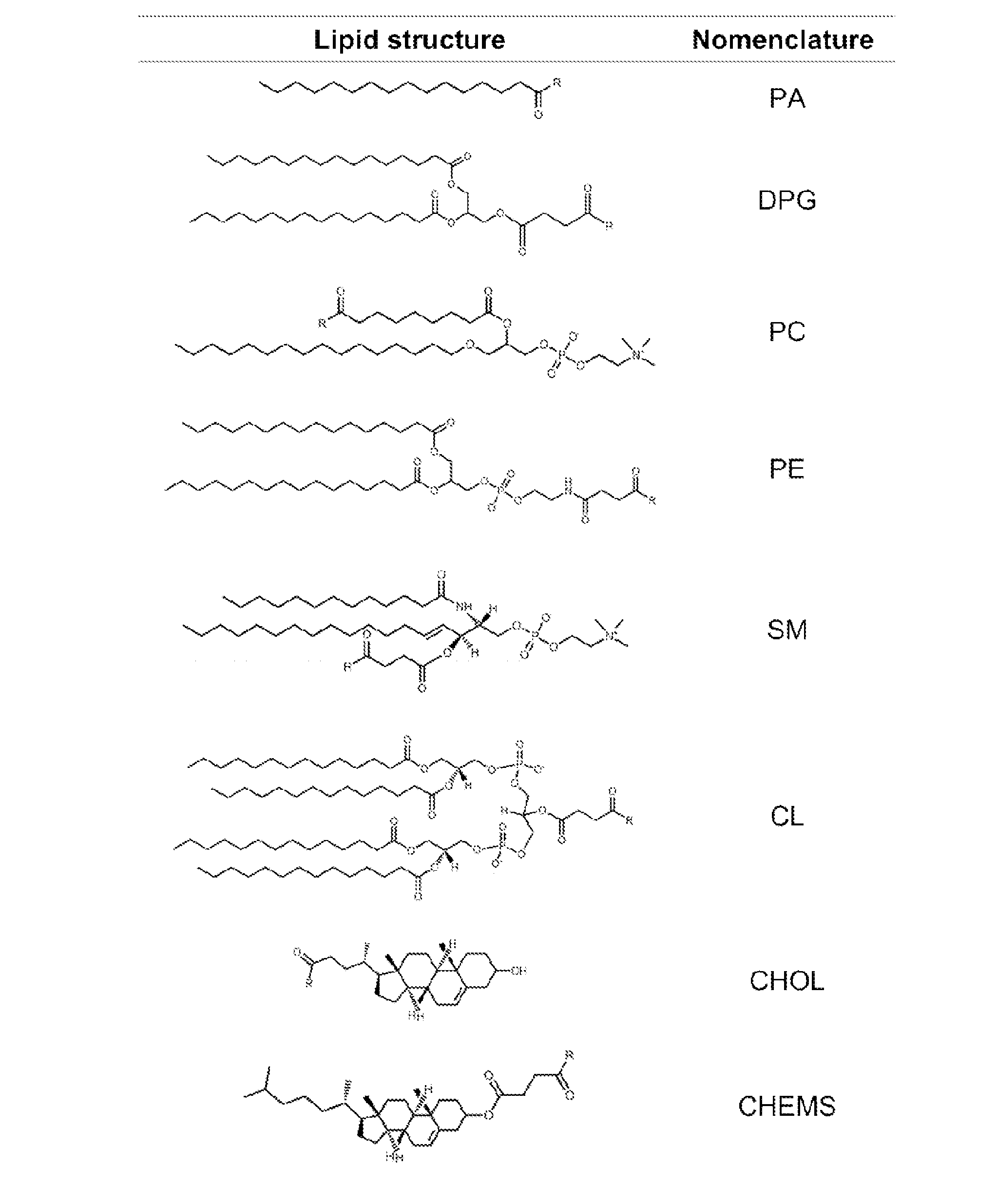
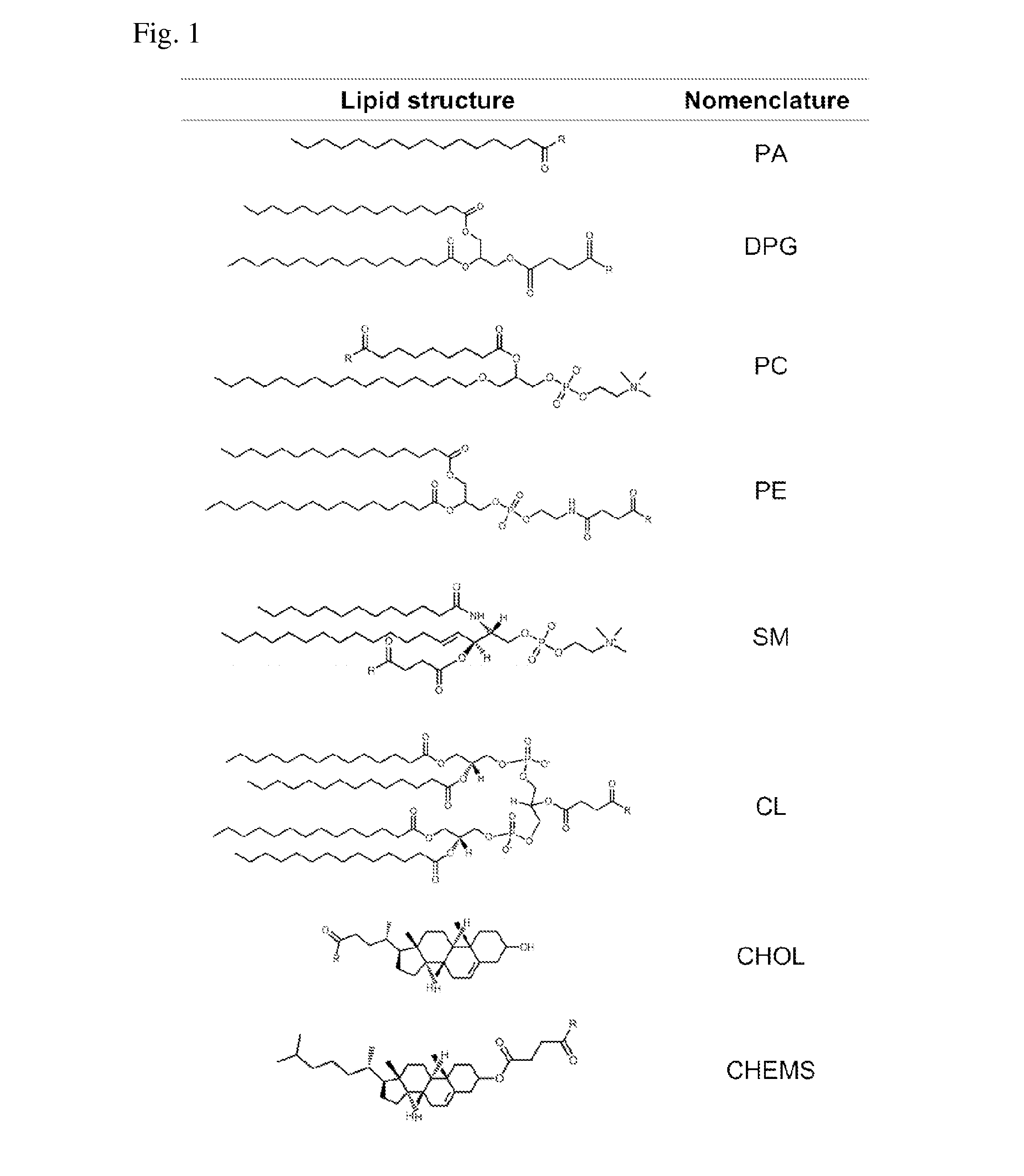
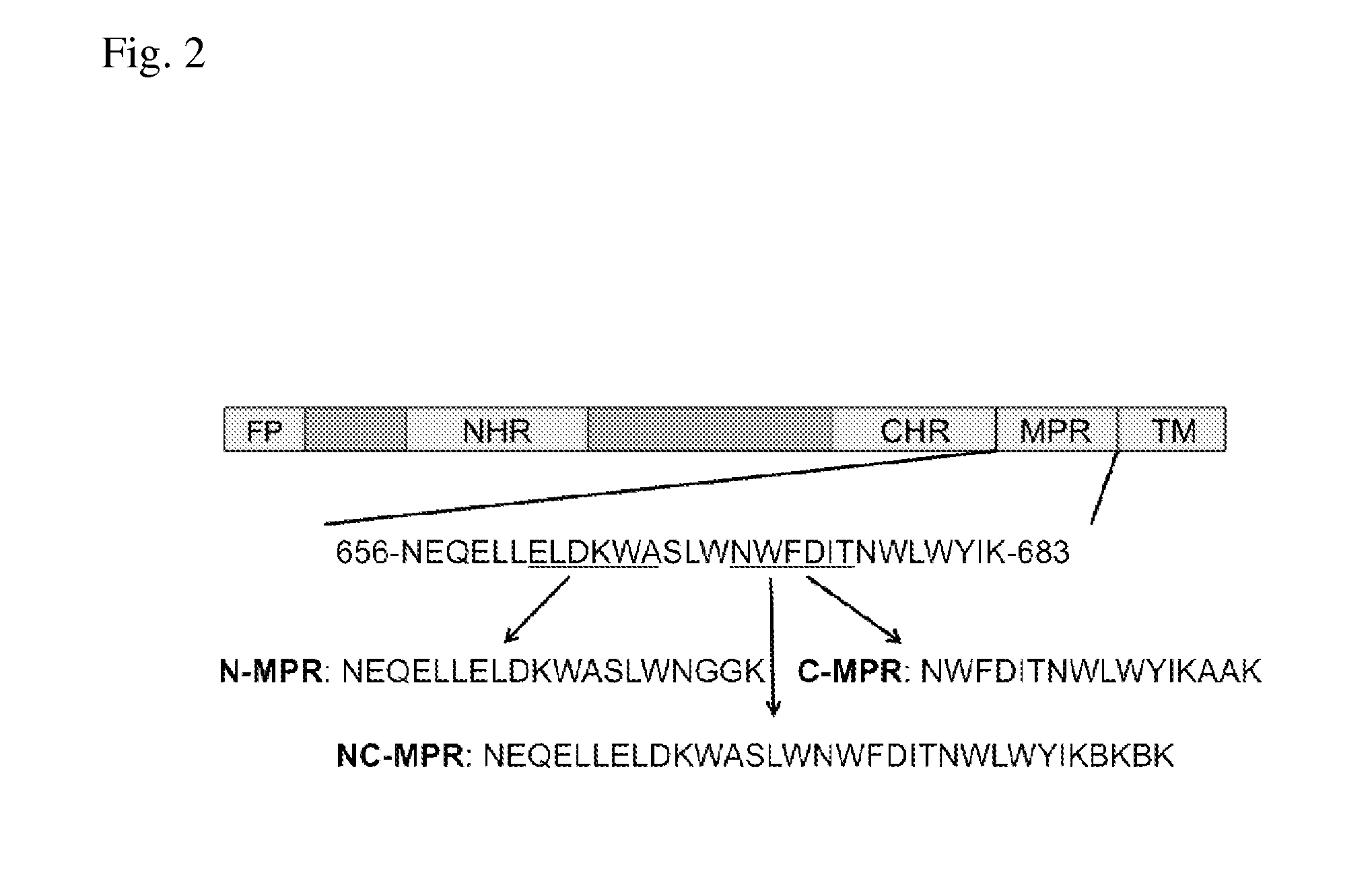
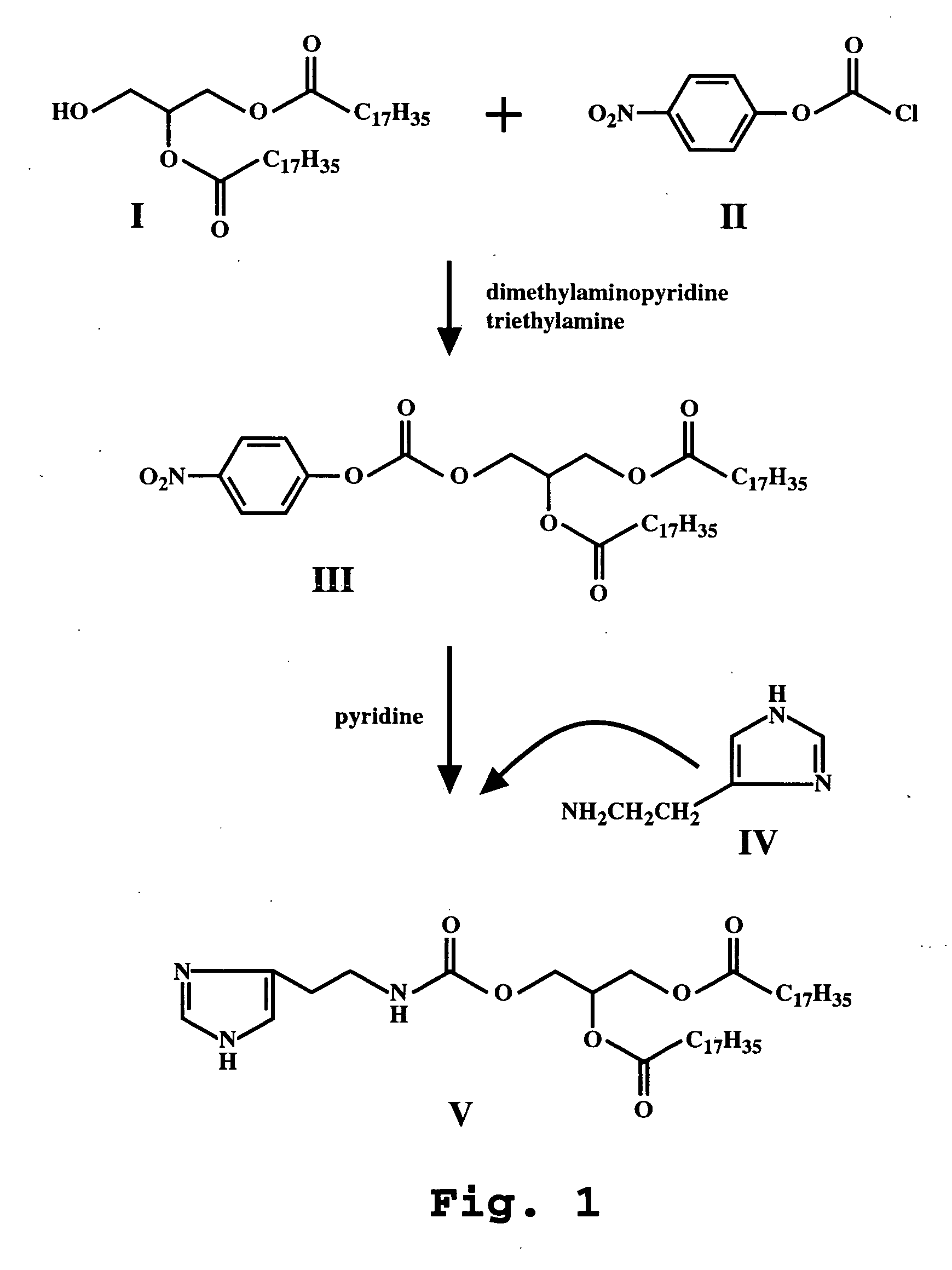
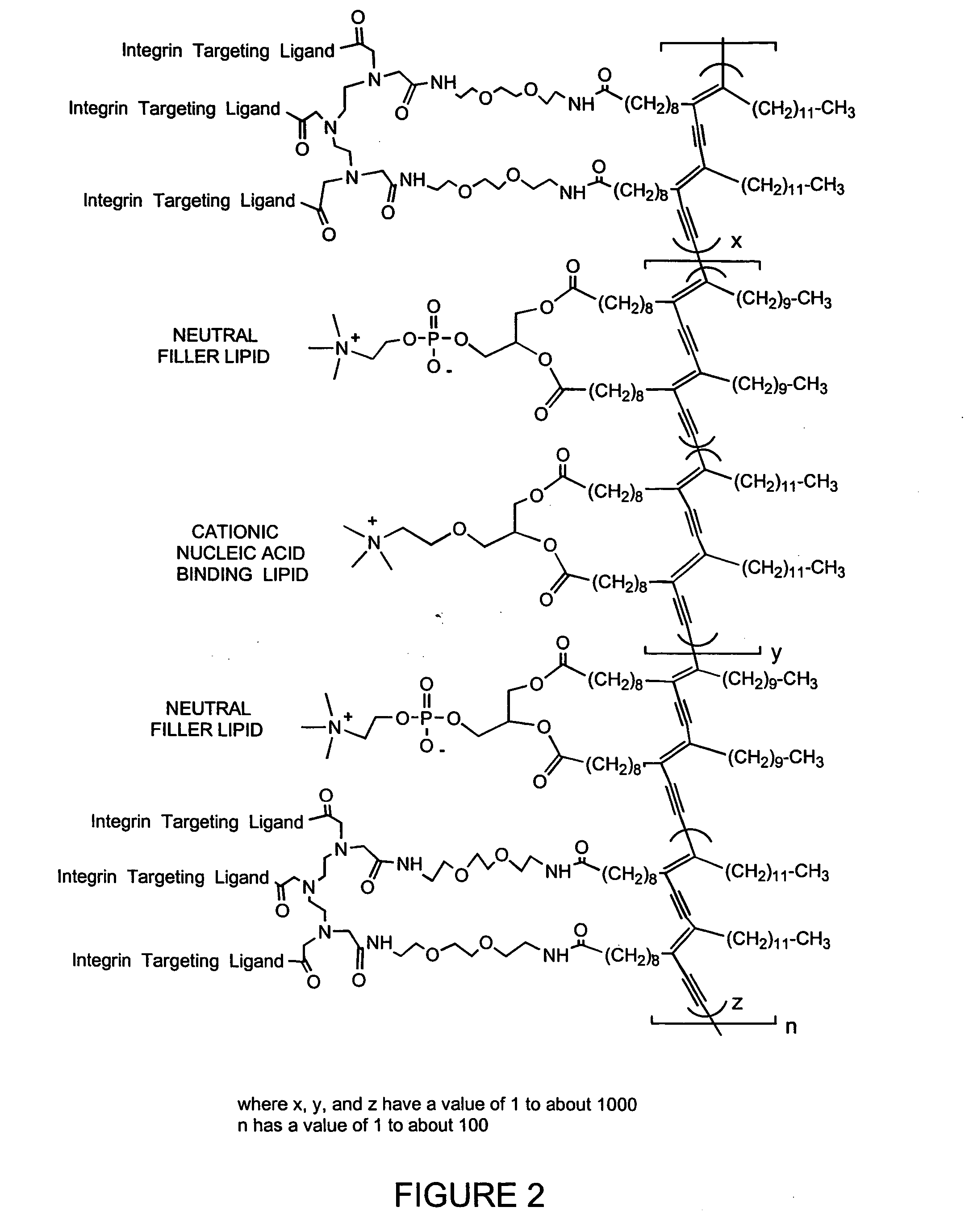
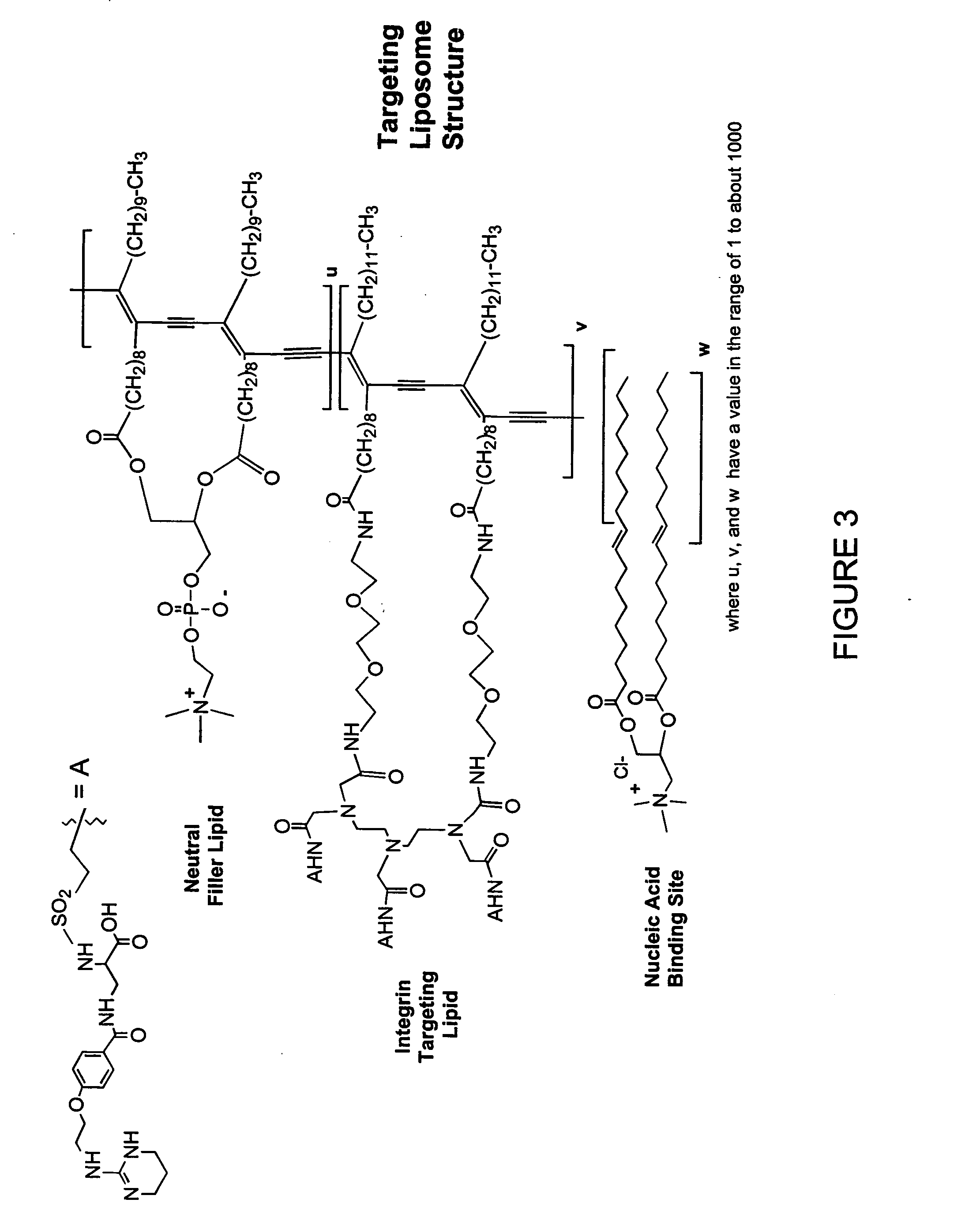
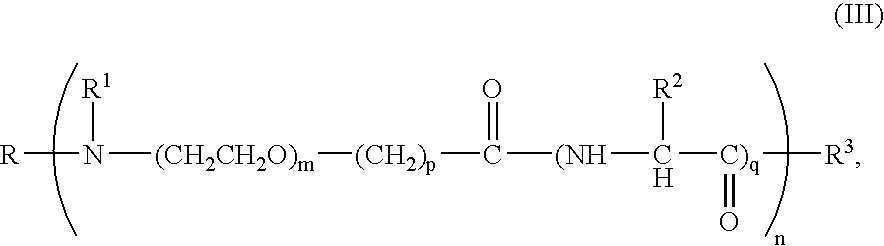
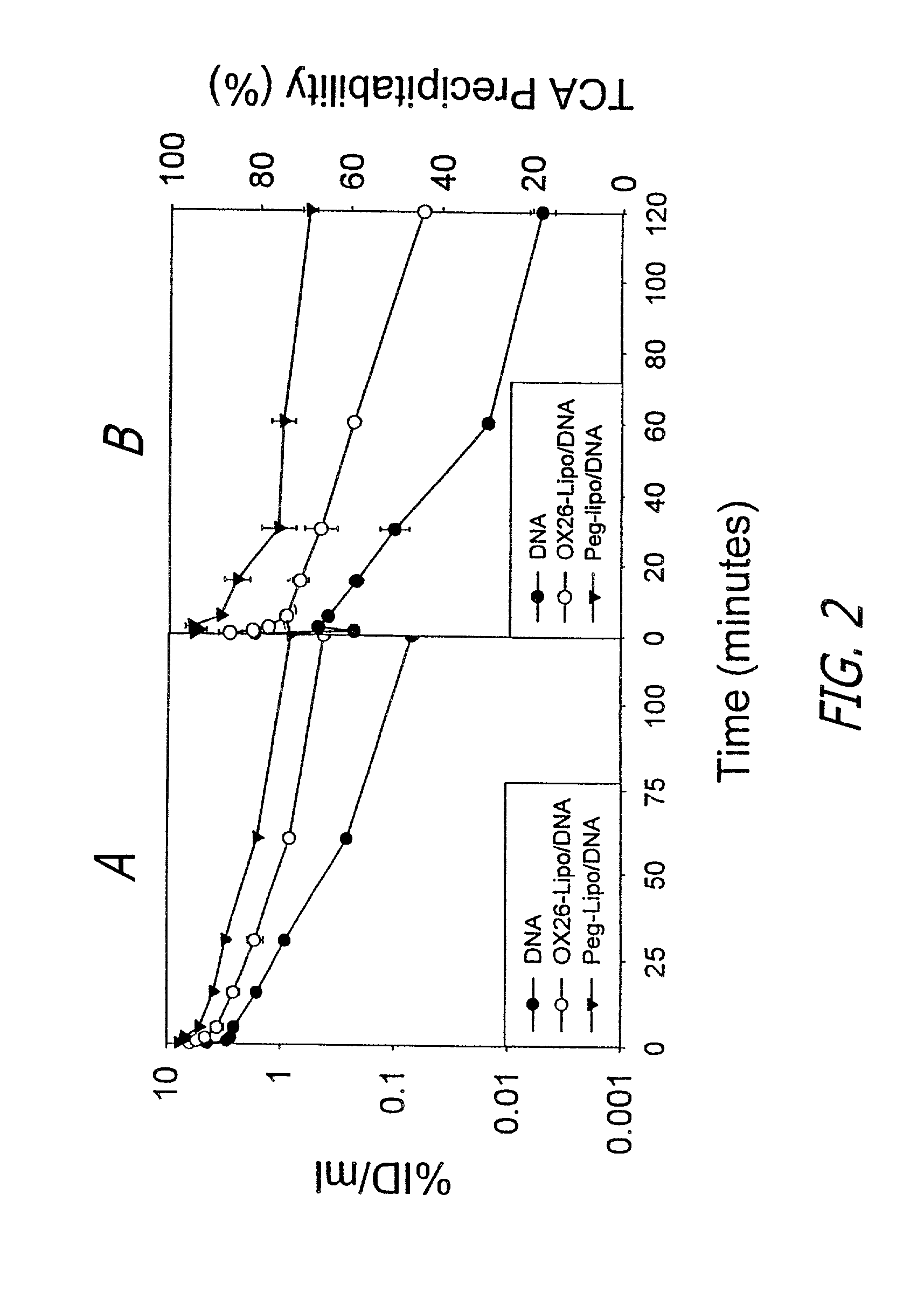
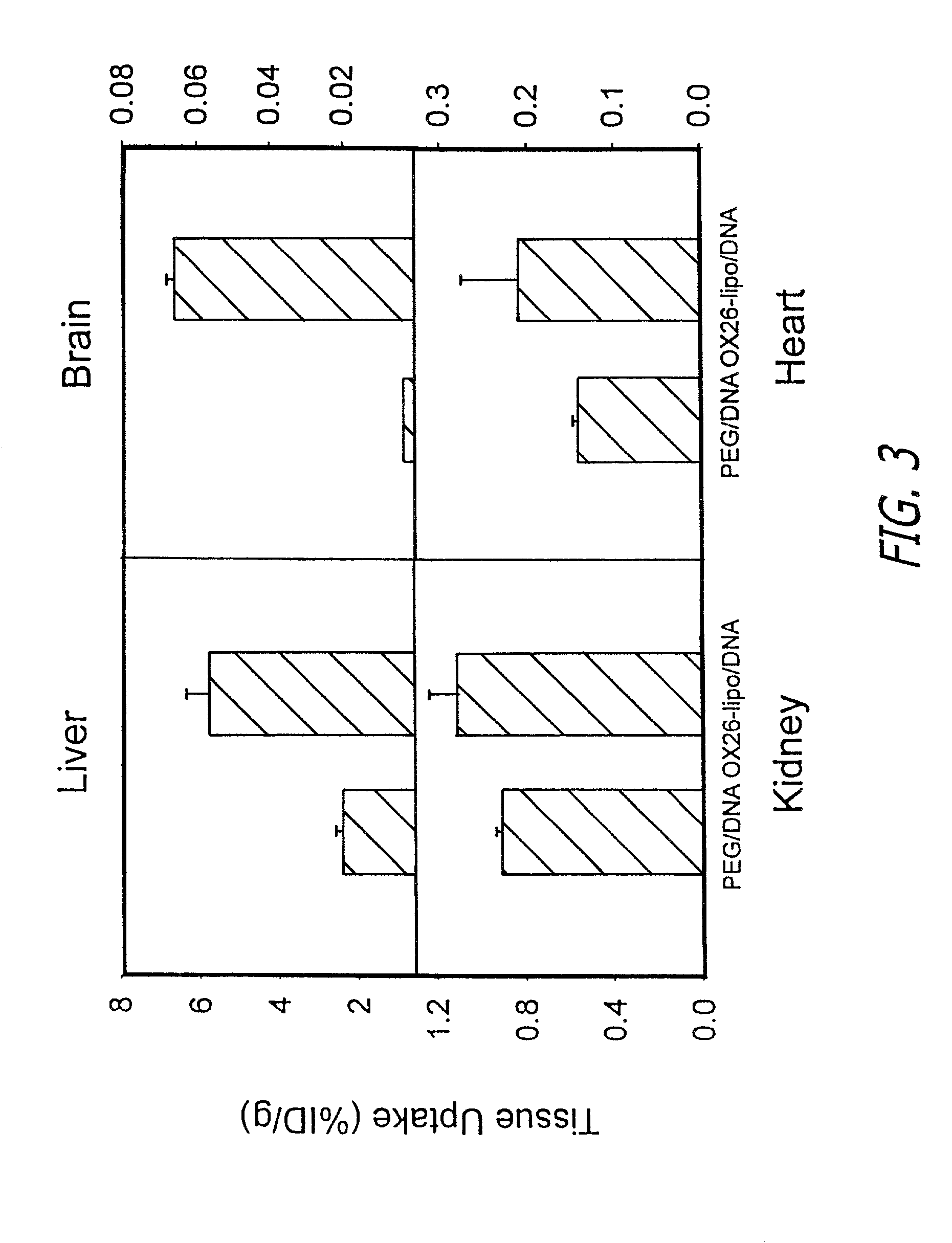
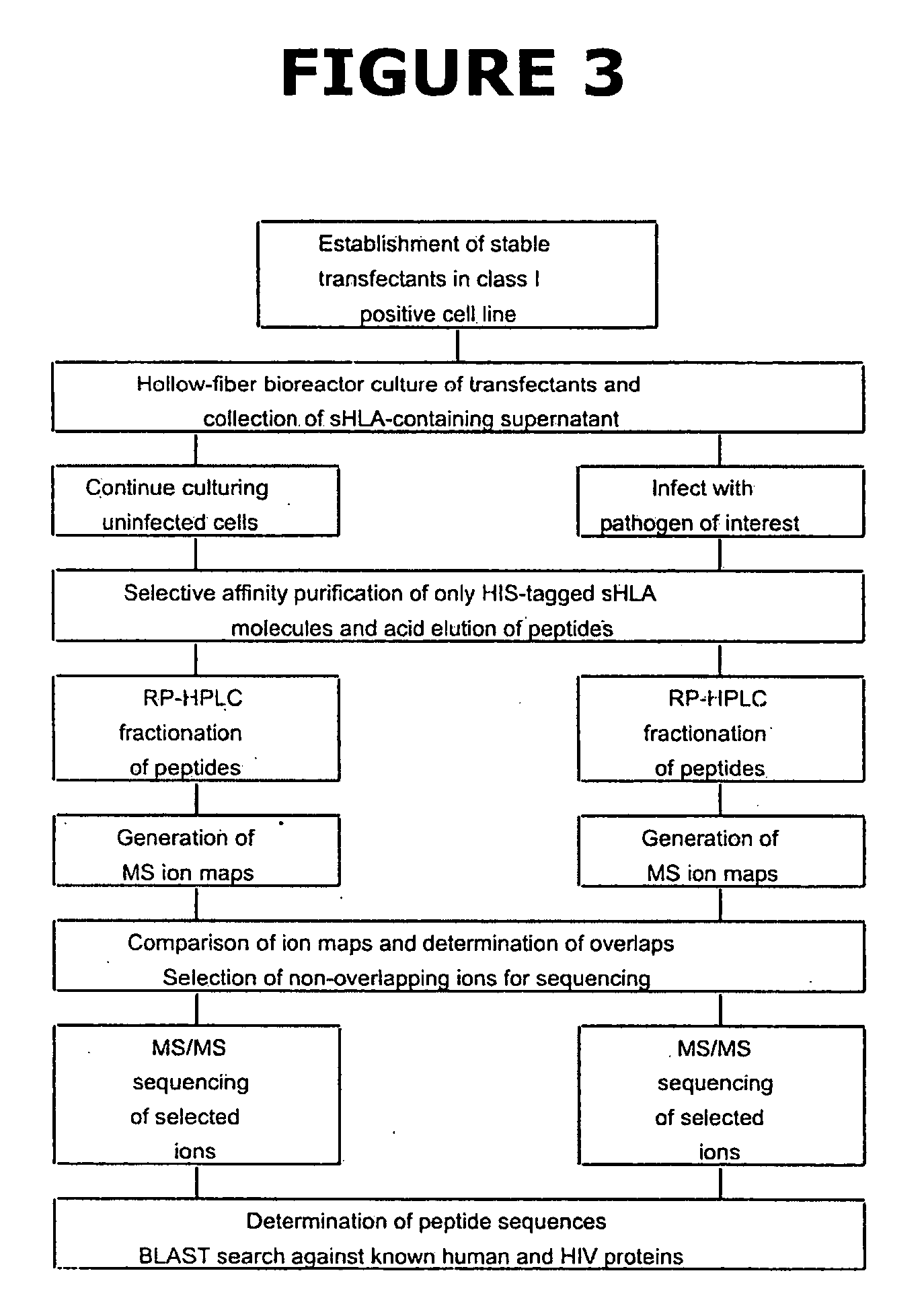
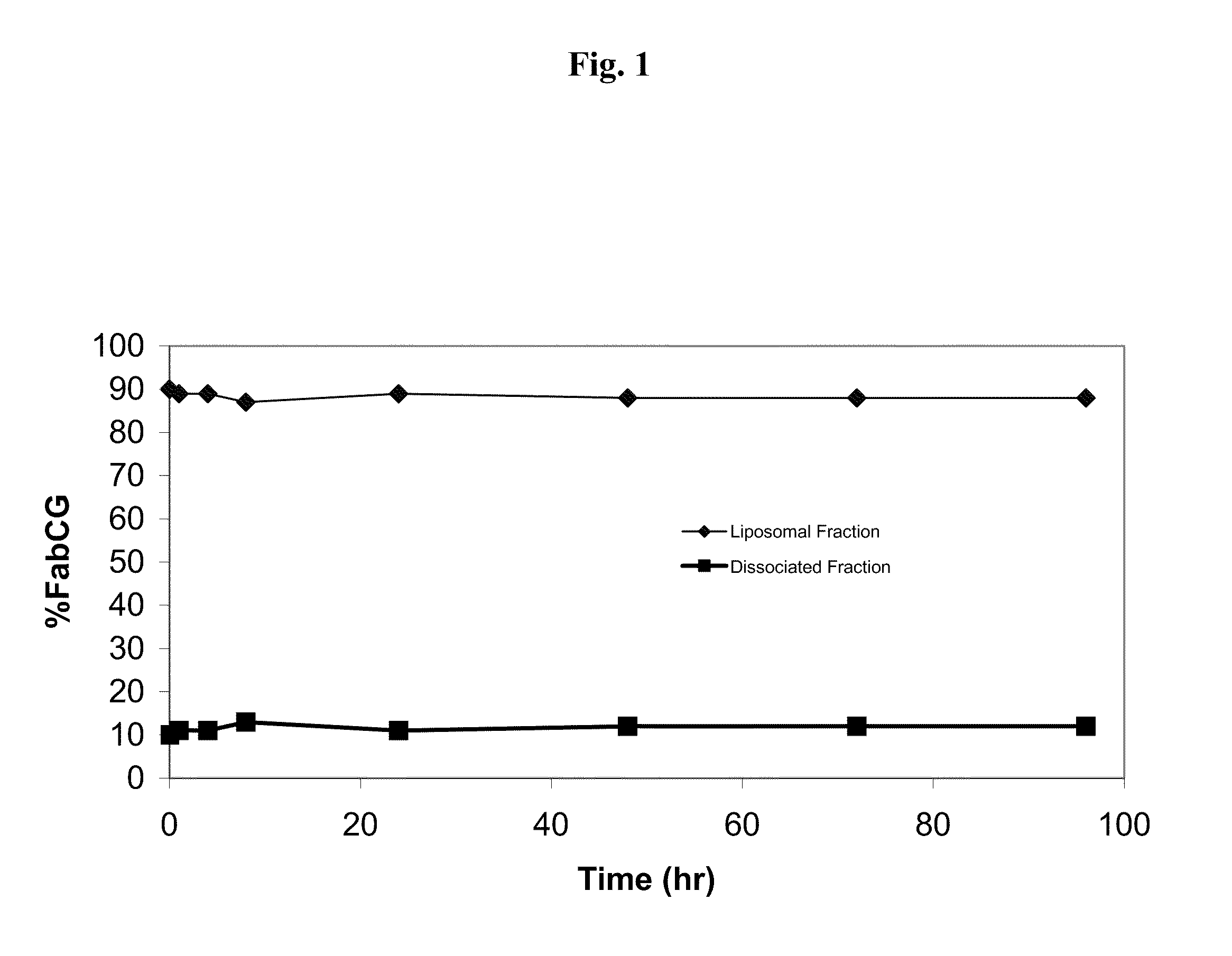
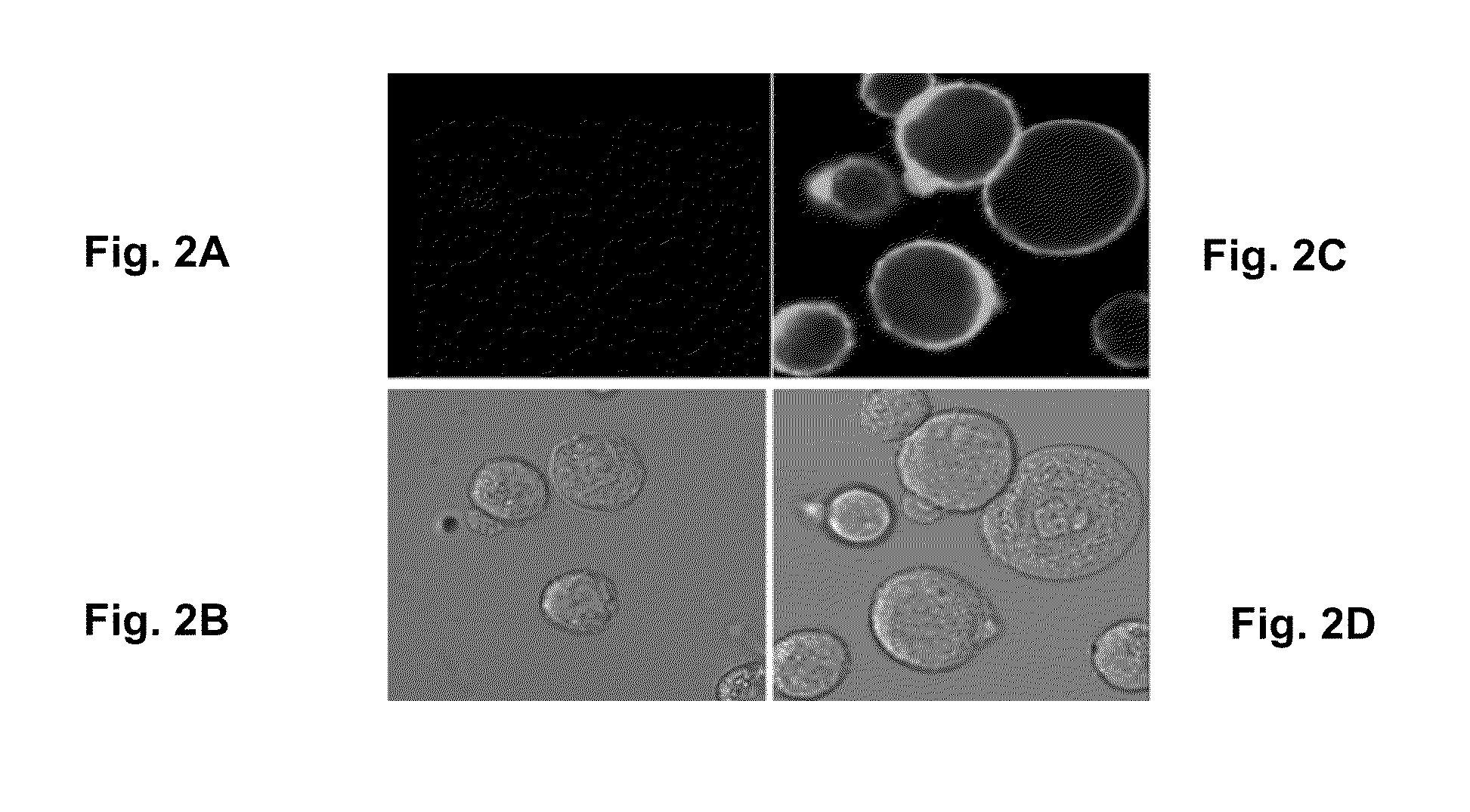
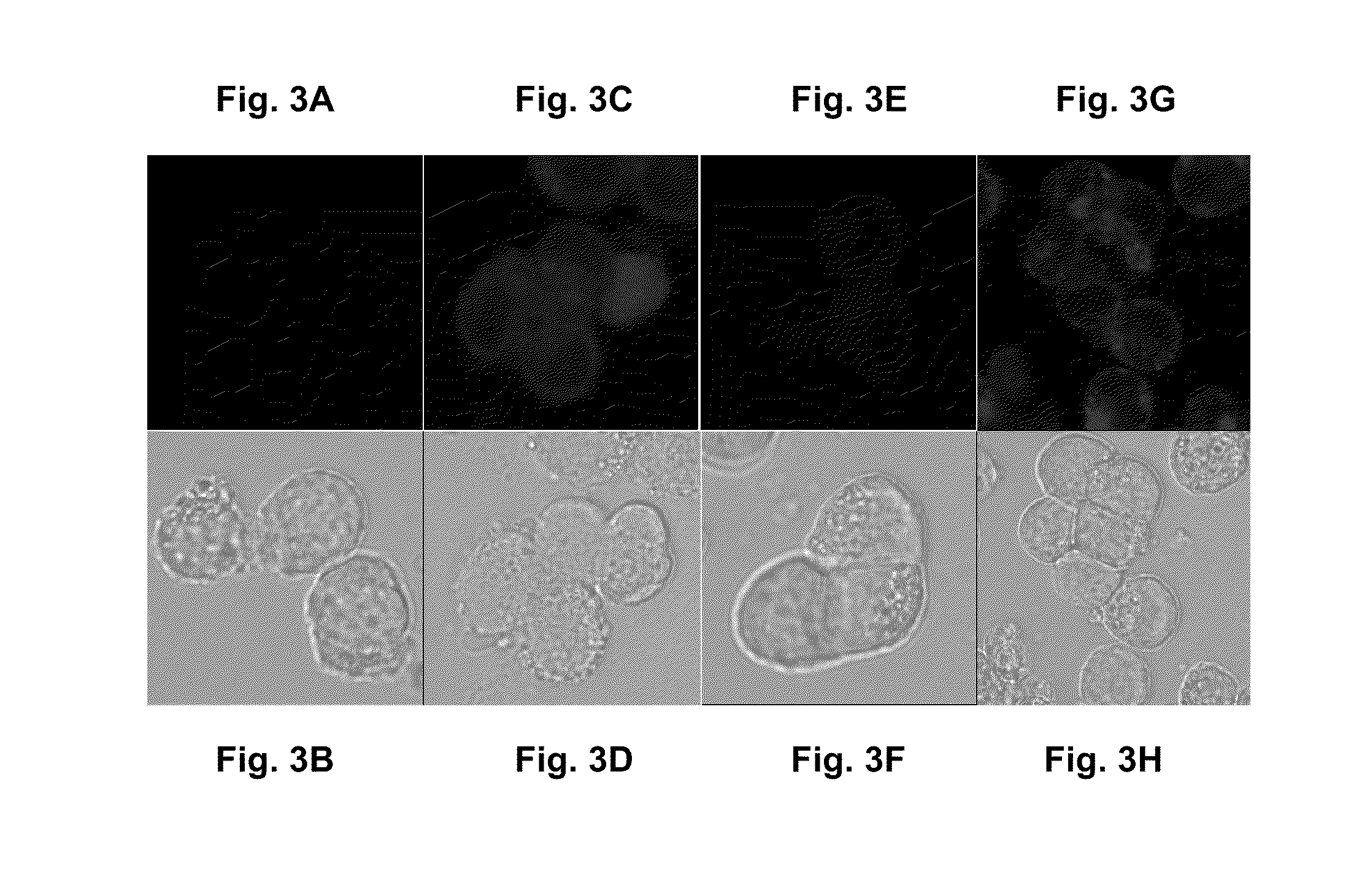
