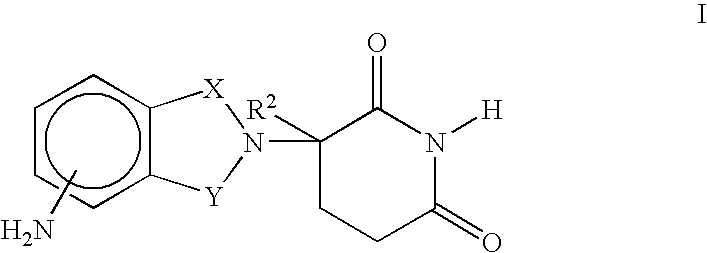
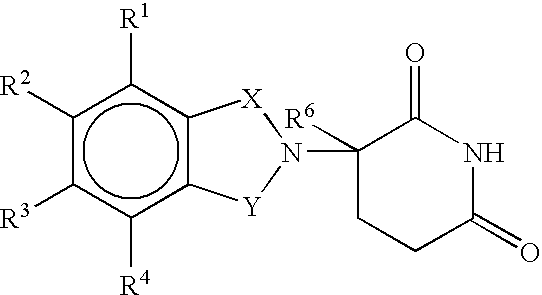
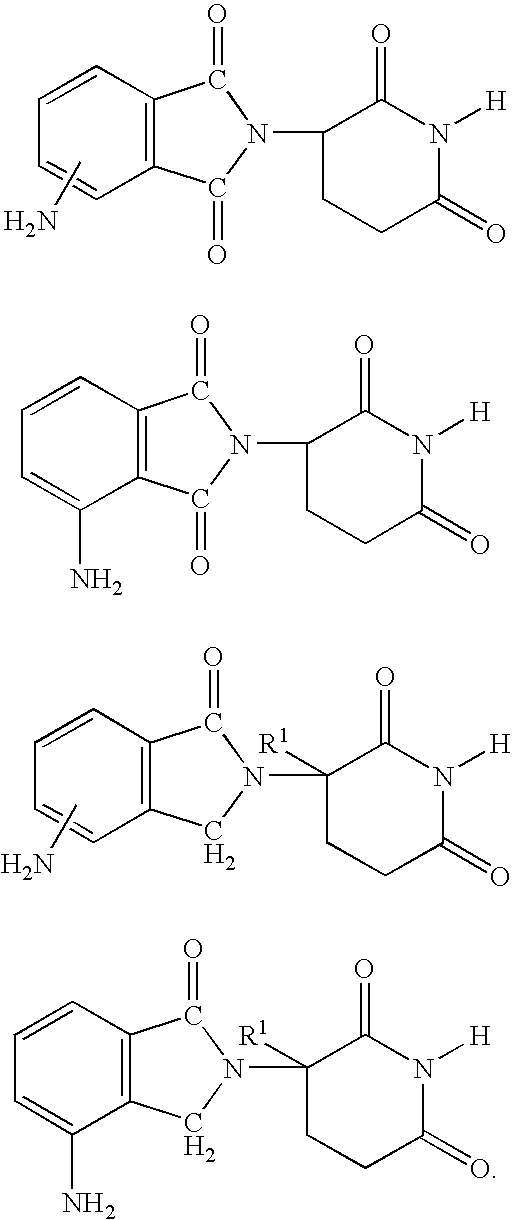
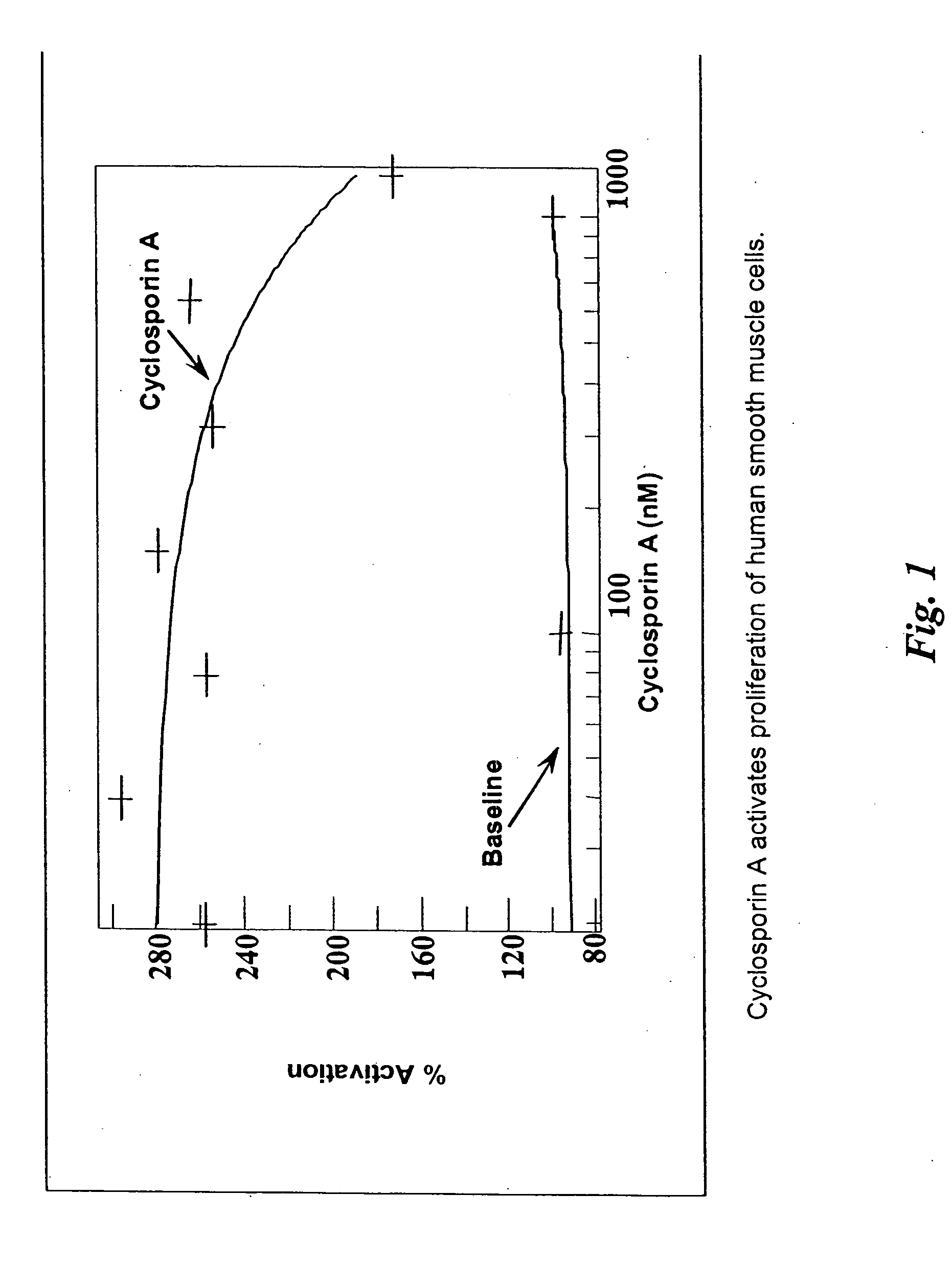
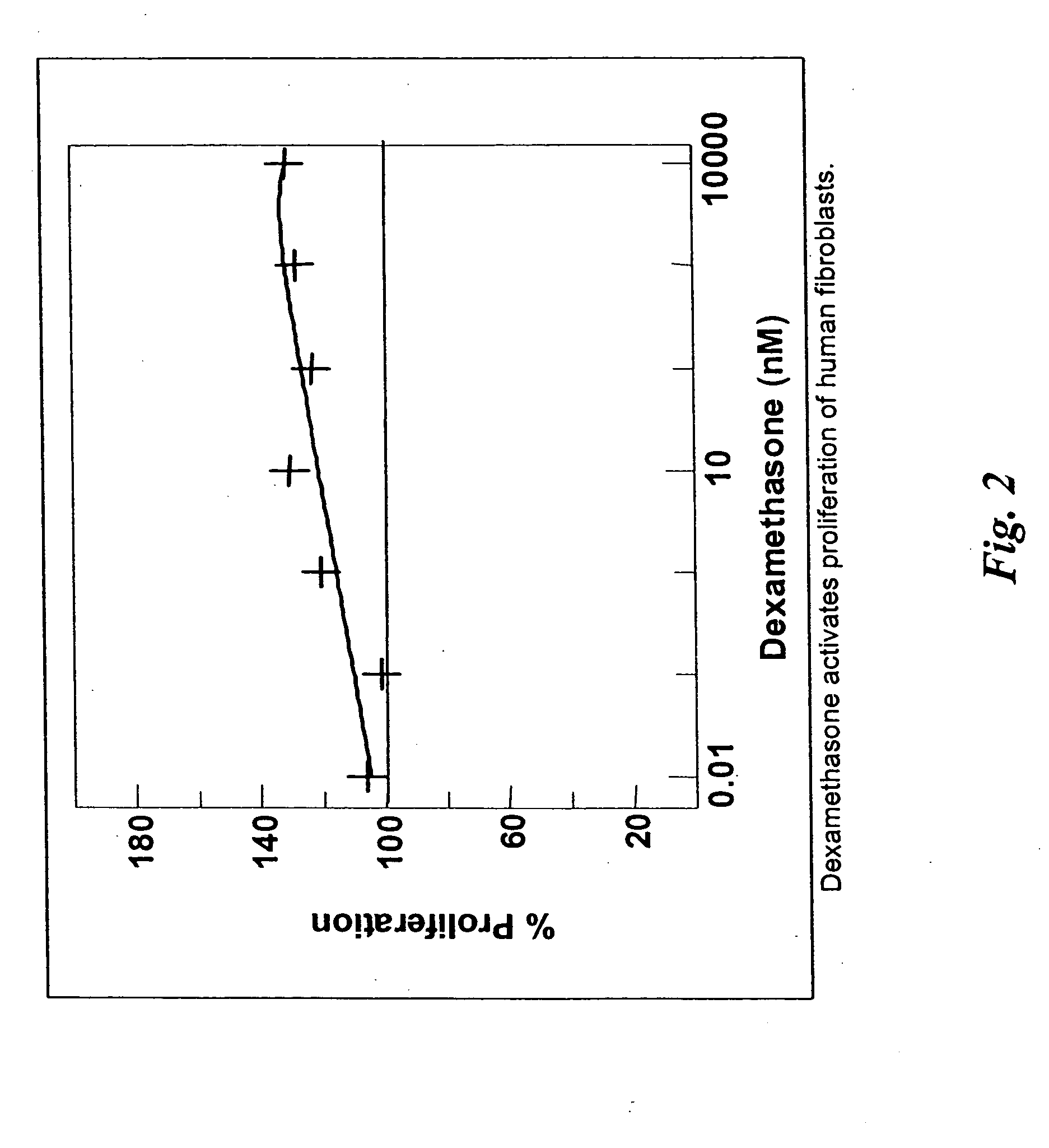
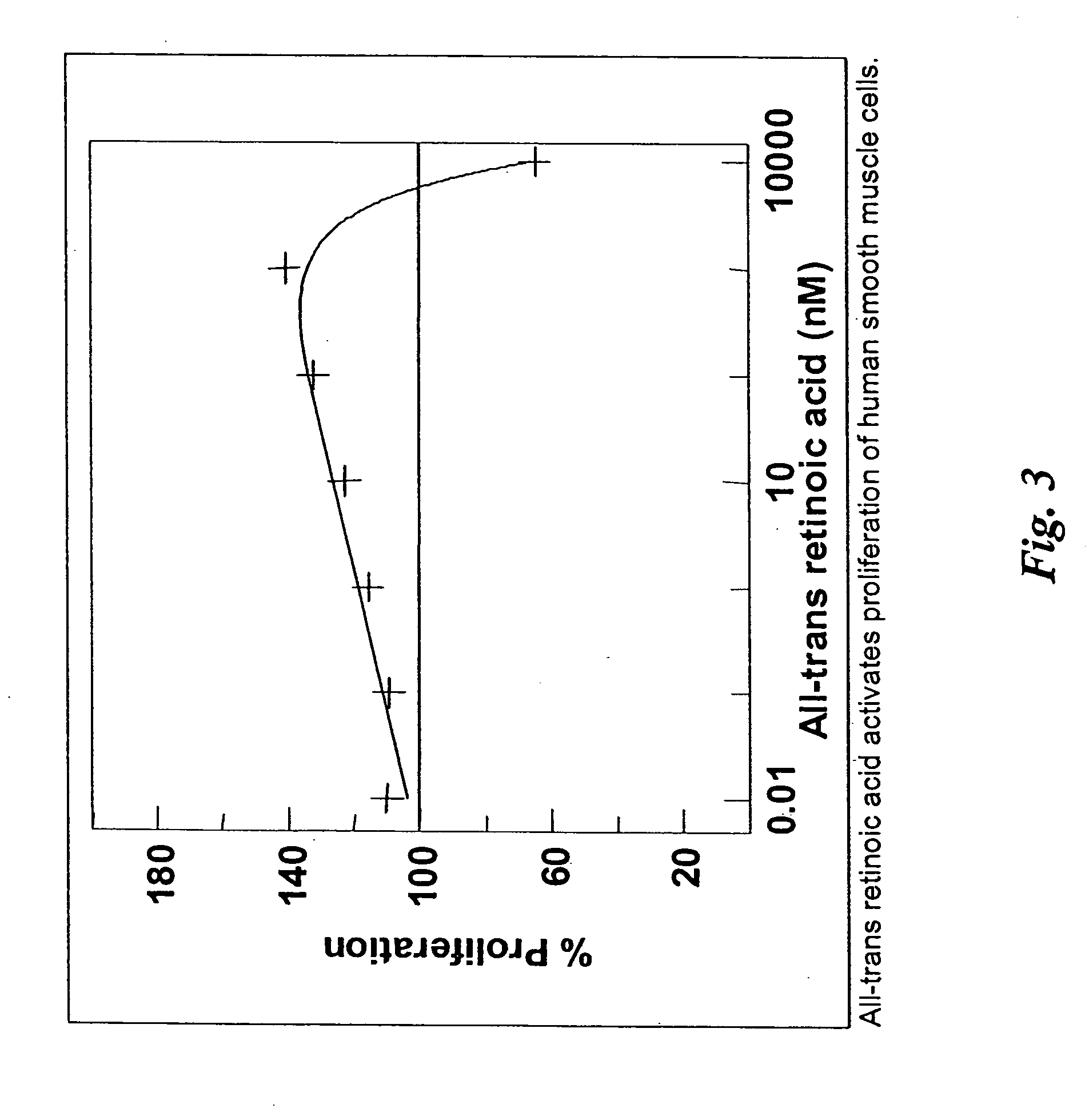
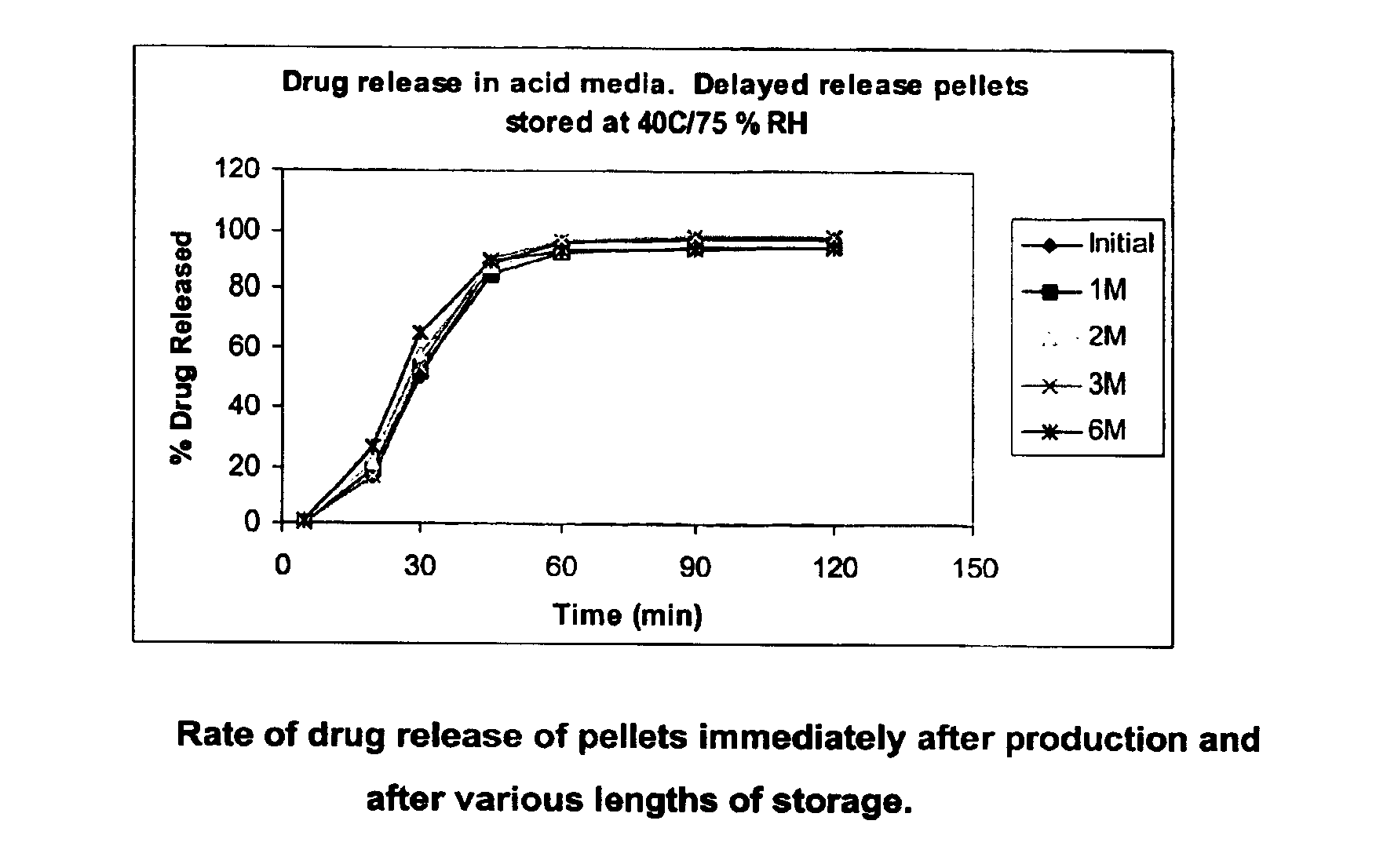
Patents
Literature
2091results about "Tetracycline active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
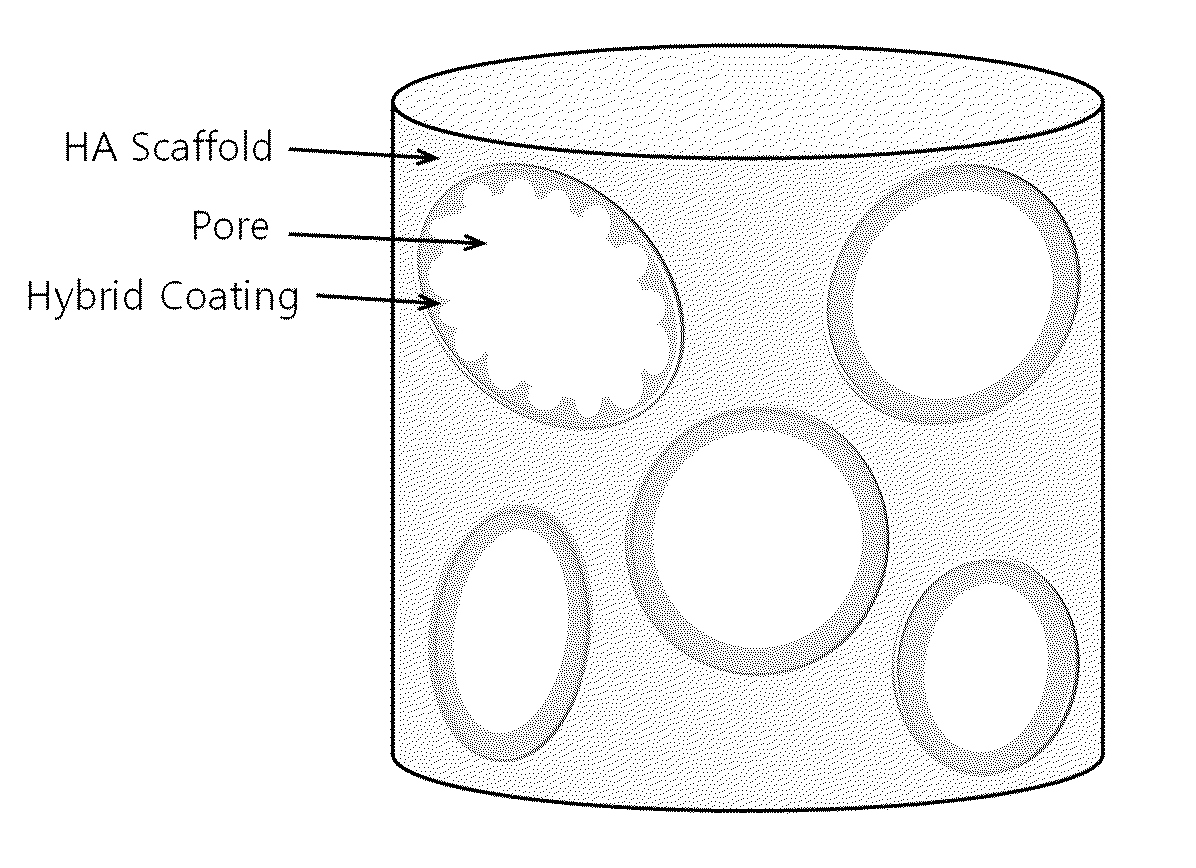
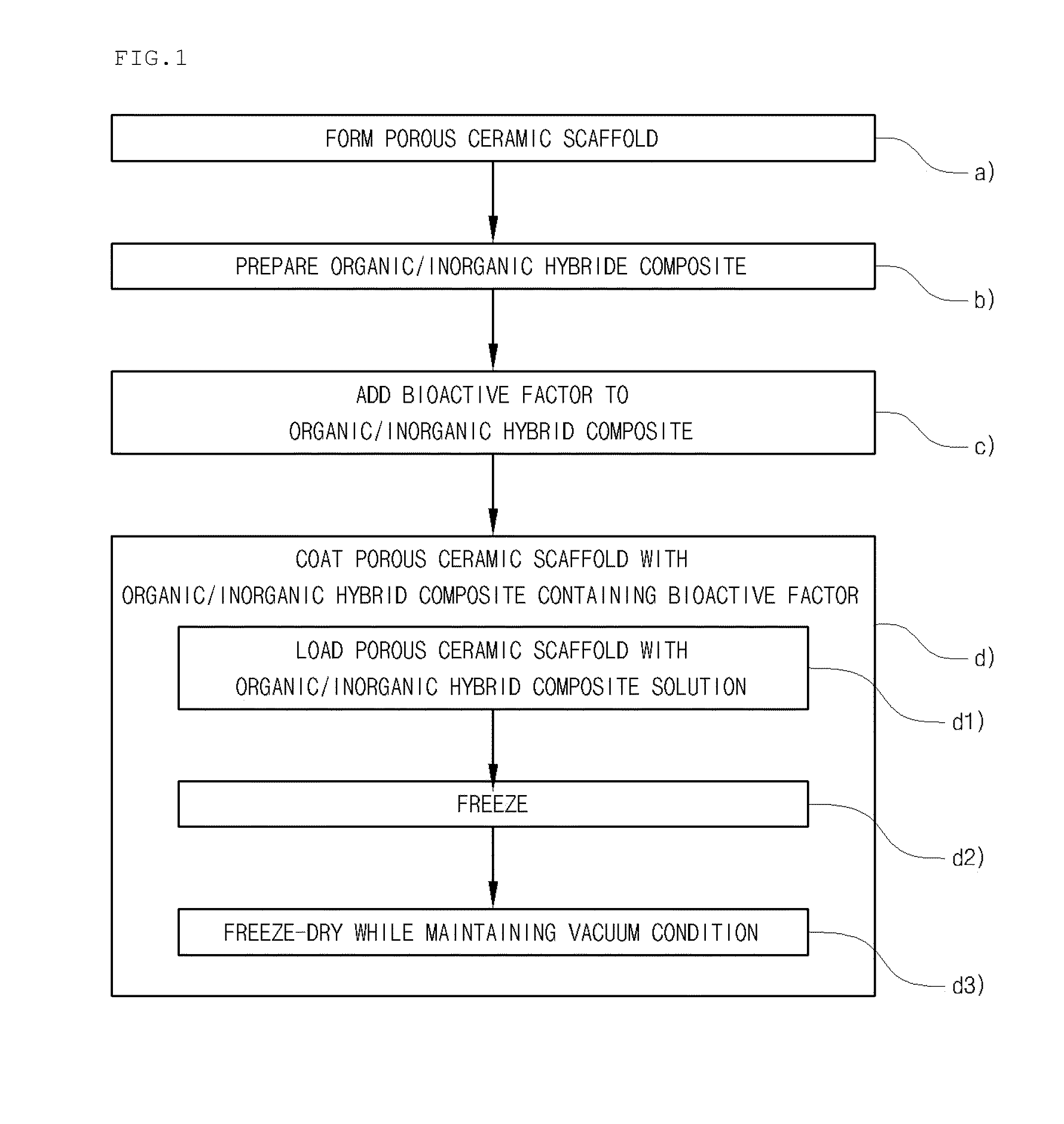
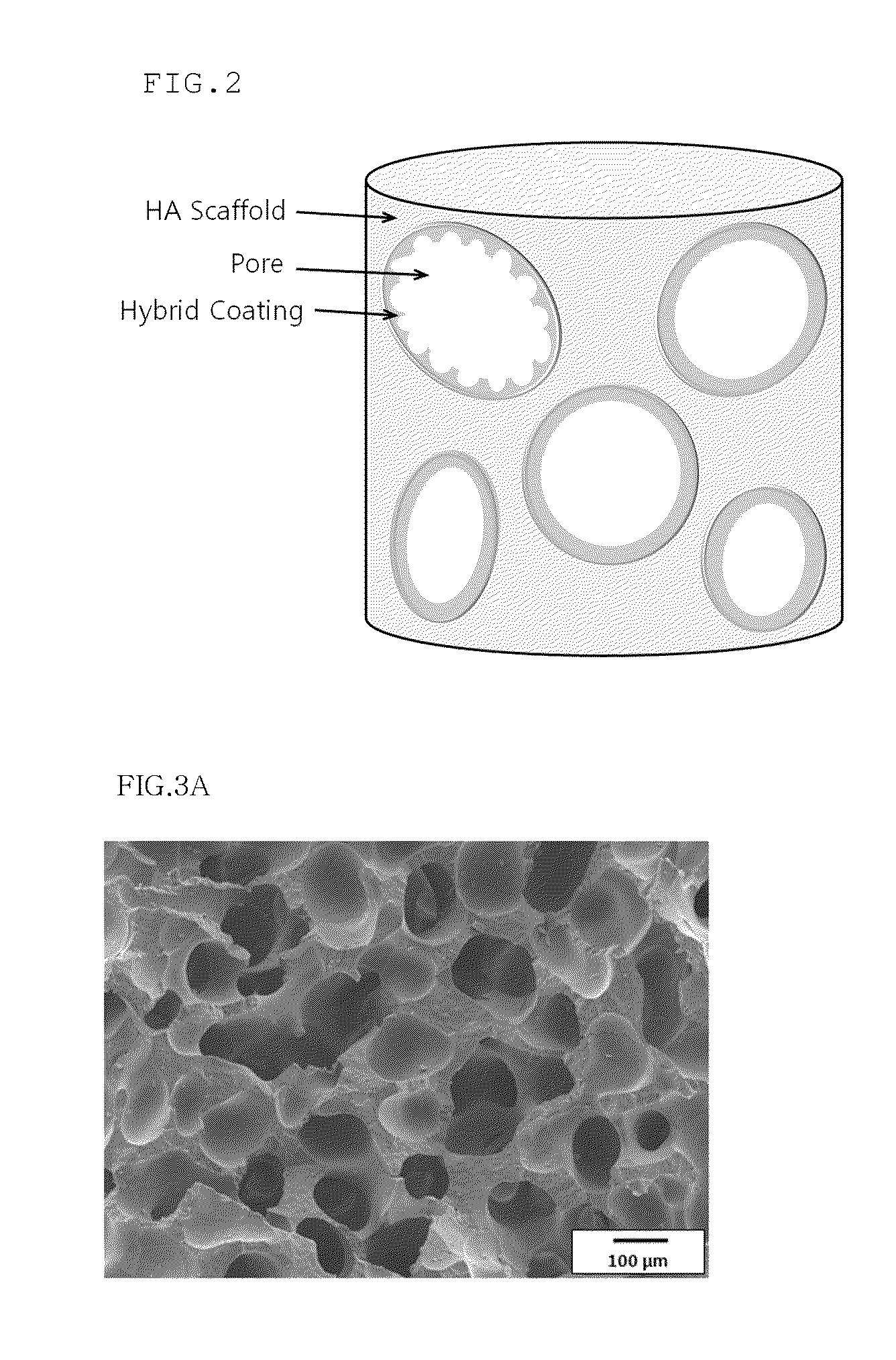
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
Glucocorticoid blocking agents for increasing blood-brain barrier permeability stan-261con
InactiveUS20050124533A1Improve breathabilityIncrease volumeAntibacterial agentsBiocideBlood brain barrier permeabilityDisease cause
Glucocorticoid blockers, including glucocorticoid receptor antagonists, are effective to prevent glucocorticoid-induced decrease in permeability of the blood-brain barrier and to increase the permeability of the blood-brain barrier. Administration of glucocorticoid blockers, including glucocorticoid receptor antagonists, concomitant with administration of drugs for treating diseases of the central nervous system increases delivery of such drugs into the central nervous system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Antibiotic treatment of age-related macular degeneration
InactiveUS6015803AImprove visual acuityBiocideTetracycline active ingredientsAntibiotic YMacrolide resistance
