Patents
Literature
7177results about "Solid electrolytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
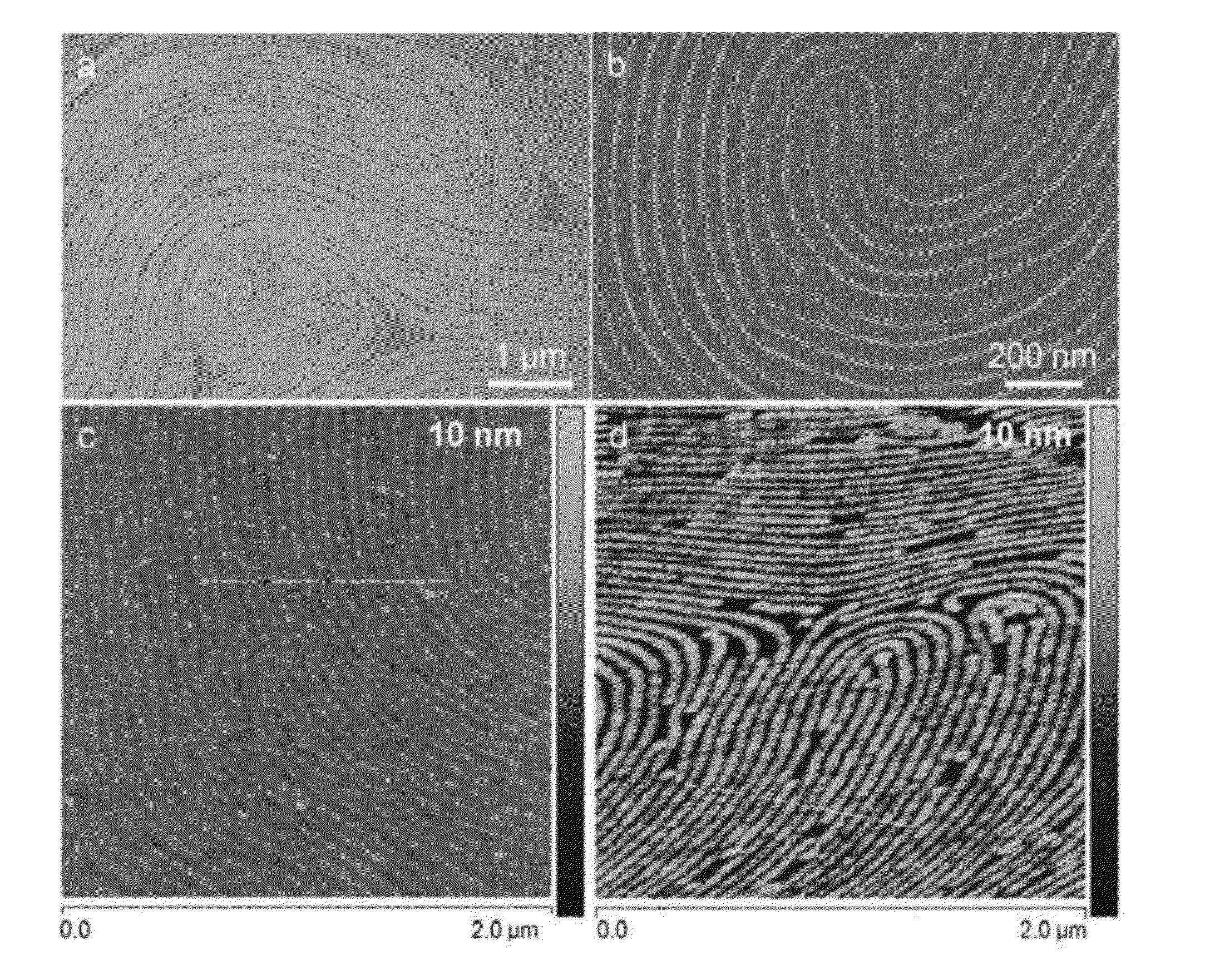
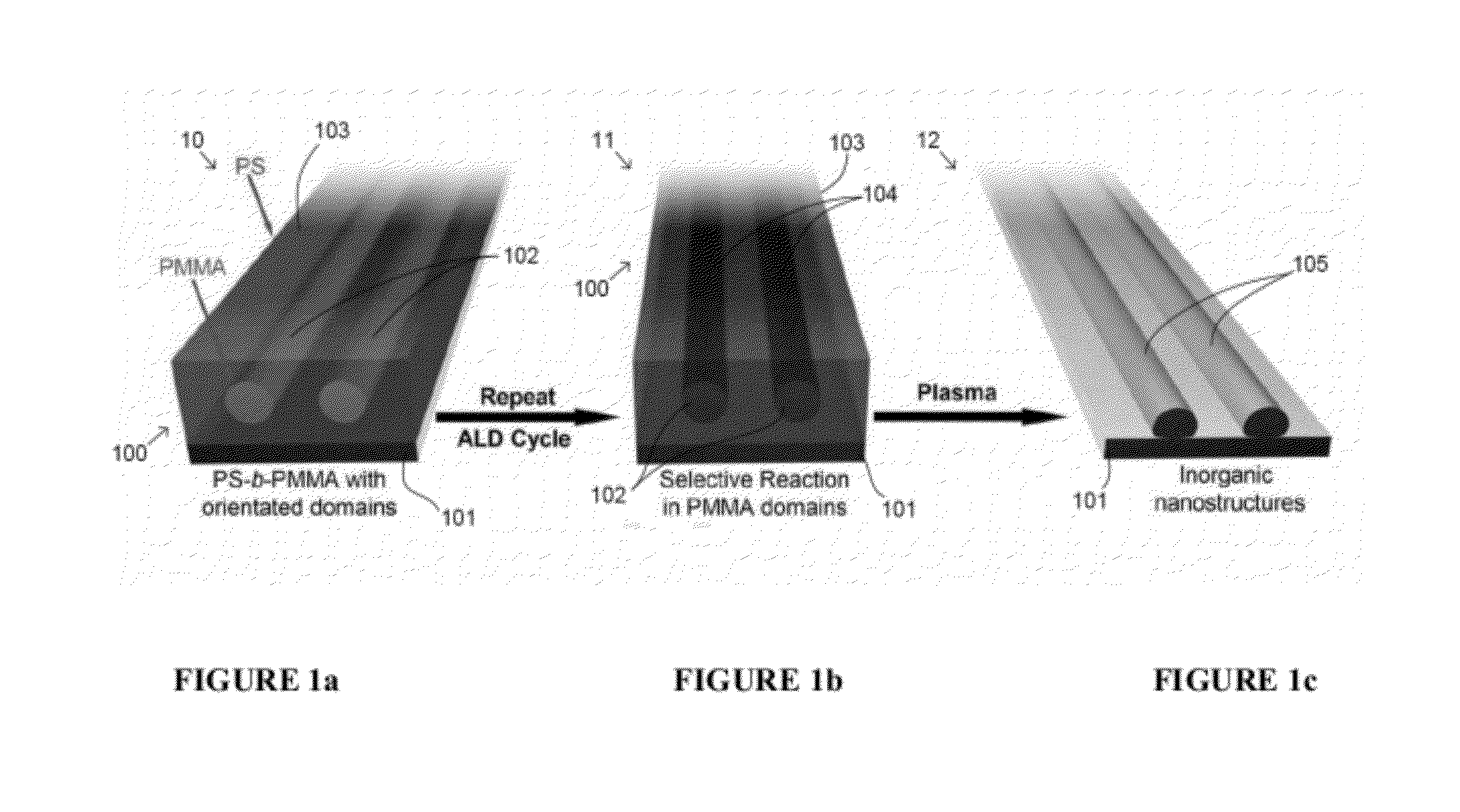
Ordered Nanoscale Domains by Infiltration of Block Copolymers
ActiveUS20120046421A1Low costHighly controllable molecularProgramme controlSolid electrolytesNanostructureAtomic layer deposition
A method of preparing tunable inorganic patterned nanofeatures by infiltration of a block copolymer scaffold having a plurality of self-assembled periodic polymer microdomains. The method may be used sequential infiltration synthesis (SIS), related to atomic layer deposition (ALD). The method includes selecting a metal precursor that is configured to selectively react with the copolymer unit defining the microdomain but is substantially non-reactive with another polymer unit of the copolymer. A tunable inorganic features is selectively formed on the microdomain to form a hybrid organic / inorganic composite material of the metal precursor and a co-reactant. The organic component may be optionally removed to obtain an inorganic feature s with patterned nanostructures defined by the configuration of the microdomain.
Owner:UCHICAGO ARGONNE LLC
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
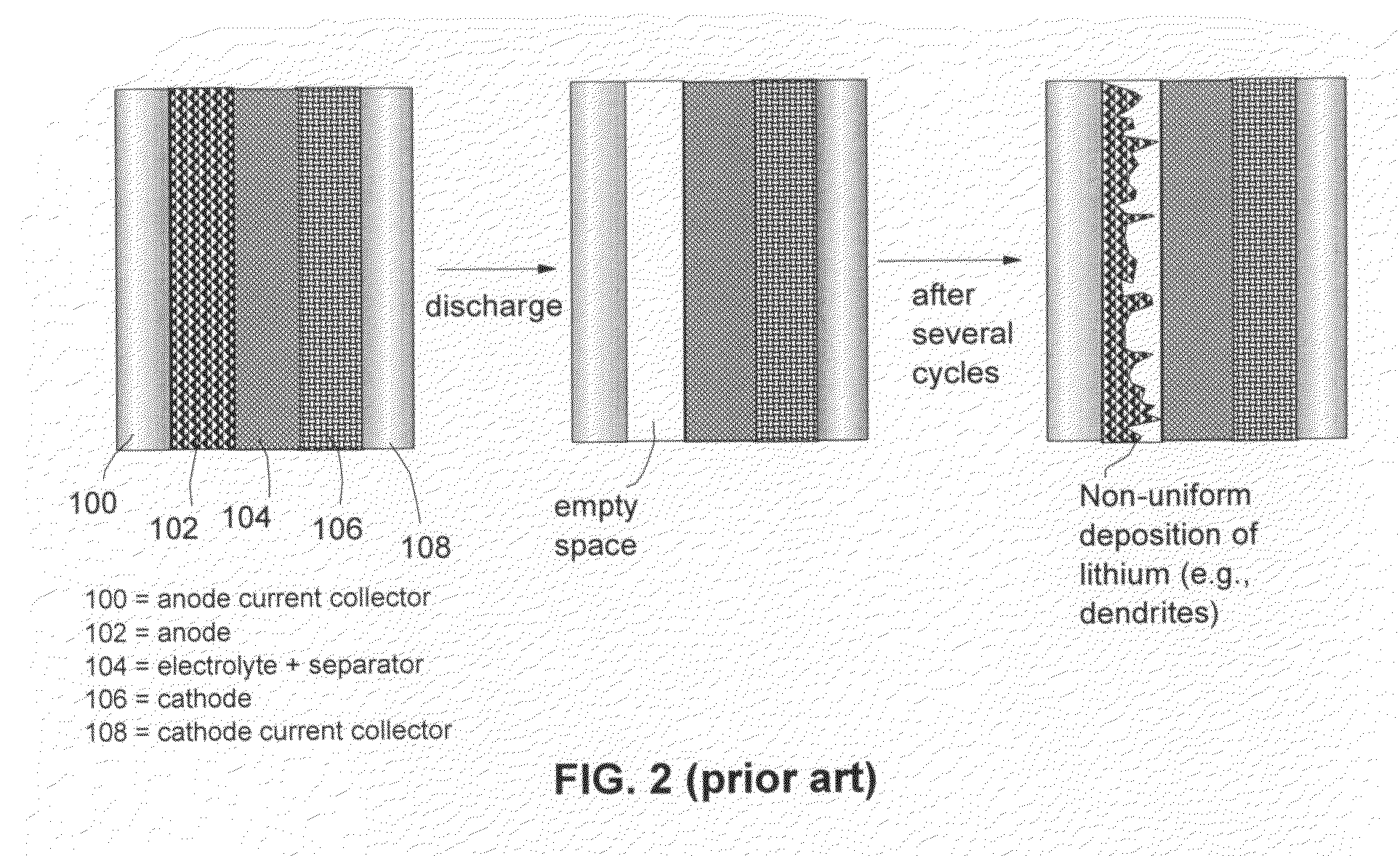
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Composite solid polymer electrolyte membranes
InactiveUS7550216B2Improve performanceLow costElectrolyte holding meansMembranesPolymer electrolytesFuel cells
