Patents
Literature
58results about How to "Extend posting time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
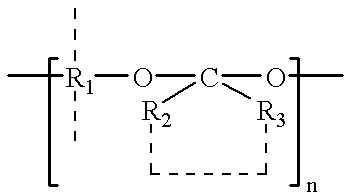
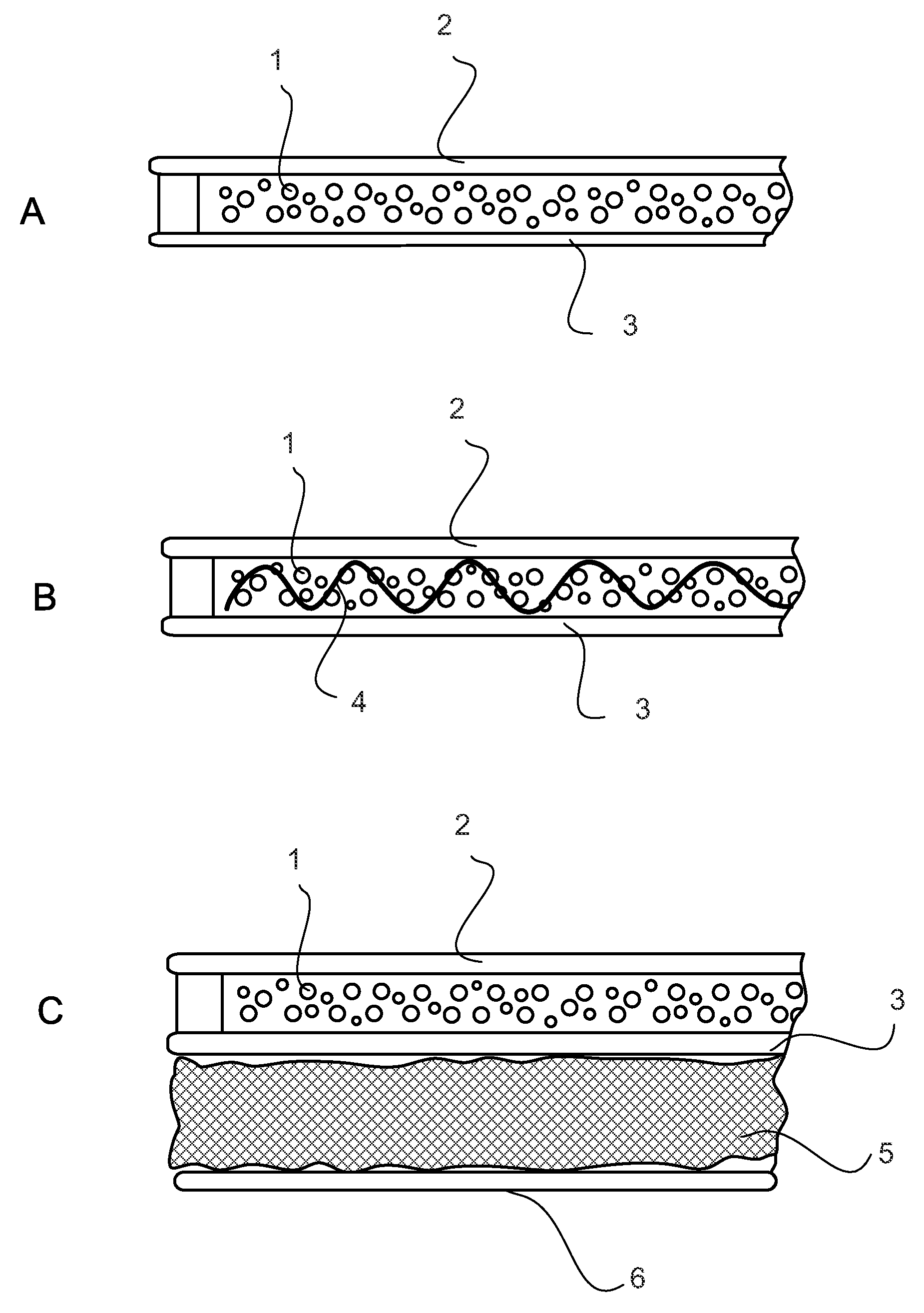
Plasticized bioerodible controlled delivery system
InactiveUS6372245B1Easy to useExtend posting timeSolution deliveryDrug compositionsControlled releaseSufficient time
A controlled release medicament delivery system comprises a plasticized bioerodible polymer, such as a polyorthoester. Medicament desirably is entrapped in the plasticized polymer. The resulting delivery system is able to release the medicament in a controlled and sustained manner. The formulation is particularly advantageous for use as a once-a-day eyedrop. During preparation, the polymer may be heated to an elevated temperature for a sufficient time to substantially reduce its molecular weight.
Owner:INSITE VISION
Porous calcium phosphate bone material
ActiveUS20070128245A1Improved controlled releaseExtend posting timeAntibacterial agentsPeptide/protein ingredientsCalcium biphosphateChemical composition
Porous calcium phosphate implant compositions that approximate the chemical composition of natural bone mineral are provided. In addition to calcium phosphate, the compositions include an effervescent agent to promote the formation of interconnected pores and a cohesiveness agent to maintain the shape and hardness of the hardened composition. When introduced at an implant site, the calcium phosphate compositions are remodeled into bone. Methods for using the calcium phosphate compositions, e.g., to repair or replace bone, are also provided.
Owner:ETEX
Sustained release intraocular implants and methods for preventing retinal dysfunction
InactiveUS20050244506A1Few and no negative side effectFacilitate obtaining successful treatment resultsPowder deliveryBiocideMicrosphereRetinal dysfunction
Biocompatible intraocular microspheres and implants include an alpha-2 adrenergic receptor agonist and a polymer associated with the alpha-2 adrenergic receptor agonist to facilitate release of the alpha-2 adrenergic receptor agonist into an eye for an extended period of time. The alpha-2 adrenergic receptor agonist may be associated with a biodegradable polymer matrix, such as a matrix of a two biodegradable polymers. The implants may be placed in an eye to treat or to prevent the occurrence of one or more ocular conditions, to reduce one or more symptoms of an ocular condition, such as an ocular neurosensory disorder and the like, to enhance normal retinal function and / or to lower intraocular pressure.
Owner:ALLERGAN INC
Medical device with drug
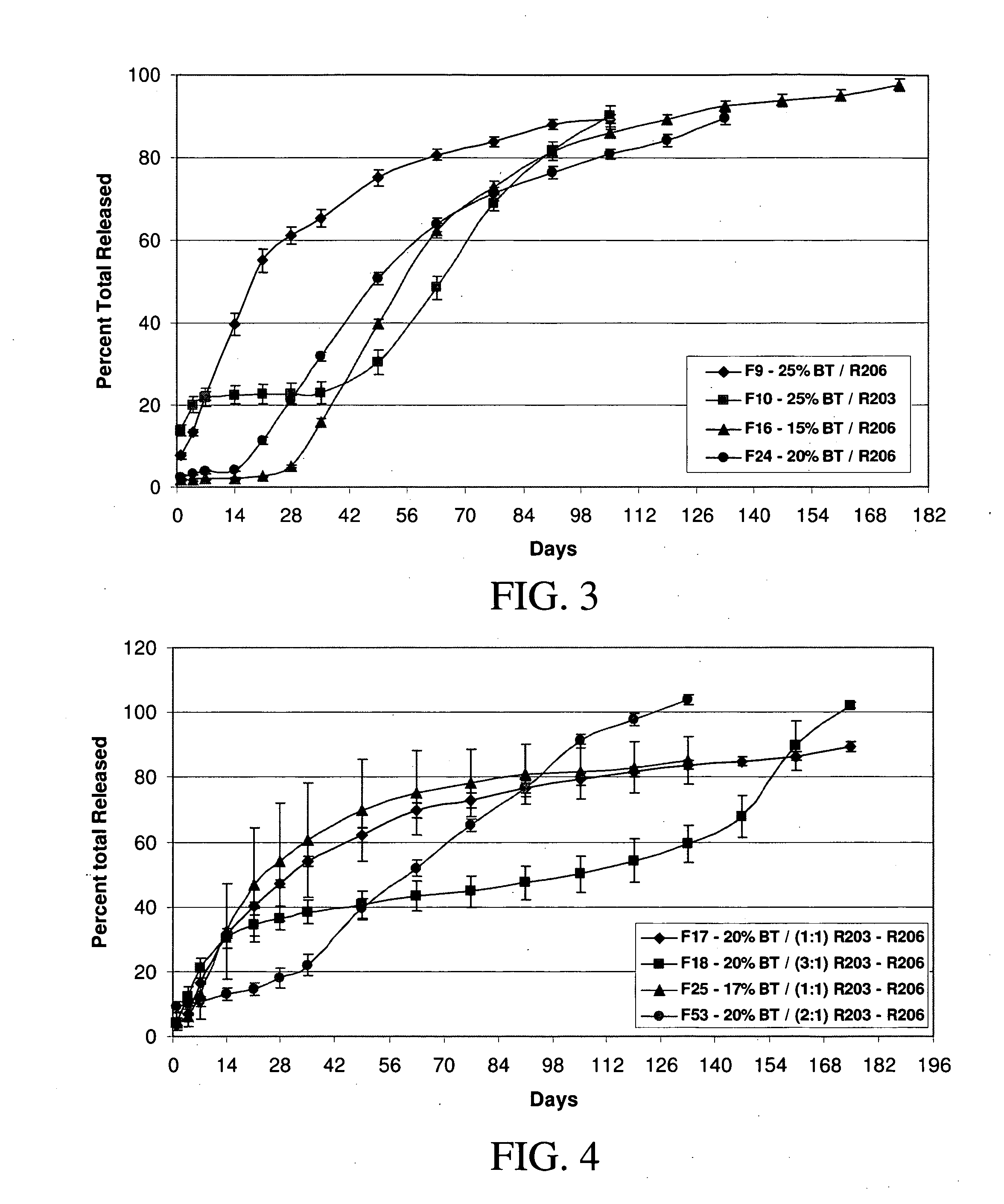
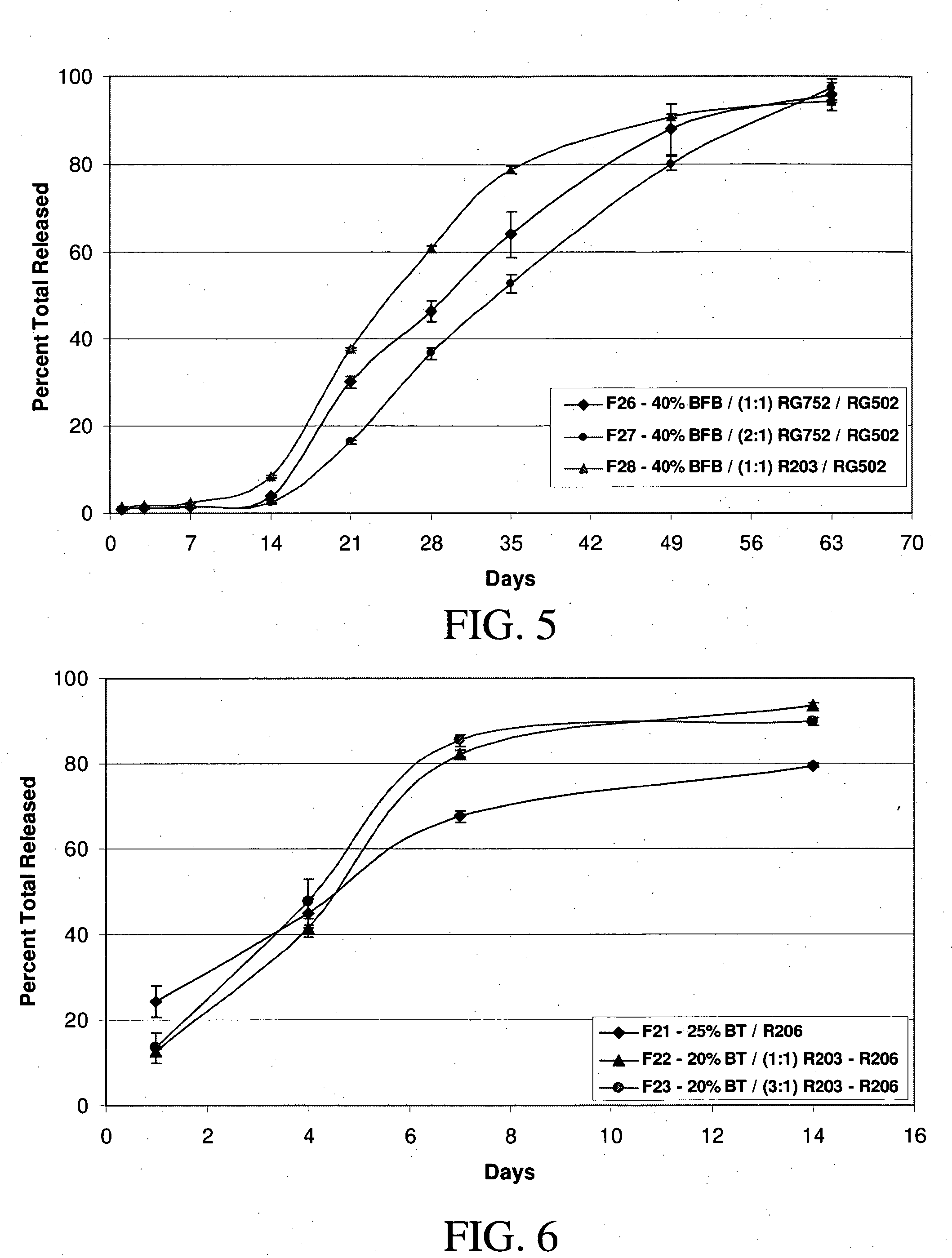
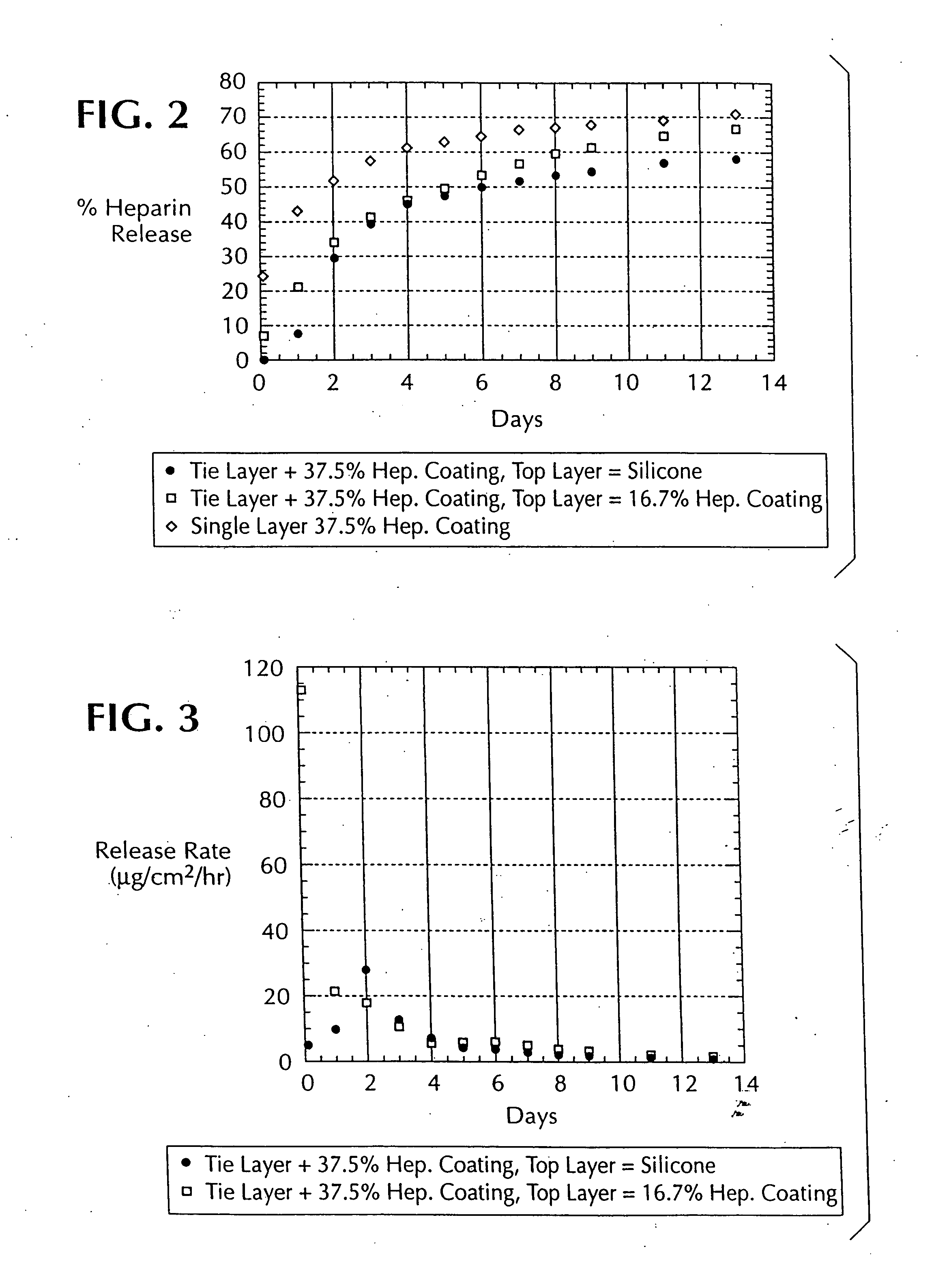
A method of coating implantable open lattice metallic stent prosthesis is disclosed which includes sequentially applying a plurality of relatively thin outer layers of a coating composition comprising a solvent mixture of uncured polymeric silicone material and crosslinker and finely divided biologically active species, possibly of controlled average particle size, to form a coating on each stent surface. The coatings are cured in situ and the coated, cured prosthesis are sterilized in a step that includes preferred pretreatment with argon gas plasma and exposure to gamma radiation electron beam, ethylene oxide, steam.
Owner:BOSTON SCI SCIMED INC
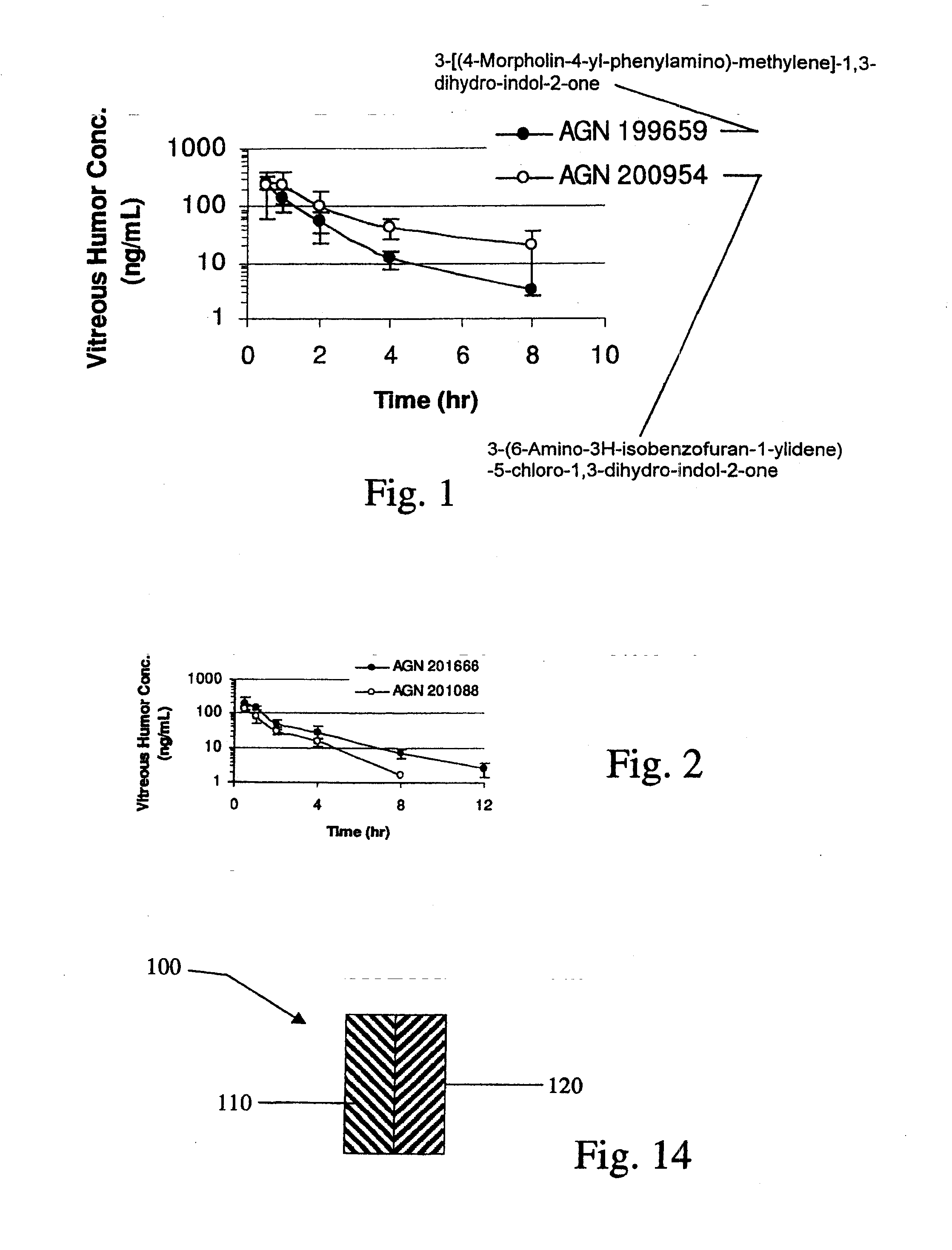
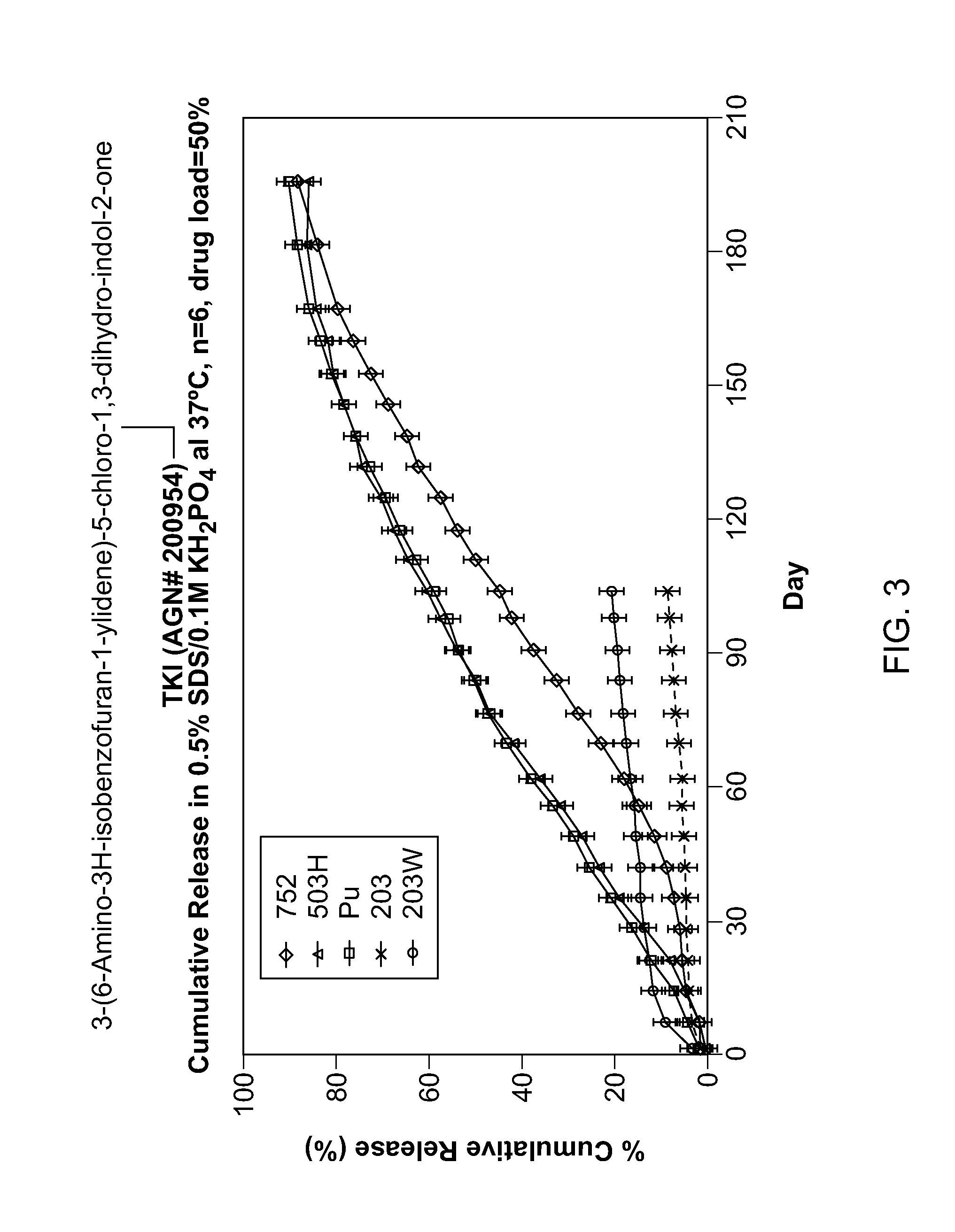
Biodegradable Intravitreal Tyrosine Kinase Implants
ActiveUS20140031408A1Reduce deliveryFacilitate obtaining successful treatment resultsBiocideSenses disorderOphthalmologyPolyvinyl alcohol
Biocompatible intraocular implants include a tyrosine kinase inhibitor and a biodegradable polymer that is effective to facilitate release of the tyrosine kinase inhibitor into the vitreous of an eye for an extended period of time. The therapeutic agents of the implants may be associated with a biodegradable polymer matrix, such as a matrix that is substantially free of a polyvinyl alcohol. The implants can be placed in an eye to treat or reduce the occurrence of one or more ocular conditions.
