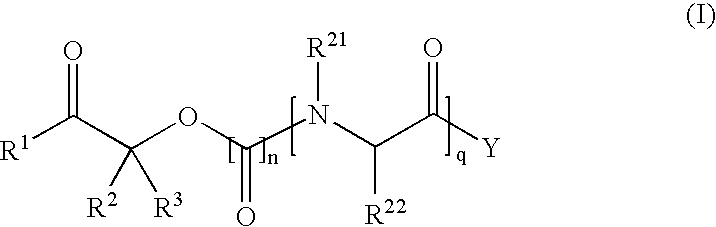
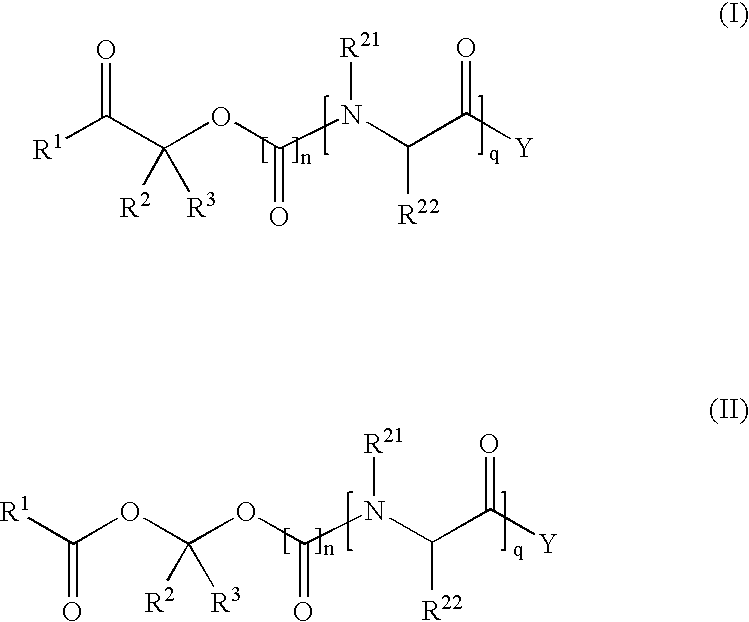
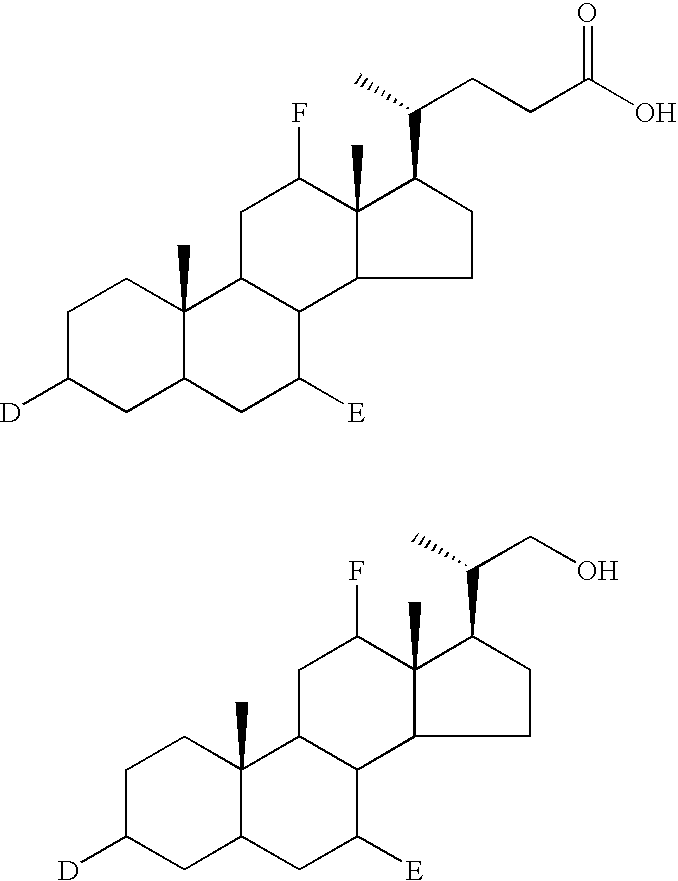
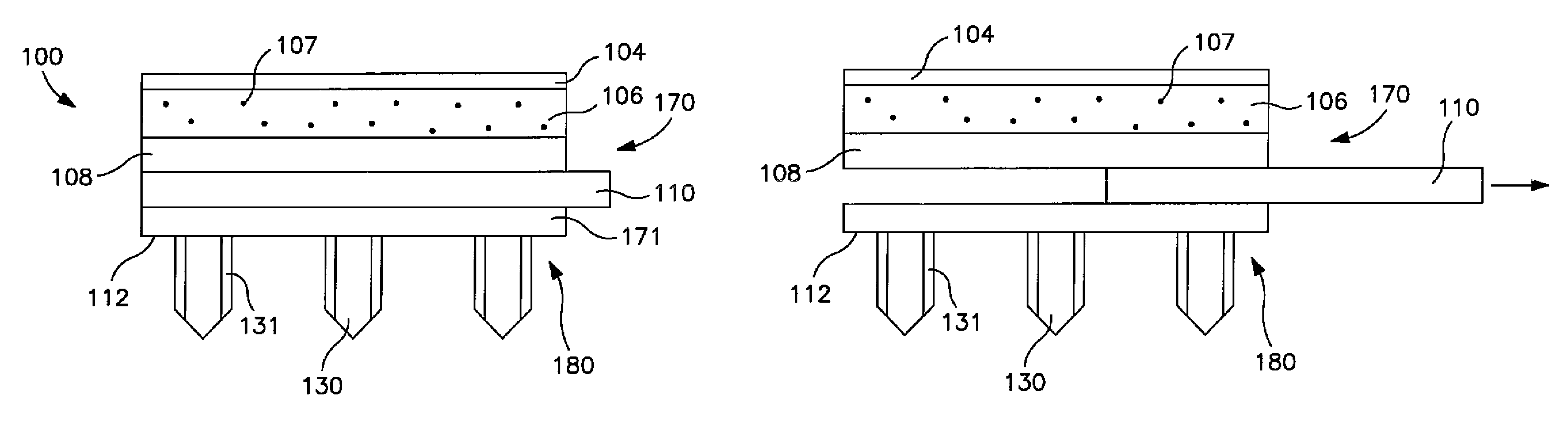
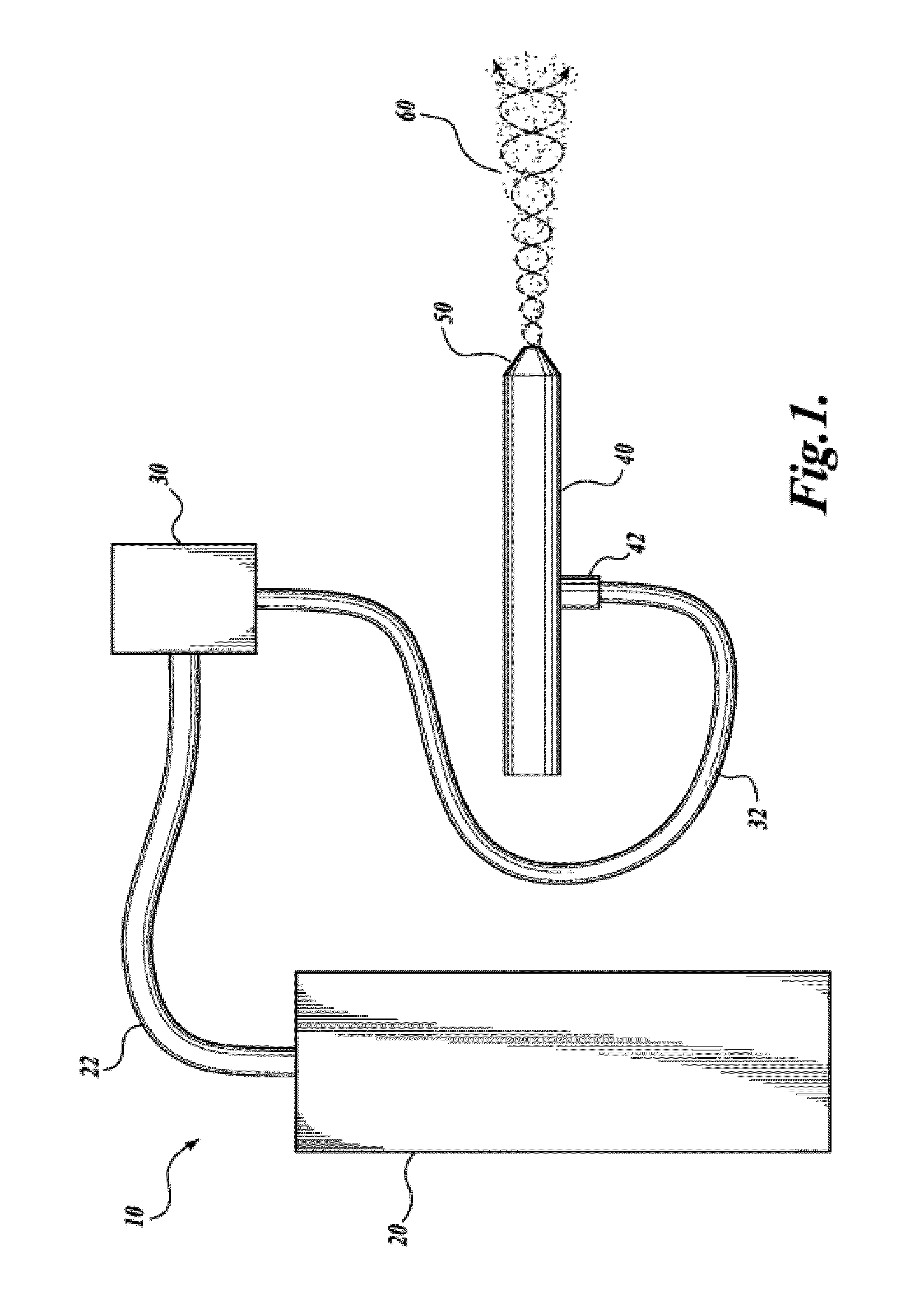
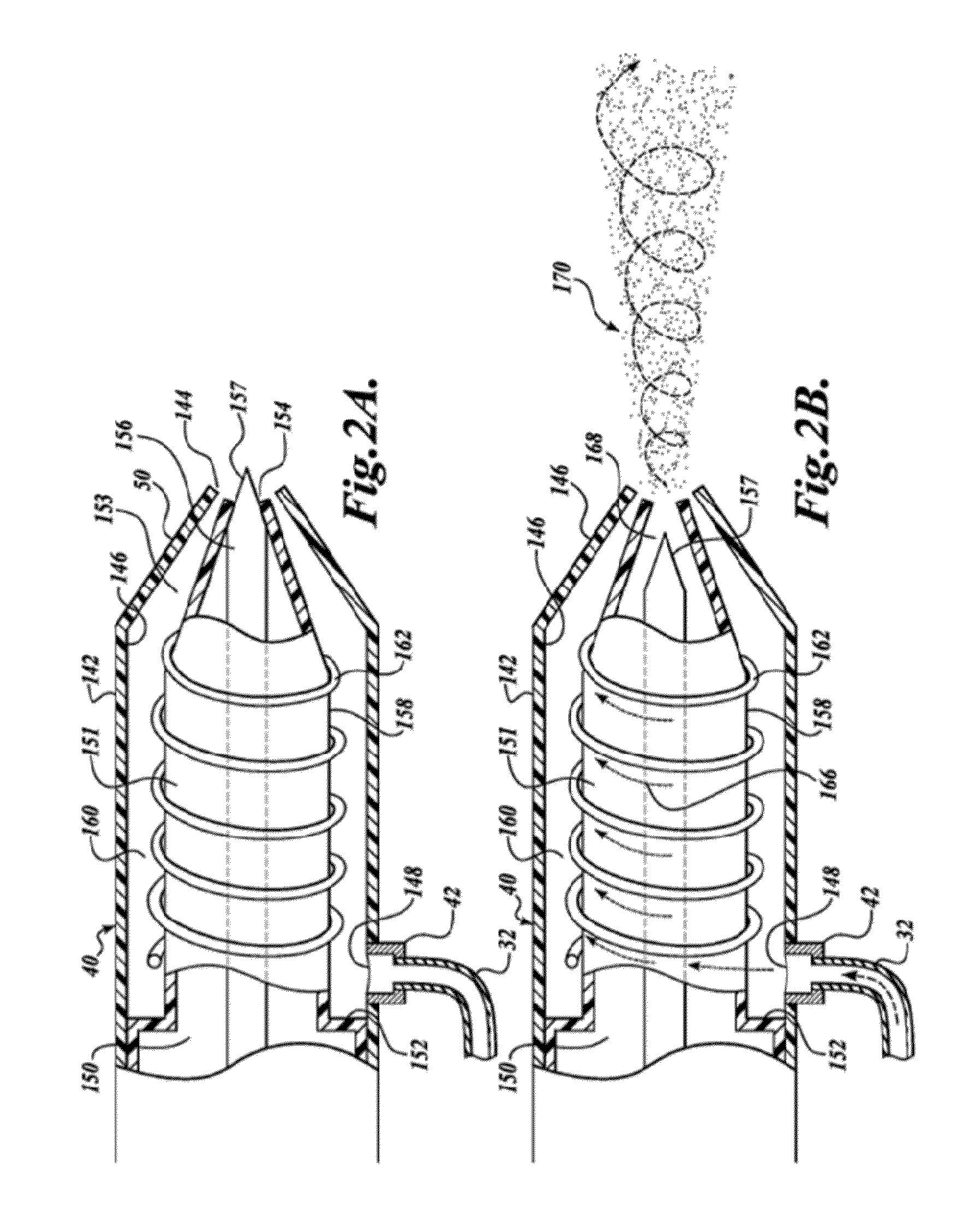
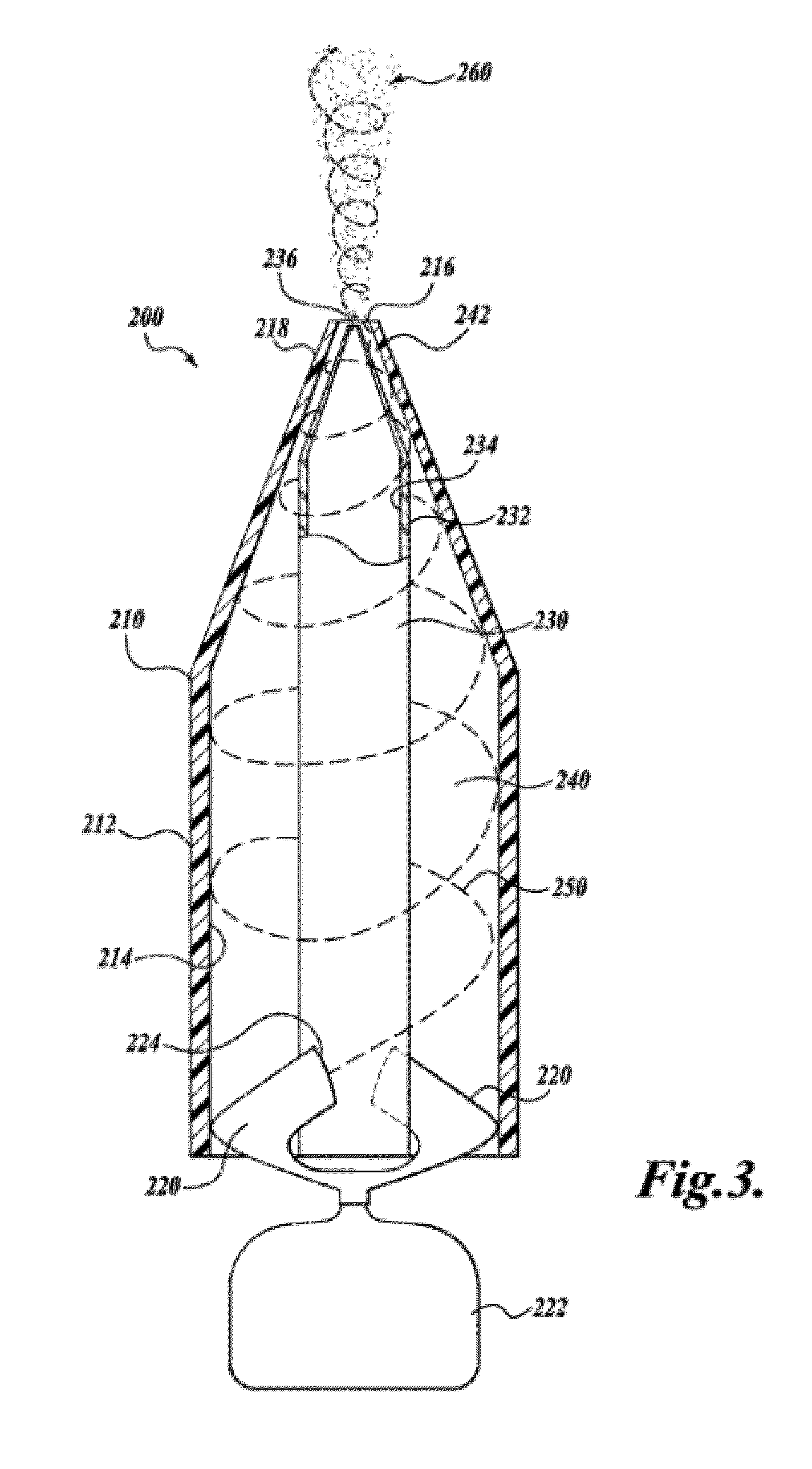
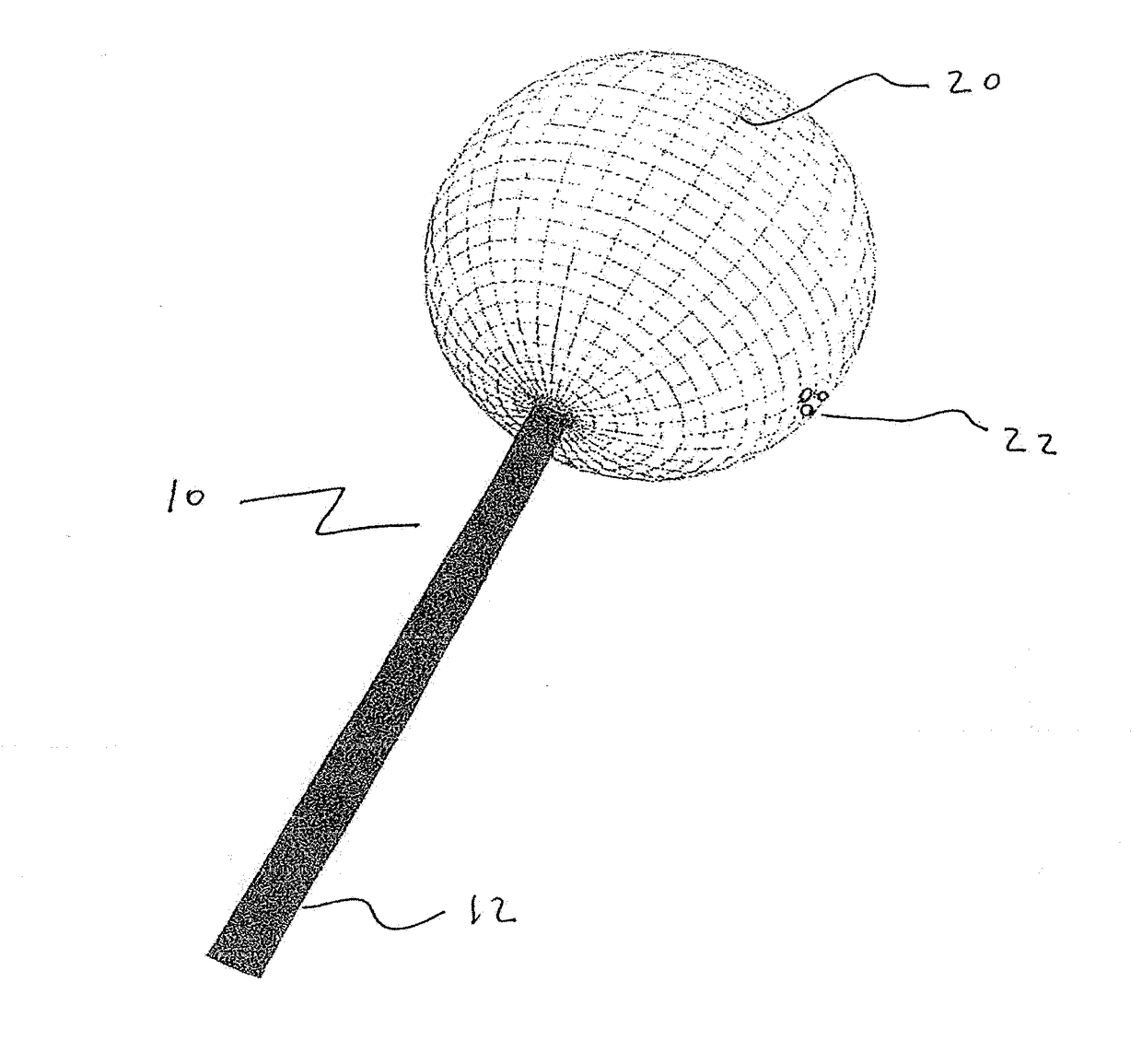
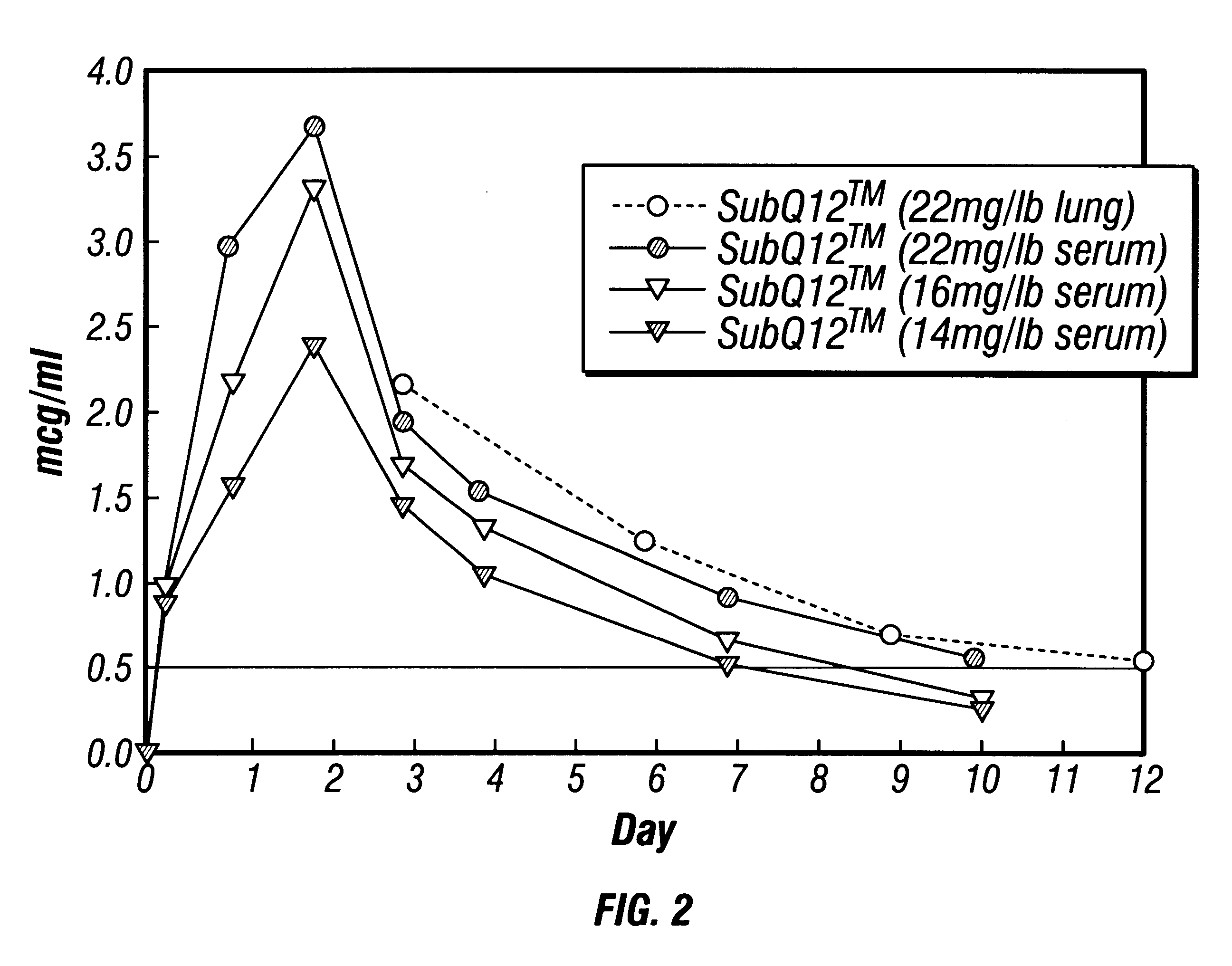
Patents
Literature