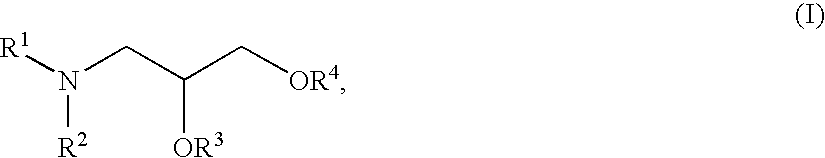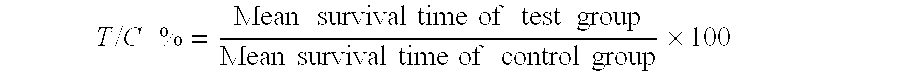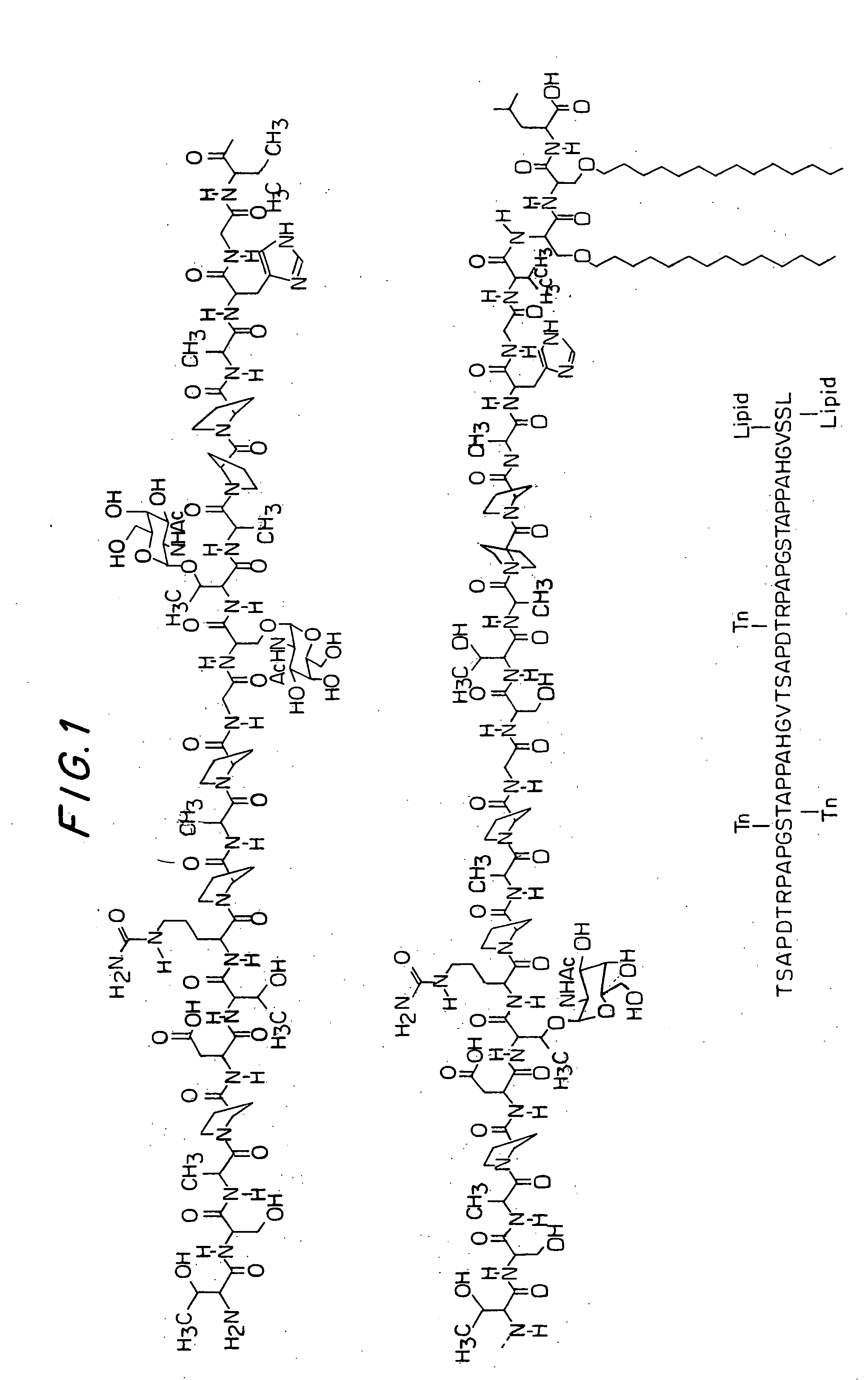Patents
Literature
789 results about "Lipofectamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lipofectamine or Lipofectamine 2000 is a common transfection reagent, produced and sold by Invitrogen, used in molecular and cellular biology. It is used to increase the transfection efficiency of RNA (including mRNA and siRNA) or plasmid DNA into in vitro cell cultures by lipofection. Lipofectamine contains lipid subunits that can form liposomes in an aqueous environment, which entrap the transfection payload, e.g. DNA plasmids.
