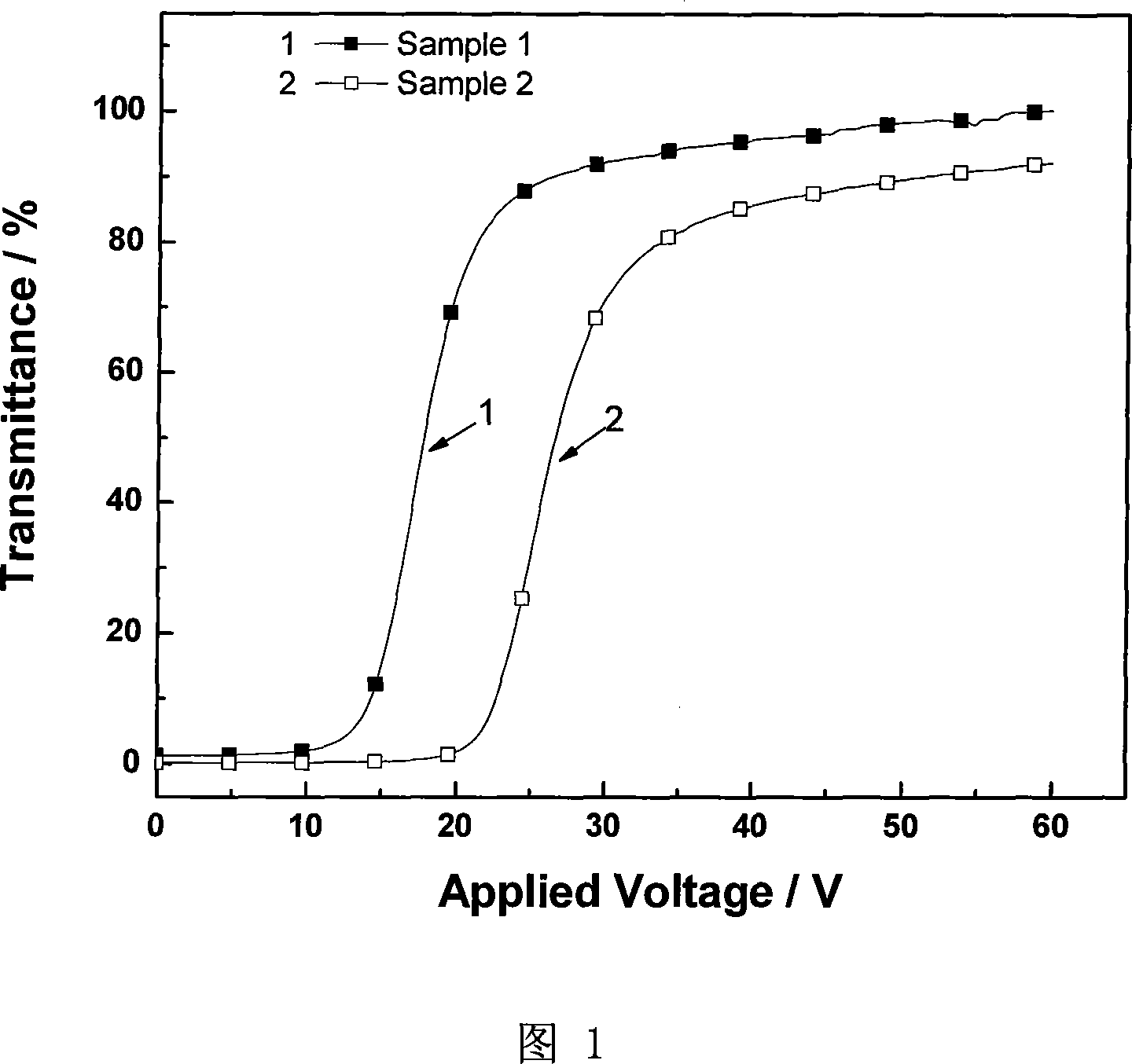
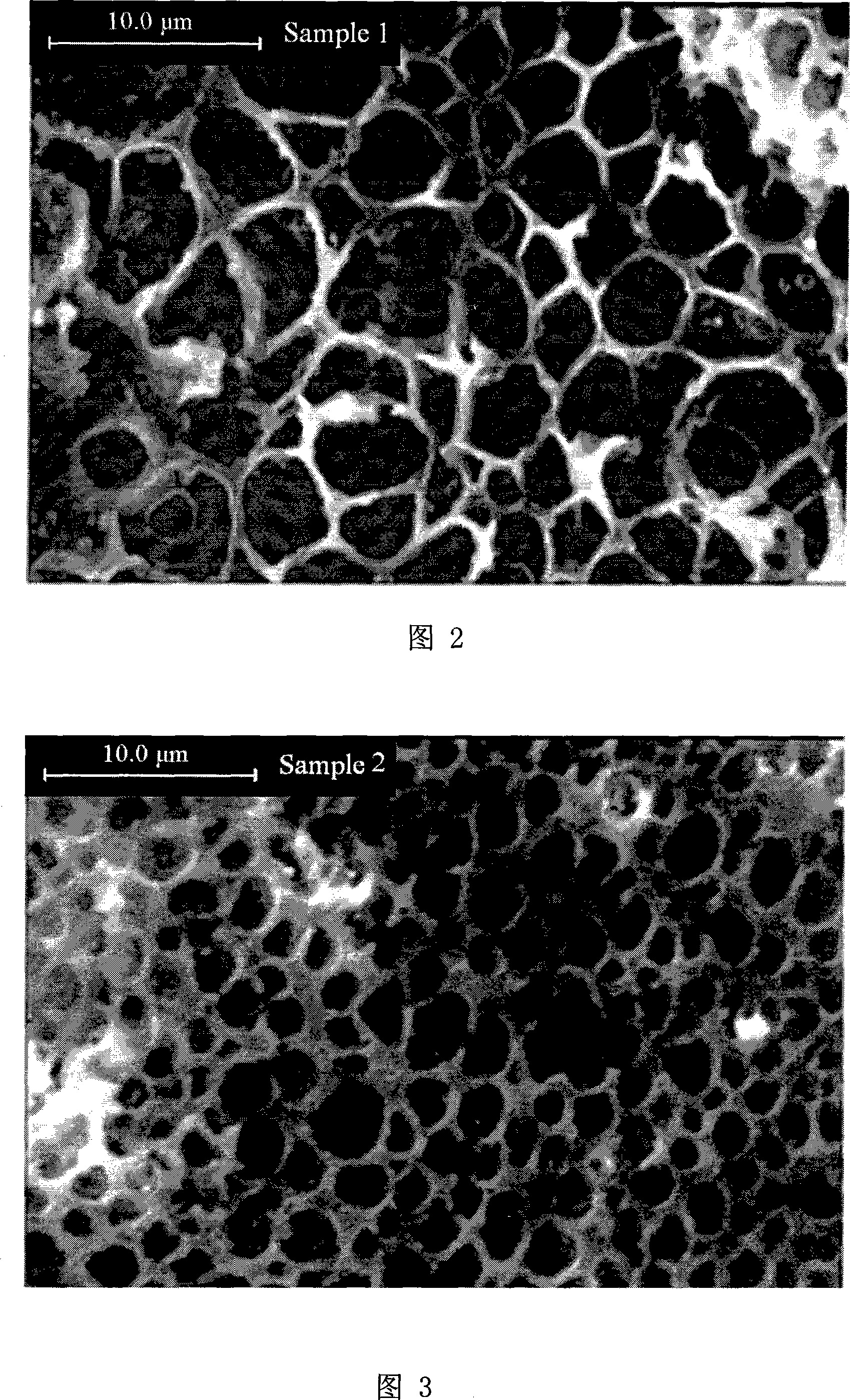
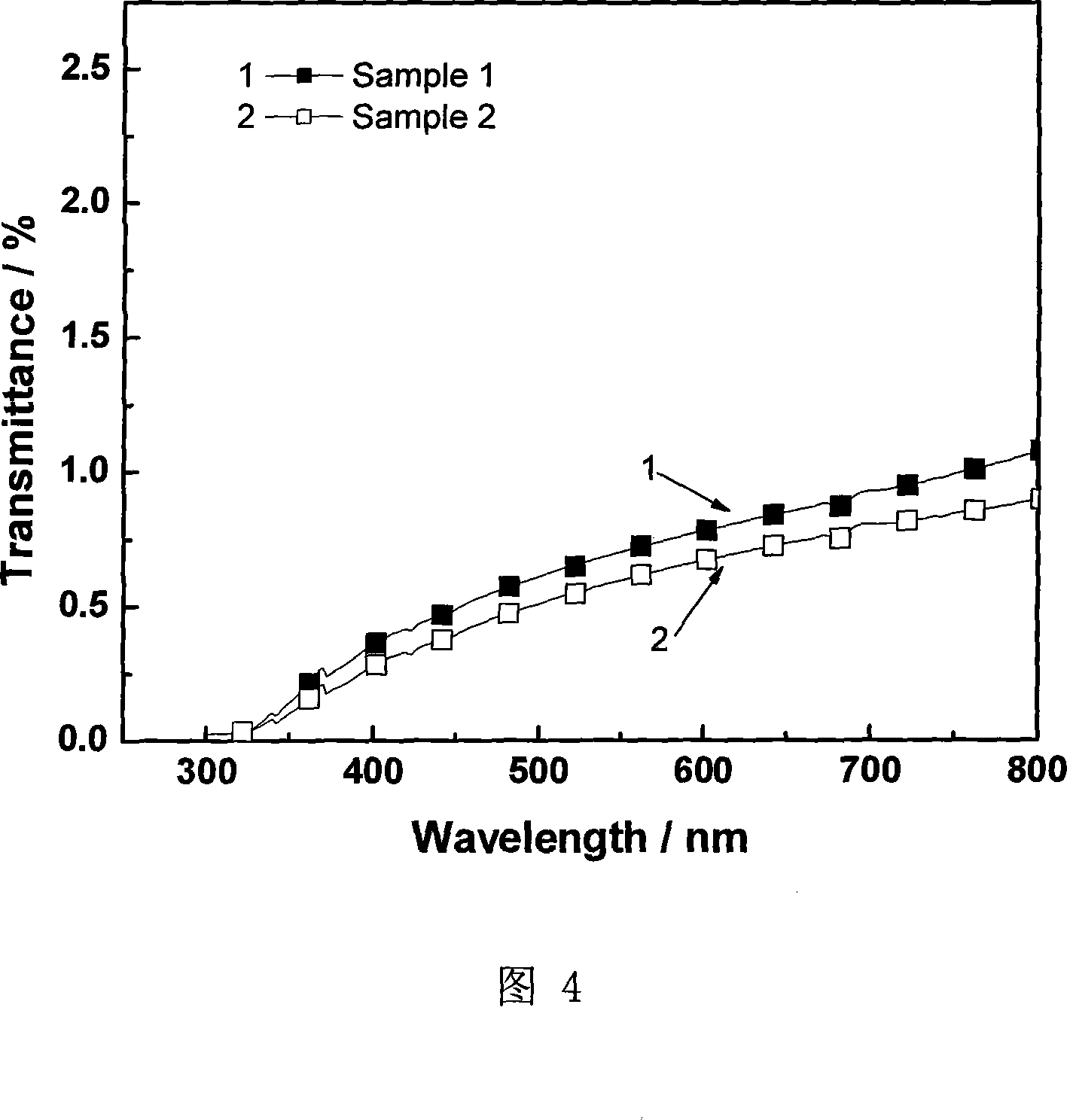
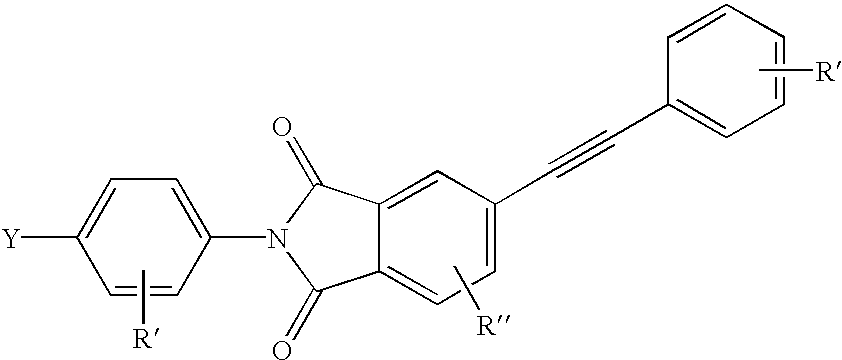
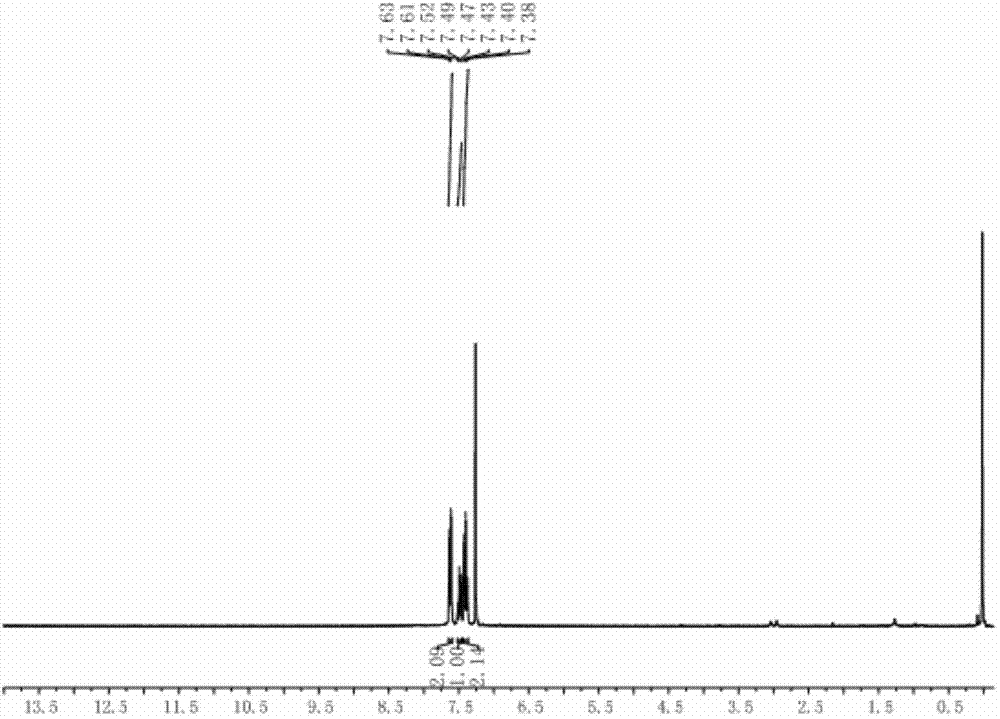
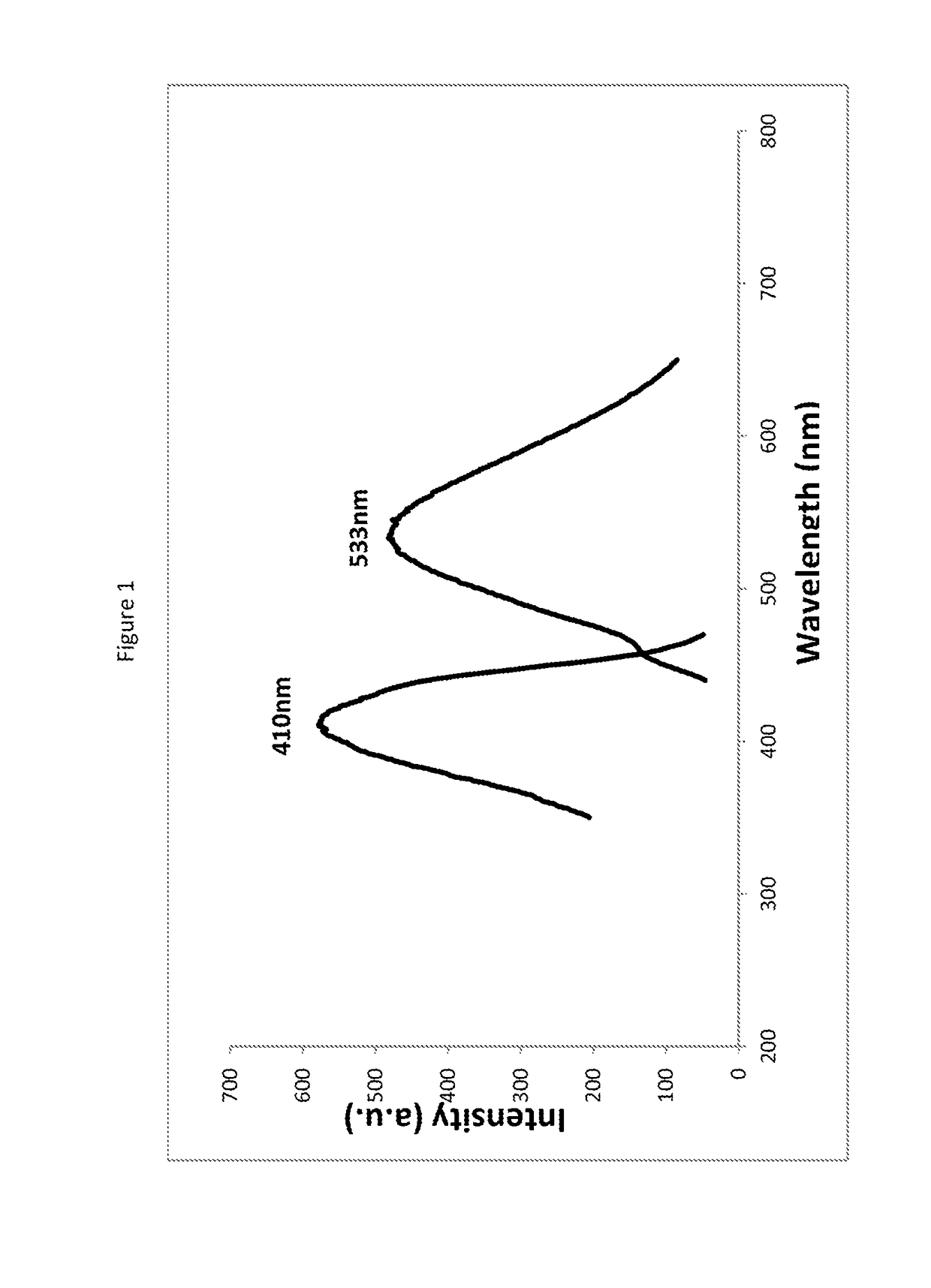
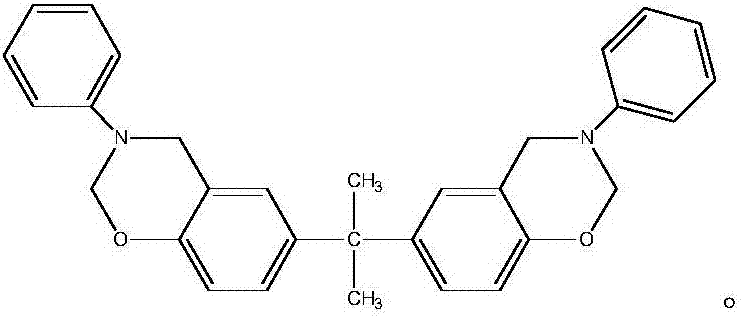
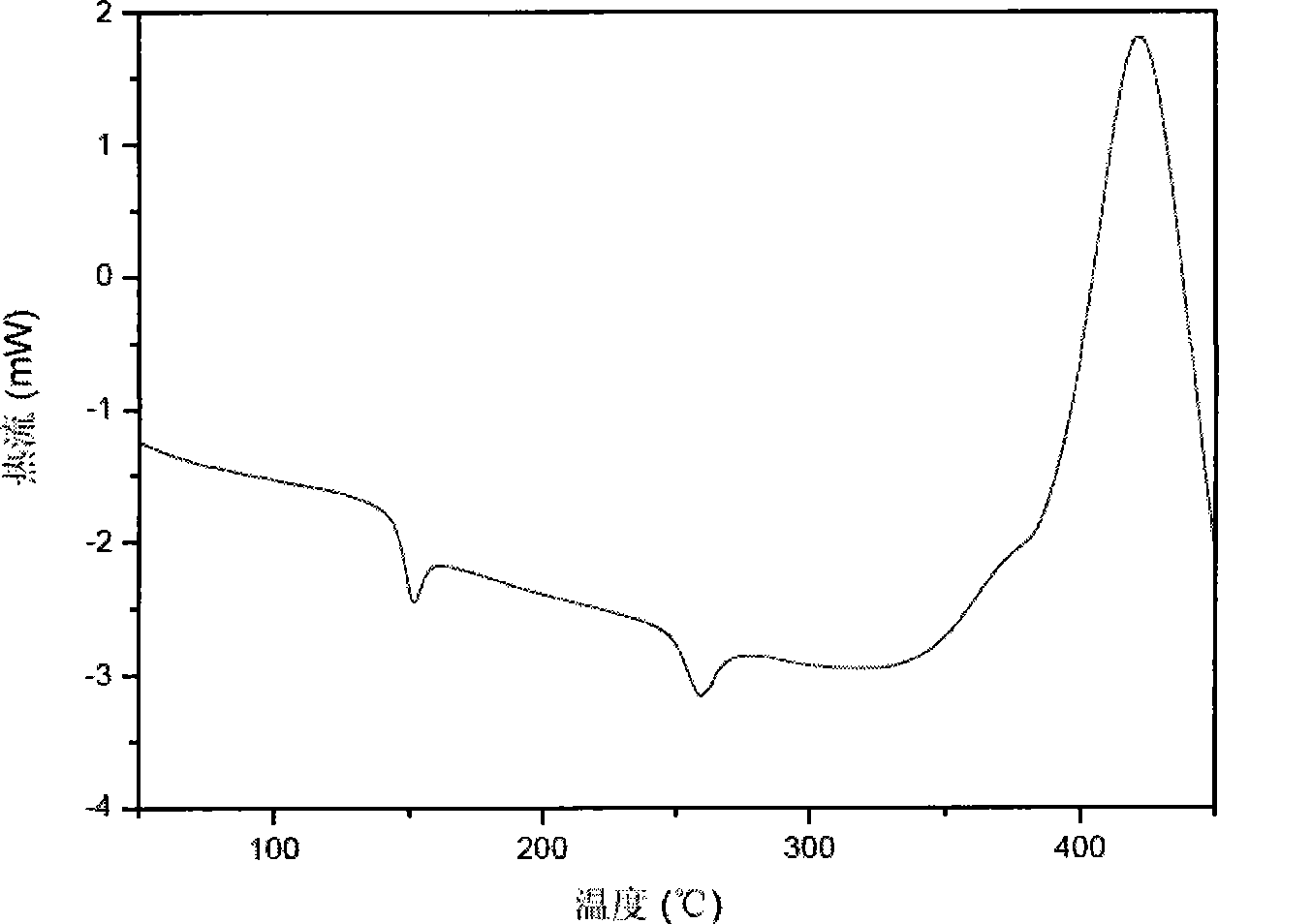
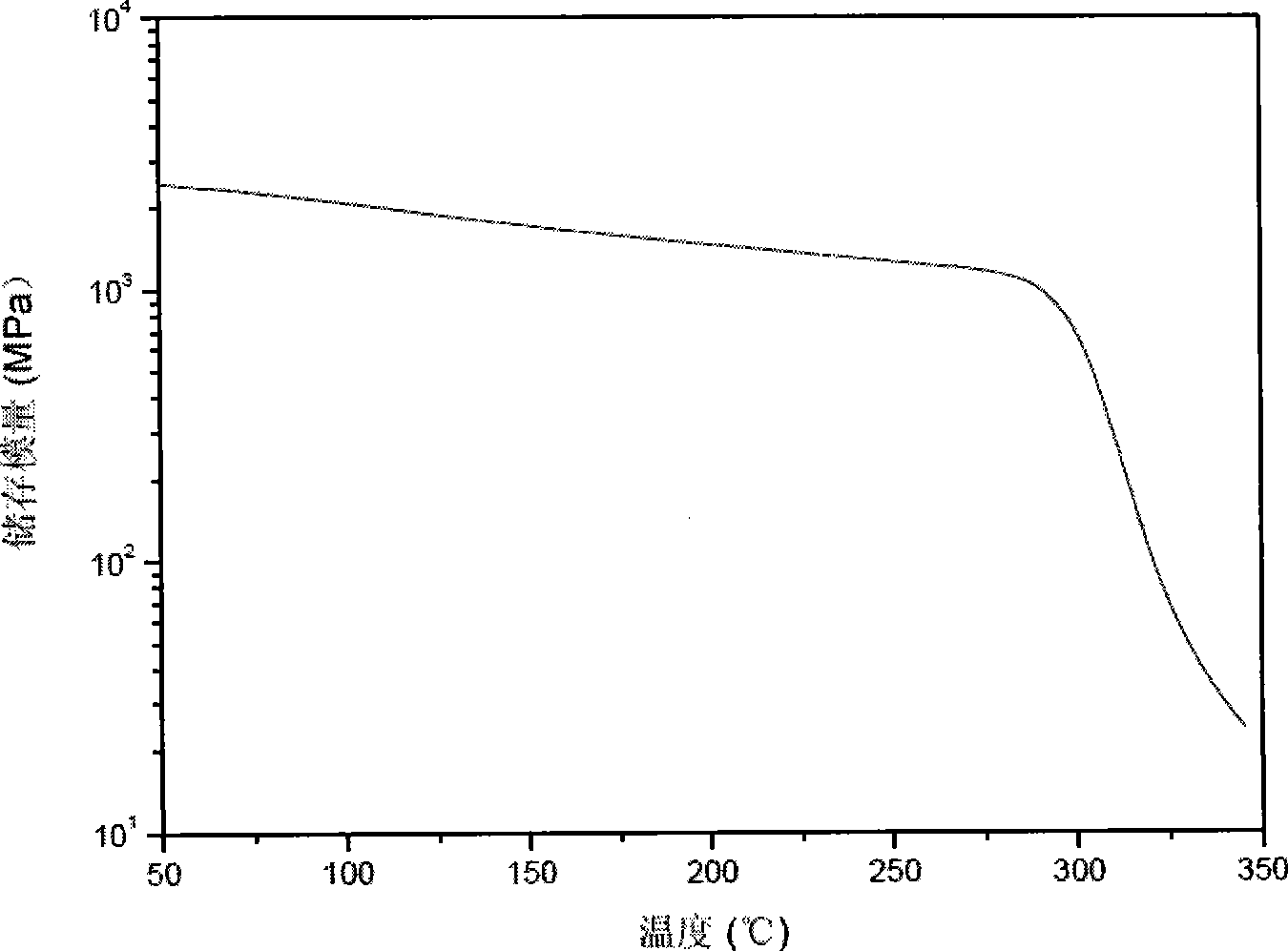
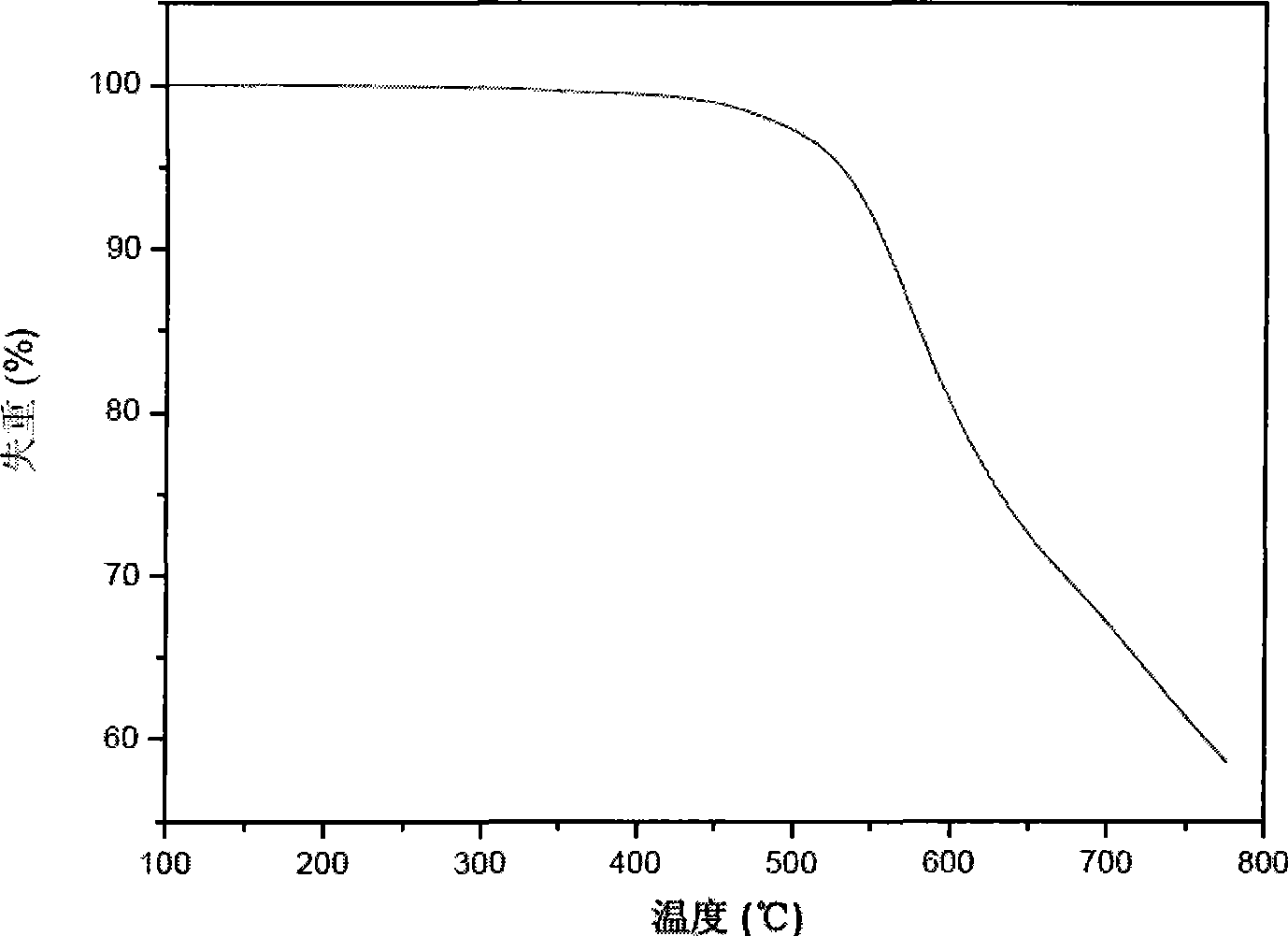
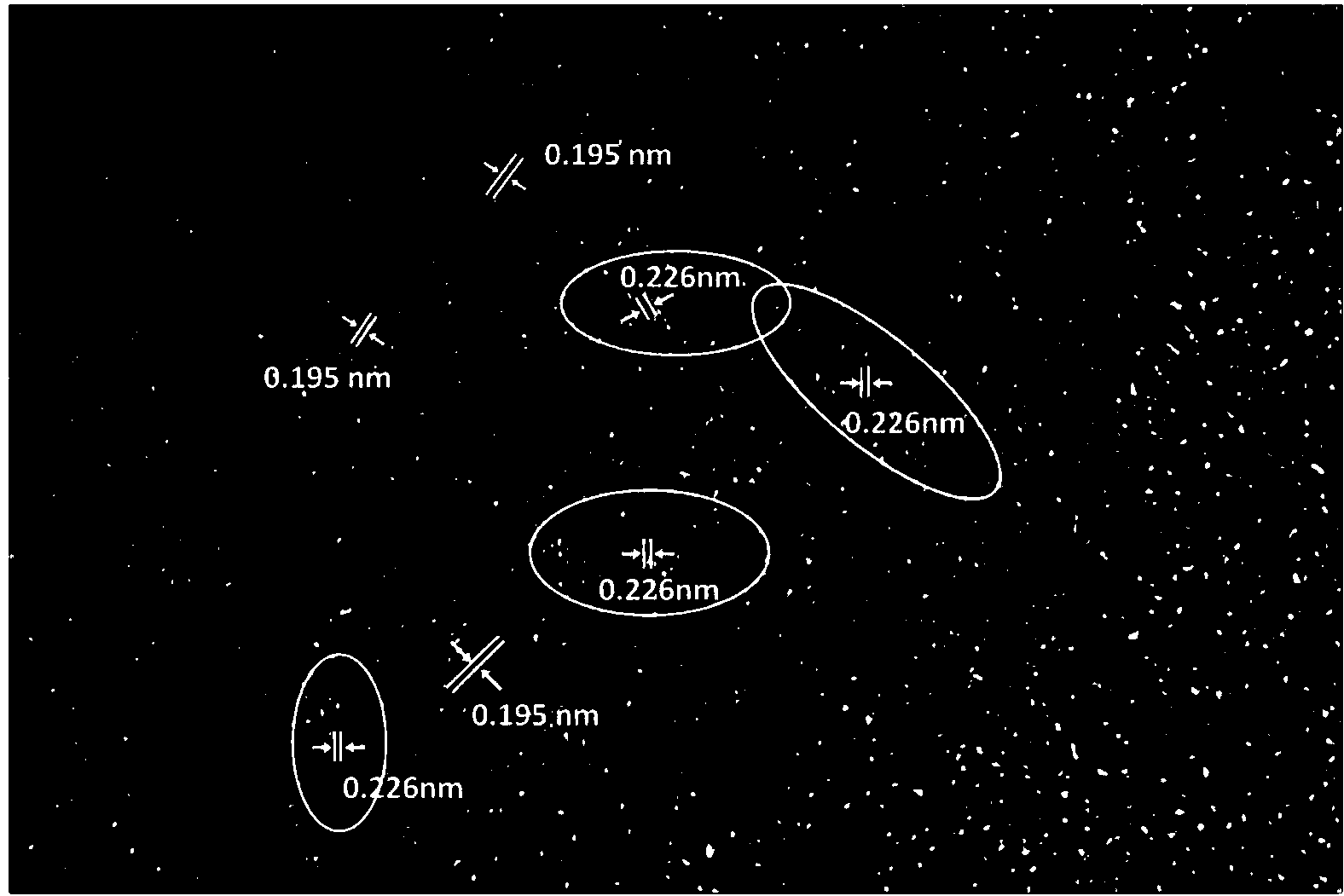
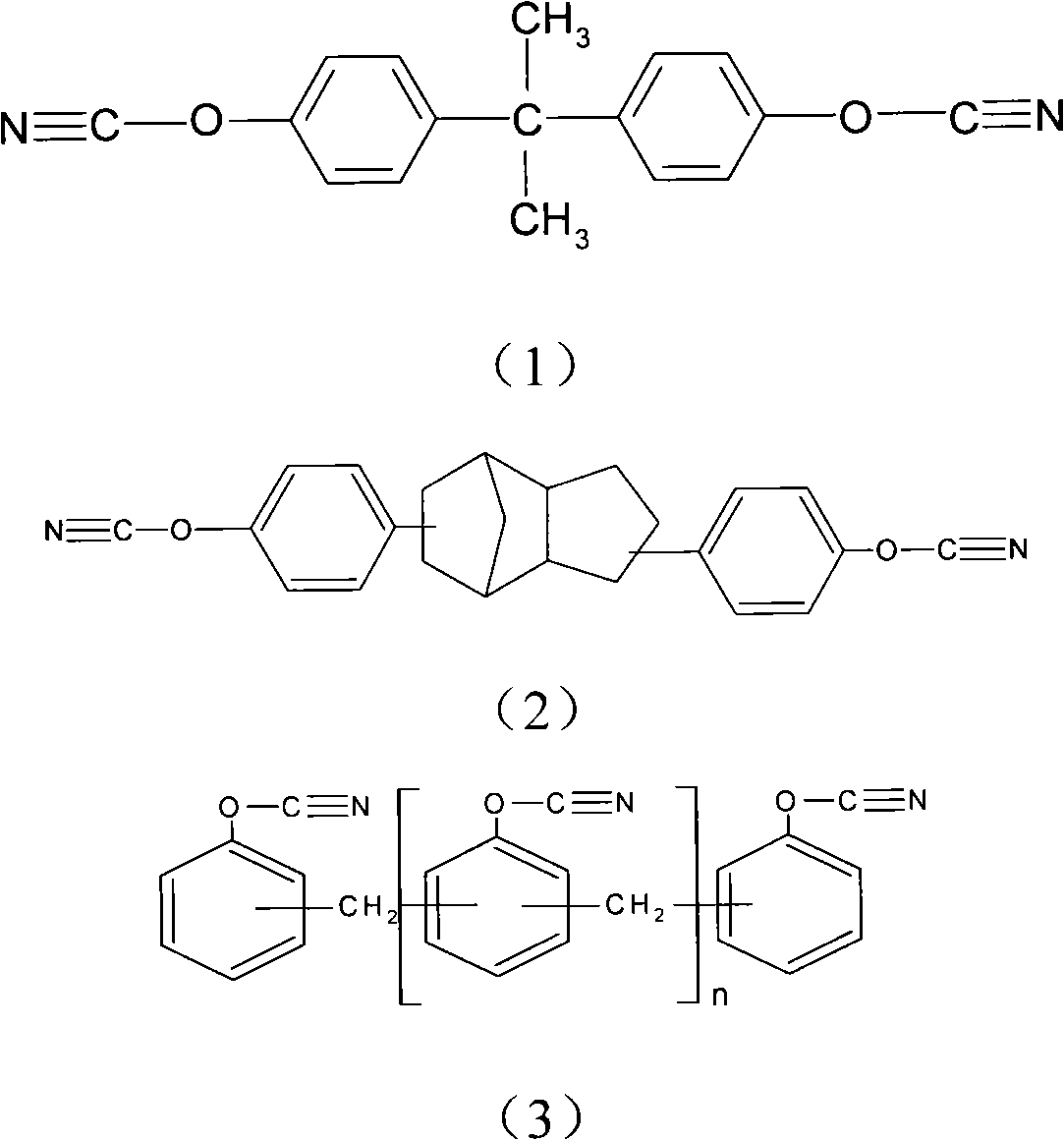
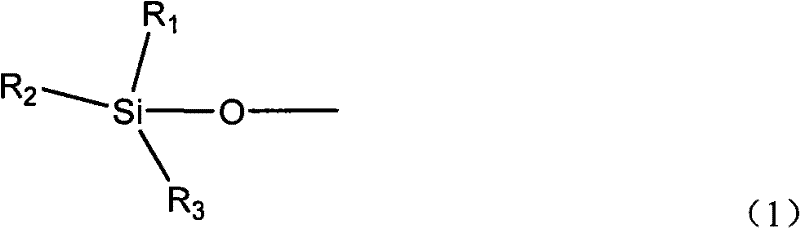Patents
Literature
379 results about "Phenylacetylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
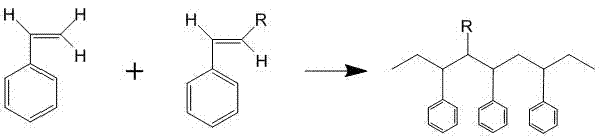
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Liquid crystalline thermosets from ester, ester-imide, and ester-amide oligomers
InactiveUS6939940B2Low viscosityLow dielectric constantLiquid crystal compositionsLiquid crystallineEnd-group
