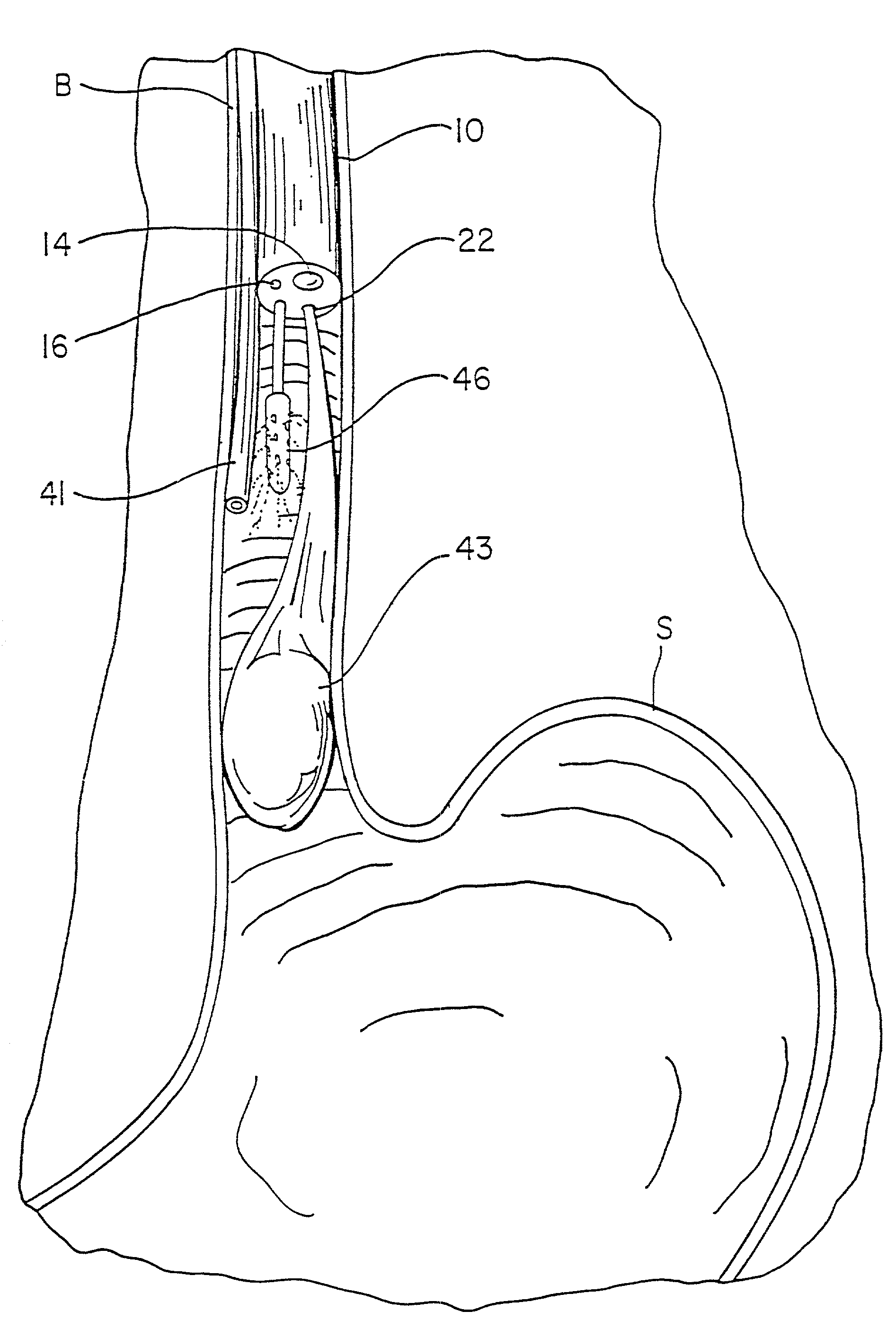
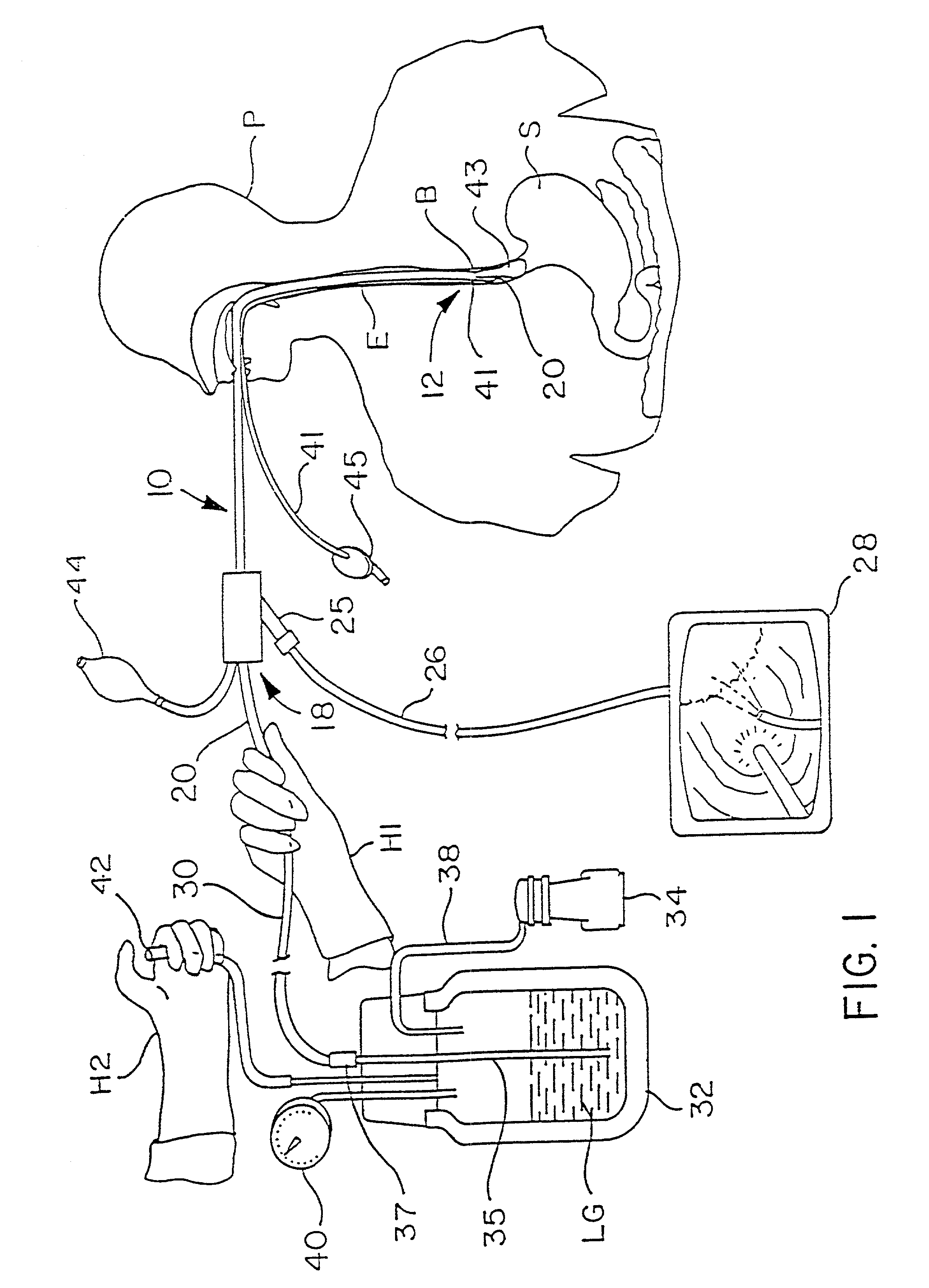
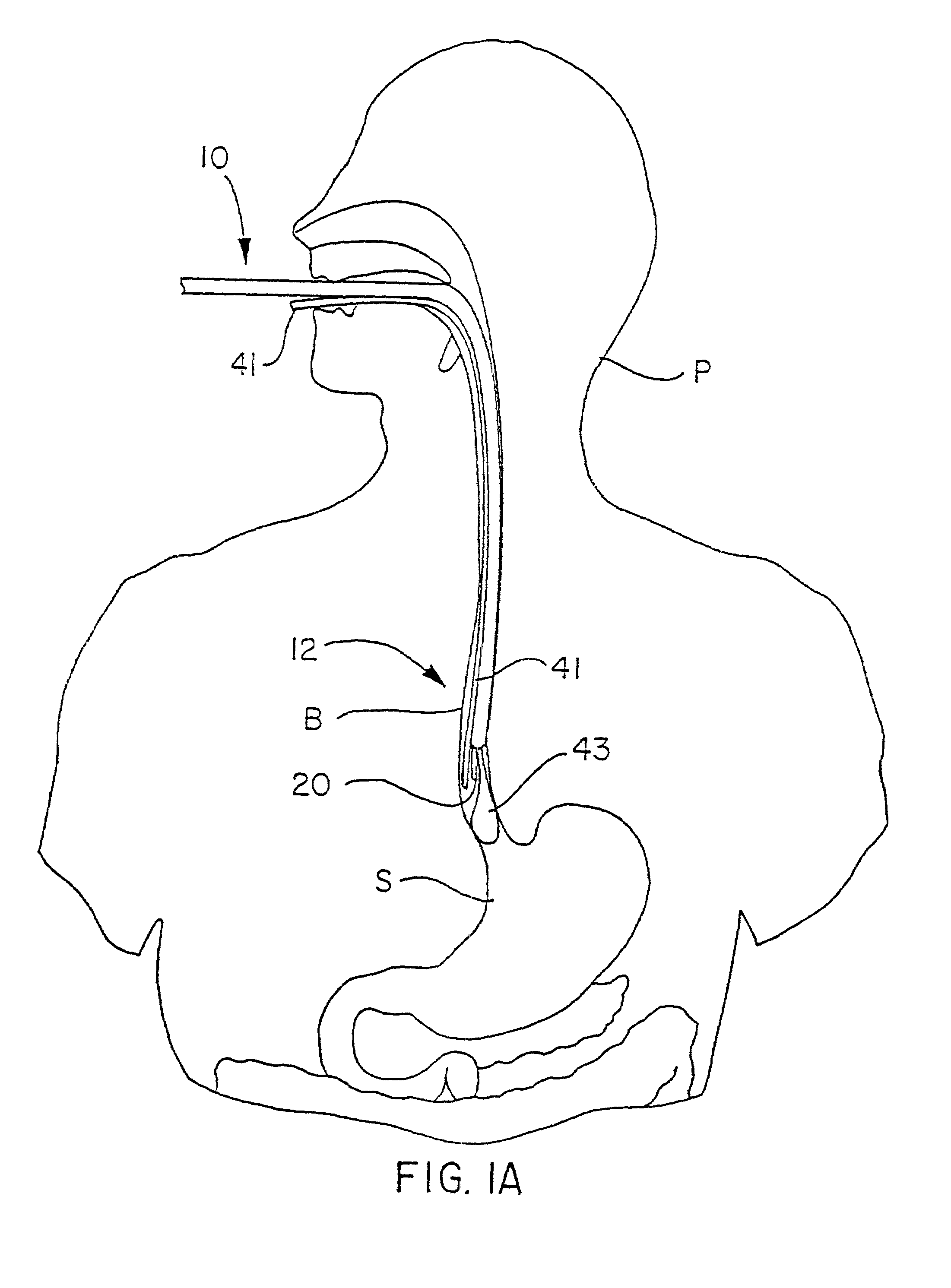
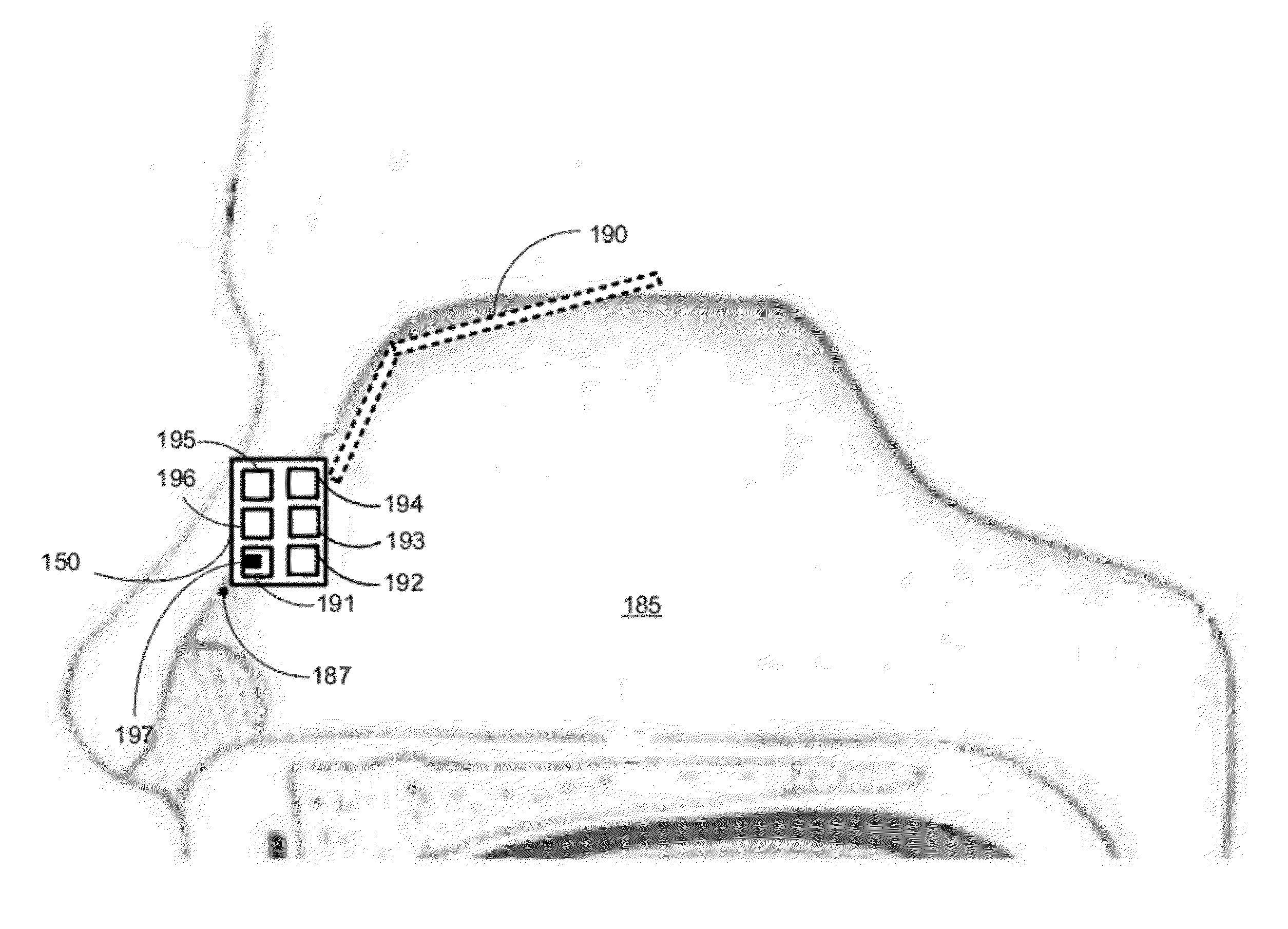
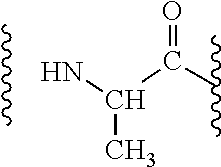
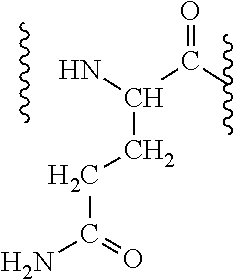
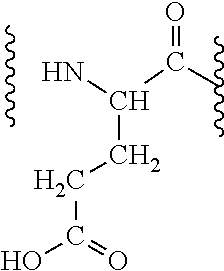
Patents
Literature
3324 results about "Mucous membrane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A mucous membrane or mucosa is a membrane that lines various cavities in the body and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It is mostly of endodermal origin and is continuous with the skin at various body openings such as the eyes, ears, inside the nose, inside the mouth, lip, vagina, the urethral opening and the anus. Some mucous membranes secrete mucus, a thick protective fluid. The function of the membrane is to stop pathogens and dirt from entering the body and to prevent bodily tissues from becoming dehydrated.
