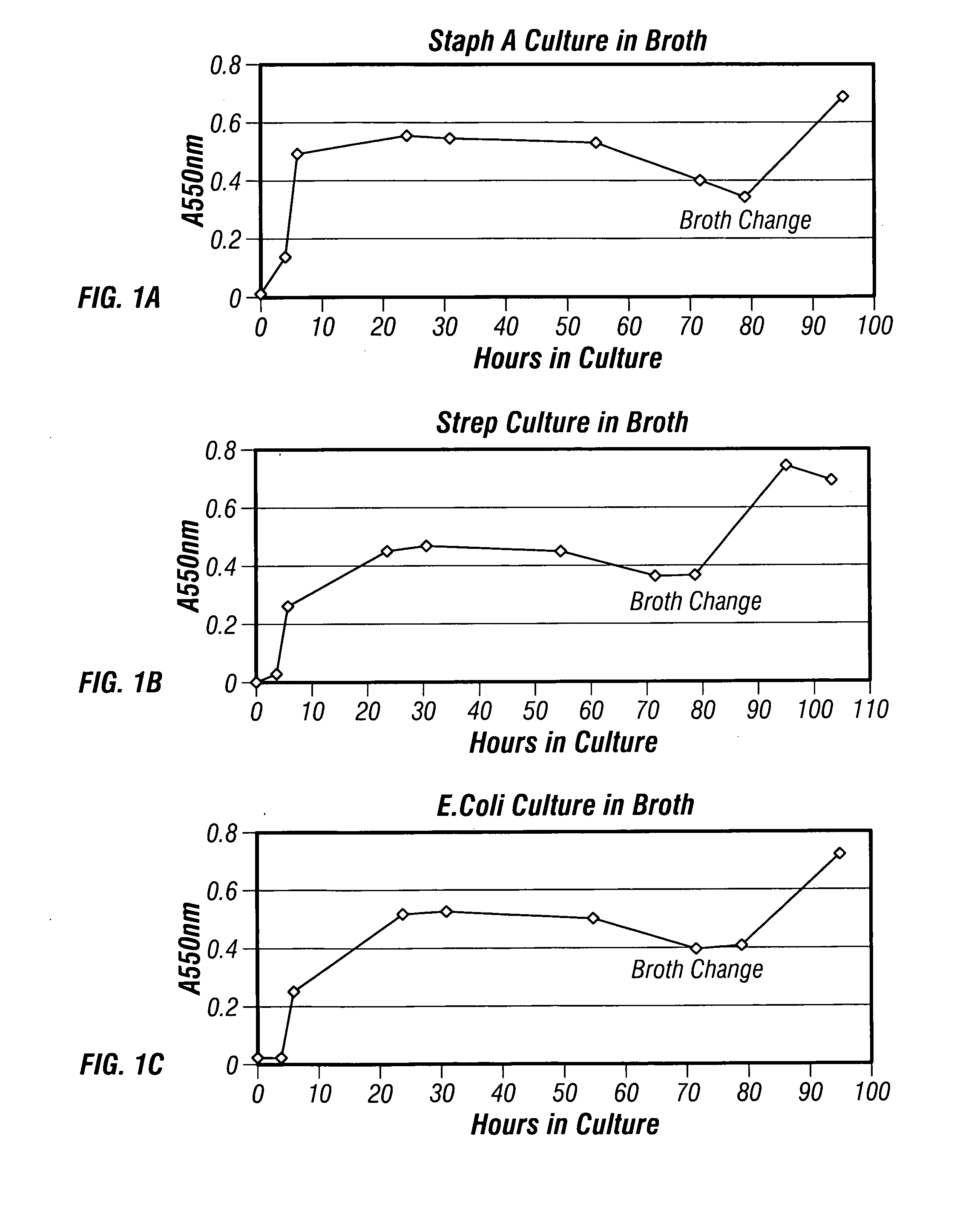
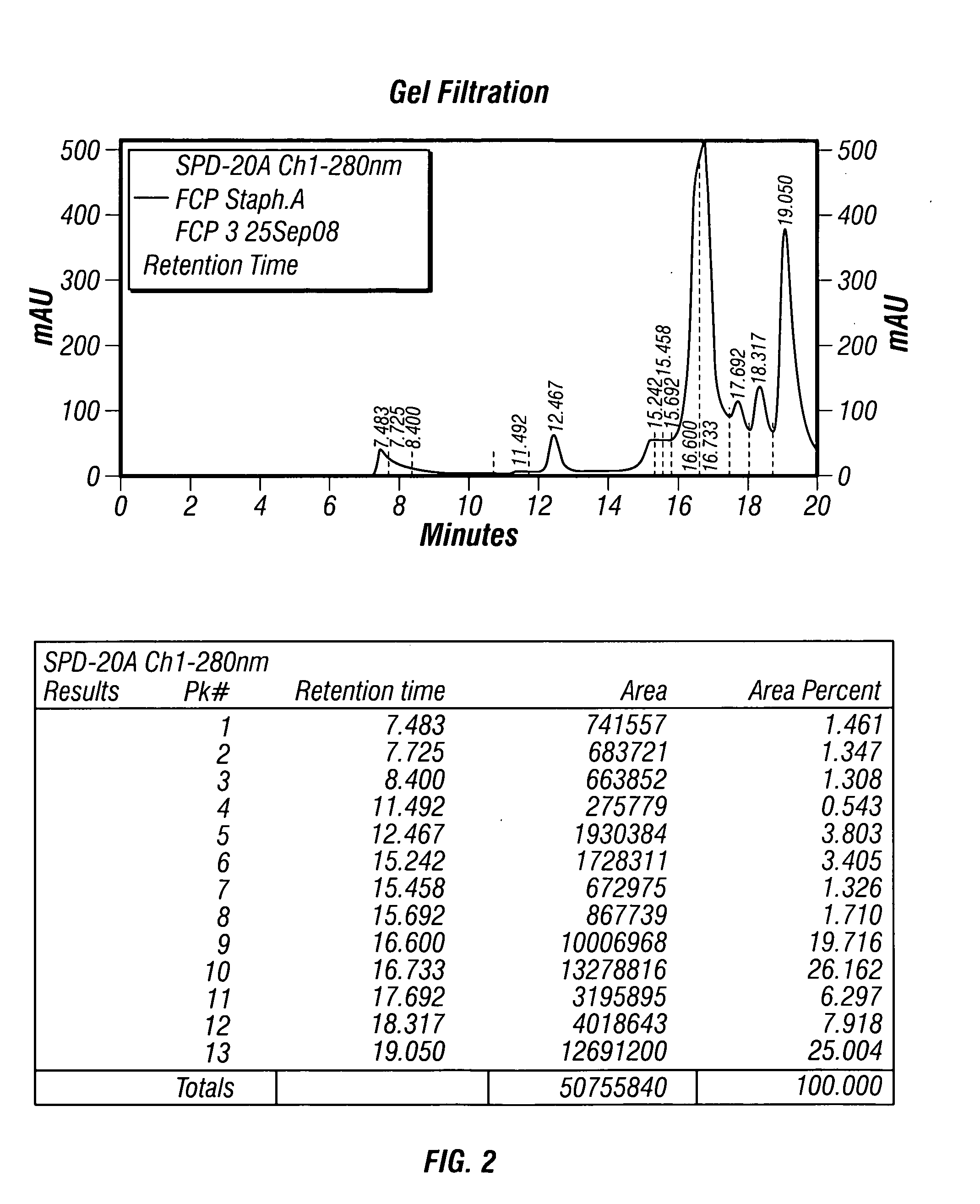
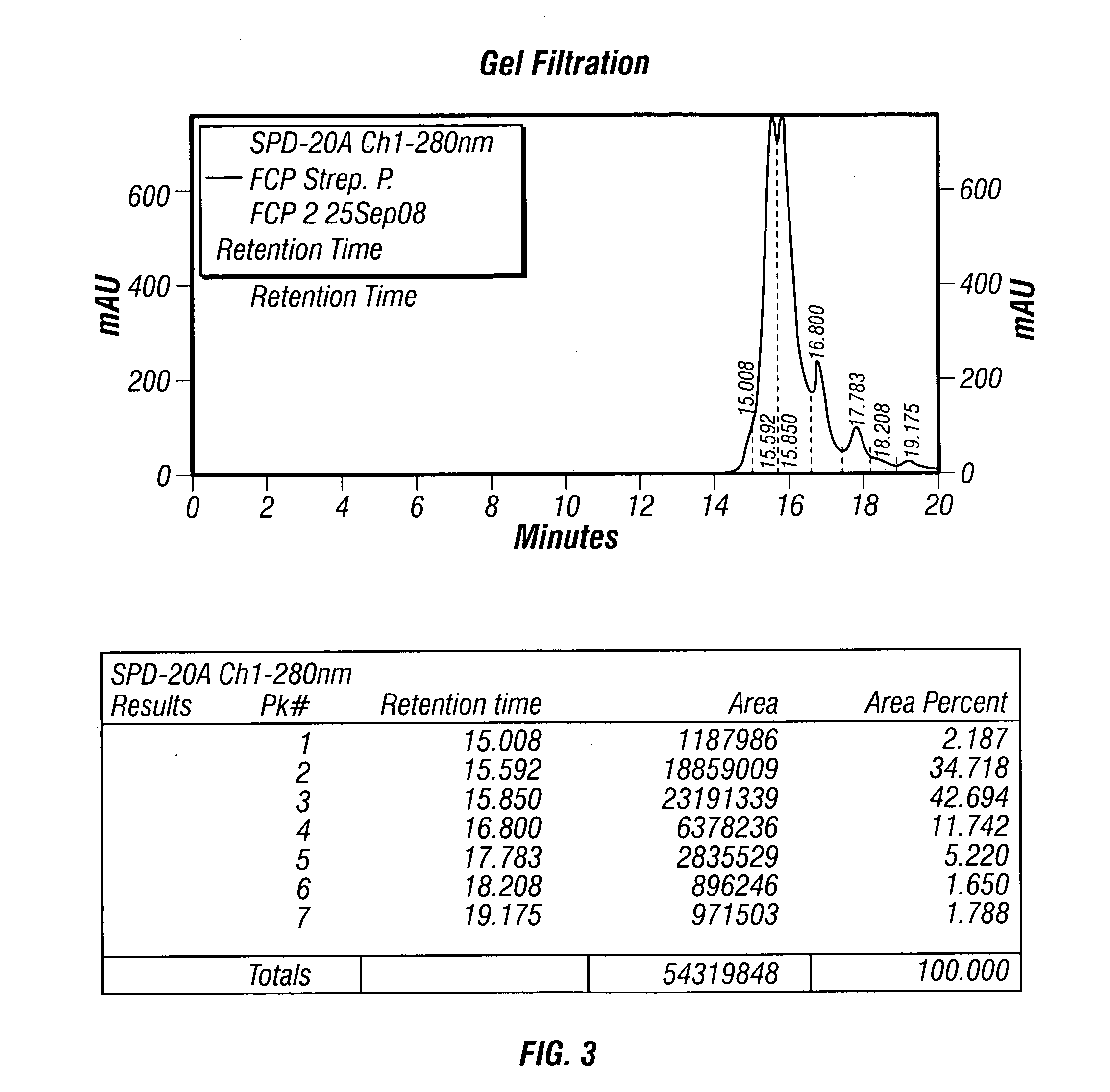
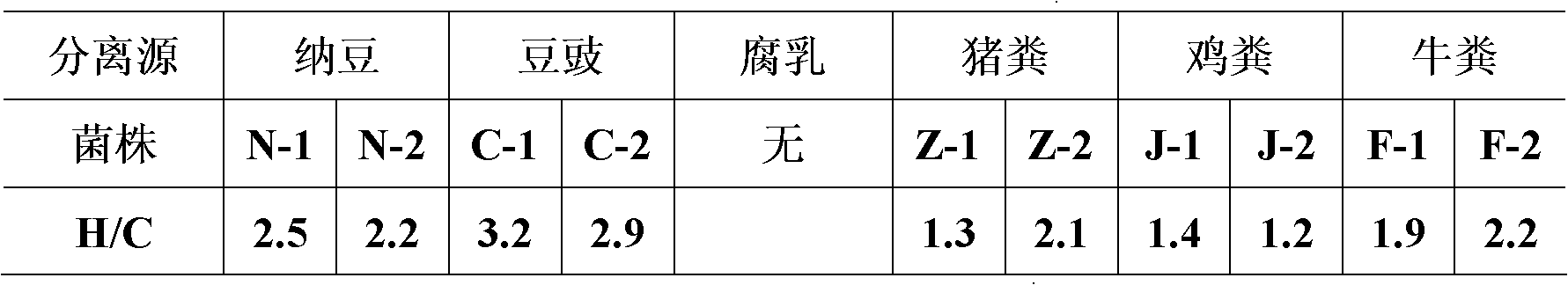
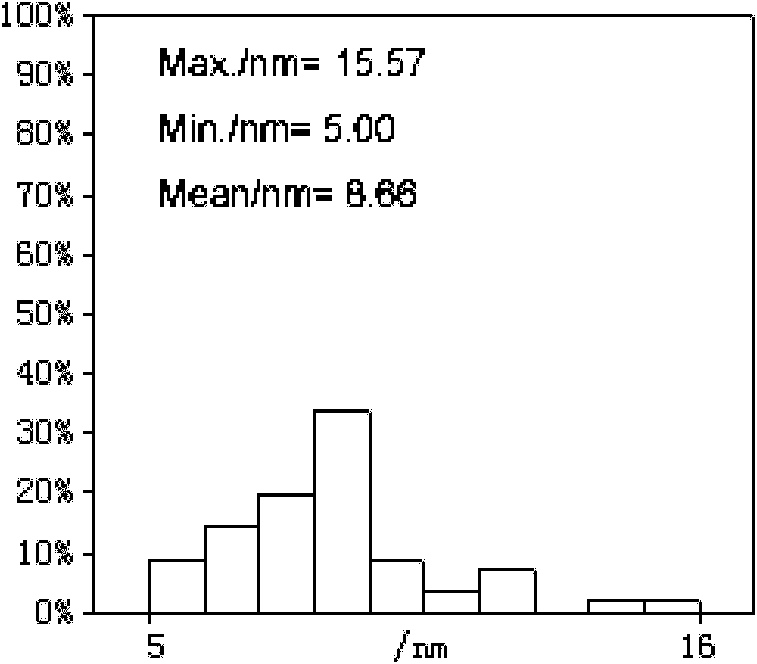
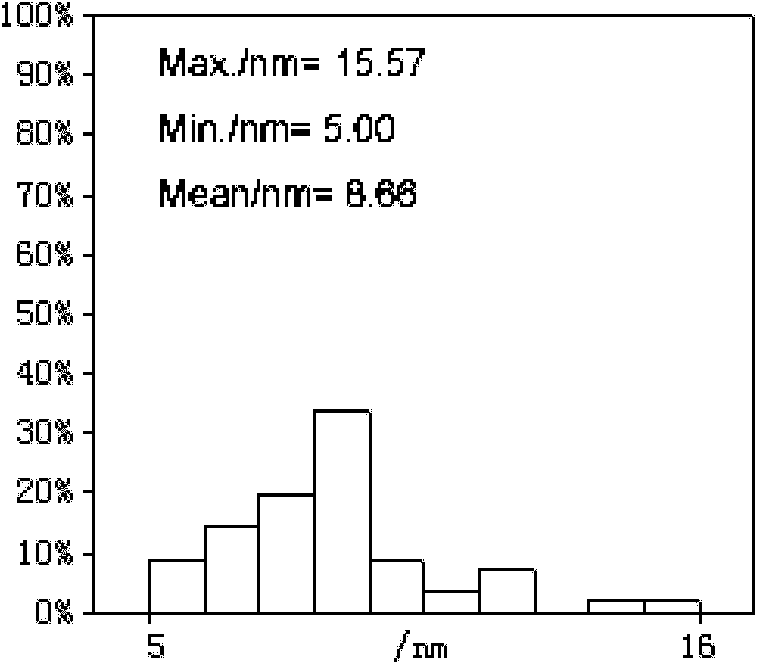
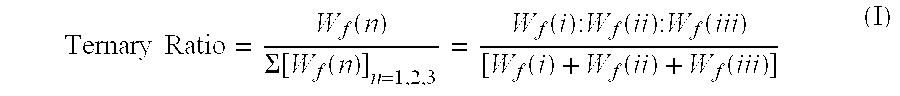Patents
Literature
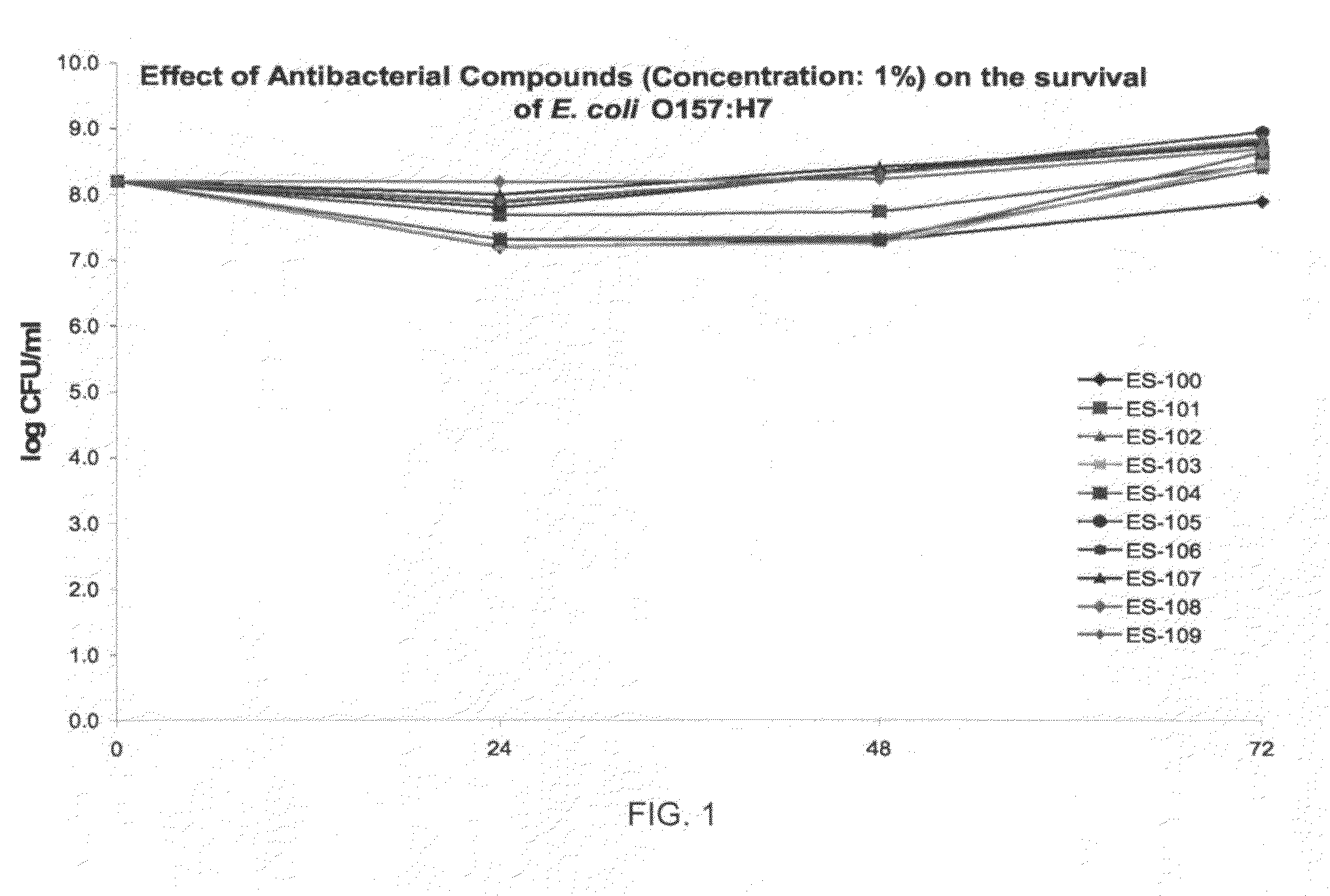
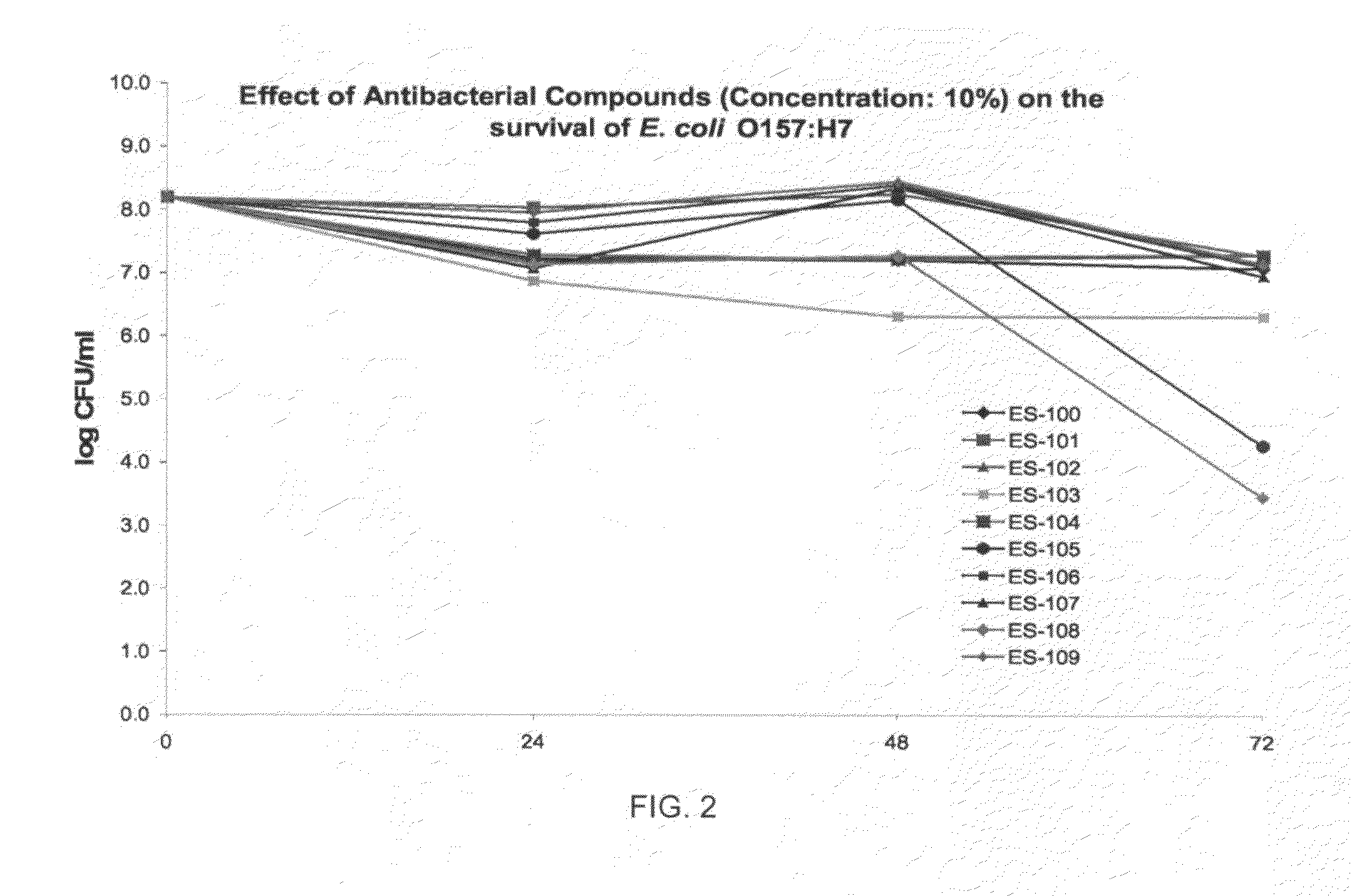
3373 results about "Staphylococcus aureus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
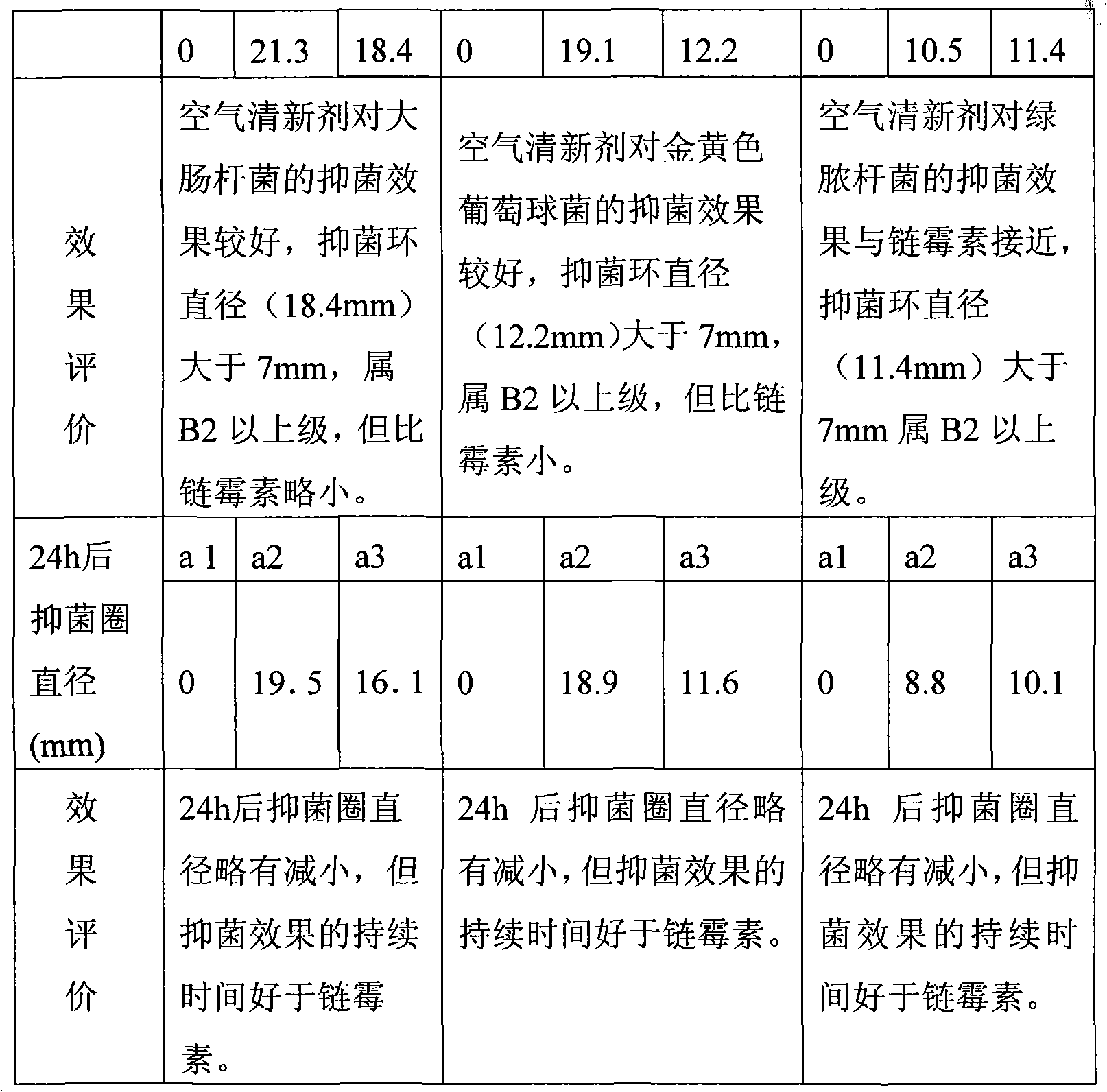
Staphylococcus aureus is a Gram-positive, round-shaped bacterium that is a member of the Firmicutes, and it is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe that can grow without the need for oxygen. Although S. aureus usually acts as a commensal of the human microbiota it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infections by producing virulence factors such as potent protein toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. The emergence of antibiotic-resistant strains of S. aureus such as methicillin-resistant S. aureus (MRSA) is a worldwide problem in clinical medicine. Despite much research and development, no vaccine for S. aureus has been approved.
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
Composition for driving and killing mosquitoes and preparation method thereof
The invention relates to a composition for driving and killing mosquitoes. The composition is characterized by comprising the following substances by portions: 20 to 50 portions of natural essential oil, 10 to 35 portions of pyrethroid, 25 to 50 portions of solvent oil, 3 to 20 portions of surface active agent and 5 to 15 portions of antioxidant. The composition has the advantages of: (1) having no toxic side effect to body, having multiple action of driving mosquitoes, sterilizing and essence, being capable of reducing the cost of the product and bringing pleasant feeling to a user; (2) having different effective components with different action mechanism for target insects and also