Owner:ALLERGAN INC
Phospholipid-coated microcrystals for the sustained release of pharmacologically active compounds and methods of their manufacture and use
InactiveUS6180136B1Long release timeExtend posting timeAntibacterial agentsPowder deliveryDiseaseDrug compound
The present invention relates to pharmaceutical compositions for the sustained release of pharmacologically active compounds and methods of their manufacture and use. Sustained release times of 10-12 days have been achieved with the present invention. The present invention provides microcrystal compositions. The microcrystals comprise pharmacologically active compounds and are contained within a phospholipid layer which contains a unique combination of phospholipids. The present invention may be applied to a wide range of pharmaceutical compositions which may be rendered suitable for injection. The microcrystals are of varying sizes. At least 50 percent of the microcrystals are from 0.5 mum to about 3.0 mum in diameter, at least ten percent of the microcrystals are from about 3.0 mum to about 10 mum in diameter, and the composition contains microcrystals which are greater than 10 mum in diameter. In preferred embodiments, at least about 1% of the microcrystals are greater than 10 mum in diameter. The compositions and methods are useful for treating respiratory diseases, infections, inflammation, and pain in a variety of mammals. The compounds and methods are also able to sharply reduce the toxicity of drug compounds.
Owner:IDEXX LABORATORIES +1
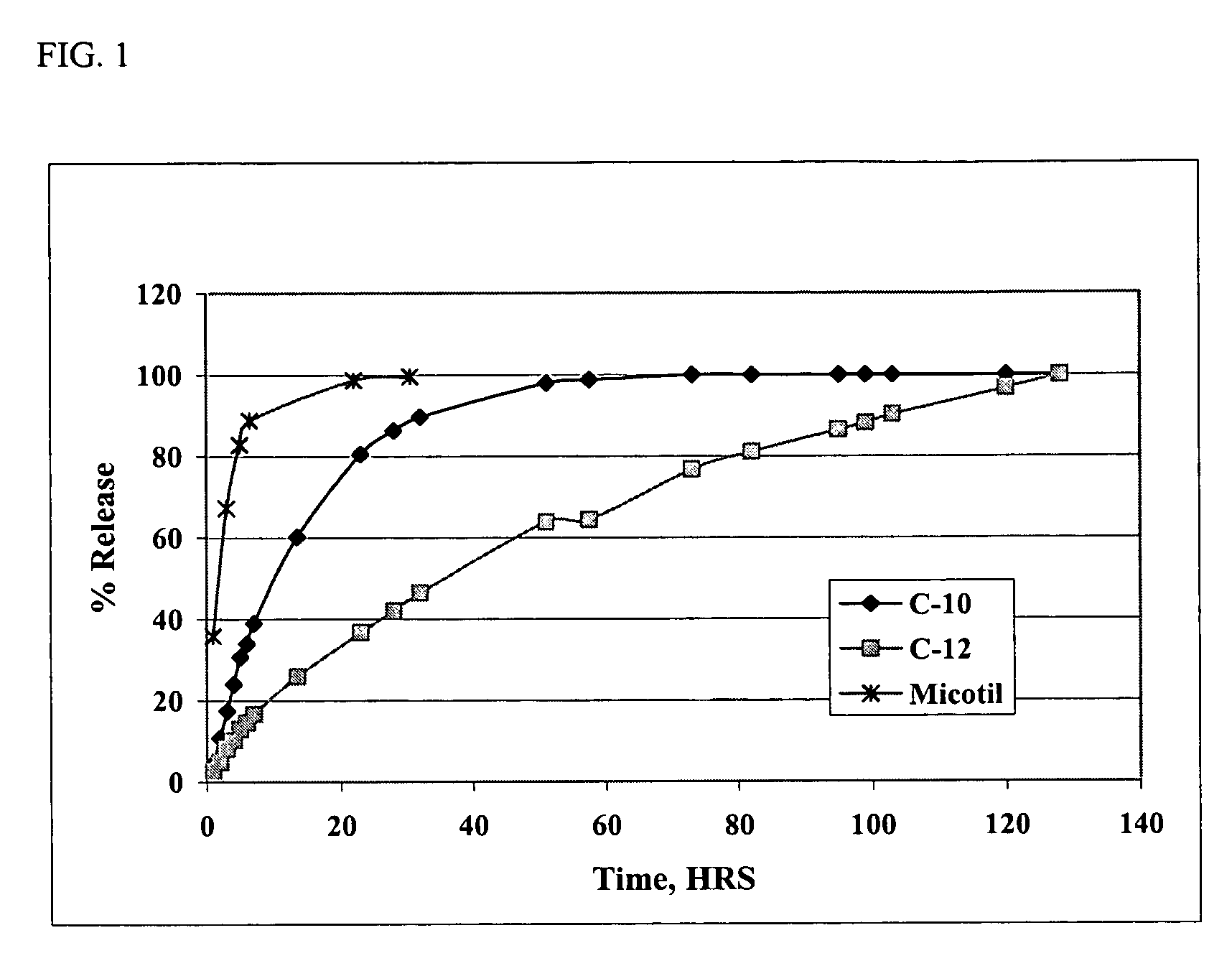
