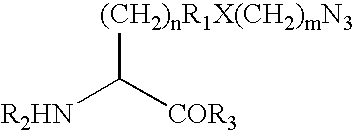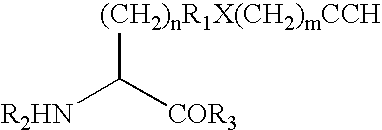Patents
Literature
2260 results about "Interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interferons (IFNs) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
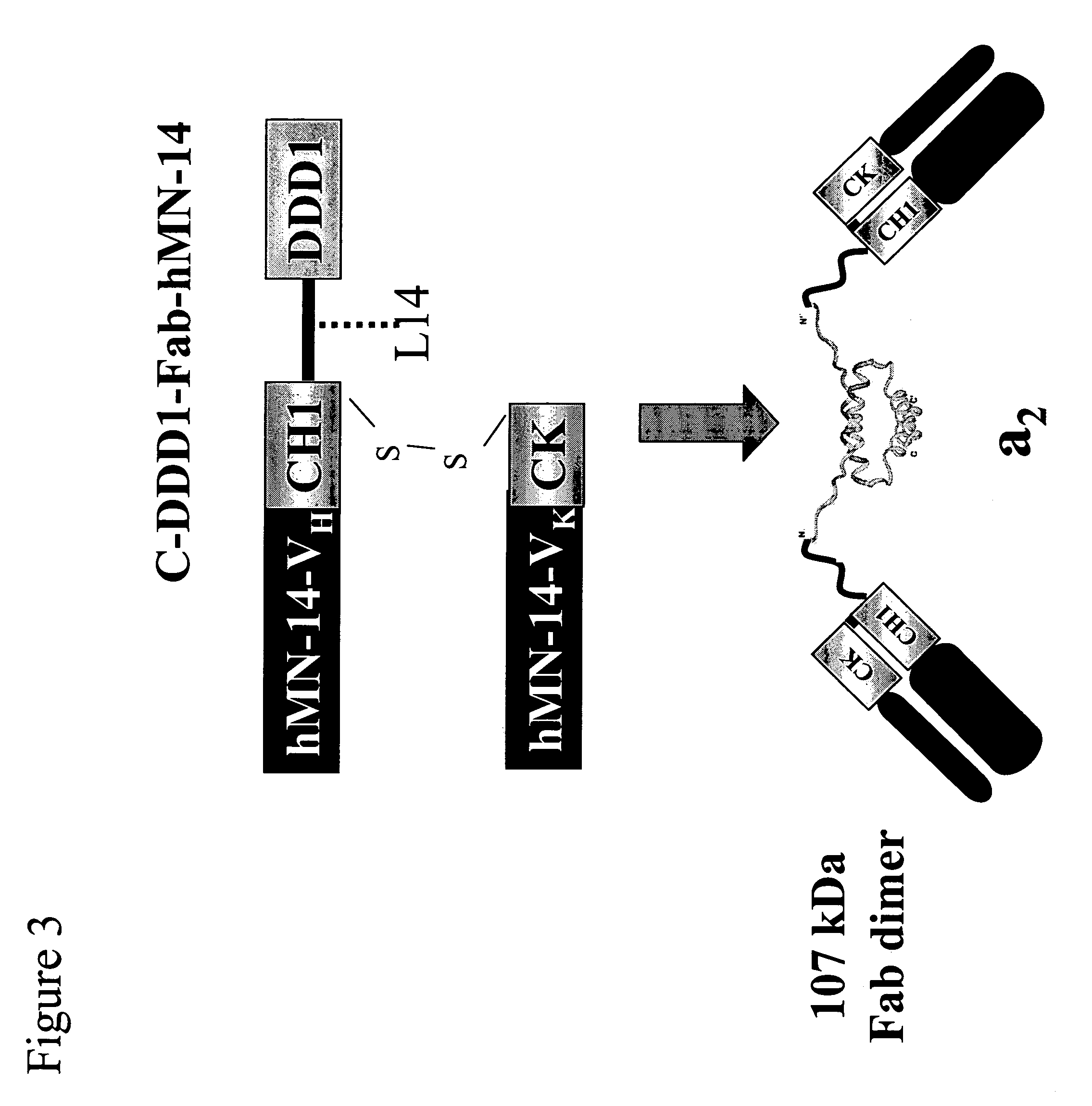
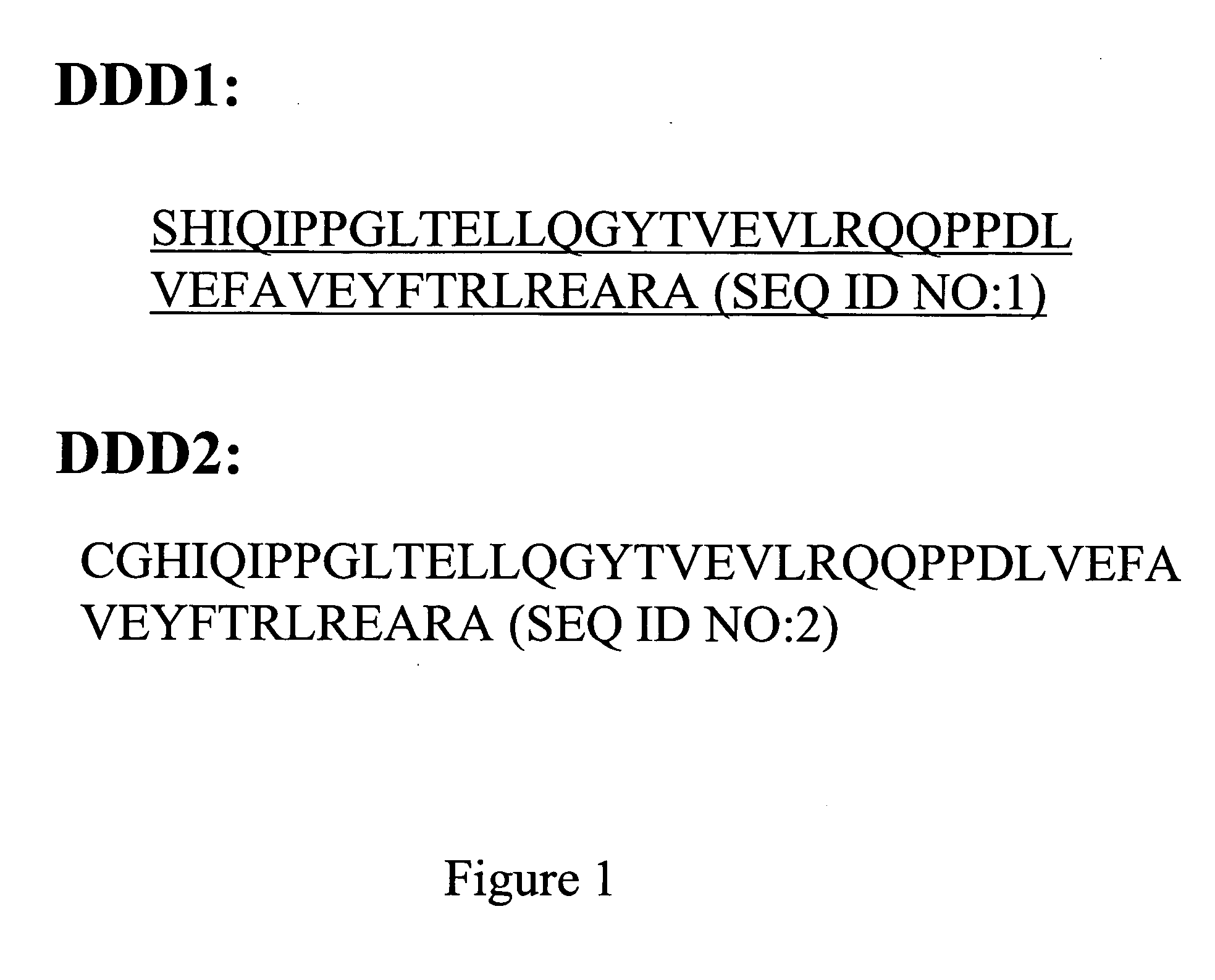
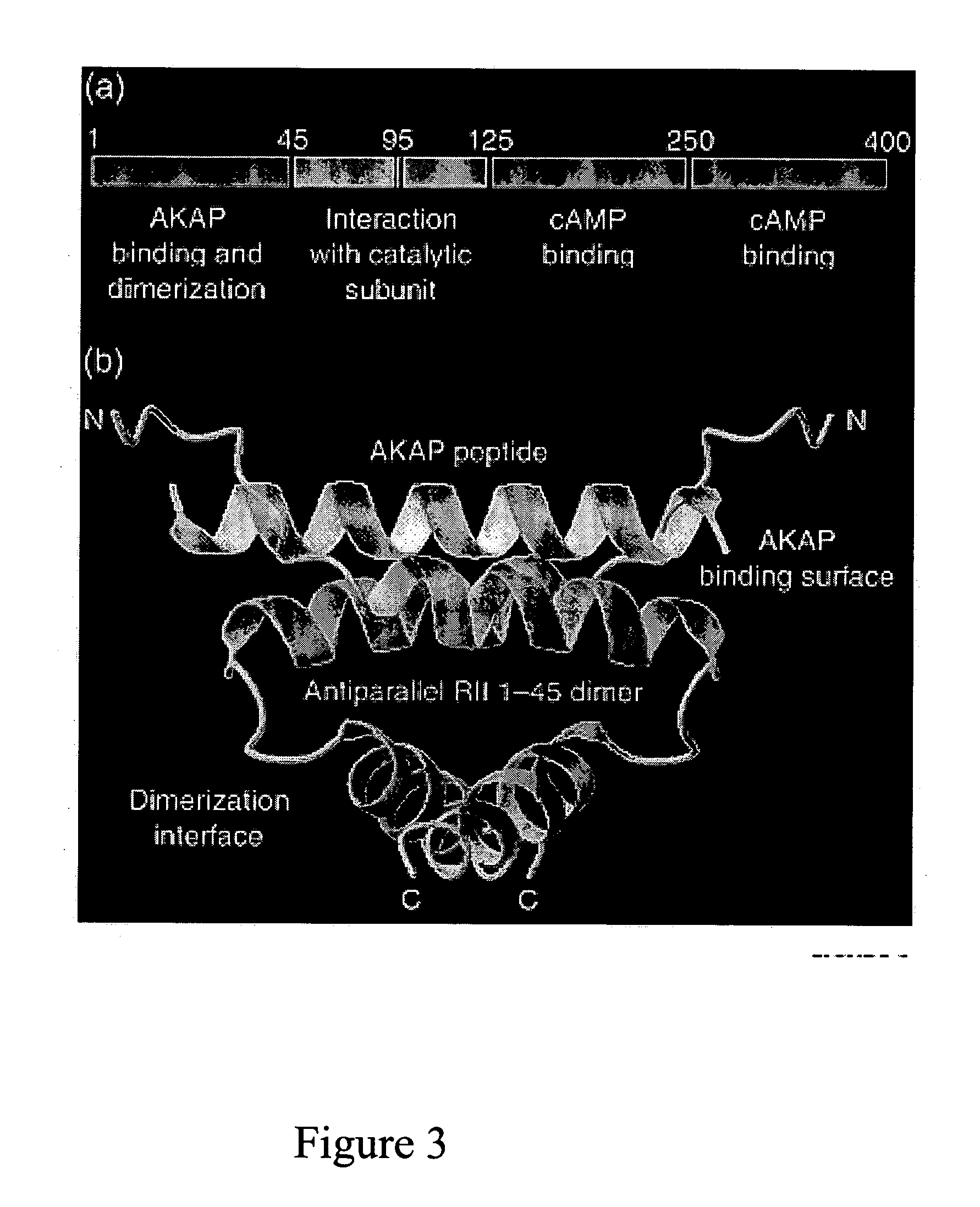
Stably tethered structures of defined compositions with multiple functions or binding specificities
Owner:IBC PHARMACEUTICALS INC
Purine derivative
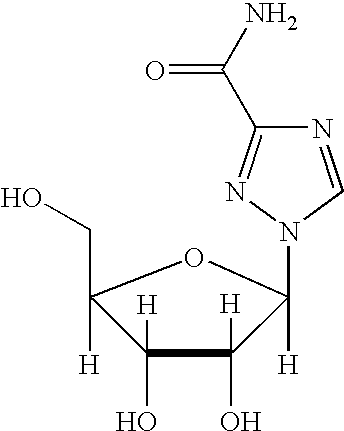
PCT No. PCT / JP97 / 02310 Sec. 371 Date Mar. 3, 1998 Sec. 102(e) Date Mar. 3, 1998 PCT Filed Jul. 3, 1997 PCT Pub. No. WO98 / 01448 PCT Pub. Date Jan. 15, 1998This invention relates to novel purine derivatives of formula (I): where R2 and R9 are hydrocarbon groups, R6 is an amino group and R8 is a hydroxyl, or acyloxy group. These purine derivatives are effective at promoting secretion of interferon in patients, and can be used to treat diseases against which interferon is effective.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Methods for generating stably linked complexes composed of homodimers, homotetramers or dimers of dimers and uses
ActiveUS7550143B2Improve functionalityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsHomotetramerAptamer
The present invention concerns methods and compositions for stably tethered structures of defined compositions, which may have multiple functionalities and / or binding specificities. Particular embodiments concern homodimers comprising monomers that contain a dimerization and docking domain attached to a precursor. The precursors may be virtually any molecule or structure, such as antibodies, antibody fragments, antibody analogs or mimetics, aptamers, binding peptides, fragments of binding proteins, known ligands for proteins or other molecules, enzymes, detectable labels or tags, therapeutic agents, toxins, pharmaceuticals, cytokines, interleukins, interferons, radioisotopes, proteins, peptides, peptide mimetics, polynucleotides, RNAi, oligosaccharides, natural or synthetic polymeric substances, nanoparticles, quantum dots, organic or inorganic compounds, etc. Other embodiments concern tetramers comprising a first and second homodimer, which may be identical or different. The disclosed methods and compositions provide a facile and general way to obtain homodimers, homotetramers and heterotetramers of virtually any functionality and / or binding specificity.
Owner:IBC PHARMACEUTICALS INC
Stably tethered structures of defined compositions with multiple functions or binding specificities
ActiveUS20060228300A1Reduce exposureReduce deliveryAntibacterial agentsSenses disorderAntibody fragmentsBinding peptide
The present invention concerns methods and compositions for stably tethered structures of defined compositions with multiple functionalities and / or binding specificities. Particular embodiments concern stably tethered structures comprising a homodimer of a first monomer, comprising a dimerization and docking domain attached to a first precursor, and a second monomer comprising an anchoring domain attached to a second precursor. The first and second precursors may be virtually any molecule or structure, such as antibodies, antibody fragments, antibody analogs or mimetics, aptamers, binding peptides, fragments of binding proteins, known ligands for proteins or other molecules, enzymes, detectable labels or tags, therapeutic agents, toxins, pharmaceuticals, cytokines, interleukins, interferons, radioisotopes, proteins, peptides, peptide mimetics, polynucleotides, RNAi, oligosaccharides, natural or synthetic polymeric substances, nanoparticles, quantum dots, organic or inorganic compounds, etc. The disclosed methods and compositions provide a simple, easy to purify way to obtain any binary compound attached to any monomeric compound, or any trinary compound.