being capable of effectively retarding the drug resistance thereof; (3) having higher safety than chemosynthetic insecticide due to easy decomposition and low residue of the natural essential oil in natural environment; (4) having obvious control effect to mosquitoes, flies and cockroaches and being capable of effectively reducing the using amount of pyrethroid due to adding of the natural essential oil; and (5) having a certain action of restraining and killing various harmful bacterium such as golden staphylococcus aureus, shigella shigae and Pseudomonas aeruginosa due to adding of natural vegetable garlic oil.
Owner:江西山峰日化有限公司
Enterococcus faecium ANSE228 and application thereof
ActiveCN102031235AIncrease production capacityReduce the death rateAntibacterial agentsBacteriaEscherichia coliStaphylococcus cohnii
The invention provides an Enterococcus faecium ANSE228 of which the collection number is CGMCC No.4082. The invention also provides application of the Enterococcus faecium ANSE228 to inhibition of salmonella pullorum and / or Escherichia coli and / or Staphylococcus aureus. The Enterococcus faecium ANSE228 is obtained by processes of repeated separation, purification, rejuvenation and the like, and has high biological activity, obvious probiotic property, high adversity resistance and the like. The invention also provides a microecological agent which contains the Enterococcus faecium ANSE228. When the microecological agent is added into drinking water and / or feeds for breeding animals, the Enterococcus faecium ANSE228 can be quickly activated and reproduced and a dominant beneficial flora can be formed after the Enterococcus faecium ANSE228 is fed into intestinal canals of the animals, and the Enterococcus faecium ANSE228 has the effects of reducing a harmful flora in the intestinal canals, adjusting microecological balance of the intestinal canals, substituting for medicaments such as antibiotic and the like, and improving weight increment of the animals and the utilization rate of the feeds.