A method is provided for the treatment of age-related macular degeneration by administering various antibiotics, such as tetracycline and its derivatives, rifamycin and its derivatives, macrolides, and metronidazole, to a patient in a therapeutically effective amount.
Owner:WIROSTKO BARBARA
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders
Methods of treating, preventing and managing an asbestos-related disease or disorder are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or chemotherapy, surgery, or radiation therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Hydrogels used to deliver medicaments to the eye for the treatment of posterior segment diseases
This invention provides a polymeric drug delivery system including a hydrogel containing one or more drugs for the treatment of a posterior segment disease. Exemplary drugs are anti-angiogenesis compounds for the treatment of macular degeneration. Allowing passive transference of this drug from a dilute solution into the hydrogel produces the delivery system. The hydrogel, when placed in contact with the eye, delivers the drug. The delivery of the drug is sustained over an extended period of time, which is of particular utility in the eye, which is periodically flushed with tears. This sustained delivery accelerates the treatment process while avoiding potential damaging effects of localized delivery of high concentrations of compounds, e.g., from eye drops.
Owner:DIRECTCONTACT
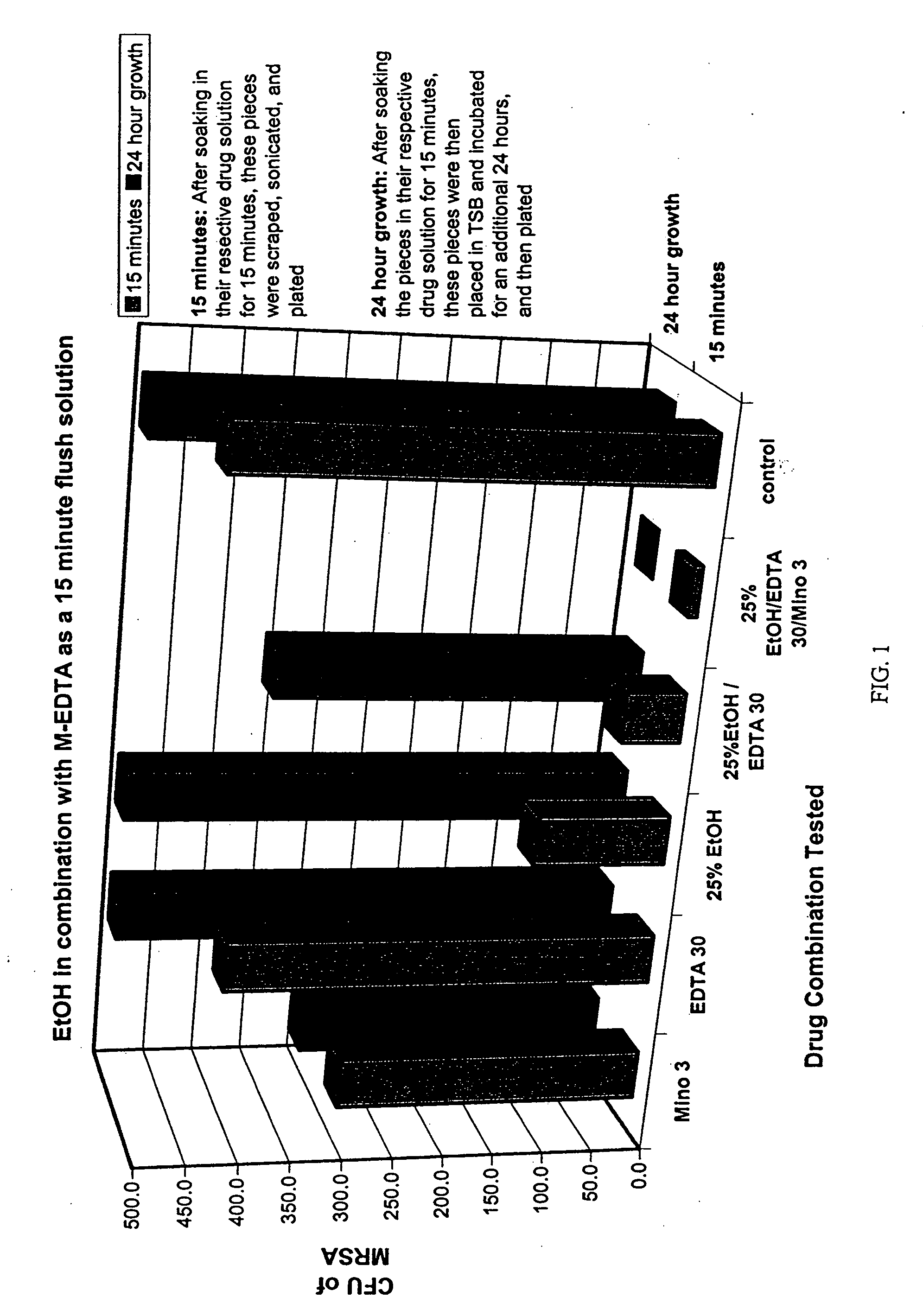
Antimicrobial flush solutions
The present invention provides antimicrobial solutions that comprise at least one alcohol, at least one antimicrobial agent and at least one chelator and / or anticoagulant. Also provided are methods for rapidly reducing a microbe or a virus from surfaces including surfaces of indwelling medical devices and organic surfaces such as skin and sutures, and inorganic surfaces such as hospital equipment, pipelines etc.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Ionic silicone hydrogels
Owner:JOHNSON & JOHNSON VISION CARE INC
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
Sprayable formulations for the treatment of acute inflammatory skin conditions
A topical spray or foam, methods of making the formulation, and methods of use thereof, has been developed. In one preferred embodiment, the composition includes one or more active agents and exhibits both antibacterial activity and antifungal activity. Excipients such as chemical disinfectants, anti-pruritic agents to minimize itching, and skin protective compounds may be added. The composition may be formulated to be dispensed as a spray or foam and the spray or foam may be administered either by a hand pump or by an aerosolizing propellant. A second single phase formulation has also been developed. The formulation comprises a first drug which is water soluble or hydrophilic and a second drug which is lipid soluble or hydrophobic, wherein at least one of the drugs is bound to an ion-exchange resin. The use of binding resins, such as ion-exchange resins, allows drugs with incompatible solvent requirements to be prepared in a single-phase formulation.
