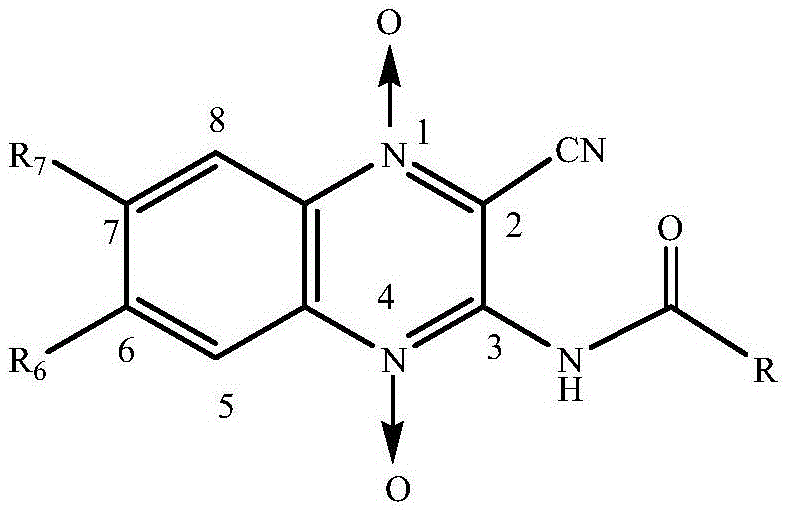
Quinoxaline-N1,N4-dioxide derivatives with antimicrobial activity
A technology of dioxide and antibacterial activity, applied in the field of quinoxaline-N1,N4-dioxide derivatives, can solve problems such as toxicity and limit its use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
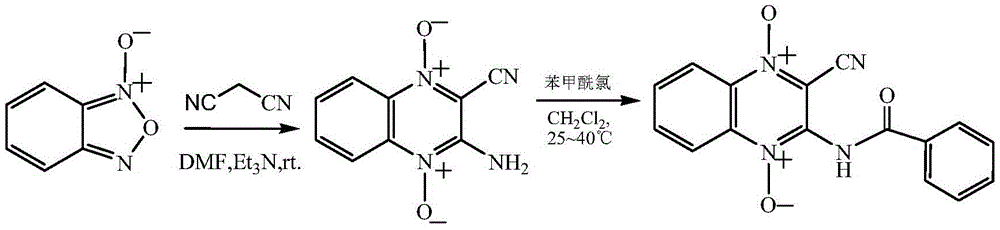
[0041] 2-cyano-3-benzamido-quinoxaline-N 1 ,N 4 -The synthetic reaction formula of dioxide oxide (1) is as follows:
[0042]
[0043] The preparation steps are as follows:
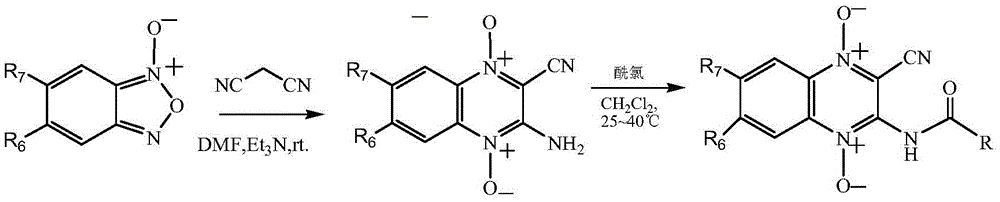
[0044] (1) Add 13.4g (0.1mol) N-benzofurazan oxide, 6.6g (0.1mol) malononitrile and 50mL DMF into a 250mL single-necked flask, stir to dissolve completely, add dropwise 2mL triethylamine, keep 25 ℃, stirred and reacted for 4h, after the reaction was complete, placed at 0°C to cool for 1 hour, filtered with suction, washed the filter cake with 95% ethanol, and dried in vacuo to obtain 2-cyano-3-amino-quinoxaline-N 1 ,N 4 - Dioxide 17.8 g, yield 88%.
[0045] (2) Add 2-cyano-3-amino-quinoxaline-N to a 50mL single-necked flask 1 ,N 4 - Dioxide 2.0g (0.01mol), 30mL dichloromethane, 1mL pyridine, stirred and dissolved, then added dropwise 1.4g (0.01mol) of benzoyl chloride, stirred at 25°C for 2 hours. The reaction solution was distilled off the solvent under reduced pressure, and then 20 mL of ethyl ...
Embodiment 2
[0047] 2-cyano-3-p-methylbenzamido-quinoxaline-N 1 ,N 4 -The synthesis reaction formula of dioxide oxide (2) is as follows:
[0048]
[0049] The preparation steps are as follows:
[0050] The benzoyl chloride in embodiment 1 is changed into p-methylbenzoyl chloride, and other reaction conditions are with embodiment 1, obtain 2-cyano group-3-p-methylbenzamido-quinoxaline-N 1 ,N 4 - Dioxide 2.65 g, 72% yield in two steps. Melting point 218.2-220.5℃; MS: [M+H] + 321.0920;IR(KBr)υcm -1 : 3420, 3253, 3083, 2224 (weak), 1675, 1526, 1335; 1 HNMR (DMSO-d 6 ,400MHz) δppm: 8.53~8.65(q,2H,H 5 +H 8 ), 7.84~7.92(m,2H,H 6 +H 7 ), 7.31~7.33(d,2H,Ar--H 2’ +H 6’ ), 7.09~7.11 (d,2H,Ar-H 3’ +H 5’ ),2.30(s,3H,-CH 3 ).
Embodiment 3
[0052] 2-cyano-3-p-fluorobenzamido-quinoxaline-N 1 ,N 4 -The synthetic reaction formula of dioxide oxide (3) is as follows:
[0053]
[0054] The preparation steps are as follows:
[0055] The benzoyl chloride in embodiment 1 is changed into p-fluorobenzoyl chloride, and other reaction conditions are with embodiment 1, obtain 2-cyano group-3-p-fluorobenzamido-quinoxaline-N 1 ,N 4 - Dioxide 2.73 g, 74% yield in two steps. Melting point 208.5-210.5℃; MS: [M+H] + 325.0772;IR(KBr)υcm -1 : 3435, 3243, 3080, 2225 (weak), 1685, 1525, 1337; 1 HNMR (DMSO-d 6 ,400MHz) δppm: 8.57~8.63(q,2H,H 5 +H 8 ), 7.92~7.94(m,2H,H 6 +H 7 ), 7.84~7.88(d,2H,Ar-H 3’ +H 5’ ), 7.07~7.11(t,2H,Ar--H 2’ +H 6’ ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com