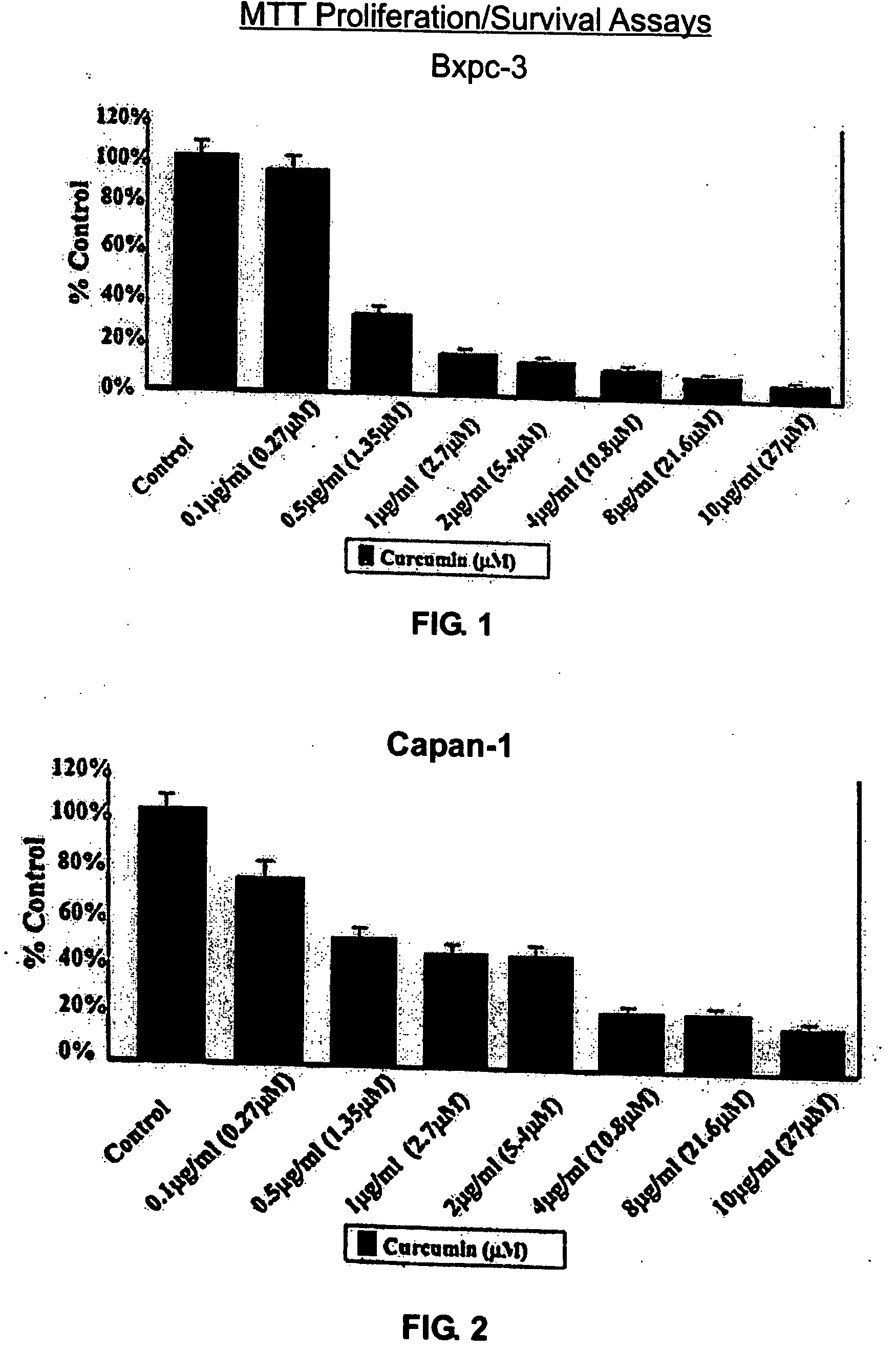
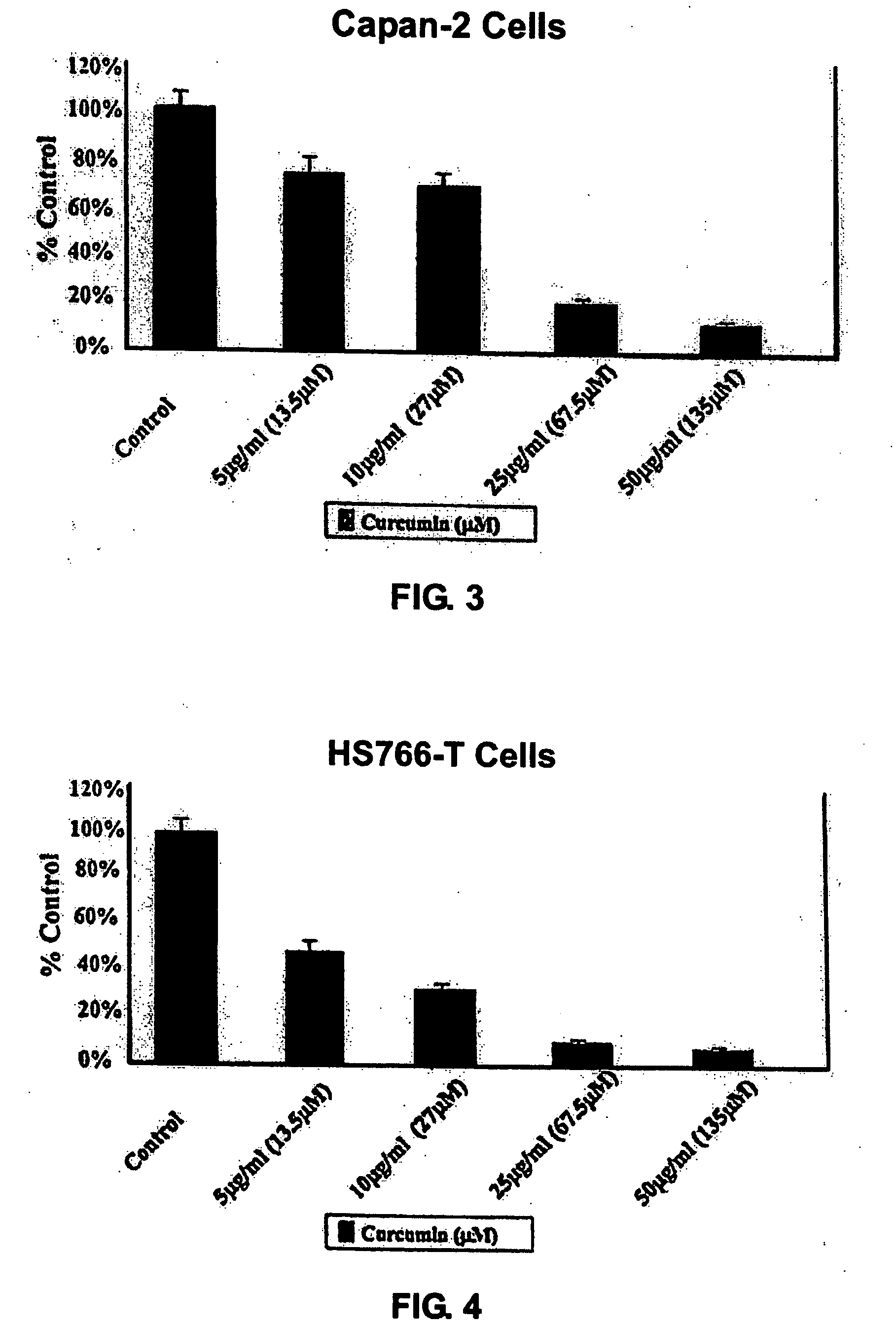
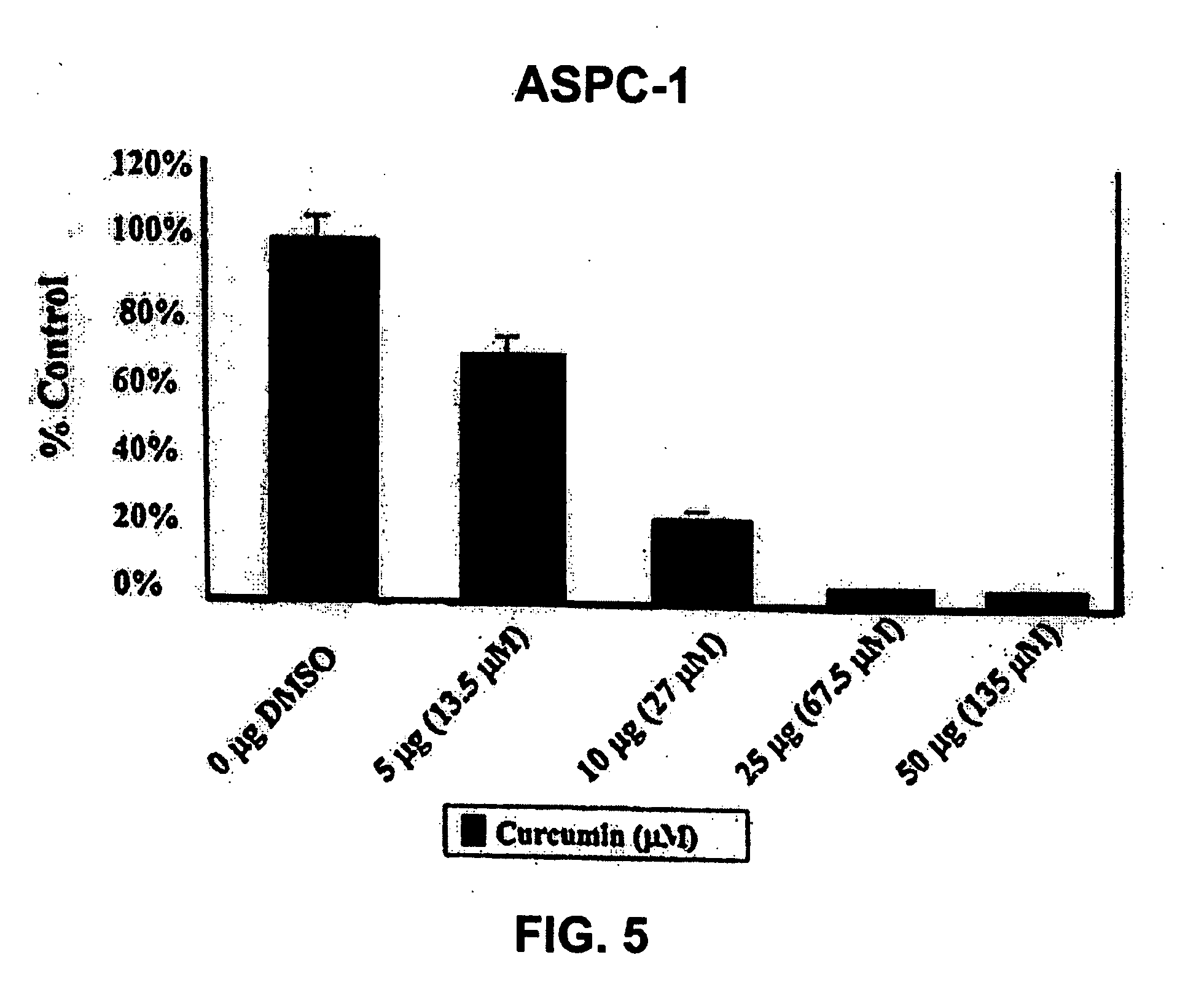
Liposomal curcumin for treatment of cancer
The present invention provides a compositions and methods for the treatment of cancer, including pancreatic cancer, breast cancer and melanoma, in a human patient. The methods and compositions of the present invention employ curcumin or a curcumin analogue encapsulated in a colloidal drug delivery system, preferably a liposomal drug delivery system. Suitable colloidal drug delivery systems also include nanoparticles, nanocapsules, microparticles or block copolymer micelles. The colloidal drug delivery system encapsulating curcumin or a curcumin analogue is administered parenterally in a pharmaceutically acceptable carrier.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
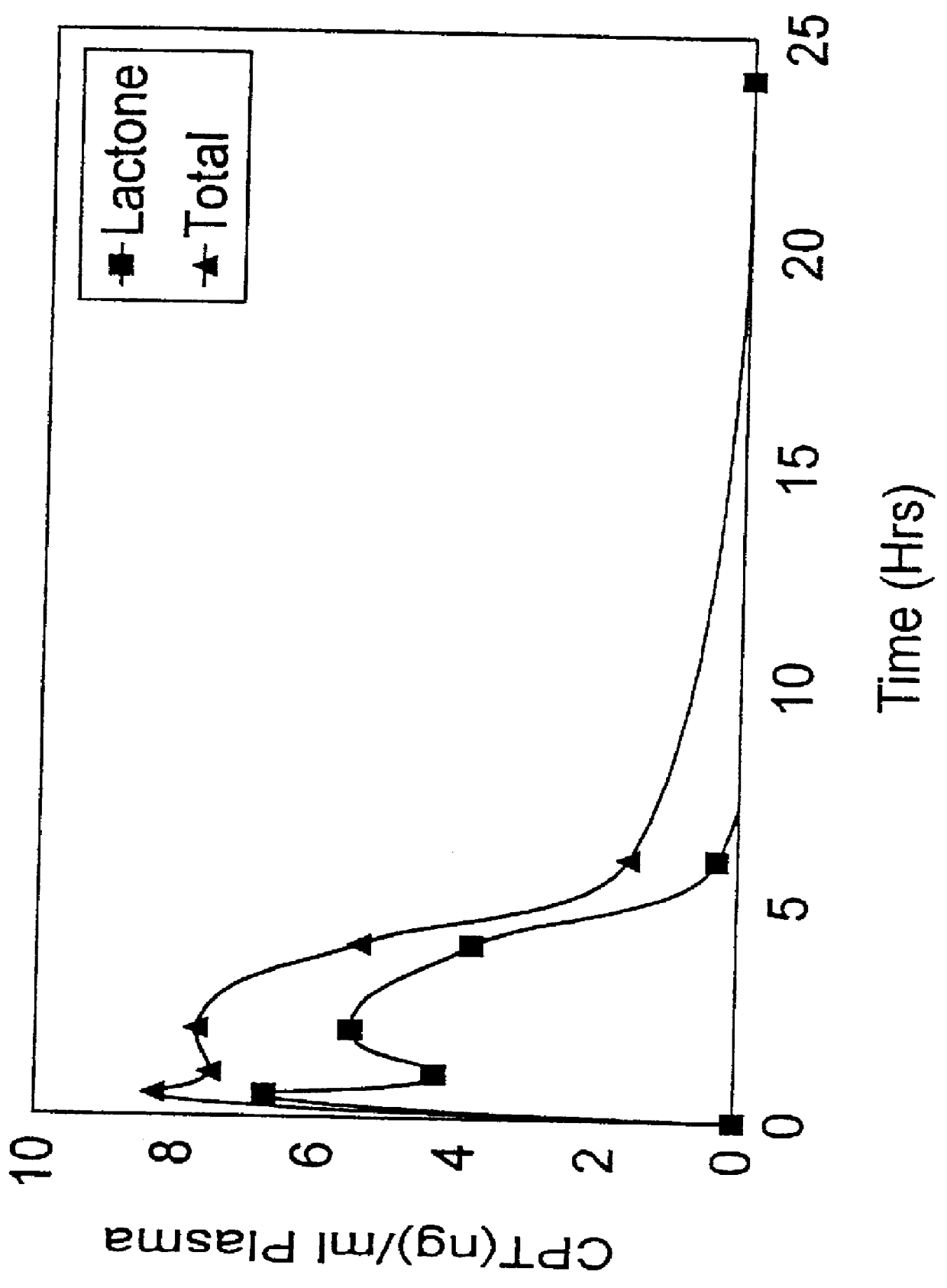
Liposomal prodrugs comprising derivatives of camptothecin and methods of treating cancer using these prodrugs
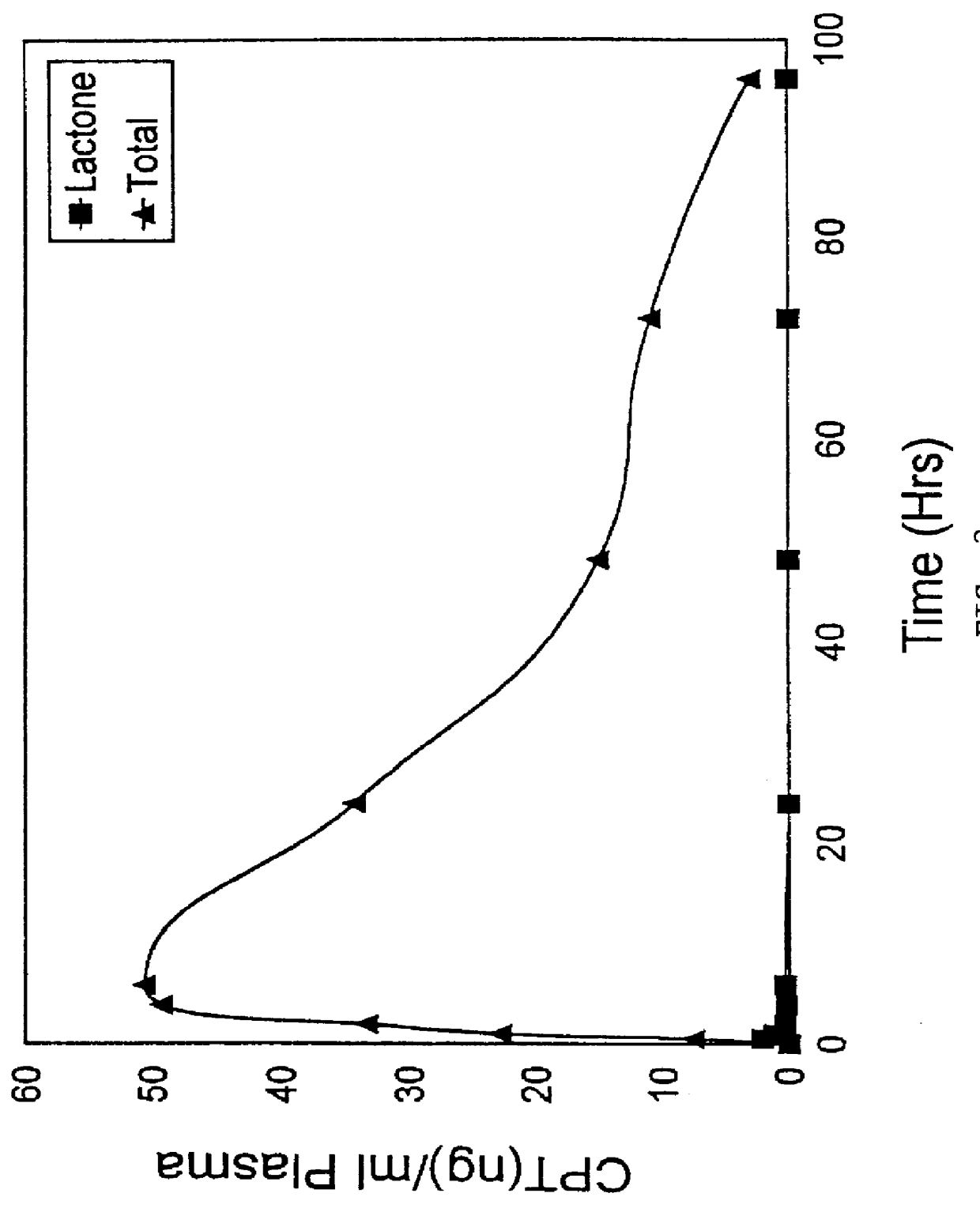
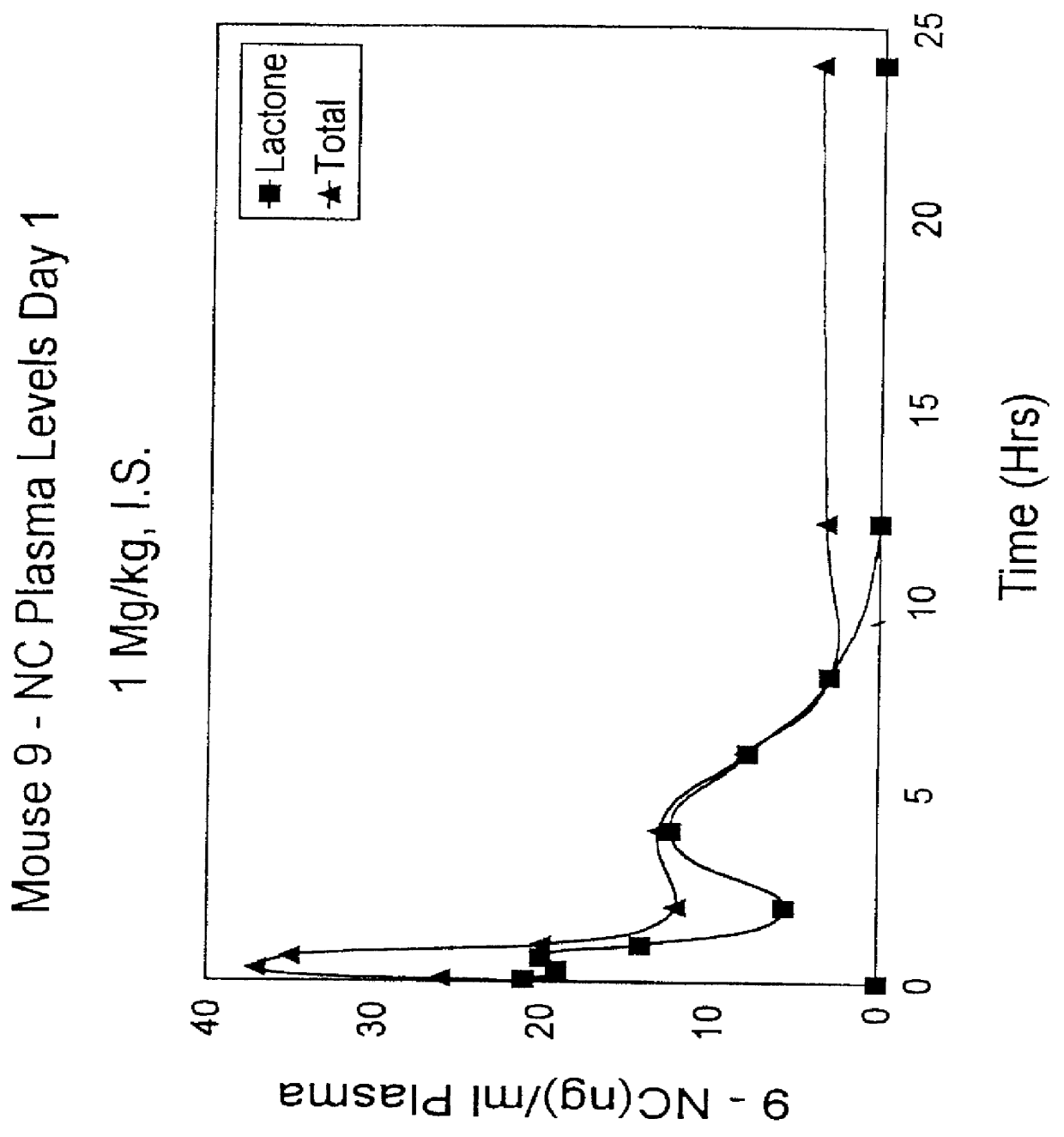
Liposomal prodrugs include specific derivatives of camptothecin restrained by a liposomal delivery system. The derivatives of camptothecin are represented by the general formula: ##STR1## wherein when R.sub.2 is H, R.sub.1 is a C.sub.2 -C.sub.4 alkyl group, a C.sub.6 -C.sub.15 alkyl group, a C.sub.3 -C.sub.8 cycloalkyl group, a C.sub.2 -C.sub.15 alkenyl group or a C.sub.2 -C.sub.15 epoxy group; and when R.sub.2 is a nitro group, R.sub.1 is a C.sub.1 -C.sub.15 alkyl group, a C.sub.2 -C.sub.15 alkenyl group, a C.sub.3 -C.sub.8 cycloalkyl group, or an epoxy group. Processes for making these prodrugs and for using them in cancer treatment are also disclosed.
Owner:THE STEHLIN FOUND FOR CANCER RES
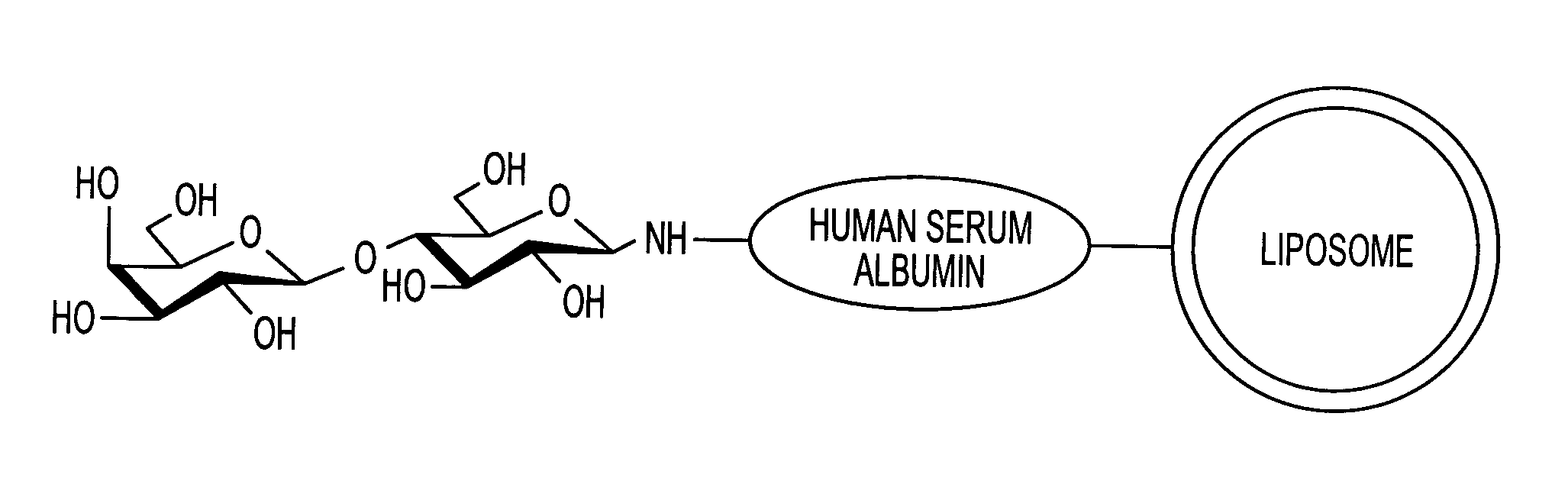
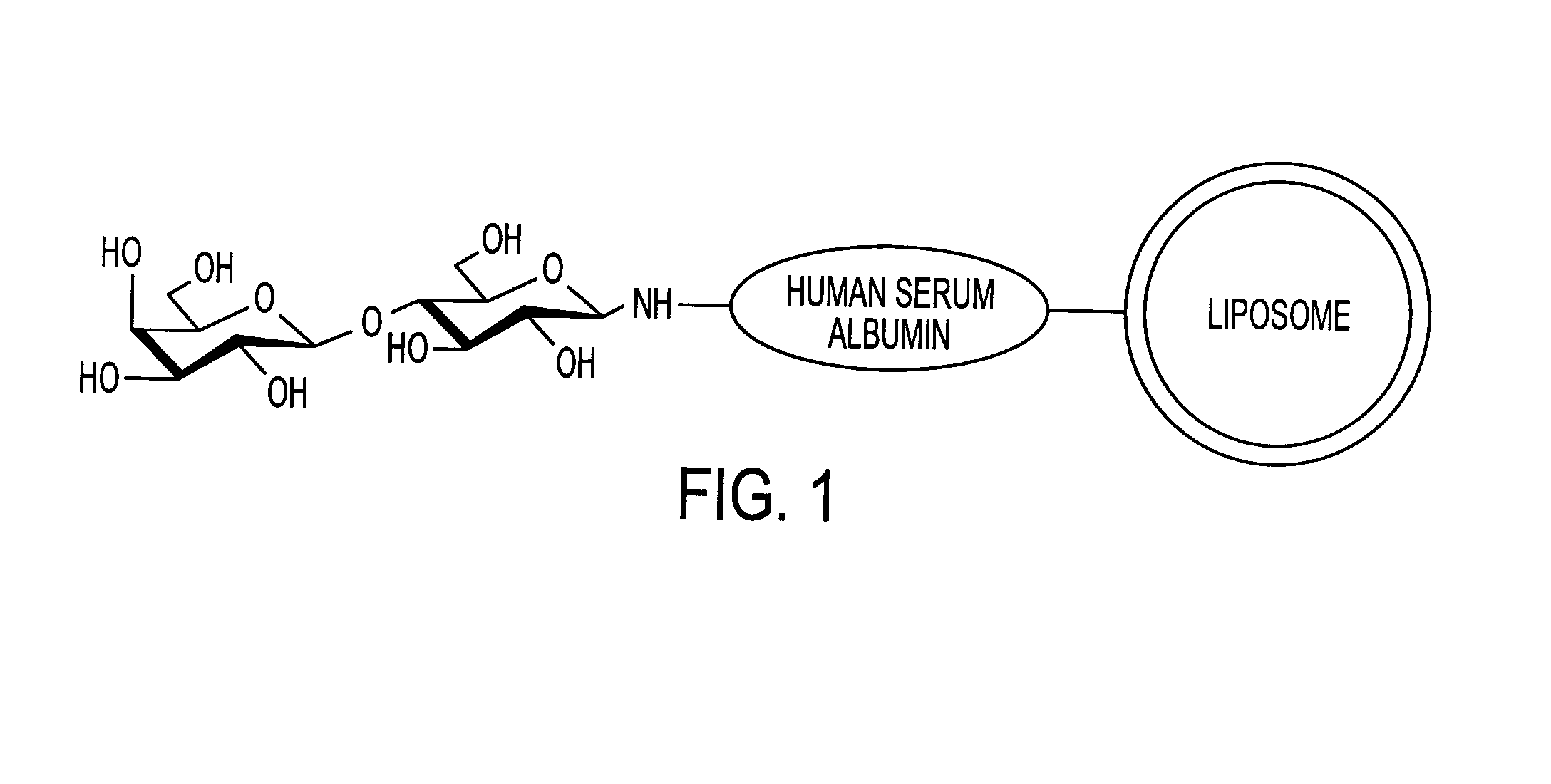
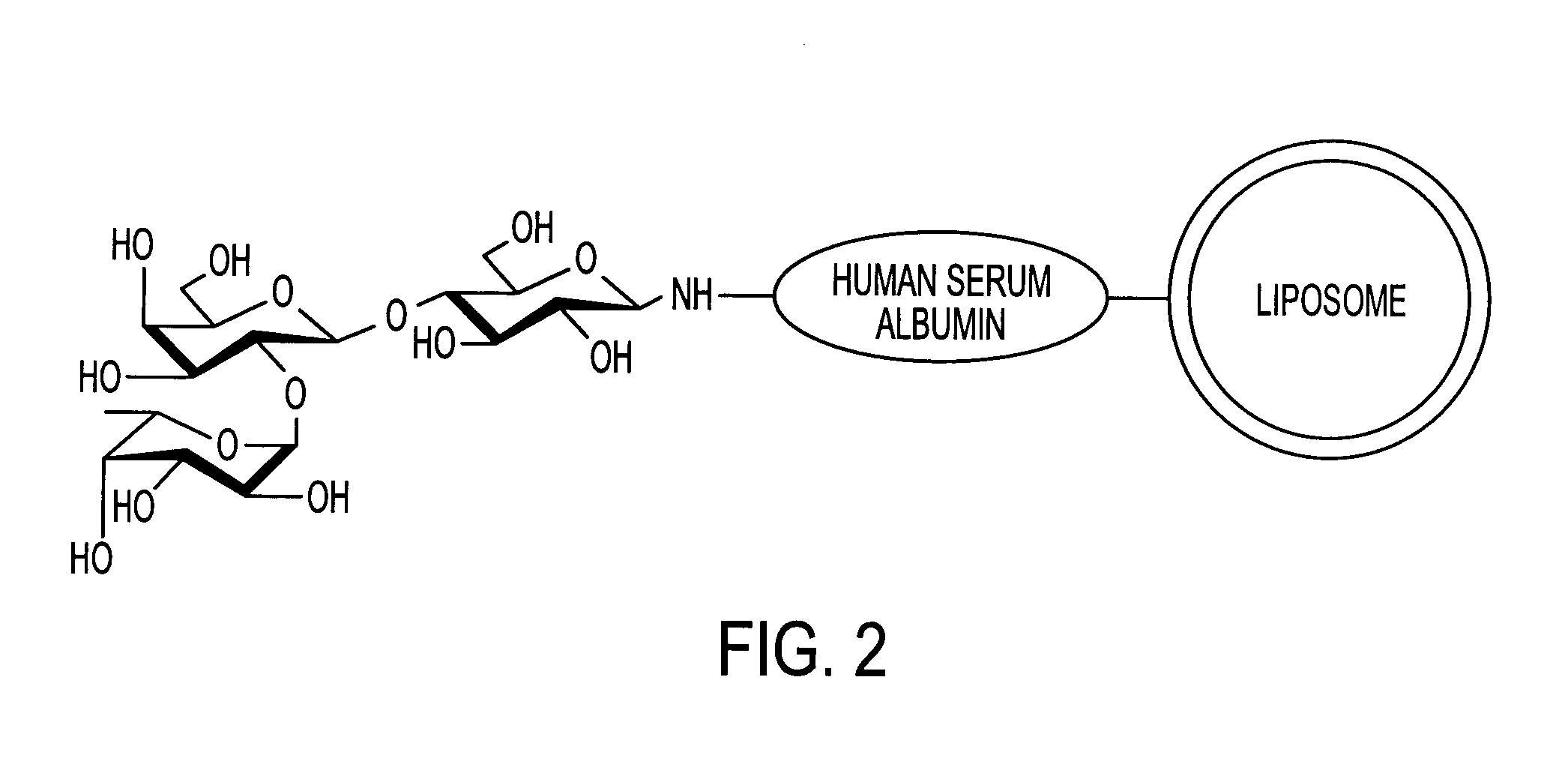
Sugar-modified liposome and products comprising the liposome
InactiveUS7070801B2Improve absorption qualityQuality improvementUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsIntestinal structureCancer cell
The present invention provides a sugar-modified liposome having a sugar chain bonded to its membrane surface, preferably through a linker protein, and having excellent absorption qualities, particularly in the intestine. The molecular structure and quantity of the sugar chain is selectively varied to allow the liposome to be delivered in a targeted manner to selected cells and tissues. The liposome is applicable to medicinal drugs, cosmetics and other various products in the medical / pharmaceutical fields, and it is especially useful in a therapeutic drug delivery system that recognizes target cells and tissues, such as cancer cells, and in the delivery of drugs or genes locally to a selected region, or in a diagnostic cell / tissue sensing probe.
