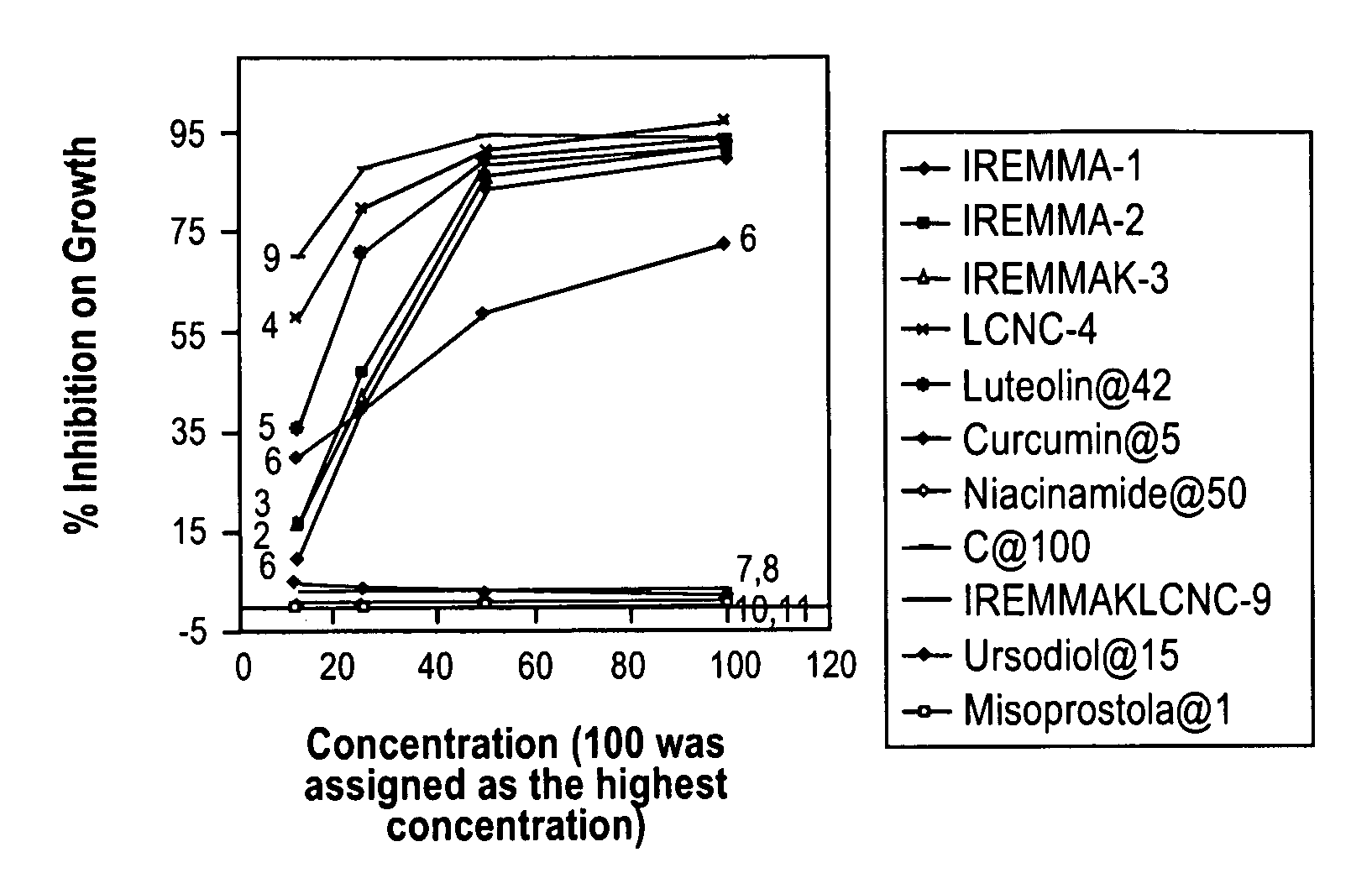
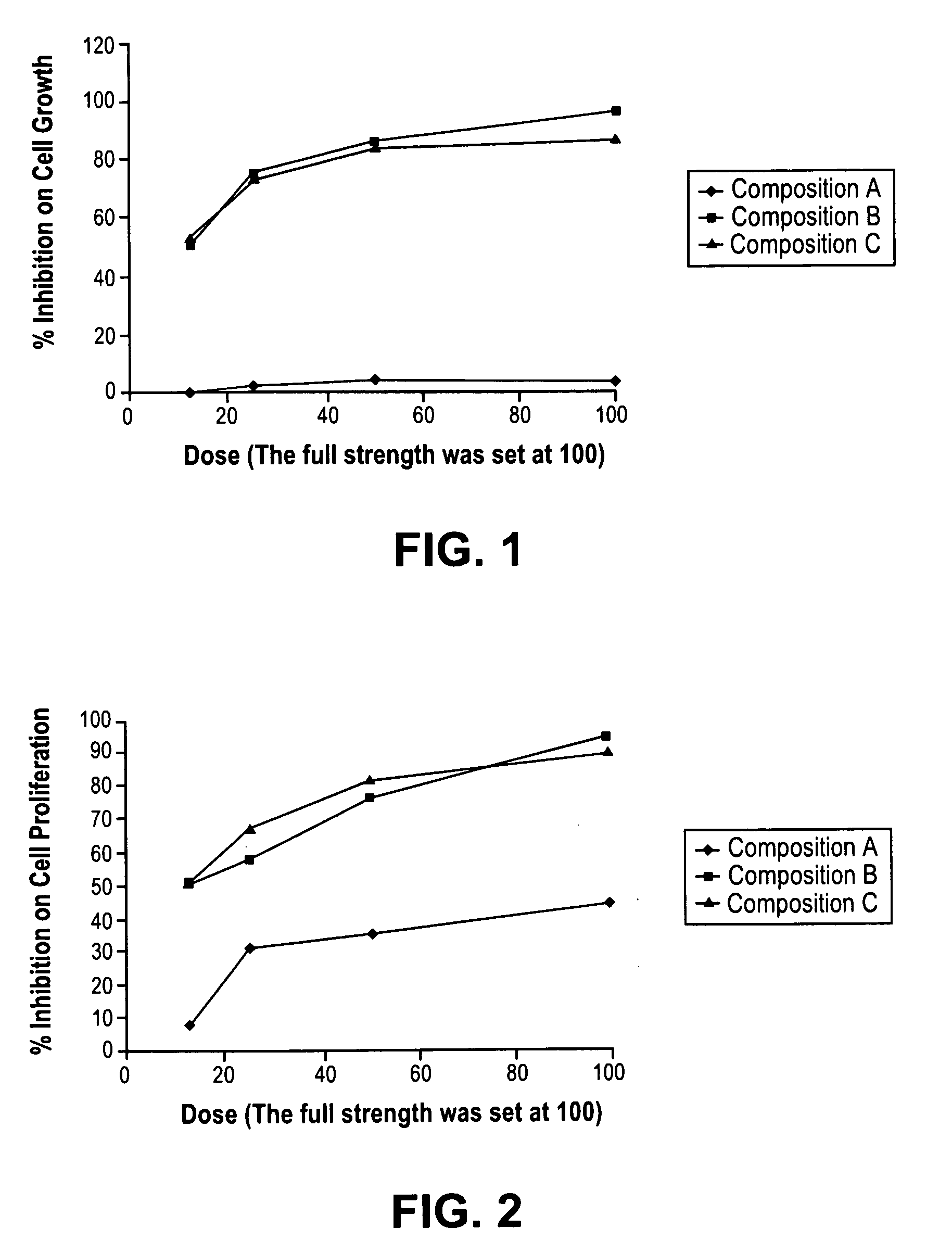
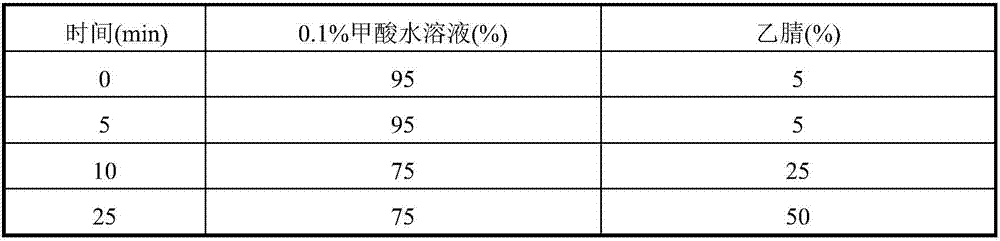
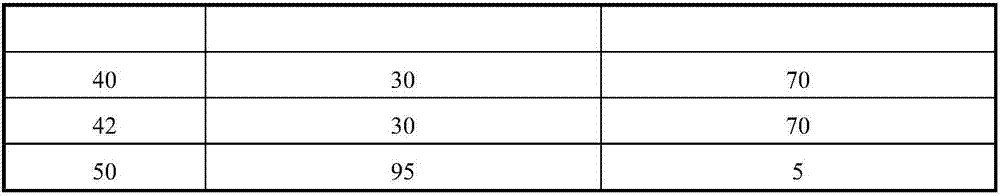
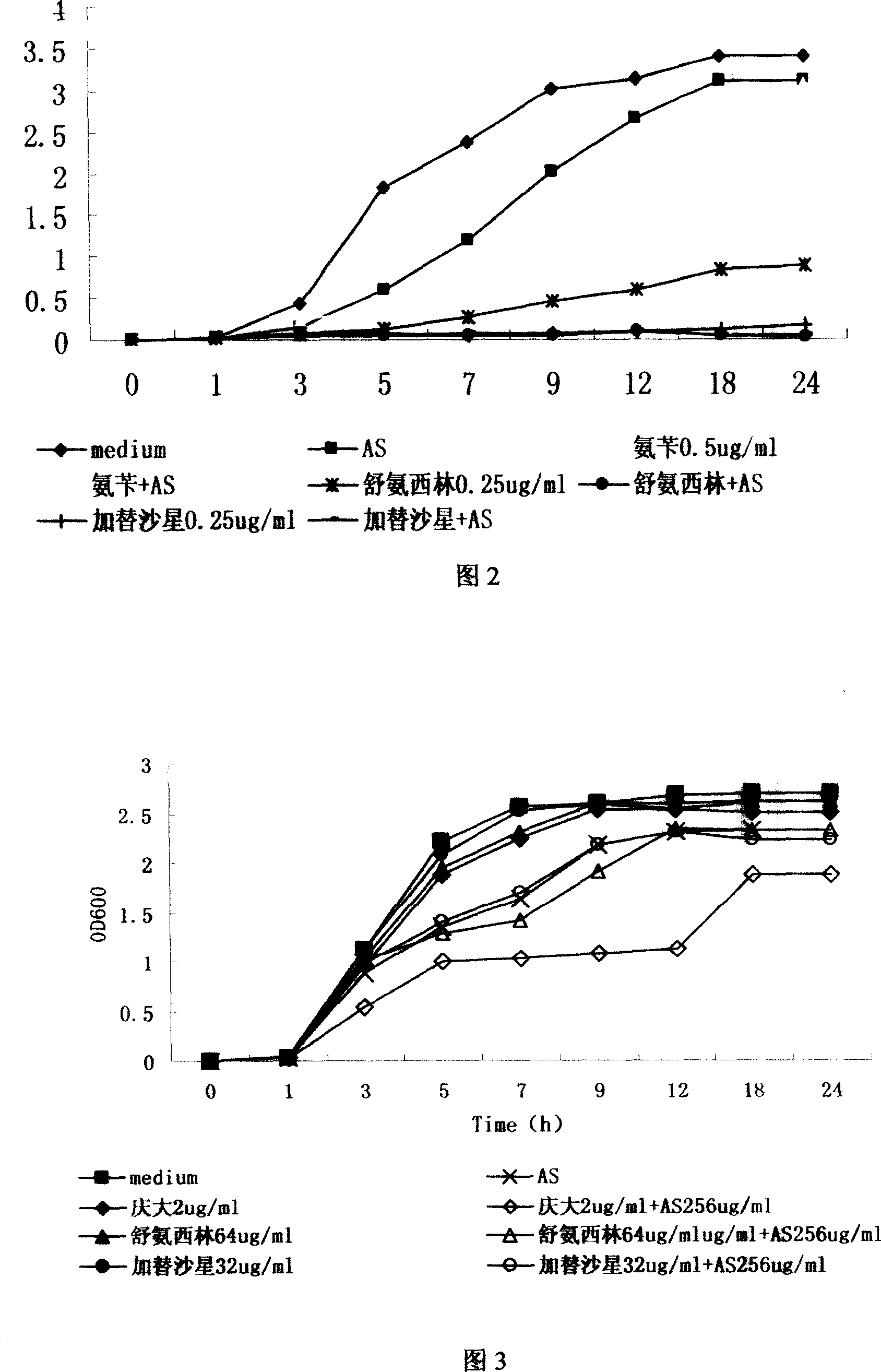
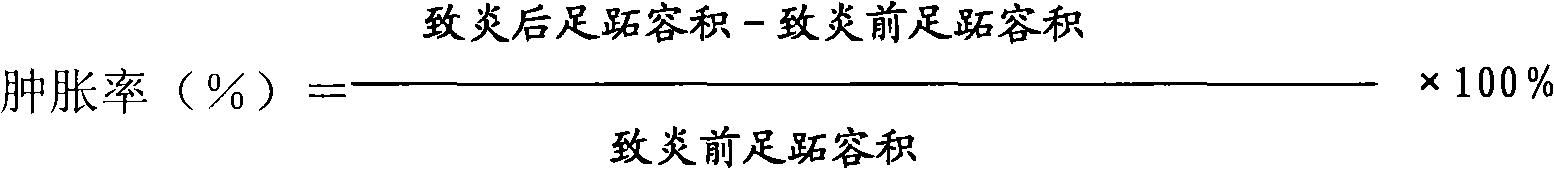
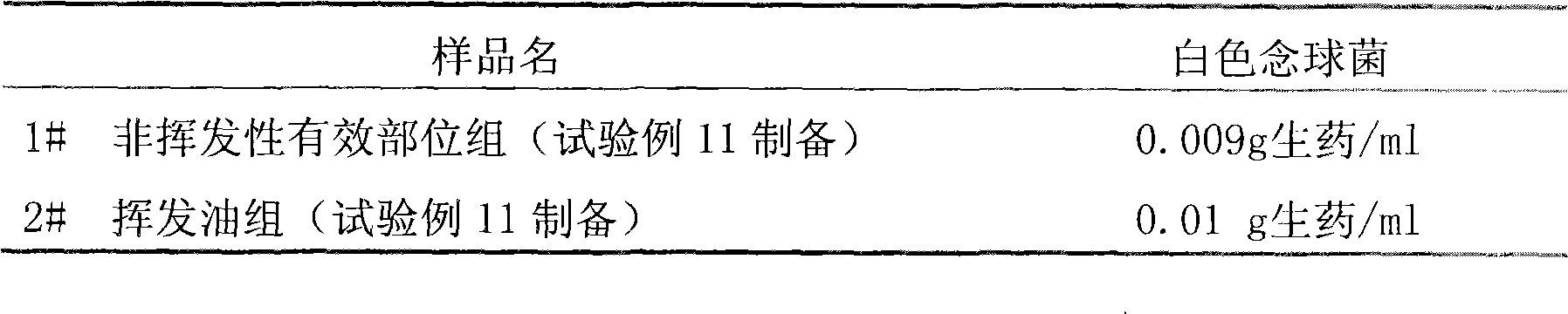
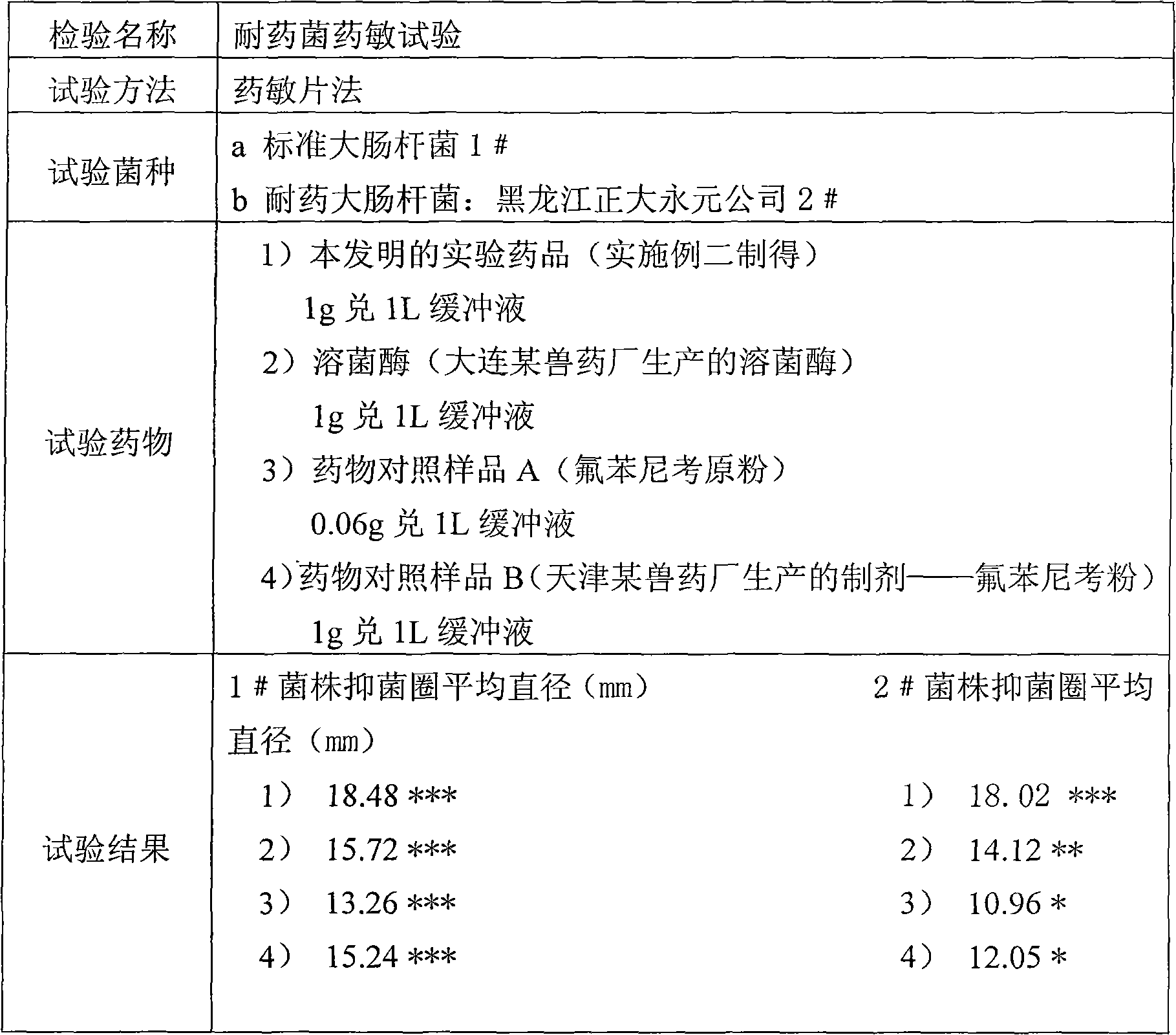
Patents
Literature
897 results about "Antimicrobial drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antimicrobial drug: A drug used to treat a microbial infection. "Antimicrobial" is a general term that refers to a group of drugs that includes antibiotics, antifungals, antiprotozoals, and antivirals.
Intravaginal drug delivery devices for the administration of an antimicrobial agent
InactiveUS6951654B2Convenient and high complianceReduce in quantityAntibacterial agentsTetracycline active ingredientsAntimicrobial drugAnti-Microbial Agents
An intravaginal antimicrobial drug delivery device is disclosed having an antimicrobial agent dispersed throughout a biocompatible elastomeric system. Also disclosed is a method of making the antimicrobial drug delivery device.
Owner:APTALIS PHARMA
3,5-substituted oxazolidinone derivative and its preparing process and application
An antibacterial 3,5-substituted oxazolidione derivative and its salt with strong activity to resist gram-position bacteria, their preparing process, their application in preparing antibacterial agents and the antibacterial medicine containing them as active components are disclosed.
