Patents
Literature
106 results about "Luciferase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
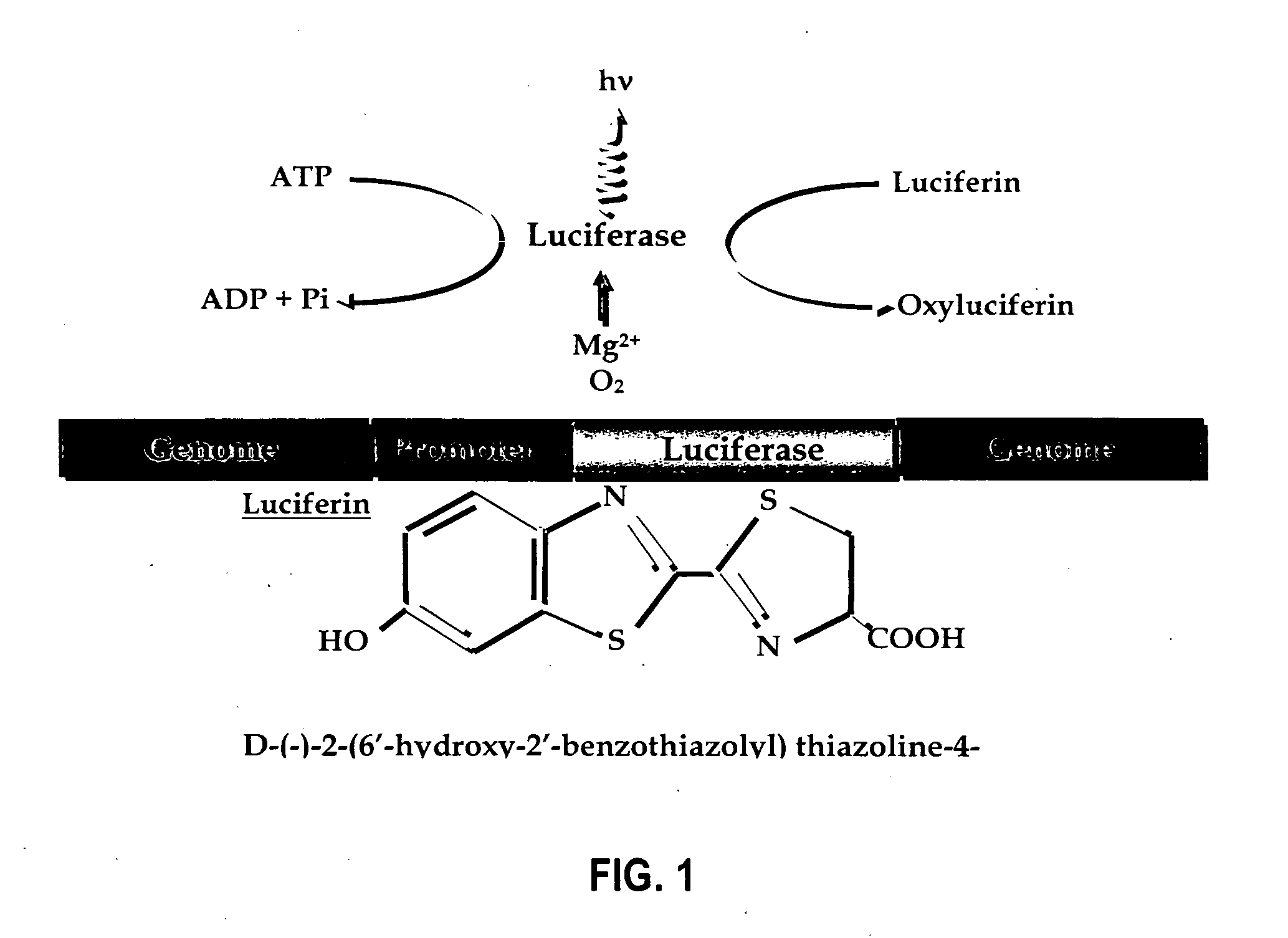
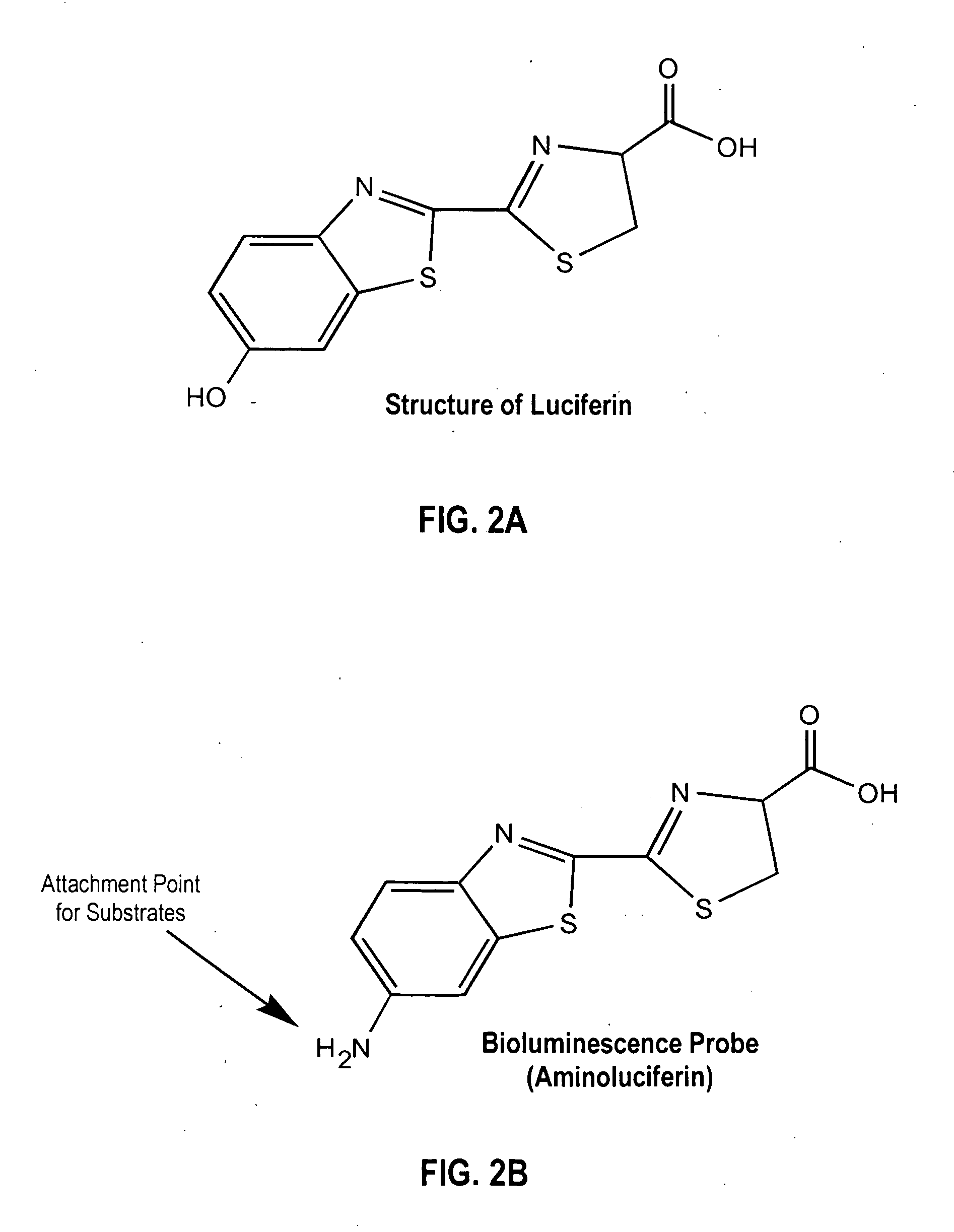
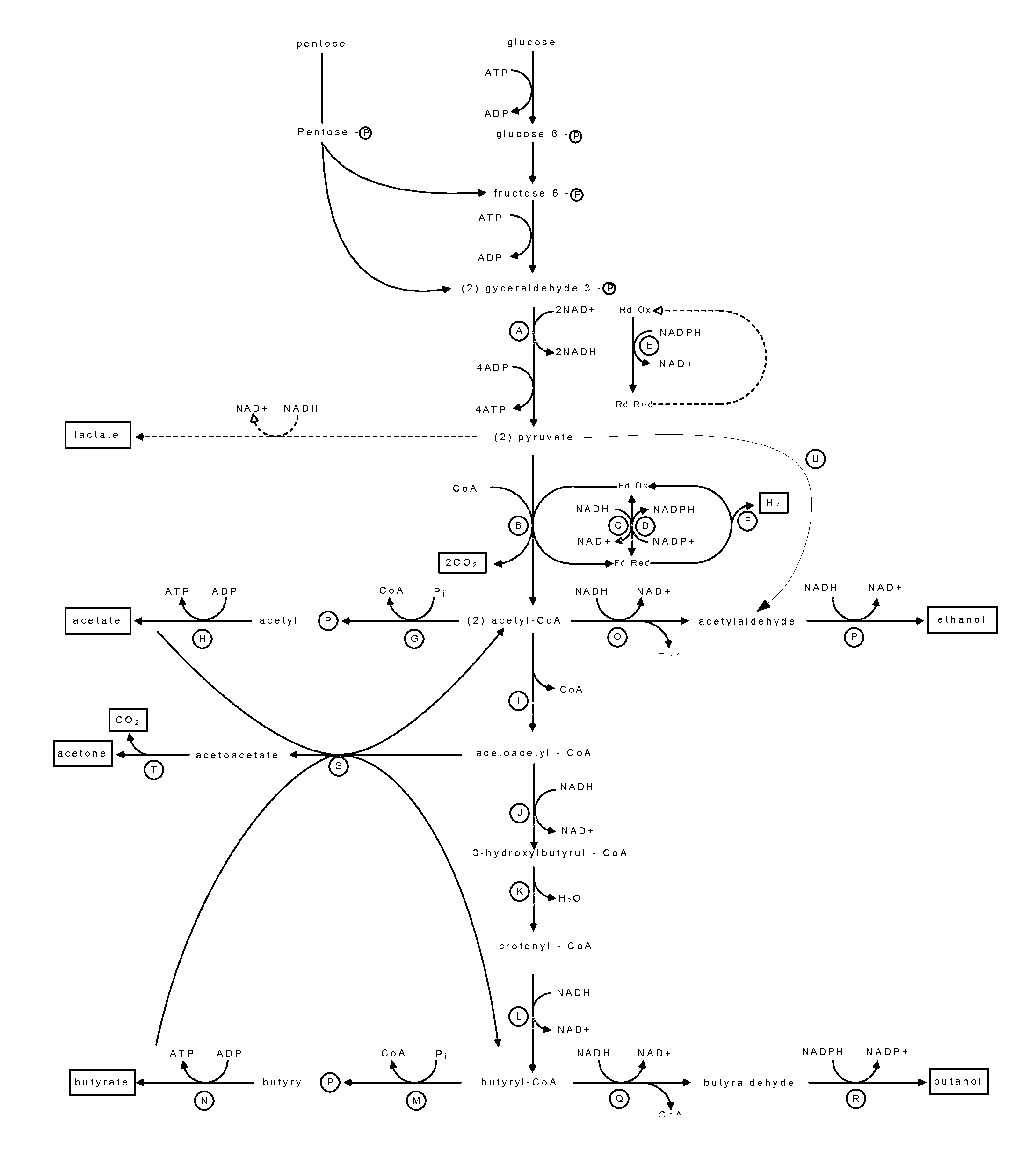
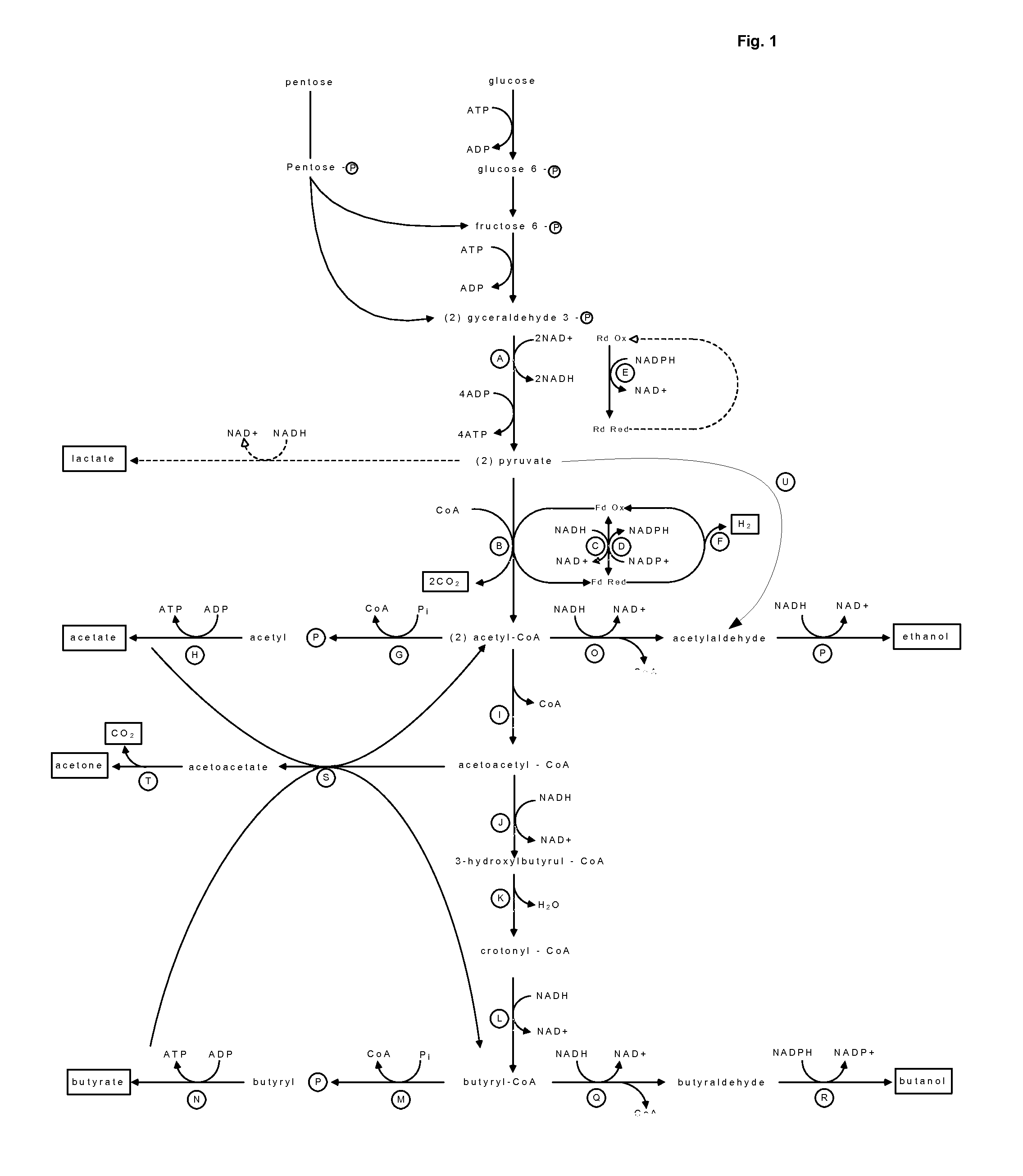
Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence, and is usually distinguished from a photoprotein. The name was first used by Raphaël Dubois who invented the words luciferin and luciferase, for the substrate and enzyme, respectively. Both words are derived from the Latin word lucifer – meaning lightbringer.
Luciferase biosensor
ActiveUS20050153310A1Inhibitory activityCompound screeningApoptosis detectionLuciferaseEnzyme protein
Owner:PROMEGA
Detection of living cells in polymers or pigments
InactiveUS20050070701A1Reliably and efficiently releasedHigh sensitivitySugar derivativesMicrobiological testing/measurementParticulatesBiochemistry
Disclosed is a process for releasing ATP from living cells in aqueous mixtures of polymers or pigments. The aqueous mixture of a polymer or pigment is agitated in the presence of a particulate disruption agent to cause rupturing of the living cells and release of the ATP contained therein. Also disclosed is a process for detecting living cells in a aqueous mixtures of polymer or pigments by detecting the ATP released by the disruption process by a luciferin / luciferase assay. A kit for the detection of living cells in aqueous mixtures of polymers or pigments also is described.