Injectable compositions for the controlled delivery of pharmacologically active compound
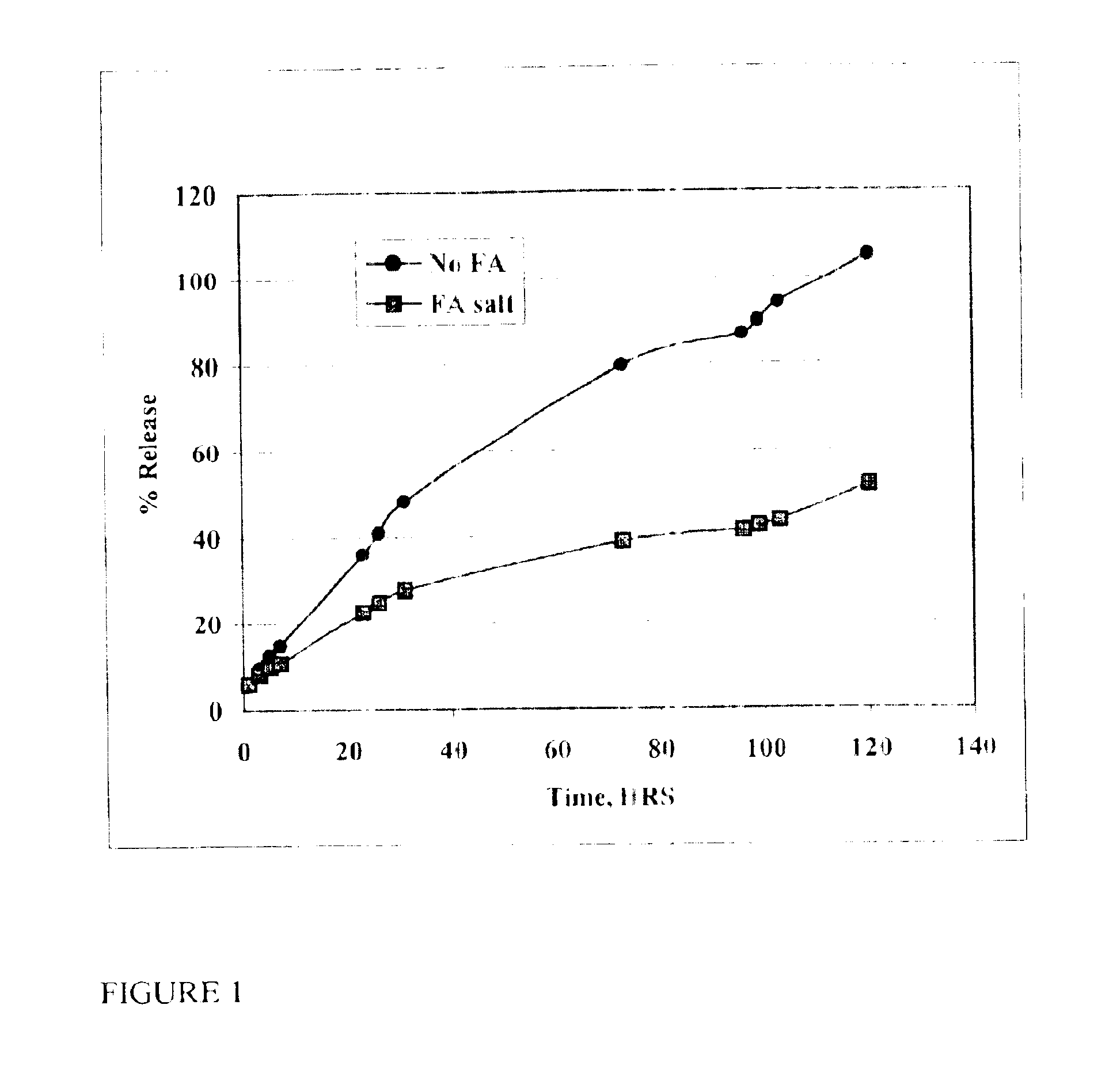
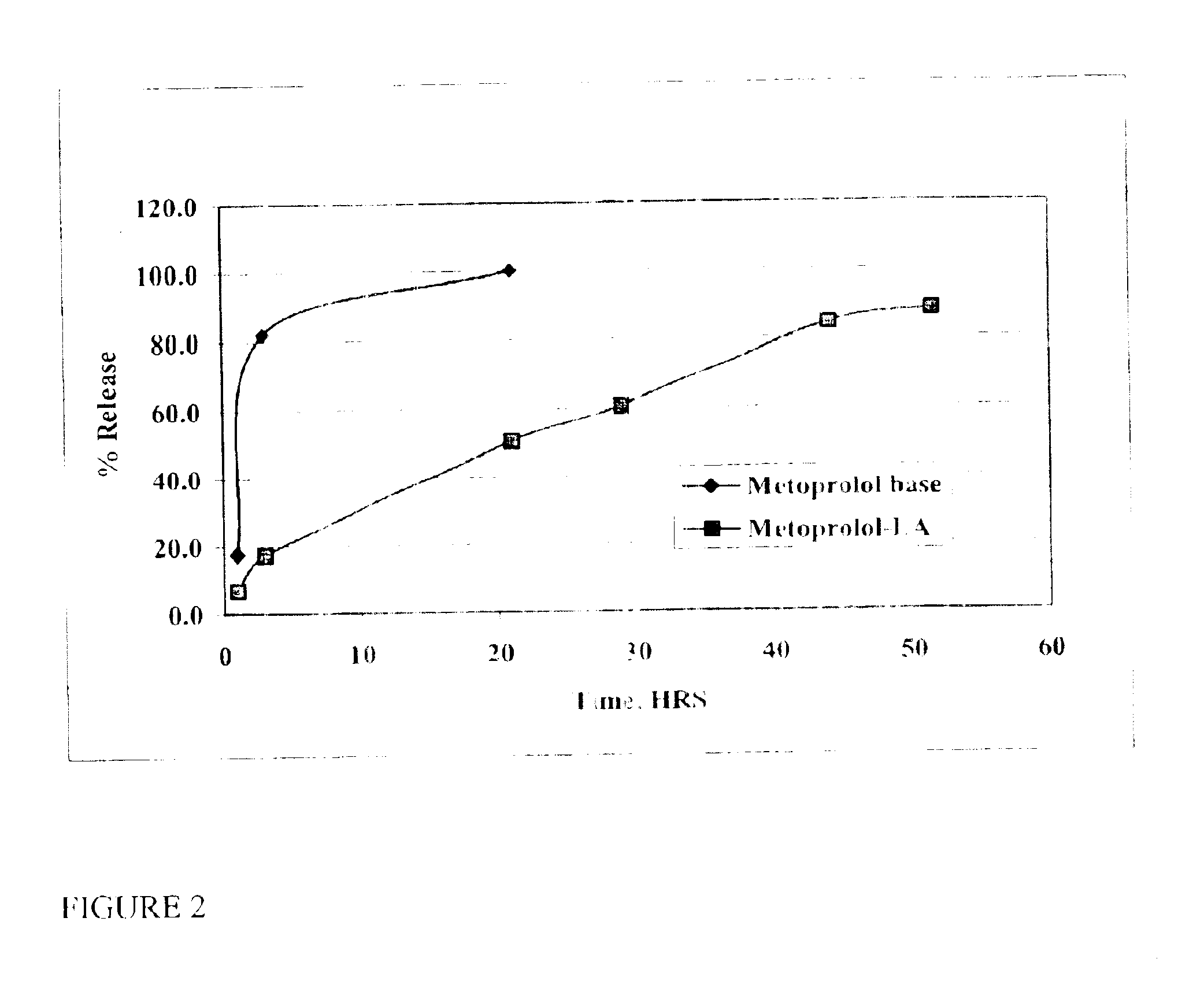
InactiveUS6887487B2Extend posting timeControl doseAntibacterial agentsBiocideRoxithromycinRelease time
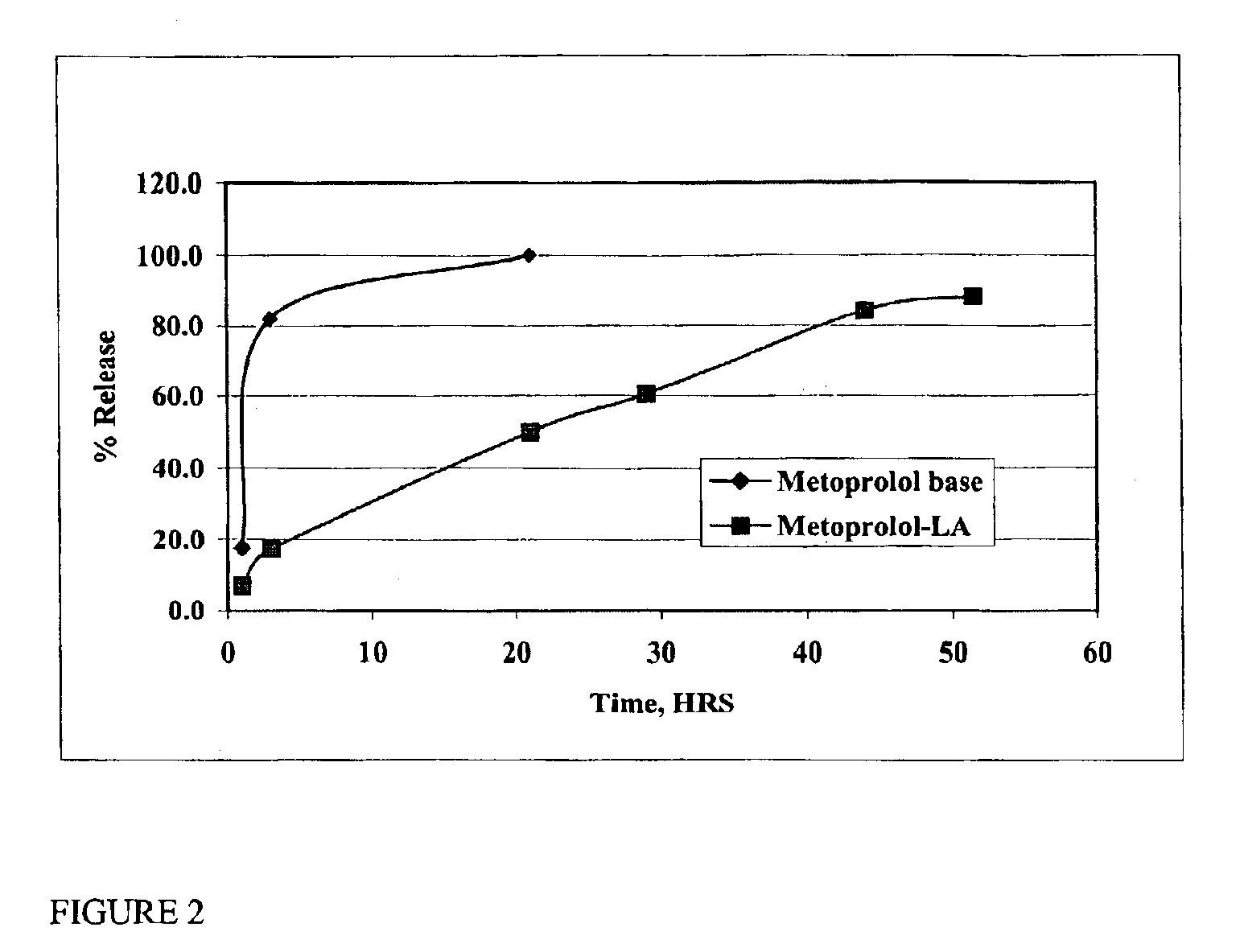
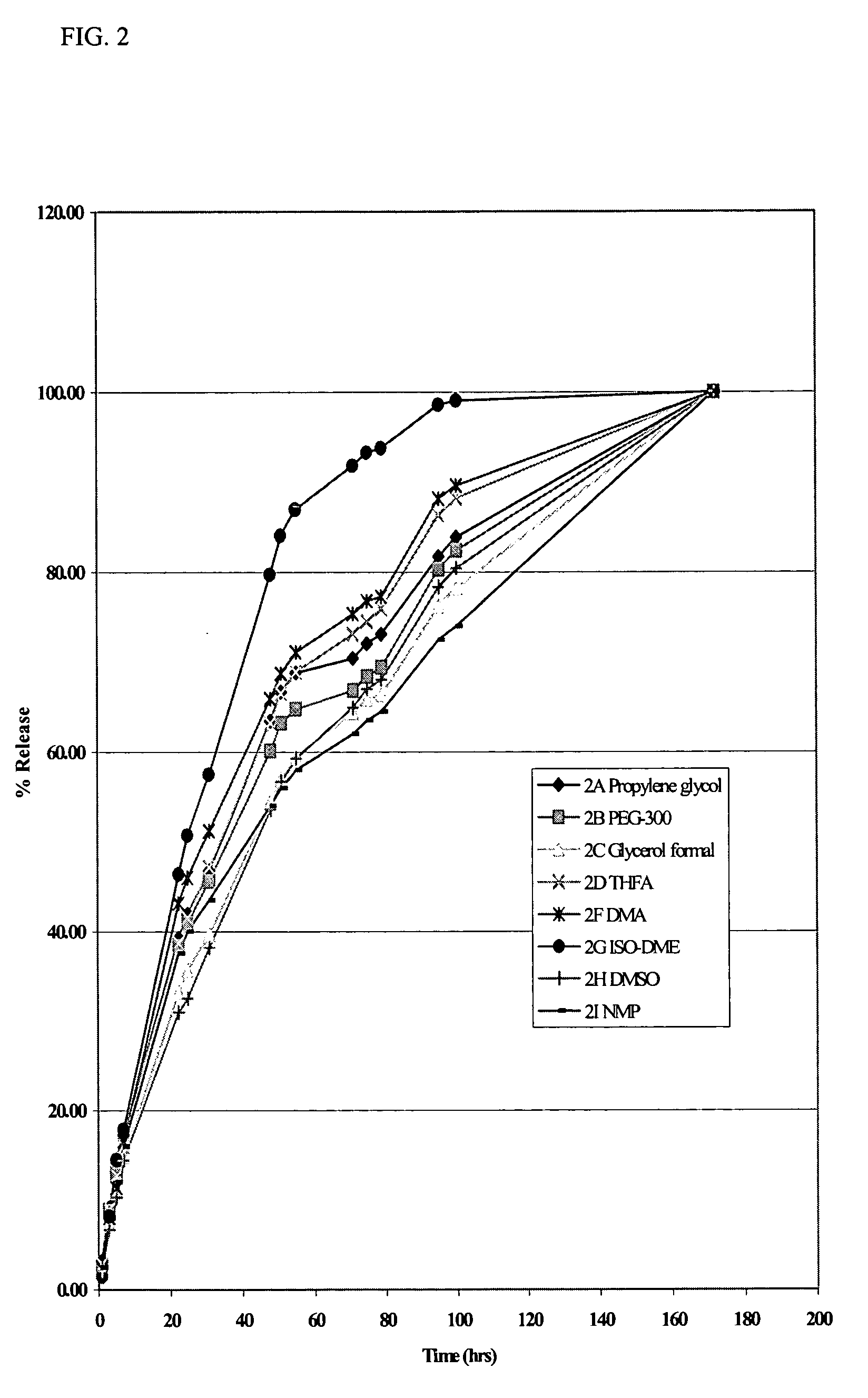
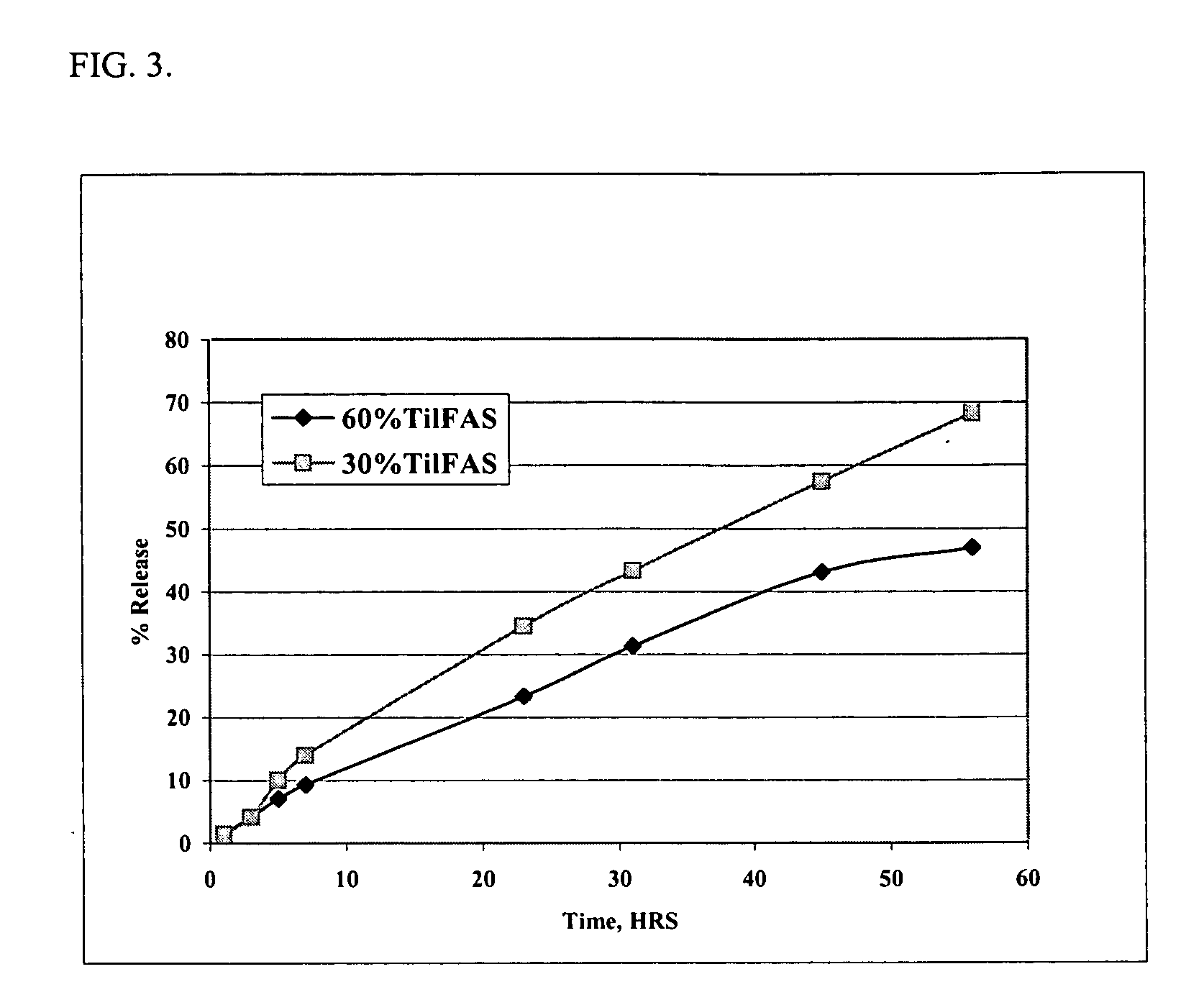
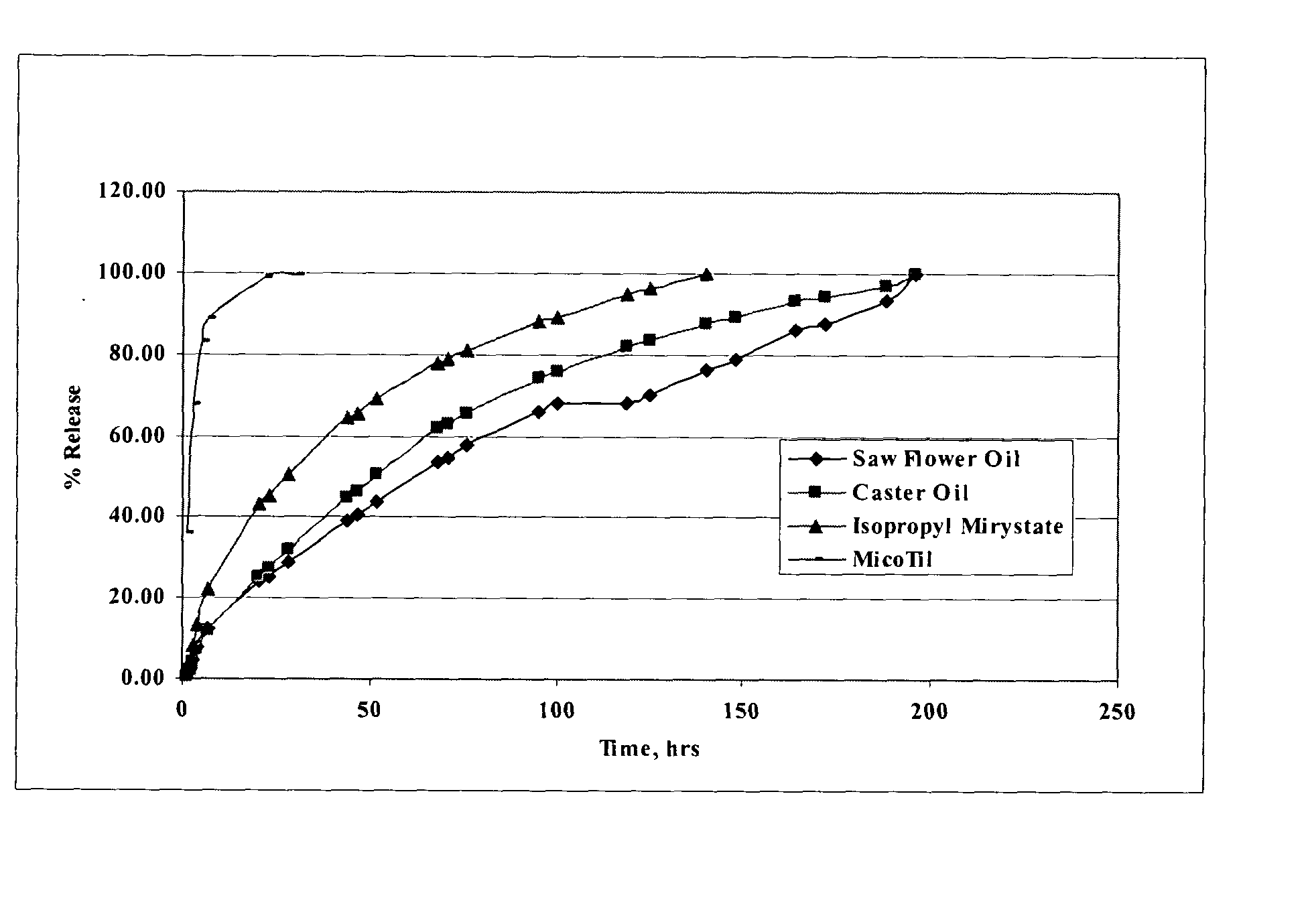
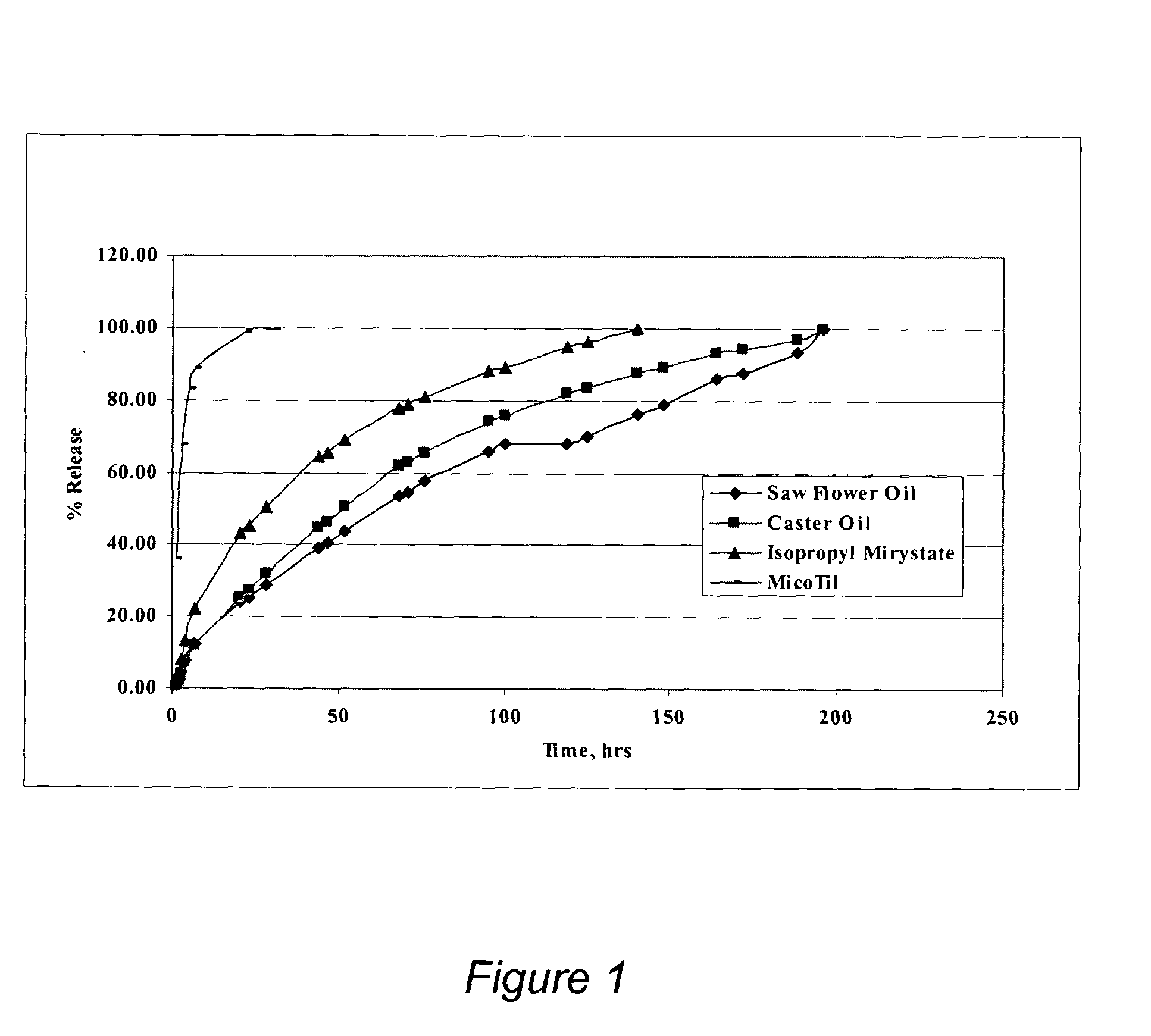
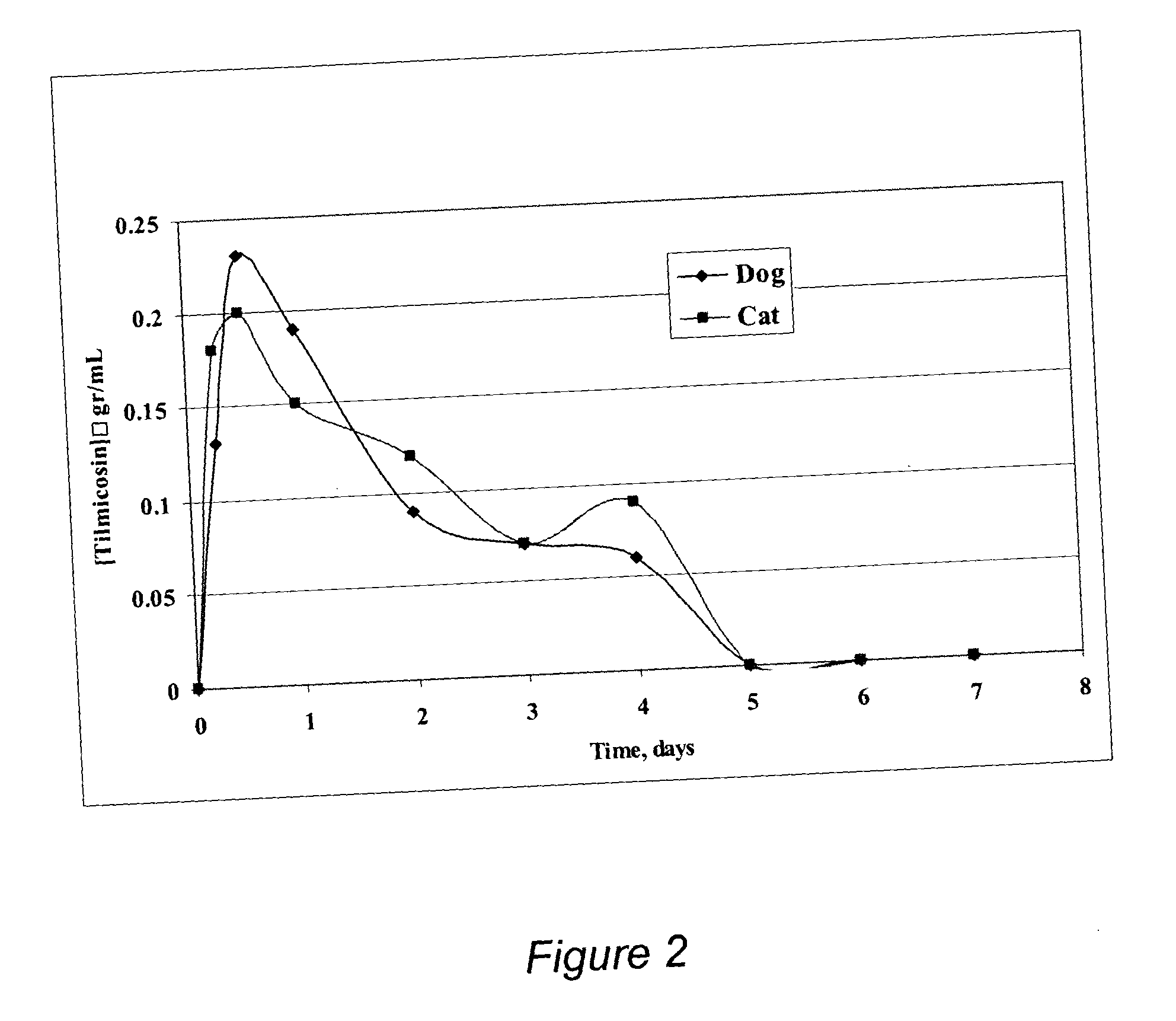
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a periods of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
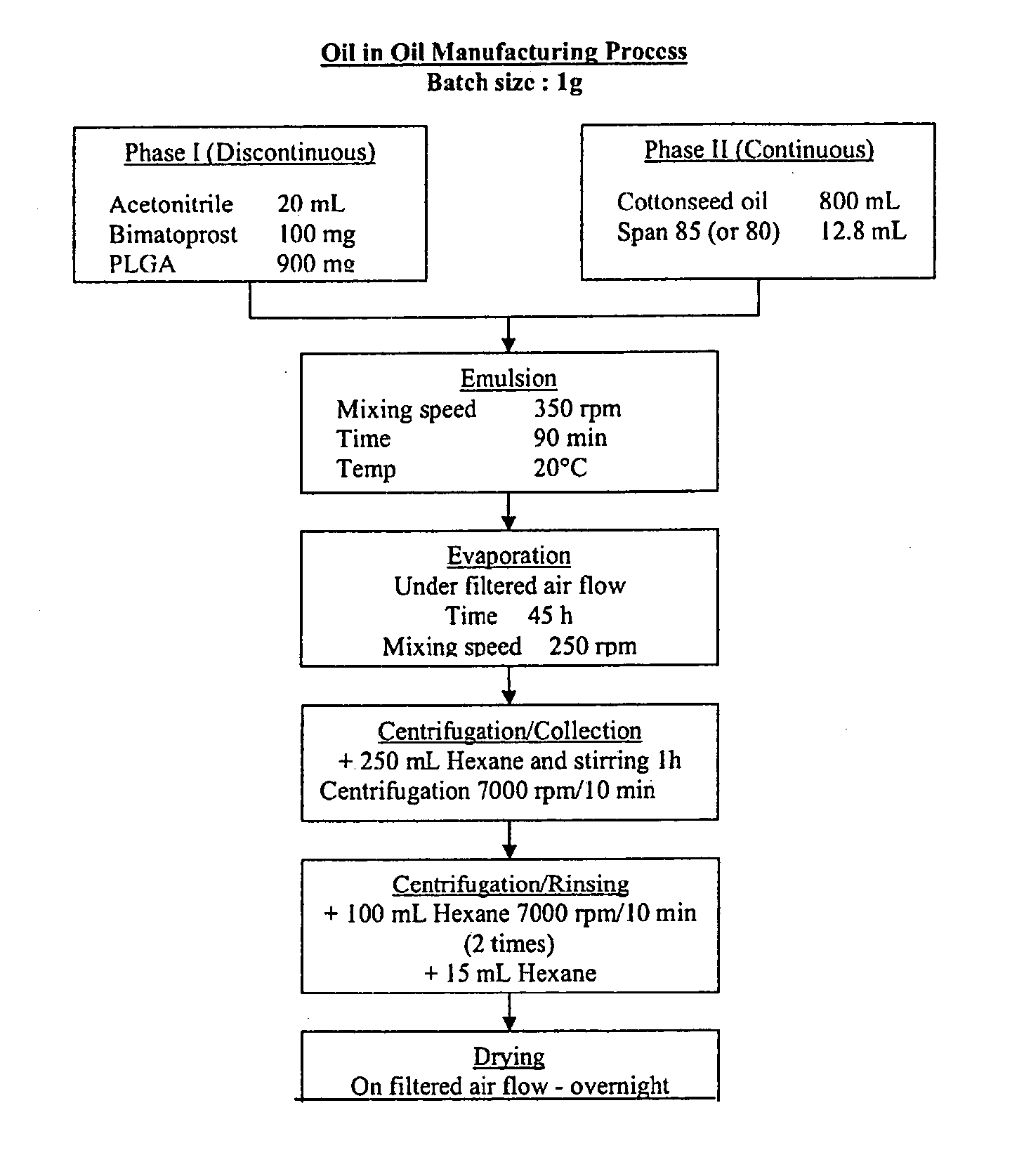
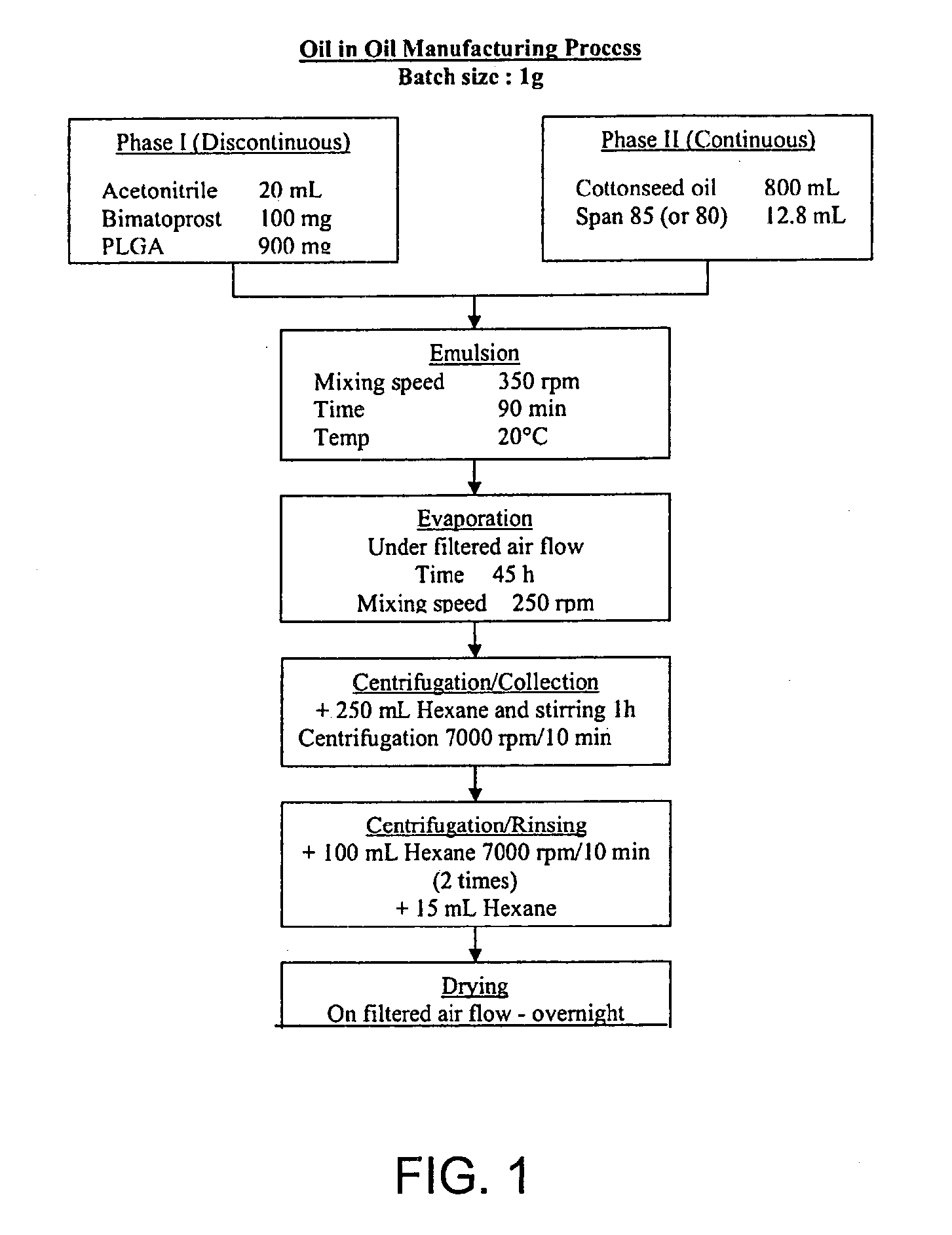
Oil-in-oil emulsified polymeric implants containing a hypotensive lipid and related methods
ActiveUS20110250285A1Facilitate obtaining successful treatment resultsExtend posting timeOrganic active ingredientsBiocideLipid formationGlaucoma
Biocompatible intraocular implants, such as microparticles, include a prostamide component and a biodegradable polymer that is effective in facilitating release of the prostamide component into an eye for an extended period of time. The prostamide component may be associated with a biodegradable polymer matrix, such as a matrix of a two biodegradable polymers. Or, the prostamide component may be encapsulated by the polymeric component. The present implants include oil-in-oil emulsified implants or microparticles. Methods of producing the present implants are also described. The implants may be placed in an eye to treat or reduce a at least one symptom of an ocular condition, such as glaucoma.
