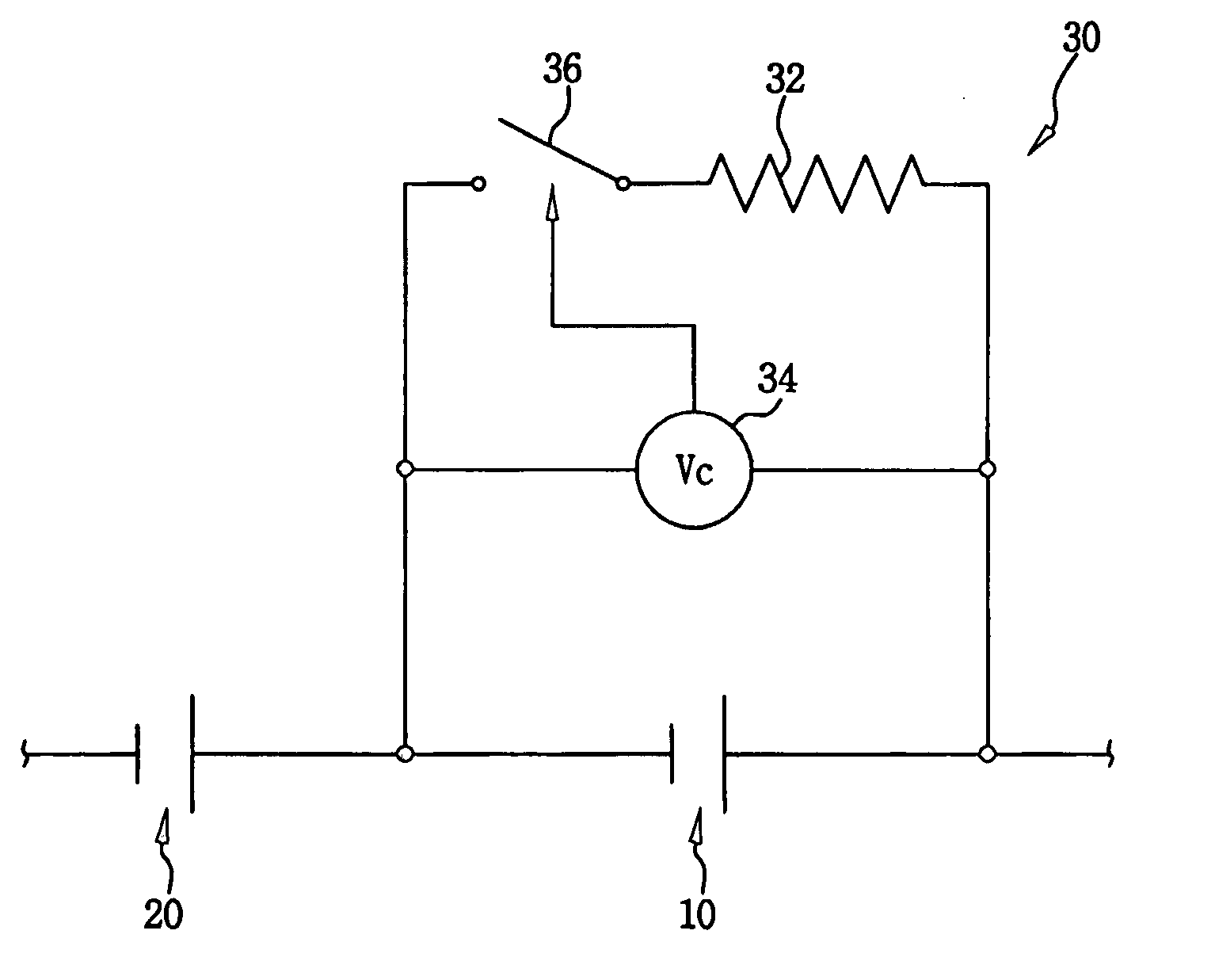
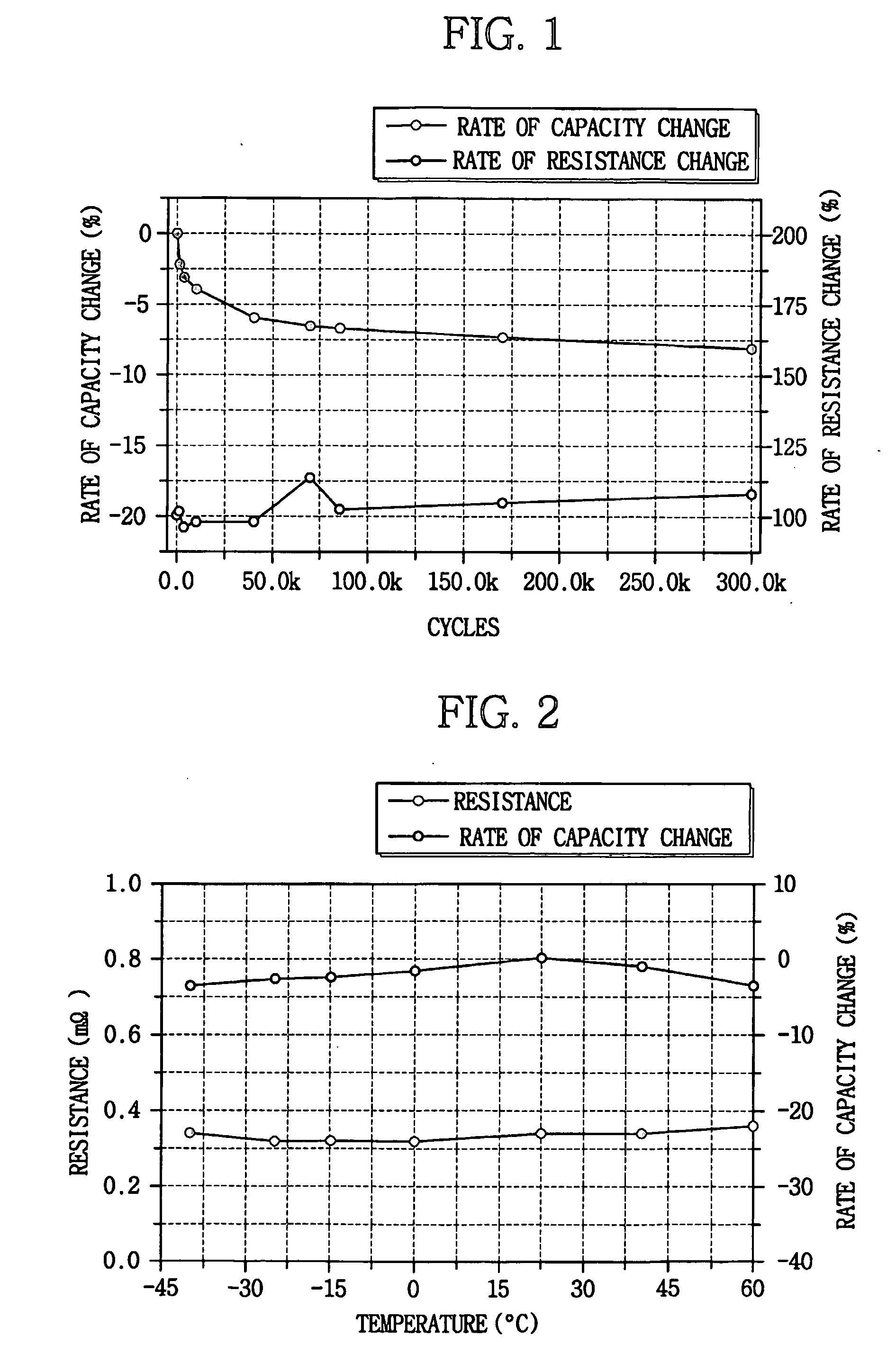
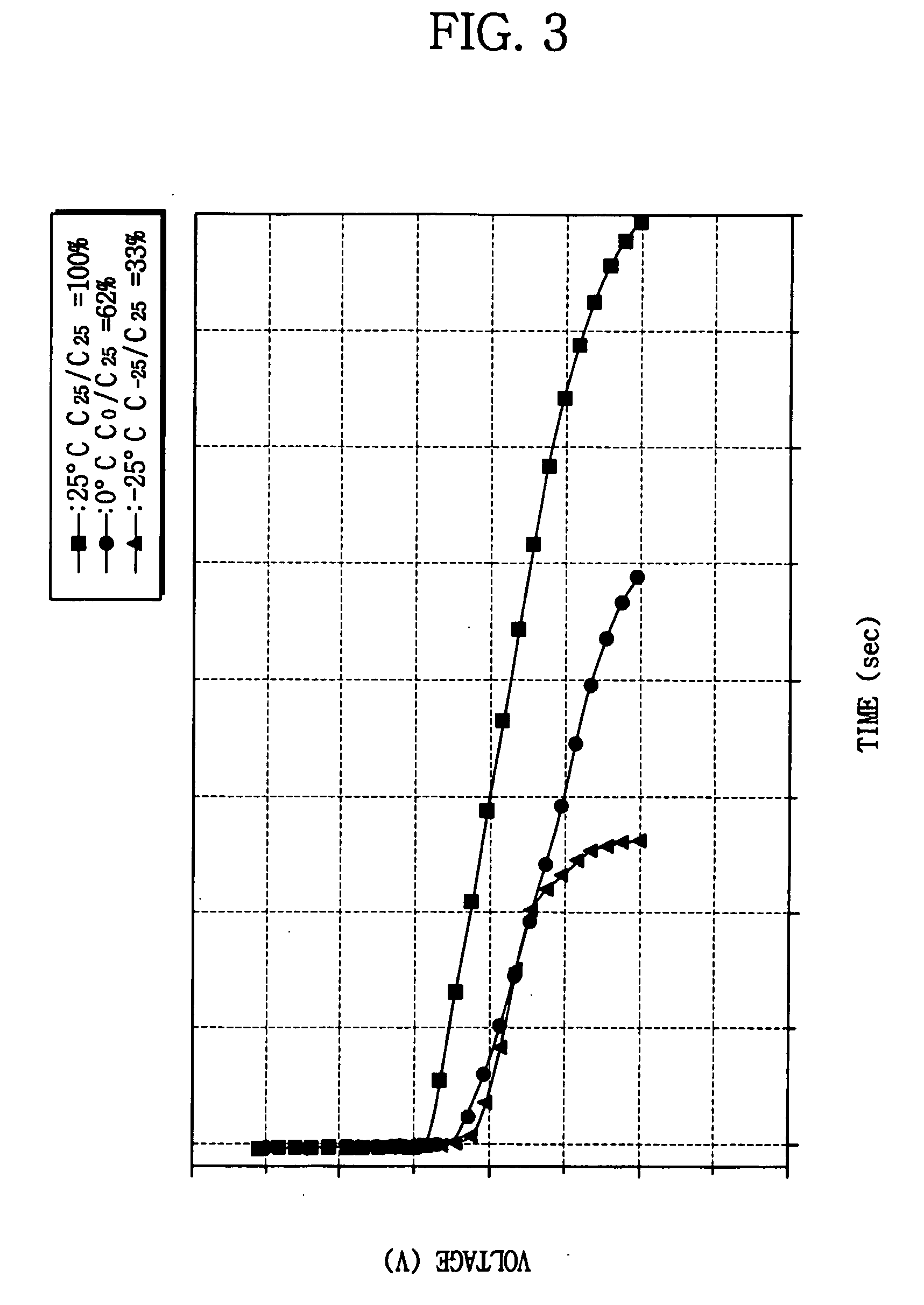
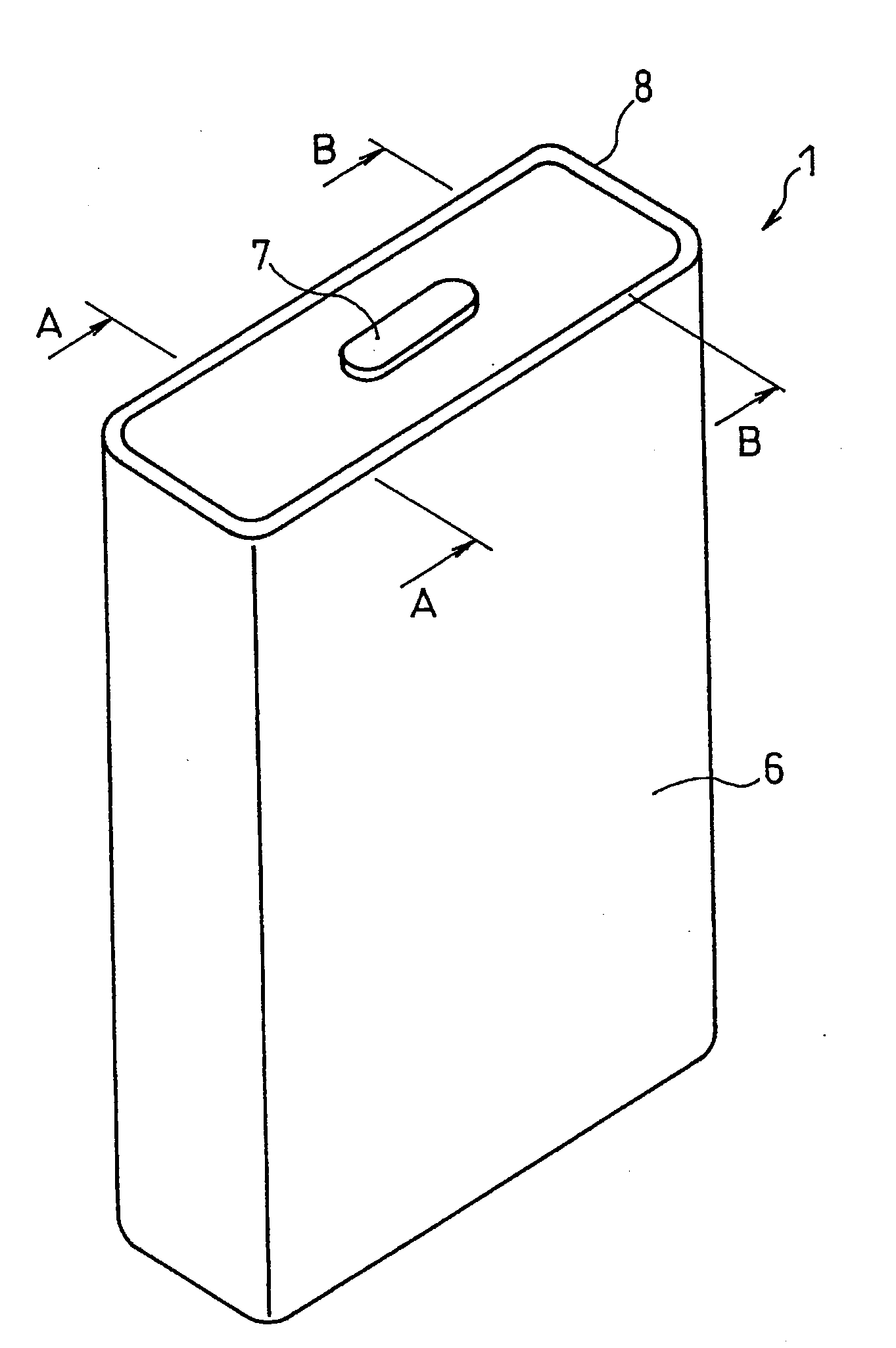
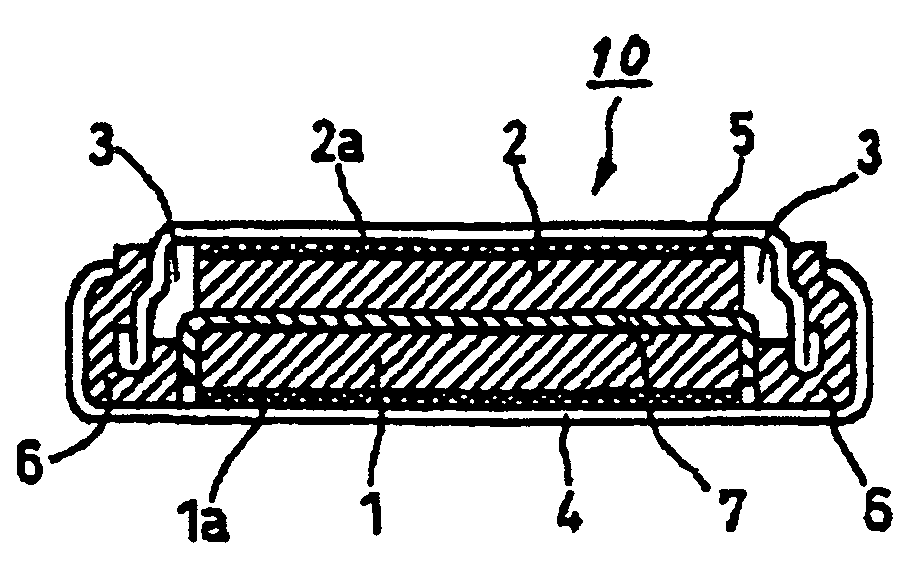
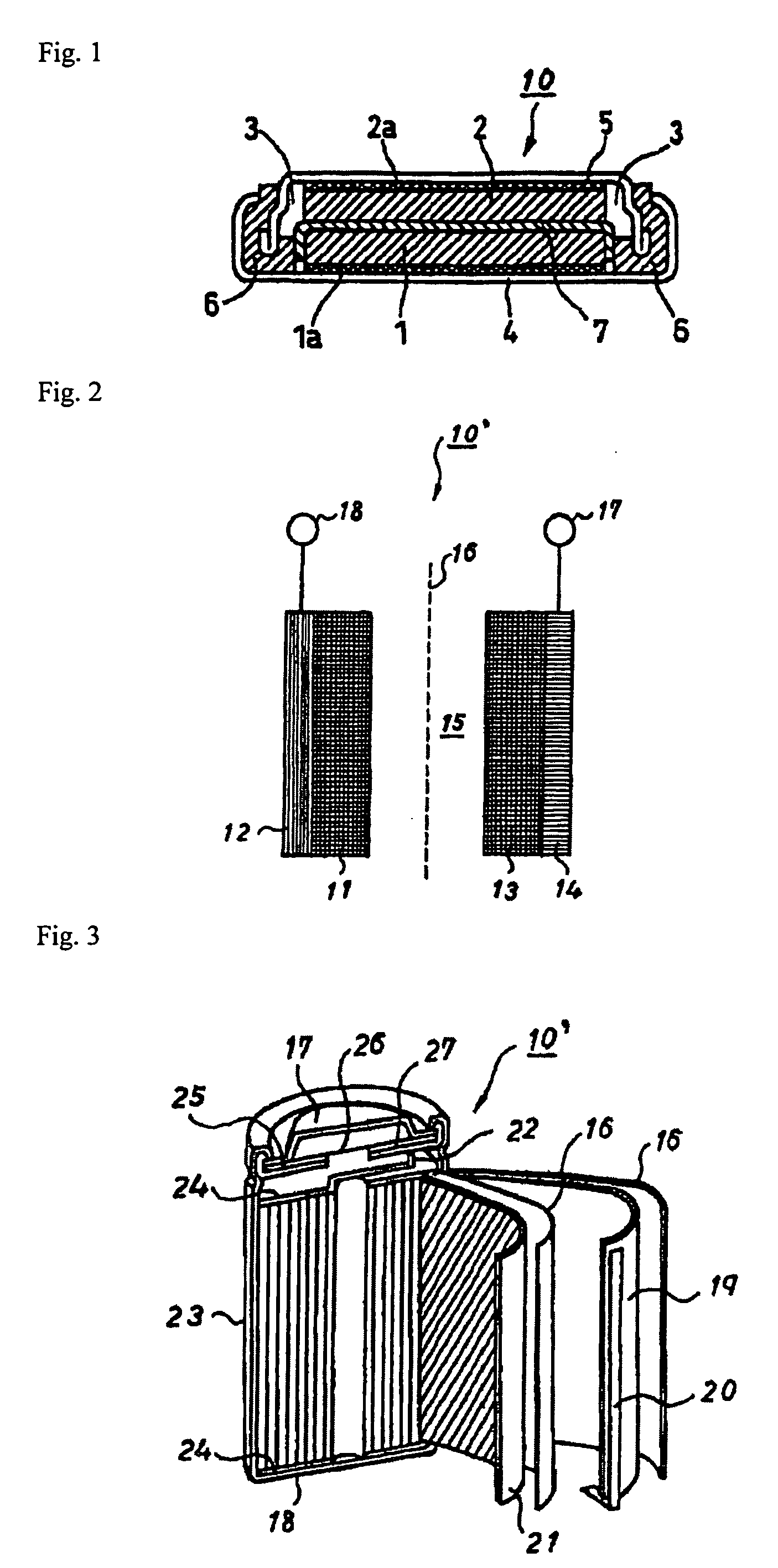
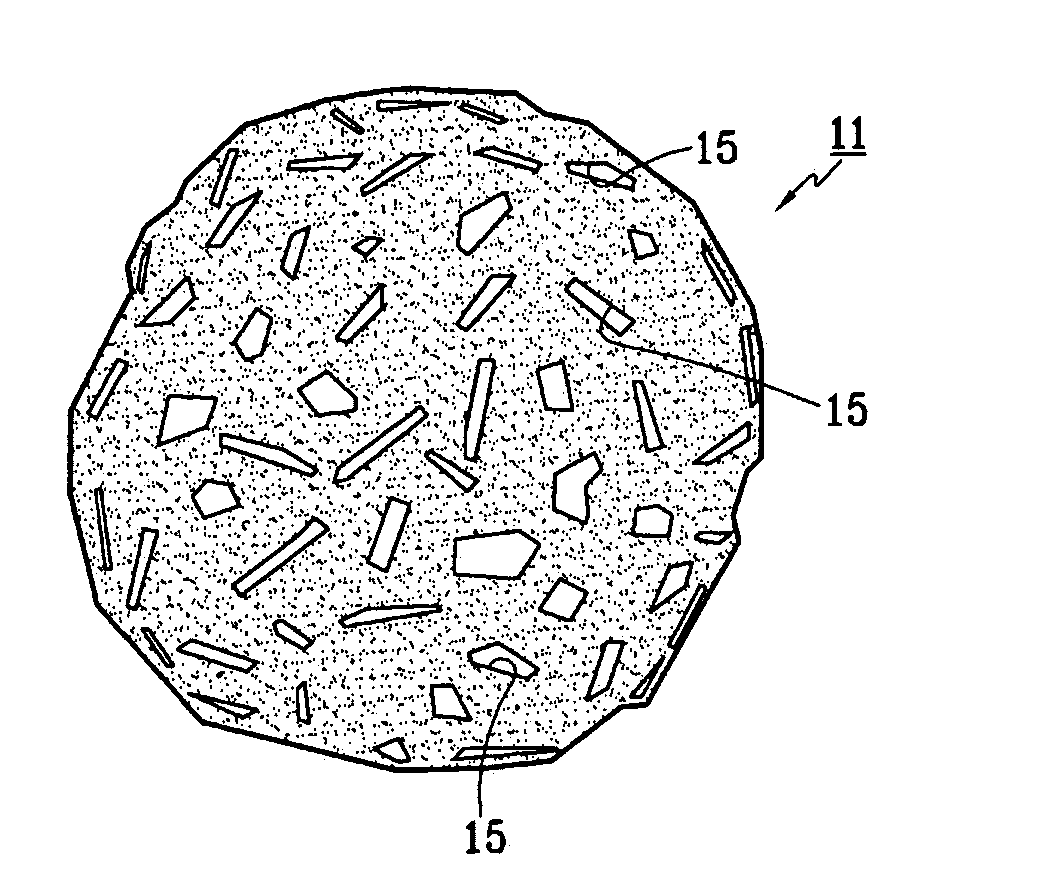
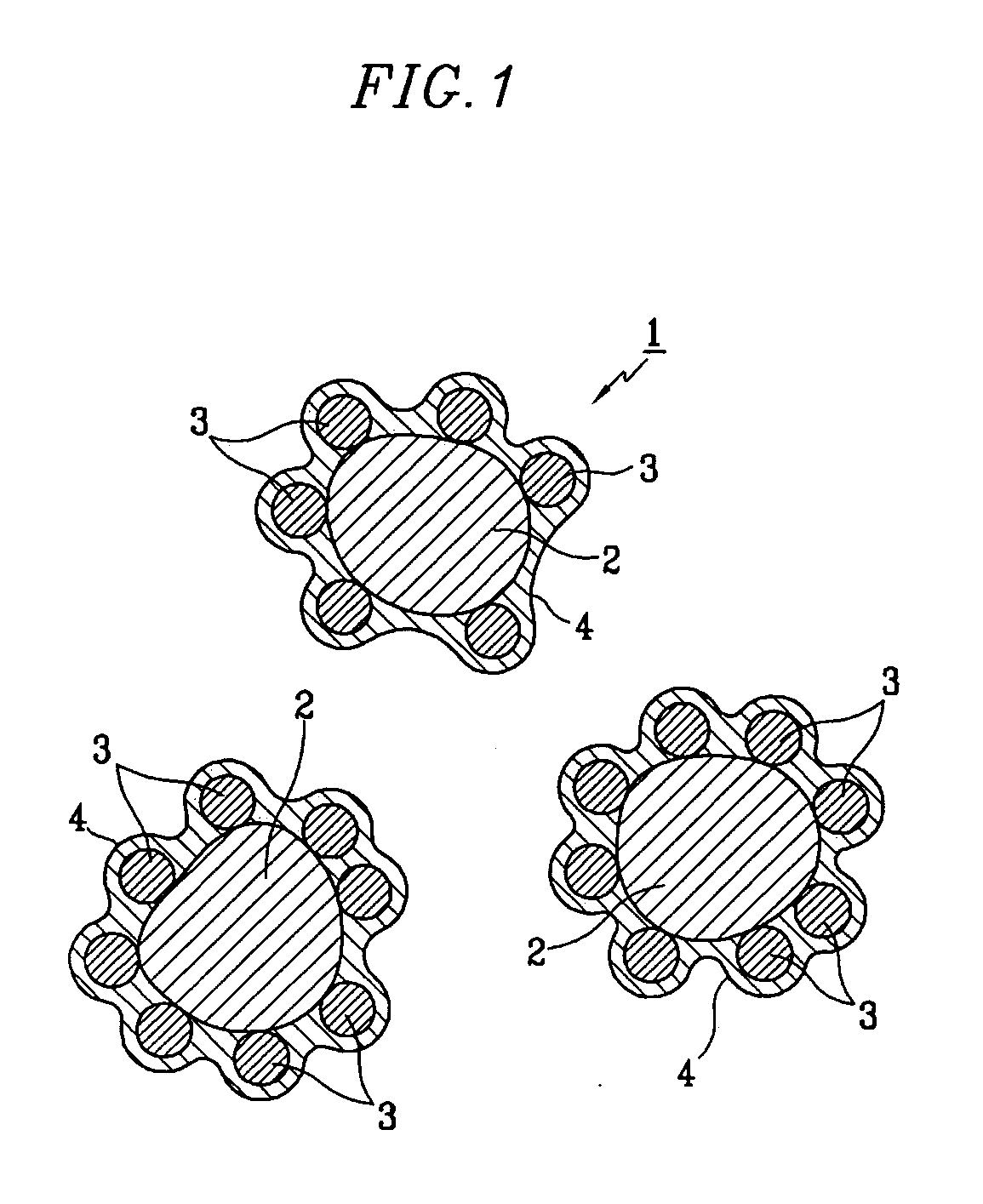
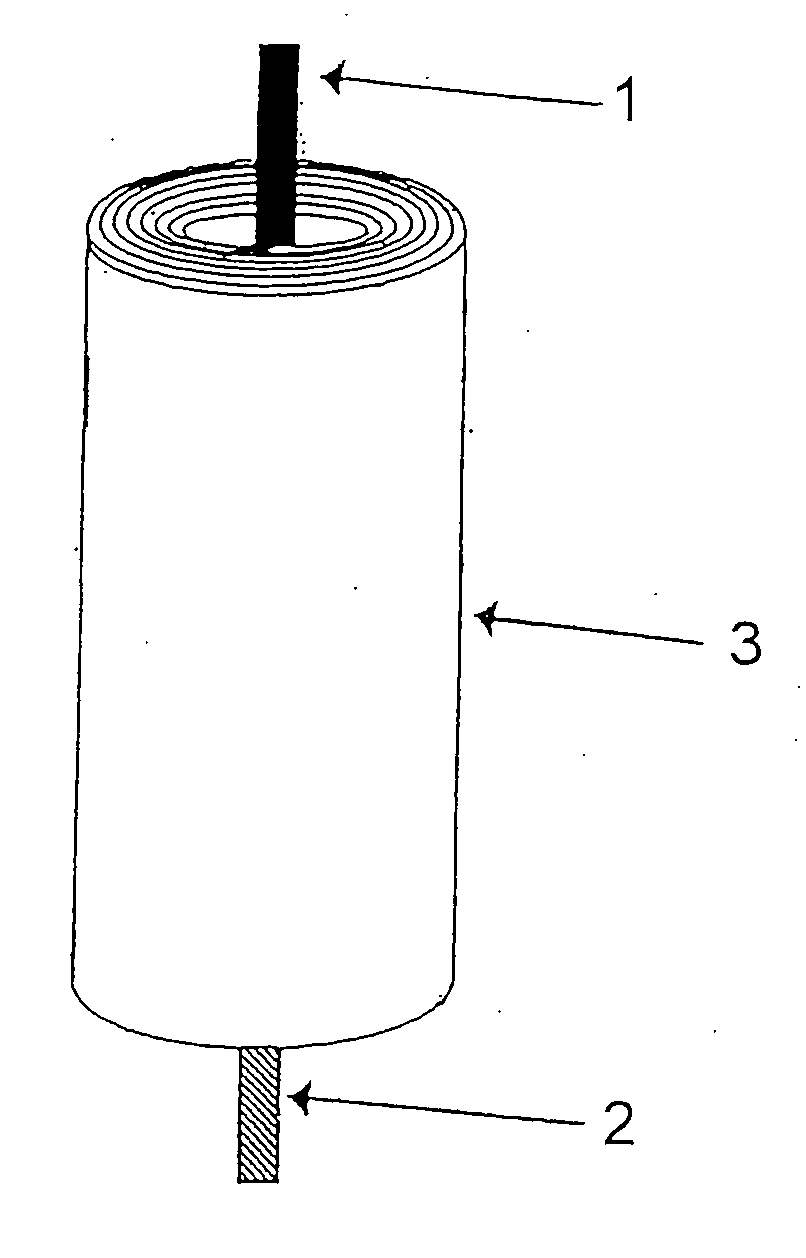
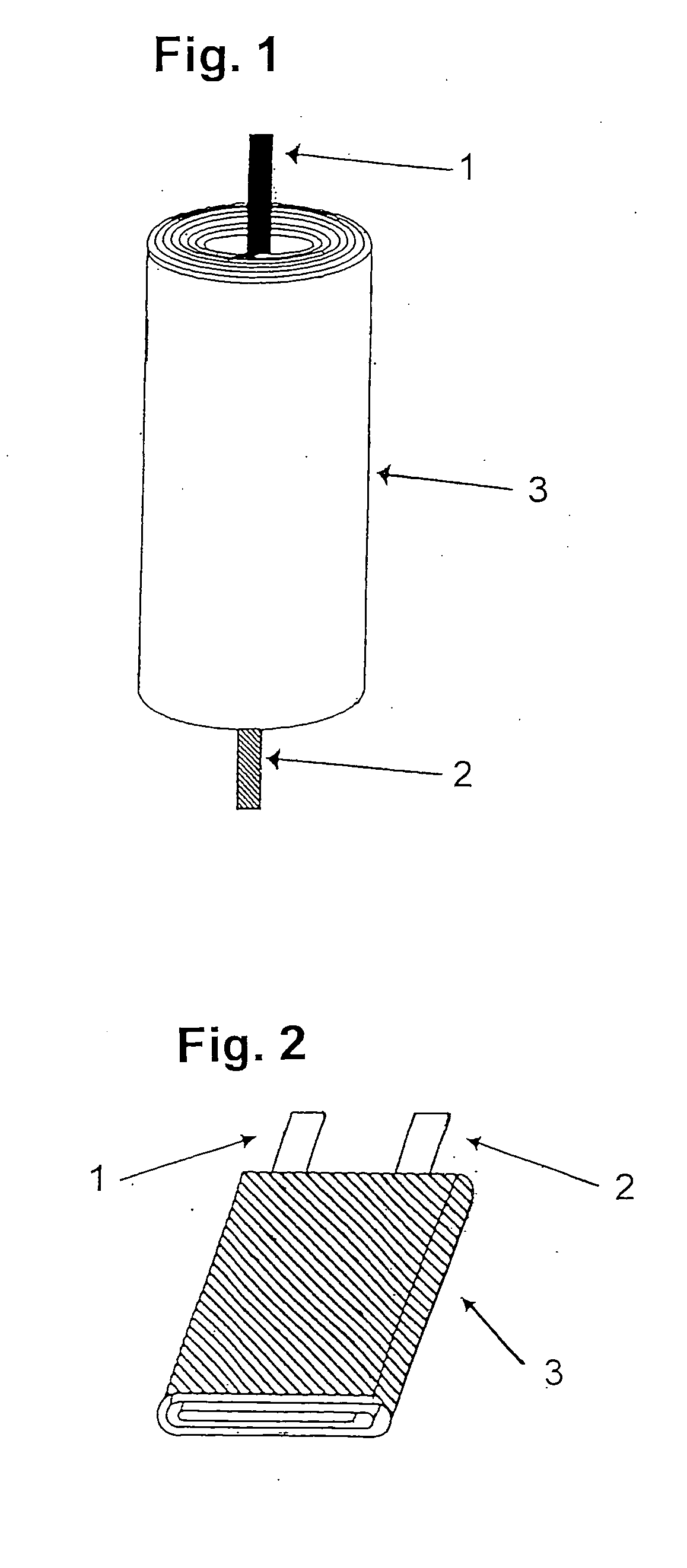
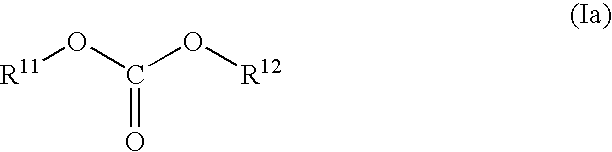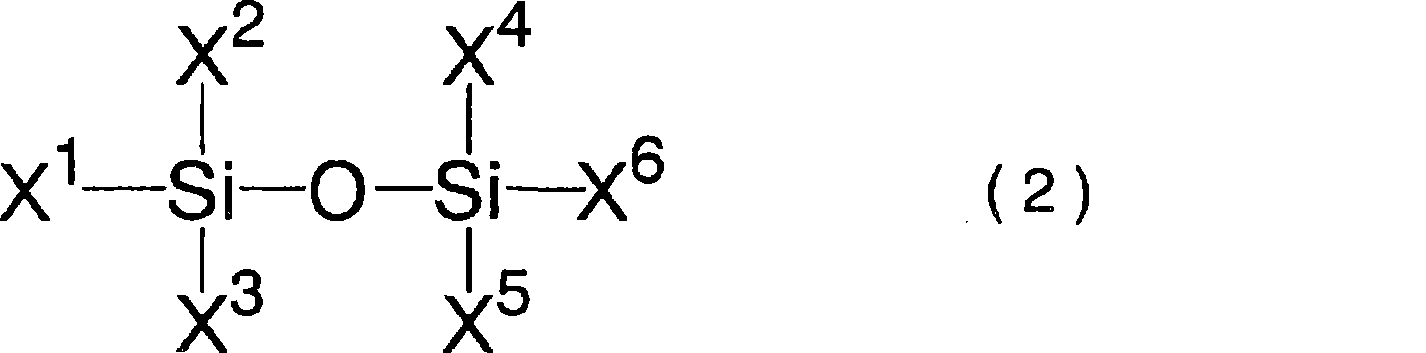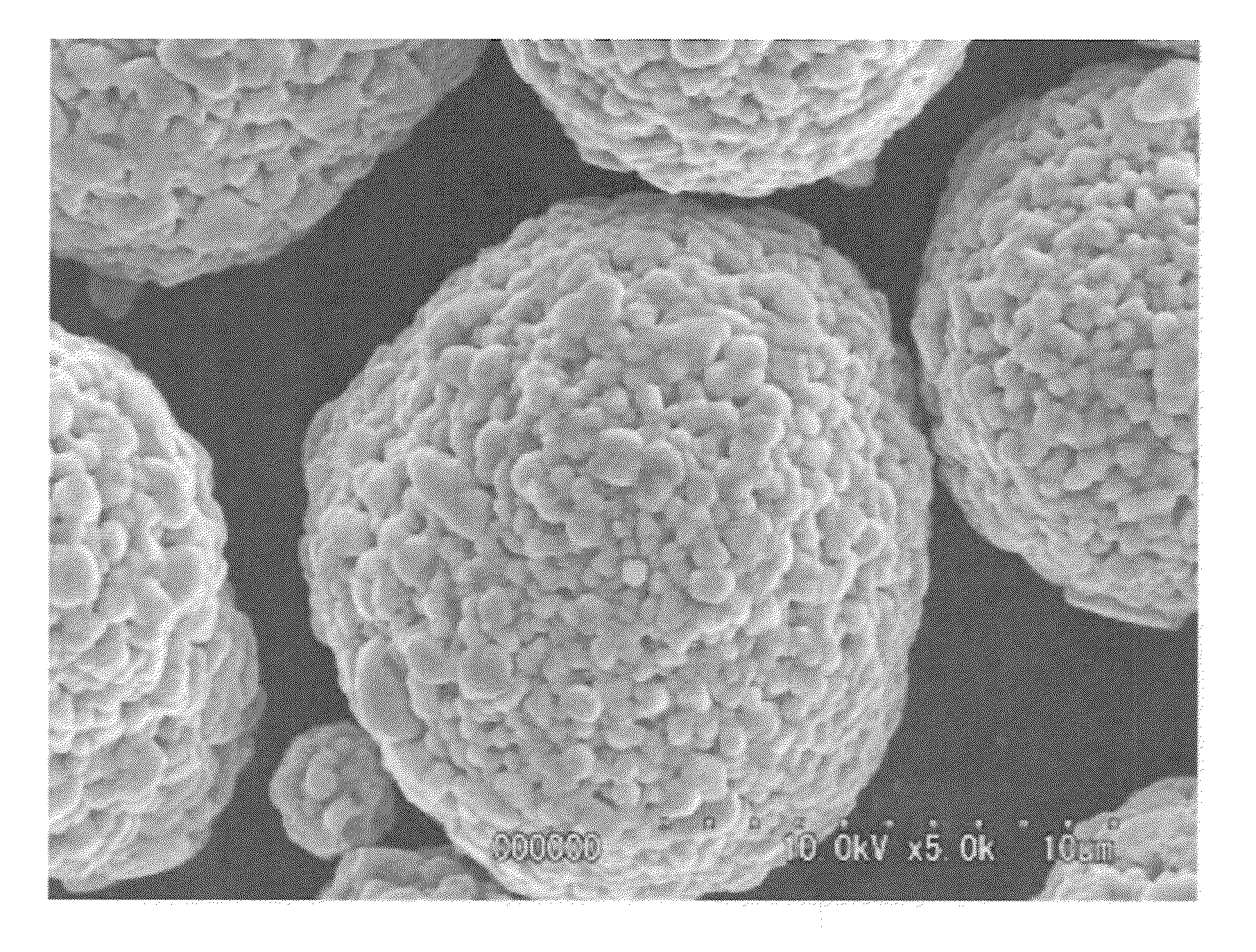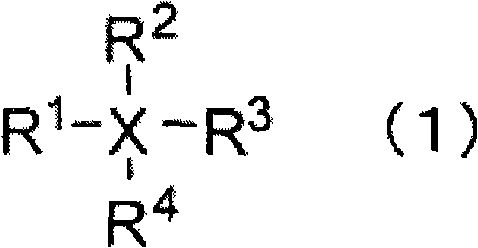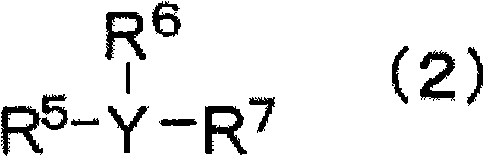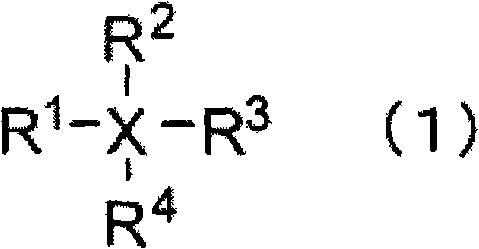Patents
Literature
1462results about How to "Excellent cycle characteristics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
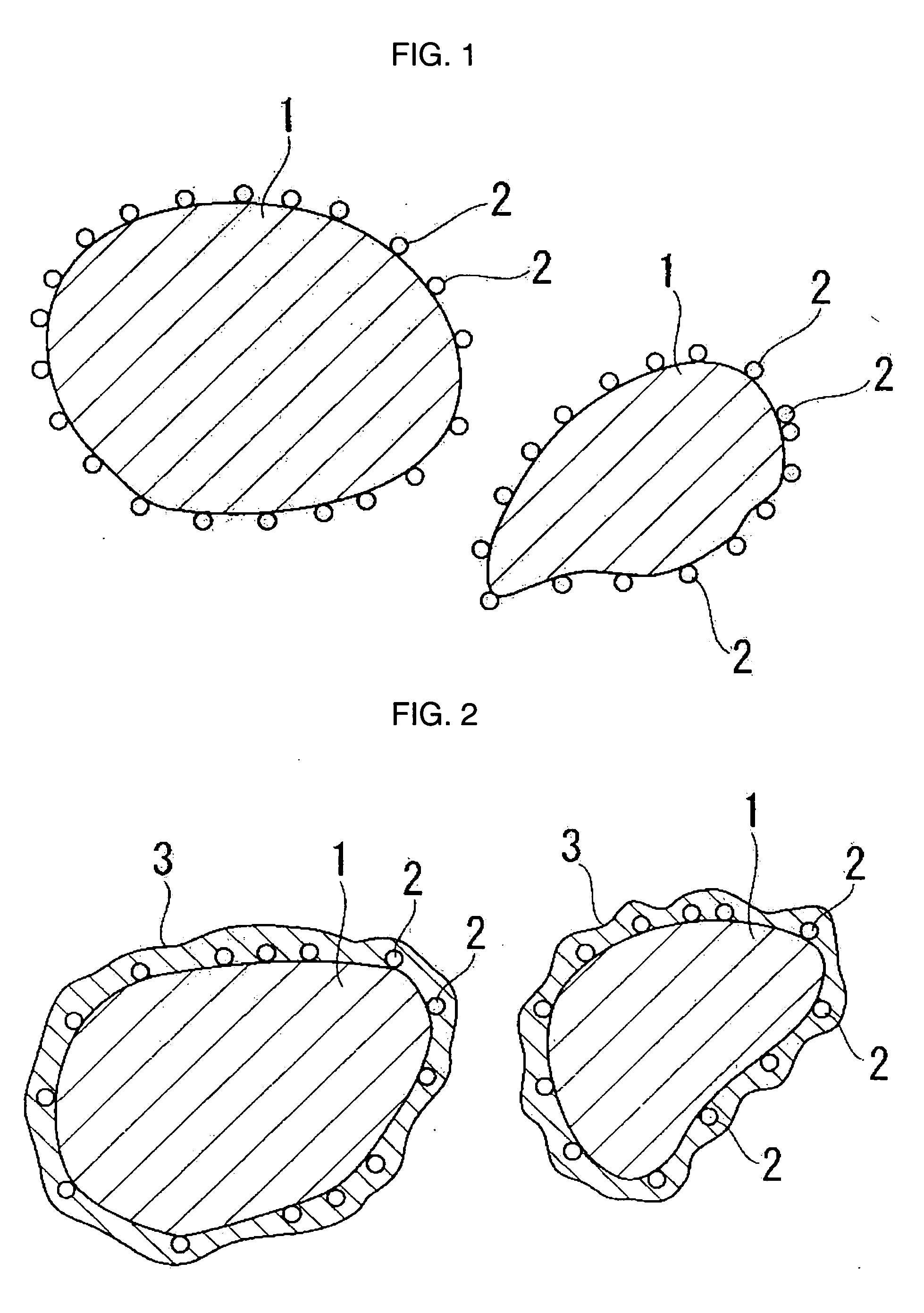
Lithium ion battery positive pole material cobalt nickel oxide manganses lithium and method for making same
ActiveCN101202343AHigh specific capacityExcellent cycle characteristicsElectrode manufacturing processesLithium compoundsLithium oxideAntioxidant
The invention relates to a nickel cobalt manganese lithium oxide material used for an anode of a li-ion battery and a preparation method. The invention belongs to the li-ion battery technical field. The nickel cobalt manganese lithium oxide material used for the anode of the li-ion battery is a li-rich laminated structure with the chemical component of Li1+zM1-x-yNixCoyO2; wherein, z is less than or equal to 0.2 and more than or equal to 0.05, x is less than or equal to 0.8 and more than 0.1, and y is less than or equal to 0.5 and more than 0.1. The preparation method of the invention is that dissoluble salt of the nickel, cobalt and manganese is taken as the raw material; ammonia or ammonium salt is taken as complexing agent; sodium hydroxide is taken as precipitator; water-dissoluble dispersant and water-dissoluble antioxidant or inert gas are added for control and protection; in a cocurrent flow type the solution is added to a reaction vessel for reaction; after alkalescence disposal, aging procedure, solid-liquid separation and washing and drying, the nickel cobalt manganese oxide is uniformly mixed with the lithium raw material; the nickel cobalt manganese lithium oxide powder is obtained by sintering the mixed powder which is divided into three temperature areas. The invention has the advantages of high specific capacity, good circulation performance, ideal crystal texture, short production period, low power loss, and being suitable for industrial production, etc.