Patents
Literature
170301 results about "Solvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A solvent (from the Latin solvō, "loosen, untie, solve") is a substance that dissolves a solute (a chemically distinct liquid, solid or gas), resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Common uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene), as paint thinners (e.g. toluene, turpentine), as nail polish removers and glue solvents (acetone, methyl acetate, ethyl acetate), in spot removers (e.g. hexane, petrol ether), in detergents (citrus terpenes) and in perfumes (ethanol). Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes.
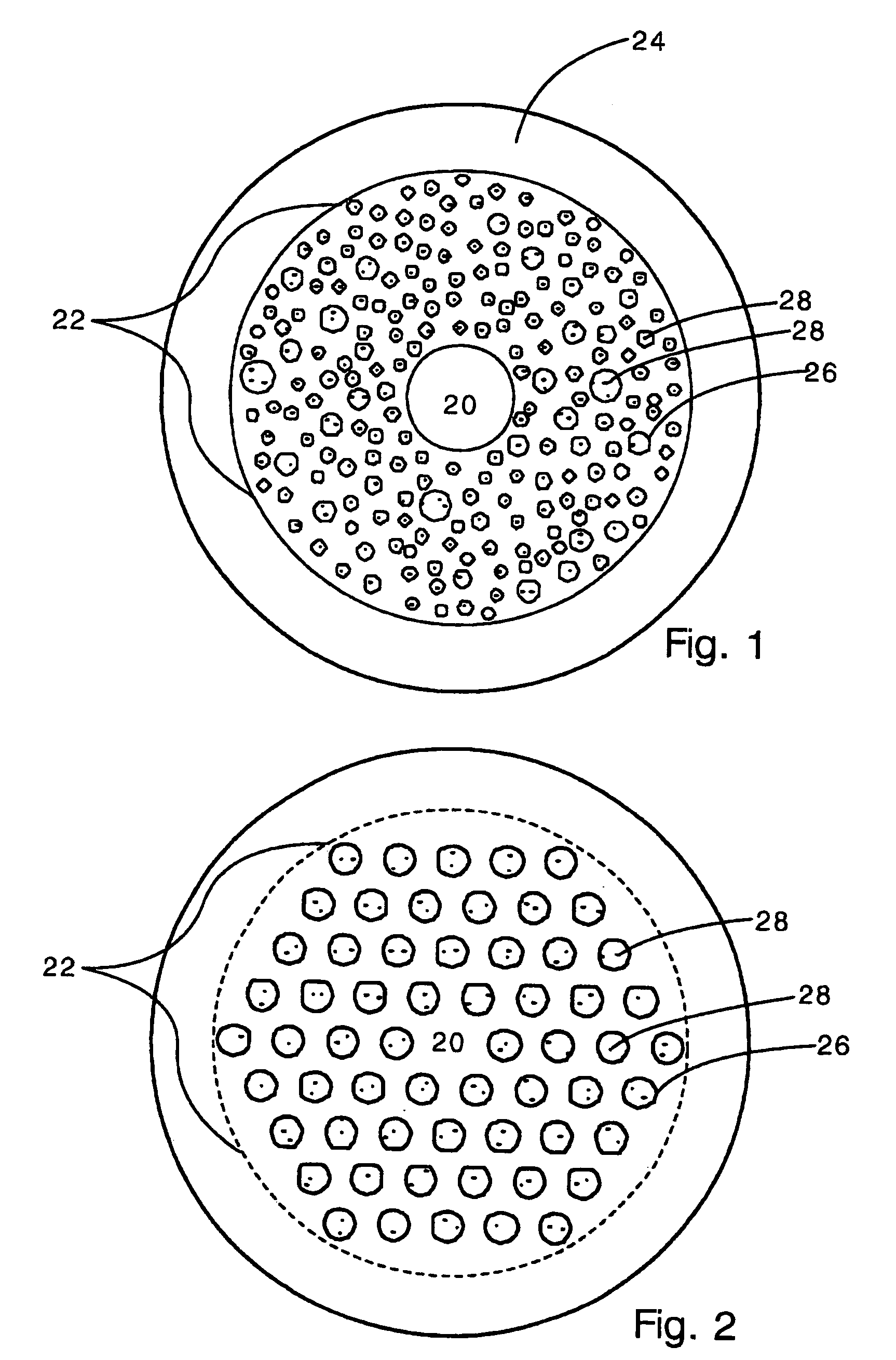
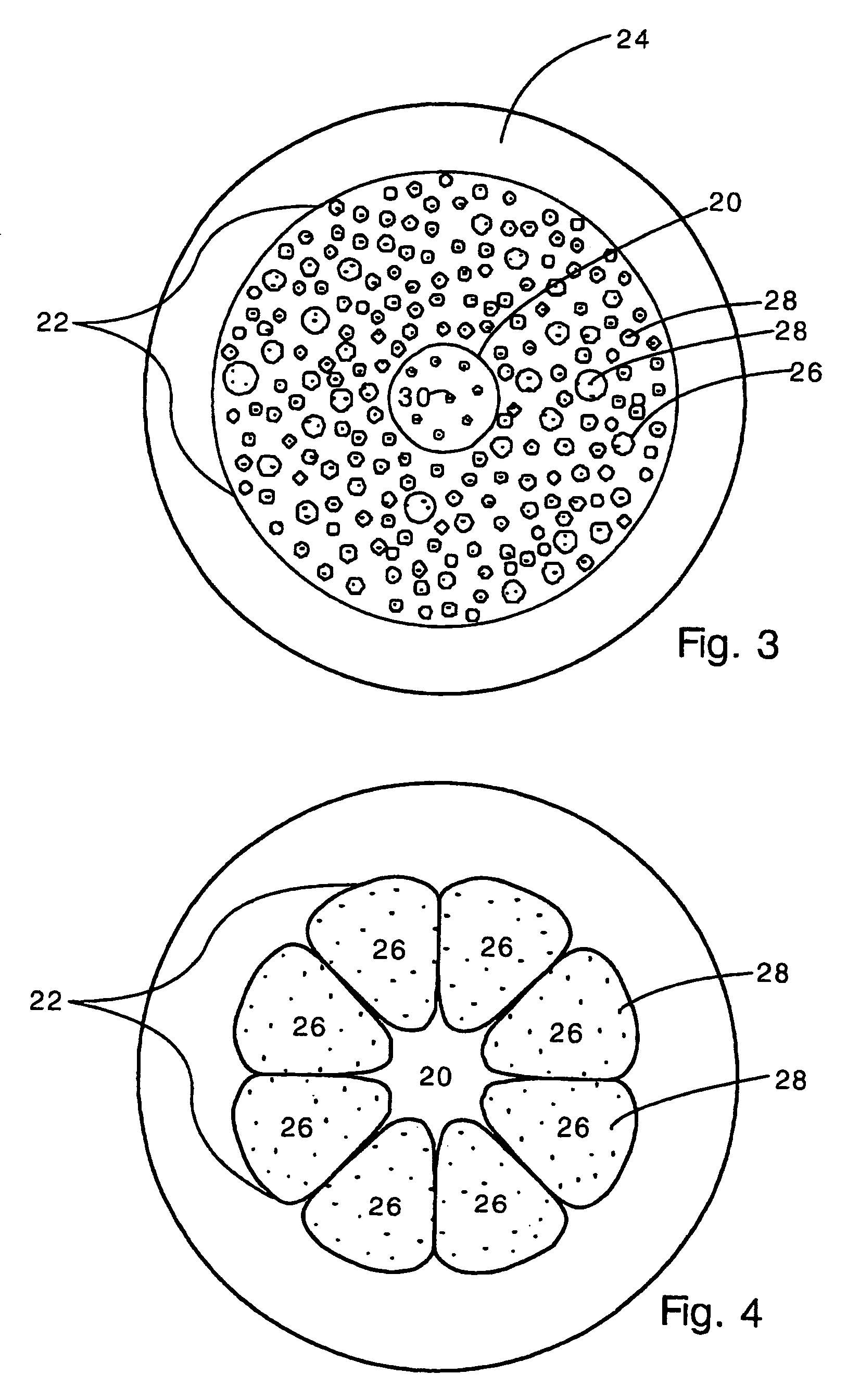
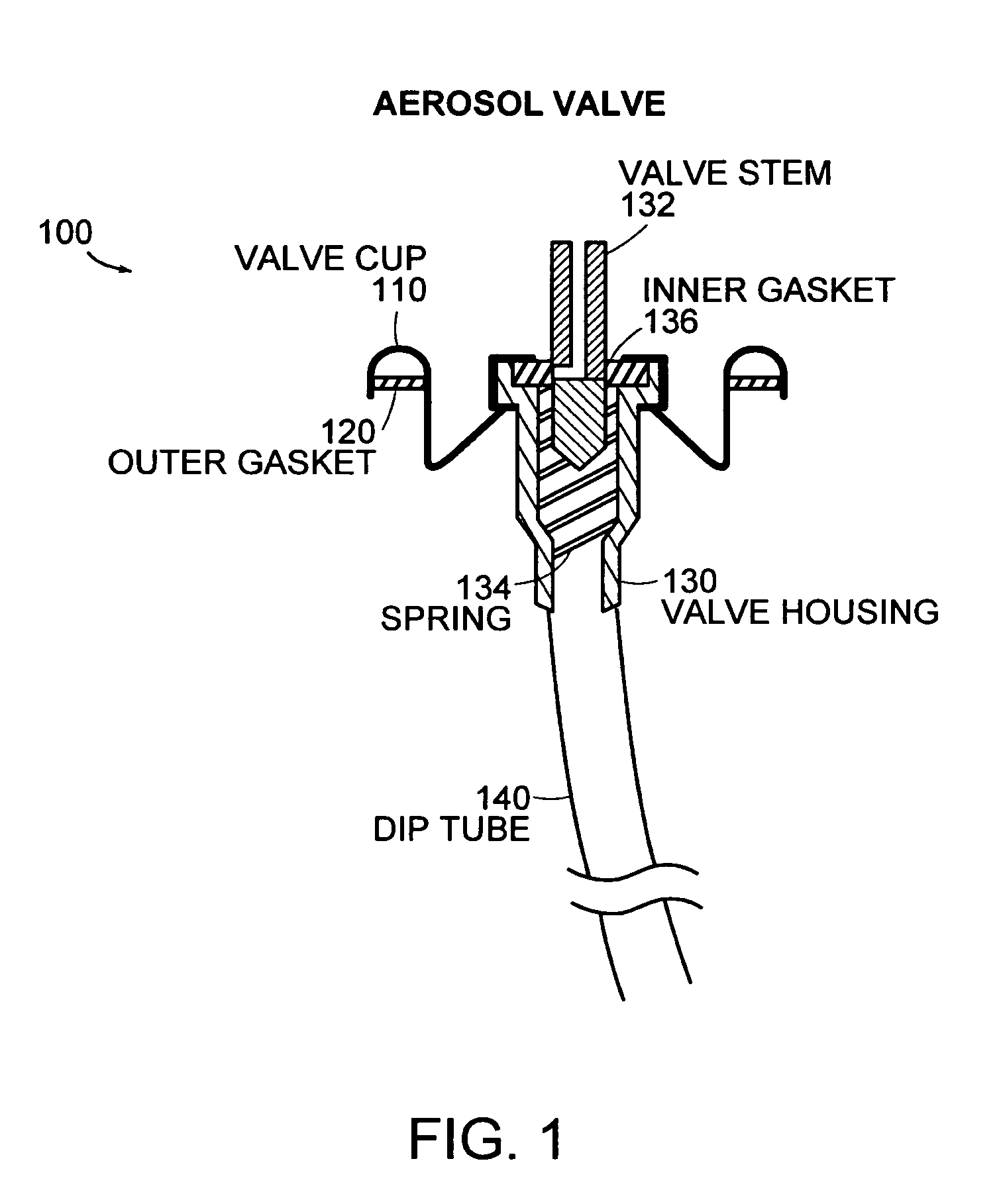
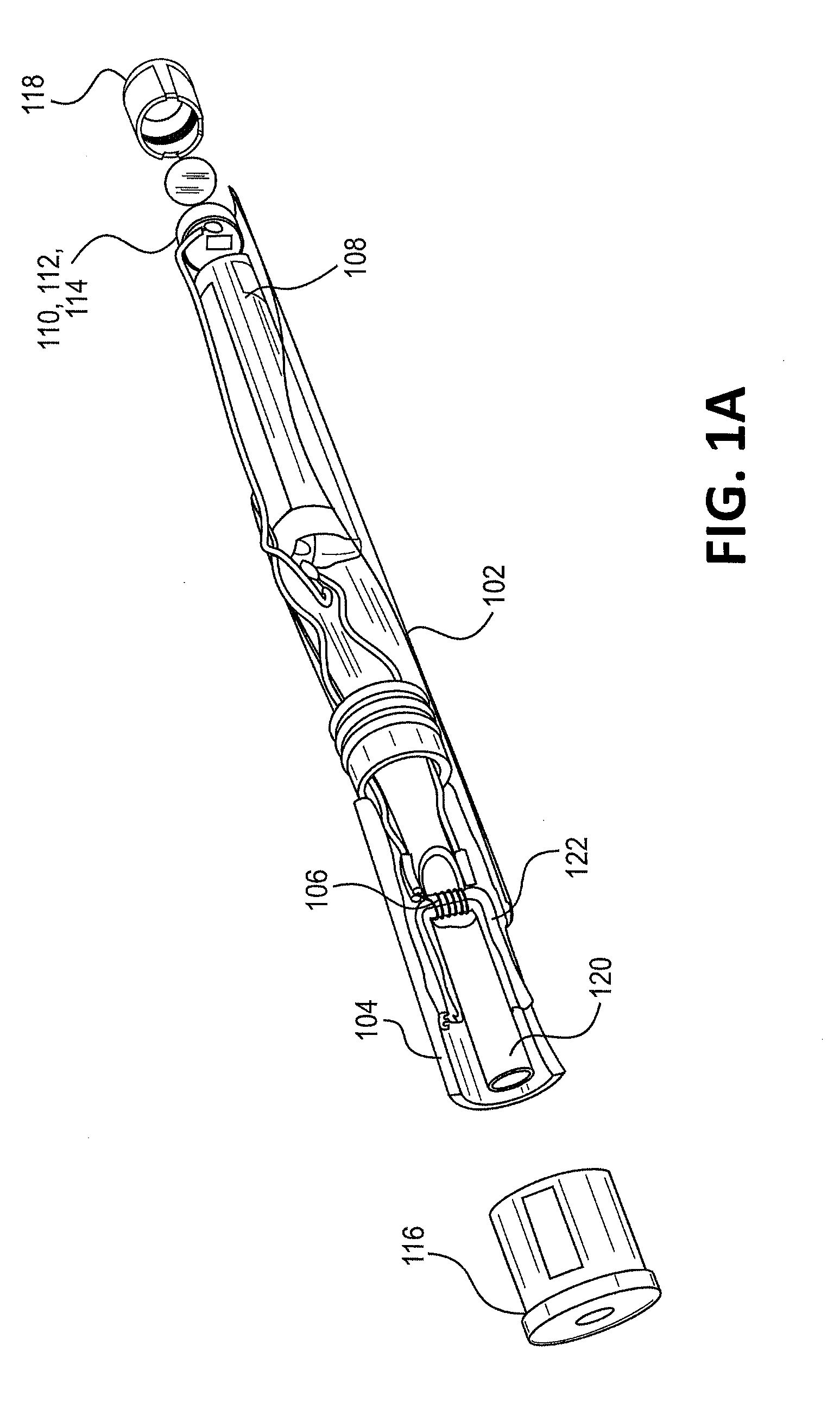
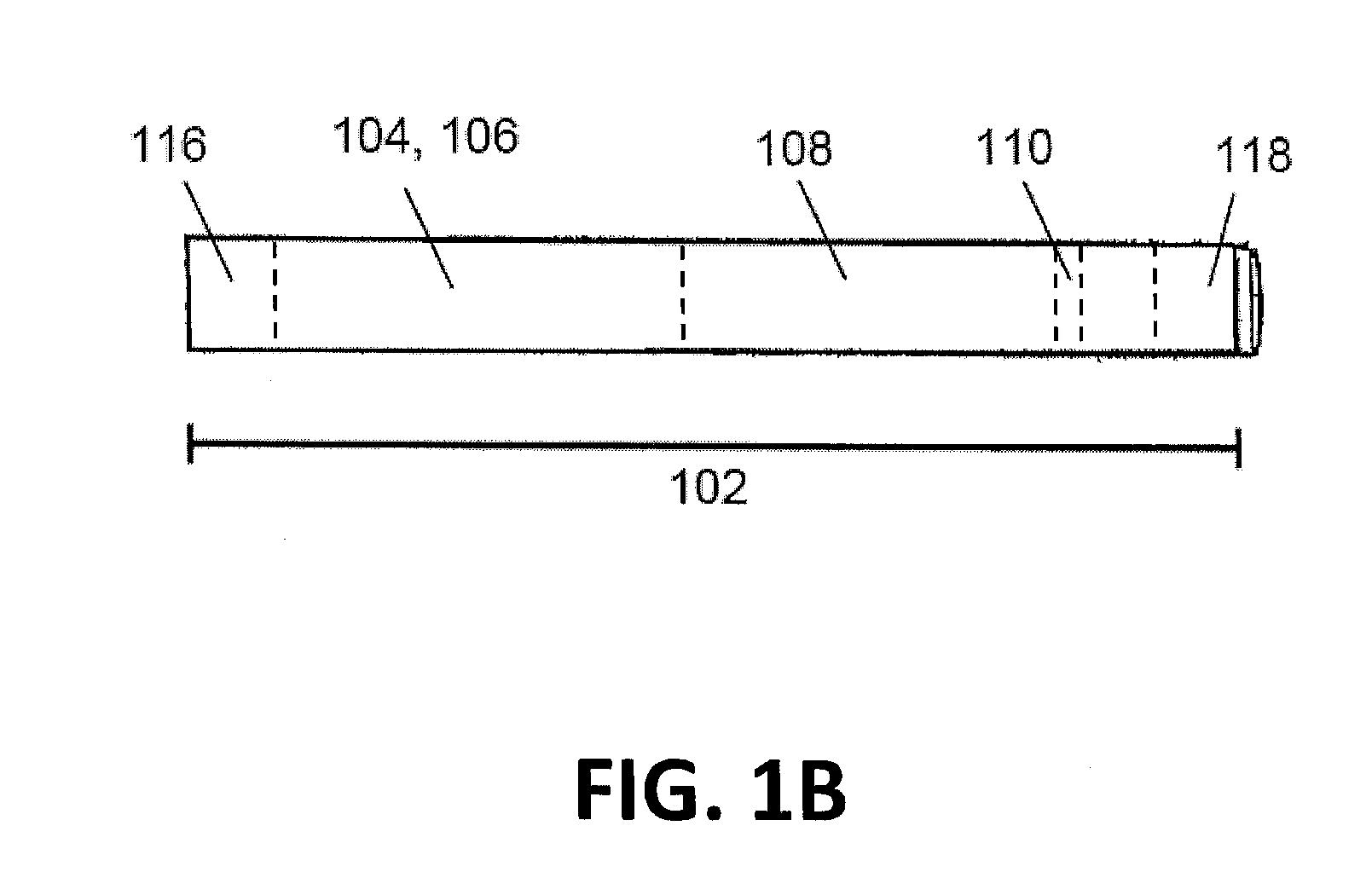
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Extraction of solvents from drug containing polymer reservoirs
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Process for obtaining a grafted elastomer having functional groups along the chain and a rubber composition
A process for obtaining a grafted diene elastomer having functional groups along the chain, a rubber composition containing this grafted elastomer and having in particular improved hysteresis properties in the cross-linked state, a preparation process for this composition, a tire tread made from this composition and a tire of reduced rolling resistance which incorporates this tread. A process for obtaining this grafted elastomer includes a radical grafting reaction carried out in solution or without a solvent by means of a reagent of the mercaptan type to graft functional groups on to the chain of a starting elastomer. The starting elastomer is treated with an antioxidant having at least one aromatic amine function before the grafting reaction, so that the grafted elastomer has a macrostructure which is practically identical to that of the starting elastomer. A rubber composition containing the grafted diene elastomer includes a reinforcing inorganic filler, and the grafted elastomer preferably has a molar ratio of units originating from conjugated dienes greater than 30%.