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Autism treatment
InactiveUS20120128683A1Treat and prevent associated lossMinimally invasiveNervous disorderPeptide/protein ingredientsMedicineNose
A safe and effective treatment to curtail and cure autism spectrum disorders has been described in this invention using insulin, IGF-1, with multiple known adjuvant therapeutic agents, as well as other pharmaceutical, biochemical, nurticeuticals, and biological agents or compounds delivered through the olfactory mucosal region of the nose and external auditory meatus.
Owner:SHANTHA TOTADA R
Antimicrobial compositions and methods
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including, in particular, an antimicrobial lipid component, such as a fatty acid ester, fatty ether, or alkoxide derivative thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Phenolic antiseptic compositions and methods of use
ActiveUS20060052452A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideDiphenyl etherSURFACTANT BLEND
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including an antiseptic selected from the group consisting of halogenated phenols, bisphenols, diphenyl ethers, anilides and derivatives thereof, and combinations thereof. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Precision ablating device
Apparatus for treating abnormal mucosa in an alimentary tract are provided. The apparatus include an ablation structure configured to be removably coupled to an endoscope and a deflection mechanism adapted to move the ablation structure with respect to the endoscope and toward a tissue surface.
Owner:TYCO HEALTHCARE GRP LP
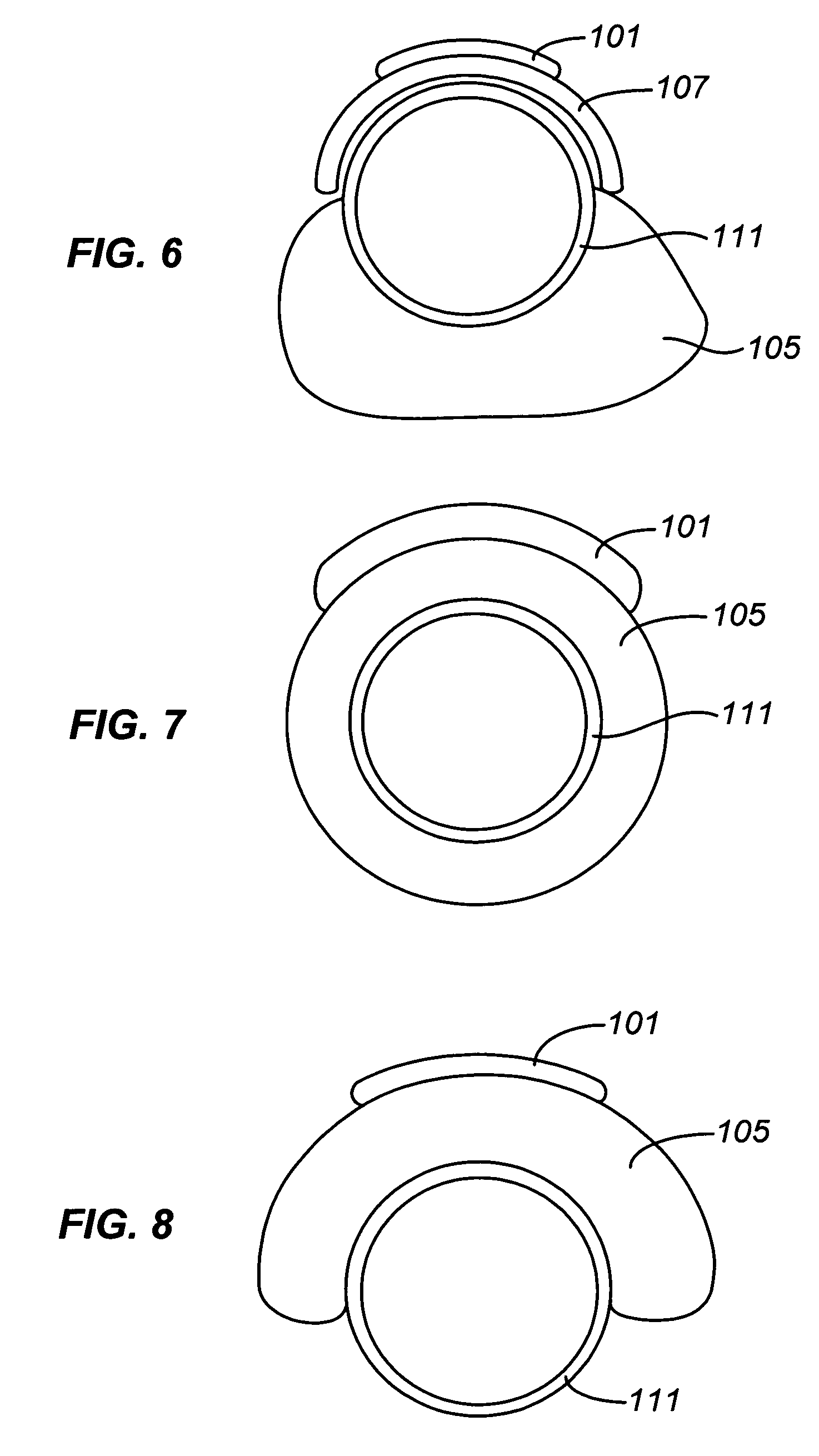
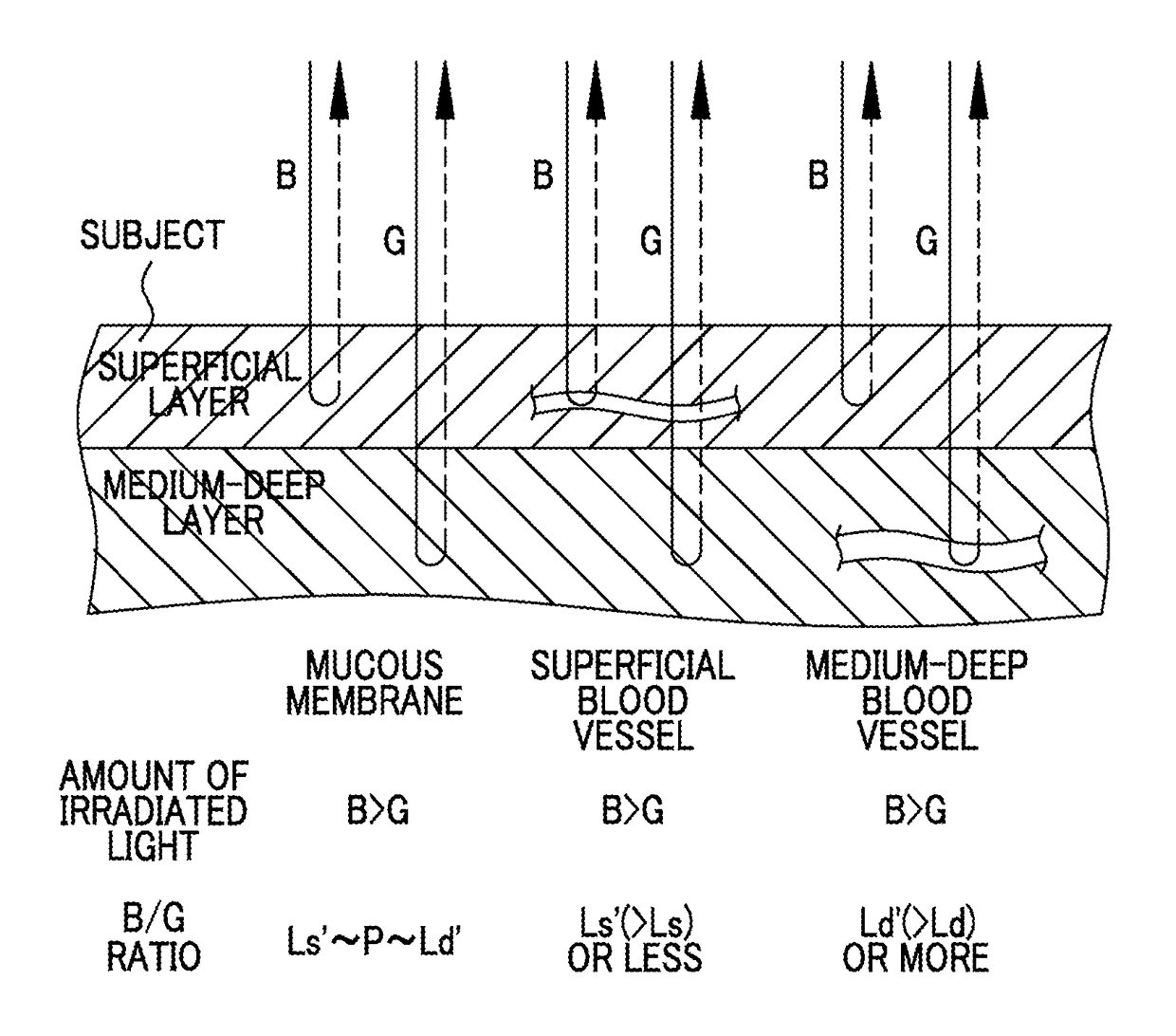
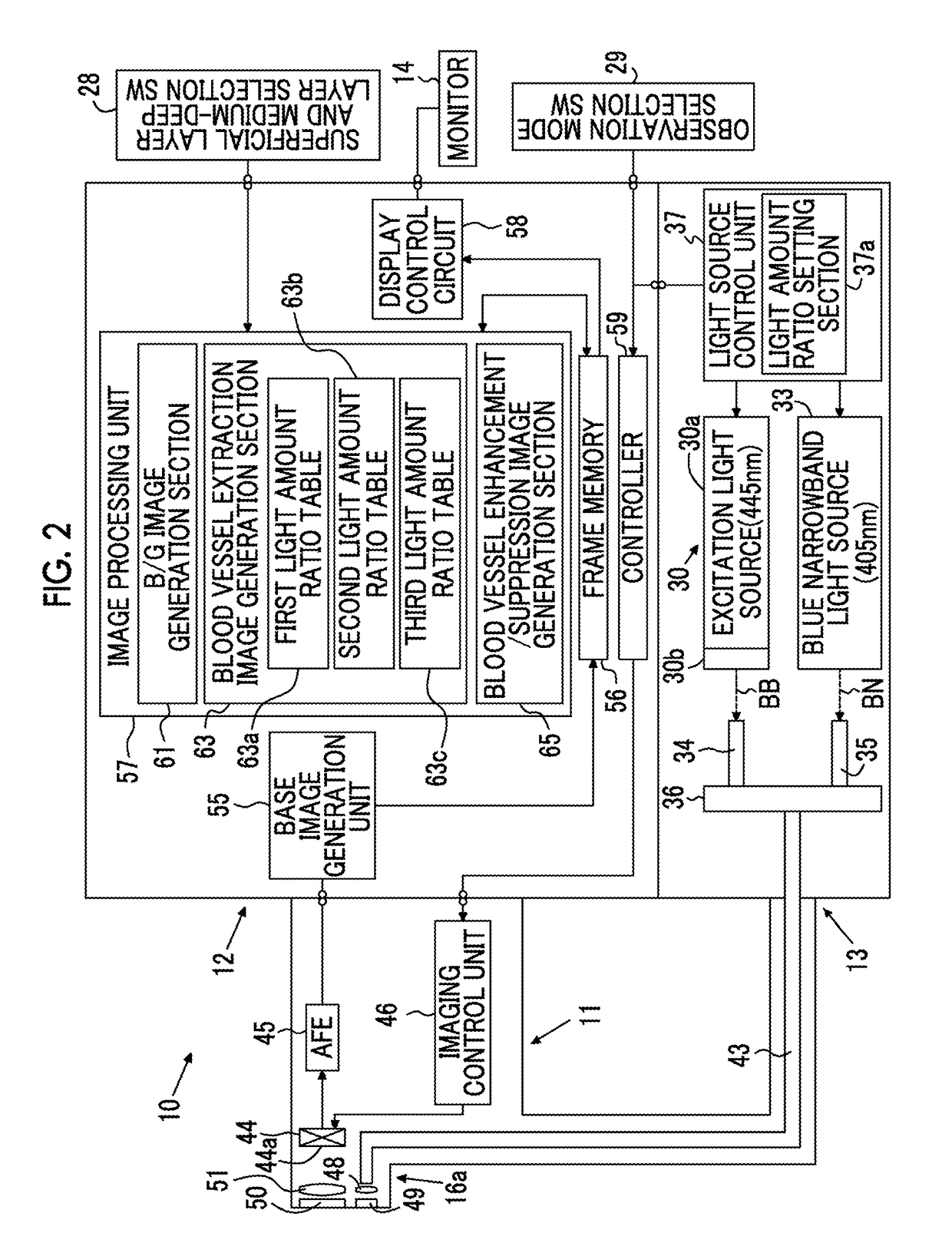
Endoscope system, processor device of endoscope system, and image processing method
ActiveUS9943230B2Reliably extract a plurality of types of blood vesselsImage enhancementImage analysisImaging processingBoundary values
Even if the ratio between blue and green components of illumination light is changed, a plurality of types of blood vessels at different depths are reliably distinguished. A blue signal B, a green signal G, a red signal R is obtained by imaging the subject using a color CCD 44. A B / G image having a B / G ratio is generated. A superficial blood vessel extraction image is obtained by extracting a pixel, in which the B / G ratio is equal to or less than a boundary value Ls between the mucous membrane and the superficial blood vessel, from the B / G image. A medium-deep blood vessel extraction image is obtained by extracting a pixel, in which the B / G ratio is equal to or greater than a boundary value Ld between the mucous membrane and the medium-deep blood vessel. The boundary values Ls and Ld differ depending on the light amount ratio.
