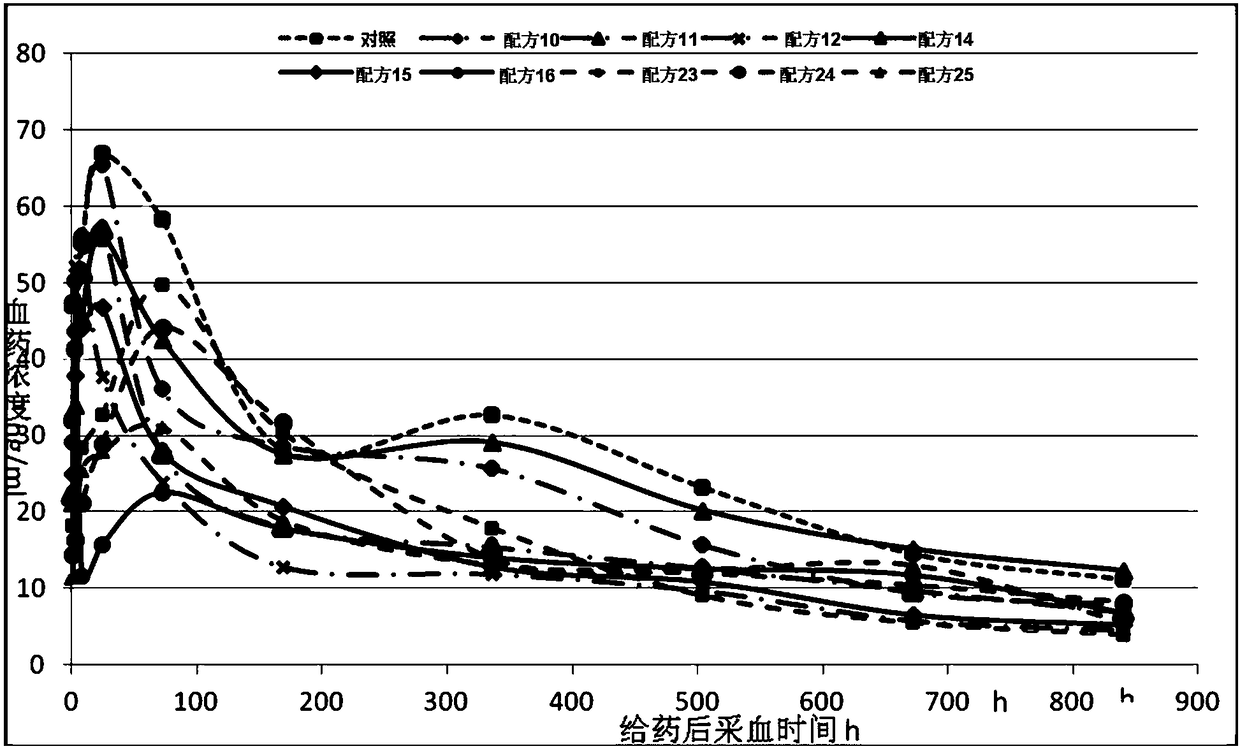
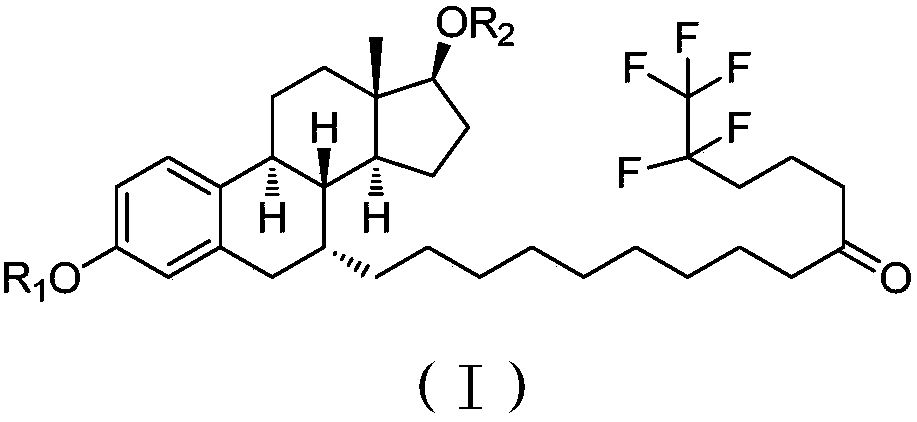
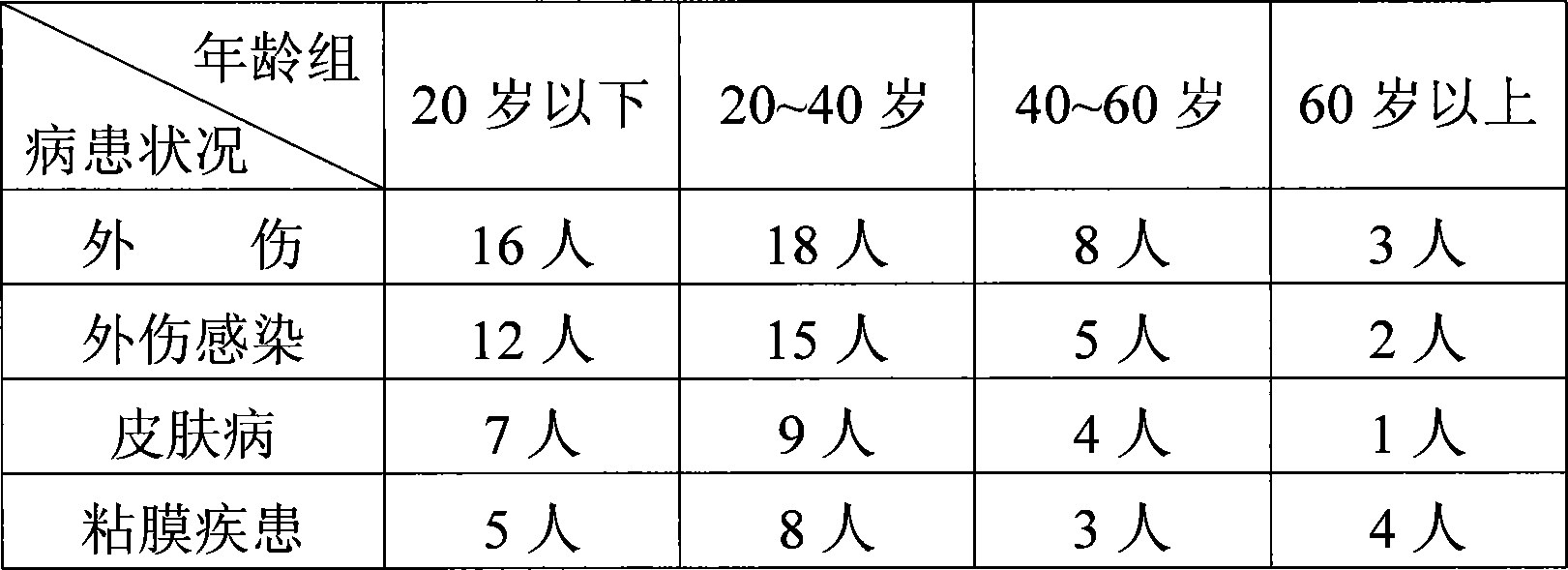
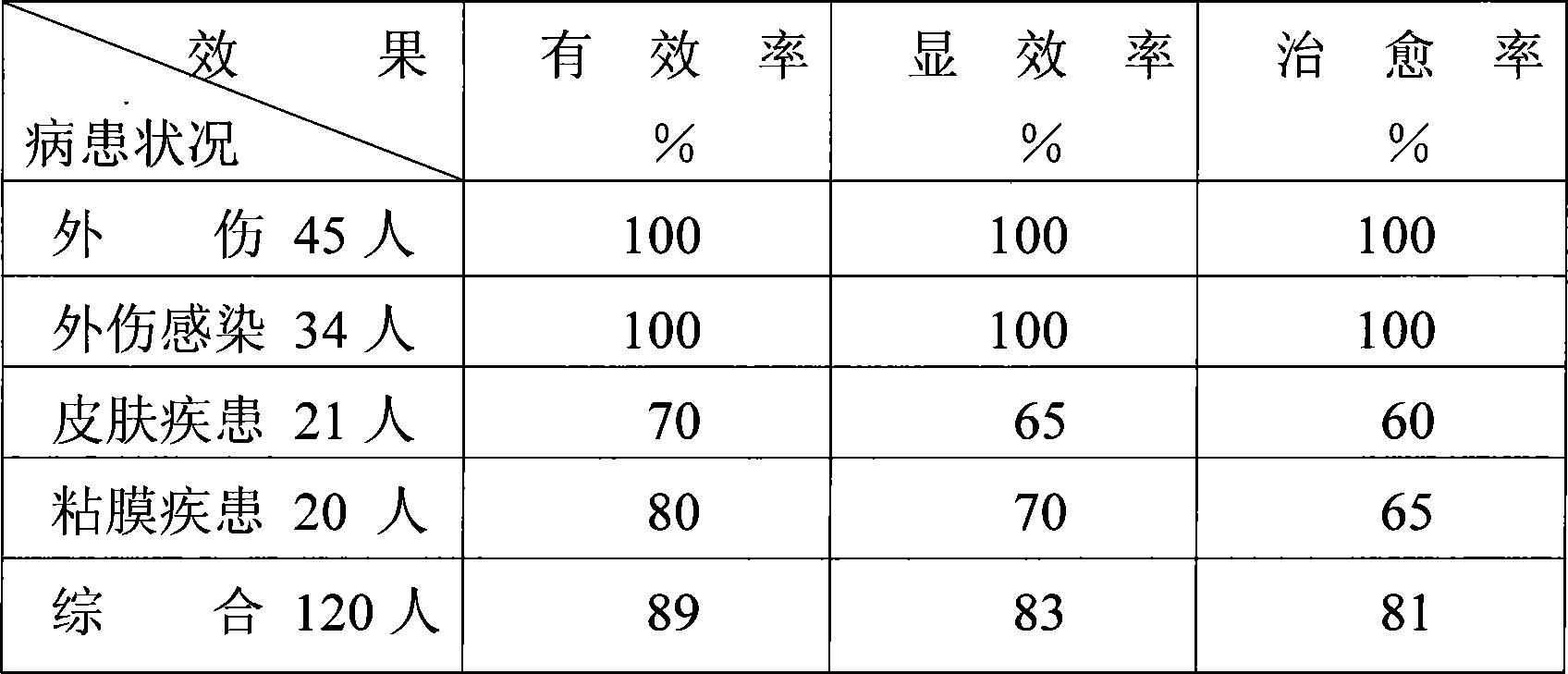
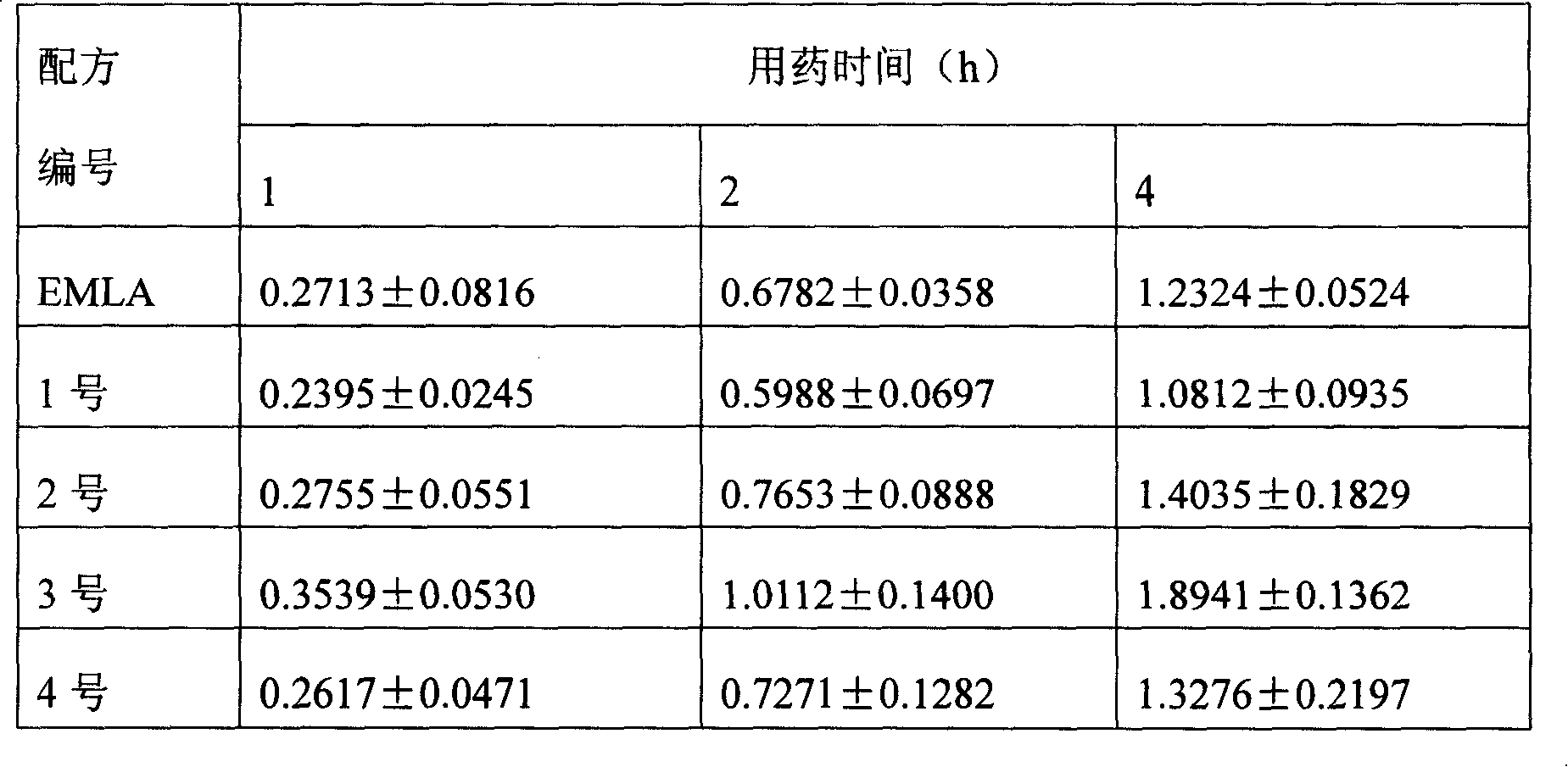
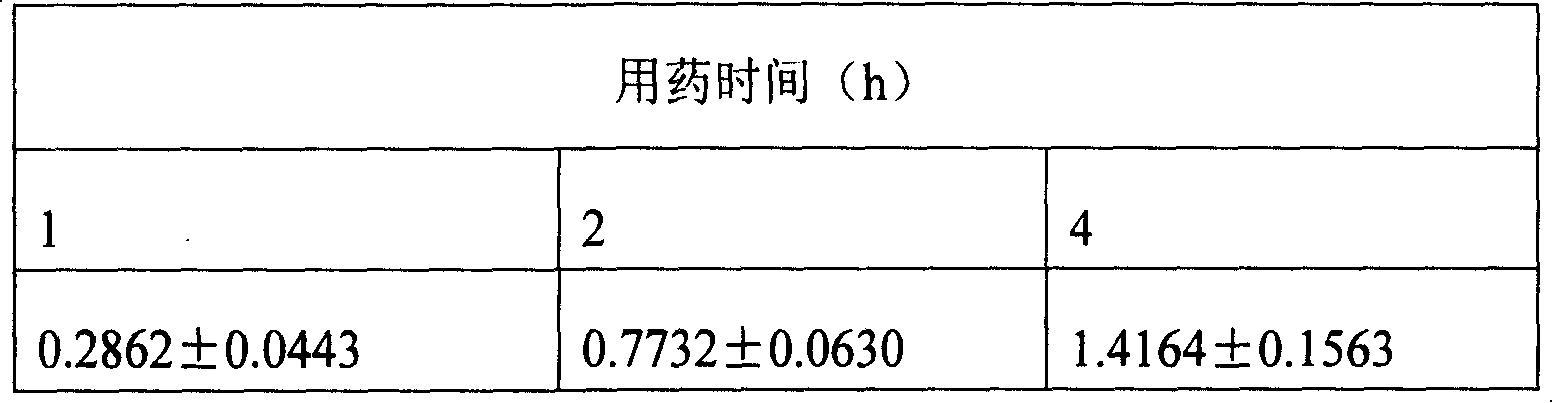
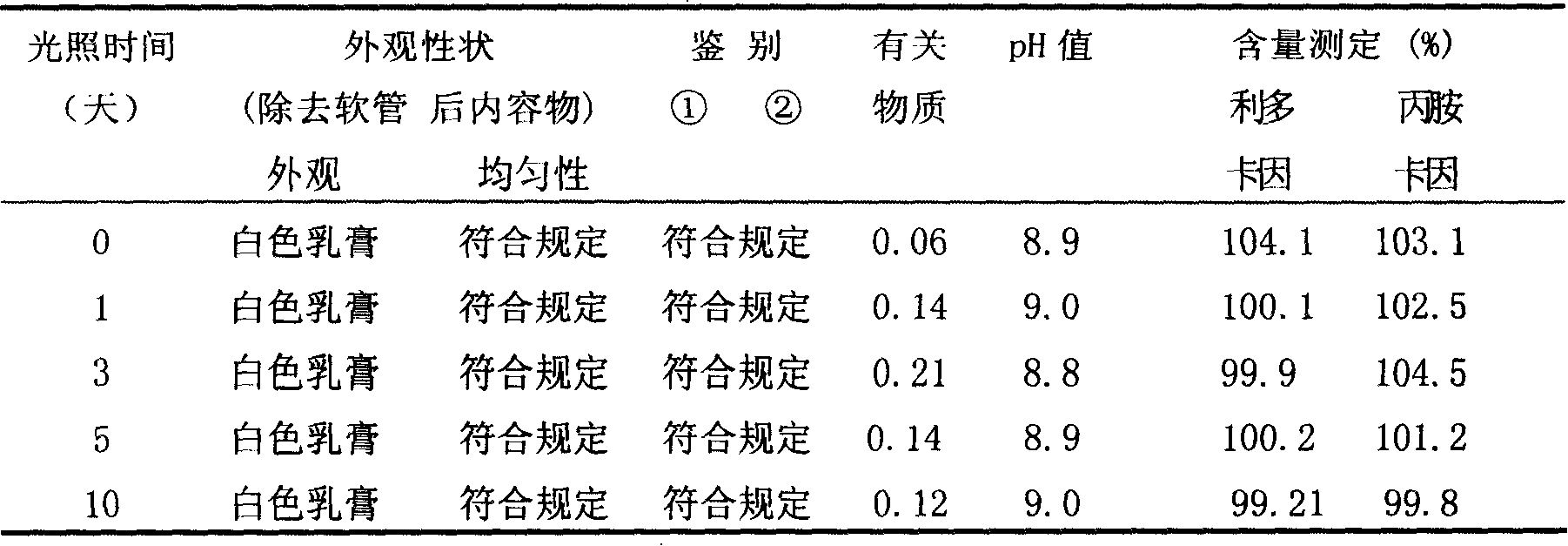
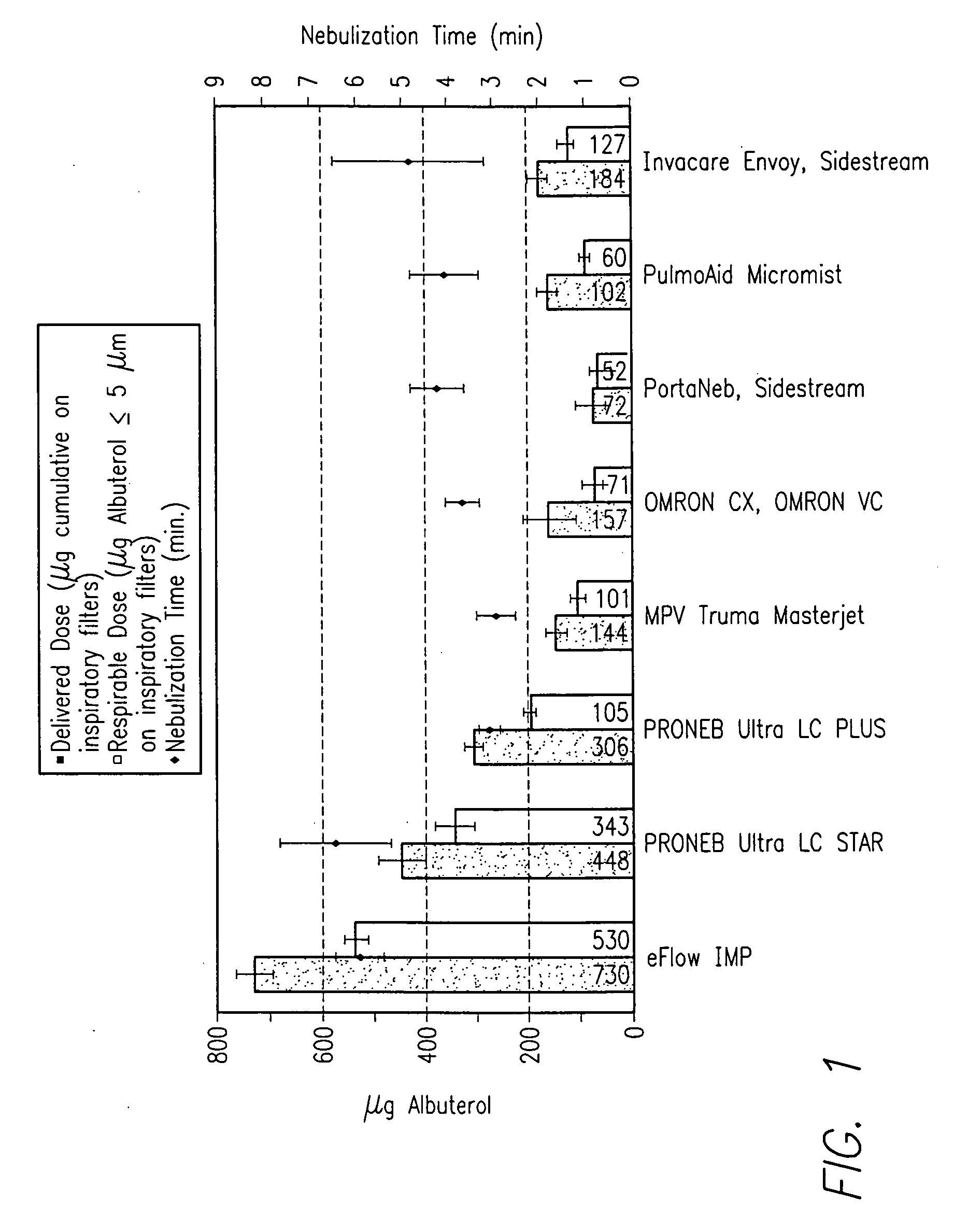
Patents
Literature
424 results about "Lidocaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lidocaine is used to temporarily numb and relieve pain from minor burns (including sunburn), skin abrasions, insect bites, and other painful conditions affecting mucous membranes. Some lidocaine products are used to numb the lining of the mouth or throat before certain medical/dental procedures. It is also used to decrease pain while dentures are being fitted and while your gums are adjusting to the dentures. It should not be used long-term to decrease pain from poorly fitting dentures.
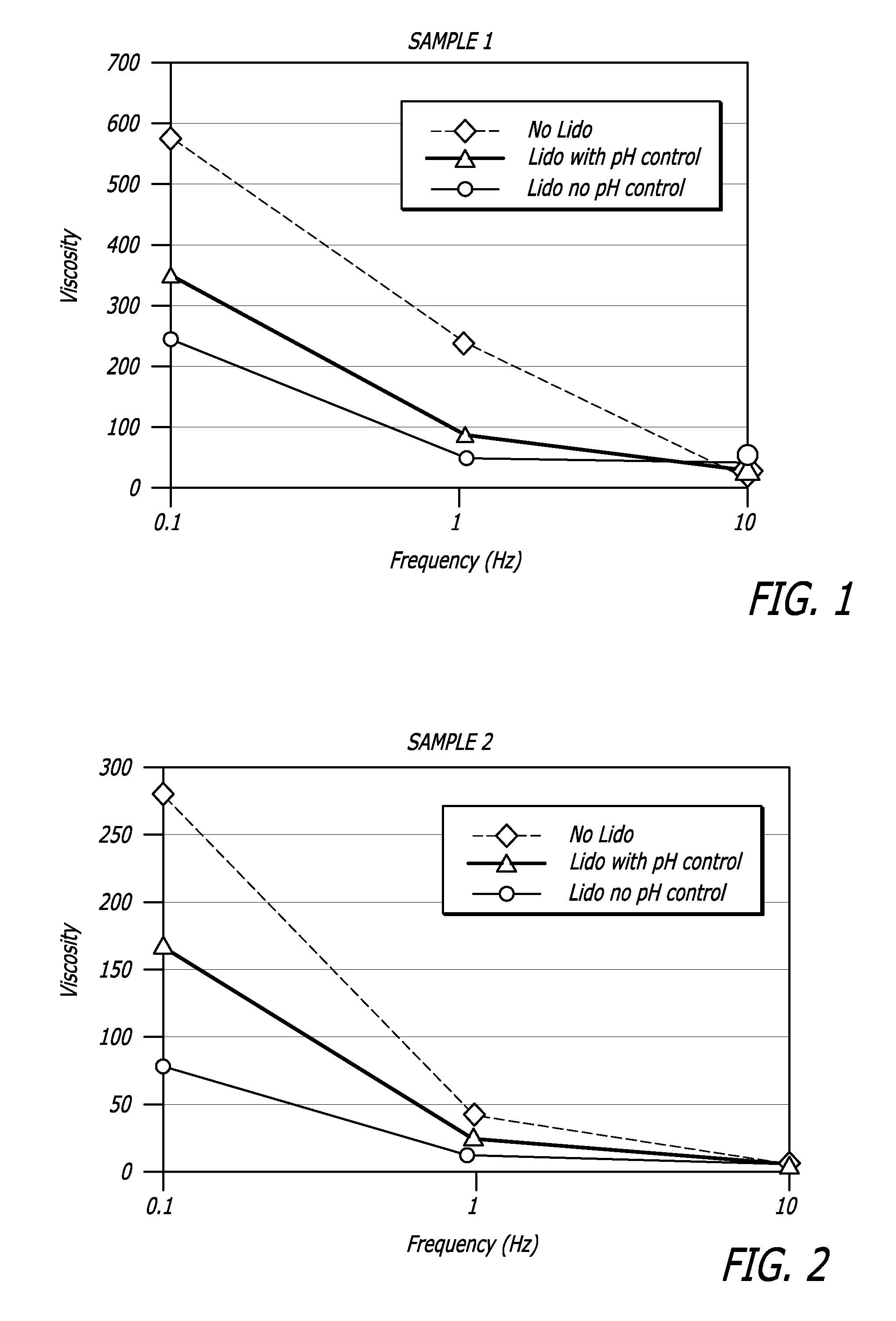
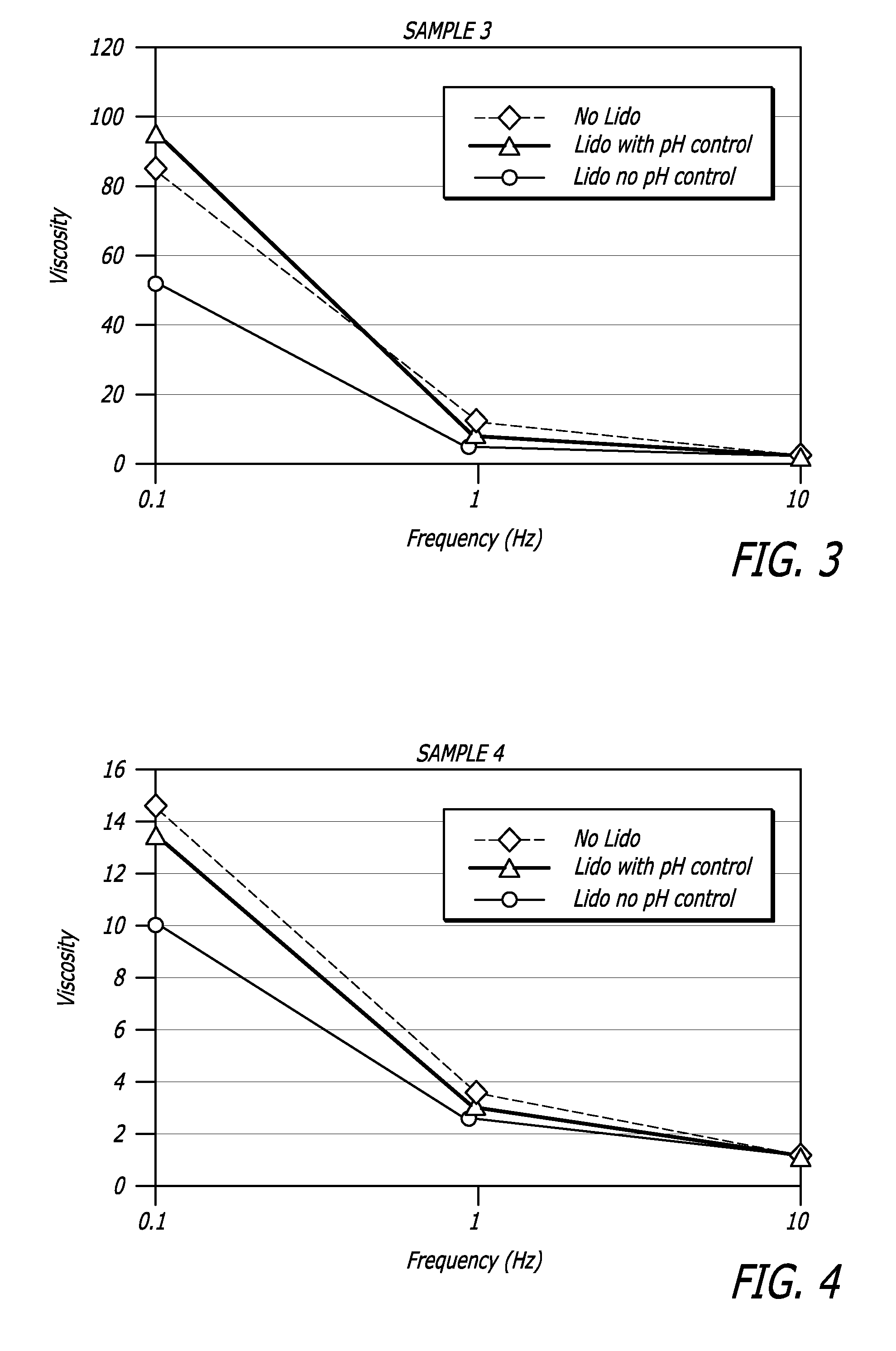
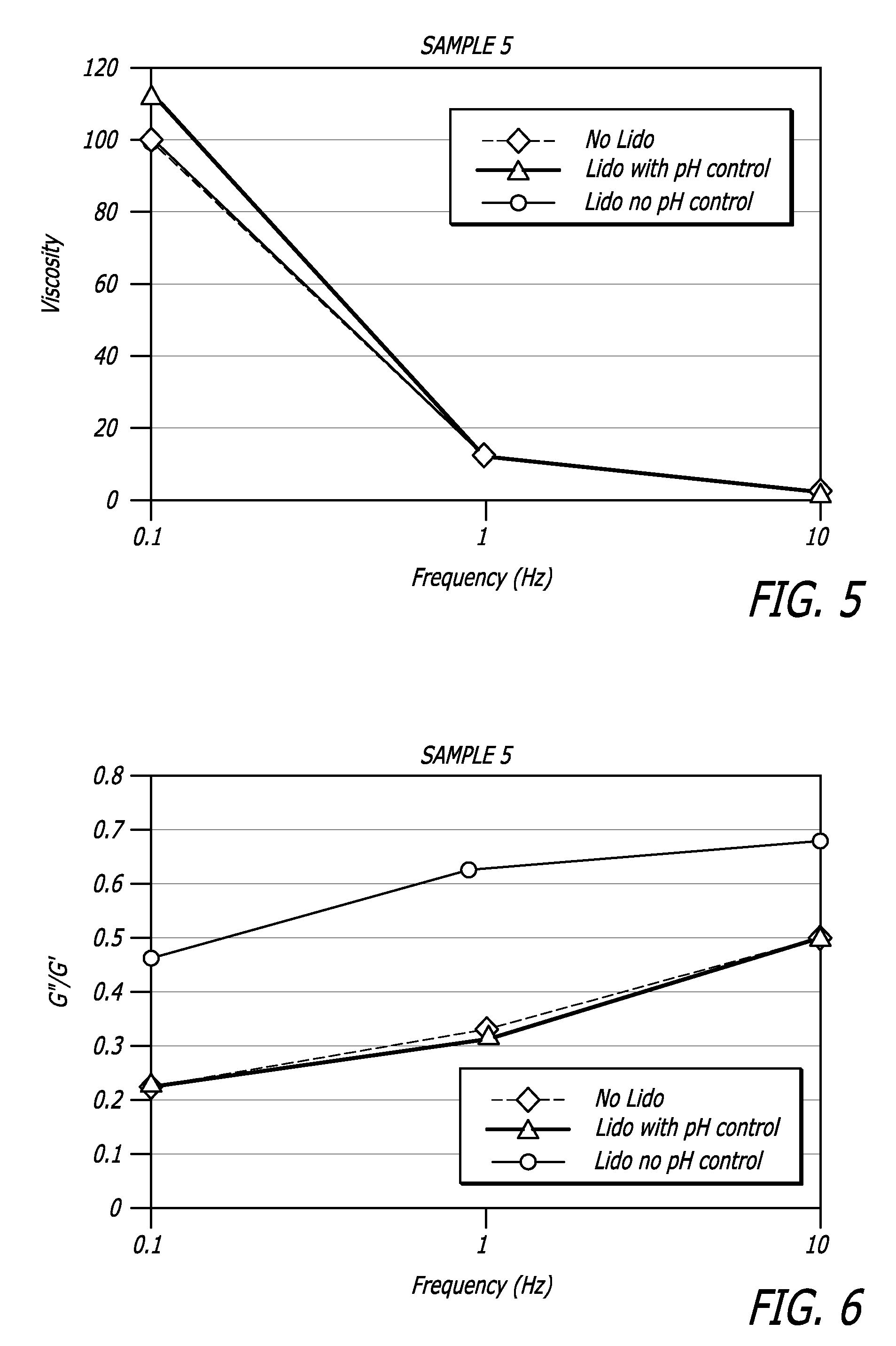
Hyaluronic acid-based gels including lidocaine
Disclosed herein are cohesive soft tissue fillers, for example, dermal and subdermal fillers, based on hyaluronic acids and pharmaceutically acceptable salts thereof. In one aspect, hyaluronic acid-based compositions described herein include a therapeutically effective amount of at least one anesthetic agent, for example, lidocaine. The present hyaluronic acid-based compositions including lidocaine have an enhanced stability and cohesivity, relative to conventional compositions including lidocaine, for example when subjected to sterilization techniques or when stored for long periods of time. Methods and processes of preparing such hyaluronic acid-based compositions are also provided.
