Molecular probe for detecting malonyl coenzyme A
A technology of malonyl coenzyme and DNA molecules, which is applied in recombinant DNA technology, microbial measurement/inspection, DNA/RNA fragments, etc. It can solve the problems of reporter gene transcription efficiency influence, inability to detect cells, inability to distinguish, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1. Preparation and Identification of Molecular Probes for Detection of Malonyl-CoA
[0105] 1. Preparation of molecular probes for detection of malonyl-CoA
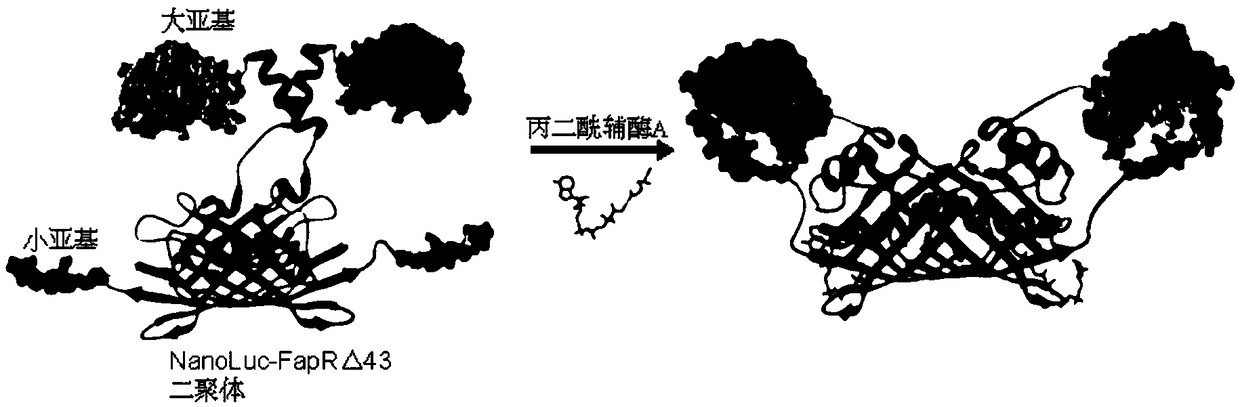
[0106] In the present invention, the molecular probe used to detect malonyl-CoA is specifically structured as figure 1 Fusion proteins indicated. From N-terminal to C-terminal, the fusion protein is NanoLuc luciferase large subunit (Lg), linker peptide I (Linker1), FapR△43 protein (FapR△43), linker peptide II (Linker2) and NanoLuc luciferase Small subunit (Sm). The amino acid sequence of the fusion protein is shown in SEQ ID No.1, wherein the 1-159th position is the NanoLuc luciferase large subunit (Lg), the 160-174th position is the connecting peptide I (Linker1), and the 175-318th position The position is FapR△43 protein (FapR△43), the 319-331 position is linker peptide II (Linker2), and the 332-342 position is NanoLuc luciferase small subunit (Sm). The fusion protein was named NanoLuc-FapRΔ43.
[0...
Embodiment 2
[0117] Example 2, detection of malonyl-CoA with the molecular probe prepared in Example 1
[0118] 1. In vitro experiment
[0119] 1. Demonstrate the reactivity of molecular probes to Malonyl-CoA
[0120] The reaction was carried out in a 96-well microplate, and the total reaction volume was 100 microliters. The reaction was divided into 8 groups, and each group replicated 5 wells. First, the purified fusion protein NanoLuc-FapRΔ43 prepared in Example 1 was added to each well so that the final concentration was 0.1 μg / ml. After that, different concentrations of Malonyl-CoA were added, and the final concentrations of each group were 0, 0.1, 1, 10, 50, 250, 1250 and 6250 μm / L. After incubating at room temperature for 10 minutes, NanoLuc's substrate Furimazine was added to a final concentration of 1 μm / L. After 10 minutes at room temperature, the fluorescence value of each well was measured with a multifunctional microplate reader.
[0121] The results showed that it was obs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com