Owner:EASTMAN CHEM CO
Luciferyl peptide substrate
InactiveUS20060073529A1Promote localizationPeptide/protein ingredientsMicrobiological testing/measurementLuciferase GeneCell membrane
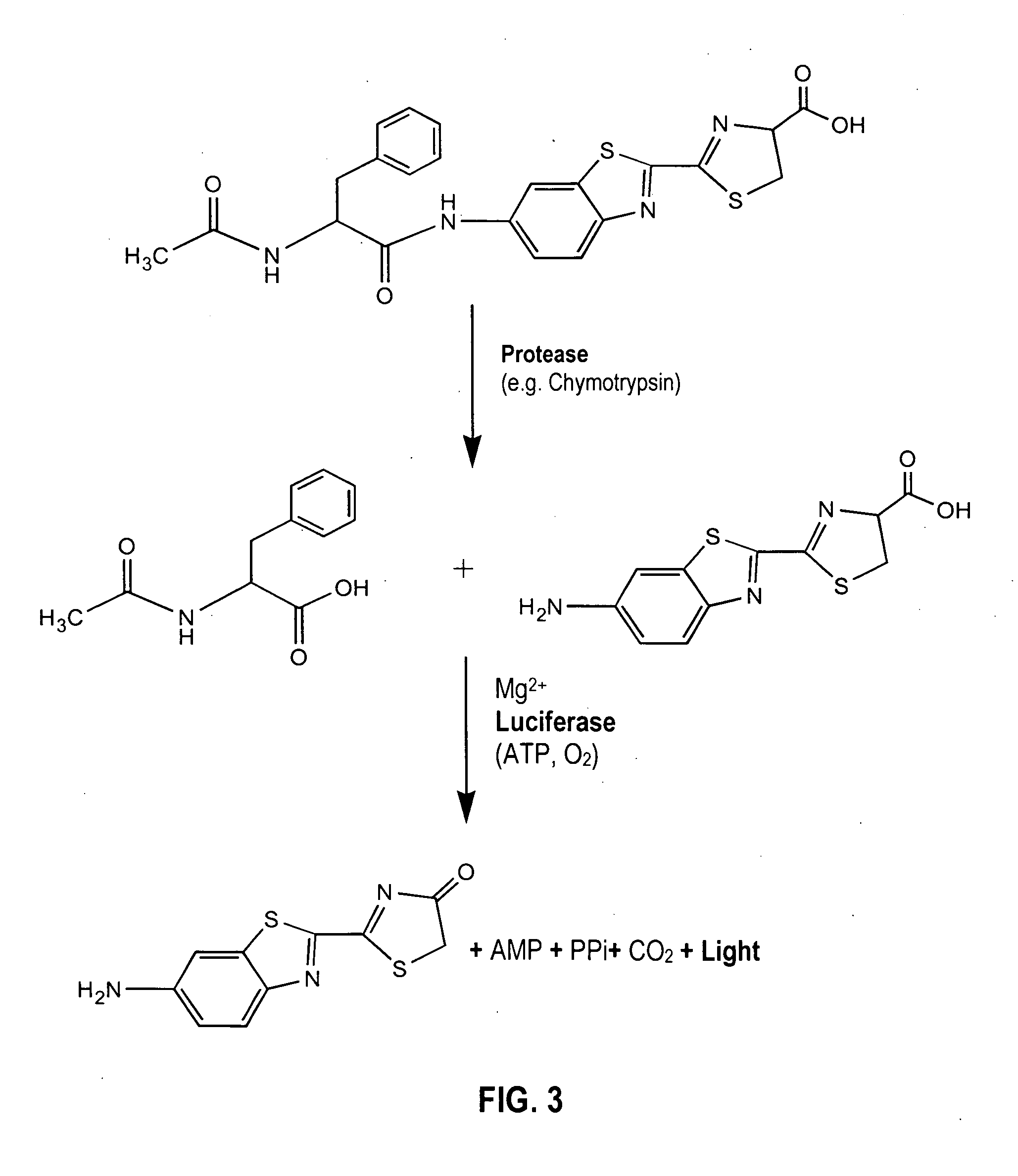
Provided are luciferyl peptide substrates that are produced by attaching specifically prepared peptide conjugates to luciferin, and / or its analogs and derivatives. The luciferyl peptide substrates are incapable of penetrating cell membranes and tissue barriers. Cleavage of the peptide conjugates from the luciferyl peptide substrates releases the luciferin, which upon contact with luciferase emits photons for easy detection. The luciferyl peptide substrates may be used in assays to detect pathogens, test protease inhibitors, probe cell physiology, assess protease activity in oncogenesis, and improve specific and regulated drug delivery.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
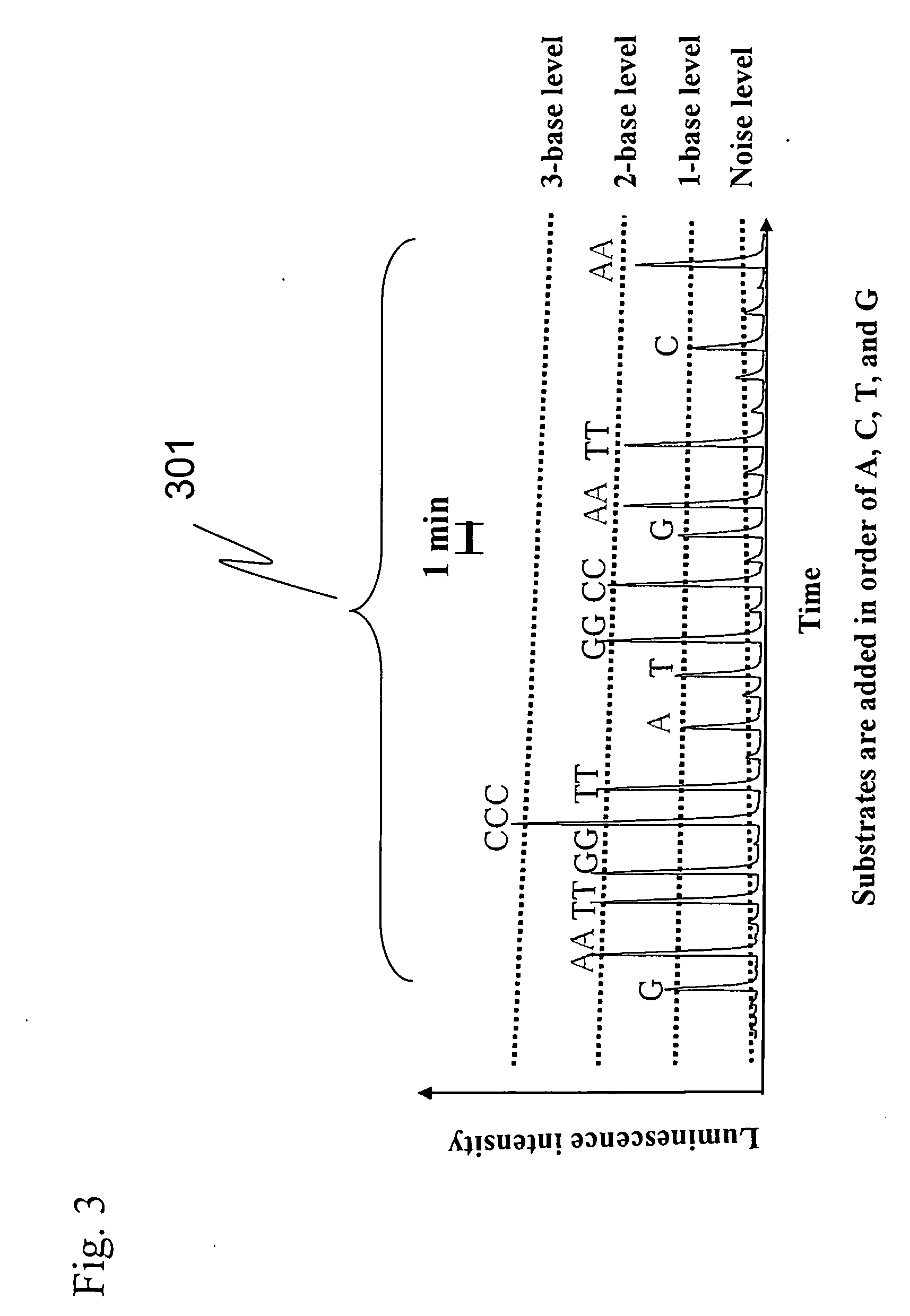
Method and reagent for sequencing
InactiveUS20070166729A1Reduce the amount requiredLow costMicrobiological testing/measurementLuciferase GenePyrophosphate
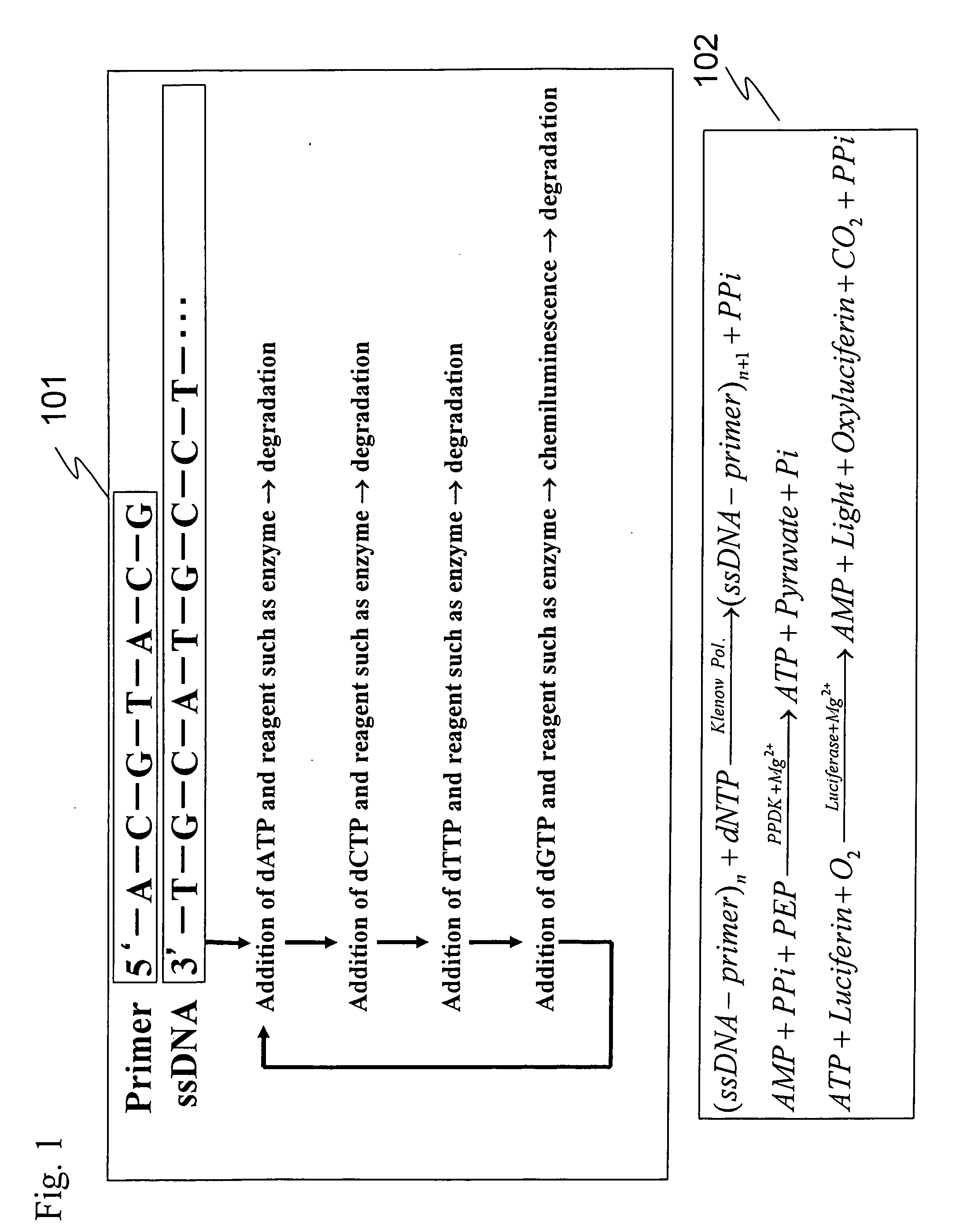
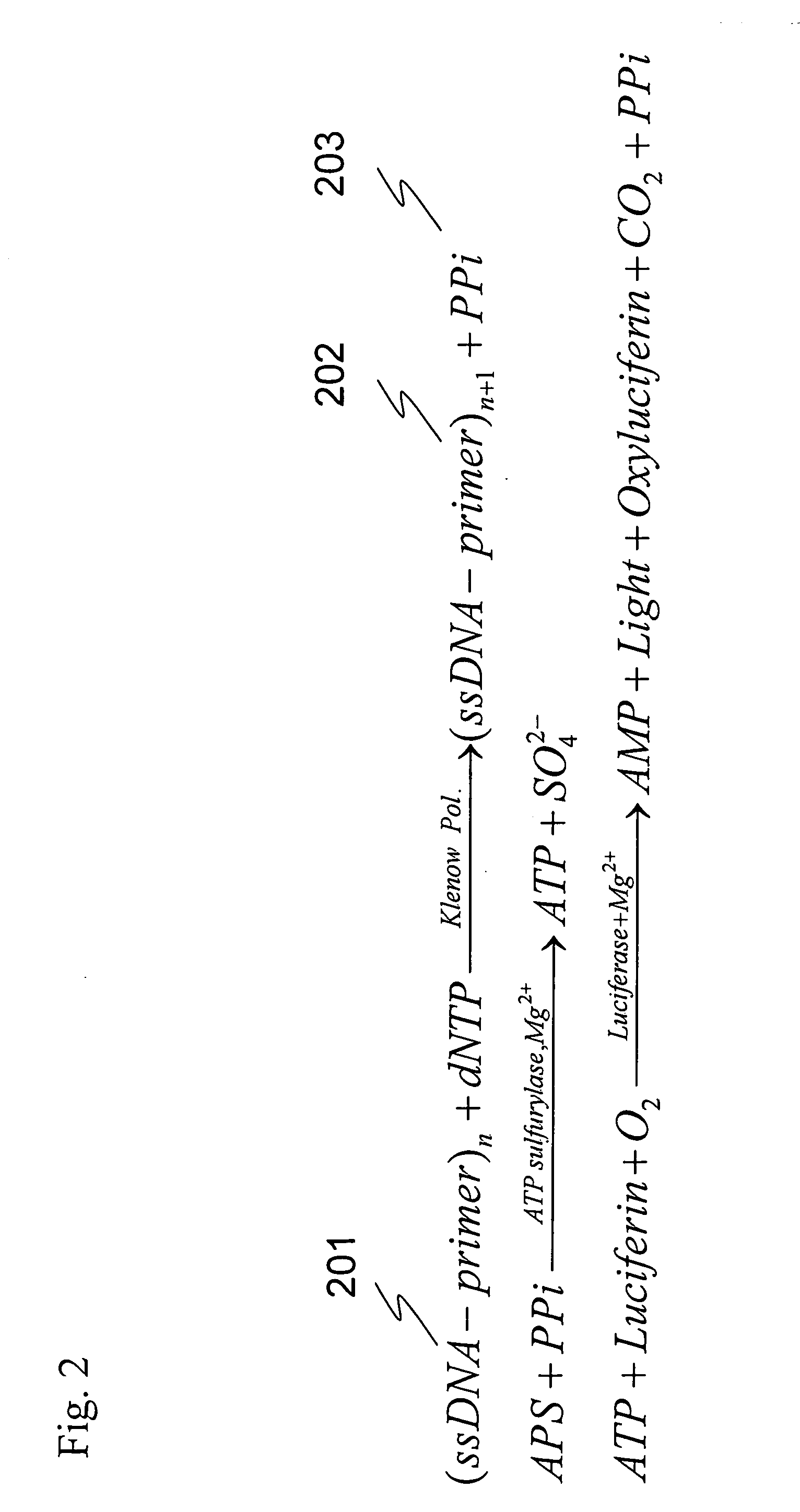
The present invention provides: a method for nucleic acid analysis including the steps of subjecting a reaction solution containing a sample nucleic acid to complementary strand synthesis with the sample nucleic acid as a template, reacting pyrophosphate produced in the complementary strand synthesis with 30 to 800 μM AMP in the coexistence of pyruvate phosphate dikinase to produce ATP, performing luciferase reaction with the ATP as a reaction substrate, and detecting chemiluminescence generated in the luciferase reaction to determine the presence or absence of the complementary strand synthesis; and a kit therefor.
