Patents
Literature
11931 results about "Glass transition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
Polymer solution for nanoimprint lithography to reduce imprint temperature and pressure
InactiveUS20040110856A1Reduce pressureReduce the temperatureNanostructure manufactureDecorative surface effectsCross-linkVitrification
A method of forming features on substrates by imprinting is provided. The method comprises: (a) forming a polymer solution comprising at least one polymer dissolved in at least one polymerizable monomer; and (b) depositing the polymer solution on a substrate to form a liquid film thereon; and then either: (c) curing the liquid film by causing the monomer(s) to polymerize and optionally cross-linking the polymer(s) to thereby form a polymer film, the polymer film having a glass transition temperature (Tg); and imprinting the polymer film with a mold having a desired pattern to form a corresponding negative pattern in the polymer film, or (d) imprinting the liquid film with the mold and curing it to form the polymer film. The temperature of imprinting is as little as 10° C. above the Tg, or even less if the film is in the liquid state. The pressure of the imprinting can be within the range of 100 to 500 psi.
Owner:HEWLETT PACKARD DEV CO LP
Shape-memory, biodegradable and absorbable material
InactiveUS6281262B1Easily and surely performSuture equipmentsCosmetic preparationsVitrificationPolymer science
Shape-memory biodegradable and absorbable materials which make it possible to easily treat vital tissues by suture, anastomosis, ligation, fixation, reconstitution, prosthesis, etc. without causing burn. These materials never induce halation in MRI or CT and never remain in vivo. Such a shape-memory biodegradable and absorbable material is a material made of a molded article of lactic acid-based polymer and can be recovered to the original shape without applying any external force thereto but heating to a definite temperature or above. It is obtained by deforming a molded article (a primary molded article) made of a lactic acid-based polymer and having a definite shape into another molded article (a secondary molded article) having another shape at a temperature higher than the glass transition temperature thereof but lower than the crystallization temperature thereof (or 100.degree. C. when the molded article has no crystallization temperature) and then fixing said molded article to the thus deformed shape by cooling it as such to a temperature lower than the glass transition temperature. When this material is heated to the above-mentioned deformation temperature or above, it is immediately recovered to the original shape. The lactic acid-based polymer is hydrolyzed and absorbed in vivo.
Owner:TAKIRON CO LTD
Integrated processing of porous dielectric, polymer-coated substrates and epoxy within a multi-chamber vacuum system confirmation
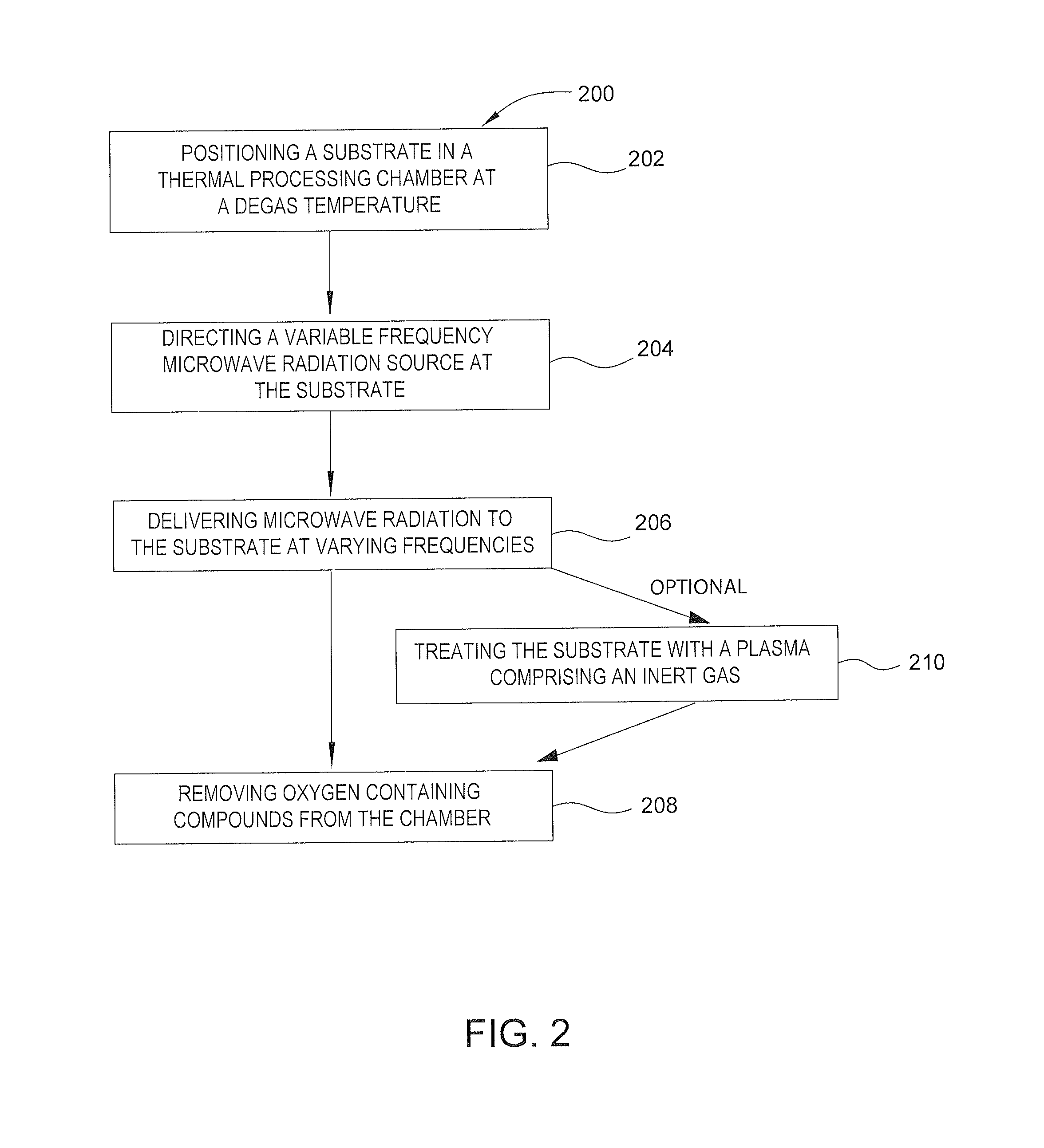
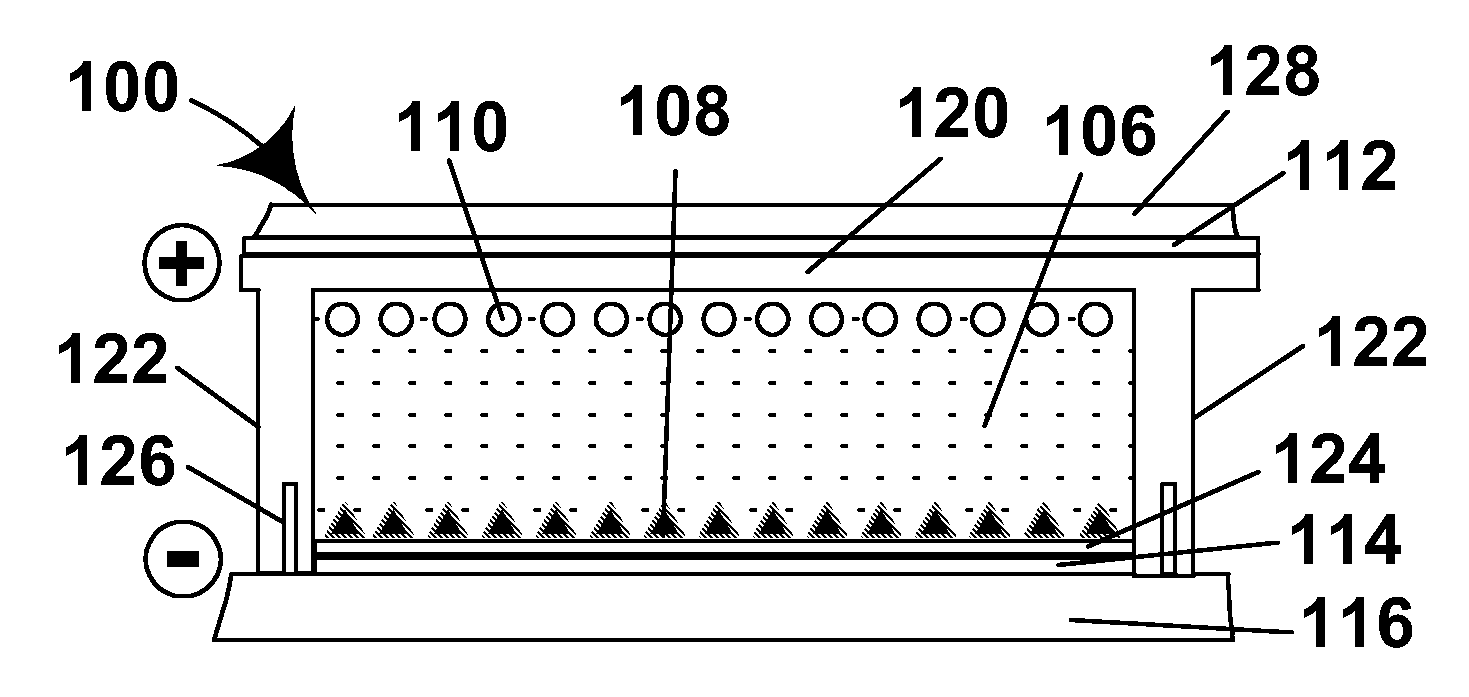
Methods and apparatus for processing a substrate are described herein. A vacuum multi-chamber deposition tool can include a degas chamber with both a heating mechanism and a variable frequency microwave source. A method for degassing a substrate can include positioning a substrate comprising a polymer or an epoxy within a processing chamber maintained between a degas temperature and a glass transition temperature, exposing the substrate to variable frequency microwave radiation, exposing the substrate to a plasma comprising an inert gas, removing oxygen containing compounds from the chamber, raising the pressure of inert gas in the chamber, and maintaining the pressure of inert gas while cooling the substrate to a temperature lower than the degas temperature.
Owner:APPLIED MATERIALS INC
Electrophoretic displays using gaseous fluids
ActiveUS7230751B2Reduce transmissionStatic indicating devicesNon-linear opticsVariable thicknessVitrification
Various improvements are provided in gas-based electrophoretic displays, including (a) the use of water getters to remove water from the gas; (b) the use of electron accepting or donating gases; (c) the use of electrophoretic polymer particles having high glass transition temperatures; (d) lateral movement of electrophoretic particles within the display; and (e) the use of variable thickness coatings on electrodes to provide for gray scale.
