Patents
Literature
35 results about "Vaccines Administered" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diphtheria and tetanus toxoids and pertussis vaccine DTP vaccine: a combination of diphtheria and tetanus toxoids and pertussis vaccine; administered intramuscularly for simultaneous immunization against diphtheria, tetanus, and whooping cough. When the pertussis vaccine is an acellular form, the combination may be abbreviated DTaP.
One dose vaccination with Mycoplasma hyopneumoniae
InactiveUS6846477B2Preventing and reducing lung lesionMaintaining immunityAntibacterial agentsBiocideDiseaseVaccines Administered
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma hyopneumoniae (M. hyo) by administering to the animal at approximately three (3) to ten (10) days of age, a single dose of an effective amount of a M. hyo vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:ZOETIS SERVICE LLC +1
One dose vaccination against mycoplasma infections of pigs
ActiveUS8444989B1Bacterial antigen ingredientsBacteriaVaccines AdministeredMycoplasma pneumoniae Infections
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Antigens and Vaccines Directed Against Human Enteroviruses
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human enterovirus B, Human enterovirus C, Human enterovirus D antigens and their use in vaccines for the prevention of an Enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Mycoplasma hyopneumoniae vaccine and methods for reducing mycoplasma bovis pneumonia in cattle
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis (M. bovis) by administering to the animal an effective amount of a Mycoplasma hyopneumoniae (M. hyo) vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Mycoplasma bovis vaccine and methods of reducing pneumonia in animals
The present invention relates to Mycoplasma bovis vaccines and methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis by administering to the animal an effective amount of a Mycoplasma bovis vaccine. The Mycoplasma bovis vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The Mycoplasma bovis vaccine administered in accordance with the present can be synthesized or recombinantly produced. The invention also relates to combination vaccines, methods of preparing Mycoplasma bovis vaccines and kits.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Recombinant fusion antigen gene, recombinant fusion antigen protein and subunit vaccine composition having the same against infection of porcine reproductive and respiratory syndrome virus
ActiveUS20160137699A1High expressionBetter protection ability without the risks of virulent spread andSsRNA viruses positive-senseAntibody mimetics/scaffoldsBaculovirus expressionEpitope
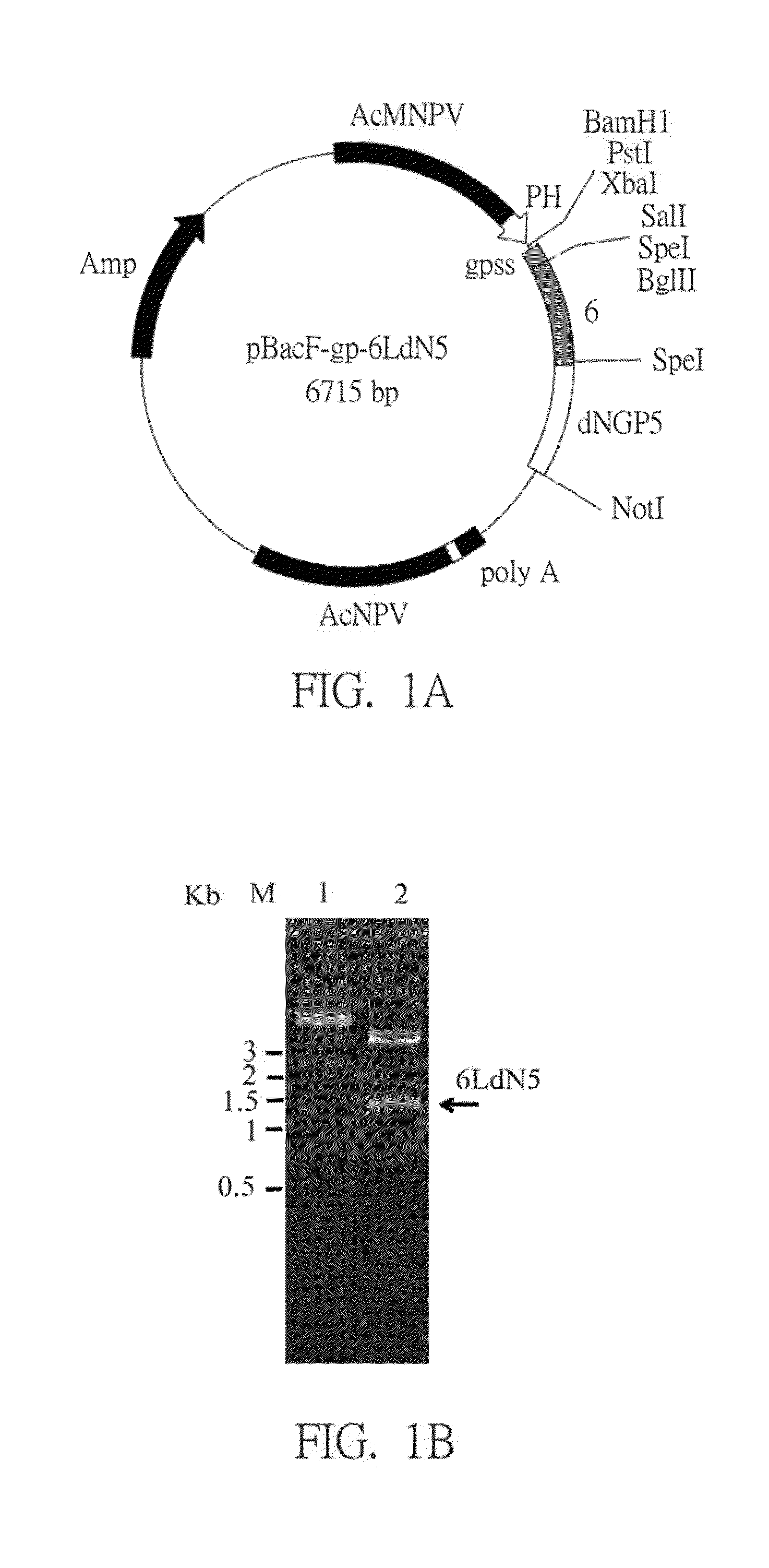