Owner:FOSTER-MILLER
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
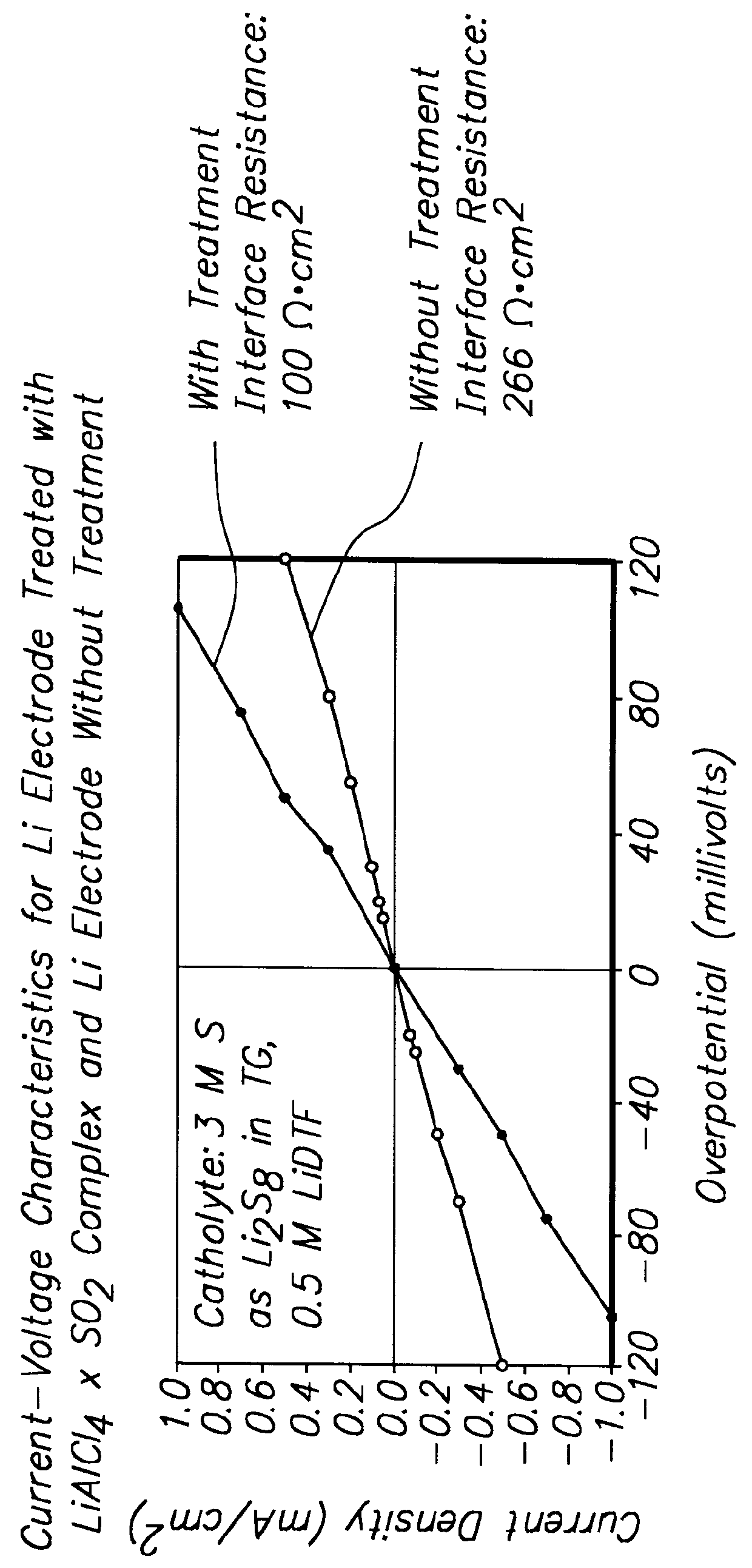
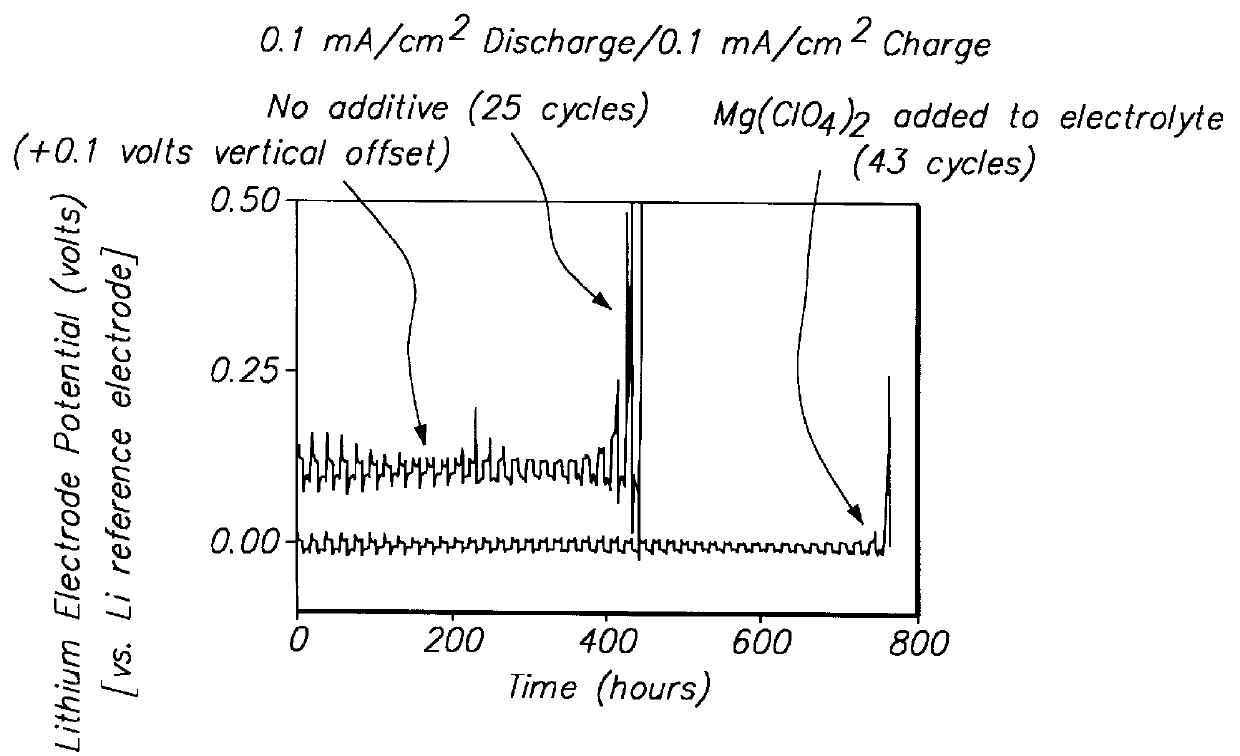
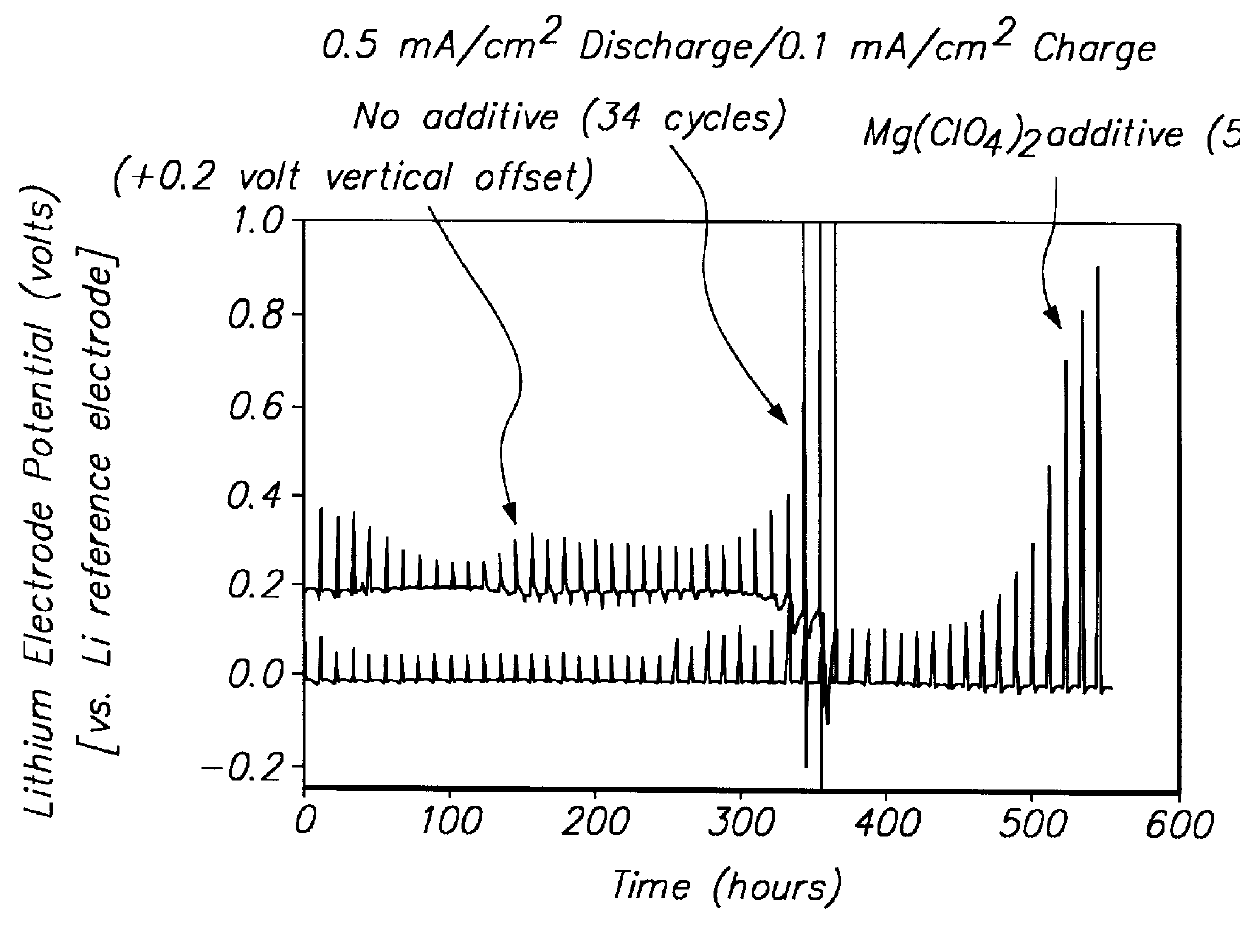
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Electrode materials with high surface conductivity
InactiveUS6855273B2Electrode manufacturing processesDouble layer capacitorsSurface conductivityIon exchange
The present invention concerns electrode materials capable of redox reactions by electrons and alkaline ions exchange with an electrolyte. The applications are in the field of primary (batteries) or secondary electrochemical generators, super capacitors and light modulating system of the super capacitor type.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
Ionically conductive membranes for protection of active metal anodes and battery cells
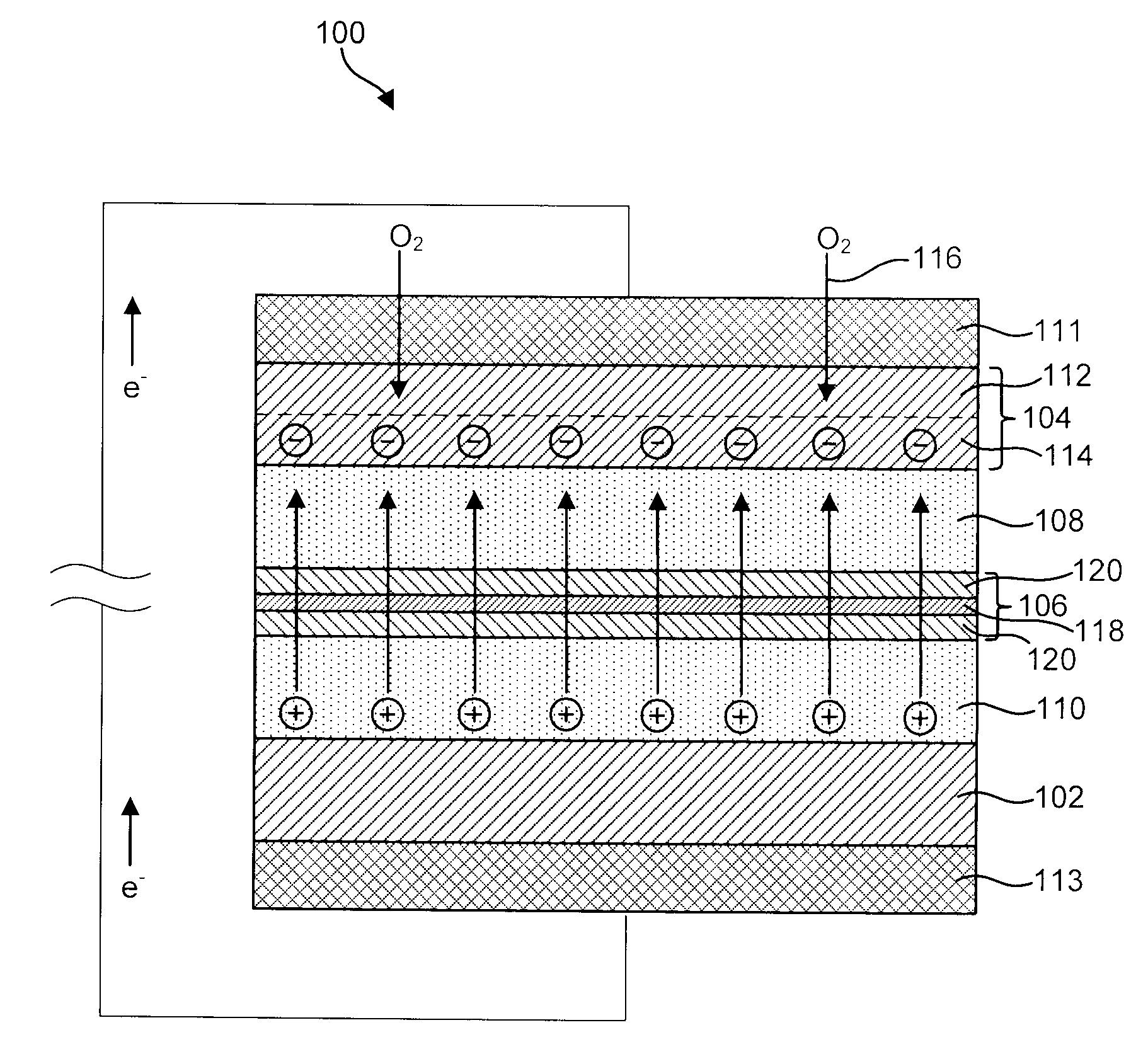
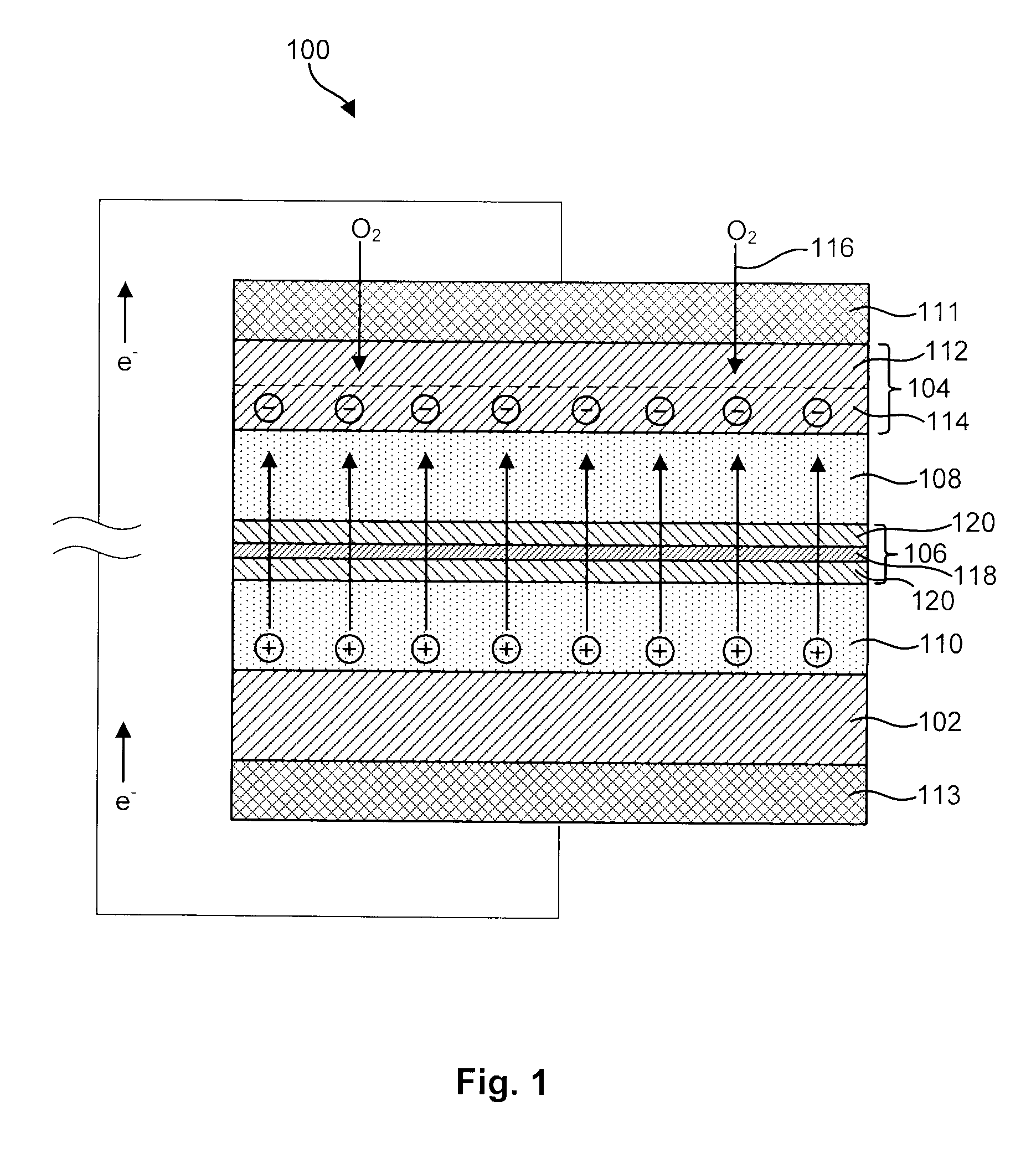
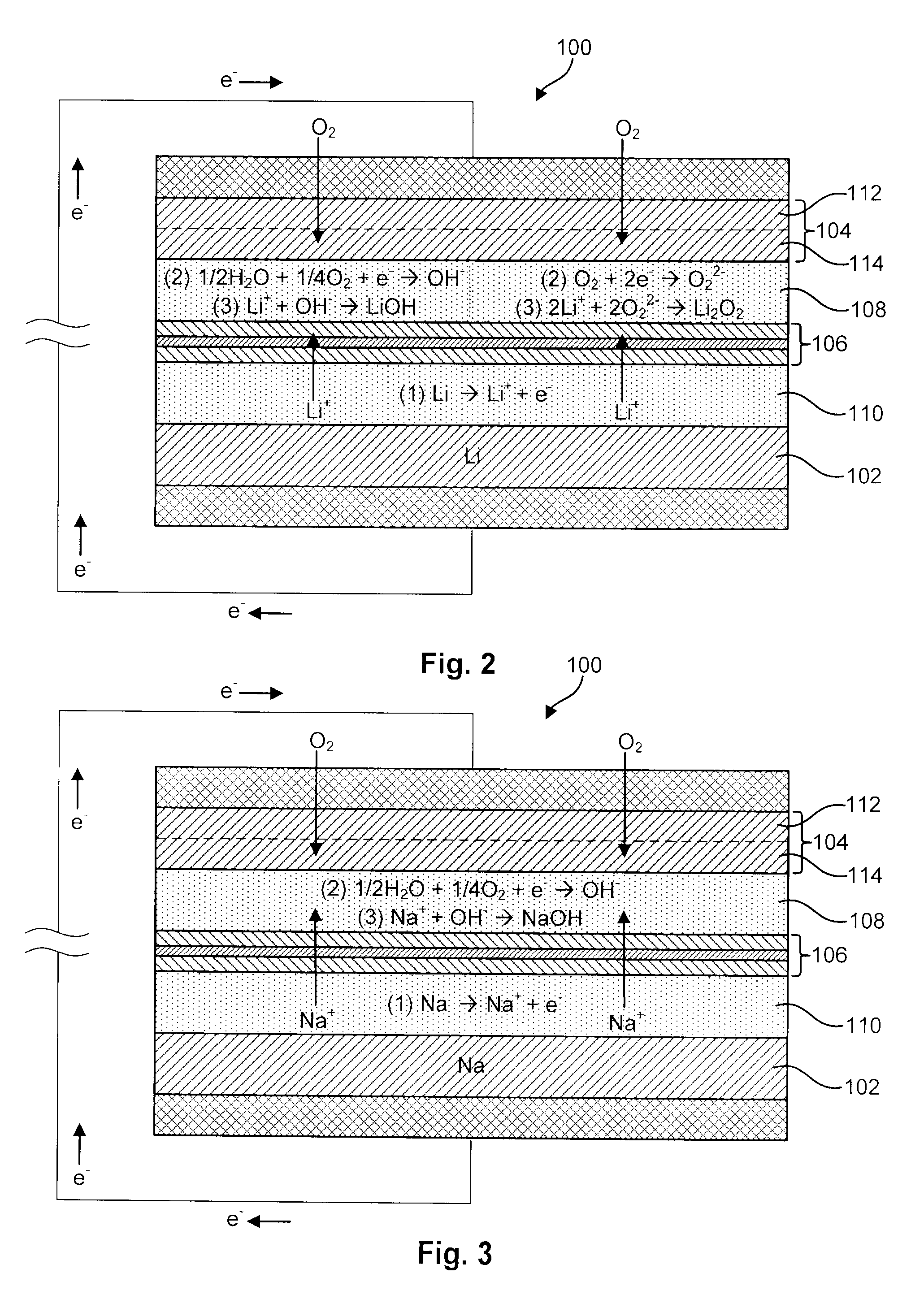
InactiveUS20040191617A1Improve propertiesImprove ionic conductivitySolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
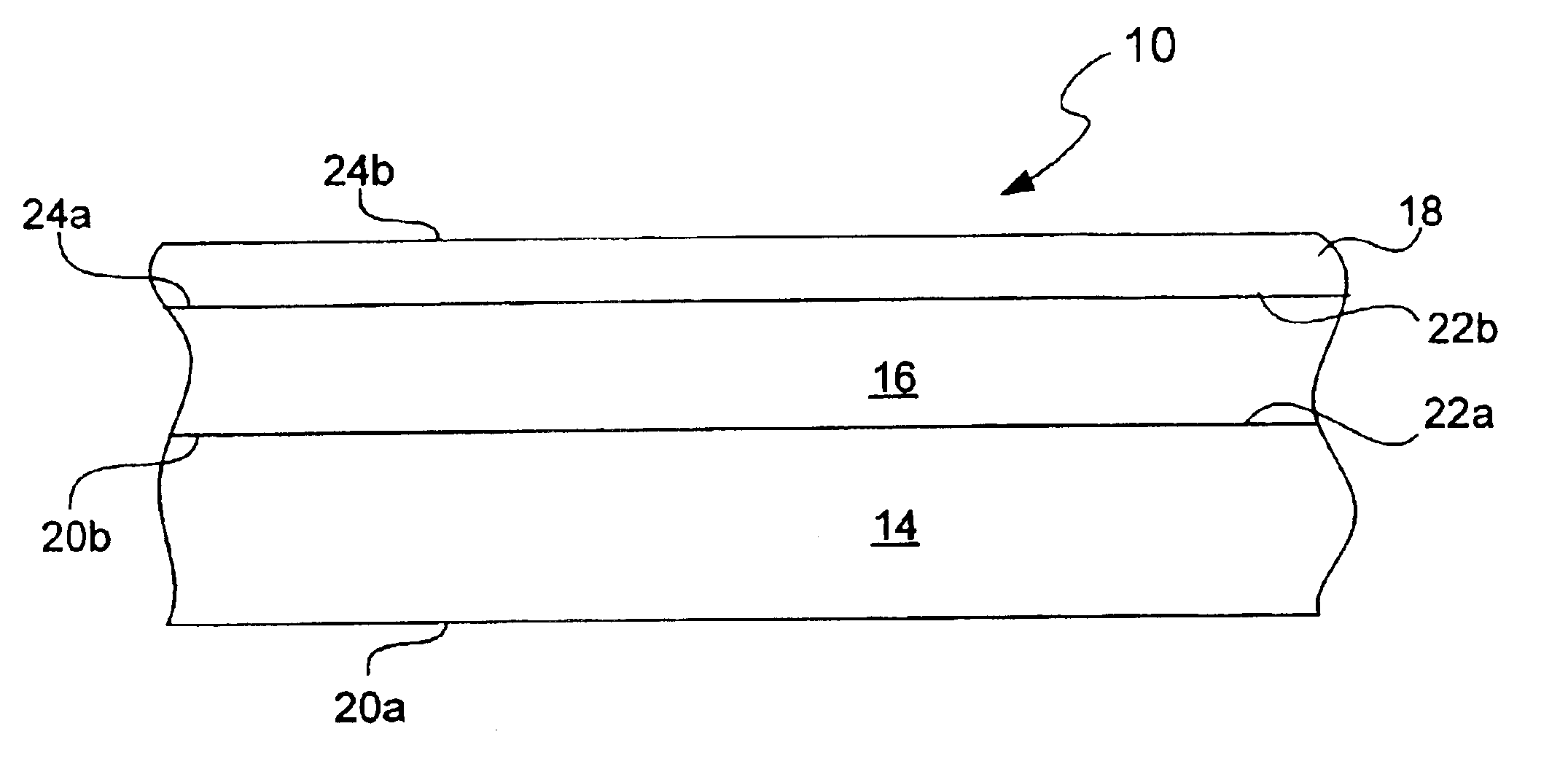
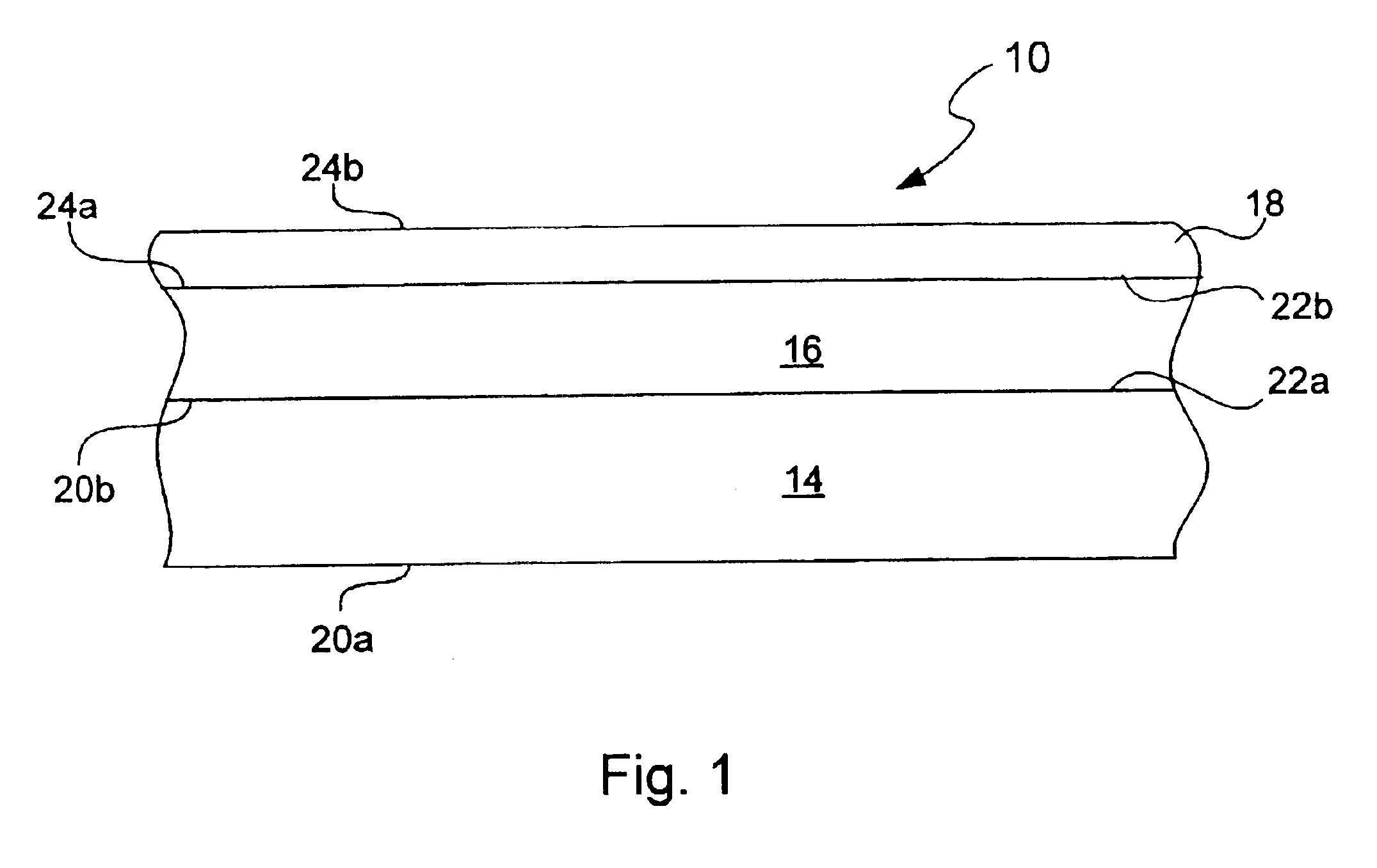
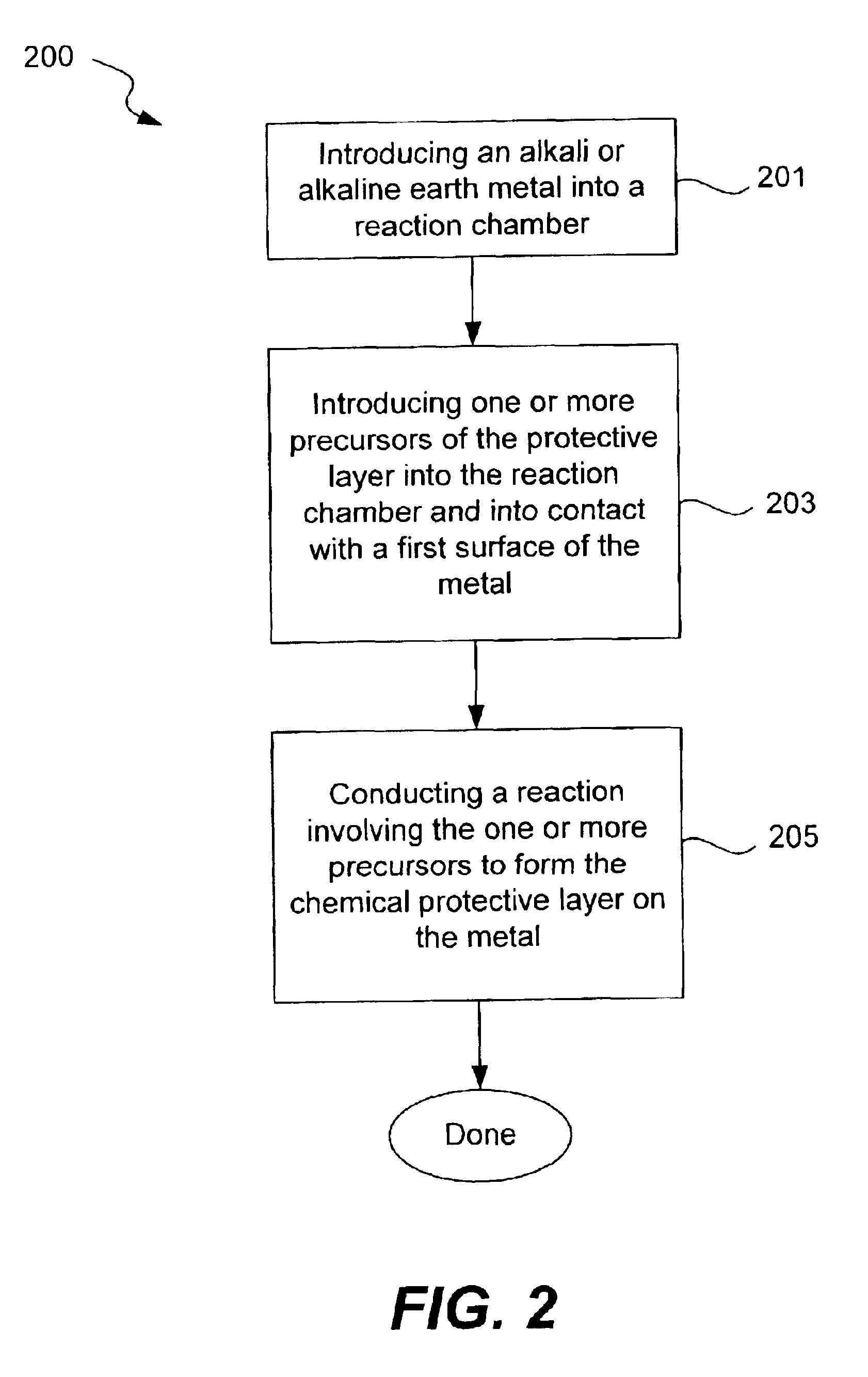
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
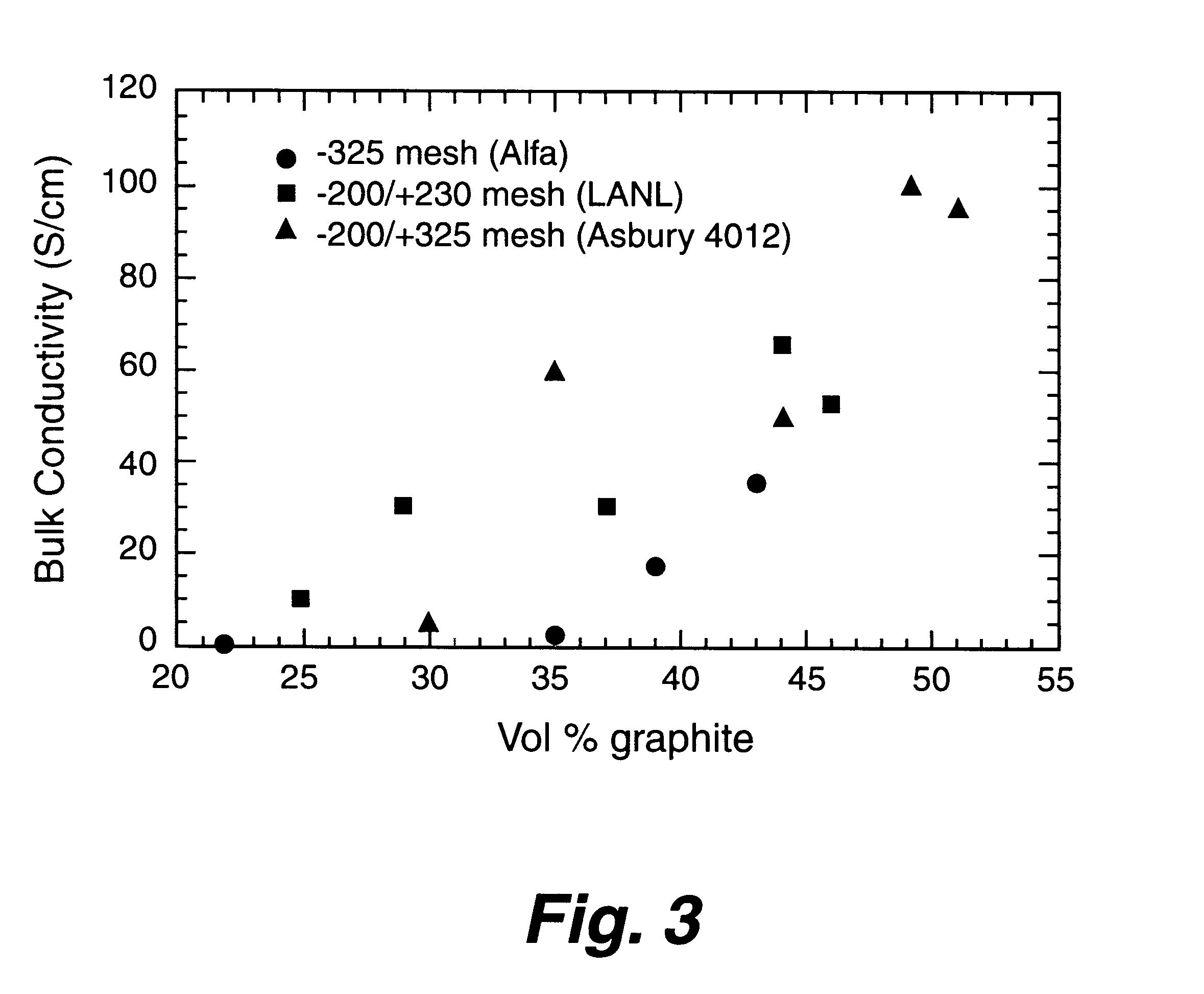
Composite bipolar plate for electrochemical cells