Owner:HITACHI LTD
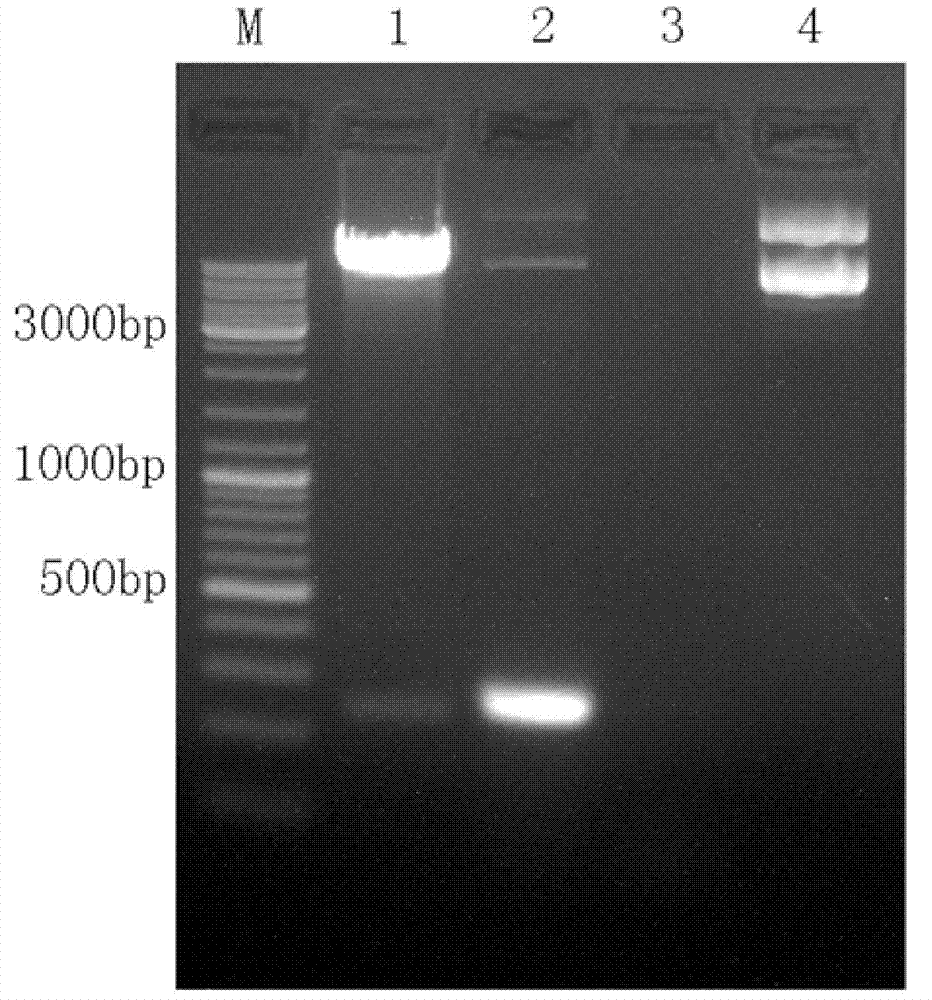
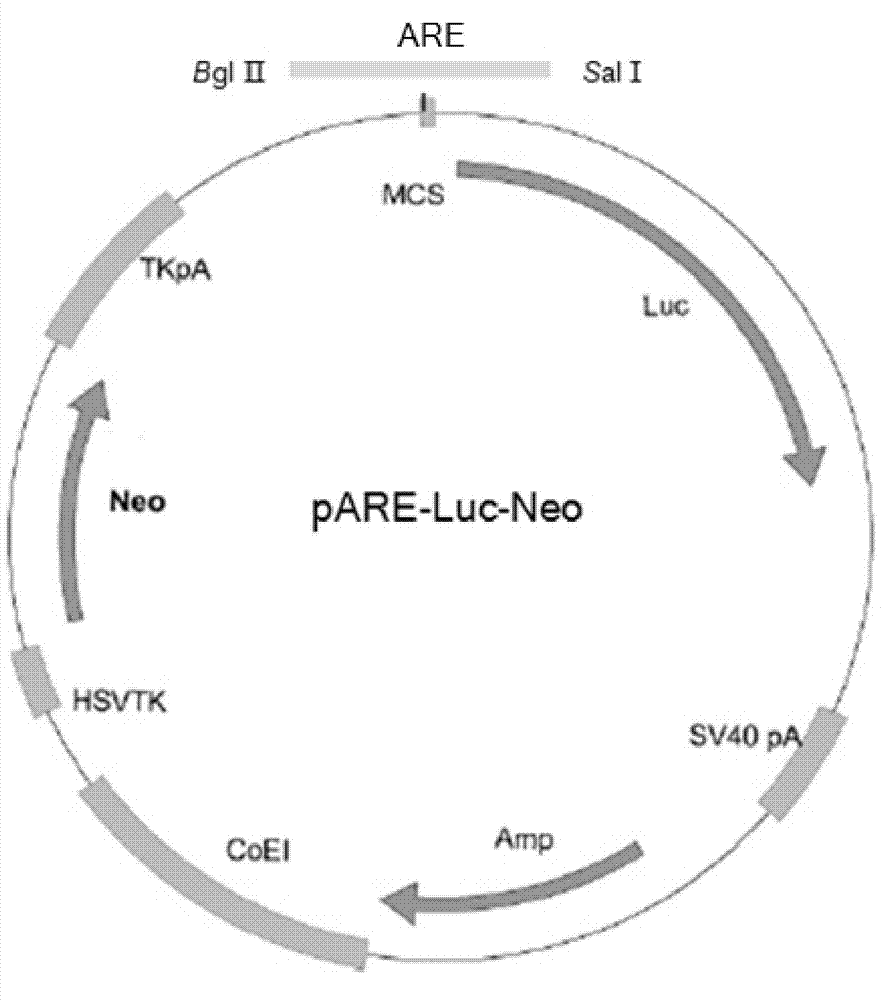
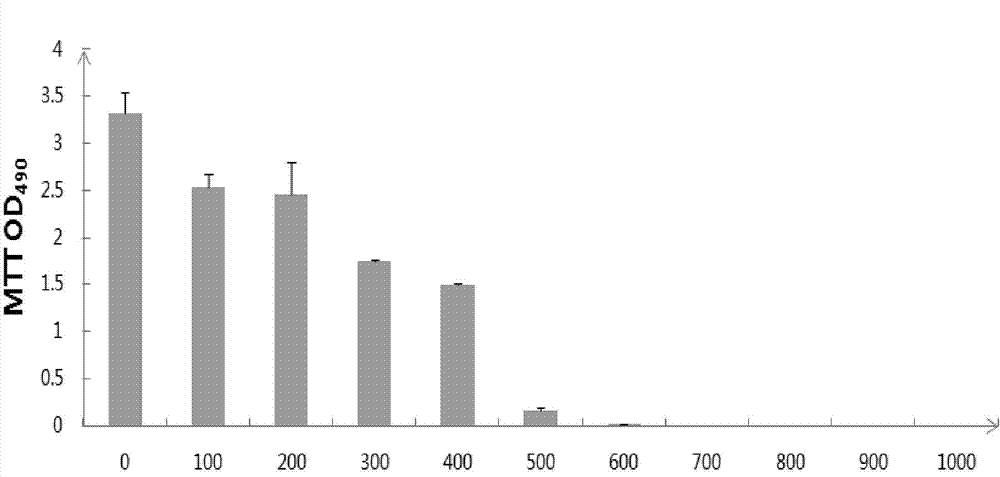
Cell line screened by anti-oxidant or chemical preventive agent, construction and application
InactiveCN102899351AIncrease vitalityIncreased sensitivityMicrobiological testing/measurementVector-based foreign material introductionAntioxidative responseNeomycin
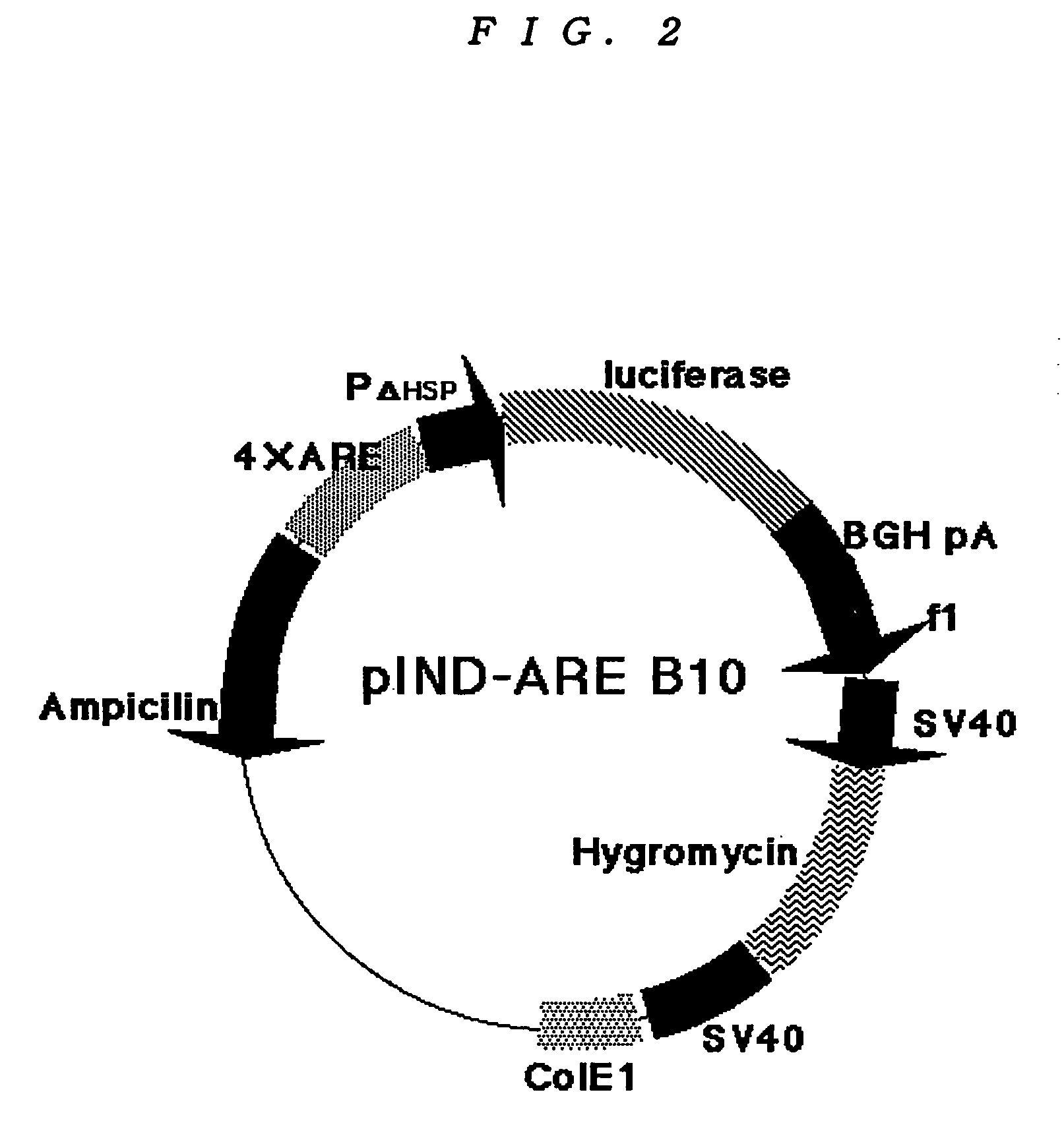
The invention discloses a recombinant plasmid and a construction method, the recombinant plasmid contains an antioxidation reaction element, luciferase gene and neomycin resistant gene. The invention also discloses a cell line screened by an anti-oxidant or a chemical preventive agent, a construction and an application, and the cell line is the cell integrated with the above recombinant plasmid. The provided cell line integrates the ARE gene in a recombinant plasmid carrier pARE-Luc-Neo, luciferase gene and neomycin resistant gene to a genome of a host cell Hek293, by following with subcultring of cell (more than 10 generation), the detection of a luciferase expression is stable, compared with a transient transfection method, and the construction provided by the invention is more convenient, sensitive and reliable.
Owner:TIANJIN YAOYU BIOLOGICAL TECH
Luciferase complementary quantum dot biosensor as well as construction method and application thereof
ActiveCN105807064AImprove performanceImprove structural stabilityBiological testingFluorescence/phosphorescenceQuantum dotLuciferase
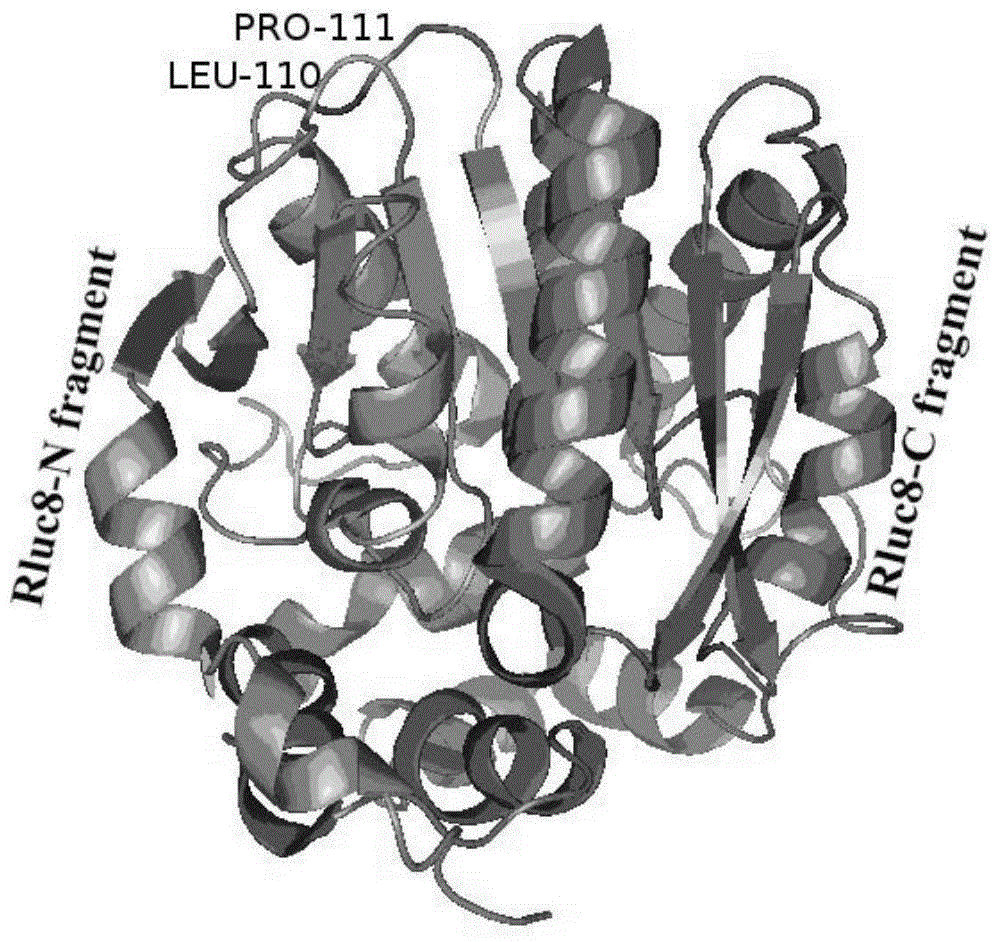
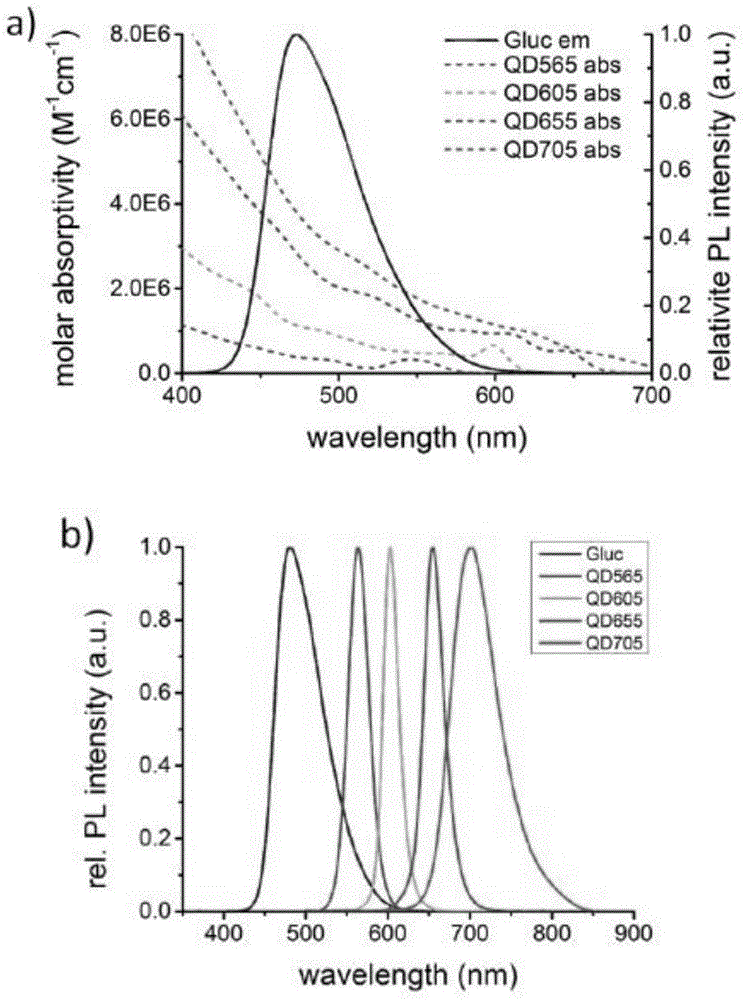
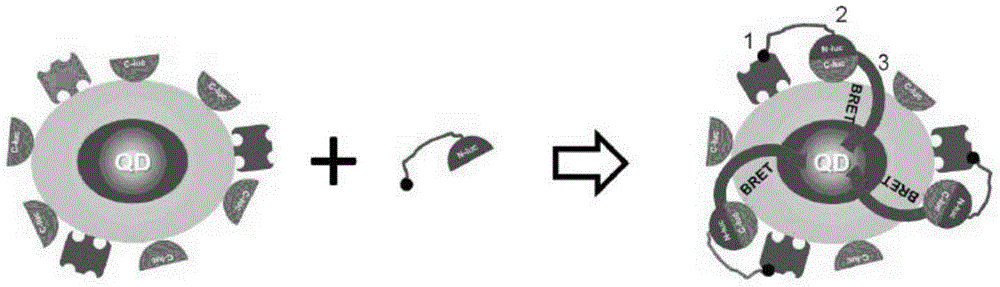
The invention discloses a luciferase complementary quantum dot biosensor as well as a construction method and application thereof, belonging to the technical field of biosensing. The luciferase complementary quantum dot biosensor comprises a quantum dot, a luciferase amino terminal fragment, a luciferase carboxyl terminal fragment, a probe of specifically recognizable determinand and a substrate which have bioluminescence reaction with luciferase. With the combination of optical advantages of the quantum dot sensor, a catalysis function can be reconstructed through induction complementation of the luciferase amino terminal fragment and the luciferase carboxyl terminal fragment on the surface of the quantum dot, a novel high-sensitivity biosensor can be constructed, and the biosensor is low in background noise, high in sensitivity, stable in structure and easy to use, can be used in high-target detection on multiple biological markers, and is applicable to accurate quantification on target testing articles in homogeneous systems.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI +1
Plasmid vector containing 3'UTR (untranslated regions) sequence of ABCB1 gene and reporter gene as well as construction method and use of plasmid vector
InactiveCN103173481AHigh sensitivityImprove featuresMicrobiological testing/measurementVector-based foreign material introductionLuciferaseMulti drug resistant
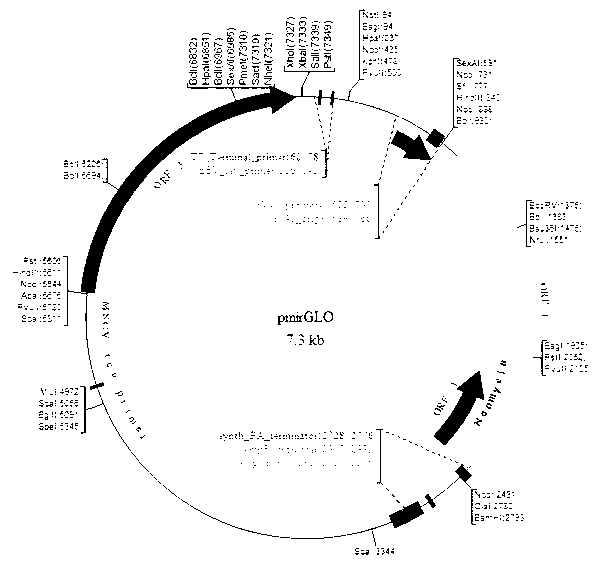
The invention relates to a plasmid vector. According to the plasmid vector, a 3'UTR (untranslated regions) sequence of an ABCB1 gene is inserted into the 6832-7349 bp segment of pmirGLODual-Luciferase-promoter plasmid vector. The invention also relates to a host cell containing the plasmid vector, a construction method of the plasmid vector and use of the plasmid vector in selection of relative microRNA (ribonucleic acid) of multi-drug resistance. The invention provides a fast, efficient, simple and practicable method for accurately forecasting microRNA which regulates human ABCB1 gene expression so that a new way for improving the sensitivity and specificity of microRNA detection, researching, developing and detecting drugs for reversing multi-drug resistance by virtue of gene regulation is opened.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Kit and method for detecting autoimmune antibody of type-I diabetes mellitus
InactiveCN103116030ASimple loading procedureImprove featuresBiological testingAutoantibody productionLuciferase
The invention provides a kit and method for detecting an autoimmune antibody of the type-I diabetes mellitus and belongs to the technical field of biochemical medicine detection. The method comprises the following steps of: adding a luciferase as an antibody fusion protein, wherein the luciferase as the antibody fusion protein is generated by 293 cell culture and can be specifically combined with a diabetes mellitus autoantibody in serum of a patient; then, adding protein-A agarose, depositing a fusion protein as an antibody compound, centrifuging, and then, absorbing the uncombined fusion protein from a supernatant liquid; and then, adding a luciferase substrate, and detecting the fluorescence intensity by using a fluorescence detection instrument to finally measure the content of the diabetes mellitus autoantibody in a sample to be detected. Compared with the traditional HPLC (High Performance Liquid Chromatography), a micro-quantitative fluorescence detection instrument used for detecting in the method has the advantages of simplicity in operation, no need of uncovering to block a pollution way, high sensitivity and signal to noise ratio, stable and reliable measured value and capability of ensuring reliable experiment result and safety of operating personnel and meeting the requirements of micro quantity and regent saving.