1255 results about "Drug compound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
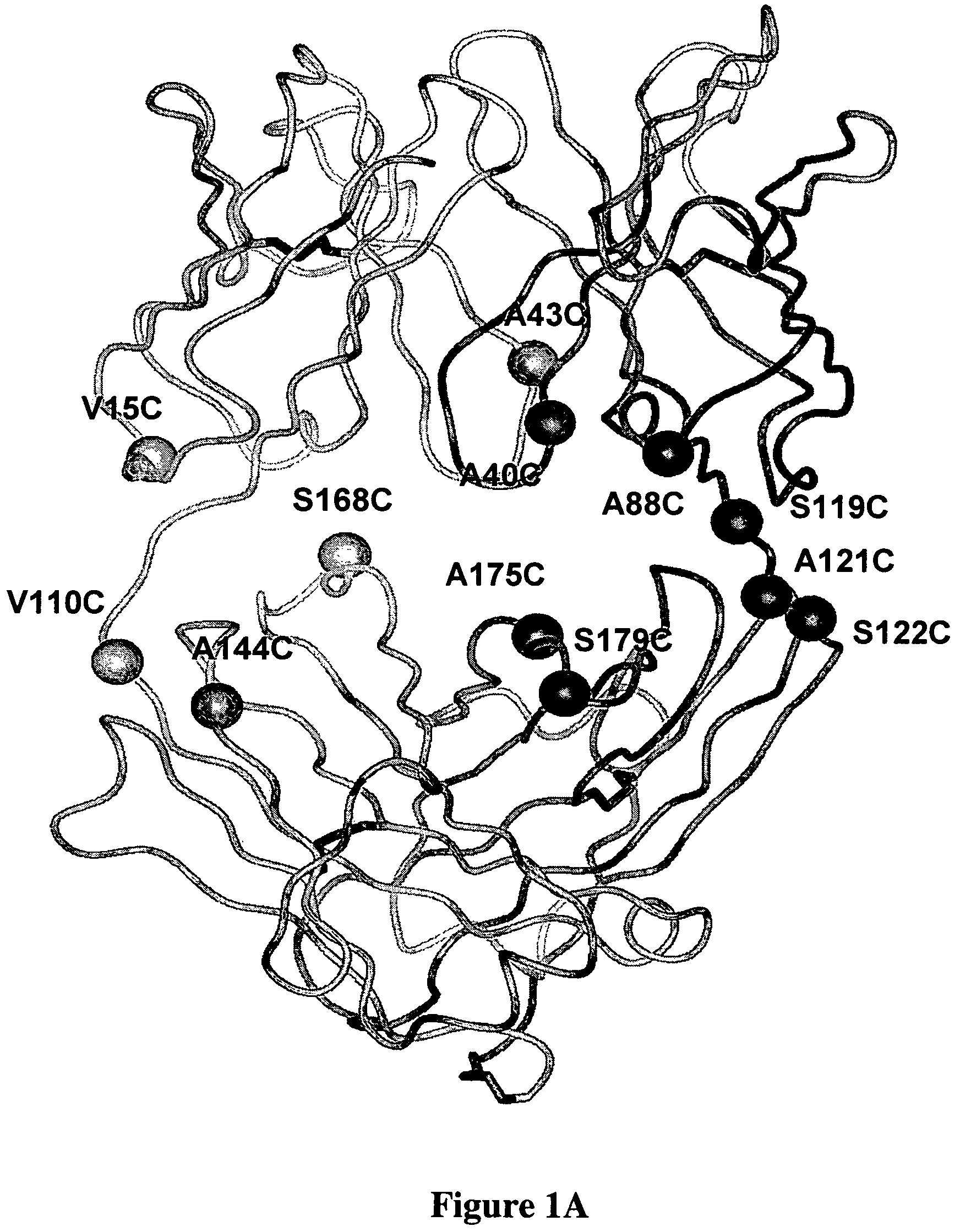
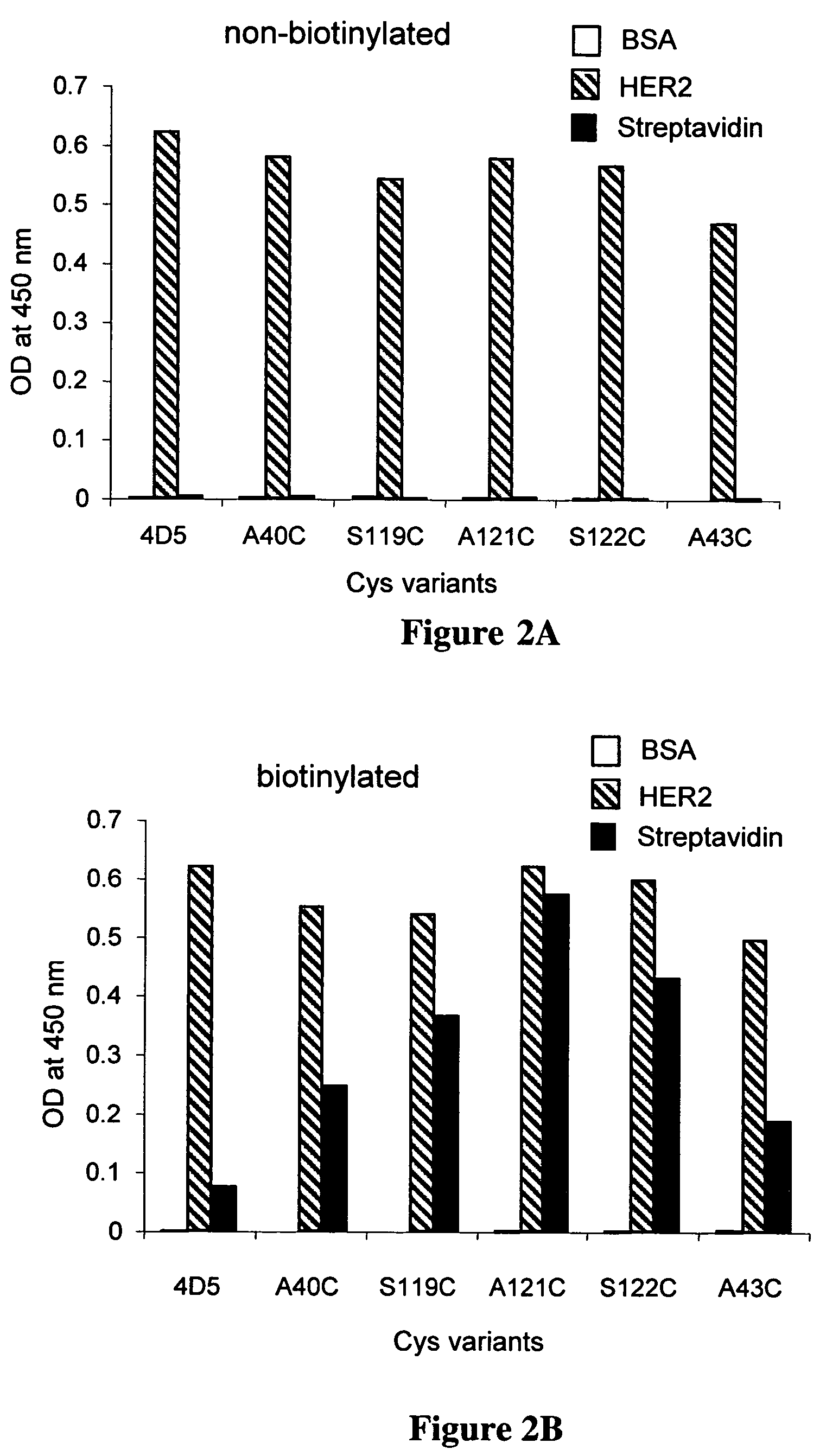
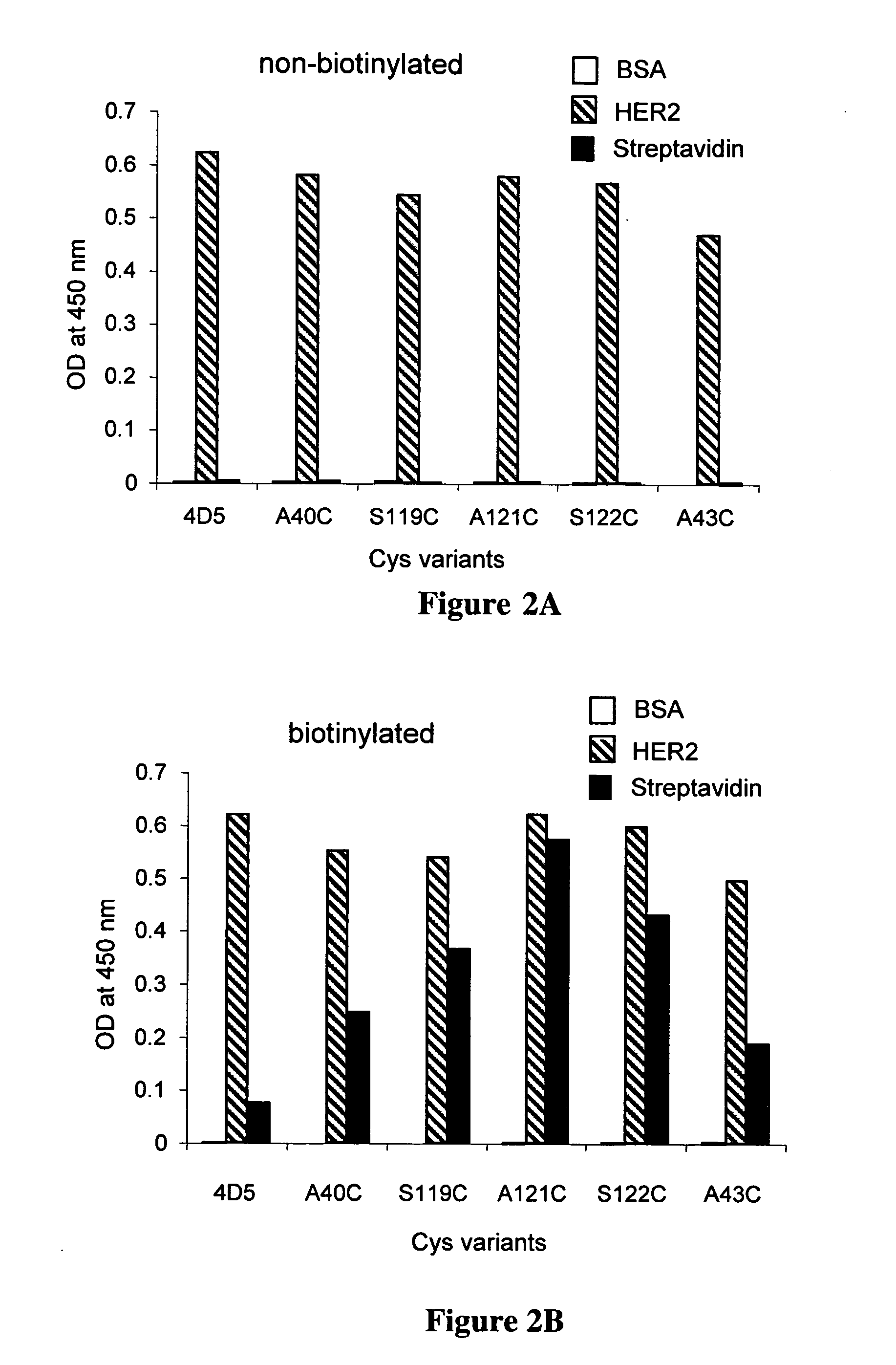
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
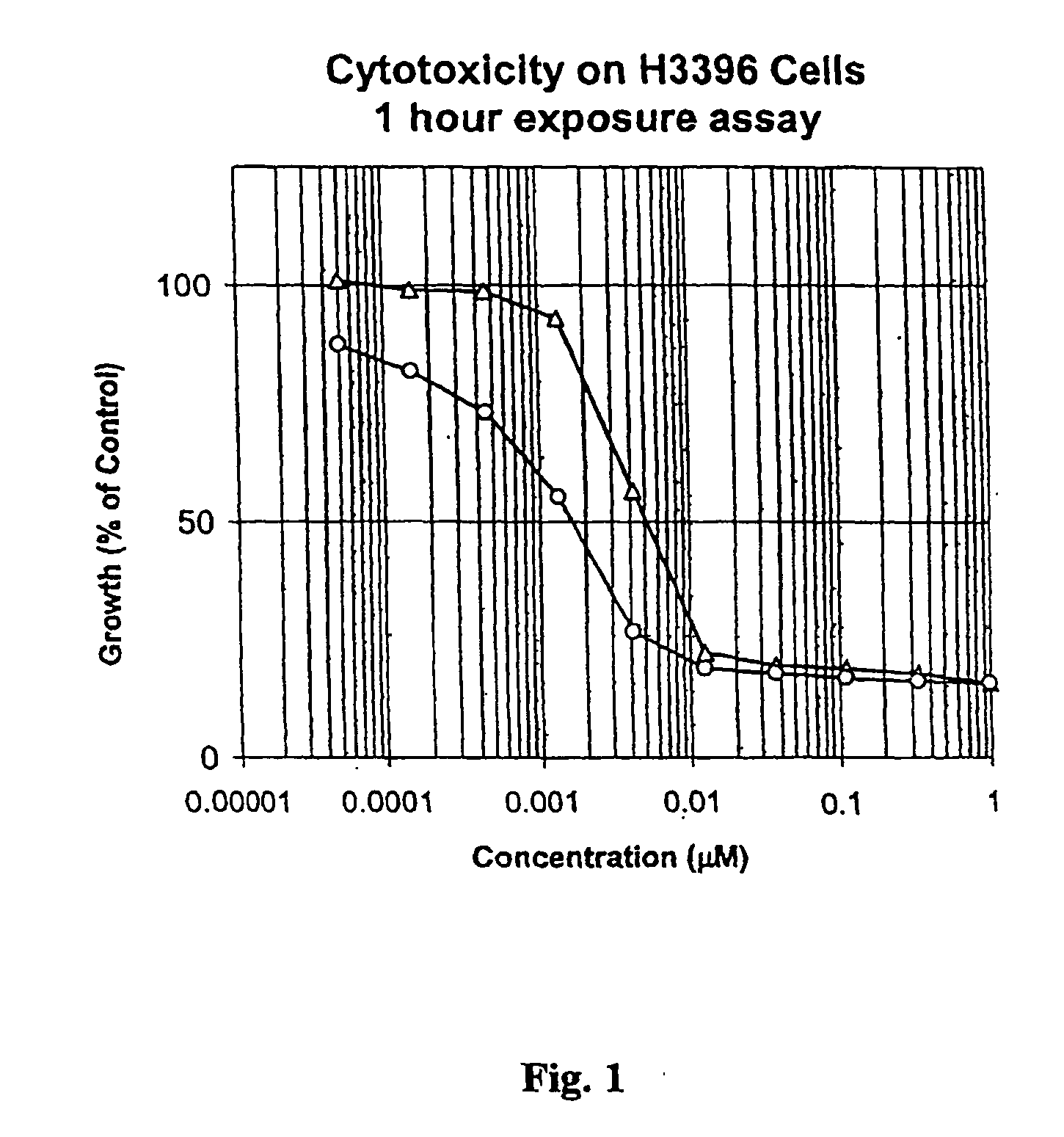
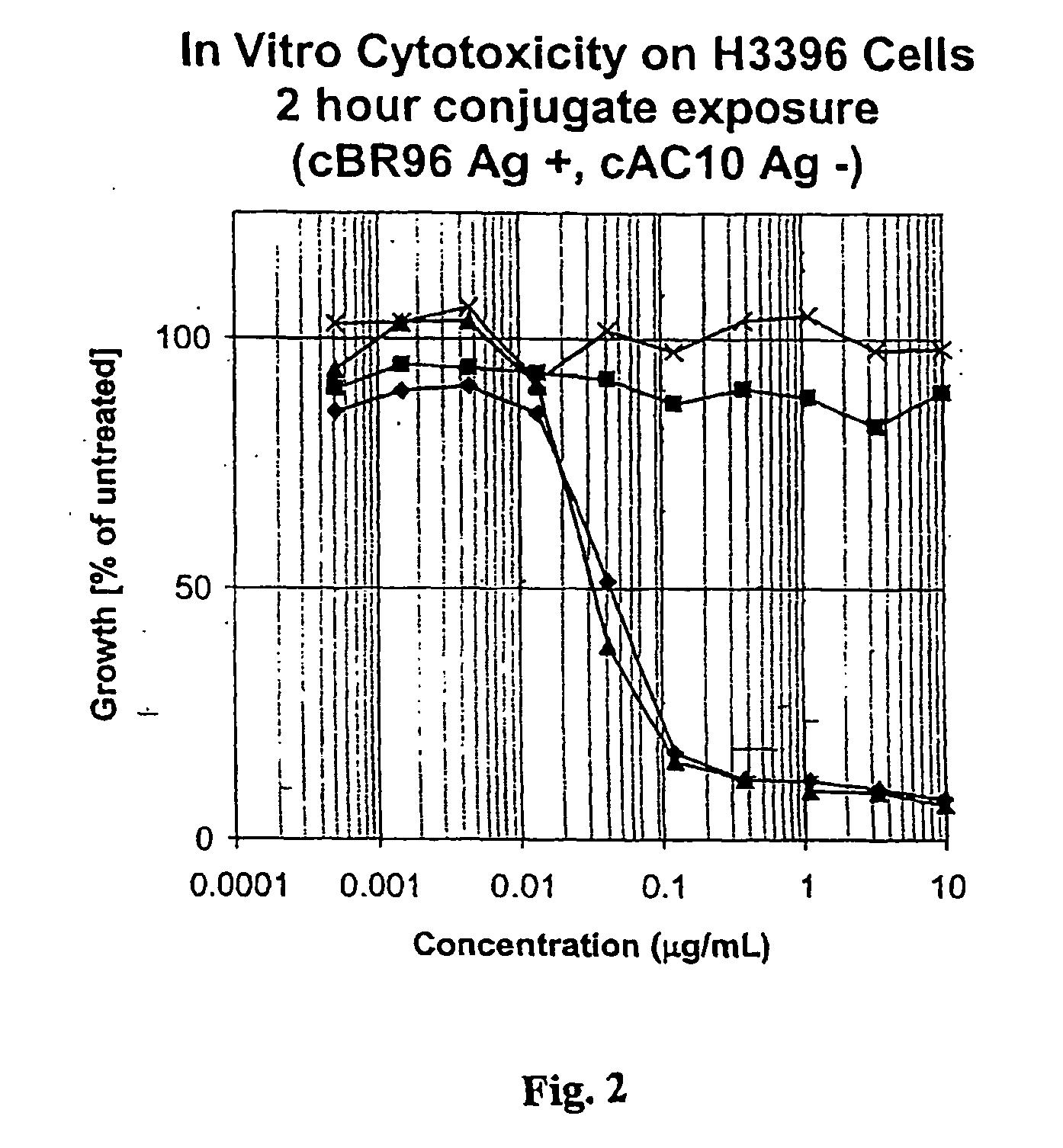
Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease
Drug-Linker-Ligand Conjugates are disclosed in which a Drug is linked to a Ligand via a peptide-based Linker unit. In one embodiment, the Ligand is an Antibody. Drug-Linker compounds and Drug compounds are also disclosed. Methods for treating cancer, an autoimmune disease or an infectious disease using the compounds and compositions of the invention are also disclosed.