Owner:FUJIFILM CORP
Therapeutic/cosmetic compositions comprising CGRP antagonists for treating sensitive human skin
Topically applicable pharmaceutical / dermatological / cosmetic compositions well suited for the therapeutic treatment or care of sensitive human skin, hair, mucous membranes, nails and / or the scalp, in particular for reducing or avoiding the skin-irritant side effects of a variety of bioactive agents, for example the alpha -hydroxy acids, comprise a therapeutically / cosmetically effective amount of at least one calcitonin gene related peptide ("CGRP") antagonist, e.g., CGRP 8-37 or an anti-CGRP antibody.
Owner:LOREAL SA
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20040102429A1Minimize and prevent irritationReduce transmissionAntibacterial agentsOrganic active ingredientsHigh concentrationFungicide
The addition of low concentrations of combinations of water-soluble organic salts of zinc to gels, creams, lotions or ointments can increase the ability of these products to reduce or prevent exogenous irritants from causing irritation of the underlying substrate. The addition of low concentrations of combinations of water-soluble organic zinc salts to these gels, creams, lotions or ointments also can reduce the irritation of skin or mucous membranes caused by the addition of potentially-irritating substances such as spermicides, microbicides, fungicides or other therapeutic agents to the gel, cream, lotion or ointment. The advantages of this anti-irritant approach over others, which generally employ high concentrations of single zinc salts, are the reduced potential for zinc toxicity, the reduced potential for toxicity related to zinc itself, and the preservation of the desirable biological properties of potentially-irritating therapeutic substances added to the gel, cream, lotion or ointment.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
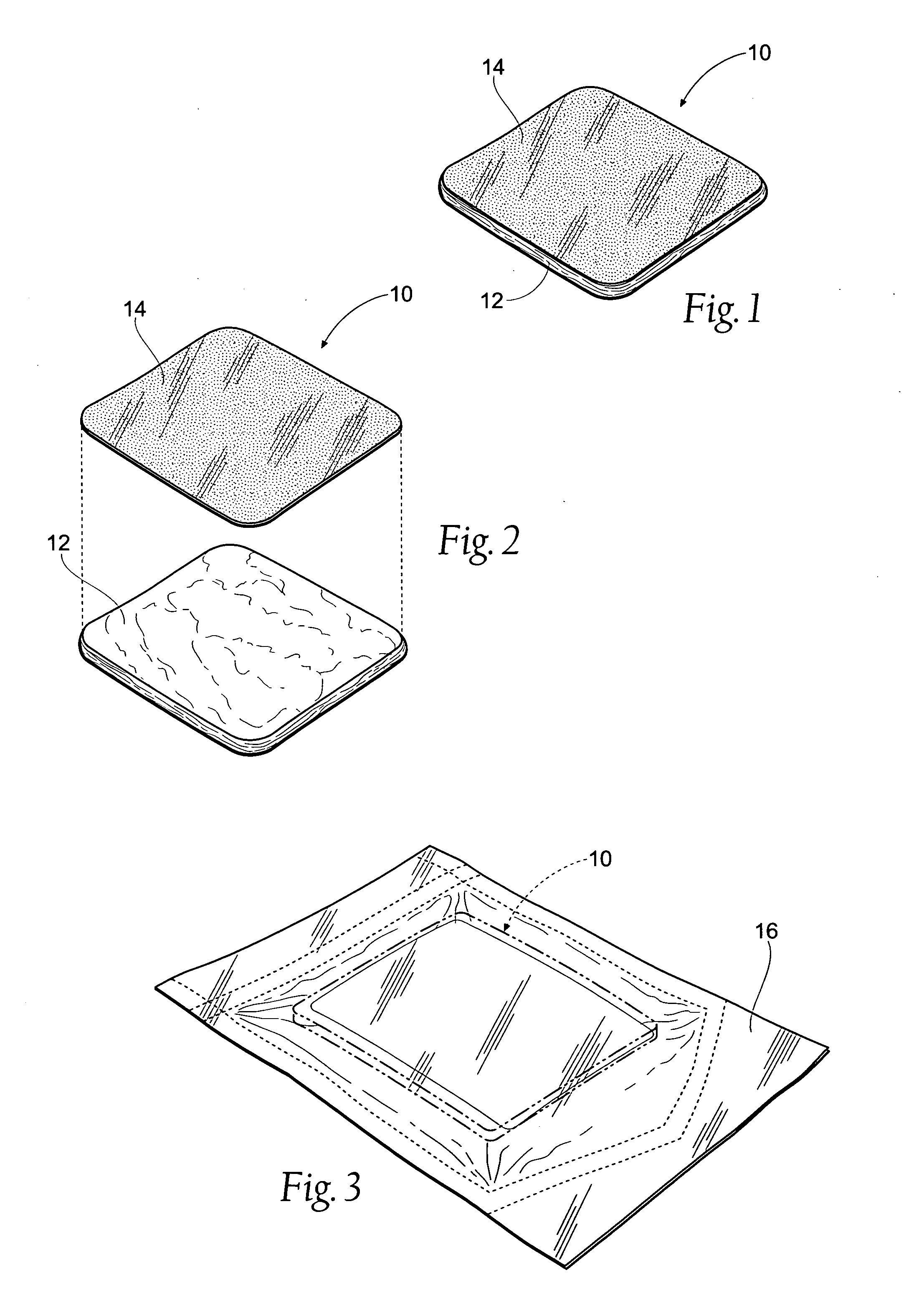
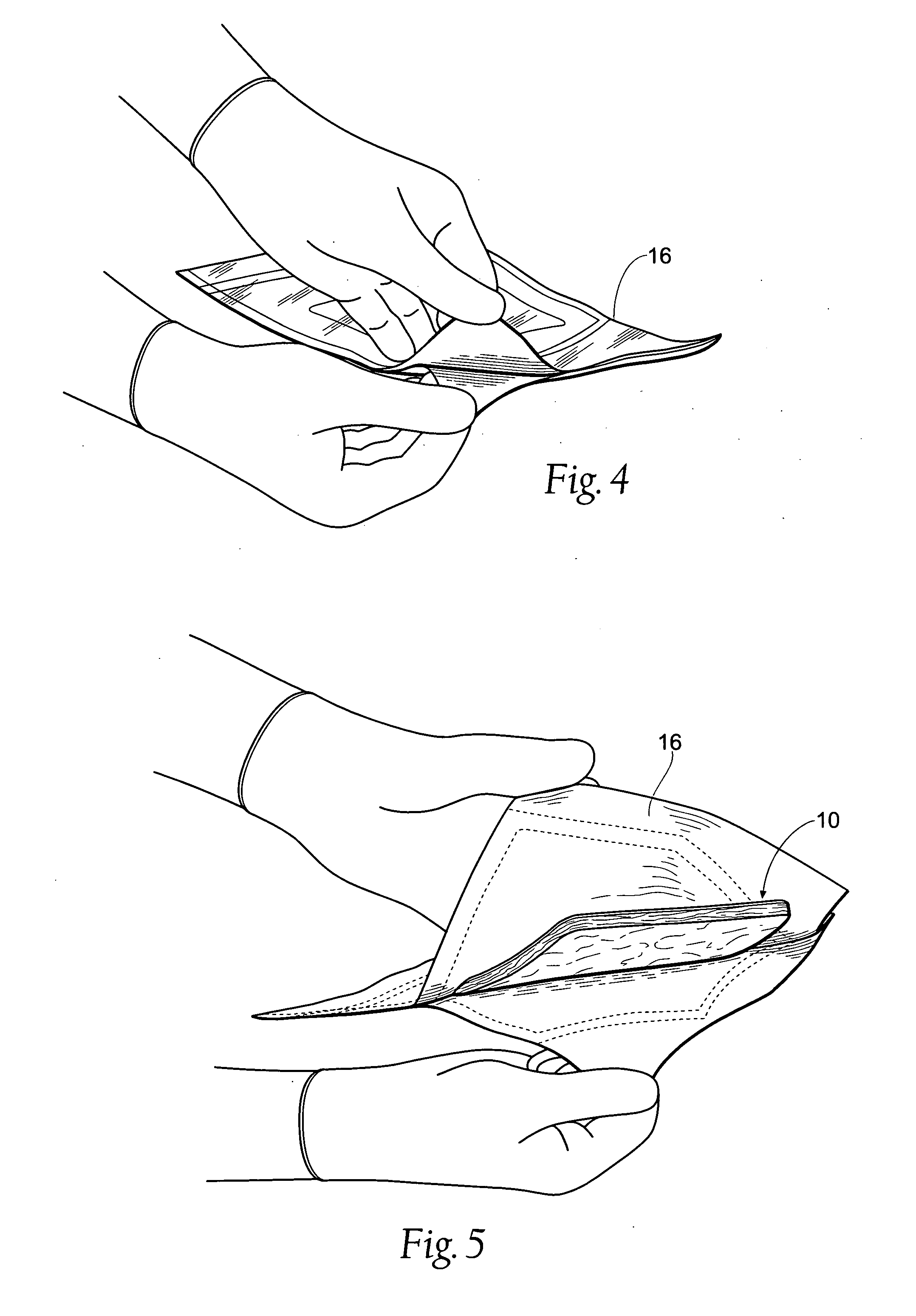
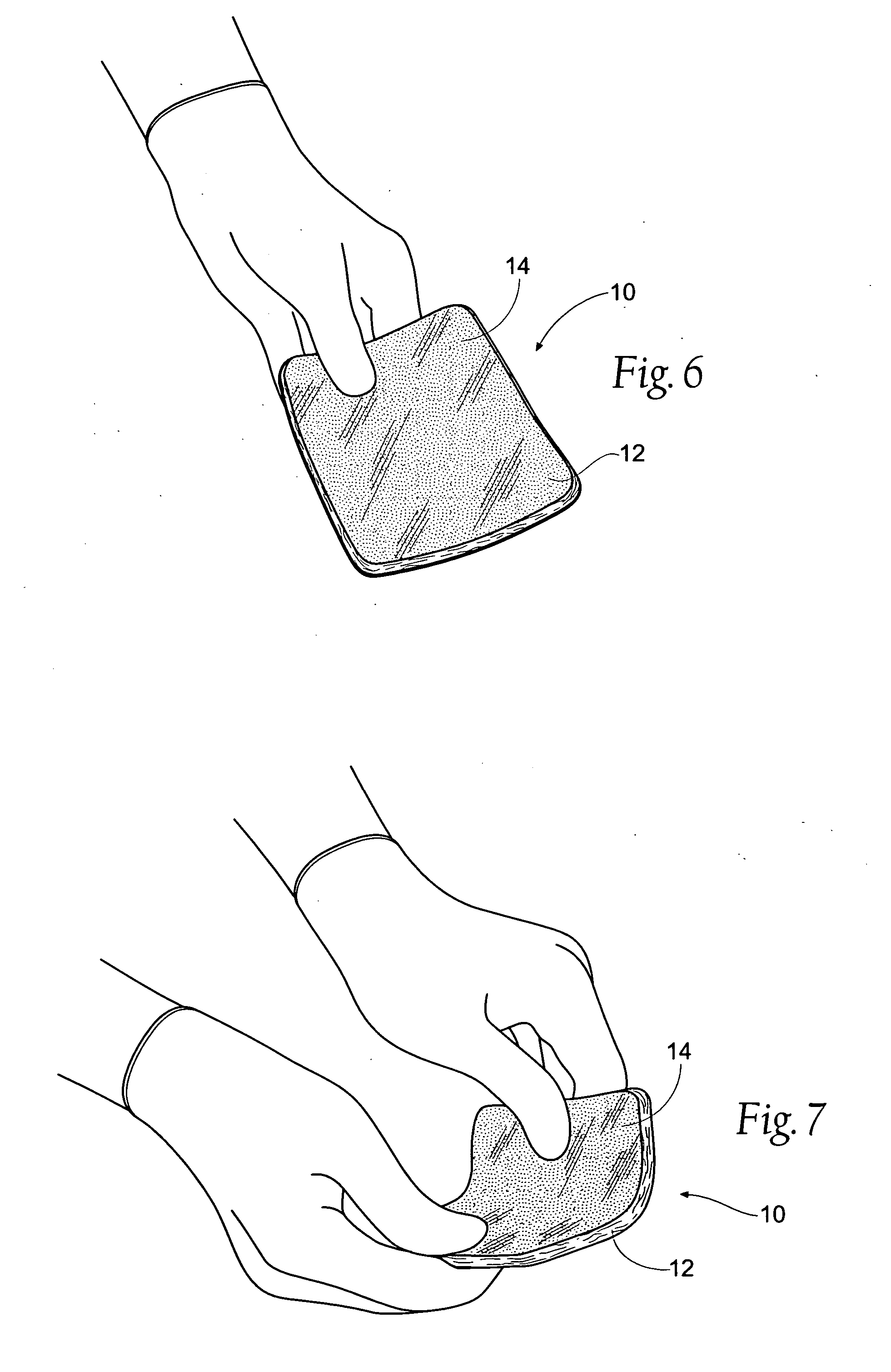
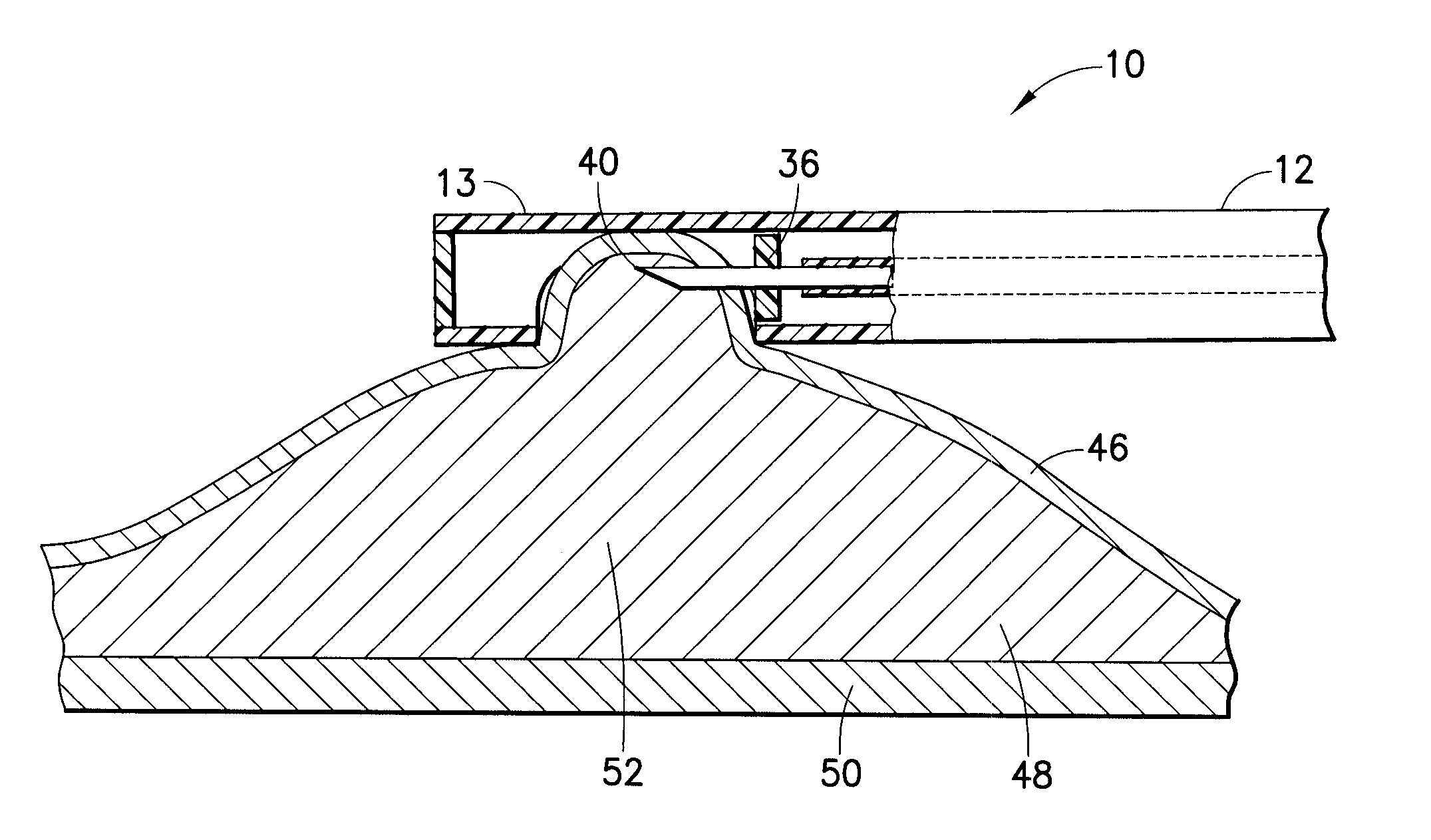