Owner:E INK CORPORATION
High-melting polyolefin copolymer elastomers, catalysts and methods of synthesis
InactiveUS6169151B1Property is limitedOrganic-compounds/hydrides/coordination-complexes catalystsElastomerPolymer science
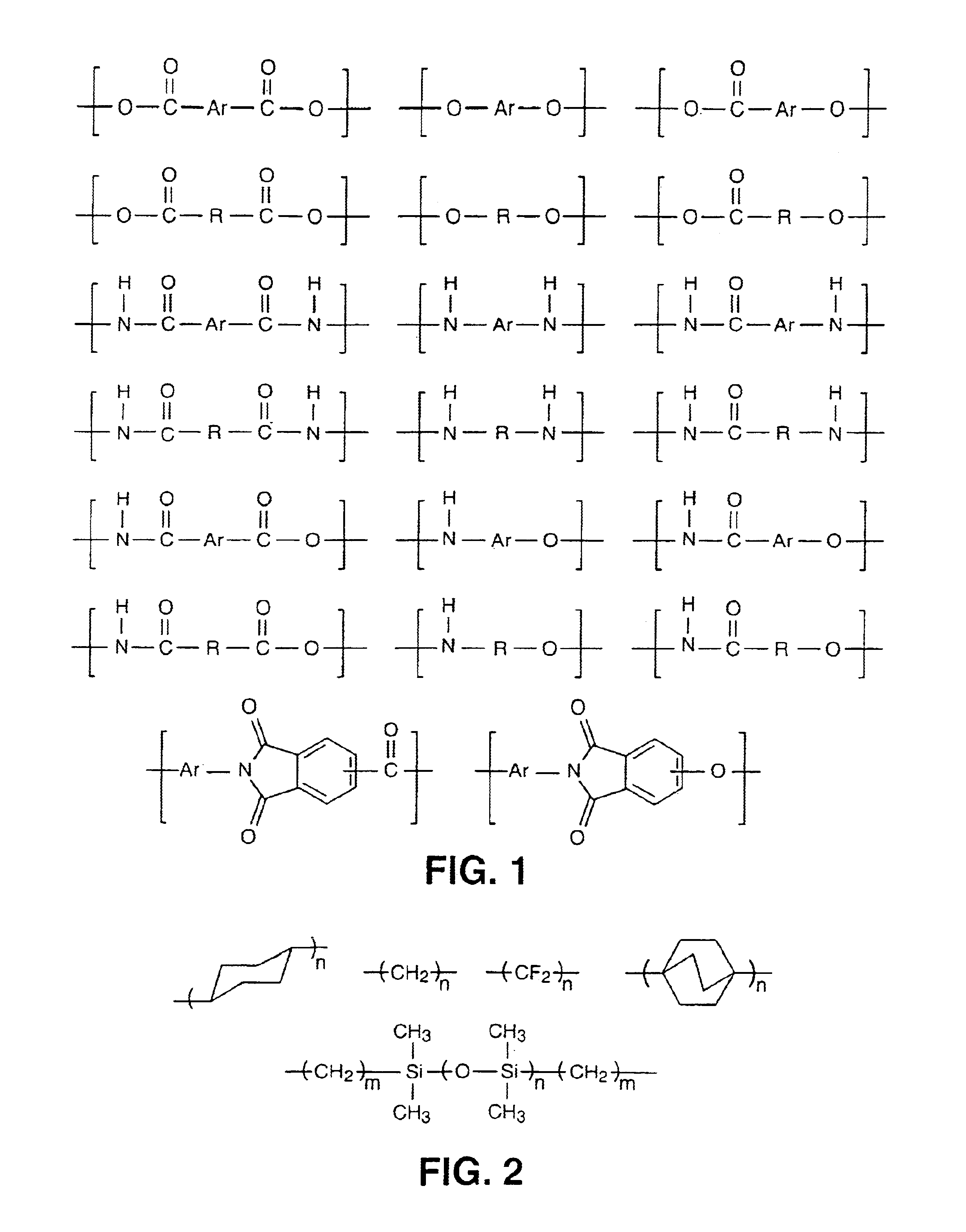
This invention relates to high melting polyolefin copolymers suitable as thermoplastic elastomers and catalysts and methods for their synthesis. These elastomeric olefin copolymers are characterized by a mole fraction of crystallizable component Xc from about 30 to about 99%; low glass transition temperatures, below -20° C., and typically below -50° C.; melting points above about 90° C.; high molecular weights; a molecular weight distribution MW / Mn< / =10; and a narrow composition distribution between chains of < / =15%. The novel copolymers of the invention range from reactor blends to multiblock copolymers that can be sequentially fractionated into fractions of differing crystallinities, which fractions nevertheless show compositions of comonomers which differ by less than 15% from the parent polymer (reactor product). The invention also relates to a process for producing such copolymers by utilizing an unbridged, substituted or unsubstituted cyclopentadienyl metallocene catalyst that is capable of interconverting between states with different copolymerization characteristics, which interconversion is controlled by selecting the substituents of the cyclopentadienyl ligands so that the rate of interconversion of the two states is within several orders of magnitude of the rate of formation of a single polymer chain. Where ri>rf the polymer can be characterized as multiblock; where ri<rf, the result is a polymer blend and where ri / rf is close to 1, the resulting polymer is a mixture of blend and multiblock. The metallocene catalysts of the invention are able to interconvert between more than two states, with embodiments of four states being shown in FIG. 2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Dimensionally stable, breathable, stretch-thinned, elastic films
A method for producing a stretch-thinned elastic article having dimensional stability over time and at elevated temperatures in which a thermoplastic block copolymer is melt-processed into a stretchable article such as a film or fiber. The article is then conditioned at an elevated temperature greater than or equal to a glass transition temperature (Tg) of a hard phase of the thermoplastic block copolymer. The article is stretch-thinned at the elevated temperature to a desired percentage stretch, forming a stretch-thinned article, after which it is cooled to a temperature below the glass transition temperature of the hard phase of the thermoplastic block copolymer. Films produced by this method are suitable for use in durable and disposable articles including personal care articles such as diapers, incontinence wear, training pants, and feminine care articles, as well as wound dressings, wipes, towels, napkins, and protective apparel.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Implantable medical devices and methods for making same
An implantable medical device is disclosed. The device is fabricated at least in part from a biocompatible, biodegradable composition. The composition is composed of a biocompatible, biodegradable polymer and a biocompatible, biodegradable wax. The concentration of the wax at the surface of the device is greater than the concentration of the wax in the body of the implantable device. The increased concentration of wax at the device surface may be attained when the device is heated to a temperature greater than the melting point of the wax and the glass transition temperature of the polymer, but lower than melting point of the polymer.
Owner:ETHICON INC
Biodegradable polymer films and sheets suitable for use as laminate coatings as well as wraps and other packaging materials
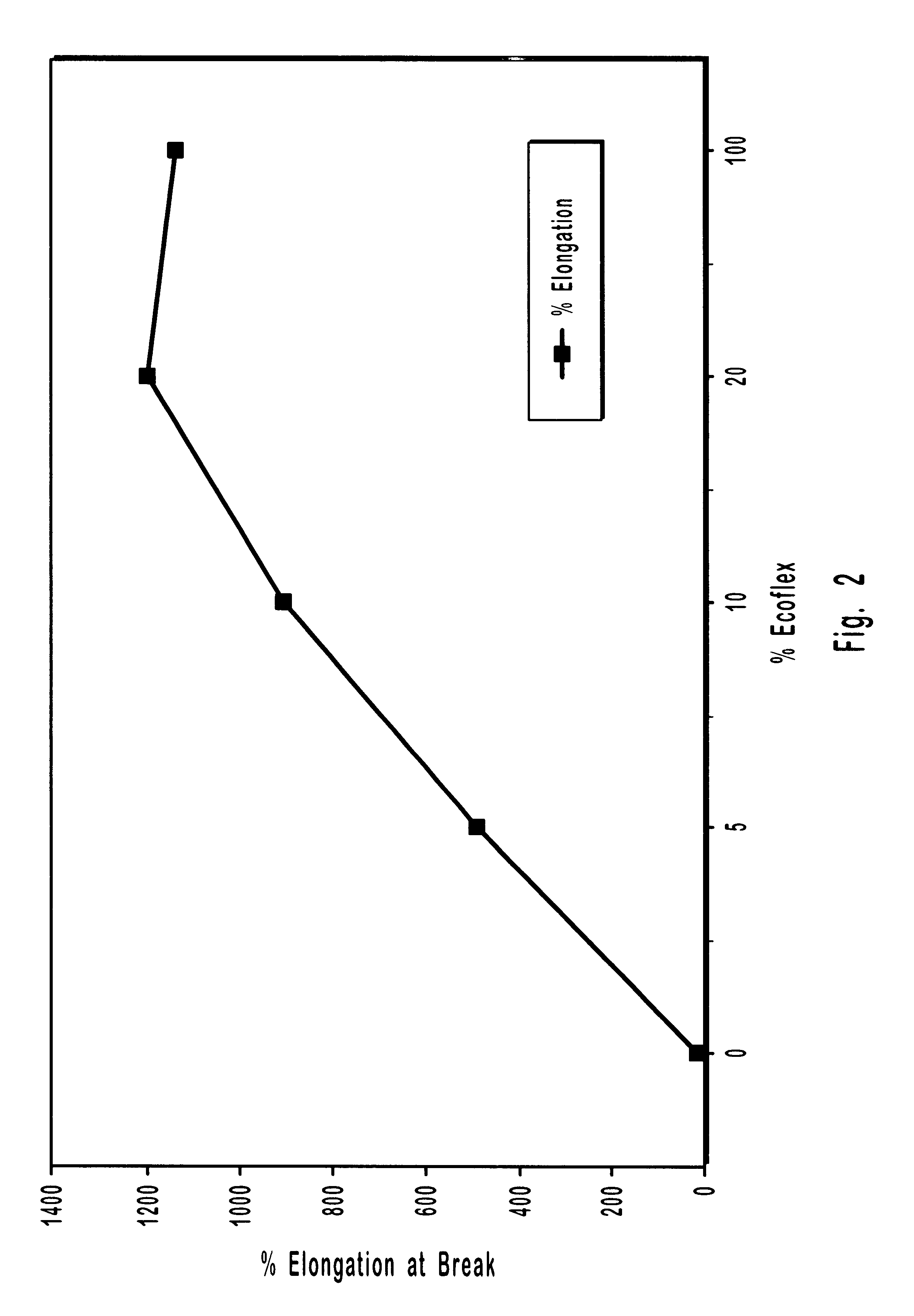
Biodegradable polymer blends suitable for laminate coatings, wraps and other packaging materials manufactured from at least one "hard" biopolymer and at least one "soft" biopolymer. "Hard" biopolymers tend to be more brittle and rigid and typically have a glass transition temperature greater than about 10° C. "Soft" biopolymers tend to be more flexible and pliable and typically have a glass transition temperature less than about 0° C. While hard and soft polymers each possess certain intrinsic benefits, certain blends of hard and soft polymers have been discovered which possess synergistic properties superior to those of either hard or soft polymers by themselves. Biodegradable polymers include polyesters, polyesteramides and thermoplastically processable starch. The polymer blends may optionally include an inorganic filler. Films and sheets made from the polymer blends may be textured so as to increase the bulk hand feel. Wraps will typically be manufactured so as to have good "dead-fold" properties so as to remain in a wrapped position and not spring back to an "unwrapped" and planar form. Laminate films will typically have good water vapor barrier properties as measured by the their Water Vapor Permeability Coefficient (WVPC).
Owner:BIO TEC BIOLOGISCHE NATURVERPACKUNGEN
Ternary systems of benzoxazine, epoxy, and phenolic resins
Low viscosity ternary mixtures of benzoxazine, epoxy and phenolic resins have been developed. The blends render homogeneous and void free cured specimen with a wide range of properties. Melt viscosity values as low as 0.3 Pa.s at 100° C. can be achieved. The phenolic resin acts as a cure accelerator to the system, besides its typical function as a hardener of epoxy resin. Glass transition temperatures Tg as high as 170° C. can also be obtained.
Owner:EDISON POLYMER INNOVATION EPIC
Synthetic fiber
InactiveUS6135987AFormed easily and efficientlyCeramic shaping apparatusBaby linensPolyesterVitrification
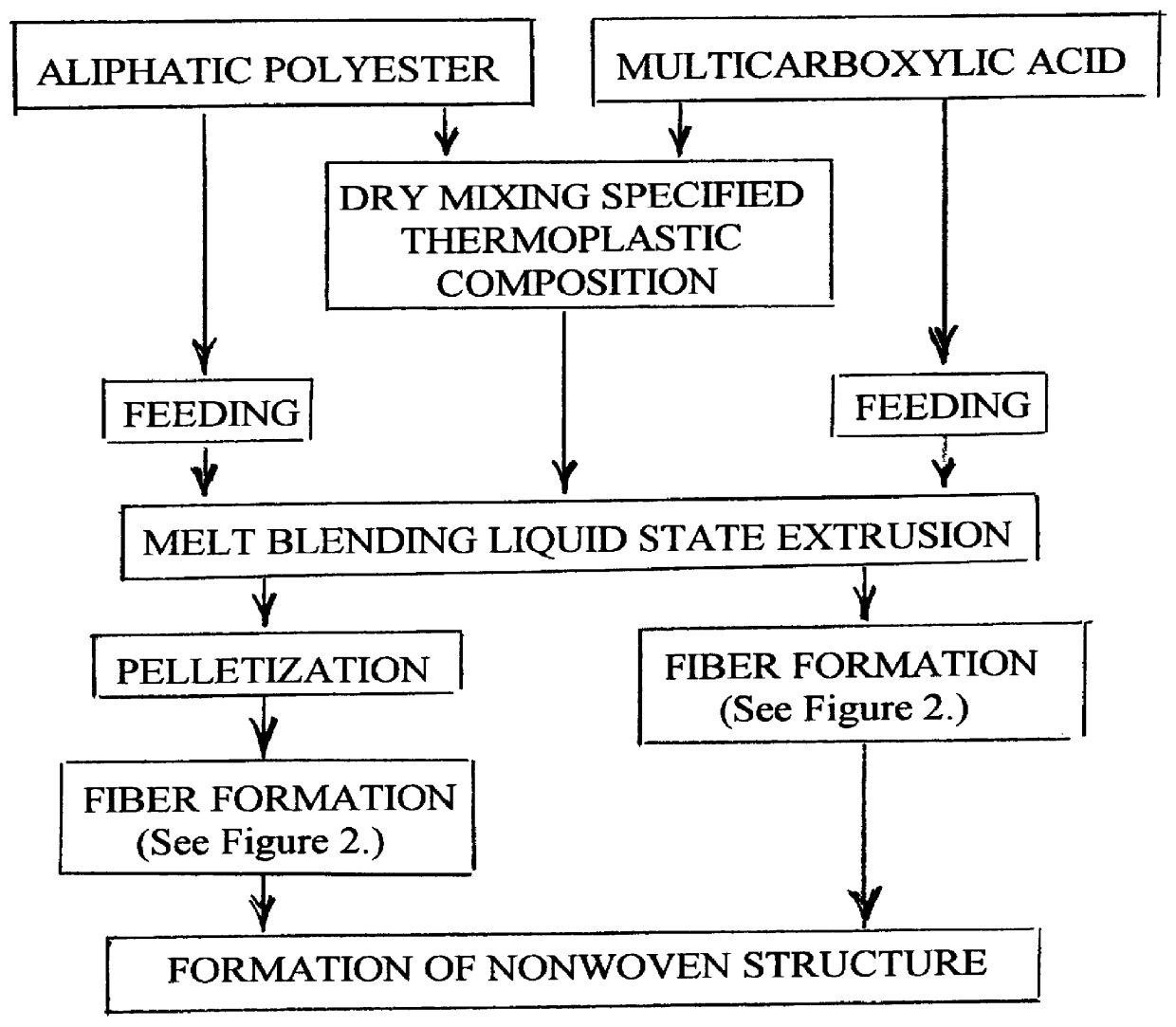
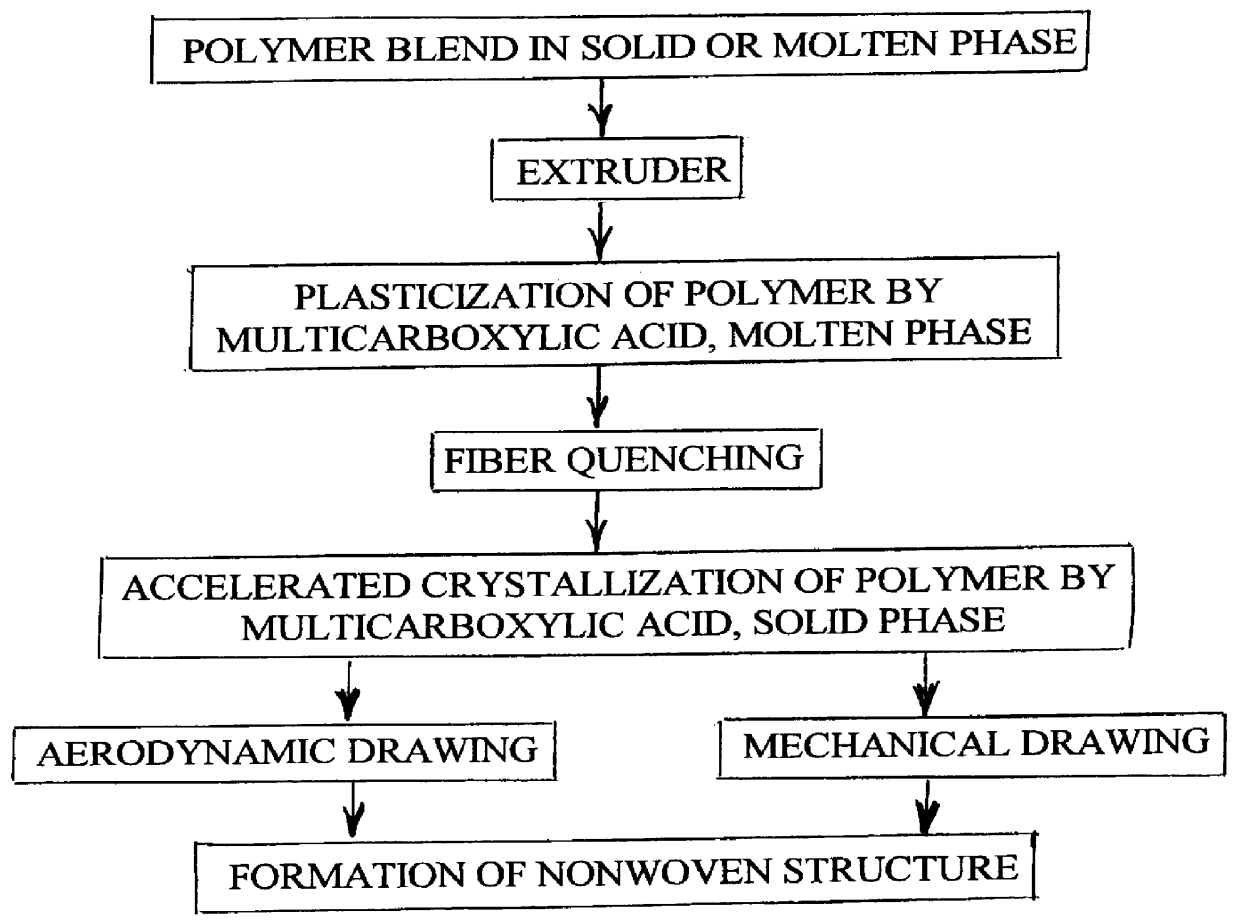
A process is disclosed for forming a synthetic fiber including providing a first component of an aliphatic polyester polymer a second component of a multicarboxylic acid, mixing the first component aliphatic polyester polymer and the second component multicarboxylic acid to form an unreacted specified thermoplastic composition, and melt blending the unreacted specified thermoplastic composition in an extruder or a mixer. The second component multicarboxylic acid lubricates the extruder and provides a nucleating agent for crystallizing the specified thermoplastic composition to form a mean crystal size less than about 120 Angstroms. Fiber composed of the specified thermoplastic composition has a mean crystal size less than about 120 Angstroms. The fiber has a glass transition temperature (Tg) less than about 55 DEG C. In one aspect, a first component of polylactic acid and a second component of adipic acid provide synthetic fibers in a nonwoven structure used in a biodegradable and compostable disposable absorbent product for the absorption and removal of body fluids.