Owner:MICHELIN & CO CIE GEN DES ESTAB MICHELIN
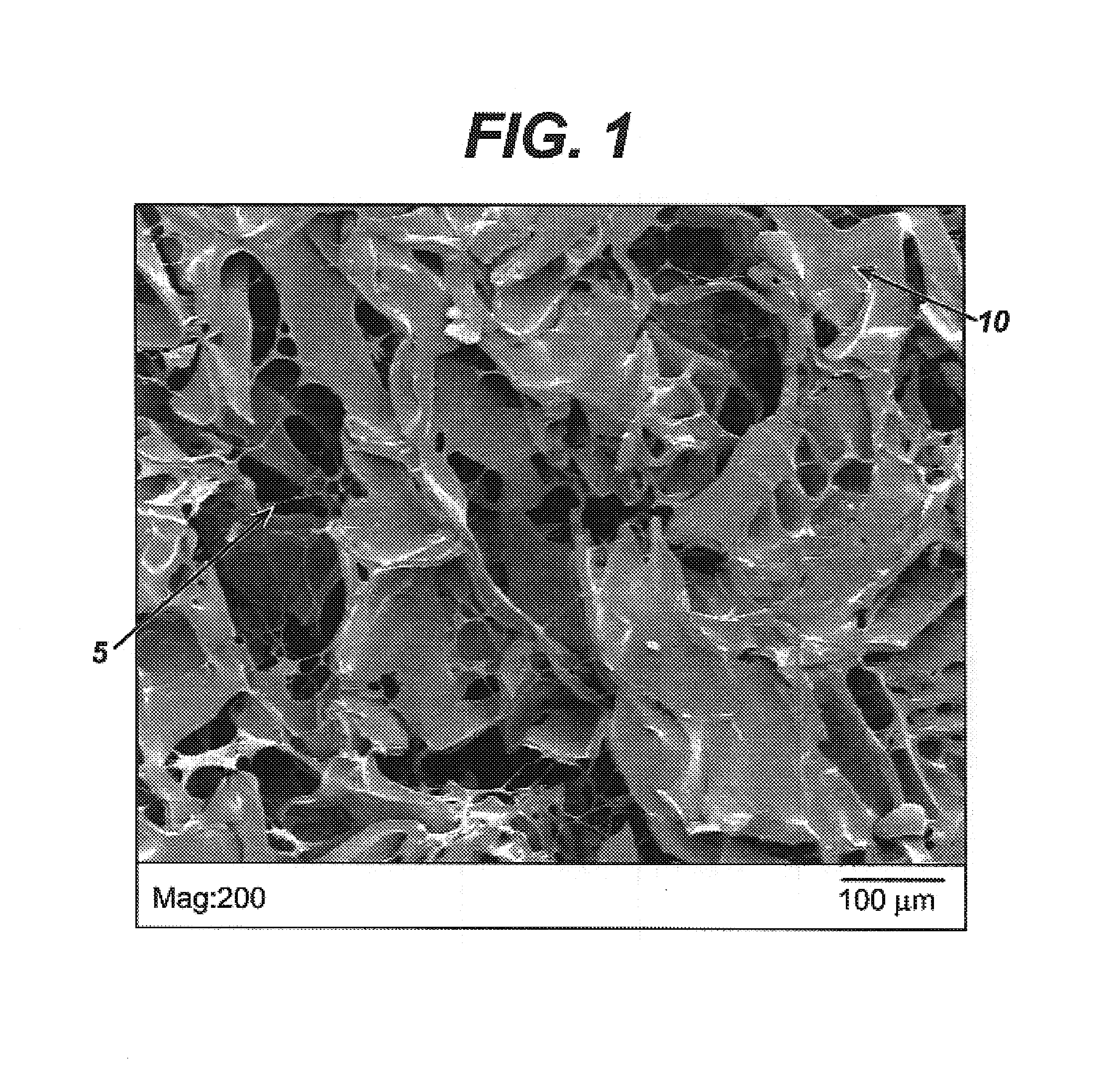
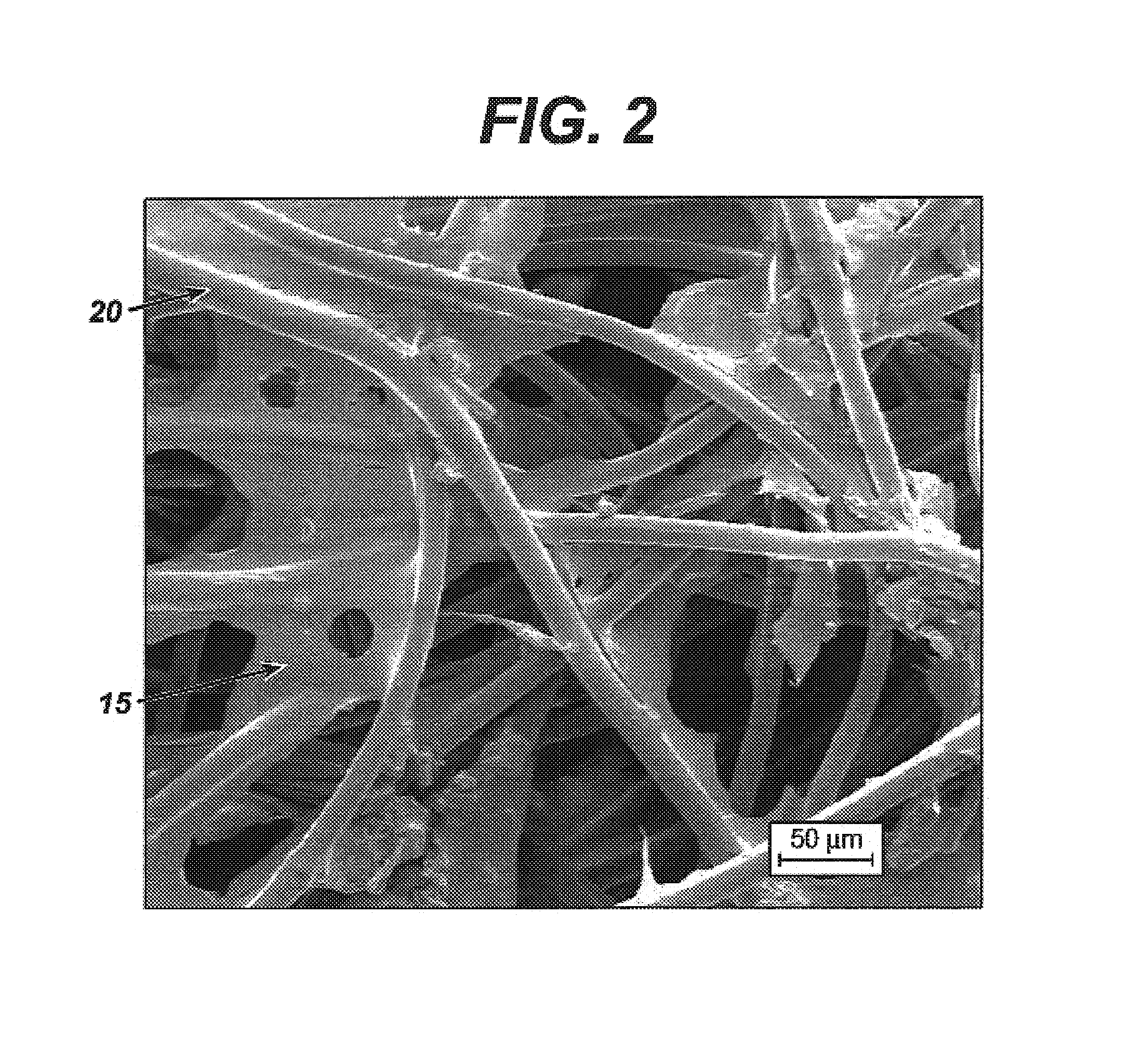
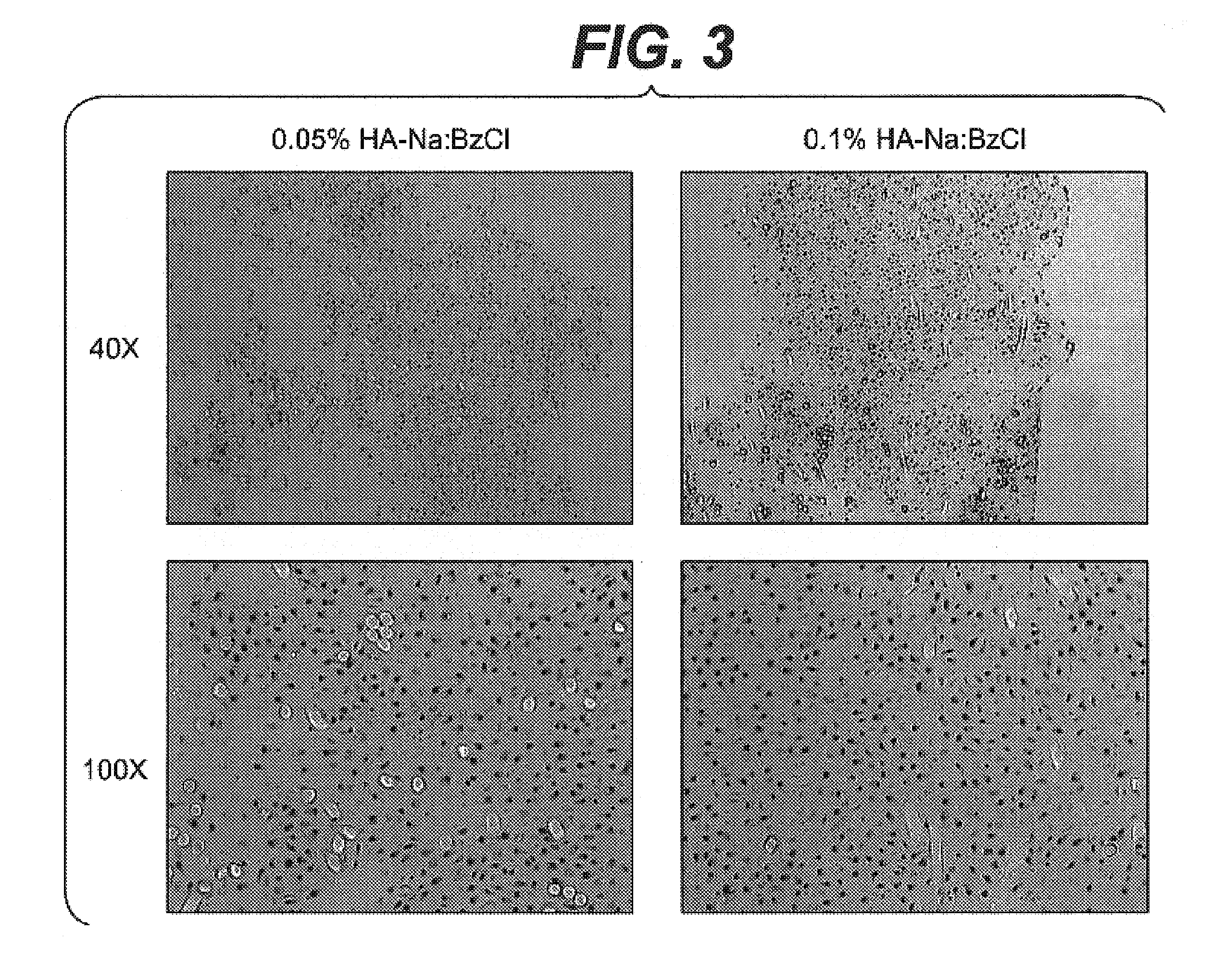
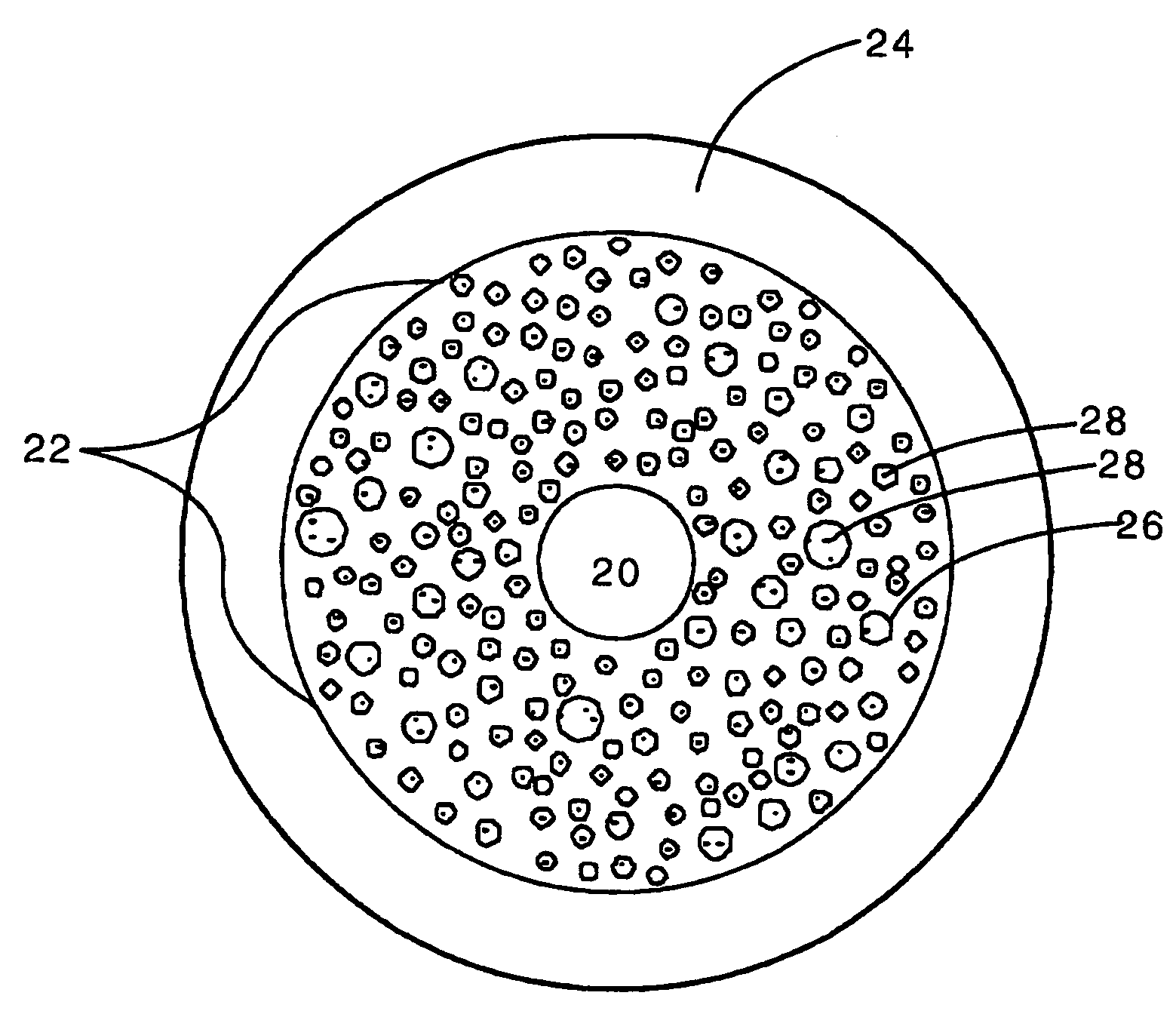
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Optical fiber with quantum dots
Holey optical fibers (e.g. photonic fibers, random-hole fibers) are fabricated with quantum dots disposed in the holes. The quantum dots can provide light amplification and sensing functions, for example. When used for sensing, the dots will experience altered optical properties (e.g. altered fluorescence or absorption wavelength) in response to certain chemicals, biological elements, radiation, high energy particles, electrical or magnetic fields, or thermal / mechanical deformations. Since the dots are disposed in the holes, the dots interact with the evanescent field of core-confined light. Quantum dots can be damaged by high heat, and so typically cannot be embedded within conventional silica optical fibers. In the present invention, dots can be carried into the holes by a solvent at room temperature. The present invention also includes solid glass fibers made of low melting point materials (e.g. phosphate glass, lead oxide glass) with embedded quantum dots.
