Double-stranded and single-stranded RNA molecules with 5 ' triphosphates and their use for inducing interferon
a technology of rna molecules and triphosphates, which is applied in the field of rna molecules, can solve the problems of inability to recognize the use and advantages of rnai, and the cost can be substantial, and achieve the effect of strong, non-sequence-specific antiviral respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
RNAs
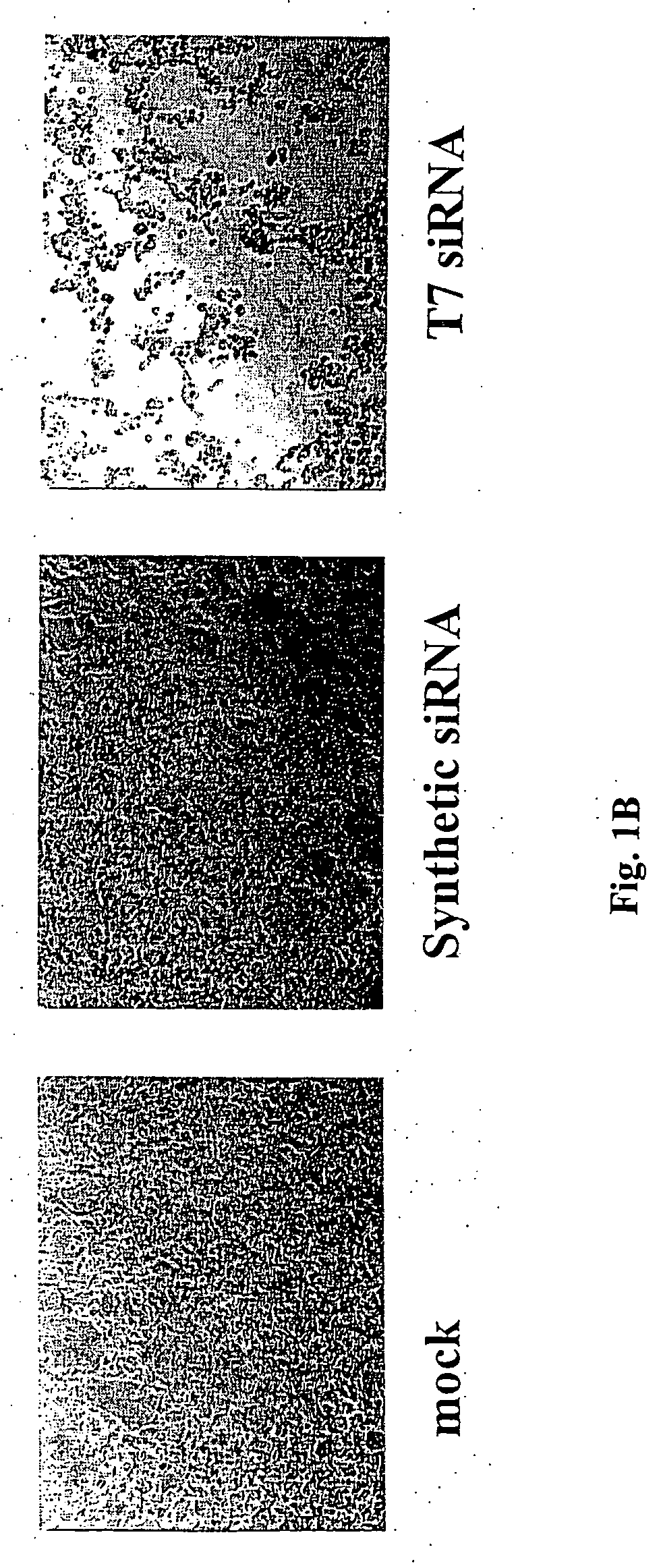
[0119] The chemically synthesized RNAs were purchased from Dharmacon. The T7 siRNAs were synthesized using the Silencer siRNA construction kit from Ambion according to the manufacturer's protocol. To transcribe RNA in vitro, T7 primer I (5′-TAATACGACTCACTAT A-3′ Q ID NO:15)) was hybridized with T7 primer II, which contains antisense sequence of each transcribed RNA and the tail sequence of 5′-CCCTATAGTGAGTCGTA-3′ Q ID NO:16). To make siRNA without interferon induction, the first AA was replaced by TT and included in the T7 primer II. For example, to make GFP #2 T7 (21-AA), two primers were used (5′-TTAAGCTGACCCTGAAGTTCATCCCCTATAGTGAGTCGTA-3′ (SEQ ID NO:17) and 5′-TTGATGAACTTCAGGGTCAGCTTCCCTATAGTGAGTCGTA-3′ (SEQ ID NO:18)). For the CIP, 20 U of RNAs (NEB) was added to the siRNA after DNase and RNase T1 digestion, and further incubated at 37° C. for 1 h. Final siRNAs were column-purified using conditions recommended for the Silencer siRNA construction kit.