Owner:KIMBERLY-CLARK WORLDWIDE INC
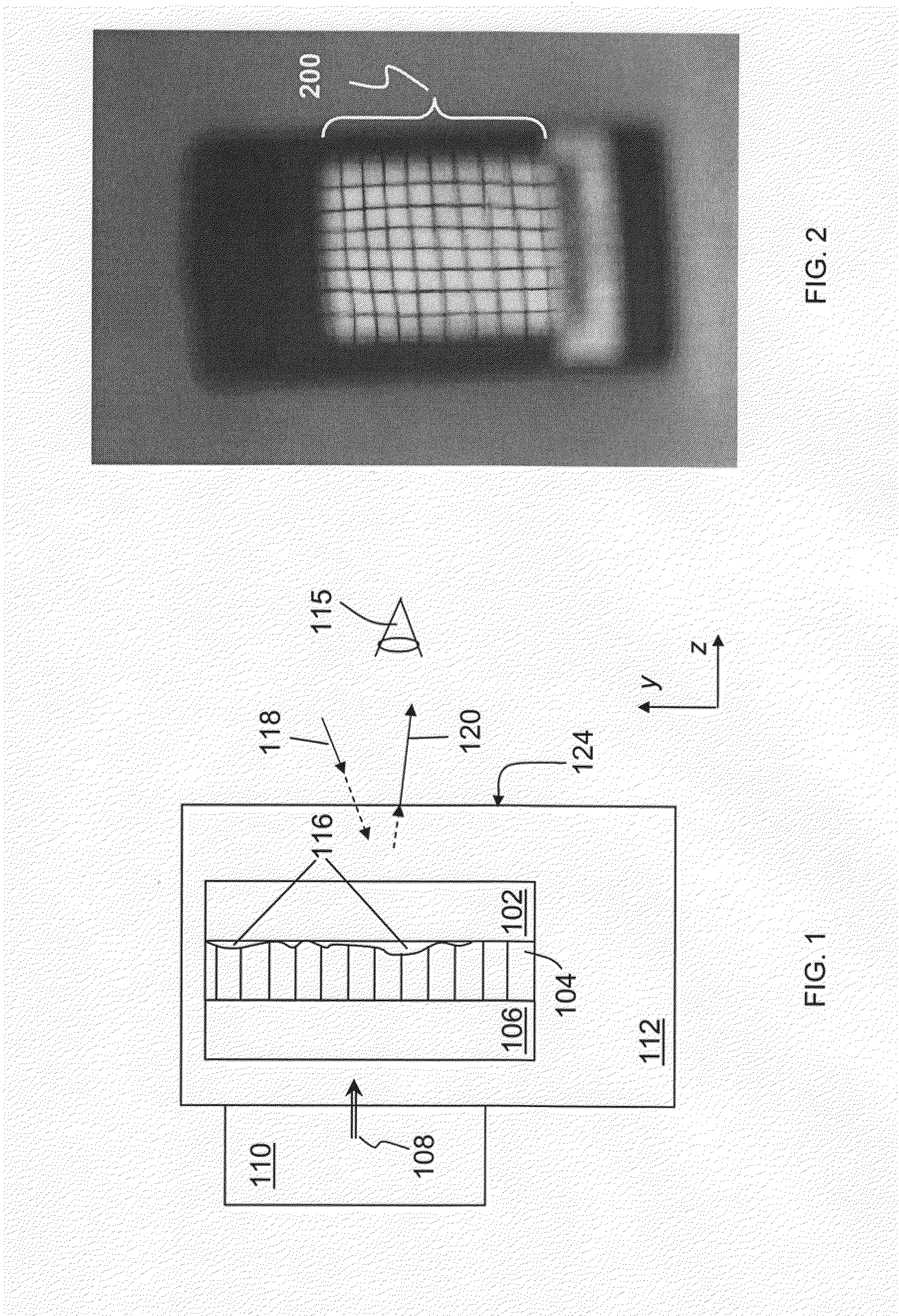
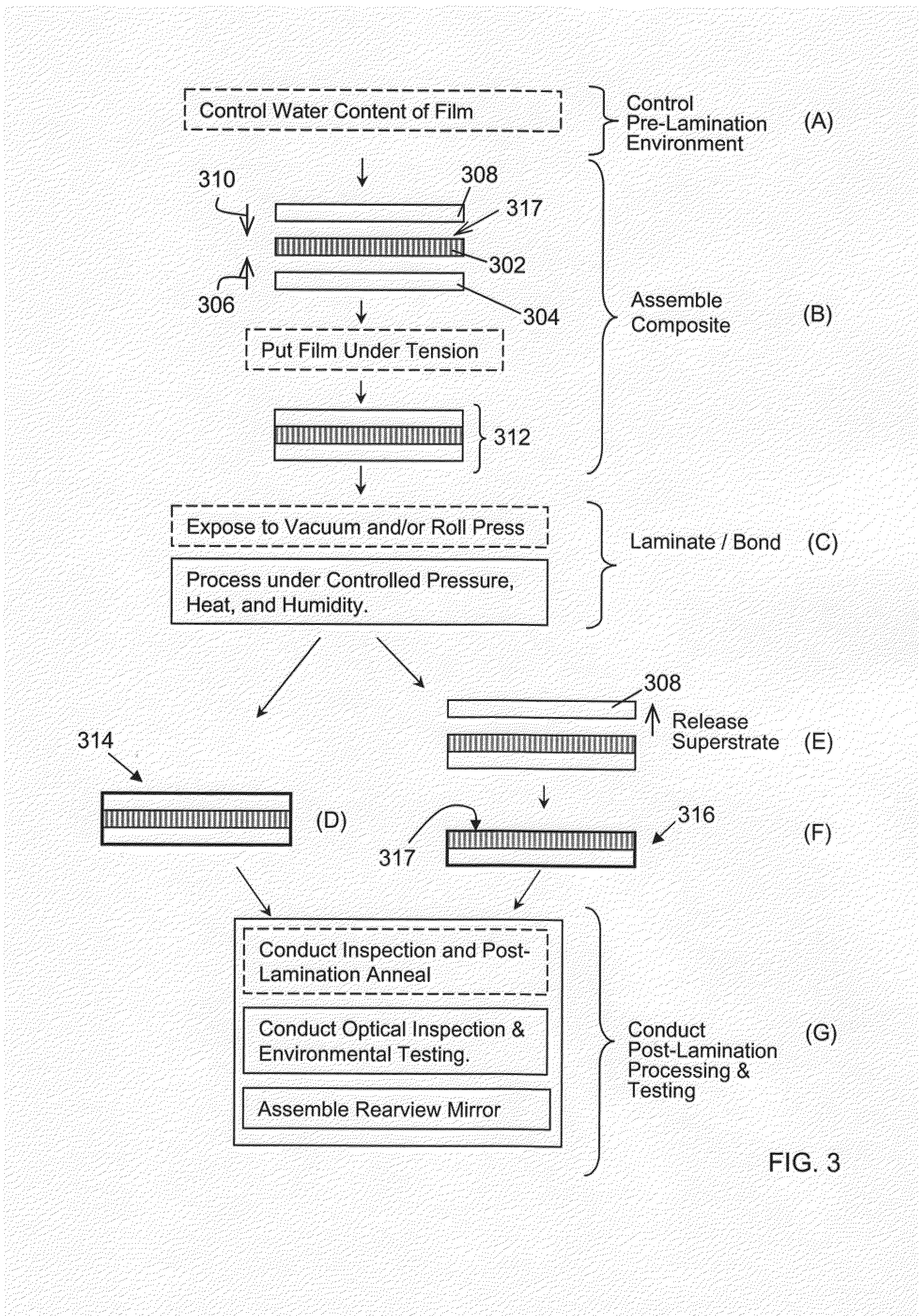
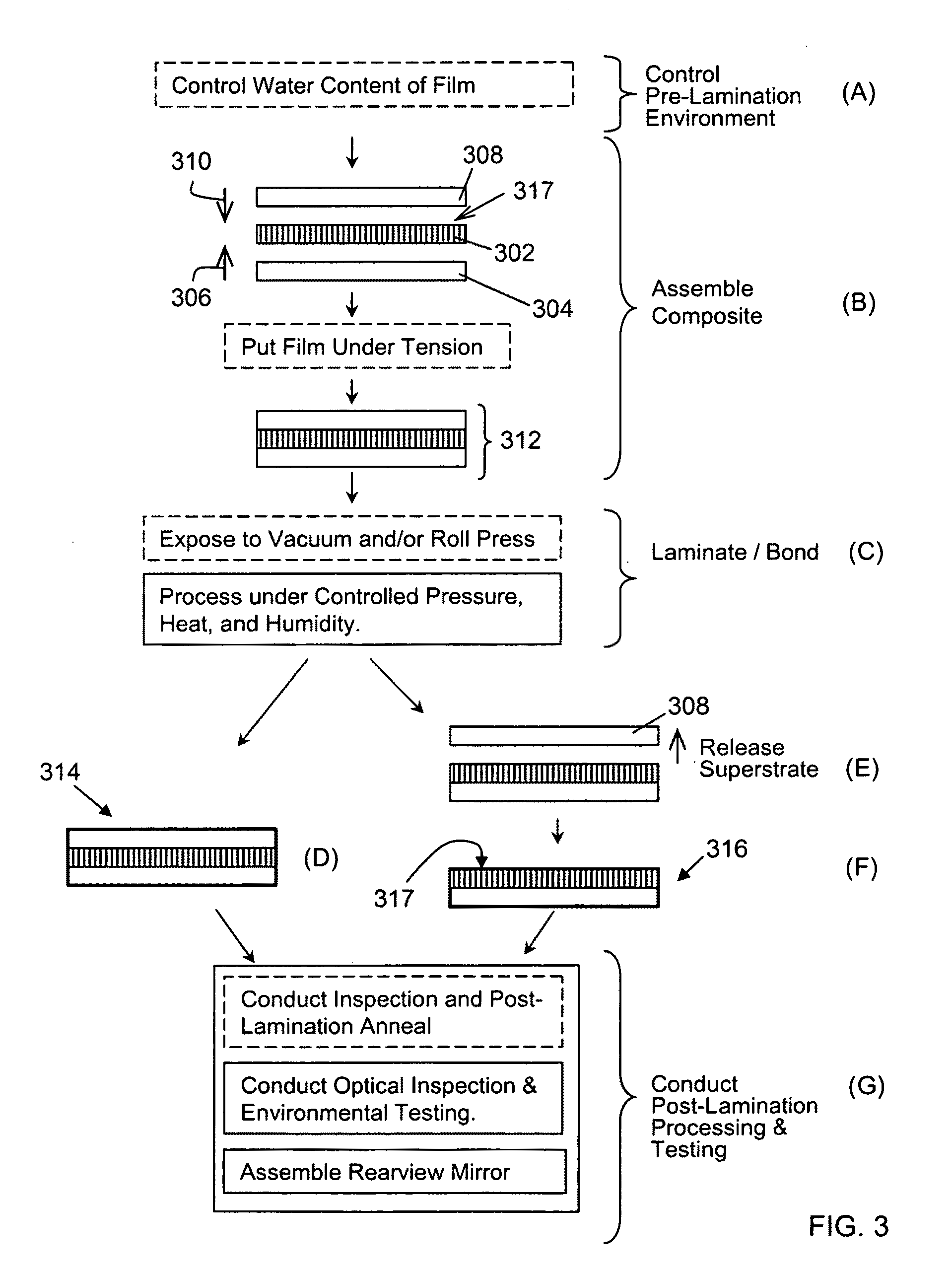
Rearview Mirror Assemblies With Anisotropic Polymer Laminates
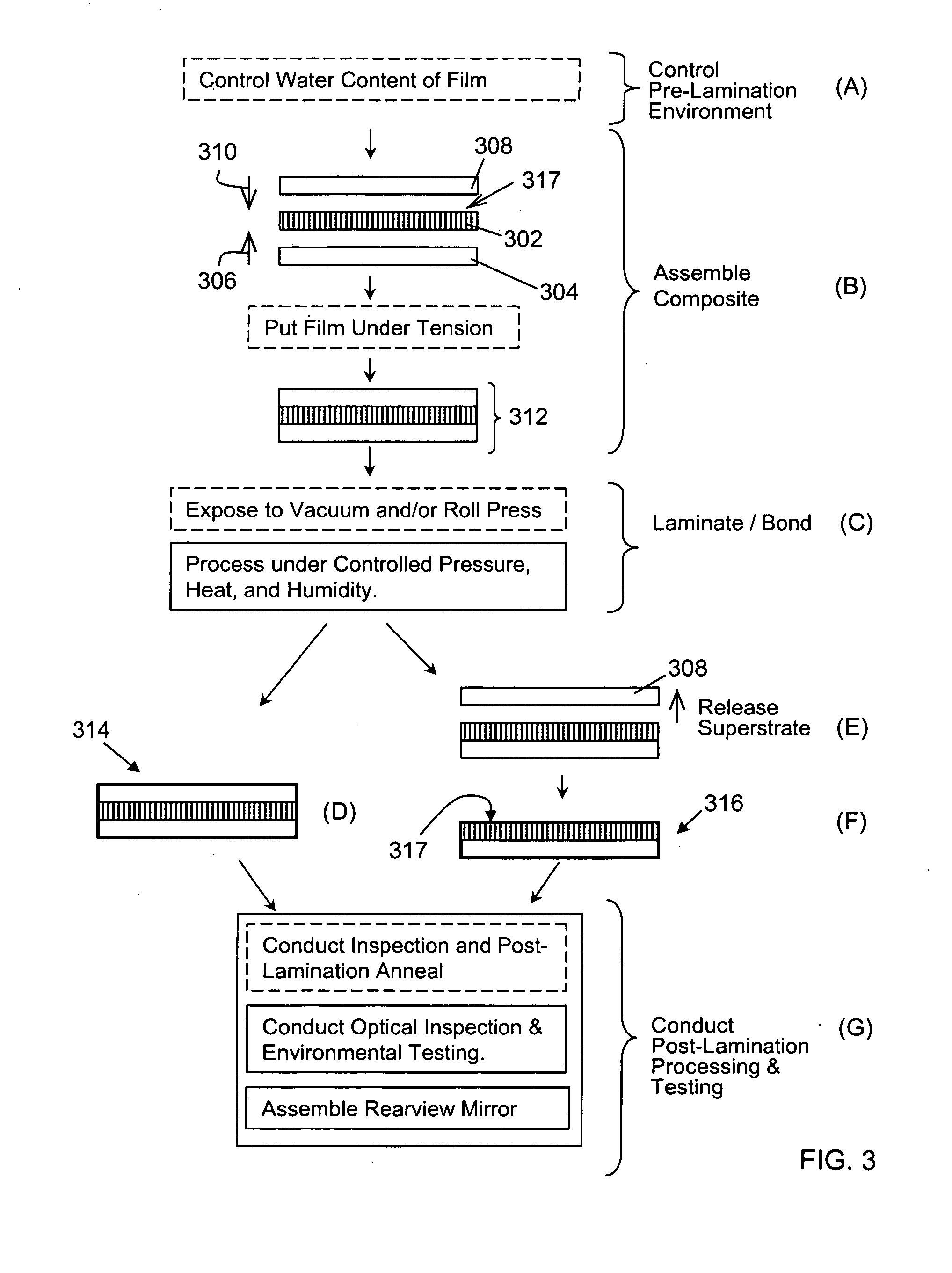
ActiveUS20100277786A1High strengthAdequate flatness of filmPolarising elementsNon-linear opticsLower limitGlass transition
Anisotropic film laminates for use in image-preserving reflectors such as rearview automotive mirror assemblies, and related methods of fabrication. A film may comprise an anisotropic layer such as a light-polarizing layer and other functional layers. The film having controlled water content is heated under omnidirectional pressure and vacuum to a temperature substantially equal to or above a lower limit of a glass-transition temperature range of the film so as to be laminated to a substrate. The laminate is configured as part of a mirror structure so as to increase contrast of light produced by a light source positioned behind the mirror structure and transmitted through the mirror structure towards a viewer. The mirror structure is devoid of any extended distortion and is characterized by SW and LW values less than 3, more preferably less than 2, and most preferably less than 1.
Owner:GENTEX CORP
Latex polymer blends for improving the permanence of ink-jet inks
InactiveUS6057384AImprove adhesionPromotes increased adhesionDuplicating/marking methodsInksGlass transitionPolymer chemistry
Ink-jet inks for ink-jet printing are provided which include a vehicle and a colorant, the colorant associated with a primer core / shell polymer to form a primer / colorant combination, and the primer / colorant combination, upon printing on a print medium, encapsulated by a durable core / shell polymer. The primer core / shell polymer serves to promote adhesion of the durable core / shell polymer to the colorant and to disperse the colorant in the ink and the durable core / shell polymer serves to provide a smear-fast film upon drying of the ink on a print medium. The primer core / shell polymer comprises a hydrophobic core and a hydrophilic shell comprising a polar component, while the durable core / shell polymer comprises a hydrophobic core comprising a first Tg component, which, when homopolymerized, has a glass trnnsition temperature, Tg, between -150 DEG C. to +25 DEG C. and a second Tg component, which, when homopolymerized, has a glass transition temperature above +25 DEG C. and a hydrophilic shell selected from the group consisting of neutral shells, cationic shells, and anionic shells.
Owner:HEWLETT PACKARD DEV CO LP
Transfer film and process for producing organic electroluminescent device using the same
InactiveUS6805979B2Decorative surface effectsCathode ray/electron stream lampsEngineeringOrganic electroluminescence
Owner:SHARP KK
Rearview Mirror Assemblies With Anisotropic Polymer Laminates
