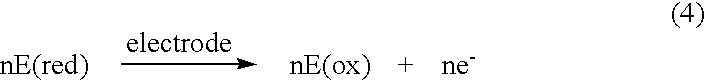Patents
Literature
46844results about "Catheter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
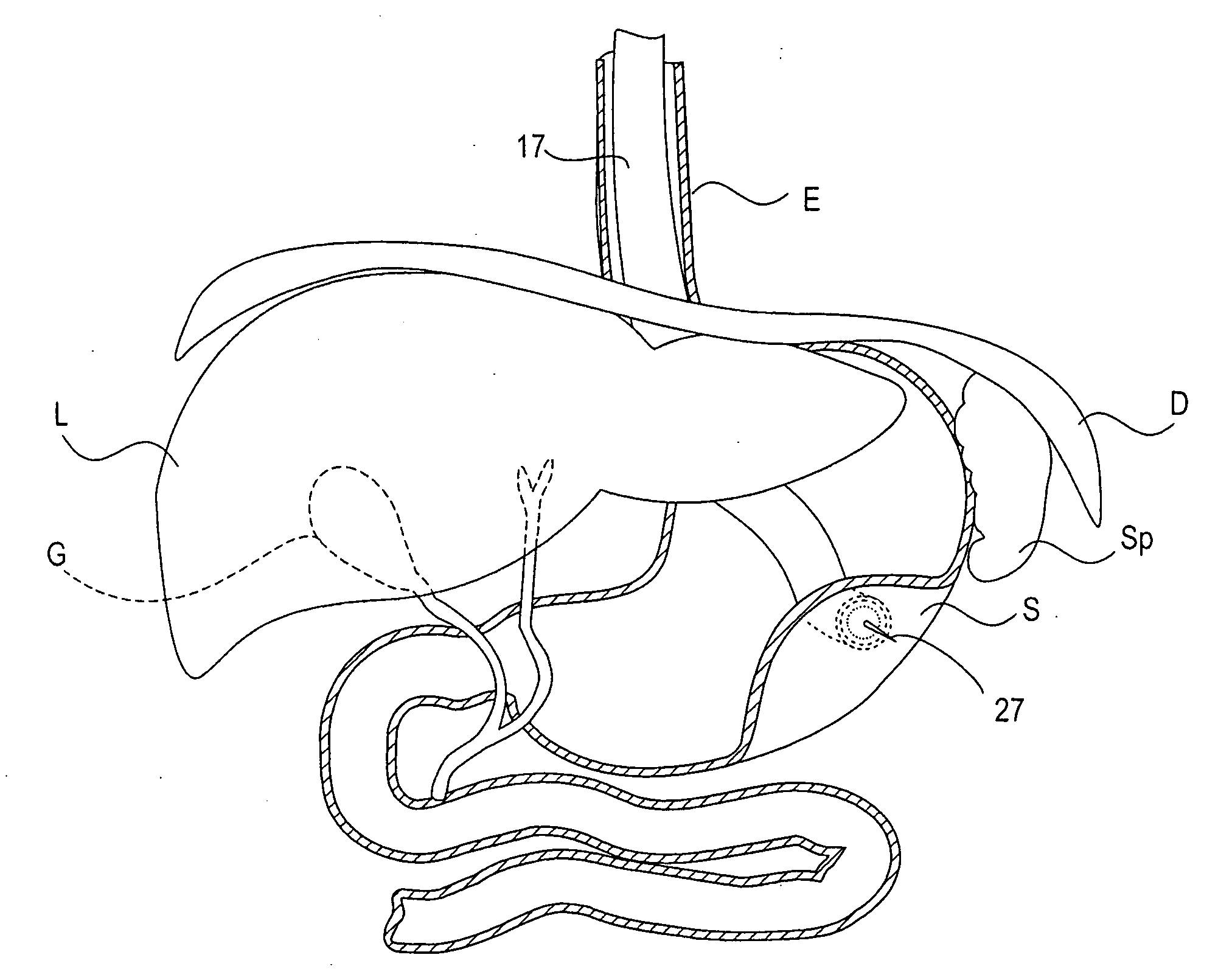
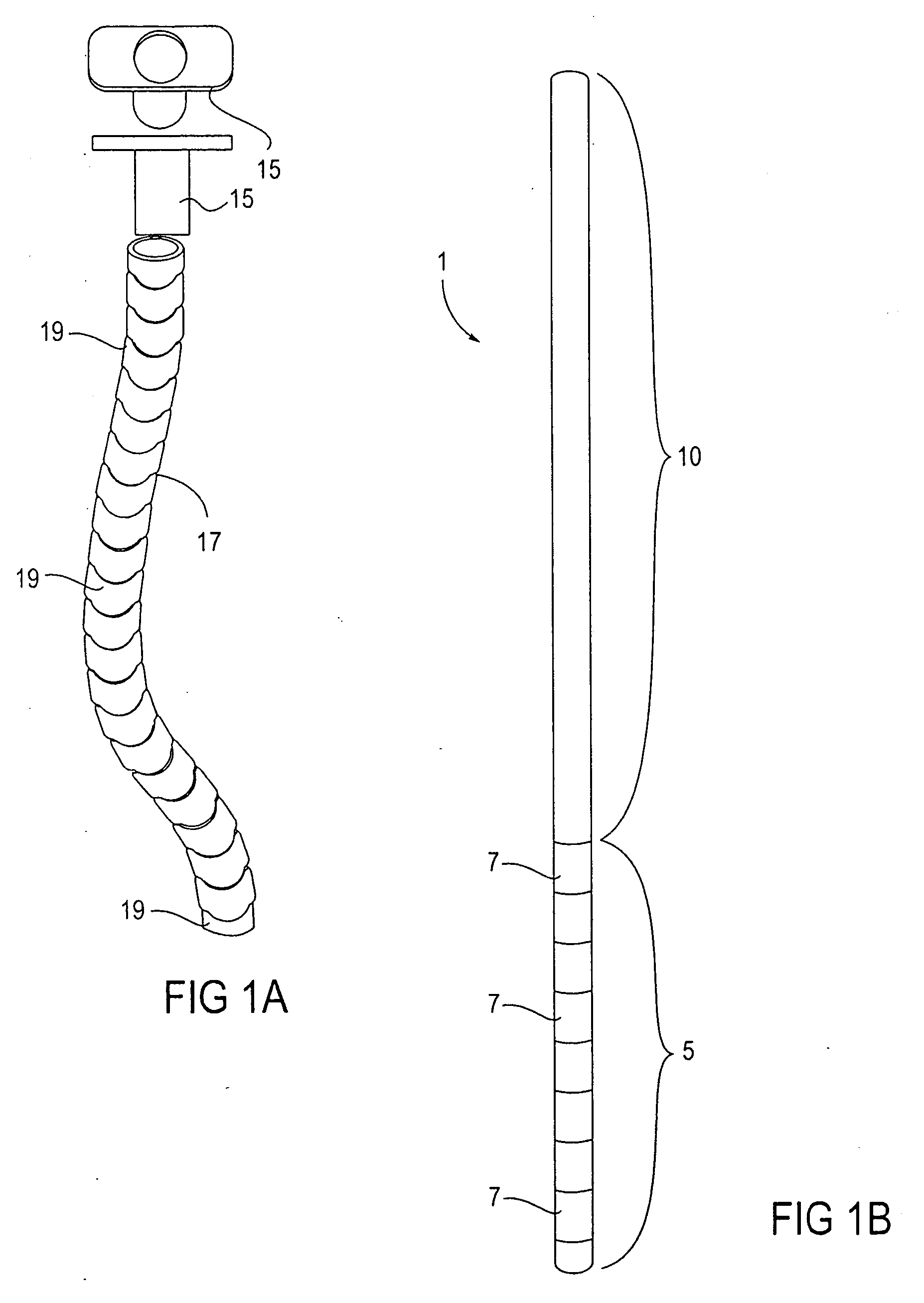
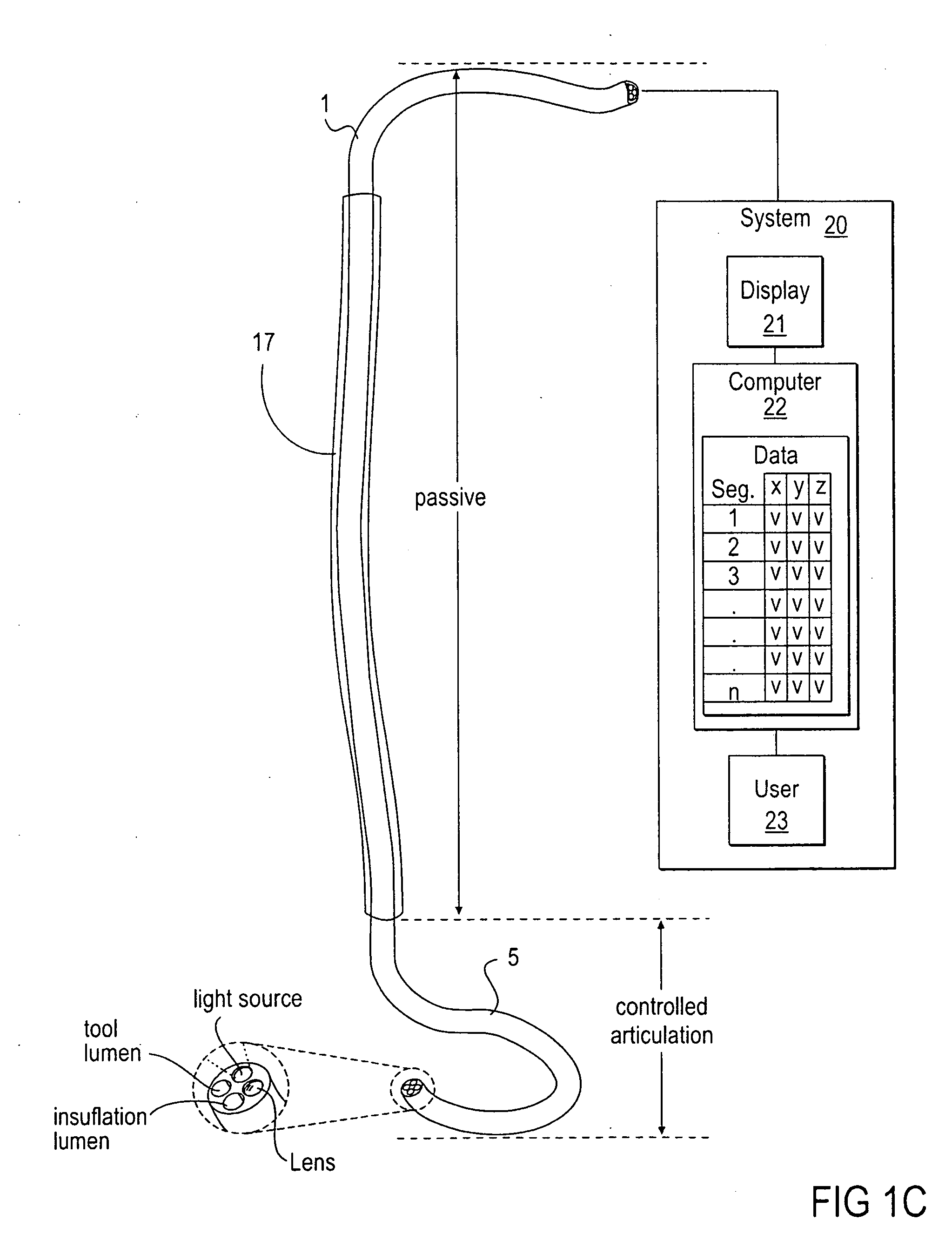
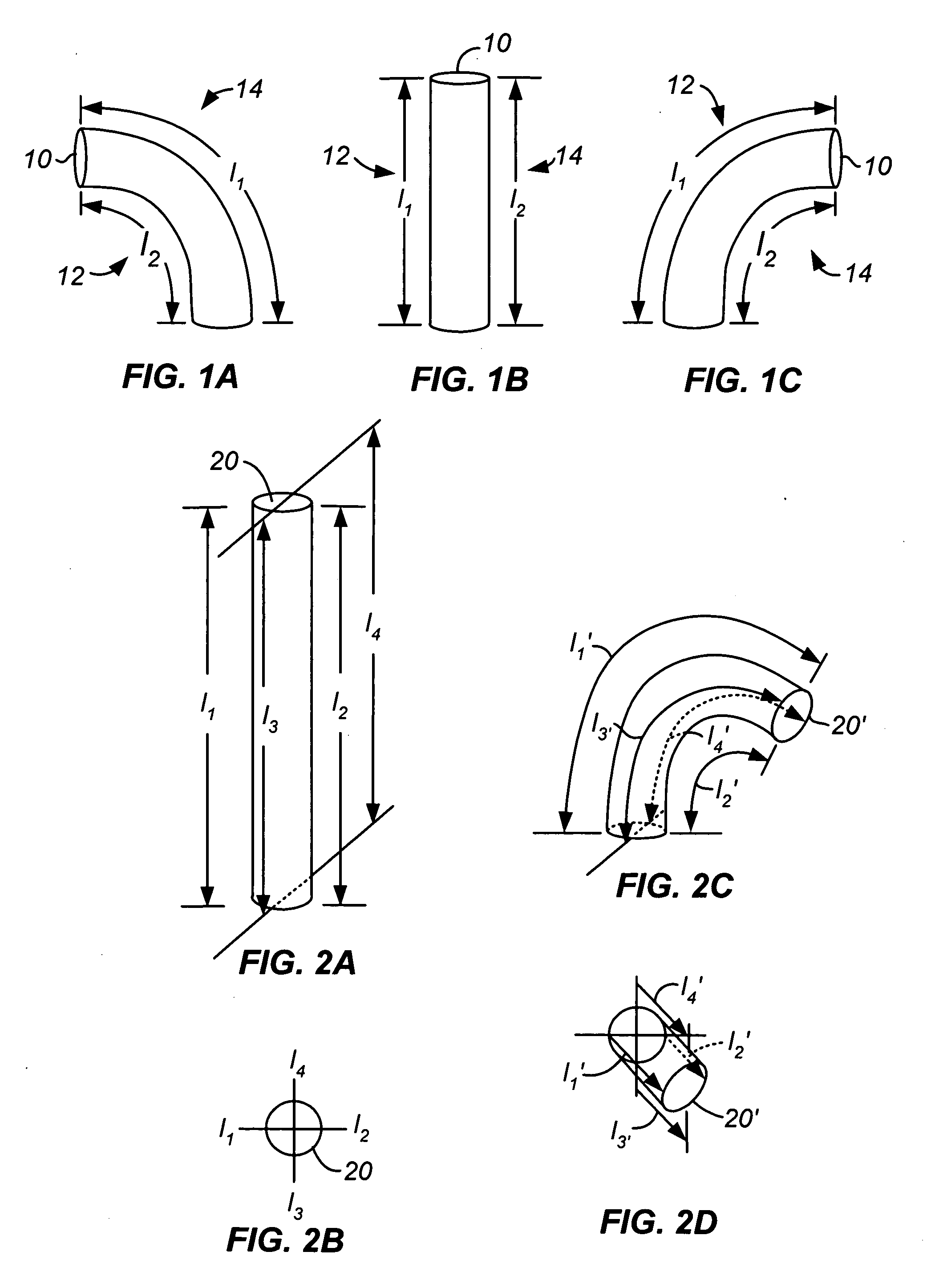
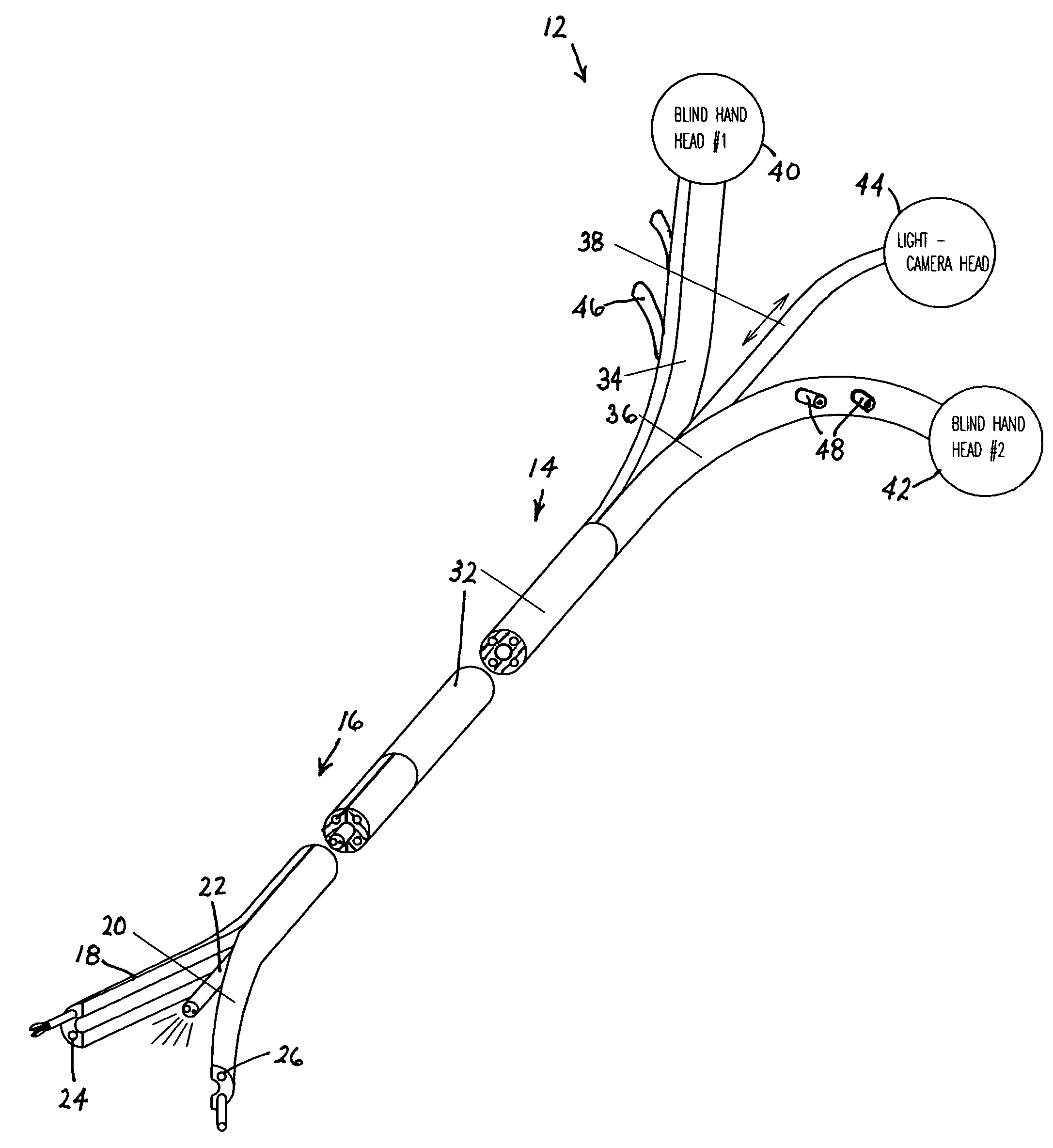
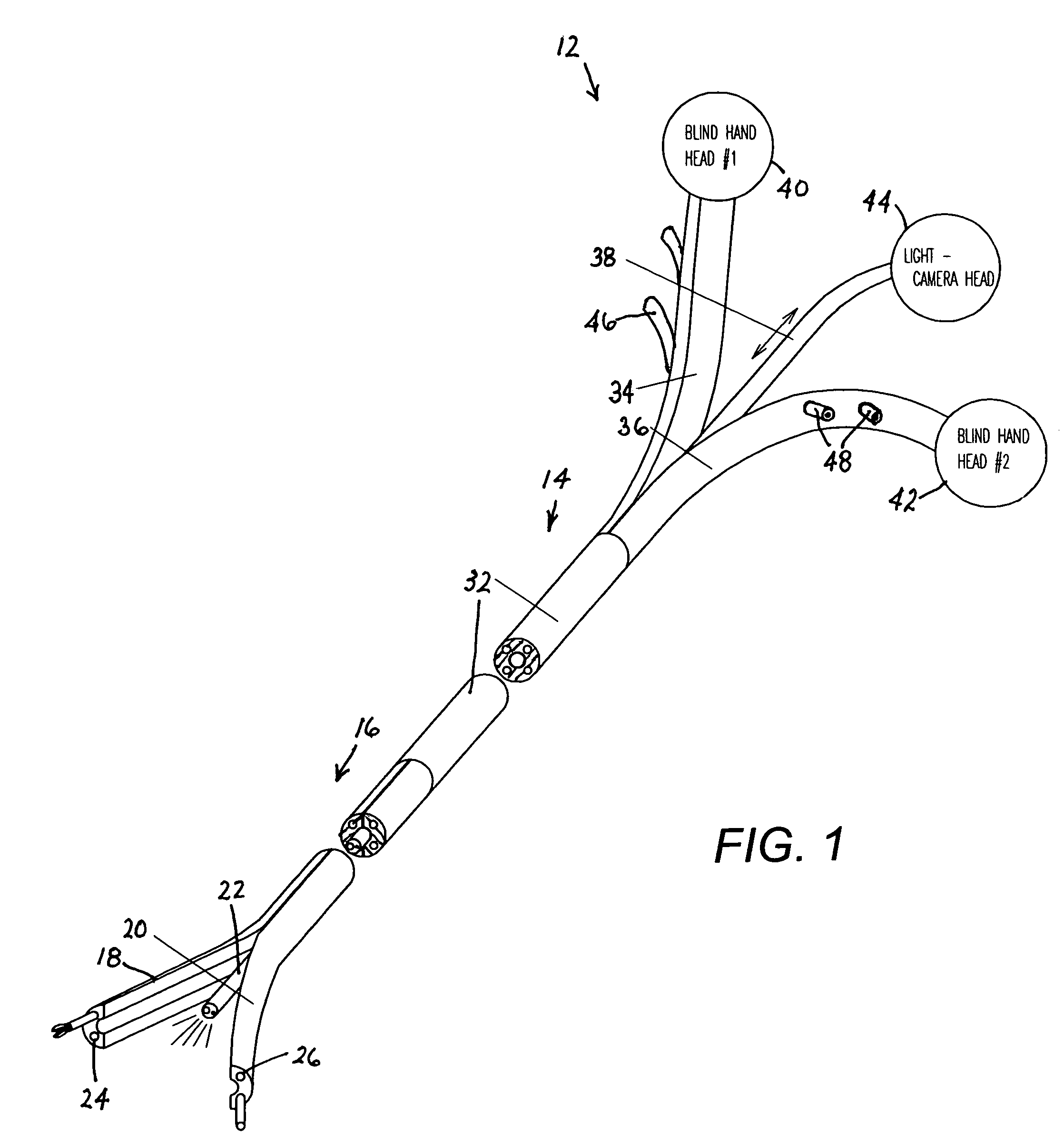
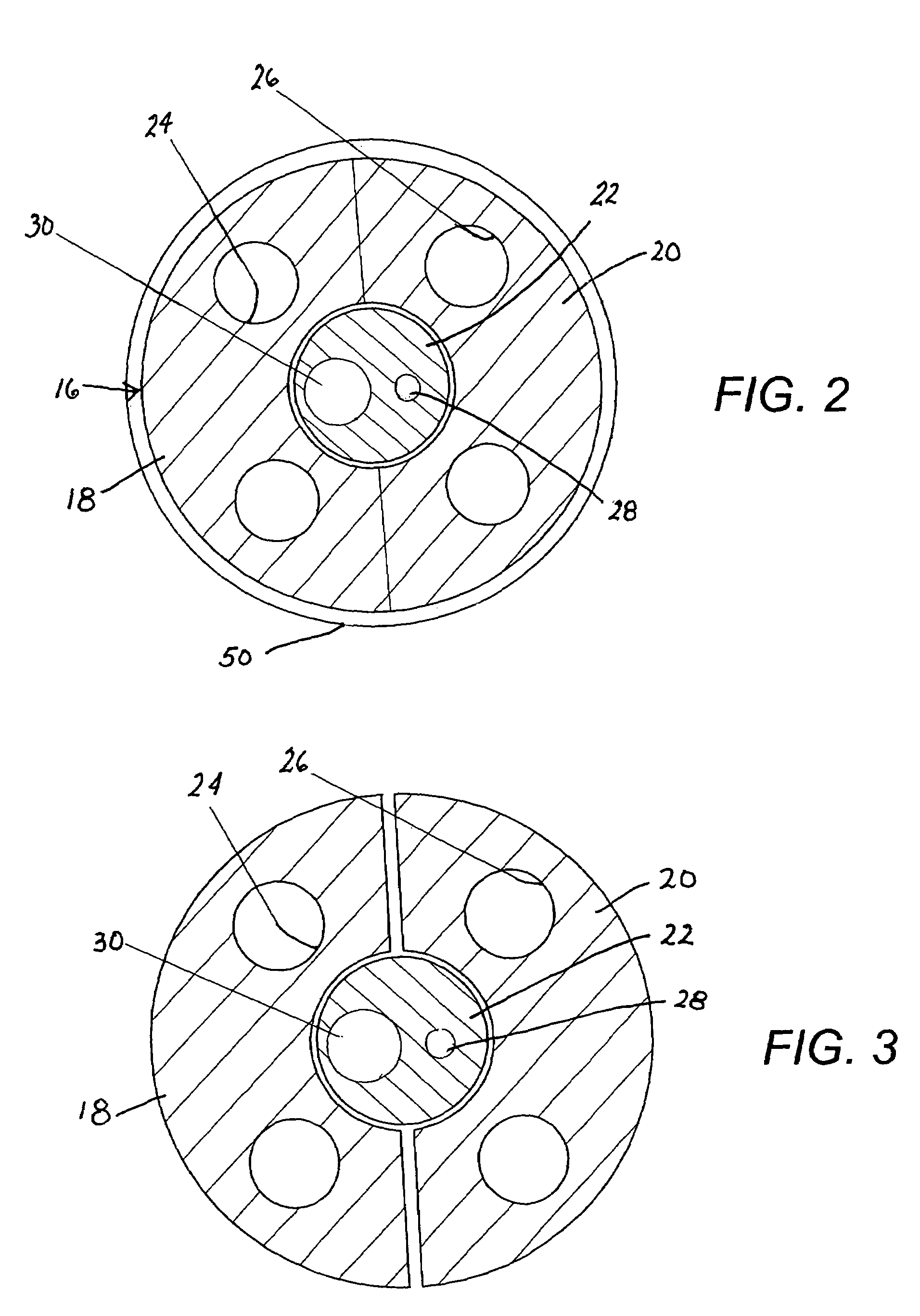
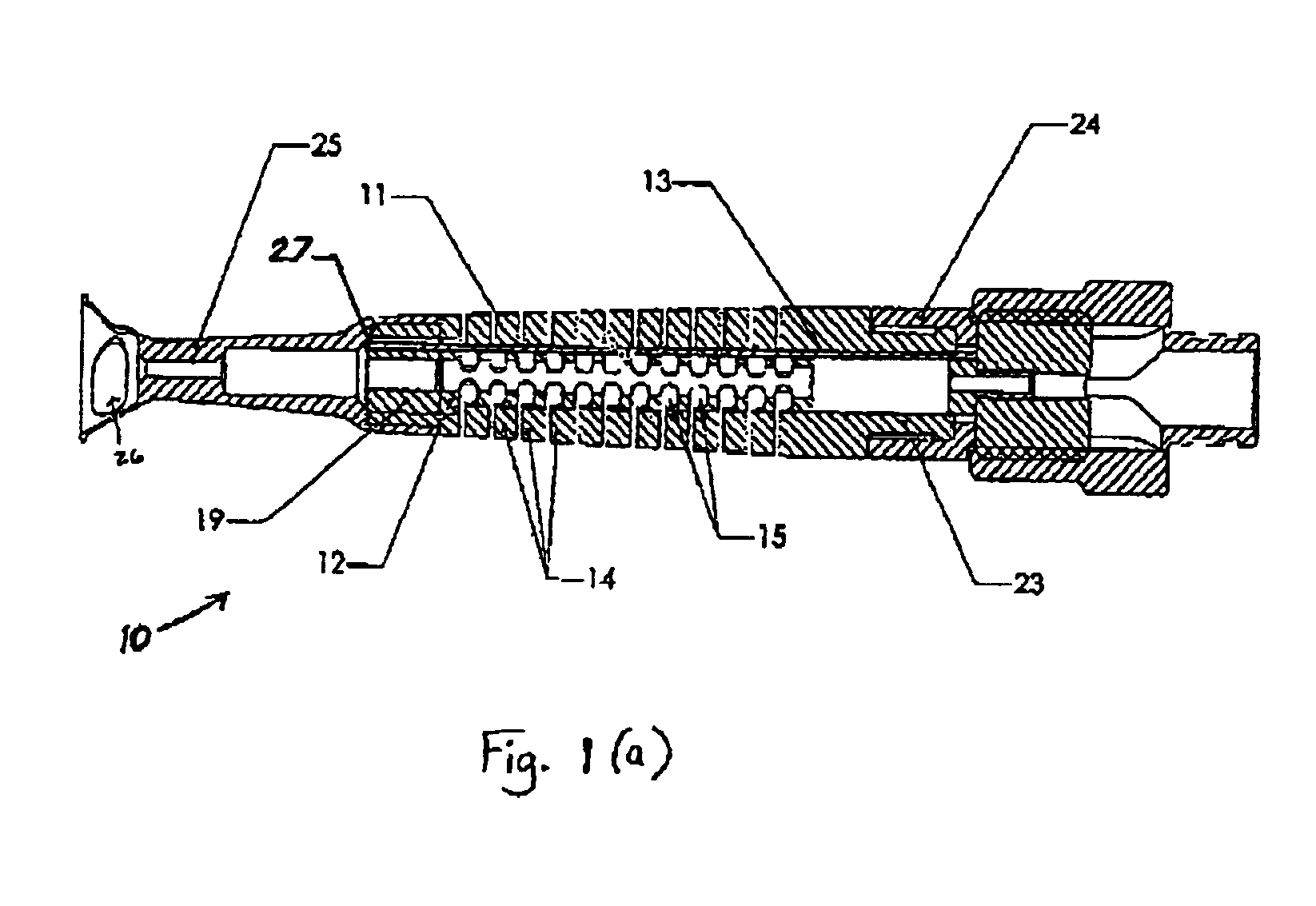
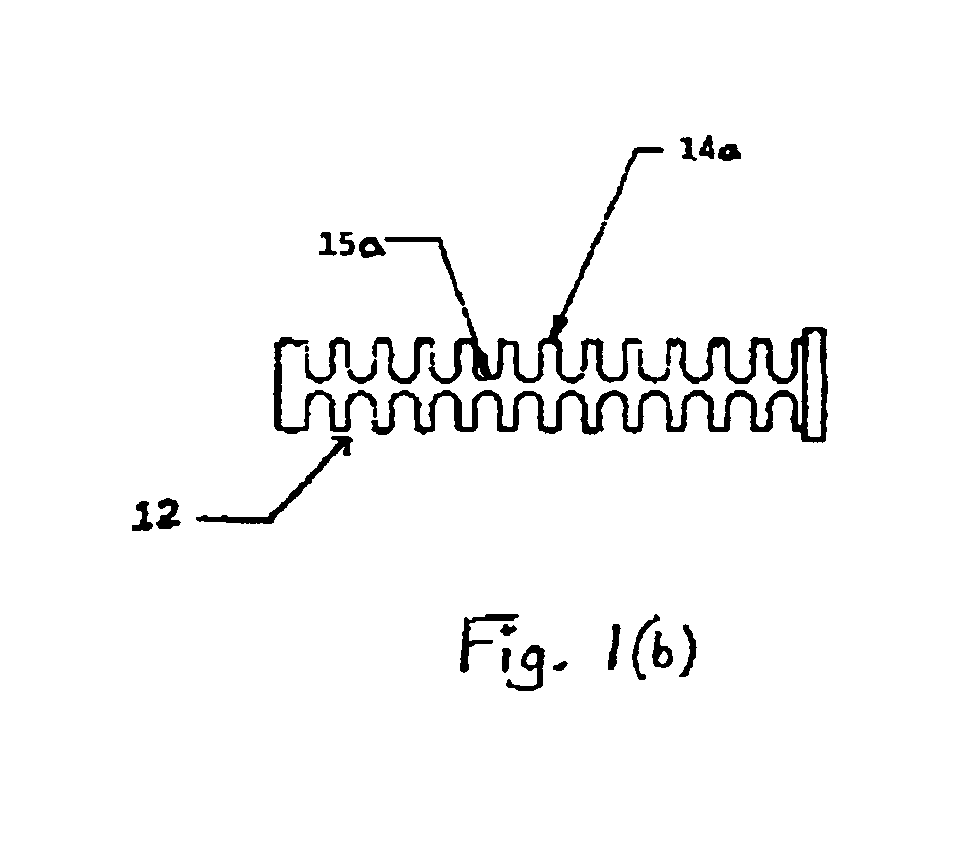
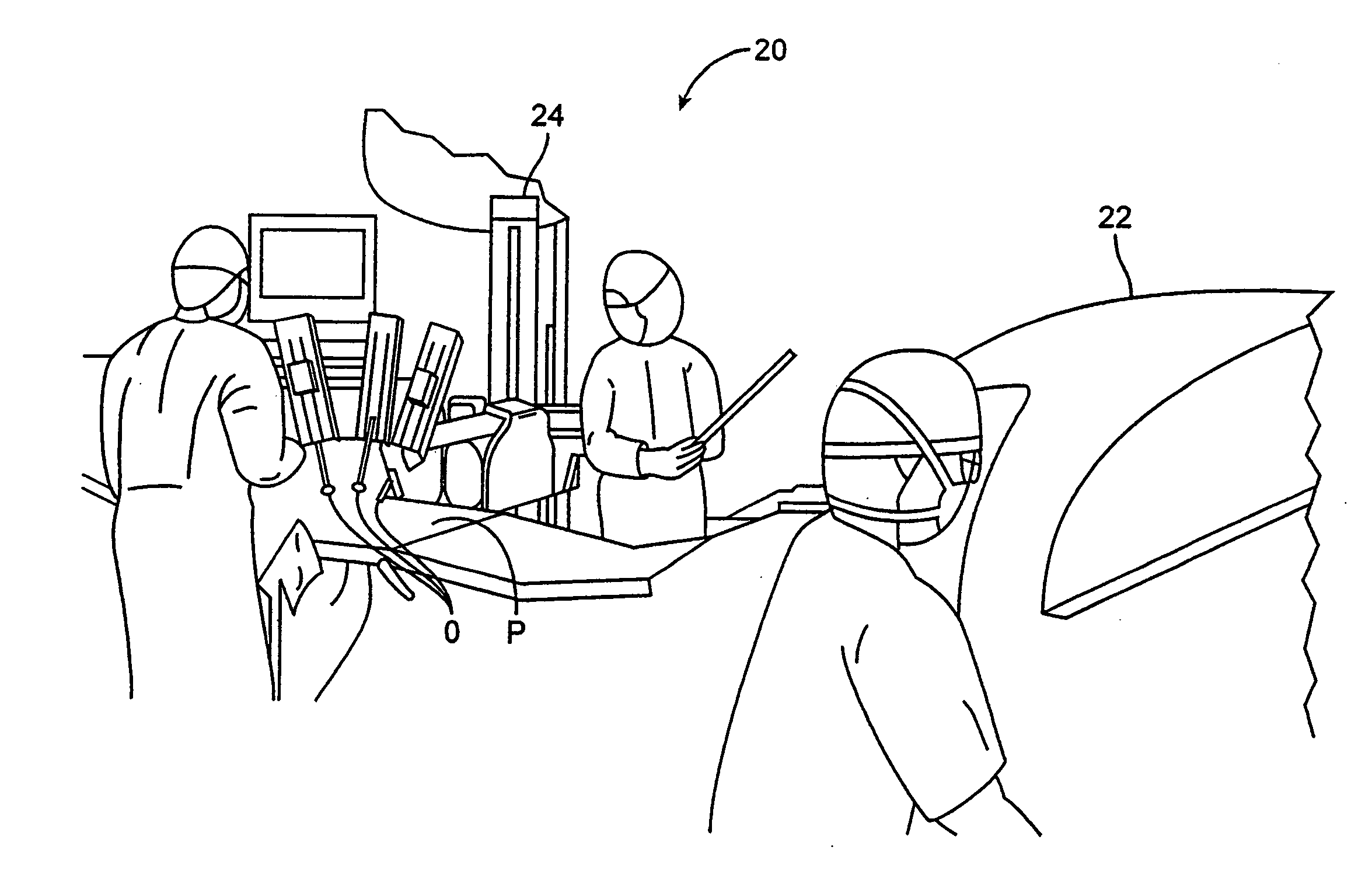
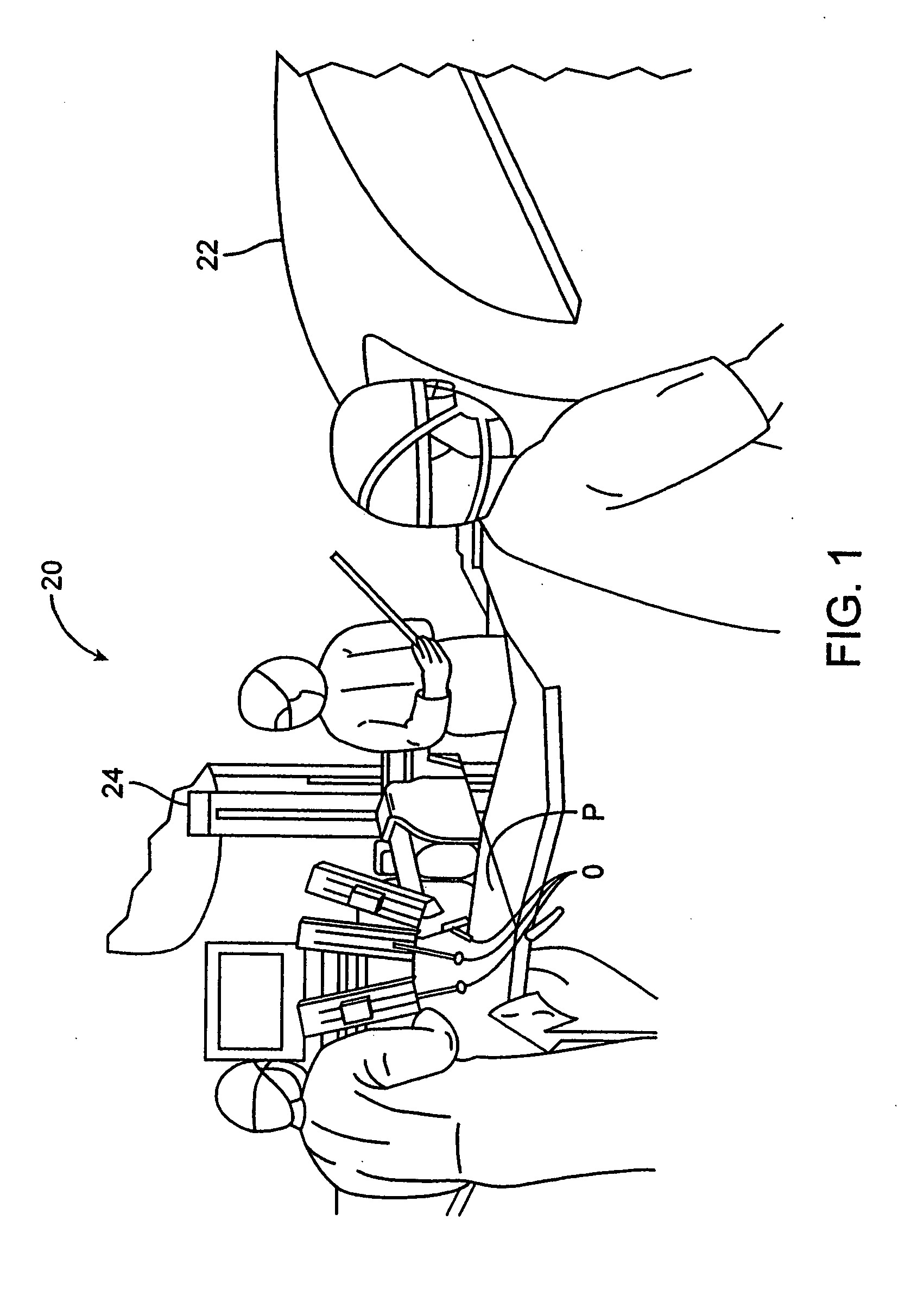
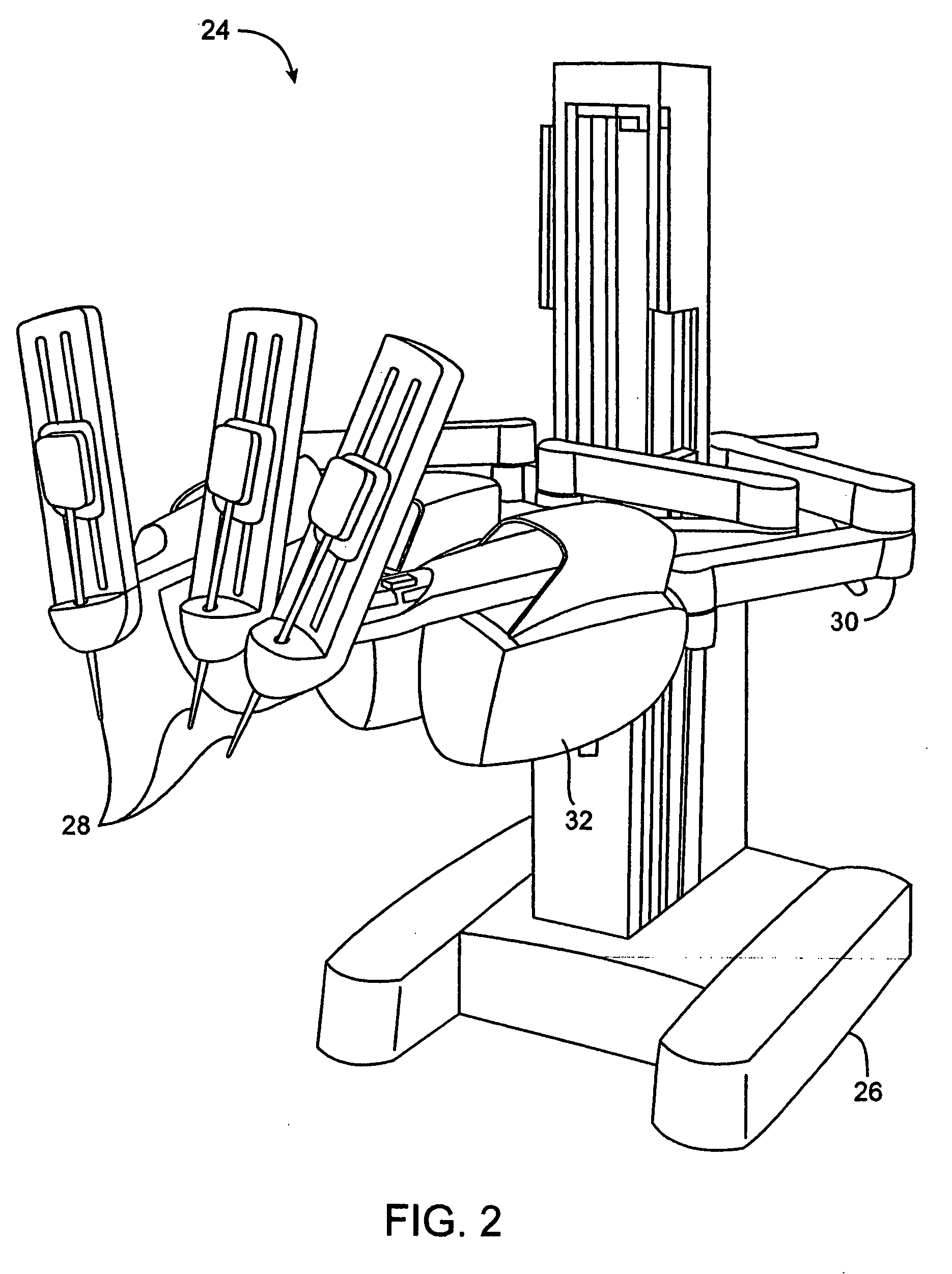
Methods and apparatus for performing transluminal and other procedures
InactiveUS20070135803A1Prevent overflowPrevent leakageSuture equipmentsEar treatmentSurgeryInstrumentation