Owner:科润生科技发展有限公司
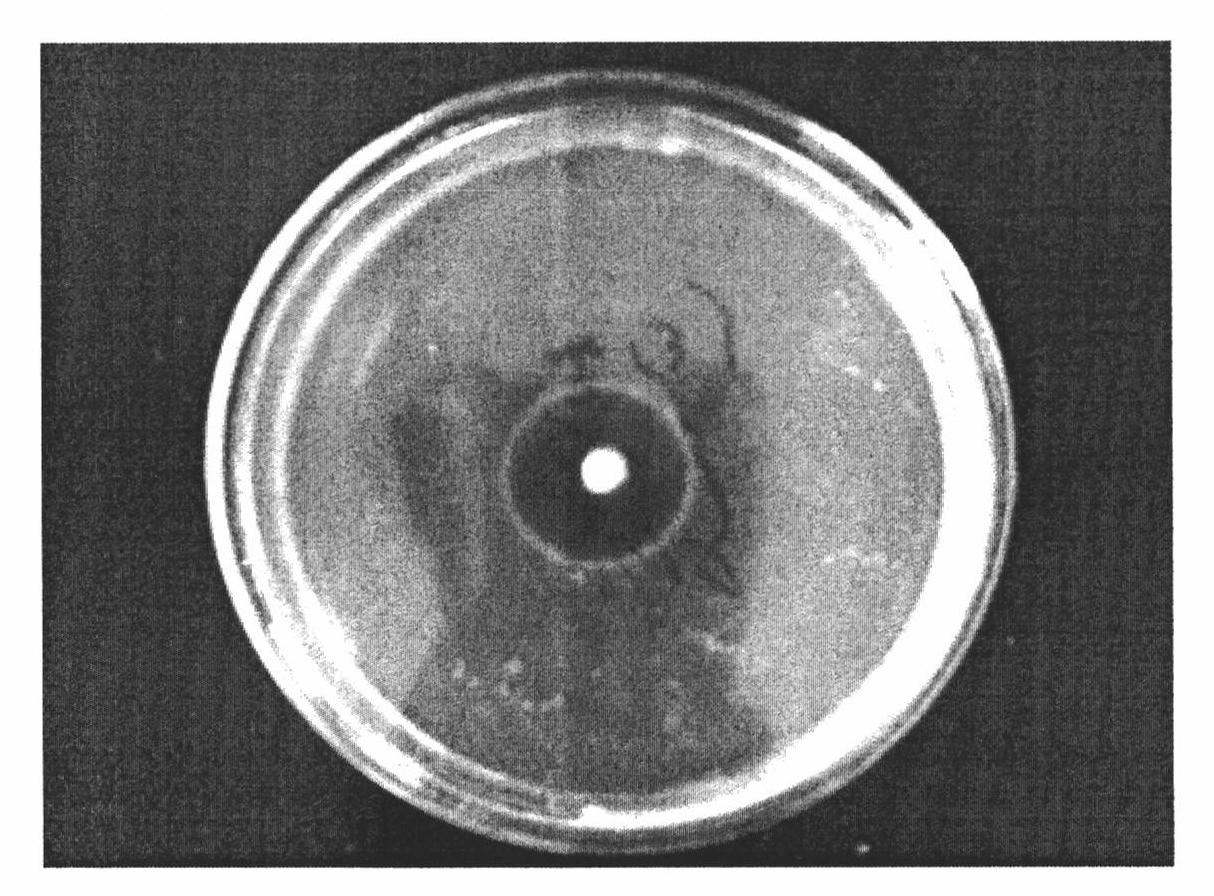
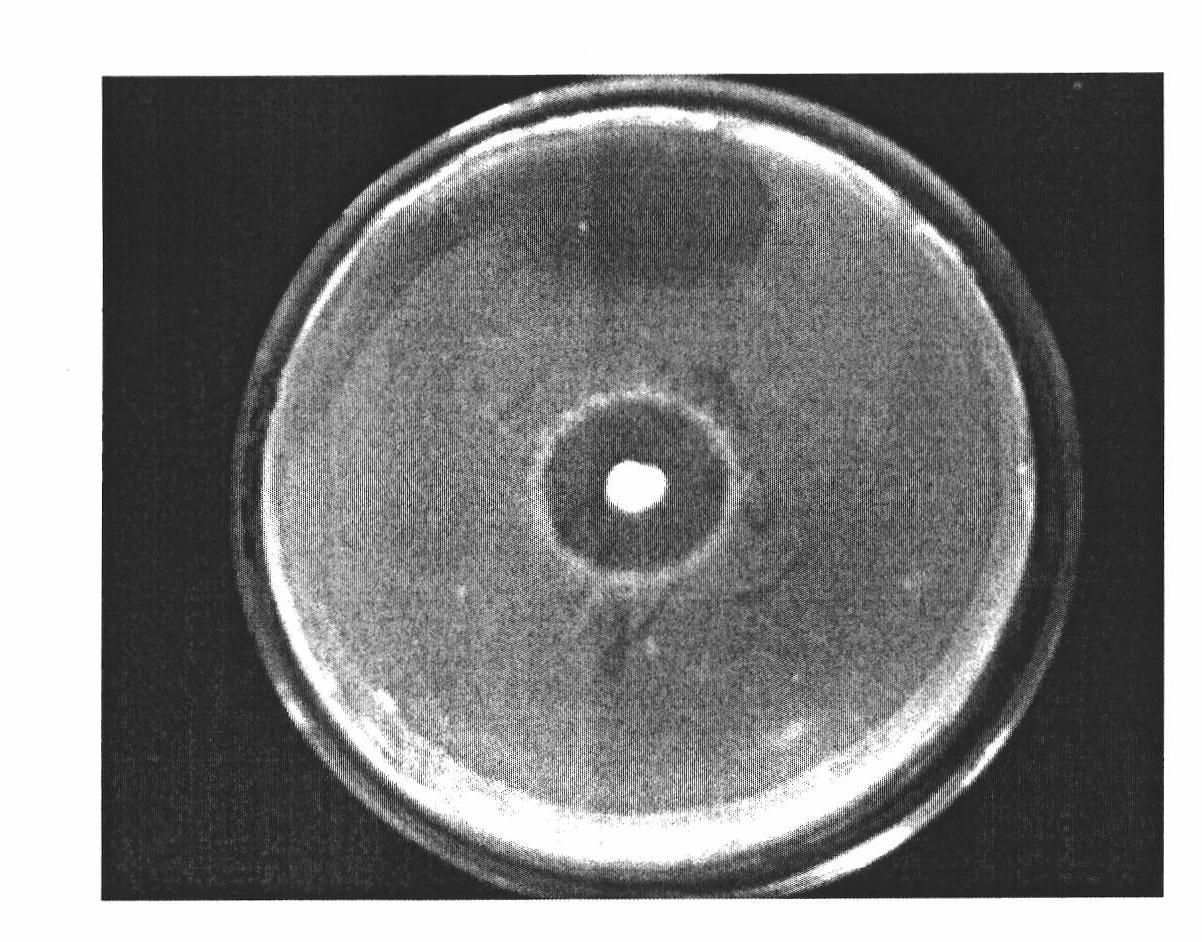
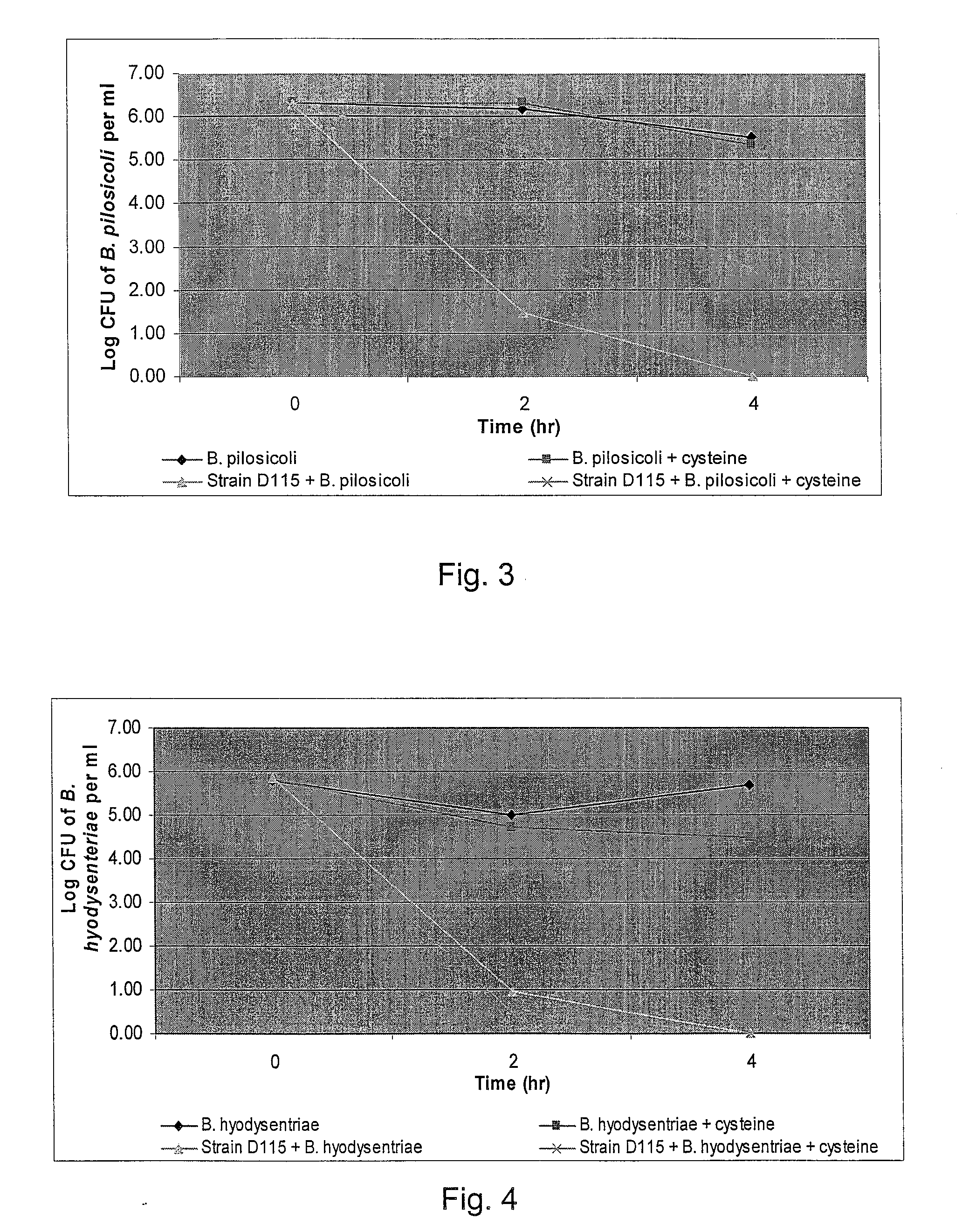
Broad-Spectrum Antibacterial and Antifungal Activity of Lactobacillus Johnsonii D115
The present invention demonstrated the potential use of Lactobacillus johnsonii D115 as a probiotic, as a prophylactic agent or as a surface treatment of materials against human and animal pathogens such as Brachyspira pilosicoli, Brachyspira hyodysenteriae, Shigella sonnei, Vibrio cholera, Vibrio parahaemolyticus, Campylobacter jejuni, Streptococcus pneumoniae, Enterococcus faecalis, Enterococcus faecium, Clostridium perfringens, Yersinia enterocolitica, Escherichia coli, Klebbsiella pneumoniae, Staphylococcus aureus, Salmonella spp., Bacillus cereus, Aspergillus niger and Fusarium chlamydosporum. The proteineous antimicrobial compound was partially characterized and found to be heat tolerant up to 121° C. for 15 min, and acid tolerant up to pH1 for 30 min at 40° C. The compound is also stable to enzymatic digestion, being able to retain more than 60% antimicrobial activity when treated with pepsin and trypsin.
Owner:KEMIN IND INC
Anti-bacterial water-based paint and preparation method thereof
InactiveCN102702889AImprove aging resistanceImprove the pulverization performanceBiocideAntifouling/underwater paintsWater basedEscherichia coli
The invention relates to anti-bacterial water-based paint and a preparation method thereof. The paint comprises the following components in parts by weight: 0.2-11 parts of anti-bacterial agent, 8-33 parts of nano material, 23-64 parts of water-based resin dispersoid and 0.75-18 parts of adhesive resin or plasticizer. The preparation method comprises the following steps: firstly preparing a nano silver anti-bacterial agent; mixing deionized water, the anti-bacterial agent, a wetting agent, a dispersing agent and a defoaming agent and uniformly mixing, adding the nano material, uniformly dispersing to obtain the water-based dispersoid; adding the obtained water-based dispersoid to the mixed emulsion or water-based resin dispersoid, then adding the adhesive resin or plasticizer and various conventional assistants, stirring and dispersing evenly; adding pigments or colorant; and supplementing water to obtain the anti-bacterial water-based paint. The long-acting broad-spectrum antibacterial water-based paint has high fungicidal efficiency (more than 99%) on escherichia coli, staphylococcus aureus, black varietas of bacillus subtilis and the like and can reduce the high concentrate of organic matters of formaldehyde to the range of specified concentration index.
Owner:ANHUI JINDUN PAINT
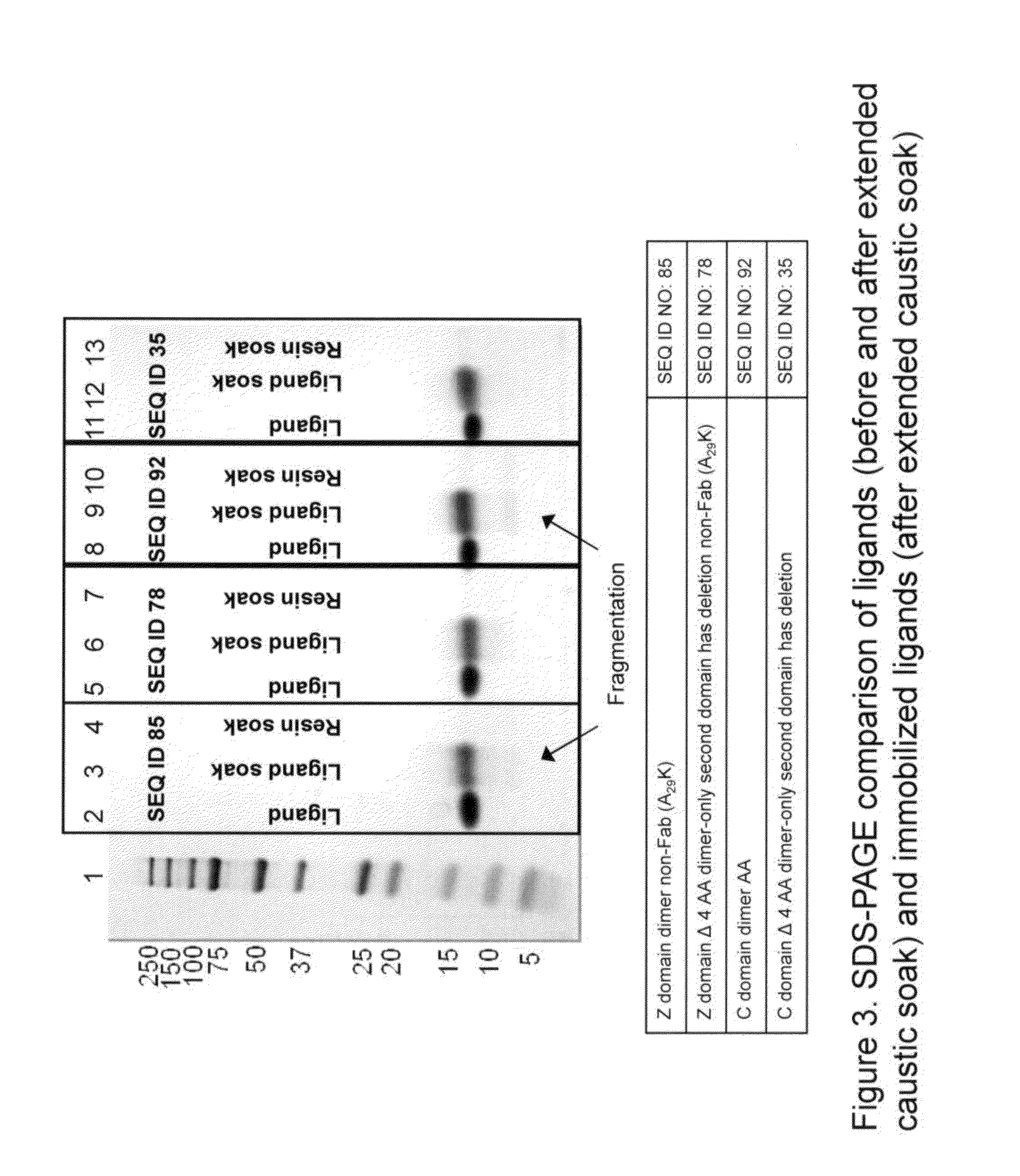
Chromatography matrices including novel staphylococcus aureus protein a based ligands
ActiveUS20130046056A1Reduce lossesLarge degree of fragmentationSolid sorbent liquid separationPeptide preparation methodsStaphylococcus aureusStaphylococcus aureus protein A
The present invention relates to chromatography matrices including ligands based on one or more domains of immunoglobulin-binding proteins such as, Staphylococcus aureus Protein A (SpA), as well as methods of using the same.
Owner:MILLIPORE CORP
Production process of high-quality ecological antibacterial health-care sock
ActiveCN103815555AGood health effectImprove antibacterial propertiesLiquid/gas/vapor article treatmentPanty-hoseYarnEscherichia coli
The invention discloses a production process of a high-quality ecological antibacterial health-care sock. The production process of the high-quality ecological antibacterial health-care sock comprises the following steps of yarn manufacturing, sock weaving, seam allowance processing, reinforcing, setting, pre-drying, water bathing, drying and package detection. The production process of the high-quality ecological antibacterial health-care sock adopts the method that natural cotton fibers, aloe fibers and modal fibers are interlaced and makes full use of the good antibacterial effect of the aloe fibers, and the bottom of the sock adopts a flat structure, so that the fabric can meet the requirements for high air permeability and comfort. The aloe fibers are used for replacing traditional common viscose, and aloe isocitric acid calcium and other matter have the functions of improving the constitution, strengthening the heart, promoting blood circulation, softening hardened arteries, lowering the cholesterol content and expanding the blood capillaries, and have a certain inhibition effect on escherichia coli and staphylococcus aureus. Compared with the prior art, the high-quality ecological antibacterial health-care sock has good health-care performance and antibacterial performance.
Owner:浙江丰悦针纺有限公司
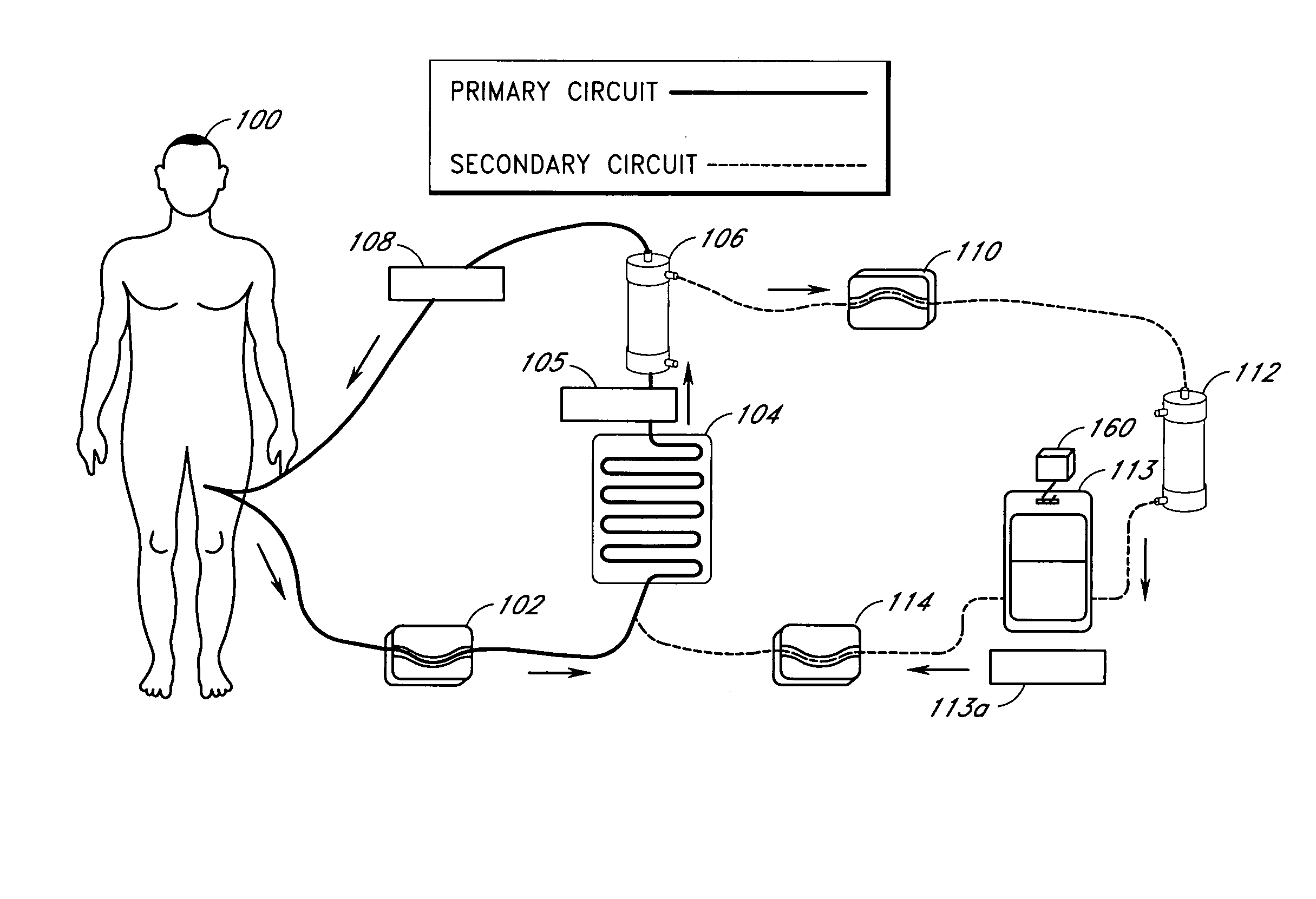
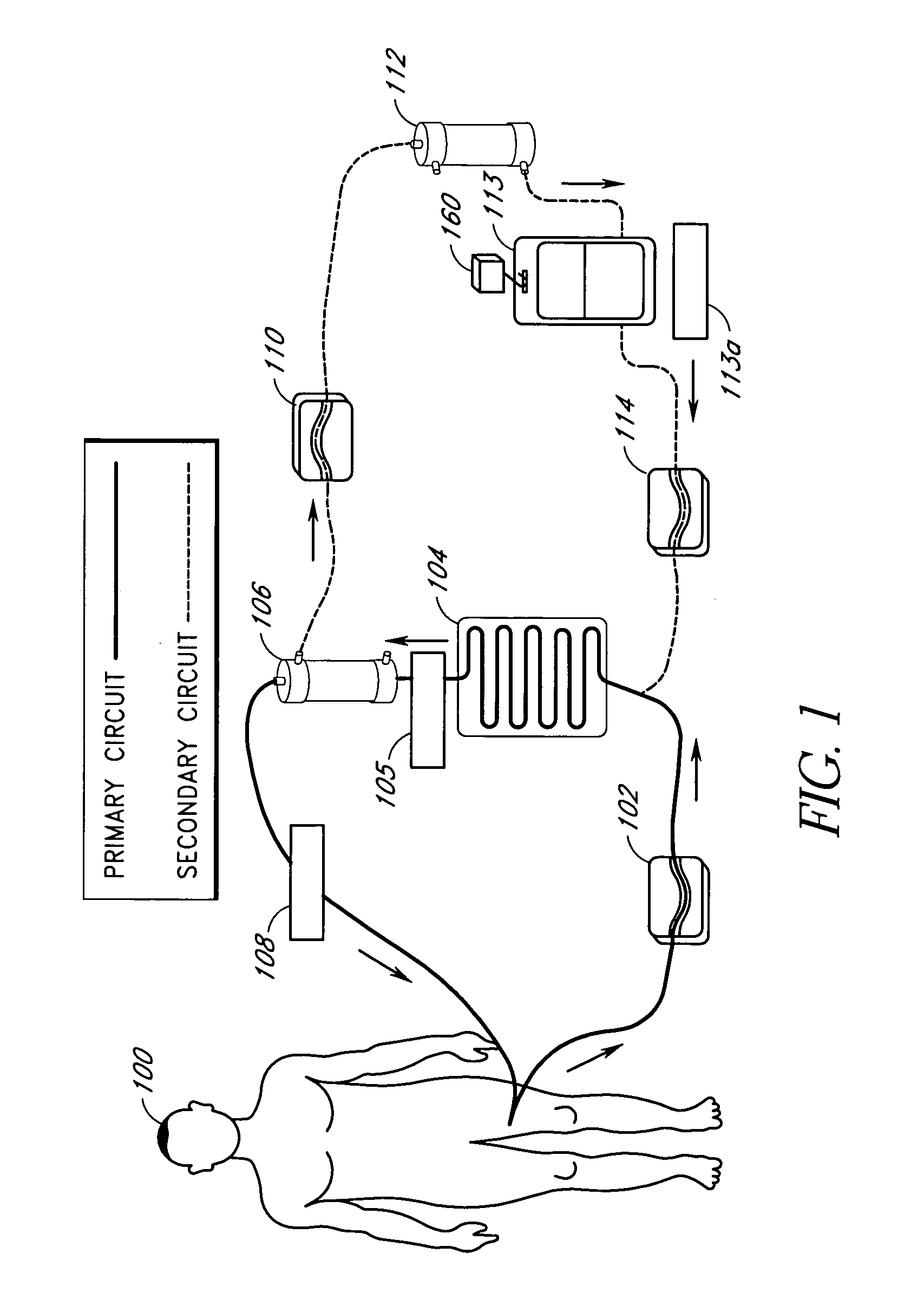
Device and method for reducing inflammatory mediators in blood
InactiveUS7201730B2Reducing free radicals in a patient's bloodReduce concentrationSemi-permeable membranesSolvent extractionInterleukin 6Staphylococcus cohnii
A method and apparatus for preventing and treating septicemia in patient blood is provided. The extracorporeal system includes an antimicrobial device to inactivate at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 50–75% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms, as well as by the patient's cells. These molecules include endotoxins from Gram negative bacteria, exotoxins from Gram negative and Gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, interleukin 6, complement proteins C3a and C5a, and bradykinin.