Owner:SEAGEN INC
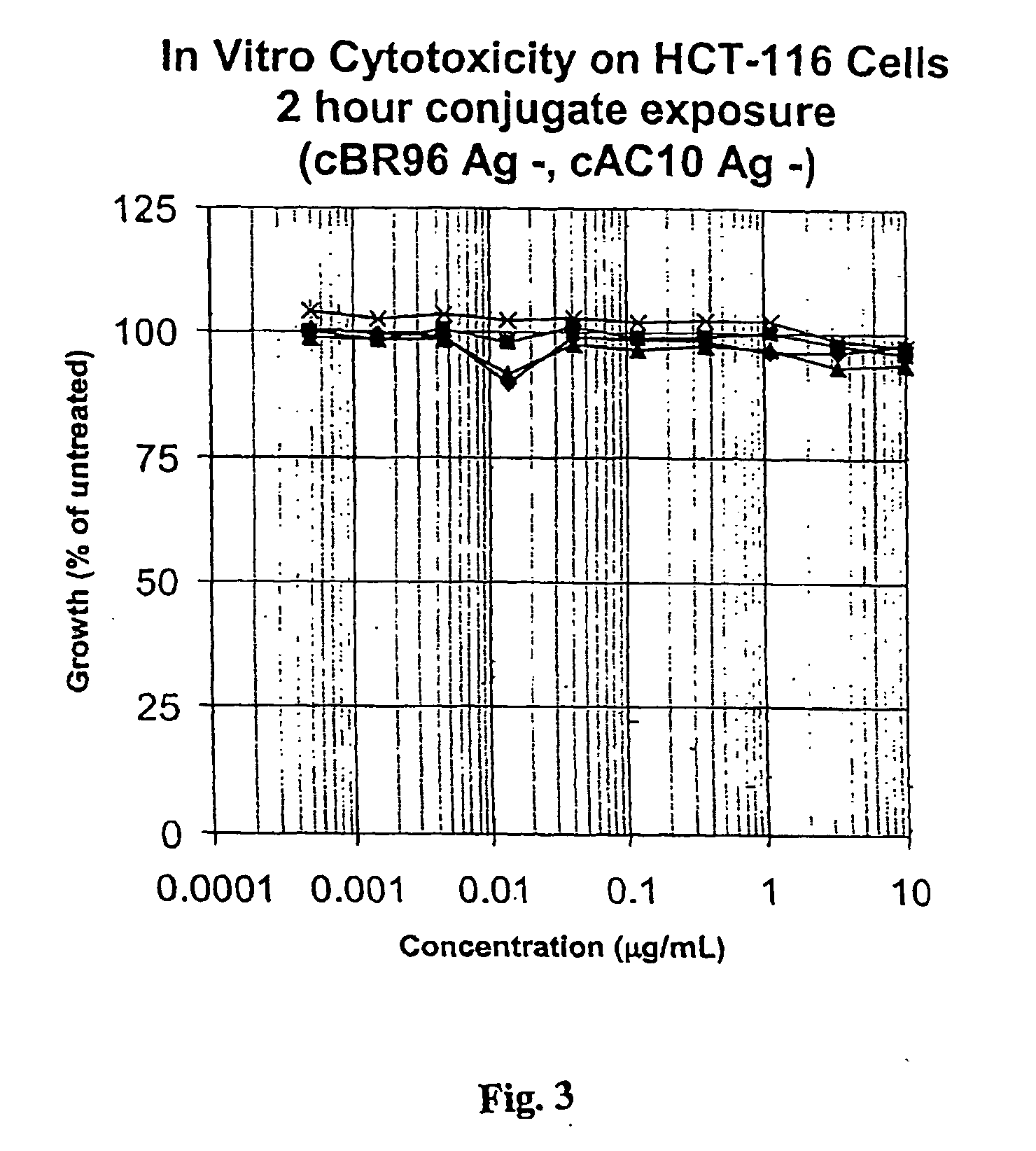
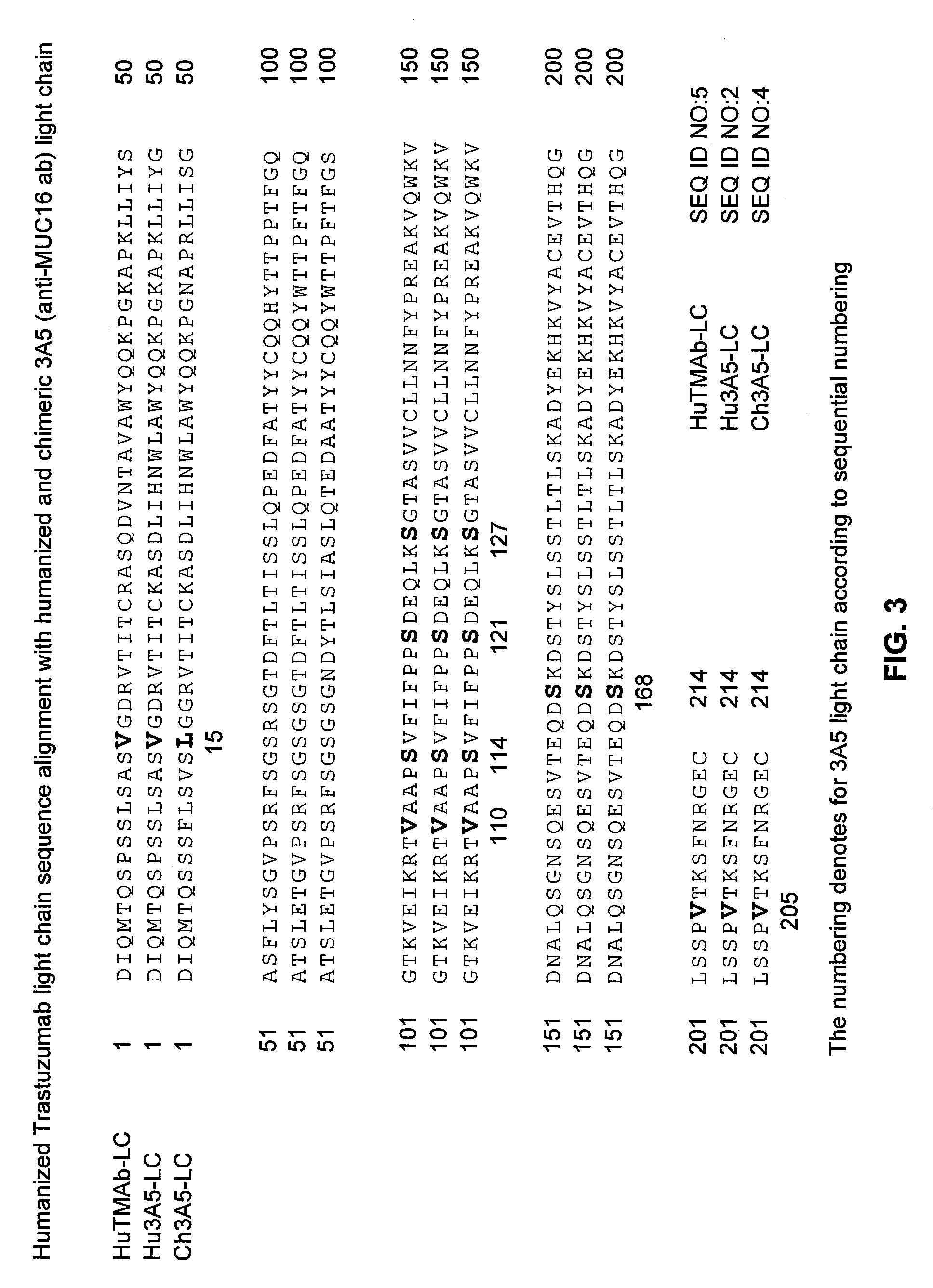
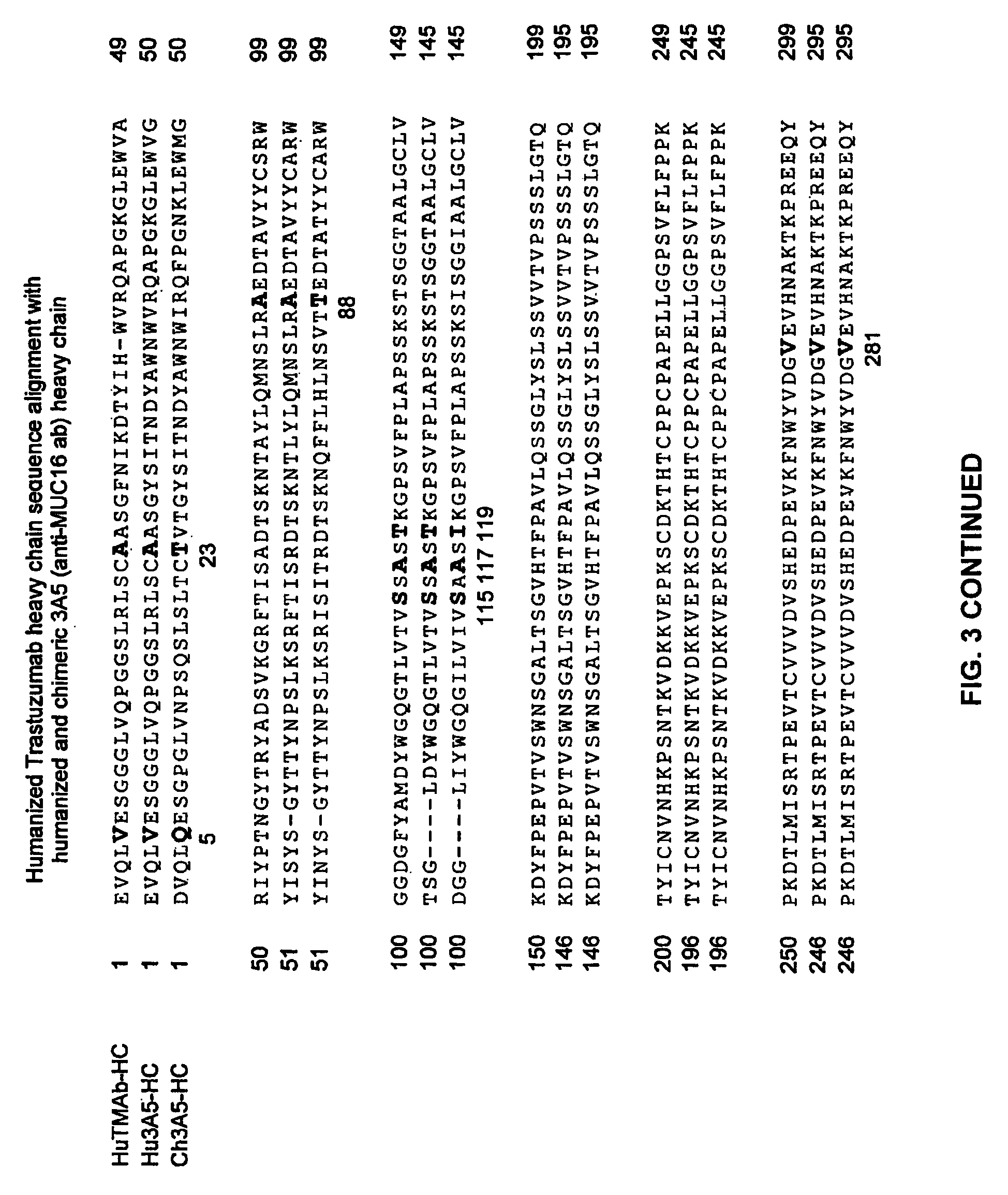
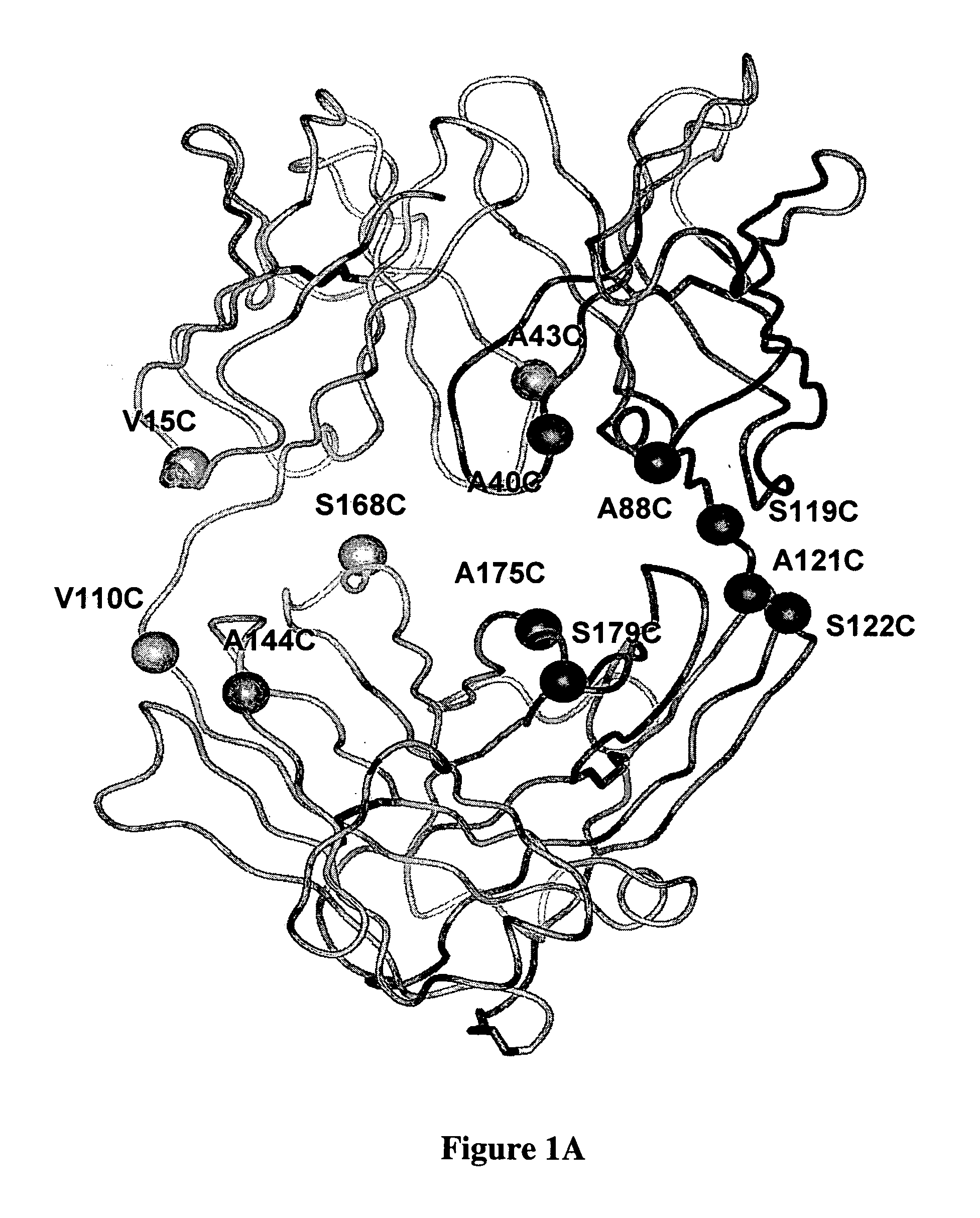
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Method and apparatus for the treatment of obesity
InactiveUS20050038415A1Improve effectivenessImprove solubilityMetabolism disorderDigestive systemDrug compoundGastrointestinal transit
The present invention includes methods and materials for manipulating the sense of satiety developed from the gastrointestinal transit of a substance in a mammal, whether the substance be a food or drug compound. The method involves administering a therapeutically effective amount, by a direct delivery route, of a pharmaceutically acceptable formulation comprising nutrients and pharmacological agents to the mammal's gastrointestinal tract. The present system is designed to maximize satiety feedback from normal intestinal sensors by small amounts of nutrients or nutrient derivatives, in essence, to “fool” body sensors that are not usually in contact with nutrients unless very large amounts are ingested.
