Patents
Literature
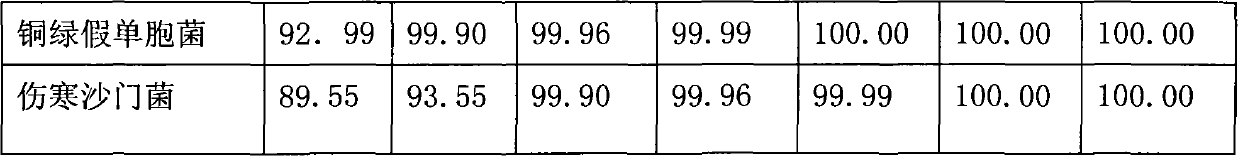
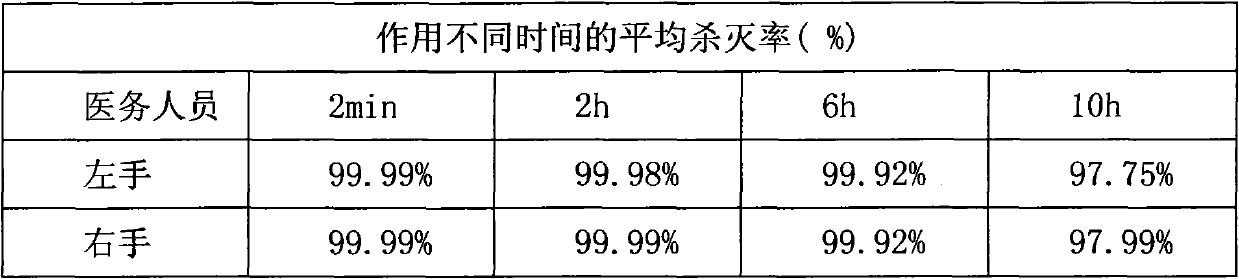
488 results about "Chlorhexidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
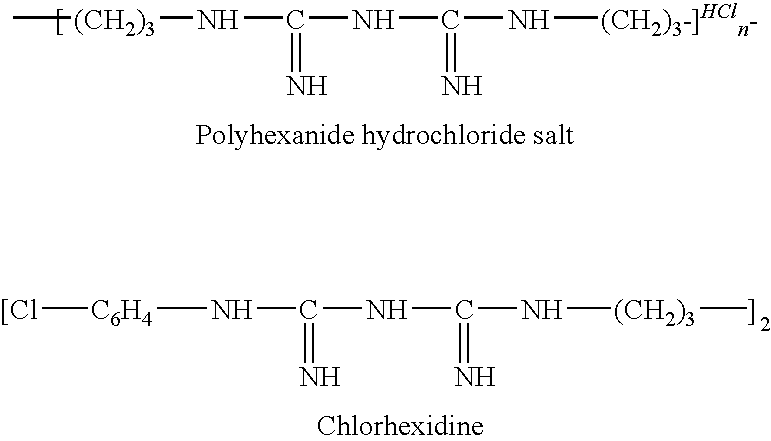
Chlorhexidine, also known as chlorhexidine gluconate (CHG), is a disinfectant and antiseptic that is used for skin disinfection before surgery and to sterilize surgical instruments. It may be used both to disinfect the skin of the patient and the hands of the healthcare providers. It is also used for cleaning wounds, preventing dental plaque, treating yeast infections of the mouth, and to keep urinary catheters from blocking. It is used as a liquid or powder.
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Triclosan and silver compound containing medical devices
InactiveUS6224579B1Preventing and inhibiting infectionAvoid adverse reactionsSuture equipmentsBiocideChlorhexidineMedical treatment
The present invention relates to polymeric medical articles comprising combinations of triclosan and silver-containing compounds. It is based, at least in part, on the discovery that these agents act synergistically, thereby permitting the use of relatively low levels of both agents. While it had been previously found that triclosan can be particularly useful when used in conjunction with chlorhexidine, it has been further discovered that medical articles having suitable antimicrobial properties may be prepared, according to the present invention, which contain triclosan without chlorhexidine. Such medical articles offer the advantage of preventing or inhibiting infection while avoiding undesirable adverse reactions to chlorhexidine by individuals that may have sensitivity to chlorhexidine.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Triclosan-containing medical devices
InactiveUS6106505ALower levelAvoiding undesirably high releaseWrappers shrinkageShrinkage connectionsTriclosanAntiinfective agent
PCT No. PCT / US96 / 20932 Sec. 371 Date Jun. 30, 1998 Sec. 102(e) Date Jun. 30, 1998 PCT Filed Dec. 23, 1996 PCT Pub. No. WO97 / 25085 PCT Pub. Date Jul. 17, 1997The present invention relates to polymeric medical articles comprising the antiinfective agents chlorhexidine and triclosan. It is based, at least in part, on the discovery that the synergistic relationship between these compounds permits the use of relatively low levels of both agents, and on the discovery that effective antimicrobial activity may be achieved when these compounds are comprised in either hydrophilic or hydrophobic polymers. It is also based on the discovery that chlorhexidine free base and triclosan, used together, are incorporated into polymeric medical articles more efficiently. Medical articles prepared according to the invention offer the advantage of preventing or inhibiting infection while avoiding undesirably high release of antiinfective agent, for example into the bloodstream of a subject.
Owner:COLUMBIA UNIV OF THE CITY OF NEW YORK TRUSTEES OF THE
Triclosan and silver compound containing medical devices
The present invention relates to polymeric medical articles comprising combinations of triclosan and silver-containing compounds. It is based, at least in part, on the discovery that these agents act synergistically, thereby permitting the use of relatively low levels of both agents. While it had been previously found that triclosan can be particularly useful when used in conjunction with chlorhexidine, it has been further discovered that medical articles having suitable antimicrobial properties may be prepared, according to the present invention, which contain triclosan without chlorhexidine. Such medical articles offer the advantage of preventing or inhibiting infection while avoiding undesirable adverse reactions to chlorhexidine by individuals that may have sensitivity to chlorhexidine.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Triclosan-containing medical devices
The present invention relates to polymeric medical articles comprising the antiinfective agents chlorhexidine and triclosan. It is based, at least in part, on the discovery that the synergistic relationship between these compounds permits the use of relatively low levels of both agents, and on the discovery that effective antimicrobial activity may be achieved when these compounds are comprised in either hydrophilic or hydrophobic polymers.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Oral and dental care agent
InactiveUS20060088482A1Strong scale-inhibiting effectGood effectCosmetic preparationsToilet preparationsChlorhexidineBULK ACTIVE INGREDIENT
The invention relates to oral and dental care agents, containing: an active ingredient combination (A) of at least one cationic component with an anti-bacterial action, (B) at least one active ingredient with an anti-tartar action, selected from the group of azacycloalkane diphosphonic acids or their phnysiologically compatible salts and (C) at least one binding agent, selected from xanthan gum, carboxymethylcellulose or a mixture of said two components. The oral and dental care anti-bacterial agents do not cause the discoloration associated with chlorohexidine and reduce or prevent discoloration to human teeth. This is unique for oral and dental care agents based on chlorohexidine. The inventive oral and dental care agents also have a tartar prevention and plaque reducing agent.
Owner:WULKNITZ PETER +1
Dentifrice Compositions for Treating Xerostomia
This invention encompasses an oral composition for treating or preventing dry mouth with following benefits: antibacterial / anti-fungal efficacy, low irritancy, and moisture retention. The compositions can be a toothpaste, a mouth rinse or a spray and include one or more non-ionic surfactants, as foaming agents, one or more broad spectrum anti-microbial ingredient, such as chlorhexidine and cetylpyridinium chloride, and one or more oral surface adhesive polysaccharides that can encage cationic actives to help the deposition and retention of the agents onto oral surfaces and thus provide antimicrobial efficacy.