The present invention is directed to a recombinant fusion antigen gene, a recombinant fusion antigen protein and a subunit vaccine composition having the same against infection of porcine reproductive and respiratory syndrome virus (PRRSV). A recombinant fusion antigen gene, which encodes glycoprotein GP5 with truncated N′-terminal decoy epitope, a linker sequence and membrane protein M, followed by codon optimization, is expressed by a baculovirus expression system in vitro, thereby enhancing a yield of the recombinant fusion antigen protein. The recombinant fusion antigen protein can be applied in a subunit vaccine composition, for providing vaccinated animals with better protection ability without the risks of virulent spread and virulent recovery.
Owner:NAT PINGTUNG UNIV OF SCI & TECH
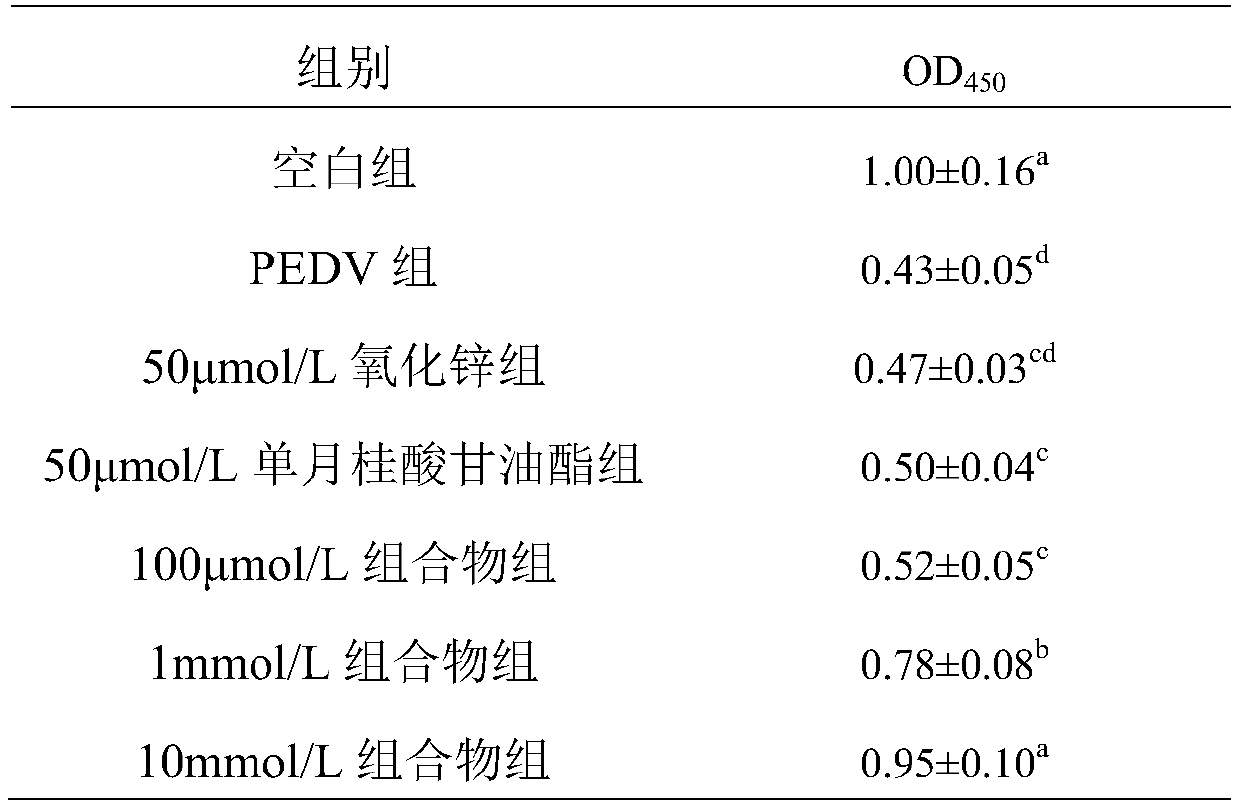
Composition resisting porcine epidemic diarrhea virus infection and application thereof
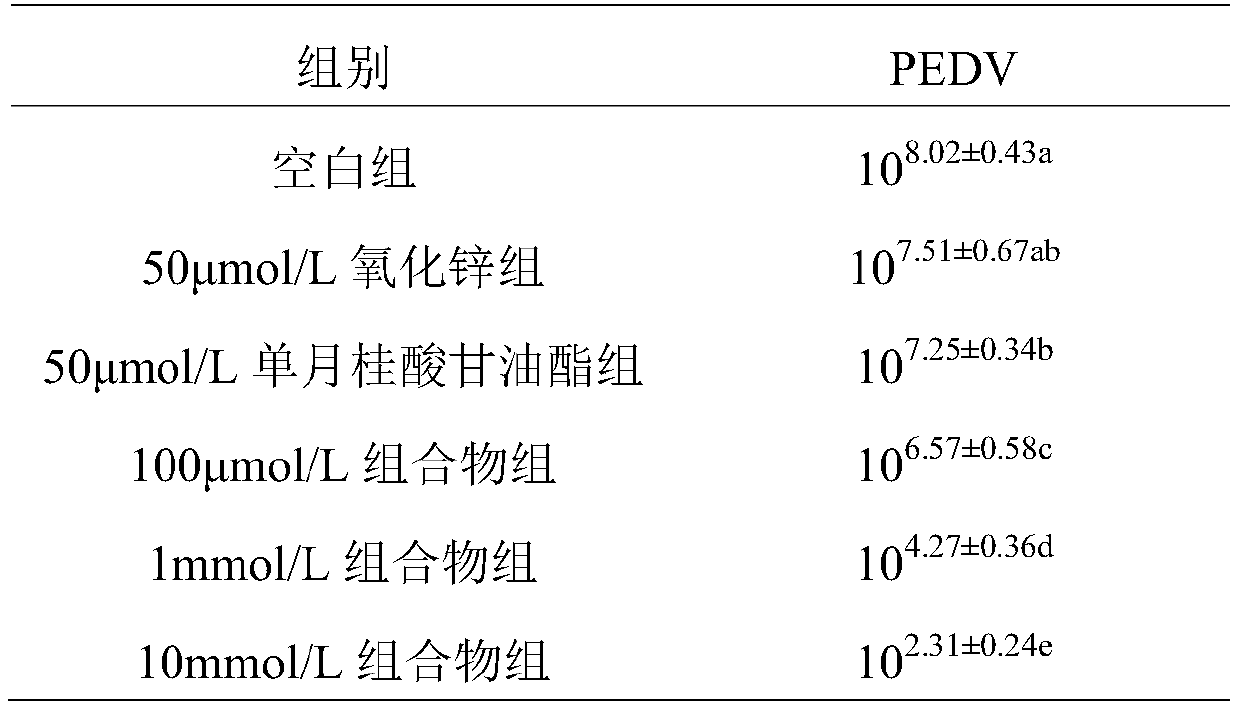
ActiveCN110354139APrevent proliferationGive full play to the synergistic anti-PEDV effectInorganic active ingredientsAntiviralsAntiviral drugSide effect
The invention discloses a composition resisting porcine epidemic diarrhea virus infection and application thereof. The composition comprises zinc oxide and glycerol monolaurate in a mass ratio of (0.5-2):1. The composition has the remarkable effects of inhibiting the replication of the porcine epidemic diarrhea virus and reducing the virulence of the porcine epidemic diarrhea virus, can serve as afeed additive or an oral agent, overcomes the defects of immunity failure possibly occurring after vaccination and virus variation caused by the use of antiviral drugs, has no toxic or side effect onanimals, and can be used safely.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Novel multivalent nanoparticle-based vaccines
Novel, nanoparticle-based vaccines are provided that elicit an immune response to a broad range of infectious agents, such as influenza viruses. The nanoparticles comprise a heterogeneous population of fusion proteins, each comprising a monomeric subunit of a self-assembly protein, such as ferritin, joined to one or more immunogenic portions of a protein from an infectious agent, such as influenza virus. The fusion proteins self- assemble to form nanoparticles that display a heterogeneous population of immunogenic portions on their surface. When administered to an individual, such nanoparticles elicit an immune response to different strains, types, subtypes and species with in the same taxonomic family. Thus, such nanoparticles can be used to vaccinate an individual against infection by different Types, subtypes and / or strains of infectious agents. Also provided are specific fusion proteins, nucleic acid molecules encoding such fusion proteins and methods of using nanoparticles of the invention to vaccinate individuals.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Systemic administration of nac for vaccination prophylaxis