Owner:IBC PHARMACEUTICALS INC
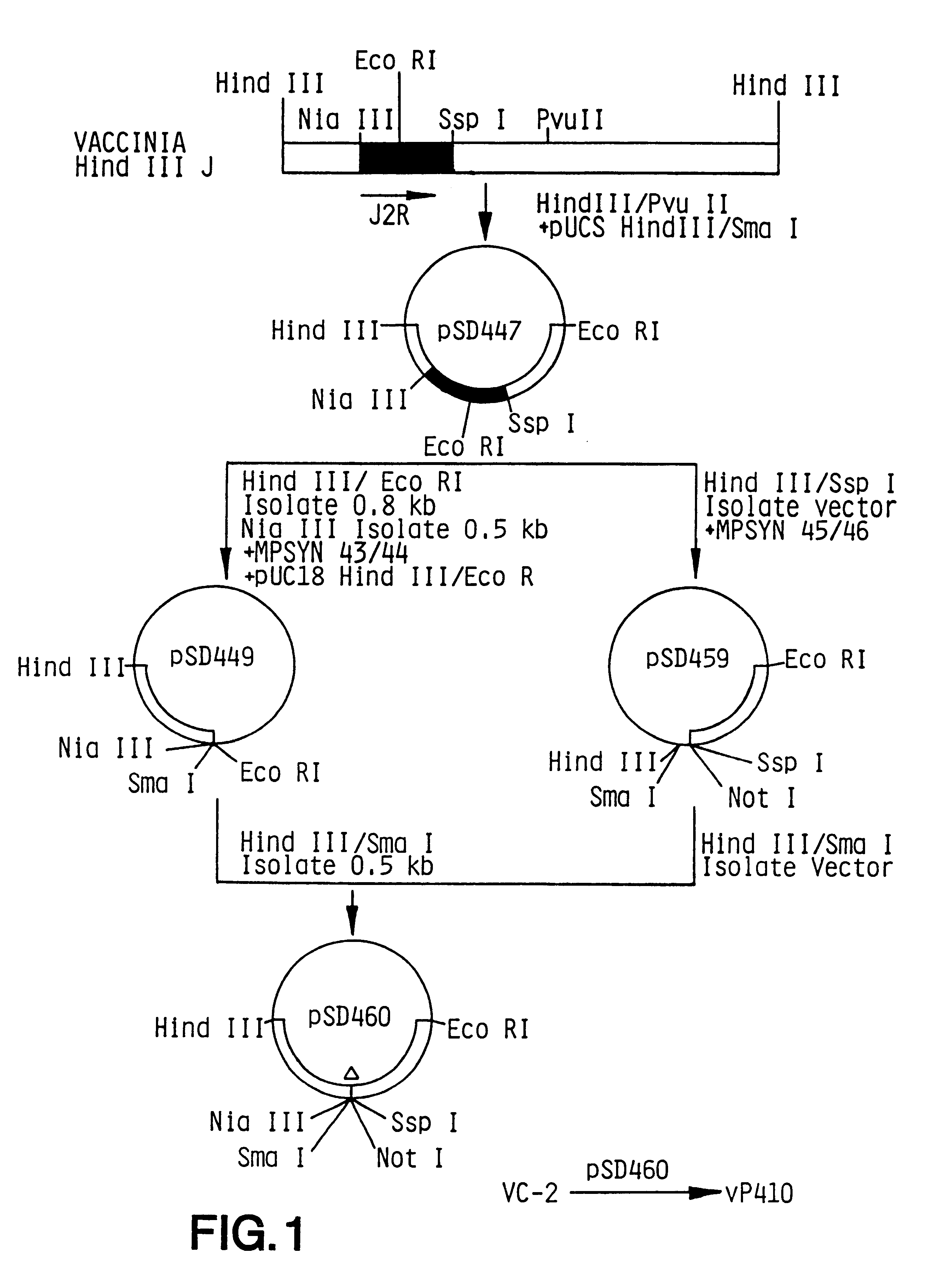
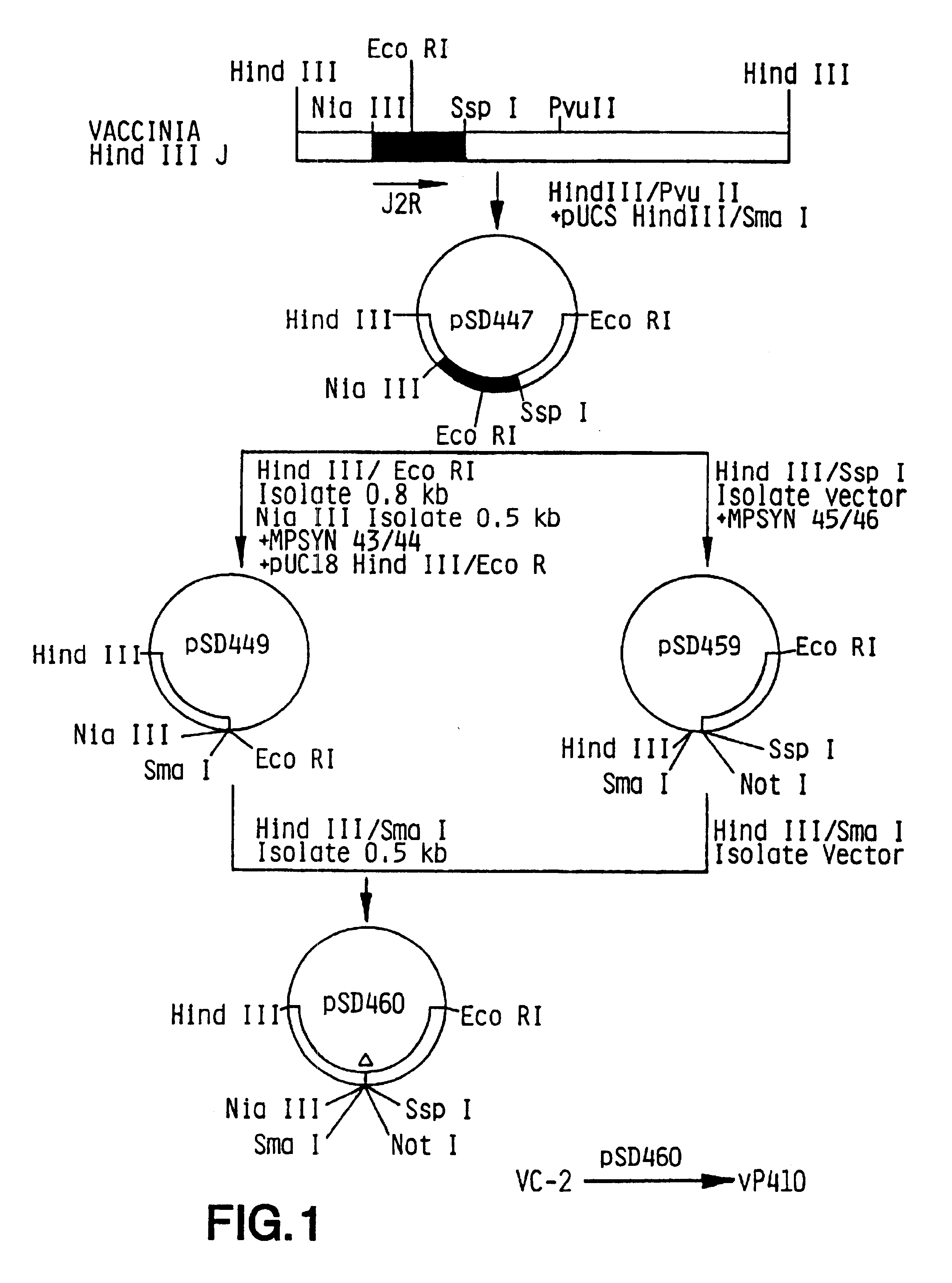
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
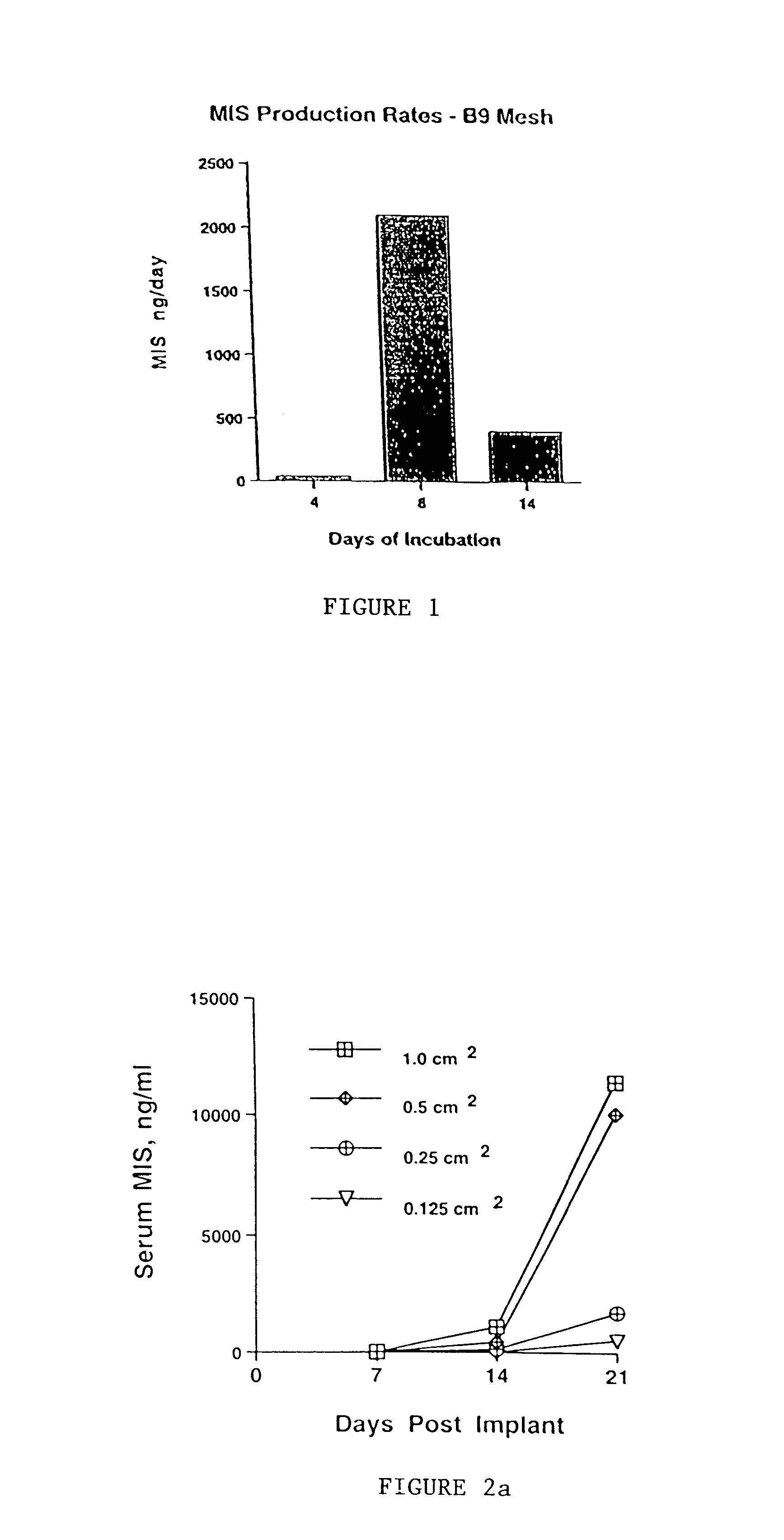
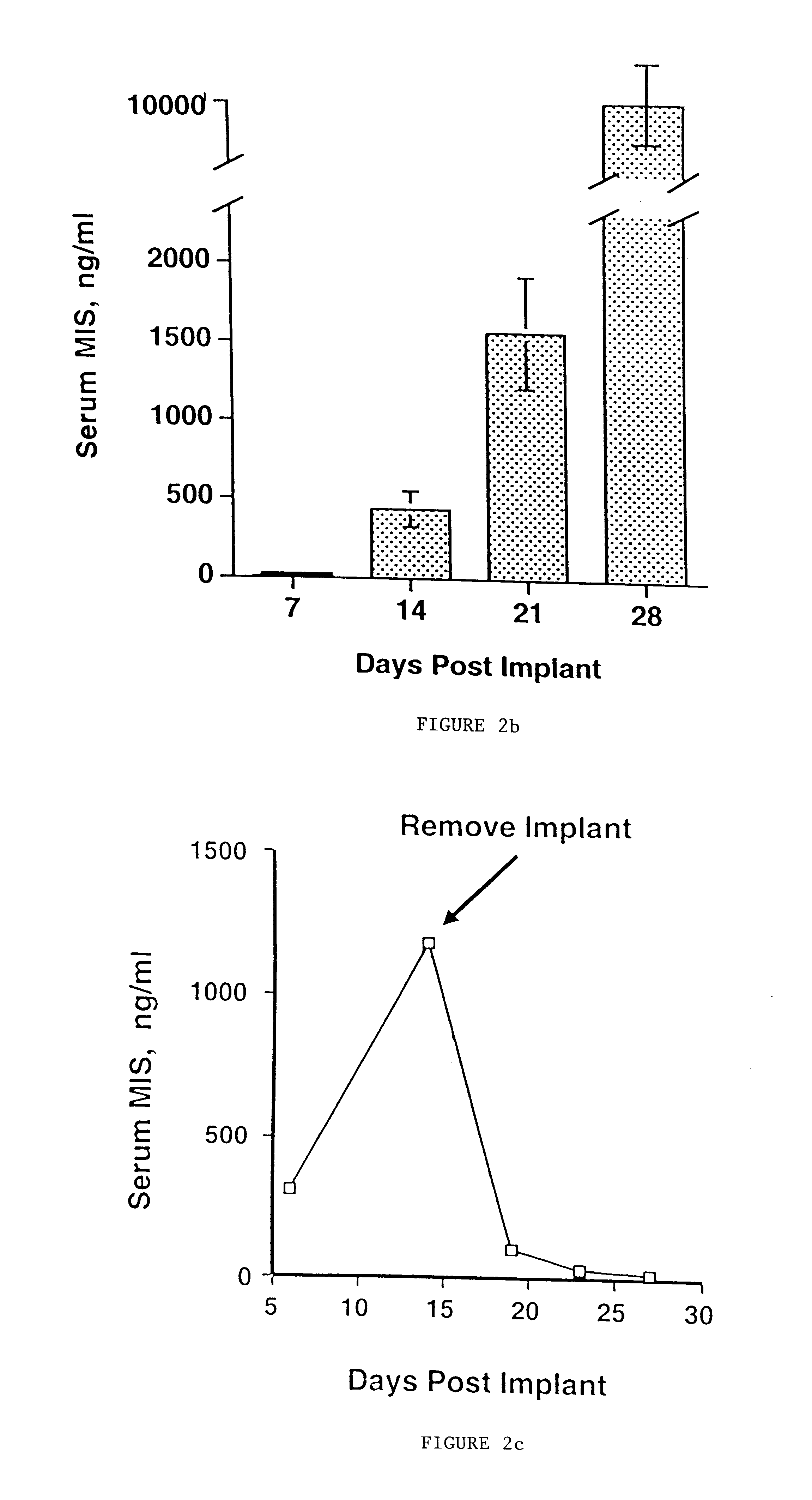
Delivery of therapeutic biologicals from implantable tissue matrices
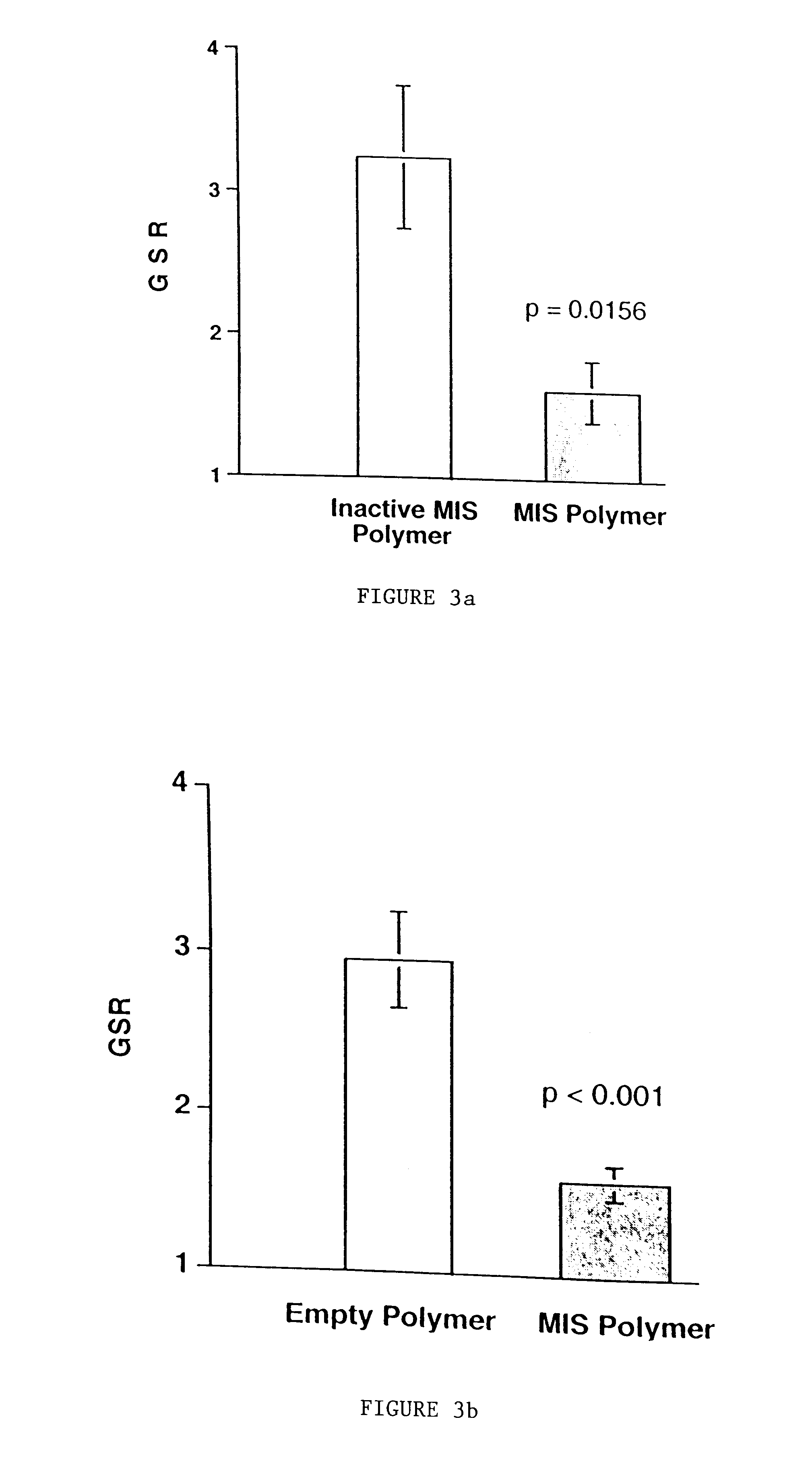
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Methods related to immunostimulatory nucleic acid-induced interferon
InactiveUS6949520B1Increased proliferationIncrease productionPeptide/protein ingredientsGenetic material ingredientsSide effectMedicine
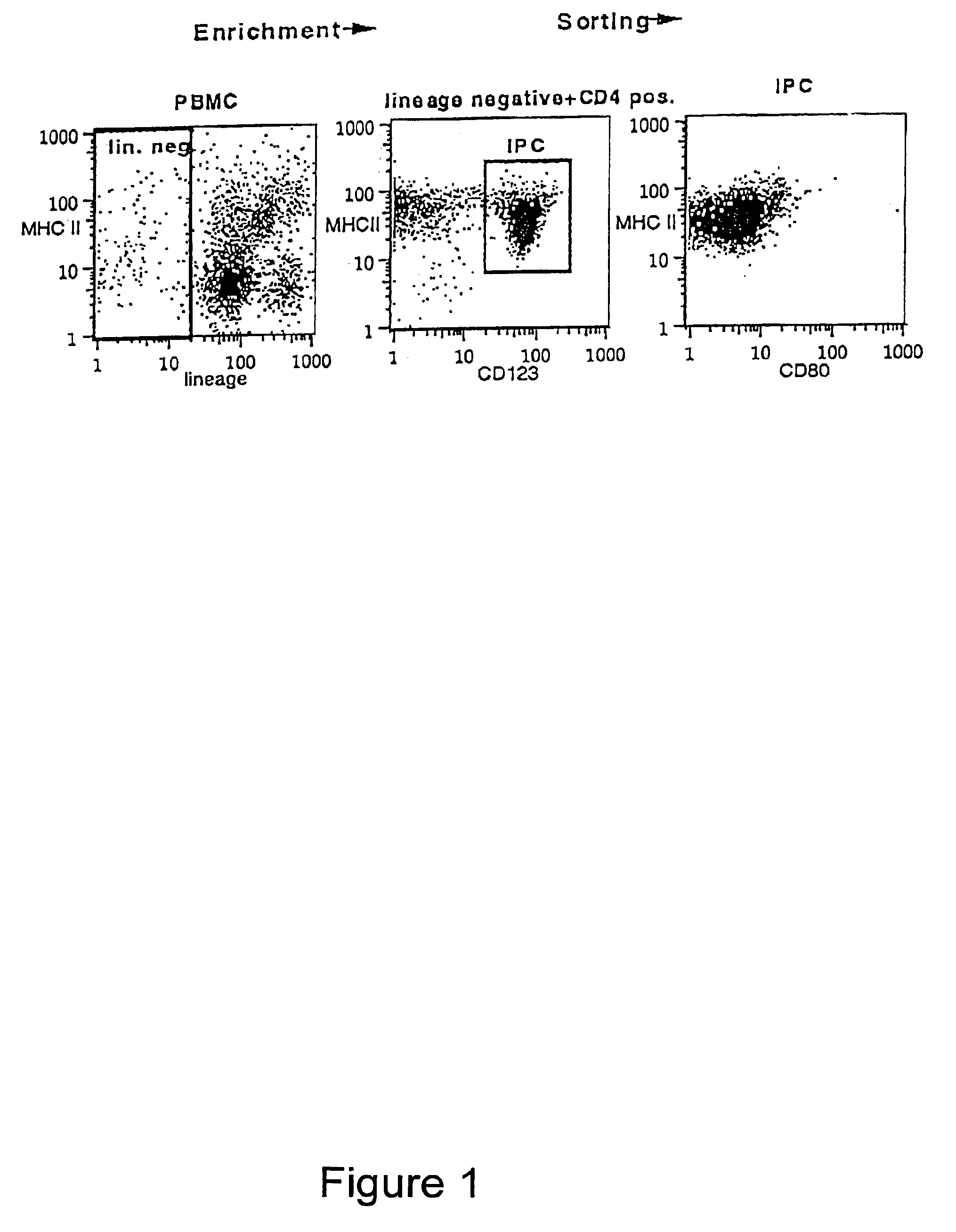
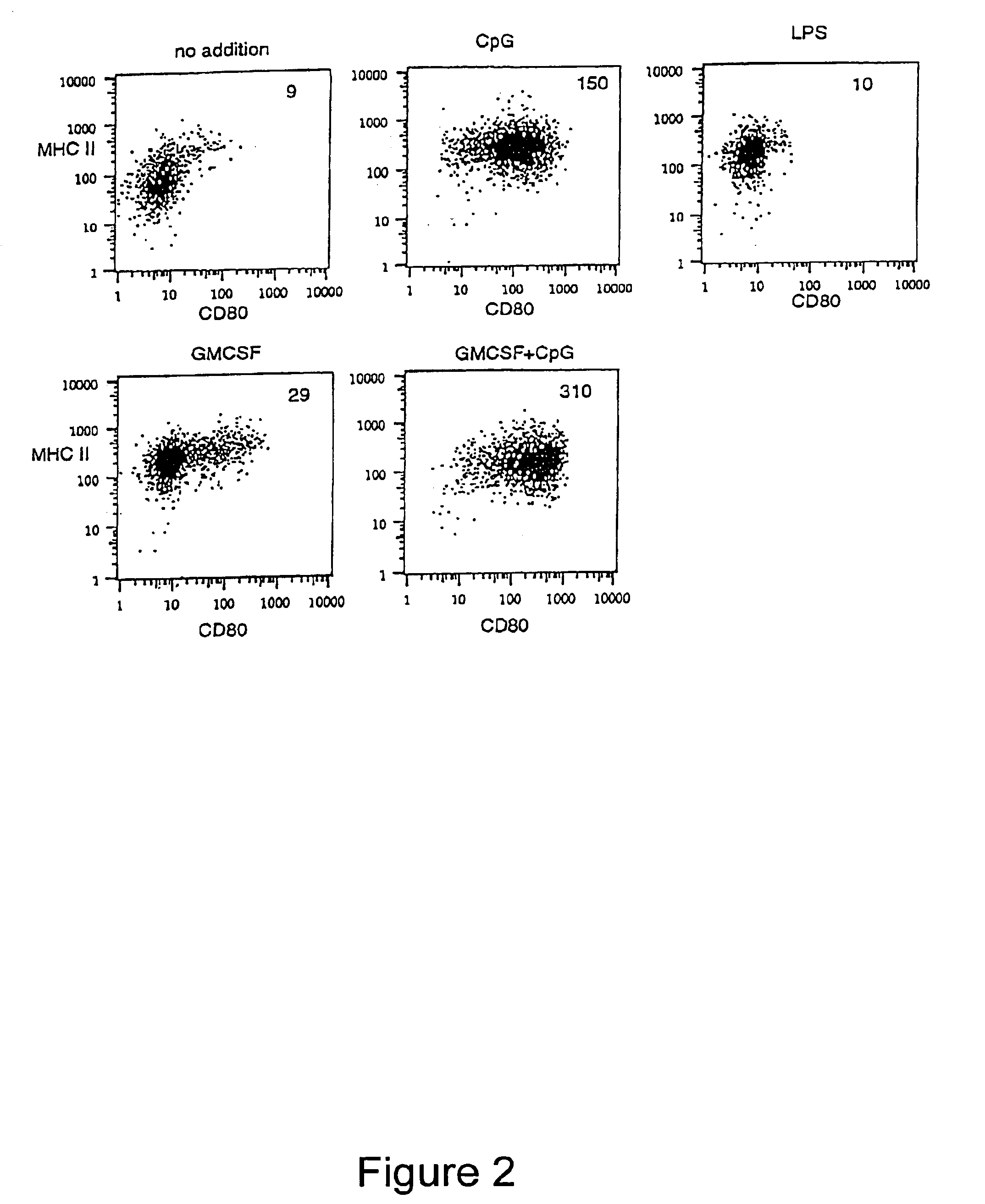
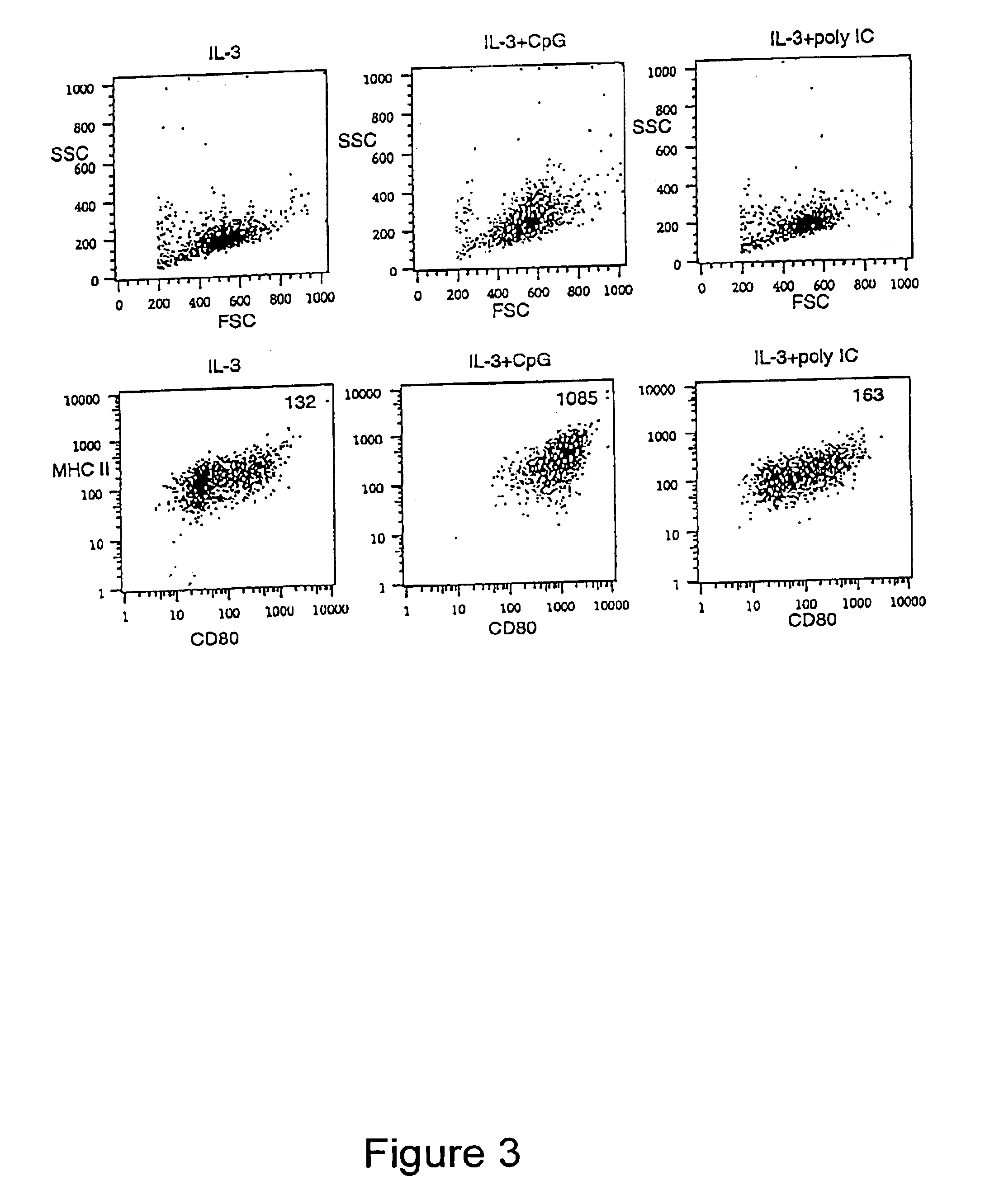