Owner:COLGATE PALMOLIVE CO
Combinations of antiseptic and antibiotic agents that inhibit the development of resistant microorganisms
InactiveUS6582719B2Toxic reductionLong-term efficacySuture equipmentsHeavy metal active ingredientsTriclosanChlorhexidine
The present invention relates to compositions comprising a combination of one or more antiseptic and an antibiotic. It is based, at least in part, on the discovery that such combinations tend to deter the formation of antibiotic-resistant organisms. In preferred, nonlimiting embodiments of the invention, the antibiotic is minocycline and the antiseptic is a chlorhexidine compound, triclosan, or benzalkonium chloride, and in particular embodiments, a silver salt or a bismuth salt is added. Examples of specific, nonlimiting embodiments of the invention include combinations of (i) minocycline, triclosan, and a bismuth salt; (ii) minocycline, a chlorhexidine compound, and a bismuth salt; and (iii) minocycline, benzalkonium chloride, and a bismuth salt. The present invention further provides for articles, such as, but not limited to, medical articles, which have been treated with or which otherwise comprise a combination of antiseptic and antibiotic.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Washing-free antibacterial hand sanitizer and preparation method thereof
InactiveCN101766552AAvoid damageSimple processAntibacterial agentsCosmetic preparationsHand sanitizerChlorhexidine
The invention discloses a washing-free antibacterial hand sanitizer and a preparation method thereof. Raw materials of the washing-free antibacterial hand sanitizer calculated at 100ml include 1-10g of chlorhexidine salt, 5-1,000ug of silver nitrate, 30-90ml of alcohol, 2-50g of skin care agent, 0.5-3g of tackifier, 0.5-3g of surfactant, 0.5-5g of p+H regulator and 15-50ml of deionized water. In the preparation method, the skin care agent and the chlorhexidine salt are added to the alcohol and dissolved under the temperature of 30-70 DEG C to form alcoholic solution; the tackifier, the surfactant and the silver nitrate are added to the deionized water to form aqueous solution; finally, the aqueous solution and the pH regulator are added to the alcoholic solution to prepare the washing-free antibacterial hand sanitizer. The washing-free antibacterial hand sanitizer contains various antibacterial components, has very strong broad-spectrum antibacterial and bactericidal effects on various bacteria and fungi, and has long-acting antibacterial effect.
Owner:ZHEJIANG KUNZHILIN BIOMEDICINE TECH
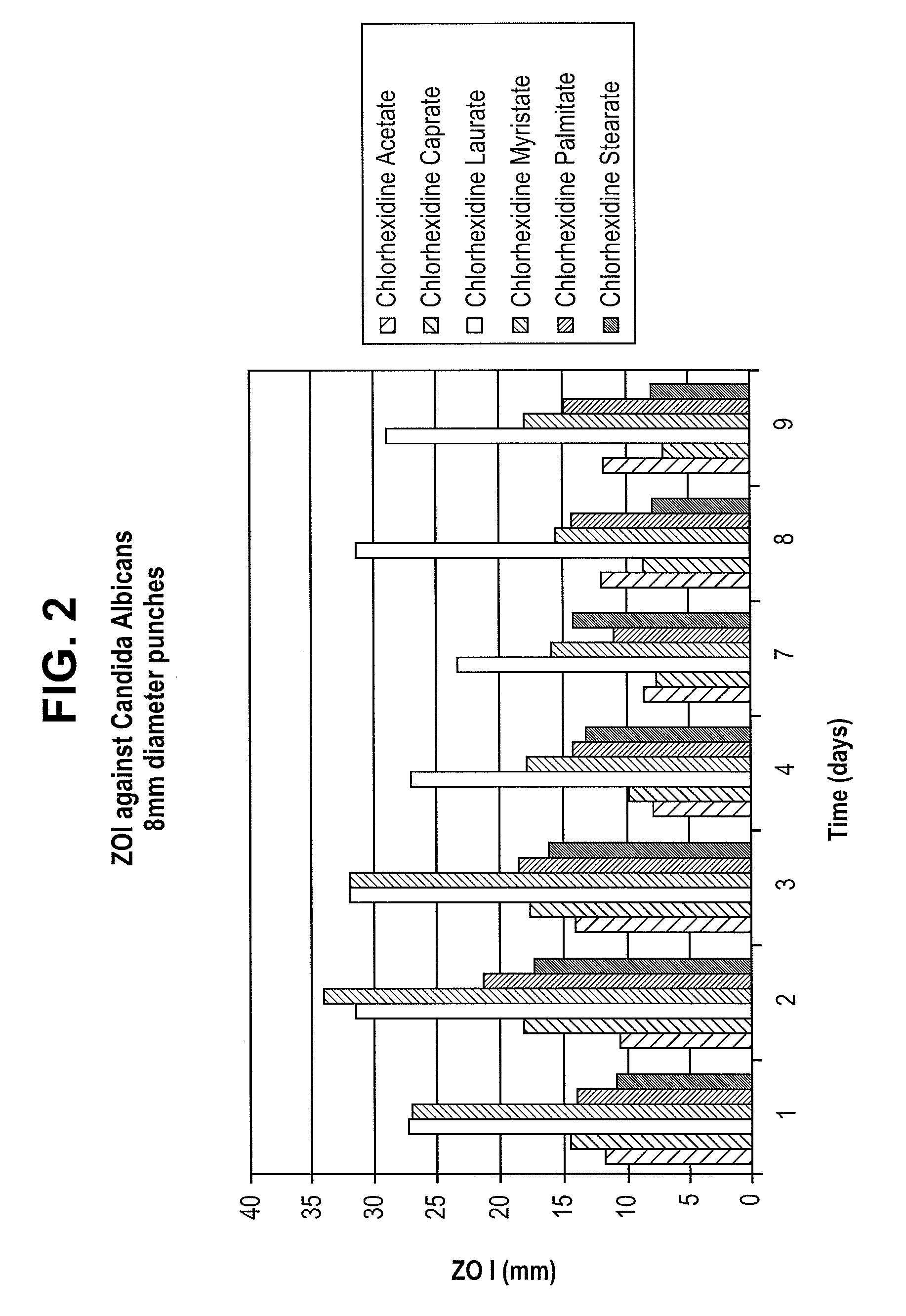
Elastomeric Devices Containing Chlorhexidine/Fatty Acid Salts Made From Fatty Acids of 12 to 18 Carbons
In a medical device, an antimicrobial agent is combined with an elastomeric polymer material. The antimicrobial agent includes at least one chlorhexidine / fatty acid salt which is a neutralization product of chlorhexidine base and a fatty acid having between 12 and 18 carbon atoms.
Owner:TELEFLEX MEDICAL INC
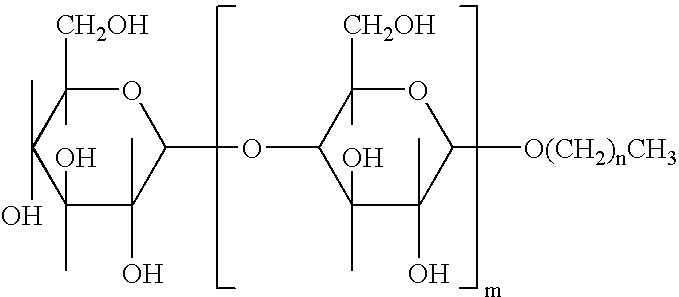
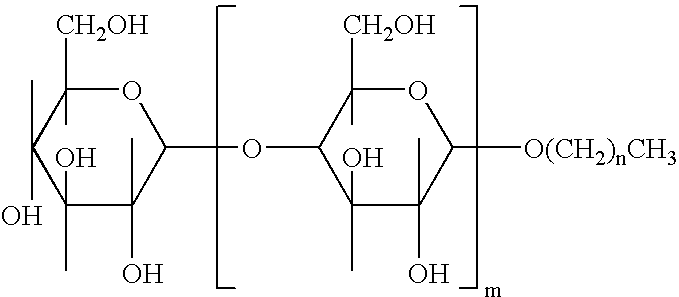
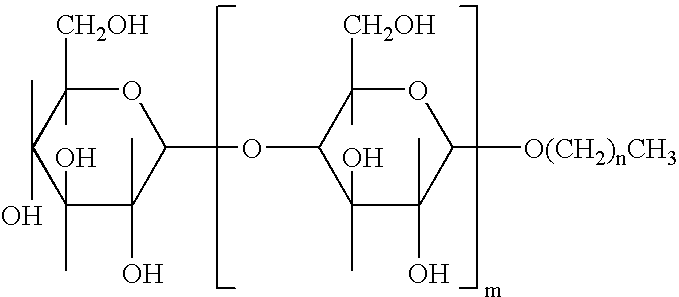
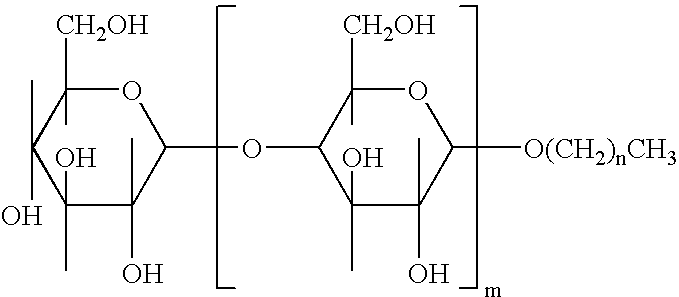
Alkylpolyglucosides containing disinfectant compositions active against pseudomonas microorganism
An antiseptic cleansing composition comprising an antimicrobial agent, an effective amount of an alkylpolysaccharide surfactant, at least one alkyl alcohol and at least one aryl alcohol. Suitable surfactant alkylpolysaccharides may contain one or more sugar units selected from the group consisting of maltose, arabinose, xylose, mannose, galactose, gulose, idose, talose, allose, altrose, sucrose, fructose, sorbose, levulose, lactose, allulose, tagatose, alloheptulose, sedoheptulose, glucoheptulose, mannoheptulose, guloheptulose, idoheptulose, galactoheptulose, taloheptulose and derivatives thereof. Suitable antimicrobial agents include chlorhexidine, chlorhexidine salt, chlorophenol derivative, octenidindihydrochloride (CH3—(CH2)7—NHON—(CH2)10—NO—NH(CH2)7—CH2 or any other salt thereof, and quaternary ammonium compounds.