Tissue dressing assemblies, systems, and methods formed from hydrophilic polymer sponge structures such as chitosan
InactiveUS20050147656A1Imparts complianceImparts flexibilityOrganic active ingredientsBiocideInlet channelHydrophilic polymers
Tissue dressing assemblies are formed from hydrophilic polymer sponge structures. The tissue dressing assemblies can be used, e.g., (i) stanch, seal, or stabilize a site of tissue injury, tissue trauma, or tissue access; or (ii) form an anti-microbial barrier; or (iii) form an antiviral patch; or (iv) intervene in a bleeding disorder; or (v) release a therapeutic agent; or (vi) treat a mucosal surface; or (vii) combinations thereof. The tissue dressing structures are made compliant, e.g., by (i) micro-fracturing of a substantial portion of the sponge structure by mechanical manipulation prior to use, or (ii) a surface relief pattern formed on a substantial portion of the sponge structure prior to use, or (iii) a pattern of fluid inlet channels formed in a substantial portion of the sponge structure prior to use, or (iv) the impregnation of a sheet material within the sponge structure.
Owner:HEMCON MEDICAL TECH
Irradiation device for therapeutic treatment of skin and other ailments
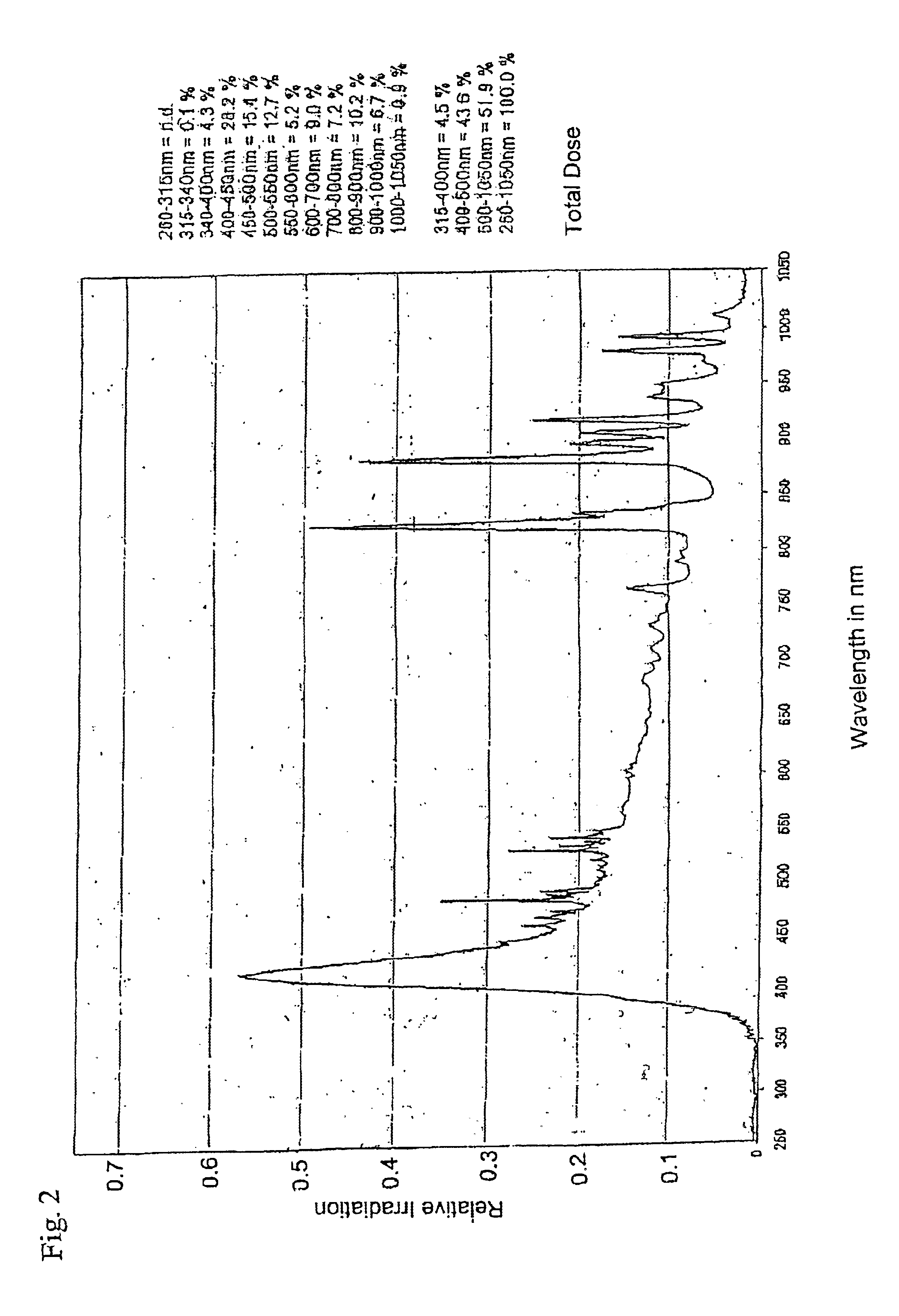
The invention relates to an irradiation device and method for the treatment of totally or partially cell-mediated inflammations of the skin, the connective tissue and the viscera, viral and other infectious diseases such as HIV and prionic infections, fungal infections of the skin and the mucous membranes, bacterial diseases of the skin and the mucous membranes as well hand eczema and anal eczema which comprises at least one irradiation device to irradiate a surface treatment area where the wavelength of the emitted radiation to a treatment area is longer than 400 nm and comprises at least one spectral band between 400-500 nm while the radiation device contains means for the generation of optical pulses towards a treatment area with a power density of the optical pulse peaks larger than 0.5 W / cm2 and smaller than 100 kW / cm2. The energy of one pulse relates to 0.05-10 J / cm2.