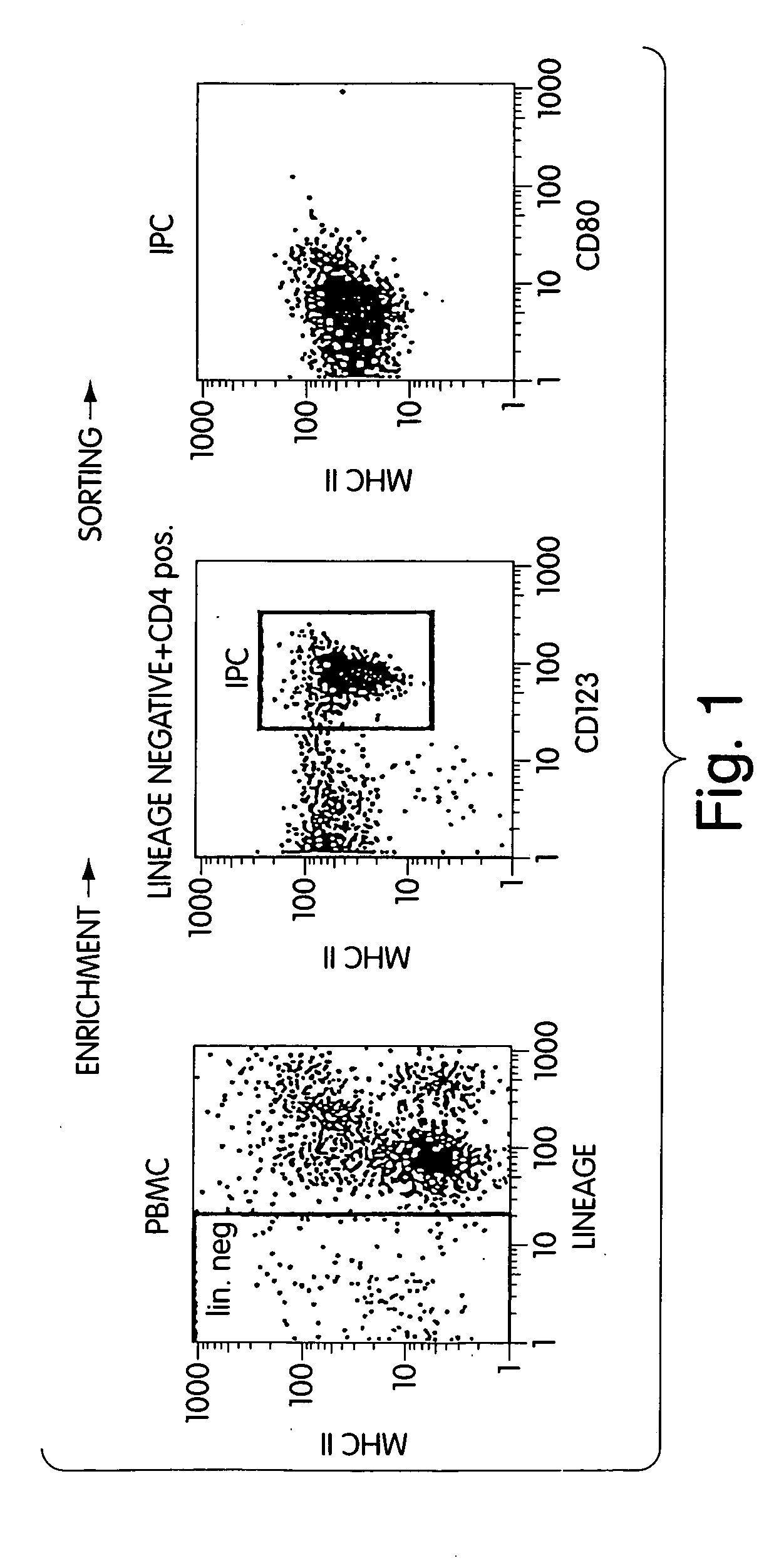
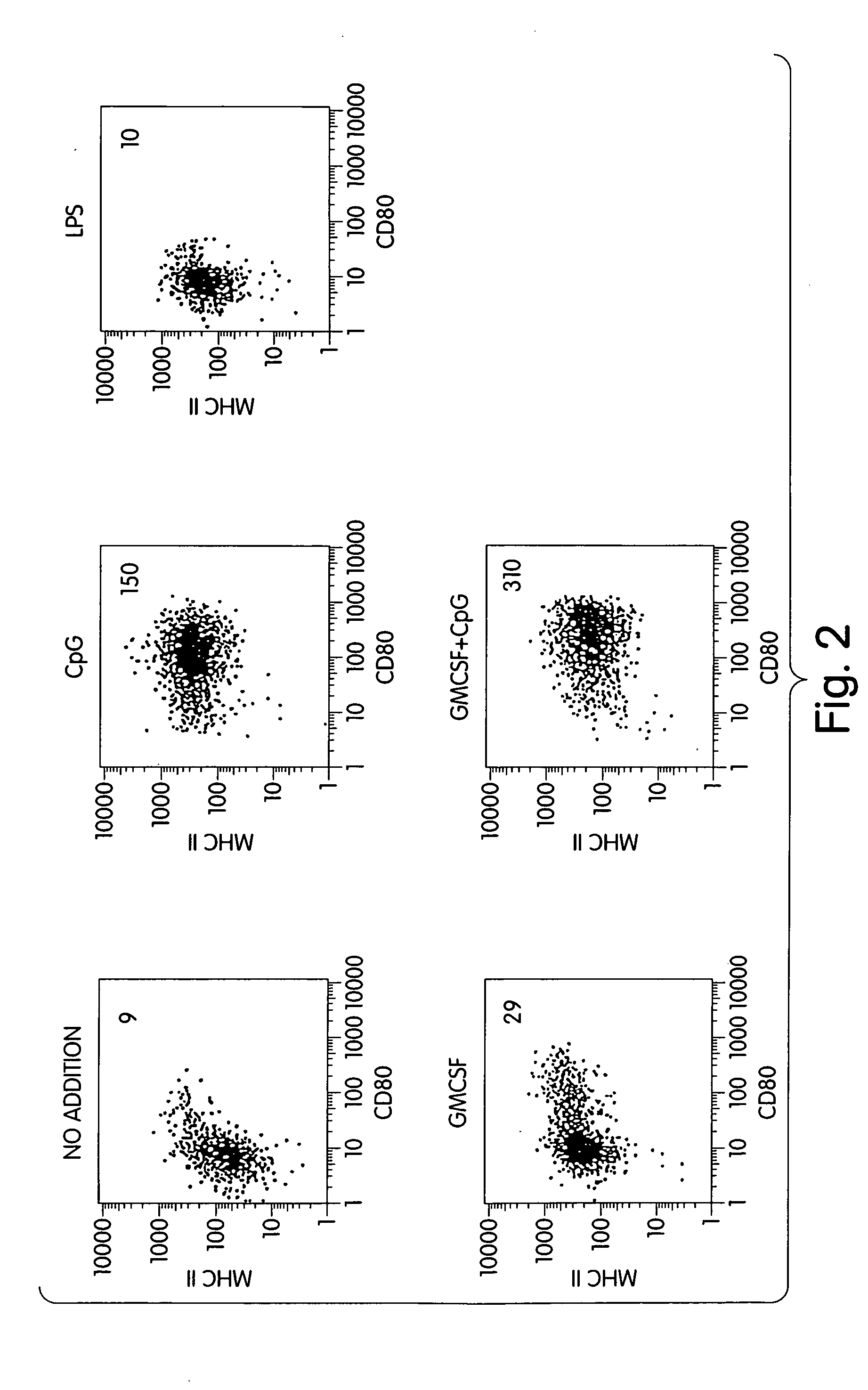
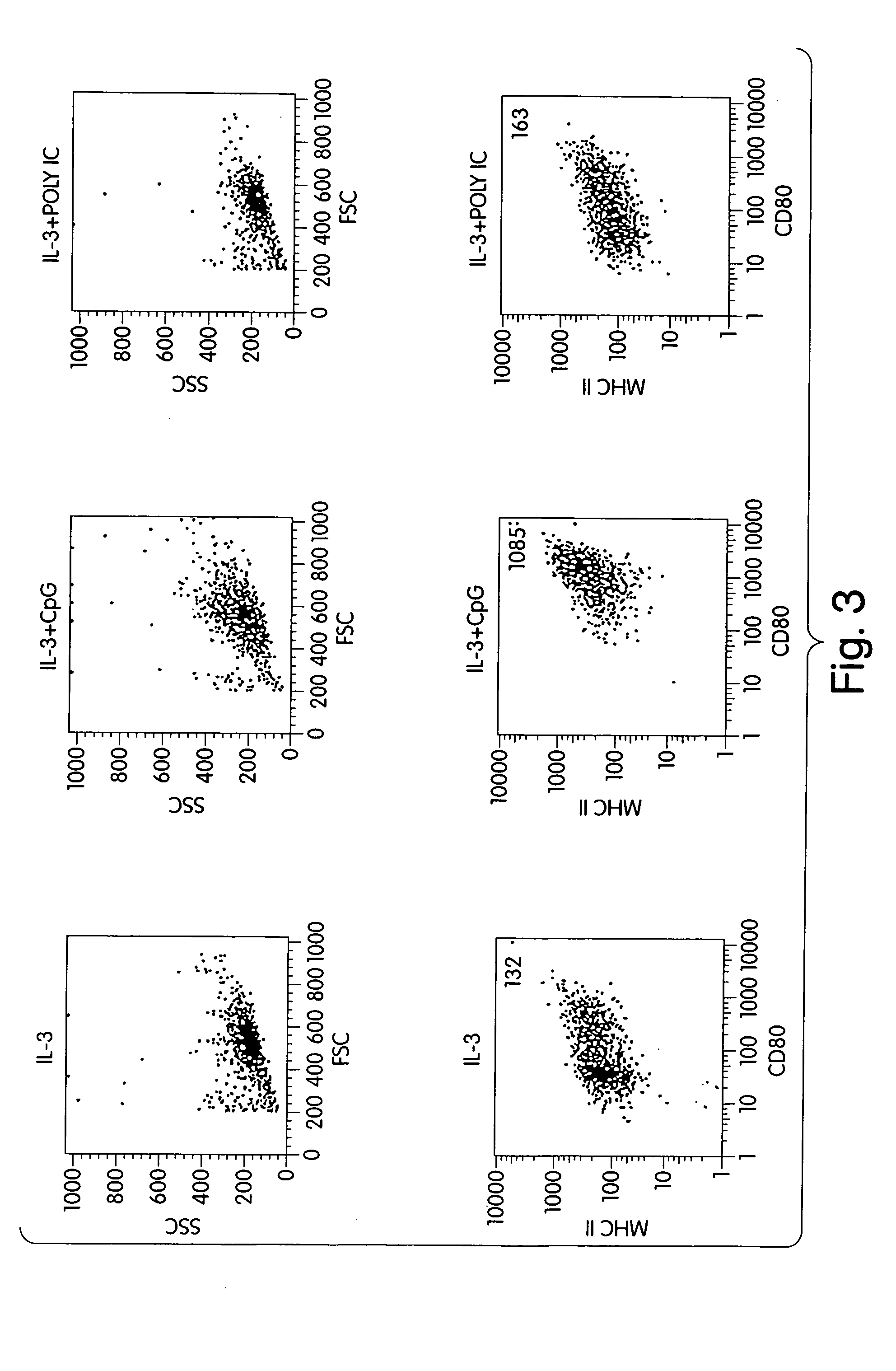
Methods and compositions are provided for extending the clinical utility of IFN-α in the treatment of a variety of viral and proliferative disorders. Among other aspects, the invention provides methods which increase the efficacy of IFN-α treatment and reduce IFN-α treatment-related side effects. In addition, methods are provided for supporting the survival and for activating natural interferon producing cells (IPCs) in vitro without exogenous IL-3 or GM-CSF. The invention is based on the discovery that certain CpG and non-CpG ISNAs promote survival and stimulation of IPCs.
