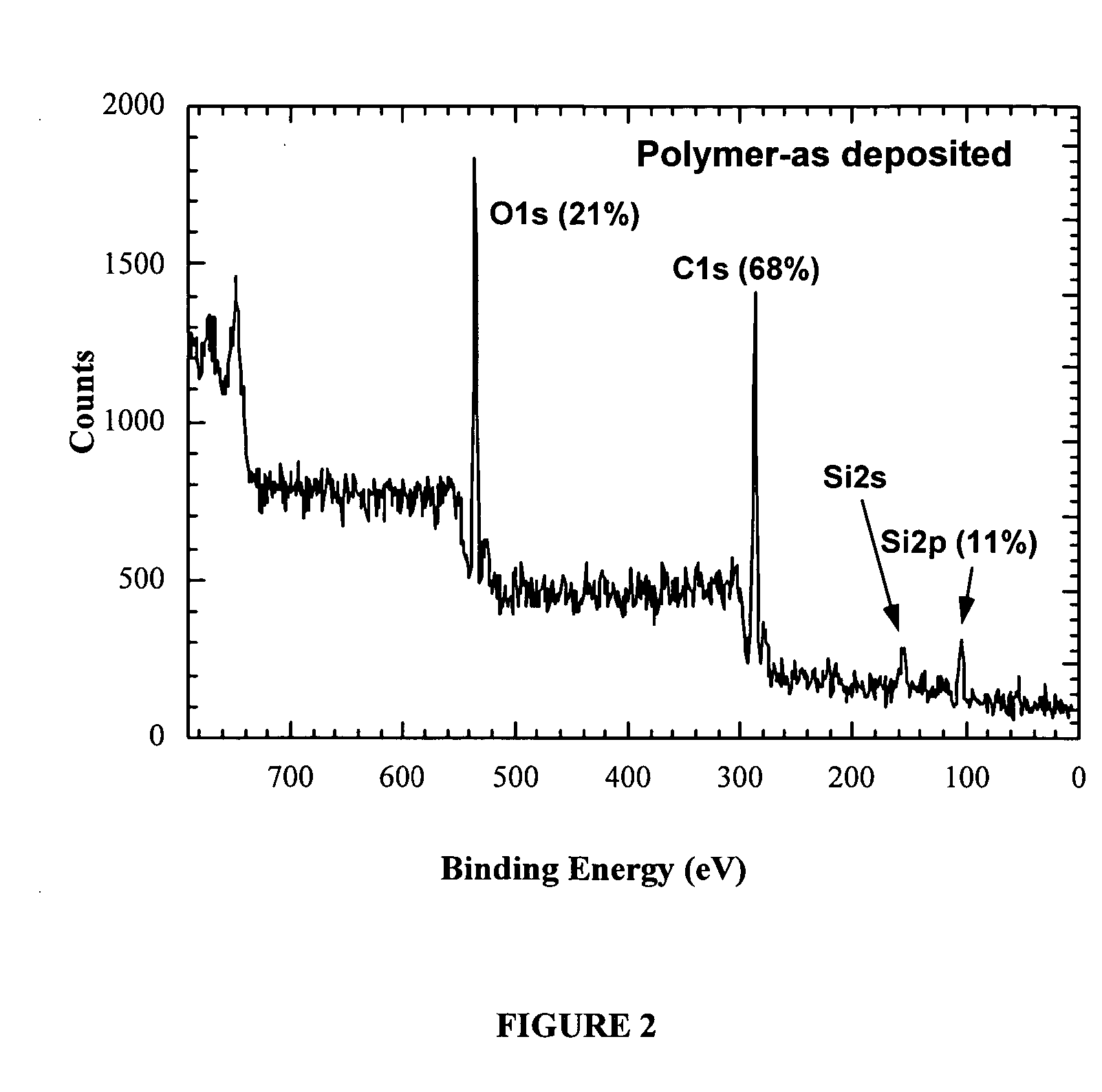
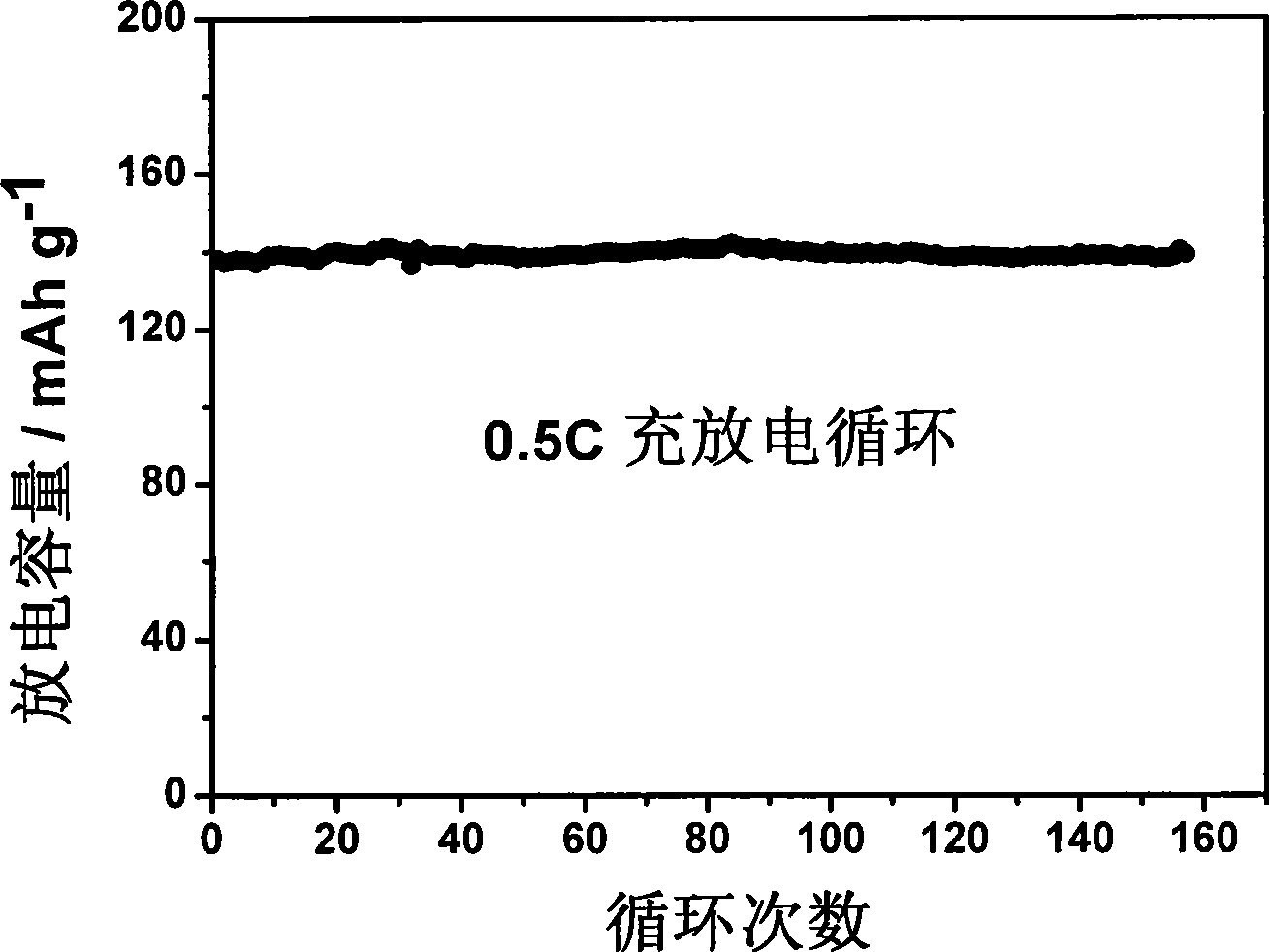
Patents
Literature
7270 results about "O-Phosphoric Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphoric acid (also known as orthophosphoric acid or phosphoric(V) acid) is a mineral (inorganic) and weak acid having the chemical formula H3PO4. Orthophosphoric acid refers to phosphoric acid, which is the IUPAC name for this compound.
Method of synthesizing zirconium phosphate particles
InactiveUS7566432B2Inhibition of agglomerationReduce moistureSemi-permeable membranesPhosphatesO-Phosphoric AcidZirconium oxychloride
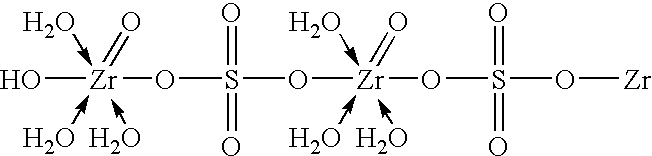
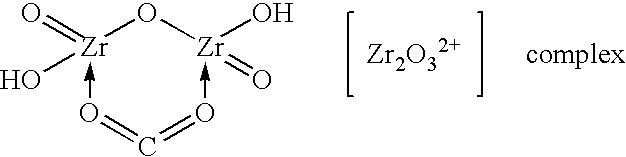
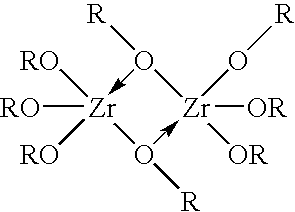
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one oxygen-containing additive to the solution, the oxygen-containing additive being selected to form a complex with zirconium ions in the solution of zirconium oxychloride and thereby reduce hydration of the zirconium ions, and combining this solution with phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:RENAL SOLUTIONS
Systems and methods for hydrogen generation from solid hydrides
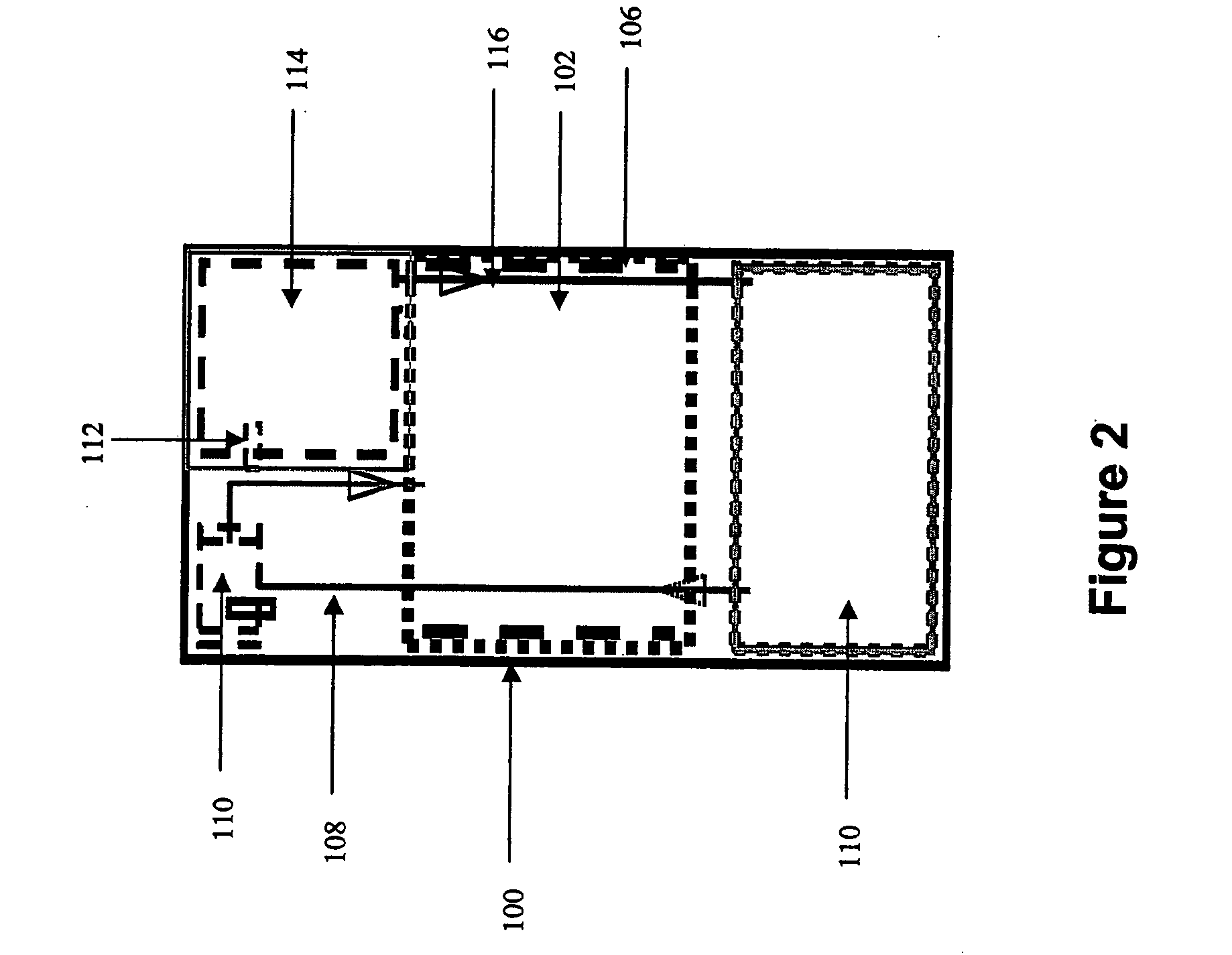
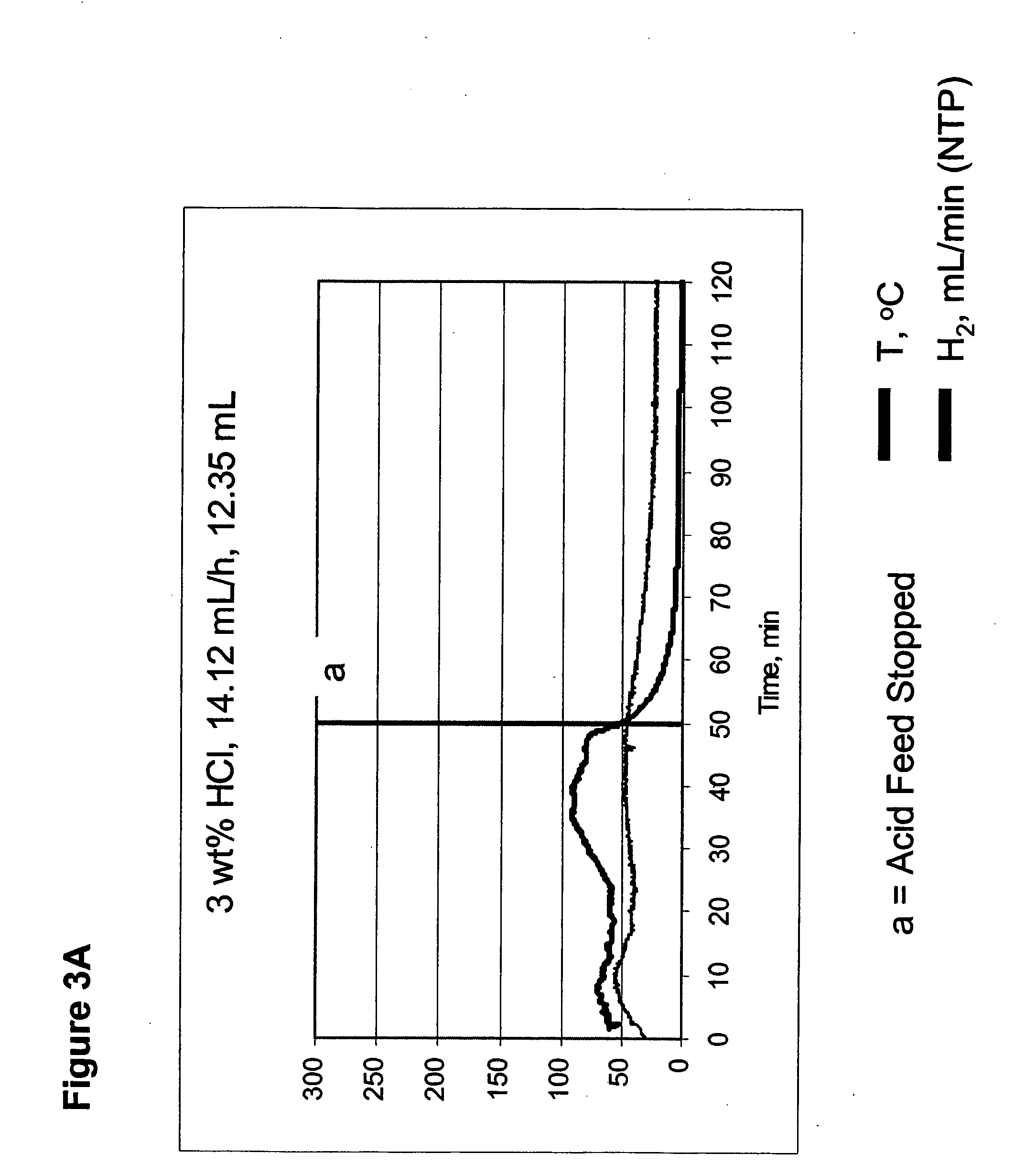
InactiveUS20050238573A1Regulate rateReactant parameters controlHydrogen productionO-Phosphoric AcidAlkaline earth metal
A system is disclosed for hydrogen generation based on hydrolysis of solid chemical hydrides with the capability of controlled startup and stop characteristics wherein regulation of acid concentration, acid feed rate, or a combination of both control the rate of hydrogen generation. The system comprises a first chamber for storing a solid chemical hydride and a second chamber for storing an acidic reagent. The solid chemical hydride is a solid metal borohydride having the general formula MBH4, where M is selected from the group consisting of alkali metal cations, alkaline earth metal cations, aluminum cation, zinc cation, and ammonium cation. The acidic reagent may comprise inorganic acids such as the mineral acids hydrochloric acid, sulfuric acid, and phosphoric acid, and organic acids such as acetic acid, formic acid, maleic acid, citric acid, and tartaric acid, or mixtures thereof.
