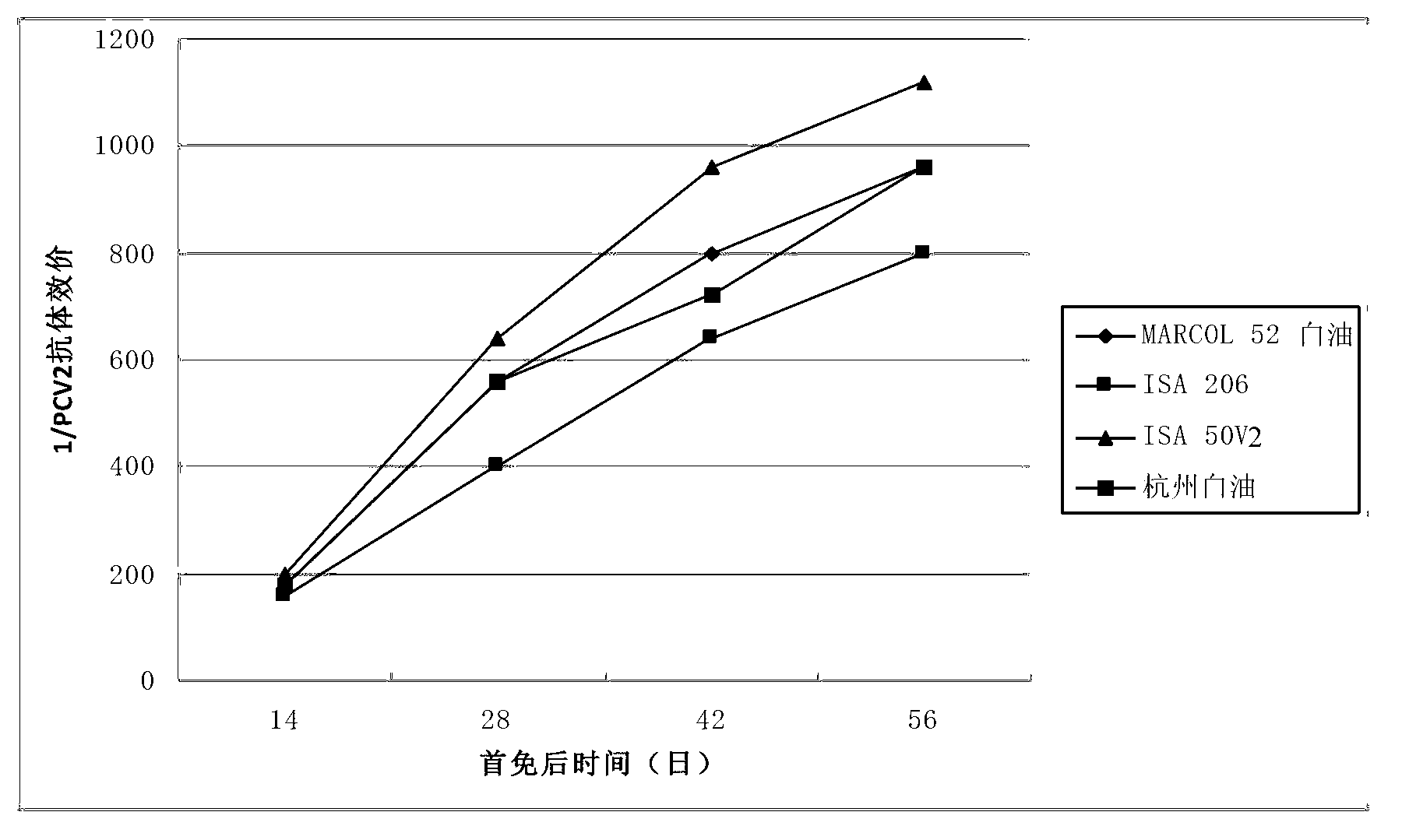
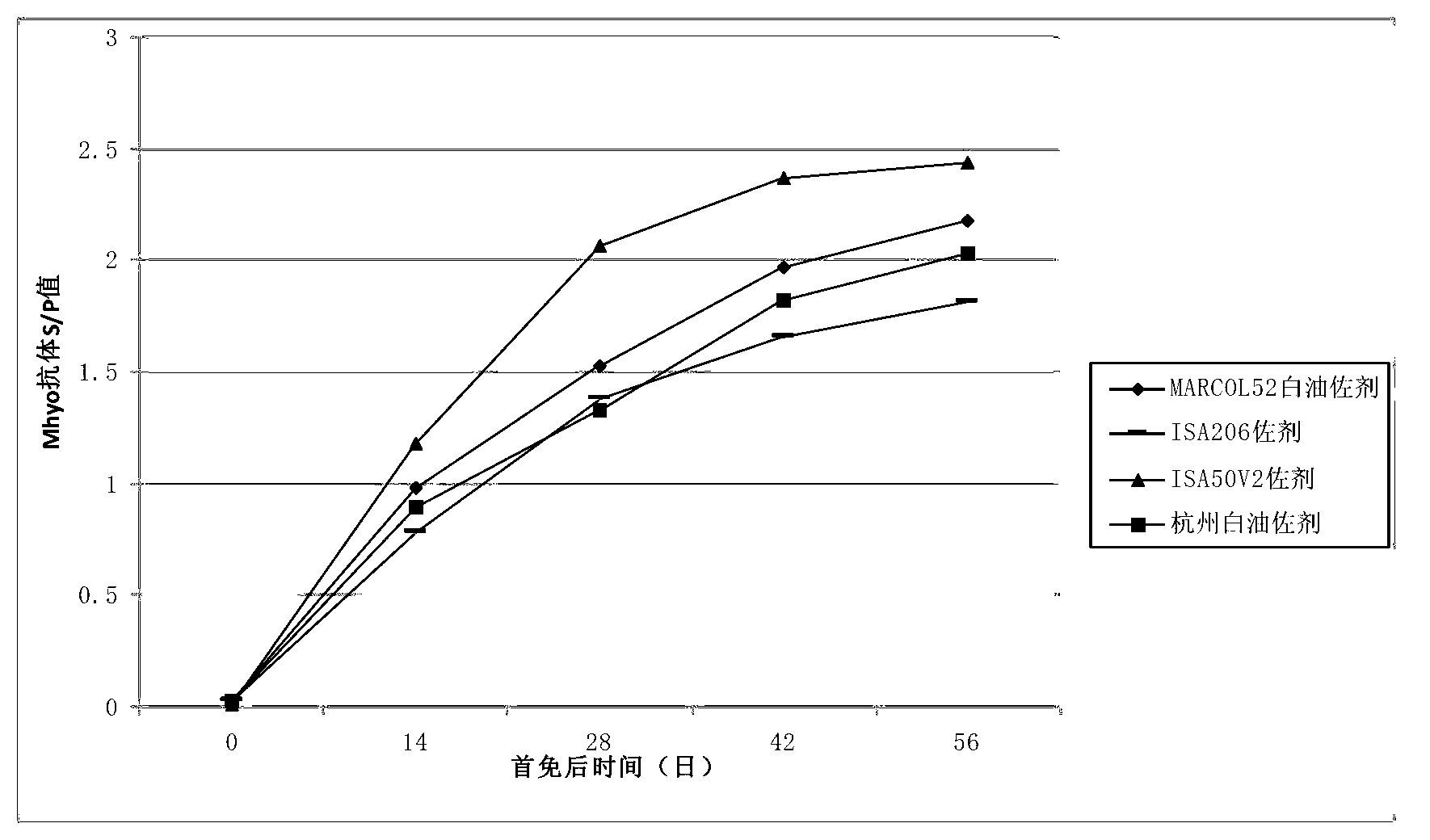
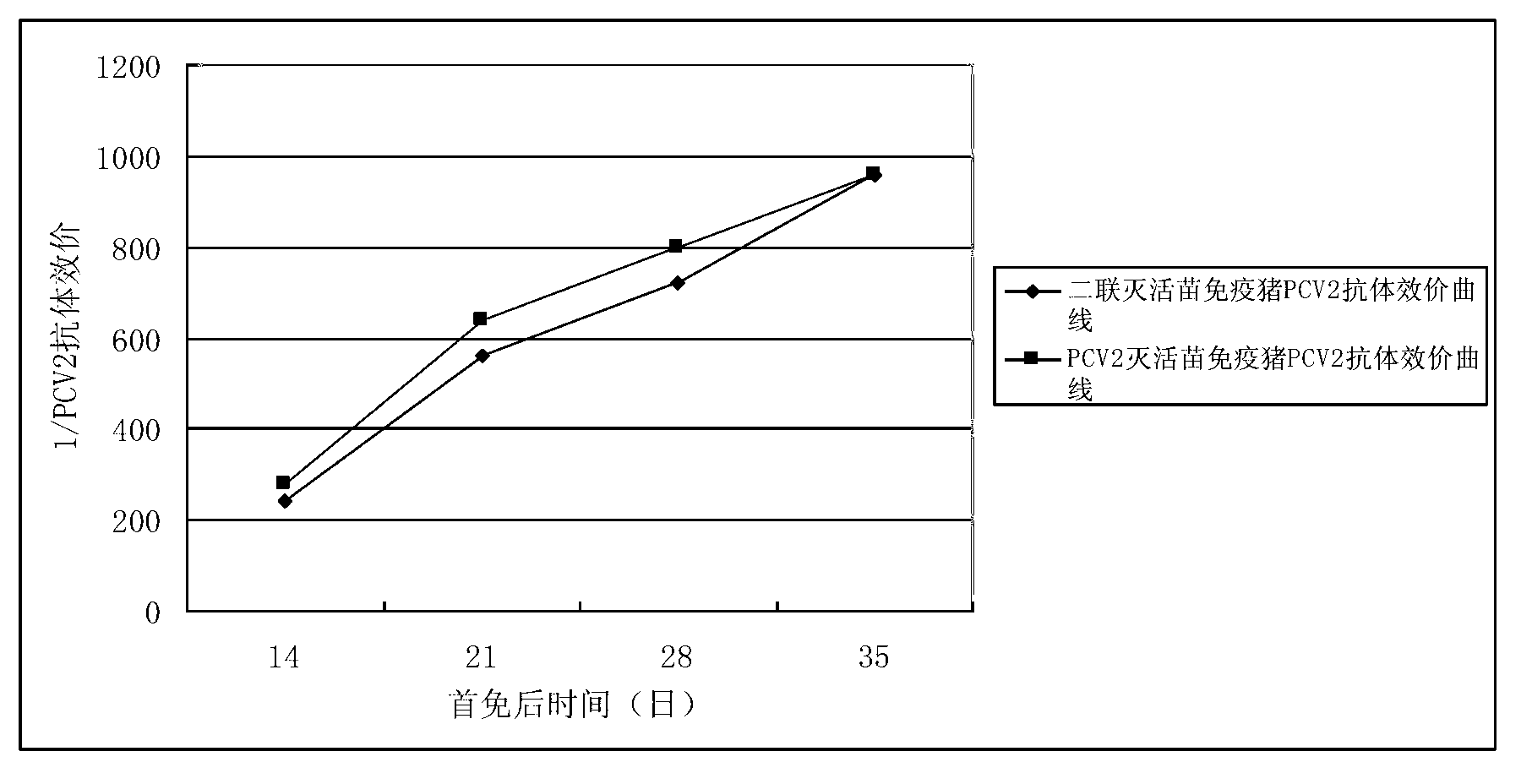
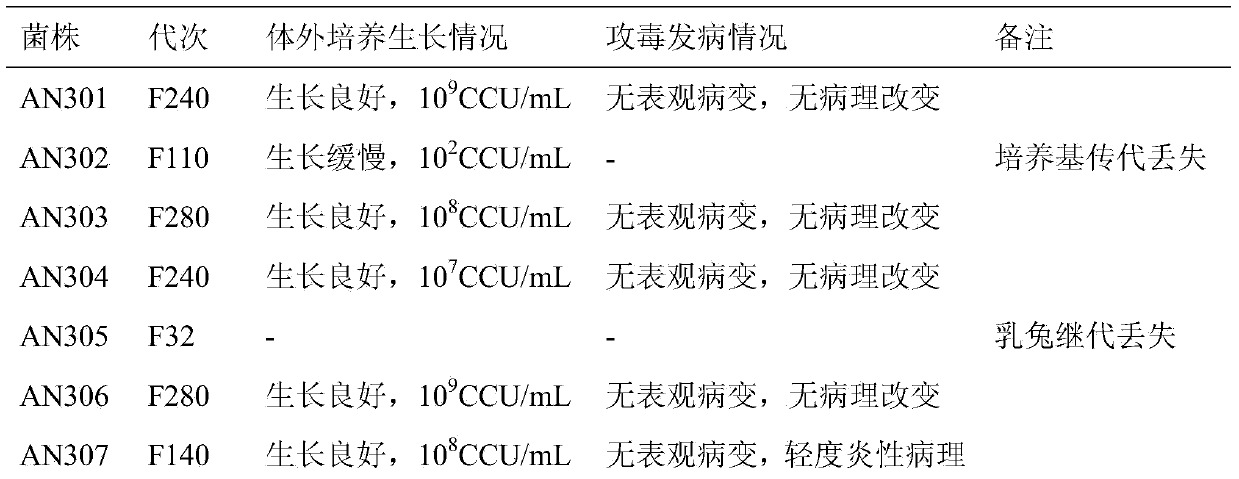
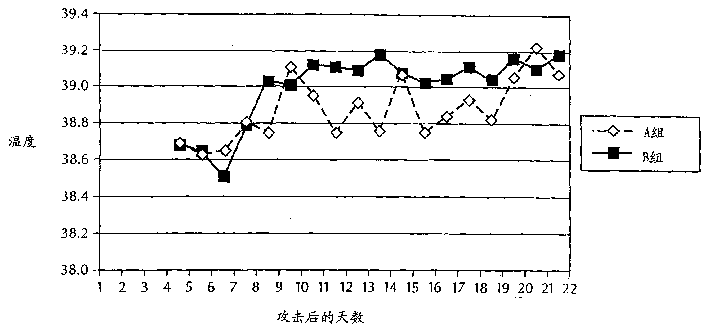
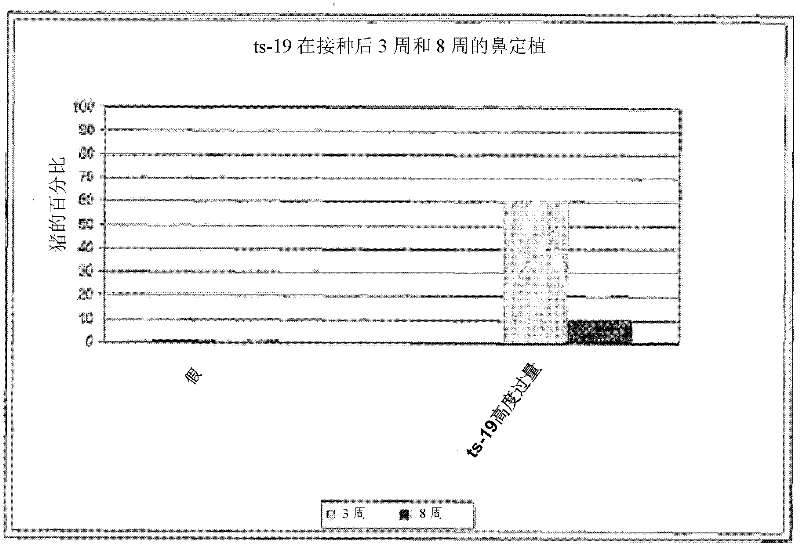
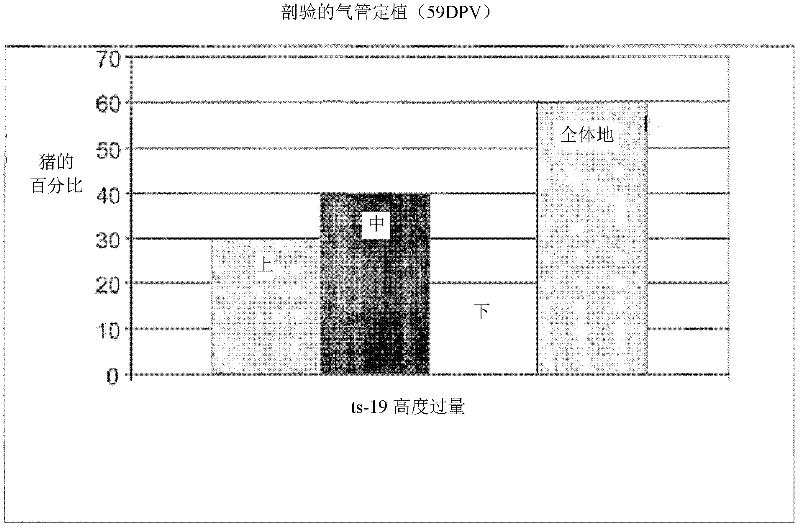
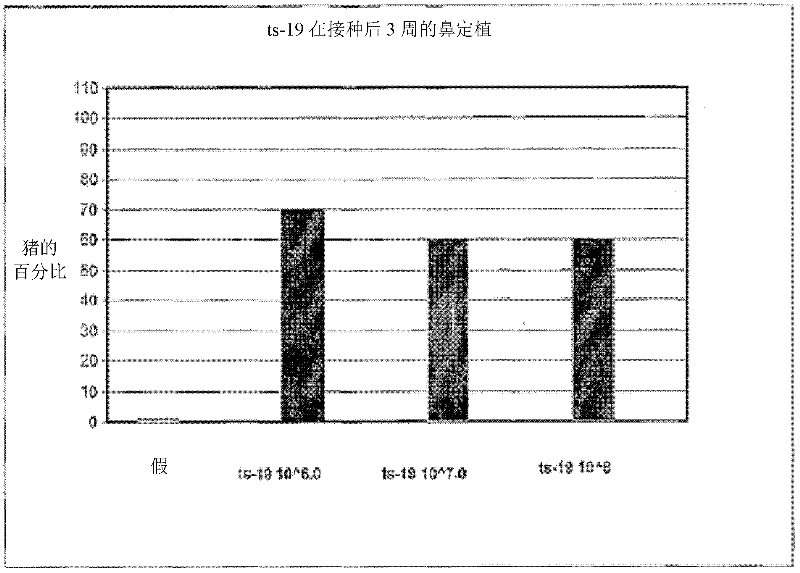
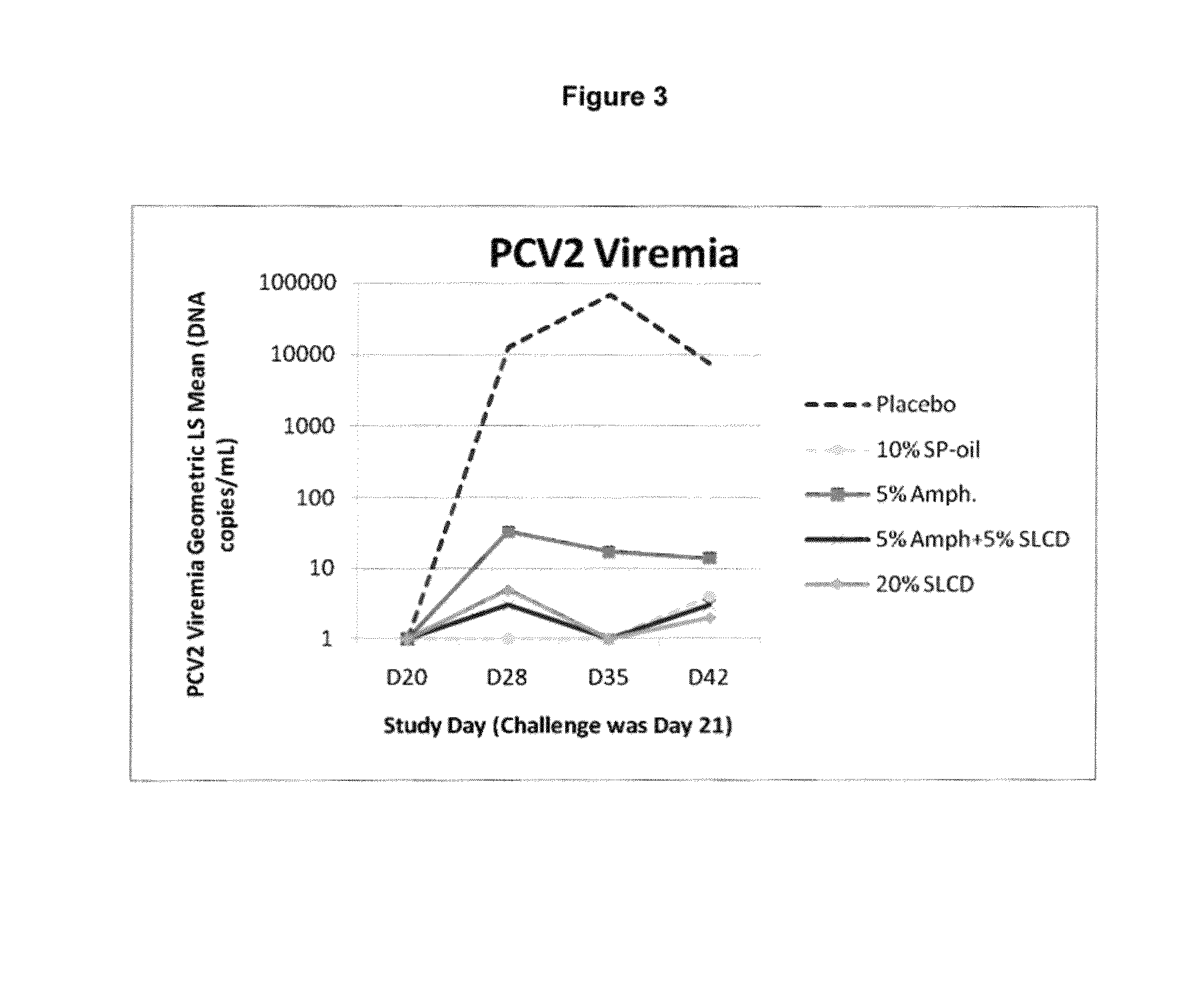
Patents
Literature
171 results about "Mycoplasma hyopneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
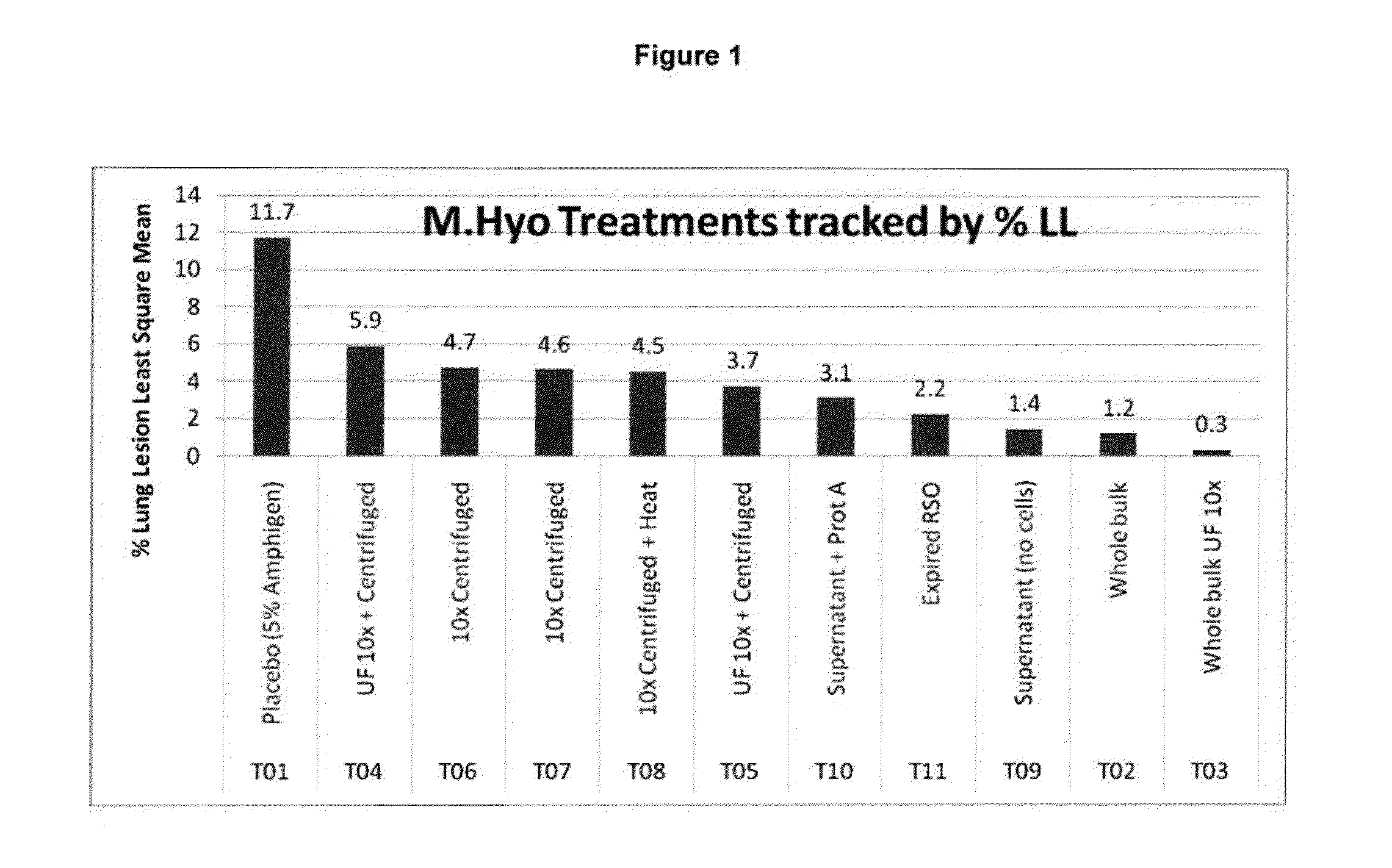
Mycoplasma hyopneumoniae is a species of bacteria known to cause the disease porcine enzootic pneumonia, a highly contagious and chronic disease affecting pigs. As with other mollicutes, M. hyopneumoniae is small in size (400–1200 nm), has a small genome (893–920 kilo-base pairs (kb)) and lacks a cell wall. It is difficult to grow in laboratories due to its complex nutritional requirements and the high chances of contamination associated with mycoplasma culture. To successfully grow the bacterium, an environment of 5–10% carbon dioxide is required, and the medium should demonstrate an acid colour shift.