Owner:INTUITIVE SURGICAL OPERATIONS INC
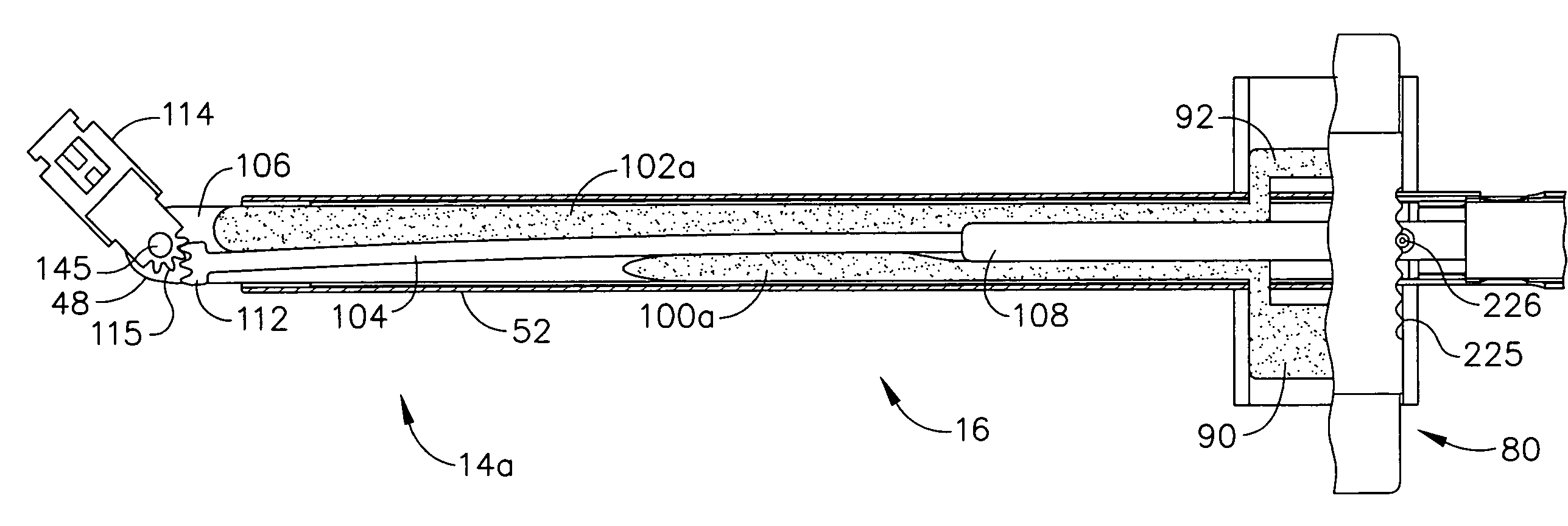
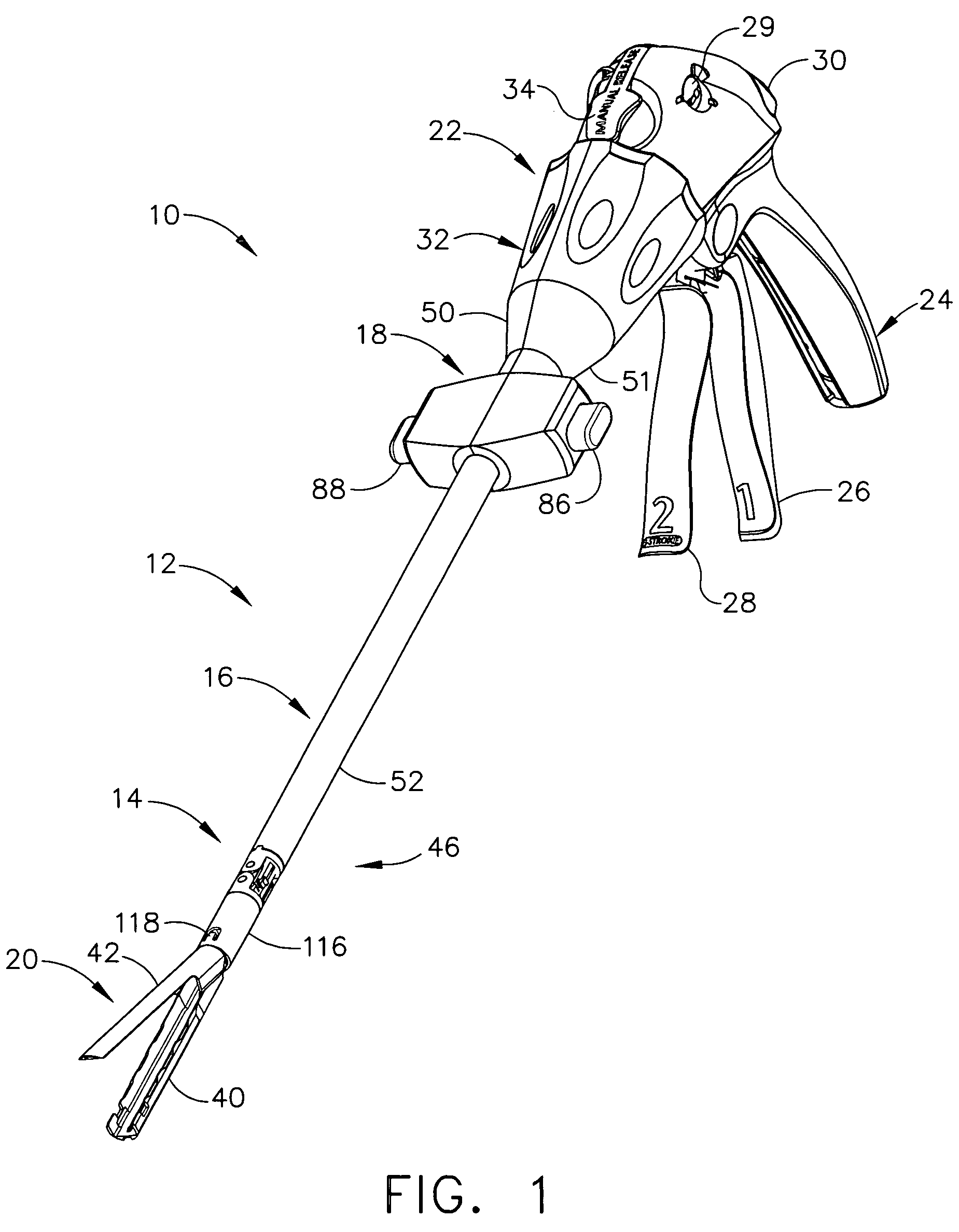
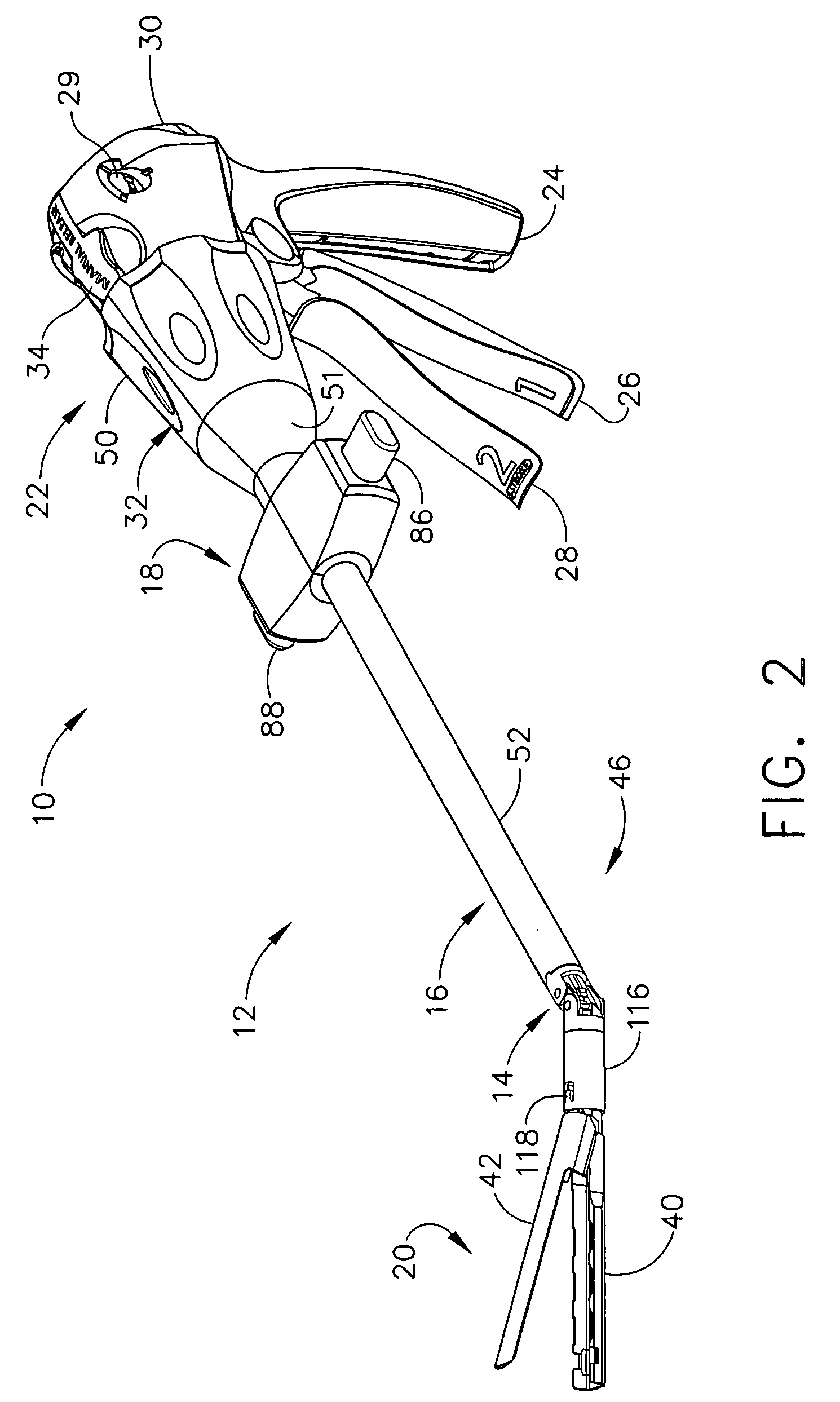
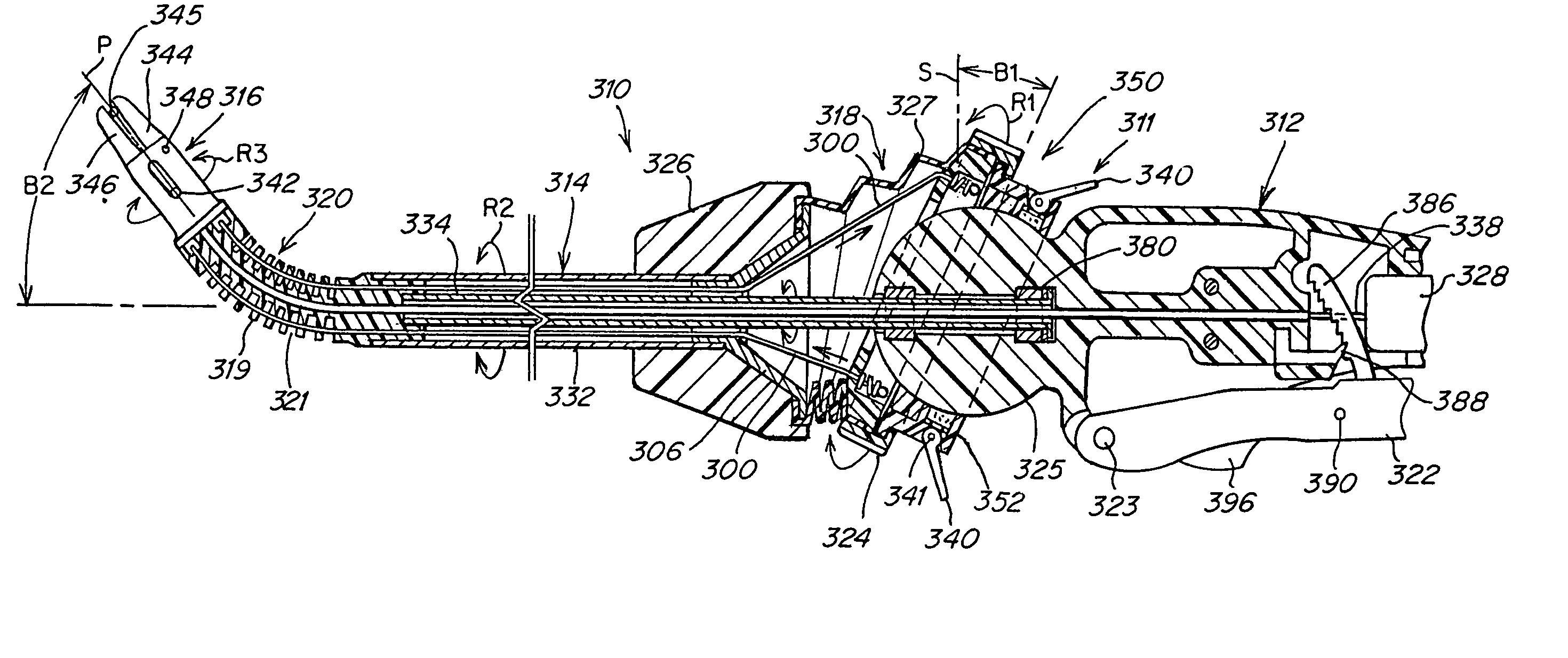
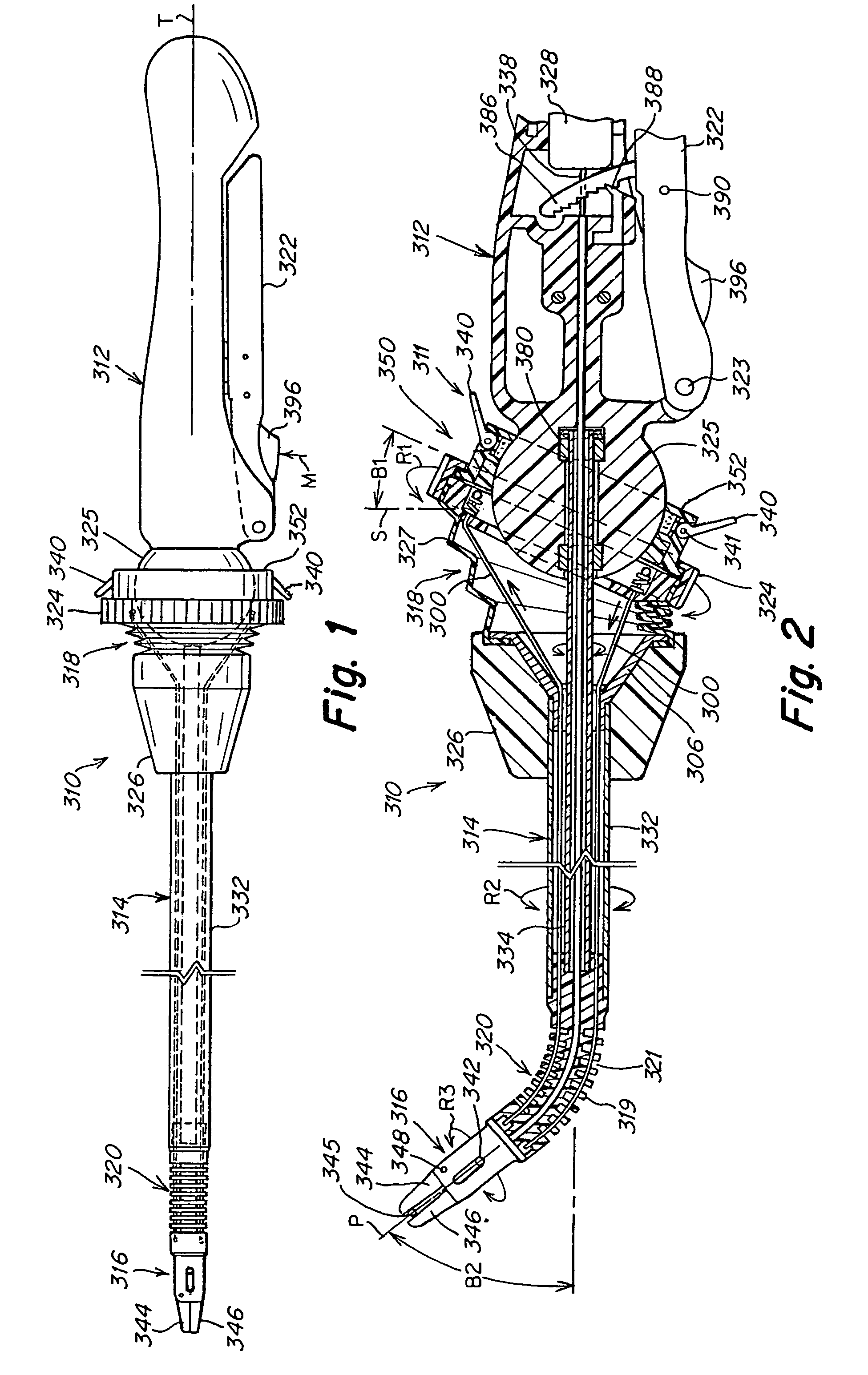
Surgical instrument with bending articulation controlled articulation pivot joint
Owner:ETHICON ENDO SURGERY INC
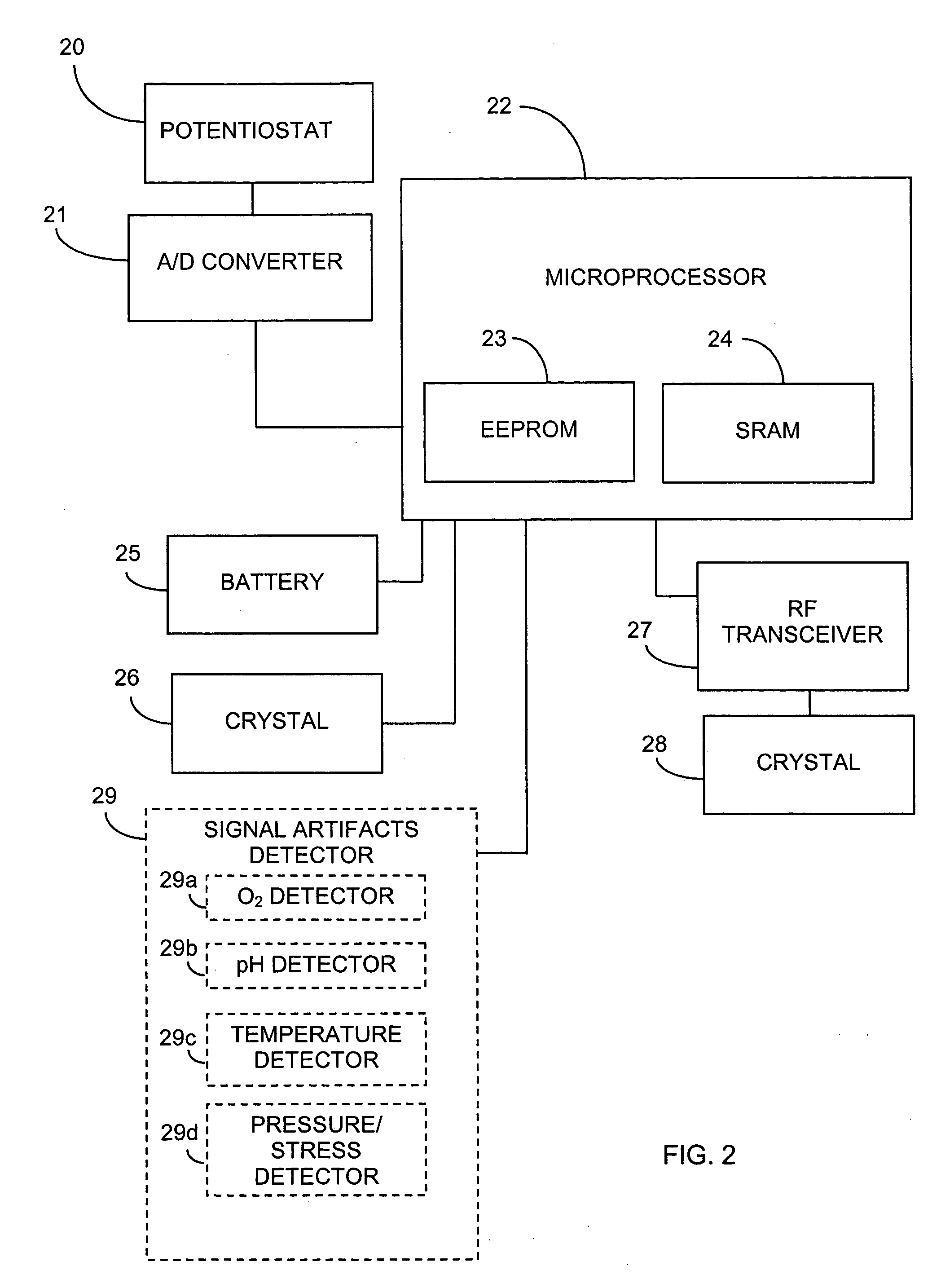
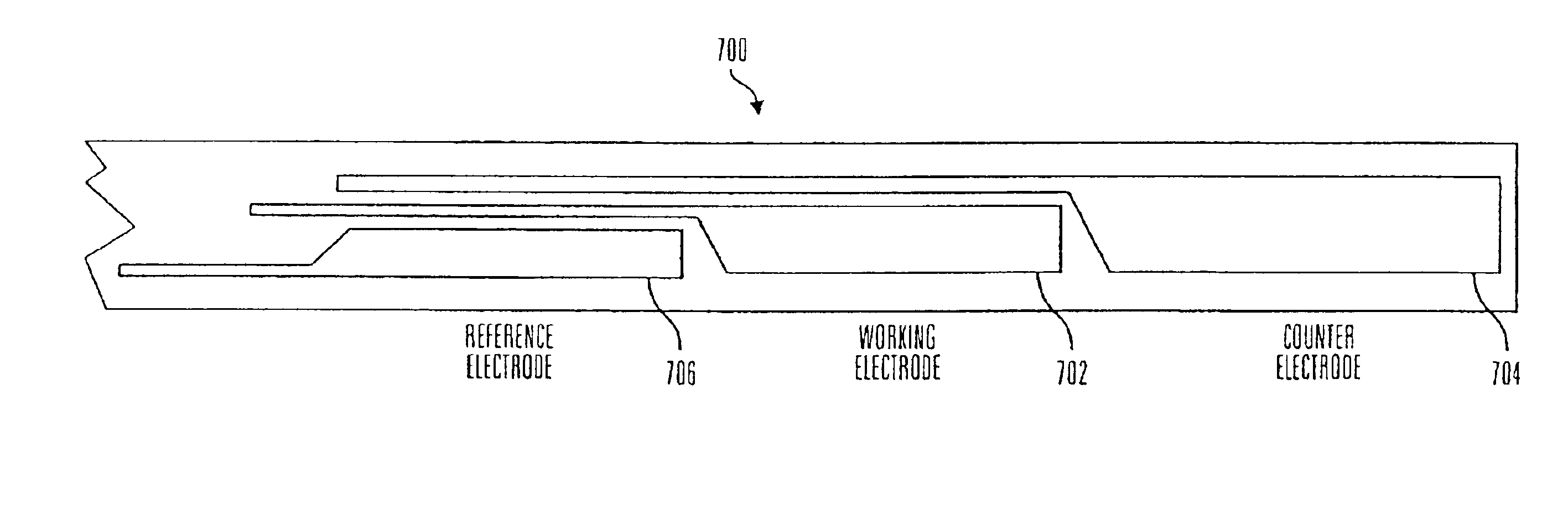
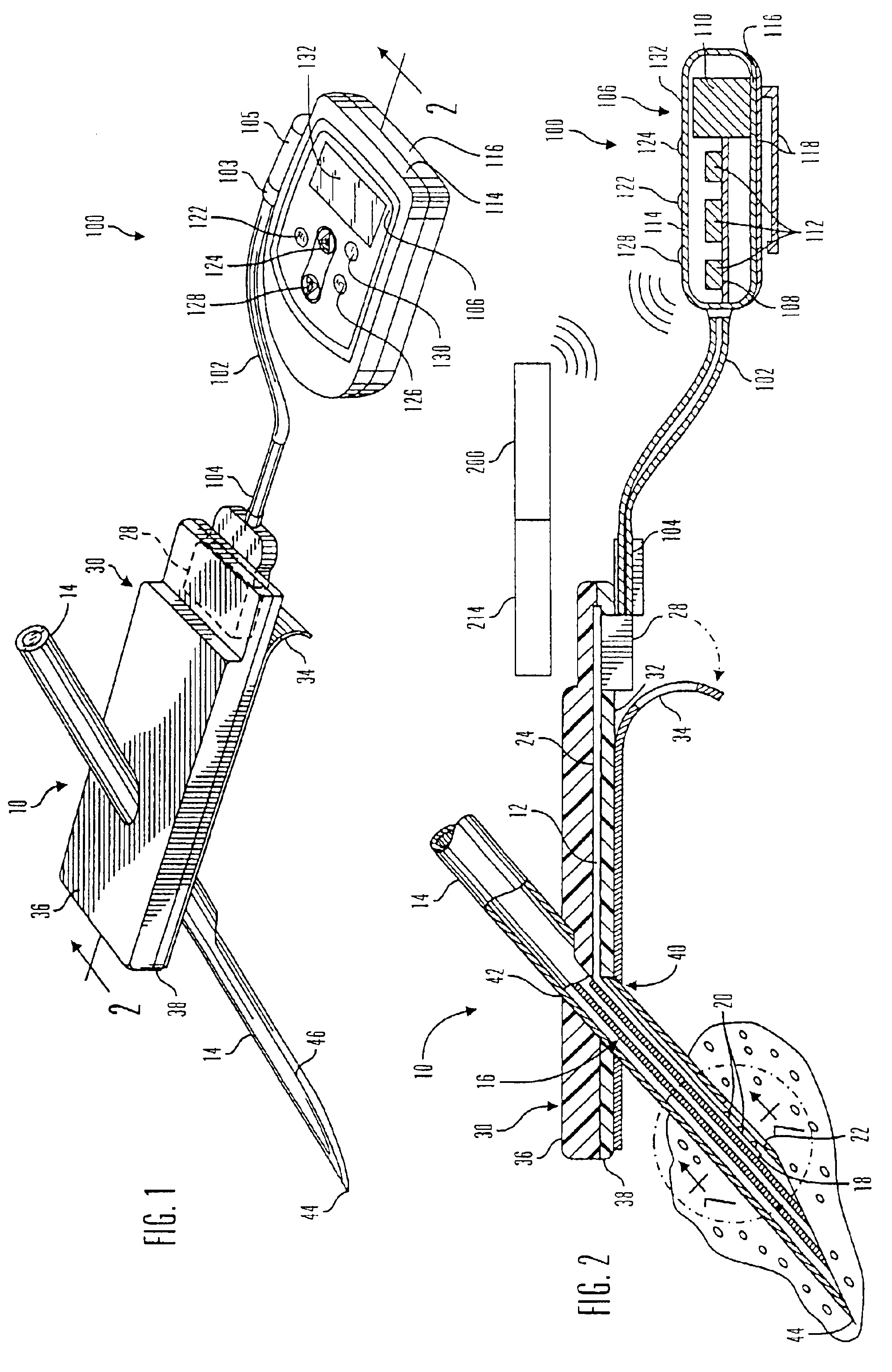
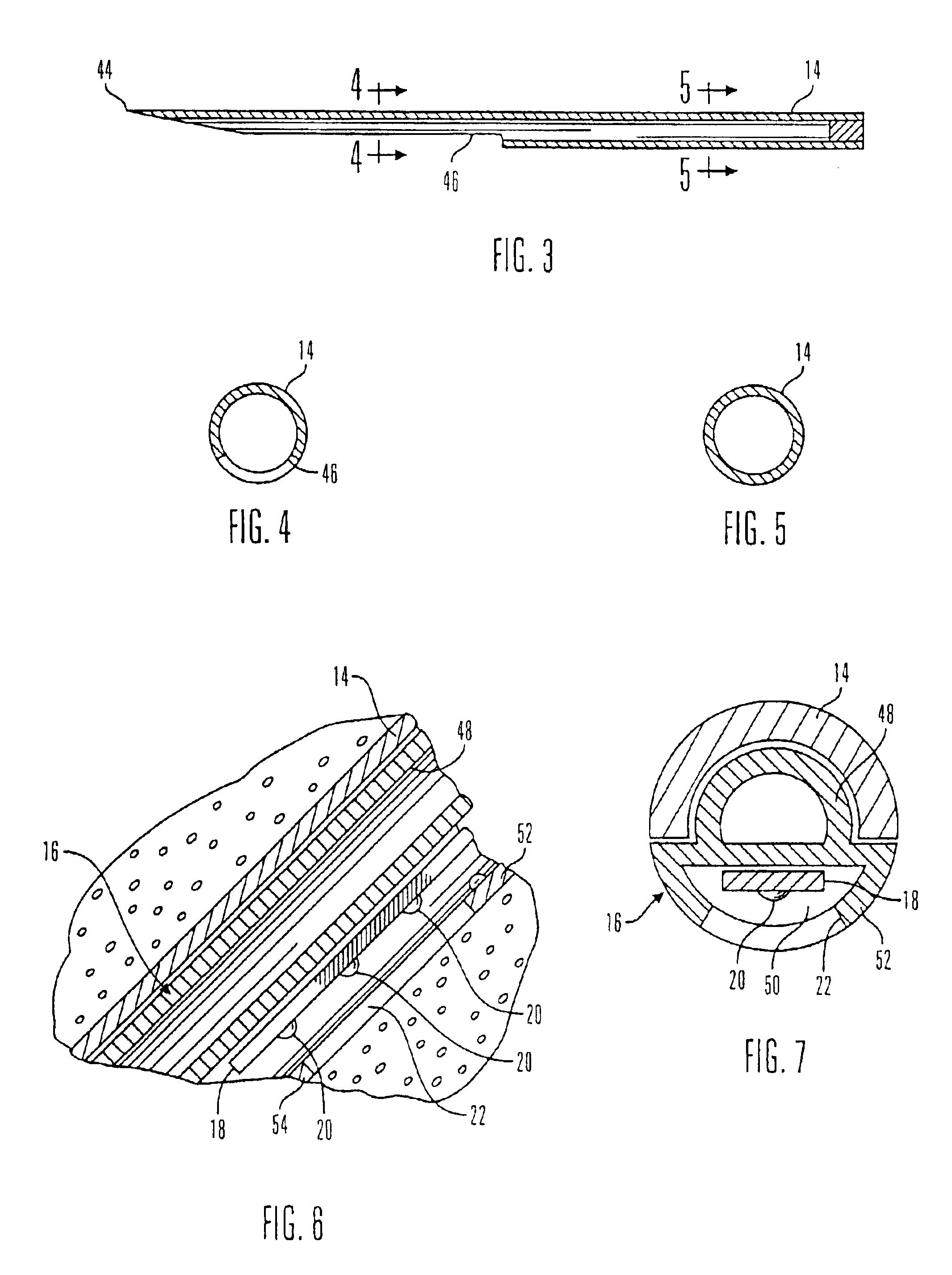
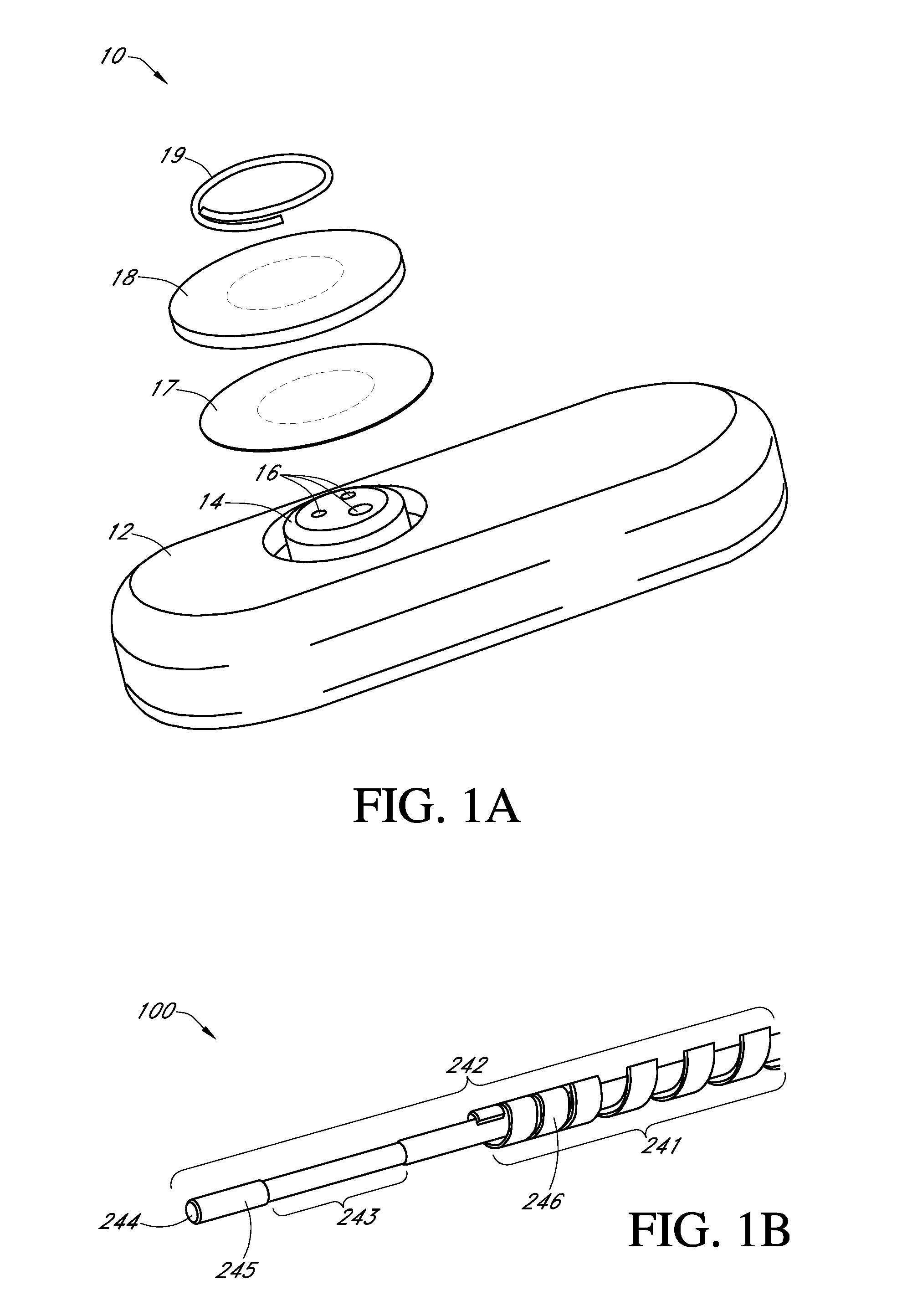
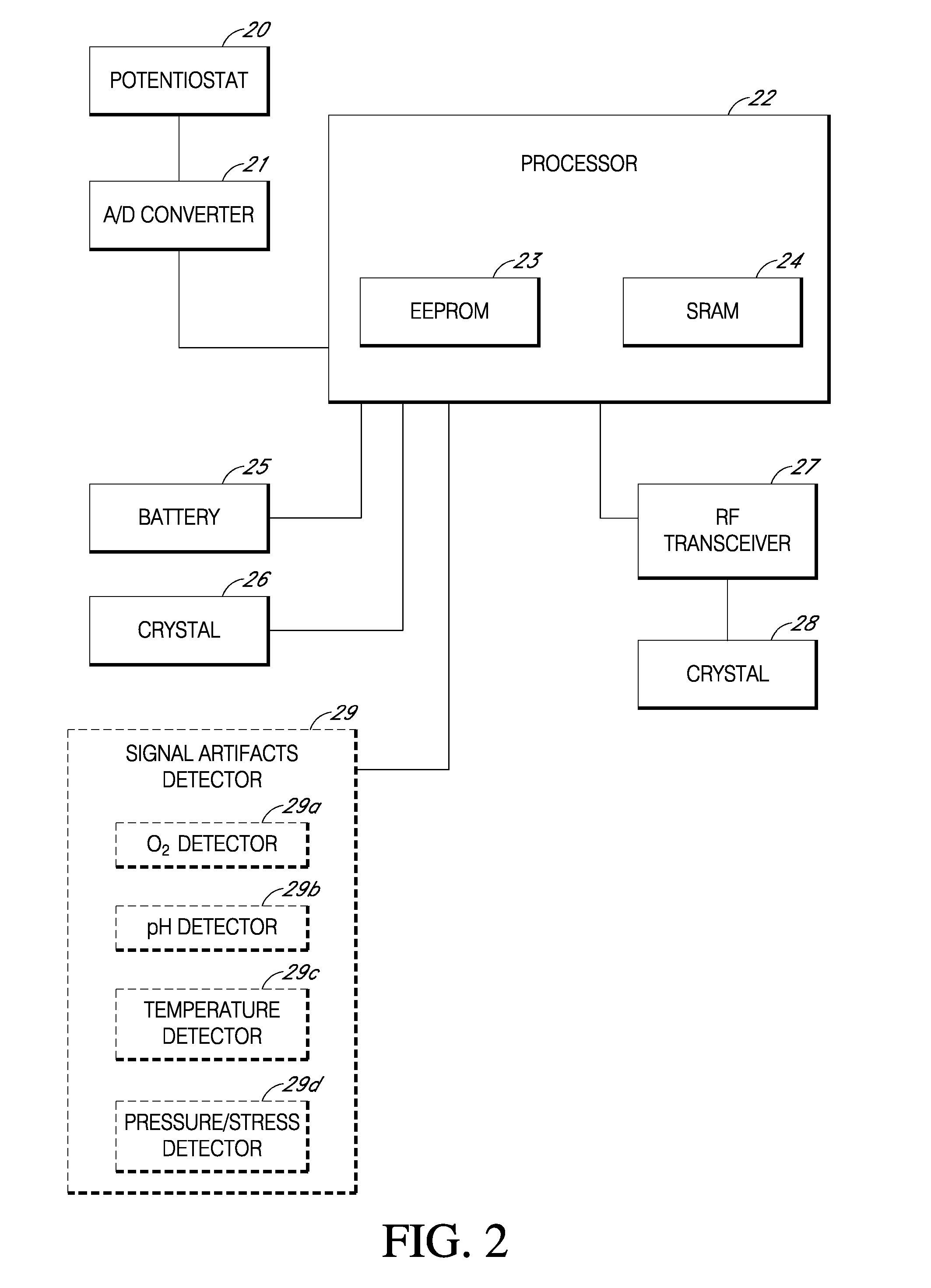
Electrochemical analyte sensor
InactiveUS6484046B1Avoid and reduce corrosionMicrobiological testing/measurementMaterial analysis by electric/magnetic meansAnalyteElectrolysis
An electrochemical analyte sensor having conductive traces on a substrate is used to determine a level of analyte in in vitro or in vivo analyte-containing fluids. The electrochemical analyte sensor includes a substrate and conductive material disposed on the substrate, the conductive material forming a working electrode. In some sensors, the conductive material is disposed in recessed channels formed in a surface of the sensor. An electron transfer agent and / or catalyst may be provided to facilitate the electrolysis of the analyte or of a second compound whose level depends on the level of the analyte. A potential is formed between the working electrode and a reference electrode or counter / reference electrode and the resulting current is a function of the concentration of the analyte in the fluid.