ActiveUS20090296190A1High strengthAdequate flatness of filmMirrorsSynthetic resin layered productsLower limitWing mirror
Anisotropic film laminates for use in image-preserving reflectors such as rearview automotive mirror assemblies, and related methods of fabrication. A film may comprise an anisotropic layer such as a light-polarizing layer and other functional layers. The film having controlled water content is heated under omnidirectional pressure and vacuum to a temperature substantially equal to or above a lower limit of a glass-transition temperature range of the film so as to be laminated to a substrate. The laminate is configured as part of a mirror structure so as to increase contrast of light produced by a light source positioned behind the mirror structure and transmitted through the mirror structure towards a viewer. The mirror structure is devoid of any extended distortion and is characterized by SW and LW values less than 3, more preferably less than 2, and most preferably less than 1.
Owner:GENTEX CORP
Foamed electrical wire and a method of producing the same
ActiveUS9142334B2Increase resistanceGood partitionPlastic/resin/waxes insulatorsInsulated cablesElectrical conductorGlass transition
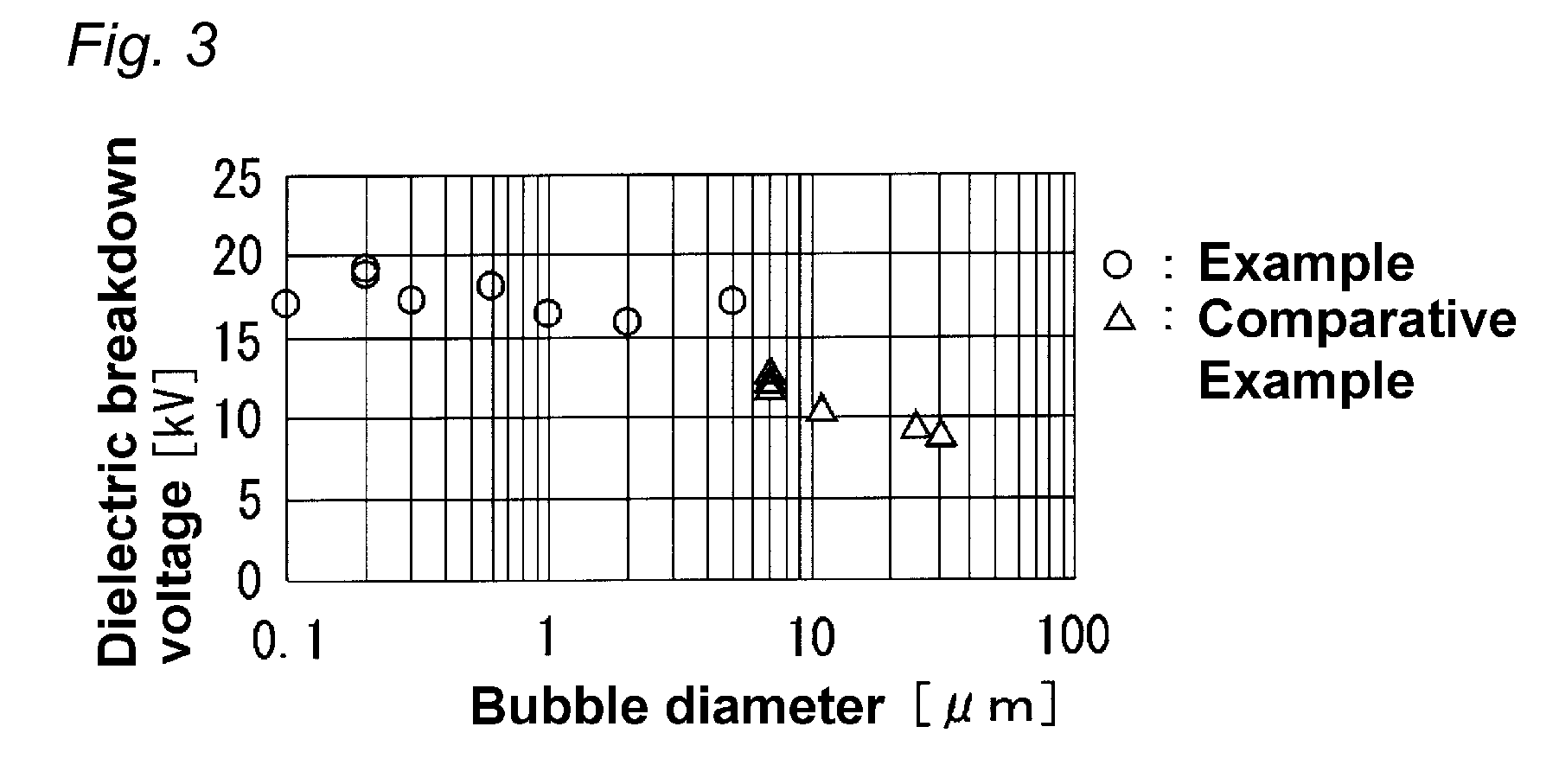
A foamed electrical wire, containing: a conductor; and a foamed insulating layer; in which the foamed insulating layer comprises a thermoplastic resin that is a crystalline thermoplastic resin having a melting point of 150° C. or more or a non-crystalline thermoplastic resin having a glass transition temperature of 150° C. or more, and the average bubble diameter of the foamed insulating layer is 5 μm or less.
Owner:ESSEX FURUKAWA MAGNET WIRE LLC
Methods for using polishing pads
Polymer-based pads useful for polishing objects, particularly integrated circuits, having interconnected porosity which is uniform in all directions, and where the solid portion of said pad consists of a uniform continuously interconnected polymer material of greater than 50% of the gross volume of the article, are produced directly to final shape and dimension by pressure sintering powder compacts of thermoplastic polymer at a temperature above the glass transition temperature but not exceeding the melting point of the polymer and at a pressure in excess of 100 psi in a mold having the desired final pad dimensions. In a preferred version, a mixture of two polymer powders is used, where one polymer has a lower melting point than the other. When pressure sintered at a temperature not to exceed the melting point of the lower melting powder, the increased stiffness afforded by incorporation of the higher melting polymer component gives improved mechanical strength to the sintered product. Conditions for producing the pads of this invention are such that the polymer powder particles from which the pads are produced essentially retain their original shape and are point bonded to form the pad.