Owner:广东赛法洛药业有限公司
Antimicrobial physical method
The invention discloses an antimicrobial physical method. An effect of killing or inhibiting microorganisms can be realized when the antimicrobial physical method is applied to a human body, or an animal or the surface of an object. The antimicrobial physical method is safe to the human body, and drug resistance caused by antibacterial agents can not be produced.
Owner:NMS TECH
Industrialized artificial seedling cultivation method for rockfishes
InactiveCN101375673AIncrease seedling yieldNeat specificationClimate change adaptationWater aerationPlant Germ CellsWater quality
The invention relates to a method of industrial and artificial breeding of Epinephelus, which is applicable to the breeding of Epinephelus malabaricus and E.coioides. The method comprises the following steps: (1) treating a nursery pond and water; (2) preparing before breeding; (3) putting larval fishes of Epinephelus or germ cells; (4) controlling the quality of the breeding water body; and (5) feeding baits. The method is applied to conduct the industrial and artificial breeding, so that the water quality of the breeding is stable, the output for the unit water body breeding is high, the bred sizes of advanced fries are uniform, no antibacterial drugs are used, no special breeding is conducted to unicellular alga, no bottom-attaching happens in the process of breeding, and the operation is simple.
Owner:HAINAN UNIVERSITY
Diagnosis and treatment of cancer related to human dormancy
InactiveUS20080160007A1Extend your lifeReverse processBioreactor/fermenter combinationsOrganic active ingredientsCancer cellAntimicrobial drug
New devices and methods for diagnosis and compositions and methods for treatment of cancers use combinations of antimicrobial agents and agents that can reverse dormancy and hibernation pathways. We unexpectedly found that surprisingly low doses of anti-hibernation compounds can substantially inhibit cancer cell growth in vitro and can successfully treat cancers, including metastatic cancer. We also unexpectedly found that antimicrobial agents and anti-HDS compounds together can increase the degree of inhibition of cancer cell growth in a synergistic fashion. We conclude that combination therapy with antimicrobial agents and anti-HDS compounds can be effective in treating human patients with cancer.
Owner:POWELL CO LTD
Ointment for treating burns and scalds and its preparation
InactiveCN1593540APromote healingPromotes new skin growthPowder deliveryAerosol deliveryBorneolCoptis
The invention relates to a pharmaceutical composition for treating burn and scald and process for its preparation, wherein the composition is prepared from coptis, corktree bark, rhubarb horsetails, garden burnet root, ragwort, alkanna tinctoria, dahurian angelica root, calamine, gypsum fibrosum preparata, sophora bud, borneol.
Owner:郭泗木
New compound and application thereof
ActiveCN101928331AStrong antibacterial activityHigh antibacterial activityAntibacterial agentsBacteriaAntibacterial activityBenzyl group
The invention provides a new compound and a preparation method and application thereof. The structure of the compound provided by the invention is shown as a formula (1). Four-potential hydroxyl of glycosyl on benzyl hydroxyl of peptide framework six-potential amino acid of the compound serves as an axial bond and the compound is obtained by fermentation and has the collection number of China General Microbiological Culture Collection Center (CGMCC) No.3053. The compound provided by the invention has high antibiotic activity, so that the compound plays a very important role in developing a new antibacterial medicament.
Owner:SHANGHAI LAIYI BIOMEDICAL RES & DEV CENT +2
Method for simultaneous determination of 92 antibacterial drug residues in water environment
ActiveCN107024548AImprove purification effectImprove accuracyComponent separationHigh fluxSolid phase extraction
The invention belongs to the technical field of determination of antibacterial drug residues in water environment, and in particular relates to a method for simultaneous determination of 92 antibacterial drug residues in the water environment. The method includes an improved solid phase extraction-liquid chromatography-tandem mass spectrometry determination method, and in particular includes (1) water sample pretreatment; (2) use of solid phase extraction for enrichment of various antibacterial drugs in a water sample; and (3) liquid chromatography-tandem mass spectrometry determination method establishment. The sample is fed by one time, can simultaneously determine 92 antibacterial drugs, reduces the detection cost, and improves detection efficiency, and is suitable for the detection of antibacterial drug residues in high flux water environment.
Owner:HUAZHONG AGRI UNIV
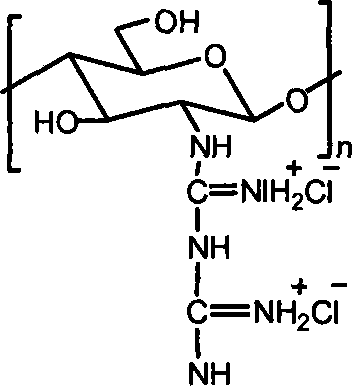
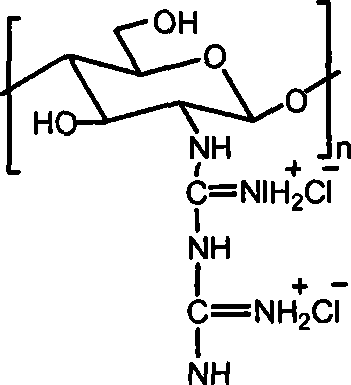
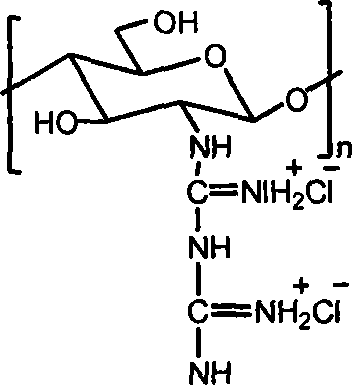
Chitosan biguanide hydrochloride, preparation method and use thereof
InactiveCN101033264AStrong and broad-spectrum antibacterial activityRich sourcesBiocideDisinfectantsSolubilitySide effect
The invention provides a new chitosan derivate-chitosan biguanide muriate, its preparation and usage, in which, the repeat unit is 2-biguanide glucose muriate, the substitution degree of guanidine is 15-50%, and the molecular weight is 0.5-25 ten thousand. This invention uses the reaction of chitosan and dicyandiamide to prepare chitosan biguanide muriate, and has advantages of mild reaction conditions and fewer side effects. The product has good antibacterial activity and water solubility, and can be widely used in medicine, textile industry, agriculture and other fields.
Owner:WUHAN UNIV
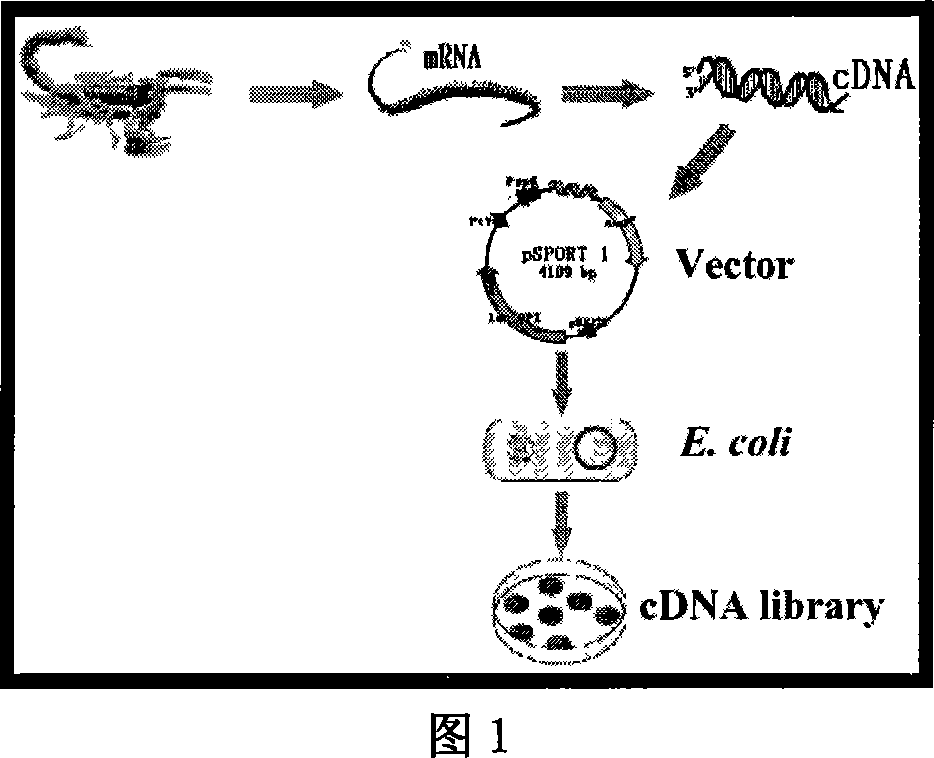
East-Asia scorpion antibiotic peptide gene and preparation method and application
ActiveCN101063102AHigh antibacterial activitySimple and efficient approach to molecular designAntibacterial agentsBacteriaChemical synthesisCDNA library
The invention discloses a preparing method of east Asia Tityus antibiotic peptide gene and appliance, which comprises the following steps: restructuring bacillus coli Escherichia coli DH5a / BmKAMP1, CCTCC NO: M207036; constructing east Asia Tityus ioterium cell cDNA library; choosing PCR method; sieving positive colony of scorpion antibiotic peptide gene from ioterium cDNA library; sequence-analyzing coding trait of antibiotic peptide gene; assuring amino acid sequence of the antibiotic peptide gene; adopting chemosynthesis antibiotic peptide; possessing inhibition of diverse density for Gram's bacterium. This antibiotic peptide possesses specificity and high active, which can be used as antibacterial drugs.
Owner:唐克煌
Slowly-released type nanometer silver antibacterial gel and the preparing method and the application thereof
InactiveCN101062055AImprove permeabilityImprove the bactericidal effectAntibacterial agentsCosmetic preparationsUrethritisCervicitis
The invention discloses an externally used nanometer silver antibiotic jel line products, which is characterized by the following: constituting with acceptable shaped agent, diluent, slow releasing agent, disperser on nanometer silver and medicinal; setting the grain size of the nanometer silver at 1-25nm; comprising push injecting agent, foam spray, aquagel cleaning solvent, spray and so on of nanometer silver antibiotic jel line products. This invention also relates to the preparing method of nanometer silver colloid and nanometer silver antibiotic jel line products. This product can be used to cure vaginitis, cervicitis, mixing infection type gynaecologic inflammation, prostatitis, prostate proliferation, prostatauxe, male urethritis, balanitis, posthitis and so on.