Owner:ALLERGAN INC
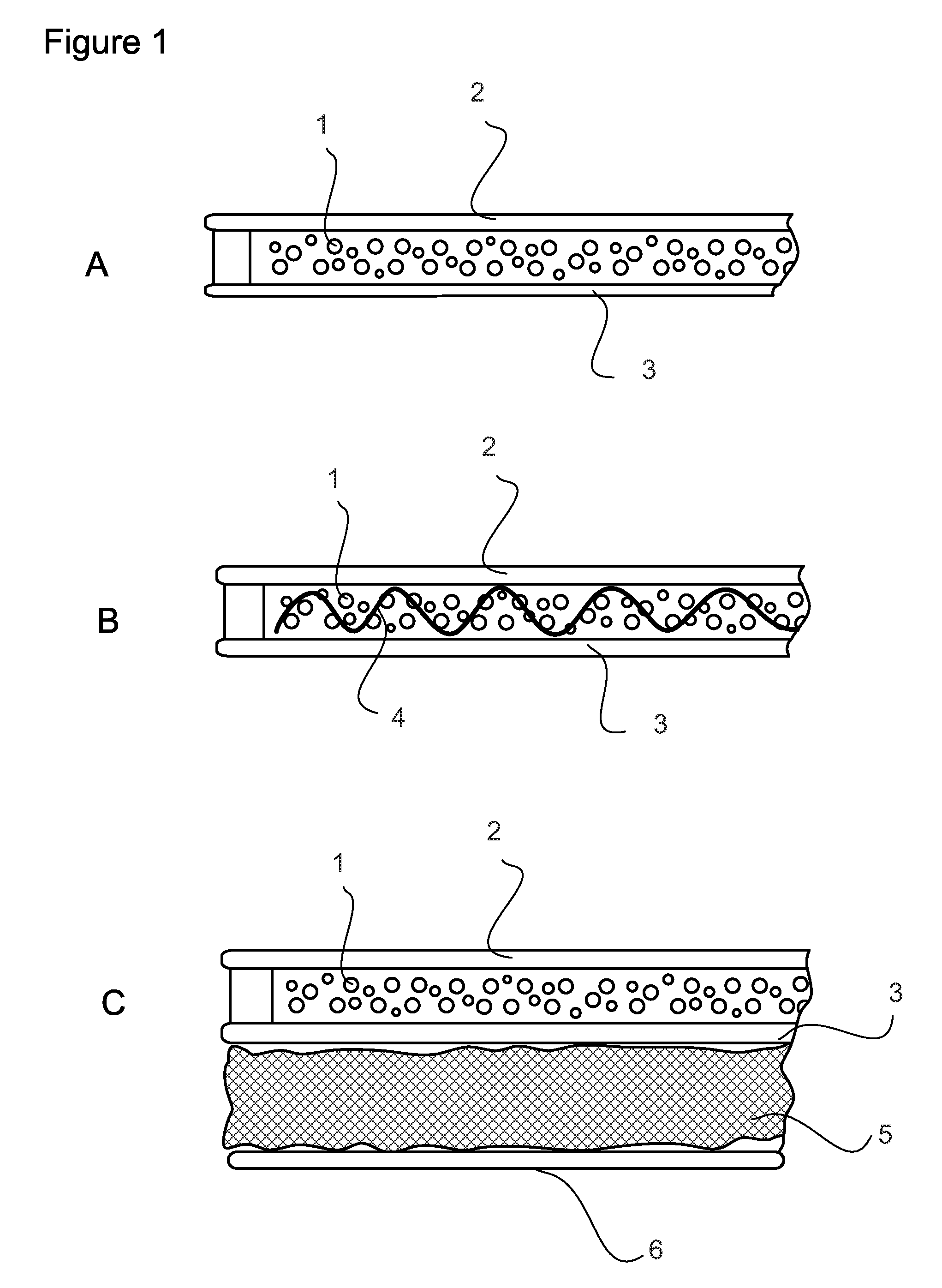
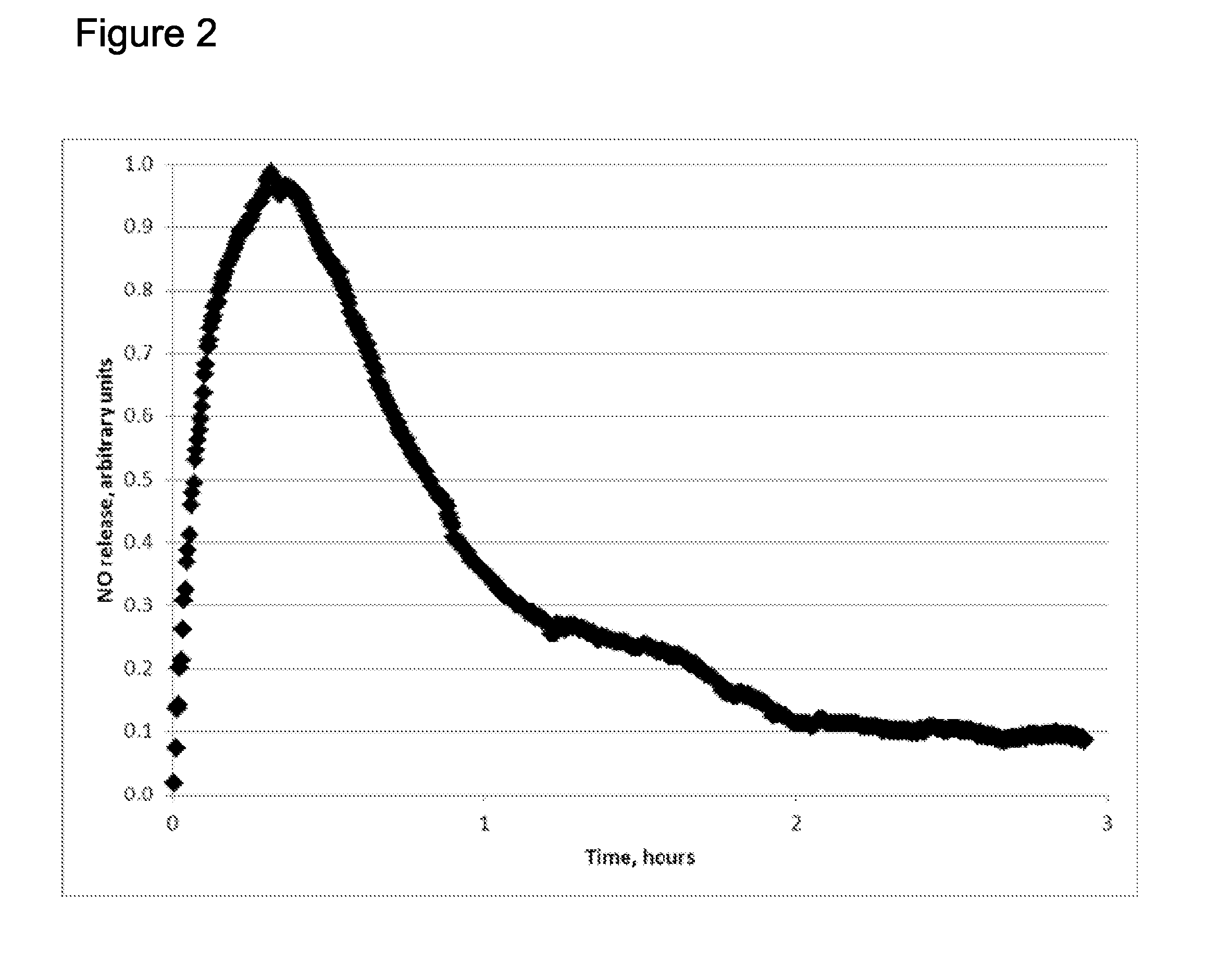
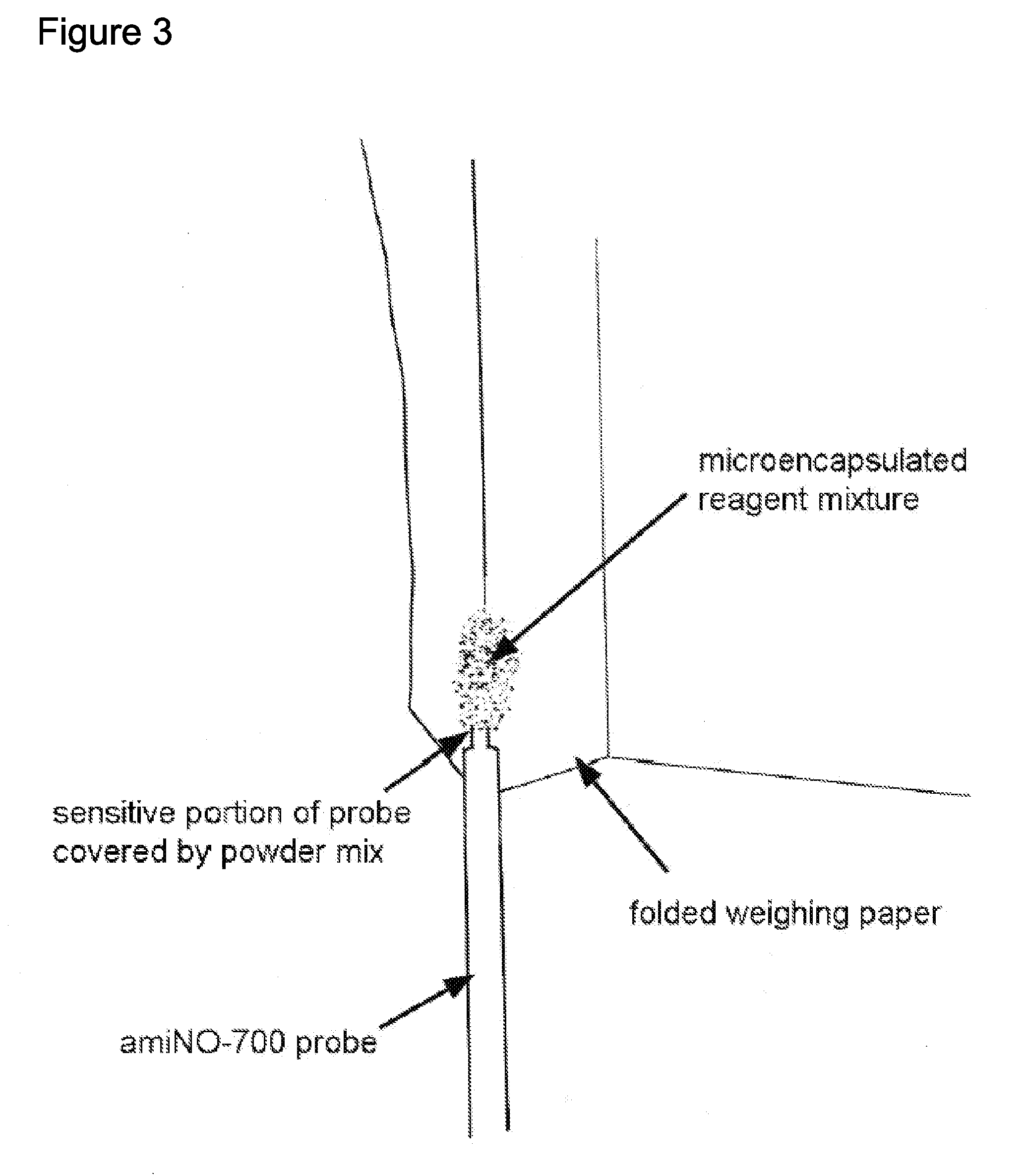
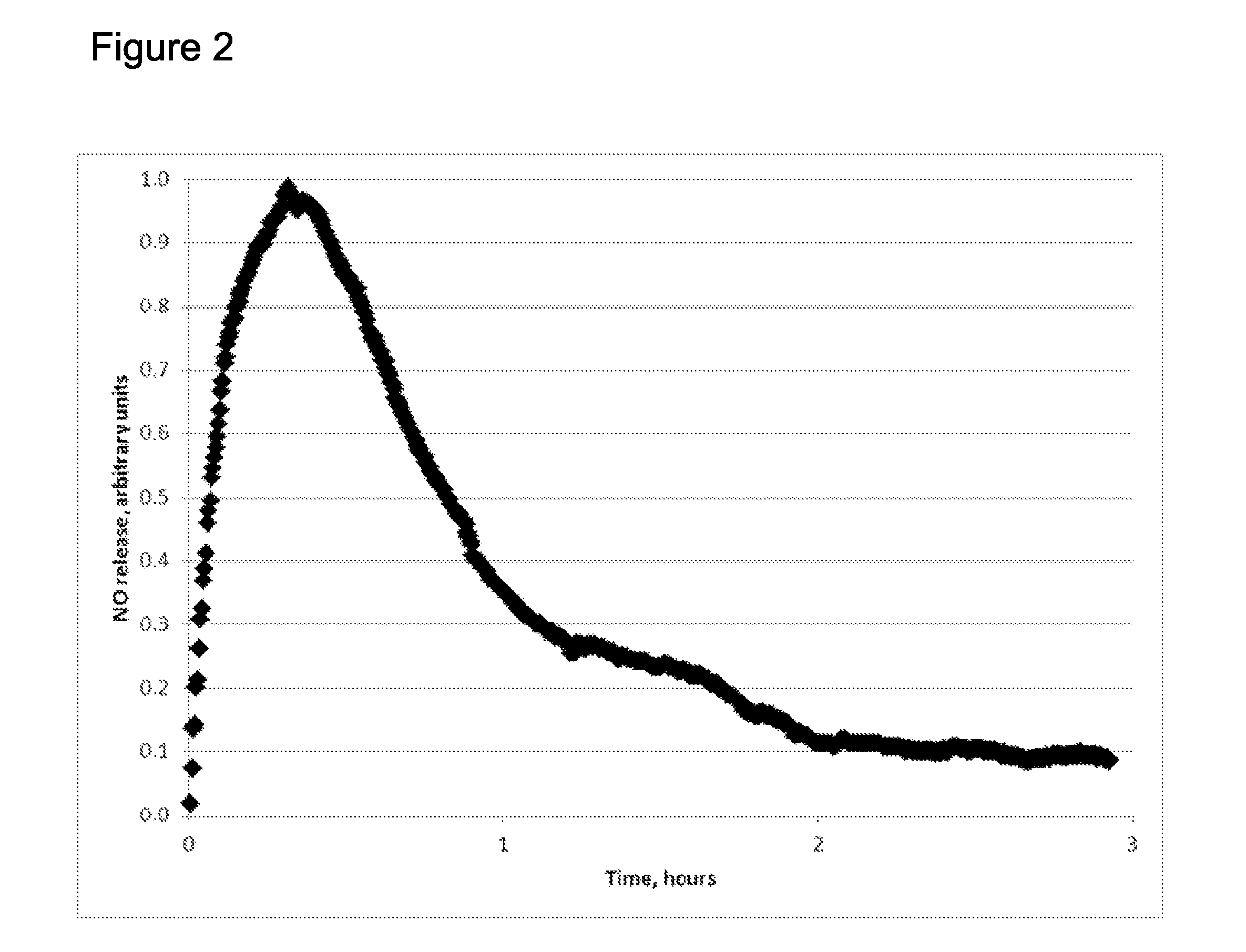
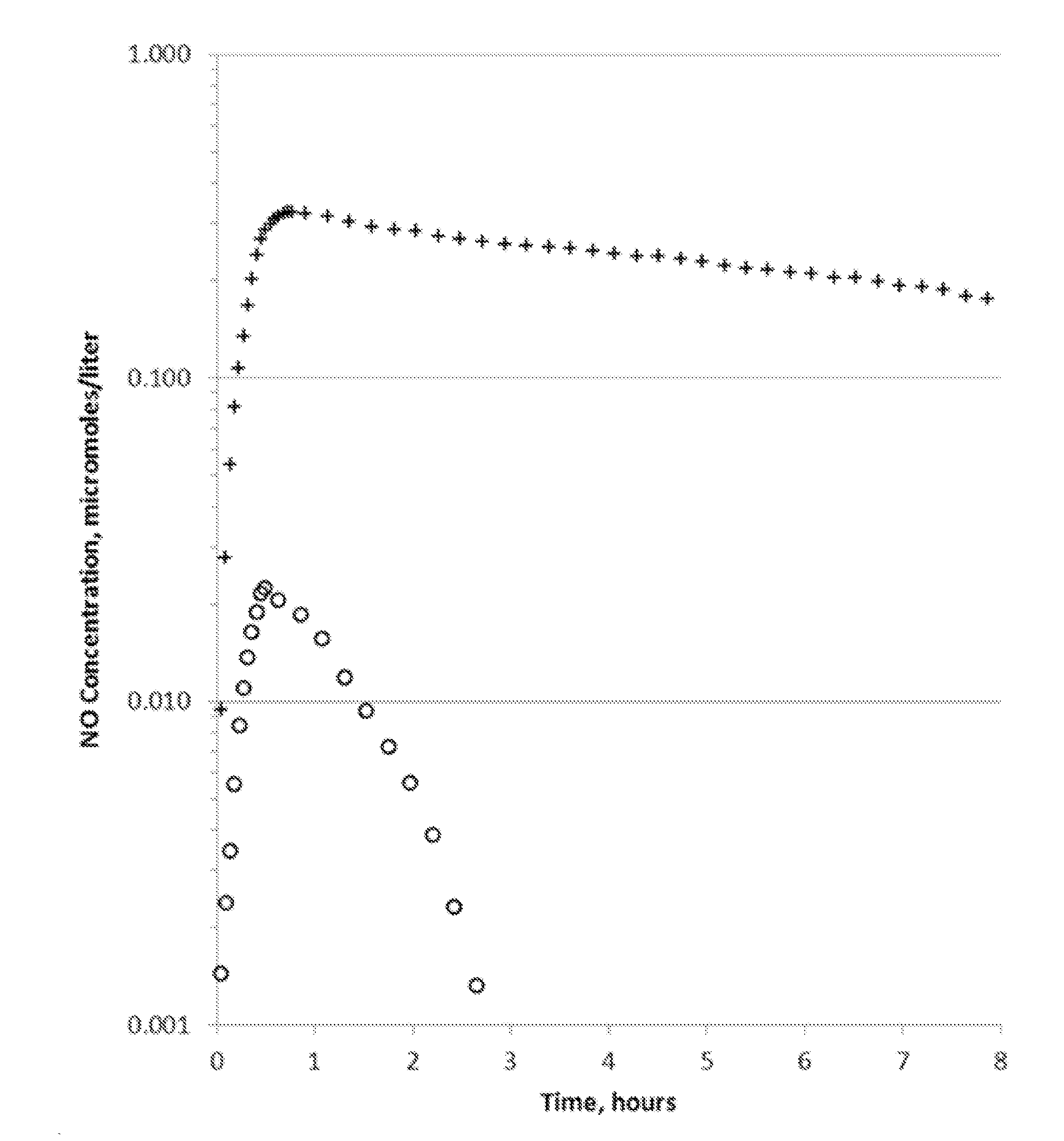
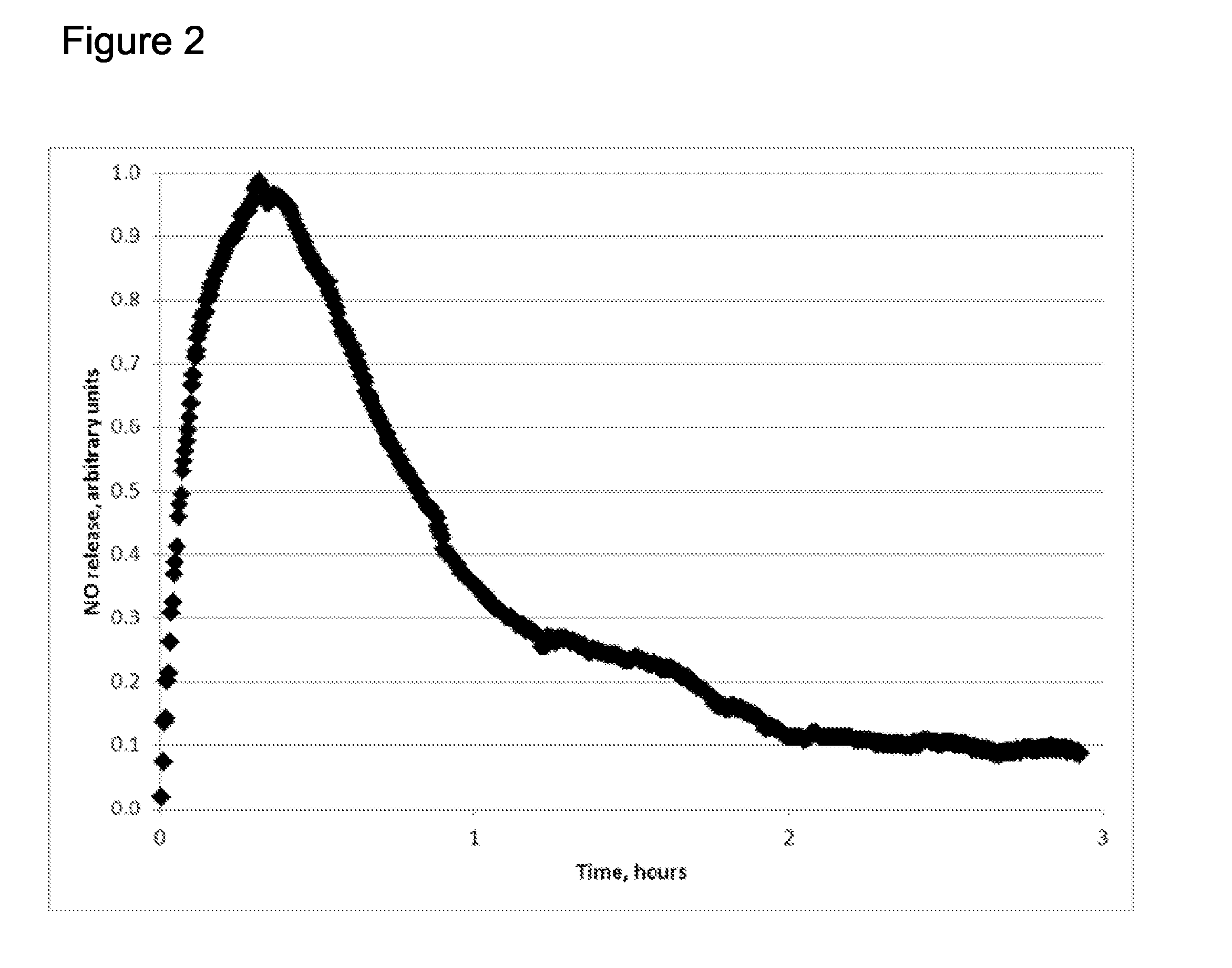
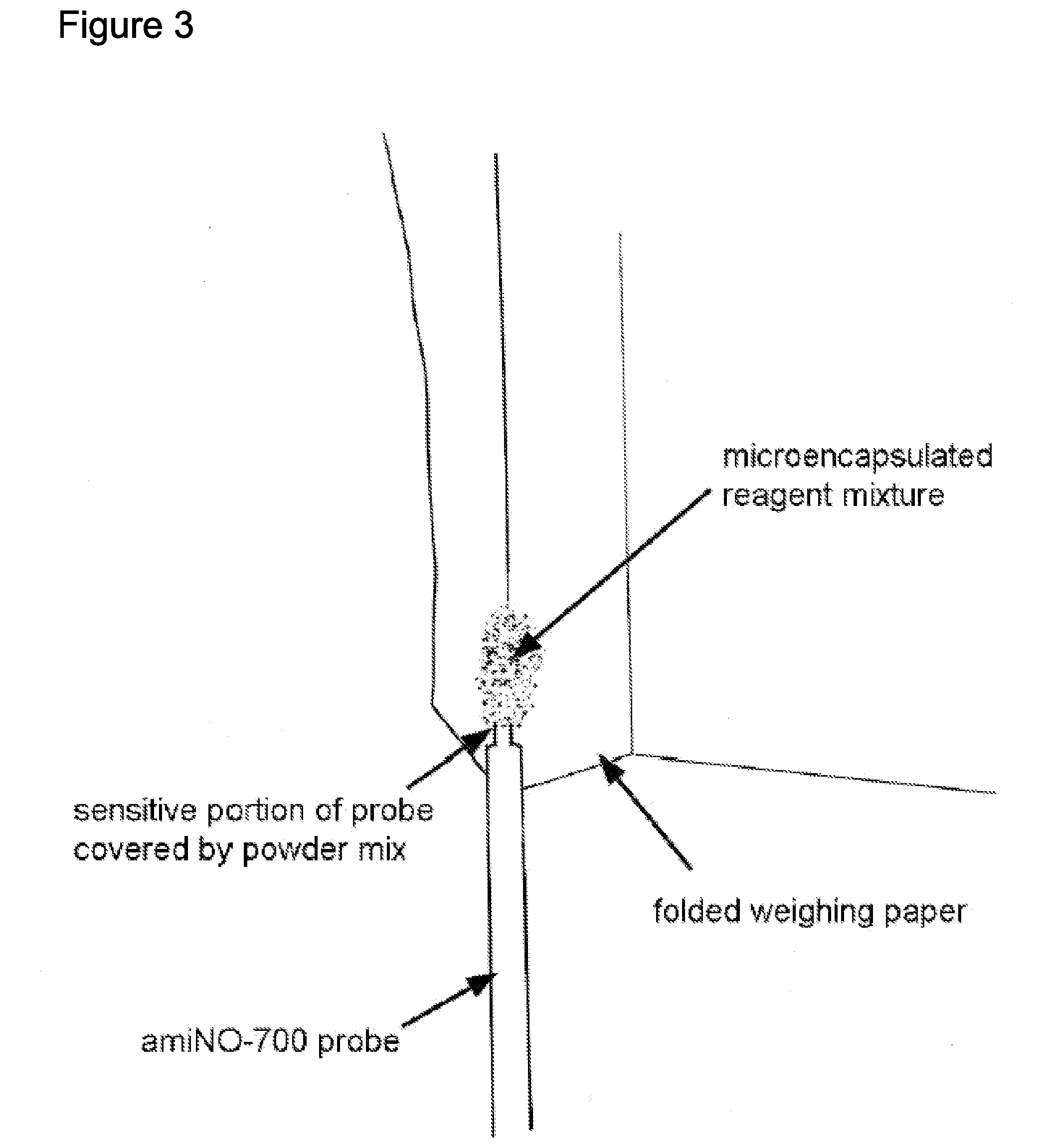
Extended production of nitric oxide from microencapsulated chemical reactants
InactiveUS20140056957A1Small volumeIncrease productionBiocidePowder deliveryCompound (substance)Nitric oxide
Methods and compositions are provided for generating and applying long-lasting therapeutic nitric oxide (NO) gas from the reaction of water-soluble chemical reactants microencapsulated in polymer matrices. In some applications the microencapsulated reactants are introduced in an aqueous gel, and in other applications they are introduced to the area of therapy either directly or in a medical device such as a therapeutic pad or dressing. In some applications, the microencapsulated chemical precursors are maintained in close physical proximity to one another in a limited volume, and using a limited amount of solvent residing within that same volume to extract and process the chemical precursors to form NO.