Owner:ROHM & HAAS ELECTRONICS MATERIALS CMP HLDG INC
Hollow sphere organic pigment for paper or paper coatings
InactiveUS6139961ATrend downHigh molecular weightSynthetic resin layered productsCellulosic plastic layered productsVitrificationPaperboard
A hollow sphere organic pigment in which a core containing a void is encapsulated by a first shell polymer having a glass transition temperature greater than 50 DEG C., the first shell having polymerized thereon a second shell polymer having a glass transition temperature of -15 DEG C. to -50 DEG C. is provided. Also provided is a paper or paperboard coating composition containing the hollow sphere organic pigment, a method for improving the strength and opacity of a paper or paperboard coating by using the coating composition dried, a coated paper or paperboard bearing the dried coating composition, and a method for improving the strength and opacity of paper or paperboard by incorporating a particular hollow sphere organic pigment into the formed wet sheet.
Owner:ROHM & HAAS CO
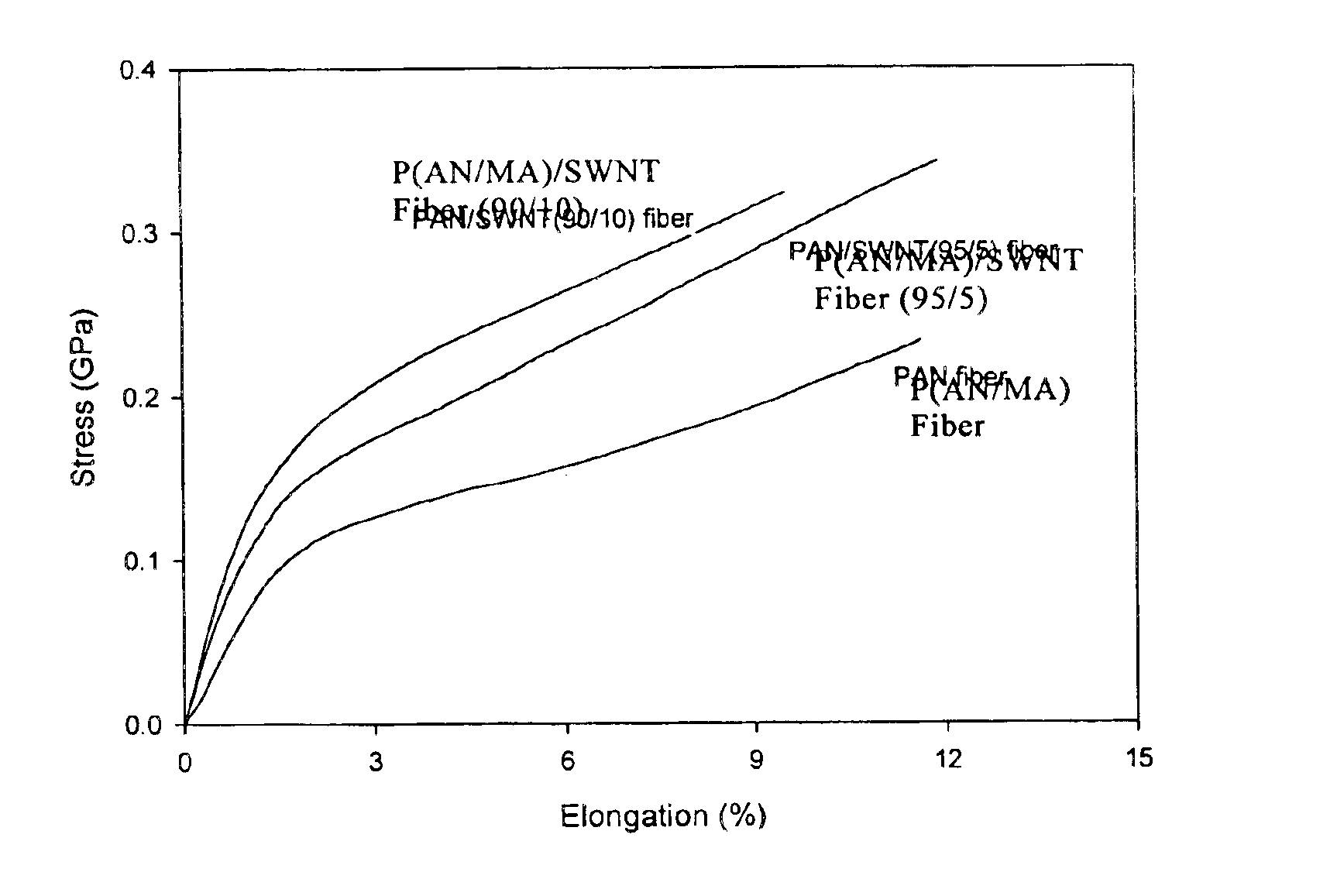
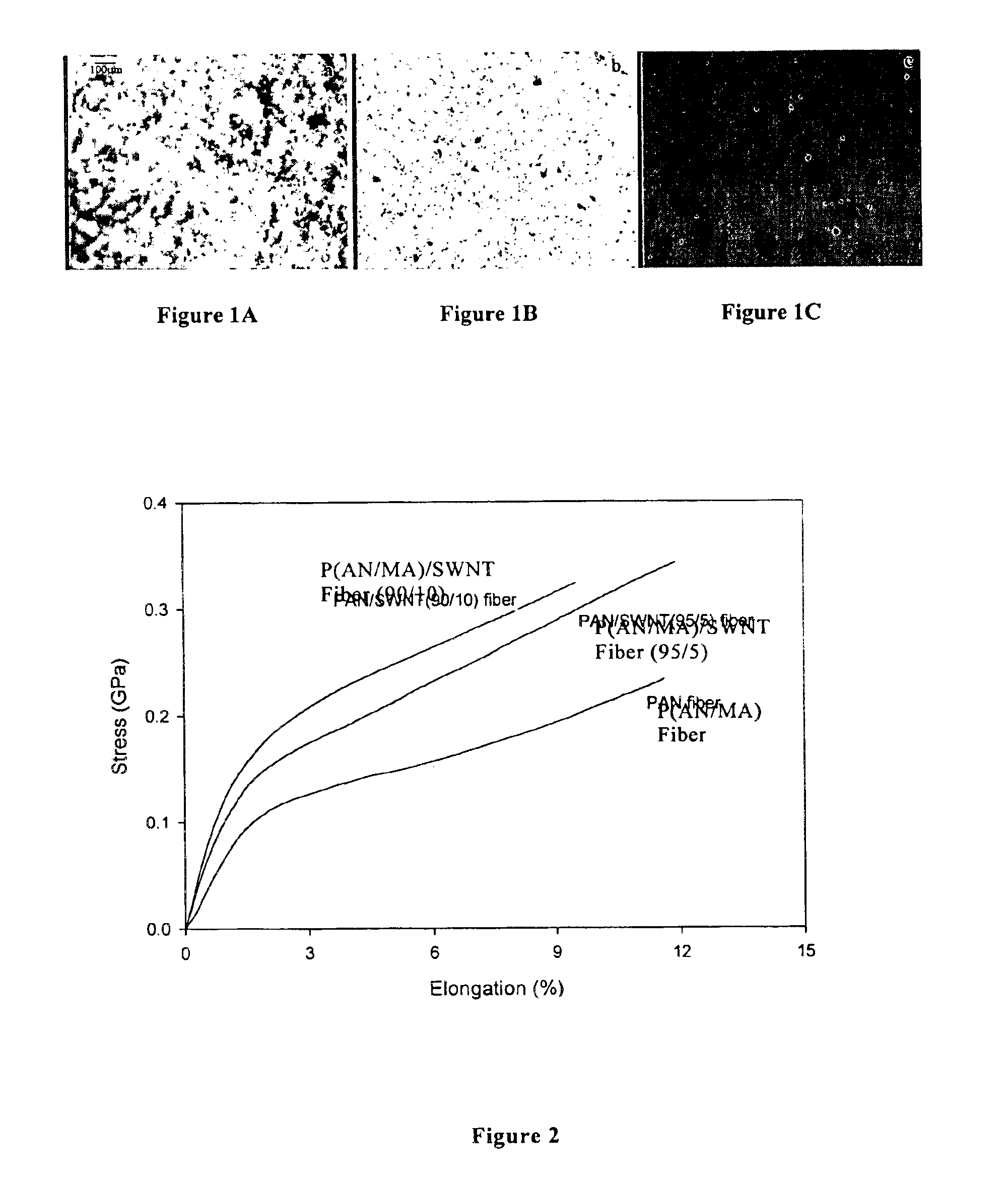
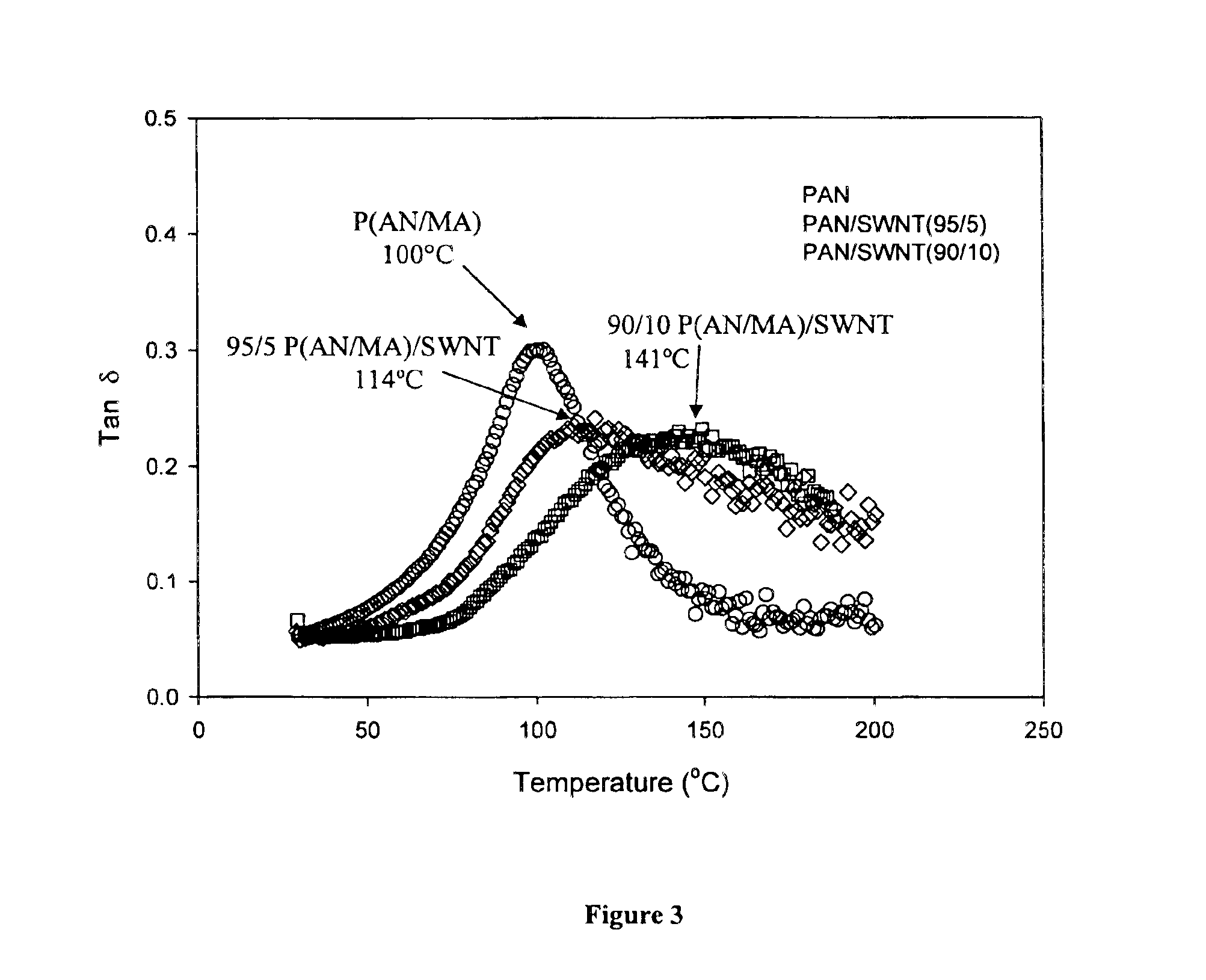
Macroscopic fiber comprising single-wall carbon nanotubes and acrylonitrile-based polymer and process for making the same
InactiveUS6852410B2Increased tensile modulusReduced thermal shrinkageMaterial nanotechnologyElectroconductive/antistatic filament manufactureVitrificationPolymer science
The present invention relates to a high modulus macroscopic fiber comprising single-wall carbon nanotubes (SWNT) and an acrylonitrile-containing polymer. In one embodiment, the macroscopic fiber is a drawn fiber having a cross-sectional dimension of at least 1 micron. In another embodiment, the acrylonitrile polymer-SWNT composite fiber is made by dispersing SWNT in a solvent, such as dimethyl formamide or dimethyl acetamide, admixing an acrylonitrile-based polymer to form a generally optically homogeneous polyacrylonitrile polymer-SWNT dope, spinning the dope into a fiber, drawing and drying the fiber. Polyacrylonitrile / SWNT composite macroscopic fibers have substantially higher modulus and reduced shrinkage versus a polymer fiber without SWNT. A polyacrylonitrile / SWNT fiber containing 10 wt % SWNT showed over 100% increase in tensile modulus and significantly reduced thermal shrinkage compared to a control fiber without SWNT. With 10 wt % SWNT, the glass transition temperature of the polymer increased by more than 40° C.