Owner:NOVAPHARM RES AUSTRALIA
Infection resistant polymers, their preparation and uses
InactiveUS7771743B1Broaden antimicrobial rangeStable materialAntibacterial agentsBiocideBiguanideChlorhexidine
A family of infection resistant and biocidal polymeric materials incorporates an infection resistant biguanide such as chlorhexidine or polyhexanide pendant to the polymer chain, chemically linked to the polymer through the biguanide group secondary nitrogen atoms. The disclosure extends to the use of such polymeric materials in articles of manufacture and particularly in medical devices, and the preparation of the materials as polymer resins from which articles can be made, as solutions and emulsions which can be used for coating performed articles, and in situ on the surface of preformed polymeric articles.
Owner:BIOINTERACTIONS
Whole Mouth Malador Control By A Combination Of Antibacterial And Deodorizing Agents
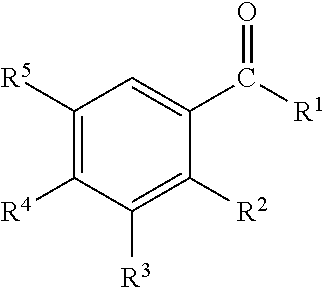
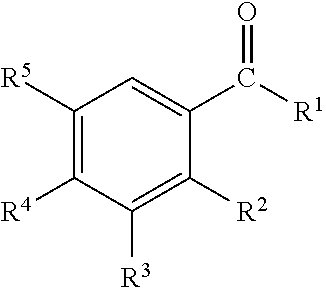
InactiveUS20110239736A1Effective controlCosmetic preparationsToilet preparationsTriclosanAmmonium compounds
Disclosed are oral care compositions effective to control mouth and breath malodour comprising in a pharmaceutically acceptable carrier, a combination of an antibacterial agent and a deodorizing or odor-neutralizing agent comprising a compound having the structurewherein R1, R2, R3, and R5 may be identical or different, each representing H, a linear or branched C1-C6 alkyl or alkenyl, phenyl, —OH or —ORa; R4 is —OH, —ORa, phenyl, or a linear or branched C1-C6 alkyl or alkenyl; and Ra is phenyl or a linear or branched C1-C6 alkyl or alkenyl. The odor-neutralizing agent may further comprise one or more of an additional odor-neutralizing compound selected from α-damascenone, α-isomethylionone, α-ionone, β-ionone, pulegone, piperitone, carvone, coenzyme Q10 or cinnamaldehyde.The antibacterial agent may comprise one or a mixture of a quaternary ammonium compound selected from cetylpyridinium chloride, tetradecylpyridinium chloride, N-tetradecyl-4-ethyl pyridinium chloride or domiphen bromide; metal ions such as stannous, zinc or copper; chlorhexidine; triclosan; triclosan monophosphate; or selected essential oils.
Owner:THE PROCTER & GAMBLE COMPANY
Liquid disinfectant
InactiveCN1476761AReduced stabilityImprove stabilityBiocideAnimal repellantsDialkyl dimethyl ammonium chlorideDisinfectant
