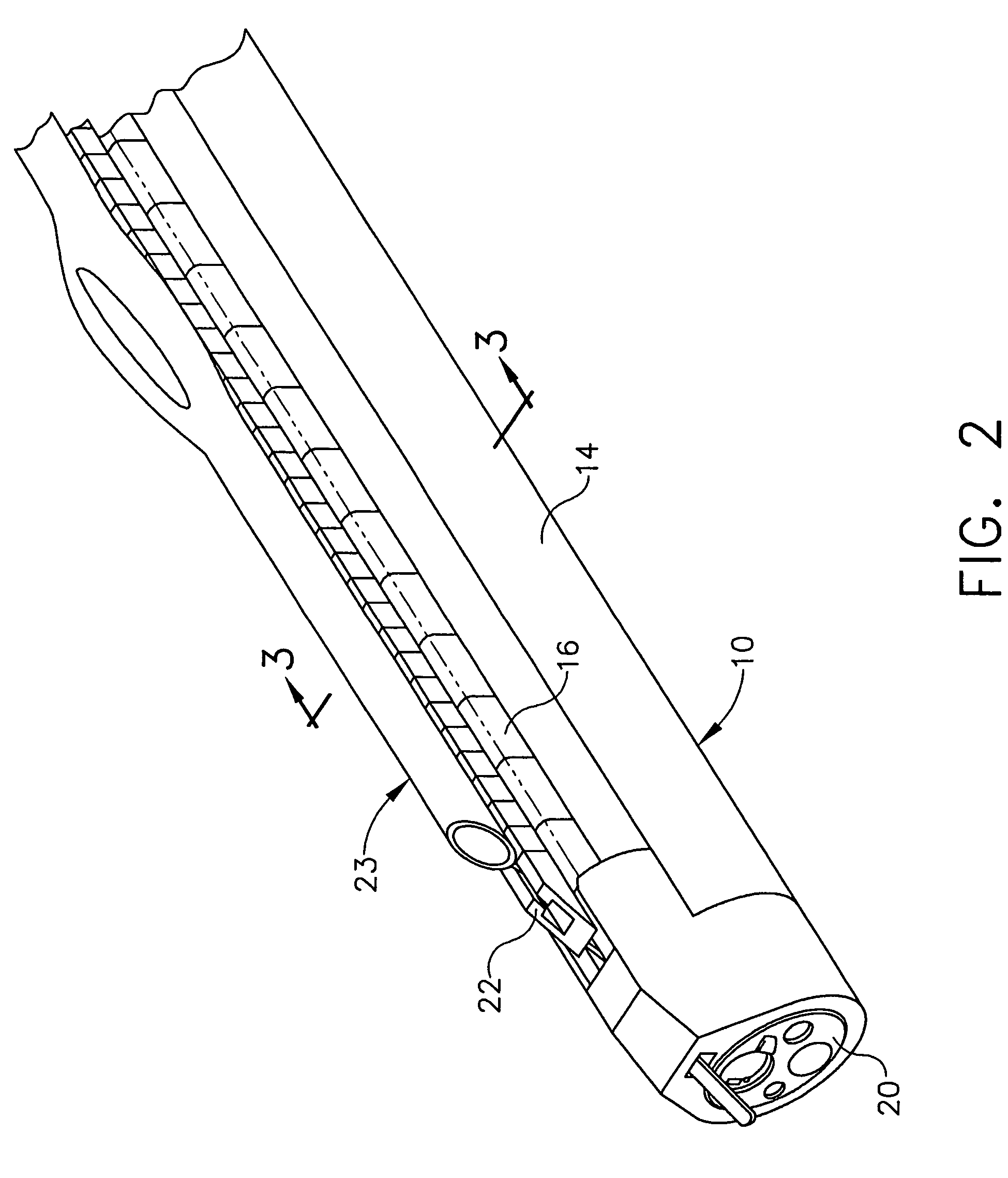
Feeding tube and track
InactiveUS20060258904A1Reduce in quantityQuickly and consistentlySurgical needlesEndoscopesFeeding tubeMedical device
A medical apparatus and method useful for positioning one or more members within the gastrointestinal tract is disclosed. The medical apparatus can include a track supported on a sheath sized to receive an endoscope, and a carrier slidable with respect to the track. A feeding tube accessory adapted to slidably engage the carrier is disclosed.
Owner:ETHICON ENDO SURGERY INC
Kit for forming implants in wall of gastrointestinal tract
InactiveUS7846085B2Inhibit injectionAvoid the needSurgical needlesMedical devicesGastrointestinal tractGeneral surgery
A kit for treating a gastrointestinal tract of a mammal and including a package and a tubular needle, an end piece and a container of an implant-forming material carried within the package. The end piece has an outer surface provided with at least one recess and an internal passageway communicating with the recess. The end piece is mounted on the distal extremity of an elongate probe member and introduced into the upper portion of the gastrointestinal tract. A portion of the wall of the gastrointestinal tract is drawn into the recess and the tubular needle is extended through the elongate probe member and the internal passageway into the portion of the wall in the recess. Material from the container is loaded into the tubular needle and injected into the portion of the wall to form an implant in the wall.
Owner:BOSTON SCI SCIMED INC
Devices and methods for pyloric anchoring
ActiveUS20050055039A1Avoiding erosion and ulcerationSuture equipmentsElectrotherapyPylorusPatient characteristics
A device for performing one or more functions in a gastrointestinal tract of a patient includes an anchoring member and at least one actuator, sensor, or combination of both coupled with the anchoring device. The anchoring device is adapted to maintain at least part of the device within a pyloric portion of the patient's stomach and to intermittently engage, without directly attaching to, stomach tissue. Actuators perform any suitable function, such as transmitting energy to tissue, acting as a sleeve to reduce nutrient absorption, occupying space in the stomach, eluting a drug and / or the like. Sensors may be adapted to sense any suitable patient characteristic within the patient's gastrointestinal tract, such as pH, temperature, bile content, nutrient content, fats, sugars, alcohol, opiates, drugs, analytes, electrolytes and / or hemoglobin.
Owner:BARONOVA
