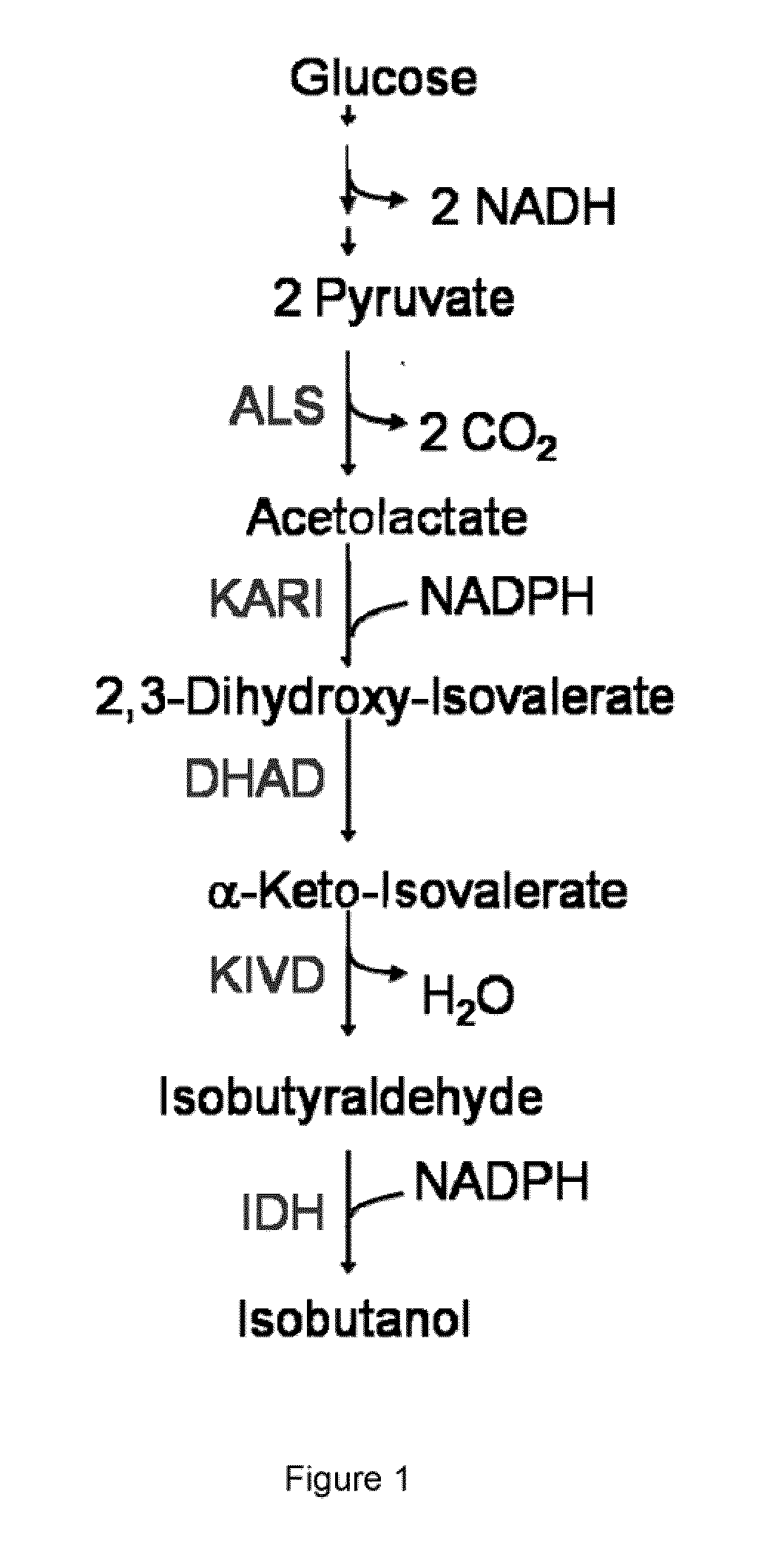Patents
Literature
197 results about "Cofactor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's activity as a catalyst, a substance that increases the rate of a chemical reaction. Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized by in an area of study called enzyme kinetics.
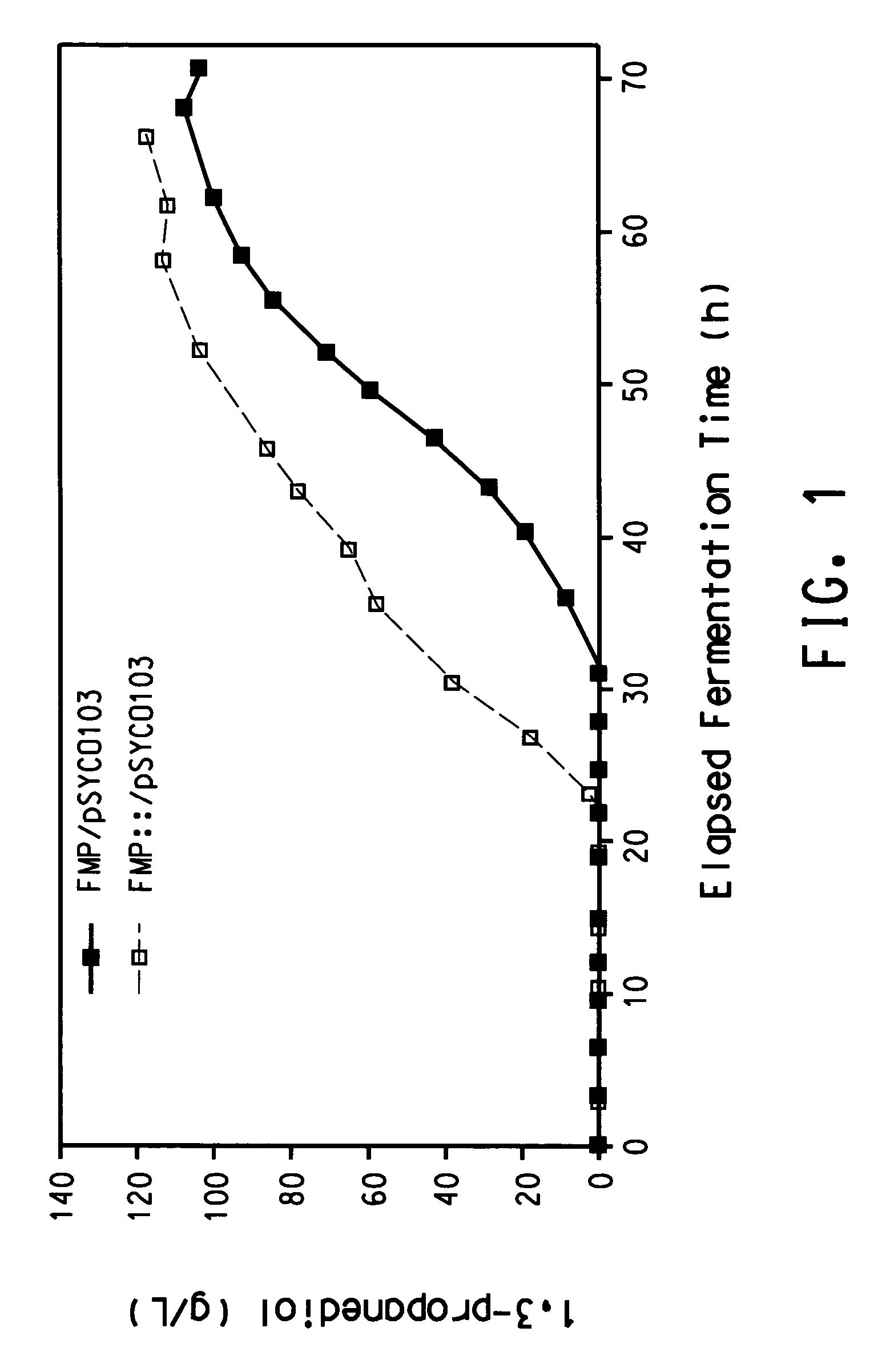
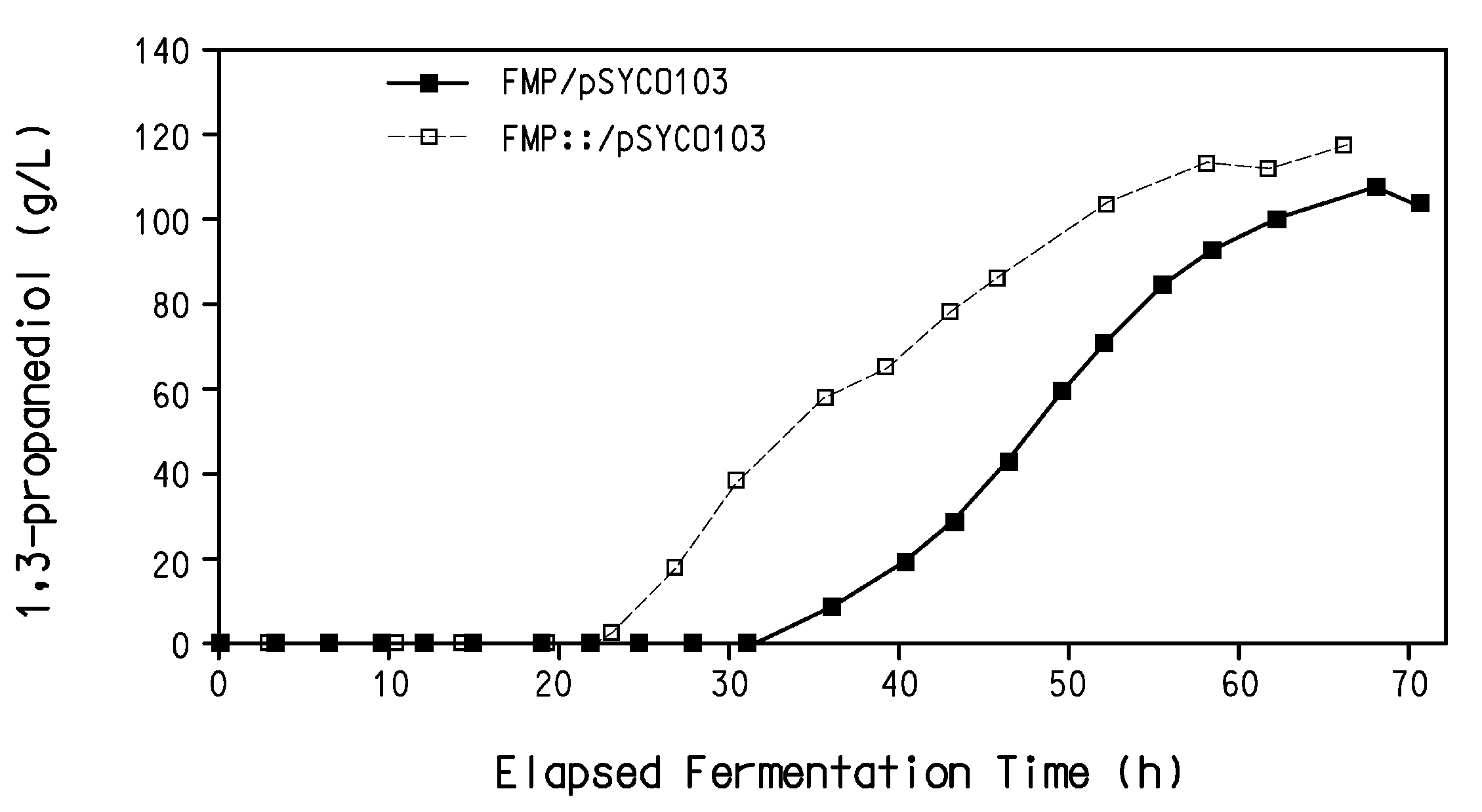
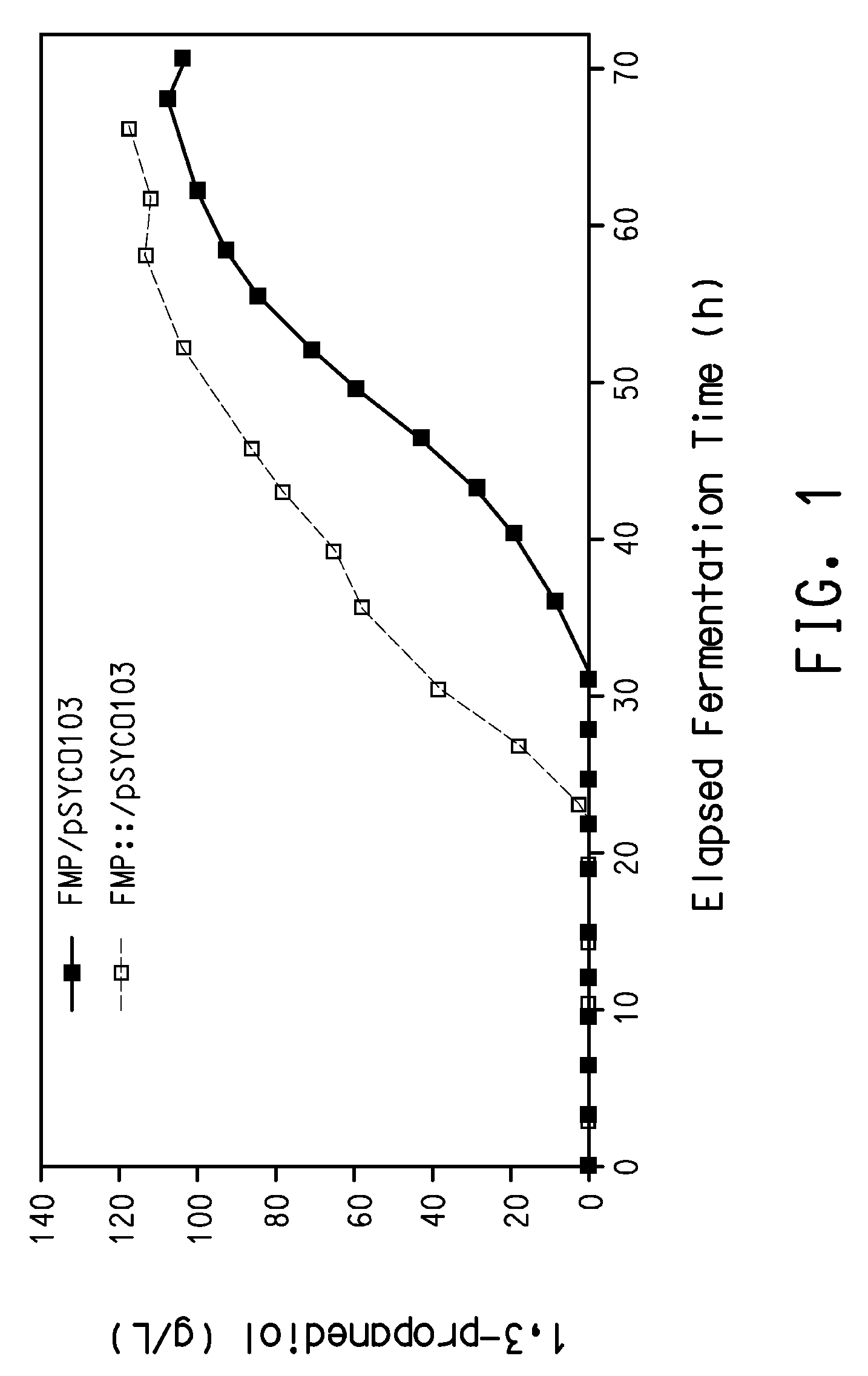
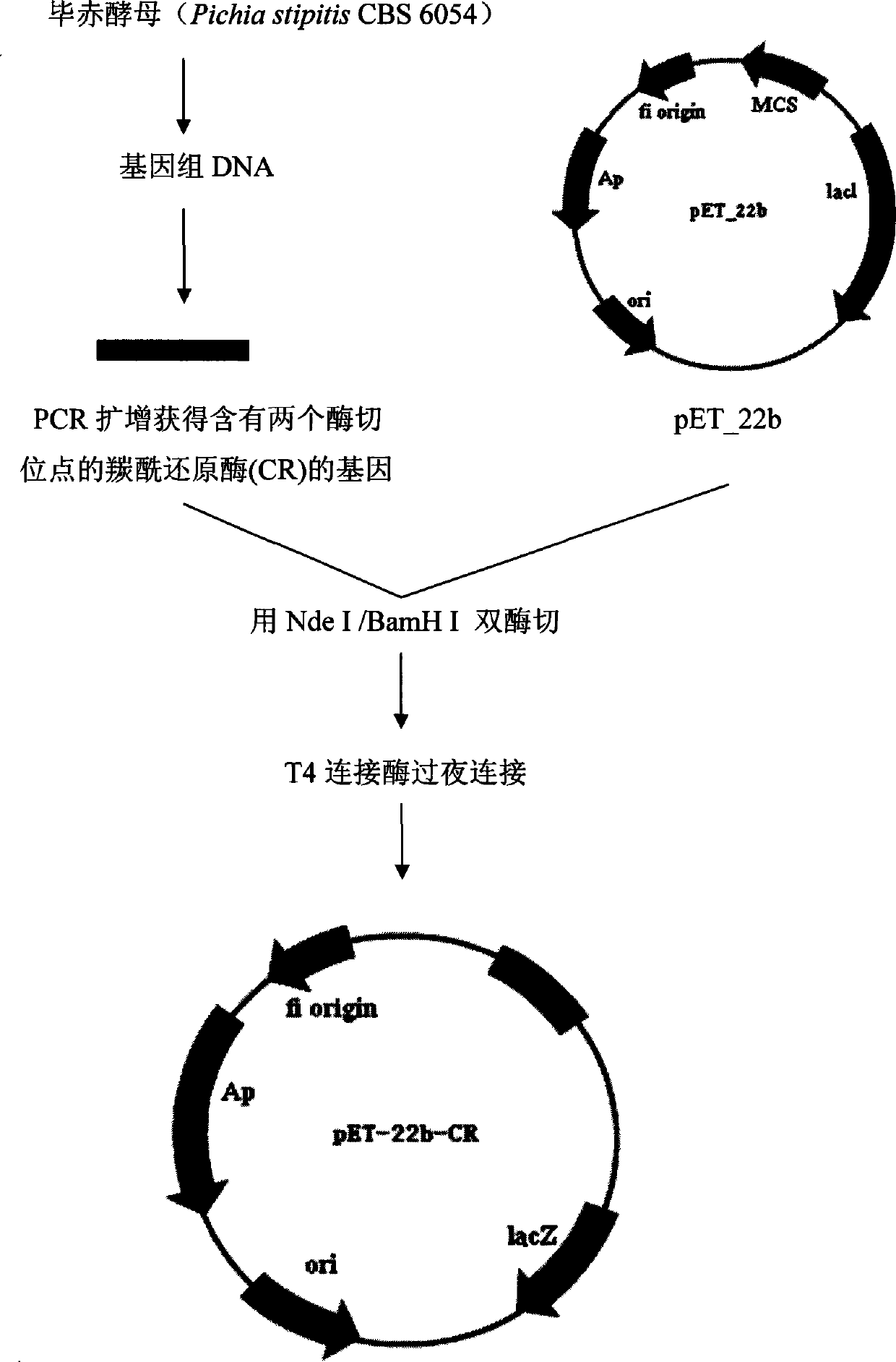
Process for the biological production of 1,3-propanediol with high yield
The present invention provides a microorganism useful for biologically producing 1,3-propanediol from a fermentable carbon source at higher yield than was previously known. The complexity of the cofactor requirements necessitates the use of a whole cell catalyst for an industrial process that utilizes this reaction sequence to produce 1,3-propanediol. The invention provides a microorganism with disruptions in specified genes and alterations in the expression levels of specified genes that is useful in a higher yielding process to produce 1,3-propanediol.