Owner:ETHICON ENDO SURGERY INC
Method for treating emotional or mental illness and emotional or mental illness concomitant with seizures
Disclosed herein is a method for treating depression associated with alcoholism in a patient comprising administering to the patient a pharmacologically effective dose of an opioid antagonist, and a pharmacologically effective dose of at least one drug compound selected from the group consisting of a tricyclic antidepressant, an a-typical antidepressant, and lithium.
Owner:DANTE LEE G
Apparatus and method for transferring data to a pharmaceutical compounding system
A system and method for use with a data entry system for providing input data to a pharmaceutical compounding device having an associated plurality of source solutions is provided. A first label comprising first indicia defining a desired pharmaceutical compound is generated. The first indicia is then provided to the pharmaceutical compounding device as an input. The pharmaceutical compounding device then prepares a pharmaceutical compound based on the first indicia and generates a second label comprising second indicia indicative of at least the contents of pharmaceutical compound. The first indicia and second indicia are provided to a comparison device where the contents of the actual pharmaceutical compound as indicated by the second indicia are compared to the desired pharmaceutical compound as indicated by the first indicia.
Owner:B BRAUN MEDICAL
Laser probes for drug permeation
InactiveUS6389313B1Improve diffusivityImprove permeabilityElectrotherapyEar treatmentHigh concentrationLaser probe
Owner:SPECTRAL BIOSYST
Delivery of tetrahydrocannabinol
InactiveUS20070104741A1Avoiding hepatic first-pass metabolismGood choiceBiocideNervous disorderChylomicronTG - Triglyceride
A self-emulsifying drug delivery system to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron delivery and optimal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY PHARMA
Process to create biomolecule and/or cellular arrays on metal surfaces and product produced thereby
InactiveUS6127129ABioreactor/fermenter combinationsMaterial nanotechnologyNucleic Acid ProbesBiomolecule
This invention provides isolated nucleic acid molecules encoding a mammalian glycine transporter, isolated nucleic acid molecules encoding a human glycine transporter, isolated proteins which are mammalian glycine transporter proteins, isolated proteins which are human glycine transporter proteins, vectors comprising isolated nucleic acid molecules encoding a mammalian or a human glycine transporter, mammalian cells comprising such vectors, antibodies directed to a mammamlian glycine transporter, antibodies directed to a human glycine transporter, nucleic acid probes useful for detecting nucleic acid encoding mammalian glycine transporter, nucleic acid probes useful for detecting nucleic acid encoding human glycine transporter, antisense oligonucleotides complementary to any sequences of a nucleic acid molecule which encodes a mammalian glycine transporter, antisense oligonucleotides complementary to any sequences of a nucleic said molecule which encodes a human glycine transporter, pharmaceutical compounds related to mammalian glycine transporter and nonhuman transporter, pharmaceutical compounds related to human glycine transporter and nonhuman transgenic animals which express DNA encoding a normal or a mutant mammalian or human glycine transorter. This invention also provides methods for determining ligand binding, detecting expression, drug screening, and treatments for alleviating abnormalities associated with mammalian glycine transporter. This invention further provides methods for determining ligand binding, detecting expression, drug screening, and treatments for alleviating abnormalities associated with human glycine transporter.
Owner:WISCONSIN ALUMNI RES FOUND
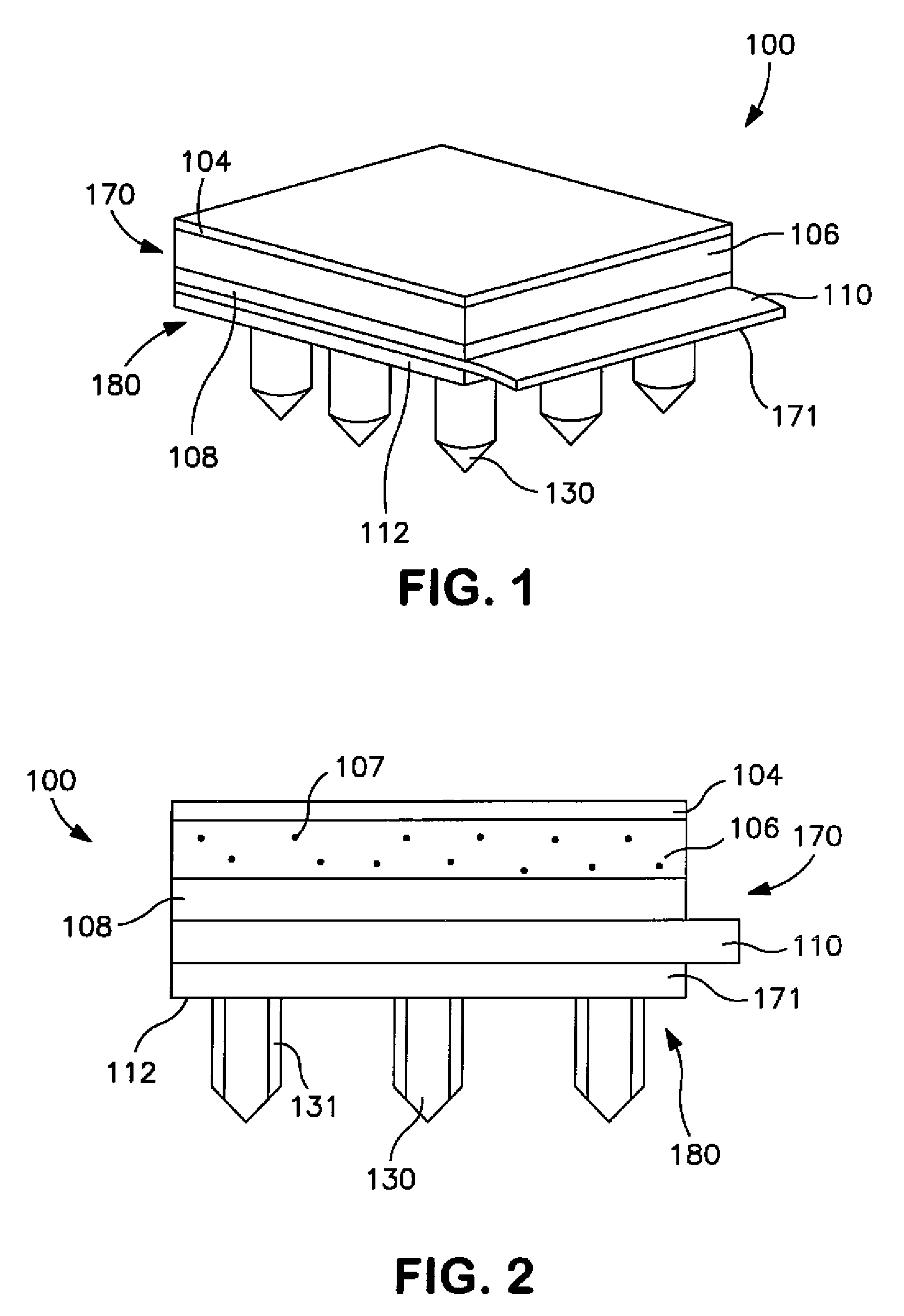
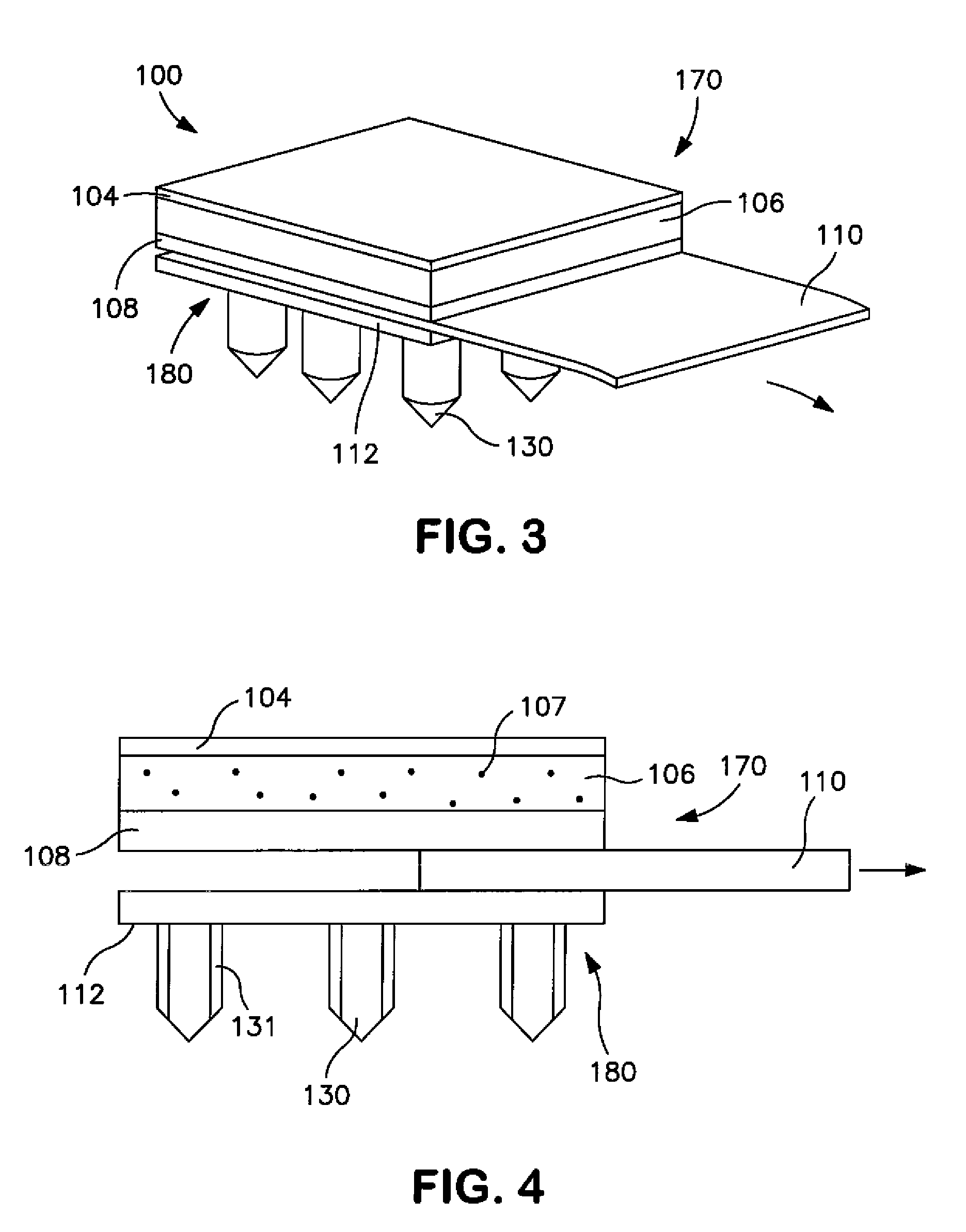
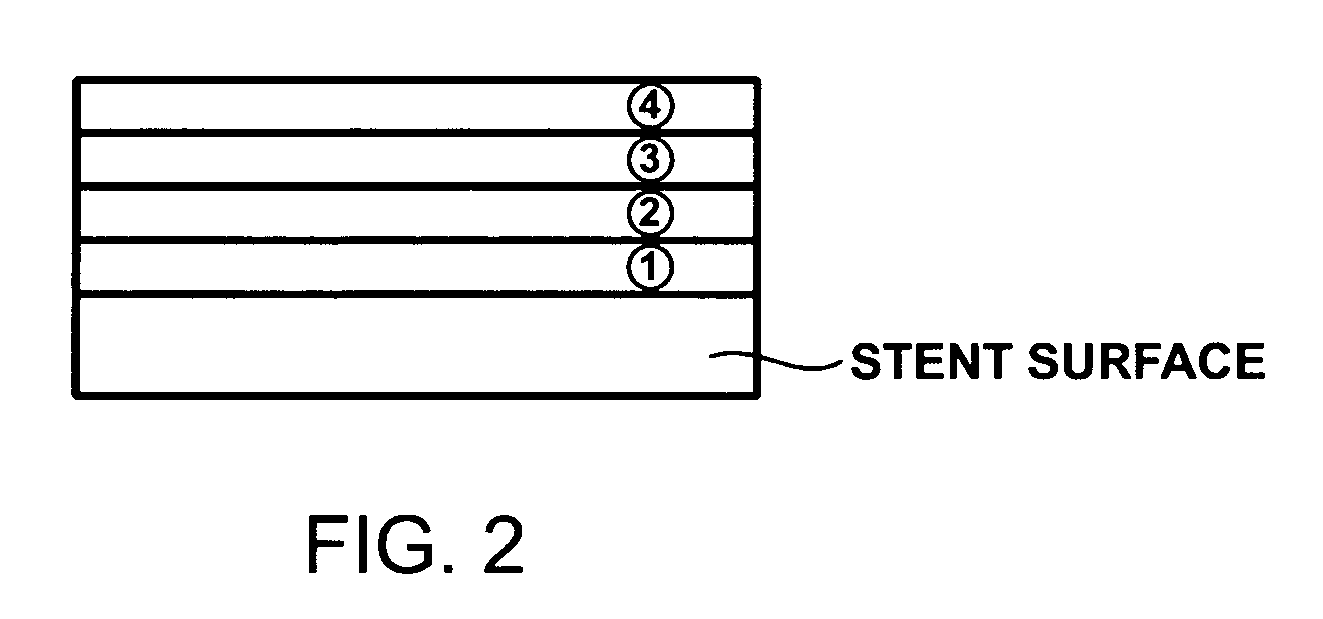
Stent coated with a sustained-release drug delivery and method for use thereof
ActiveUS7279175B2Reduce deliveryReduce solubilitySuture equipmentsAntibacterial agentsSustained release drugDrug compound
An intraluminal medical device comprises a stent having a coating applied to at least part of an interior surface, an exterior surface, or both. The coating comprises a sustained release formulation of a combination of pharmaceutical compounds dispersed within a biologically tolerated polymer composition. The choice of the combination of pharmaceutical compounds are intended to reduce neointimal hyperplasia restenosis.