Owner:MILLENNIUM CELL
Strontium-apatite-cement-preparations, cements formed therefrom, and uses thereof
ActiveUS20050142211A1Facilitated releaseTo promote metabolismBiocideSurgical adhesivesO-Phosphoric AcidPowder mixture
Calcium-strontium-hydroxyphosphate (strontium-apatite-) cement preparations are described, comprising a powder mixture, which contains molar quantities of the components calcium (Ca), strontium (Sr) and phosphate (P) in the mixture in the ranges 1.00<Ca / P≦1.50 and 0<Sr / P<1.5, together with an alkali salt or an ammonium salt of phosphoric acid, and with water and / or an aqueous solution. The powder mixture particularly contains, as the Ca-component, Ca3(PO4)2 (TCP), and as the Sr-component SrHPO4 and / or Sr3(PO4)2 and optionally additional SrCO3. As the aqueous mixing solution for the formation of the strontium-apatite cement, an aqueous solution of an alkali salt or an ammonium salt of the phosphoric acid is suitable.
Owner:KYPHON
Gypsum-containing board and tile, and method for producing same
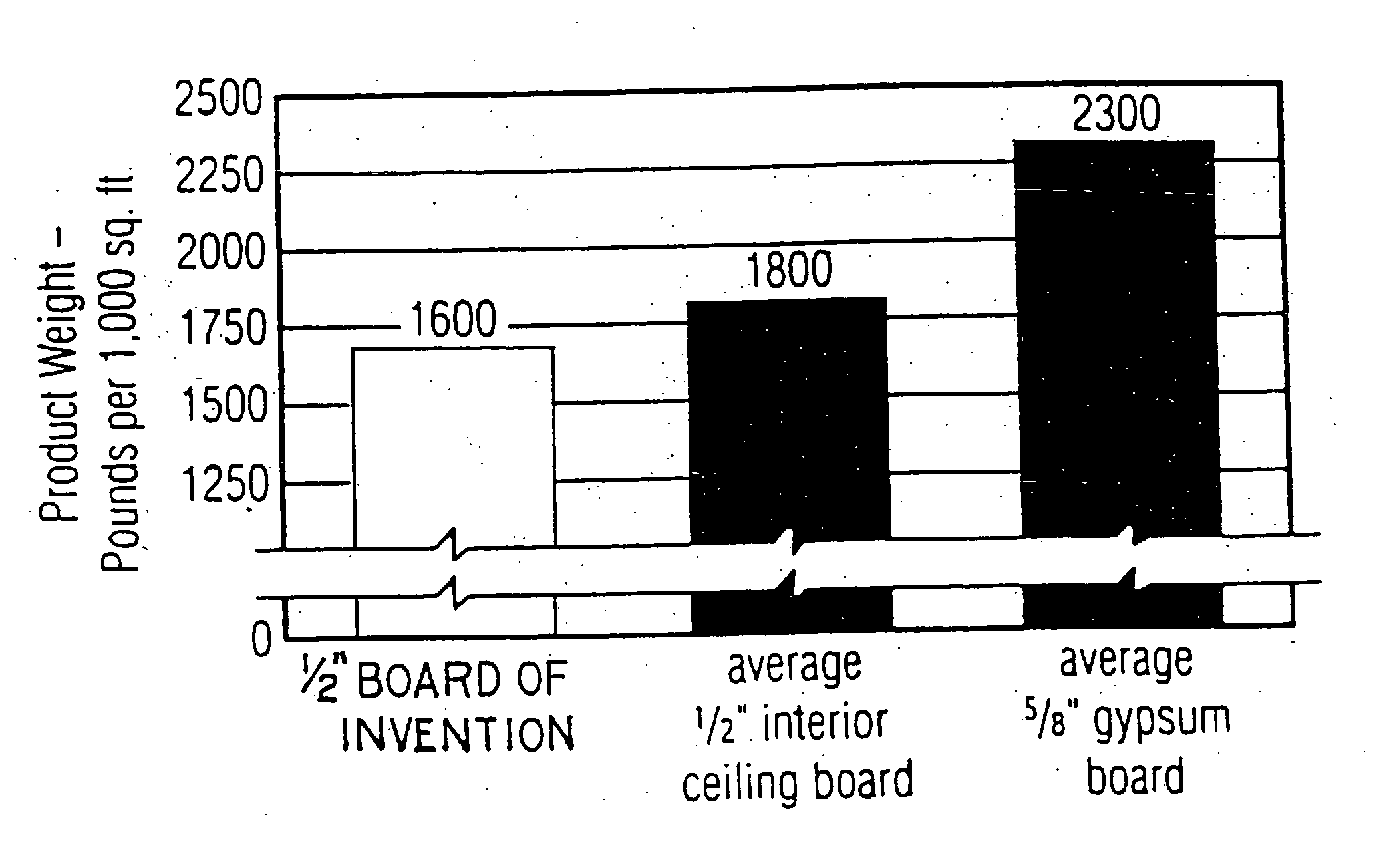
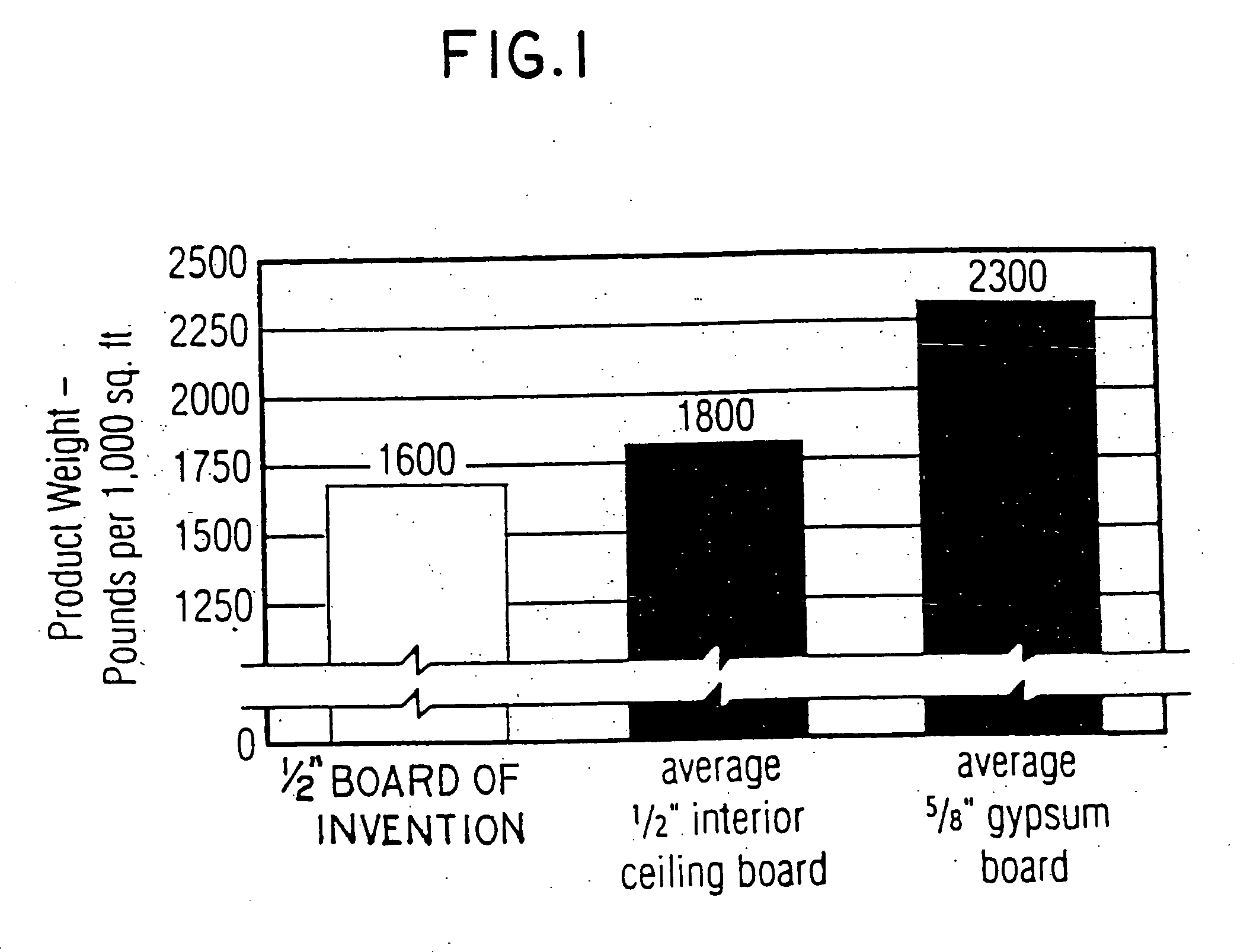
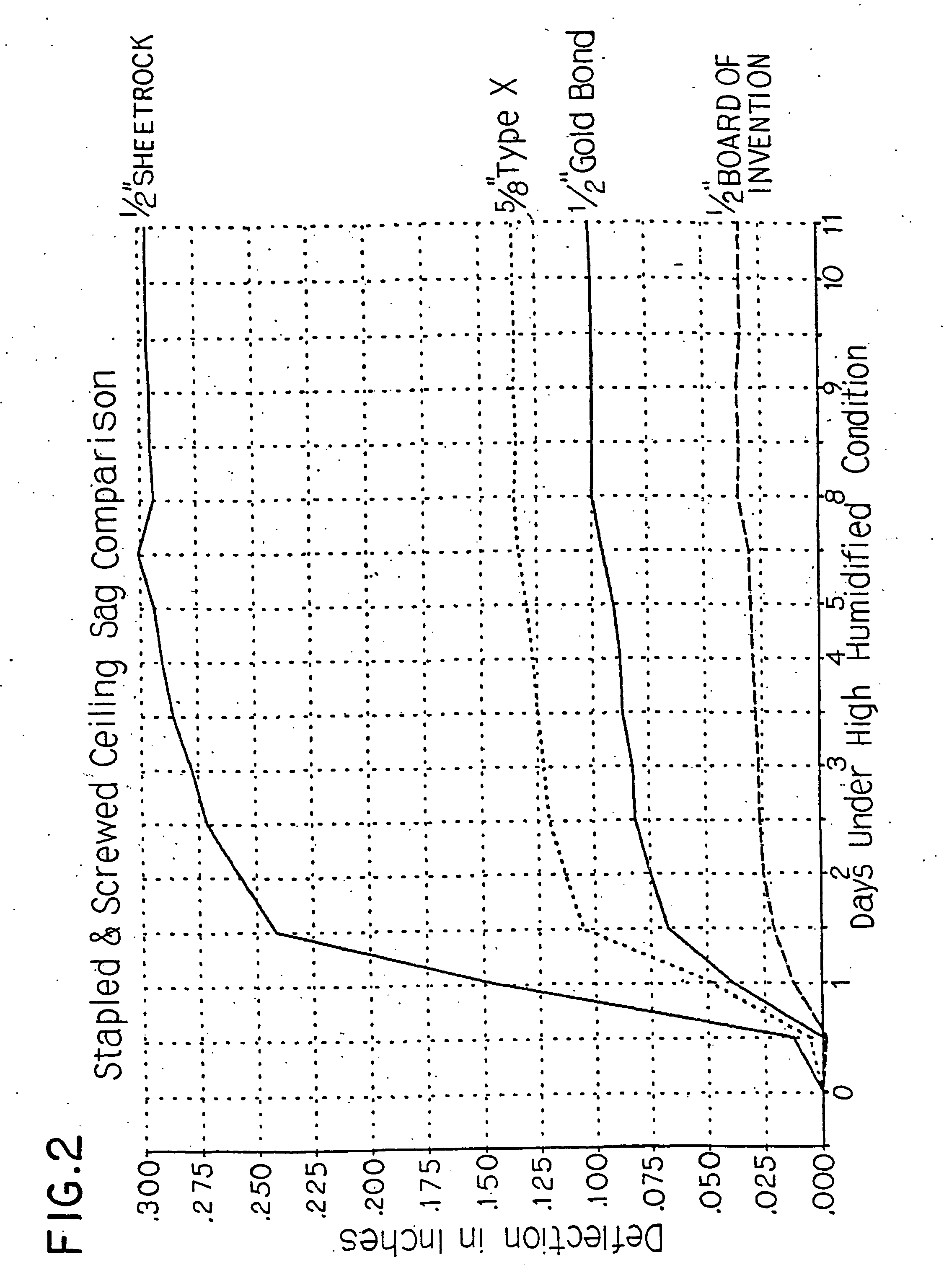
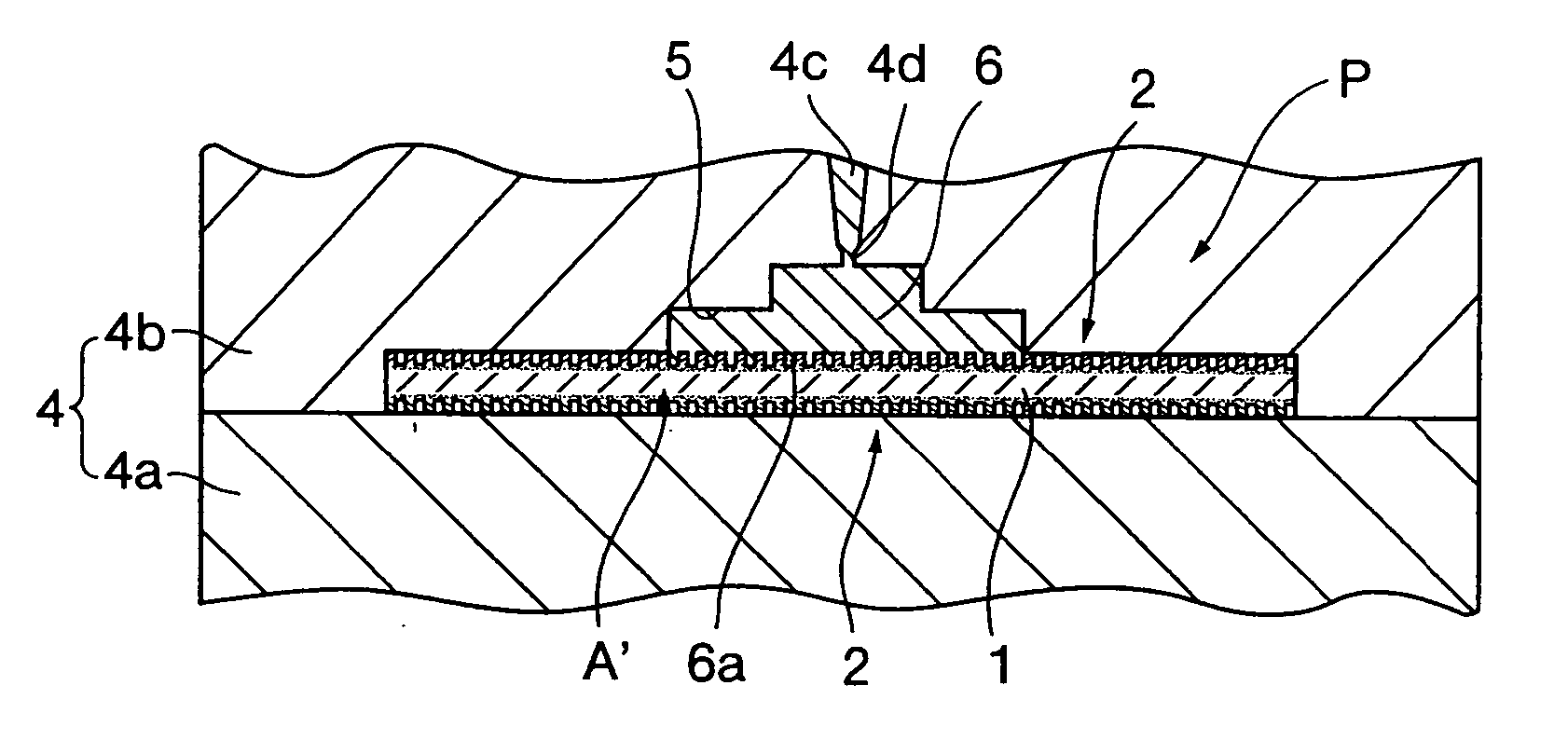
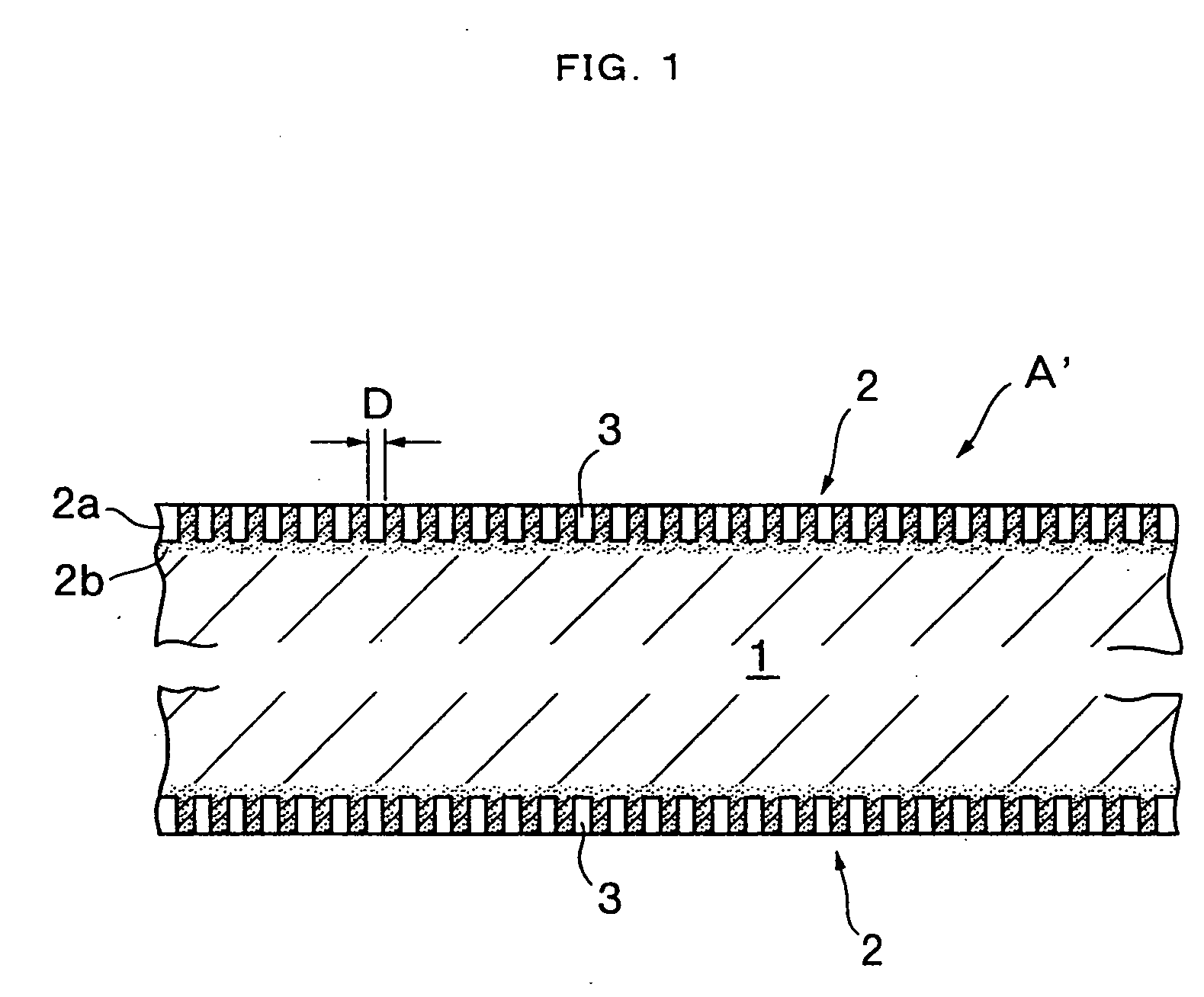
InactiveUS20050019618A1Improve the immunitySave a lot of costLiquid surface applicatorsConstruction materialO-Phosphoric AcidBrick
A method for producing a set gypsum-containing product comprising an interlocking matrix of set gypsum wherein said method comprises applying to said set gypsum one or more enhancing materials selected from the group consisting of phosphoric acid; condensed phosphoric acids, each of which comprises 2 or more phosphoric acid units; and salts or ions of condensed phosphates, each of which comprises 2 or more phosphate units, and monobasic salts or monovalent ions of orthophosphates.