A bipolar separator plate for fuel cells consists of a molded mixture of a vinyl ester resin and graphite powder. The plate serves as a current collector and may contain fluid flow fields for the distribution of reactant gases. The material is inexpensive, electrically conductive, lightweight, strong, corrosion resistant, easily mass produced, and relatively impermeable to hydrogen gas. The addition of certain fiber reinforcements and other additives can improve the properties of the composite material without significantly increasing its overall cost.
Owner:TRIAD NAT SECURITY LLC
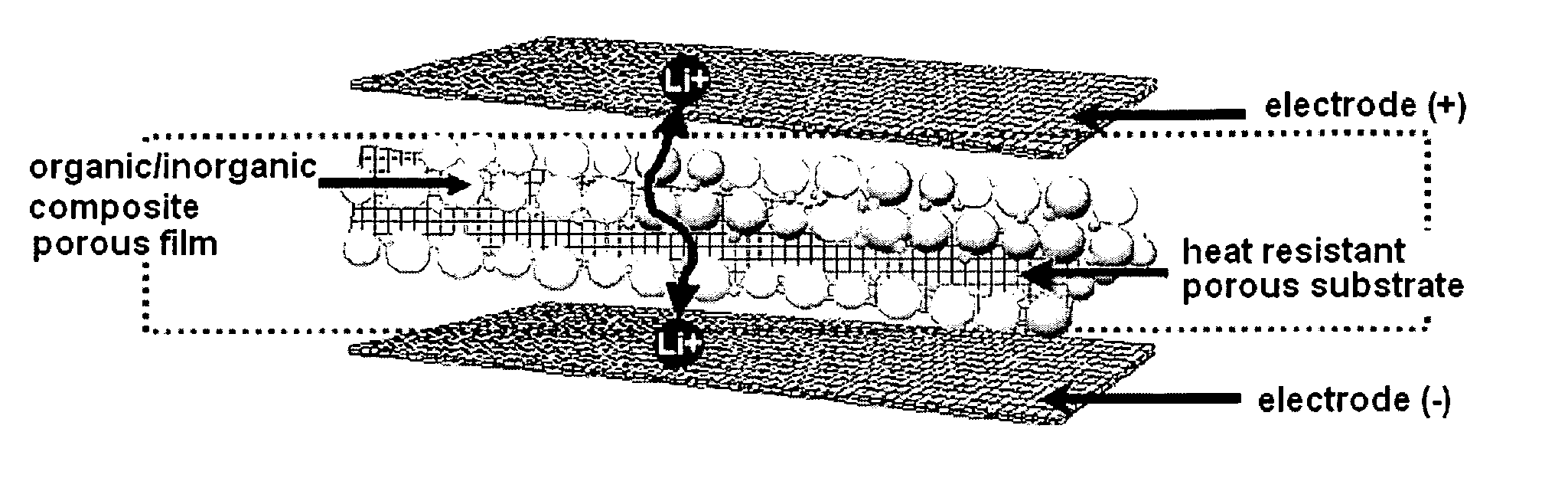
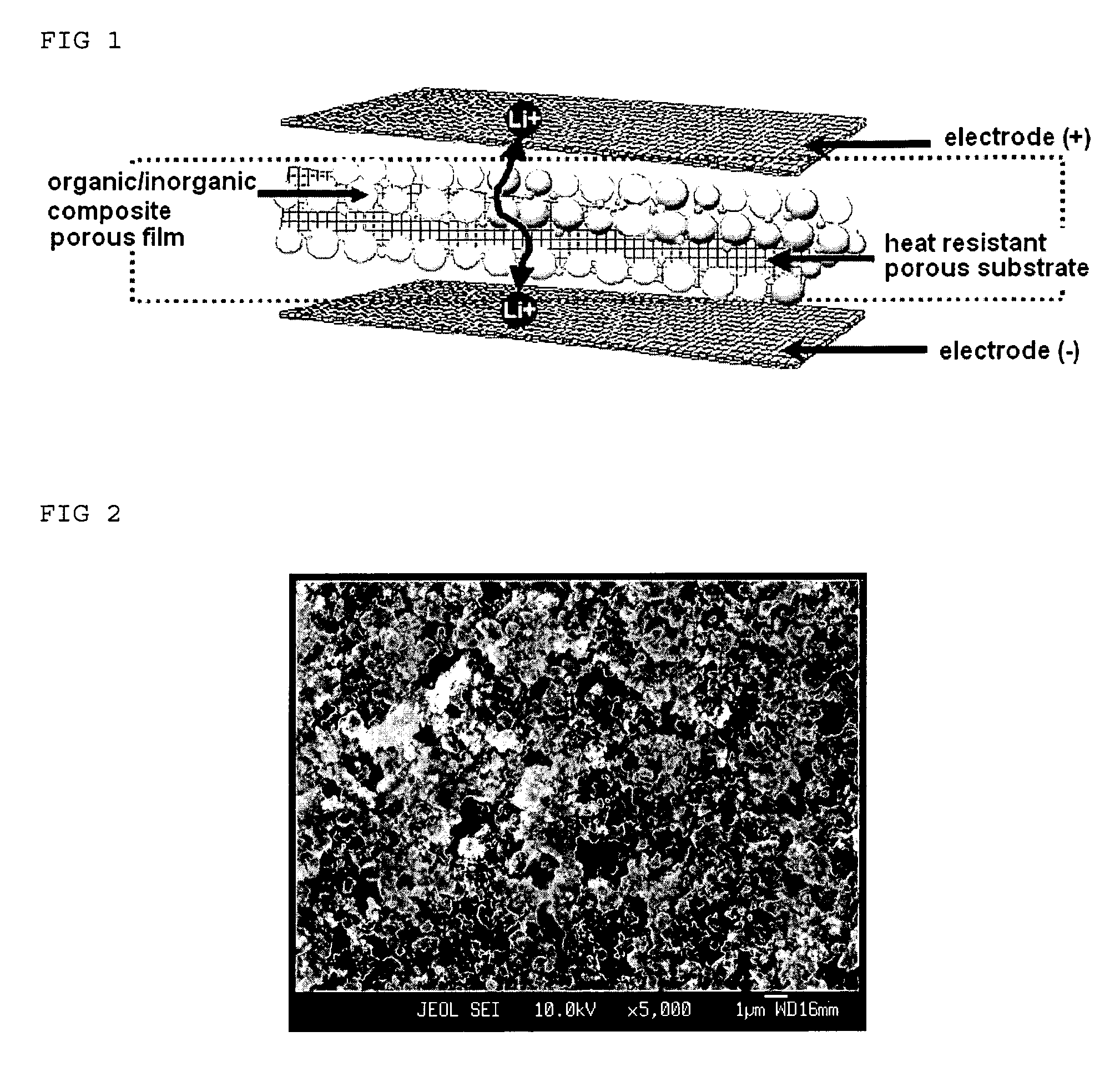
Organic/inorganic composite porous film and electrochemical device prepared thereby
ActiveUS20060008700A1Improve thermal safetyImprove adhesionSolid electrolytesLi-accumulatorsPorous substrateInorganic particle
Disclosed is an organic / inorganic composite porous film comprising: (a) a porous substrate having pores; and (b) an active layer formed by coating a surface of the substrate or a part of the pores in the substrate with a mixture of inorganic particles and a binder polymer, wherein the inorganic particles in the active layer are interconnected among themselves and are fixed by the binder polymer, and interstitial volumes among the inorganic particles form a pore structure. A method for manufacturing the same film and an electrochemical device including the same film are also disclosed. An electrochemical device comprising the organic / inorganic composite porous film shows improved safety and quality, simultaneously.
