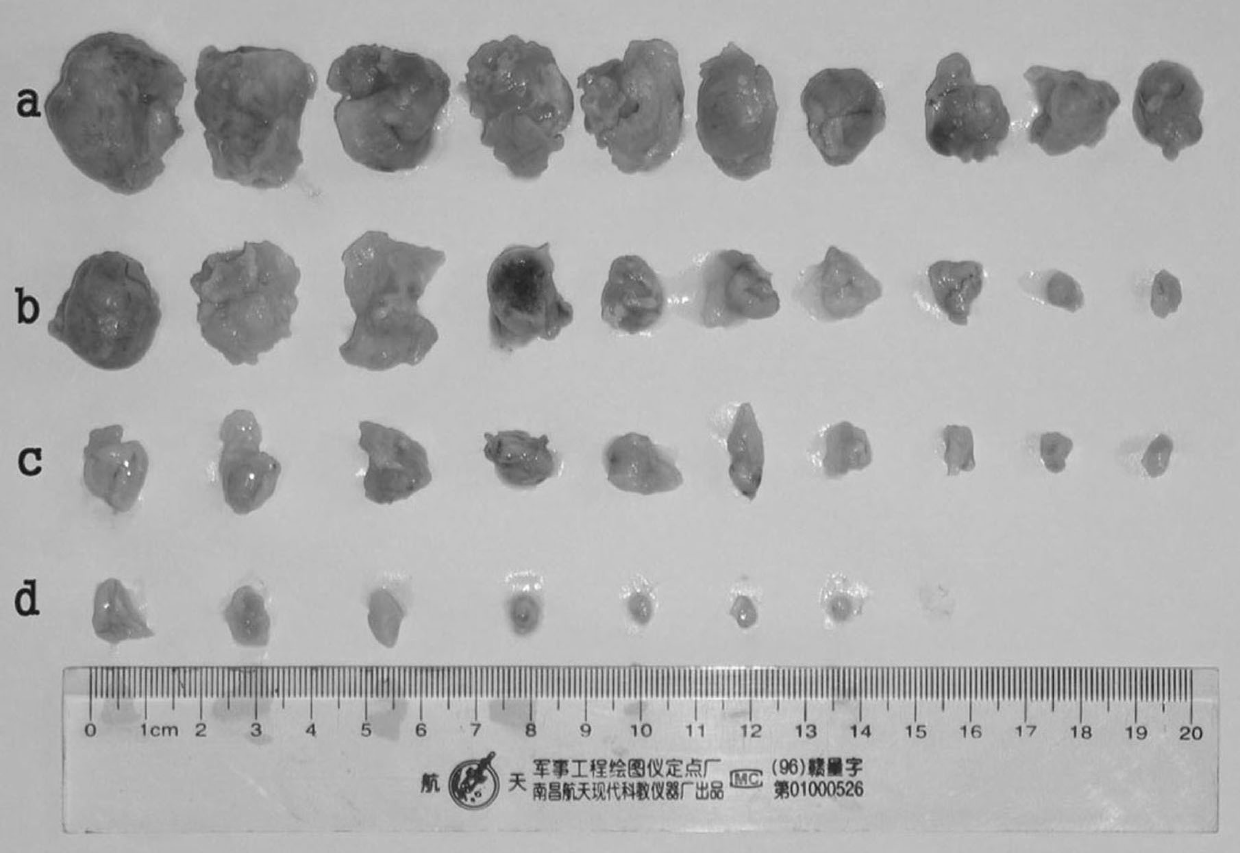
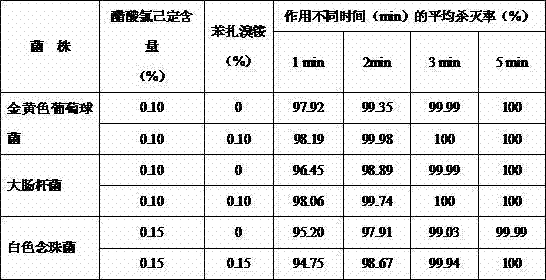
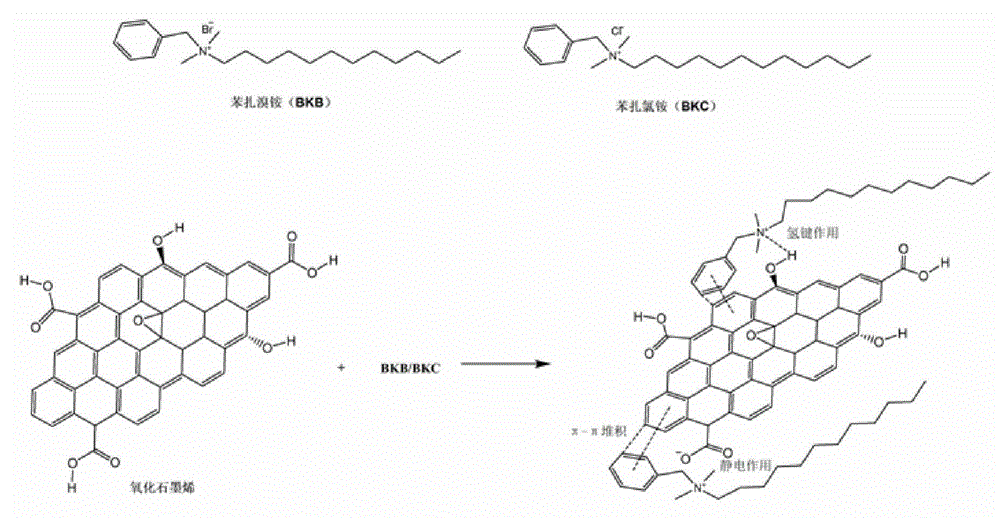
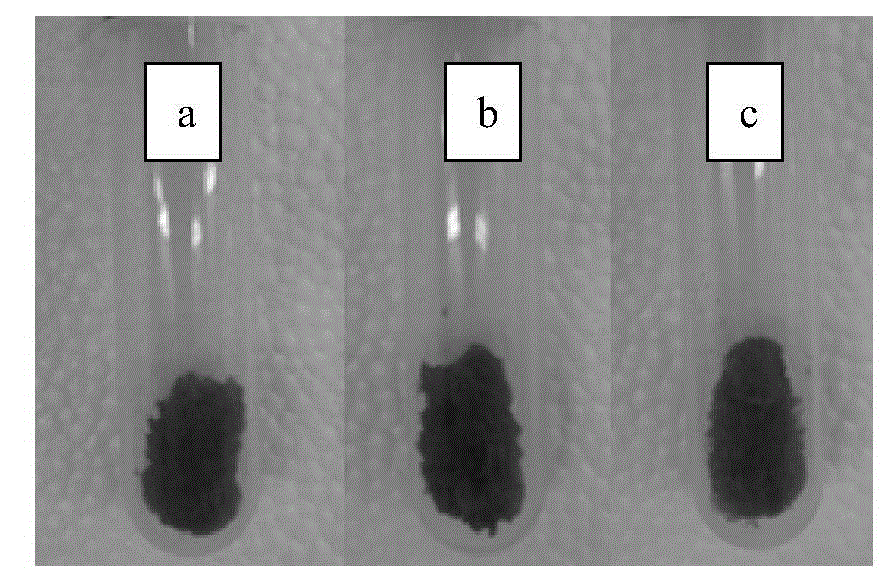
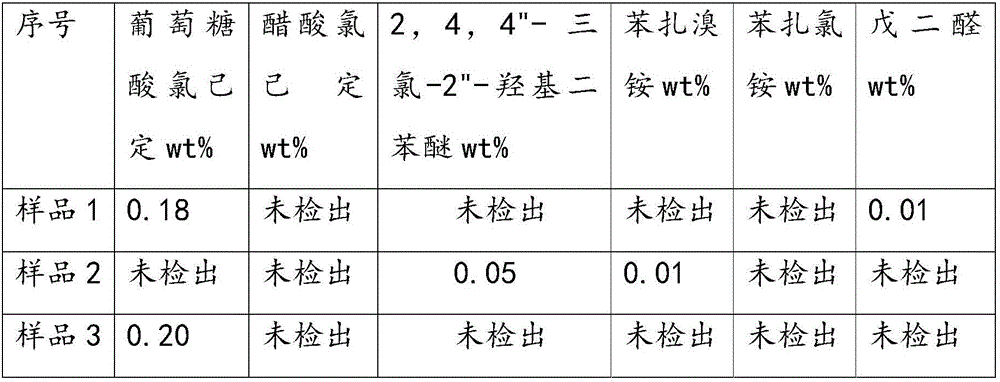
Patents
Literature
313 results about "BENZALKONIUM BROMIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzalkonium is a quaternary ammonium cationic surfactant that exhibits antimicrobial activity. Benzalkonium is clinically used as a disinfectant; it displays antibacterial activity by inducing dissociation of lipid membrane bilayer components and allowing cellular leakage.
Disinfection sterilizing type medical supersonic couplant and method of preparing the same
ActiveCN101249268AIncrease concentrationImprove the bactericidal effectIn-vivo testing preparationsUltrasonographyChlorhexidine Acetate
The invention discloses a disinfection sterilization medical ultrasonic coupling medium with the bactericidal function and used in the ultrasonic detection and treatment of skin and cavitary mucosa and a preparation method of the coupling medium, wherein, the ultrasonic coupling medium comprises propylene glycol, de-ionized water and one viscosity modifier of the hydroxy ethyl cellulose, carbomer and hydroxy propyl cellulose. The coupling medium further comprises a fungicide of 0.05 to 0.15 percent and the constituents of the fungicide are any one of polyhexamethylene biguanide, benzalkonium chloride, benzalkonium bromide, chlorhexidine acetate, trichlorobenzene phenoxy diphenyl ether and nanometer silver or any combination of the olyhexamethylene biguanide, the benzalkonium chloride, the benzalkonium bromide, the chlorhexidine acetate, the trichlorobenzene phenoxy diphenyl etherand, and the nanometer silver; the preparation method used for preparing the ultrasonic coupling medium is as follows: dispersing the hydroxy ethyl cellulose in the propylene glycol, stirring and dispersing the hydroxy ethyl cellulose by adding enough water, heating to 75 to 80 DEG C, preserving the heat for 30 minutes to cool the hydroxy ethyl cellulose and the propylene glycol to 45 DEG C, adding the fungicide to stir and cool the fungicide, the hydroxy ethyl cellulose and the propylene glycol to obtain the filled coupling medium. Tests show that the products of the coupling medium have fast and long sterilization effect and meet the requirements of various related standards.
Owner:广州市一杰医药科技有限公司