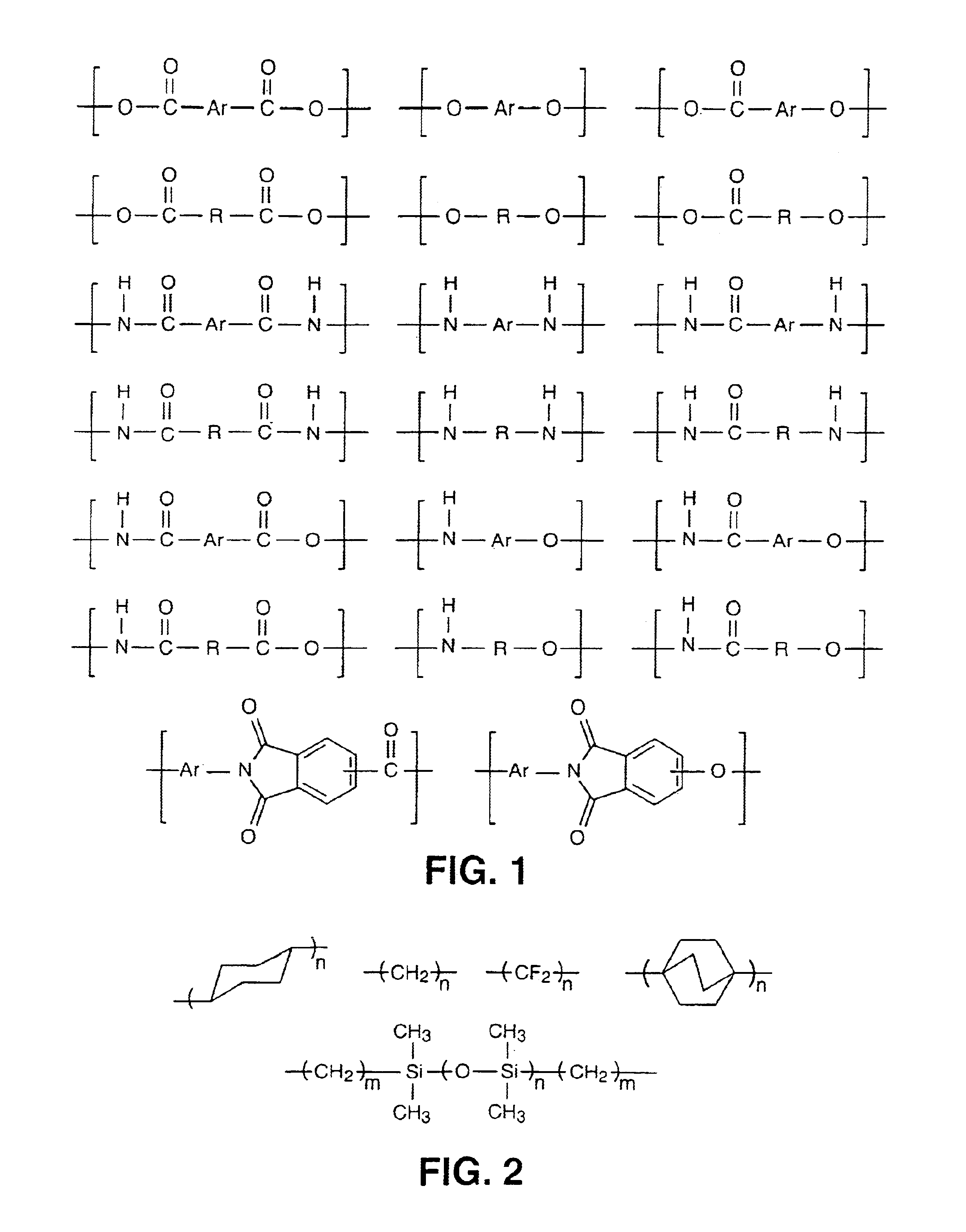
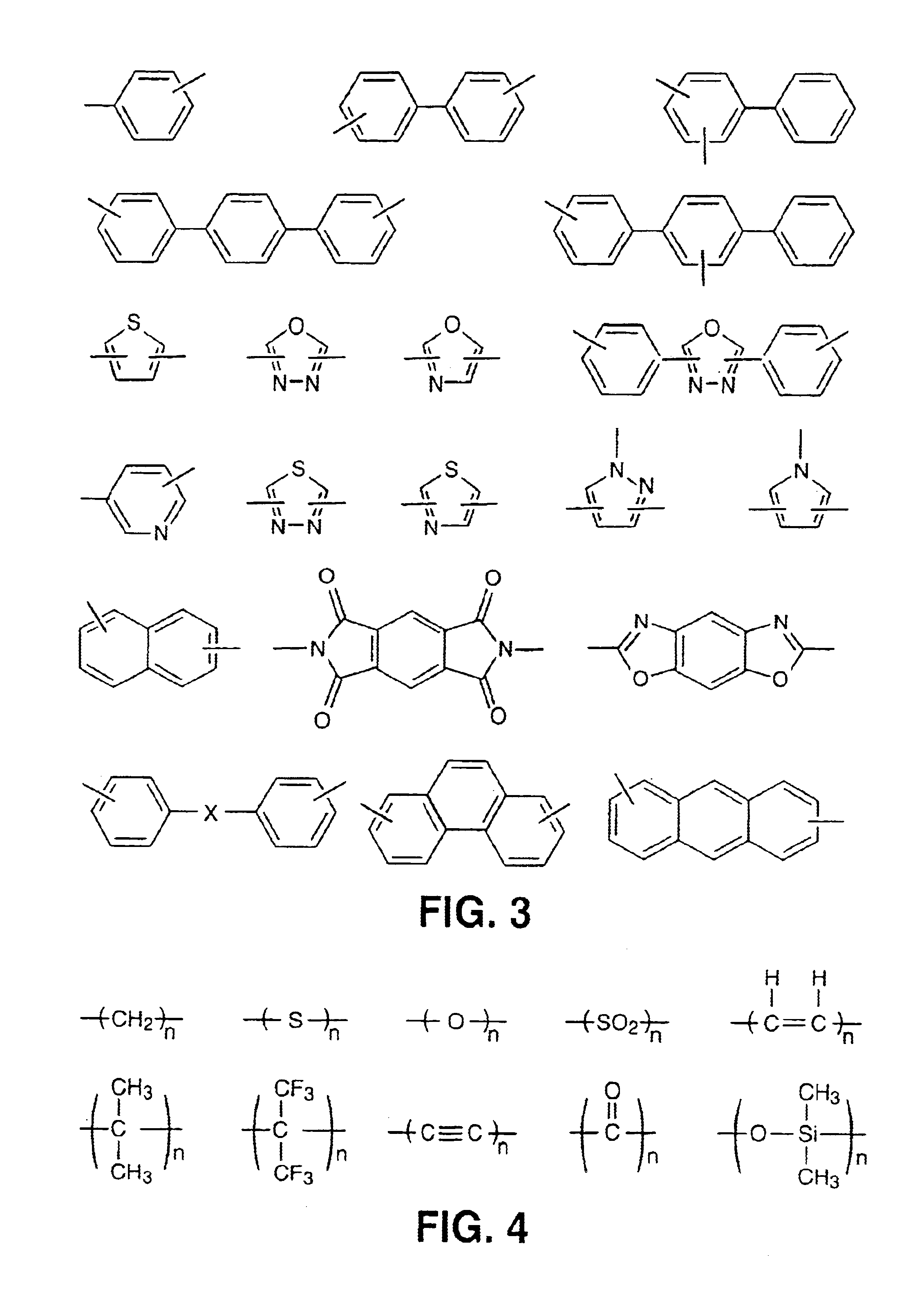
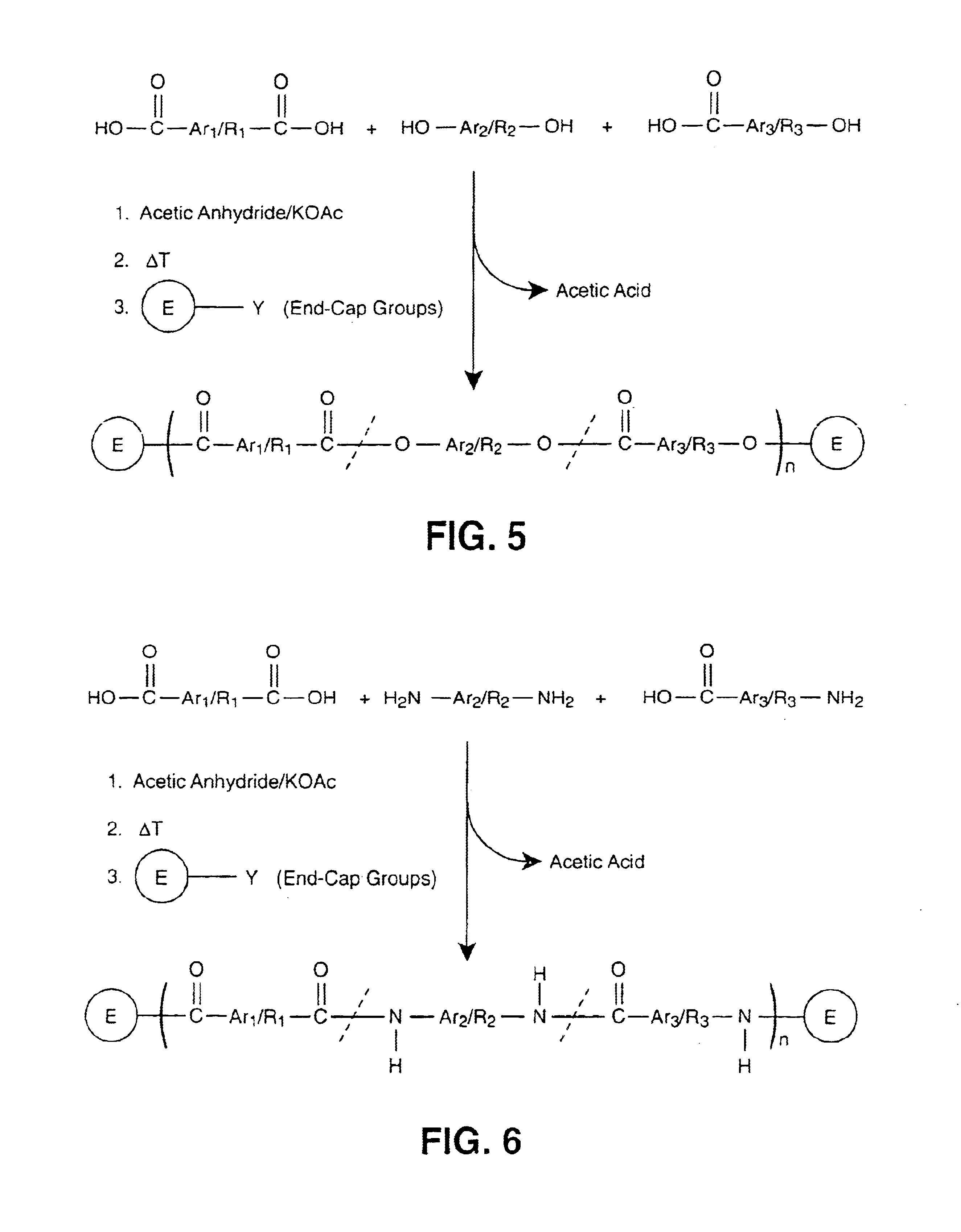
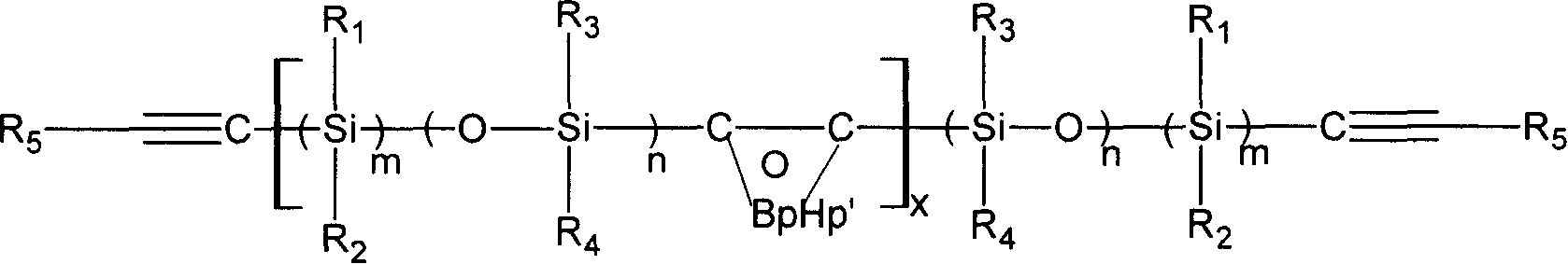
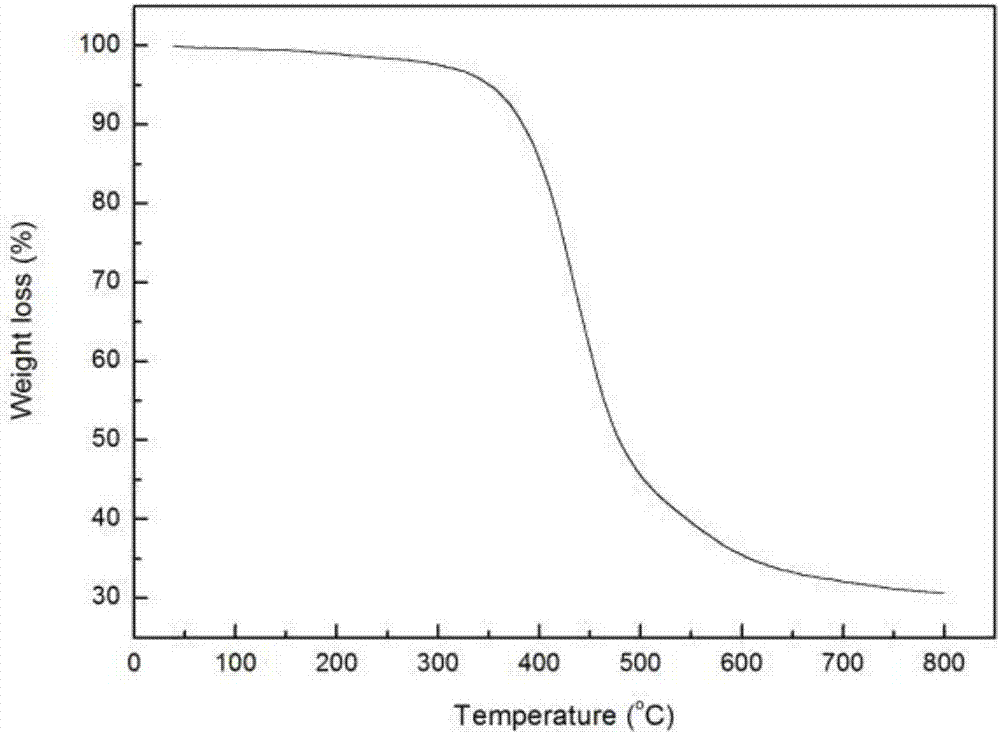
Main chain thermotropic liquid crystal esters, ester-imides, and ester-amides were prepared from AA, BB, and AB type monomeric materials and were end-capped with phenylacetylene, phenylmaleimide, or nadimide reactive end-groups. The resulting reactive end-capped liquid crystal oligomers exhibit a variety of improved and preferred physical properties. The end-capped liquid crystal oligomers are thermotropic and have, preferably, molecular weights in the range of approximately 1000-15,000 grams per mole. The end-capped liquid crystal oligomers have broad liquid crystalline melting ranges and exhibit high melt stability and very low melt viscosities at accessible temperatures. The end-capped liquid crystal oligomers are stable for up to an hour in the melt phase. These properties make the end-capped liquid crystal oligomers highly processable by a variety of melt process shape forming and blending techniques including film extrusion, fiber spinning, reactive injection molding (RIM), resin transfer molding (RTM), resin film injection (RFI), powder molding, pultrusion, injection molding, blow molding, plasma spraying and thermo-forming. Once processed and shaped, the end-capped liquid crystal oligomers were heated to further polymerize and form liquid crystalline thermosets (LCT). The fully cured products are rubbers above their glass transition temperatures. The resulting thermosets display many properties that are superior to their non-end-capped high molecular weight analogs.
