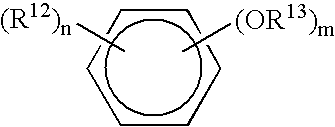
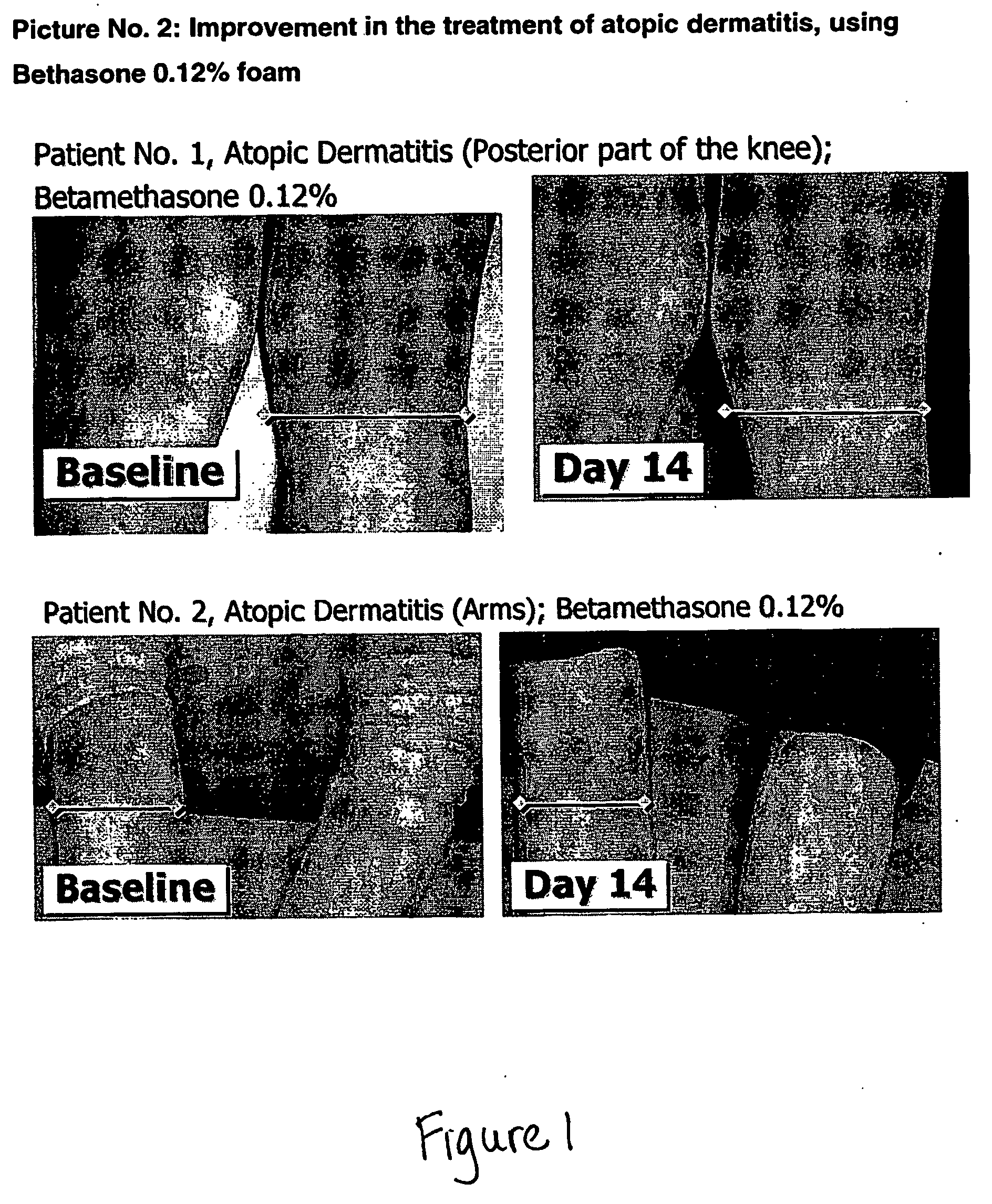
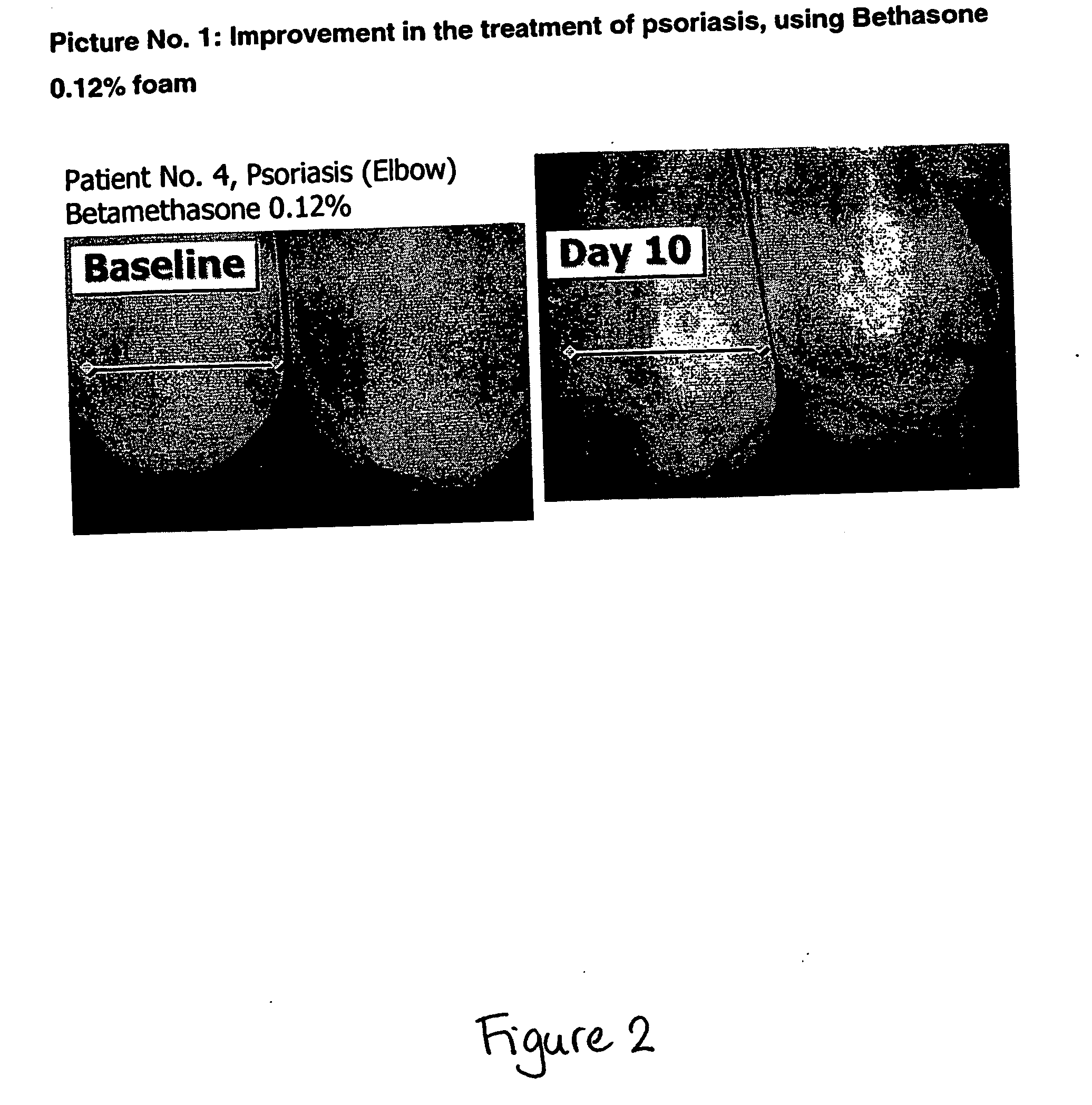
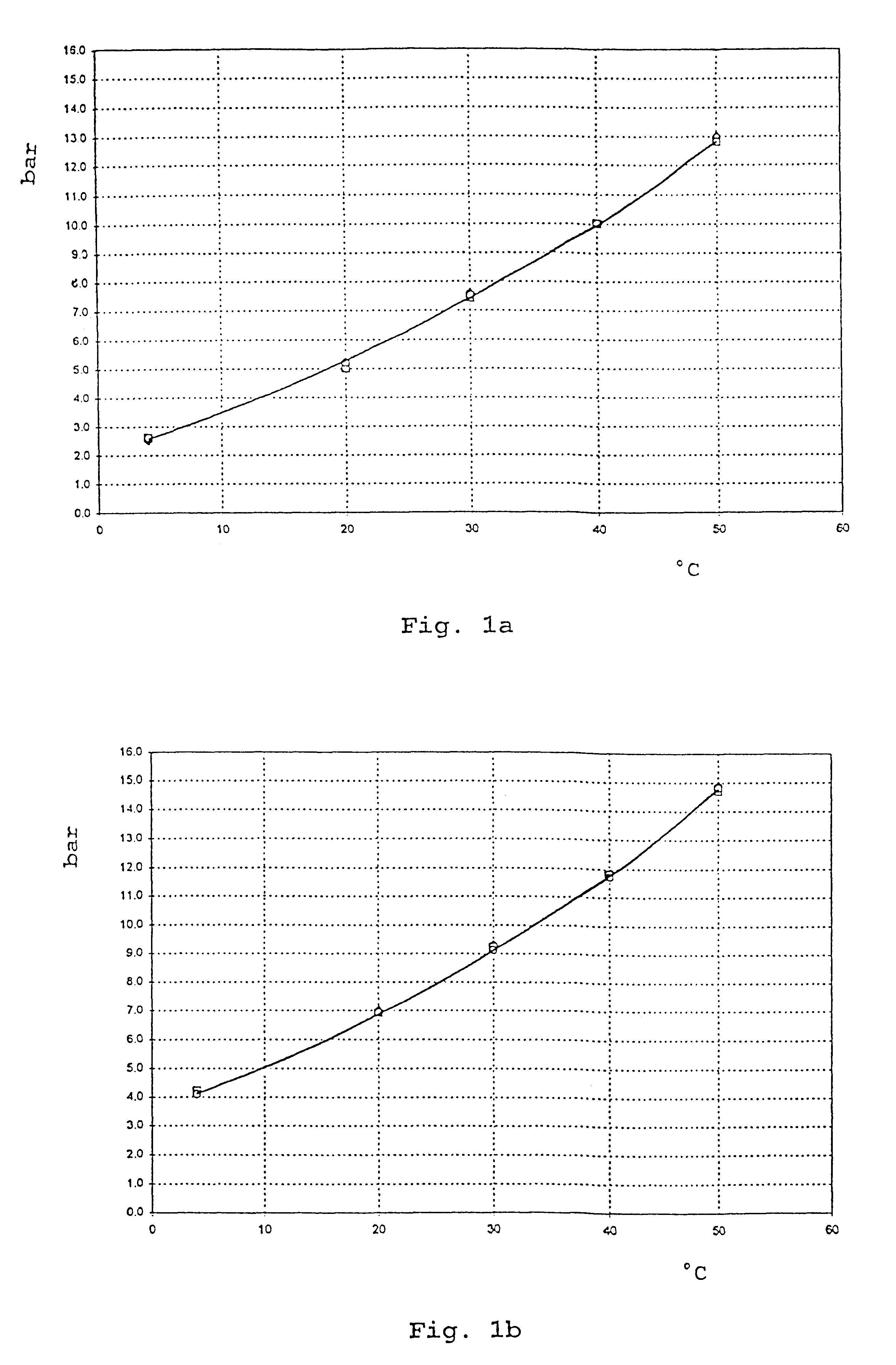
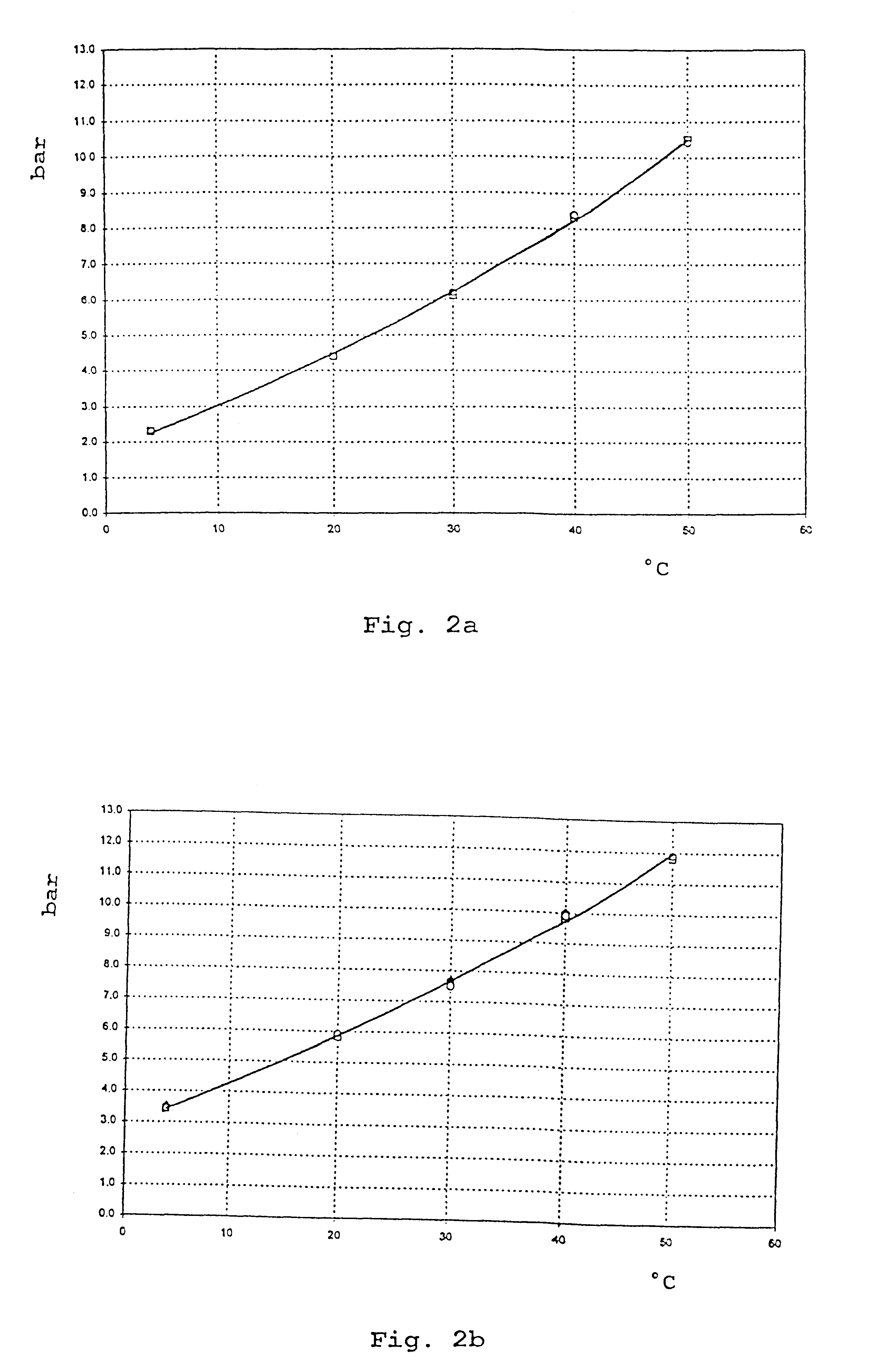
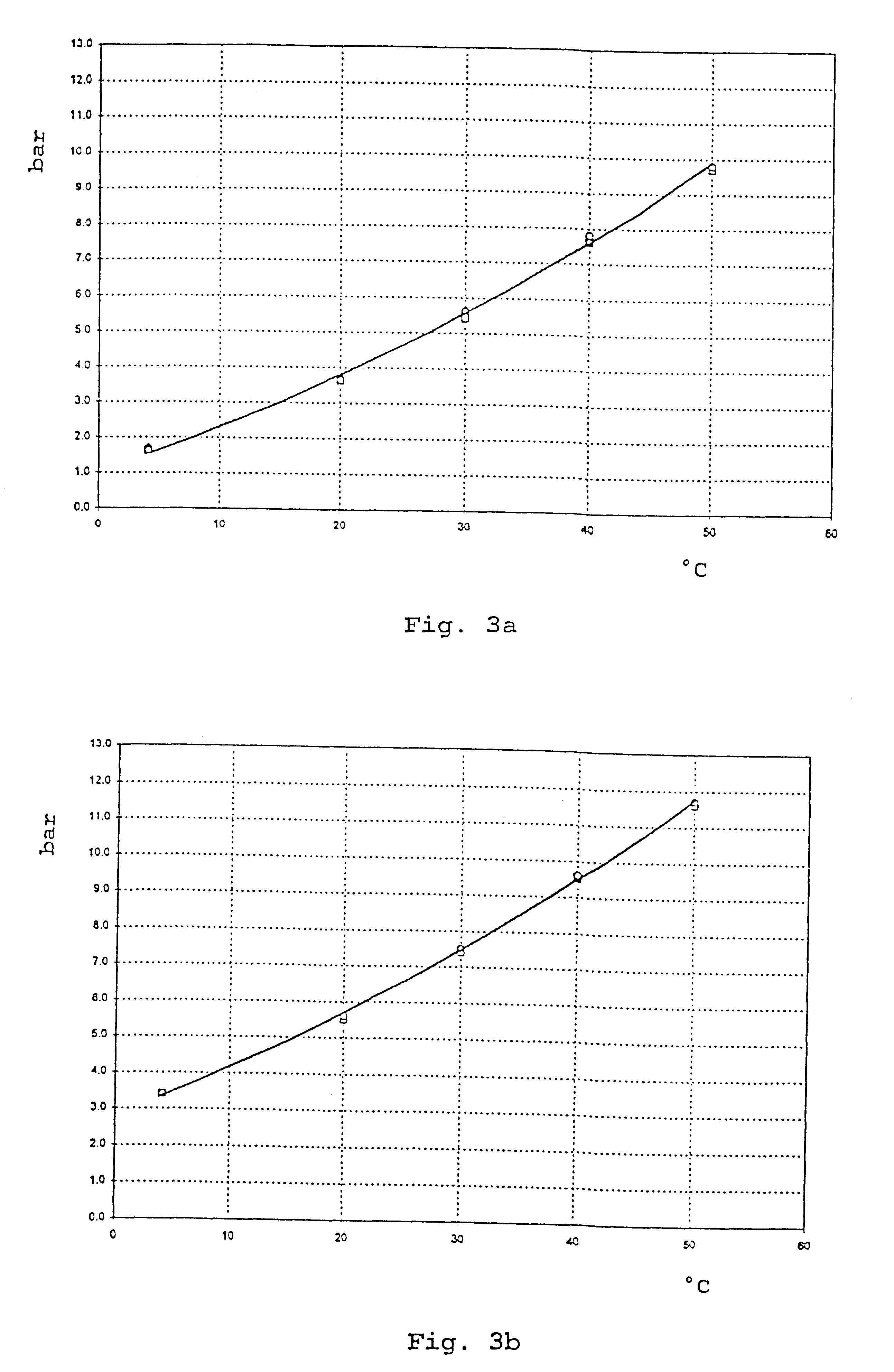
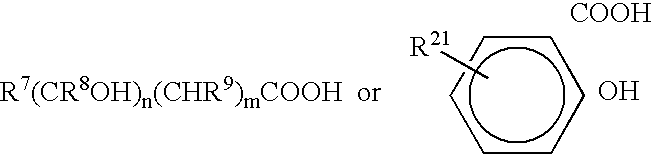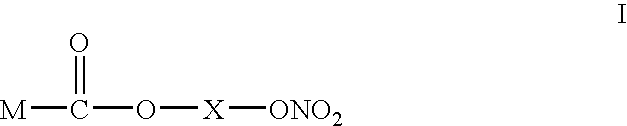Patents
Literature
3864results about "Organic non-active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Antibiotic kit and composition and uses thereof
The present invention relates to a therapeutic kit to provide a safe and effective dosage of an antibiotic agent, including an aerosol packaging assembly including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam, wherein the pressurized product comprises a foamable composition including: an antibiotic agent; at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, an organic polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight, a surface-active agent, about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent, water; and liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Lipids, lipid compositions, and methods of using them