Owner:山东东兴生物科技股份有限公司
Recombinant plasmid for screening Nrf2 activating agent and construction method and application thereof
ActiveCN106282229AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementNucleic acid vectorHigh-Throughput Screening MethodsNrf2 activation
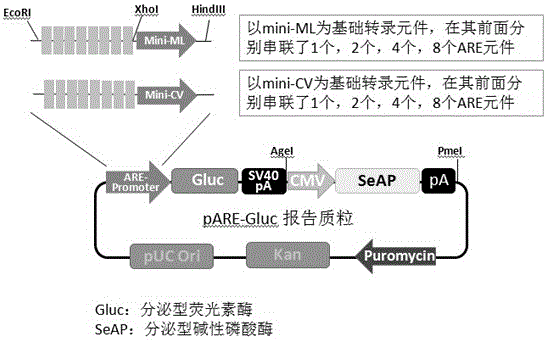
The invention relates to the field of biological medicine, in particular to recombinant plasmid for screening an Nrf2 activating agent and a construction method and application thereof. The recombinant plasmid comprises one or two or above series antioxidant reaction elements, basal transcription elements, secretion-type luciferase genes and secretion-type alkaline phosphatase genes. Compared with the prior art, as secretion-type luciferase is used as reporter genes, the detection sensitivity is high, cells do not need to be pyrolysed when the activity of the luciferase genes is detected, high-throughput screening and comparing and detection of the long-term effect of an antioxidant are achieved, and an efficient, rapid, simple and convenient method is provided for screening the antioxidant or detecting the activity of the antioxidant, and the recombinant plasmid can be applied to screening of natural medicine with the antioxidation effect and artificially-synthesized compounds.
Owner:广州市皮肤病防治所
ATP releasing agent and germ fast detection reagent containing ATP releasing agent
ActiveCN103483229ADoes not affect enzyme activityImprove enzyme thermostabilityMicrobiological testing/measurementSulfonic acids salts preparationSulfonateMicroorganism
The invention provides an ATP releasing agent and a method for preparing the ATP releasing agent which is 5-(4-ortho-pelargonic alkyl-phenyl group)-1-hexane sodium sulfonate. The invention further provides a germ fast detection reagent which comprises a germ collecting swab, luciferase, a fluorescein substrate and reaction buffer which are immersed with the ATP releasing agent. The invention relates to ATP releasing and measuring, wherein the processes of ATP releasing and measuring are finished at one step, the number of the steps of ATP releasing is reduced, the measuring method is simpler, the process is faster, and the result can be obtained within minutes. The ATP releasing agent has the advantages of being simple in preparing process, low in cost, obvious in effect and the like, and can be suitable for detecting microorganisms better.
Owner:HANGZHOU CLONGENE BIOTECH
Detecting infection in reduced pressure wound treatment
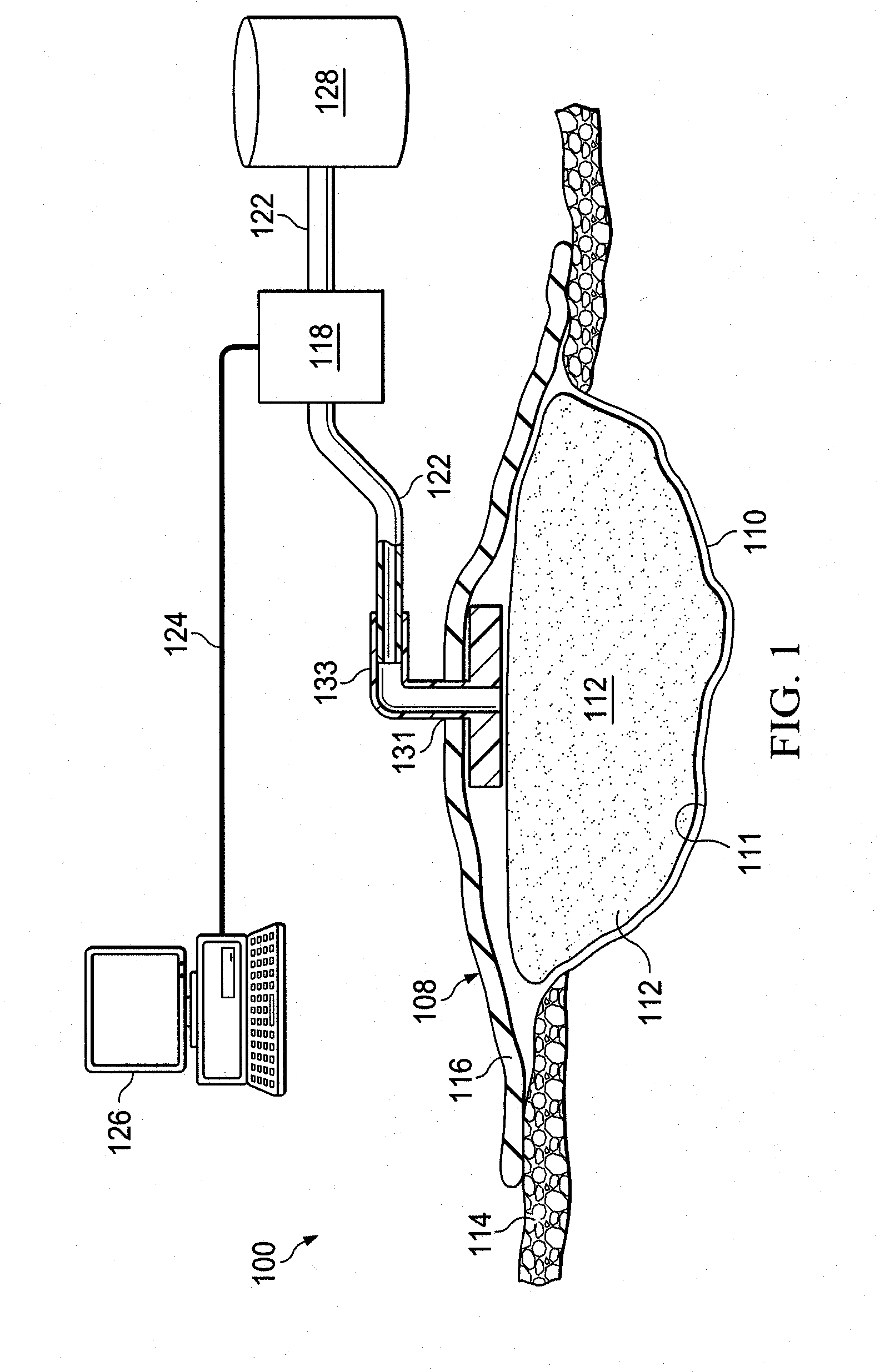
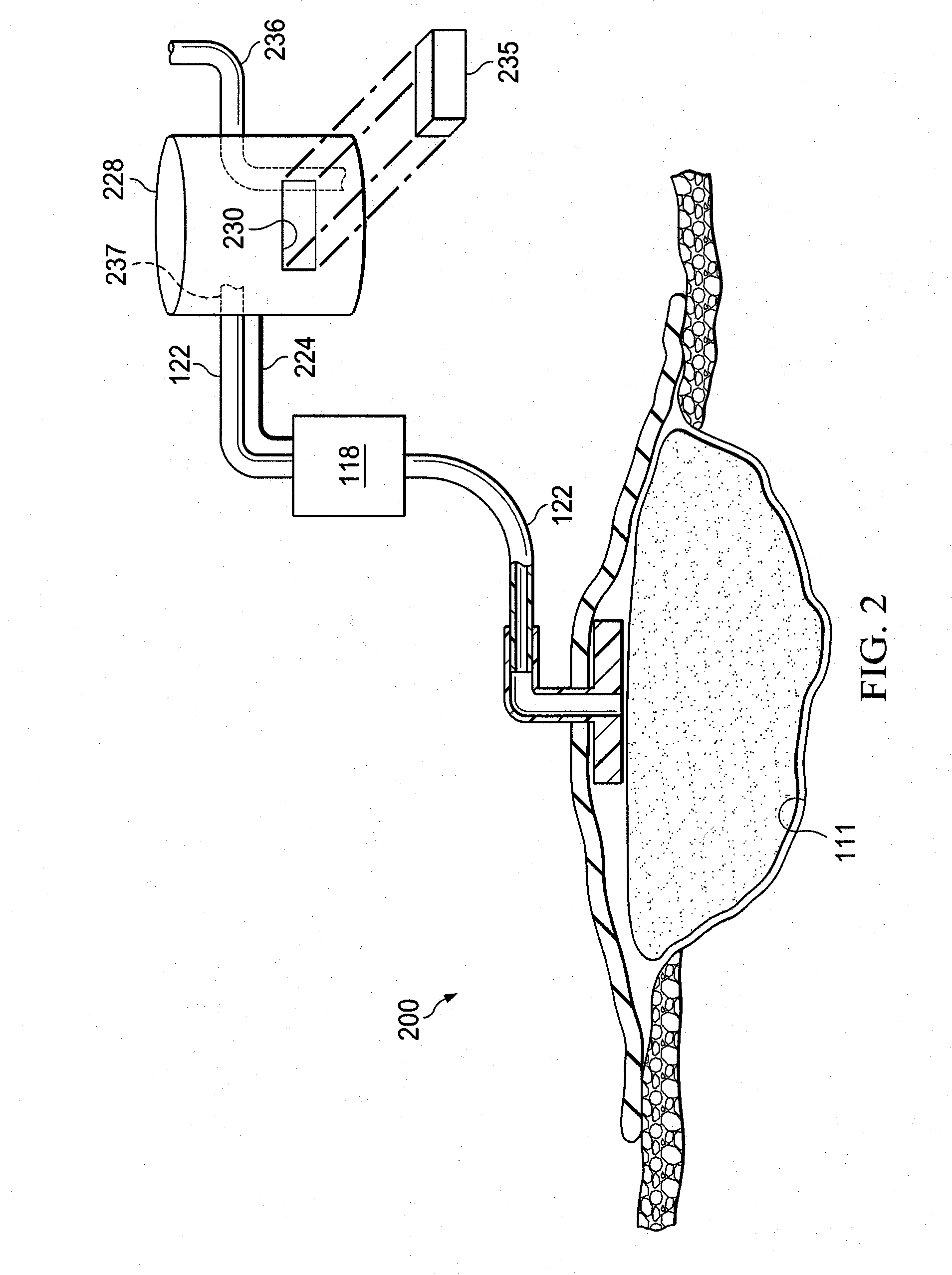
ActiveUS20090326416A1Bioreactor/fermenter combinationsBiological substance pretreatmentsWound siteLuciferase
Provided is a method of detecting infection in a wound caused by an infecting organism at a wound site. Also provided is a system for detecting an infection in a wound at a wound site. Additionally, a porous pad comprising luciferase is provided.