Owner:HEMAVATION
Affinity purified human polyclonal antibodies and methods of making and using them
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
Bacillus subtilis and feed additive and fermenting agent thereof
ActiveCN102178057AStrong stress resistanceImprove immunityAnimal feeding stuffEscherichia coliBiotechnology
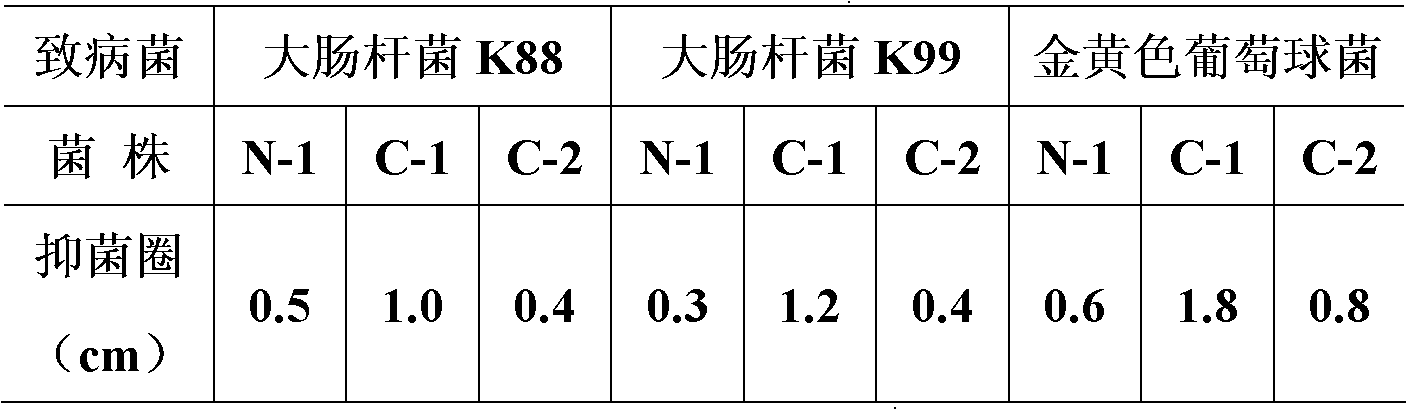
The invention belongs to the field of biological techniques and relates to a bacillus subtilis strain and a feed additive and a fermenting agent thereof. The bacillus subtilis has high stress resistance and probiotic effect, so the bacillus subtilis can tolerate artificial gastric juice with a pH value of 2.0 and a concentration of 1 percent, artificial cholate at a concentration of 0.3 percent and a granulating temperature of 80 DEG C; and the bacillus subtilis has a strong inhibiting effect on Escherichia coli K88, Escherichia coli K99 and staphylococcus aureus, high cellulase producing capacity and ability of degrading cellulose. The biological feed additive prepared by using the bacillus subtilis provided by the invention can be used in place of part of antibiotics in livestock and aquatic product culture, improve immunity in animal, improve feed conversion rate and lower culture cost. The bacillus subtilis also can be used in fermentation of bean pulp, cotton meal, vegetable mealand the like, prevent the feed from mildewing, promote the digestion of cellulose in feed and improve the utilization rate of nutrients in the feed.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
Antibacterial finishing method for textile containing cellulose
ActiveCN103614927AImprove stabilityLess irritatingVegetal fibresMicroballoon preparationStaphylococcus aureusCellulose fiber
The invention relates to an antibacterial finishing method for textile containing cellulose. The method uses natural polymer chitosan and sodium alginate as wall materials; traditional Chinese medicine extracts are used as a core material and are subjected to encapsulation by a micro capsule technology; then an afterfinishing method is employed to treat the microcapsule to the partially carboxymethylated textile containing cellulose, so as to endow the textile with antibacterial performance and good wash fastness through electrostatic attraction between the carboxyl on cellulose fiber and chitosan, as well as the crosslinking effect of the cross-linking agent. The raw materials employed by the invention are green, environment-friendly, safe and high-efficiency, and the obtained textile has good antibacterial effect on common staphylococcus aureus, Eschierichia coli and bacillus subtilis.
Owner:成都艾蒂浮兰科技有限公司
Antibacterial mildewproof polylactic acid composition and preparation method thereof
ActiveCN102453315AImprove antibacterial propertiesGood anti-mildew effectEscherichia coliSodium Pyrithione
The invention relates to an antibacterial mildewproof polylactic acid composition and a preparation method thereof. The composition comprises the following mixed components: polylactic acid, an antibacterial mildewproof agent, and a dispersant; wherein on a basis of 100 parts by weight of polylactic acid, the composition comprises 0.3-2 parts by weight of antibacterial mildewproof agents, and 0-0.5 parts by weight of dispersants; the antibacterial mildewproof agent is a polyguanidine pyrithione antibacterial mildewproof agent which is obtained by mixing an aqueous solution of water-soluble polyguanidine inorganic acid salts or organic acid salts and an aqueous solution of sodium pyrithione; the molar ratio of the water-soluble polyguanidine inorganic acid salts or organic acid salts and sodium pyrithione is 1:(0.1-5). The polylactic acid composition of the invention has good antibacterial and mildewproof effect. The antibacterial effect on staphylococcus aureus and escherichia coli is up to 99.9%; the mildewproof grade for mold such as aspergillus niger, aspergillus terreus, paecilomyces varioti bainier, and the like reaches 0 grade; the composition has good water resistant stability, and has wide application prospects.
Owner:CHINA PETROLEUM & CHEM CORP +1
Air freshener and preparation method and application thereof
ActiveCN101884801AIrritatingIncrease relative volatilityGaseous substancesDeodrantsChemical synthesisSide effect
The invention discloses an air freshener and a preparation method and application thereof. The active ingredients of the air freshener comprise thyme essential oil, mint essential oil and tea tree oil. Bacteriostatic experiments prove the bacteriostatic effect of the air freshener, and the experimental results prove that the air freshener has the advantages of safety, high efficiency, no toxin, no irritation and environmental protection, has strong bacteriostatic and antibacterial effects, and can purify the air and improve the air quality. The air freshener can effectively suppress the activities of colibacillus and staphylococcus aureus in the air, has broad-spectrum antimicrobial activity, can play roles in completely improving the air quality and keeping indoor air fresh and clean, and has bacteriostatic activity similar to the streptomyces avermitilis. The air freshener is prepared from aromatic plant essential oil, such as lavender essential oil and rosemary essential oil instead of chemically synthesized essence, is non-toxic and harmless to human body, and has no irritating harm, sensitivity or other side effects.
Owner:INST OF BOTANY CHINESE ACAD OF SCI
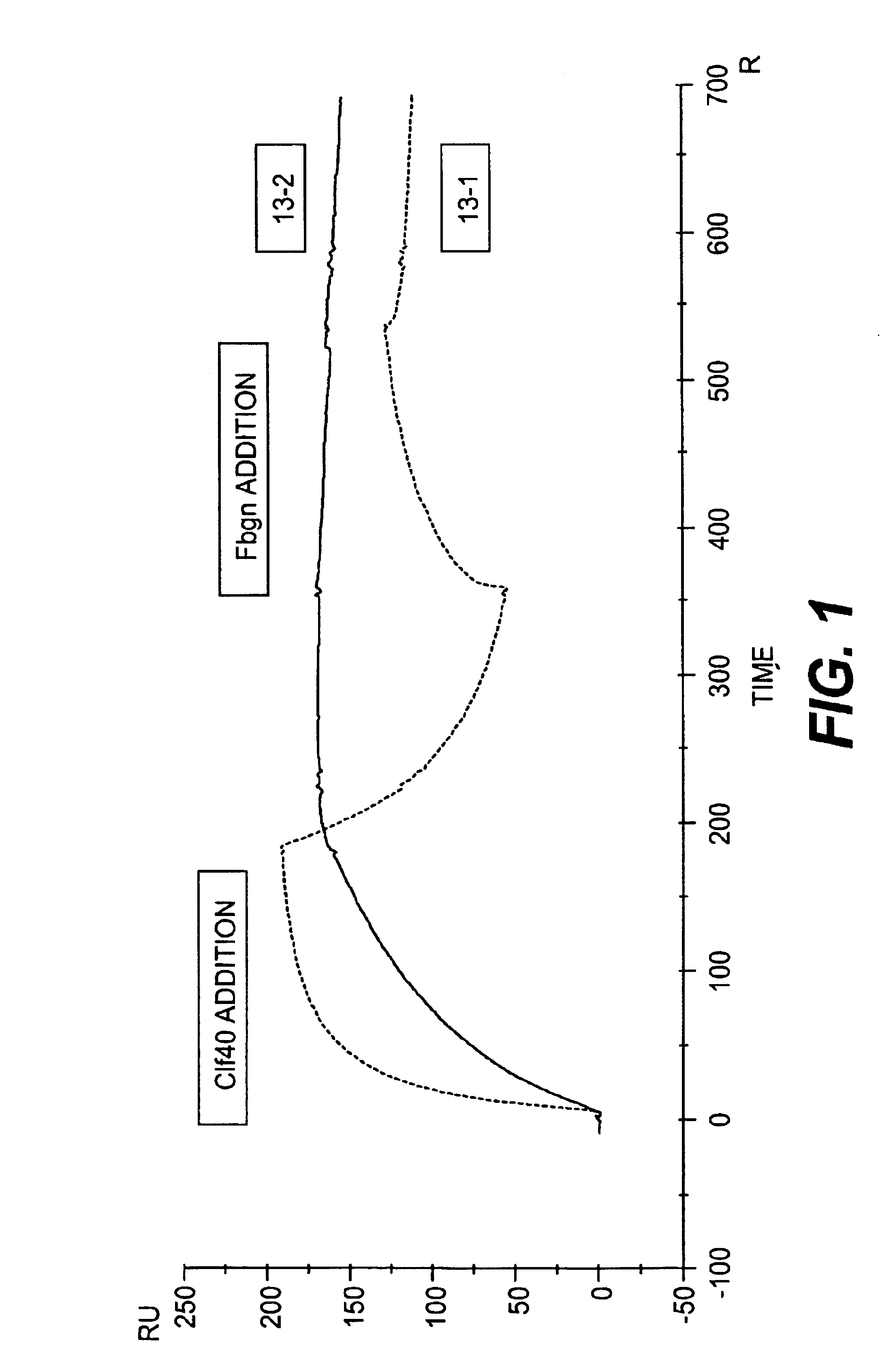
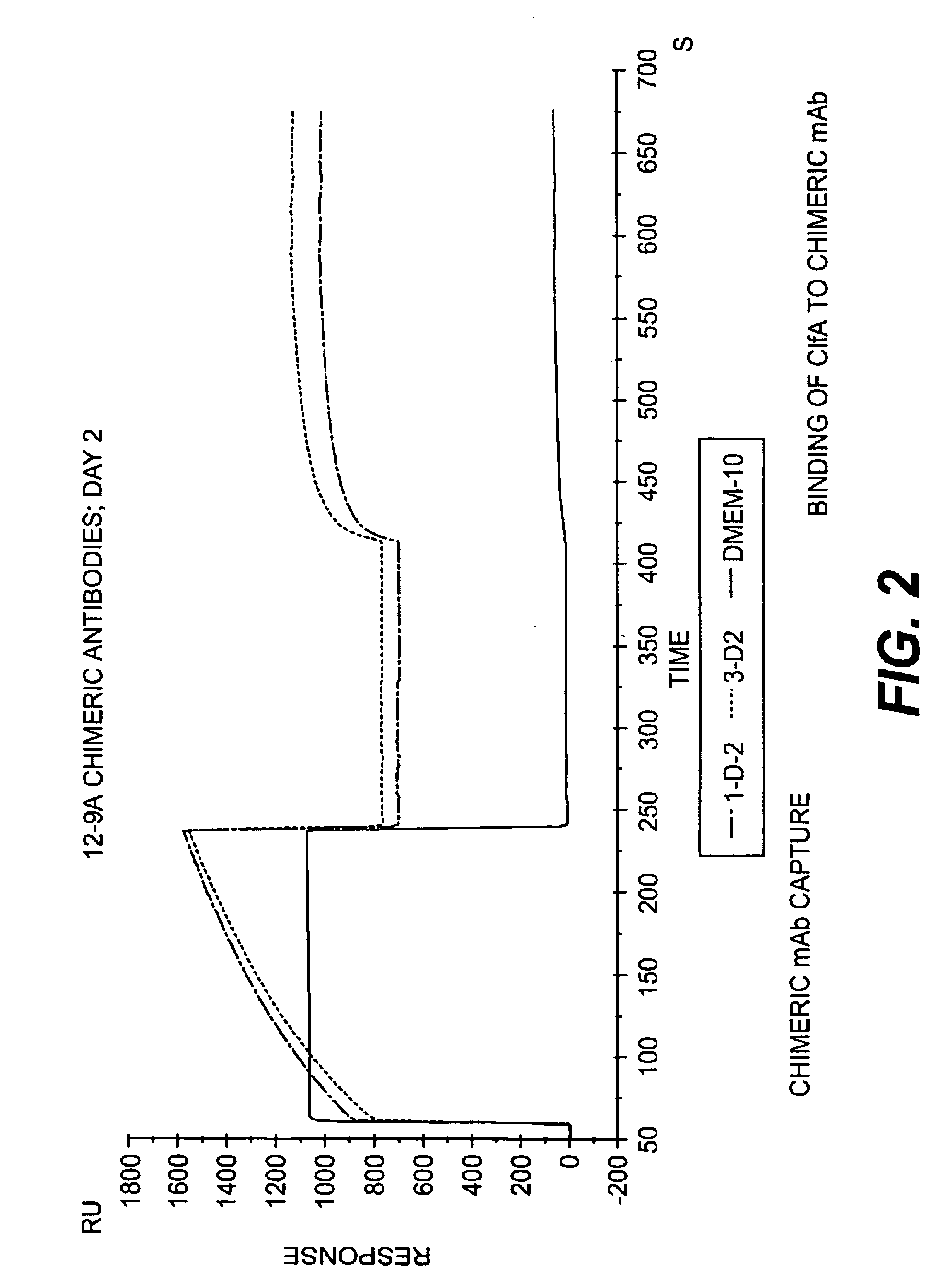
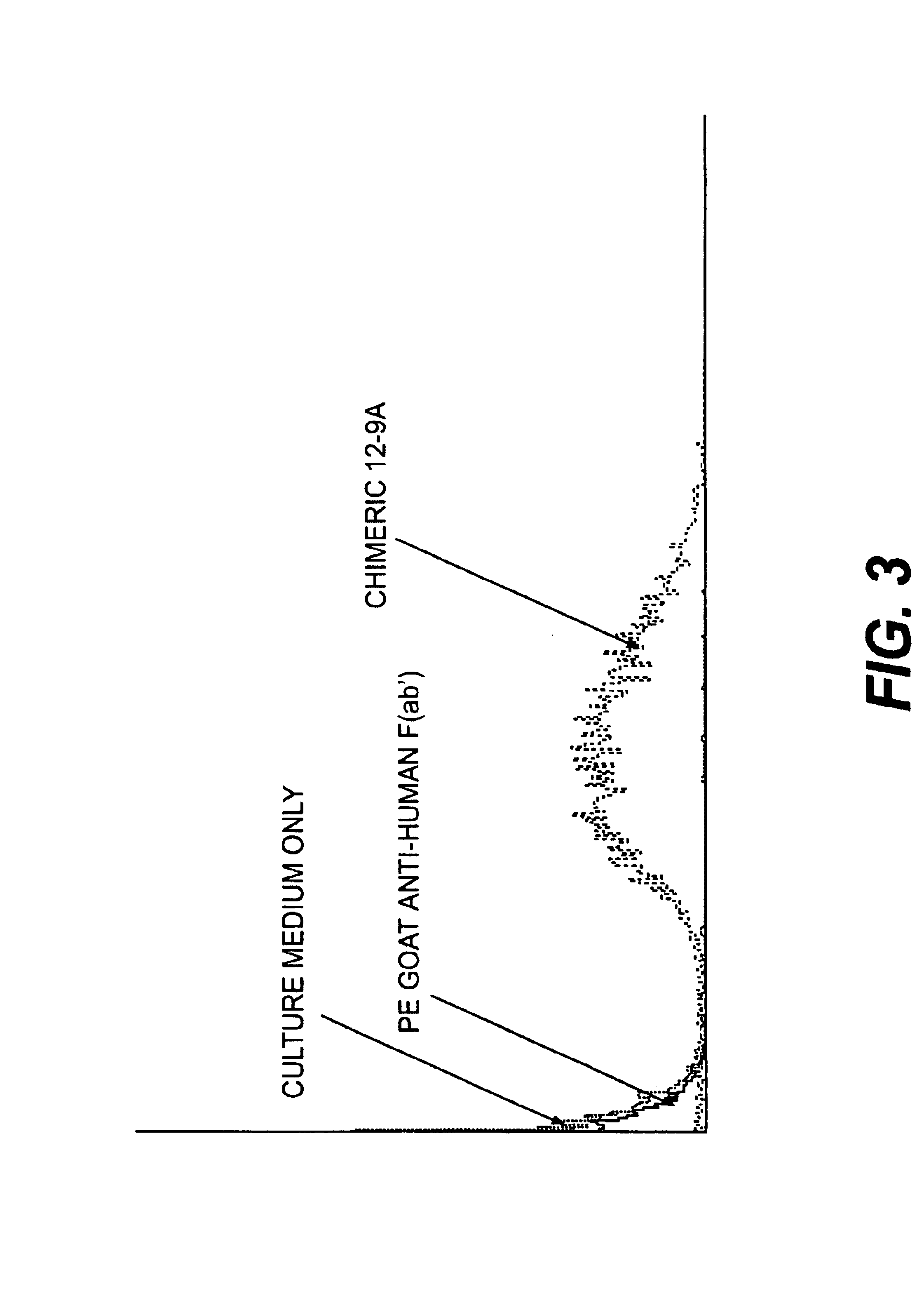
Monoclonal antibodies to the ClfA protein and method of use in treating or preventing infections
InactiveUS6979446B2Avoid stickingInhibiting or impairing the binding of the ClfA proteinAntibacterial agentsBacterial antigen ingredientsBacteroidesStaphylococcus cohnii
Monoclonal antibodies which can bind to the ClfA protein and which are generated from binding subdomains or active fragments of the ClfA protein from Staphylococcus aureus, including the active fragments proteins from its fibrinogen binding domain such as Clf40 protein, the Clf33 protein, or ClfA N3, are provided which can be useful in the treatment and protection against infection from staphylococcal bacteria such as Staphylococcus aureus. In addition, medical instruments can be treated using the monoclonal antibodies of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the antibodies of the present invention are advantageous because they can prevent adherence of the bacteria to host cells by impairing or inhibiting the ability of S. aureus ClfA to bind to fibrinogen or fibrin, and thus can be utilized in methods or treating or preventing staphylococcal inventions.