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Mycoplasma hyopneumoniae bacterin vaccine
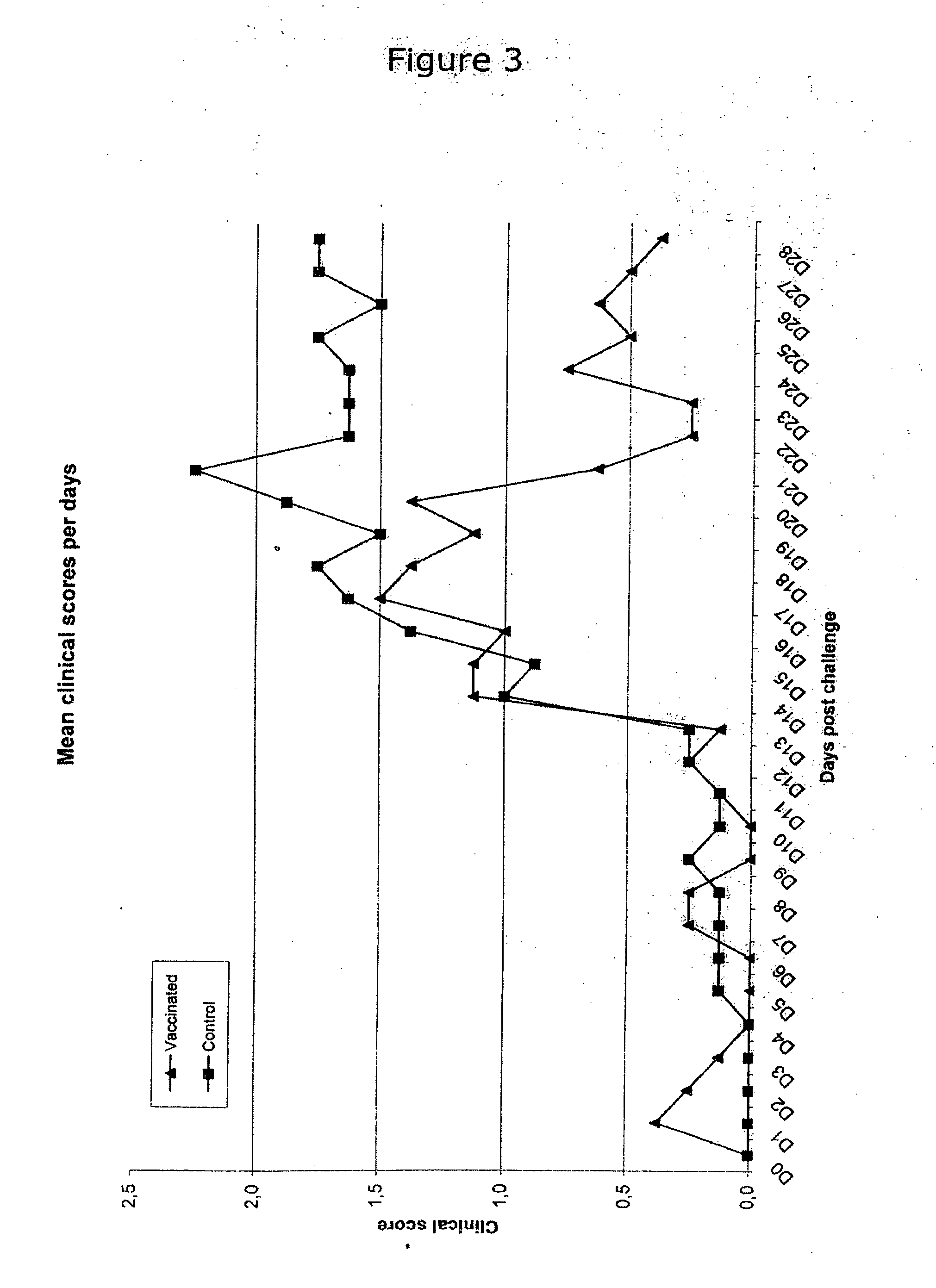
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
One dose vaccination with Mycoplasma hyopneumoniae
InactiveUS6846477B2Preventing and reducing lung lesionMaintaining immunityAntibacterial agentsBiocideDiseaseVaccines Administered
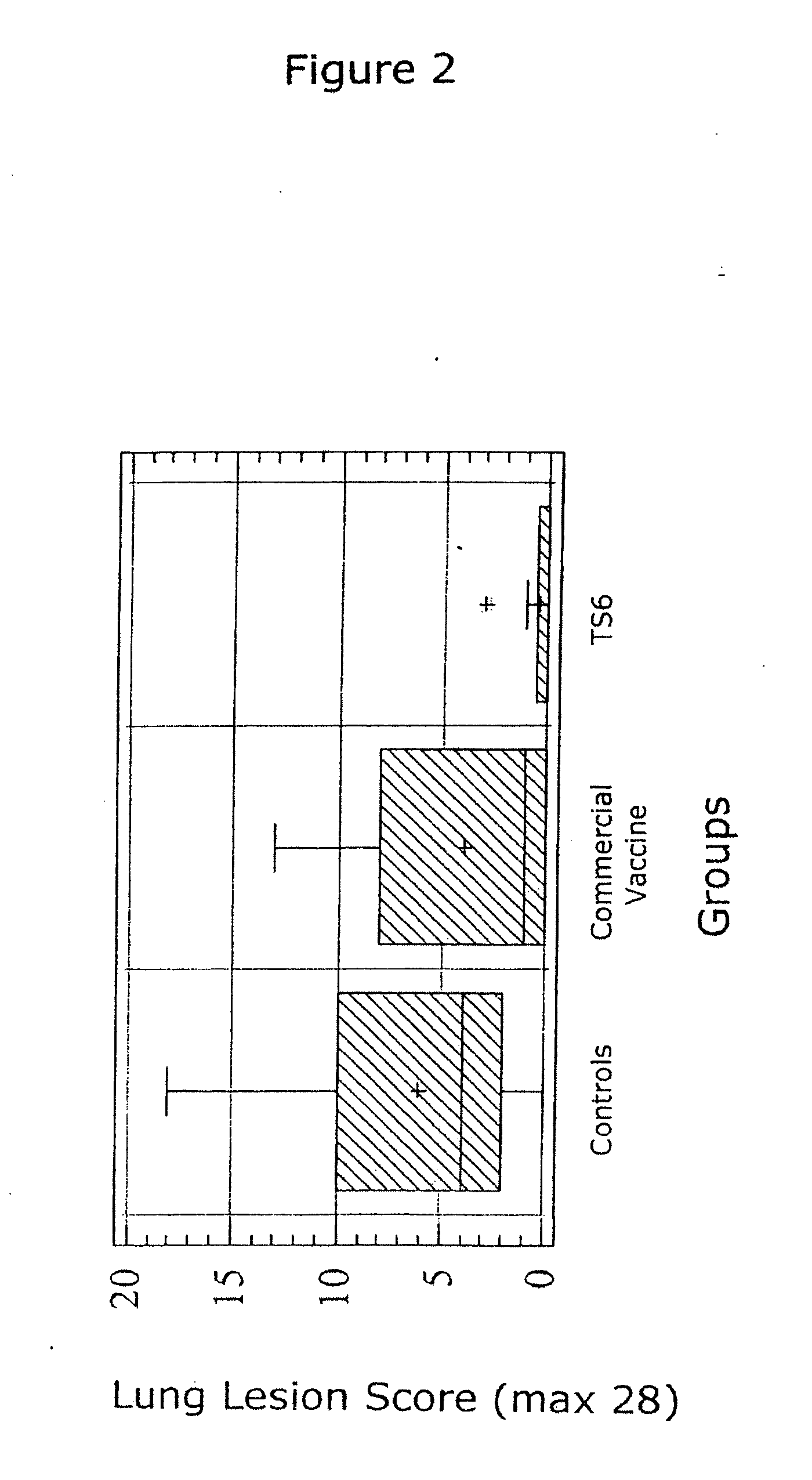
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma hyopneumoniae (M. hyo) by administering to the animal at approximately three (3) to ten (10) days of age, a single dose of an effective amount of a M. hyo vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:ZOETIS SERVICE LLC +1
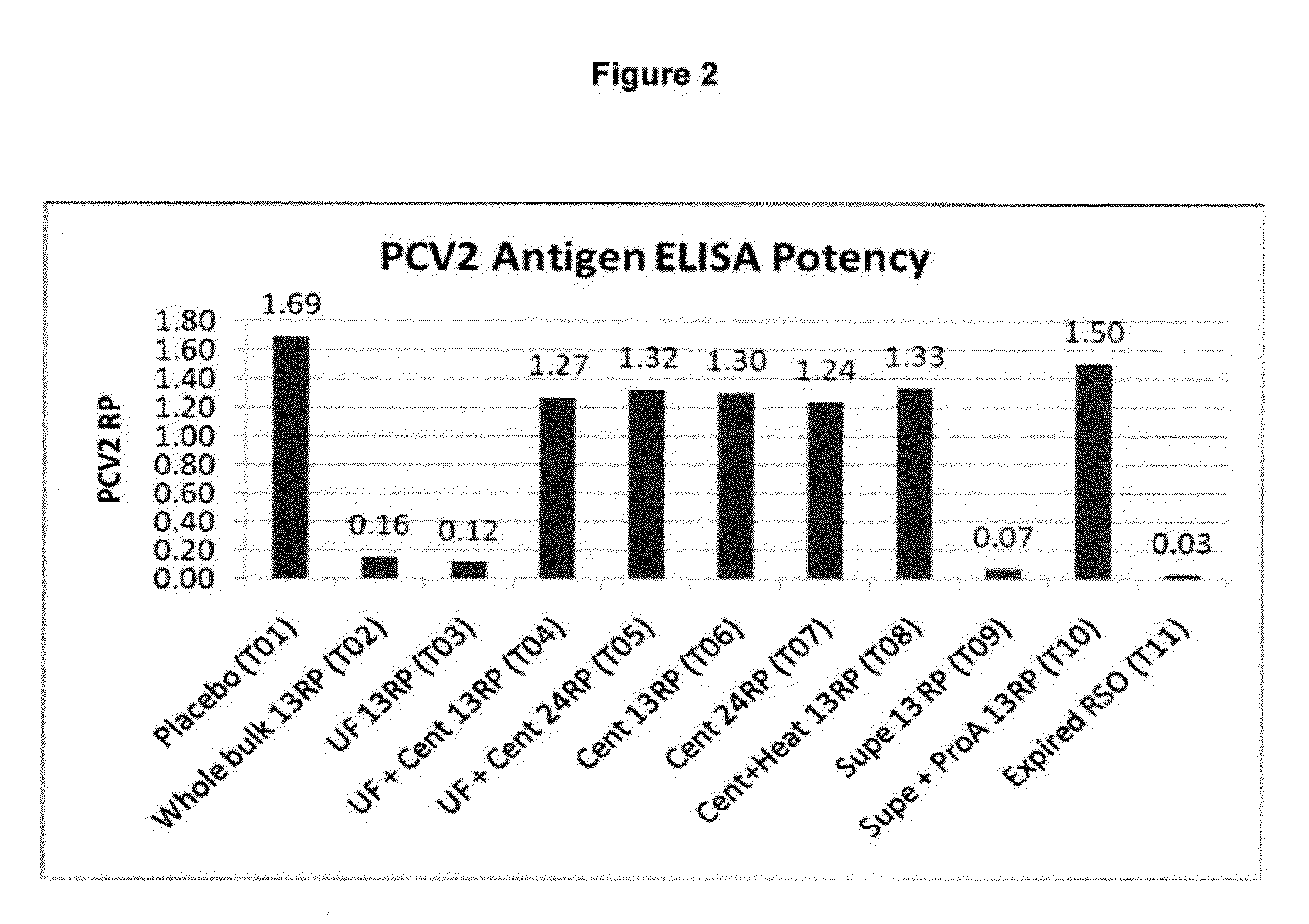
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
InactiveUS20090317423A1Reduce incidenceEffective immunityViral antigen ingredientsAntiinfectivesDiseaseActive component
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Immunogenic Mycoplasma hyopneumoniae polypeptides
Mycoplasma hyopneumoniae polypeptides and nucleic acids, as well as nucleic acid expression vectors and host cells containing nucleic acid vectors are provided. In addition, compositions containing M. hyopneumoniae polypeptides and nucleic acids are provided for use in methods of treating swine to prevent enzootic pneumonia. Furthermore, the invention provides diagnostic tests for the detecting of M. hyopneumoniae infection in swine herds.
