Method for preparing amorphous nanometer rhodium palladium alloy and catalytic application thereof
An amorphous, nano-rhodium technology, applied in nanotechnology, nanotechnology, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as unsatisfactory selectivity, and achieve high selectivity and high yield , the effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
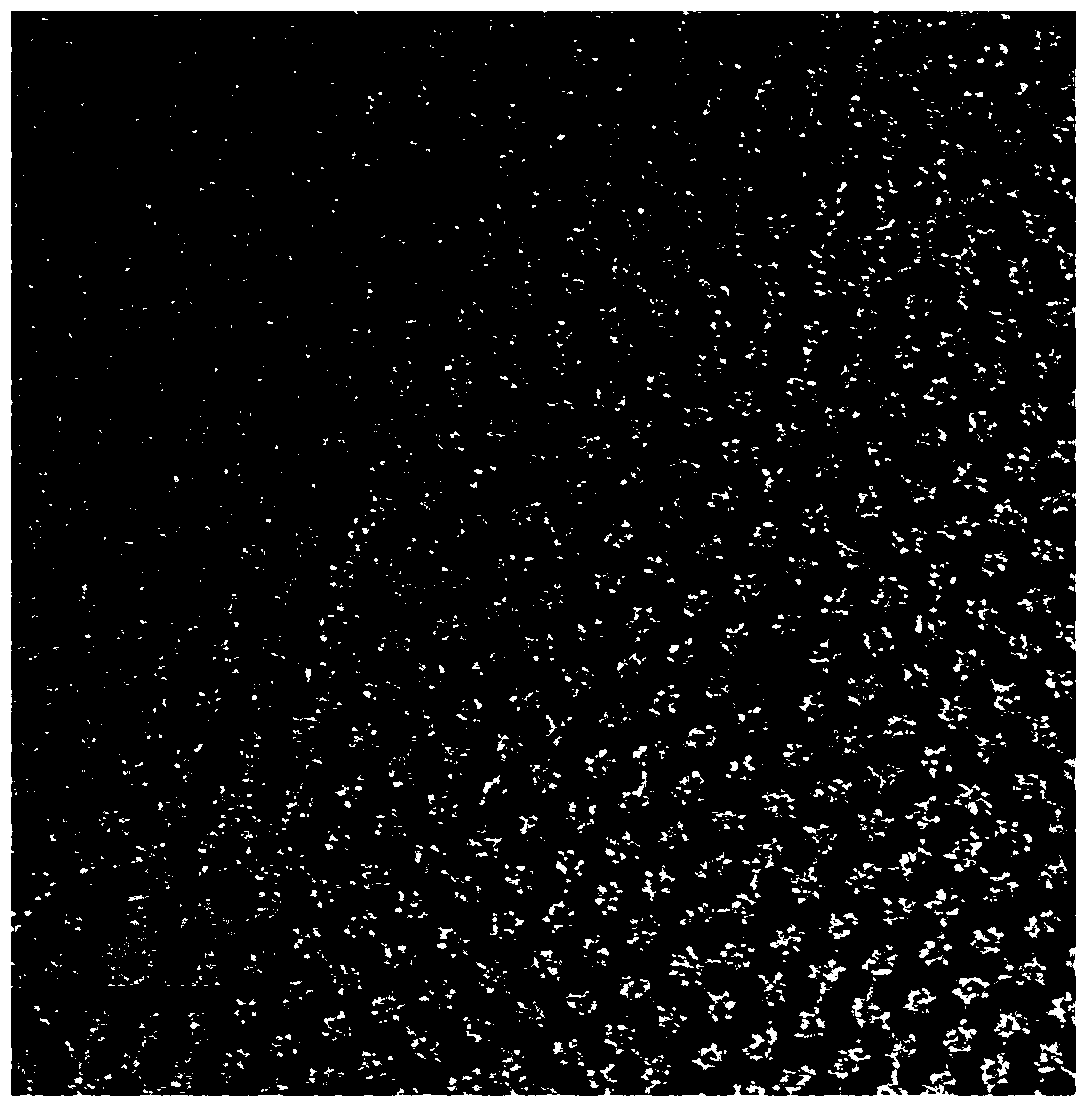
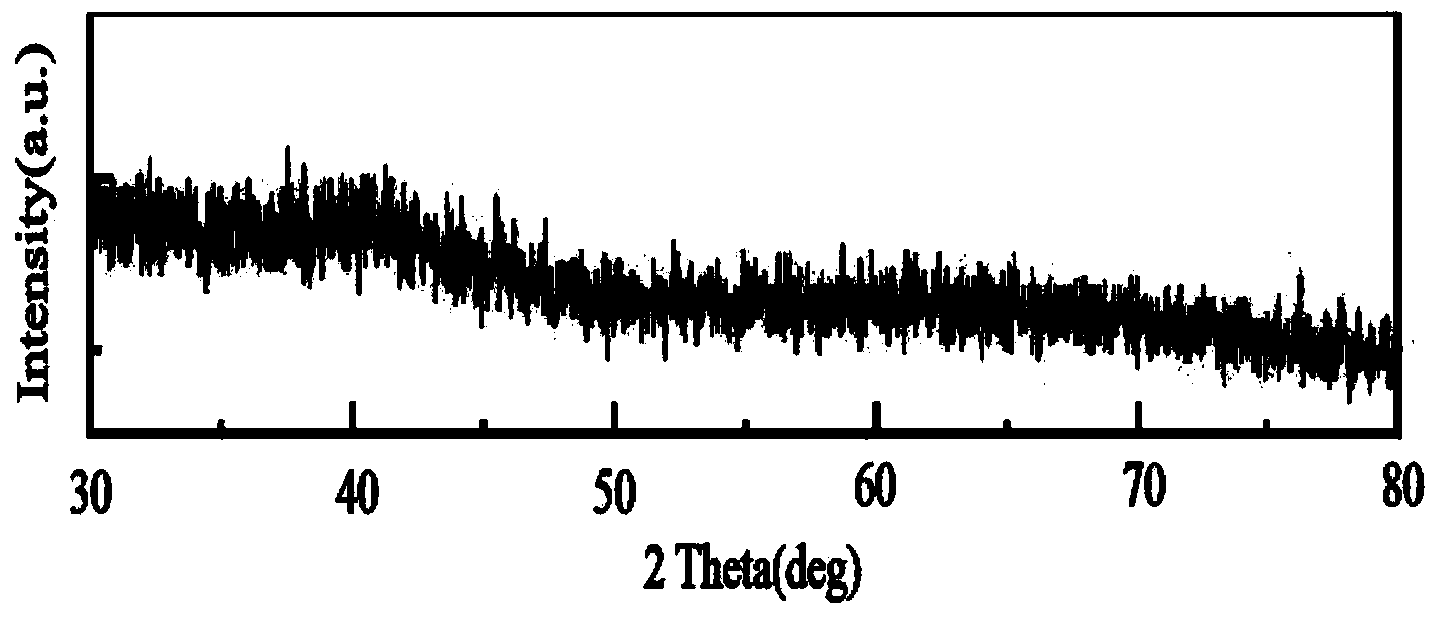
[0014] At 25°C, weigh 2mg of rhodium acetylacetonate and 1.5mg of palladium acetylacetonate, add them to 5ml of oleylamine, then add 60μL of tri-n-octylphosphine, put the above mixture into a 25ml beaker, and ultrasonicate for 20min at 25°C, then Stir magnetically at 25°C for 20 minutes; transfer the reaction solution to a 10ml autoclave, react at 200°C for 16 hours, and centrifuge the slurry after reaction for 3 times with a mixed solvent of ethanol and cyclohexane with a volume ratio of 10:1 at 70°C , the precipitate after centrifugation was dispersed in 4mL of cyclohexane to obtain an amorphous nano-rhodium-palladium alloy, the particle size of which was 5nm, and the dispersion was uniform.
[0015] The amorphous nano-rhodium-palladium alloy prepared above is used as a catalyst to catalyze the hydrogenation of phenylacetylene to prepare styrene. The reaction conditions are: the reaction temperature is 30 ° C, the reaction pressure is normal pressure, the catalyst amorphous n...
Embodiment 2
[0017] At 25°C, weigh 1.3mg of rhodium acetylacetonate and 2mg of palladium acetylacetonate, add them to 5ml of oleylamine, then add 60μL of tri-n-octylphosphine, put the above substances into a 25ml beaker, and ultrasonically for 20min at 25°C, then Stir magnetically at 25°C for 20 minutes; transfer