InactiveUS20110200582A1Reducing and inhibiting and ameliorating diseaseLower Level RequirementsBiocideOrganic active ingredientsLipidomeActive agent
Owner:NOVARTIS AG
Pharmaceutical compositions for buccal and pulmonary application
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth.
Owner:GENEREX PHARMA INC +1
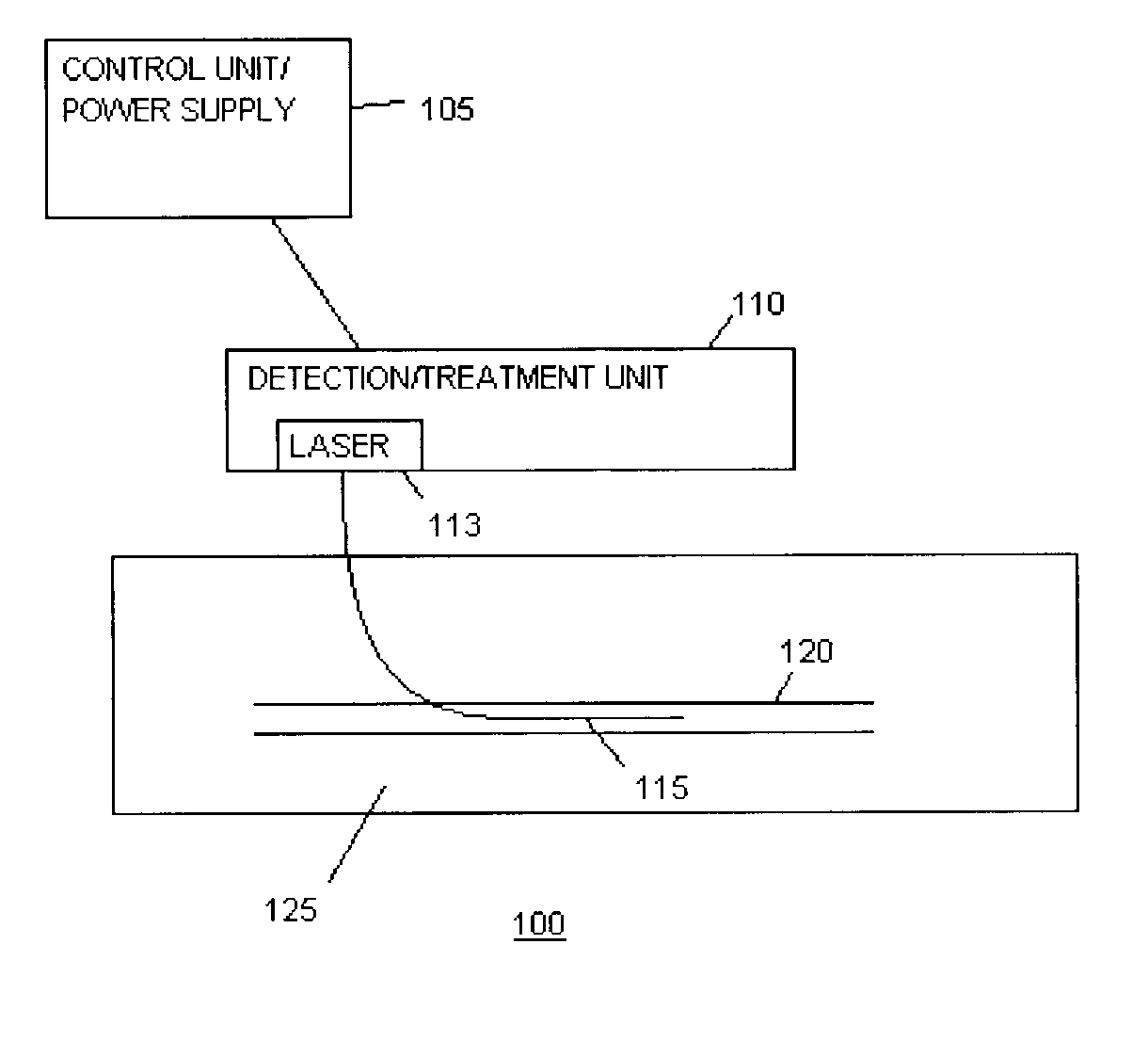
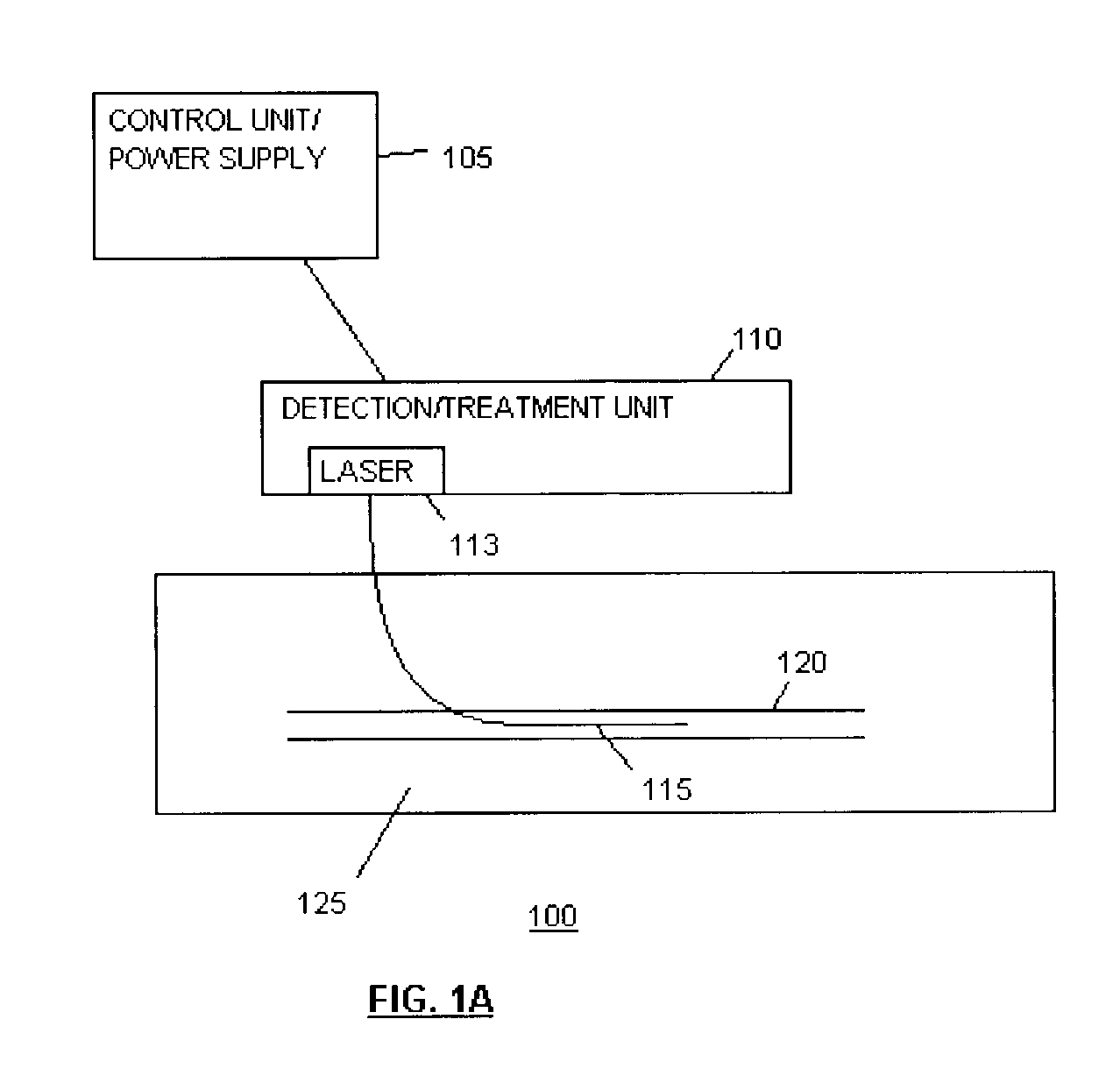
Methods and devices for detection and therapy of atheromatous plaque
InactiveUS20030082105A1Easy to detectHigh sensitivityUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsVulnerable plaqueFluorescence
The present invention relates to devices for detection and therapy of active atheromatous plaque and / or thin-capped fibro-atheroma ("vulnerable plaque"), using selectively targeted fluorescent, radiolabeled, or fluorescent and radiolabeled compositions. The present invention further relates to methods and devices for detection and theraphy of active atheromatous plaques and / or vulnerable plaques, using selectively targeted beta-emitting compositions, optionally comprising fluorescent compositions.
Owner:THE GENERAL HOSPITAL CORP