Owner:NASA
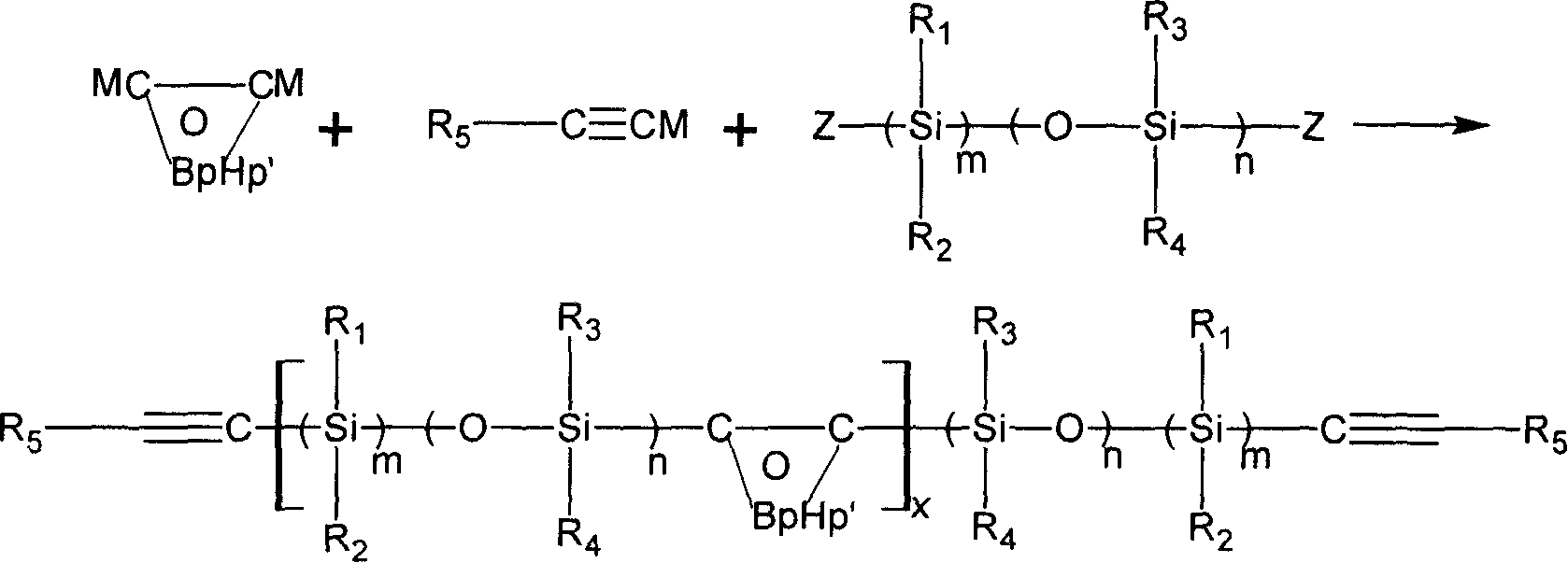
Novel carborane- (siloxane or silane)- ethinyl high temperature resistant polymer and its preparation method
InactiveCN1884343AThe synthesis process is simpleRaw materials are easy to getFireproof paintsSilanesFunction group
The invention discloses a new kind of carborane- silane- phenylacetylene polymer and the method for preparing the same. Said polymer employs methylchlorosilane, phenylacetylene, carborane and organic lithium agent as raw material, anhydrous tetrahydrofuran (THF) as dissolvent and preparing with protection of nitrogen. The invention moderates molar ratio between simple function group ethynyl compound and carborane to easily control molecular weight of polymer, crosslinking density and carborane content, and realizes purpose of adjustable polymer property. The invention is characterized by simple process and easy operation. The prepared polymer forms thermostable thermosetting material with excellent thermal oxidation performance by heat, light and chemical initiation. Said thermosetting material can be produced to ceramic structure by heating on air or inactive gas. The polymer can be used as base resin and thermo-stable coating for advanced complex material and for preparing ceramic pioneer body.
Owner:EAST CHINA UNIV OF SCI & TECH
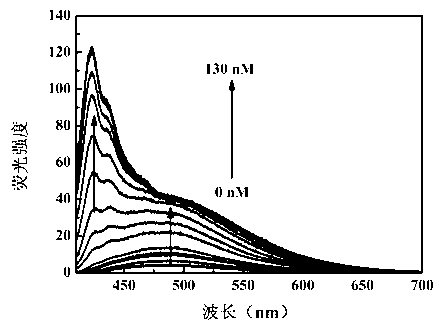
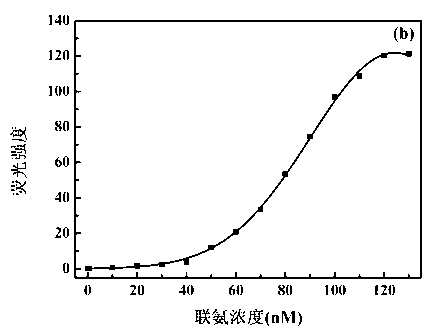
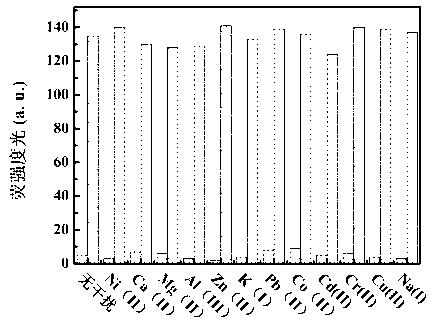
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
Preparation method for polymer dispersion liquid crystal thin film
InactiveCN101121887AFacilitated DiffusionIncrease the driving voltageLiquid crystal compositionsPolymer networkBright spot
The invention pertains to liquid crystal material application and relevant fields, a method to prepare polymer dispersed liquid crystal (PDLC) film. 1,2-bipositive olefin butyl phenylacetylene compound, rigid double functional group acrylic acid ester polymerizable monomer and methyl metharcrylate are evenly mixed according to a mass ratio 8:2:3 to 8:3:2, and the mixture (taken as polymerized monomer of PDLC film) is evenly mixed with nematic liquid crystal according to a mass ratio 2:8 to 8:2, and a isotropic liquid is achieved. The isotropic liquid, photoinitiator and nano micro-beads are mixed evenly and laid between two transparent conducting films coated with ITO, and rolled evenly to form a membranous layer; the bright spots of isotropic liquid are irradiated with 365nm UV-lights under a temperature within 0.5 to 3 DEG C to achieve a PDLC film. The invention is capable to improve the driving voltage of PDLC materials, increase the mesh strength of polymers; as a result, the electro-optical stability of PDLC film is improved.
Owner:JIANGSU SENRAN CHEM
Liquid crystalline thermosets from ester, ester-imide, and ester-amide oligomers
InactiveUS20020132933A1Good physical propertiesLiquid crystal compositionsLiquid crystallineEnd-group
Main chain thermotropic liquid crystal esters, ester-imides, and ester-amides were prepared from AA, BB, and AB type monomeric materials and were end-capped with phenylacetylene, phenylmaleimide, or nadimide reactive end-groups. The resulting reactive end-capped liquid crystal oligomers exhibit a variety of improved and preferred physical properties. The end-capped liquid crystal oligomers are thermotropic and have, preferably, molecular weights in the range of approximately 1000-15,000 grams per mole. The end-capped liquid crystal oligomers have broad liquid crystalline melting ranges and exhibit high melt stability and very low melt viscosities at accessible temperatures. The end-capped liquid crystal oligomers are stable for up to an hour in the melt phase. These properties make the end-capped liquid crystal oligomers highly processable by a variety of melt process shape forming and blending techniques including film extrusion, fiber spinning, reactive injection molding (RIM), resin transfer molding (RTM), resin film injection (RFI), powder molding, pultrusion, injection molding, blow molding, plasma spraying and thermo-forming. Once processed and shaped, the end-capped liquid crystal oligomers were heated to further polymerize and form liquid crystalline thermosets (LCT). The fully cured products are rubbers above their glass transition temperatures. The resulting thermosets display many properties that are superior to their non-end-capped high molecular weight analogs.
Owner:NASA
Preparation method of silver nano particle loaded metal organic framework complex catalyst
InactiveCN104117390AEasy to makeImprove solubilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystMetal-organic framework
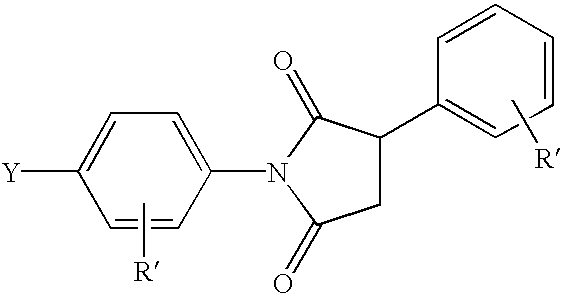
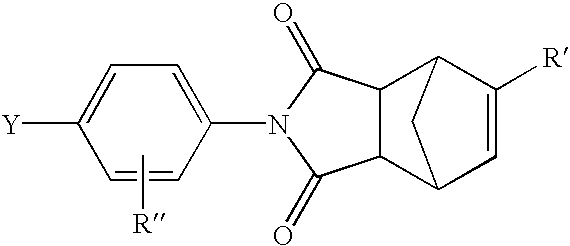
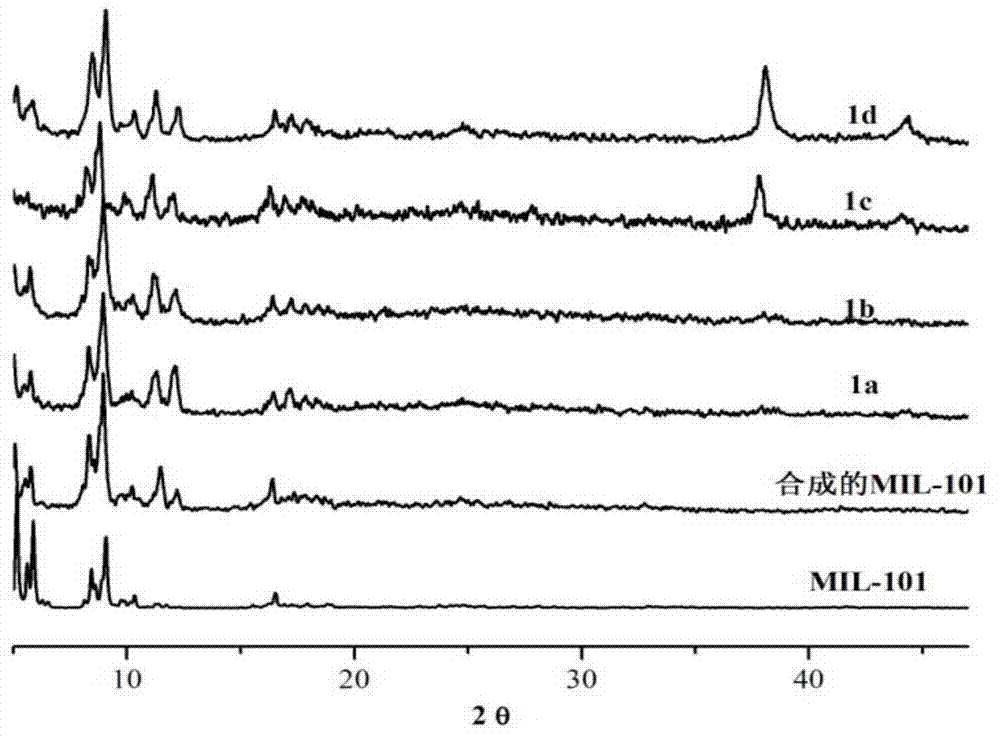
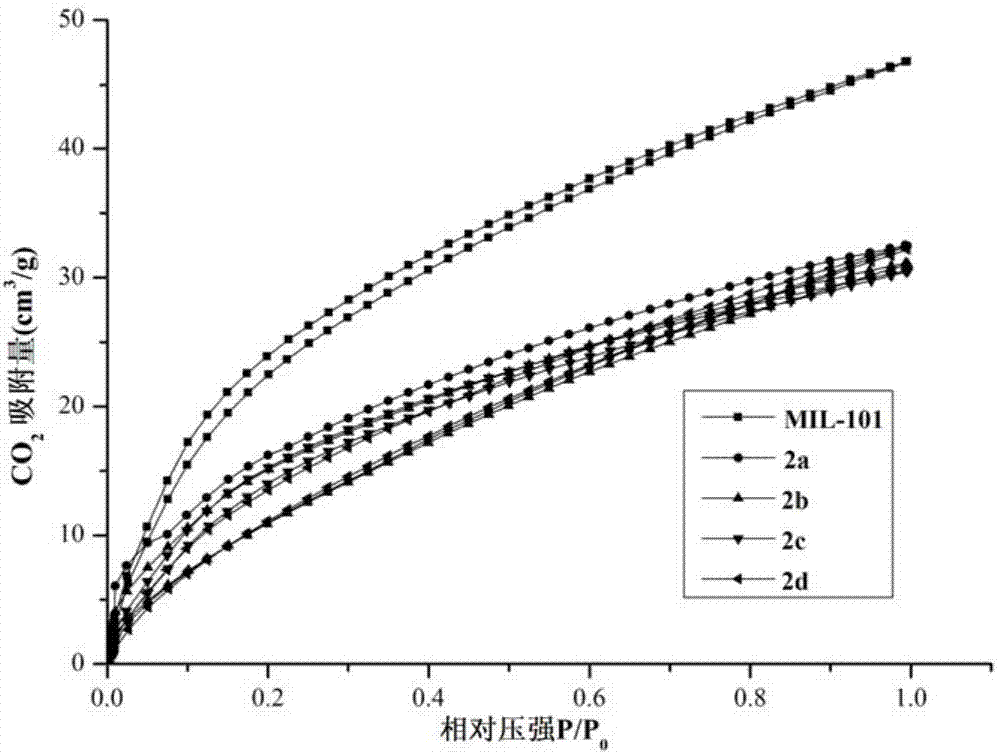
The invention discloses a preparation method of a silver nano particle loaded metal organic framework complex catalyst. The preparation method of the silver nano particle loaded metal organic framework complex catalyst by adopting an impregnation method comprises the following steps: firstly, synthesizing a metal organic framework complex MIL-101; and then, preparing the silver nano particle loaded metal organic framework complex catalyst Ag@MIL-101, wherein the silver nano particle loaded metal organic framework complex catalyst is used for converting CO2 into phenylpropiolic acid via the reaction of CO2 and phenylacetylene under a mild reaction condition. The preparation method of the silver nano particle loaded metal organic framework complex catalyst, which is provided by the invention, has the advantages that the catalyst can adsorb a CO2 molecule so as to improve the solubility of the CO2 molecule in a solvent, thereby improving the reaction velocity; the catalyst is simple to prepare, belongs to a heterogenous catalyst, avoids the difficulty that a homogeneous catalyst is difficult to separate, and has an obvious technical effect.