Owner:ABBOTT DIABETES CARE INC
Integrated delivery device for continuous glucose sensor
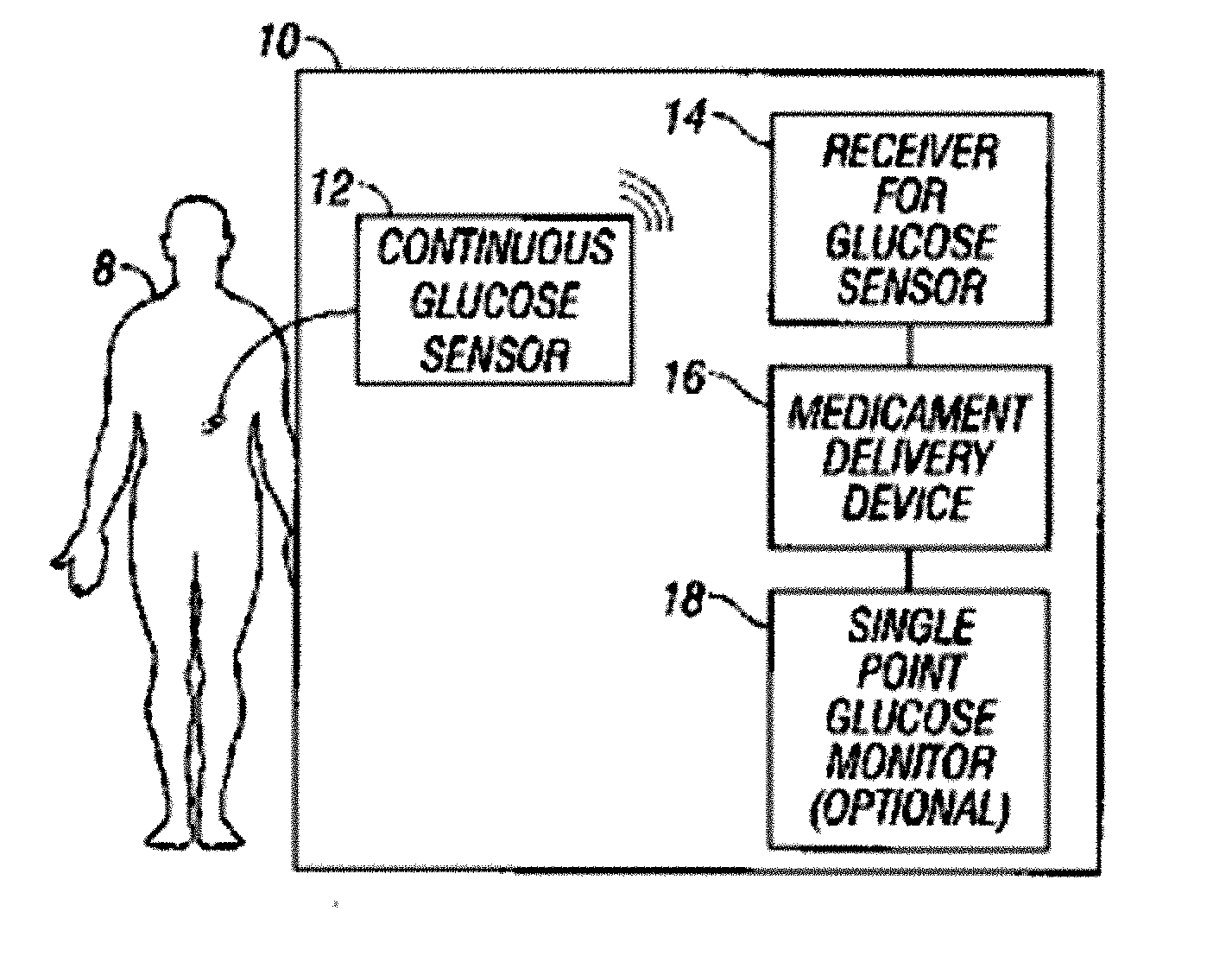
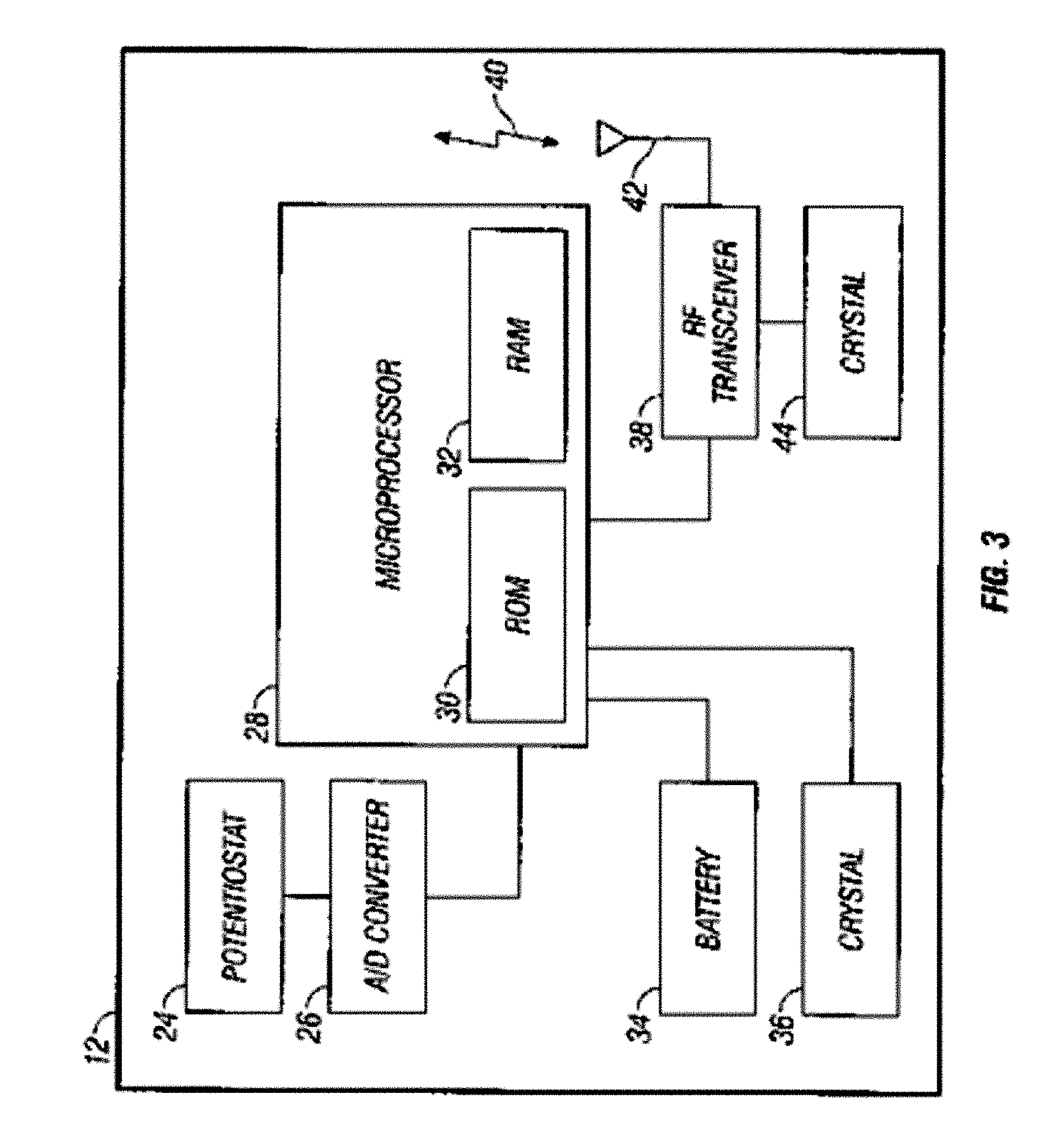
Abstract of the DisclosureSystems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
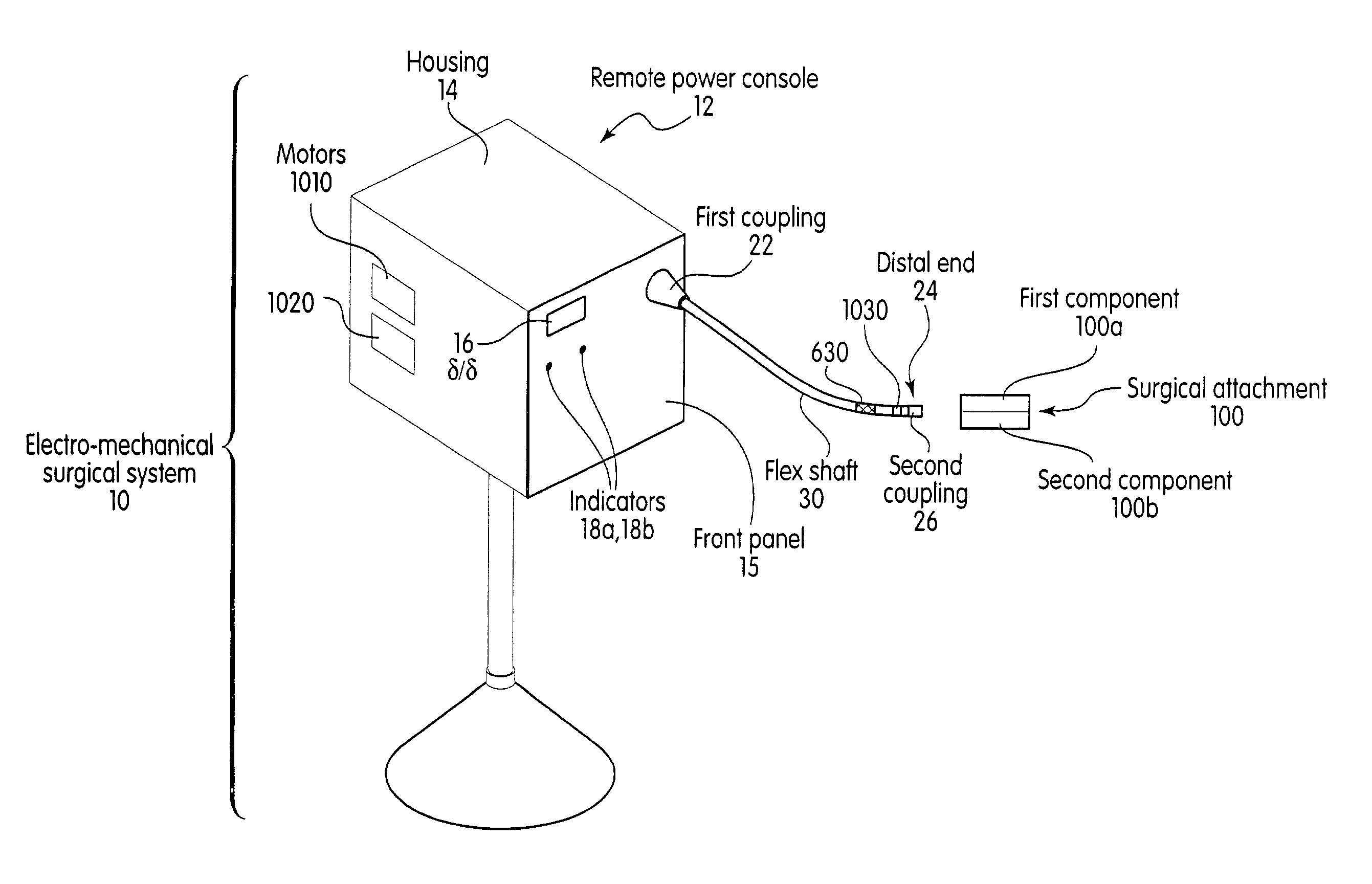
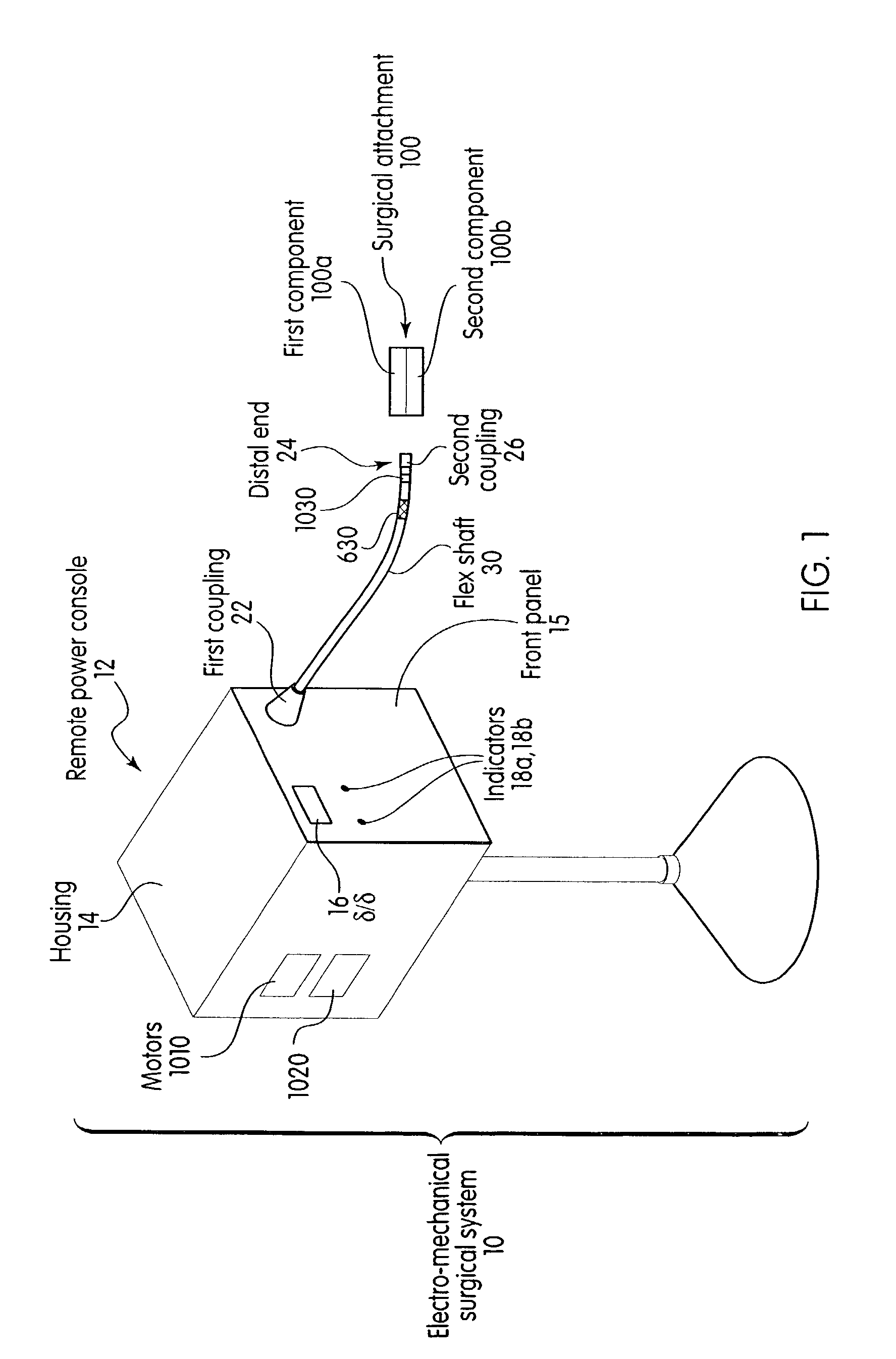
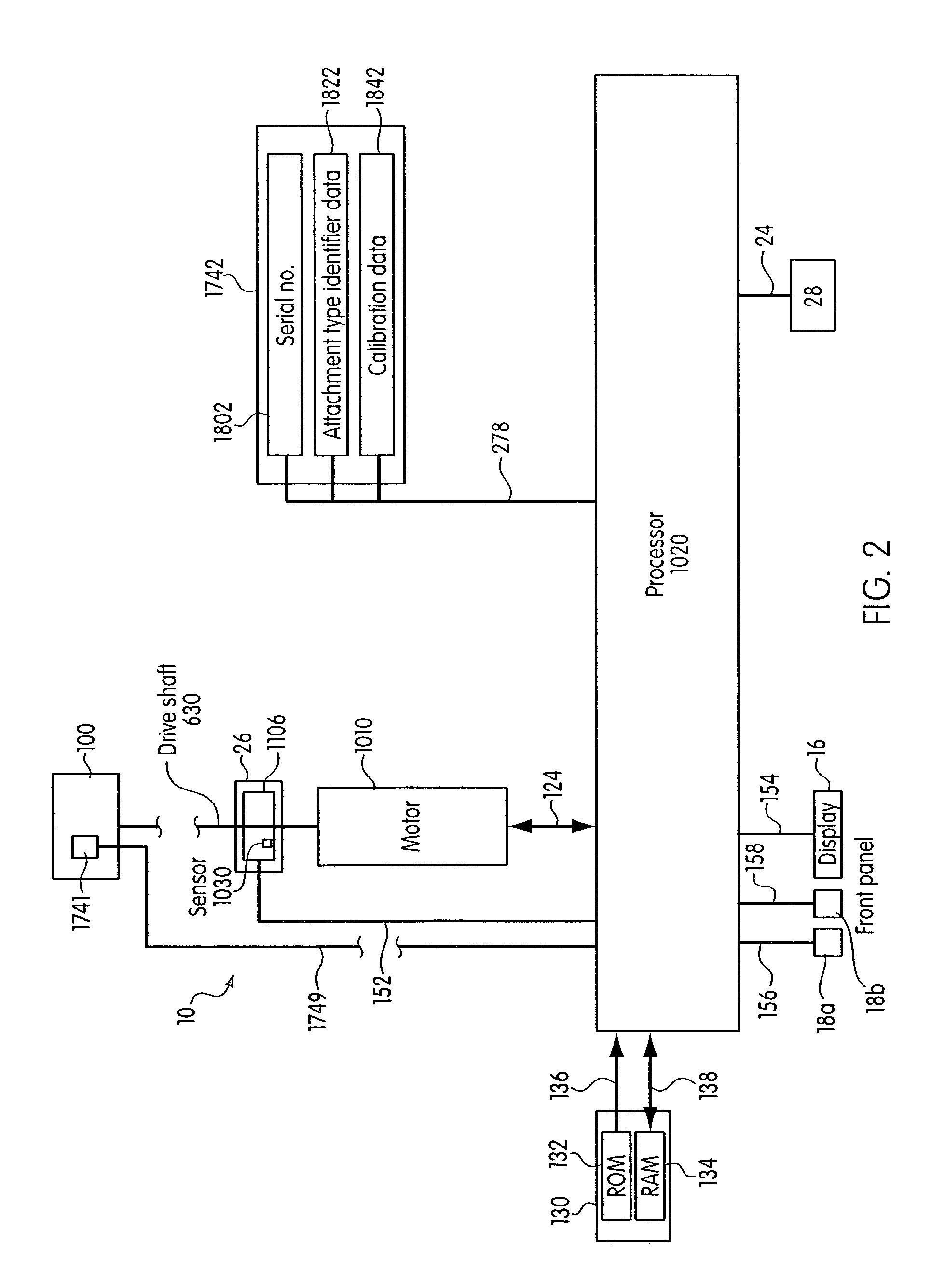
System and method for calibrating a surgical instrument
A calibration system for a surgical instrument. The calibration system includes an actuator, such as a motor system and a flexible shaft. The calibration system also includes a surgical instrument actuatable by the actuator. The calibration system also include calibration data corresponding to the surgical instrument. A processor is configured to process the calibration data for determining a position of the surgical instrument. The calibration system may include a sensor configured to provide a signal corresponding to a movement of the actuator, the processor being further configured to process the signal for determining a position of the surgical instrument.