Owner:COLLEGIUM PHARMA INC
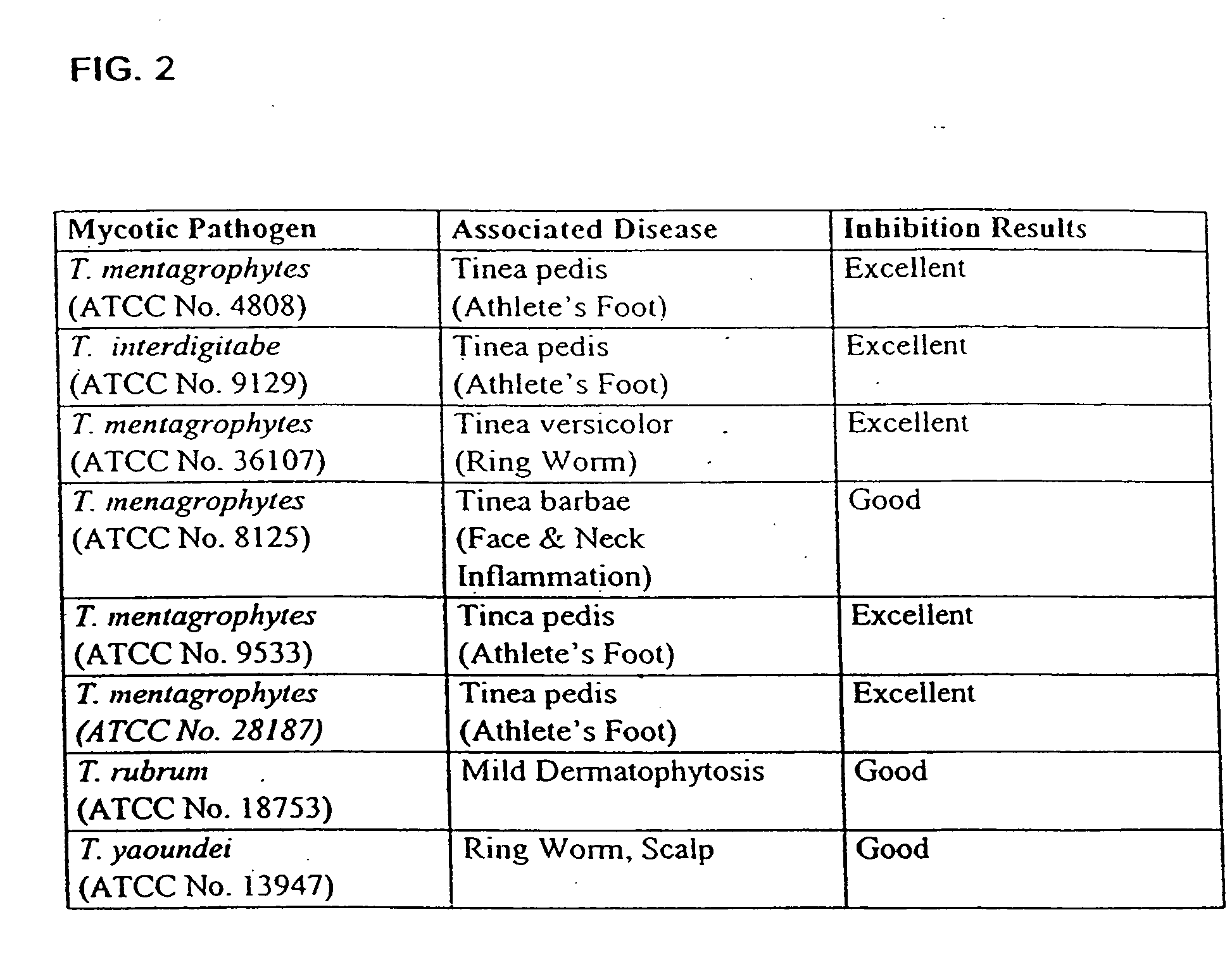
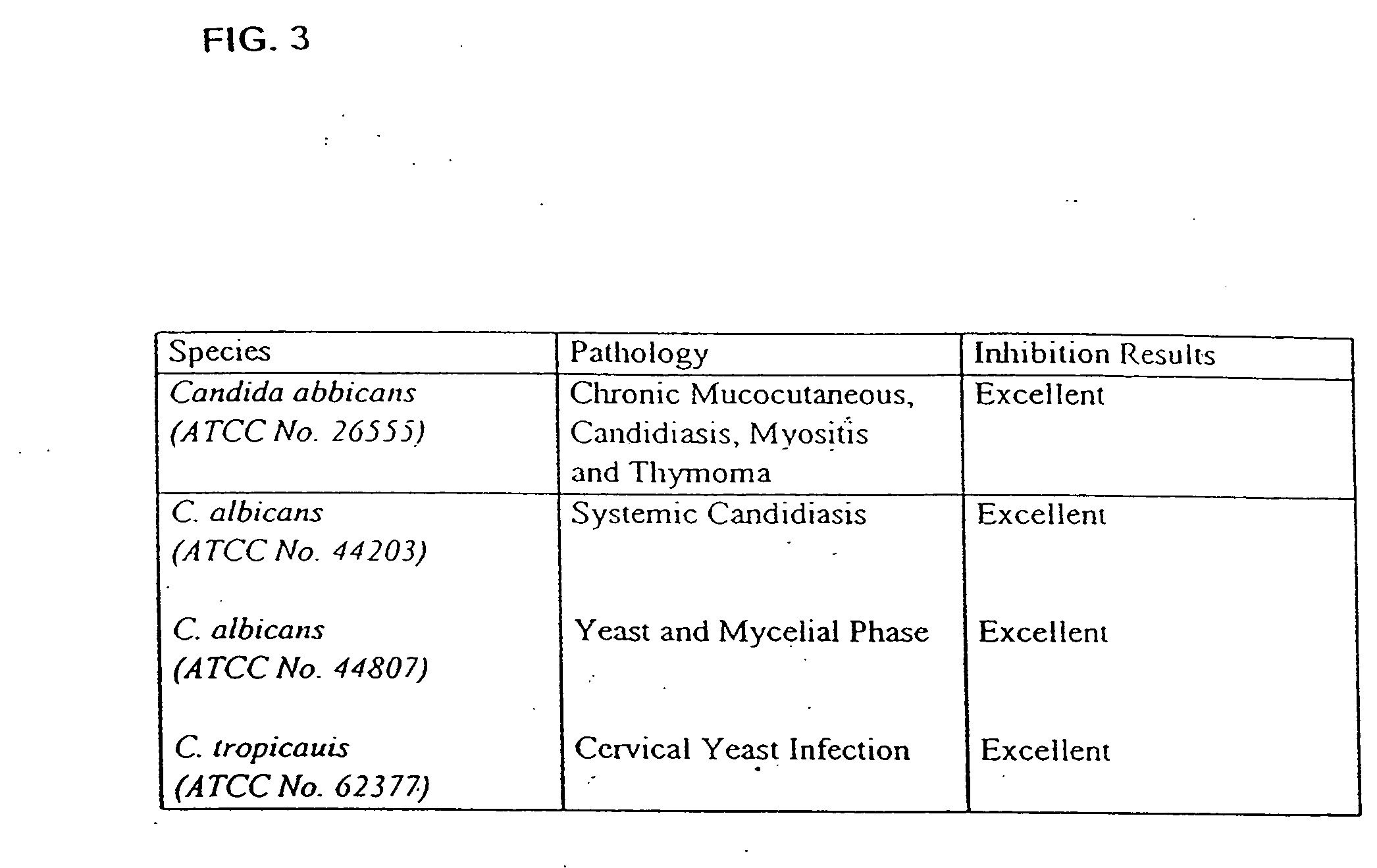
Substituted tetracycline compounds as antifungal agents
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Intravaginal drug delivery devices for the administration of an antimicrobial agent
InactiveUS6951654B2Convenient and high complianceReduce in quantityAntibacterial agentsTetracycline active ingredientsAntimicrobial drugAnti-Microbial Agents
An intravaginal antimicrobial drug delivery device is disclosed having an antimicrobial agent dispersed throughout a biocompatible elastomeric system. Also disclosed is a method of making the antimicrobial drug delivery device.
Owner:APTALIS PHARMA
Biodegradable, Polymer Coverings for Breast Implants
ActiveUS20080241212A1Inhibit and reduce formation of scar tissueInhibiting and reducingAntibacterial agentsBiocideBreast implantMedicine
A biodegradable, flexible covering for a breast implant is provided which comprises one or more biodegradable polymer layers dimensioned and shaped to cover at least a portion of the breast implant. The implant can be inserted into an opening of the covering immediately prior to surgery, but alternate configurations and times of insertion are contemplated as well as open or sheet type devices. The coverings can optionally contain one or more drugs for delivery at the surgical site, particularly for treating or preventing infection, pain, inflammation, capsular contracture, scarring or other complications associated with breast augmentation or breast reconstruction.
Owner:MEDTRONIC INC
Methods and compositions using immunomodulatory compounds for treatment and management of parasitic diseases
Methods of treating, preventing and / or managing various protozoan parasitic disease and disorders are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone, or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with conventional anti-parasitic treatments which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:HENSEL JENNIFER L