Owner:NANKAI UNIV
Selective hydrogenation method for phenylacetylene in the presence of phenylethylene
ActiveCN101475438AMolecular sieve catalystsHydrocarbon by hydrogenationLoss rateReaction temperature
The invention relates to a method for selective hydrogenation of phenylacetylene in the presence of styrene, which mainly solves the problem of high loss rate of the styrene in the prior art. The method adopts a technical proposal that hydrocarbon fractions containing the phenylacetylene are taken as raw materials, the raw materials are contacted with carbonic oxide catalyst under the condition that the reaction temperature is between 15 and 100 DEG C, the weight space velocity is between 0.01 and 100 h, the mol ratio of hydrogen to the phenylacetylene is 1-30:1, and the reaction pressure is between -0.08 and 5.0 MPa, the phenylacetylene in reaction effluent is hydrogenated into styrene, wherein carbon content in the carbonic oxide catalyst is between 0.02 and 8 in percentage by weight. The method better solves the problem, and can be applied in industrial production for removing the phenylacetylene by hydrogenation in the presence of the styrene.
Owner:CHINA PETROLEUM & CHEM CORP +1
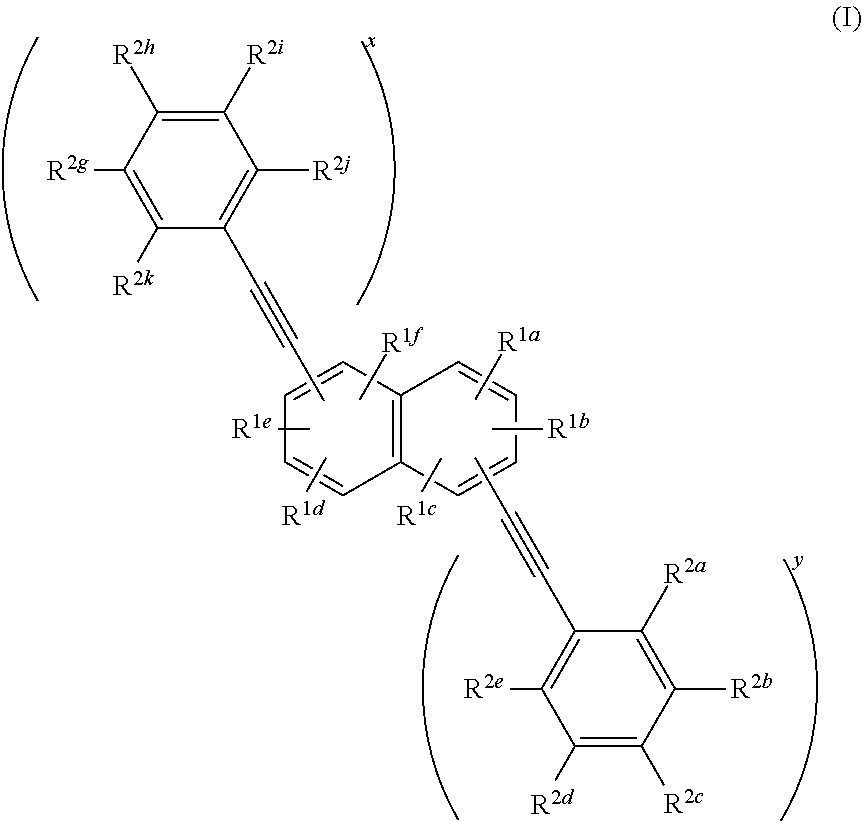
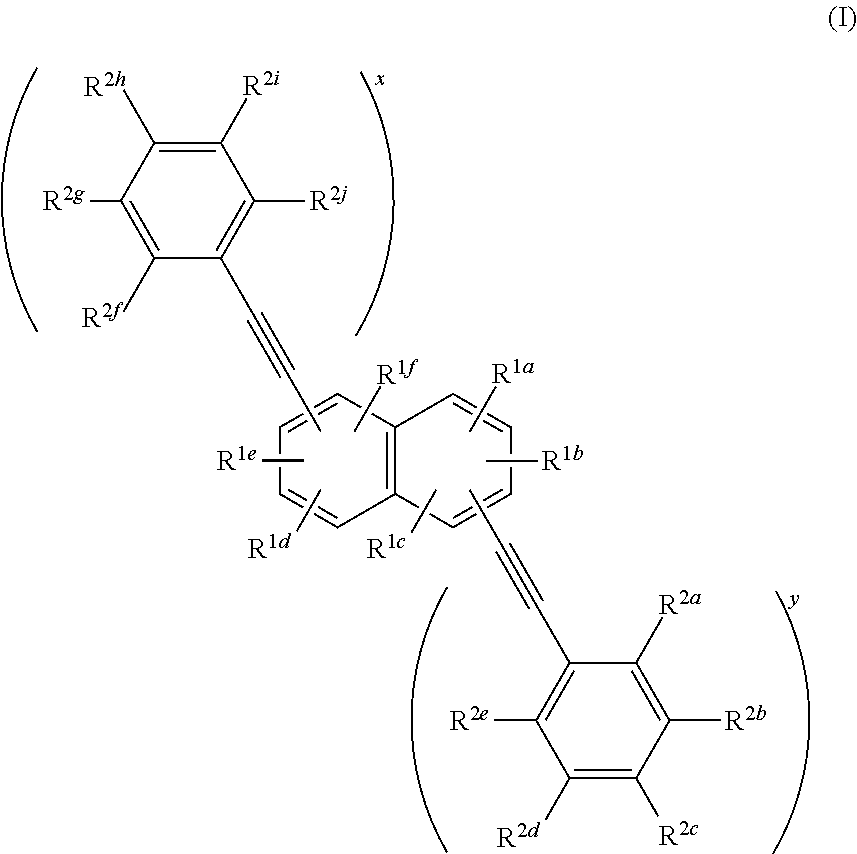
Phenylethynylnaphthalene dyes and methods for their use
ActiveUS20180237641A1Methine/polymethine dyesPhotosensitive materialsStereochemistryChemical compound
Compounds useful as fluorescent or colored dyes are disclosed. The compounds have the following structure (I), including salts thereof, wherein R1a, R1b, R1c, R1d, R1e, R1f, R2a, R2b, R2c, R2d, R2e, R2f, R2g, R2h, R2i, R2j, x and y are as defined herein. Methods associated with preparation and use of such compounds are also provided.
Owner:SONY CORP
Processable thermally stable addition polyimide for composite applications
A novel polyimide resin consisting essentially of 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA), 3,4,3′,4′-biphenyltetracarboxylic dianhydride (BPDA), 2,2 bis (3′,4′-dicarboxy phenyl) hexafluoro propane dianhydride (6FDA), 2-(3,4-dicarboxyphenyl)-1-phenylacetylene anhydride (4-PEPA) and an aromatic diamine.
Owner:THE UNITED STATES OF AMERICA AS REPRESETNED BY THE SEC OF THE AIR FORCE
Low dielectric constant materials with improved thermo-mechanical strength and processability
InactiveUS6987147B2Synthetic resin layered productsSemiconductor/solid-state device manufacturingPolymer networkDiamantane
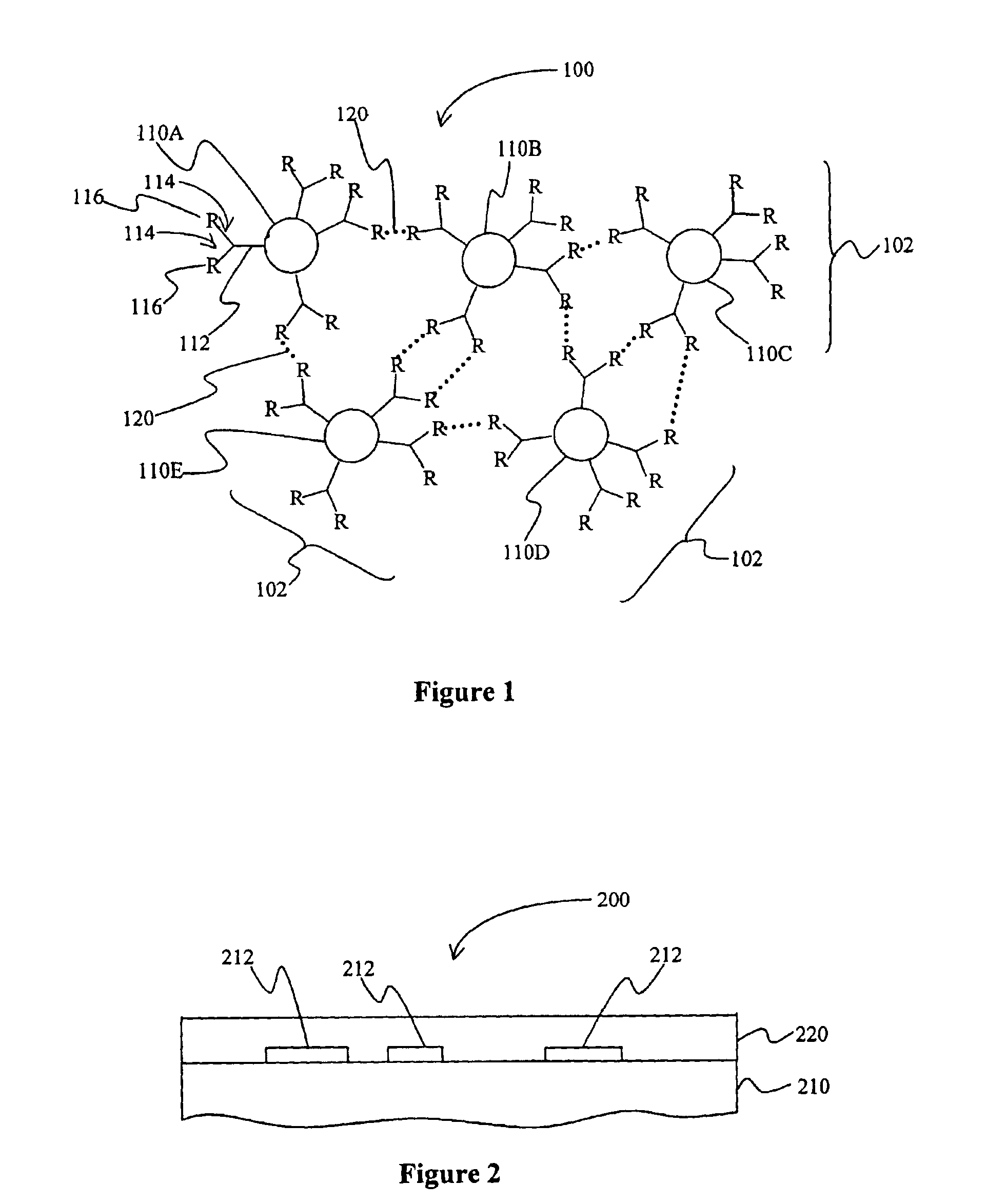
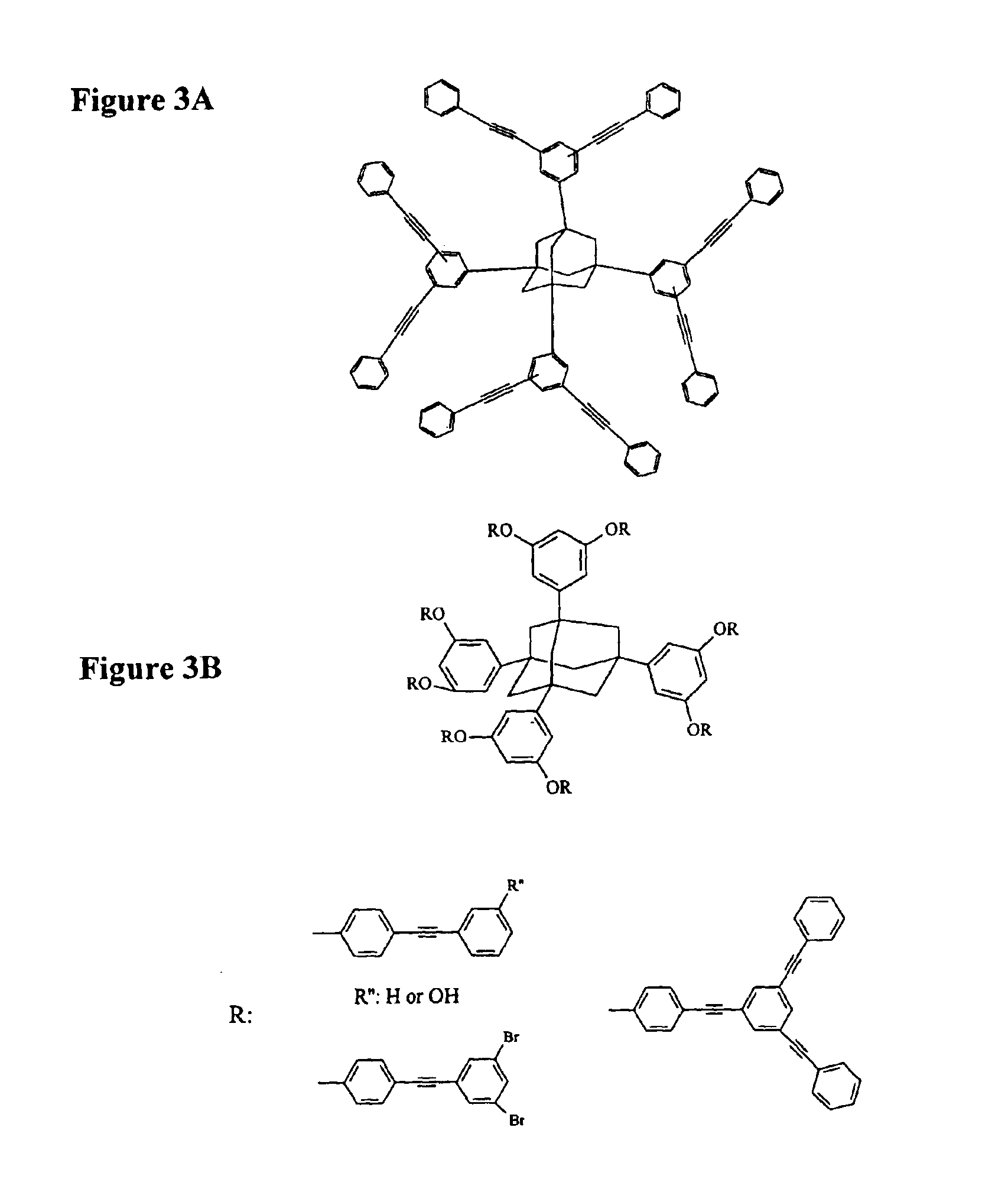
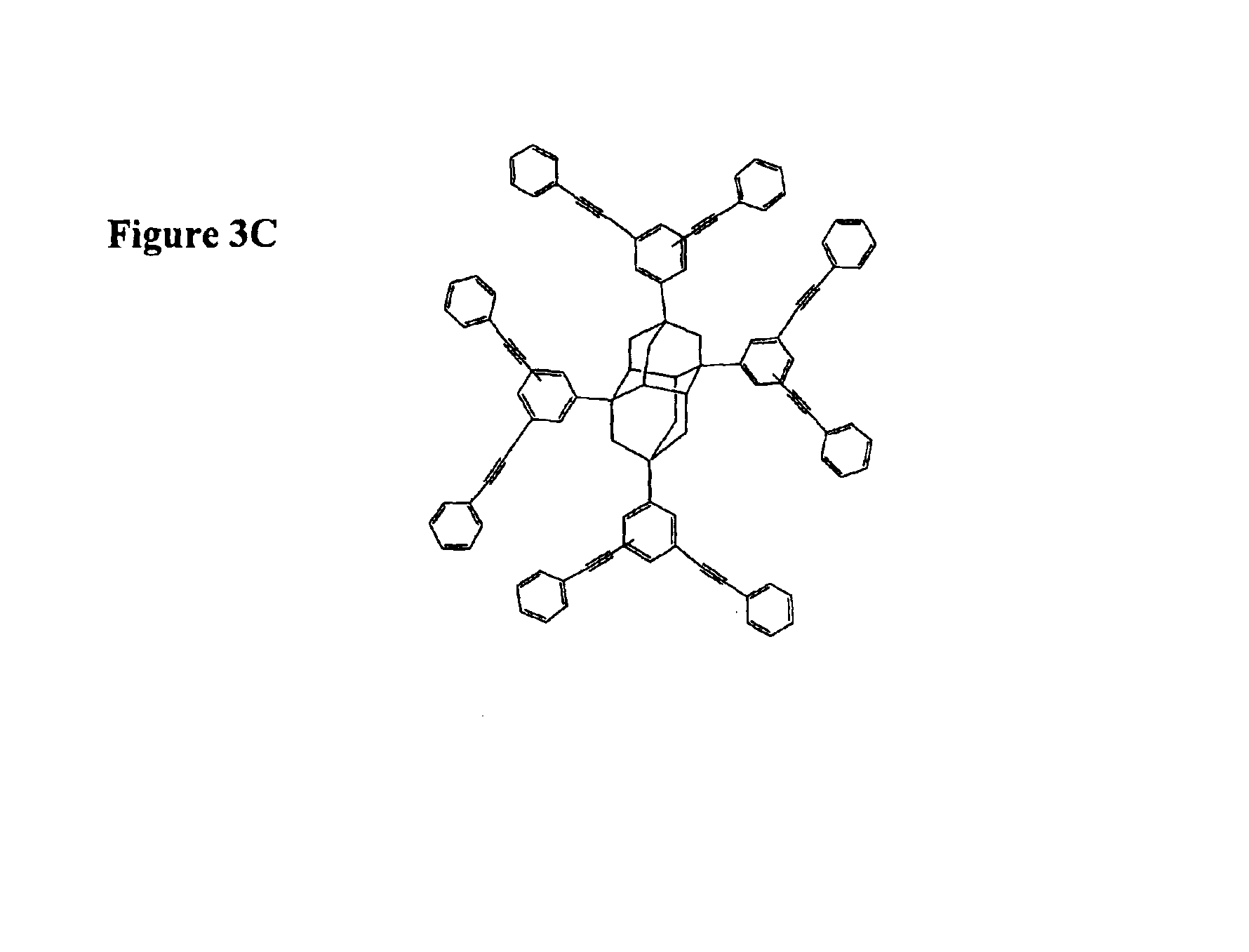
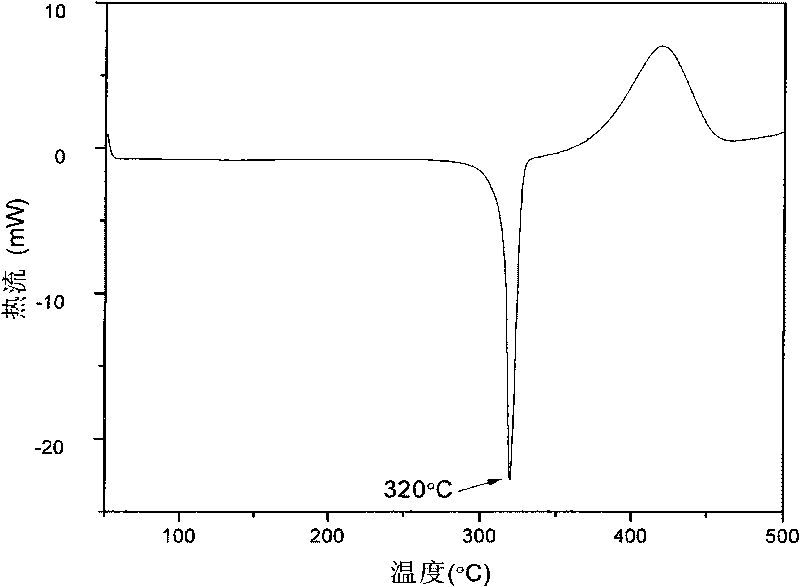
A polymeric network comprises a plurality of monomers that include a cage compound with at least three arms, wherein at least one of the arms has two or more branches, and wherein each of the branches further comprises a reactive group. Monomers in contemplated polymeric networks are covalently coupled to each other via the reactive groups. Particularly contemplated cage compounds include adamantane and diamantane, and especially contemplated branched arms comprise ortho-bis(phenylethynyl)phenyl. Especially contemplated polymeric networks have a dielectric constant of no more than 3.0, and are formed on the surface of a substrate.