Owner:UNITED STATES GYPSUM CO
Composite of aluminium material and synthetic resin molding and process for producing the same
ActiveUS20060055084A1Stable and fast compositeHigh tensile strengthAnodisationNatural mineral layered productsO-Phosphoric AcidSynthetic resin
The present invention is to provide a process for producing a composite of an aluminum material and a synthetic resin molding that can be produced at a high efficiency and to provide a stable and fast composite that is large in a peel strength and a mechanical strength. The process for producing a composite according to the present invention is characterized in that an aluminum raw material is oxidized in an electrolytic bath of phosphoric acid or sodium hydride, thereby an anodic oxidation coating provided with innumerable pores 3 having a diameter of 25 nm or more made open in the surface thereof is formed thereon, and a synthetic resin mold 6 is coupled with the anodic oxidation coating 2 in such a condition that the part 6a thereof is intruded in the innumerable pores.
Owner:CHERRY CHIEF MAF CORP
Flame retardant thermoplastic composition and articles comprising the same
A flame retardant thermoplastic composition comprises: a poly(arylene ether); an impact modifier; a polyolefin; a phosphoric acid salt selected from the group consisting of melamine phosphate, melamine pyrophosphate, melamine orthophosphate, diammonium phosphate, monoammonium phosphate, phosphoric acid amide, melamine polyphosphate, ammonium polyphosphate, polyphosphoric acid amide, and combinations of two or more of the foregoing; a metal hydroxide; and an organic phosphate wherein the amount of phosphoric acid salt by weight is greater than or equal to the amount of organic phosphate by weight. The flame retardant composition is may be used in the production of electrical wires.
Owner:SHPP GLOBAL TECH BV
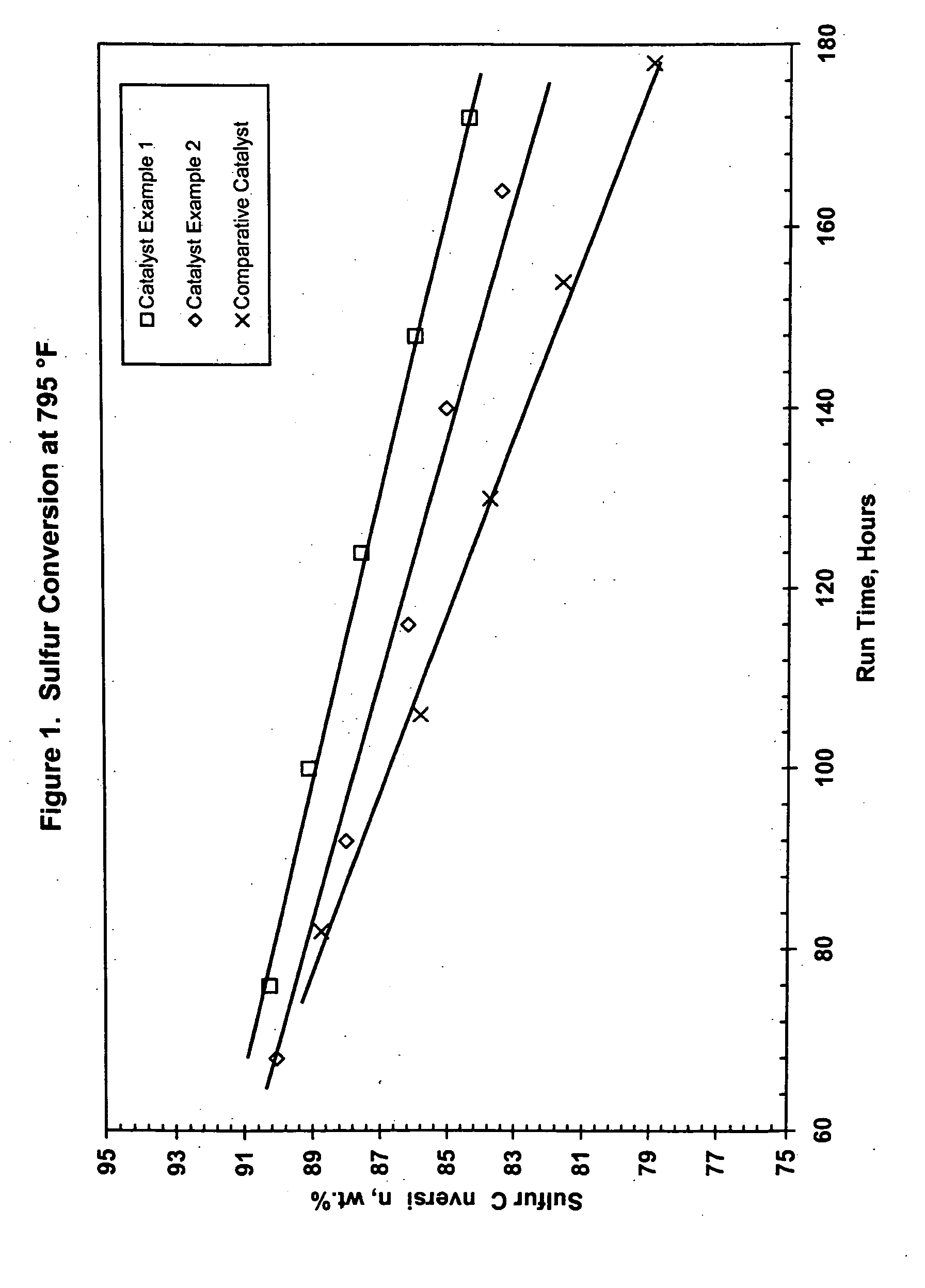
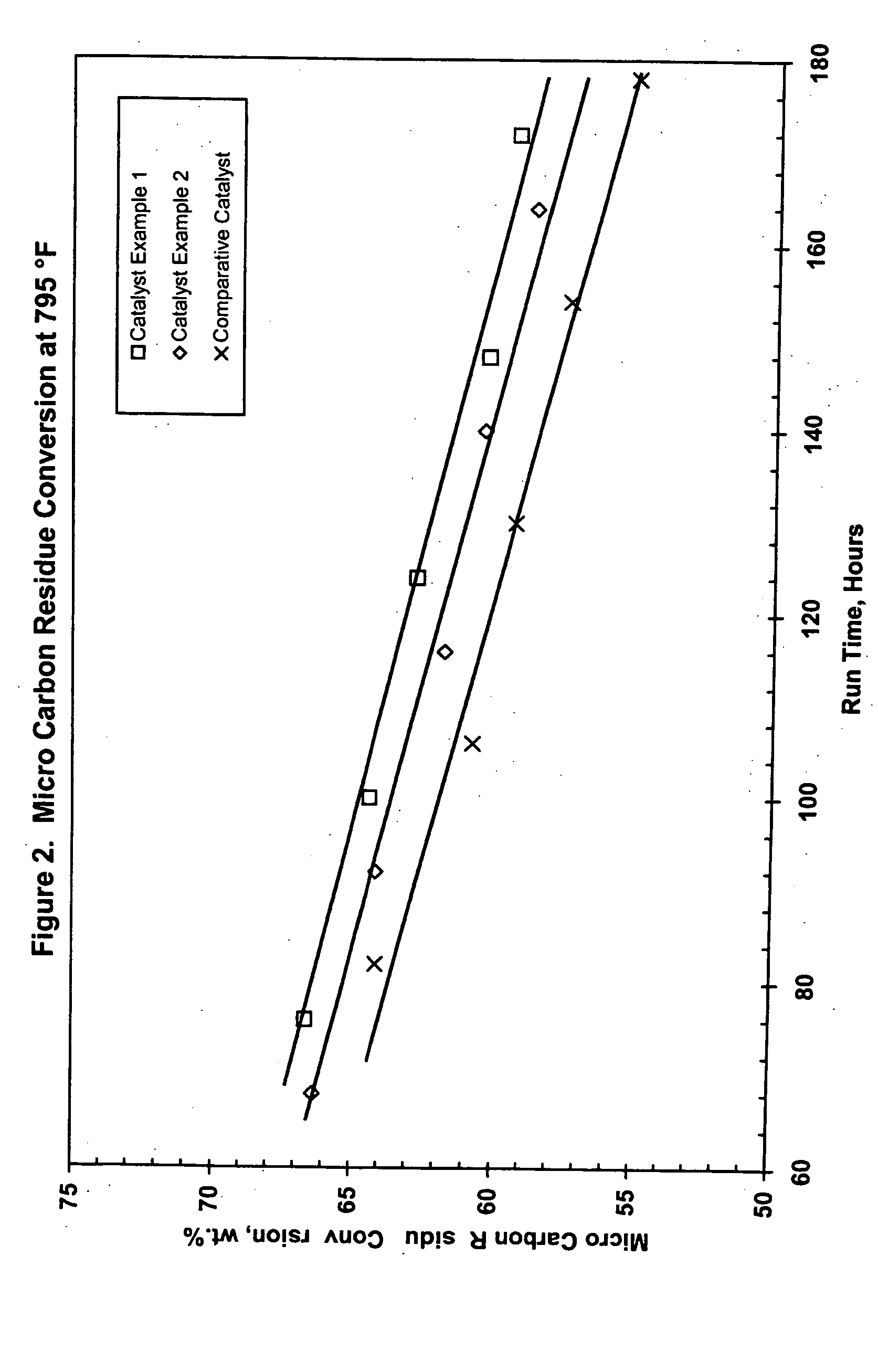
Hydroconversion catalysts and methods of making and using same
InactiveUS20050109674A1Improve the level ofImprove performanceCatalyst activation/preparationHydrocarbon oil crackingHigh concentrationO-Phosphoric Acid
Stable catalyst carrier impregnating solutions can be prepared using a component of a Group VIB metal, e.g., molybdenum, at high concentration, a component of a Group VIII metal, e.g., nickel, at low concentration, and a phosphorous component, e.g., phosphoric acid, at low concentration, provided that the Group VIII metal is in a substantially water-insoluble form and a particular sequence of addition of the components is followed, even when a substantially water-insoluble form of the Group VIB component is used. The resulting stabilized impregnating solution can be supplemented with additional Group VIII metal in water-soluble form to achieve increased levels of such metal in the final catalyst. Furthermore, uncalcined catalyst carriers impregnated with stable solution and subsequently shaped, dried and calcined, have unexpectedly improved performance when used in the hydroprocessing of heavy hydrocarbon feedstocks. High conversion can be achieved at reduced levels of sediment, especially in comparison to standard commercial catalysts.
