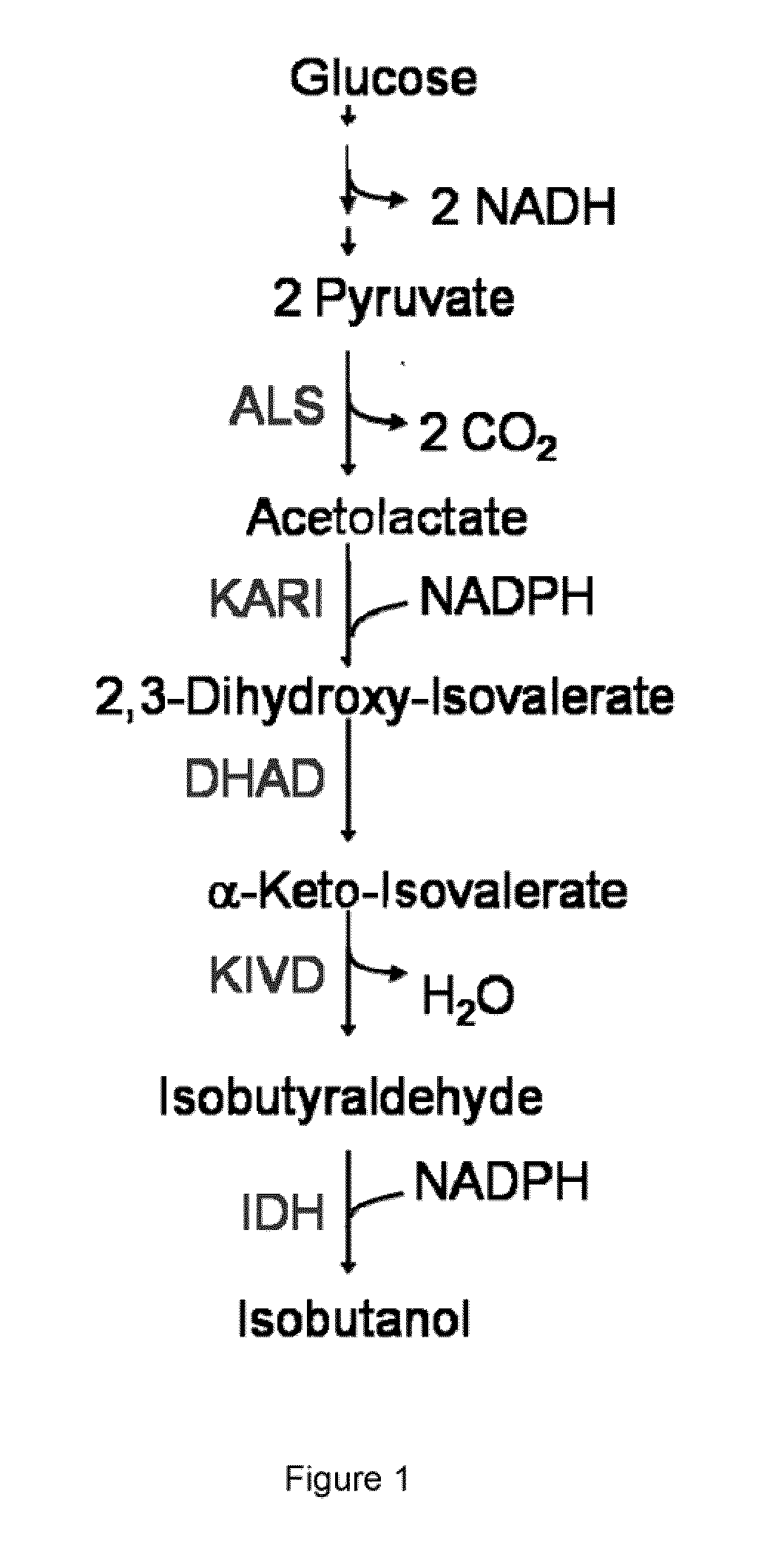Engineered microorganisms capable of producing target compounds under anaerobic conditions
a technology of anaerobic conditions and microorganisms, applied in microorganisms, biofuels, enzymes, etc., can solve the problems of increasing the demand for domestically produced biofuels, increasing the cost of fuel, increasing the demand for alternative fuels, etc., and achieves the effect of increasing the rate, titer and productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
EXAMPLE 1
Low-Level Anaerobic Production of Isobutanol
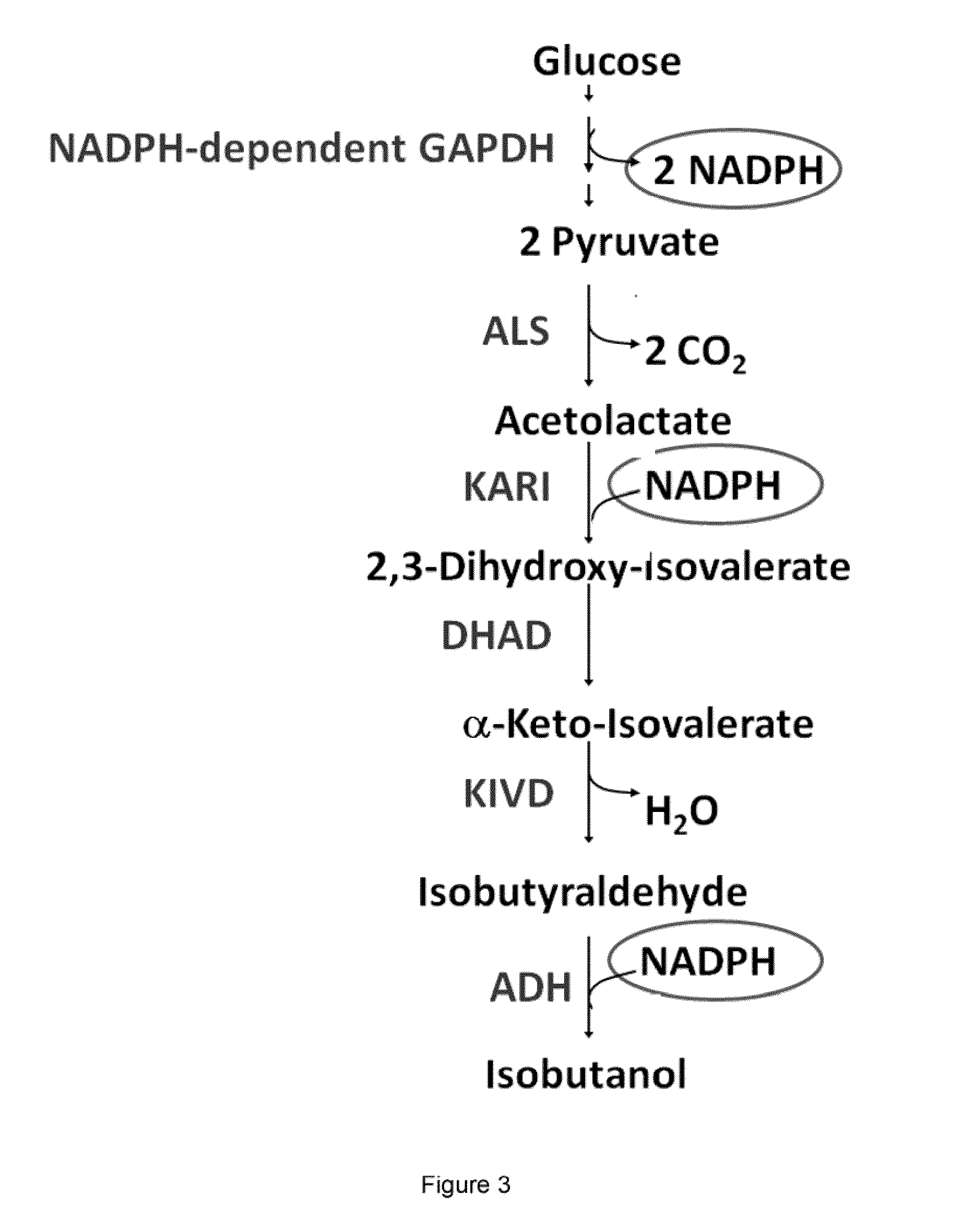
[0471]This example illustrates that a modified microorganism which is engineered to overexpress an isobutanol producing pathway produces a low amount of isobutanol under anaerobic conditions.
[0472]Overnight cultures of GEVO1859 were started from glycerol stocks stored at −80° C. of previously transformed strains. These cultures were started in 3 mL M9 minimal medium (Miller, J. H. A Short Course in Bacterial Genetics: A laboratory manual and handbook for Escherichia coli and related bacteria. 1992. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.), supplemented with 10 g / L yeast extract, 10 μM ferric citrate and trace metals, containing 8.5% glucose and the appropriate antibiotics in snap cap tubes about 14 h prior to the start of the fermentation. Isobutanol fermentations were then carried out in screw cap flasks containing 20 mL of the same medium that was inoculated with 0.2 mL of the overnight culture. The cells ...