InactiveUS20100233193A1Organic active ingredientsSnake antigen ingredientsWhole bodyAntiviral therapy
Owner:GUILFORD F TIMOTHY +1
Methods for Diagnosing Lyme Disease
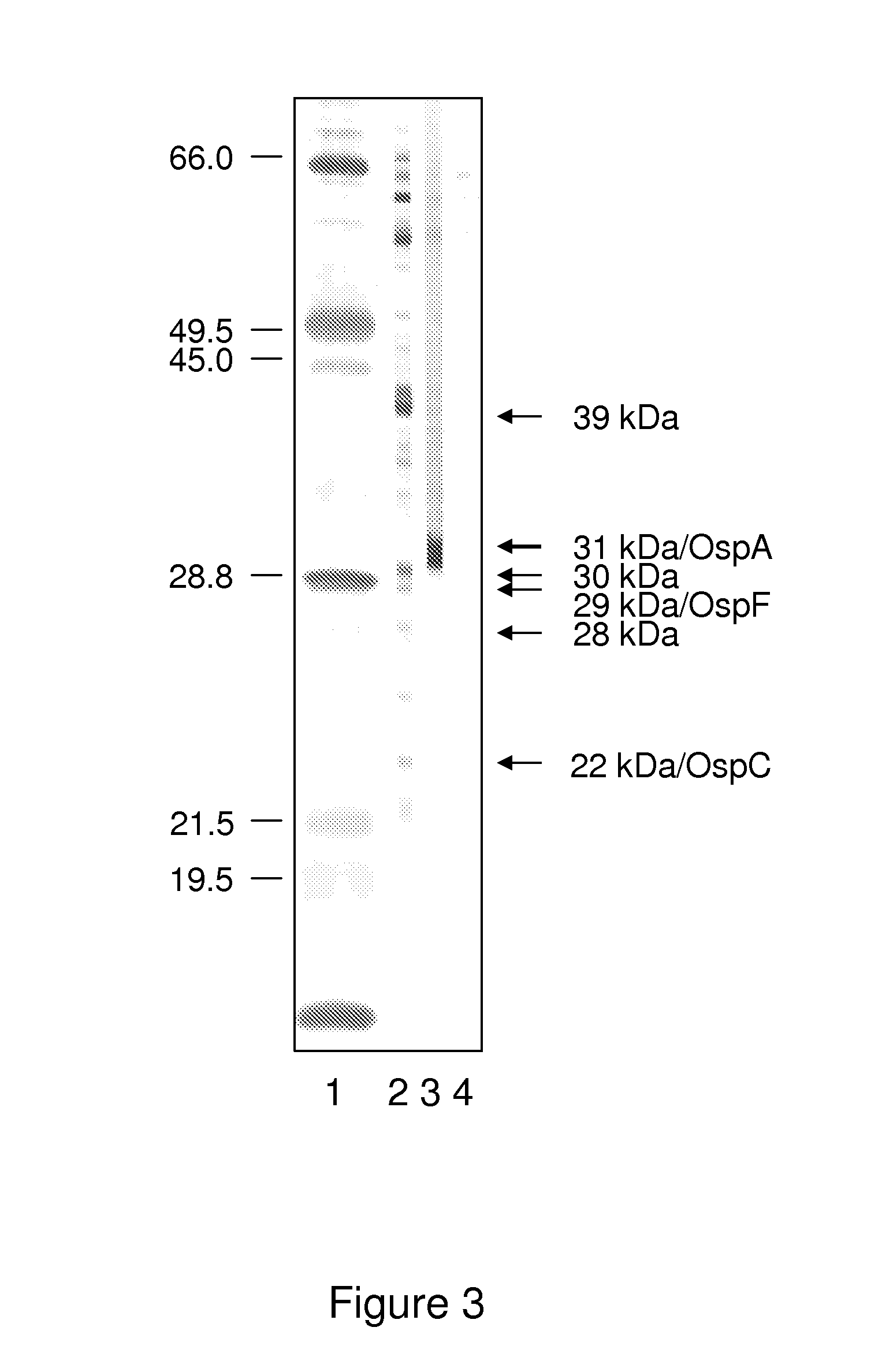
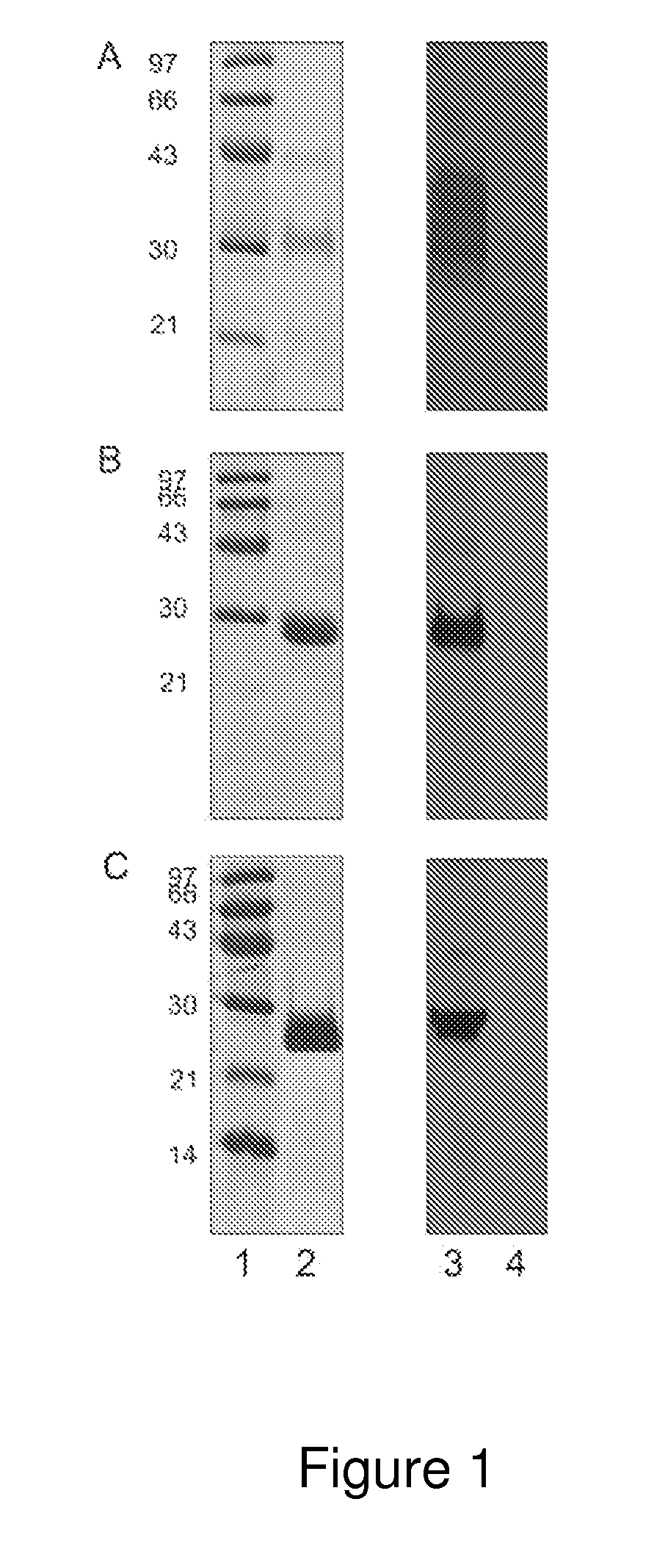
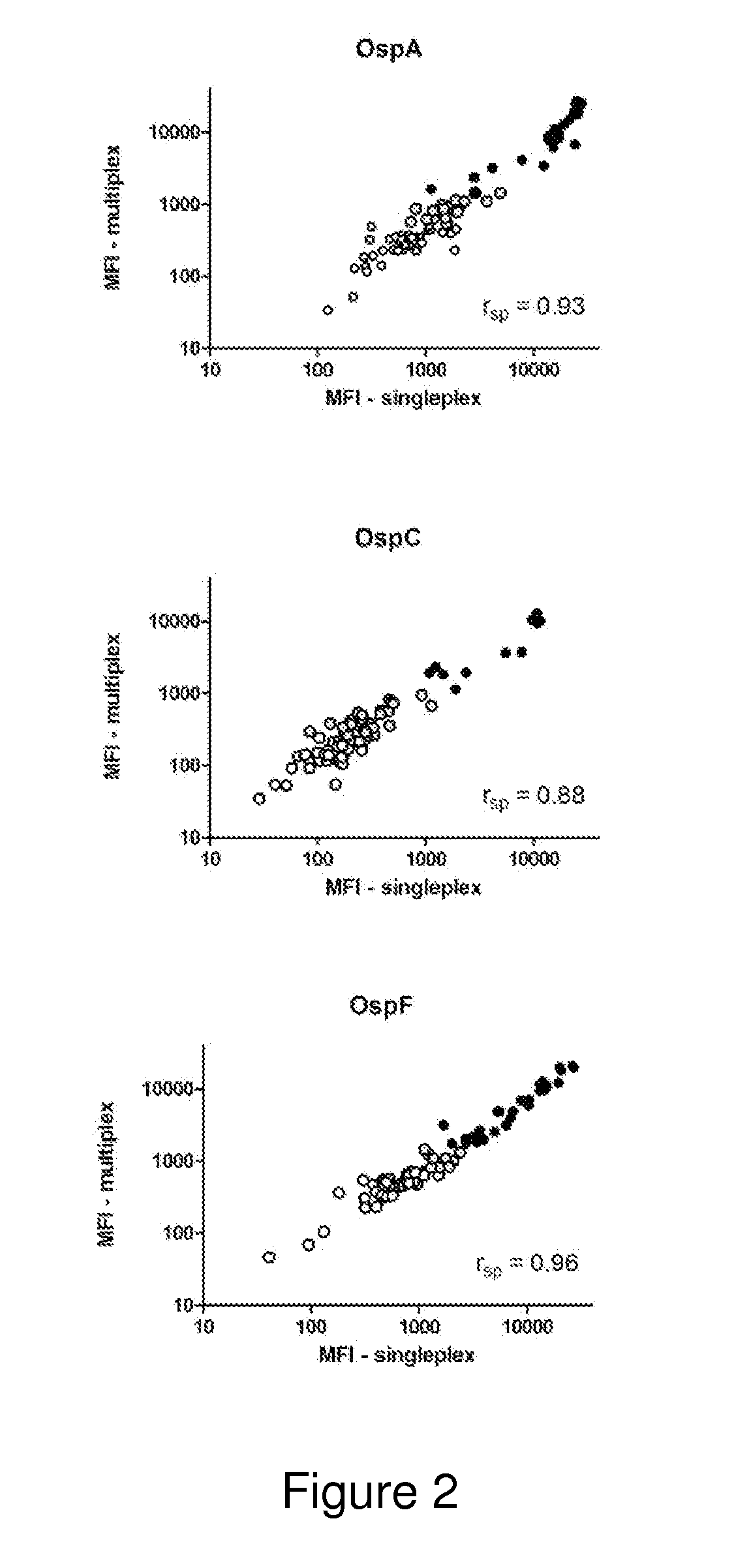
ActiveUS20130273572A1Biological material analysisPeptide preparation methodsOuter surface proteinBorrelia burgdorferi
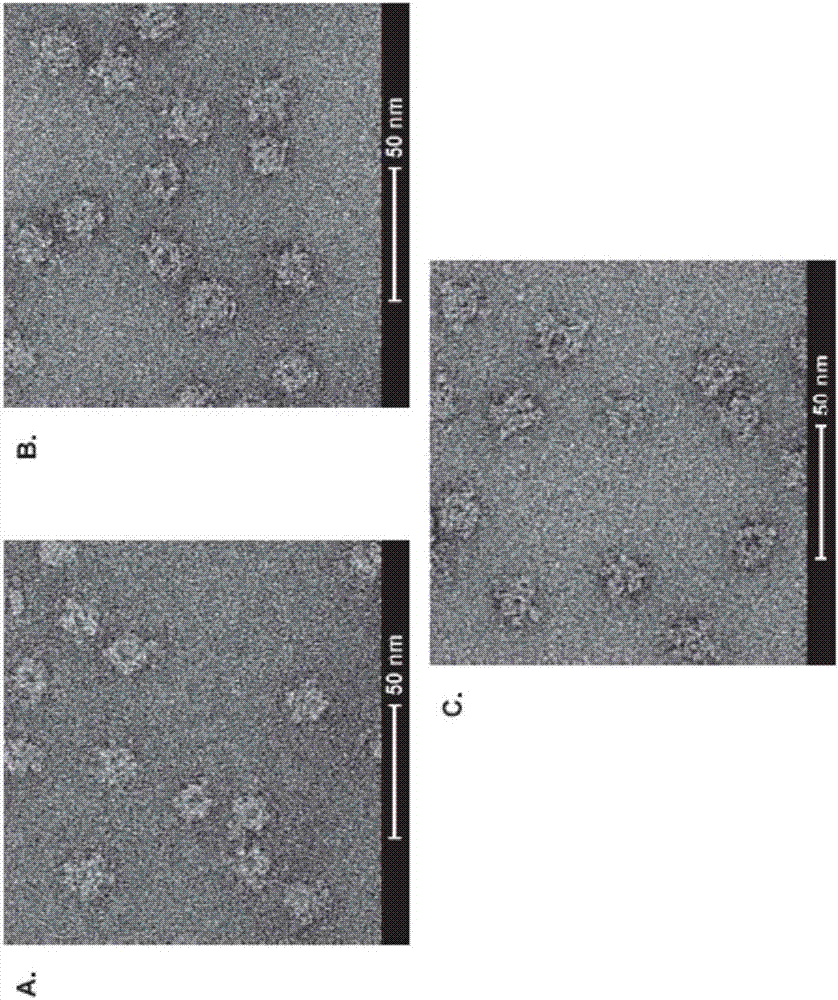
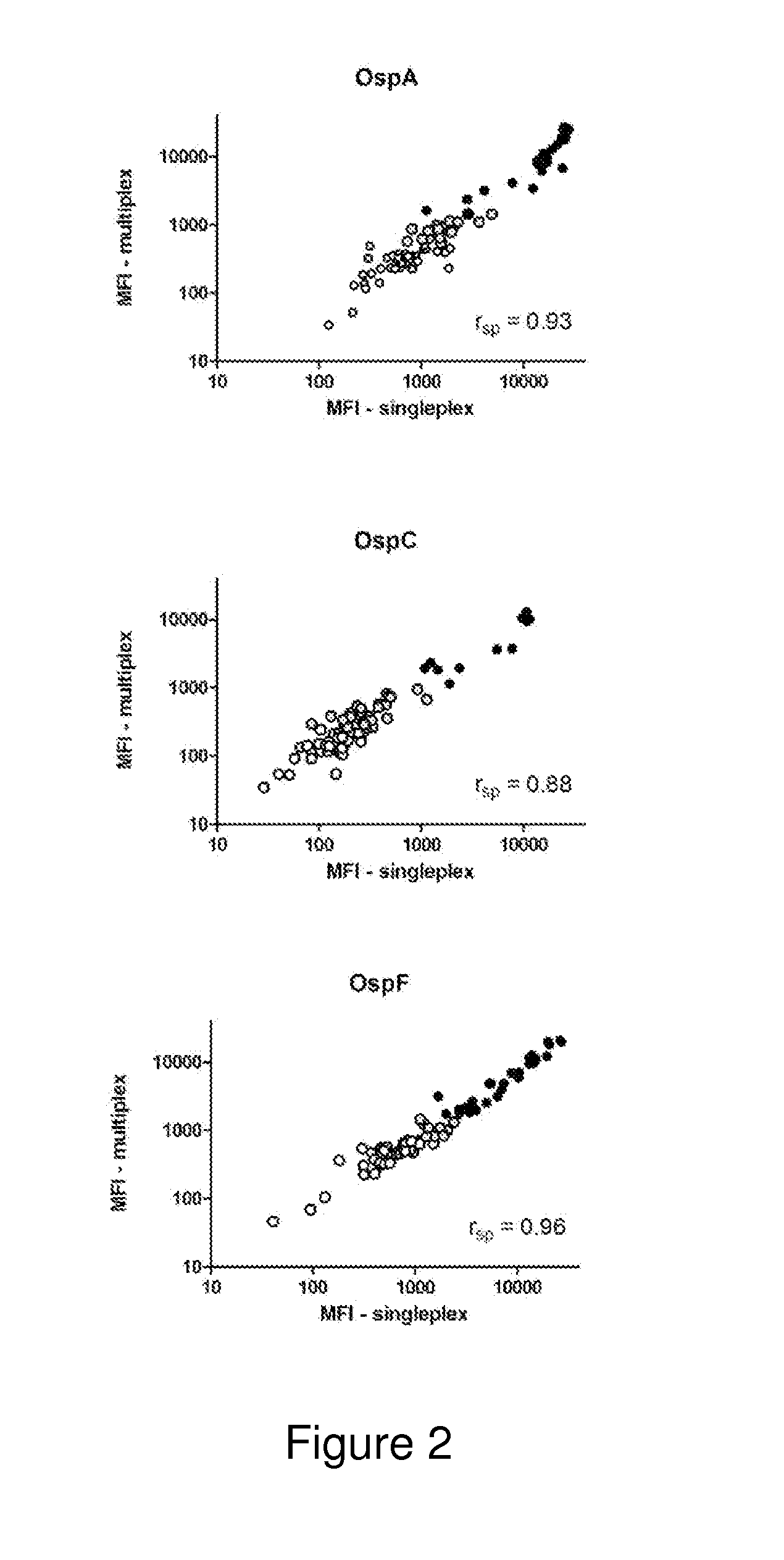
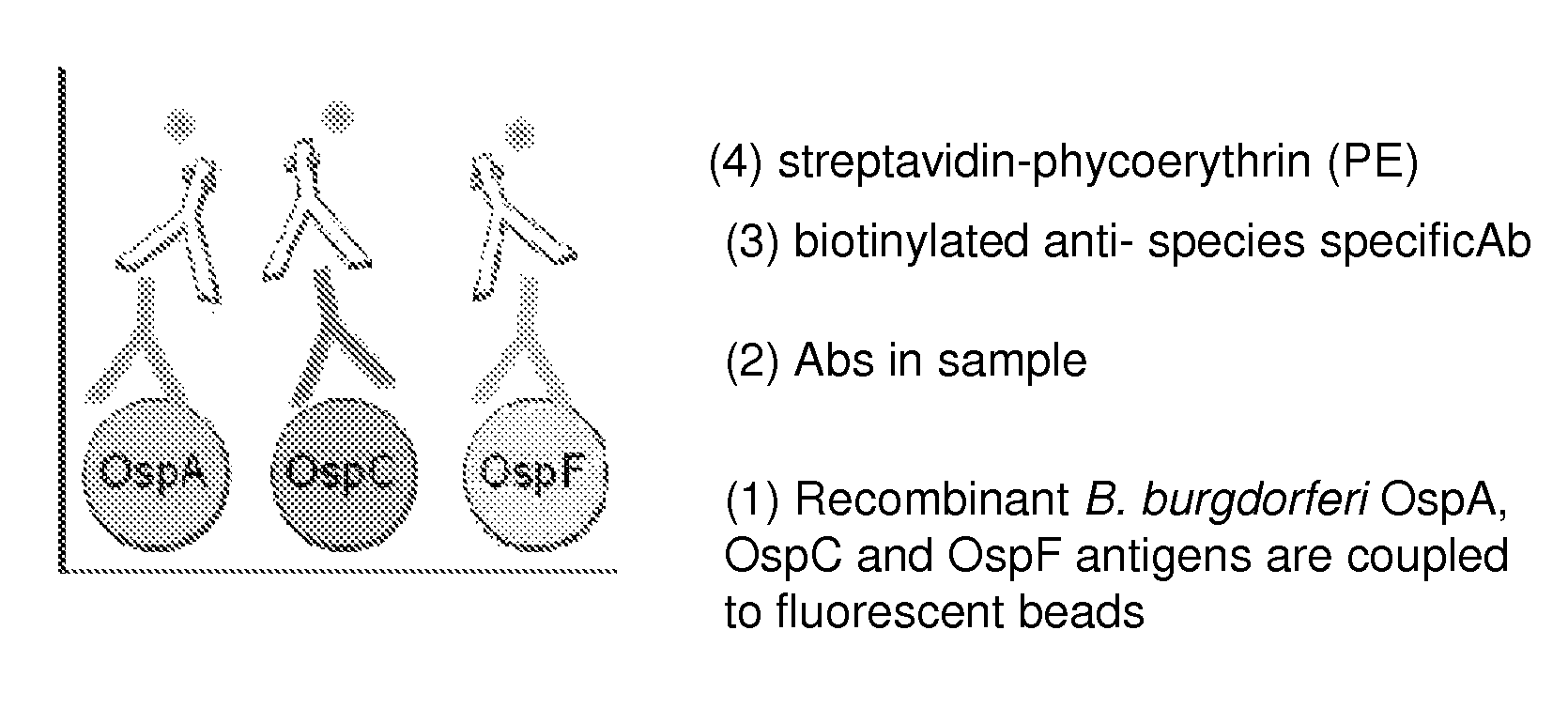
A method for diagnosing Lyme disease status in a mammal is provided. The method entails, in a biological sample obtained or derived from a mammal, determining antibodies to Borrelia burgdorferi (B. burgdorferi) outer surface proteins (Osp) OspA, OspC, and OspF. Based upon determining the OspA, OspC, and OspF antibodies, the mammal can be diagnosed as vaccinated, not vaccinated, infected or not infected with B. burgdorferi. Mammals that have early, intermediate or chronic B. burgdorferi infection can also be identified. The method is particularly suited for use with horses and dogs. Isolated or recombinant B. burgdorferi antigens and compositions that contain them are also provided.
Owner:CORNELL UNIVERSITY
Antigens and Vaccines Directed Against Human Enteroviruses
InactiveUS20150140027A1SsRNA viruses positive-senseViral antigen ingredientsEnteroviral infectionsProviding material
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human enterovirus B, Human enterovirus C, Human enterovirus D antigens and their use in vaccines for the prevention of an Enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Nanodisk-associated immunogen super polyvalent vaccines
InactiveUS20160089431A1Efficient and economical productionEasy to produceSsRNA viruses negative-sensePeptide librariesEpitopePolyvalent Vaccine
Disclosed are compositions of matter consisting of collections of nanodisks associated with polypeptide immunogens. Each said collection contains multiple epitopes of at least one immunogen. When used as a component of a vaccine, these epitopes are chosen to confer immunity, in the vaccinated subject, against a spectrum of possible infective strains of the target pathogen. This immunity is derived from the various antibodies, to the various epitopes of the immunogen, produced by the adaptive immune system of the subject in response to exposure to said vaccine. In this manner, said vaccine may confer immunity against the entire spectrum of possible infective strains of the target pathogen.
