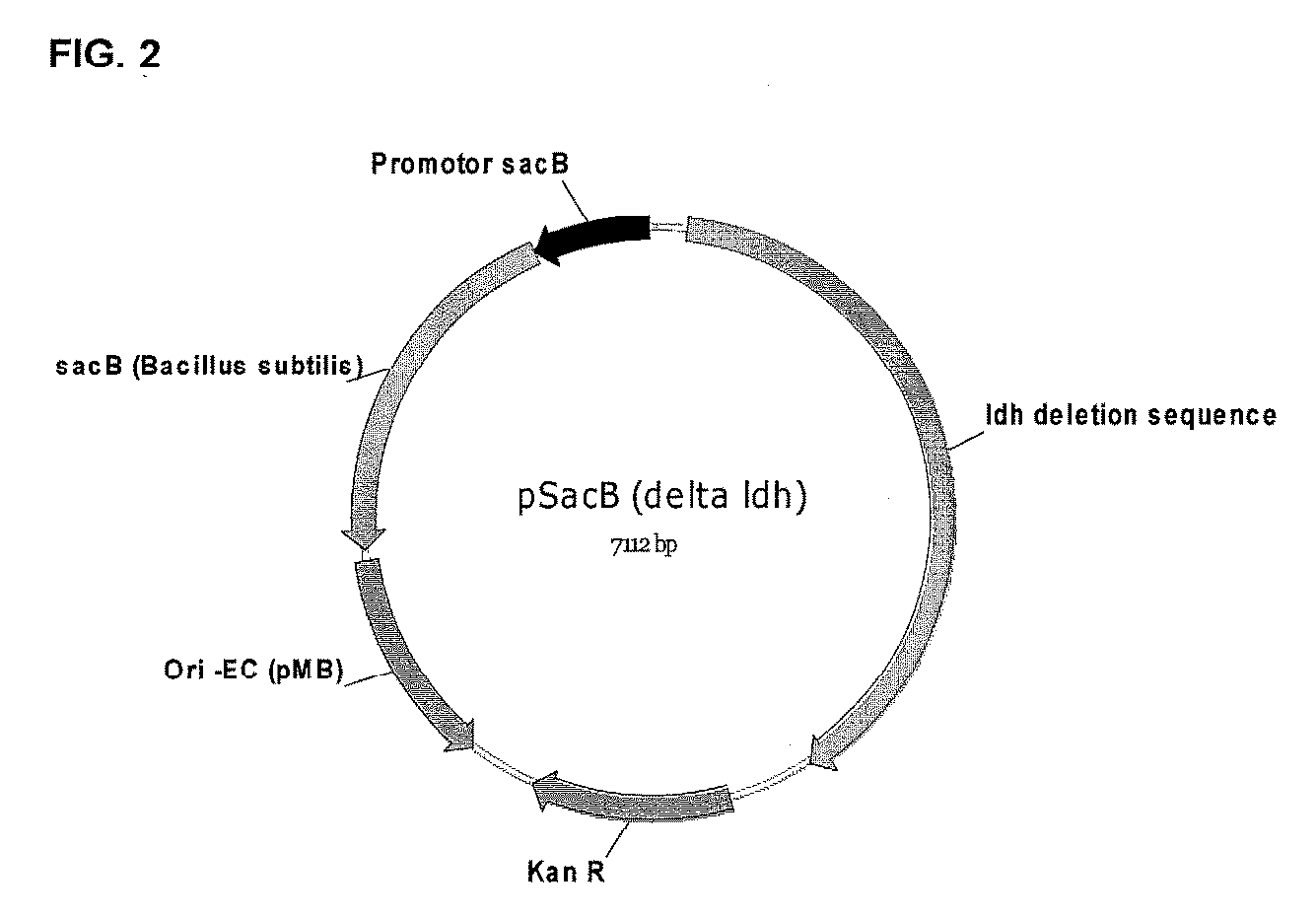
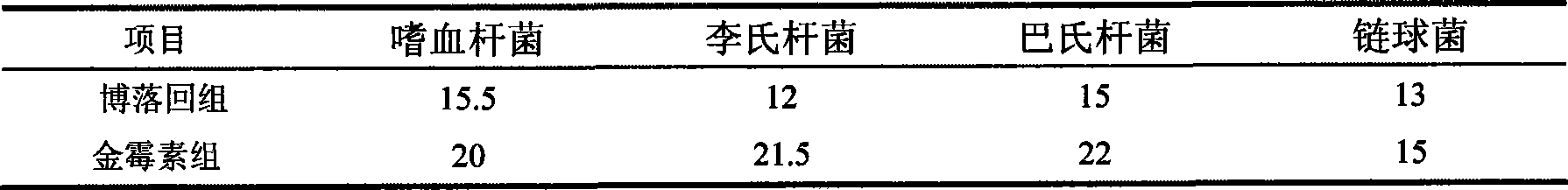
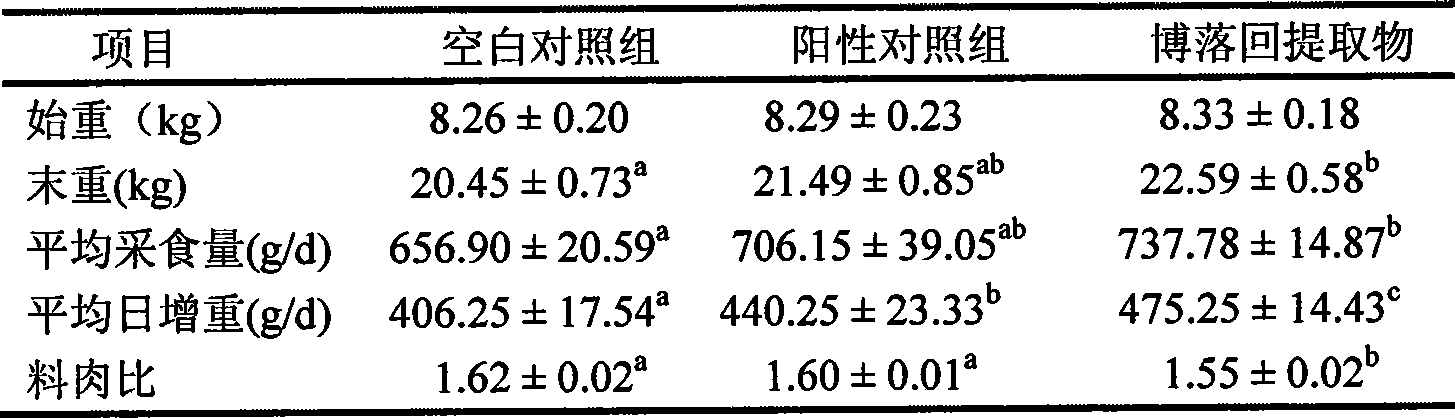
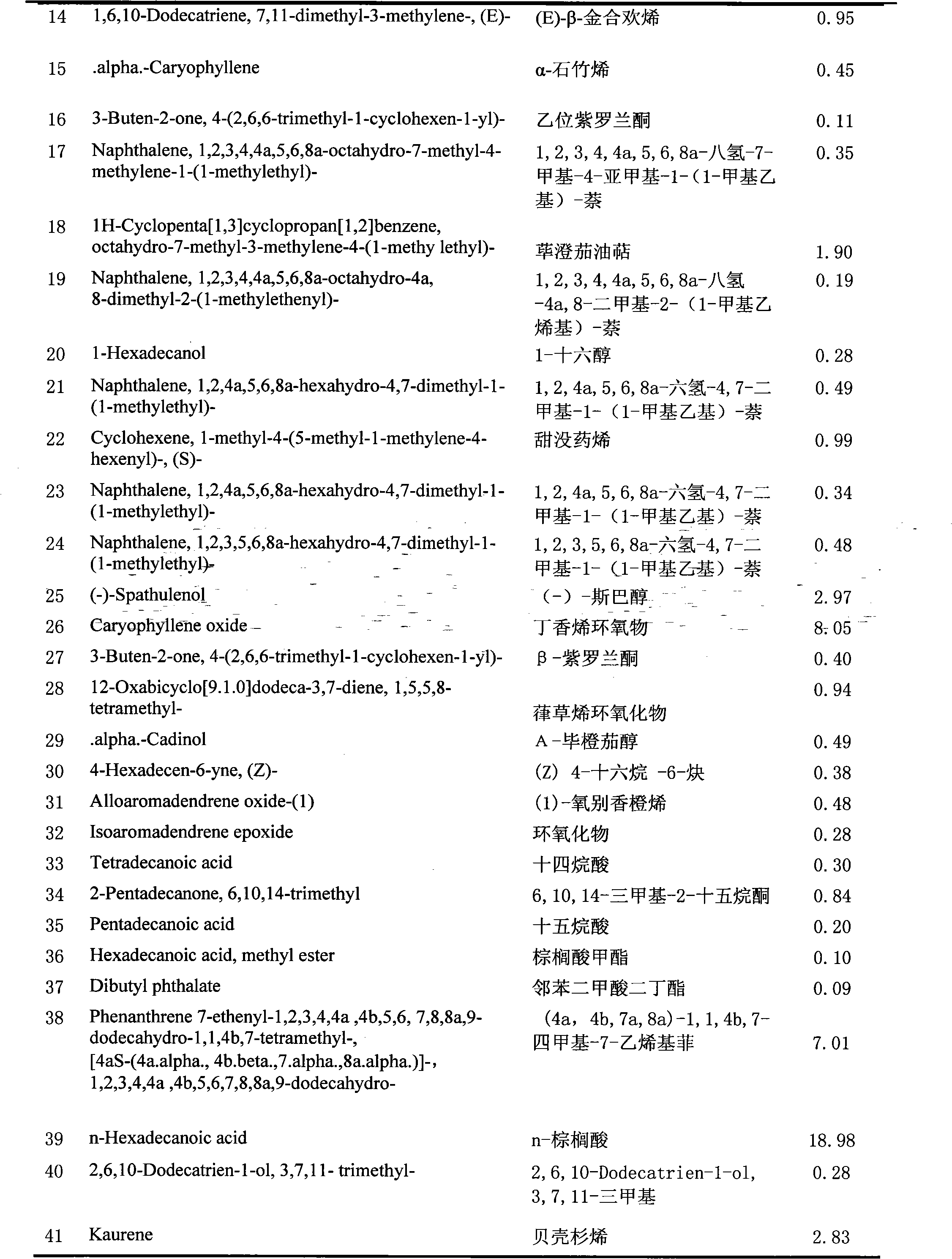Patents
Literature
135 results about "Pasteurella" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pasteurella is a genus of Gram-negative, facultatively anaerobic bacteria. Pasteurella species are nonmotile and pleomorphic, and often exhibit bipolar staining ("safety pin" appearance). Most species are catalase- and oxidase-positive. The genus is named after the French chemist and microbiologist, Louis Pasteur, who first identified the bacteria now known as Pasteurella multocida as the agent of chicken cholera.
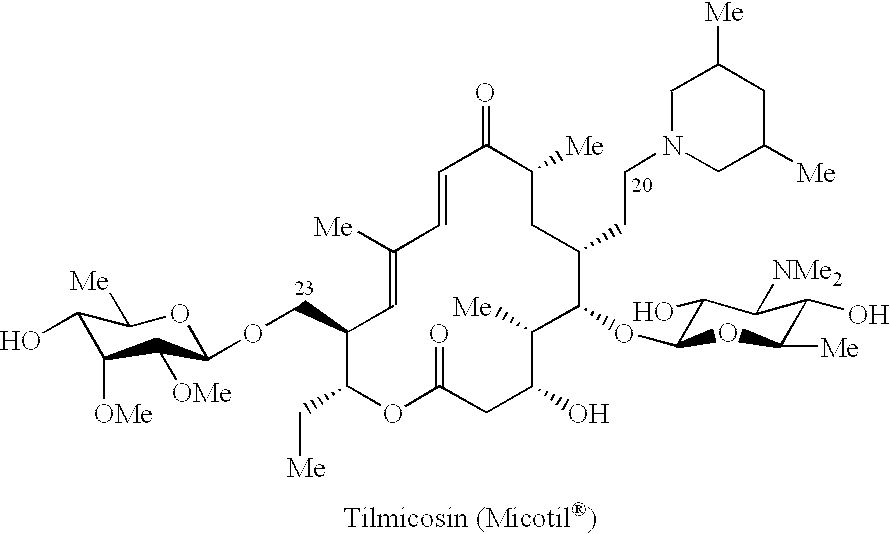
Macrolide antibiotics and treatment and prophylaxis of pasteurellosis using the same
ActiveUS6514946B1High antibacterial activityReduced activityAntibacterial agentsBiocideTreatment fieldAntibacterial activity
20,23-disubstituted mycaminosyltylonolide derivatives and use of the same in the field of the prophylaxis and treatment of pasteurellosis are disclosed. The di-substituents are peperidino optionally substituted with one or two methyl groups. The derivatives have selective antibacterial activity against Pasteurella.