Owner:YAMAZAKI DDS
Remote detection of substance delivery to cells
InactiveUS20050112065A1Highly preventive effectIncreasing effect on proton relaxivityDiagnostics using lightDispersion deliveryLipid formationElectrochemical gradient
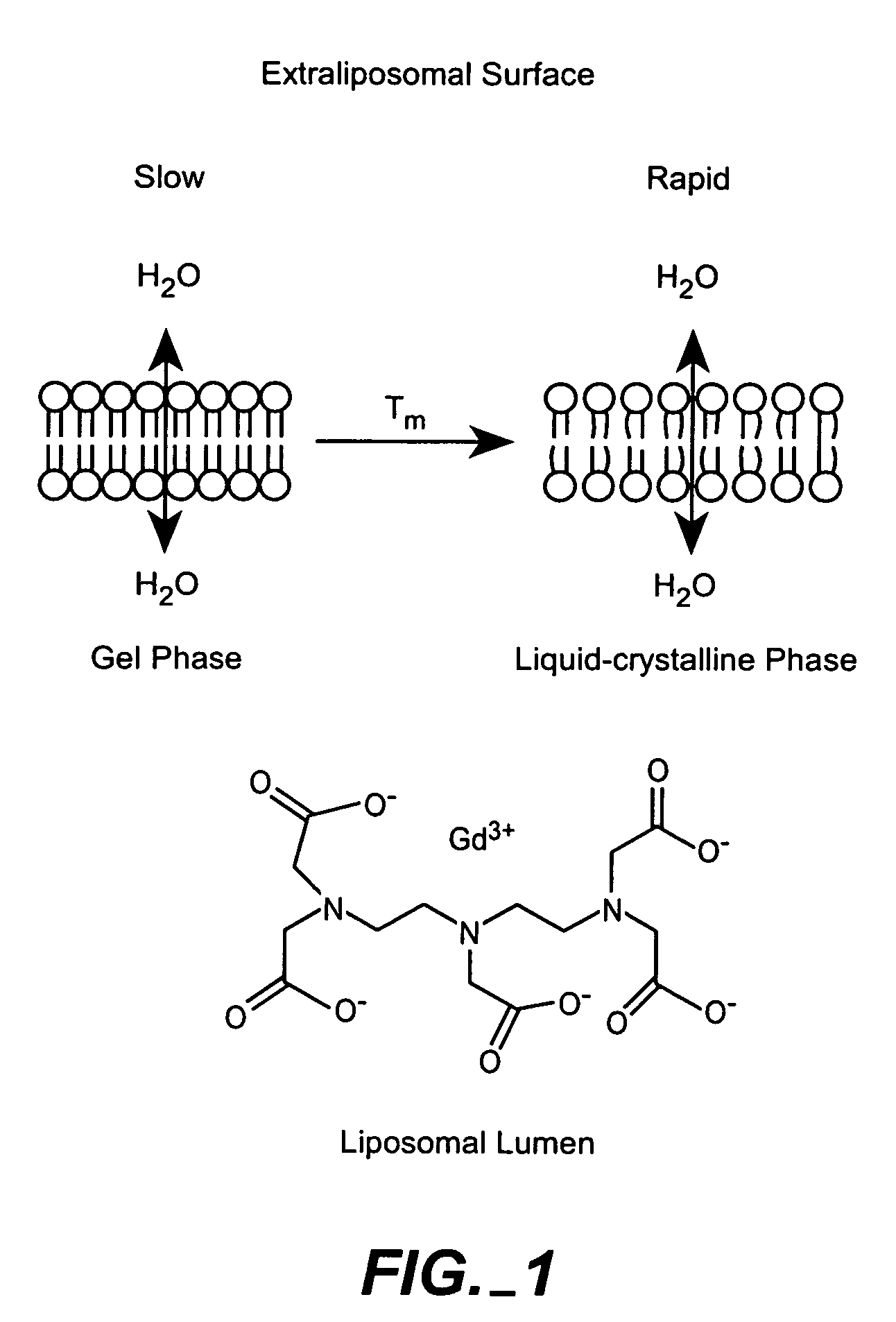
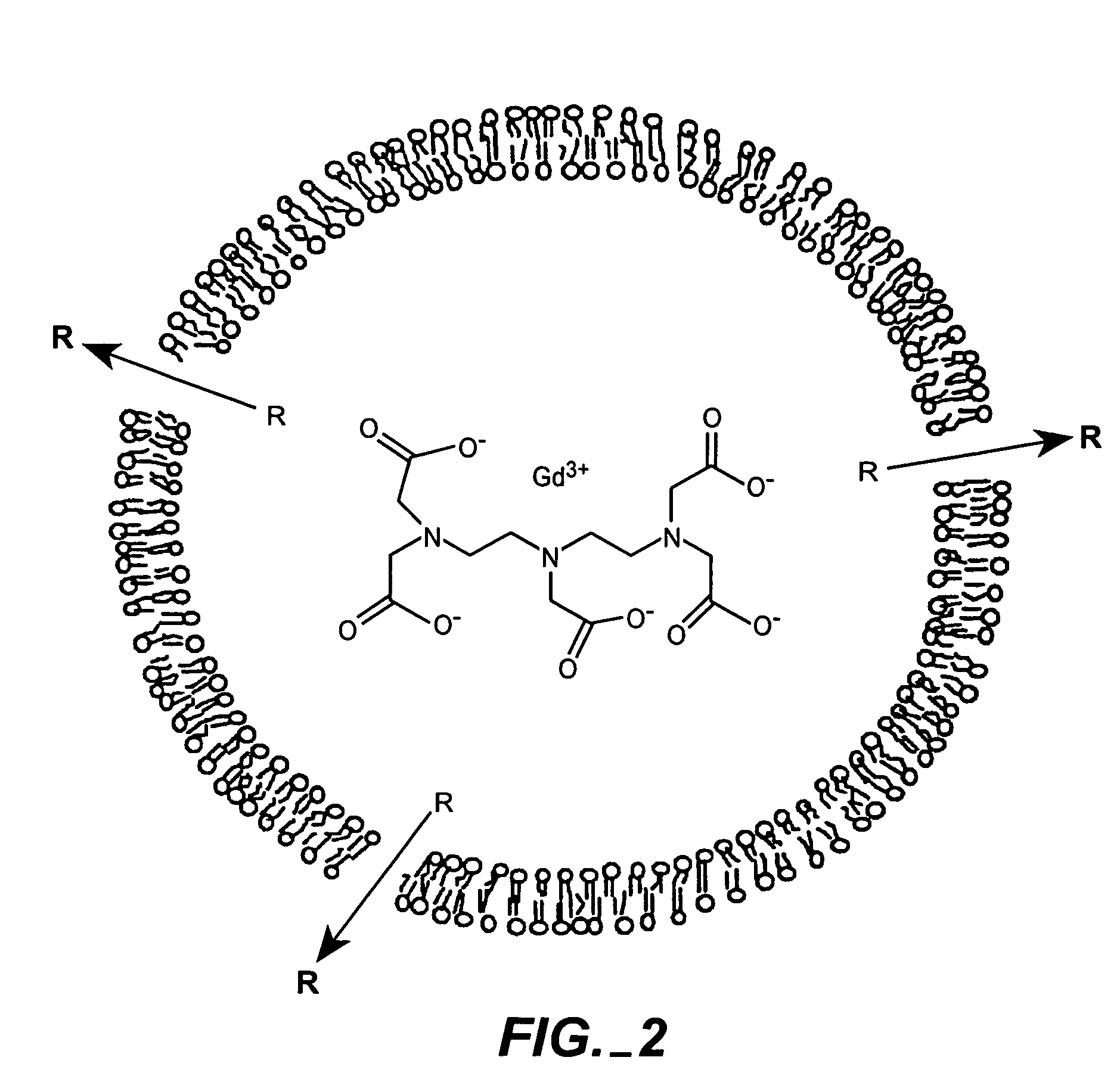
The present invention provides for the development of endocytosis-sensitive probes, and a remote method for measuring cellular endocytosis. These probes are based on the reduced water permeability of a nanoparticle or liposomal delivery system, and inherent degradability or disruption of barrier integrity upon endocytosis. The invention also provides for liposomes having combined therapeutic and diagnostic utilities by co-encapsulating ionically coupled diagnostic and therapeutic agents, in one embodiment, by a method using anionic chelators to prepare electrochemical gradients for loading of amphipathic therapeutic bases into liposomes already encapsulating an imaging agent. The invention provides for imaging of therapeutic liposomes by inserting a lipopolymer anchored, remotely sensing reporter molecules into liposomal lipid layer. The invention allows for an integrated delivery system capable of imaging molecular fingerprints in diseased tissues, treatment, and treatment monitoring.
Owner:SUTTER WEST BAY HOSPITALS
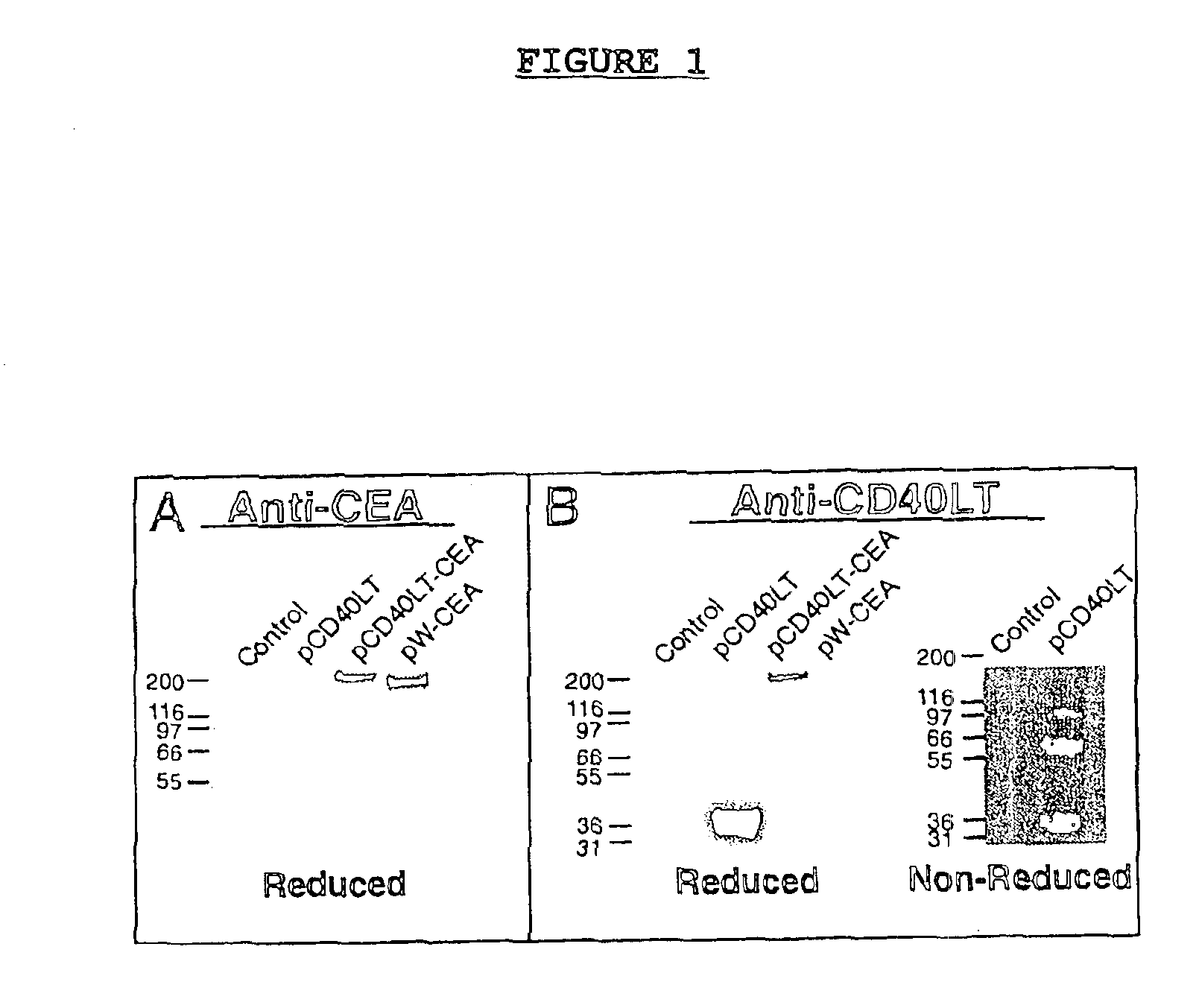
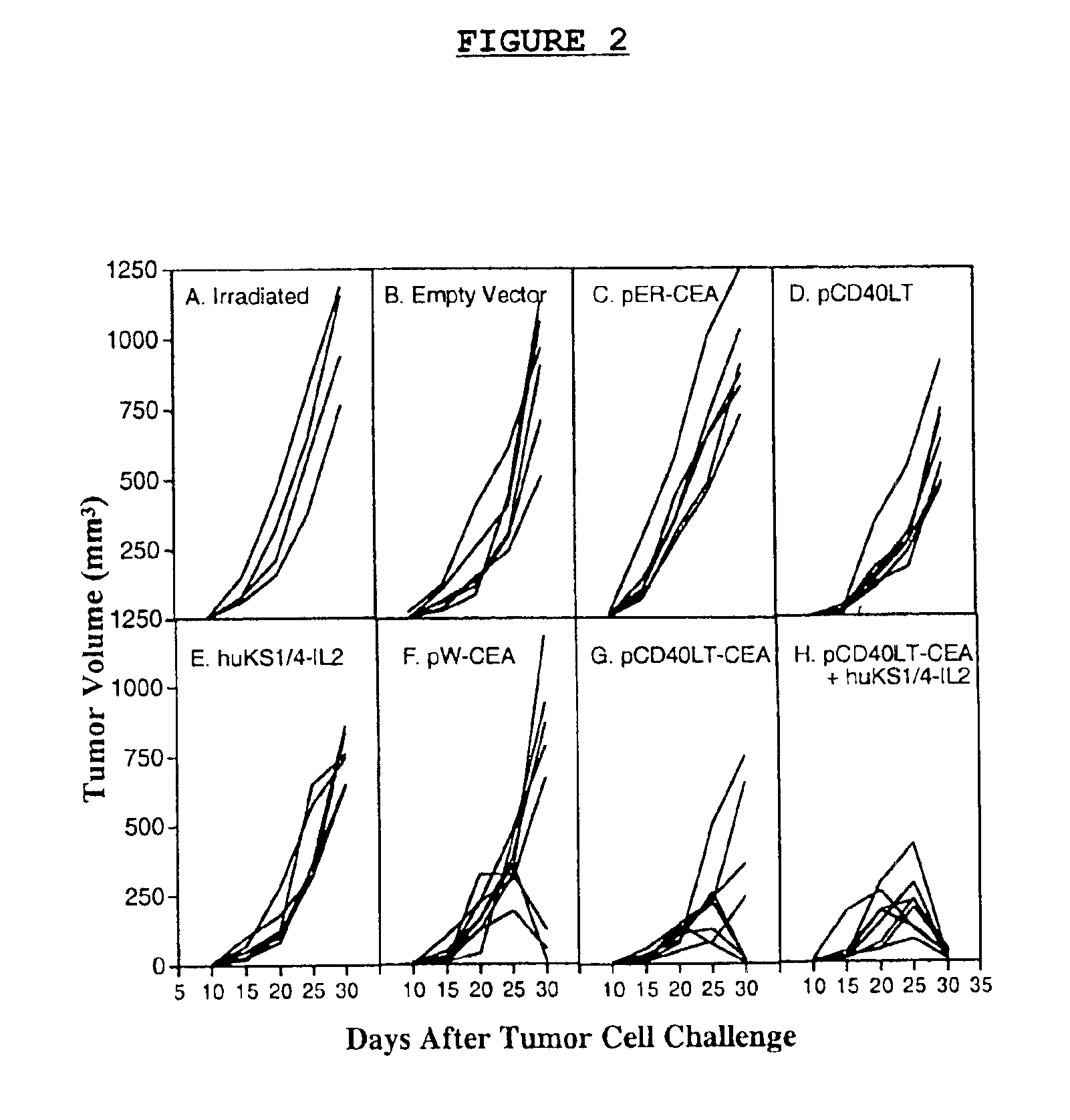
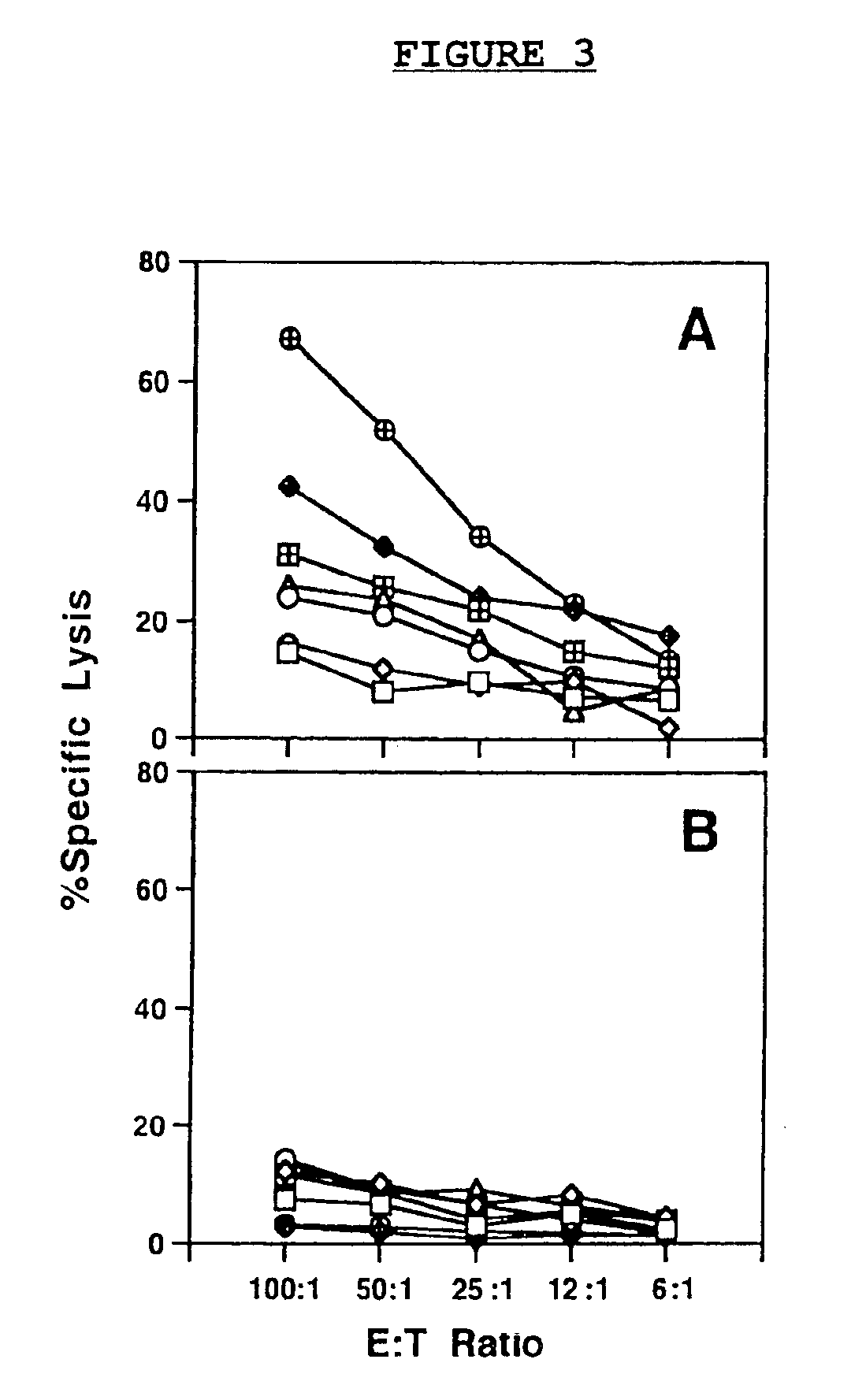
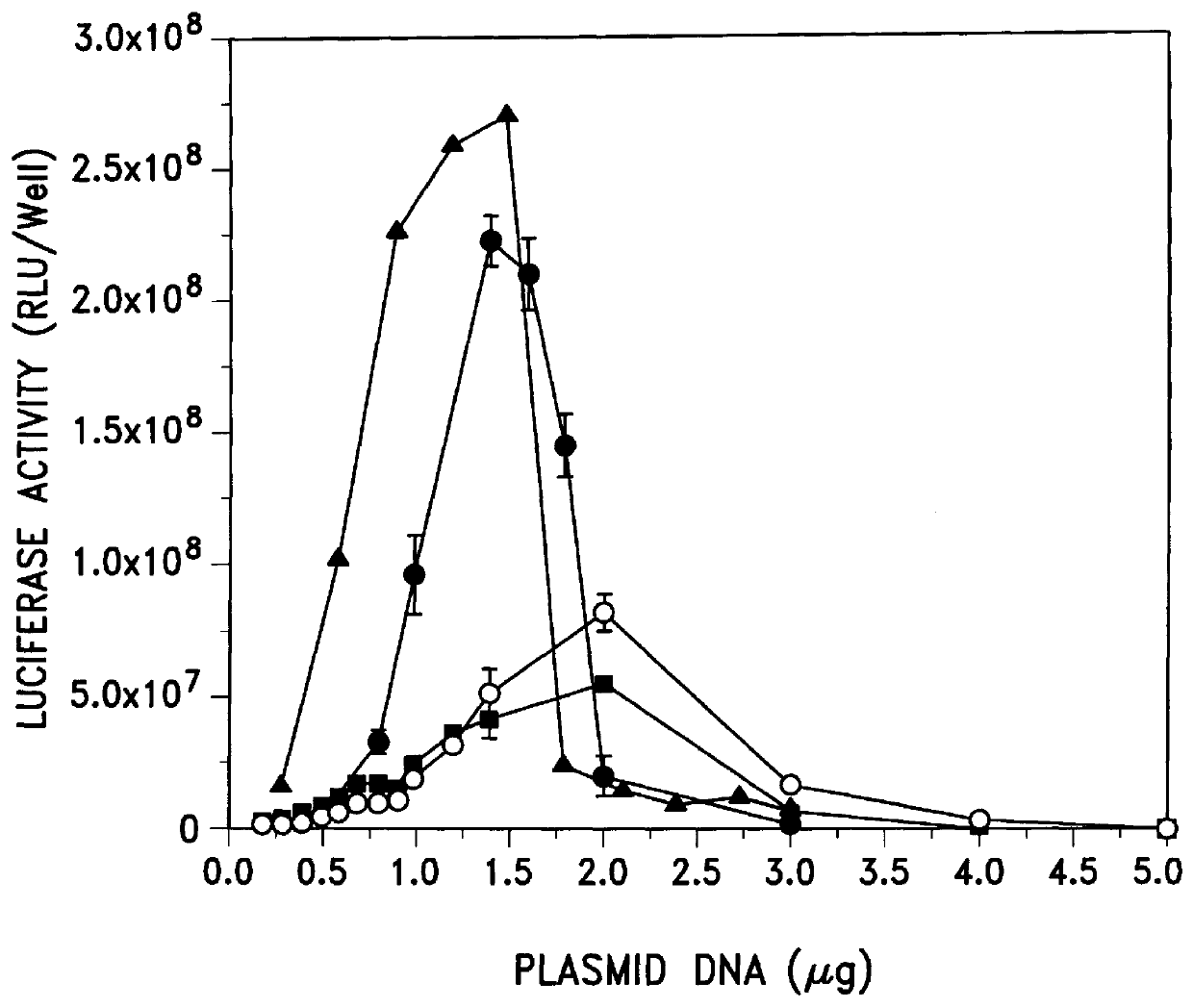
DNA vaccines encoding CEA and a CD40 ligand and methods of use thereof
InactiveUS6923958B2Enhance immune responseEffective immune responseOrganic active ingredientsPeptide/protein ingredientsLipofectamineMammal
A DNA vaccine effective for eliciting an immune response against cells that present a carcinoembryonic antigen (CEA) comprises a DNA operably encoding a CEA and a DNA operably encoding a CD40 ligand, SEQ ID NO:1 and SEQ ID NO: 2, respectively, or its homotrimer, CD40LT. The DNA vaccine can be incorporated in a delivery vector such as an attenuated live bacterium or virus, or a liposome carrier. In a method embodiment, the DNA vaccine is administered orally to a mammal, such as a human, to elicit an immune response against CEA presenting cells such as colon cancer cells. A preferred method embodiment includes the additional step of treating the mammal with recombinant antibody fusion protein huKS1 / 4-IL2 to enhance the immune response effectiveness of the vaccine.
Owner:THE SCRIPPS RES INST
Emulsion and micellar formulations for the delivery of biologically active substances to cells
InactiveUS6120794AChange concentrationReduce deliveryPeptide/protein ingredientsGenetic material ingredientsLipid formationVaccine delivery
New emulsion and micelle formulations are described as are complexes of these formulations with biologically active substances. The novel formulations are different from cationic lipid vectors such as cationic liposomes in that the complexes formed between biologically active substances and the emulsion and micellar formulations of this invention are physically stable and their transfection activity is resistant to the presence of serum. These novel formulations are disclosed to be useful in areas such as gene therapy or vaccine delivery.
Owner:UNIVERSITY OF PITTSBURGH
Modular targeted liposomal delivery system
InactiveUS7060291B1Improve abilitiesUltrasonic/sonic/infrasonic diagnosticsBiocideLipid bilayerOrganic chemistry
A liposome including a fusogenic liposome, a linking moiety and a targeting moiety. The fusogenic liposome is a lipid bilayer encapsulating contents. The linking moiety is electrostatically bound to the lipid bilayer, and the targeting moiety is covalently bound to the linking moiety. The liposome may also include a stabilizing moiety interposed between the linking and targeting moieties, and covalently bound to both. Alternatively, the stabilizing and targeting moieties may be covalently bound to separate linking moieties, the linking moieties being electrostatically bound to the lipid bilayer.