Owner:LG ENERGY SOLUTION LTD +1
All-solid lithium battery
ActiveUS20090081554A1Improve output performanceLayer formationSolid electrolytesElectrode carriers/collectorsRedoxSulfide
Owner:NAT INST FOR MATERIALS SCI
Fuel cell platelet separators having coordinate features
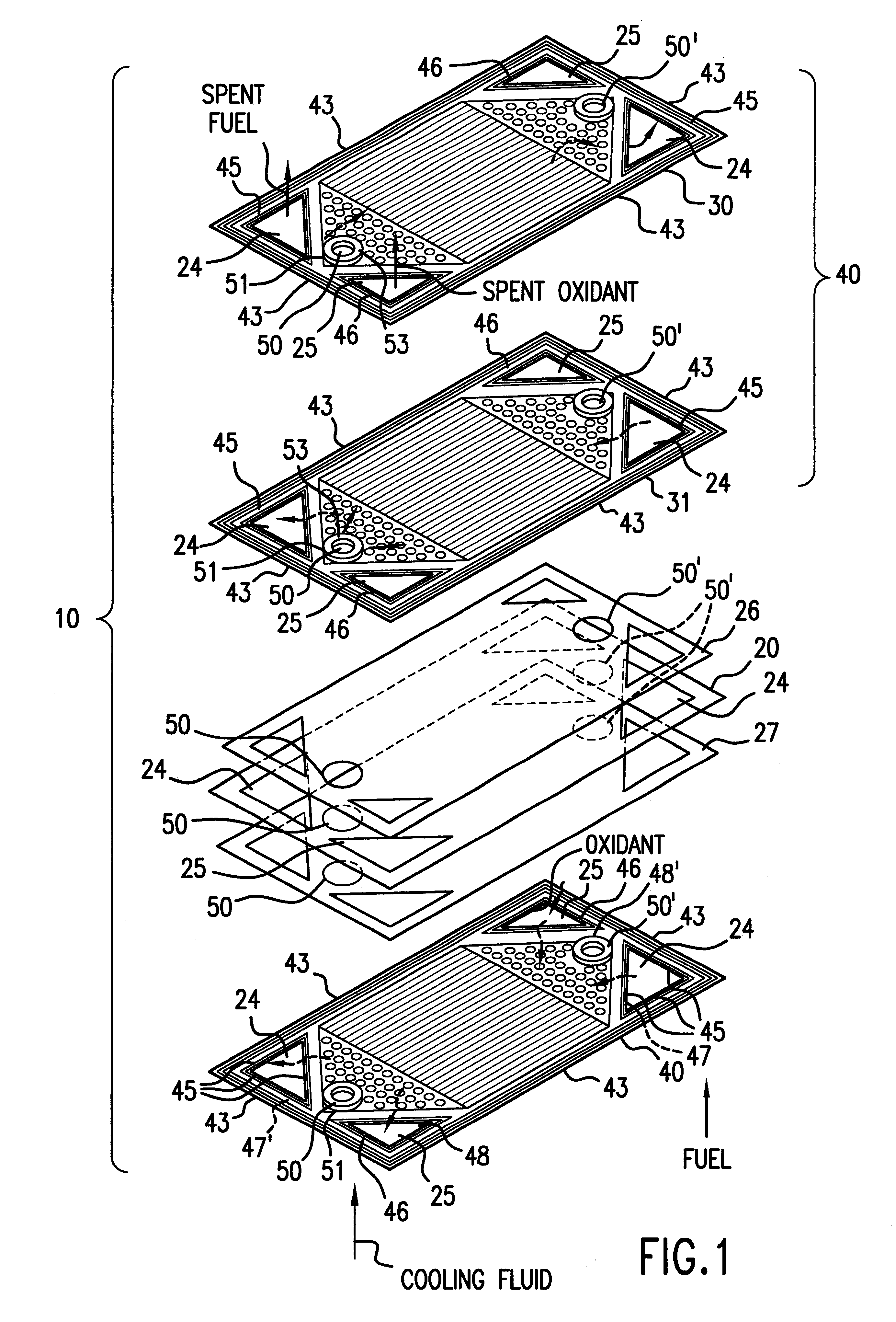
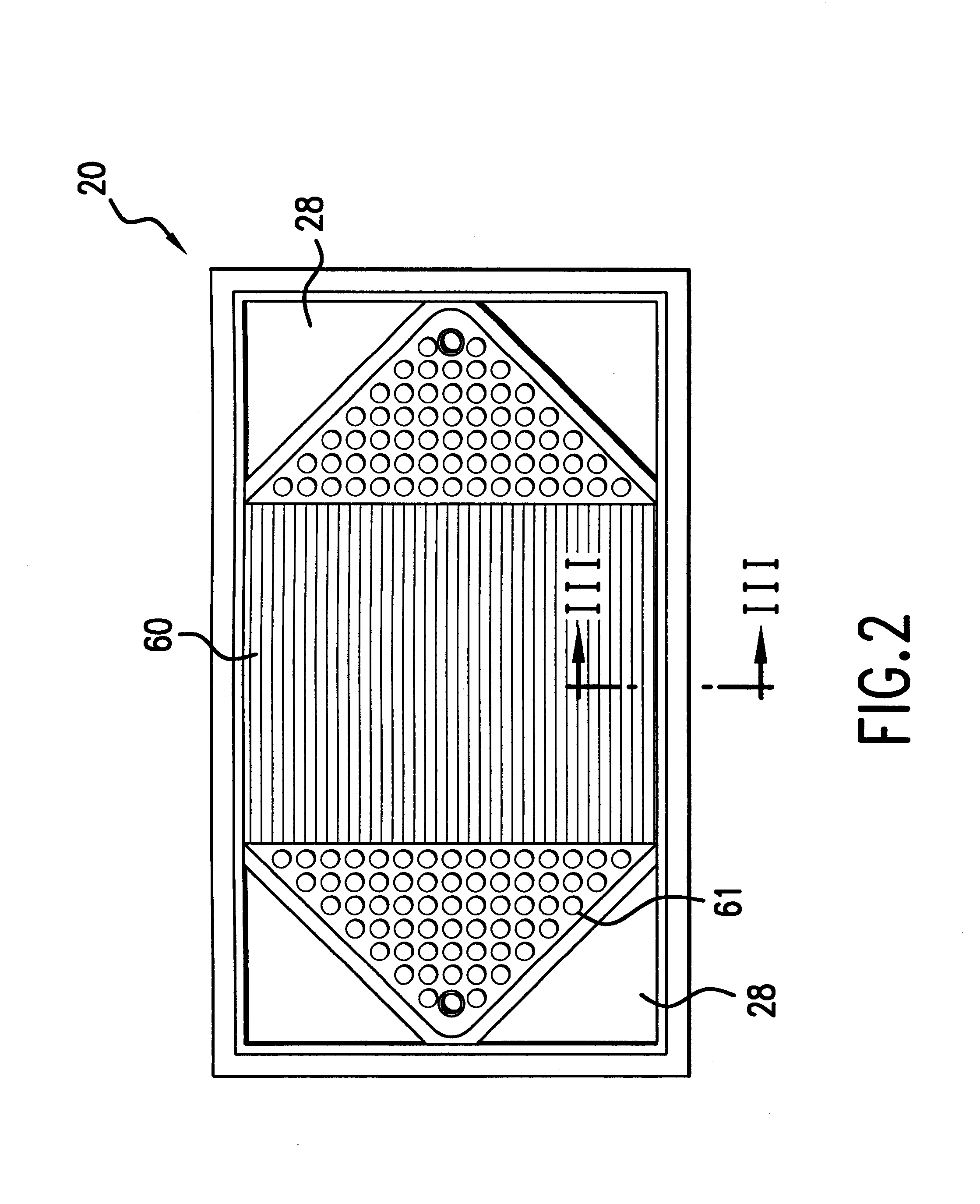
InactiveUS6051331ASimple designEvenly distributedSolid electrolytesFuel cells groupingLaser etchingFuel cells
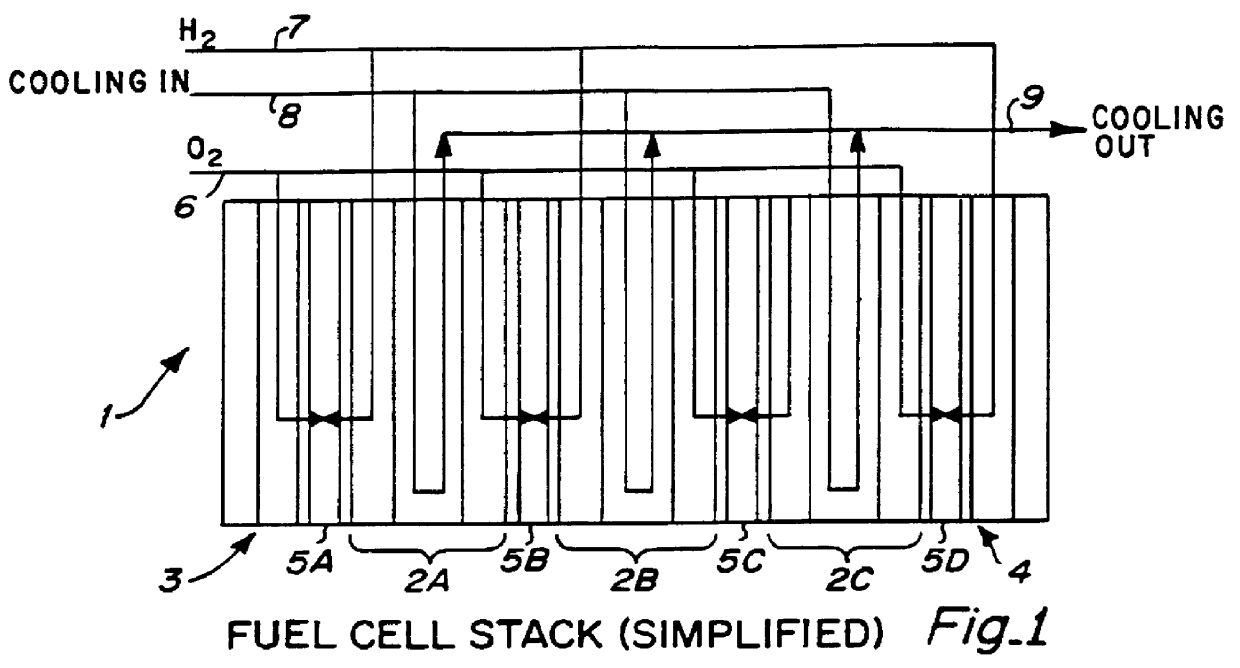
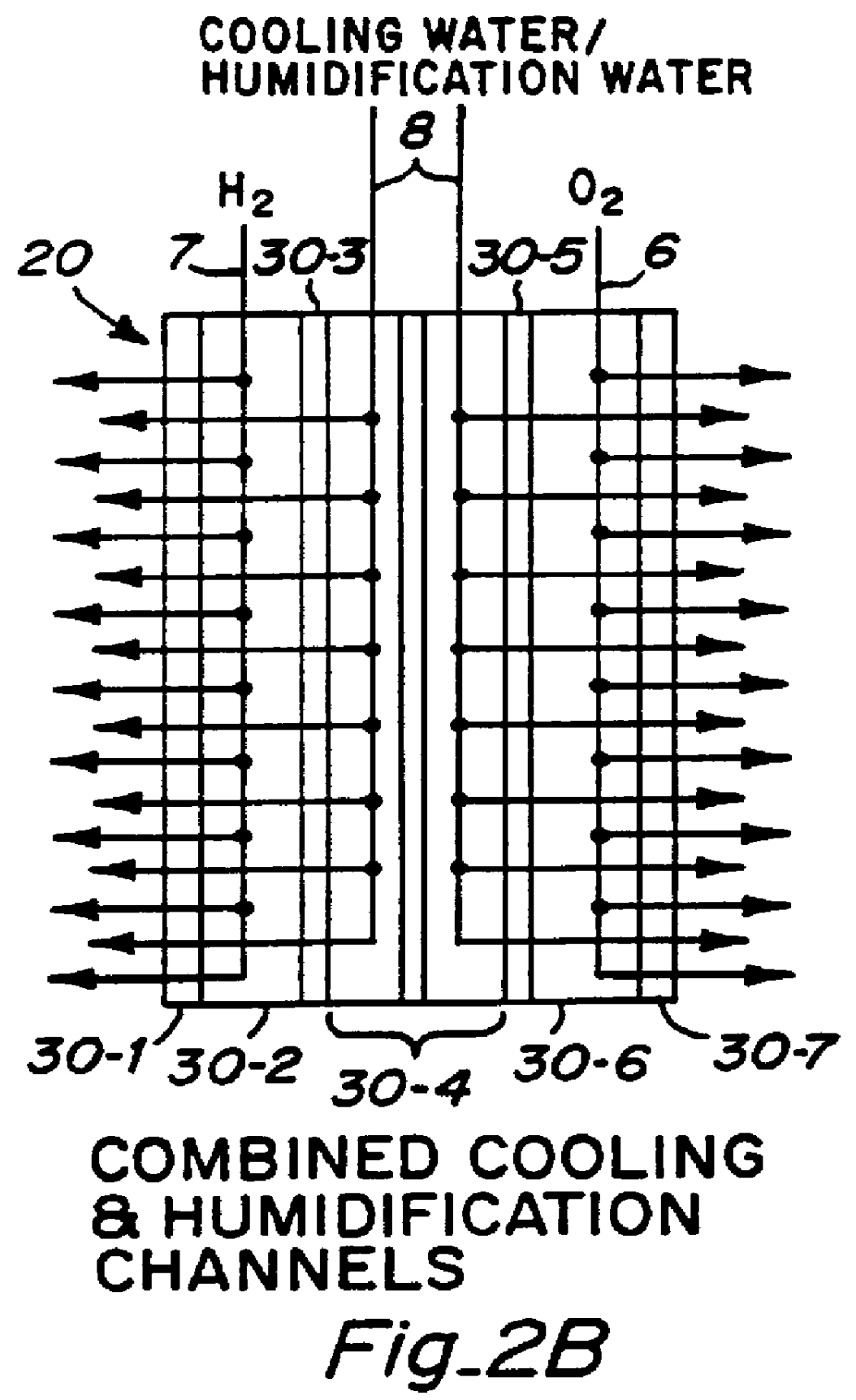
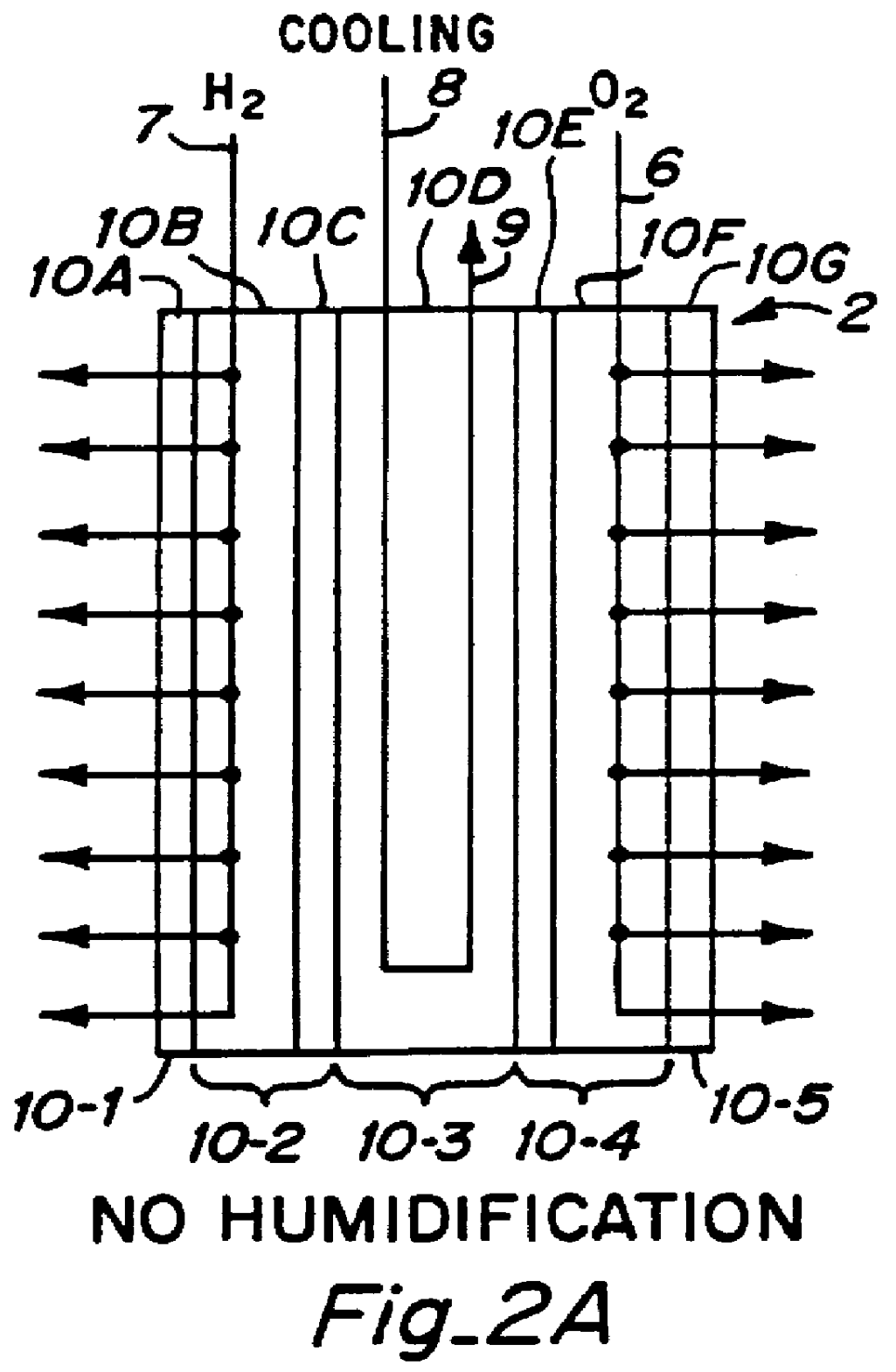
PCT No. PCT / US95 / 13325 Sec. 371 Date Sep. 28, 1997 Sec. 102(e) Date Sep. 28, 1997 PCT Filed Oct. 10, 1995 PCT Pub. No. WO96 / 12316 PCT Pub. Date Apr. 25, 1996Fuel cell stacks comprising stacked separator / membrane electrode assembly fuel cells in which the separators comprise a series of thin sheet platelets, having individually configured serpentine micro-channel reactant gas humidification active areas and cooling fields therein. The individual platelets are stacked with coordinate features aligned in contact with adjacent platelets and bonded to form a monolithic separator. Post-bonding processing includes passivation, such as nitriding. Preferred platelet material is 4-25 mil Ti, in which the features, serpentine channels, tabs, lands, vias, manifolds and holes, are formed by chemical and laser etching, cutting, pressing or embossing, with combinations of depth and through etching preferred. The platelet manufacturing process is continuous and fast. By employing CAD based platelet design and photolithography, rapid change in feature design can accommodate a wide range of thermal management and humidification techniques. One hundred H2-O2 / PEM fuel cell stacks of this IFMT platelet design will exhibit outputs on the order of 0.75 kW / kg, some 3-6 times greater than the current graphite plate PEM stacks.
Owner:H POWER
Ionically conductive composites for protection of active metal anodes
ActiveUS7282302B2Good chemical stabilityImprove ionic conductivitySolid electrolytesCell seperators/membranes/diaphragms/spacersChemical stabilityBattery cell
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
Chemical protection of a lithium surface
InactiveUS20050186469A1Easy to produceEasy to processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process is the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Advanced Metal-Air Battery Having a Ceramic Membrane Electrolyte Background of the Invention
ActiveUS20080268327A1Reduce oxygenReduce layeringFuel and primary cellsSolid electrolytesOxygenCeramic membrane
A metal-air battery is disclosed in one embodiment of the invention as including a cathode to reduce oxygen molecules and an alkali-metal-containing anode to oxidize the alkali metal (e.g., Li, Na, and K) contained therein to produce alkali-metal ions. An aqueous catholyte is placed in ionic communication with the cathode to store reaction products generated by reacting the alkali-metal ions with the oxygen containing anions. These reaction products are stored as solutes dissolved in the aqueous catholyte. An ion-selective membrane is interposed between the alkali-metal containing anode and the aqueous catholyte. The ion-selective membrane is designed to be conductive to the alkali-metal ions while being impermeable to the aqueous catholyte.