Corrosive for displaying austenitic stainless steel grain boundary of fine grains and method for preparing corrosive
ActiveCN102888608AAccelerated corrosionReduce corrosionPreparing sample for investigationAcetic acidThermal treatment
The invention discloses a corrosive for displaying austenitic stainless steel grain boundaries of fine grains and a method for preparing the corrosive. The corrosive is characterized by comprising the following components by weight: 80-150ml of H2O, 10-20ml of HCl, 15-25g of FeCl3, 10-15g of CuCl2 / H2O, 2.0-7.0ml of glacial acetic acid and 1-5 drops of benzalkonium bromide. The method comprises the steps of: placing CuCl2.H2O in a beaker; then adding FeCl3, and uniformly stirring after adding HCl and H2O; and uniformly mixing after adding glacial acetic acid and benzalkonium bromide finally. According to the corrosive for the austenitic stainless steel of the fine grains, the grain boundaries of the austenitic stainless steel of the fine grains in the original state and the thermal treatment state can be clearly displayed, the 100 times of rating on grain size can be easily performed, the grain boundaries are clear, the interference of a texture to the grain boundaries is small, and the corrosion effect is good.
Owner:SHANGHAI BOILER WORKS
Bactericidal laundry detergent containing traditional Chinese medicine components and preparation method thereof
InactiveCN104178370AAvoid stratificationChange mobilityOrganic detergent compounding agentsAmpholytes/electroneutral surface-active compoundsBetaineGlycan
The invention discloses a bactericidal laundry detergent containing traditional Chinese medicine components. The bactericidal laundry detergent containing traditional Chinese medicine components comprises the following raw materials in parts by weight: 10-20 parts of benzalkonium bromide, 20-25 parts of fatty alcohol-polyoxyethylene ether, 20-25 parts of glycine betaine, 1-6 parts of potassium sorbate, 1-4 parts of sodium alginate, 1-4 parts of glycan, 10-35 parts of deionized water, 2-10 parts of a pH adjusting agent and 2-5 parts of a traditional Chinese medicine extract, wherein the traditional Chinese medicine extract is an extract with a concentration of 2g / ml; the extract is prepared from rheum officinale and radix scutellariae according to a mass ratio of (2-3) to 1. The bactericidal laundry detergent containing traditional Chinese medicine components has the advantages of good bactericidal and antibacterial effects and strong cleaning power.