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST +1
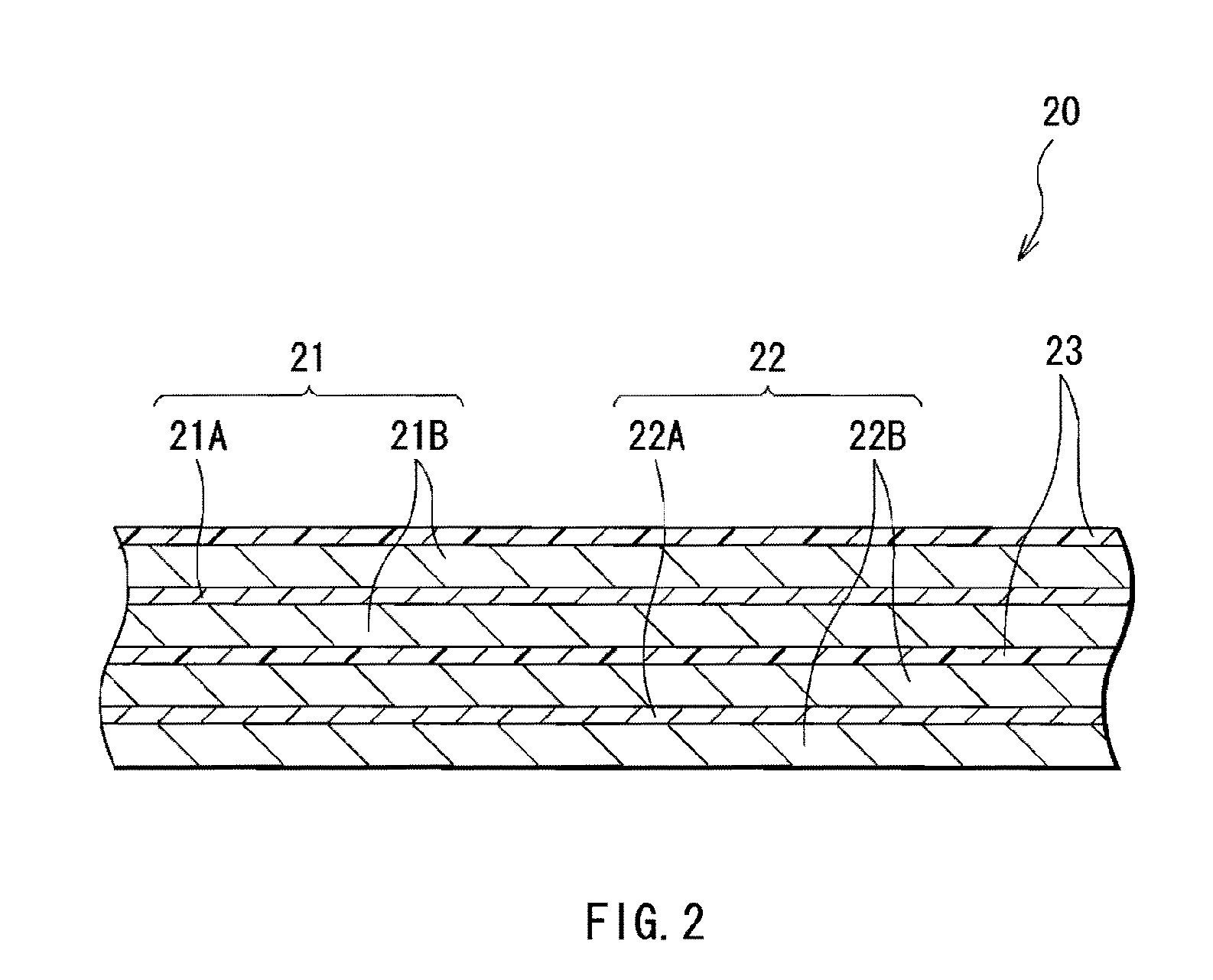
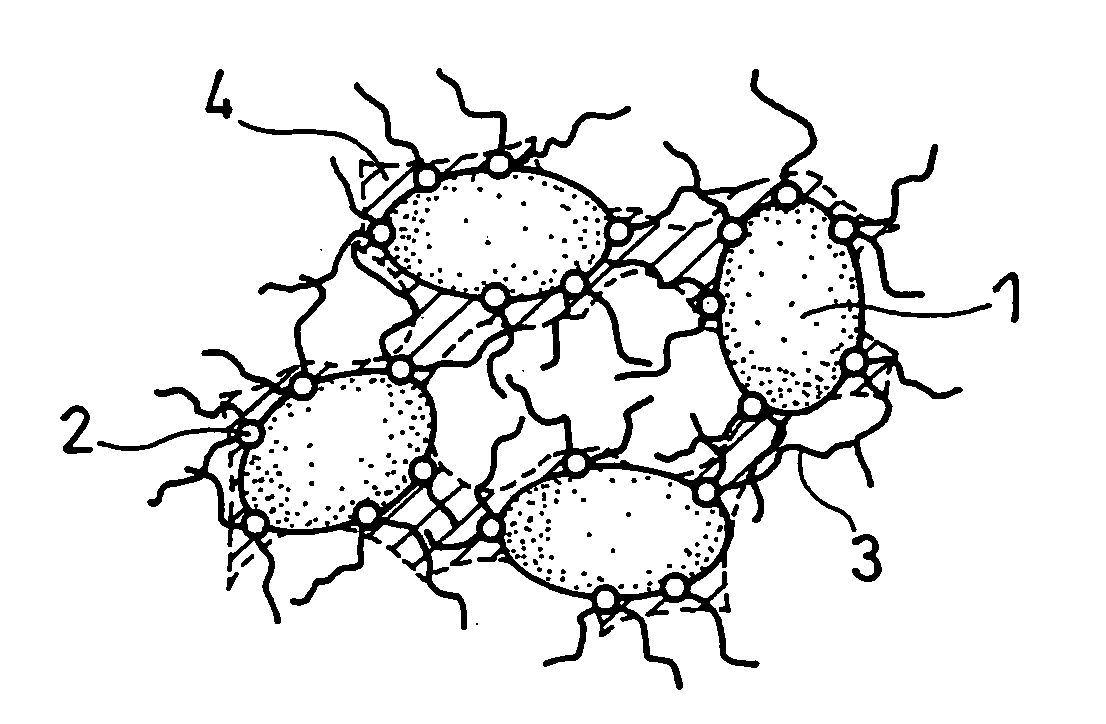
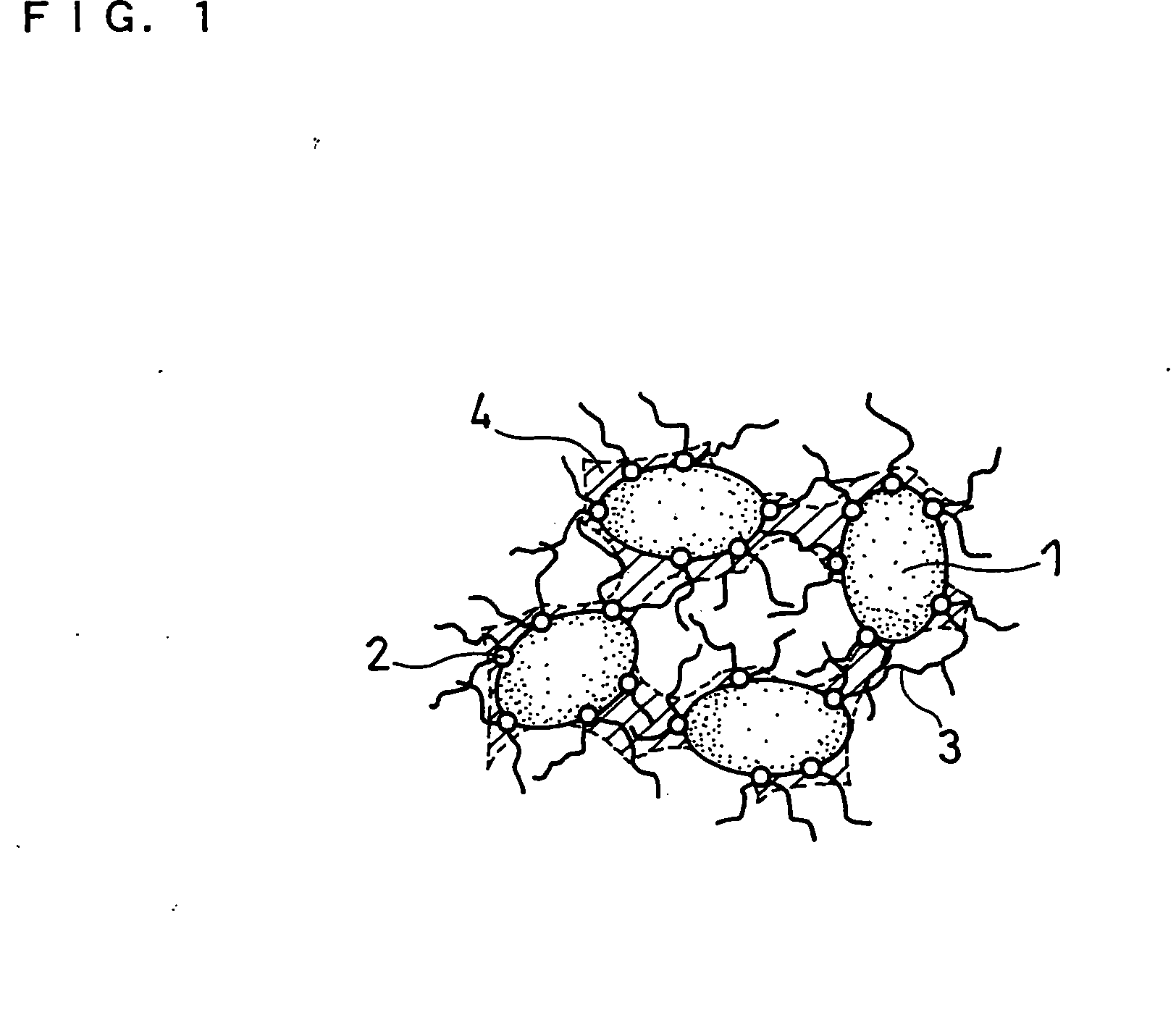
Anode and secondary battery
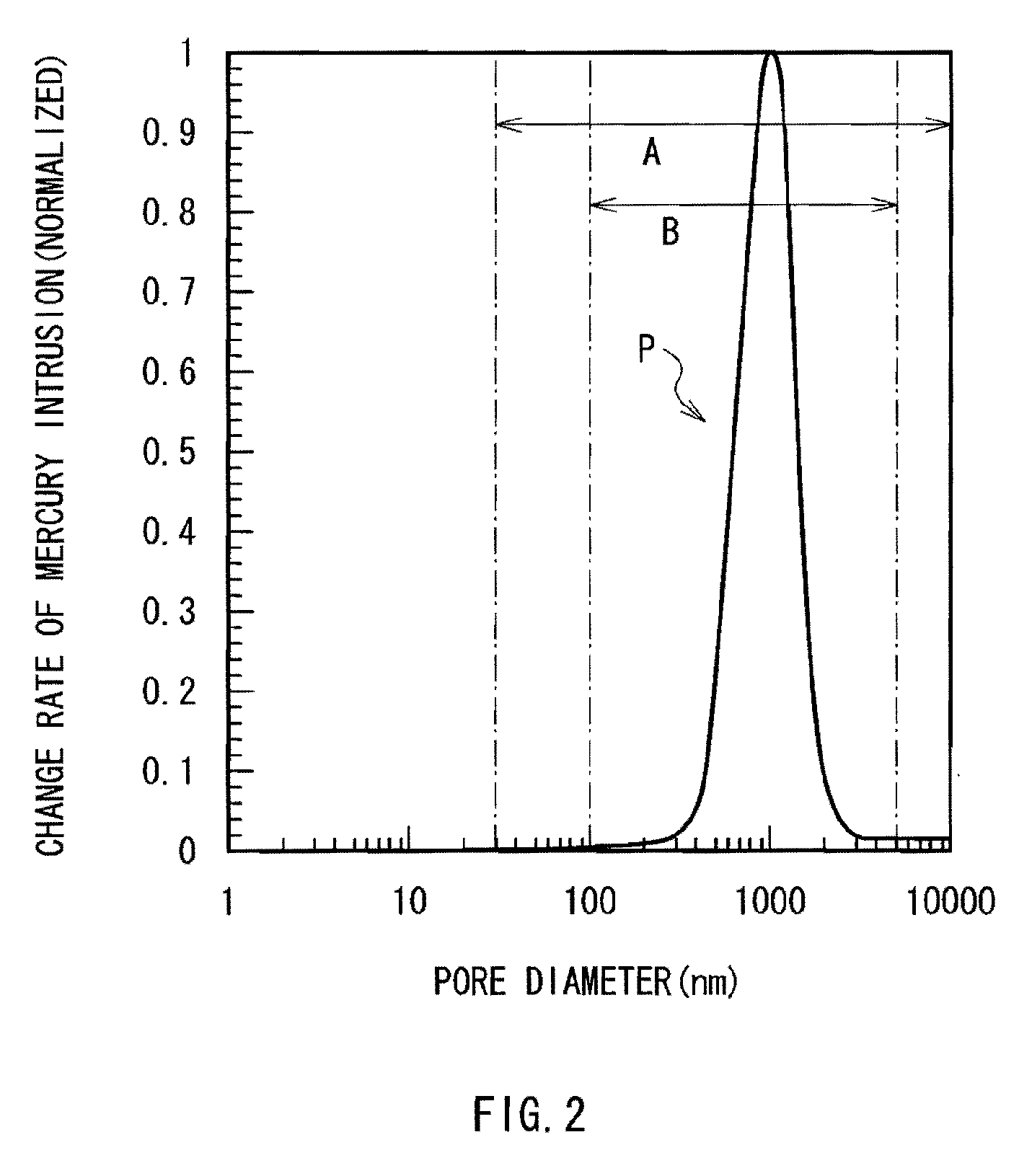
ActiveUS20090253033A1Improve performanceExcellent cycle characteristicsAlkaline accumulatorsFinal product manufacturePore diameterMaterials science
A secondary battery capable of improving the cycle characteristics and the swollenness characteristics is provided. The secondary battery includes a cathode, an anode, and an electrolytic solution. The anode includes an anode active material layer having a plurality of fine pores on an anode current collector. The anode active material layer contains an anode active material and an anode binder. A change rate of a mercury intrusion into the plurality of fine pores measured by mercury penetration technique is distributed to show a peak in the pore diameter range from 30 nm to 10000 nm, both inclusive.
Owner:MURATA MFG CO LTD
Negative active material for rechargeable lithium battery, method of preparing same and rechargeable lithium battery using same
InactiveUS20050074672A1Excellent cycle characteristicsAvoid excessive changesNegative electrodesNon-aqueous electrolyte accumulator electrodesLithiumEvaporation
Disclosed is a negative active material for a rechargeable lithium battery including a composite of a graphite particle and at least one supermicroparticle, wherein the supermicroparticle has a diameter in the range of 1 nm to 100 nm, is produced using an evaporation method under a gas atmosphere, and includes elements alloyable with lithium.
Owner:SAMSUNG SDI CO LTD
Anode and method of manufacturing the same, and secondary battery
ActiveUS20090111020A1Increase battery capacityExcellent cycle characteristicsAlkaline accumulatorsElectrode manufacturing processesDischarge efficiencySilicon