Owner:崔彬
Screening method of 79 antibacterial agents in animal foods
InactiveCN107228912APromote healthy developmentReduce testing costsComponent separationHigh-Throughput Screening MethodsScreening method
The invention discloses a screening method of 79 antibacterial agents in animal foods. The screening method comprises preparation of a standard working solution, analysis pretreatment on a sample to be tested, drawing of a standard curve, qualitative analysis and quantitative analysis. A high throughput screening and quantitative detection platform is built so that the detection of a single compound is developed into the simultaneous detection of a plurality of compounds in the field. Compared with the prior art, the screening method realizes a detection cost about 1 / 2 the original cost, shortens a sample detection period by 1 / 2 and greatly improves work efficiency. The screening method plays an effective role in the veterinary drug residue monitoring program, the pollution-free animal product testing program and the national animal product routine testing program implemented by the Ministry of Agriculture.
Owner:河南省兽药饲料监察所
Topical spray for burn treatment and anti-infection
InactiveUS6987133B2Function increaseReduce disadvantagesBiocideCosmetic preparationsCross-linkAntimicrobial drug
This invention relates to a topical spray preparation for burn treatment and microbial infections on human being or animals. This non-aerosol preparation contains an antimicrobial drug, i.e., silver sulfadiazine, as is dispersed or solubilized in a cream or lotion base matrix which can be sprayed directly from a common trigger spray device. The key component of the matrix can be characterized by it having a suitable molecular weight polymer of cross-linked acrylic acid, such as Carbomers or non-ionic surfactants such as polyoxyethylene alkyl ethers, or any combination of the above materials.
Owner:SAGE PHARMA
Compositions and methods for treating pathological infections
InactiveUS20060003969A1High recurrence rateEfficient infectionAntibacterial agentsBiocideYeastActive agent
The present invention relates to compositions and methods for treating pathological infections. An embodiment of the present invention is directed to a topical composition useful for the treatment of yeast, fungal or bacterial infections of a patient in need of such treatment which includes a pharmaceutically effective amount of an anti-yeast, anti-fungal or antibacterial pharmaceutically active agent; and a pharmaceutically acceptable carrier.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Preparation method for silver-containing carbon dot and application of silver-containing carbon dot to preparation of antibacterial agent