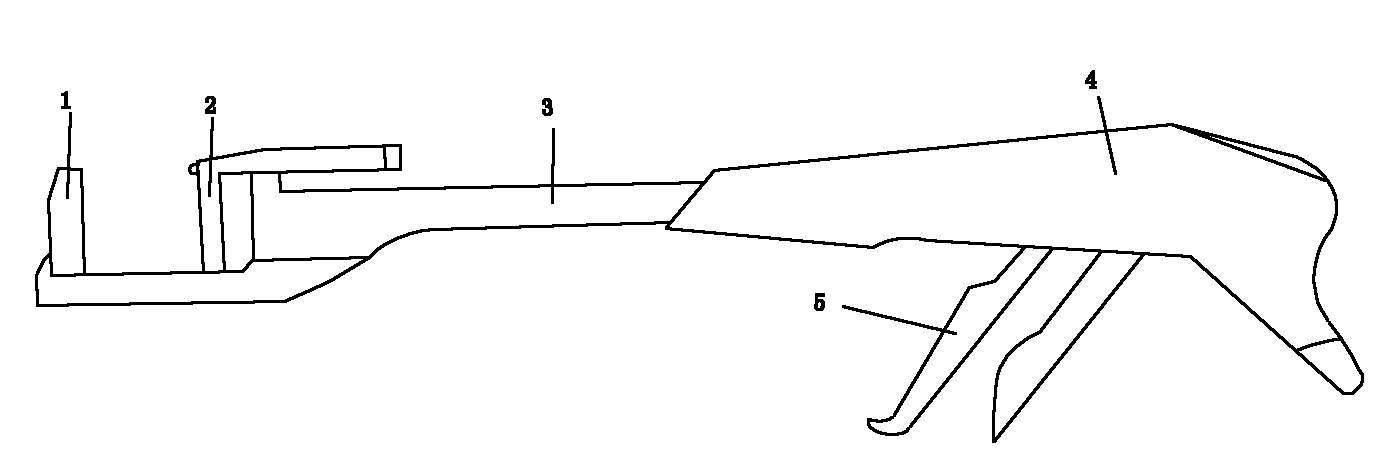
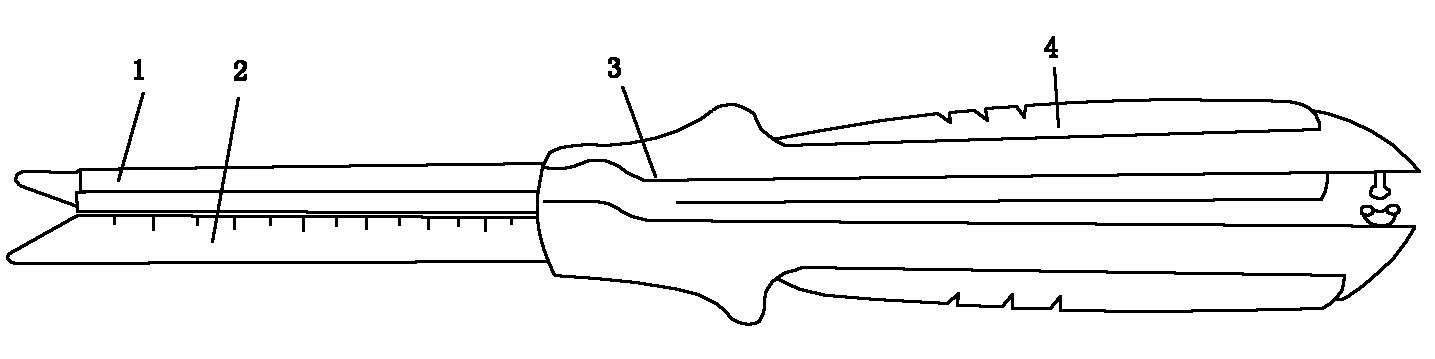
Sandwiched stapler type alimentary tract anastomosis dissecting sealer
The invention discloses a sandwiched stapler type alimentary tract anastomosis dissecting sealer, relating to the surgical instruments. The invention provides a sandwiched stapler type alimentary tract anastomosis dissecting sealer which can prevent the alimentary tract anastomotic stoma from tearing, leaking and errhysis from the cut section in surgical operations. The sandwiched stapler type alimentary tract anastomosis dissecting sealer is provided with a nail anvil (which is also called as a bottom needle holder), a nail cartridge (which is also called as a nail anvil box), a sealer body, a movable handle, an adjusting button, a cutter cushion and an anastomosing nail (which is also called as a suturing nail), wherein the nail anvil is connected with the front end of the sealer body, the adjusting button is arranged at the tail part of the sealer body, the movable handle is arranged at the back of the sealer body, a safety button is arranged at the connection of the movable handle and the sealer body, the cutter cushion is arranged on the nail anvil, the anastomosing nail is arranged at the front end part of the sealer body, a membrane is arranged on the surface of the nail anvil (which is also called as the bottom needle holder), and a membrane is also arranged on the surface of the nail cartridge (which is also called as the nail anvil box); and after stapling, a sandwich structure consisting of the membrane, an intestinal wall and the membrane is formed.
Owner:刘忠臣
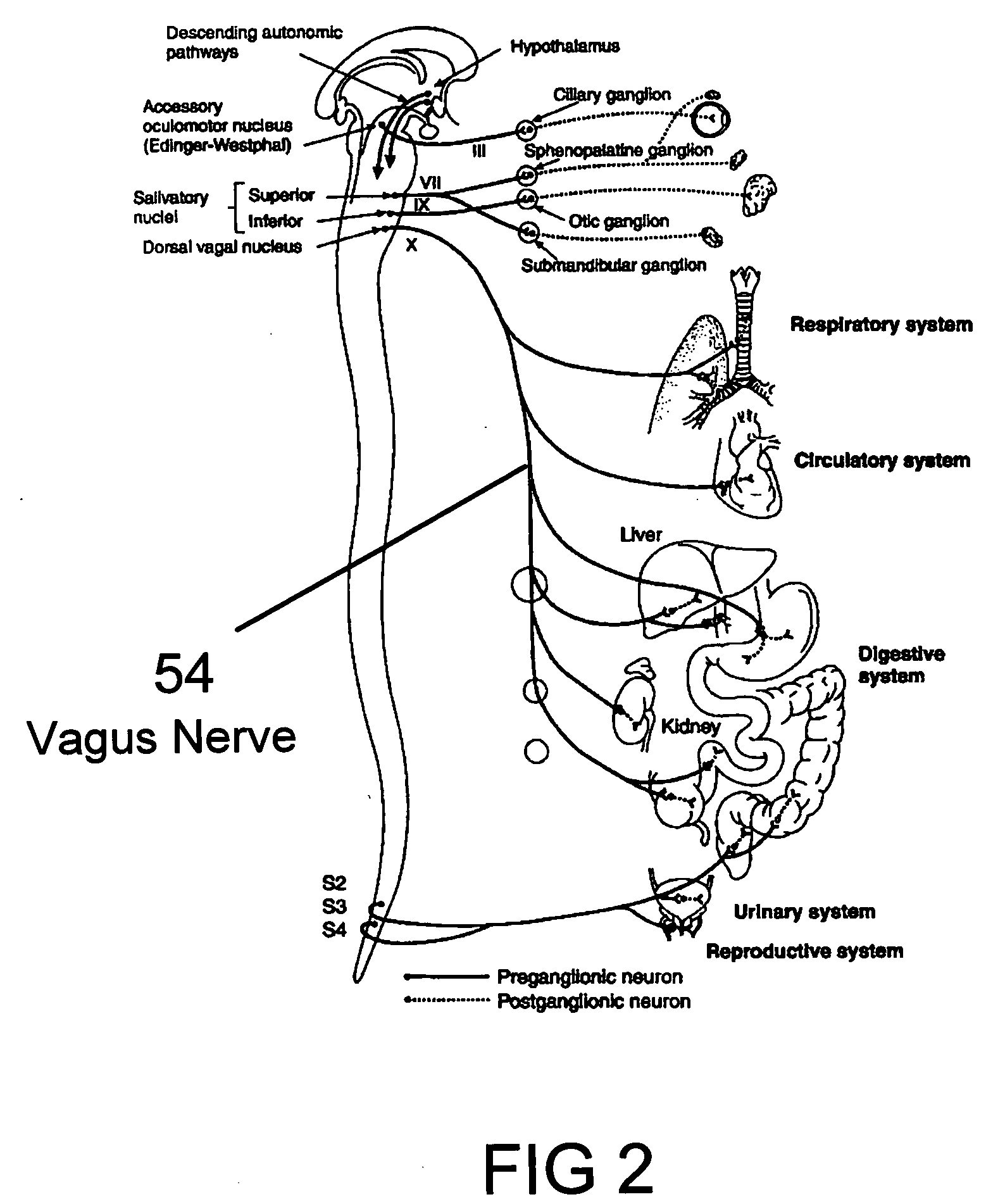
Method, apparatus, and surgical technique for autonomic neuromodulation for the treatment of disease
InactiveUS20060167498A1Reduce or prevent conditionReducing and preventing symptomSpinal electrodesSurgical needlesSplanchnic nervesDisease
The present invention teaches a method and apparatus for physiological modulation, including neural and gastrointestinal modulation, for the purposes of treating several disorders, including obesity, depression, epilepsy, and diabetes. This includes chronically implanted neural and neuromuscular modulators, used to modulate the afferent neurons of the sympathetic nervous system to induce satiety. Furthermore, this includes neuromuscular stimulation of the stomach to effect baseline and intermittent smooth muscle contraction to increase gastric intraluminal pressure, which induces satiety, and stimulate sympathetic afferent fibers, including those in the sympathetic trunk, splanchnic nerves, and greater curvature of the stomach, to augment the perception of satiety.