An anode and a secondary battery capable of improving the charge and discharge efficiency are provided. The anode includes an anode current collector, and an anode active material layer provided on the anode current collector. The anode active material layer has a plurality of anode active material particles containing at least one of a simple substance of silicon, a compound of silicon, a simple substance of tin and a compound of tin, and has a coat containing an oxo acid salt in at least part of the surface of the anode active material particles.
Owner:MURATA MFG CO LTD
Anode and battery
InactiveUS20050153208A1Improved characteristicDestruction of structureElectrode carriers/collectorsNegative electrodesCurrent collectorCharge and discharge
The invention provides an anode capable of relaxing stress due to expansion and shrinkage of an anode active material layer associated with charge and discharge, or an anode capable of reducing structural destruction of the anode active material layer and reactivity between the anode active material layer and an electrolyte associated with charge and discharge, and a battery using it. The anode active material layer contains an element capable of forming an alloy with Li, for example, at least one from the group consisting of simple substances, alloys, and compounds of Si or Ge. An interlayer containing a material having superelasticity or shape-memory effect is provided between an anode current collector and the anode active material layer. Otherwise, the anode current collector is made of the material having superelasticity or shape-memory effect. Otherwise, a thin film layer containing the material having superelasticity or shape-memory effect is formed on the anode active material layer.
Owner:SONY CORP
Non-aqueous electrolyte secondary battery
InactiveUS20070099081A1Improve reliabilityHigh charge and discharge capacityMaterial nanotechnologyNon-aqueous electrolyte accumulatorsPolyetherimidePolyamide