Owner:CHENGDU SHUNFA DISINFECTANT & WASHING TECH
Chitosan contraceptive gel and preparation method thereof
InactiveCN101889974AWon't hurtAvoid infectionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationPolyethylene glycol
The invention relates to a chitosan contraceptive gel and a preparation method thereof. The contraceptive gel consists of 0.5 to 1.5 percent of chitosan, 0.5 to 1.5 percent of carbomer, 0.5 to 3.0 percent of triethanolamine, 10 to 30 percent of ethanol, 5 to 20 percent of propylene glycol, 0.3 to 1.0 percent of benzalkonium chloride, 0.5 to 2.0 percent of glycerin, 0.5 to 2 percent of citric acid, 0.5 to 2 percent of polyethylene glycol 400, 0.3 to 1.0 percent of benzalkonium bromide, 0.1 to 0.5 percent of ethylparaben, 0.1 to 0.5 percent of methylparaben and the balance of purified water. The preparation method comprises the following steps of: spraying the chitosan and the carbomer on the liquid surface of the purified water, standing for a whole night to form a gel substrate, and removing bubbles under vacuum after standing; and then uniformly mixing the triethanolamine, the ethanol, the propylene glycol, the benzalkonium chloride, the glycerin, the citric acid, the polyethylene glycol, the benzalkonium bromide and the ethylparaben, and gradually adding into the gel substrate for uniformly mixing to prepare the chitosan contraceptive gel. The contraceptive gel is used in the vagina of a woman, has the effects of contraception, antibacterium and lubrication, avoids the influences on the vaginal pH, and maintains the normal balance of vaginal microflora; and the contraceptive gel also has the advantages of the antibacterium, inflection prevention, no damage to a genital mucosa, small irritation, low allergy and the like.
Owner:林一文 +1
External composition, and preparation and application thereof
ActiveCN103182070AStickyAvoid secondary cross-infectionPeptide/protein ingredientsAerosol deliveryOfficinalChlorhexidine
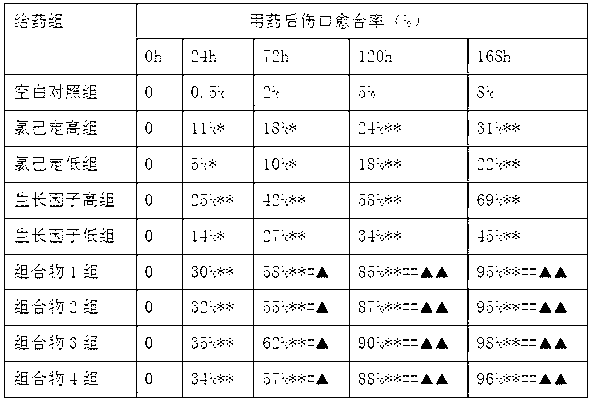
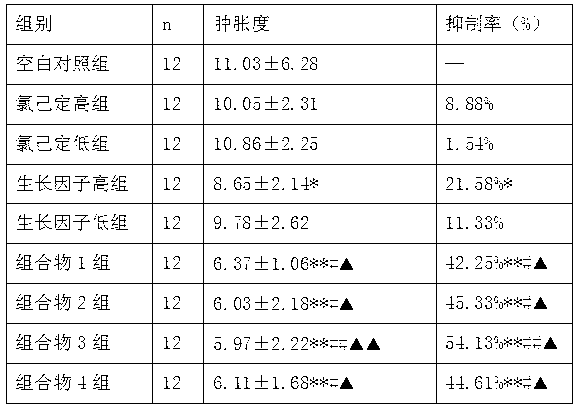
The invention relates to an external composition, and a preparation and an application thereof and belongs to the field of medicines. The external composition provided by the invention is a medicine composition which contains chlorhexidine or officinal salt thereof, or benzalkonium bromide or officinal salt thereof and a recombinant human epidermal growth factor or a recombinant cattle alkali fibroblast growth factor. According to the invention, the medicine composition is also further prepared into gel, liniment, ointment or paste, and the gel further comprises chitosan or officinal salt thereof and hyaluronic acid or officinal salt thereof. When being used for treating vagina inflammation, the external composition disclosed by the invention can improve a vagina microenvironment, promote growth of granulation tissue and repair vagina micro damage; and when being used for skin wound atraumatic restorative treatment, the external composition also can speed up restoration and regeneration of a wound tissue, promote healing and reduce scar formation, so that the external composition has a wide medical application prospect.
Owner:JIANGSU DIWO BIOLOGICAL PROD
Compound quaternary ammonium salt disinfection solution, and preparation method and application thereof
InactiveCN106035361AAvoid defectsNo stimulationBiocideDead animal preservationHexamethylenetetramineDisinfectant
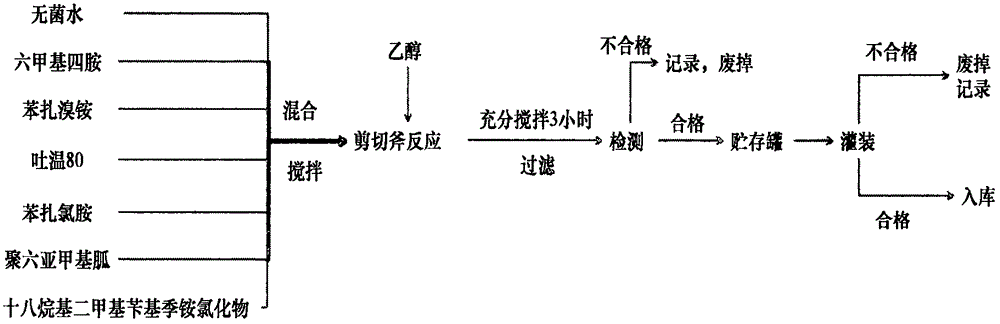
The invention discloses a quaternary ammonium salt disinfection solution, and a preparation method and application thereof. The quaternary ammonium salt disinfection solution comprises the following components: hexamethylenetetramine, benzalkonium bromide, benzalkonium chloride, polyhexamethylene guanidine, Tween 80, octadecyldimethylbenzyl chloride, isopropanol and sterile water. The invention further discloses a method for preparing the disinfection solution. According to the invention, hexamethylenetetramine, benzalkonium bromide, benzalkonium chloride, polyhexamethylene guanidine, octadecyldimethylbenzyl chloride and Tween 80 are combined together according to a specific mixing proportion, and the components have synergistic effects, so a variety of disadvantages of a single disinfectant variety or a simply-mixed disinfectant are overcome, and a variety of bacteria, fungi, spores and viruses can be thoroughly killed; meanwhile, the disinfection solution is safe to human body, is free of toxicity and stimulation, is convenient to use, can be directly used by being diluted with water, realizes one-step completion of disinfection and cleaning, has no corrosion to equipment and no harm on the environment, and is biodegradable.