Owner:FIELD UPGRADING USA INC
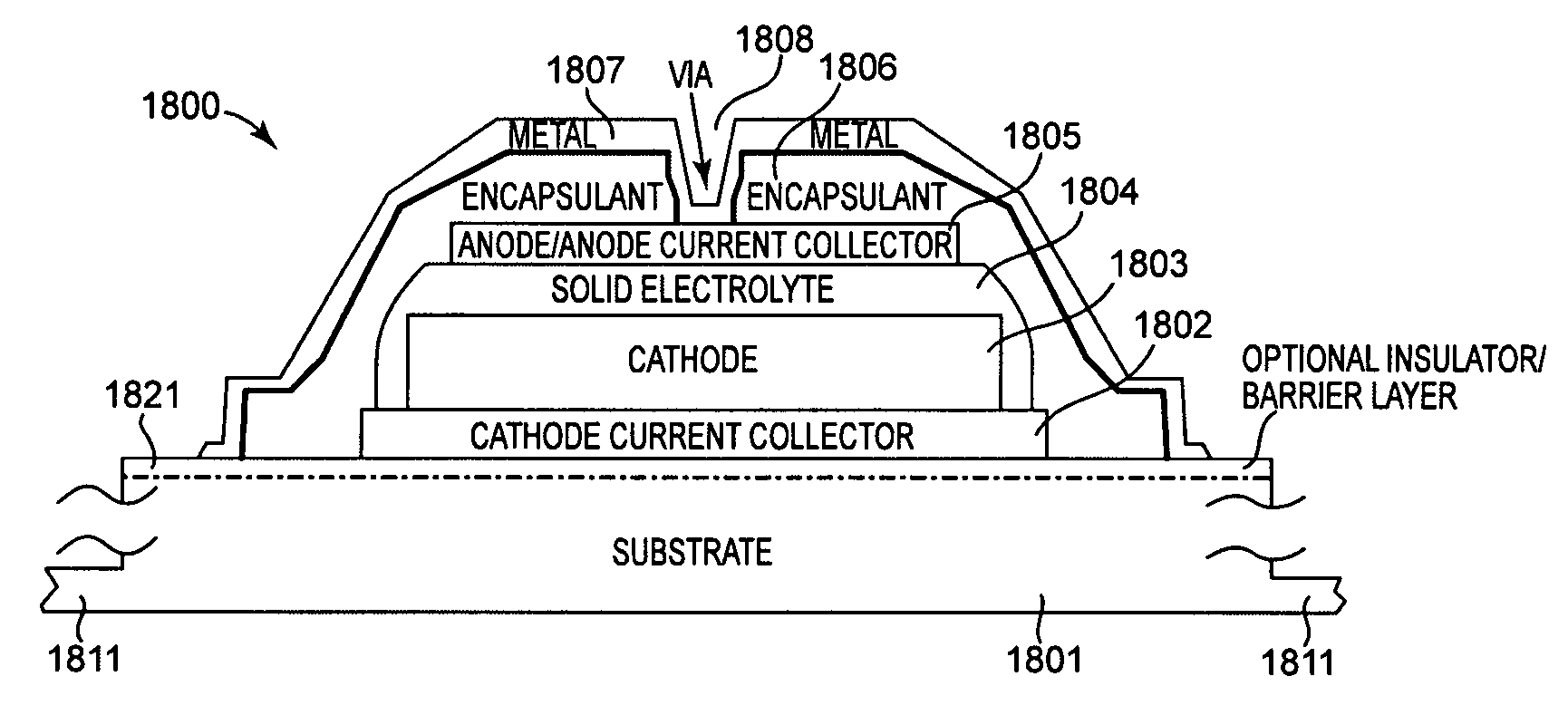
Method and apparatus for solid-state microbattery photolithographic manufacture, singulation and passivation
InactiveUS20080032236A1Efficient and economical manufactureReduced number of stepSolid electrolytesDecorative surface effectsChemical treatmentPhotoresist
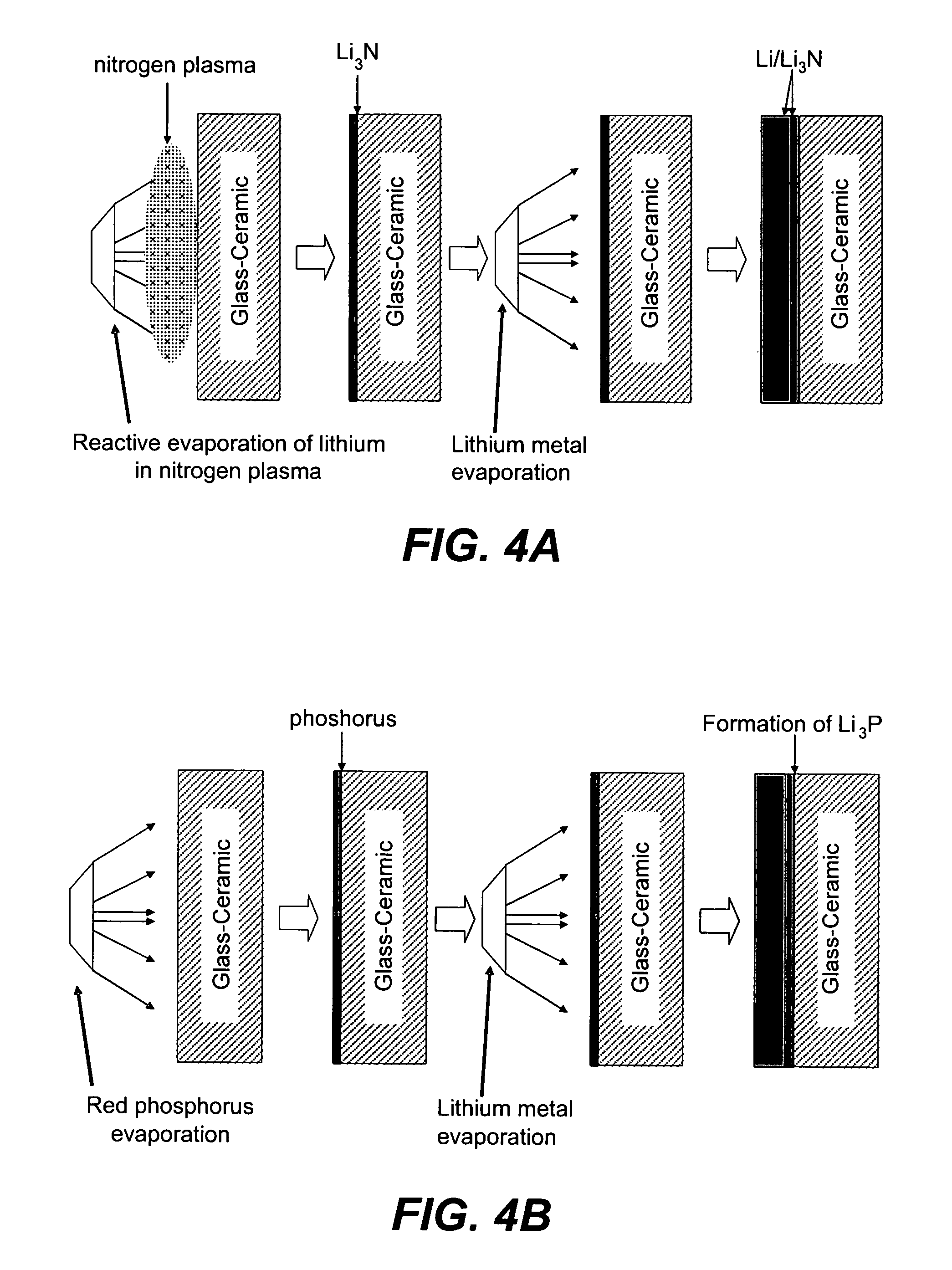
A method for producing a thin film lithium battery is provided, comprising applying a cathode current collector, a cathode material, an anode current collector, and an electrolyte layer separating the cathode material from the anode current collector to a substrate, wherein at least one of the layers contains lithiated compounds that is patterned at least in part by a photolithography operation comprising removal of a photoresist material from the layer containing lithiated compounds by a process including a wet chemical treatment. Additionally, a method and apparatus for making lithium batteries by providing a first sheet that includes a substrate having a cathode material, an anode material, and a LiPON barrier / electrolyte layer separating the cathode material from the anode material; and removing a subset of first material to separate a plurality of cells from the first sheet. In some embodiments, the method further includes depositing second material on the sheet to cover the plurality of cells; and removing a subset of second material to separate a plurality of cells from the first sheet.
Owner:CYMBET CORP
Sheet metal bipolar plate design for polymer electrolyte membrane fuel cells
InactiveUS6261710B1Compact designIncrease the number ofSolid electrolytesFuel cells groupingPolymer electrolytesFuel cells
A separator plate for a polymer electrolyte membrane fuel cell stack constructed of at least two coextensive sheet metal elements shaped to promote the distribution of reactant gases to the electrodes of the fuel cell units of the fuel cell stack. The coextensive sheet metal elements are nestled together and form a coolant flow space therebetween.
Owner:INST OF GAS TECH
Nano-structured ion-conducting inorganic membranes for fuel cell applications
InactiveUS20060078765A1Easy to operateIncrease moistureSolid electrolytesElectrode carriers/collectorsIonChemistry
An inorganic proton-conducting membrane and a fuel cell comprising this membrane. The fuel cell comprises a fuel anode, an oxidant cathode, and an inorganic proton-conducting membrane disposed between the anode and the cathode. The membrane is composed of a nano-structured network of proton-exchange inorganic particles. The particles form a sufficiently high density of proton-conducting nanometer-scaled channels with at least one dimension smaller than 100 nanometers so that ionic conductivity of the membrane is no less than 10−6 S / cm (mostly greater than 10−4 S / cm ) at 25° C. or no less than 10−4 S / cm (mostly greater than 10−2 S / cm) at 200° C. This inorganic membrane allows a hydrogen-oxygen fuel cell to operate at a higher temperature without the need (or with a reduced need) to maintain the membrane in a highly hydrated state. A higher operating temperature also implies a fast electro-catalytic reaction of a fuel (e.g., mixture of methanol and water) at the anode permitting a lesser amount of fuel to cross-over the membrane and, hence, a higher fuel utilization efficiency.
Owner:YANG LAIXIA +2
Ionically conductive membranes for protection of active metal anodes and battery cells
InactiveUS7390591B2Improve conductivityEasy to manufactureSolid electrolytesSolid electrolyte cellsChemical stabilityBattery cell