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Cosmetic and pharmaceutical foam
InactiveUS20080031907A1Efficient ConcentrationReduce sensitivityAntibacterial agentsBiocideAlcohol freeVegetable oil
The invention relates to uses of an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent as a flame retardant or flame resistant foam. The hydrophobic solvent is preferably mineral oil; medium chain triglycerides; isopropyl myristearate or octyl dodecanol, silicone oil or vegetable oil or mixtures thereof. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, also making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil-soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Foamable composition combining a polar solvent and a hydrophobic carrier
The present invention relates to a foamable vehicle or cosmetic or pharmaceutical composition, comprising: (1) an organic carrier, at a concentration of 10% to 70% by weight, wherein said organic carrier concurrently comprises: (i) at least one hydrophobic organic carrier, and (ii) at least one polar solvent; (2) at least one surface-active agent; (3) water; and (4) at least one liquefied or compressed gas propellant at a concentration of 3% to 25% by weight of the total composition. The present invention further provides a method of treating, alleviating or preventing a disorder of mammalian subject, comprising administering the above-mentioned compositions to an afflicted target site.
Owner:VYNE THERAPEUTICS INC
Antimicrobial compositions and methods
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including, in particular, an antimicrobial lipid component, such as a fatty acid ester, fatty ether, or alkoxide derivative thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Cosmetic and pharmaceutical foam
InactiveUS20060140984A1Lower yield strengthRubbing easy and efficientCosmetic preparationsBiocideAlcohol freeAdjuvant
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a hydrophobic solvent, a foam adjuvant agent, a surface-active agent and a water gelling agent. The cosmetic or pharmaceutical foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble pharmaceutical and cosmetic agents.
Owner:FOAMIX PHARMACEUTICALS LIMITED
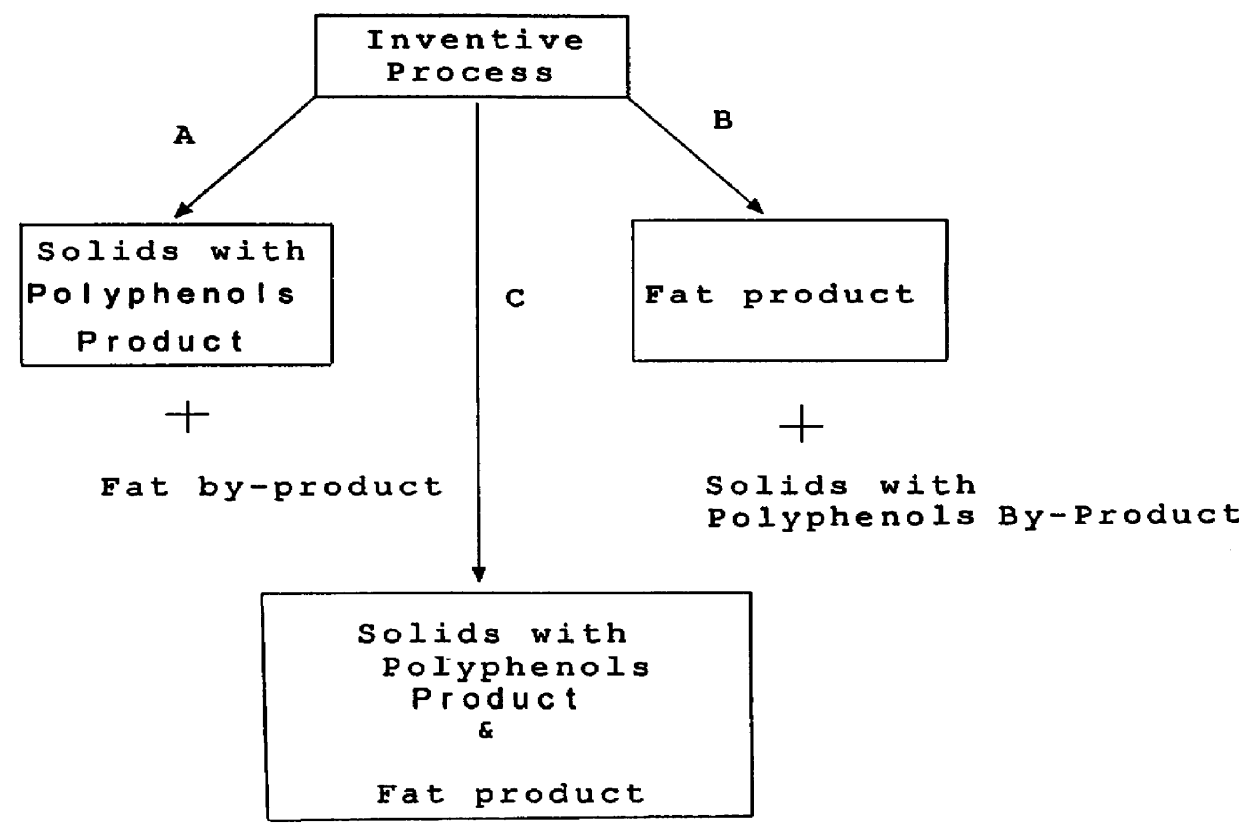
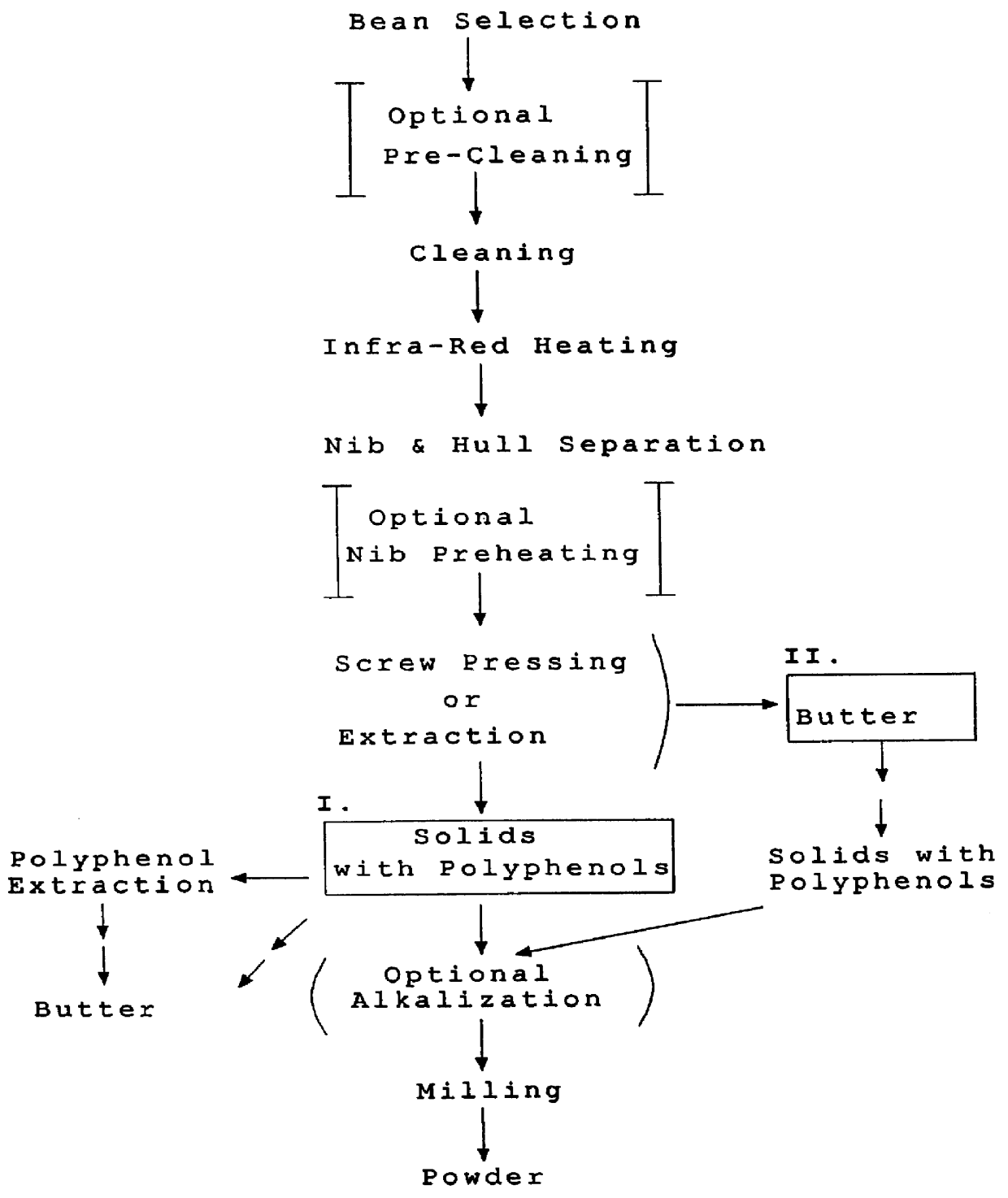
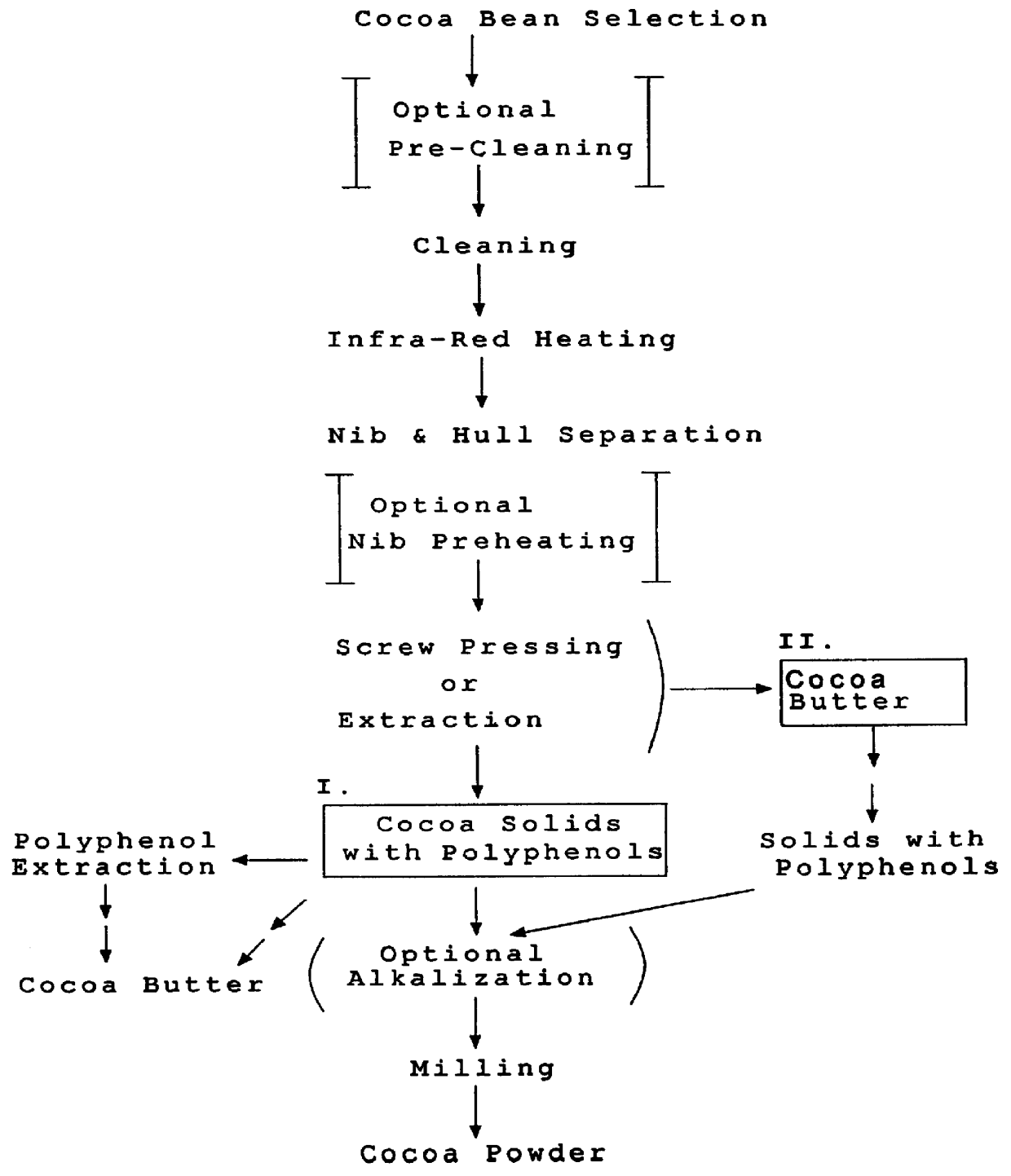
Method for producing fat and/or solids from cocoa beans
InactiveUS6015913AHighly conserved levelReduce moisture contentBiocideDough treatmentPolyphenolCOCOA BEAN
The present invention is directed to a method of processing a fat-containing bean, e.g., cocoa beans, for producing solids comprising active polyphenols and / or fat-containing products, comprising extracting the fat to produce solids and fat-containing products. Additionally, the inventive method also provides cocoa compositions comprising at least one active polyphenol, wherein the concentration of the polyphenol(s) with respect to the nonfat solids is conserved with respect to the concentration of the active polyphenol(s) in the bean from which the compositions are derived.
Owner:MARS INC +1
Compositions containing fluorine substituted olefins
The use to e of tetrafluoropropenes, particularly (HFO-1234) in a variety of applications, including refrigeration equipment, is disclosed. These materials are generally useful as refrigerants for heating and cooling, as blowing agents, as aerosol propellants, as solvent composition, and as fire extinguishing and suppressing agents.