Owner:IOWA STATE UNIV RES FOUND +1
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably Carbopol, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polypropylene block copolymer such as Pluronic®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another emodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
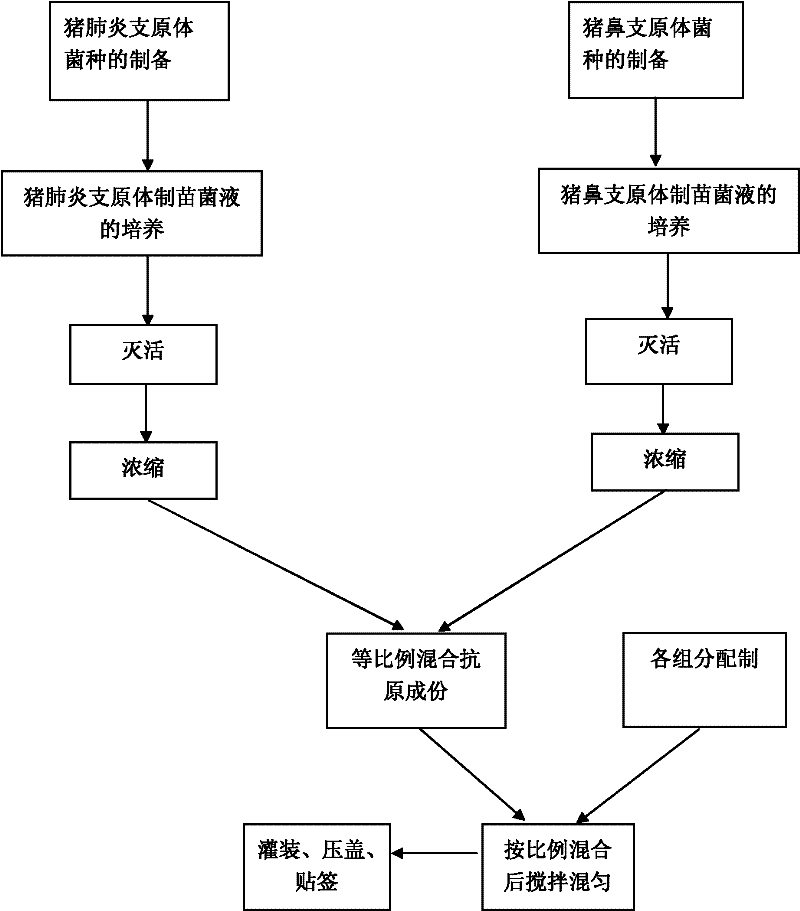
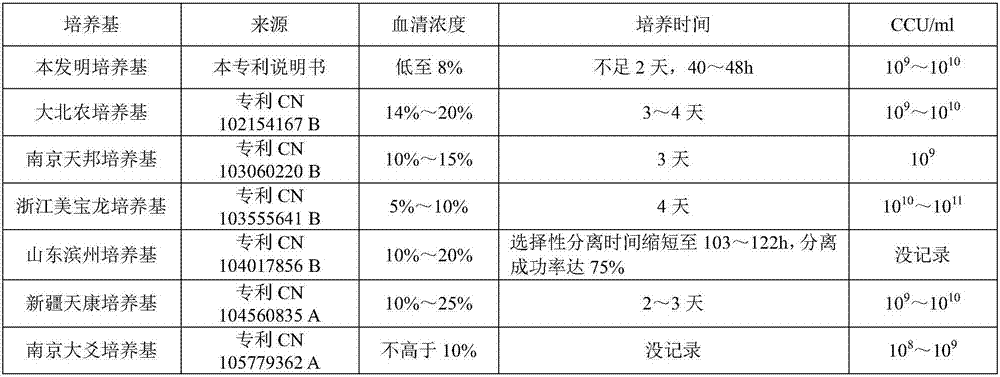
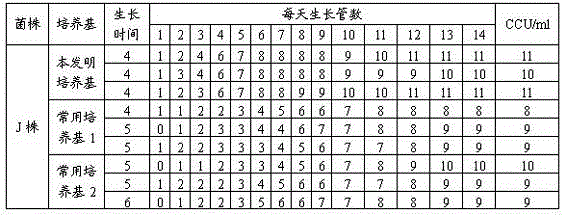
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN102154167ARich in nutrientsIncrease the titer of live bacteriaBacteriaMicroorganism based processesPenicillinMycoplasma culture
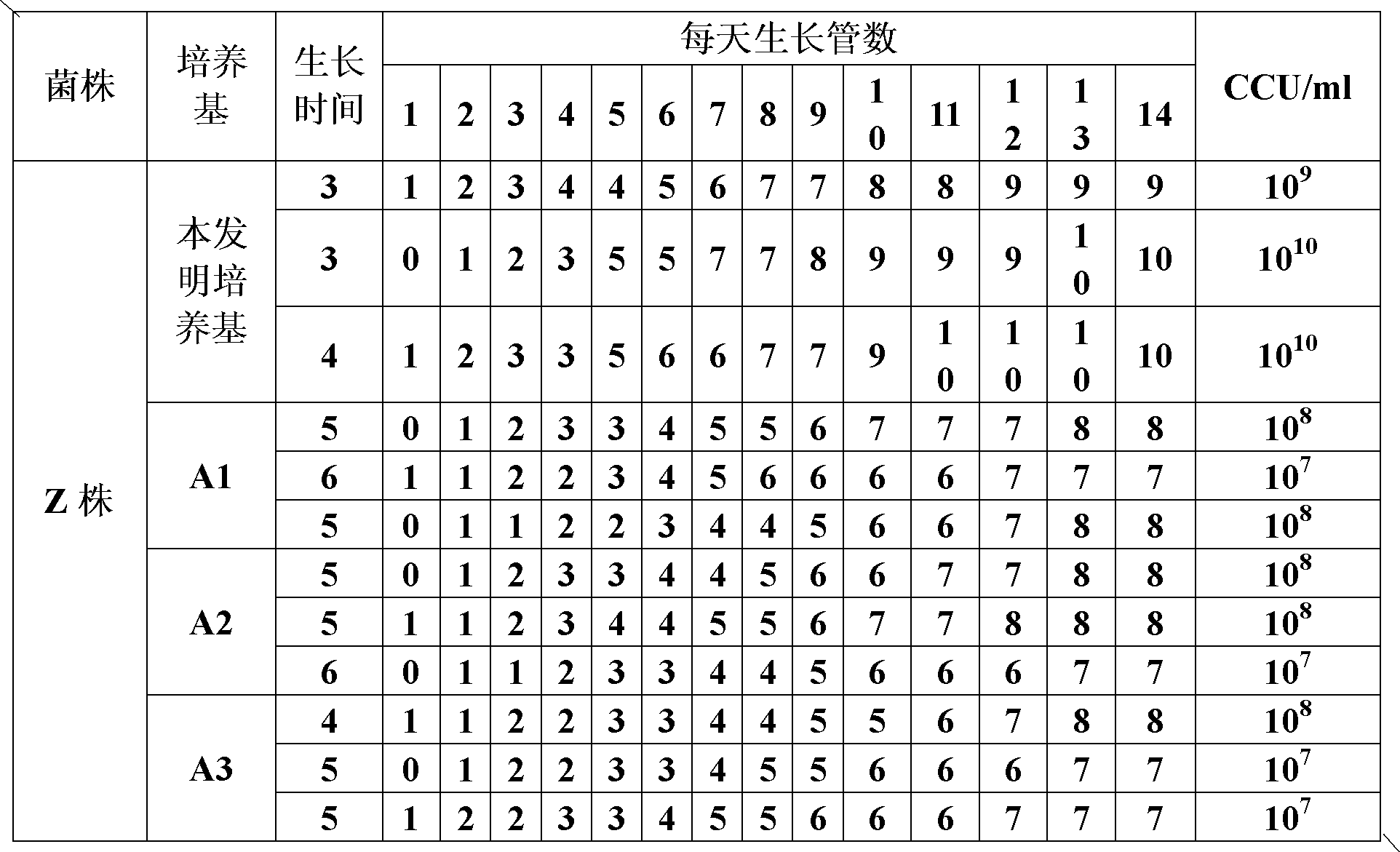
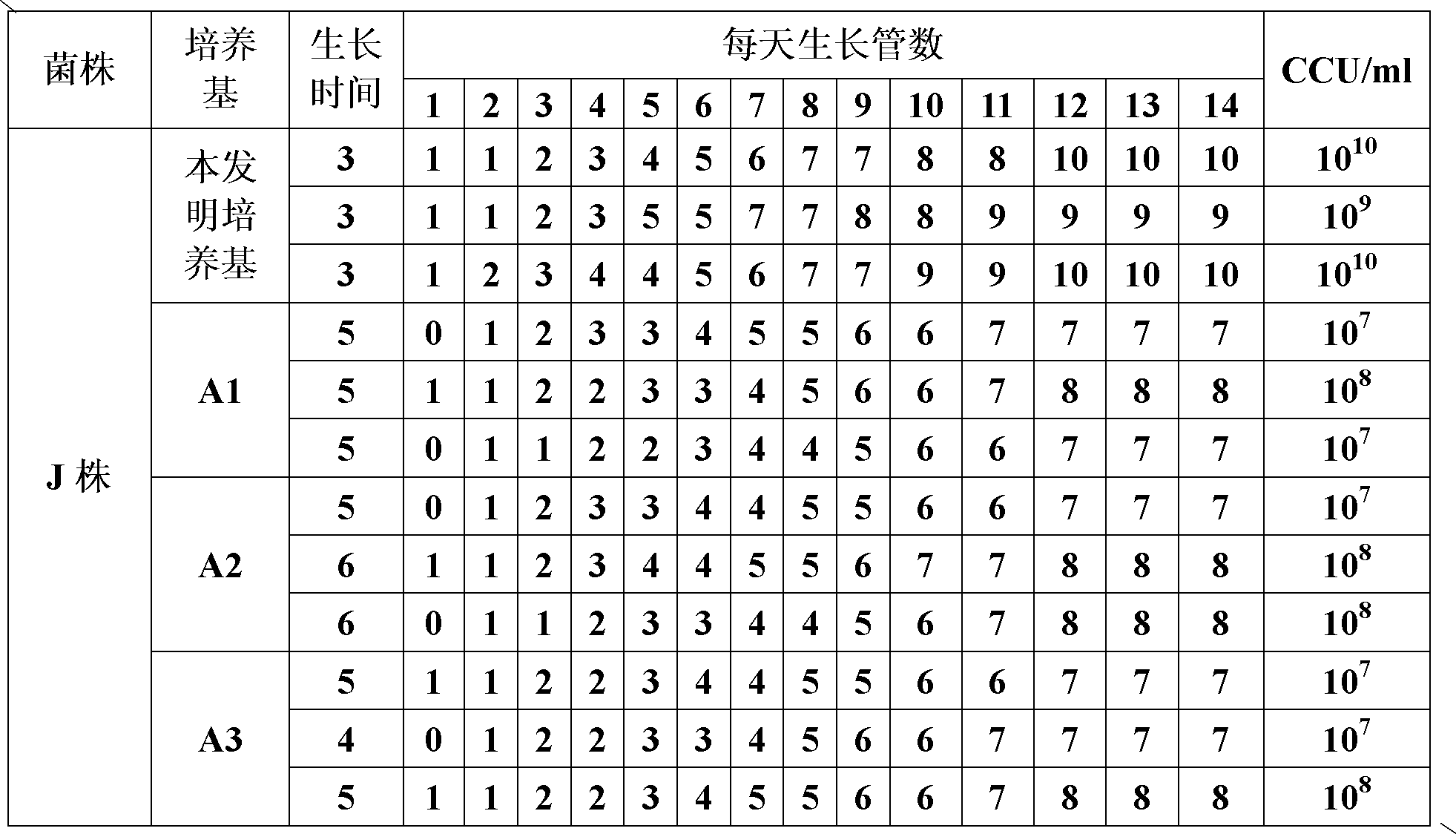
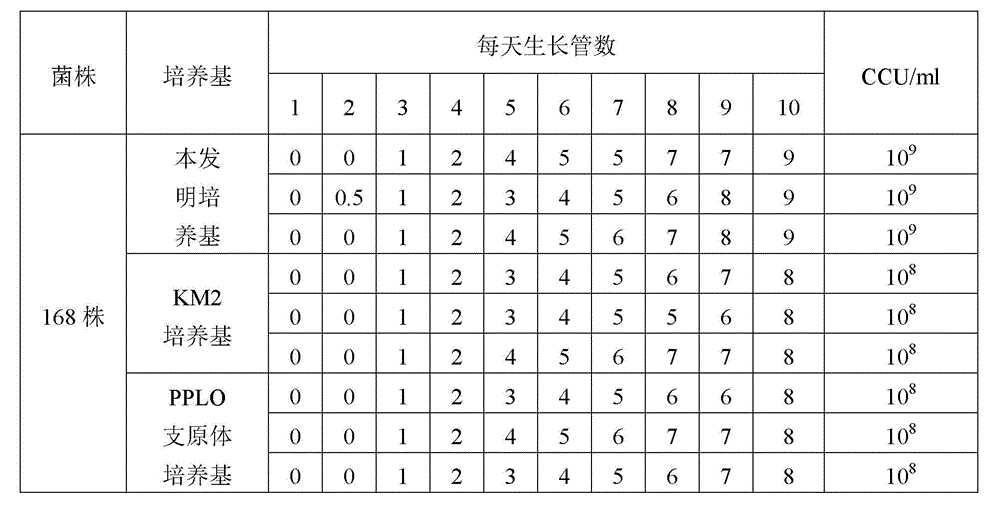
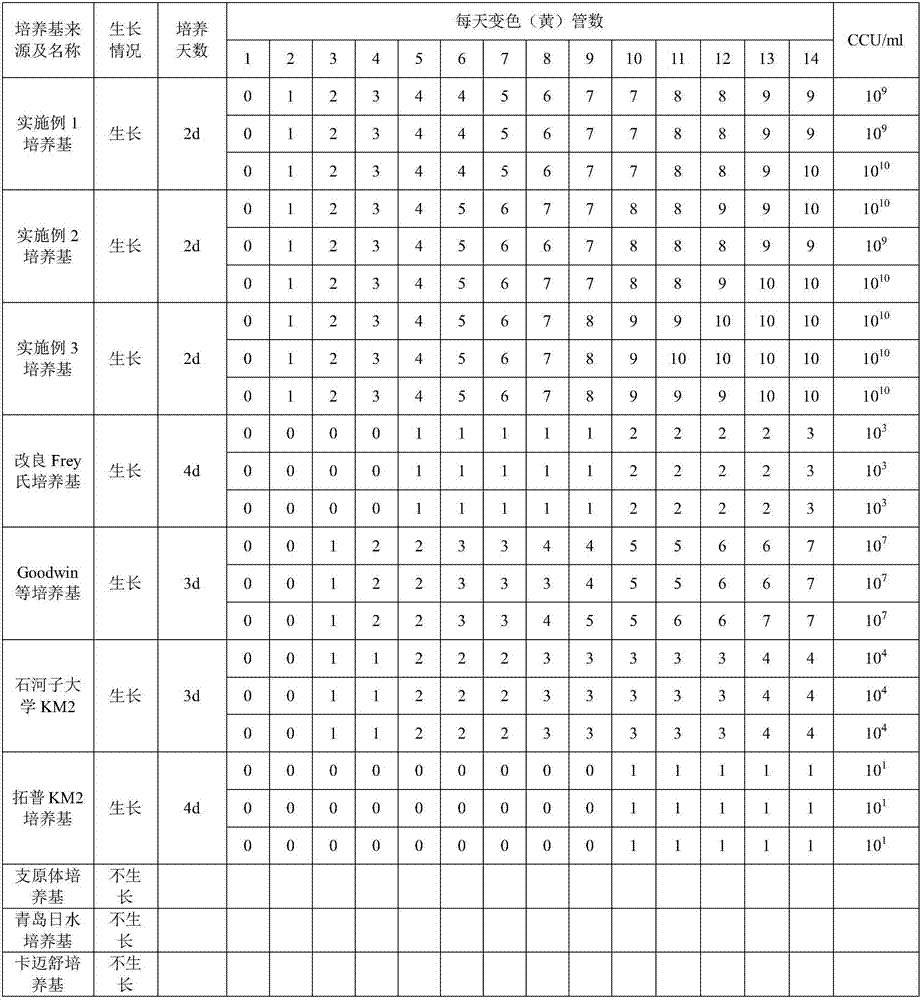
The invention provides a mycoplasma hyopneumoniae culture medium and a preparation method thereof, belonging to the technical field of veterinary biology. The mycoplasma hyopneumoniae liquid culture medium comprises the components as follows: brain heart infusion, lactalbumin hydrolysate, PPLO (pleuropneumonia-like organism) broth, yeast extract powder, proteose peptone, sodium thiosulfate, Hank's liquid, sodium pyruvate, 0.1% phenol red solution, penicillin and deionized water. The preparation method comprises the following steps of: adding health horse serum before using, and adding agar into the liquid culture medium to obtain a solid culture medium of mycoplasma hyopneumoniae. The viable bacteria titer of the mycoplasma hyopneumoniae culture medium can reach 1*109CCU / ml-1*1010CCU / ml; the viable bacteria titer and the separation sensibility are far higher than those of the existing culture medium, and the mycoplasma hyopneumoniae is fast in growth speed and high in the separation sensibility; and the preparation method of the culture medium is simple in technology, strong in operability, and suitable for industrial large-scale production.
Owner:兆丰华生物科技(南京)有限公司 +1
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
One dose vaccination against mycoplasma infections of pigs
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
One dose vaccination against mycoplasma infections of pigs
ActiveUS8444989B1Bacterial antigen ingredientsBacteriaVaccines AdministeredMycoplasma pneumoniae Infections
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
ActiveCN102258776AStrong specificityGood immune effectAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