Owner:SHIVA SCI & TECH GRP
Method of inducing an enhanced immune response against hiv
InactiveUS20050106123A1Enhance immune responseIncrease probabilityBiocideGenetic material ingredientsTreatment effectMammal
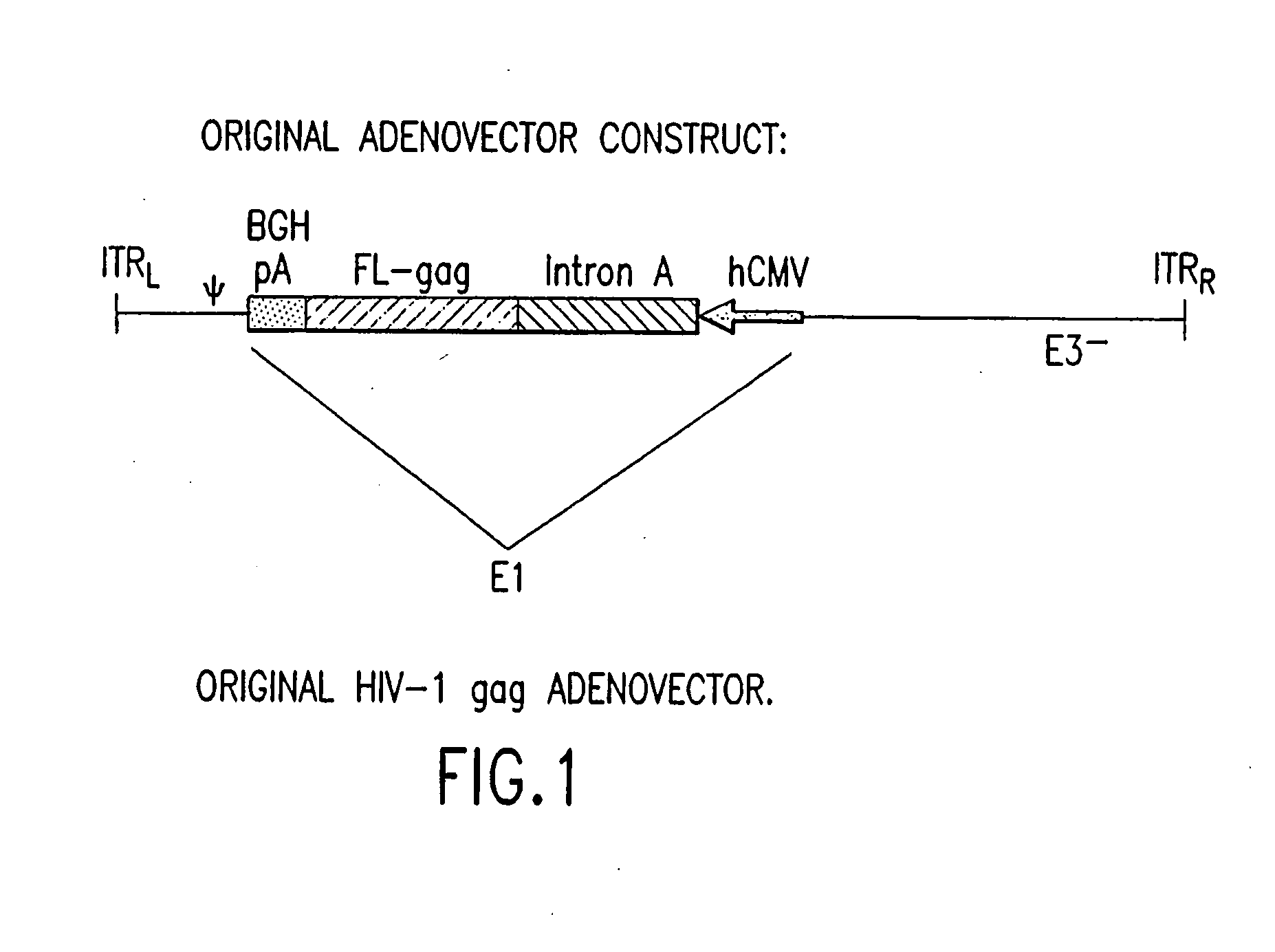
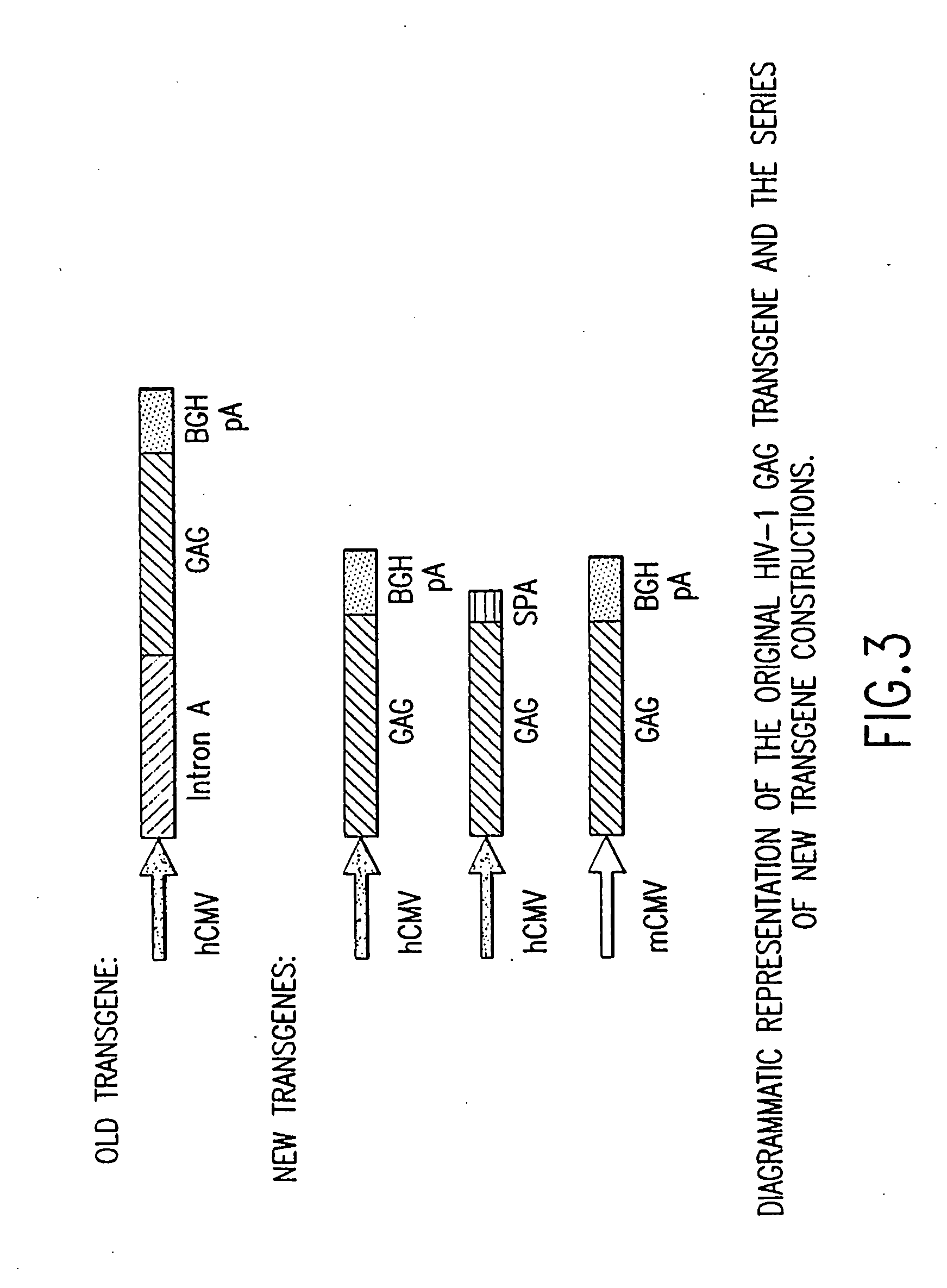
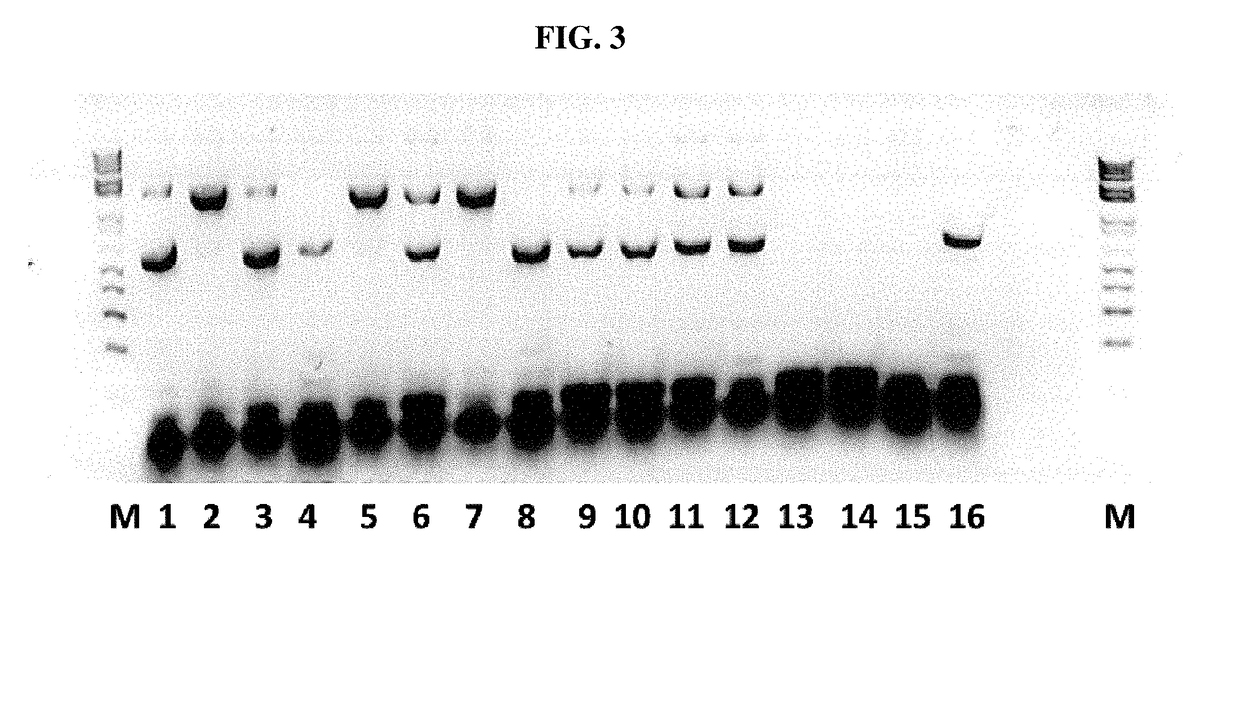
An efficient means of inducing an immune response against human immunodeficiency virus (“HIV”) utilizing specific prime-boost regimes is disclosed. The specific prime-boost regimes employ a heterologous prime-boost protocol wherein recombinant adenoviral and poxvirus vectors comprising exogenous genetic material encoding a common HIV antigen are administered in that order. Vaccines administered into living vertebrate tissue in accordance with the disclosed regimes, preferably a mammalian host such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV-1 antigen (e.g., Gag), inducing a cellular immune response which specifically recognizes HIV-1. It is believed that the disclosed prime / boost regime will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:EMINI EMILIO +5
Preparation method of inactivated vaccine for preventing duck reovirus
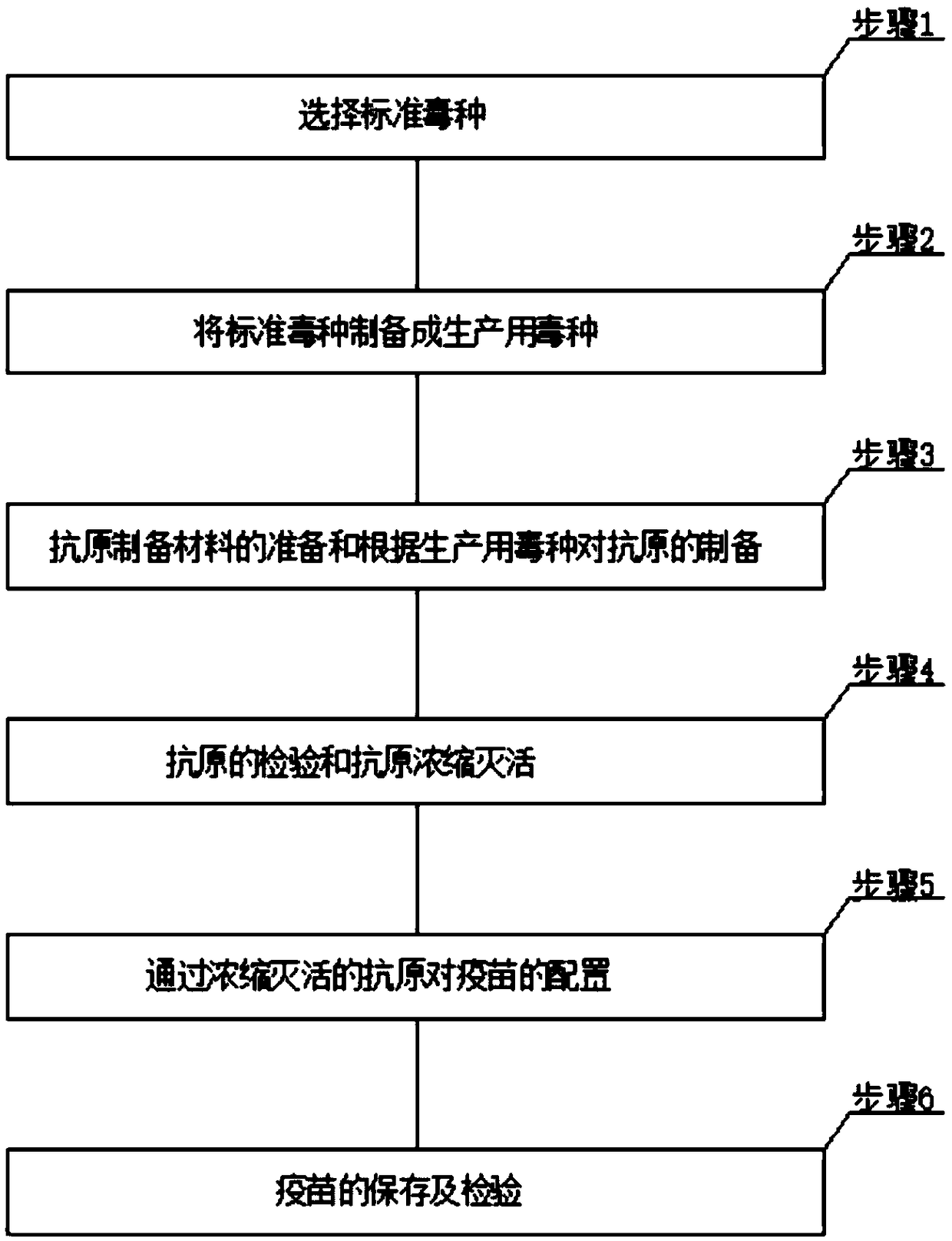
InactiveCN108743932AImprove survival rateEffective preventionViral antigen ingredientsAntiviralsAntigenAdjuvant
The invention is applicable to the technical field of vaccine preparation, and provides a preparation method of inactivated vaccine for preventing duck reovirus. The method comprises the following steps: step 1, selecting standard viral species; step 2, preparing the standard viral species into viral species for production; step 3, preparing an antigen preparation material and preparing antigen according to the viral species for production; step 4, detecting the antigen and performing concentration inactivation on the antigen. The method has the advantages that inoculation is only required tobe performed before breed ducks start laying, the inoculation dosages of two vaccines are respectively 1ml and 0.8ml for one duck, after vaccination is performed about 21 days, hatching eggs can be collected for hatching, by taking that the protection rate of duck reovirus of ducklings is greater than 98 percent as a standard, hatching egg utilization cycles of the two vaccines after vaccination are respectively 6 weeks and 9 weeks after vaccination, immune vaccines are enhanced again after 6 weeks and 9 weeks after vaccination of the two vaccines, and the dosages are the same, the vaccines are repeatedly inoculated till the breed ducks are eliminated, and the preventive effect on liver and spleen necrosis caused by infection of the duck reovirus of the two vaccines with two types of adjuvant are exact.