Owner:SPECTROMETRIX OPTOELECTRONICS SYST
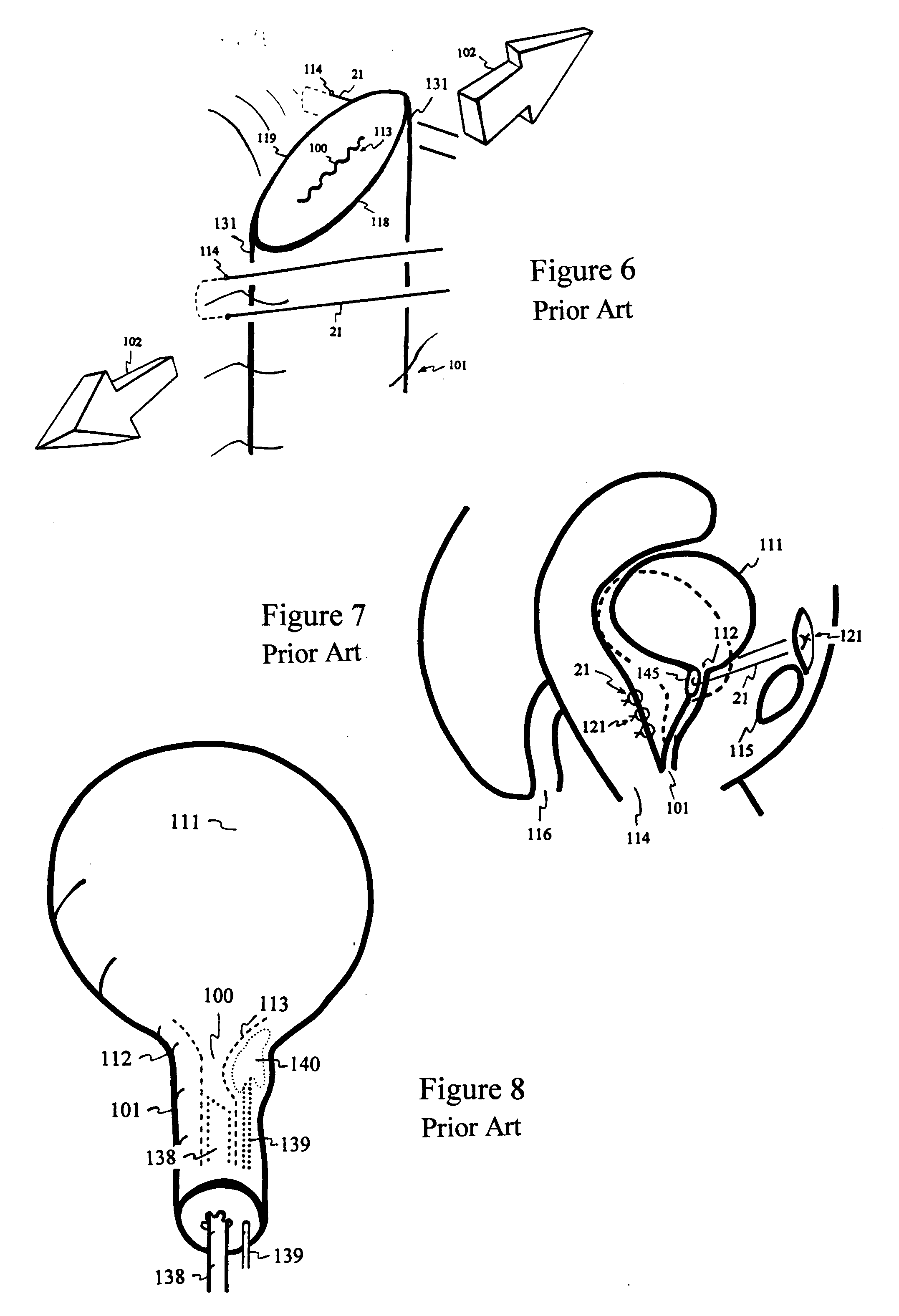
Urethral muscle controlled micro-invasive sphincteric closure device
InactiveUS20040030217A1Minimize device migrationIncrease lumensAnti-incontinence devicesSurgical needlesAnterior urethraMucous membrane
A rod-like or tube-like sphincteric closure device is micro-invasively implanted beneath the surface of the posterior mucosal wall to elevate the mucosa and close the lumen, by using a delivery device operated through the urethra. To initiate voiding, the muscle-rich anterior urethral wall contracts and widens the lumen beyond the closing or elevating range of the sphincteric closure device. For urethral obstruction, repelling magnets are micro-invasively implanted beneath the surface of mucosa with the delivery device through the urethra, to widen the lumen with magnetic force.
Owner:YEUNG JEFFREY E +1
Methods and Systems for Performing Submucosal Medical Procedures
InactiveUS20100217151A1Avoid damageSurgical needlesBlunt dissectorsMucosal resectionSubmucosal dissection
Instruments, systems and methods are provided for performing submucosal medical procedures in a desired area of the digestive tract using endoscopy. Instruments include safe access needle injection instruments incorporating electrosurgical capability, a submucosal tunneling instrument, a submucosal dissection instrument, a mucosal resection device and submucosal biopsy system. Systems include a combination of one or more of such instruments with or without injectable agents. Embodiments of various methods for performing the procedures are also provided.
Owner:APOLLO ENDOSURGERY INC
Antimicrobial compositions containing synergistic combinations of quaternary ammonium compounds and essential oils and/or constituents thereof
InactiveUS20050019431A1Synergistic antimicrobial effectAntibacterial agentsBiocideAmmonium compoundsCompound (substance)
The present invention relates to compositions comprising quaternary ammonium compounds and essential oils or individual constituents thereof which exhibit enhanced antimicrobial effects. Such combinations may be comprised in lotions, gels, creams, soaps, etc. for application to skin or mucous membranes. The invention is based, at least in part, on the observation that synergistic antimicrobial effects are achieved with combinations of essential oils or individual constituents thereof and low concentrations of quaternary ammonium compounds.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
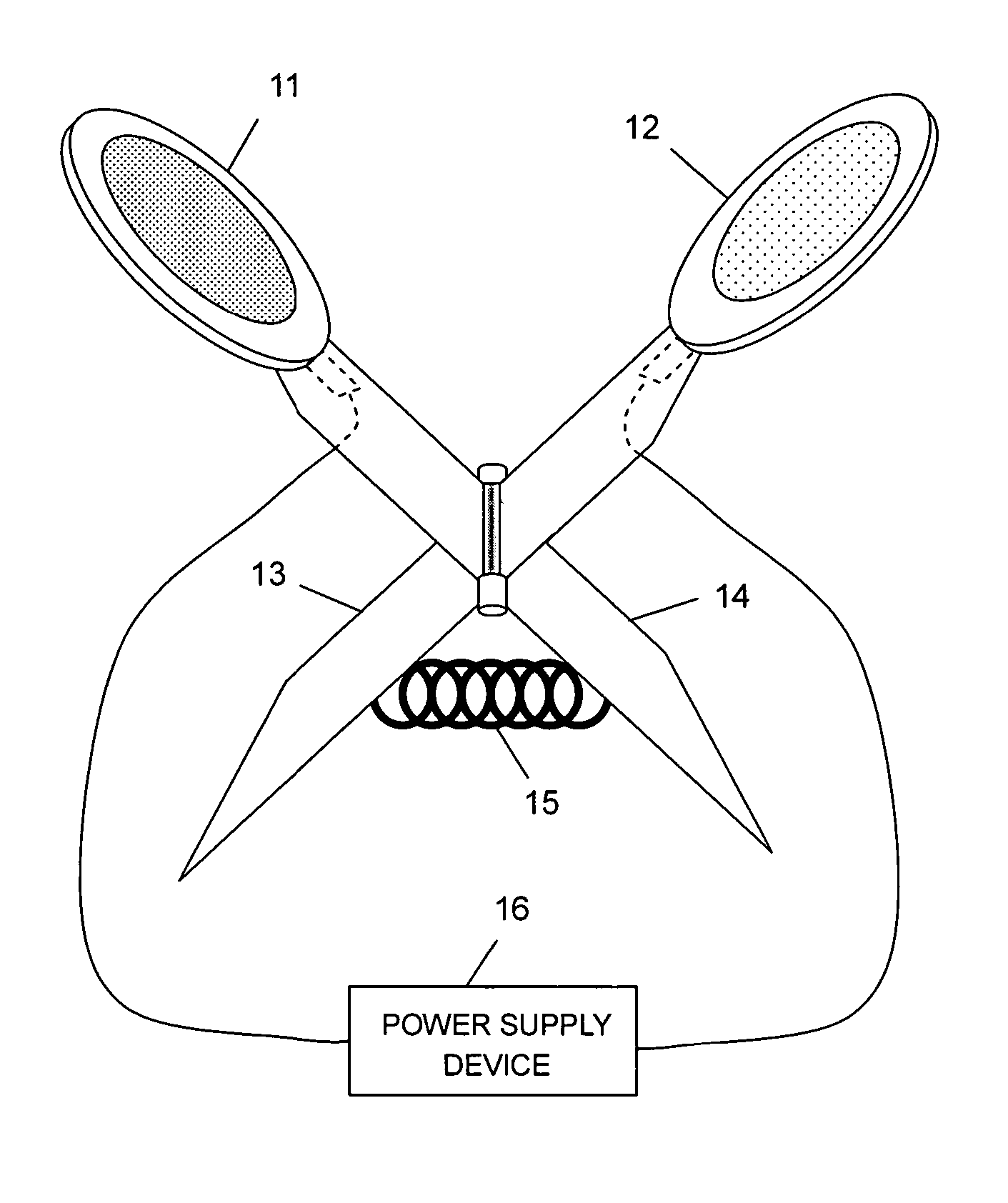
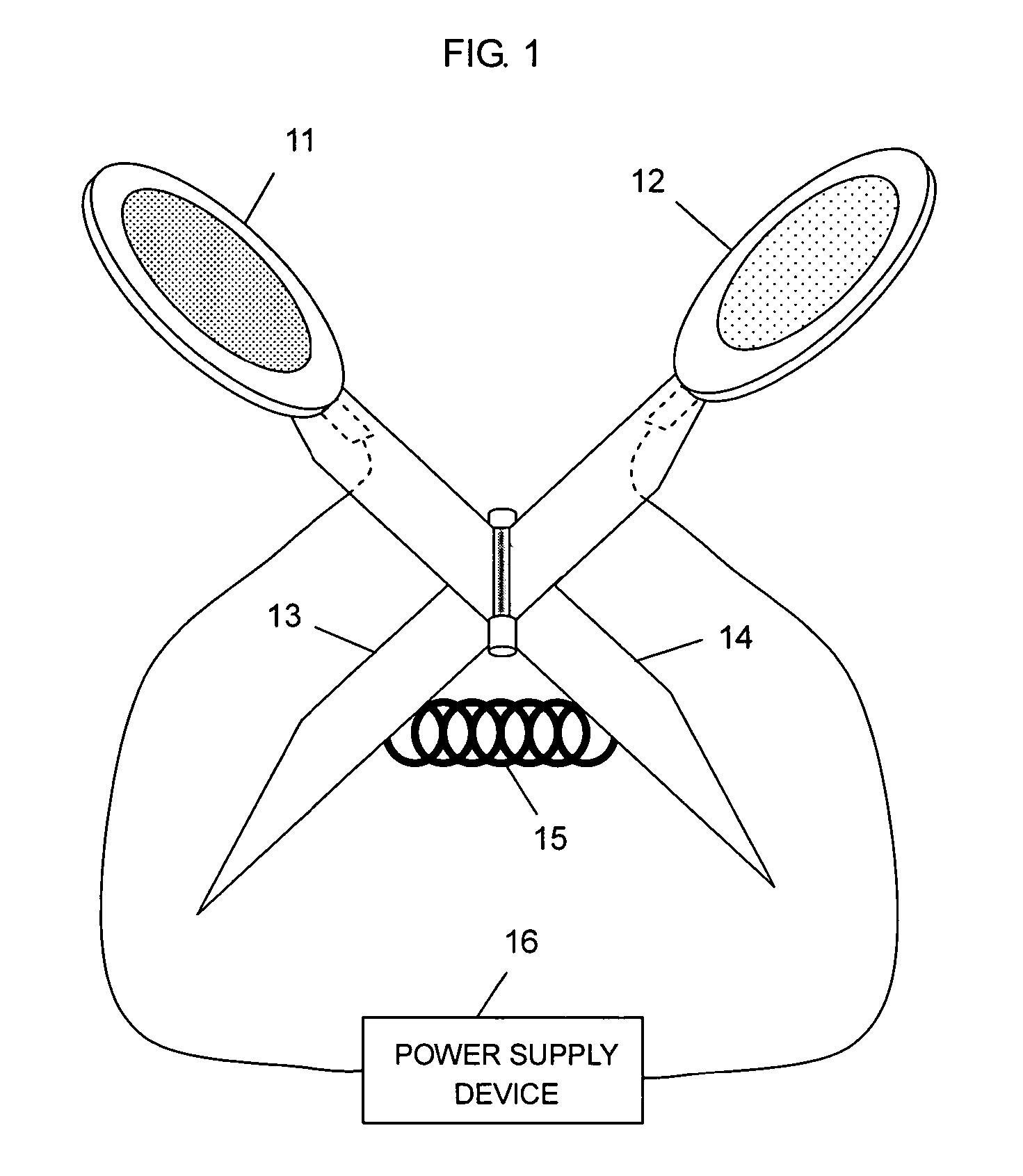
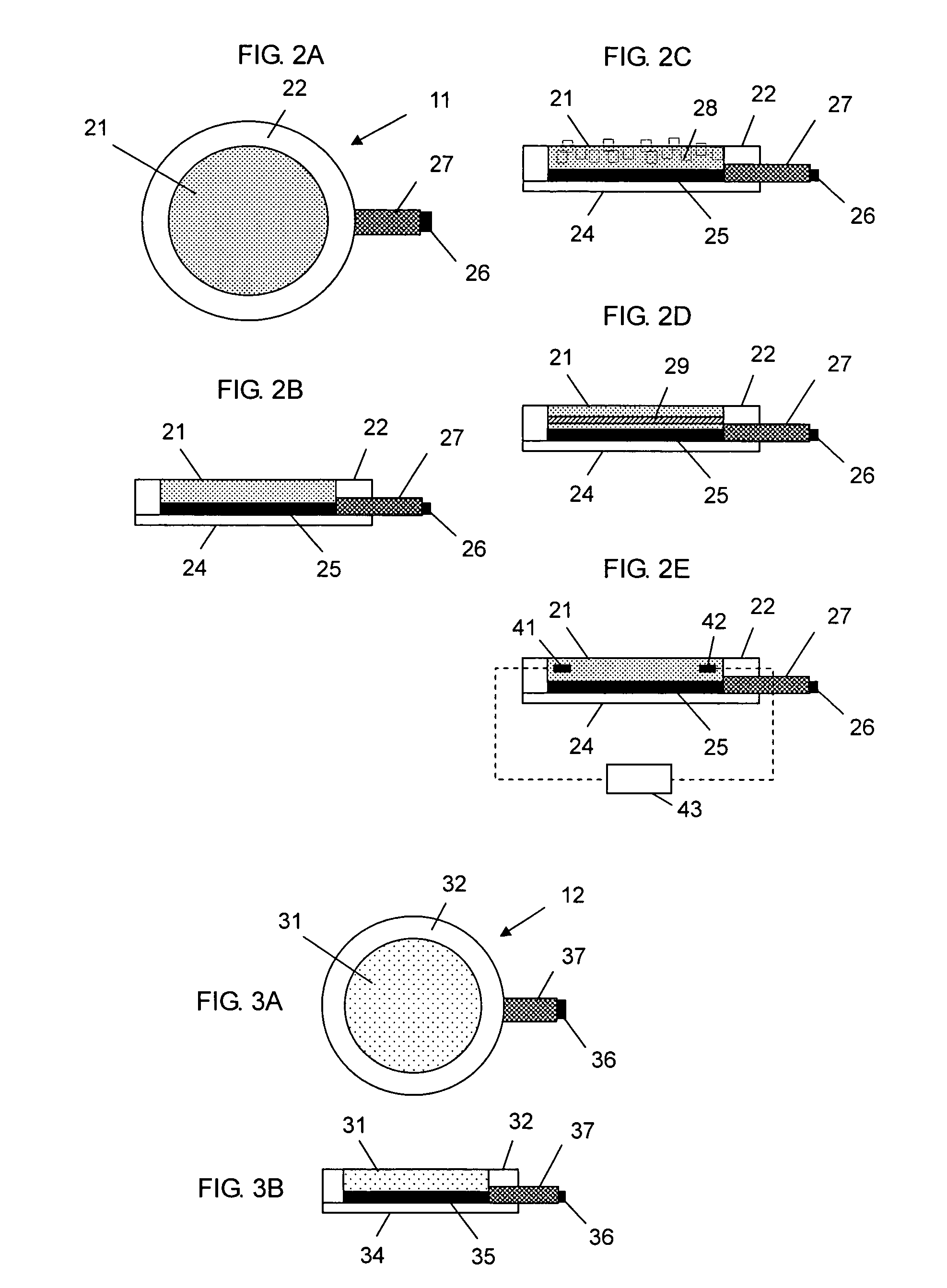
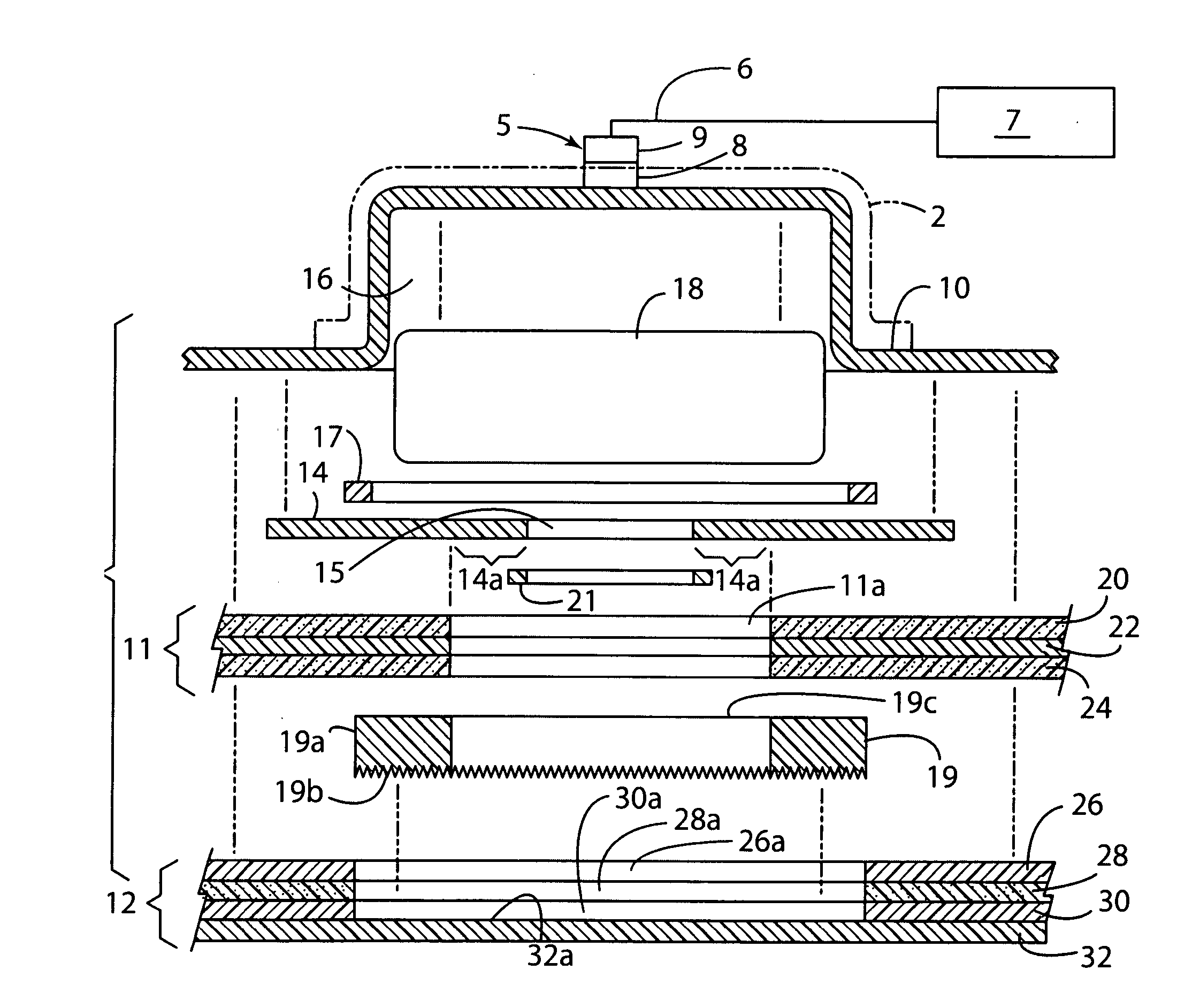
Iontophoresis system
The present invention provides an iontophoresis system for non-invasively taking a physiological substance out of the living body, the system being suitably used for the mucous membrane. The present iontophoresis system non-invasively takes a physiological substance out of a living body. The system includes a plurality of electrode structures and a power supply device connected to the electrode structures. At least one of the electrode structures has a physiological substance extraction pad applied to the mucous membrane. In the present system, the time to apply electric energy to the living body by the power supply device is set between 30 seconds and 20 minutes. The physiological substance extraction pad which is provided in the electrode structure is applicable to the mucous membrane of the mouth and can be used, for example, to monitor glucose in the living body or an amount of drug administered.