The present invention discloses a liquid high-effective disinfectant. It is formed from (wt%) 1-2% of hyamine which is the mixture of alkyl dimethyl benzyl ammonium chloride and dialkyl dimethyl ammonium chloride, 0.1-0.5% of biguanide which is one of chlorhexidine and polyhexamethylene biguanide salt or their mixture, 3-8% of aldehyde which is one of glutaraldehyde, citrial, jasminal and myristic aldehyde or mixture of more than two kinds of them, 5-10% of surfactant, 1-5% of urea, 40-50% of hydrophilic solvent, 0.1% of EDTA sodium salt, 2.5-35% of water and 0-3% of selectable components, such as aromatic compound, colouring matter and viscosity-regulating agent.
Owner:刘瑞源
Combinations of antiseptic and antibiotic agents containing medical devices
InactiveUS20050192547A1Toxic reductionLong efficacyBiocideDead animal preservationTriclosanChlorhexidine
The present invention relates to compositions comprising a combination of one or more antiseptic and an antibiotic. It is based, at least in part, on the discovery that such combinations tend to deter the formation of antibiotic-resistant organisms. In preferred, nonlimiting embodiments of the invention, the antibiotic is minocycline and the antiseptic is a chlorhexidine compound, triclosan, or benzalkonium chloride, and in particular embodiments, a silver salt or a bismuth salt is added. Examples of specific, nonlimiting embodiments of the invention include combinations of (i) minocycline, triclosan, and a bismuth salt; (ii) minocycline, a chlorhexidine compound, and a bismuth salt; and (iii) minocycline, benzalkonium chloride, and a bismuth salt. The present invention further provides for articles, such as, but not limited to, medical articles, which have been treated with or which otherwise comprise a combination of antiseptic and antibiotic.
Owner:MODAK SHANTA M +2
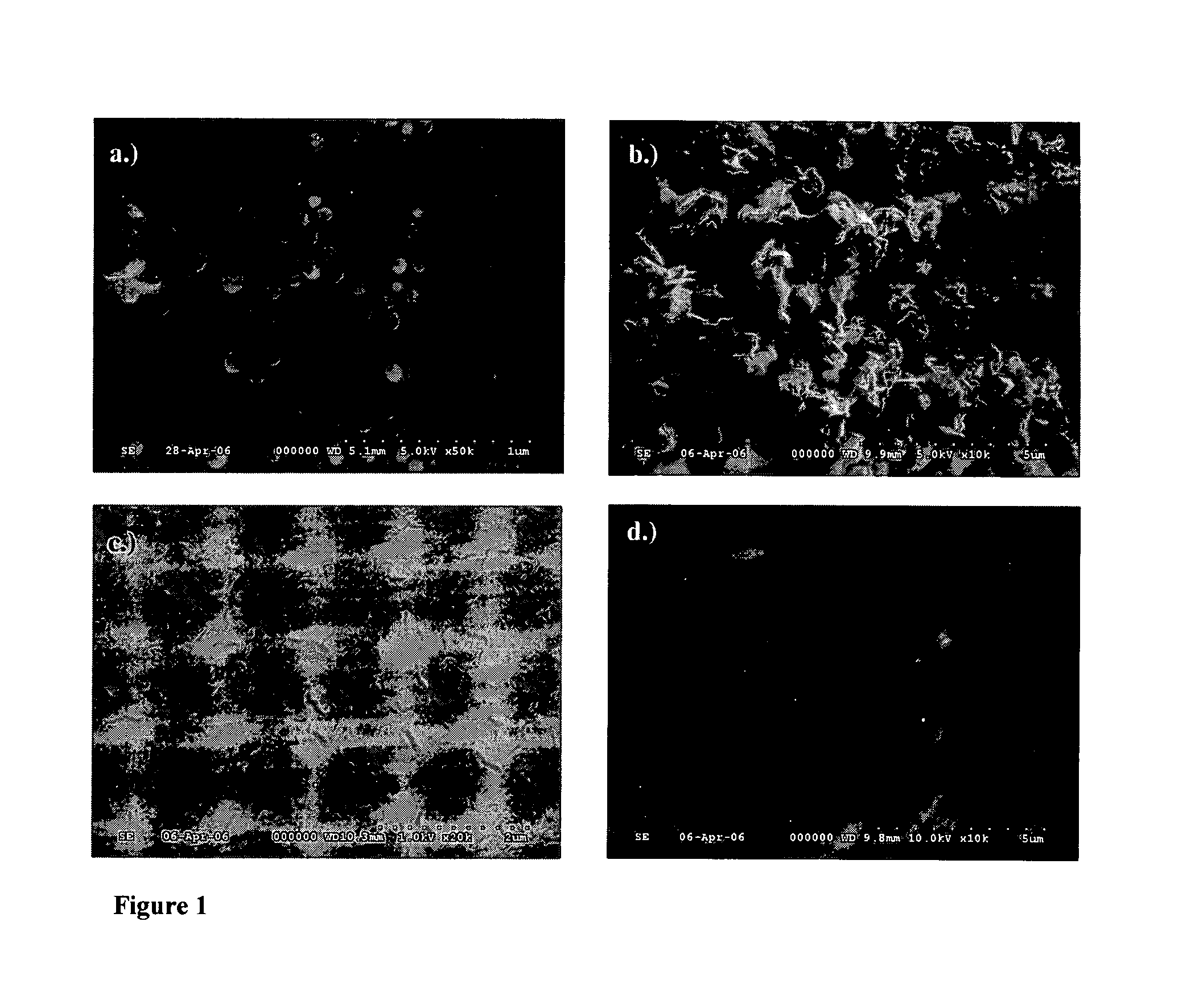
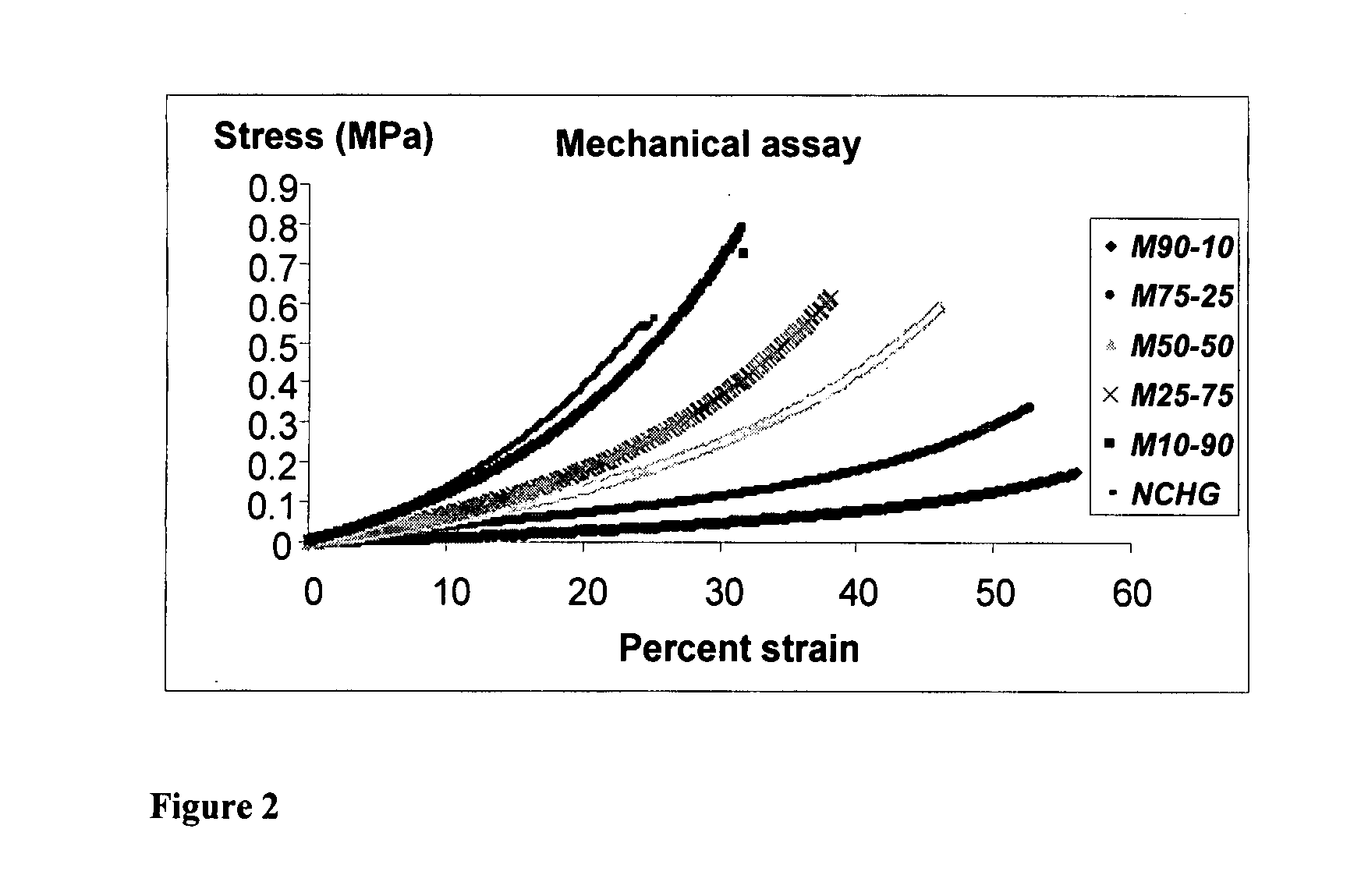
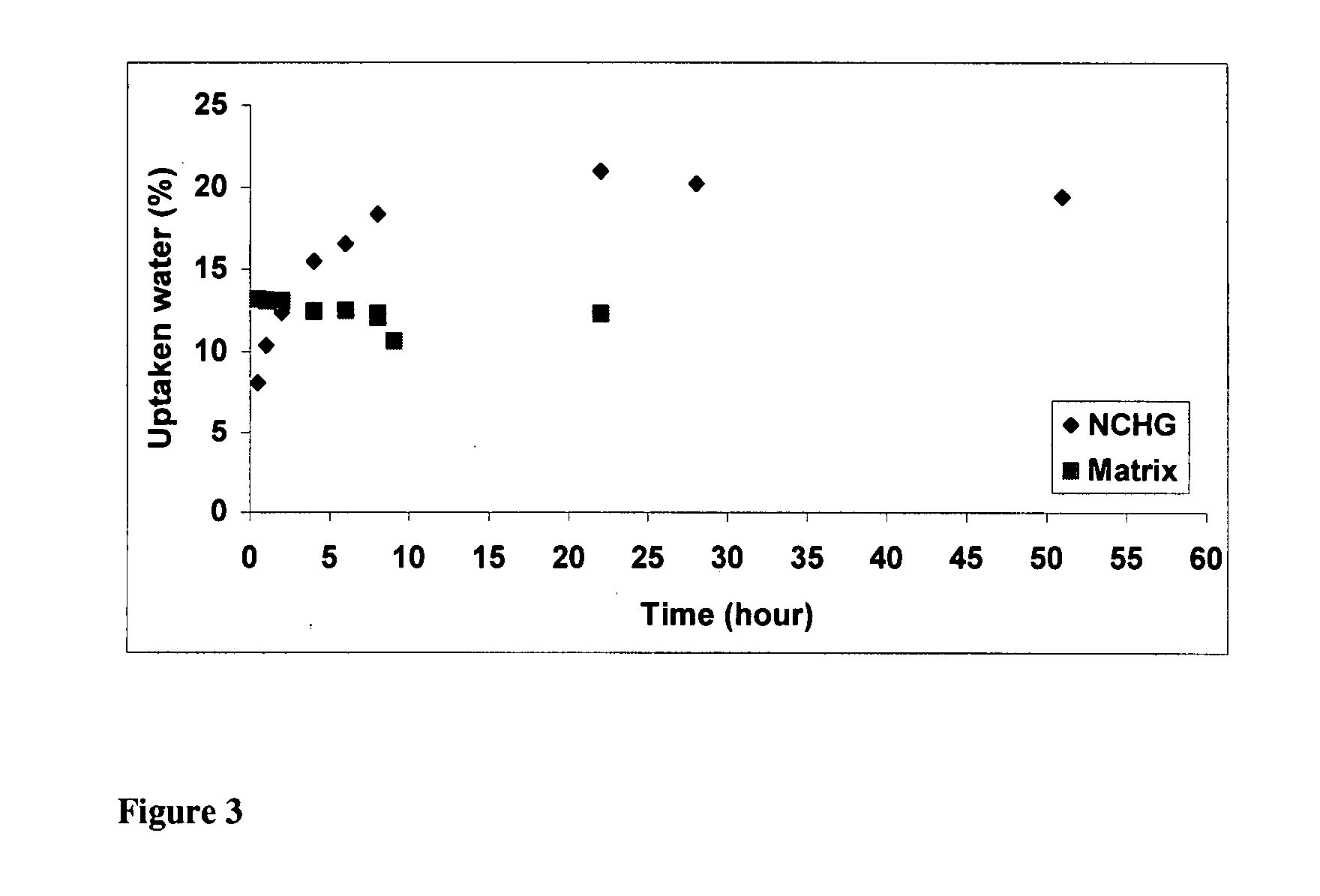
Synthesis of biocompatible nanocomposite hydrogels as a local drug delivery system
InactiveUS20070212419A1Slow drug releaseHigh compressive strengthOrganic active ingredientsBiocide(Hydroxyethyl)methacrylateModel system
Nanocomposite biocompatible hydrogels (NCHGs) may be synthesised as model systems for in situ cured local drug delivery devices for treatment of inter alia periodontal infections. The composite includes the following components: nanoparticles (NPs), a matrix gel, and chlorhexidine (CHX) or other antibacterial drug. The NPs were obtained by free radical initiated copolymerization of the monomers, 2-hydroxyethyl methacrylate (HEMA) and polyethyleneglycol dimethacrylate (PEGDMA), in aqueous solution. The same monomers were used to prepare crosslinked matrices by photopolymerization. NCHGs were obtained by mixing NPs, monomers, and drug in an aqueous solution then crosslinked by photopolymerization.