Owner:重庆永健生物技术有限责任公司
One dose vaccination with mycoplasma hyopneumoniae
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma hyopneumoniae (M. hyo) by administering to the animal at approximately three (3) to ten (10) days of age, a single dose of an effective amount of a M. hyo vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:ZOETIS SERVICE LLC
Marked Bovine Viral Diarrhea Virus Vaccines
InactiveUS20080305130A1SsRNA viruses positive-senseViral antigen ingredientsDNA unwinding enzymeWild type
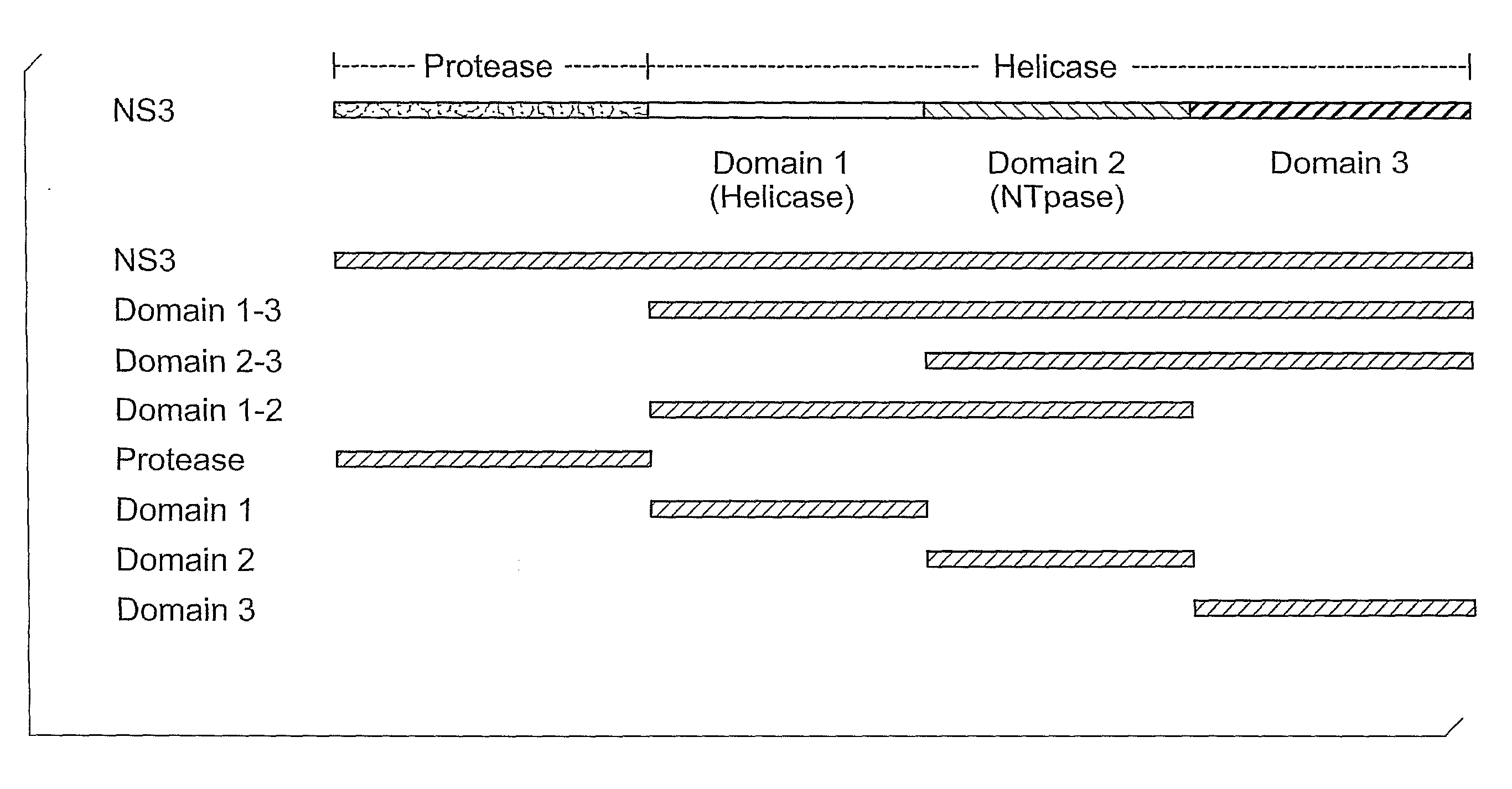
The present invention is directed to a bovine viral diarrhea virus comprising at least one helicase domain amino acid mutation wherein the mutation in the NS3 domain results in a loss of recognition by a monoclonal antibody raised against wild-type NS3 but wherein viral RNA replication and the generation of infectious virus is retained. The present invention is useful, for example, to produce a marked bovine viral diarrhea virus vaccine or to differentiate between vaccinated and infected or unvaccinated animals.
Owner:PFIZER INC +1
Marker system, in particular for baculovirus-expressed subunit antigens
ActiveUS20160258953A1SsRNA viruses negative-senseVirus peptidesBaculovirus expressionVaccines Administered
The present invention belongs to the field of compliance markers and marker vaccines which allow for the differentiation between infected and vaccinated individuals. In particular, it relates to a method of determining whether an individual has received an immunogenic composition comprising a recombinant protein produced by a baculovirus expression system in cultured insect cells.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Marker system, in particular for baculovirus-expressed subunit antigens
ActiveUS10168330B2SsRNA viruses negative-senseVirus peptidesBaculovirus expressionVaccines Administered
The present invention belongs to the field of compliance markers and marker vaccines which allow for the differentiation between infected and vaccinated individuals. In particular, it relates to a method of determining whether an individual has received an immunogenic composition comprising a recombinant protein produced by a baculovirus expression system in cultured insect cells.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Method of inducing an enhanced immune response against hiv
InactiveUS20060165664A1Enhance immune responseIncrease probabilityBiocideGenetic material ingredientsTreatment effectMammal
An efficient means of inducing an immune response against human immunodeficiency virus (HIV) utilizing specific prime-boost regimes is disclosed. The specific prime-boost regimes employ a heterologous prime-boost protocol employing recombinant adenoviral vectors of alternative and distinct serotypes comprising exogenous genetic material encoding a common HIV antigen. Vaccines administered into living vertebrate tissue in accordance with the disclosed regimes, preferably a mammalian host, such as a human or a non-human mammal of commercial or domestic veterinary importance, express the HIV-1 antigen (e.g., Gag), inducing a cellular immune response which specifically recognizes HIV-1. It is believed that the disclosed prime / boost regime will offer a prophylactic advantage to previously uninfected individuals and / or provide a therapeutic effect by reducing viral load levels within an infected individual, thus prolonging the asymptomatic phase of HIV-1 infection.
Owner:MERCK & CO INC
Live Attenuated Antigenically Marked Classical Swine Fever Vaccine
Controlling Classical Swine Fever Virus (CSFV) involves either prophylactic vaccination or non-vaccination and elimination of infected herds depending on the epidemiological situation. Marker vaccines allowing distinction between naturally infected from vaccinated swine could complement “stamping out” measures. Previously, we reported the development of FlagT4v, a double antigenic marker live attenuated CSFV strain. FlagT4v was later shown as not to be completely stable in terms of its attenuation when assessed in a reversion to virulence protocol. We have developed a modified version of the FlagT4v where changes in the codon usage of genomic areas encoding for Flag and T4 were introduced to rectify the reversion to the virulent genotype. The new virus, FlagT4-mFT-Gv, possesses the same amino acid sequence as FlagT4v except for one substitution, Asparagine is replaced by Glycine at position 852 of the CSFV polypeptide. FlagT4-mFT-Gv protected swine against challenge with Brescia virulent virus at 21 days post vaccination.
Owner:UNIV OF CONNECTICUT +1
Pcv2 orf2 carrier platform
ActiveUS20180344835A1SsRNA viruses positive-senseViral antigen ingredientsVirus-like particleVaccines Administered
The present invention relates to an immunogen-carrier, wherein the immunogen-carrier is preferably a virus-like particle (VLP) composed of a plurality of a modified PCV2 ORF2 protein. In particular, the present invention belongs to the field of compliance markers and marker vaccines which allow for the differentiation between infected and vaccinated individuals. In particular, it relates to a compliance marker for vaccines including a subunit antigen, and a DIVA (Differentiating Infected from Vaccinated Animals) system which makes it possible to differentiate between animals infected with a pathogen and animals treated with a subunit antigen derived from said pathogen.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Nanoemulsion vaccines
ActiveCN102596243ASsRNA viruses negative-senseViral antigen ingredientsMorbillivirusPreventative Medicine
The present invention provides methods and compositions for the stimulation of immune responses. Specifically, the present invention provides immunogenic nanoemulision compositions and methods of using the same to induce immune responses (e.g., immunity (e.g., protective immunity)) against a pathogenic virus of the paramyxoviridae family (e.g., a Paramyxovirinae virus (e.g., Paramyxovirus, Rubulavirus and / or Morbillivirus) and / or a Pneumovirinae virus (e.g., respiratory syncytial virus))). Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:RGT UNIV OF MICHIGAN
Antigens and vaccines directed against human enteroviruses
InactiveUS9987350B2SsRNA viruses positive-senseViral antigen ingredientsRNA Virus InfectionsProviding material
The instant invention provides materials and methods for producing immunologically active antigens derived from members of the Picornaviridae virus family. The picornavirus antigens of the invention may be in a form for use as a vaccine administered to a subject in a therapeutic treatment or for the prevention of a picornavirus infection. The picornavirus antigens of the invention may be in the form of an immunogenic composition for use in vaccines which are administered for the prevention of an Enterovirus infection. The instant invention further encompasses immunogenic compositions comprising Human enterovirus A, Human Enterovirus B, Human enterovirus C, Human Enterovirus D antigens and their use in vaccines for the prevention of an enterovirus infection.