Owner:ALLERGAN IND
Electrically assisted lidocaine and epinephrine delivery device having extended shelf-stability
InactiveUS20050228336A1Great confidenceFewer returnsOrganic active ingredientsElectrotherapyLidocaineAnesthetic
Highly shelf-stable electrically assisted transdermal drug delivery systems for delivering epinephrine, typically with an anesthetic such as lidocaine, are provided along with methods for making the highly shelf-stable epinephrine-containing transdermal delivery device. Highly shelf-stable packaged electrode assemblies for transdermal delivery of epinephrine also are provided.
Owner:VYTERIS
Inhalable lidocaine formulation for treatment of asthma and for reducing the need for corticosteroids in asthmatic patients
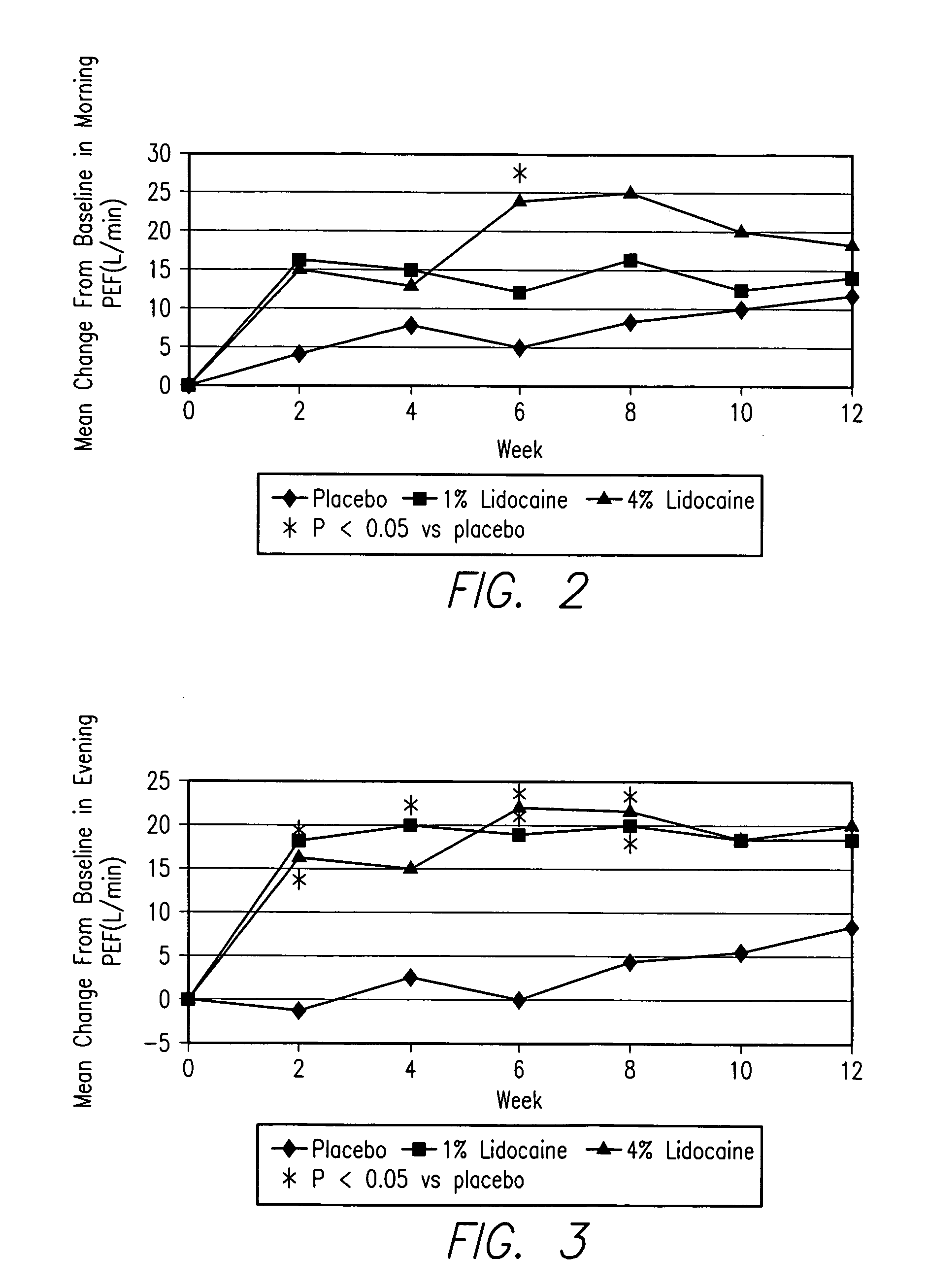
An inhalable lidocaine solution for treatment of asthma and a method for treatment of asthma with the reduced need for concurrent administration of oral corticosteroids in asthmatic patients. The lidocaine solution or lidocaine dry powder is delivered by an electronic nebulizer or by dry powder inhaler or dose meter one to several times a day.
Owner:CORUS PHARMA
Nutrigenomics methods and compositions