Disclosed are ionically conductive membranes for protection of active metal anodes and methods for their fabrication. The membranes may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the membrane has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The membrane is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the membrane is incorporated.
Owner:POLYPLUS BATTERY CO INC
Metal-air battery with ion-conducting inorganic glass electrolyte
InactiveUS20060063051A1Safe and reliable solid-stateIncrease energy densityFuel and primary cellsSolid electrolytesMetal–air electrochemical cellIonic conductivity
A solid-state metal-air electrochemical cell comprising: (A) a metal-containing electro-active anode; (B) an oxygen electro-active cathode; and (C) an ion-conducting glass electrolyte disposed between the metal-containing anode and the oxygen electro-active cathode. The cathode active material, which is oxygen gas, is not stored in the battery but rather fed from the environment. The oxygen cathode is preferably a composite carbon electrode which serves as the cathode current collector on which oxygen molecules are reduced during discharge of the battery to generate electric current. The glass electrolyte typically has an ion conductivity in the range of 5×10−5 to 2×10−3 S / cm. The electrolyte layer is preferably smaller than 10 μm in thickness and further preferably smaller than 1 μm. The anode metal is preferably lithium or lithium alloy, but may be selected from other elements such as sodium, magnesium, calcium, aluminum and zinc.
Owner:JANG BOR Z
Low Cost Solid State Rechargeable Battery and Method of Manufacturing Same
InactiveUS20090092903A1Solid electrolytesElectrode thermal treatmentOptoelectronicsConductive materials
A solid state Li battery and an all ceramic Li-ion battery are disclosed. The all ceramic battery has a solid state battery cathode comprised of a mixture of an active cathode material, an electronically conductive material, and a solid ionically conductive material. The cathode mixture is sintered. The battery also has a solid state battery anode comprised of a mixture of an active anode material, an electronically conductive material, and a solid ionically conductive material. The anode mixture is sintered. The battery also has a solid state separator positioned between said solid state battery cathode and said solid state battery anode. In the solid state Li battery the all ceramic anode is replaced with an evaporated thin film Li metal anode.
Owner:JOHNSON IP HLDG LLC
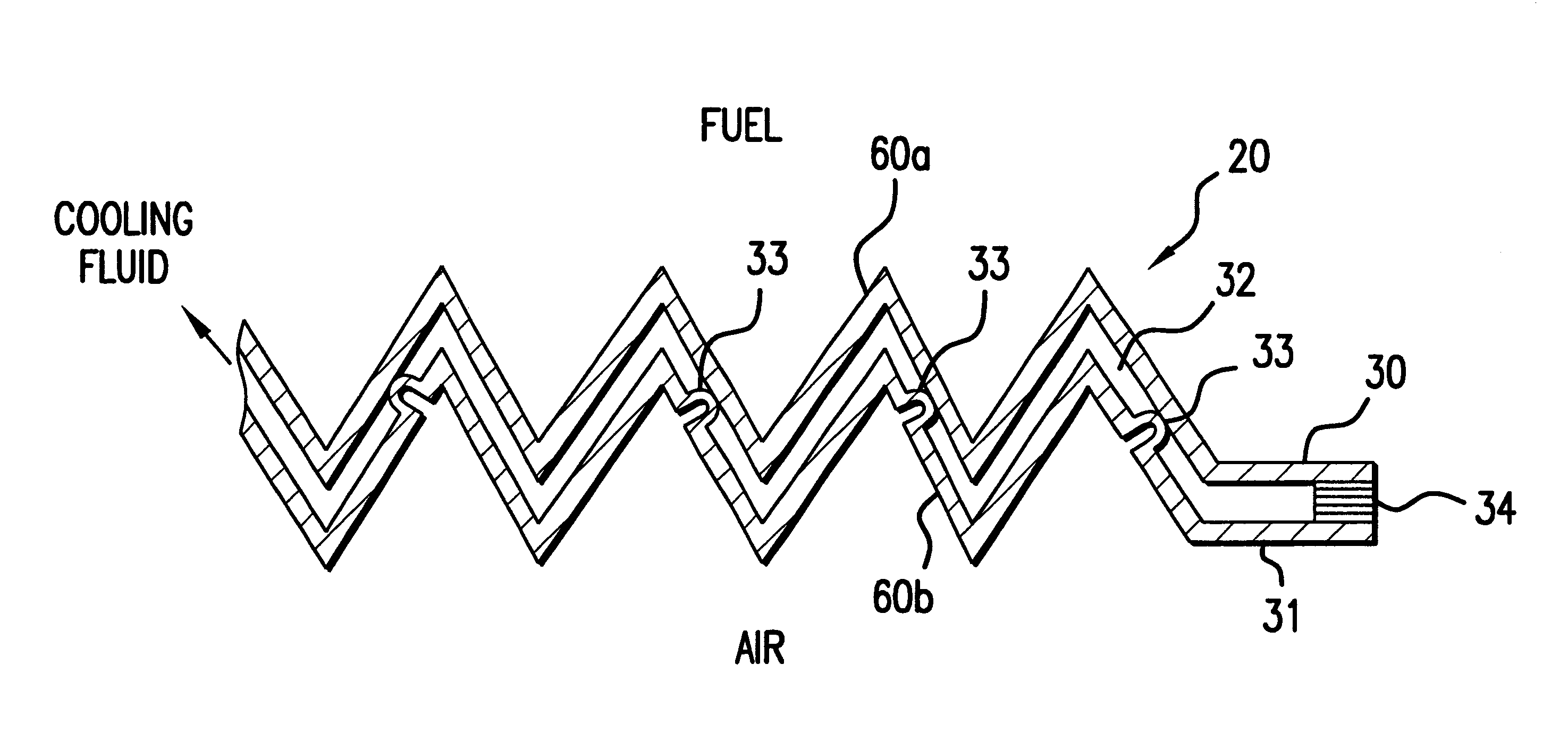
Steam producing hydrocarbon fueled power plant employing a PEM fuel cell
InactiveUS6120923AReduce pressureIncrease steam temperatureHydrogenSolid electrolytesFuel cellsPower station
The present invention relates to a method and apparatus for creating steam from the cooling stream of a proton exchange membrane (PEM) fuel cell. As the cooling stream exits the PEM fuel cell, a portion of the cooling fluid is extracted from the circulating cooling stream, thereby creating a secondary stream of cooling fluid. This secondary stream passes through a restriction, which decreases the pressure of the secondary stream to its saturation pressure, such that when the secondary stream enters a flash evaporator it transforms into steam. Creating steam from the cooling stream of a PEM fuel cell power plant provides the fuel processor with a co-generated source of steam without adding a significant amount of auxiliary equipment to the power plant.
Owner:INT FUEL CELLS
Solid polymer fuel cell with improved voltage reversal tolerance