Owner:LAMBDA LABORATORY INSTRUMENTS +1
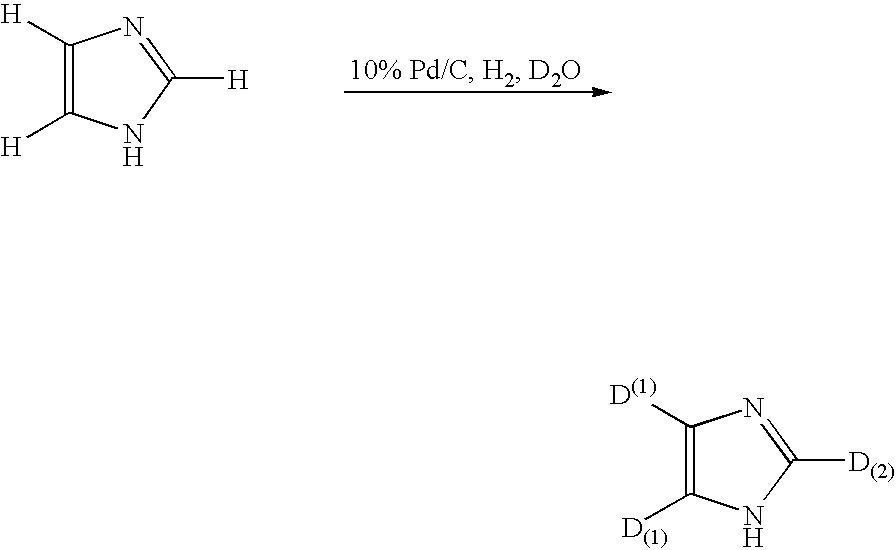
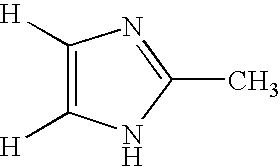
Method for deuteration of a heterocyclic ring
ActiveUS7517990B2Isotope introduction to heterocyclic compoundsSugar derivativesNickel catalystHydrogen atom
The present invention relates to a method for deuteration of a heterocyclic ring, which comprises subjecting a compound having a heterocyclic ring to sealed refluxing state in a deuterated solvent in the presence of an activated catalyst selected form a palladium catalyst, a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst. In accordance with a method of the present invention, a hydrogen atom belonging to a heterocyclic ring of a compound having a heterocyclic ring can be very efficiently deuterated because temperature of deuteration reaction can be maintained at higher than boiling point of the solvent.Further, a method for deuteration of the present invention can be applied widely to deuteration of various compounds having a heterocyclic ring which are liable to decomposition under supercritical conditions or acidic conditions, leading to industrial and efficient deuteration of a compound having a heterocyclic ring.
Owner:FUJIFILM WAKO PURE CHEM CORP
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
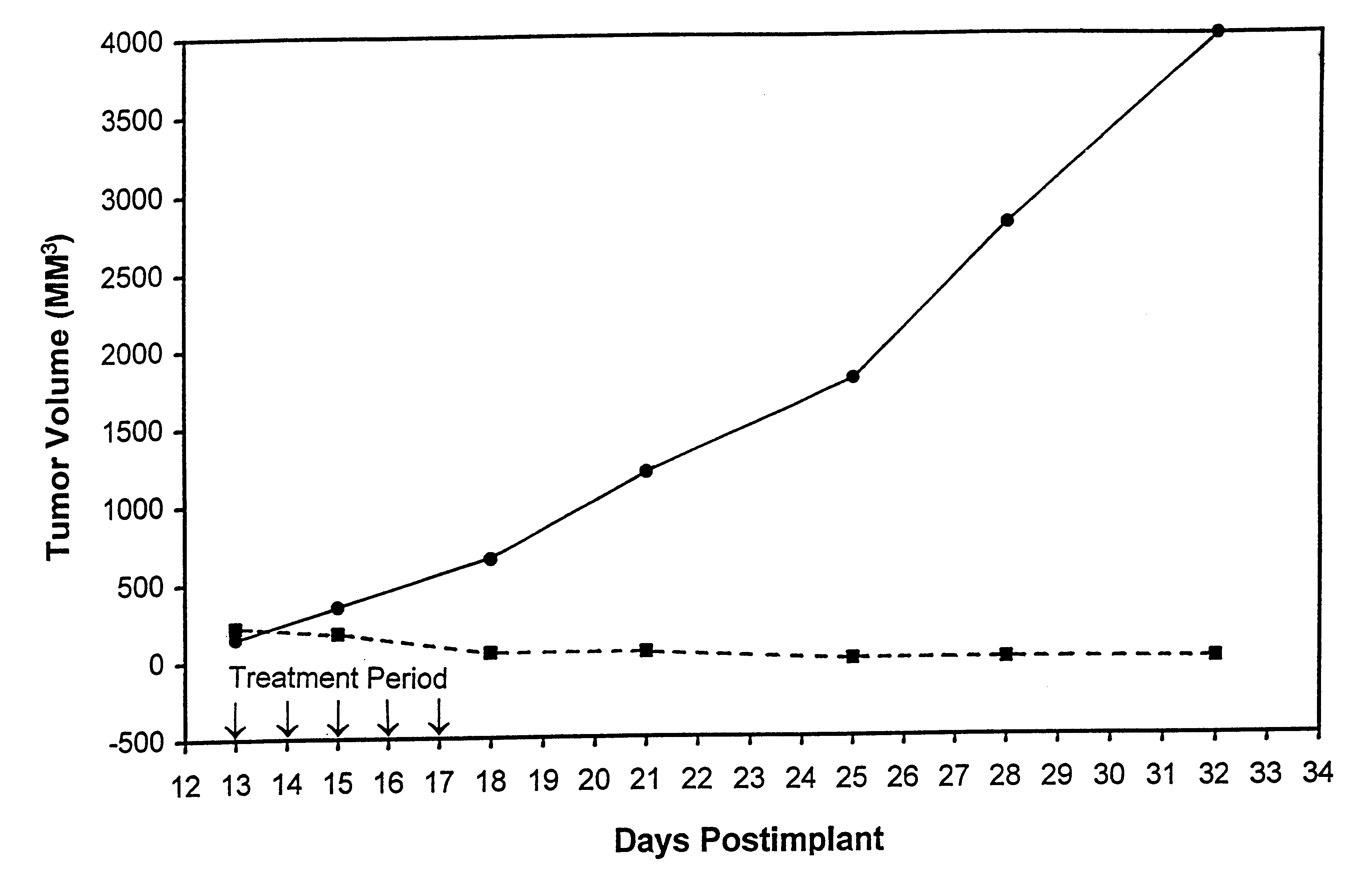
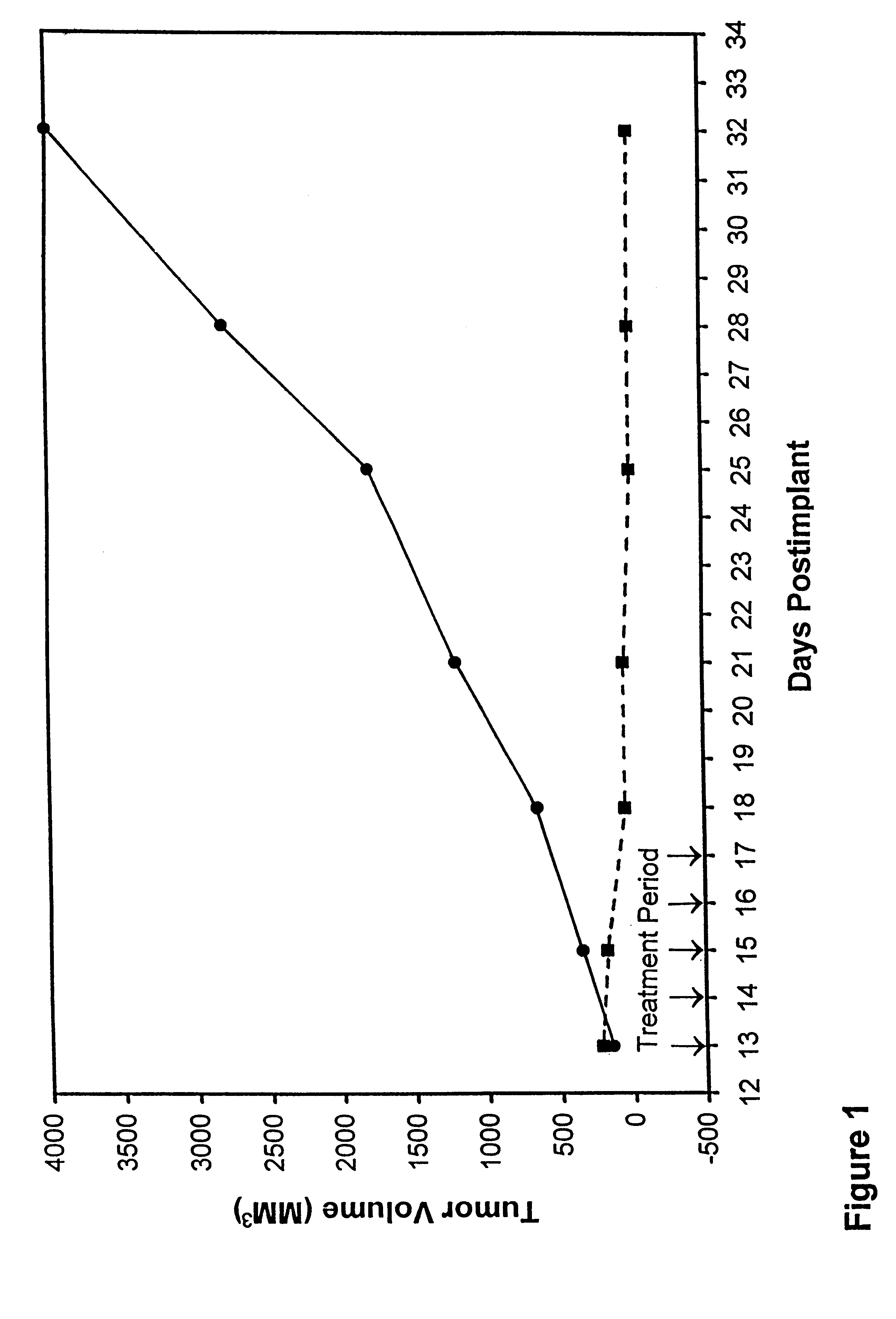
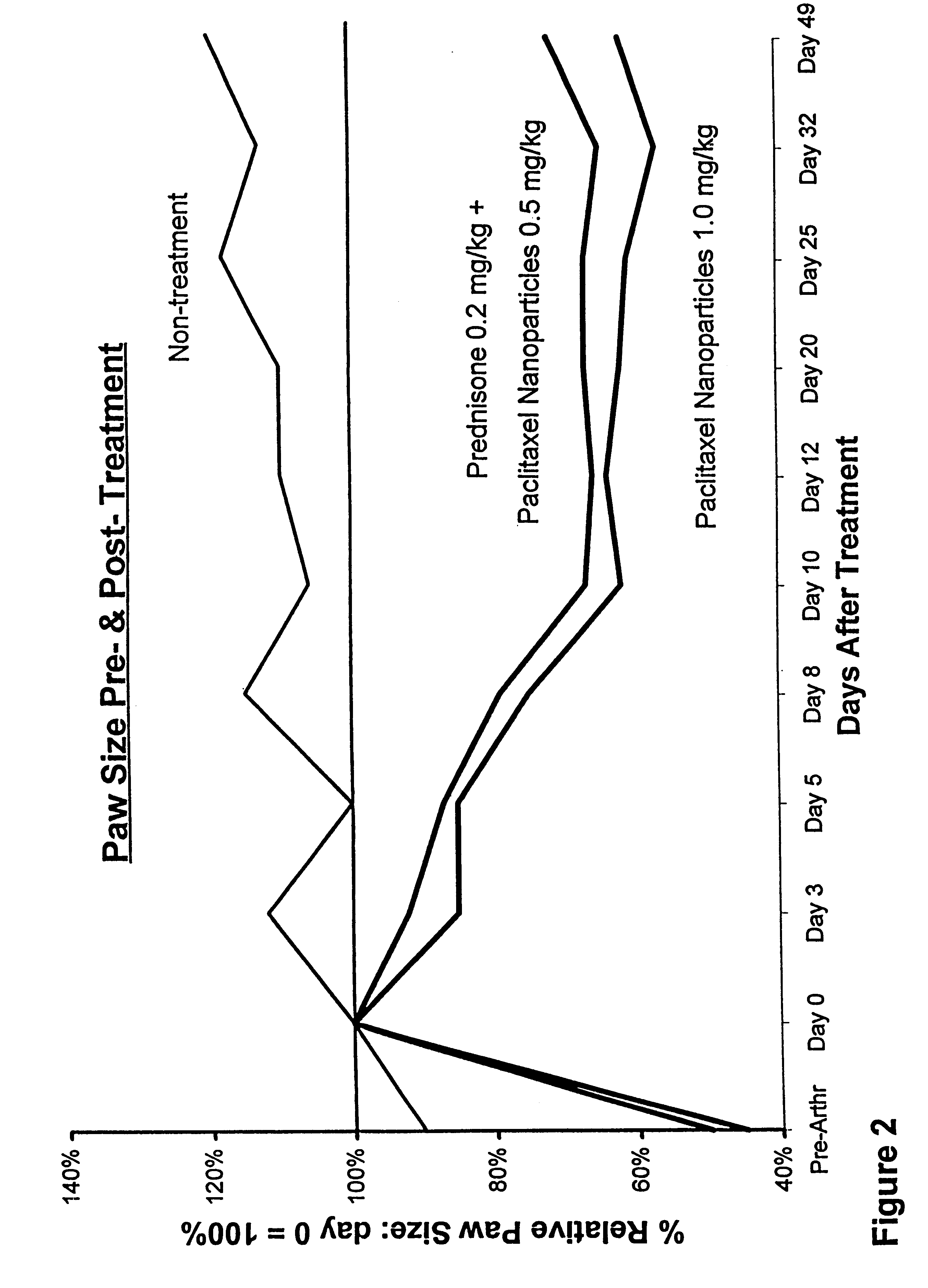
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Continuous analyte sensors and methods of making same
InactiveUS20110027458A1Low production costMinimize changesHot-dipping/immersion processesVacuum evaporation coatingAnalyteSolvent
Owner:DEXCOM
Porous polymeric matrices made of natural polymers and synthetic polymers and optionally at least one cation and methods of making
A porous polymeric matrix containing at least one natural polymer and at least one synthetic polymer and optionally at least one cation. Furthermore, a method of making a porous polymeric matrix involving mixing at least one natural polymer and inorganic salts with a solution comprising at least one solvent and at least one synthetic polymer to form a slurry, casting the slurry in a mold and removing the solvent to form solid matrices, immersing the solid matrices in deionized water to allow natural polymer cross-linking and pore creation to occur simultaneously, and drying the matrices to create a porous polymeric matrix; wherein the matrix contains a cation. Also, a method of making a porous polymeric matrix, involving mixing at least one natural polymer in an aqueous solvent and mixing at least one synthetic polymer in an organic solvent, combining the mixtures and casting in a mold, and separately removing said aqueous solvent and said organic solvent to form a porous polymeric matrix; wherein the porous polymeric matrix does not contain a cation.