Owner:3M INNOVATIVE PROPERTIES CO
Screen method of sensitization tumour cell pharmaceutical product and use thereof
InactiveCN101457251AToxicAlleviate or even reverse drug resistancePeptide/protein ingredientsMicrobiological testing/measurementCoralyne sulfoacetateCancer cell
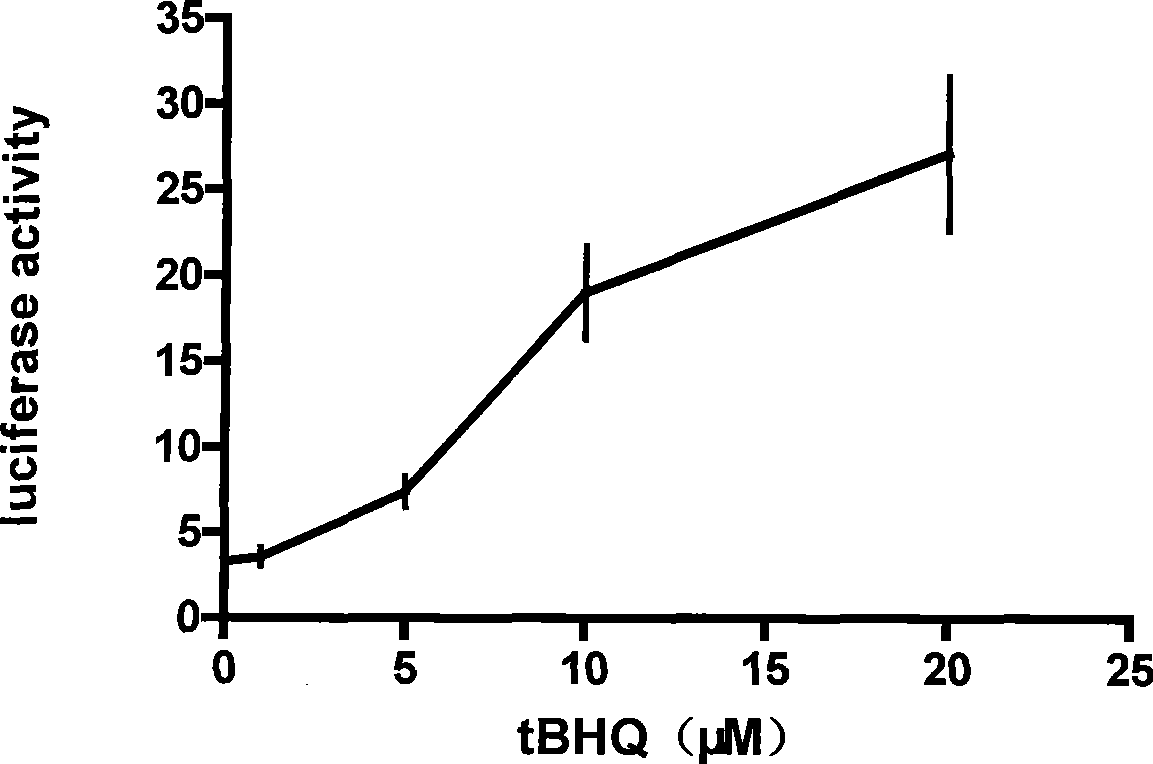
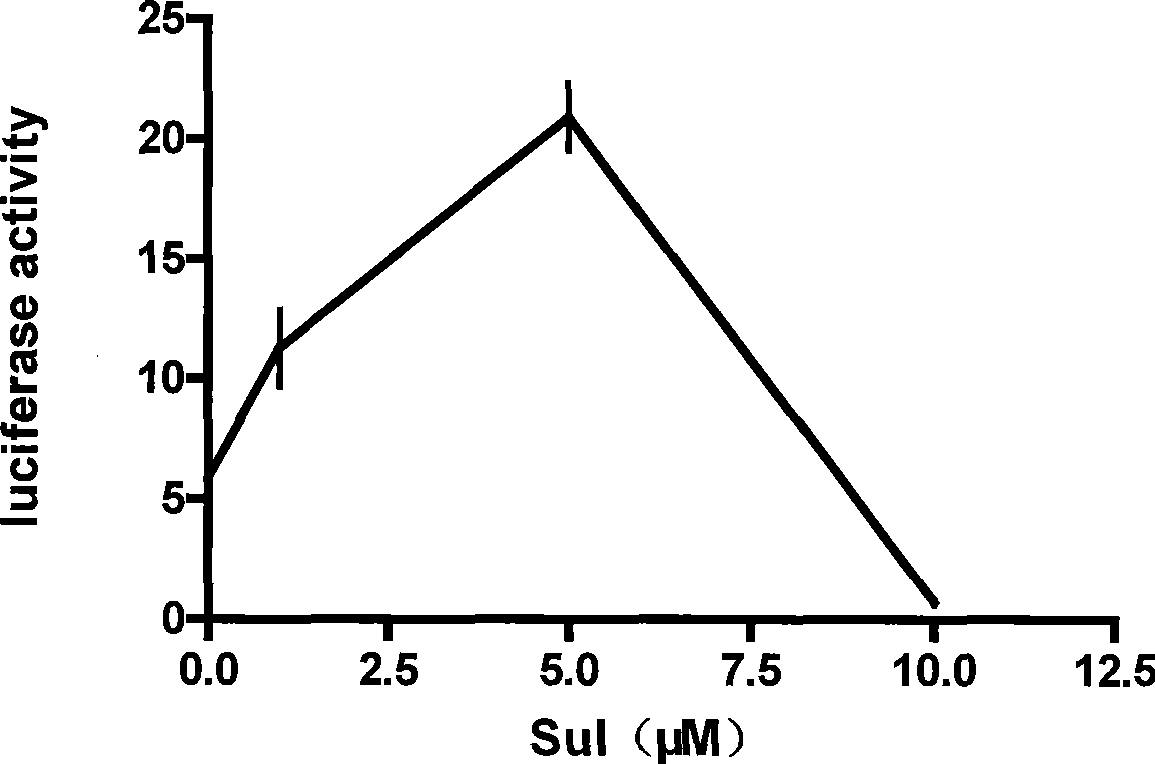
The invention discloses an enhancement tumor cell medicament screening method and its application mainly for effectively overcoming the multidrug resistance to improve the anti-cancer drug treatment effect in a tumor chemotherapy process. The invention screens an inhibitor which can effectively inhibiting the induction action through luciferase activity detection by using an Nrf2 as a cell drug screen platform of a molecular target and a tBHQ as an Nrf2 inducer, and the inhibitor is a medicament of the enhancement tumor cell. The medicament obtained by the screen is a small molecular compound ZDAK02 (coralyne sulfoacetate coralyne thioacetate). The combined use of the medicament of the invention with the anti-cancer drug Chlorambucil or anti-cancer drug oxaliplatin can enhance the toxicity of the anti-cancer drug to the cancer cells and be helpful for easing even reversing the multidrug resistance to the anti-cancer drugs.
Owner:ZHEJIANG UNIV
Method for establishing inflammation-based pancreatic cancer animal model
InactiveCN105039410AConfirmed existenceFermentationGenetic engineeringPancreas CancersPancreatic cancer cell
The invention provides a method for establishing an inflammation-based pancreatic cancer animal model, which comprises the following steps: 1. constructing a pLVX-luciferase-IRES-ZsGreen1 plasmid; 2. establishing a slow-virus-mediated luciferase overexpression system; 3. establishing a pancreatic cancer cell line for expressing luciferase; 4. establishing a nude mouse pancreatic gland orthotopic transplantation tumor model; 5. establishing an inflammation model; and 6. establishing the inflammation-based pancreatic cancer animal model in the nude mouse body by continuing breeding the nude mouse for 30 days. The pancreatic cancer animal model established by the method can catalyze the substrate to emit light by using the luciferase, and the size of the tumor can be evaluated after the light is detected by the corresponding instrument and equipment.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Luciferase complementary image detection method and carrier kit for detecting protein interaction
The invention discloses a luciferase complementary image detection method and a carrier kit for detecting protein interaction, belonging to a technology of protein research. The method is characterized in that a gateway technique is introduced into the luciferase complementary image detection method, thus a gateway expression carrier, a kit and an optimized protein interaction detection method for luciferase complementary image detection can be obtained, and the interaction of protein can be effectively and rapidly detected with high throughput.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Method for performing low-dosage radiant biology early warning by utilizing luminous bacteria
The invention relates to a method for performing low-dosage radiant biology early warning by utilizing luminous bacteria. According to the method, a dosage-effect relationship between a radiant dosage acceptable by the bacteria and a luminous intensity can be established by utilizing a sensitivity of the luminous bacteria to the low-dosage radiation, and the luminous intensity of the bacteria has positive correlation with the activity of luminous elements such as fluorescein, luciferase and ATP in the bacterial cells. Therefore, through calculation of a luminous intensity suppression ratio of the luminous bacteria, the comprehensive toxicity of the low-dosage radiation for the luminous bacteria can be estimated, and a biology early warning method is established. The method for performing low-dosage radiant biology early warning by utilizing the luminous bacteria has multiple advantages of being convenient, simple and fast to operate, low in cost, high in sensitivity, good in accuracy, low in environment risk and the like.
Owner:NANHUA UNIV
RGA (Report Gene Assay) method for detecting biological activity of exendin-4-HAS Byetalog
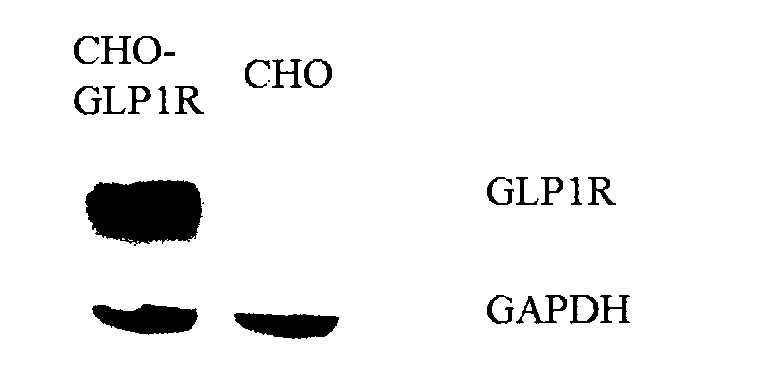
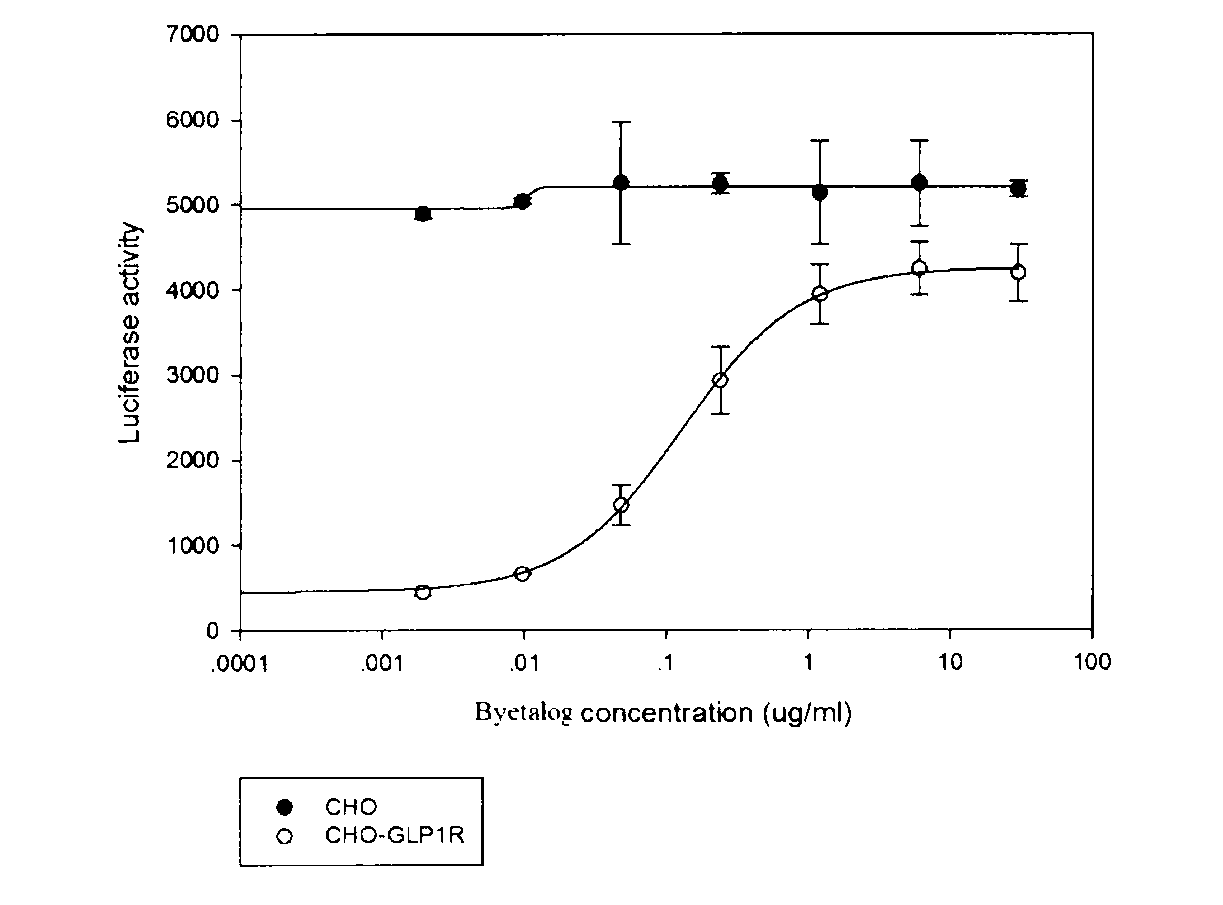
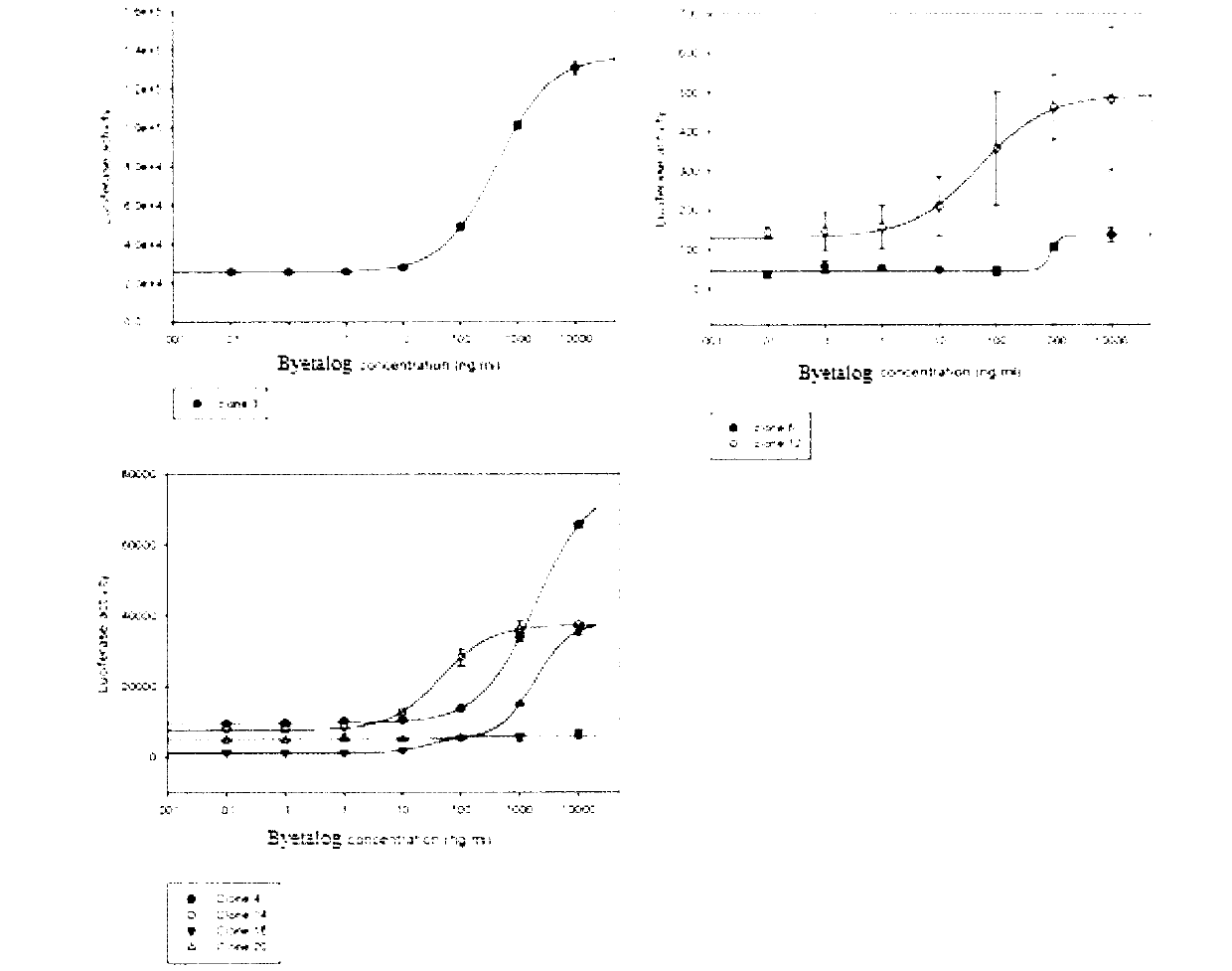
ActiveCN103293315APromote research and developmentQuality is easy to controlBiological testingForeign genetic material cellsDrug biological activityGene carrier
The invention provides a novel biological activity determination method of exendin-4-HAS Byetalog. The basic principle of the method is that a GLP-1R (Glucagon-Like Peptide-1R) and a cAMP (cyclic Adenosine Monophosphate) response element (CER) report gene carrier are used for co-transfection of a CHO-K1 cell, and the steps of pressurization, sieving and monoclonal separation and cultivation are carried out to obtain a stable monoclonal cell strain CHOglplr / crec14. After the GLP-1R is combined with a ligand, a series of signal transduction is carried out to stimulate the expression of CRE reporter gene luciferase, and the activity of the Byetalog is quantified by detecting the change of the expression of the luciferase after the Byetalog stimulates. The specific detection steps are as follows: 6000 CHOglplr / crec14 cells are placed on each pore of a 96-pore plate to be cultured for 16-18 hours; the Byetalog with different concentrations is added to stimulate the cells for 6 hours; the activity of the luciferase is detected. The method is simple to operate, sensitive in reaction and small in variability, and has very important meaning to quality control and clinical application of the Byetalog.