Owner:GENERAL HOSPITAL OF CHINESE PEOPLES ARMED POLICEFORCES
Amnion eye drops for curing cornea alkali burn
ActiveCN101658491AImprove stabilitySolubility temperature increasesSenses disorderPharmaceutical delivery mechanismSide effectIrritation
The invention provides amnion eye drops for curing cornea alkali burn and a preparation method thereof. The preparation method comprises the steps of: taking amnion homogenate supernate as main activeconstituent; and adding with trehalose, hyaluronic acid or salt thereof, heparin or salt thereof, sodium chloride, benzalkonium bromide, vitamin, etc. The eye drops belongs to an ophthalmology drug delivery system, has a good lubrication function, and can provide a conglutination-resistant surface; and the drug delivery system is added with the other pharmacological active constituents, thereby being capable of increasing the viscocity of medical liquid, prolonging the residence time of the drug in eye surface, improving the bioavailability of the drug, reducing the side effect of the drug, reducing the drug irritation, and having a slow release effect. The amnion eye drops is an effective ophthalmology new drug, and has good clinic application foreground.
Owner:HARBIN MEDICAL UNIVERSITY
Skin mucous membrane disinfectants and preparation method thereof
ActiveCN101642449AEasy to prepareReduce manufacturing costAntibacterial agentsBiocideDisinfectantAdditive ingredient
The invention provides various skin mucous membrane disinfectants which are prepared by adopting double-chain quaternary ammonium salt and benzalkonium bromide (C20H40BrN) as main germicidal ingredients and using triclosan (C12H7Cl3O2), polyethylene glycol (HOCH2(CH2OCH2)nCH2OH), glycerol (C3H8O3), Tween 80, menthol, borneol, medical alcohol and the like as adjuvant ingredients, can be used in thefields such as the disinfection of skin and mucosa, the disinfection of surgical hand washing and the disinfection of medical apparatus and instruments in the hospital, the disinfection of tools anddevices in public places and different production industries, the mold prevention of industrial products and agricultural grains, the health disinfection of poultry houses, the aquatic product field,the aquaculture, the killing of alga, the preparation of plastic antibacterial agent, the preparation of compound disinfectant and the like and has effective bactericidal action to staphylococcus aureus, colibacillus, candida albicans, HIV and the like ; in addition, the preparation method is simple and the production cost is lower.
Owner:CHENGDU SHUNFA DISINFECTANT & WASHING TECH
Surgical flush fluid and its preparing process and application
InactiveCN1839875AGood effectImprove stabilityOrganic active ingredientsSurgical drugsCelluloseGlycine Irrigation Solution
The invention provides a flushing fluid for surgery, which comprises solute and solvent, wherein the solute is one or two selected from cellulose glycollic ether, carboxymethyl chitosan or carboxymethyl chitosan, the solvent being water for injection, physiological saline solution, sodium chloride flushing fluid, mannitol flushing fluid, glucose flushing fluid, chlorhexidine flushing fluid, gentamicin physiological saline flushing fluid, active iodine flushing fluid, benzalkonium bromide flushing fluid, aminoacetic acid flushing fluid, metronidazole flushing fluid or physiological equilibrium liquid comprising phosphates cushioning liquid, NaHCO3, sodium gluconate and mannitol.
Owner:刘万顺
Compound quaternary ammonium salt disinfectant liquid and preparation method thereof
InactiveCN101647469ASuitable for antibacterial disinfectionWide range of sterilizationBiocideAntiinfectivesAdditive ingredientDisinfectant
The invention relates to a compound quaternary ammonium salt disinfectant liquid, which is prepared by compounding single-double chain quaternary ammonium salt benzalkonium bromide and didodecyl dimethyl ammonium chloride used as raw components and povidone-iodine, dissolving the three components by using ethanol with the concentration of 95 percent as a solvent and mixing evenly. The compound quaternary ammonium salt disinfectant liquid comprises the following components by weight percent: 0.2-0.6 percent of benzalkonium bromide, 5-15 percent of didodecyl dimethyl ammonium chloride, 5-13 percent of povidone-iodine, 25-35 percent of ethanol with the concentration of 95 percent, and the balance of purified water. The implementation steps are as follows: (1), weighing benzalkonium bromide, didodecyl dimethyl ammonium chloride and povidone-iodine according to the proportion and placing into a container; (2), weighing the ethanol with the proportional concentration of 95 percent into the container; and (3) sufficiently and evenly mixing the components in the steps (1) and (2) to be dissolved, fixing volume by using purified water, separately packaging and checking according to the routine, and warehousing the qualified products. The invention has the advantages of simple preparation, safe use, no toxicity, no pollution for environment, stable medicine property and obvious effect.
Owner:天津市海纳德动物药业有限公司
Sterilizing and deodorizing agent for pets
InactiveCN105724439AReasonable formulaLong-term stabilityBiocideDead animal preservationWater bathsDistilled water
The invention relates to a sterilizing and deodorizing agent for pets. The sterilizing and deodorizing agent for pets comprises 0.1-0.4 part of di-octadecyl dimethyl ammonium chloride, 0.5-1 part of didecyl dimethyl ammonium chloride, 15-25 parts of benzalkonium bromide, 0.1-0.3 part of glutaraldehyde, 15-25 parts of pure plant extract, 0.05-0.15 part of plant essence and an appropriate amount of distilled water. A preparation method of the sterilizing and deodorizing agent includes: using the distilled water as the solvent to dissolve the di-octadecyl dimethyl ammonium chloride through water-bath heating, then mixing with other components, and well mixing to obtain the sterilizing and deodorizing agent. The sterilizing and deodorizing agent has the advantages that the sterilizing and deodorizing agent is reasonable in formula, safe and nontoxic, evident in sterilizing and deodorizing effect, wide in action range, long in duration, convenient to use and pleasant in smell; the sterilizing and deodorizing agent diluted with water can be sprayed to pet cages, positions with urine and excrement or in the air of parts requiring sterilizing and deodorizing, and effects of sterilizing and deodorizing can be achieved.