Owner:BAKO JOZSEF +6
Flavoured mouth wash composition
InactiveUS20060210491A1Growth inhibitionReduced effectivenessCosmetic preparationsBiocideDamasconePyrophosphate
The present invention provides a flavoured product comprising four or more flavour materials having antimicrobial properties and selected from the group comprising nonanol, decanol, nonanal, decanal, amyl propionate, anethole synthetic, anisic aldehyde, basil oil, benzyl benzoate, benzyl butyrate, benzyl formate, camomile oil, cinnamic aldehyde, cis-3-hexenol, clove bud oil, damascone, ethyl acetoacetate, eucalyptus oil, ginger, isoamyl acetate, menthol laevo, methyl cinnamate, methyl salicylate, orange oil, rosemary oil, tarragon, Tea Tree oil, and peppermint oil; and one or more antimicrobial agents selected from the group comprising triclosan, pyrophosphates, zinc salts, cetylpyridinium chloride, parabens, stannous salts, sodium dodecyl sulphate, chlorhexidine, copper salts, strontium salts, peroxides and sanguinarine.
Owner:QUEST INTERNATIONAL
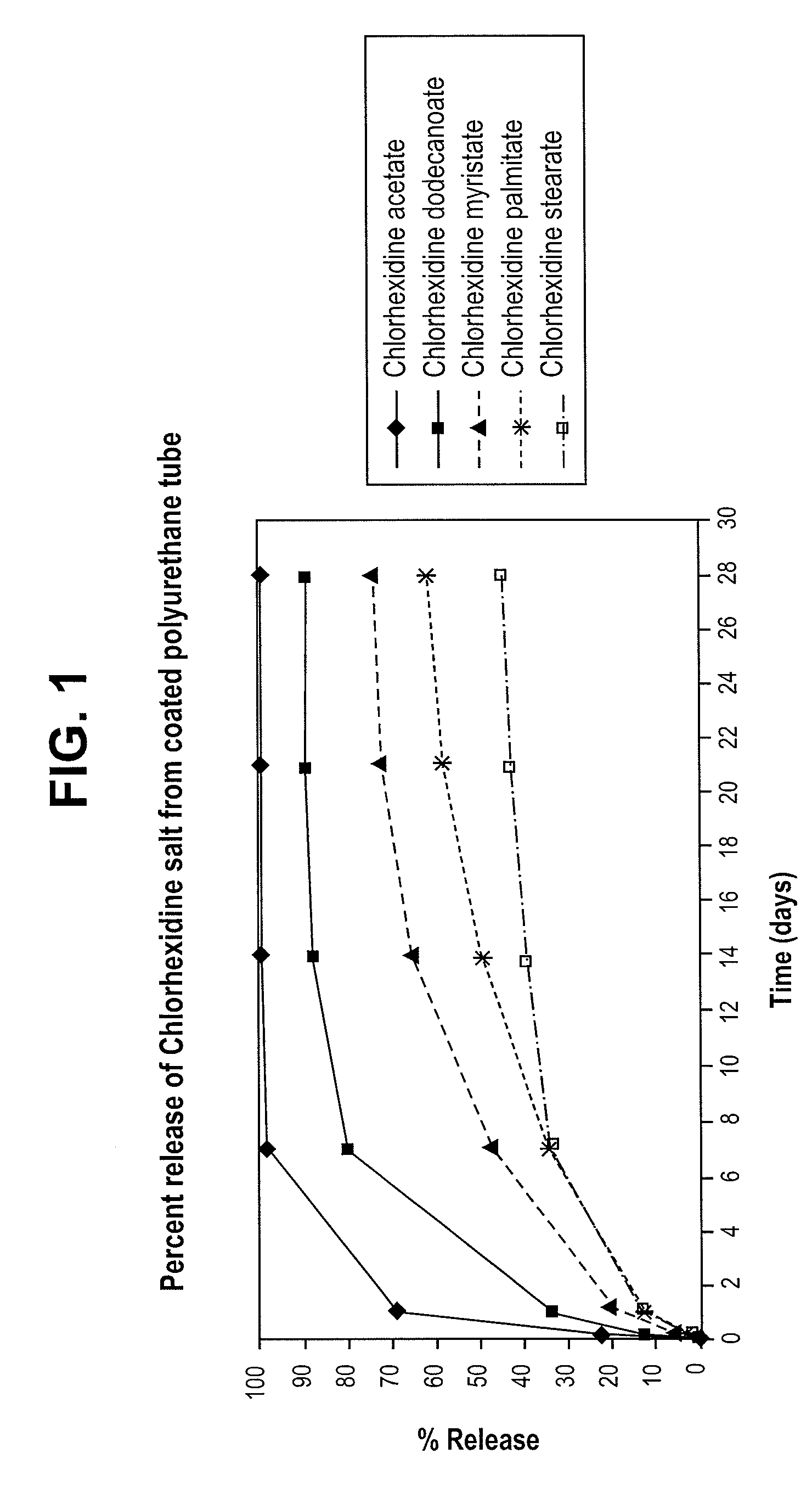
Stable melt processable chlorhexidine compositions
InactiveUS20100234815A1Reduce microbial growthOrganic active ingredientsBiocideChlorhexidineMedical device
In a medical device having an antimicrobial agent, the medical device includes a base material and an amount of chlorhexidine or a pharmaceutically acceptable salt thereof disposed in the base material sufficient to reduce microbial growth. The base material is melt processed together with the chlorhexidine to generate the medical device which is substantially free of destabilized chlorhexidine.
Owner:TELEFLEX MEDICAL INC
Aqueous Antiseptic Solution and Compatible Anionic Dye for Staining Skin
InactiveUS20070254854A1Without reducing efficacyBiocideOrganic active ingredientsStaining skinPreservative
Aqueous antiseptic solutions and compatible dyes and methods for making and using such solutions are provided. More specifically, in one embodiment, the present invention relates to an aqueous antiseptic solution comprising an aqueous solution of chlorhexidine or a salt thereof, an anionic dye in an amount sufficient to stain a patient's skin when the aqueous solution is applied thereon, and a cationic excipient.
Owner:CAREFUSION 2200 INC
Compositions for the relief of xerostomia and the treatment of associated disorders
InactiveUS7198779B2Organic active ingredientsCosmetic preparationsSodium bicarbonateBenzalkonium chloride
New liquid compositions are described for the relief of the xerostomia and the treatment of associated disorders, in which the compositions contain: a) saline saliva substitute agents selected from the group consisting of sodium chloride, potassium chloride, sodium bicarbonate, monobasic potassium phosphate, and dibasic phosphate potassium; b) saliva production stimulation agents selected from the group consisting of citric acid or its alkali metal salts and malic acid or its alkali metal salts; c) oral antiseptics selected from the group consisting of triclosan, chlorhexidine and its salts, benzalkonium and its salts, and cetylpyridinium chloride; d) anticariogenic agents selected from the group consisting of fluoride sodium, sodium monofluorophosphate, and xylitol; and e) oral mucosa protective agents selected from the group consisting of vitamin E acetate, panthenol, dipotassium glycyrrhizinate and extracts of aloe vera.The aforementioned compositions are presented in the form of mouthwashes, sprays, oral gels, and toothpastes.