Owner:HONEYWELL INT INC
Body cavity foams
The invention relates to an alcohol-free cosmetic or therapeutic foam carrier comprising water, a hydrophobic organic carrier, a foam adjuvant agent, a surface-active agent and a gelling agent. The cosmetic or therapeutic foam carrier does not contain aliphatic alcohols, making it non-irritating and non-drying. The alcohol-free foam carrier is suitable for inclusion of both water-soluble and oil soluble therapeutic and cosmetic agents.
Owner:VYNE THERAPEUTICS INC
Pharmaceutical Dosage Form For Oral Administration Of Tyrosine Kinase Inhibitor
InactiveUS20090203709A1BiocideOrganic non-active ingredientsOral medicationTyrosine-kinase inhibitor
A pharmaceutical dosage form comprises a solid dispersion product of at least one tyrosine kinase inhibitor, at least one pharmaceutically acceptable polymer, and at least one pharmaceutically acceptable solubilizer.
Owner:ABBVIE INC
Medical aerosol formulations
InactiveUS6461591B1Improve dosage accuracyIncrease doseBiocideDispersion deliveryAlkaneAerosol spray
A pressure-liquefied propellant mixture for aerosols, comprising a fluorinated alkane, in particular 1,1,1,2-tetrafluoroethane and / or 1,1,1,2,3,3,3-heptafluoropropane, and carbon dioxide, makes possible an improvement of the wetting properties of pharmaceutically active compounds, with which the formulation problems existing with hydrofluoroalkanes in relation to suspension as well as solution aerosols can be overcome and thus improved medicinal aerosol formulations can be obtained. With the aid of carbon dioxide, it is also possible to specifically influence the pressure and thus the particle size distribution and also by displacement of oxygen from the hydrofluoroalkanes to improve the storage stability of oxidation-sensitive active compounds.
Owner:JAGOTEC AG
Composition
A new pharmaceutical composition in the form of lipoglobules which comprises (a) one or more NO-releasing NSAID(s); (b) one or more surfactant(s); and (c) an aqueous phase, as well as a process for the preparation of such composition and the use of such composition in the treatment of pain and inflammation.
Owner:ASTRAZENECA AB
Alcohol-free transdermal analgesic composition and processes for manufacture and use thereof
InactiveUS7052715B2Reduced shelf lifeImprove permeabilityOrganic active ingredientsBiocideAlkaneAlcohol free
The instant invention is directed toward a dermal delivery system composition comprising an aqueous base vehicle including American Emu oil, Isopropyl Palmitate (PROTACHEM IPP), PEG-8 (a polyethylene glycol available under the tradename PROTACHEM 400), methylsulfonylmethane (MSM) and SEPIGEL 305 (a combination including polyacrylamide / C13–C14 Iso-paraffin and Laureth-7), in combination with an analgesic composition, such as ibuprofen, and to processes for the manufacture and use thereof.
Owner:ALL NATURAL FMG
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Novel lipids and compositions for the delivery of therapeutics
ActiveUS20110311583A1Adequate therapeutic indexSimple compositionAntibacterial agentsOrganic active ingredientsLipid formationAryl
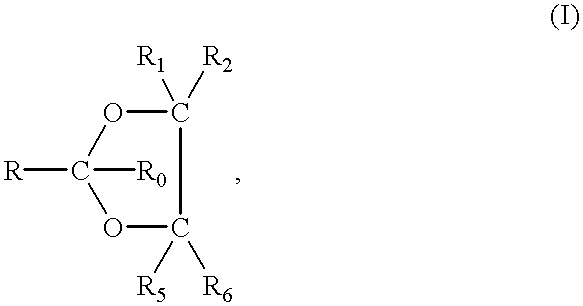
(A1) Translate this text The present invention provides lipids that are advantageously used in lipid particles for the in vivo delivery of therapeutic agents to cells. In particular, the invention provides lipids having the following structure (I) wherein R1 and R2 are each independently for each occurrence optionally substituted C10-C30 alkyl, optionally substituted C10-C30 alkenyl, optionally substituted C10-C30 alkynyl, optionally substituted C10-C30 acyl, or -linker-ligand; R3 is H, optionally substituted C1-C10 alkyl, optionally substituted C2-C10 alkenyl, optionally substituted C2-C10 alkynyl, alkylhetrocycle, alkylphosphate, alkylphosphorothioate, alkylphosphorodithioate, alkylphosphonates, alkylamines, hydroxyalkyls, ?-aminoalkyls, ?-(substituted)aminoalkyls, ?-phosphoalkyls, ?-thiophosphoalkyls, optionally substituted polyethylene glycol (PEG, mw 100-40K), optionally substituted mPEG (mw 120-40K), heteroaryl, heterocycle, or linker-ligand; E is O, S, N(Q), C(O), N(Q)C(O), C(0)N(Q), (Q)N(CO)O, O(CO)N(Q), S(O), NS(O)2N(Q), S(O)2, N(Q)S(O)2, SS, O═N, aryl, heteroaryl, cyclic or heterocycle; and, Q is H, alkyl, ?-aminoalkyl, ?-(substituted)aminoalky, ?-phosphoalkyl or ?-thiophosphoalkyl.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Nicotine-containing pharmaceutical compositions giving a rapid transmucosal absorption