Owner:PSIVIDA US INC
Apparatus and method for transferring data to a pharmaceutical compounding system
InactiveUS20050086008A1Drug and medicationsComputer-assisted medicine prescription/deliveryDrug compoundEngineering
A system and method for use with a data entry system for providing input data to a pharmaceutical compounding device having an associated plurality of source solutions is provided. A first label comprising first indicia defining a desired pharmaceutical compound is generated. The first indicia is then provided to the pharmaceutical compounding device as an input. The pharmaceutical compounding device then prepares a pharmaceutical compound based on the first indicia and generates a second label comprising second indicia indicative of at least the contents of pharmaceutical compound. The first indicia and second indicia are provided to a comparison device where the contents of the actual pharmaceutical compound as indicated by the second indicia are compared to the desired pharmaceutical compound as indicated by the first indicia.
Owner:B BRAUN MEDICAL
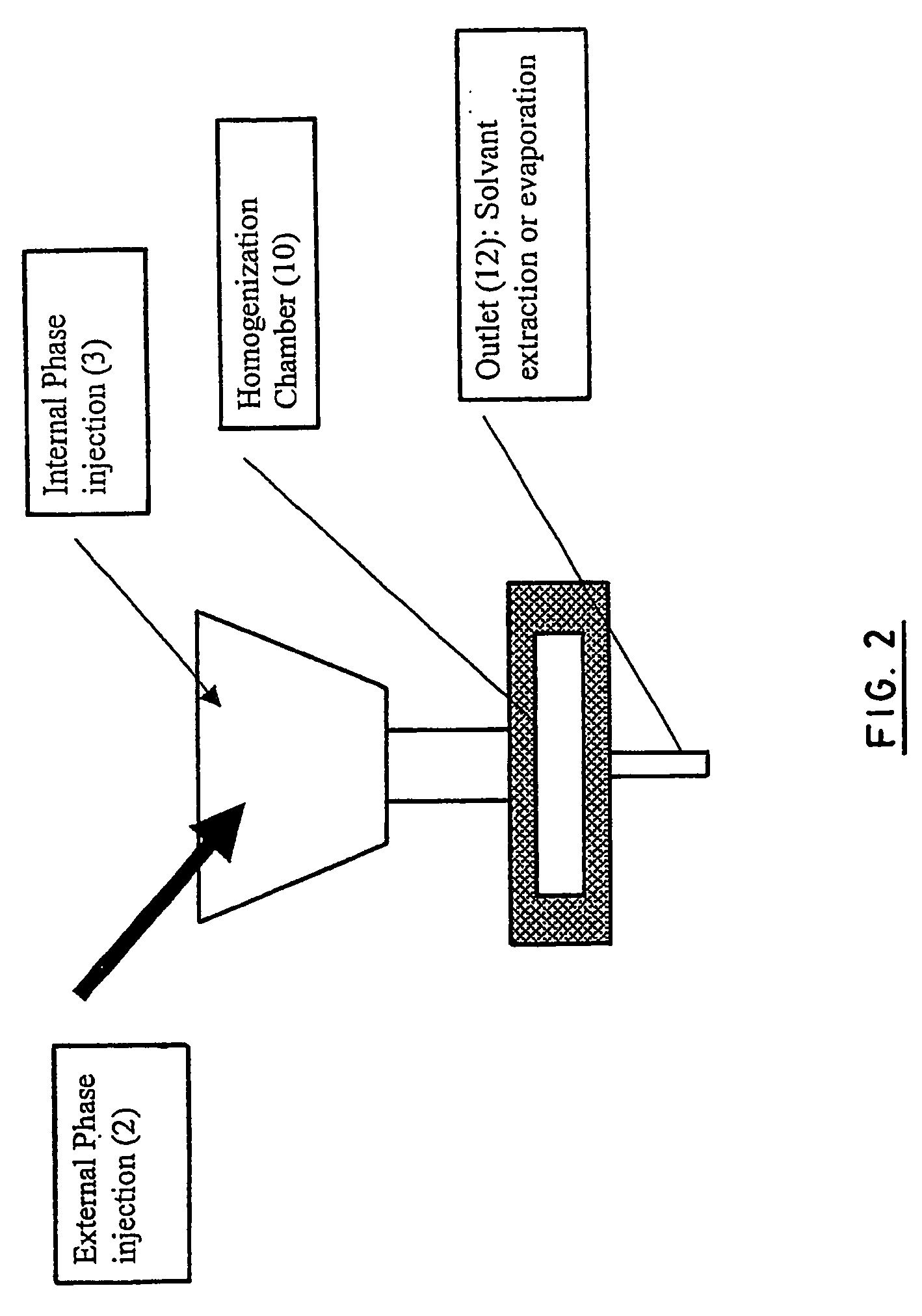
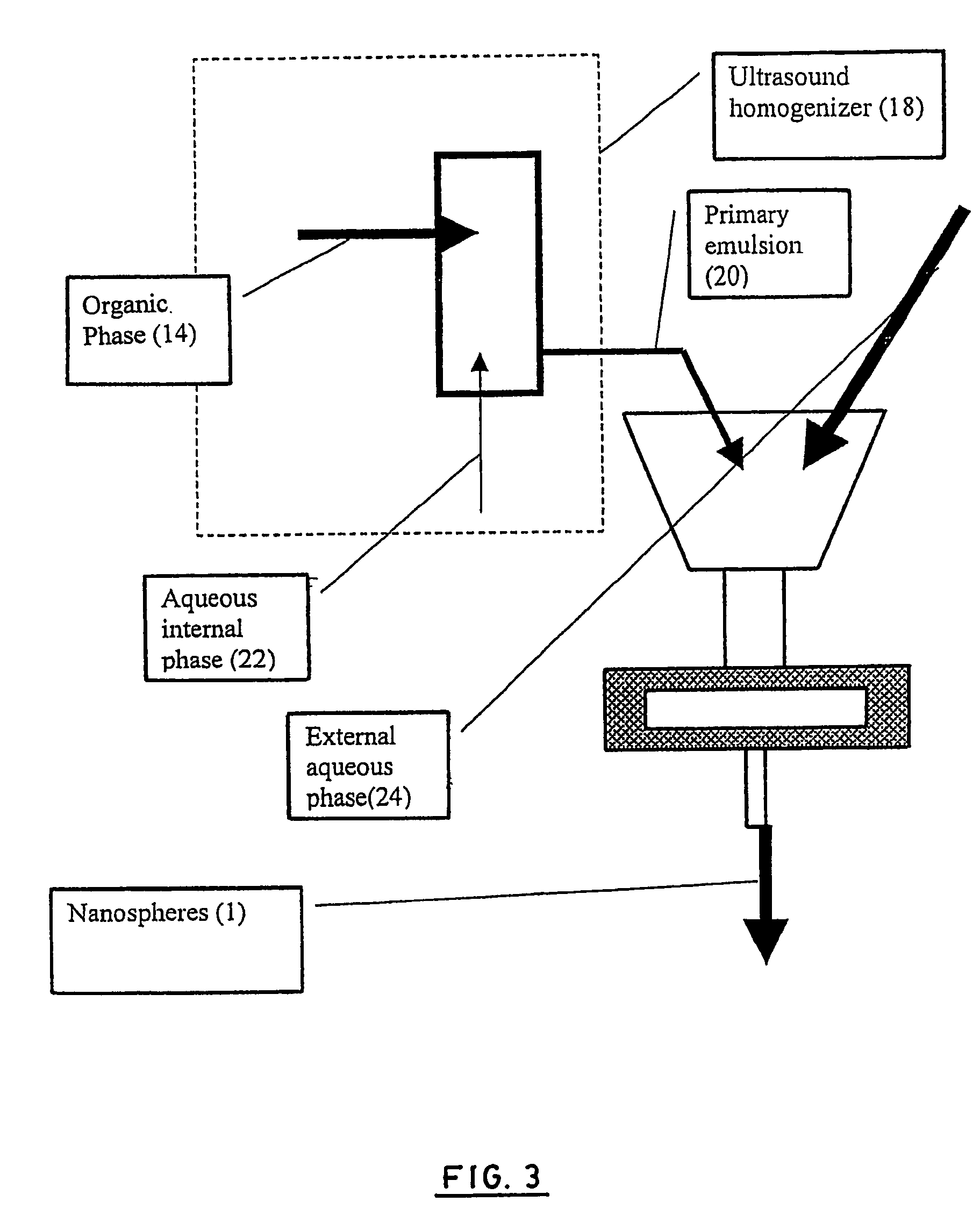
Stealthy polymeric biodegradable nanospheres and uses thereof
InactiveUS20060165987A1Maintain stable propertiesImprove drug deliverySynthetic resin layered productsCellulosic plastic layered productsPolyesterDrug compound
Disclosed herein are stealthy polymeric biodegradable nanospheres each comprising: (i) a polyester-polyethylene multiblock copolymer; (ii) optionally a polyester entangled with the multiblock copolymer to give rigidity to the nanospheres; and (iii) optionally a pharmaceutical compound incorporated therein. Also disclosed is the use of such nanospheres for the preparation of a medicament having a long-term and non-toxic release of a pharmaceutical compound into a mammal, and the method for preparing a stealthy polymeric biodegradable nanospheres.
Owner:VALORISATION RECH SOC & COMMANDITE
Pharmaceutical carrier device suitable for delivery of pharmaceutical compounds to mucosal surfaces
InactiveUS7579019B2Improve the situationPromote healing, asepty, scarificationOrganic active ingredientsPowder deliveryDrug compoundDrug carrier
The present invention relates to a pharmaceutical delivery device for application of a pharmaceutical to mucosal surfaces. The device comprises an adhesive layer and a non-adhesive backing layer, and the pharmaceutical may be provided in either or both layers. Upon application, the device adheres to the mucosal surface, providing localized drug delivery and protection to the treatment site. The kinetics of erodability are easily adjusted by varying the number of layers and / or the components.
Owner:ARIUS TWO
Methods for synthesis of prodrugs from 1-acyl-alkyl derivatives and compositions thereof
InactiveUS20030171303A1High yieldProviding some amountBiocideCarbamic acid derivatives preparationDrug compoundStereochemistry
The present invention provides a method for synthesizing 1-(acyloxy)-alkyl derivatives from 1-acyl-alkyl derivatives, which typically proceeds stereospecifically, in high yield, does not require the use of activated intermediates and / or toxic compounds and is readily amenable to scale-up. The current invention also provides 1-acyl-alkyl derivatives of known drug compounds and methods for synthesizing these 1-acyl-alkyl derivatives.
