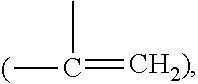
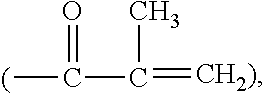
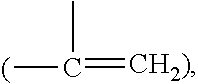
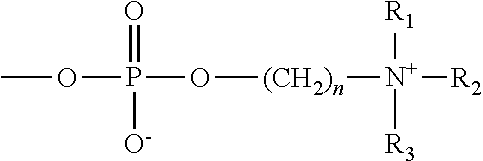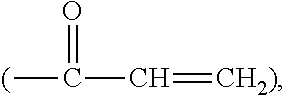Patents
Literature
3126results about "Package sterilisation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
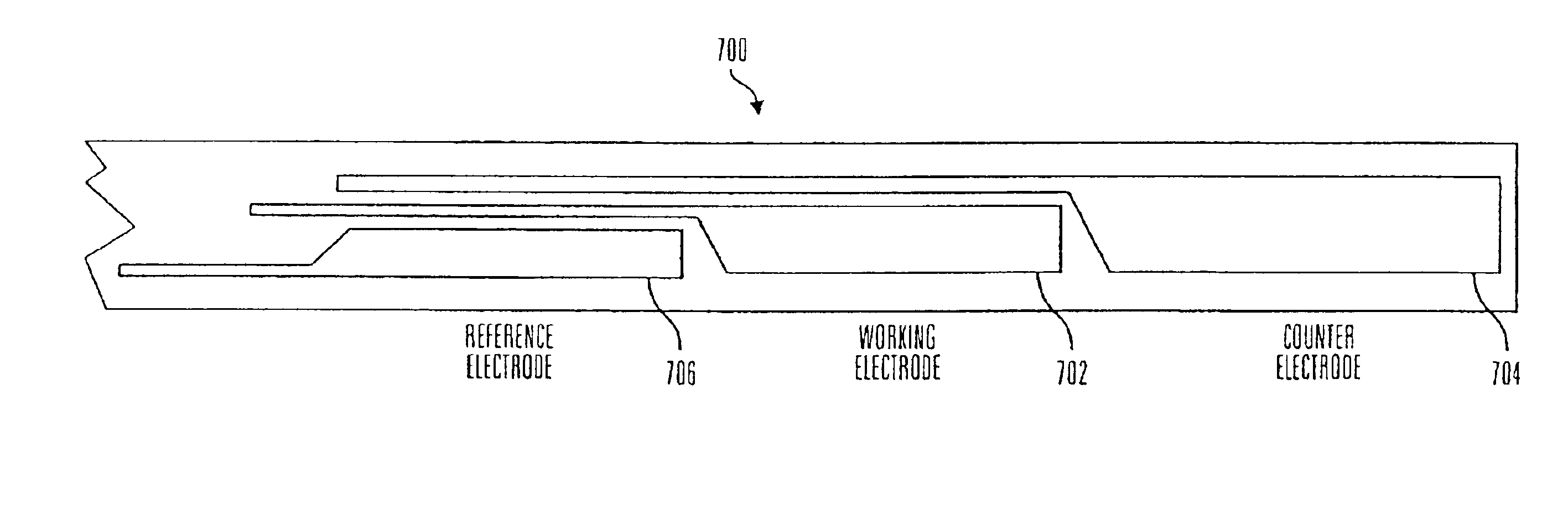
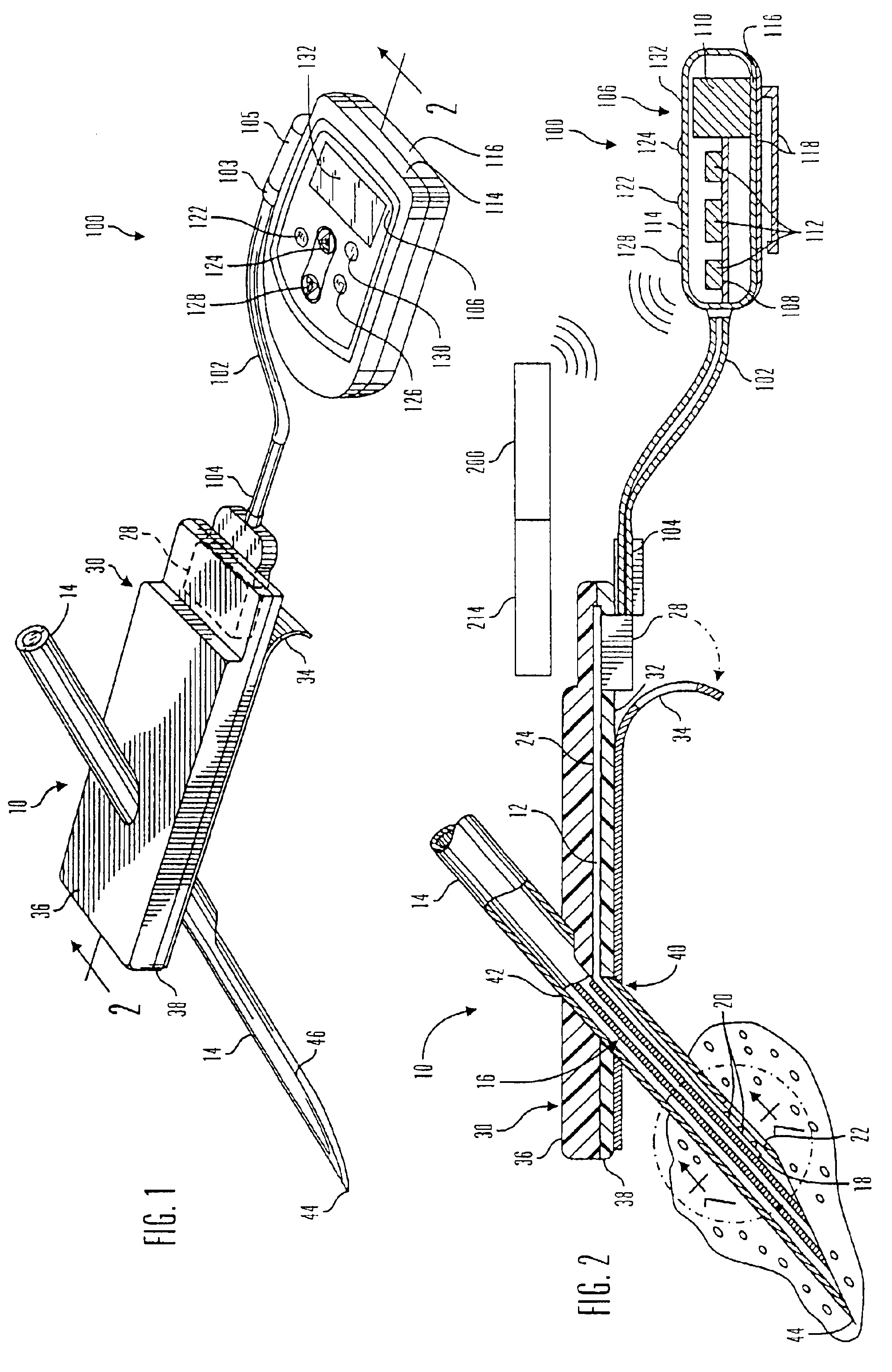