Owner:TYCO HEALTHCARE GRP LP
Activated polymer articulated instruments and methods of insertion
An electro-polymeric articulated endoscope and method of insertion are described herein. A steerable endoscope having a segmented, elongated body with a manually or selectively steerable distal portion and an automatically controlled proximal portion can be articulated by electro-polymeric materials. These materials are configured to mechanically contract or expand in the presence of a stimulus, such as an electrical field. Adjacent segments of the endoscope can be articulated using the electro-polymeric material by inducing relative differences in size or length of the material when placed near or around the outer periphery along a portion of the endoscope.
Owner:INTUITIVE SURGICAL
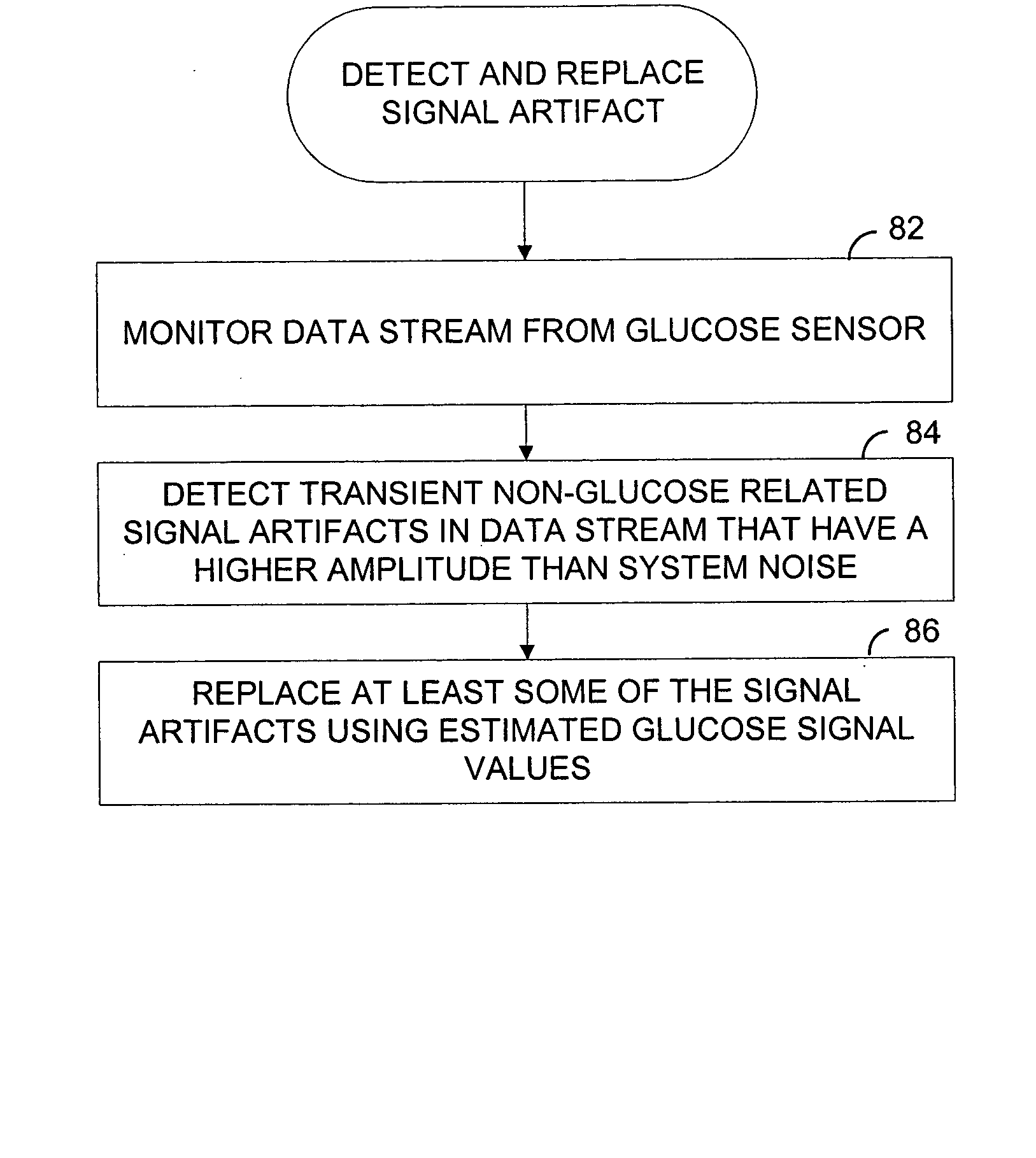
Systems and methods for replacing signal artifacts in a glucose sensor data stream
ActiveUS20050043598A1Accurate detectionAccurate replacementCatheterTemperature sensorsRate limitingData stream
Systems and methods for minimizing or eliminating transient non-glucose related signal noise due to non-glucose rate limiting phenomenon such as ischemia, pH changes, temperatures changes, and the like. The system monitors a data stream from a glucose sensor and detects signal artifacts that have higher amplitude than electronic or diffusion-related system noise. The system replaces some or the entire data stream continually or intermittently including signal estimation methods that particularly address transient signal artifacts. The system is also capable of detecting the severity of the signal artifacts and selectively applying one or more signal estimation algorithm factors responsive to the severity of the signal artifacts, which includes selectively applying distinct sets of parameters to a signal estimation algorithm or selectively applying distinct signal estimation algorithms.
Owner:DEXCOM
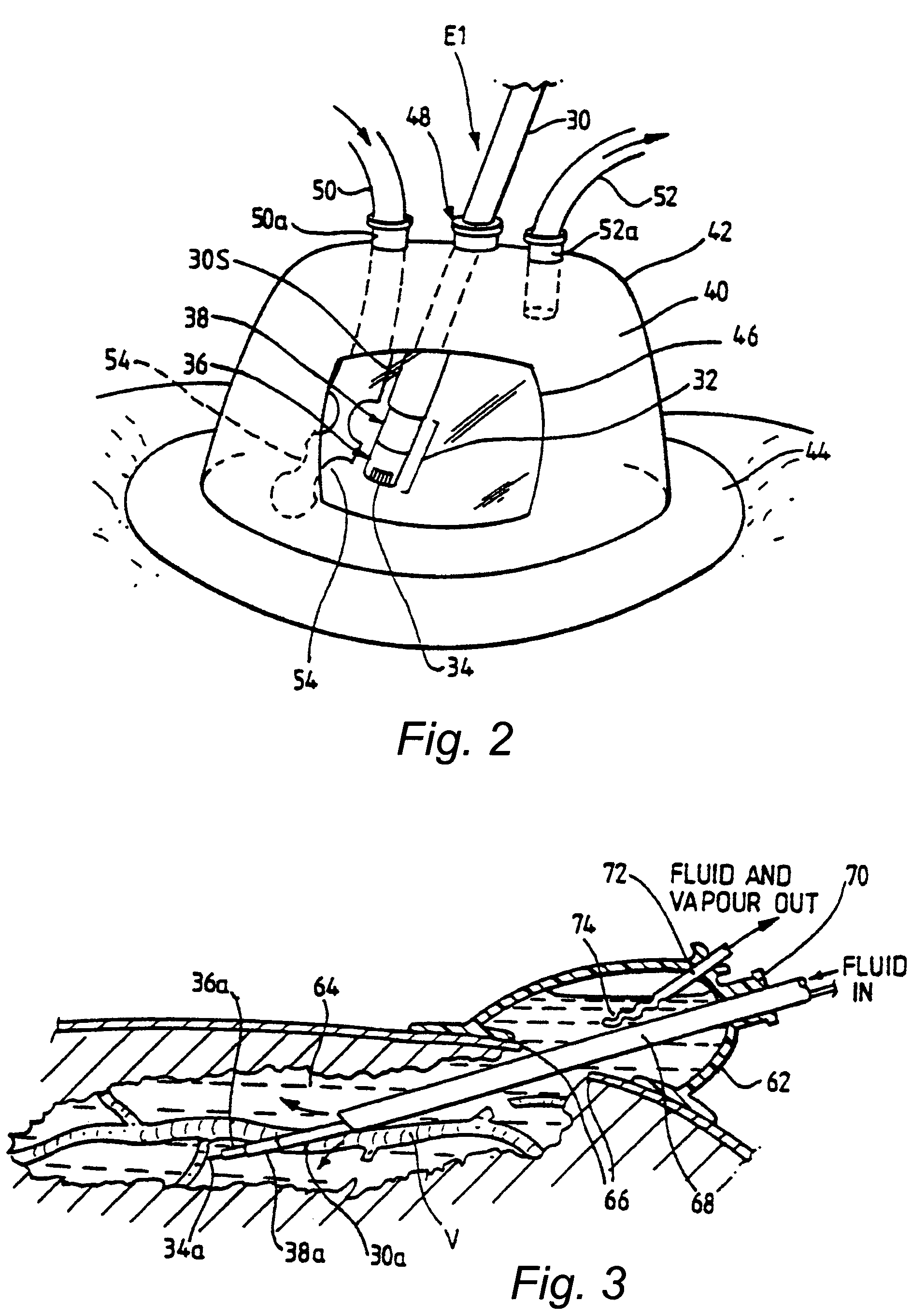
Fluid-assisted medical devices, systems and methods
Surgical devices, systems and methods for treating tissue are provided. An exemplary surgical device comprises a tip portion including first and second jaws each having a tissue grasping surface, at least one of the jaws being movable toward the other jaw. The tissue grasping surface of each jaw has includes an electrically insulative surface. The device also includes first and second electrodes connectable to different terminals of an RF generator to generate electrical current flow therebetween, with each of the electrodes having an electrode surface. One of the electrode surfaces is located on one of the jaws separated from one edge of the tissue grasping surface, and the other of the electrode surfaces is located on one or the other of the jaws separated from the other edge of the tissue grasping surface. The device also includes at least one fluid passage being connectable to a fluid source.
Owner:MEDTRONIC ADVANCED ENERGY
Endoscope having multiple working segments
InactiveUS7029435B2Improve performanceEasy to insertSuture equipmentsCannulasEngineeringIntroduction procedure
A flexible fiberoptic endoscope has an insertion member with a distal end portion split longitudinally into a plurality of independently operable working segments each provided with at least one longitudinally extending working channel. Visualization optics are provided in the working segments or a separate central segment surrounded by the working segments. A sheath temporarily joins the working segments to one another during an introduction procedure. The sheath is removed upon insertion of the endoscope, so that the working segments may be / separated from one another and independently maneuvered via proximal control heads to facilitate the performance of an endoscopic surgical procedure.
Owner:GRANIT MEDICAL INNOVATION
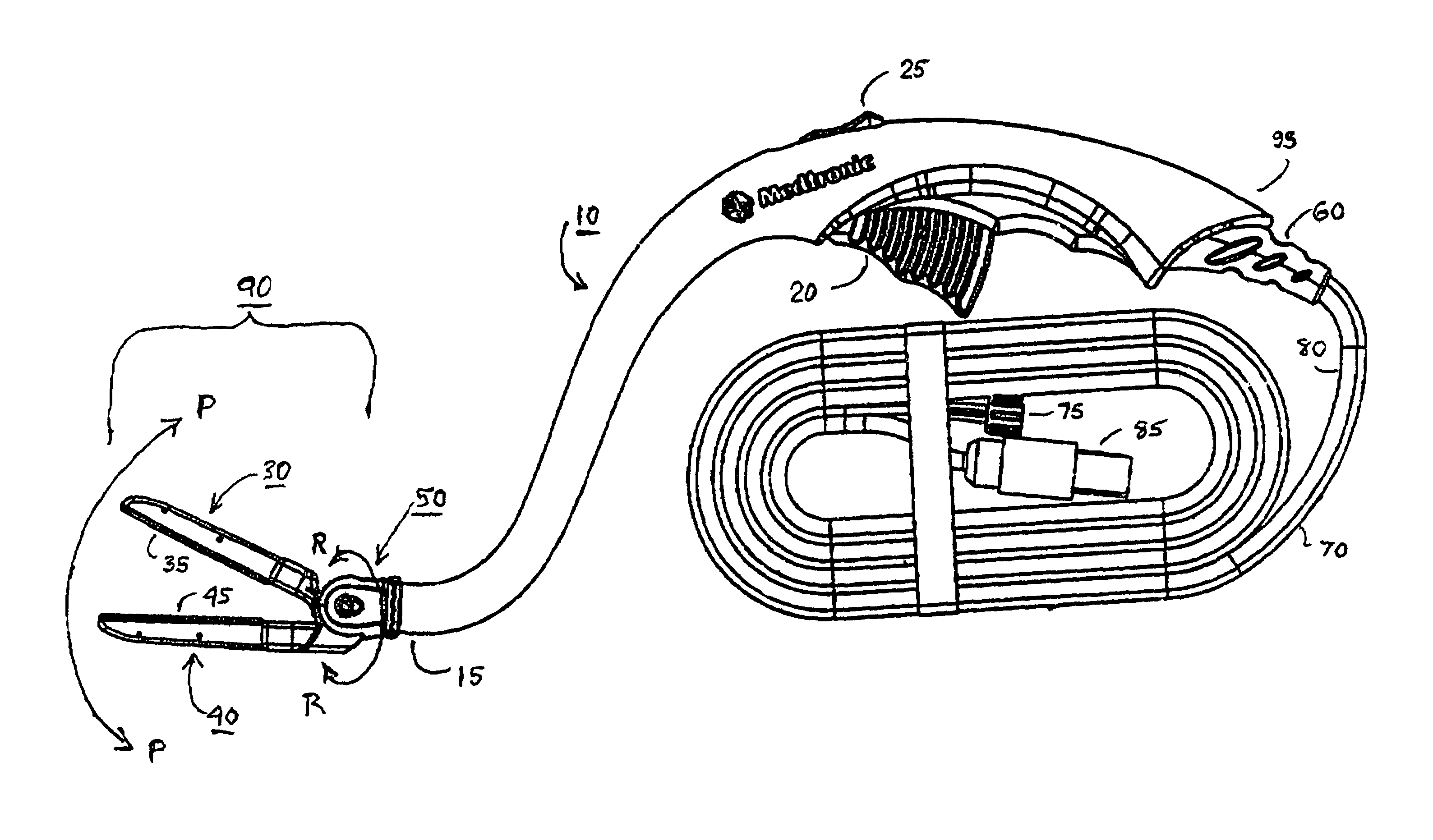
Electrosurgical hemostat
InactiveUS7083620B2Reduce usageImprove performanceCatheterSurgical instruments for heatingDetentEffective treatment
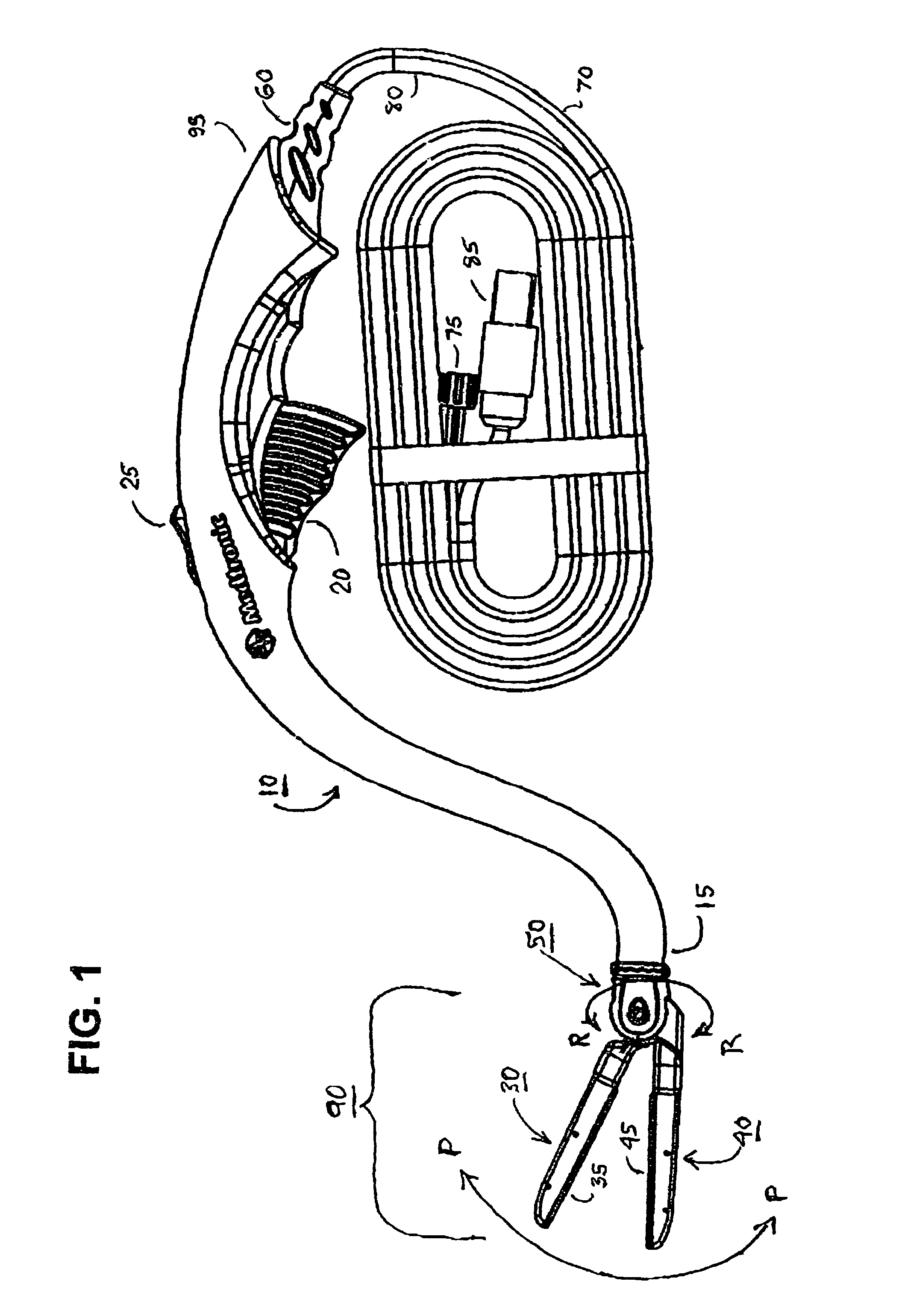
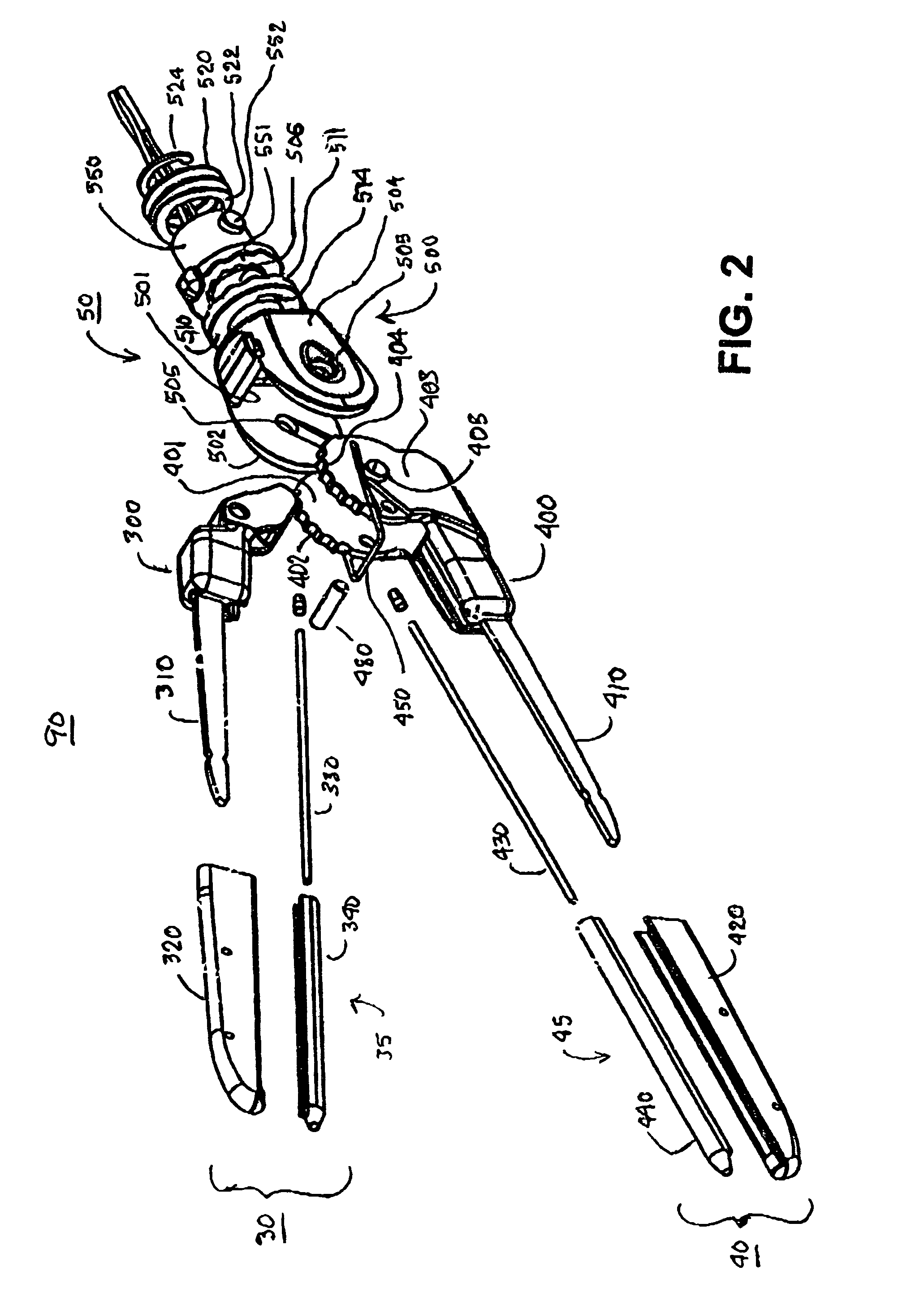
A hemostat-type device for ablative treatment of tissue, particularly for treatment of atrial fibrillation, is constructed with features that provide easy and effective treatment. A swiveling head assembly can allow the jaws to be adjusted in pitch and roll. Malleable jaws can permit curved lesion shapes. A locking detent can secure the jaws in a closed position during the procedure. An illuminated indicator provides confirmation that the device is operating. A fluid delivery system simplifies irrigated ablation procedures.
Owner:MEDTRONIC INC
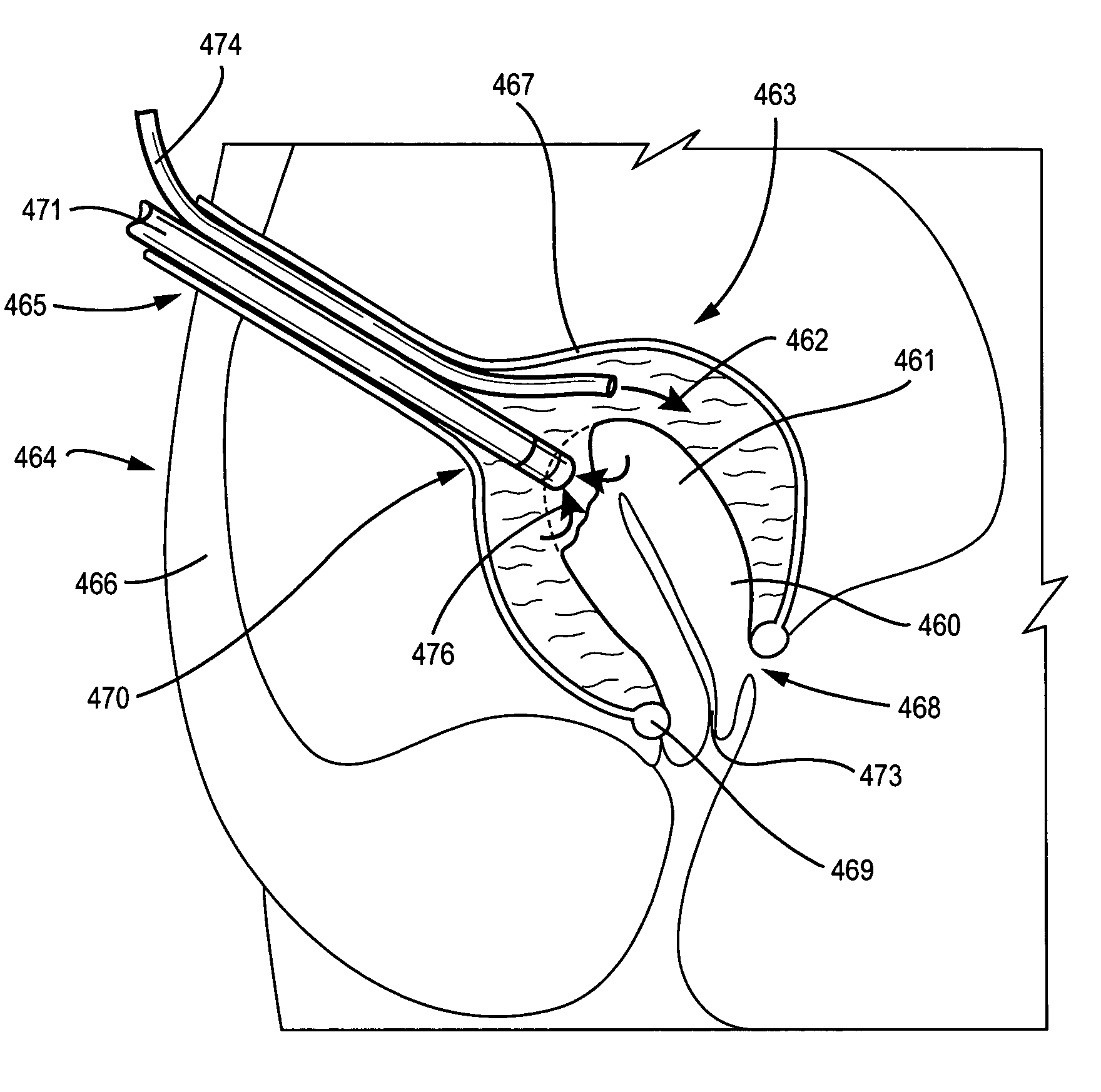
Fundoplication apparatus and method
An endoscopic device for the partial fundoplication for the treatment of GERD, comprises: a) a distal bending portion and a flexible portion suitable to be positioned in extended shape within the esophagus of a subject; b) a positioning assembly comprising two separate elements, one of which is located on said distal bending portion, and the other on said flexible portion; c) a stapling assembly comprising a staple ejecting device, wherein said staple ejecting device is located on either said bending portion or on said flexible portion, said staple ejecting devices being in working positioned relationship when said two separate elements of said positioning assembly are aligned; and d) circuitry for determining when said two separate elements of said positioning assembly are aligned.