Owner:ZH BISEIBUTSU KAGAKU KENYKU KAI
Production of defined monodisperse heparosan polymers and unnatural polymers with polysaccharide synthases
InactiveUS20080109236A1Surgical adhesivesPharmaceutical delivery mechanismPolysaccharide synthesisSugar
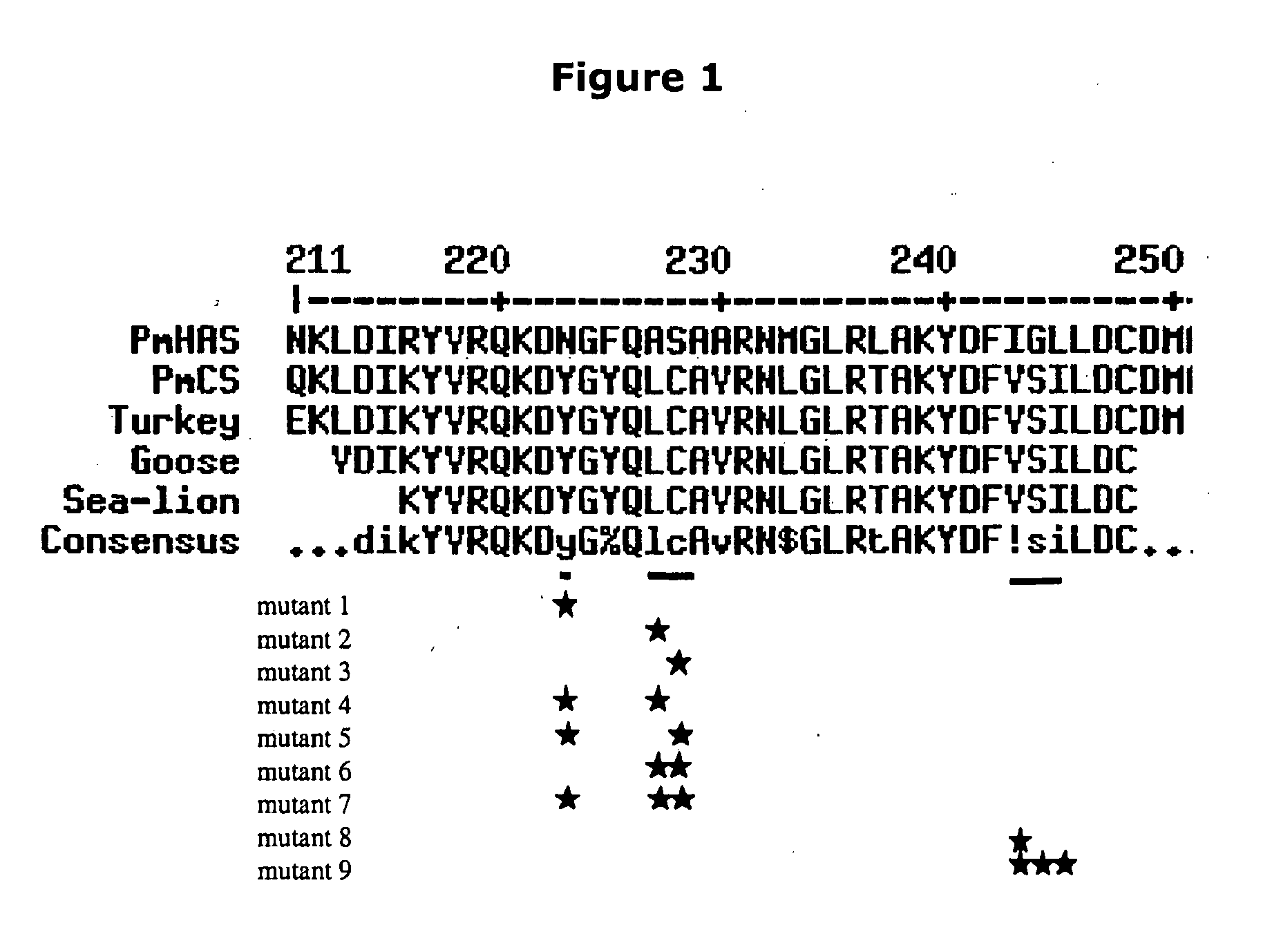
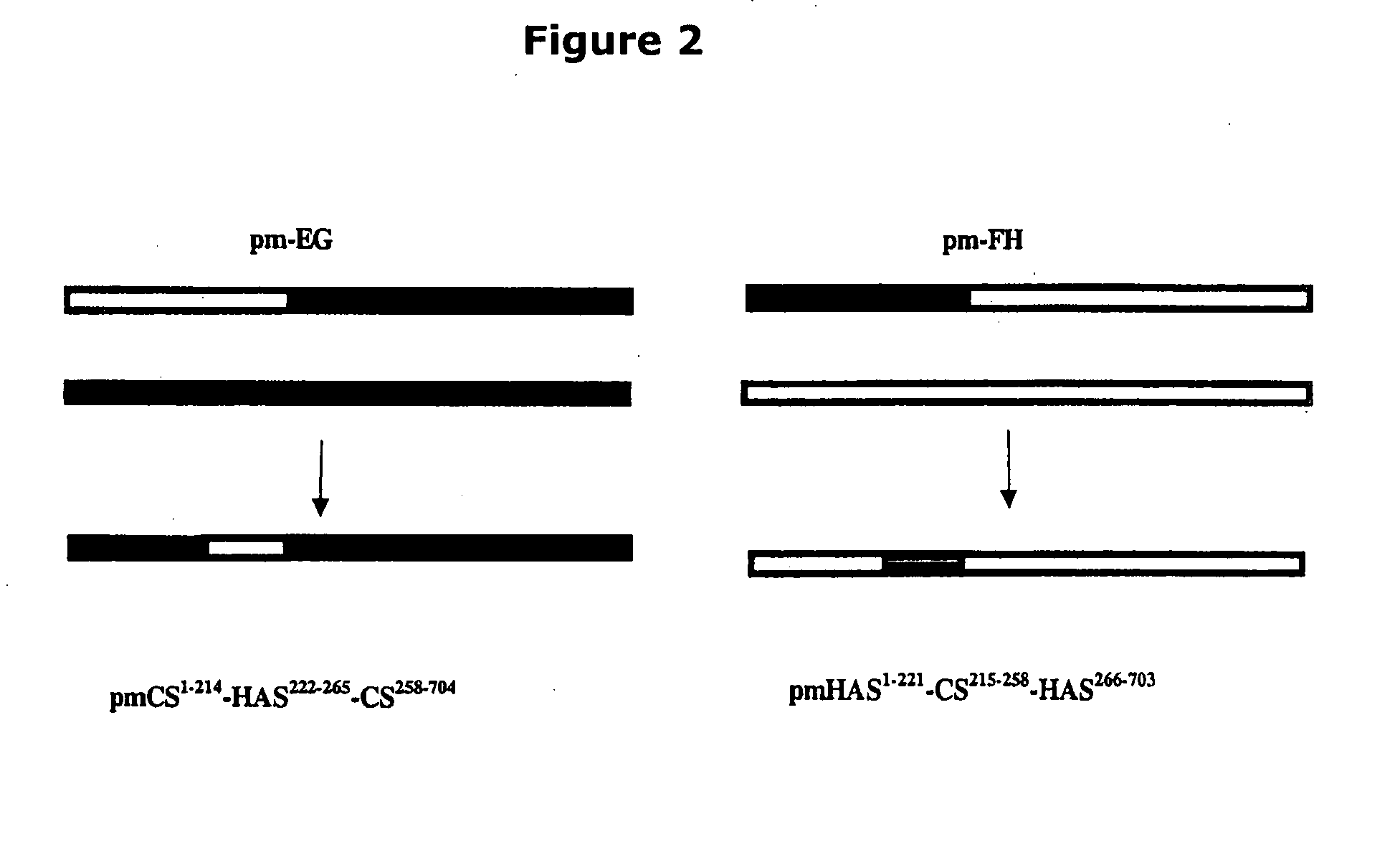
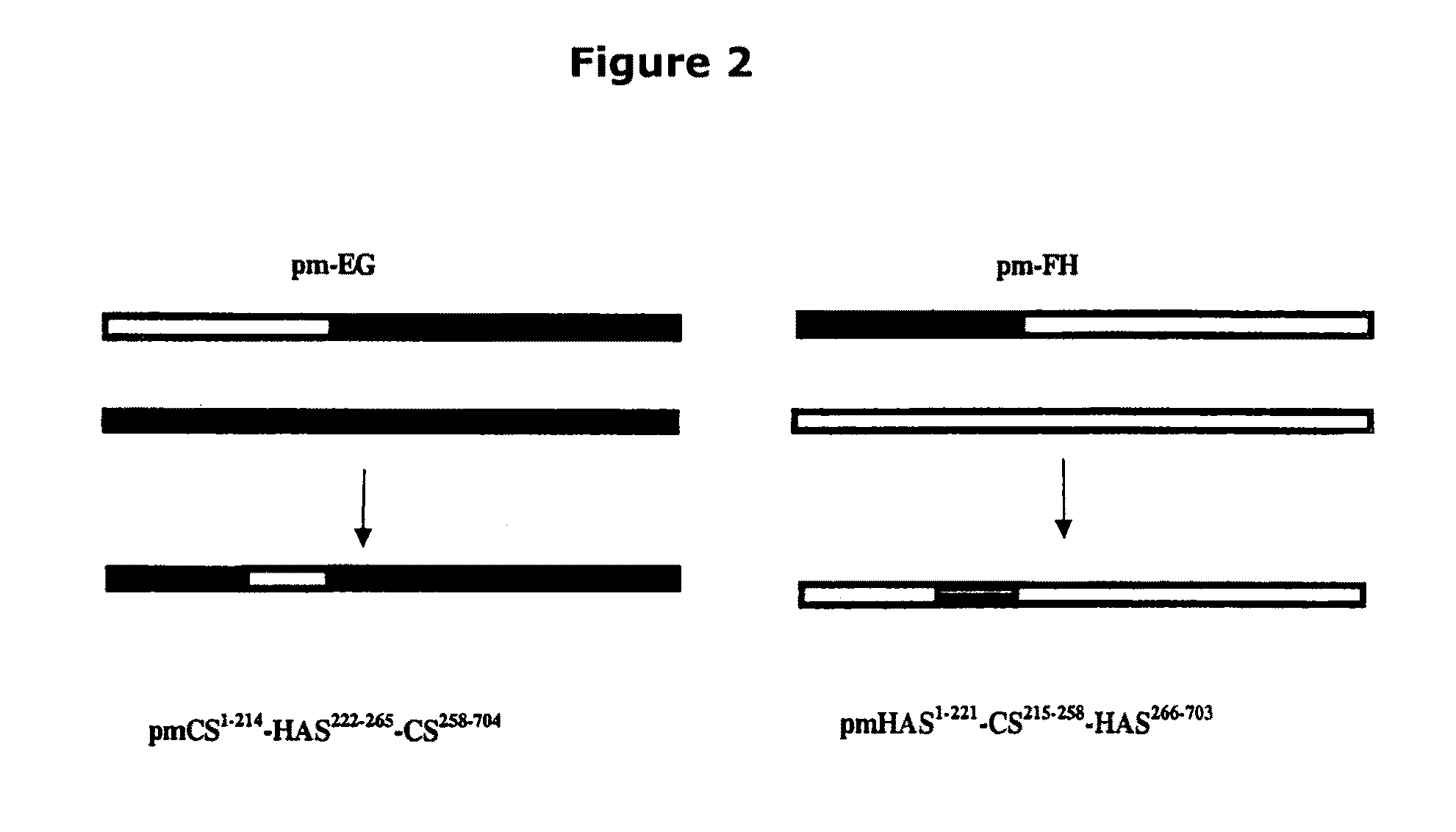
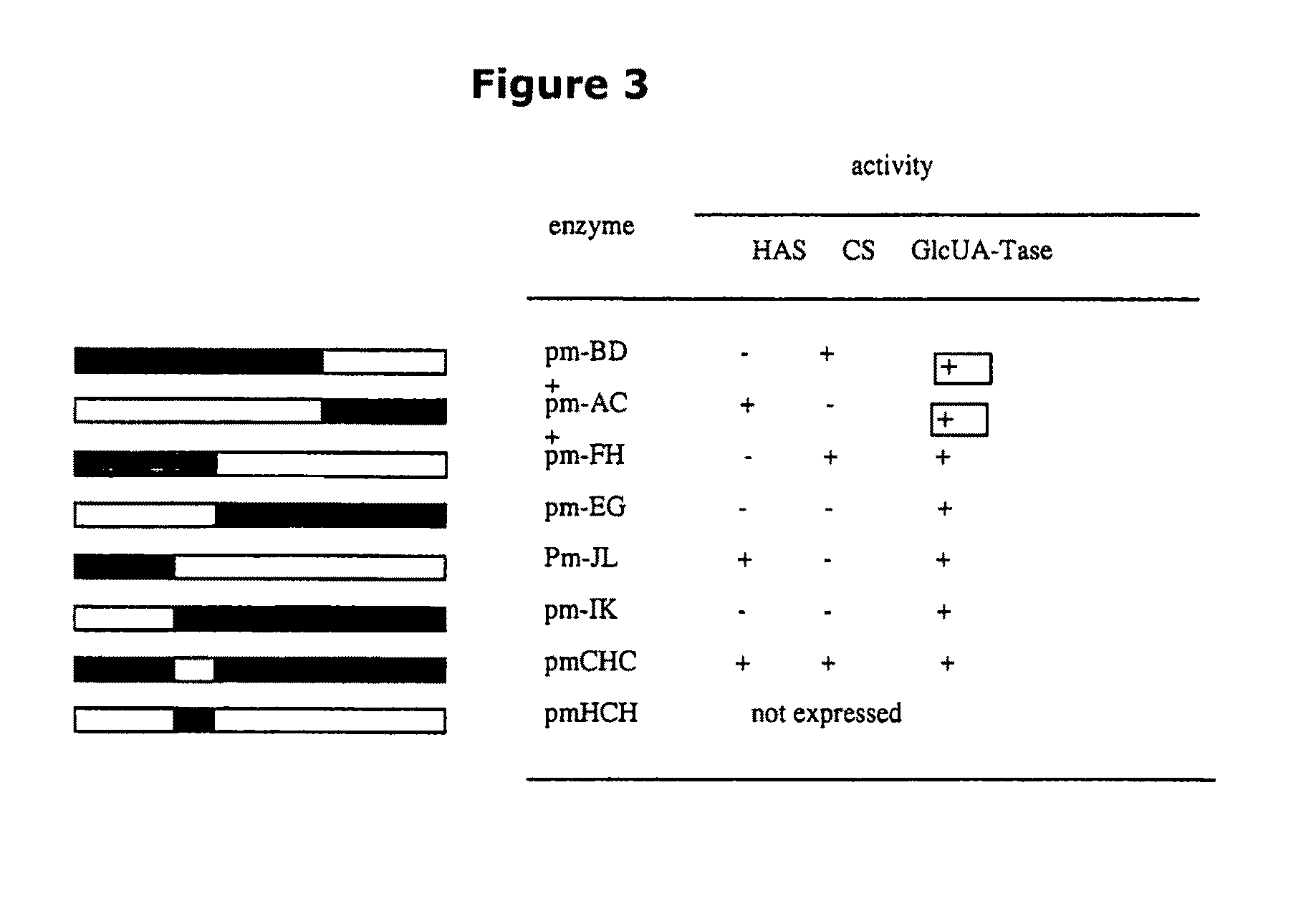
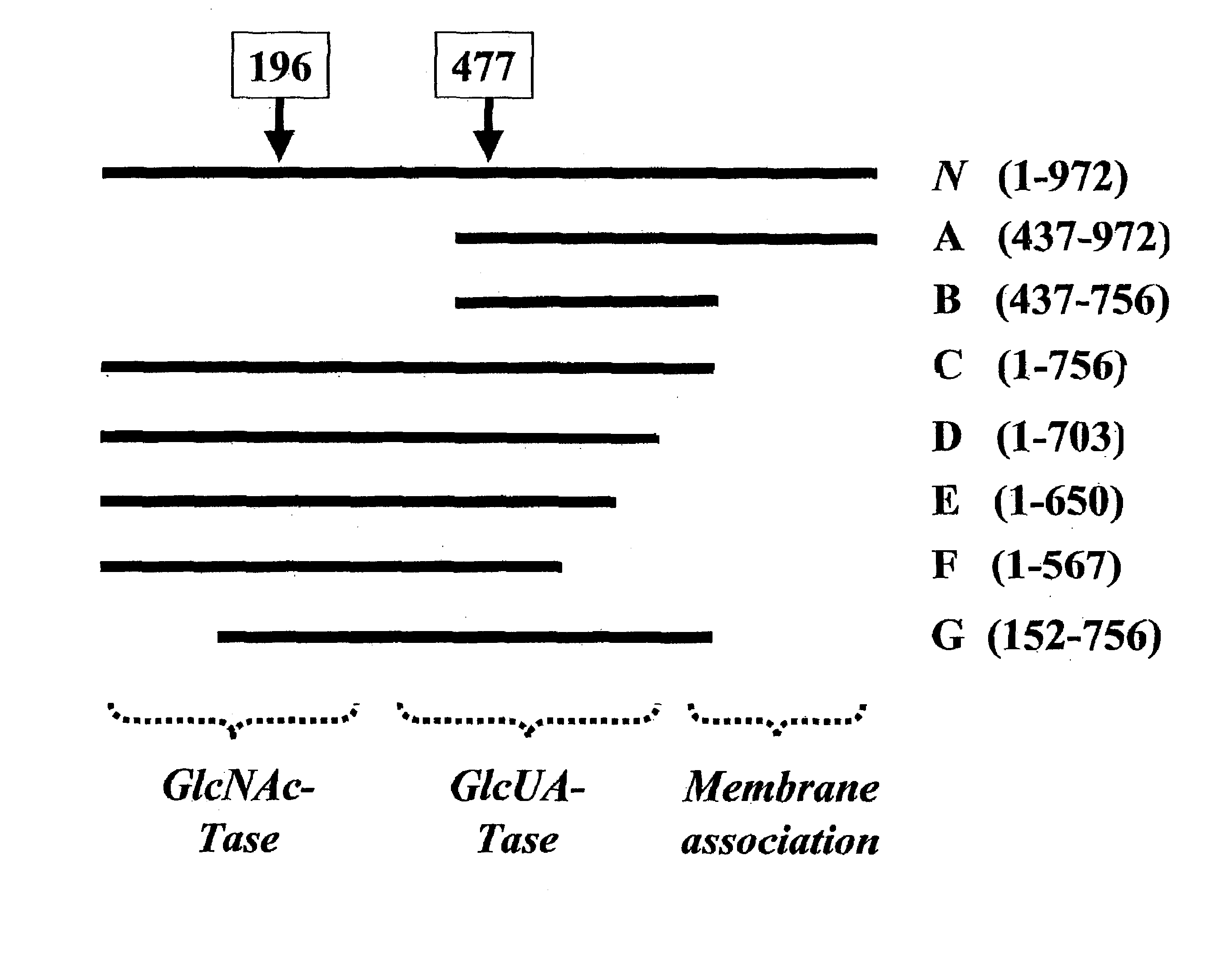
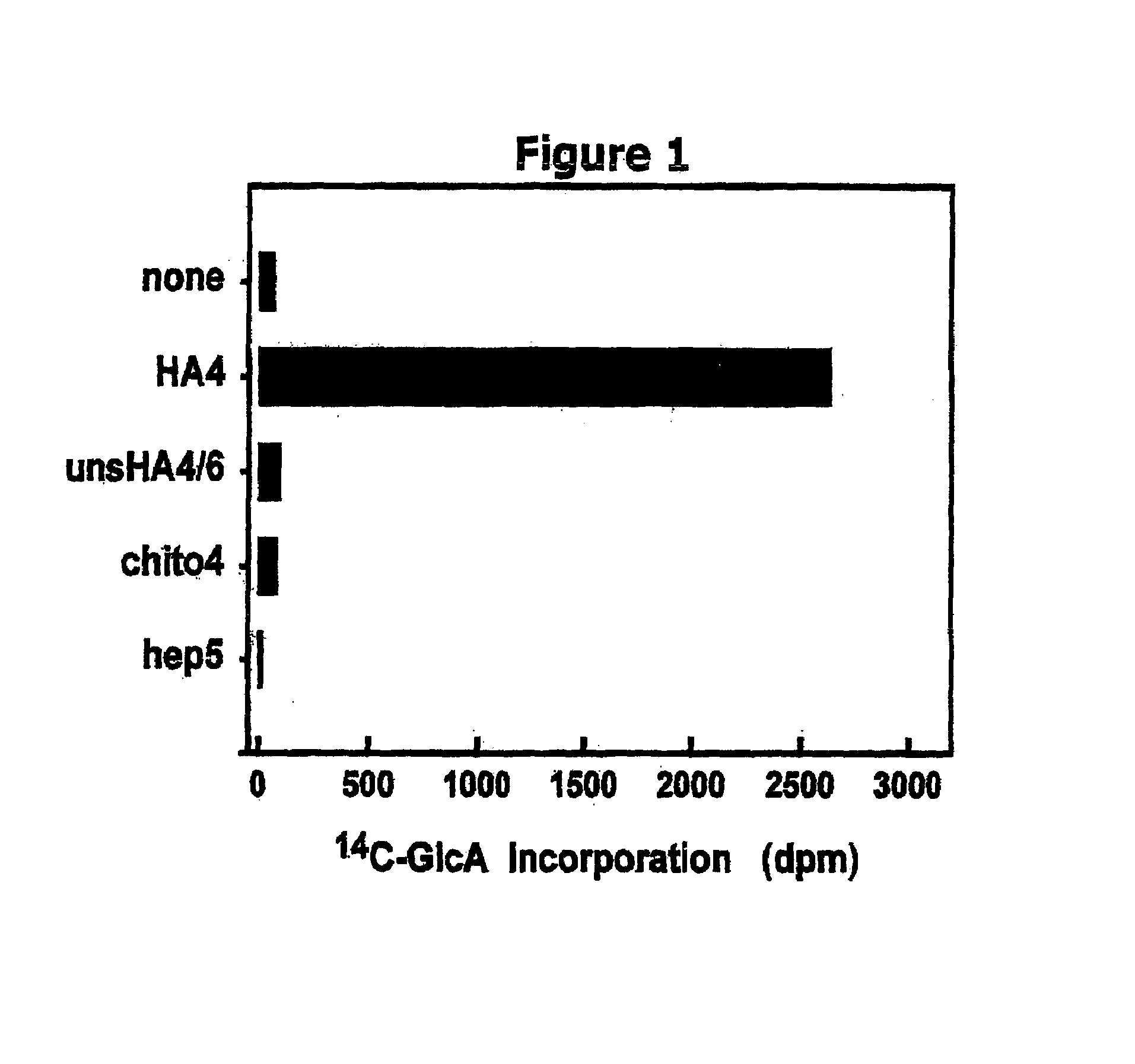
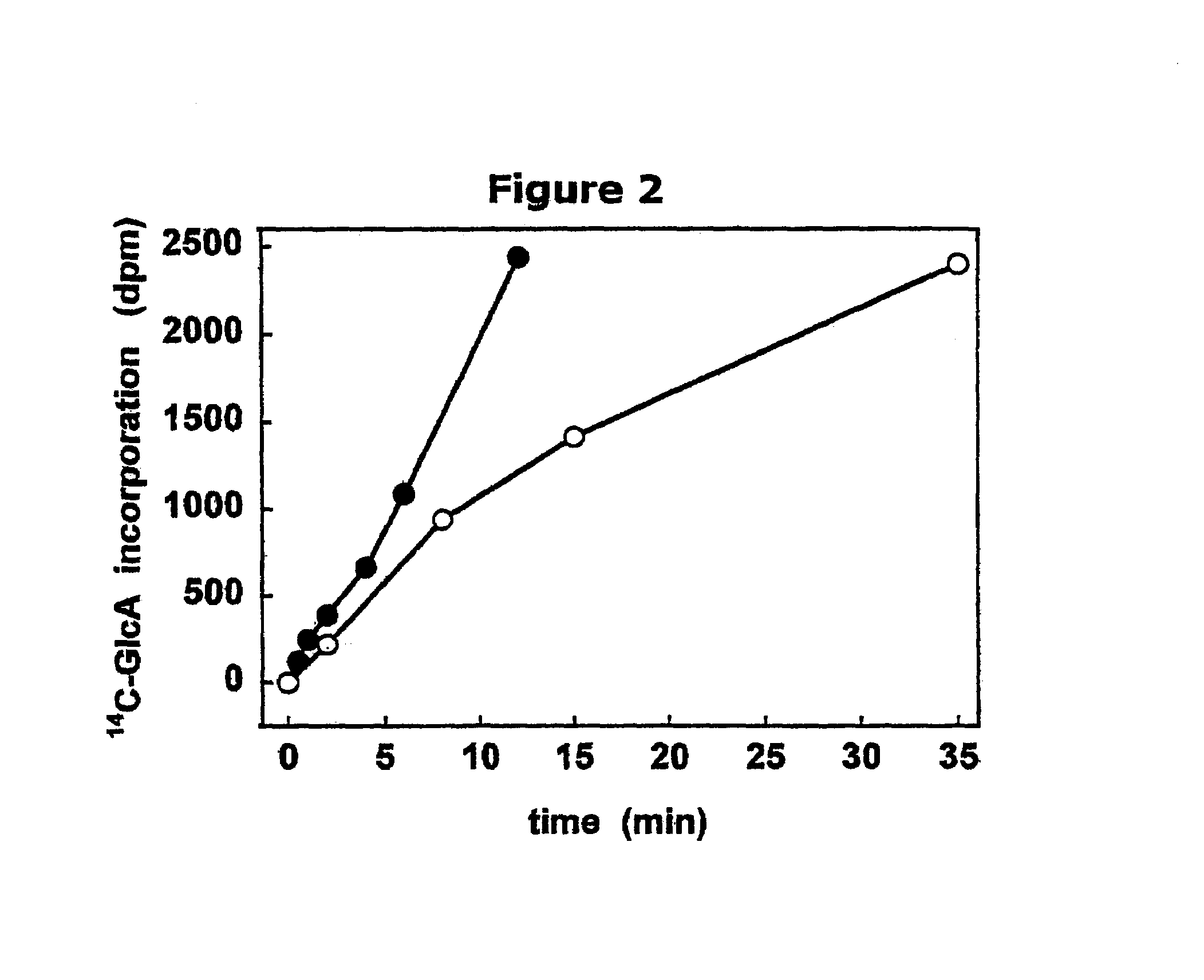
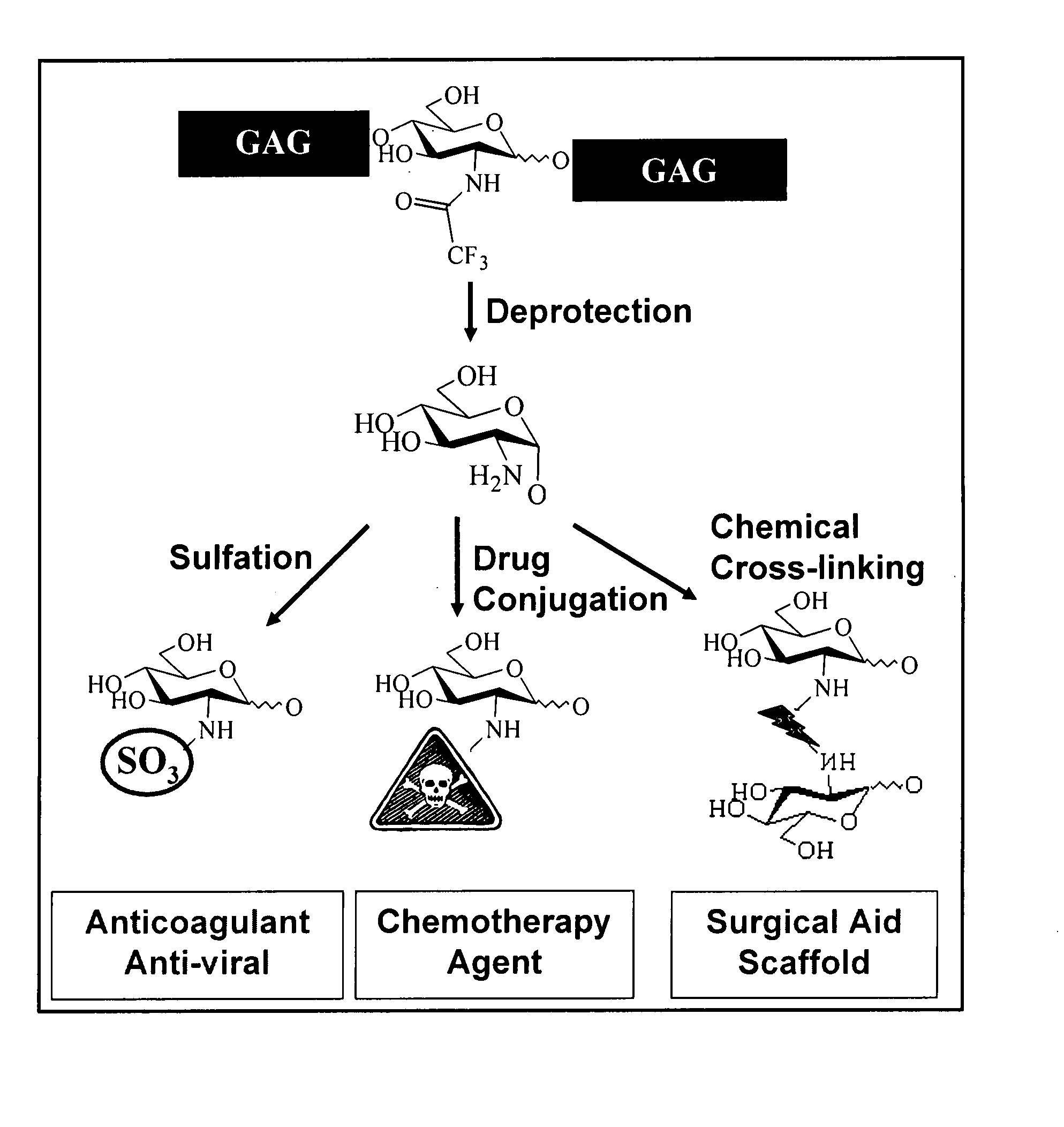
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size. The present invention also relates to methodology for polymer grafting by a polysaccharide synthase to form glycosaminoglycan polymers having an unnatural structure.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Chinese medicinal herb additive for meat chickens and application
InactiveCN101543261AImprove immunityFormulation ScienceFood processingAnimal feeding stuffCoccidiosisEscherichia coli
The invention discloses a Chinese medicinal herb additive for meat chickens and application. The Chinese medicinal herb additive comprises heterophylly falsestarwort root, nutgrass galingale rhizome, amur corktree bark, coptis root, medicated leaven, sanguisorba, hawthorn, Indian Buead, officinal magnolia bark, largehead atractylodes rhizome, tangerine peel, malt, indigowoad root, swordlike atractylodes rhizome, indigowoad leaf, cyrtomium fortune, chatoyancy and calamus; and mixture of the compositions is added with calcium bicarbonate, phytase, mulitivitamin for birds and lysine. The Chinese medicinal herb additive effectively promotes absorption