Owner:NUTRITION & BIOSCIENCES USA 4 INC +1
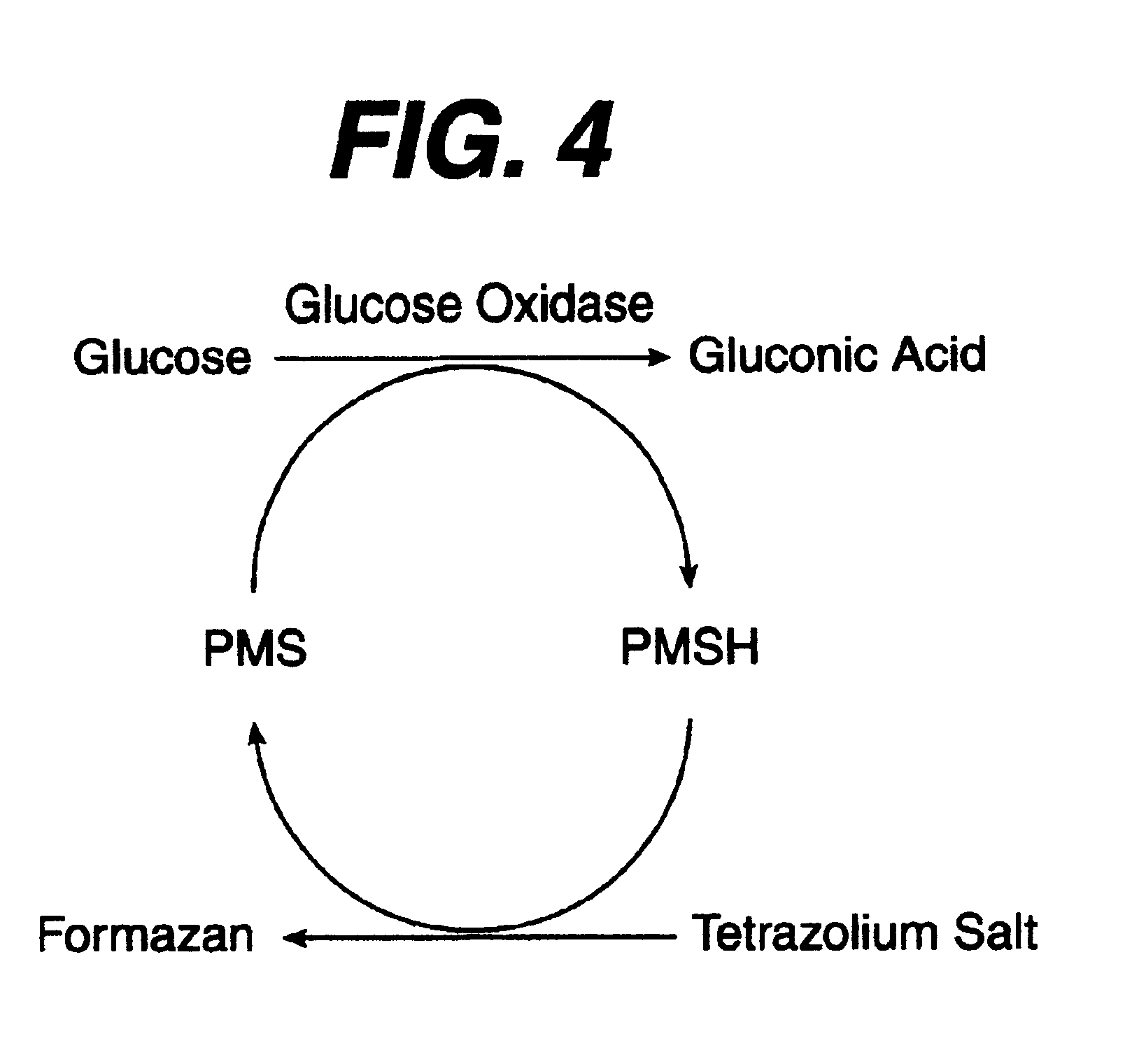
Diagnostics based on tetrazolium compounds
InactiveUS6656697B1Bioreactor/fermenter combinationsBiological substance pretreatmentsNon enzymaticElectron transfer
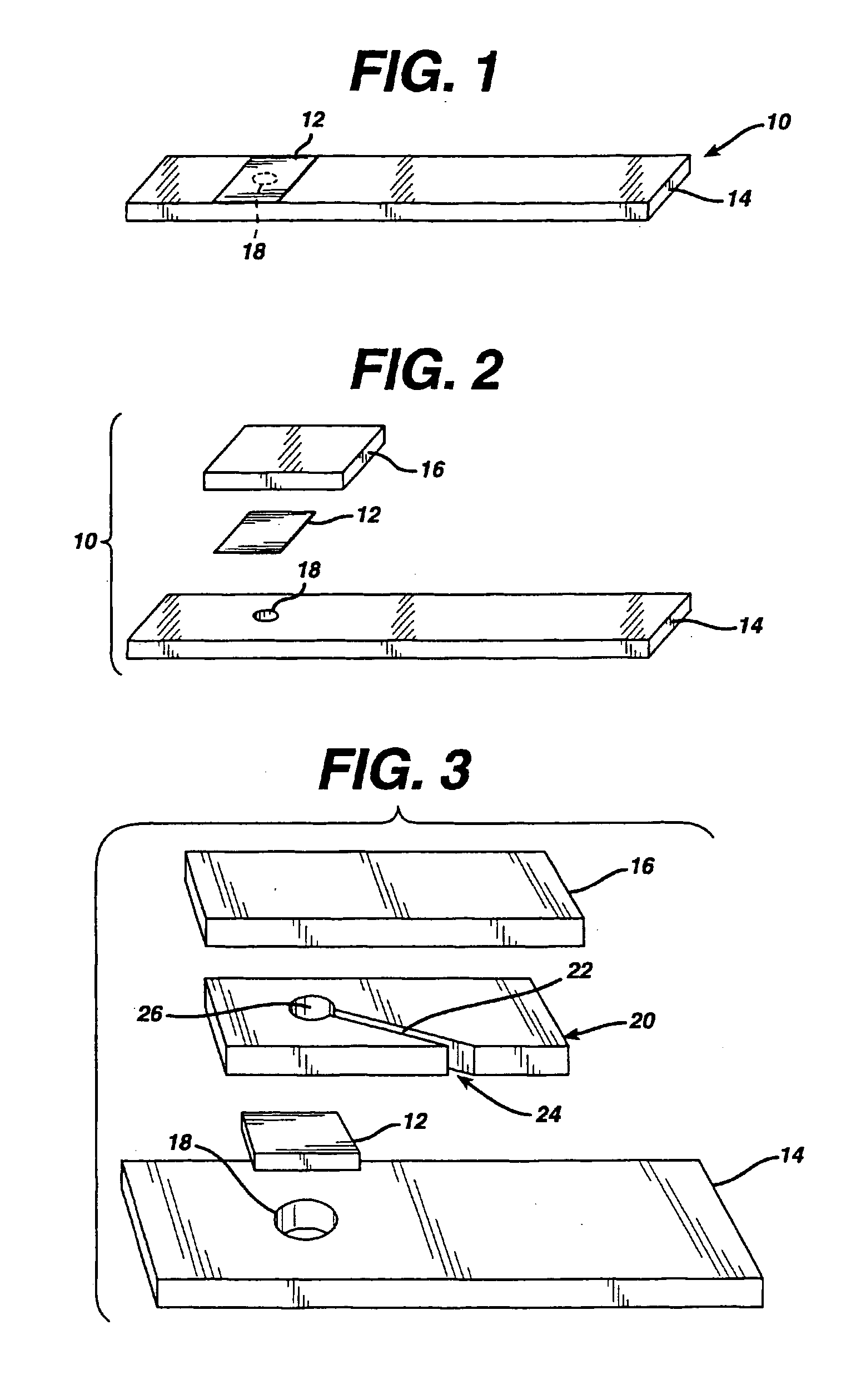
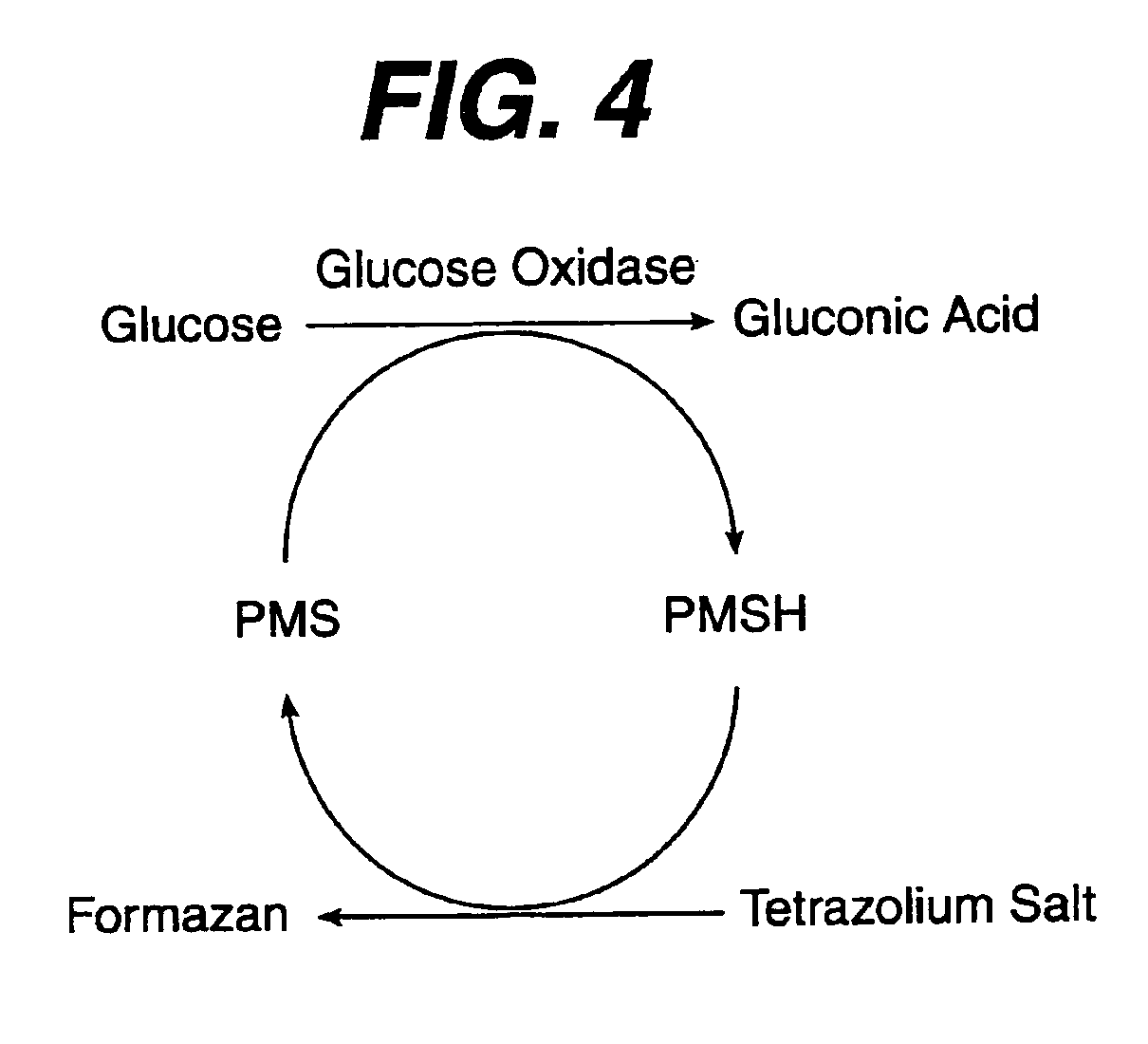
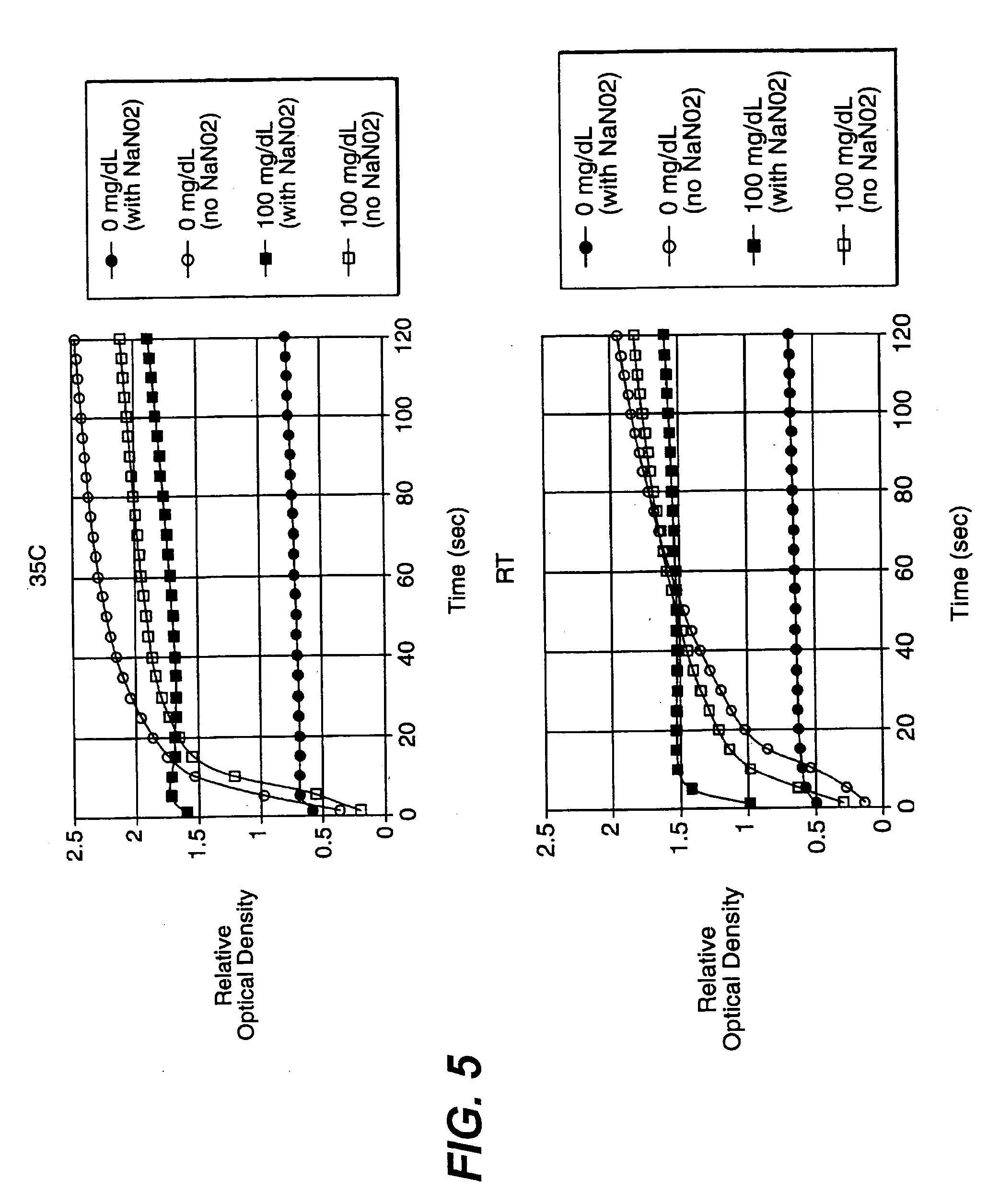
A reagent is suitable for measuring the concentration of an analyte in a hemoglobin-containing biological fluid, such as whole blood. The reagent comprises a flavin-dependent enzyme that has specificity for the analyte, a flavin cofactor if, and only if, a flavin is not bound to the enzyme, a tetrazolium dye precursor, an electron transfer agent, and a nitrite salt. The reagent causes dye formation that is a measure of the analyte concentration. The nitrite salt suppresses interfering dye formation caused non-enzymatically by the hemoglobin. Preferably, the reagent is used in a dry strip for measuring glucose in whole blood.
Owner:LIFESCAN IP HLDG LLC
Diagnostics based on tetrazolium compounds
InactiveUS20040053352A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementEnzyme bindingElectron transfer
A reagent is suitable for measuring the concentration of an analyte in a hemoglobin-containing biological fluid, such as whole blood. The reagent comprises a flavin-dependent enzyme that has specificity for the analyte, a flavin cofactor if, and only if, a flavin is not bound to the enzyme, a tetrazolium dye precursor, an electron transfer agent, and a nitrite salt. The reagent causes dye