Owner:ADVANCED REFINING TECH
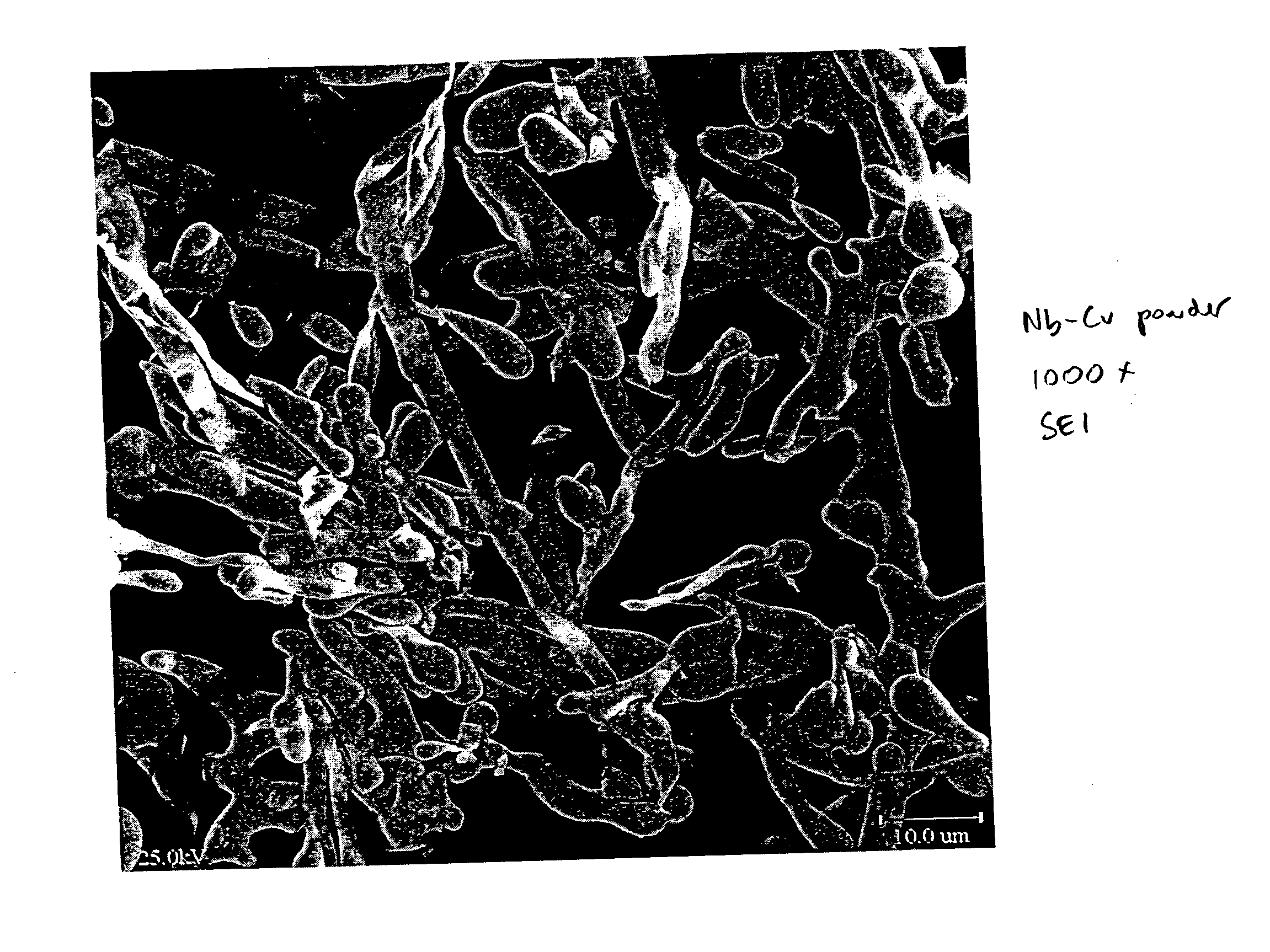
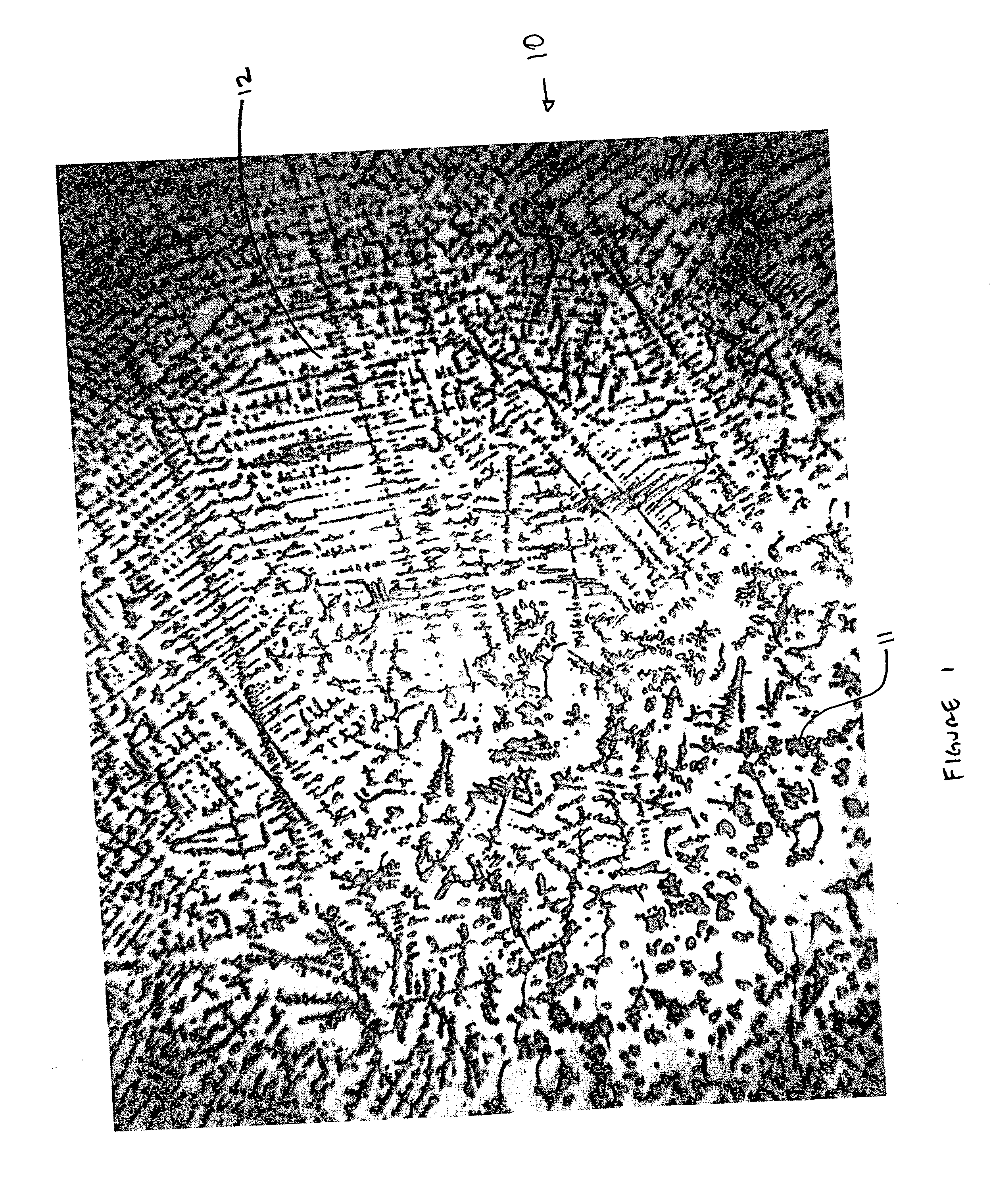
Method for producing metal fibers
InactiveUS20050000321A1Transportation and packagingMetal-working apparatusO-Phosphoric AcidMetal fibers
A method of producing metal fibers including melting a mixture of at least a fiber metal and a matrix metal, cooling the mixture to form a bulk matrix comprising at least a fiber phase and a matrix phase and removing at least a substantial portion of the matrix phase from the fiber phase. Additionally, the method may include deforming the bulk matrix. In certain embodiments, the fiber metal may be at least one of niobium, a niobium alloy, tantalum and a tantalum alloy and the matrix metal may be at least one of copper and a copper alloy. The substantial portion of the matrix phase may be removed, in certain embodiments, by dissolving of the matrix phase in a suitable mineral acid, such as, but not limited to, nitric acid, sulfuric acid, hydrochloric acid and phosphoric acid.
Owner:ATI PROPERTIES
Method for producing metal fibers
Owner:ATI PROPERTIES
Aluminum phosphate or polyphosphate particles for use as pigments in paints and method of making same
InactiveUS20060045831A1PhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesAluminium sulfateOrganic acid
An aluminum phosphate or polyphosphate-based pigment product is made by a process comprising contacting phosphoric acid with aluminum sulfate and an alkaline solution to produce an aluminum phosphate based product; and optionally calcining the aluminum phosphate based product at an elevated temperature, wherein the process is substantially free of an organic acid. The aluminum phosphate or polyphosphate-based pigment is amorphous. The amorphous aluminum phosphate or polyphosphate characterized by a bulk density of less than 2.30 grams per cubic centimeter and a phosphorus to aluminum mole ratio of greater than 0.8. The composition is useful in paints and as a substitute for titanium dioxide.
Owner:BUNGE AMORPHIC SOLUTIONS +1
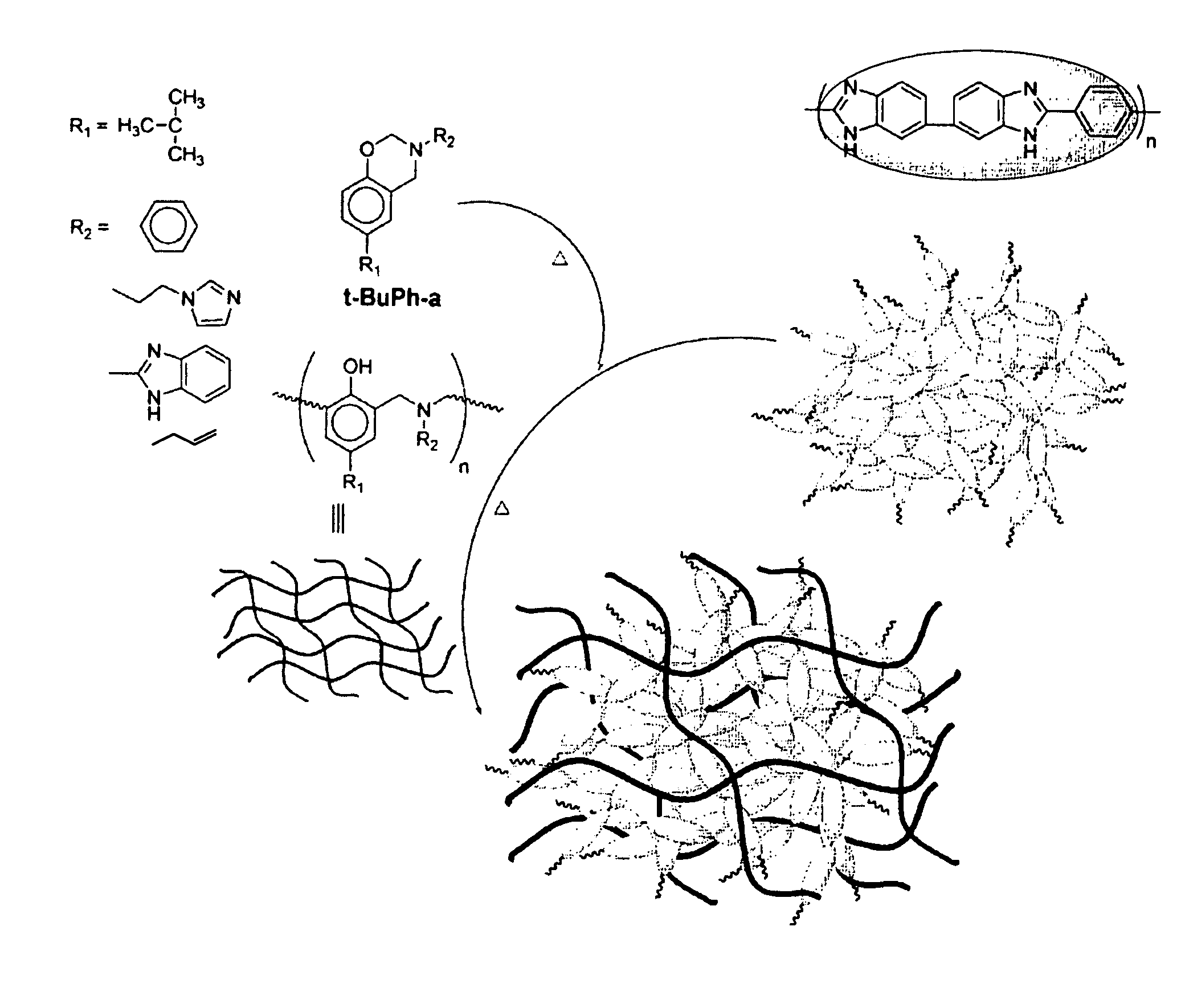
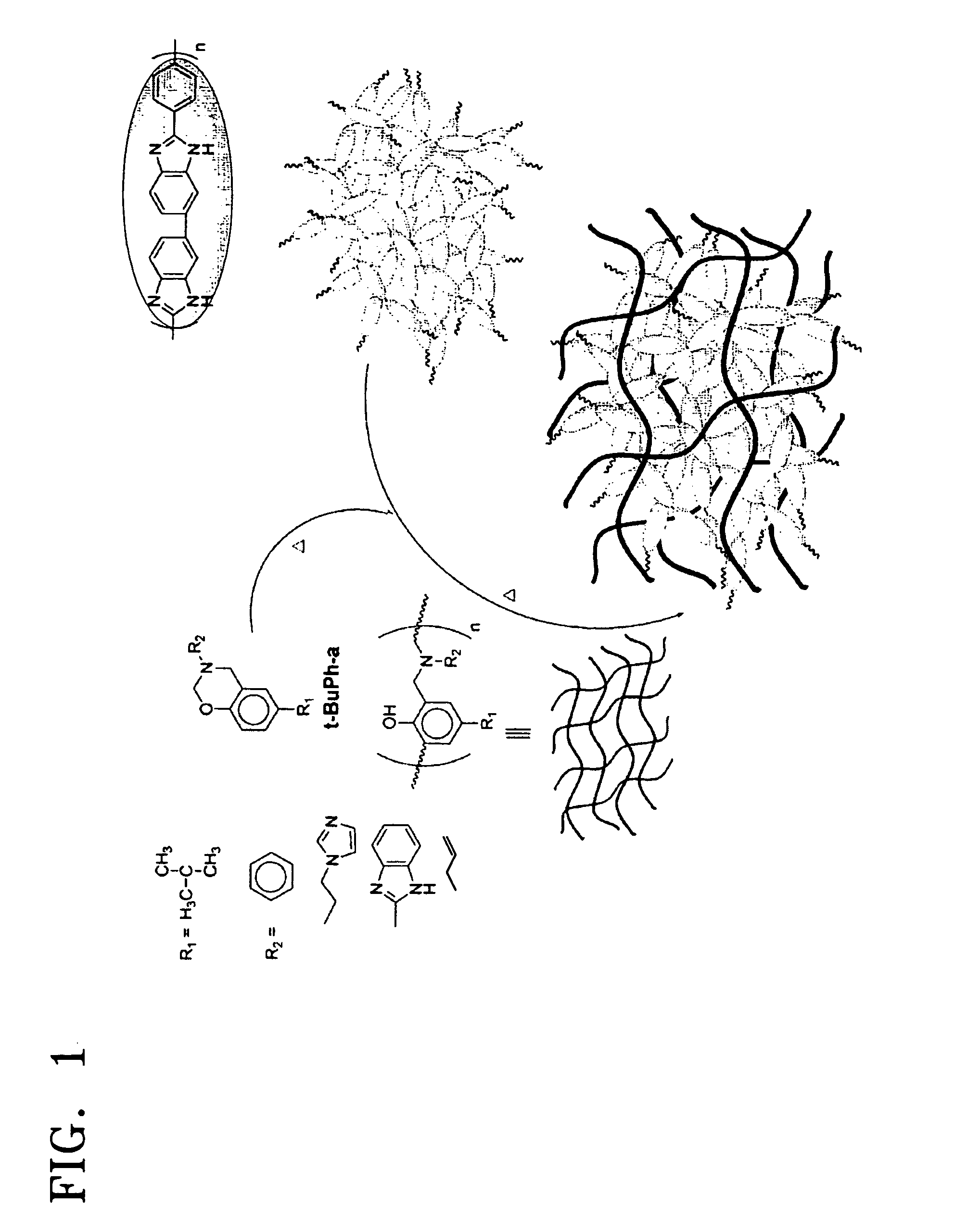
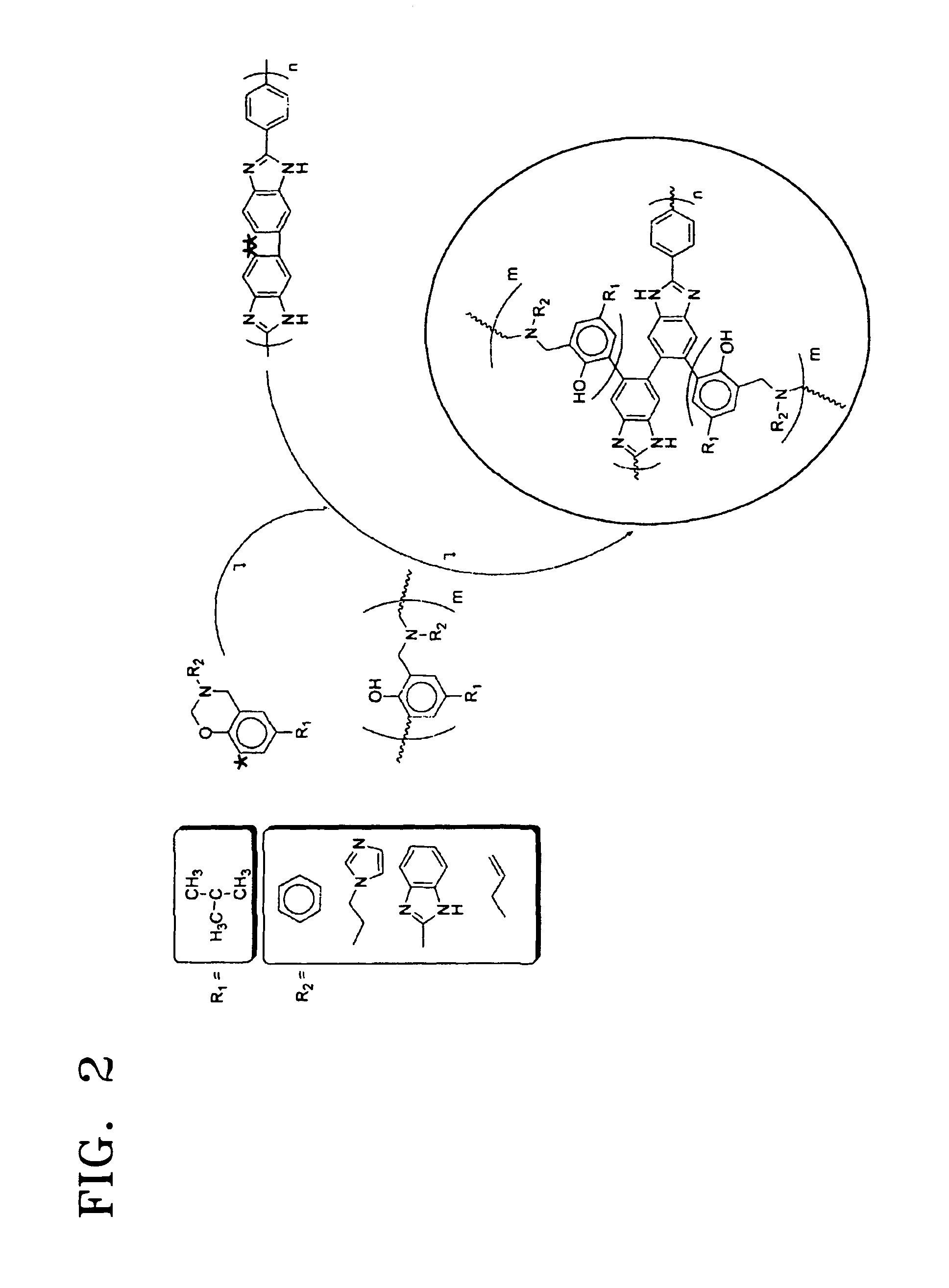
Electrolyte membrane using polybenzoxazine based compound and method of manufacturing the same
An electrolyte membrane includes a cross-linked reaction product of a benzoxazine monomer and a cross-linkable compound. The electrolyte membrane is impregnated with 300 to 600 parts by weight of phosphoric acid based on 100 parts by weight of the electrolyte membrane, and has a yield strain 0.5% or less, and a yield stress 0.3 Mpa or less. The cross-linked material has a strong acid trapping ability with respect to the benzoxazine compound and excellent mechanical properties due to a cross-linkage. Also, the solubility of the cross-linked material in polyphosphoric acid is low, thereby showing excellent chemical stability. Accordingly, when the cross-linked material is used, an electrolyte membrane having an excellent liquid supplementing ability and excellent mechanical and chemical stability at a high temperature can be obtained. The cross-linked material can be obtained by a simple polymerization process by combining a benzoxazine monomer and a crosslinkable compound and by using heat instead of using a polymerization initiator or a cross-linking agent.