Owner:MEDIGUS LTD
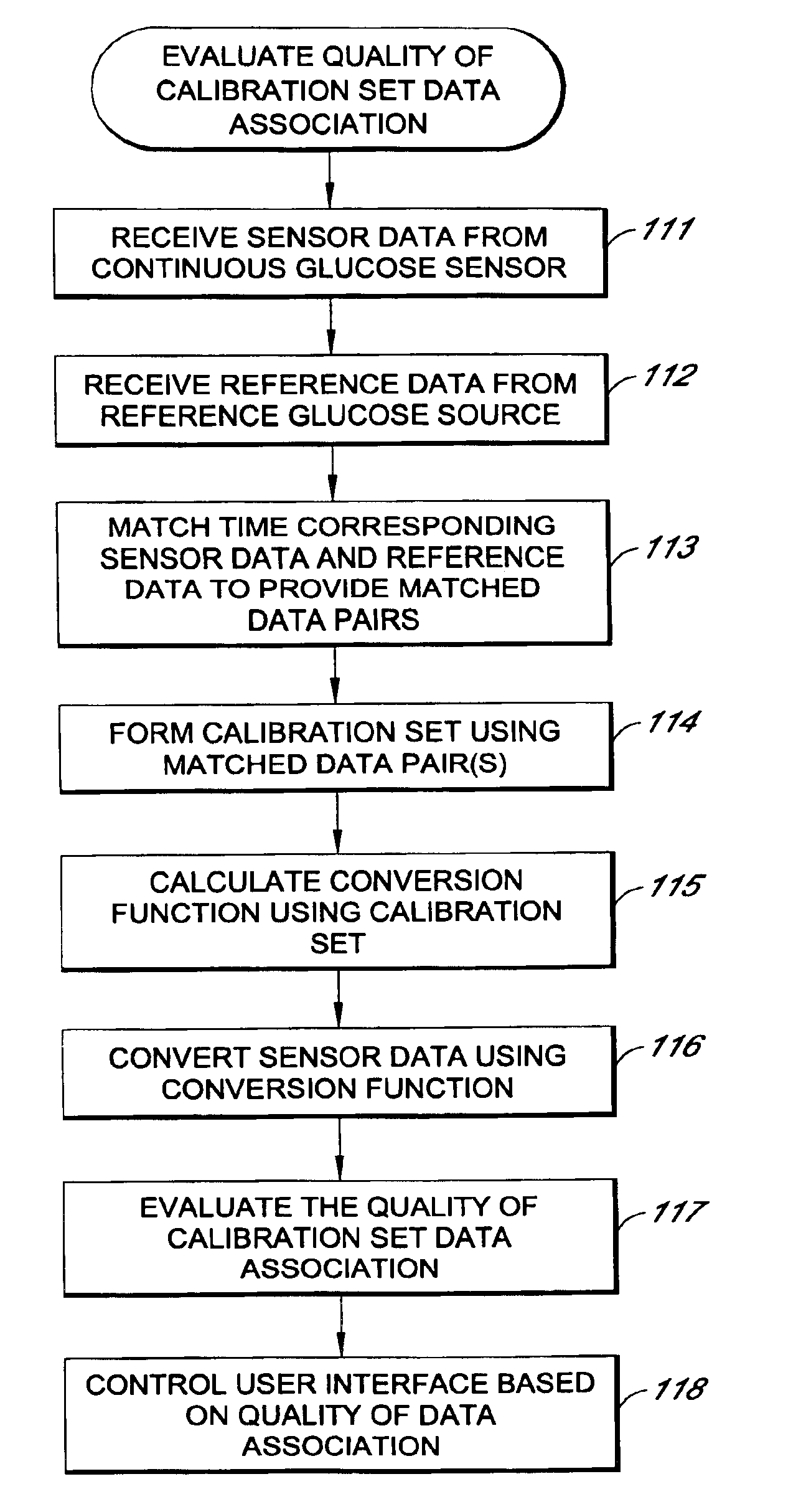
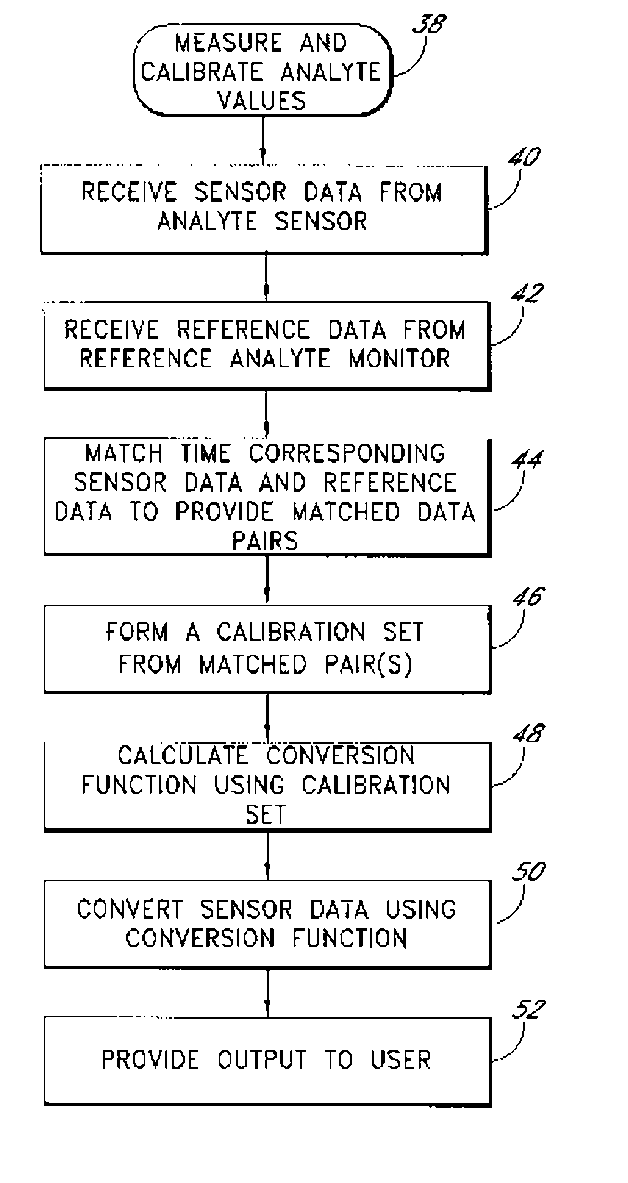
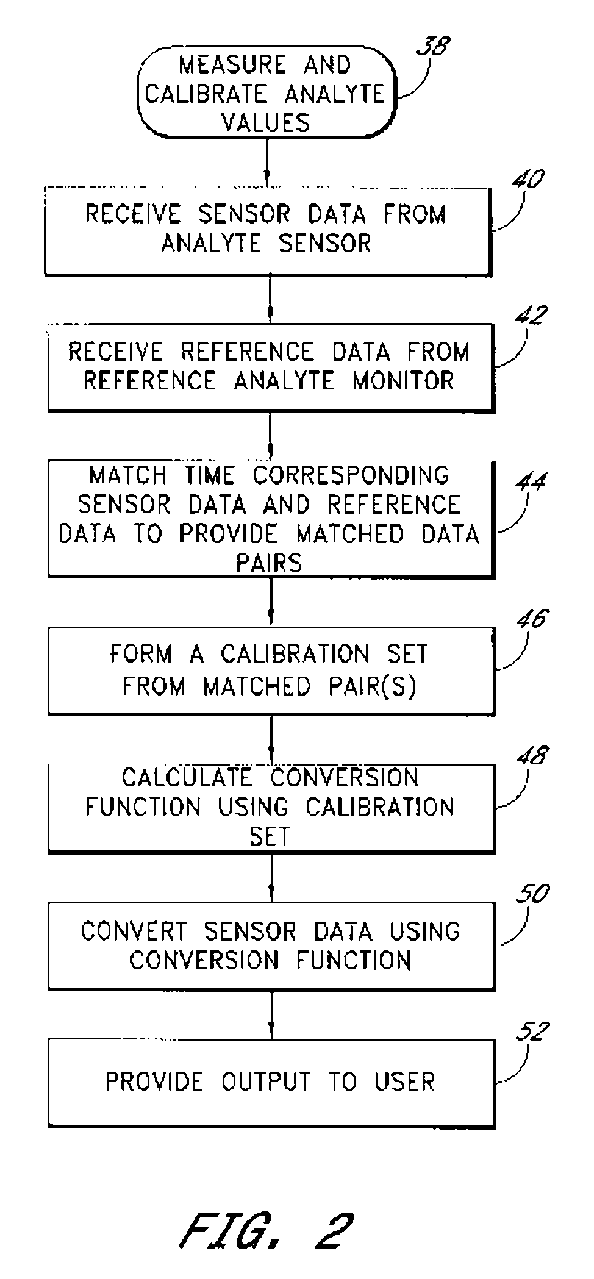
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
Systems and methods for manufacture of an analyte-measuring device including a membrane system
InactiveUS20060015020A1Minimized in sizeMaximize adhesionLaminationLamination apparatusAnalyteBiomedical engineering
Abstract of the DisclosureSystems and methods for manufacture of an analyte-measuring device, including adhering a membrane system that allows the passage of the analyte therethrough to a sensing mechanism. The implantable analyte-measuring device includes a body formed from a material that is substantially similar to the membrane system so as to enable sufficiently strong adhesion therebetween, which enables a sufficiently strong adhesive joint capable of withstanding in vivo cellular forces. In some embodiments, the device body includes an insert to which the membrane system is adhered, wherein the insert is formed from a material substantially similar to the membrane system to enable strong adhesion therebetween. The analyte-measuring device is designed with optimized device sizing and maximum membrane adhesion and longevity to enable controlled transport of analytes through the membrane system in vivo with improved device performance.
Owner:DEXCOM
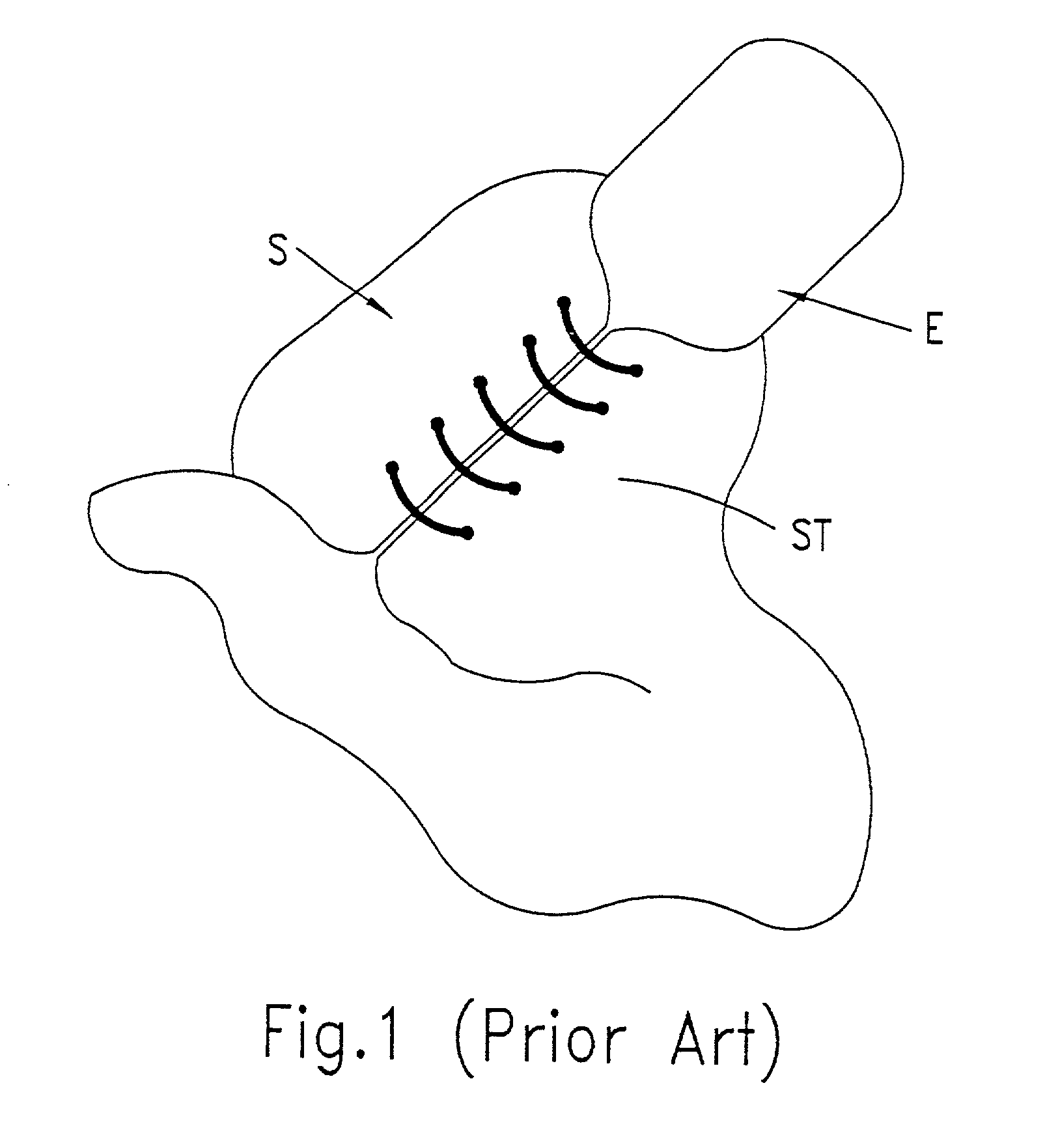
Drug releasing anastomosis devices and methods for treating anastomotic sites
ActiveUS7108701B2Reduce drug toxicityGood curative effectSuture equipmentsSurgical needlesBiological bodyReady to use
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned.
Owner:WYETH
Systems and methods for processing analyte sensor data
The present invention relates generally to systems and methods for measuring an analyte in a host. More particularly, the present invention relates to systems and methods for processing sensor data, including calculating a rate of change of sensor data and / or determining an acceptability of sensor or reference data.
Owner:DEXCOM
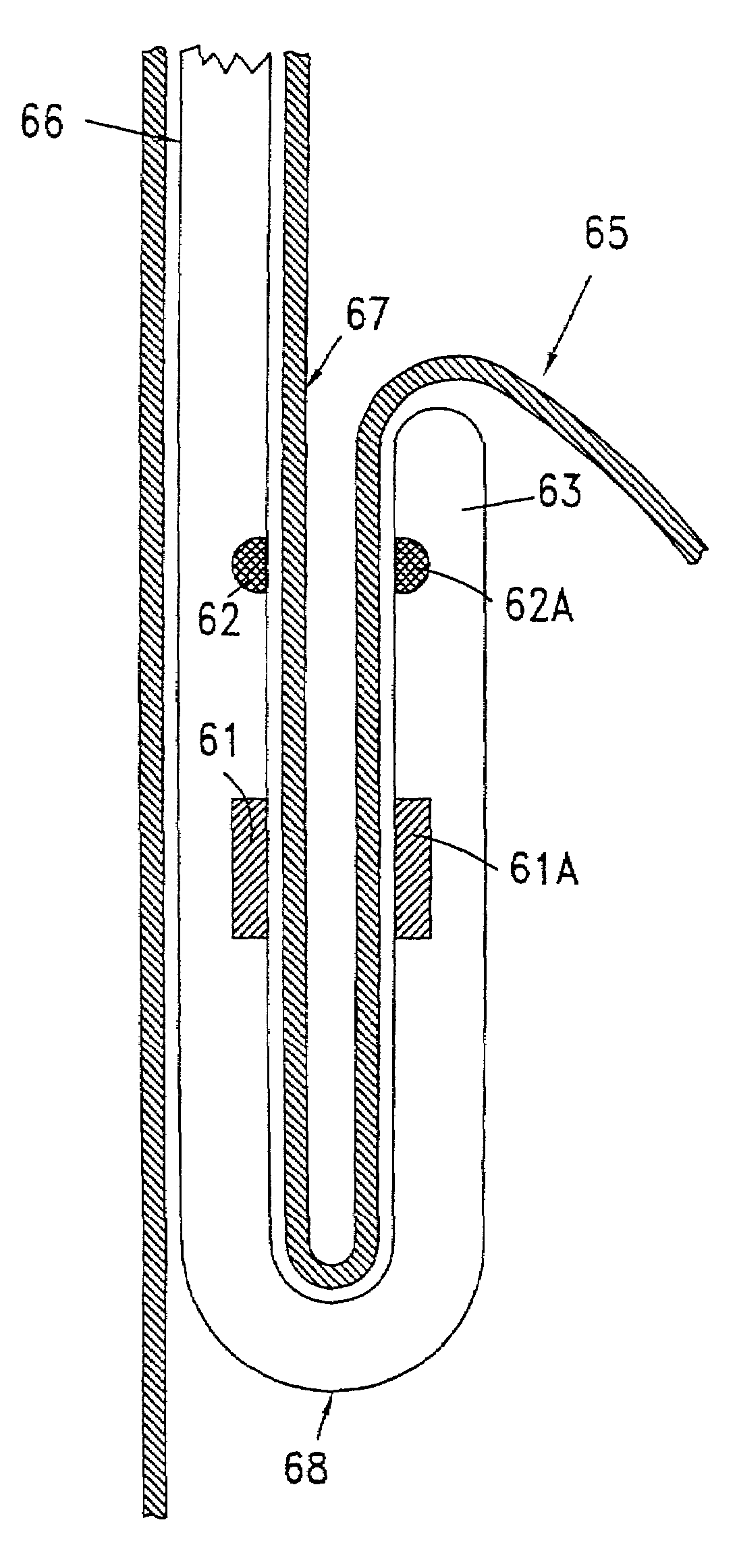
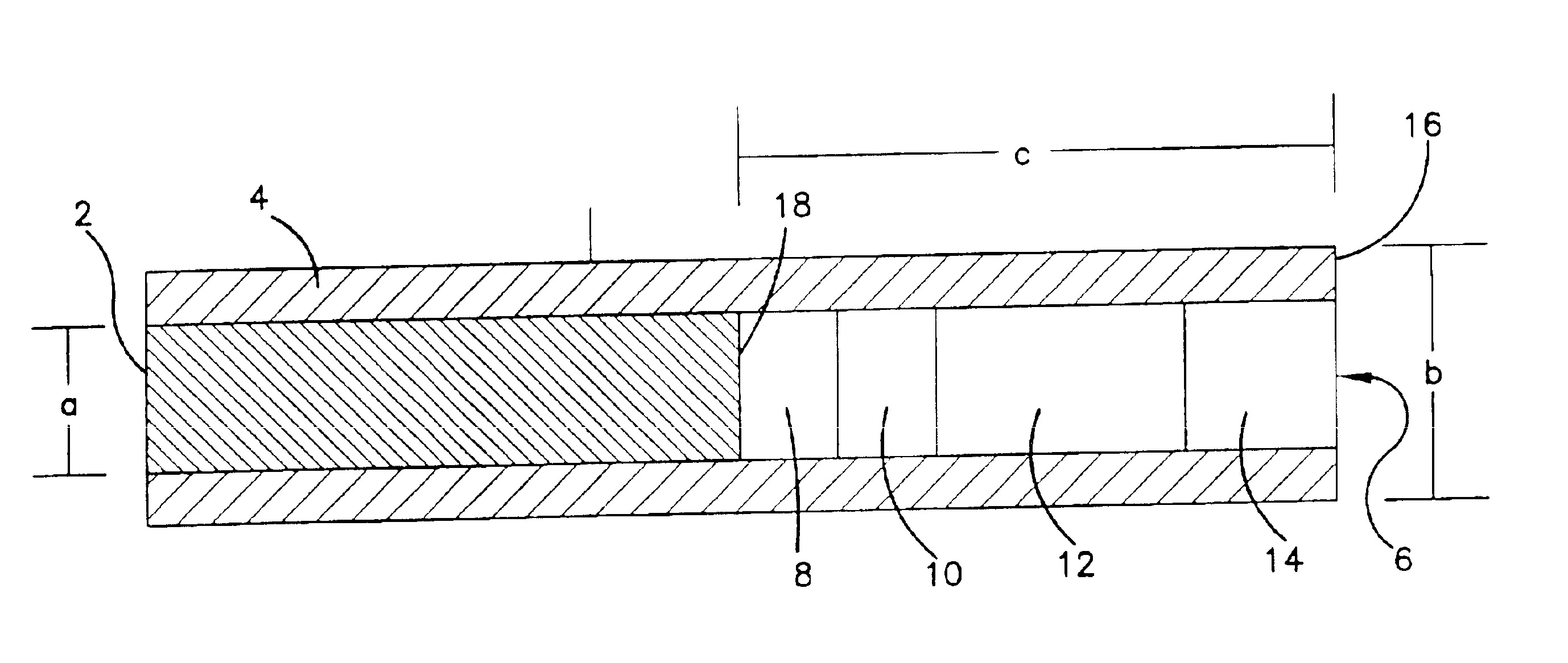
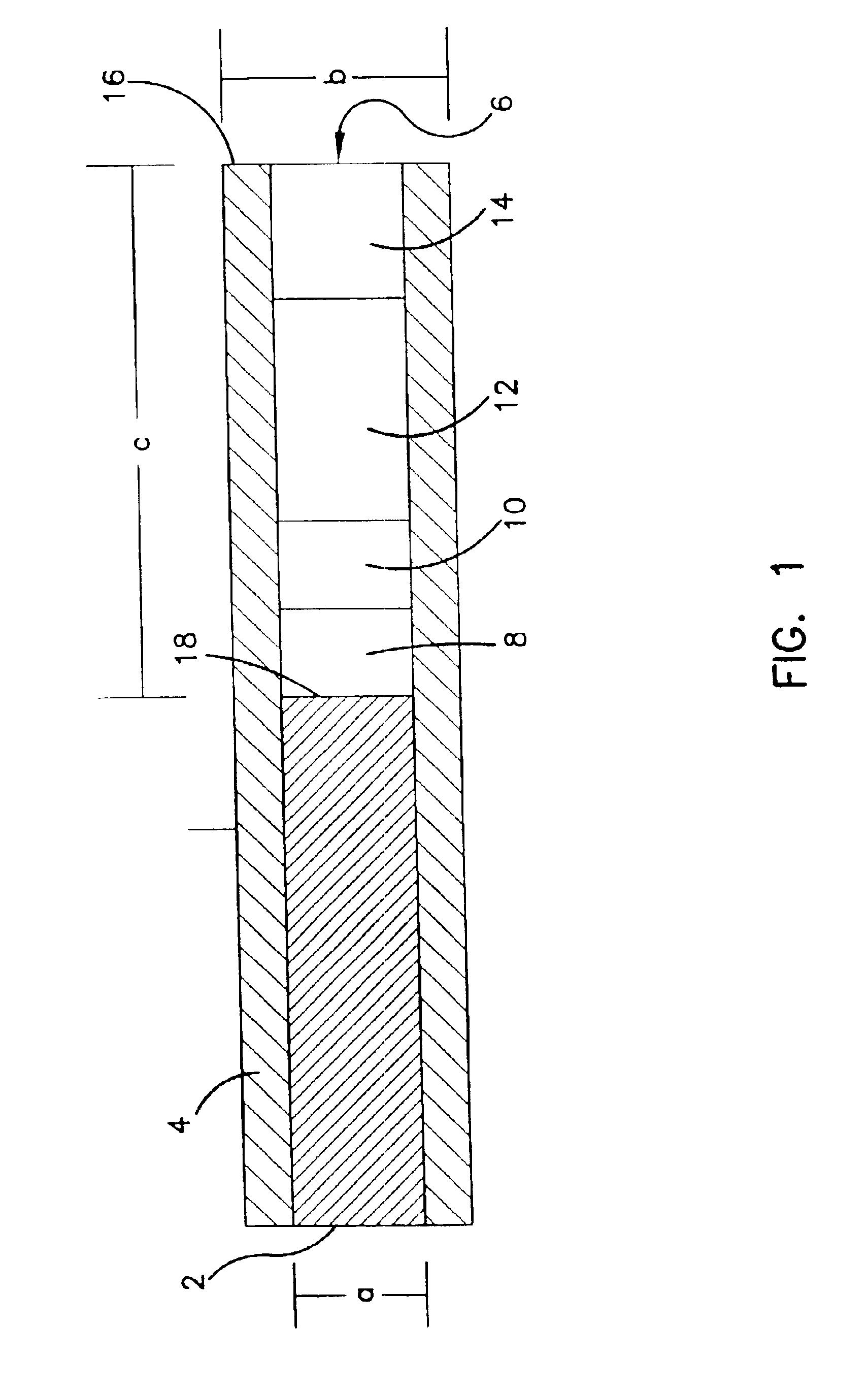
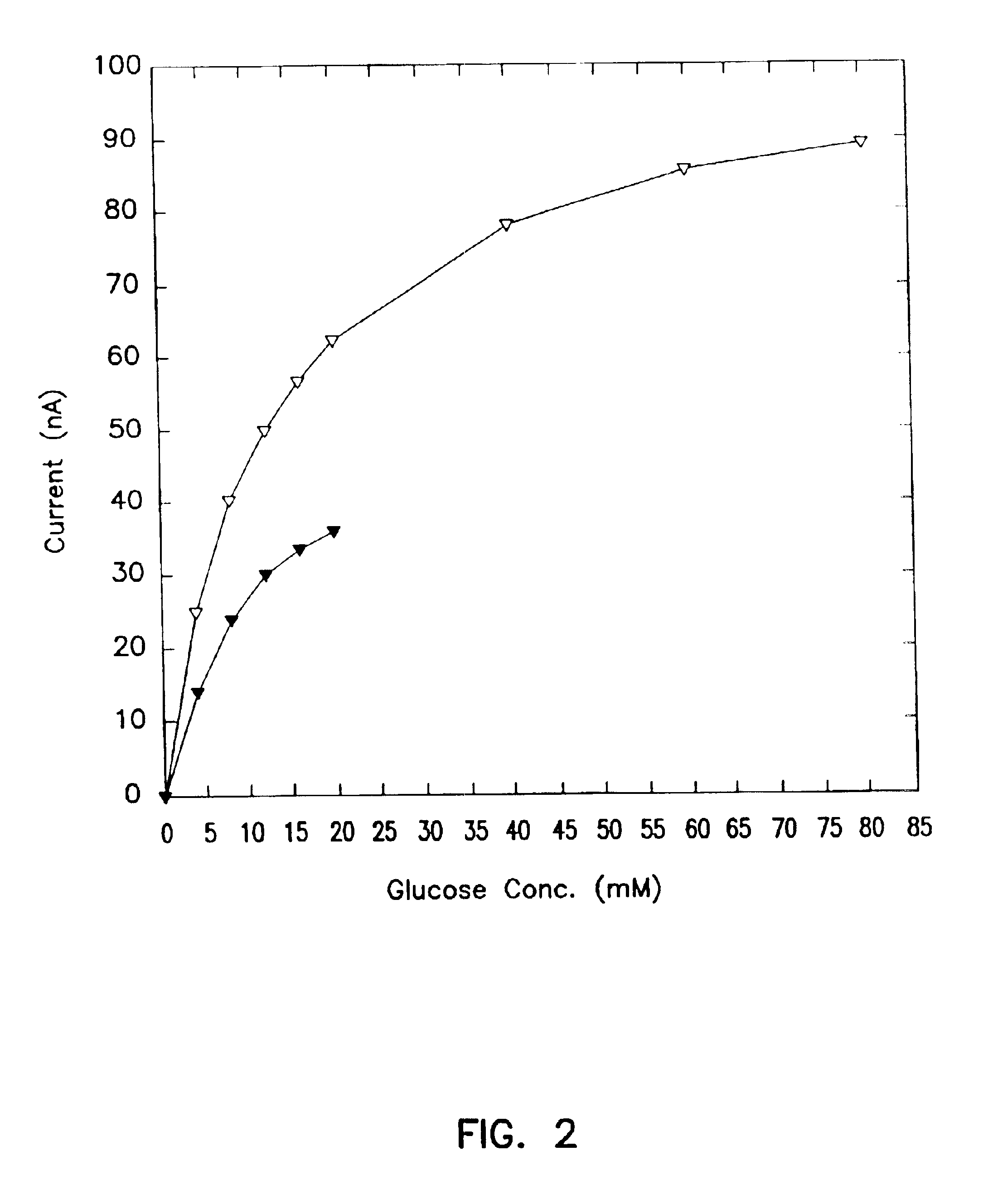
Subcutaneous glucose electrode
InactiveUS6881551B2Reduce transportationAccurate measurementBioreactor/fermenter combinationsBiological substance pretreatmentsConcentrations glucosePolyamide
A small diameter flexible electrode designed for subcutaneous in vivo amperometric monitoring of glucose is described. The electrode is designed to allow “one-point” in vivo calibration, i.e., to have zero output current at zero glucose concentration, even in the presence of other electroreactive species of serum or blood. The electrode is preferably three or four-layered, with the layers serially deposited within a recess upon the tip of a polyamide insulated gold wire. A first glucose concentration-to-current transducing layer is overcoated with an electrically insulating and glucose flux limiting layer (second layer) on which, optionally, an immobilized interference-eliminating horseradish peroxidase based film is deposited (third layer). An outer (fourth) layer is biocompatible.