Owner:COLEY PHARMA GMBH +2
Inhibitors and methods of use thereof
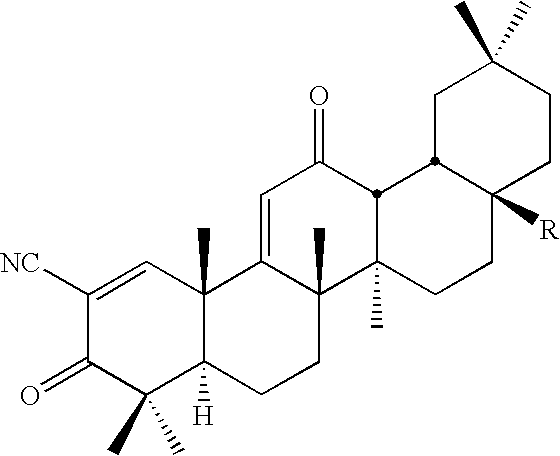
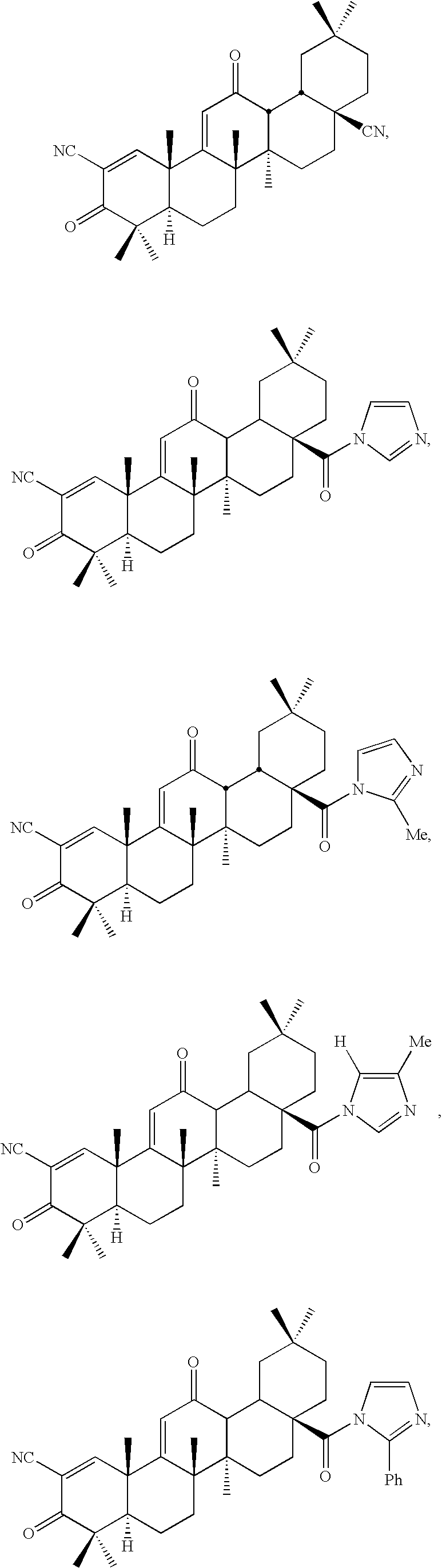
New triterpenoid derivatives with various substituents at the C-17 position of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were synthesized. Among them, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (CNDDO), 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl) imidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-2-methylimidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-4-methylimidazole show extremely high inhibitory activity (IC50=0.01-1 pM level) against production of nitric oxide induced by interferon-γ in mouse macrophages. These compounds can be used in the prevention or treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, multiple sclerosis, rheumatoid arthritis, and other inflammatory diseases. All the new triterpenoid derivatives are more potent than previously known CDDO.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Fully human antibody Fab fragments with human interferon-gamma neutralizing activity
InactiveUS7084257B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsDNA-binding domainAntigen binding
Selective binding agents of interferon-gamma (IFNγ) are provided by the invention. More particularly, the invention provides for antibodies and antigen binding domains which selectively bind to IFNγ and may be used to prevent or treat conditions relating to autoimmune and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis. Nucleic acid molecules encoding said antibodies and antigen binding domains, and expression vectors and host cells for the production of same are also provided.
Owner:AMGEN INC
Screening methods to identify agents that selectively inhibit hepatitis C virus replication
InactiveUS6030785APrevent dimerizationBlock viral inhibitionFungiSsRNA viruses positive-senseCellular defenseViral infection
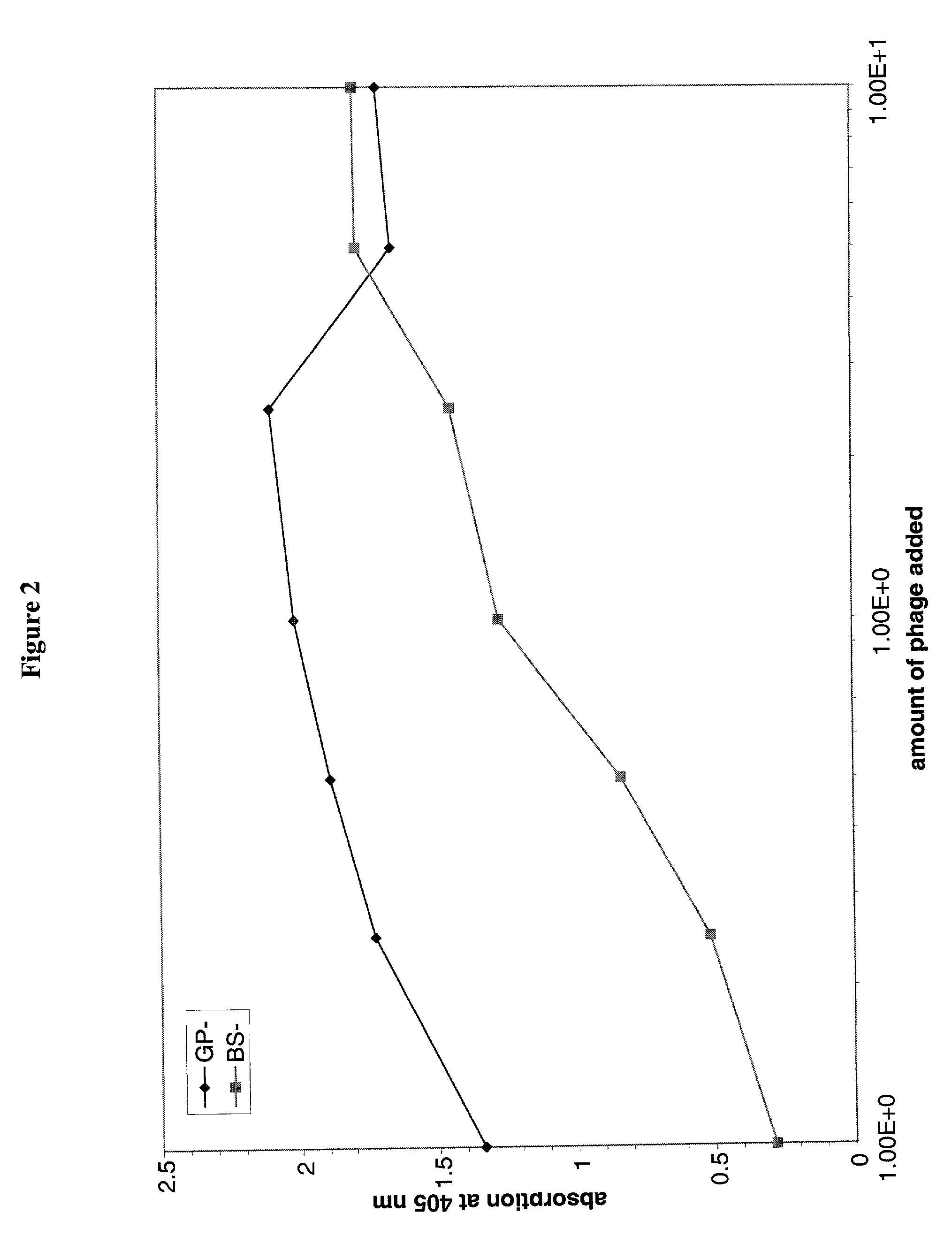
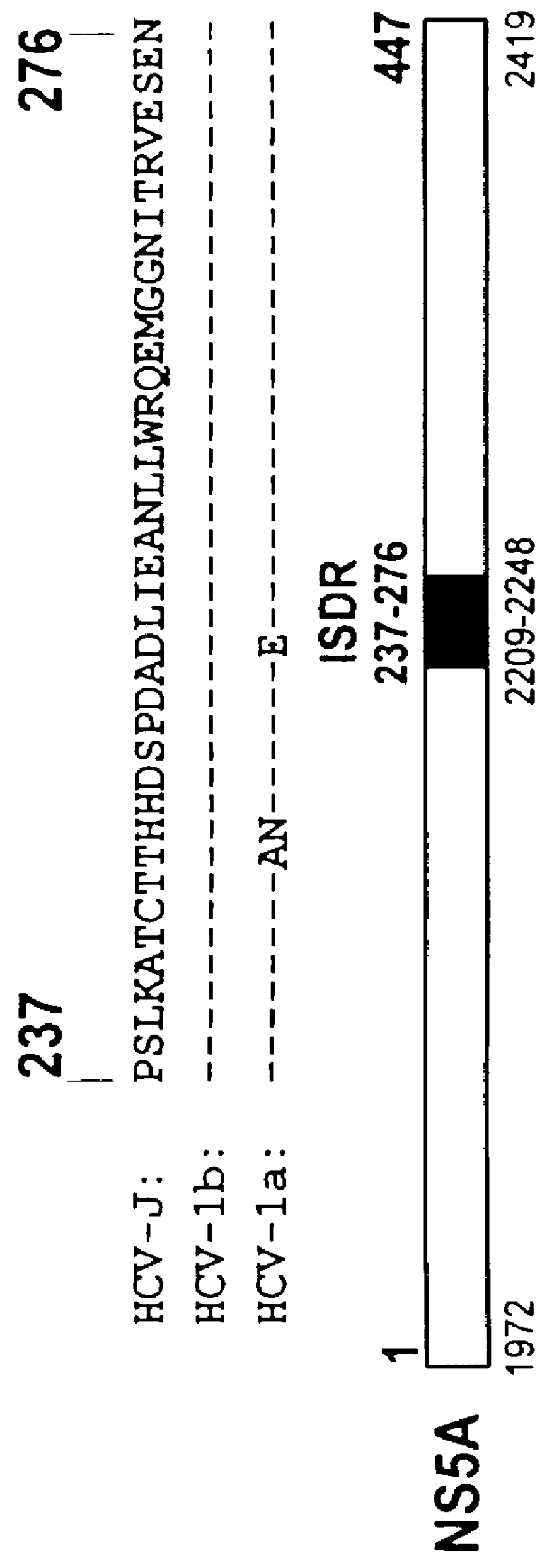
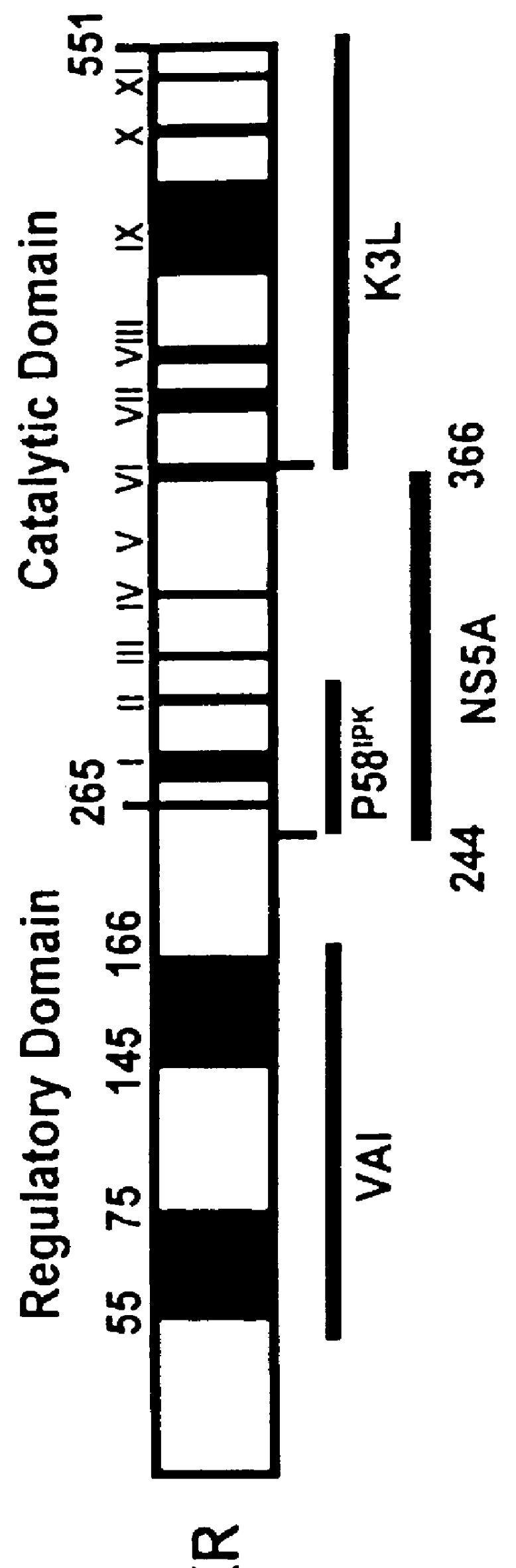
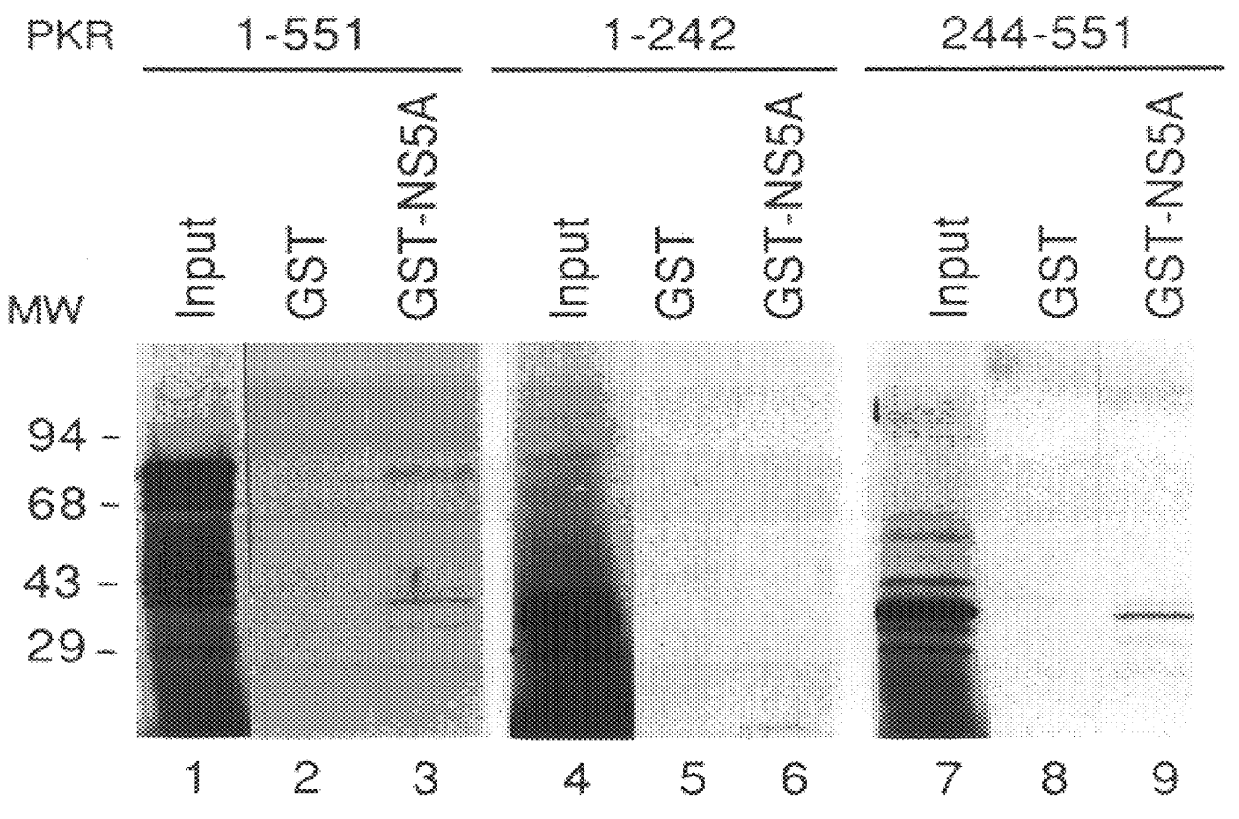
The present invention relates to novel methods for identifying antiviral agents which selectively interfere with viral proteins that override the interferon(IFN)-induced cellular defense mechanisms against viral infection. In particular, the present invention relates to screening assays that identify agents which selectively inhibit the interaction between viral proteins containing an interferon sensitivity determining region (ISDR) and IFN-induced PKR protein kinase. The present invention more particularly relates to screening assays that identify agents which selectively inhibit the interaction between hepatitis C virus (HCV) nonstructural 5A protein (NS5A), which contains an ISDR, and IFN-induced PKR protein kinase. The interaction between the viral ISDR and IFN-induced PKR protein kinase results in the override of IFN-induced cellular defense mechanisms to combat viral infection. Therefore the agents identified using the assays of the invention may have utility as antiviral agents.