formation that is a measure of the analyte concentration. The nitrite salt suppresses interfering dye formation caused non-enzymatically by the hemoglobin. Preferably, the reagent is used in a dry strip for measuring glucose in whole blood.
Owner:LIFESCAN IP HLDG LLC
Engineered microorganisms capable of producing target compounds under anaerobic conditions
InactiveUS20100143997A1Improve abilitiesHigh catalytic efficiencyFungiBacteriaMicroorganismMetabolite
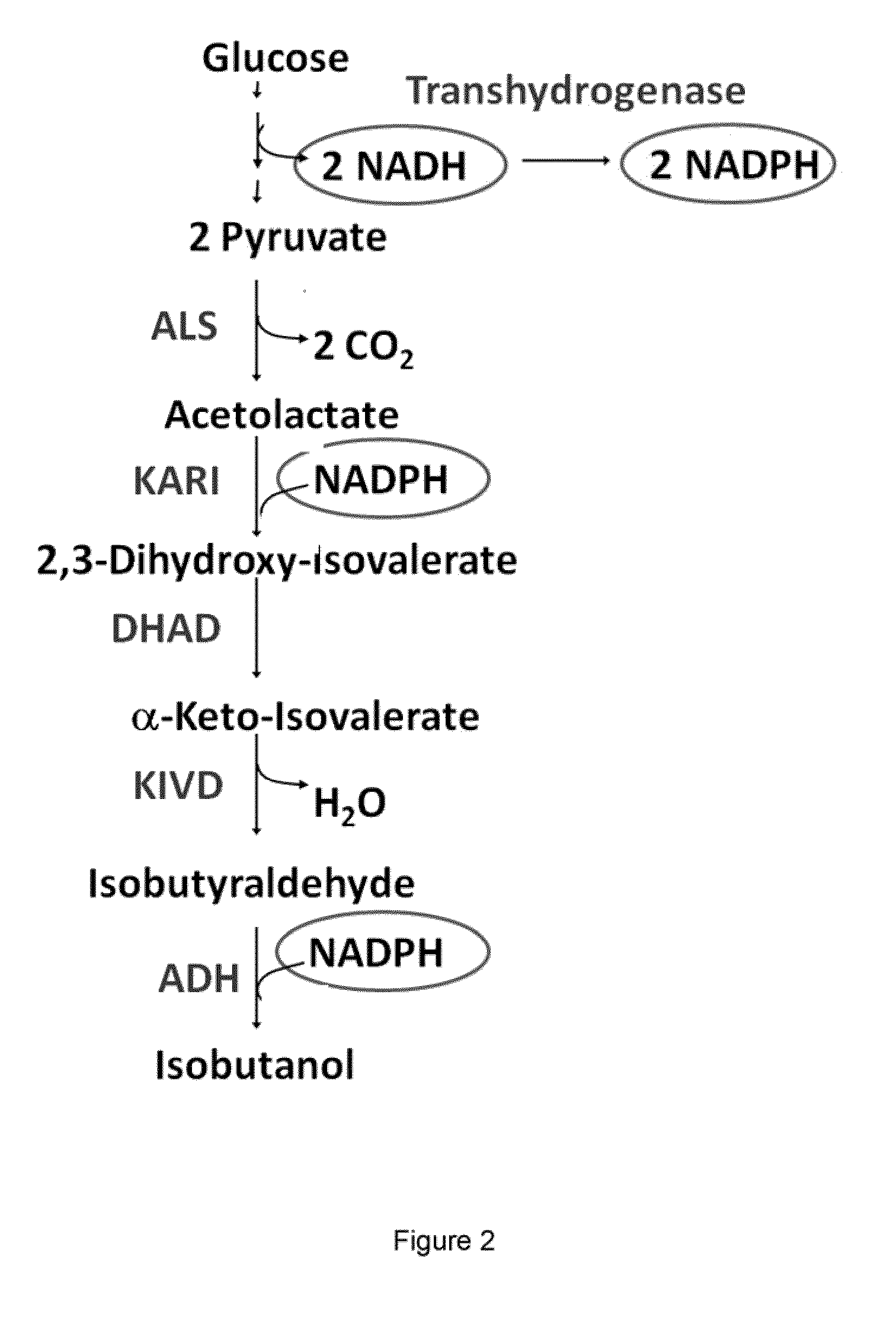
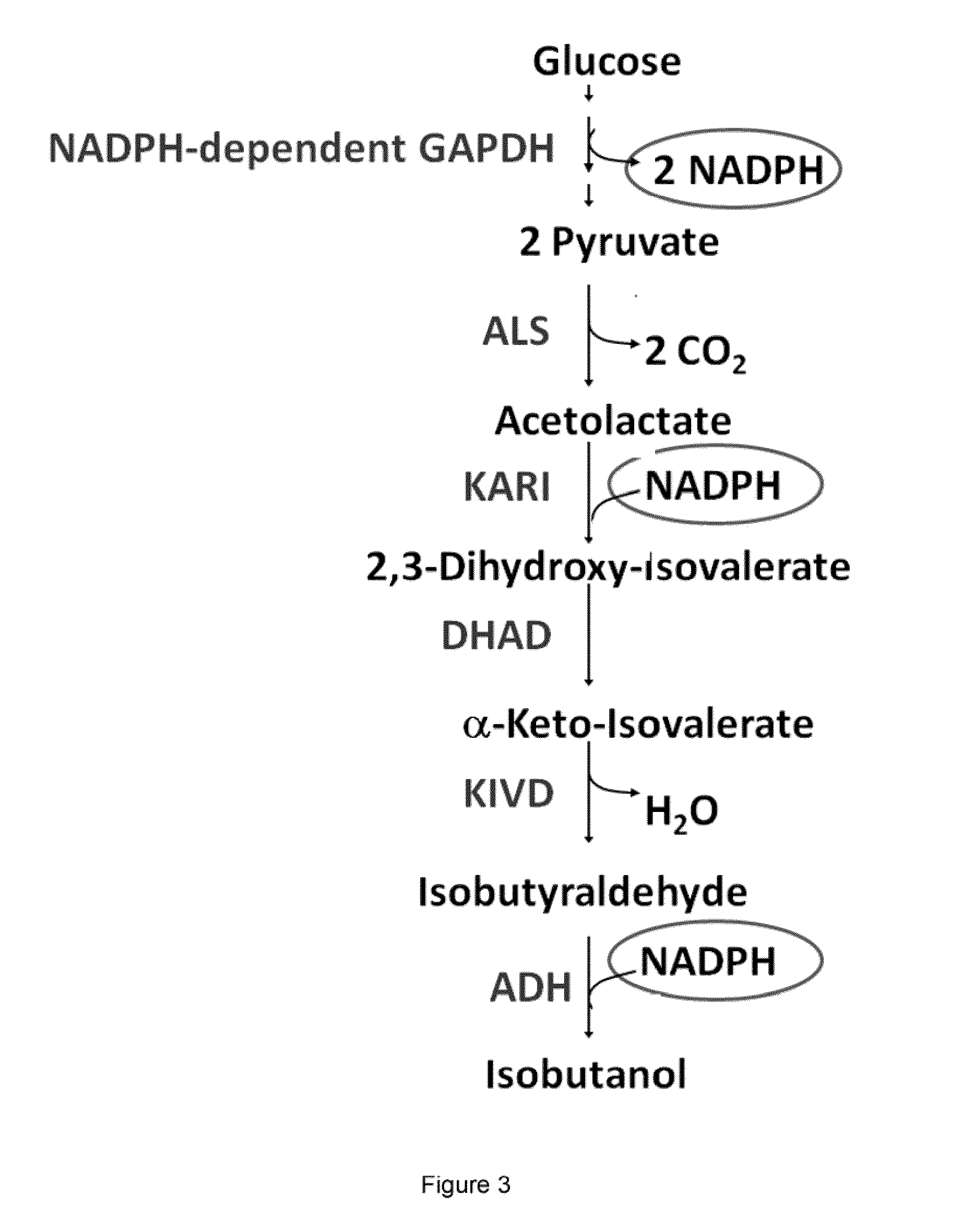
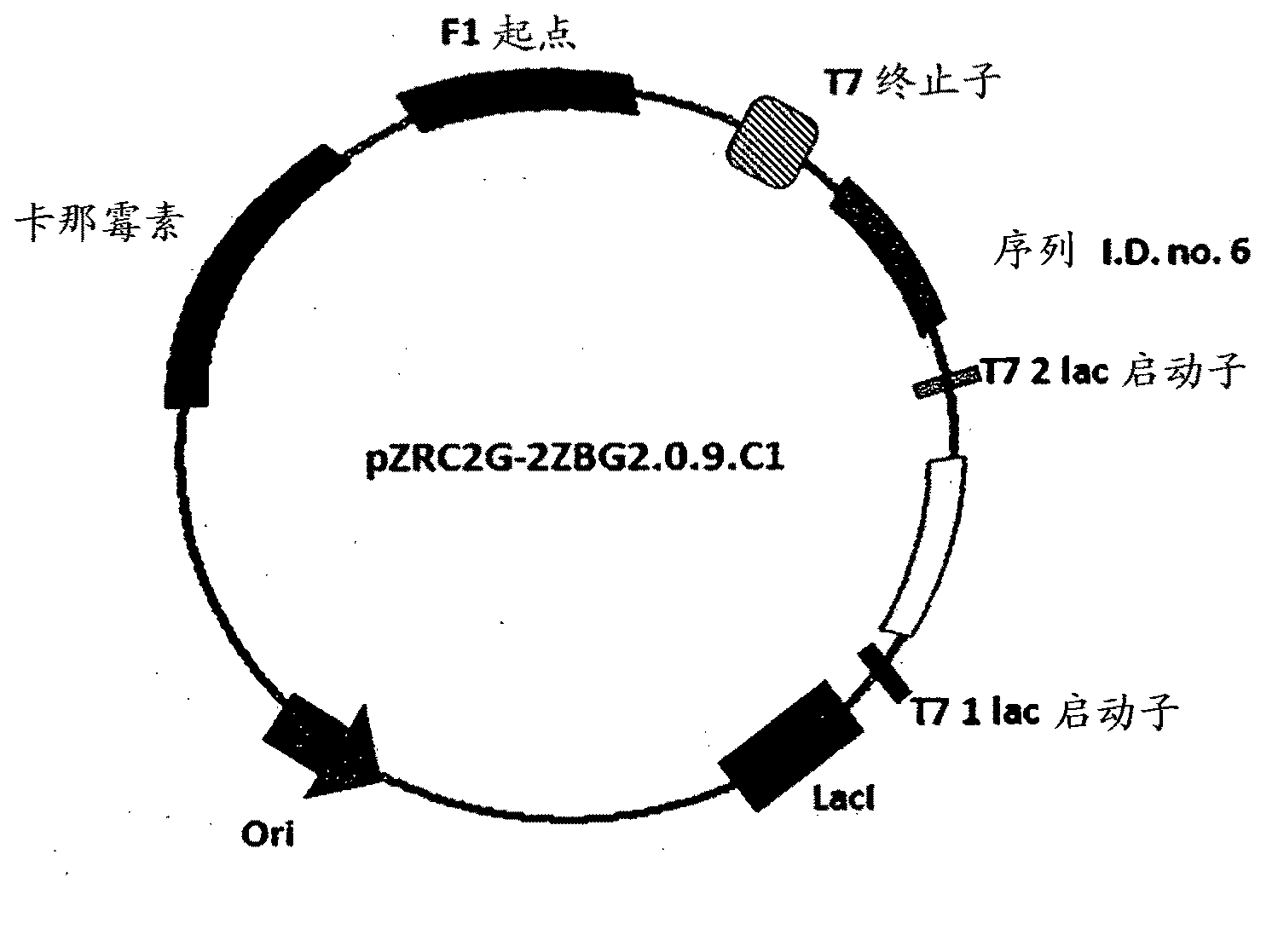
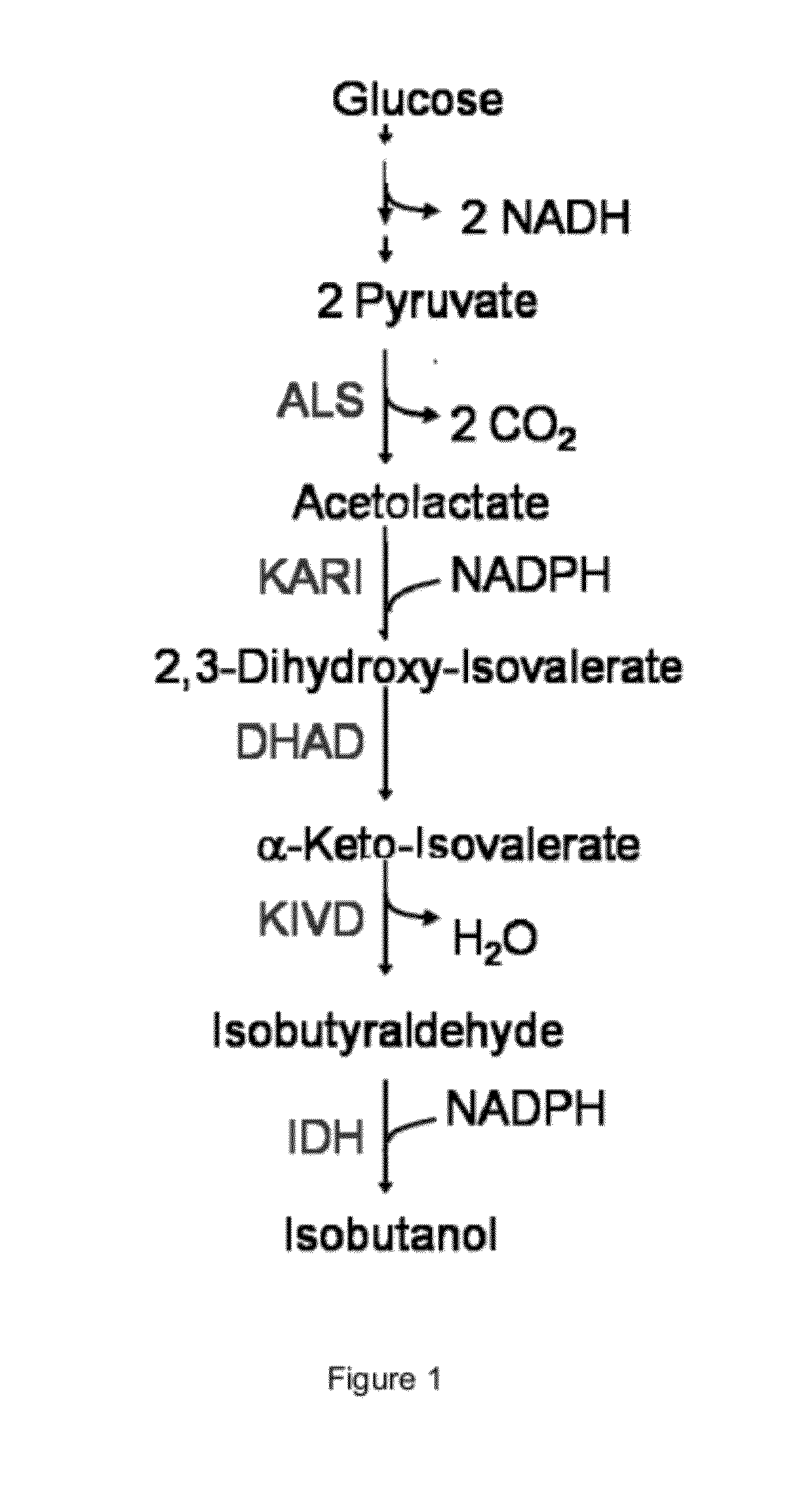
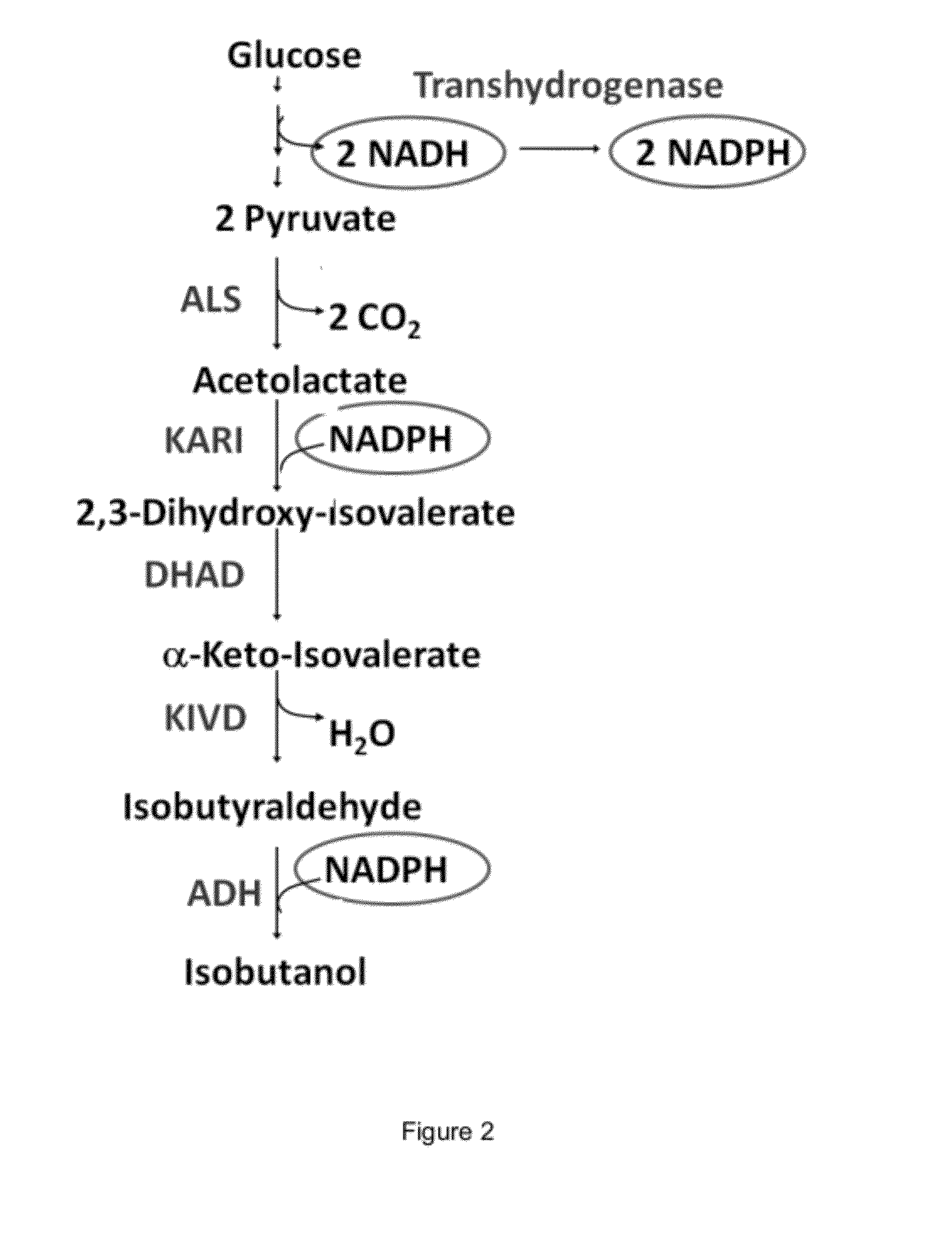
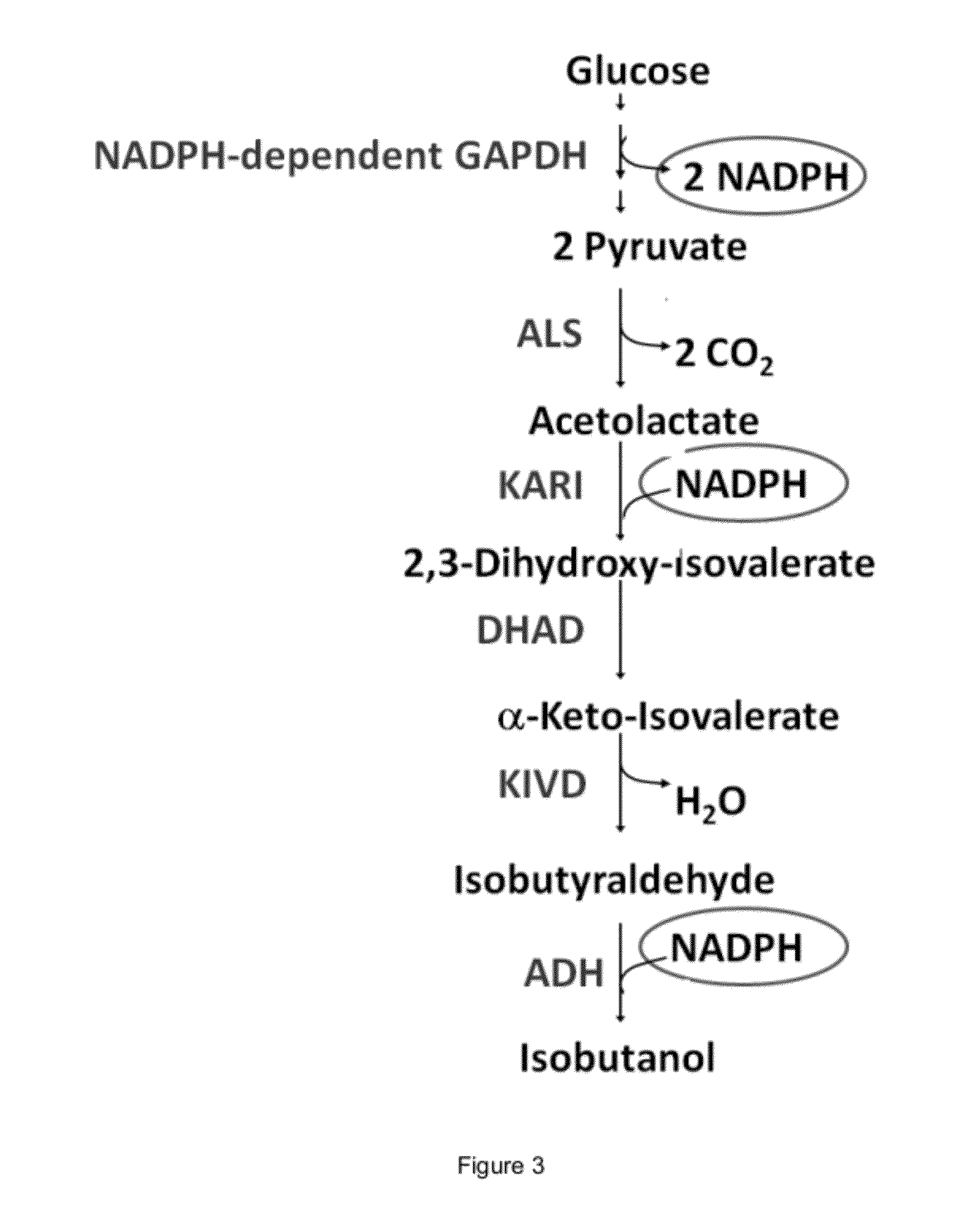
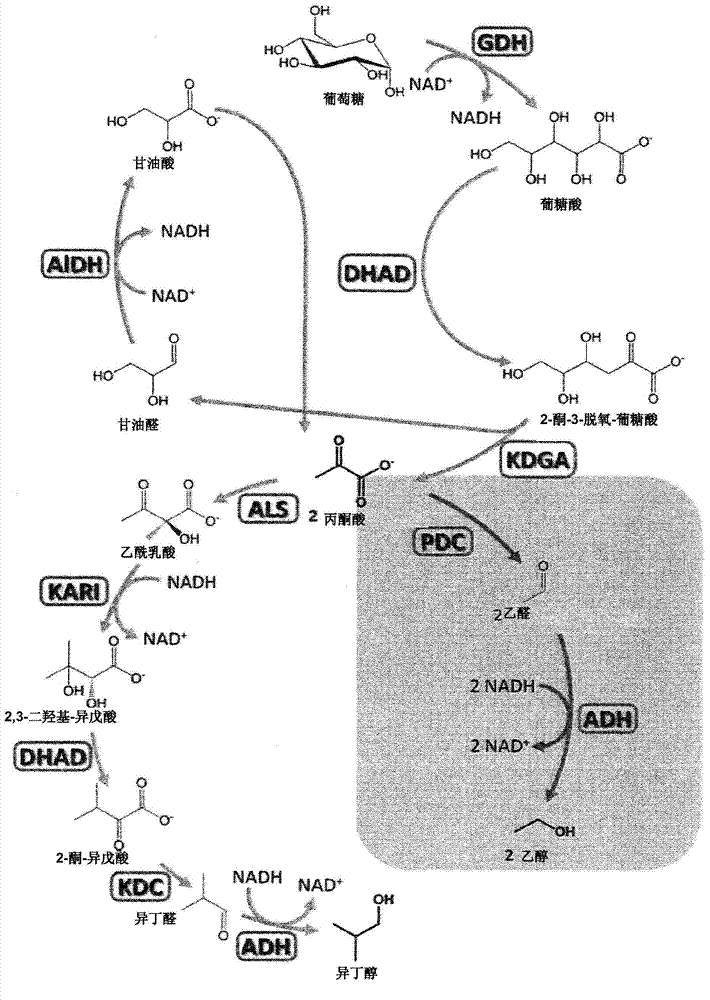
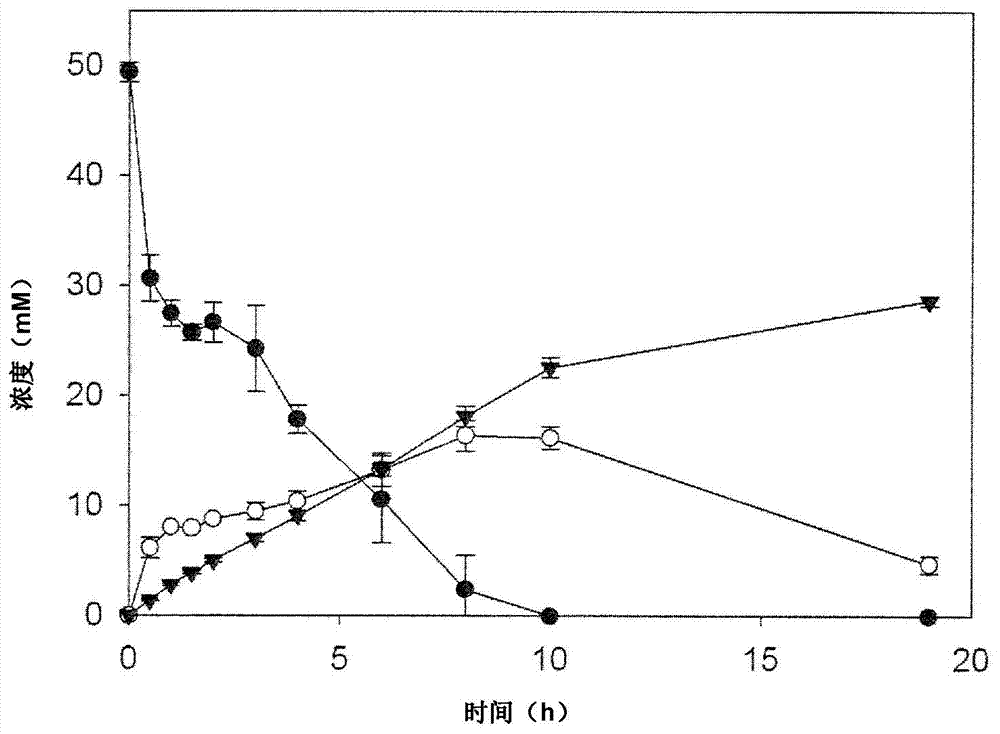
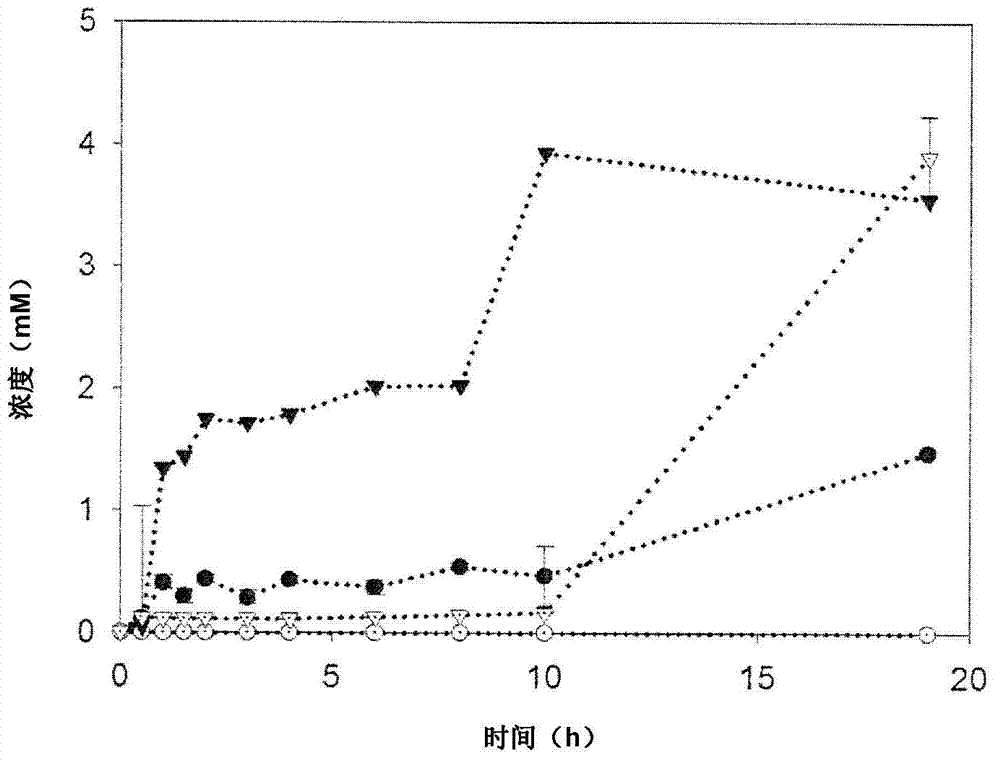
The present invention is generally provides recombinant microorganisms comprising engineered metabolic pathways capable of producing C3-C5 alcohols under aerobic and anaerobic conditions. The invention further provides ketol-acid reductoisomerase enzymes which have been mutated or modified to increase their NADH-dependent activity or to switch the cofactor preference from NADPH to NADH and are expressed in the modified microorganisms. In addition, the invention provides isobutyraldehyde dehydrogenase enzymes expressed in modified microorganisms. Also provided are methods of producing beneficial metabolites under aerobic and anaerobic conditions by contacting a suitable substrate with the modified microorganisms of the present invention.