Owner:NIOXX
Methods for the controlled delivery of pharmacologically active compounds
InactiveUS6946137B2Low toxicitySmall investmentBiocideTetracycline active ingredientsRoxithromycinRelease time
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. The compounds precipitate in aqueous environments. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a period of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
Vehicle controller and control method
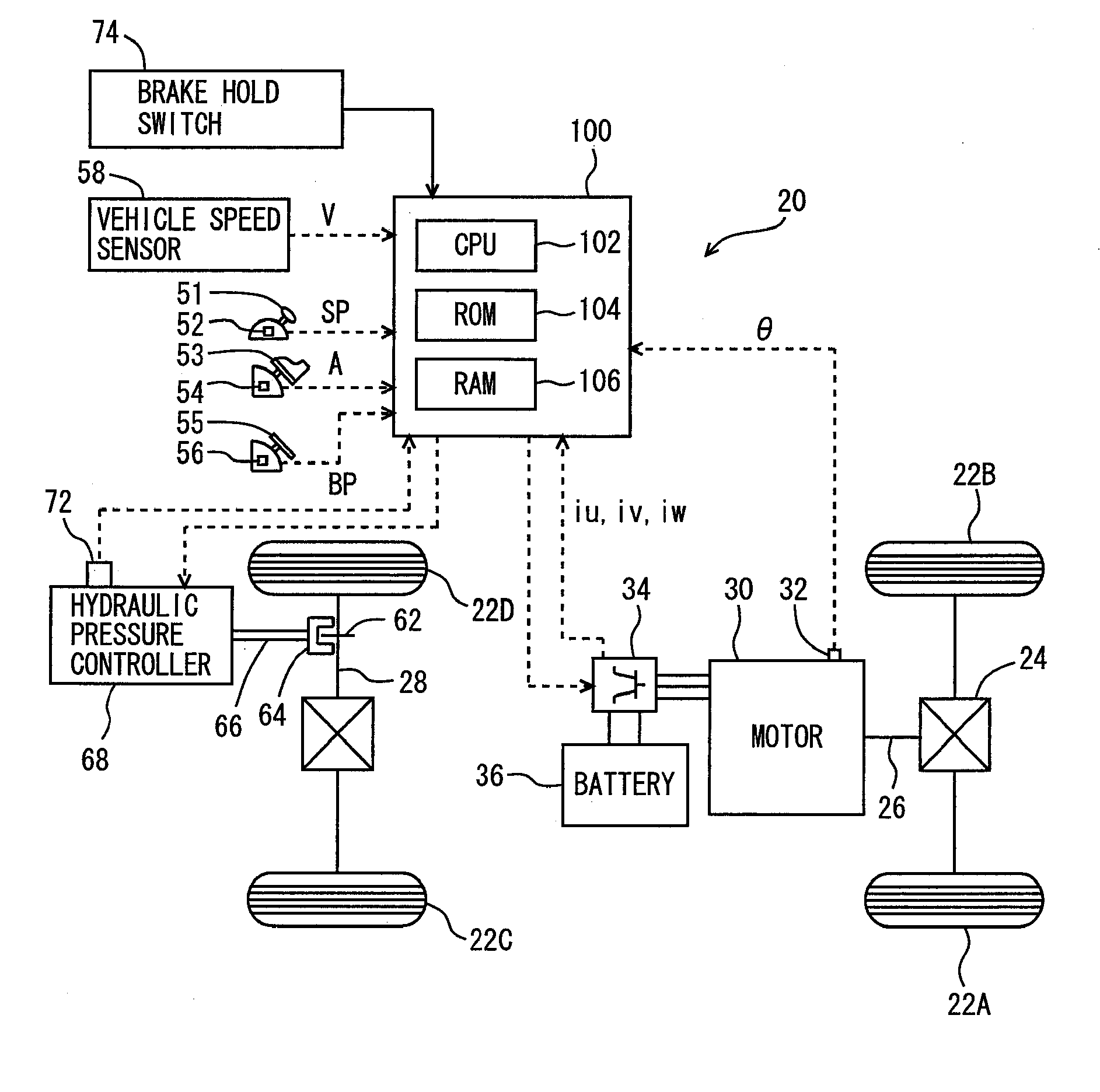
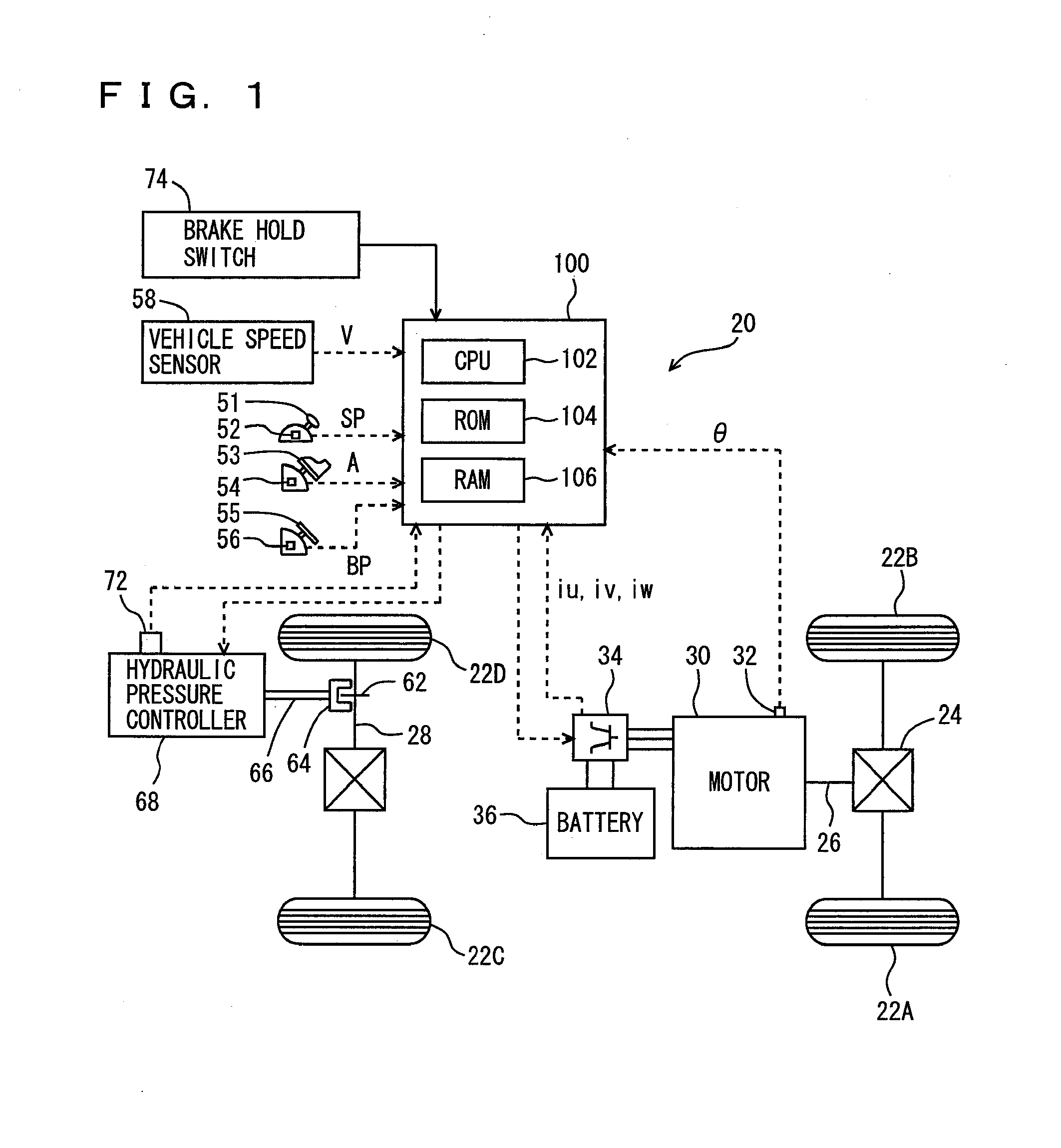
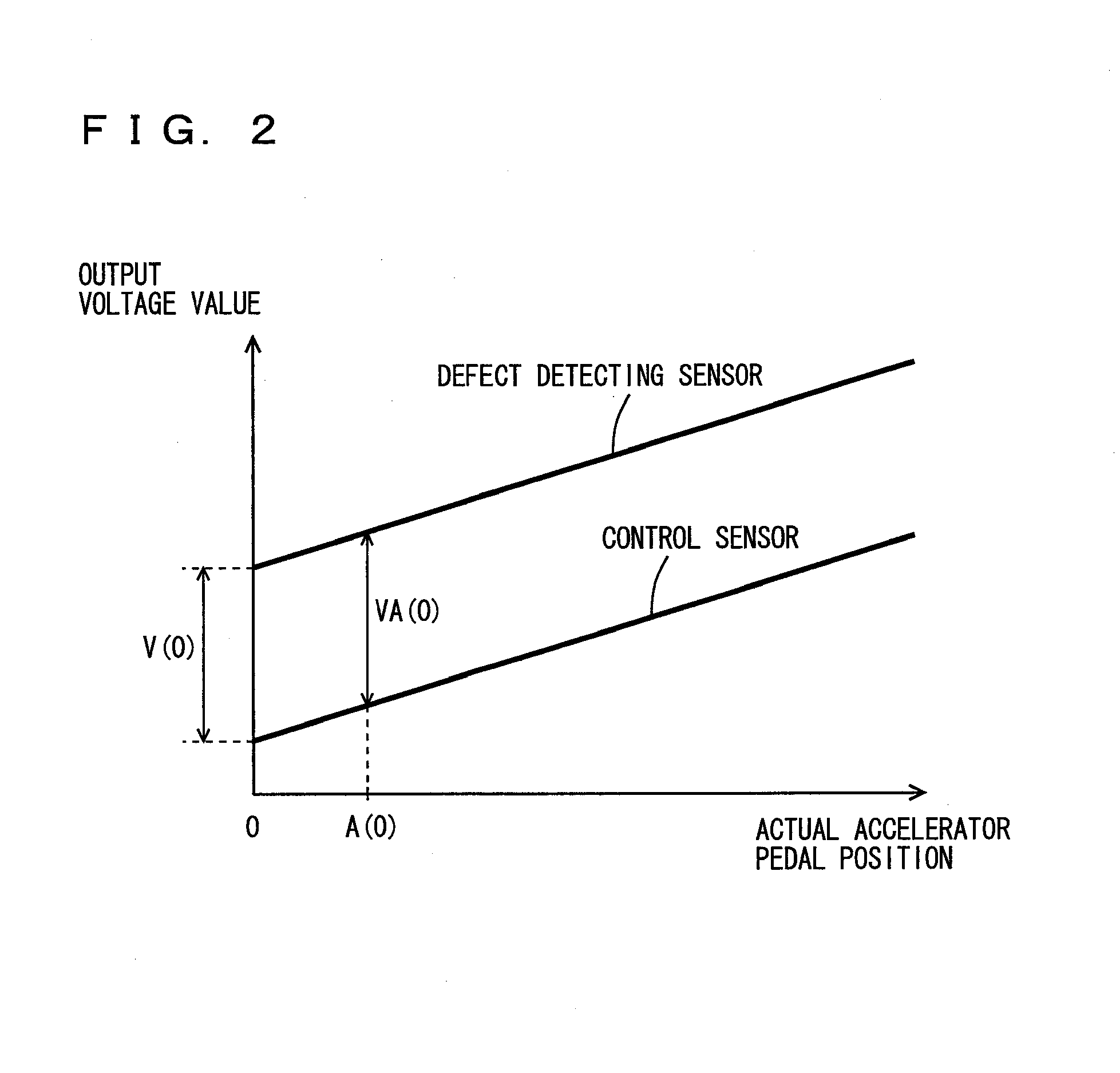
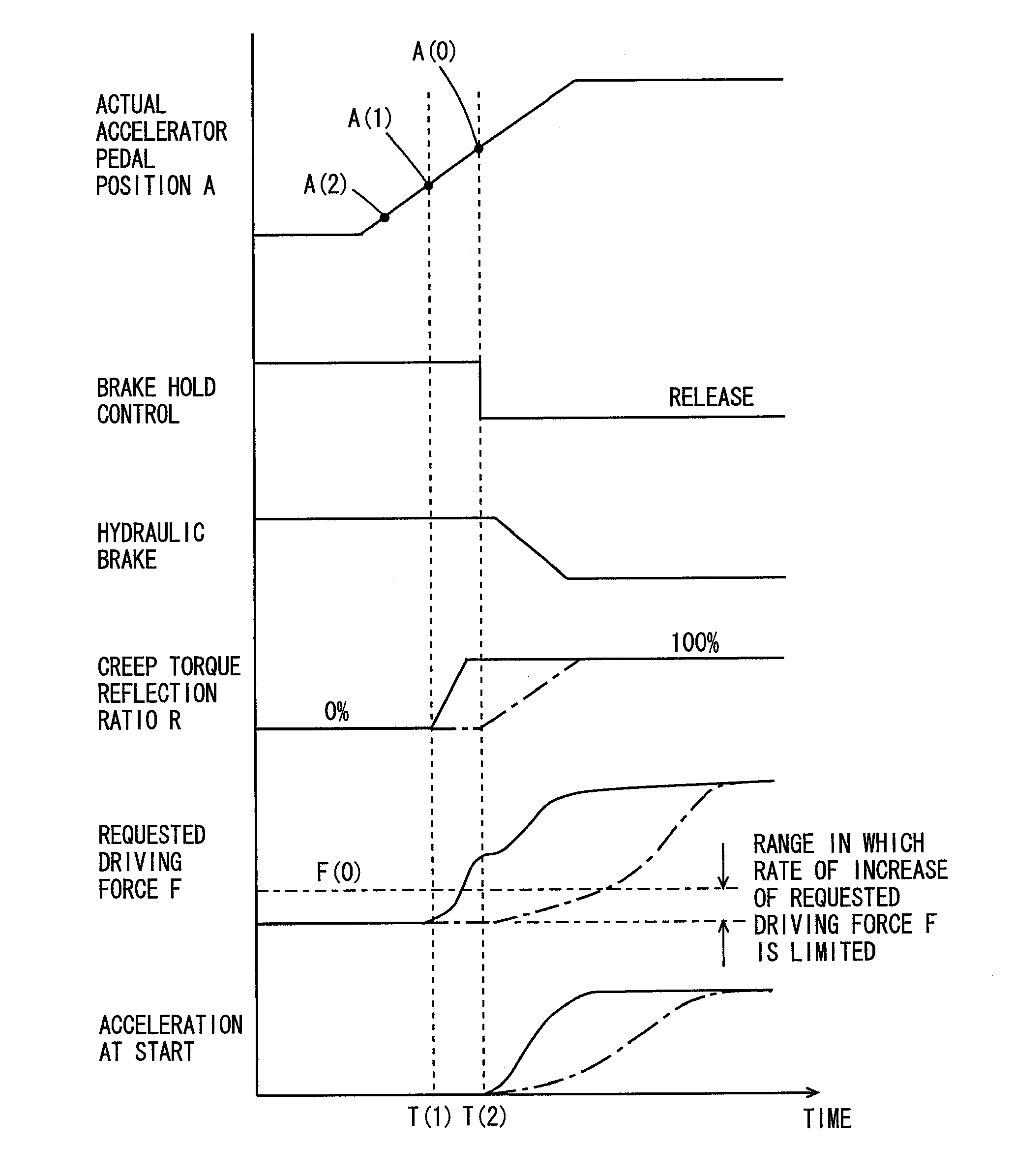
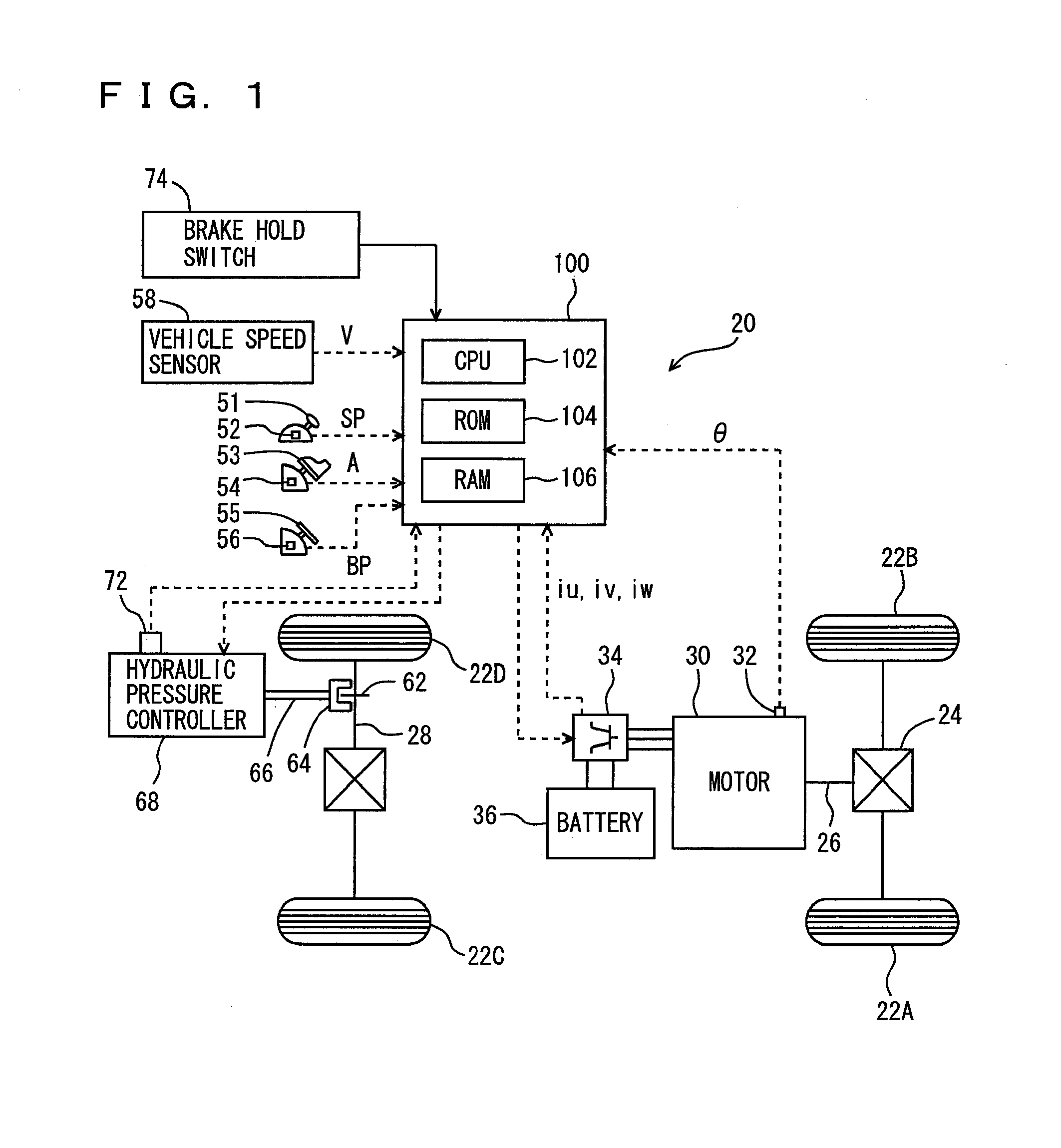
ActiveUS20090112432A1Improve starting characteristicsImprove featuresSpeed controllerAnalogue computers for trafficControl theory
An ECU releases brake hold control when an actual accelerator pedal position A exceeds a predetermined position A(0) while the brake hold control is being executed. Further, the ECU determines whether or not the actual accelerator pedal position A is larger than a position A(1), which is a value smaller than the predetermined position A(0), and if it is larger than the predetermined position A(1), executes a process for increasing a creep torque reflection ratio R to recover creep force that has been stopped.
Owner:TOYOTA JIDOSHA KK
Porous calcium phosphate bone material
ActiveUS8147860B2Improved controlled releaseExtend posting timeBiocideHeavy metal active ingredientsCalcium biphosphateChemical composition
Porous calcium phosphate implant compositions that approximate the chemical composition of natural bone mineral are provided. In addition to calcium phosphate, the compositions include an effervescent agent to promote the formation of interconnected pores and a cohesiveness agent to maintain the shape and hardness of the hardened composition. When introduced at an implant site, the calcium phosphate compositions are remodeled into bone. Methods for using the calcium phosphate compositions, e.g., to repair or replace bone, are also provided.
Owner:ETEX
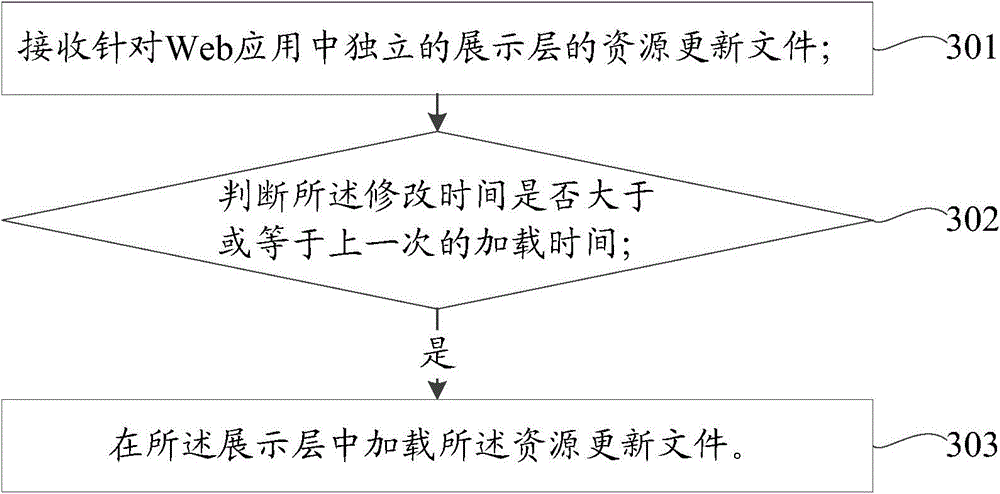
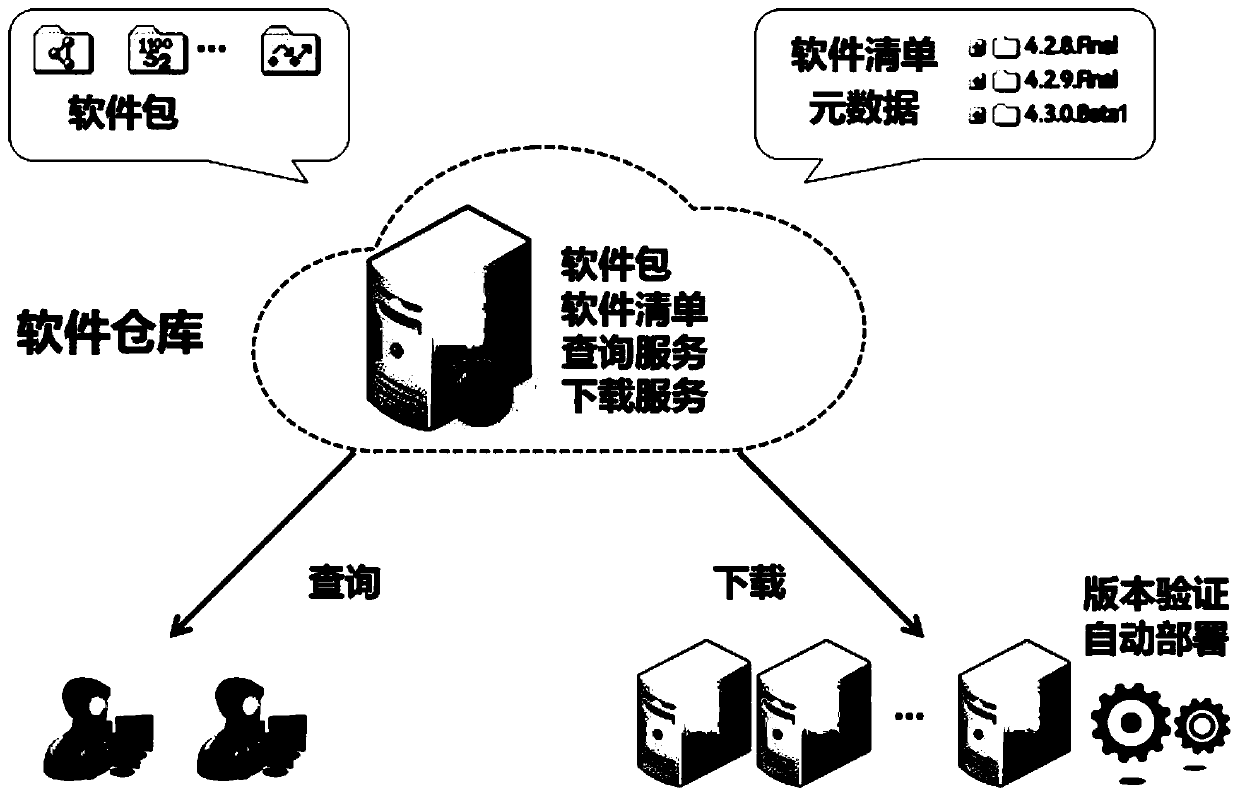
Web application updating method, apparatus and system
ActiveCN105630522AReduce the release processSpeed up release timeProgram loading/initiatingSoftware deploymentRelease timeLoad time
Embodiments of the invention provide a Web application updating method, apparatus and system. The method comprises the steps of receiving a resource updating file for an independent display layer in a Web application, wherein the display layer contains a resource file, the resource file has loading time, and the resource updating file has modification time; judging whether the modification time is greater than or equal to previous loading time; and if yes, loading the resource updating file in the display layer. According to the embodiments of the invention, the display layer is independently managed, and when the display layer is updated, the resource updating file can be modified and released directly in the display layer, the updating of back-end codes does not need to be concerned and the complicated conventional release processes of code combination, compilation, packaging and the like are not required, so that the release processes are greatly reduced, the release time is shortened, the updating risk is lowered, the stability of the display layer is improved, and the updating efficiency is greatly improved.