[0120] To synthesize th...
example 2
Transfection and RNAi Assay
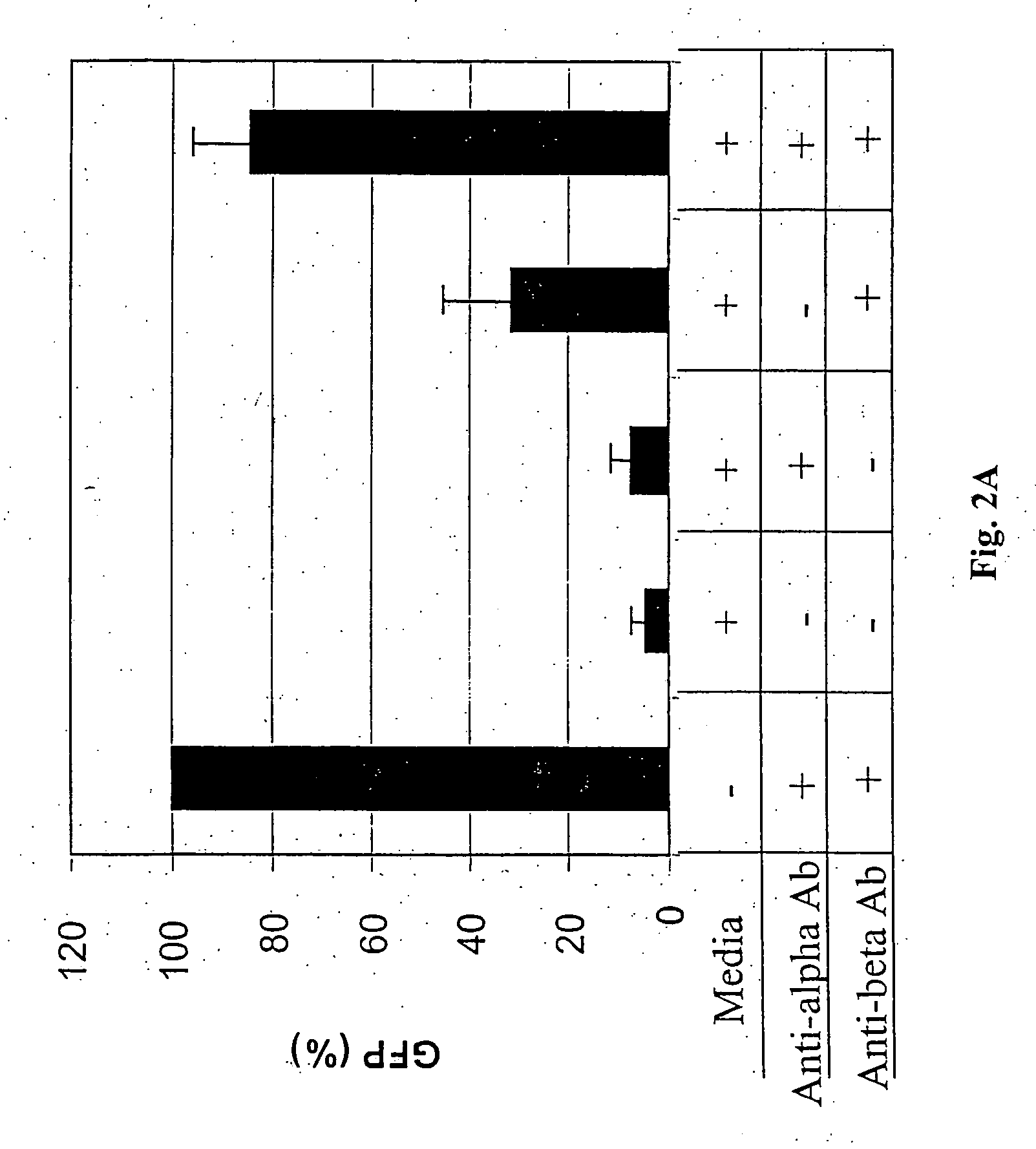
[0122] All transfection assays were done using Lipofectamine 2000 following the manufacturer's protocol (Invitrogen). HEK-293 cells at ninety percent confluency were transfected in 24-well plates with the reporter genes and siRNAs. 250 ng of the pLEGFP-C1 vector (Clontech) and 10 nM of each anti-EGFP siRNA were cotransfected. EGFP expression levels were determined 24 h later from the mean number of EGFP-fluorescent cells determined by fluorescence-activated cell sorting (FACS) analyses. Percentages of EGFP expression were determined relative to nonspecific controls.