Owner:TRANSAVE
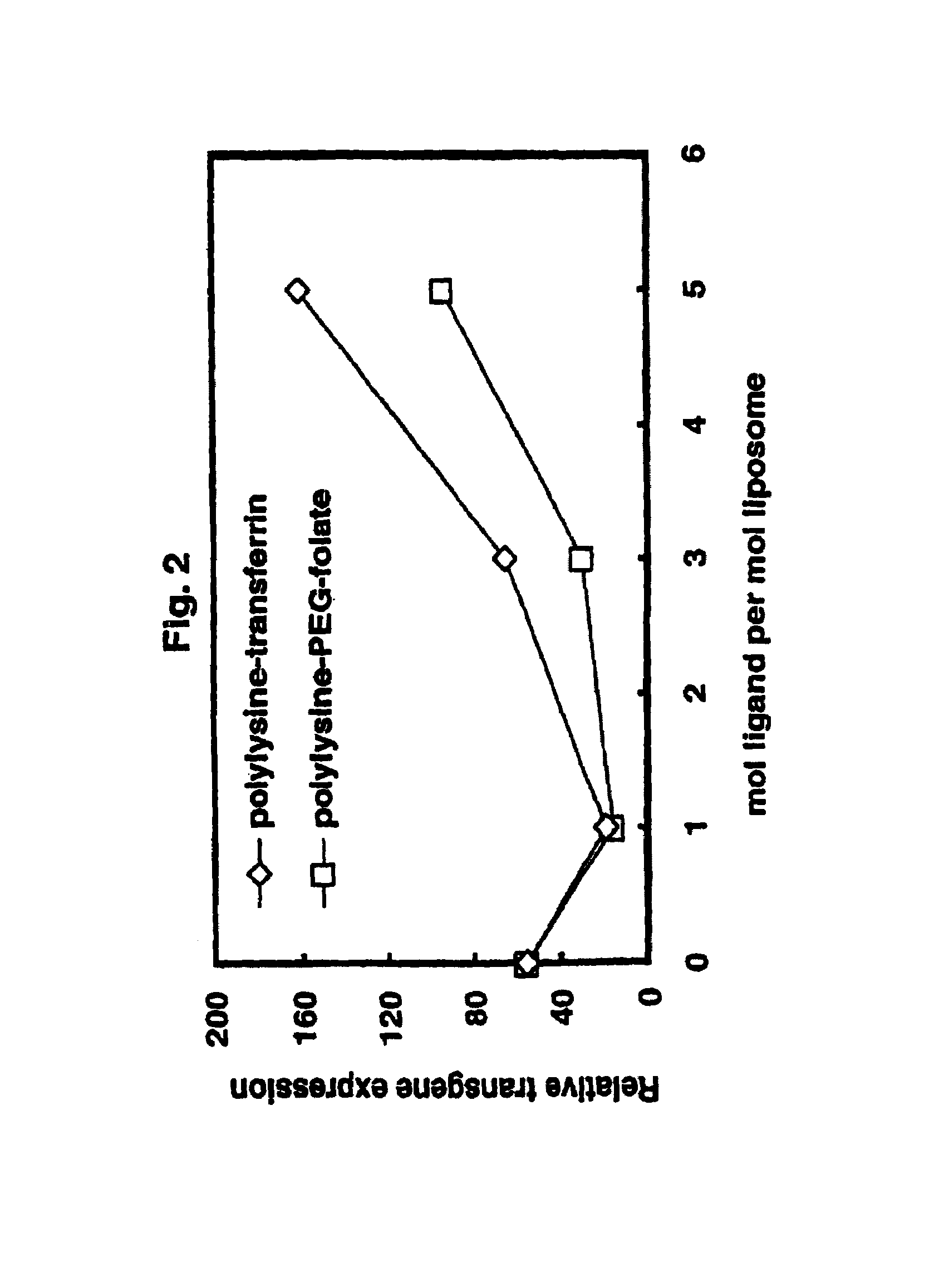
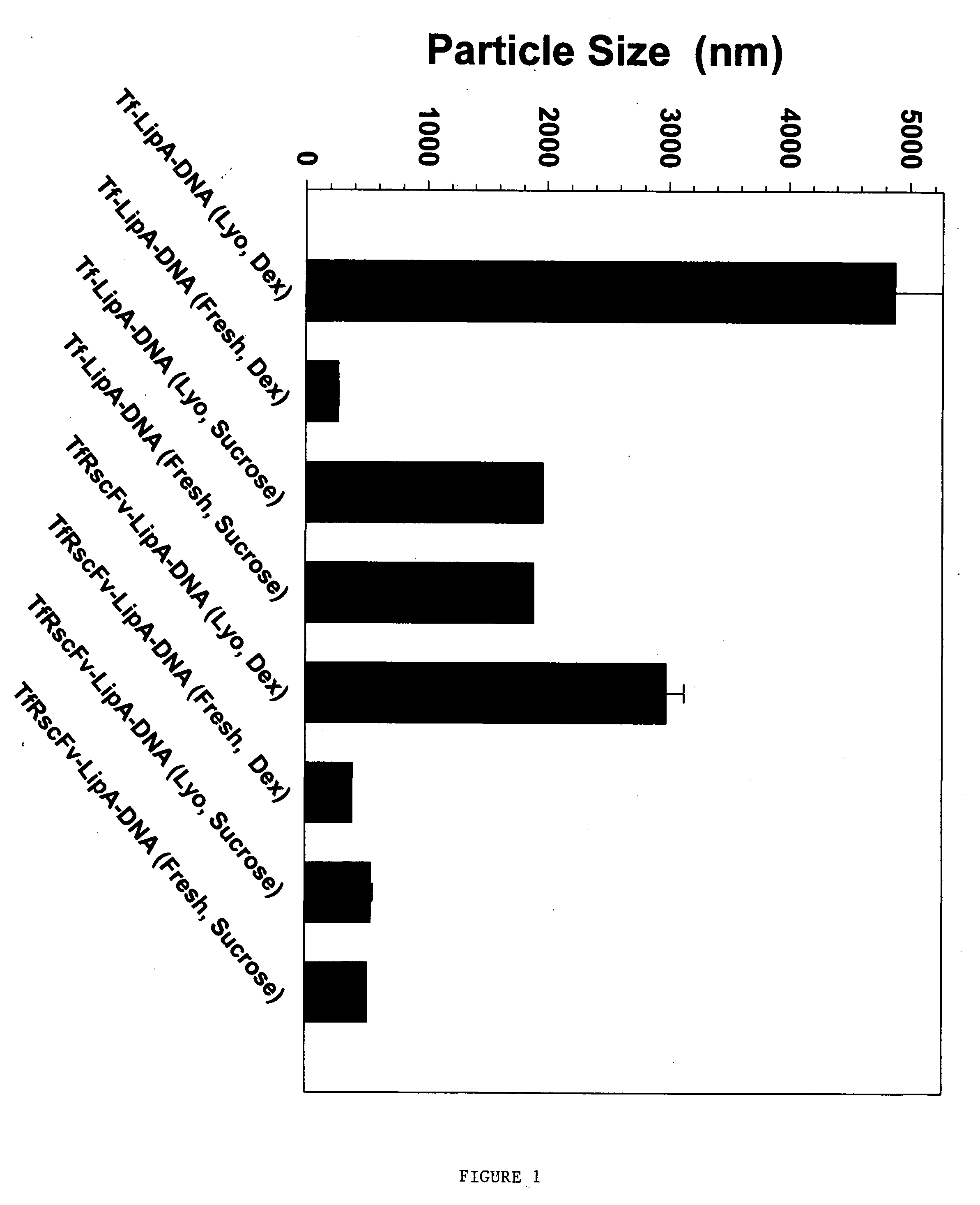
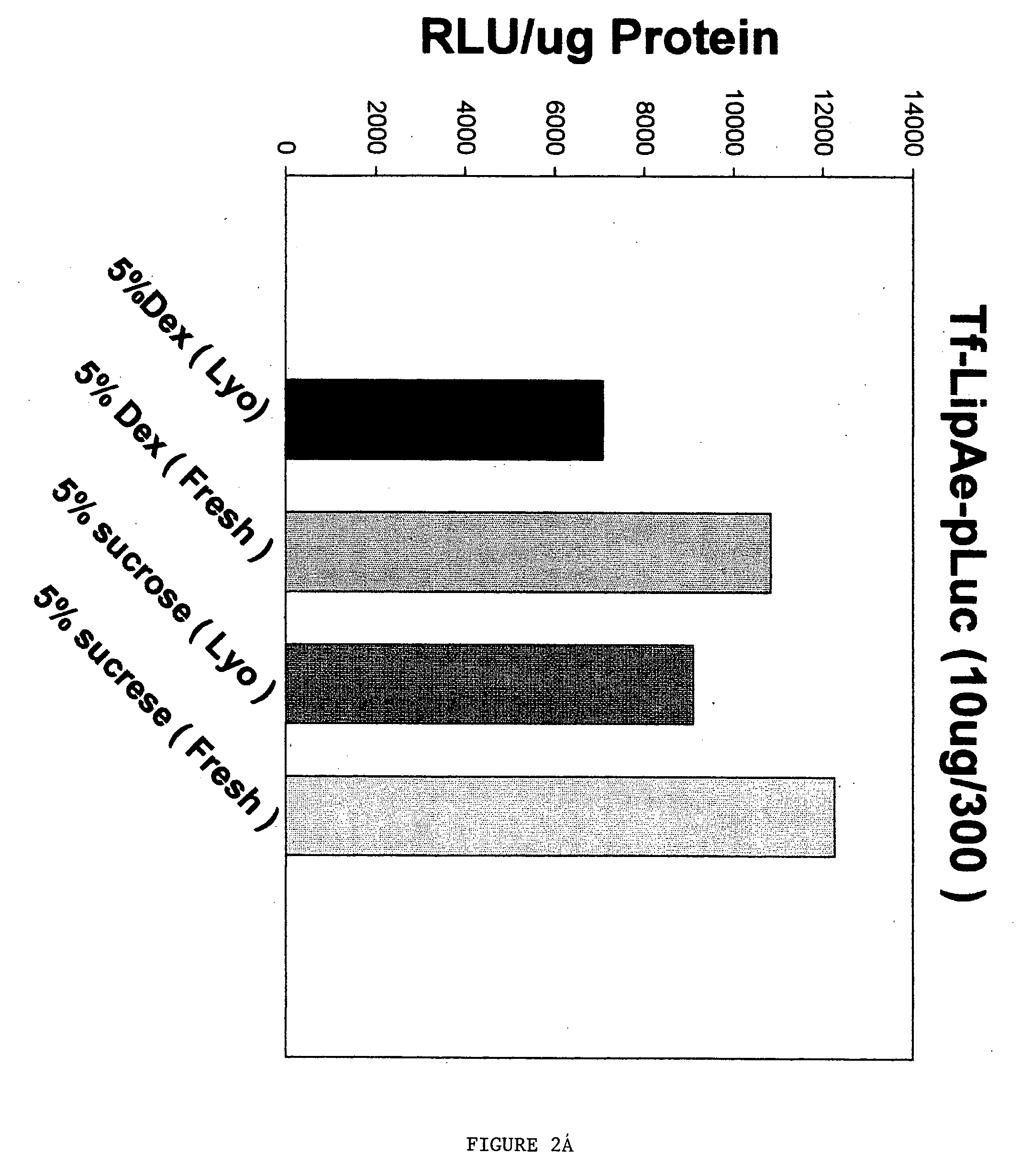
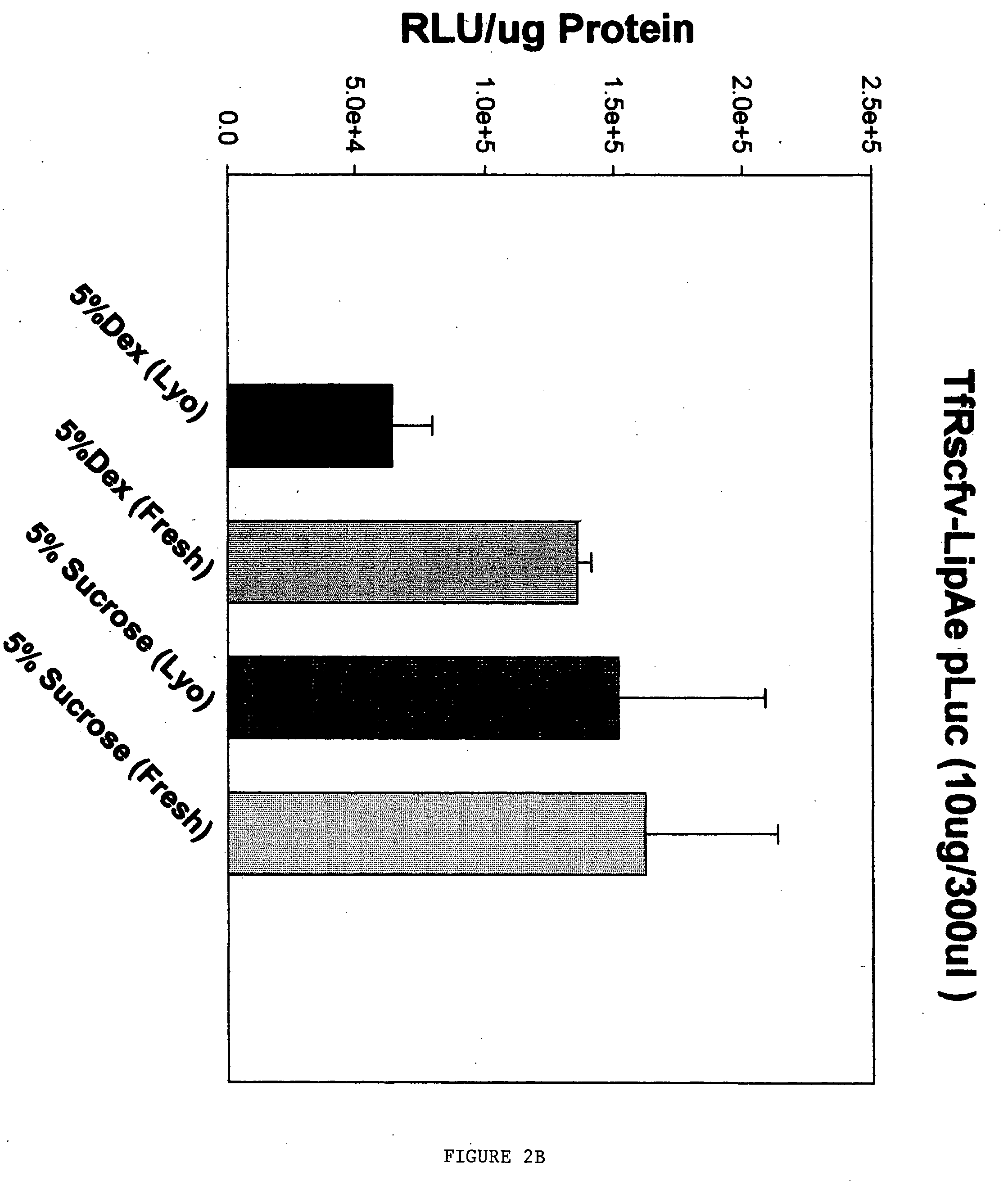
Method for improving stability and shelf-life of liposome complexes
A method for preparing a stable cell-targeting complex comprising a ligand and a cationic liposome encapsulating a therapeutic or diagnostic agent comprises (a) combining the complex with a solution comprising a stabilizing amount of sucrose and (b) lyophilizing the resultant solution to obtain a lyophilized preparation; wherein, upon reconstitution, the preparation retains at least about 80% of its pre-lyophilization activity.
Owner:GEORGETOWN UNIV
Methods and compositions for liposomal formulation of antigens and uses thereof
The present invention relates to liposomal vaccine compositions, methods for the manufacture thereof, and methods for the use thereof to stimulate an immune response in an animal. These compositions comprise dimyristoylphosphatidylcholine (“DMPC”); either dimyristoylphosphatidylglycerol (“DMPG”) or dimyristoyltrimethylammonium propane (“DMTAP”) or both DMPC and DMTAP; and at least one sterol derivative providing a covalent anchor for one or more immunogenic polypeptide(s) or carbohydrate(s).
Owner:RGT UNIV OF CALIFORNIA +1
Gene delivery mediated by liposome-DNA complex with cleavable PEG surface modification
A liposome composition and method for delivery of a nucleic acid in vivo or ex vivo is described. The liposomes in the composition are comprised of (i) a cationic lipid and (ii) a lipid joined to a hydrophilic polymer by a releasable linkage. The liposomes are associated with a nucleic acid for delivery to a cell.
Owner:ALZA CORP
Small rna-dependent translational regulatory system in cell or artificial cell model
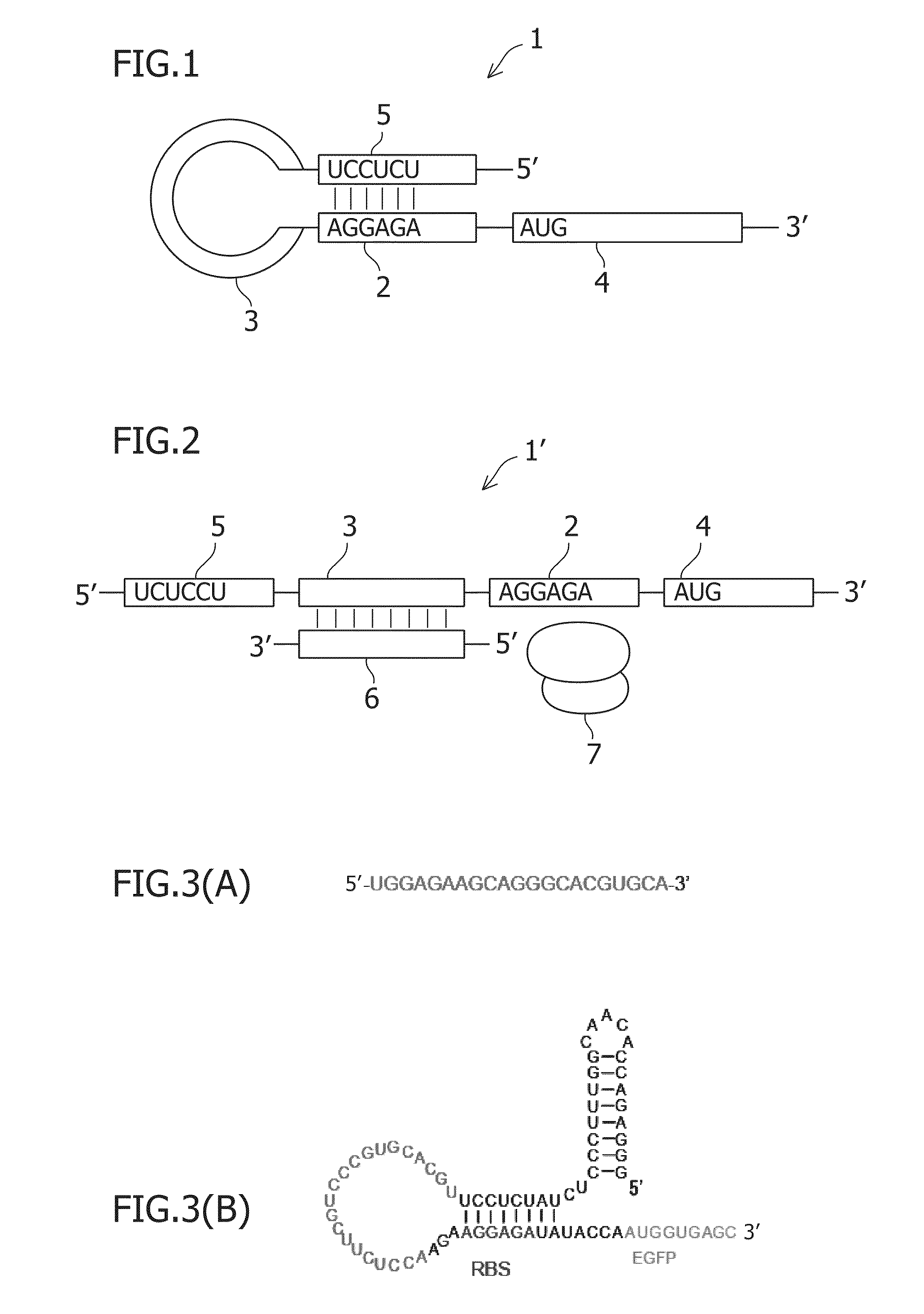
An object of the present invention is to construct an mRNA which specifically responds to a short RNA sequence and can activate, repress, and regulate the translation of the desired gene, and to construct an artificial cell model system using a liposome comprising the mRNA and a cell-free translational system encapsulated therein. The present invention provides: an mRNA comprising a target RNA-binding site located immediately 5′ to the ribosome-binding site, and a nucleotide sequence located 5′ to the target RNA-binding site, the nucleotide sequence being complementary to the ribosome-binding site; an mRNA comprising a small RNA-binding site located 3′ to the start codon, and a nucleotide sequence located 3′ to the small RNA-binding site, the nucleotide sequence encoding a protein; and a liposome comprising any of these mRNAs encapsulated therein.
Owner:JAPAN SCI & TECH CORP
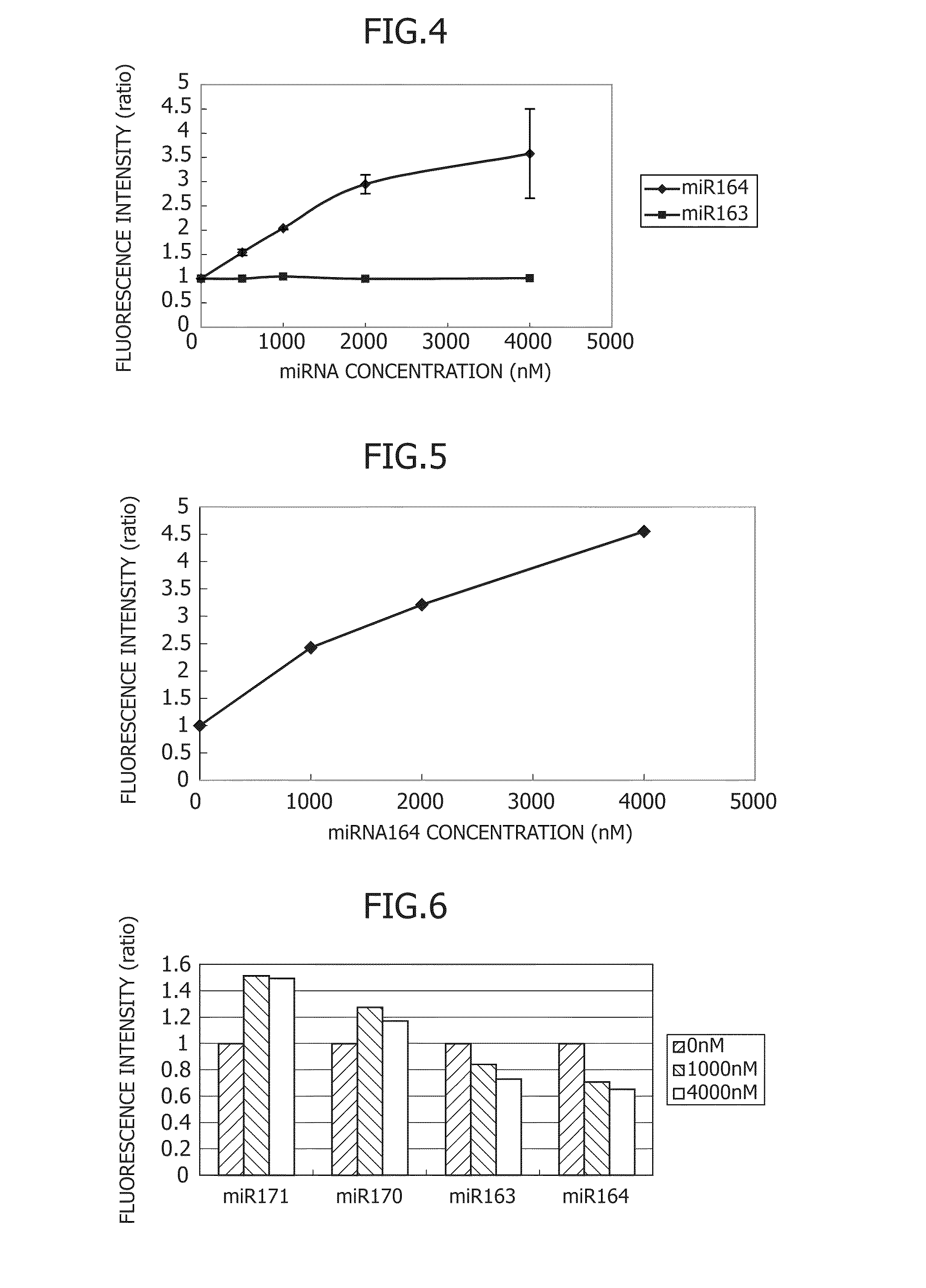
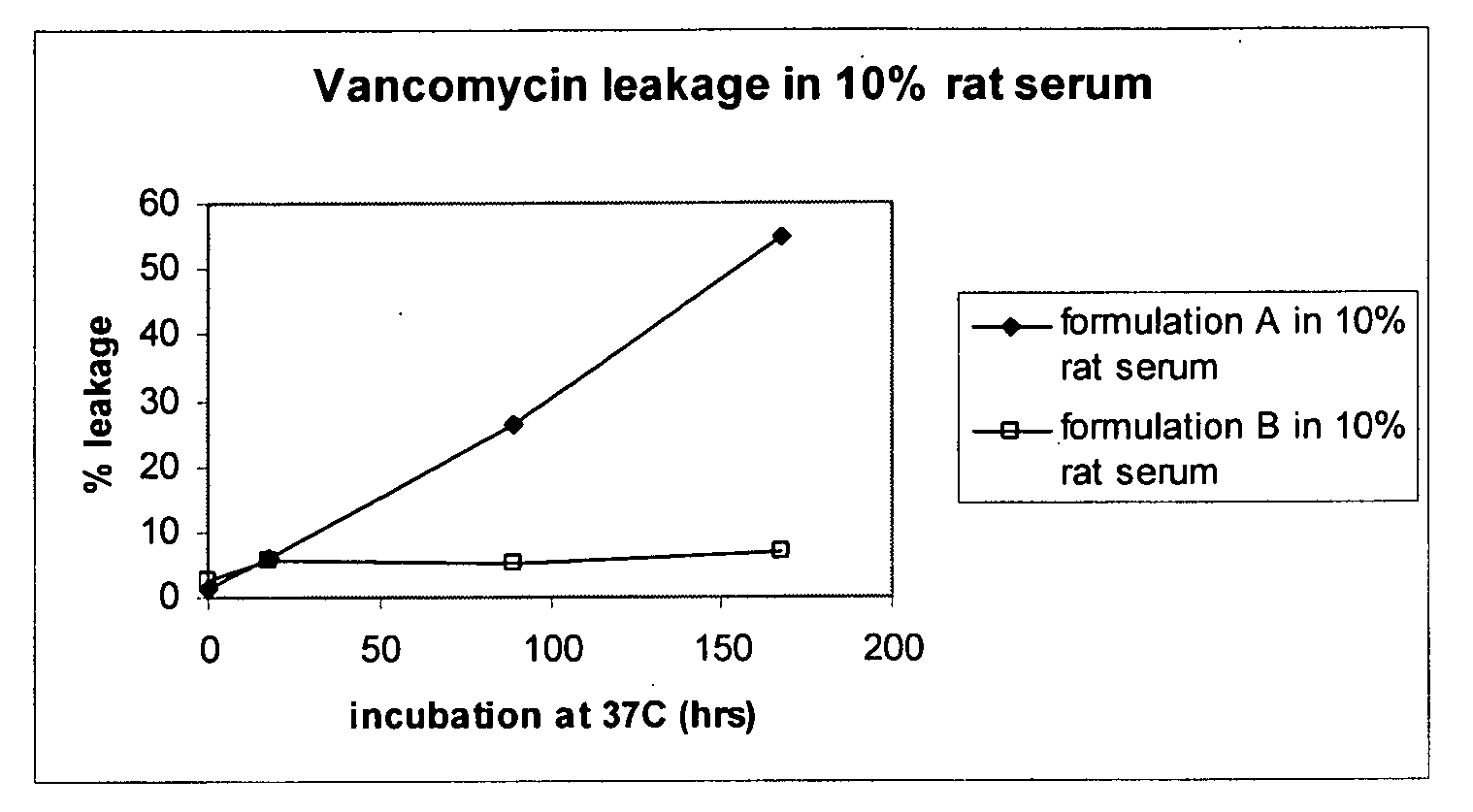
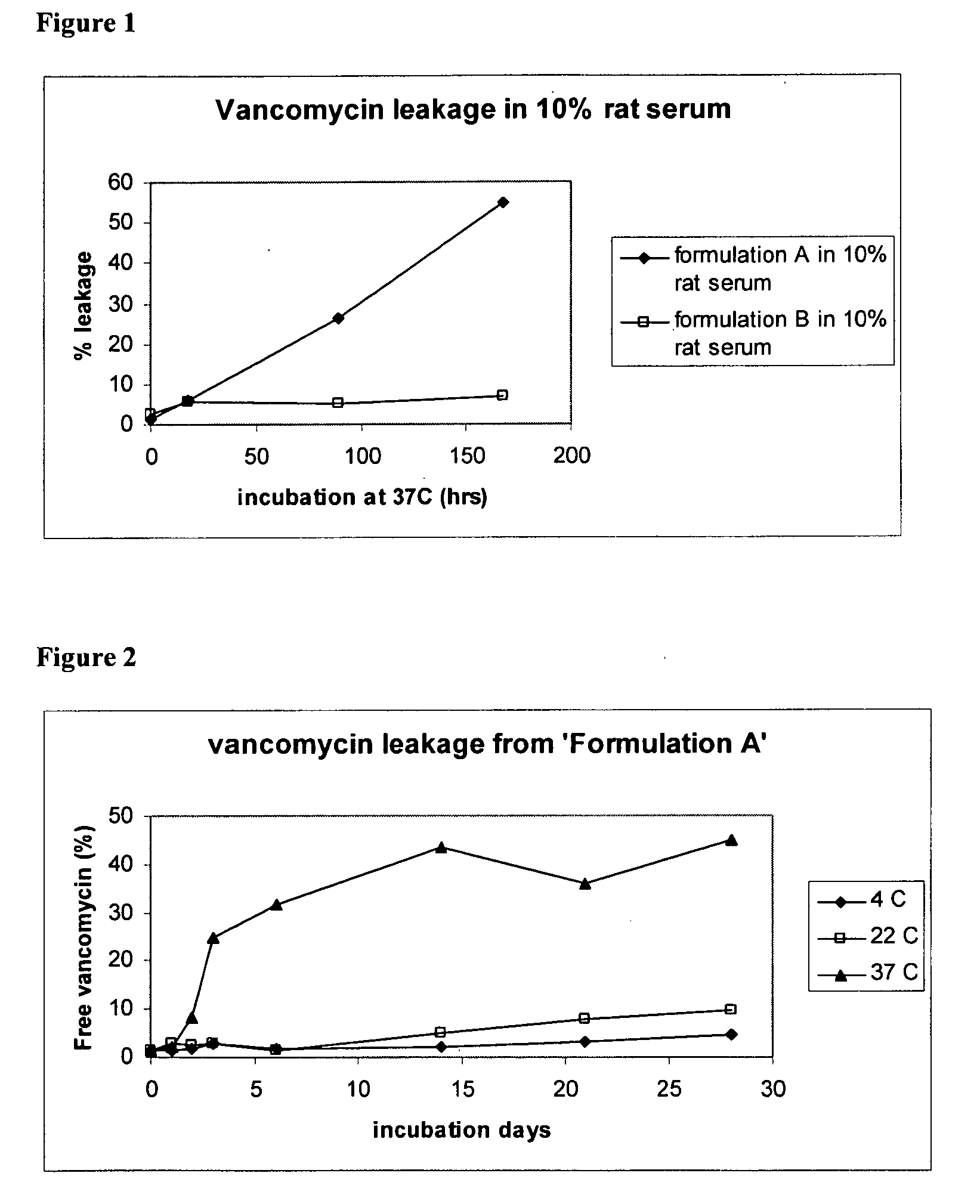
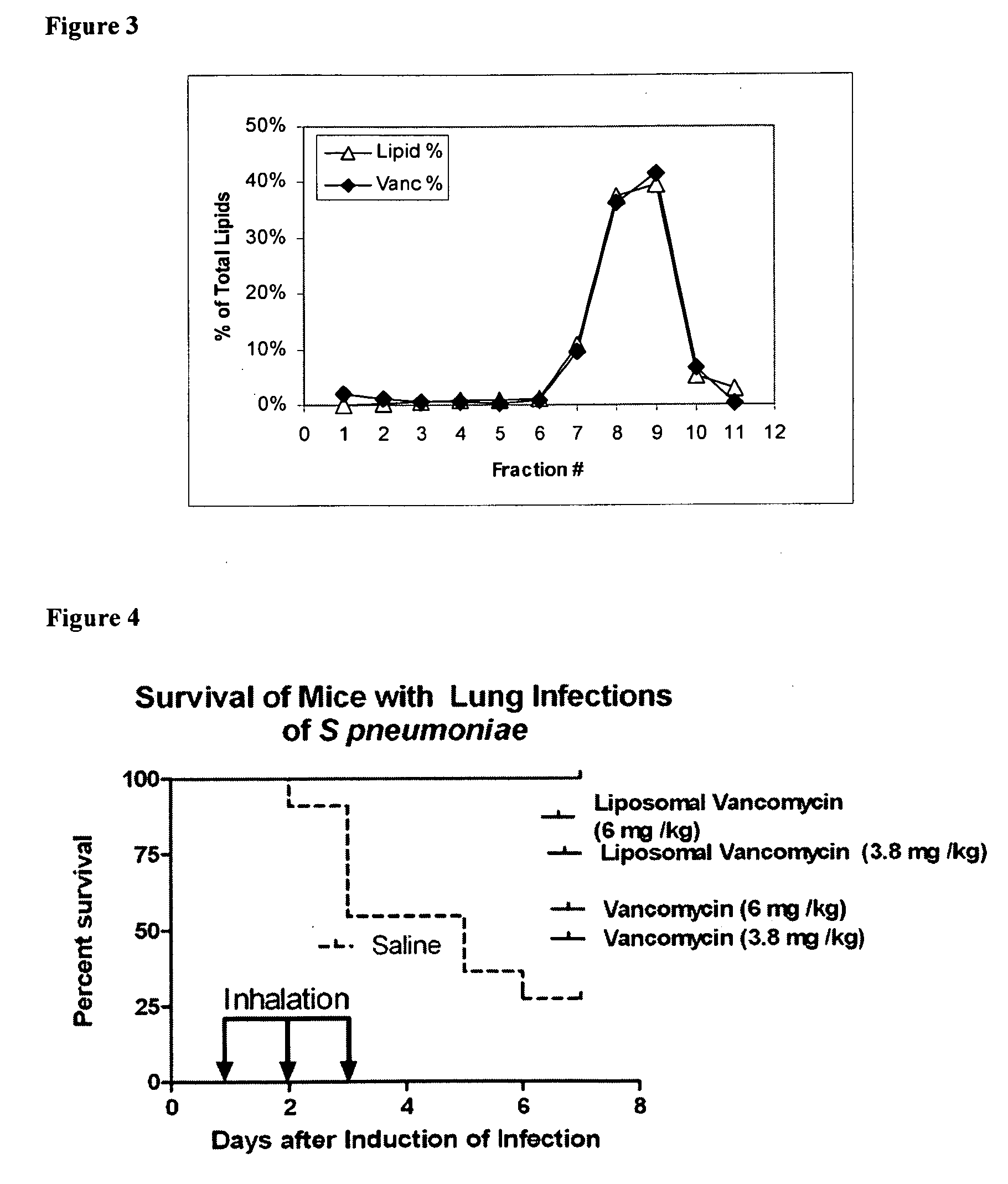
Liposomal Vancomycin Formulations
The present disclosure relates in part to liposomal vancomycin compositions having low lipid to drug ratios and high concentration of vancomycin. The present disclosure also relates in part to methods of making such compositions.
