Patents
Literature
13883 results about "Current collector" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
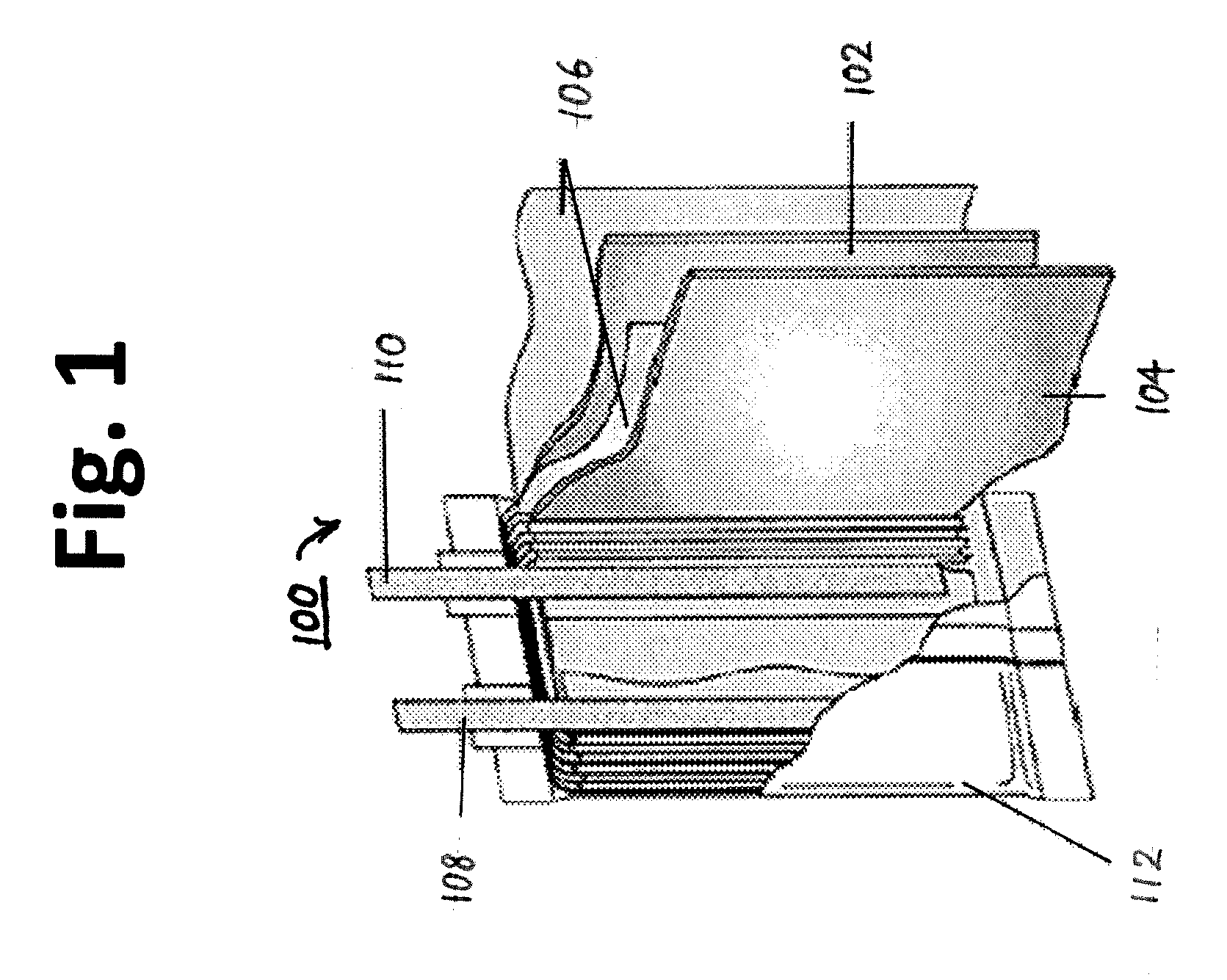
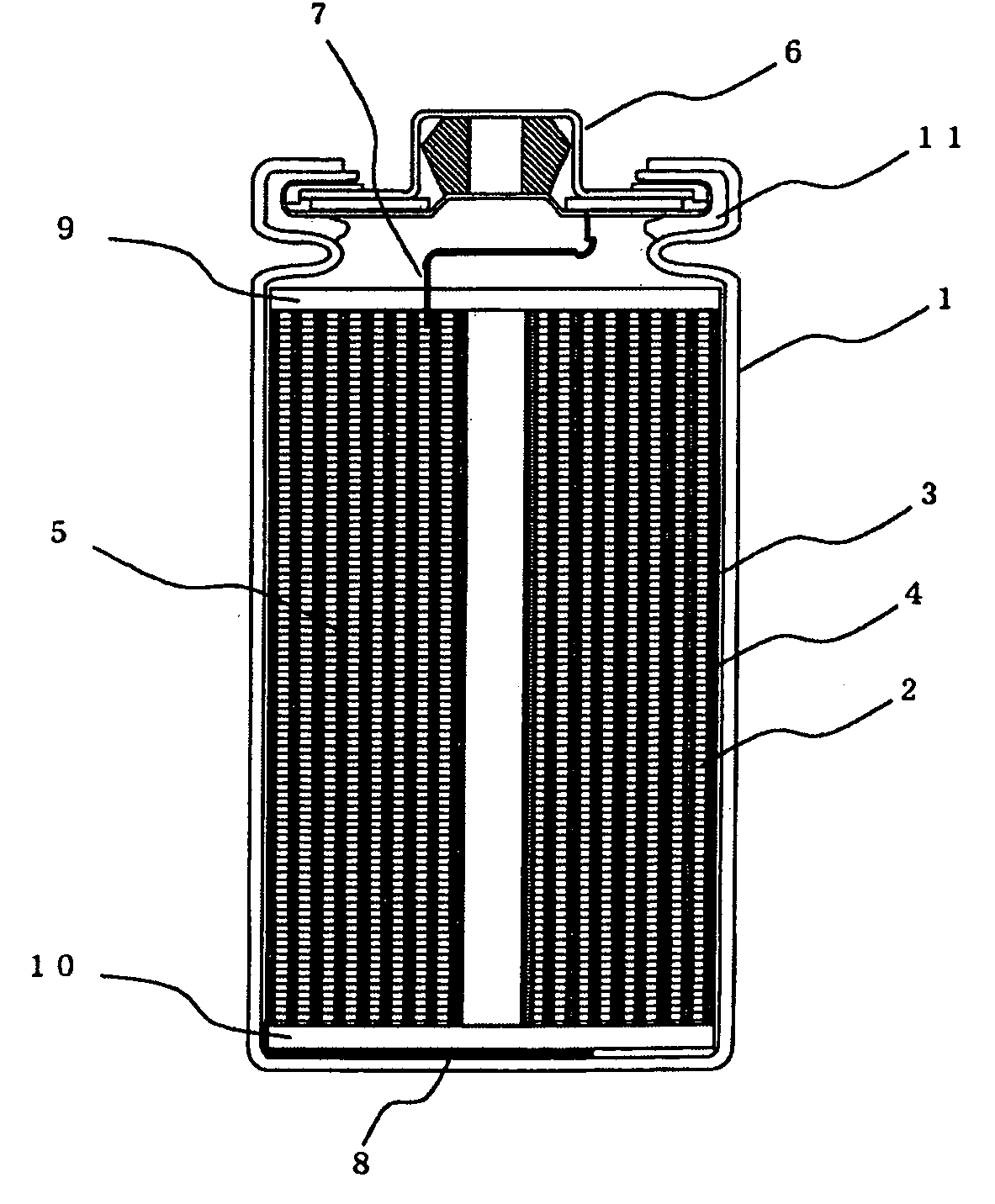
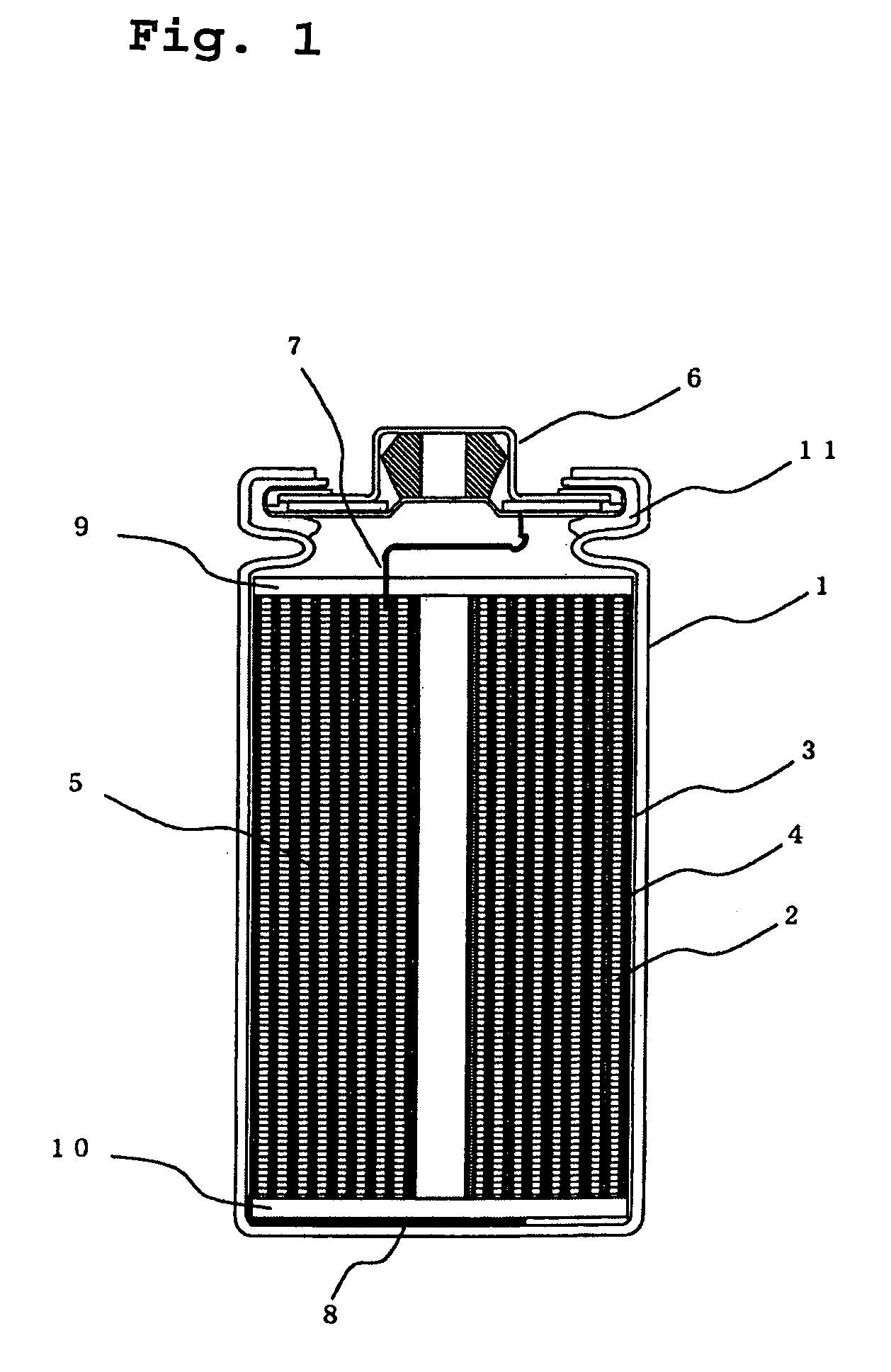
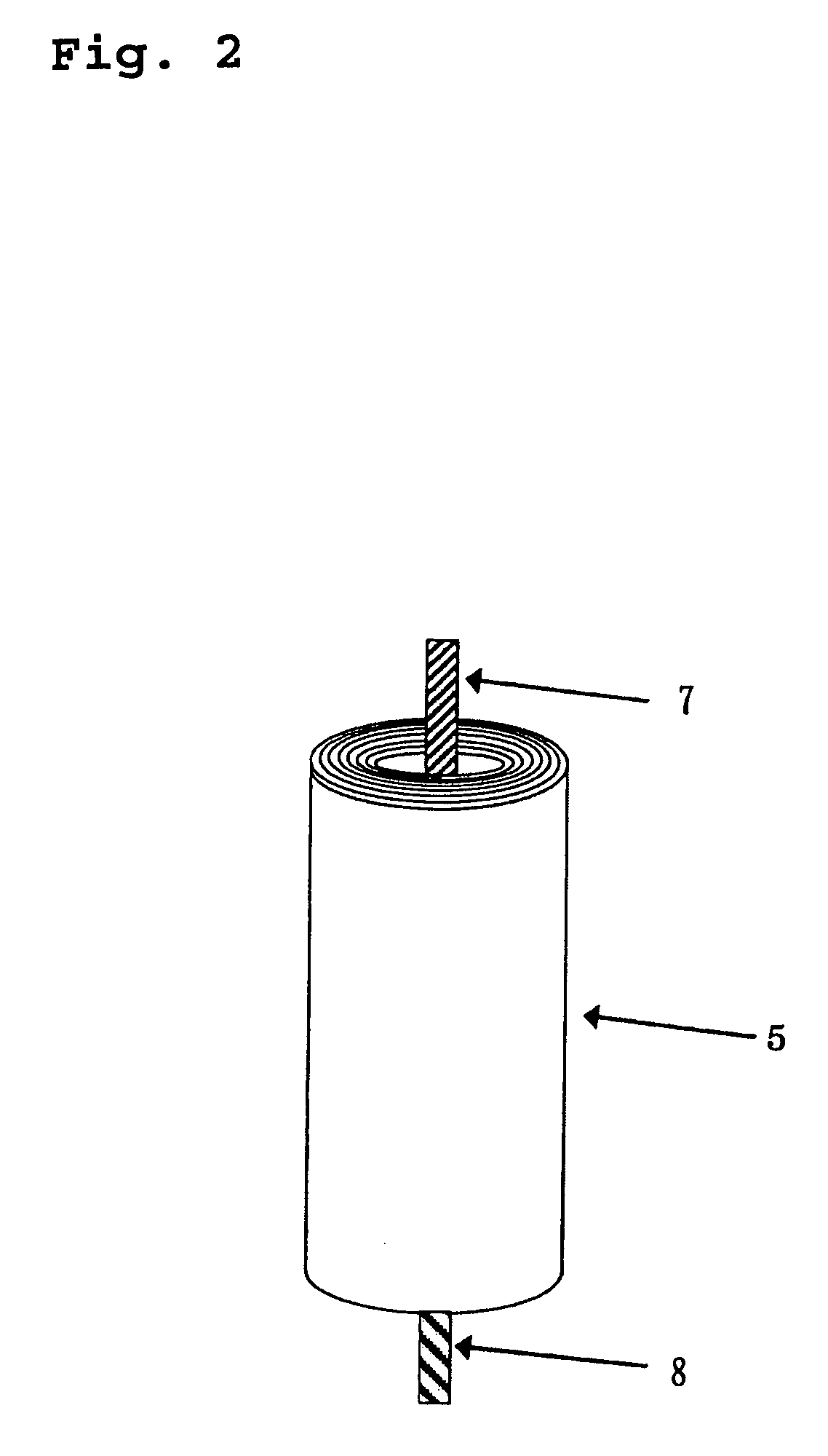
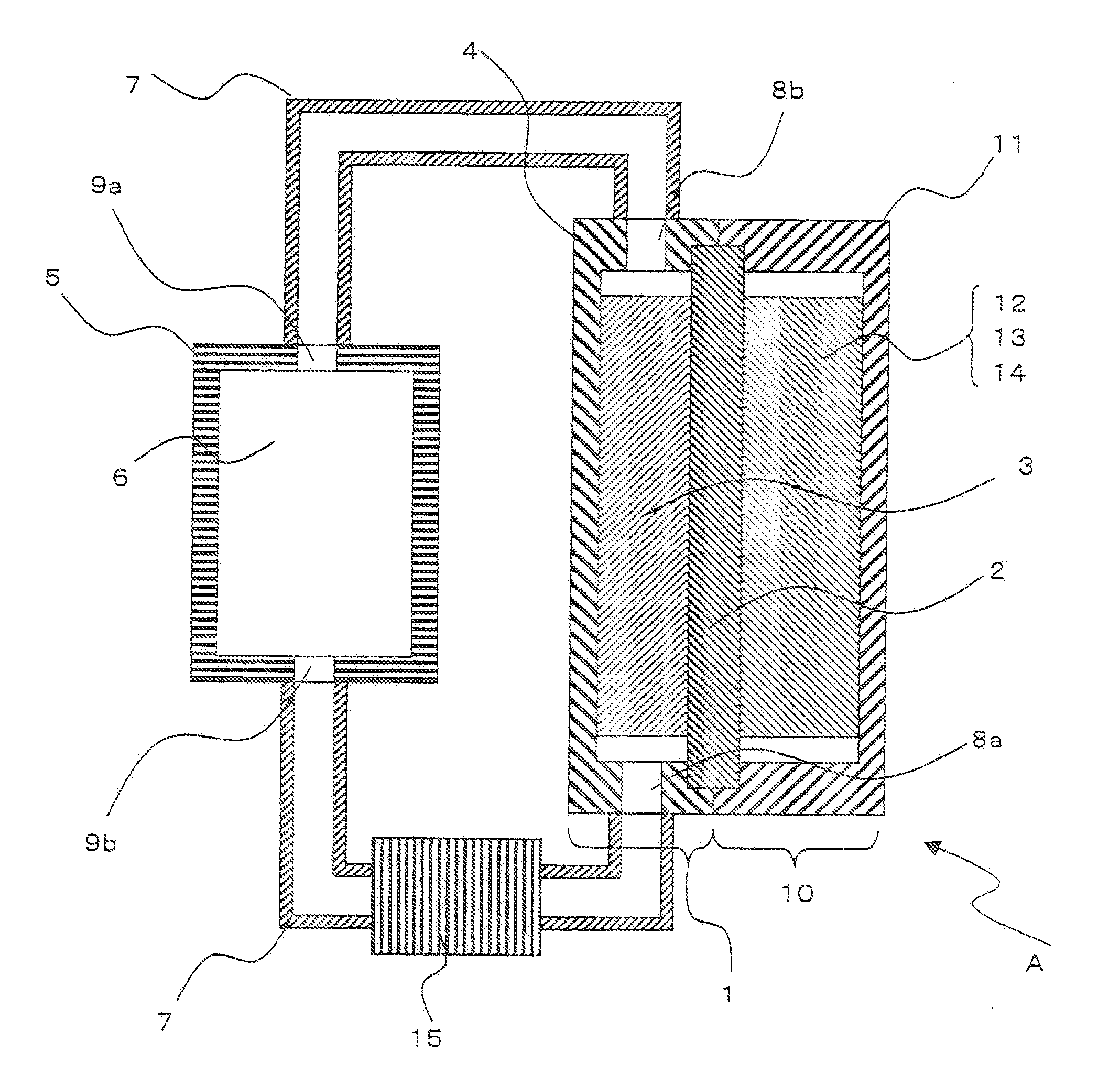
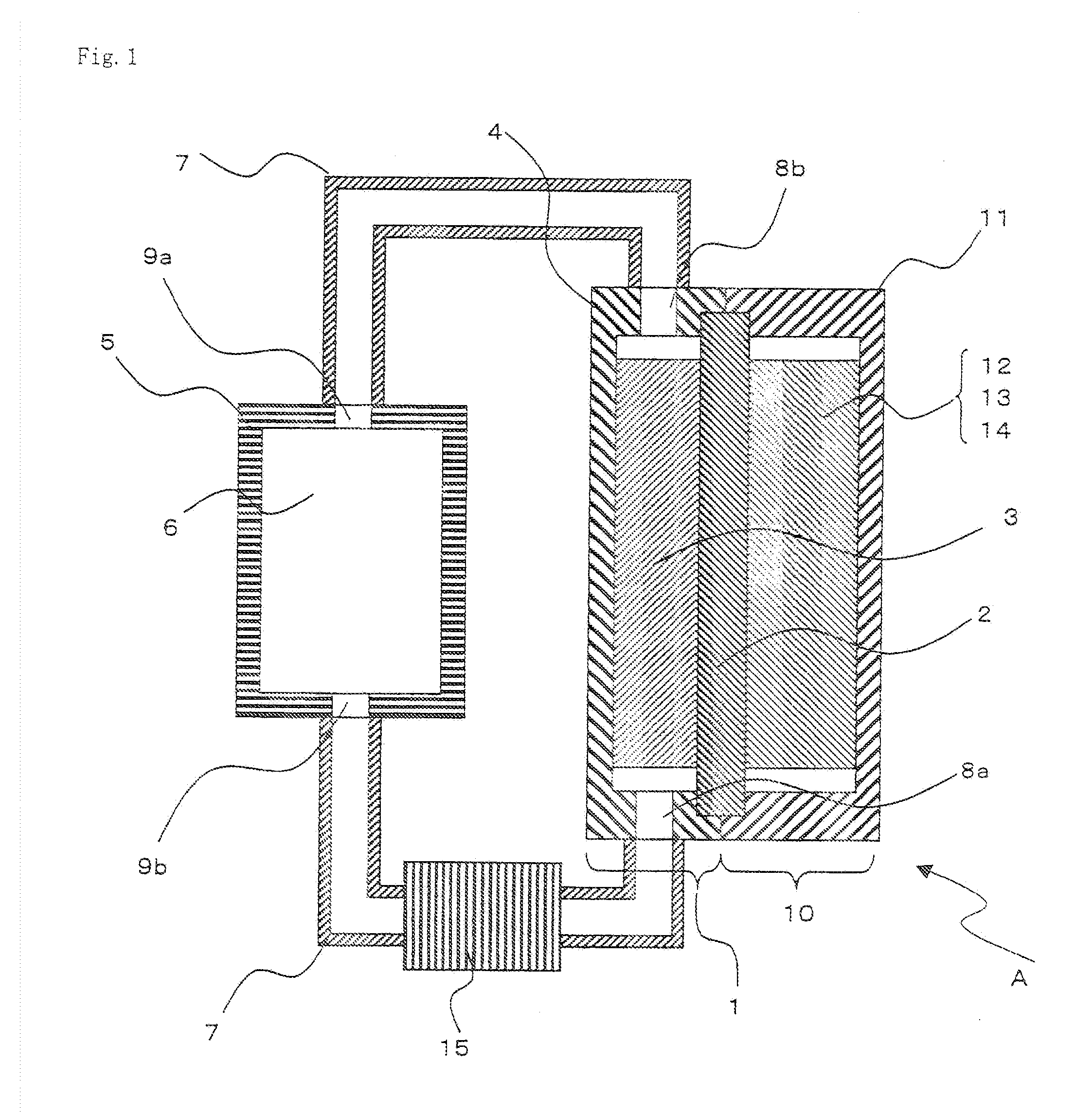
Electric current collectors are used by trolleybuses, trams, electric locomotives or EMUs to carry electrical power from overhead lines or electrical third rails to the electrical equipment of the vehicles. Those for overhead wires are roof-mounted devices, those for third rails are mounted on the bogies.