Owner:UNIV OF WASHINGTON
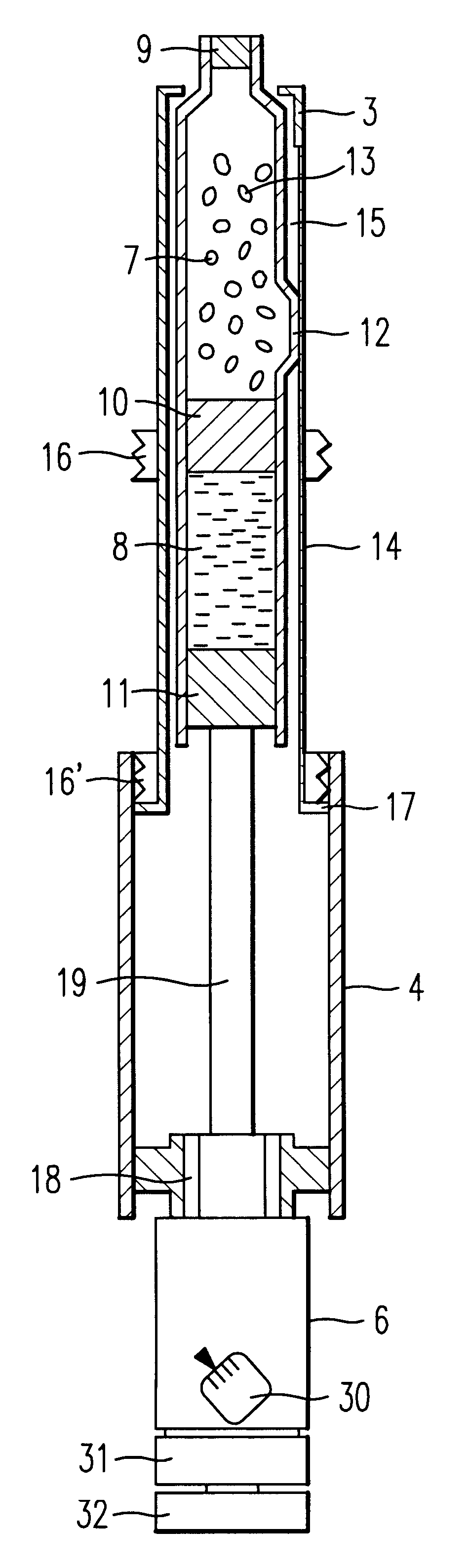
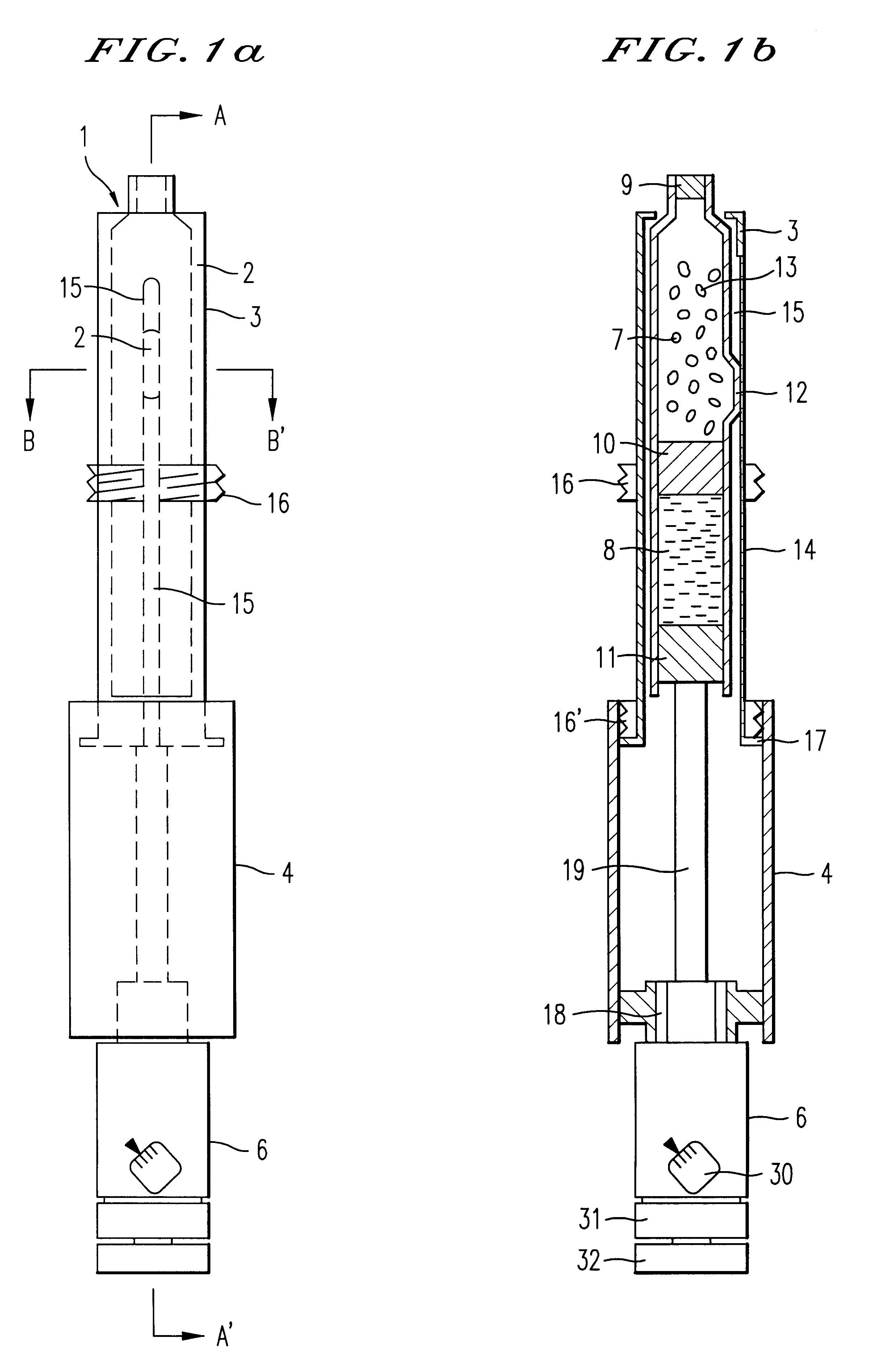
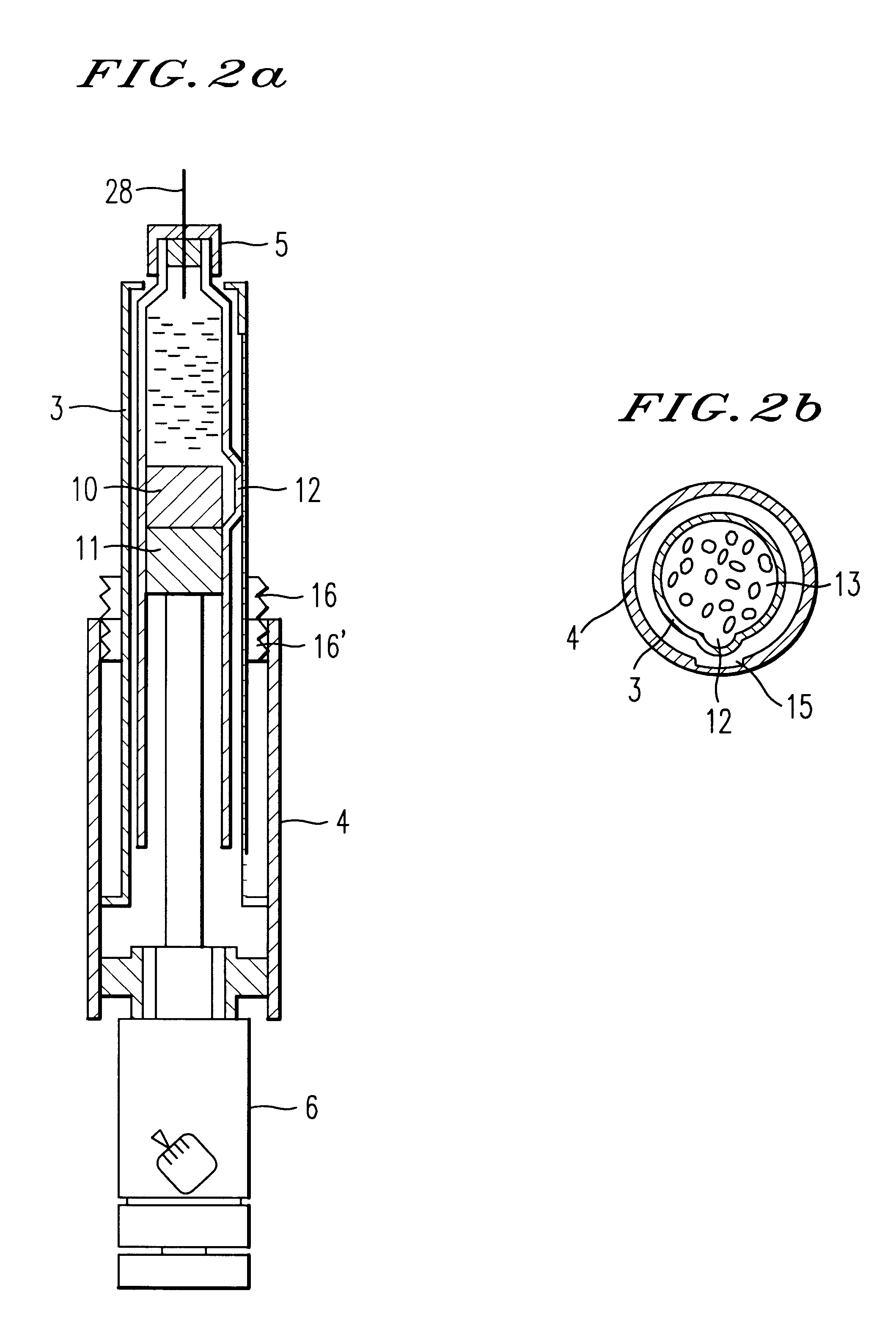
Injection syringe including device for preparation of injection
An injection syringe including a device for preparation of an injection in situ, useful to the injection which is liable to suffer chemical changes if left for a long time in the state of solution or dispersion ready to inject. The injection syringe is portable by a patient who may prepare a necessary injection in situ with the use of a device included in the syringe which will automatically perform an injection with a prescribed dosage. The injection syringe includes a device for preparation of an injection, whereby mechanical impacts affecting the medicine and consequently, chemical changes with a medicine would be minimized during the step of dissolving the medicine. The syringe as noted is especially useful for preparation of injection and injection of human growth hormones, interferon and various polypeptides which are of an environmentally sensitive nature and liable to suffer chemical changes if left for a long time in the state of solution or dispersion.
Owner:JCR PHARMA
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
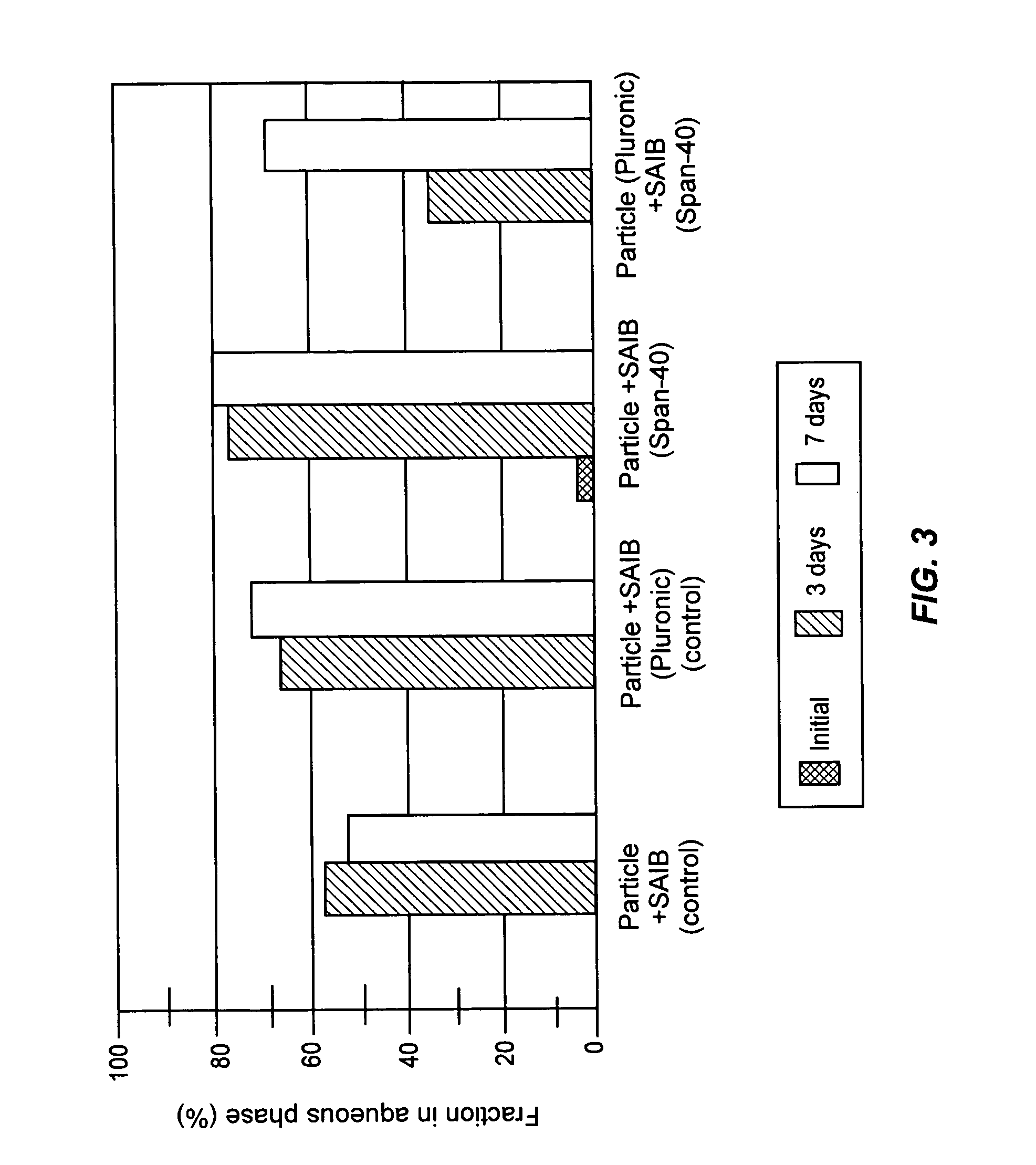
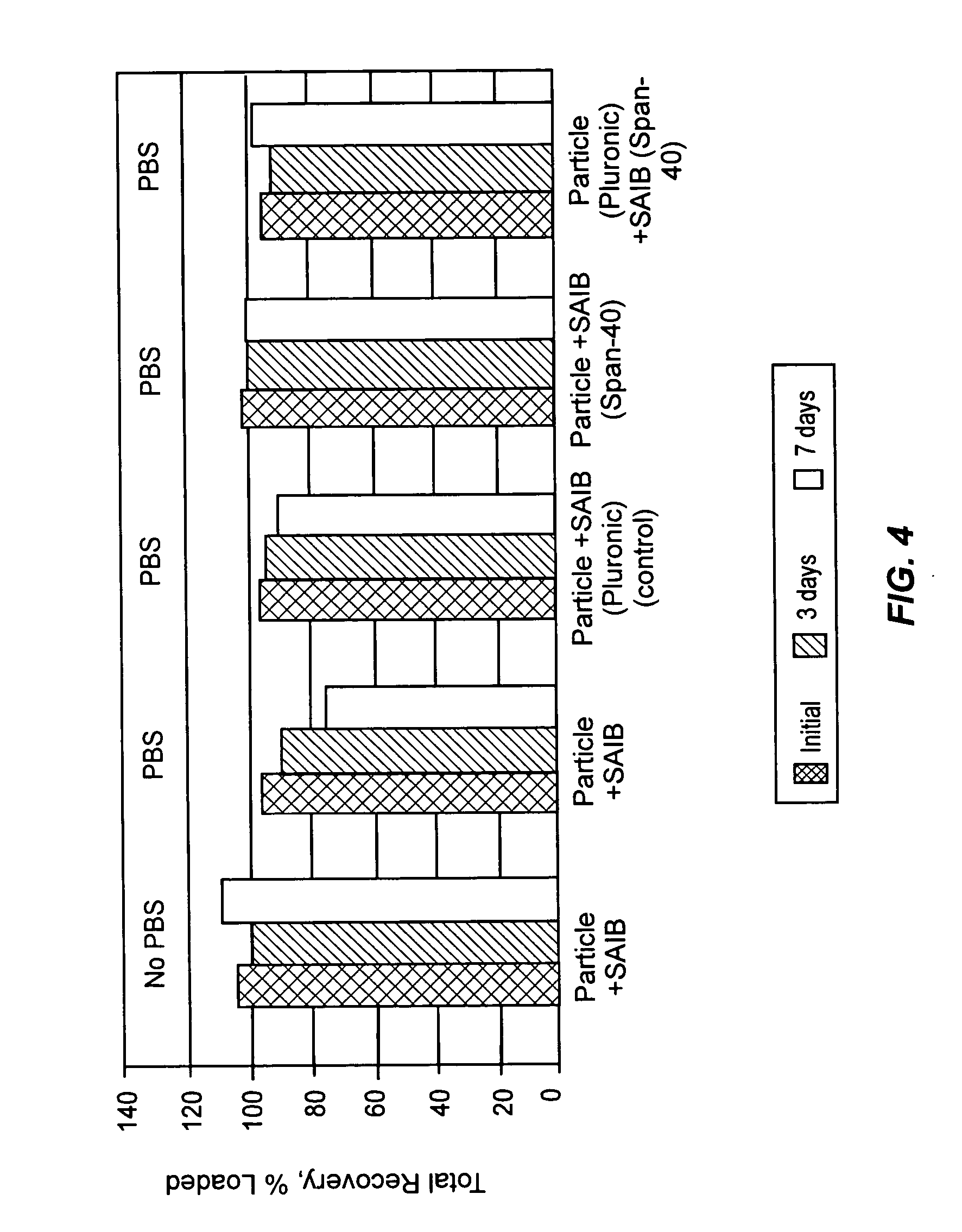
Formulations having increased stability during transition from hydrophobic vehicle to hydrophilic medium
InactiveUS20050266087A1Sufficient amountFacilitated releaseBiocideOrganic active ingredientsAntioxidantCompound (substance)
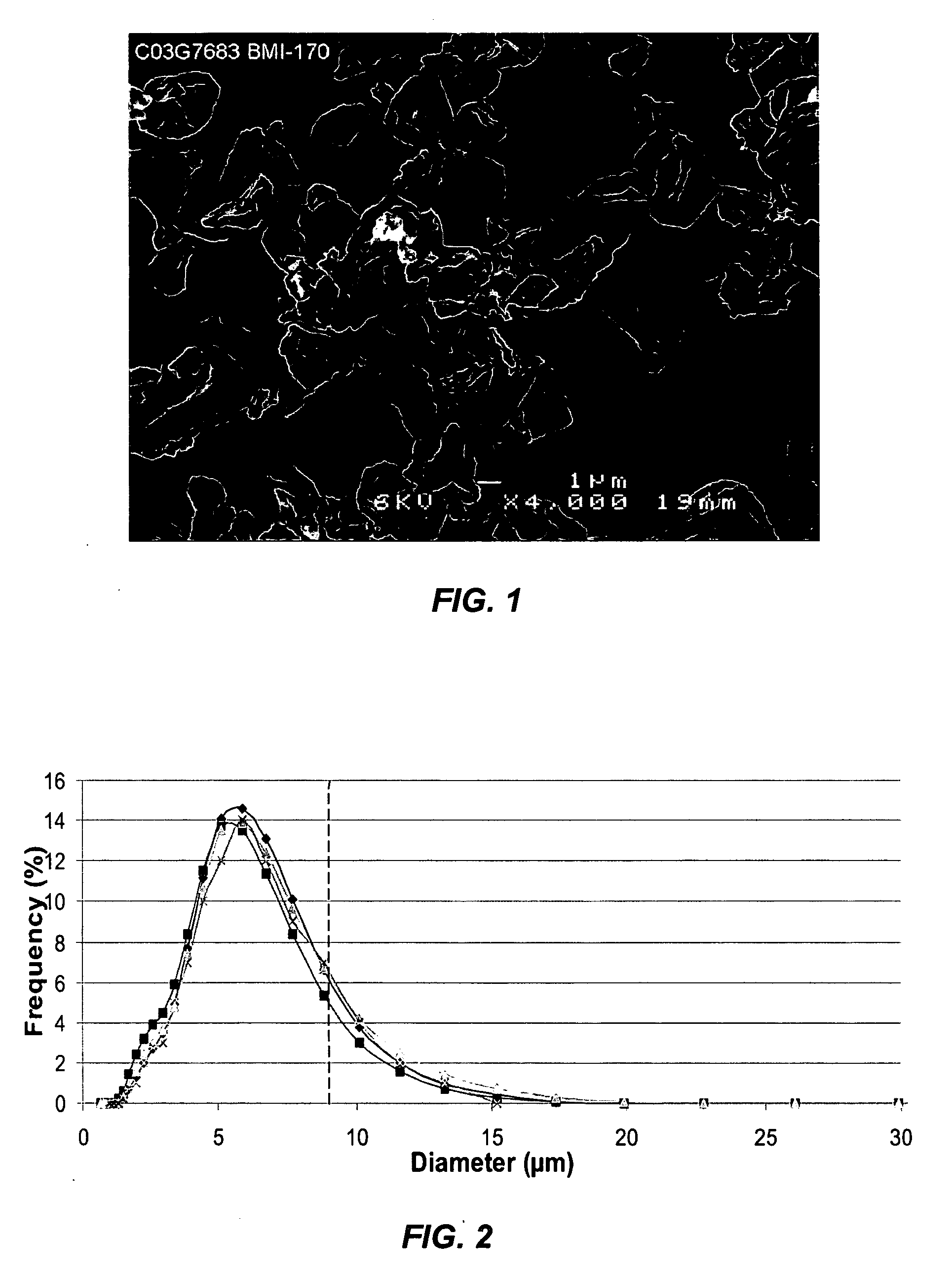
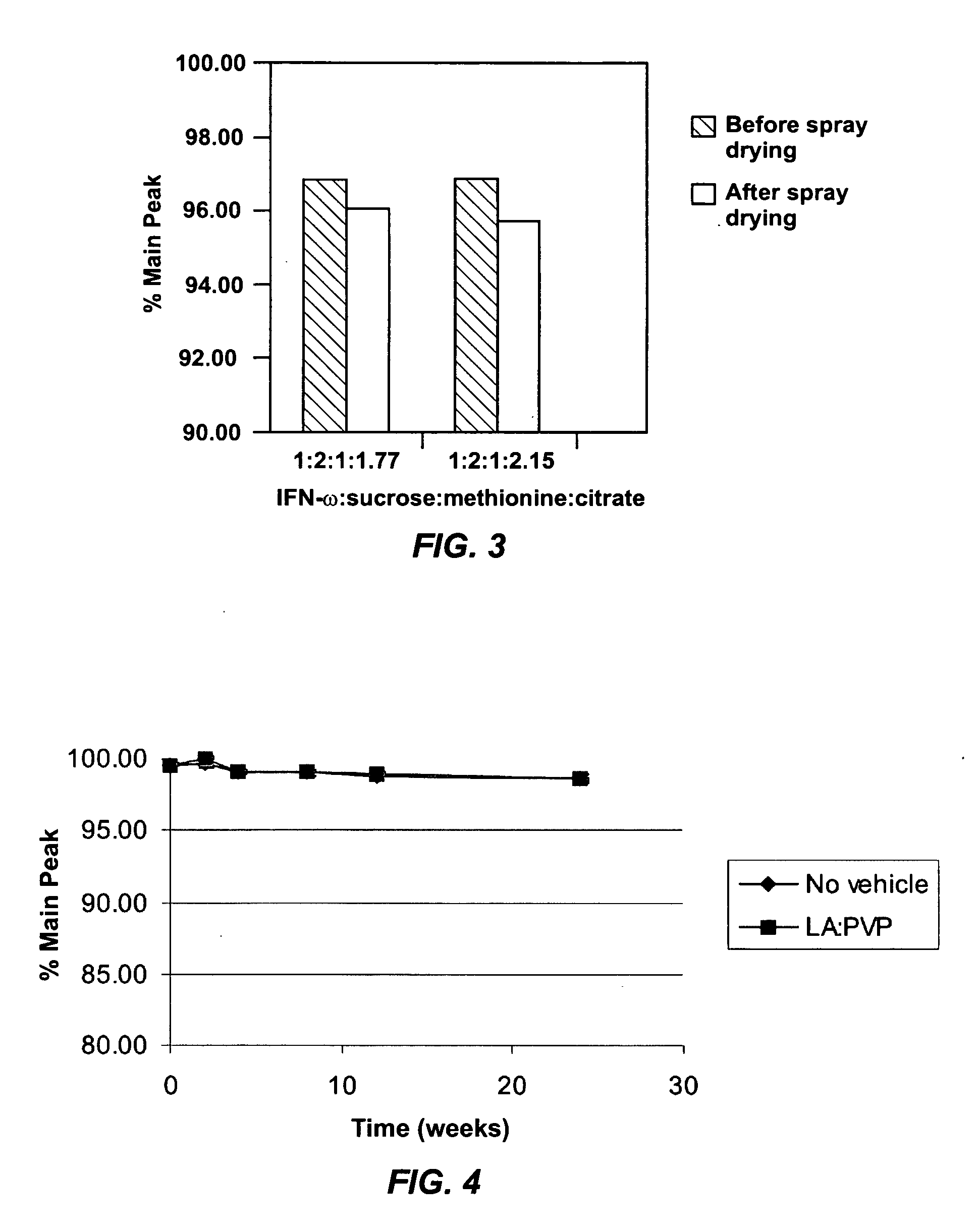
A suspension formulation for therapeutic use includes a non-aqueous, hydrophobic vehicle exhibiting viscous fluid characteristics, a dry particle formulation comprising a biomolecule dispersed in the vehicle, and a surfactant incorporated in at least one of the vehicle and dry particle formulation. A dry particle formulation includes an interferon, a buffer, a surfactant, and one or more stabilizers selected from the group consisting of a carbohydrate, an antioxidant, and an amino acid.
Owner:INTARCIA THERAPEUTICS INC
Modified human interferon polypeptides and their uses