Owner:INSMED INC
Compound liposome containing anti-tumor drugs and preparation method and application thereof
InactiveCN102091036ASolve the problem of poor wettabilityReduce harmMacromolecular non-active ingredientsAntineoplastic agentsDiseaseLiposome
The invention belongs to a liposome, in particular relates to a compound liposome preparation with high targeting property of tumor tissues and efficient penetrability of tumor cells. The invention is characterized in that hyaluronic acid or sodium hyaluronate, tumor cell selective penetrating peptides and a liposome are used for together constructing a compound liposome. The compound liposome is characterized by utilizing the dual-tumor cell targeting property of the hyaluronic acid and tumor cell selective penetrating peptides to effectively solve the problem that because a conventional liposome and traditional cell penetrating peptides have no selectivity on normal cells and tumor cells, the toxicity is increased when the drug effect is increased in the therapeutic process, thereby fully meeting the clinical requirements of high efficiency and low toxicity when anti-tumor drugs are prepared into preparations for treating diseases.
Owner:CHINA PHARM UNIV
Delivery system for nucleic acids
Owner:THE SCRIPPS RES INST +1
Glucocorticoid modulation of nucleic acid-mediated immune stimulation
InactiveUS20070054873A1Minimize and inhibit immune responseOrganic active ingredientsBiocideLipid formationDosing regimen
The present invention provides methods for minimizing or inhibiting immune responses to immunostimulatory nucleic acids by pretreating with one or more doses of a glucocorticoid prior to nucleic acid administration. The nucleic acids are typically administered using a lipid-based carrier system such as a nucleic acid-lipid particle or liposome. As a result, patients following a glucocorticoid dosing regimen advantageously benefit from nucleic acid therapy without suffering any of the immunostimulatory side-effects associated with such therapy.
Owner:PROTIVA BIOTHERAPEUTICS
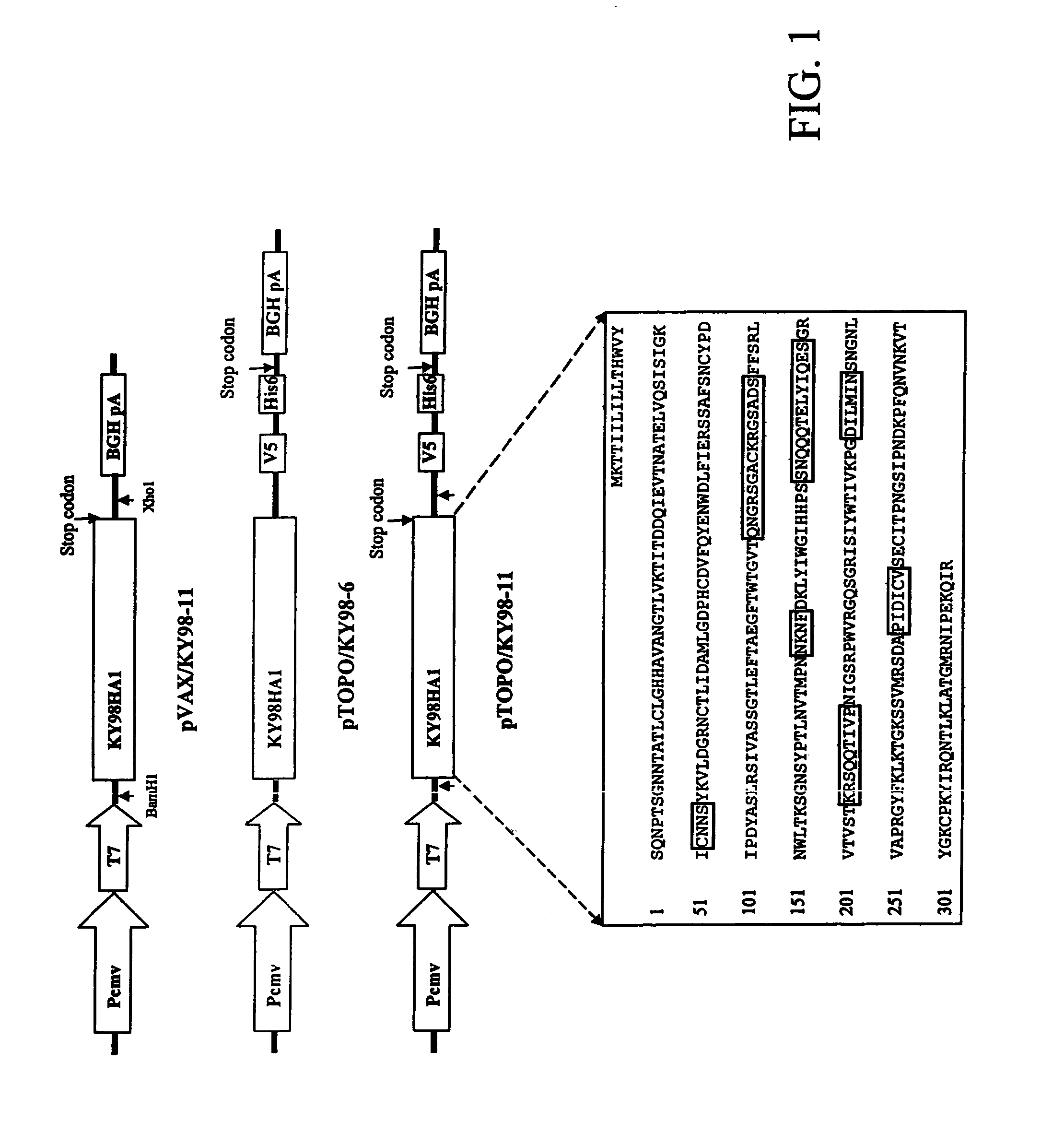
DNA vaccine expressing HA1 of equine-2 influenza virus
InactiveUS7244435B2Reduce riskReduce dosageSsRNA viruses negative-senseViral antigen ingredientsHemagglutininA-DNA
The invention is for a DNA vaccine expressing the hemagglutinin (HA1) gene of equine-2 influenza virus. By engineering a stop codon within HA1, expression of HA1 is ensured. By encapsulation of the DNA vaccine in liposome and by intranasal inoculation, it is sufficient to elicit protective immunity at a significantly lower dosage compared to a DNA vaccine expressing the full length HA gene. Lower dosage reduces the risk of induction of anti-DNA antibodies. Intranasal inoculation directly to the respiratory epithelial cells reduces the risk of DNA integration. The inventive vaccine is advantageous over current inactivated or live attenuated vaccines, as updating of the vaccine requires only the replacement of the encoding sequence with the new virus.
Owner:BOARD OF REGENTS FOR OKLAHOMA STATE UNIVERSITY
Non-invasive gene targeting to ocular cells
Liposomes containing therapeutic genes are conjugated to multiple targeting agents to provide transport of the encapsulated gene across the blood-retinal barrier and the plasma membrane of ocular cells. Once across the blood-retinal barrier and ocular cell membrane, the encapsulated gene expresses the encoded therapeutic agent within the ocular cells to provide diagnosis and / or treatment of disease.
Owner:RGT UNIV OF CALIFORNIA
Non-pegylated long-circulating liposomes
InactiveUS20050142178A1Slow tumor growthBiocideCarbohydrate active ingredientsAbnormal tissue growthPegylated Liposomal Doxorubicin Hydrochloride
The present invention provides a long circulating non-pegylated liposomal doxorubicin hydrochloride composition for parenteral administration and a process for its preparation. The circulation time in Swiss albino mice is at least 25 times longer than conventional non-liposomal formulations. The non-pegylated liposomes are stable, exhibit low toxicity and have been found to be efficacious in different tumor models.
Owner:BHARAT SERUMS & VACCINES
Synthetic glyco-lipo-peptides as vaccines
A glycolipopeptide comprising at least one disease-associated epitope, and characterized by at least one lipidated interior amino acid or by the presence of a MUC1 epitope, may be used in a vaccine, preferably in conjunction with a liposome.
Owner:ONCOTHYREON
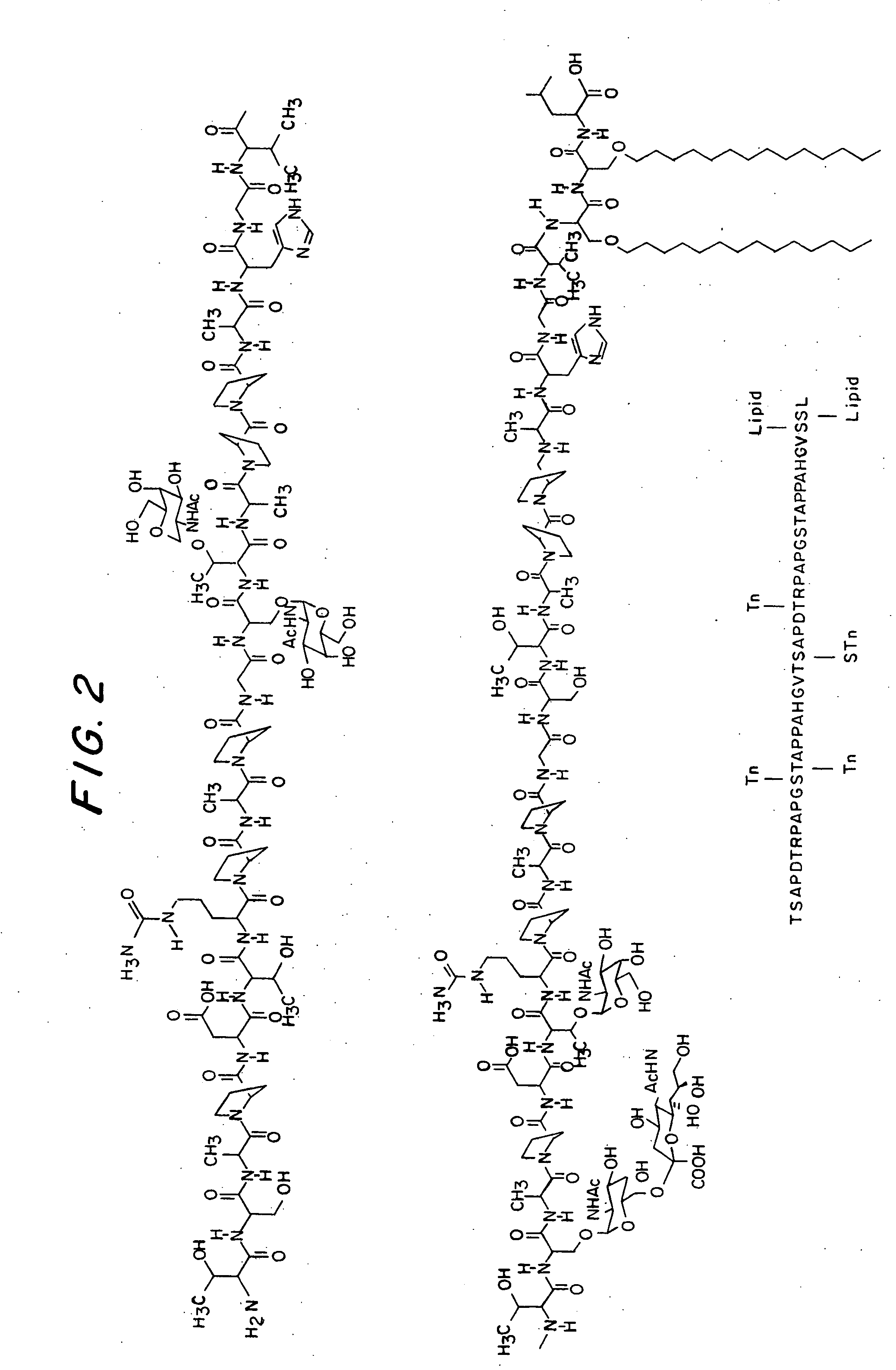
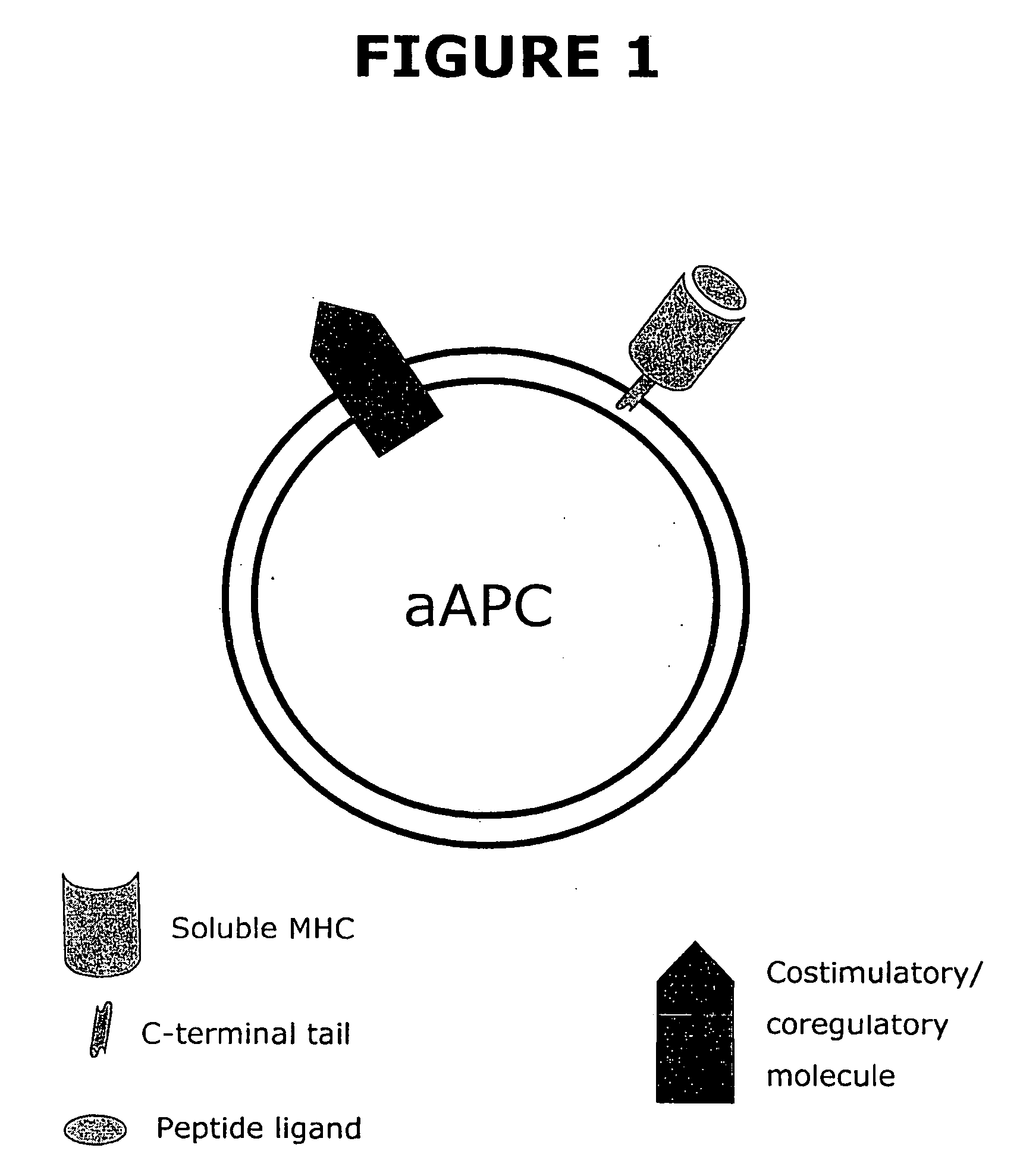
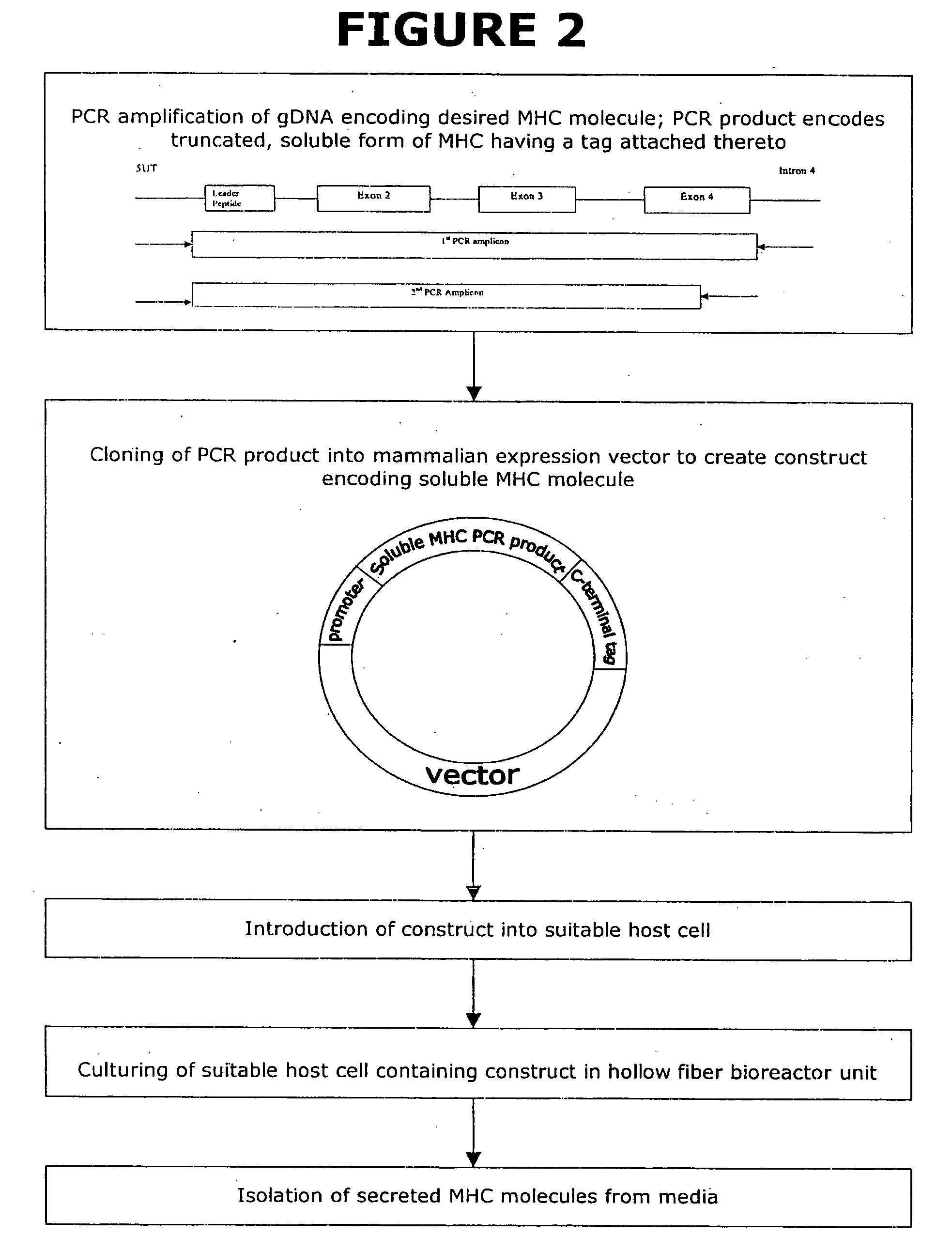
Soluble MHC artificial antigen presenting cells
InactiveUS20060034865A1Carrier-bound antigen/hapten ingredientsPharmaceutical non-active ingredientsLipofectamineSignalling molecules
An artificial antigen presenting cell includes a liposome having at least one recombinant soluble MHC-peptide complex incorporated therein. The artificial antigen presenting cell may also include at least one additional signal molecule incorporated therein for manipulating the intensity and quality of the immune response. The recombinant soluble MHC molecule is obtained by a method utilizing PCR amplification of gDNA or cDNA, and a tag is attached thereto for anchoring the recombinant soluble MHC molecule to the liposome.
Owner:HILDEBRAND WILLIAM H +1
Novel polypeptide modified tumor targeted liposome of targeted integrin receptor
InactiveCN103417480AIncrease intakeAntitumor effectMacromolecular non-active ingredientsHybrid peptidesTumor targetCholesterol
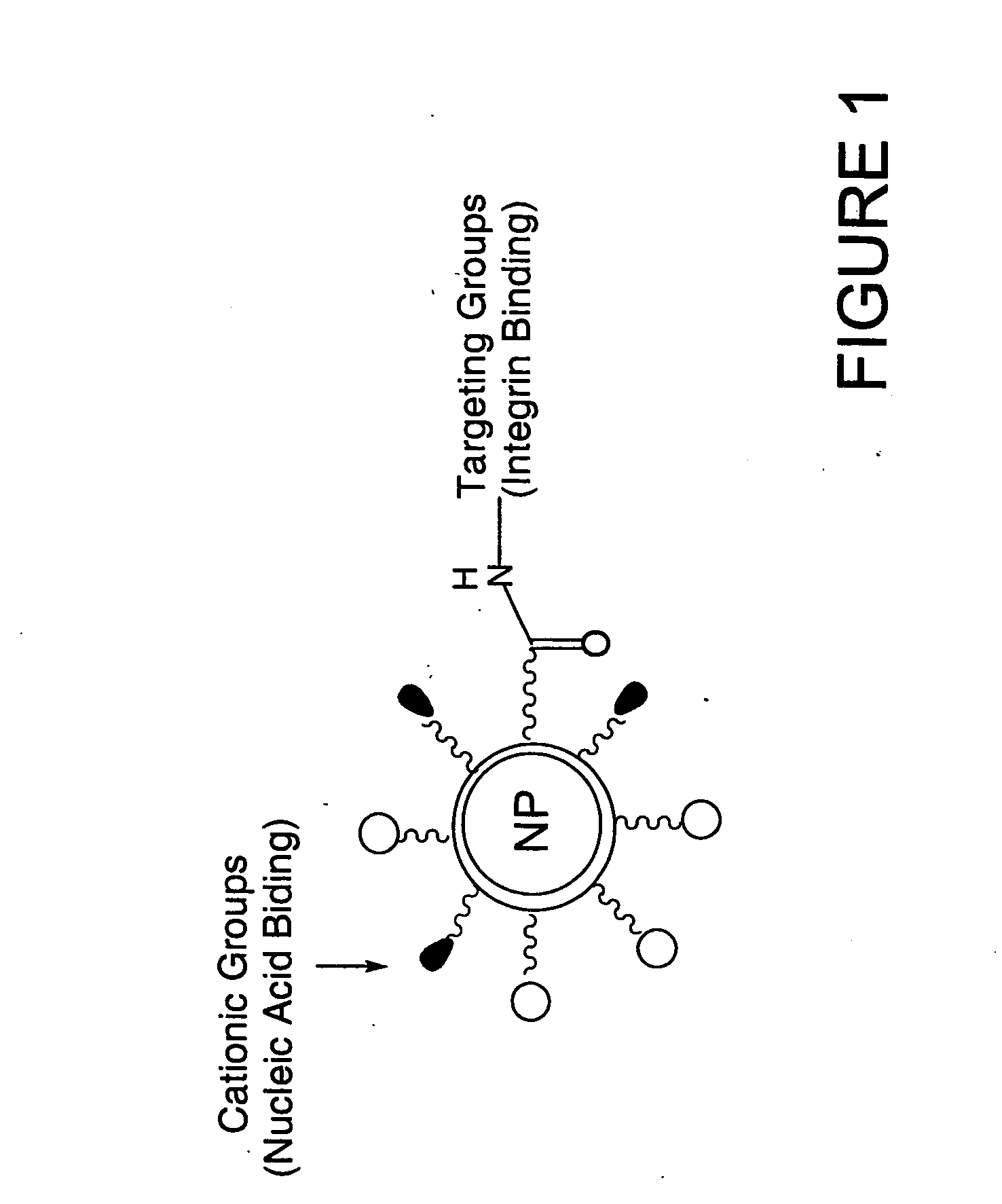
The invention provides a novel polypeptide modified tumor targeted liposome of a targeted integrin receptor. The novel polypeptide is mainly formed by covalent linkage of ring-shaped RGD and cell penetrating peptides, and not only has the selective targeting capability of an integrin receptor, but also can achieve mediated endocytosis; the liposome mainly comprises phospholipid, cholesterol, lipid-polyethyleneglycol and lipid-polyethyleneglycol-novel polypeptide ligand chimeric substance, and is a very potential tumor targeted treatment carrier.
Owner:SICHUAN UNIV
Anti-alpha v immunoliposome compositions, methods and uses
InactiveUS20110268655A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsInternalizationTargeting ligands
An immunoliposome composition targeted to the alphaV-integrin subunit of integrin receptors comprised of ligand-targeted liposomes bearing at least one targeting-ligand derived from an antibody and having binding specificity for at least one integrin receptor comprising an alpha V subunit including αvβ1, αvβ3 αvβ5, αvβ6, or αvβ8 integrin cell receptors is described. The antibody-derived targeting ligand may be a Fab′ fragment, a scFv, or the like. Binding of the immunoliposome to αv-integrin expressing cells, preferably results in internalization of the immunoliposome for cytoplasmic delivery of a liposome-entrapped agent.
Owner:CENTOCOR ORTHO BIOTECH
Targeted ligand-PEG (polyethylene glycol)-cholesterol/tocopherol derivative, and preparation method and application of derivative
InactiveCN103623416AGenetic material ingredientsPharmaceutical non-active ingredientsTumor targetCholesterol
The invention belongs to the field of pharmaceutic preparations, and relates to a targeted ligand-PEG (polyethylene glycol)-cholesterol / tocopherol derivative, and a preparation method and an application of the derivative, in particular to an application of the derivative in a composite mechanism mediate tumor targeted drug delivery system. According to the derivative, the preparation method and the application, a drug delivery system consisting of the targeted ligand-PEG-cholesterol / tocopherol derivative with a targeting function, a long circulating function and a pH (power of hydrogen) sensitive function as a functional material, a cation liposome and a drug can simultaneously carry anticancer polypeptide and gene / chemotherapy drugs. According to the system, different drugs are jointly entrapped into the lipid nano drug delivery system and delivered into a targeted cell by utilizing a physicochemical property difference between components or by in-vivo specific targeted recognition, signal conduction blocking and pH triggering PEG chain breaking charge reversion methods, so that a targeted tumor therapeutic effect is improved. According to the derivative, the preparation method and the application, the advantages of the system in directional delivery, co-delivery and the like are proved by in-vivo and in-vitro activity evaluation, the anticancer activity is improved significantly, and definite synergic therapeutic and immunity effects are provided.