In a solid polymer fuel cell series, various circumstances can result in the fuel cell being driven into voltage reversal. For instance, cell voltage reversal can occur if that cell receives an inadequate supply of fuel (for example, fuel starvation). In order to pass current during fuel starvation, reactions other than fuel oxidation may take place at the fuel cell anode, including water electrolysis and oxidation of anode components. The latter may result in significant degradation of the anode. Such fuel cells can be made more tolerant to cell reversal by promoting water electrolysis over anode component oxidation at the anode. This can be accomplished by incorporating a catalyst composition at the anode to promote the water electrolysis reaction, in addition to the typical anode electrocatalyst for promoting fuel oxidation.
Owner:BALLARD POWER SYSTEMS +1
Lithium ion conductive solid electrolyte and method for manufacturing the same
ActiveUS20070087269A1Increase battery capacityImprove discharge characteristicsSolid electrolytesPhosphatesLithiumPorosity
Owner:OHARA
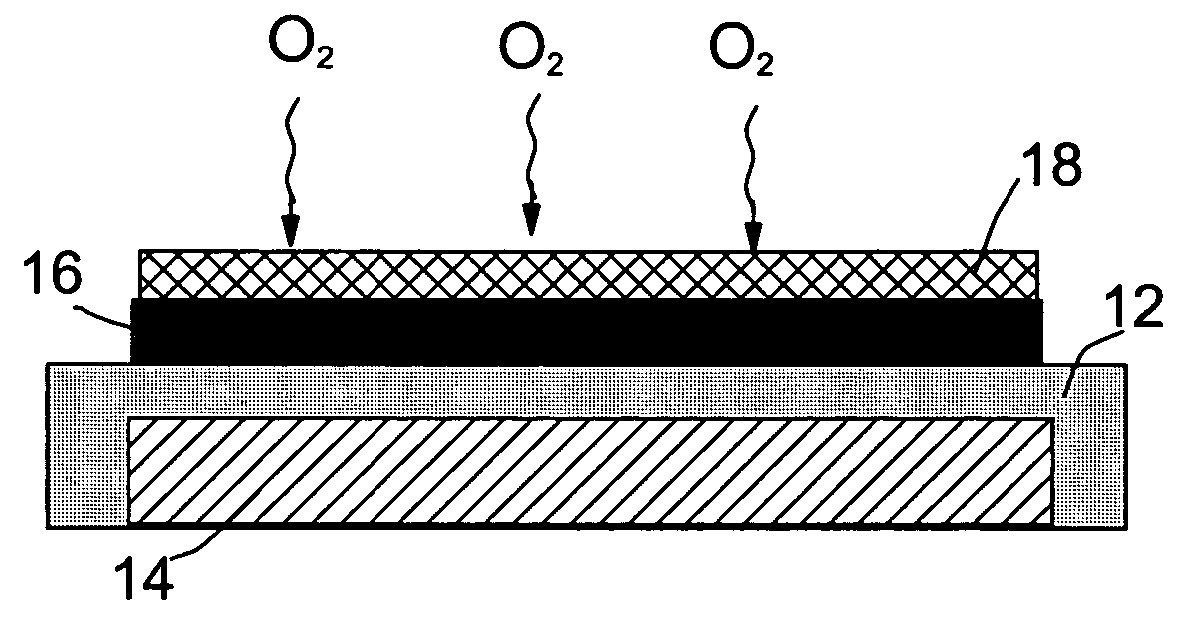
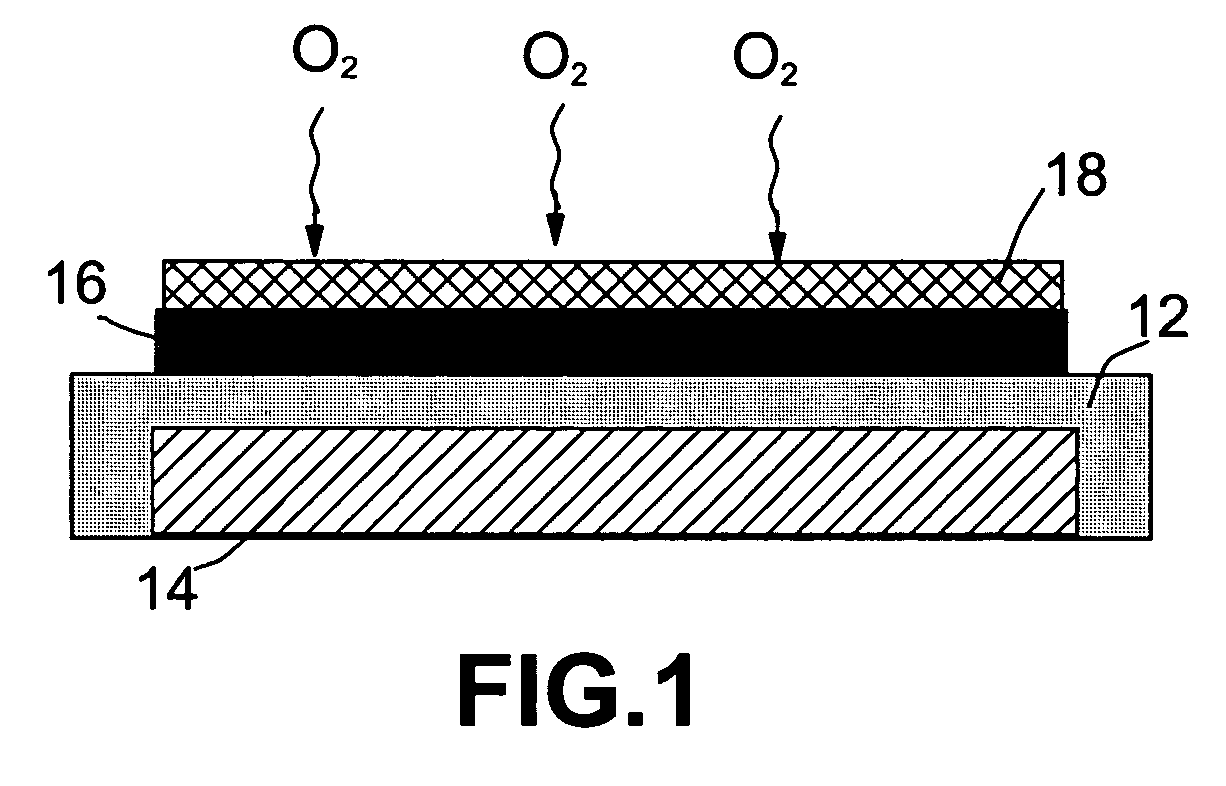
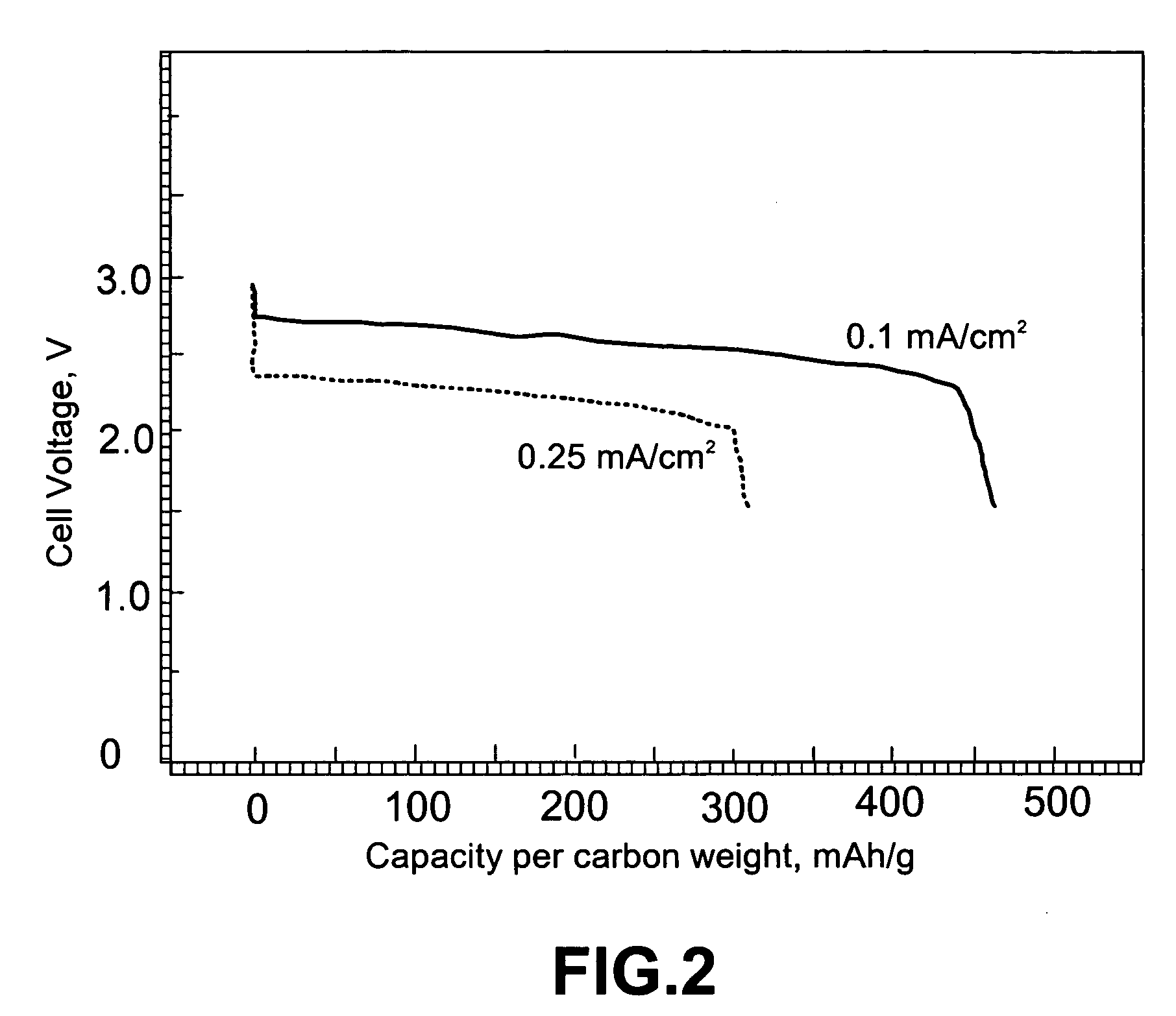
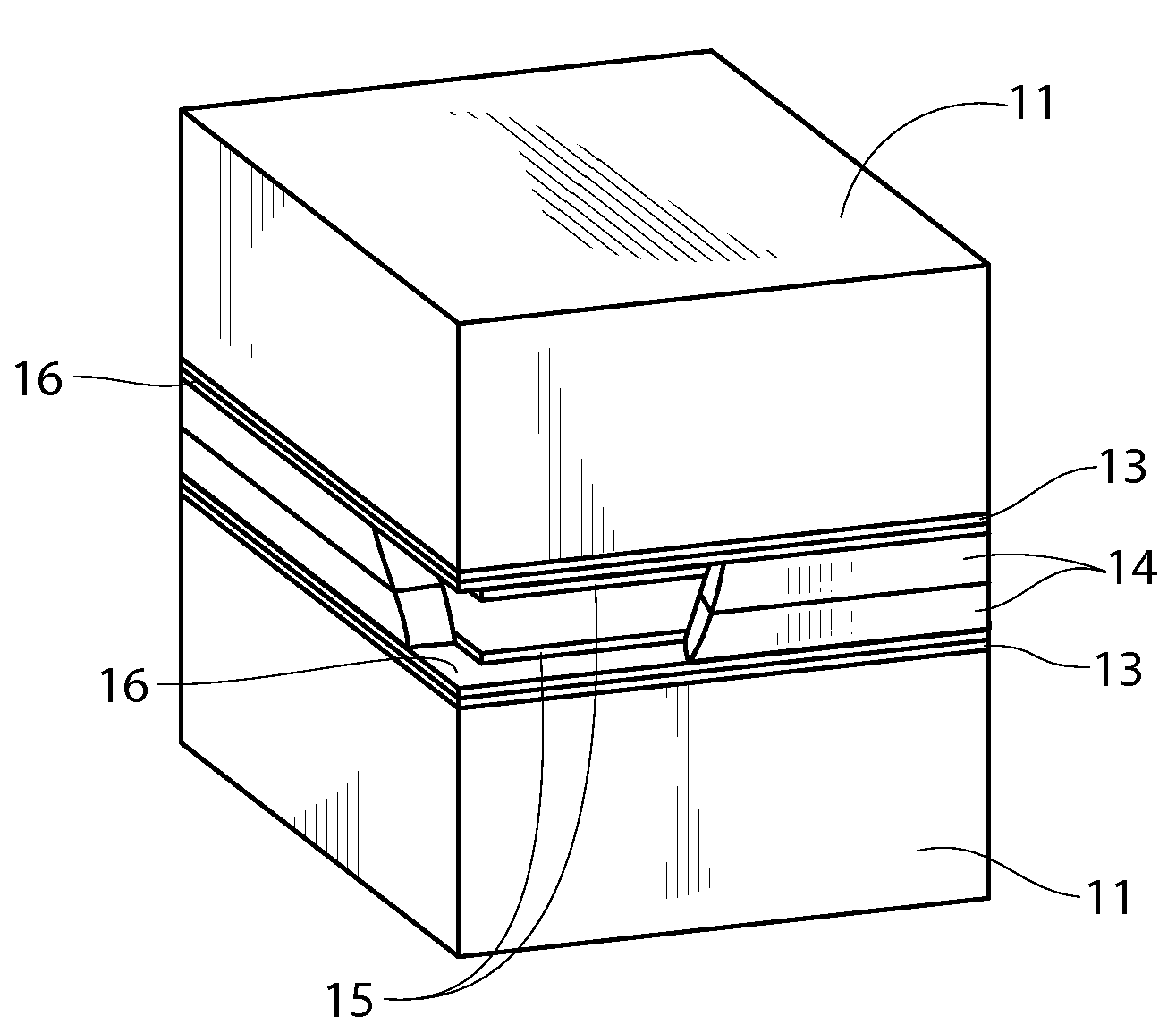
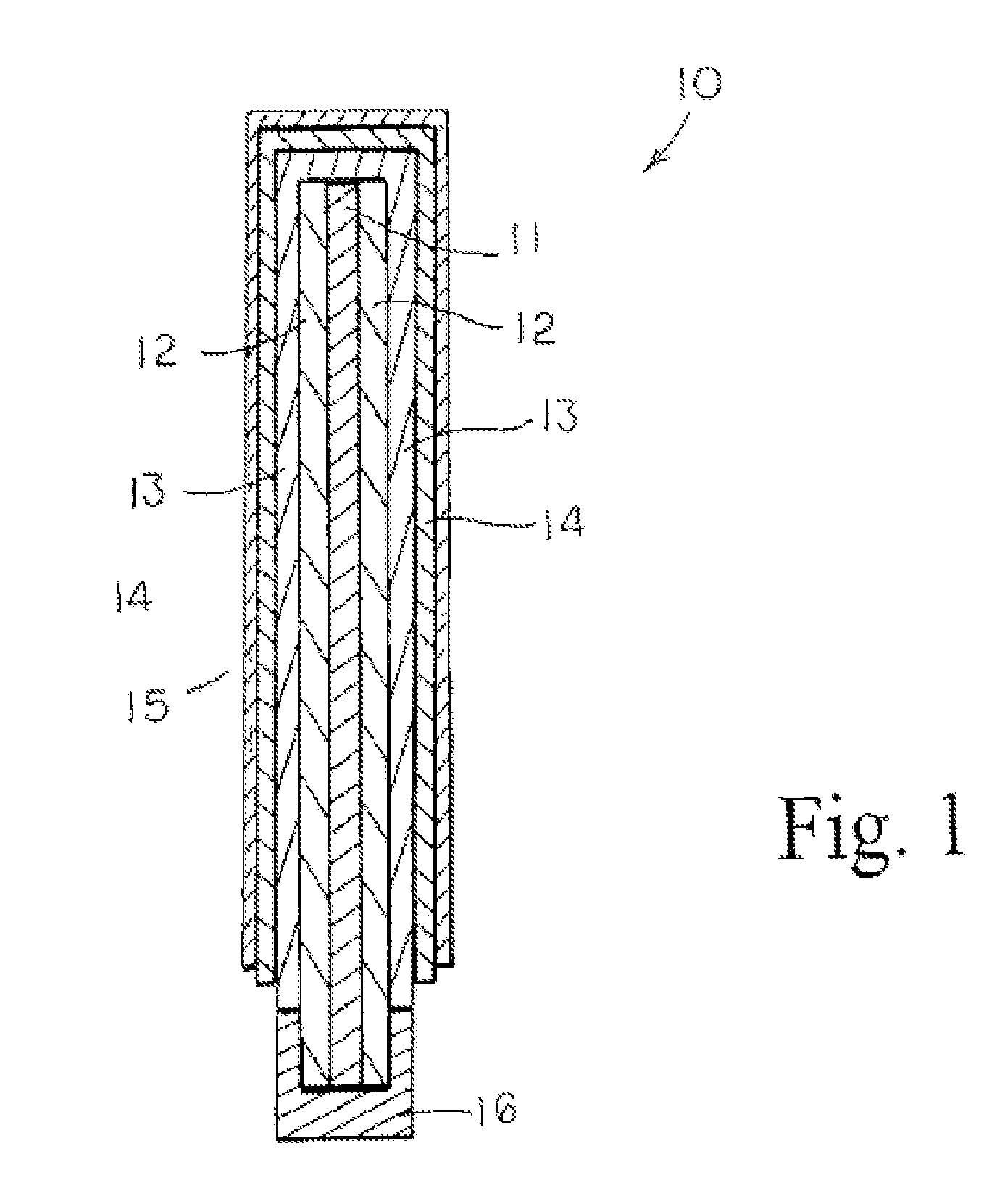
Lithium oxygen batteries and method of producing same
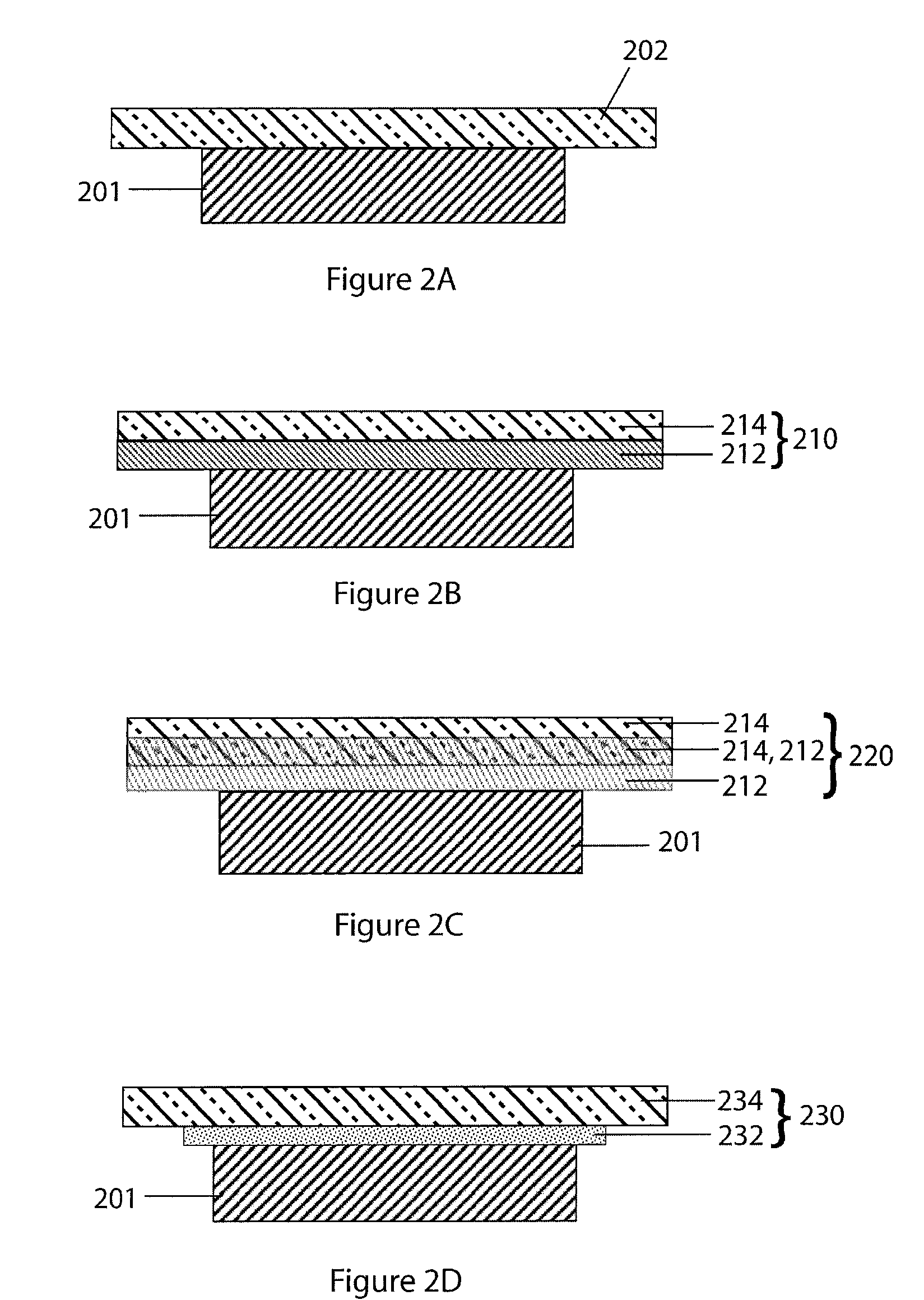
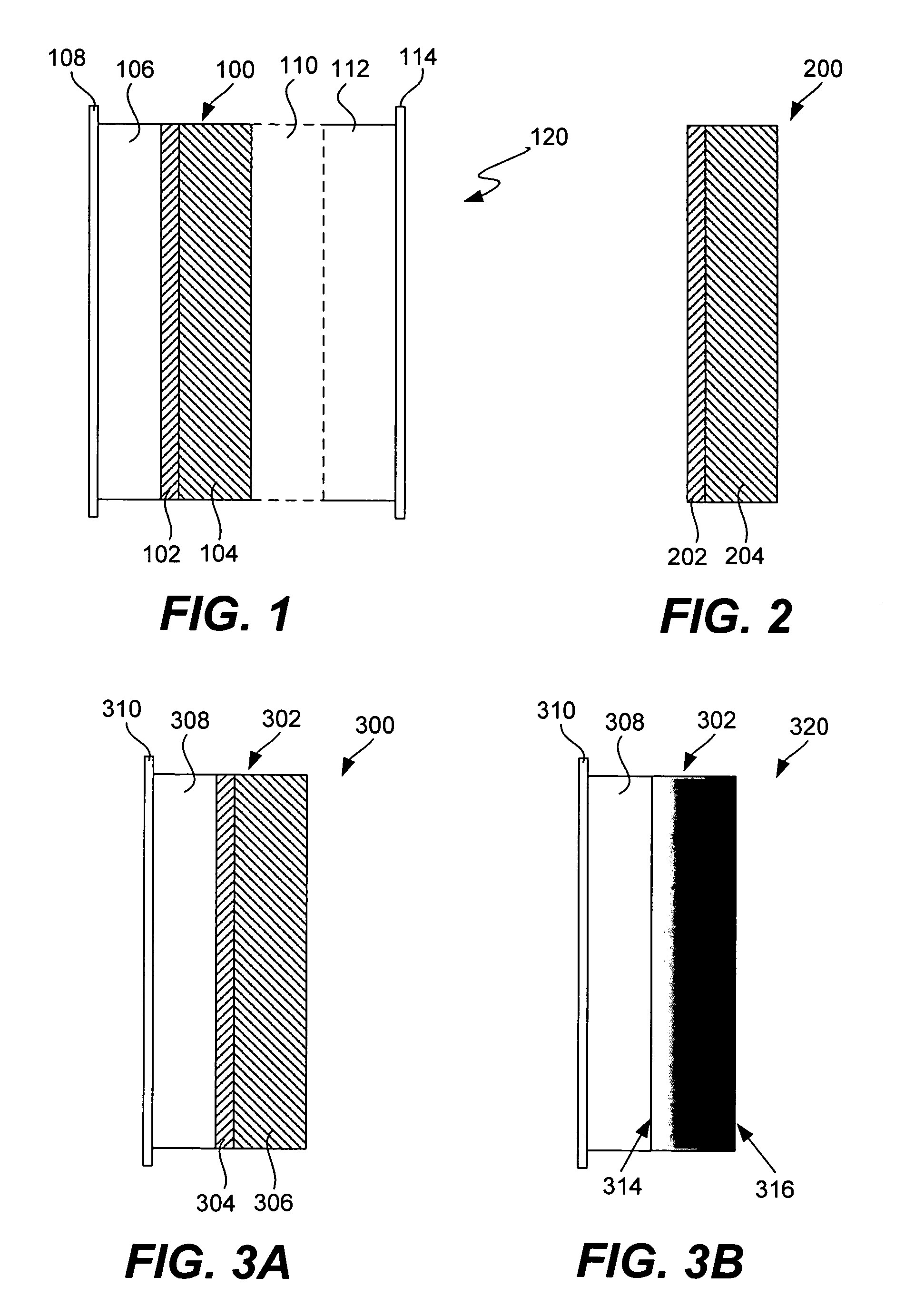
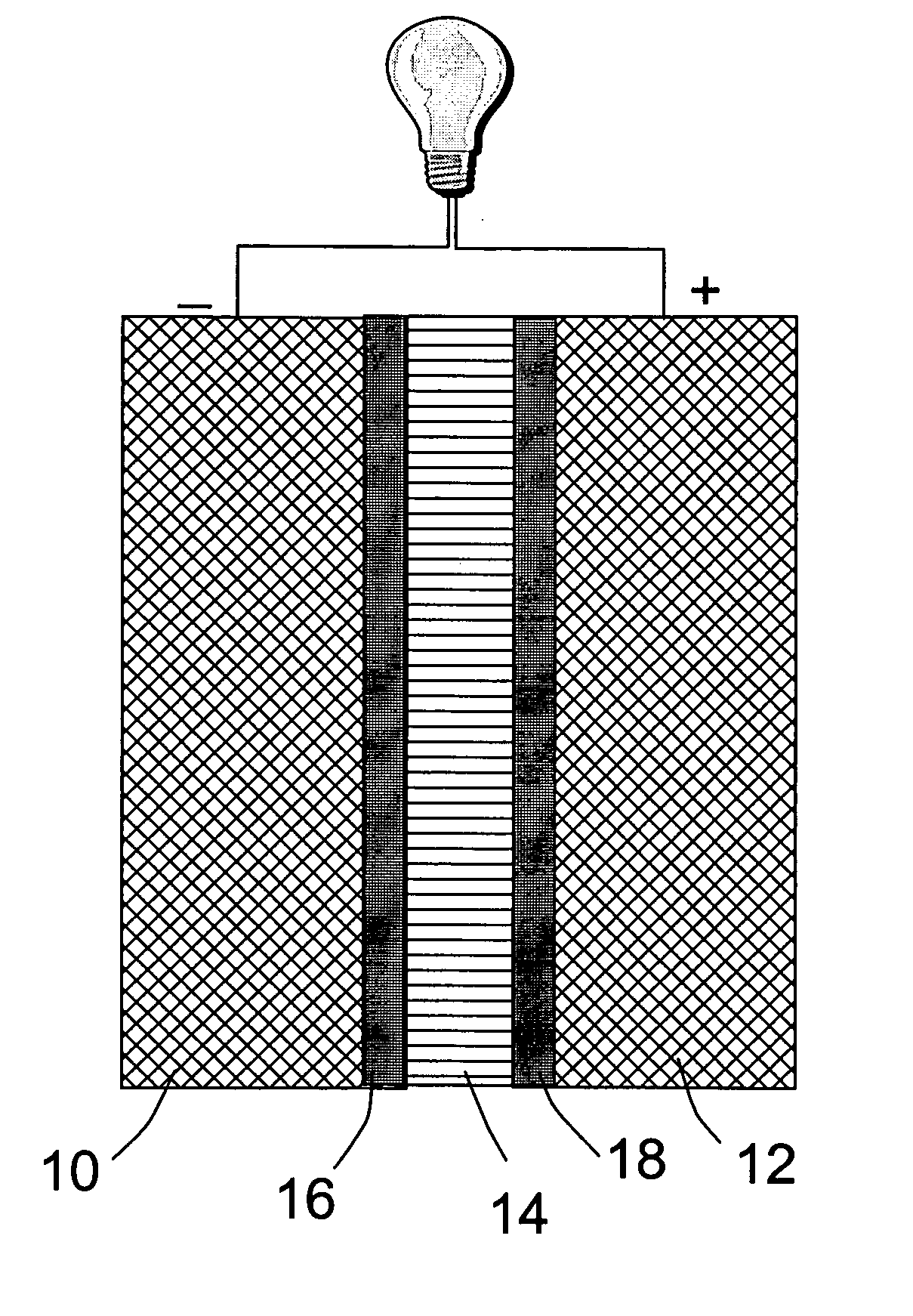
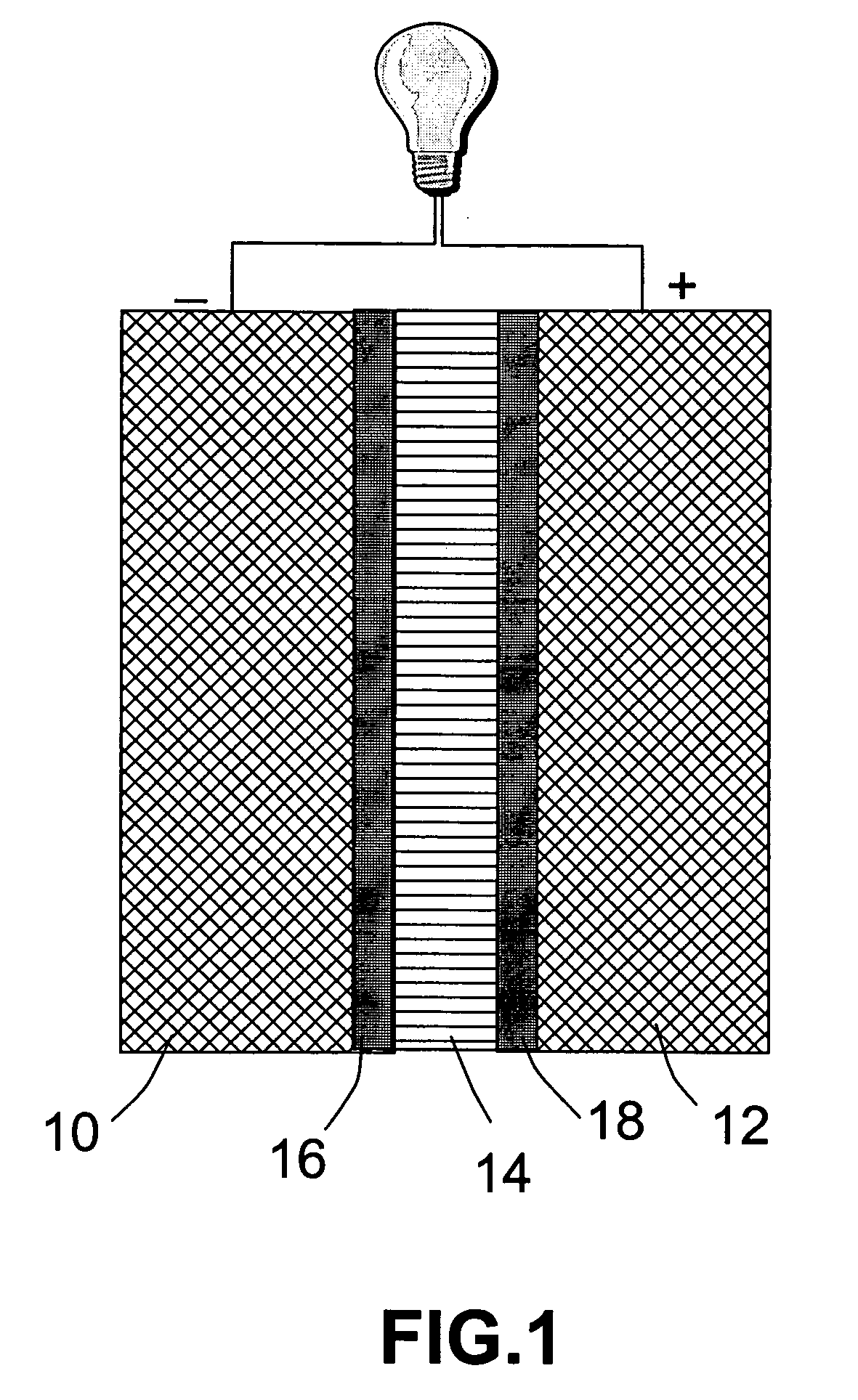
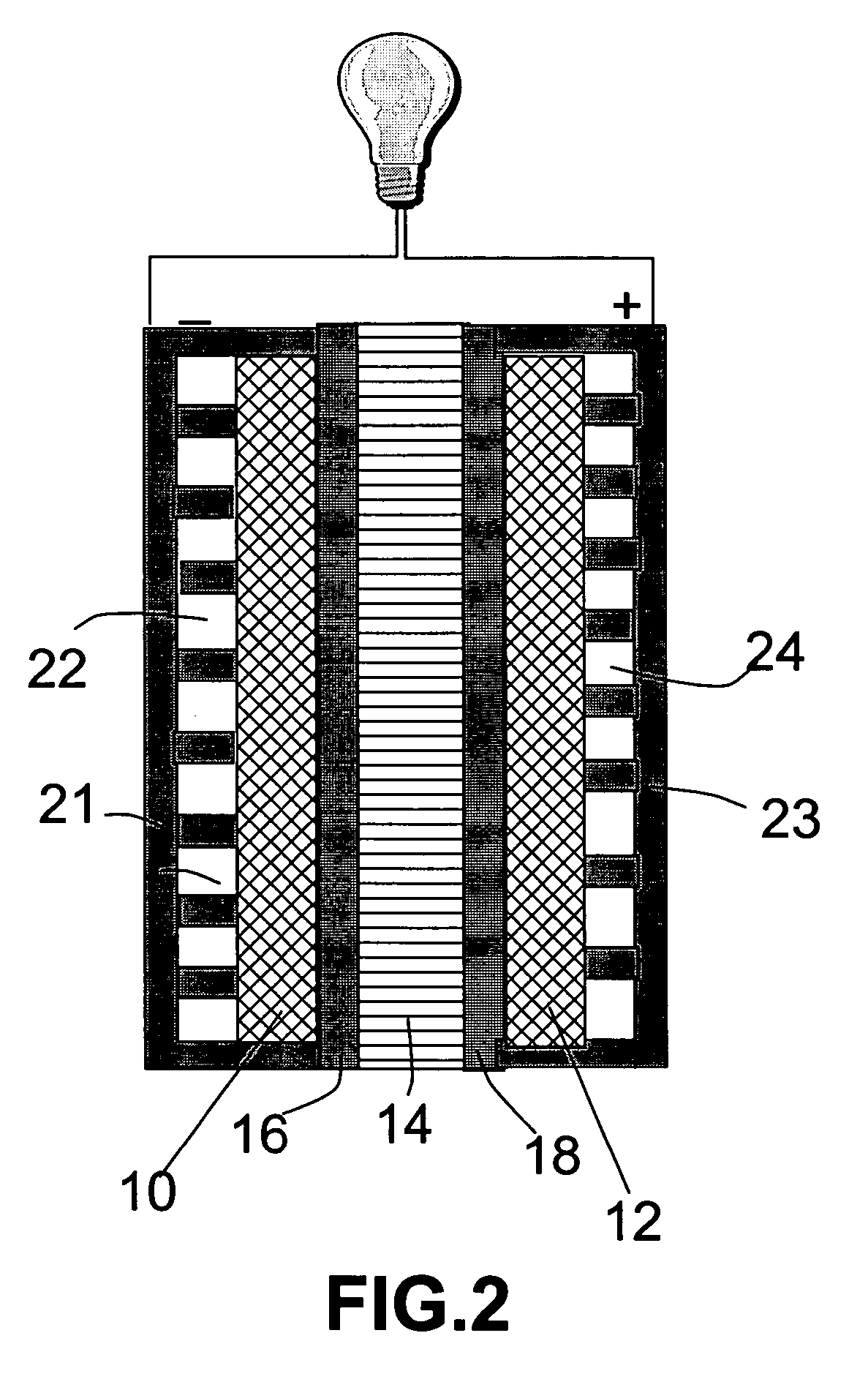
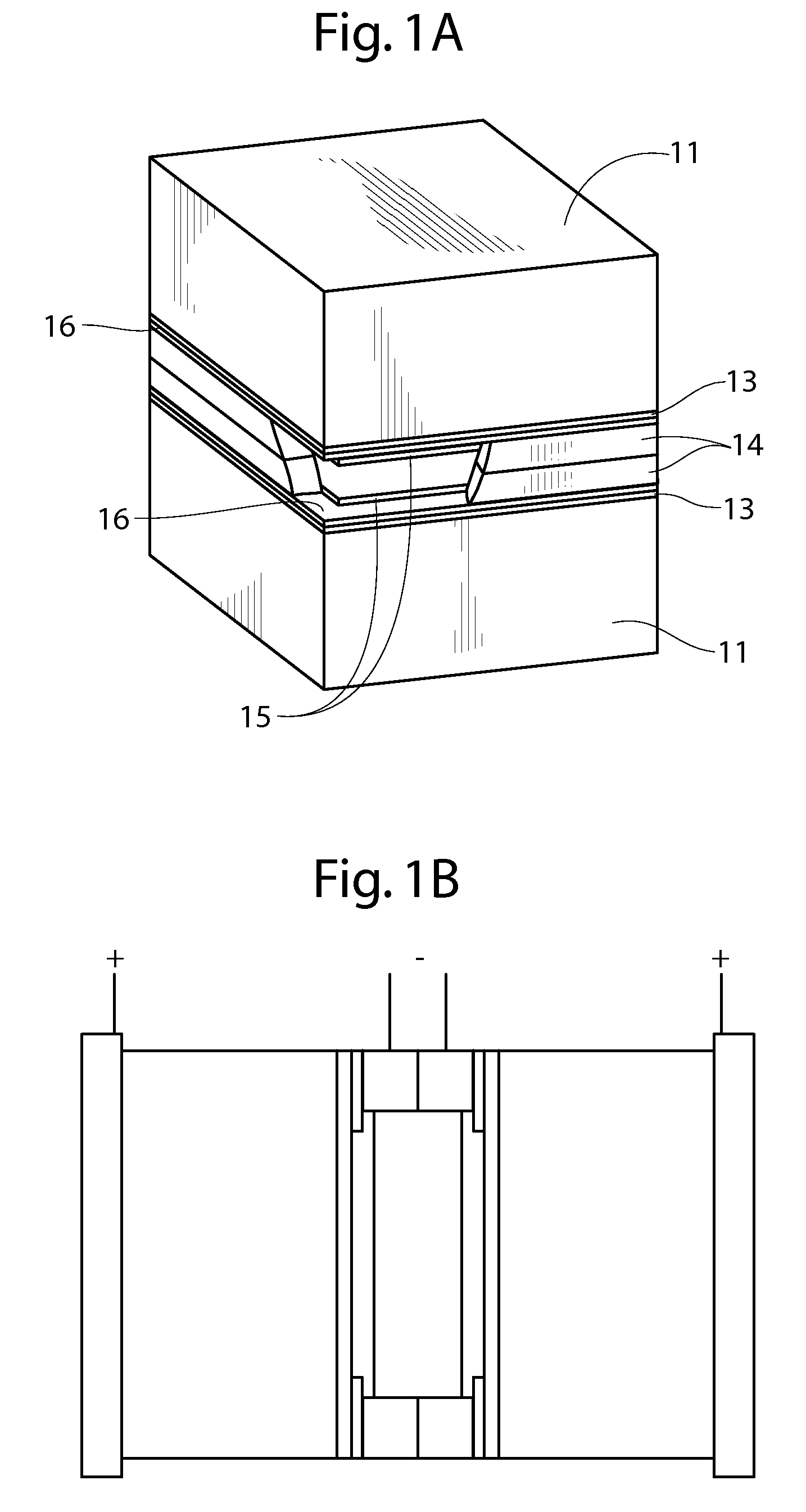
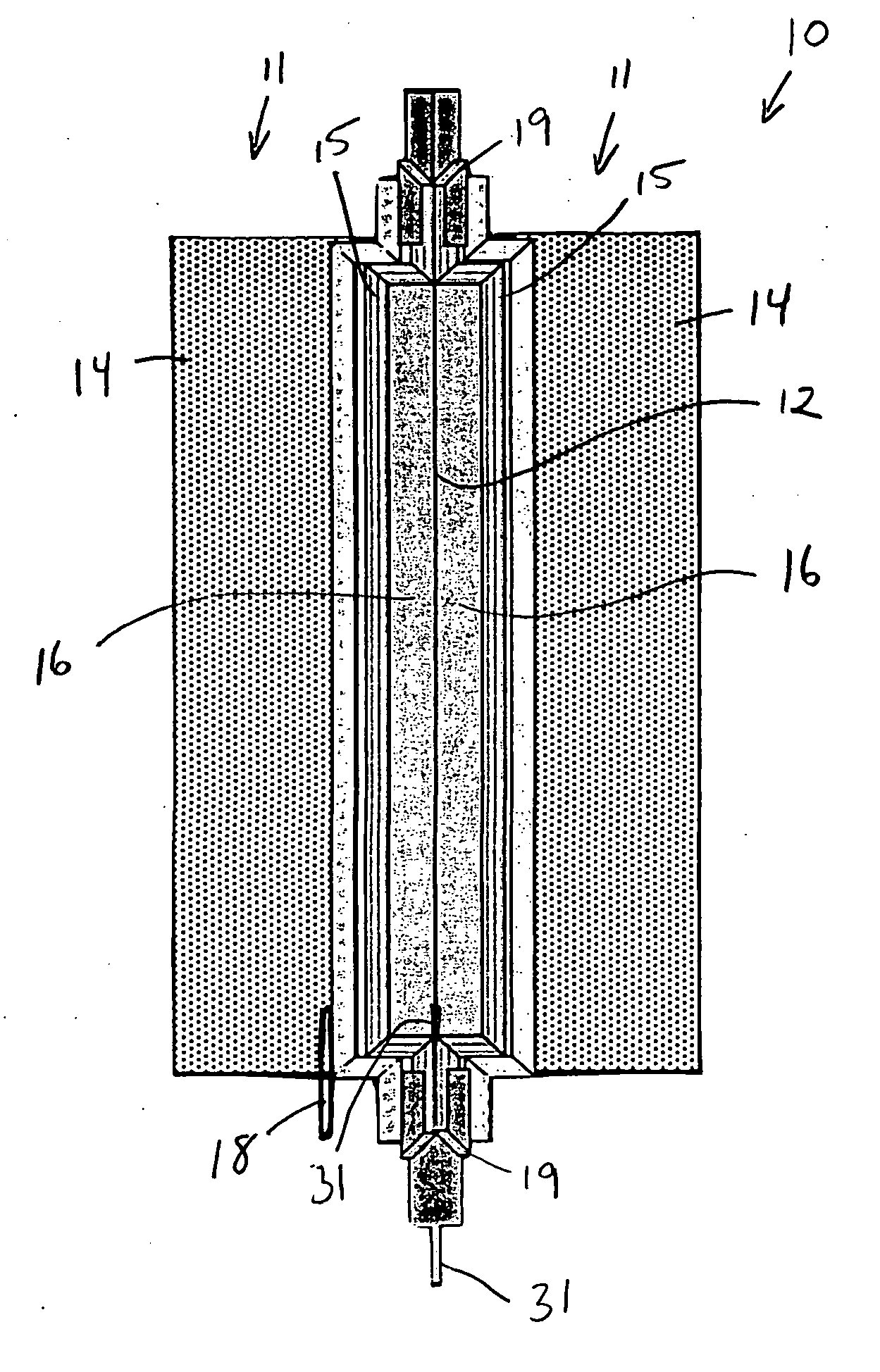
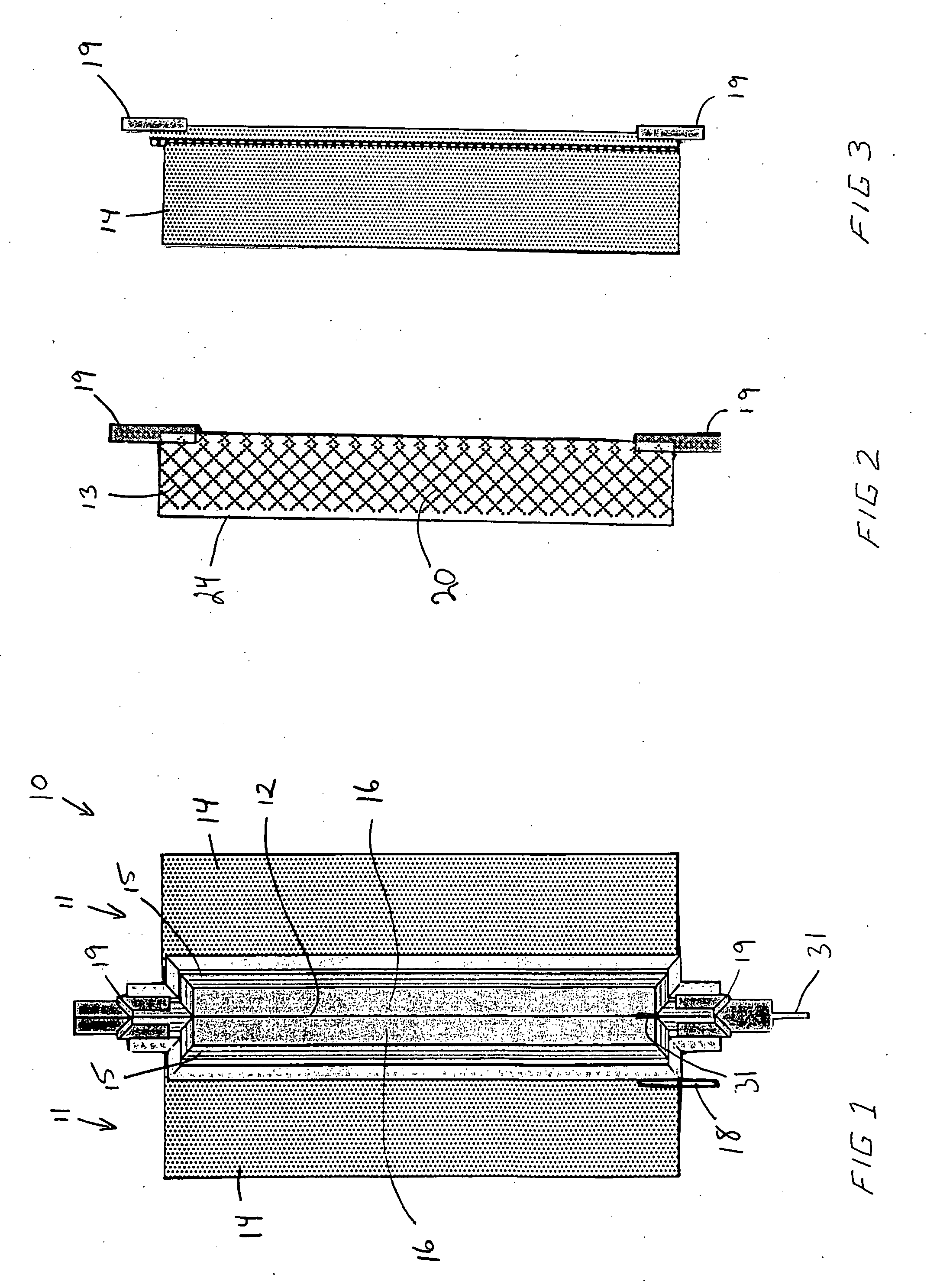
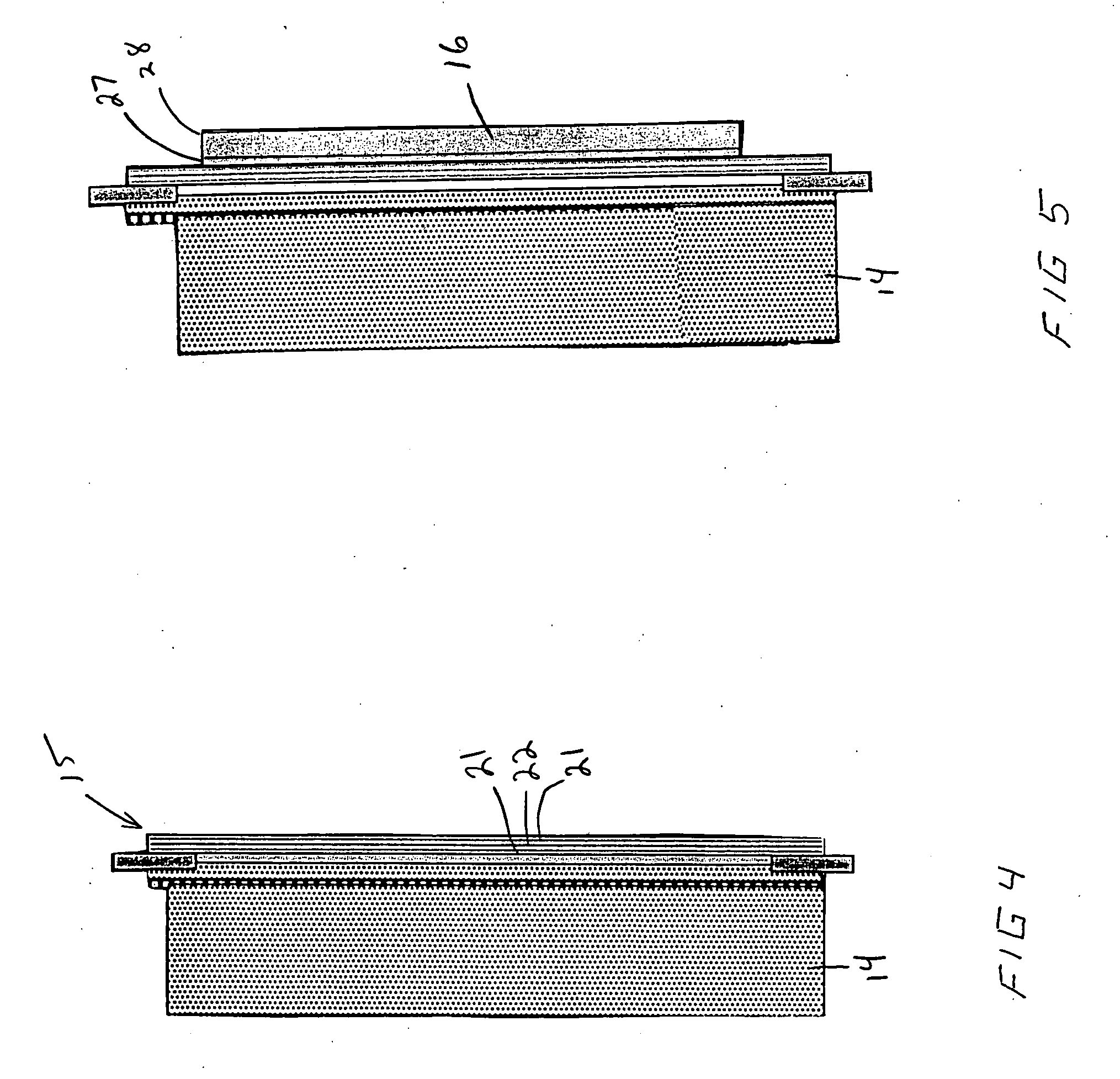
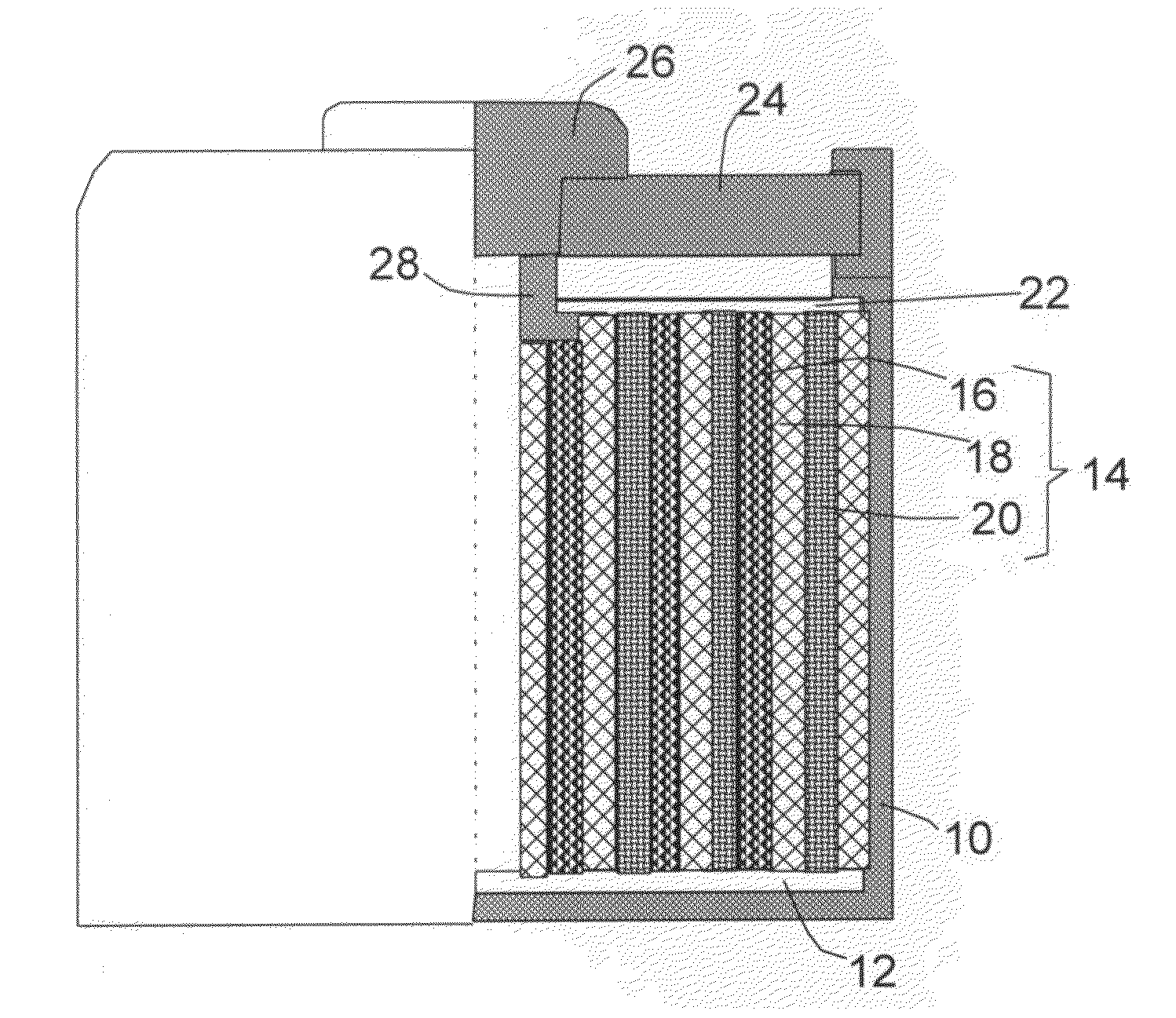
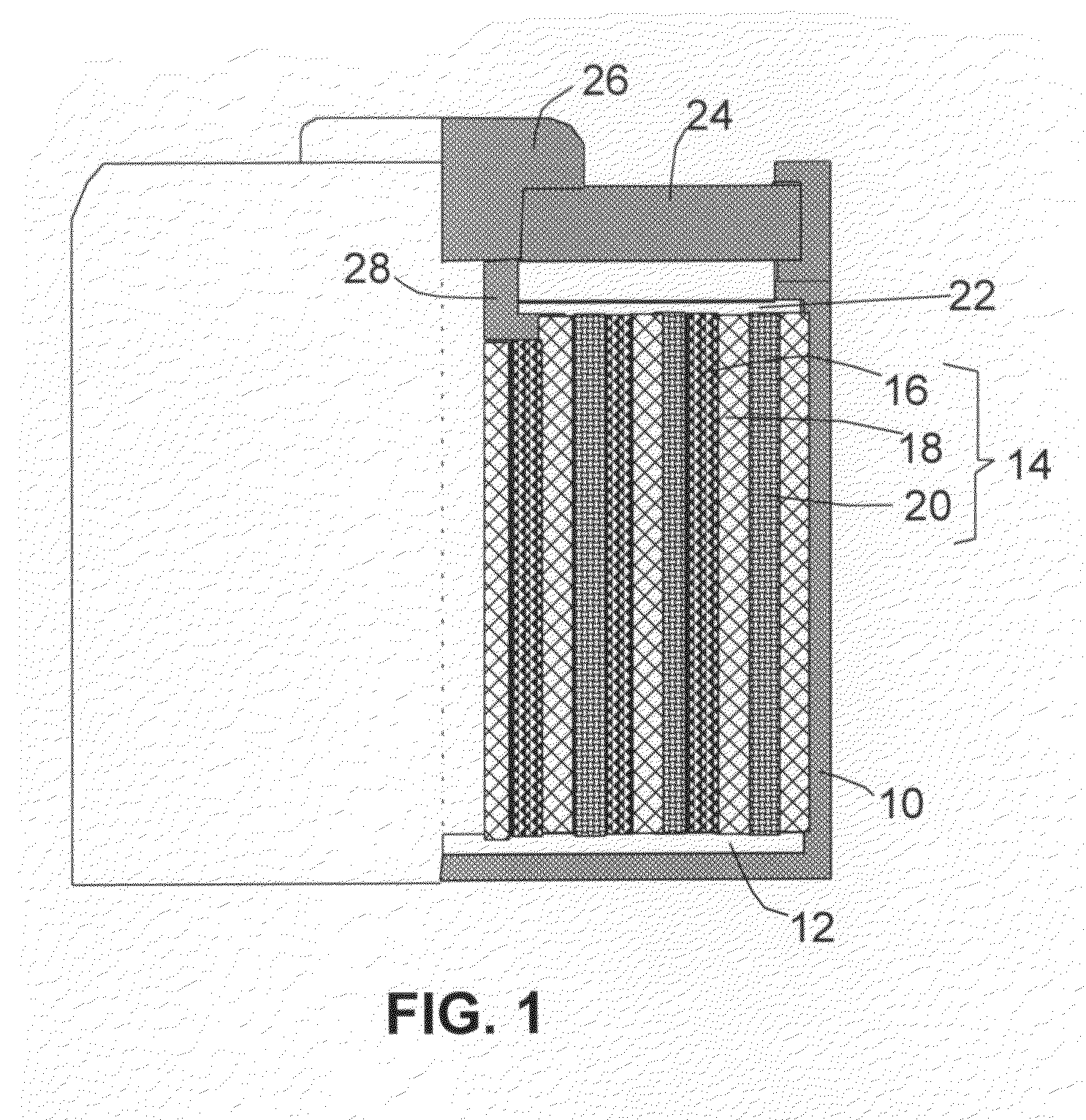
An air lithium battery (10) is provided having two equal halves (11) that are joined together along a centerline (12). Each half includes a substrate (13), a carbon based cathode (14), a solid electrolyte (15), an anode (16), an anode current collector (17), and end seals (19). The solid electrolyte includes alternating layers of ion conductive glass (21) and ion conductive polymer (22) materials.
Owner:JOHNSON IP HLDG LLC
Thin-film batteries with soft and hard electrolyte layers and method
InactiveUS20070015060A1Improve environmental resistanceInhibition formationFinal product manufactureConductive materialLithium metalPolymer gel
A method and apparatus for making thin-film batteries having composite multi-layered electrolytes with soft electrolyte between hard electrolyte covering the negative and / or positive electrode, and the resulting batteries. In some embodiments, foil-core cathode sheets each having a cathode material (e.g., LiCoO2) covered by a hard electrolyte on both sides, and foil-core anode sheets having an anode material (e.g., lithium metal) covered by a hard electrolyte on both sides, are laminated using a soft (e.g., polymer gel) electrolyte sandwiched between alternating cathode and anode sheets. A hard glass-like electrolyte layer obtains a smooth hard positive-electrode lithium-metal layer upon charging, but when very thin, have randomly spaced pinholes / defects. When the hard layers are formed on both the positive and negative electrodes, one electrode's dendrite-short-causing defects on are not aligned with the other electrode's defects. The soft electrolyte layer both conducts ions across the gap between hard electrolyte layers and fills pinholes.
Owner:CYMBET CORP
Reactive deposition for electrochemical cell production