Owner:NAT INST FOR FOOD & DRUG CONTROL
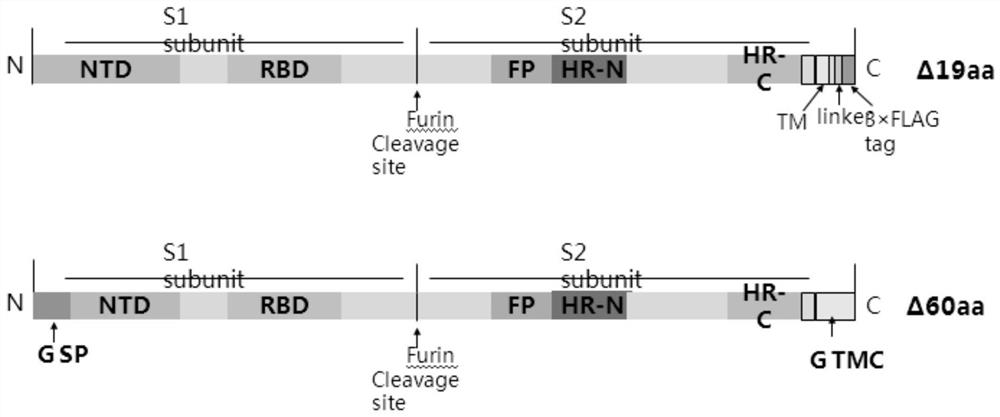
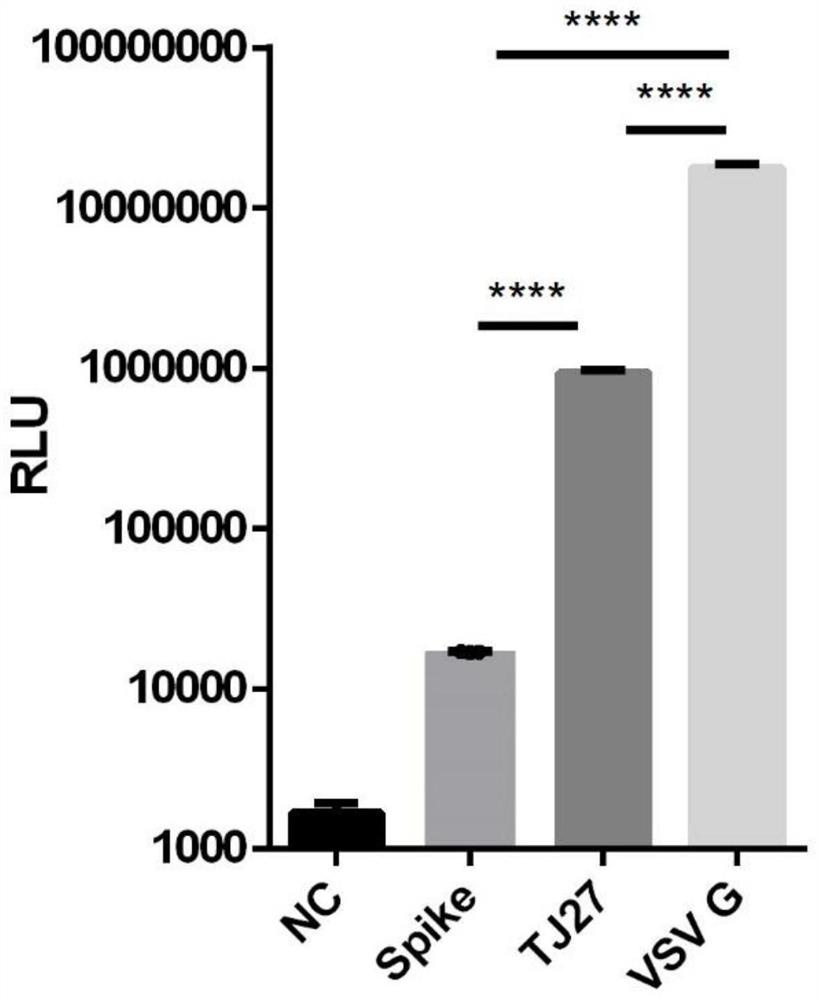
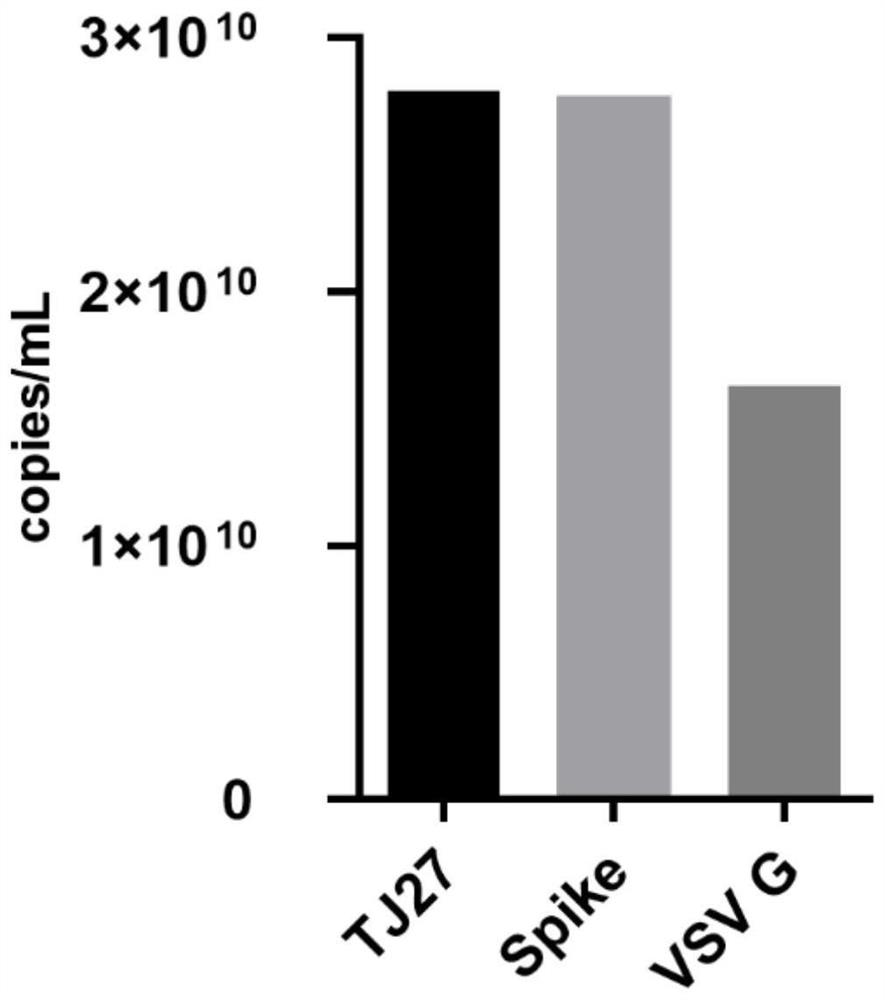
Pseudovirus of COVID-19 coronavirus as well as preparation method and application of pseudovirus
InactiveCN112375768AWill not cause diseaseEvaluate the effect of immunizationCompound screeningSsRNA viruses positive-senseLuciferaseTGE VACCINE
The invention discloses a pseudovirus of COVID-19 coronavirus as well as a preparation method and application of the pseudovirus, and belongs to the technical field of biological medicines. Accordingto the pseudovirus, COVID-19 membrane protein participates in packaging of a defective virus genome with a reporter gene, one or more sections of exogenous sequences for encoding bioactive substancescan be included under the condition that the reporter gene sequence is carried or not carried, the reporter gene is selected from enhanced green fluorescent protein (EGFP) or luciferase. the defectivevirus genome or a portion thereof are derived from VSV, HIV, SARS and other viruses, and one or more structural genes contained in the defective virus genome can be removed or regulated to be inactivated. The pseudovirus can reduce the risk of virus research to the maximum extent, and can also be used for screening antiviral drugs, determining the titer of a neutralizing antibody in the body of an infected person, searching for an epitope combined with the neutralizing antibody on a COVID-19 coronavirus surface antigen and evaluating the immune effect of vaccines.
Owner:TONGJI UNIV
Preparation method for synthesizing microbial self-luminous biosensor by using self-luminous operon, corresponding biosensor and application thereof
ActiveCN113005070AEnables real-time fluorescence detectionEasy to operateBacteriaMicrobiological testing/measurementMicroorganismFluorescence
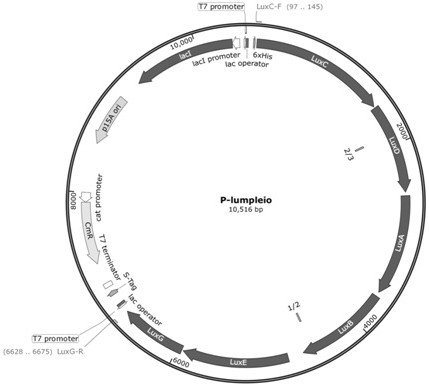
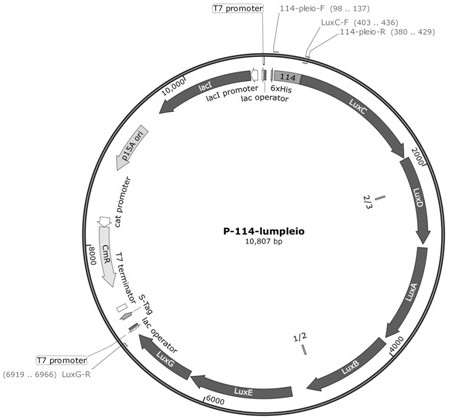
The invention discloses a preparation method for synthesizing a microbial self-luminous biosensor by using a self-luminous operon, and a corresponding biosensor and application thereof. The microbial self-luminous biosensor comprises a reporting element containing a self-luminous operon luxABCDE operon and a sensing element containing a promoter 114. The microbial self-luminous biosensor can sense explosive molecules with different concentrations to generate self-luminescence with different RLU values, and the concentration of the explosive molecules and the self-luminous RLU values are coupled, so that real-time fluorescence detection of the explosive molecules is realized, and the defect that luciferase is required to generate fluorescence for detection can be overcome. Compared with other detection methods, the microbial self-luminous biosensor has the advantages that the operation of detecting the explosive molecules is simpler and more convenient, the signal collection is easier, and the explosive molecules with a wider concentration range can be detected, so that the microbial self-luminous biosensor has a very good application prospect.
Owner:QINGDAO AGRI UNIV +1
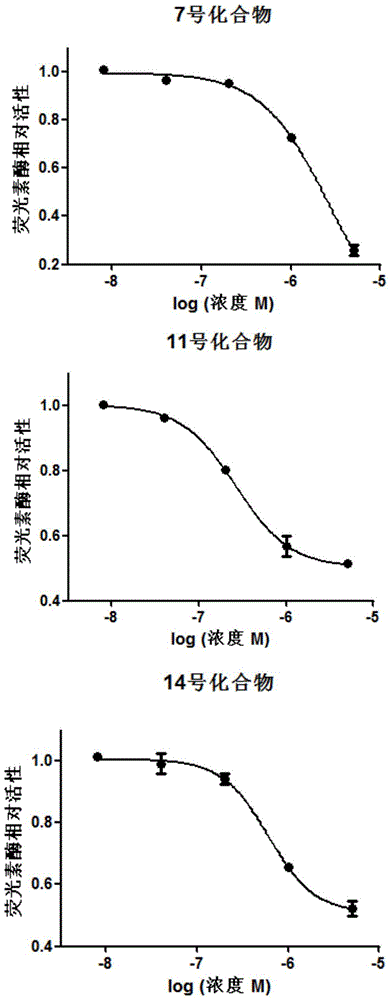
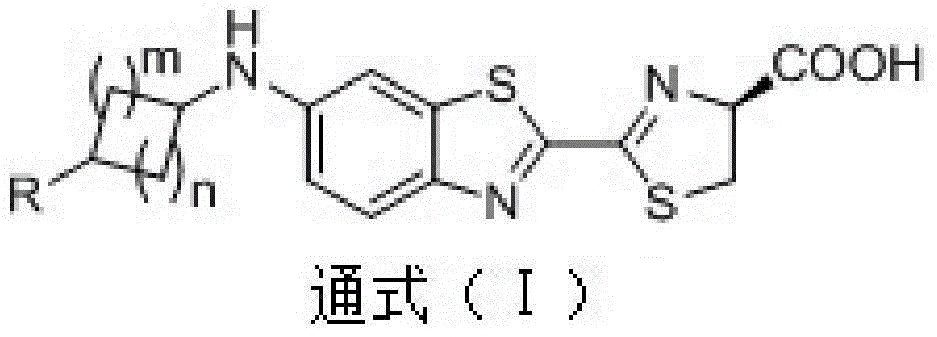
Application of 4N heterocyclic compound as inhibitor for Th17 cell differentiation
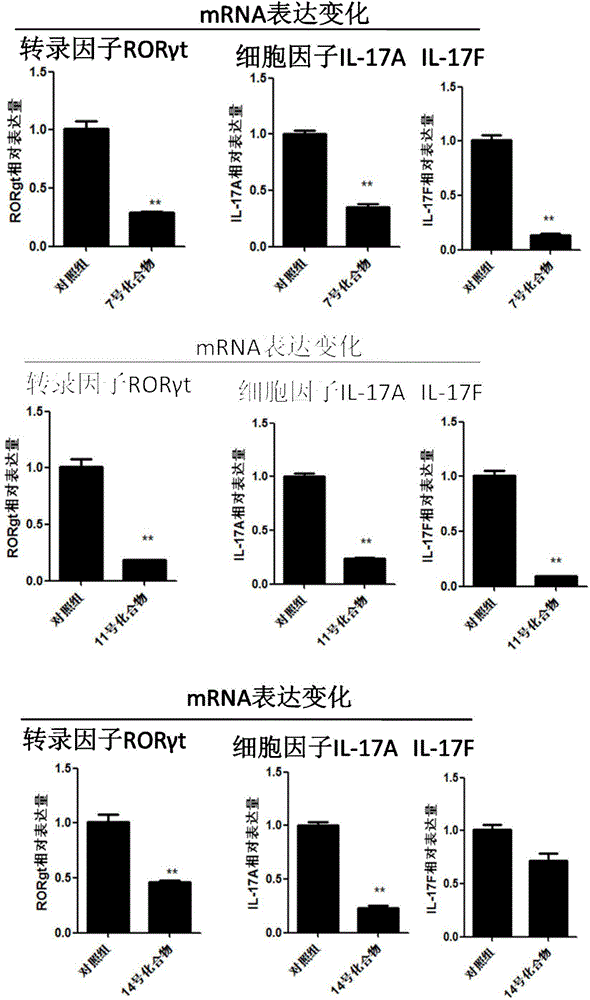
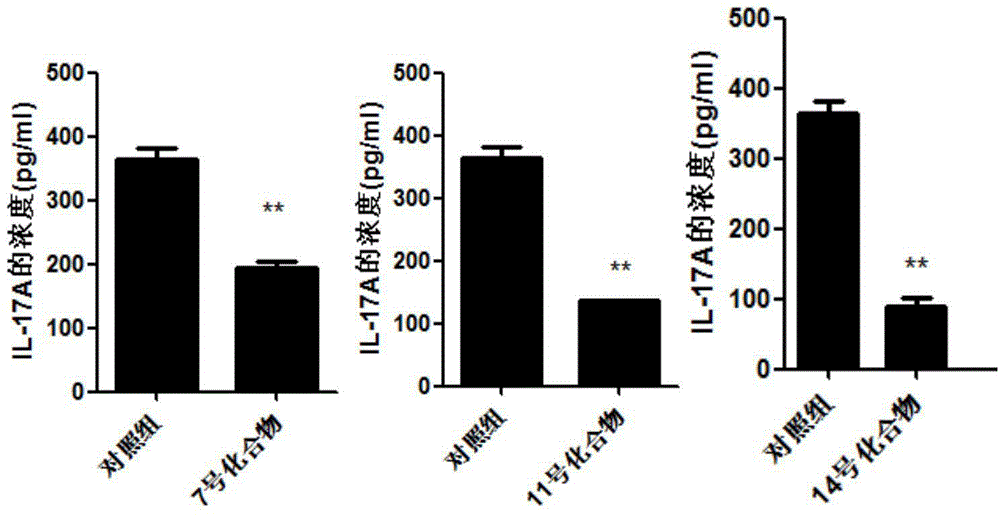
ActiveCN104814962AOrganic active ingredientsNervous disorderAutoimmune conditionTranscription factor activity
The invention discloses application of a 4N heterocyclic compound as an inhibitor for Th17 cell differentiation. In virtue of a luciferase activity screening system based on the activity of transcription factors, it is found that the 4N heterocyclic compound with a general formula as described in the specification has capability in inhibiting the key transcription factor ROR gamma t of Th17 cell differentiation and the inhibition capability EC50 is in a range of 0.2 to 1.5 [mu]M. In-vitro Th17 cell differentiation experiments prove that the 4N heterocyclic compound is capable of inhibiting Th17 differentiation, including substantially reducing the transcription expression of ROR gamma t, inhibiting the transcription expression of the effector molecules IL17A and IL17F of Th17 and obviously inhibiting the secretion level of IL17A cytokines in a culture. The 4N heterocyclic compound is expected to be developed into a drug used for treating autoimmune diseases, especially psoriasis, systematic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, asthma or inflammatory intestinal diseases.