Formulations of nicotine for use in nicotine replacement therapy. The formulations are intended for application in the oral cavity where upon the uptake of nicotine mainly takes place through the buccal mucosa. The formulations essentially comprise apolar, polar and surface-active components. The formulations may be administered in combination with other nicotine formulations.
Owner:MCNEIL AB
Topically applied clostridium botulinum toxin compositions and treatment methods
InactiveUS20030113349A1Efficient use ofBacterial antigen ingredientsPeptide/protein ingredientsChromhidrosisSeborrheic dermatitis
Hyperactive glandular conditions are treated using topically formulated botulinum toxin compositions. In the preferred embodiment of the invention, topical botulinum preparations are applied directly to the skin by a patient as needed to suppress his or her hyperhidrosis, bromhidrosis, chromhidrosis, nevus sudoriferous, acne, seborrhiec dermatitis or other glandular condition. In other embodiments, topical botulinum toxins are applied with the aid of mechanical, electrical, and / or chemical transdermal delivery enhancers.
Owner:COLEMAN WILLIAM P III
Mixture for Transdermal Delivery of Low and High Molecular Weight Compounds
InactiveUS20080154210A1Low and high molecular weightPromote recoveryOrganic active ingredientsCosmetic preparationsHigh molecular massDrug
Owner:ORIX +1
Peroxide removal from drug delivery vehicle
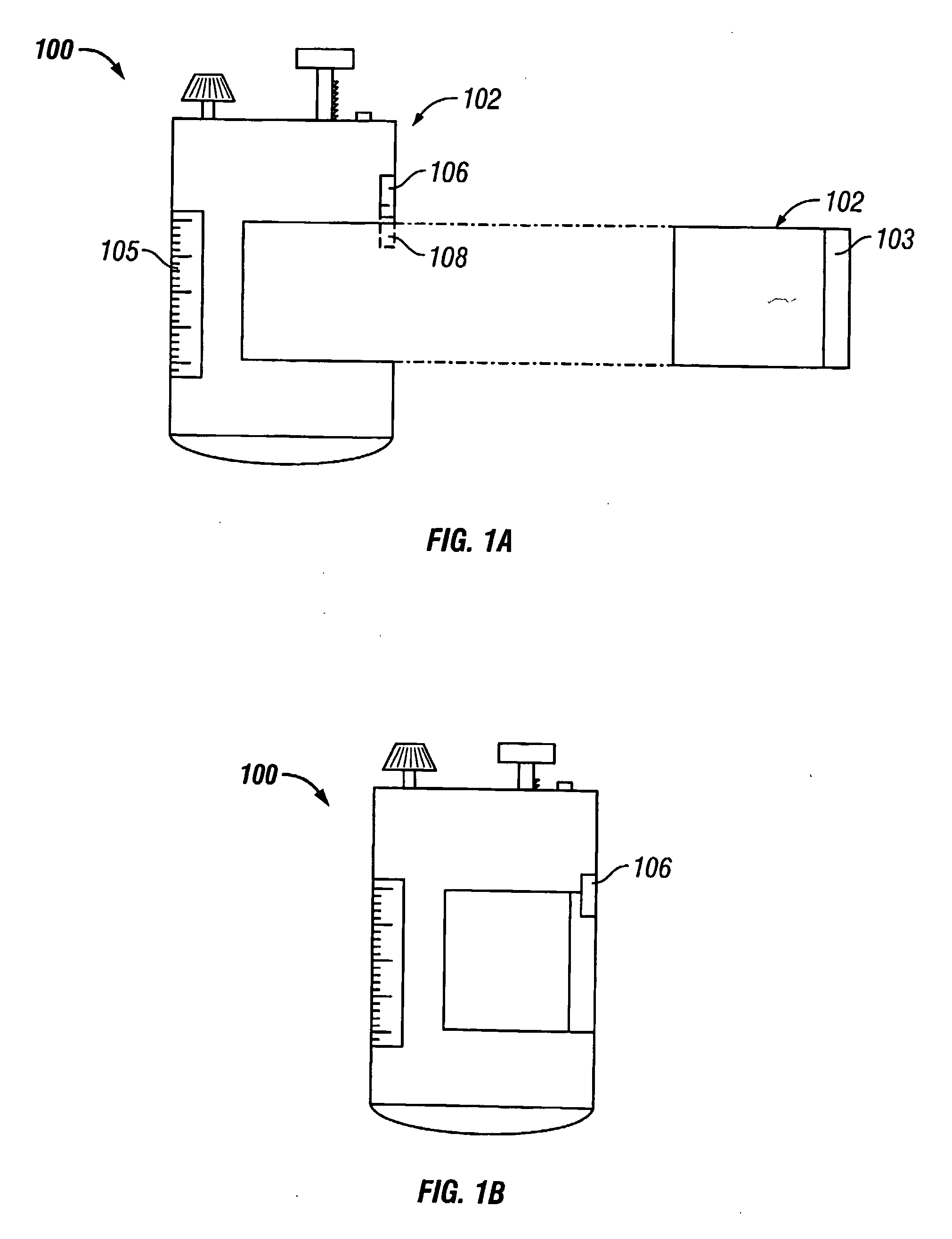
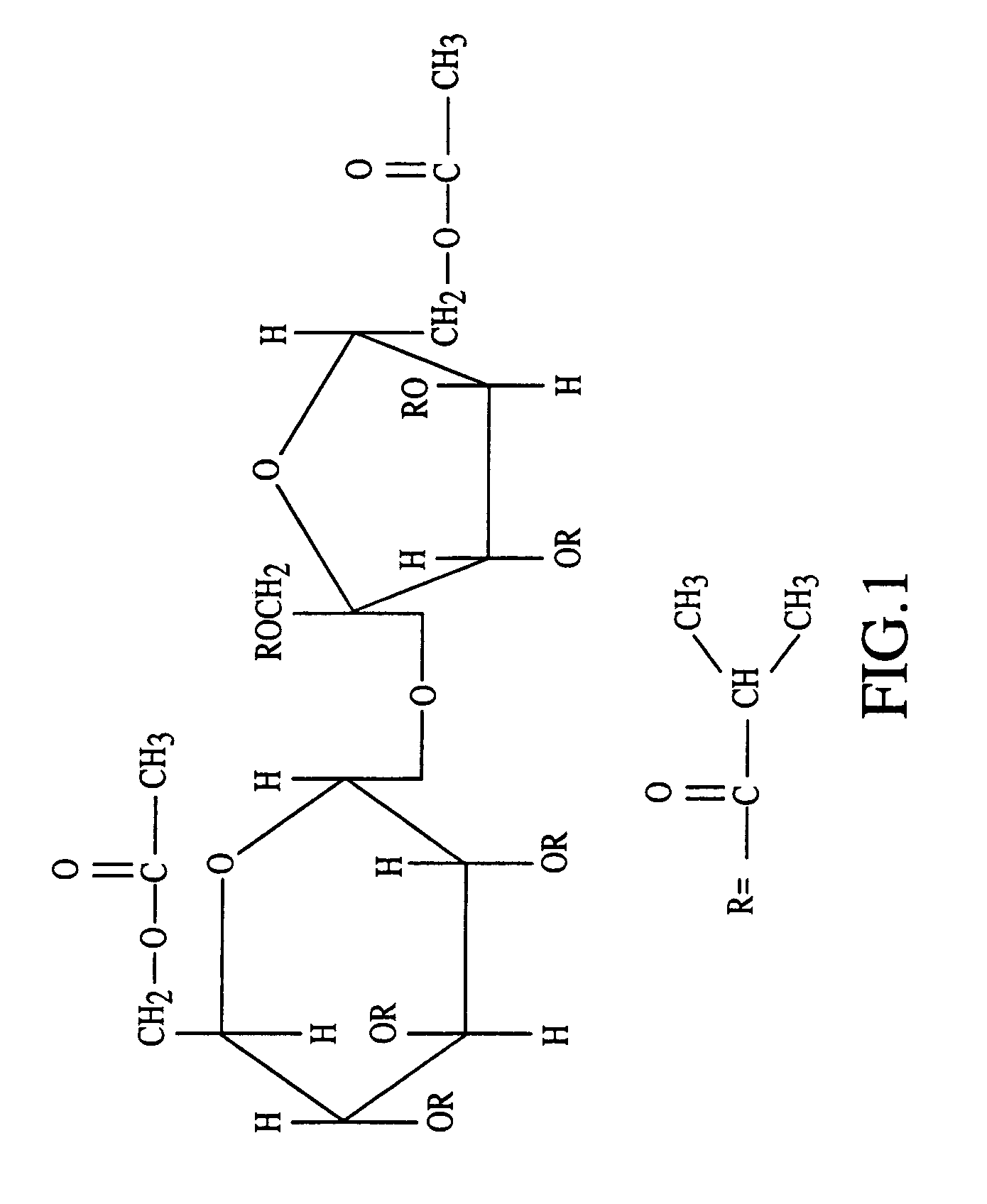
InactiveUS20070027105A1Improve drug stabilityReduced level of peroxideOrganic active ingredientsBiocideMedicineSucrose acetate isobutyrate
Owner:DURECT CORP
Compositions and methods
Non-aerosol spray-on skin patch compositions as described comprising at least one substantially water insoluble film forming agent, at least one film plasticizer agent, at least one water soluble compound, and at least one organic solvent, the composition forming a flexible, porous and physiologically compatible skin patch when sprayed on to skin and allowed to dry. Also described are methods of improving wound healing by administering a physiologically active ingredient to a patient in need of such treatment.
Owner:KO THOMAS SAI YING
Morinda citrifolia (Noni) enhanced animal food product
The present invention advances prior art animal food products by providing an animal food product formulated with Morinda Citrifolia, or Noni, from the Indian Mulberry plant. The addition of Noni to the animal food product of the present invention serves to provide significant health advantages not found in prior art animal food products.
Owner:TAHITIAN NONI INT INC
Antifungal nail lacquer and method using same
InactiveUS6224887B1Effective preventionEffective treatmentBiocideCosmetic preparationsAntifungalLacquer
A nail lacquer effective for the treatment or prevention of fungal infections, such as, onychomycosis, includes fungicidally effective amount of ciclopirox, econazole, or other antifungal agent in a clear, stable, film-forming lacquer vehicle which includes a water-insoluble film-forming polymer; 2-n-nonyl-1,3-dioxolane or similar penetration enhancer; and volatile solvent. A plasticizer for the film-forming polymer which is also compatible with the other components may be included although the preferred penetration enhancers may also function as plasticizer. The composition, when applied to the nails provides a hard, clear, water-resistant film containing the antifungal agent. The film is resistant to multiple washings and is effective in the treatment of onychomycosis.