Owner:ADVANCED NEW TECH CO LTD
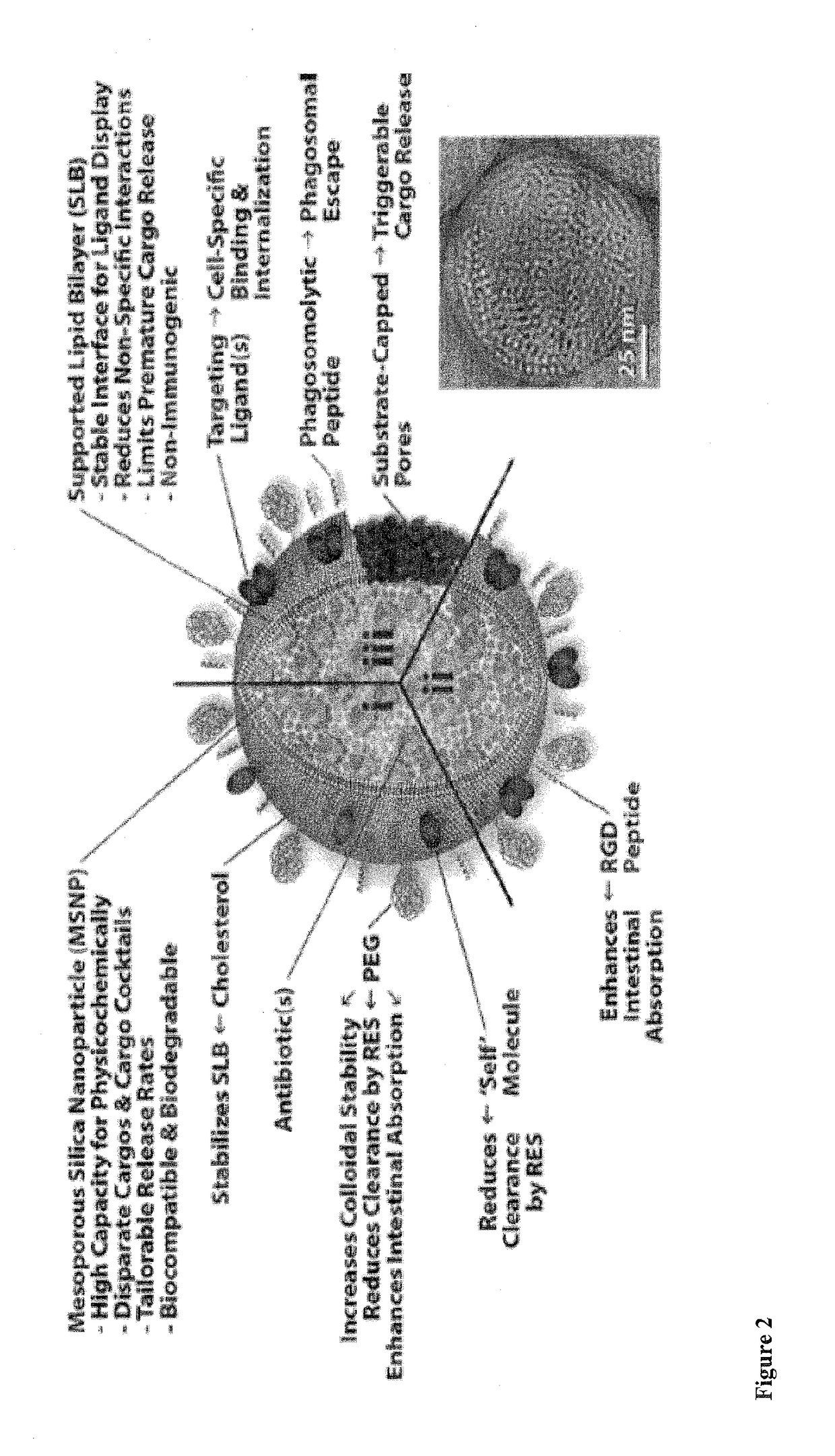
Antibiotic protocells and related pharmaceutical formulations and methods of treatment
InactiveUS20170165375A1Highly flexible and modularRelease rate can be optimizedPowder deliveryTetracycline active ingredientsDrugMultilamellar liposomes
The invention provides novel antibiotic protocells comprising mesoporous nanoparticles encapsulated within a lipid bi- or multilayer. The nanoparticles have pore sizes and surface chemistries that enable facile adsorption and intracellular presentation of antibiotics which are effective in the treatment of a wide variety of bacterial infections, including F. tularensis, B. pseudomallei and P. aeruginosa-related infections. Related pharmaceutical compositions and methods of treatment are also provided.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Extended production of nitric oxide from microencapsulated chemical reactants
Methods and compositions are provided for generating and applying long-lasting therapeutic nitric oxide (NO) gas from the reaction of water-soluble chemical reactants microencapsulated in polymer matrices. In some applications the microencapsulated reactants are introduced in an aqueous gel, and in other applications they are introduced to the area of therapy either directly or in a medical device such as a therapeutic pad or dressing. In some applications, the microencapsulated chemical precursors are maintained in close physical proximity to one another in a limited volume, and using a limited amount of solvent residing within that same volume to extract and process the chemical precursors to form NO.
Owner:NIOXX
Method for treating bacterial infections in horses or pigs with tilmicosin
InactiveUS20060094669A1Low toxicitySmall investment in timeAntibacterial agentsBiocideSolventTilmicosin
The invention relates to methods of treating a bacterial infection in a horse or a pig. The method involves administering to the horse or pig a composition comprising (i) a salt made from tilmicosin and a lipophilic acid and (ii) a pharmaceutically acceptable solvent combined together to form an injectable composition that precipitates when injected into water
Owner:IDEXX LABORATORIES
Methods for the controlled delivery of pharmacologically active compounds
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water immiscible solvent. In one embodiment the compositions are provided as injectable compositions. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C8 fatty acid. The compositions are released over time when administered to a mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for periods of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
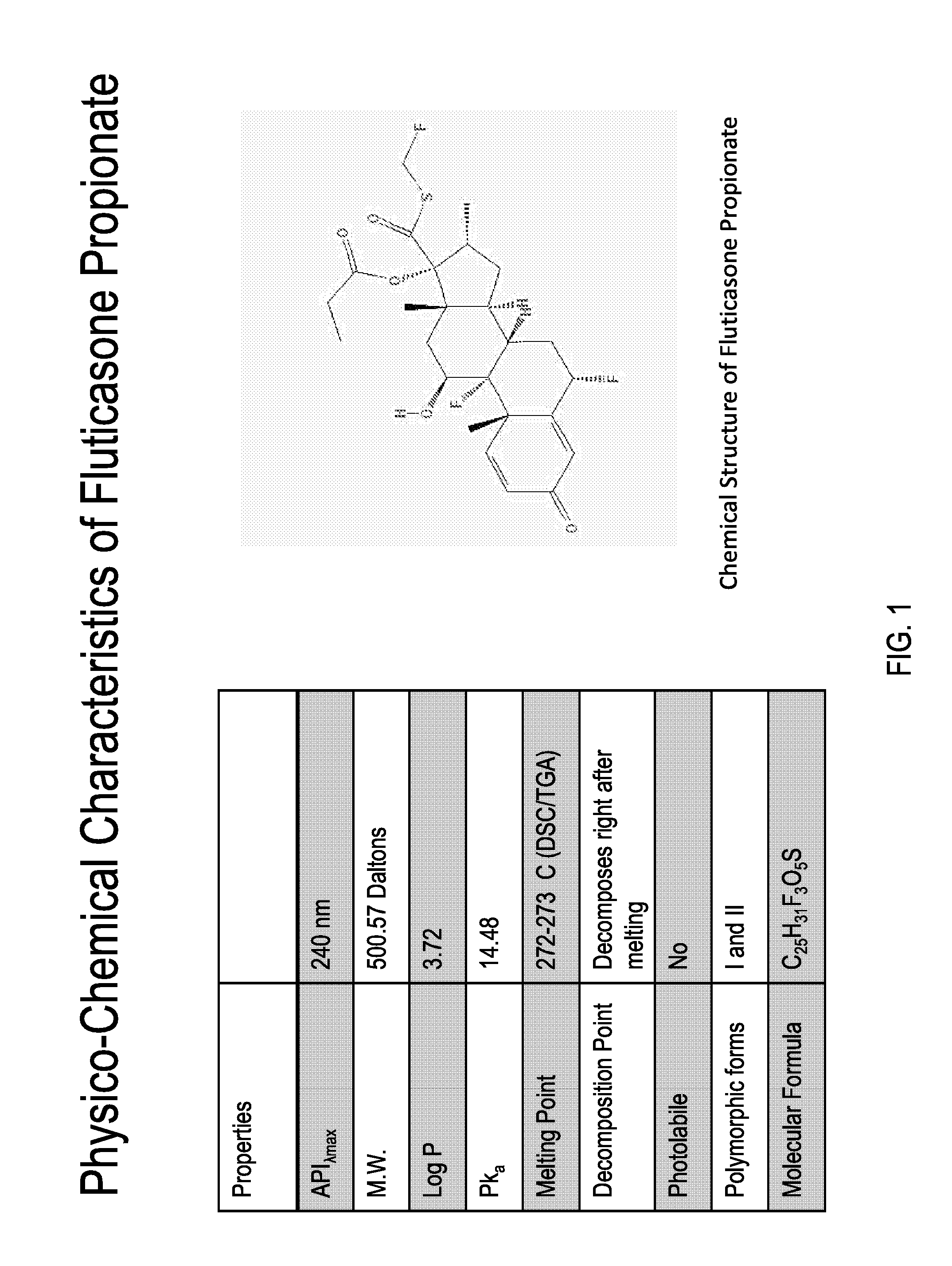
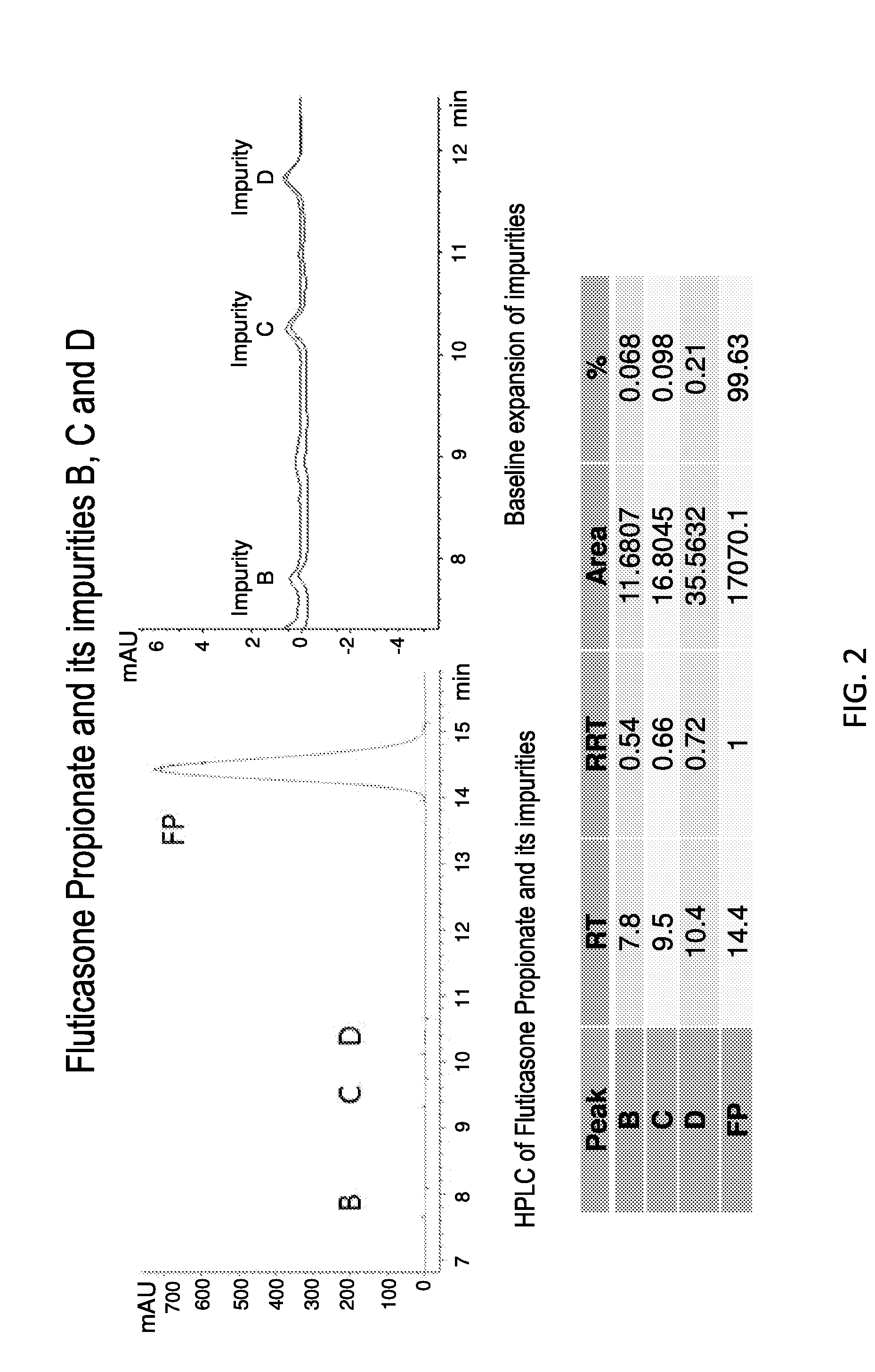
ActiveUS20150337006A1Modifies release timeExtend posting timeOrganic active ingredientsSynthetic resin layered productsDiseaseRespiratory disease
The present invention further provides method of preparing nanocrystals or microcrystals of a hydrophobic therapeutic agent such as fluticasone or triamcinolone, pharmaceutical compositions (e.g., topical or intranasal compositions) thereof and methods for treating and / or preventing the signs and / or symptoms of disorders such as blepharitis, meibomian gland dysfunction or skin inflammation or a respiratory disease (e.g., asthma).
Owner:NICOX OPHTHALMICS
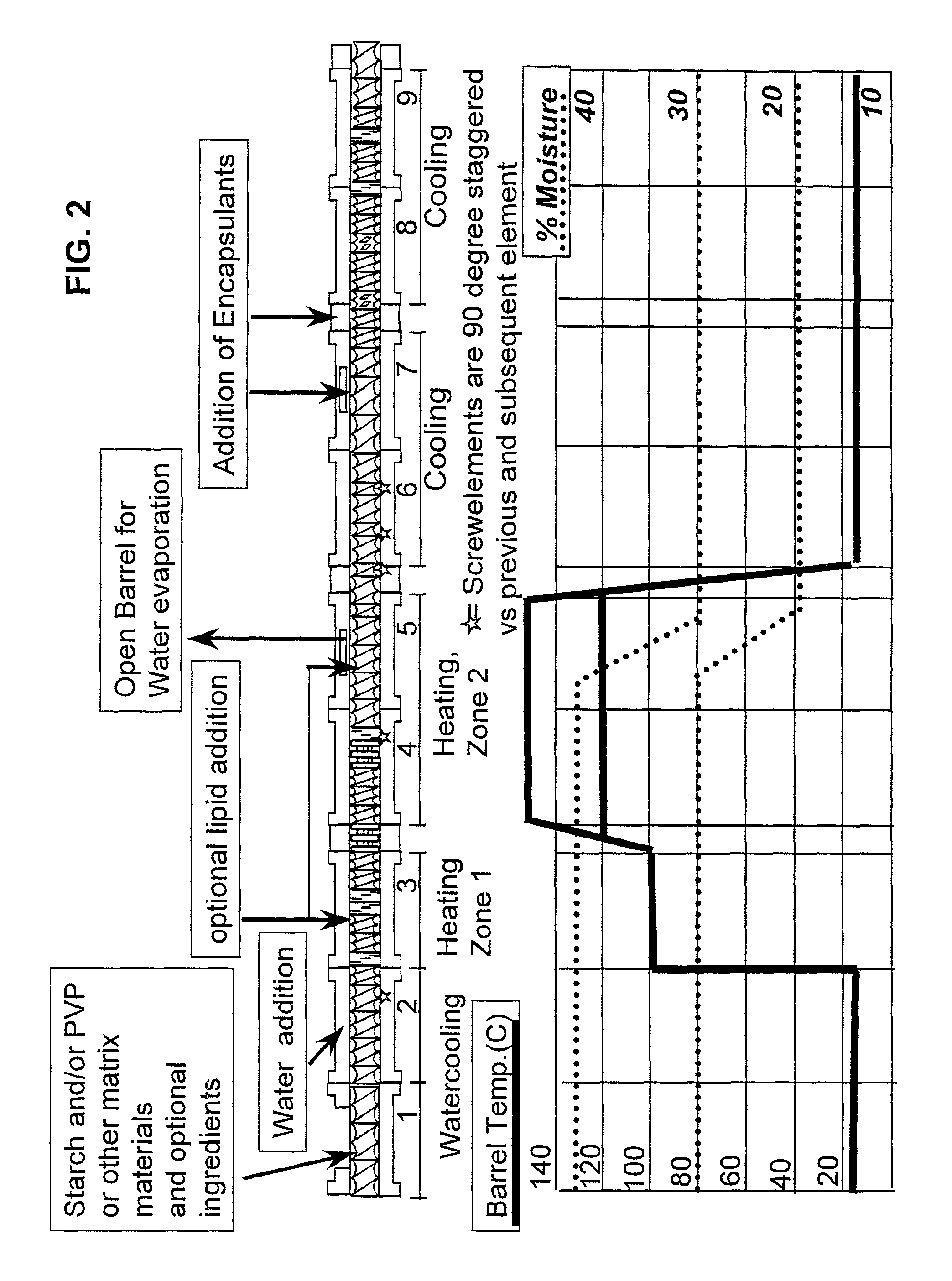
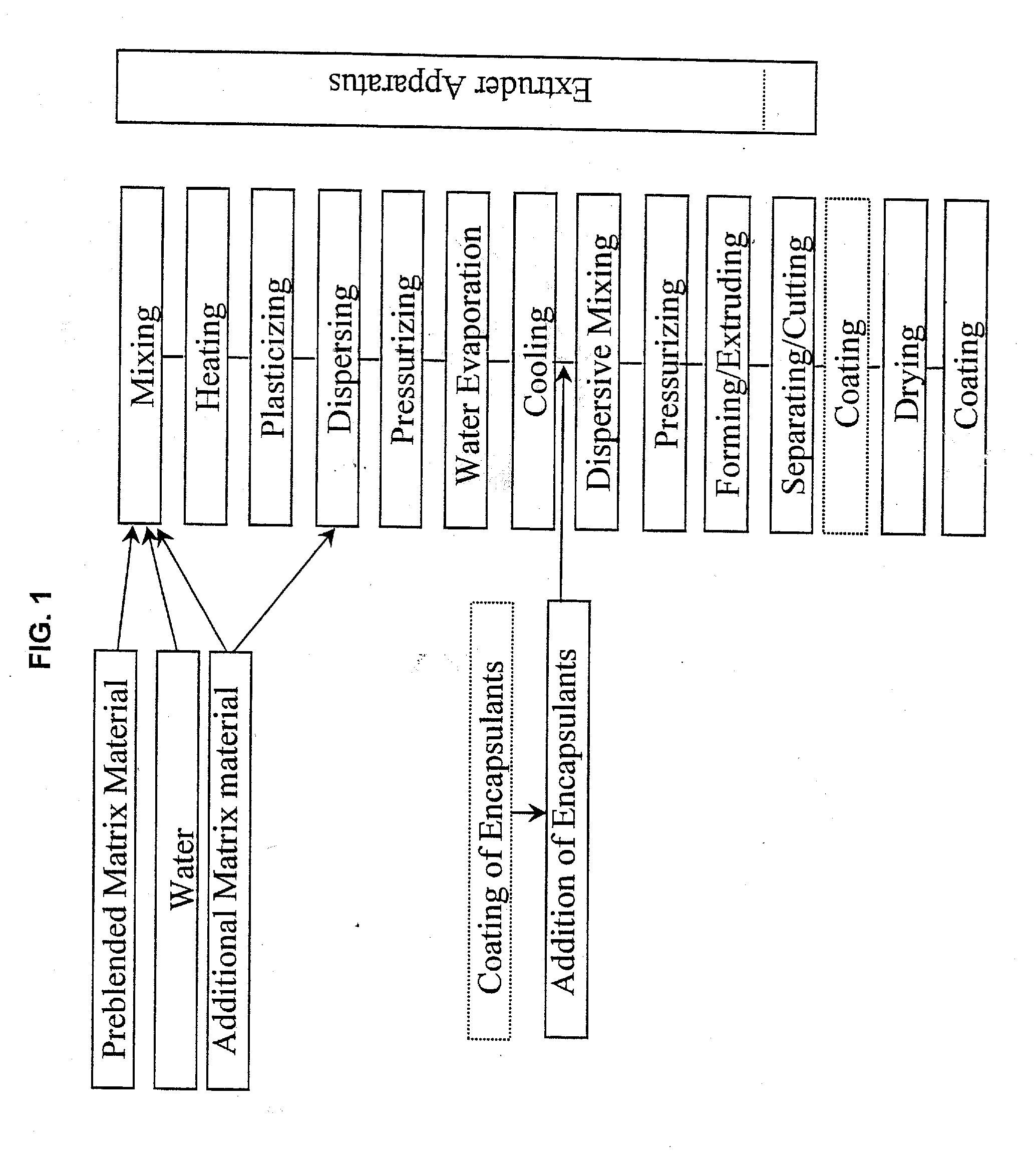
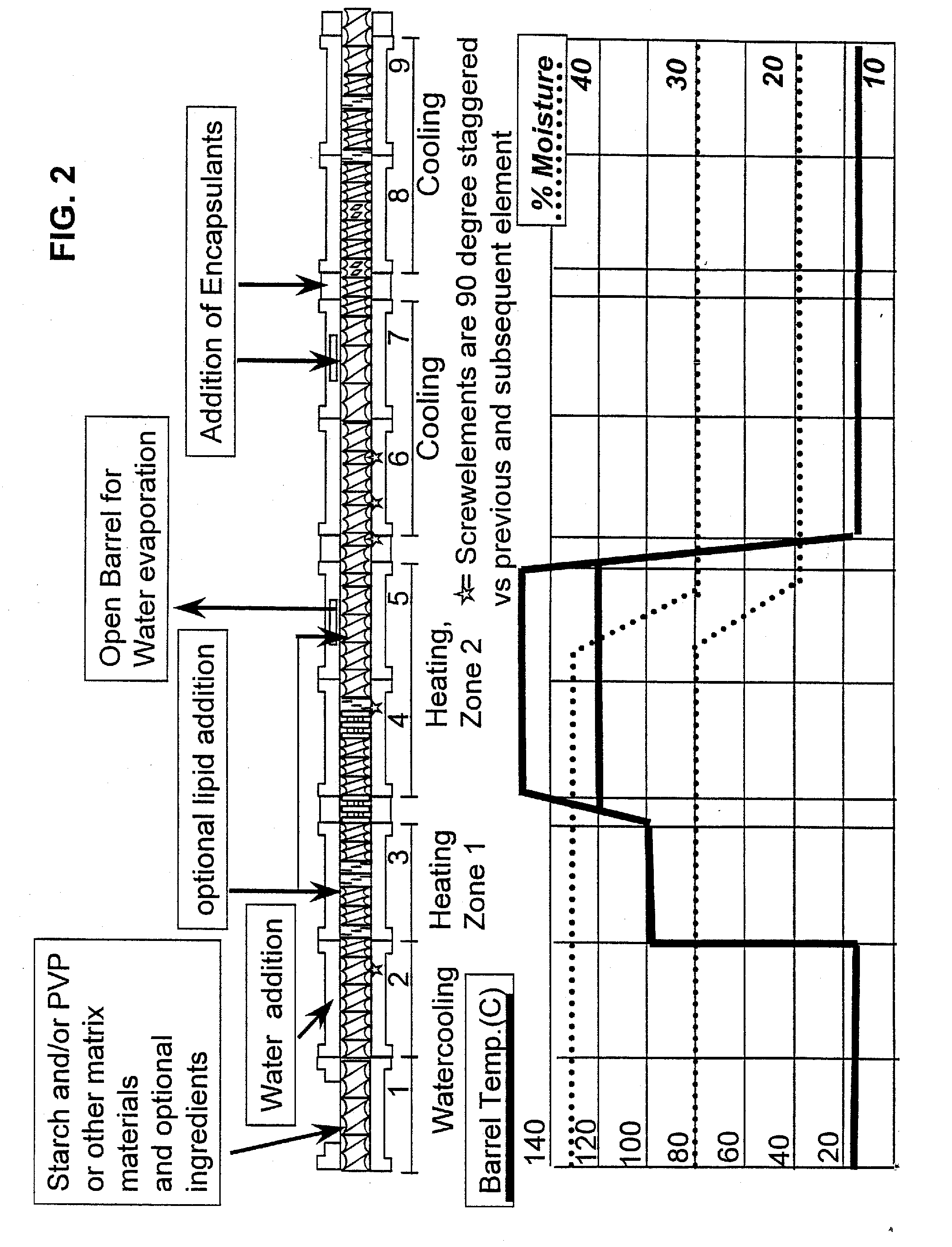
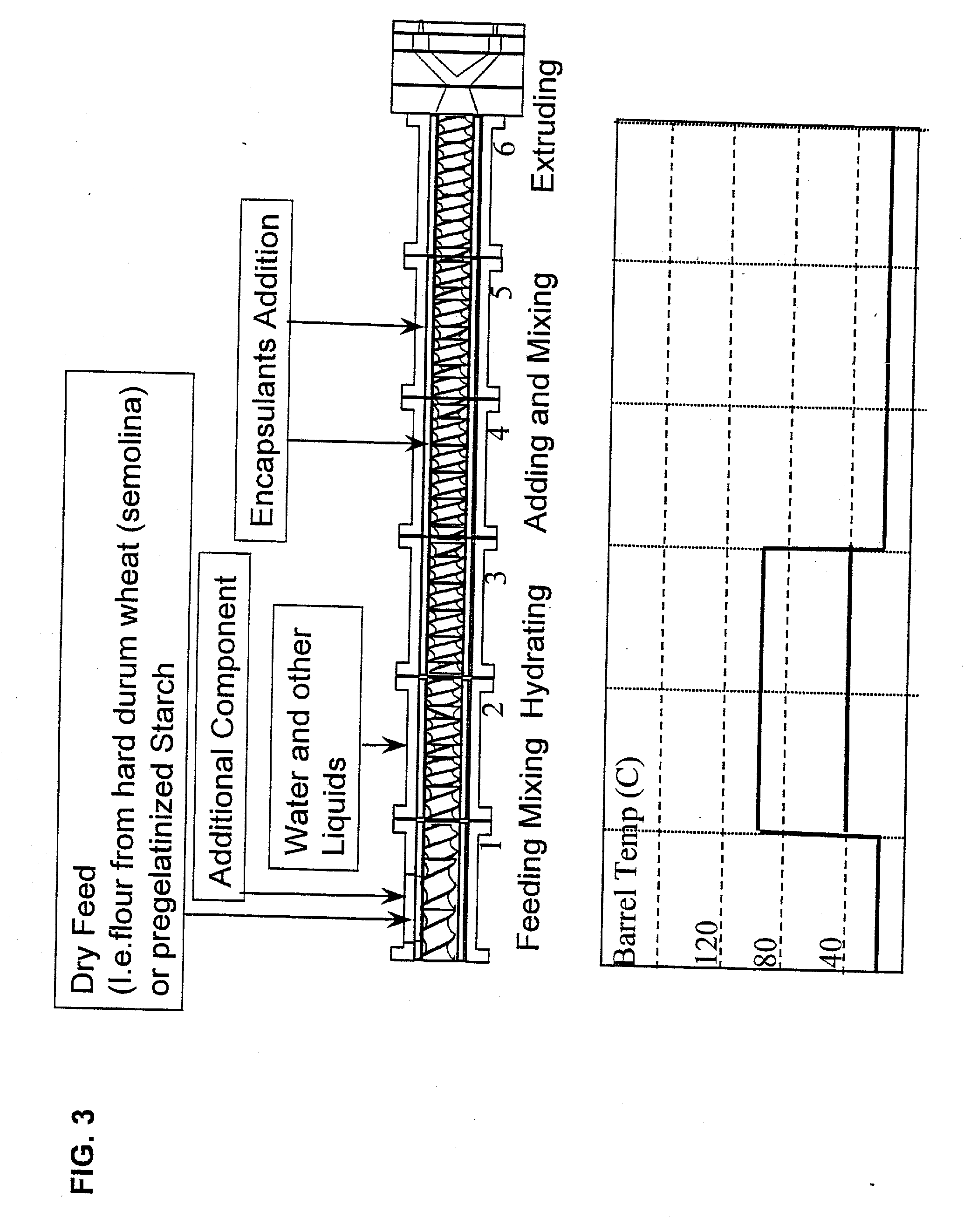
Embedding and encapsulation of sensitive components into a matrix to obtain discrete controlled release particles
InactiveUS8828432B2Avoid substantial destruction of and volatilizationFacilitate substantial gelatinization of starchBiocidePill deliveryRelease timeHeat sensitive
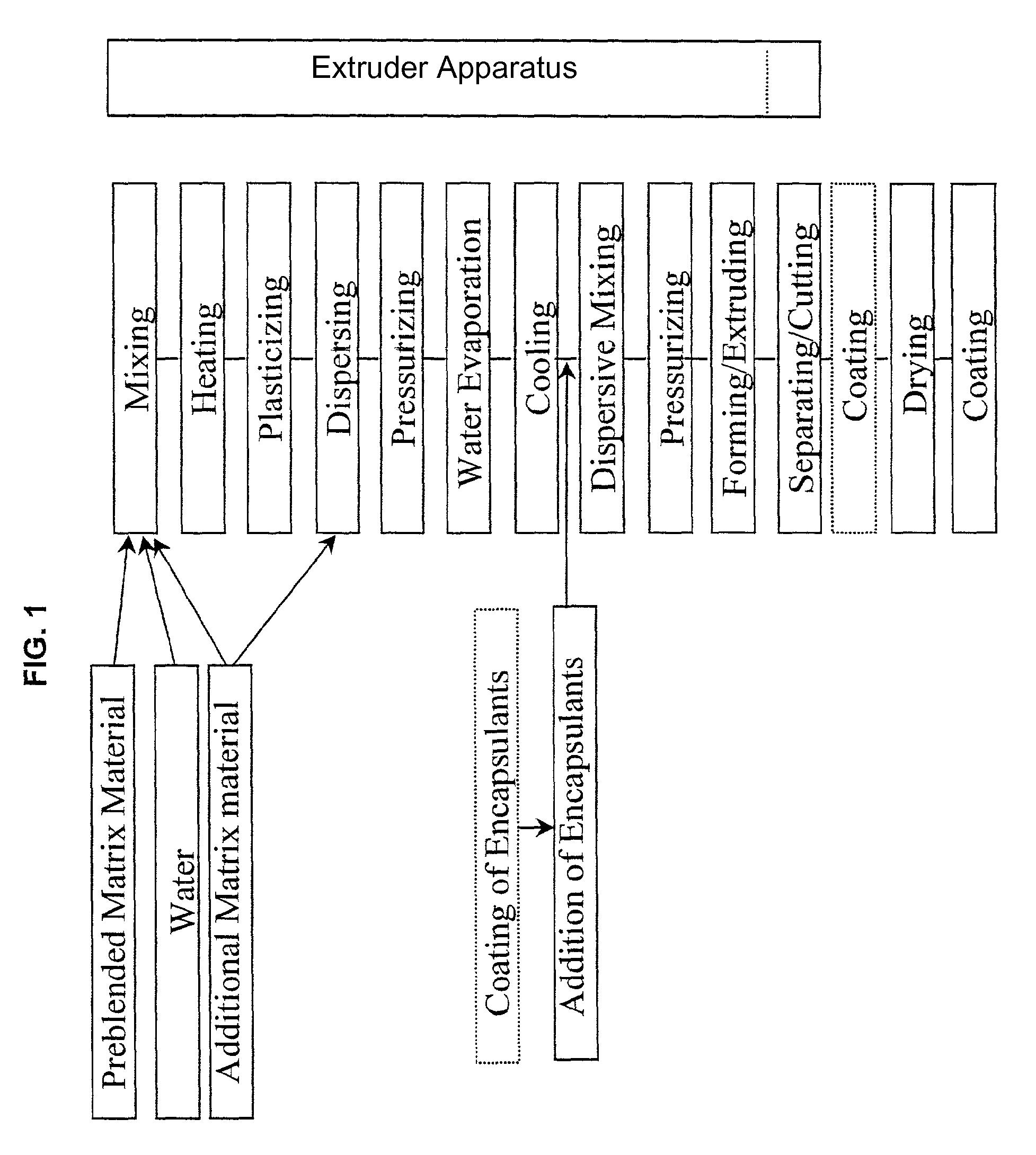
Controlled release, discrete, solid particles which contain an encapsulated and / or embedded component such as a heat sensitive or readily oxidizable pharmaceutically, biologically, or nutritionally active component are continuously produced without substantial destruction of the matrix material or encapsulant. A release-rate controlling component is incorporated into the matrix to control the rate of release of the encapsulant from the particles. The additional component may be a hydrophobic component or a high water binding capacity component for extending the release time. The plasticizable matrix material, such as starch, is admixed with at least one plasticizer, such as water, and at least one release-rate controlling component under low shear mixing conditions to plasticize the plasticizable material without substantially destroying the at least one plasticizable material and to obtain a substantially homogeneous plasticized mass. The plasticizer content is substantially reduced and the temperature of the plasticized mass are substantially reduced prior to admixing the plasticized mass with the encapsulant to avoid substantial destruction of the encapsulant and to obtain a formable, extrudable mixture. The mixture is extruded through a die without substantial or essentially no expansion and cut into discrete, relatively dense particles. Release properties may also be controlled by precoating the encapsulant and / or coating the extrudate particles with a film-forming component.