Owner:SHANGHAI SHENYA ANIMAL HEALTH PROD FUYANG CO LTD
Cadaver-preservative fluid and method for producing the same
InactiveCN1839691AUnique formulaImprove sterilization effectDead animal preservationGlycerolDistilled water
Disclosed is a cadaver-preservative fluid and method for preparation, characterized in that, it is prepared from 50% of glutaric dialdehyde, aliphatic alcohol polyoxyethylene ether, ethanol, benzalkonium bromide, antiseptic germicide, Iodopropynyl butylcarbamate, glycerin, deionized water or distilled water.
Owner:赵铎
Preparation technology of nanometer aluminum hydroxide adjuvant
InactiveCN101991850AHigh densityGood dispersionImmunological disordersAntibody medical ingredientsAdjuvantAluminium hydroxide
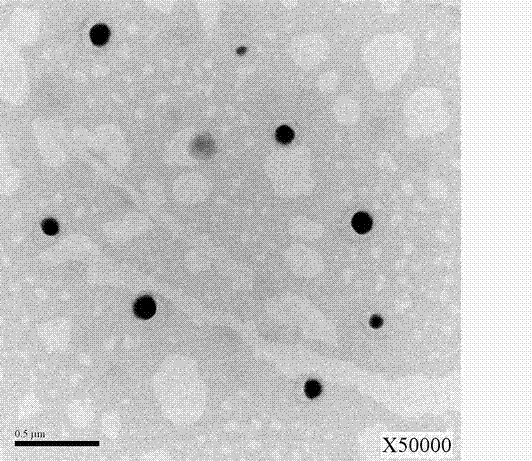
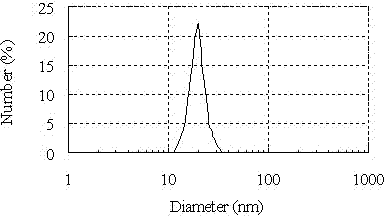
The invention discloses a preparation technology of nanometer aluminum hydroxide adjuvant, comprising the following steps: taking benzalkonium bromide, n-caprylic alcohol and cyclohexane at equal ratio; stirring mixture at high speed, and pouring the mixture into a triangular flask; putting in a stir bar; putting the triangular flask on a magnetic stirrer, adding a proper quantity of AlCl3 solution, and stirring; slowing dropwise adding ammonia water into a separating funnel to regulate dripping rate; causing a reaction system to react for 2 hours at the pH of 10; after the reaction ends, adding acetone for demulsification and centrifugation; abandoning supernatant; respectively adding absolute ethyl alcohol and deionized water; evenly stirring by a glass rod; washing for three times, and decentralizing; collecting decentralized sediment into a beaker; drying for 12 hours in a drying oven to obtain fluffy Al(OH)3 adjuvant. The prepared nanometer aluminum hydroxide adjuvant has small grain diameter, good dispersibility and stability and favourable biocompatibility. In an animal experiment, the anti-tumor effect of nanometer aluminium adjuvant adsorption autovaccine is obviously superior to the curative effect obtained by using common aluminium adjuvant adsorption autovaccine.
Owner:SOUTHEAST UNIV
Porous antibiotic air filter paper and making method thereof
InactiveCN104005278AHas antibacterial propertiesImprove adsorption capacityNon-fibrous pulp additionPaper/cardboardAir filterOxygen
The invention discloses a porous antibiotic air filter paper. The porous antibiotic air filter paper is made by using the following raw materials, by weight, 53-56 parts of a flax pulp, 43-45 parts of a softwood pulp, 0.2-0.3 parts of benzalkonium bromide, 0.3-0.5 parts of petroleum ether, 2-3 parts of soybean powder, 3-4 parts of nanometer porcelain clay, 1-2 parts of crushed horseradish, 2-3 parts of zircon, 1-2 parts of macro-porous silicon powder, 0.6-0.8 parts of calcium peroxide, 4-7 parts of chlorinated paraffin, 0.6-0.8 parts of turkey red oil, 0.3-0.4 parts of xanthan gum, 7-9 parts of a polyacrylate emulsion, 1-2 parts of an assistant and a proper amount of water. Horseradish in the above raw materials has a certain antibiosis effect; the addition of calcium peroxide realizes slow oxygen release after meeting water, and forms minimal holes on the filter paper layer, so the adsorption effect is increased; and the addition of the assistant is safe and environmentally friendly, and forms minimal holes on the surface of filter paper, so the adsorptivity is increased.
Owner:BENGBU DEMO FILTRATION TECH
Disinfectant wipes
InactiveCN104434652ASoft textureGlossy surfaceCosmetic preparationsToilet preparationsChlorhexidine AcetateDisinfectant
The invention discloses disinfectant wipes. The disinfectant wipes comprises a wipes body, wherein a mixed liquid is dissolved in the wipes body, and is prepared from a humectant, a solubilizer, a disinfectant, a preservative, a flavor enhancer and deionized water; the humectant is one or more of potassium sorbate, xylitol and chloroacetic acid; the solubilizer is one or more of sodium carboxymethyl cellulose, sodium dodecyl sulfate, polyvinylpyrrolidone, dipotassium glycyrrhizinate and benzalkonium bromide; the disinfect is one or more of alcohol, paeonol, chlorhexidine acetate, baicalin, aloe extract and citric acid; the preservative is one or more of methylparaben, ethylparaben, diacetyl acetoacetate, salicylic acid and aluminum silicate; and the flavor enhancer is one or more of vanillin, menthol, hexanal and leaf acetate. The wipes have soft texture, bright surface and fragrant smell, and the storage time of the wipes can be prolonged.