InactiveUS20050220762A1Improve stabilityGood water solubilitySenses disorderPeptide/protein ingredientsInterferon
Owner:AMBRX
Methods related to immunostimulatory nucleic acid-induced interferon
InactiveUS20050169888A1Avoid side effectsIncreased proliferationPeptide/protein ingredientsGenetic material ingredientsSide effectMedicine
Methods and compositions are provided for extending the clinical utility of IFN-α in the treatment of a variety of viral and proliferative disorders. Among other aspects, the invention provides methods which increase the efficacy of IFN-α treatment and reduce IFN-α treatment-related side effects. In addition, methods are provided for supporting the survival and for activating natural interferon producing cells (IPCs) in vitro without exogenous IL-3 or GM-CSF. The invention is based on the discovery that certain CpG and non-CpG ISNAs promote survival and stimulation of IPCs.
Owner:UNIV OF IOWA RES FOUND +2
Media and method for treating pathological syndrome
InactiveUS7572441B2Energy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsNatural antibodyUltra low dose
A medicament based on antibodies contains an activated form of monoclonal, polyclonal, or natural antibodies to interferon in low or ultra-low doses prepared by multiple consecutive dilutions and exposure to external factors, preferably in accordance with homeopathic technology. In order to obtain antibodies, human or heterologous interferon alpha, beta, or gamma, including recombinant interferon, is used; a mixture of various, mostly centimal, homeopathic dilutions being employed. A method of treating a pathologic syndrome, whose formation is affected by interferon, consists in the use of activated forms of antibodies to interferon alpha, beta, or gamma in low or ultra-low doses obtained by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Methods for treating cancer using cytokine-expressing polynucleotides
InactiveUS7268120B1Improved in vivo polypeptide expressionMinimizing adverse side effectBiocideOrganic active ingredientsMammalSodium phosphates
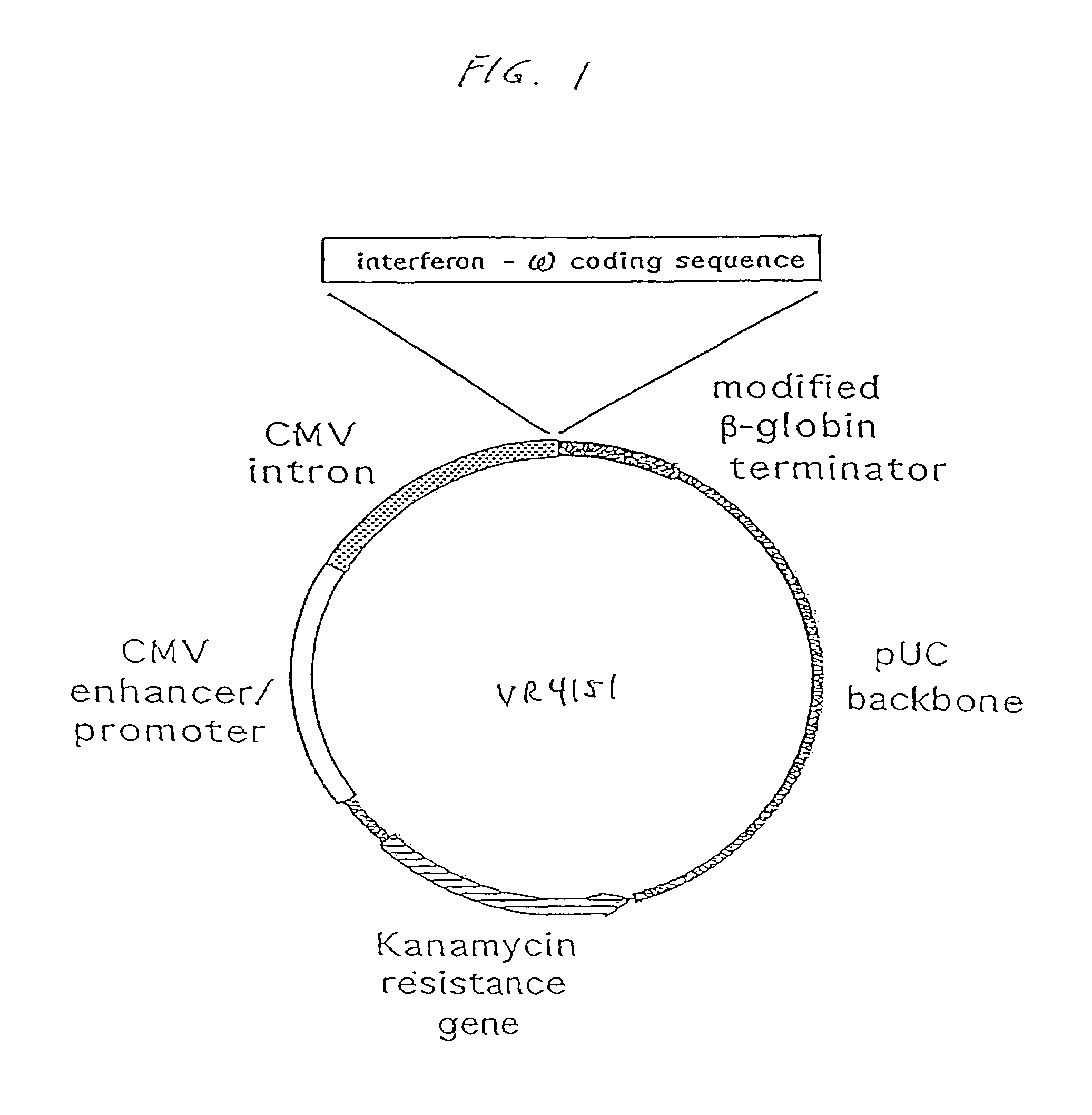
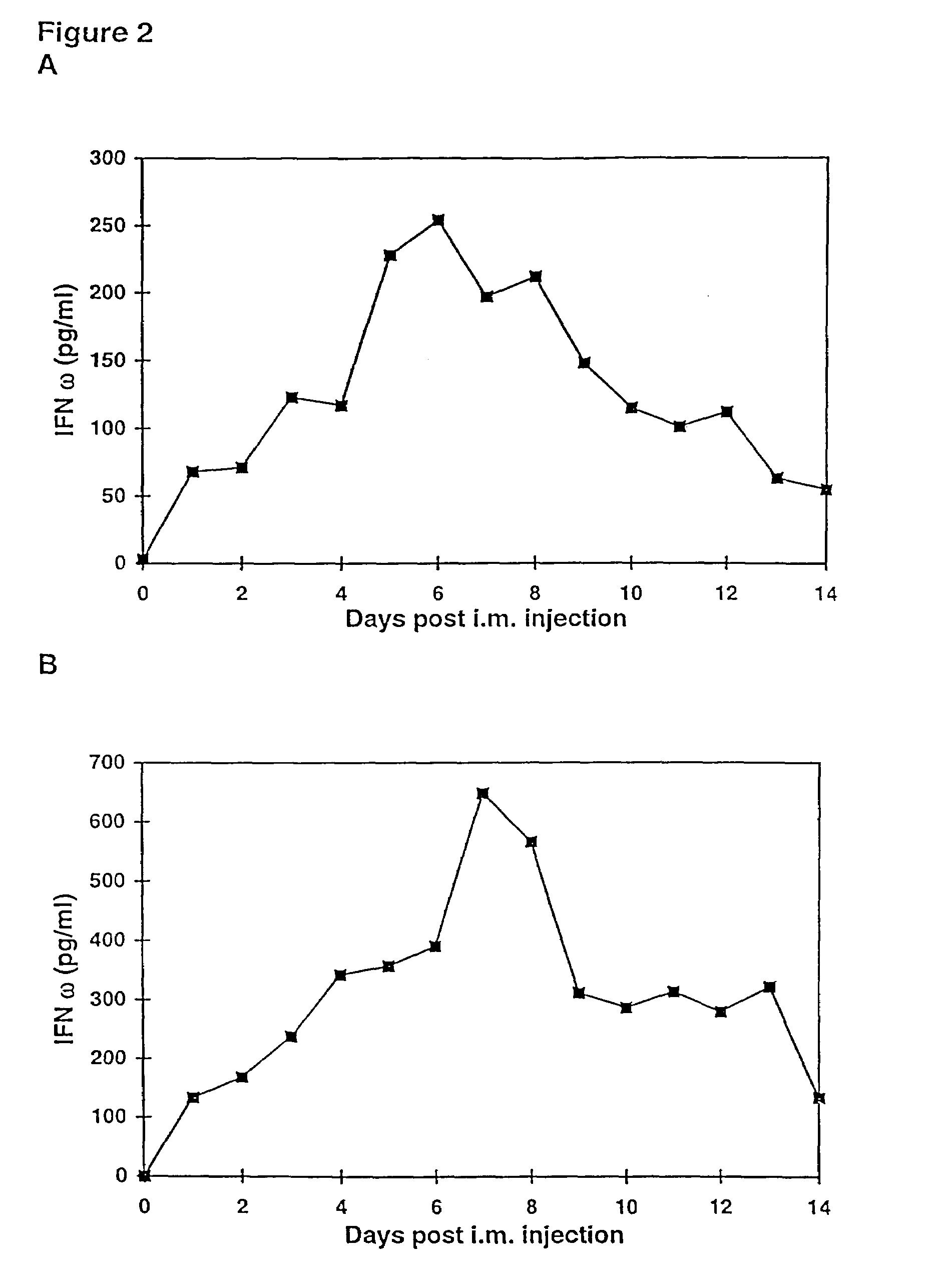
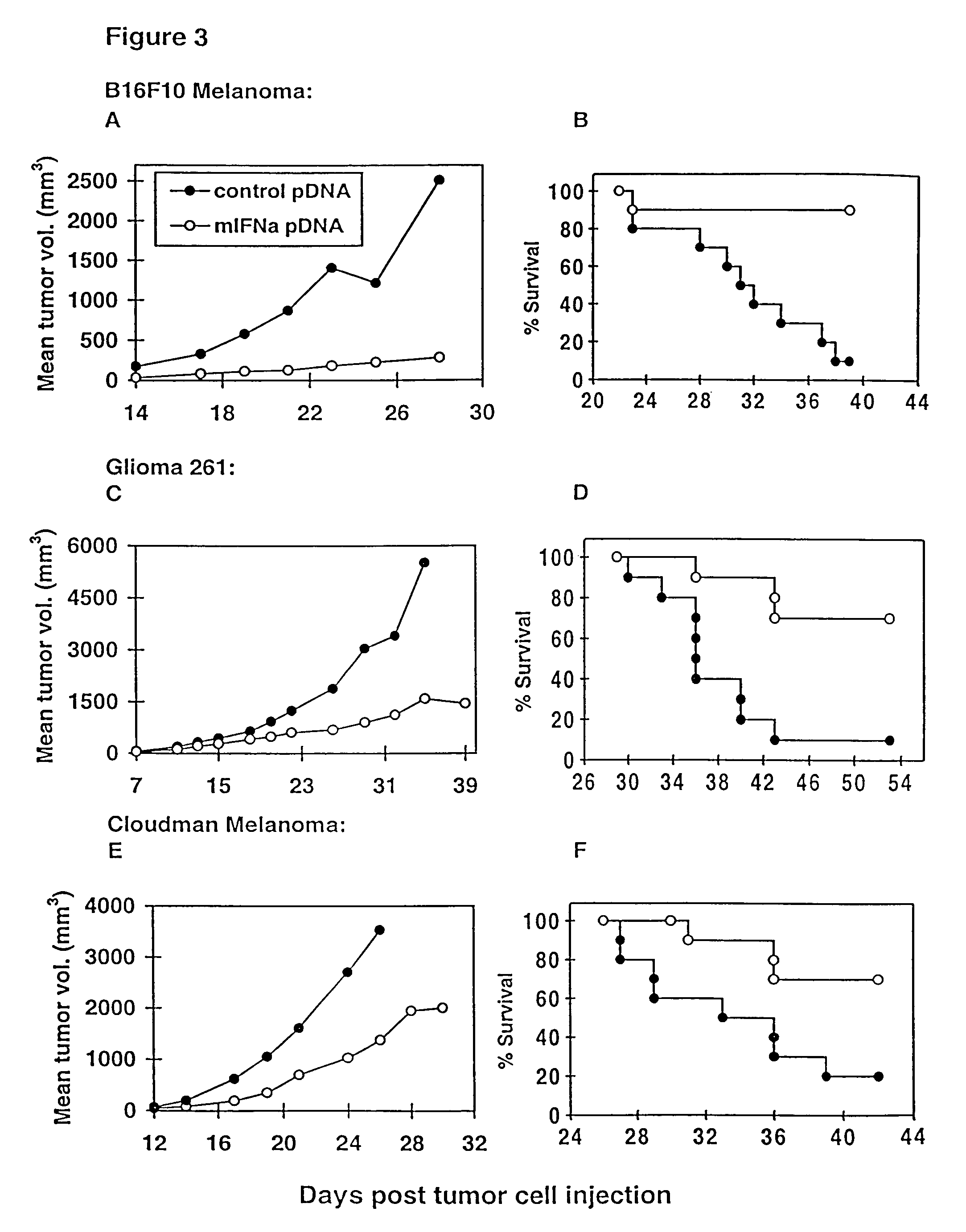