Owner:GEVO INC
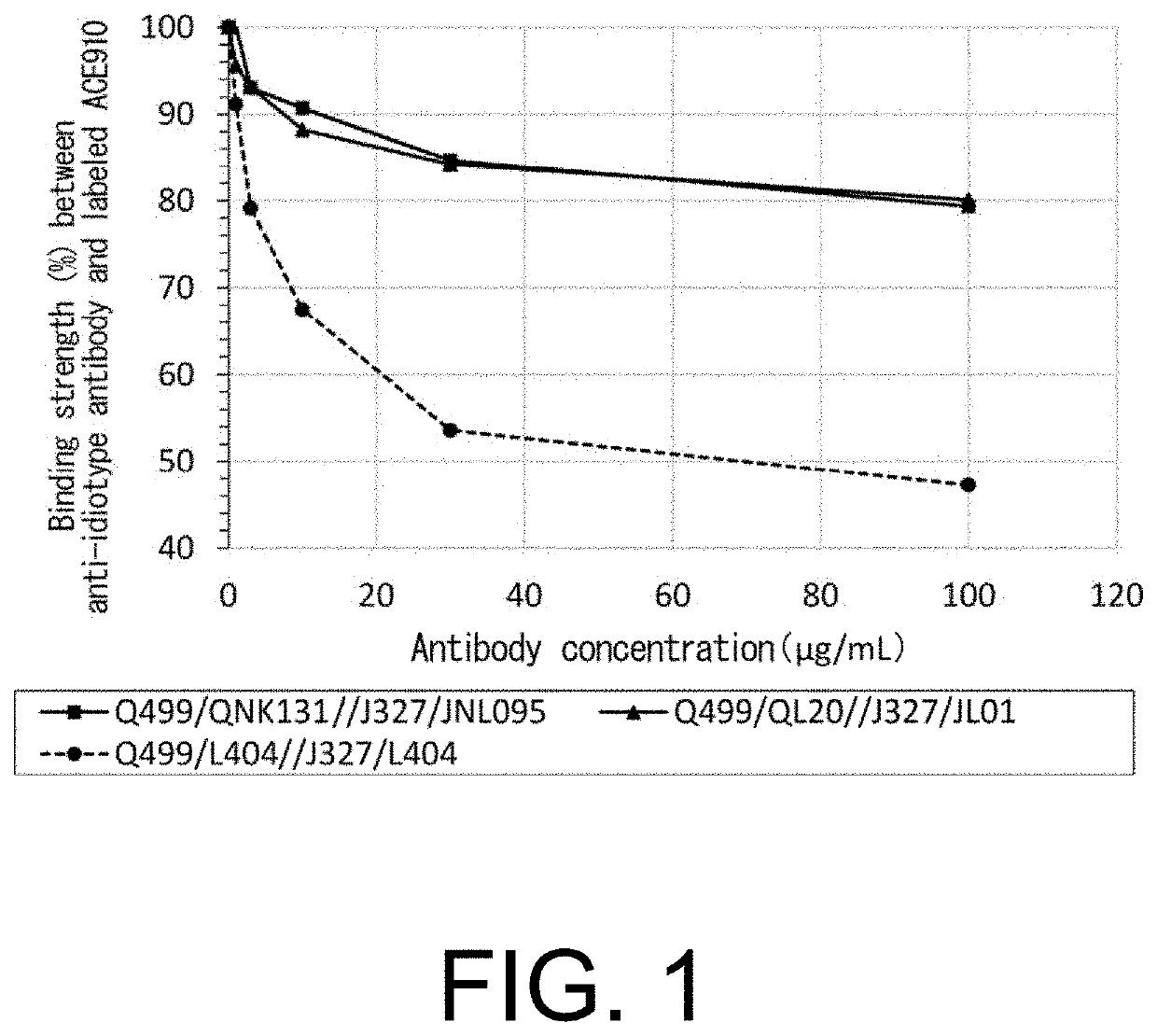
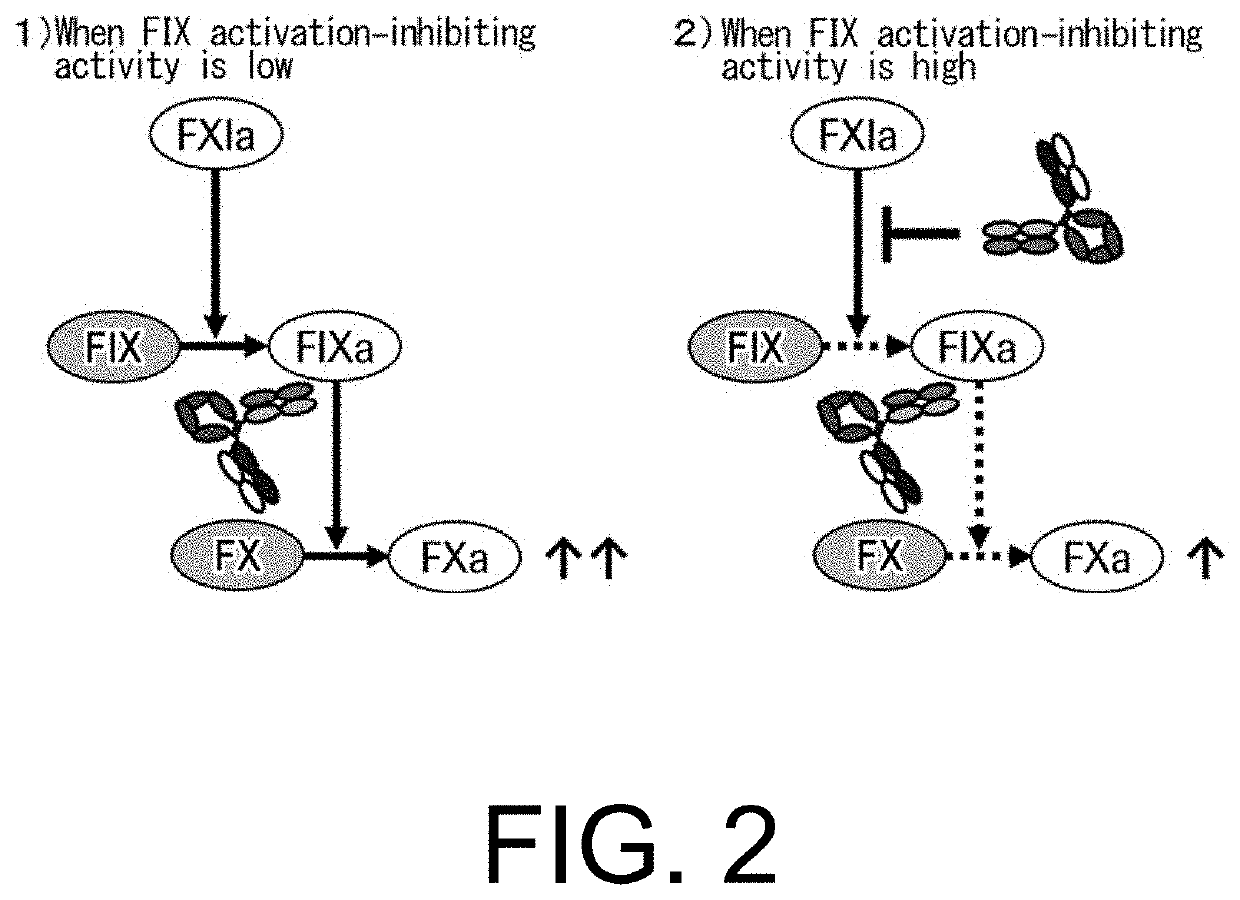
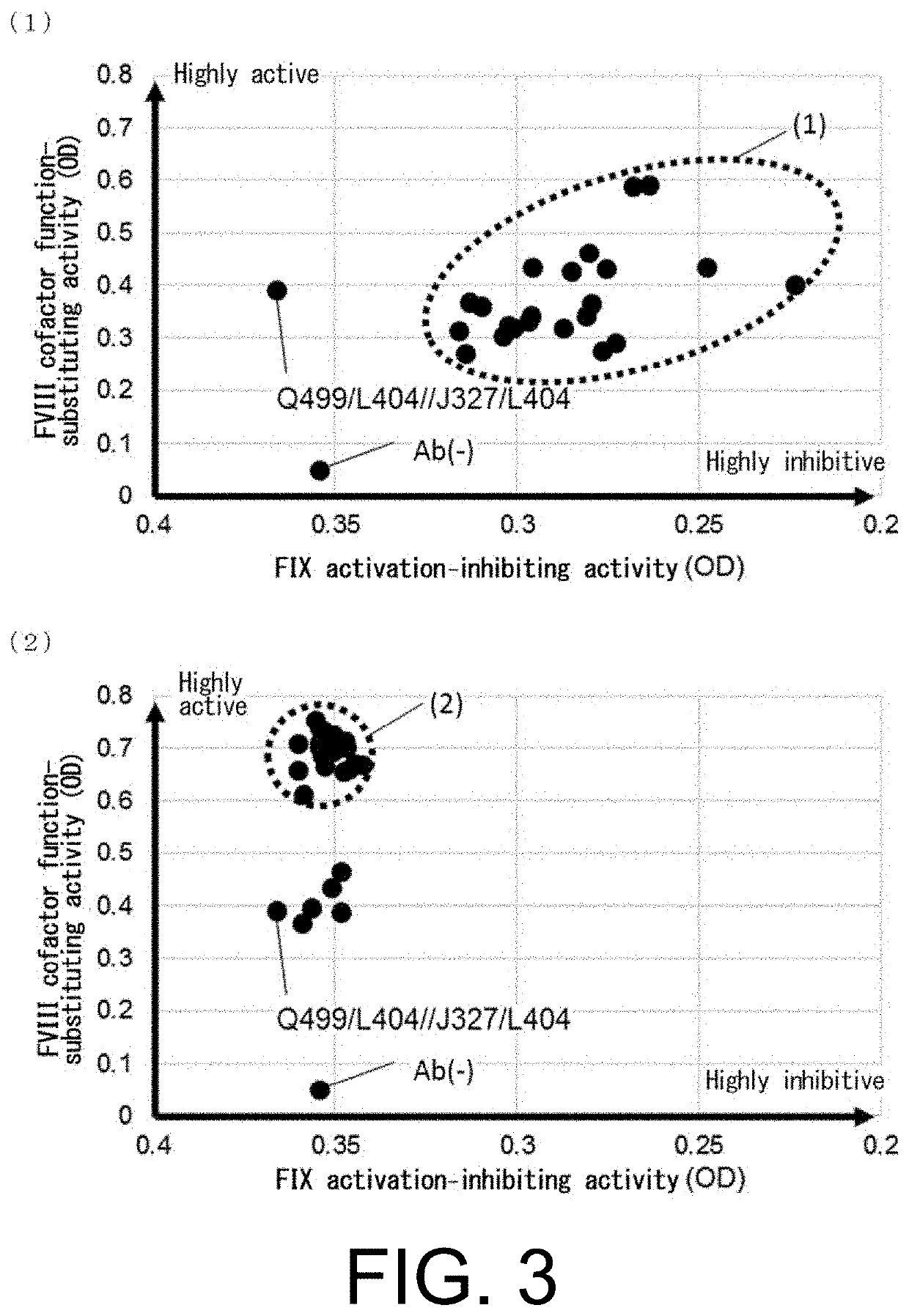
Antibody Substituting for Function of Blood Coagulation Factor VIII
InactiveUS20100003254A1Enhances enzymatic reactionShorten clotting timeImmunoglobulins against blood coagulation factorsAntibody ingredientsBlood coagulation factor VIIIAntiendomysial antibodies
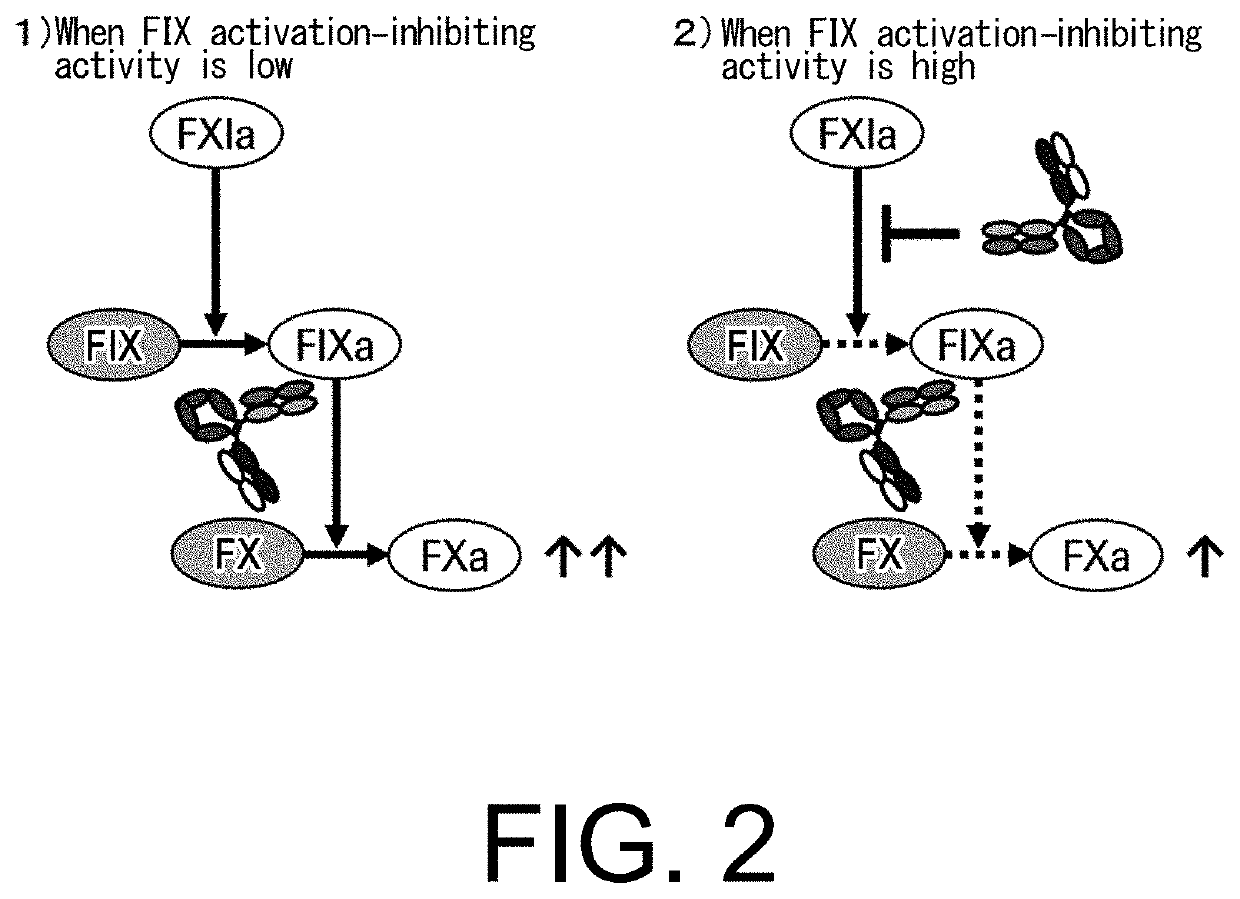
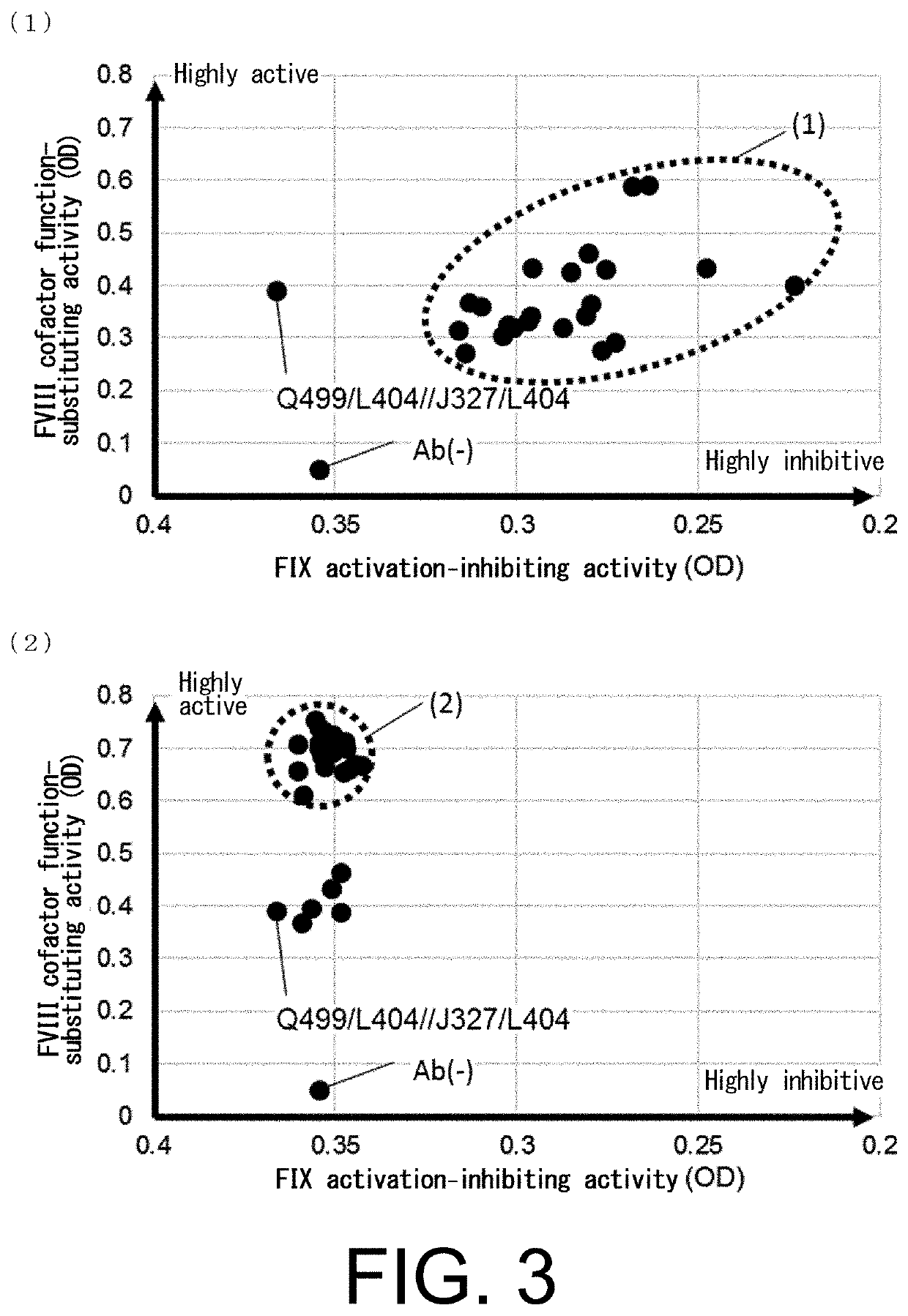
The present inventors produced a variety of bispecific antibodies that specifically bind to both F. IX / F. IXa and F. X, and functionally substitute for F. VIIIa, i.e., have a cofactor function to promote F. X activation via F. IXa. Among these antibodies, the antibody A44 / B26 reduced coagulation time by 50 seconds or more as compared to that observed when the antibody was not added. The present inventors produced a commonly shared L chain antibody from this antibody using L chains of A44, and showed that A44L can be used as commonly shared L chains, although the activity of the resulting antibody is reduced compared to the original antibody (A44HL-B26HL). Further, with appropriate CDR shuffling, the present inventors successfully produced highly active multispecific antibodies that functionally substitute for coagulation factor VIII.
Owner:CHUGAI PHARMA CO LTD
Biosensor carrying redox enzymes
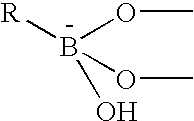
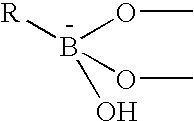
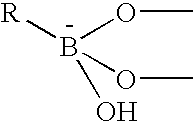
The present invention concerns an electrode carrying immobilized redox enzymes such that electric charge can flow between an electron mediator group to the enzyme cofactor by the use of boronic acid or a boronic acid derivative that acts as a linker moiety between the cofactor and the electron mediator group. The invention also concerns devices and systems that make use of the electrode of the invention, such as bio-sensors and fuel cells, the electrode being one of the components thereof.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Methods of coating a device using anti-thrombin heparin
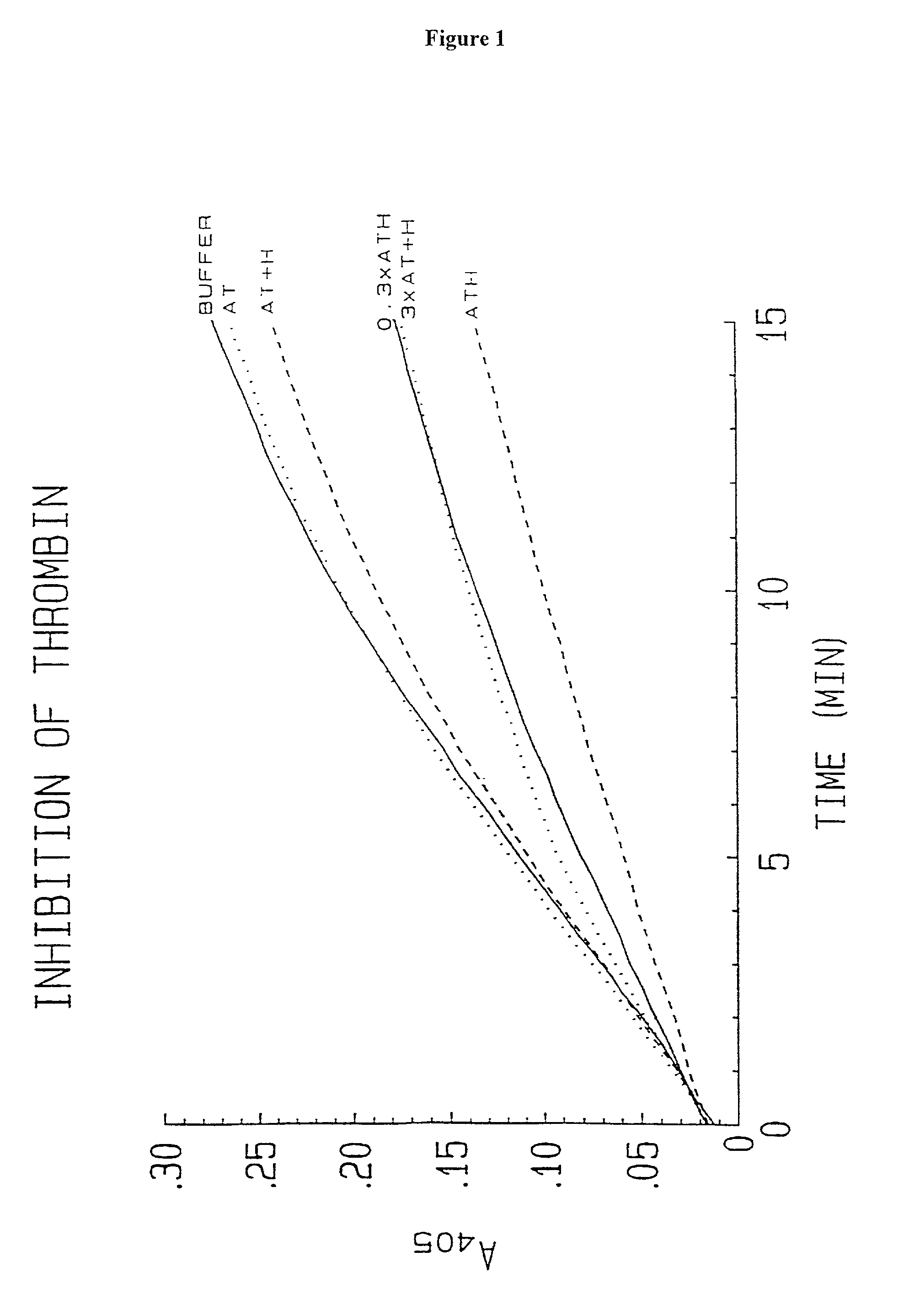
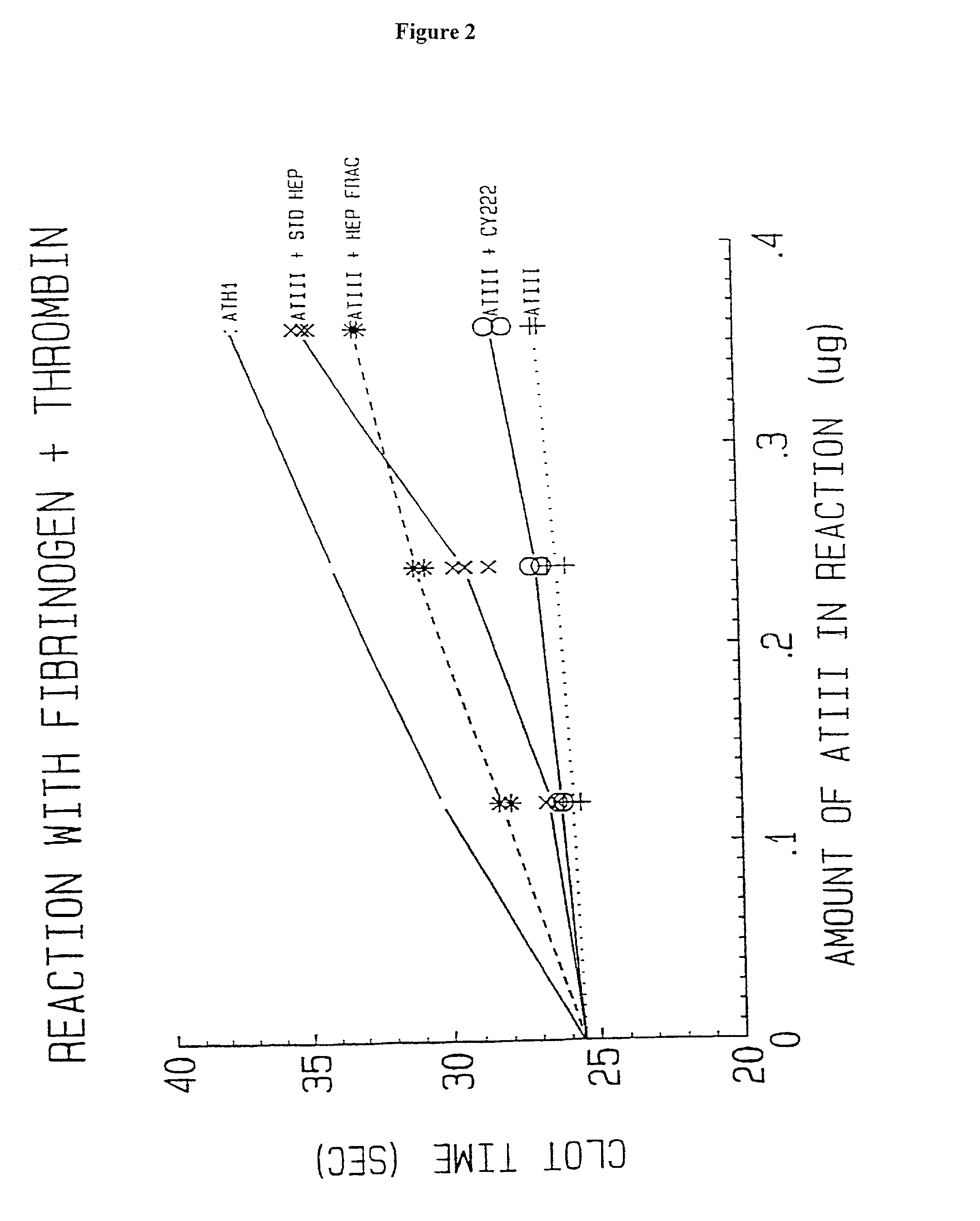
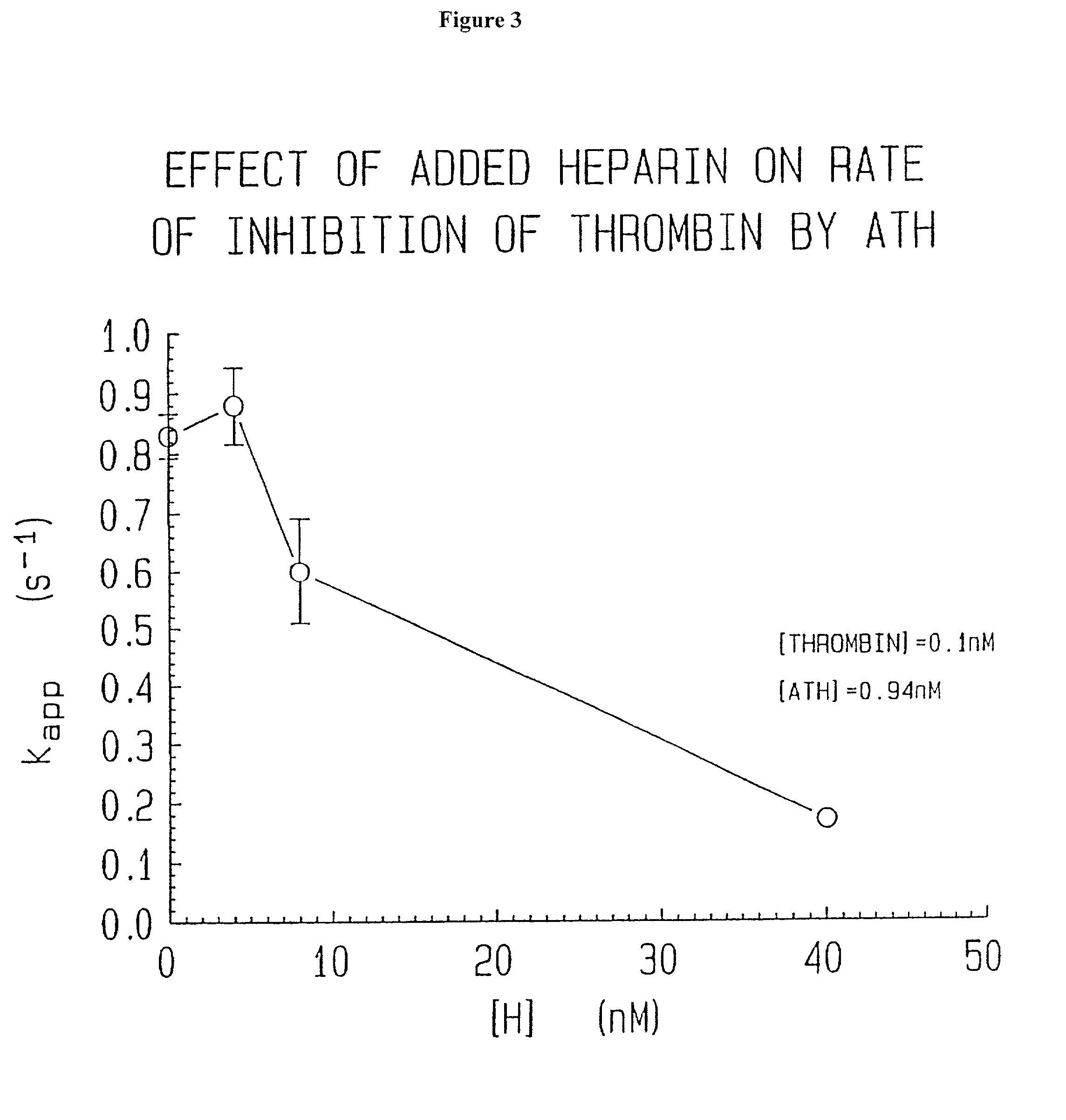
Novel conjugates of glycosaminoglycans, particularly heparin and dermatan sulfate, and amine containing species and therapeutic uses thereof are described. In particular, mild methods of conjugating heparins to proteins, such as antithrombin III and heparin cofactor II, which provide covalent conjugates which retain maximal biological activity are described. Uses of these conjugates to prevent thrombogenesis, in particular in lung airways, such as found in infant and adult respiratory distress syndrome, and on surfaces in contact with blood are also described.