A non-aqueous electrolyte secondary battery including a positive electrode, a negative electrode, a separator interposed between the positive and negative electrodes, and a non-aqueous electrolyte. The negative electrode includes composite particles and a binder. Each of the composite particles includes: a negative electrode active material including an element capable of being alloyed with lithium; carbon nanofibers that are grown from a surface of the negative electrode active material; and a catalyst element for promoting the growth of the carbon nanofibers. The binder comprises at least one polymer selected from the group consisting of polyimide, polyamide imide, polyamide, aramid, polyarylate, polyether ether ketone, polyether imide, polyether sulfone, polysulfone, polyphenylene sulfide, and polytetrafluoroethylene.
Owner:PANASONIC CORP
Synthesis and surface modification method of lithium excessive laminar oxide anode material
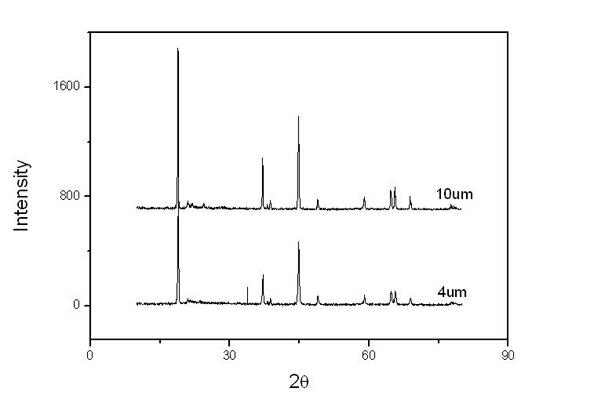
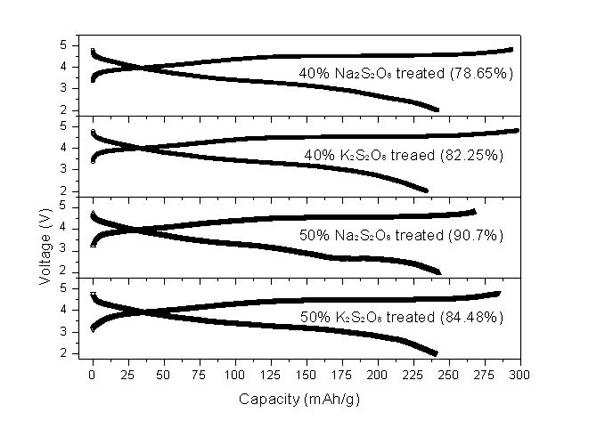
The invention relates to a synthesis and surface modification method of a lithium rich anode material Li1+xM1-xO2 (M is one or more of Ni, Co and Mn, and X is more than or equal to 0 and less than or equal to 1 / 3) for a lithium ion battery. The method comprises the following steps of: synthesizing a precursor by using a carbonate precipitation method, mixing the precursor and a lithium salt, and calcining for 2 to 20 hours at the temperature of between 800 and 1,100 EG C to obtain a lithium rich material, wherein the prepared lithium rich material has controllable particle size and higher reversible capacity; and dissolving persulfate or sulfate in an amount which is 5 to 80 mass percent of the lithium rich material into deionized water, adding the lithium rich material, stirring for 2 to 100 hours at the temperature of between 25 and 80 DEG C, heating the materials to the temperature of between 100 and 500 DEG C in a muffle furnace, calcining the materials for 2 to 20 hours, fully filtering the obtained materials, and washing off impurities to obtain the surface modified anode material Li1+x-yM1-xO2. The synthesized lithium rich material has controllable particle size; the first charge / discharge efficiency of the lithium rich material and the discharge specific capacity and the cyclical stability under high magnification can be improved; and the method is simple, low in cost, convenient for operation and suitable for industrialized production.