Owner:HONEYWELL INT INC
Phenylacetylene-capped polyether-ether-ketone oligomer and preparation method thereof
InactiveCN101759546ALow viscosityReduce melt viscosityOrganic compound preparationCarbonyl compound preparationPolymer scienceSolvent
The invention relates to phenylacetylene capped polyether ether ketone oligomer and a preparation method thereof, belonging to the technical field of high polymer materials and syntheses thereof. The invention relates to a polyether ether ketone oligomer having higher glass transition temperature, good heat resistance and low viscosity, and the polymerization degree n of the oligomer is 1, 2 or 3. The synthesis of the oligomer can adopt a one-step method or a two-step method. The preparation method of the oligomer comprises the following steps of: adding biluorine and bisphenol monomers to anorganic solvent, after reacting water into salts for 2 to 3 hours and polymerizing for 4 to 5 hours, adding a phenylacetylene end capping agent, and then continuing to reacting for 2 to 4 hours; discharging an obtained capped polyether ether ketone oligomer material to a hydrochloric acid aqueous solution; and cleaning and drying to finally obtain a powdered polyether ether ketone oligomer capable of self crosslinking. The polyether ether ketone oligomer is a polymer having self heat crosslinking property and has the processability of thermoplastic resin and the high heat stability of thermosetting resin.
Owner:JILIN UNIV
Novel phenylacetylene end capacity capped polyimide prepolymer and preparation method thereof
The invention belongs to the field of polymer materials, and in particular relates to a performed polyimide polymer of two novel phenylacetylene sealed ends and a preparation method thereof. The preparation method is to use diamine 1, 3-di(3, aminobenzene oxyl-4'-formacyl) benzene and triamine 1, 3, 5-tri(3- aminobenzene oxyl-4'-formacyl) benzene respectively with an end-capping reagent - phenylacetylene anhydride to prepare performed polyimide polymer powder of the phenylacetylene sealed ends by the acetone method, the one-step method and the two-step method. The method for preparing the performed polyimide polymer has the advantages of high yield, good quality, high purity and no necessity of performing recrystallization. The performed polyimide polymer of the phenylacetylene sealed ends can be used for preparing high-performance crosslinking materials and has a wide processing window.
Owner:JILIN UNIV
Prepolymer, oriented film, preparation method for oriented film, and liquid crystal display device
InactiveCN102634020AImprove alignment performanceReduce pretiltNon-linear opticsPolymer scienceLiquid-crystal display
The invention provides a polyimide prepolymer, an oriented film, a preparation method for the oriented film and a liquid crystal display device. The polyimide prepolymer has repetitive units shown in a formula (1) and is blocked by blocking agent with a phenylacetylene group, wherein Ar is selected from one structure shown in a formula (2) or (3), and n is an integer from 3 to 8.
Owner:BOE TECH GRP CO LTD +1
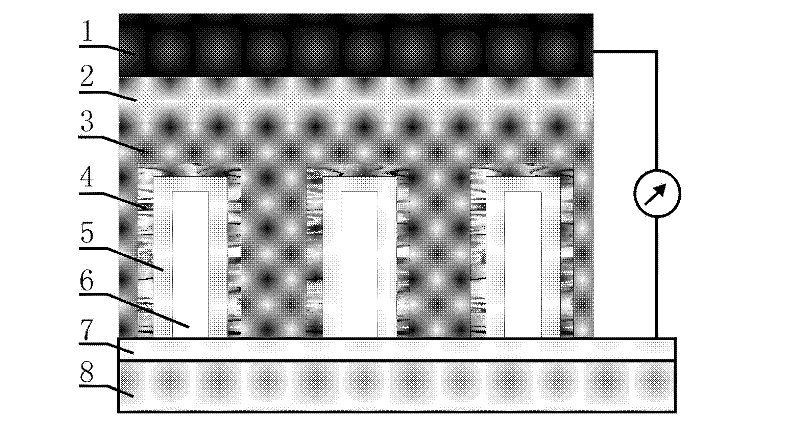
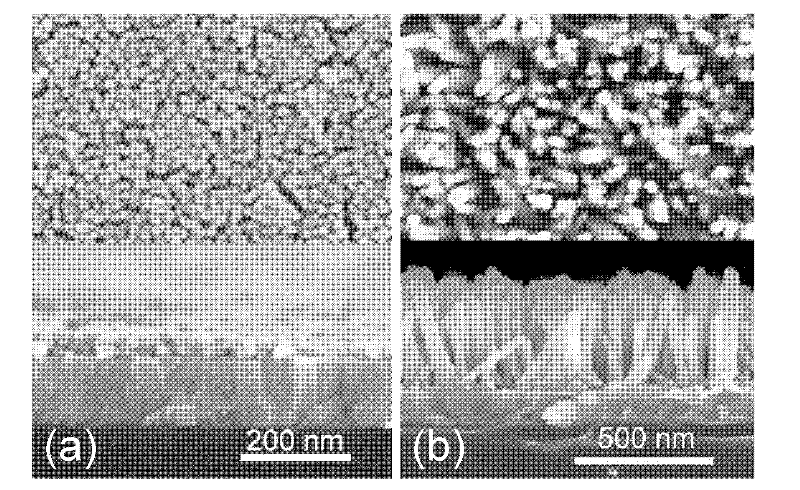
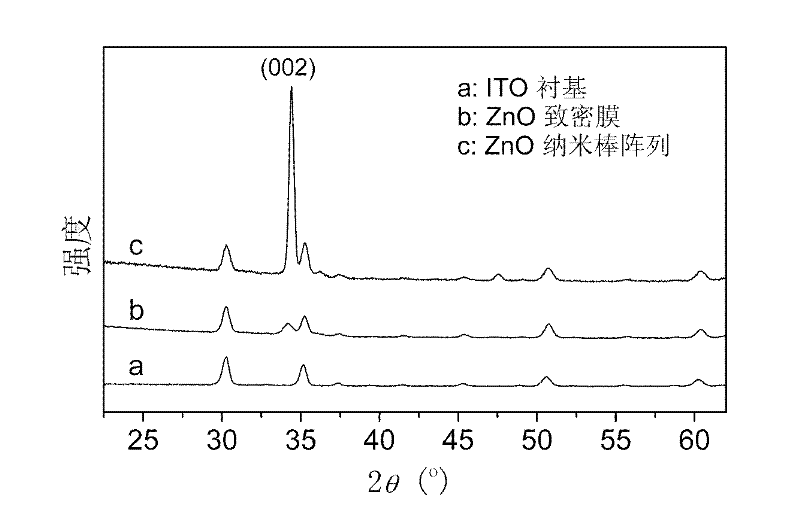
Organic/inorganic hybridization solar cell based on zinc oxide (ZnO) homogeneous core-shell structure nanorod array and production method thereof
InactiveCN102544378AImprove performanceIncrease the open circuit voltageSolid-state devicesSemiconductor/solid-state device manufacturingSolar cellPolymer
The invention discloses an organic / inorganic hybridization solar cell and a production method thereof, wherein a zinc oxide (ZnO) nanorod array ZnO-sodium (Na) which is vertically grown on an indium tin oxide (ITO) lining base is utilized as a template, a nanorod array ZnO-natural rubber (NR)-quantum dot (QD)-NA which takes a ZnO nanorod as a core and a polycrystal film which consists of ZnO quantum dots as an inorganic finishing shell layer is prepared, an organic matter N719 is utilized to modify the ZnO-NR-QD-NA, so a homogeneous core-shell structure nanorod array ZnO-NR-QD-N719-NA which is modified by the N719 is obtained, a polymer and the ZnO-NR-QD-N719-NA are compounded to produce a finished product, the open-circuit voltage of the cell reaches 0.62V, the short-circuit current of the cell is 3.79mA / cm<2>, and the conversion efficiency of the cell reaches 0.84%. Compared with a phenylacetylene (MEH-PPV) / ZnO-NA cell, the open-circuit voltage of the cell is improved by 88%, the short-circuit current is increased by 192%, and the conversion efficiency is improved by 425%.
Owner:INST OF PLASMA PHYSICS CHINESE ACAD OF SCI
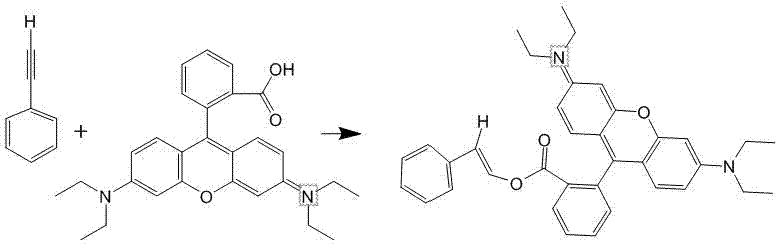
Solid fluorescent nanometer microsphere as well as preparation method and application thereof
ActiveCN103940798AHigh fluorescence intensityHigh signal magnificationNanotechnologyFluorescence/phosphorescenceBenzenePolystyrene
The invention discloses a solid fluorescent nanometer microsphere as well as a preparation method and an application thereof, and belongs to the technical field of fluorescence detection. The solid fluorescent nanometer microsphere disclosed by the invention is prepared from rhodamine B-styrene which is prepared by carrying out covalent addition on carboxyl rhodamine B and phenylacetylene and polymerizing the rhodamine B-styrene and styrene according to the molar ratio of 1:(1-12). A fluorescent molecule is embedded into a space network of polystyrene, and the obtained solid fluorescent microsphere has the characteristics of being uniform and consistent in fluorescence intensity among the microspheres, high in fluorescence intensity, high in signal amplification factor and the like. No hinder of the fluorescent molecule exists on the surface of the microsphere, so that the microsphere can be coupled to protein with the high efficiency and the high productivity. The fluorescent background generated by the microsphere is low, the accuracy of the detection result is not affected, and the solid fluorescent nanometer microsphere can be widely applied to a diagnostic reagent.
Owner:WUHAN NEWCANDO BIOTECH CO LTD
Compound having phenylacetylene structure, liquid crystal composition, polymer, optically anisotropic product, optical or liquid crystal element, dibenzothiophene compound, intermediate, and process for producing the same
InactiveUS6849202B2Large refractive index anisotropyWell mixedLiquid crystal compositionsHalogenated hydrocarbon preparationCrystallographyAlkoxy group
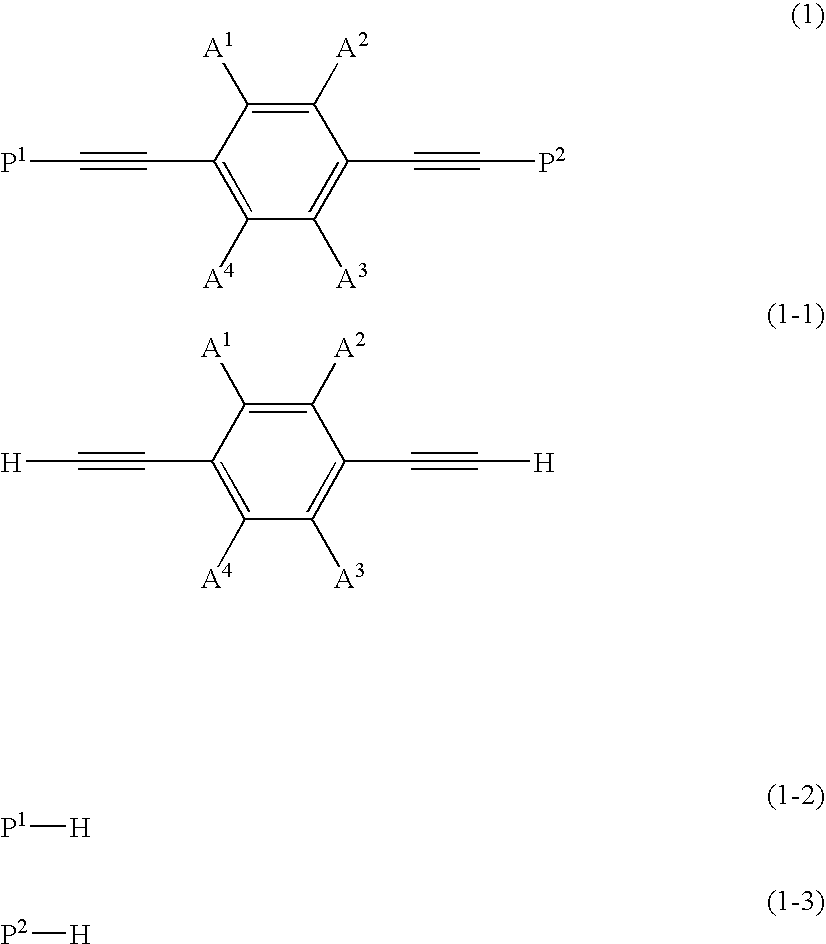
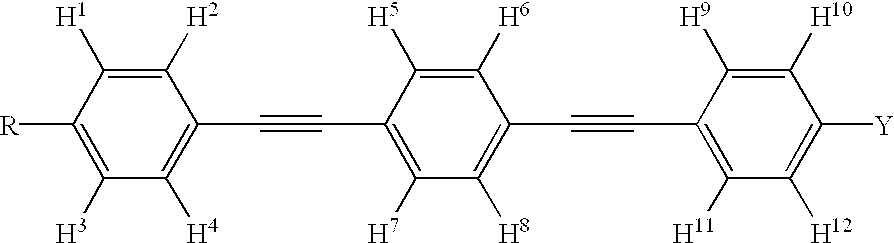
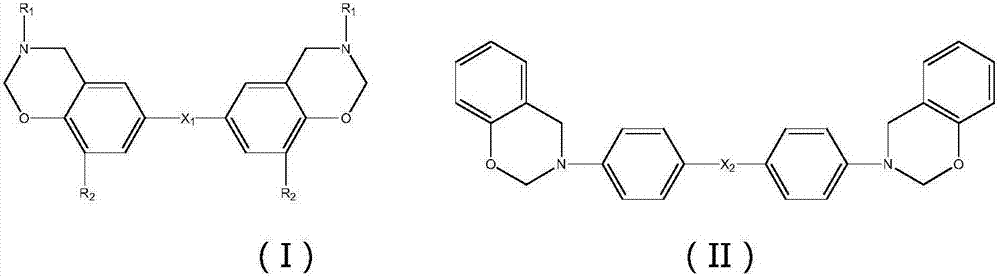
There are disclosed novel compounds, liquid crystal compositions, polymers, optically anisotropic products, and optical or liquid crystal elements that have large refractive index anisotropy, mix easily with other liquid crystals, have advantageous stability against light, and exhibit absorption at practically short wavelength in the ultraviolet region. The compounds are represented by the formula (1) and have a phenylacetylene structure, wherein difference ΔE in energy of HOMO of parts (1-1), (1-2) and (1-3) calculated by the method of molecular orbitals is not less than 0.3 electronvolt, and the polarizability anisotropy Δα of a molecule represented by the formula (1) calculated in the same way is not lower than 500 A.U.: (A1 to A4: H, F, alkyl or alkoxy group of C1 to C10 optionally substituted with F; P1, P2: structure fulfilling the conditions of HOMO energy and polarizability).