Light reactive deposition can be adapted effectively for the deposition of one or more electrochemical cell components. In particular, electrodes, electrolytes, electrical interconnects can be deposited form a reactive flow. In some embodiments, the reactive flow comprises a reactant stream that intersects a light beam to drive a reaction within a light reactive zone to produce product that is deposited on a substrate. The approach is extremely versatile for the production of a range of compositions that are useful in electrochemical cells and fuel cell, in particular. The properties of the materials, including the density and porosity can be adjusted based on the deposition properties and any subsequent processing including, for example, heat treatments.
Owner:NANOGRAM
Novel composite cathodes, electrochemical cells comprising novel composite cathodes, and processes for fabricating same
The present invention pertains to composite cathodes suitable for use in an electrochemical cell, said cathodes comprising: (a) an electroactive sulfur-containing cathode material, wherein said electroactive sulfur-containing cathode material, in its oxidized state, comprises a polysulfide moiety of the formula —Sm—, wherein m is an integer equal to or greater than 3; and, (b) an electroactive transition metal chalcogenide composition, which encapsulates said electroactive sulfur-containing cathode material, and which retards the transport of anionic reduction products of said electroactive sulfur-containing cathode material, said electroactive transition metal chalcogenide composition comprising an electroactive transition metal chalcogenide having the formula MjYk(OR)l wherein: M is a transition metal; Y is the same or different at each occurrence and is oxygen, sulfur, or selenium; R is an organic group and is the same or different at each occurrence; j is an integer ranging from 1 to 12; k is a number ranging from 0 to 72; and l is a number ranging from 0 to 72; with the proviso that k and l cannot both be 0. The present invention also pertains to methods of making such composite cathodes, cells comprising such composite cathodes, and methods of making such cells.
Owner:SION POWER CORP
Anode compositions for lithium secondary batteries
ActiveUS20110165462A1High specific capacityHigh reversible capacitySolid electrolytesFuel and secondary cellsNano structuringElectrical battery
A lithium secondary battery comprising a cathode, an anode, and a separator-electrolyte assembly or electrolyte layer disposed between the cathode and the anode, wherein the anode comprises: (a) an integrated nano-structure of electrically conductive nanometer-scaled filaments that are interconnected to form a porous network of electron-conducting paths comprising interconnected pores, wherein the filaments have a transverse dimension less than 500 nm; and (b) a foil of lithium or lithium alloy as an anode active material. The battery exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
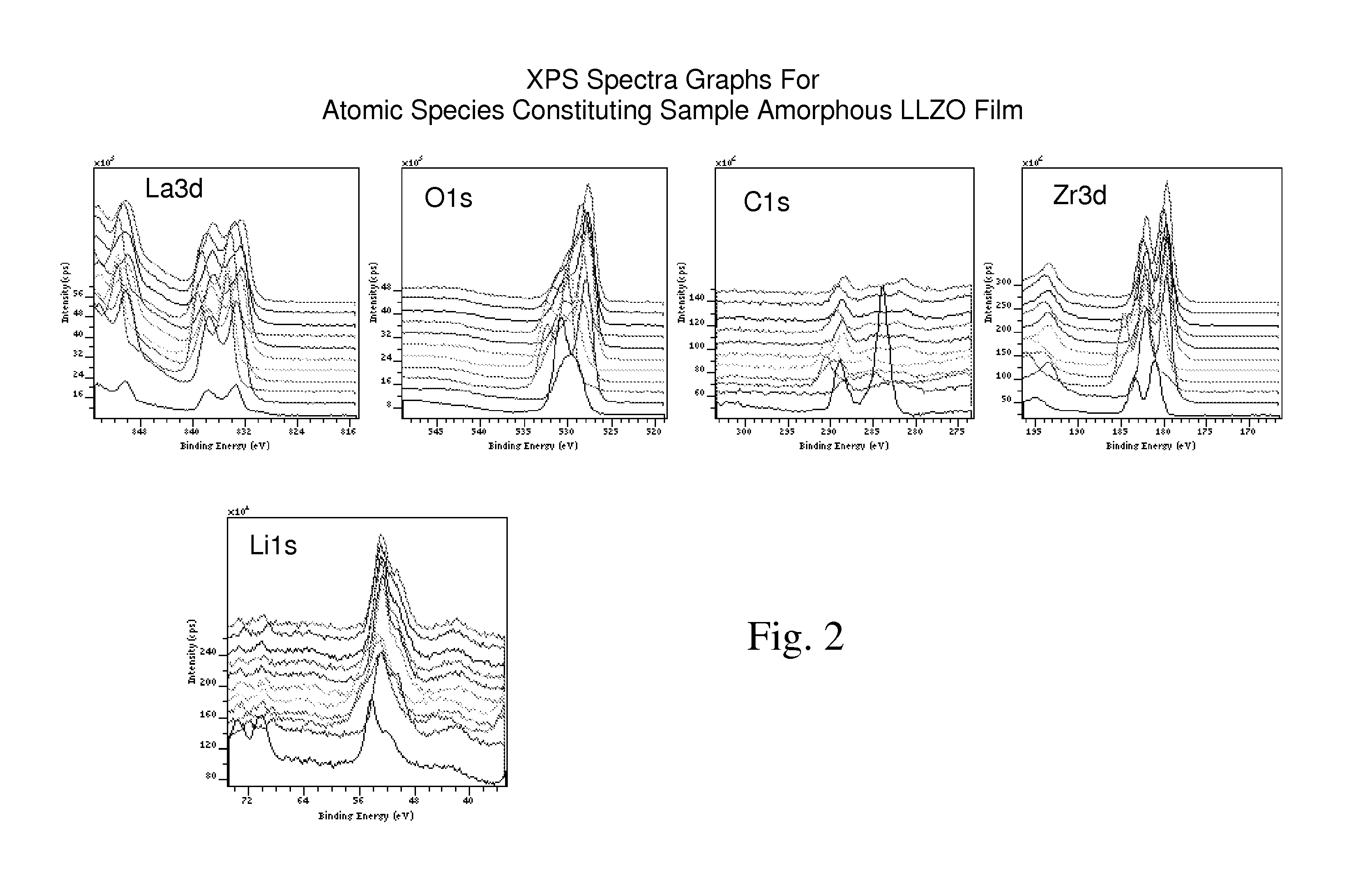
Ionically-conductive amorphous lithium lanthanum zirconium oxide
Amorphous lithium lanthanum zirconium oxide (LLZO) is formed as an ionically-conductive electrolyte medium. The LLZO comprises by percentage of total number of atoms from about 0.1% to about 50% lithium, from about 0.1% to about 25% lanthanum, from about 0.1% to about 25% zirconium, from about 30% to about 70% oxygen and from 0.0% to about 25% carbon. At least one layer of amorphous LLZO may be formed through a sol-gel process wherein quantities of lanthanum methoxyethoxide, lithium butoxide and zirconium butoxide are dissolved in an alcohol-based solvent to form a mixture which is dispensed into a substantially planar configuration, transitioned through a gel phase, dried and cured to a substantially dry phase.
Owner:JOHNSON IP HLDG LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com