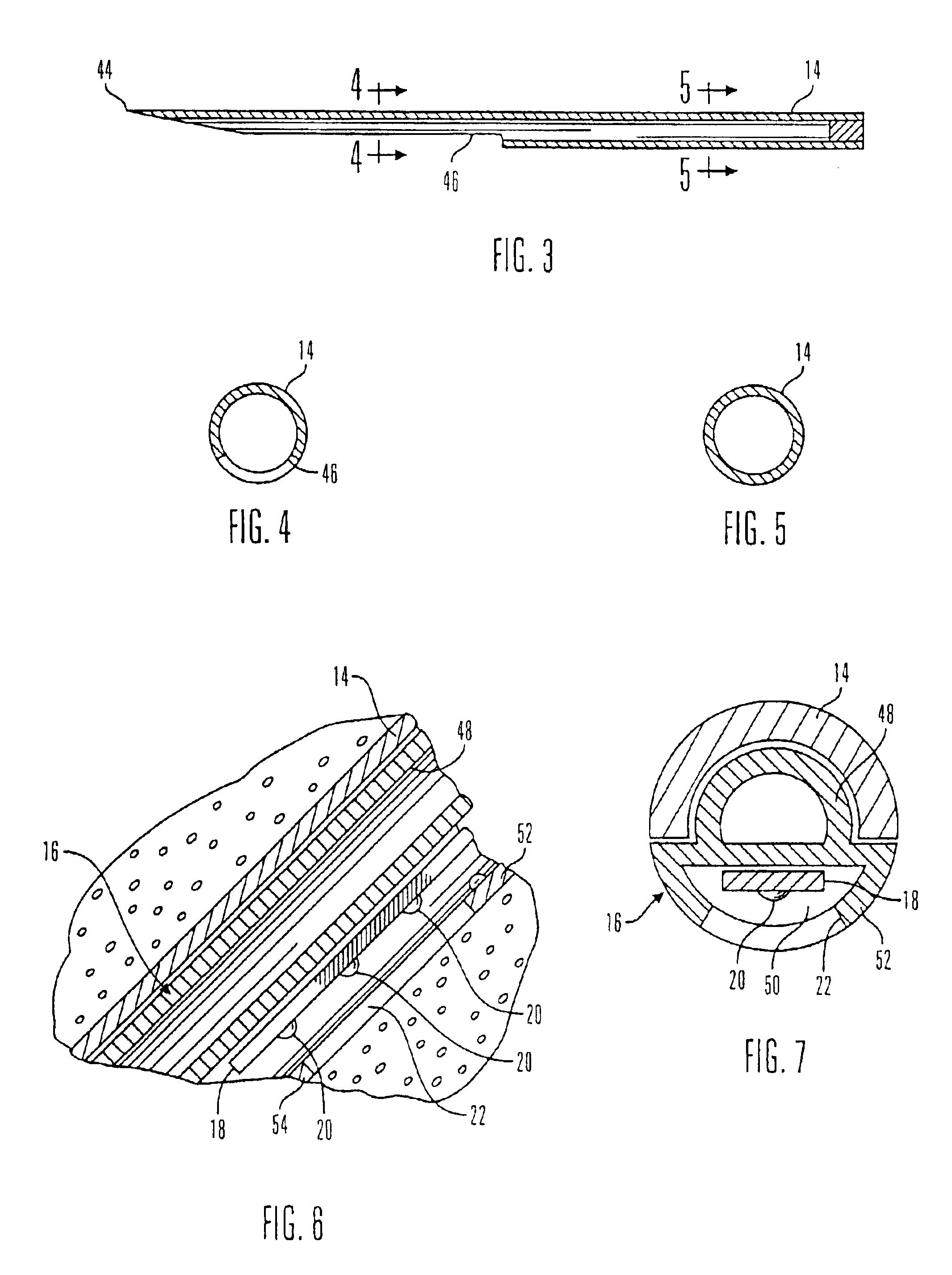
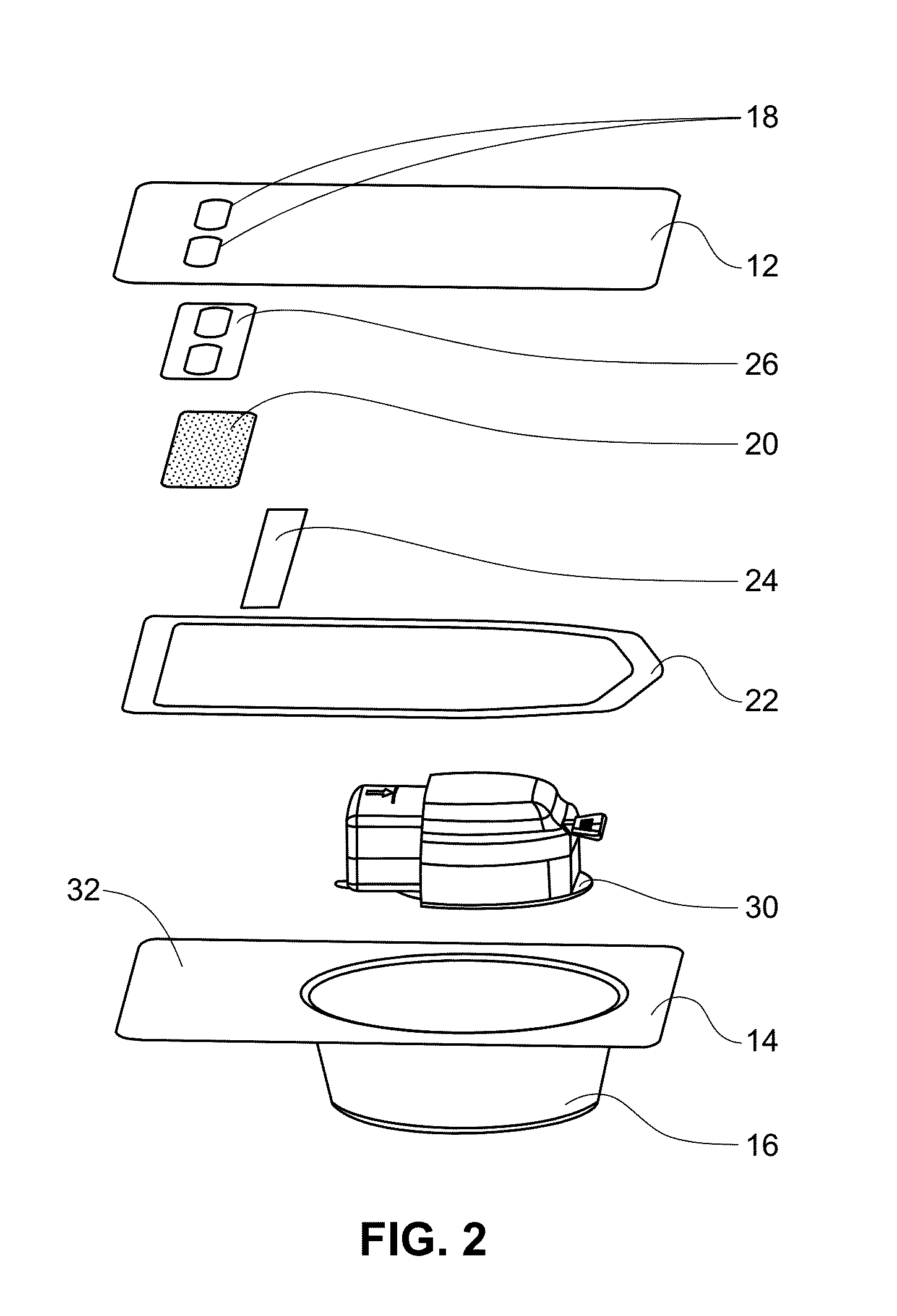
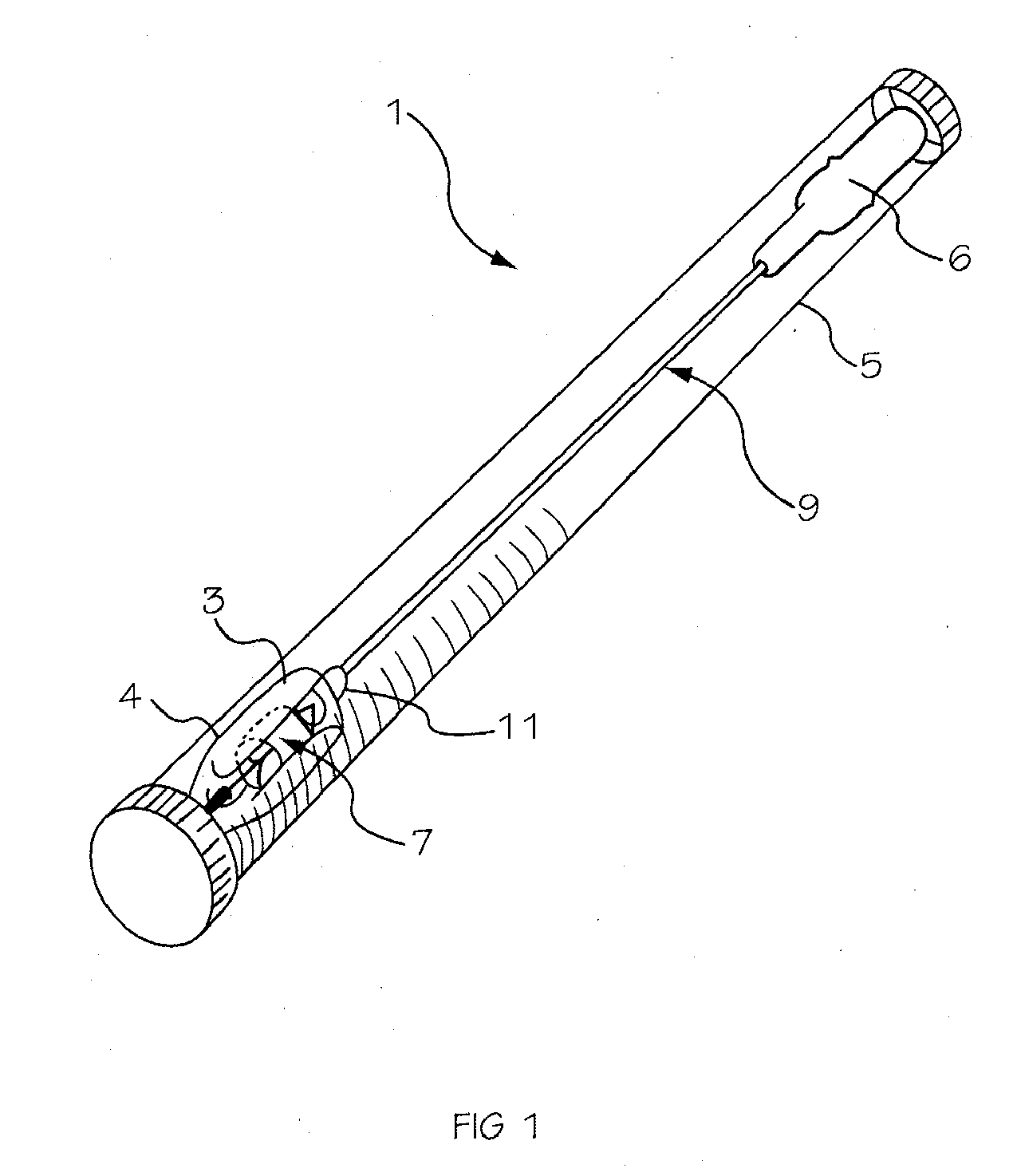