Laminated lithium ion battery, battery pack comprising same and pole piece of laminated lithium ion battery
InactiveCN104882635AImprove cooling effectReduce welding processFinal product manufactureElectrode carriers/collectorsInternal resistanceElectrical battery
Disclosed are a laminated lithium ion battery, a battery pack comprising the same and a pole piece of the laminated lithium ion battery. The pole piece comprises a current collector and an active material layer, wherein the current collector is coated with the active material layer. A section of continuous uncoated area is arranged at the tail end of a first width end portion of the pole piece, the top face and the bottom face of the uncoated area are not coated with the active material layer, the current collector is exposed in the uncoated area, and the exposed current collector serves as a pole lug of the pole piece. By the pole piece, internal resistance of the lithium ion battery is reduced and heat dissipation performance of the battery is improved.
Owner:SHENZHEN GREPOW BATTERY CO LTD
Porous silicon particulates for lithium batteries
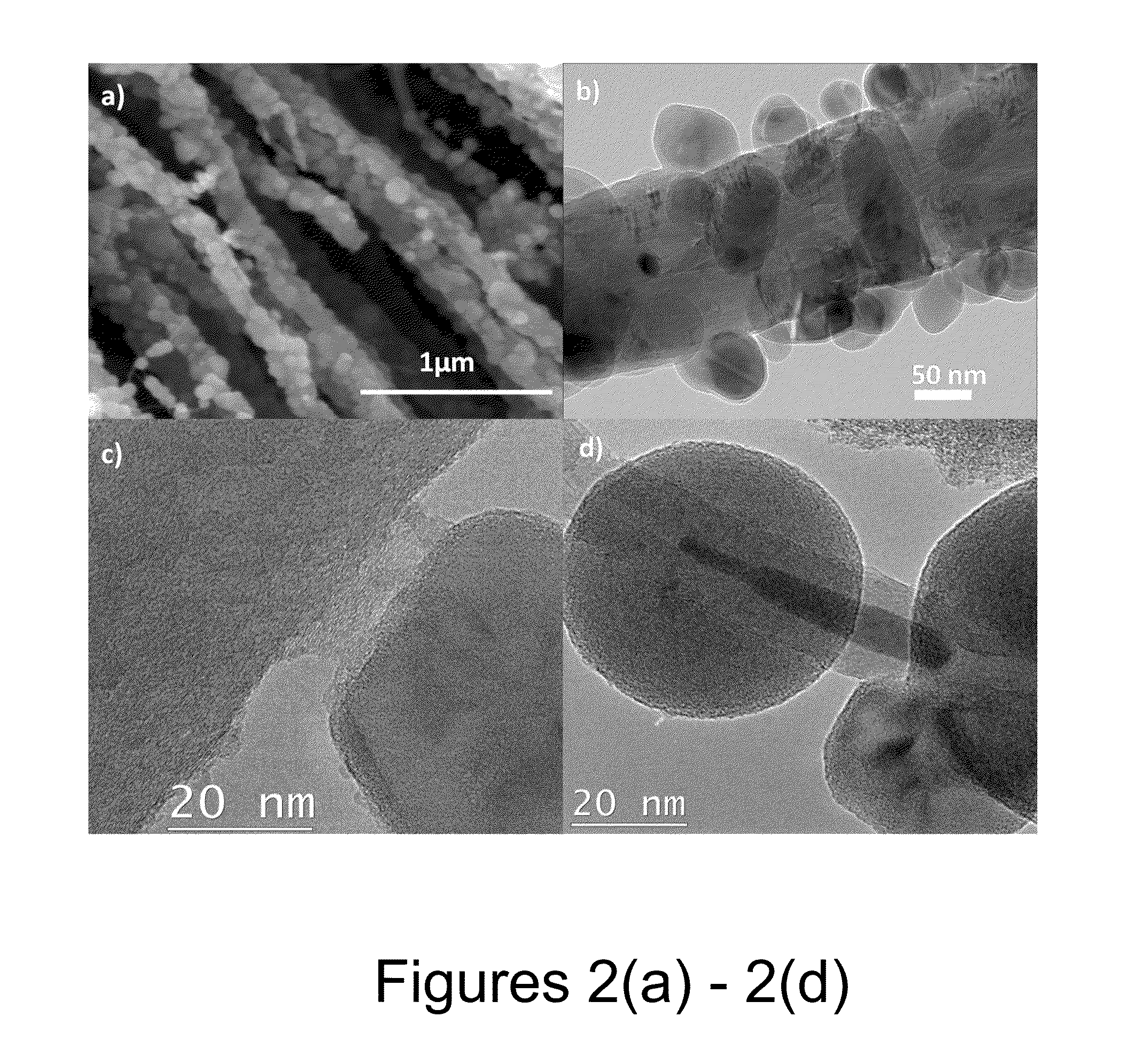
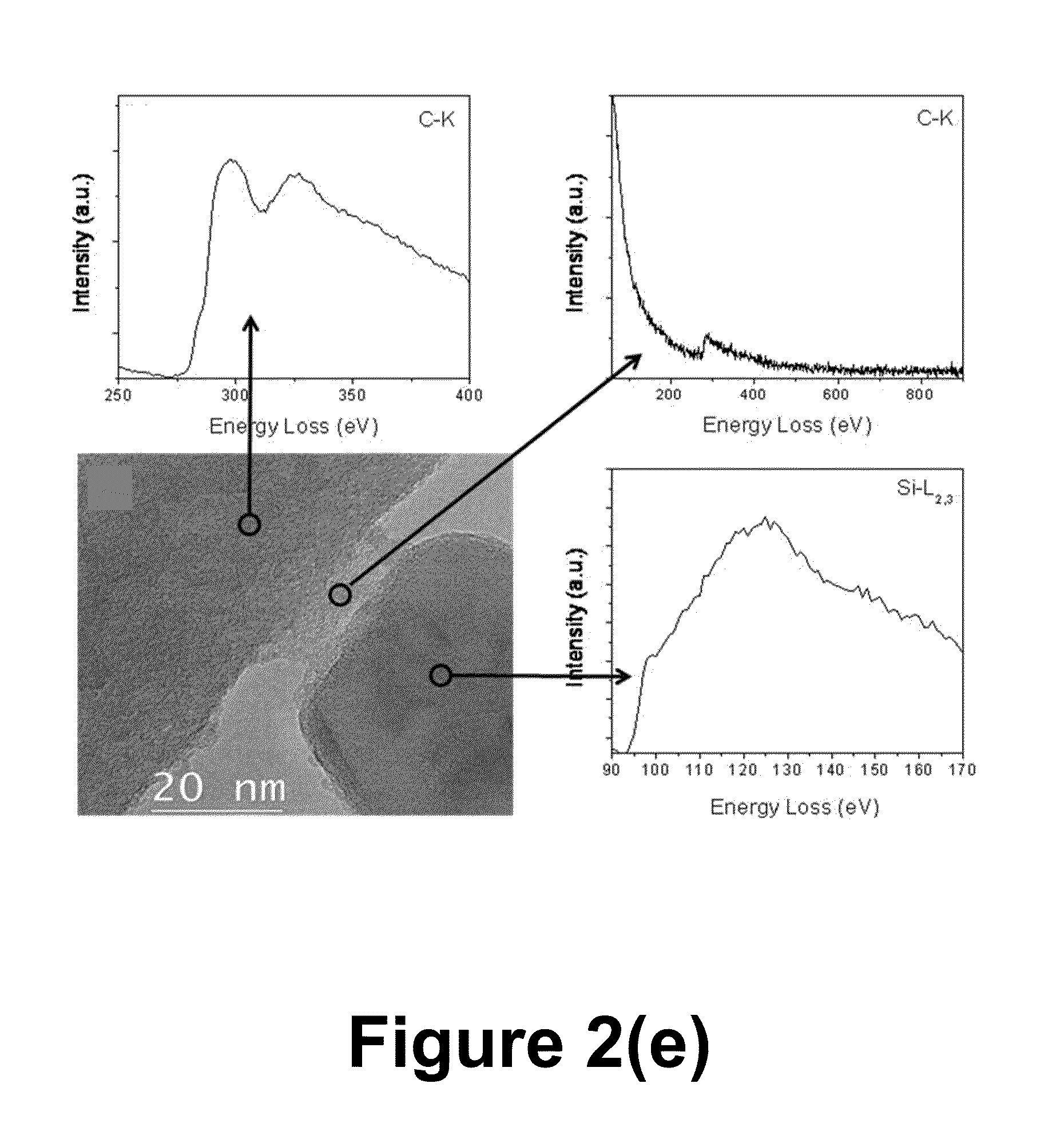
An anode structure for lithium batteries includes nanofeatured silicon particulates dispersed in a conductive network. The particulates are preferably made from metallurgical grade silicon powder via HF / HNO3 acid treatment, yielding crystallite sizes from about 1 to 20 nm and pore sizes from about 1 to 100 nm. Surfaces of the particles may be terminated with selected chemical species to further modify the anode performance characteristics. The conductive network is preferably a carbonaceous material or composite, but it may alternatively contain conductive ceramics such as TiN or B4C. The anode structure may further contain a current collector of copper or nickel mesh or foil.
Owner:TIEGS TERRY N
High energy lithium ion batteries with particular negative electrode compositions
ActiveUS20090305131A1Degree of crystallinity of will decreaseAlkaline accumulatorsElectrode manufacturing processesHigh energyMetal alloy
Combinations of materials are described in which high energy density active materials for negative electrodes of lithium ion batteries. In general, metal alloy / intermetallic compositions can provide the high energy density. These materials can have moderate volume changes upon cycling in a lithium ion battery. The volume changes can be accommodated with less degradation upon cycling through the combination with highly porous electrically conductive materials, such as highly porous carbon and / or foamed current collectors. Whether or not combined with a highly porous electrically conductive material, metal alloy / intermetallic compositions with an average particle size of no more than a micron can be advantageously used in the negative electrodes to improve cycling properties.
Owner:IONBLOX INC
Supercapacitor having electrode material comprising single-wall carbon nanotubes and process for making the same
InactiveUS7061749B2Avoid shortingHybrid capacitor electrolytesHybrid capacitor electrodesSupercapacitorNanotube
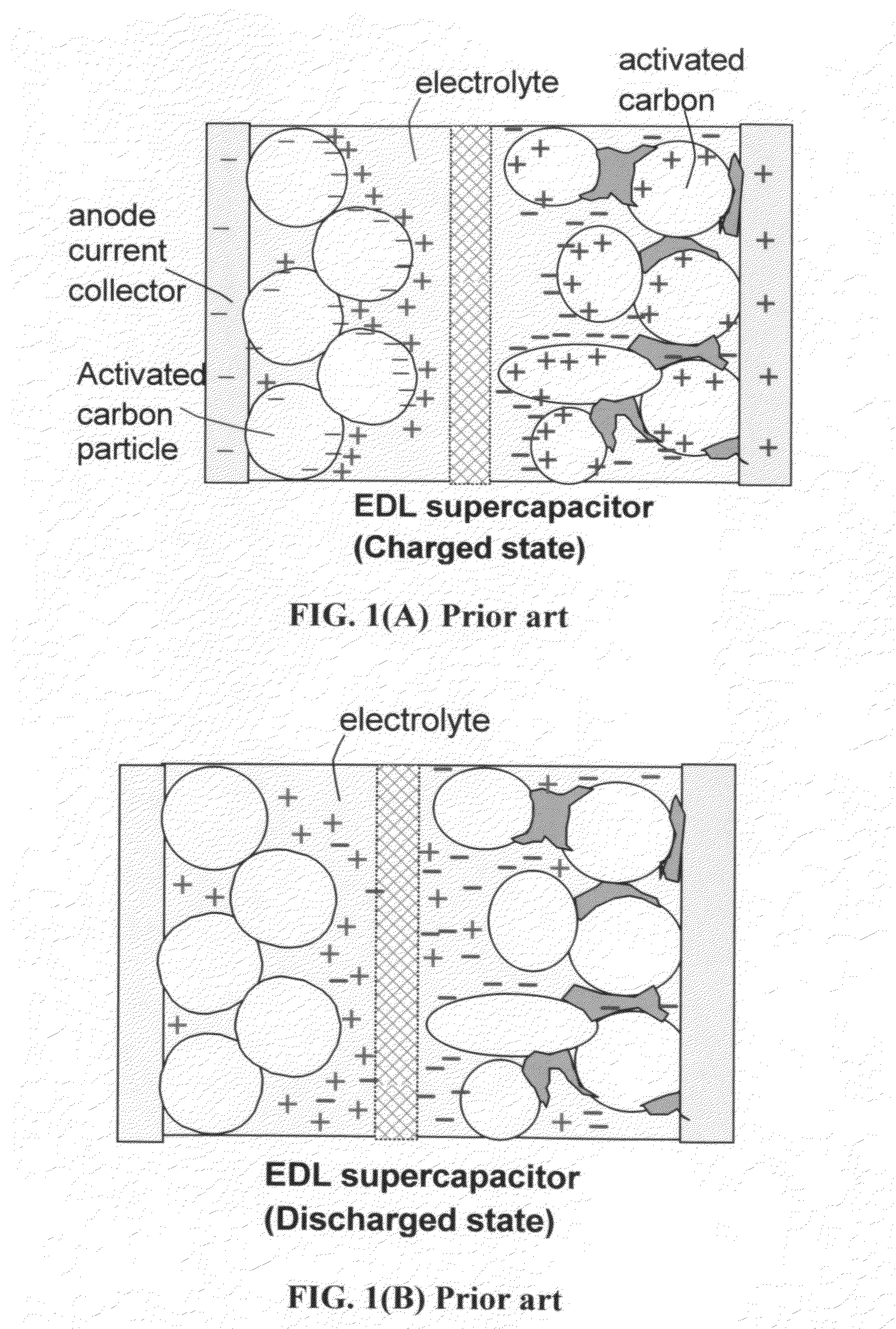
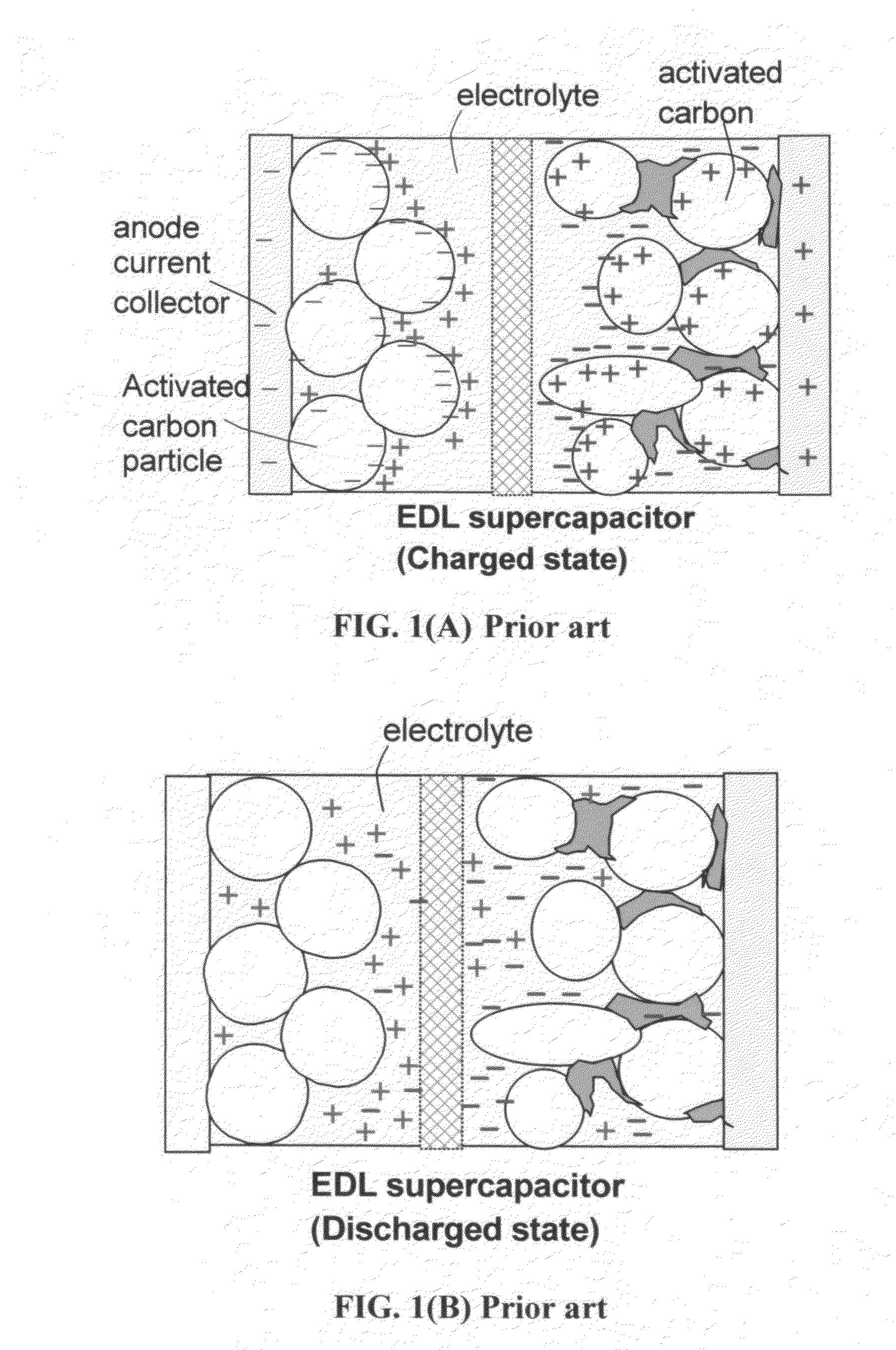
The present invention relates to a supercapacitor, also known as an electrical double-layer capacitor or ultracapacitor, having electrode material comprising single-wall carbon nanotubes. The carbon nanotubes can be derivatized with functional groups. The electrode material is made by preparing a polymer-nanotube suspension comprising polymer and nanotubes, forming the polymer-nanotube suspension into a polymer-nanotube composite of the desired form, carbonizing the polymer-nanotube composite to form a carbonaceous polymer-nanotube material, and activating the material. The supercapacitor includes electrode material comprising activated carbonaceous polymer-nanotube material in contact with current collectors and permeated with an electrolyte, which may be either fluid or solid. In the case of a fluid or compressible electrolyte, an electrolyte-permeable separator or spacer is interposed between the electrodes to keep the electrodes from shorting. The supercapacitor made with electrodes comprising underivatized single-wall carbon nanotubes and polymer that has been carbonized and activated appears to operate as a non-Faradaic supercapacitor.