Owner:DILORENZO BIOMEDICAL
Capsule and method for treating or diagnosing the intestinal tract
ActiveUS7160258B2Comprehensive knowledgeElectrotherapyPerson identificationMedicineElectrical stimulations
A device and method for mapping, diagnosing and treating the intestinal tract is provided using a capsule passing through the intestinal tract. Further, a capsule tracking system is provided for tracking a capsule's location along the length of an intestinal tract as various treatment and / or sensing modalities are employed. In one variation, an acoustic signal is used to determine the location of the capsule. A map of sensed information may be derived from the pass of a capsule. Capsules may be subsequently passed through to treat the intestinal tract at a determined location along its length. One variation uses an electrical stimulation capsule to treat and / or diagnose a condition in the intestinal tract.
Owner:ENTRACK INC
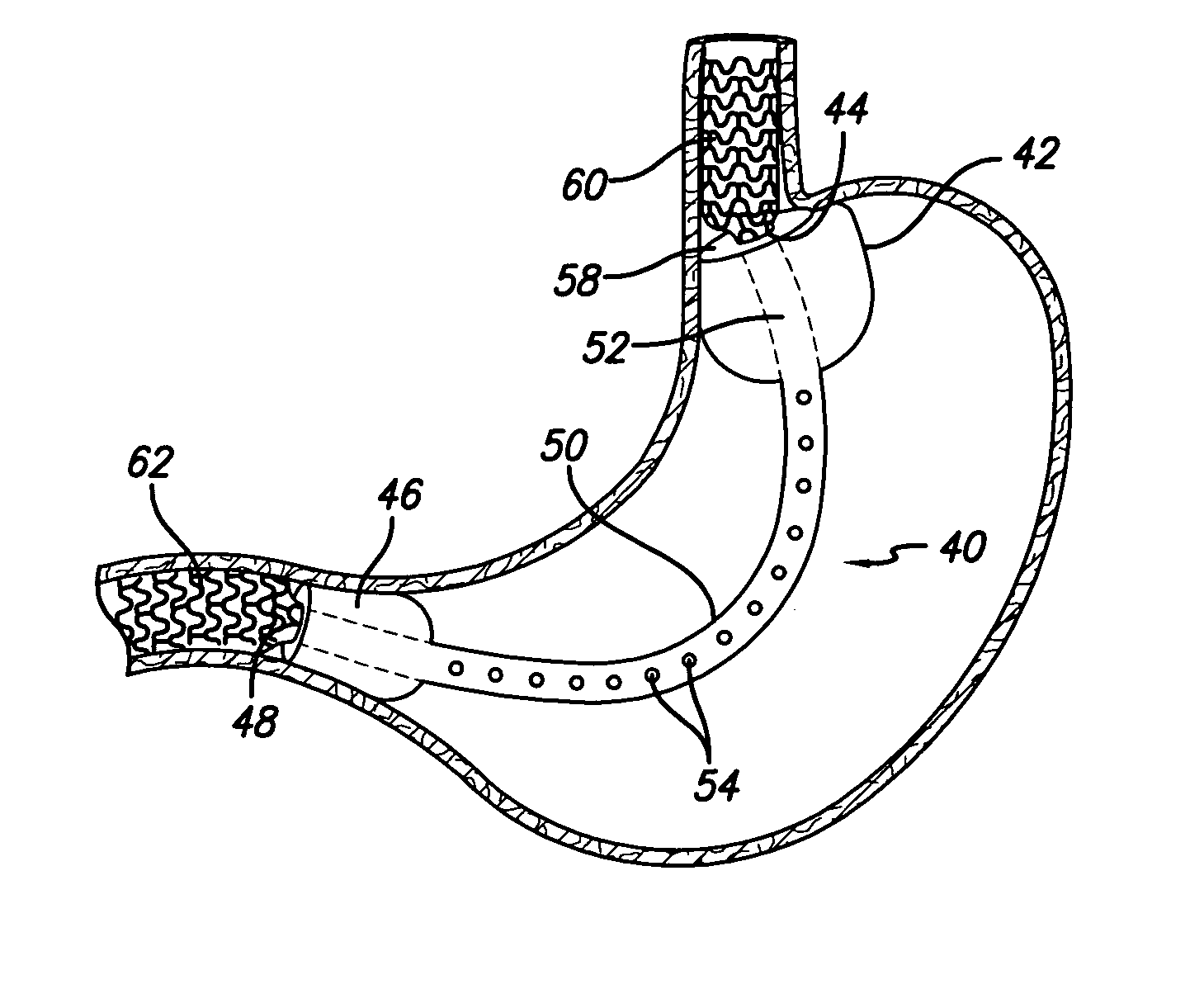
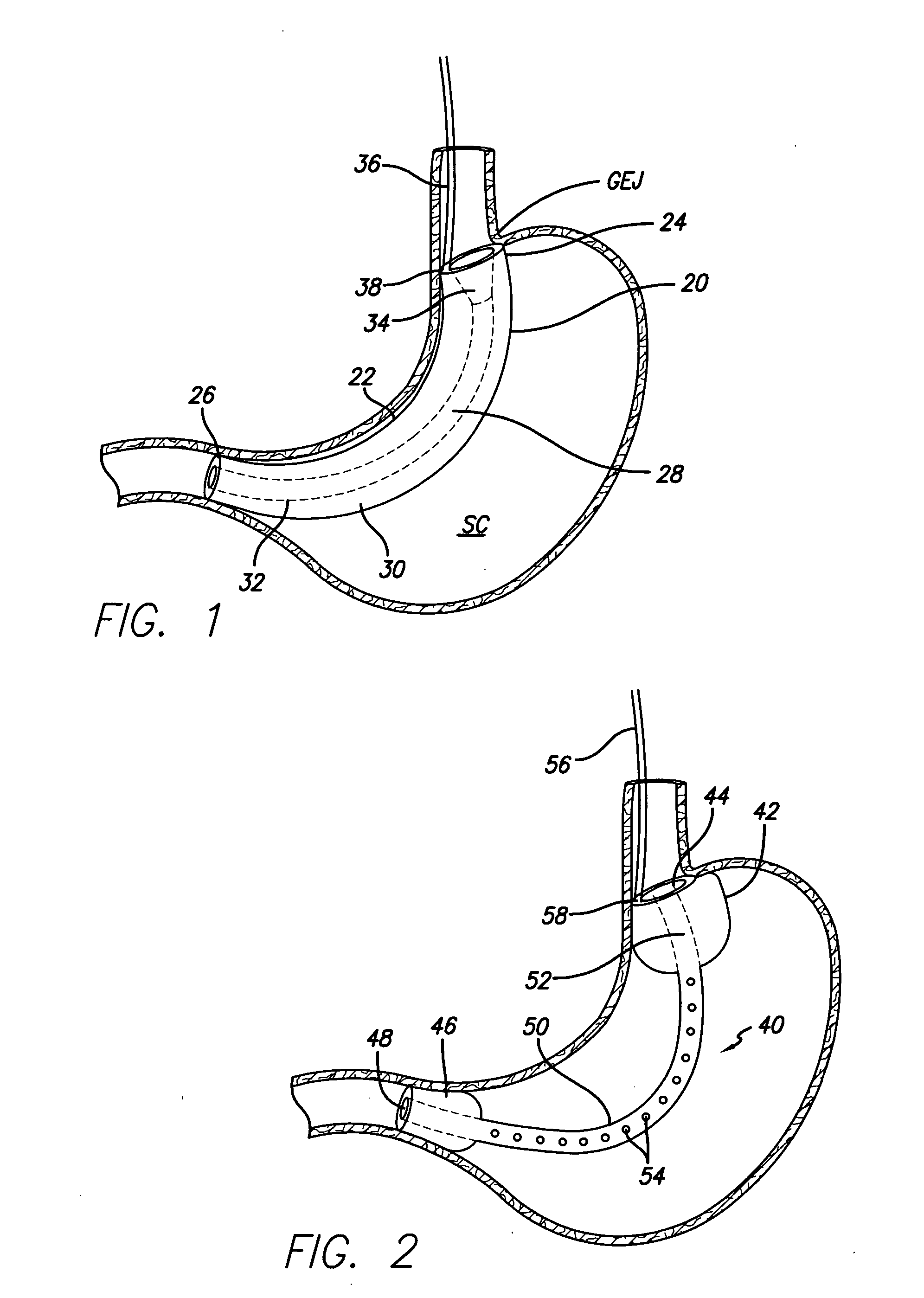
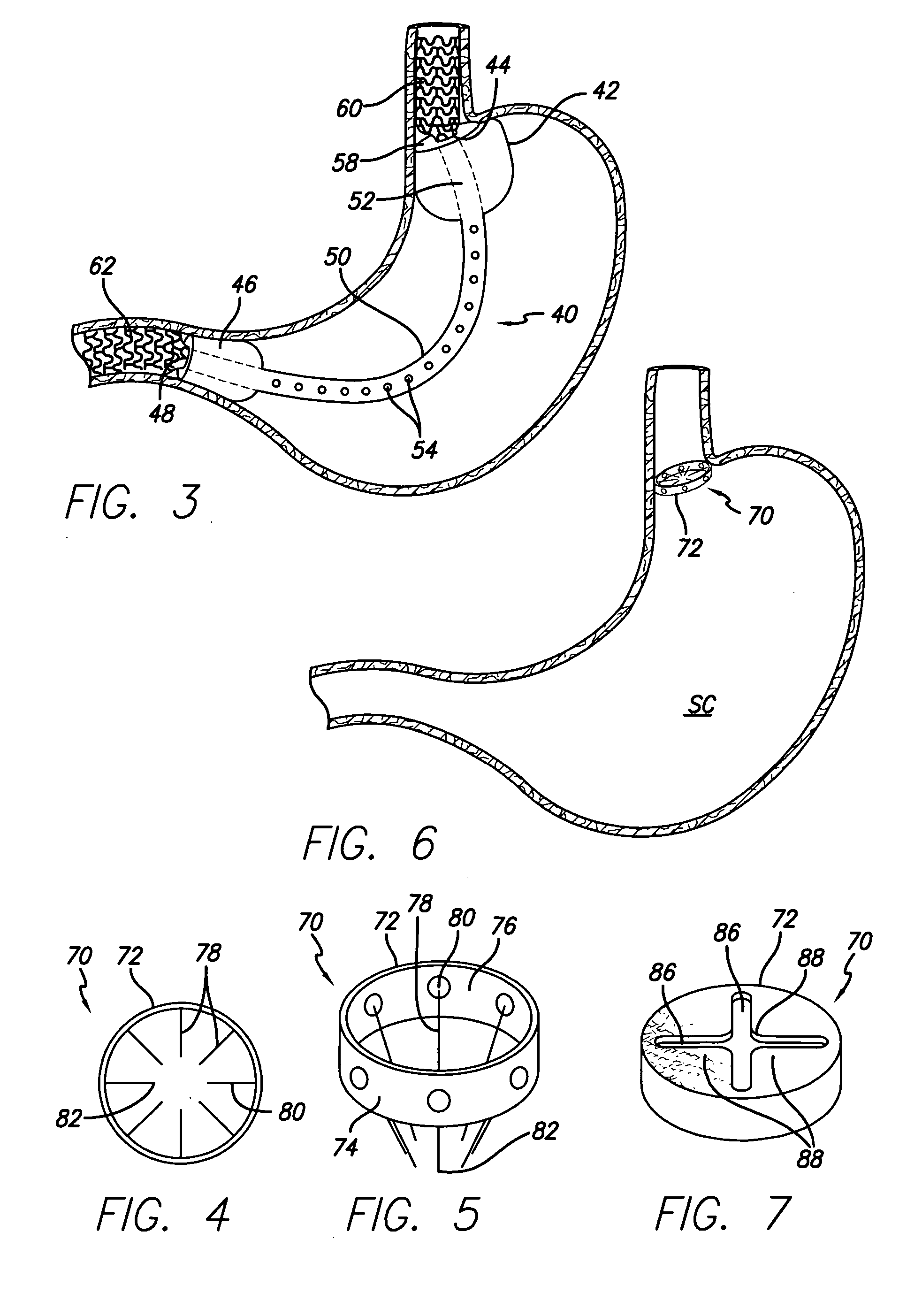
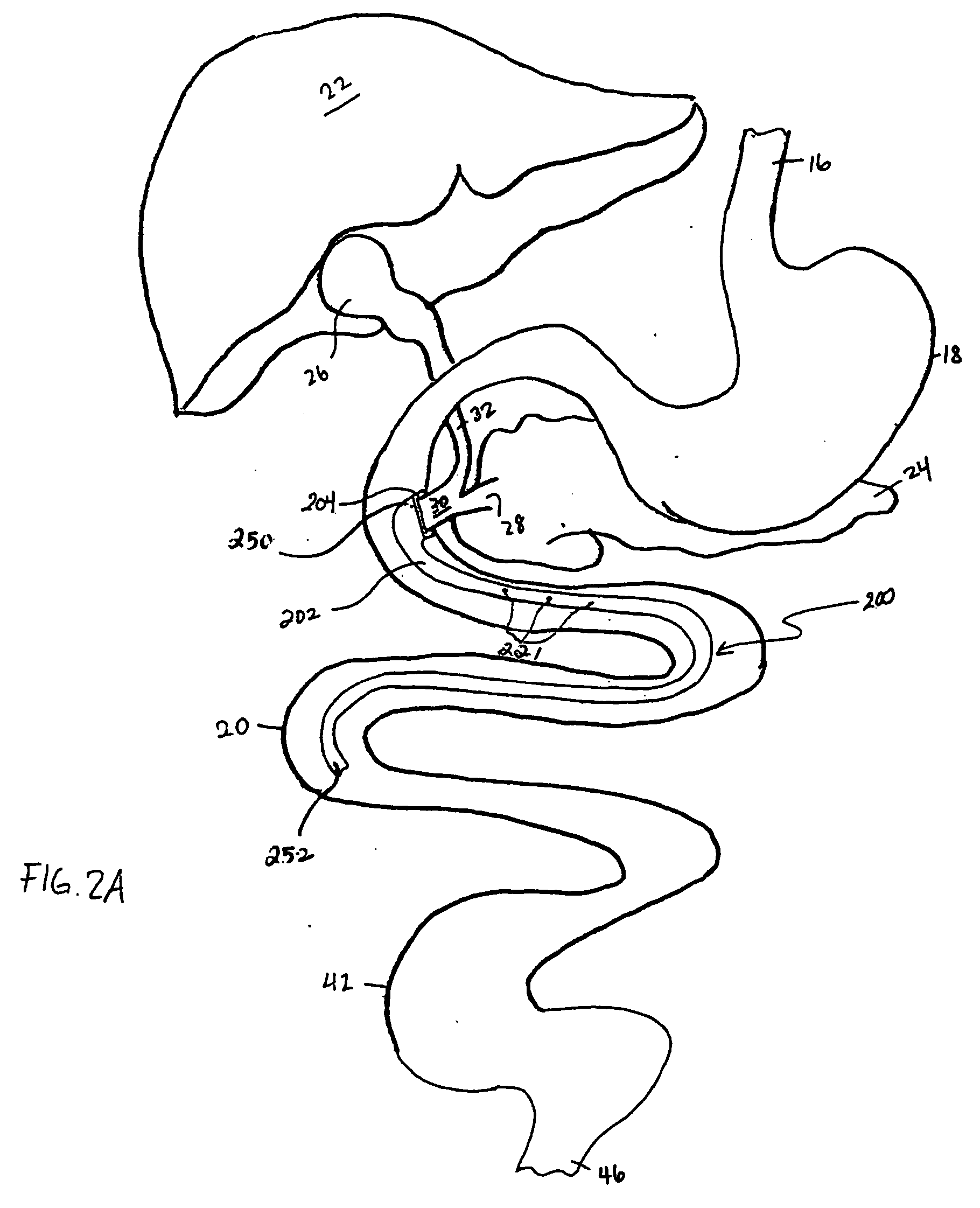
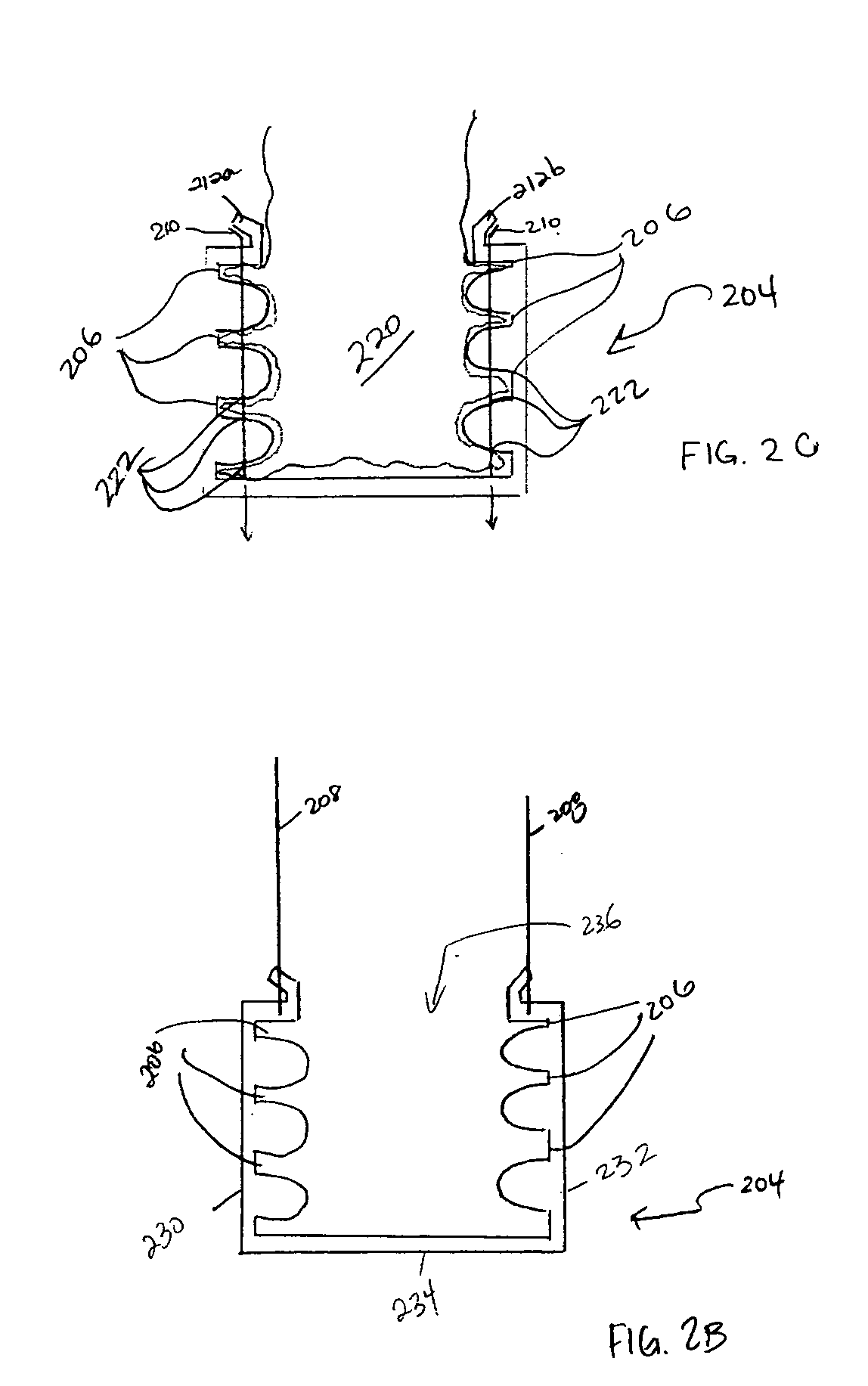
Methods and apparatus for anchoring within the gastrointestinal tract
ActiveUS20050125020A1Minimize traumaLarge caliberSuture equipmentsStentsIntestinal structureMedical device
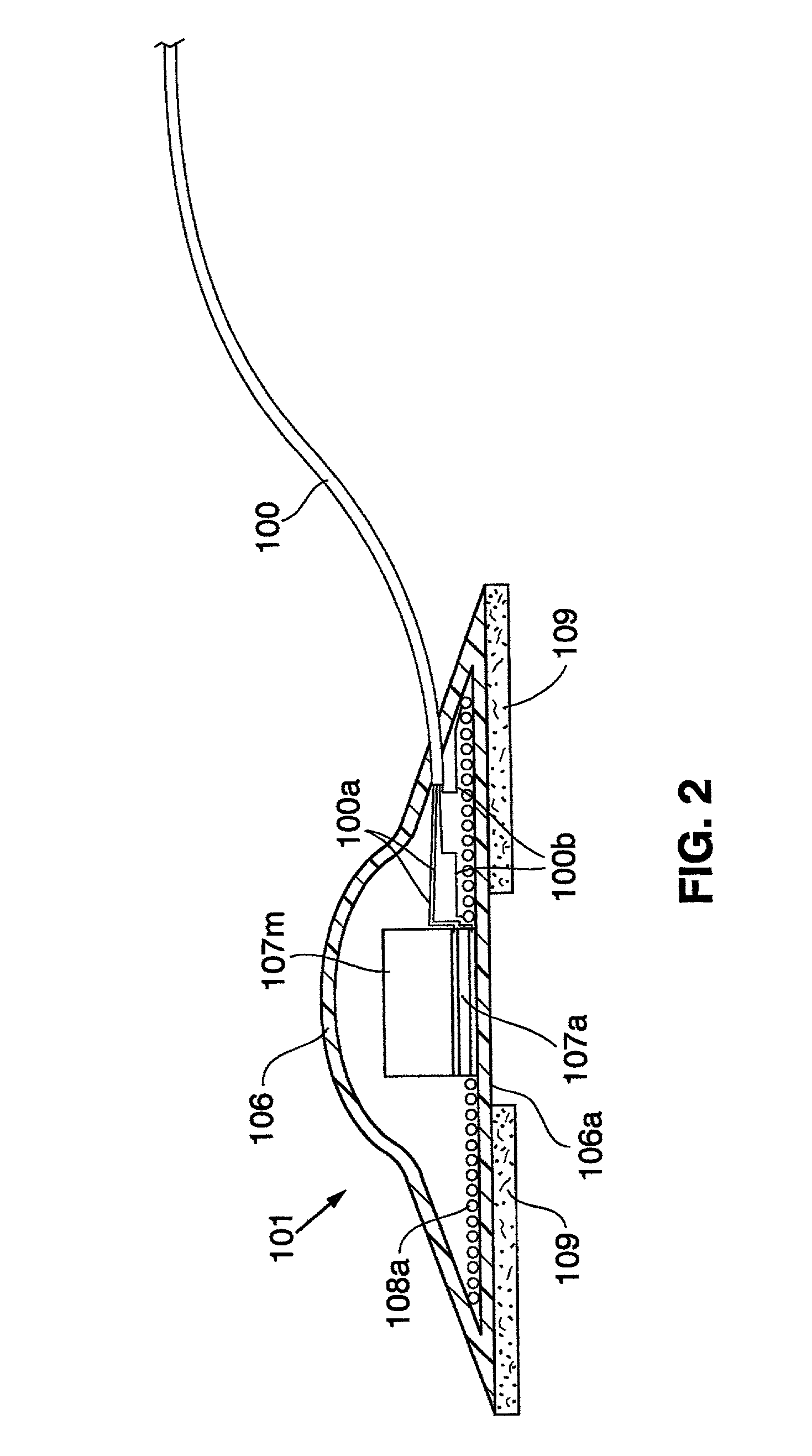
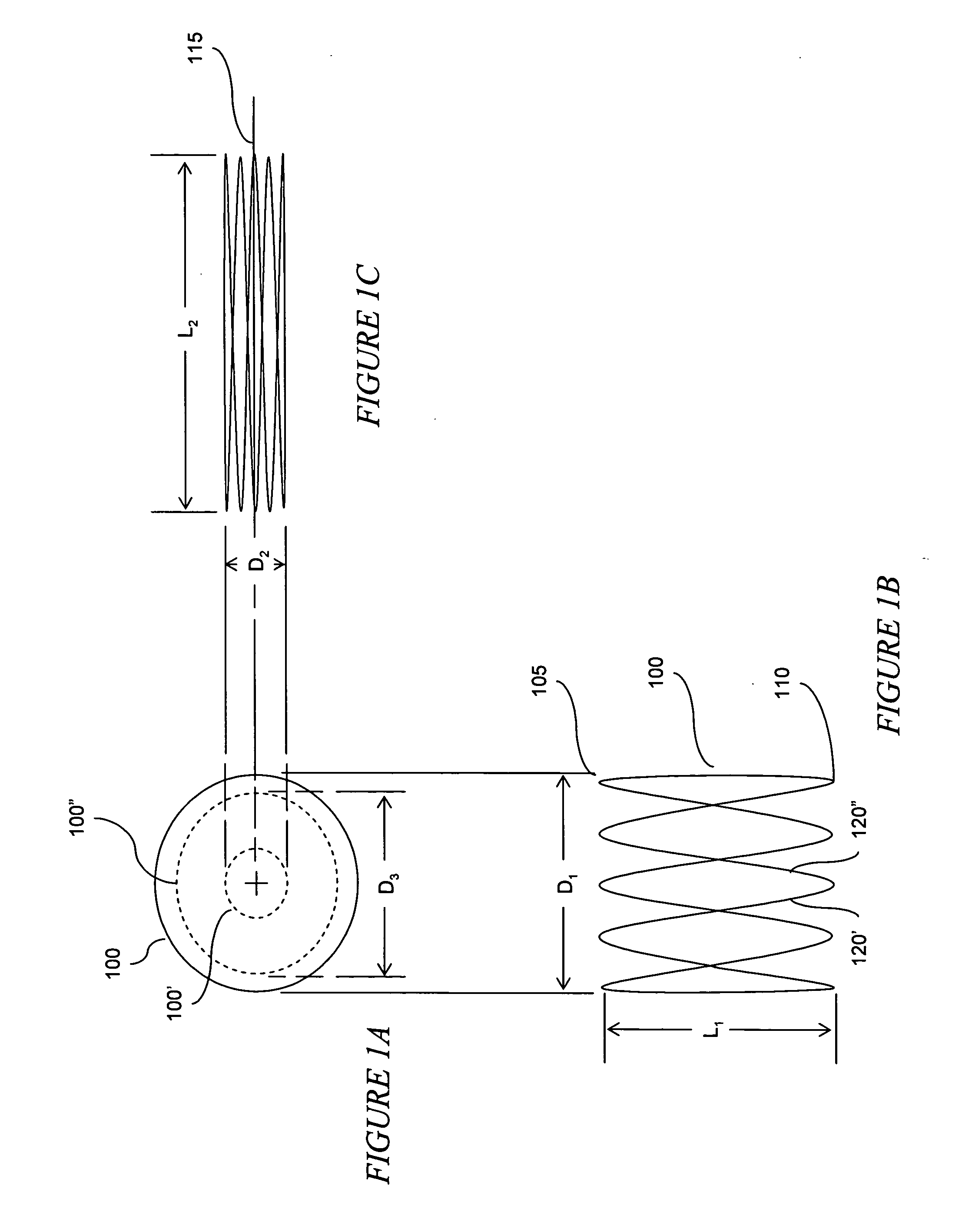
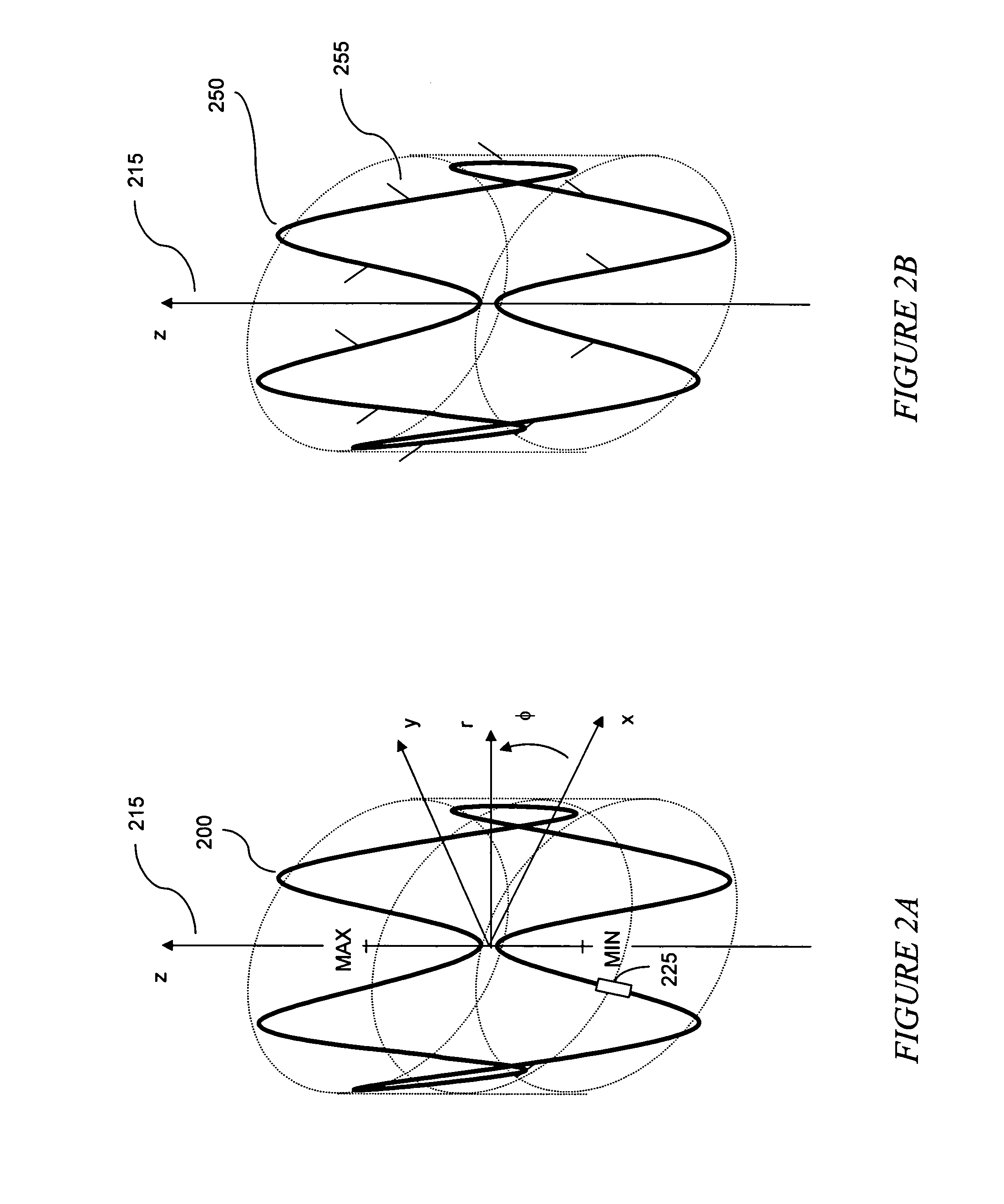
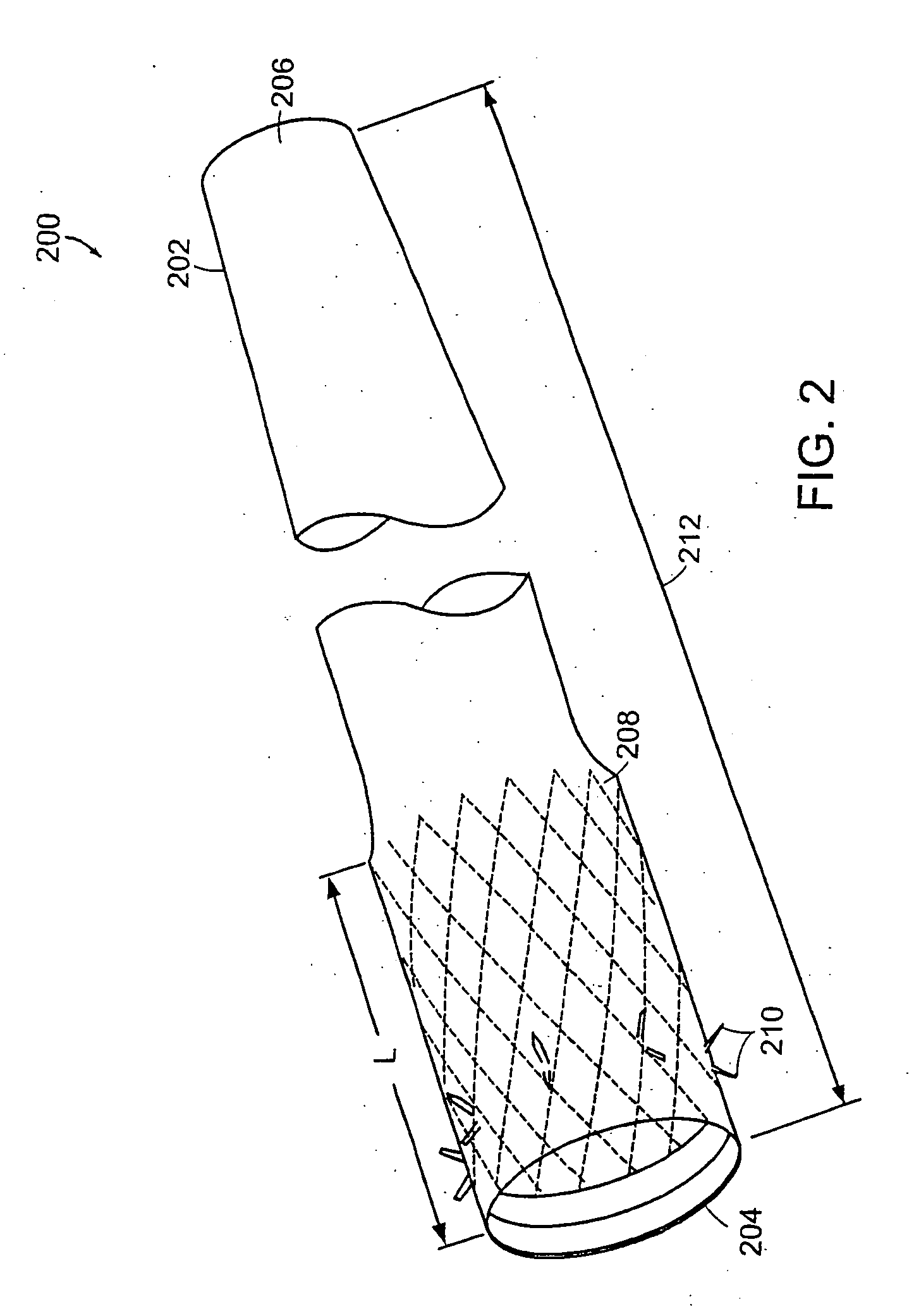
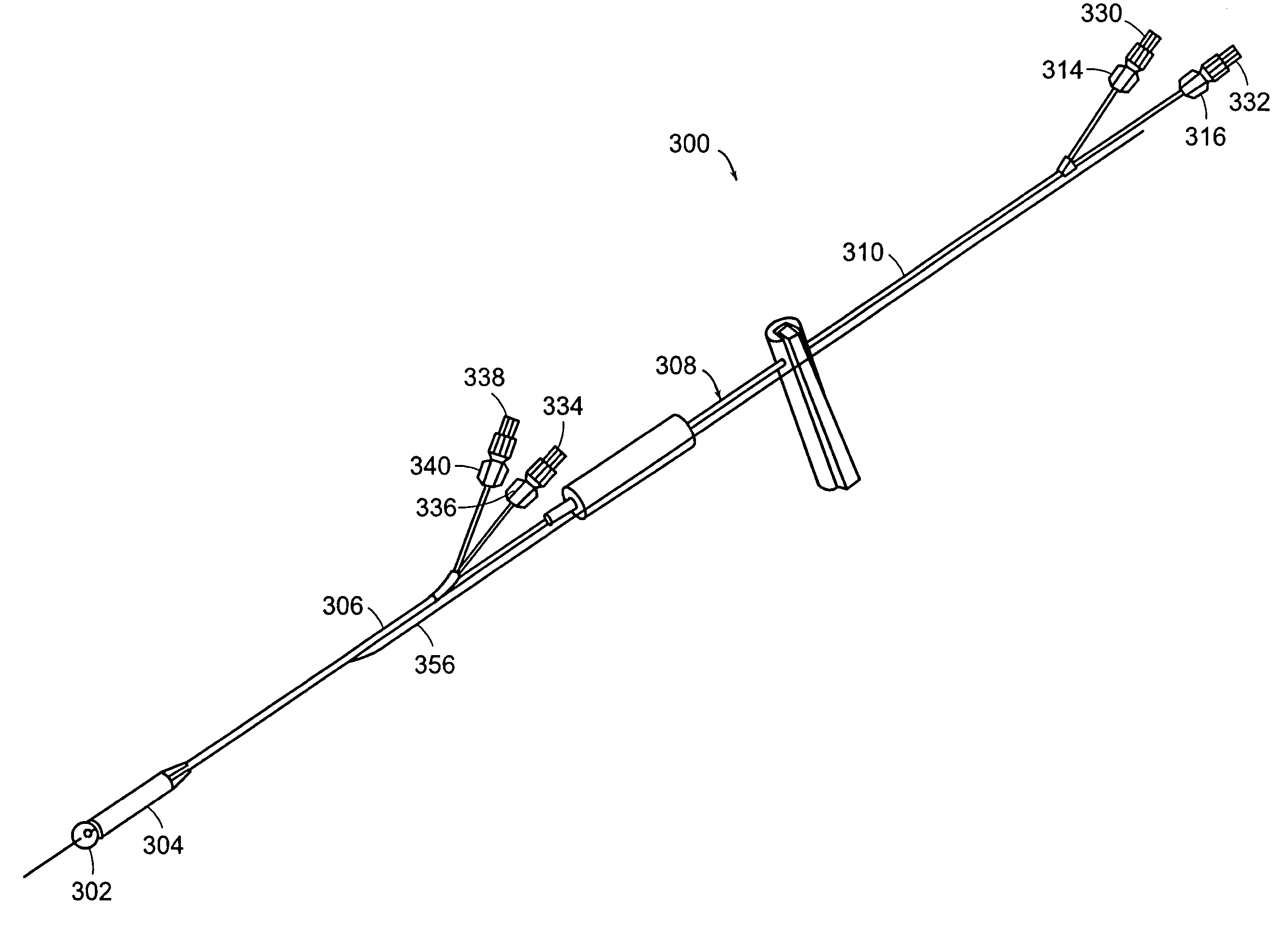
The present invention relates to an anchor configured for minimally-invasive implantation and sized to remain securely positioned within at least a portion of the gastrointestinal tract of an animal. The anchor includes a radial spring formed from an elongated resilient member shaped into an annular wave pattern about a central axis. The anchor defines a central lumen and provides an outward radial force, while allowing for substantial flexure about its perimeter. The anchor is generally removable, but can include fasteners, such as barbs, to further secure it to the surrounding anatomy. In some embodiments, the anchor includes a connector coupling a fixed portion to a removable portion. Further, the anchor can be used to secure a medical device within the body, such as a flexible sleeve within the intestine.
Owner:GI DYNAMICS
Systems and methods for treating obesity
ActiveUS20050228504A1Good for weight lossStimulatesIntravenous devicesTubular organ implantsPylorusGastric emptying