of nutrient, can resist bacteria and virus, strengthen immunity of the meat chickens, effectively prevents bird flu, has obvious curative effect on diseases having respiratory tract symptoms as the major symptom, and has inhibiting function on familiar newcastle disease, colon bacillus, Pasteurella, enteritis, diarrhea, feather pecking, hair shedding, anus pecking, coccidiosis and the like. The additive cannot restrict the growth and disease resistance of the meat chickens, the meat quality of the meat chickens fed by the additive is obviously better than that of the meat chickens fed by the prior feed additive, the average daily gain of the meat chickens is improved by 23 to 28 percent than that of the meat chickens fed by the prior feed additive, the cultivation cycle is obviously shortened, and no harmful substance is deposited.
Owner:CHONGQING KAIZHOU JIUDING ANIMAL HUSBANDRY SCI & TECH DEV
Bacterial cells having a glyoxylate shunt for the manufacture of succinic acid
The present invention is concerned with bacteria for the production of succinic acid. Specifically, the invention relates to a bacterial cell of the genus Pasteurella comprising a heterologous polypeptide having isocitrate lyase activity and a heterologous polypeptide having malate synthase activity. Further, the present invention contemplates a polynucleotide comprising a nucleic acid encoding a polypeptide having isocitrate lyase activity and a nucleic acid encoding a polypeptide having malate synthase activity. Finally, the present invention relates to the use of a bacterial cell of the invention for the manufacture of succinic acid.
Owner:BASF AG
Application of pink plumepoppy herb extract in veterinary drugs of economic animals