Owner:GEORGIA TECH RES CORP
Hybrid electrode and surface-mediated cell-based super-hybrid energy storage device containing same
PendingUS20130171502A1Primary cell to battery groupingMaterial nanotechnologyHigh energyLithium metal
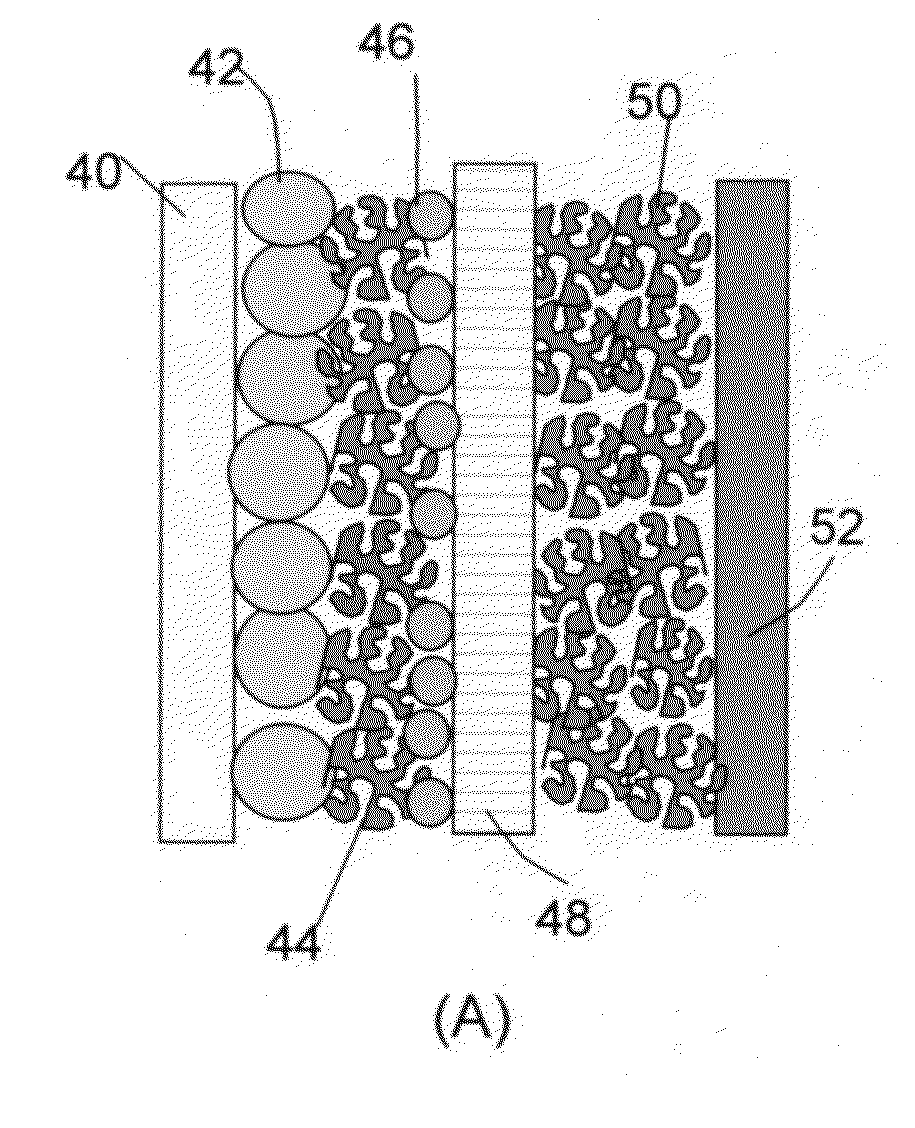
The present invention provides a multi-component hybrid electrode for use in an electrochemical super-hybrid energy storage device. The hybrid electrode contains at least a current collector, at least an intercalation electrode active material storing lithium inside interior or bulk thereof, and at least an intercalation-free electrode active material having a specific surface area no less than 100 m2 / g and storing lithium on a surface thereof, wherein the intercalation electrode active material and the intercalation-free electrode active material are in electronic contact with the current collector. The resulting super-hybrid cell exhibits exceptional high power and high energy density, and long-term cycling stability that cannot be achieved with conventional supercapacitors, lithium-ion capacitors, lithium-ion batteries, and lithium metal secondary batteries.
Owner:GLOBAL GRAPHENE GRP INC +1
Composite bipolar plate for electrochemical cells
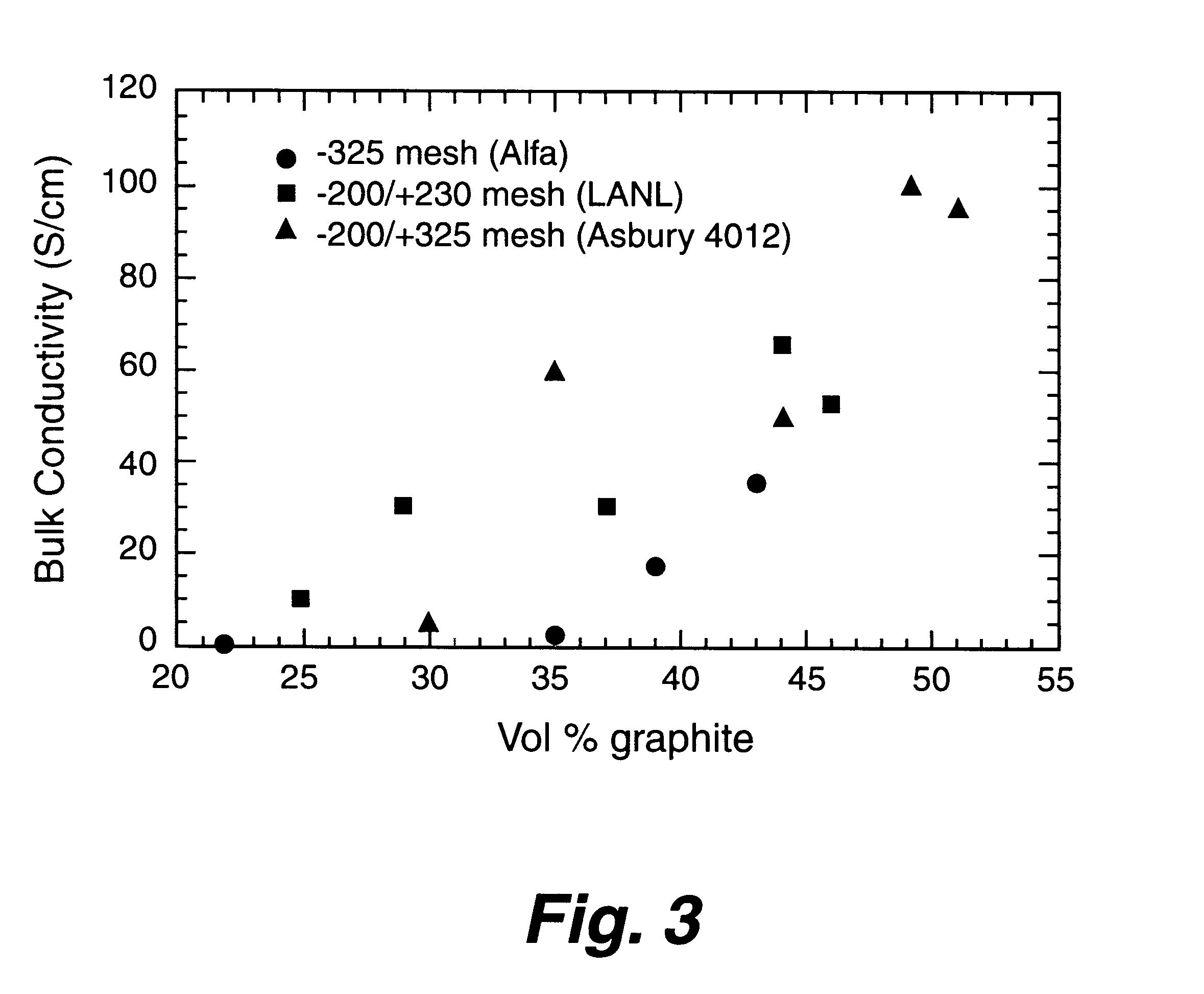
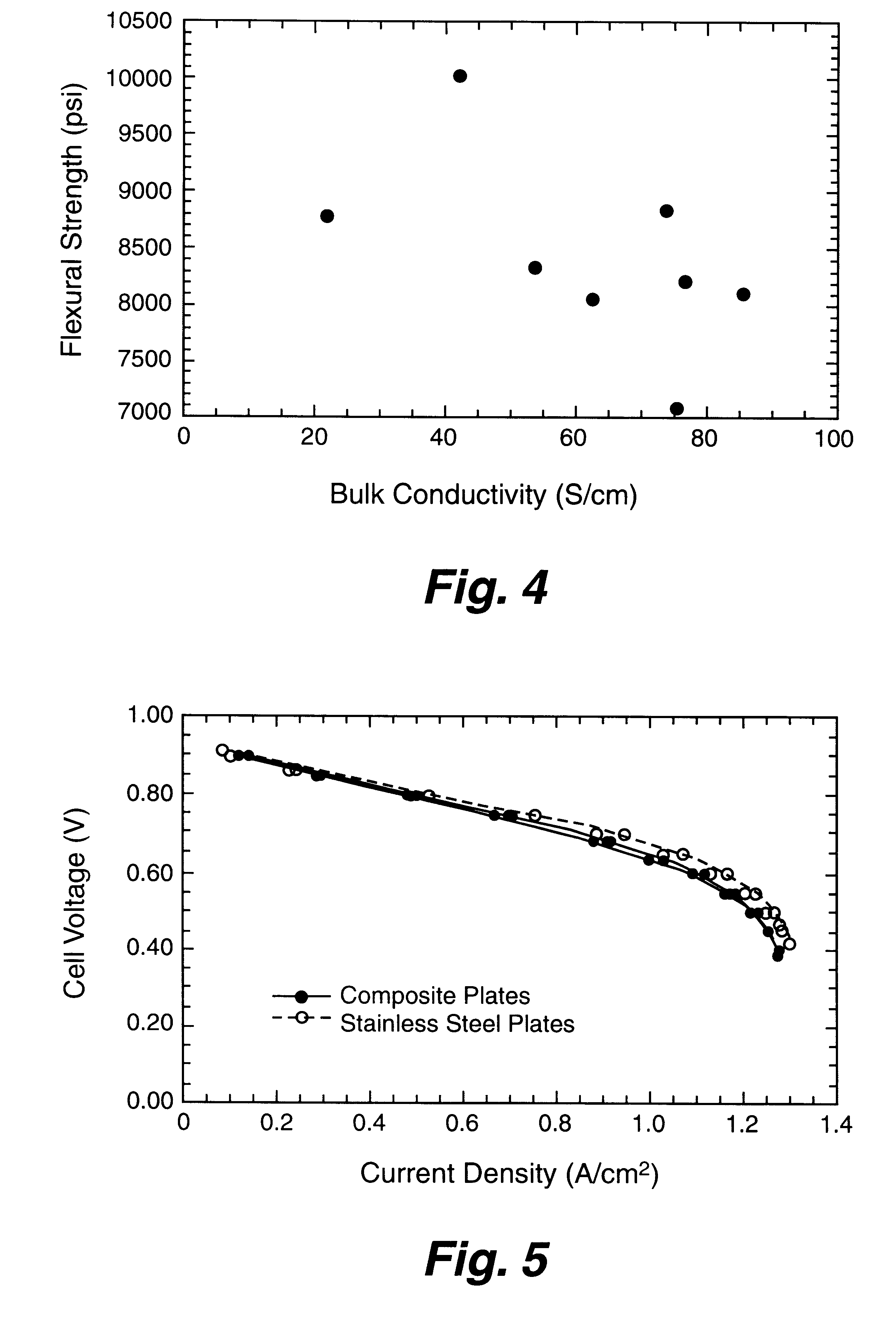
A bipolar separator plate for fuel cells consists of a molded mixture of a vinyl ester resin and graphite powder. The plate serves as a current collector and may contain fluid flow fields for the distribution of reactant gases. The material is inexpensive, electrically conductive, lightweight, strong, corrosion resistant, easily mass produced, and relatively impermeable to hydrogen gas. The addition of certain fiber reinforcements and other additives can improve the properties of the composite material without significantly increasing its overall cost.
Owner:TRIAD NAT SECURITY LLC
Lithium ion secondary battery
InactiveUS7335448B2Improve securityFinal product manufactureElectrode carriers/collectorsLithiumEngineering
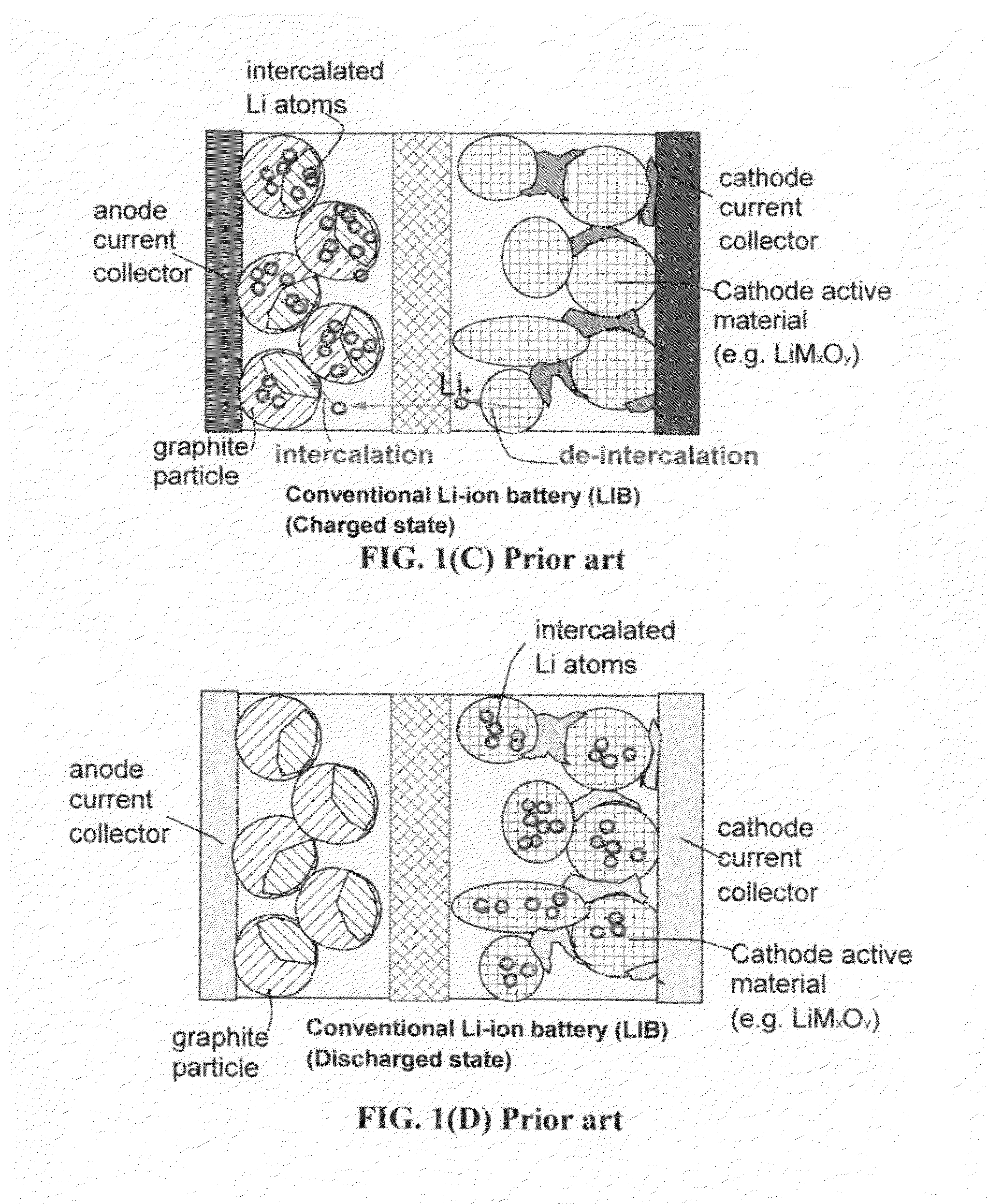
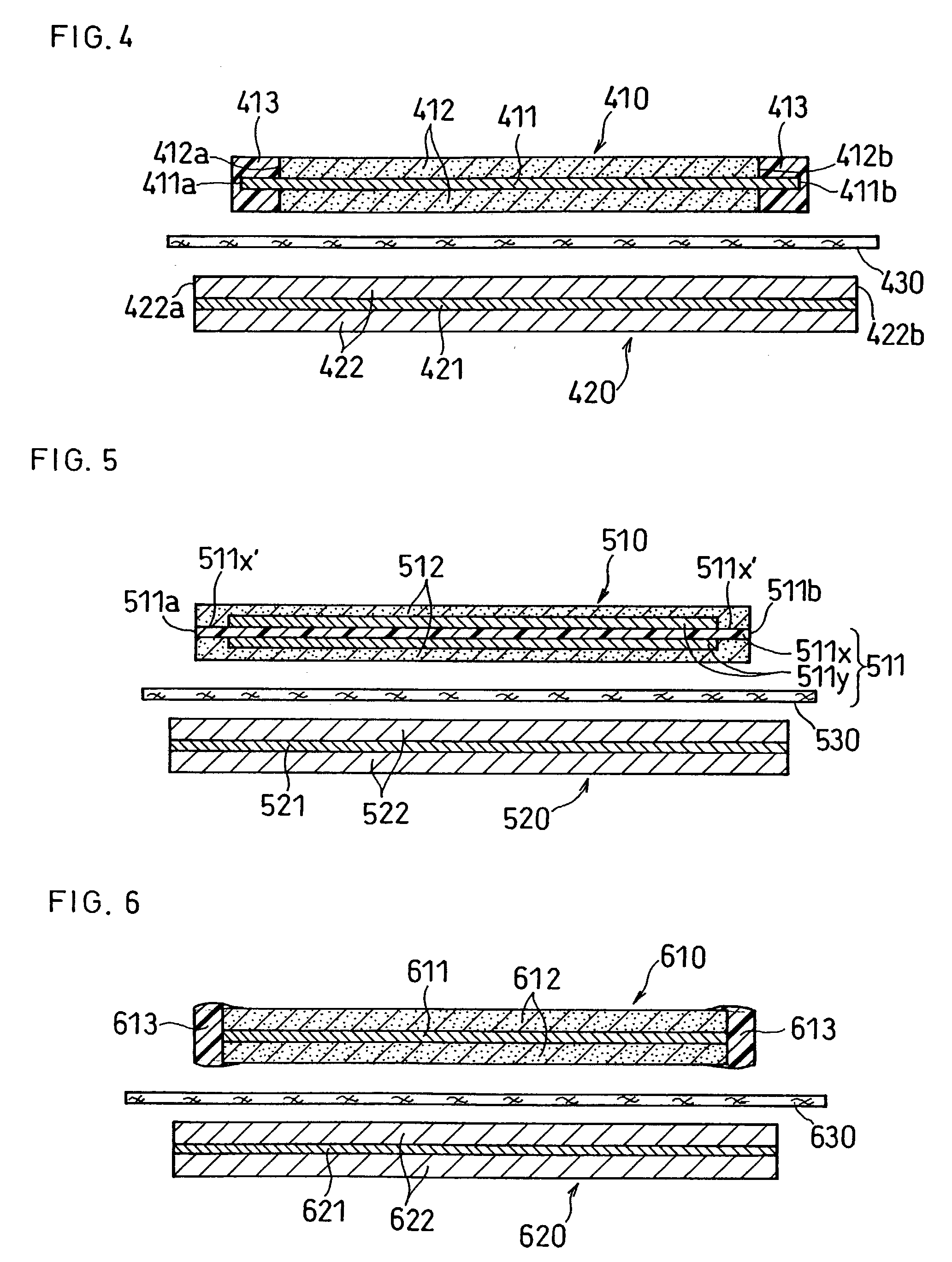
A lithium ion secondary battery includes: (a) a positive electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a positive electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (b) a negative electrode plate comprising an active material part and a current collector carrying the active material part, the active material part comprising a negative electrode active material capable of absorbing or desorbing a lithium ion during charge and discharge; (c) a separator interposed between the positive and negative electrode plates; (d) an electrolyte; and (e) a battery case accommodating the positive and negative electrode plates, the separator, and the electrolyte. The positive and negative electrode plates are wound with the separator interposed therebetween, thereby to form an electrode plate assembly. The electrode plate assembly is so configured that each lengthwise edge of the positive electrode current collector is positioned on an outer side of each lengthwise edge of the negative electrode active material part.