Owner:铜陵麟安生物科技股份有限公司
A environmental friendly spray type compound iodine disinfectant and its preparation method
InactiveCN1689409AImprove sterilization efficiencyReduced tinting abilityBiocideAnimal repellantsDisinfectantIrritation
The present invention relates to one kind of environment friendly sprayed composite iodine disinfectant and its compounding process. The disinfectant consists of non-ionic surfactant Tween, iodine, potassium iodide solution and benzalkonium bromide and other synergist and stabilizer. Owing to the novel iodine carrier, the synergist and the stabilizer, the disinfectant has the features of fast iodine releasing and acting, less iodine color residue, raised permeability and no irritation to skin and mucous membrane. Therefore, the present invention may be used in effective disinfection of air and object surface and for clinical disinfection. The present invention may be disintegrated naturally and is environment friendly.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES +1
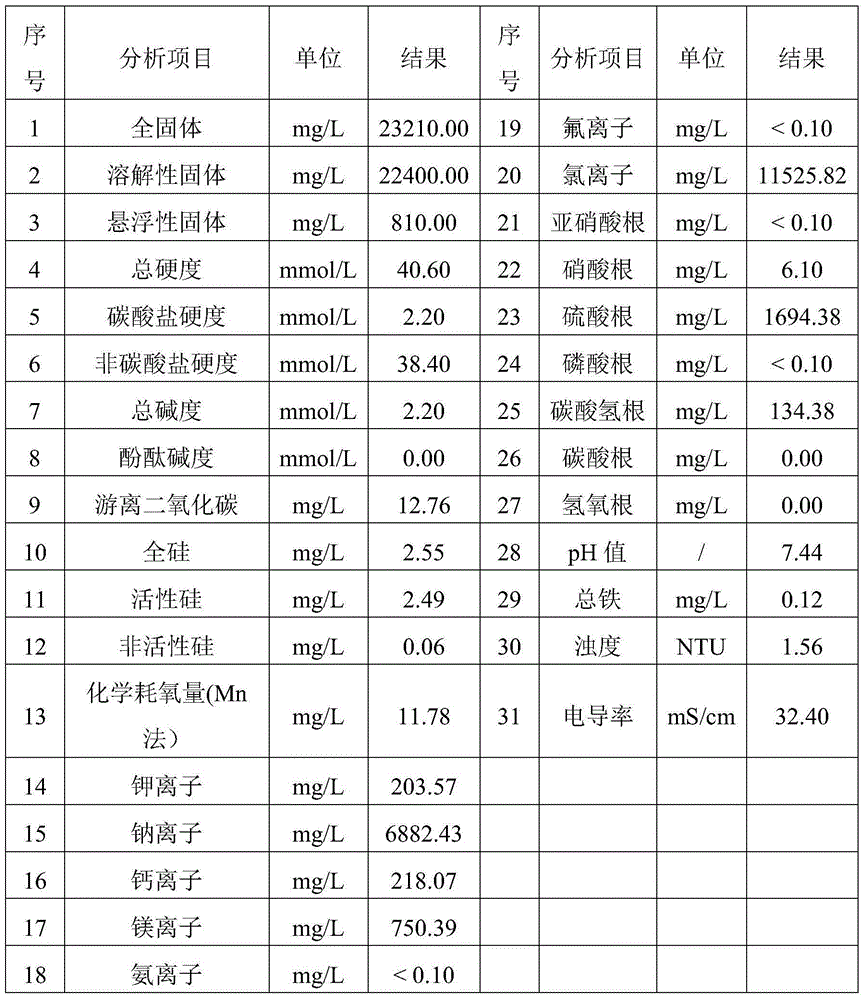
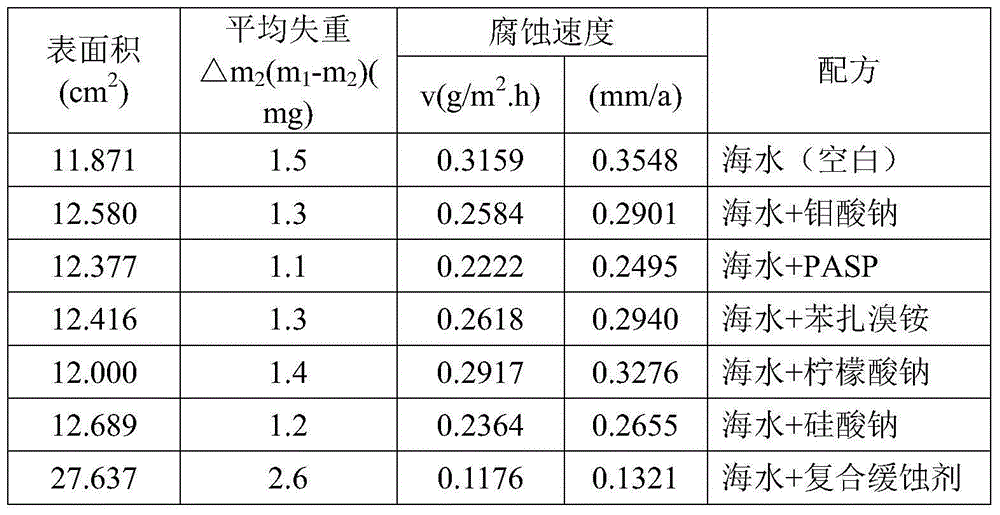
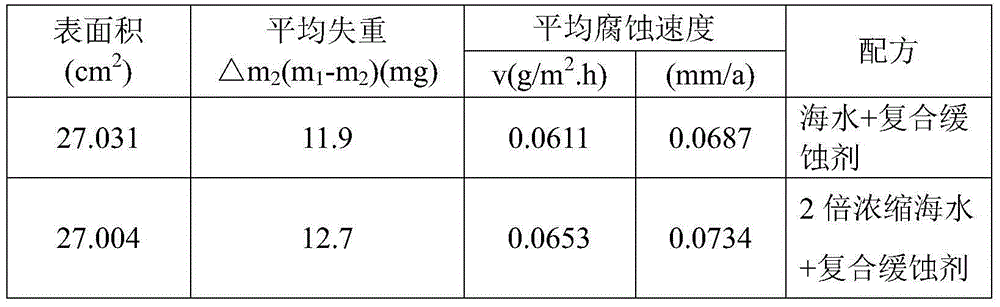
Composite corrosion inhibitor for seawater circulation cooling system carbon steel material anticorrosion
The invention discloses a composite corrosion inhibitor for seawater circulation cooling system carbon steel material anticorrosion. The composite corrosion inhibitor comprises sodium molybdate, polyaspartic acid (PASP), benzalkonium bromide, sodium citrate and sodium silicate according to a mass ratio of 0.8-1.2:1.5-2.5:3.5-4.5:3.5-4.5:7.5-8.5. The corrosion inhibitor does not contain phosphorus (phosphine), difficultly decomposed polymers or heavy metal salts, so the corrosion inhibitor is harmless to environment; the corrosion inhibitor can reach a corrosion inhibition effect under a small dosage, so the use cost is reduced; and the corrosion inhibitor can control the carbon steel corrosion speed in a national standard allowed range in seawater and 2 times concentrated seawater, so the application range of the corrosion inhibitor is wide.