Owner:US SEC AGRI
Continuous analyte sensors and methods of making same
InactiveUS20110024043A1Low production costMinimize changesHot-dipping/immersion processesPretreated surfacesAnalyteSolvent
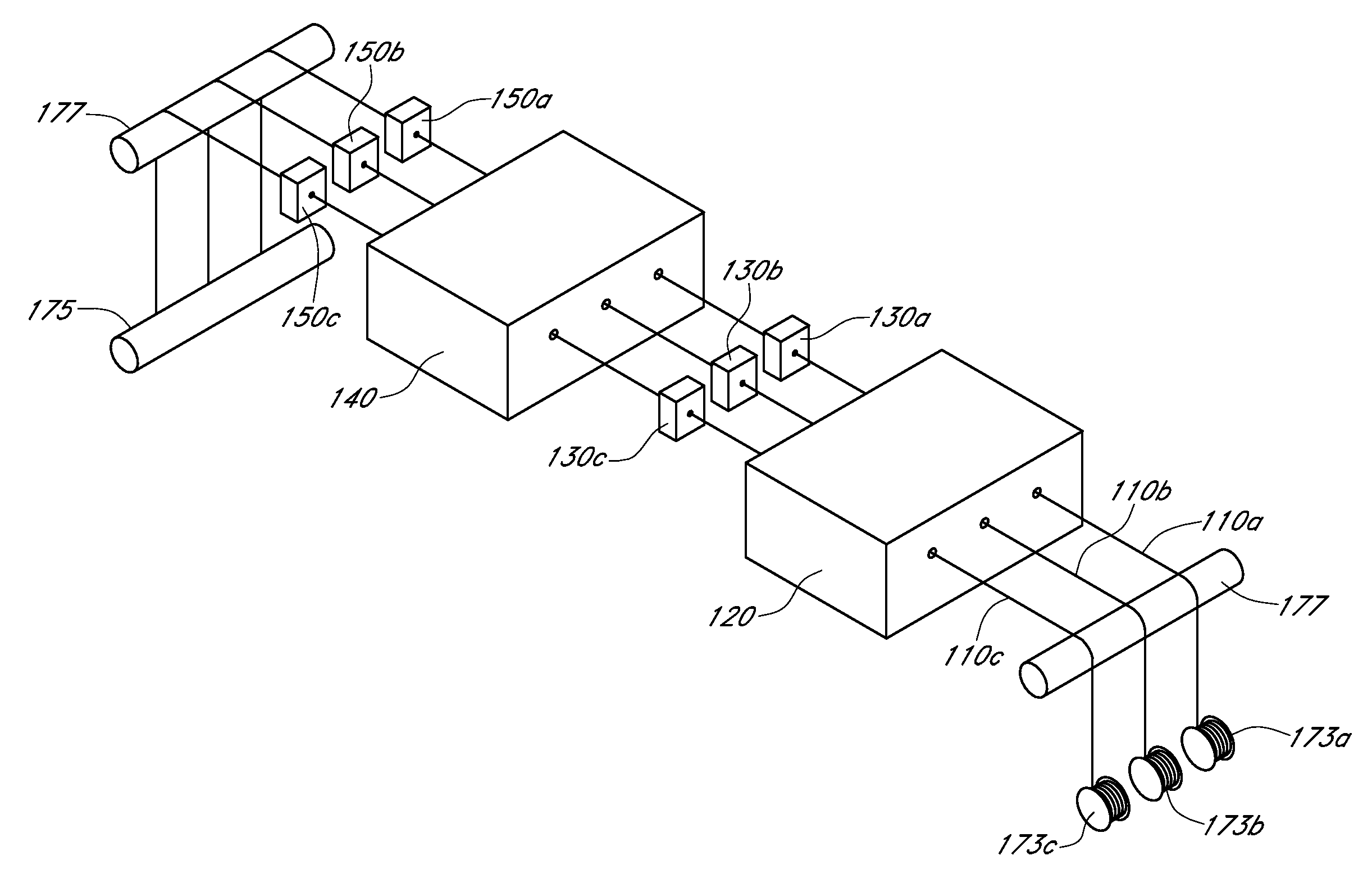
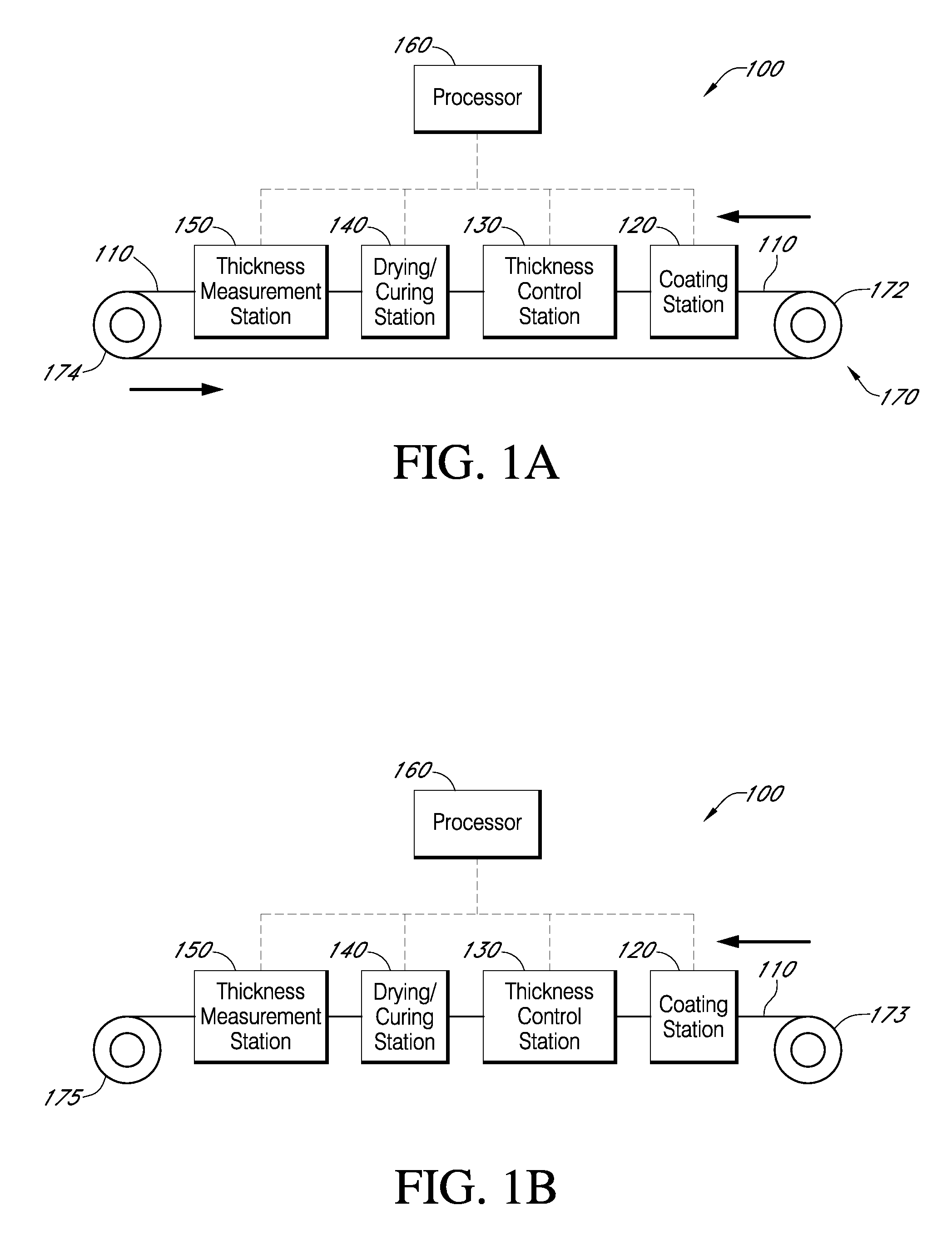
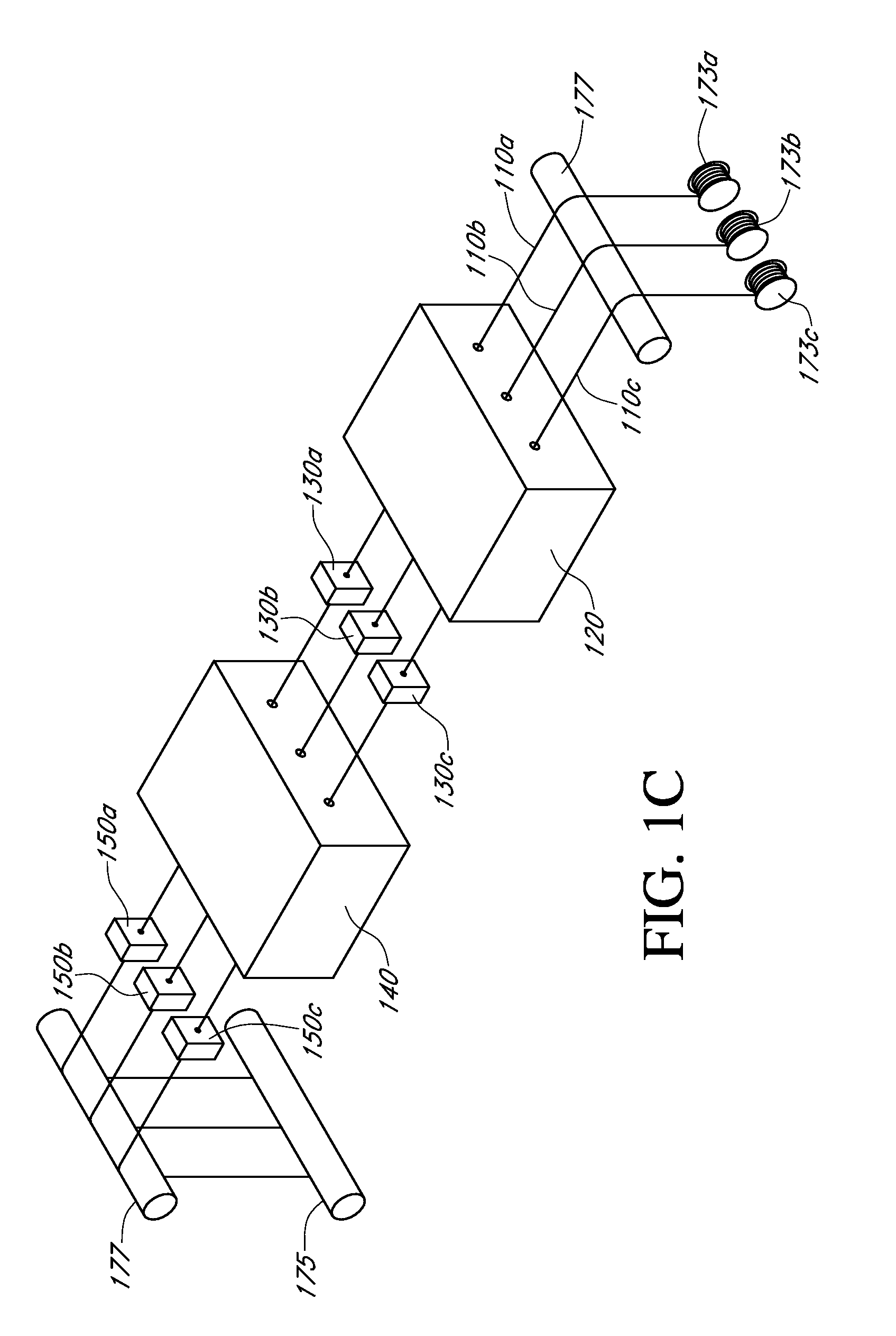
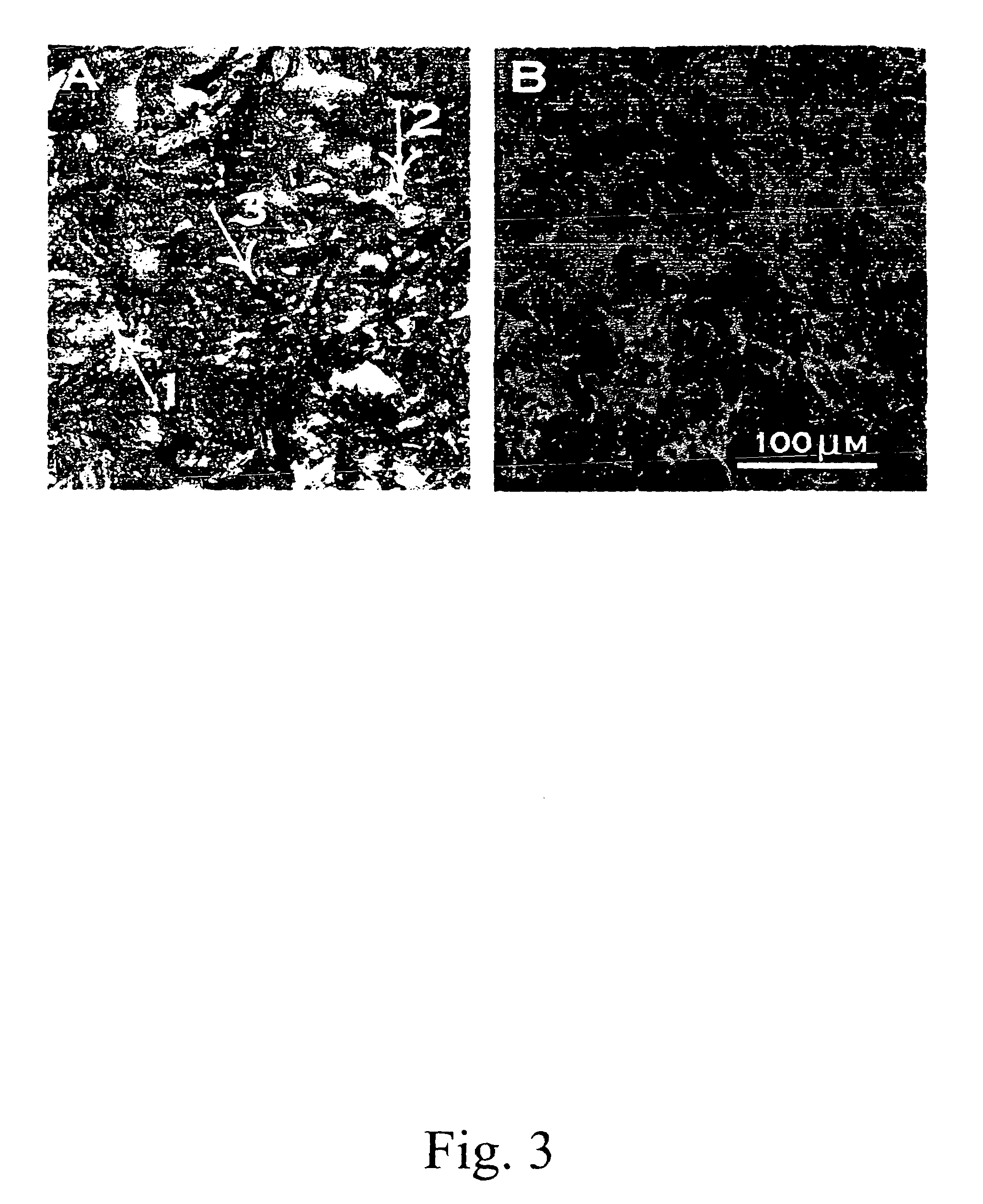
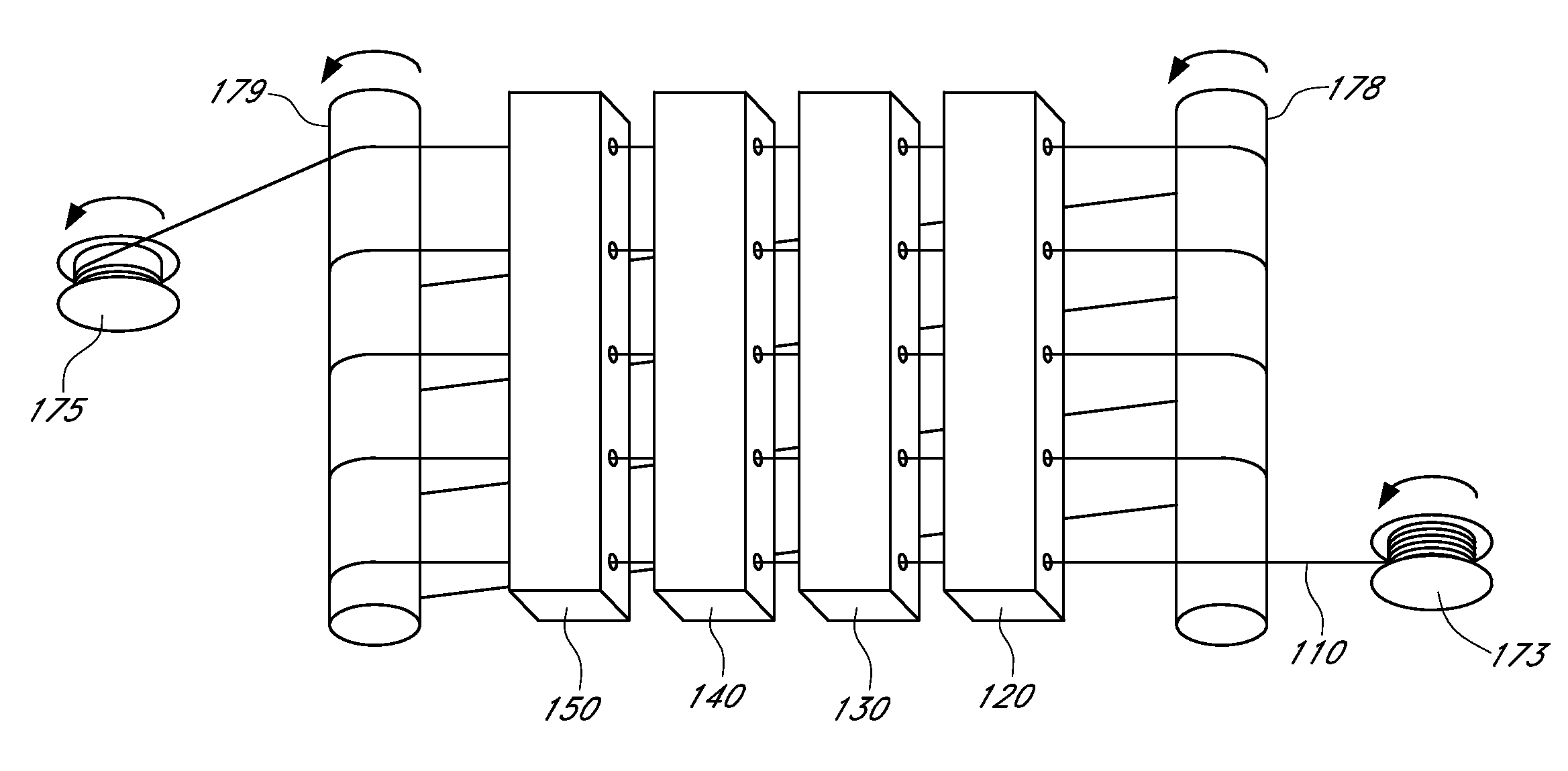
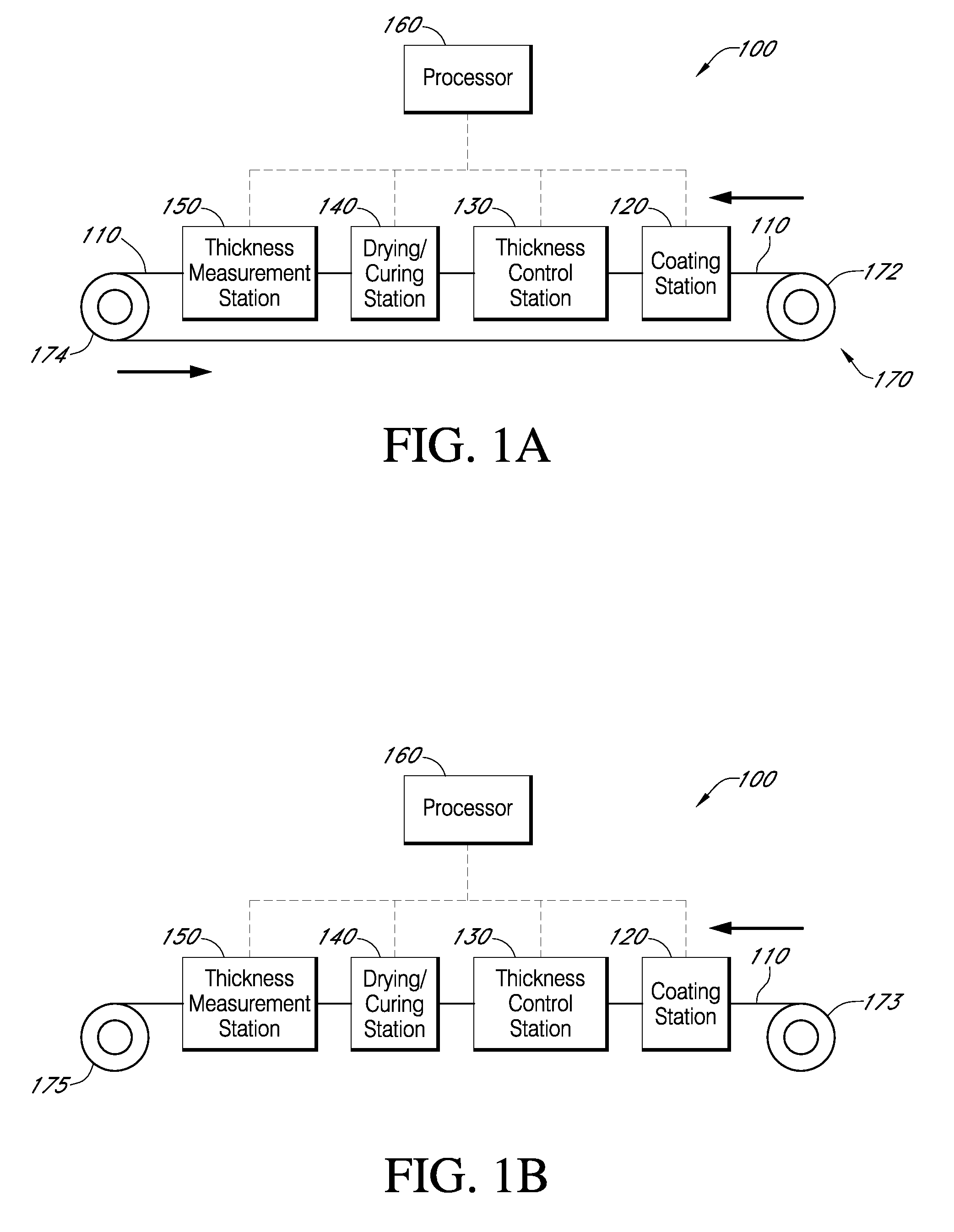
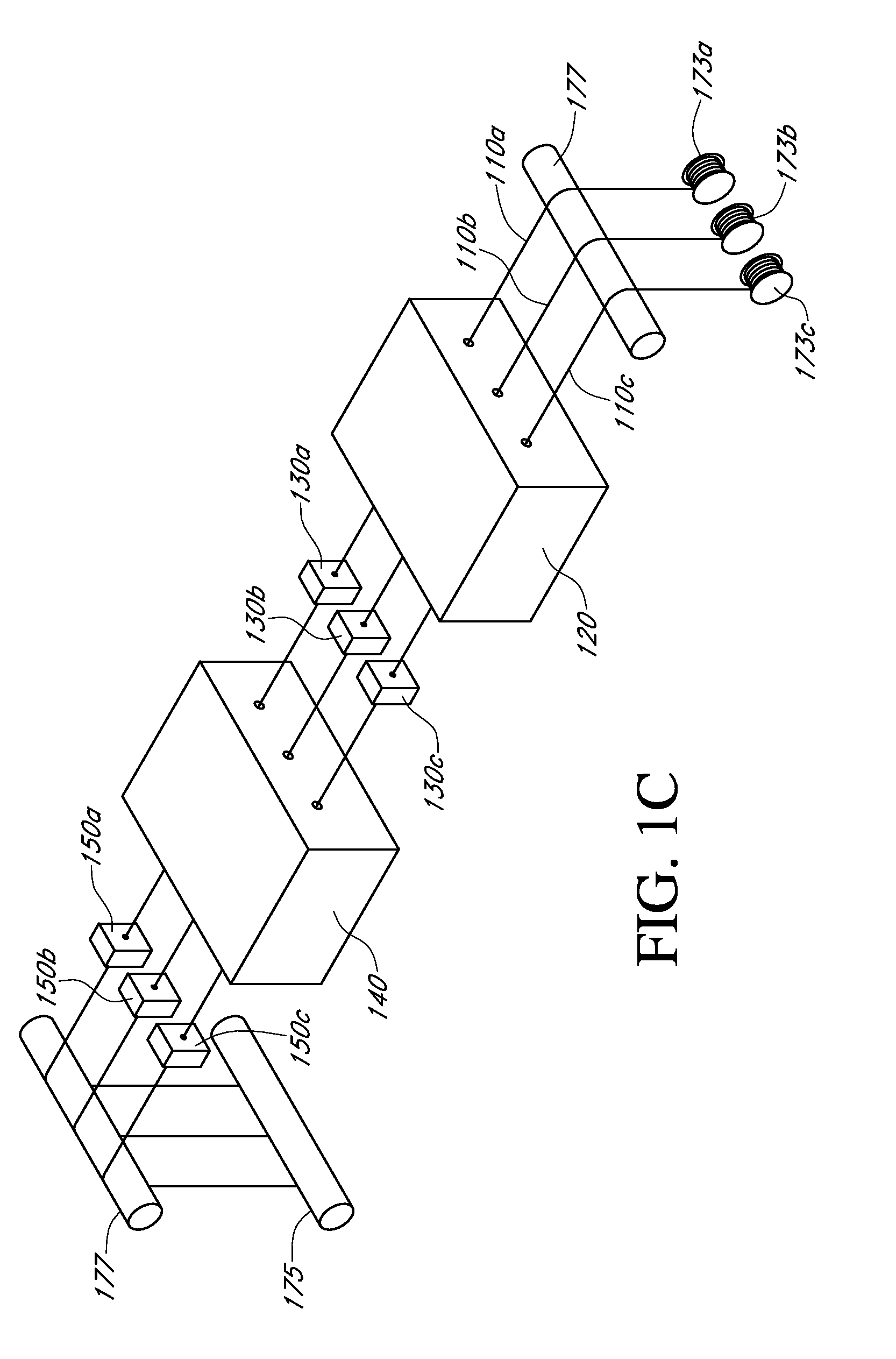
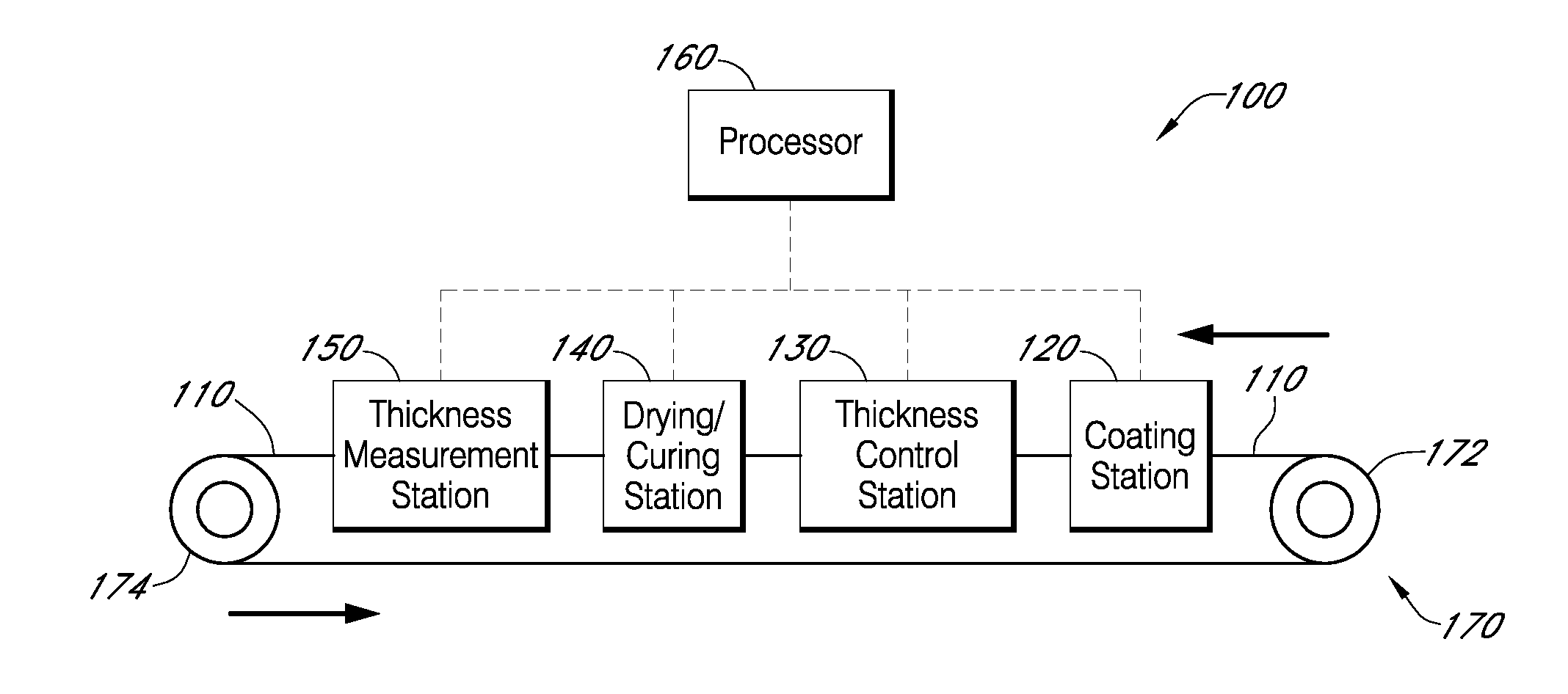
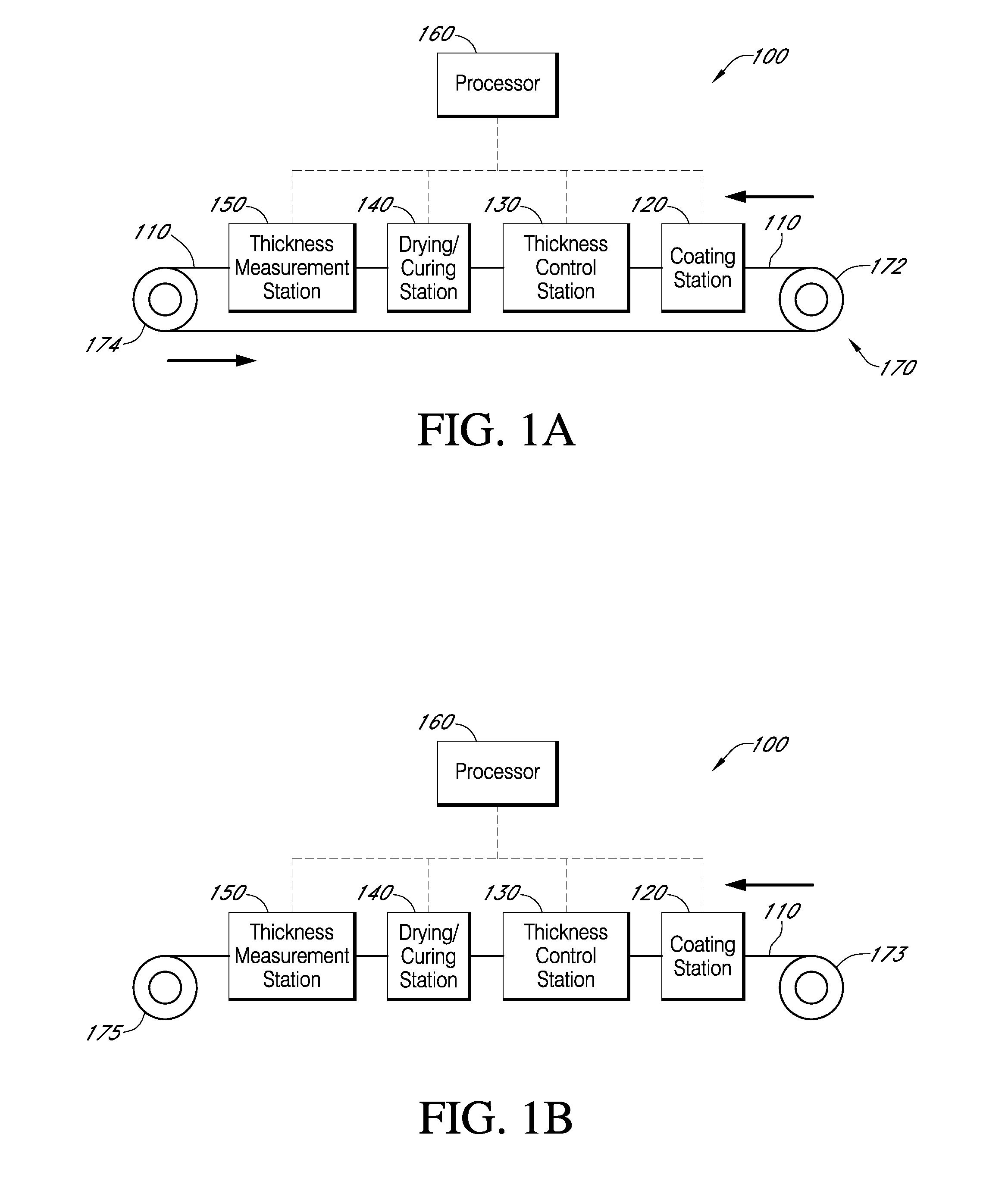
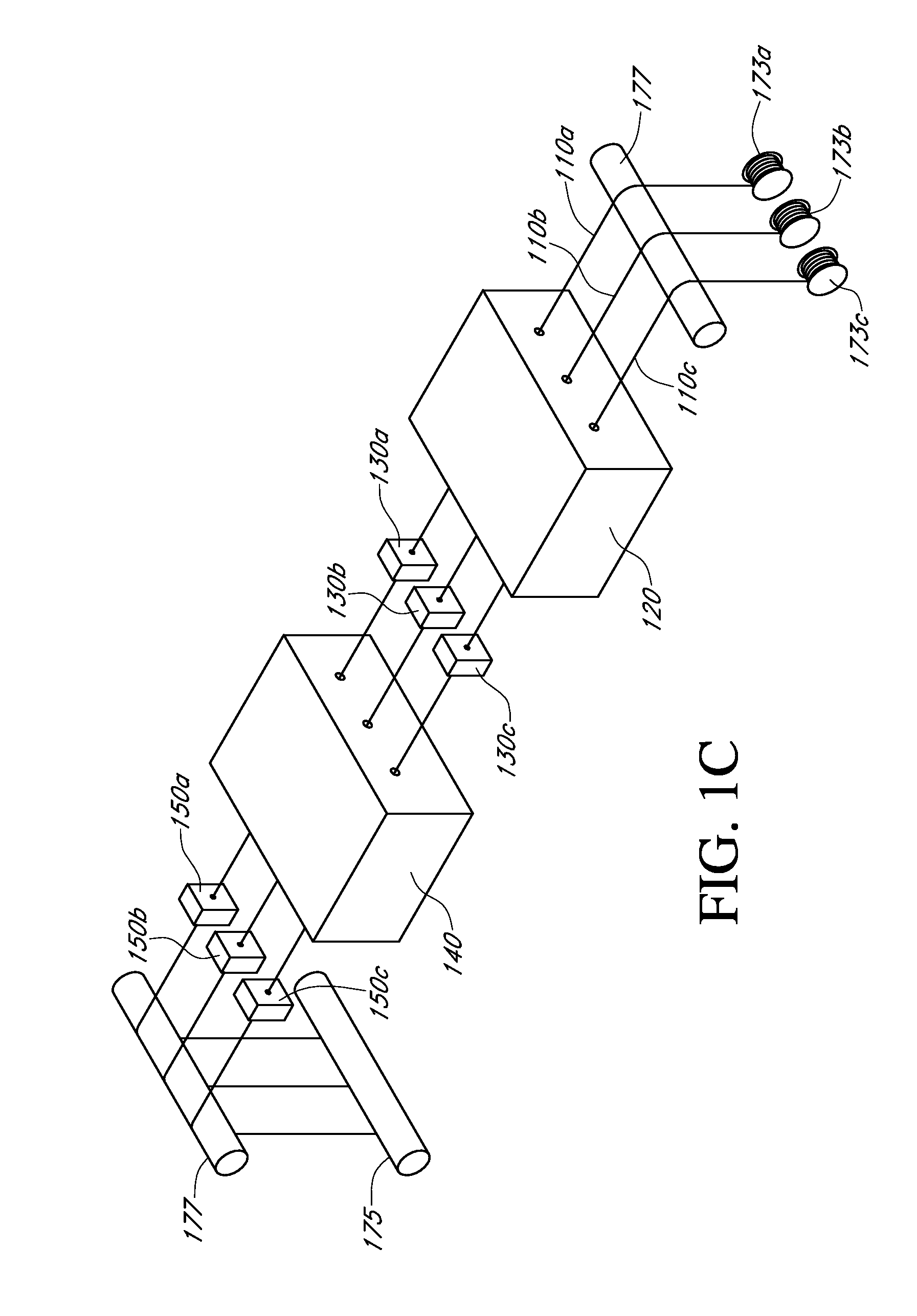
Described here are embodiments of processes and systems for the continuous manufacturing of implantable continuous analyte sensors. In some embodiments, a method is provided for sequentially advancing an elongated conductive body through a plurality of stations, each configured to treat the elongated conductive body. In some of these embodiments, one or more of the stations is configured to coat the elongated conductive body using a meniscus coating process, whereby a solution formed of a polymer and a solvent is prepared, the solution is continuously circulated to provide a meniscus on a top portion of a vessel holding the solution, and the elongated conductive body is advanced through the meniscus. The method may also comprise the step of removing excess coating material from the elongated conductive body by advancing the elongated conductive body through a die orifice. For example, a provided elongated conductive body 510 is advanced through a pre-coating treatment station 520, through a coating station 530, through a thickness control station 540, through a drying or curing station 550, through a thickness measurement station 560, and through a post-coating treatment station 570.
Owner:DEXCOM
Continuous analyte sensors and methods of making same
InactiveUS20110027453A1Low production costMinimize changesHot-dipping/immersion processesPharmaceutical containersAnalyteSolvent
Described here are embodiments of processes and systems for the continuous manufacturing of implantable continuous analyte sensors. In some embodiments, a method is provided for sequentially advancing an elongated conductive body through a plurality of stations, each configured to treat the elongated conductive body. In some of these embodiments, one or more of the stations is configured to coat the elongated conductive body using a meniscus coating process, whereby a solution formed of a polymer and a solvent is prepared, the solution is continuously circulated to provide a meniscus on a top portion of a vessel holding the solution, and the elongated conductive body is advanced through the meniscus. The method may also comprise the step of removing excess coating material from the elongated conductive body by advancing the elongated conductive body through a die orifice. For example, a provided elongated conductive body 510 is advanced through a pre-coating treatment station 520, through a coating station 530, through a thickness control station 540, through a drying or curing station 550, through a thickness measurement station 560, and through a post-coating treatment station 570.