Owner:XENOPORT
Transdermal patch containing microneedles
A transdermal patch that can easily deliver a controlled volume of a fluidic drug compound to skin is provided. More particularly, the patch contains a microneedle assembly that is configured to be placed in fluid communication with a drug delivery assembly. The microneedle assembly contains a support and a plurality of microneedles that extend outwardly from the support. The microneedles are formed with one or more channels of a certain dimension such that passive capillary flow drives a flow of the drug compound. The drug delivery assembly contains a reservoir for the drug compound that is in fluid communication with a rate control membrane that helps control a flow rate of the drug compound by modulating a pressure of the drug compound, downstream from the reservoir. A release member is also positioned adjacent to the microneedle and drug delivery assemblies. Prior to use, the release member acts as a barrier to the flow of the drug compound and thus inhibits premature leakage. In this manner, the patch can initially be provided in an “inactive” configuration in which the drug compound is securely retained. When it is desired to release the drug compound, the patch can simply be activated by at least partially separating the release member from the drug delivery and microneedle assemblies.
Owner:SORRENTO THERAPEUTICS INC
Controllable drug releasing gradient coatings for medical devices
InactiveUS20070078513A1Prevent vessel occlusionSlow and more prolonged releaseOrganic active ingredientsSurgerySolubilityMedicine
Implantable medical devices having a polymer gradient coating capable of controllably releasing at least one pharmaceutical compound to a localized area are disclosed. More specifically, the gradient coatings comprise at least two layers where at least one of these layers incorporates at least one pharmaceutical compound. Each of the layers of the gradient coating has at least one physical property affecting the releasability of the pharmaceutical compound incorporated therein that differs from that of at least one other layer. These physical properties include, but are not limited to, solubility constants, molecular weights, elution profiles, and bonding strengths.
Owner:MEDTRONIC VASCULAR INC
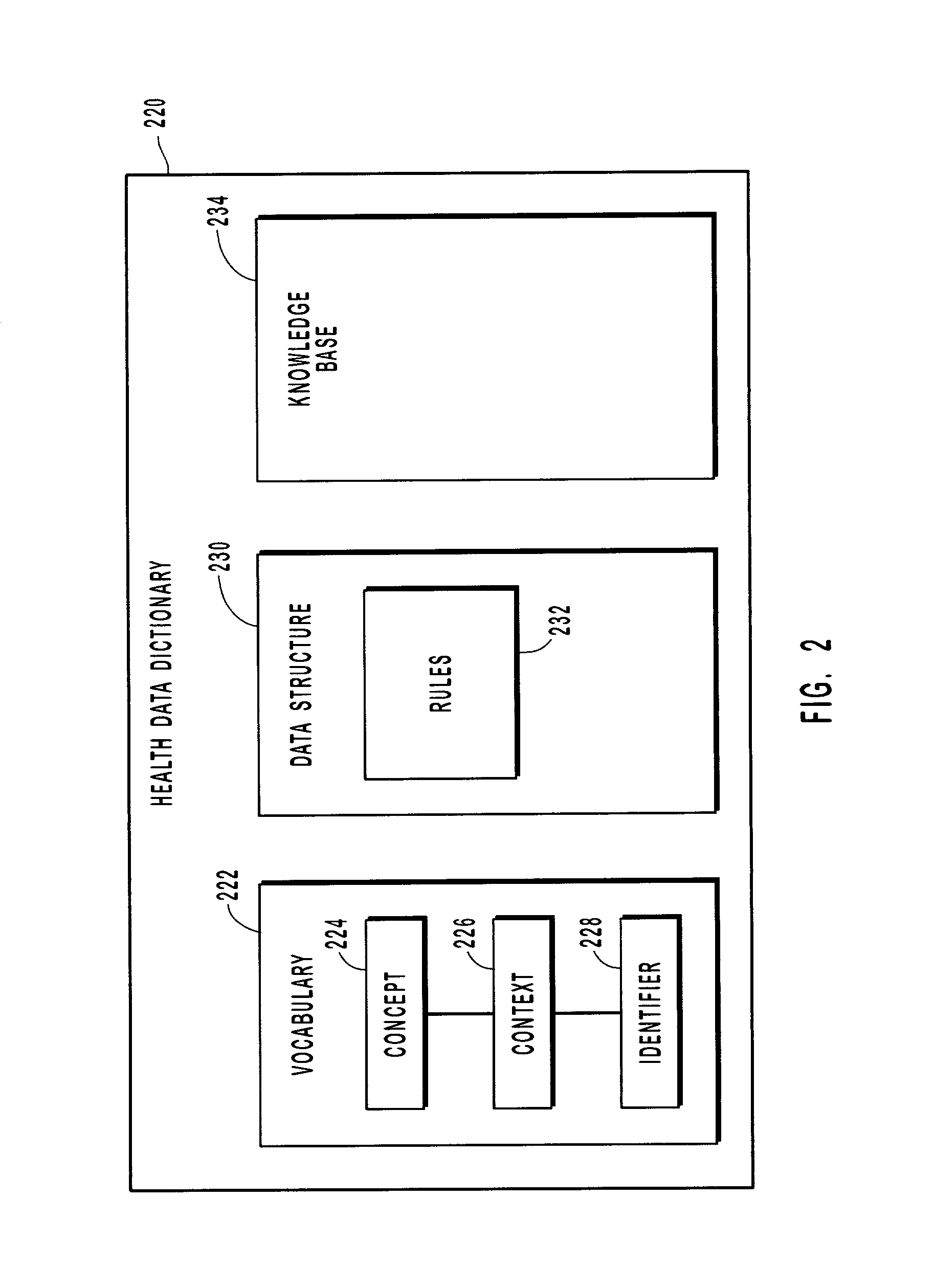
Mapping clinical data with a health data dictionary
InactiveUS20020128861A1Automate processingOffice automationMedical practises/guidelinesExact matchDrug compound
Systems, methods, and computer program products for mapping clinical data including insurance data and pharmaceutical data with a health data dictionary. The health data dictionary provides a vocabulary that identifies insurance companies and pharmaceutical compounds including drugs in a normalized and standard form. The clinical data generated at a legacy system is compared with standard clinical data stored in the health data dictionary. Based on this comparison a partial or exact match is identified for the clinical data. After a match is selected, the normalized clinical data can be stored in a data repository. For unmatched clinical data, new concepts can be created and added to the health data dictionary for future use.
Owner:3M INNOVATIVE PROPERTIES CO
Pharmaceutical carrier device suitable for delivery of pharmaceutical compounds to mucosal surfaces
InactiveUS20050048102A1Improve the situationPromote healing, asepty, scarificationPowder deliveryAdhesive dressingsDrug compoundDrug carrier
The present invention relates to a pharmaceutical delivery device for application of a pharmaceutical to mucosal surfaces. The device comprises an adhesive layer and a non-adhesive backing layer, and the pharmaceutical may be provided in either or both layers. Upon application, the device adheres to the mucosal surface, providing localized drug delivery and protection to the treatment site. The kinetics of erodability are easily adjusted by varying the number of layers and / or the components.
Owner:ARIUS TWO
Medical use of 2 alpha, 3 beta-dihydroxy-5, 11(13)- diallyl eudesmane-12 acid for inhibiting hepatitis B virus
InactiveCN1923188APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsDigestive systemDiseaseDrug compound
The invention relates to a sesterterpane as formula (1) and relative medical drug or solvent, and relative drug compound, which can reduce hepatitis B surface antigen and restrain hepatitis B HBVDNA copy activity. Wherein, said invention has strong restrain on hepatitis B surface antigen (HBsAg) generated by HepG2.2.15 cell and the copy of hepatitis B deoxyribonucleic acid (HBV-DNA), while it restrain ability is higher than positive contrast difuradin; and the copy restrain activity at large amount (100 mug / mL) and middle amount (20 mug / mL) on the hepatitis B HBV-DNA are both higher then difuradin.
Owner:赵昱
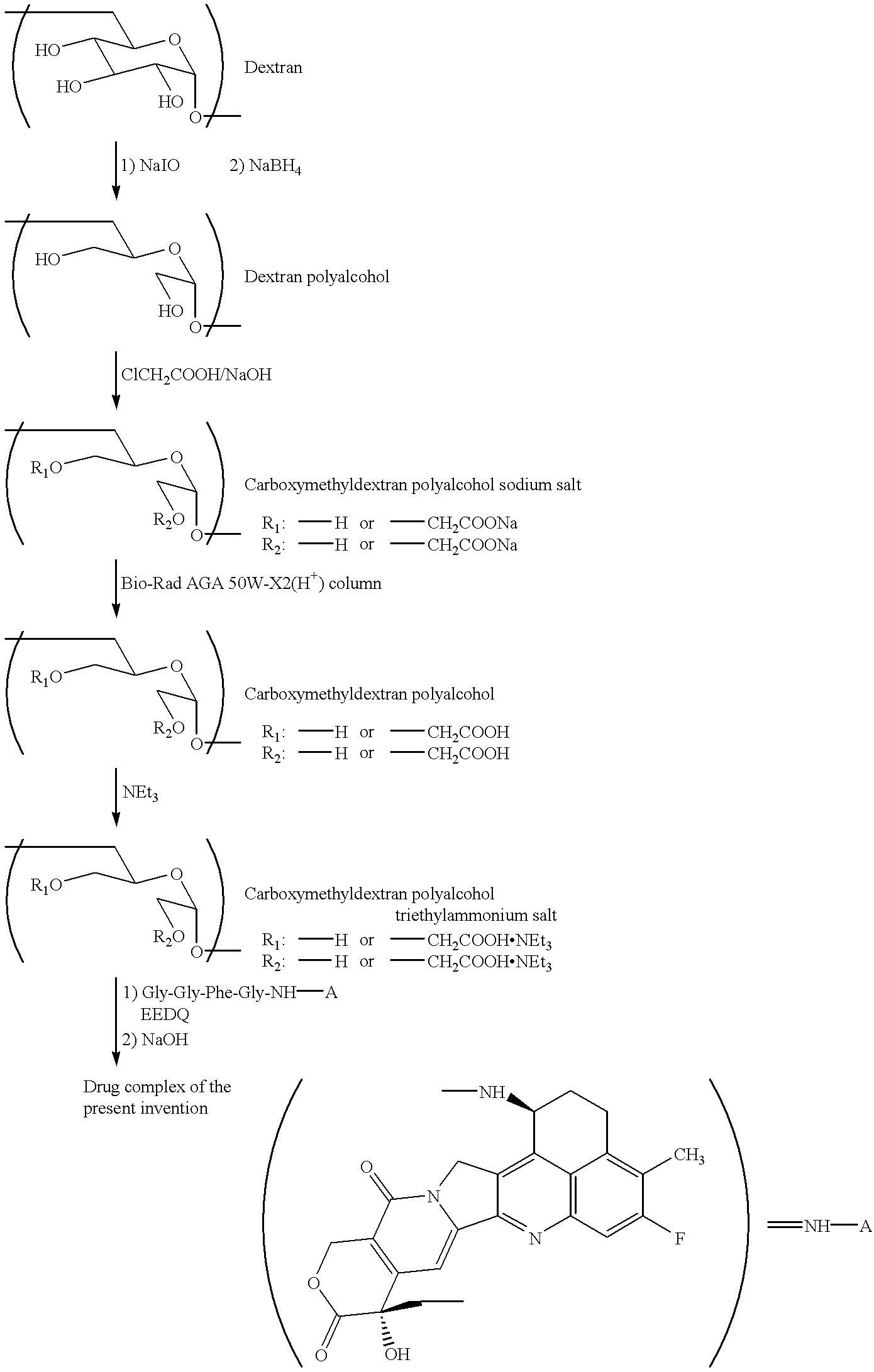
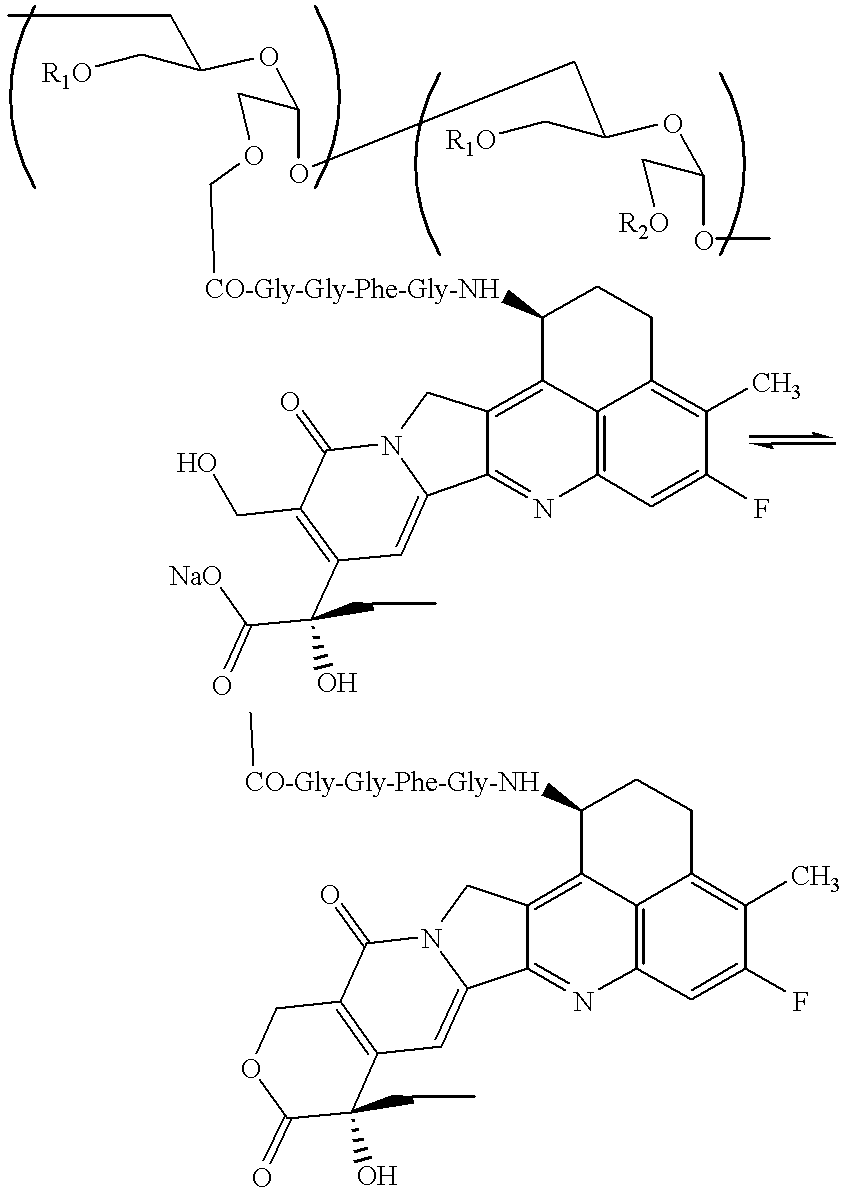
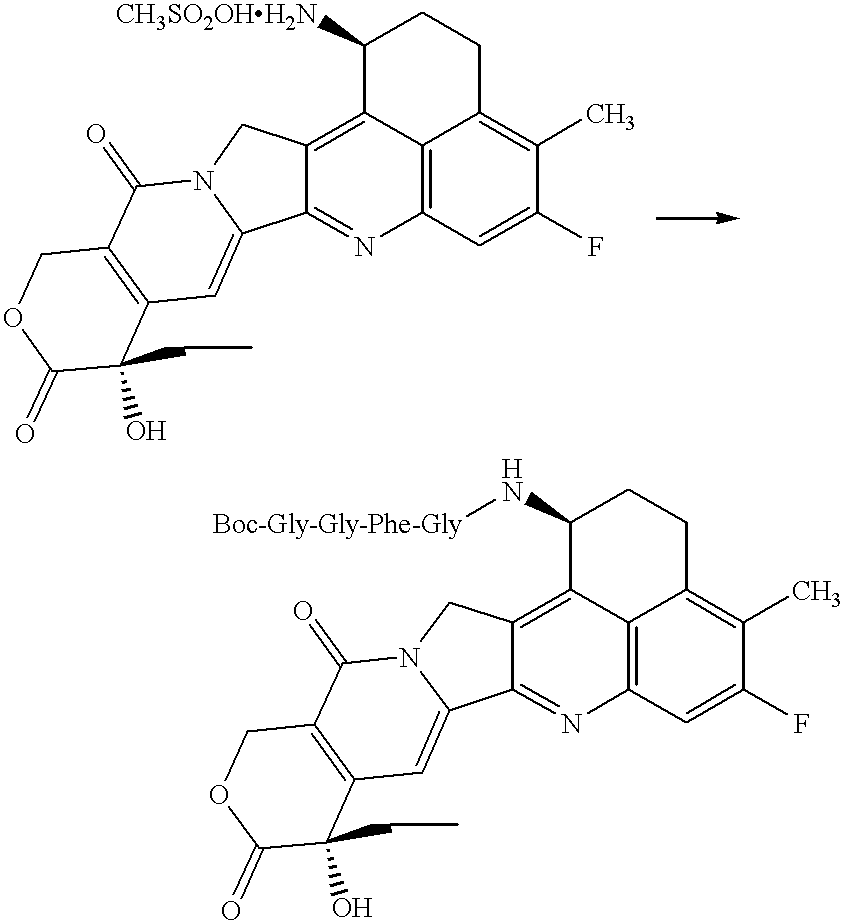
Process for producing drug complexes
InactiveUS6291671B1Efficient productionProduce significantEsterified saccharide compoundsSugar derivativesDrug compoundSide reaction
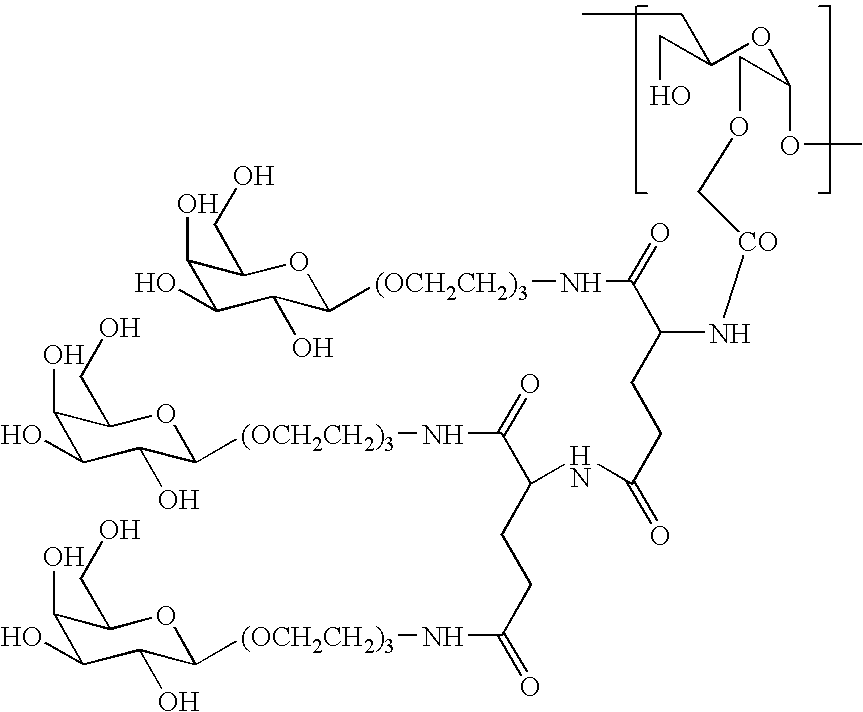
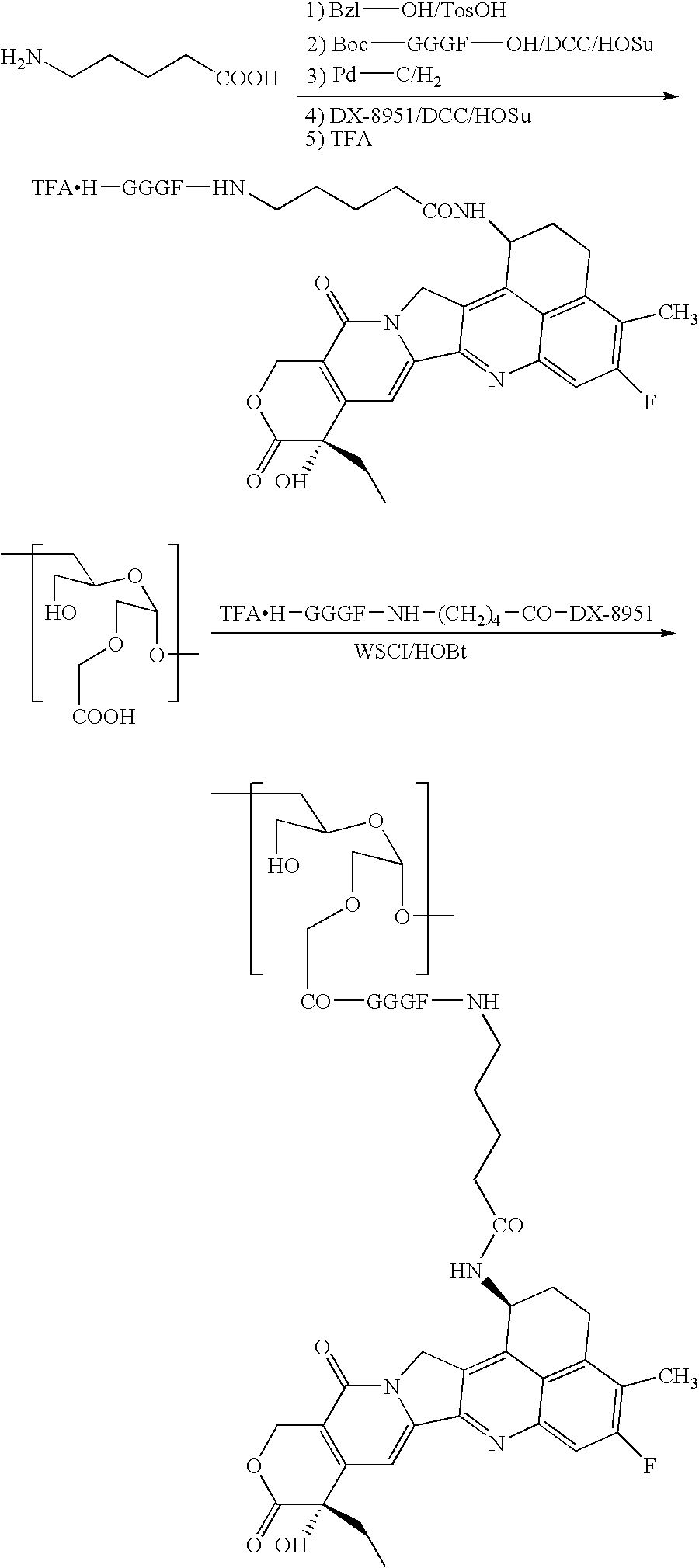
A method for preparing a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other by means of a spacer comprising an amino acid or a spacer comprising peptide-bonded 2 to 8 amino acids, or a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other without the spacer, characterized in that an organic amine salt of the polysaccharide derivative having carboxyl groups is reacted with the drug compound or the spacer bound to the drug compound in a non-aqueous system. The reaction between the polysaccharide derivative having carboxyl groups and the drug compound bound with the spacer or the like can be carried out in high yields, and when a drug compound having a lactone ring is subjected to the reaction, side reactions can be reduced.
Owner:DAIICHI PHARMA CO LTD
System, Device and Method for Electroporation of Cells