The present invention provides a proprietary compositions and systems to modulate genetic and metabolomic contributing factors affecting disease diagnosis, stratification, and prognosis, as well as the metabolism, efficacy and / or toxicity associated with specific vitamins, minerals, herbal supplements, homeopathic ingredients, and other ingredients for the purposes of customizing a subject's nutritional supplement formulation to optimize specific health outcomes. Specific to this invention the utilization of certain known polymorphic genes associated with Substance Use Disorder (SUD) are analyzed to target certain genetic anomalies that lead to a high risk and predisposition to SUD. The genotypic patterns are then utilized to provide certain nutritional customized solutions especially related to the attenuation of aberrant abuse of physician prescribed narcotic pain medication across all pain conditions. A priority GENOPROFILE is measured and directs the customization of a subsequent nutraceutical to act as a therapeutic modality. Specifically the treatment includes slow attenuation of the pain medication by incorporating orals (shakes, liquid beverages, pills, tablets, troche, ointments etc.), Intramuscular, Intravenous, intra-rectal and any form necessary to deliver a sufficient amount of an anti-craving and anti-stress nutraceutical. Moreover, the invention includes examples of novel analgesic ointments coupling Synaptamine and such analgesic and other anesthetic compounds including but not limited to Gabapentin, Ketamine, Baclofen, Ketoprofen, Amitriptyline, Lidocaine, Cyclobenzapine, Diclofenac, Menthol, Camphor and Capsaicin. The GENOPROFILE will be used to determine pain sensitivity Intolerance.