The present invention provides a pharmaceutical composition, comprising a non-infectious, non-integrating polynucleotide construct comprising a polynucleotide encoding an interferon ω and one or more cationic compounds. The present invention also provides methods of treating cancer in a mammal, comprising administering into a muscle of the mammal a non-infectious, non-integrating DNA polynucleotide construct comprising a polynucleotide encoding a cytokine. In addition, the present invention also relates to the methodology for selective transfection of malignant cells with polynucleotides expressing therapeutic or prophylactic molecules in intra-cavity tumor bearing mammals. More specifically, the present invention provides a methodology for the suppression of an intra-cavity dissemination of malignant cells, such as intraperitoneal dissemination. Furthermore, the invention relates to compositions and methods to deliver polynucleotides encoding polypeptides to vertebrate cells in vivo, where the composition comprises an aqueous solution of sodium phosphate.
Owner:VICAL INC
Modified vaccinia virus ankara for the vaccination of neonates
Owner:BAVARIAN NORDIC AS
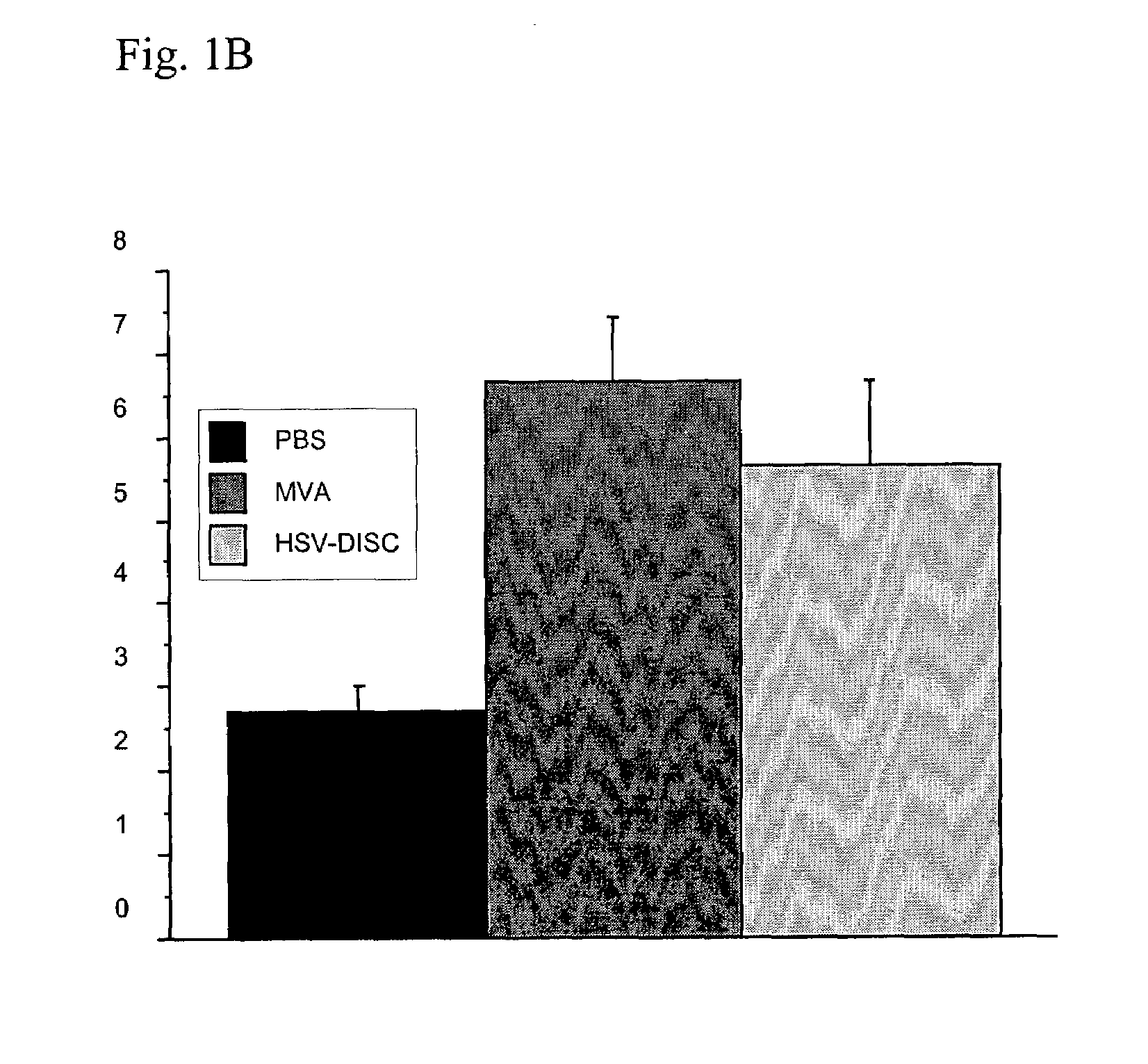
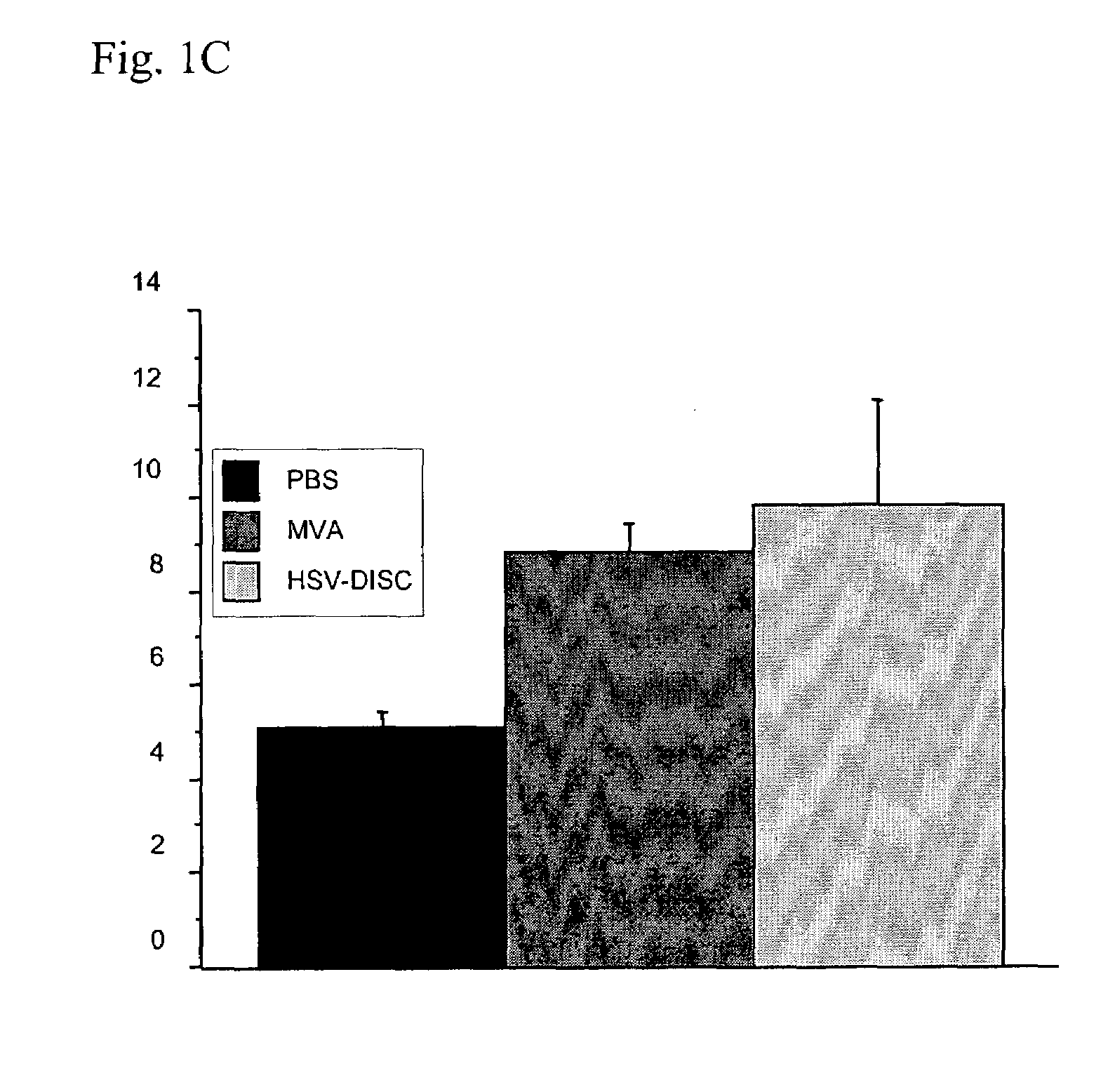
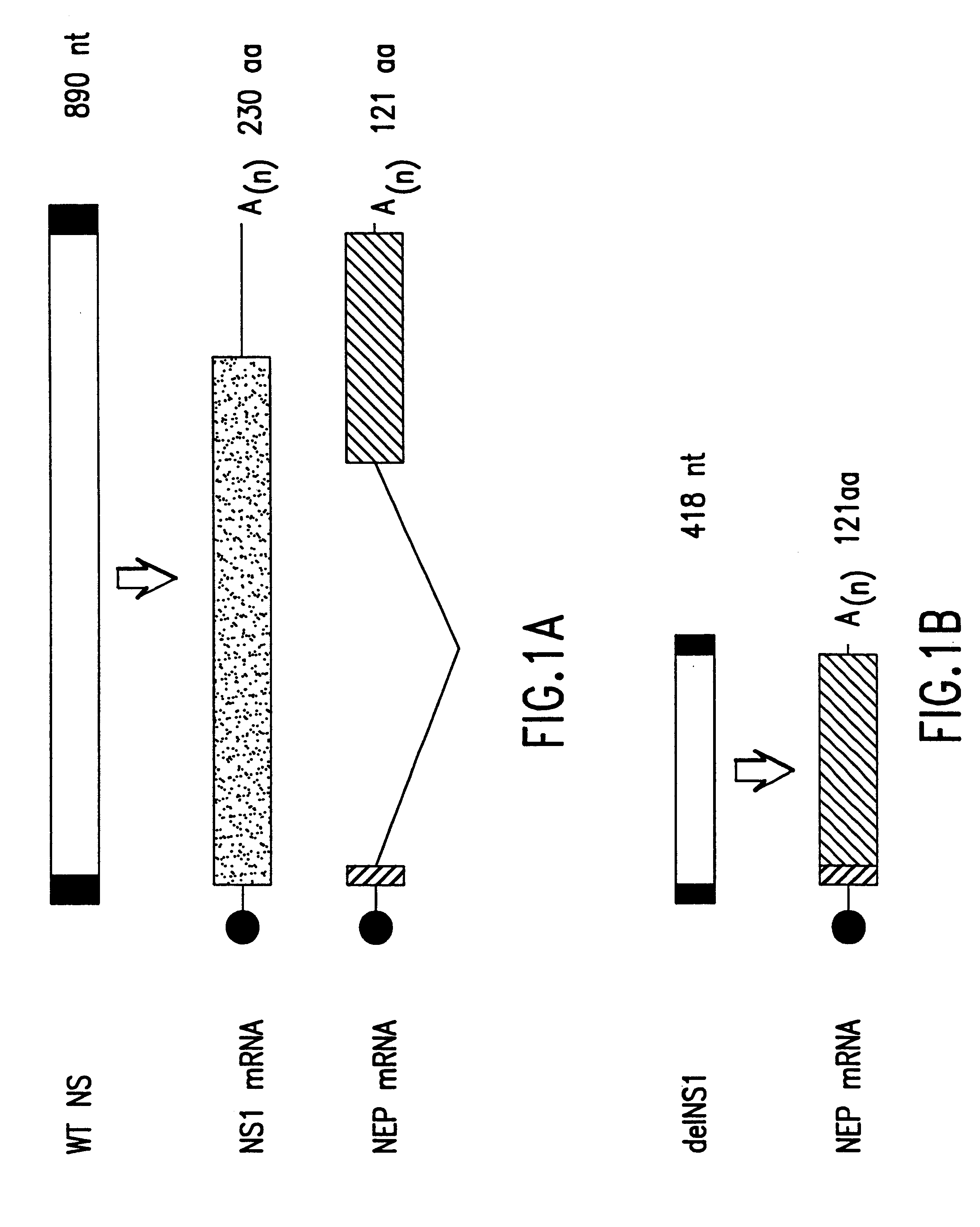
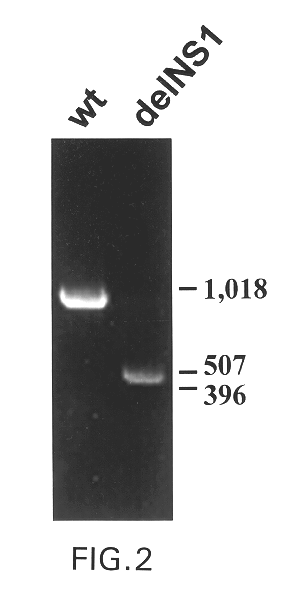
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Interferon alpha antibodies and their uses
ActiveUS20070014724A1Inhibit biological activityInhibiting surface expressionPeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides isolated anti-interferon alpha monoclonal antibodies, particularly human monoclonal antibodies, that inhibit the biological activity of multiple interferon (IFN) alpha subtypes but do not substantially inhibit the biological activity of IFN alpha 21 or the biological activity of either IFN beta or IFN omega. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting the biological activity of IFN alpha using the antibodies of the invention, as well as methods of treating disease or disorders mediated by IFN alpha, such as autoimmune diseases, transplant rejection and graft versus host disease, by administering the antibodies of the invention.