Owner:ELECTRIC POWER RES INST OF GUANGDONG POWER GRID +1
Compound disinfectant for livestocks as well as preparation method and application thereof
The invention belongs to the technical field of disinfectants, and particularly relates to a compound disinfectant for livestocks as well as a preparation method and an application thereof. The compound disinfectant containing the following components by weight percentage: 3.0-15.0% of glutaraldehyde, 5.0-15.0% of benzalkonium bromide, 0.5-1.0% of turpentine, 1.0-10.0% of ethanol, and the balance of pure water. The compound disinfectant for the livestocks can well play roles of disinfecting and virus killing, can be used as drinking water for the livestocks, can greatly improve the safety of the disinfectant, and has functions of reducing ammonia concentration in animal houses and purifying air.
Owner:HENAN XINZHENGHAO BIO ENG
Lidocaine and chlorhexidine aerosol
ActiveCN101785766AReduce dosageReduce adverse reactionsAerosol deliveryOrganic non-active ingredientsTreatment effectChlorhexidine Acetate
In order to overcome the defects of current medicines for treating mosquito / bug bite, skin abrasion, soft tissue injury and minor burn, shorten treatment period, reduce adverse reaction, achieve convenient carrying, enhance curative effect and lower the use amount of medicine, the invention obtains a novel Lidocaine aerosol after optimized screening, which contains Lidocaine, chlorhexidine acetate, benzalkonium bromide, vinyl pyrrolidone-vinyl acetate copolymer, acetic ester, ethanol and propellant. The aerosol plays a good role of treating mosquito / bug bite and skin injury and simultaneously reduces the stimulation on skin.
Owner:江苏天济药业有限公司
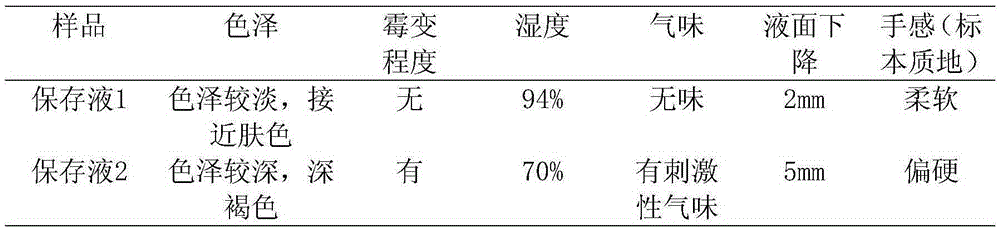
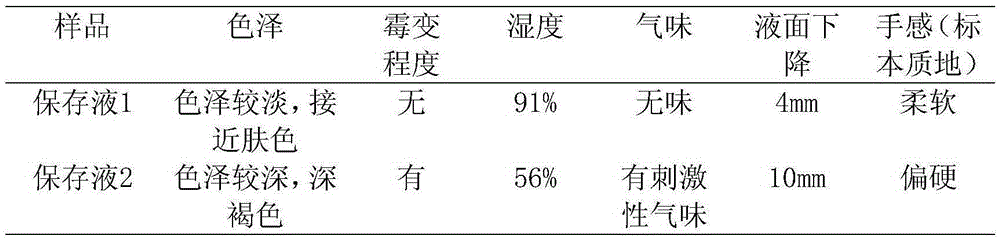
Specimen preservation fluid good in preservation effect and preparation method of specimen preservation fluid
The invention discloses specimen preservation fluid good in preservation effect and a preparation method of the specimen preservation fluid. The preservation fluid is mainly prepared from 50% glutaraldehyde, polyoxyethylene aliphatic alcohol ether, industrial alcohol, benzalkonium bromide, phenol, glycerinum, ethylenediamine tetraacetic acid disodium salt and water. The prepared preservation fluid is free of formaldehyde, slow in volatilization, free of pungent gas, quite low in toxicity, high in safety, good in preservation effect and capable of making a specimen good in color and luster, preventing the specimen from mildew after long-term preservation and making the specimen soft in texture.
Owner:GUIYANG COLLEGE OF TRADITIONAL CHINESE MEDICINE
Sterilizing agent, preparation method and use thereof
ActiveCN1729778AImprove the bactericidal effectBroad spectrum antibacterialBiocideCosmetic preparationsSkin surfaceChlorhexidine
The invention relates to a sterilizing and disinfecting agent, its preparing process and use thereof, wherein the sterilizing and disinfecting agent comprises water and compound water-soluble mother liquor, the mother liquor comprises (by weight parts) acetic chlorhexidine 8-12, benzalkonium bromide 2-4, sulfonyl acetic ammonium 0.5-1.5, OP emulsifying agent 4-6 and water 10.5-17.5, the mother liquor is diluted with water till the content of the acetic chlorhexidine is 0.15-0.25%.
Owner:吴吉平 +1
Leather antiseptic
InactiveCN104388603AExtended service lifeEasy to sterilizeLeather surface finishingPolymer sciencePyrrolidinones
Owner:QINGDAO TOPLINK INFORMATION TECH
Disinfection sterilizing type medical supersonic couplant and method of preparing the same
ActiveCN101249268BIncrease concentrationImprove the bactericidal effectIn-vivo testing preparationsCelluloseChlorhexidine Acetate
The invention discloses a disinfection sterilization medical ultrasonic coupling medium with the bactericidal function and used in the ultrasonic detection and treatment of skin and cavitary mucosa and a preparation method of the coupling medium, wherein, the ultrasonic coupling medium comprises propylene glycol, de-ionized water and one viscosity modifier of the hydroxy ethyl cellulose, carbomerand hydroxy propyl cellulose. The coupling medium further comprises a fungicide of 0.05 to 0.15 percent and the constituents of the fungicide are any one of polyhexamethylene biguanide, benzalkonium chloride, benzalkonium bromide, chlorhexidine acetate, trichlorobenzene phenoxy diphenyl ether and nanometer silver or any combination of the olyhexamethylene biguanide, the benzalkonium chloride, thebenzalkonium bromide, the chlorhexidine acetate, the trichlorobenzene phenoxy diphenyl etherand, and the nanometer silver; the preparation method used for preparing the ultrasonic coupling medium is as follows: dispersing the hydroxy ethyl cellulose in the propylene glycol, stirring and dispersing the hydroxy ethyl cellulose by adding enough water, heating to 75 to 80 DEG C, preserving the heat for 30 minutes to cool the hydroxy ethyl cellulose and the propylene glycol to 45 DEG C, adding the fungicide to stir and cool the fungicide, the hydroxy ethyl cellulose and the propylene glycol to obtain the filled coupling medium. Tests show that the products of the coupling medium have fast and long sterilization effect and meet the requirements of various related standards.