The invention provides a combined inactivated vaccine against mycoplasma hyopneumoniae (MHP) and mycoplasma hyorhinis. The combined inactivated vaccine contains the MHP and the mycoplasma hyorhinis with preferred content as well as a Carbomer adjuvant with concentration of 10% (V / V). The invention further provides a preparation method of the combined inactivated vaccine against the MHP and the mycoplasma hyorhinis. The preparation method comprises the following steps: preparing production strains; preparing bacterium solution for producing seedlings; and inactivating, concentrating and blending to obtain the vaccine. The combined inactivated vaccine has the advantages of strong specificity and good immunity, thus solving the problem of specific infection caused by the MHP in the current domestic breeding farm and obtaining the mycoplasma hyorhinis vaccine under a blank state at home and abroad at present. The combined inactivated vaccine has the beneficial effects that the step of vaccine inoculation is simplified, trouble caused by a plurality of inoculation and easily produced side effects are avoided, and vaccine cost is saved, thus being especially applicable to preventing andtreating mixed infection diseases in the breeding farms at home and abroad and the like; and compared with the existing single vaccine, application range is widened and immune effect is enhanced.
Owner:PU LIKE BIO ENG
Low-serum culture medium for efficiently culturing mycoplasma hyopneumoniae and preparation method thereof
ActiveCN103060220AIncrease the titer of live bacteriaReduce allergic reactionsAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureOrganism
The invention relates to an efficient mycoplasma hyopneumoniae culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The efficient mycoplasma hyopneumoniae culture medium comprises an A liquid and a B liquid mainly consisting of MEM, yeast leaching powder, tryptone, glucose, inorganic salt and the like. The prepared culture medium of the invention has the main advantages of low serum content which is only 10%-15%, while the serum content in common culture medium is 20% even more. The culture medium prepared by the low serum relieves the pig allergic to the stress reaction, meanwhile gives consideration to the biosafety of animals. Besides the valence of the semi-finished bacterial solution prepared by the method is up to 109CCU / ml, which is much higher than the culture medium prepared by the common technology.