Owner:BLUM KENNETH +3
Medicament formula for relieving pain caused by infusion medicament and preventing phlebitis
InactiveCN102579458ARelief the painRelieve and prevent phlebitisAntipyreticAnalgesicsFormularySodium phosphates
The invention discloses a medicament formula for relieving pain caused by infusion medicament and preventing phlebitis. The medicament formula can be used for relieving pain caused by intravenous supplementing potassium, infusion mannitol, azithromycin, fructose diphosphate sodium and chemotherapeutic medicaments, and can be used for effectively preventing and treating extravasation phlebitis caused by long-term use of a remaining needle, infusion of vein high-nutrition medicaments, infusion of chemotherapeutic medicaments, infusion extravasation and the like. According to the medicament formula, 20-35ng of anisodamine, 6-13ml of lidocaine, 15-26mg of dexamethasone and 4-6ml of glycerin are arranged in a sterile kidney basin, sterile gauze is coated above a puncture part after being uniformly wetted, and an external preservative film is wound on the gauze. The medicinal formula has the advantages of continually and efficiently relieving local pain caused by infusion medicaments, and effectively preventing and treating local inflammation, swelling pain and stripped cable change caused by infusion.
Owner:GENERAL HOSPITAL OF JIZHONG ENERGY FENGFENG GRPCO
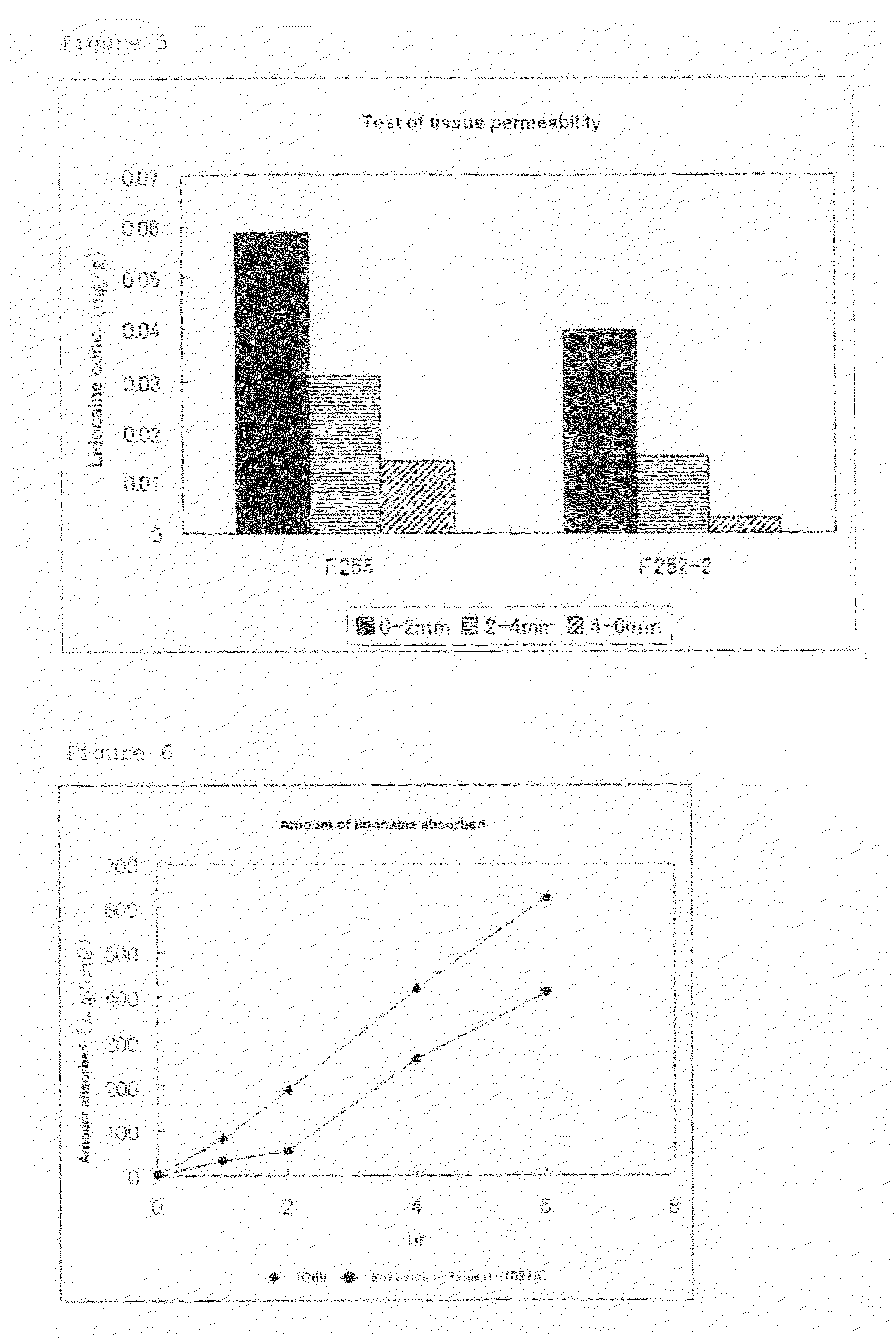
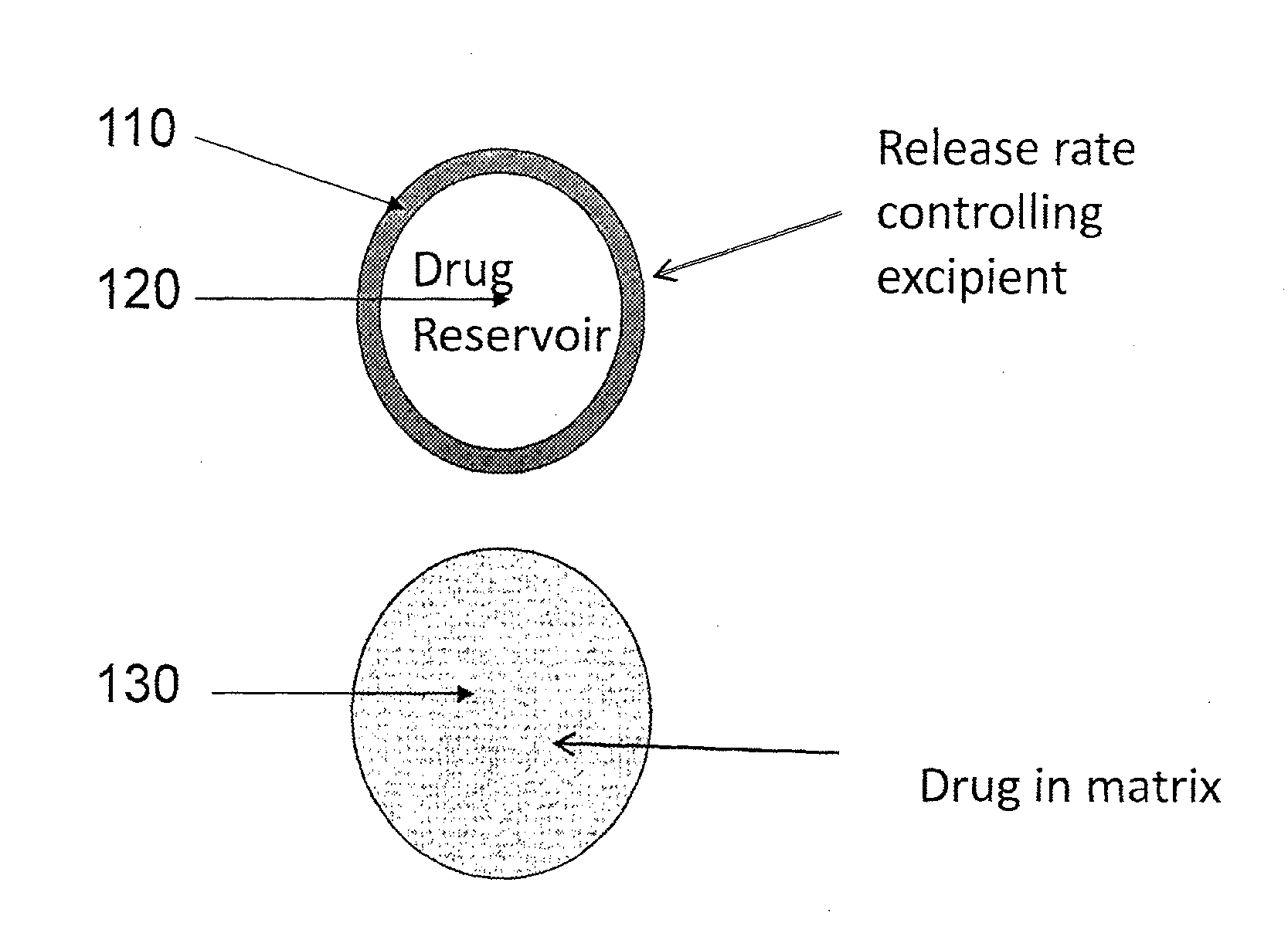
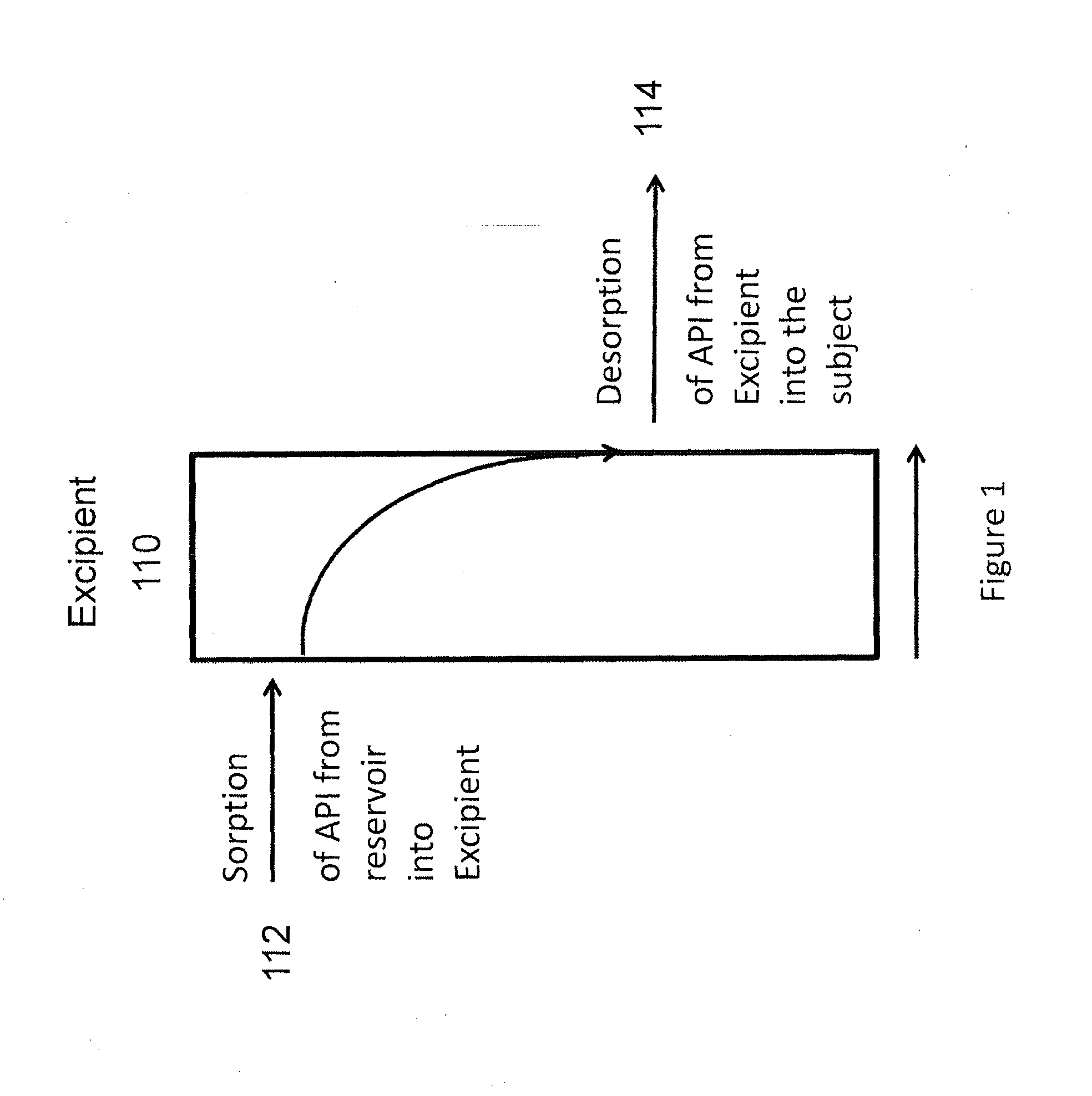
Lidocaine tape preparation
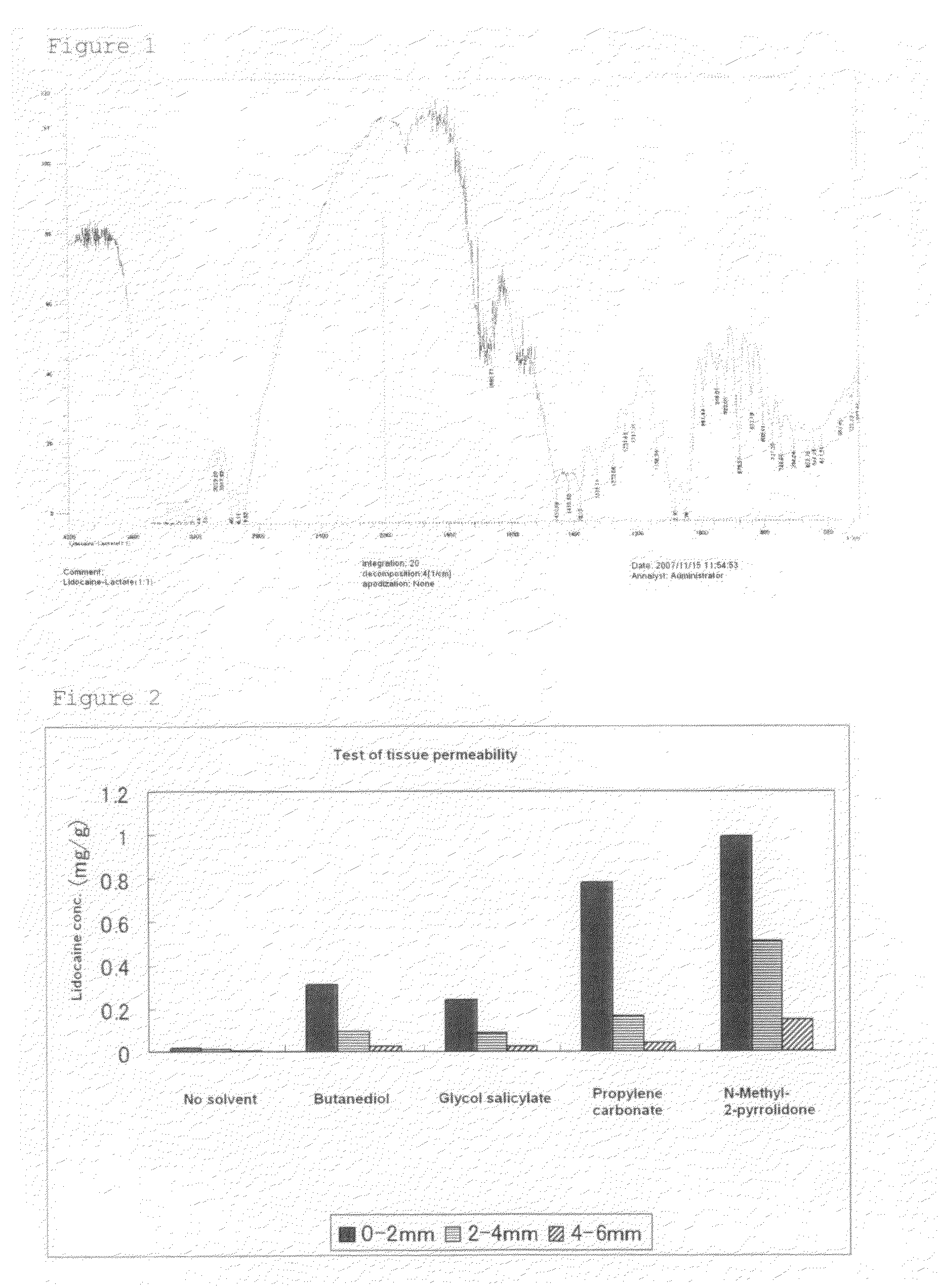
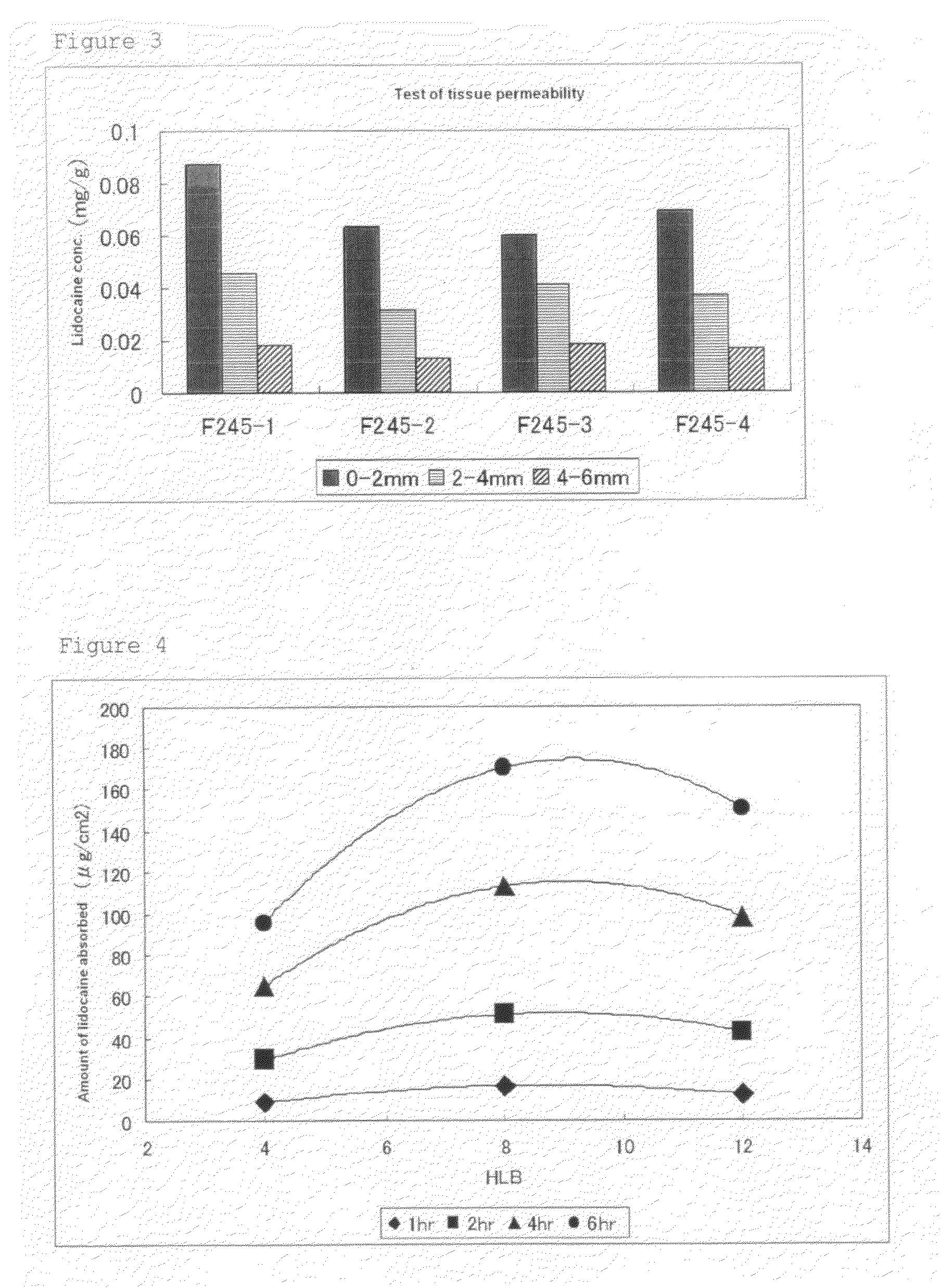
ActiveUS8722065B2Excellent and well-balanced absorbabilityExcellent and well-balanced and permeabilityOrganic active ingredientsAntipyreticHigh concentrationPharmacology
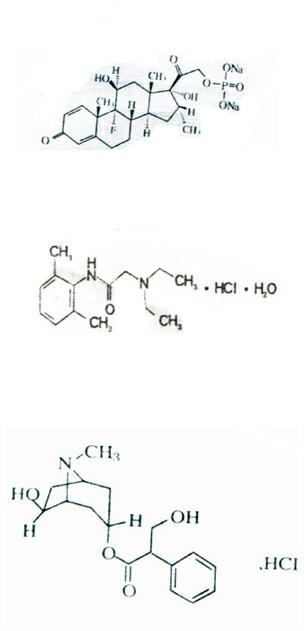
The present invention discloses a novel tape preparation containing lidocaine at a high concentration. A tape preparation containing lidocaine at a high content, which has a lidocaine content of 10 w / w % or more, can be produced by using a lactic acid salt of lidocaine, while preventing the precipitation of a crystal of lidocaine.
Owner:MEDRX CO LTD
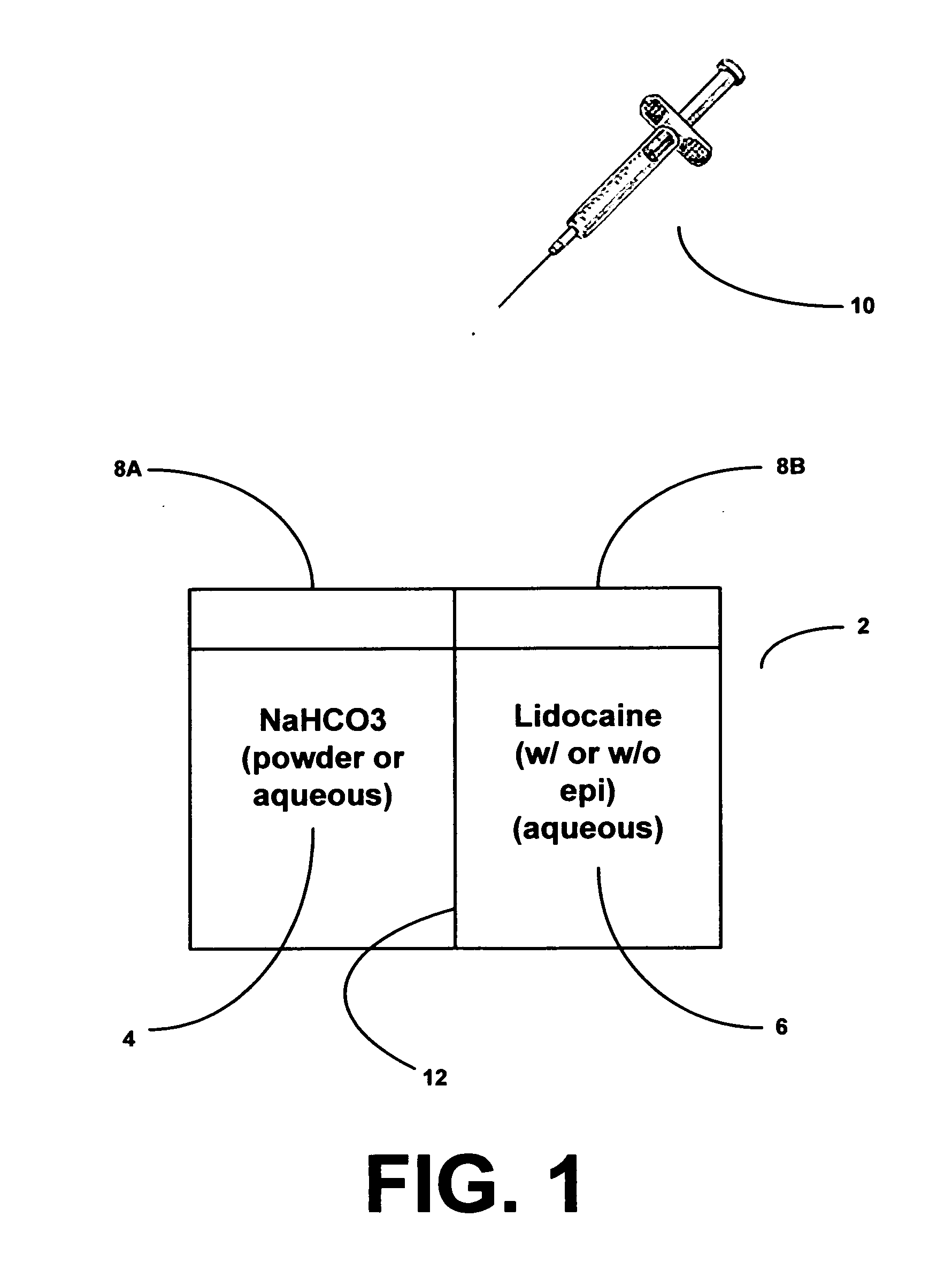
Method and apparatus for providing therapeutically effective dosage formulations of lidocaine with and without epinephrine