Owner:HISAMITSU PHARM CO INC
Transdermal delivery device
A dermal, transdermal, mucosal or transmucosal delivery device includes a backing layer overlying an ingredient containing reservoir, and having a microprotrusion array attached thereto, a cover for the reservoir having at least one opening therethrough, an adhesive layer and a liner layer. Upon removal of the liner layer, the device may be placed over the desired area of the skin or mucosa and adhesively applied thereto allowing the ingredients to flow from the reservoir through the at least one opening to the skin or mucosa.
Owner:CORIUM PHARMA SOLUTIONS INC
Endoscopic mucosal resection method and associated instrument
InactiveUS20060064113A1Accurate removalReduce the possibilityVaccination/ovulation diagnosticsSurgical instrument detailsMucosal resectionThin layer
An endoscopic tissue resection device and related method is used in conjunction with a flexible or rigid endoscope. Tissue is resected by shaving thin layers of tissue for diagnostic and therapeutic purposes.
Owner:GRANIT MEDICAL INNOVATION
Diindolylmethane-based compositions and methods of use thereof for promoting oral mucosal and bone health
InactiveUS20060264497A1Improve bioavailabilityEffective oral systemic useBiocideCosmetic preparationsDiseaseDental flossing
The present invention includes compositions and methods for the treatment and prevention of oral mucosal disorders and for promotion of bone health. In particular, the present invention describes new therapeutic and preventative uses for 3,3′-diindolylmethane (DIM), or a DIM-related indole, alone or in combination with anti-inflammatory agents and / or antibacterial agents, to treat oral mucosal disorders and promote bone health. The compositions of the invention are used to prevent and reverse oral mucosal disorders and bone loss (osteopenia and osteoporosis) associated with aging and chronic inflammation. Oral mucosal disorders include Periodontitis, gingivitis and related oral mucosal inflammation. Formulations of the compositions of the invention include capsules, tablets, toothpastes, oral gels, mouthwashes, mouth rinses, lozenges, chewing gum, dental floss, and dental topical formulations, and fortified foods.
Owner:BIORESPONSE
Method and apparatus for cryogenic spray ablation of gastrointestinal mucosa
InactiveUS7025762B2Readily and inexpensivelyOvercomes drawbackSurgical furnitureDiagnosticsCryoablationSphincter of Oddi
A method and apparatus to treat Barrett's tissue, a pre-cancerous condition, by removing the epithelium above the lower esophageal sphincter through cryo-ablation. An endoscope with fiber optics is used to view the operation, and a catheter for supplying liquid nitrogen is passed through the lumen of the endoscope. Liquid nitrogen at low pressure is sprayed directly onto the Barrett's tissue through the catheter while the physician views the operation through the fiberoptics of the endoscope and controls the spray via a valve. Freezing is indicated by whiteness and shows that the epithelium has been cryoablated. The apparatus can also be used to treat various other gastrointestinal tract lesions. The catheter is insulated to withstand extremely cold temperatures without becoming stiff and without affecting the inherent flexibility and maneuverability of the endoscope.