Owner:GK BRIDGE 1
Electrode for rechargeable lithium battery and rechargeable lithium battery
InactiveUS7192673B1Improve charge and discharge cycle characteristicsInhibition formationElectrode manufacturing processesSmall-sized cells cases/jacketsAmorphous siliconMaterials science
An electrode for a rechargeable lithium battery which includes a thin film composed of active material that expands and shrinks as it stores and releases lithium, e.g., a microcrystalline or amorphous silicon thin film, deposited on a current collector, characterized in that said current collector exhibits a tensile strength (=tensile strength (N / mm2) per sectional area of the current collector material×thickness (mm) of the current collector) of not less than 3.82 N / mm.
Owner:SANYO ELECTRIC CO LTD
Nonaqueous electrolyte secondary battery and battery module
ActiveUS20050069777A1Excellent in battery capacity characteristic and cycle performanceEasy dischargeFinal product manufactureElectrode carriers/collectorsAlloyAluminum foil
A nonaqueous electrolyte secondary battery includes a case, a nonaqueous electrolyte provided in the case, a positive electrode provided in the case, and a negative electrode provided in the case, the negative electrode comprising a negative electrode current collector and a negative electrode layer that is carried on the negative electrode current collector and contains negative electrode active material particles, and the negative electrode current collector comprising an aluminum foil having an average crystal grain size of 50 μm or less or an aluminum alloy foil having an average crystal grain size of 50 μm or less.
Owner:KK TOSHIBA
Micro electrochemical energy storage cells
InactiveUS6197450B1Improve performanceIncrease capacityPrimary cell to battery groupingFinal product manufactureThin layerOptoelectronics
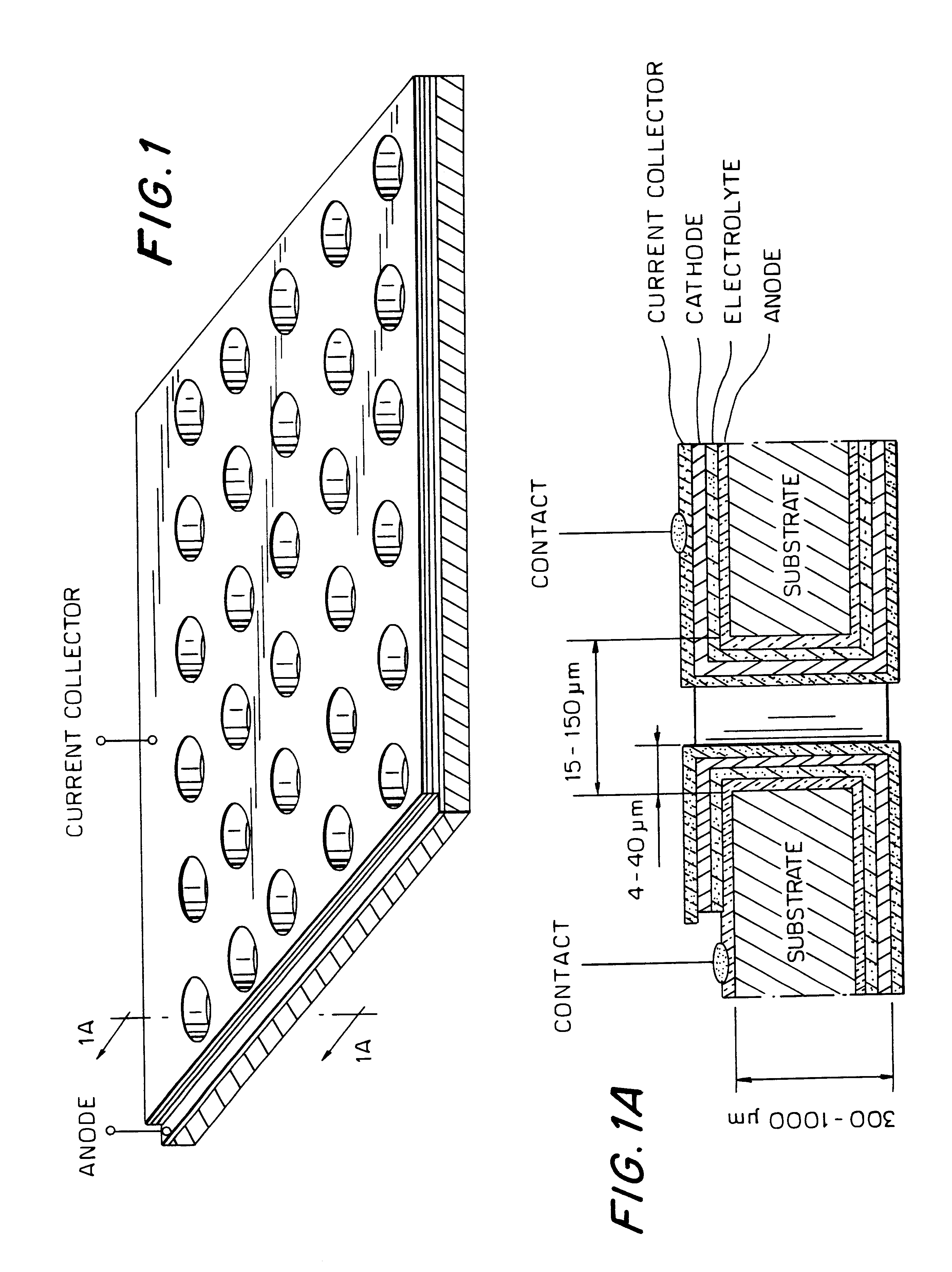
Thin-film micro-electrochemical energy storage cells (MEESC) such as microbatteries and double-layer capacitors (DLC) are provided. The MEESC comprises two thin layer electrodes, an intermediate thin layer of a solid electrolyte and optionally, a fourth thin current collector layer; said layers being deposited in sequence on a surface of a substrate. The MEESC is characterized in that the substrate is provided with a plurality of through cavities of arbitrary shape, with high aspect ratio. By using the substrate volume, an increase in the total electrode area per volume is accomplished.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Lithium-ion cell having a high-capacity anode and a high-capacity cathode
ActiveUS20130224603A1Easy dischargeImprove power densityMaterial nanotechnologyHybrid capacitor electrodesLithiumHigh energy
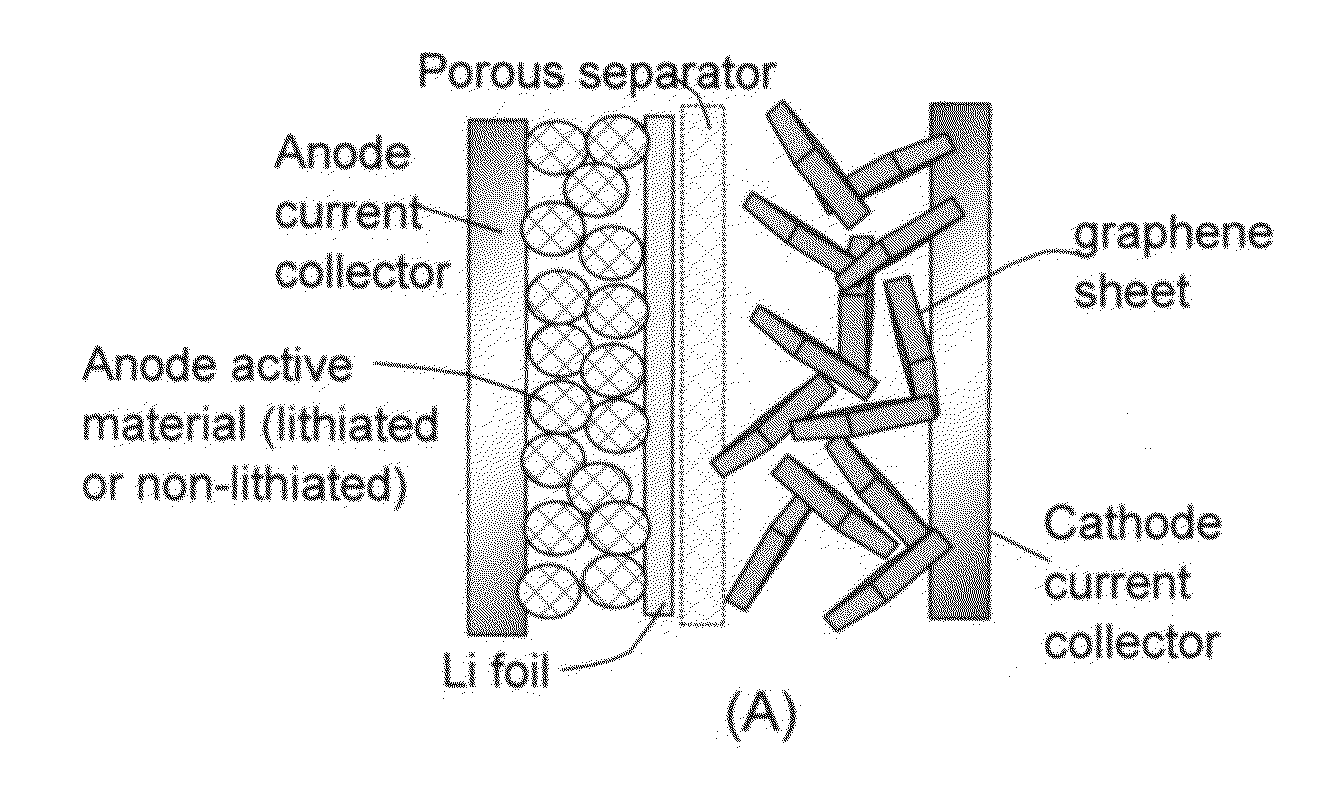
A lithium-ion cell comprising: (A) a cathode comprising graphene as the cathode active material having a surface area to capture and store lithium thereon and wherein said graphene cathode is meso-porous having a specific surface area greater than 100 m2 / g; (B) an anode comprising an anode active material for inserting and extracting lithium, wherein the anode active material is mixed with a conductive additive and / or a resin binder to form a porous electrode structure, or coated onto a current collector in a coating or thin film form; (C) a porous separator disposed between the anode and the cathode; (D) a lithium-containing electrolyte in physical contact with the two electrodes; and (E) a lithium source disposed in at least one of the two electrodes when the cell is made. This new Li-ion cell exhibits an unprecedentedly high energy density.
Owner:GLOBAL GRAPHENE GRP INC
Prelithiated current collector and secondary lithium cells containing same
ActiveUS20130045427A1Reduce capacityMaterial nanotechnologyHybrid capacitorsLithium metalLithium sulfur
The present invention provides a battery or supercapacitor current collector which is prelithiated. The prelithiated current collector comprises: (a) an electrically conductive substrate having two opposed primary surfaces, and (b) a mixture layer of carbon (and / or other stabilizing element, such as B, Al, Ga, In, C, Si, Ge, Sn, Pb, As, Sb, Bi, Te, or a combination thereof) and lithium or lithium alloy coated on at least one of the primary surfaces, wherein lithium element is present in an amount of 1% to 99% by weight of the mixture layer. This current collector serves as an effective and safe lithium source for a wide variety of electrochemical energy storage cells, including the rechargeable lithium cell (e.g. lithium-metal, lithium-ion, lithium-sulfur, lithium-air, lithium-graphene, lithium-carbon, and lithium-carbon nanotube cell) and the lithium ion based supercapacitor cell (e.g, symmetric ultracapacitor, asymmetric ultracapacitor, hybrid supercapacitor-battery, or lithium-ion capacitor).
Owner:GLOBAL GRAPHENE GRP INC
Release system for electrochemical cells
InactiveUS20110068001A1Active material electrodesElectrical-based machining electrodesEngineeringElectrochemical cell
Electrochemical cells, and more specifically, release systems for the fabrication of electrochemical cells are described. In particular, release layer arrangements, assemblies, methods and compositions that facilitate the fabrication of electrochemical cell components, such as electrodes, are presented. In some embodiments, methods of fabricating an electrode involve the use of a release layer to separate portions of the electrode from a carrier substrate on which the electrode was fabricated. For example, an intermediate electrode assembly may include, in sequence, an electroactive material layer, a current collector layer, a release layer, and a carrier substrate. The carrier substrate can facilitate handling of the electrode during fabrication and / or assembly, but may be released from the electrode prior to commercial use.
Owner:SION POWER CORP
Compositions Including Nano-Particles and a Nano-Structured Support Matrix and Methods of preparation as reversible high capacity anodes in energy storage systems
The present invention relates to compositions including nano-particles and a nano-structured support matrix, methods of their preparation and applications thereof. The compositions of the present invention are particularly suitable for use as anode material for lithium-ion rechargeable batteries. The nano-structured support matrix can include nanotubes, nanowires, nanorods, and mixtures thereof. The composition can further include a substrate on which the nano-structured support matrix is formed. The substrate can include a current collector material.