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
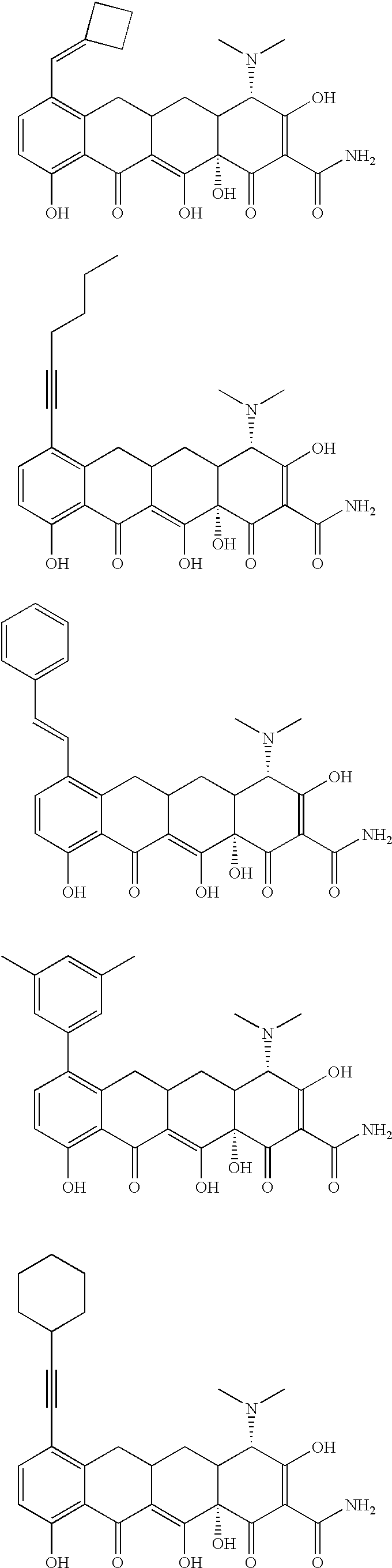
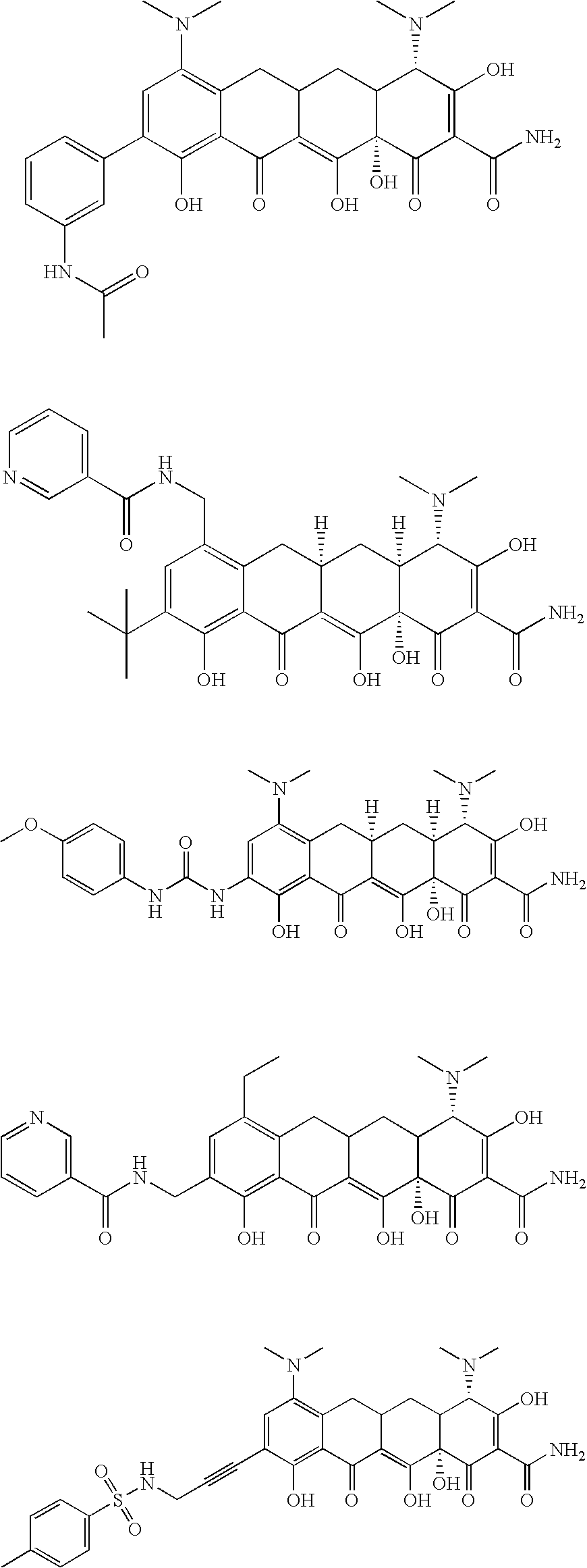
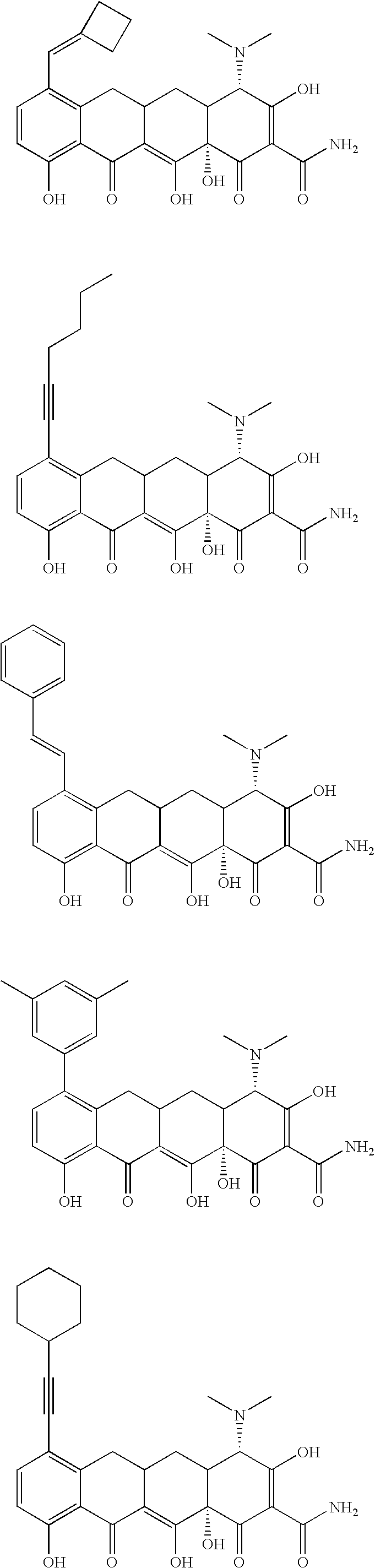
Tetracycline compositions for topical administration
Pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
Substituted tetracycline compounds as synergistic antifungal agents
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Tetracycline compositions for topical administration
Multi-part pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
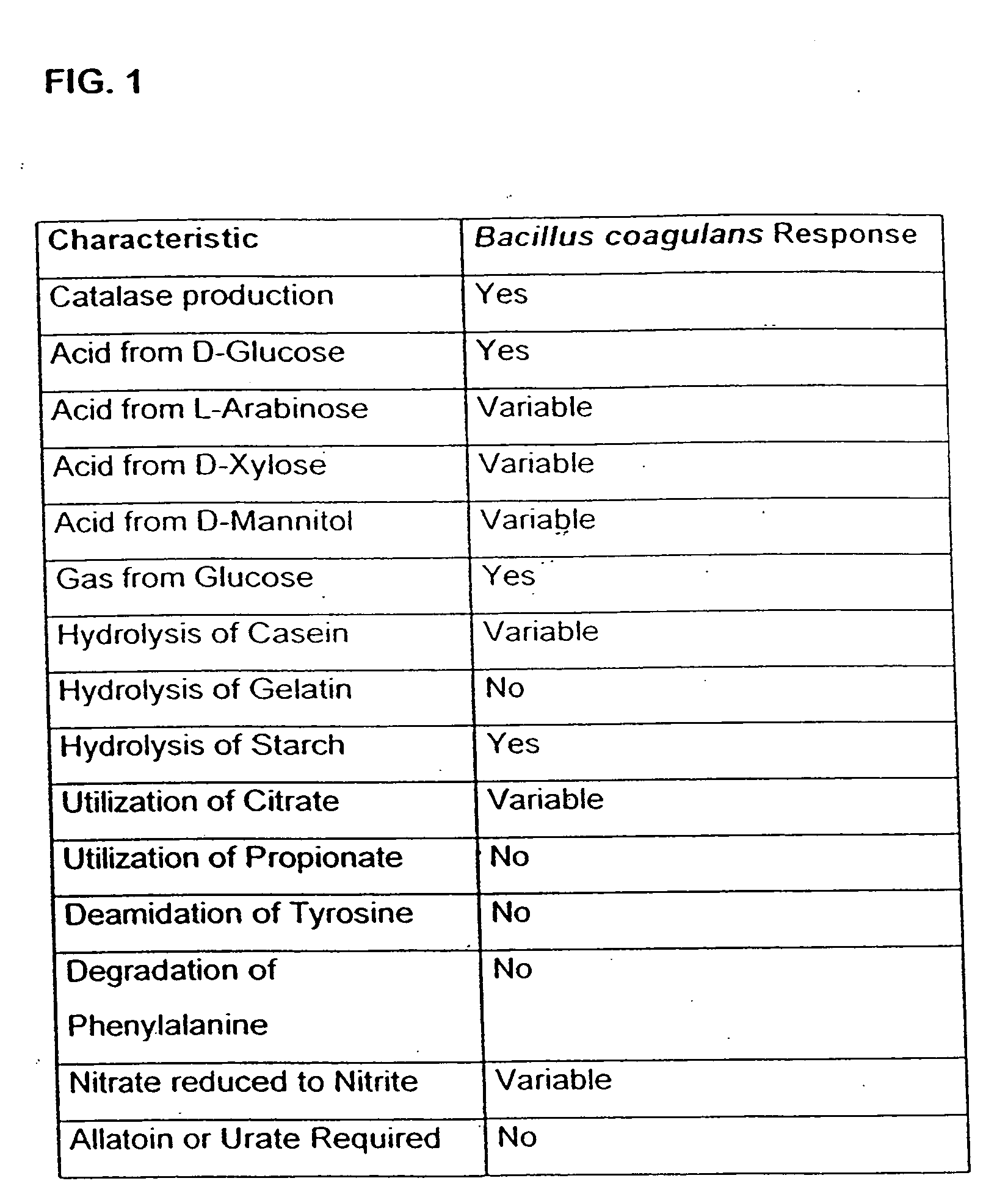
Probiotic, lactic acid-producing bacteria and uses thereof
InactiveUS20060099197A1Good curative effectMitigating deleterious side-effectsAntibacterial agentsBiocideMicrobial agentAnti fungal
The present invention discloses compositions and methodologies for the utilization of probiotic organisms in therapeutic compositions. More specifically, the present invention relates to the utilization of one or more species or strains of lactic acid-producing bacteria, preferably strains of Bacillus coagulans, for the control of gastrointestinal tract pathogens, including antibiotic-resistant gastrointestinal tract pathogens, and their associated diseases by both a reduction in the rate of colonization and the severity of the deleterious physiological effects of the colonization of the antibiotic-resistant pathogen. In addition, the present invention relates to the utilization of therapeutic compounds comprised of lactic acid—producing bacteria and anti-microbial agents such as antibiotics, anti-fungal compounds, anti-yeast compounds, or anti-viral compounds. The present invention also discloses methodologies for: (i) the selective breeding and isolation of probiotic, lactic acid-producing bacterial strains which possess resistance or markedly decreased sensitivity to anti-microbial agents (e.g., antibiotics, anti-fungal agents, anti-yeast agents, and anti-viral agents); and (ii) treating or preventing bacteria-mediated