An apparatus for providing therapeutically effective mixtures of buffered lidocaine (with and without epinephrine) while simultaneously increasing the shelf life, having a two-chambered medicine vial, in which one chamber isolates up to 20 ml of a lidocaine solution (1% with or without epinephrine) and the other chamber isolates up to 2 ml of sodium bicarbonate (8.4%), with a fracturable wall therebetween such that the wall is fractured by bending or twisting, for mixing the lidocaine and sodium bicarbonate to create a buffered lidocaine for administration in therapeutically effective amounts, and further comprising stopper means, like a rubber stopper, for the insertion of a syringe for extraction of the mixture for administration. Also shown is a method for isolating the components for shelf life and providing mixing for an effective composition.
Owner:GIRGIS AKRAM +1
Analgesic composition for topical use
An analgesic composition, is disclosed which comprises a mixture of piroxicam, dexamethasone, ketamine, lidocaine injection, dimethyl sulfoxide, gabapentin and Vanicream™, preferably in the form of a cream or ointment. The composition is applied topically for the relief of pain of arthritis, neuropathy, post-herpetic (shingles) conditions, sore muscles, tendons and ligaments, and local reactions to insect bites or stings.
Owner:CATHCART CELEVATORON H
Implantable drug delivery compositions and methods of treatment thereof
Owner:BRAEBURN PHARMA INC
Method for treating nerve injury pain associated with shingles
Methods and compositions are offered for reducing nerve injury pain associated with shingles (herpes-zoster and post-herpetic neuralgia), where intradermal delivery of lidocaine is maintained for a predetermined period of time. The lidocaine appears to specifically affect the damaged nerve fibers, while leaving the undamaged and normal nerve fibers with retention of response to other stimuli. Lidocaine formulations are provided which allow for the necessary dosage of the lidocaine in the dermis during the period of treatment. The formulation may be covered with an occlusive or non-occlusive dressing, which protects the lidocaine formulation from mechanical removal and enhances the transport of the lidocaine into the dermis. Long term relief is realized after maintenance of the administration of lidocaine has been terminated.