The invention discloses a preparation method for a silver-containing carbon dot and application of the silver-containing carbon dot to preparation of an antibacterial agent. The preparation method comprises the following steps: with polyethyleneimine (PEI), citric acid and silver nitrate as raw materials, synthesizing a silver-doped functionalized carbon dot solution (a PEI-Ag carbon dot solution)by using a hydrothermal process or microwave process; and then carrying out centrifugation and drying so as to obtain the solid PEI-Ag carbon dot. The preparation method provided by the invention isenvironment-friendly in preparation and uses cheap and easily available raw materials; and the prepared PEI-Ag carbon dot has good biocompatibility and high quantum yield, can be individually used orcooperatively used with antibacterial agents like tetracycline, gentamycin and cefoxitin, and show inhibitory effect on Staphylococcus aureus, Escherichia coli, Candida albicans and the like.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Composition containing porous microparticle impregnated with biologically-active compound for treatment of infection
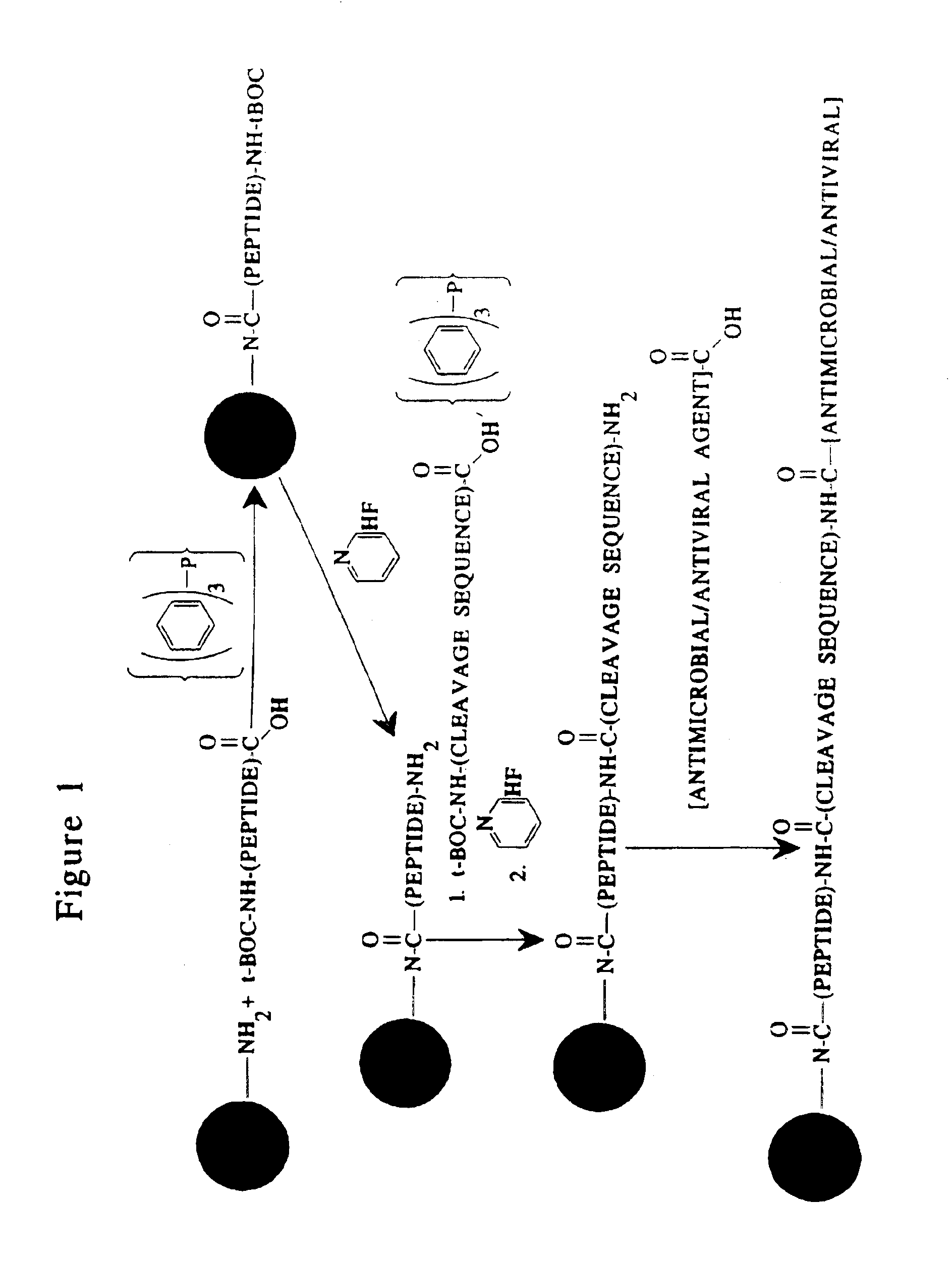
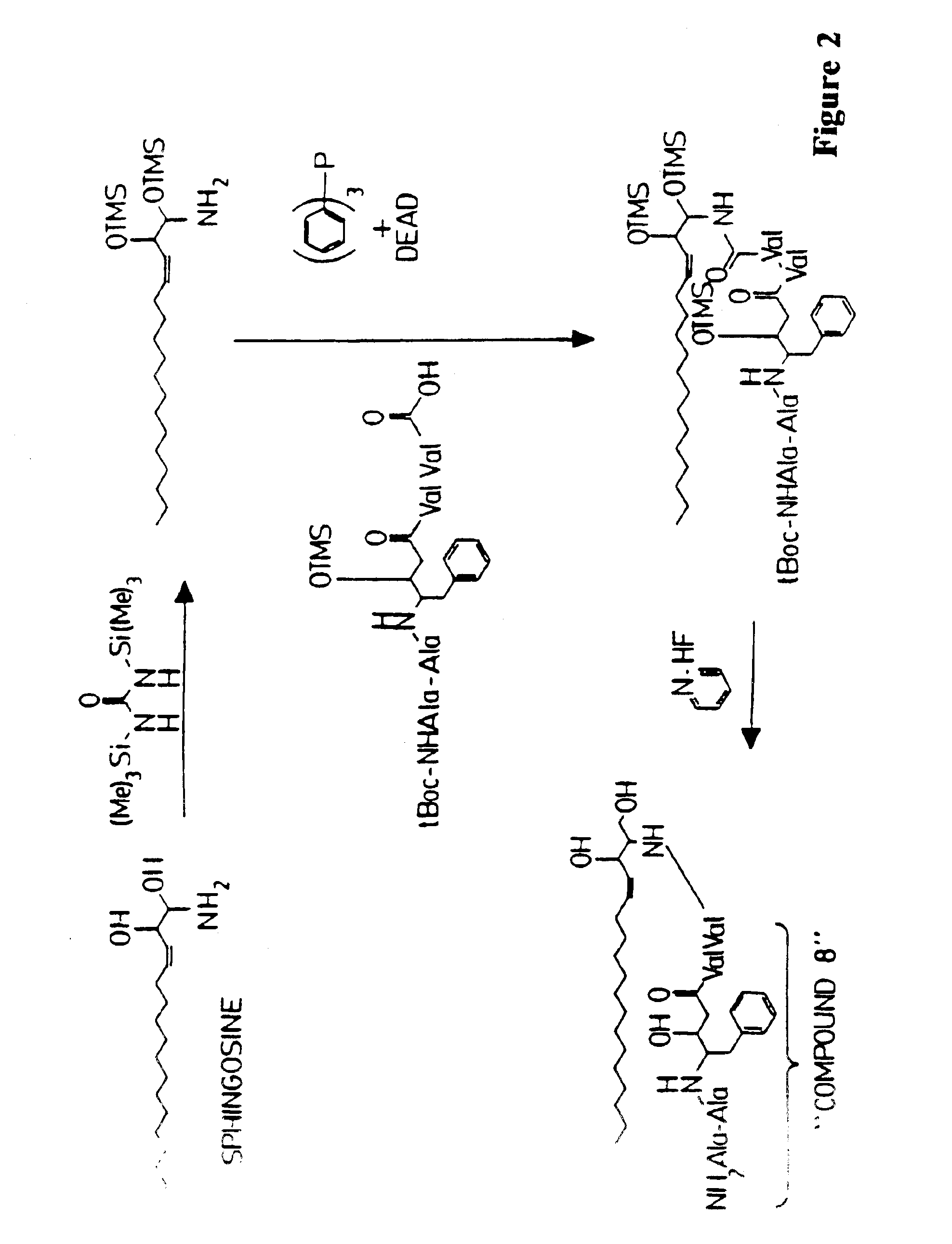
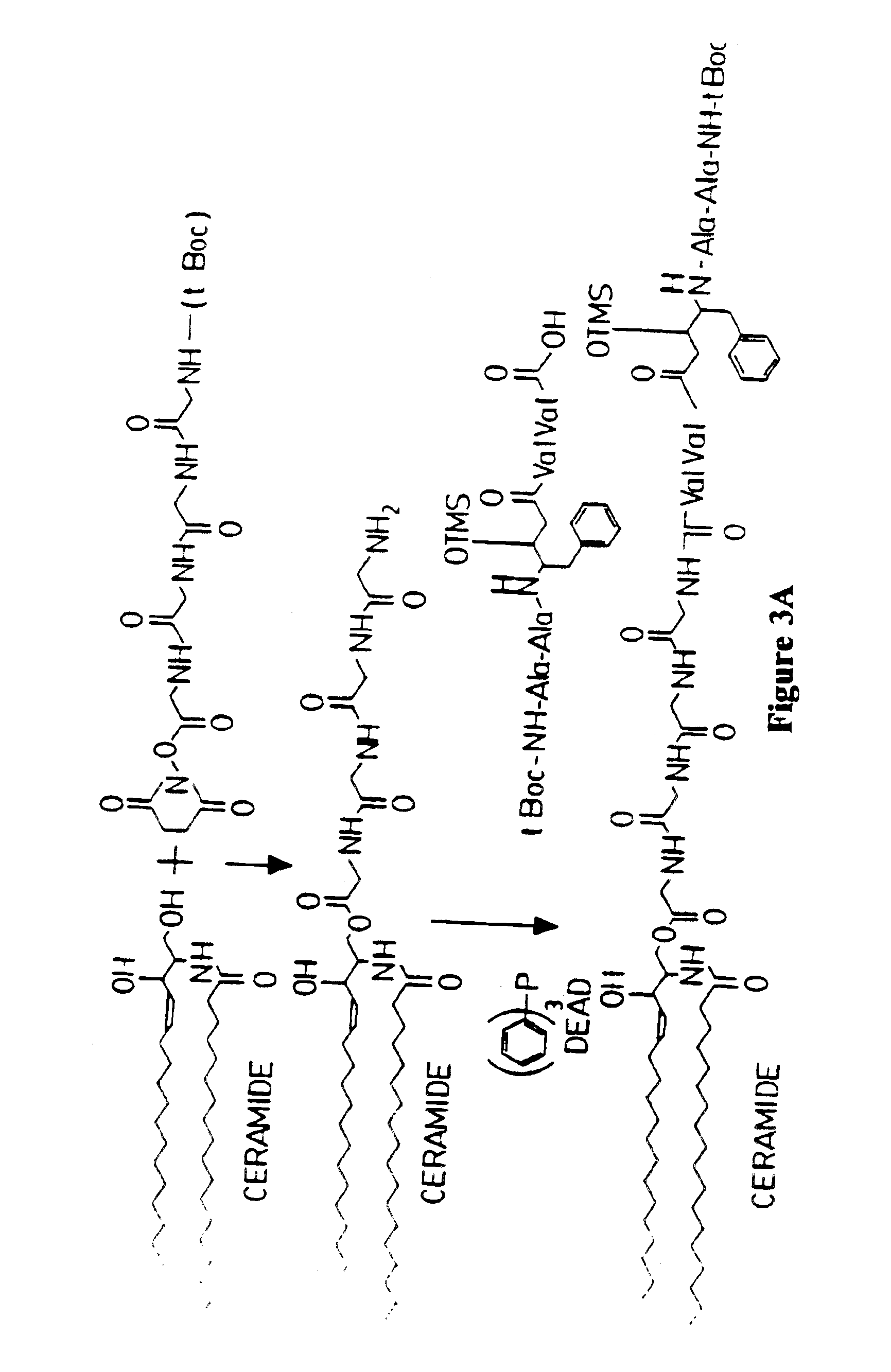
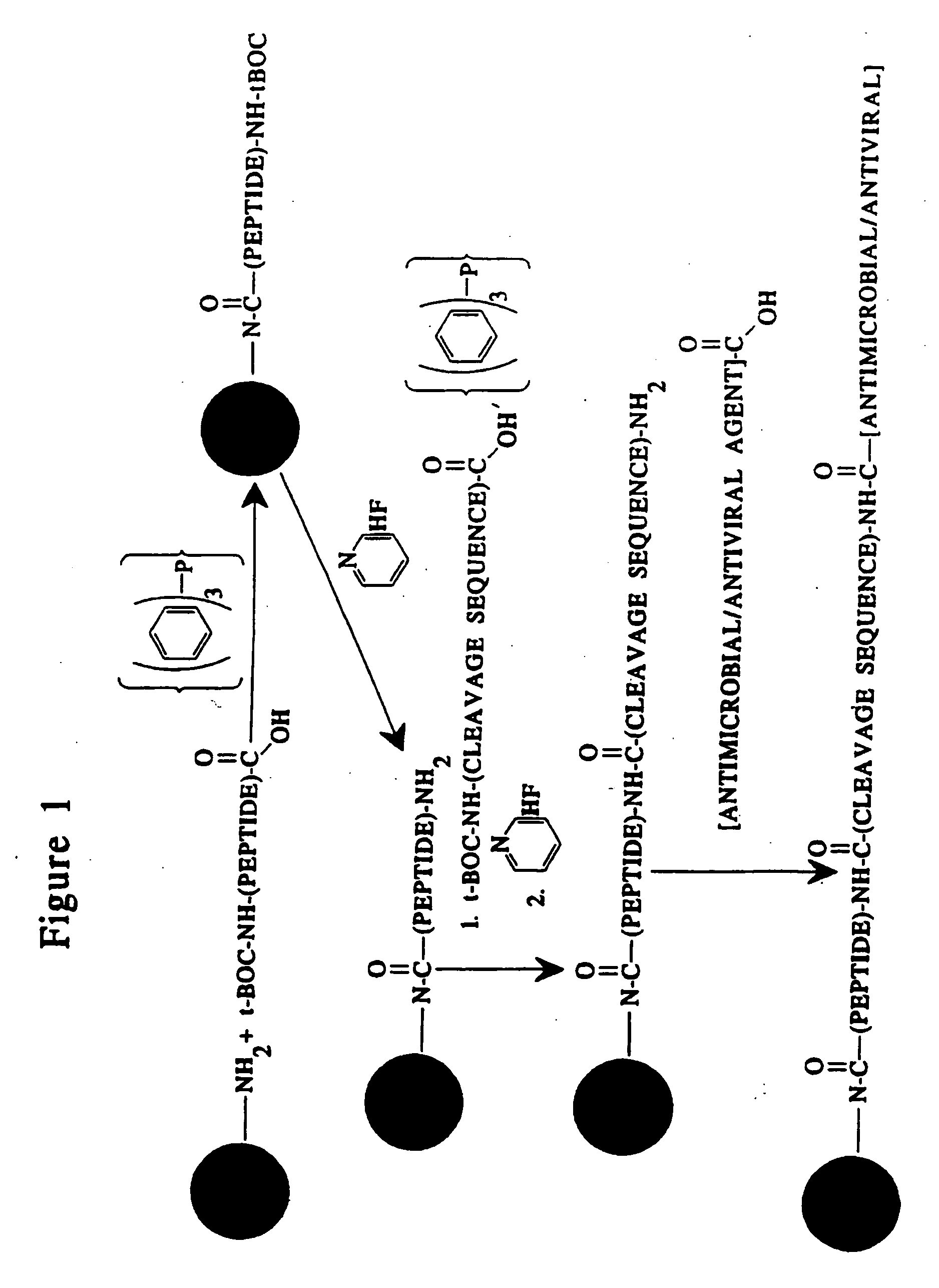
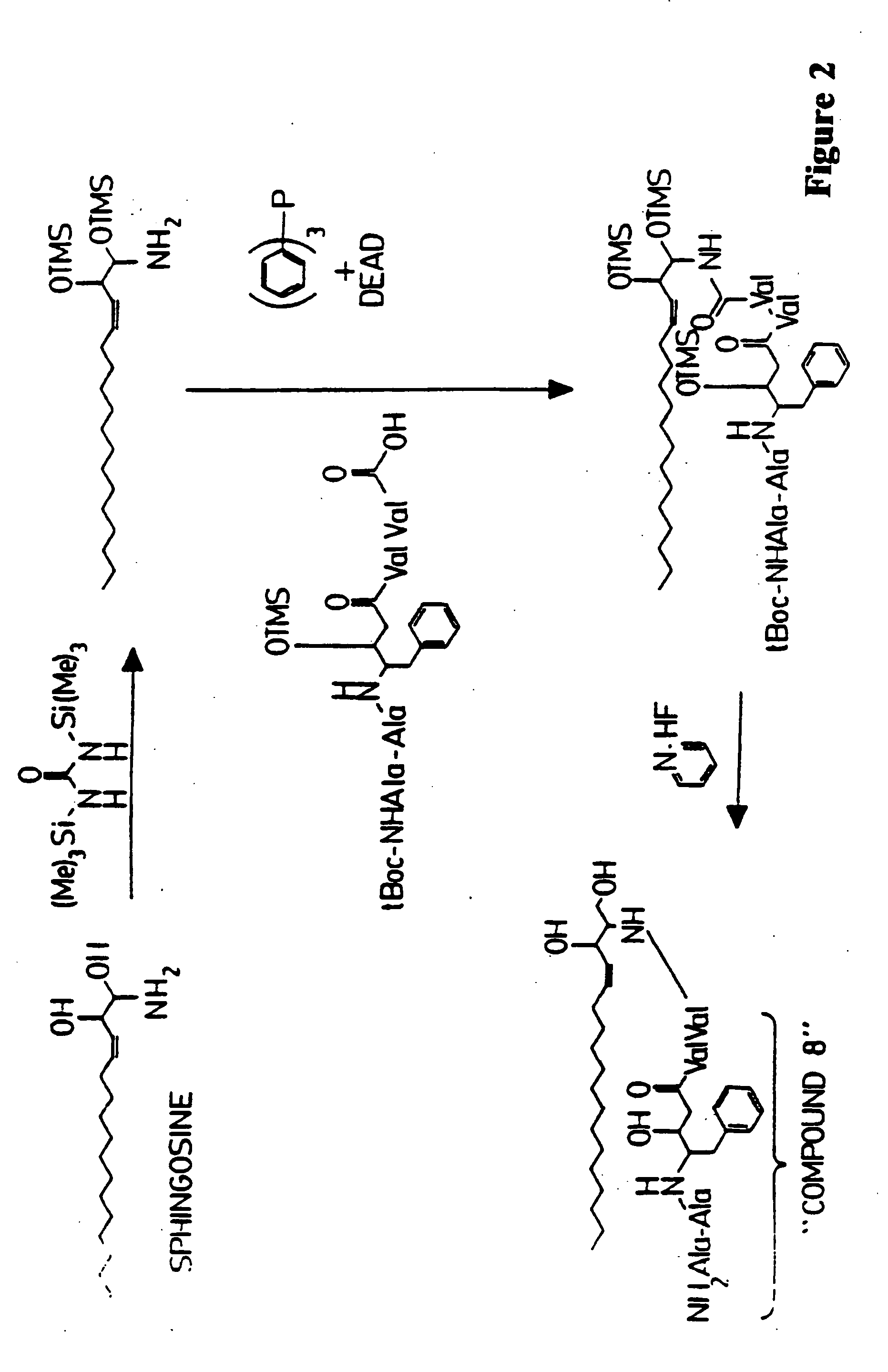
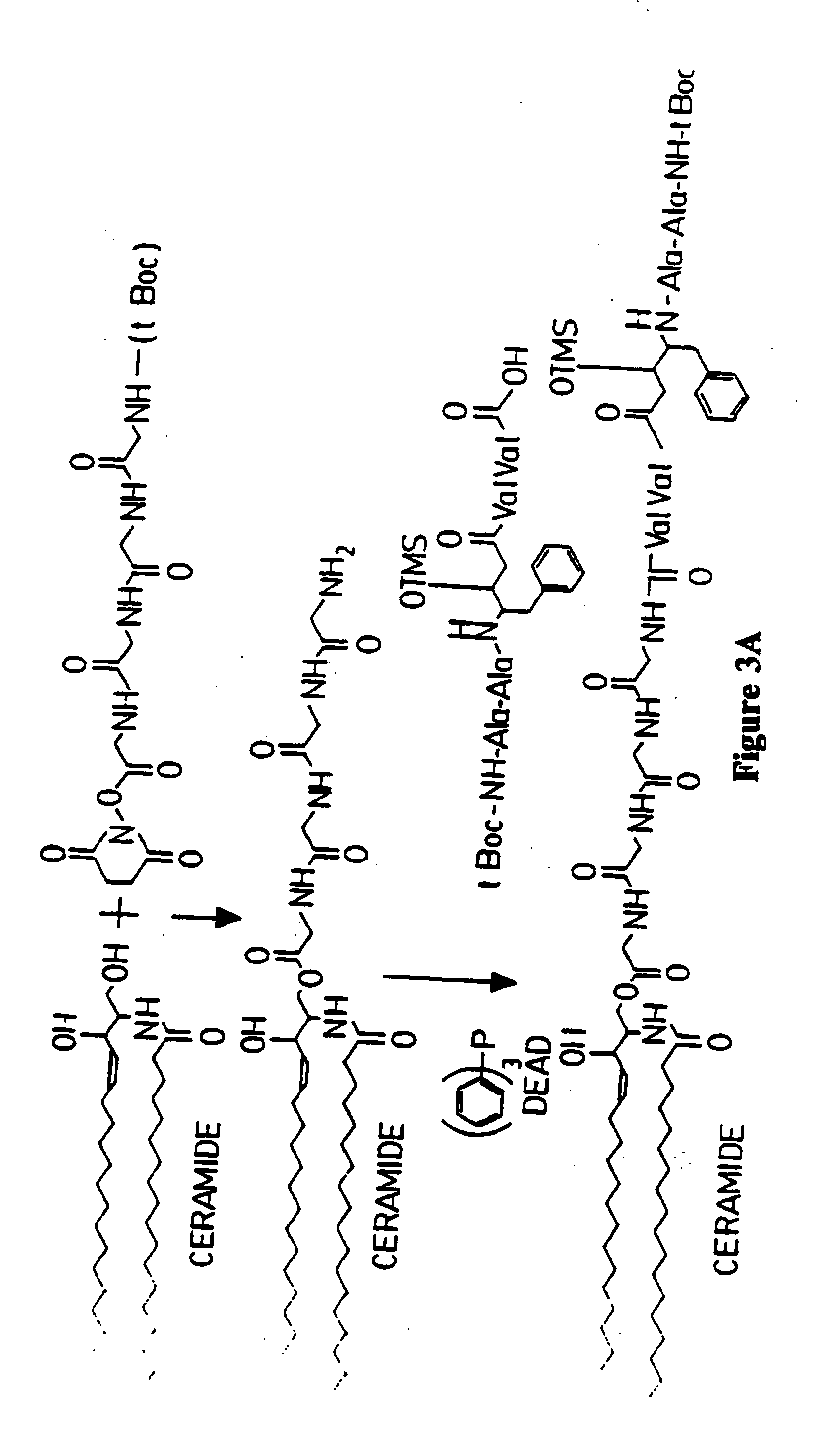
Methods and reagents are provided for specifically targeting biologically active compounds such as antiviral and antimicrobial drugs, or prodrugs containing the biologically active compound to specific sites such as specific organelles in phagocytic mammalian cells. The biologically active compound or prodrug is linked to a microparticle with a linker that is non-specifically or specifically cleaved inside a phagocytic mammalian cell. Alternatively, the biologically active compound or prodrug is impregnated into a porous microparticle or coated on a nonporous microparticle, and then coated with a coating material that is non-specifically or specifically degraded inside a phagocytic mammalian cell. The prodrug contains the biologically active compound linked to a polar lipid such as ceramide with a specific linker such as a peptide that is specifically cleaved to activate the prodrug in a phagocytic mammalian cell infected with a microorganism. A microparticle linked antimicrobial drug or prodrug may be used for killing a microorganism infecting a phagocytic mammalian cell in vivo or in vitro.
Owner:OREGON HEALTH & SCI UNIV
Super-hydrophilic coating with long-acting antibacterial property and preparation method thereof
ActiveCN108816689AResistance adhesionGood antibacterial propertiesSurgeryPharmaceutical delivery mechanismIonNitrogen gas
The invention provides a super-hydrophilic coating with a long-acting antibacterial property and a preparation method thereof. The preparation method comprises the following steps that the surface ofa substrate material is pretreated; the pretreated substrate material is put into a slight acidic buffer solution, then a polyphenolic substance, a multi-amine compound, an antibacterial agent and anoxidizing agent are added to react; a reaction product is immersed in deionized water, ultrasonic