Owner:INHIBITEX INC
Composite nanometer antibiotic material, preparation method and products thereof
PendingCN1568704AHas broad-spectrum antibacterial functionGood killing effectBiocideAnimal repellantsStaphylococcus aureusStaphylococcus aureus bacteria
A combined nano antiseptic material and its preparation method and products are disclosed. The advantages of the invention lie in 1.combining different antiseptic material and preparing combined nano antiseptic material, taking advantage of synergism and coupling effect of between nano particle, promoting the antiseptic ability 2.The combined antiseptic material has wide spectrum antiseptic function and killing effect to staphylococcus aureus etc. 3.The method can be used in producing sorts of bacteria and virus killing antiseptic material ,such as antiseptic fabric, latex etc.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Nucleic acid arrays for detecting multiple strains of a non-viral species
InactiveUS20070031850A1Bioreactor/fermenter combinationsBiological substance pretreatmentsStaphylococcus aureusPolynucleotide
Nucleic acid arrays and methods of using the same for concurrent or discriminable detection of different strains of a non-viral species. In many embodiments, the nucleic acid arrays of the present invention include probes that are specific to different respective strains of a non-viral species. In many other embodiments, the nucleic acid arrays of the present invention include probes that are common to two or more different strains of the non-viral species. In one embodiment, the non-viral species is Staphylococcus aureus, and the different Staphylococcus aureus strains include COL, N315, Mu50, EMRSA-16, MSSA-476, and 8325 strains. In another embodiment, a nucleic acid array of the present invention includes polynucleotide probes capable of hybridizing under stringent or nucleic acid array hybridization conditions to respective sequences selected from SEQ ID NOs: 1 to 7,852, or the complements thereof.
Owner:WYETH
Bacillus subtilis and use thereof
ActiveCN103614327AEnhanced inhibitory effectStrong amylase production abilityBacteriaMicroorganism based processesEscherichia coliStaphylococcus aureus
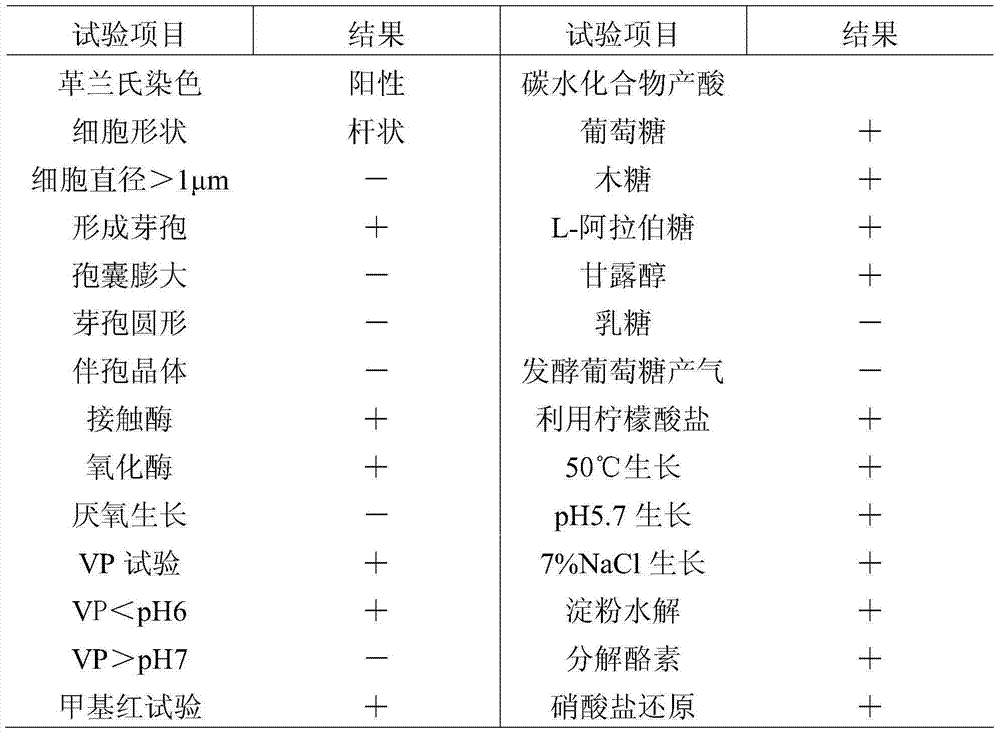
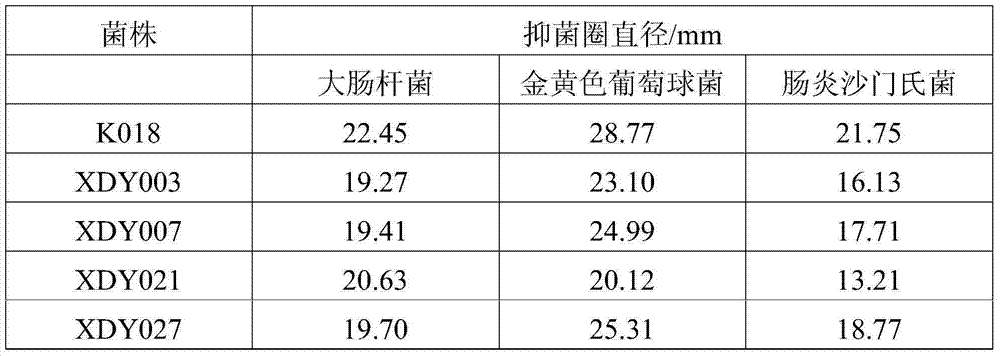
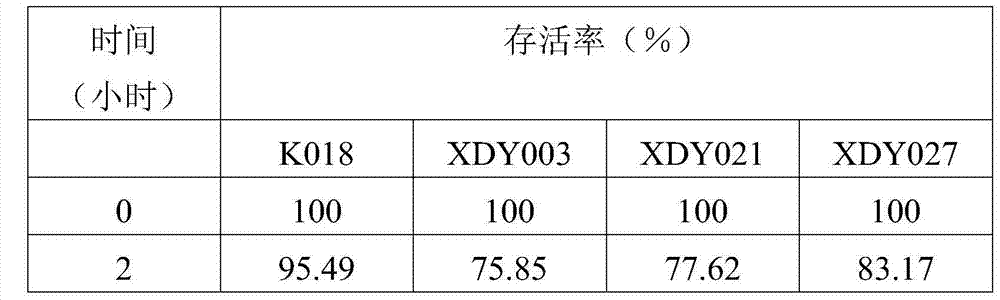
The invention provides bacillus subtilis K018 which is strong in stress resistance, excellent in probiotics function and safe and reliable, a biological feed additive containing the bacillus subtilis K018 and use thereof. The bacillus subtilis K018 provided by the invention can resist artificial gastric acid, cholate, artificial intestinal juice and high temperature, has stronger inhibiting effects to pathogenic staphylococcus aureus, escherichia coli, salmonella enteritidis and the like in the intestinal tract, is stronger in amylase and cellulase generating capacity, and can degrade starch and cellulose.
Owner:北京昕大洋科技发展有限公司
Coating composition with antimicrobial and air purification function
ActiveCN101245211AGood dispersionLong-acting antibacterialDeodrantsCoatingsEscherichia coliStaphylococcus aureus
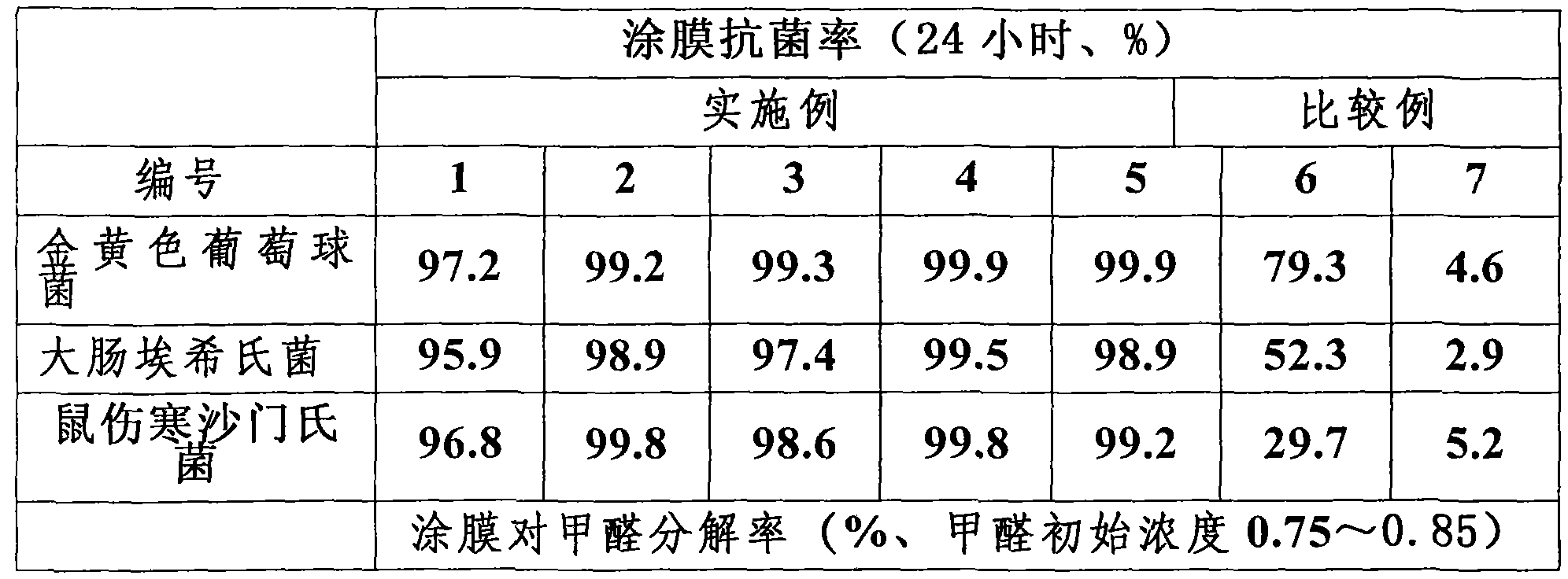
The invention provides a coating composition with antibacterial and air purification function, which comprises 1 to 10 parts by weight of doping four acicular zinc oxide whisker, 1 to 10 parts by weight of mesoporous filler, 40 to 55 parts by weight of acrylate emulsion, 10 to 30 parts by weight of titanium dioxide, 10 to 25 parts by weight of filler talcum powder, and 0.5 to 10 parts by weight of addition agent, that are calculated according to part by weight; wherein, the doping ion is one or more than one elements among Fe<3+>, Cu<2+>, Ag<+> or V<5+>. The coating composition constructed by ordinary spraying or brushing has long-term antibacterial and air purification effects after curing and film forming; the antibacterial effect to staphylococcus aureus, escherichia coli, salmonella typhimurium and other ordinary bacteria in indoor air reaches more than 95 percent, and the degradation rate to formaldehyde with thickness of 0.75 to 0.85 in 12h, 24h, 48h and 72h reaches respectively to 50 percent, 70 percent, 82 percent and more than 85 percent. The coating composition with antibacterial and air purification function is mainly used as indoor coating.