Owner:LACER SA
Disinfectant and antiseptic composition
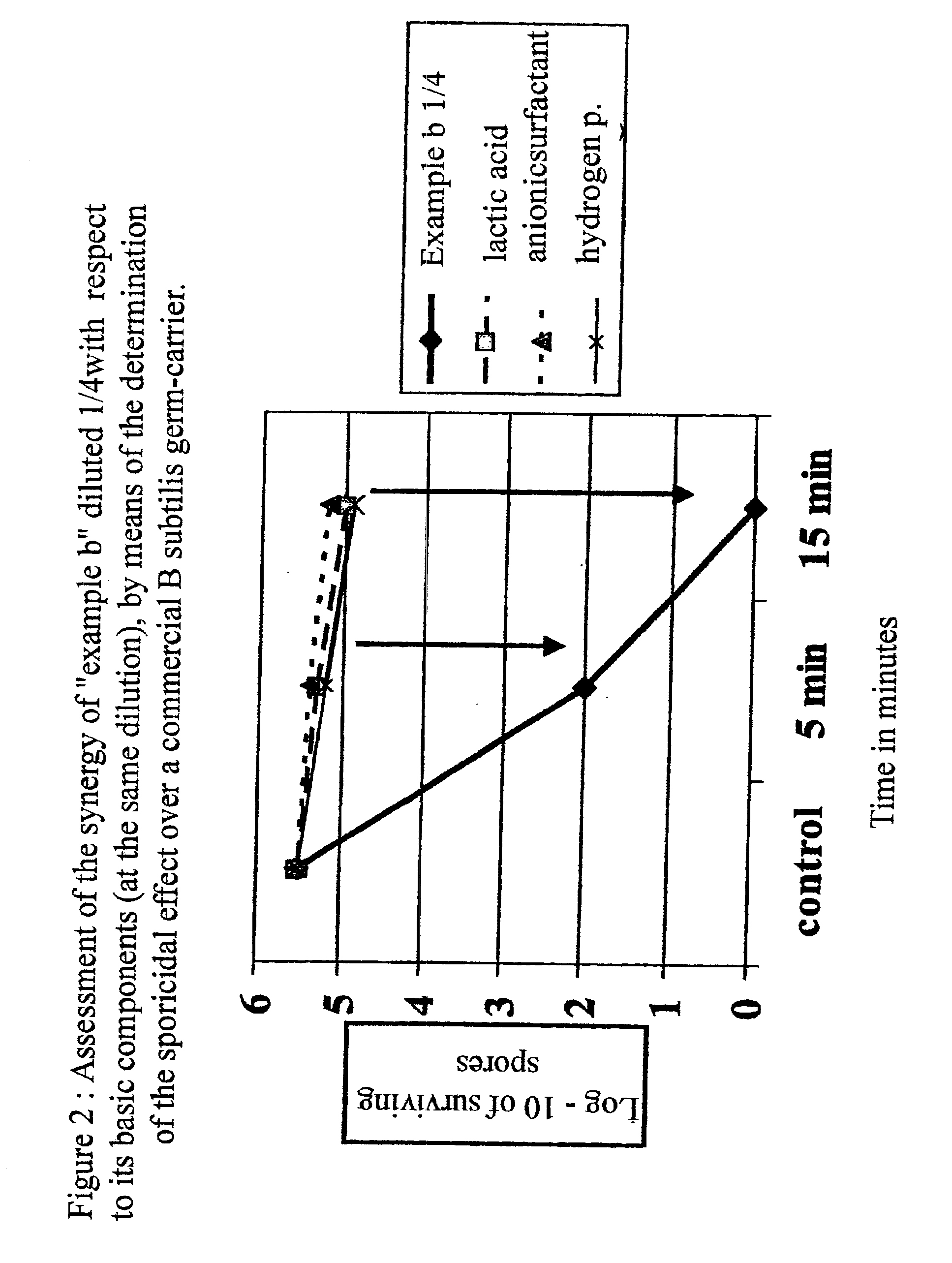
Wide spectrum disinfecting and antiseptic composition for use in the fields of human medicine, veterinary science and industry, characterized because it includes:Hydrogen peroxide, lactic acid and halogen salts (Br, I) and / or salts of heavy metals (for example, silver halides) with surfactant agents, either cationic, like chlorhexidine and / or quaternary ammonium salts, like didecyl-methyl-polyoxy-ethyl-ammonium propionate, chlorides of ammonium or compounds of ammonium propylamide or anionic, like lauryl sulphate, dodecyl sulphate or alkyl succinic salts, with suitable excipients, some of which may be ethyl or isopropyl alcohol, chlorhexidine, non-chlorinated quaternary ammonium salts, like didecyl-methyl-polyoxy-ethyl-ammonium propionate, combined or not with iodine, and / or its salts, together with excipients, some of which may be ethyl or isopropyl alcohol.
Owner:OFTRAI
Antimicrobial medical devices
InactiveUS20020122876A1Improve antibacterial propertiesFacilitated releaseSuture equipmentsOrganic active ingredientsMedicineChlorhexidine
The present disclosure invention relates to medical devices treated with a solution comprising one or more solvents and a combination of chlorhexidine free base and a water-soluble chlorhexidine salt in a weight / weight ratio of between about 1:1 to about 1:5, preferably about 1:1.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Alkylpolyglucosides containing disinfectant compositions active against pseudomonas microorganism
An antiseptic cleansing composition comprising an antimicrobial agent, an effective amount of an alkylpolysaccharide surfactant, at least one alkyl alcohol and at least one aryl alcohol. Suitable surfactant alkylpolysaccharides may contain one or more sugar units selected from the group consisting of maltose, arabinose, xylose, mannose, galactose, gulose, idose, talose, allose, altrose, sucrose, fructose, sorbose, levulose, lactose, allulose, tagatose, alloheptulose, sedoheptulose, glucoheptulose, mannoheptulose, guloheptulose, idoheptulose, galactoheptulose, taloheptulose and derivatives thereof. Suitable antimicrobial agents include chlorhexidine, chlorhexidine salt, chlorophenol derivative, octenidindihydrochloride (CH3—(CH2)7—NHON—(CH2)10—NO—NH(CH2)7—CH2 or any other salt thereof, and quaternary ammonium compounds.
Owner:NOVAPHARM RES AUSTRALIA
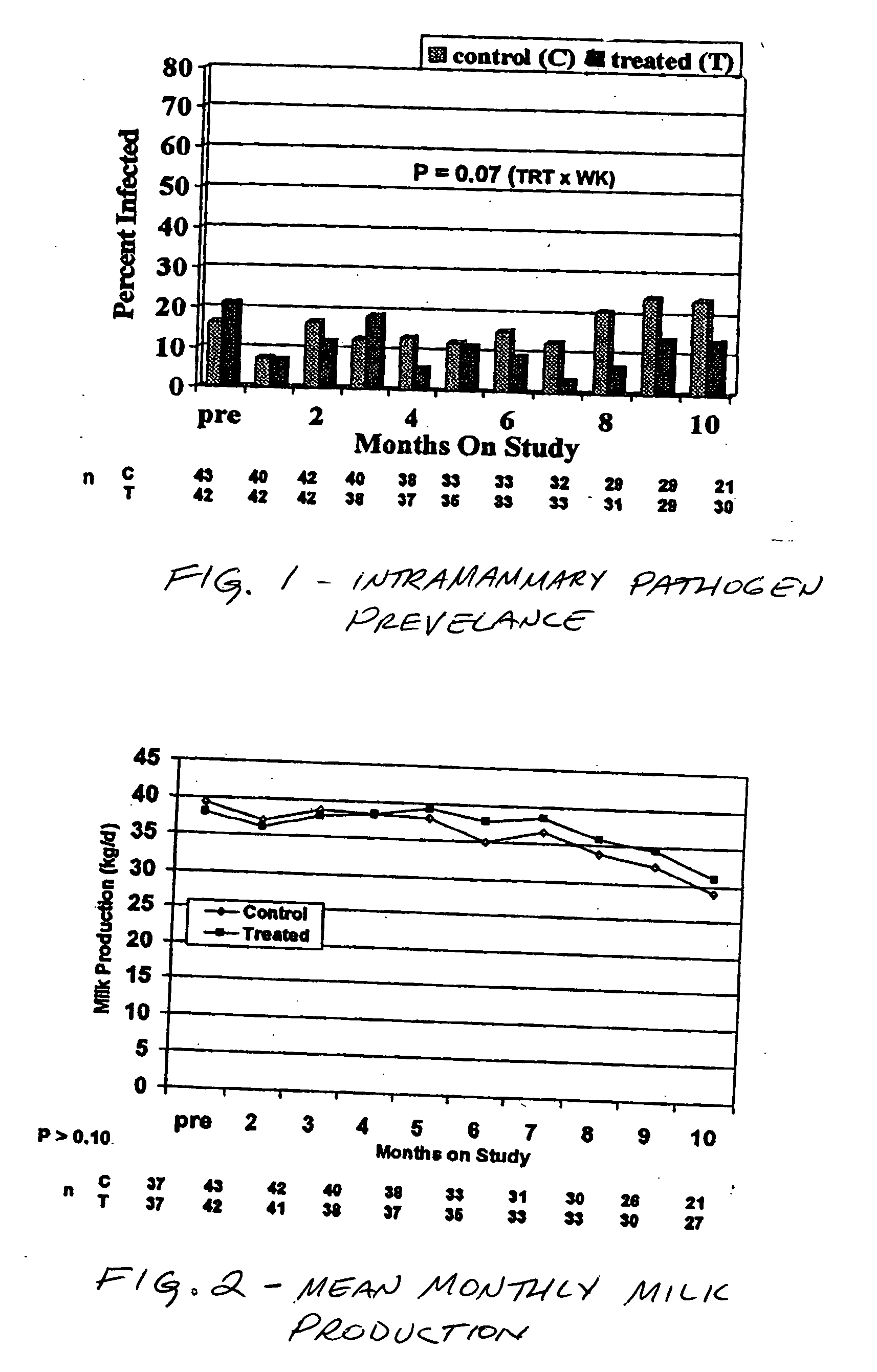
Teat disinfectant having zinc EDTA, and methods
Compositions, systems, and methods for the prevention of mastitis in milk producing animals are provided. In particular, the compositions comprise zinc EDTA and chlorhexidine. The composition is applied to the teat of a milk producing animal such as a cow either by dipping the teat therein or spraying the composition thereon. The composition is particularly suited for application by aerosol.