cleaning is carried out, then drying is carried out under a nitrogen condition to prepare the coating.The super-hydrophilic coating with the long-acting antibacterial property and the preparation method thereof have the advantages that the operation is simple, the reaction condition is mild; the prepared coating material contains the polyphenolic substance, and a large number of carboxyl, phenolic hydroxyl group, quinonyl, amidogen and other functional groups and metal ions or agents which has the antibacterial property, so that the metal ions and the antibacterial agents achieve the long-term existence of the antibacterial agent through the mutual effect with the phenolic hydroxyl group function group or the accumulation of a pi-pi system. The coating can be applied to the preparation of wound dressing materials in the field of medical materials.
Owner:JILIN VENUS HAOYUE MEDICAL LTD
Method for efficiently expressing antibacterial peptide NZ2114 in recombinant pichia pastoris
ActiveCN103045636ARealize large-scale productionFungiMicroorganism based processesPichia pastorisChemical synthesis
The invention provides a method for efficiently expressing an antibacterial peptide NZ2114 in recombinant pichia pastoris. An expression vector comprising a DNA (Deoxyribose Nucleic Acid) sequence of the antibacterial peptide NZ2114 which is subjected to optimization coding by yeast preferred codons is constructed, pichia pastoris is transformed, the obtained recombinant pichia pastoris secretes to generate the antibacterial peptide NZ2114 by fermentation cultivation. According to the invention, by optimizing the genic sequence of the antibacterial peptide NZ2114 and constructing the special expression vector, efficient expression of the antibacterial peptide NZ2114 in the pichia pastoris is realized for the first time; yield breaks through the gram-grade level; the problem of excessively low yield or excessively high chemical synthesis cost in the large-scale production is solved; a perfect purification system is established; scale production can be realized; the method can be applied to the fields of development of antibacterial agents, development of feed additives and the like and has high application values and broad market prospects.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Scorpion analgesic antibacterial active peptide and preparation method thereof
ActiveCN101041692AEasy to prepareSugar derivativesPeptide/protein ingredientsChemical synthesisAntimicrobial drug
The invention discloses a structure, function and making method of karsch analgesic antibiotic active peptide, whose amino acid sequence is NGYLLDKYTGCKVWCVINNESCNSECKIRGG-YYGYCYFWK LACFCQGARNSELWNYNTNKCNGKL, wherein the active peptide or derivant or analog can be synthesized through gene engineering technique, which solves the difficulty to separate and purify peptide; the active peptide is mixed with multiple carriers as medical acceptable agent, which is fit for clinical.