Owner:CHINA PAINT MFG CO SHENZHEN
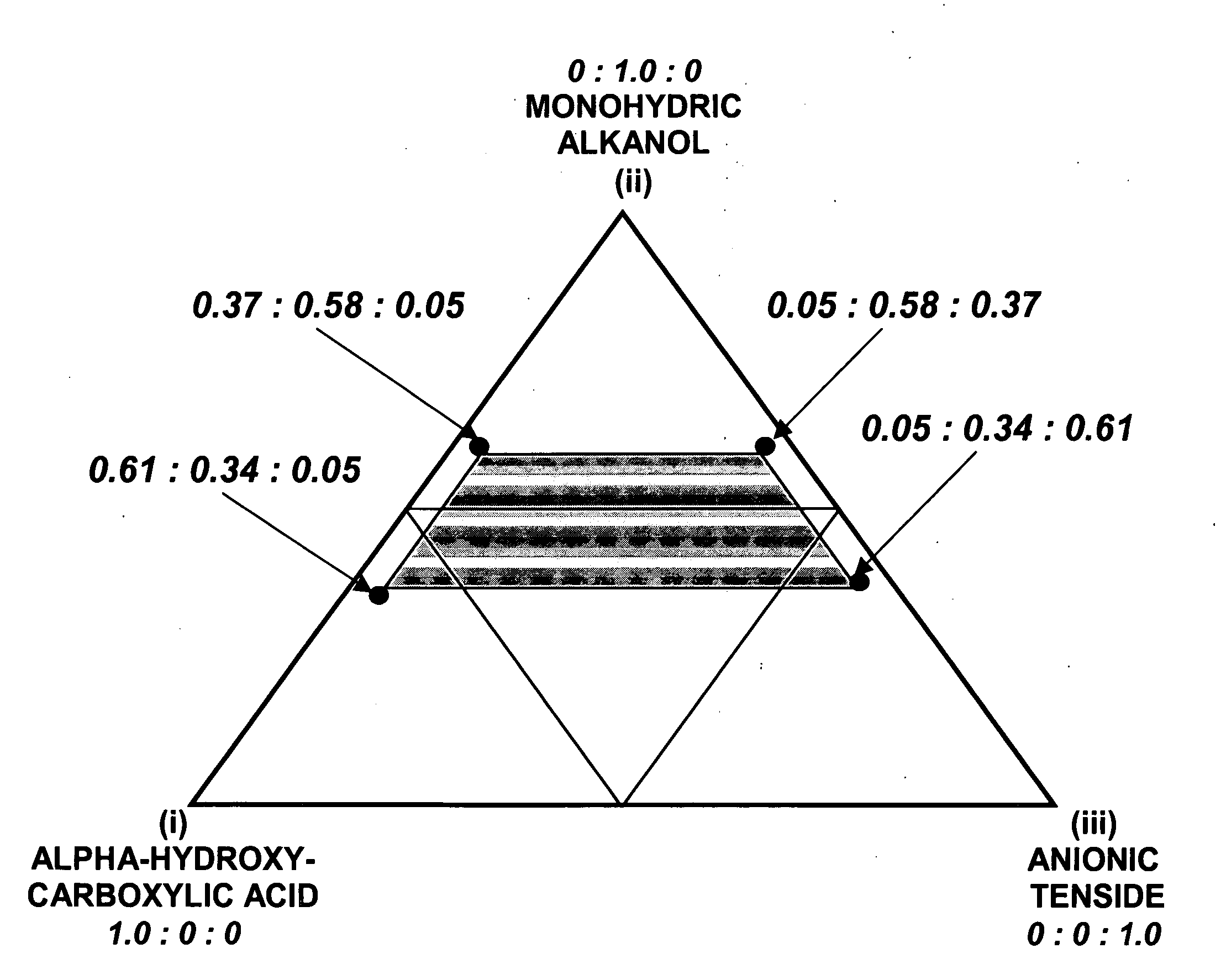
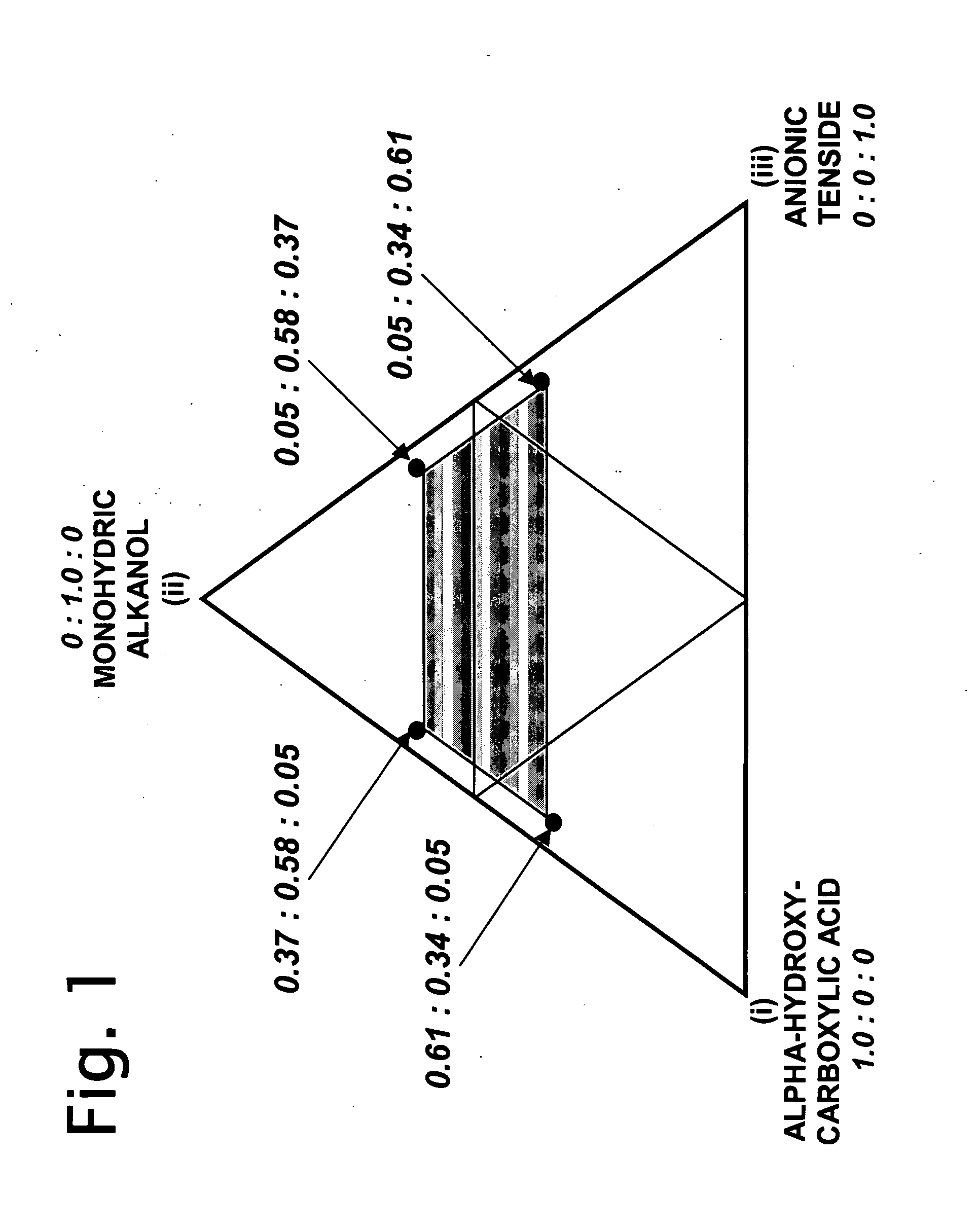
Synergistic acidic ternary biocidal compositions
InactiveUS20060293214A1Great anti-microbial activitySurprising synergistic antimicrobial efficacyBiocideAnionic surface-active compoundsBiotechnologySalmonella kiel
Aqueous dilutions of acidic ternary compositions having unusually synergistic antimicrobial activity against Staphylococcus aureus and Salmonella choleraesuis microorganisms are disclosed. The compositions encompass ternary component mixtures of an alpha-hydroxycarboxylic acid, a monohydric water soluble alkanol and an anionic tenside having a Ternary Ratio residing within a defined phase region wherein synergistic antimicrobial activity is observed; whereas mixtures outside of the defined phase region and those lacking any one of the components do not exhibit synergistic activity. Compositions, methods and articles employing the inventive ternary synergistic compositions, and their utility in sanitizing and disinfecting hard surfaces are described.
Owner:THE CLOROX CO
Screening and application of probiotic Enterococcus faecium
ActiveCN102747003AGood antibacterial effectImprove securityAntibacterial agentsBacteriaDiseaseSynechococcus
The invention belongs to the technical field of veterinary microbial additive preparation, and specifically relates to a strain of separated and screened Enterococcus faecium providing significant bacterial inhibition effects for common enteropathogenic bacteria such as staphylococcus aureus, escherichia coli and salmonella in breeding animals, and an application thereof. The probiotic Enterococcus faecium of the present invention is characterized in that: the strain is Enterococcus faecium HDRsEf1, and is preserved in the China General Microbiological Culture Collection Center (CGMCC), and the preservation number is CCTCC NO:M2011031. The probiotic Enterococcus faecium of the present invention has characteristics of fast growth, high acid production capacity, strong stress resistance, safety, disease resistance and growth promotion, and can be used as the microbial feed additive for livestock and poultry feeds.
Owner:武汉华大瑞尔科技有限公司 +1
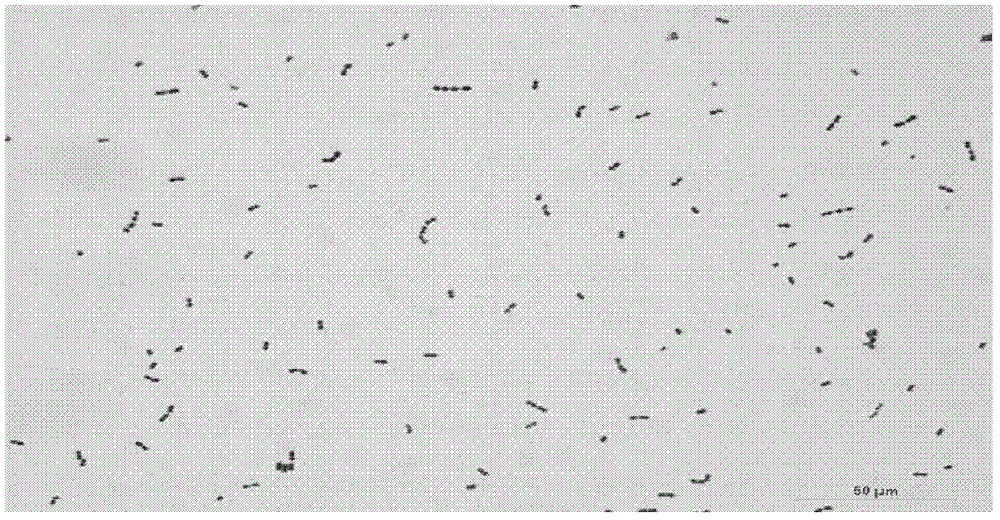
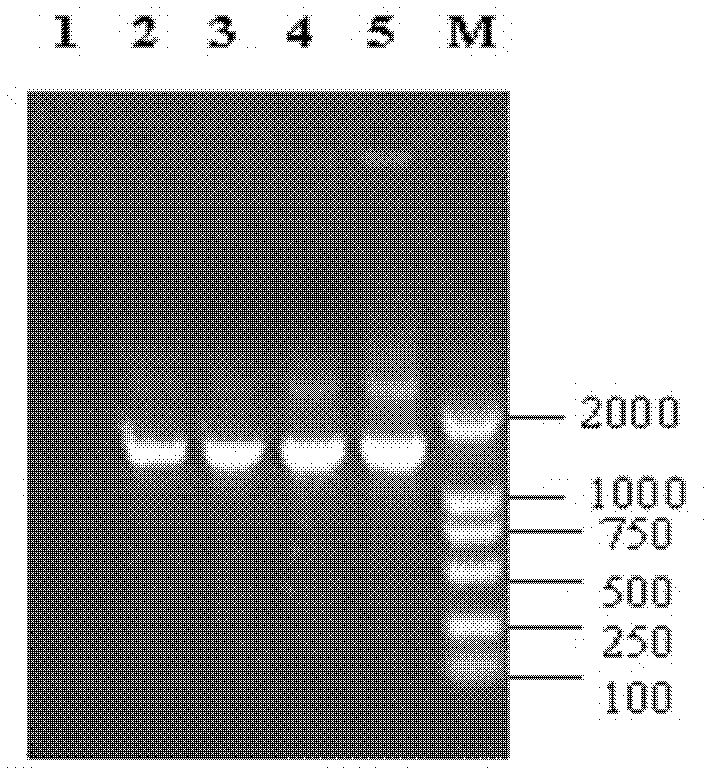
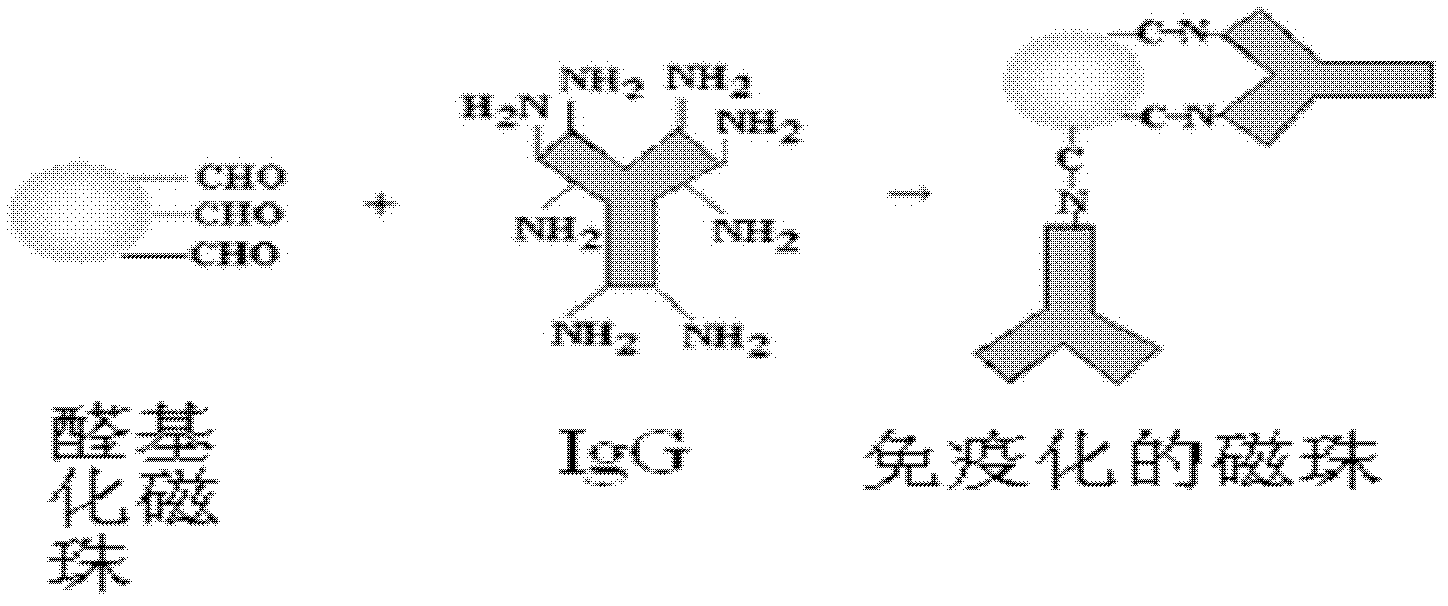
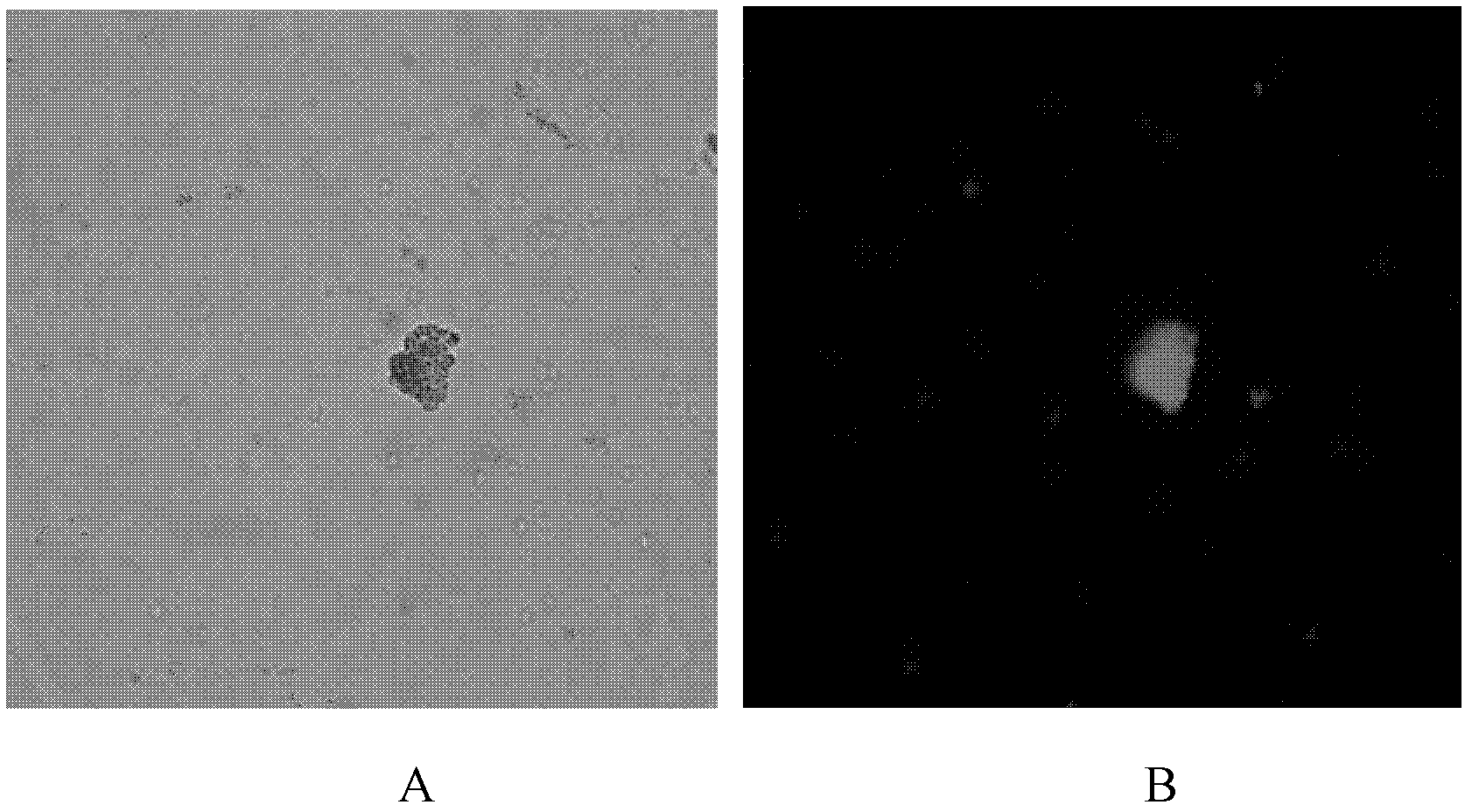
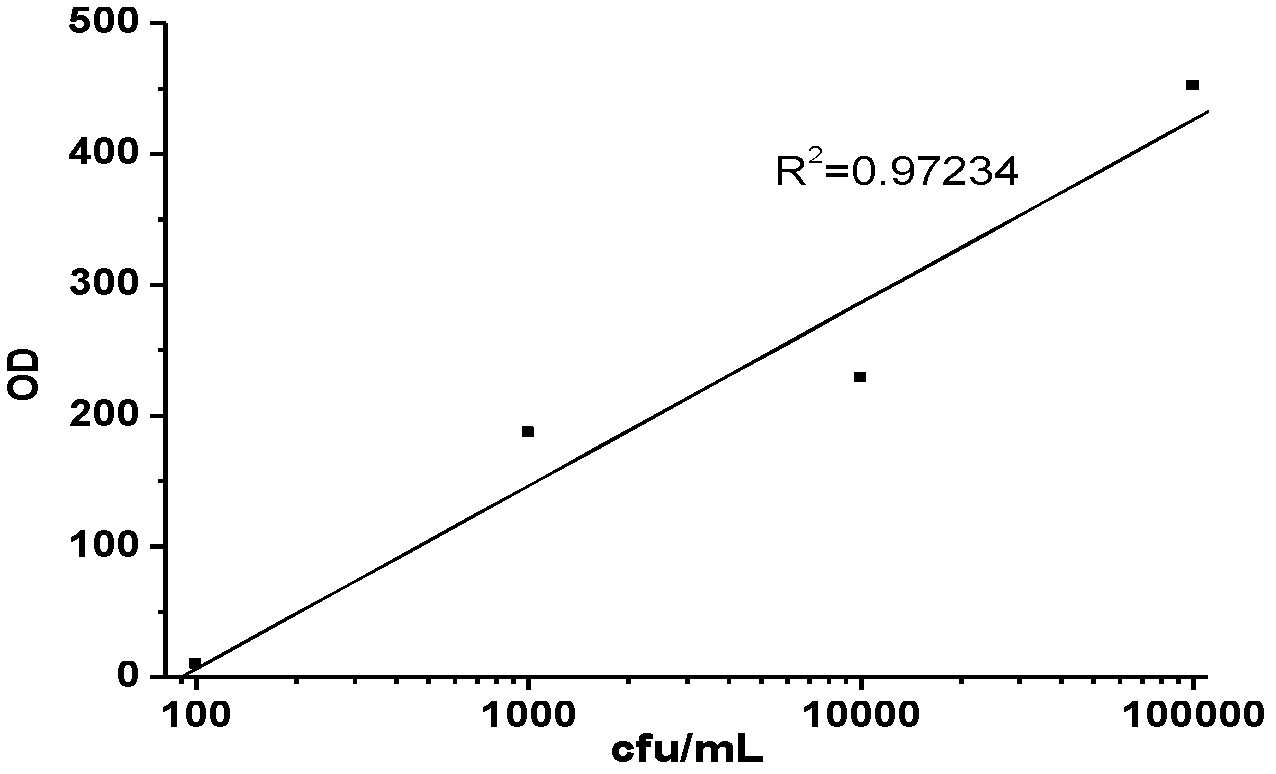
Method for rapidly detecting and screening staphylococcus aureus
InactiveCN102590506ALower requirementEasy to operateMaterial analysisMicroorganismStaphylococcus cohnii
The invention relates to the detection field of microorganism foodborne pathogens and discloses a method for rapidly detecting and screening staphylococcus aureus, which adopts bio-functionalized superparamagnetic nano particles and immunized quantum dots to immunologically recognize target microorganisms to realize to specificity detection on the foodborne pathogens (staphylococcus aureus). According to the method, target bacteria can be rapidly separated from various samples, and enrichment is also performed efficiently; and besides, bacteria separated by the enrichment can be marked rapidly by fluorescence, so that the bacteria can be qualitatively identified by a fluorescence microscope and can be quantitatively detected by a fluorescence photometer. The method is convenient and simple to operate, and has high reliability and a low requirement on corollary equipment.