Owner:WESTFALL GEOFFREY J
Dressing device for use wtih a cannula or a catheter
InactiveUS20130110025A1Reduce morbidityIncrease infectionPharmaceutical delivery mechanismAbsorbent padsWound dressingChlorhexidine
A wound dressing device for use with a transcutaneous medical device such as a cannula or a catheter comprises a polyurethane matrix which may be covered with a film backing. Chlorhexidine di- gluconate and a polyanhydroglucuronic salt are contained in the polymer matrix. The wound dressing device prevents microbial colonization of the dressing and stops bleeding from the insertion site. The device provides combined haemostatic and antimicrobial effects at the insertion site but without adversely affecting wound healing.
Owner:BARD ACCESS SYST
Compositions used to alleviate xerostomia and to treat disorders associated with same
New liquid compositions are described for the relief of the xerostomia and the treatment of associated disorders, in which the compositions contain: a) saline saliva substitute agents selected from the group consisting of sodium chloride, potassium chloride, sodium bicarbonate, monobasic potassium phosphate, and dibasic phosphate potassium; b) saliva production stimulation agents selected from the group consisting of citric acid or its alkali metal salts and malic acid or its alkali metal salts; c) oral antiseptics selected from the group consisting of triclosan, chlorhexidine and its salts, benzalkonium and its salts, and cetylpyridinium chloride; d) anticariogenic agents selected from the group consisting of fluoride sodium, sodium monofluorophosphate, and xylitol; and e) oral mucosa protective agents selected from the group consisting of vitamin E acetate, panthenol, dipotassium glycyrrhizinate and extracts of aloe vera. The aforementioned compositions are presented in the form of mouthwashes, sprays, oral gels, and toothpastes.
Owner:LACER SA
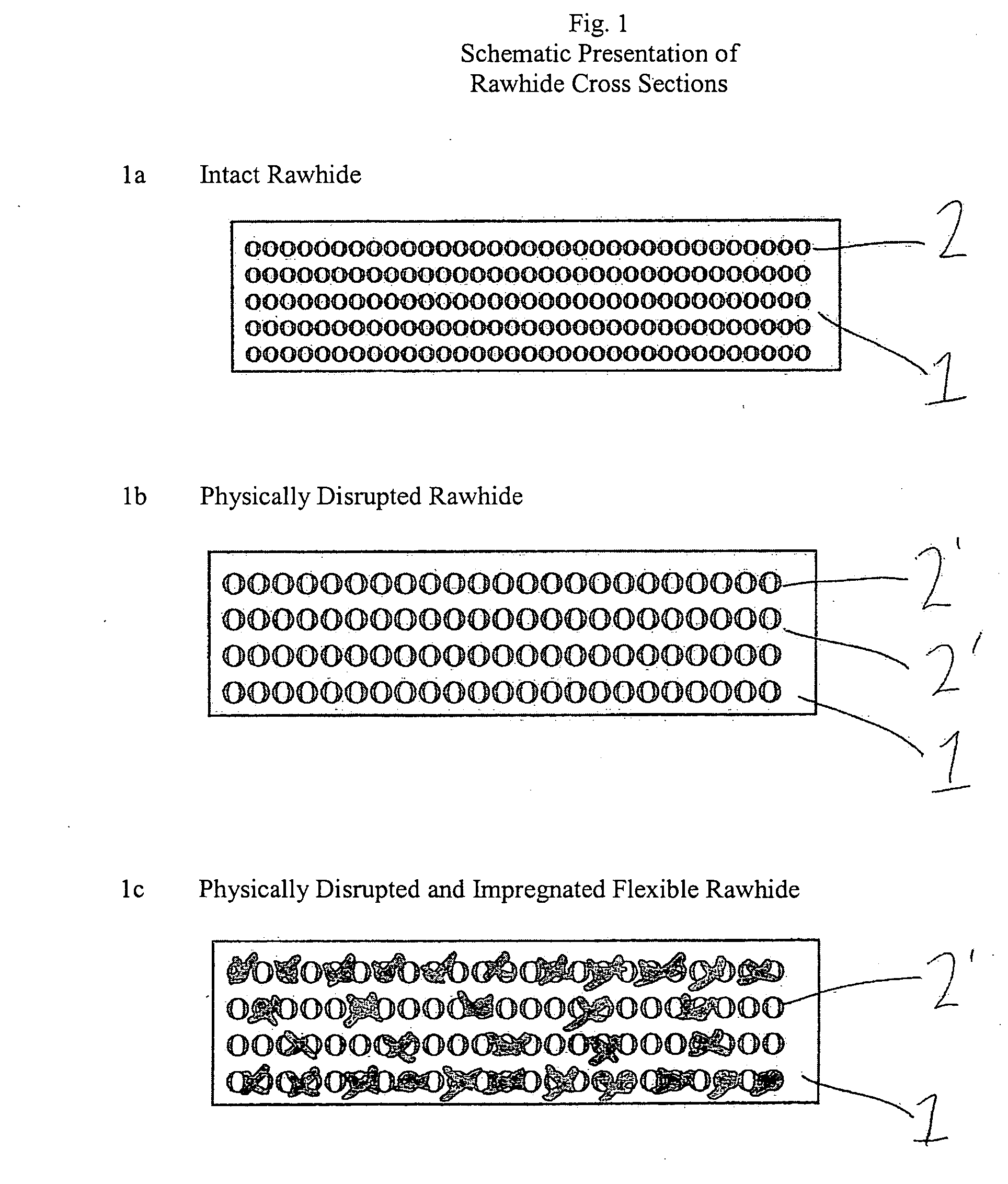
Impregnated, flexible, rawhide pet chews containing antimicrobially active chlorhexdine
InactiveUS20050064019A1Excellent physical cleaning actionEffective controlAnimal feeding stuffBiofilmEmulsion
Therapeutic, flexible, tough, rawhide pet chews impregnated with an emulsion and / or surfactant containing antimicrobially active chlorhexidine suitable for releasing said antimicrobially active chlorhexidine over the chew-life of the chew to: help control, disrupt and remove biofilms, treat fetid breath and treat gum disease in pets.