Owner:MCMASTER UNIV
Gene recombination bacterium and application thereof in preparing chiral pure acetoin and 2,3-butanediol
InactiveCN101565685AEasy to operateAchieve productionBacteriaMicroorganism based processesEscherichia coliGram
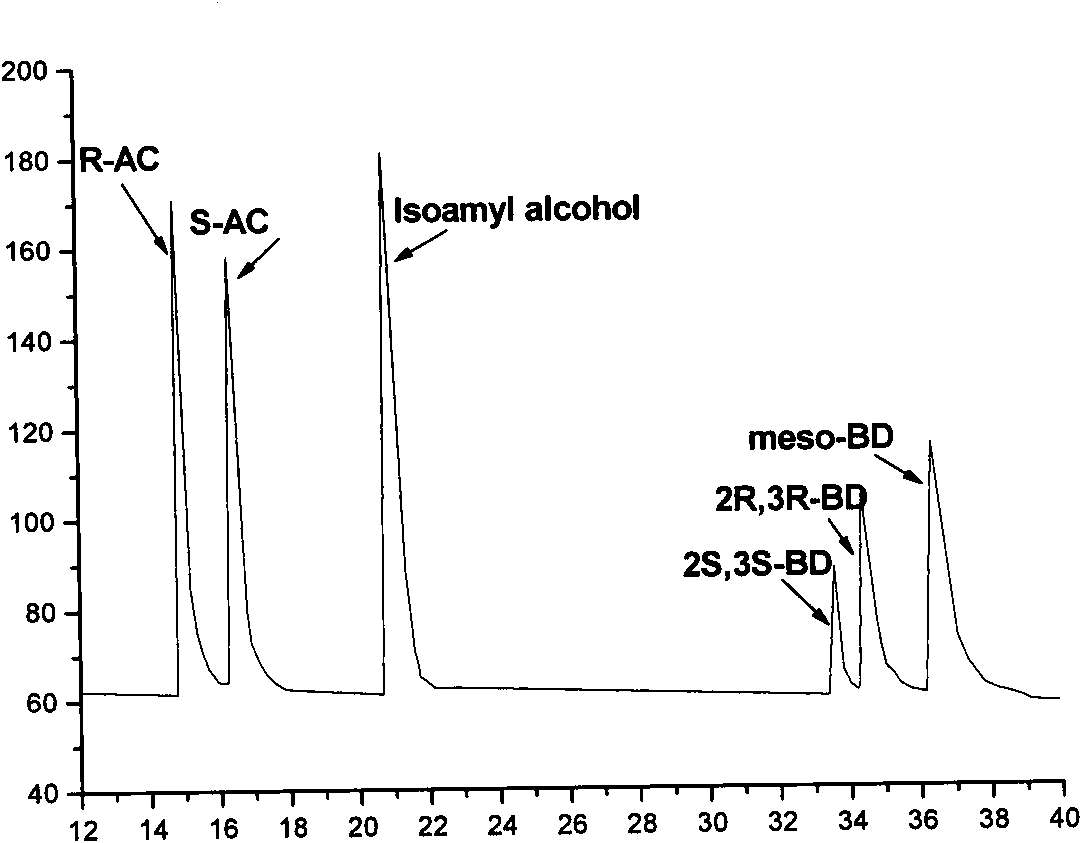
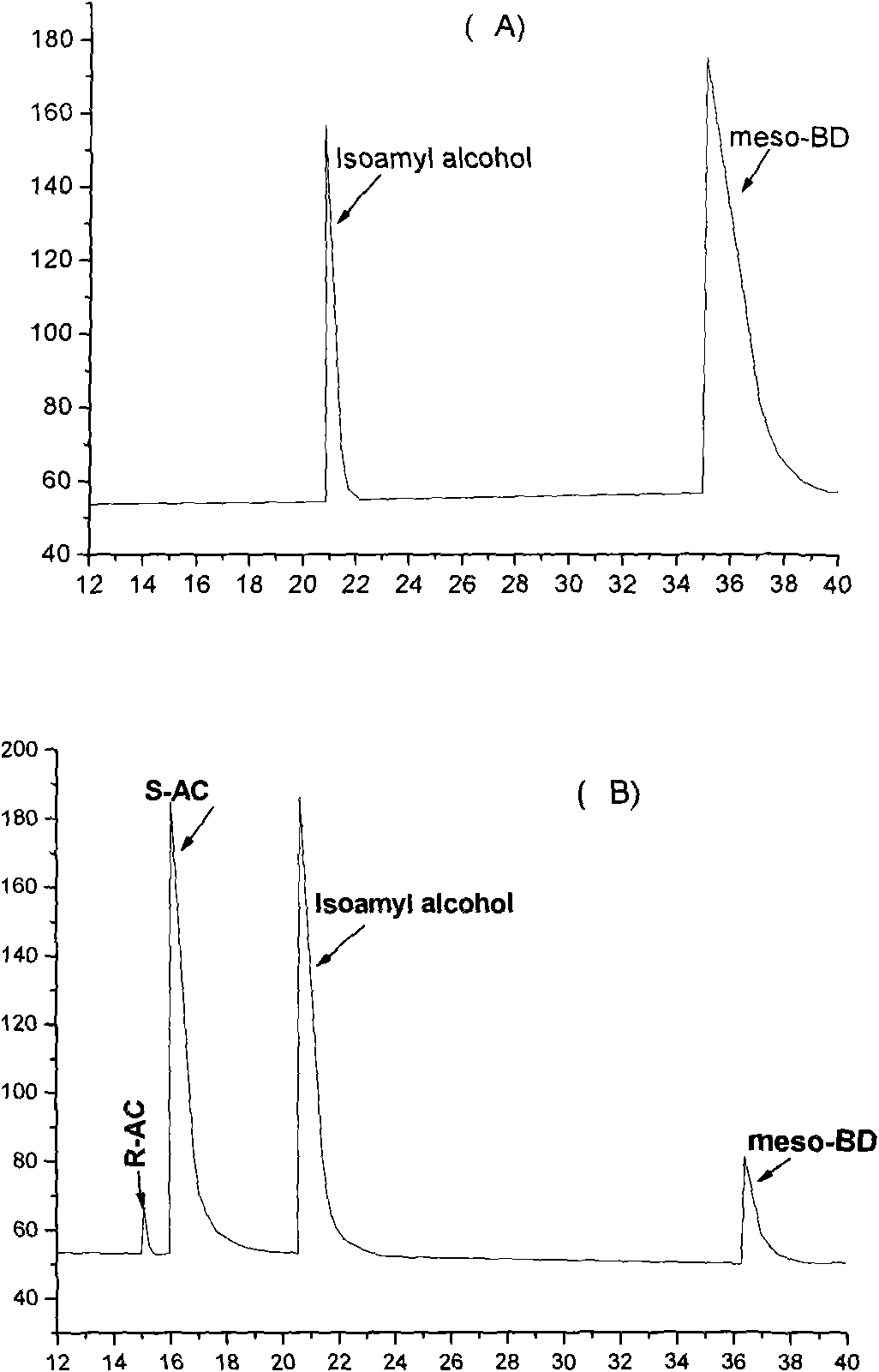
The invention discloses an E. coli BL21(pETDuet-ydjLnox) containing a 2R,3R-butanediol dehydrogenase gene ydjL and an NADH oxidase gene nox, wherein the strain is preserved in the 'China Center for Type Culture Collection' on December 23 in 2008, and the preservation number is CCTCC NO: M 208259. The invention also discloses application of a gene recombination bacterium in producing chiral S-AC by catalyzing meso-BD, producing chiral R-AC by catalyzing 2R,3R-BD and producing chiral pure 2S,3S-BD by splitting a 2,3-BD mixture. The concentration of a chiral AC prepared from the recombinant E. coli can reach over 6 grams per liter (the ee value is more than or equal to 96 percent); and the ee value of the chiral pure 2S,3S-BD is more than or equal to 98 percent, and the regeneration of a cofactor is achieved, thus the gene recombination bacterium has great industrial application prospect.
Owner:SHANDONG UNIV
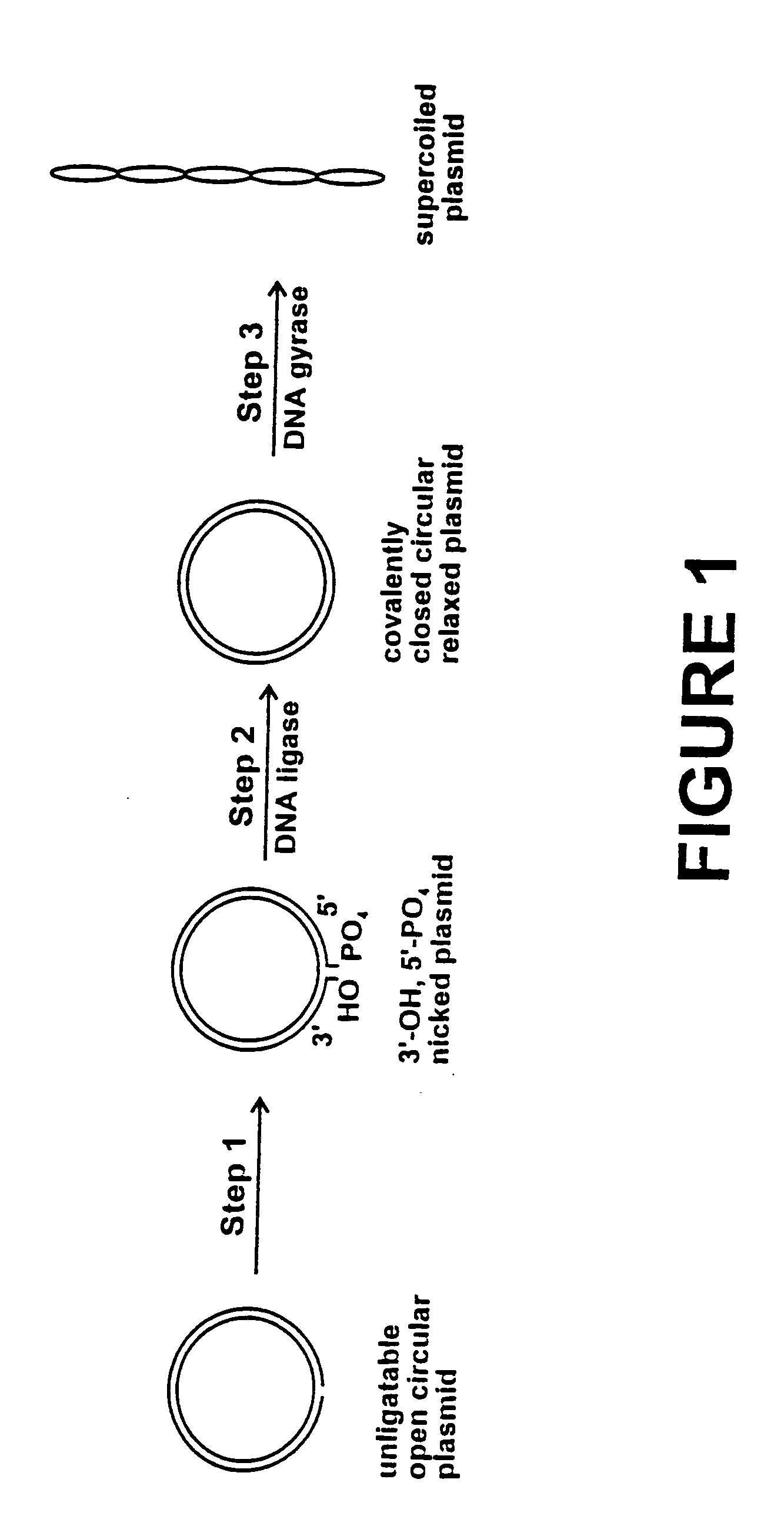
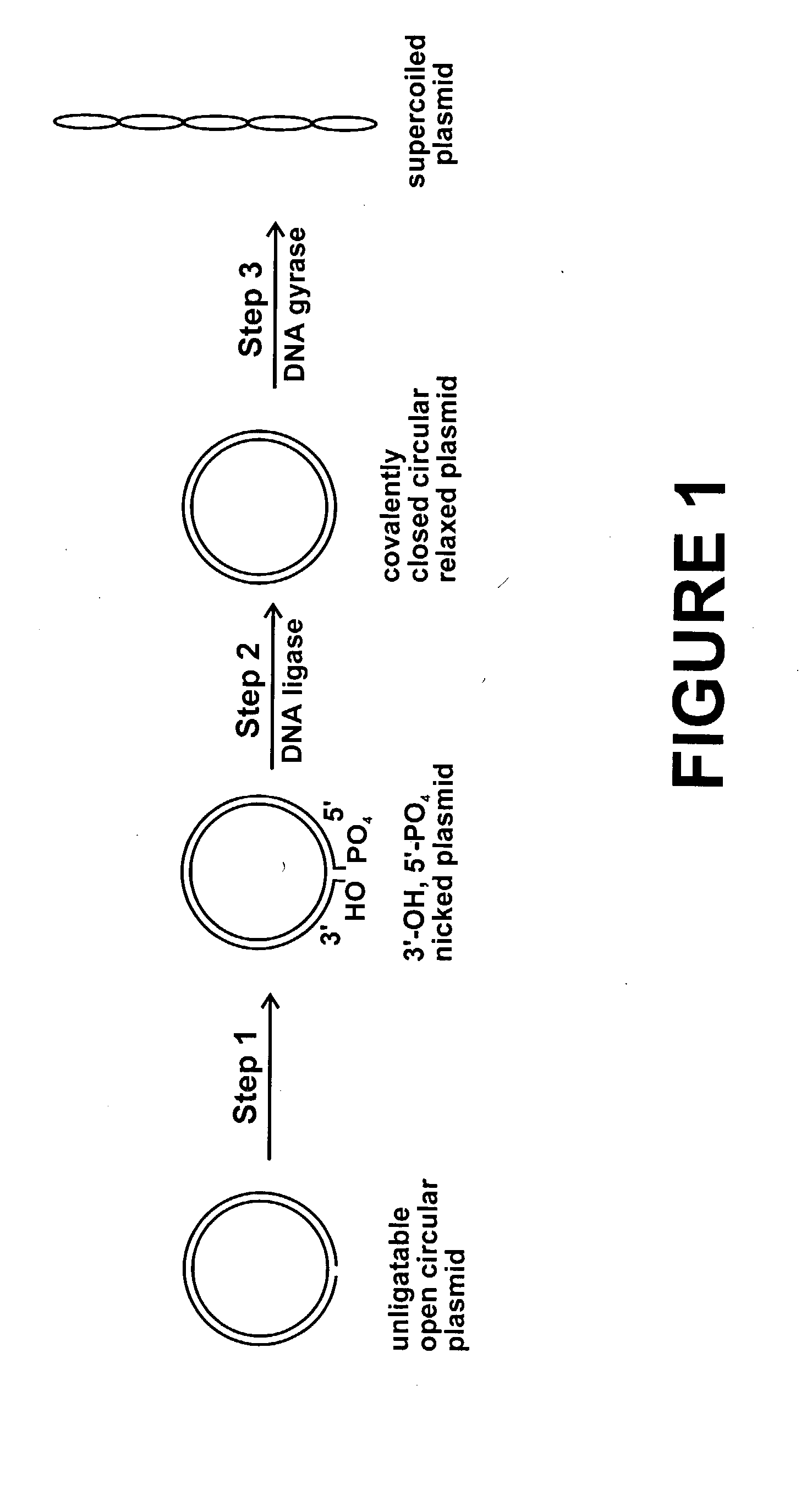
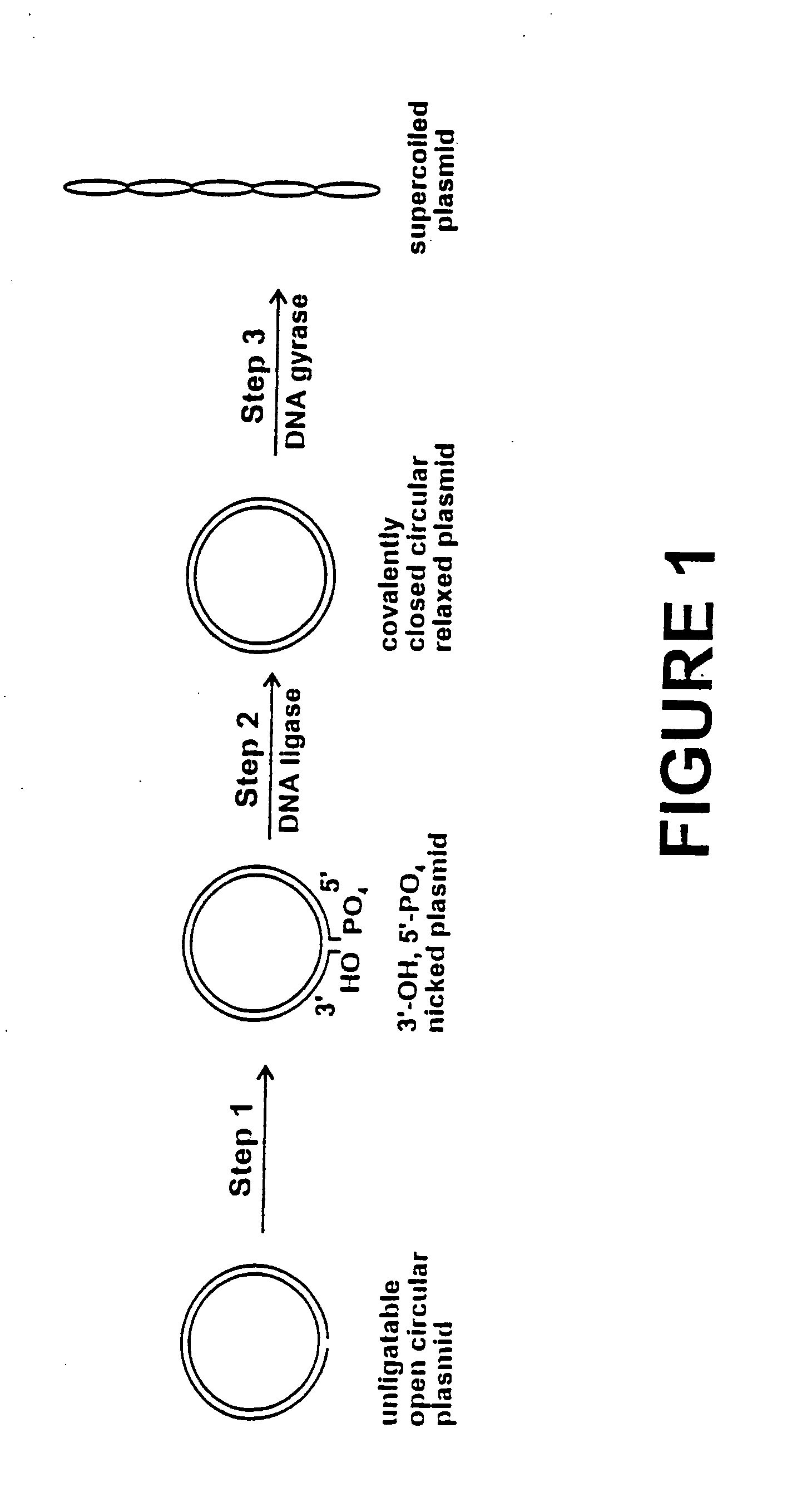
Method for plasmid preparation by conversion of open circular plasmid to supercoiled plasmid
InactiveUS20050069991A1Increase productionUniversal procedureHydrolasesMicrobiological testing/measurementPhosphoric acidDNA Gyrase Inhibitors
In one embodiment of the invention, a method is provided for preparing plasmid from host cells which contain the plasmid, comprising: (a) providing a plasmid solution comprised of unligatable open circular plasmid; (b) reacting the unligatable open circular plasmid with one or more enzymes and appropriate nucleotide cofactors, such that unligatable open circular plasmid is converted to 3′-hydroxyl, 5′-phosphate nicked plasmid; (c) reacting the 3′-hydroxyl, 5′-phosphate nicked plasmid with a DNA ligase and DNA ligase nucleotide cofactor, such that 3′-hydroxyl, 5′-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) reacting the relaxed covalently closed circular plasmid with a DNA gyrase and DNA gyrase nucleotide cofactor, such that relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. In other embodiments, DNA gyrase is replaced by reverse DNA gyrase or reaction (d) is not performed.
Owner:HYMAN EDWARD D
Process for the biological production of 1,3-propanediol with high yield
Owner:NUTRITION & BIOSCIENCES USA 4 INC
Application of alcohol dehydrogenase in catalytic generation of ethyl (R)-4-chloro-3-hydroxy butyrate
InactiveCN103160547AHigh yieldHigh optical activityMicroorganism based processesFermentationHydroxybutyric acidPtru catalyst
The invention discloses application of alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction. By using alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 as a catalyst, ethyl 4-chloroacetoacetate as a substrate and NADH (nicotinamide adenine dinucleotide) as a cofactor, asymmetric reduction is carried out to prepare the ethyl (R)-4-chloro-3-hydroxy butyrate. The invention applies the alcohol dehydrogenase with amino acid sequence disclosed as SEQ ID NO:2 in preparing ethyl (R)-4-chloro-3-hydroxy butyrate from ethyl 4-chloroacetoacetate by asymmetric reduction for the first time, and has favorable effect. The enzyme activity is up to 5.6 U / mg, the yield of the substrate is up to 94%, and the enantiomeric excess value of the product is 100%. The yield is high, and the production cost is greatly lowered.