Owner:广州市一杰医药科技有限公司
Compound chlorhexidine acetate microemulsion disinfectant and its preparation method
InactiveCN103081940AGood water solubilityImprove solubilityAntibacterial agentsBiocideChloraPrepChlorhexidine Acetate
The invention relates to a disinfectant, and aims to provide a compound chlorhexidine acetate microemulsion disinfectant having the advantages of good water solubility, strong disinfection effect, long disinfection effect maintenance time and good stability, and its preparation method. The compound chlorhexidine acetate microemulsion disinfectant is prepared through using the following raw materials, by weight, 0.02-11.0 parts of chlorhexidine acetate, 0-11.0 parts of benzalkonium bromide, 18.5-30.2 parts of a surfactant, 0-18.6 parts of a cosurfactant, 2.7-5.0 parts of oil and 36.6-67.2 parts of distilled water. The compound chlorhexidine acetate microemulsion disinfectant has the advantages of high chlorhexidine acetate content, use convenience, simple production technology, low production cost, and convenience for the scale production.
Owner:HENAN INST OF SCI & TECH
Compound antibacterial gel, preparation method thereof and applications thereof
InactiveCN103598179AEasy to recycleEasy to filterBiocideDisinfectantsAntibacterial activityGram-positive bacterium
Owner:广东海尔斯激光医疗科技有限公司
Infantile eczema nursing wet tissue
InactiveCN102824514ARelieve symptomsRelieve itchingOrganic active ingredientsDermatological disorderSide effectVitamin C
The invention discloses an infantile eczema nursing wet tissue and relates to the field of infantile eczema prevention nursing materials. The invention aims to solve the problem that traditional medicines for external application and medicines for oral administration cannot be conveniently used or have side effects. The infantile eczema nursing wet tissue comprises wet tissue liquid and a carrier of the wet tissue liquid, and is characterized in that the wet tissue liquid is prepared from the following raw material components in weight ratio: 0.05-0.1 percent of vitamin B, 0.05-0.1 percent of vitamin C, 0.05-0.1 percent of vitamin E, 0.2-0.4 percent of benzalkonium bromide, 0.05-0.1 percent of rehmannia extract, 0.05-0.1 percent of angelica sinensis extract, 1-2 percent of propylene glycol, 0.5-1 percent of aloe extract and the balance of water. The infantile eczema nursing wet tissue disclosed by the invention is simple and convenient in use and is suitable for preventing and nursing infantile eczema. Pure cotton non-woven fabric which is non-irritant to human bodies is used as the carrier, so that a solution for treating the eczema directly acts on an affected part. Nursing, cleaning and treatment are combined into a whole. On the basis of treating the eczema, itching-relieving traditional Chinese medicines are also added in the infantile eczema nursing wet tissue, thus, itching feelings brought to infants by the eczema can be effectively relieved. The vitamins capable of improving human immunity are also used, and thus, the eczema symptom can be effectively relieved.
Owner:TONGLING JIEYA BIOLOGIC TECH
Method for simultaneously measuring contents of multiple skin disinfectants in wet tissue
InactiveCN105954397AGuaranteed separation effectEfficient analysisComponent separationChlorhexidine AcetateDisinfectant
The invention provides a method for simultaneously measuring the contents of multiple skin disinfectants in the wet tissue, and aims at providing the method which has simple operating method for simultaneously measuring the contents of multiple skin disinfectants in the wet tissue. The method uses a high performance liquid chromatography method to simultaneously measure the contents of chlorhexidine gluconate, chlorhexidine acetate, 2,4,4'-trichlorine-2'-phenoxyphenol, benzalkonium bromide, benzalkonium chloride and glutaraldehyde in the wet tissue. The invention belongs to the technical field of chemical detection.
Owner:WUZHOU INST FOR FOOD & DRUG CONTROL
Cream composition and paper comprising same
The invention provides a cream composition which comprises bacteriostatic agents, glycerin and deionized water. The mass ratio of the bacteriostatic agents to the glycerin to the deionized water is 0.1-22:10-97:3-9. The bacteriostatic agents are at least one type of polyhexamethylene guanidine hydrochloride, polyhexamethylene biguanidine hydrochloride, iodopropynyl butylcarbamate, 2-methyl-4-amino-6-methoxy s-triazine, benzalkonium bromide, chloretone, sulfoacid pyrimidine sodium, diclofenac sodium, chlorhexidine and myristylpicolinum bromide. The invention further provides paper comprising the cream composition. The cream composition is formed on the outer surface of the paper, so that the paper realizes moisture retention, sterilization and bacteriostasis effects when used for wiping the skin of a user, and feels comfortable and soft.
Owner:GOLD HONG YE PAPER
Composite agricultural sterilizing agent and preparation method of composite agricultural sterilizing agent
ActiveCN102617226AImprove the bactericidal effectBroad-spectrum and high-efficiency sterilizationBiocideFungicidesAdditive ingredientButyl carbamate
The invention discloses a composite agricultural sterilizing agent and a preparation method of the composite agricultural sterilizing agent. The sterilizing agent comprises the following materials and ingredients in parts by weight: 65 to 90 parts of dodecyl dimethyl benzyl ammonium bromide (benzalkonium bromide), 8 to 20 parts of iodo propynyl butyl carbamate (IPBC) and 3 to 15 parts of absorption promoters, wherein the absorption promoters are one kind of materials selected from citric acid, urea, potassium phosphate, potassium nitrate, amino acid and chitosan or are a mixture of the citric acid, the urea, the potassium phosphate, the potassium nitrate, the amino acid and the chitosan. The sterilizing agent prepared by the method provided by the invention is efficient and safe, virus, germs and fungi cannot generate the drug resistance, and the environment cannot be polluted.
Owner:石家庄卫科生物科技有限公司
Salt-water liquid for irrigating nasal cavity
InactiveCN101028337ALow priceReduce dosagePharmaceutical non-active ingredientsRespiratory disorderNasal cavityUse medication
A saline liquid for treating allergic rhinitis by using it to flush nasal cavity contain proportionally sodium chloride, mint oil, polyoxyvinyl sorbitan monooleate, benzalkonium bromide and distilled water.
Owner:卢育华
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com