Methods and devices for simulating a gastric bypass and reducing the volume of the stomach involve placing a tubular liner along the lesser curve of the stomach cavity. Also, methods and devices for slowing gastric emptying involve placing valves within the stomach cavity near the gastrointestinal junction and / or the pylorus. These methods and devices may prevent a patient from drinking and eating large volumes at one time and from eating slowly all day.
Owner:ETHICON ENDO SURGERY INC
Bariatric sleeve
InactiveUS20060161265A1Increased axial stabilityLess movementSuture equipmentsStentsIntestinal structureGastrointestinal device
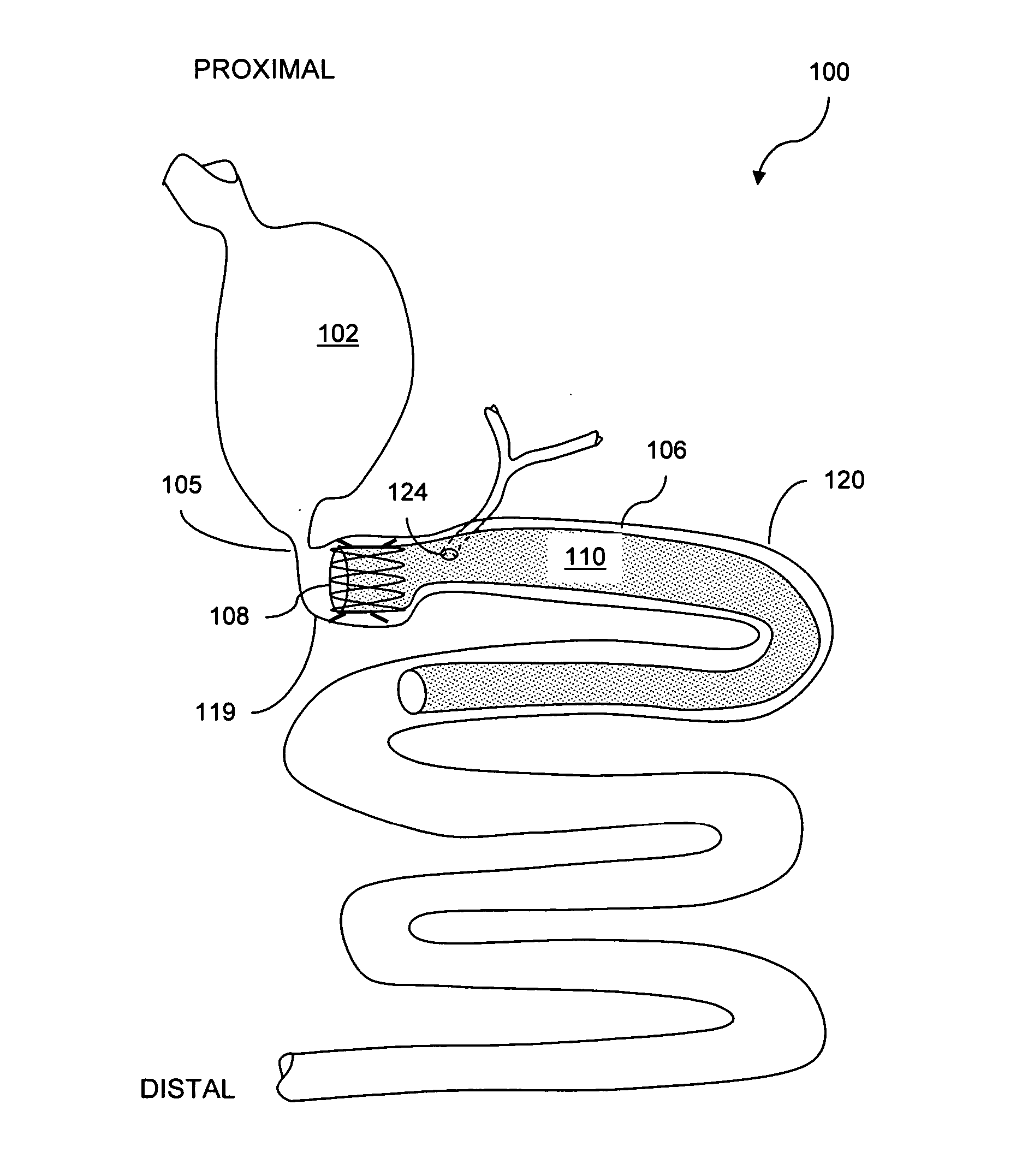
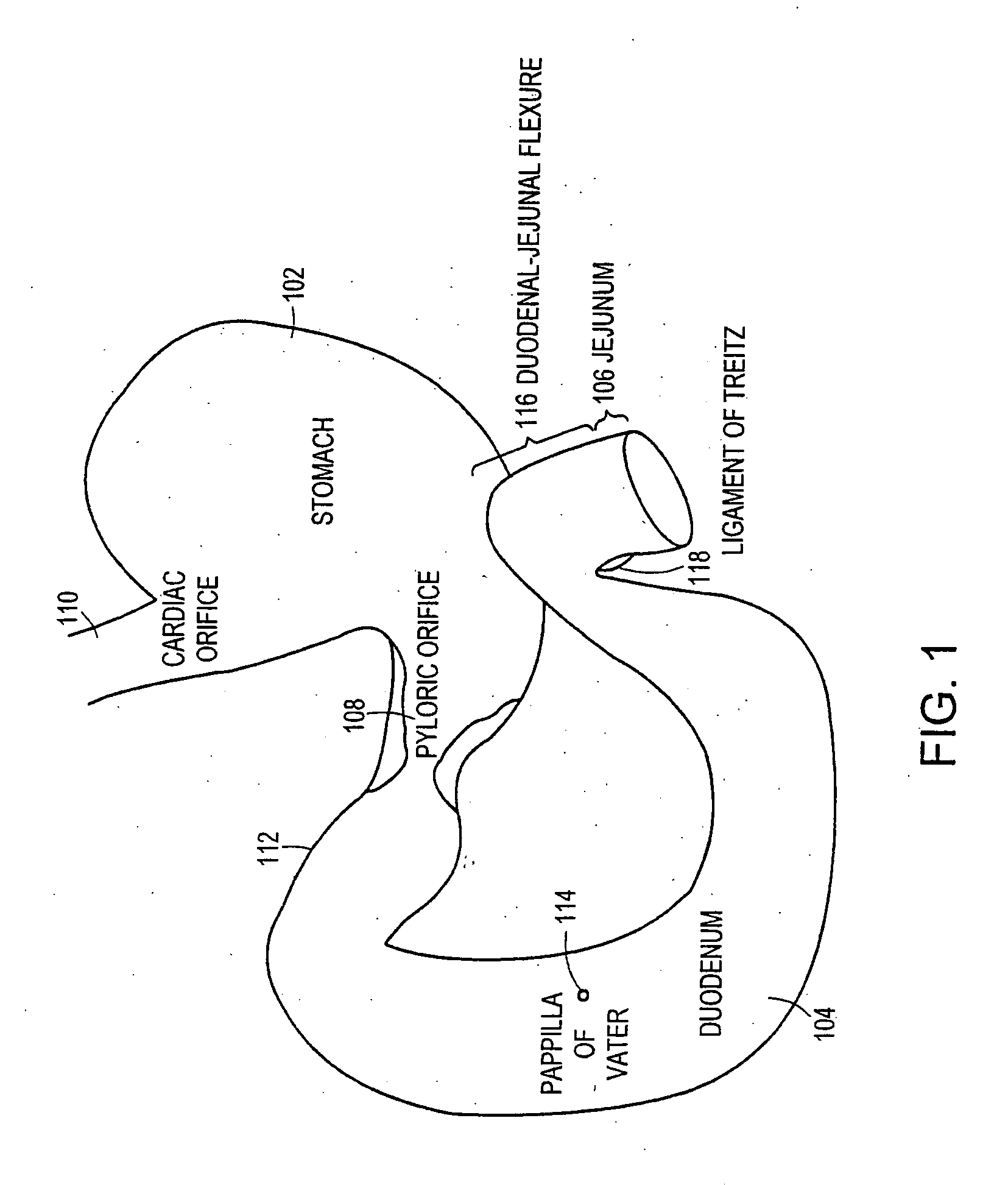
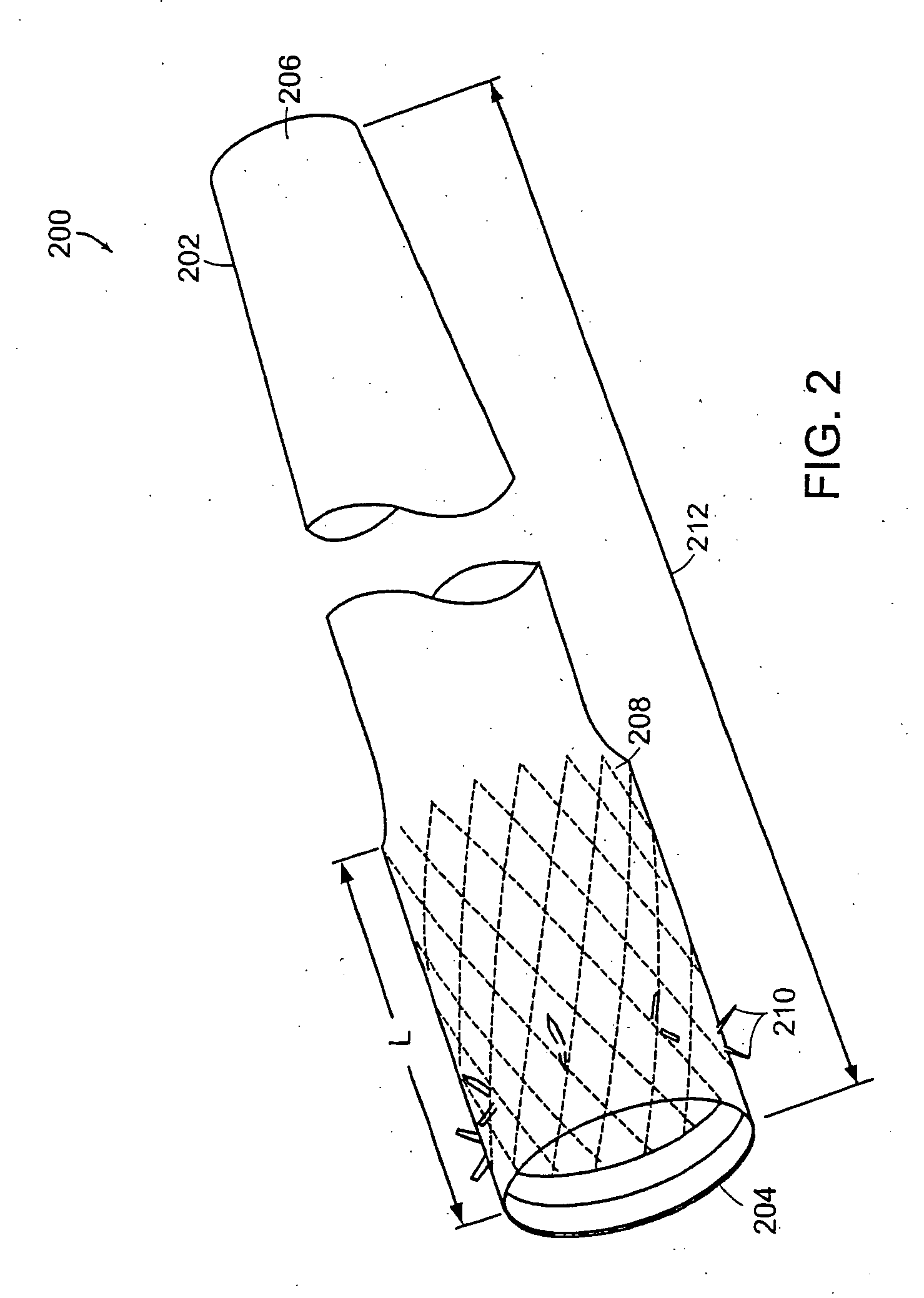
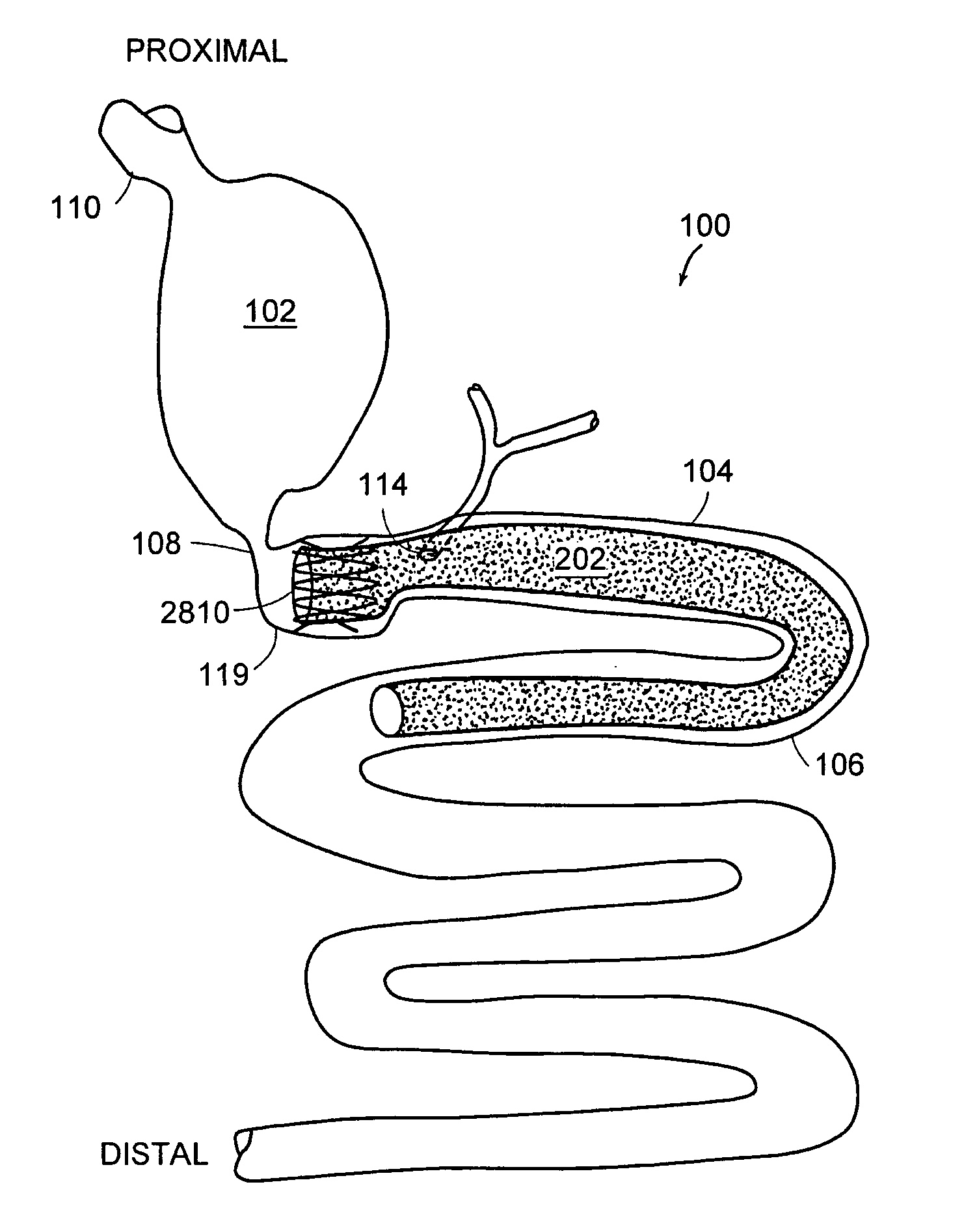
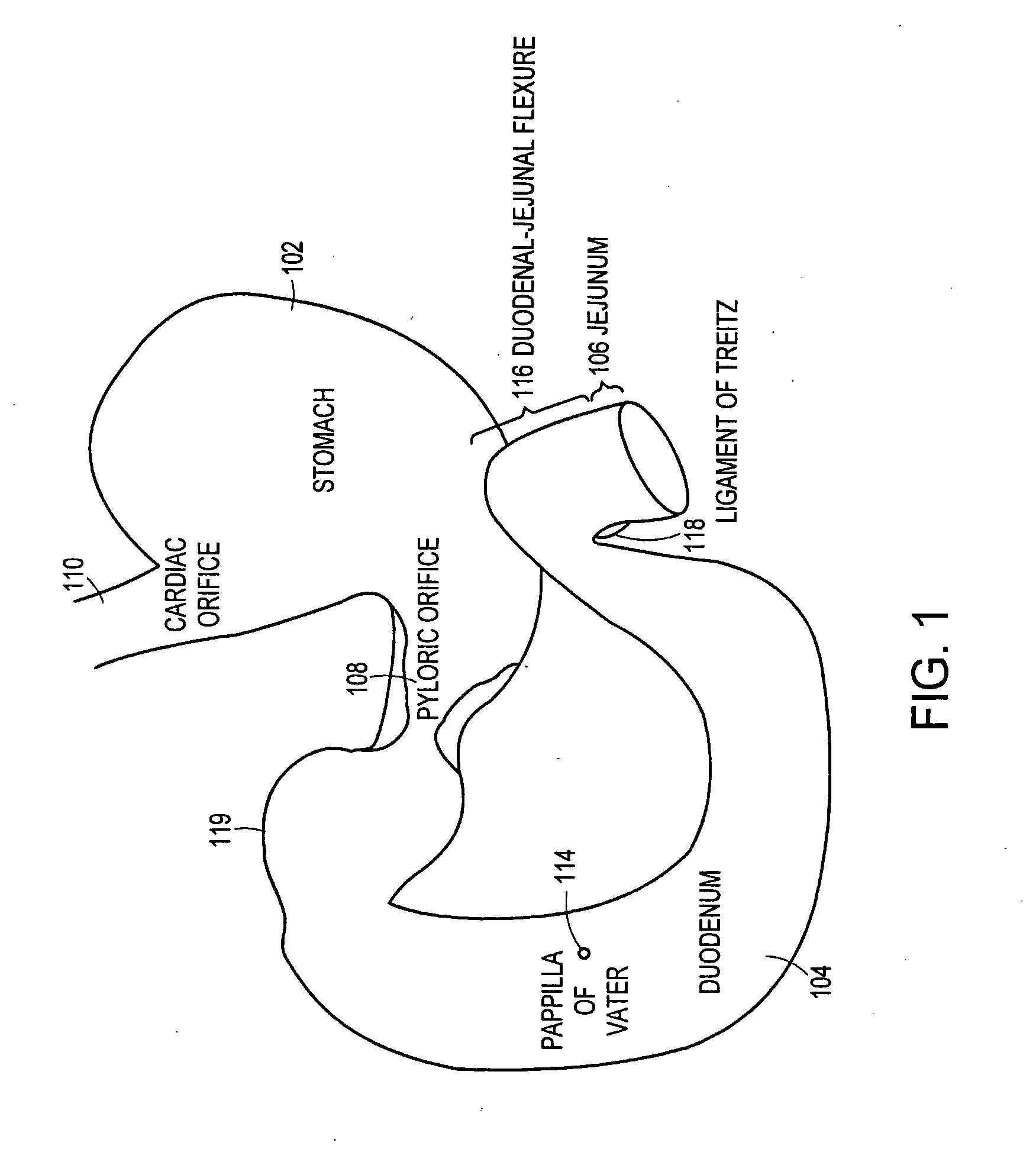
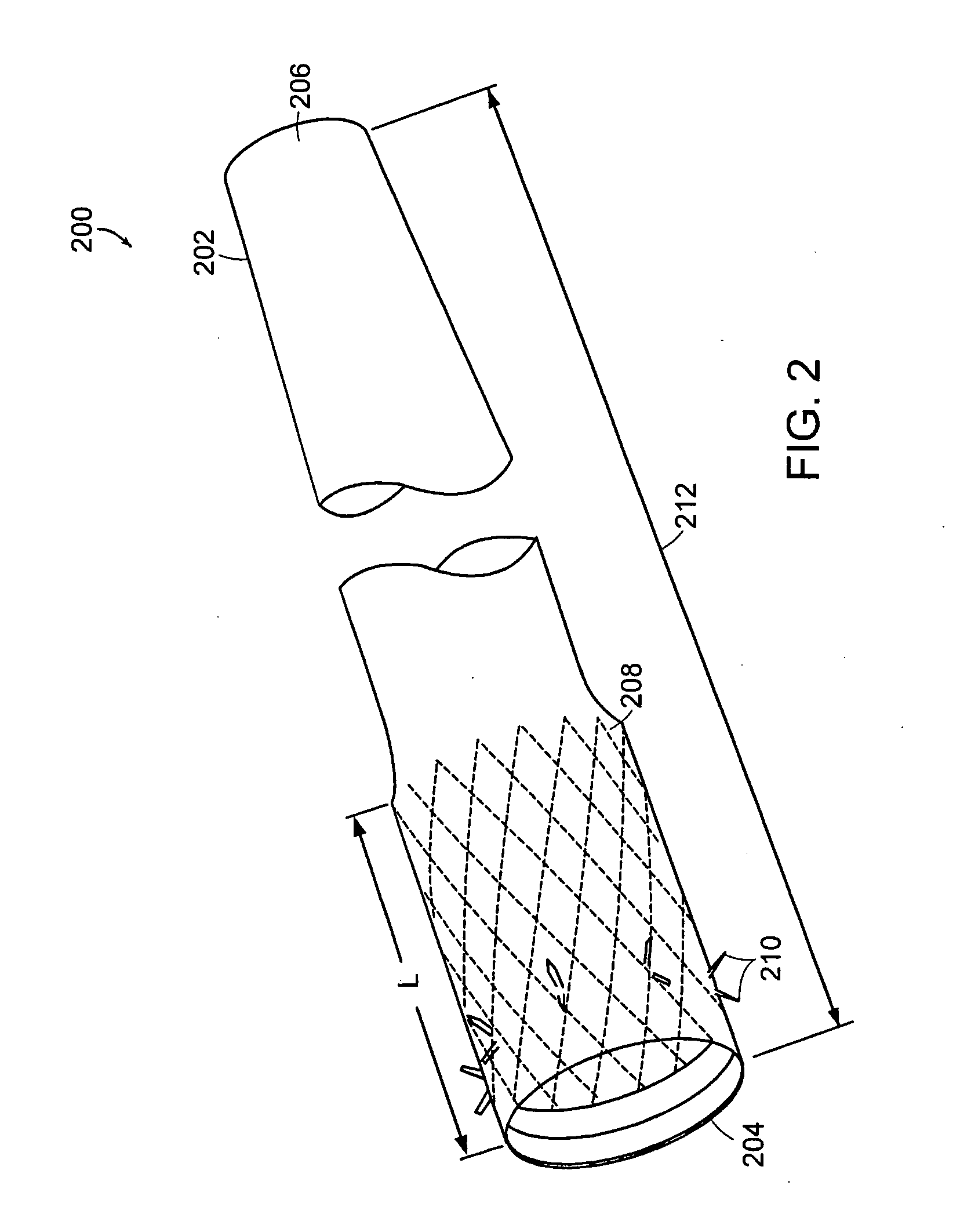
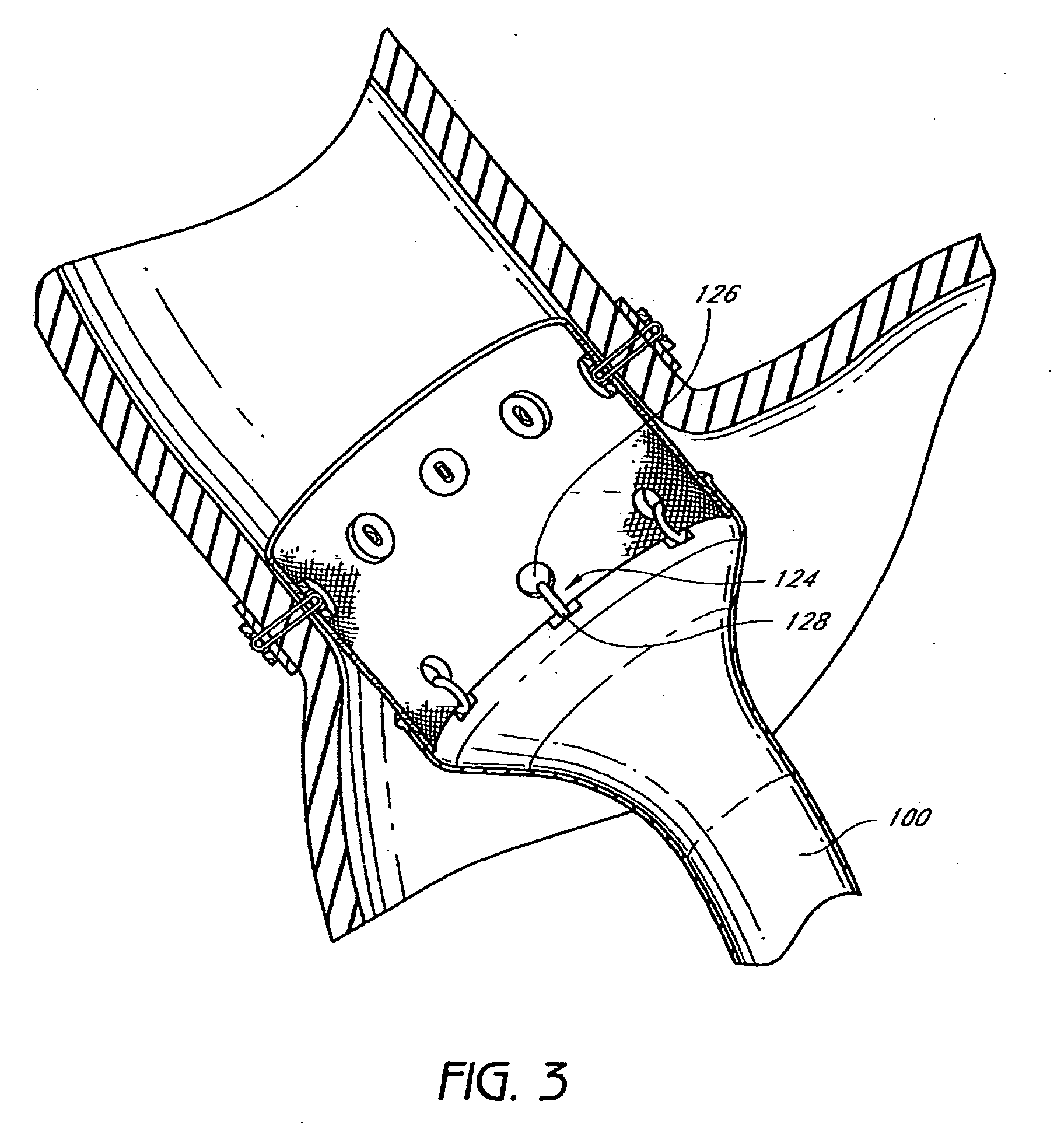
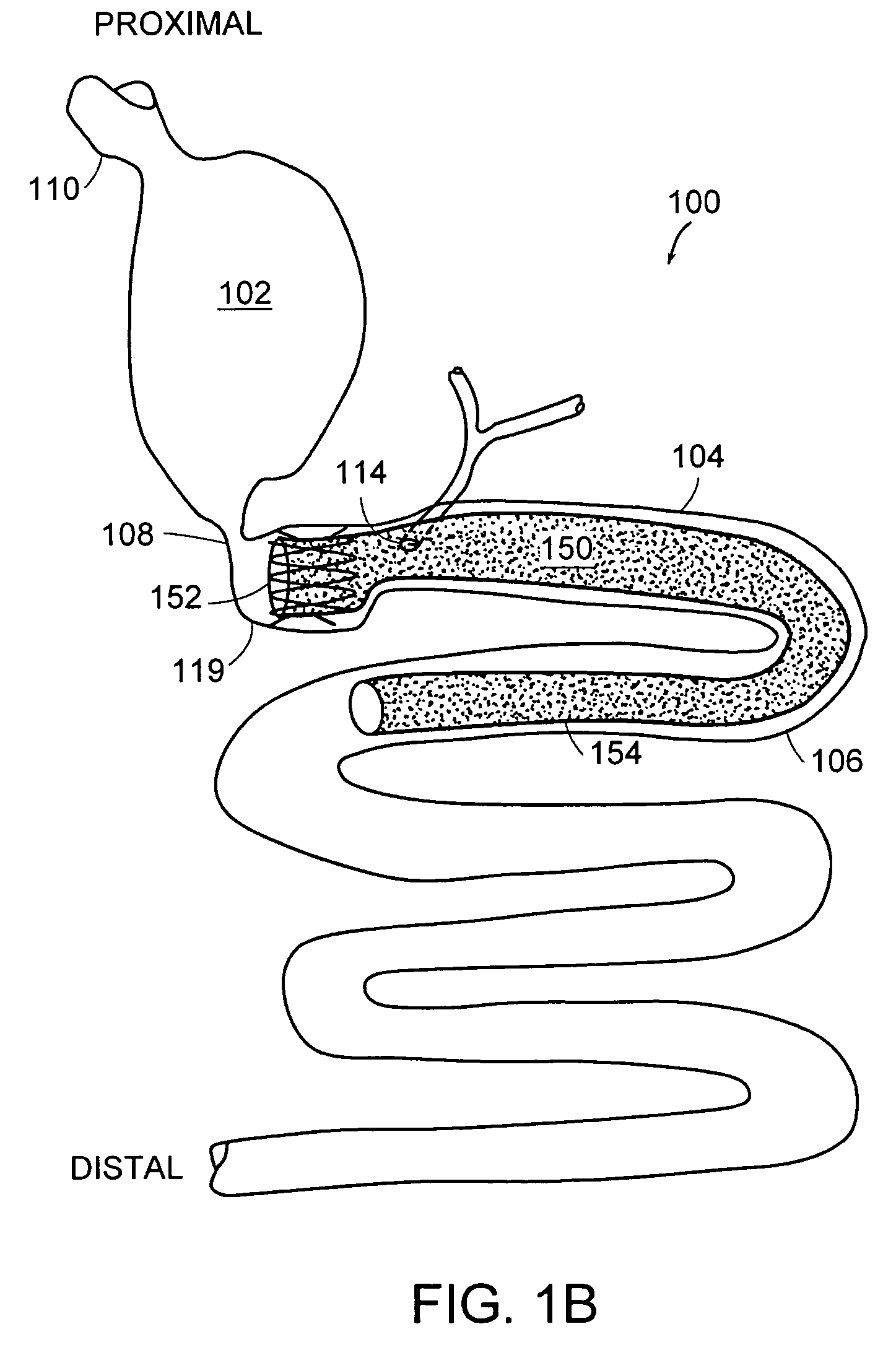
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve. When implanted within the intestine, the sleeve can limit the absorption of nutrients, delay the mixing of chyme with digestive enzymes, altering hormonal triggers, providing negative feedback, and combinations thereof. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
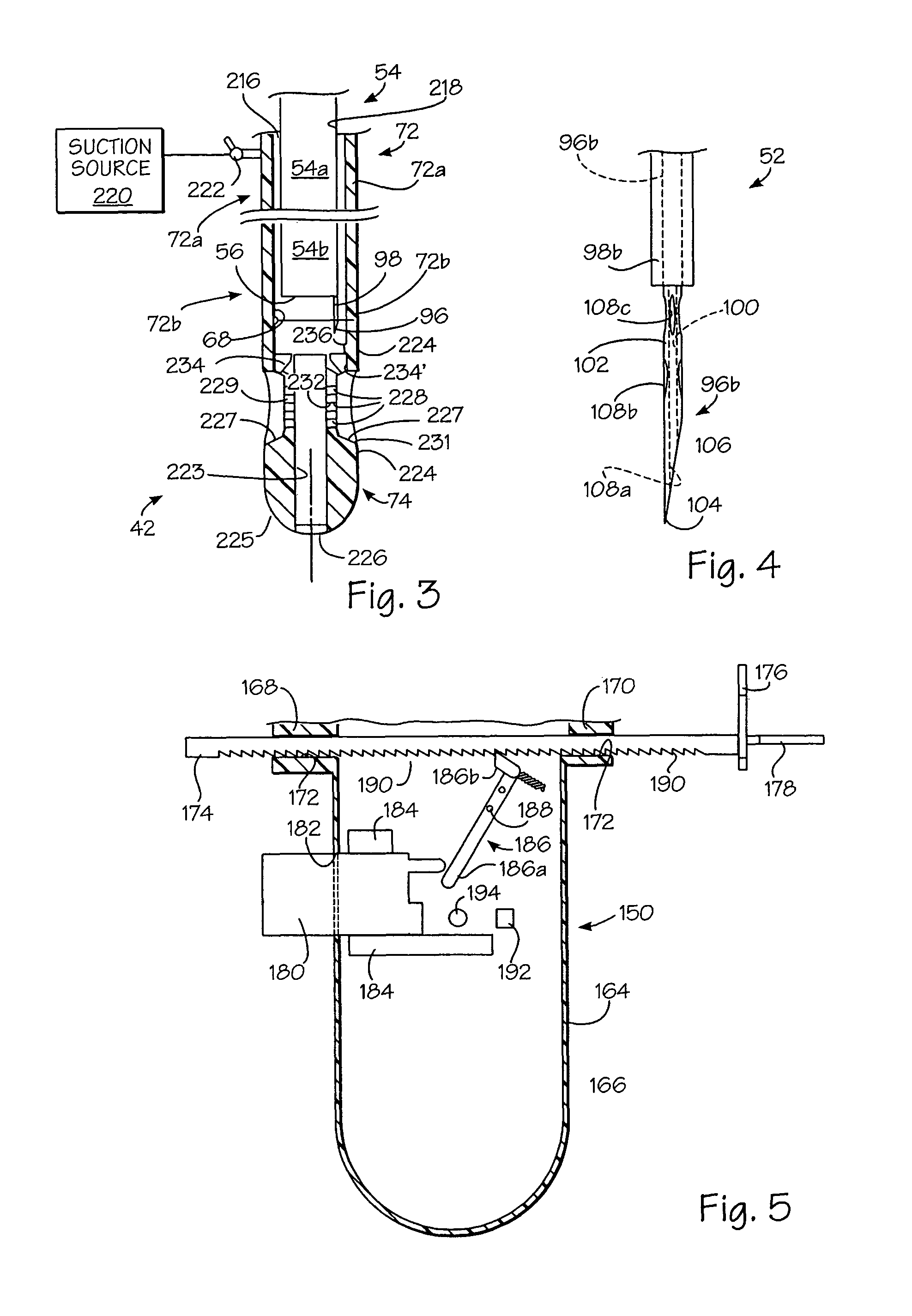
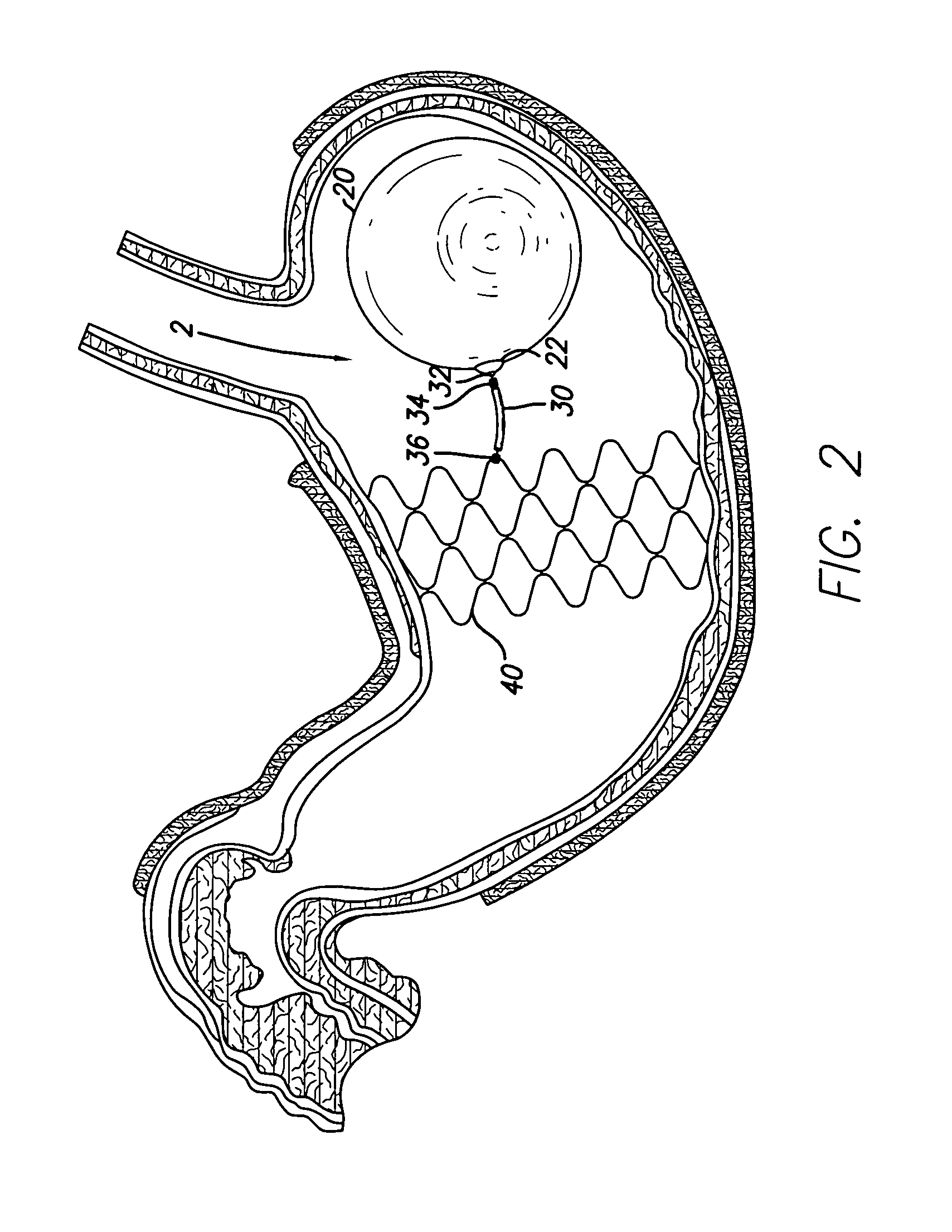
Stented anchoring of gastric space-occupying devices
InactiveUS7033384B2Easy accessReduce riskBalloon catheterDiagnosticsInsertion stentGastrointestinal tract
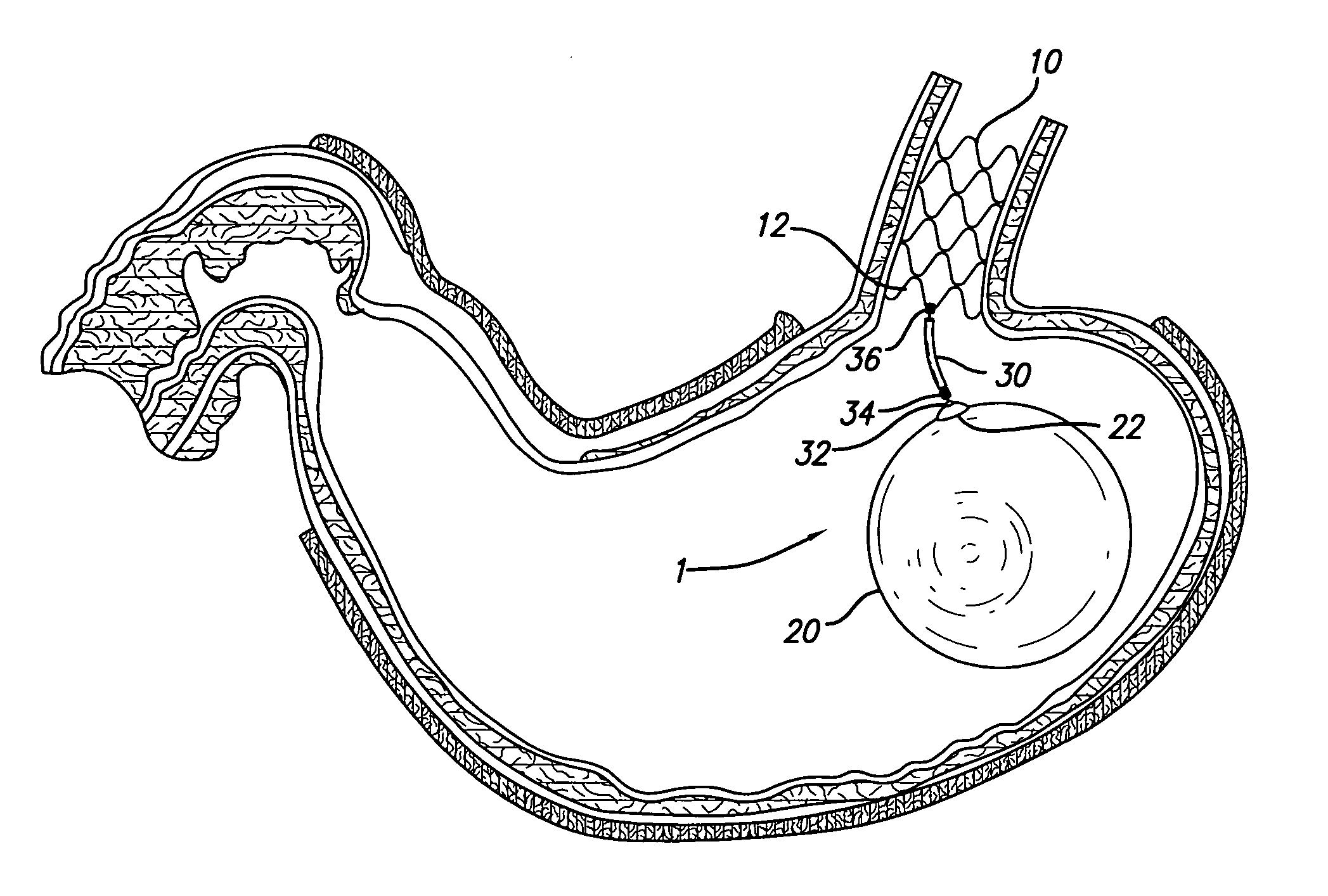
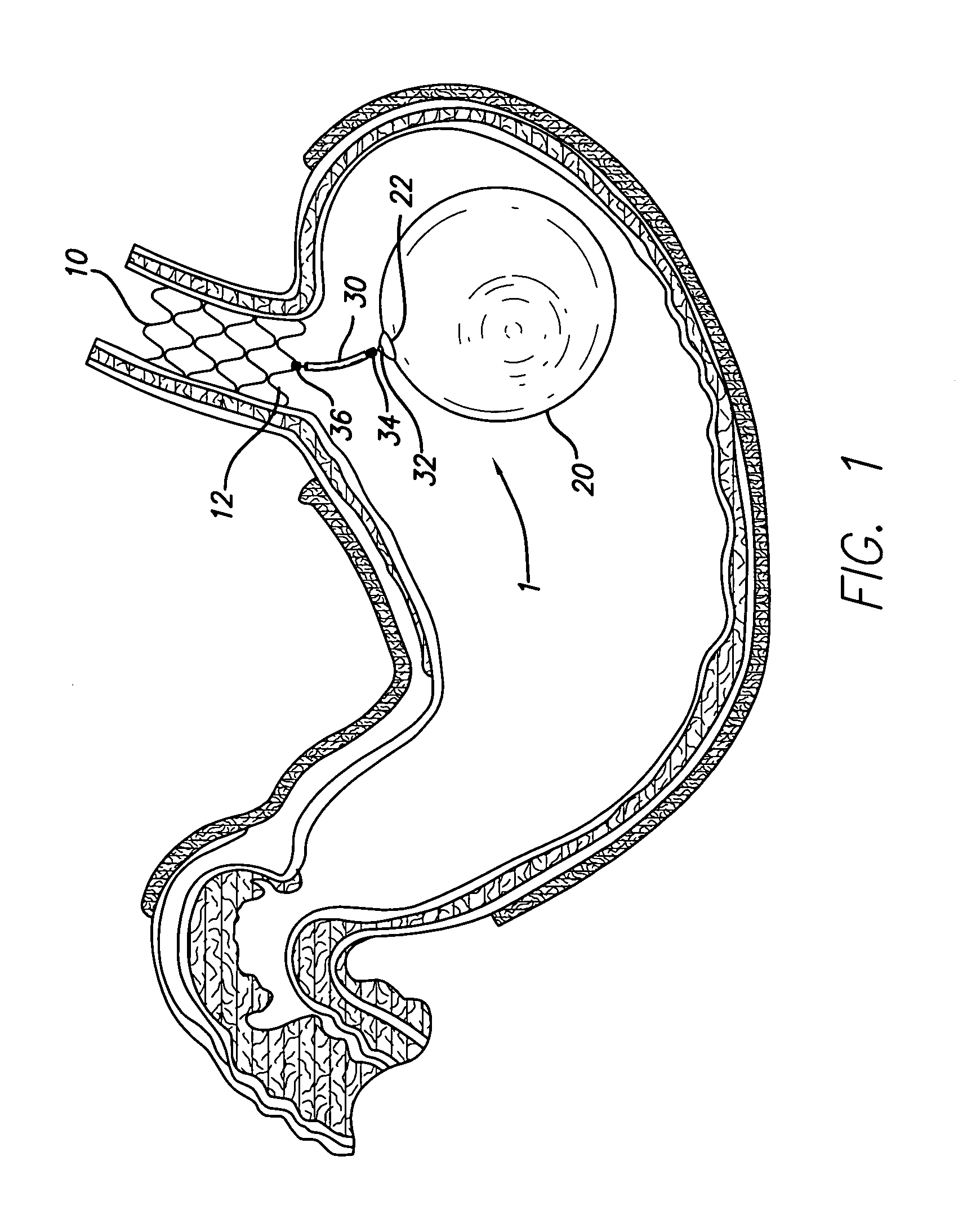
Gastric space occupying devices are provided that include a stent configured for deployment in the gastrointestinal tract of a patient, and in particular, for deployment in the esophagus or the stomach. Secured to the stent is an expandable member that is adapted to reside within the patient's stomach. When expanded, the expandable member occupies a predefined volume within the patient's stomach and is further tethered to the deployed stent, thereby retaining or anchoring the expandable member within the stomach. Methods and systems for the deploying the space occupying devices are also provided.
Owner:ETHICON ENDO SURGERY INC
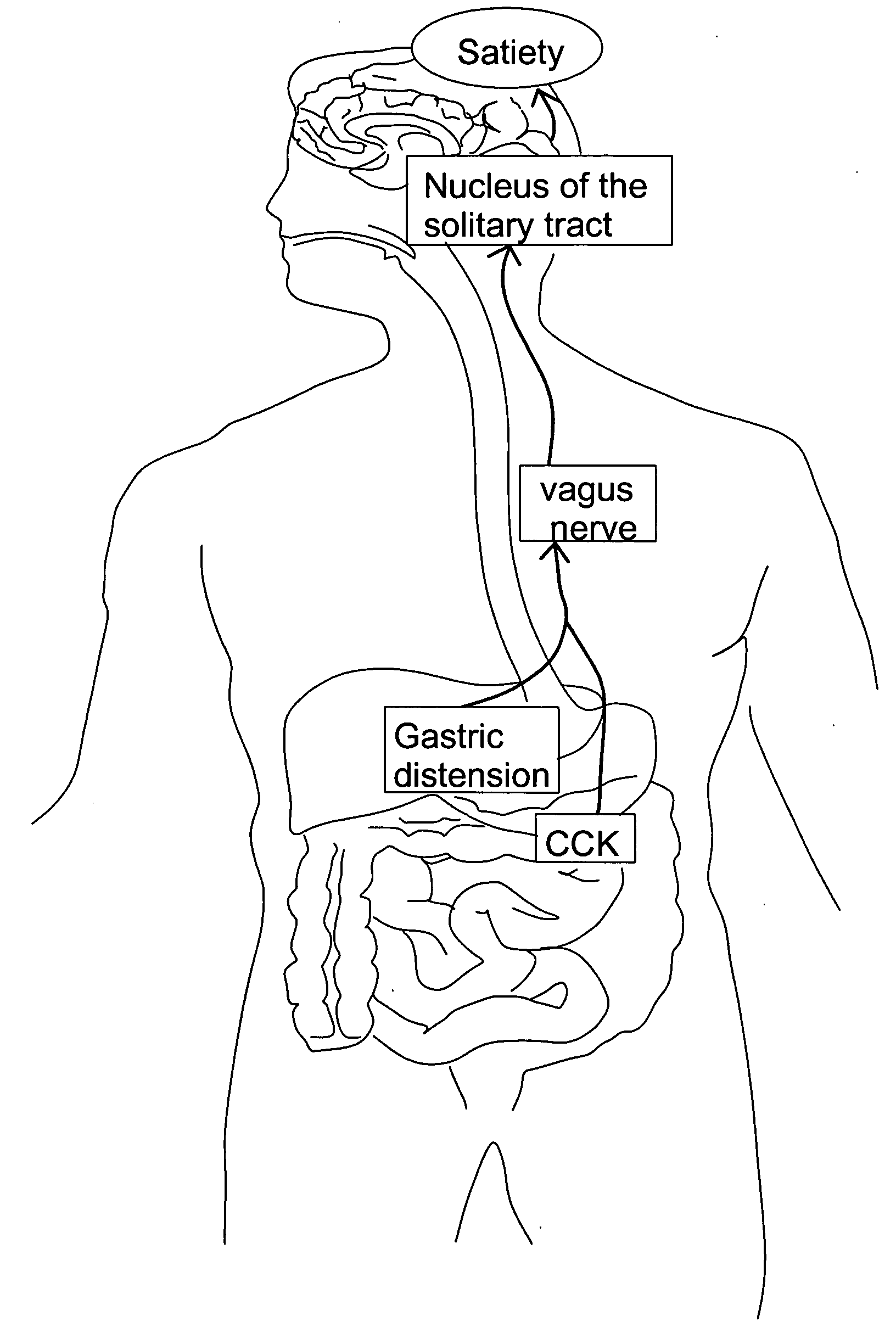
Method and system for vagal blocking and/or vagal stimulation to provide therapy for obesity and other gastrointestinal disorders
InactiveUS20050137644A1Eliminates repeated surgeryOvercomes shortcomingElectrotherapyArtificial respirationDiseaseDisease irritable bowel