Owner:MACROCHEM CORP
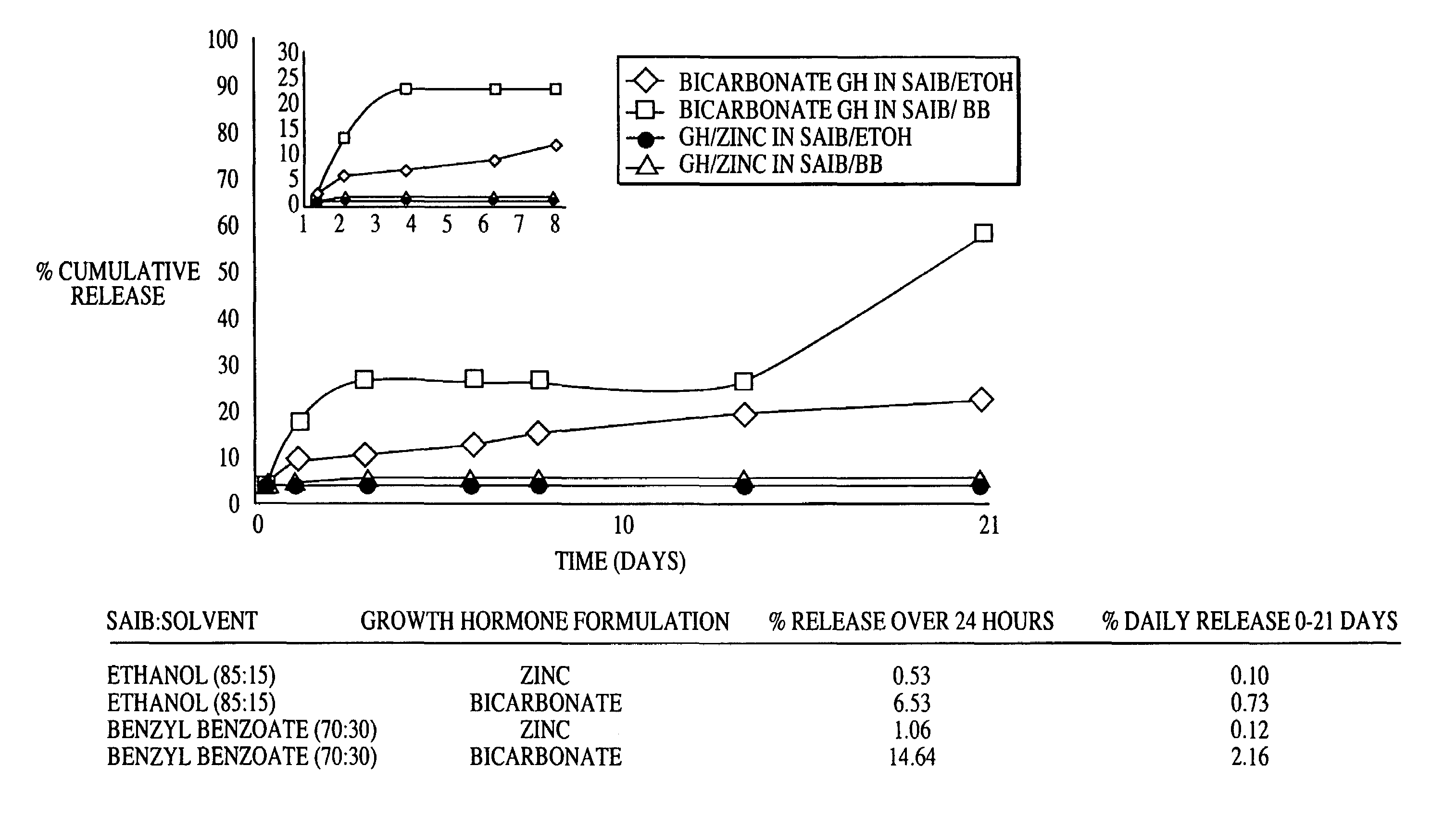
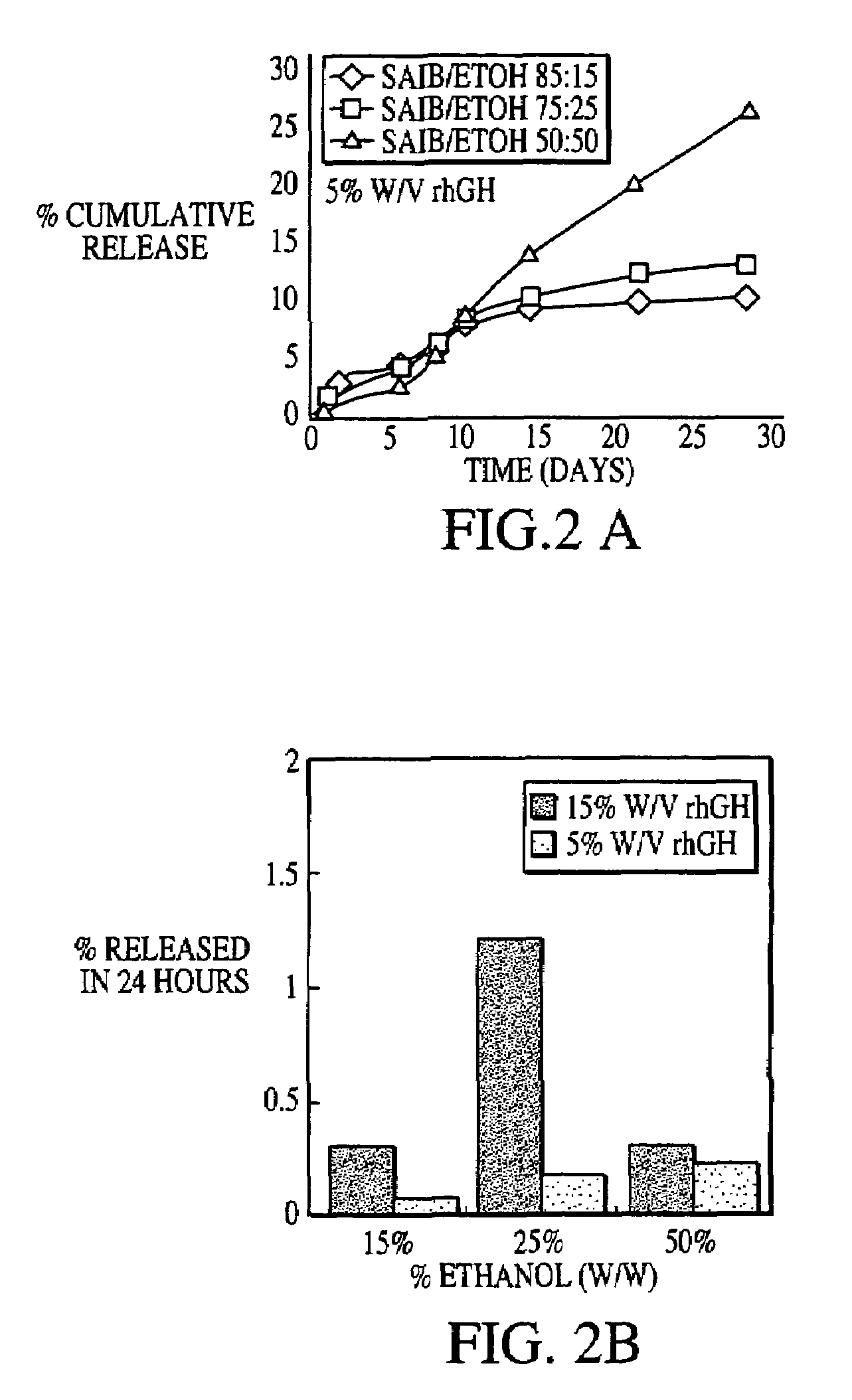
Sustained release formulations
A composition for sustained release comprises a carrier material containing a non-polymeric, non-water soluble liquid material having a viscosity of at least 5,000 cP at 37° C. that does not crytallize neat under ambient physiological conditions, a multivalent metal cation, and growth hormone.
Owner:DURECT CORP
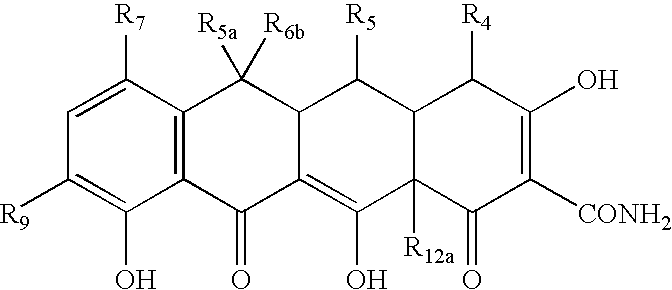
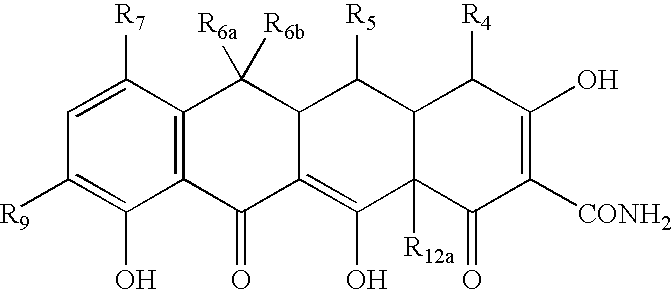
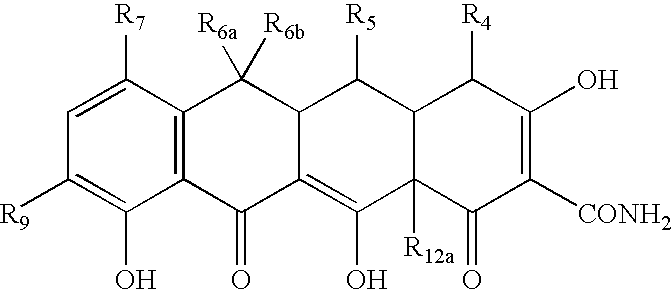
Tetracycline compositions for topical administration
Pharmaceutical formulations containing tetracycline for topical administration, as well as methods of making and administering the same, are disclosed.
Owner:WARNER CHILCOTT CO LLC
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com