infections of the gastrointestinal tract by use of the aforementioned probiotic bacterial strains with or without the concomitant administration of antibiotics. While the primary focus is on the treatment of gastrointestinal tract infections, the therapeutic compositions of the present invention may also be administered to buccal, vaginal, optic, and like physiological locations.
Owner:GANEDEN BIOTECH
Substituted tetracycline compounds as synergistic antifungal agents
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Kit for flushing medical devices and method of preparation
InactiveUS6187768B1Easy and inexpensiveEasy to packBiocideTetracycline active ingredientsAntithrombotic AgentAnticoagulant
A kit and for flushing a medical device a method of preparing the kit is disclosed. The kit includes a container containing a mixed solution a unit dose of a pharmacologically effective amount of an antimicrobial agent and a second agent. The mixed solution has been mixed in a carrier solution and lyophilized. The second agent is an anticoagulant, an antithrombotic agent or a chelating agent. The kit and method are useful for maintaining the patency of indwelling medical devices such as catheters and for preventing infections caused by bacterial growth in catheters.
Owner:BECTON DICKINSON & CO
Substituted tetracycline compounds as antifungal agents
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
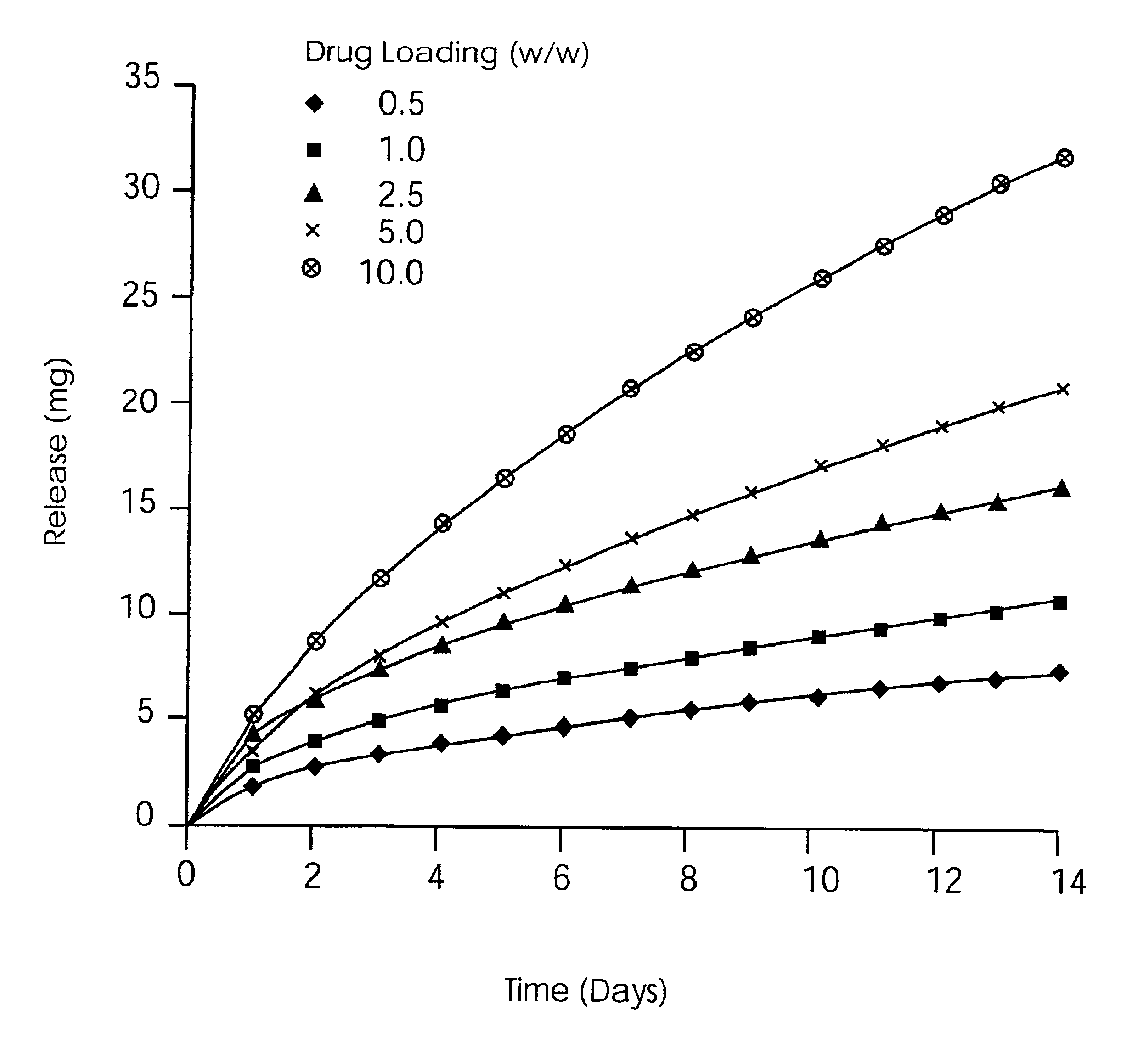
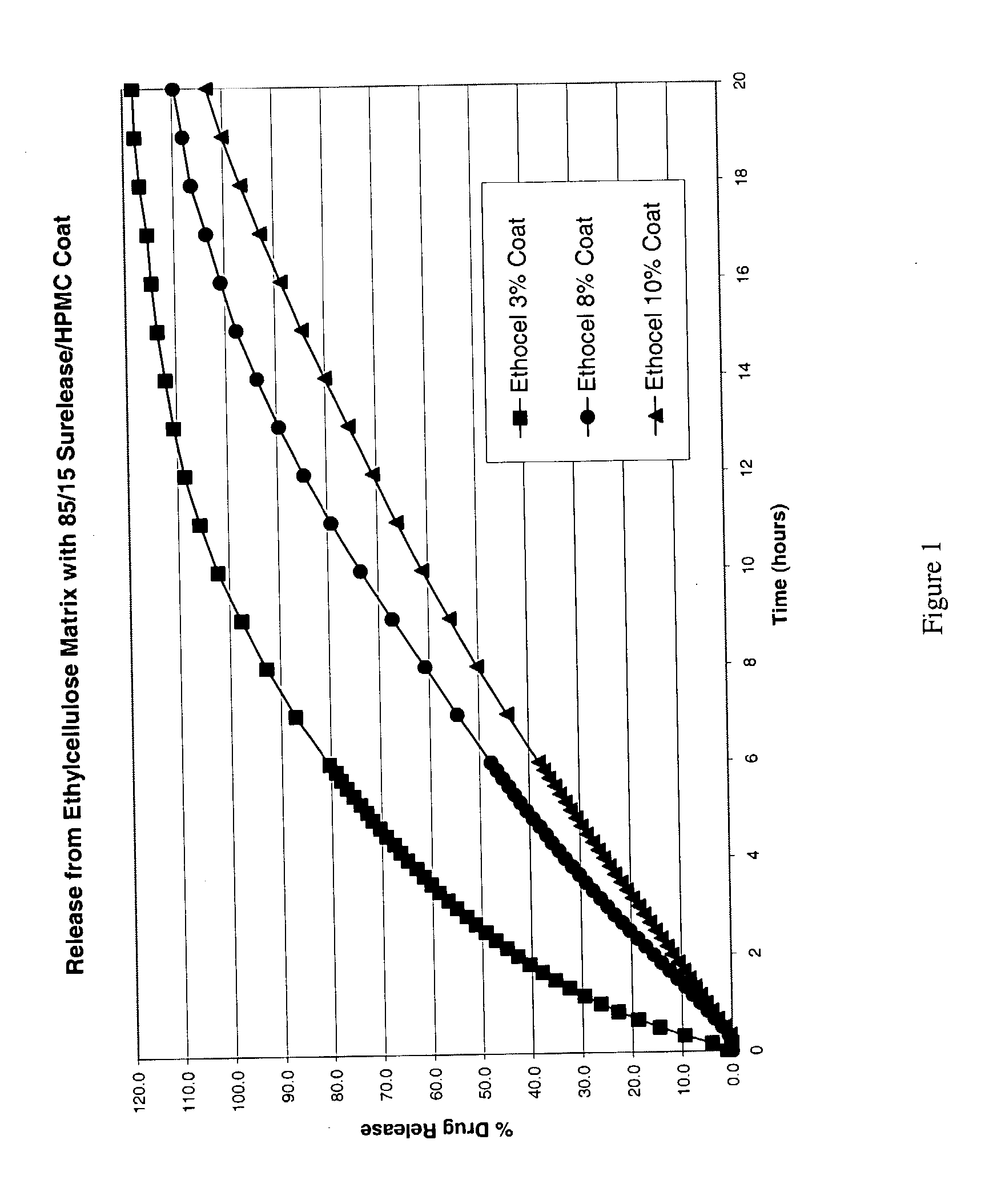
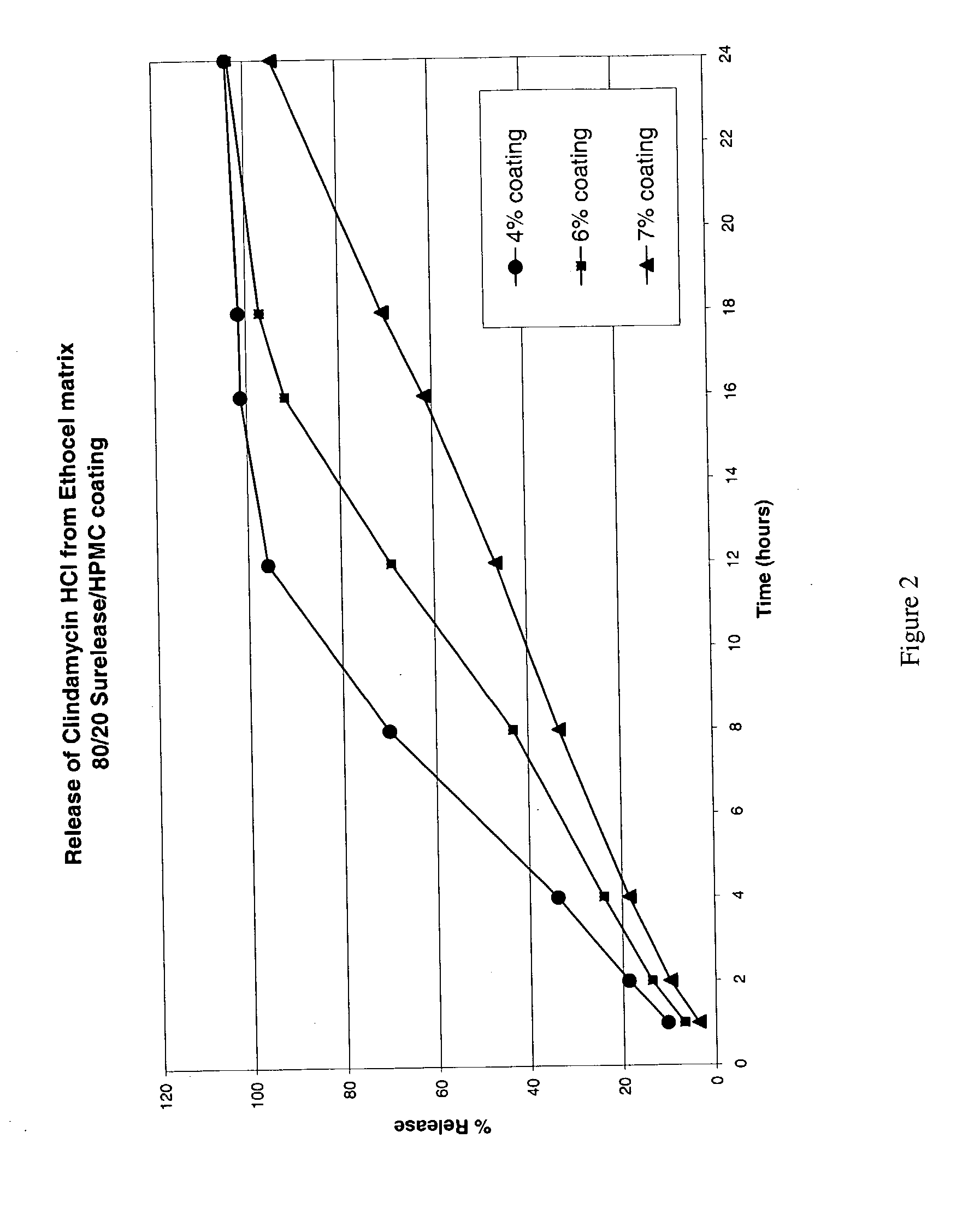
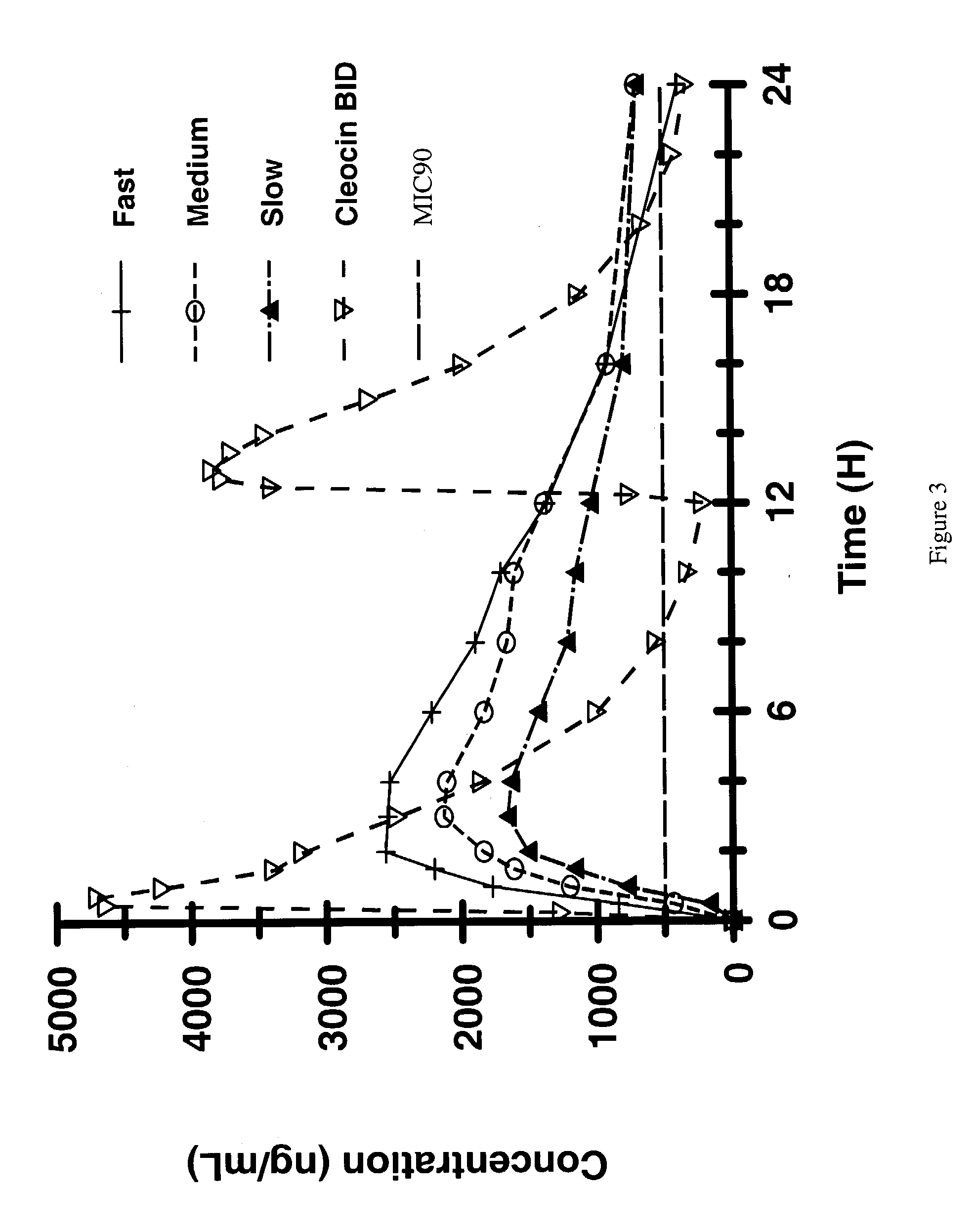
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
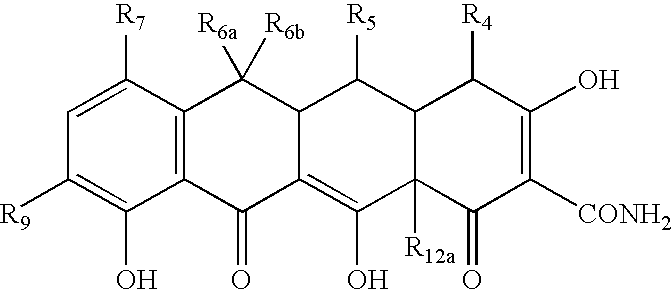
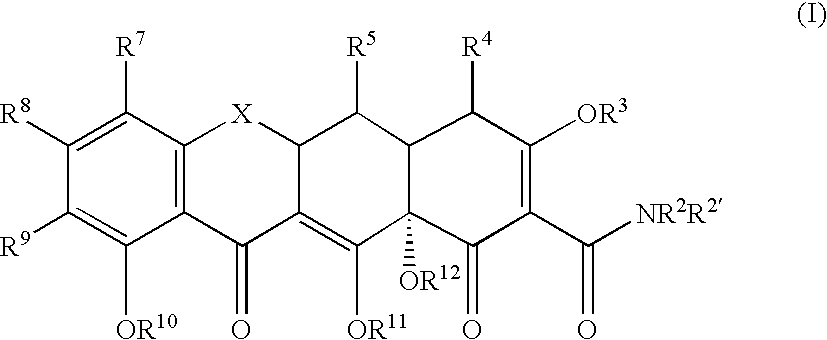
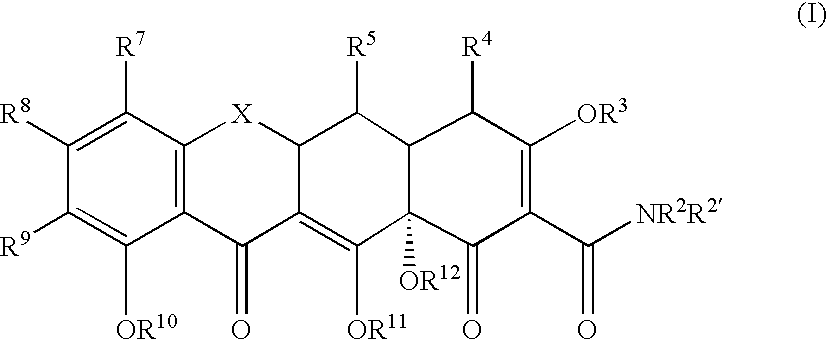
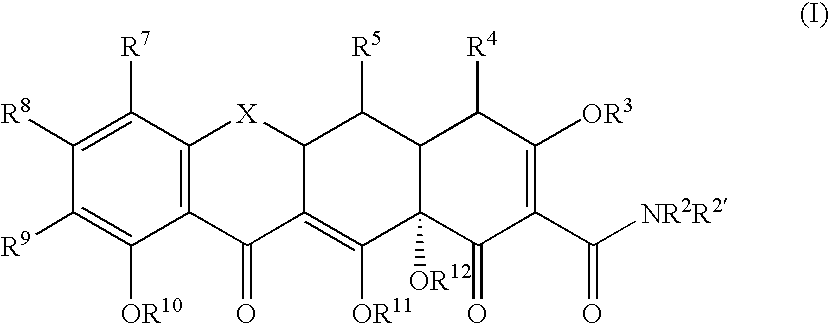
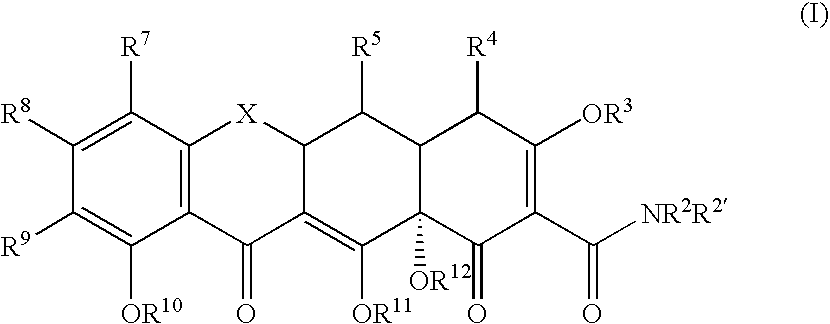
Tetracyline compounds having target therapeutic activities
Methods and compounds for treating diseases with tetracycline compounds having a target therapeutic activity are described.