Owner:SENTINEXT THERAPEUTICS
Compositions and methods for treatment of cancer
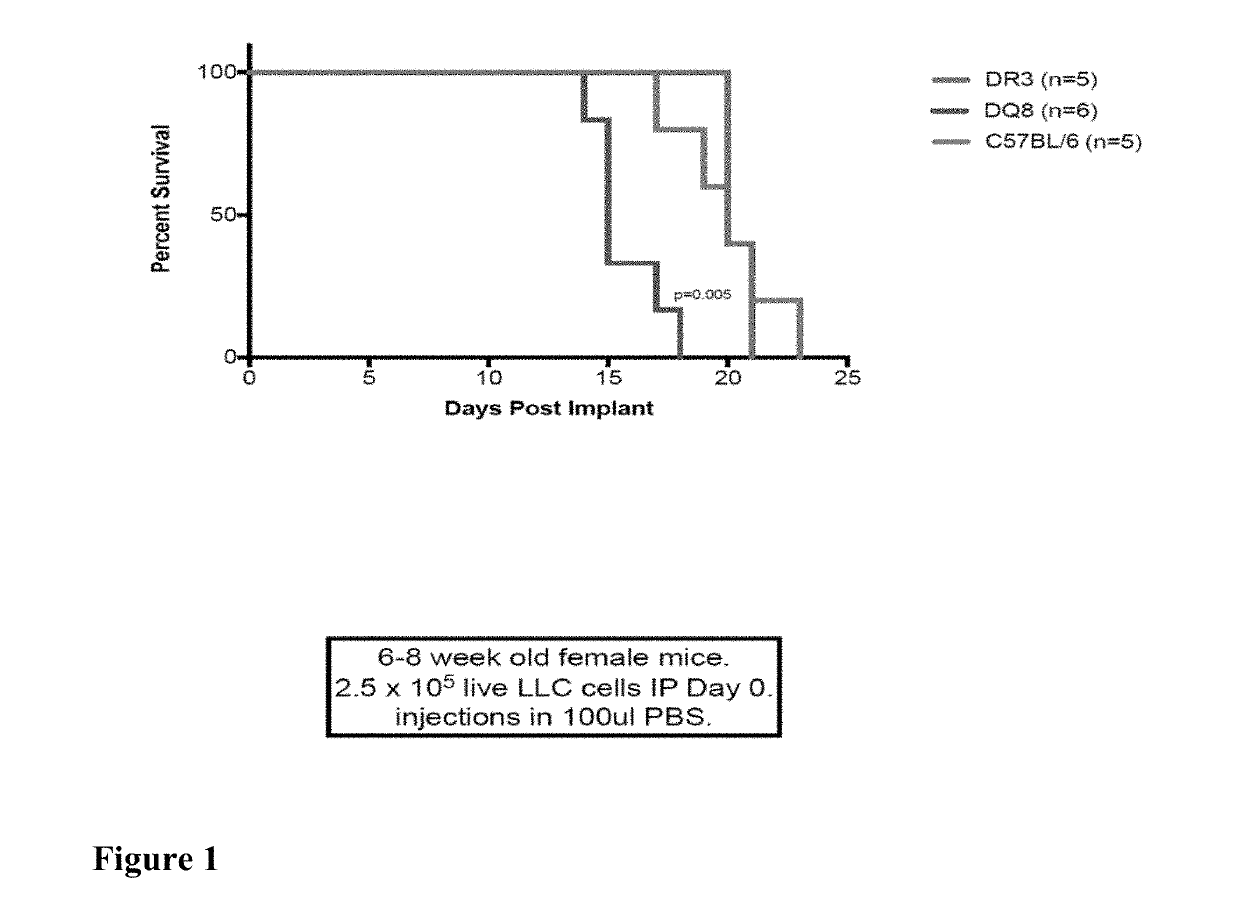
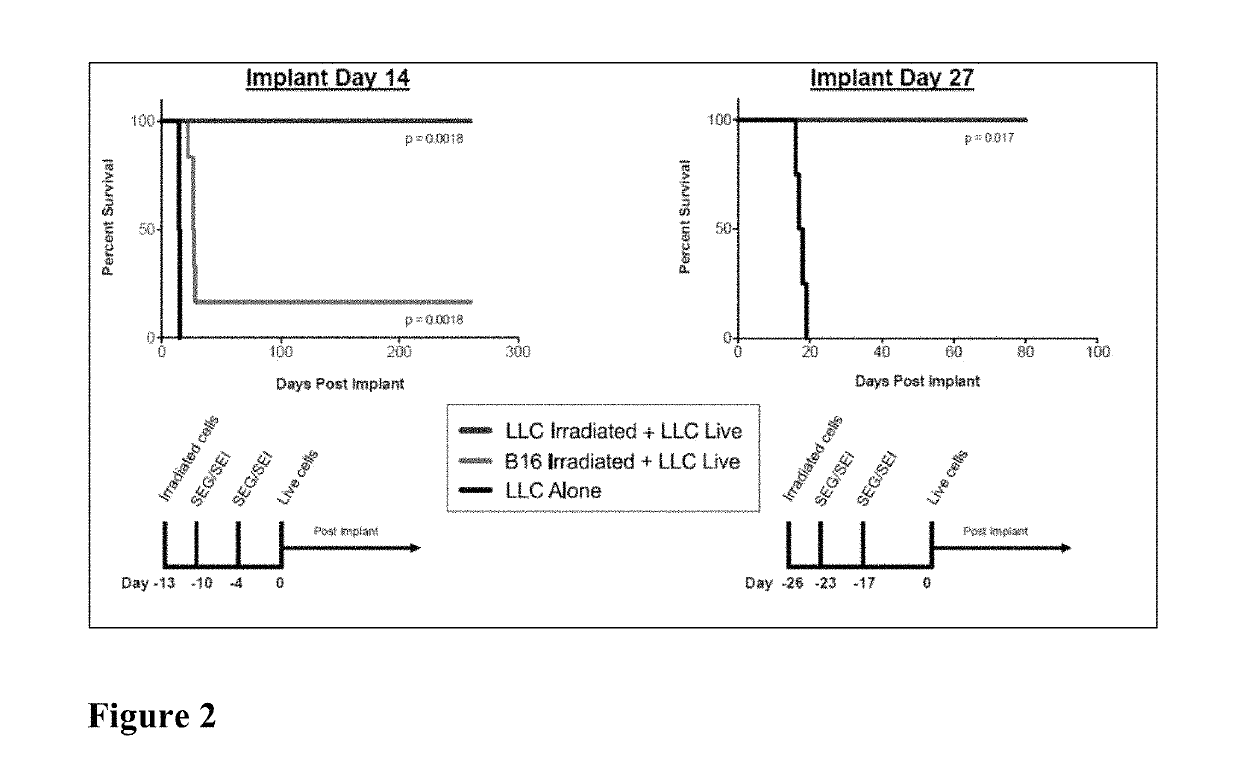
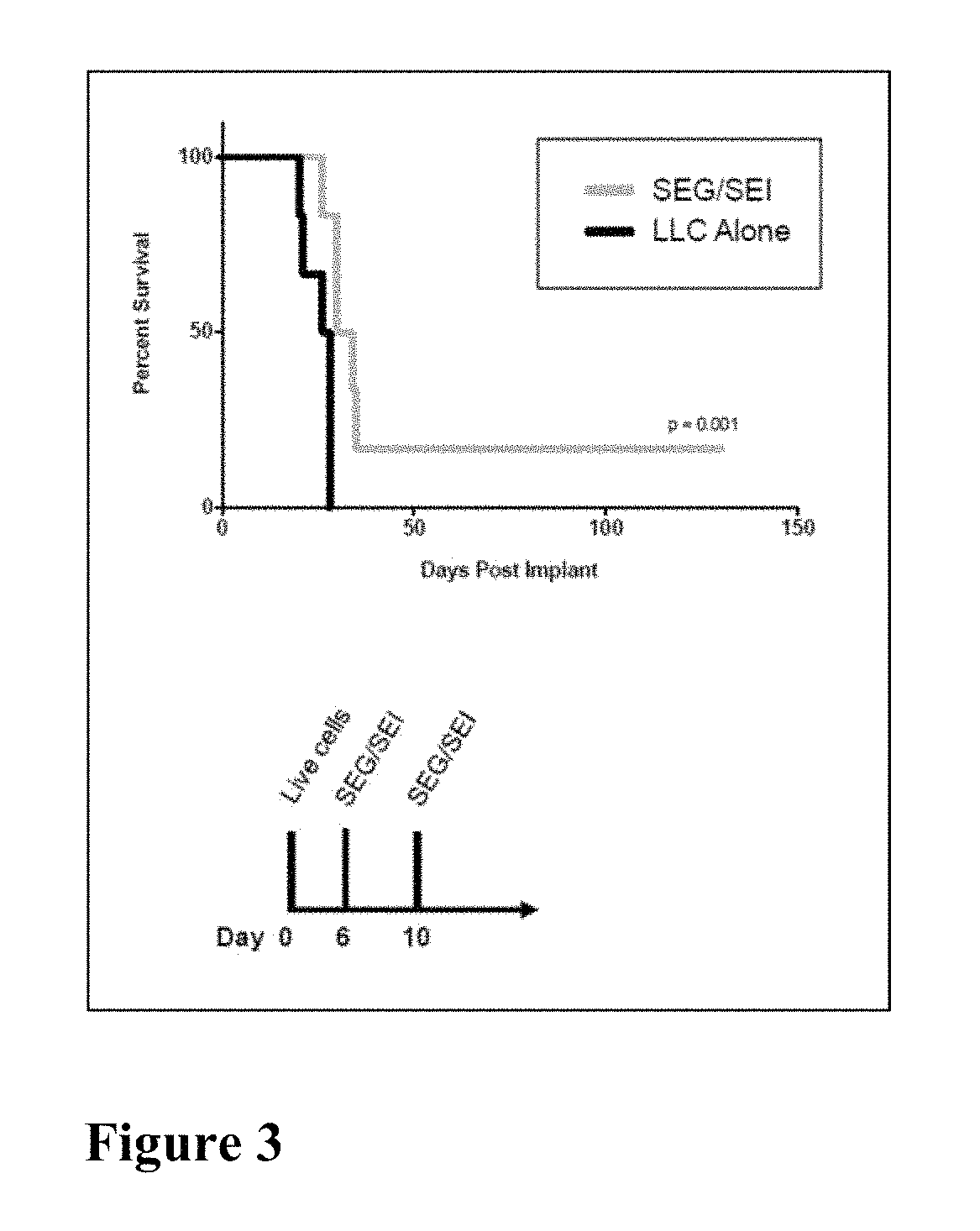
Here we show that SEG / SEI presented from a HLA-DQ8 (HLA-DQB*0302 and HLA-DQA*0301) platform prevent the de novo outgrowth (vaccination) of Lewis lung carcinoma (LLC) and B16-F10 melanoma and retard the growth of established tumors with no significant toxicity. Vaccination of DQ8 tg mice with irradiated LLC or B16-F10 melanoma followed by SEG / SEI immunization and live tumor challenge resulted in 100 and 66% survival respectively for 200 days compared to a median survival of 20 days for untreated controls (p<0.001). In vaccination studies, DQ8 tg mice showed a surge in IFNγ serum levels reaching 3000 fold above baseline devoid of a parallel spike in TNFα levels above baseline levels. Presentation of the SEG / SEI superantigen from a MHC-DQ8 platform, therefore, augments the therapeutic index of these SAgs inducing a tumoricidal response against Lewis lung carcinoma and B16 melanoma accompanied by a sharp increase of therapeutic IFNγ levels absent toxic levels of TNFα.
Owner:TERMAN DAVID S +1
Methods for diagnosing lyme disease
ActiveUS8946393B2Bacterial antigen ingredientsPeptide/protein ingredientsAntigenOuter surface protein
A method for diagnosing Lyme disease status in a mammal is provided. The method entails, in a biological sample obtained or derived from a mammal, determining antibodies to Borrelia burgdorferi (B. burgdorferi) outer surface proteins (Osp) OspA, OspC, and OspF. Based upon determining the OspA, OspC, and OspF antibodies, the mammal can be diagnosed as vaccinated, not vaccinated, infected or not infected with B. burgdorferi. Mammals that have early, intermediate or chronic B. burgdorferi infection can also be identified. The method is particularly suited for use with horses and dogs. Isolated or recombinant B. burgdorferi antigens and compositions that contain them are also provided.
Owner:CORNELL UNIVERSITY
Vaccine against streptococcus agalactiae infection using native or recombinant s. agalactiae glyceraldheyde-3-phosphate dehydrogenase (GAPDH) as a target antigen
The present invention regards a vaccine against Streptococcus agalactiae infection, a leading cause of neonatal pneumonia, sepsis and meningitis. The vaccine is composed by glyceraldheyde-3-phosphate dehydrogenase (GAPDH) protein obtained from culture supernatants of S. agalactiae cells, or by recombinant GAPDH (rGAPDH) obtained from the gene coding for the S. agalactiae GAPDH cloned and expressed in a heterologous system. The vaccine administered in a submitogenic dose of GAPDH, or rGAPDH, protects the host against S. agalactiae infection. Vaccination is used as a preventive approach and is administered by intravenous and / or intradermic and / or subcutaneous and / or mucosal route. Therefore, the invention field is in the area of the pharmaceutical industry.
