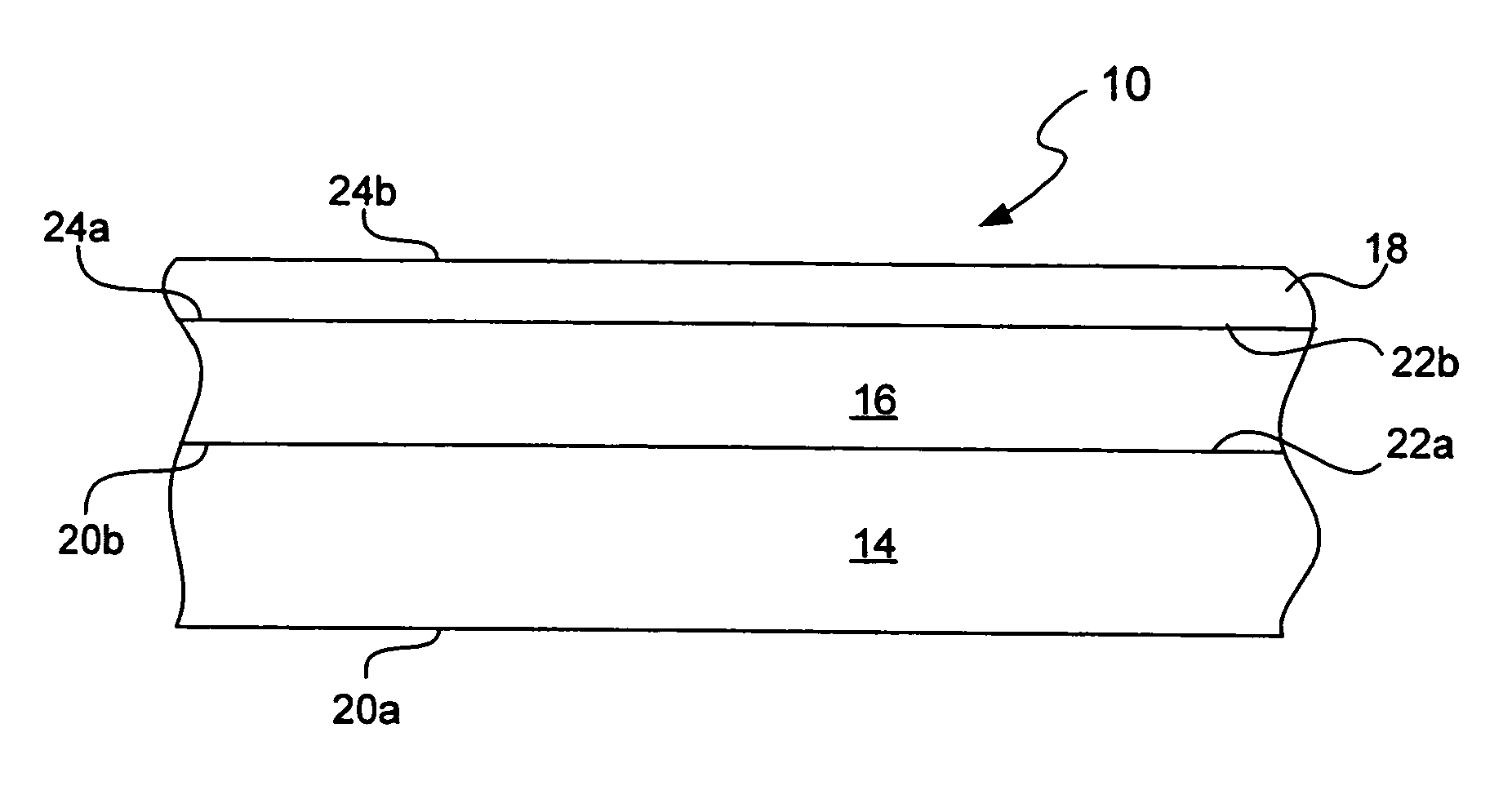
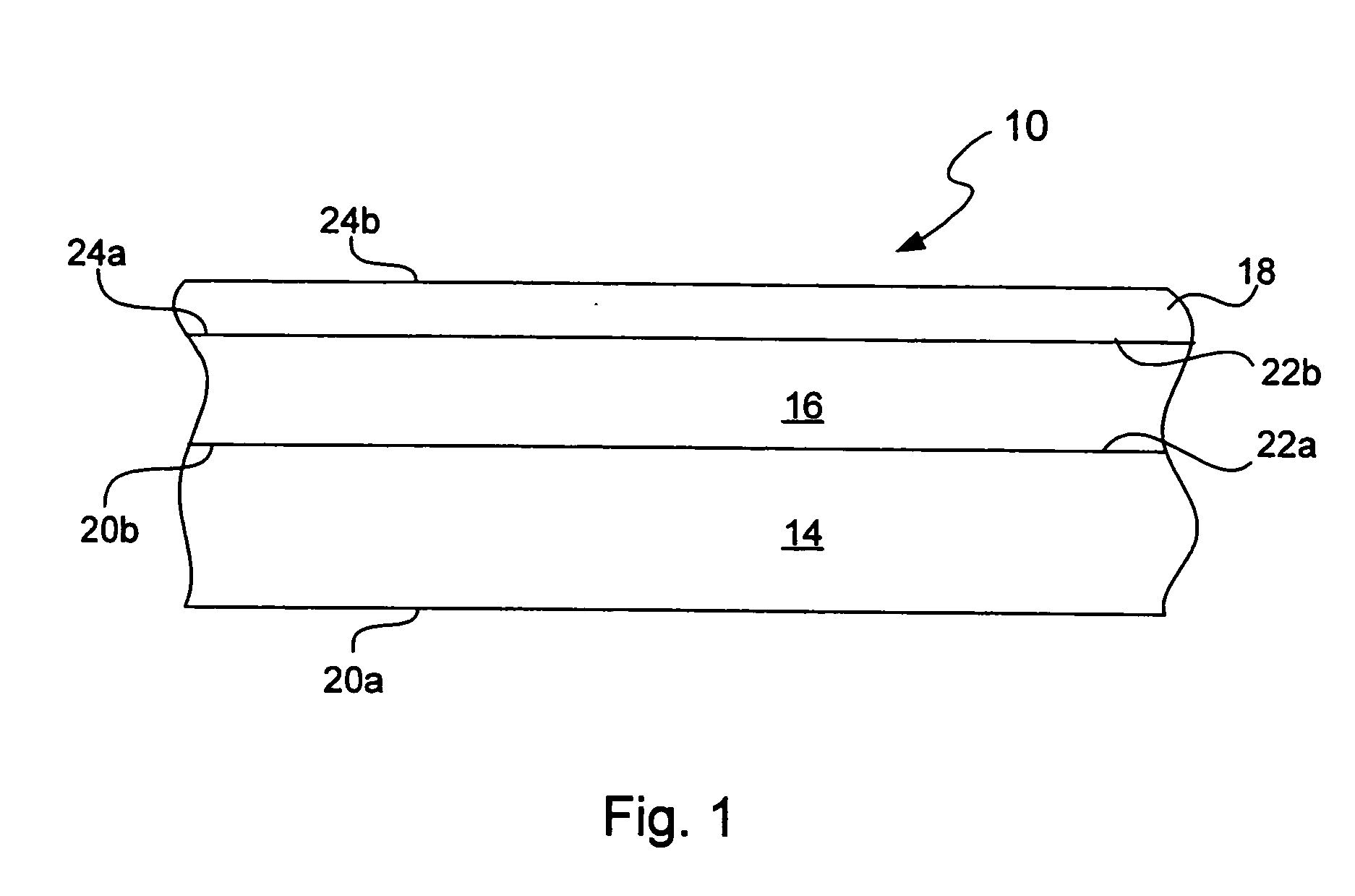
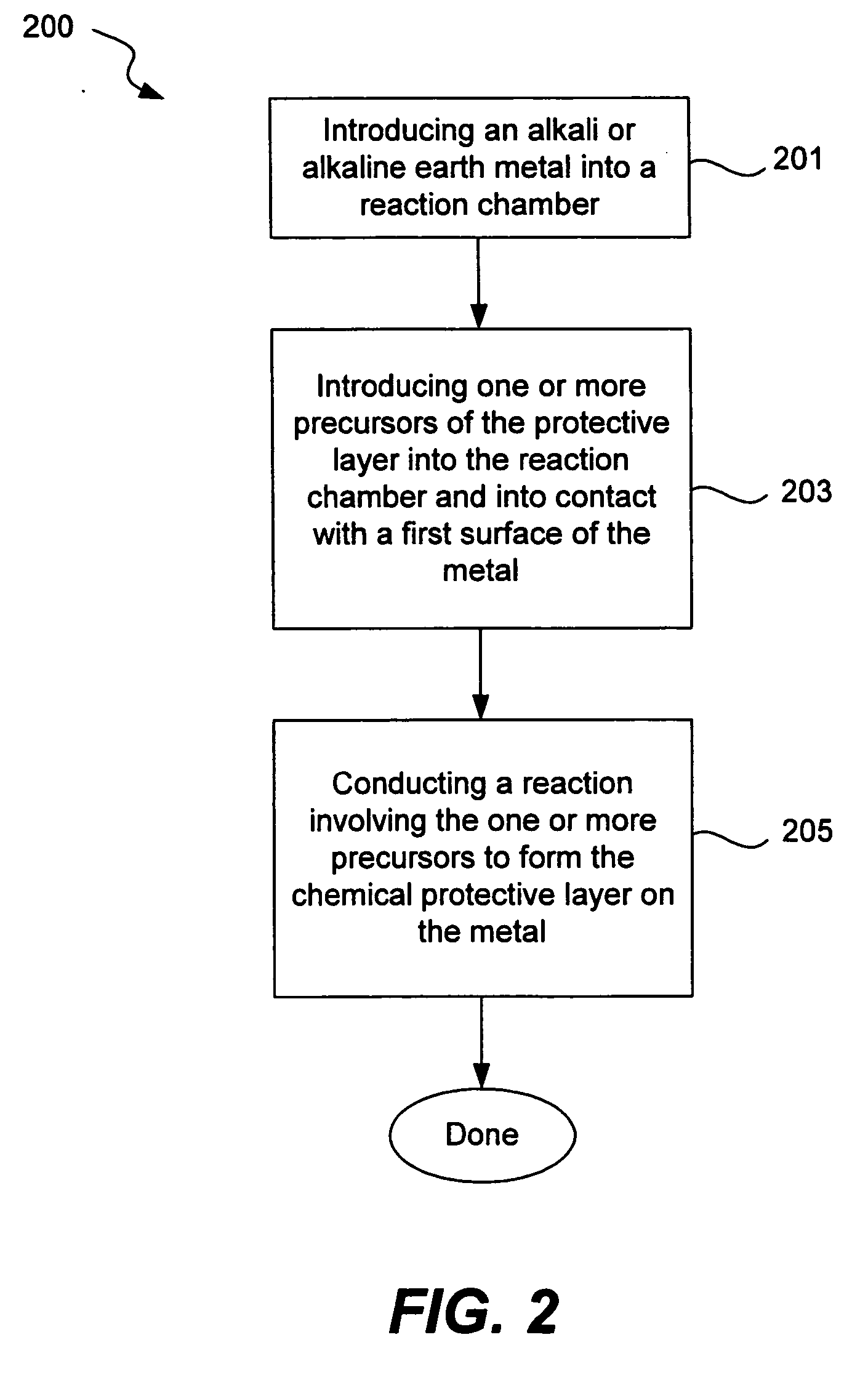
Chemical protection of a lithium surface
a lithium surface and surface treatment technology, applied in the field of surface treatment, can solve the problems of lithium being highly reactive, lithium may adversely react with incompatible materials in the processing environment, and requires special handling, and achieve the effect of reducing the complexity of lithium production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Protective Lithium Phosphate Film by Li Surface Treatment with Phosphoric Acid
[0079] The Li electrode surface (125 micron foil from Cyprus Foote) was treated with dry DME containing anhydrous phosphoric acid (1500 ppm) for a treatment time of 45 seconds. Surface treatment was conducted by coating of the Li foil pressed onto SS current collector with this solution followed by DME evaporation. About 1.0 ml of the solution was put on Li surface. After Li reaction with phosphoric acid and formation of lithium phosphate layer on the Li surface, DME was allowed to evaporate