Owner:SAMSUNG ELECTRONICS CO LTD +1
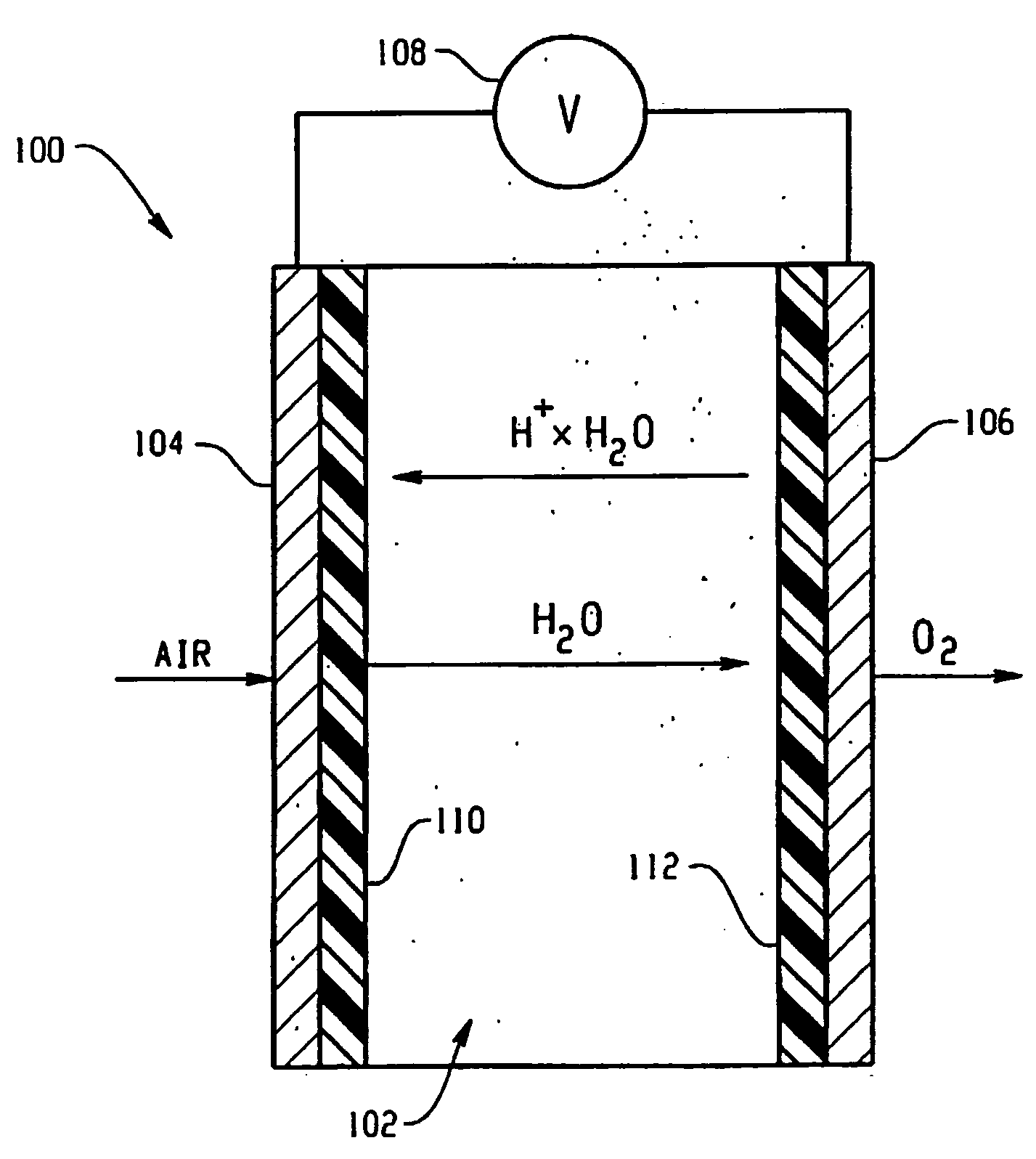
Oxygen producing device for woundcare
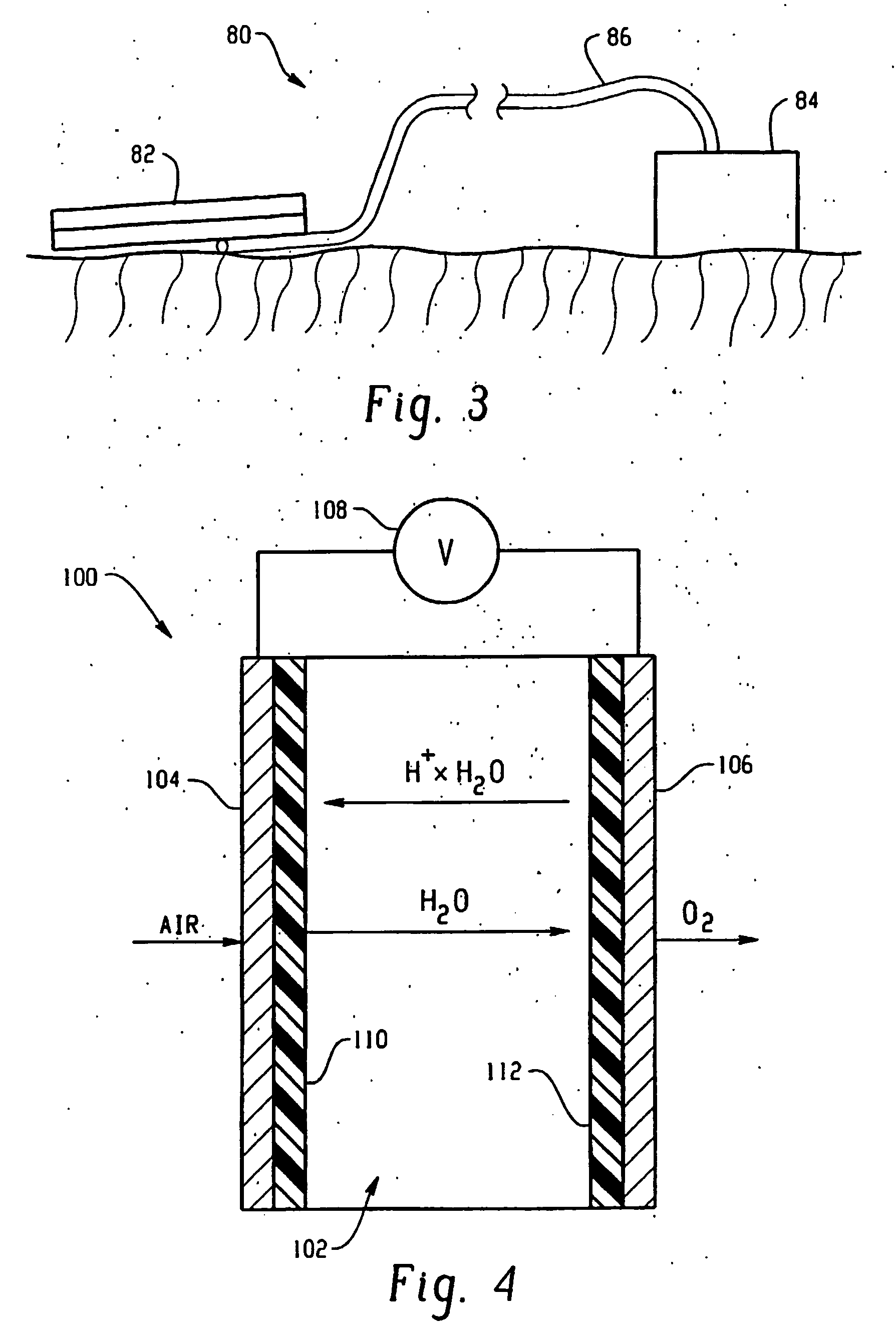
InactiveUS20060287632A1High ionic resistanceElectrotherapyElectrolysis componentsWound healingTissue repair
A device for the application of oxygen to promote wound healing and tissue repair. The device includes a portable oxygen generating device (18), which includes a cathode (10), an anode (16), and a phosphoric acid treated ion conducting membrane (14).
Owner:NEOGENIX LLC
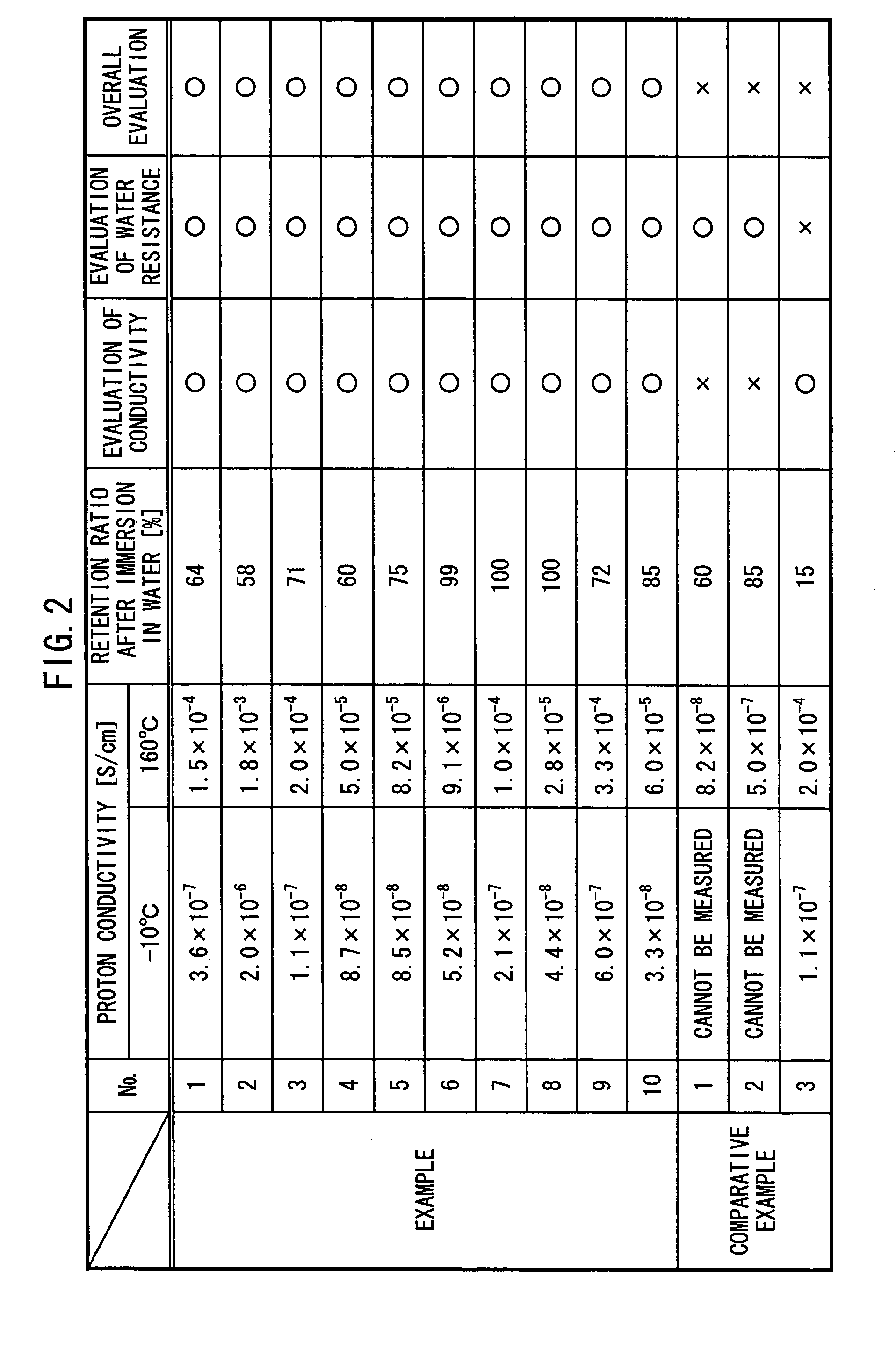
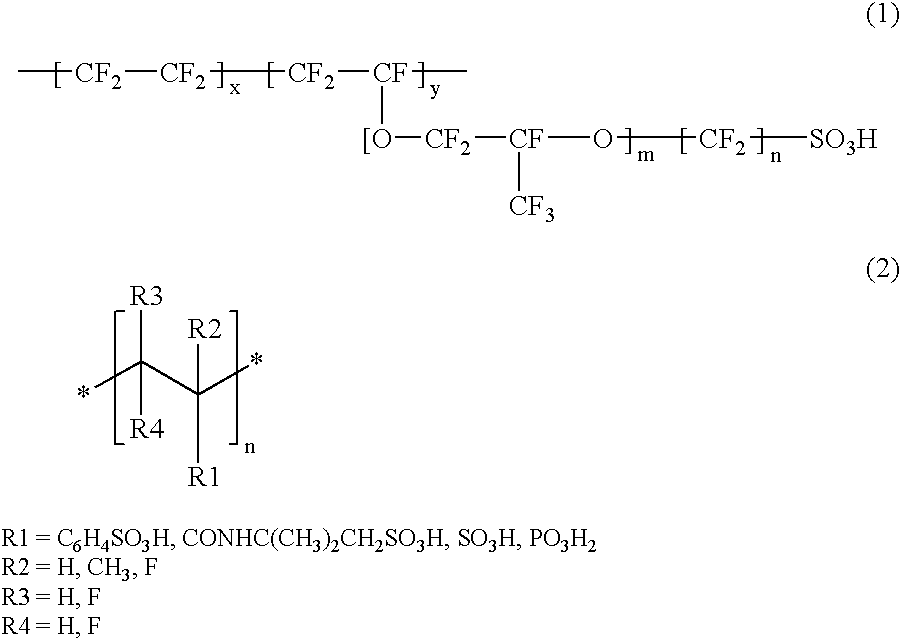
Proton conductor and method for producing the same
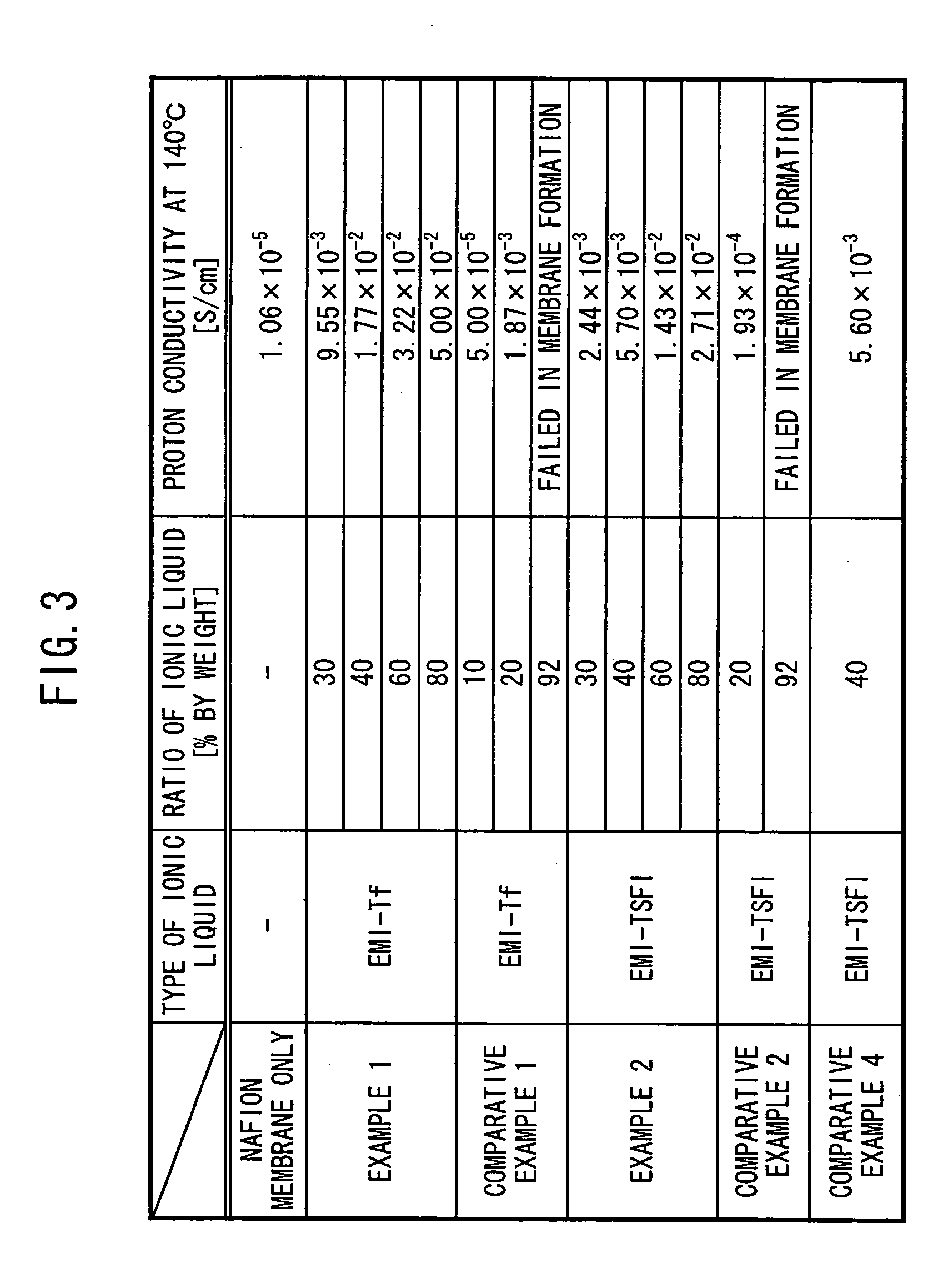
InactiveUS20050106440A1Improve featuresExcellent in its ability to retain a large amount of ionic liquidElectrolyte holding meansSolid electrolytesPolymer scienceO-Phosphoric Acid
An acidic group-containing solid polymer, having an acidic group such as a sulfonic acid group, a phosphoric acid group, and / or a phosphonic acid group, is dissolved in an organic solvent other than methanol. An ionic liquid is added to the solution to prepare a casting liquid. The casting liquid is subjected to casting in a cavity formed by an opening of a frame and a sheet member, each of which is composed of PTFE (fluorine-containing polymer material). Thereafter, the solvent is removed to yeild a proton conductor membrane.