Owner:GUANGZHOU HKUST FOK YING TUNG RES INST
Lithium Secondary Battery
InactiveUS20030190530A1Excellent cycle characteristicsPrevent materialCell seperators/membranes/diaphragms/spacersOrganic electrolyte cellsHydrofluoric acidOrganic base
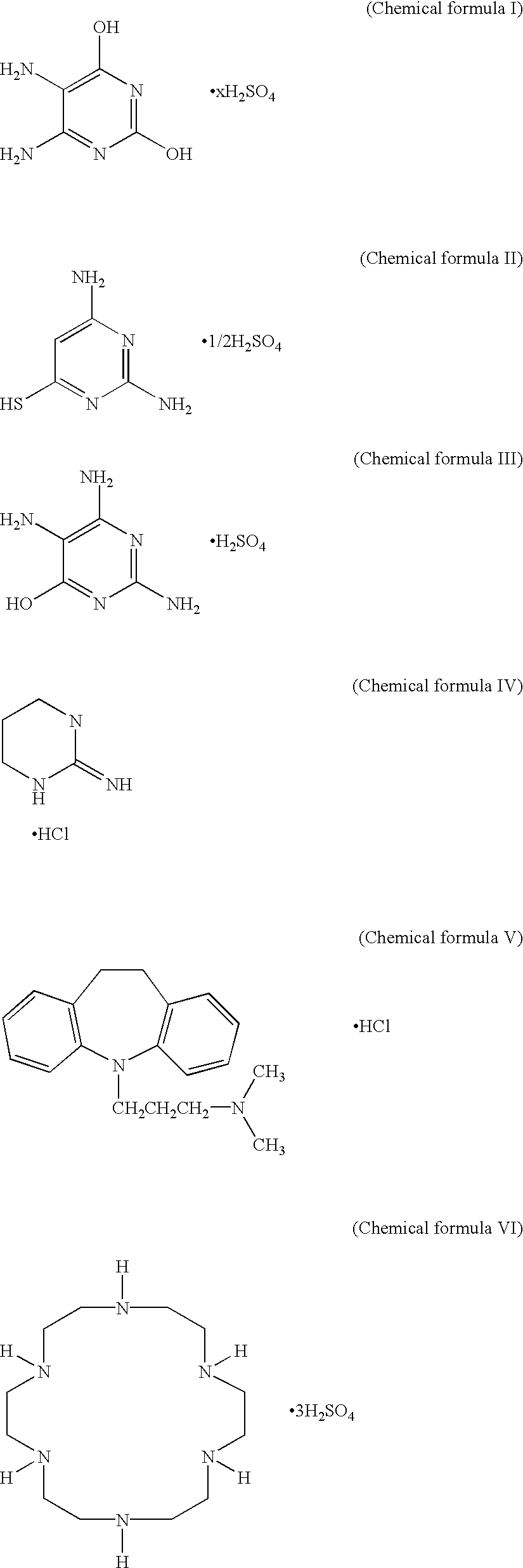
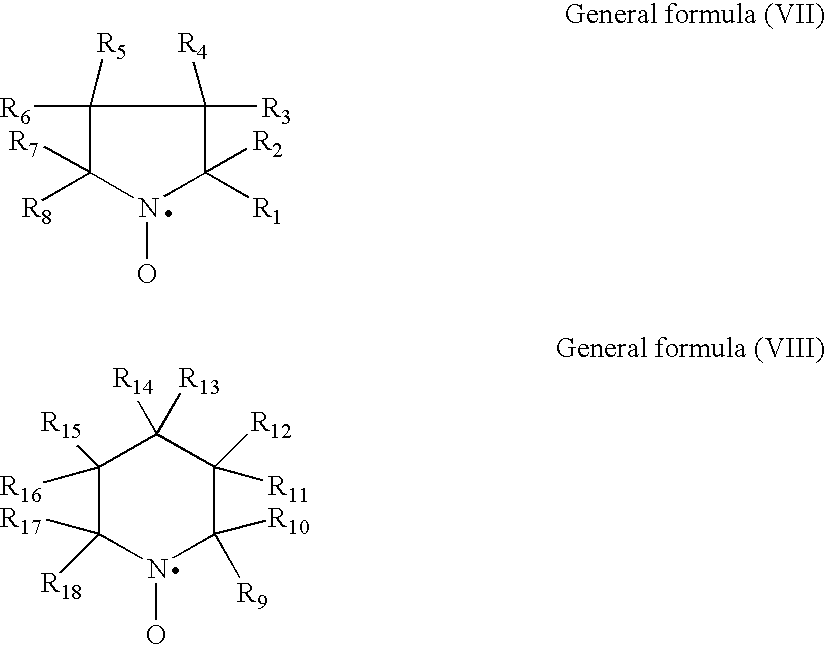
A lithium secondary battery includes: an electrode body having a positive electrode, a negative electrode, and a separator, the positive electrode and the negative electrode being wound or laminated by means of the separator; and a nonaqueous electrolyte solution containing a lithium compound as a electrolyte. At least one of the positive electrode, the negative electrode, the separator, the nonaqueous electrolyte solution contains at least one of: (a) an organic and / or inorganic inhibitor, which functions as a Cu-corrosion inhibitor or a Cu-trapping agent, (b) a compound having an organic base and an inorganic acid which are unitarily combined in a molecule, (c) a cyclic compound containing a N-O radical in a molecular structure, (d) a cyclic compound which becomes a Mn<2+> supplier in the nonaqueous electrolyte solution, (e) a compound containing an atom showing Lewis acidity and an atom showing Lewis basisity in one molecule, (f) a three-dimensional siloxane compound, and (g) a nonionic surfactant; or the nonaqueous electrolyte solution contains: (h) a water-extracting agent, or (i) a hydrofluoric acid-extracting agent. This lithium secondary battery exhibits an excellent effect that self-discharge property, cycle characteristics, long period stability and reliability can be planned.
Owner:NGK INSULATORS LTD
Nonaqueous electrolyte for rechargeable battery, and rechargeable battery with nonaqueous electrolyte
InactiveCN101652894AExcellent discharge load characteristicsImprove featuresFinal product manufactureCell electrodesHigh current densityHigh temperature storage
This invention provides a nonaqueous electrolyte which is excellent in discharge load properties, as well as in high-temperature storage stability, cycling characteristics, high capacitance, continuous charge properties, storage stability, gas evolution suppression in continuous charging, charge / discharge properties at a high current density, discharge load properties and the like, and a rechargeable battery with a nonaqueous electrolyte. The nonaqueous electrolyte contains a monofluorophosphate and / or a difluorophosphate and further contains a compound having a specific chemical structure orspecific properties.
Owner:MITSUBISHI CHEM CORP
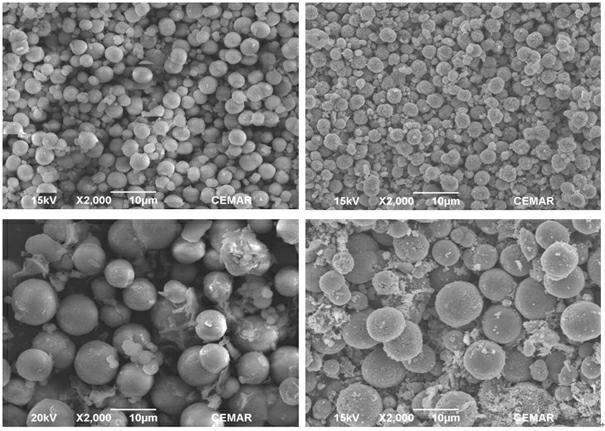
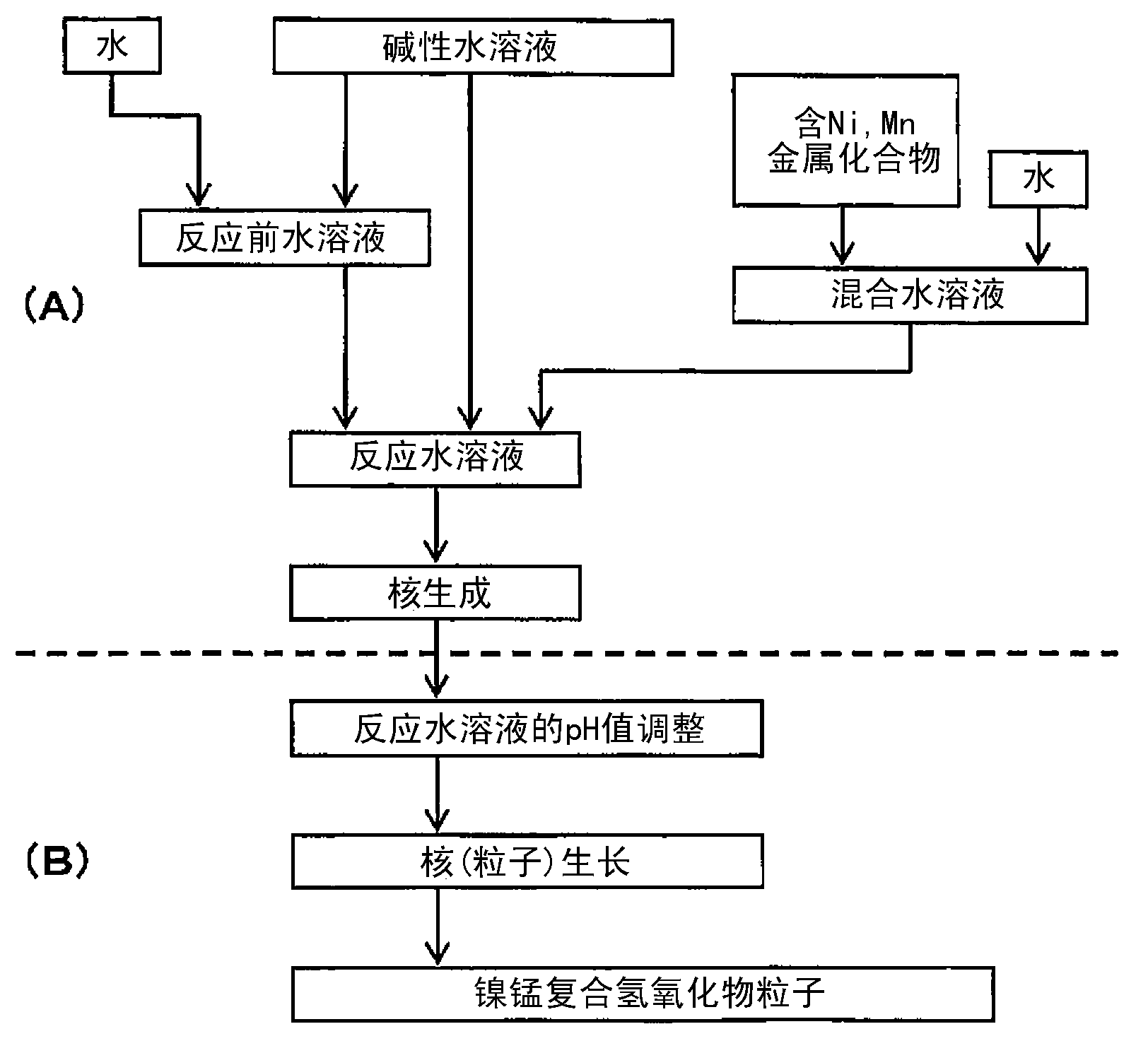
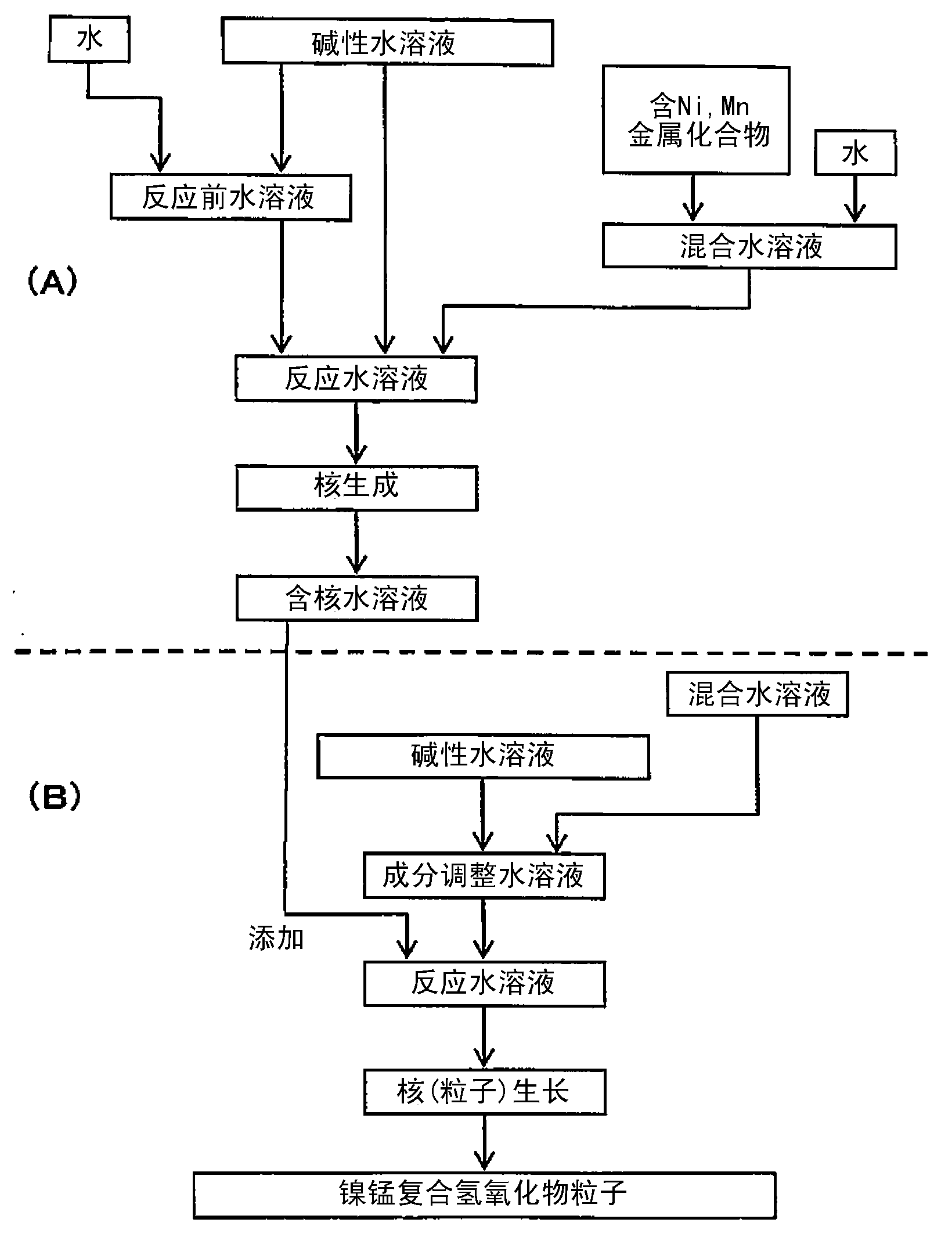
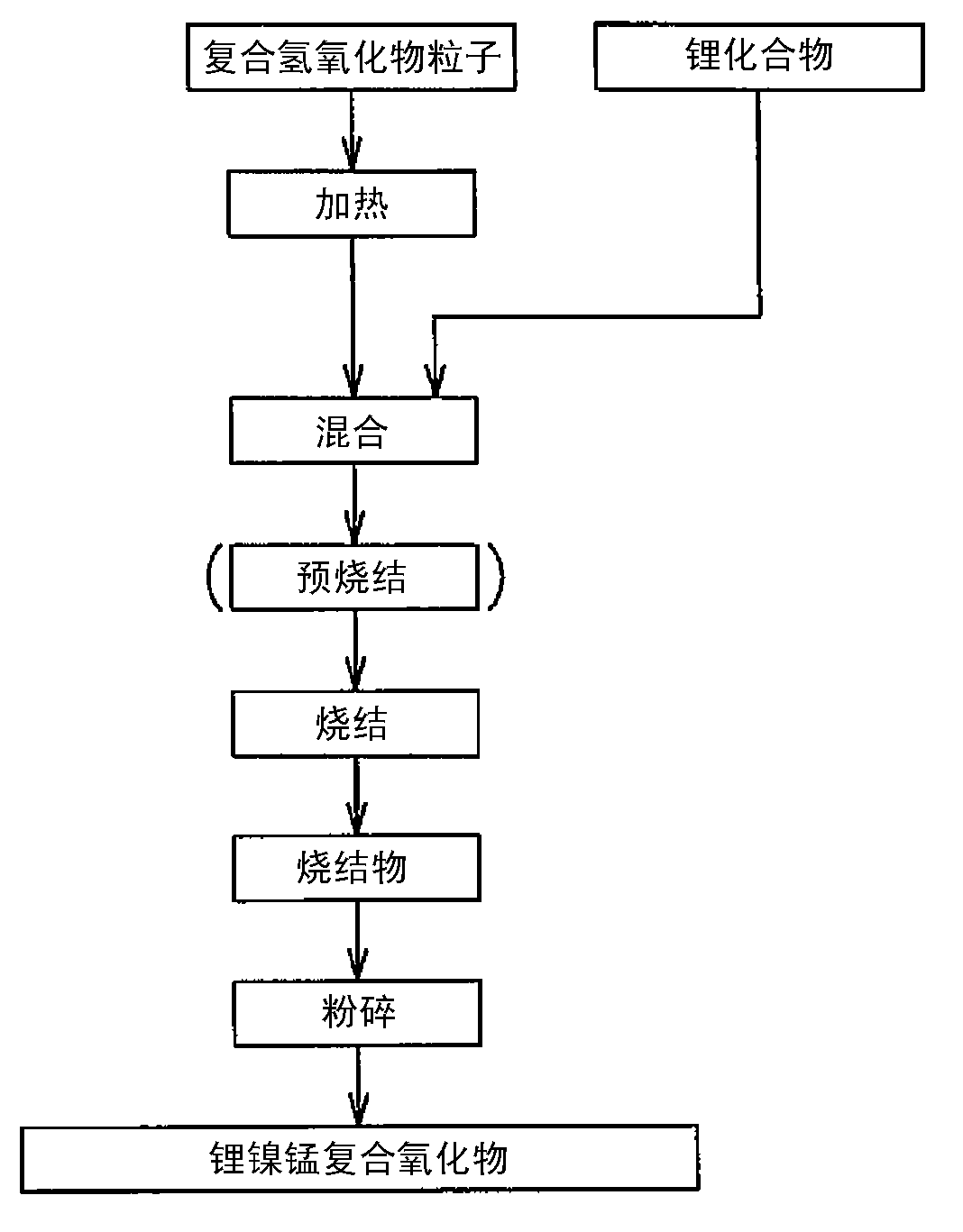
Nickel-manganese composite hydroxide particles, method for producing same, positive electrode active material for nonaqueous electrolyte secondary batteries, method for producing said positive electrode active material, and nonaqueous electrolyte secondary battery
ActiveCN102884659ASmall particle sizeImprove particle size uniformityFinal product manufactureCylindrical casing cells/batteryComplex ionsComposite oxide
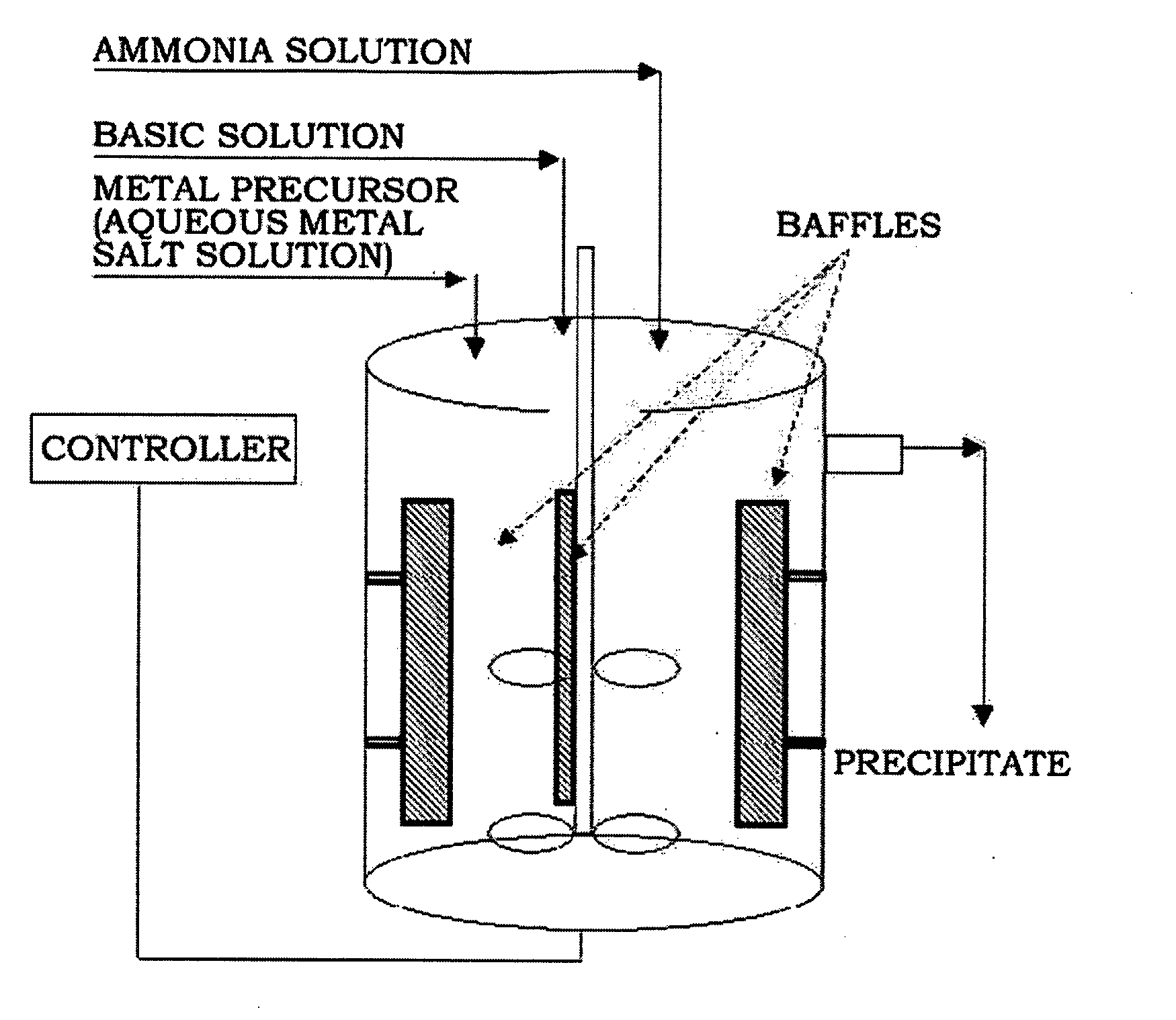
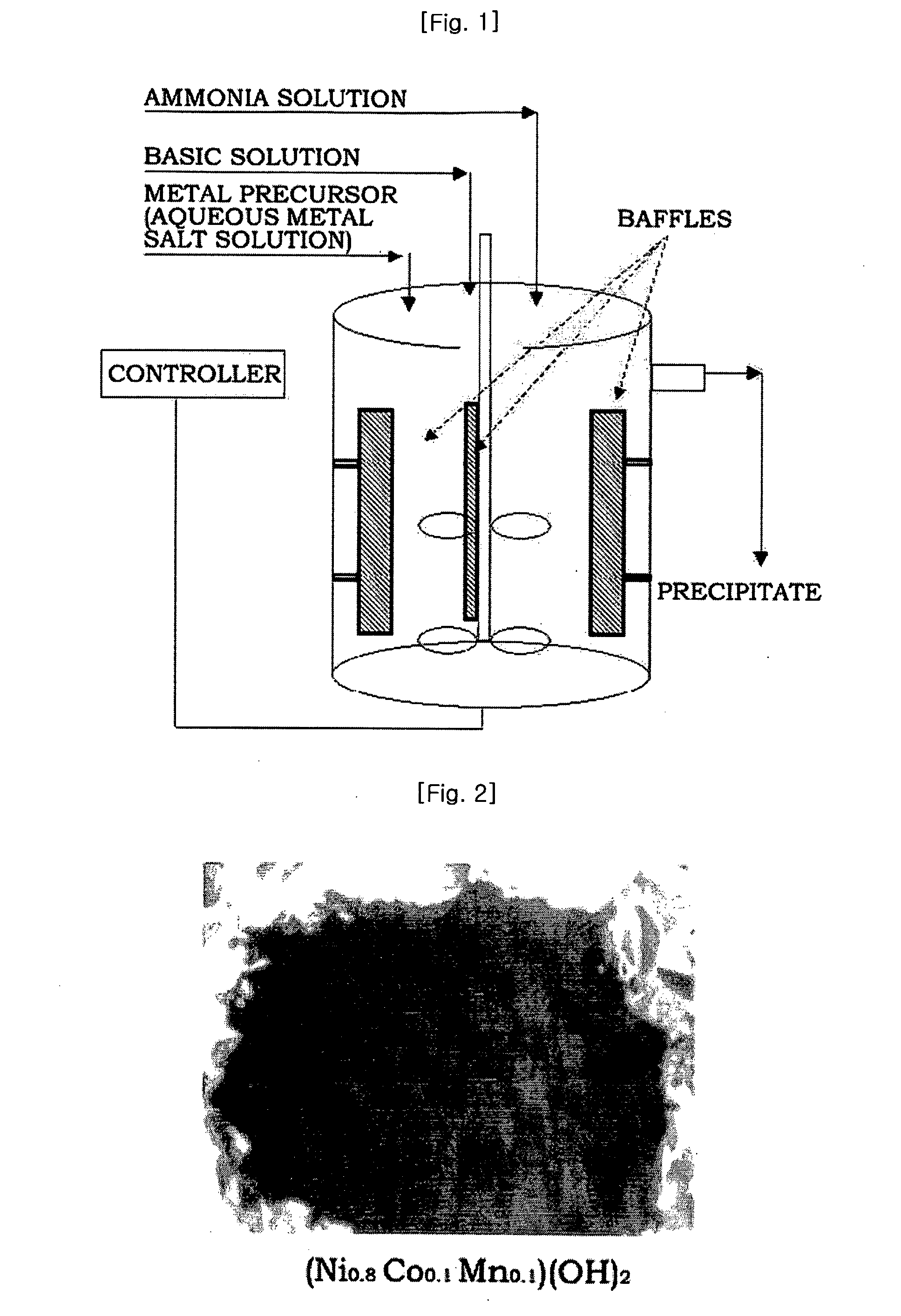
Provided are nickel manganese composite hydroxide particles that are a precursor for forming cathode active material comprising lithium nickel manganese composite oxide having hollow structure of particles having a small and uniform particle size for obtaining a non-aqueous electrolyte secondary battery having high capacity, high output and good cyclability. When obtaining the nickel manganese composite hydroxide particles from a crystallization reaction, an aqueous solution for nucleation, which includes at least a metallic compound that contains nickel and a metallic compound that contains manganese, and does not include a complex ion formation agent that forms complex ions with nickel, manganese and cobalt, is controlled so that the temperature of the solution is 60 DEG C or greater, and so that the pH value that is measured at a standard solution temperature of 25 DEG C is 11.5 to 13.5, and after nucleation is performed, an aqueous solution for particle growth, which includes the nuclei that were formed in the nucleation step and does not substantially include a complex ion formation agent that forms complex ions with nickel, manganese and cobalt, is controlled so that the temperature of the solution is 60 DEG C or greater, and so that the pH value that is measured at a standard solution temperature of 25 DEG C is 9.5 to 11.5, and is less than the pH value in the nucleation step.
Owner:SUMITOMO METAL MINING CO LTD
Negative electrode for non-aqueous electrolyte secondary batteries, non-aqueous electrolyte secondary battery having the electrode, and method for producing negative electrode for non-aqueous electrolyte secondary batteries
InactiveUS20070111102A1Excellent cycle characteristicsPrevent collapseMaterial nanotechnologyFinal product manufactureFiberManganese
A negative electrode for non-aqueous electrolyte secondary batteries has a mixture layer including a composite negative electrode active material which is composed of active material cores capable of charging and discharging at least lithium ions; carbon nanofibers; and catalyst elements. The carbon nanofibers are attached to the surfaces of the active material cores. The catalyst elements are at least one selected from the group consisting of copper, iron, cobalt, nickel, molybdenum, and manganese, and promote the growth of the carbon nanofibers. The active material cores have the carbon nanofibers therebetween.