Owner:HIND HEALTH CARE INC
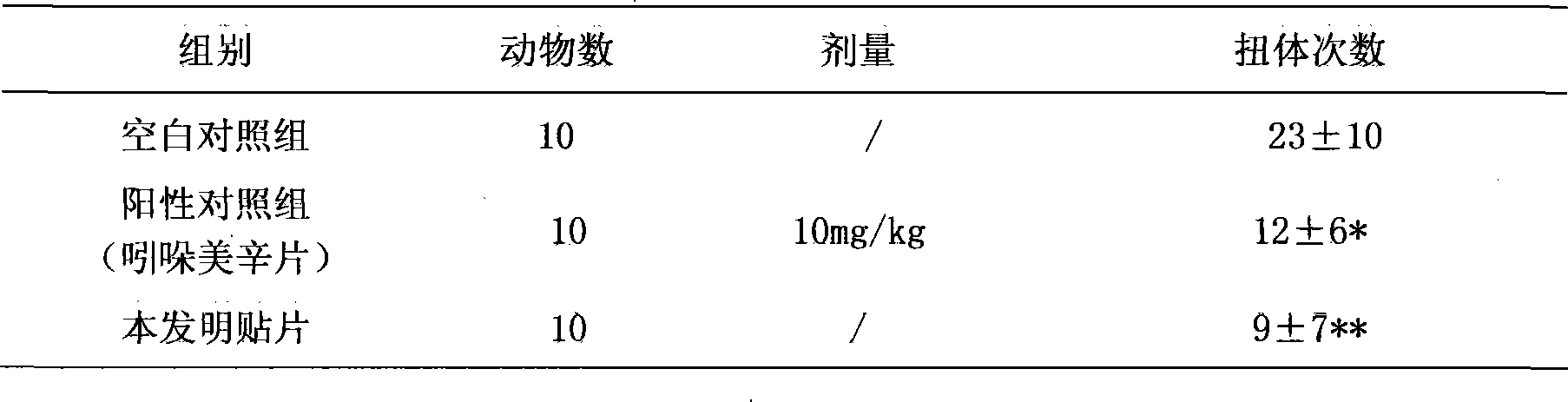
Compound transdermal patch used for curing acute and chronic inflammatory pain
InactiveCN101530401AImprove stabilityPromote absorptionOrganic active ingredientsAntipyreticTransdermal patchTreatment effect
The invention discloses a compound transdermal patch used for curing acute and chronic inflammatory pain. The patch comprises a compound penetrating agent and a pressure sensitive adhesive and is characterized by also comprising Xylocaine or hydrochloride thereof and sodium salt or sylvite of diclofenac. The transdermal patch contains the following components by weight percentages: 1-20 percent of Xylocaine or the hydrochloride thereof, 1-20 percent of the sodium salt or sylvite of diclofenac, 1-30 percent of compound penetrating agent and 40-49 percent of pressure sensitive adhesive. Aiming at overcoming the defects of the existing medicinal preparations in treatment and use, the invention provides a compound transdermal patch that is used for curing acute and chronic inflammatory pain, can act on local human body and quickly permeate, and is good in treatment effect, safe and convenient in use.
Owner:中山健康基地孵化器管理有限公司
Multifunctional microemlusion gel preparation and preparation process thereof
InactiveCN103655459AImprove skin penetrationImprove performanceAntimycoticsAntipyreticIndometacinActive agent
The invention discloses a multifunctional microemlusion gel preparation and a preparation process thereof, and belongs to the technical field of medicines. The preparation mainly comprises bulk pharmaceutical chemicals (such as non-steroidal anti-inflammatory drugs-diclofenac sodium, ibuprofen, indometacin, antifungal drugs-ornidazole, antiviral drugs-ganciclovir, hormone drugs-dexamethasone, local anesthesia drugs-lidocaine and irritants-menthol), a cationic polymer and a microemlusion, can further comprise gel or a thickener, and can be used for transdermal drug delivery and local drug delivery. The preparation process is simple, convenient, good in stability and pollution-free. Compared with existing cream and gel, the preparation has the advantages that a novel action mechanism is adopted, the accumulative penetration amount of unit area of drugs is remarkably increased, a certain slow-release effect is achieved, and the drug delivery frequency and the drug delivery amount can be reduced; a chemical penetration enhancer and a conventional preservative are not added, a certain bacterial inhibition effect is achieved, the skin irritation is avoided, and the use safety of the drugs is improved.
Owner:CHINA PHARM UNIV
Method for extracting mesenchymal stem cells from trace human fatty tissues and massively culturing
InactiveCN101974486ARelieve painOvercoming barriers to access to adipose tissueSkeletal/connective tissue cellsAnimal productBiology
The invention belongs to the field of biomedicine, and in particular discloses a method for extracting mesenchymal stem cells from trace human fatty tissues and massively culturing. The method for extracting mesenchymal stem cells from trace human fatty tissues comprises the steps of: locally narcotizing in small area by injecting lidocaine, extracting trace fatty tissues of a patient by using a micro draining needle; then soaking with a phosphate buffer, centrifugally washing and obtaining fatty tissues in a sterile state; and digesting with collagenase, centrifuging with gradient and filtering and then culturing in an in-vitro culturing system. The invention effectively reduces the pain of the patient, eliminates the barrier that the thin patient can not obtain the fatty tissues, avoids using animal products in the whole cell culturing system, especially serum-free products, and meets the demands on future clinical safety application.
Owner:王泰华
Slow-release preparation for postoperation analgesia and preparation method of slow-release preparation
ActiveCN108379269AProlong the action timeImprove bioavailabilityAntipyreticAerosol deliveryProcaineSide effect
The invention discloses a slow-release preparation for postoperation analgesia and a preparation method of the slow-release preparation. The slow-release preparation is mainly prepared from the following raw materials: 10-15 parts of phospholipid, 4-7 parts of glyceride, 0.3-5 parts of poloxamer, 0.8-5 parts of a cosolvent and 0.5-2 parts of a local anesthetic mixture of cocaine, procaine and lidocaine, wherein the mass ratio of the cocaine to the procaine to the lidocaine in the local anesthetic mixture is 1:1:(4-7.5). The phospholipid is phosphatidylcholine, and preferably soybean phosphatidylcholine; the glyceride is dinolin; the cosolvent is ethanol; the slow-release preparation is easy to inject, and medicines can be slowly released in situ; the action time of the medicines is prolonged, the administration times are reduced, and the bioavailability of the medicines is improved; meanwhile, toxic and side effects caused by too high medicine concentrations can be avoided.
Owner:武汉百纳礼康生物制药有限公司
Method for preparing lidocaine
ActiveCN102070483AImprove responseHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideAcetyl chloride
The invention provides a method for preparing lidocaine. The method comprises the following steps: using 2,6-dimethylaniline and chloroacetic chloride as raw materials to prepare an intermediate, namely acetyl chloride-2,6-dimethylaniline, and using the prepared intermediate and diethylamine to react and obtain lidocaine, wherein acetone is used as solvent and carbonate is used as catalyst in thereaction process. The method of the invention has simple synthetic technology and does not require the complicated step that the intermediate is washed with acid firstly and washed with base secondlyin the post-treatment, thus avoiding unnecessary loss. Therefore, the yields of the intermediate and lidocaine prepared by the method are higher; and the prepared lidocaine has high purity which is more than 99%, and good industrial application prospect. In addition, the method of the invention uses acetone as solvent, thus the solvent is non-toxic basically and environmentally friendly, has no stimulation and can be recycled.
Owner:BENGBU BBCA MEDICINE SCI DEV
Compositions and methods for treating neuropathic sensory loss
The present invention relates to methods of reducing the effects of neuropathically induced negative sensory phenomena (NSP). NSP are manifested as the decreased ability to feel light touch, pain, proprioception, vibration, warmth / heat, and coolness / cold. The NSP are treated by application of an anesthetic. The anesthetic is preferably a benzoic acid-based anesthetic. Specifically, a patch containing about 5% lidocaine may be used. The anesthetic is transdermally administered to a patient suffering from NSP at or near the locus of the negative sensory phenomena.
Owner:ENDO PHARMA INC
LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
InactiveCN101961316AImprove hydrophobicityHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsWater bathsMatrix solution
The invention discloses an LID-PEG-PLGA controlled-release nano microsphere and a preparation method thereof. The microsphere is the controlled-release microsphere which contains medicinal lidocaine and a degradable carrier. The degradable carrier contains polylactic-glycolic acid and PEG-2000. The mass percentage of the lidocaine in the controlled-release microsphere is 30 to 35 percent. The preparation method comprises the following steps of: preparing the carrier into matrix solution; dispersing the lidocaine into the matrix solution and preparing the lidocaine into an oil phase; mixing the oil phase and the aqueous solution of polyvinyl alcohol, and performing ultrasonic emulsification on the mixture under a water bath condition to obtain W / O-type protogala; mixing the W / O-type protogala and the aqueous solution of polyvinyl alcohol again, and further emulsifying the mixture into W / O / W-type complex emulsion; volatilizing the emulsion by reducing pressure at the normal temperature to obtain cured lidocaine-carried nano microsphere; and scattering, blending, packaging, freezing, sterilizing and the like. The medicament loading rate of the controlled-release nano microsphere can be up to 15 to 22 percent; the entrapment rate can be up to 68 to 78 percent; and the half-life period can be prolonged to 3 to 4 days. Therefore, the microsphere has relatively good effect of burst in the first day after the microsphere is taken and good effect of slow release in later days.
Owner:ARMY MEDICAL UNIV
Production of bitter principle derivatives
PendingUS20190185416A1Carbamic acid derivatives preparationOrganic compound preparationCarboxylic acidBitter taste
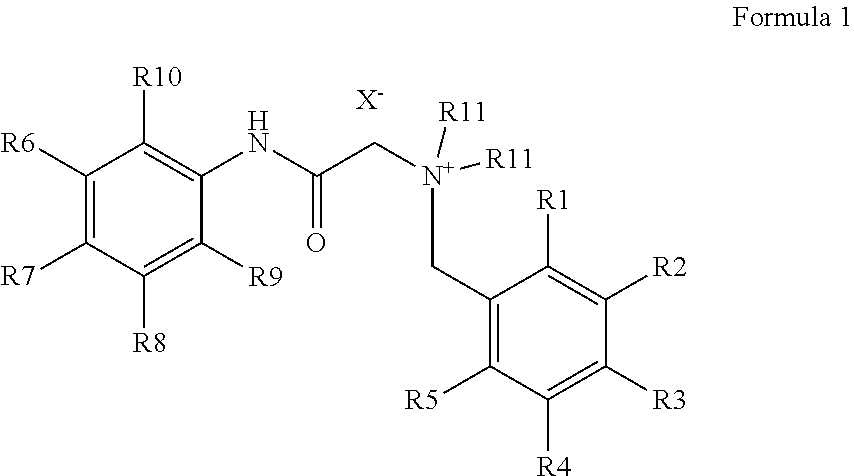
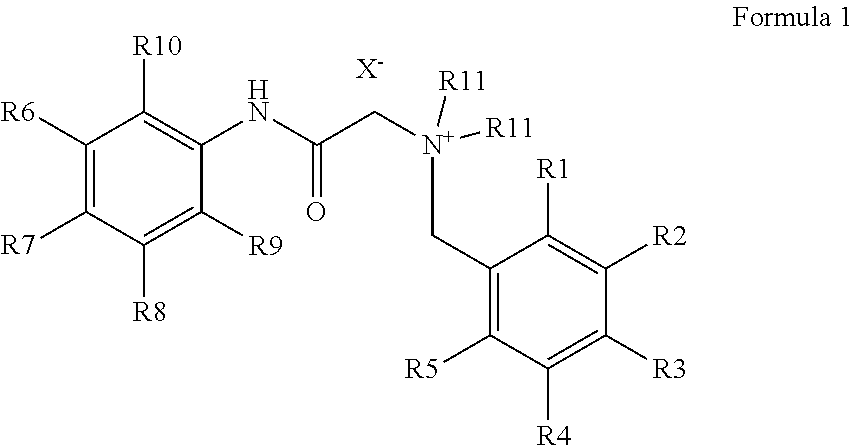
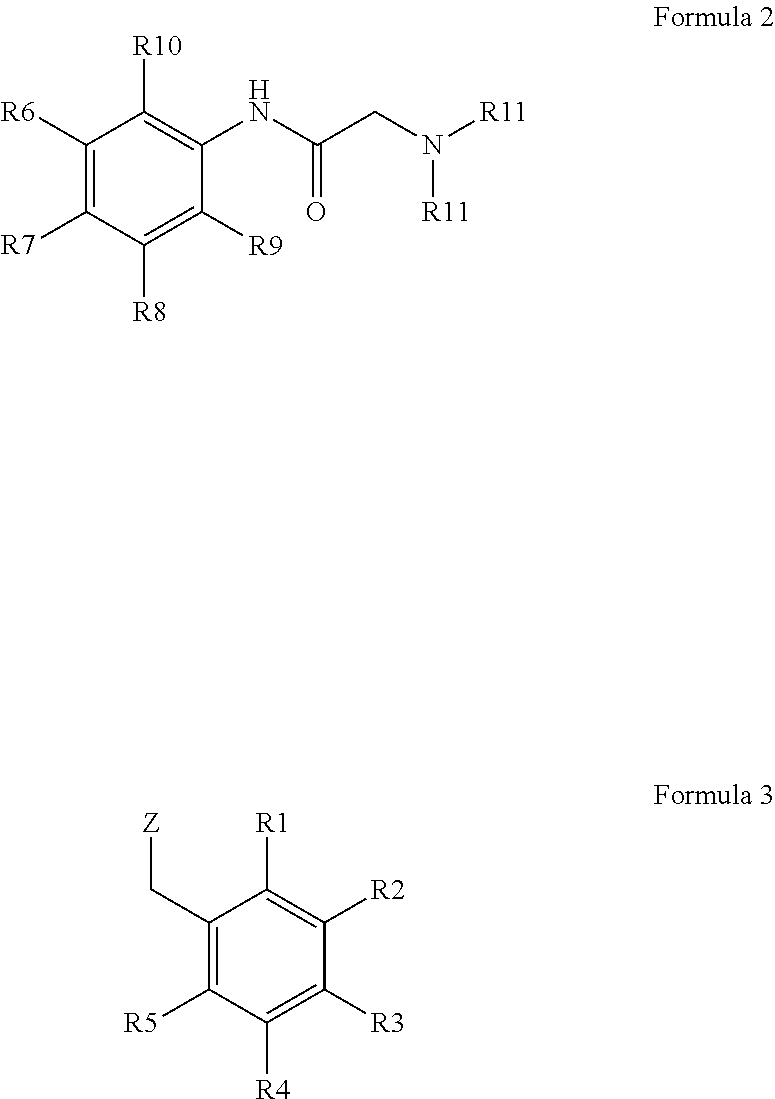
It is an object of the present invention to introduce carboxylic acid-functionalities suitable for coupling into the denatonium structure by means of simple synthesis, namely the synthesis of bitter principle derivatives based on the denatonium structure according to formula 1:For example, according to the invention, lidocaine derivatives may be reacted with carboxylated benzyl halogenides. The carboxylated denatonium derivatives of the present invention are especially applied in medicine, biology, medical engineering as well as cosmetics, the pharmaceutical, chemical, and foodstuff industry.