example 2
EXAMPLE 2
Determination of Transhydrogenase Activity
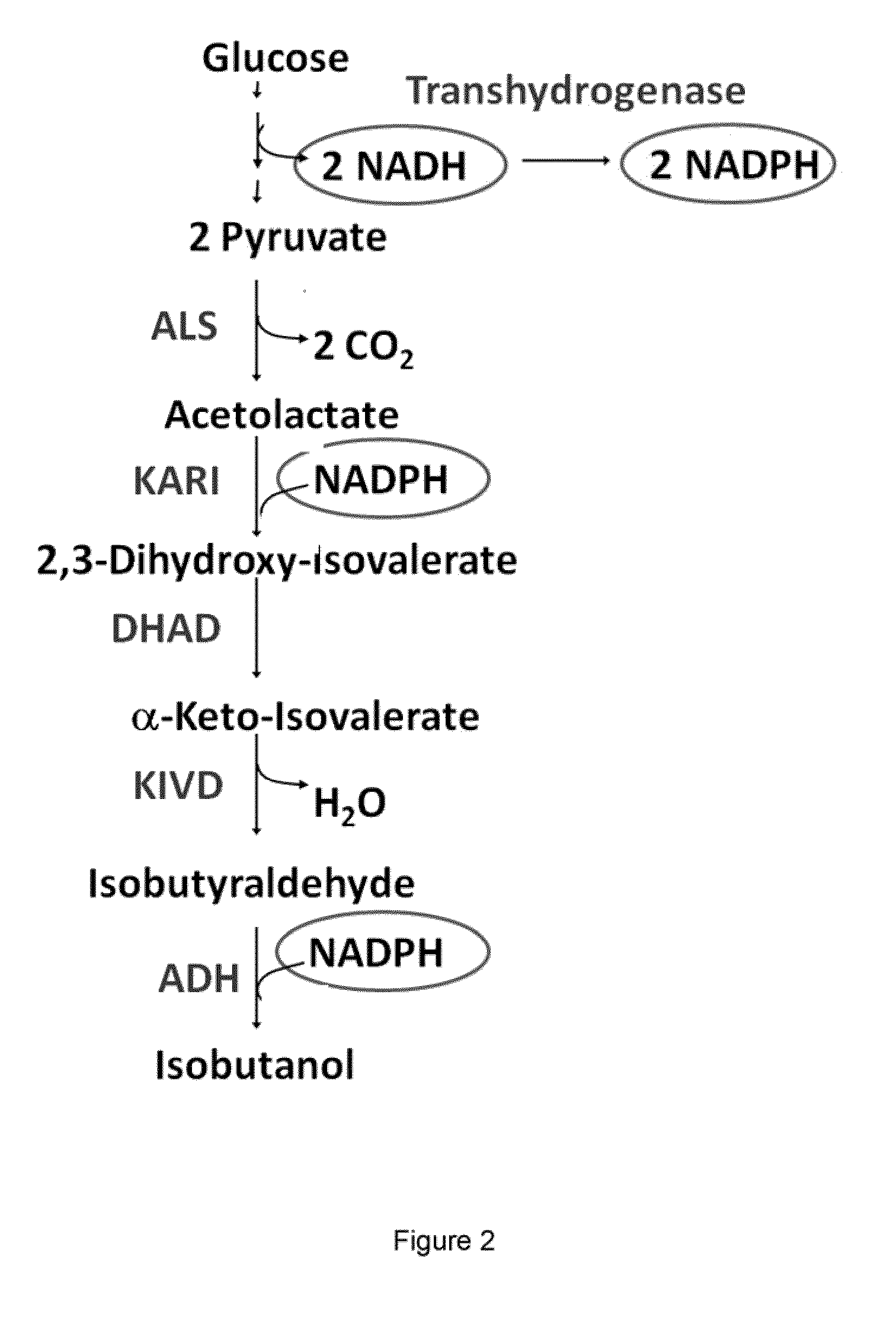
[0477]This example illustrates that an isobutanol producing microorganism which carries a plasmid for the expression of the E. coli PntAB transhydrogenase (SEQ ID NO: 2 and SEQ ID NO: 4) contains increased transhydrogenase activity.
[0478]A fermentation was performed with a strain expressing the tet repressor (GEVO1385) and carrying the plasmids pGV1655 (SEQ ID NO: 109) and pGV1698 (SEQ ID NO: 112) for expression of the isobutanol pathway. The E. coli transhydrogenase PntAB was expressed from a third plasmid pGV1685 (SEQ ID NO: 111), which contained the E. coli pntAB genes under control of the PLtet promoter. The appropriate empty vector control carries the plasmid pGV1490 (SEQ ID NO: 104).
[0479]GEVO1385 was transformed with pGV1698, pGV1655, and either pGV1685 or pGV1490. Transformed cells were plated on LB-plates containing the appropriate antibiotics and the plates were incubated overnight at 37° C. Overnight cultures were starte...
example 3
EXAMPLE 3
Overexpression of pntAB Improves Isobutanol Fermentation Performance
[0481]This example illustrates that overexpression of a transhydrogenase, exemplified by the E. coli pntAB operon (SEQ ID NO: 1 and SEQ ID NO: 3) on a low copy plasmid improves isobutanol production under micro-aerobic conditions.
[0482]GEVO1748 was transformed with plasmids pGV1698 (SEQ ID NO: 112) and one of either pGV1720 (SEQ ID NO: 115) (control) or pGV1745 (SEQ ID NO: 117) (E. coli pntAB).
[0483]The aforementioned strains were plated on LB-plates containing the appropriate antibiotics and incubated overnight at 37° C. Overnight cultures were started in 3 ml. EZ-Rich medium (Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J Bacteriol. 119:736-47) containing 5% glucose and the appropriate antibiotics in snap cap tubes about 14 h prior to the start of the fermentation. Isobutanol fermentations were then carried out in EZ-Rich Medium containing 5% glucose and the ap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| OD | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com