Owner:SHENYANG PHARMA UNIVERSITY
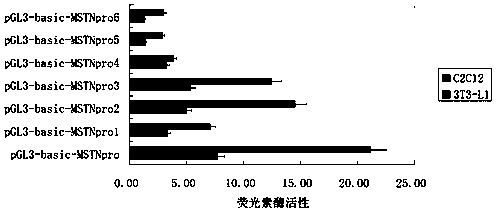
Method for detecting promoter activity by utilizing double luciferase reporter genes
InactiveCN103382505AConvenient researchAvoid influenceMicrobiological testing/measurementLipofectaminePromoter activity
The invention discloses a method for detecting promoter activity by utilizing double luciferase reporter genes. The method for detecting the promoter activity by utilizing the double luciferase reporter genes comprises step 1, building a pGL3-basic-MSTNpro recombinant which contains 7 sections of MSTN gene 5' control region fragments with different lengths; step 2, performing cultivation and planking on target cells, configuring a mixture of the pGL3-basic-MSTNpro of the step 1 and a lipidosome and enabling a renilla luciferase carrier pRL-TK to be served as an internal reference to perform cell co-transfection; step 3, performing detection on luciferase activity through the double luciferase reporter genes.
Owner:GUIZHOU UNIV
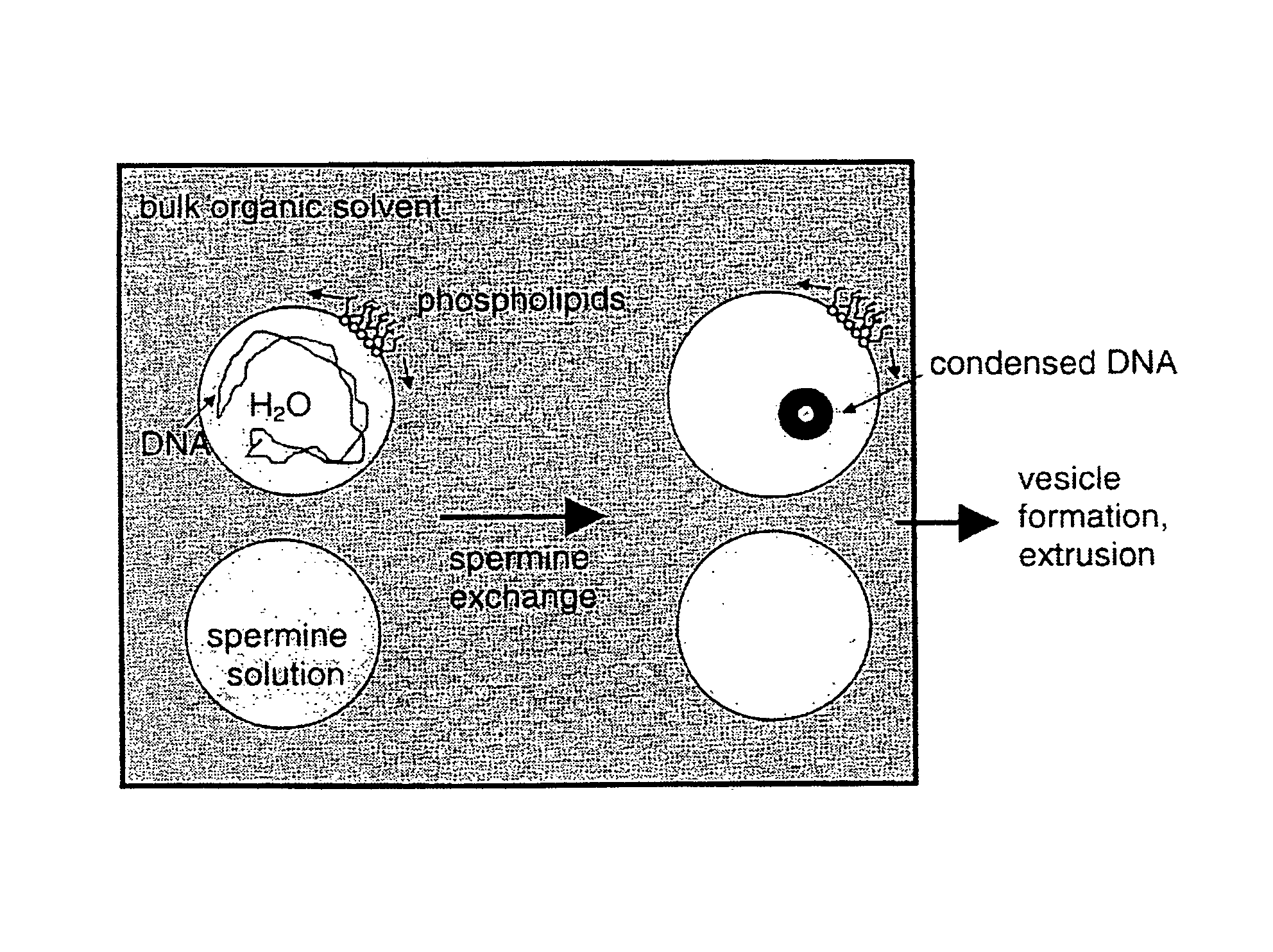
Encapsulation of bioactive complexes in liposomes
InactiveUS7491409B1Easily through lipid bilayerNot stably sequesteredAntineoplastic agentsLiposomal deliveryActive agentLiposome
This invention provides a method to prepare liposome-encapsulated bioactive agents such as nucleic acids, comprising complexation of the bioactive agents in reverse micelles prior to forming liposomes, as well as methods of using the liposomes so formed and formulations to deliver nucleic acids to cells.
Owner:LIPOSOME
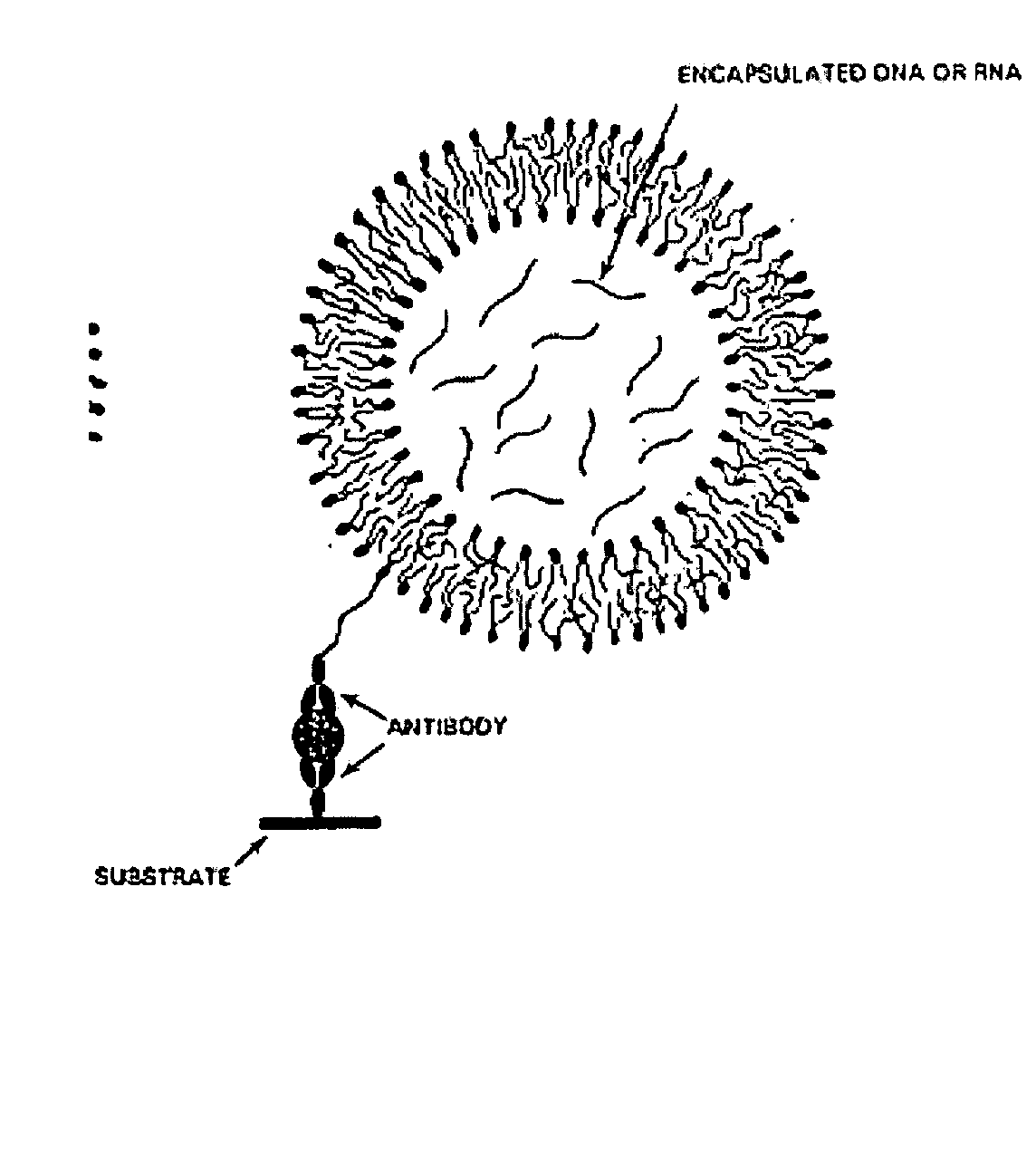
Immunoliposome-nucleic acid amplification (ILNAA) assay
InactiveUS20050158372A1Convenience to mergeIncrease rangeSugar derivativesMicroencapsulation basedLipofectamineAntigen
Immunoliposomes and use thereof in highly specific and sensitive nucleic acid amplification assays relying on amplification of specific nucleic acid sequences released from encapsulation within a liposome after a receptor on the liposome couples with a targeted analyte / antigen immobilized on a select surface. The immunoliposome nucleic acid amplification assay permits both quantitative and qualitative analyte detection.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
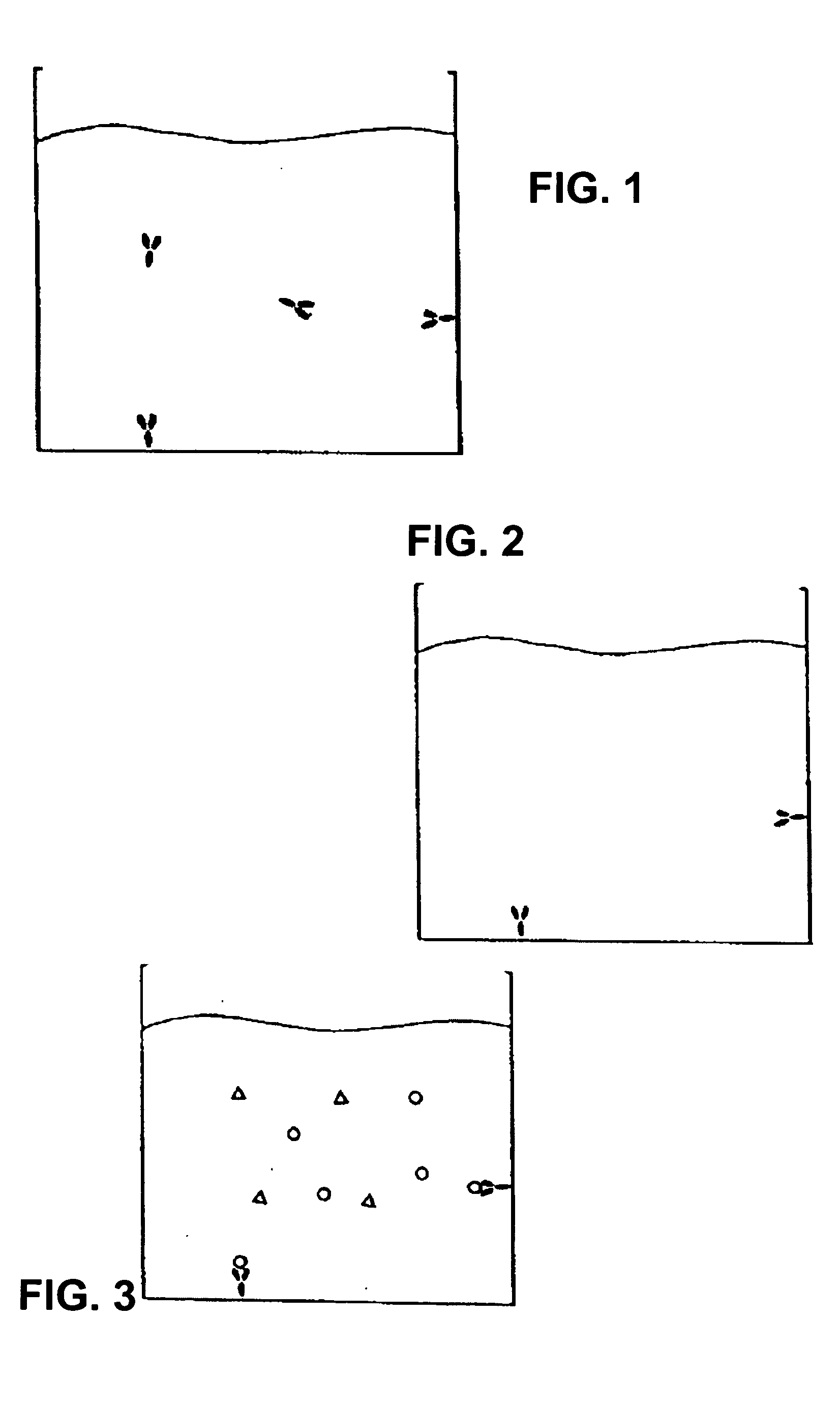
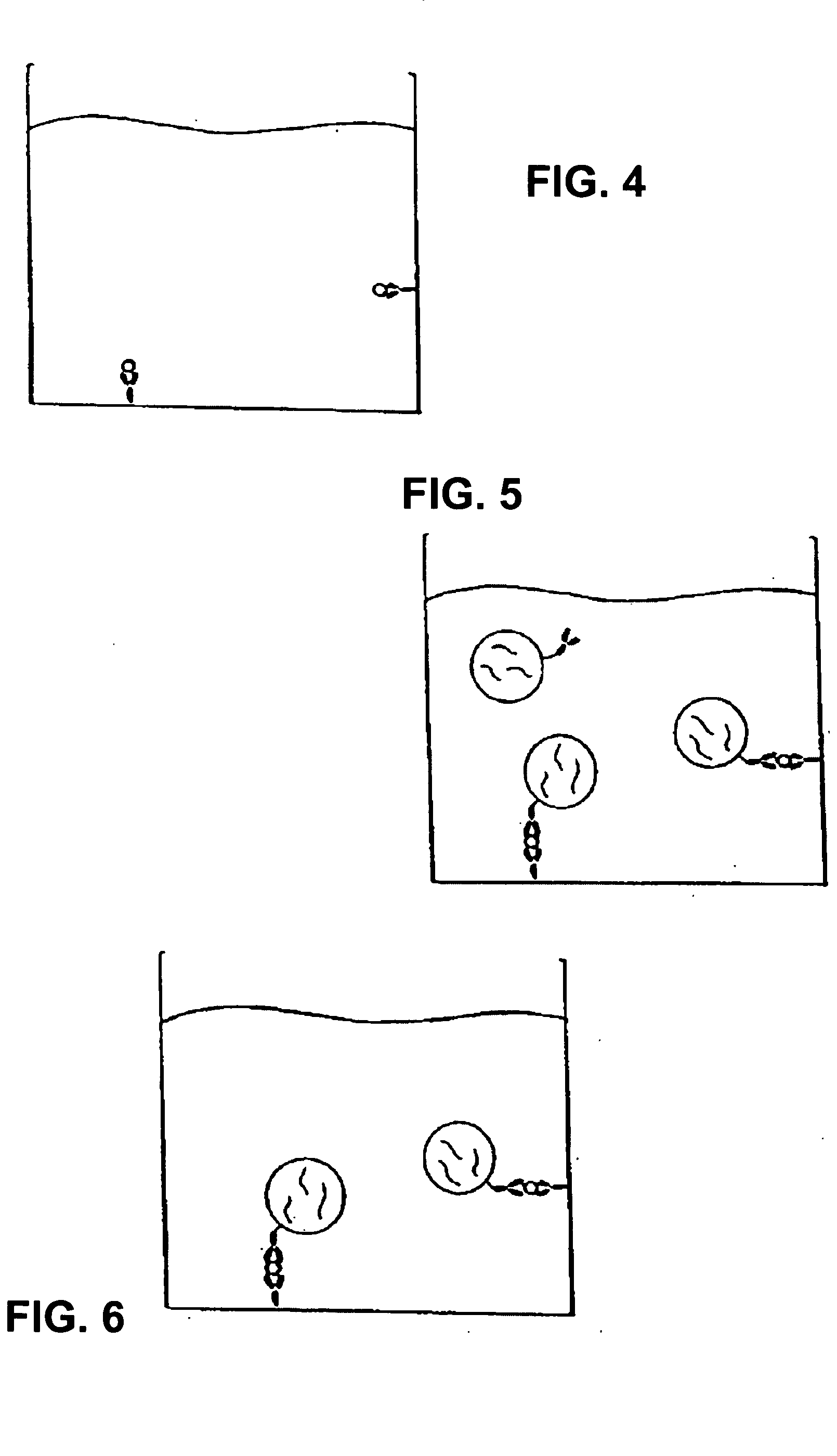
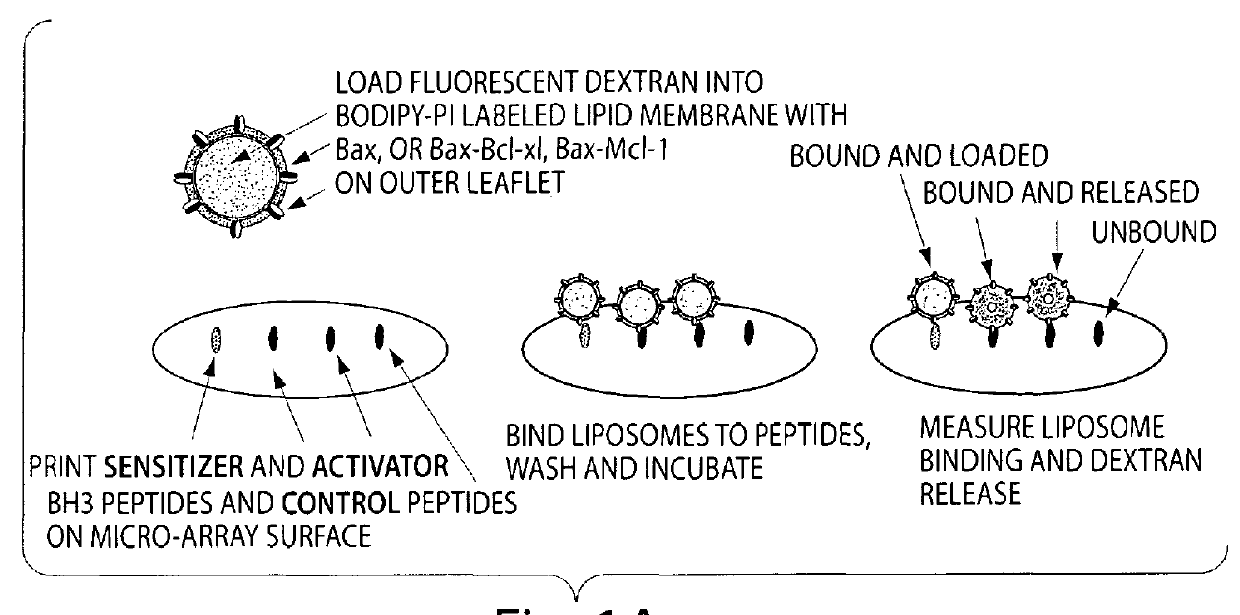
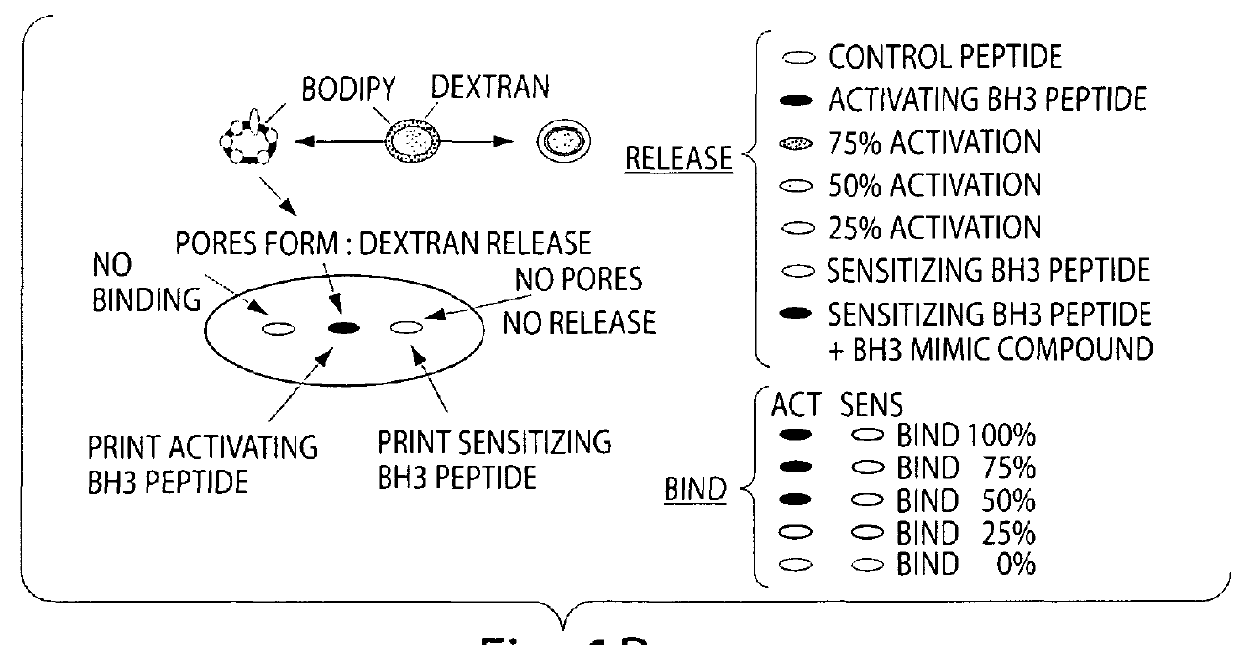
Assay system to identify therapeutic agents
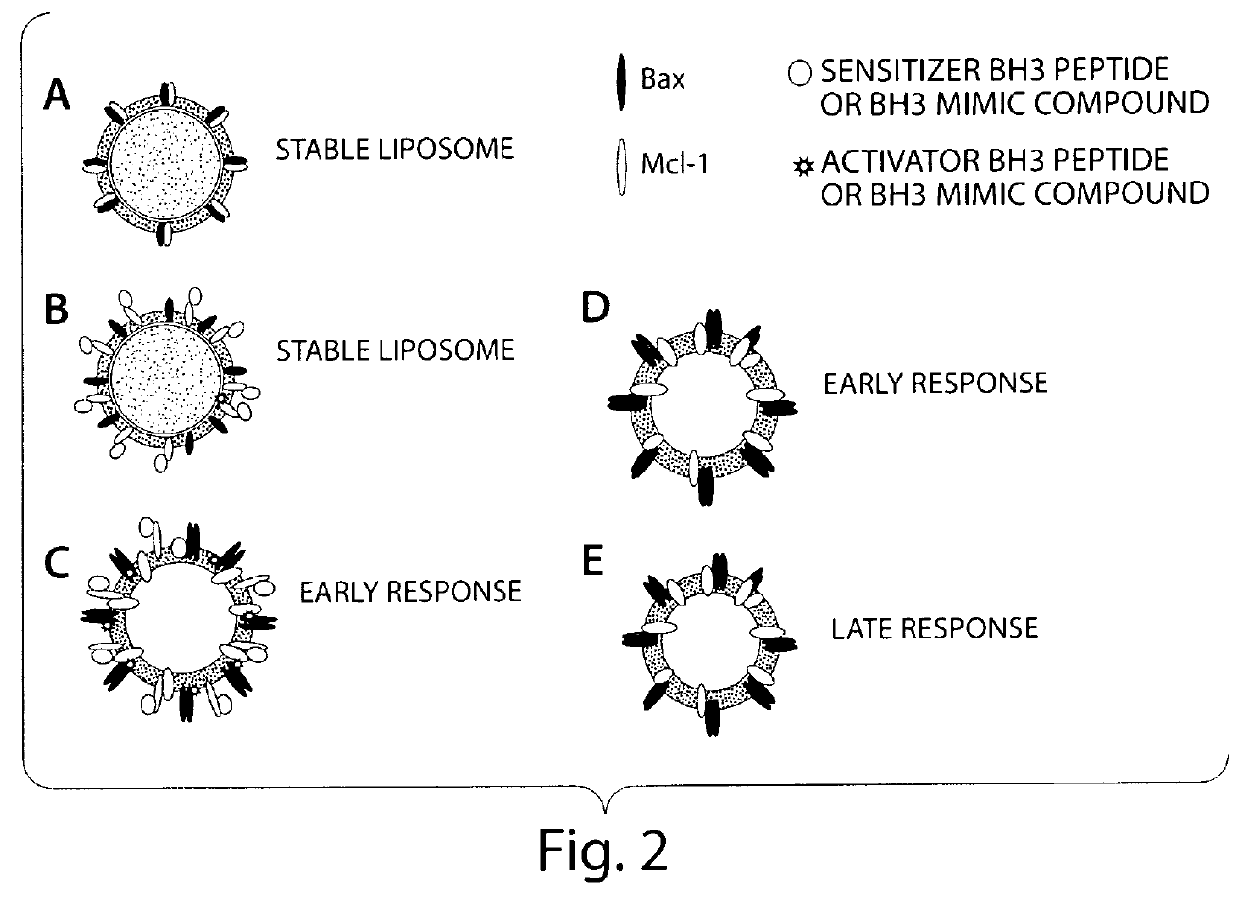
The invention is an assay designed to identify agents that modulate apoptosis. The invention provides an assay system and methods for screening for inhibitors of the Bcl-2 family of proteins. In various aspects the invention provides an assay system containing a liposome reagent and an immobilized BH3 domain peptide. In further aspects the invention provides an assay system containing mitochondria, an immobilized BH3 domain peptide and a mitochondrial binding agent, e.g. an anti-VDAC anti-body.