Owner:UNIVERSITY OF PITTSBURGH
Electrode for use in lithium battery and rechargeable lithium battery
InactiveUS7235330B1High charge and discharge capacityImprove featuresElectrode manufacturing processesSmall-sized cells cases/jacketsAmorphous siliconSurface roughness
Owner:SANYO ELECTRIC CO LTD
Particle filter system incorporating nanofibers
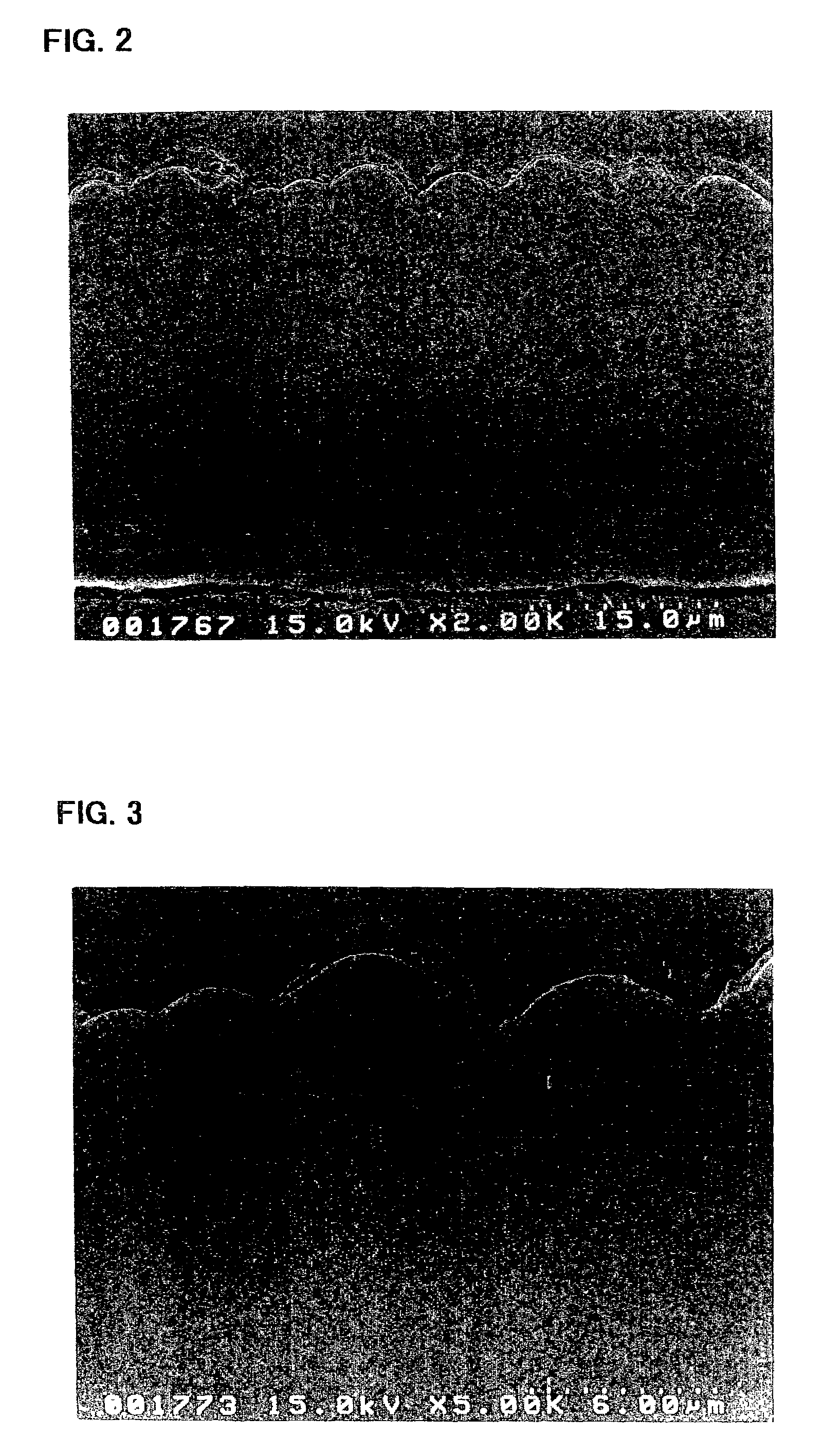
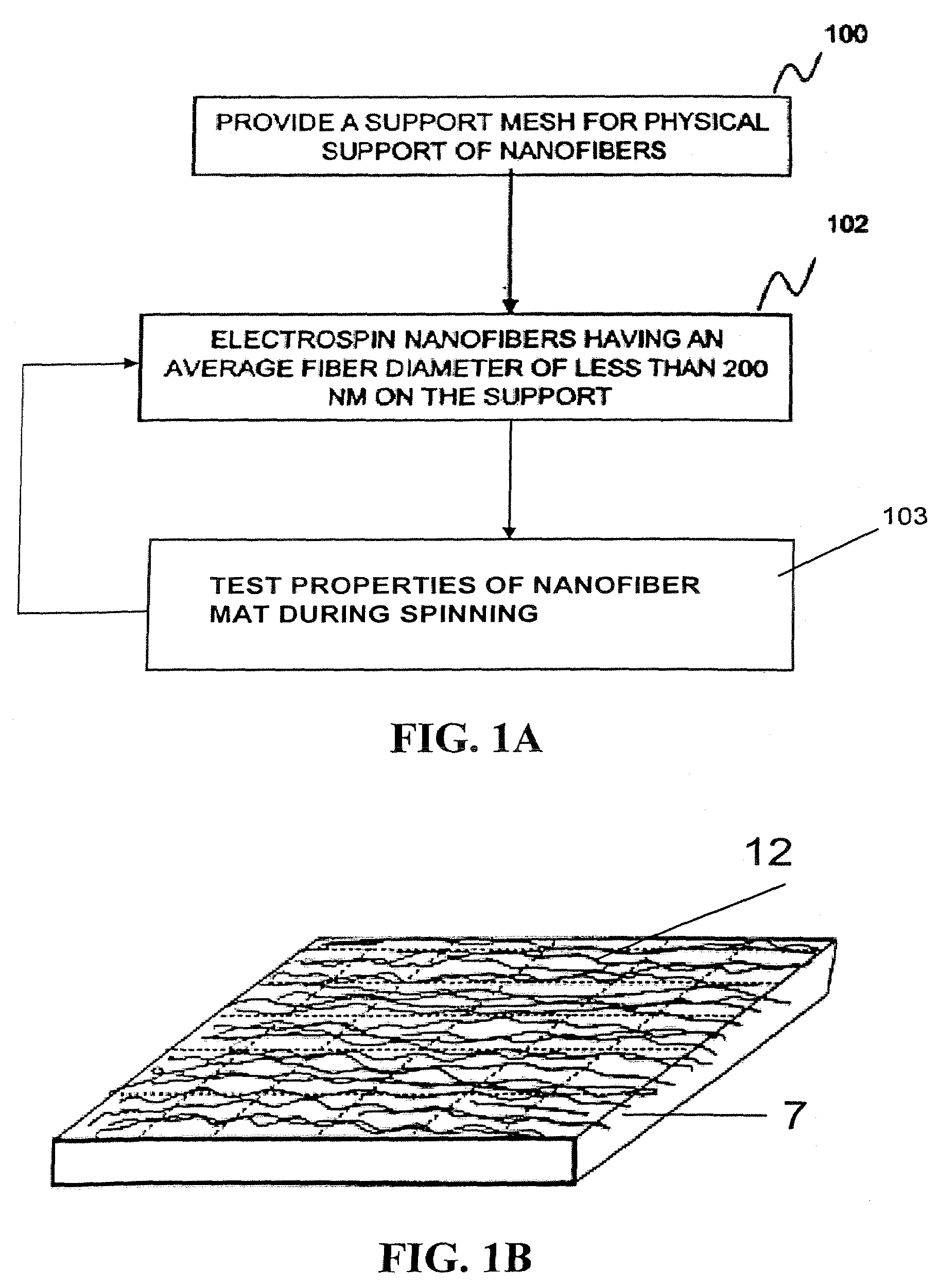
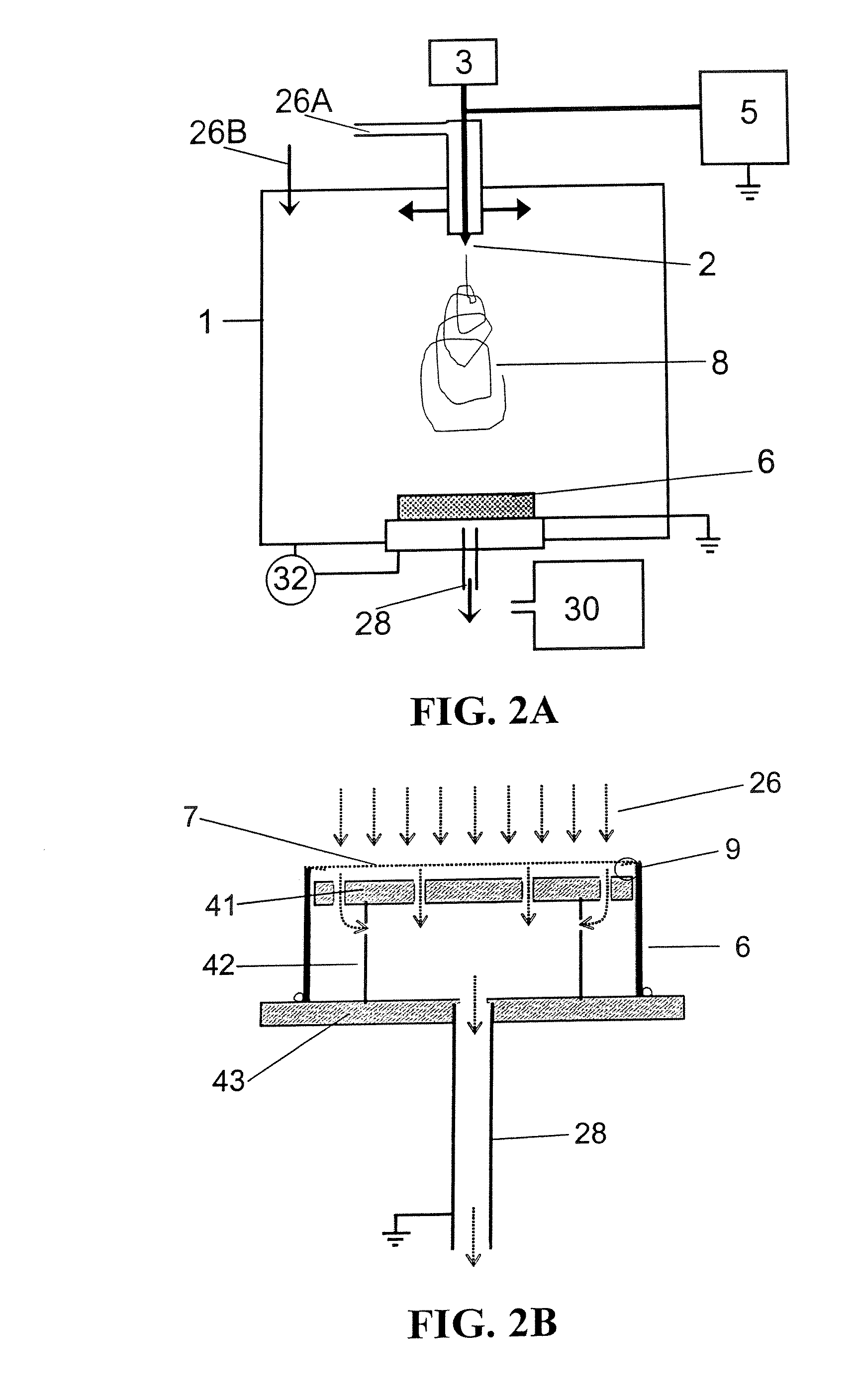
ActiveUS20080110342A1Electric discharge heatingFilament/thread formingElectric field modulationFiber
A filtration device including a filtration medium having a plurality of nanofibers of diameters less than 1 micron formed into a fiber mat in the presence of an abruptly varying electric field. The filtration device includes a support attached to the filtration medium and having openings for fluid flow therethrough. A device for making a filter material. The device includes an electrospinning element configured to electrospin a plurality of fibers from a tip of the electrospinning element, a collector opposed to the electrospinning element configured to collect electrospun fibers on a surface of the collector, and an electric field modulation device configured to abruptly vary an electric field at the collector at least once during electrospinning of the fibers. A method for making a filter material. The method provides a support having openings for fluid flow therethrough, electrospins nanofibers across an entirety of the openings, and abruptly varies an electric field at the collector at least once during electrospinning of the fibers.
Owner:RES TRIANGLE INST
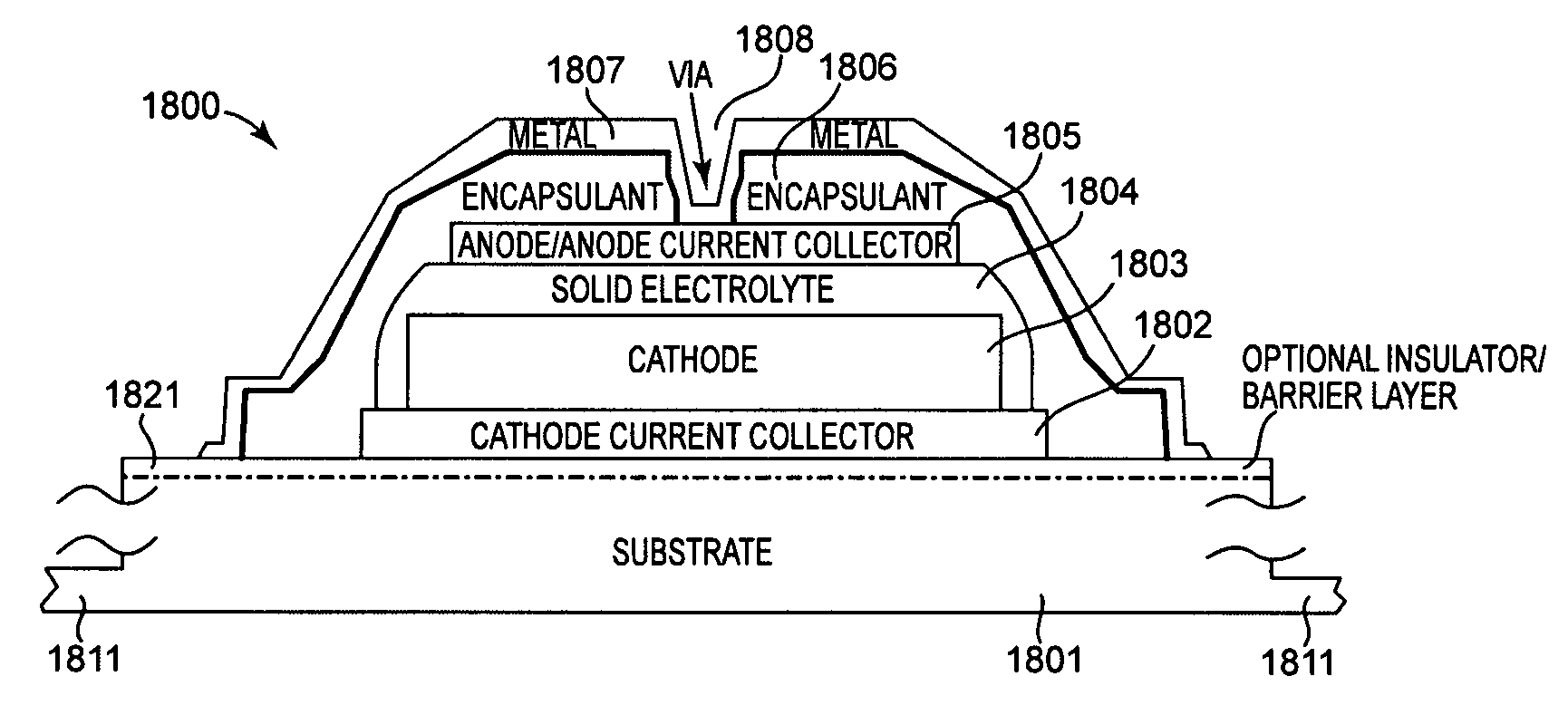
Method and apparatus for solid-state microbattery photolithographic manufacture, singulation and passivation
InactiveUS20080032236A1Efficient and economical manufactureReduced number of stepSolid electrolytesDecorative surface effectsChemical treatmentPhotoresist
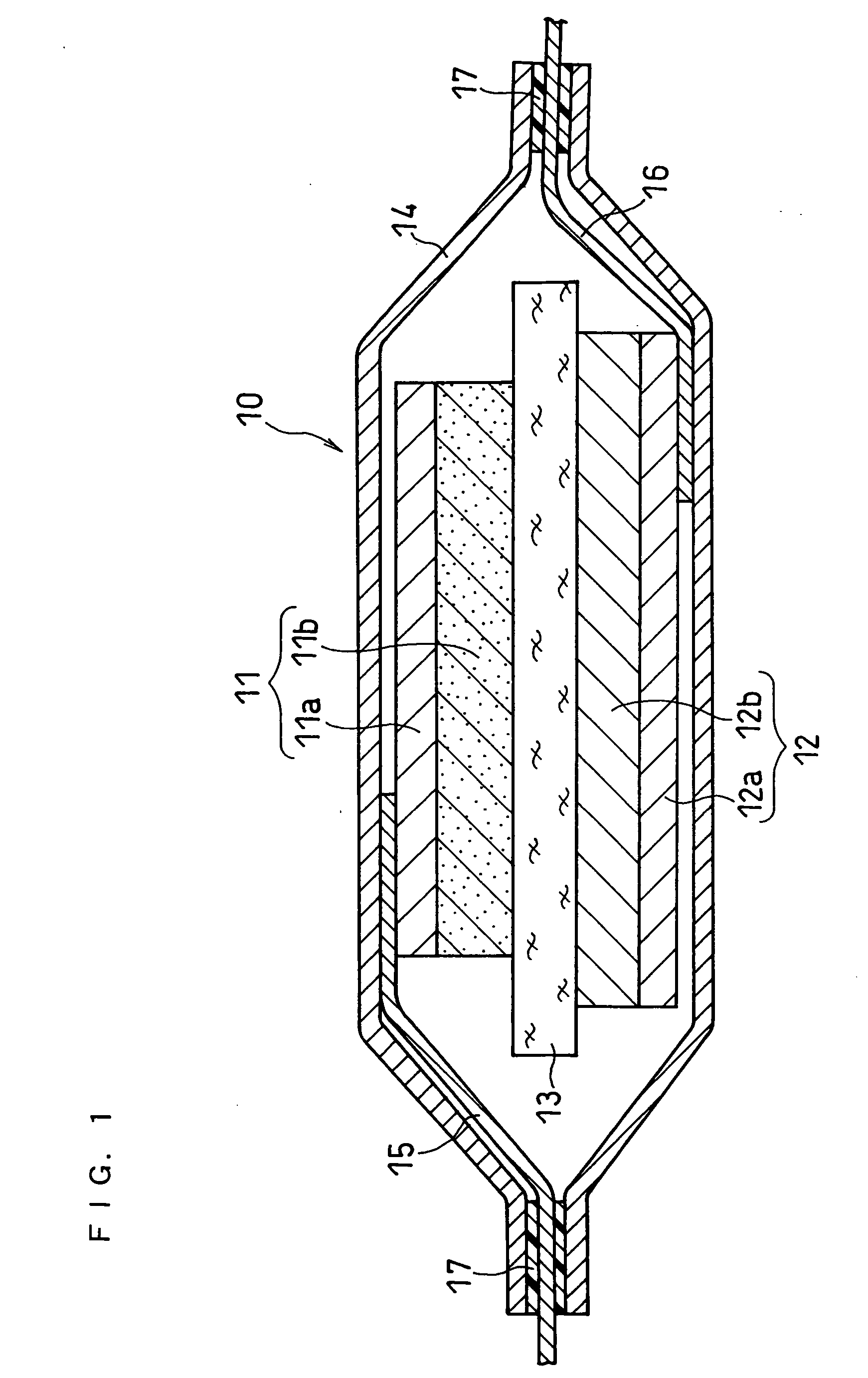
A method for producing a thin film lithium battery is provided, comprising applying a cathode current collector, a cathode material, an anode current collector, and an electrolyte layer separating the cathode material from the anode current collector to a substrate, wherein at least one of the layers contains lithiated compounds that is patterned at least in part by a photolithography operation comprising removal of a photoresist material from the layer containing lithiated compounds by a process including a wet chemical treatment. Additionally, a method and apparatus for making lithium batteries by providing a first sheet that includes a substrate having a cathode material, an anode material, and a LiPON barrier / electrolyte layer separating the cathode material from the anode material; and removing a subset of first material to separate a plurality of cells from the first sheet. In some embodiments, the method further includes depositing second material on the sheet to cover the plurality of cells; and removing a subset of second material to separate a plurality of cells from the first sheet.