Method and system to provide therapy for obesity and gastrointestinal disorders such as FGIDs, gastroparesis, gastro-esophageal reflex disease (GERD), pancreatitis and ileus comprises vagal blocking and / or vagal stimulation. Vagal blocking may be in the afferent or efferent direction, and may be with or without stimulation pulses. Blocking may be provided by one of a number of different electrical blocking techniques. Electrical signals may be provided with an external stimulator in conjunction with an implanted stimulus-receiver, or an implanted stimulus-receiver comprising a high value capacitor for temporary power source. In one embodiment, the external stimulator may comprise an optional telemetry unit. The addition of the telemetry unit to the external stimulator provides the ability to remotely interrogate and change stimulation programs over a wide area network, as well as other networking capabilities,
Owner:NEURO & CARDIAC TECH
Atraumatic delivery devices
InactiveUS20060155312A1Limit absorptionReducing hormone triggersSuture equipmentsStentsImplanted deviceGastrointestinal tract
Methods and apparatus for delivering an implant device within the digestive system of an animal are presented. An delivery device includes an outer sheath, or container, for storing a proximal portion of the implant device. The outer sheath is moveable relative to the stored portion of the implant device to release the proximal portion from within the outer sheath. The delivery device also includes an inner sheath defining a lumen therein that extends distal to the outer sheath, a moveable element adapted to secure the distal end of the implant to the inner sheath, and a release mechanism coupled to the moveable element for releasing the distal end of the implant. The device also includes a atraumatic tip, or ball, coupled at its distal end to facilitate guiding the delivery device through the gastrointestinal tract.
Owner:GI DYNAMICS
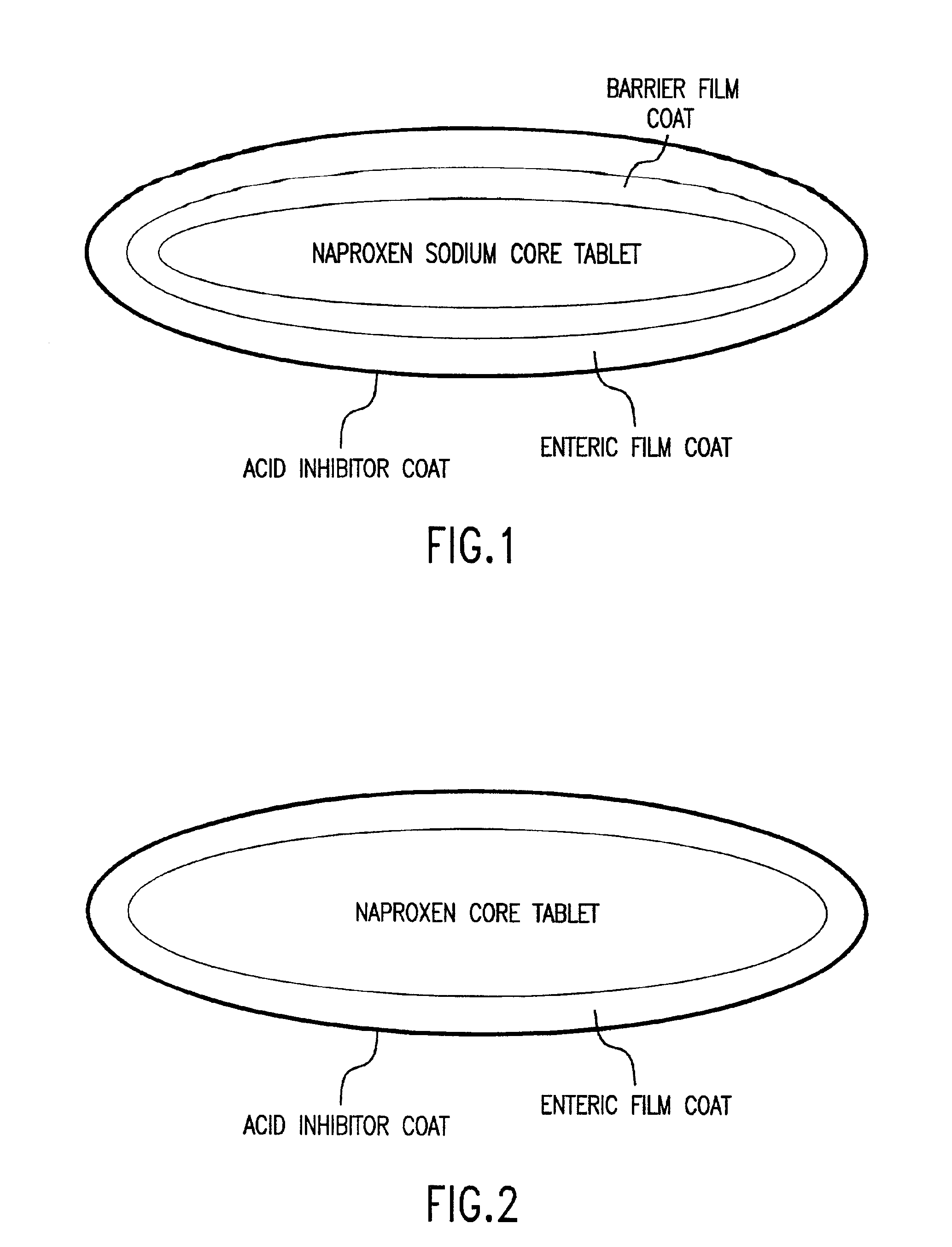
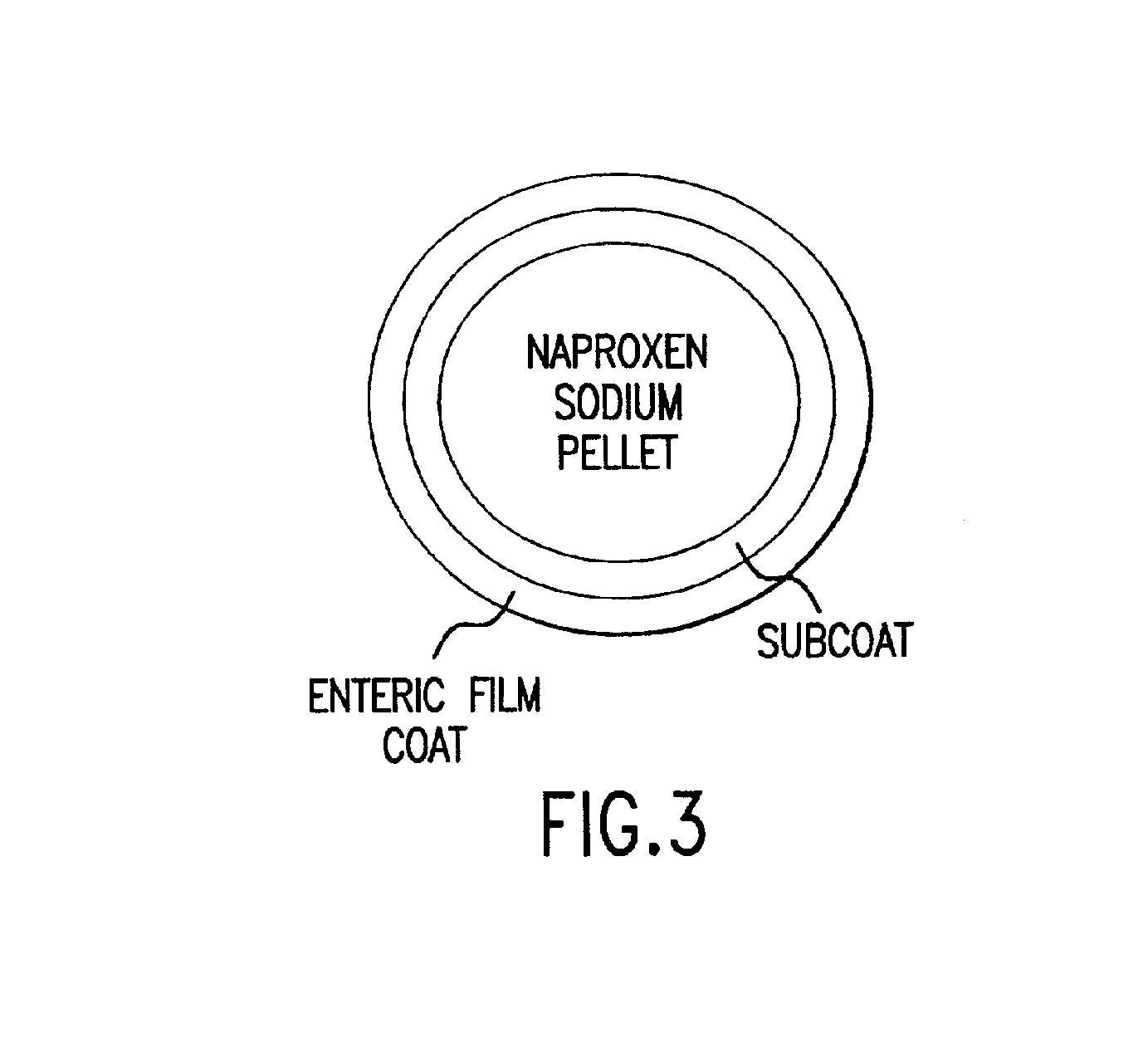
Pharmaceutical compositions for the coordinated delivery of NSAIDs
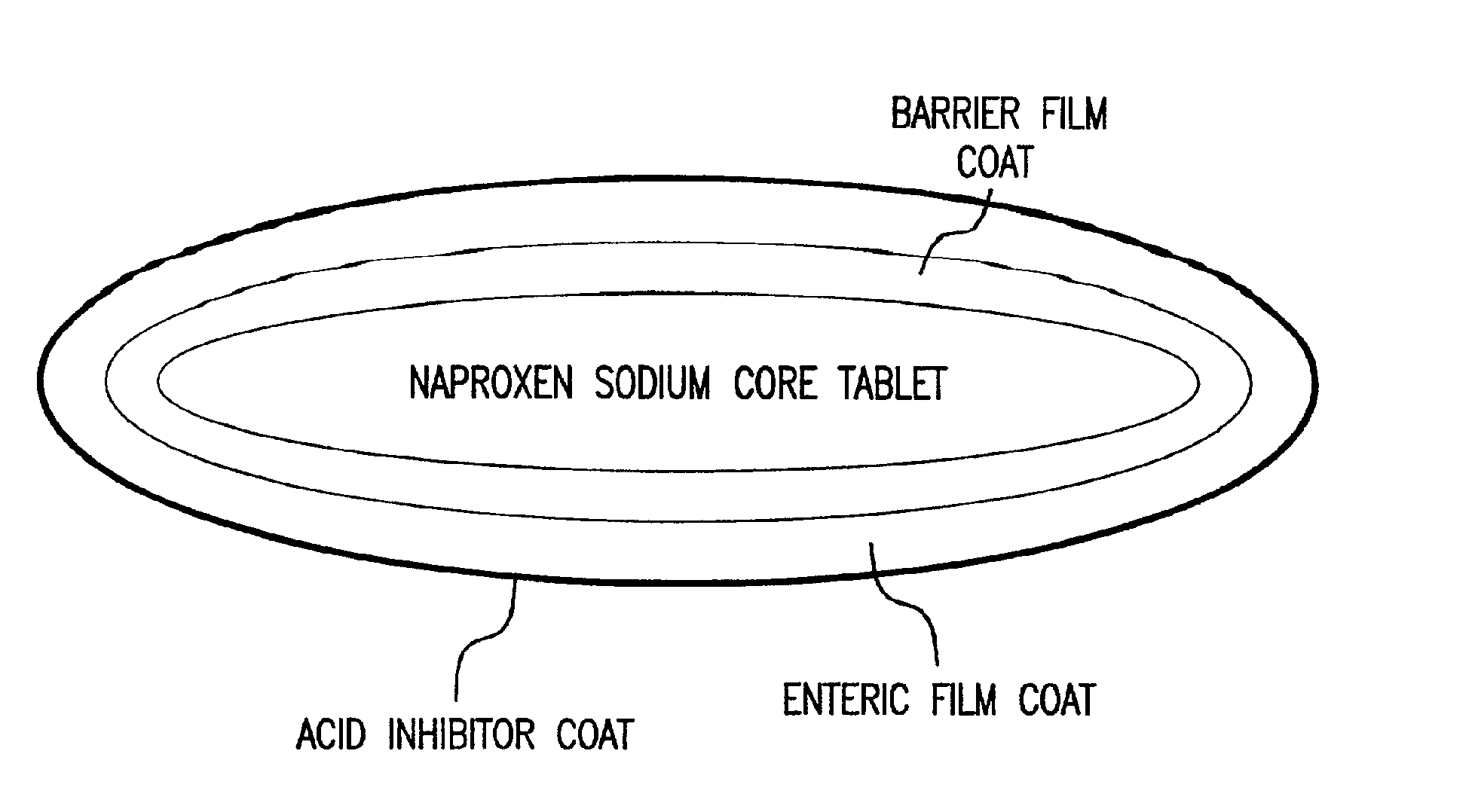
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Anti-obesity devices
InactiveUS20050085923A1Controlled absorptionChange habitsSuture equipmentsStentsGastrointestinal deviceLigament structure
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the stomach and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for anchoring the device to the stomach and a flexible sleeve to limit absorption of nutrients in the duodenum. The anchor is collapsible for endoscopic delivery and removal.