Owner:SHENYANG PHARMA UNIVERSITY
Microparticle-drug conjugates for biological targeting
InactiveUS20060008461A1Control releasePowder deliveryTripeptide ingredientsAntimicrobial drugMicroparticle
This invention provides novel methods and reagents for specifically delivering biologically active compounds to phagocytic mammalian cells. The invention also relates to specific uptake of such biologically active compounds by phagocytic cells and delivery of such compounds to specific sites intracellularly. The invention specifically relates to methods of facilitating the entry of antiviral and antimicrobial drugs and other agents into phagocytic cells and for targeting such compounds to specific organelles within the cell. The invention specifically provides compositions of matter and pharmaceutical embodiments of such compositions comprising conjugates of such antimicrobial drugs and agents covalently linked to particulate carriers generally termed microparticles. In particular embodiments, the antimicrobial drug is covalently linked to a microparticle via a cleavable linker moiety that is non-specifically cleaved in a phagocytic cell. In additional embodiments, the biologically-active compound is provided in an inactive, prodrug form that is activated by a chemical or enzymatic activity specific for cells infected by a microorganism, particularly a pathological or disease-causing microorganism. Thus, the invention provides cell targeting of drugs wherein the targeted drug is only activated in cells infected with a particular microorganism. Alternative embodiments of such specific drug delivery compositions also contain polar lipid carrier molecules effective in achieving intracellular organelle targeting in infected phagocytic mammalian cells. Particular embodiments of such conjugates comprise antimicrobial drugs covalently linked both to a microparticle via a cleavable linker molecule and to a polar lipid compound, to facilitate targeting of such drugs to particular subcellular organelles within the cell. Also provided are porous microparticles impregnated with antiviral and antimicrobial drugs and agents wherein the surface or outside extent of the microparticle is covered with a degradable coating that is degraded within a phagocytic mammalian cell. Also provided are nonporous microparticles coated with an antiviral or antimicrobial drug and further coated wherein the surface or outside extent of the microparticle is covered with a degradable coating that is degraded within a phagocytic mammalian cell. Methods of inhibiting, attenuating, arresting, combating and overcoming microbial infection of phagocytic mammalian cells in vivo and in vitro are also provided.
Owner:OREGON HEALTH & SCI UNIV
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Hernia patch solid-supported with antibiotic and preparation method
InactiveCN102205151AGood biocompatibilityNon-immunogenicAntibacterial agentsPowder deliveryHerniaMicrosphere
The invention relates to a hernia patch solid-supported with antibiotics and a preparation method. The antibiotic hernia patch solid-supported with drug-loaded microspheres comprises drug-loaded microspheres, a patch, and a supporting agent. The sandwich-type antibiotic hernia patch comprises a patch layer, a drug layer, and a film layer. The antibiotic hernia patch solid-supported with drug-loaded microspheres is prepared by solid-supporting the drug-loaded microspheres onto the surface of the patch material by the supporting agent. The drug-loaded microspheres are loaded with water-soluble drugs by a solvent evaporation method. The sandwich-type antibiotic hernia patch is prepared by sandwiching the drug layer between the patch layer and the film layer through a layer-by-layer superposition principle. The antibiotic hernia patch of the invention has a simple and practical preparation method, is suitable for different clinical medication requirements, has obvious slow release performance, has significant antibiotic effect in rats, solves the problem of acute and delayed infection after herniorrhaphy, has good market prospects, and is worthy of popularization and utilization.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Preparation method of sulfadoxine
A preparation method of sulfadoxine belongs to the field of sulfanilamide antimicrobial drug preparation. Cyclization reaction comprises the following steps of: firstly pouring a sodium methoxide solution into a reactive pan, then successively adding methanamide and methyl ethyl methoxymalonate, keeping warm, recovering methanol, cooling for crystallization, drying by centrifugation, discharging,and drying to obtain 5-methoxy-4,6-disodium dihydroxypyrimidine; Chlorination reaction comprises the following steps of: firstly putting phosphorus oxychloride into a reaction vessel for heating, adding 5-methoxy-4,6-disodium dihydroxypyrimidine into the reaction vessel to react, decompressing and recovering phosphorus oxychloride until the material is dry, cooling, adding trichloro ethylene withuniformly stirring, putting into a hydrolysis pan for hydrolyzation, collecting a trichloro ethylene layer after standing and delaminating, followed by a neutralization reaction, controlling pH value, washing, removing a water layer, recovering trichloro ethylene, and releasing crystals to obtain 5-methoxy-4,6-dichloropyrimidine. The preparation method provided by the invention can be used to guarantee the product purity, prolong the service life of equipment, avoid the damage to the environment and human body, reduce emission, and save energy, and accords with foreign pharmacopoeia standard requirements.
Owner:CHANGSHU JINSHEN MEDICAL PROD CO LTD
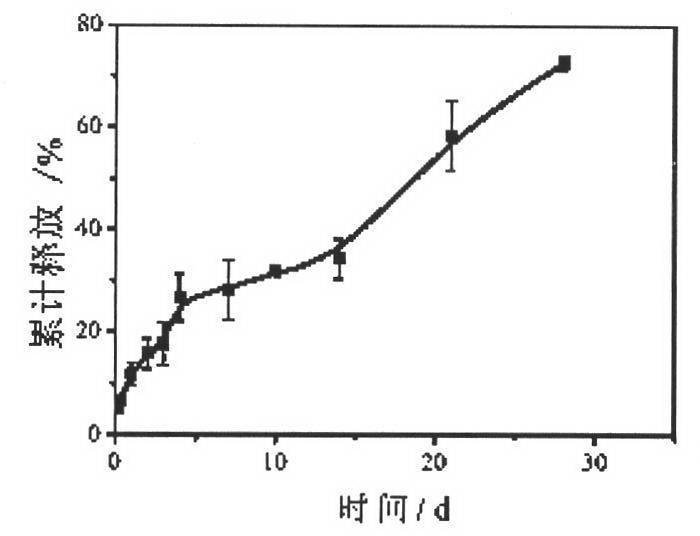
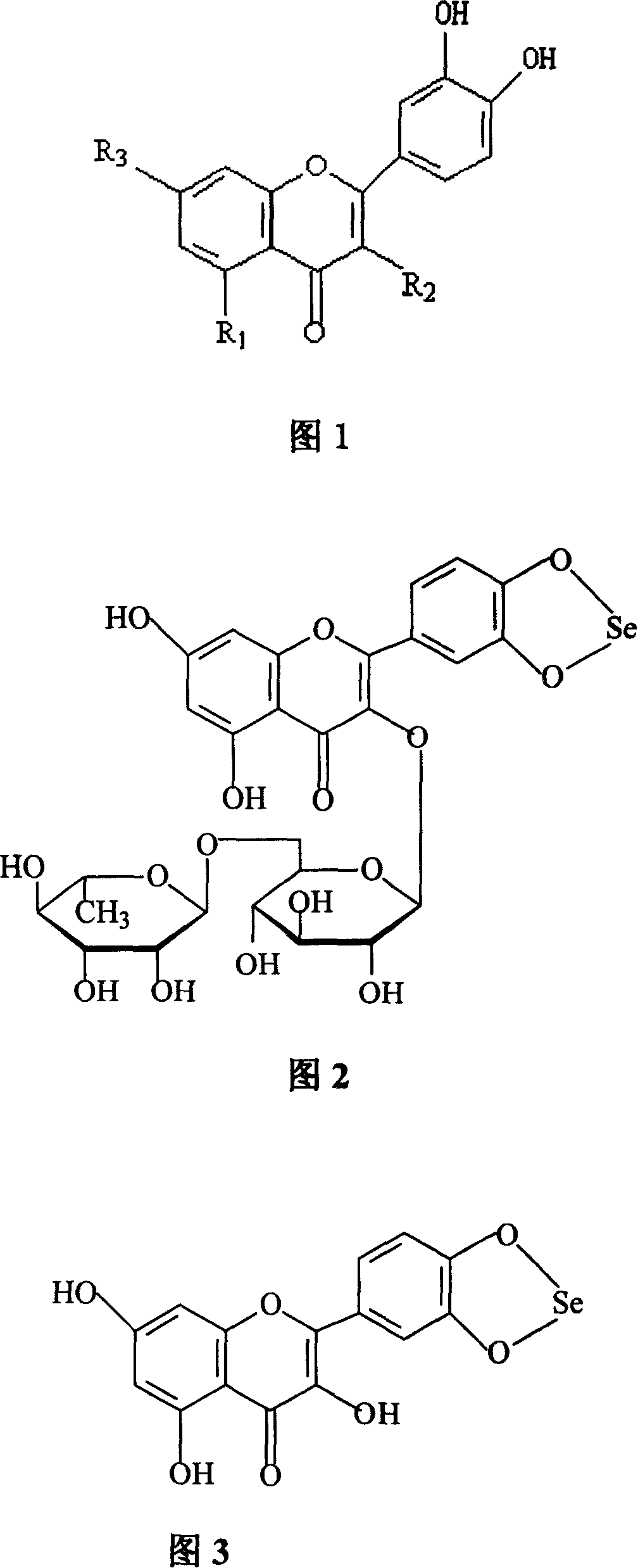
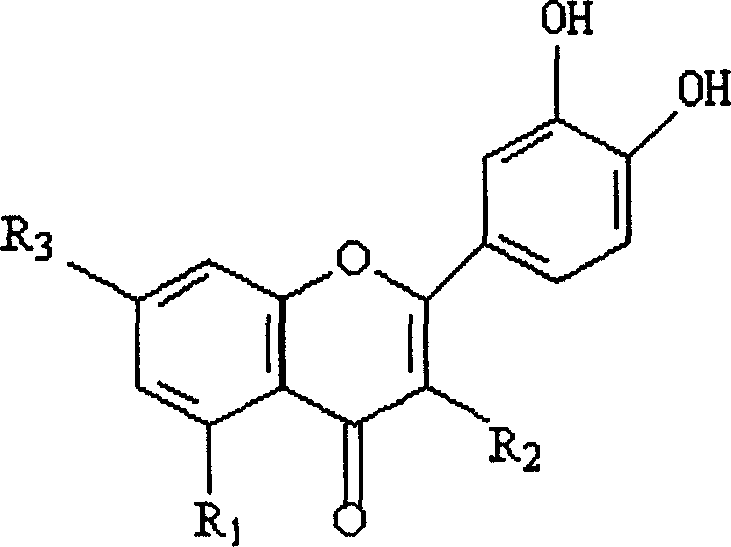
Method for preparing o-dihydroxyflavone-selenium complexes and medical use
The present invention relates to synthesis process and medicinal use of o-dihydroxyflavone-selenium complex. The o-dihydroxyflavone-selenium complex in the structure as shown may be applied in preparing health product, pesticide, plant growth regulator, feed additive, immunomodulator, antitumor medicine, antibacterial medicine, etc.