at room temperature. Residual unreacted phosphoric acid on the surface was rinsed out by a large volume of DME. In some experiments before treatment with phosphoric acid Li surface was polished with Tyvek fabric (1509 B). All described operations were conducted in an argon-filled glove box.
[0080] Electrochemical cells containing a Li electrode coated with a lithium phosphate chemical protective underlay...
example 2
Production of Protective Lithium Phosphate Underlayer by Li Surface Treatment with Phosphoric Acid
[0081] The Li electrode surface (125 um foil from Cyprus Foote) was treated with dry DME containing anhydrous phosphoric acid as described in Example 1. After the pre-treatment Li foil was transferred to the sputtering chamber for reactive RF sputtering of LiPON glass layer using lithium phosphate target of 8 inch diameter in the presence of nitrogen. RF power was 100 W, and duration of sputtering was about 1.5 hrs. No evidence of reaction between nitrogen and Li and formation of black lithium nitride reaction product was observed and the LiPON layer was successfully deposited onto Li surface. In experiments where described Li pre-treatment with acid was not used, the Li surface was attacked with nitrogen and almost immediately covered with black lithium nitride film. Therefore, Li chemical treatment with phosphoric acid creates a protective underlayer that allows for direct reactive s...
example 3
Production of Protective Lithium Phosphate Underlayer by Sputtering of Lithium Phosphate onto Li Surface
[0083] The Li electrode (125 micron foil from Cyprus Foote) was transferred to the sputtering chamber and lithium phosphate was sputtered onto the Li surface. Sputtering was conducted in an atmosphere of pure Ar at RF power 100 W. After about 1 hr of sputtering, nitrogen was introduced into the chamber and the LiPON layer about 0.1 micron thick was sputtered onto the Li surface. No evidence of reaction between nitrogen and Li and formation of black lithium nitride reaction product was observed. This demonstrates that Li surface coating with dense lithium phosphate underlayer protects Li surface from nitrogen attack and allows for direct LiPON sputtering onto Li.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com