Owner:THERASENSE
Signal processing for continuous analyte sensor
Abstract of the DisclosureSystems and methods for dynamically and intelligently estimating analyte data from a continuous analyte sensor, including receiving a data stream, selecting one of a plurality of algorithms, and employing the selected algorithm to estimate analyte values. Additional data processing includes evaluating the selected estimative algorithms, analyzing a variation of the estimated analyte values based on statistical, clinical, or physiological parameters, comparing the estimated analyte values with corresponding measure analyte values, and providing output to a user. Estimation can be used to compensate for time lag, match sensor data with corresponding reference data, warn of upcoming clinical risk, replace erroneous sensor data signals, and provide more timely analyte information encourage proactive behavior and preempt clinical risk.
Owner:DEXCOM
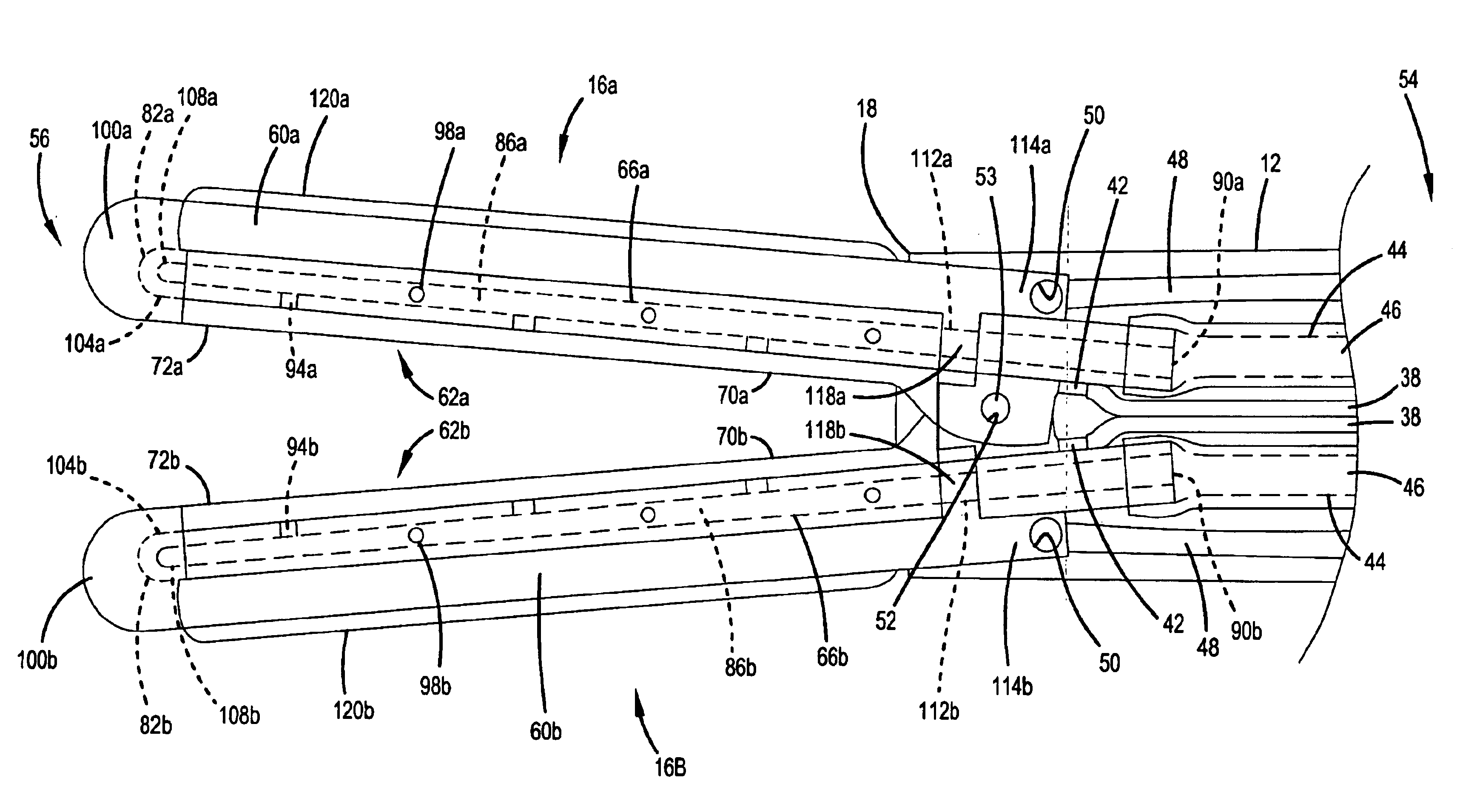
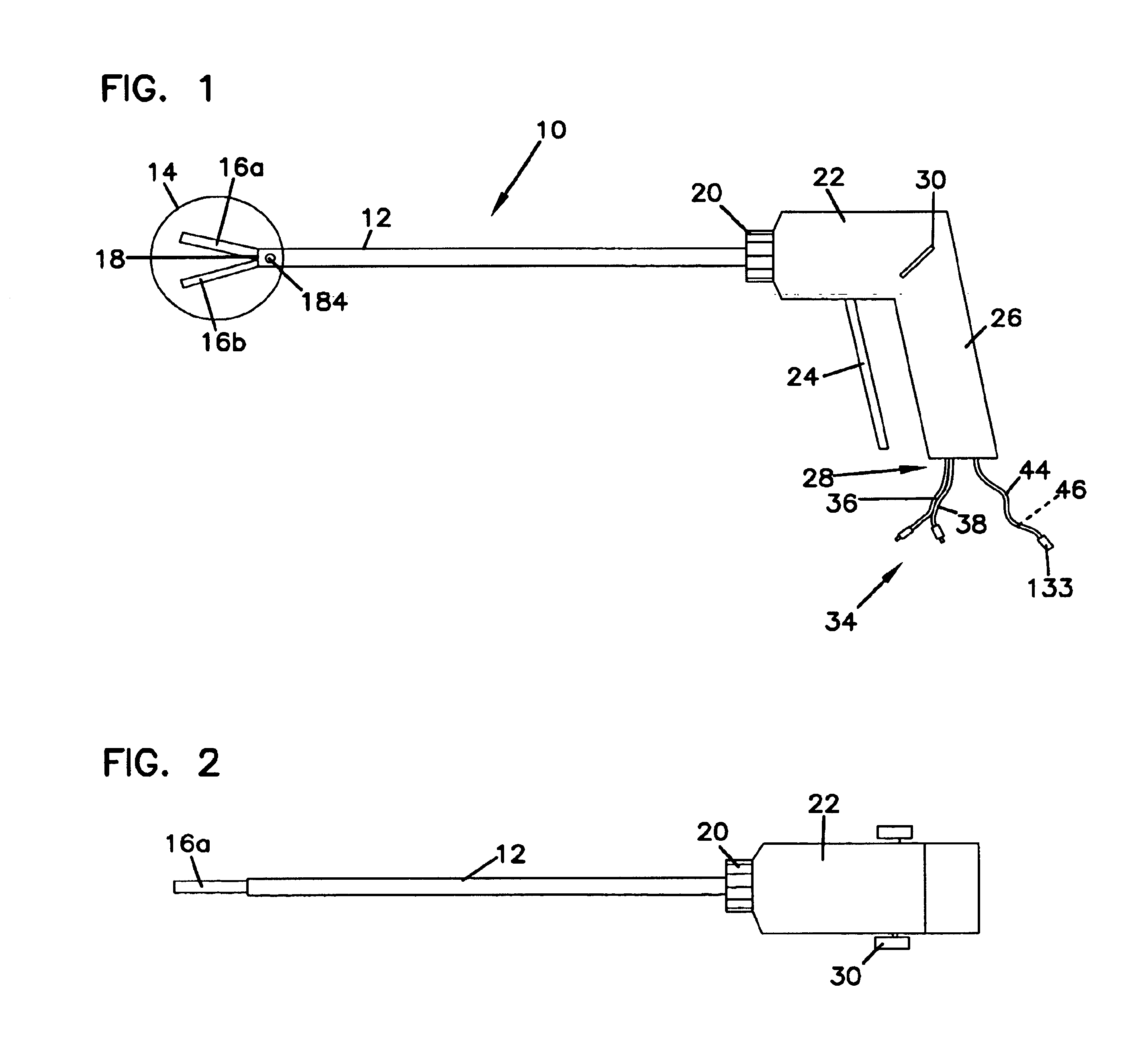
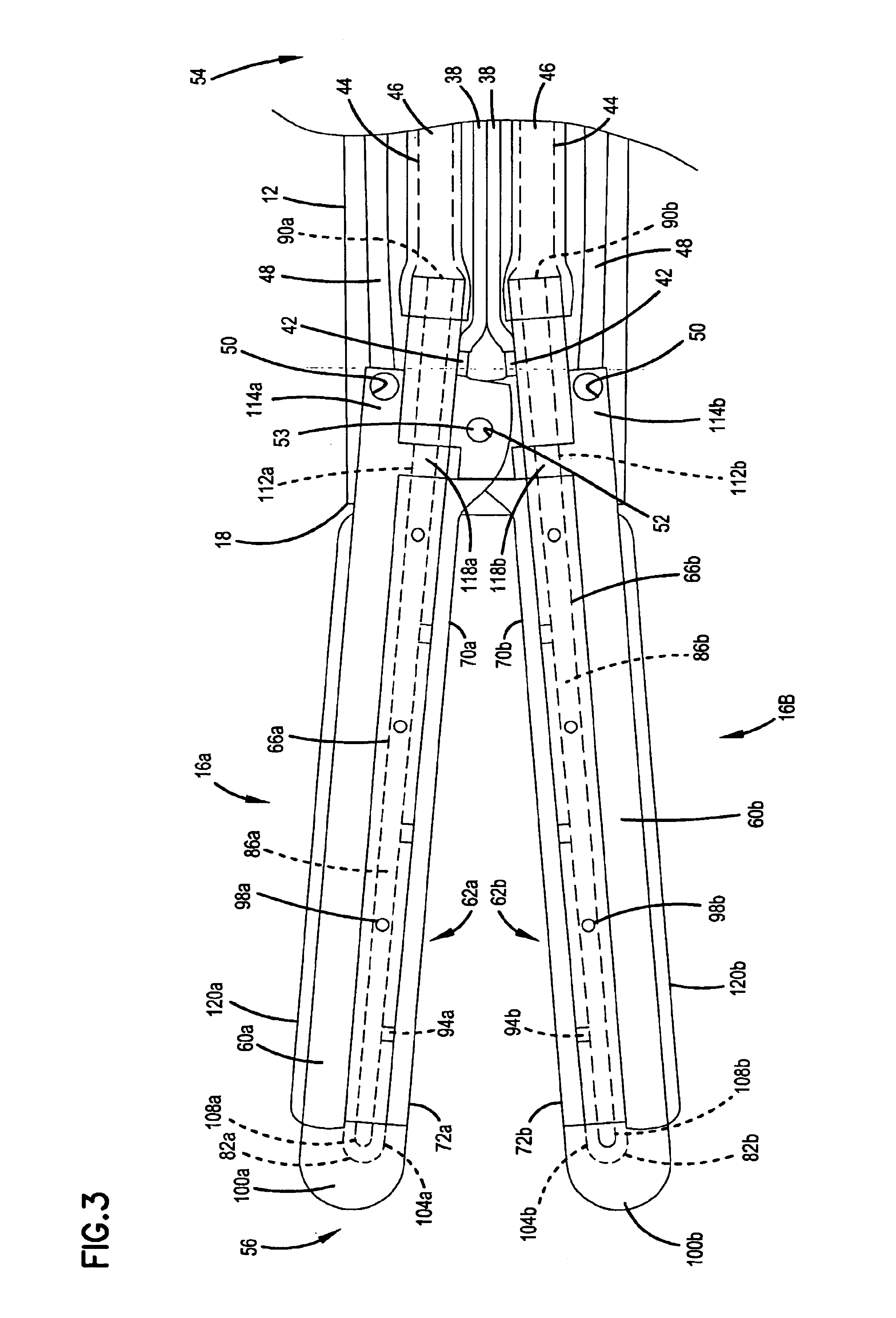
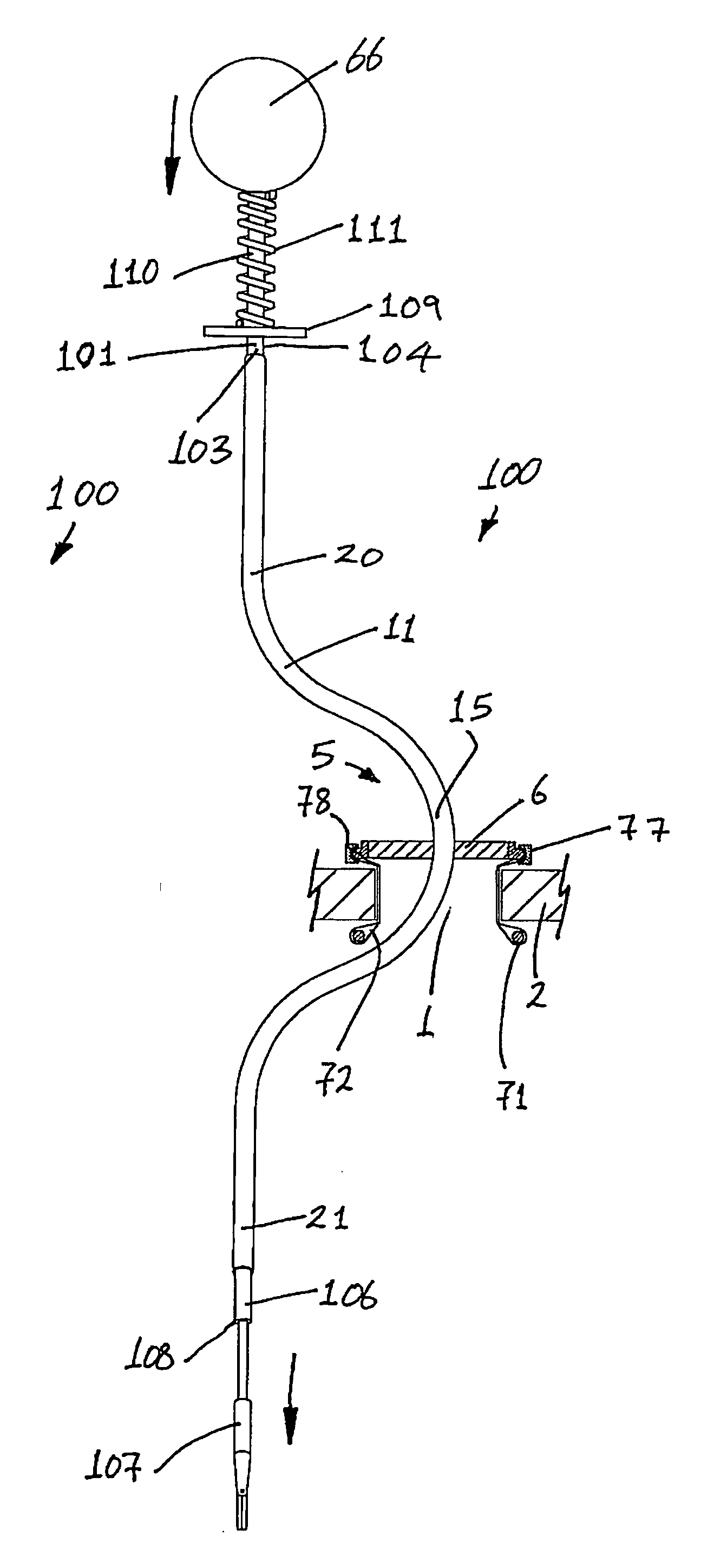
Surgical instrument
InactiveUS20070049966A1Maintain positionMinimise incisionSuture equipmentsCannulasActuatorAccess port
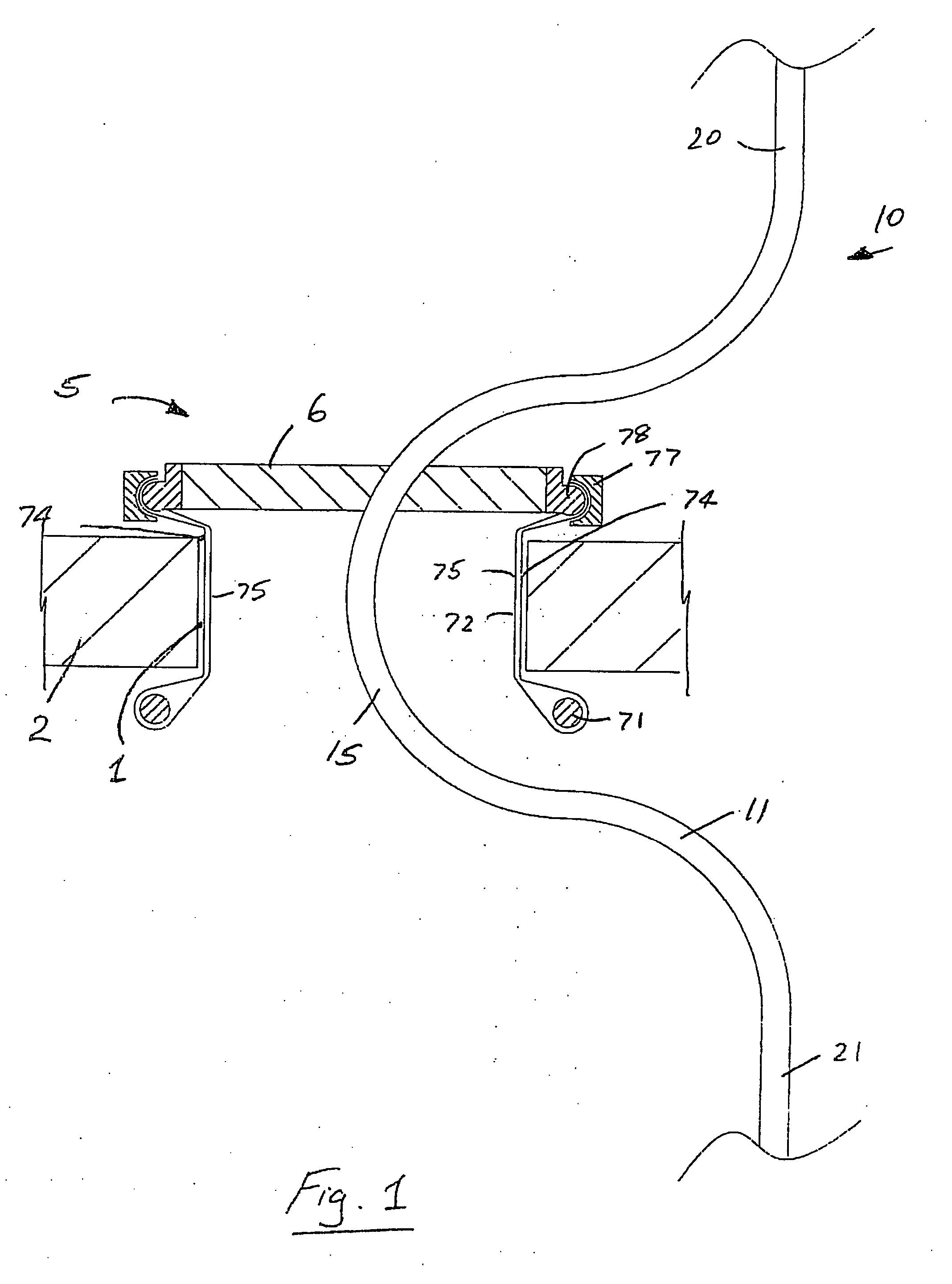
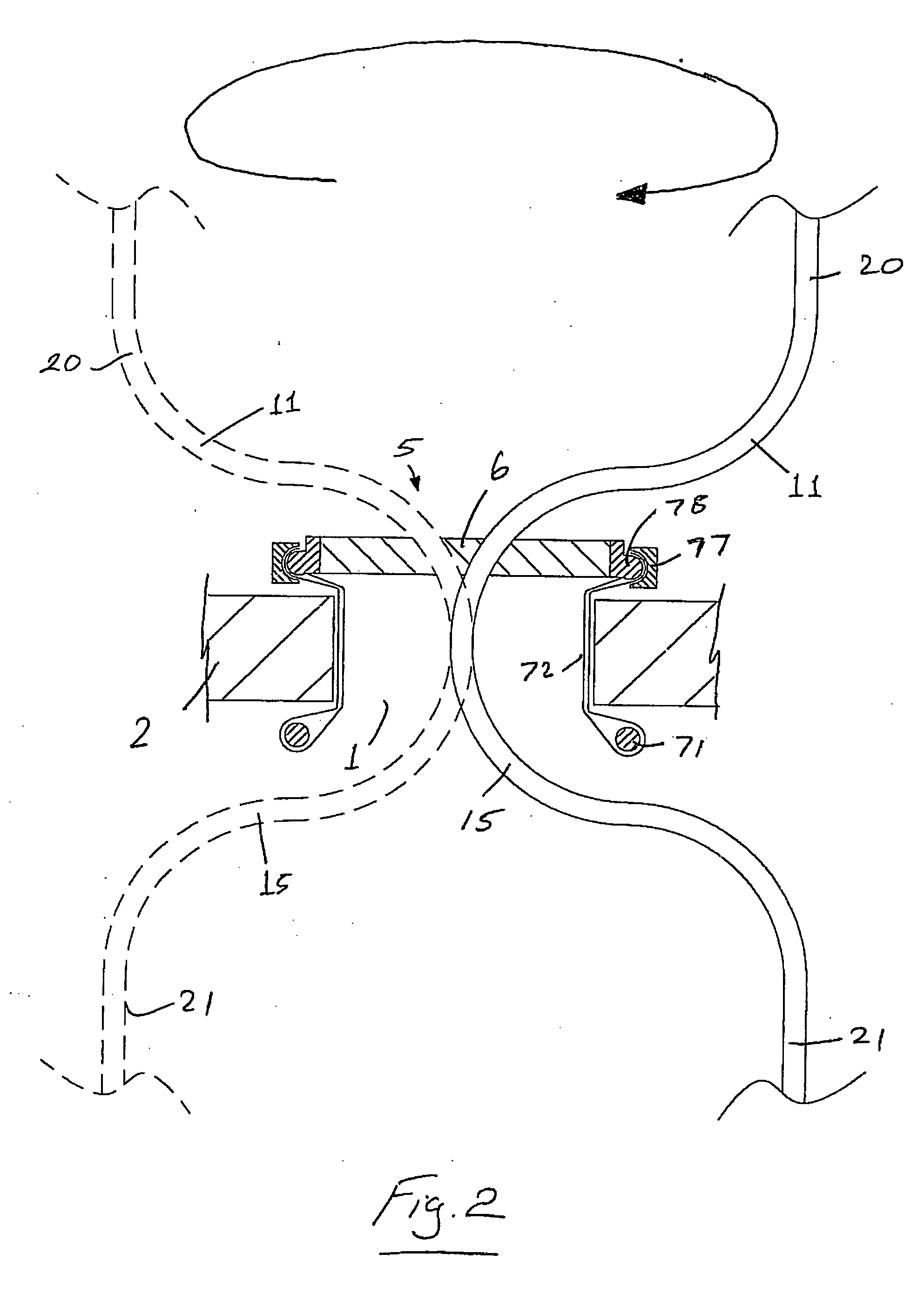
A surgical access system (100) comprises an access port (5), a rigid cannula having a shaft (11) and a laparoscopic surgical instrument (101). The access port (5) comprises a seal (6) and a retractor. The retractor comprises a distal O-ring (71), an outer proximal ring member (77), an inner proximal ring member (78) and a sleeve (72). The sleeve (72) extends distally from the inner proximal ring member (78) to the distal O-ring (71) in a first layer, is looped around the distal O-ring (71), and extends proximally in a second layer between the inner proximal ring member (78) and the outer proximal ring member (77). The instrument (101) comprises a shaft (103) with a rigid proximal region (104), a flexible intermediate region (105), and a rigid distal region (106). The instrument shaft (103) may be inserted through the cannula shaft (11). The instrument (101) has a rigid end effector (107) releasably coupled to the distal end (108) of the instrument shaft (103). An actuator (109) for actuating the end effector (107) is provided at the proximal end (110) of the instrument shaft (103). The actuator (109) is movable along the instrument shaft (103) parallel to the longitudinal axis of the instrument shaft (103).