Owner:兆丰华生物科技(南京)有限公司 +1
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
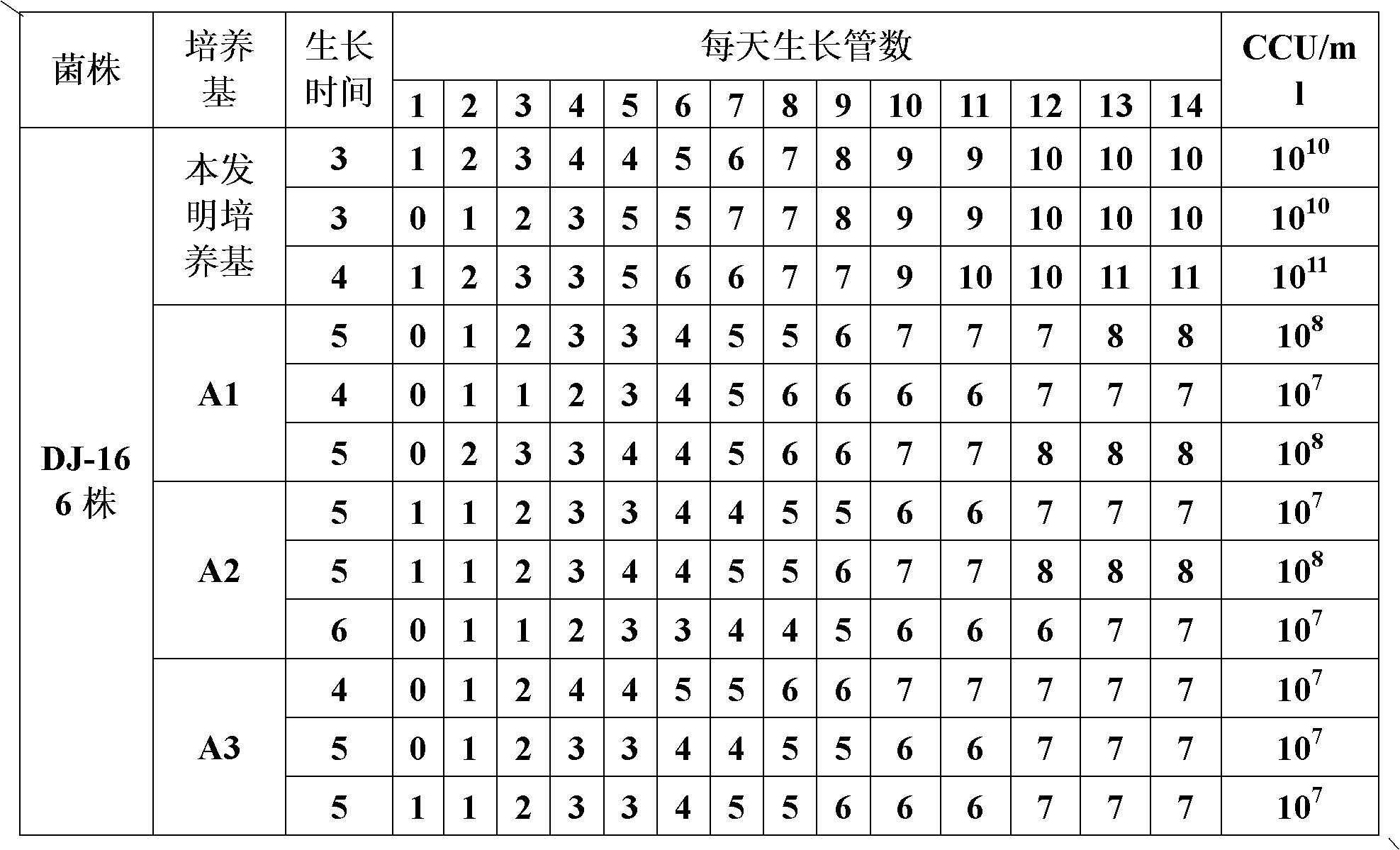
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
Pig mycoplasma pneumonia live attenuated vaccine and application thereof
ActiveCN103740625AAvoid infectionSimple and fast operationAntibacterial agentsBacterial antigen ingredientsAdjuvantPneumonia mrsa
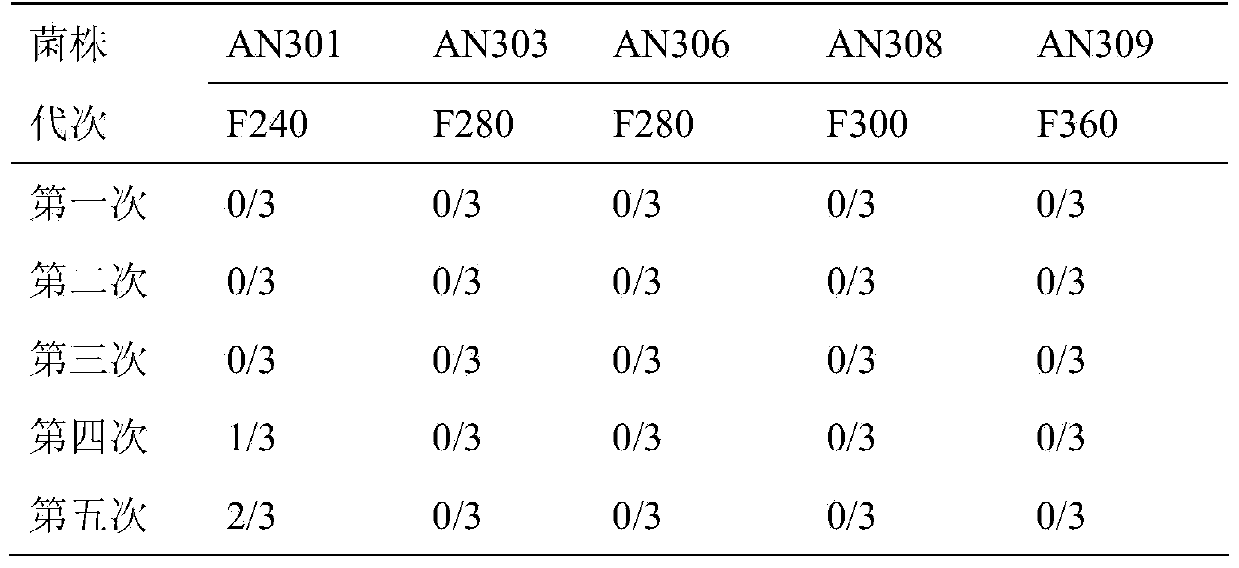
The invention discloses a pig mycoplasma pneumonia live attenuated vaccine and application thereof. A pig lung disease material infected by typical pig mycoplasma hyopneumoniae and not infected by other pathogens is screened and then is subcultured to the 100th generation through the lung of a baby rabbit, then pig mycoplasma hyopneumoniae strains are separated and are continuously subcultured through a culture medium; meanwhile, the mycoplasma hyopneumoniae AN306 is obtained through screening of a plurality of strains, and the preservation number of the mycoplasma hyopneumoniae AN306 is CCTCC M2012431. The invention also relates to a pig mycoplasma pneumonia live vaccine preparation based on the preparation of the attenuated vaccine strain. The pig mycoplasma pneumonia live vaccine preparation comprises a live attenuated vaccine strain, a pharmaceutically acceotable carrier or auxiliary ingredient and an adjuvant, and further comprises immunogens of other pathogens. The pig mycoplasma pneumonia live vaccine disclosed by the invention can be immunized in multiple ways and can enable animals to gain protection ability for resisting pig mycoplasma hyopneumoniae infection.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Mycoplasma hyopneumoniae avirulent adjuvanted live vaccine
InactiveCN101883581AAntibacterial agentsBacterial antigen ingredientsDiseaseVirulent characteristics
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS W LLC
PCV/Mycoplasma Hyopneumoniae Combination Vaccine
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Mycoplasma hyopneumoniae DJ-166 strain and application thereof
ActiveCN103184171AReduce manufacturing costImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsMicroorganismCell free
The invention belongs to the field of veterinary microbial technology, and particularly relates to a mycoplasma hyopneumoniae DJ-166 strain and an application thereof. The mycoplasma hyopneumoniae can be used for preparing veterinary biological products or veterinary drugs for prevention. Experiments demonstrate that the mycoplasma hyopneumoniae DJ-166 strain has high active bacterium titer reaching 10<10-11> CCU / mL in a prepared cell-free culture medium, can greatly reduce production cost, and has good immunogenicity with an average pneumonia pathology reduction ratio reaching over 80%.
Owner:兆丰华生物科技(南京)有限公司 +1
Mycoplasma Hyopneumoniae Vaccine
ActiveUS20130266601A1Antibacterial agentsSsRNA viruses positive-senseImmunoglobulin IgEMycoplasma hyopneumoniae
This invention provides an immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M.hyo) whole cell preparation, wherein the soluble portion of the M.hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Mycoplasma hyopneumoniae vaccine and methods for reducing mycoplasma bovis pneumonia in cattle
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis (M. bovis) by administering to the animal an effective amount of a Mycoplasma hyopneumoniae (M. hyo) vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
A temperature sensitive vaccine strain of mycoplasma hyopneumoniae and uses thereof
ActiveCN102458462AAntibacterial agentsBacterial antigen ingredientsGene listMycoplasma hyopneumoniae
The present invention relates to a Mycoplasma hyopneumoniae vaccine strain comprising a mutation in at least one of the genes listed or as deposited with the National Measurements Institute (Australia) under accession number NM04 / 41259, which strain is temperature sensitive and attenuated, a vaccine comprising such strains and methods and uses thereof.
Owner:BIOPROPERTIES +1
Mycoplasma hyopneumoniae vaccine
Owner:ZOETIS SERVICE LLC
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
ActiveUS20150174233A1Reduce incidenceReduce severityBacterial antigen ingredientsViral antigen ingredientsDiseaseMultivalent Vaccine
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Mycoplasma hyopneumoniae culture medium and preparation method thereof
The invention relates to a mycoplasma hyopneumoniae culture medium and a preparation method thereof. The mycoplasma hyopneumoniae culture medium consists of a basic culture medium and an auxiliary culture medium, wherein the basic culture medium has major components of Hank's liquid, lactoprotein hydrolysate, a yeast extract and an ox heart extract, sterilization can be performed through autoclaving, and a pollution risk caused by filtration sterilization is greatly reduced; and the auxiliary culture medium comprises pig serum, argenine, cysteine, phenol red solution, penicillium and the like, the pig serum is sterilized through cobalt radiation, and the rest components are mixed with inactivated pig serum after filtration sterilization. The titer of a lapinized attenuated strain of the mycoplasma hyopneumoniae cultured with the culture medium provided by the invention is 109-1010 CCU, the minimum culture time may be 40 h, the generation of old bacteria and aged bacteria is greatly reduced, the usage amount of the pig serum can be as low as 8%, the allergic stress reaction caused by the pig serum is reduced, and the production cost of an enterprise is lowered.
Owner:JIANGSU NANNONG HI TECH
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN103555641AImprove enduranceReduce apoptosis rateBacteriaMicroorganism based processesBacteroidesMycoplasma culture
The invention discloses a mycoplasma hyopneumoniae culture medium and a preparation method thereof, which belong to the technical field of veterinary biological products. Each 1000 ml of phosphate buffer contains 20-50 g of PPLO (pleuropneumonia-like organism), 2-10 g of yeast powder, 3-15 g of glucose, 0.5-4 ml of 1% phenol red, 2-10 g of amino acid composition, 15-30 g of compound traditional Chinese medicine polysaccharide and 50-100 ml of pig serum. When culturing the mycoplasma hyopneumoniae, the mycoplasma hyopneumoniae culture medium disclosed by the invention can improve the tolerance of the mycoplasma hyopneumoniae and reduce the apoptosis rate of the mycoplasma hyopneumoniae; the mycoplasma hyopneumoniae culture medium disclosed by the invention can increase the amount of culture bacteria of the mycoplasma hyopneumoniae, the concentration of the culture bacteria liquid reaches 10<10-11> / ml, which is increased by more than 5-10 times compared with that of a common culture medium; the mycoplasma hyopneumoniae culture medium disclosed by the invention can inhibit the growth of other bacteria when culturing the mycoplasma hyopneumoniae so as to reduce the culture pollution risk of culturing the mycoplasma hyopneumoniae.