ActiveCN101530475APromote growthImprove conversion rateAntibacterial agentsAnimal feeding stuffFeed conversion ratioFeed additive
The invention relates to an application of pink plumepoppy herb extract in veterinary drugs of economic animals; the pink plumepoppy herb extract is used for preparing medical feed additives of the veterinary drugs which are used for enhancing the growth of the economic animals and increasing conversion rate of the feed or veterinary drugs which has better inhibiting action and disinfecting action to common pathogenic bacteria in livestock and poultry, such as listeria monocytogenes, haemophilus and pasteurella.
Owner:MICOLTA BIORESOURCE INC LTD
Production of defined monodisperse heparosan polymers and unnatural polymers with polysaccharide synthases
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size. The present invention also relates to methodology for polymer grafting by a polysaccharide synthase to form glycosaminoglycan polymers having an unnatural structure.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Application of macleaya cordata extractive in veterinary drugs for economic animals
ActiveCN101972309APromote growthImprove conversion rateAntibacterial agentsDigestive systemPoultry diseaseHaemophilus
The invention relates to the application of a macleaya cordata extractive in veterinary drugs for economic animals, in particular to the application of the macleaya cordata extractive in the preparation of veterinary drugs with favorable inhibition effect and killing effect on common livestock and poultry diseases, such as protoblem listeria monocytogenes, haemophilus and pasteurella.