Owner:DEXCOM
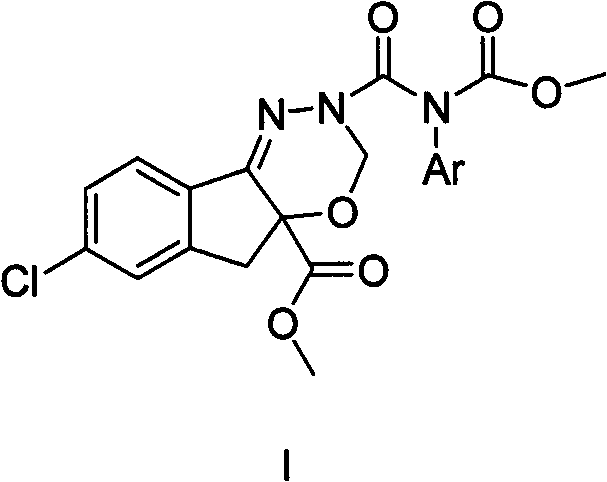
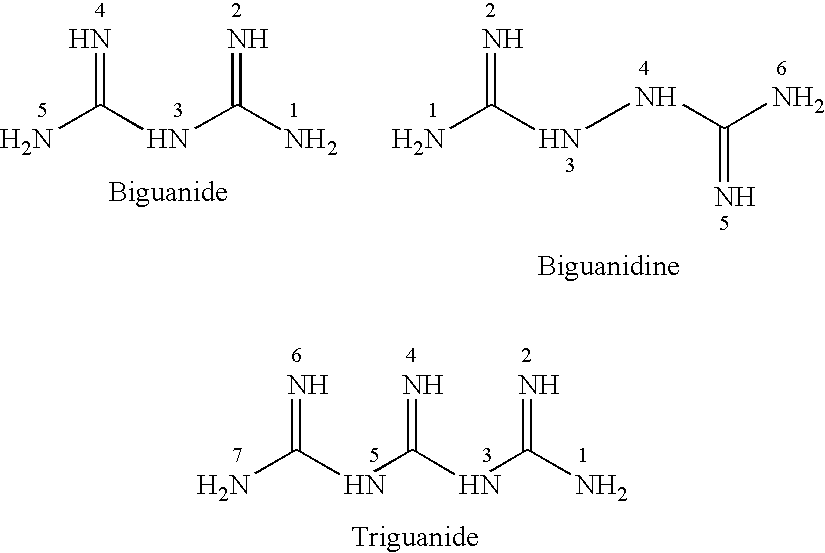
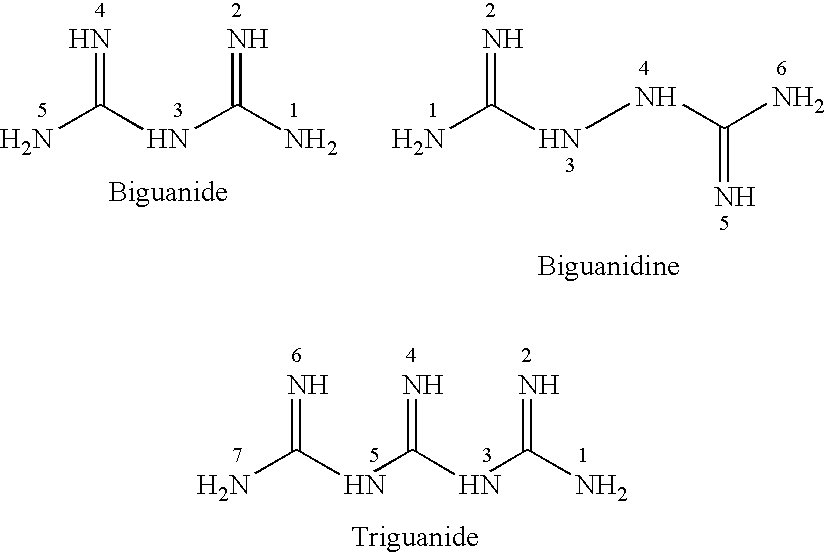
N-substituted dioxazine compound as well as preparation method and application thereof
The invention relates to an N-substituted dioxazine compound as well as a preparation method and application thereof. The N-substituted dioxazine compound has the following chemical structure general formula (I), wherein Ar in the chemical structure general formula (I) is a benzene ring, a naphthalene ring, and a pyridine or pyrimidine ring system. The compound is prepared with the following two steps: (1) taking 2-(benzyl)-7-benzindene[1,2-e][1,3-4] dioxazine-2,4a(3H,5H)-dicarboxylic acid-4a-methoxycarbonyl group and hydrogen as raw materials, taking Pd / C in solvent a as catalyst a, and carrying out hydrogenation, thus obtaining an intermediate B after reaction is finished; (2) taking (carbonylchloride) (aryl) methyl carhamate as the intermediate C, and carrying out (carbonylchloride) (aryl) methyl carhamate with the intermediate B obtained in the step (1) in the presence of a catalyst b in the solvent b; and obtaining the product N-substituted dioxazine compound. Compared with the prior art, the insecticidalactivity on the armyworm by the N-substituted dioxazine compound is 80-100%.
Owner:SHANGHAI JIAO TONG UNIV +1
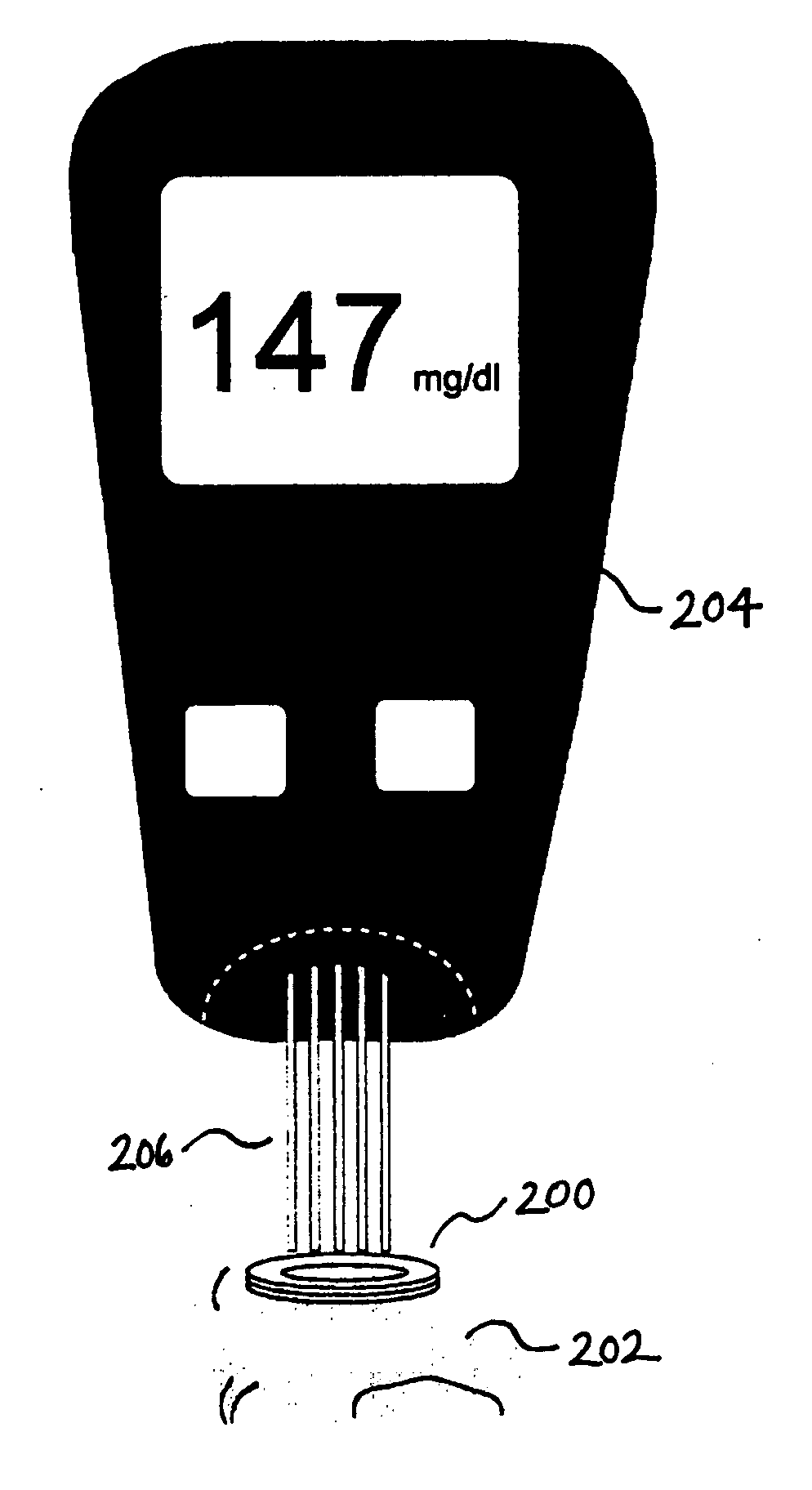
Devices, methods, and kits for non-invasive glucose measurement
InactiveUS20060004271A1Minimize impactMicrobiological testing/measurementSurgeryMeasurement deviceDisplay device
Described are devices, methods, and kits for non-invasively measuring glucose. In general, the devices comprise skin patches for placement on a skin surface and measurement devices for measuring glucose collected in the patches. The patches may include an adhesive material, a collection layer, an interface layer, and a sweat-permeable membrane. The sweat-permeable membrane is configured to act as a barrier to epidermal contaminants and glucose brought to the skin surface via diffusion. In this way, non-correlatable skin surface glucose will not be measured. The patches may further include components to induce a local sweat response. The measurement device typically includes a display, a processor, and a measurement mechanism. The methods typically include the steps of wiping the skin surface with a wipe containing at least one solvent for removing glucose, placing a patch on a skin surface, and measuring glucose collected in the patch. Kits comprising the patch and measurement device are also described.
Owner:VIVOMEDICAL INC
Method for forming silazane-based dielectric film
A method of forming a dielectric film includes: introducing a source gas essentially constituted by Si, N, H, and optionally C and having at least one bond selected from Si—N, Si—Si, and Si—H into a reaction chamber where a substrate is placed; depositing a silazane-based film essentially constituted by Si, N, H, and optionally C on the substrate by plasma reaction at −50° C. to 50° C., wherein the film is free of exposure of a solvent constituted essentially by C, H, and optionally O; and heat-treating the silazane-based film on the substrate in a heat-treating chamber while introducing an oxygen-supplying source into the heat-treating chamber to release C from the film and increase Si—O bonds in the film.
Owner:ASM JAPAN
Methods of making garments having stretchable and conductive ink
InactiveUS20140318699A1Stable and continuous positioningRobust detectionPrinted circuit manufactureResistor manufactureAdhesiveSolvent
Methods of forming garments having one or more stretchable conductive ink patterns. Described herein are method of making garments (including compression garments) having one or more highly stretchable conductive ink pattern formed of a composite of an insulative adhesive, a conductive ink, and an intermediate gradient zone between the adhesive and conductive ink. The conductive ink typically includes between about 40-60% conductive particles, between about 30-50% binder; between about 3-7% solvent; and between about 3-7% thickener. The stretchable conductive ink patterns may be stretched more than twice their length without breaking or rupturing.
Owner:L I F E
Method of making an organic thin film transistor
A process for fabricating thin film transistors in which the active layer is an organic semiconducting material with a carrier mobility greater than 10-3 cm2 / Vs and a conductivity less than about 10-6 S / cm at 20 DEG C. is disclosed. The organic semiconducting material is a regioregular (3-alkylthiophene) polymer. The organic semiconducting films are formed by applying a solution of the regioregular polymer and a solvent over the substrate. The poly (3-alkylthiophene) films have a preferred orientation in which the thiophene chains has a planar stacking so the polymer backbone is generally parallel to the substrate surface.
Owner:BELL SEMICON LLC +2
Method for forming silazane-based dielectric film
A method of forming a dielectric film includes: introducing a source gas essentially constituted by Si, N, H, and optionally C and having at least one bond selected from Si—N, Si—Si, and Si—H into a reaction chamber where a substrate is placed; depositing a silazane-based film essentially constituted by Si, N, H, and optionally C on the substrate by plasma reaction at −50° C. to 50° C., wherein the film is free of exposure of a solvent constituted essentially by C, H, and optionally O; and heat-treating the silazane-based film on the substrate in a heat-treating chamber while introducing an oxygen-supplying source into the heat-treating chamber to release C from the film and increase Si—O bonds in the film.
Owner:ASM JAPAN
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Method of preparing nano-structured surface coatings and coated articles
InactiveUS7892606B2Improve adhesionMore readily manufacturableMaterial nanotechnologyNanostructure manufactureCross-linkNano structuring
Owner:DSM IP ASSETS BV
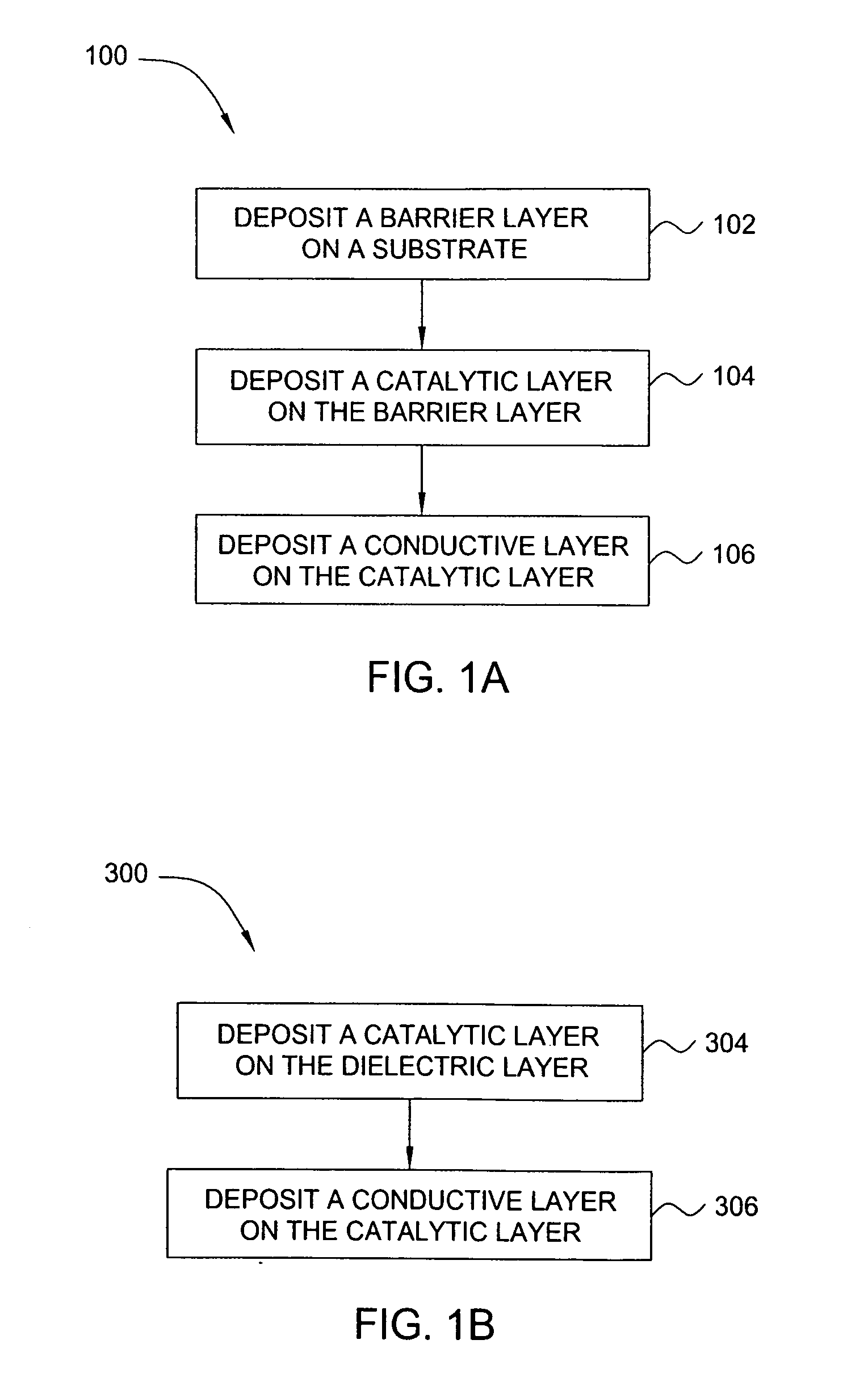
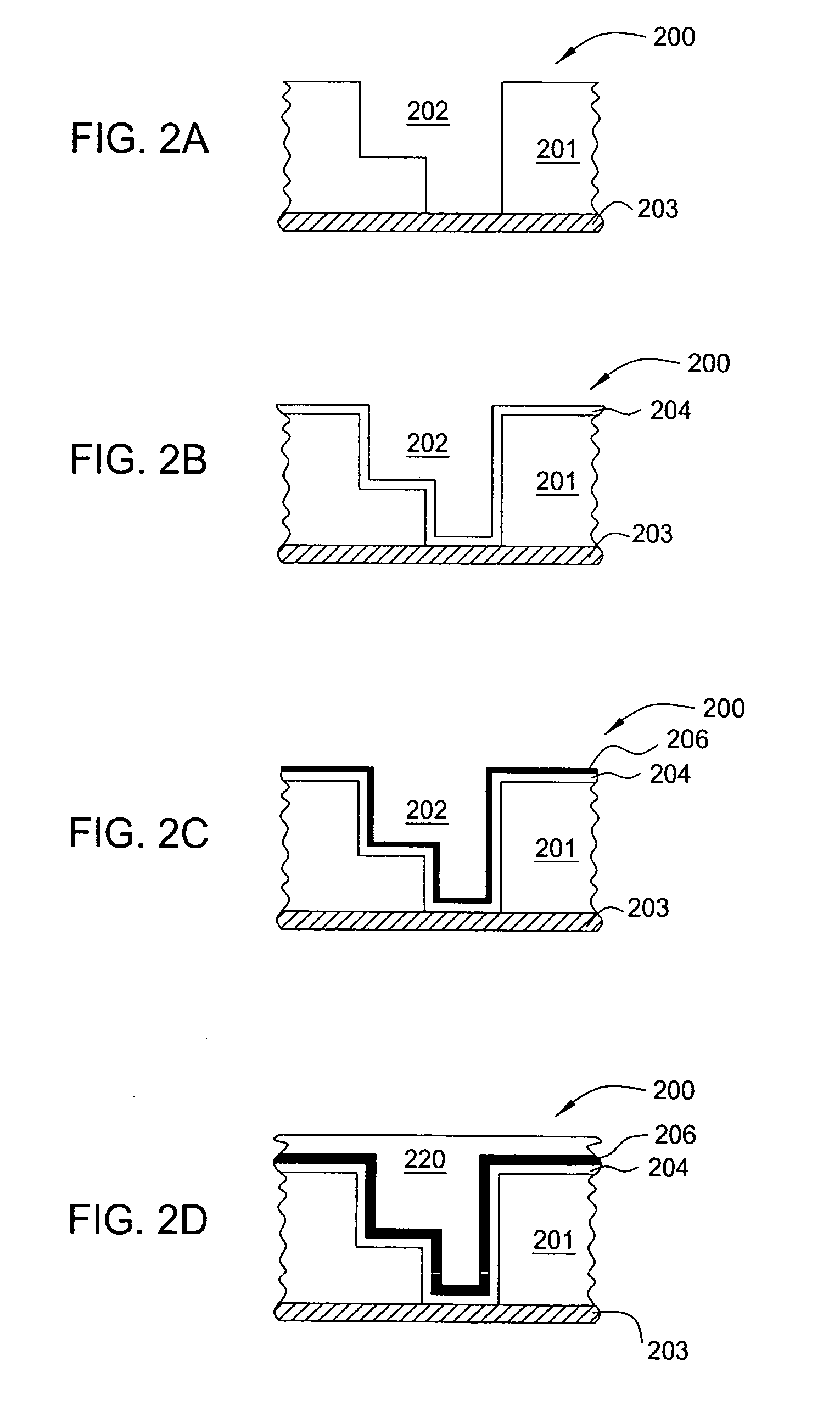
Ruthenium containing layer deposition method
ActiveUS20060165892A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingTemperature controlSource material
An exemplary apparatus and method of forming a ruthenium tetroxide containing gas to form a ruthenium containing layer on a surface of a substrate is described herein. The method and apparatus described herein may be especially useful for fabricating electronic devices that are formed on a surface of the substrate or wafer. Generally, the method includes exposing a surface of a substrate to a ruthenium tetroxide vapor to form a catalytic layer on the surface of a substrate and then filling the device structures by an electroless, electroplating, physical vapor deposition (PVD), chemical vapor deposition (CVD), plasma enhanced CVD (PECVD), atomic layer deposition (ALD) or plasma enhanced ALD (PE-ALD) processes. In one embodiment, the ruthenium containing layer is formed on a surface of a substrate by creating ruthenium tetroxide in an external vessel and then delivering the generated ruthenium tetroxide gas to a surface of a temperature controlled substrate positioned in a processing chamber. In one embodiment, a ruthenium tetroxide containing solvent formation process is used to form ruthenium tetroxide using a ruthenium tetroxide containing source material. In one embodiment, of a ruthenium containing layer is formed on a surface of a substrate, using the ruthenium tetroxide containing solvent. In another embodiment, the solvent is separated from the ruthenium tetroxide containing solvent mixture and the remaining ruthenium tetroxide is used to form a ruthenium containing layer on the surface of a substrate.