Owner:CELLREGEN BEIJING LIFE SCI & TECH CO LTD
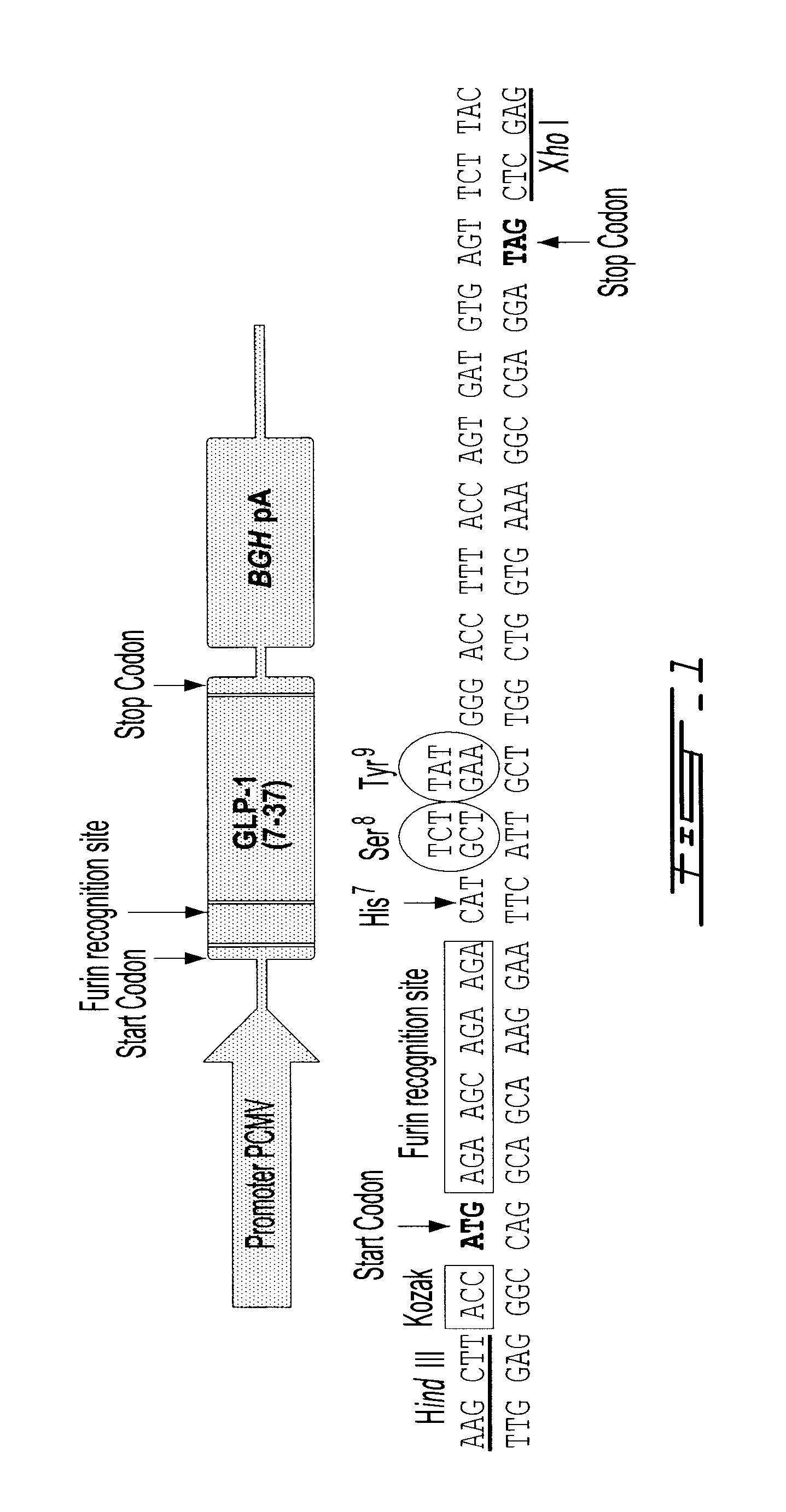
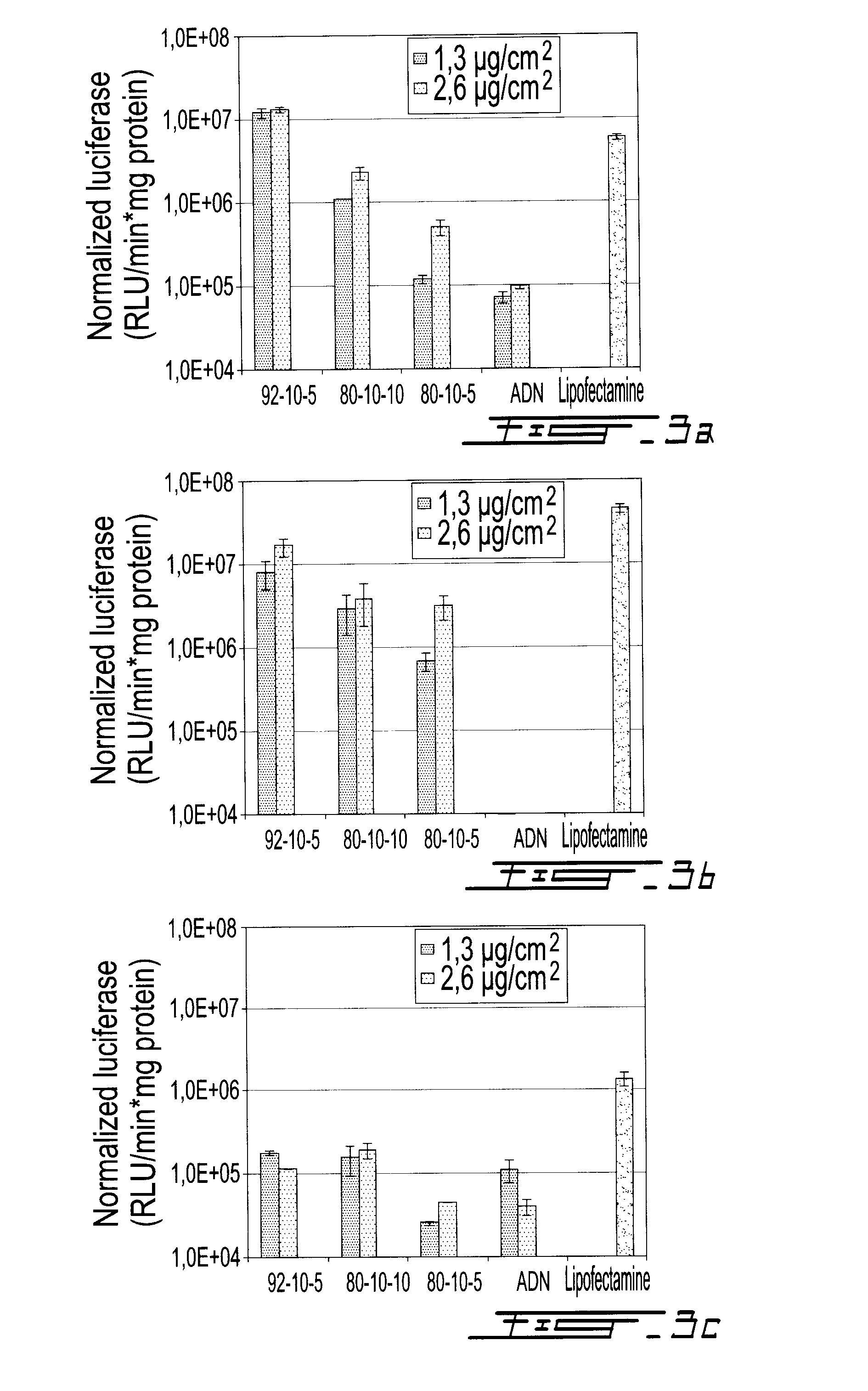
Gene therapy for diabetes with chitosan-delivered plasmid encoding glucagon-like peptide 1
InactiveUS20130210717A1Lower blood sugar levelsLimit metabolismOrganic active ingredientsPeptide/protein ingredientsPhosphateGlucagon-like peptide-1
Chitosan delivers a plasmid encoding Glucagon-Like Peptide 1 (GLP-1) to cells in a patient for gene therapy of diabetes. Chitosan is optimized for plasmid transfection by modulating three of its physico-chemical properties: degree of deacetylation (DDA), molecular weight (MW), and ratio of amines on chitosan to phosphates on DNA (N:P ratio), Chitosan 92-10-5 (DDA-MW-N:P) is more efficient than chitosans 80-10-10 and 80-80-5 in delivering a plasmid encoding luciferase or GLP-1(7-37) to cells. In the Zucker Diabetic Fatty (ZDF) rat model of diabetes, chitosan-delivered pVax plasmid encoding GLP-1 lowers glucose levels, increases insulin production and reduces weight gain.
Owner:POLYVALOR LP
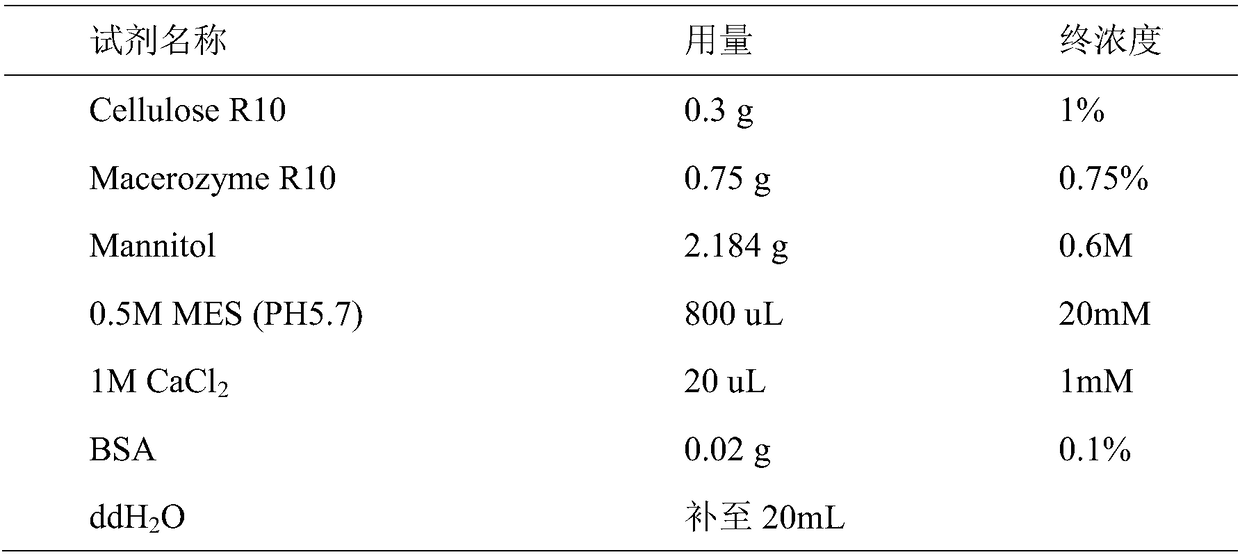
Preparation method of sorghum protoplast, and conversion method of prepared protoplast
InactiveCN108546670ASimple methodEfficient methodGenetically modified cellsPlant cellsGerminationGene expression
The invention discloses a preparation method of a sorghum protoplast, and a conversion method of the prepared protoplast. The preparation method comprises the following steps: (1) taking sorghum seedsinto water, accelerating germination, and sowing and cultivating obtained buds to form etiolated seedlings; (2) shearing the well-grown sorghum etiolated seedling, pretreating the sheared etiolated seedlings with mannitol, taking middle leaf sheaths, and cutting the sheaths by a blade to form thin strips; (3) performing enzymatic digestion on the cut leaf sheaths by using a protoplast enzymatic hydrolysate to cleave sorghum protoplasts; and (4) performing solid-liquid separation on the cleaved sorghum protoplasts to obtain the sorghum protoplast. A preparation and conversion system for the sorghum protoplast is established for the first time, the methods are simple and effective, and the sorghum protoplast can be used for studying sorghum protein subcellular localization, sorghum proteininteraction and protein purification and luciferase-labeled gene expression.
Owner:GUIZHOU UNIV
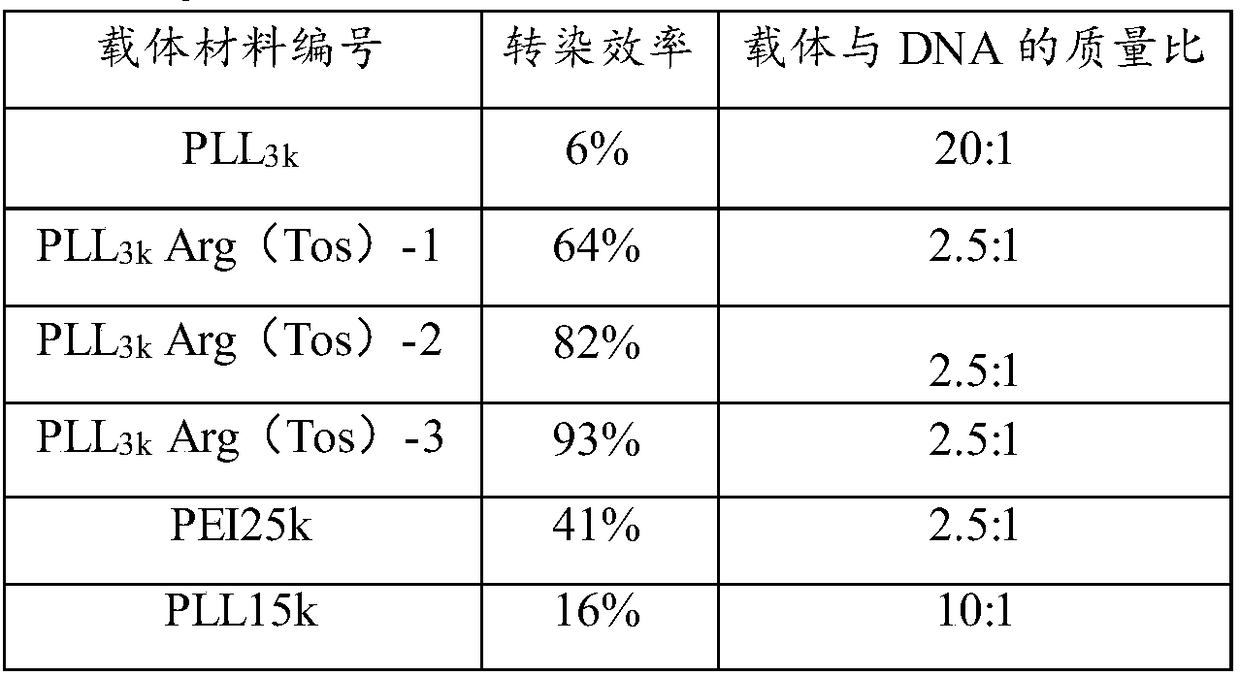
Cationic gene vector with high transfection efficiency and low cytotoxicity and preparation method thereof
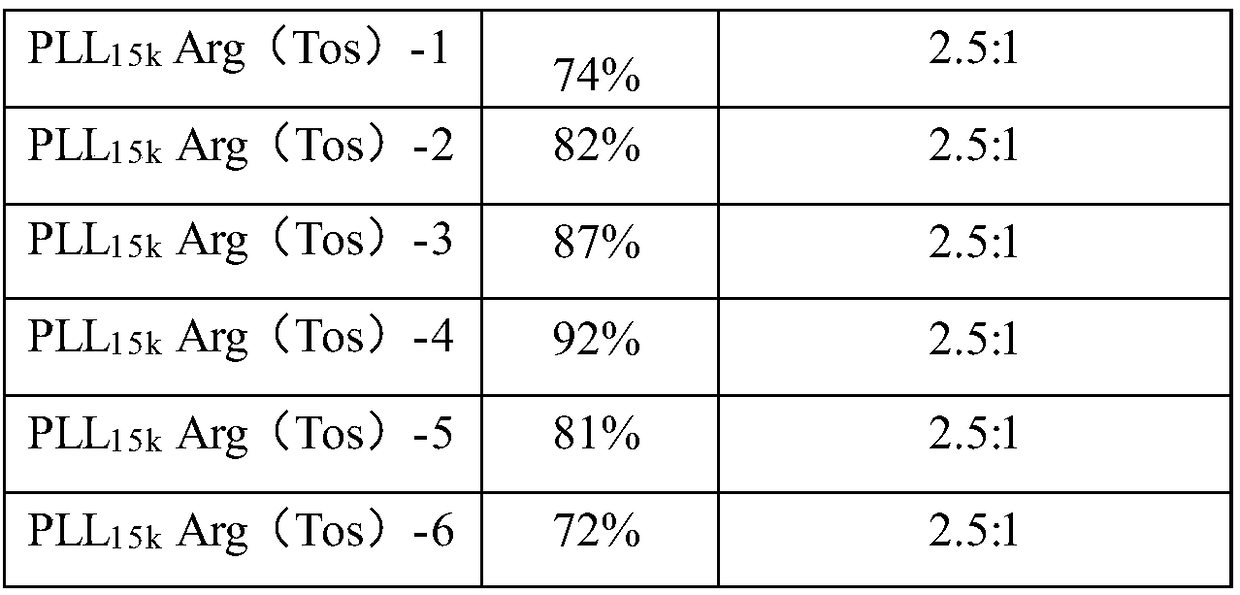
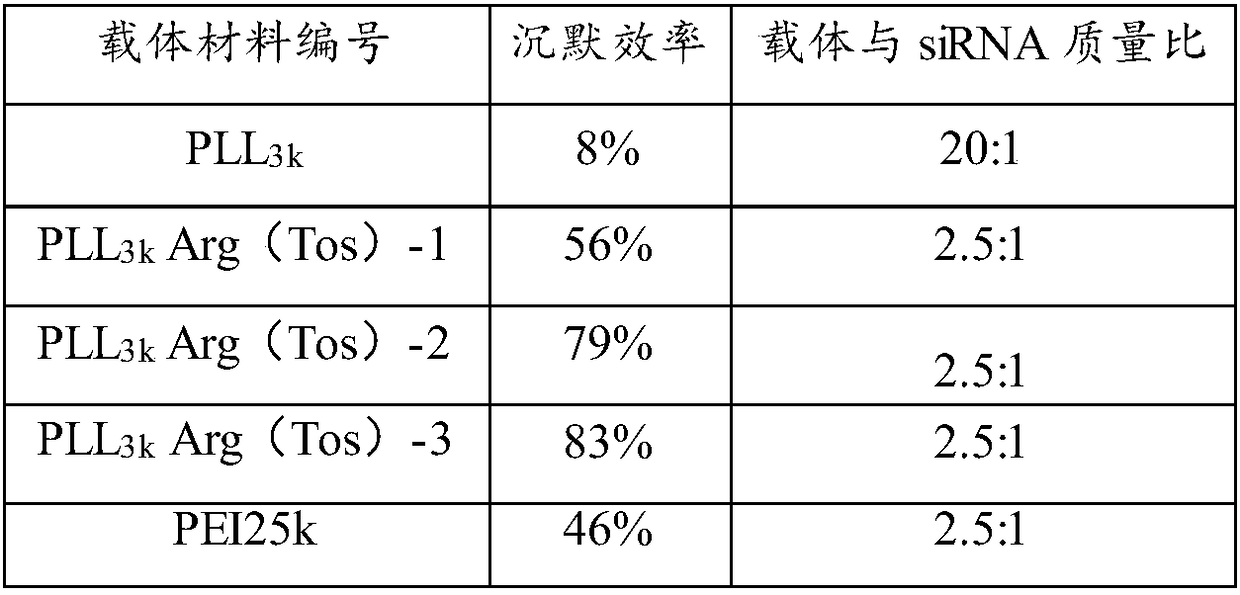
ActiveCN108384810AHigh transfection efficiencyImprove gene silencing efficiencyNucleic acid vectorOther foreign material introduction processesArginineCytotoxicity
The invention provides a cationic gene vector with high transfection efficiency and low cytotoxicity. The cationic gene vector comprises linear poly-alpha-lysine (PLL) and tosyl protected arginine (Arg(Tos)) grafted on linear poly-alpha-lysine, wherein the molar ratio of linear poly-alpha-lysine to tosyl protected arginine is 1 to (5 to 120). The optimal transfection efficiency of the cation genevector PLL-Arg(Tos) is more than ten times of the optimal transfection efficiency of a cationic gene vector gold standard PEI25k; when the cationic gene vector PLL-Arg(Tos) has the optimal transfection efficiency, the survival rate in cells is 90 percent or above. Furthermore, the optimal gene silencing efficiency of the PLL-Arg(Tos) on HuH-7Luc capable of constantly expressing luciferase can reach 80 percent or above. The invention further provides a preparation method of the cation vector with high transfection efficiency and low cytotoxicity.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Non-radioactive method for determining the cytolitic activity of an agent with respect to target cells, use thereof and associated kit
InactiveUS20180051317A1Microbiological testing/measurementBiological testingFluorescenceComplement-dependent cytotoxicity
A non-radioactive method for the direct in vitro determination (control and quantification) of the cytolytic activity of an active agent with respect to target cells and / or a medium surrounding target cells, comprising the steps of genetically transforming target cells to express an enzyme exogenous to said target cells, exposing said genetically transformed target cells to the active agent and / or to said surrounding medium to be tested, which can result in the lysis of at least a portion of the target cells by releasing said exogenous enzyme into the extracellular medium, and measuring the activity of the exogenous enzyme released during the lysis of said target cells, characterised in that said exogenous enzyme is an enzyme having a molar mass less than or equal to 45 kDa (e.g. an Oplophorus gracilirostris luciferase), and of which the activity can be detected by luminescence or fluorescence. Application to the measurement of antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) and / or the measurement of apoptosis.