Owner:EUTROPICS PHARMA
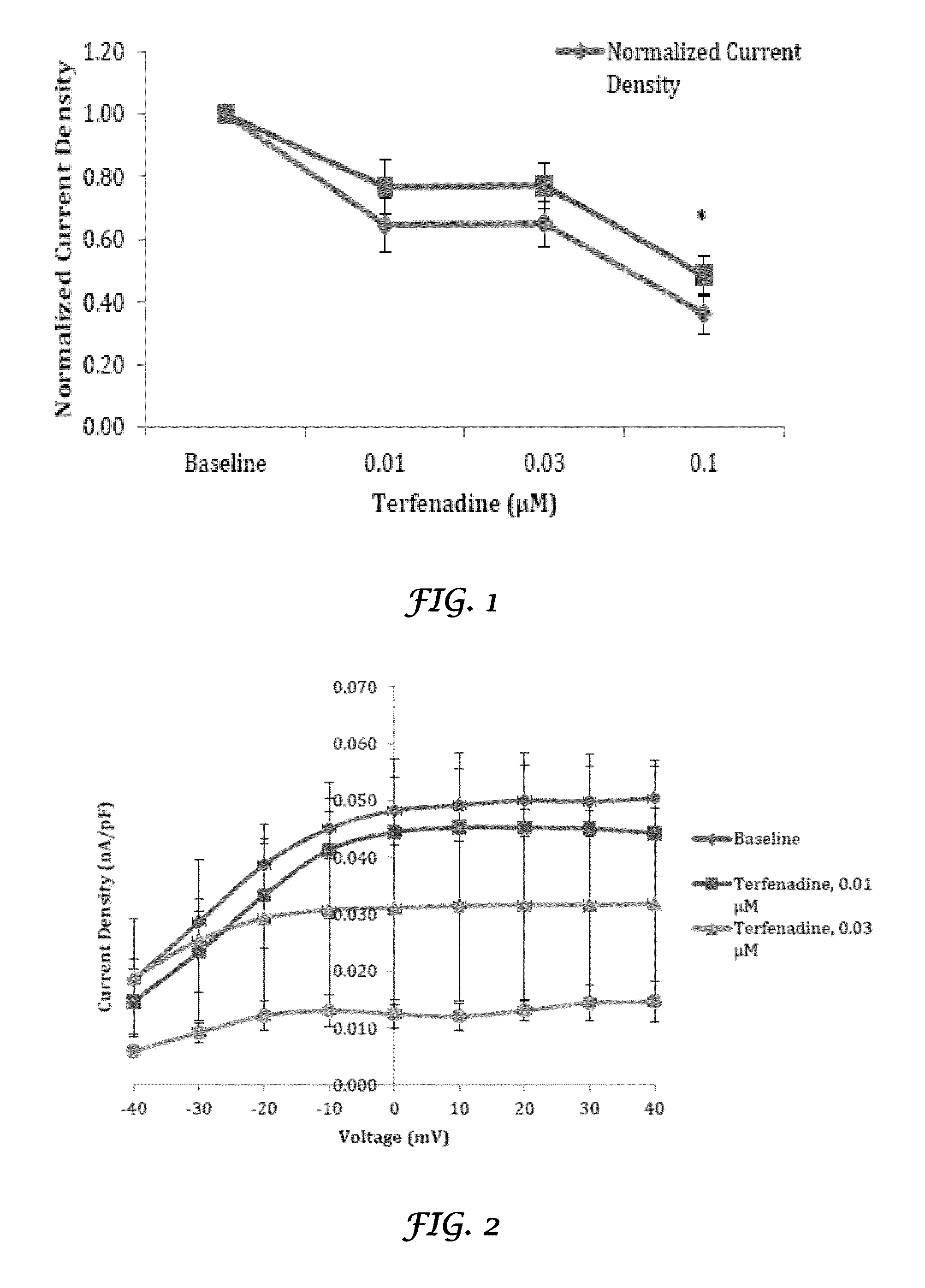
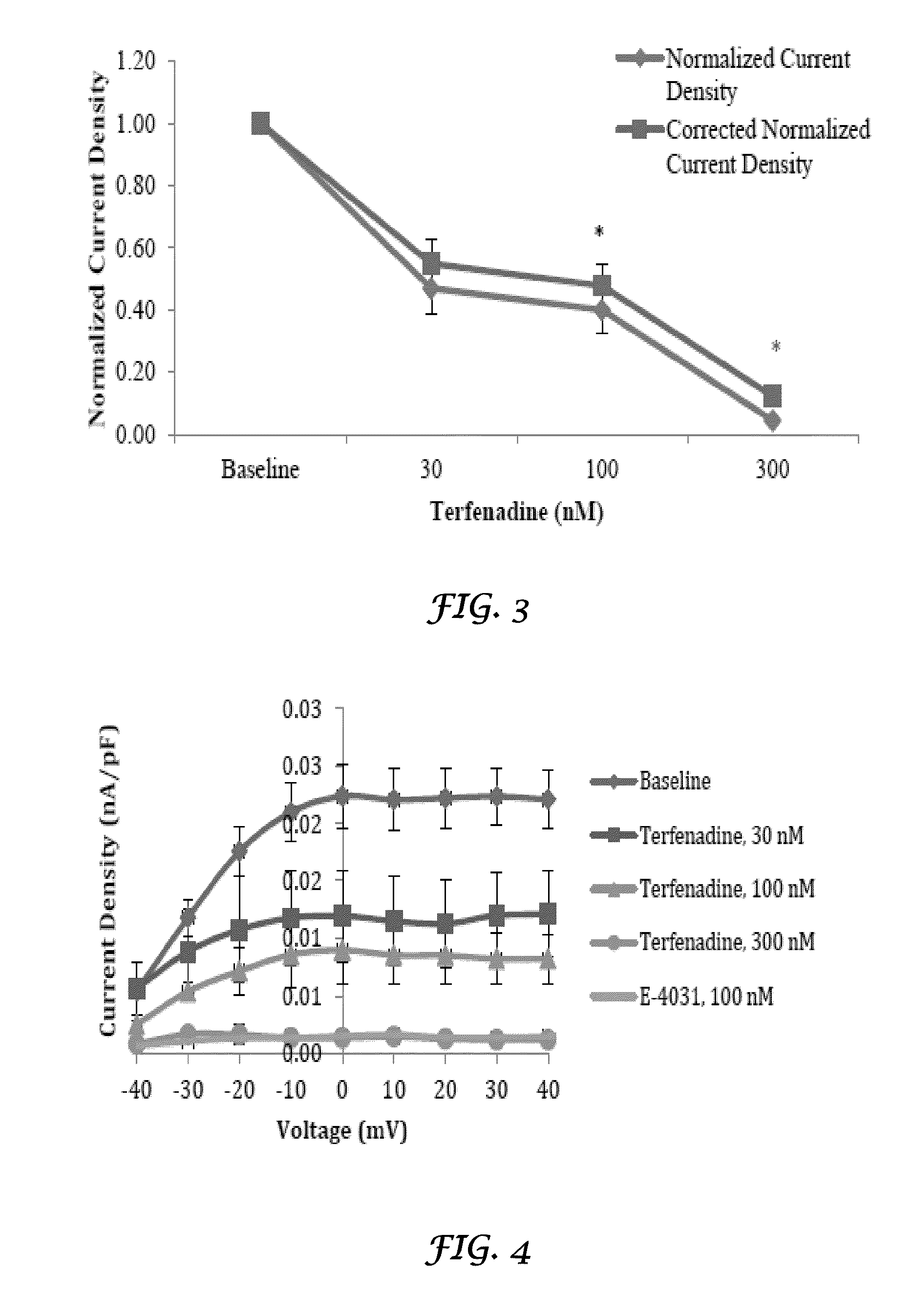
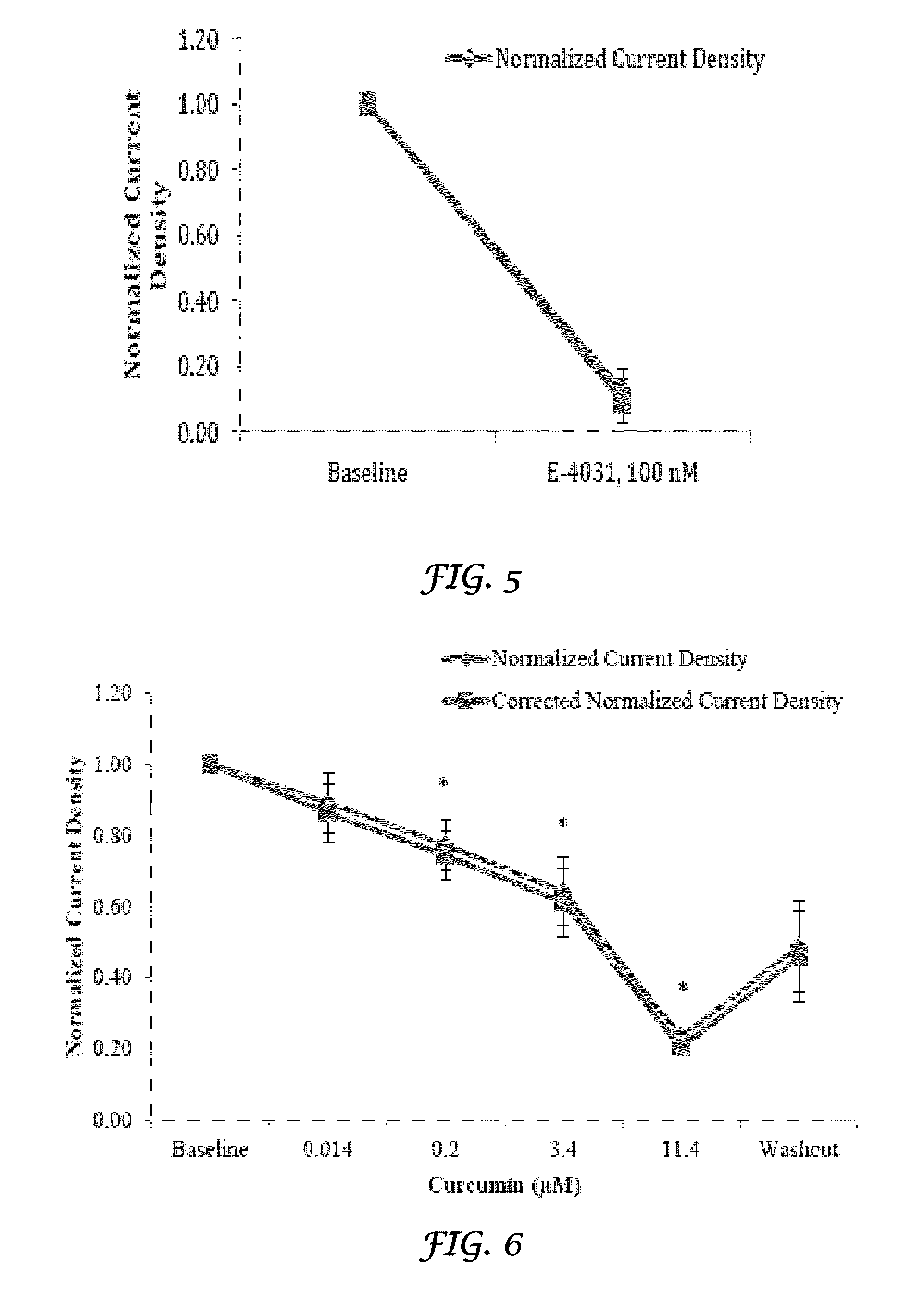
Liposomal mitigation of drug-induced inhibition of the cardiac ikr channel
Compositions and methods are provided for preventing one or more cardiac channelopathies or conditions resulting from irregularities or alterations in cardiac patterns, or both, in a human or animal subject comprising: one or more pharmacologically active agents that causes at least one of IKr channel inhibition or QT prolongation by inhibiting the activity of an ether-a-go-go-related gene (hERG); and one or more liposomes, wherein the liposomes are empty liposomes and administered prior to, concomitantly, or after administration of the pharmacologically active agent.
Owner:SIGNPATH PHARMA INC
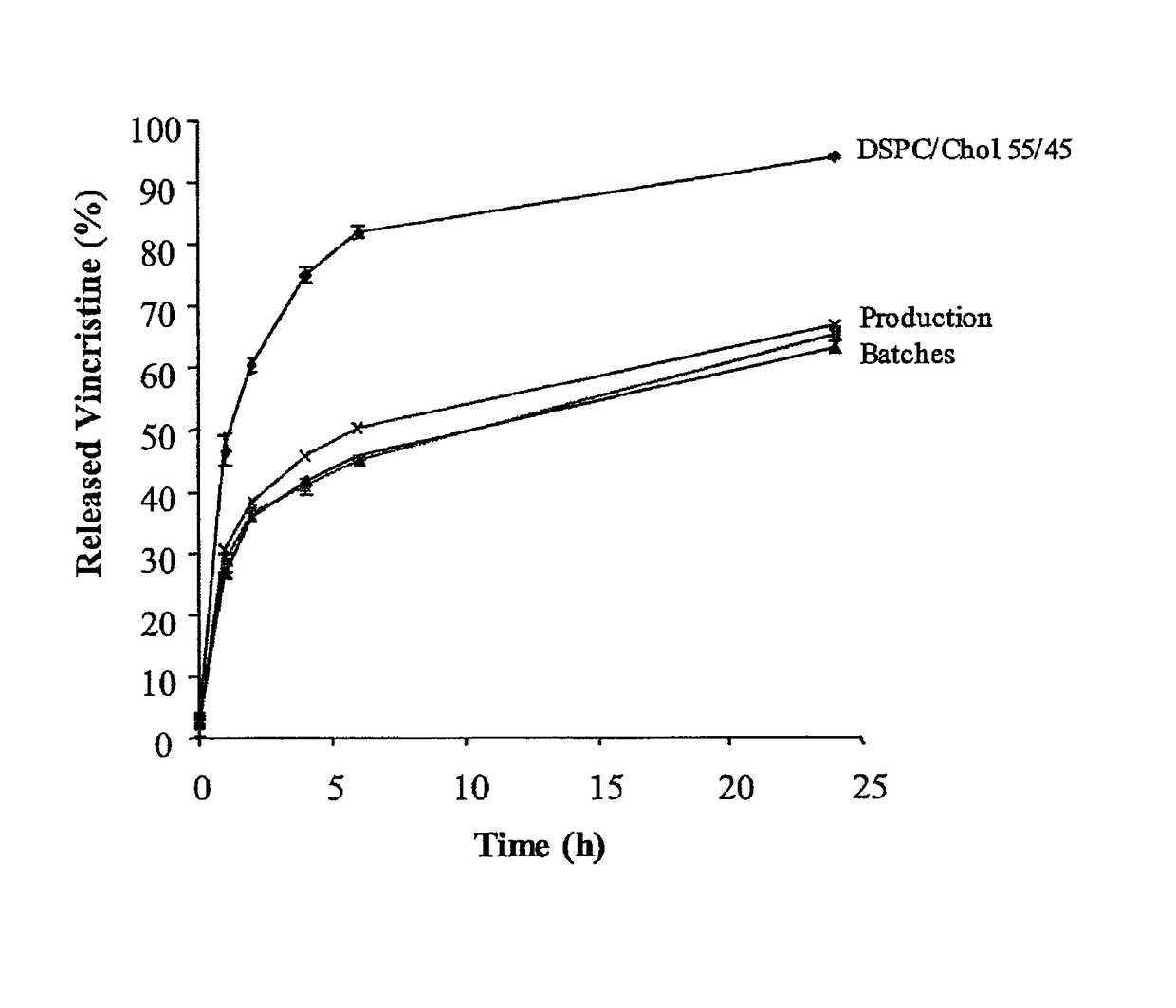
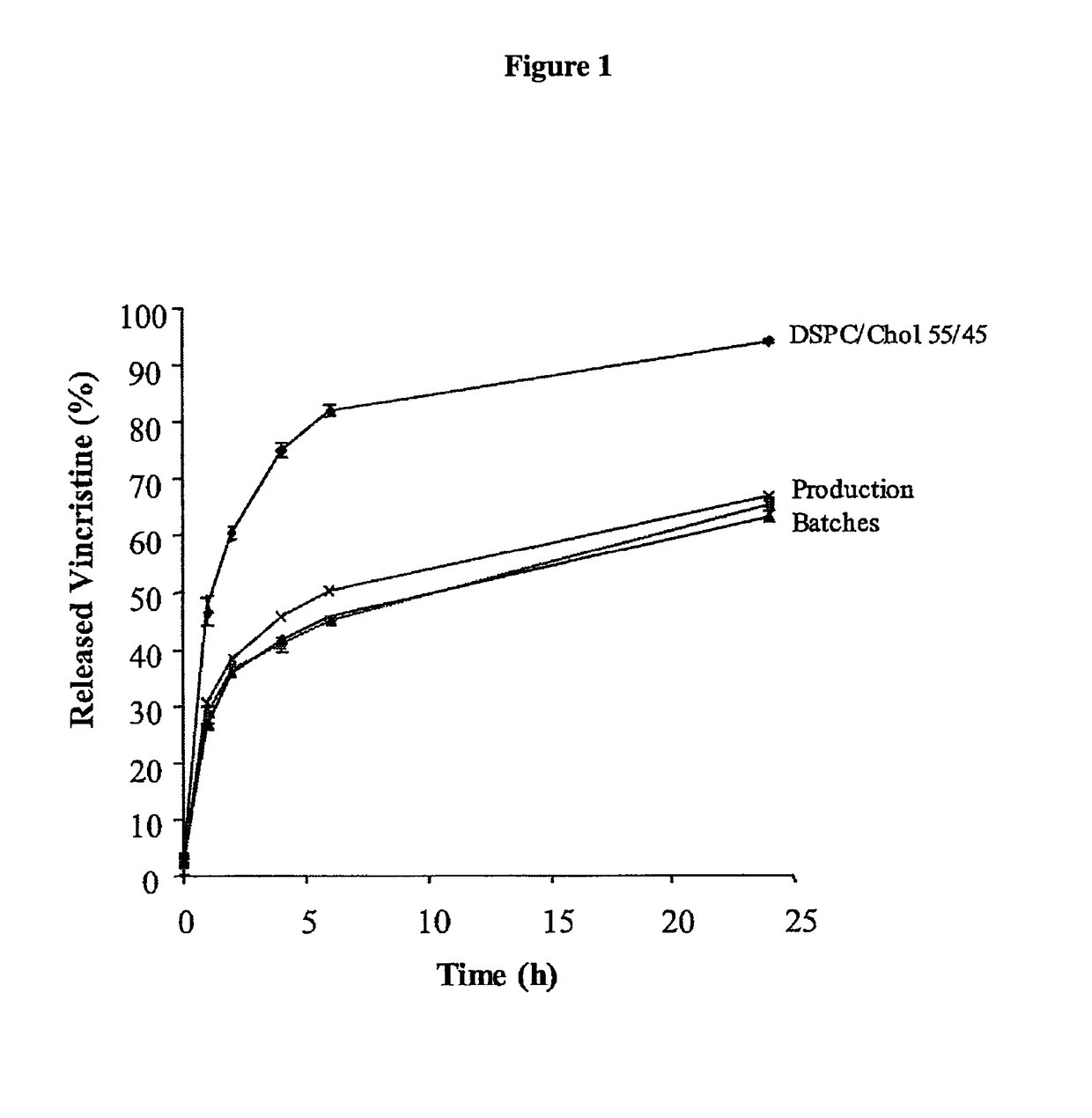
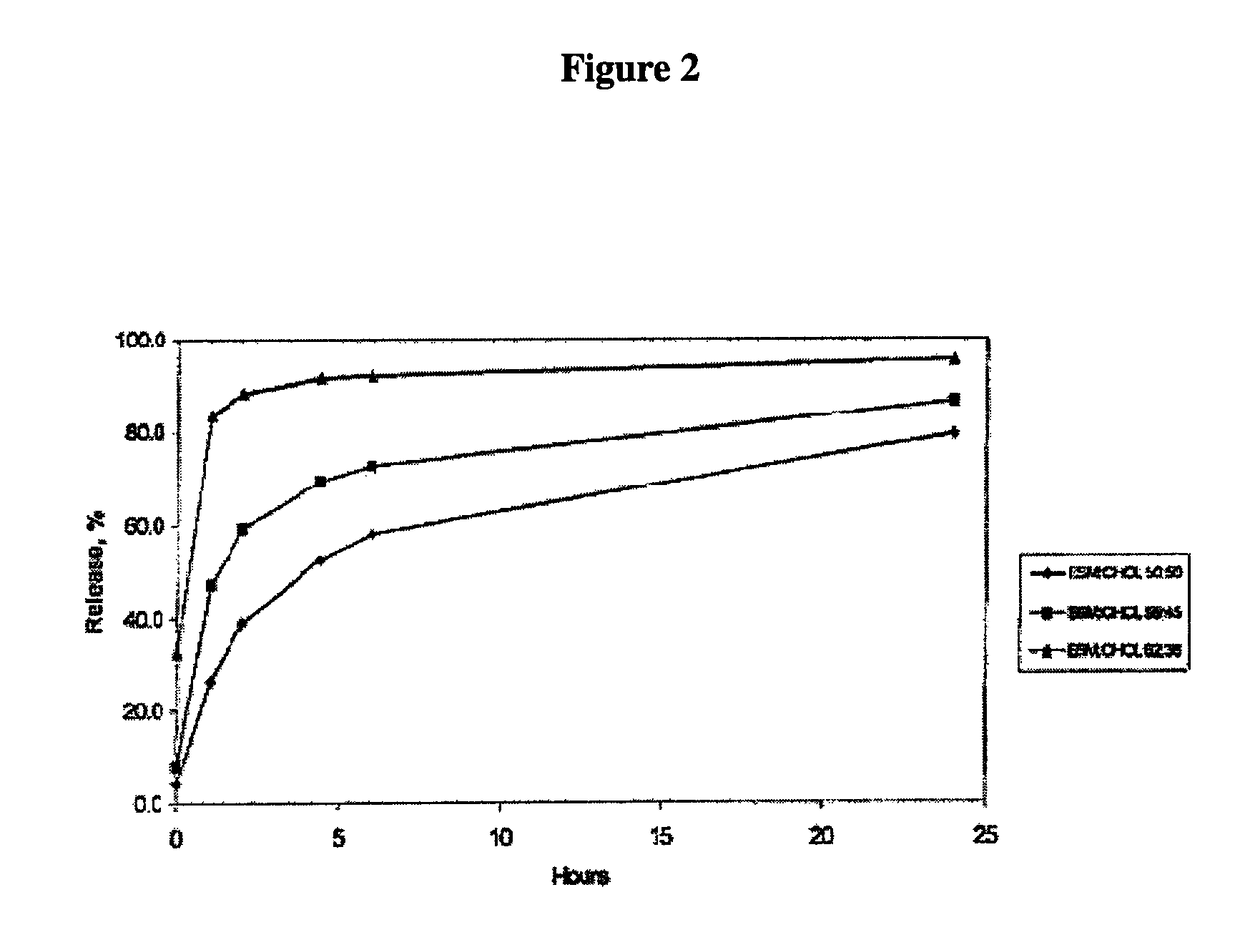
In vitro release assay for liposome encapsulated vincristine
Owner:ARBUTUS BIOPHARMA CORPORAT ION
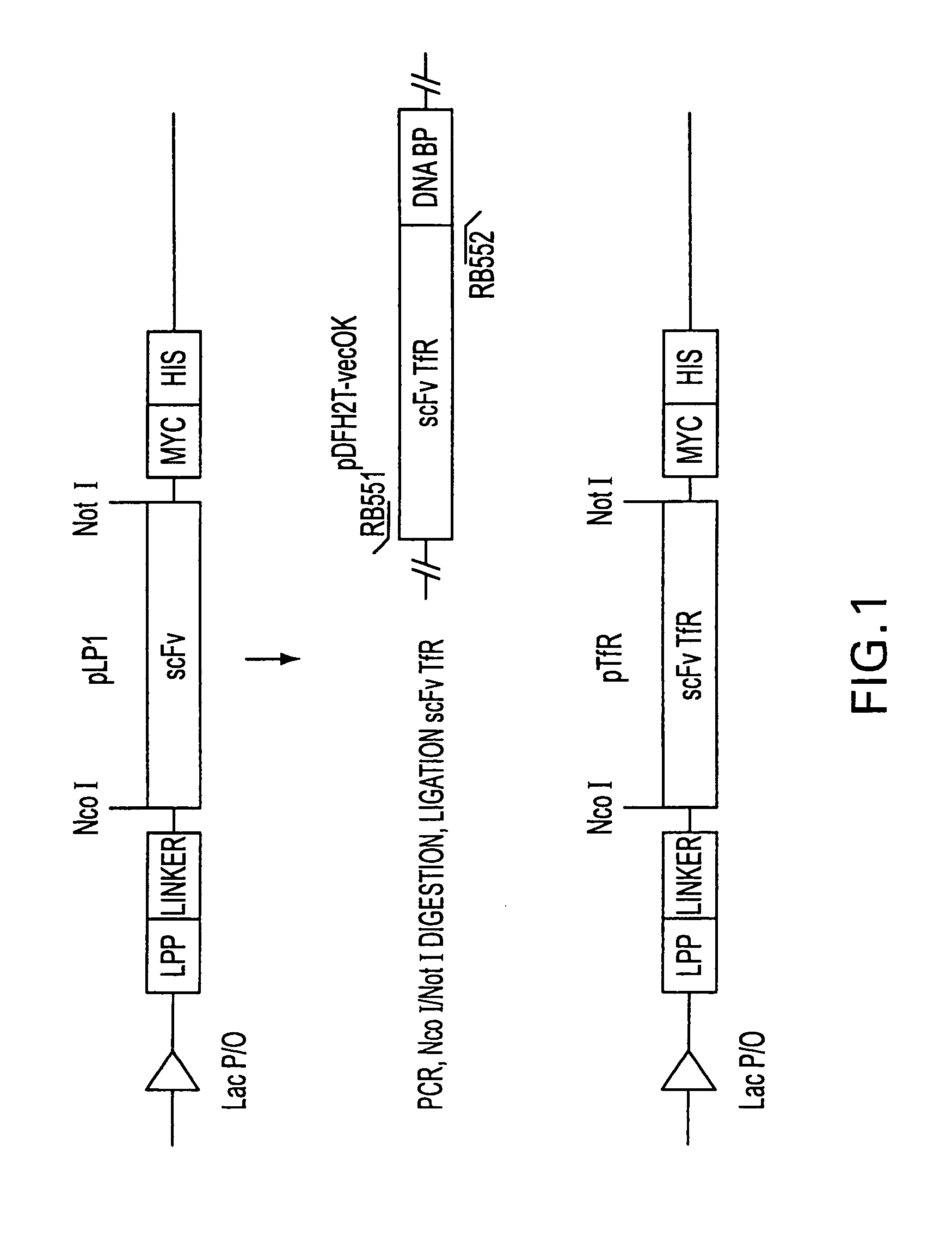
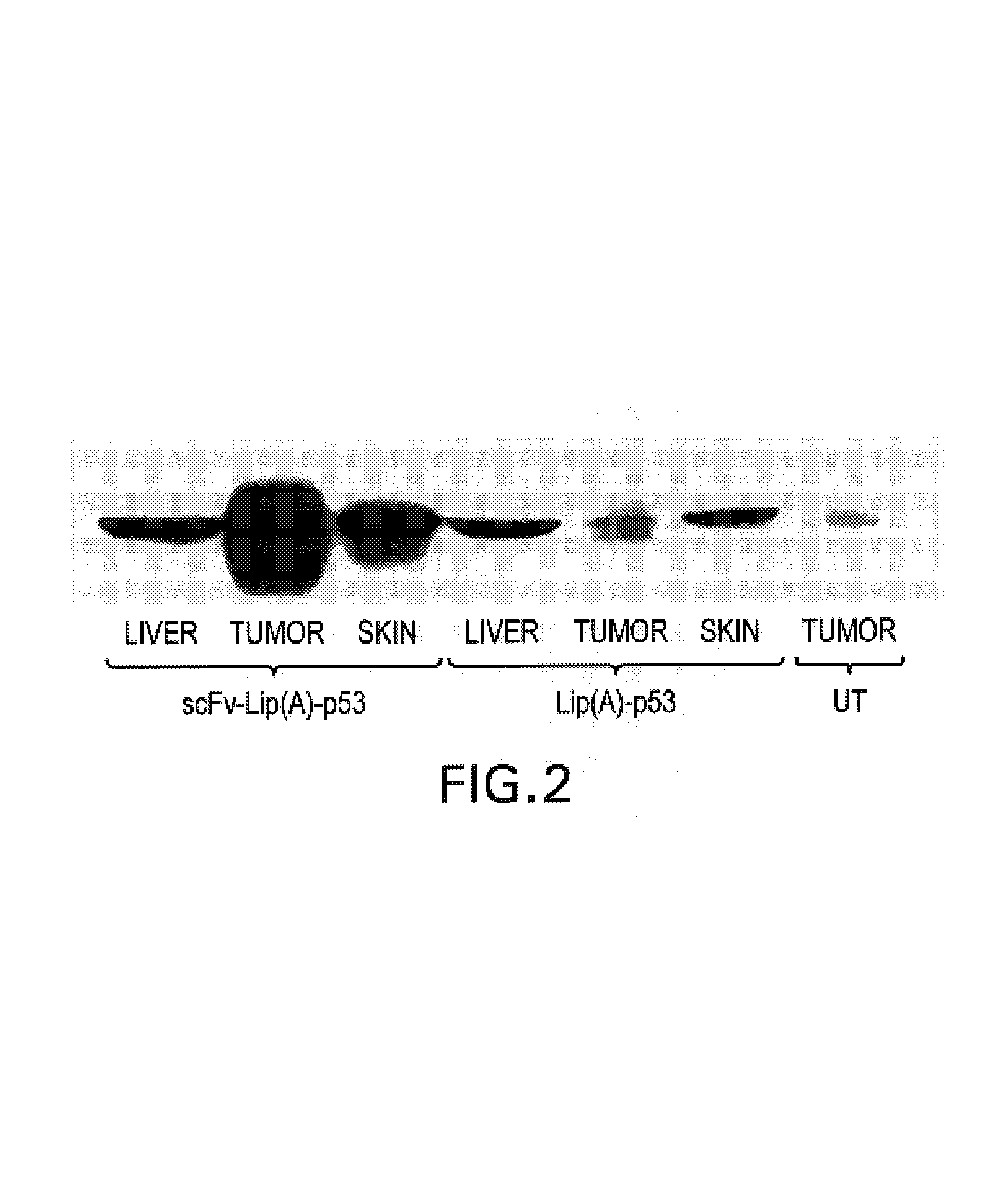
Antibody fragment-targeted immunoliposomes for systemic gene delivery
InactiveUS7479276B1Improve the level ofEfficient transfectionVectorsAntibody ingredientsGene deliveryAntiendomysial antibodies
Nucleic acid-immunoliposome compositions useful as therapeutic agents are disclosed. These compositions preferably comprise (i) cationic liposomes, (ii) a single chain antibody fragment which binds to a transferrin receptor, and (iii) a nucleic acid encoding a wild type p53. These compositions target cells which express transferrin receptors, e.g., cancer cells. These compositions can be used therapeutically to treat persons or animals who have cancer, e.g., head and neck cancer, breast cancer or prostate cancer.
Owner:SYNERGENE THERAPEUTICS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com