Owner:APPLIED MATERIALS INC
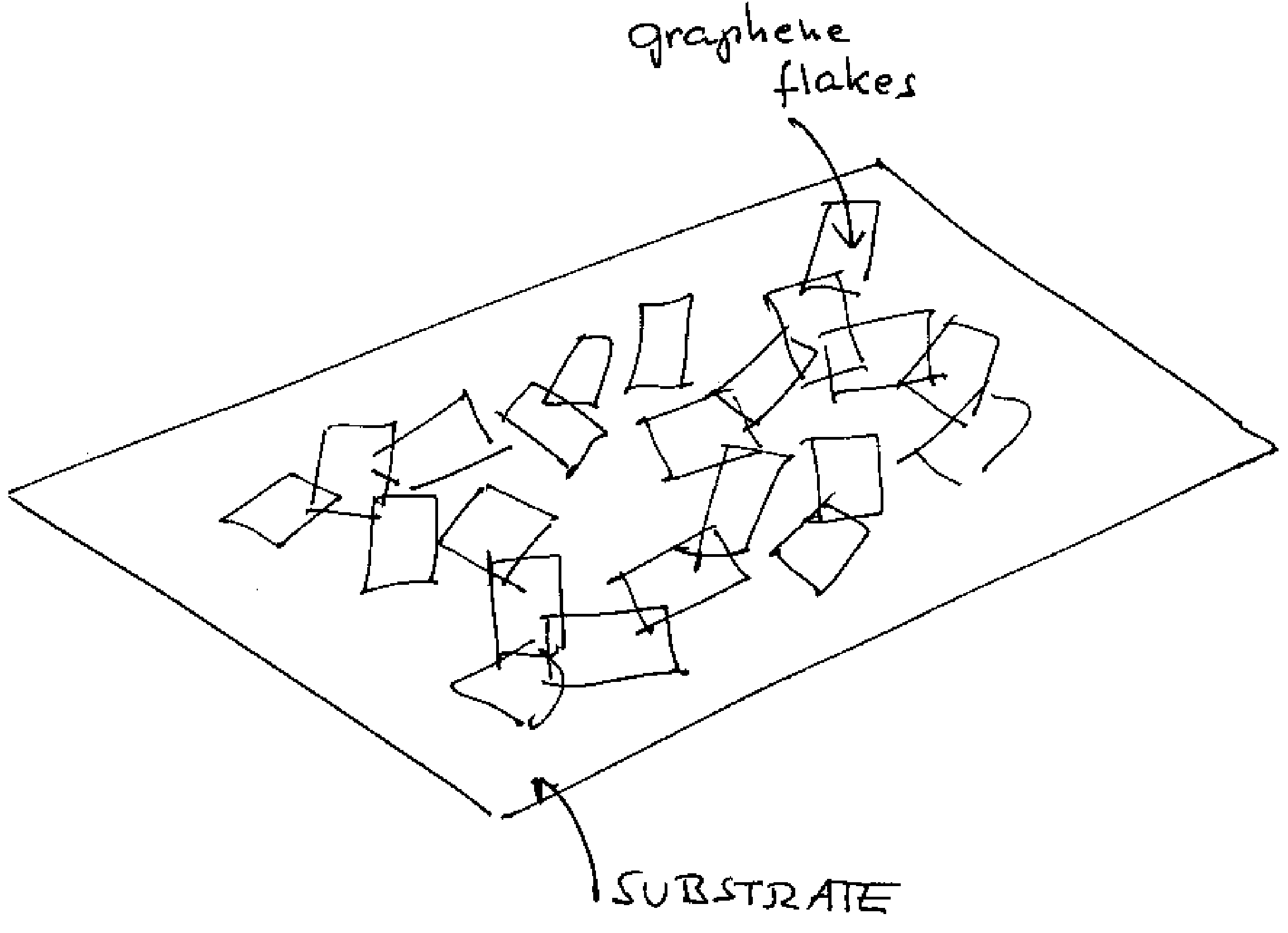
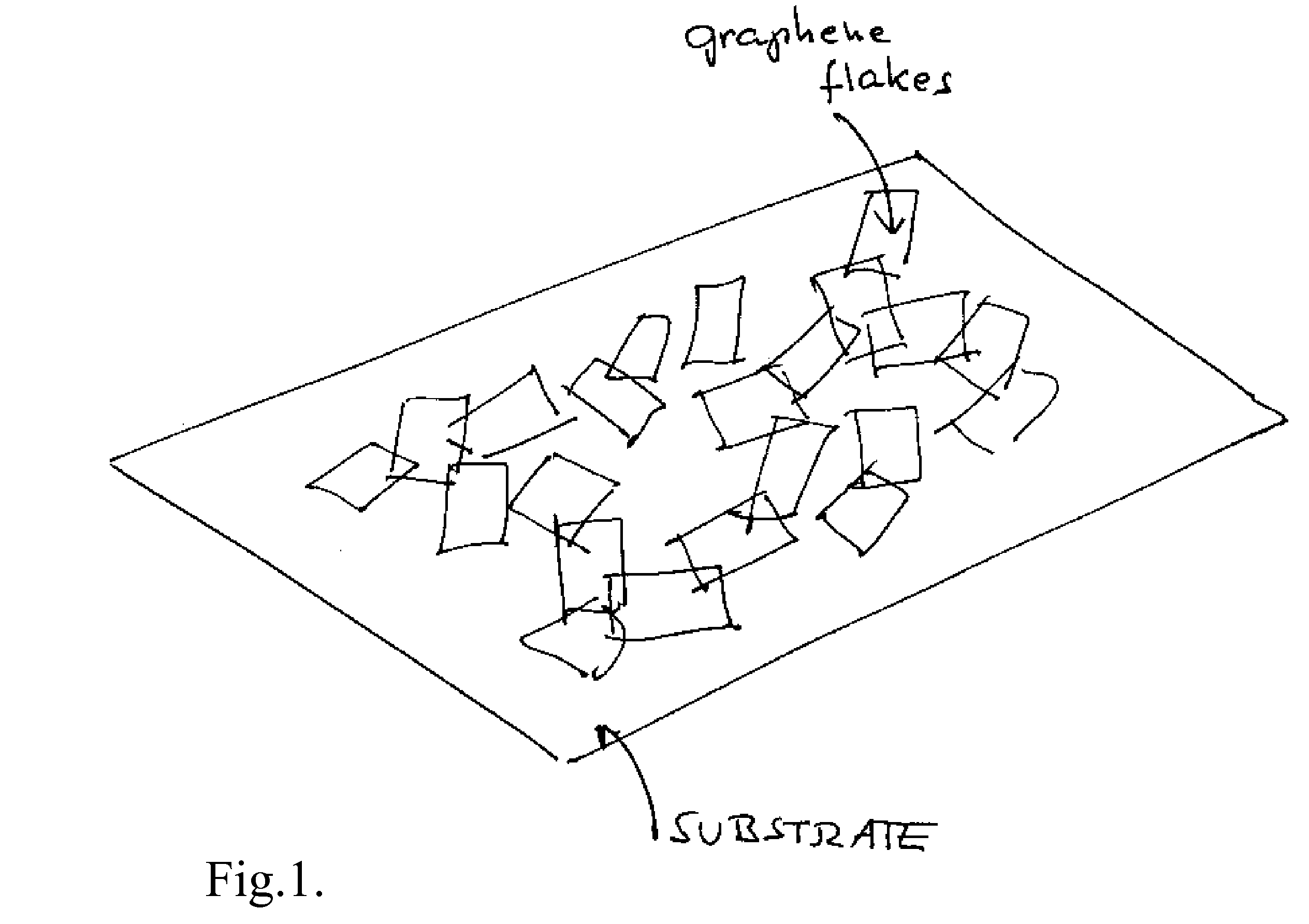
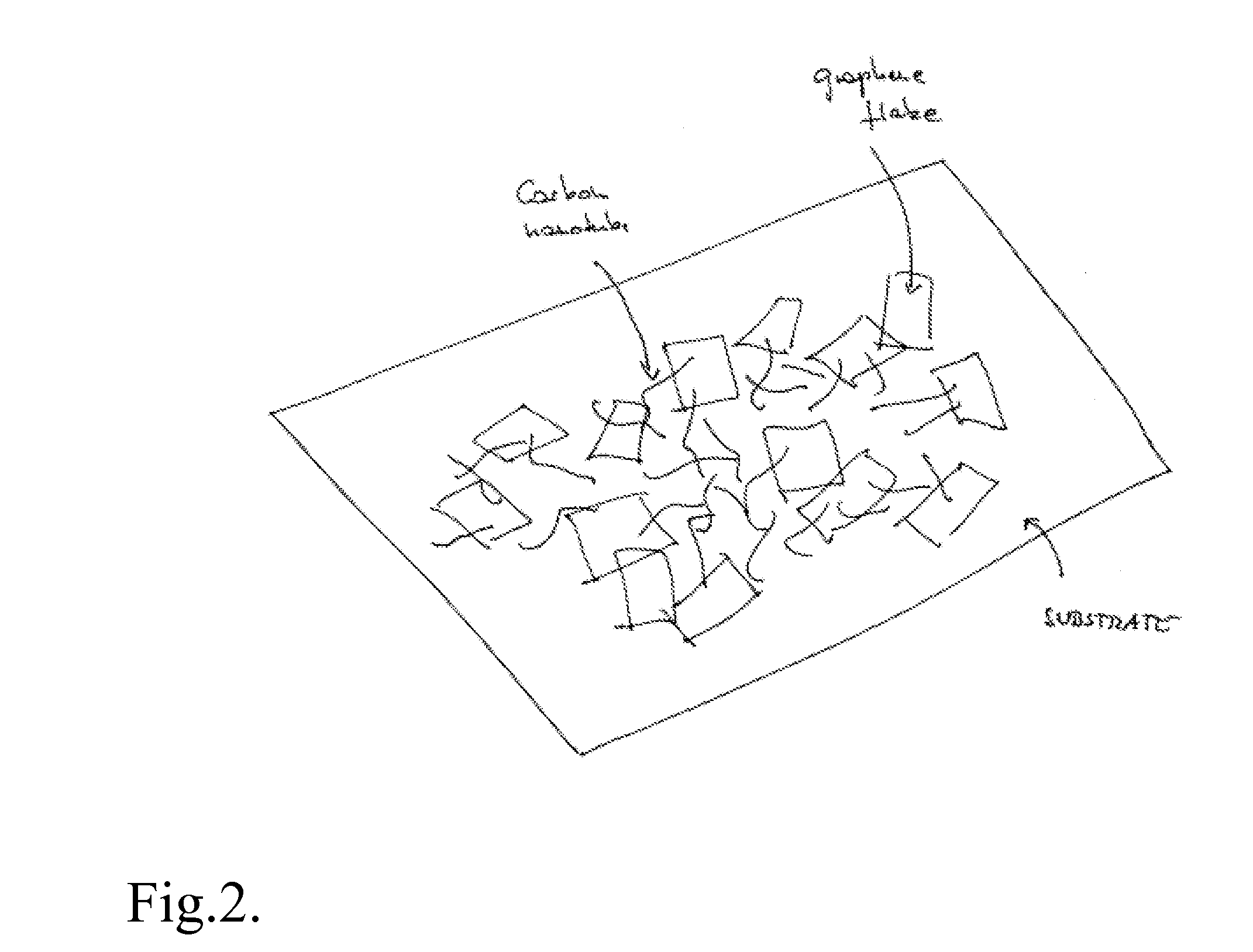
Graphene film as transparent and electrically conducting material
ActiveUS20070284557A1Material nanotechnologyConductive materialGraphene flakeTransparent conducting film
A transparent and conductive film comprising at least one network of graphene flakes is described herein. This film may further comprise an interpenetrating network of other nanostructures, a polymer and / or a functionalization agent(s). A method of fabricating the above device is also described, and may comprise depositing graphene flakes in solution and evaporating solvent therefrom.
Owner:SAMSUNG ELECTRONICS CO LTD
Antibiotic kit and composition and uses thereof
The present invention relates to a therapeutic kit to provide a safe and effective dosage of an antibiotic agent, including an aerosol packaging assembly including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam, wherein the pressurized product comprises a foamable composition including: an antibiotic agent; at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, an organic polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight, a surface-active agent, about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent, water; and liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Antimicrobial silver compositions
The present invention comprises methods and compositions for antimicrobial silver compositions comprising silver nanoparticles. The present invention further comprises compositions for preparing silver nanoparticles comprising at least one stabilizing agent, one or more silver compounds, at least one reducing agent and a solvent. In one aspect, the stabilizing agent comprises a surfactant or a polymer. The polymer may comprise polymers such as polyacrylamides, polyurethanes, and polyamides. In one aspect, the silver compound comprises a salt comprising a silver cation and an anion. The anion may comprise saccharinate derivatives, long chain fatty acids, and alkyl dicarboxylates. The methods of the present invention comprise treating devices with the silver nanoparticle compositions, including, but not limited to, such devices as woven wound care materials, catheters, patient care devices, and collagen matrices. The present invention further comprises treatment of humans and animals wacr6ith the antimicrobial devices described herein.
Owner:AVENT INC
Composition for forming resist overlayer film for EUV lithography
ActiveUS20130209940A1High resolutionSemiconductor/solid-state device manufacturingCoatingsLithographic artistLithography process
There is provided a composition for forming an EUV resist overlayer film that is used in an EUV lithography process, that does not intermix with the EUV resist, that blocks unfavorable exposure light for EUV exposure, for example, UV light and DUV light and selectively transmits EUV light alone, and that can be developed with a developer after exposure. A composition for forming an EUV resist overlayer film used in an EUV lithography process including a resin containing a naphthalene ring in a main chain or in a side chain and a solvent, in which the resin may include a hydroxy group, a carboxy group, a sulfo group, or a monovalent organic group having at least one of these groups as a hydrophilic group.
Owner:NISSAN CHEM IND LTD
Materials and methods of forming controlled void
ActiveUS20080038934A1Solid-state devicesSemiconductor/solid-state device manufacturingPorous layerSolvent
The present invention is a process for forming an air gap within a substrate, the process comprising: providing a substrate; depositing a sacrificial material by deposition of at least one sacrificial material precursor; depositing a composite layer; removale of the porogen material in the composite layer to form a porous layer and contacting the layered substrate with a removal media to substantially remove the sacrificial material and provide the air gaps within the substrate; wherein the at least one sacrificial material precursor is selected from the group consisting of: an organic porogen; silicon, and a polar solvent soluble metal oxide and mixtures thereof.
Owner:VERSUM MATERIALS US LLC
Positive resist composition and pattern-forming method
ActiveUS20080248425A1Suppress generationPhotosensitive materialsRadiation applicationsPolymer scienceActinic Rays
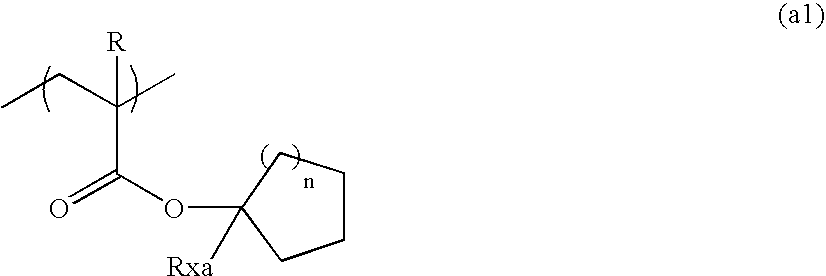
A positive resist composition comprises: (A) a resin that has a repeating unit represented by general formula (a1) and increases its solubility in an alkali developer by action of an acid; (B) a compound which generates an acid upon irradiation with an actinic ray or a radiation; and (C) a resin that has at least one of a fluorine atom and a silicon atom and has a group selected from the group consisting of (x), (y) and (z); and (D) a solvent:(x) an alkali-soluble group;(y) a group capable that decomposes by action of an alkali developer to undergo an increase in a solubility of the resin (C) in an alkali developer; and(z) a group that decomposes by action of an acid,wherein R represents a hydrogen atom or a methyl group, Rxa represents an alkyl group or a cycloalkyl group, and n represents an integer of 1 to 8.
Owner:FUJIFILM CORP
Imageable element with solvent-resistant polymeric binder
InactiveUS20050003285A1Enhances on-press solvent resistanceProlong lifeRadiation applicationsSemiconductor/solid-state device manufacturingSolventPendant group
The present invention provides an imageable element including a lithographic substrate and an imageable layer disposed on the substrate. The imageable layer includes a radically polymerizable component, an initiator system capable of generating radicals sufficient to initiate a polymerization reaction upon exposure to imaging radiation, and a polymeric binder having a hydrophobic backbone and including both constitutional units having a pendant cyano group attached directly to the hydrophobic backbone, and constitutional units having a pendant group including a hydrophilic poly(alkylene oxide) segment. When the imageable element is imaged and developed, the resulting printing plate may exhibit improved on-press solvent resistance and longer press life.