Owner:CYMBET CORP
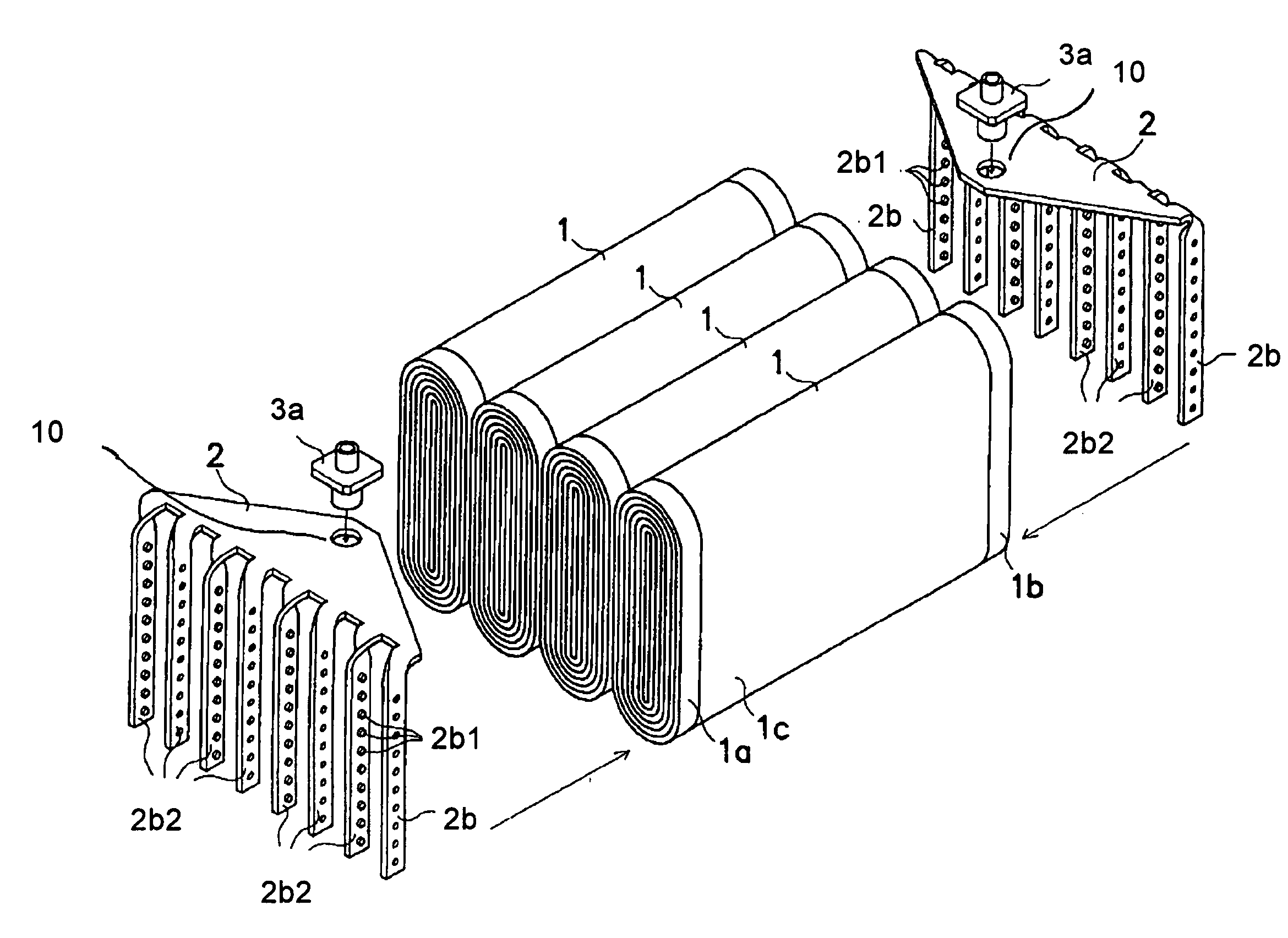
Battery
ActiveUS20060051664A1Simple working processImprove reliabilityFinal product manufactureActive material electrodesMetal foilEngineering
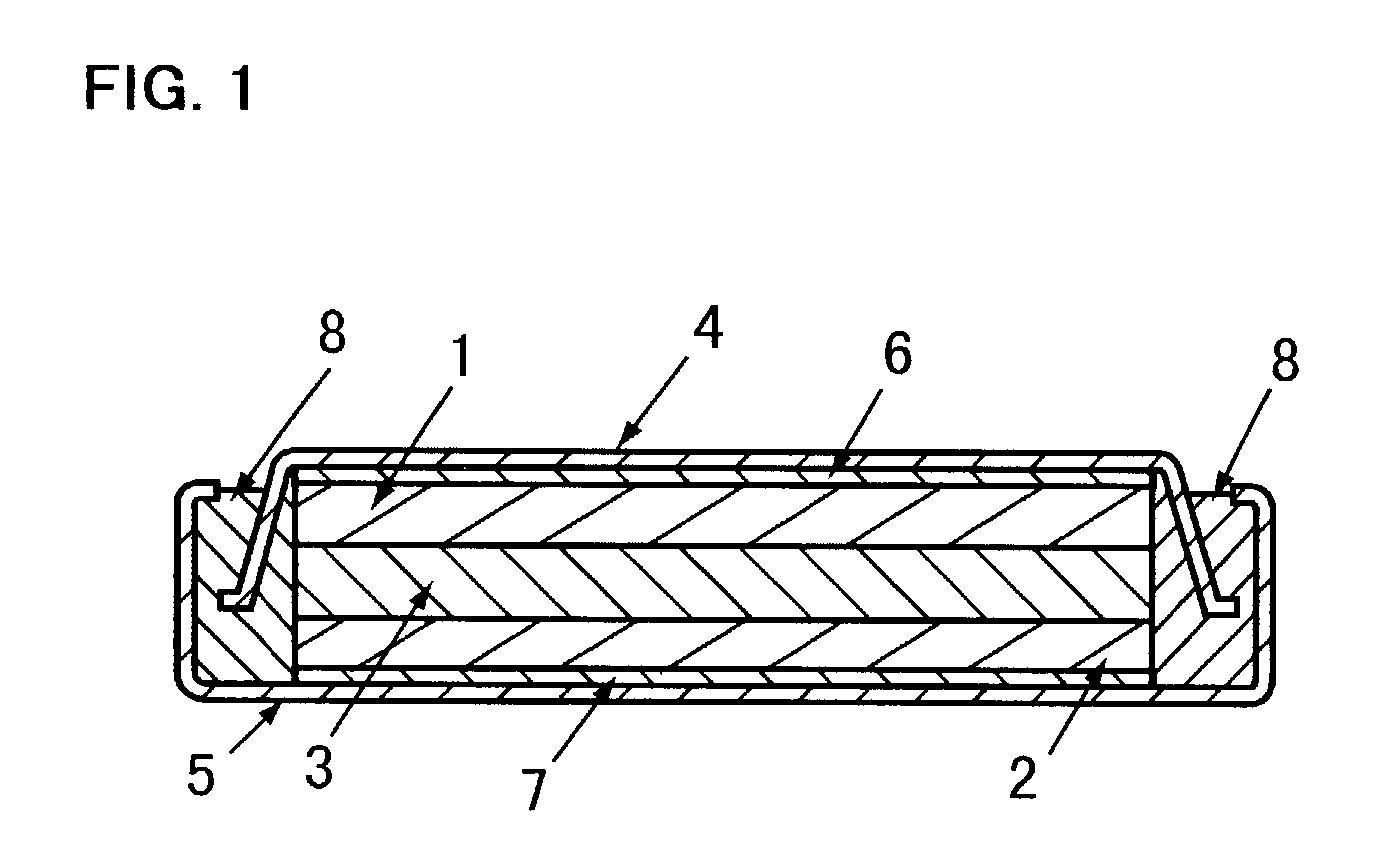
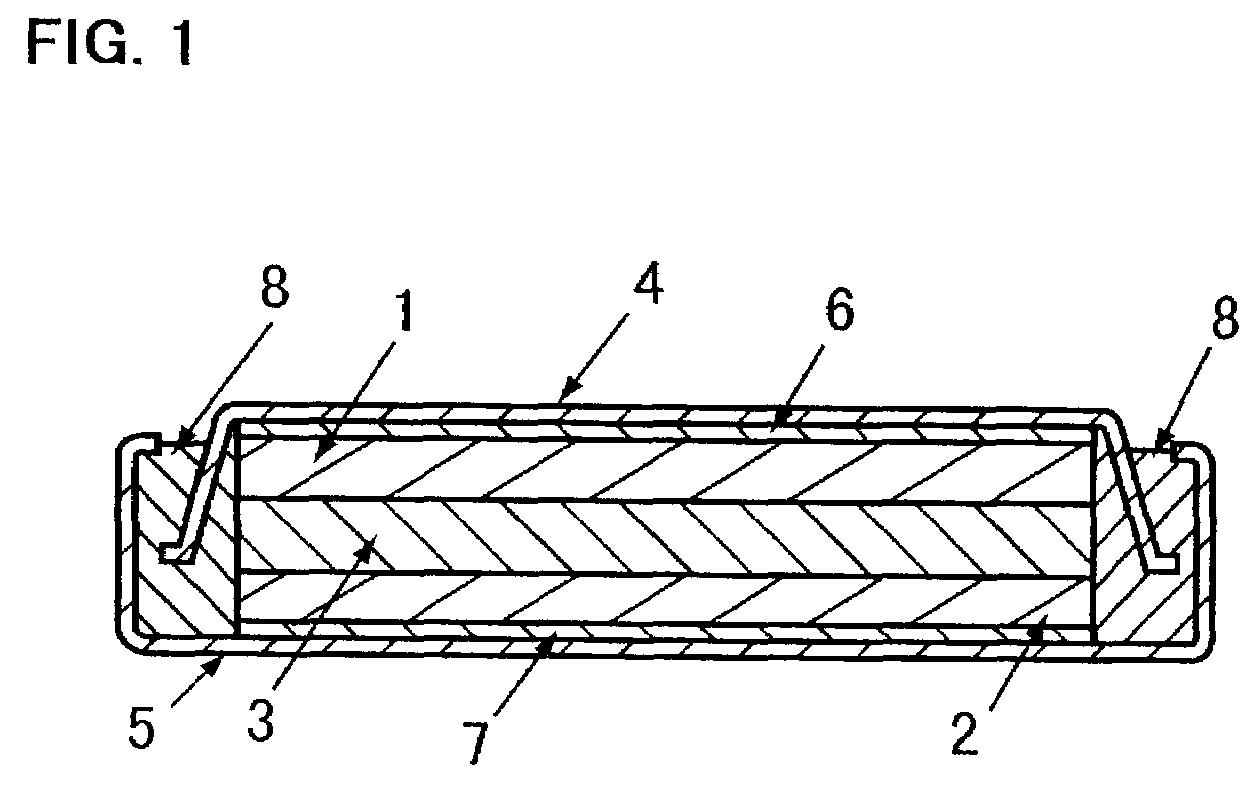
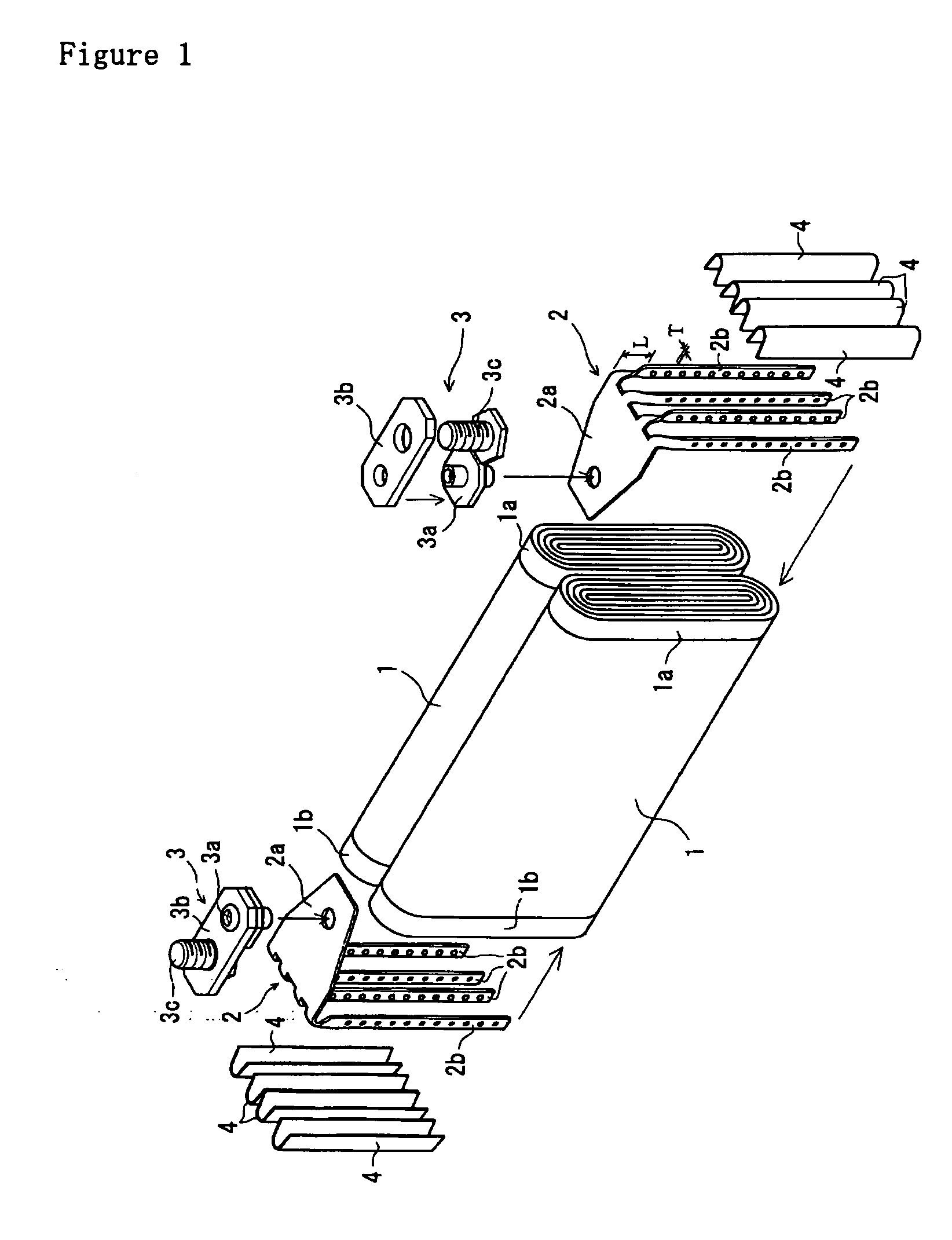
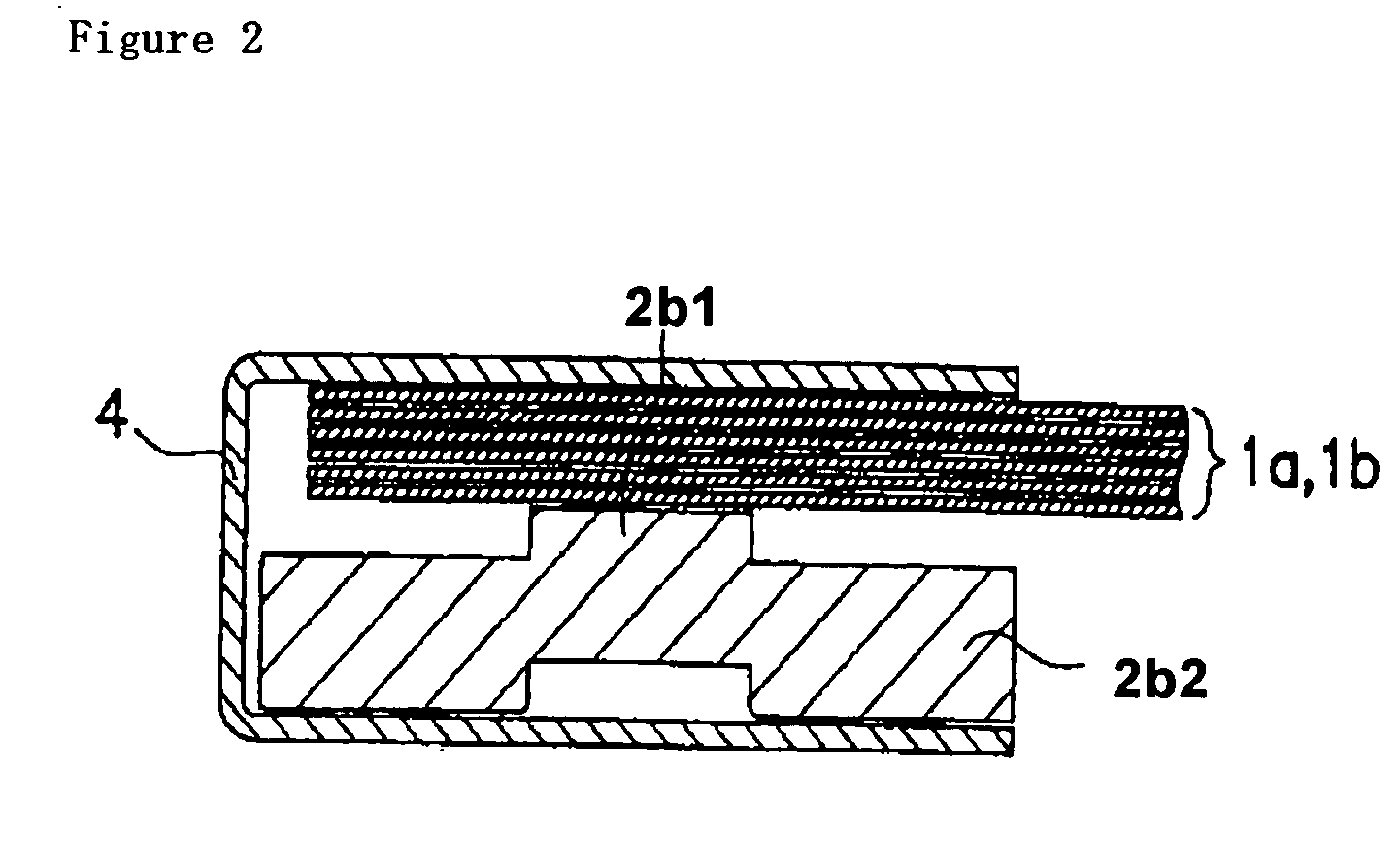
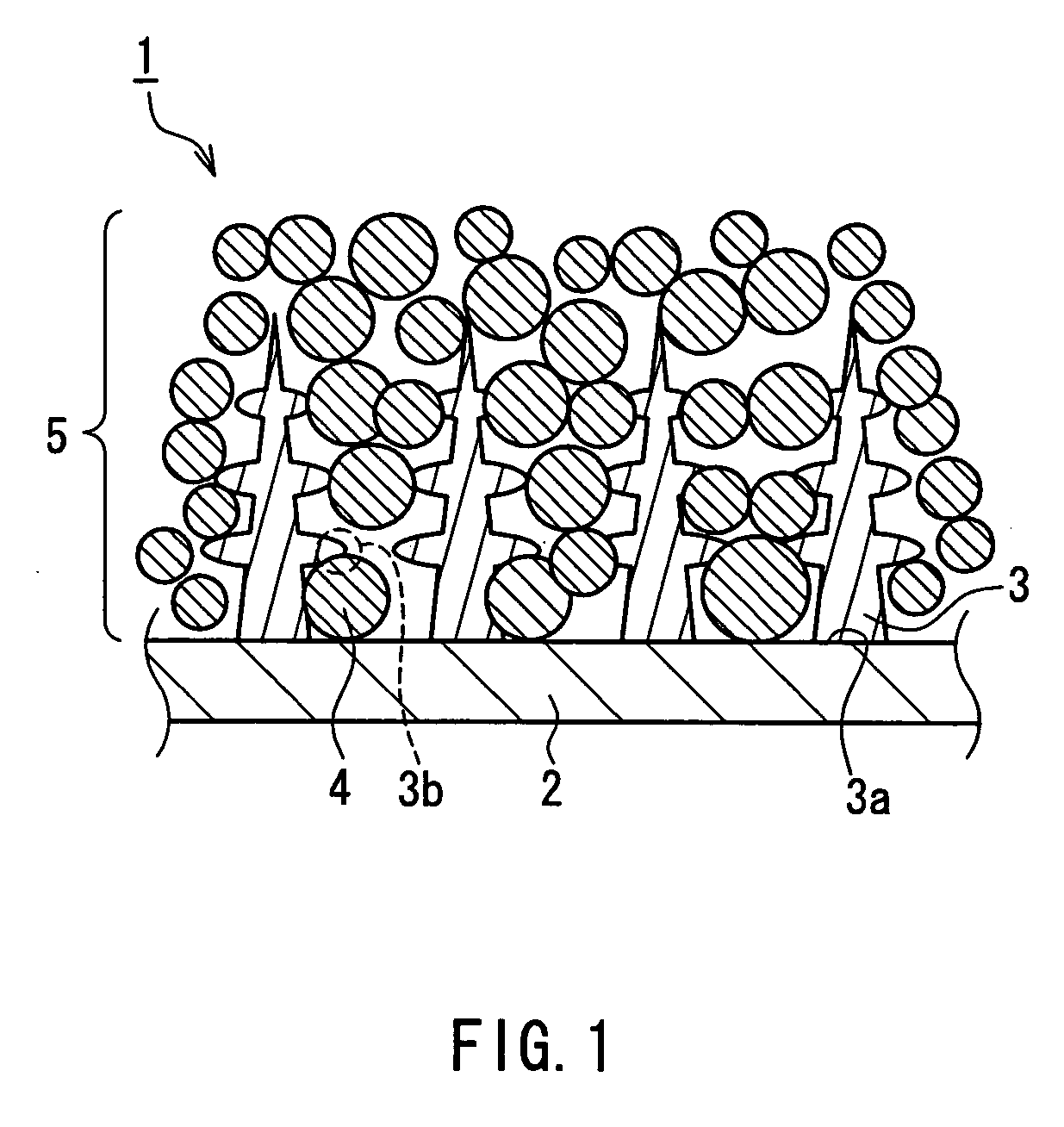
The metal foil of the positive electrode 1a or the negative electrode 1b in the power generating element 1 is connected along the connecting plate portion 2b which is folded, twisted, and provided in a protruding condition from the main portion 2a of the current-collector connector 2; hence the shape of the current-collector connector 2 becomes easy to form, and a battery capable of enhancing current collection efficiency, reliability and workability can be provided.