Owner:MICOLTA BIORESOURCE INC LTD
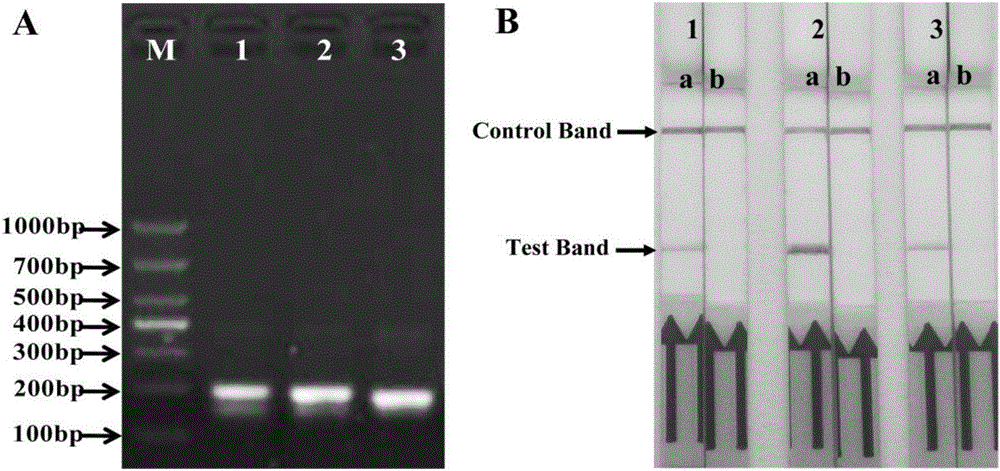
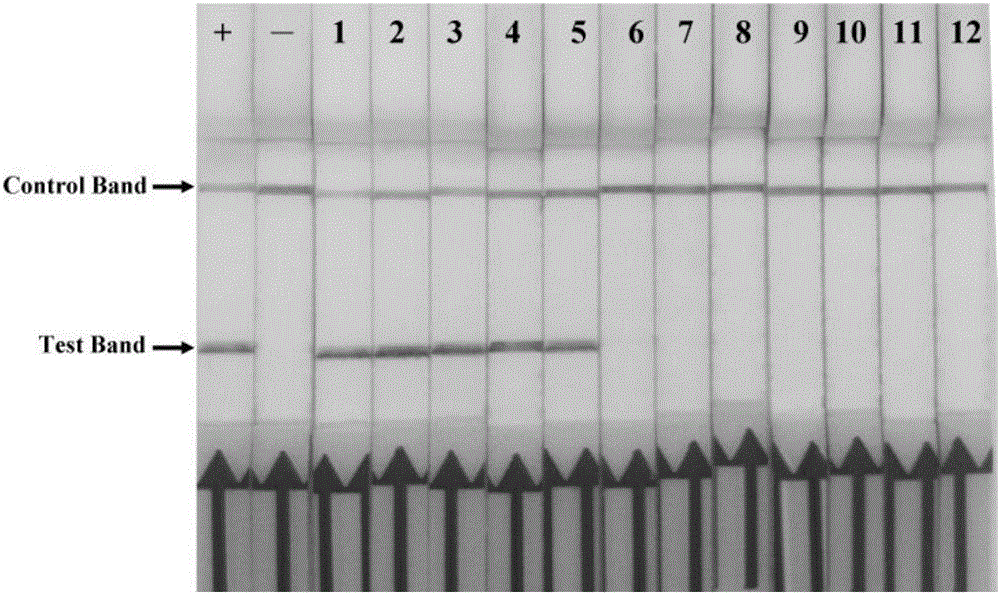
Primer, probe and kit for rapidly detecting pasteurella mutocida on site
InactiveCN106811541AExcellent detection timeNo cross reactionMicrobiological testing/measurementMicroorganism based processesForward primerCrude lysate
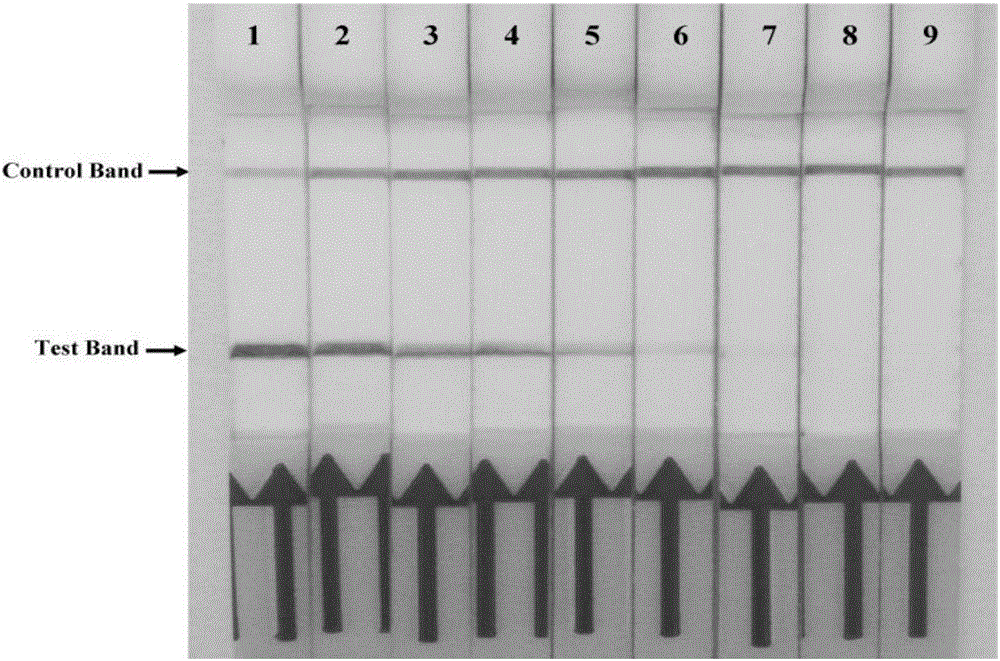
The invention discloses a combination of primer and probe for rapidly detecting pasteurella multocida on site by RPA-LFD. A forward primer sequence is shown in SEQ ID No. 1; a reverse primer sequence is shown in SEQ ID No. 2; a probe sequence is shown in SEQ ID No. 3. The invention also discloses a kit for detecting pasteurella multocida. The pasteurella multocida RPA-nfo detection combination of primer and probe and kit have high sensitivity and strong specificity, at least can detect six copied / reacted Pasteurella multocida DNAs, and can perform sensitive, specific and rapid detection of Pasteurella multocida DNA on crude lysate of a sample to be detected within 25 min by means of a constant-temperature water bath kettle or human armpit temperature without special instrument and equipment, so as to be suitable for the diagnosis of the pasteurella mutocida disease on site or base.
Owner:SHANDONG NORMAL UNIV
Targeted glycosaminoglycan polymers by polymer grafting and methods of making and using same
InactiveUS7223571B2Narrow size distributionReduce immunoreactivityCell receptors/surface-antigens/surface-determinantsSurgical adhesivesPolymer scienceSugar
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Soluble and stable tilmicosin composition
ActiveCN101496811AStable in natureSolve the problem of water solubilityAntibacterial agentsOrganic active ingredientsEscherichia coliDisease
The invention relates to a soluble and stable Tilmicosin composition. The soluble preparation consists of the following components: Tilmicosin, a latent solvent, a pH value stabilizing agent and proper auxiliary materials. The Tilmicosin obtained by the invention can be quickly dissolved into water and the effective components cannot be degraded with the prolonging of the placing time of an aqueous solution. The preparation composition can be combined with other substances for use so as to strengthen the drug effect. The composition is mainly used for treating respiratory tract infection, mixed infection of mycoplasma and Escherichia coli, other various pneumonia and pulmonary infectious diseases of livestock and poultry caused by Pasteurella, the mycoplasma, and the like.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Targeted glycosaminoglycan polymers by polymer grafting and methods of making and using the same
InactiveUS20070128703A1Firmly attachedEasy to addBiocideOrganic active ingredientsPolymer scienceSugar
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
Targeted glycosaminoglycan polymers by polymer grafting and methods of making and using same
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size. The present invention also relates to methodology for polymer grafting by a polysachharide synthase to form glycosaminoglycan polymers having an unnatural structure.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Complex microbial inoculant for controlling root-knot nematodes of crops and preparation method of complex microbial inoculant
ActiveCN110577916AGood control effectHigh activityBiocideFungiVerticillium speciesBacillus thuringiensis
The invention relates to the field of biologically-derived insecticidal preparations, in particular to a complex microbial inoculant for controlling root-knot nematodes of crops and a preparation method of the complex microbial inoculant. The complex microbial inoculant for controlling the root-knot nematodes of the crops and the preparation method of the complex microbial inoculant are characterized in that microorganism is composed of bacillus subtilis, bacillus sphaericus, pseudomonas fluorescens, bacillus thuringiensis, brevibacillus laterosporus, paenibacillus polymyxa, pasteurella pneumoniae, paecilomyces lilacinus, verticillium chalamydosporium, beauveria bassiana and aspergillus fumigatus. The novel multi-bacteria same-pot fermentation technology is adopted, the formula is clever and reasonable, the disadvantages of mutual antagonism in the liquid deep fermentation culture of compound bacteria are eliminated, the compound bacteria has high activity, and the complex microbial inoculant for controlling the root-knot nematodes of the crops has a good control effect on tomatoes and cucumber root-knot nematodes.
Owner:四川郎三实业发展有限公司
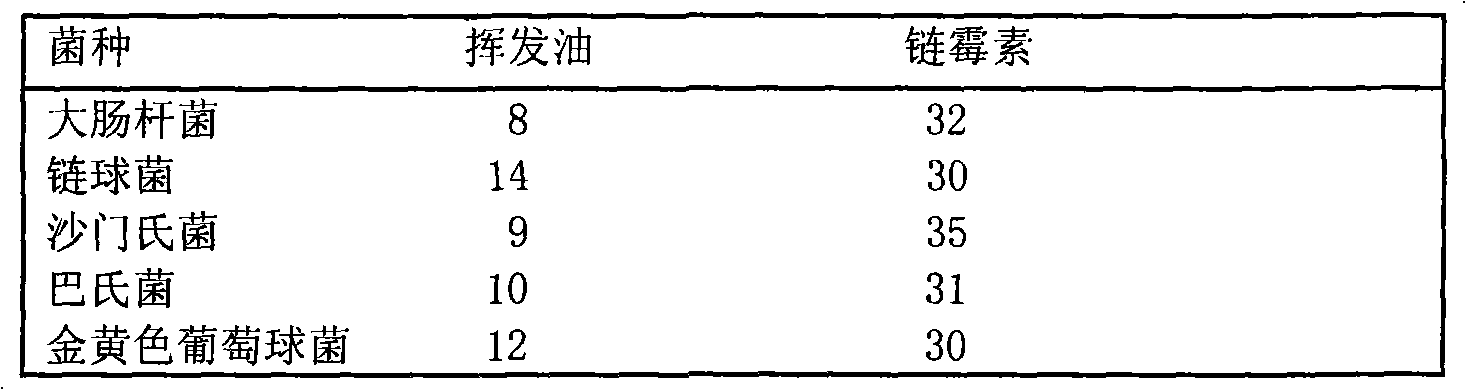
Separation and identification of syringa amurensis rupr. bark volatile oil components and novel application
InactiveCN101612175ADifferent degrees of inhibitionInhibition hasAntibacterial agentsComponent separationEscherichia coliGas phase
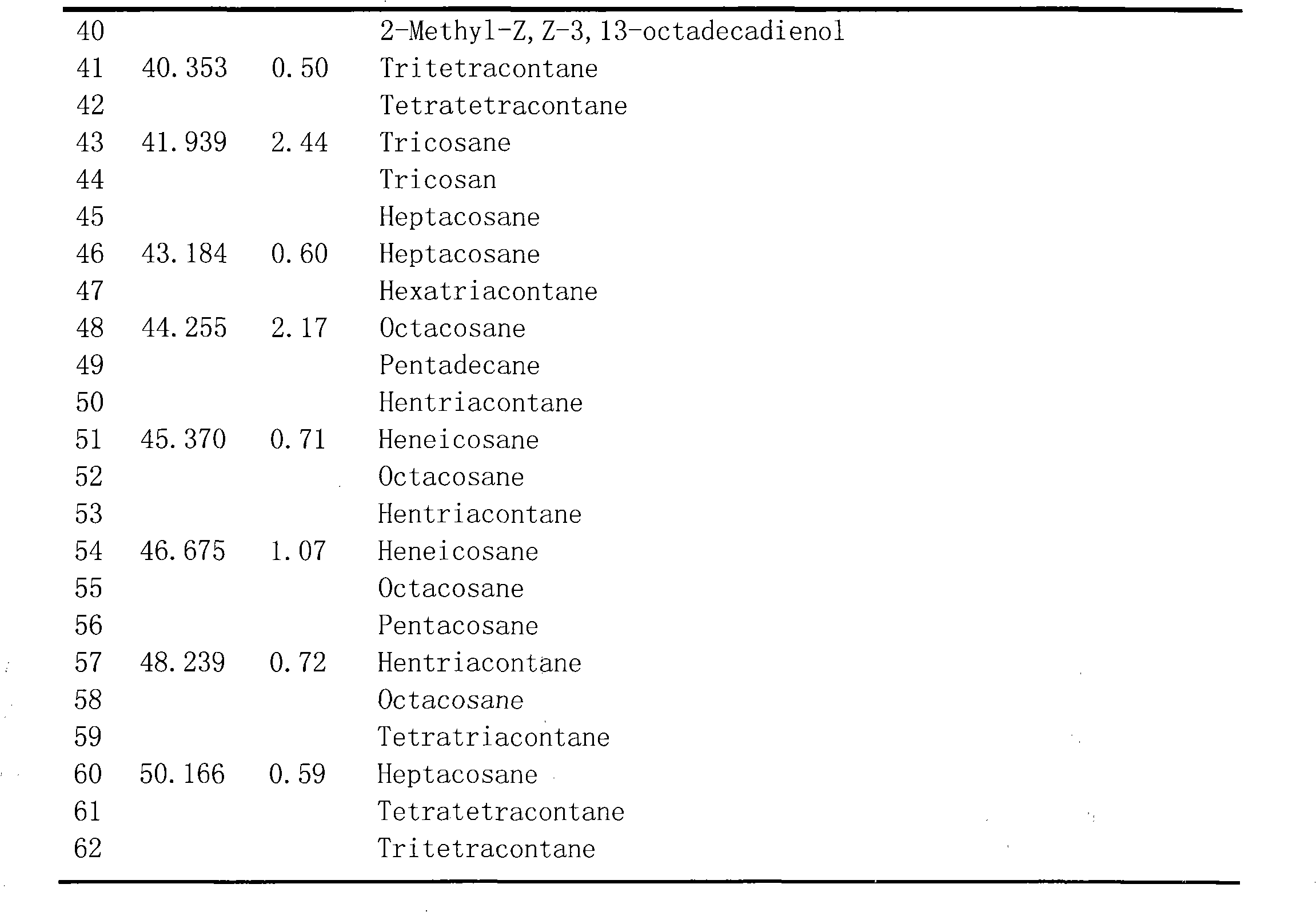
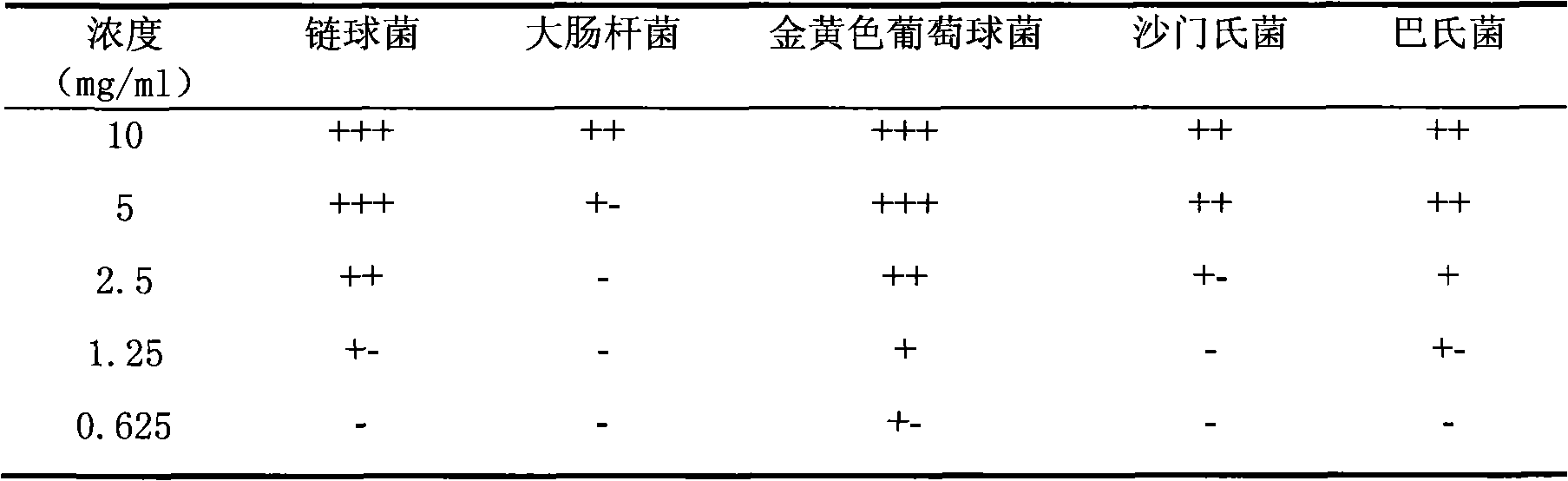
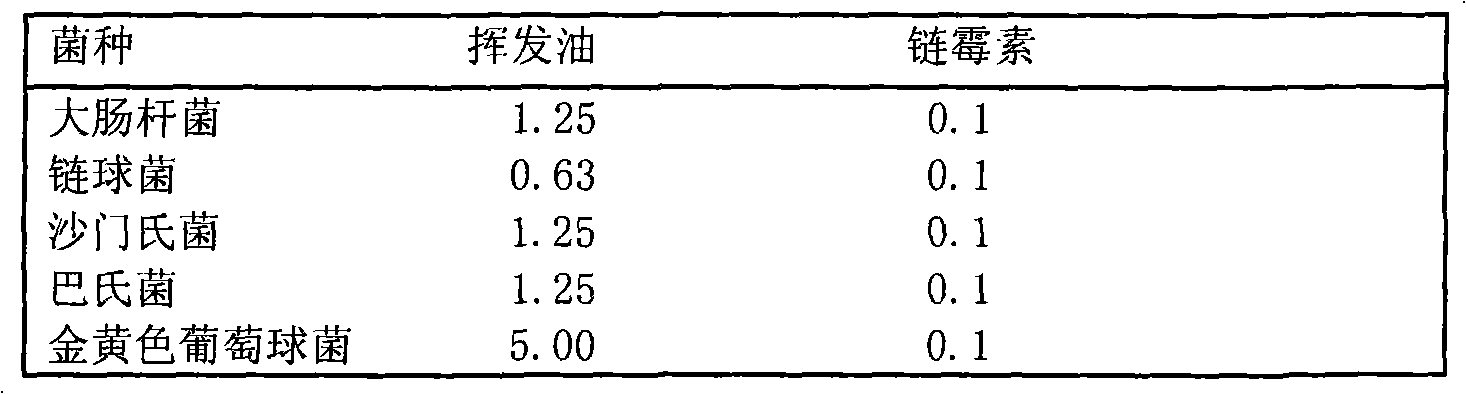