Owner:NANJING UNIV OF TECH
A genetically engineered bacterium producing 2-phenylethanol and an applying method thereof
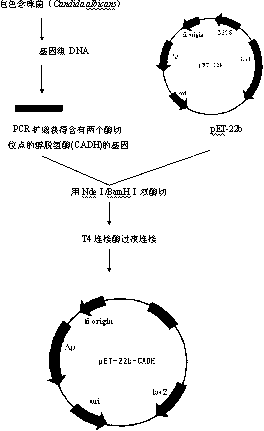
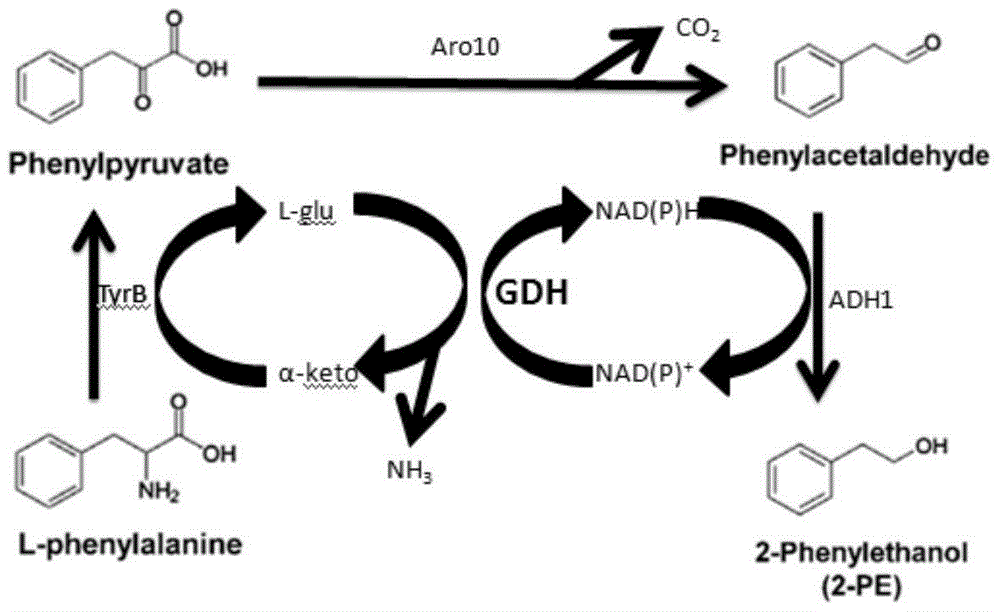
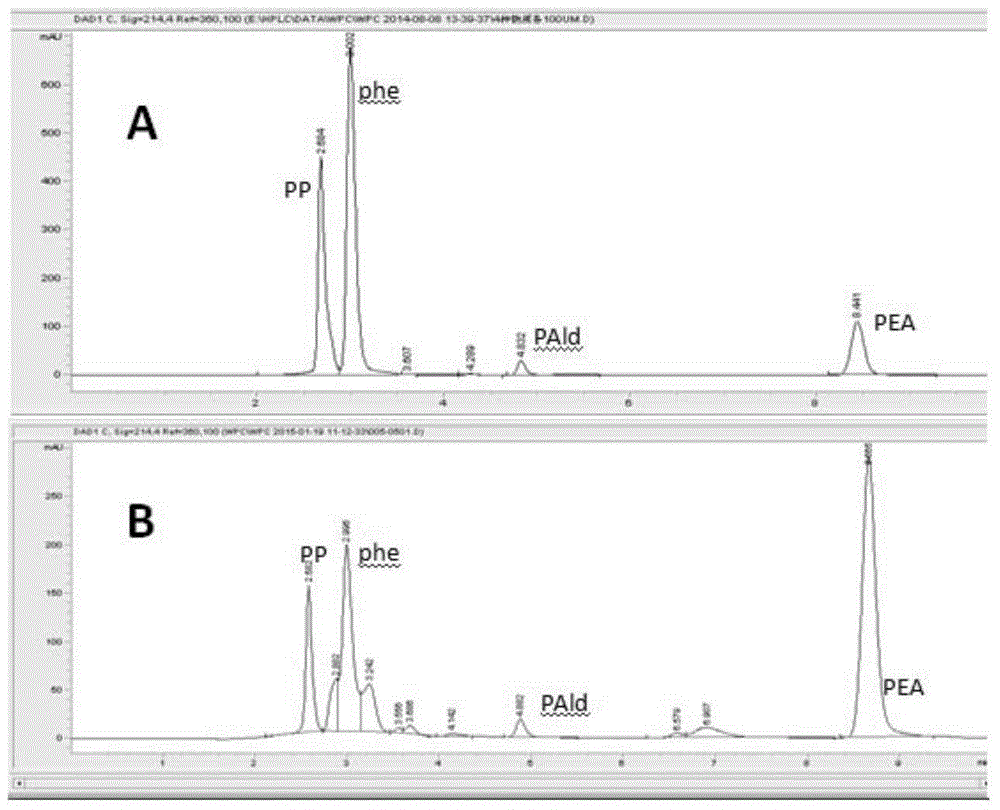
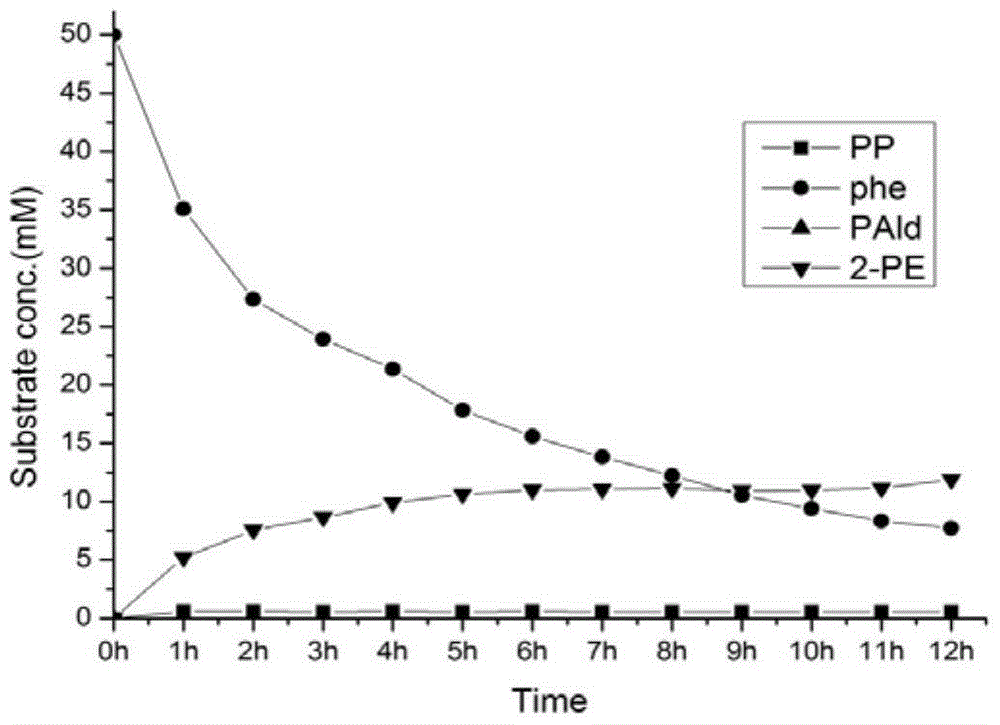
ActiveCN106566794AImprove cycle efficiencyDisinhibitionBacteriaMicroorganism based processesPhenethyl alcoholPhenylpyruvate decarboxylase
A genetically engineered bacterium producing 2-phenylethanol and an applying method thereof are disclosed. The recombinant bacterium is constructed by coexpressing aromatic amino acid aminotransferase and exogenous phenylpyruvate decarboxylase (Aro10) (or indole-3-pyruvic acid decarboxylase), phenylethanol dehydrogenase or phenylacetaldehyde reductase in escherichia coli to obtain the recombinant bacterium producing the 2-phenylethanol. Glutamate dehydrogenase is coexpressed on this basis to achieve self-balancing cofactor circulation, thus increasing the yield of the 2-phenylethanol. The conversion ratio of the 2-phenylethanol prepared through the recombinant bacterium is high. The bacterium and the method have important application value in production of the 2-phenylethanol in the future.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Cell culture process
InactiveUS20050069979A1High degree of sialylationHigh degreePeptide/protein ingredientsImmunoglobulinsBiotechnologyLipid formation
The invention provides a method for producing a recombinant polypeptide of interest which method comprises: (a) providing a host cell which comprises a nucleotide sequence which encodes the recombinant polypeptide of interest and which directs expression of the recombinant polypeptide of interest in the host cell; (b) providing a serum-free culture medium which comprises (i) water, a plant-derived peptone, an osmolality regulator, a buffer, an energy source, at least one amino acid, a lipid source or precursor, a source of iron, non-ferrous metal ions and optionally one or more vitamins and cofactors; and (ii) does not contain any full-length polypeptides; and (c) culturing the host cell in the culture medium under conditions that allow for expression of the recombinant polypeptide of interest.
Owner:ZENG STEFFEN +4
Use of carbonyl reductase in (S)-4-chloro-3 hydroxy butyric ether production
The invention discloses an application of carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 to prepare (S)-4-chloro-3-hydroxybutanoic ethyl ester by asymmetric reduction of 4-chloracetyl ethyl acetate. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is taken as a catalyst, the ethyl 4-chloracetyl ethyl acetate is taken as a substrate, and NADPH is taken as a cofactor, and the (S)-4-chloro-3-hydroxybutanoic ethyl ester is prepared by the asymmetric reduction. The carbonyl reductase with the amino acid sequence as shown in SEQ ID NO:2 is applied to preparing the (S)-4-chloro-3-hydroxybutanoic ethyl ester by the asymmertric reduction of the 4-chloroacetyl ethyl acetate for the first time, which produces good effect; the yield of the substrate is up to 95%, and the enantiomer excess value of the product is 100%, the yield is high, and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
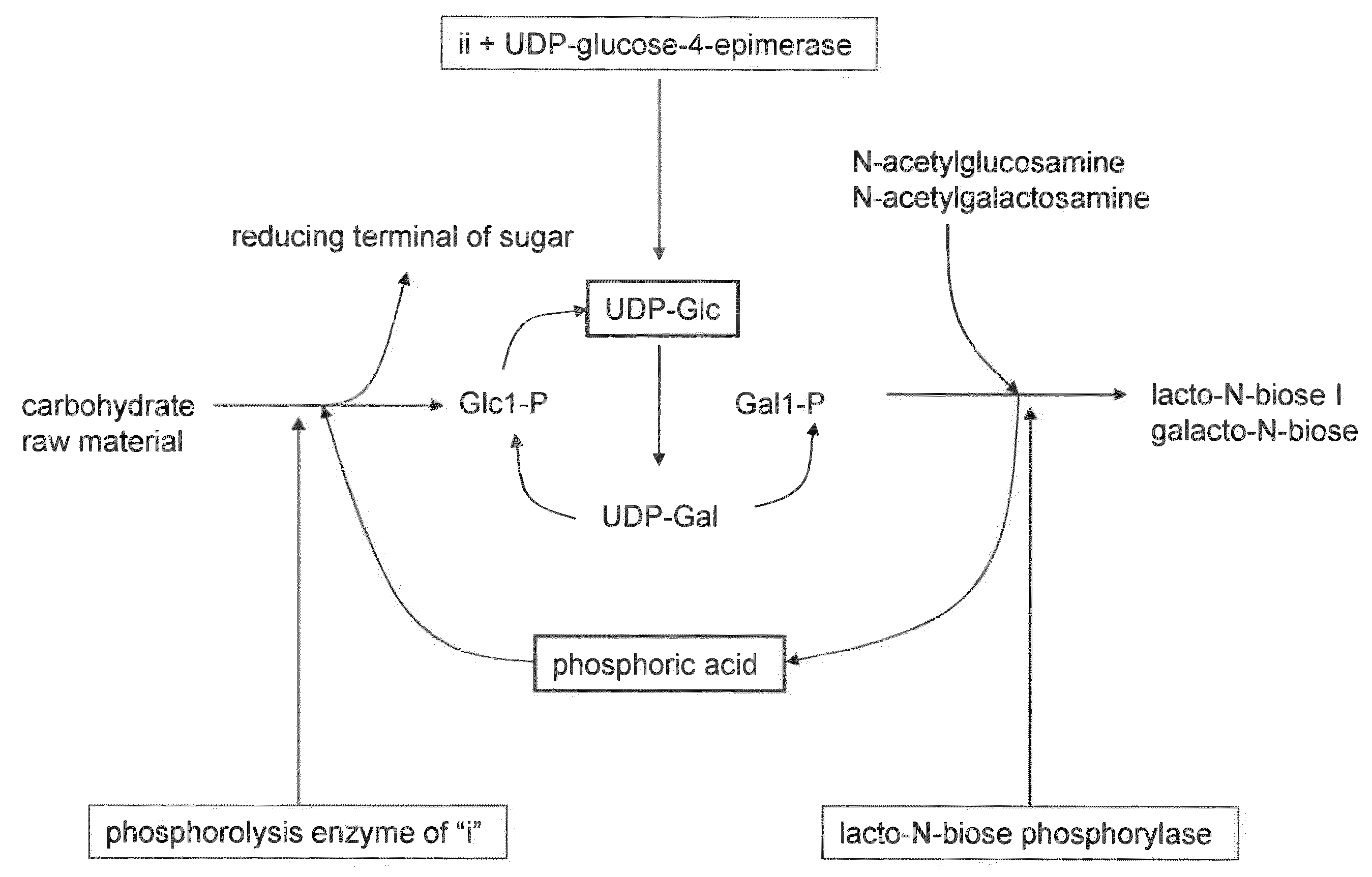
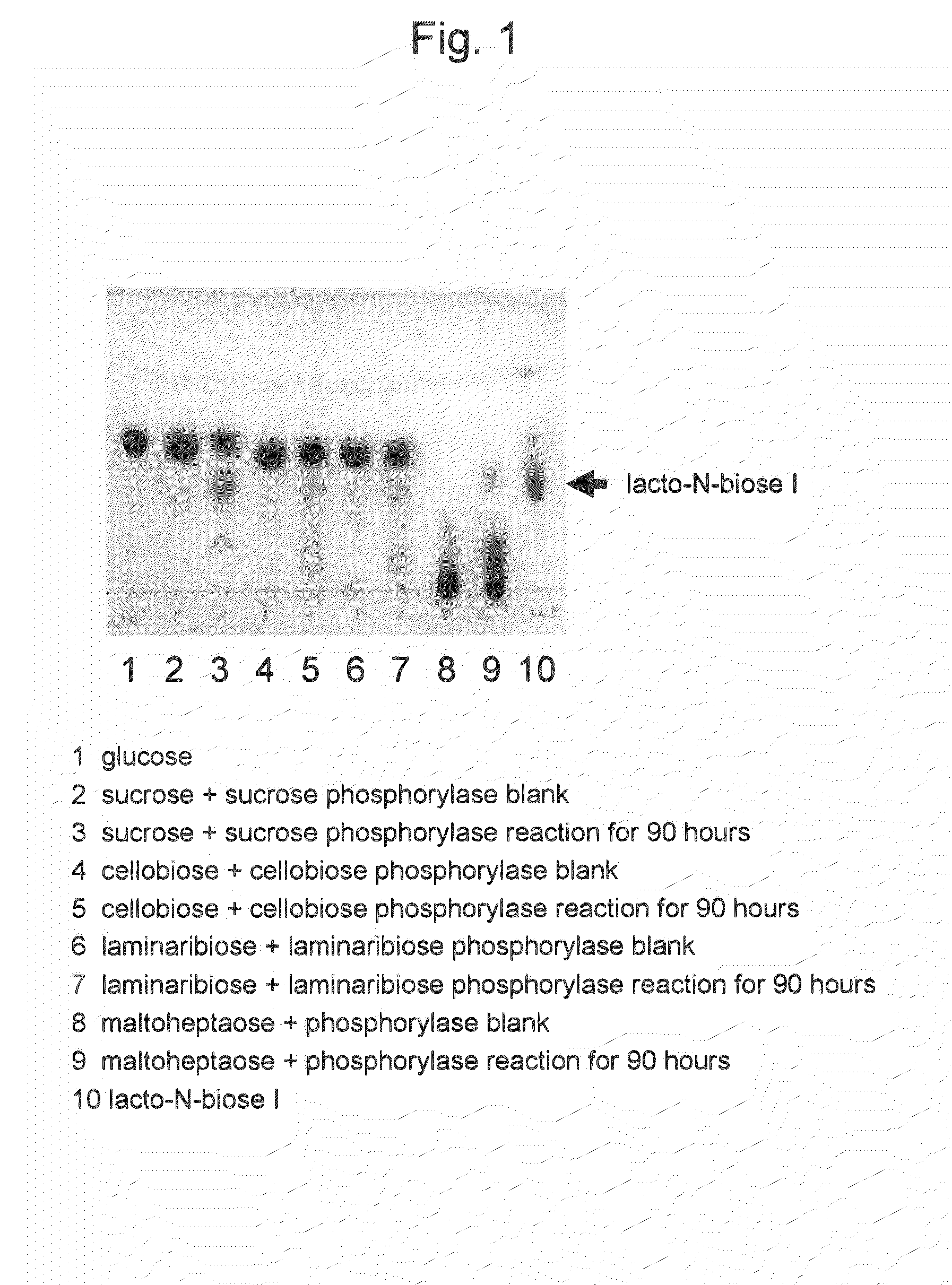
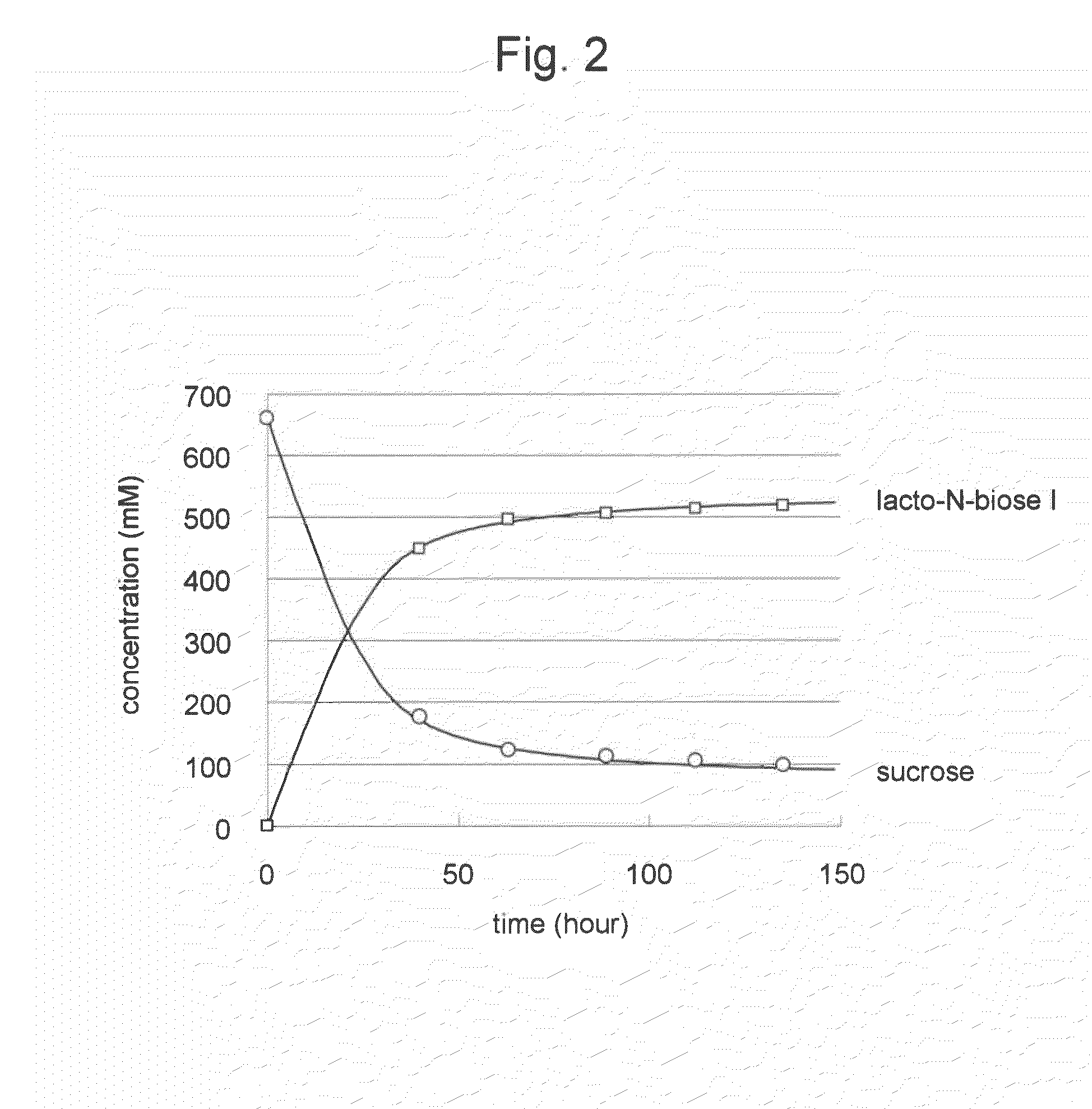
Method for producing lacto-n-biose i and galacto-n-biose
ActiveUS20100120096A1Easily and conveniently carry-outGood value for moneyImmobilised enzymesHydrolasesO-Phosphoric AcidPhosphorylation
A method for producing lacto-N-biose I and galacto-N-biose inexpensively and conveniently is provided. The method for producing lacto-N-biose I or galacto-N-biose, characterized in that the method comprises causing: (i) a combination of a carbohydrate raw material with an enzyme that catalyzes phosphorolysis of the carbohydrate raw material to give a-glucose-1-phosphate; and (ii) a combination of an enzyme that converts α-glucose-1-phosphate to UDP-glucose and an enzyme that converts UDP-galactose to galactose-1-phosphate with their cofactors, and / or a combination of an enzyme (UDP-Gly synthase) that converts α-glucose-1-phosphate and UDP-galactose to UDP-glucose and α-galactose-1-phosphate, respectively, with its cofactor(s) to act in the presence of N-acetylglucosamine or N-acetylgalactosamine, phosphoric acid, lacto-N-biose phosphorylase (EC 2.4.1.211), and UDP-glucose-4-epimerase (EC 5.1.3.2).
Owner:NAT AGRI & FOOD RES ORG
Application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate
InactiveCN104726507ADisinhibition effectImprove conversion abilityBacteriaOxidoreductasesHydroxybutyric acidPtru catalyst
The invention discloses an application of aldo-keto reductase in catalytic generation of (R)-4-chlorine-3-hydroxy ethyl butyrate. With aldo-keto reductase of which an amino acid sequence is as shown in SEQ ID NO:2 as a catalyst, with 4-ethyl acetoacetate as a substrate and NADPH as a cofactor, the (R)-4-chlorine-3-hydroxy ethyl butyrate is prepared by asymmetric reduction. The aldo-keto reductase of which the amino acid sequence is as shown in SEQ ID NO:2 is applied to the 4-ethyl acetoacetate to prepare the (R)-4-chlorine-3-hydroxy ethyl butyrate in an asymmetric reduction manner for the first time, so that a good effect is obtained; the enzyme activity can be up to 8.1U / mg; the conversion ratio of the aldo-keto reductase on the substrate can be up to 99.8%; the enantiomer excess value of the product is greater than 995; the yield is high; and the production cost is greatly reduced.