Owner:浙江美保龙生物技术有限公司
Mycoplasma hyopneumoniae P97R1 gene recombined Pichia pastoris and expression protein
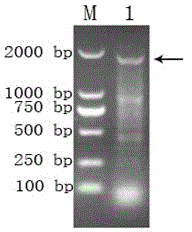
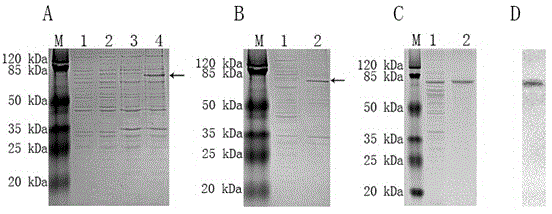
InactiveCN101565680ASecretion efficiently guidesGood antigenicityFungiMicroorganism based processesProtein targetGenetic engineering
The invention discloses Mycoplasma hyopneumoniae P97R1 gene recombined Pichia pastoris and expression protein, and relates to the field of bioprotein preparation. The invention is mainly obtained by gene acquisition, establishment of an expression vector and expression of target protein. A method for preparing the protein comprises the following steps that: a PCR method and uses a designed primer to obtain the target protein from Mycoplasma hyopneumoniae genome DNA by amplification; the target gene is cloned into a yeast expression vector pPICZalpha-A through enzyme restriction and connection; a secretion type recombined yeast expression vector pPICZalpha-A / P97R1 is established; and the pPICZalpha-A / P97R1 is electrically converted into a Pichia pastoris strain GS115 through Sac I enzyme restriction and linearization. The recombined Pichia pastoris expresses the P97R1 protein through methanol induction and secretion. The Mycoplasma hyopneumoniae P97R1 protein prepared by the method is pure, has good immunoreaction, and can be used for researching Mycoplasma hyopneumoniae P97 protein and developing Mycoplasma hyopneumoniae immunodetection kits and genetic engineering vaccines.
Owner:JIANGSU ACAD OF AGRI SCI
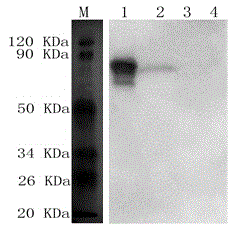
Anti-swine SC protein monoclonal antibody and application of monoclonal antibody in preparing mycoplasma hyopneumoniae SIgA antibody ELISA detection kit
ActiveCN104877027AAvoid cross interferenceAvoid interferenceTissue cultureImmunoglobulinsImmune effectsProtein.monoclonal
The invention provides an anti-swine SC protein monoclonal antibody and an application of the monoclonal antibody in preparing a mycoplasma hyopneumoniae SIgA antibody ELISA detection kit, and relates to the technical field of animal virological and epizootiological detection. The anti-swine SC protein monoclonal antibody is secreted by a hybridoma cell strain 4H11 and the collection number of the anti-swine SC protein monoclonal antibody is CCTCC NO: C201526. The invention also discloses the monoclonal antibody and the application of the monoclonal antibody in preparing the mycoplasma hyopneumoniae SIgA antibody ELISA detection kit. The kit has high specificity, high stability and high sensitivity; a detection sample can be sampled conveniently; and the kit is capable of distinguishing porcine mycoplasma pneumonia inactivated vaccine immunization and natural infection, and can be applied to the early diagnosis of pneumonic porcine mycoplasma infection and the evaluation of the immune effect after attenuated live vaccine immunization.
Owner:JIANGSU ACAD OF AGRI SCI
Mycoplasma hyopneumoniae fusion gene and application
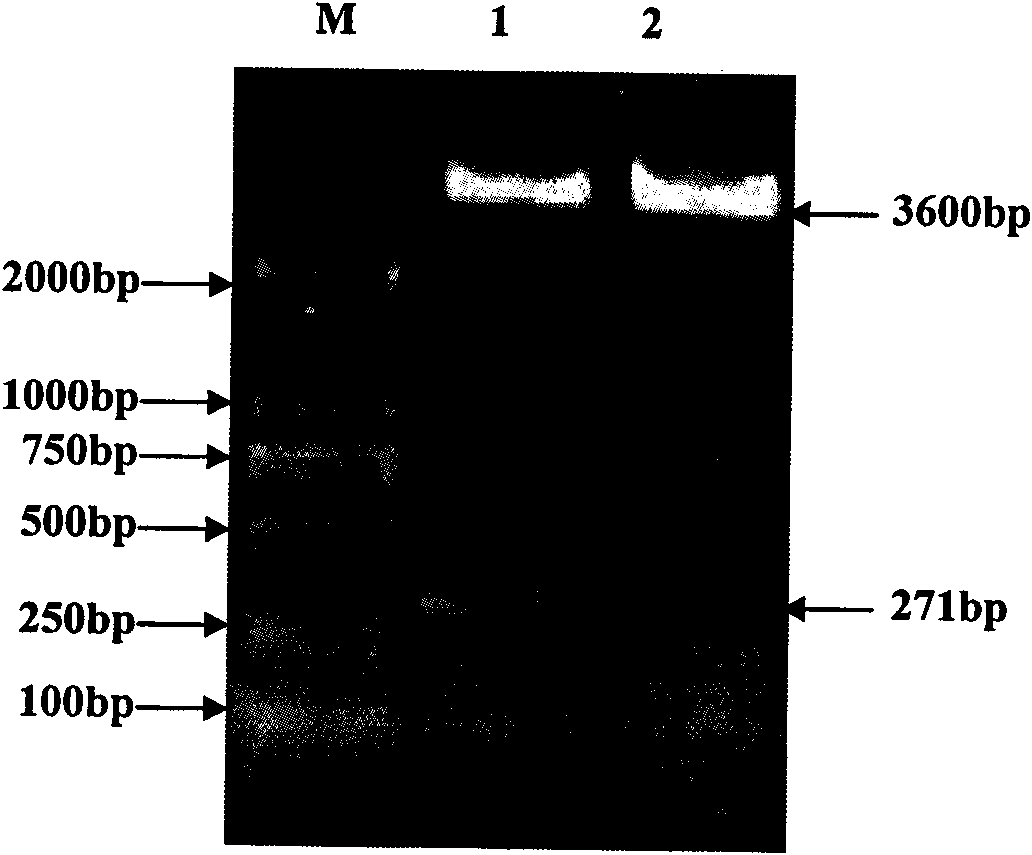