The invention belongs to the technical field of the research and development of medical products, and relates to the separation and identification of syringa amurensis rupr. bark volatile oil and a novel application. In the invention, syringa amurensis rupr. barks are taken, cut up after being dried, and processed by ultrasonic for one hour after being soaked by using distilled water for 6 to 8 hours, the temperature is 30 DEG C, and the power is 80W; medicinal materials and soaking liquid are poured into a volatile oil extractor together and are extracted for 4 to 5 hours by adopting a steam distillation method; a water layer is discharged, and the volatile oil extractor is repeatedly washed by ether to enable syringa amurensis rupr. volatile oil to be fully dissolved into the ether; and ether solution dissolved with the volatile oil is poured into a beaker, then anhydrous sodium sulfate is added into the beaker to suck up water, pure volatile oil is obtained after the ether is recovered, and the pure volatile oil is put into a refrigerator of 4 DEG C and preserved for standby. The syringa amurensis rupr. bark volatile oil is analyzed by using a 6890 / 5973N gas phase color spectrum and mass spectrum united instrument, and comprises the main chemical components of 27.46% of n-hexadecanoic acid, 28.52% of 9,12-octadecadienoic acid (Z,Z), 14.16% of 9,17-octadecadienal and 2.44% of tricosane. The syringa amurensis rupr. bark volatile oil has a suppressing function with different degrees for colibacillus, staphyiococcus aureus, pasteurella, streptococci and salmonella, can be developed into a natural antimicrobial agent applied to the industries of foods, medicines and the like, and can prepare various forms of products with a bacteriostatic function.
Owner:SHANDONG UNIV AT WEIHAI
Bovine capsular serotype A Pasteurella mutocida, validation identification and application thereof
The invention discloses bovine capsular serotype A Pasteurella mutocida and application thereof. The bovine capsular serotype A Pasteurella mutocida is separated from disease samples with haemorrhagic septicomia of cattle in six provinces (cities) with the microbial preservation number of CGMCC No.3619. The bovine capsular serotype A Pasteurella mutocida is cultured by BHI, oil adjuvant is adopted to prepare inactivated immunogen, and a mouse model proves that the bovine capsular serotype A Pasteurella mutocida has complete protecting function on serotype A Pm velogenic attck, and does not have crossing protection with serotype B Pm. Testing results prove that vaccine can be prepared by the bovine capsular serotype A Pasteurella mutocida for preventing the haemorrhagic septicomia of cattle caused by the capsular serotype A Pasteurella mutocida. The strain cannot cause diseases when inoculated to chicks, which proves that the strain is chicken isolated capsular serotype A Pasteurella mutocida.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Traditional Chinese medicine oral liquid for treating calf diarrhea and preparation method of traditional Chinese medicine oral liquid
ActiveCN104491038AImprove immunityWide variety of sourcesDigestive systemPharmaceutical delivery mechanismEscherichia coliLicorice roots
The invention belongs to the field of veterinary medicines, and in particular relates to traditional Chinese medicine oral liquid for treating calf diarrhea and a preparation method of the traditional Chinese medicine oral liquid. The traditional Chinese medicine oral liquid is prepared from 19-20 parts of golden threads, 20-30 parts of Chinese pulsatilla roots, 27-29 parts of honeysuckle flowers, 30-32 parts of scutellaria baicalensis, 30-31 parts of licorice roots, 25-30 parts of sweet wormwood herbs and 27-30 parts of oriental wormwoods. A Chinese herbal compound can be prepared into yellow and white honeysuckle flower oral liquid, so that the functions of dispelling dampness, cooling down a fever, resisting bacteria and eliminating inflammation, effectively inhibiting pathogenic bacteria such as escherichia coli, salmonella and pasteurella can be achieved while the spleen and stomach can be protected and the immunity of the organism is increased; under the combination of prevention and control, the calf diarrhea can be effectively treated. A clinical test proves that by using the traditional Chinese medicine oral liquid, the cure rate of the calf diarrhea reaches 86.7 percent; in addition, the medicinal raw materials of traditional Chinese medicine oral liquid are wide in sources and low in cost; as a whole, the cost of the traditional Chinese medicine composition is reduced.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Haemophilus parasuis detection kit and detection method thereof
ActiveCN104711359AHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesPasteurellaBacilli
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Compound microbial fertilizer and preparation method thereof
The invention discloses compound microbial fertilizer and a preparation method thereof and belongs to the technical field of agricultural bio-fertilizer. The fertilizer comprises dried chicken manure, rice chaff, cane sugar, urea, calcium superphosphate, potassium sulphate, zeolite powder, ATCC144712 azotobacter chroococcum, CICC20026 rhizobium astragali, ATCC6542 bacillus pasteurella, ATTC842 paenibacillus polymyxa, ACCC10168 bacillus megatherium, ATTC527 saccharomycetes and ATTC161 filamentous bacteria. According to the compound microbial fertilizer, through innovative technologies such as screening and matching, solid fermentation, negative pressure low temperature drying, two-section pelleting and particle high polymer material peridium for microbial bacteria, the novel biological environment-friendly type fertilizer integrating organic constituents and inorganic constituents is developed out, and the fertilizer has the advantages that the fertilizer consumption is reduced, the fertilizer utilization rate is raised, the agricultural product quality is improved, the environment is protected, and the fertilizer serves as a production means of green foodstuff.