Owner:GS YUASA INT LTD
Sulfur-carbon nanocomposites and their application as cathode materials in lithium-sulfur batteries
ActiveUS20110052998A1Minimize formationEasy to transportAlkaline accumulatorsElectrode thermal treatmentCarbon compositesPorous carbon
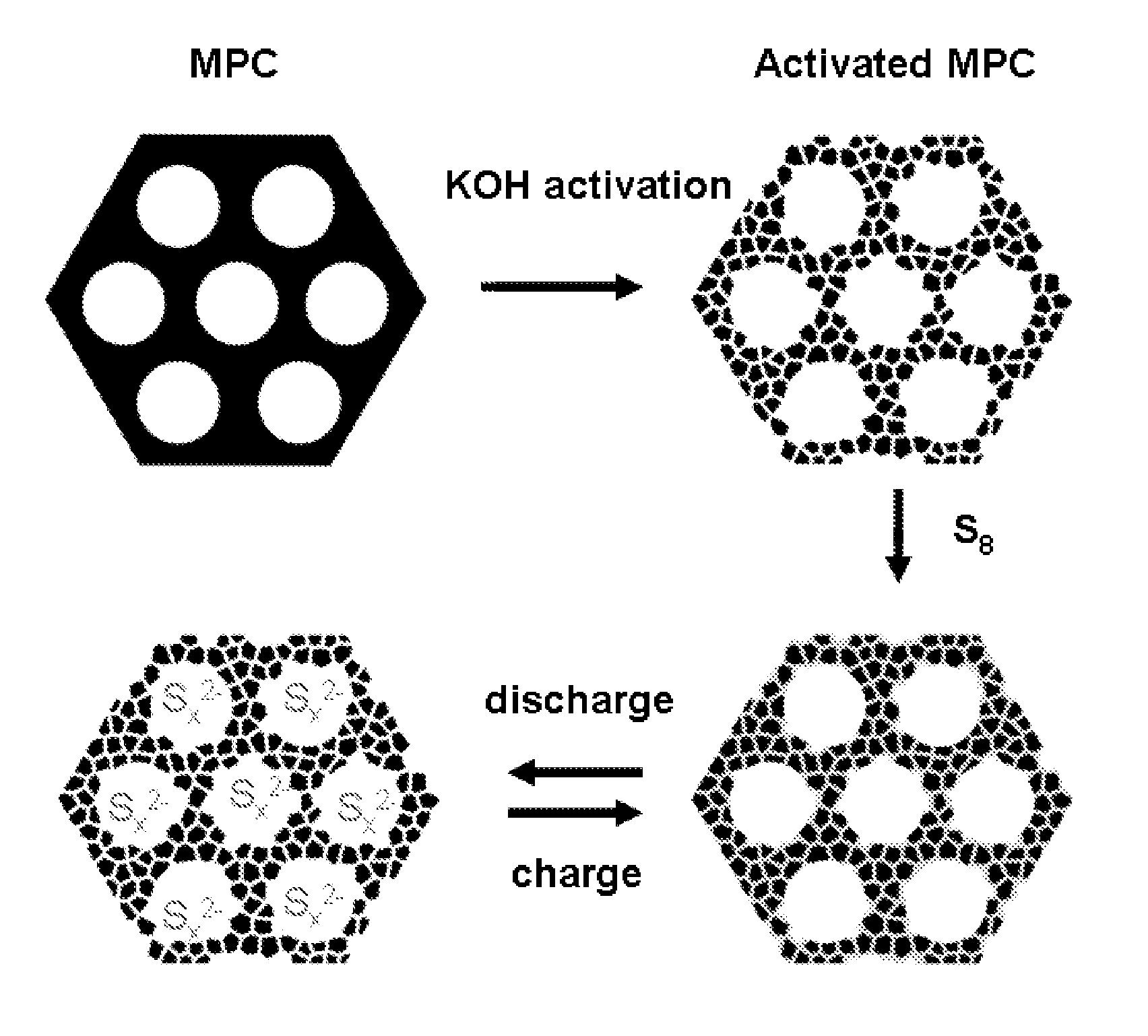
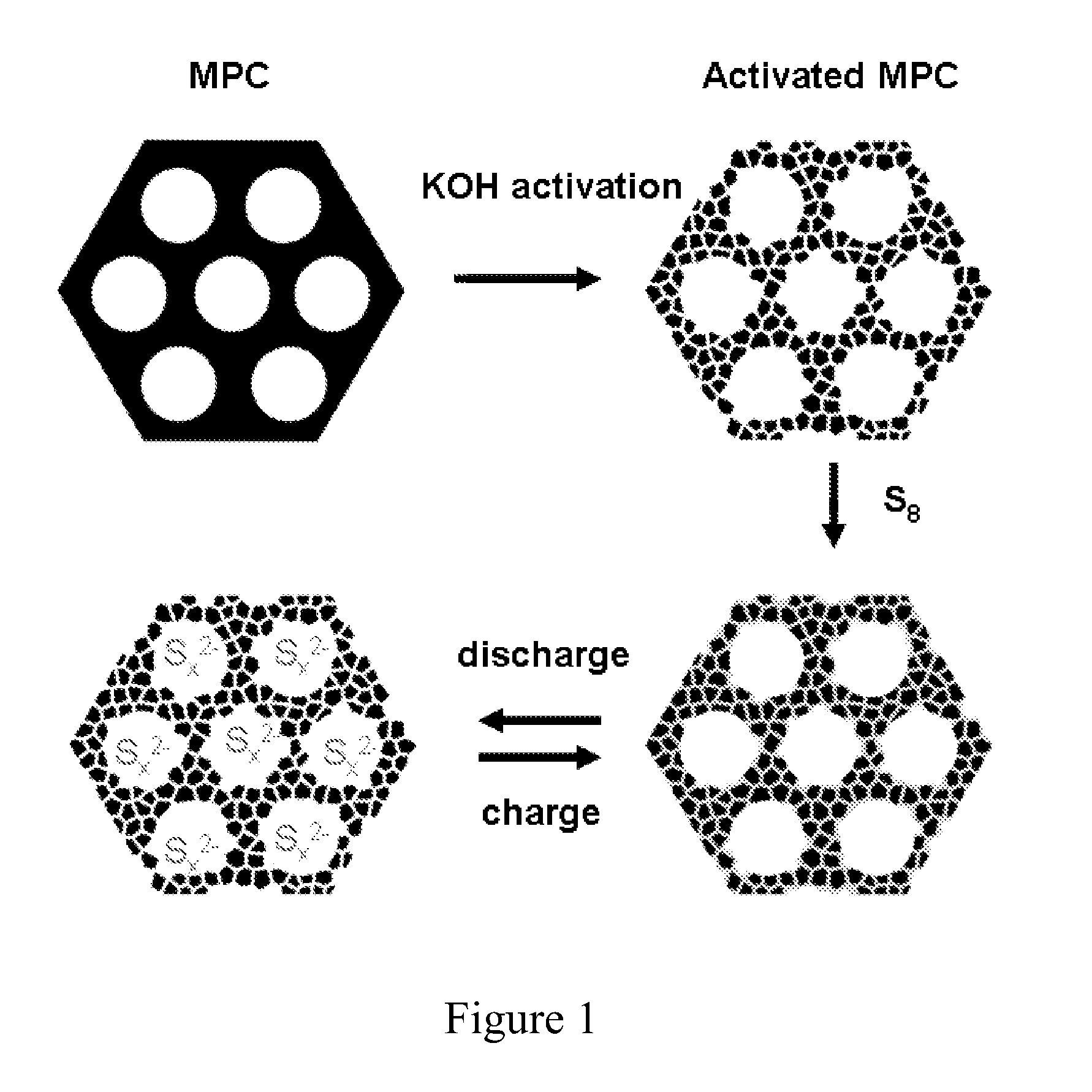
The invention is directed in a first aspect to a sulfur-carbon composite material comprising: (i) a bimodal porous carbon component containing therein a first mode of pores which are mesopores, and a second mode of pores which are micropores; and (ii) elemental sulfur contained in at least a portion of said micropores. The invention is also directed to the aforesaid sulfur-carbon composite as a layer on a current collector material; a lithium ion battery containing the sulfur-carbon composite in a cathode therein; as well as a method for preparing the sulfur-composite material.
Owner:UT BATTELLE LLC
Primer for battery electrode
ActiveUS20100291442A1Electrode carriers/collectorsActive material electrodesPolyvinyl alcoholCrosslinked polymers
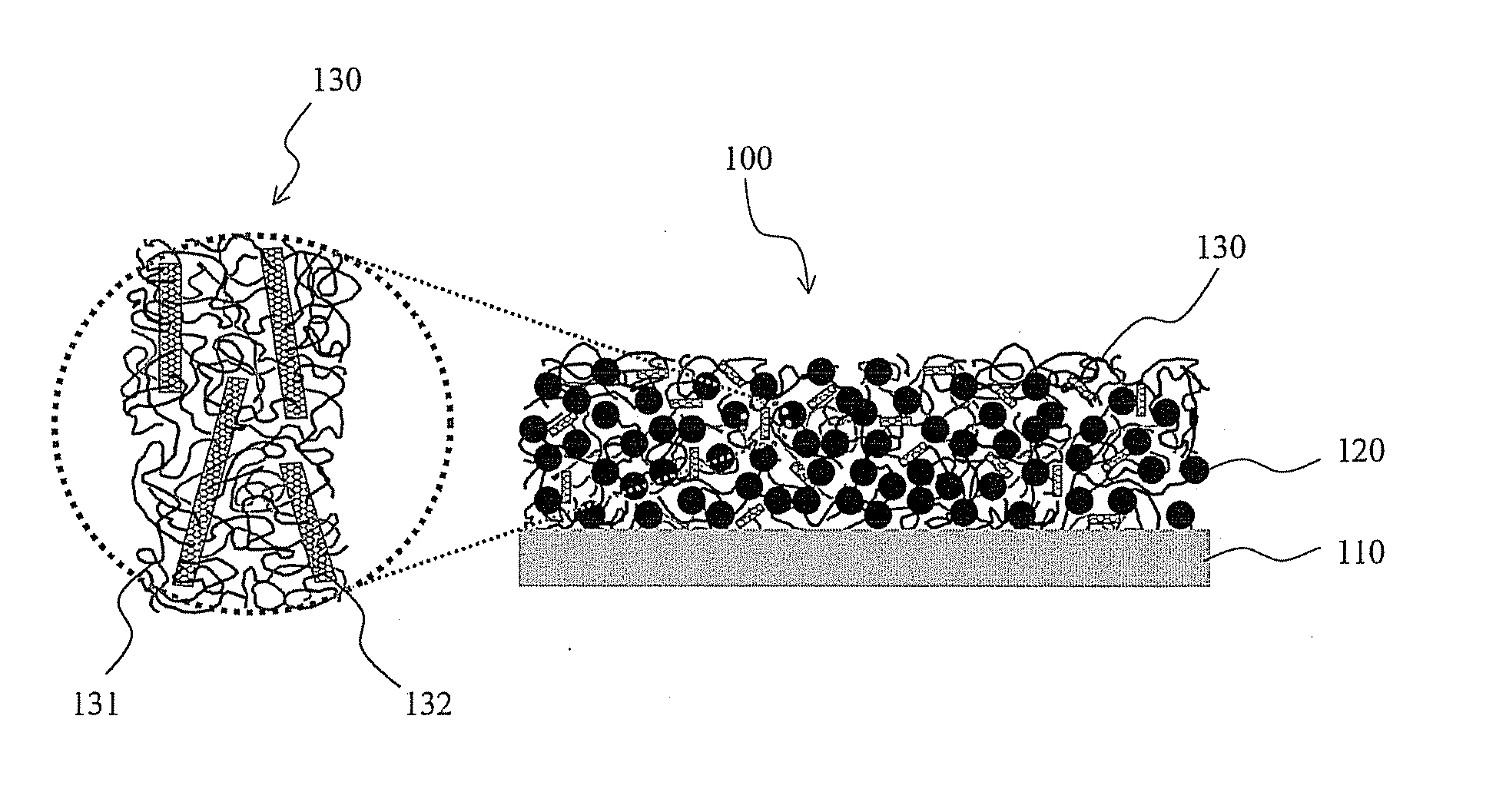
Primer arrangements that facilitate electrical conduction and adhesive connection between an electroactive material and a current collector are presented. In some embodiments, primer arrangements described herein include first and second primer layers. The first primer layer may be designed to provide good adhesion to a conductive support. In one particular embodiment, the first primer layer comprises a substantially uncrosslinked polymer having hydroxyl functional groups, e.g., polyvinyl alcohol. The materials used to form the second primer layer may be chosen such that the second primer layer adheres well to both the first primer layer and an electroactive layer. In certain embodiments including combinations of first and second primer layers, one or both of the first and second primer layers comprises less than 30% by weight of a crosslinked polymeric material. A primer including only a single layer of polymeric material is also provided.
Owner:SION POWER CORP
Supercapacitor having electrode material comprising single-wall carbon nanotubes and process for making the same
InactiveUS20060098389A1Avoid shortingHybrid capacitor electrolytesHybrid capacitor electrodesSupercapacitorNanotube
The present invention relates to a supercapacitor, also known as an electrical double-layer capacitor or ultracapacitor, having electrode material comprising single-wall carbon nanotubes. The carbon nanotubes can be derivatized with functional groups. The electrode material is made by preparing a polymer-nanotube suspension comprising polymer and nanotubes, forming the polymer-nanotube suspension into a polymer-nanotube composite of the desired form, carbonizing the polymer-nanotube composite to form a carbonaceous polymer-nanotube material, and activating the material. The supercapacitor includes electrode material comprising activated carbonaceous polymer-nanotube material in contact with current collectors and permeated with an electrolyte, which may be either fluid or solid. In the case of a fluid or compressible electrolyte, an electrolyte-permeable separator or spacer is interposed between the electrodes to keep the electrodes from shorting. The supercapacitor made with electrodes comprising underivatized single-wall carbon nanotubes and polymer that has been carbonized and activated appears to operate as a non-Faradaic supercapacitor.
Owner:GEORGIA TECH RES CORP
Plating technique for electrode
ActiveUS20130017441A1Electrode manufacturing processesFinal product manufactureElectrical batteryEngineering
Articles and methods for forming protected electrodes for use in electrochemical cells, including those for use in rechargeable lithium batteries, are provided. In some embodiments, the articles and methods involve an electrode that does not include an electroactive layer, but includes a current collector and a protective structure positioned directly adjacent the current collector, or separated from the current collector by one or more thin layers. Lithium ions may be transported across the protective structure to form an electroactive layer between the current collector and the protective structure. In some embodiments, an anisotropic force may be applied to the electrode to facilitate formation of the electroactive layer.
Owner:SION POWER CORP
Metal-air battery with ion-conducting inorganic glass electrolyte
InactiveUS20060063051A1Safe and reliable solid-stateIncrease energy densityFuel and primary cellsSolid electrolytesMetal–air electrochemical cellIonic conductivity
A solid-state metal-air electrochemical cell comprising: (A) a metal-containing electro-active anode; (B) an oxygen electro-active cathode; and (C) an ion-conducting glass electrolyte disposed between the metal-containing anode and the oxygen electro-active cathode. The cathode active material, which is oxygen gas, is not stored in the battery but rather fed from the environment. The oxygen cathode is preferably a composite carbon electrode which serves as the cathode current collector on which oxygen molecules are reduced during discharge of the battery to generate electric current. The glass electrolyte typically has an ion conductivity in the range of 5×10−5 to 2×10−3 S / cm. The electrolyte layer is preferably smaller than 10 μm in thickness and further preferably smaller than 1 μm. The anode metal is preferably lithium or lithium alloy, but may be selected from other elements such as sodium, magnesium, calcium, aluminum and zinc.
Owner:JANG BOR Z
Nonaqueous electrolyte
ActiveUS20050221188A1Excellent characteristicsNon-aqueous electrolyte accumulatorsElectrode carriers/collectorsLithiumMaterials science
A nonaqueous electrolyte battery includes a positive electrode containing an active material, a negative electrode, and a nonaqueous electrolyte, the negative electrode including a current collector and a negative electrode active material supported by the current collector, the negative electrode active material having a Li insertion potential not lower than 0.2V (vs. Li / Li+) and an average primary particle diameter not larger than 1 μm, and a specific surface area of the negative electrode, excluding a weight of the current collector, as determined by the BET method falls within a range of 3 to 50 m2 / g.
Owner:KK TOSHIBA
Lithium secondary battery
InactiveUS20090087731A1Improve charge-discharge cycle performanceCell electrodesSecondary cellsSilicon alloyComposite oxide
A lithium secondary battery includes: a positive electrode having a positive electrode active material layer disposed on a positive electrode current collector, the positive electrode active material layer containing a positive electrode binder and a positive electrode active material containing a layered lithium-transition metal composite oxide; a negative electrode having a negative electrode current collector and a negative electrode active material layer disposed on the negative electrode current collector, the negative electrode active material layer containing a negative electrode binder and a negative electrode active material containing particles of silicon and / or a silicon alloy; and a non-aqueous electrolyte. Al2O3 particles are firmly adhered to a surface of the lithium-transition metal composite oxide so that a BET specific surface area of the positive electrode active material after the adherence of the Al2O3 particles is from 1.5 times to 8 times greater than that before the adherence of the Al2O3 particles
Owner:SANYO ELECTRIC CO LTD
Composite binder containing carbon nanotube and lithium secondary battery employing the same
ActiveUS20070202403A1Improve mechanical propertiesGood strength propertiesNon-metal conductorsMaterial nanotechnologyLithiumCarbon nanotube
Provided is a nanocomposite binder for an electrode mix of a secondary battery, comprising carbon nanotubes in a photo- and / or thermo-polymerizable material or polymer, or a mixture thereof; and a lithium secondary battery comprising the same. The carbon nanotube-containing composite binder according to the present invention and a lithium secondary battery comprising the same employs a novel nanocomposite, prepared by combination of carbon nanotubes with a conventional binder material, as a binder of an anode. As a result, the present invention provides advantages such as improved conductivity of the anode due to decreased electrical resistance of the binder, and enhanced mechanical properties of the binder and thereby being capable of preventing the separation of an anode active material layer from a current collector due to volume changes occurring upon charge / discharge cycles.