Owner:CRYMED TECH
Apparatus and methods for delivery of therapeutic agents to mucous or serous membrane
ActiveUS20120298105A1Medical devicesImplantable neurostimulatorsMedical deviceBiomedical engineering
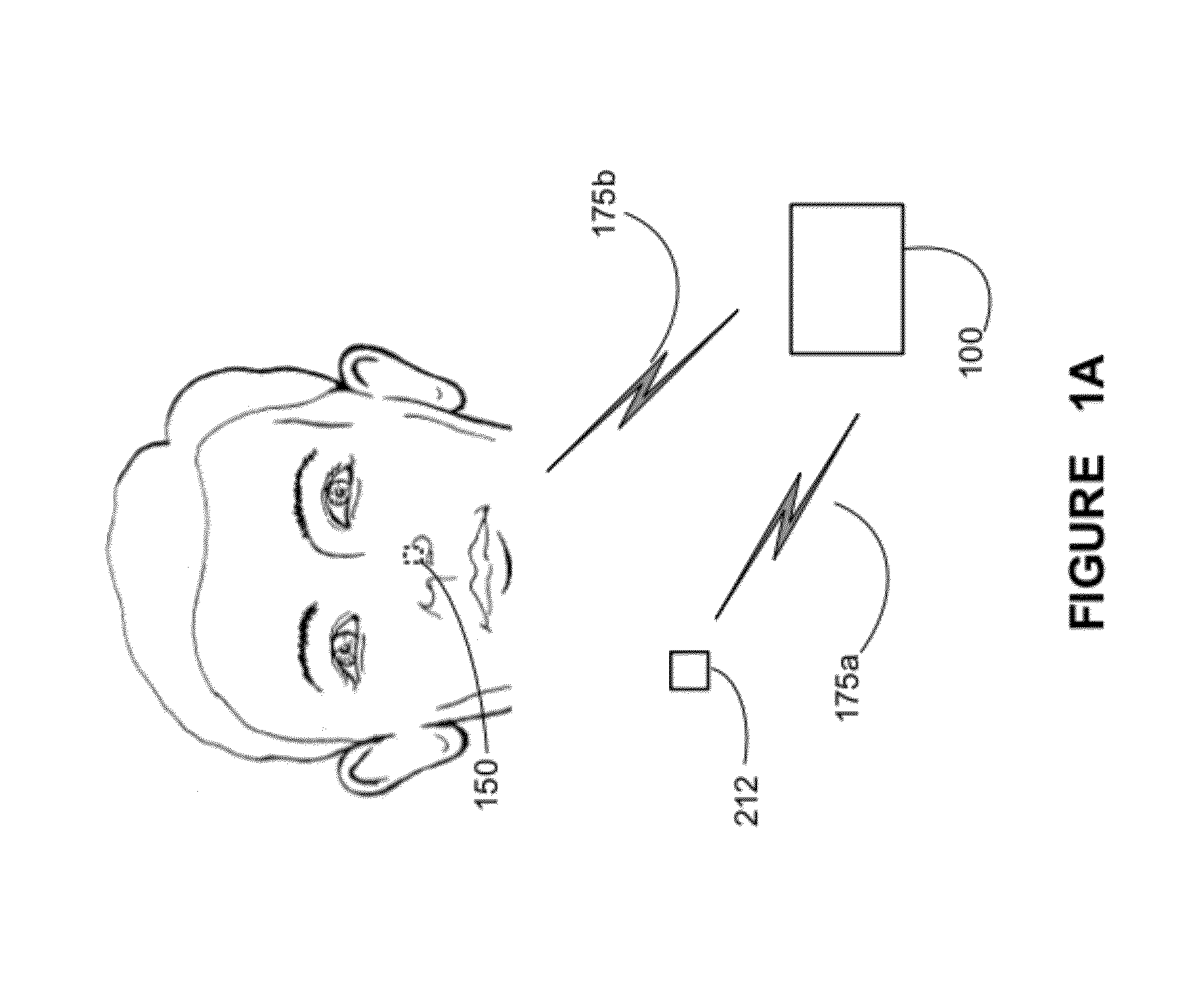
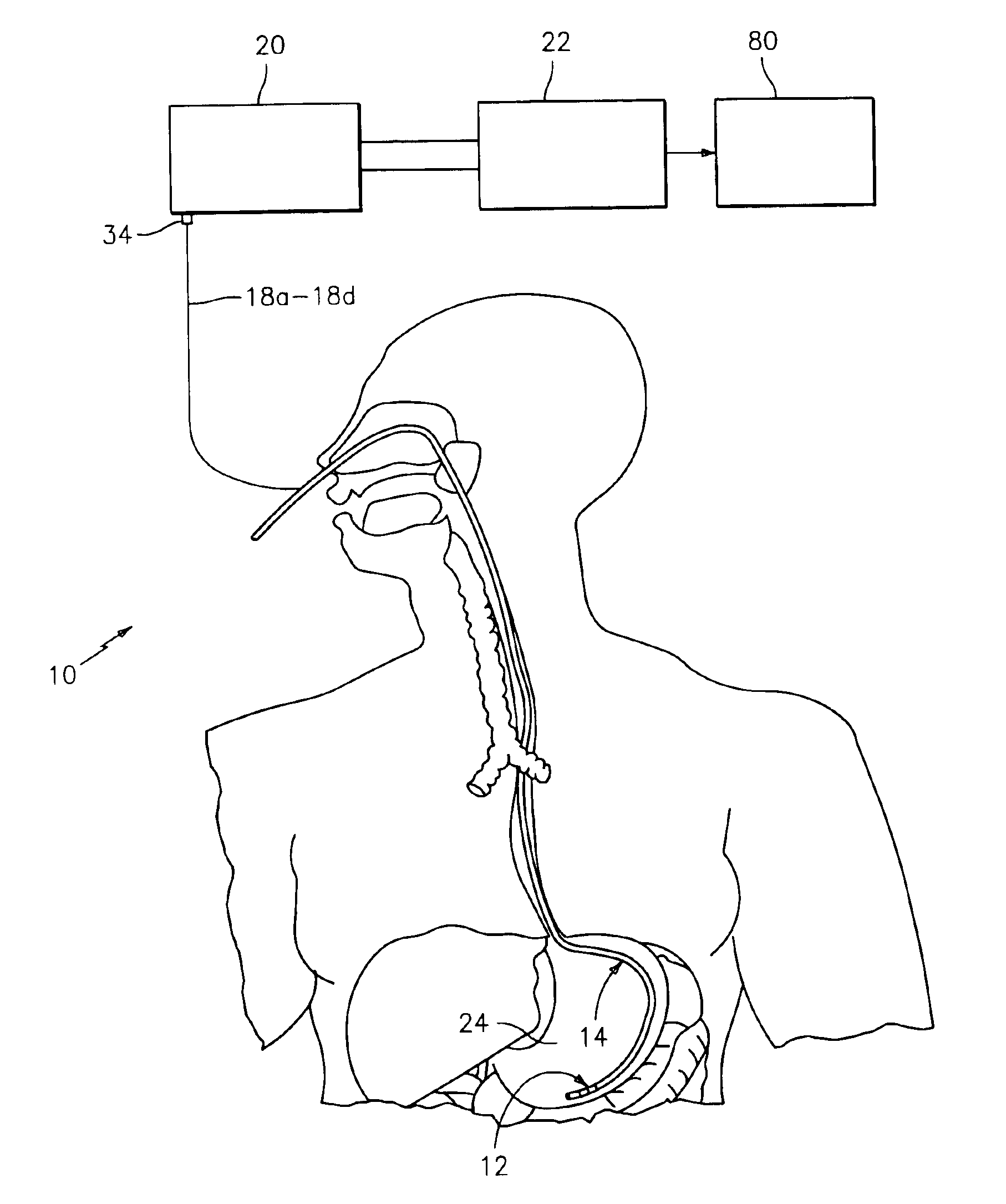
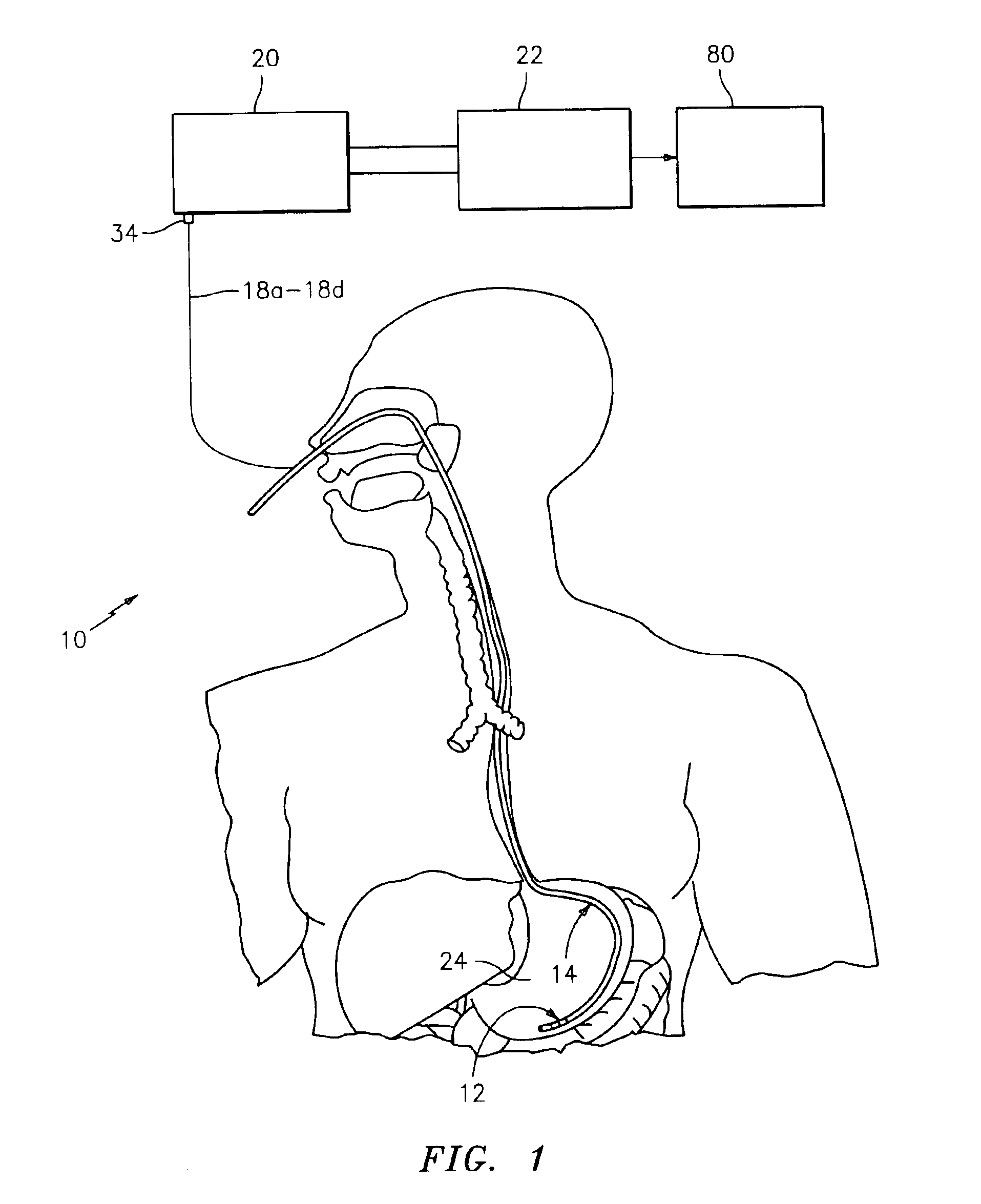
A method, apparatus, and system are provided for mucous membrane therapy. The method includes receiving at least one body signal from a patient; detecting a condition of the patient based on the body signal; and administering the therapy to at least one of a mucous membrane or a serous membrane of the patient. A medical device system configured to implement the method is provided. A computer-readable storage device for storing instructions that, when executed by a processor, perform the method is also provided.
Owner:OSORIO IVAN
Impedance spectroscopy system and catheter for ischemic mucosal damage monitoring in hollow viscous organs
InactiveUS6882879B2ElectrocardiographyDevices for locating reflex pointsSpectroscopyImpedance spectrum
An impedance spectroscopy system for monitoring ischemic mucosal damage in hollow viscous organs comprises a sensor catheter and an impedance spectrometer for electrically driving the catheter to obtain a complex tissue impedance spectrum. Once the catheter is in place in one of a patient's hollow viscous organs, the impedance spectrometer obtains the complex impedance spectrum by causing two electrodes in the tip of the catheter to inject a current into the mucosal tissue at different frequencies, while two other electrodes measure the resulting voltages. A pattern recognition system is then used to analyze the complex impedance spectrum and to quantify the severity of the mucosal injury. Alternatively, the complex impedance spectrum can be appropriately plotted against the spectrum of normal tissue, allowing for a visual comparison by trained personnel.
Owner:CRITICAL PERFUSION
Non-irritating compositions containing zinc salts
InactiveUS20070020342A1Minimize prevent irritationPromoting rapid killBiocideCosmetic preparationsIrritationMedicine
The present invention relates to methods and compositions which employ low concentrations of combinations of zinc salts to prevent the irritation of skin or mucous membranes that may be caused by therapeutic agents, by personal hygiene products, by articles such as gloves or condoms, or by various physical, chemical, mechanical, or biological irritants.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Treating instrument for endoscope
A flexible sheath is removably inserted into an instrument-inserting channel of an endoscope. A treating member for applying a treatment to mucous membrane in a body cavity is projected from and withdrawn into the distal end of the sheath by an operation conducted at the proximal end of the sheath. An exposed electrode is provided at the distal end surface of the sheath. An electrically conductive member extending through the sheath is electrically connected at a distal end thereof to the electrode and at a proximal end thereof to a high-frequency power supply at the proximal end of the sheath.
Owner:ASAHI KOGAKU KOGYO KK
Peptides useful in the treatment and care of the skin and mucous membranes and their use in cosmetic or pharmaceutical compositions
ActiveUS20140120141A1Promotes collagen synthesisIncrease moistureCosmetic preparationsBiocideDiseasePharmaceutical drug
Peptides of general formula (I), their stereoisomers, mixtures thereof and / or their cosmetically or pharmaceutically acceptable salts, a preparation process, cosmetic or pharmaceutical compositions which contain them and their use for the treatment and / or care of conditions, disorders and / or diseases of the skin and / or mucous membranes.
Owner:LIPOTEC SA
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20050281762A1Minimize and prevent irritationReduce transmissionBiocideCosmetic preparationsMedicineIrritation
The present invention relates to methods and compositions which employ low concentrations of combinations of zinc salts to prevent the irritation of skin or mucous membranes that may be caused by therapeutic agents, by personal hygiene products, or by various physical, chemical, mechanical, or biological irritants, including infectious agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Pharmaceutical composition for oral use with improved absorption
InactiveUS7008640B2Promote absorptionDifficult to absorbBiocideNervous disorderBULK ACTIVE INGREDIENTAcid substances
The present invention presents a pharmaceutical composition for oral use with improved absorption, which comprises drug, aminoalkyl methacrylate copolymer E, and acidic substance and is obtained by bringing said 3 components together and uniformly mixing at least this polymer and this acidic substance, and a method of improving oral absorption by using this pharmaceutical composition. Moreover, the present invention presents an agent for improving oral absorption that increases drug permeability of the digestive tract mucous membrane and / or mucous layer present on the surface of this membrane, whose active ingredient is aminoalkyl methacrylate copolymer E. In addition, the present invention presents an oral agent for improving absorption by increasing drug permeability of the digestive tract mucous membrane and / or the mucous layer distributed over this mucous membrane, whose effective component is aminoalkyl methacrylate copolymer E.
Owner:ASTELLAS PHARMA INC
Moistening preparation
InactiveUS6156293ALow salivationGreat tasteCosmetic preparationsSenses disorderActive agentMucous membrane
PCT No. PCT / FI98 / 00008 Sec. 371 Date Aug. 26, 1999 Sec. 102(e) Date Aug. 26, 1999 PCT Filed Jan. 2, 1998 PCT Pub. No. WO98 / 29090 PCT Pub. Date Jul. 9, 1998The present invention relates to preparations alleviating the symptoms caused by drying of the mucous membranes of the body. The preparations contain trimethylglycine as an active agent. The invention also relates to the use of trimethylglycine as an agent alleviating the symptoms of dry mucous membranes in different preparations intended for the body care and hygiene, and to a method of alleviating the symptoms, caused by drying, appearing on the mucous membranes of the body.