the slurry to a 10ml reactor, and react at 200°C for 16 hours. The precipitate after centrifugation was dispersed in 4 mL of cyclohexane to obtain an amorphous nano-rhodium-palladium alloy, the particle size of which was 4.9 nm, and the dispersion was uniform.
[0018] The amorphous nano-rhodium-palladium alloy prepared above is used as a catalyst to catalyze the hydrogenation of phenylacetylene to prepare styrene. The reaction conditions are: the reaction temperature is 30 ° C, the reaction pressure is normal pressure, the catalyst amorphous nano-rhodium-palladium alloy and the reaction substrate phenylacetylene The molar ratio is 1:1200, and the reaction time is 2.5h.
[0019] ...
Embodiment 3
[0020] At 25°C, weigh 2.6 mg of rhodium acetylacetonate and 1 mg of palladium acetylacetonate, add them to 5 ml of oleylamine, and then add 100 μL of tri-n-octylphosphine, add the above substances into a 25 ml beaker, and ultrasonicate for 20 minutes at 25°C, then Stir magnetically at 25°C for 20 minutes; transfer the slurry to a 10ml reactor, and react at 200°C for 16h. After the reaction, the slurry is centrifuged and washed 3 times with a mixed solvent of ethanol and cyclohexane with a volume ratio of 10:1 at 65°C. The precipitate after centrifugation was dispersed in 4 mL of cyclohexane to obtain an amorphous nano-rhodium-palladium alloy, the particle size of which was 5.2 nm, and the dispersion was uniform.
[0021] The amorphous nano-rhodium-palladium alloy prepared above is used as a catalyst to catalyze the hydrogenation of phenylacetylene to prepare styrene. The reaction conditions are: the reaction temperature is 30 ° C, the reaction pressure is normal pressure, the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com