Owner:SUMITOMO CHEM CO LTD
Brake pad made of composite material
InactiveCN106009495AImprove high temperature resistanceImprove heat stabilityFriction liningActuatorsCarbon fibersSisal fiber
The invention discloses a brake pad made of a composite material. The brake pad comprises a steel sheet and a friction block, and the friction block is prepared from boron-modified phenolic resin, organic silicone-modified phenolic resin, barium phenolic resin, phenylacetylene phenolic resin, epoxy-terminated liquid reaction type nitrile rubber, butadiene rubber, molybdenum disulfide, sisal fiber, bamboo carbon fiber, aluminum fiber, molybdenum fiber, poly-p-phenylene benzodioxazole fiber, zinc oxide crystal whiskers, montmorillonite, vermiculite, nanometer aluminum oxide, cerium oxide, zirconium boride, aluminum silicate hollow spheres, graphene oxide, copper powder, carbon nano tubes, nano carbon black, butadiene styrene rubber powder, ammonium molybdate and octaphenyl silsesquioxane. The brake pad made of the composite material is high in high temperature resistance, excellent in heat stability, high in abrasion resistance and long in service life.
Owner:NINGGUO FEIYING AUTO SPARE PARTS
Method for preparing amorphous nanometer rhodium palladium alloy and catalytic application thereof
ActiveCN103785858ASimple stepsHigh yieldMaterial nanotechnologyHydrocarbon by hydrogenationSolventPhenylacetylene
The invention discloses a method for preparing amorphous nanometer rhodium palladium alloy, and belongs to the technical field of manufacturing of nanometer material. According to the method, oleylamine is selected as solvent and reducing agent, tri-n-octylphosphine is selected as protecting agent, and even and disperse amorphous nanometer rhodium palladium alloy is manufactured with a pyrosol reduction method. The method has the advantages of being simple in step and high in yield, and the prepared nanometer particles are small and even in particle diameter and good in reproducibility. The prepared amorphous nanometer rhodium palladium alloy serves as catalyst to be applied into a reaction for manufacturing styrene through phenylacetylene hydrogenation, the reactant conversion rate is high, the selectivity of products is high, and stability of the catalyst is good.
Owner:BEIJING UNIV OF CHEM TECH
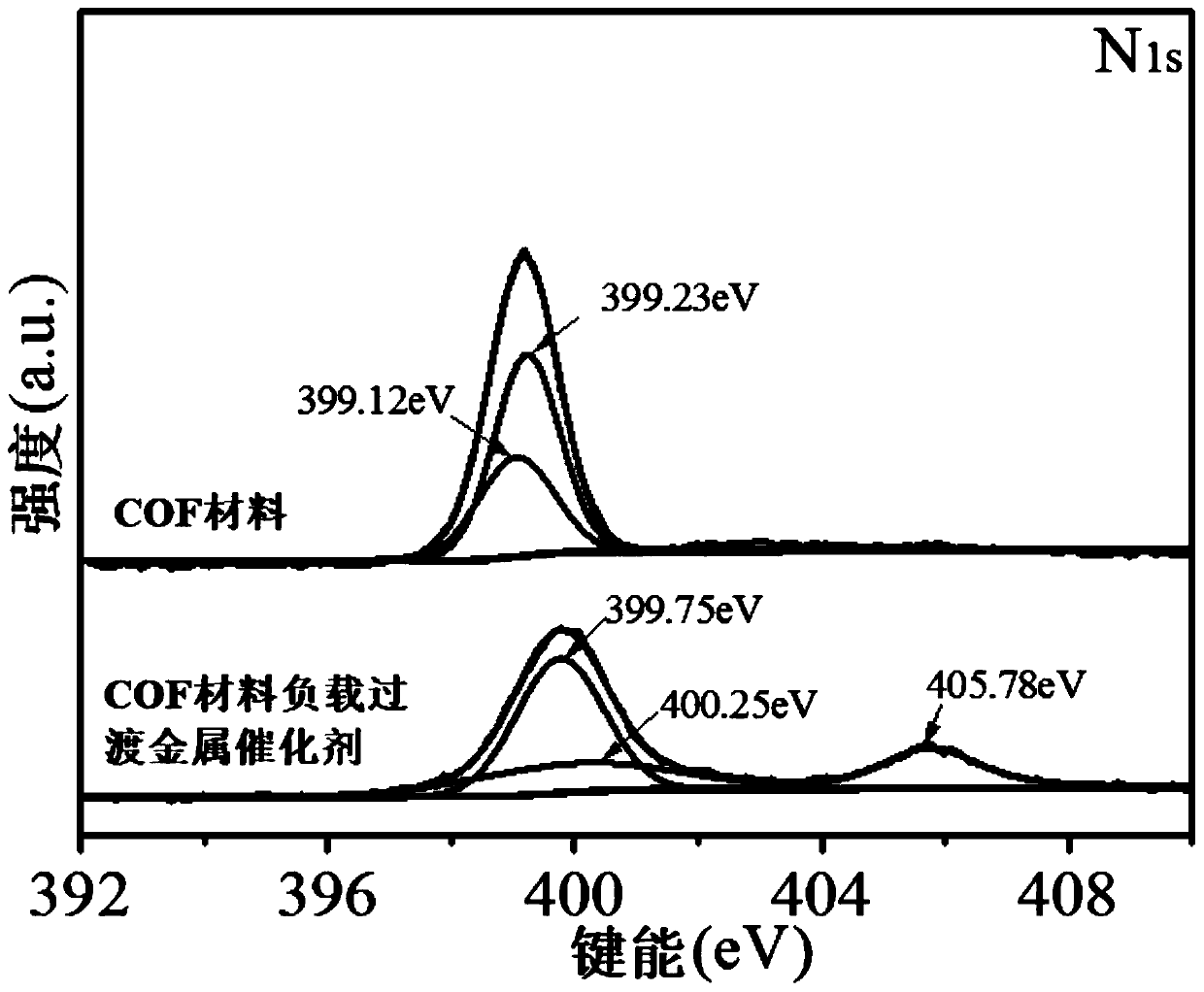
COF material-loaded transition metal catalyst, and preparation method and application thereof
ActiveCN111111785AHigh catalytic activityGood choiceCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystAlcohol
The invention discloses a COF material-loaded transition metal catalyst, and a preparation method and application thereof. The COF material-loaded transition metal catalyst comprises a COF material and Pd<2 +> or Ni <2+> loaded on the COF material. The preparation method comprises the following steps: 1) mixing an aldehyde precursor, an amine precursor and absolute ethyl alcohol, carrying out a full reaction, and separating and purifying a reaction product to obtain a COF material; and 2) mixing the COF material, a soluble Pd salt or soluble Ni salt and dichloromethane, carrying out impregnation loading, and separating and purifying the obtained product. The COF material-loaded transition metal catalyst has the advantages of high catalytic activity, good selectivity, wide substrate application range, easiness in recycling, mild reaction conditions, usage of green and environment-friendly reaction solvents and the like when used for a semi-hydrogenation reaction of phenylacetylene compounds, and is simple in preparation process, easily available in raw materials and low in production cost.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Process for the selective hydrogenation of phenylacetylene
InactiveUS7105711B2Reduce contentIncrease contentHydrocarbon by hydrogenationHydrocarbon purification/separationBenzeneHydrogen
A process for the reduction of a phenylacetylene contaminant in the presence of a styrene monomer. A styrene monomer stream containing a minor amount of phenylacetylene is supplied to a hydrogenation reactor. A hydrogenation gas comprising hydrogen is also supplied to the hydrogenation reactor. The styrene monomer stream and the hydrogen are brought into contact with a catalyst bed containing a catalyst comprising a reduced copper compound on a theta alumina support. The hydrogenation reactor is operated at a temperature of at least 60° C. and a pressure of at least 30 psig to hydrogenate phenylacetylene to styrene. A product is recovered from the hydrogenation reactor having a substantially reduced phenylacetylene content and an enhanced styrene content. The hydrogenation gas comprises a mixture of nitrogen and hydrogen.
Owner:FINA TECH
Synthesis of biphenyl type polyimide resin with phenyl ethyne dead-end
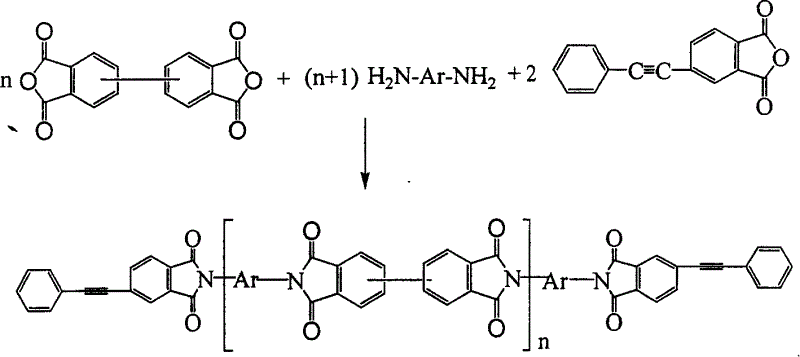
A process for synthesizing the phenylacetylene dead-end biphenylpolyimide resin with high thermal stability and mechanical performance and low viscosity of molten body includes such steps as proportionally adding diphenyldianhydride, bibasic amine and phenylacetylene phenylanhydride to polar solvent, stirring while reacting, adding acetic anhydride and triethylamine, stirring while reacting, depositing, washing the deposit and drying.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Modified bismaleimide resin prepolymer and preparation method thereof
The invention provides a modified bismaleimide resin prepolymer. Bismaleimide is subjected to copolymerization, chain extension and toughening modification by a melting polymerization process and by using an allyl compound, aminophenylacetylene and benzoxazine; the obtained modified bismaleimide resin prepolymer has excellent processing property while maintaining high heat resistance of the bismaleimide, and can greatly improve the mechanical property; furthermore, the prepolymer is moderate in melt viscosity, low in volatile component, high in paving and covering property, long in storage period and low in cost, and is an ideal adhesive film material for prepreg; and the modified bismaleimide resin prepolymer can serve as matrix resin of a composite material, a high-temperature-resistant adhesive, an electronic appliance copper-clad plate insulating material and the like, and is applicable in the technical fields of aerospace, electronic appliances, traffic and transportation and the like.
Owner:WUHAN UNIV OF TECH
High performance and low cost polyimide preformed polymer and preparation method thereof
The invention pertains to the field of polymer materials, in particular to a polyimide prepolymer with comparatively high vitrification transformation temperature (270 DEG C to 320 DEG C), comparatively high stability of thermal oxidization, comparatively low viscosity and comparatively low cost, and a preparation method of the prepolymer. 1, 4-(para-aminophenoxy)-2-phenylbenzene is firstly added into an organic solvent and completely dissolved, then pyromellitic dianhydride is added into the organic solvent and reacts at room temperature for 3 hours to 10 hours, and then an end capping agent, 4-(2-phenylacetylenel) phthalic anhydride is added and the reaction continues for 1 hour to 3 hours. An obtained polyamide acid solution is put into an oven and the solvent is removed; under the condition of vacuum, the temperature is increased for thermal imidization and an obtained product is crushed to obtain the powdered polyimide prepolymer with a structure formula shown as follows.
Owner:JILIN UNIV
Hydrotalcite-loaded palladium catalyst for preparing styrene through selective hydrogenation of phenylacetylene and preparation method thereof and application
ActiveCN111054333AEasy to manufactureSuitable for mass productionOrganic reductionOrganic compound preparationPolymer sciencePtru catalyst
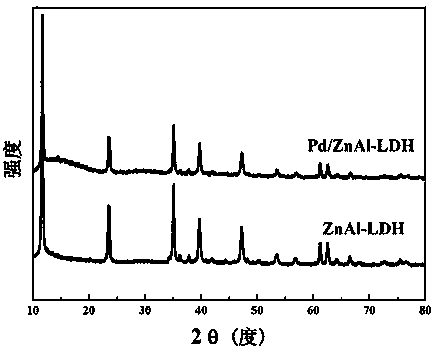
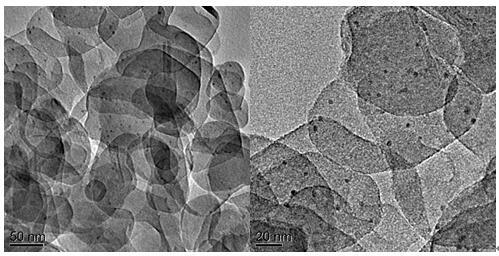
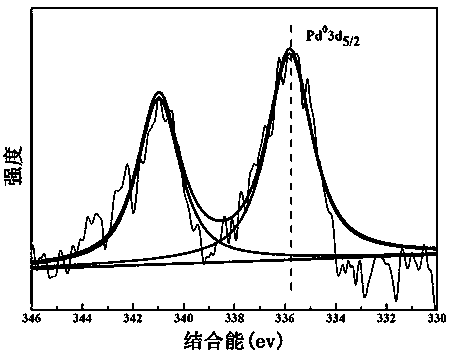
The invention discloses a hydrotalcite-loaded palladium catalyst for preparing styrene through selective hydrogenation of phenylacetylene and a preparation method thereof and application. According tothe catalyst, hydrotalcite is used as a carrier, and palladium is dispersed on the surface of the carrier in a nanoparticle form. The carrier is any one of ZnAl, NiAl, CoAl and NiFe hydrotalcite. Theactive component is palladium, and the mass fraction of palladium is 0.5-1.5%. The preparation method comprises the following steps of: dispersing hydrotalcite in mixed alcohol, adding a noble metalsalt solution, stirring for several hours, centrifuging and drying to obtain the hydrotalcite-loaded palladium nano-catalyst. The preparation method of the catalyst is simple and suitable for large-scale production. The obtained catalyst has extremely high activity and styrene selectivity in a selective hydrogenation reaction of phenylacetylene, and also has good stability and substrate universality.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Preparation method and application of eggshell type molybdenum carbide catalyst
ActiveCN103657633AImprove performanceAchieve eggshell distributionHydrocarbon by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsEggshellAlkyne
The invention discloses a fast reaction film formation method to prepare an eggshell type molybdenum carbide catalyst and belongs to the technical field of industrial catalysis. The method comprises the following steps: a carrier with a molybdenum oxide perssad adsorbed on the surface is added into a melamine solution, a film composed of a molybdenum carbide precursor is rapidly generated on the surface of the carrier through the fast reaction of melamine and the molybdenum oxide perssad, and the eggshell type molybdenum carbide catalyst is formed via pyrolysis of the film. According to the invention, the technology is simple, the thickness of the shell layer can be controlled, the energy consumption during the process is low, and the utilization rate and the stability of the active components in the prepared eggshell type molybdenum carbide catalyst are high and excellent. The eggshell type molybdenum carbide catalyst has excellent industrial application prospect and can be used for alkene-alkyne selective hydrogenation reaction and other reactions represented by phenylacetylene selective hydrogenation.