Owner:PET ORAL CARE INT
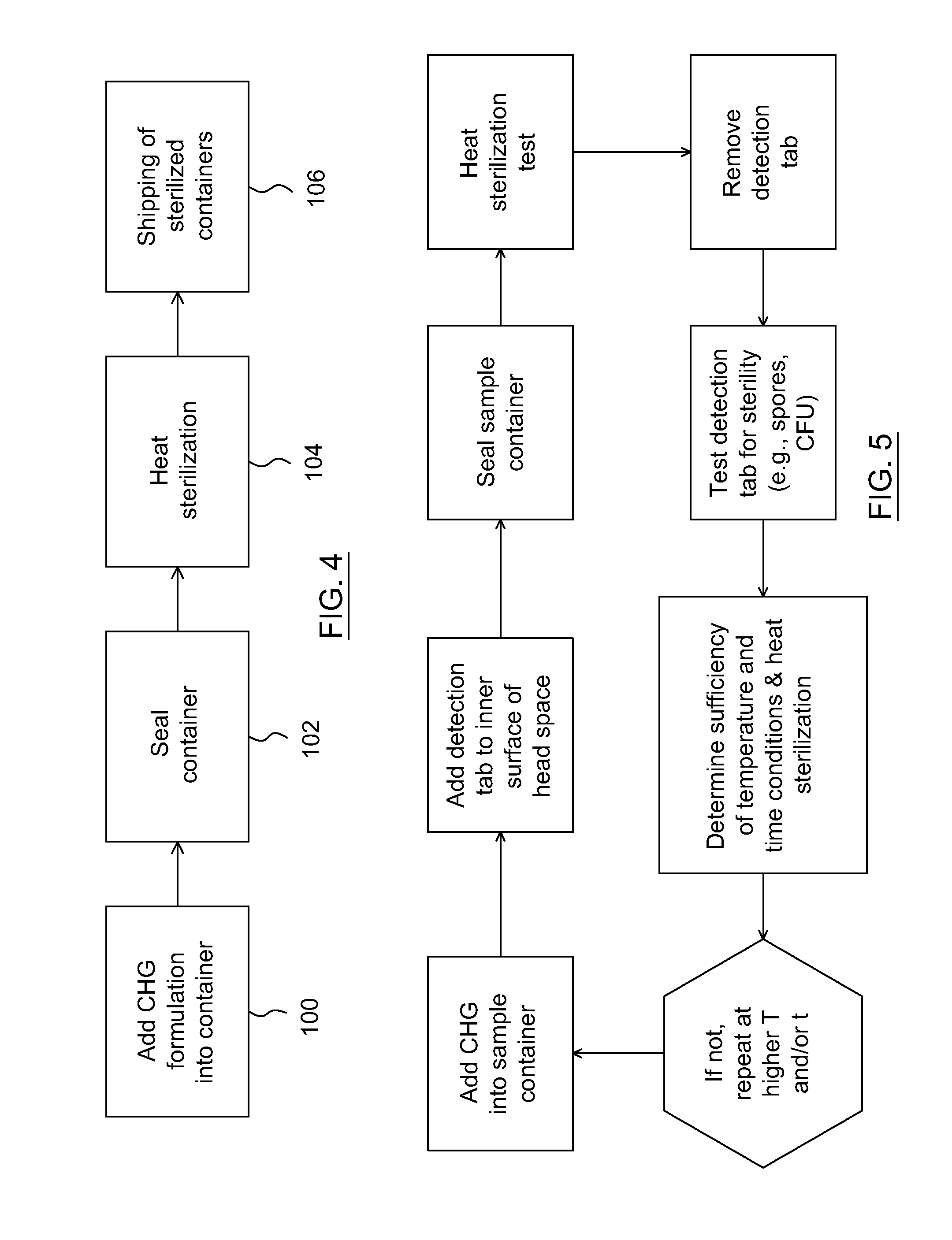
Heat sterilization techniques for chlorhexidine based antiseptic formulations
Techniques for sterilizing chlorhexidine based antiseptic formulations include exposing a sealed container containing the formulation to heat at a temperature and heating time sufficient to sterilize the chlorhexidine based antiseptic formulation and the hermetically sealed interior of the container, which may be an applicator, a bottle, a swab stick, or a pad-containing pouch.
Owner:LERNAPHARM LORIS
External composition, and preparation and application thereof
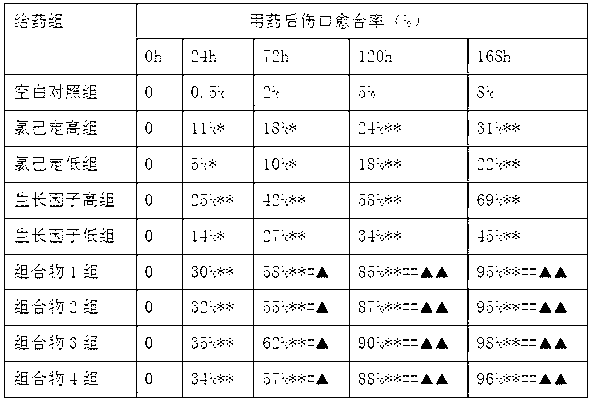
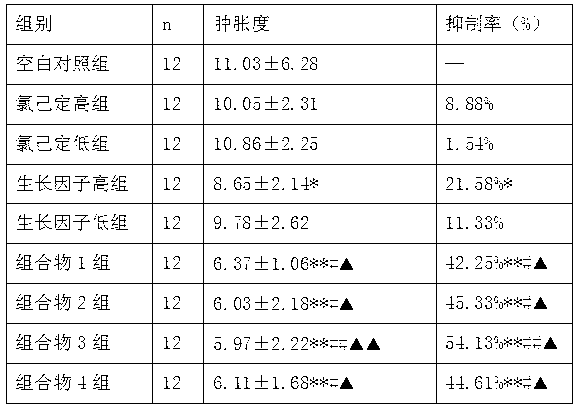
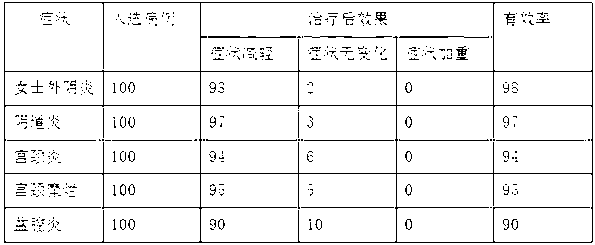
ActiveCN103182070AStickyAvoid secondary cross-infectionPeptide/protein ingredientsAerosol deliveryOfficinalChlorhexidine
The invention relates to an external composition, and a preparation and an application thereof and belongs to the field of medicines. The external composition provided by the invention is a medicine composition which contains chlorhexidine or officinal salt thereof, or benzalkonium bromide or officinal salt thereof and a recombinant human epidermal growth factor or a recombinant cattle alkali fibroblast growth factor. According to the invention, the medicine composition is also further prepared into gel, liniment, ointment or paste, and the gel further comprises chitosan or officinal salt thereof and hyaluronic acid or officinal salt thereof. When being used for treating vagina inflammation, the external composition disclosed by the invention can improve a vagina microenvironment, promote growth of granulation tissue and repair vagina micro damage; and when being used for skin wound atraumatic restorative treatment, the external composition also can speed up restoration and regeneration of a wound tissue, promote healing and reduce scar formation, so that the external composition has a wide medical application prospect.
Owner:JIANGSU DIWO BIOLOGICAL PROD
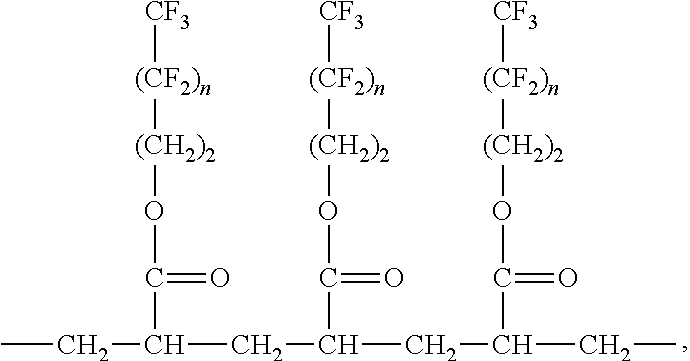
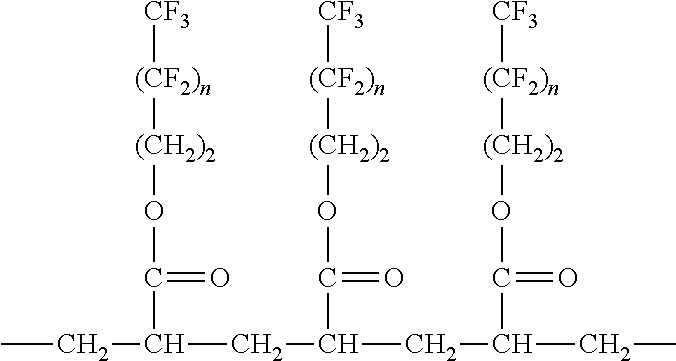
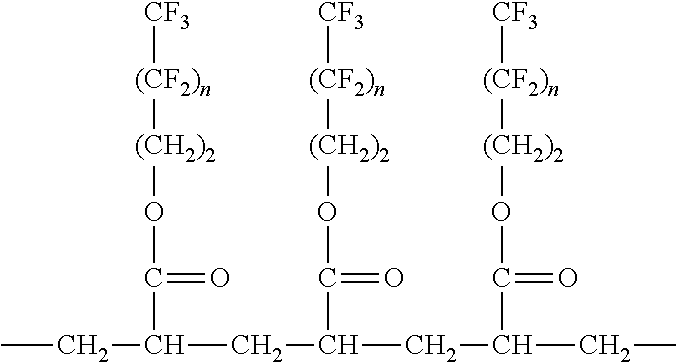
Nonwoven materials coated with fluoropolymer and antimicrobial agent
An article having a nonwoven material and a coating contacting the nonwoven material, the coating comprising at least one fluorochemical and at least one antimicrobial agent. The fluorochemical comprises at least one fluoropolymer. The add-on value of the at least one antimicrobial agent on the nonwoven material is less than 0.75% and the coating provides at least a 4-log reduction within 5 minutes when measured using AATCC Method 100. The at least one antimicrobial agent is selected from the group consisting of a benzalkonium salt, a benzethonium salt, a octenidine salt, a chlorhexidine salt, free base chlorhexidine, triclosan and combinations thereof. The coating provides at least a 2-log reduction within 5 minutes when measured using AATCC Method 100.
Owner:ALLEGIANCE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com