Owner:NANJING UNIV OF TECH
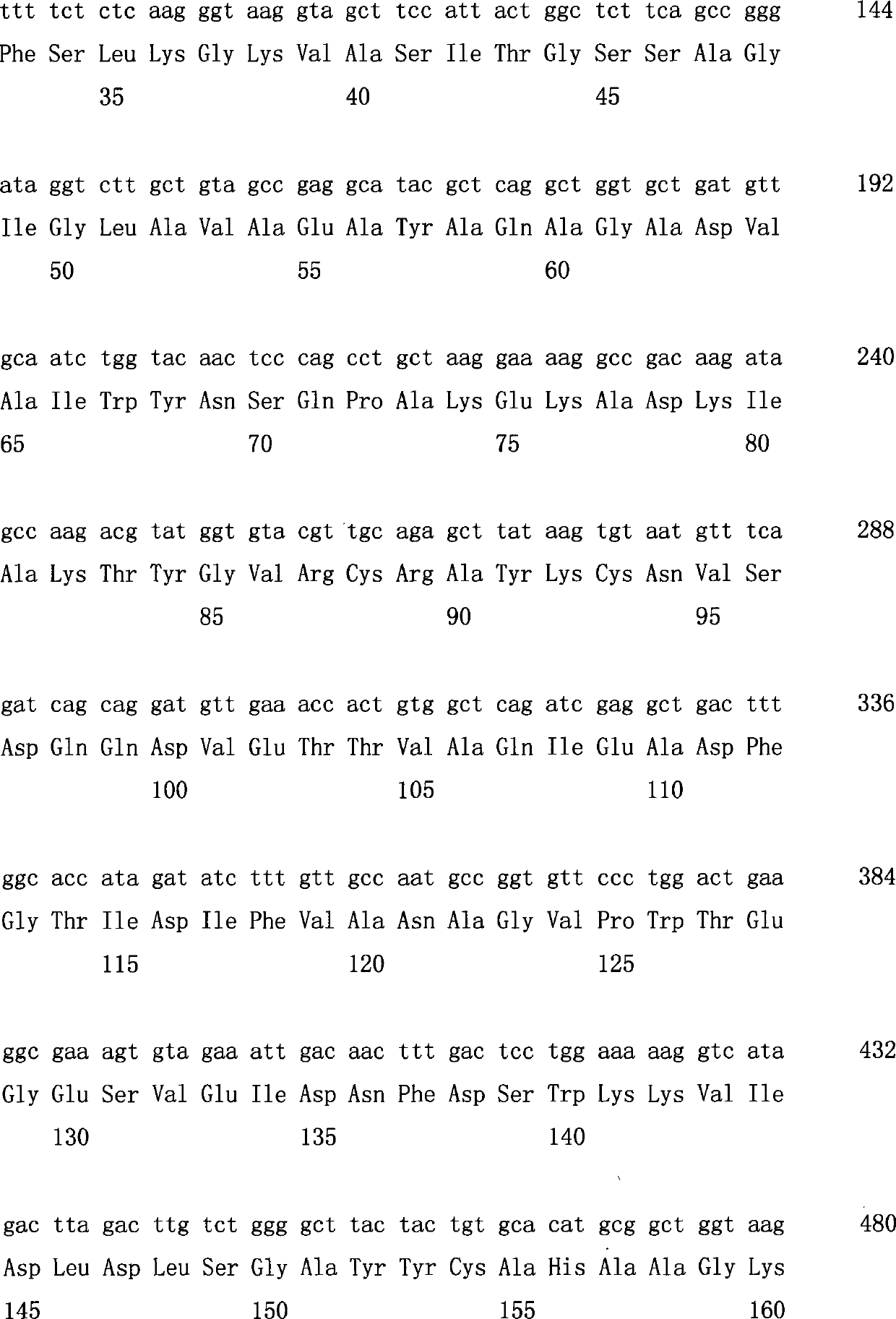
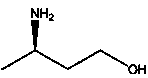
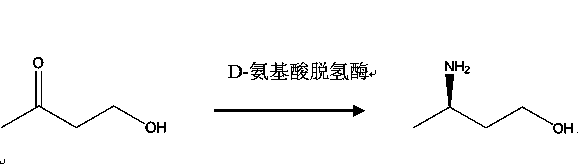
Method for producing R-3-aminobutanol
The invention relates to a method for producing R-3-aminobutanol. The method comprises the following steps: artificially designing a sequence shown in SEQID NO: 1, carrying out full gene synthesis, cloning the synthesized gene fragments into an expression vector pET22b to prepare a recombinant expression vector, transferring the recombinant expression vector into Escherichia coli to prepare a genetically engineered bacterium of a recombinant D-amino acid dehydrogenase, culturing the genetically engineered bacterium to prepare the recombinant D-amino acid dehydrogenase, sequentially adding a substrate of which the final concentration is 10-300mmol / L, a 2-15wt% cosolvent, a cofactor of which the final concentration is 0.1-1mmol / L, an amino donor of which the final concentration is 0.02-1mmol / L and the 0.01-1wt% D-amino acid dehydrogenase into a reaction solution to constitute a reaction system, reacting, after the reaction is completed, and extracting R-3-aminobutanol in the reaction solution. The method disclosed by the invention has the advantages that the cost is low, the conversion rate is greater than 95%, the product yield is greater than 85%, no by-product is generated and method is more suitable for industrial applications.
Owner:洛阳华荣生物技术有限公司
Process for preparing an intermediate of sitagliptin via enzymatic conversion
The invention provides a process for preparing 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one (Formula I), into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms comprising: a) reacting 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one of formula (III) with a suitable oxidoreductase enzymes or its suitable variants in the presence of suitable conditions and co-factor; and b) isolating 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one, into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms.
Owner:CADILA HEALTHCARE LTD
Construction of coenzyme efficient regeneration system and application thereof
InactiveCN105238807AImprove conversion efficiencyLow costFermentationVector-based foreign material introductionEscherichia coliThreonine
The invention relates to a method for preparing alpha-aminobutyric acid by utilizing series connection amino acid dehydrogenase and codon optimization formate dehydrogenase recombinant escherichia coli. First, codon optimization is performed on a Candida boidinii formate dehydrogenase gene sequence according to the codon preference of the escherichia coli. After codon optimization on formate dehydrogenase, the expression quantity of formate dehydrogenase in the escherichia coli can be improved remarkably, the yield of alpha-aminobutyric acid is increased, and in the escherichia coli, co-expression formate dehydrogenase and L-amino acid dehydrogenase can promote circulation of cofactors in mycetome without needing the adding of any exogenous cofactors. In addition, the process of utilizing whole-cell transformation bulk chemical L-threonine to produce alpha-aminobutyric acid is simple and quick, and the cost is low. In a 5 L fermentation tank, the yield of alpha-aminobutyric acid obtained through the method can be 81.5 g / L, and an actually effective strategy is provided for industrial production of alpha-aminobutyric acid.
Owner:ANHUI HUAHENG BIOTECH
Engineered microogranisms capable of producing target compounds under anaerobic conditions
The present invention is generally provides recombinant microorganisms comprising engineered metabolic pathways capable of producing C3-C5 alcohols under aerobic and anaerobic conditions. The invention further provides ketol-acid reductoisomerase enzymes which have been mutated or modified to increase their NADH-dependent activity or to switch the cofactor preference from NADPH to NADH and are expressed in the modified microorganisms. In addition, the invention provides isobutyraldehyde dehydrogenase enzymes expressed in modified microorganisms. Also provided are methods of producing beneficial metabolites under aerobic and anaerobic conditions by contacting a suitable substrate with the modified microorganisms of the present invention.
Owner:CALIFORNIA INST OF TECH +1
Method for plasmid preparation by conversion of open circular plasmid
InactiveUS20040191871A1Increase percentageFermentationVector-based foreign material introductionNucleotidePhosphoric acid
In accordance with the invention, there is provided a method for preparing plasmid from host cells which contain the plasmid, comprising the steps: (a) preparing a cleared lysate of the host cells, wherein the cleared lysate comprises unligatable open circular plasmid, wherein the open circular plasmid is not 3'-hydroxyl, 5-phosphate nicked plasmid; (b) incubating the unligatable open circular plasmid with one or more enzymes in the presence of their appropriate nucleotide cofactors, whereby the unligatable open circular plasmid is converted to 3'-hydroxyl, 5'-phosphate nicked plasmid; (c) incubating the 3'-hydroxyl, 5'-phosphate nicked plasmid with DNA ligase in the presence of DNA ligase nucleotide cofactor, whereby 3'-hydroxyl, 5'-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) incubating the relaxed covalently closed circular plasmid with DNA gyrase in the presence of DNA gyrase nucleotide cofactor, whereby relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. Preferably, the enzymatic steps (b), (c), and (d) are performed in a single step using an enzyme mixture comprising DNA polymerase, DNA ligase, and DNA gyrase. Preferably, the mixture further comprises a 3' terminus deblocking enzyme, such as exonuclease III or 3'-phosphatase. Preferably, the mixture further comprises one or more regenerating enzymes and a high energy phosphate donor, whereby the nucleotide by-products of the nucleotide cofactors generated by DNA ligase and DNA gyrase are converted to back to nucleotide cofactor. Preferably, the enzyme mixture further comprises one or more exonucleases, such as ATP dependent exonuclease, whereby linear chromosomal DNA is selectively degraded.
Owner:HYMAN EDWARD DAVID
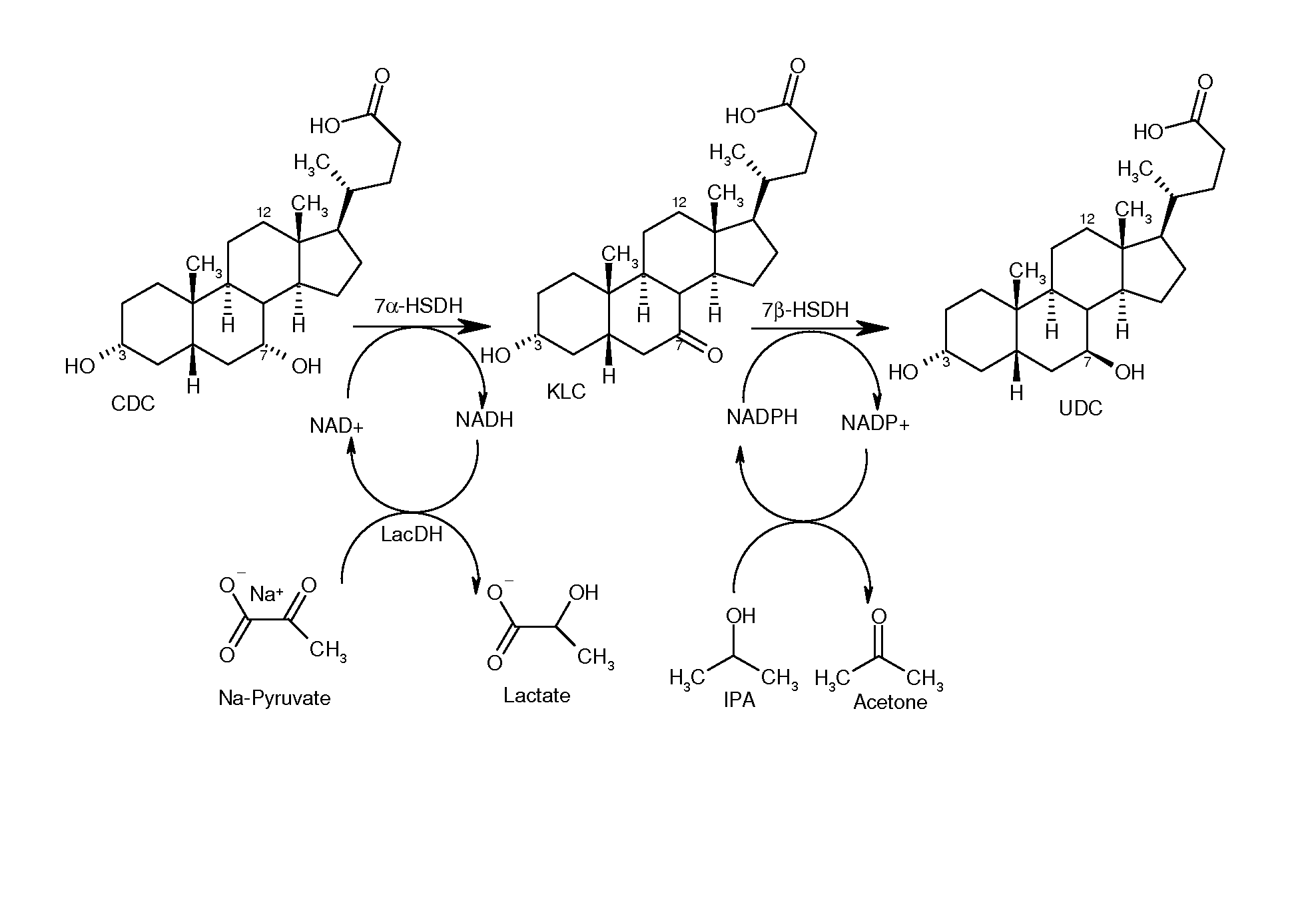
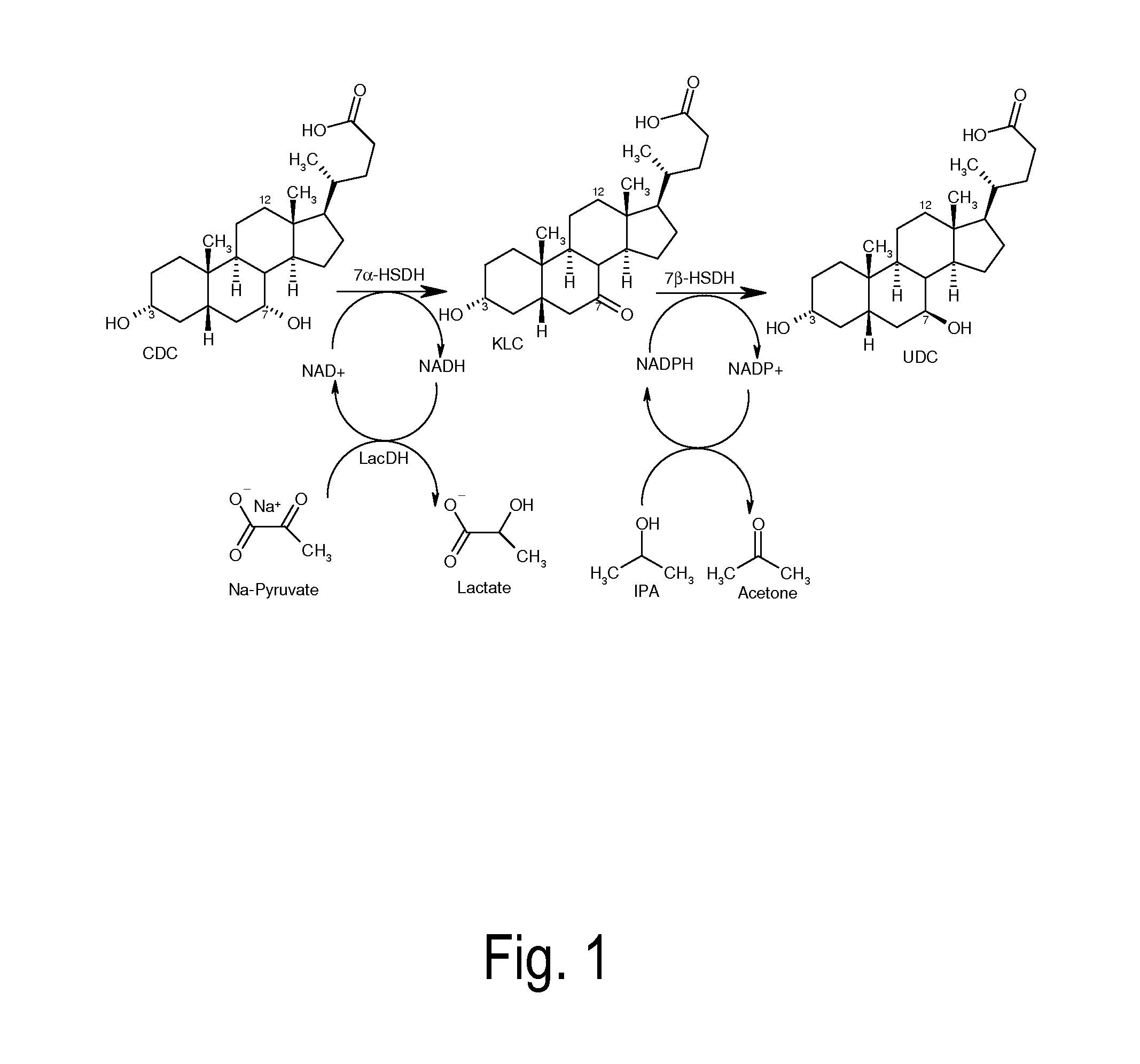
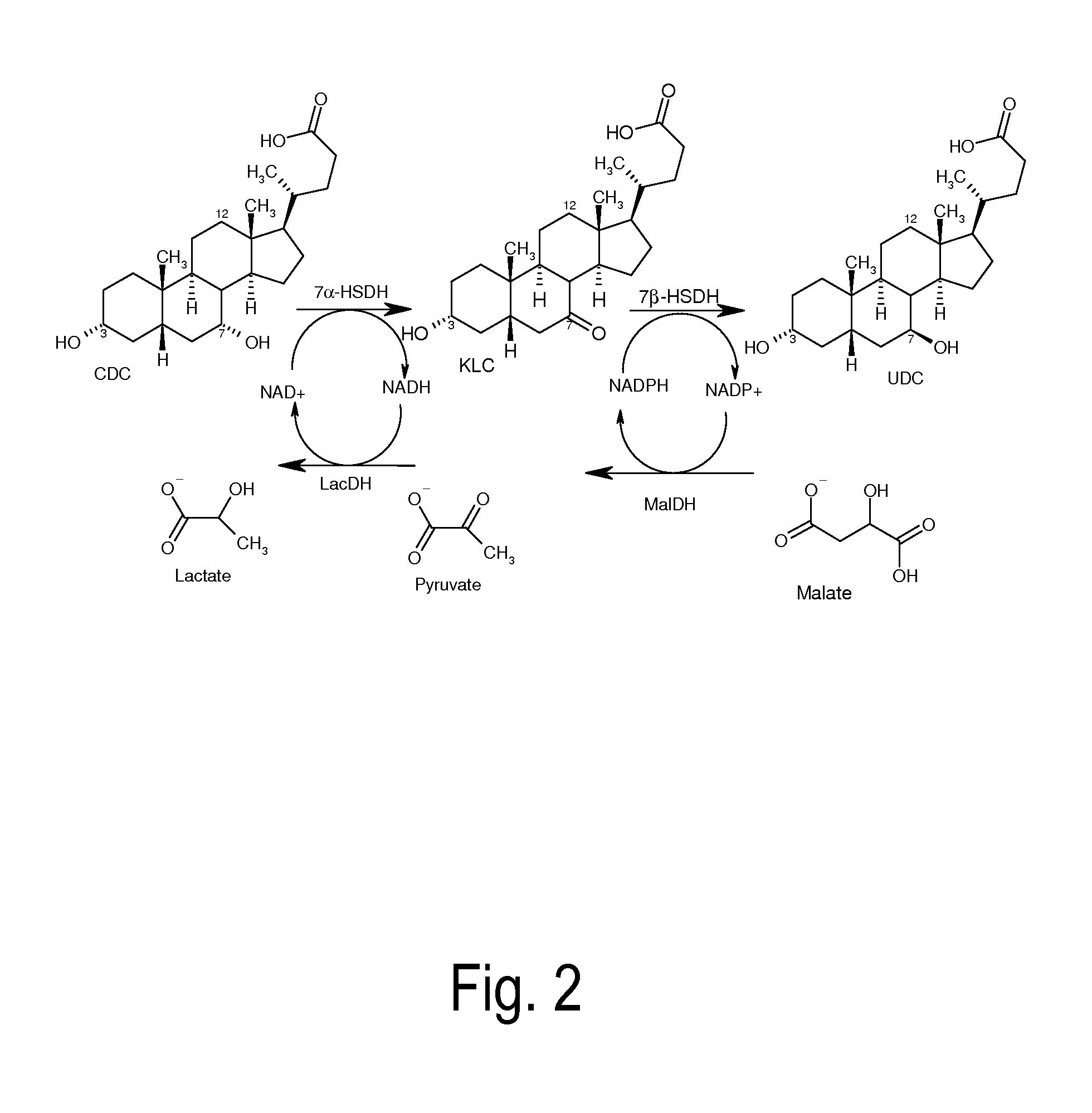
Process for the enzymatic regeneration of redox cofactors
A process for the enzymatic regeneration of the redox cofactors NAD+ / NADH and NADP+ / NADPH in a one-pot reaction, wherein, as a result of at least two further enzymatically catalyzed redox reactions proceeding in the same reaction batch (product-forming reactions), one of the two redox cofactors accumulates in its reduced form and, respectively, the other one in its oxidized form, characterized in that a) in the regeneration reaction which reconverts the reduced cofactor into its original oxidized form, oxygen or a compound of general formula R1C(O)COOH is reduced, and b) in the regeneration reaction which reconverts the oxidized cofactor into its original reduced form, a compound of general formula R2CH(OH)R3 is oxidized and wherein R1, R2 and R3 in the compounds have different meanings.
Owner:ANNIKKI GMBH
Application of a kind of sorbose reductase in biological preparation of ethyl (s)-4-chloro-3-hydroxybutyrate
InactiveCN102286556AHigh yieldHigh optical activityMicroorganism based processesFermentationHydroxybutyric acidPtru catalyst
The invention discloses application of sorbose reductase in preparation of (S)-4-chloro-3-hydroxyethyl butyrate by a biological method. Sorbose reductase of which the amino acid sequence is disclosed as SEQ ID NO:2 is used as a catalyst, 4-chloracetylethyl acetate is used as a substrate, and NADPH (nicotinamide adenine dinucleotide phosphate) is used a cofactor to prepare the (S)-4-chloro-3-hydroxyethyl butyrate in an asymmetric reduction mode. In the invention, the sorbose reductase of which the amino acid sequence is disclosed as SEQ ID NO:2 is applied to preparation of (S)-4-chloro-3-hydroxyethyl butyrate in an asymmetric reduction mode for the first time, which has a good effect. The enzyme activity of the sorbose reductase is up to 5.6U / mg, the yield for substrate is up to 90%, and the enantiomeric excess of the product is 100%. Besides, the invention has the advantage of high yield, and greatly lowers the production cost.