Owner:CLEAN CELLS
Real time monitoring of microbial enzymatic pathways
InactiveUS20120264107A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismBiological body
Owner:COBALT TECH INC
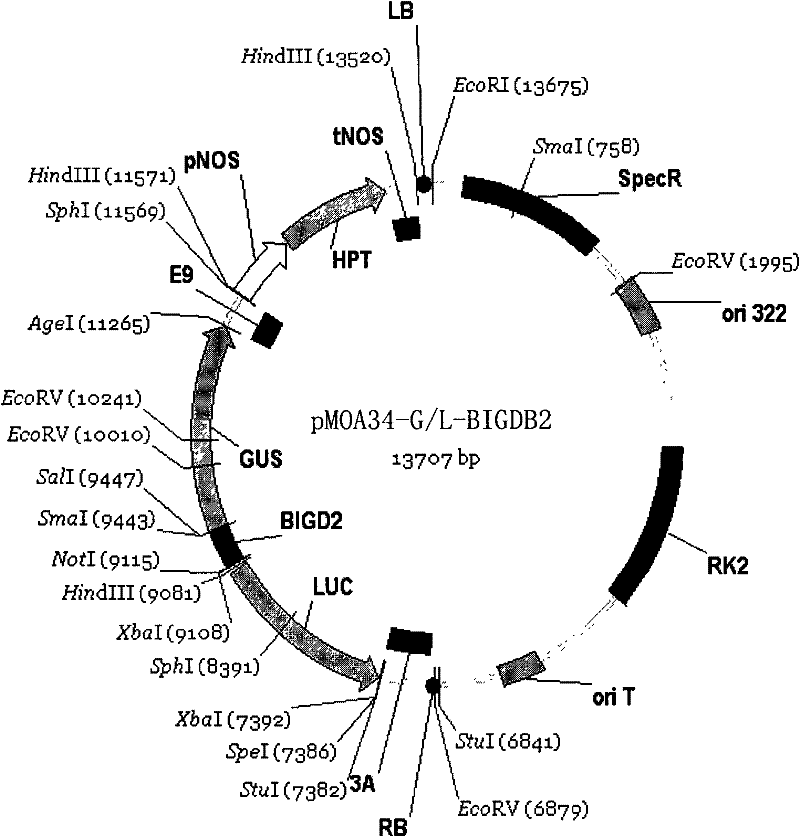
Plant bidirectional promoter BIGDB2
The invention discloses a plant bidirectional promoter BIGDB2. The nucleotide sequence of the promoter is shown as a sequence 1 in a sequence table. Experiments prove that: the BIGDB2 can promote glucuronidase (GUS) expression after being inserted into the space between GUS and luciferase (LUC) in a vector pMOA34-G / L, and the BIGDB2 can also promote the GUS expression after being inserted into the space between the GUS and the LUC in the pMOA34-G / L reversely, so that the BIGDB2 has a bidirectional promoting function in arabidopsis thaliana.
Owner:INST OF GENETICS & DEVELOPMENTAL BIOLOGY CHINESE ACAD OF SCI
Naphthenic-base mono substituted amino fluorescein compound as well as preparation method and application thereof
ActiveCN104311504AQuick responseIncreased sensitivityOrganic chemistryFluorescence/phosphorescencePharmacologic actionEthyl group
The invention discloses a naphthenic-base mono substituted amino fluorescein compound as well as a preparation method and application thereof. The naphthenic-base mono substituted amino fluorescein compound has a structural general formula (described in the specification) or a free form thereof, wherein n is 0-9, and m is 1-9; when n is equal to m, R is mono substituted groups such as a hydrogen group, methyl, ethyl, propyl and halogen; and when n is not equal to m, R is the hydrogen group. The naphthenic-base mono substituted amino fluorescein compound can be used for detecting distribution and imaging of luciferase in vitro, cells and vivo by detecting the presence and quantity (including enzyme level, cellular level and animal level) of the luciferase by virtue of bioluminescence, can be also used for detecting distribution and imaging of ATP in vitro, cells and vivo by detecting the presence and quantity (including enzyme level, cellular level and animal level) of ATP and can be used for detecting pharmacological action of a medicine in enzyme level, cellular level and animal level as a report signal in the presence of luciferase, ATP and Mg<2+>.
Owner:SHANDONG UNIV
Method and reagent for gene sequence analysis
InactiveUS20120270210A1Easy to analyzeEliminate the effects ofSugar derivativesMicrobiological testing/measurementNucleotidePurine
Provided is a nucleic acid substrate which has nucleic acid substrate characteristics equivalent to those of dATP, has a low substrate specificity for luciferase, exerts no negative effect on enzymatic reactions such as a complementary-strand synthesis, and therefore is particularly suitable for the pyrosequencing method. As a nucleic acid substrate complementary to nucleotide T, a 7-substituted deoxyribonucleotide triphosphate whose 7-position of a purine group is modified by a substituent is used as a substitute for a nucleotide α-thiotriphosphate analog.
Owner:HITACHI LTD +1
Hyperbranched polyethyleneimine-grafted poly L-glycine copolymer and preparation method thereof
InactiveCN108250431AHigh transfection efficiencyImprove gene silencing efficiencyOther foreign material introduction processesVector-based foreign material introductionCytotoxicityCarboxylic acid
The invention provides hyperbranched polyethyleneimine-grafted poly L-glycine copolymer with a structure shown in formula (I). Hyperbranched polyethyleneimine is taken as a macroinitiator and is dissolved in an organic solvent, and L-glycine N-carboxylic acid anhydride ring opening polymerization is initiated in a waterless and oxygen-free condition, so that the hyperbranched polyethyleneimine-grafted poly L-glycine copolymer is obtained. The polymer belongs to a polycation gene carrier, and is high in transfection efficiency, the maximum transfection efficiency of polymer to MCF7 and CHO cell-mediated luciferase plasmid is 10 times and 8 times that of the commercial PE125K, and the cell survival rate is 80% or higher within a transfection proportional range; when the polymer is applied tosiRNA transfection, the maximum gene silencing efficiency of the polymer to Huh7 and CT26 cells for constantly expressing luciferase can reach 80% or higher, and the polymer has high gene silencing efficiency and small cytotoxicity for a commercial transfection reagent PE125K.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Molecular probe for detecting malonyl coenzyme A
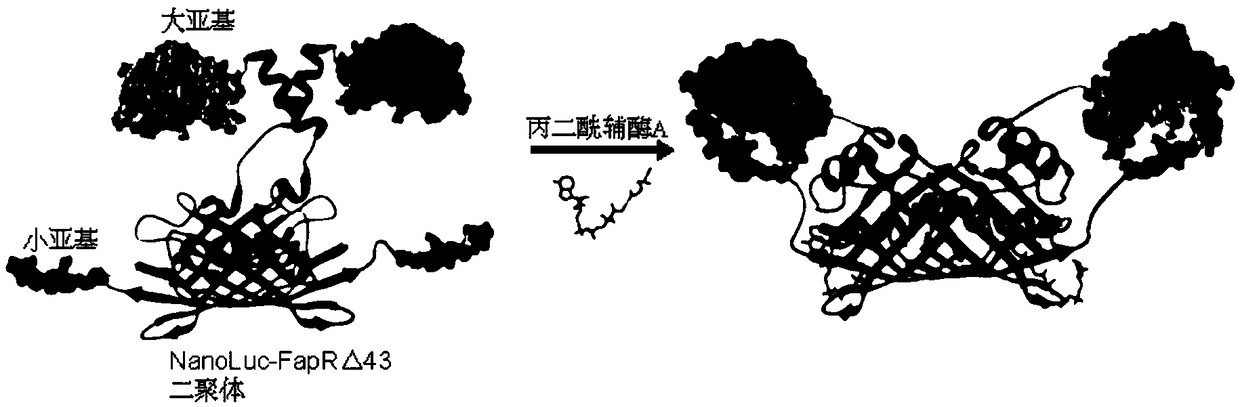
ActiveCN108752484AAntibody mimetics/scaffoldsMicrobiological testing/measurementMolecular probeMalonyl Coenzyme A
The invention discloses a molecular probe for detecting malonyl coenzyme A. The moklecular probe contains fusion protein of NanoLuc luciferase large subunit, FapRdelta43 protein and NanoLuc luciferasesubunit from the N end to the C end. The molecular probe can both detect the malonyl coenzyme A in the extract of sample in vitro and detect and perform detection and dynamic observation on the malonyl coenzyme A in living cells; most importantly, the malonyl coenzyme A can be detected in different organelles of the living cells, simpleness in operation is achieved, and therefore, the fusion protein has the potential to become a commercial product.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Reporter gene assay method
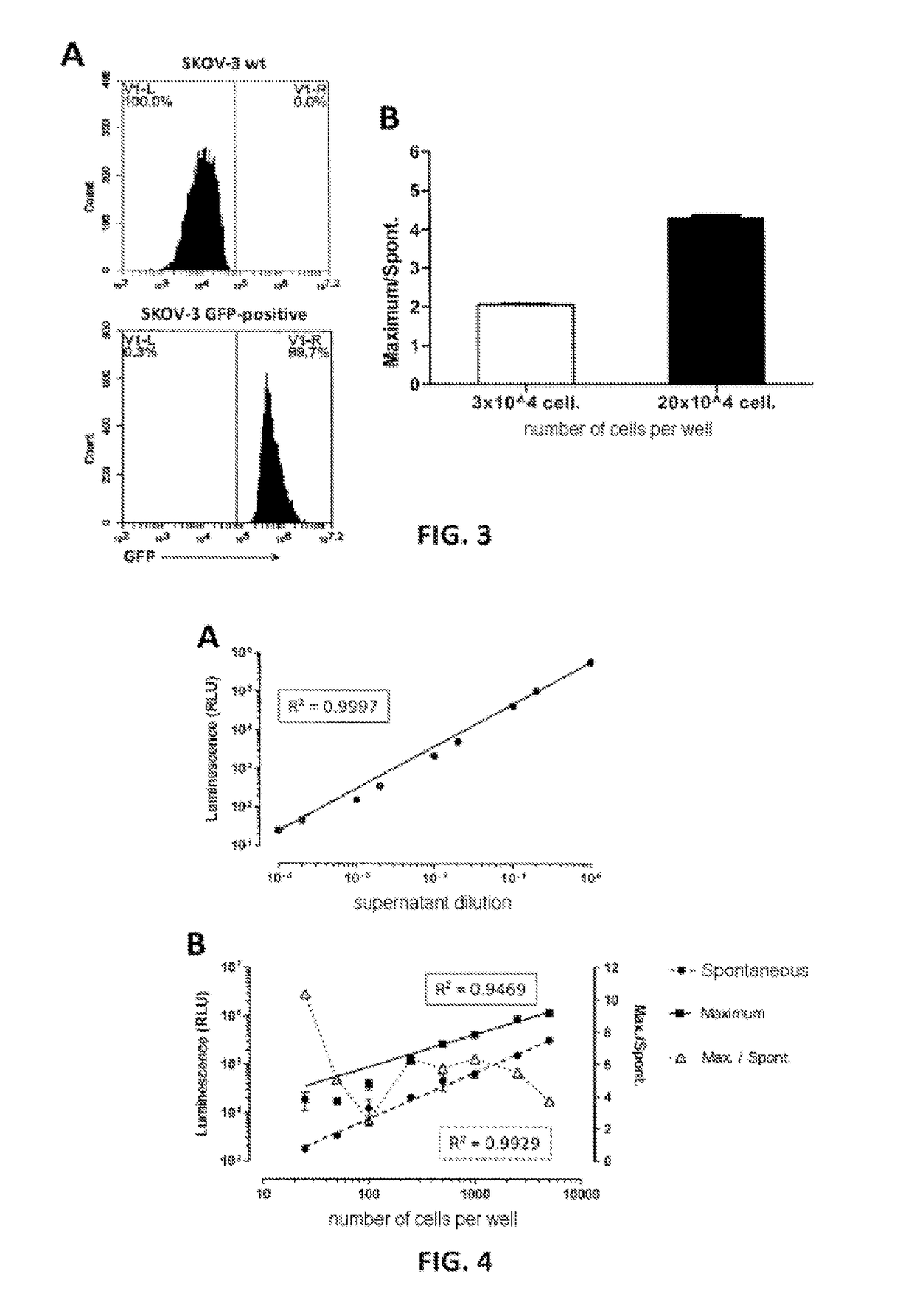
InactiveUS8609342B2Accurate identificationGood correlationAnimal cellsMaterial analysis by observing effect on chemical indicatorCell activityHormones regulation
The invention provides a reporter gene assay method by which the sex hormone-like activity inherent in a test substance can be accurately assayed excluding the effects caused by a decrease in cell activity (protein expressing capacity). The assay method is a reporter gene assay method using luciferase-expressing cells which comprises further transferring a GFP gene into the luciferase-expressing cells, measuring the luciferase expression level and GFP expression level, and making a judgment about the thus-measured luciferase expression level using the decrease in GFP expression level as an indication of the decrease in cell activity.
Owner:OTSUKA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com