Owner:JULIUS MAXIMILIANS UNIV WURZBURG
Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
InactiveCN108159055AEnsure safetyIncrease concentrationOrganic active ingredientsPharmaceutical delivery mechanismProcainePhospholipid
The invention provides a long acting drug delivery system, which comprises fulvestrant or derivatives thereof and takes phospholipid as a sustained release material. The invention mainly discloses a high concentration formula of fulvestrant or derivatives thereof. The main component is fulvestrant or derivatives thereof. The sustained release material is different phospholipids or a mixture of phospholipids and different kinds of plant oil. The solvent is at least one of ethanol, ethyl lactate, 1,2-propylene glycol, and ethyl acetate. The analgesic is benzyl alcohol, lidocaine, procaine, or ropivacaine. The viscosity of the formula is 20-45 mPa.s, and the concentration of the formula is 60-300 mg / mL. The antioxidant of the formula is Ve, lipoic acid, and the like. The phospholipids can increase mutual solubility and has a sustained release function. The preparation is prepared in an aseptic filtration mode.
Owner:XIAN LIBANG PHARMA TECH
Compound externally applied tetrodotoxin ointment and its prepn
InactiveCN101066340AEliminate swellingPrevent seepageHydroxy compound active ingredientsAerosol deliveryMyrrhVitamin C
The externally applied compound tetrodotoxin ointment is prepared with globe fish liver oil, vaseline, frankincense, myrrh, dragon's blood, notoginseng, safflower, catechu, borneol, vitamin B1, vitamin B2, vitamin C, moroxydine, dexamethasone and lidocaine in certain weight proportion. It has very high curative rate on trauma and traumatic infection, including intractable ulcer and fistula, high effective rate and curative rate on skin diseases caused by bacteria, fungi, viruses, etc, and certain treating effect on serious furuncle, luetic balanitis, etc.
Owner:苏振芳
Autologous-repair nutrient injection and application method thereof
PendingCN109528642ASolve potential safety hazardsImprove timelinessOrganic active ingredientsHydrolysed protein ingredientsVitamin CMedicine
The invention discloses autologous-repair nutrient injection. The injection comprises the following main components: collagen and physiological saline. By further improvement, the injection can further comprise lidocaine, reduced glutathione, vitamin C, beta thymosin, and hyaluronic acid with low molecular weight. The invention solves the problems that absorbable injectants have no lasting effectand need repeated injection, and also solves the problem that the non-absorbable injectants have potential safety hazards. The injection completely performs autologous repair on the growing skin, causes no rejection and allergy, and has long timeliness and natural effect. The injection is most suitable for people who pursue safe and natural beautifying, people who seek to recover within a short period without influencing daily work and life, and people who seek to maintain their own characteristics with exquisite beautifying.
Owner:上海欧邦医疗管理有限公司
Compound lignocaine emulsifiable paste and preparing technique
InactiveCN101209250AImprove transdermal absorption rateReduce waiting timeOrganic active ingredientsAerosol deliveryMedicineLidocaine
The invention provides a compound lidocaine cream and the preparation technique. The invention selects a new cream formulation and technique. The product which is prepared by the technique has stable quality, the formulation and the technique are reasonable and stable, and the method overcomes the shortcomings of the original technique and can meet the needs of industrial large-scale production. The product of the invention can shorten the anesthesia time when in clinical use, thus greatly reducing the waiting time of the patients and being much easier to be accepted by the patients.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Stimulant/desensitizer swabs
InactiveUS20060029648A1Quickly stimulate the penisQuick installationBiocideAnimal repellantsPenisStimulant
A method and pharmaceutical composition for treating the sexual dysfunction of premature ejaculation is disclosed. The pharmaceutical composition consists of a solution of one or more stimulants and a desensitizer. The stimulant is selected from the group comprising spearmint solution, peppermint solution, cool mint solution, pepper solution, alcohol, rubbing alcohol, ether, or any solution that will stimulate the penis to cause an erection. The desensitizer is selected from the group comprising benzocaine and lidocaine. The pharmaceutical composition is enclosed within a cotton swab applicator and is applied directly to the penis with the cotton swab applicator.
Owner:TSAUR GARRY
Targeted delivery of lidocaine and other local anesthetics and a method for treatment of cough and tussive attacks
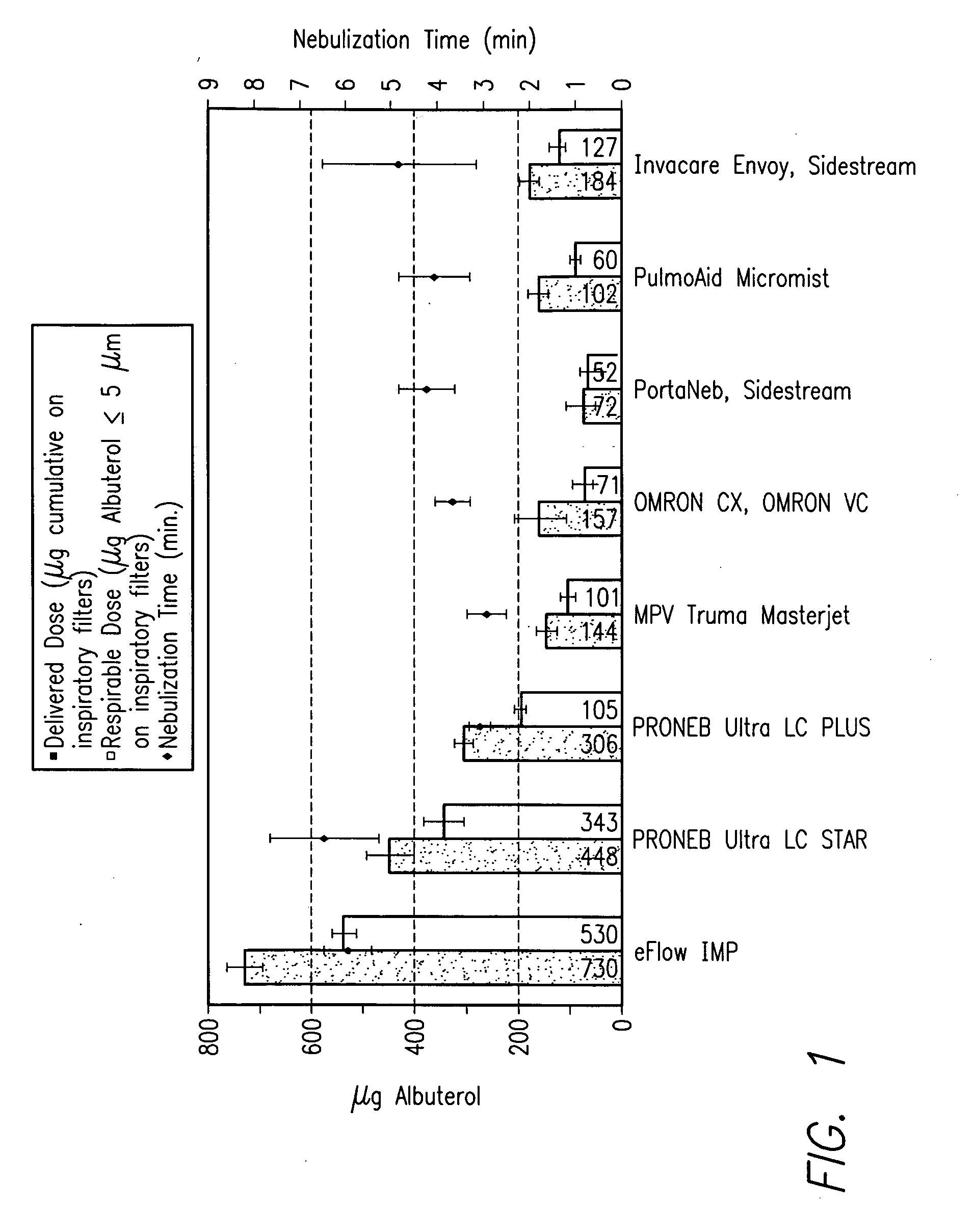
An anti-tussive nebulized solution for targeted delivery of lidocaine into conducting and central airways. A method for treatment of cough and tussive attacks or episodes using said lidocaine solution. A nebulized lidocaine solution administered in daily dose from about 10 mg to 80 mg of lidocaine dissolved in a saline and nebulized into an aerosol having a mass median aerodynamic diameter 3 μm to 10 μm and a geometric standard deviation less than 1.7 using an electronic nebulizer.
Owner:GILEAD SCI INC
Disinfecting paste for dissolving wart and eliminating wart
The invention relates to cream for dissolving wart, relieving neoplasm and detoxicating, which comprises the following components by weight portion: 0 to 3.0 portions of licorice, 0 to 3.0 portions of scutellaria, 0 to 3.0 portions of phellodendron amurense, 0 to 3.0 portions of rhubarb, 0 to 1.0 portion of menthol, 2 to 60 portions of sodium hydroxide (or potassium hydroxide), 0 to 10 portions of calcium hydroxide, and 0.75 to 1.0 portion of levobupivacaine hydrochloric acid (or toad venom, bupivacaine hydrochloric acid, lidocaine, dicaine or procaine or other local anesthetics). The sodium hydroxide (or the potassium hydroxide) and the calcium hydroxide of proper concentration can quickly dissolve protein, and melt the wart, the neoplasm and various inflammatory tissues in time from 1 minute to tens of minutes; the scutellaria, the phellodendron amurense and the rhubarb resist bacteria and virus; the licorice and the scutellaria adjust immune function, and inhibit adverse tissue reaction; the toad venom has anti-inflammatory, anti-cancer and local anesthetic functions; the menthol is fresh and cool, and can deodorize and stop pain; and the levobupivacaine hydrochloric acid or other local anesthetics has local anesthetic and paregoric functions.
Owner:谭国梁
Freeze dry preparation containing diclofenac salt and lidocaine and its preparation method
InactiveCN1689561AReduce volumeSolve solubilityOrganic active ingredientsPowder deliveryOrganic solventAlcohol
The freeze dried powder for injection containing diclofenac salt and lidocaine is prepared with Tween-80, pharmaceutically acceptable solution pH regulator, solution of diclofenac salt and lidocaine of pH 7.0 and in effective treating amount, and through freeze drying. The solution may contain other pharmaceutically acceptable supplementary material. The preparation of the present invention has stable performance, easy transportation, long storage period, and no bad reaction caused by alcohol and other organic solvent.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Dermal medicaments application enhancer
InactiveUS20060182790A1High activityImprove bioavailabilityBiocideOrganic active ingredientsVitamin E AcetateCholesterol
A topical medicament for use in treating tissues comprising: an enhancer for facilitating non-invasive, transdermal delivery and / or enhancing metabolic effect of a therapeutic dosage of LEVULAN® KERASTICK™ (aminolevulinic acid HCl) into a tissue. The present invention provides a topical, transdermal medicament for use in treating tissues comprising an enhancer for facilitating non-invasive, transdermal delivery of a therapeutic dosage of comprising LEVULAN® KERASTICK™ (aminolevulinic acid HCl) into a tissue for example wherein the enhancing agent is comprising L.M.X.4®The enhancer may be selected from the group consisting of lidocaine, benzyl alcohol, carbomer 940, cholesterol, hydrogenated lecithin, polysorbate 80, propylene glycol, trolamine, vitamin E acetate and water or combinations thereof. The present invention, also, provides a pharmaceutical composition useful for treating tissues in humans and animals which comprises a therapeutically effective amount of LEVULAN® KERASTICK™ (aminolevulinic acid HCl) or pharmaceutically acceptable salt thereof in combination with a synergistically effective amount of at least one enhancer or pharmaceutically acceptable carrier wherein said enhancer is selected from the group consisting of lidocaine, benzyl alcohol, carbomer 940, cholesterol, hydrogenated lecithin, polysorbate 80, propylene glycol, trolamine, vitamin E acetate and water. The invention, also, provides a method of treating or preventing a human or animal afflicted by a diseases comprising topically administering a pharmaceutical composition which comprises of a therapeutically effective amount of aminolevulinic acid HCl in combination with an enhancer and / or a pharmaceutically acceptable carrier. The invention, also, provides a method wherein the composition further comprises a synergistically effective amount of an enhancer and / or pharmaceutically acceptable salt thereof wherein said enhancer is selected from the group consisting of lidocaine, benzyl, carbomer 940, cholesterol, hydrogenated lecithin, polysorbate 80, propylene glycol, trolamine, vitamin E acetate, water and combinations thereof.