Owner:PANASONIC CORP
Secondary battery
ActiveUS20070154815A1Avoid decompositionExcellent cycle characteristicsFinal product manufactureOrganic electrolyte cellsSolventComposite oxide
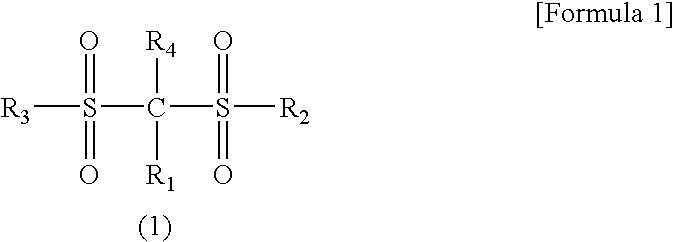
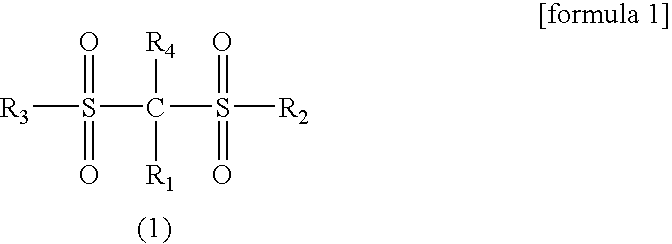
A lithium secondary battery which has excellent characteristics such as energy density and electromotive force and is excellent in cycle life and storage stability is provided. The secondary battery comprises a positive electrode, a negative electrode, and an electrolyte solution comprising an aprotic solvent having at least an electrolyte dissolved therein, wherein the positive electrode comprises a lithium-manganese composite oxide having a spinel structure as a positive electrode active material, and the electrolyte solution comprises a compound represented by the general formula (1).
Owner:NEC CORP
Nonaqueous Electrolyte Solution and Lithium Secondary Battery Using Same
ActiveUS20070224514A1Large capacityImprove storage characteristicsCell electrodesOrganic electrolyte cellsSolventOxygen
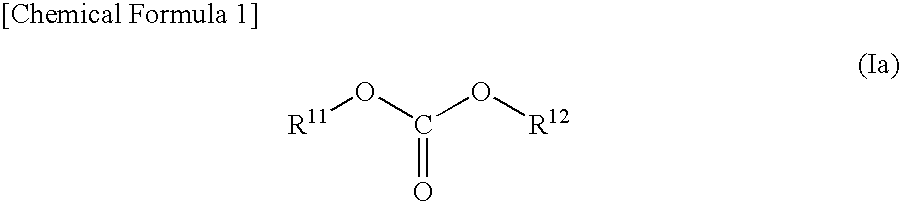
A non-aqueous electrolyte solution is provided that realizes a large capacity, exhibits high storage characteristics and cycle characteristics, and is capable of inhibiting gas generation. The non-aqueous electrolyte solution comprises a lithium salt and a non-aqueous solvent, and further comprises: a cyclic carbonate compound having an unsaturated bond in a concentration of 0.01 weight % or higher and 8 weight % or lower; and a compound expressed by general formula (Ia) in a concentration of 0.01 weight % or higher and 5 weight % or lower. (in the formula (Ia), R11 and R12 represent, independently of each other, an organic group that is composed of one or more carbon atoms and hydrogen atoms and may optionally contain one or more oxygen atoms but excludes unsaturated bonds, provided that at least either R11 or R12 has an ether linkage. The total number of carbon atoms of R11 and R12 is between 3 and 18, and the total number of oxygen atoms contained in R11 and R12 is between 1 and 6.)
Owner:MU IONIC SOLUTIONS CORP +1
Electric Energy Storage Device and Method of Charging and Discharging the Same
ActiveUS20080013224A1Excellent cycle characteristicsGood temperature characteristicsElectrolytic capacitorsElectric powerCharge and dischargeElectric double-layer capacitor
Disclosed are an electric energy storage device having a good cycle characteristic and a temperature characteristic and a method of charging and discharging the electric energy storage device. The electric energy storage device including a capacitor and a secondary battery combined in series is provided. When the capacitor of the electric energy storage device is an electric double layer capacitor, the capacitor is used to the voltage of OV or less to increase an available energy usage. When this energy storage device is used as a power in a place where a rapid keeping and repairing is difficult such as out in the fields including in the mountain, at the sea, on the road, the cost for keeping and repairing can be largely reduced.
Owner:MAXWELL TECH KOREA CO LTD
Non-aqueous electrolyte secondary battery
InactiveUS20090233176A1Improve conductivityImprove thermal stabilitySecondary cellsNon-aqueous electrolyte accumulator electrodesThermal stabilityMaterials science
An object of the present invention is to provide a non-aqueous electrolyte secondary battery that is excellent in cycle characteristics even in a high-temperature environment and high in thermal stability. The non-aqueous electrolyte secondary battery of the present invention comprises at least one of an active material A and an active material C, and an active material B as positive electrode active materials. The active material A is LixCoO2 (0.9≦x≦1.2). The active material B is LixNiyMnzM1-y-zO2 (0.9≦x≦1.2, 0.1≦y≦0.5, 0.2≦z≦0.5, 0.2≦1−y−z≦0.5 and 0.9≦y / z≦2.5; and M is at least one selected from the group consisting of Co, Mg, Al, Ti, Sr, Ca, V, Fe, Y, Zr, Mo, Tc, Ru, Ta, W and Re). The active material C is LixCo1-aMaO2 (0.9≦x≦1.2 and 0.005≦a≦0.1; and M is at least one selected from the group consisting of Mg, Al, Ti, Sr, Mn, Ni, Ca, V, Fe, Y, Zr, Mo, Tc, Ru, Ta, W, Re, Yb, Cu, Zn and Ba).
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
Electrolyte solution for secondary battery and secondary battery using same
ActiveUS20060292452A1Sufficient battery characteristicImprove efficiencyOrganic chemistryLight-sensitive devicesPhysical chemistrySolvent
There is provided a lithium secondary battery which has excellent characteristics such as energy density and electromotive force and is excellent in cycle life and storage stability. An electrolyte solution for secondary battery comprising at least an aprotic solvent having an electrolyte dissolved therein and a compound represented by the general formula (1).
Owner:NEC CORP
Negative electrode for lithium metal battery and lithium metal battery including the same
ActiveCN106299244AImprove adhesionKeep depositingElectrode thermal treatmentFinal product manufactureMetallic electrodePolyvinyl alcohol
A negative electrode for a lithium metal battery, the negative electrode including: a lithium metal electrode; and a protection film disposed on at least a portion of the lithium metal electrode, wherein the protection film includes at least one first polymer selected from a polyvinyl alcohol graft copolymer, a crosslinked copolymer formed from the polyvinyl alcohol graft copolymer, a polyvinyl alcohol crosslinked copolymer, and a blend thereof.