Owner:SHANGHAI NORMAL UNIVERSITY
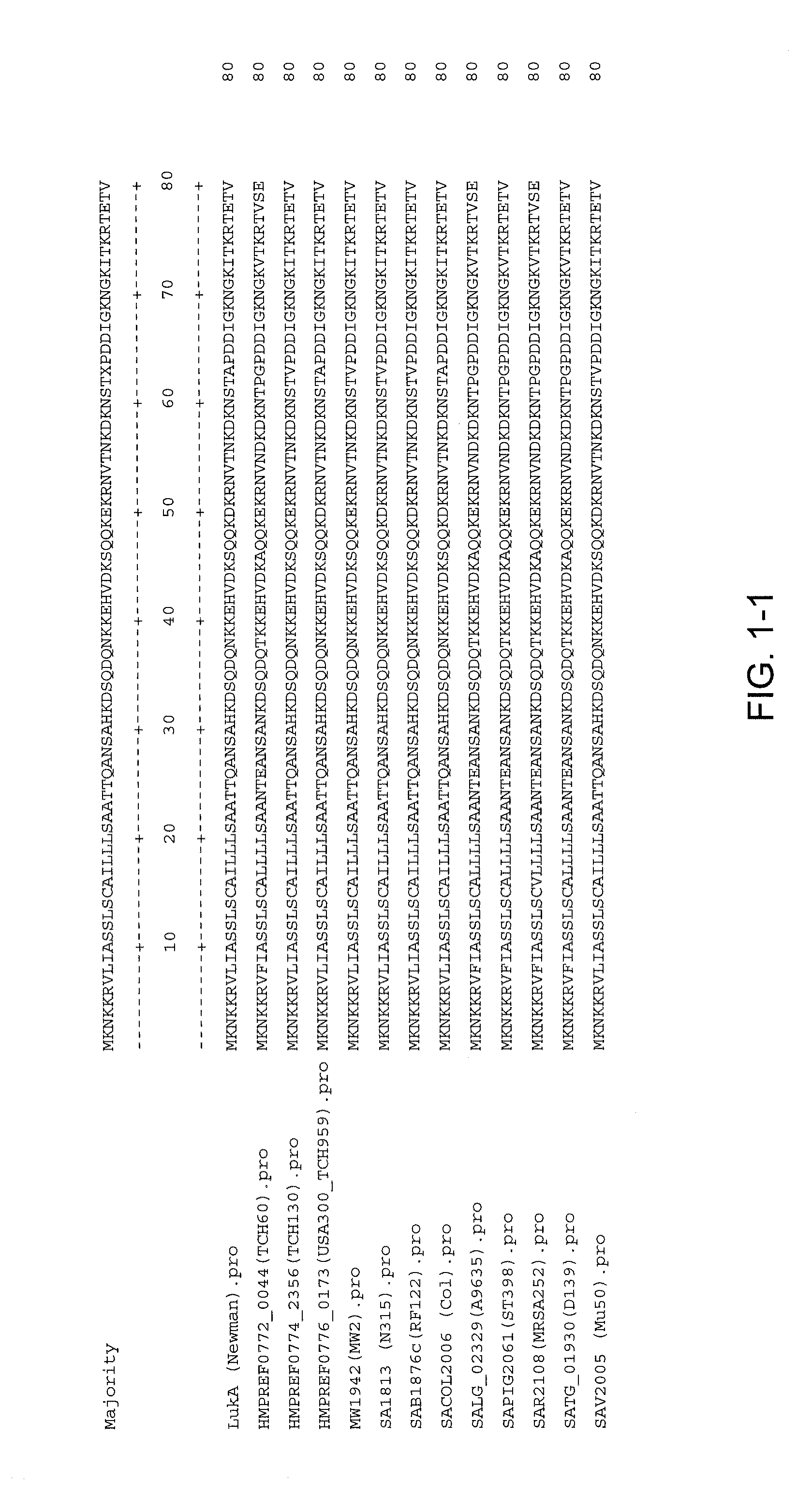
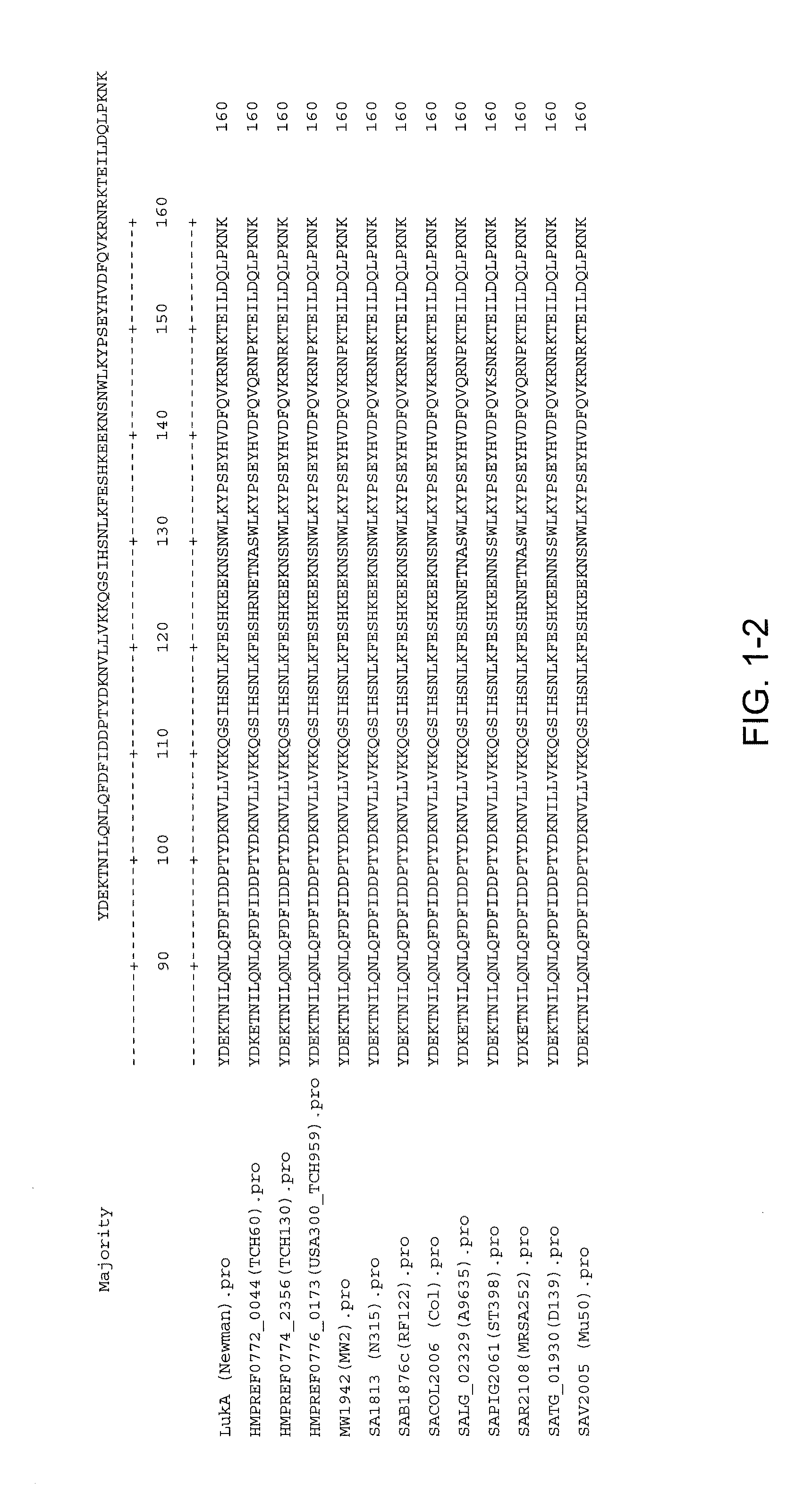
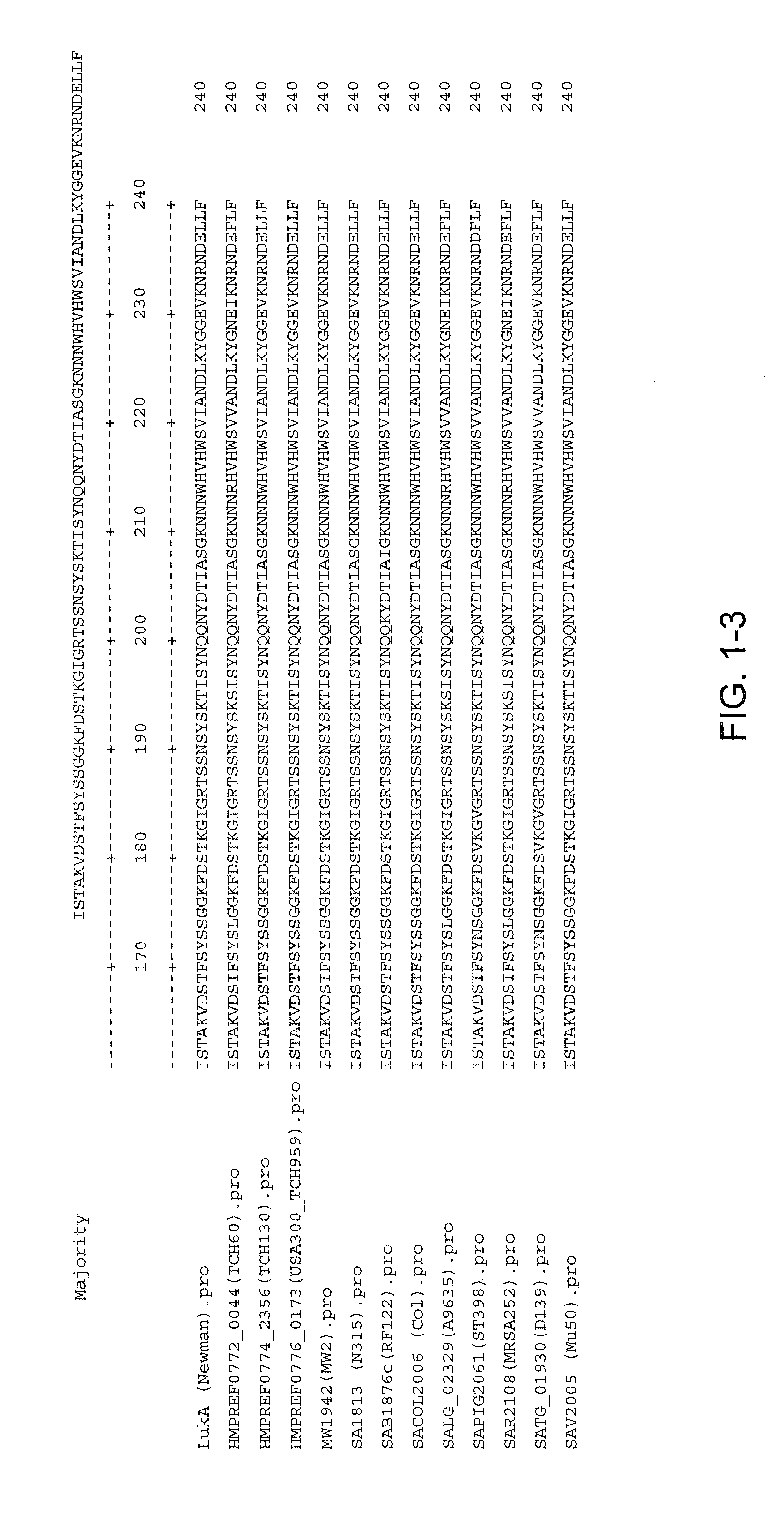
Staphylococcus aureus leukocidins, therapeutic compositions, and uses thereof
ActiveUS20110274693A1Reduce in quantityReduce severityAntibacterial agentsPeptide/protein ingredientsPhagocyteWhite blood cell
Disclosed herein are isolated and purified Staphylococcus aureus bi-component leukocidin, referred to herein as LukAB, and its components LukA and LukB, antibodies specific to LukA, antibodies specific to LukB, therapeutic compositions containing LukA and / or LukB, or anti-LukA and / or anti-LukB antibodies, uses of the compositions to treat acute inflammatory conditions or S. aureus infection, methods for identifying inhibitors of LukAB-mediated cytotoxicity of human phagocytes, and methods for using LukAB as a marker to predict severity of S. aureus infection.
Owner:NEW YORK UNIV
Lactobacillus plantarum and application thereof
ActiveCN105132322AIncrease production capacityGood for healthBacteriaMicroorganism based processesEscherichia coliFeed conversion ratio
The invention discloses lactobacillus plantarum GLM101 and an application thereof in preparing a feed additive. The strain is preserved in the China General Microbiological Culture Collection Center (CGMCC) with the preservation number CGMCC No. 11156. The lactobacillus plantarum GLM101 has very strong inhibiting ability for escherichia coli, staphylococcus aureus, salmonella typhi, vibrio vulnificus and aeromonas hydrophila, and also has excellent acid-resisting and cholate-resisting abilities. The lactobacillus plantarum GLM101 can be used for adjusting the microecological balance inside the intestines of animals, has the action of enhancing the nonspecific immunity function to prevent diseases, and also can provide trophic factors, promote digestive absorption of nutrients, promote growth of animals, and improve the feed conversion ratio.