Owner:GENERAL MILLS INC
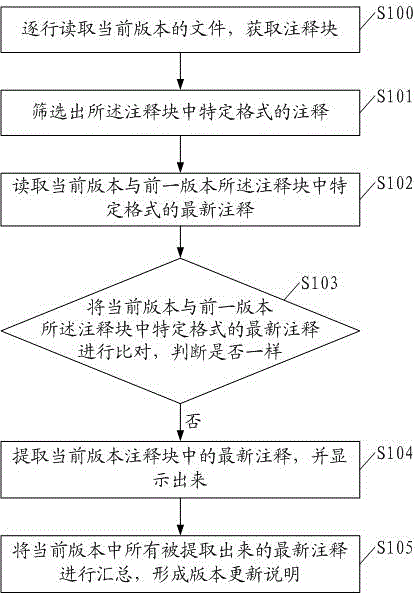
Method and device for automatically generating software integration version updating description
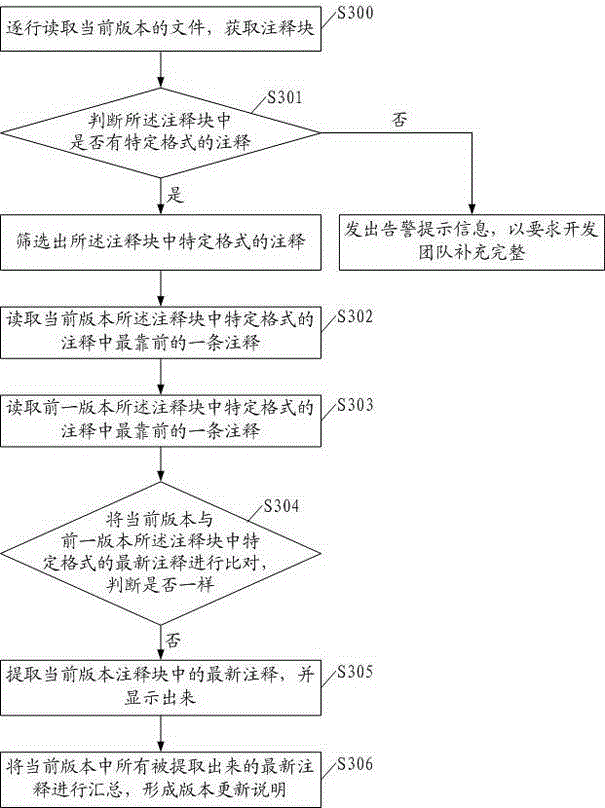
InactiveCN104156198AReduce workloadLow costSpecific program execution arrangementsSource code fileSoftware update
The invention discloses a method for automatically generating software integration version updating description. The method includes the steps that a file of a current version is read line by line so that an annotation block can be obtained; annotations in specific formats are screened from the annotation block, and the specific formats include SOA fields; newest annotations in the specific formats in the annotation block of the current version and newest annotations in the specific formats in an annotation block of a previous version are read; when the newest annotations in the specific formats in the annotation block of the current version and the newest annotations in the specific formats in the annotation block of the previous version are compared and judged whether to be the same or not, and if not, the newest annotations in the specific formats in the annotation block of the current version are extracted and displayed; all the extracted newest annotations of the current version are collected, and the version updating description is formed. The invention further discloses a device for automatically generating software integration version updating description. Due to the method and device, the version updating description can be automatically generated and corresponds to actually-modified source code files one by one, workloads of a development team can be reduced, software updating and issuing cost is reduced, and issuing is performed in advance.
Owner:GUANGDONG POWER GRID CO LTD INFORMATION CENT
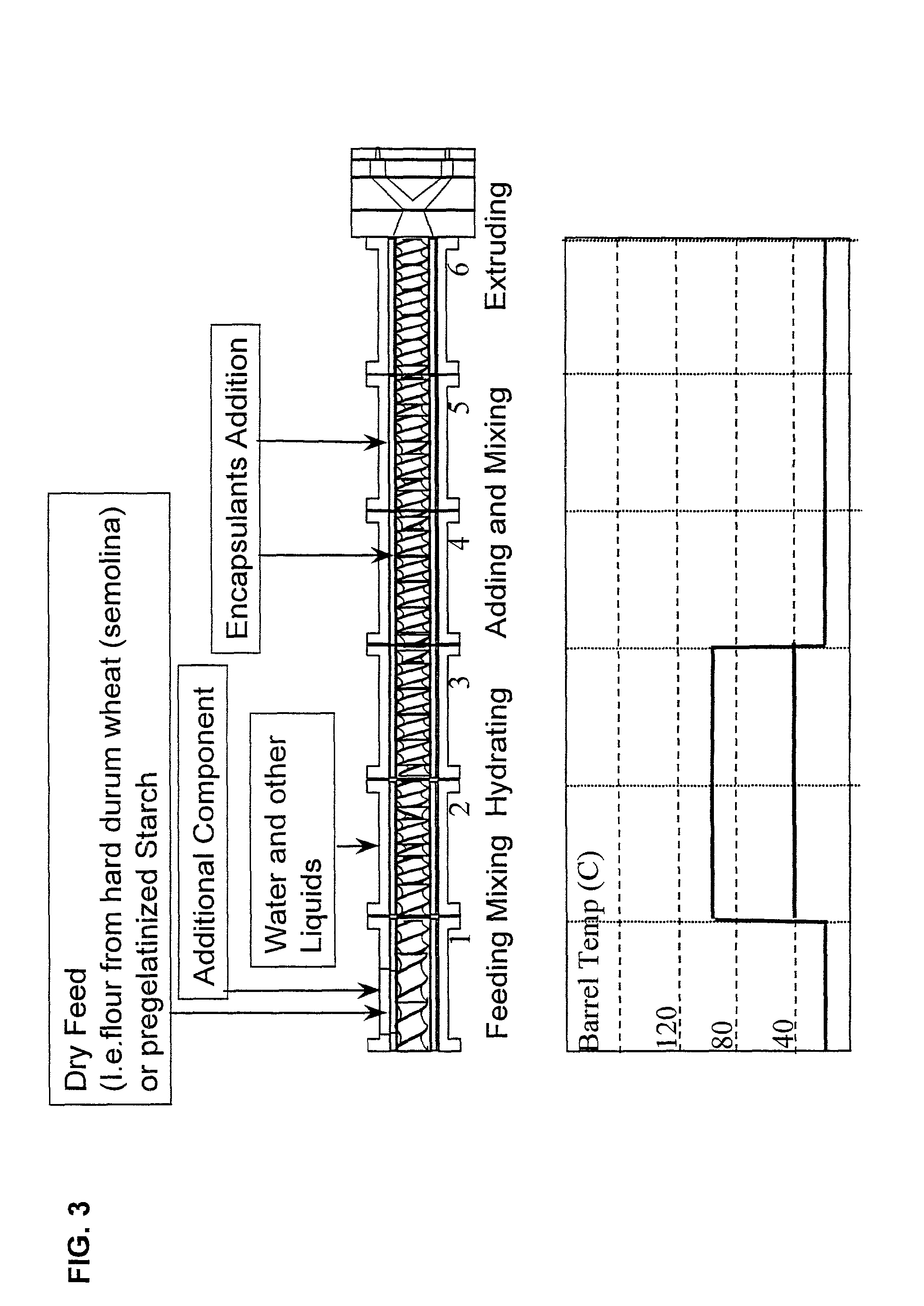
Method for the embedding and encapsulation of components
InactiveUS20140147501A1Avoid substantial destruction of and volatilizationFacilitate substantial gelatinization of starchConfectionerySweetmeatsControlled releasePlasticizer
Controlled release, discrete, solid particles which contain an encapsulated and / or embedded component such as a heat sensitive or readily oxidizable pharmaceutically, biologically, or nutritionally active component are continuously produced without substantial destruction of the matrix material or encapsulant. A release-rate controlling component is incorporated into the matrix to control the rate of release of the encapsulant from the particles. The additional component may be a hydrophobic component or a high water binding capacity component for extending the release time. The plasticizable matrix material, such as starch, is admixed with at least one plasticizer, such as water, and at least one release-rate controlling component under low shear mixing conditions to plasticize the plasticizable material without substantially destroying the at least one plasticizable material and to obtain a substantially homogeneous plasticized mass. The plasticizer content is substantially reduced and the temperature of the plasticized mass are substantially reduced prior to admixing the plasticized mass with the encapsulant to avoid substantial destruction of the encapsulant and to obtain a formable, extrudable mixture. The mixture is extruded through a die without substantial or essentially no expansion and cut into discrete, relatively dense particles. Release properties may also be controlled by precoating the encapsulant and / or coating the extrudate particles with a film-forming component.
Owner:GENERAL MILLS INC
Preparations of hydrophobic therapeutic agents, methods of manufacture and use thereof
ActiveUS20150126483A1Maintaining osmolalityMaintain purityOrganic active ingredientsOrganic chemistry methodsDiseaseRespiratory disease
Owner:NICOX OPHTHALMICS
Extended production of nitric oxide from a microencapsulated nitrite salt and an aqueous acidified gel
Methods and compositions are provided for generating and applying long-lasting therapeutic nitric oxide (NO) gas from the reaction of a least one microencapsulated nitrite salt and an activating volume of an aqueous acidified gel that has sufficient acidity to convert the nitrite salt to a nitric oxide (NO) and further provides a reducing property that retains the NO in bioactive form.
Owner:NIOXX
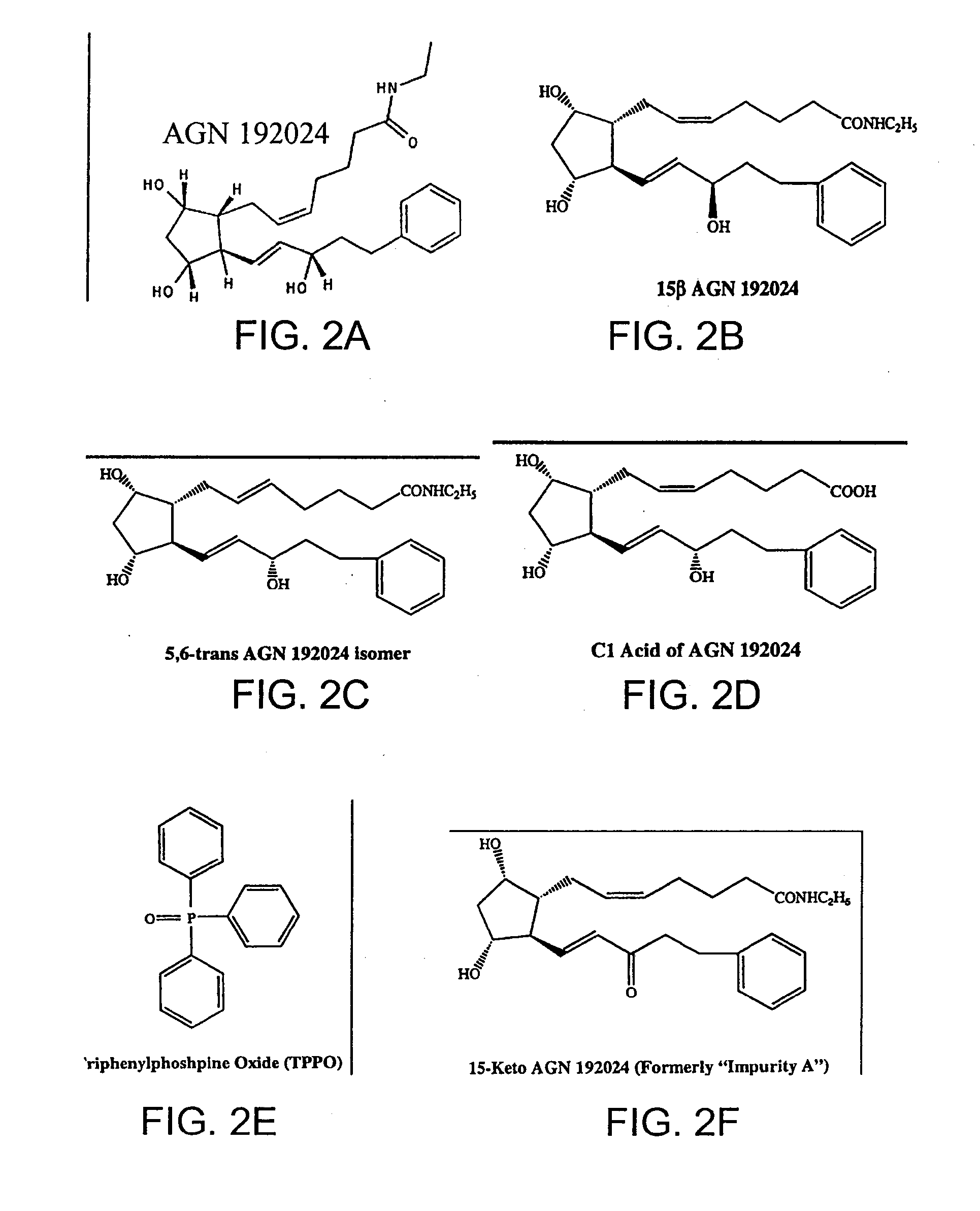
Prostaglandin and prostamide drug delivery systems and intraocular therapeutic uses thereof
Biocompatible, bioerodible implants and microspheres include latanoprost and a biodegradable polymer effective, when placed intraocular (such as into the subtenon space) to treat glaucoma.