Owner:GI DYNAMICS
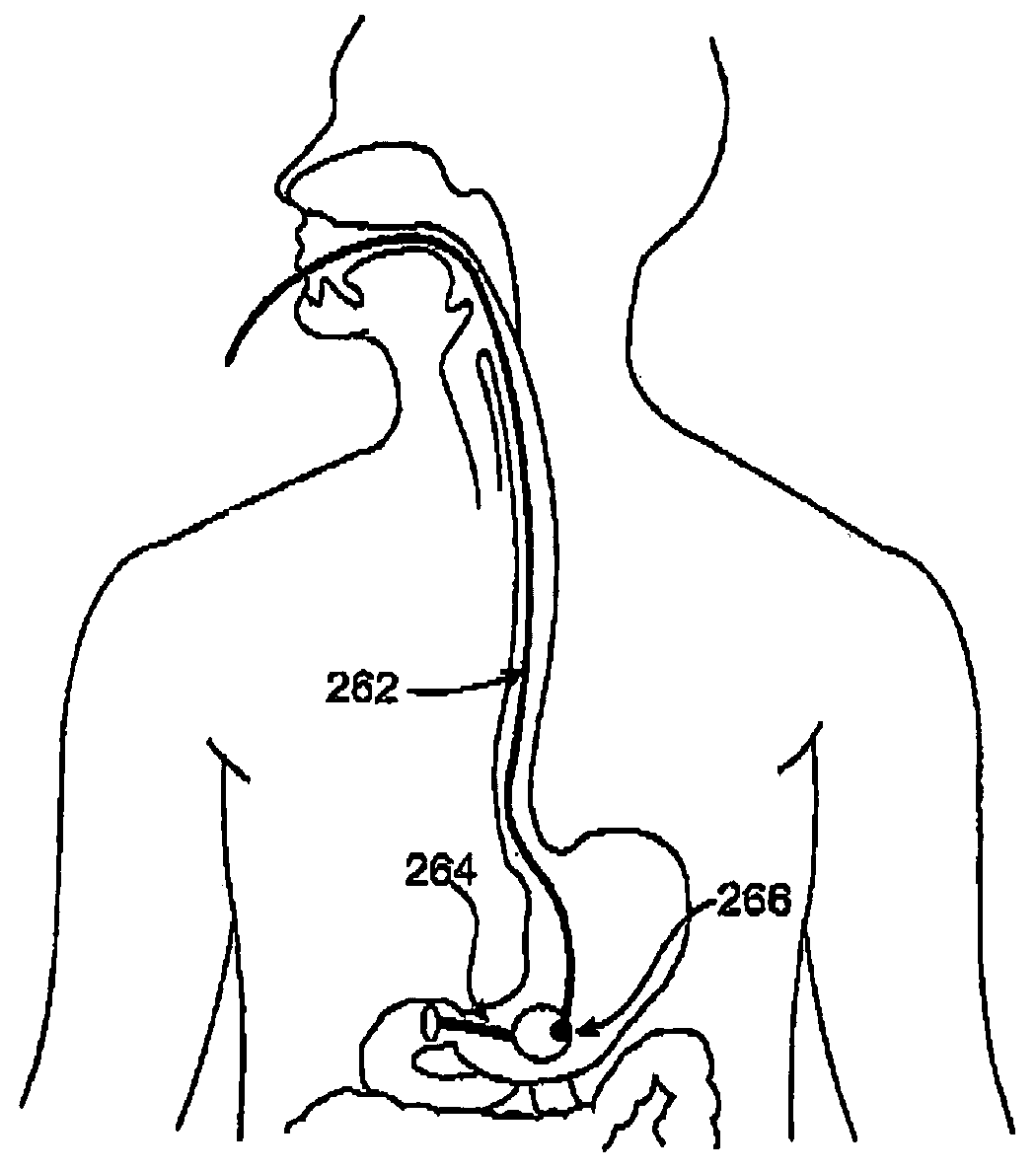
Minimally invasive gastrointestinal bypass
A solution is provided for modifying the location at which bodily fluids interact with nutrients in a gastrointestinal tract having a conduit with a first end and a second end. The first end is configured to divert bodily fluids from an entrance within a gastrointestinal tract to a location downstream from the entrance. The solution also provides for a means for attaching the second end to the entrance.
Owner:LAUFER MICHAEL D
Active drug delivery in the gastrointestinal tract
InactiveUS20050058701A1Easy accessPromote absorptionInternal electrodesBody temperature measurementMedicineDrug administration
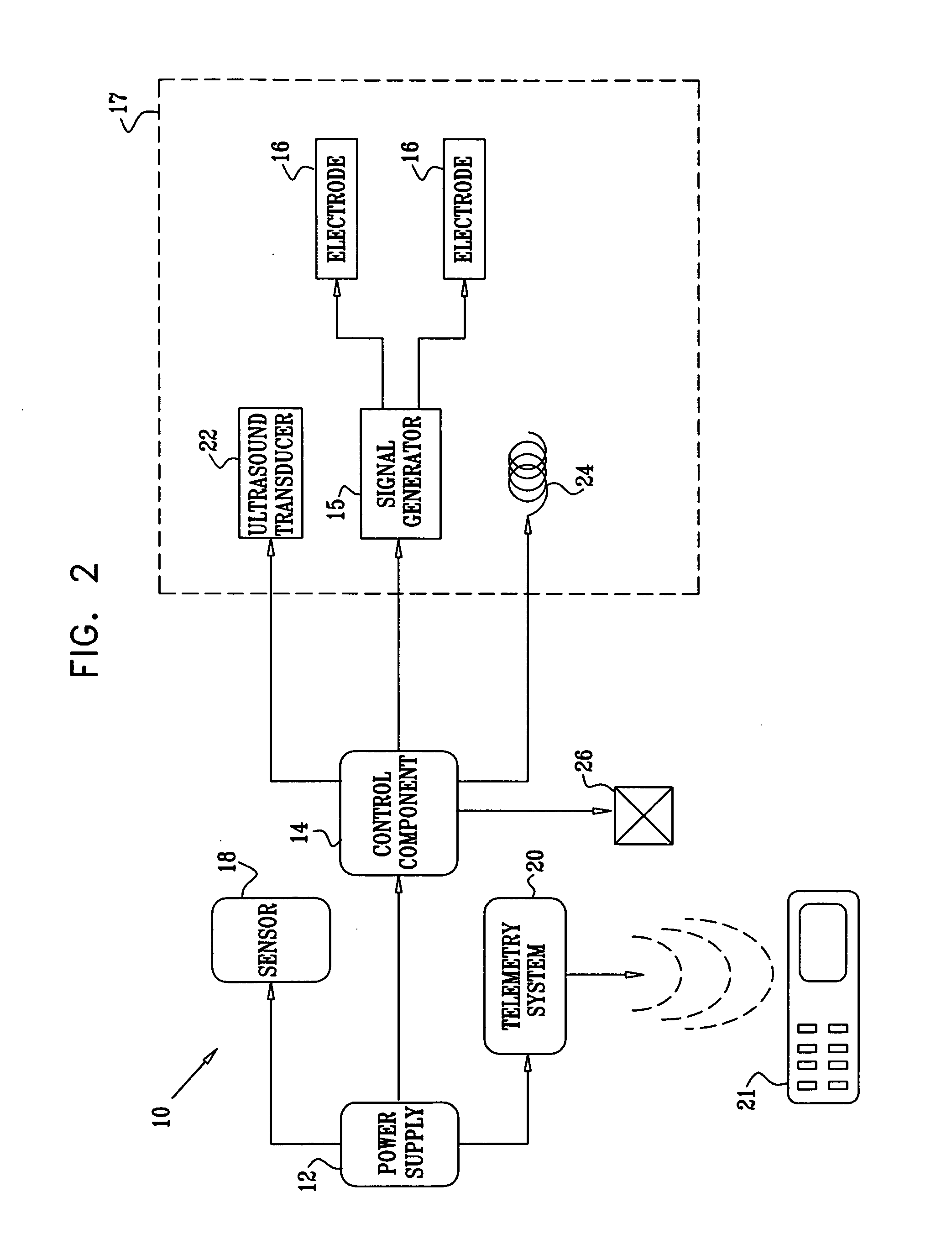
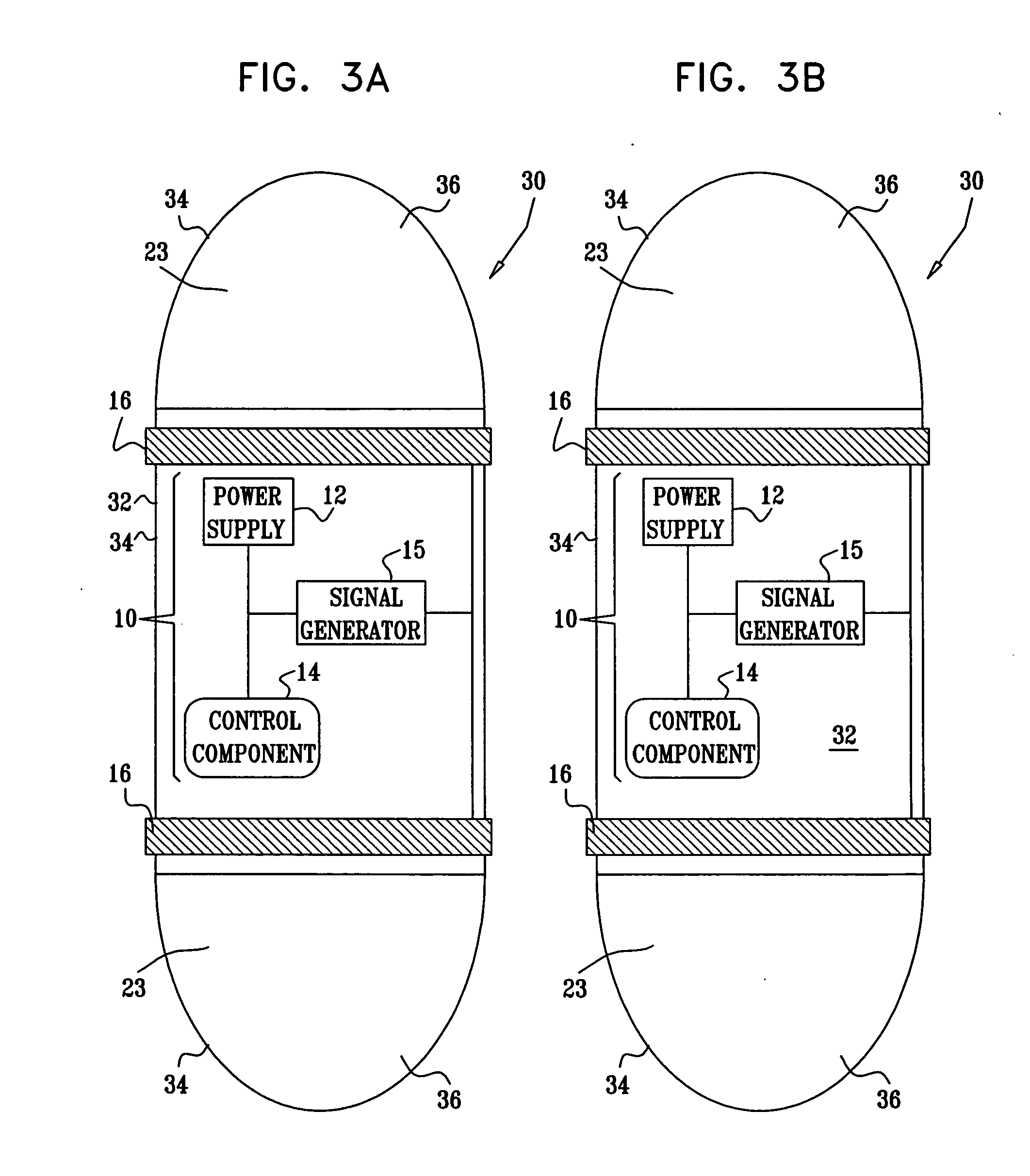
Apparatus for drug administration is provided, including an ingestible capsule, which includes a drug, stored by the capsule, and an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject. The capsule further includes first and second electrodes, and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, through an epithelial layer of the GI tract by driving the first and second electrodes to apply a series of pulses at a current of less than about 5 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds.
Owner:E PILL PHARMA
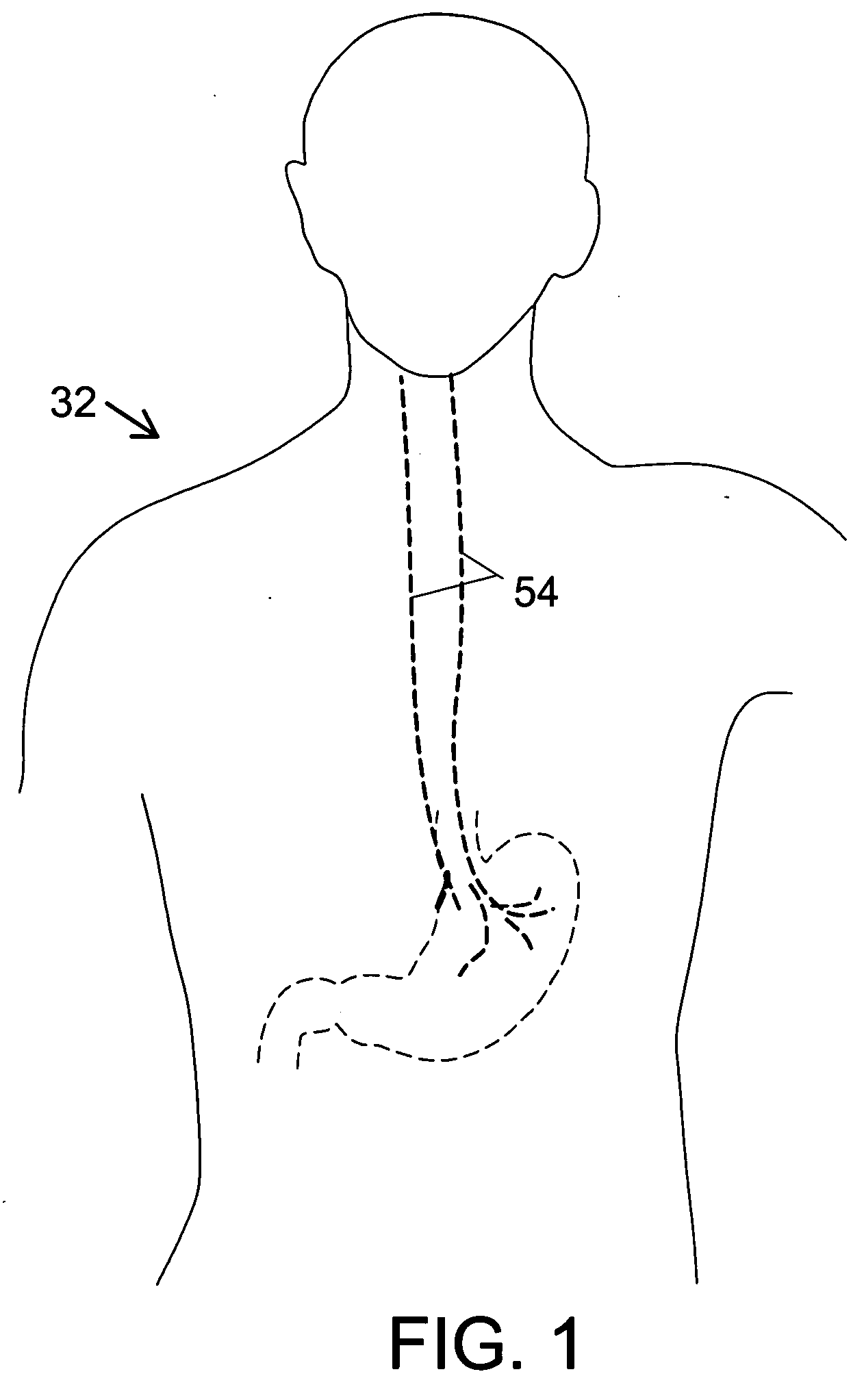
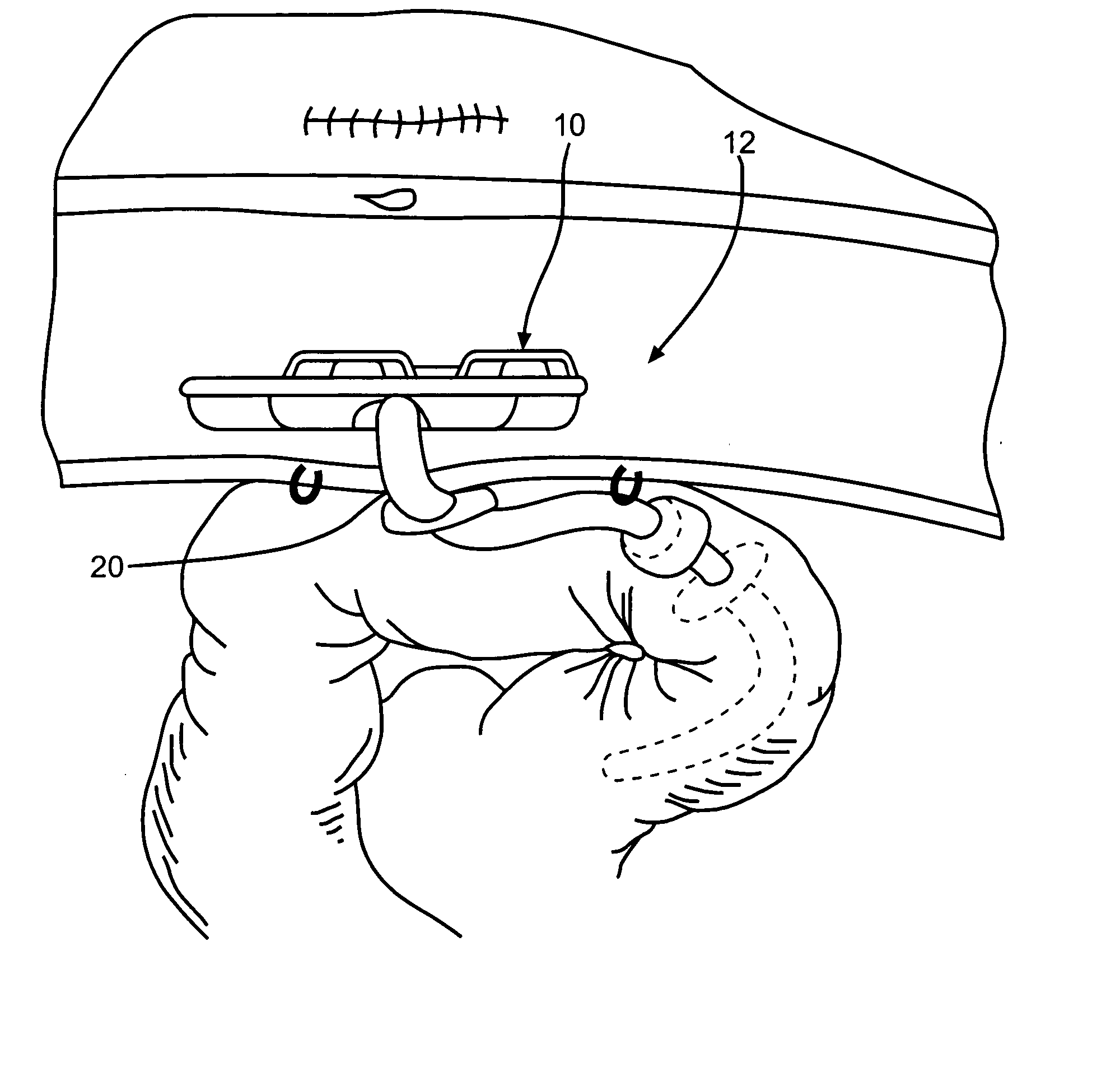
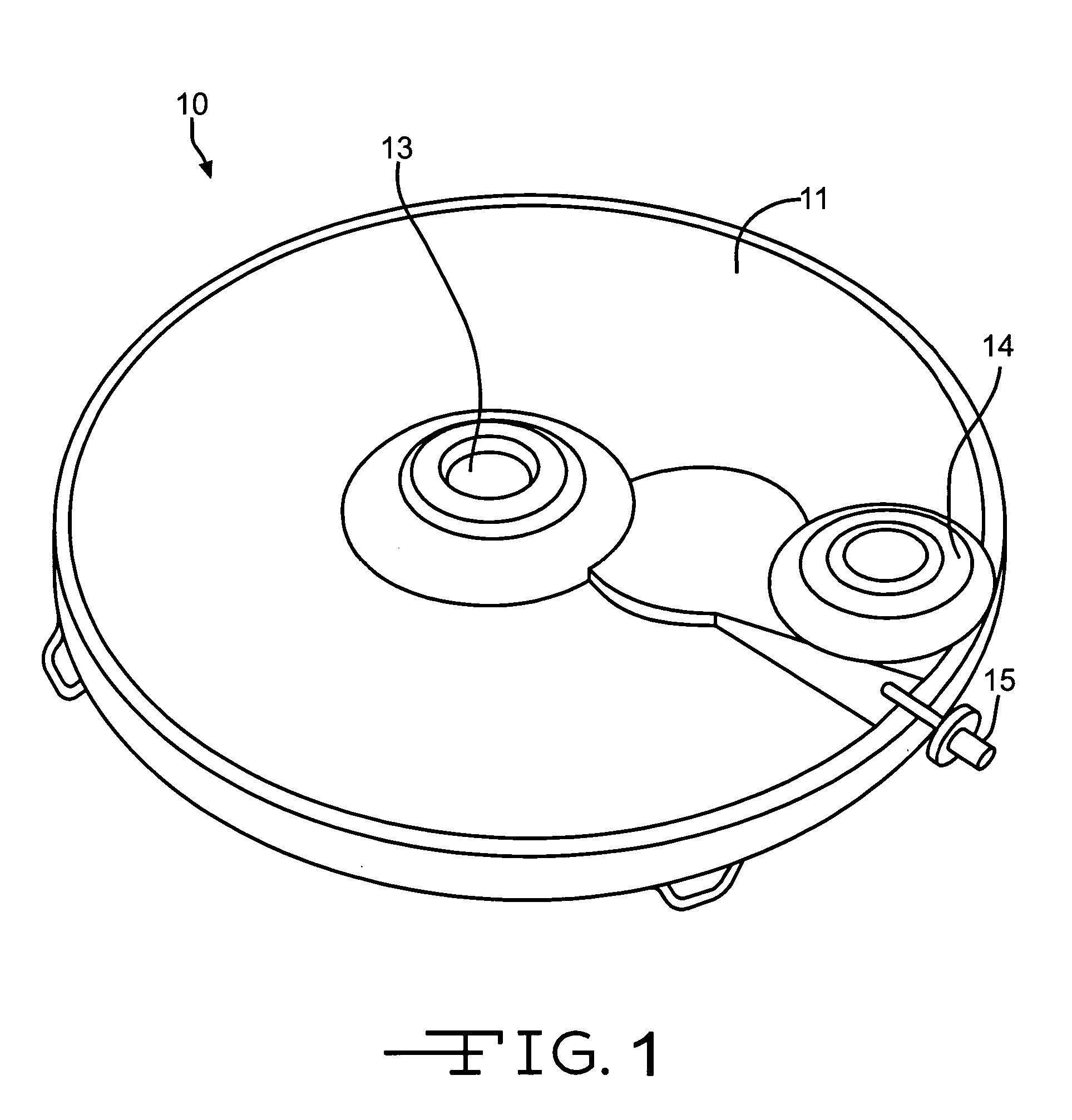
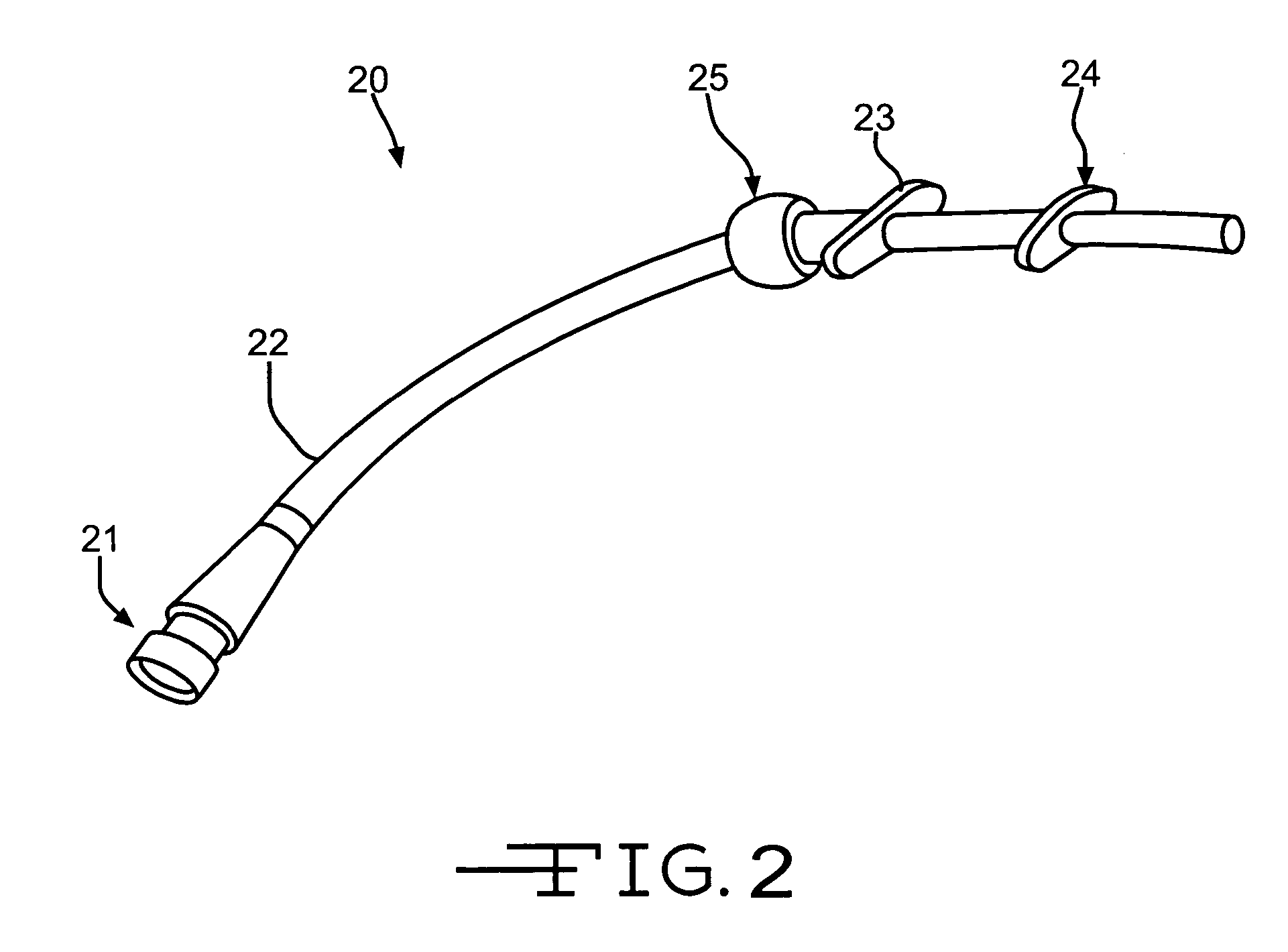