Owner:WENZHOU MEDICAL UNIV
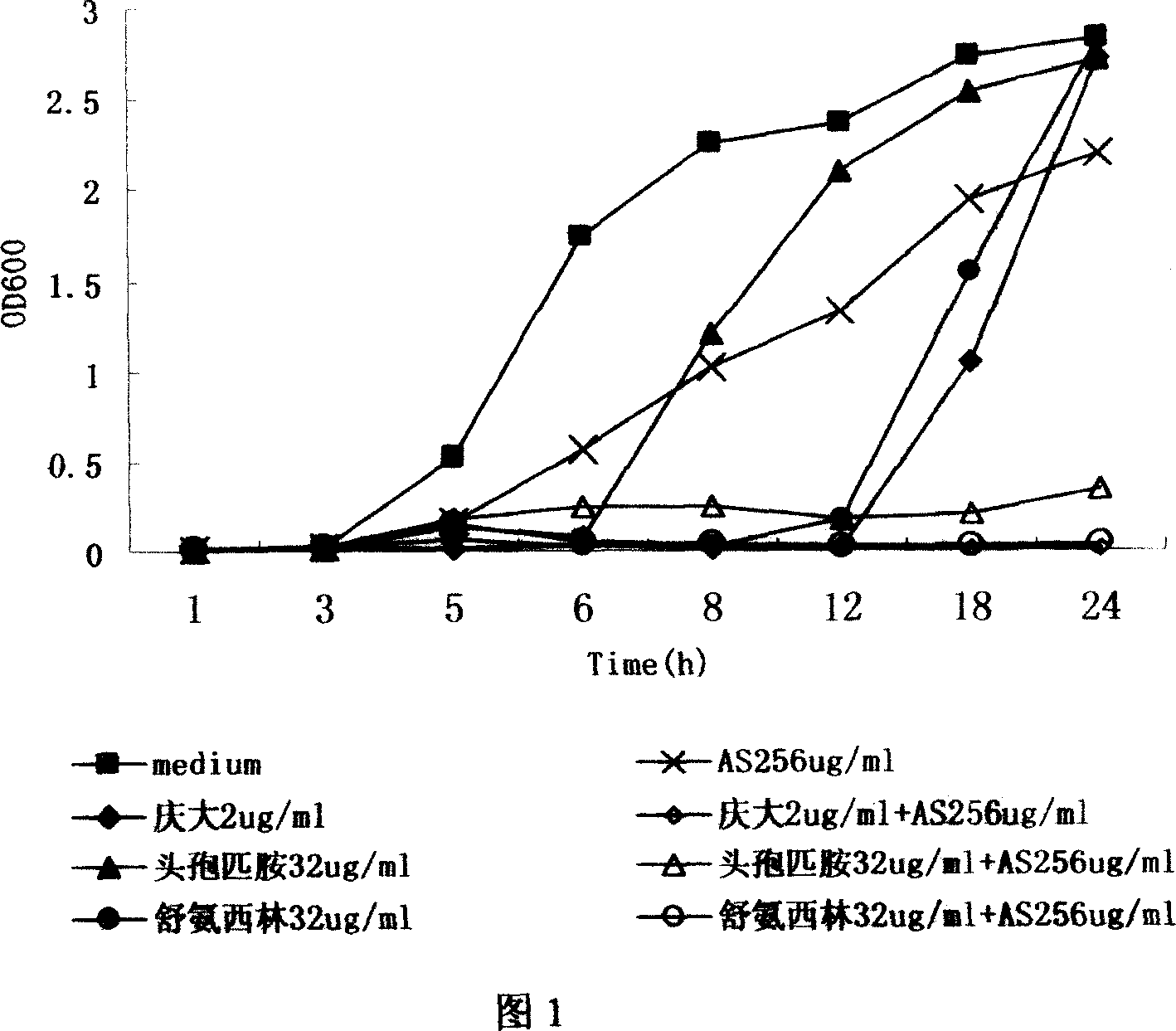
Combined application of artemisinin and its derivative and antibiotic medicine
InactiveCN101020056AIncrease useHigh antibacterial efficacyAntibacterial agentsHeterocyclic compound active ingredientsAntimicrobial drugMedicine
The present invention discloses the new use of available antimalarial arteannuin and its derivatives dihydro arteannuin, antiannuic methyl ether, antiannuic ethyl ether and antiannuic amber. Arteannuin and its derivatives are used through combination with antibiotic medicine to inhibit bacterial growth and enhance the antibiotic effect of available antibiotic medicine. Especially, antiannuic amber is applied in preventing and treating bacterial infection diseases except being used as antimalarial.
Owner:ARMY MEDICAL UNIV
Method and compositions for transdermal administration of antimicrobial medications
InactiveUS20060222692A1Avoid gastrointestinal side effectsReduce the possibilityAntibacterial agentsBiocideSerum concentrationMedicine
The present invention relates to methods and compositions for improved efficacy and delivery of time-dependent antimicrobial drug compositions to a patient. Transdermal dosage forms and methods for steady-state delivery of drug to produce and maintain a serum concentration of drug above the minimum inhibitory concentration or minimum microbicidal concentration are provided.
Owner:FAIRFIELD CLINICAL TRIALS
Preparation method of chimonanthus nitens valid target, production method and use of formulation thereof
ActiveCN101357146AReduce dosageEasy to takeAntibacterial agentsAntimycoticsDiseaseAdditive ingredient
The invention relates to a preparation method of effective fractions of shining wintersweet leaf, a preparation method and a use of a preparation thereof, in particular to an application of the effective fractions of the shining wintersweet leaf and the preparation thereof in drugs used for preventing and treating respiratory system diseases as well as in anti-cold drugs, antimicrobial drugs, anti-inflammation drugs, antiviral drugs, antifungal drugs, etc. In the invention, the volatile effective fractions and non-volatile effective fractions are added to the crude drug of the shining wintersweet medicinal material with a weight ratio of the actual extraction amount of the shining wintersweet to the effective fractions being 1.0-2.6:0.5-3.5, thus the effective fractions of the shining wintersweet leaf are obtained. A preparation of the effective fractions of the shining wintersweet can be made into granules, soft capsules, capsules, drop pills, tablets, compound capsules, dispersible tablets, etc. In the invention, the preparation of the shining wintersweet is deeply developed, which ensures that the preparation is rich in active ingredients, has definite effective ingredients, obvious pharmacological action and significant improvement of drug action. Besides, the preparation has stable performance and controllable quality, is safe and reliable, has less dosage, and is favorable for transportation and storage of the medicine as well as for patients to take.
Owner:江西佑美制药有限公司 +1
Veterinary antibacterial drug composition containing lysozyme and oligosaccharide and application thereof
InactiveCN101934070ALower resistanceBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsDiseaseEscherichia coli
The invention relates to a veterinary antibacterial drug composition containing lysozyme and oligosaccharide and an application thereof. The composition can be prepared into powder, granules, premixes, suspensions and oral solutions by mixing a pharmaceutically acceptable drug carrier with the main drugs of the lysozyme, the oligosaccharide and an antibacterial drug, as well as the auxiliary ingredients of biotin, phytic acid, trehalose, glycine and the like which are used for coordination, according to a conventional preparation method. The composition not only can enhance the self immunity of animals, equalize the absorption of amino acids and mineral substances in vivo and broaden the antimicrobial spectrum, but also has high efficiency, safety and no toxic and side effect and is not easy to lead to drug tolerance and drug resistance. The composition is used for preventing and treating intestinal diseases and respiratory diseases, such as colibacillosis, salmonellosis, pasteurella multocida, staphylococosis and the like, of livestock and poultry. Meanwhile, the developed drugs can be used for effectively substituting the traditional veterinary antibiotic drugs which are easy to lead to drug tolerance.
Owner:TIANJIN RINGPU BIO TECH
Dihydroisoquinoline compounds and application of dihydroisoquinoline compounds for preparing antibacterial agents for plants
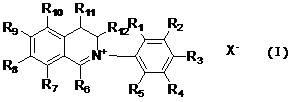
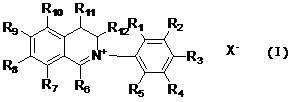
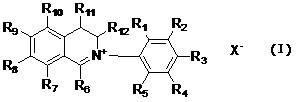
InactiveCN102603629AExcellent antibacterial activityExcellent antibacterialBiocideOrganic chemistryCarboxyl radicalSulfate radicals
The invention relates to dihydroisoquinoline compounds and an application of the dihydroisoquinoline compounds for preparing antibacterial agents for plants. The dihydroisoquinoline compounds have remarkable bacteriostatic activity on various phytopathogens. The dihydroisoquinoline compounds disclosed by the invention have the molecular structure characteristics shown as follows, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R10, R11, and R12 are same or different groups, such as hydrogen, alkyl, cycloalkyl, alkenyl, alkinyl, unsaturated monocycloalkyl, alkoxyl, halogen, hydroxyl, nitryl, cyano, trifluoromethyl, heterocyclic substituent, carboxyl, ester group, acylamino, acyl or aldehyde group; and X<-> is sulfate radical, halogen anion, carbonate radical, bicarbonate radical, phosphate radical, hydrophosphate radical, acid radical of fatty acid, sulfonate radical or tetraphenylborate radical.
Owner:NORTHWEST A & F UNIV
Selective antibacterials for clostridium difficile infections
ActiveUS8796292B2Reduce the possibilityHigh activityAntibacterial agentsBiocideDiseaseClostridium difficile infections
The invention features compounds of formula (I): The compounds are useful as antibacterial agents, especially again Clostridium difficile-associated diseases.
Owner:ACURX PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com