Owner:GUANGZHOU GLAM BIOTECH
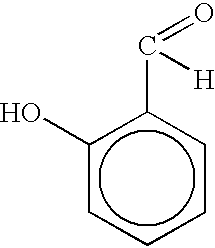
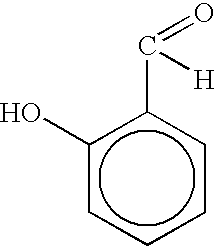
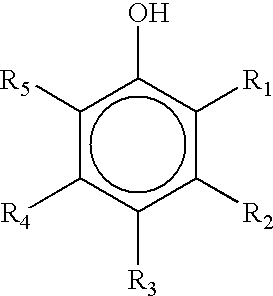
Salicylaldehyde-containing composition having antimicrobial and fragrancing properties and process for using same
InactiveUS6495512B1Alter aromaEffective amountBiocideCosmetic preparationsEscherichia coliSalicylaldehyde
Described are synergistic antimicrobial-fragrance compositions including broad spectrum antimicrobial compositions containing salicylaldehyde and at least one organoleptically-compatible antimicrobial synergism cofactor substance. The weight ratio range of salicylaldehyde:synergism cofactors substance is from 1:10 up to 10:1. The cofactor substance is such that the degree of synergism of the resultant mixture is defined according to the IFF Antimicrobial Synergism Test wherein the difference between the actual and expected antimicrobial values of the mixture is greater than or equal to a multiple of (i) 0.05 and (ii) the expected antimicrobial value of the mixture. Cofactor substances include phenolics such as cresol, caravacrol and thymol; ethyl vanillin; benzyl alcohol; indol; beta-orcinol; and terpinenol-4. Microorganisms against which the synergistic compositions are effective include:Escherichia coli;Enterococcus hirae;Pseudomonas aeruginosa;Staphylococcus aureus; andSaccharomyces cerevisae.The compositions have application in all-purpose cleaning compositions, gel-type toilet rim articles, liquid-type toilet rim articles, personal shower cleaning compositions, and body and hair care products including shower gel compositions, shampoo compositions and foam bath compositions.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES +2
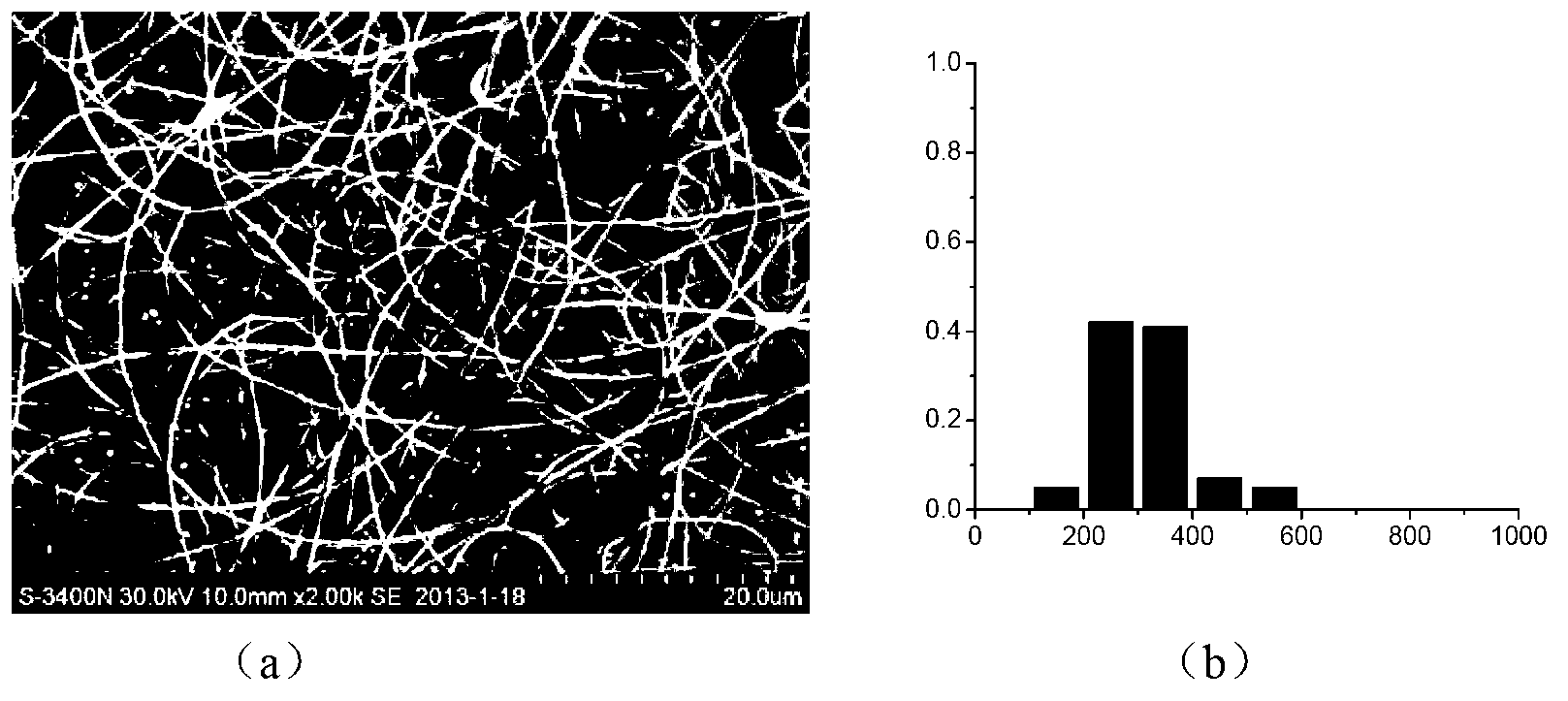
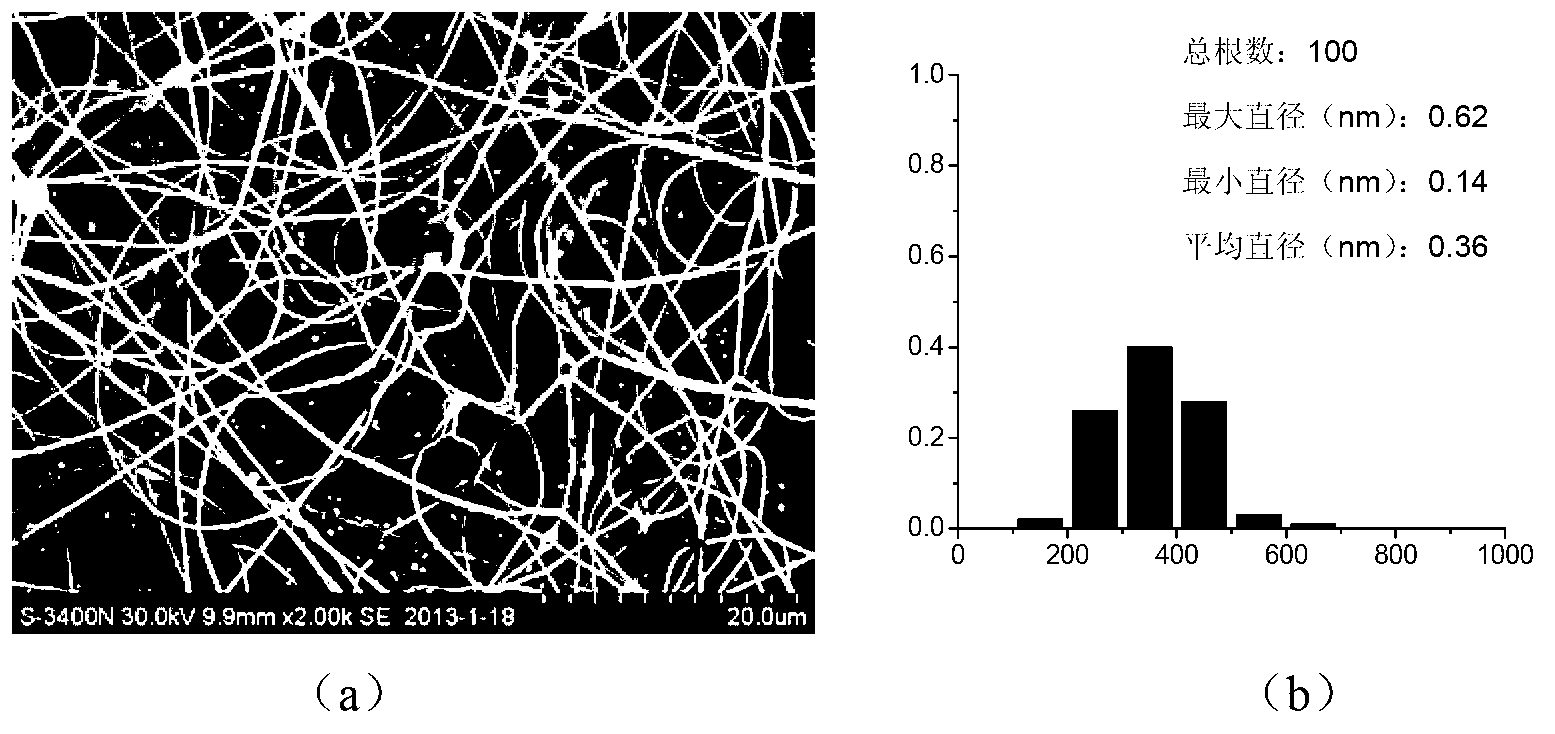
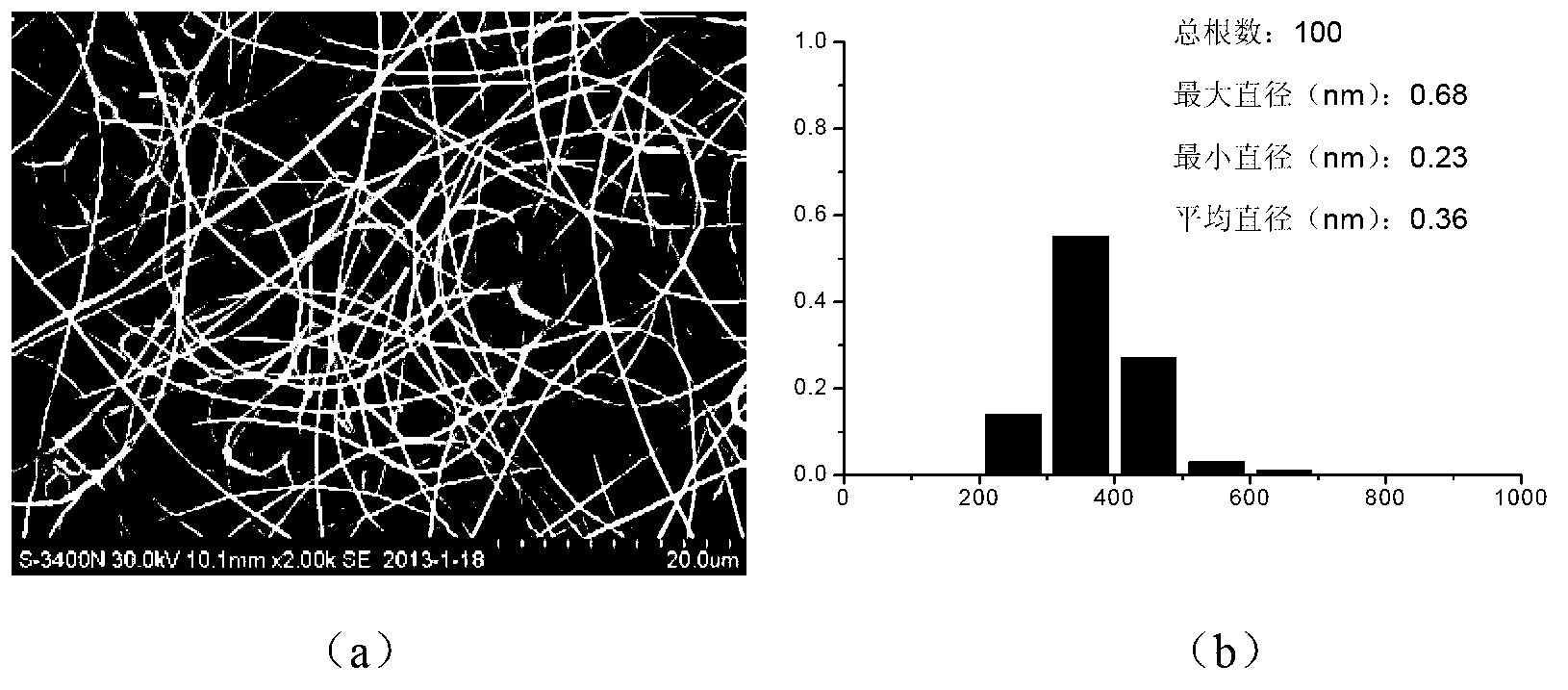
Preparation of antibacterial silver/chitosan nano fiber membrane
InactiveCN101297976AImprove mechanical propertiesRemain biodegradableSurgeryFilament/thread formingFiberStaphylococcus aureus
The invention discloses a preparation method of antibacterial silver / chitosan nano fibrous membranes, pertaining to the preparative technologies of nano composite fibrous membranes. The process of the method includes that a chitosan hexanoic acid solution, a silver nitrate aqoeous solution, a sodium borohydride water solution and an ethylene epoxide hexanoic acid solution are prepared and mixed to form compounded latex according to the volume ratios of the chitosan hexanoic acid solution and the silver nitrate aqoeous solution as well as an NaBH4 aqueous solution. The compounded latex and the ethylene epoxide hexanoic acid solution are mixed according to the volume ration to prepare a spinning solution, then the spinning solution is added into an injector in an electrostatic spinning device and electrostatic spinning is carried out to form the fibrous membrane. Crosslinking treatment is carried out to the fibrous membrane to obtain the antibacterial silver / chitosan nano fibrous membrane. The antibacterial silver / chitosan nano fibrous membrane of the invention has the advantages that the preparation process is simple; the prepared membrane material has broad-spectrum bactericidal property and comparatively high fatality rate to Bacillus coli, Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa for 24 hours.
Owner:TIANJIN UNIV
Antiviral and antibacterial activity from medicinal mushrooms
Compounds having unique antiviral and antibacterial properties are prepared from medicinal mushroom mycelium, extracts and derivatives. The compositions are derived from Fomitopsis, Piptoporus, Ganoderma, Inonotus, Trametes, Pleurotus, and blends of medicinal mushroom species and are useful in preventing and treating viruses including Poxyiridae and Orthopox viruses, flu viruses including bird flu (H5N1), SARS and Hepatitis C(HCV), as well as infections from Mycobacterium tuberculosis, Staphylococcus aureus and Escherichia coli.
Owner:TURTLE BEAR HLDG LLC
Nanofiber composite membrane containing plant source antibacterial agents, preparation method and application of nanofiber composite membrane
InactiveCN103266424AGood water solubilityGood biocompatibilityFilament/thread formingMonocomponent synthetic polymer artificial filamentEscherichia coliPolyvinyl alcohol
The invention belongs to the field of nanometer materials and food packaging materials, and discloses a nanofiber composite membrane containing plant source antibacterial agents, a preparation method and application of the nanofiber composite membrane. The preparation method of the nanofiber composite membrane includes a first step of preparing 6%-10%, by mass, of polyving akohol solution, a second step of cooling the obtained polyving akohol solution down to 30-50 DEG C, adding 1%-3% of beta-cyclodextrin, carrying out magnetic stirring for 0.5-1 hour, and then cooling the mixtures down to indoor temperatures, a third step of adding 2%-8% of cinnamon essential oil to the solution of the second step, carrying out magnetic stirring for 1-2 hours at 25-40 DEG C, and obtaining spinning solution, and a fourth step of carrying out electrostatic spinning on the obtained spinning solution, carrying out vacuum drying, and obtaining the nanofiber composite membrane containing the plant source antibacterial agents. The sterilizing rate of the nanofiber composite membrane for staphylococcus aureus and escherichia coli is larger than or equal to 90%, and the nanofiber composite membrane has good tenacity, biocompatibility and degradability, and has wide application prospect in the field of the food packaging materials.
Owner:SOUTH CHINA UNIV OF TECH
Andrographolide derivatives and application of the same in pharmacy
The invention relates to an andrographolide derivative, which has the structure as shown in a general formula I: wherein, R1, R2 and R3 are the same or different hydrogen, substituted or non-substituted organic acid radical, inorganic acid radical, alkyl, aryl or heteroaryl, while at least one of R1, R2 and R3 is R-lipoic acid or S-lipoic acid or the mixture of R-lipoic acid and S-lipoic acid or the corresponding dihydrolipoic acid or acetylcysteine radical of R-lipoic acid or S-lipoic acid; the derivative has good anti-tumor effect, and the derivative can cause apoptosis of tumor cells, directly eliminate gram positive bacteria, staphylococcus aureus and sensitivities MRSA5676 and MRSA5677, inhibit the QS system of gram negative bacteria and pseudomonas aeruginosa and inhibit and damage the formation of the bio-film of pseudomonas aeruginosa; the product has prominent hypoplycemic effect and is suitable for the preparation of the medicines that can cure cancers, inflammations, diabetes mellitus and bacterial and viral infection.
Owner:JINAN UNIVERSITY
Nano-silver solution and preparation method thereof
ActiveCN101999412AImprove antibacterial propertiesAvoid infectionBiocideDisinfectantsEscherichia coliAntibiosis
The invention relates to nano-silver solution with the nano-silver concentration between 250 and 3,200ppm and the average sliver grain diameter between 2 and 12nm. The nano-silver solution also comprises medicine accessory level dispersing agents and medicine level reducing agents. The preparation method mainly comprises the following steps of: dissolving the medicine accessory level dispersing agents into deionized water to prepare dispersing agent solution; adding and dissolving silver nitrate to obtain solution I; taking and adding the medicine accessory level reducing agent solution into the solution I; mixing the medicine accessory level reducing agent solution with the solution I to obtain solution II; placing the solution II under an ultraviolet lamp to be irradiated; and stirring the solution II for reaction to obtain the nano-silver solution. The nano-silver solution has stronger antibiosis performance. When the nano-silver solution is diluted to 10ppm to detect and evaluate the antibiosis effect on staphylococcus aureus, colibacillus and pseudomonas aeruginosa, the antibiosis efficiency reaches higher than 99.9 percent in two minutes. The preparation method has simple process, can avoid the use of various strong acid, strong base and strong reducing agents and has good medical safety.
Owner:SHENZHEN LANDO BIOMATERIALS
Method for preparing nanofibre membrane
InactiveCN101638830AHas antibacterial functionPromote wound healing functionFilament/thread formingAbsorbent padsComposite nanofibersNanofiber
The invention discloses a method for preparing a nanofibre membrane, belonging to a technique for preparing a nanofibre compound membrane. The method comprises the following steps: preparing curcuminethanol solution and chitosan acetum, mixing the curcumin ethanol solution and the chitosan acetum by the volume ratio thereof to prepare spinning solution which is injected into an injector of an electrostatic spinning device for electrostatic spinning to form a compound nanofibre membrane and to obtain a curcumin / chitosan nanofibre membrane of antibacterial property, wherein the diameter of thecurcumin / chitosan nanofibre membrane is 200-400 nm. The invention has the advantages that the preparation process is simple, the prepared membrane material heals wound, has broad spectrum bactericidalproperty and has higher bacterial inhibition rate to colibacillus and staphylococcus aureus for 24h.
Owner:JIANGNAN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com