ActiveCN104293816AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
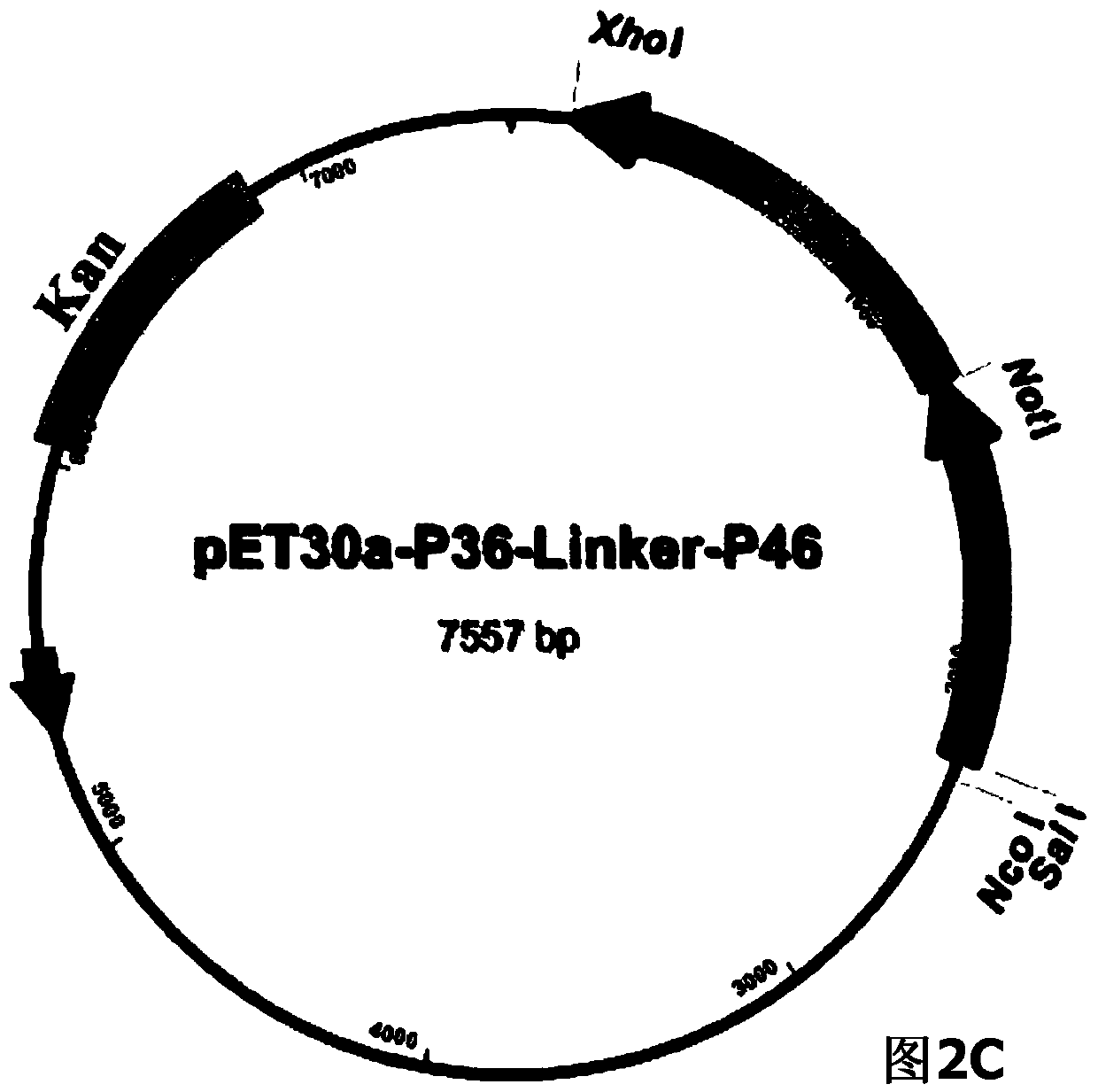
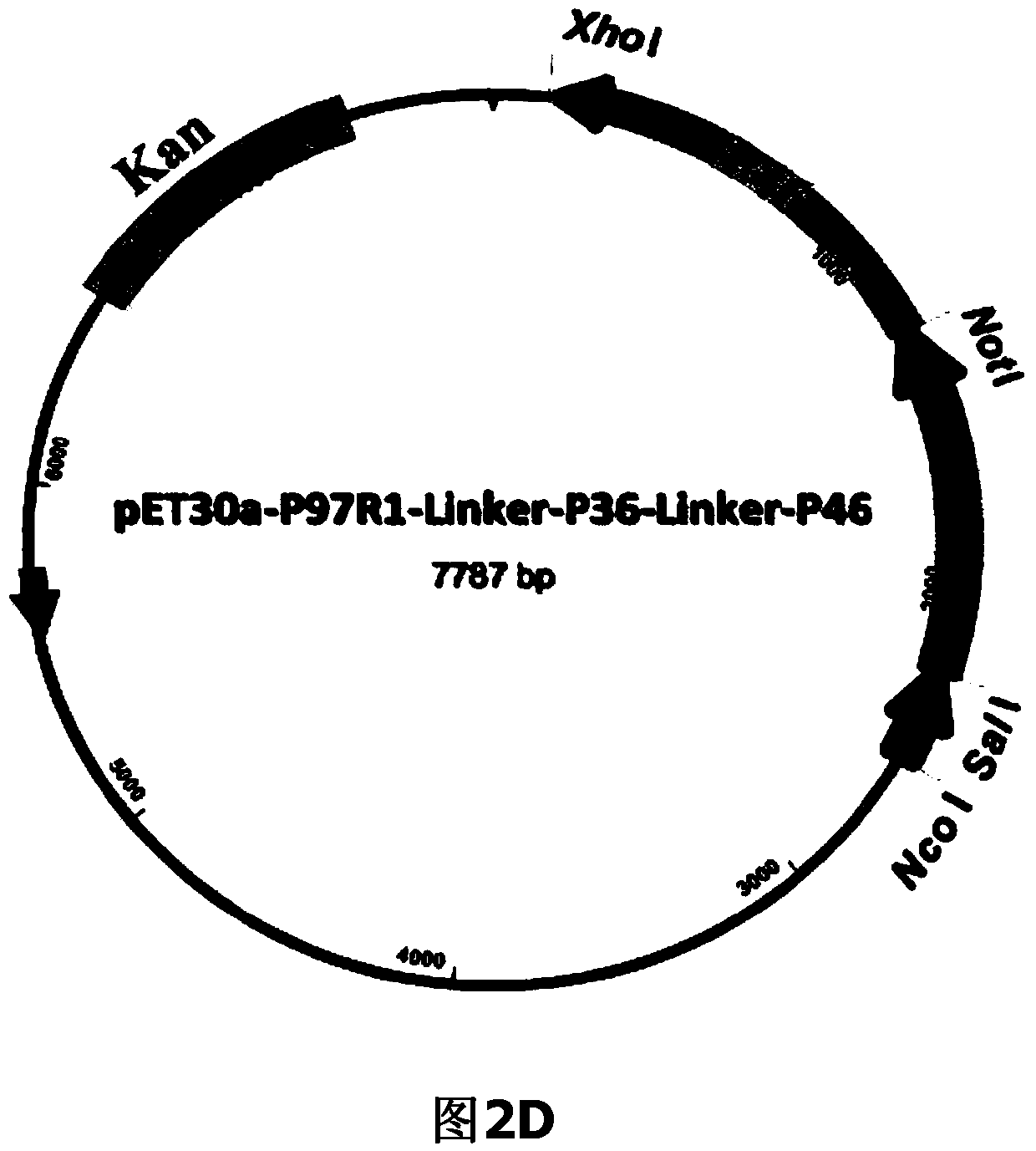
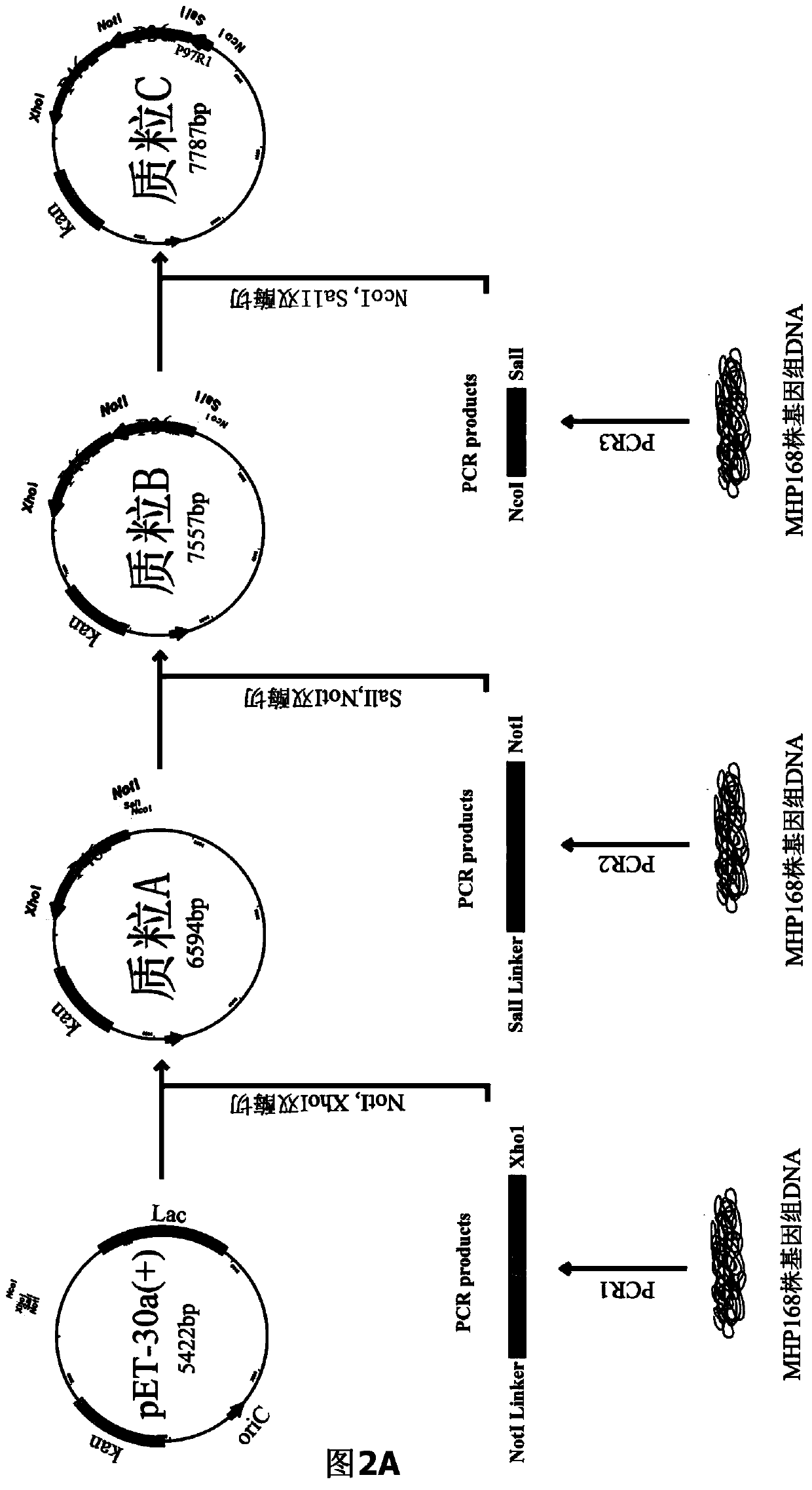
The invention belongs to the field of animal gene engineering, and in particular relates to a synthetic fusion gene for expressing mycoplasma hyopneumoniae and application. The fusion gene is characterized in that a mycoplasma hyopneumoniae P97R1 gene is connected in series with a P36 gene through a Linker, the termination codon of the P36 gene is deleted, the P46 gene with signal peptide removed is fused at the C end of the P36 gene through Linker, and then the fusion gene P97R1-P36-P46 is obtained, wherein the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The fusion gene is included in a prokaryotic expression plasmid, and escherichia coli BL21 / pET30a-P97R1-Linker-P36-Linker-P46 containing the plasmid is collected in CCTCC with the collection number CCTCC NO: M2014269. The invention further discloses immune efficacy evaluation of the fusion gene and application of the fusion gene in a novel vaccine.
Owner:HUAZHONG AGRI UNIV
Mycopasma hyopneumoniae strain
InactiveCN103484414AReduce generation costImproving immunogenicityBacteriaMicroorganism based processesMicroorganismMycoplasma hyopneumoniae
The invention discloses a mycoplasma hyopneumoniae strain which is preserved in the Ordinary Microorganism Center of the Chinese Microorganism Culture Collection and Management Committee which is located in #3, No.1 Yard, Beichen Road, Chaoyang District, Beijing, the preservation number is CGMCC No.8096, and the preservation date is August 15th, 2013. The name of the strain is mycoplasma hyopneumoniae HDZK-Mhp57. According to the mycoplasma hyopneumoniae HDZK-Mhp57, the viable bacteria titer of a prepared acellular culture medium is as high as 1012CCU / mL, the immunogenicity is good, the pneumonia lesion is reduced by more than 90%, the generation cost of an animal biological product is greatly lowered, the stain can be prepared into preventing animal biological products or veterinary medicines, and a foundation for researching and preparing mycoplasma hyopneumoniae inactivated vaccines, genetic engineering vaccines and mycoplasma hyopneumoniae diagnosis kits is laid.
Owner:HEILONGJIANG UNIV
PCV/mycoplasma hyopneumoniae/PRRS combination vaccine
Owner:ZOETIS SERVICE LLC
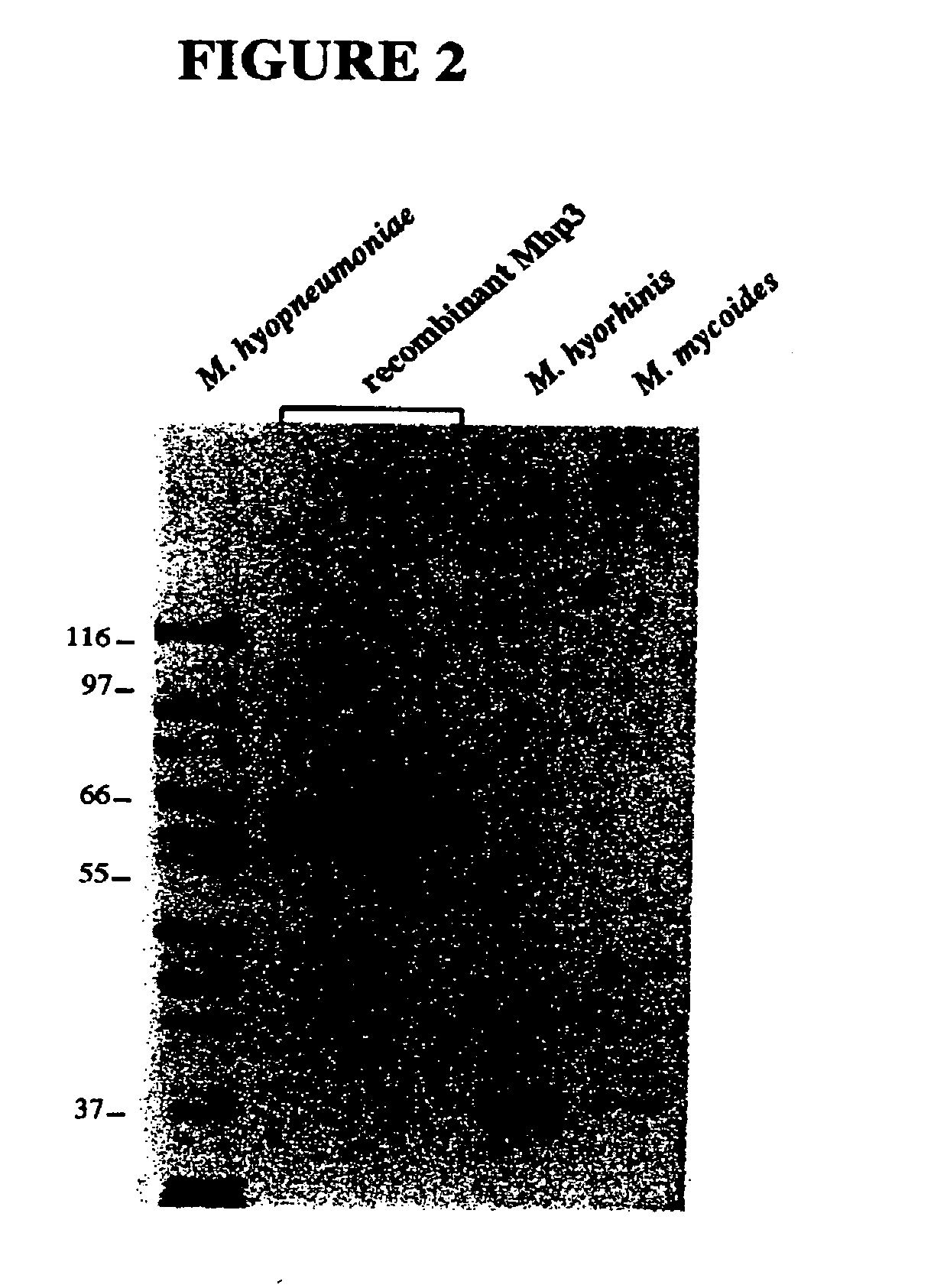
Nucleic acids and proteins of the mycoplasma hyopneumoniae mhp3 gene and uses thereof
InactiveUS20060233823A1Increase stringencyAntibacterial agentsBacteriaAntigenMycoplasma hyopneumoniae
The present invention relates to mhp3 nucleic acids and proteins encoded by the foregoing. The present invention further relates to novel apoprotein antigens encoded by mhp3 for use in vaccines to prevent and treat diseases caused by infection with Mycoplasma hyopneumoniae. The invention further relates to methods for the recombinant production of such antigens.
Owner:KING KENDALL +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com