Owner:KODAK POLYCHROME GRAPHICS
Compositions for rapid and non-irritating transdermal delivery of pharmaceutically active agents and methods for formulating such compositions and delivery thereof
InactiveUS6444234B1Effective therapeutic doseReduced barrier effectBiocideCosmetic preparationsSolventActive agent
Pharmaceutical compositions for the transdermal administration of a medicament or other active agent by topical application of the composition to the skin of humans or other animals are described. Methodology for formulating such compositions which provide for very rapid uptake of the medicament and transmigration into and through the skin to either fatty tissues or the vascular system, while minimizing irritation to the skin and / or immunological response, is based on a transdermal delivery system (TDS) wherein the medicament is modified to form a true solution in a complex formed from particular solvents and solvent and solute modifiers in combination with skin stabilizers. Uptake of the medicament is further facilitated and made more rapid by including Forskolin or other source of cellular energy, namely induction of cAMP or cGMP. Selection of specific solvents and solvent and solute modifiers and other functional ingredients and the amounts thereof are chosen such that there is a balance between the sum of the mole-moments [(molar amount of each individual ingredient)x(dipole moment of that ingredient)] of the delivery system and the sum of the moler moments of the composition in which the medicament is dissolved. Preferably, the van der Waals forces of the delivery system is also similarly matched to the van der Waals forces of the total composition, namely, delivery system plus active agent.
Owner:TRANSDERMAL DELIVERY SOLUTIONS
Recovery of organic acids
InactiveUS20090281354A1Promote recoveryOvercome consumptionPreparation from carboxylic acid saltsOrganic compound preparationOrganic acidReactive distillation
A method is disclosed for the recovery of an organic acid from a dilute salt solution in which the cation of the salt forms an insoluble carbonate salt. An amine and CO2 are introduced to the solution to form the insoluble carbonate salt and a complex between the acid and the amine. The acid / amine complex is thermally dissociated, or “cracked”, in the presence of a water immiscible solvent in which the amine is selectively soluble and in which the acid is not appreciably soluble. The organic acid may then be recovered from the water by any suitable means such as distillation, reactive distillation, extraction, or reactive extraction.
Owner:ZEACHEM
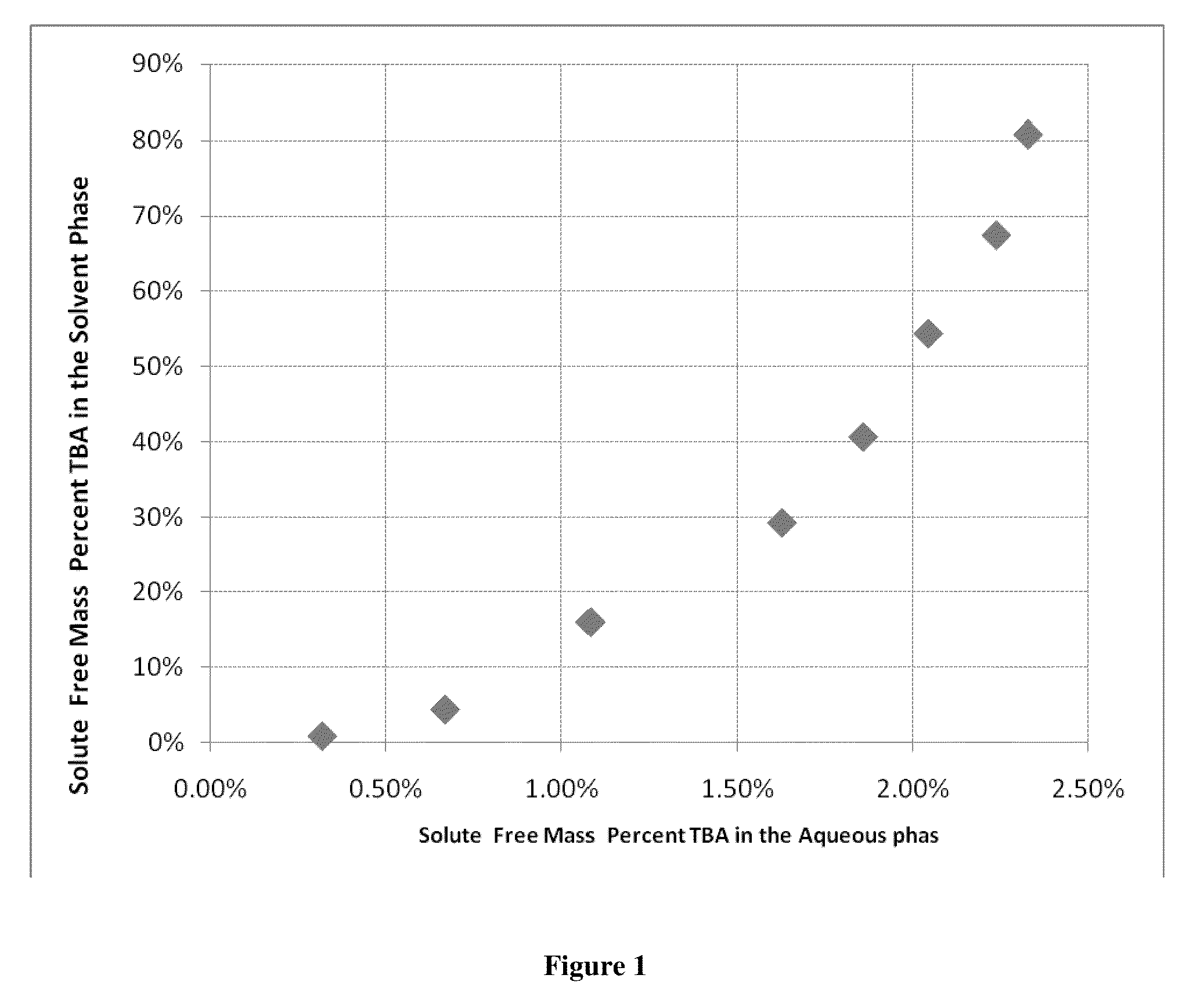
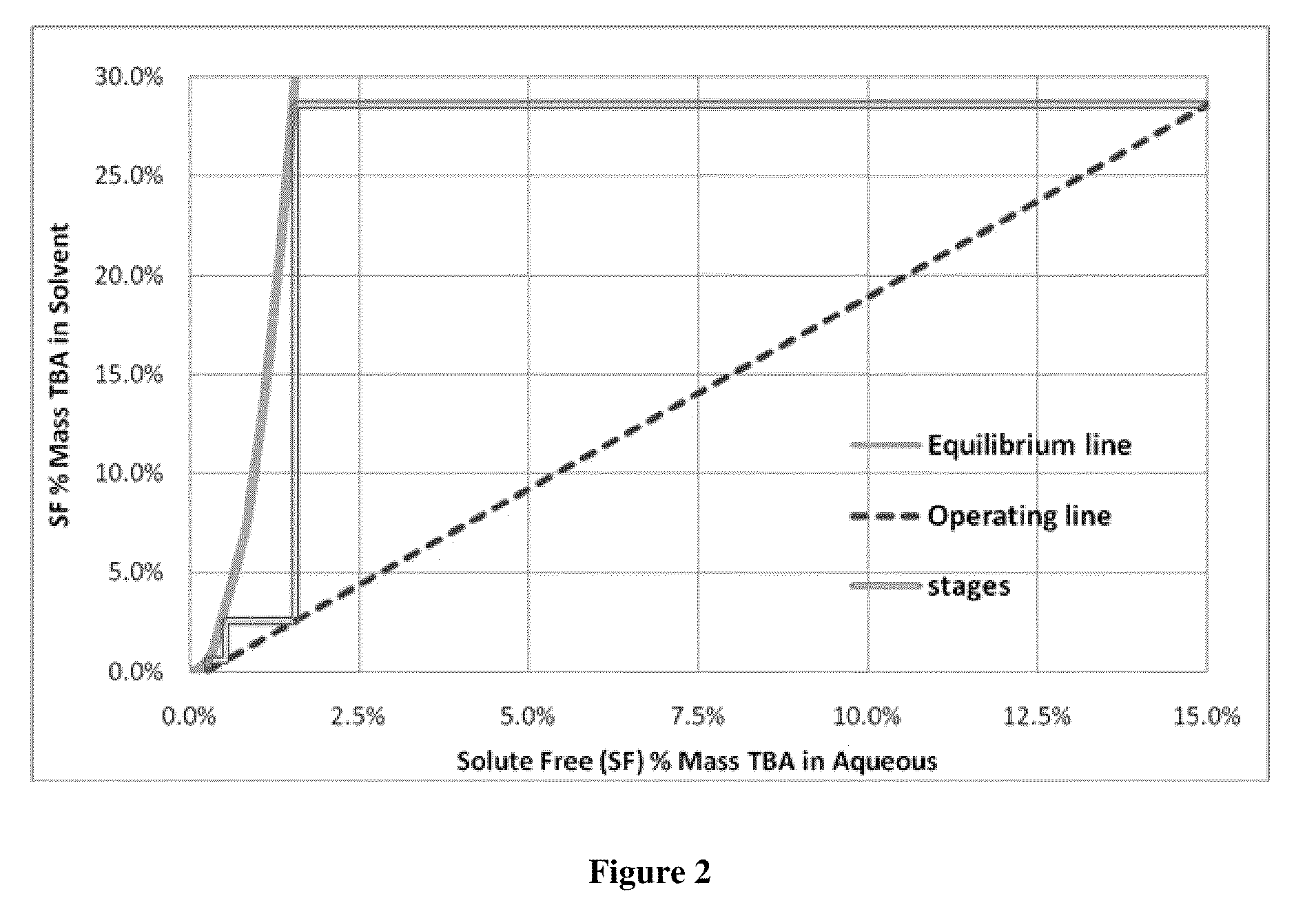
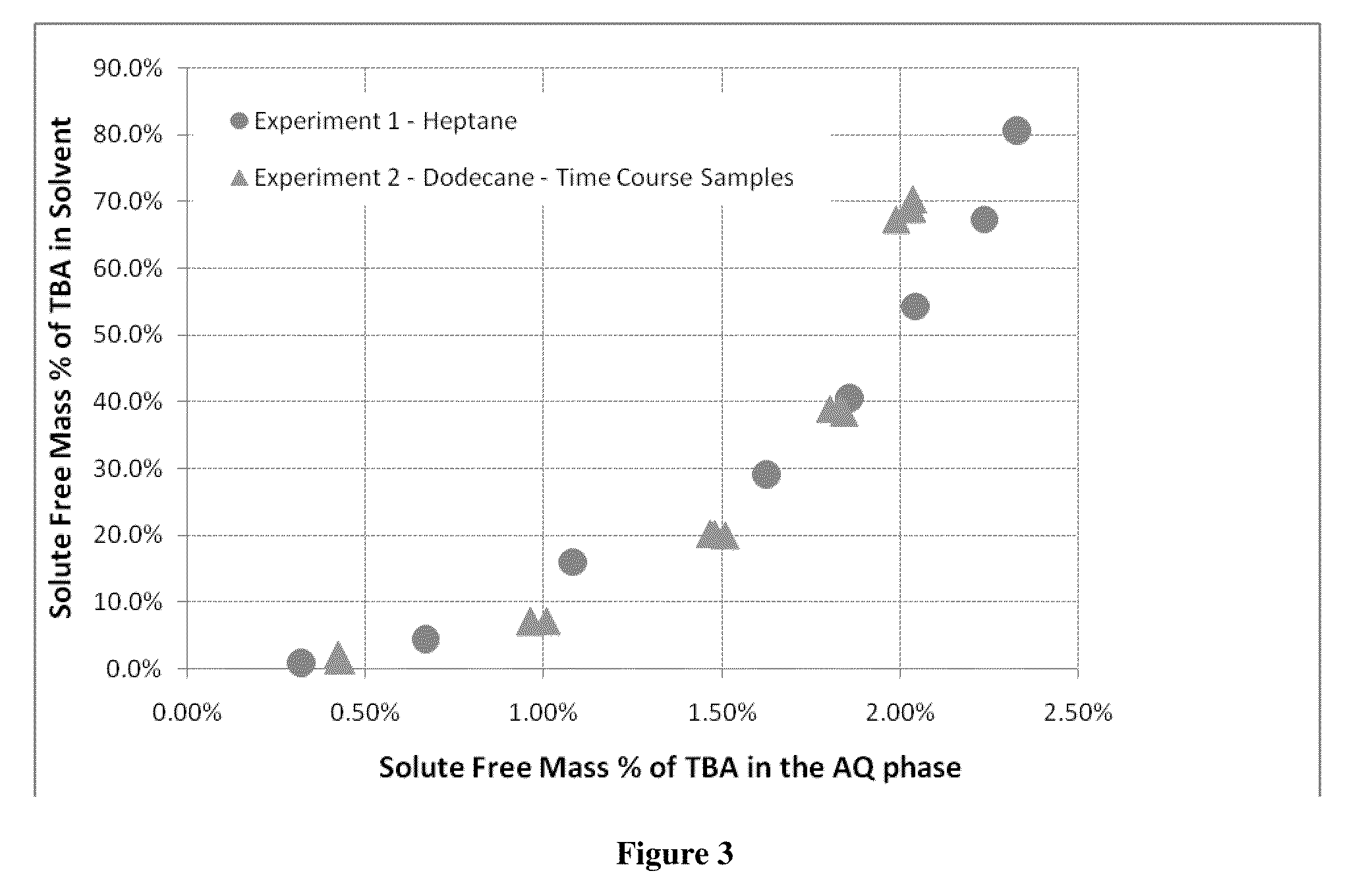
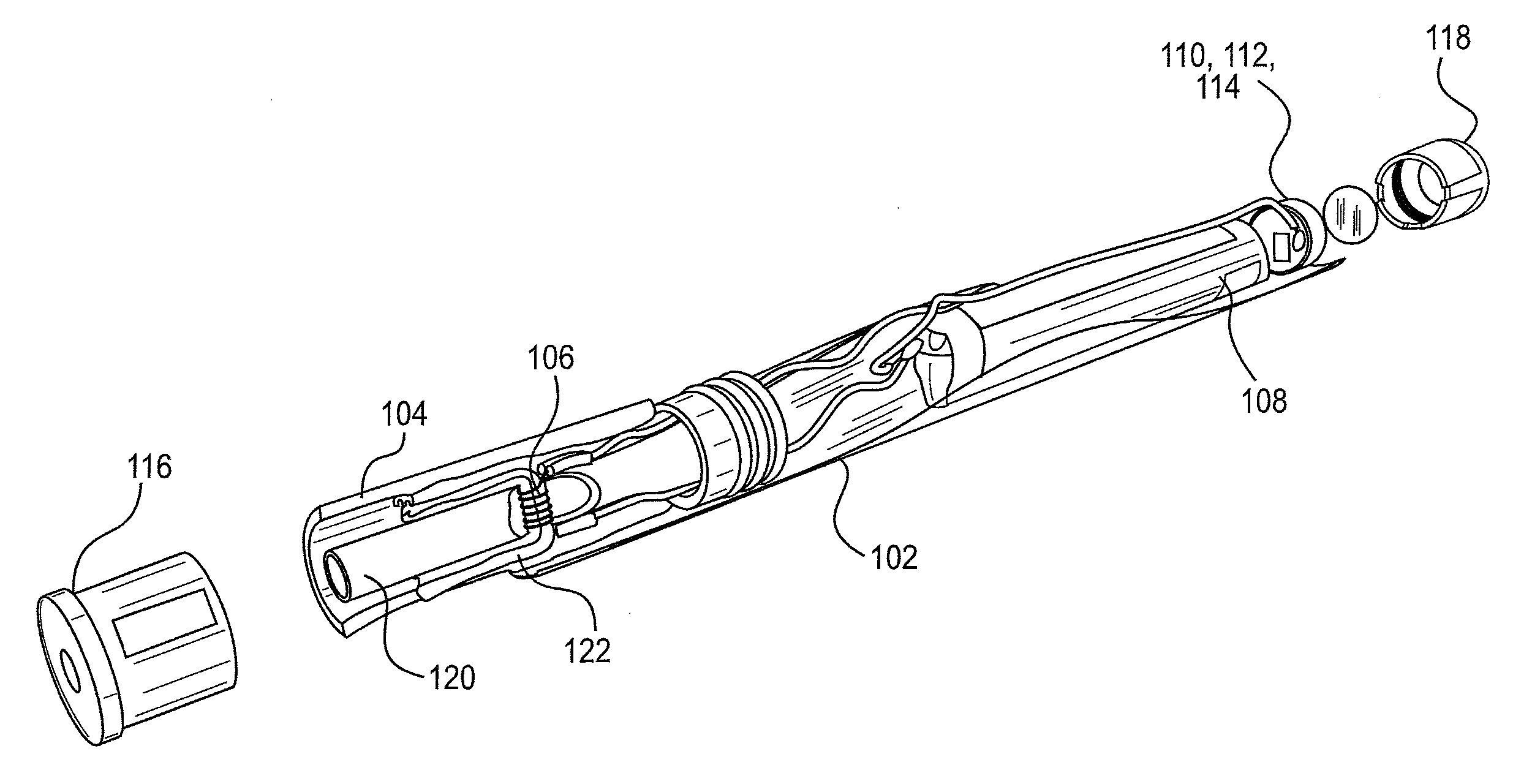
Compositions, devices, and methods for nicotine aerosol delivery
ActiveUS20140345635A1Reduce degradationConstant efficiencyTobacco treatmentTobacco devicesSolventElectron
The present disclosure generally relates to compositions, and related devices and methods, useful in vaporizing devices such as electronic cigarettes. The composition may comprise nicotine, at least one solvent, and at least one ion pairing agent, and may be vaporized to form a condensation aerosol, wherein inhalation of the aerosol allows for deposition of nicotine with the respiratory system, including deep lung deposition. The vaporizing device may comprise a vaporization unit, a battery, and an integrated circuit coupled to the battery, wherein the integrated circuit is configured to control the battery for rapid initial vaporization without overheating, producing thermal degradation products, or draining battery energy. The battery may operate with pulse width modulation for at least a portion of the time the vaporizing device is being used.
Owner:NJOY LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com