Owner:ATROPOS LTD
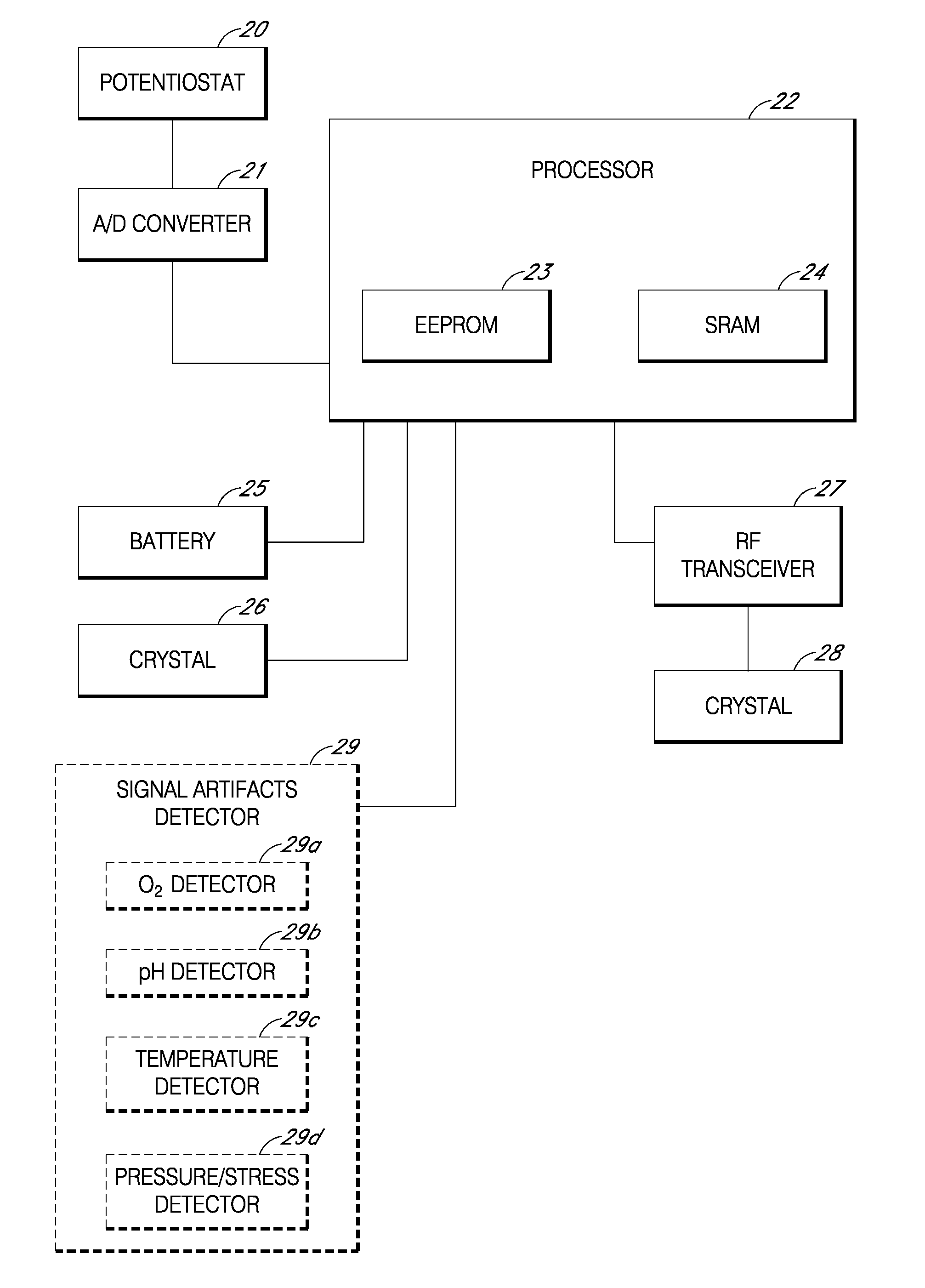
Remotely powered and remotely interrogated wireless digital sensor telemetry system
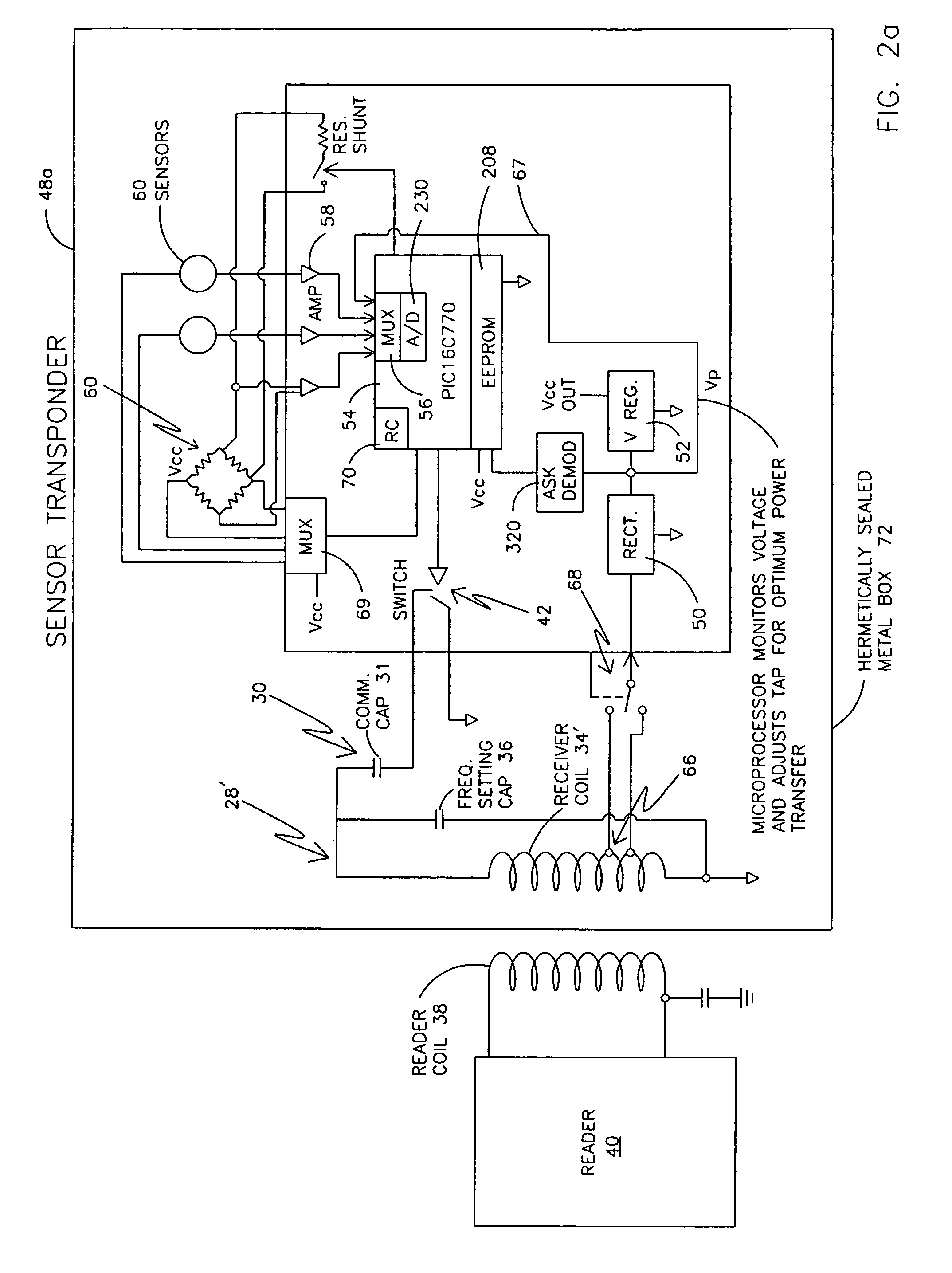
ActiveUS7256695B2More powerElectric signal transmission systemsElectrotherapyElectronic systemsPower flow
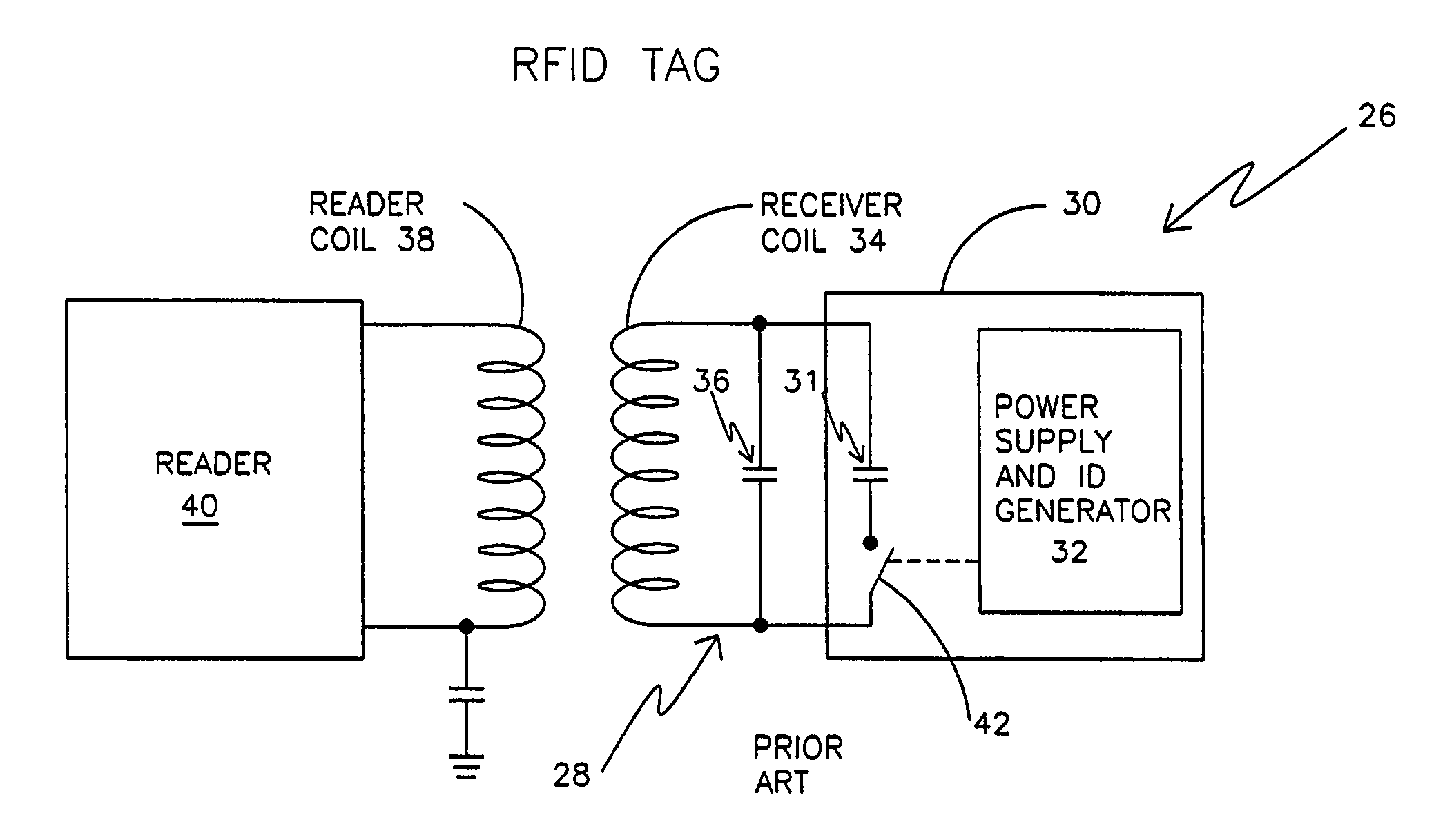
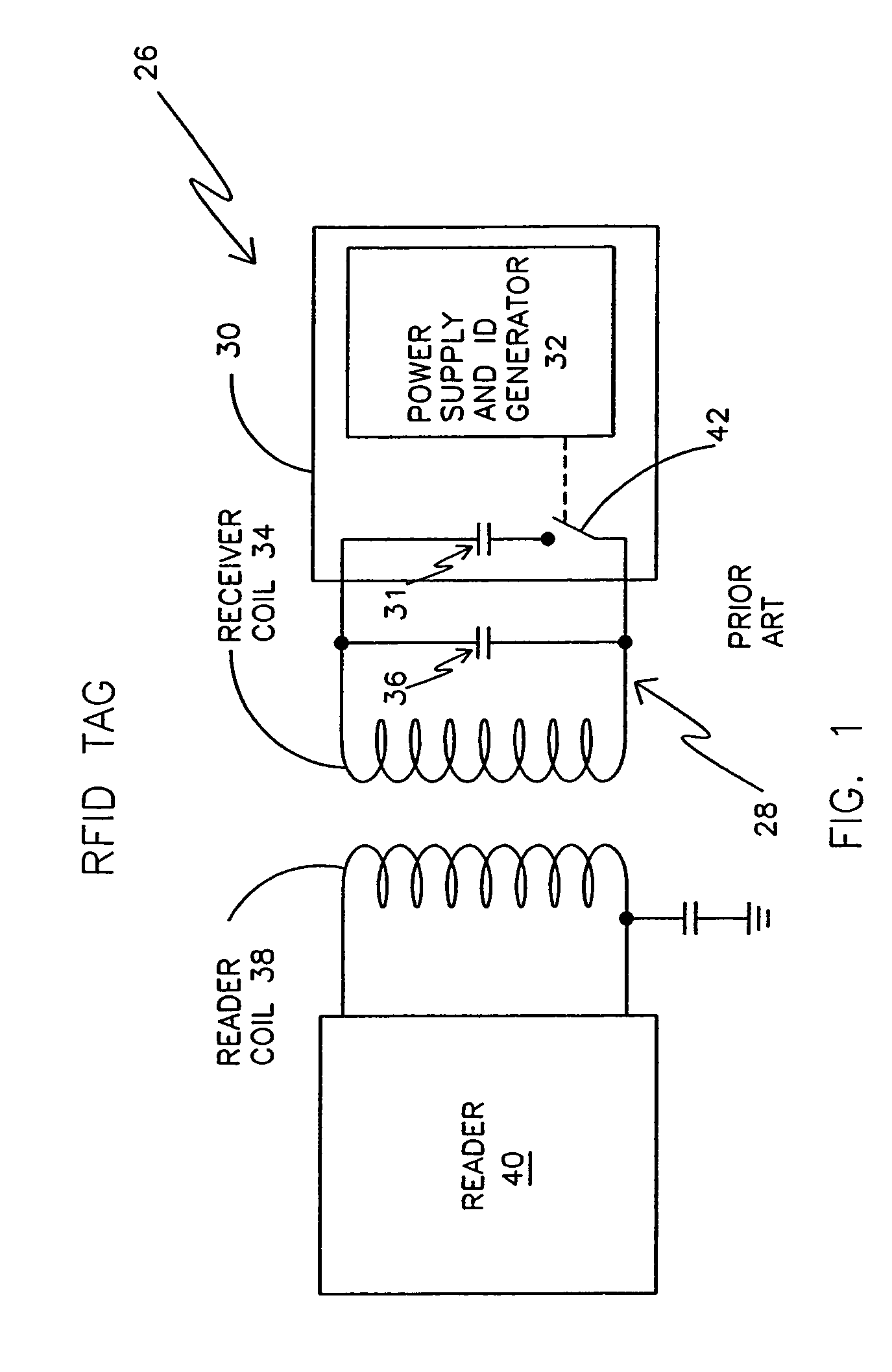
An electronic system includes a reader and a remotely powered and remotely interrogated sensor transponder. The sensor transponder includes a coil or an antenna, a switched reactance circuit, a processor, and a sensor. The sensor transponder receives power radiated from the reader to the coil or antenna. The sensor uses the power for sensing. The sensor transponder is capable of processing sensor data in the processor and transmitting the sensor data to the reader using the switched reactance circuit. In one embodiment, the receiver coil or antenna is part of a resonant tank circuit which includes an impedance matching circuit. The impedance matching circuit is connected to the receiver coil or antenna to provide greater current to the sensor or other power-using device than would be available to the sensor or other power-using device if the sensor or other power-using device were connected between the first and second end. The impedance matching circuit can be two or more taps to the coil or antenna.
Owner:LORD CORP
Methods and systems for inserting a transcutaneous analyte sensor
The present invention relates generally to systems and methods for measuring an analyte in a host. More particularly, the present invention relates to systems and methods for transcutaneous measurement of glucose in a host.
Owner:DEXCOM INC
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
Articulation mechanism for medical devices
An improved articulating device for use with a medical insertion instrument comprising in part a first tube having a plurality of ribs defining a plurality of bending segments, a second tube axially disposed within the first tube, and means for transmitting an axial deflecting pull load. Such device has improved controlled for positioning an end of the device at a selected position within the body. The articulating device has a generally constant moment of inertia and a polar moment of inertia that generally decreases from its proximal to distal end.
Owner:EDWARDS LIFESCIENCES CORP
Surgical instrument
The surgical instrument includes a distal tool, a rigid or flexible elongated shaft that supports the distal tool, and a proximal handle or control member, where the tool and the handle are coupled to the respective distal and proximal ends of the elongated shaft via distal and proximal bendable motion members. Actuation means extends between said distal and proximal members whereby any deflection of said control handle with respect to said elongated instrument shaft causes a corresponding bending of said distal motion member for control of said working member. The proximal movable member comprises a movable ring assembly supported from the handle and adapted for three dimensional motion relative to the handle. A manually rotatable member may be arranged adjacent to the control handle for manually rotating the instrument shaft and working member relative to the control handle. A locking member may be supported from the control handle. The locking member is manually operable by a user and includes a follower the position of which is responsive to the position of the movable members.
Owner:CAMBRIDGE ENDOSCOPIC DEVICES
Real time self-adjusting calibration algorithm
Owner:MEDTRONIC MIMIMED INC
Bipolar cauterizing instrument
A bipolar surgical instrument that includes opposing grips that can engage the tissue. A current is delivered from an electrosurgical power source to electrodes disposed on the grips to cauterize the tissue. The electrode configurations provide efficient cauterization of the tissue. In some embodiments, the positive and negative electrodes will be offset from each other to prevent shorting and to provide a thin line of coagulation heating to the gripped tissue. In some embodiments the electrodes are removably coupled to the grips through nonconductive sleeves. In some embodiments, the first electrode is disposed in a groove and the second electrode is disposed on a boss.
Owner:INTUITIVE SURGICAL
Glucose sensor package system
A glucose sensor package system that includes a glucose sensor and a protective package that indicates exposure to temperature changes to indicate proper temperature control. Also covered are methods of transporting and sterilizing the package. In addition, glucose sensors directed to various sizing and positioning of the electrodes on the glucose sensor are covered.
Owner:MEDTRONIC MIMIMED INC
Systems and methods for replacing signal data artifacts in a glucose sensor data stream
Systems and methods for detecting noise episodes and processing analyte sensor data responsive thereto. In some embodiments, processing analyte sensor data includes filtering the sensor data to reduce or eliminate the effects of the noise episode on the signal.
Owner:DEXCOM
Device and method for determining analyte levels
InactiveUS6862465B2Reduce in quantityControl volumeImmobilised enzymesBioreactor/fermenter combinationsAnalyteImplanted device
Devices and methods for determining analyte levels are described. The devices and methods allow for the implantation of analyte-monitoring devices, such as glucose monitoring devices that result in the delivery of a dependable flow of blood to deliver sample to the implanted device. The devices include unique architectural arrangement in the sensor region that allows accurate data to be obtained over long periods of time.
Owner:DEXCOM
Electrosurgical instrument
InactiveUS7278994B2Lower impedanceReduced effectivenessCannulasDiagnosticsGynecologyPeritoneal cavity
A system and method are disclosed for removing a uterus using a fluid enclosure inserted in the peritoneal cavity of a patient so as to enclose the uterus. The fluid enclosure includes a distal open end surrounded by an adjustable loop, that can be tightened, a first proximal opening for inserting an electrosurgical instrument into the fluid enclosure, and a second proximal opening for inserting an endoscope. The loop is either a resilient band extending around the edge of the distal open end or a drawstring type of arrangement that can be tightened and released. The fluid enclosure is partially inserted into the peritoneal cavity of a patient in a deflated condition and then manipulated within the peritoneal cavity over the body and fundus of the uterus to the level of the uterocervical junction. The loop is tightened around the uterocervical junction, after which the enclosure is inflated using a conductive fluid. The loop forms a pressure seal against the uterocervical junction to contain the conductive fluid used to fill the fluid enclosure. Endoscopically inserted into the fluid enclosure is an electrosurgical instrument that is manipulated to vaporize and morcellate the fundus and body of the uterus. The fundus and body tissue that is vaporized and morcellated is then removed from the fluid enclosure through the shaft of the instrument, which includes a hollow interior that is connected to a suction pump The fundus and body are removed after the uterus has been disconnected from the tissue surrounding uterus.
Owner:GYRUS MEDICAL LTD
Medical device introducer and obturator
An introducer having an elongate tubular member and a device connector releasably attached to a proximal end of the tubular member. The introducer allows exchange of medical instruments, such as a blood filter and cardioplegia catheter, through a single lumen. An obturator having a retractable blade for making incision on a tissue is insertable through the lumen of the introducer. Methods of using the obturator and the introducer for introducing medical device(s) into body cavity, such as a vessel or cardiac tissue, are also disclosed.
Owner:EDWARDS LIFESCIENCES CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com