Synthesis of fluorescence enhanced fluorescent molecular probe for detecting mercapto-containing amino acids, and application of probe
A technology of fluorescent molecular probes and mercapto amino acids, which is applied in the field of chemical analysis and detection, and achieves the effects of high sensitivity, fast response speed and stable optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: 2, the preparation of 4-dinitrobenzenesulfonic acid-7-hydroxyquinoline ester
[0024]Take 7-hydroxyquinoline (145mg, 1mmol) and 2,4-dinitrobenzenesulfonyl chloride (267mg, 1mmol) and dissolve in anhydrous dichloromethane, then add triethylamine (150mg, 1.5mmol) dropwise, room temperature Under stirring for 12 hours, after the reaction ended, dichloromethane was distilled off, and dichloromethane was used as eluent to obtain 2,4-dinitrobenzenesulfonic acid 7-hydroxyquinoline ester through column chromatography, 273mg, the yield was 73%.
Embodiment 2
[0025] Embodiment 2: Preparation of molecular fluorescent probe
[0026] 7-hydroxyquinoline 2,4-dinitrobenzenesulfonate (75mg, 0.2mmol) and dimethyl sulfate (252mg, 2mmol) were dissolved in 15mL of anhydrous dichloromethane, stirred at room temperature for 5 days, a large amount of White precipitate. A white solid was obtained by suction filtration, and recrystallized from a mixed solvent of dichloromethane:methanol=1:1 (volume ratio) to obtain 30 mg of white solid fluorescent probe with a yield of 30%. 1HNMR (301MHz, DMSO): δ ppm =9.57(d, J=5.8Hz, 1H), 9.33(d, J=8.3Hz, 1H), 9.17(d, J=2.3Hz, 1H), 8.65(dd, J=8.7, 2.3Hz, 1H) , 8.60(d, J=9.1Hz, 1H), 8.45(d, J=2.1Hz, 1H), 8.40(d, J=8.7Hz, 1H), 8.24(dd, J=8.4, 5.8Hz, 1H) , 7.90(dd, J=9.0, 2.2Hz, 1H), 4.55(s, 3H), 3.36(s, 3H), 2.50(dt, J=3.6, 1.8Hz, 3H).
Embodiment 3
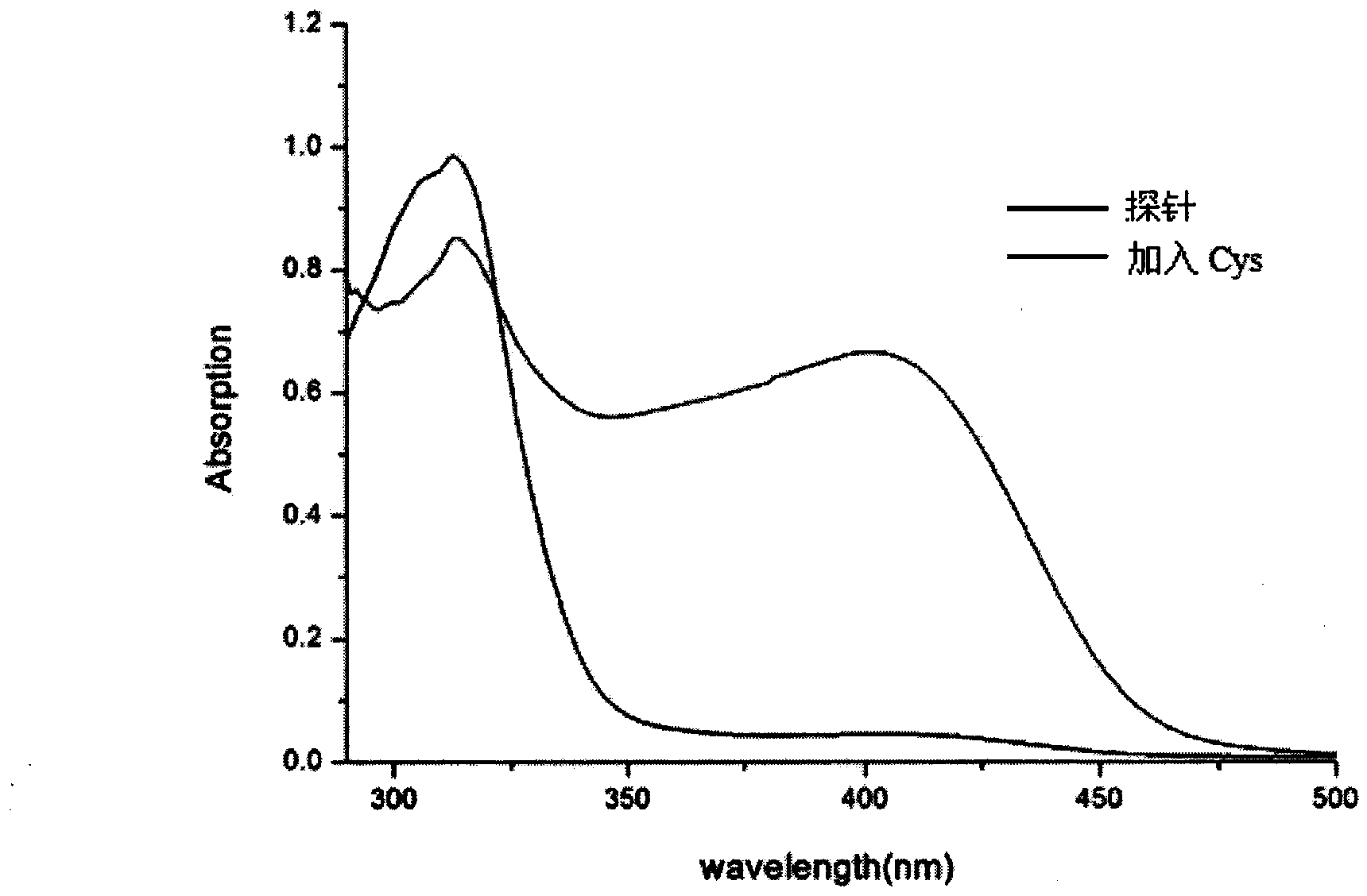
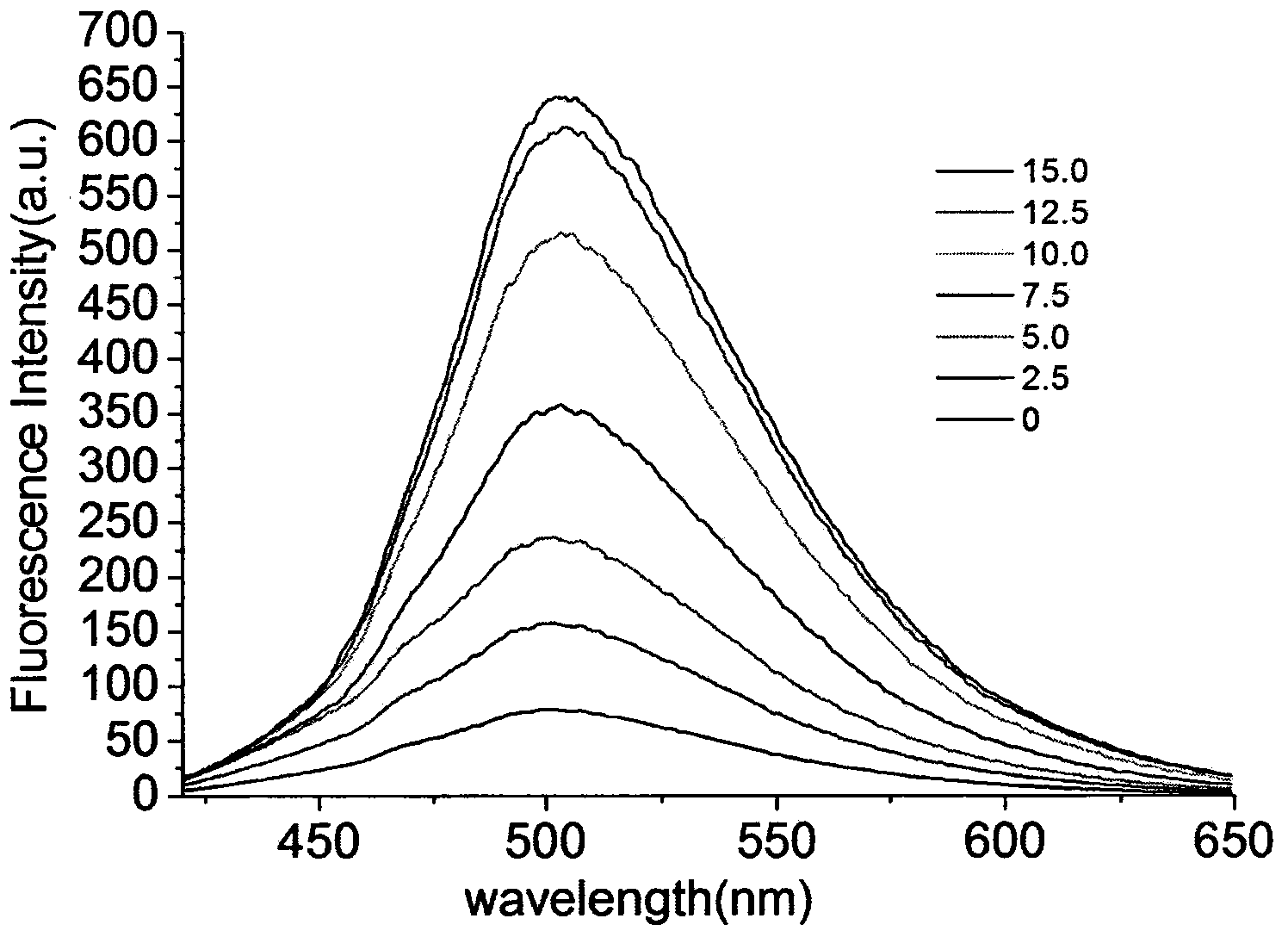
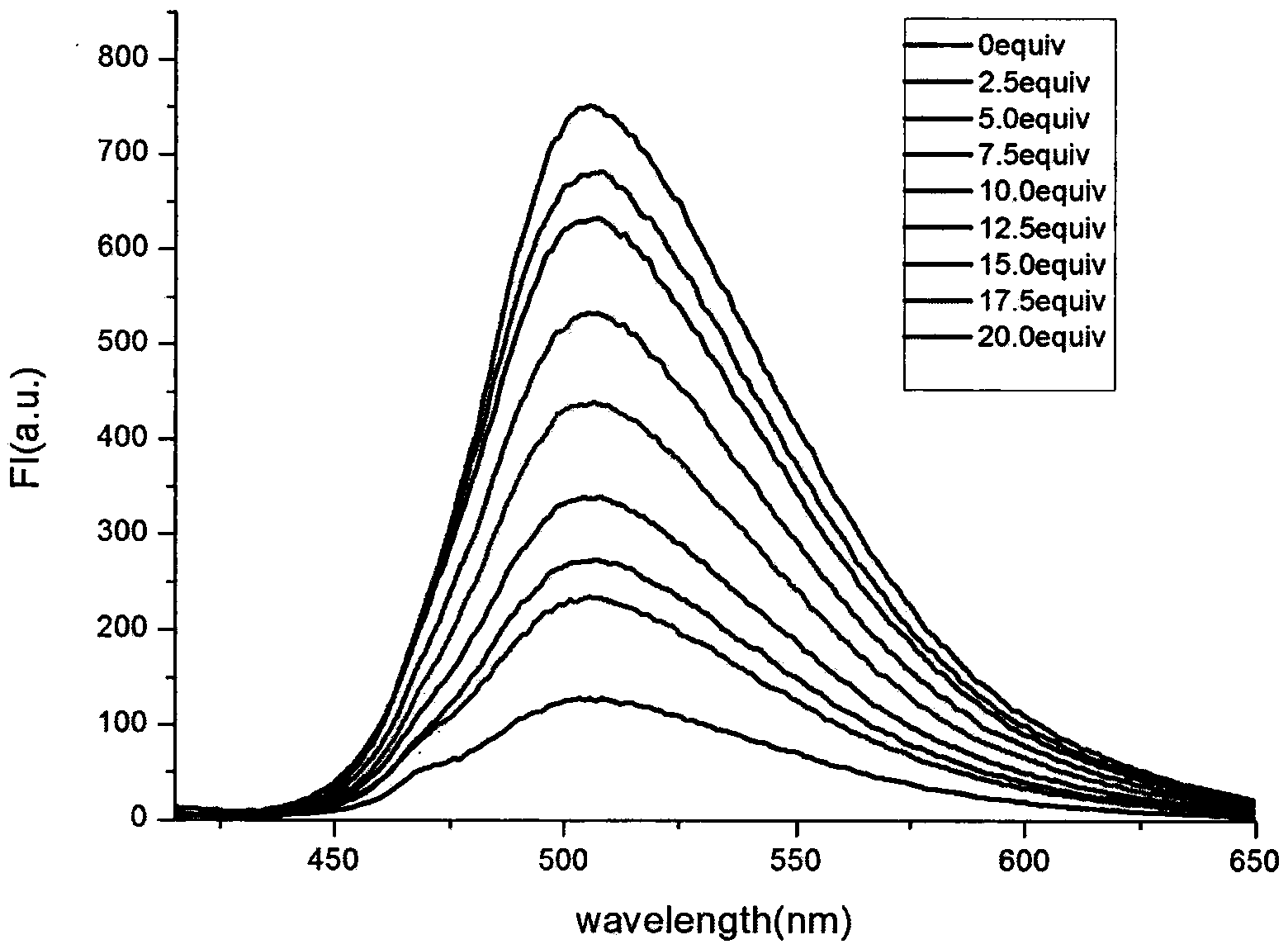
[0027] Example 3: Application of naked-eye and fluorescence-enhanced detection of thiol-containing amino acid fluorescent probes
[0028] The probe was dissolved in DMSO / PBS (pH=7.4) buffer solution, and the changes of its ultraviolet absorption spectrum and fluorescence spectrum were tested. Figure 1 to Figure 5 It shows that the probe has high selectivity to amino acids containing thiol in the ultraviolet spectrum and fluorescence spectrum. With the increase of the concentration of amino acid containing thiol, its ultraviolet spectrum and fluorescence spectrum change obviously, and the color of the solution changes significantly. It is suitable for At the same time, after adding mercapto amino acid, the fluorescence intensity is enhanced by 130 times, and the probe is not affected by some other amino acids, such as: cysteine (Cys), aspartic acid (Asp), alanine ( Ala), Valine (Val), Phenylalanine (Phe), Histidine (His), Isoleucine (Ile), Lysine (Lys), Arginine (Arg), Proli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com