Owner:SAMSUNG ELECTRONICS CO LTD
Nonaqueous electrolyte and nonaqueous electrolyte secondary battery using the same
InactiveUS20060269843A1Increase capacitanceIncrease internal resistanceCell electrodesOrganic electrolyte cellsEtherPhenyl group
A nonaqueous electrolyte containing a silicon compound of formula (1) or (2) and a nonaqueous electrolyte secondary battery using the nonaqueous electrolyte and excellent in cycle characteristics and low temperature characteristics, wherein R1 and R2 each represent alkyl, cycloalkyl, alkoxy or halogen; R3 represents alkenyl; and X represents halogen, wherein R4, R5, R6, and R7 each represent alkyl, alkoxy, alkenyl, alkenyloxy, alkynyl, alkynyloxy, phenyl or phenoxy, each of which may have an ether bond in its chain; R8 represents halogen, halogen-substituted aryl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl; a trifluoromethyl group, an acyloxy group having 5 to 8 carbon atoms, a sulfonate group having 1 to 8 carbon atoms, an isocyanyl group an isothianyl or a cyano group, R9 represents halogen, a trifluoromethyl group,an acyloxy group having 5 to 8 carbon atoms, a sulfonate group having 1 to 8 carbon atoms, an isocyanyl group an isothianyl or a cyano group: halogen-substituted aryl; n represents 1 or 2; and Y represents a single bond, oxygen, alkylene, alkylenedioxy, alkenylene, alkenylenedioxy, alkynylene, alkynylenedioxy, arylene or arylenedioxy; provided that the number of groups having an unsaturated bond in R4, R5, R6, R7, R8, and R9 is zero or one.
Owner:DENSO CORP +1
Rechargeable lithium battery
InactiveUS20060008706A1Excellent cycle characteristicsReduce generationSolid electrolyte cellsLi-accumulatorsParticulatesSolvent
The present invention provides a lithium secondary battery having high-capacity as well as good cycle characteristics. The lithium secondary battery includes a positive electrode comprising a positive active material, a negative electrode comprising a negative active material and an electrolyte. The negative active material includes graphite particles combined to Si particulate. The electrolyte includes a solvent, a polyether modified silicone oil where a linear polyether chain is linked to a polysiloxane chain, and a solute comprising a lithium salt.
Owner:SAMSUNG SDI CO LTD
Nonaqueous electrolytic solution and nonaqueous secondary battery
InactiveUS6872493B2Excellent cycle characteristicsIncrease internal resistanceSilicon organic compoundsTin organic compoundsOrganic solventAlkoxy group
A nonaqueous electrolytic solution having an electrolyte salt dissolved in an organic solvent, which contains a silicon compound having an unsaturated bond which is represented by formula (I): wherein R1, R2, R3, R4, R5, and R6 each represent an alkyl group, an alkoxy group, an alkenyl group, an alkenyloxy group, an alkynyl group, an alkynyloxy group, an aryl group or an aryloxy group, each of which may have an ether bond in the chain thereof; n represents a number of from 0 to 5; when n is 1 to 5, X represents a single bond, an oxygen atom, an alkylene group, an alkylenedioxy group, an alkenylene group, an alkenylenedioxy group, an alkynylene group, an alkynylenedioxy group, an arylene group or an arylenedioxy group; provided that at least one of R1, R2, R3, R4, R5, R6, and X represents a group containing an unsaturated bond,an organotin compound or an organogermanium compound and a nonaqueous secondary battery having the same.
Owner:ADEKA CORP +1
Nonaqueous electrolytic solution and nonaqueous electrolyte secondary battery
InactiveUS20100035146A1Increase energy densityImprove featuresCell electrodesLi-accumulatorsHydrogenHydrogen atom
A nonaqueous electrolytic solution that can provide a high energy density nonaqueous electrolyte secondary battery having a high capacity, excellent storage characteristics, and excellent cycle characteristics and suppressing the decomposition of an electrolytic solution and the deterioration thereof when used in a high-temperature environment includes an electrolyte, a nonaqueous solvent, and a compound represented by general formula (1):wherein R1, R2, and R3 each independently represent a hydrogen atom, a cyano group, or an optionally halogen atom-substituted hydrocarbon group having 1 to 10 carbon atoms, with the proviso that R1 and R2 do not simultaneously represent hydrogen atoms.
Owner:MITSUBISHI CHEM CORP
Lithium difluorophosphate, electrolytic solution containing lithium difluorophosphate, process for producing lithium difluorophosphate, process for producing nonaqueous electrolytic solution, nonaqueo
ActiveCN101507041AEasy and efficient to prepareHigh purityCell electrodesLi-accumulatorsElectrolytic agentElectrical battery
A difluorophosphate salt, which is expensive and not readily available, can be produced with a high purity readily and efficiently from inexpensive and readily available materials. A nonaqueous electrolyte secondary battery that exhibits low-temperature discharge and heavy-current discharge characteristics and high-temperature preservation and cycle characteristics without impairing the battery safety. A hexafluorophosphate salt is reacted with a compound having a bond represented by the following formula (1) in the molecule: Si-O-Si (1). A nonaqueous electrolyte used for nonaqueous electrolyte secondary batteries including a negative electrode and a positive electrode that can occlude and discharge ions, and a nonaqueous electrolyte is prepared from a mixture obtained by mixing at least one nonaqueous solvent, a hexafluorophosphate salt and a compound having a bond represented by the following formula (1), and removing low-boiling compounds newly formed in the system, the low-boiling compounds having a lower boiling point than that of the compound having the bond represented by the formula (1): Si-O-Si (1).
Owner:MITSUBISHI CHEM CORP +1
Polyethylene multilayer microporous membrane, battery separator using same, and battery
InactiveCN101208198AImprove permeabilityExcellent mechanical propertiesSynthetic resin layered productsSecondary cellsVitrificationSurface layer
A multi-layer, microporous polyethylene membrane having at least three layers comprising (a) a first microporous layer amount of a polyethylene resin and constituting at least both surface layers, and (b) at least one second microporous layer made of a polyethylene resin, a heat-resistant resin having a melting point or glass transition temperature of 150°C or higher and a filler, and sandwiched by both surface layers.
Owner:东丽东燃机能膜合同会社
Porous membrane for secondary battery and secondary battery
InactiveUS20120189897A1Improve output characteristicsImprove characteristic long-term cycleFinal product manufactureElectrode carriers/collectorsVitrificationLithium
Disclosed is a porous membrane for a secondary battery, which has further improved output characteristics and long-term cycle characteristics when compared with conventional porous membranes. The porous membrane for a secondary battery is used for a lithium ion secondary battery or the like. Specifically disclosed is a porous membrane for a secondary battery, which contains polymer particles A that have a number average particle diameter of 0.4 μm or more but less than 10 μm and a glass transition temperature of 65° C. or more and polymer particles B that have a number average particle diameter of 0.04 μm or more but less than 0.3 μm and a glass transition temperature of 15° C. or less. It is preferable that the polymer particles B have a crystallization degree of 40% or less and a main chain structure that is composed of a saturated structure.
Owner:ZEON CORP
Negative-electrode active material for nonaqueous electrolyte secondary battery, and negative electrode and nonaqueous electrolyte secondary battery using the same
InactiveUS20070207381A1Excellent cycle characteristicsIncrease energy densityNegative electrodesSilicon hydridesLithiumHydrogen
A negative-electrode active material for nonaqueous electrolyte secondary battery, comprising a silicon compound capable of inserting and extracting lithium ion, wherein the silicon compound contains silicon-hydrogen bonds and the silicon-hydrogen bonds are introduced into the compound by reduction of at least one compound selected from the group consisting of silicon oxide, silicon nitride and silicon carbide with hydrogen, and a negative electrode for nonaqueous electrolyte secondary battery having a layer containing the negative-electrode active material in the above arrangement formed on a current collector.
Owner:PANASONIC CORP
Double-Layer Cathode Active Materials for Lithium Secondary Batteries, Method for Preparing The Active Materials, and Lithium Secondary Batteries Using the Active Materials
ActiveUS20080160410A1High-capacity characteristicImprove thermal safetyElectrode thermal treatmentConductive materialLithiumThermal safety
Disclosed herein are double-layer cathode active materials comprising a nickel-based cathode active material as an inner layer material and a transition metal mixture-based cathode active material as an outer layer material facing an electrolyte. Since the nickel-based cathode active material as an inner layer material has high-capacity characteristics and the transition metal mixture-based cathode active material as an outer layer material facing an electrolyte has superior thermal safety, the double-layer cathode active materials have high capacity, high charge density, improved cycle characteristics and superior thermal safety.
Owner:IUCF HYU (IND UNIV COOP FOUND HANYANG UNIV)
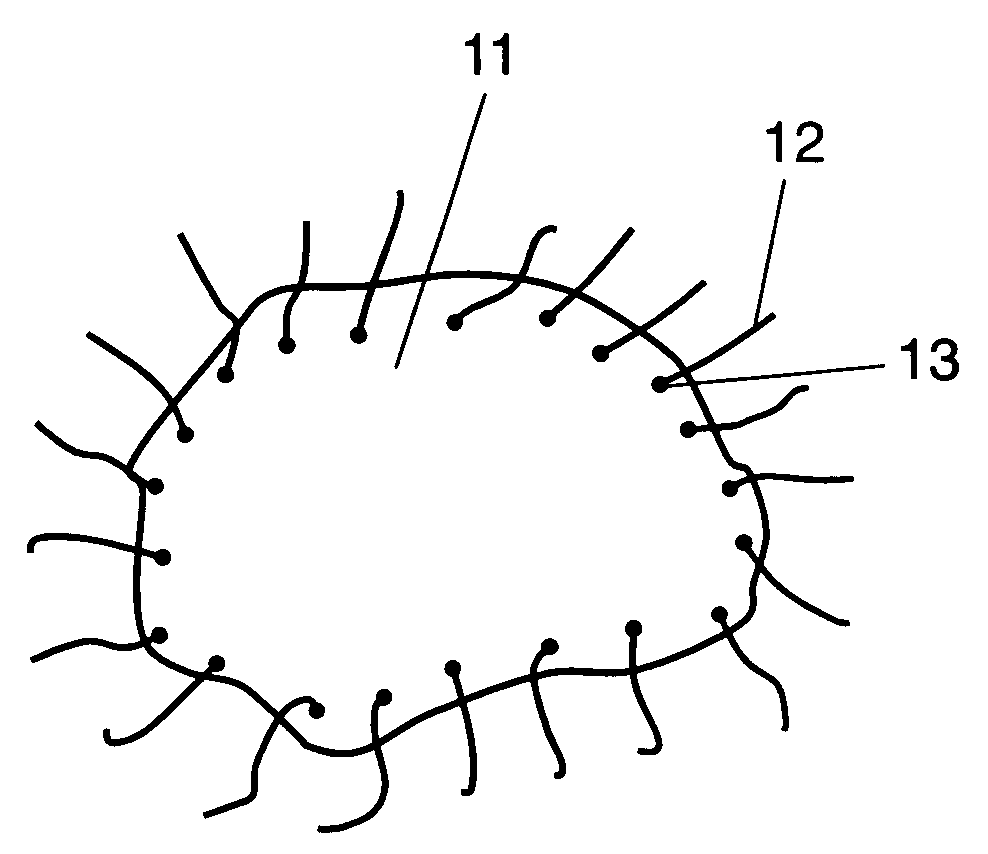
Nickel-cobalt-maganese-based compound particles and process for producing the nickel-cobalt-manganese-based compound particles, lithium composite oxide particles and process for producing the lithium composite oxide particles, and non-aqueous electrolyte secondary battery
ActiveUS20130045421A1Good thermal stabilityLess thermal stabilitySynthetic resin layered productsCellulosic plastic layered productsComposite oxideChemistry
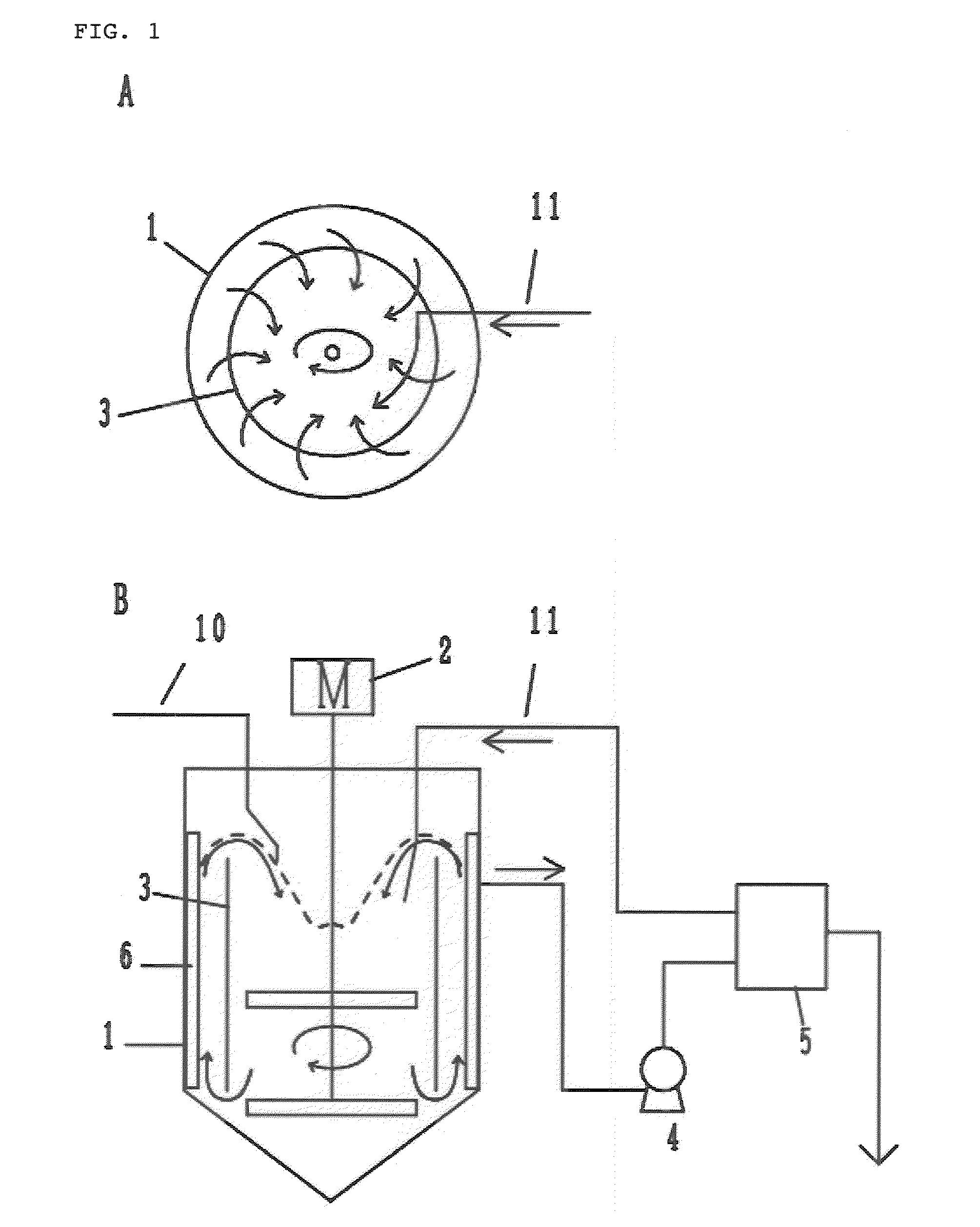
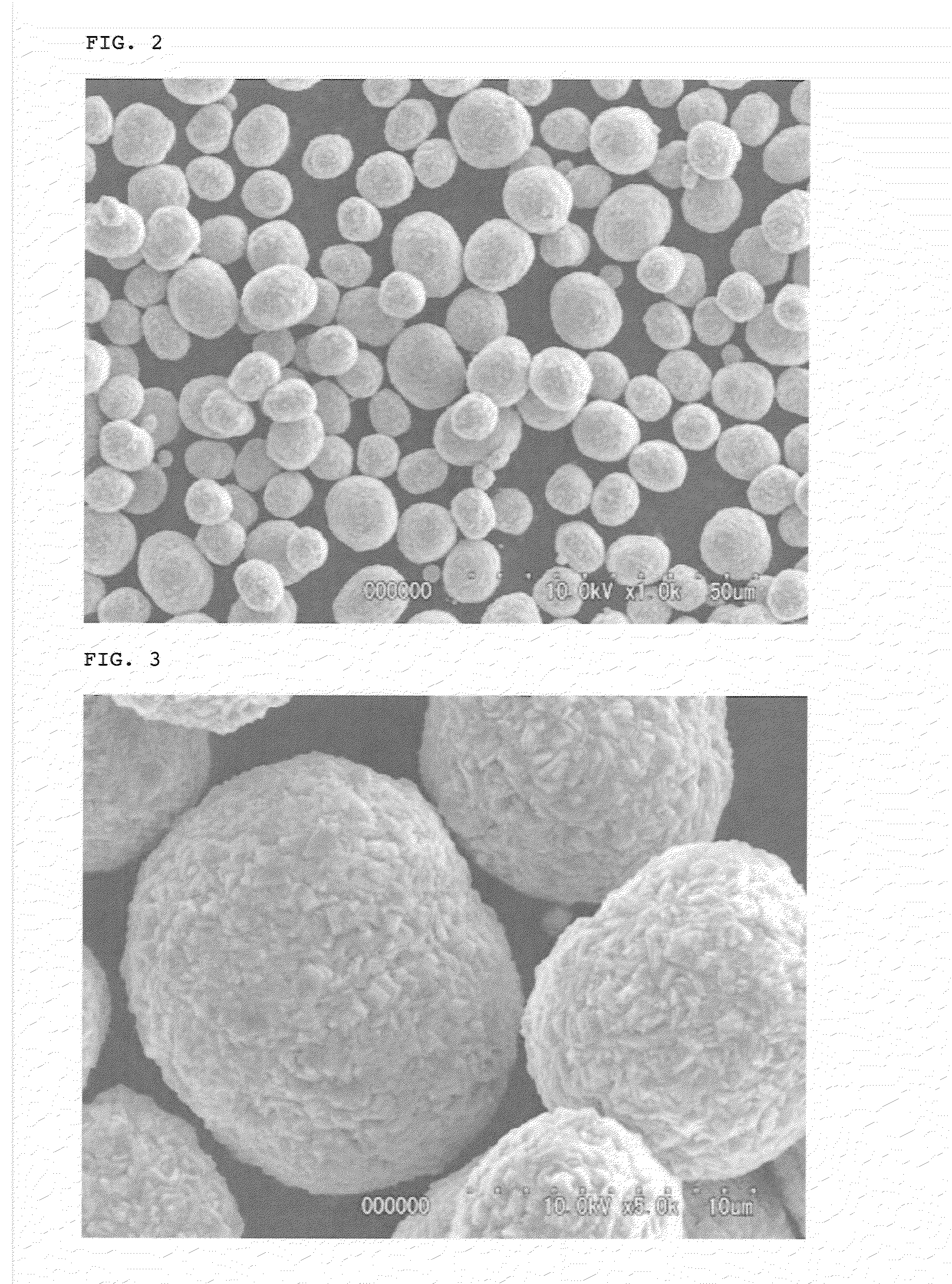
The present invention relates to nickel-cobalt-manganese-based compound particles which have a volume-based average secondary particle diameter (D50) of 3.0 to 25.0 μm, wherein the volume-based average secondary particle diameter (D50) and a half value width (W) of the peak in volume-based particle size distribution of secondary particles thereof satisfy the relational formula: W≦0.4×D50, and can be produced by dropping a metal salt-containing solution and an alkali solution to an alkali solution at the same time, followed by subjecting the obtained reaction solution to neutralization and precipitation reaction. The nickel-cobalt-manganese-based compound particles according to the present invention have a uniform particle size, a less content of very fine particles, a high crystallinity and a large primary particle diameter, and therefore are useful as a precursor of a positive electrode active substance used in a non-aqueous electrolyte secondary battery.