Owner:ALLERGAN INC
Communication-based service processing method and device
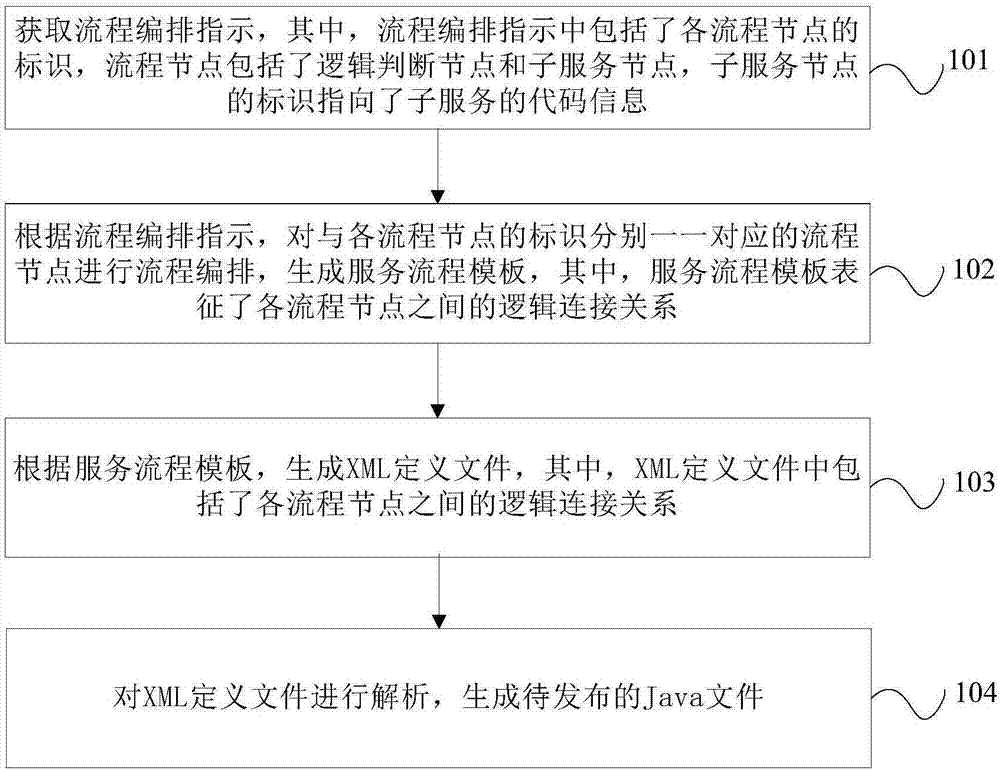
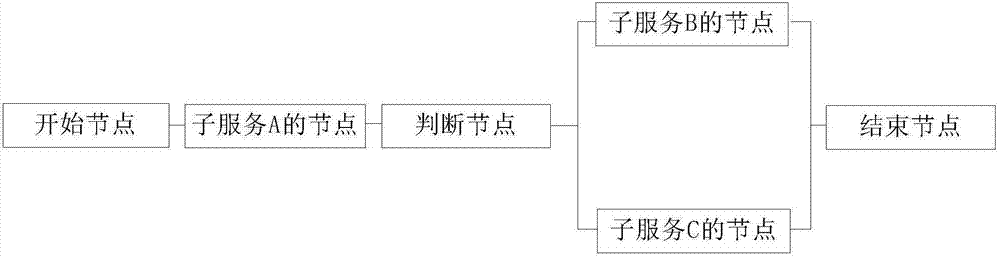
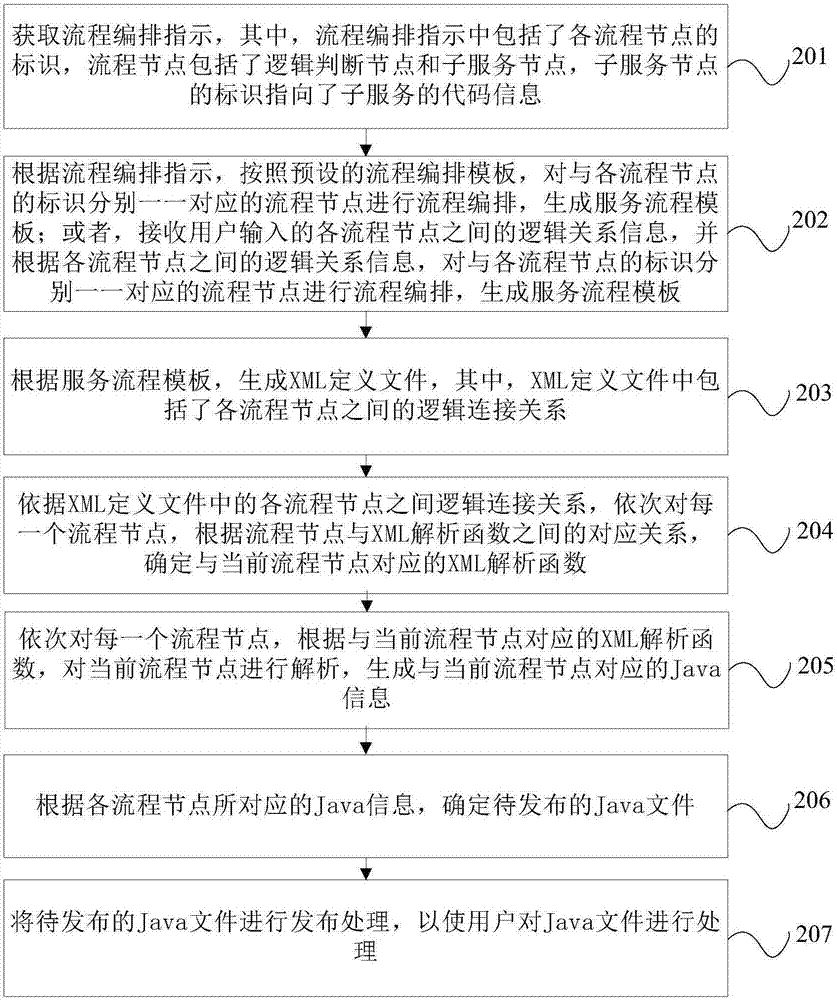
InactiveCN107133049AReduce chance of errorImprove efficiencySoftware designVisual/graphical programmingService processExtensible markup
The invention provides a communication-based service processing method and device. The method comprises the following steps: obtaining a process arrangement indication, wherein the process arrangement indication comprises an identification of each process node, and each process node comprises a logic judgement node and a subservice node; performing process arrangement on the process nodes corresponding to the identifications of the process nodes one to one, and generating a service process template, wherein the service process template expresses a logic connection relation among all the process nodes; generating an XML (eXtensible Markup Language) definition file, wherein the XML definition file comprises the logic connection relation among all the process nodes; analyzing the XML definition file, and generating a Java file to be published. An arrangement bug is difficult to cause, and the error possibly after combined service is put on line is reduced; the file obtained by arrangement does not need to be subjected to lots of function tests; therefore, the efficiency is improved, and the version publishing time is speeded up.
Owner:CHINA UNITED NETWORK COMM GRP CO LTD
Extended production of nitric oxide from a microencapsulated nitrite salt and an aqueous acidified gel
Methods and compositions are provided for generating and applying long-lasting therapeutic nitric oxide (NO) gas from the reaction of a least one microencapsulated nitrite salt and an activating volume of an aqueous acidified gel that has sufficient acidity to convert the nitrite salt to a nitric oxide (NO) and further provides a reducing property that retains the NO in bioactive form
Owner:NIOXX
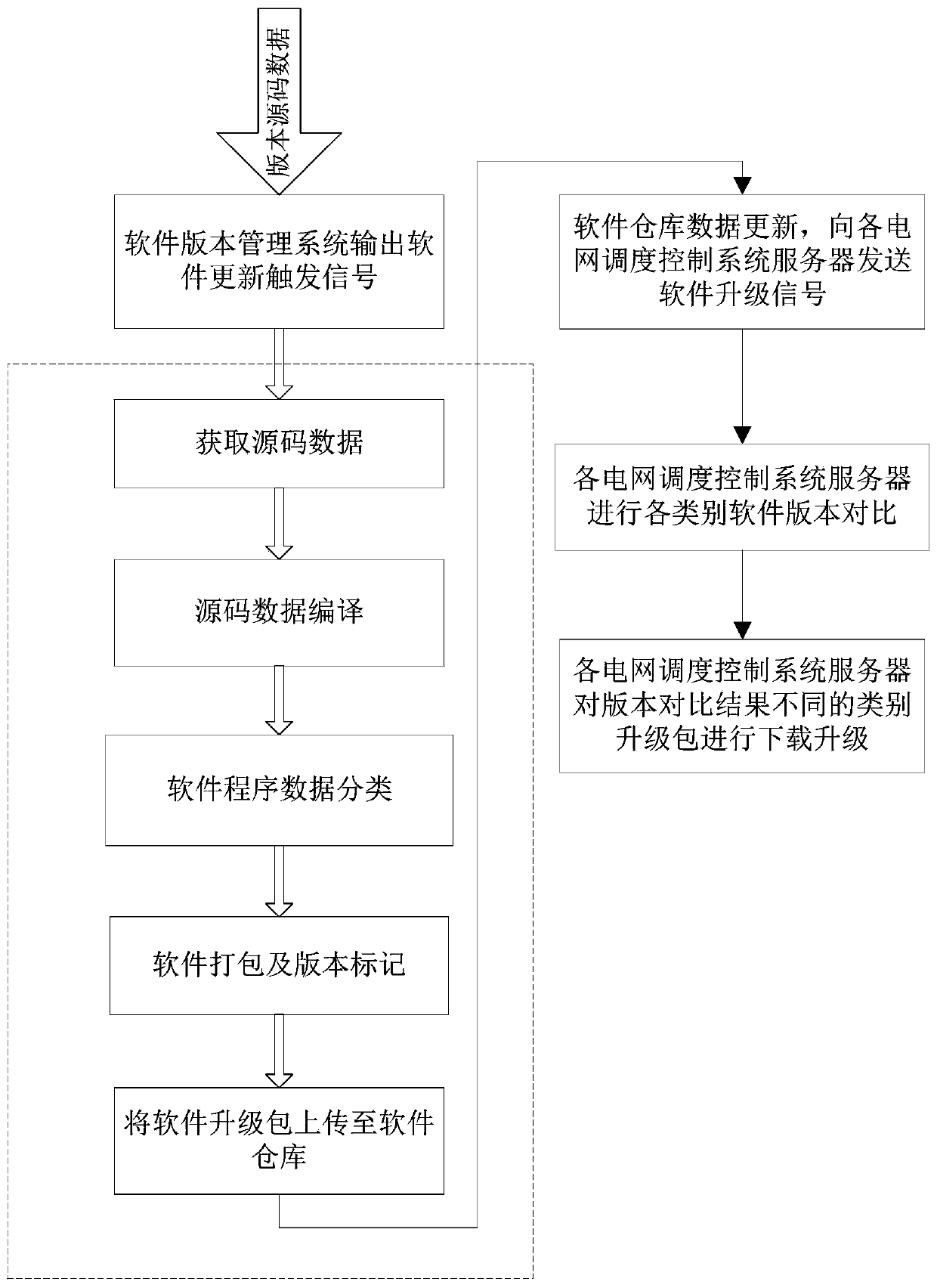
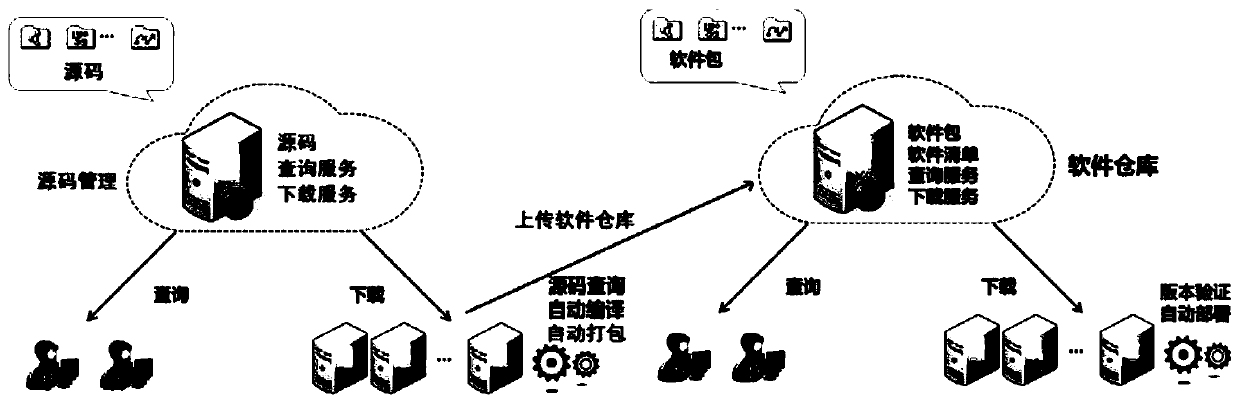
Software operation and maintenance method, device and system of power grid dispatching control system
The invention discloses a software operation and maintenance method, a device and a system of a power grid dispatching control system. The method comprises the following steps: responding to an external software updating triggering signal, and obtaining the latest version source code data in a software version management system; compiling, classifying, packaging and version marking are conducted on the received source code data, and a software upgrading package is obtained; uploading the software upgrade package to a software warehouse of a software version management system; the software version management system responds to software warehouse updating and sends a software upgrading signal to each power grid dispatching control system server; and each power grid dispatching control systemserver performs version comparison on the local multi-class software and the latest version software upgrade package of the corresponding class in the software warehouse, and downloads the corresponding software upgrade package and then performs local software upgrade when the comparison result is different. Intelligent upgrading of power grid dispatching control system software can be achieved,the software publishing efficiency is improved, and the software publishing period is shortened.
Owner:NARI TECH CO LTD +5
Vehicle controller and control method
InactiveUS8010270B2Improve featuresReduce unnecessary energy consumptionSpeed controllerAnalogue computers for trafficControl theory
An ECU releases brake hold control when an actual accelerator pedal position A exceeds a predetermined position A(0) while the brake hold control is being executed. Further, the ECU determines whether or not the actual accelerator pedal position A is larger than a position A(1), which is a value smaller than the predetermined position A(0), and if it is larger than the predetermined position A(1), executes a process for increasing a creep torque reflection ratio R to recover creep force that has been stopped.
Owner:TOYOTA JIDOSHA KK
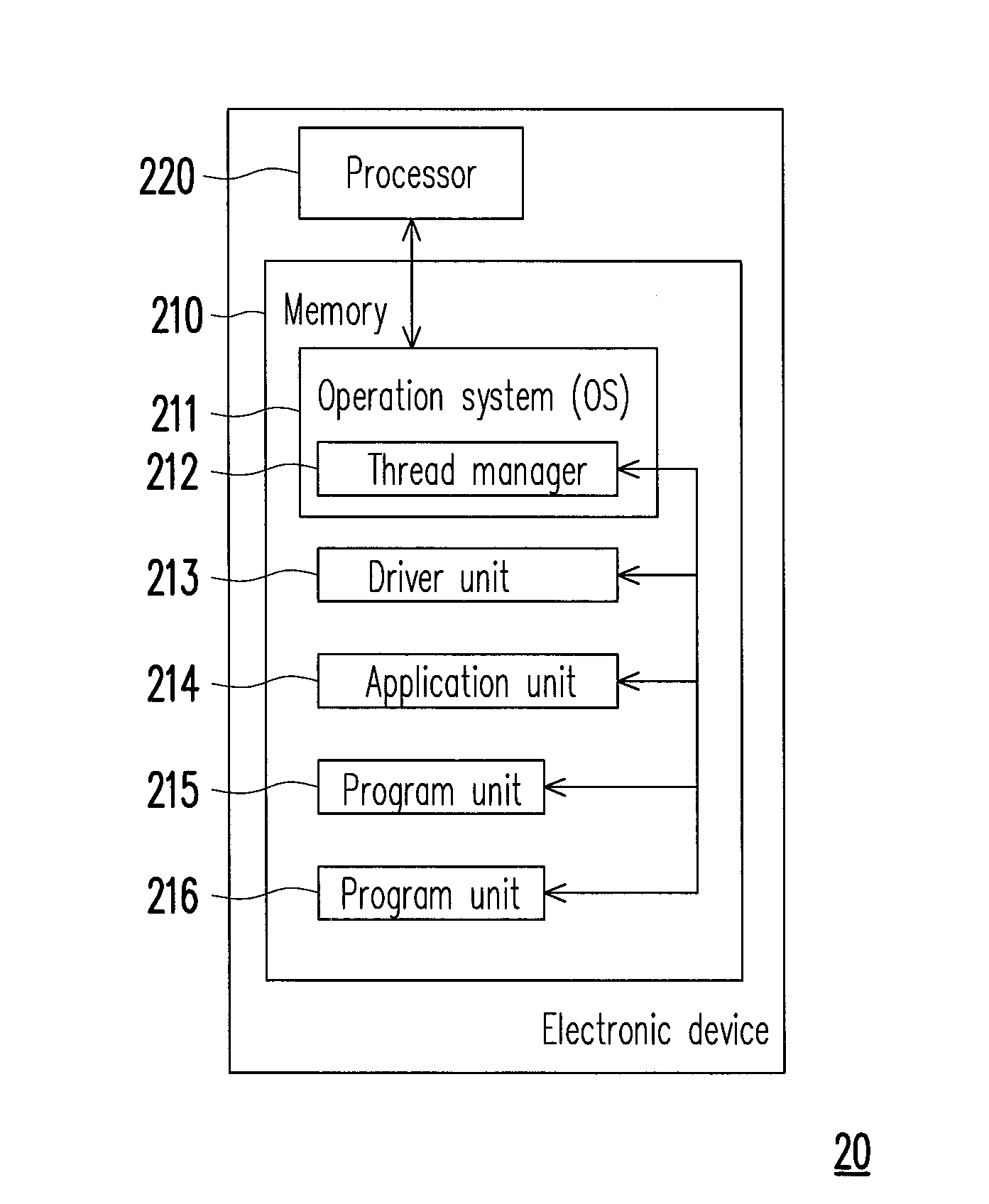
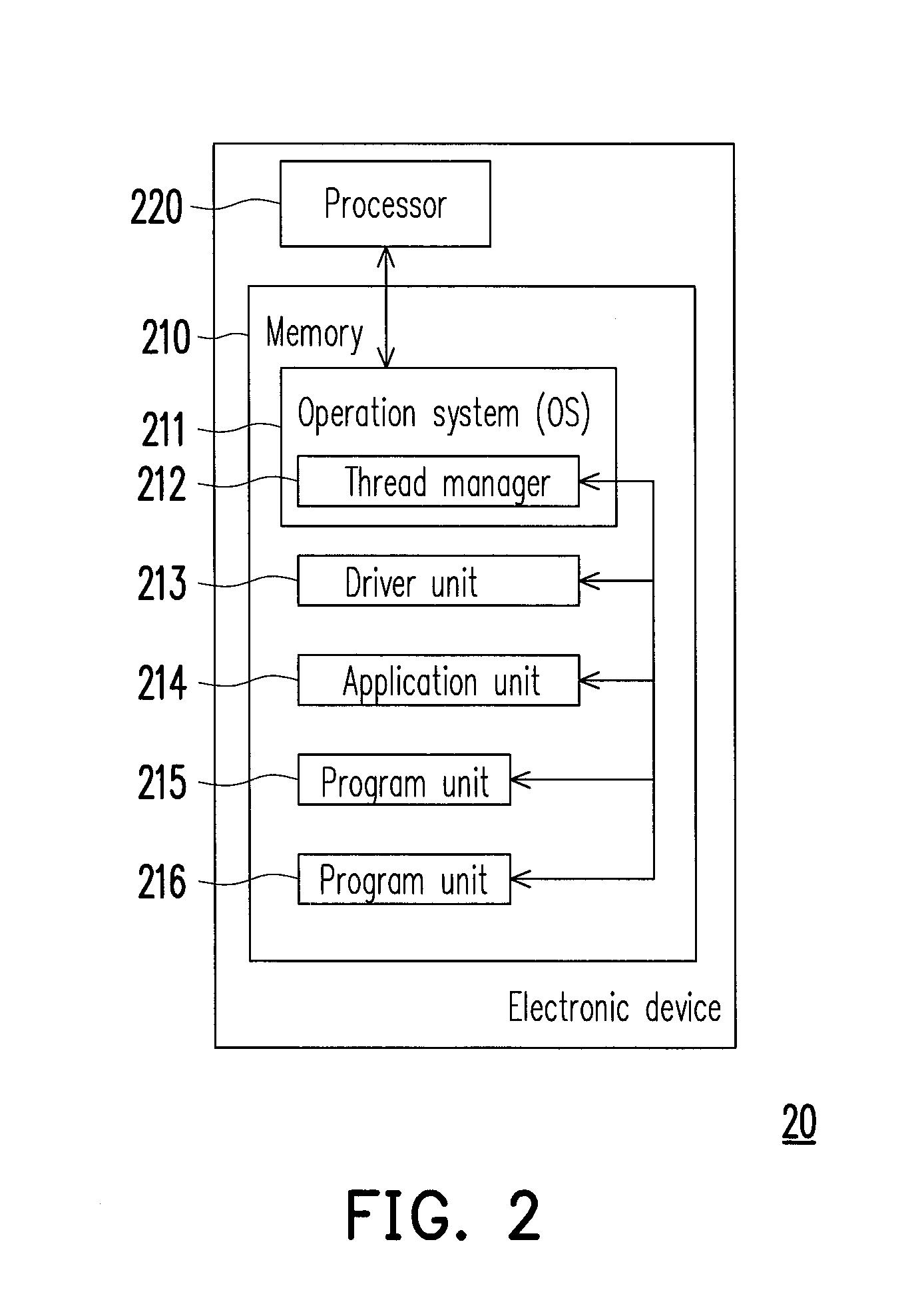
Method for managing threads and electronic device using the same method
ActiveUS20140149988A1Lower processor frequencyReduce unnecessary power consumptionMultiprogramming arrangementsEnergy efficient computingCurrent timeReal-time computing
A method for managing threads and an electronic device using the method are provided. In the method, a current time is obtained. A time interval from now to a time for the processor to wake up next time is calculated. The processor is released until reaching the end of the time interval. When the end of the time interval is reached or a first notice signal of the processor is received, a first newest time is obtained to update a current time, and the current time is logged as a basis time. It is respectively checked whether the current time satisfies a plurality of predetermined time conditions of the registered threads against a plurality of registered threads in the threads. When the current time satisfies the predetermined time condition of a first registered thread among the registered threads, the first registered thread is waked up.
Owner:HTC CORP
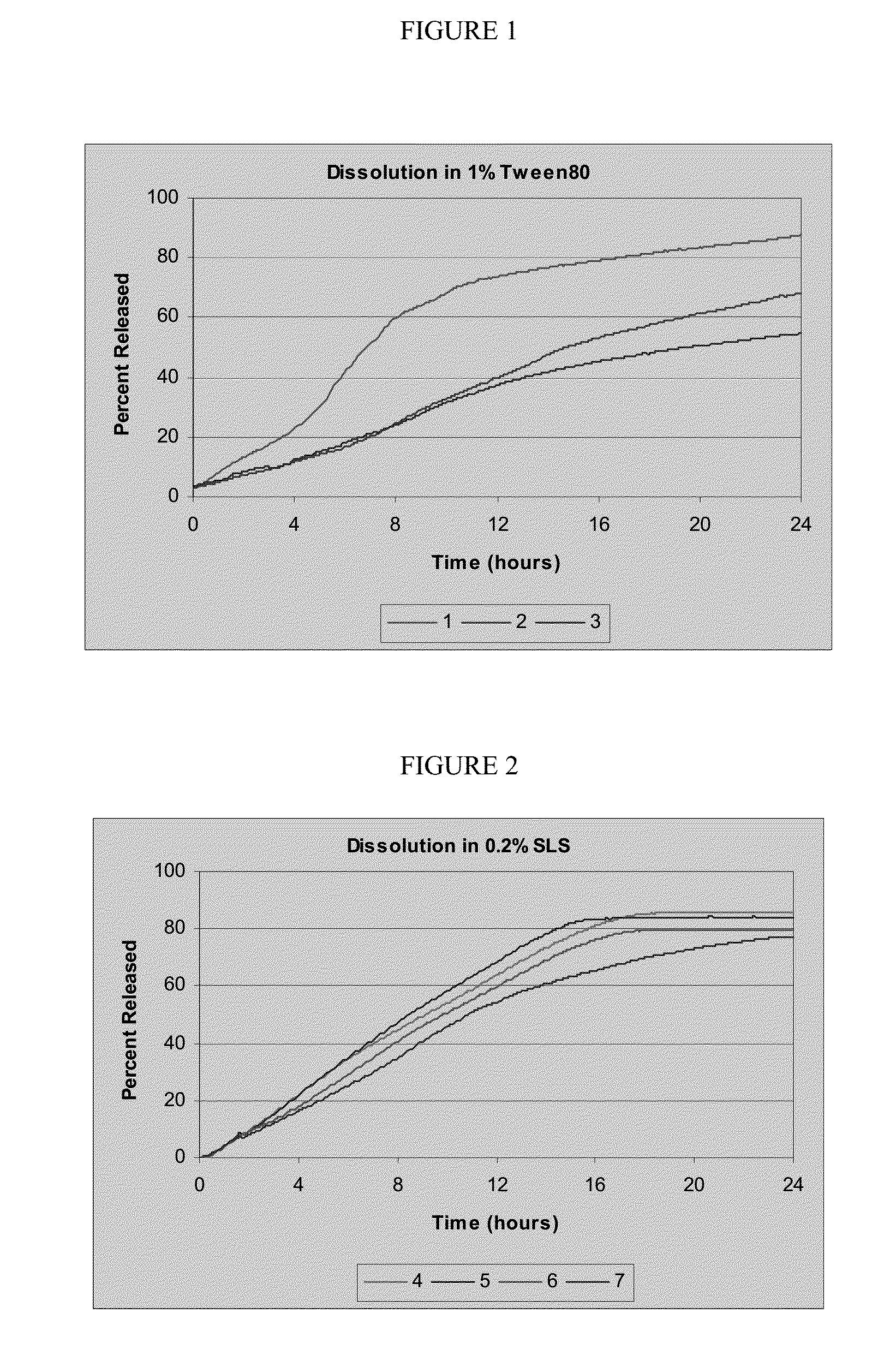
Controlled release oral dosage forms of poorly soluble drugs and uses thereof
ActiveUS20160128981A1Improve bioavailabilityExtend posting timeBiocidePill deliveryDiseaseControl release
Provided herein are controlled release oral dosage forms of poorly soluble drugs, methods of making the dosage forms, and methods of their use for the treatment of various diseases and / or disorders.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com