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Method for decreasing low density lipoprotein
The present invention relates to a method for decreasing elevated serum / plasma LDL-cholesterol levels or LDL-cholesterol levels and CRP levels in a mammal in need thereof. The methods comprises administering an effective amount of a tetracycline formulation. In one embodiment, the tetracycline formulation is a non-antibacterial tetracycline. In another embodiment, the tetracycline formulation is an antibacterial tetracycline at a sub-antibacterial amount.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +2
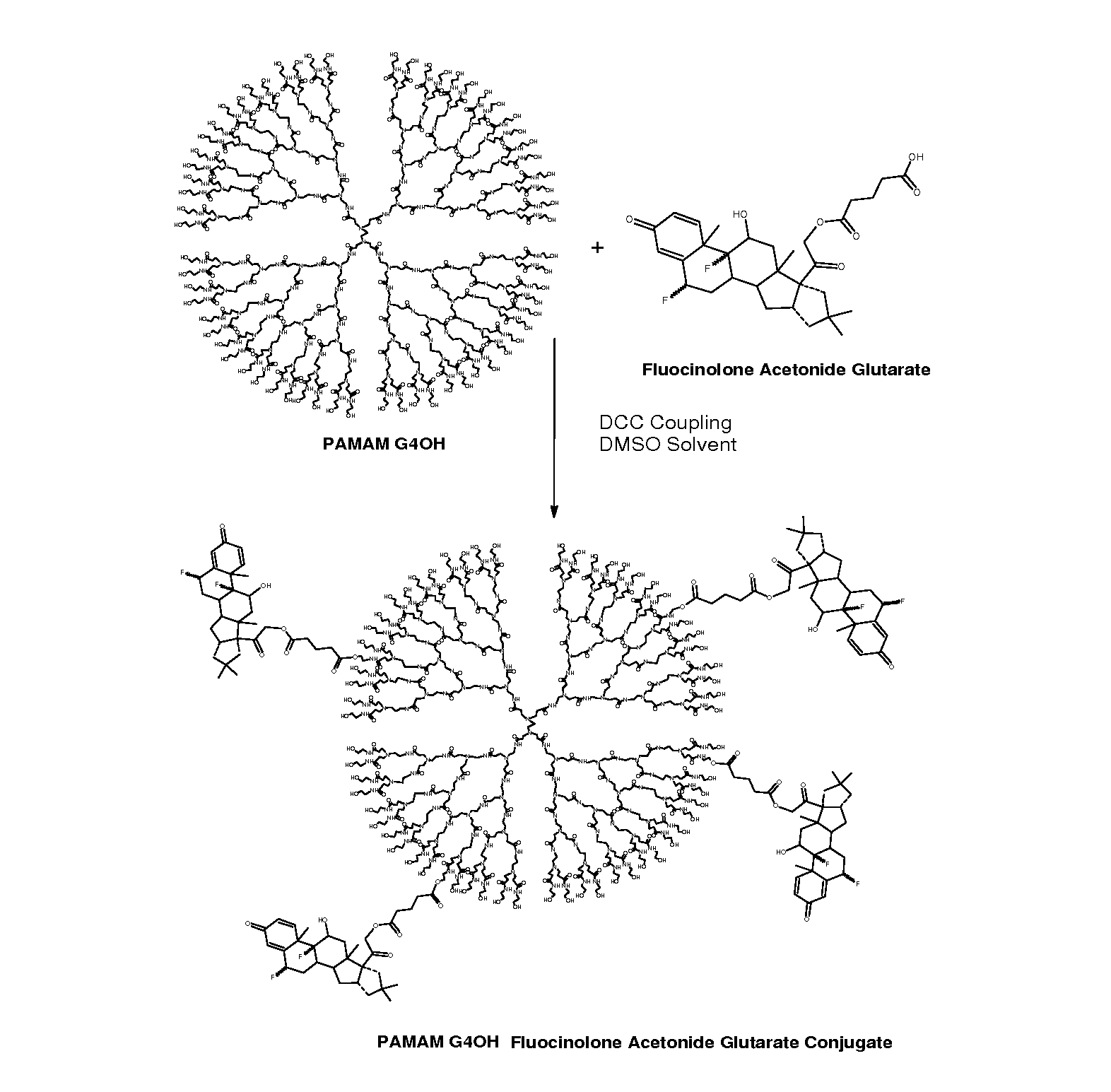
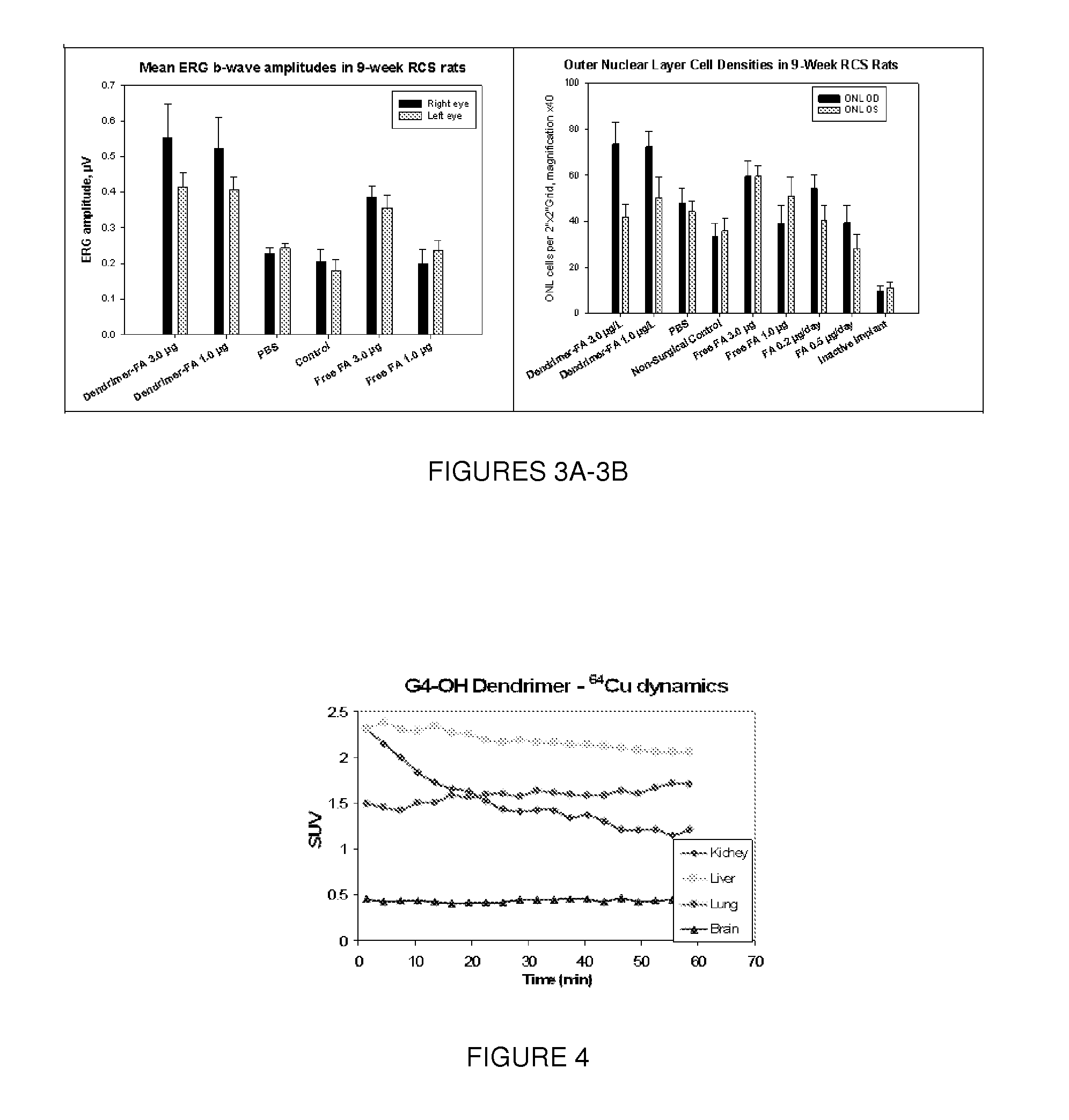
Dendrimers for sustained release of compounds
Dendrimer-based compositions and methods are provided, that are useful for administering pharmaceutical compositions to target cells and tissues for treatment of ocular diseases including macular degeneration, diabetic retinopathy, and retinitis pigmentosa.
Owner:WAYNE STATE UNIV
Medical implants and fibrosis-inducing agents
InactiveUS20050169959A1Good curative effectInduce adhesion or fibrosis in the surrounding tissueHeavy metal active ingredientsInternal osteosythesisHost tissueIncreased fibrosis
Owner:ANGIOTECH INT AG (CH)
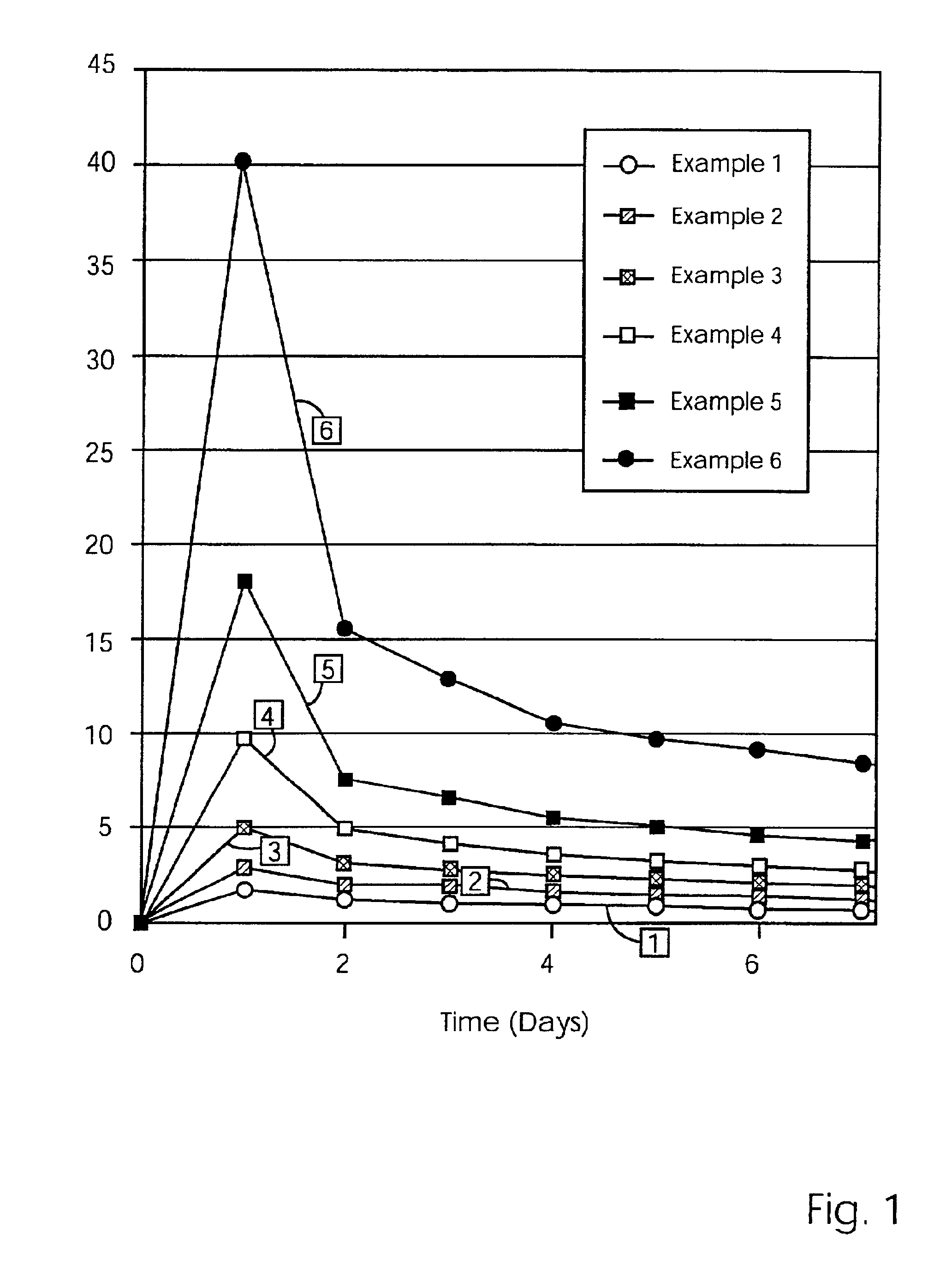
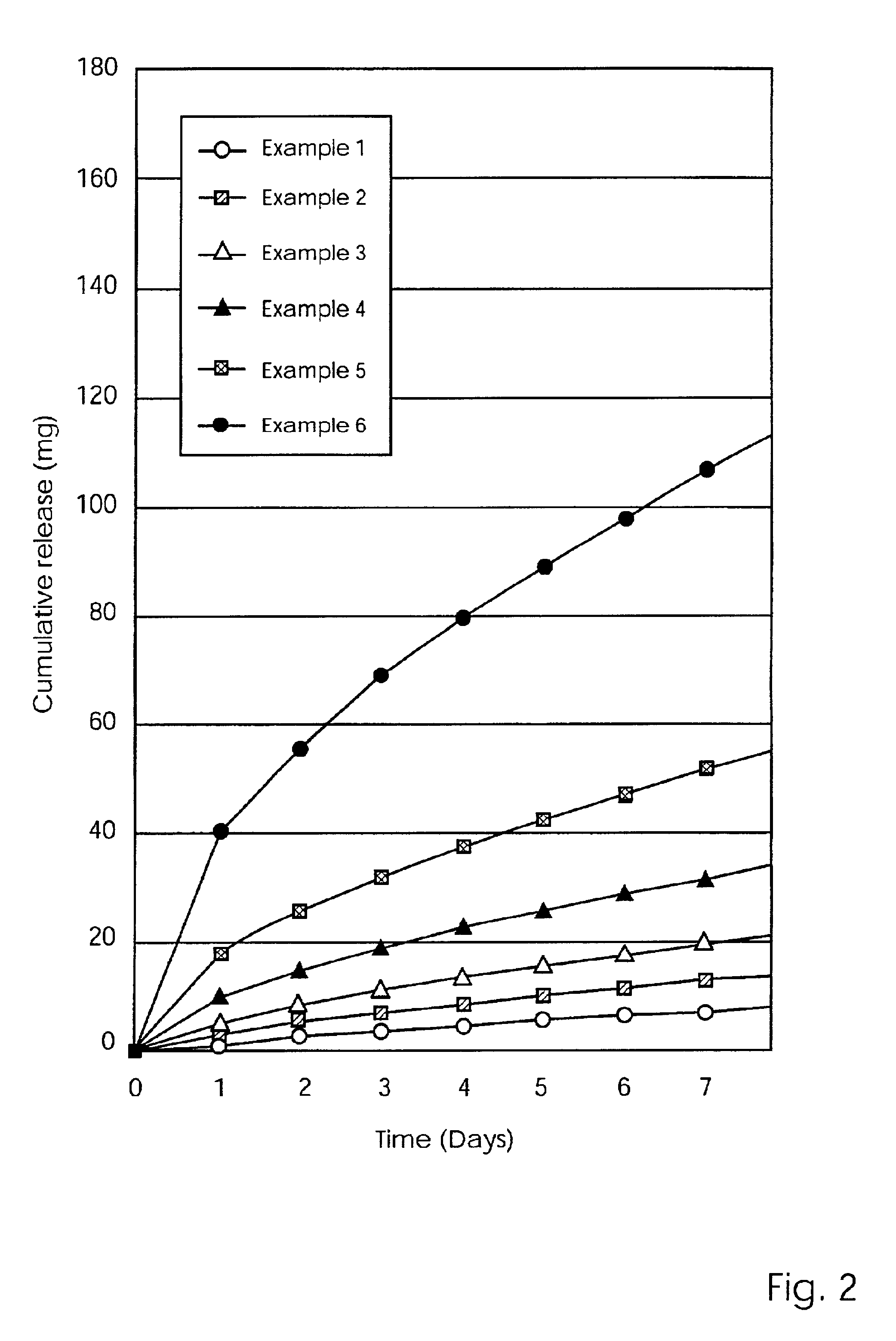
Modified release coated drug preparation
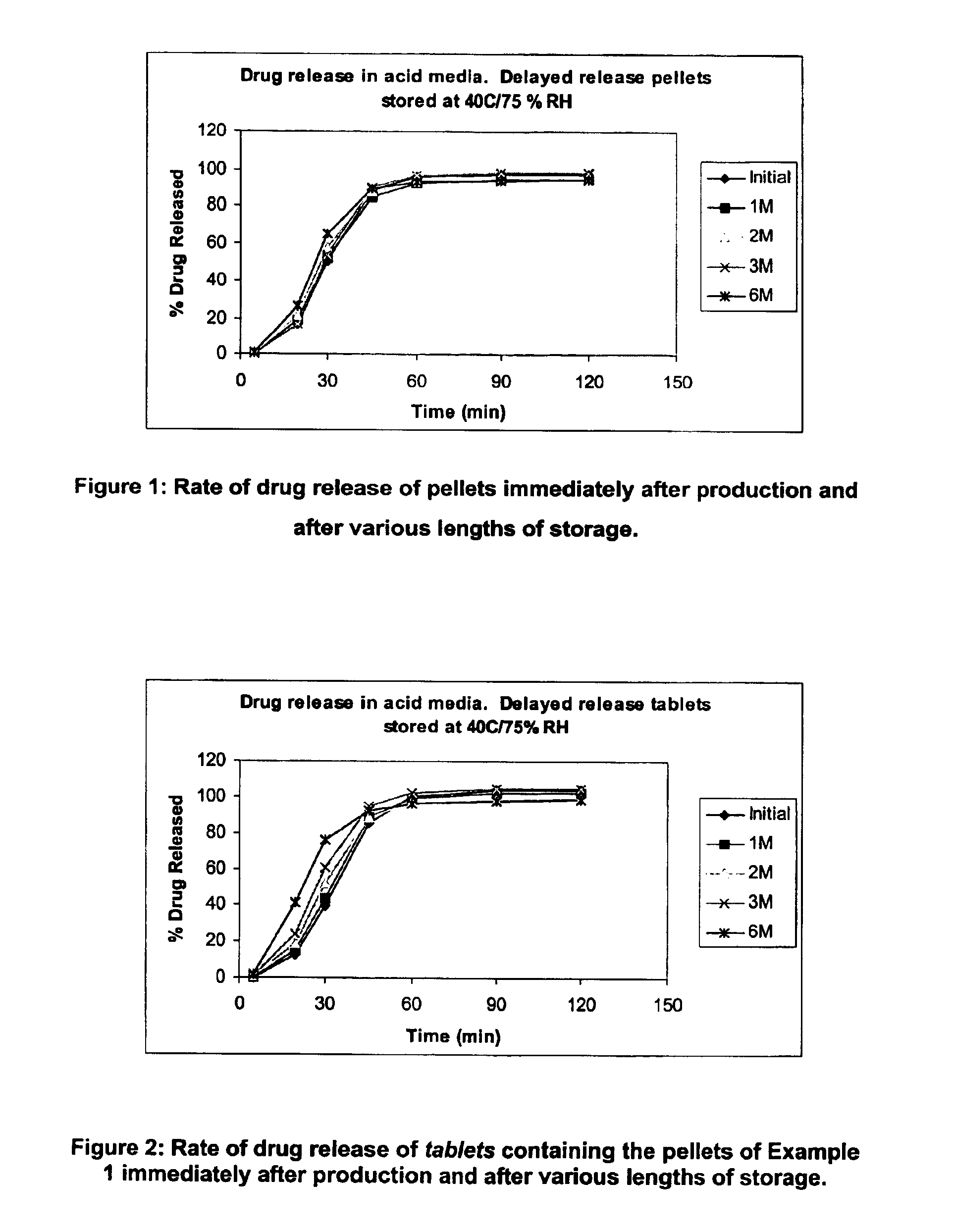
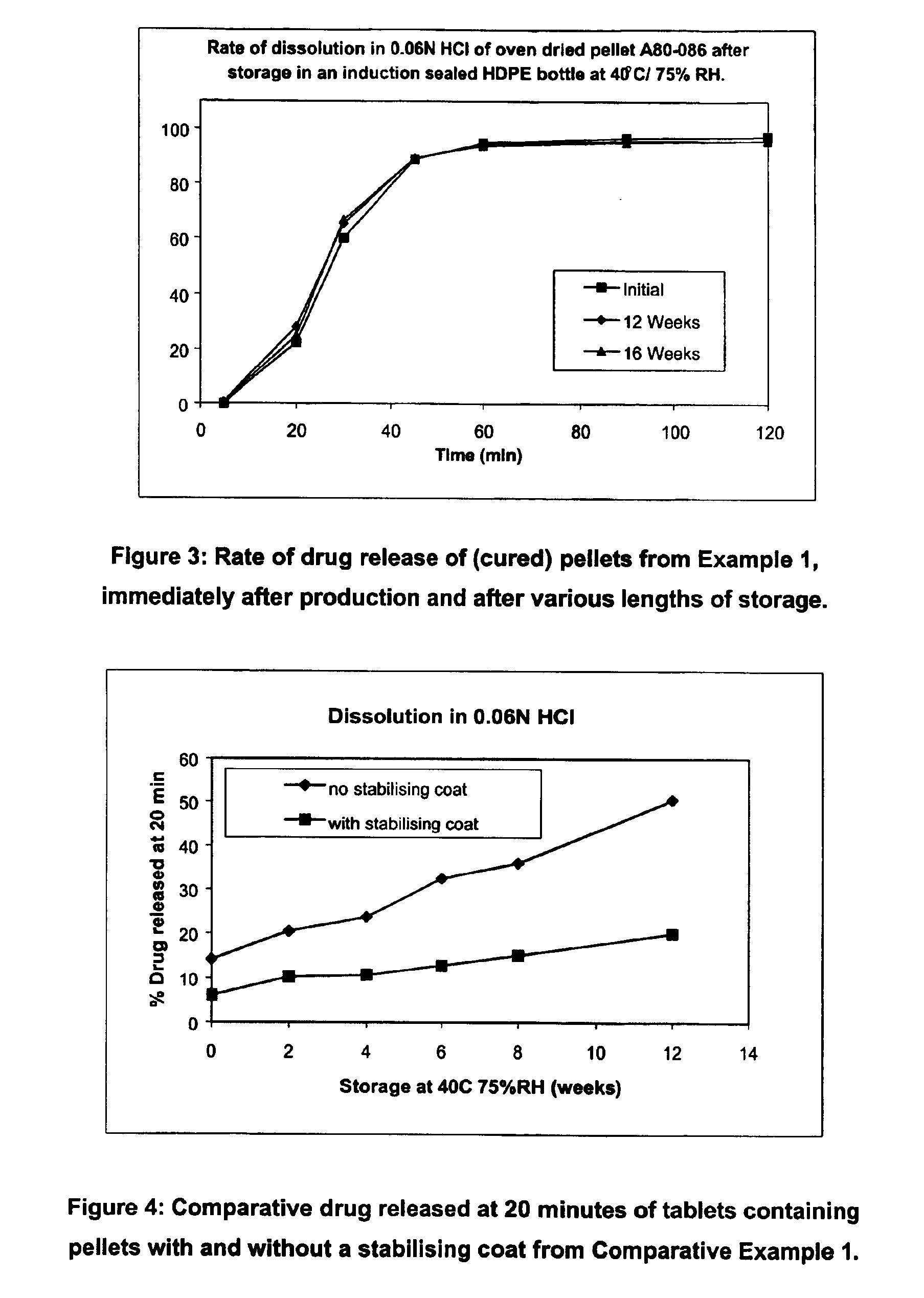
InactiveUS6958161B2Extension of timeImprove trustBiocideTetracycline active ingredientsDissolutionBULK ACTIVE INGREDIENT
A modified release preparation having one or more coated core elements, each core element including an active ingredient and having a modified release coating, wherein a stabilising coat is provided between each core element and its modified release coating so that, upon in vitro dissolution testing, the amount of active ingredient released at any time on a post-storage dissolution profile is within 40 percentage points of the amount of active ingredient released at any time on a pre-storage dissolution profile.