Owner:UNIV DO PORTO
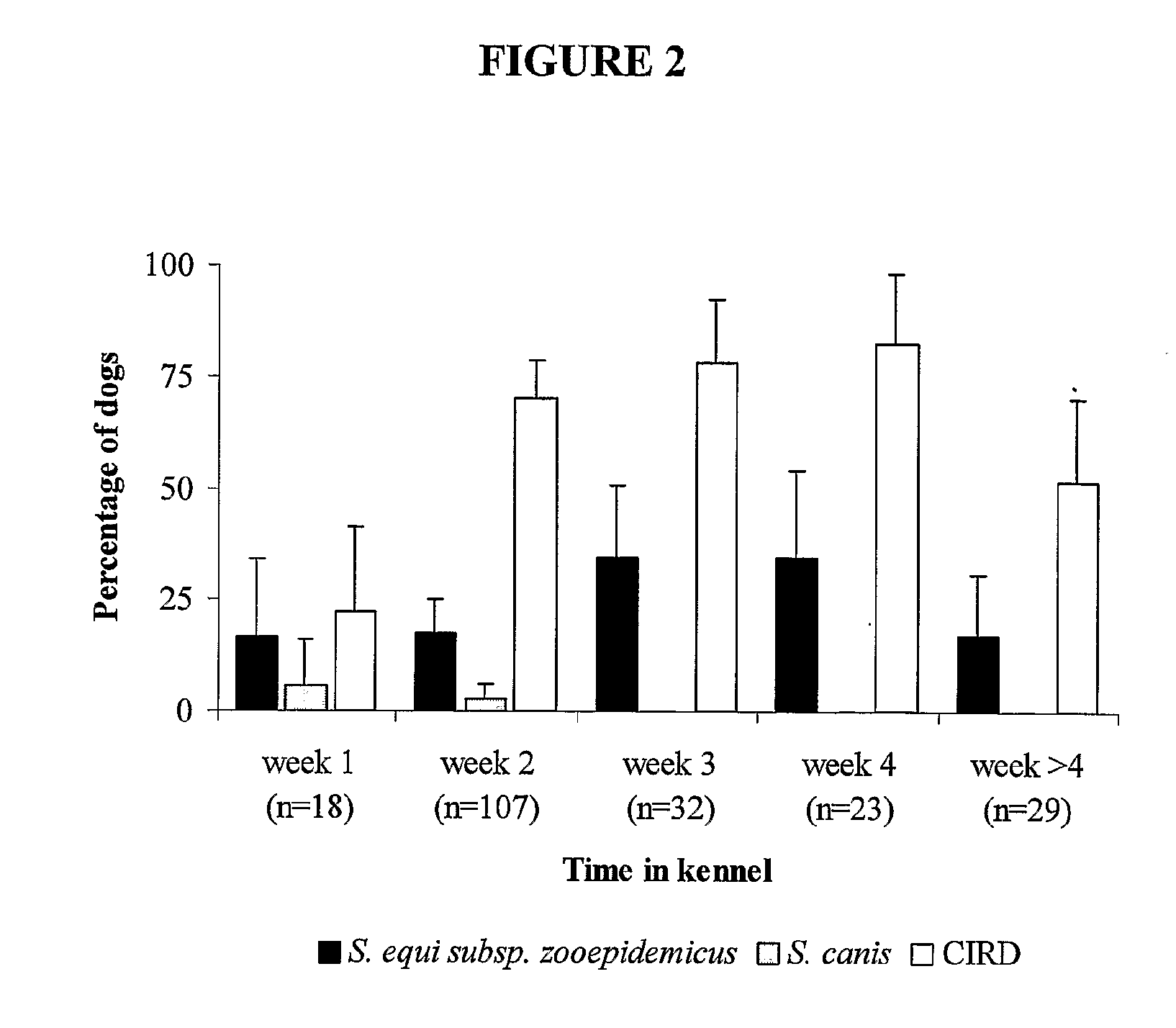
Vaccine composition for vaccinating dogs against canine infectious respiratory disease (CIRD)
InactiveUS20110059128A1Reducing pathogenic changeEnhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseChlamydophila
A vaccine composition for vaccinating dogs comprising any one or more of (a) an agent capable of raising an immune response against Streptococcus equi sub species zooepidemicus in a dog, (b) an agent capable of raising an immune response against Mycoplasma cynos in a dog, and (c) an agent capable of raising an immune response against a Chlamydophila in a dog.
Owner:THE ROYAL VETERINARY COLLEGE
AIDS virus vaccines using Sendai virus vector
InactiveUS8217019B2Easy to set upImprove performanceSsRNA viruses negative-senseBiocideFeline immunodeficiency virusControl animal
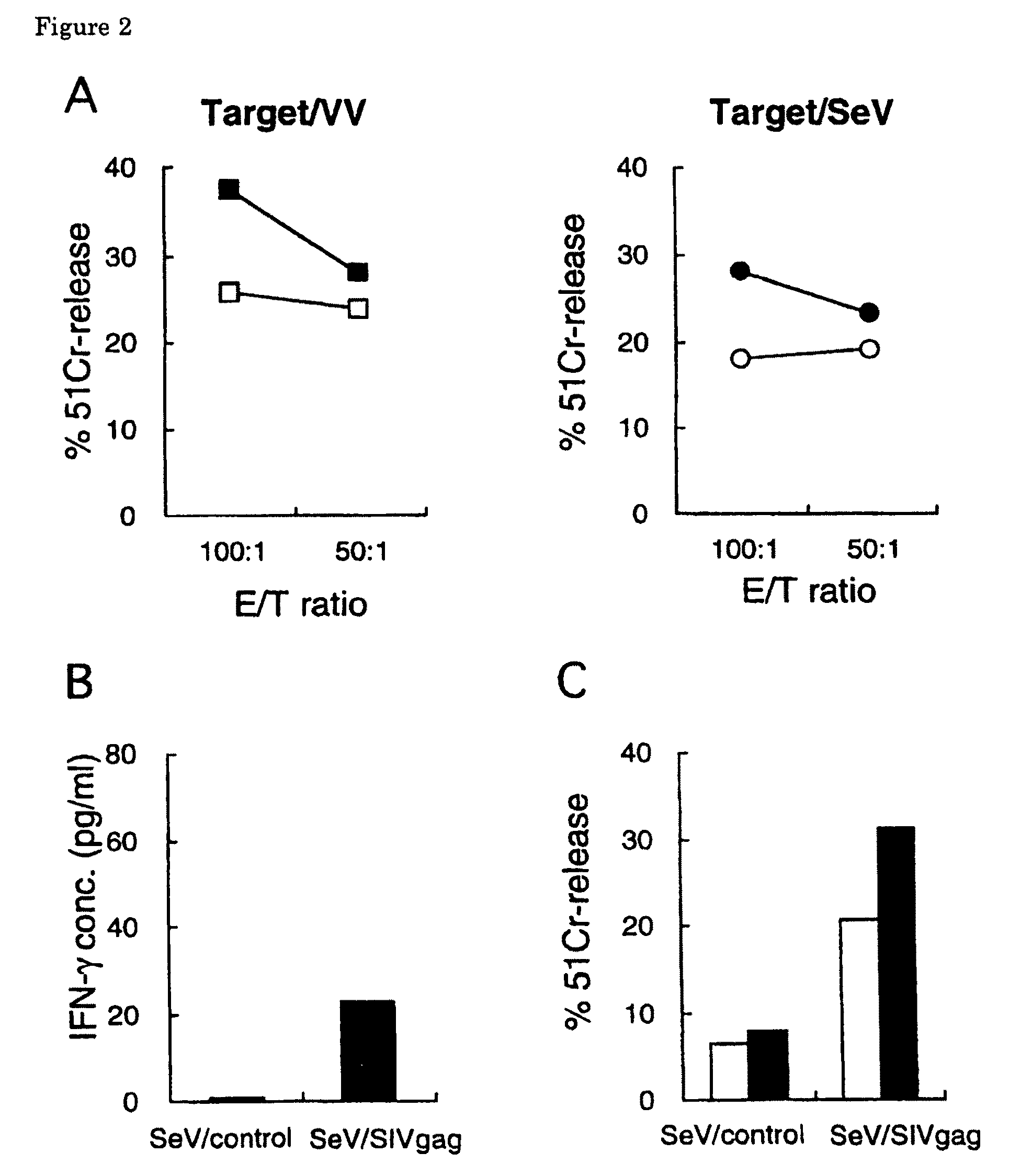
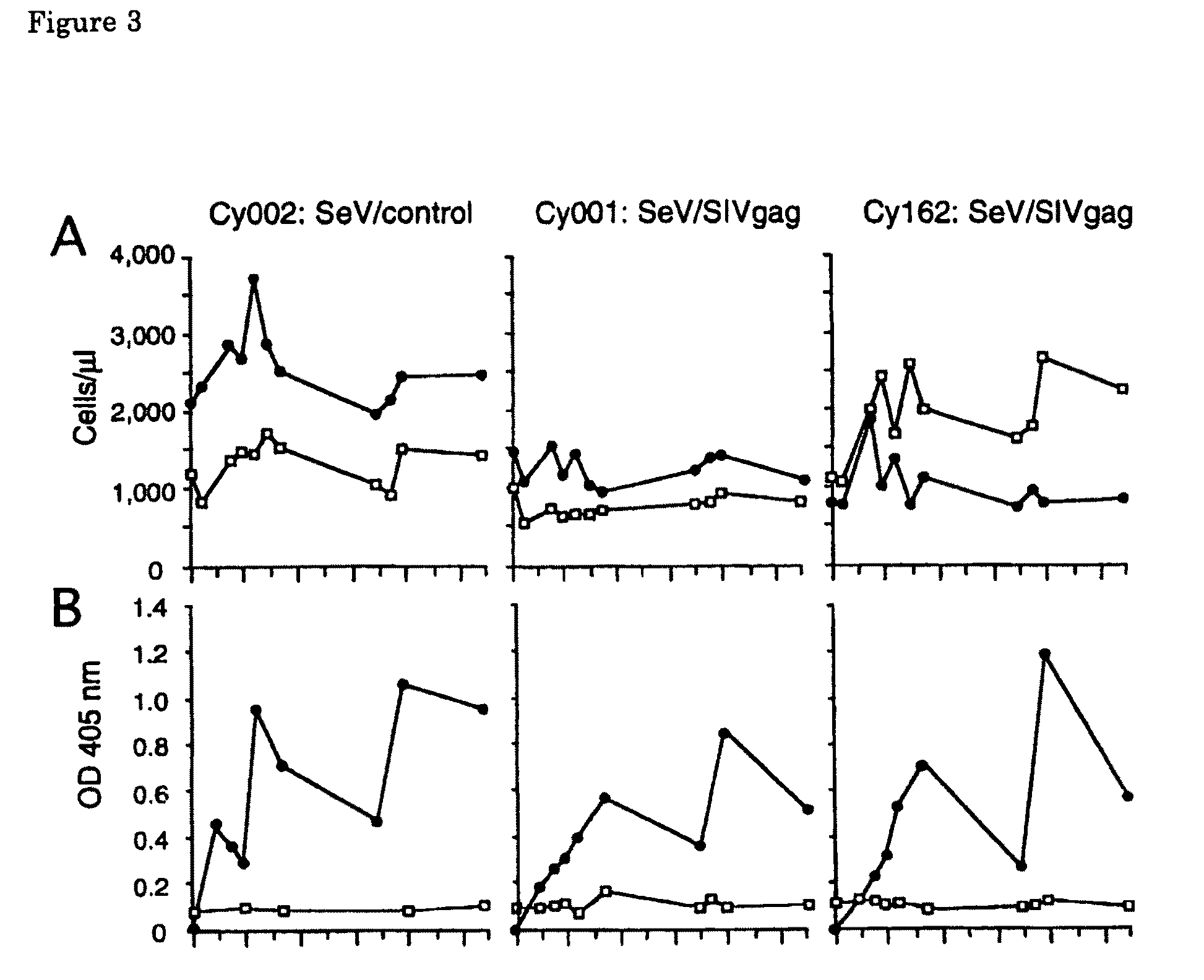
The present invention provides a vaccine containing a Sendai virus vector encoding a virus protein of an immunodeficiency virus. By intranasally administering a Sendai virus encoding a virus protein of an immunodeficiency virus to a macaque monkey, the present inventors have succeeded in efficiently inducing protective immunity against an immunodeficiency virus. As a result of intranasal inoculation of vaccine, expression of an antigen protein mediated by Sendai virus vector was detected in intranasal mucous membrane and local lymph nodes and antigen-specific cellular immune response was induced at a significant level. No pathological symptom by vaccination was observed. After vaccination, exposure of simian immunodeficiency virus was performed and the effect was examined. As a result, the amount of virus in plasma significantly decreased, compared with that of the control animal. The present invention provides a promising vaccine as an AIDS vaccine.