Owner:MEDAREX LLC
Method for administering a cytokine to the central nervous system and the lymphatic system
InactiveUS6991785B2Provide effectModulate immune and inflammatory responseBiocideNervous disorderImmunologic disordersInterferon alpha
The present invention is directed to a method for delivering cytokines to the central nervous system and the lymphatic system by way of a tissue innervated by the trigeminal nerve and / or olfactory nerve. Cytokines include tumor necrosis factors, interleukins, interferons, particularly interferon-β and its muteins such as IFN-βser17. Such a method of delivery can be useful in the treatment of central nervous system disorders, brain disorders, proliferative, viral, and / or autoimmune disorders such as Sjogren's disorder.
Owner:CHIRON CORP
Supplemented and unsupplemented tissue sealants, methods of their production and use
InactiveUSRE39321E1Decreasing thrombogenicityLow antigenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant dressing, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, antiinflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant dressing.
Owner:AMERICAN NAT RED CROSS
Double-stranded and single-stranded RNA molecules with 5 ' triphosphates and their use for inducing interferon
InactiveUS20060178334A1Strong responseInhibits and prevents viral infectionSugar derivativesGenetic material ingredientsSingle-Stranded RNADouble stranded
Double-stranded and single-stranded RNA molecules, and their use in methods for inducing interferon are provided. The interferon induction provides anti-viral and other medically useful effects, such as anti-cancer effects. Also provided are methods for reducing or inhibiting interferon induction exhibited by such molecules, particularly siRNA and shRNA molecules produced in vitro.
Owner:CITY OF HOPE
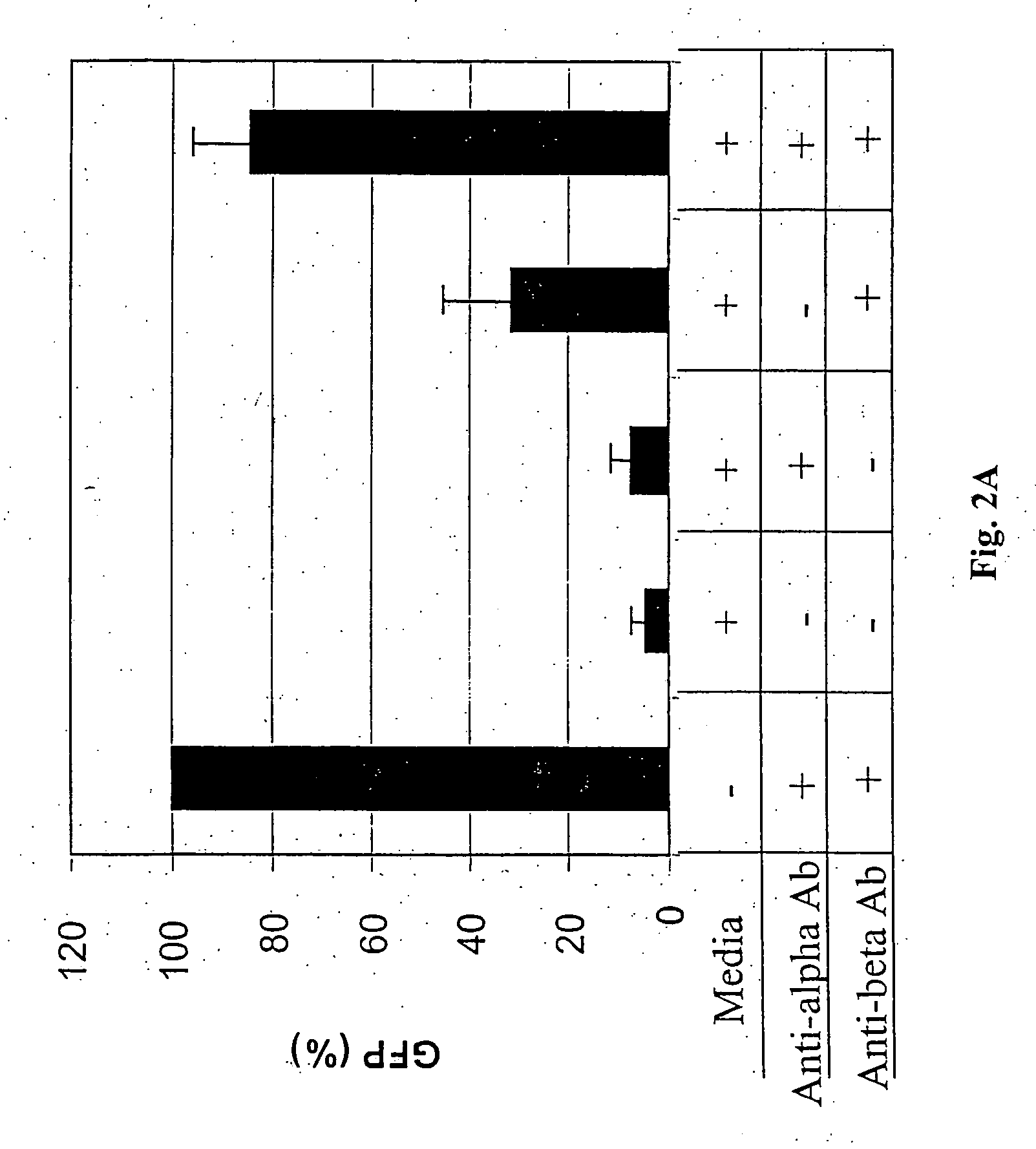
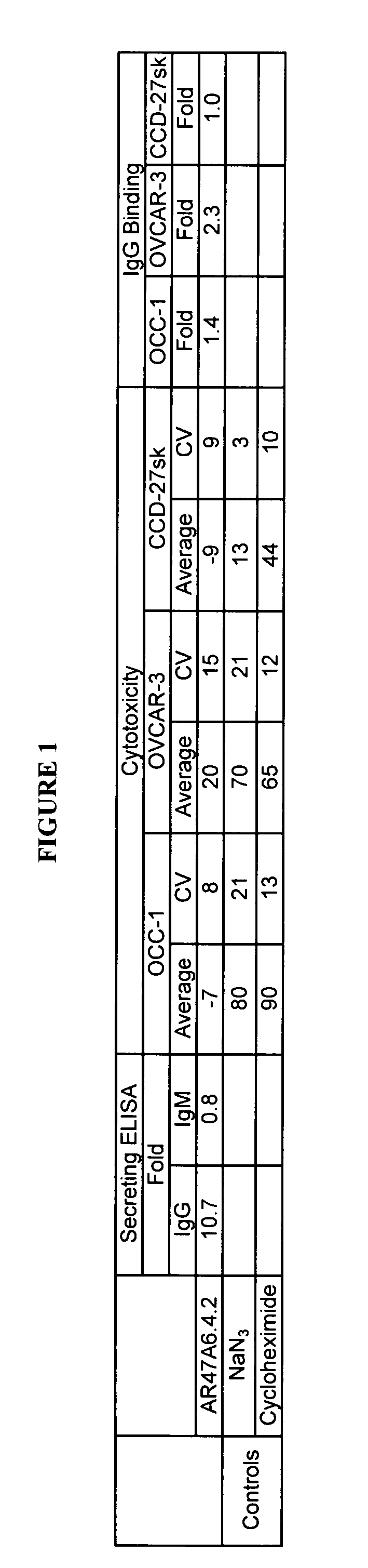
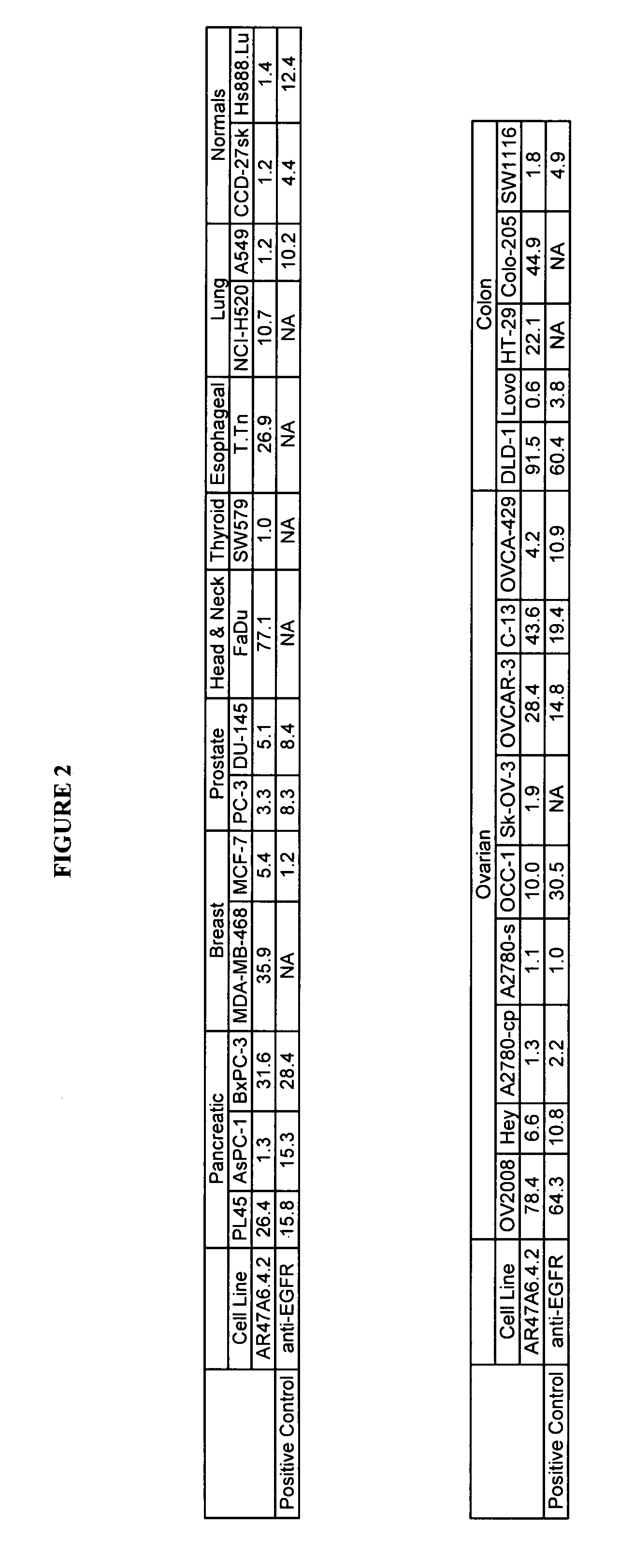
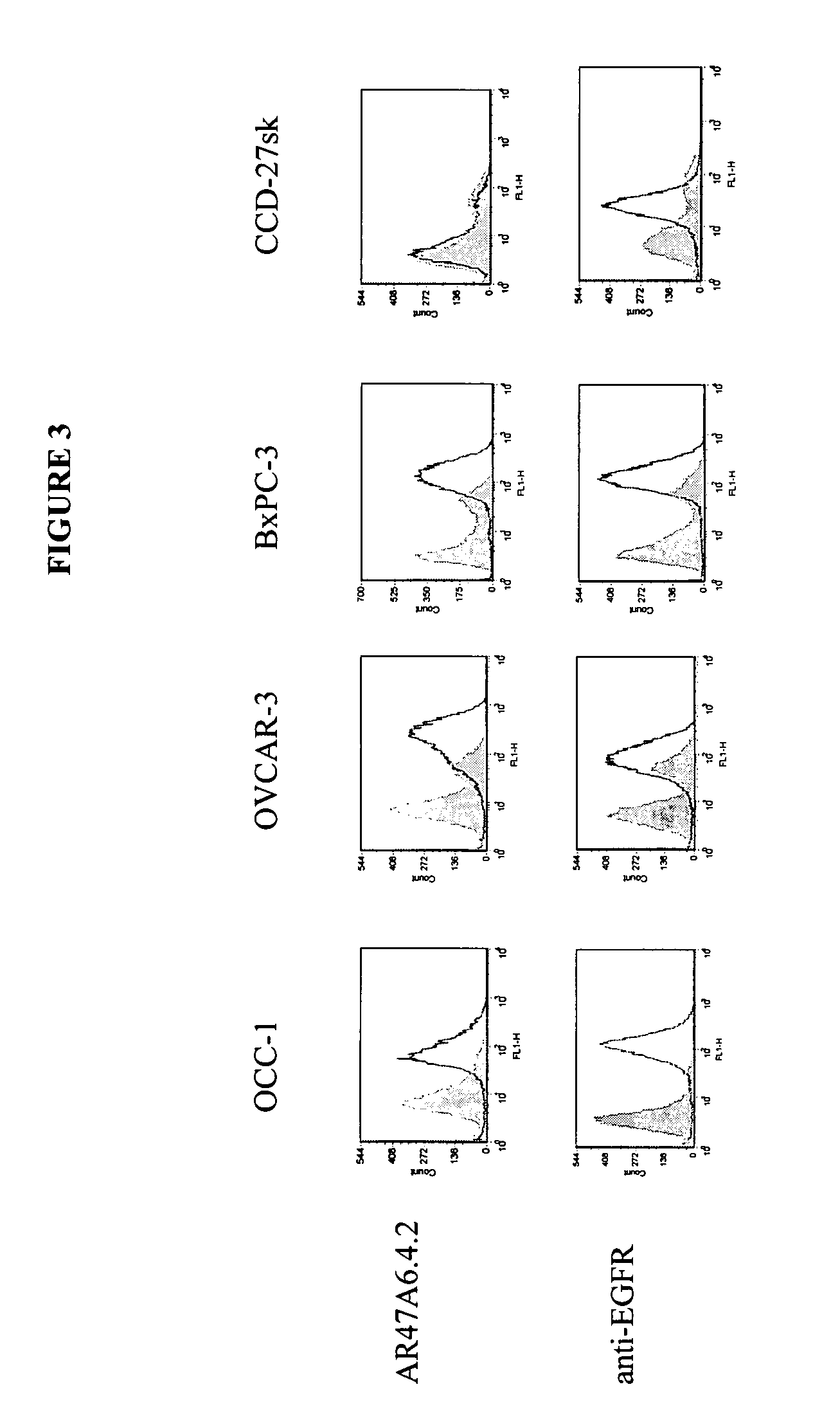
Cytotoxicity mediation of cells evidencing surface expression of TROP-2
InactiveUS7420040B2Reduce the likelihood of problemsProlong survival timeImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationDiseaseHematopoietic cell
The present invention relates to a method for producing cancerous disease modifying antibodies using a novel paradigm of screening. By segregating the anti-cancer antibodies using cancer cell cytotoxicity as an end point, the process makes possible the production of anti-cancer antibodies for therapeutic and diagnostic purposes. The antibodies can be used in aid of staging and diagnosis of a cancer, and can be used to treat primary tumors and tumor metastases. The anti-cancer antibodies can be conjugated to toxins, enzymes, radioactive compounds, cytokines, interferons, target or reporter moieties and hematogenous cells.
Owner:F HOFFMANN LA ROCHE & CO AG
Implantable device for continuous delivery of interferon
An implantable device includes a reservoir containing a suspension of an interferon in an amount sufficient to provide continuous delivery of the interferon at a therapeutically effective rate of 1 ng / day to 600 μg / day to maintain and achieve therapeutic blood or plasma levels of the interferon throughout a substantial period of the administration period.
Owner:INTARCIA THERAPEUTICS INC
Methods for treating wound tissue and forming a supplemented fibrin matrix
InactiveUS7196054B1Low antigenicityDecreasing thrombogenicityOrganic active ingredientsSurgical adhesivesTissue sealantVascular dilatation
Owner:AMERICAN NAT RED CROSS
Drug for reducing side effects in ribavirin interferon combination therapy
InactiveUS20060088502A1Eliminate side effectsReduces side effects found in ribavirin/IFNBiocideElcosanoid active ingredientsChronic viral hepatitis CSide effect
There is provided a drug for reducing side effects, anemia in particular, in combination therapy of chronic hepatitis C with ribavirin and interferon, which contains as the active ingredient at least one member selected from the group consisting of eicosapentaenoic acid (EPA) and pharmaceutically acceptable salts and esters thereof.
Owner:MOCHIDA PHARM CO LTD
Vaccina virus comprising cytokine and/or tumor associated antigen genes
InactiveUS6537594B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Owner:VIROGENETICS
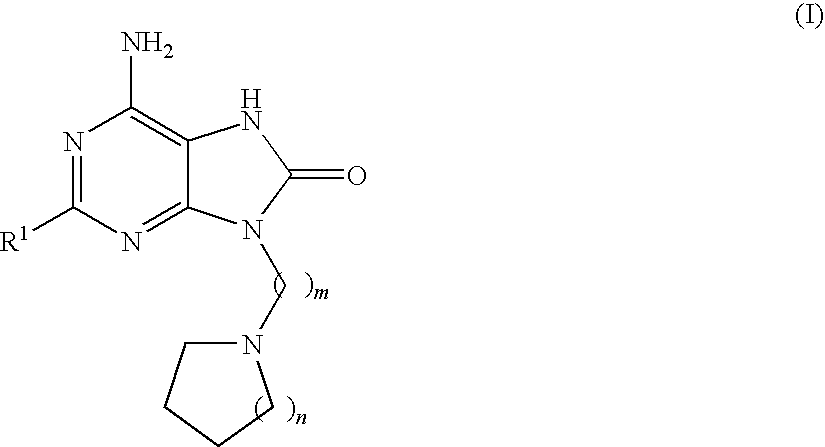
Compounds
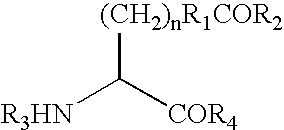
Compounds of formula (I):wherein R1 is C1-6alkylamino, C1-6alkoxy, or C3-7cycloalkyloxy; m is an integer having a value of 3 to 6; n is an integer having a value of 0 to 4; and salts thereof are inducers of human interferon. Compounds which induce human interferon may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com