Owner:GEORGIA TECH RES CORP
Compatibilized blends of biodegradable polymers with improved rheology
This invention relates to a blend of biodegradable polymers comprising: (A) about 5% to about 95% by weight of at least one flexible biodegradable polymer (A) having a glass transition less than about 0° C., (B) about 5% to about 95% by weight of at least one rigid biodegradable polymer (B) having a glass transition greater than about 10° C., and (C) about 0.25 to about 10 weight % of at least one compatibilizer (C), said percentages being based on the total weight of the polymer blend; where the polymer blend has a higher zero shear melt viscosity than polymers (A) and (B) separately.
Owner:NOVAMONT SPA
Solar cell module
InactiveUS20050199279A1Easy to solveImprove moisture resistancePV power plantsPhotovoltaic energy generationVitrificationSilver paste
A solar cell module of the present invention was made to improve adhesion between electrodes, which is formed with thermosetting resin containing silver paste, of a solar cell element and connecting tabs coated with lead (Pb) free solder. To achieve this purpose, the solar cell module is comprised of a front surface member, a rear surface protective member, a plurality of solar cell elements provided between the front surface member and the rear surface protective member, and connecting tabs for electrically connecting the solar cell elements to each other through electrodes with the use of lead free solder. The electrodes of the solar cell elements are made of silver paste containing thermosetting resin and silver powder. The thermosetting resin contains epoxy resin at volume ratio of 70% or more having a glass transition rate of 80° C. to 200° C. measured by a TMA method. The connecting tabs coated with lead free solder are soldered to the electrodes.
Owner:PANASONIC INTELLECTUAL PROPERTY MANAGEMENT CO LTD
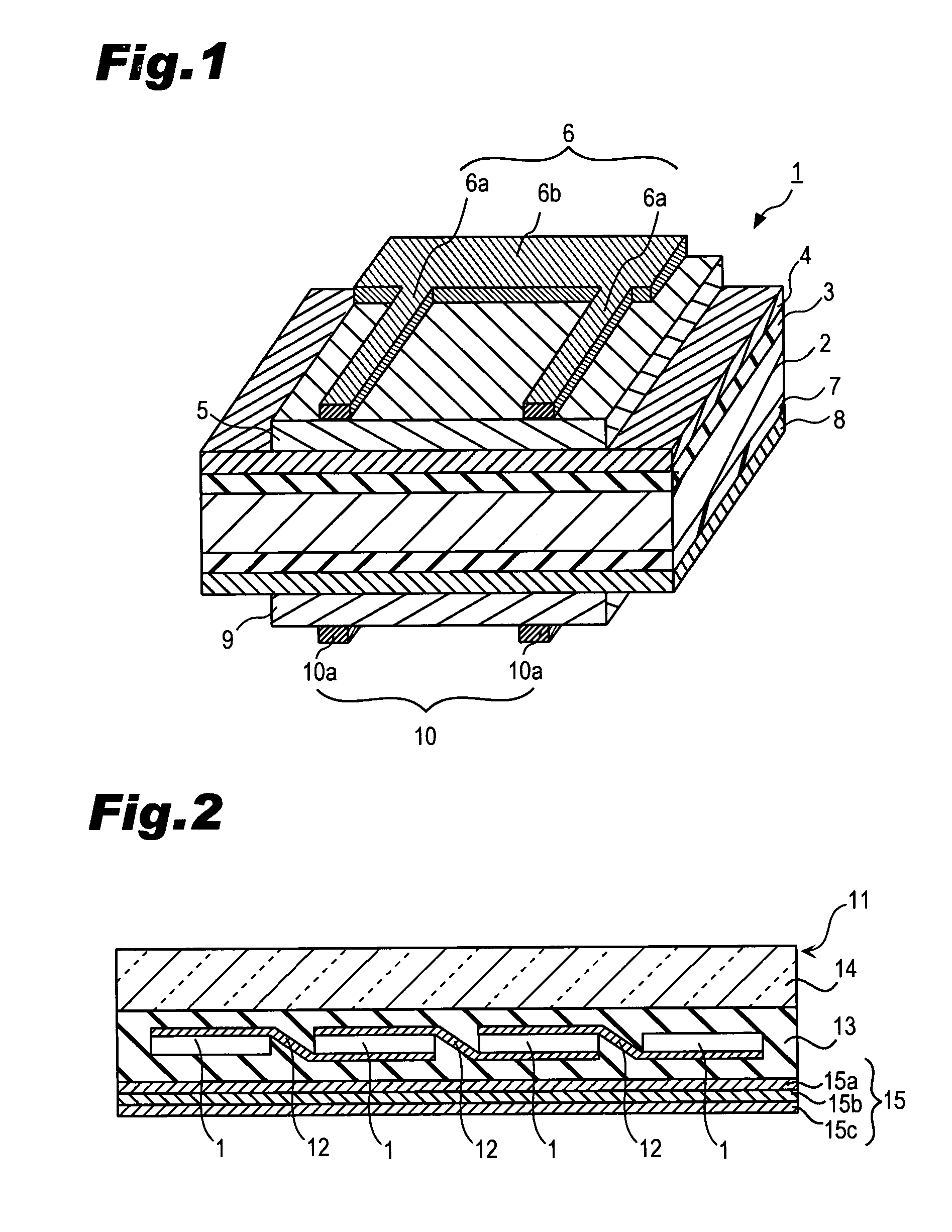
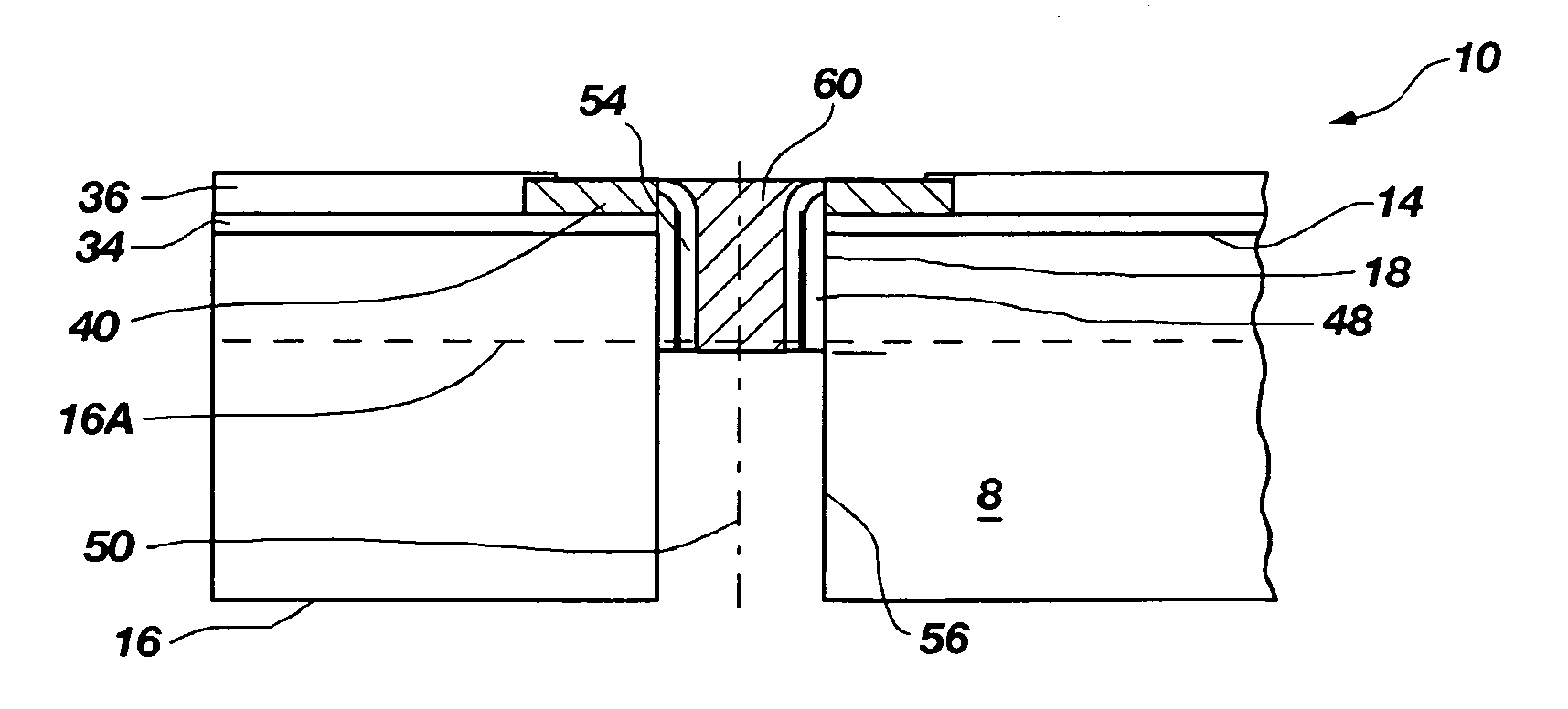
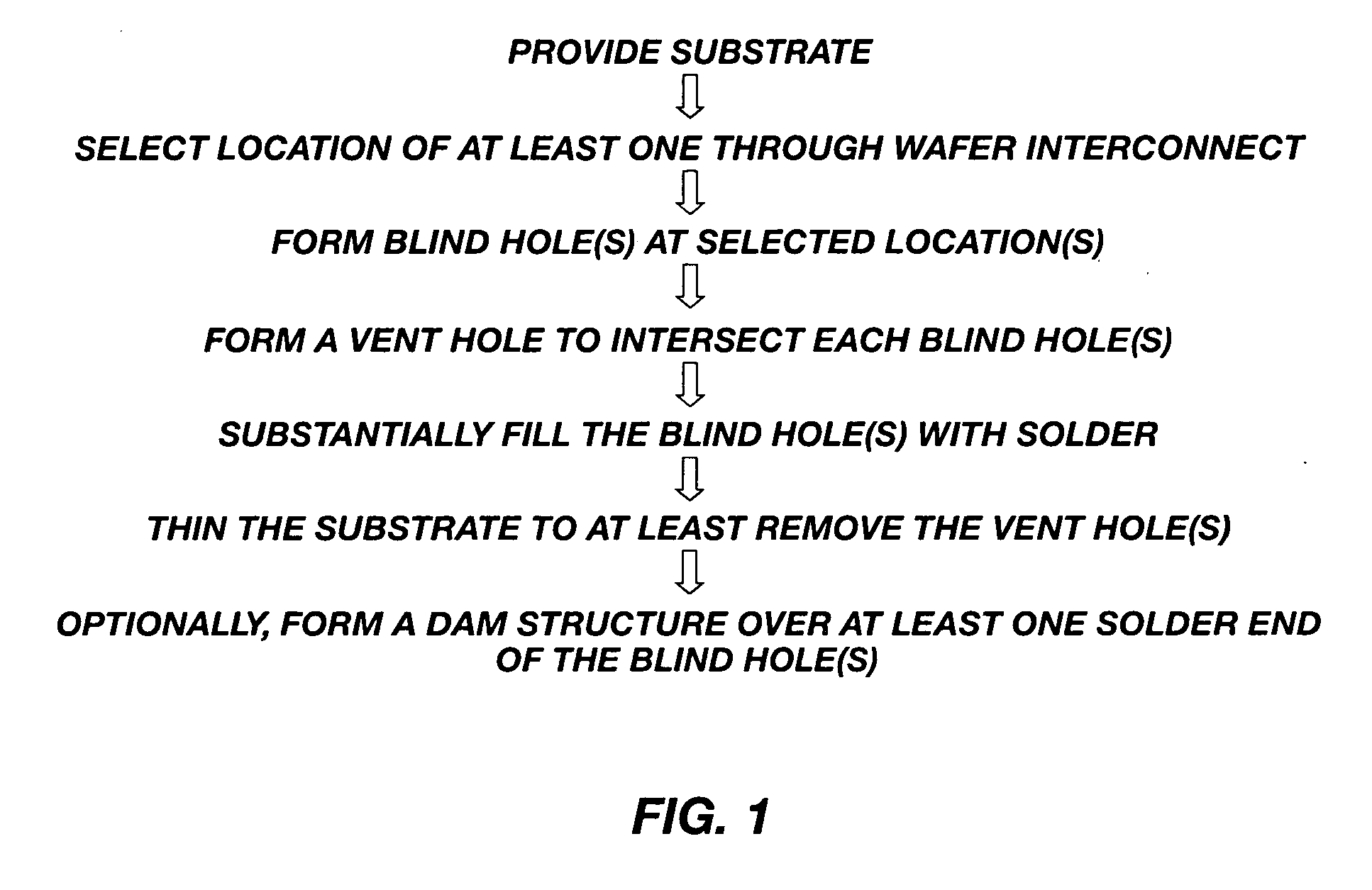
Methods for forming through-wafer interconnects, intermediate structures so formed, and devices and systems having at least one solder dam structure
ActiveUS20070045779A1Conveniently attachedEliminate cross-contaminationSemiconductor/solid-state device detailsSolid-state devicesVitrificationDevice material
A method for forming through-wafer interconnects (TWI) in a substrate of a thickness in excess of that of a semiconductor die such as a semiconductor wafer. Blind holes are formed from the active surface, sidewalls thereof passivated and coated with a solder-wetting material. A vent hole is then formed from the opposite surface (e.g., wafer back side) to intersect the blind hole. The blind hole is solder filled, followed by back thinning of the vent hole portion of the wafer to a final substrate thickness to expose the solder and solder-wetting material at both the active surface and the thinned back side. A metal layer such as nickel, having a glass transition temperature greater than that of the solder, may be plated to form a dam structure covering one or both ends of the TWI including the solder and solder-wetting material to prevent leakage of molten solder from the TWI during high temperature excursions. Intermediate structures of semiconductor devices, semiconductor devices and systems are also disclosed.