Owner:HONDA MOTOR CO LTD
Strontium-apatite-cement-preparations, cements formed therefrom, and uses thereof
ActiveUS7273523B2Facilitated releaseTo promote metabolismBiocideSurgical adhesivesPowder mixturePhosphate
Calcium-strontium-hydroxyphosphate (strontium-apatite-) cement preparations are described, comprising a powder mixture, which contains molar quantities of the components calcium (Ca), strontium (Sr) and phosphate (P) in the mixture in the ranges 1.00<Ca / P≦1.50 and 0<Sr / P<1.5, together with an alkali salt or an ammonium salt of phosphoric acid, and with water and / or an aqueous solution. The powder mixture particularly contains, as the Ca-component, Ca3(PO4)2 (TCP), and as the Sr-component SrHPO4 and / or Sr3(PO4)2 and optionally additional SrCO3. As the aqueous mixing solution for the formation of the strontium-apatite cement, an aqueous solution of an alkali salt or an ammonium salt of the phosphoric acid is suitable.
Owner:KYPHON
Chemical cleaning for membranes
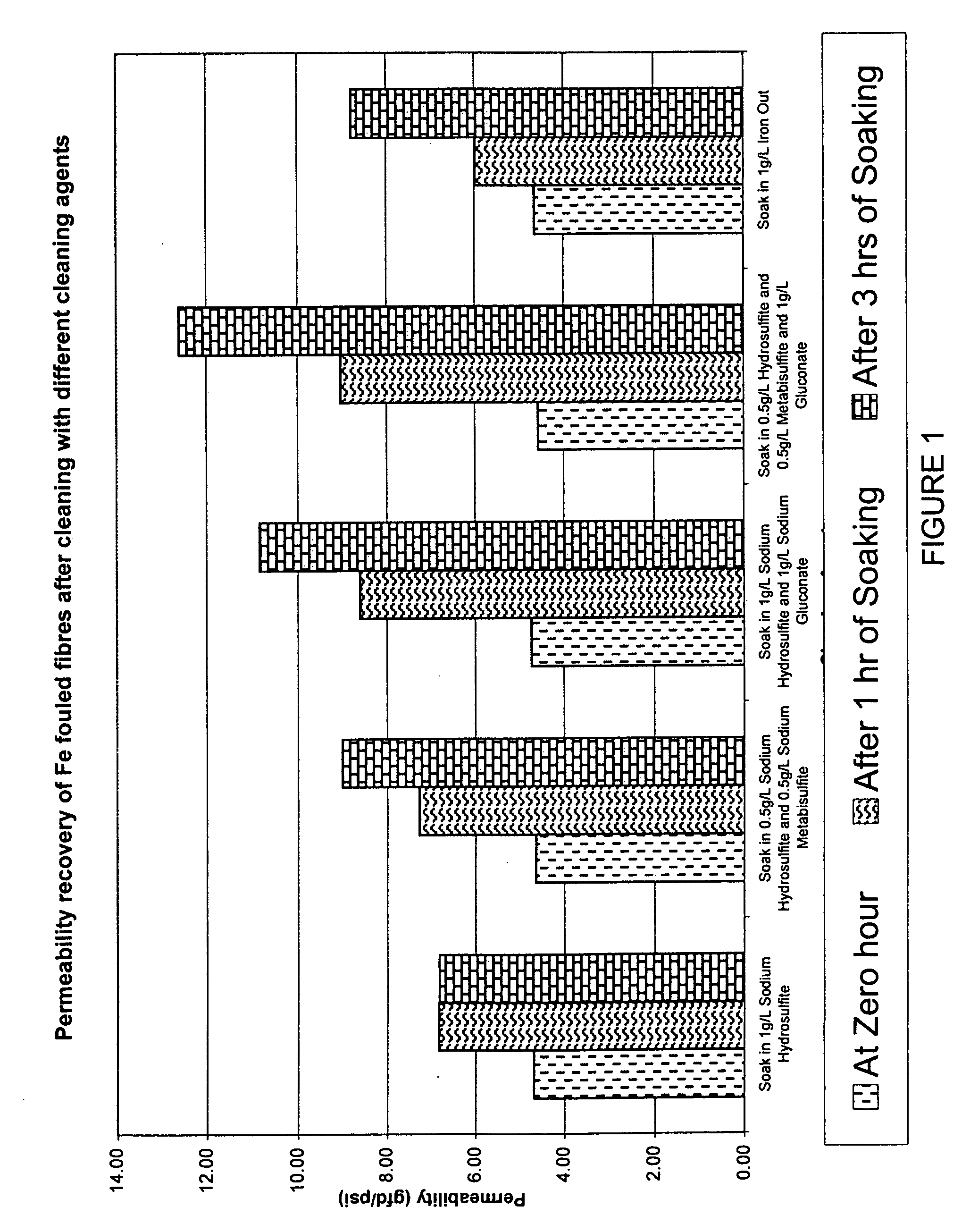
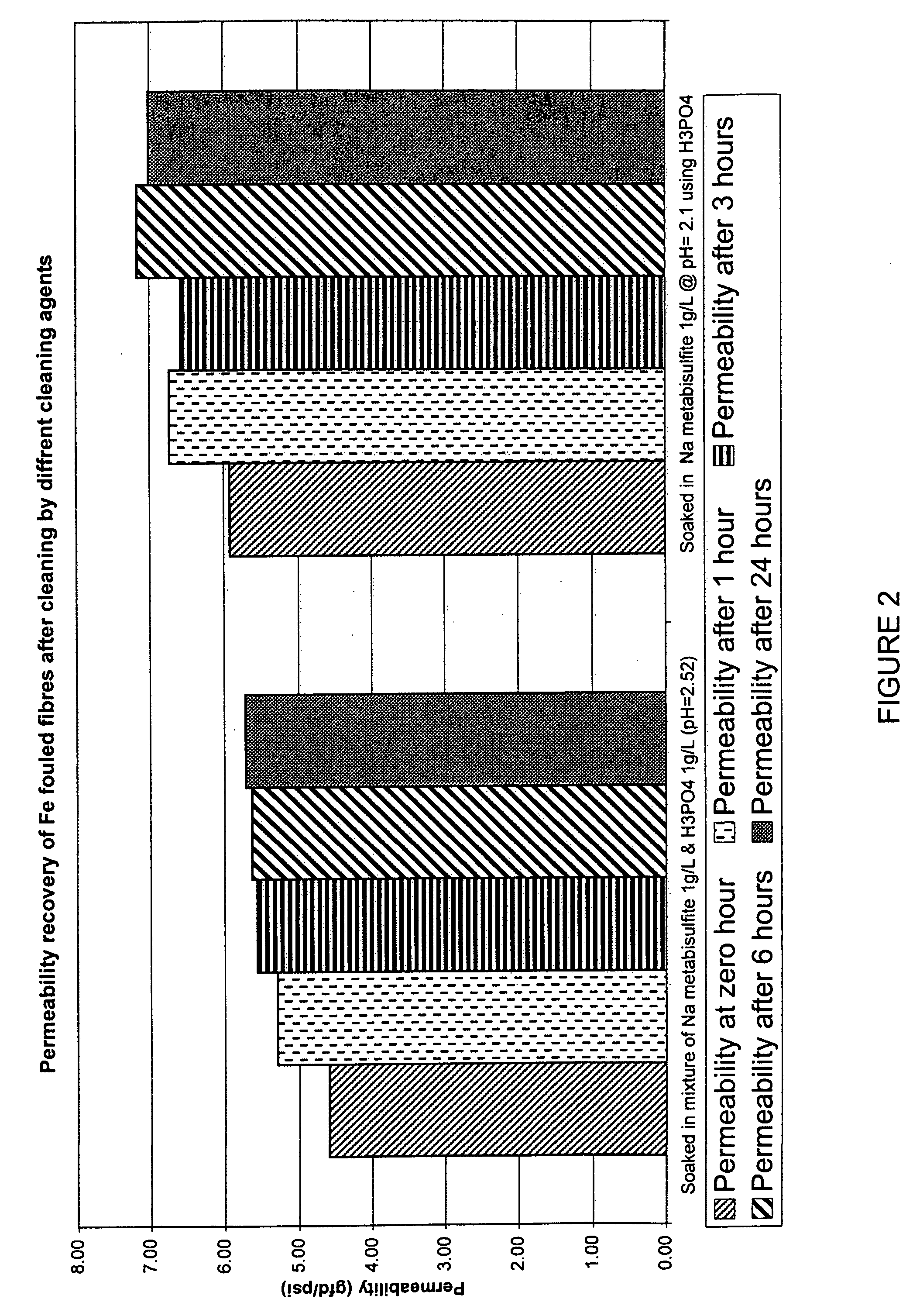
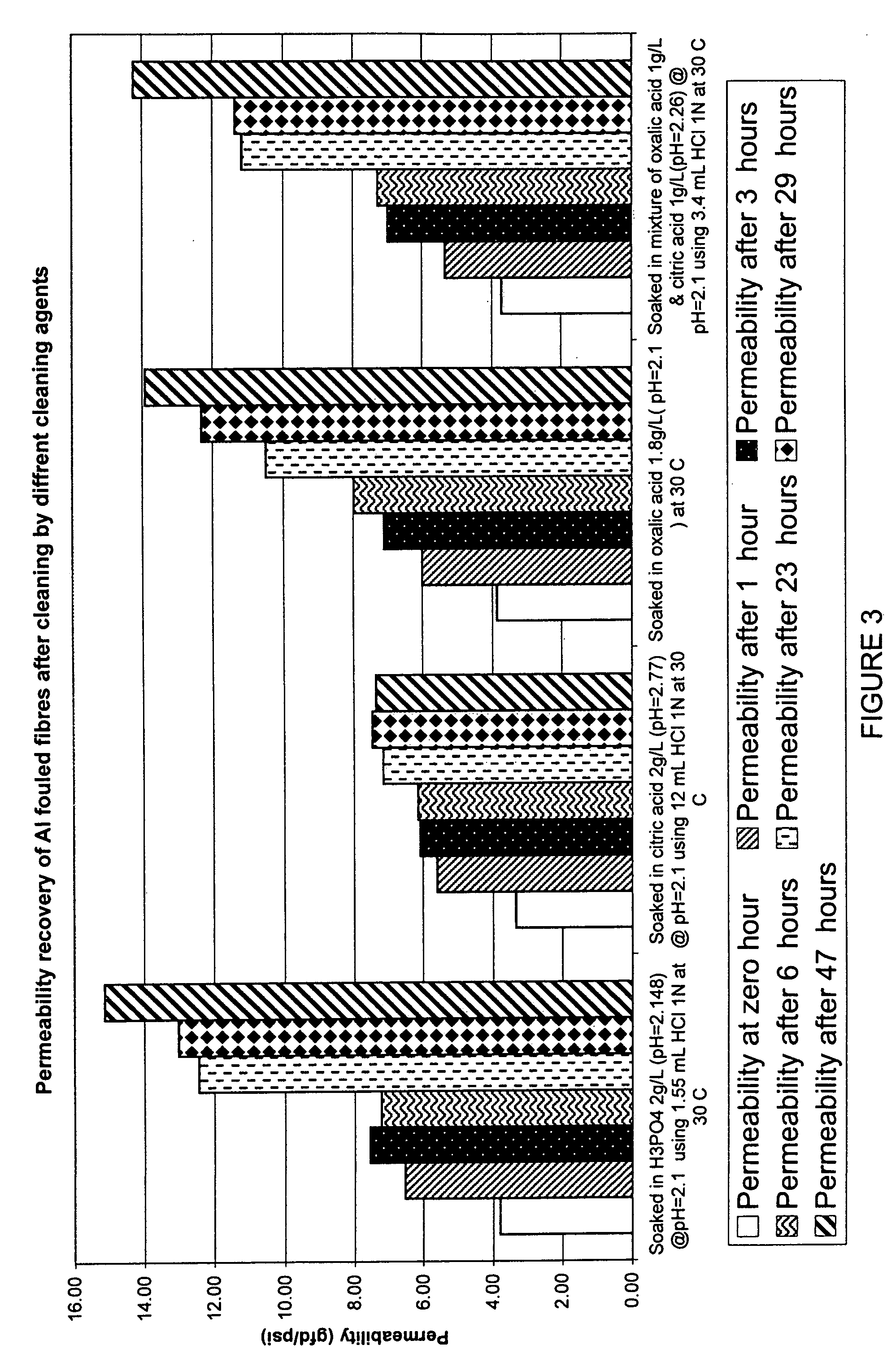
Membranes, for example immersed polymeric ultrafiltration or microfiltration membranes, are cleaned by contacting them with a chemical cleaner comprising one or more of a gluconate, a hydrosulfite, a metabisulfite or an acid, for example phosphoric acid. The membranes may have been exposed to organic or inorganic foulants, some of which may have resulted from pretreatment involving adding coagulants, for example coagulants containing iron or aluminum, to water to be treated. Methods for treating a waste cleaning solution were described.
Owner:SYED MURTUZA ALI +3
Flame retardant thermoplastic composition and articles comprising the same
ActiveUS20070261878A1Decreasing and eliminating plate-outDecreasing and eliminating and migrationPlastic/resin/waxes insulatorsDyeing processElectrical conductorPolyolefin
A flame retardant thermoplastic composition comprising a poly(arylene ether), a block copolymer, a liquid polyolefin, and a flame retardant additive composition. The flame retardant additive composition comprises a metal hydroxide, an organic phosphate, and a phosphoric acid salt selected from the group consisting of melamine phosphate, melamine pyrophosphate, melamine orthophosphate, melem polyphosphate, melam polyphosphate, diammonium phosphate, monoammonium phosphate, phosphoric acid amide, melamine polyphosphate, ammonium polyphosphate, polyphosphoric acid amide, and combinations of two or more of the foregoing. The block copolymer comprises a block that is a controlled distribution copolymer having terminal regions that are rich in alkylene units and a center region that is rich in aryl alkylene units. The flame retardant composition may be used in the production of covered conductors.