[0123] For monitoring of cell death, the medium from transfected cells was changed 24 h after transfection. Additional media changes were made after 48 h, and the plates were examined microscopically after another 48 h incubation.
example 3
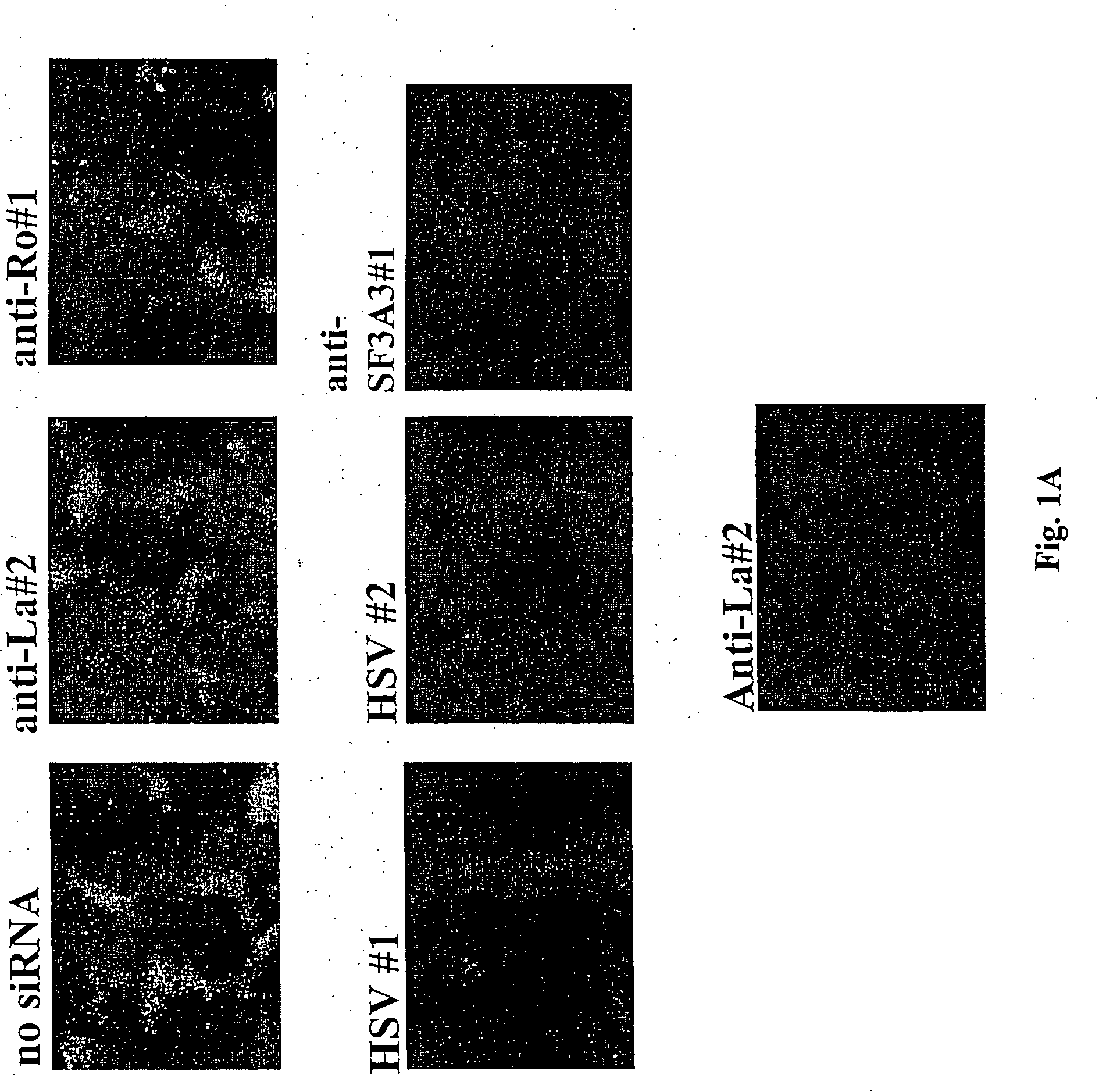
[0124] Anti-HSV-1 Assays
[0125] 60% confluent HEK-293 cells were transfected in 24-well dishes with siRNA or ssRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The cells were placed in fresh medium 18 h after transfection. The cells were infected 6 h later with HSV-1 expressing EGFP at an MOI of 1. Cells were subject to FACS analyses 24 h after infection to determine levels of EGFP expression.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com