Owner:MAYORAL FLOR
Injection for curing angiomatous
InactiveCN101284127AOrganic active ingredientsPeptide/protein ingredientsDexamethasoneCurative effect
The invention relates to an injection for treating hemangioma, which is prepared from interferon, dexamethasone, adrenaline, lidocaine and water for injection. The preparation method comprises dissolving pingyangmycin (8 mg) into 2% lidocaine injection to reach volume of 3 ml; dissolving interferon (3,000,000 unit) into water for injection to reach volume of 3 ml; mixing diluted pingyangmycin (2 ml), diluted interferon (1 ml), dexamethasone (1 ml), adrenaline (0.5 ml), 2% lidocaine (2.5 ml) and water for injection (3 ml); and making into injection. The injection has good curative effect on hemangioma, convenient application, and no restriction to medical treatment condition.
Owner:广水市第一人民医院
Suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid
ActiveCN104013574AStrong penetrating powerImprove bioavailabilityOrganic active ingredientsSolution deliveryCelluloseChlorhexidine
The invention discloses a suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid. The formula comprises a sulfadiazine metallic compound, a hyaluronic acid substance, an analgetic, a dispersing agent aid, a suspension aid and water, wherein the sulfadiazine metallic compound comprises sulfadiazine silver and sulfadiazine zinc; the hyaluronic acid substance is sourced from biological fermentation and animal tissue extraction and the like and can be hyaluronic acid or hyaluronate or crosslinked hyaluronic acid or crosslinked hyaluronate; the analgetic comprises ropivacaine, bupivacaine, levobupivacaine, lidocaine and other local anesthetics or combination of the local anesthetics; the dispersing agent aid comprises chlorhexidine, Tween series, oleic acid or sodium oleate; the suspension aid can comprise glycerinum, celluloses, poloxamer series and hyaluronic acid substances. The suspension preparation is mainly used for burn, scald or the surface of a wound caused by explosion or anabrosis, has the main clinical characteristics that pain is rapidly relieved, the surface of the wound is subjected to rapid film formation and rapid healing of the wound is promoted, and the conventional main administration modes refer to spraying, smearing and coating.
Owner:LIPONT PHARMA
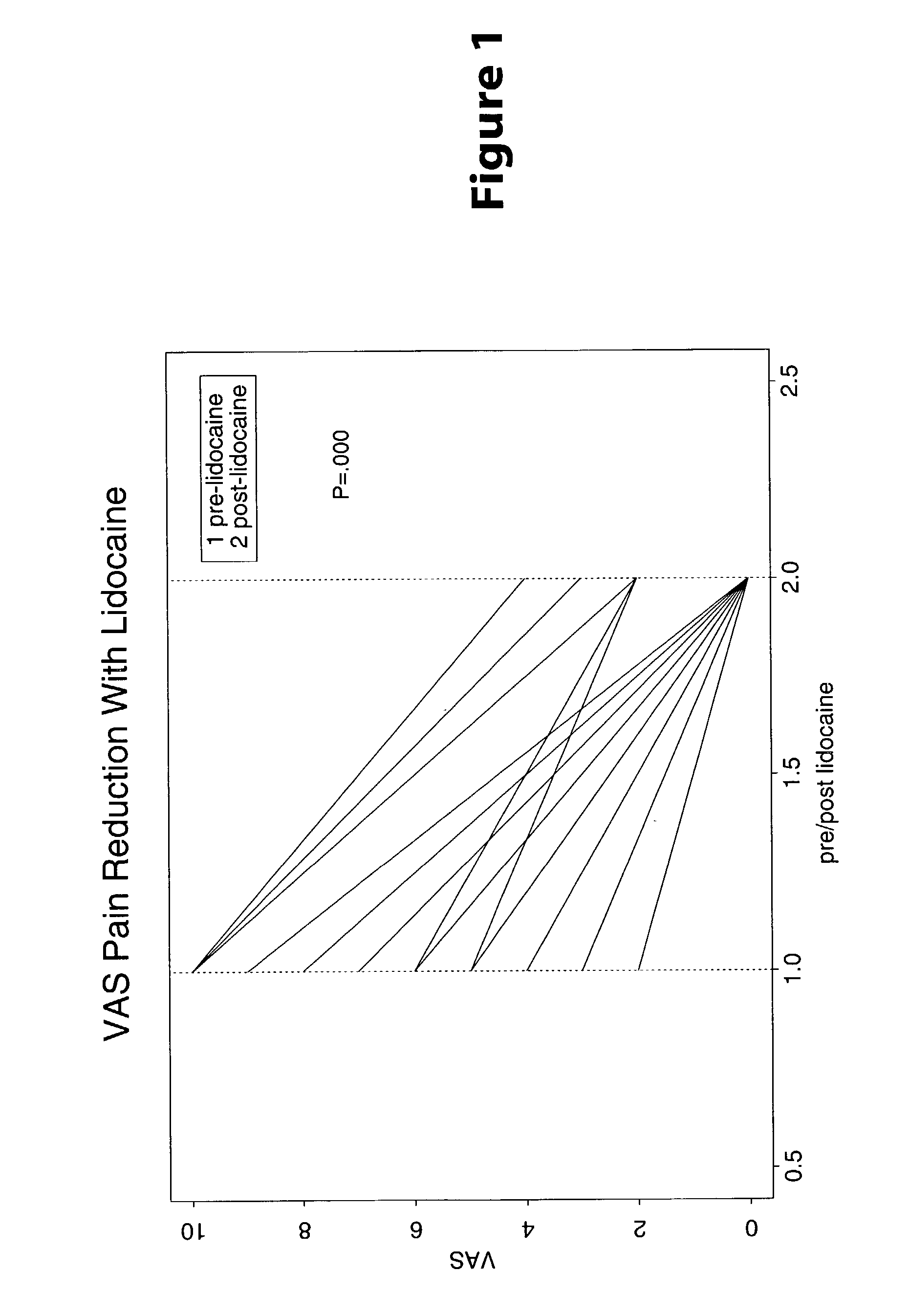
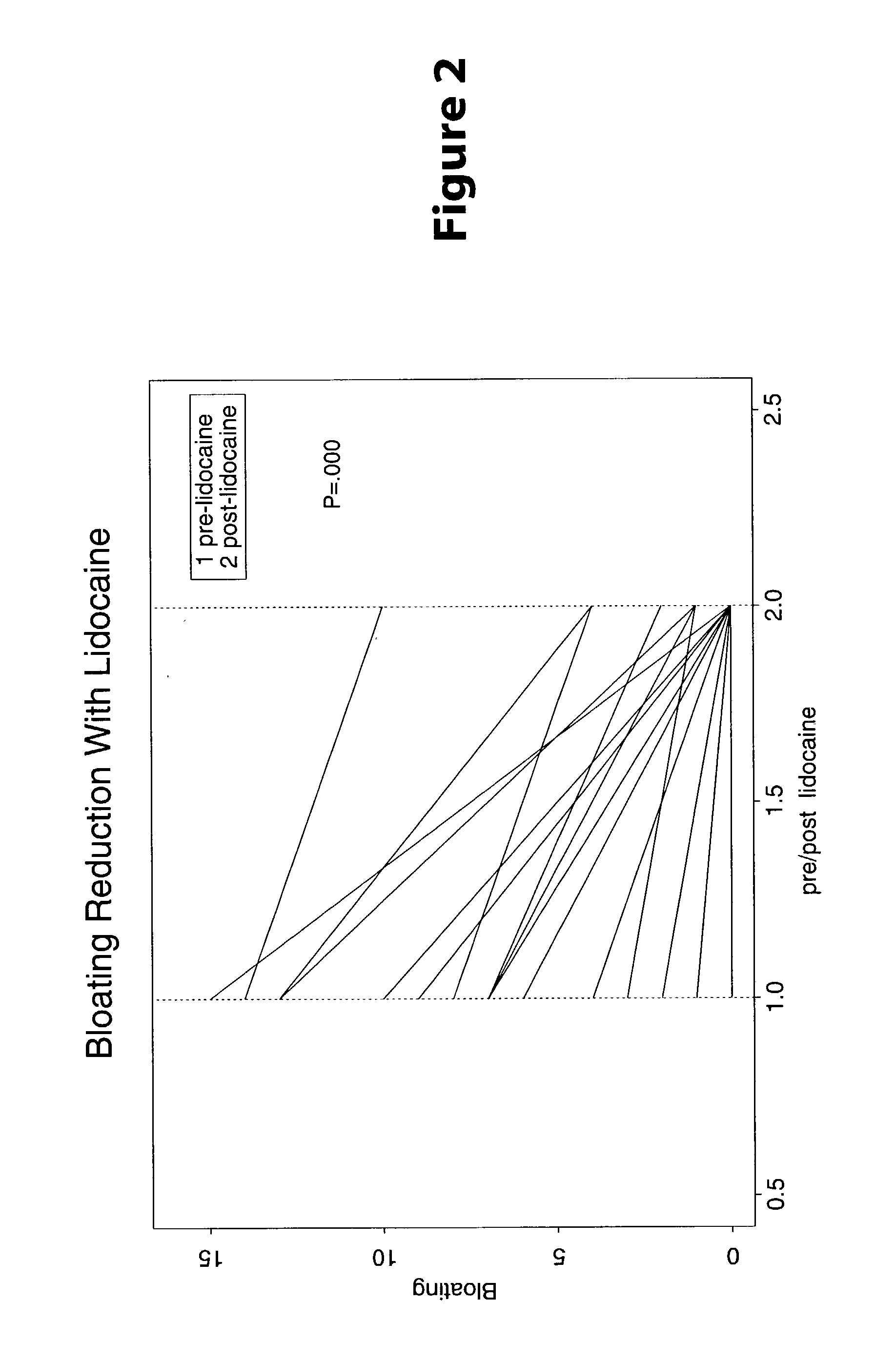
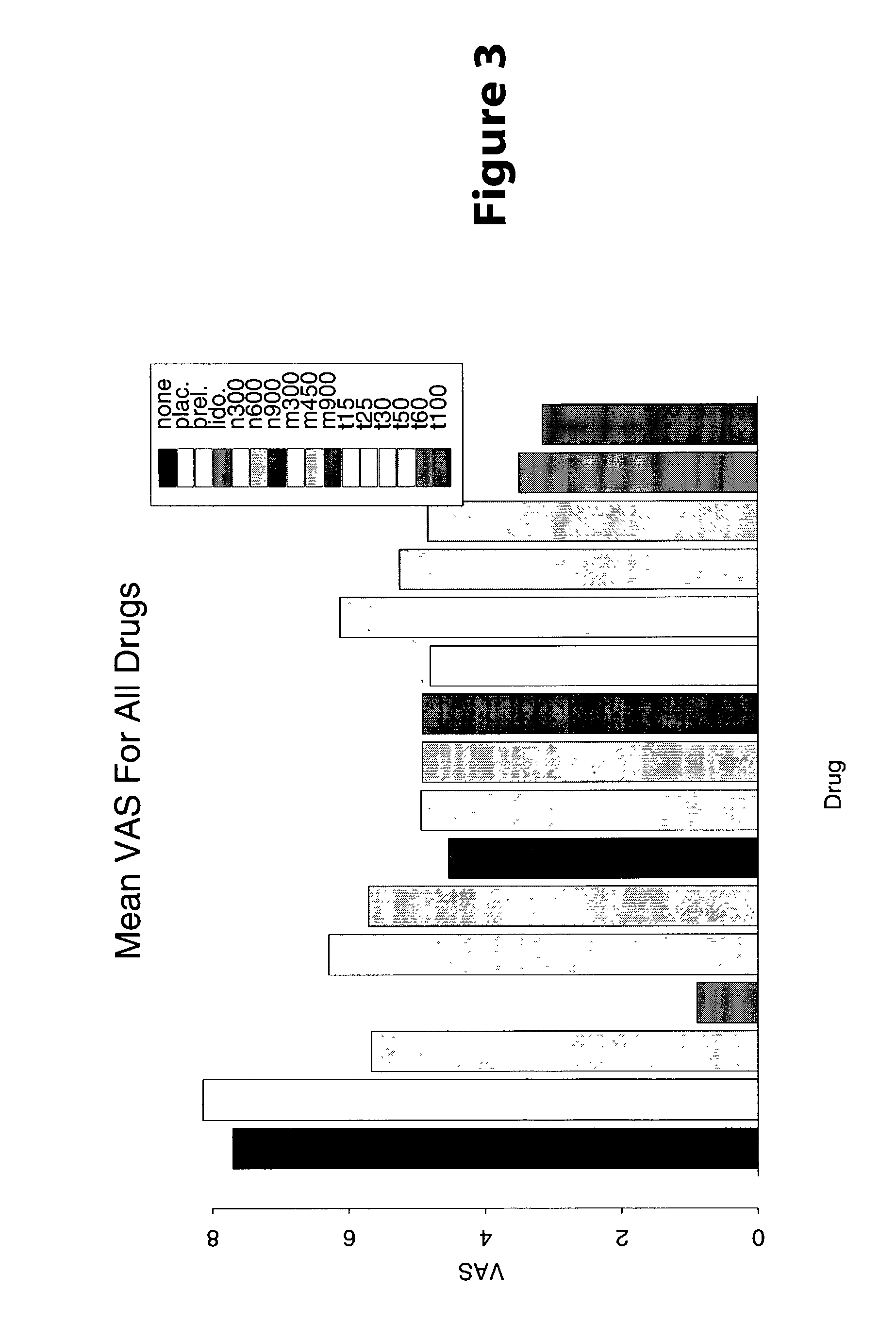
Treatment of visceral pain, E.G., irritable bowel syndrome with nerve-acting agents
Methods are provided for use in treating humans suffering from irritable bowel syndrome. In the subject methods, an effective amount of a nerve-acting agent, e.g., lidocaine, topiramate, mexiletine and gabapentin, etc., is administered to a human suffering from irritable bowel syndrome. Also provided are pharmaceutical compositions and kits for use in practicing the subject methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com