Owner:MICRON TECH INC
Titanium group powder metallurgy
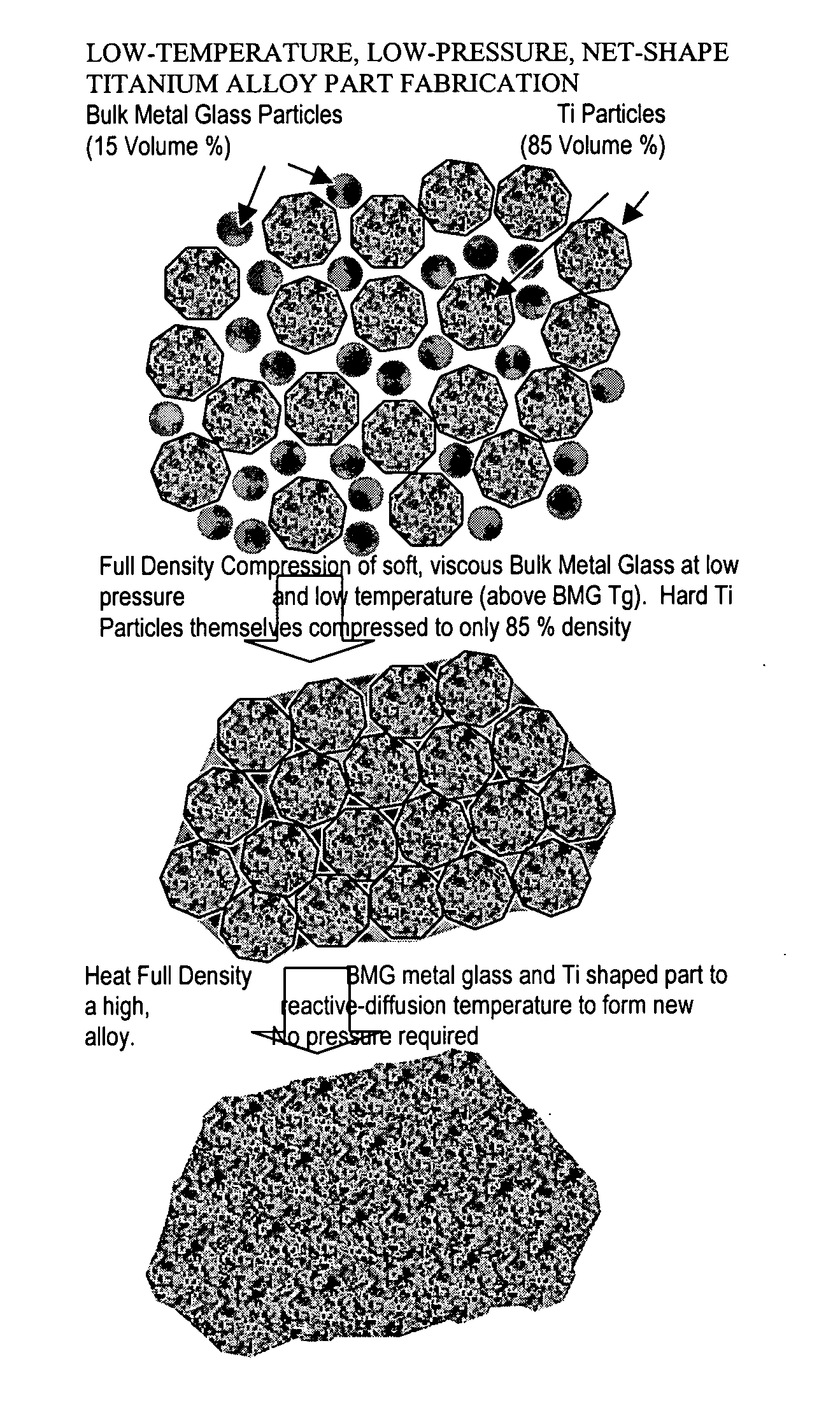
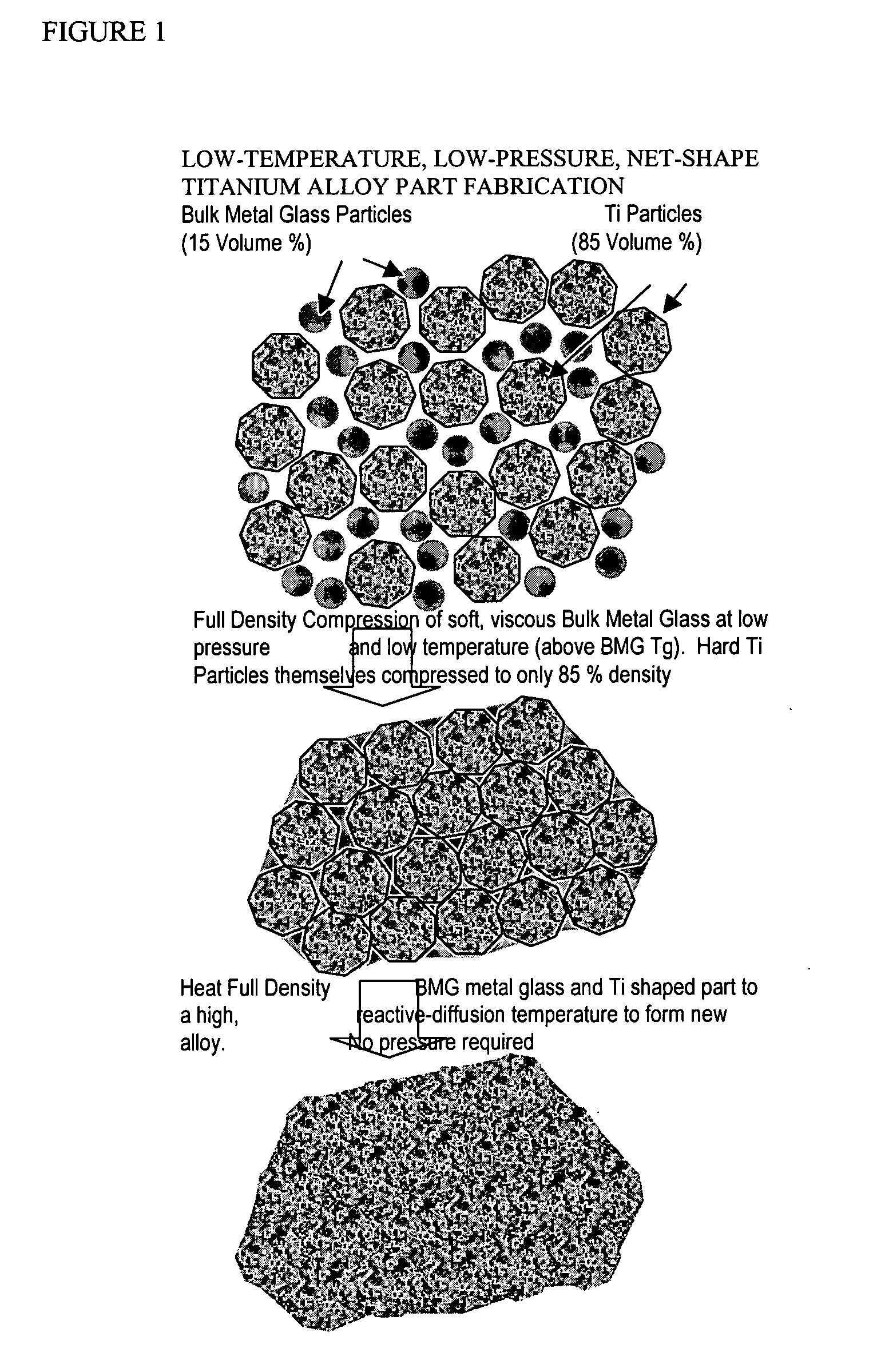
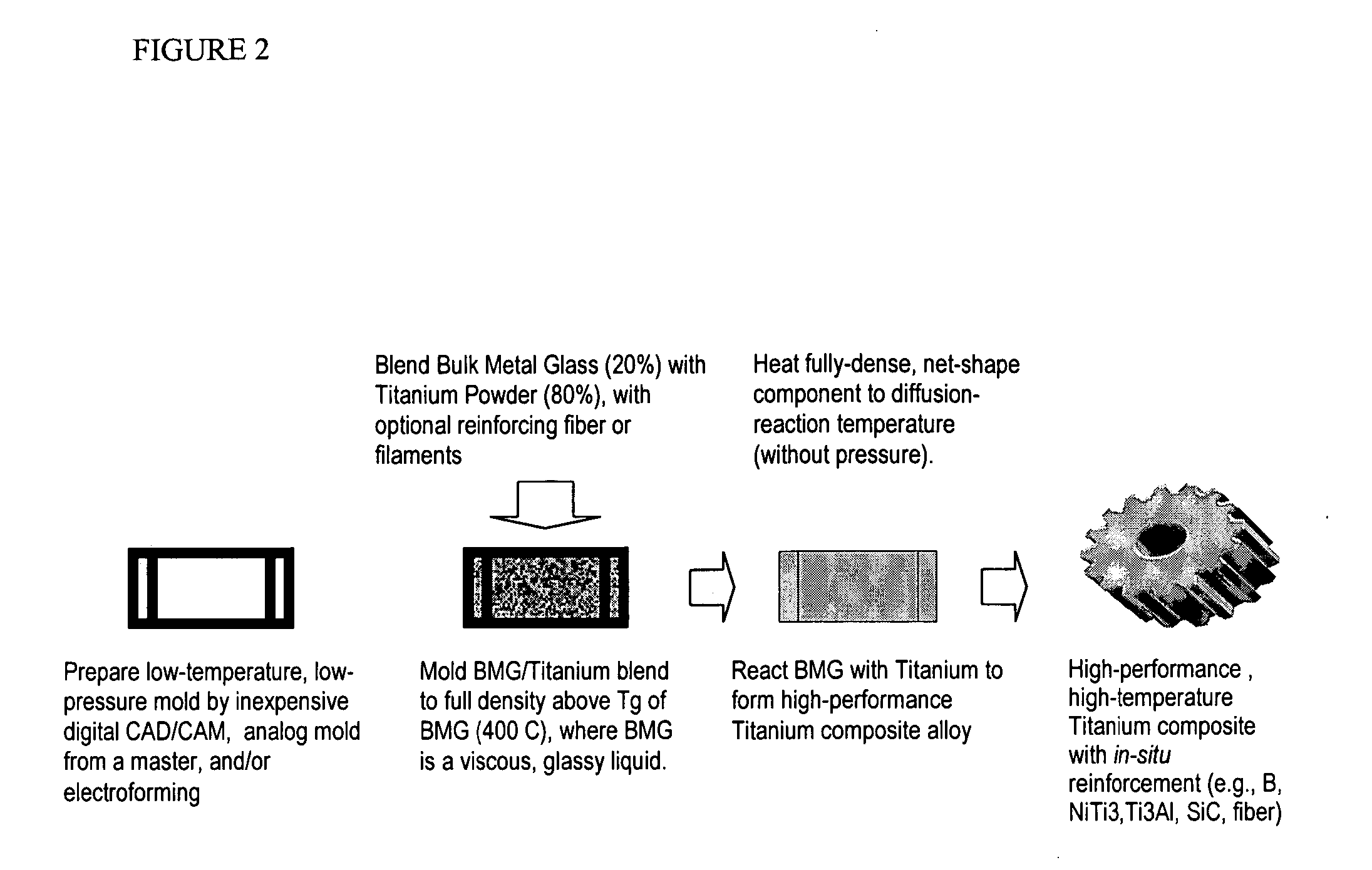
InactiveUS20050084407A1Excellent cold formabilityImprove hardenabilityVitrificationVolumetric Mass Density
Methods and compositions relating to powder metallurgy in which an amorphous-titanium-based metal glass alloy is compressed above its glass transition temperature Tg with a titanium alloy powder which is a solid at the compression temperature, to produce a compact with a relative density of at least 98%.
Owner:MYRICK JAMES J
Polyimide sulfones, method and articles made therefrom
InactiveUS7041773B2Synthetic resin layered productsCeramic shaping apparatusVitrificationPolymer science
Polyimide sulfone resins are provided with a glass transition temperature of from 200–350° C., residual volatile species concentration of less than 500 ppm and a total reactive end group concentration of less than about 120 milliequivalents / kilogram resin. The resins have high heat capability and good melt stability. Methods to prepare the said resins and articles made from the resins are also provided.
Owner:SHPP GLOBAL TECH BV
Thermoset materials with improved impact resistance
The present invention relates to a thermoset material with improved impact resistance comprising:99 to 20% of a thermoset resin, and1 to 80% of an impact modifier comprising at least one copolymer comprising S-B-M, B-M and M-B-M blocks,wherein:each block is connected to the other by means of a covalent bond or of an intermediate molecule connected to one of the blocks via a covalent bond and to the other block via another covalent bond,M is a PMMA homopolymer or a copolymer comprising at least 50% by weight of methyl methacrylate,B is incompatible with the thermoset resin and with the M block and its glass transition temperature Tg is less than the operating temperature of the thermoset material, andS is incompatible with the thermoset resin, the B block and the M block and its Tg or its melting temperature is greater than the Tg of B.S is advantageously polystyrene and B polybutadiene. The thermoset resin advantageously originates from the reaction of a thermosetting epoxy resin and of a hardener.