Owner:NANJING UNIV OF TECH
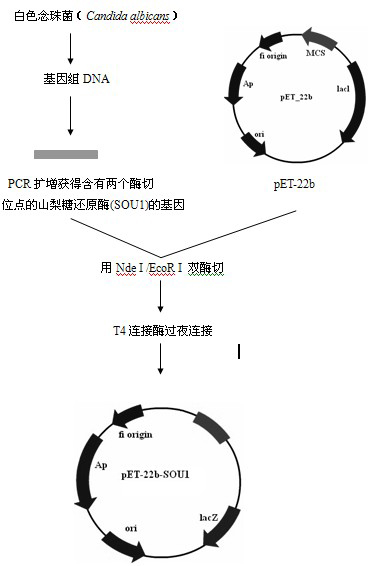
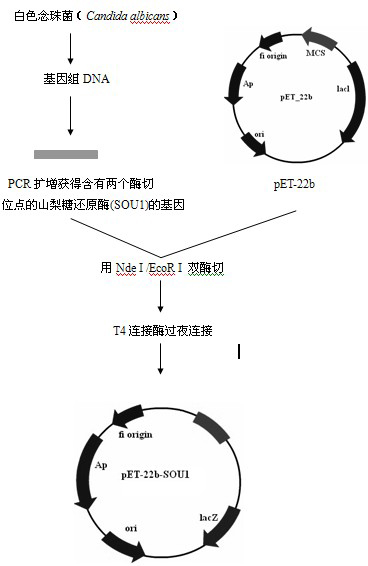
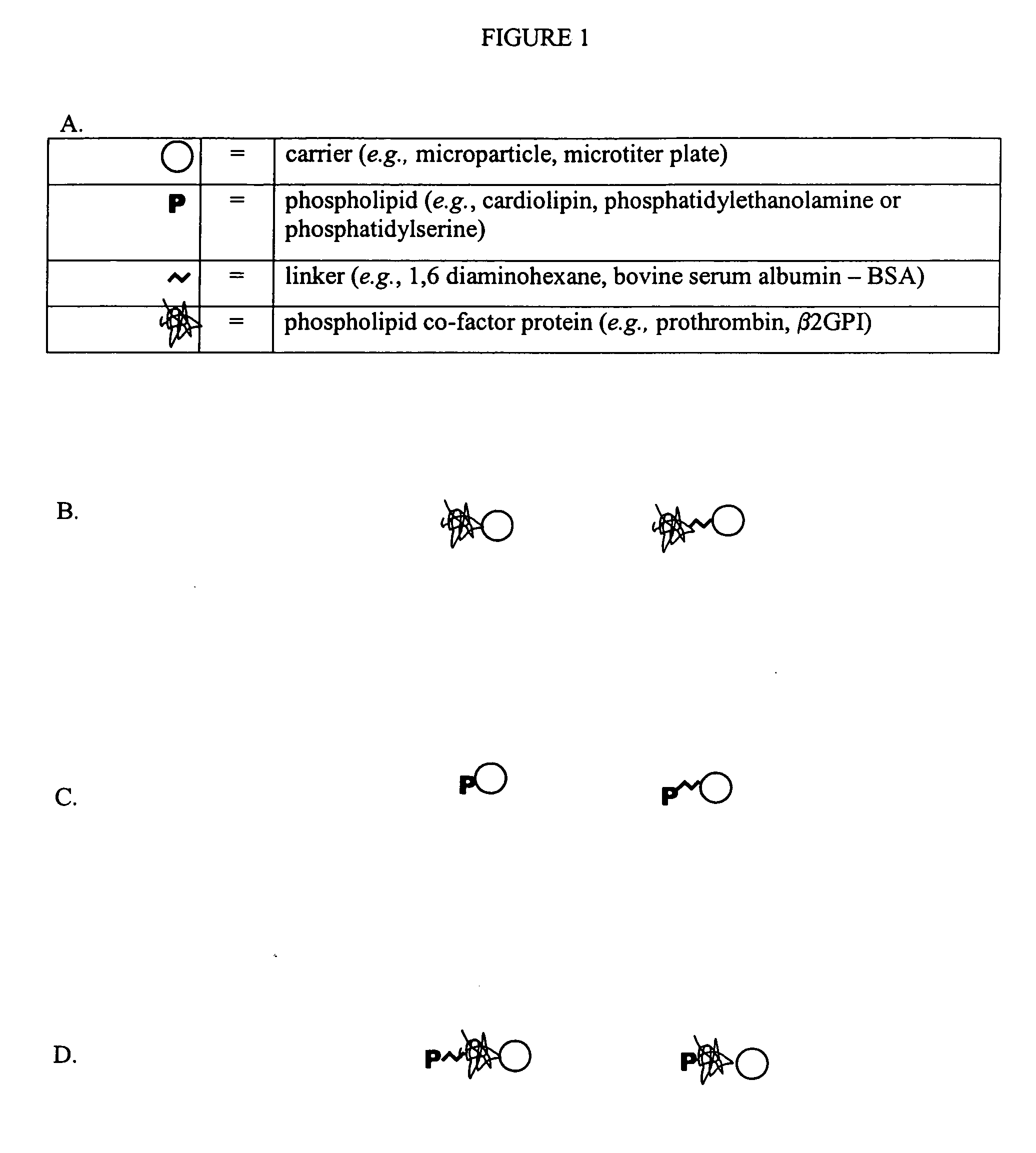
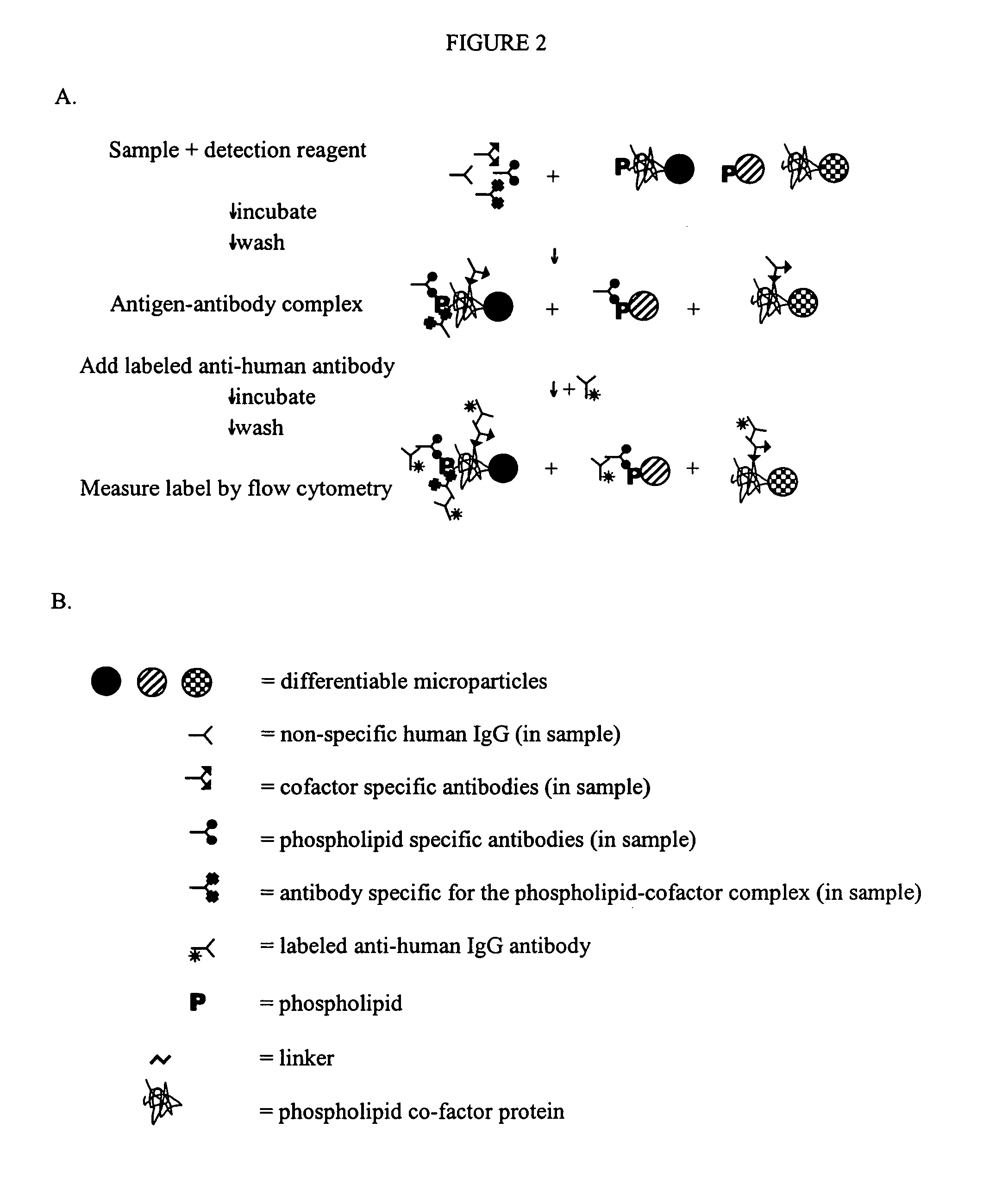
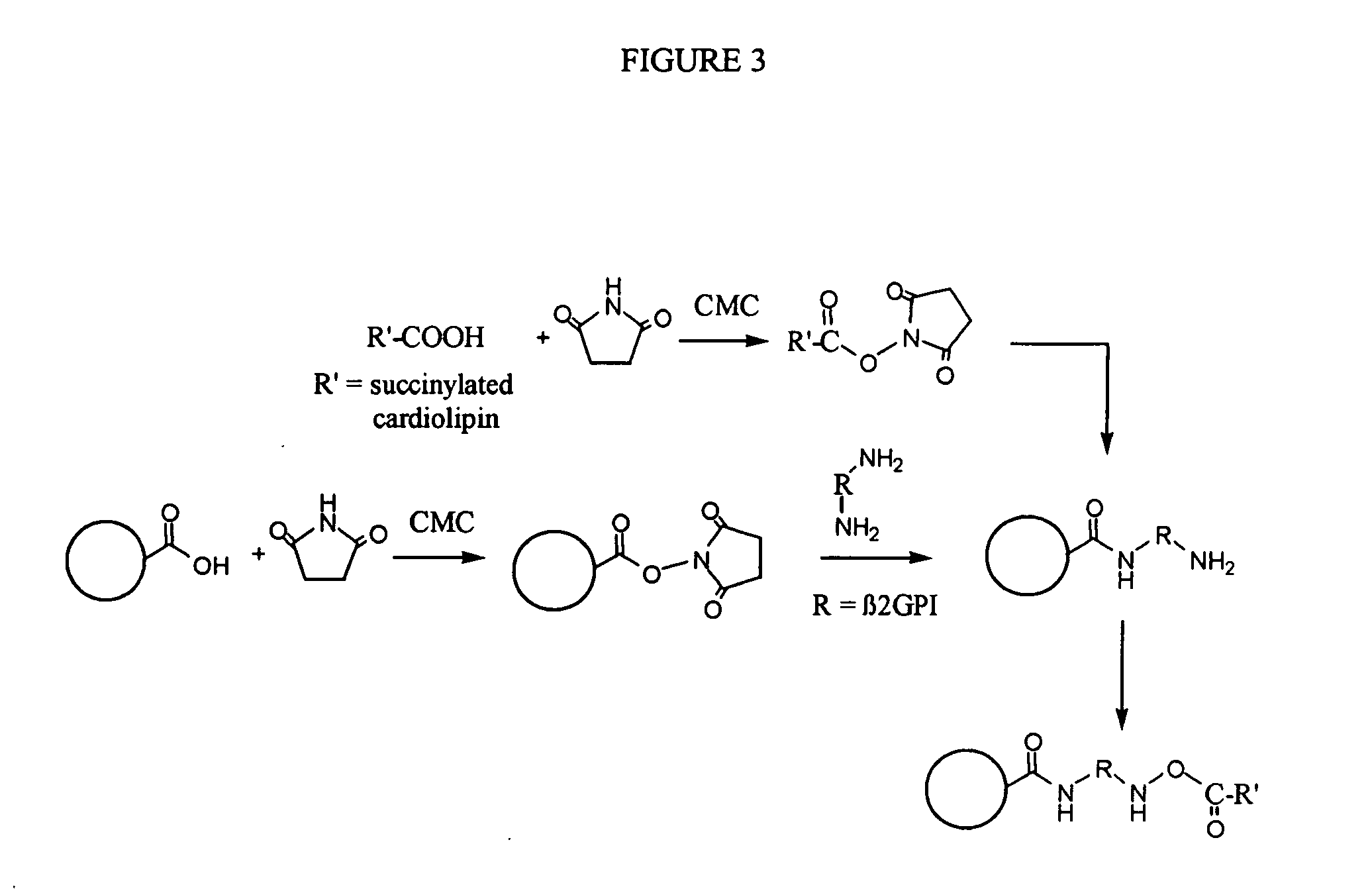
Solid phase immobilization of phospholipids and cofactor proteins via covalent attachment
The present invention provides methods and reagents for detecting anti-phospholipid-cofactor protein-antibodies.
Owner:BIO RAD LAB INC
Multispecific antigen-binding molecules having blood coagulation factor VIII (FVIII) cofactor function-substituting activity and pharmaceutical formulations containing such a molecule as an active ingredient
ActiveUS10759870B2Efficiently formedImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIAntigen
Bispecific antibodies whose FIX activation-inhibiting activity is not elevated and whose FVIII cofactor function-substituting activity is elevated have been successfully discovered.
Owner:CHUGAI PHARMA CO LTD
Cell-free and minimized metabolic reaction cascades for the production of chemicals
Provided are enzymatic processes for the production of chemicals from carbon sources. In particular, a process for the production of a target chemical is disclosed using a cell-free enzyme system that converts carbohydrate sources to the intermediate pyruvate and subsequently the intermediate pyruvate to the target chemical.
Owner:CLARIANT PROD DEUT GMBH
Method for plasmid preparation by conversion of open circular plasmid to supercoiled plasmid
InactiveUS20050084938A1Increase productionUniversal procedureHydrolasesMicrobiological testing/measurementPhosphateGenetics
In one embodiment of the invention, a method is provided for preparing plasmid from host cells which contain the plasmid, comprising: (a) providing a plasmid solution comprised of unligatable open circular plasmid; (b) reacting the unligatable open circular plasmid with one or more enzymes and appropriate nucleotide cofactors, such that unligatable open circular plasmid is converted to 3′-hydroxyl, 5′-phosphate nicked plasmid; (c) reacting the 3′-hydroxyl, 5′-phosphate nicked plasmid with a DNA ligase and DNA ligase nucleotide cofactor, such that 3′-hydroxyl, 5′-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) reacting the relaxed covalently closed circular plasmid with a DNA gyrase and DNA gyrase nucleotide cofactor, such that relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. In other embodiments, DNA gyrase is replaced by reverse DNA gyrase or reaction (d) is not performed.
Owner:HYMAN EDWARD D
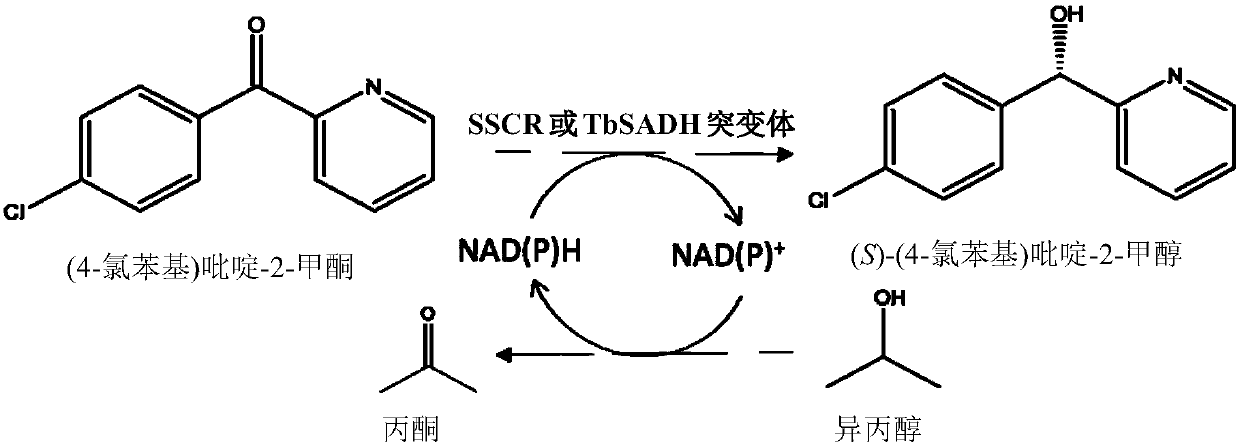
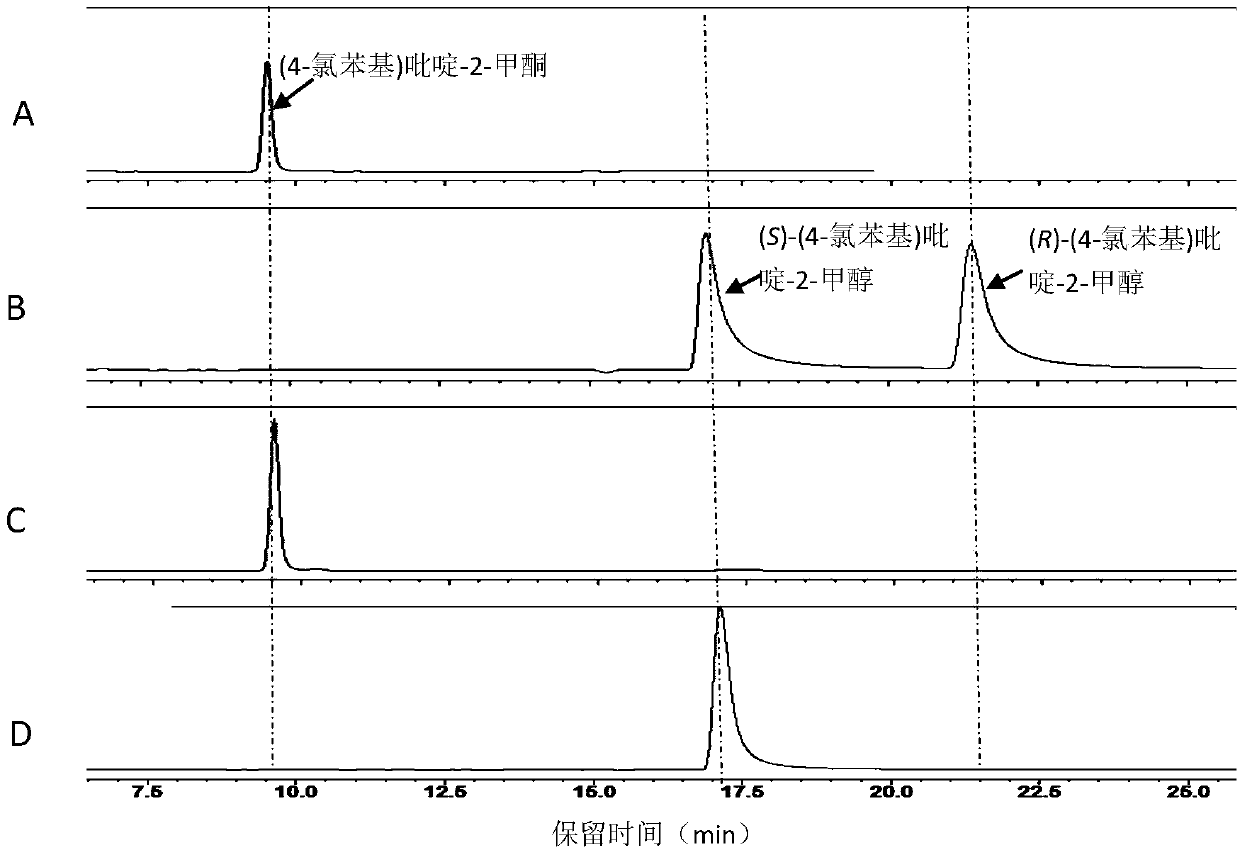
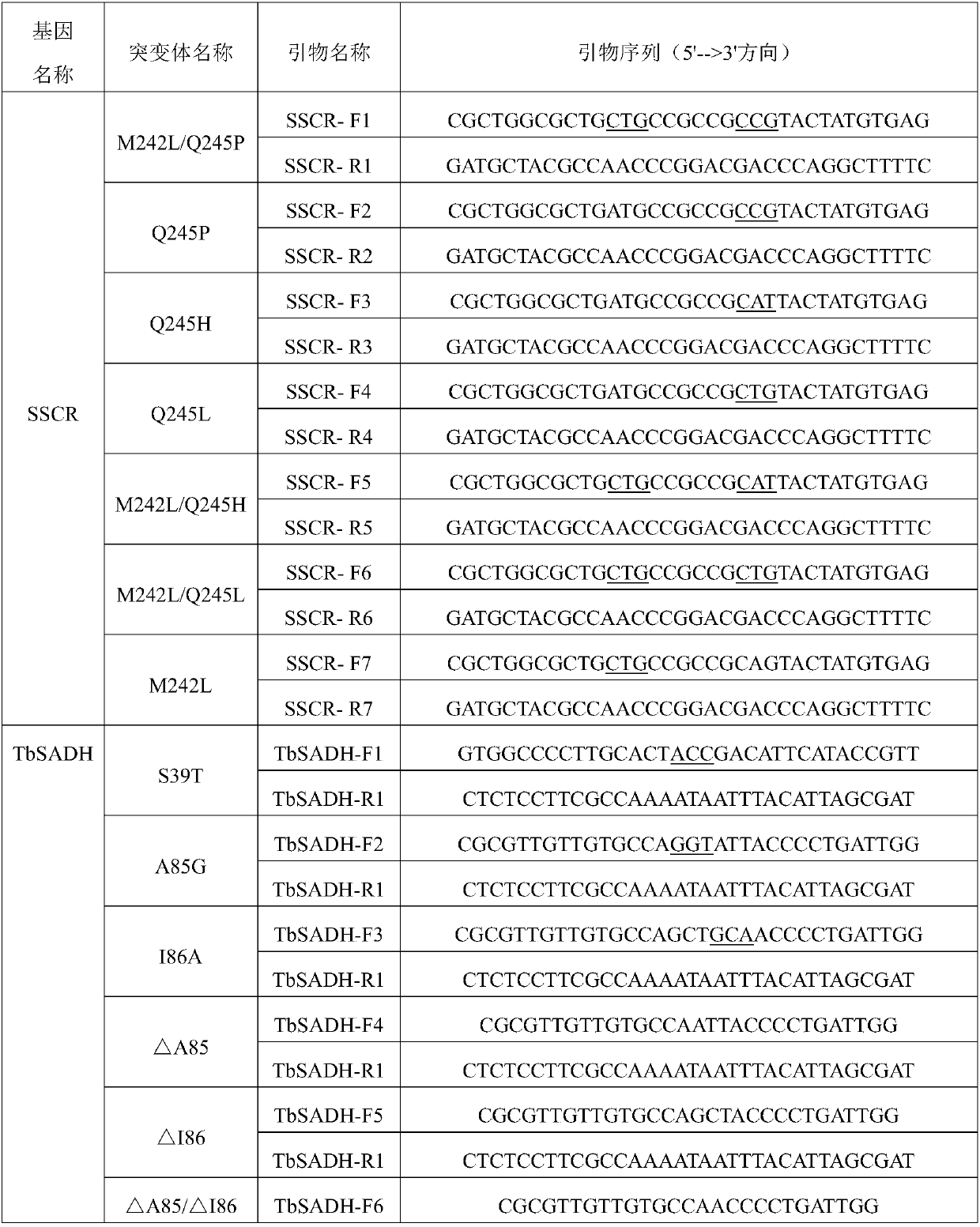
Method for synthesizing chiral bisaryl alcohol compound
ActiveCN109837317AReduce usageHigh yieldOxidoreductasesGenetic engineeringAlcoholSporobolomyces species
The invention discloses a method for synthesizing a chiral bisaryl alcohol compound. The method provided by the present invention specifically uses a mutant of a carbonyl reductase SSCR derived from Sporobolomyces Salmonicolor and a mutant of alcohol dehydrogenase TbSADH derived from Thermoanaerobacter brockii to catalyze asymmetric reduction of (4-chlorophenyl)pyridine-2-ketone to form (S)-(4-chlorophenyl) pyridine-2-methanol. The experiment proves that the method provided by the invention has high yield and high ee value, can reduce the addition amount of coenzyme, and does not need to add enzyme such as glucose alcohol dehydrogenase for cofactor circulation, the reaction condition is mild, and the operation is simple.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Multispecific antigen-binding molecules having blood coagulation factor viii (FVIII) cofactor function-substituting activity and pharmaceutical formulations containing such a molecule as an active ingredient
PendingUS20200354473A1Undesirable CH1 and CL association can be suppressedEfficiently formedImmunoglobulins against blood coagulation factorsHybrid immunoglobulinsBlood coagulation factor VIIIAntigen
Bispecific antibodies whose FIX activation-inhibiting activity is not elevated and whose FVIII cofactor function-substituting activity is elevated have been successfully discovered.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com