Owner:FINPHIDE
Methods for protecting the skin from radiation insults
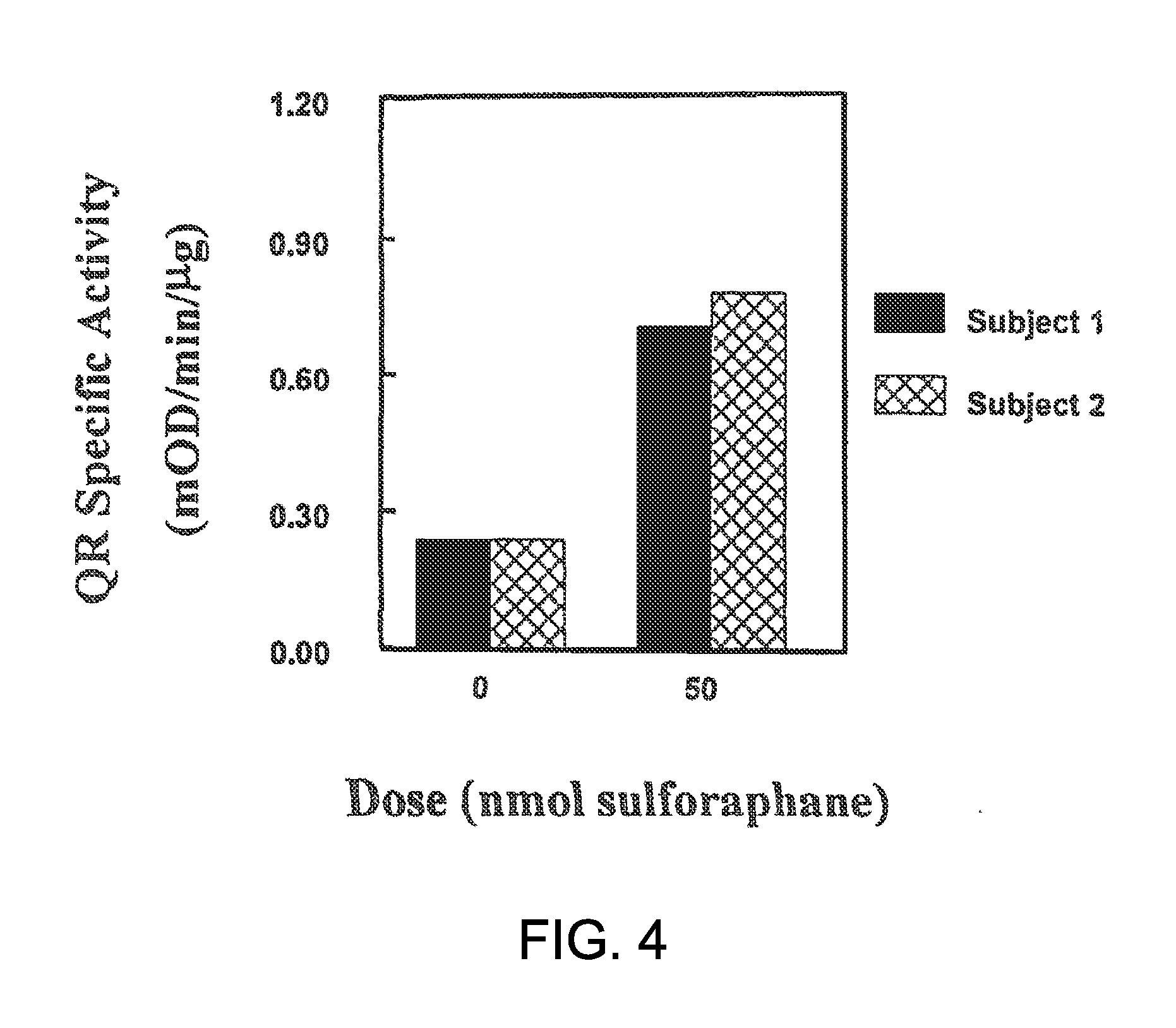
The present invention relates to methods and compositions for the protection of skin and mucous membranes from undesirable side effects of ionizing radiation in a patient undergoing ionizing radiation therapy. In particular, the application describes compositions and methods comprising the topical use of Nrf2 inducers.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
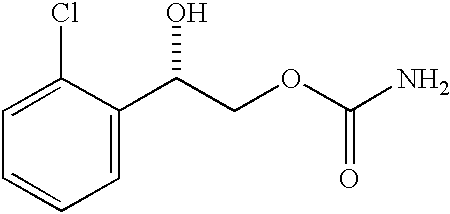
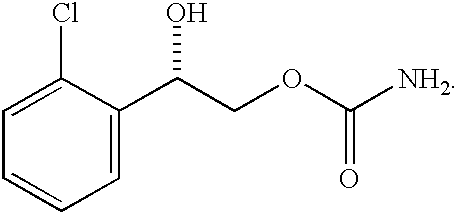
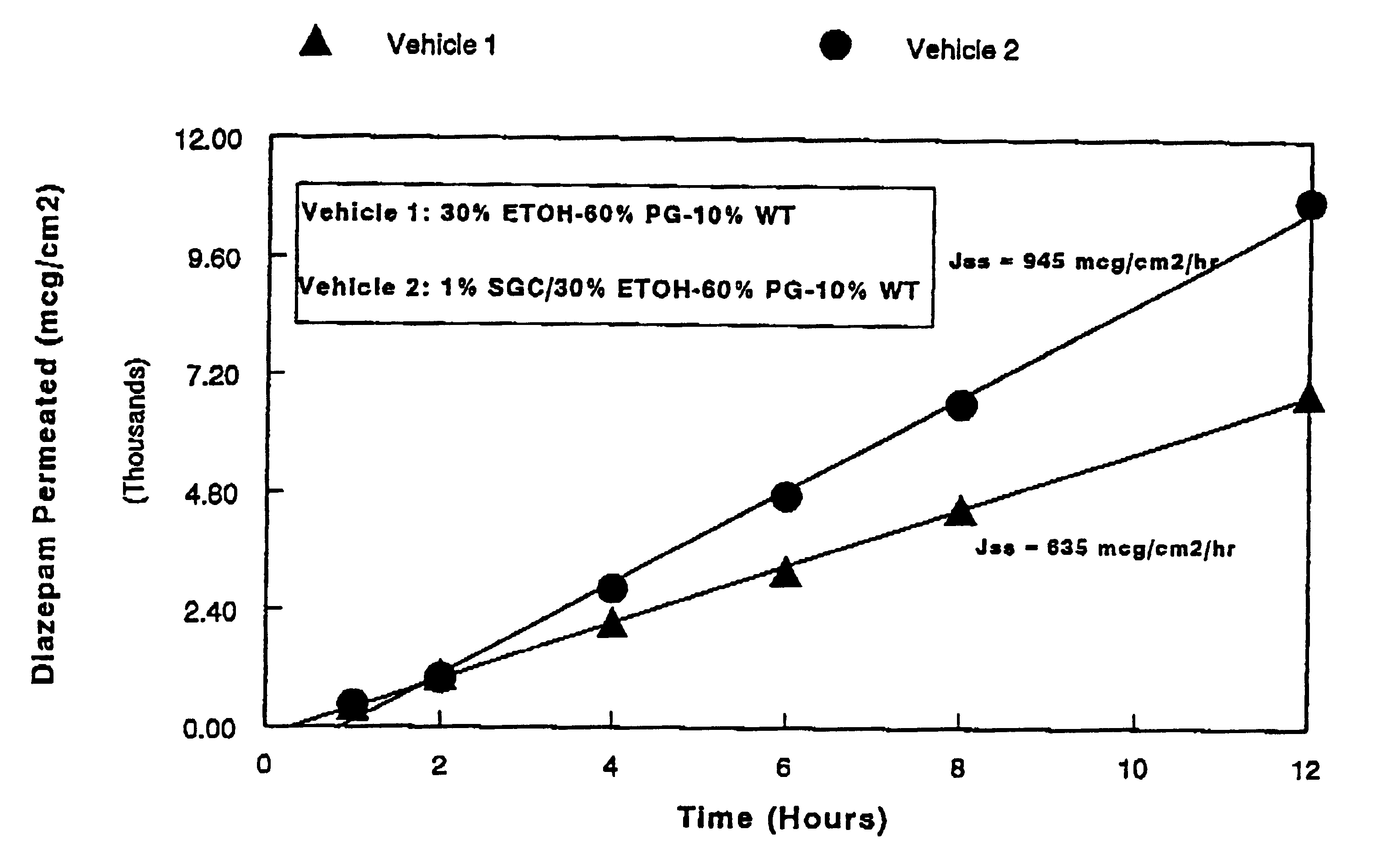
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
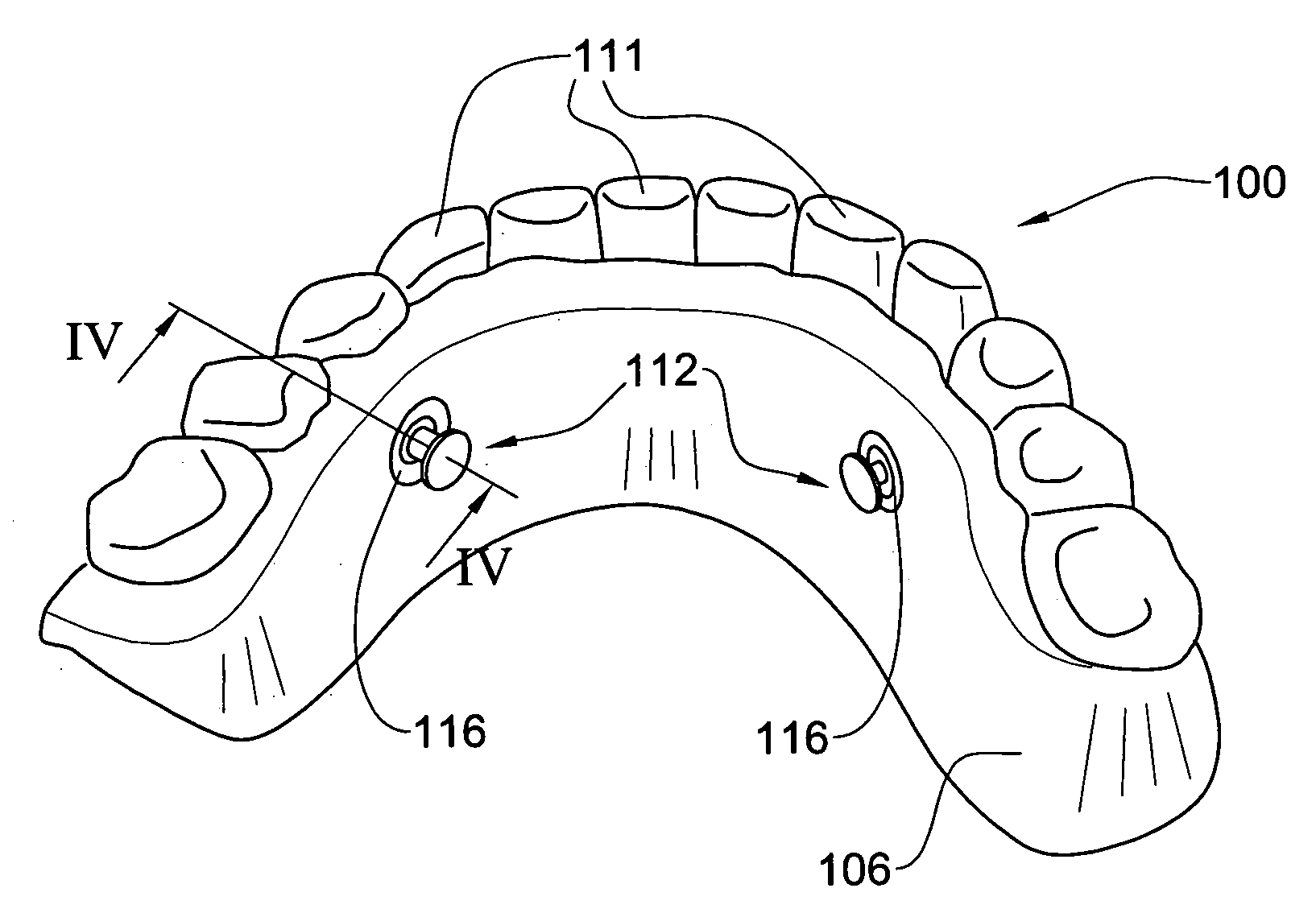
Method and system for fixing removable dentures
ActiveUS20060223029A1Preventing unintentional disengagementTight toleranceDental implantsFastening prosthesisEngineeringDental implant
A removable denture system comprising a support beam fixedly attached to the individual's alveolar ridge above the mucous membrane by a plurality of dental implants, and a denture generally conforming with the dental parameters of the individual and integrated with a supper-structure. The supper-structure comprises at least a portion shaped in confirmation with the support beam, and a denture locking arrangement for removably though fixedly articulating the denture to the support beam preventing unintentional disengagement of the denture.
Owner:BIO DENTAL SOLUTIONS
Method for preparing SIS tissue repair material
The invention provides a method for preparing an SIS tissue restoring material. The method comprises the following steps: carrying out sterilizing treatment, degreasing treatment, cell free treatment and absterging treatment according to an arbitrary sequence at the lower layer of small intestinal mucosa; and finally preparing the material through freezing and drying. The SIS tissue restoring material can be used as an independent and common support vector for a growth factor, a cell, a drug and the like, and becomes a tissue engineering material through in-vitro culture. The material is a natural and nontoxic biological material without antigenicity and immunity. The SIS tissue restoring material can be used for restoring hernia, defect of skin, urinary system tissue defect, mucosa defect and other periphery and internal parenchyma defects.
Owner:BEIJING DATSING BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com