Owner:TODA IND
Nonaqueous electrolyte secondary battery
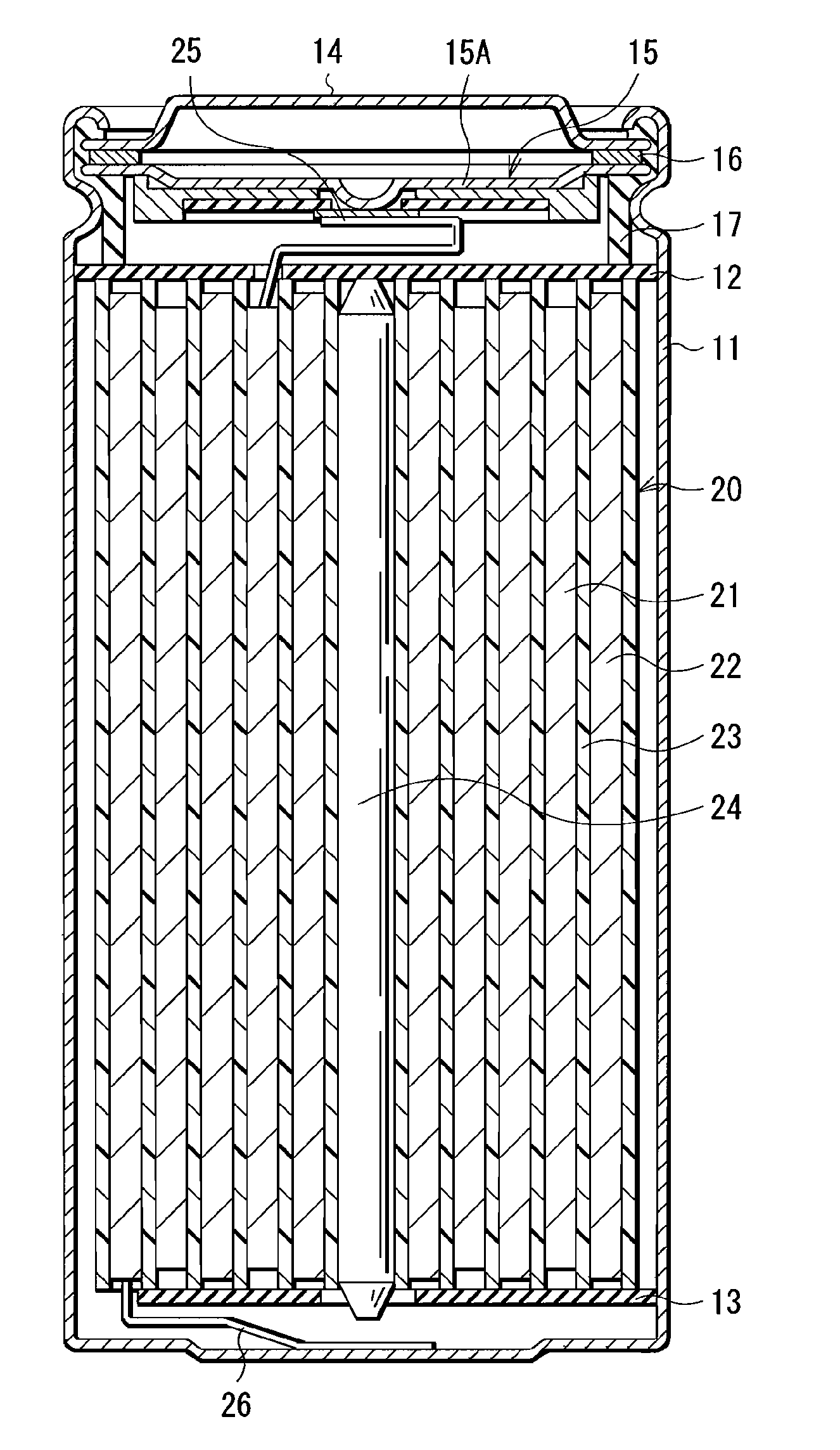
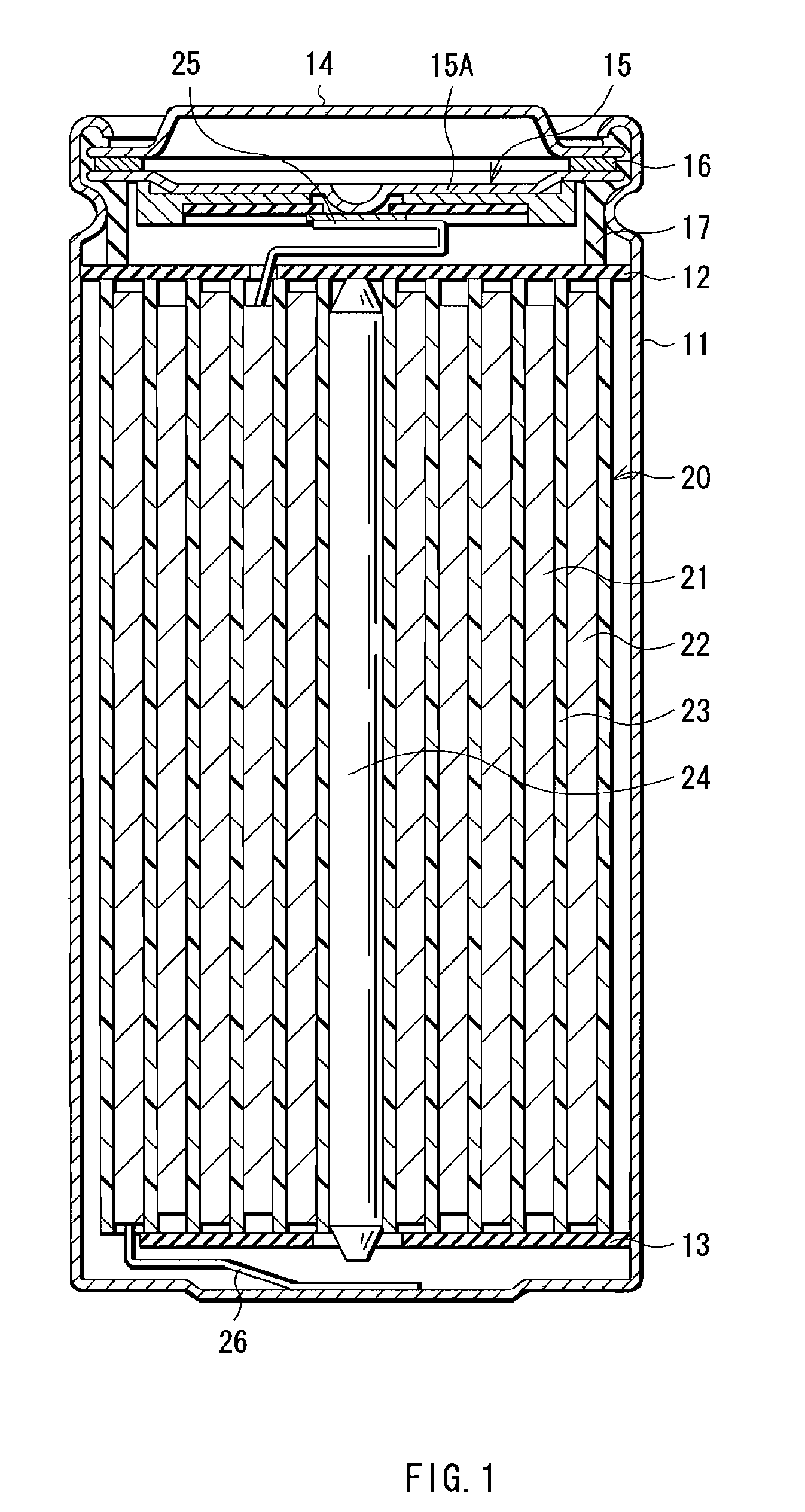
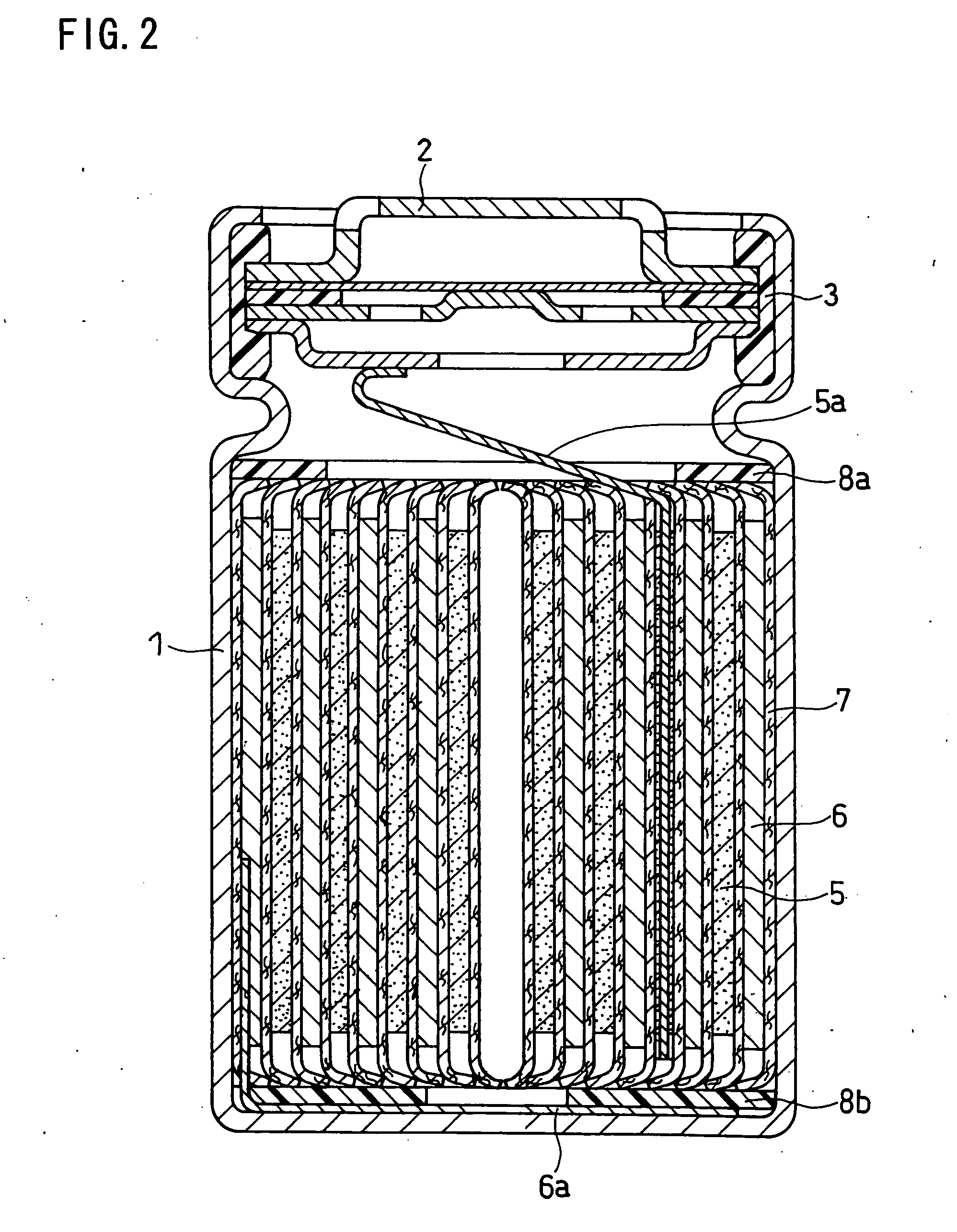
InactiveUS20080241703A1Increase energy densityExcellent cycle characteristicsAlkaline accumulatorsElectrode thermal treatmentLithiumHigh energy
A nonaqueous electrolyte secondary battery which includes a positive electrode, a negative electrode and a separator stacked and wound in a cylindrical configuration, and which achieves a high energy density and superior cycle performance characteristics.The nonaqueous electrolyte secondary battery includes a positive electrode containing a positive active material, a negative electrode containing a negative active material, a separator interposed between the positive electrode and the negative electrode, and a nonaqueous electrolyte containing a solvent and a solute. Those positive electrode, negative electrode and separator are stacked and wound in a cylindrical configuration. Characteristically, the negative active material comprises a material that stores lithium via alloying with lithium, the solvent in the nonaqueous electrolyte contains a fluorinated cyclic carbonate, and the nonaqueous electrolyte has a viscosity of not higher than 2.5 mPas.
Owner:SANYO ELECTRIC CO LTD
Nonaqueous electrolyte, and rechargeable battery with the nonaqueous electrolyte
InactiveCN101663790AIncrease capacityExcellent cycle characteristicsSecondary cellsHigh current densityChemical structure
A subject is to provide a nonaqueous electrolyte excellent in cycle performances such as capacity retention after cycling, output after cycling, discharge capacity after cycling, and cycle discharge capacity ratio, output characteristics, high-temperature storability, low-temperature discharge characteristics, heavy-current discharge characteristics, high-temperature storability, safety, high capacity, high output, high-current-density cycle performances, compatibility of these performances, etc. Another subject is to provide a nonaqueous-electrolyte secondary battery employing the nonaqueouselectrolyte. The subjects have been accomplished with a nonaqueous electrolyte which contains a monofluorophosphate and / or a difluorophosphate and further contains a compound having a specific chemical structure or specific properties.
Owner:MITSUBISHI CHEM CORP
Negative electrode for non-aqueous electrolyte secondary battery, production method thereof and non-aqueous electrolyte secondary battery
InactiveUS20050048369A1Large capacityImprove cycle lifeElectrode carriers/collectorsActive material electrodesMaterials scienceNon aqueous electrolytes
A negative electrode capable of giving a non-aqueous electrolyte secondary battery which has high capacity, long cycle life and excellent safety, and exhibits an excellent cycle characteristic even when charging / deep-discharging are repeated. The negative electrode comprises a current collector sheet and an active material layer deposited on the surface of the current collector sheet, wherein the active material layer comprises SiOx satisfying: 0.7≦x≦1.3, and does not include a binder.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com