Owner:MAYNE PHARMA INT
Janus kinase inhibitors for treatment of dry eye and other eye related diseases
InactiveUS20120301464A1High expressionIncrease productionBiocideSenses disorderDiseaseOcular disease
Owner:INCYTE HLDG & INCYTE
Low Viscosity Liquid Polymeric Delivery System
InactiveUS20090181068A1Heavy metal active ingredientsNervous disorderPolymer sciencePolymer solution
Owner:DUNN RES & CONSULTING
Compositions & formulations for preventing and treating chronic diseases that cluster in patients such as cardiovascular disease, diabetes, obesity, polycystic ovary syndrome, hyperlipidemia and hypertension, as well as for preventing and treating other diseases and conditions
InactiveUS20140271923A1Good for healthImproving well-beingHeavy metal active ingredientsBiocideSide effectPolycystic ovary
Patients inflicted with various clustering chronic diseases require treatment with multiple drugs having distinct mechanisms of action. Accordingly, patients with multiple conditions suffer from cumulative side effects of multiple drugs as well as drug-drug interactions. Embodiments, agents, compounds or drugs of the present invention, such as sesquiterpenes, e.g., Zerumbone, replace an equal or larger number of approved drugs during patient treatment. Examples of disorders prevented or ameliorated by administration of the formulations of this invention include but are not limited to inflammatory diseases that may be, oncological, genetic, ischemic, infectious, neurological, hematological, ophthalmological, rheumatoid, orthopedic, neurological, hematological, kidney, vascular, dermatological, gynecological, or obstetric. The present invention further relates to a method of identifying agents, compounds or drugs useful in preventing or treating CDCP related diseases and conditions as well as other disorders, diseases and conditions treatable or preventable by the same agents, compounds or drugs.
Owner:REID CHRISTOPHER BRIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com