Owner:LG CHEM LTD
Battery structures, self-organizing structures and related methods
InactiveUS7579112B2Maximizes electrode interface areaDistance minimizationFinal product manufactureElectrode carriers/collectorsElectrical batteryCurrent collector
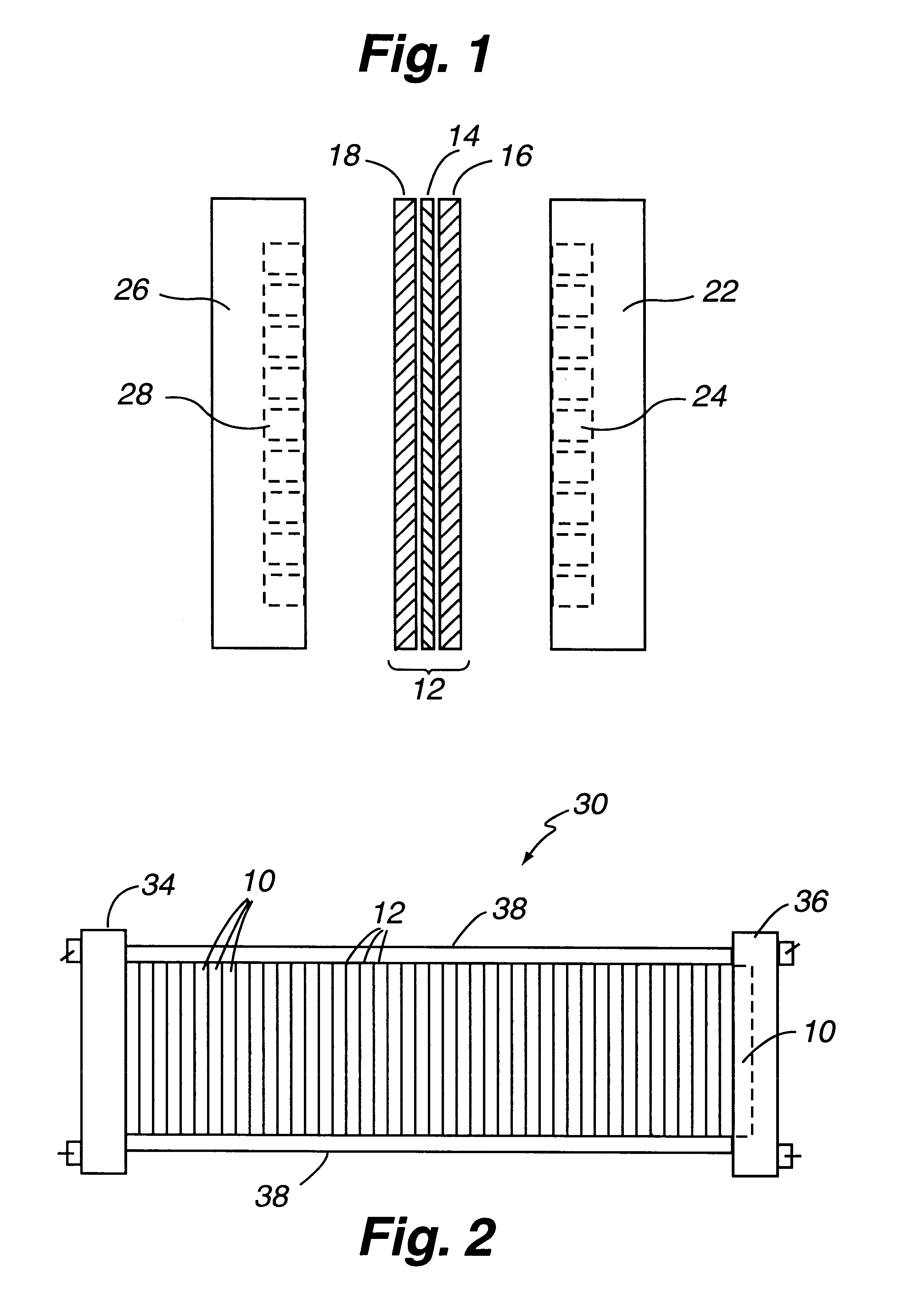
An energy storage device includes a first electrode comprising a first material and a second electrode comprising a second material, at least a portion of the first and second materials forming an interpenetrating network when dispersed in an electrolyte, the electrolyte, the first material and the second material are selected so that the first and second materials exert a repelling force on each other when combined. An electrochemical device, includes a first electrode in electrical communication with a first current collector; a second electrode in electrical communication with a second current collector; and an ionically conductive medium in ionic contact with said first and second electrodes, wherein at least a portion of the first and second electrodes form an interpenetrating network and wherein at least one of the first and second electrodes comprises an electrode structure providing two or more pathways to its current collector.
Owner:A123 SYSTEMS LLC +1
Redox flow battery
InactiveUS20120135278A1High charge-discharge efficiencyIncrease energy densityCell electrodesFinal product manufactureRedoxSlurry
A redox flow battery comprising an electrode cell including a negative electrode cell, a positive electrode cell and a separator for separating them, in which at least one of the negative electrode cell and the positive electrode cell includes a slurry type electrode solution, a porous current collector and a casing; a tank for storing the slurry type electrode solution; and a pipe for circulating the slurry type electrode solution between the tank and the electrode cell.
Owner:SHARP KK
Battery and non-aqueous electrolyte secondary battery using the same
InactiveUS20050064291A1Increase energy densityExcellent cycle characteristicsElectrode carriers/collectorsNegative electrodesEngineeringNon aqueous electrolytes
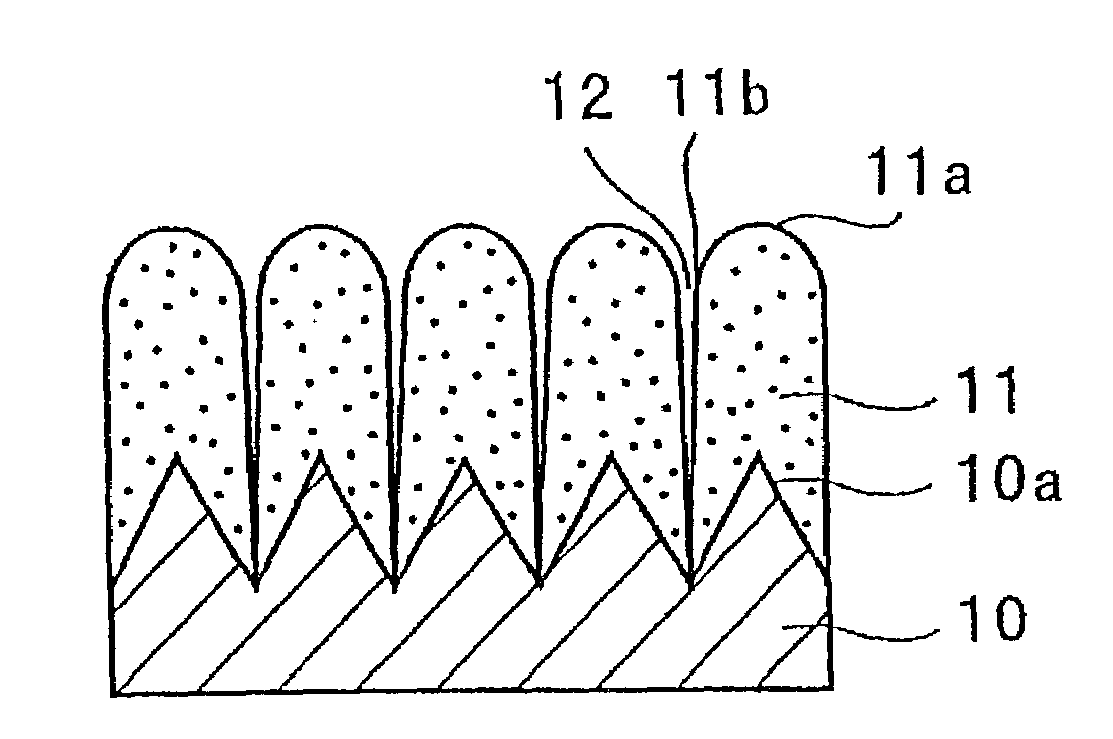
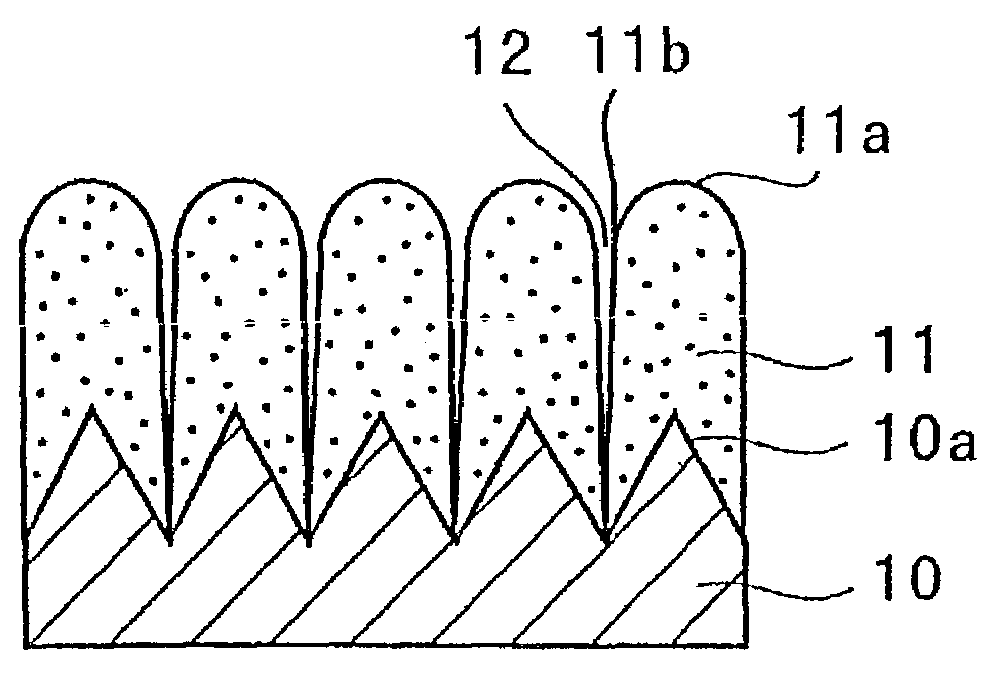
A negative electrode for a non-aqueous electrolyte secondary battery including a current collector, and an electrode material layer including an electrode material capable of reversibly absorbing and desorbing Li ions is provided. The electrode material includes at least one element selected from the group consisting of Si, Sn and Al; the surface of the current collector is provided with protrusions; the electrode material layer is disposed on the surfaces of the current collector and the protrusions; and the protrusion has a portion facing the surface of the current collector other than a portion that is brought into contact with the current collector. Thus, a negative electrode for a non-aqueous electrolyte battery having high properties such as an energy density, charging / discharging cycle property, and the like, and a non-aqueous electrolyte secondary battery can be provided.
Owner:PANASONIC CORP
Lithium secondary battery
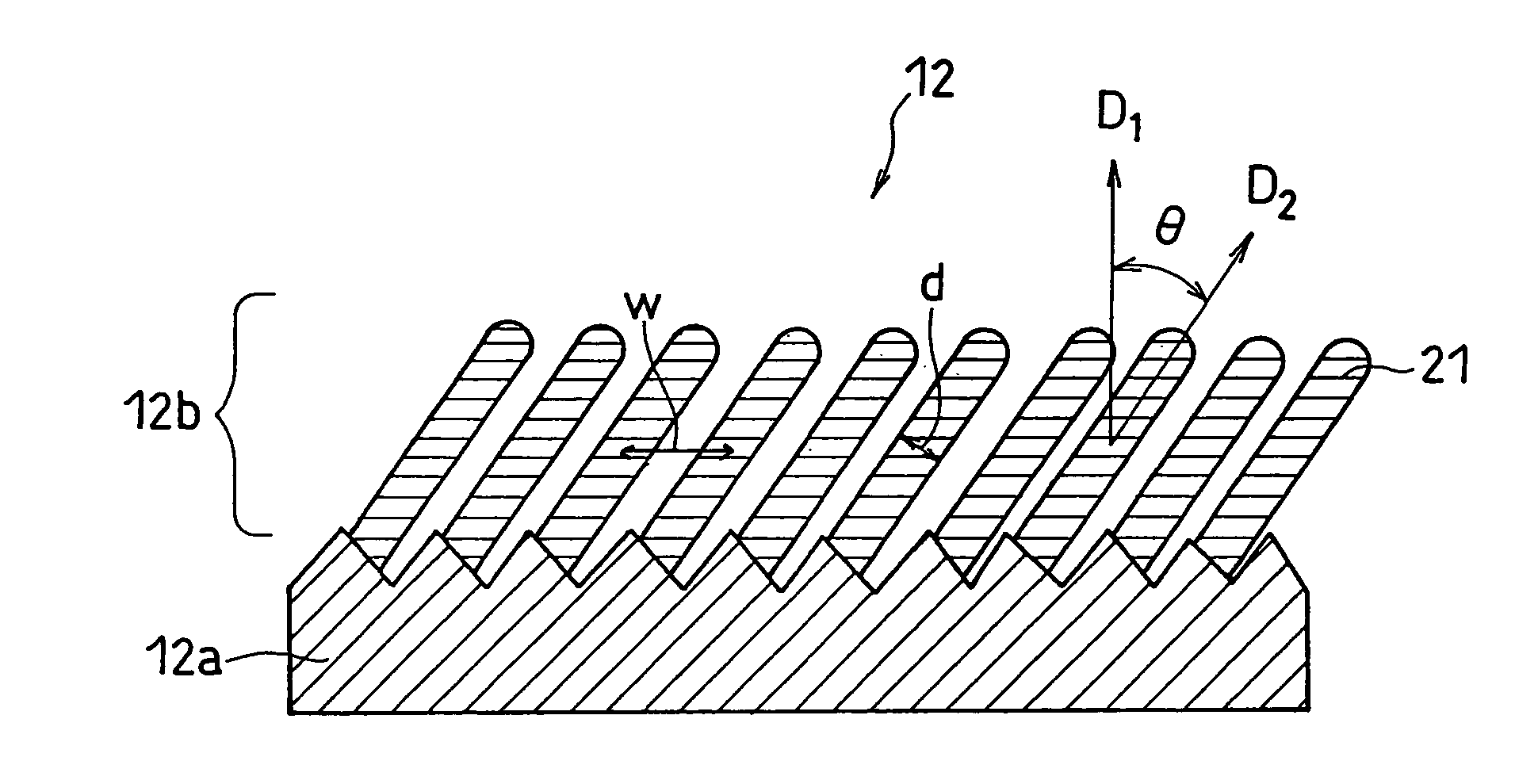
InactiveUS20070031733A1Negative currentEasy dischargeFinal product manufactureElectrode carriers/collectorsLithiumSilicon
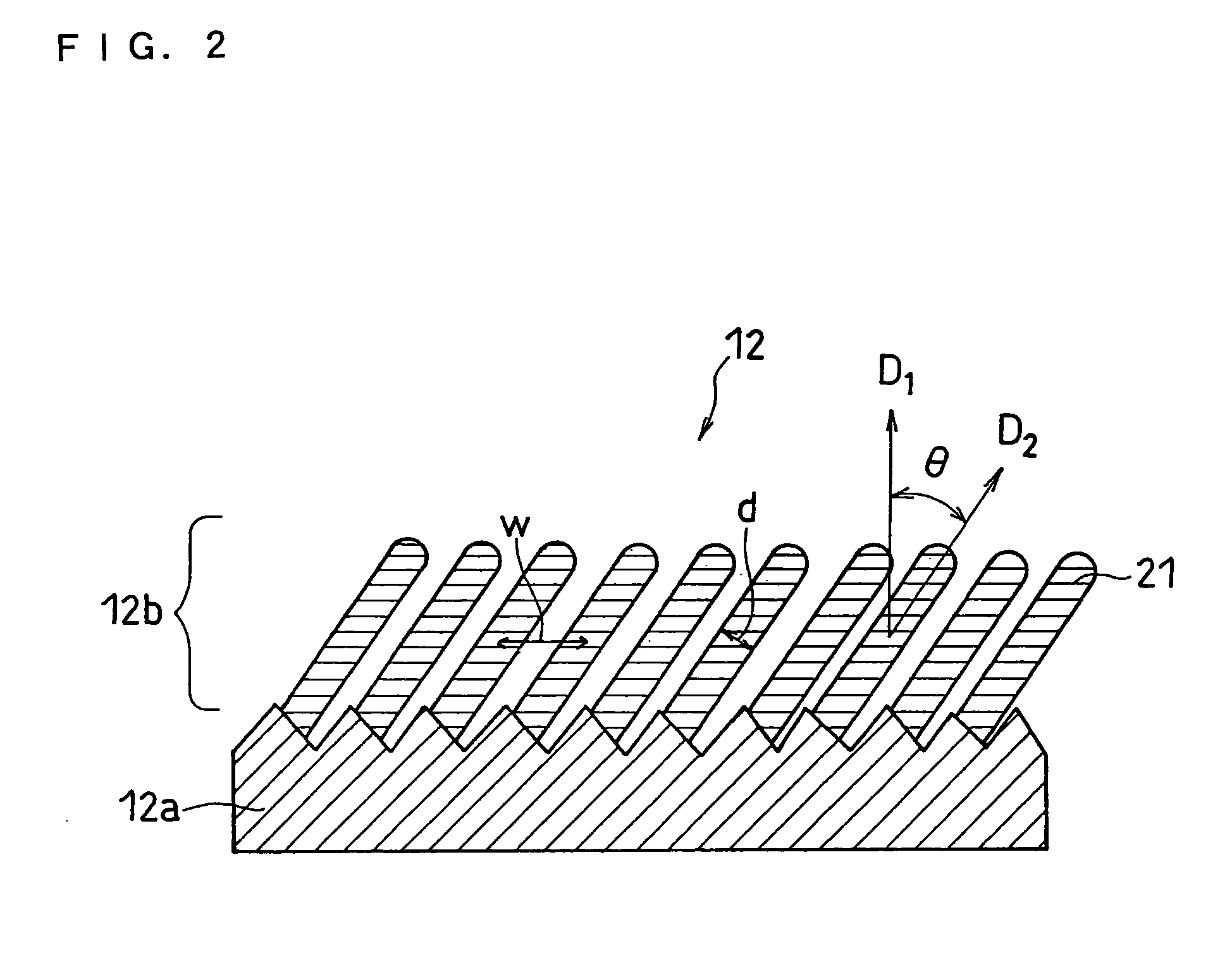
In order to enhance charge and discharge efficiency and to improve cycle characteristics by increasing a facing area between a positive electrode active material and a negative electrode active material, in a negative electrode for lithium secondary battery having a current collector and an active material layer carried on the current collector, the active material layer includes a plurality of columnar particles. The columnar particles include an element of silicon, and are tilted toward the normal direction of the current collector. Angle θ formed between the columnar particles and the normal direction of the current collector is preferably 10°≦θ<90°.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com