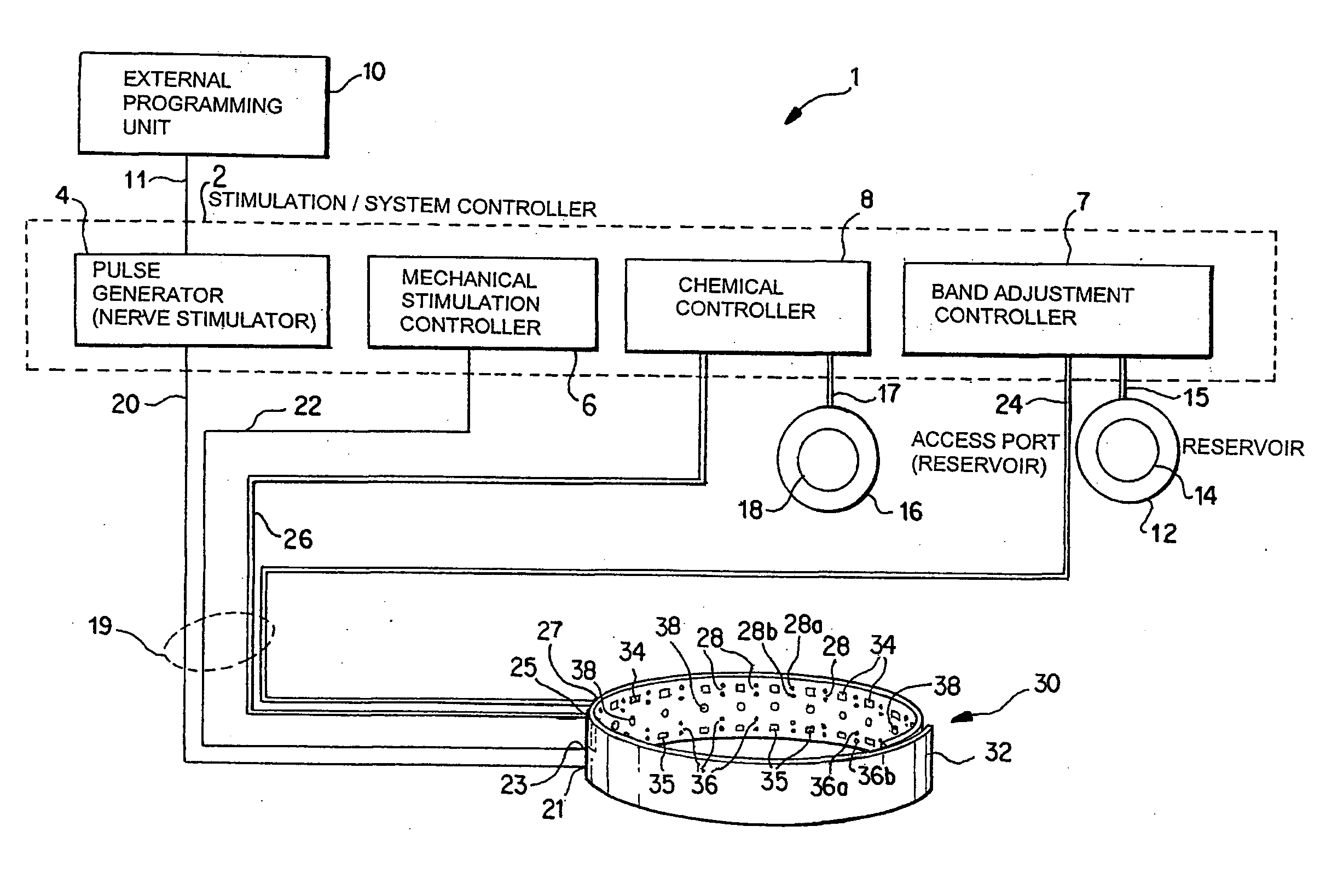
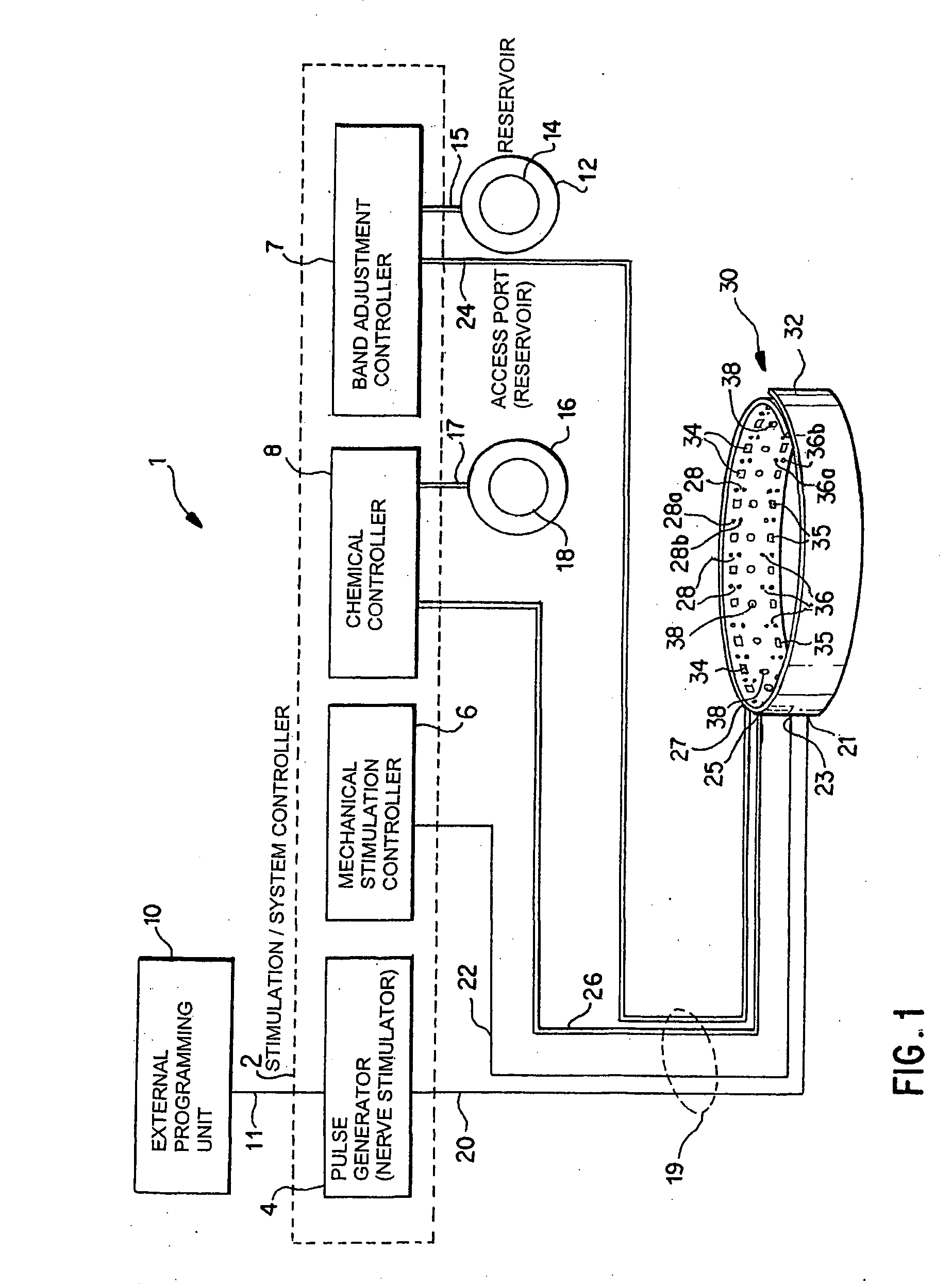
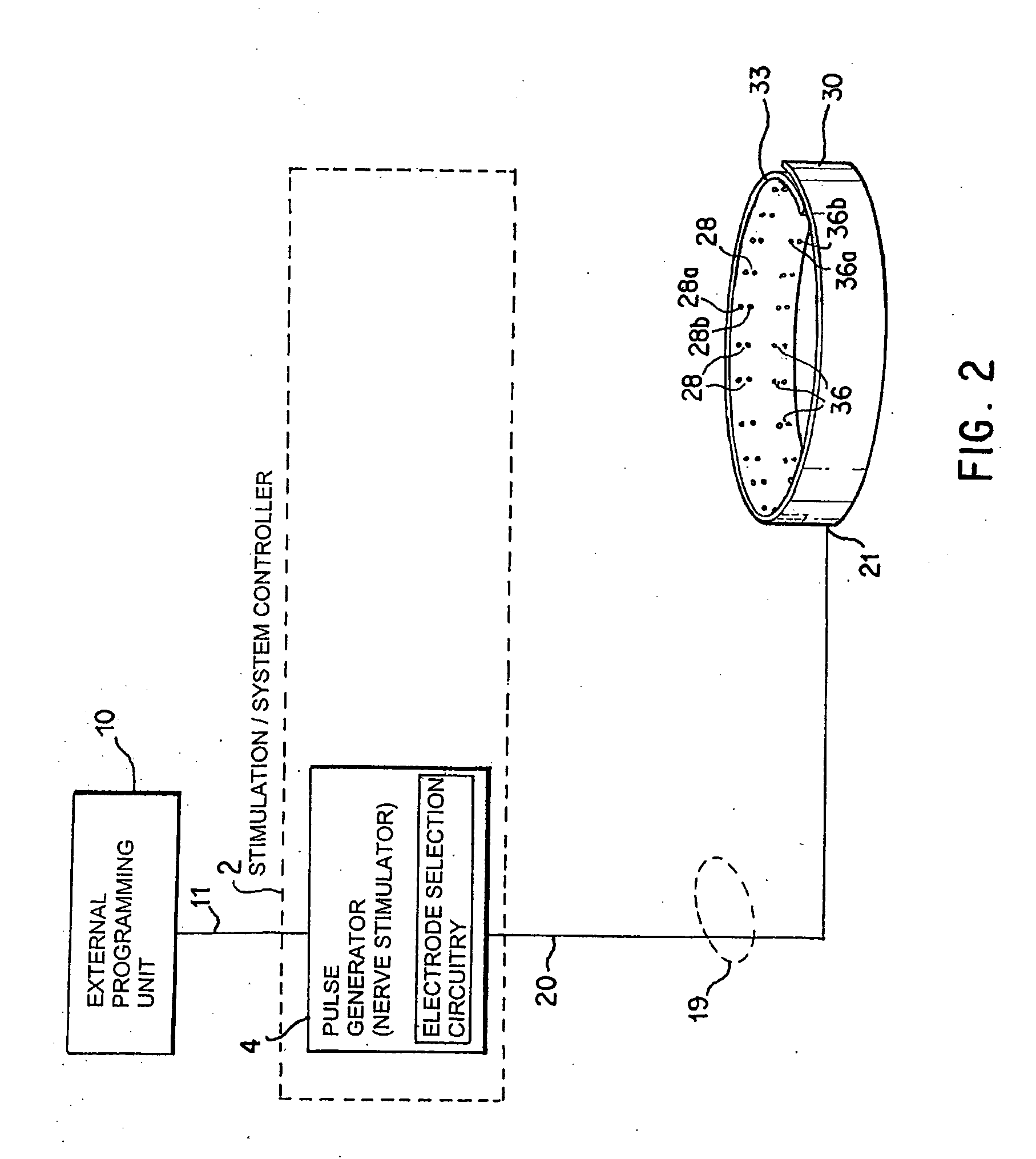
Noninvasively adjustable gastric band
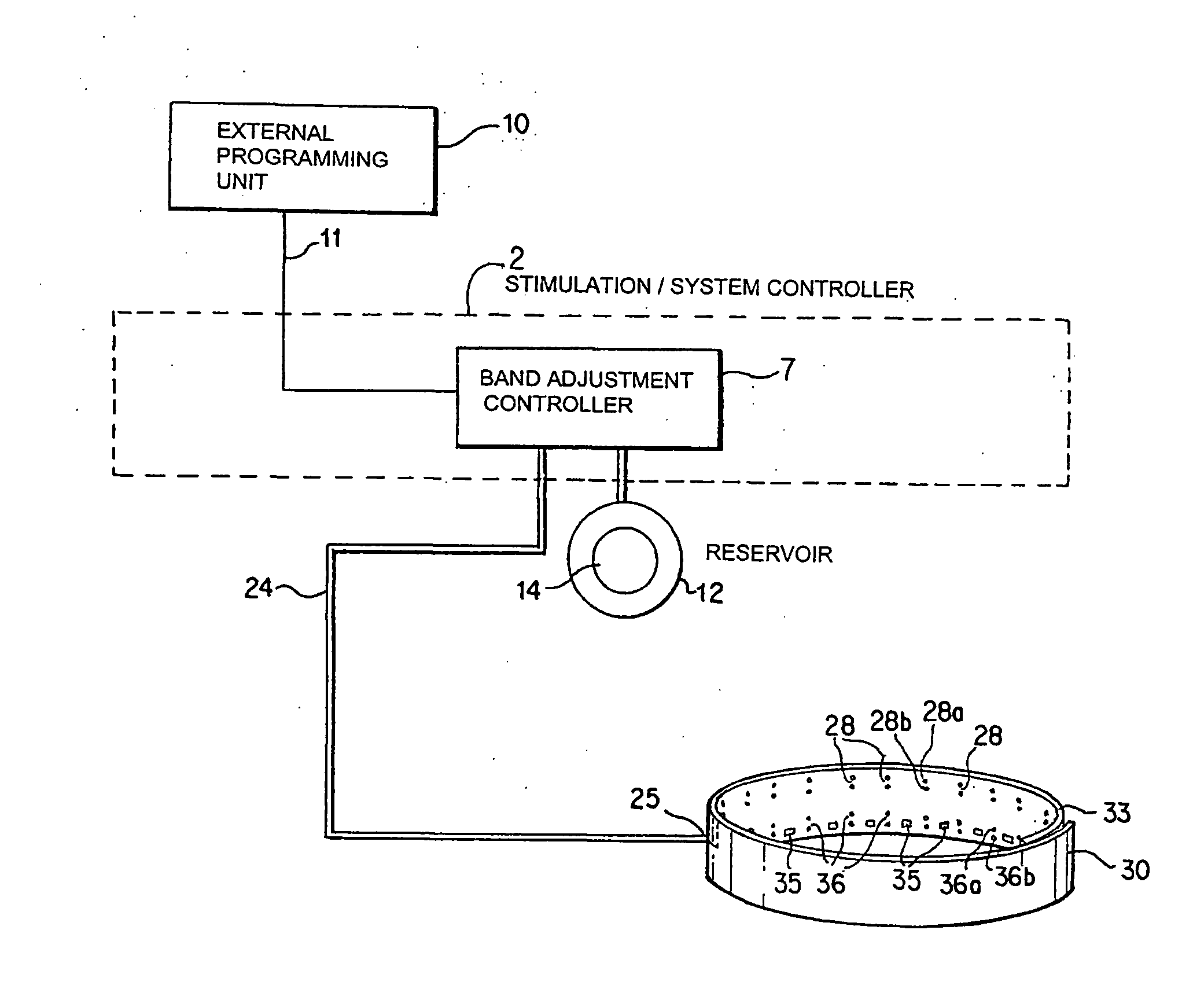
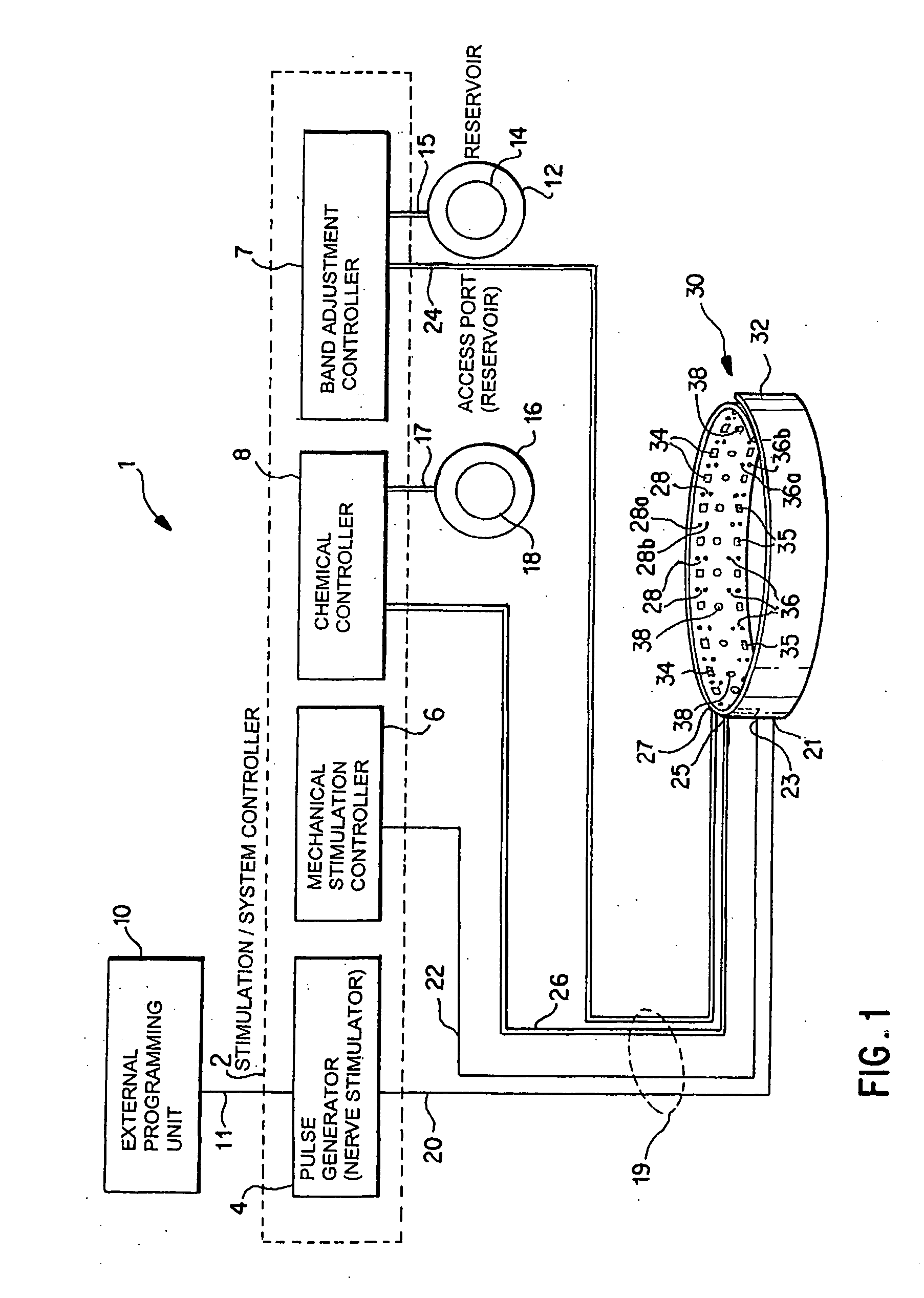
A method and apparatus for treatment of an eating disorder includes electrically, mechanically and / or pharmaceutically / chemically stimulating a of the vagus nerve of the lower esophagus, cardia, esophageal / cardia junction, cardia / fundus junction or upper stomach so as to induce afferent action potentials on the vagus nerve. The device may be noninvasively adjusted after implantation to provide increased or decreased restriction on the patient's gastrointestinal tract. Each stimulus may be administered as a series of programmed pulses of defined amplitude, duration and period, to evoke a responsive signal to the brain by the target nerve, effective for producing a temporary feeling of satiety in the person. An implantable stimulus generator may be operatively coupled to a nerve electrode, pressure device or chemical outlet to apply a defined signal to a selected nerve branch. The implantable stimulus generator is programmable to allow clinician programming of defined signal parameters effective to treat the eating disorder of the patient. Methods are also provided to identify electrodes nearest to a branch of the vagus nerve to apply an electrical stimulation signal with improved efficiency.