ActiveUS20170283761A1Avoid blockingHigh strengthBioreactor/fermenter combinationsBiological substance pretreatmentsDrug compoundCell membrane
A system, device and method for electroporation of living cells and the introduction of selected molecules into the cells utilizes a fluidic system where living cells and biologically active molecules flow through a channel that exposes them to electric fields, causing the molecules to be transferred across the cell membrane. The device is structured in a manner that allows precise control of the cells location, motion, and exposure to electric fields within the flow channel device. The method is particularly well suited for the introduction of DNA, RNA, drug compounds, and other biologically active molecules into living cells.
Owner:CYTEQUEST INC
Medical Device Having Coating With Zeolite Drug Reservoirs
A medical device having a drug-eluting coating that includes a pharmaceutical compound or, more generally, a therapeutic material housed within pores of a zeolite carrier. The zeolite carrier has an open porous structure with reservoirs for holding the therapeutic material. The therapeutic material loaded zeolites may be suspended or dispersed within a bioerodible polymer matrix to provide controlled delivery of the therapeutic material. Zeolite drug carriers may have enhanced or optimally engineered pore sizes for a particular therapeutic material and release profile. Along with a therapeutic material, reservoirs of a zeolite drug delivery system may include a release agent. The release agent may be used to entrap the therapeutic material until such time as a triggering condition is met that prompts the release agent to activate and thereby release the therapeutic material from the zeolite reservoir.
Owner:MEDTRONIC VASCULAR INC
Circumferential Aerosol Device for Delivering Drugs to Olfactory Epithelium and Brain
InactiveUS20130142868A1Improve consistencyImprove efficiencyAnaesthesiaAerosol deliveryDrug compoundNanoparticle
Methods of delivering a pharmaceutical compounds directly to the olfactory epithelium of a mammal by providing a pharmaceutical aerosol suspension comprising an aerosol and the pharmaceutical compound; aerosolizing the suspension to generate a stream of droplets, the stream having a rotational component, and, delivering the droplets directly to the olfactory epithelium, wherein at least 15% of the droplets are delivered directly to the olfactory deposition. The pharmaceutical compound may be encapsulated within a liposome nanoparticle.
Owner:UNIV OF WASHINGTON
Peptides having ligand activities on APJ that is an orphan G protein-coupled receptor, and use thereof
Owner:TAKEDA PHARMA CO LTD
Alternative use for hydrogel intrasaccular occlusion device with an umbrella member for structural support
ActiveUS20180193025A1Expand coverageReduce penetrationStentsBalloon catheterAneurysm recurrenceTreatment field
The present disclosure relates to the field of endovascular treatment. More particularly, the present invention uses a modified hydrogel intrasaccular occlusion device designed to implement an endovascular treatment to ameliorating or eliminating aneurysm recurrence, which hydrogel may optionally be impregnated with pharmaceutical compounds. The present invention also teaches the use of thin hydrogel coatings to ameliorate endovascular treatment related difficulties.
Owner:WALZMAN INNOVATIONS LLC
DDS compound and method for measurement thereof
InactiveUS7041818B2High organ selectivityExcellent liver selectivityBiocidePharmaceutical non-active ingredientsPolyolHydrolysate
A DDS compound comprising a carboxy(C1-4)alkyldextran polyalcohol modified with a saccharide compound and a residue of drug compound bound to the carboxy(C1-4)alkyldextran polyalcohol, and a method for measuring a DDS compound in which a polymer carrier and a residue of drug compound are bound to each other by means of a spacer comprising 2 to 8 amino acids linked by peptide bond(s), which comprises the steps of treating the DDS compound with a peptidase, and measuring the resulting hydrolysate.
Owner:DAIICHI PHARMA CO LTD
Drug conjugates and their use for treating cancer, an autoimmune disease or an infectious disease
Drug-Linker-Ligand Conjugates are disclosed in which a Drug is linked to a Ligand via a peptide-based Linker unit. In one embodiment, the Ligand is an Antibody. Drug-Linker compounds and Drug compounds are also disclosed. Methods for treating cancer, an autoimmune disease or an infectious disease using the compounds and compositions of the invention are also disclosed.
Owner:SEAGEN INC
Phospholipid-coated microcrystals for the sustained release of pharmacologically active compounds and methods of their manufacture and use
InactiveUS6180136B1Long release timeExtend posting timeAntibacterial agentsPowder deliveryDiseaseDrug compound
The present invention relates to pharmaceutical compositions for the sustained release of pharmacologically active compounds and methods of their manufacture and use. Sustained release times of 10-12 days have been achieved with the present invention. The present invention provides microcrystal compositions. The microcrystals comprise pharmacologically active compounds and are contained within a phospholipid layer which contains a unique combination of phospholipids. The present invention may be applied to a wide range of pharmaceutical compositions which may be rendered suitable for injection. The microcrystals are of varying sizes. At least 50 percent of the microcrystals are from 0.5 mum to about 3.0 mum in diameter, at least ten percent of the microcrystals are from about 3.0 mum to about 10 mum in diameter, and the composition contains microcrystals which are greater than 10 mum in diameter. In preferred embodiments, at least about 1% of the microcrystals are greater than 10 mum in diameter. The compositions and methods are useful for treating respiratory diseases, infections, inflammation, and pain in a variety of mammals. The compounds and methods are also able to sharply reduce the toxicity of drug compounds.
Owner:IDEXX LABORATORIES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com