Owner:DNAVEC RES +1
Vaccine for preventing sheep from brucellosis
InactiveCN107412758AWill not cause dispersionLower doseAntibacterial agentsPowder deliveryVaccines AdministeredBrucellosis
The invention discloses a vaccine for preventing sheep from brucellosis. The vaccine is an eye drop vaccination and comprises a brucellosis live vaccine and a vaccine diluent. By application of the vaccine, which is the eye drop vaccination and comprises the brucellosis live vaccine and the vaccine diluent, through eye drop vaccination, the dosage is small, the vaccination mode is simple and safe, the vaccine liquid cannot be spread into the environment a lot, and accordingly, the biological safety risk is reduced.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Cytotoxic T Lymphocyte Inducing Immunogens For Prevention Treatment and Diagnosis of Dengue Virus Infection
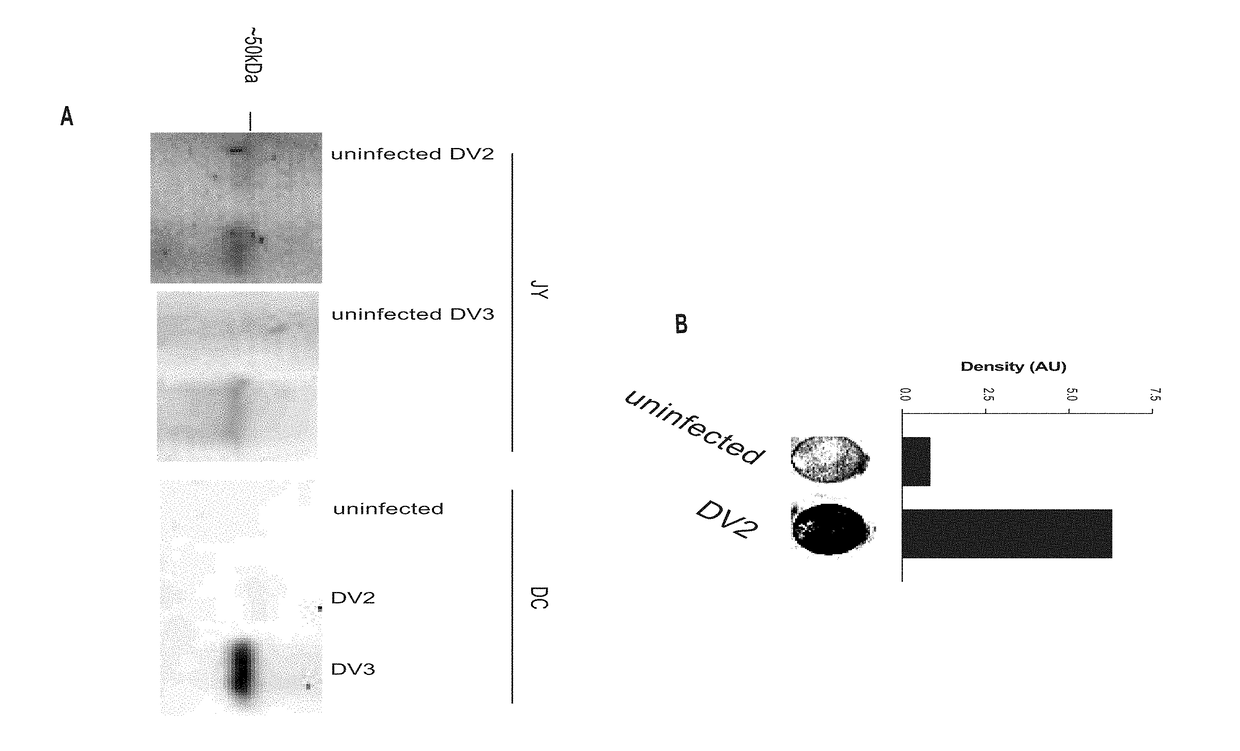
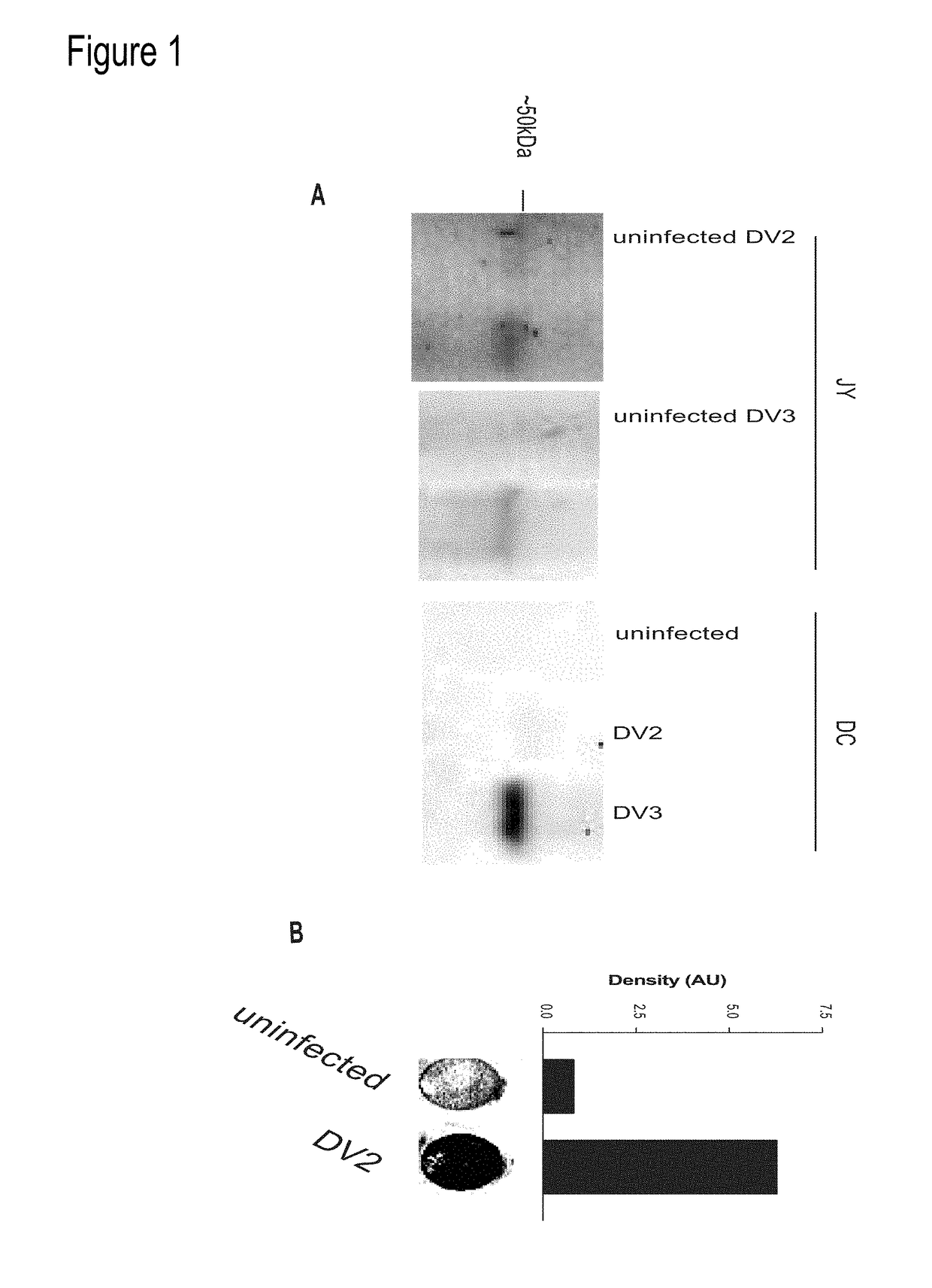
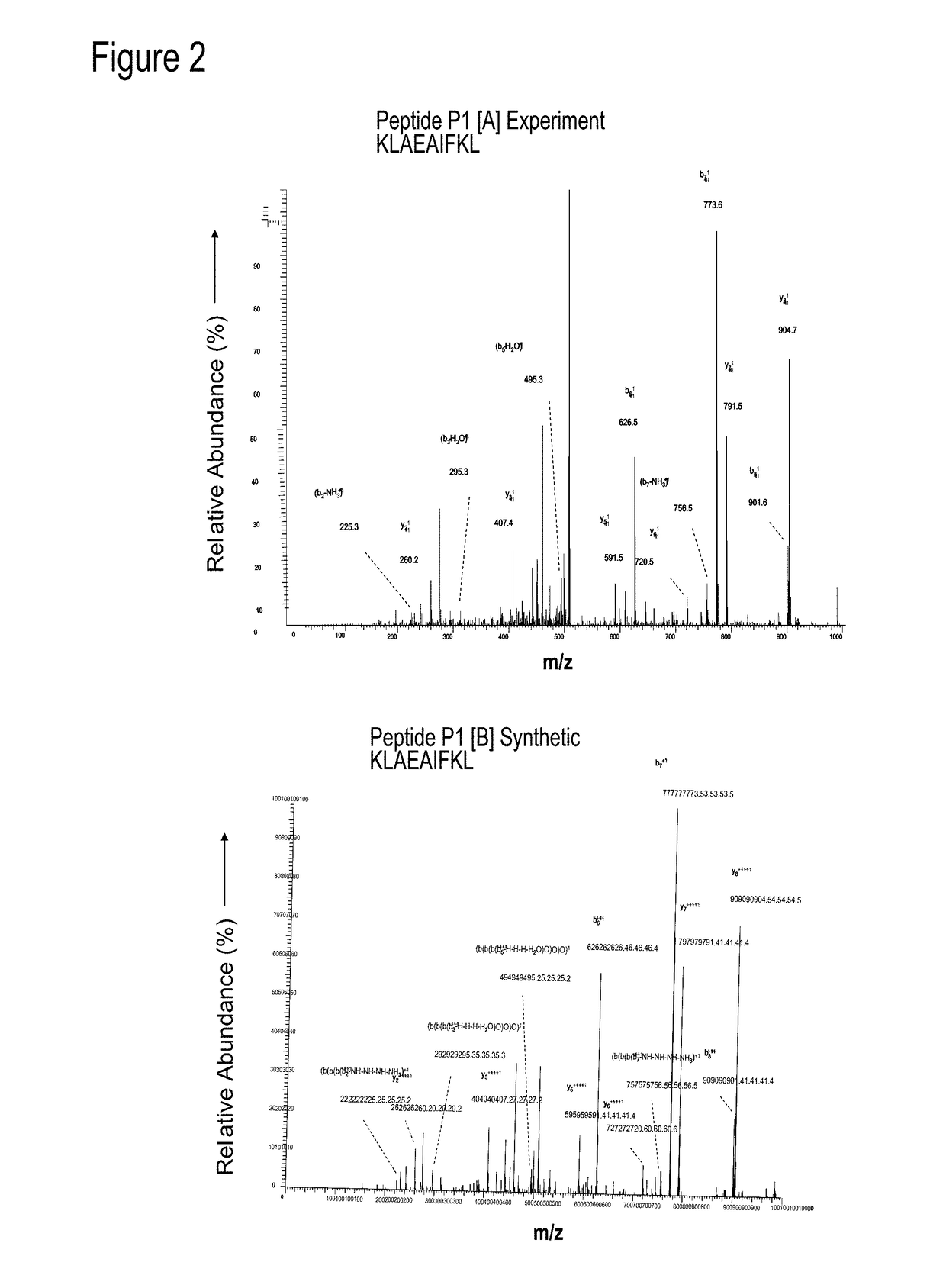
Dengue Fever (DF) and Dengue Hemorrhagic Fever (DHR) are significant global public health problems and understanding the overall immune response to infection will contribute to appropriate management of the disease and its potentially severe complications. Live attenuated and subunit vaccine candidates, which are under clinical evaluation, induce primarily an antibody response to the virus and minimal cross-reactive T cell responses. Currently, there are no available tools to assess protective T cell responses during infection or post vaccination. The present invention incorporates immunoproteomics to uncover novel HLA-A2 specific epitopes derived from Dengue Virus (DV)-infected cells. These epitopes are conserved with epitope-specific CTLs cross-reacting against all four DV serotypes. These epitopes have potential as new informational and diagnostic tools to characterize T cell immunity in Dengue virus (DV) infection, and serves as a universal vaccine candidate complementary to current vaccines.
Owner:EMERGEX VACCINES HLDG LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com