Owner:ATOFINA
Liquid crystalline thermosets from ester, ester-imide, and ester-amide oligomers
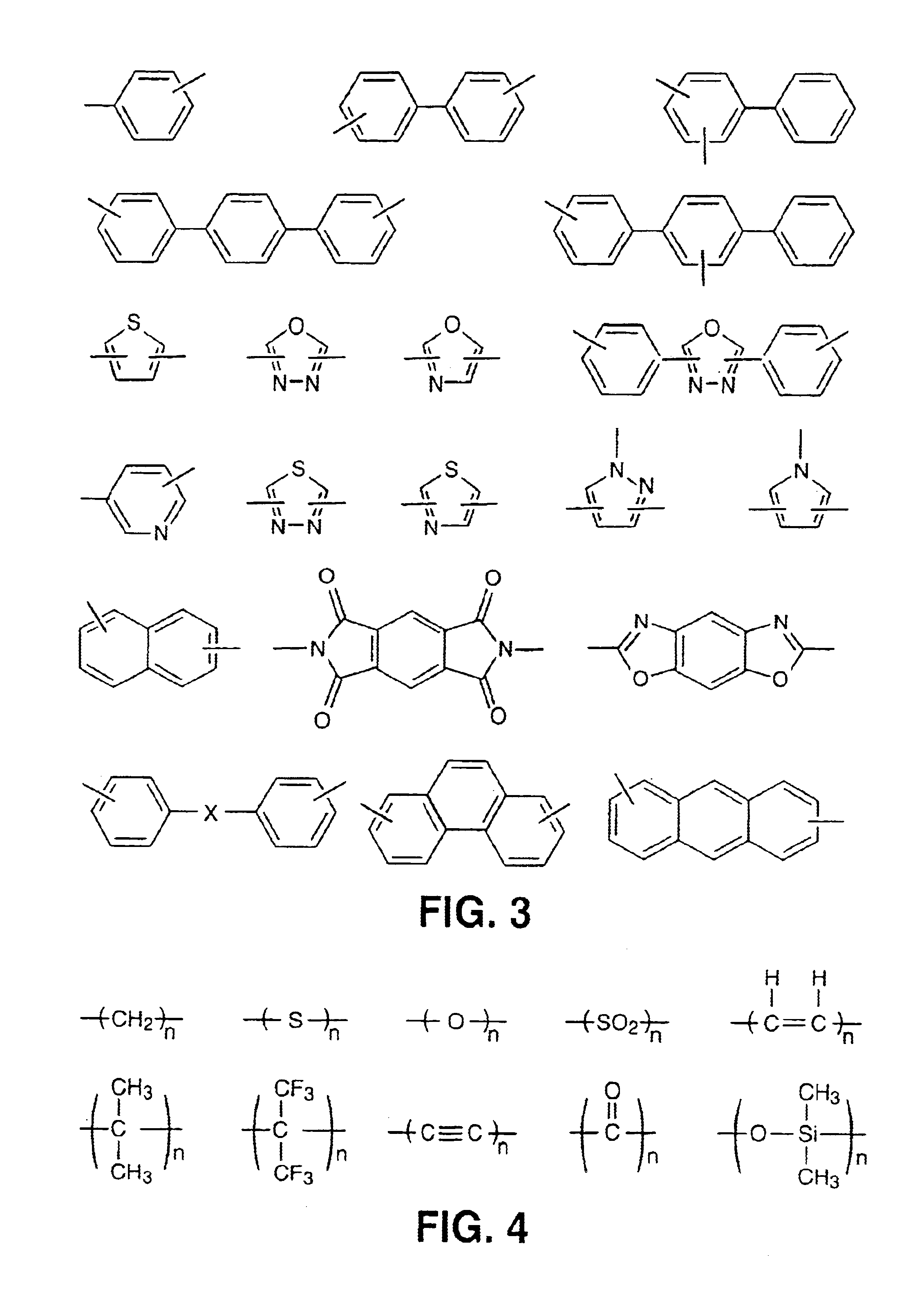
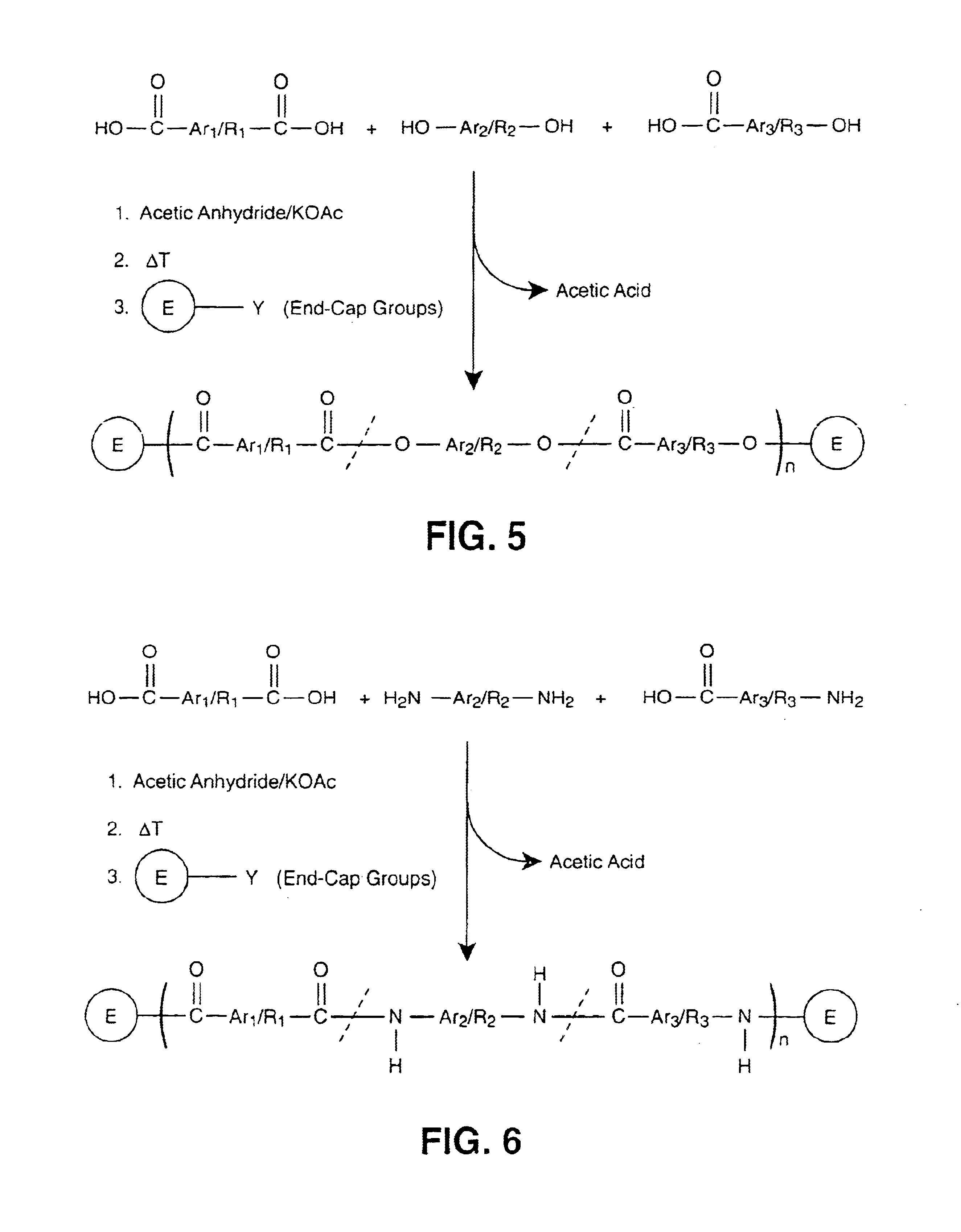
InactiveUS6939940B2Low viscosityLow dielectric constantLiquid crystal compositionsLiquid crystallineEnd-group
Main chain thermotropic liquid crystal esters, ester-imides, and ester-amides were prepared from AA, BB, and AB type monomeric materials and were end-capped with phenylacetylene, phenylmaleimide, or nadimide reactive end-groups. The resulting reactive end-capped liquid crystal oligomers exhibit a variety of improved and preferred physical properties. The end-capped liquid crystal oligomers are thermotropic and have, preferably, molecular weights in the range of approximately 1000-15,000 grams per mole. The end-capped liquid crystal oligomers have broad liquid crystalline melting ranges and exhibit high melt stability and very low melt viscosities at accessible temperatures. The end-capped liquid crystal oligomers are stable for up to an hour in the melt phase. These properties make the end-capped liquid crystal oligomers highly processable by a variety of melt process shape forming and blending techniques including film extrusion, fiber spinning, reactive injection molding (RIM), resin transfer molding (RTM), resin film injection (RFI), powder molding, pultrusion, injection molding, blow molding, plasma spraying and thermo-forming. Once processed and shaped, the end-capped liquid crystal oligomers were heated to further polymerize and form liquid crystalline thermosets (LCT). The fully cured products are rubbers above their glass transition temperatures. The resulting thermosets display many properties that are superior to their non-end-capped high molecular weight analogs.
Owner:NASA
Void-containing polyester shrink film
InactiveUS20050119359A1Improve performanceHigh opacitySynthetic resin layered productsLabelsPolyesterVolumetric Mass Density
Disclosed are polyester shrink films comprising a voiding agent dispersed within a continuous polyester phase. The voiding agent comprises at least one first polymer and at least one second polymer, in which the polymer components have selected physical properties such as glass transition temperature, melting point, tensile modulus, surface tension, and melt viscosity. The resulting shrink films have high opacity, a low coefficient of friction, lower density, low shrink force, and good printability. The films are useful for sleeve label and other shrink film applications, and their lower density allows them to be readily separated from soft drink bottles, food containers and the like during recycling operations. Also disclosed is a process for separating a void-containing polyester from a mixture of polymers.
Owner:SHELBY MARCUS DAVID +2
Rearview Mirror Assemblies with Anisotropic Polymer Laminates
ActiveUS20100110553A1High strengthAdequate flatness of filmMirrorsNon-linear opticsLower limitWing mirror
Anisotropic film laminates for use in image-preserving reflectors such as rearview automotive mirror assemblies, and related methods of fabrication. A film may comprise an anisotropic layer such as a light-polarizing layer and other functional layers. The film having controlled water content is heated under omnidirectional pressure and vacuum to a temperature substantially equal to or above a lower limit of a glass-transition temperature range of the film so as to be laminated to a substrate. The laminate is configured as part of a mirror structure so as to increase contrast of light produced by a light source positioned behind the mirror structure and transmitted through the mirror structure towards a viewer. The mirror structure is devoid of any extended distortion and is characterized by SW and LW values less than 3, more preferably less than 2, and most preferably less than 1.
Owner:GENTEX CORP
Process for producing liquid polyalphaolefin polymer, metallocene catalyst therefor, the resulting polymer and lubricant containing same
InactiveUS6858767B1Eliminate needHydrocarbons from unsaturated hydrocarbon additionHydrocarbonsPolymer scienceHydrogen
A liquid polyalphaolefin homo- or copolymer, preferably 1-decene, which is substantially amorphous is obtained by a polymerization process employing hydrogen and a particular type of metallocene catalyst. Additionally, liquid polyalphaolefin homo- or copolymer containing from 2 to about 12 carbon atoms possess a unique combination of properties, i.e., low molecular weight (Mw), low polydispersity index (Mw / Mn controllable kinematic viscosity (Kv100), low Iodine Number (I2) and low glass transition temperature (Tg) and are substantially amorphous. The liquid polyalphaolefin homo- or copolymers provided herein are useful for manufacturing a variety of products including lubricating oils in which the polyalphaolefin functions as a viscosity modifier.
Owner:DEUT BANK AG NEW YORK BRANCH
Aqueous dispersions containing multi-stage emulsion polymers
Aqueous dispersions are disclosed, having a minimum film formation temperature no greater than about 50° C., that include a multi-stage emulsion polymer made by a process that includes a first polymerization stage, in which a first monomer mixture having a calculated glass transition temperature of at least about 50° C. is polymerized via free radical emulsion polymerization to obtain a first-stage emulsion polymer, and a second polymerization stage, in which a second monomer mixture, having a calculated glass transition temperature from about −30° C. to about 10° C., is polymerized via free radical emulsion polymerization, in the presence of the first-stage emulsion polymer. The dispersions are useful in a variety of coating compositions that exhibit improved block resistance.
Owner:HEXION INC
High-melting polyolefin copolymer elastomers, catalysts and methods of synthesis
InactiveUS6518378B2Property is limitedOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationElastomerPolyolefin
This invention relates to high melting polyolefin copolymers suitable as thermoplastic elastomers and catalysts and methods for their synthesis. These elastomeric olefin copolymers are characterized by a mole fraction of crystallizable component Xc from about 30 to about 99%; low glass transition temperatures, below -20° C., and typically below -50° C.; melting points above about 90° C.; high molecular weights; a molecular weight distribution MW / Mn< / =10; and a narrow composition distribution between chains of < / =15%. The novel copolymers of the invention range from reactor blends to multiblock copolymers that can be sequentially fractionated into fractions of differing crystallinities, which fractions nevertheless show compositions of comonomers which differ by less than 15% from the parent polymer (reactor product). The invention also relates to a process for producing such copolymers by utilizing an unbridged, substituted or unsubstituted cyclopentadienyl metallocene catalyst that is capable of interconverting between states with different copolymerization characteristics, which interconversion is controlled by selecting the substituents of the cyclopentadienyl ligands so that the rate of interconversion of the two states is within several orders of magnitude of the rate of formation of a single polymer chain. Where ri>rf the polymer can be characterized as multiblock; where ri<rf, the result is a polymer blend and where ri / rf is close to 1, the resulting polymer is a mixture of blend and multiblock. The metallocene catalysts of the invention are able to interconvert between more than two states, with embodiments of four states being shown in FIG. 2.
Owner:BP AMOCO CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com