Owner:SHPP GLOBAL TECH BV
Medicament for treating composite water in hot water boiler as well as preparation method and use method thereof
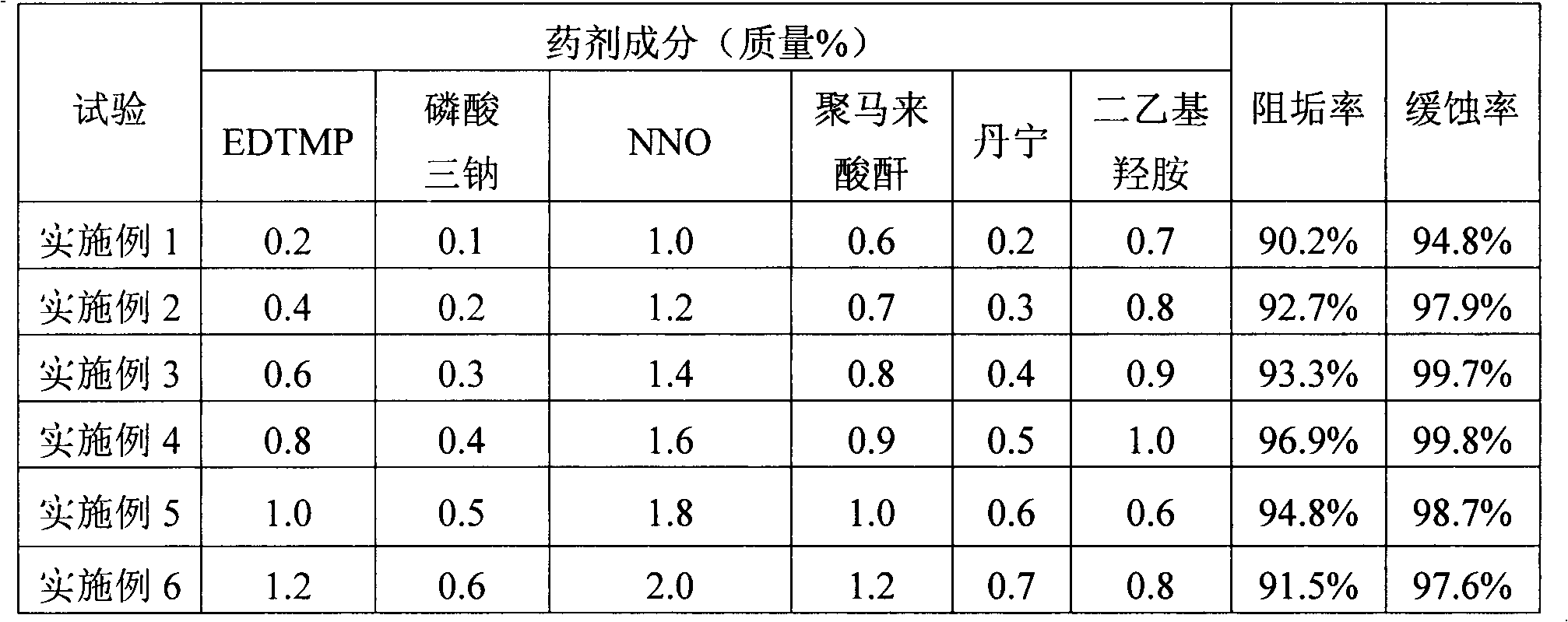
ActiveCN103183417ADoes not reduce corrosion inhibitionWill not reduce the effect of anti-scalingScale removal and water softeningInorganic phosphatePhosphoric acid
The invention discloses a medicament for treating composite water in a hot water boiler. The medicament comprises the following components by mass percent: 0.1-3% of inorganic phosphate, 0.1-2% of organic phosphoric acid and / or organic phosphate, 0.1-4% of component A, 0.1-3% of component B, 0.6-1.0% of organic amine deoxidizer, 0.1-2% of tannin and / or chitosan and the balance of water, wherein the mass percent of the components is 100%, the component A is one or more of an organic antisludging agent, an organic dispersant and an organic dispersible antisludging agent, and the component B is one or more of an organic carboxylic acid corrosion inhibitor, an organic carboxylate corrosion inhibitor and sodium methylene dinaphthalenesulfonate. The invention further discloses a preparation method and a use method of the medicament, the preparation process is clean, and the medicament is low in toxicity for body health and environment in the using process. The medicament is excellent in corrosion resistance and scale inhibition effects and simple and convenient to store and transport, and the corrosion and scale inhibition effects cannot be reduced after the medicament is stored and transported for a long term.
Owner:SHANGHAI EMPEROR OF CLEANING HI TECH
Carbon fluoride particles, preparation process and users of the same
InactiveUS6068921AGood effectReduce developmentNitrogen compoundsCell electrodesO-Phosphoric AcidFluoride
Carbon fluoride particles in which a number-average particle size is 0.01 to 50 mu m, a content of particles having such a diameter that the particles size distribution falls with in range of the number-average particle size + / -20% amounts to at least 50% of the whole, a true specific gravity is 1.7 to 2.5, a F / C as a whole is 0.001 to 0.5, and a F / C at the surface is always larger than the F / C as a whole and is 0.1 to 2. 0. These carbon fluoride partilces are obtainable by reacting carbon particles with fluorine at 350 DEG to 600 DEG C. for one minute to six hours. These carbon fluoride particles have an excellent dispersibility and a powder flowability, and are usable solely or in the form of a composite, as water- and oil-repellents, non-tackifying agents, solid lubricants, agents for imparting electric conductivity, additives to toner for developing electrostatic image, additives to coating of carrier for developing electrostatic image, composit materials for fixing roller, phosphoric acid fuel cells, zinc / air batteries and nickel / hydride storage batteries.
Owner:DAIKIN IND LTD
Microlitographic projection exposure apparatus and immersion liquid therefore
InactiveUS20060244938A1Transparent highLittle chemical effectMicroscopesPhotomechanical exposure apparatusSimple Organic CompoundsO-Phosphoric Acid
An immersion liquid for a microlithographic projection exposure apparatus is enriched with heavy isotopes. This reduces the chemical reactivity, which leads to an extension of the lifetime of optical elements which come in contact with the immersion liquid. For example, heavy water (D2O), deuterated sulfuric acid, (D2SO4) or deuterated phosphoric acid D3P16O4 may be used. Organic compounds such as perfluoro polyethers, which have been deuterated or enriched with heavy oxygen (18O), are furthermore suitable.
Owner:CARL ZEISS SMT GMBH
Resin composition, process for preparing the same, and laminate containing layer of said resin composition
InactiveUS6174949B1Dispersed uniformly and efficientlyBottlesSynthetic resin layered productsAcetic acidPolymer science
A resin composition is provided which comprises (A) a saponified ethylene-vinyl acetate copolymer (referred to as EVOH for short) with an ethylene content of 20 to 60 mole percent and a saponification degree of not less than 90 mole percent, and contains therein (B) a boron compound as an essential component, (C) acetic acid as an optional component, and at least one compound selected from among (D) an acetic acid salt and (E) a phosphoric acid compound as an essential component, the contents of the respective additive components per 100 parts by weight of EVOH (A) being as follows:boron compound (B): 0.001 to 1 part by weight on the boron basis;acetic acid (C): 0 to 0.05 part by weight;acetic acid salt (D): 0.001 to 0.05 part by weight on the metal basis; andphosphoric acid compound (E): 0.0005 to 0.05 part by weight on the phosphate radical basis.This resin composition is typically produced by bringing EVOH (A) with a water content of 20 to 80% by weight into contact with an aqueous solution containing the respective additive components mentioned above and then subjecting the thus-treated EVOH to fluidized state drying and then to stationary state drying.
Owner:THE NIPPON SYNTHETIC CHEM IND CO LTD
Surface-treated metallic material, method of surface treating therefor and resin coated metallic material, metal can and can lid
InactiveUS20050175798A1Number of step is increasedCorrosion resistance is not sufficientEnvelopes/bags making machineryDead plant preservationElectrolysisO-Phosphoric Acid
A surface-treated metal material is obtained by forming an inorganic surface-treating layer containing at least Zr, O and F as chief components but without containing phosphoric acid ions on the surfaces of a metal by the cathodic electrolysis or by forming an inorganic surface-treating layer containing at least Zr, O and F as chief components and having an atomic ratio of P and Zr of 0≦P / Zr<0.6 in the uppermost surface on the surfaces of the metal by the cathodic electrolysis at a low cost featuring high productivity, environmental friendliness, scar resistance, adhesion, workability and intimate fitting. By using a metal material obtained by coating the surface-treated metal material with an organic resin and, particularly, with a polyester resin, a metal can or a can lid exhibits excellent adhesion and corrosion resistance even at portions that are worked to a high degree. Further, the can lid exhibits excellent opening performance even after the sterilization by heating.
Owner:TOYO SEIKAN KAISHA LTD
Chemical treatment of material surfaces
InactiveUS20050239295A1Lower contact angleImprove adhesionPrinted circuit assemblingSemiconductor/solid-state device manufacturingChemical treatmentSemiconductor structure
A method for treating the surfaces of materials to improve wettability and adhesion of subsequently deposited polymer layers is disclosed. Suitable materials for practice of the method include polymeric materials and silicon-containing materials is disclosed. The method involves contacting at least a portion of the surface of the material with an aqueous solution of sulfuric acid or phosphoric acid, followed by rinsing with water. After the acid treatment, the contact angle of the surface decreases, and subsequently deposited polymer coatings easily wet the material's surface and exhibit enhanced adhesion. The method may be used to fabricate useful structures, such as semiconductor structures, optical waveguide structures, and coated articles.
Owner:POLYSET +1
Spherical calcium carbonate and method for producing thereof
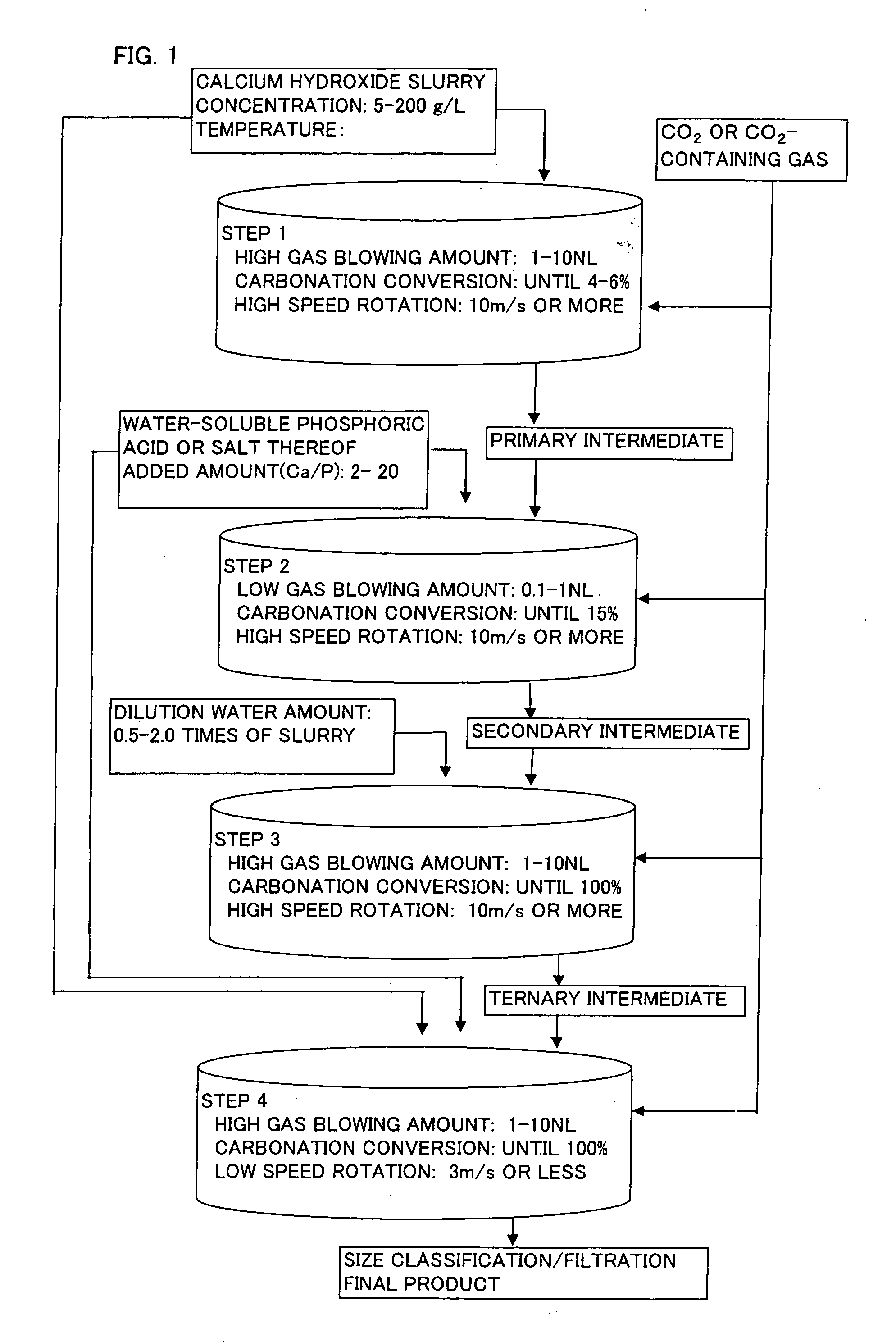
InactiveUS20060165583A1Inferior physical propertyLow blowing rateCalcium/strontium/barium carbonatesCosmetic preparationsCalcium hydroxideO-Phosphoric Acid
When spherical calcium carbonate is produced by blowing a carbon dioxide gas or a carbon dioxide-containing gas into an aqueous suspension containing calcium hydroxide to react them, after start of the reaction, an aqueous solution or suspension of a water-soluble phosphoric acid compound or a water-soluble salt thereof is added to the reaction mixture when carbonation ratio reaches 2 to 10%, and the reaction is further allowed to continue at a low gas blowing rate of 1.0 NL / minute or lower (step (a)). Subsequently, an aqueous suspension containing calcium hydroxide and an aqueous solution or suspension of a water-soluble phosphoric acid compound or a water-soluble salt thereof are added to the reaction mixture, and a carbon dioxide gas or a carbon dioxide-containing gas is introduced to allow to react and thereby produce spherical calcium carbonate having a mean particle diameter of 10 μm or larger. This production method is performed under high velocity revolution from the start of the reaction to the end of the step (a) This method provides calcite type spherical calcium carbonate showing high brightness and small friction coefficient, and having a shape comparatively close to a true sphere and a mean particle diameter of 10 μm or larger.
Owner:OKUTAMA IND
Preparation of lithium iron phosphate nano composite microsphere
ActiveCN101475157AImprove conductivityHigh tap densityElectrode manufacturing processesPhosphorus compoundsMicrospherePhosphoric acid
The invention discloses a method for preparing a lithium iron phosphate nano composite microsphere for a lithium-ion secondary battery anode material by a hydrothermal method, which comprises the following steps: evenly mixing a lithium source, an iron source and a phosphoric acid source according to the stoichiometric ratio of 1-1.05:1:1 to prepare an aqueous solution with certain concentration, adding proper amount of reducing agent, carbonizing agent, ion dopant and the like into the aqueous solution, growing and curing crystals under high-temperature hydrothermal condition, removing a solvent from a hydrothermal product, and burning the hydrothermal product in inert or reducing atmosphere to obtain a final product. The lithium iron phosphate anode material prepared by the method has a regular nano composite microsphere structure, has a particle diameter of between 2 and 4 mu m, even distribution, tap density up to 1.3 to 1.6 g / cm, and has excellent cycle performance and multiplying power performance. The method has simple and easily-controlled process, can use cheap trivalent iron as the iron source, can obtain rich raw materials, and is a practical technique for preparing lithium iron phosphate.
Owner:湖北金泉新材料有限公司
Proton conductor
InactiveUS20050197467A1Easy to evaporateExcellent proton conductionSolid electrolytesConductive materialSimple Organic CompoundsElectrical conductor
An acidic group-containing polymer which has an acidic group such as sulfonic acid group, phosphoric acid group, and phosphonic acid group, and a proton acceptor which has a boiling point at 1 atmosphere higher than 100° C. and which functions as a medium for conducting proton dissociated from the acidic group are retained in pores of a porous member. Preferred examples of the proton acceptor include a salt structure composed of an anion and a cation derived from a basic organic compound, a basic organic compound, and a dissociation-facilitating polymer which facilitates dissociation of proton. Any one of the acidic group-containing polymer and the proton acceptor may be retained first, or both may be retained simultaneously.