Owner:CYBERONICS INC
Oral devices and methods for controlled drug release
Drug dosage forms, which are housed in oral devices, and methods for controlled drug release are provided. The oral devices are permanently or removably inserted in the oral cavity and refilled or replaced as needed. The controlled drug release may be passive, based on the dosage form, or electronically controlled, for a high-precision, intelligent, drug delivery. Additionally, the controlled release may be any one of the following: release in accordance with a preprogrammed schedule, release at a controlled rate, delayed release, pulsatile release, chronotherapeutic release, closed-loop release, responsive to a sensor's input, release on demand from a personal extracorporeal system, release in accordance with a schedule specified by a personal extracorporeal system, release on demand from a monitoring center, via a personal extracorporeal system, and release in accordance with a schedule specified by a monitoring center, via a personal extracorporeal system. Drug absorption in the oral cavity may be assisted by an electrotransport mechanism. The oral devices require refilling or replacement at relatively long intervals of weeks or months, maintain a desired dosage level in the oral cavity, hence in the gastrointestinal tract, for extended periods, address situations of narrow drug therapeutic indices, and by being automatic, ensure adherence to a prescribed medication regimen.
Owner:WOLFAF ANDY +1
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Methods for treating gastrointestinal disorders
A small implantable stimulator(s) having at least two electrodes is implanted adjacent to a gastrointestinal nerve and / or muscle for the stimulation treatment of gastrointestinal disorders, including gastrointestinal motility, sphincteric disorders, and / or eating disorders. The stimulator provides a means of stimulating tissue at a stimulation site when desired, and may be implanted via a minimal surgical procedure.
Owner:BOSTON SCI NEUROMODULATION CORP
Intestinal sleeve
ActiveUS20050125075A1Controlled absorptionChange habitsSuture equipmentsStentsGastrointestinal deviceInsertion stent
Method and apparatus for limiting absorption of food products in specific parts of the digestive system is presented. A gastrointestinal implant device is anchored in the duodenum and extends beyond the ligament of Treitz. All food exiting the stomach is funneled through the device. The gastrointestinal device includes an anchor for attaching the device to the duodenum and an unsupported flexible sleeve to limit absorption of nutrients in the duodenum. The anchor can include a stent and / or a wave anchor and is collapsible for catheter-based delivery and removal.
Owner:GI DYNAMICS
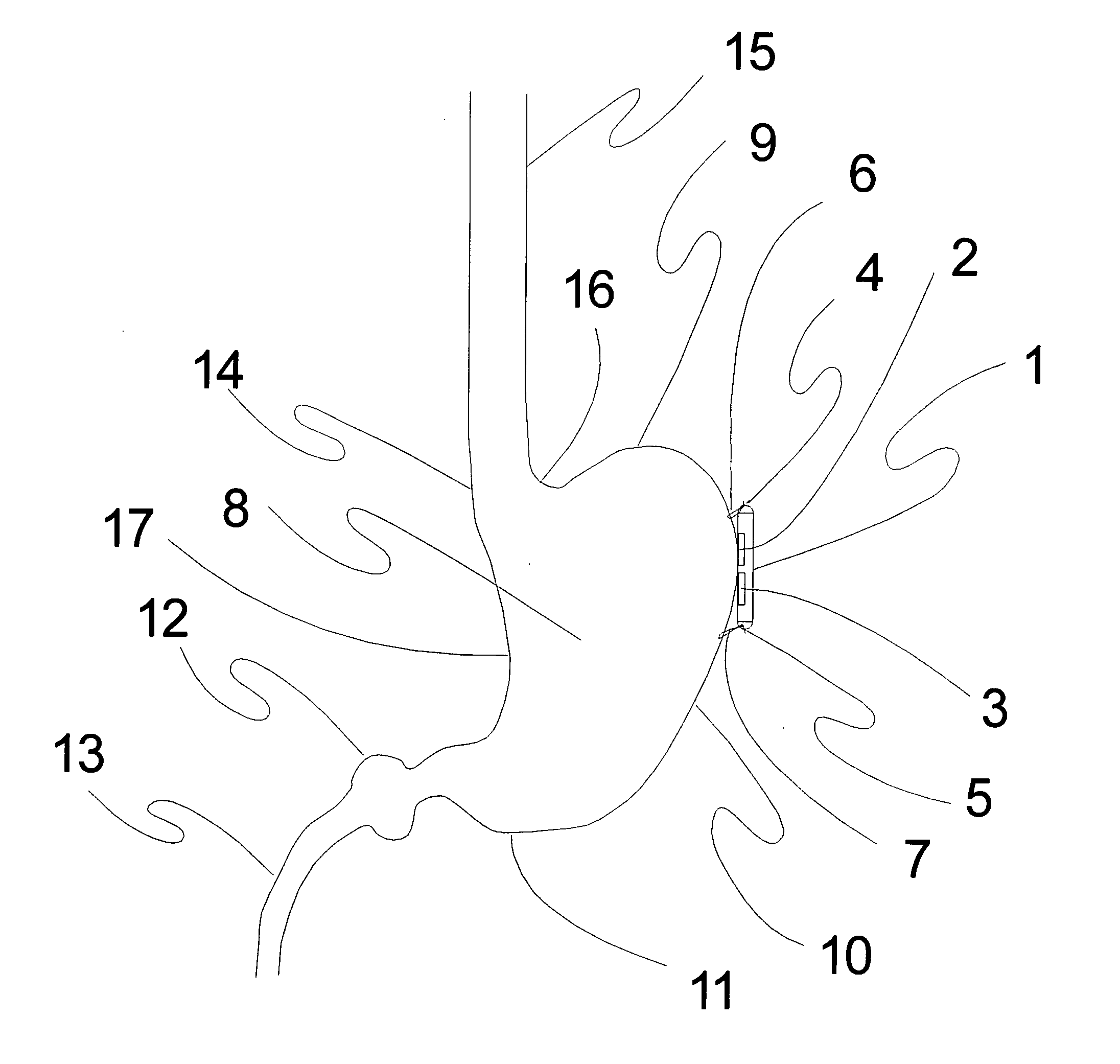
Fluid-assisted medical devices, systems and methods
A medical device (5) is provided which comprises a catheter tube having a distal end and a lumen, and configured to assist in applying tamponage to a bleeding source in a gastrointestinal tract when flexed. A catheter tip having a catheter tip outer surface is assembled with the tube adjacent the distal end of the tube. The catheter tip comprises a probe body comprising an electrically insulative material, at least one electrode pair located on the probe body which comprises a first electrode spaced from a second electrode, and a fluid distribution manifold to direct a fluid from inside the probe body towards the tip outer surface. The manifold comprises a central passage within the probe body and a plurality of lateral passages which extend from the central passage towards the tip outer surface. An extendable injection needle is housed within the central passage to provide treatment to tissue.
Owner:MEDTRONIC ADVANCED ENERGY
Treatment of gastro-intestinal disorders
InactiveUS6645530B1Dampen bacterial inactivationAcid secretion in the stomach could also be pharmacologically suppressedBiocideMilk preparationEscherichia coliDisease
A method of treating chronic disorders associated with the presence of abnormal microflora or an abnormal distribution of microflora in the gastrointestinal tract involves removing the host's existing enteric microflora and substitution of feces from a disease screener donor or composition comprising microorganism selected from the group consisting of Bacteroides and E. coli.
Owner:CRESTOVO LLC
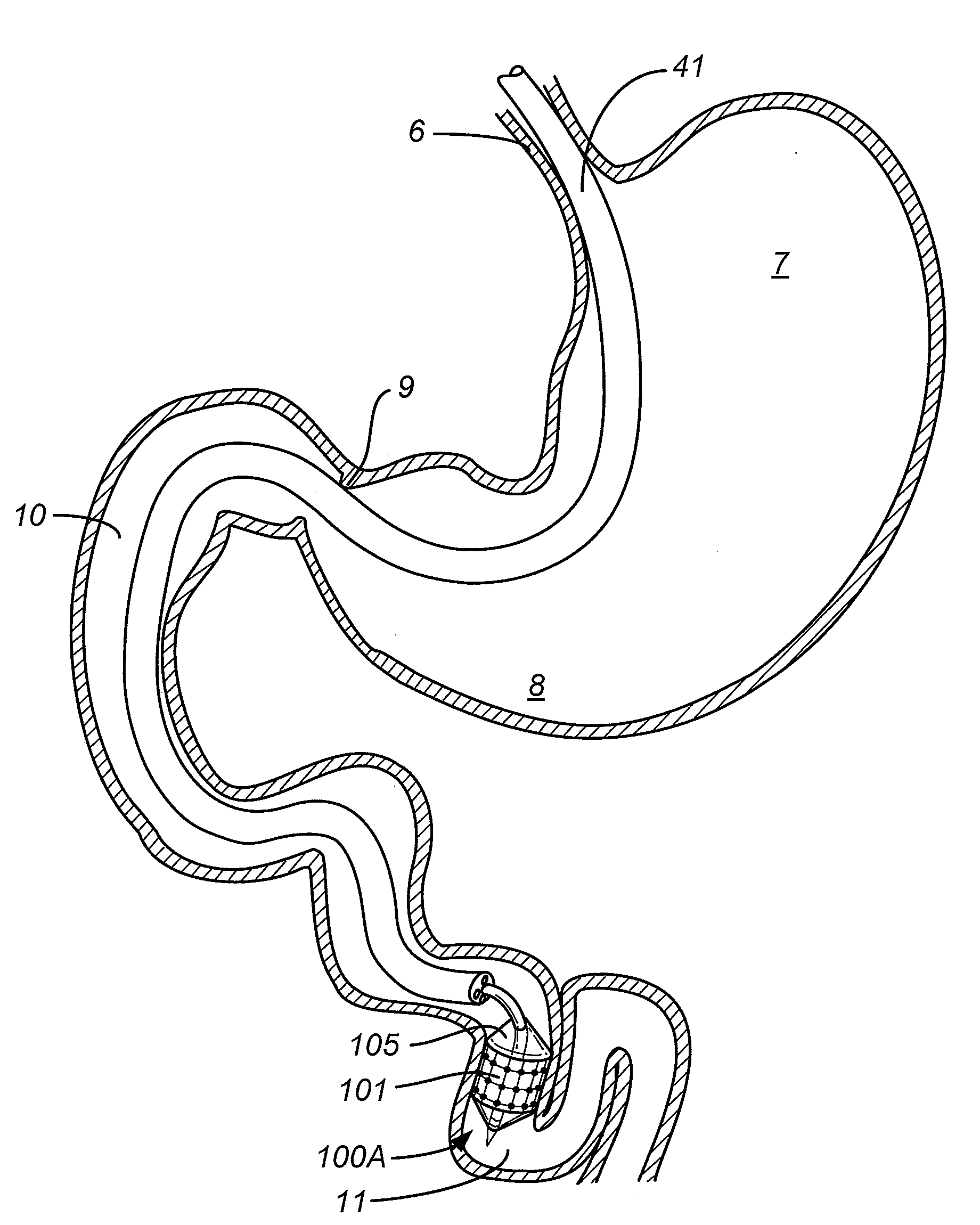
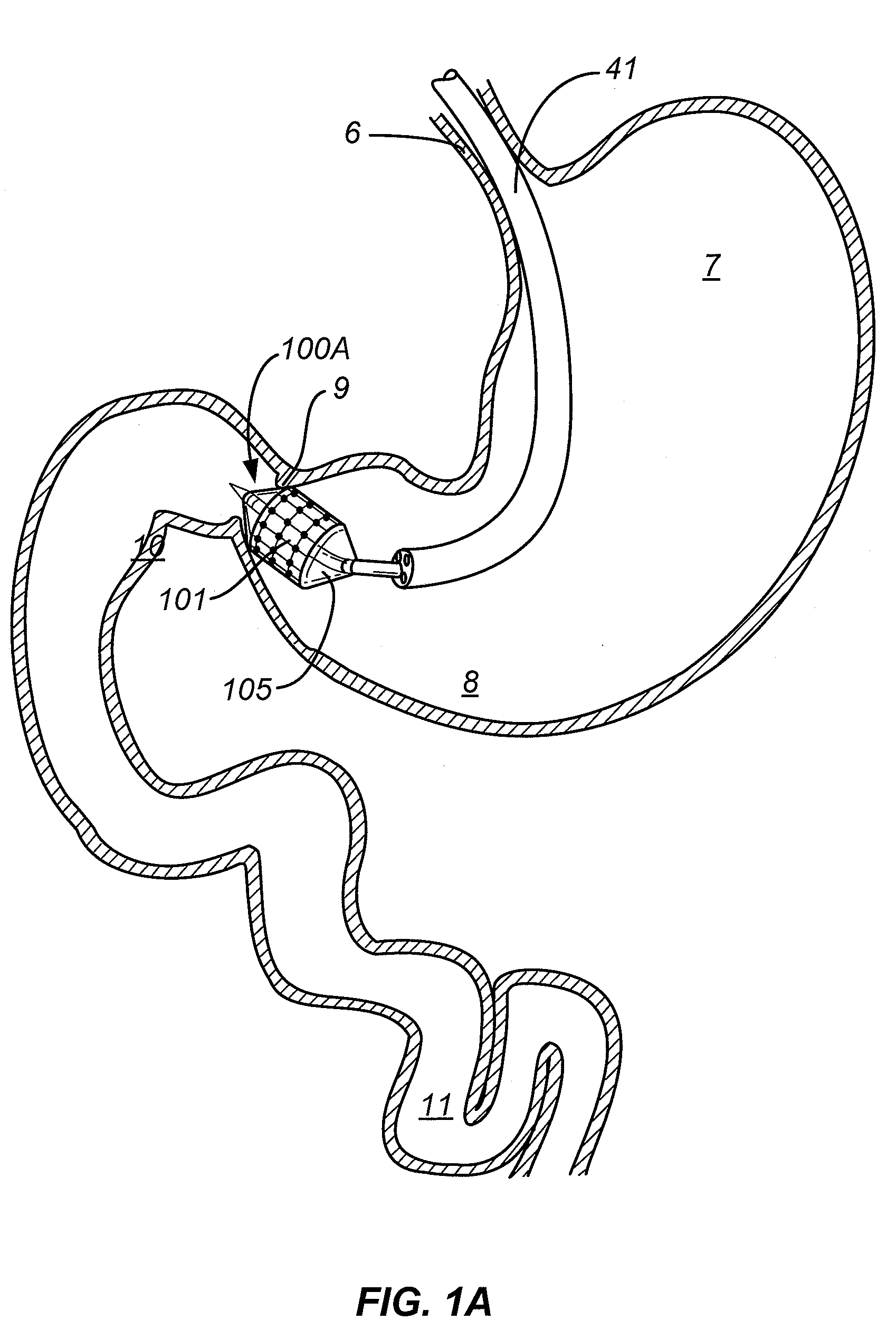
Gastrointestinal implant system
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:DANN MITCHELL +4
Methods and devices for placing a gastrointestinal sleeve
Methods and systems for delivering or placing a gastrointestinal implant device into a mammal. The gastrointestinal implant device can be used to limit absorption of food products in specific parts of the digestive system and can include a gastrointestinal sleeve having an anchor portion and a barrier or sleeve portion. The methods include endoluminal delivery of the device.
Owner:GI DYNAMICS
Method and apparatus for the treatment of obesity
InactiveUS20050038415A1Improve effectivenessImprove solubilityMetabolism disorderDigestive systemDrug compoundGastrointestinal transit
The present invention includes methods and materials for manipulating the sense of satiety developed from the gastrointestinal transit of a substance in a mammal, whether the substance be a food or drug compound. The method involves administering a therapeutically effective amount, by a direct delivery route, of a pharmaceutically acceptable formulation comprising nutrients and pharmacological agents to the mammal's gastrointestinal tract. The present system is designed to maximize satiety feedback from normal intestinal sensors by small amounts of nutrients or nutrient derivatives, in essence, to “fool” body sensors that are not usually in contact with nutrients unless very large amounts are ingested.
Owner:ETHICON ENDO SURGERY INC
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
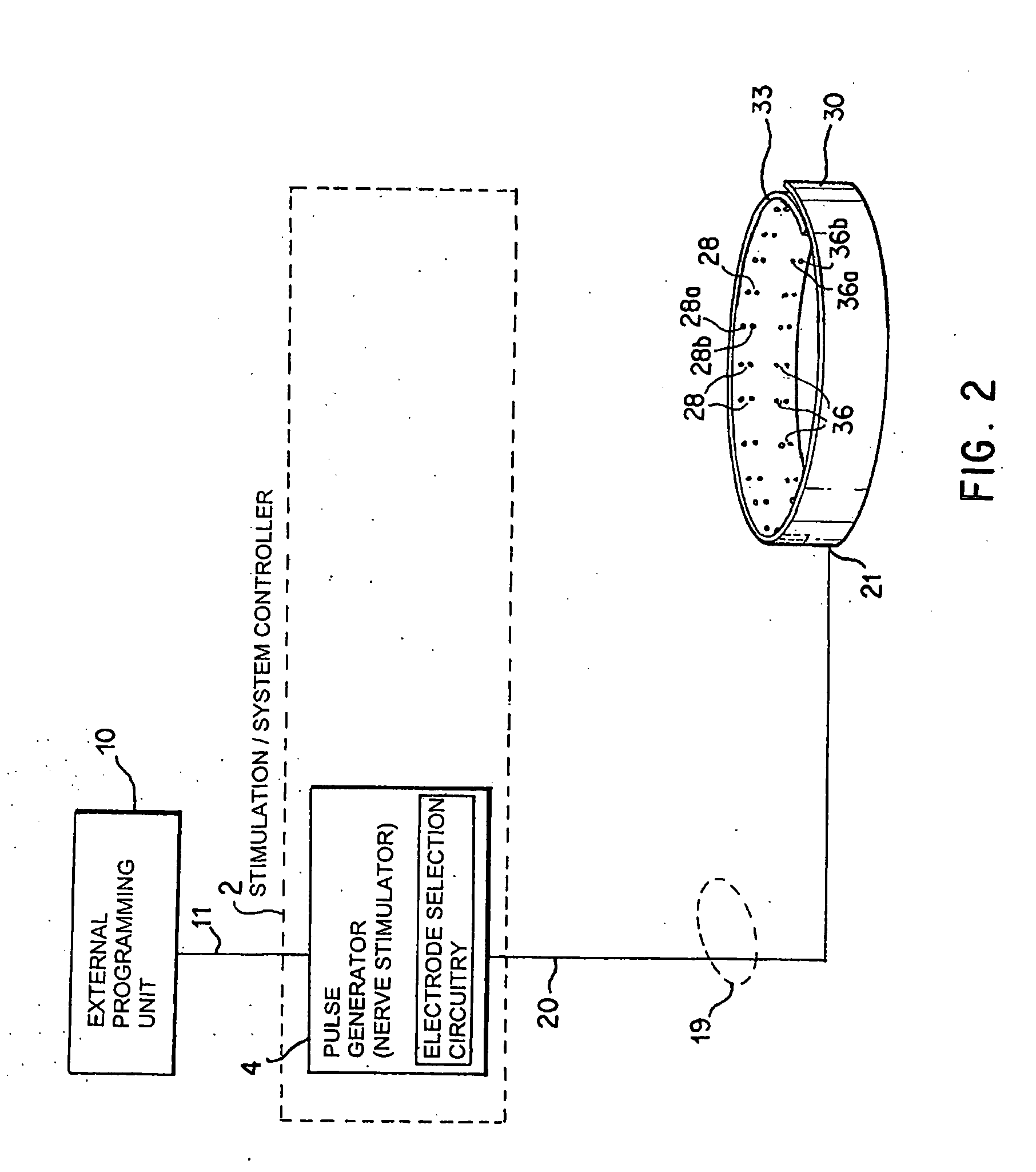
Identification of electrodes for nerve stimulation in the treatment of eating disorders
A method and apparatus for treatment of an eating disorder includes electrically, mechanically and / or pharmaceutically / chemically stimulating a of the vagus nerve of the lower esophagus, cardia, esophageal / cardia junction, cardia / fundus junction or upper stomach so as to induce afferent action potentials on the vagus nerve. The device may be noninvasively adjusted after implantation to provide increased or decreased restriction on the patient's gastrointestinal tract. Each stimulus may be administered as a series of programmed pulses of defined amplitude, duration and period, to evoke a responsive signal to the brain by the target nerve, effective for producing a temporary feeling of satiety in the person. An implantable stimulus generator may be operatively coupled to a nerve electrode, pressure device or chemical outlet to apply a defined signal to a selected nerve branch. The implantable stimulus generator is programmable to allow clinician programming of defined signal parameters effective to treat the eating disorder of the patient. Methods are also provided to identify electrodes nearest to a branch of the vagus nerve to apply an electrical stimulation signal with improved efficiency.
Owner:LIVANOVA USA INC
Method and apparatus for gastrointestinal tract ablation for treatment of obesity
ActiveUS20080275445A1Reduce rateSlowing of gastric emptyingCatheterSurgical instruments for heatingBiological activationElectrode array
Devices and methods for ablating tissue in the wall of various organs of the gastrointestinal tract of a patient in order to cure or ameliorate metabolic pathophysiological conditions such as obesity, insulin resistance, or type 2 diabetes mellitus are provided. Ablational treatment of target areas may be fractional or partial, rendering a post-treatment portion of target tissue ablated and another portion that is substantially intact. Fractional ablation is achieved by controlling the delivery of ablational energy across the surface area being treated, and controlling the depth of energy penetration into tissue. Surface area control of energy delivery may controlled by the spatial pattern of distributed ablation elements or by the selective activation of a subset of a dense pattern of ablation elements. Embodiments of the device include an ablational electrode array that spans 360 degrees and an array that spans an arc of less than 360 degrees.
Owner:TYCO HEALTHCARE GRP LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com