Owner:山东普金肥料有限公司
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
Isolation and identification as well as application of volatile oil elements of Parasenecio firmus leaf
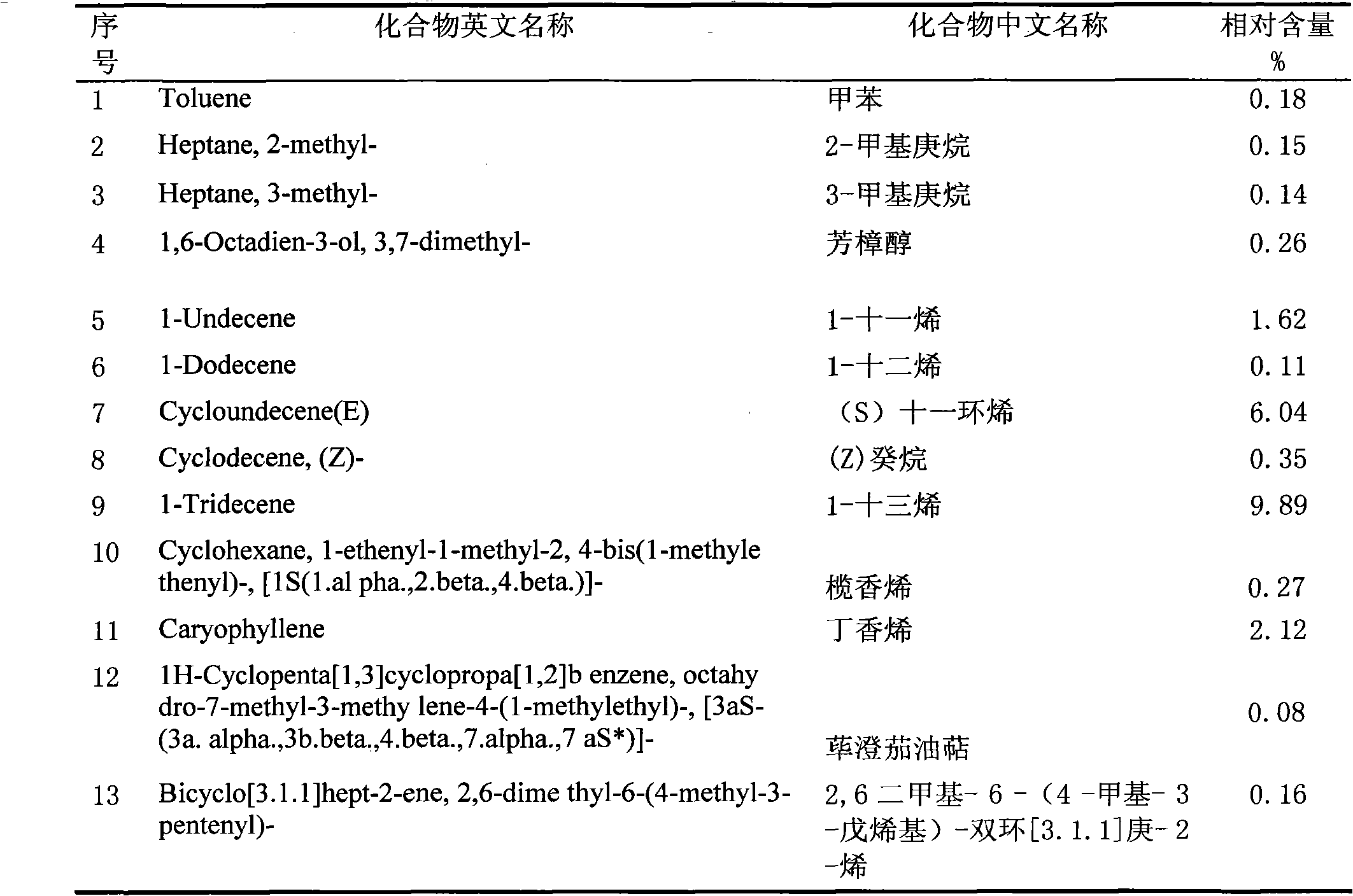
The invention belongs to the technical field of medicine production research development, and relates to isolation and identification as well as application of volatile oil elements of Parasenecio firmus leaf. The isolation and identification of the volatile oil elements of the Parasenecio firmus leaf are realized by the following steps of: weighing dried Parasenecio firmus leaf, and extracting ultrasonically by a water steam distillation method to obtain the volatile oil; and analyzing the obtain volatile oil by utilizing a 6890 / 5973N gas chromatograph-mass spectrometer. The obtain volatile oil mainly comprises the following chemical elements in proportion: 18.98% of n-palmitic acid, 9.89% of 1-tridecylene, 8.05% of caryophyllene epoxide, 6.04% of (S)-undeca-cycloolefin, 3.84% of 9,12,15-octadecatrienoic acid, 2.97% of (-)-spathulenol, 2.83% of kaurene and 2.12% of caryophyllene. The volatile oil is used for preparing pharmaceutical preparation capable of restraining escherichia coli, streptococcus, Salmonella, Pasteurella and staphylococcus aureus.
Owner:SHANDONG UNIV AT WEIHAI
Composite medicine preparation for prevention and treatment of bacterium and virus polyinfection diseases of livestock as well as preparation method and application thereof
ActiveCN103655593AImprove immunityStrengthen and broaden the efficacyAntibacterial agentsTetracycline active ingredientsDiseaseBacterial virus
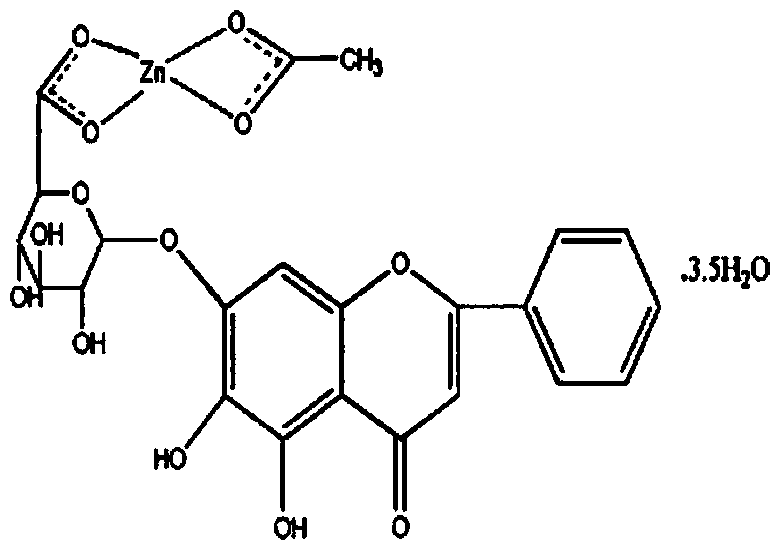
The invention provides a composite medicine preparation for the prevention and treatment of bacterium and virus polyinfection diseases of livestock as well as a preparation method and application thereof. By adopting an admixture and dissolution method, the composite medicine preparation is mainly prepared from the following components in parts by weight: 5-30 parts of doxycycline hydrochloride and 10-30 parts of baicalin zinc, wherein the components in the composite medicine preparation are scientific in formula and stable in property, cooperate with each other to act, and have remarkable effect on the prevention and treatment of serious respiratory tract and digestive tract diseases caused by the polyinfection of bacteria and viruses such as mycoplasma, colibacillus, salmonella and pasteurella, of the livestock,; in addition, the composite medicine is simple in preparation method, lowers the production cost, is convenient to use, and satisfies demand for large scale industrial production.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Novel lactic acid bacterial strain, composition containing same and use thereof
ActiveCN102978126AImprove immunityImprove survival rateAntibacterial agentsBacteriaMicroorganismBacillus badius
Provided is novel lactic acid bacterial strain, a composition containing the same and use thereof.The lactic acid bacterial straincomprises novel lactic acid pediococcus pentosaceus strain, or subculture descendant of the strain. The lactic acid bacterial strain is preserved in the general microbiology center of China microorganism culture preservation management council. Preservation number is CGMCC No.5235. The bacterial strain can restrain growing of photobacterium damselae (or called pasteurella) and other pathogenic bacteria. Thecomposition containing the lactic acid bacteria PP49 is also provided. Thecomposition is in a form of an animal feed additive or a medical treatment composition used for animals.
Owner:SYNBIO TECH INC +1
New preparation technology and new applications of syringa amurensis rupr bar volatile oil
InactiveCN101574093ADifferent degrees of inhibitionInhibition hasAntibacterial agentsBiocideEscherichia coliMedical product
The invention relates to a new preparation technology and new applications of syringa amurensis rupr bark volatile oil, belonging to the technical field of research and development on medical products. The preparation technology comprises the steps of: drying and cutting up syringa amurensis rupr bark, soaking the syringa amurensis rupr bark in distilled water for 12 hours and conducting ultrasonic treatment for 30 minutes at temperature of 30 DEG C and with power of 80W; pouring medicinal materials together with soak solution into a volatile oil extractor and conducting extraction for 3 hours by adopting a water vapour distillation method; releasing water-yielding stratum and washing the volatile oil extractor repeatedly with ether so as to lead the syringa amurensis rupr volatile oil to be dissolved in the ether completely; and pouring the ether solution dissolved with volatile oil into a beaker, adding anhydrous sodium sulfate in the beaker to suck dry moisture and recycling the ether to obtain the purified volatile oil, and then storing the volatile oil in a refrigerator at temperature of 4 DEG C for standby. The syringa amurensis rupr bark volatile oil has inhibitory actions with different degrees on colon bacillus, staphylococcus aureus, pasteurella, streptococcus and salmonella, therefore the volatile oil can be developed into natural antibacterial agent used for industries of food and medicament, and the like, and prepare various products with antibacterial effects.
Owner:JILIN AGRICULTURAL UNIV
Traditional Chinese medicine preparation for preventing and treating livestock bowel diseases
ActiveCN102671174AAnthropod material medical ingredientsDigestive systemBiotechnologyIntestinal tract diseases
The invention discloses a traditional Chinese medicine preparation for preventing and treating livestock bowel diseases. According to the principle of treating both principal and secondary aspect of diseases, the traditional Chinese medicine preparation with double functions for healthcare is capable of preventing and treating livestock bowel diseases by strengthening the body resistance to eliminate pathogenic factors, suppressing bacterial growth in intestinal canals, killing intestinal bacteria and reducing damages to intestinal mucosa. On the basis of the secret prescription handed down from the inventor's ancestors and the traditional Chinese medicine theory, the traditional Chinese medicine preparation is prepared according to the monarch-minister-assistant-guide composition principle of a traditional Chinese medicine prescription, wherein clove, star anise, camuning, aspongopus and nasturtium are taken as monarchs, sward beans, saururus chinensis, common burreed rhizome, dried ginger, dried lacquer and pericarpium arecae are taken as ministers, liriope spicata, rhizoma kaempferiae, hawthorn leaves, semen cannabis and radix polygonati officinalis are taken as assistants, and juncus roemerianus, pepper, rice sprouts, sea-buckthorn, terminalia fruits and musk are taken as guides. The traditional Chinese medicine preparation which is prepared by scientific compositions and processes has high effects of killing and suppressing livestock escherichia coli, salmonella and pasteurella.
Owner:潍坊新希望六和饲料科技有限公司
Veterinary compound hydrochloric acid injection and preparation method thereof
InactiveCN102151263AGood effectEasy to prepareAntibacterial agentsOrganic active ingredientsProtozoaDisease
The invention relates to veterinary compound hydrochloric acid injection and a preparation method thereof. The veterinary compound hydrochloric acid injection consists of oxytetracycline dihydrate injection, imidocarb and diclofenac sodium; the veterinary compound hydrochloric acid injection per 100 ml comprises the required raw materials of: 10-25g of oxytetracycline dihydrate injection, 1.5-5g of diclofenac sodium, 0.1-0.2g of imidocarb, 0.2-0.6g of sodium formaldehyde sulphoxylate, 5-15g of magnesium chloride, 5-13 ml of ethanolamine and 60-81 ml of organic solvents, wherein the allowance is water for injection. The veterinary compound hydrochloric acid injection has remarkable effect when being used for treating acute respiratory infection caused by eperythrozoon suis, babesiosis and other blood protozoa diseases, porcine respiratory disease complex (PRDC) and swine influenza virus (SIV), airway inflammation induced by porcine reproductive and respiratory syndrome (PRRS), haemophilus parasuis, pasteurella, pleuropneumonia and mycoplasma diseases; and the preparation method is simple and easy to operate, and is suitable for batch production.