Owner:HONDA MOTOR CO LTD
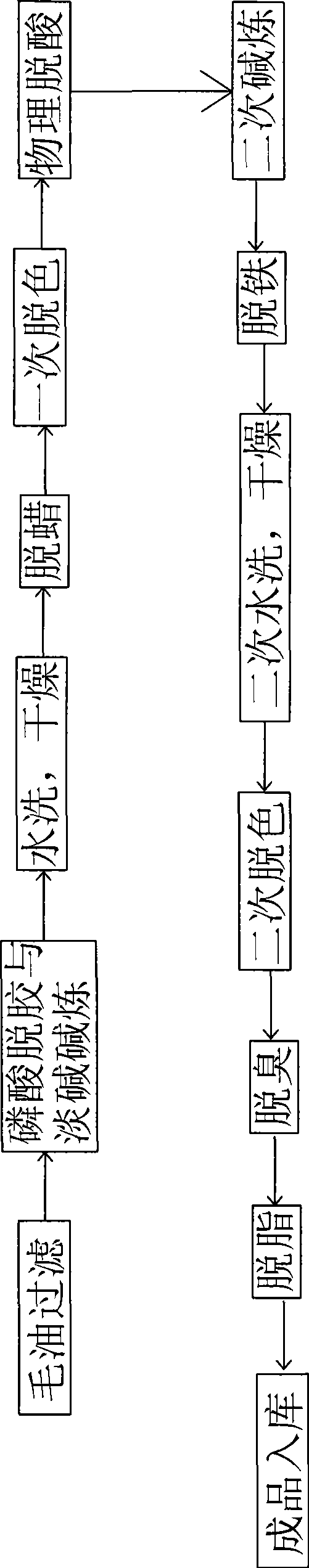
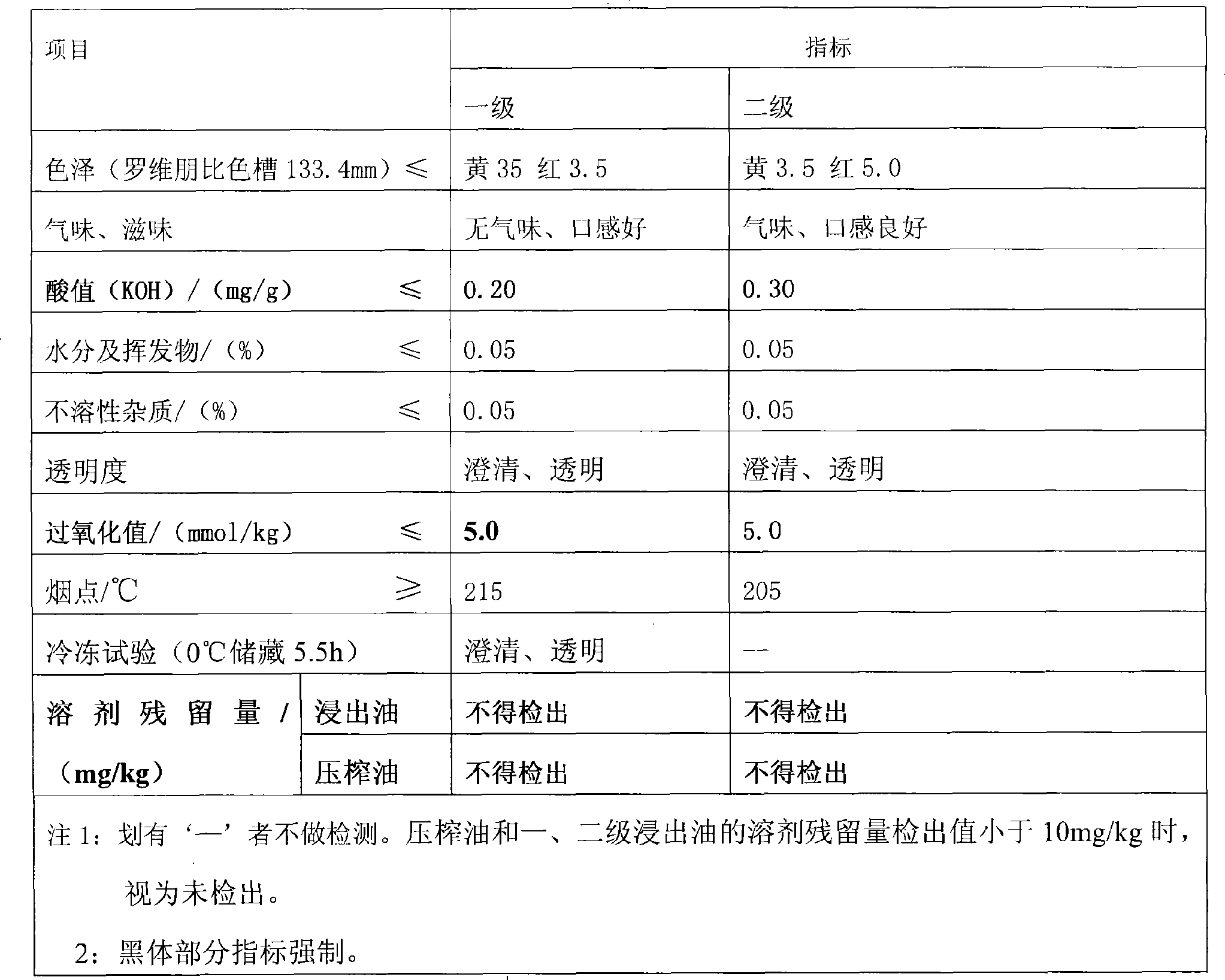
Rice bran first-level oil refinement production method
ActiveCN101455242ARealize large-scale productionReduce pollutionEdible oils/fatsFood preparationSocial benefitsO-Phosphoric Acid
The present invention relates to a manufacturing method of refining rice bran top oil, which can effectively refining top oil from the rice bran and satisfy the people requirement to the health dieting. The invention includes the following steps: (1) crude oil filtering; (2) phosphoric acid degumming and light alkali alkali refining; (3) washing, drying; (4) dewaxing; (5) a primary decolorizing; (6) physical deacidification; (7) secondary alkali refining; (8) iron removing; (9) washing; (10) drying; (11) secondary decolorizing; (12) deodorization; (13) degreasing. The invention adopts a mode of combining the physical refining and chemical refining to realize a large-scale production of the rice bran top oil, provides a favorable technique to the rice bran deep-processing, improves the extraction rate from traditionally 55-60% to 75-80%, saves materials and reduces environmental pollution, has huge economic and social benefits. The invention is a major innovation to the edible rice bran oil production.
Owner:河南华泰粮油机械股份有限公司
Process for producing iron phosphate for producing iron lithium phosphate material
The preparation method of iron phosphate used for preparing lithium iron phosphate material in the present invention relates to heavy metal phosphate, and the steps are: dissolving analytically pure soluble iron salt in distilled water to prepare a 0.05-5M aqueous solution, and the added mass is the iron salt mass 0.01~3% of anionic surfactant, then add analytically pure phosphoric acid according to the molar ratio of Fe3+:PO43-=1:0.8~1.2 and stir evenly, and slowly add alkaline solution with a concentration of 1~9M under stirring , the feeding time is more than 1 hour, until the pH value of the solution reaches 6-7, the iron phosphate precipitate is filtered, and the filtered iron phosphate is washed 3-5 times with distilled water 2-5 times its weight. Dry at ~90°C to obtain the product FePO4·2H2O powder. The iron phosphate product with two crystal waters prepared by the method of the invention has high reactivity, and the performance of the lithium iron phosphate material made by using it is better than that of the lithium iron phosphate material made by commercially available iron phosphate products.
Owner:HEBEI UNIV OF TECH
Electrically conductive polymer compositions
Electrically conducting polymer compositions are provided. The compositions comprise homopolymers and copolymers of polythiophene, polypyrrole, polyaniline, and polycyclic heteroaromatics, and combinations of those, in admixture with an organic solvent wettable fluorinated acid polymer. The acid polymers are fluorinated or highly fluorinated and have acidic groups including carboxylic acid groups, sulfonic acid groups, sulfonimide groups, phosphoric acid groups, phosphonic acid groups, and combinations thereof. The compositions may be used in organic electronic devices (OLEDs).
Owner:DUPONT DISPLAY +1
Method for preparing graphical sapphire substrate for nitrifier epitaxial growth
InactiveCN101330002AFree from dry etch damageLow costLaser detailsFinal product manufactureHydrofluoric acidPhosphoric acid
The invention discloses a production method of the patterned sapphire substrate for the nitride epitaxial growth, which comprises the following steps: a silica coating is deposited on the sapphire substrate used for the nitride epitaxial growth; a general photetch technology is utilized to prepare a mask of photetch patterns; the photetch patterns are etched on the silica coating by utilizing the doped liquid of hydrofluoric acid, ammonium fluoride and H2O; with the patterned silica coating as a mask, the sapphire substrate are etched by adopting the doped liquid of sulphuric acid and phosphoric acid in a wet manner so that the patterns are etched on the sapphire substrate; a dilute hydrofluoric acid solution is used for wet etching so as to remove the residual silica coating and clean the sapphire substrate, and then the preparation of the patterned sapphire substrate is completed. The production method has the advantages of low cost, preventing the sapphire substrate from being damaged by dry etching, etc. The patterned sapphire substrate can be used for the epitaxial growth of nitrides with low dislocation density and high crystal weight.
Owner:INST OF SEMICONDUCTORS - CHINESE ACAD OF SCI
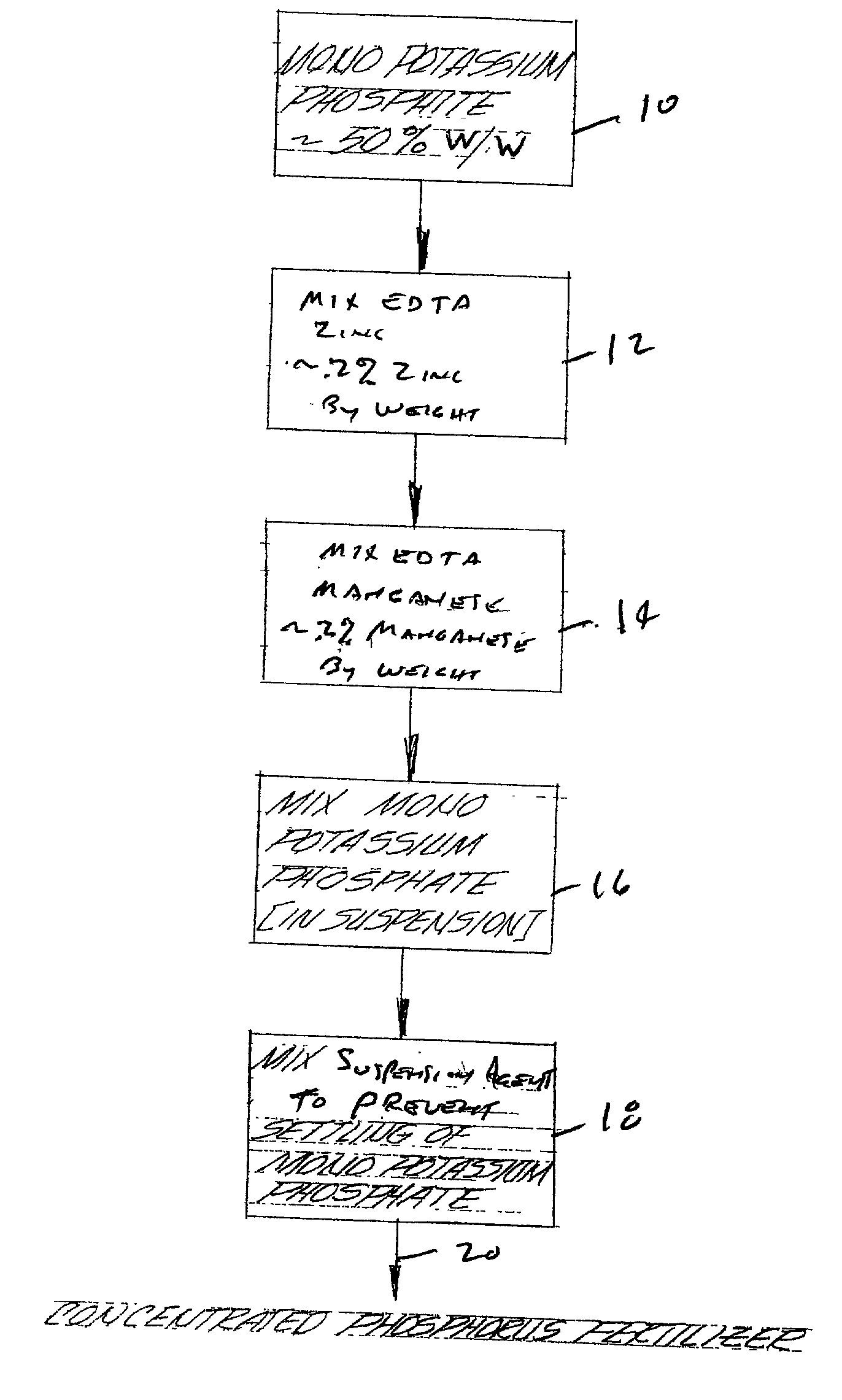
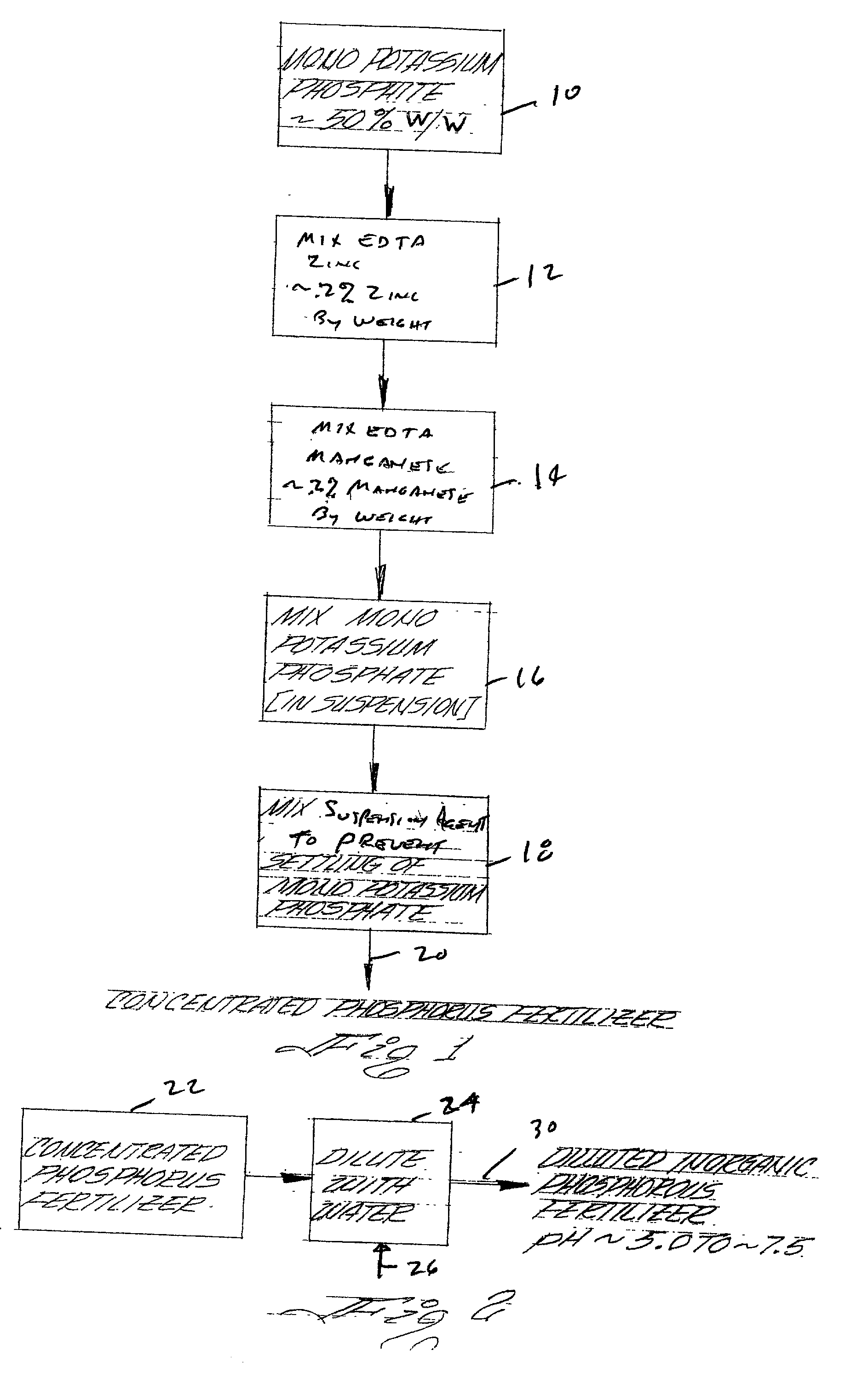
Concentrated phosphorus fertilizer
InactiveUS20020129632A1Easy to diluteImproving phosphorus uptakeAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersPhosphorous acidSalt content
A concentrated fluid phosphorus fertilizer is shown. In the preferred embodiment, the concentrated fluid phosphorus fertilizer is an aqueous suspension fertilizer composition. The composition comprises one or more phosphorus-containing acids or salts thereof selected from the group consisting of phosphoric acid, polyphosphoric acid, phosphorous acid, polyphosphorous acid, hypophosphorous acid and polyhypophosphorous acid, and salts thereof. A suspension agent maintains undissolved solids in substantially homogeneous suspension wherein the suspension has a total acid and salt content of about 50% w / w to about 80% w / w. The concentrated fluid phosphorus fertilizer is a stable suspension and when diluted with water a substantially fully soluble fertilizer having acceptable pH for phosphorus uptake is formed. Methods for improving the phosphorus uptake and improving the growth rate of plants is also shown.
Owner:INTERFARM
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com