Owner:DALIAN UNIV OF TECH
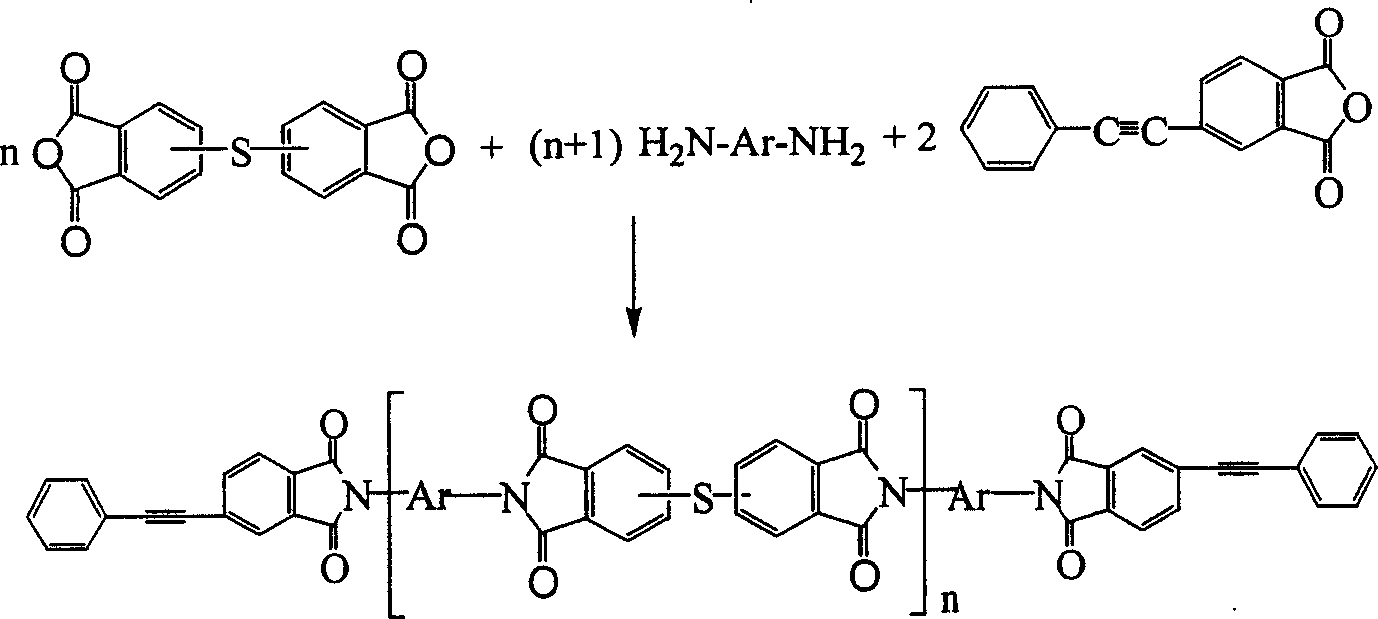
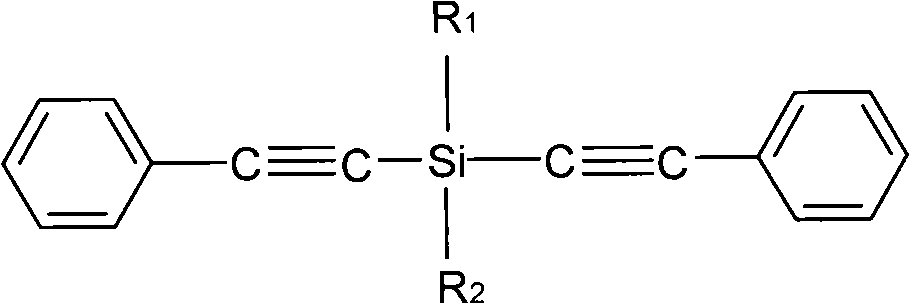
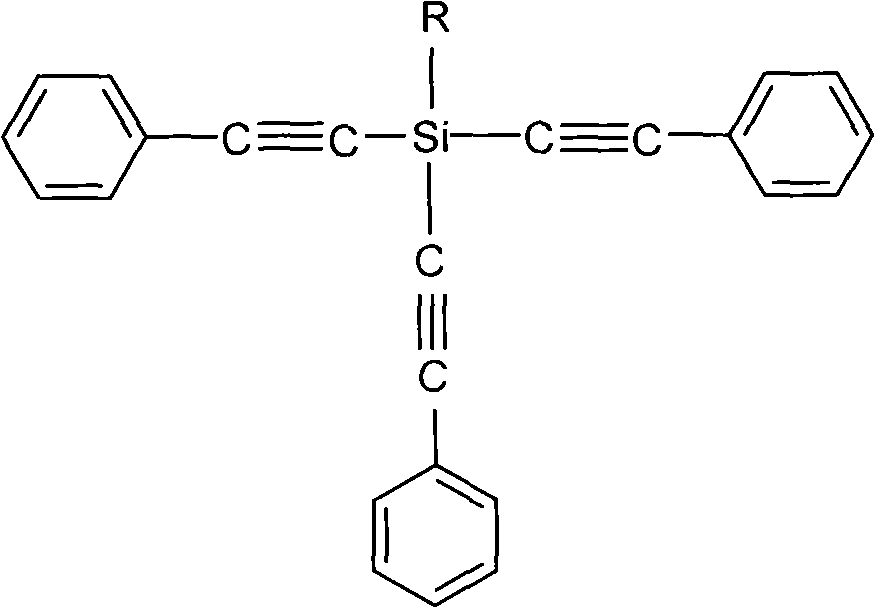
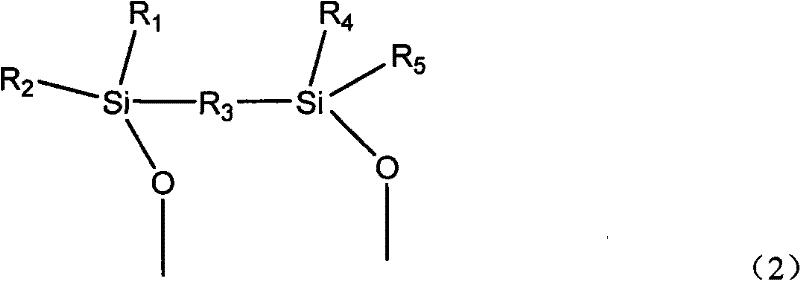
Cyanate resin modified by phenylacetylene base silane resin and preparation method thereof
The invention relates to cyanate resin modified by phenylacetylene base silane resin and a preparation method thereof. The preparation method is technically characterized by comprising the steps of: mixing 100 parts by weight of the cyanate resin and 1-100 parts by weight of the phenylacetylene base silane resin in a solvent, then, distilling to remove the solvent, and curing according to a certain curing process to form a three-dimensional network structure. Compared with the traditional modification method of the cyanate resin, in the preparation method, the phenylacetylene base silane resin is soluble in the common organic solvent and has favorable compatibility with the cyanate resin, which can effectively improve the heat resistance and the thermal oxidation property of the cyanate resin. The modification system prepared in the invention has excellent heat resistance, favorable mechanical property and dielectric property and can be sued as matrix resin of the heat resistant and wave-transmitting material.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for removing phenylacetylene in presence of styrene
ActiveCN102408299AReduce generationExtended service lifeHydrocarbon purification/separationCatalyst activation/preparationHydrocarbon mixturesSilanes
The invention relates to a method for removing phenylacetylene in the presence of styrene. According to the requirements of adaptability to water or water content fluctuation of a reaction system and inhibition of carbon deposit in the selective hydrogenation process of the phenylacetylene at present, a hydrogen / phenylacetylene hydrocarbon mixture in a certain molar ratio is introduced into a reactor and contacted with a hydrogenation catalyst to remove the phenylacetylene at the inlet temperature of between 10 and 120 DEG C under the pressure of 0.1 to 4.0MPa, wherein the hydrogenation catalyst comprises a carrier, metal active ingredients and a silane group which is grafted by silylation and accounts for 0.1 to 15 weight percent of the catalyst. Compared with the prior art, the method has the advantages that: the method is high in raw material applicability on the premise of ensuring that the catalyst in the method has high activity and selectivity, and the catalytic performance of the selective hydrogenation catalyst is almost not influenced by trace water; meanwhile, the carbon deposit on the surface of the catalyst can be obviously inhibited, the service life of the catalyst is prolonged, and the catalyst has a long stable operation period.
Owner:CHINA PETROLEUM & CHEM CORP +1
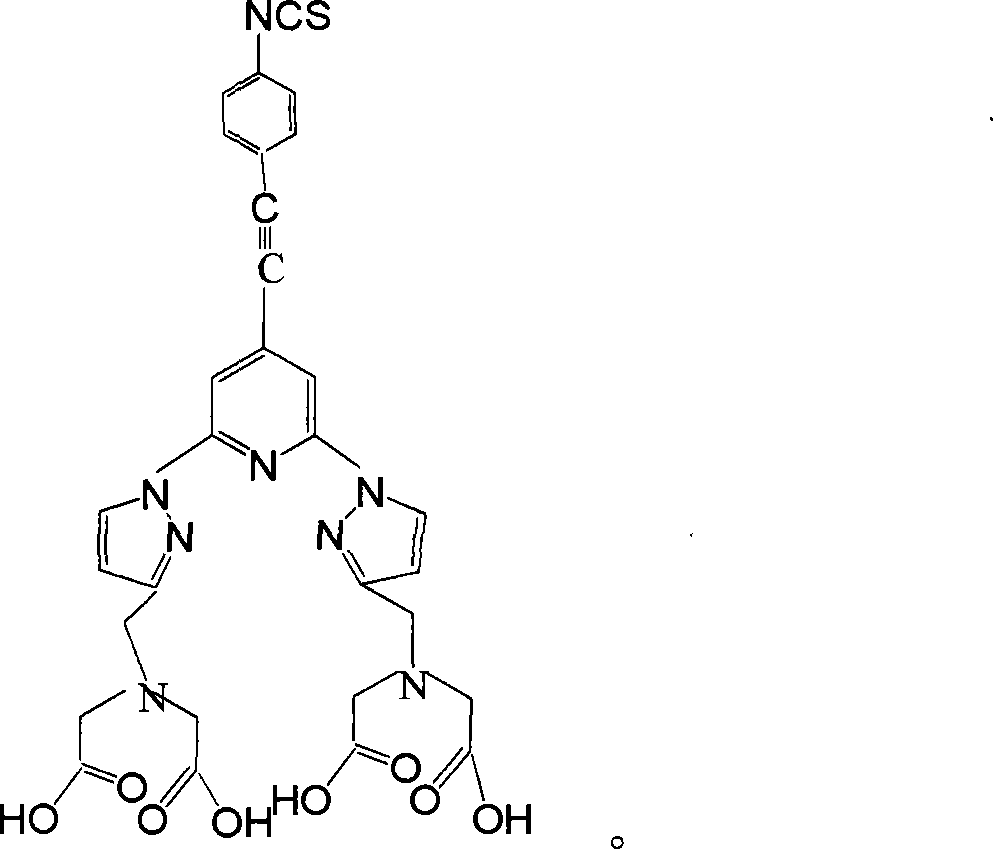
Homogeneous phase time discrimination fluorescence immunity analysis chelating agent and its preparing method
InactiveCN101221169AAdvantage designAdvantage structureBiological testingLuminescent compositionsSolubilityTriplet state
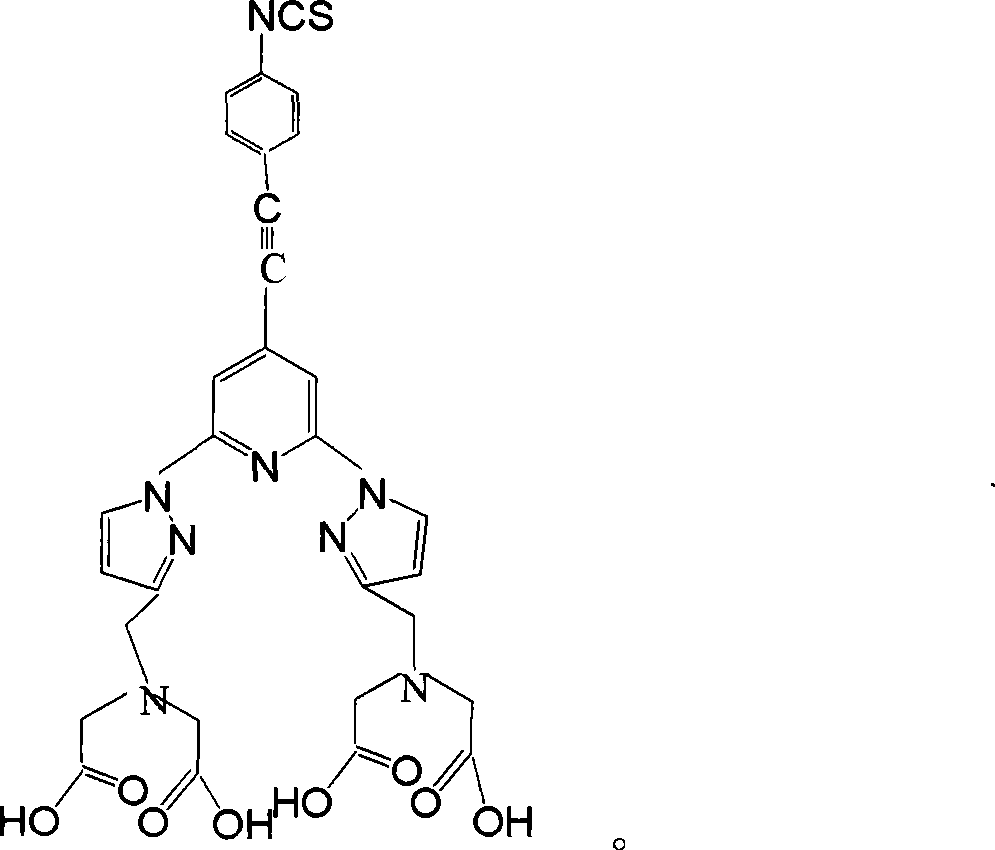
The invention relates to a homogeneous time resolved fluorescence immunoassay chelating agent and the preparation method thereof. The chelating agent is N, N, N', N'-((2, 6-di(3'-aminomethyl)-1-pyrazole)-4-(4-isothiocyanate(phenylacetylene)-pyridine))-tetraacetic acid. A luminous group is 2, 6-di(pyrazole) pyridine; the invention has high triplet-state energy level, the excitation wavelength after the combination with rare earth ions is 320nm, the emission wavelength is 520 to 620nm, the luminous service life is 2.99ms, the quantum yield is 0.58, the invention is in line with the requirements of homogeneous TRFIA to be combined with phenylacetylene at the pyridine 4-position, and the acetylene bond can block protein macromolecules from consuming luminous energy. The phenyl 4-linking isothiocyanate at the pyridine 4-position can be coupled with protein without injury, so as to be conductive to the high-efficient measurement of the luminescence of the chelating agent. The polyammonia polycarboxyl structure of 11 coordination site can exclude the quenching function of water molecules on rare earth luminescence and improve the solubility of the chelating agent in water and the dynamic stability of the chelating agent in the water r solution.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Novel polytriazoles imide resin and preparation method thereof
The invention discloses polyimide with triazole rings in a main chain structure, i.e. polytriazoles imide resin and a preparation method thereof. The method comprises the following steps: firstly, designing and synthesizing binary azides; then, using binary anhydride and m-phenylacetylene as raw materials for designing and synthesizing terminal alkynyl imide compounds; and thirdly, using the terminal alkynyl imide compounds and azides with equimolar amounts to take 1, 3-Dipolar cycloaddition polymerization reaction in a solvent or adopting thermal polymerization reaction, or adopting redox catalysed polymerization for synthesizing novel polytriazoles imide. The polytriazoles imide resin prepared by the method of the invention has good processing performance, has high thermal stability, simultaneously has good mechanical property and electric performance, and embodies the characteristics of high corrosion resistance performance, high binding power and the like of the polytriazoles imide resin. The invention has wide application prospects in the fields of aviation, astronavigation, micro-electronics, automobiles, shipping industries and the like.
Owner:安徽中科达美新材料科技有限公司
MOF-derived Pt1@CeO2 monatomic catalyst as well as preparation method and application thereof
ActiveCN112871154AEasy to makeEasy to manufactureHydrocarbon by hydrogenationCatalyst activation/preparationPlatinumPtru catalyst
The invention provides a platinum monatomic catalyst, a preparation method thereof and an application of the platinum monatomic catalyst in a reaction for preparing styrene through selective hydrogenation of phenylacetylene. The selectivity of the styrene can reach 99.5% under a mild condition. The catalyst is composed of a carrier with MOF as a precursor and an active component loaded on the carrier, the active component is precious metal platinum, and platinum single atoms are uniformly dispersed on the carrier. The catalyst is high in precious metal utilization rate and high in atom economy, and the cost of the catalyst can be effectively reduced. The reaction system is simple, the reaction conditions are mild, and the catalyst and the solvent are easy to separate and recover. The platinum monatomic catalyst provided by the invention is novel in structure, metal is uniformly dispersed on the carrier, and the platinum monatomic catalyst has good phenylacetylene hydrogenation activity and relatively high styrene selectivity, and has a wide application prospect in the field of preparation of styrene by selective hydrogenation of phenylacetylene.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com