Owner:XUCHANG TIANYUAN BIOLOGICAL TECH CO LTD
Subunit vaccine of pasteurella multocida in veterinary uses
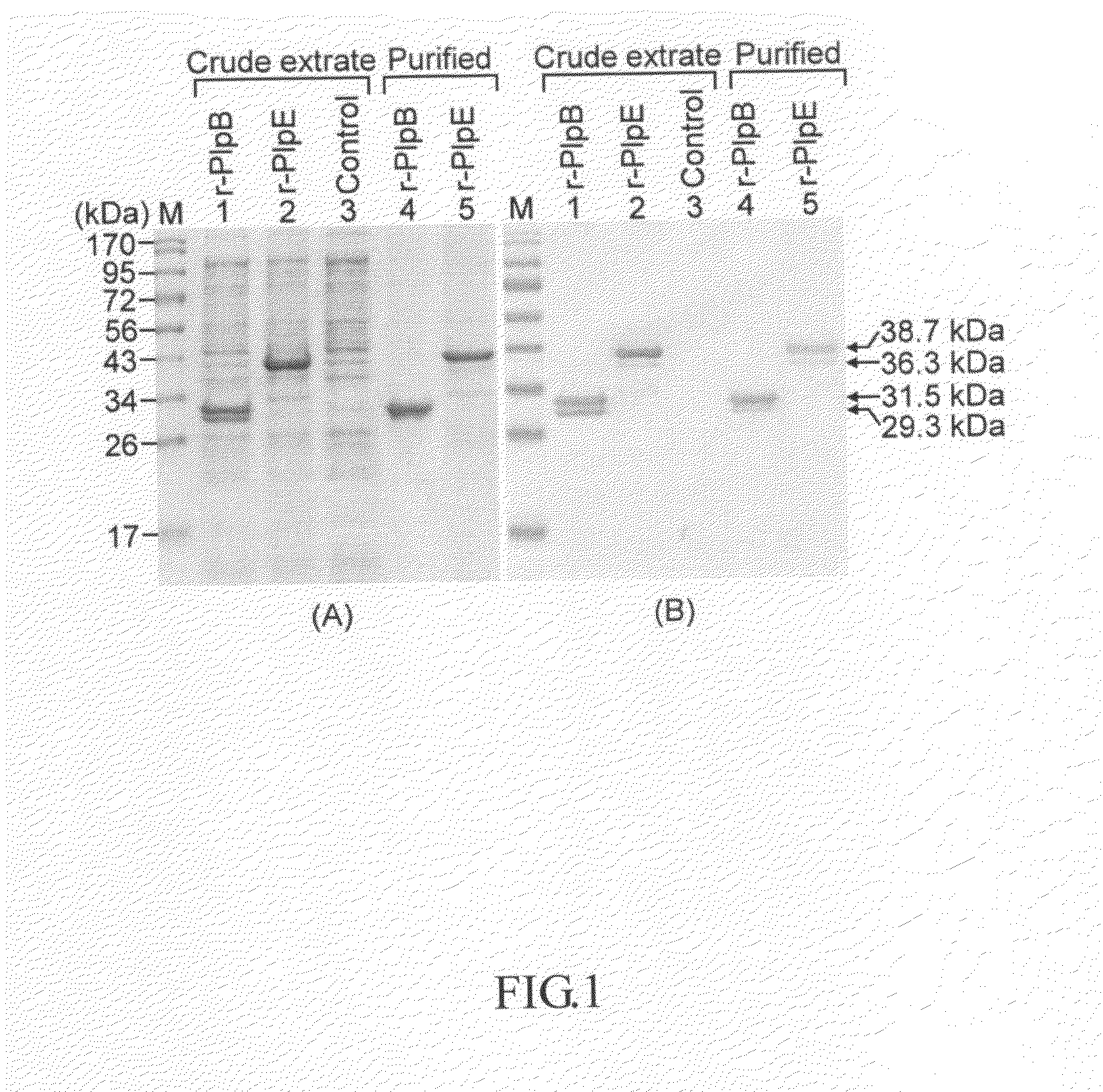
The present invention declaims the use of Pasteurella lipoprotein E (PlpE) as a subunit vaccine and the use of vaccines containing PlpE to protect animals from diseases caused by P. multocida. The results of vaccination and challenge experiments showed that mice and chickens immunized with PlpE were completely protected animals from challenge infection with 101-103 LD50 of P. multocida and no adverse effect was observed.
Owner:NATIONAL CHUNG HSING UNIVERSITY
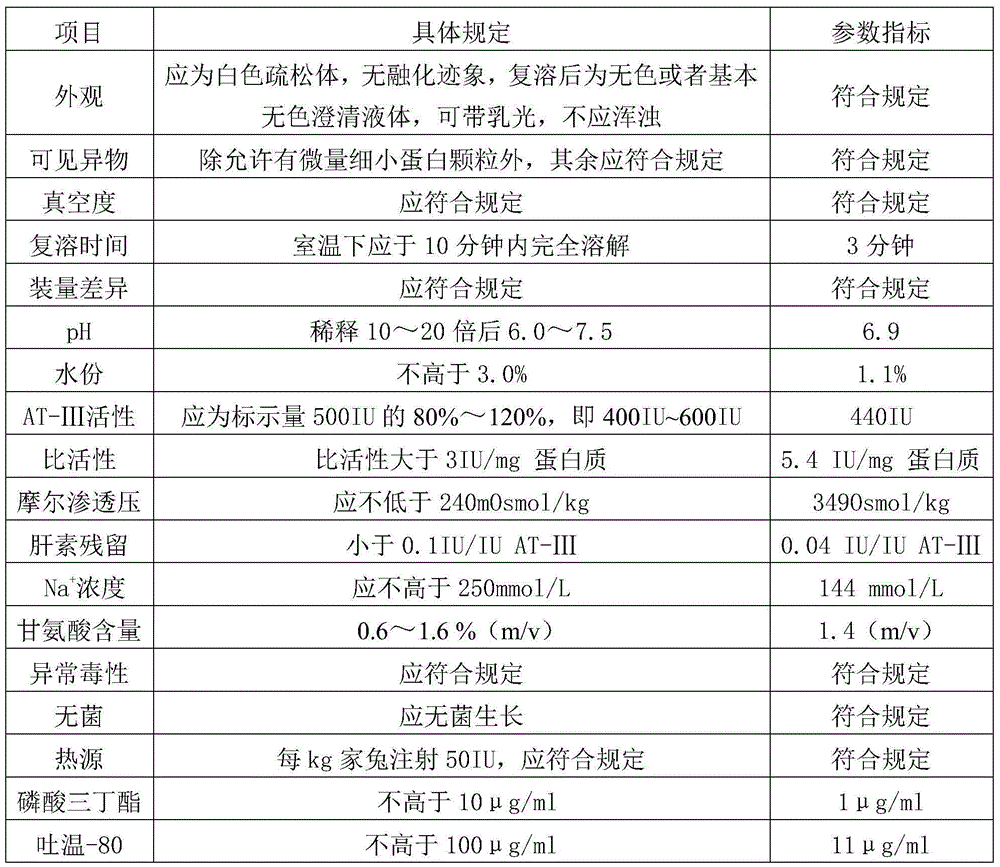
Method for separating and purifying human antithrombin-III (AT-III) from human plasma
InactiveCN104672326AThe process steps are simple to operateEasy to operatePeptide preparation methodsProtease inhibitorsFiltration membraneFreeze-drying
The invention discloses a method for separating and purifying human antithrombin-III from human cryoprecipitate-reduced plasma. The method comprises the operating steps of performing chromatography on the human cryoprecipitate-reduced plasma, performing S / D (solvent / detergent) virus inactivation, and performing affinity chromatography for the second time; performing ultrafiltration; performing sterilizing filtration; filtering with a 20nm nano-filtration membrane to remove viruses; subpackaging; performing freeze-drying; performing dry heat virus inactivation; separating and purifying human antithrombin-III from the human cryoprecipitate-reduced plasma; performing pre-filtration treatment, and then directly separating and purifying antithrombin-III (AT-III) out of the plasma by adopting an affinity chromatography technology; adding an appropriate amount of a protective agent, replacing pasteurella virus inactivation process with an S / D virus inactivation process to reduce the loss and activity and shorten the preparation time; then performing affinity chromatography for the second time to further purify the AT-III, and finally performing the steps of ultrafiltration, virus removal through nano-filtration with a 20nm nano-membrane, freeze-drying and the like, wherein the purity of the final product is more than 95%, the specific activity is more than 6IU / mg, the virus safety is high, and blood plasma can not be influenced during preparation to produce a downstream product.
Owner:GUIZHOU TAIBANG BIOLOGICAL PROD +1
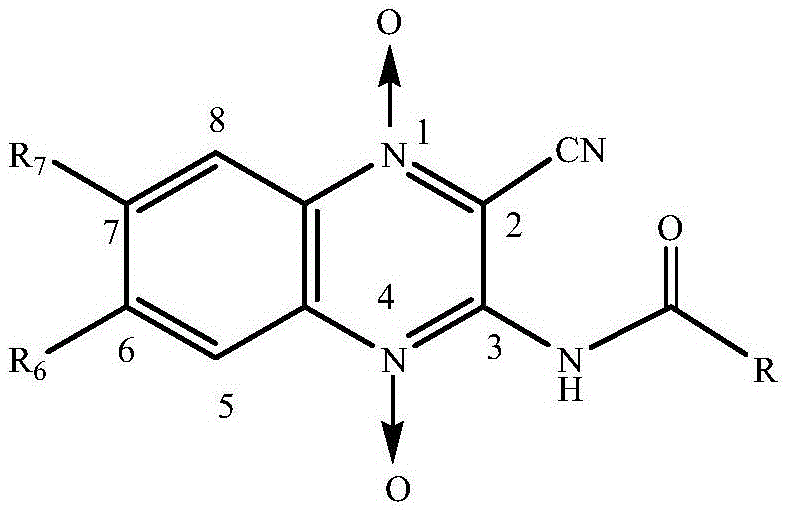
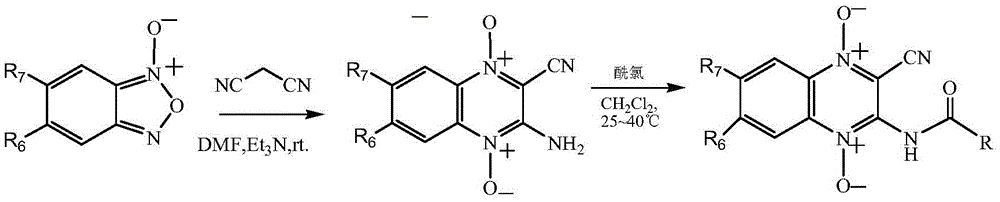
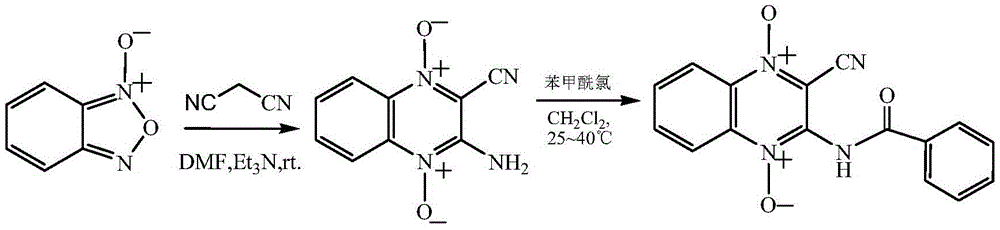
Quinoxaline-N1,N4-dioxide derivatives with antimicrobial activity
The invention belongs to the technical field of pharmaceutical chemosynthesis, and particularly relates to quinoxaline-N1,N4-dioxide derivatives with antimicrobial activity. The invention also relates to preparation of the derivatives and antimicrobial activity testing of the derivatives. The new synthetic compounds are prepared by the following steps: carrying out Beirut reaction on the raw material N-benzofuroxan oxide and malononitrile under alkaline conditions to obtain 3-amino-2-cyano-quinoxaline-N1,N4-dioxide, and reacting with appropriate acyl chloride to obtain a series of 2-cyano-amidoquinoxaline-N1,N4-dioxides. The in-vitro antibacterial activity test result indicates that the quinoxaline-N1,N4-dioxide derivatives have favorable antibacterial activity for mycobacterium hominis and mycobacterium bovis and also have antibacterial activity for Staphylococcus aureus, Streptococcus pneumoniae and Pasteurella. The invention also discloses a structural formula of the compounds used as a target antibacterial drug.
Owner:HUAZHONG AGRI UNIV
Bacillus subtilis, its combination preparation and method for preparing combination preparation
The invention relates to a bacilli, its compound preparation and the preparing method for the compound preparation. Its name is Bacillus subtilis LY-35, which is preserved in the general microbe center of Micro Germ conservation Management committee of China, and its conservation number is:CGMCC N0.1222. The preparation is a mixture of lambda phage of culture of bacilli and staphylococcus, streptococcus, colibacillus, bacillus proteus, pseudomonad, salmonella, pasteurella and klebsiella, can cure and prevent all kinds of illness caused by conditional pathogen,is a micro biological preparation which can be used by being mixed with antibiotics or replace antibiotics without any remainder and pollution, with good curative effect and safety.
Owner:烟台绿云生物工程研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com