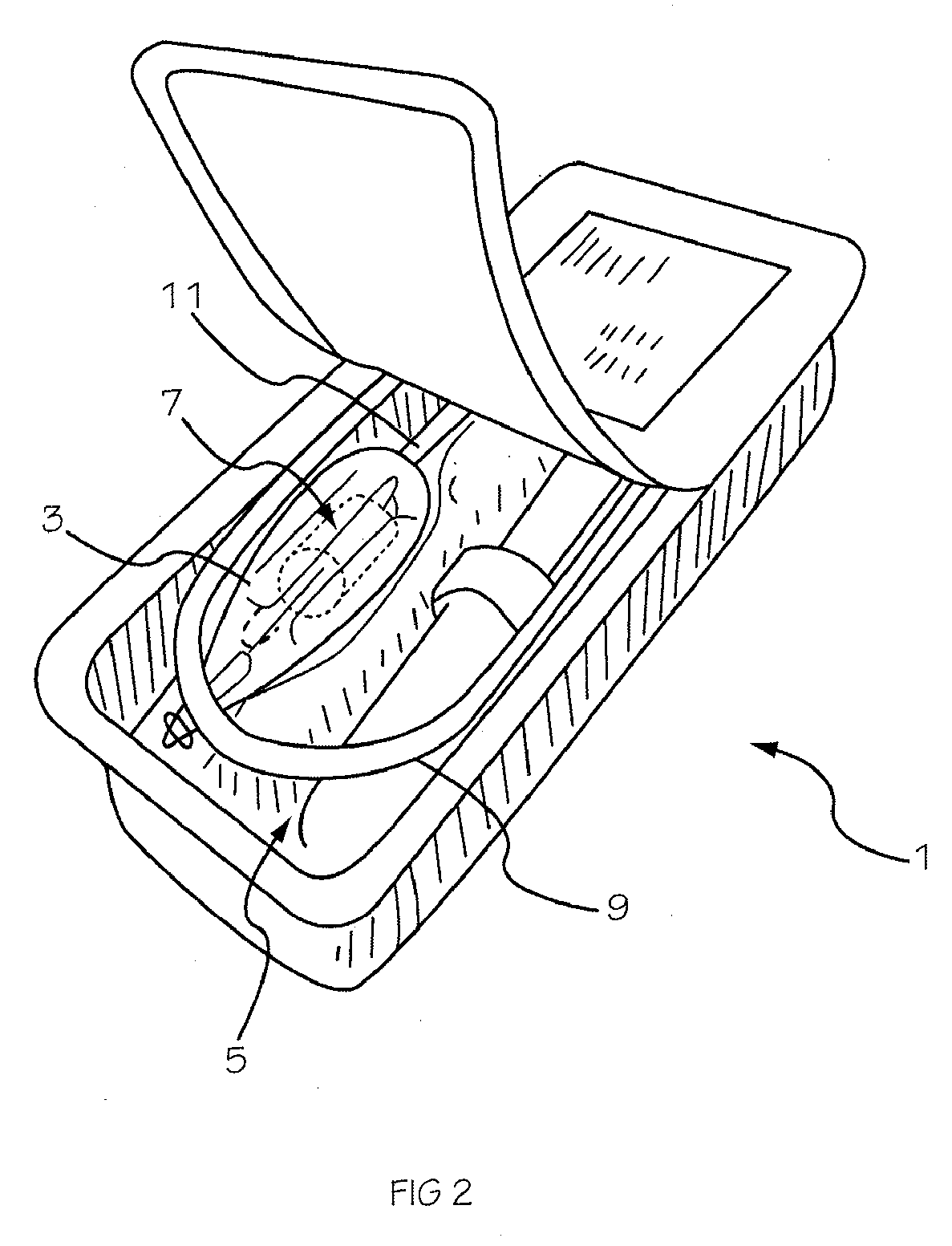
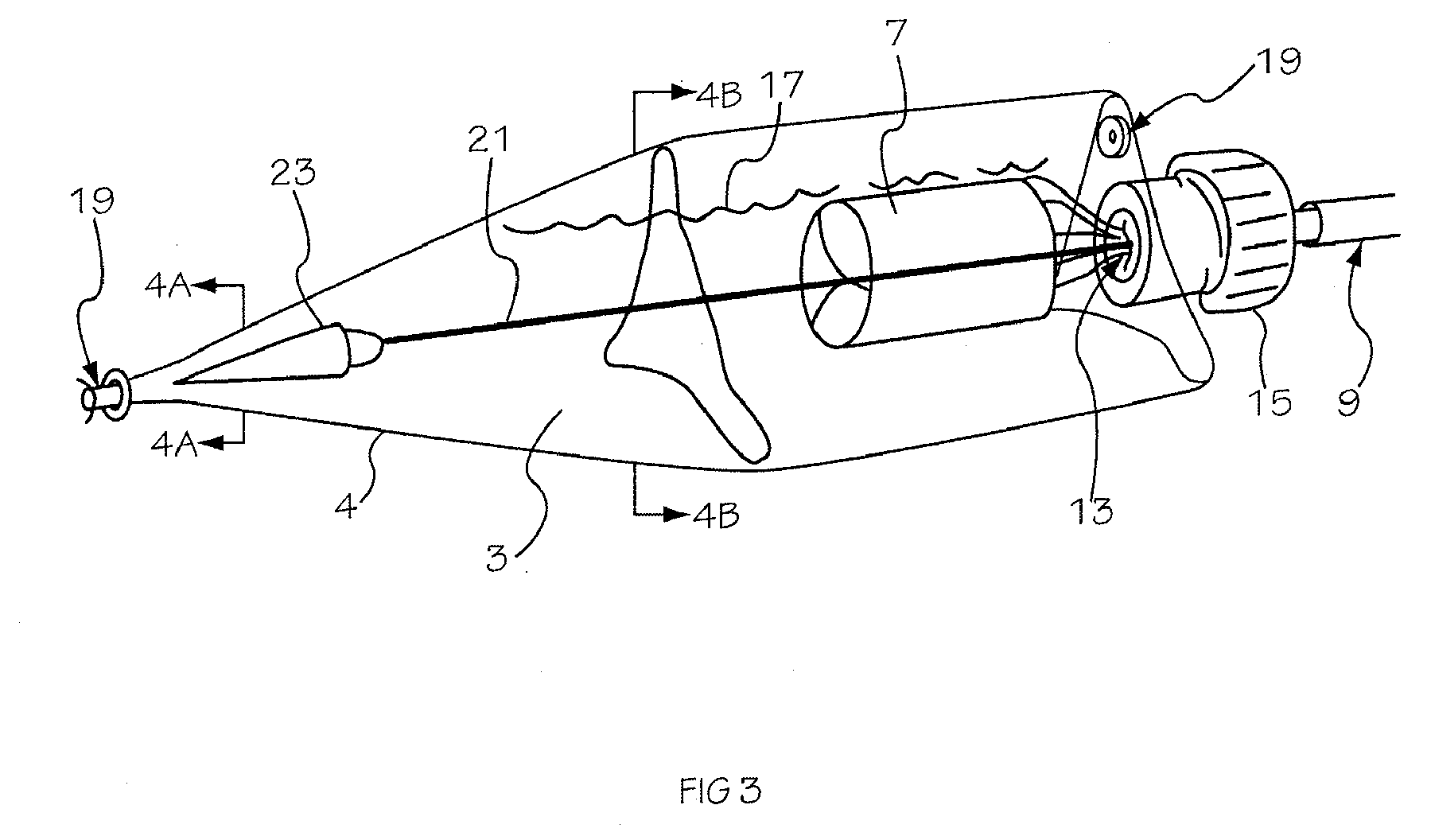
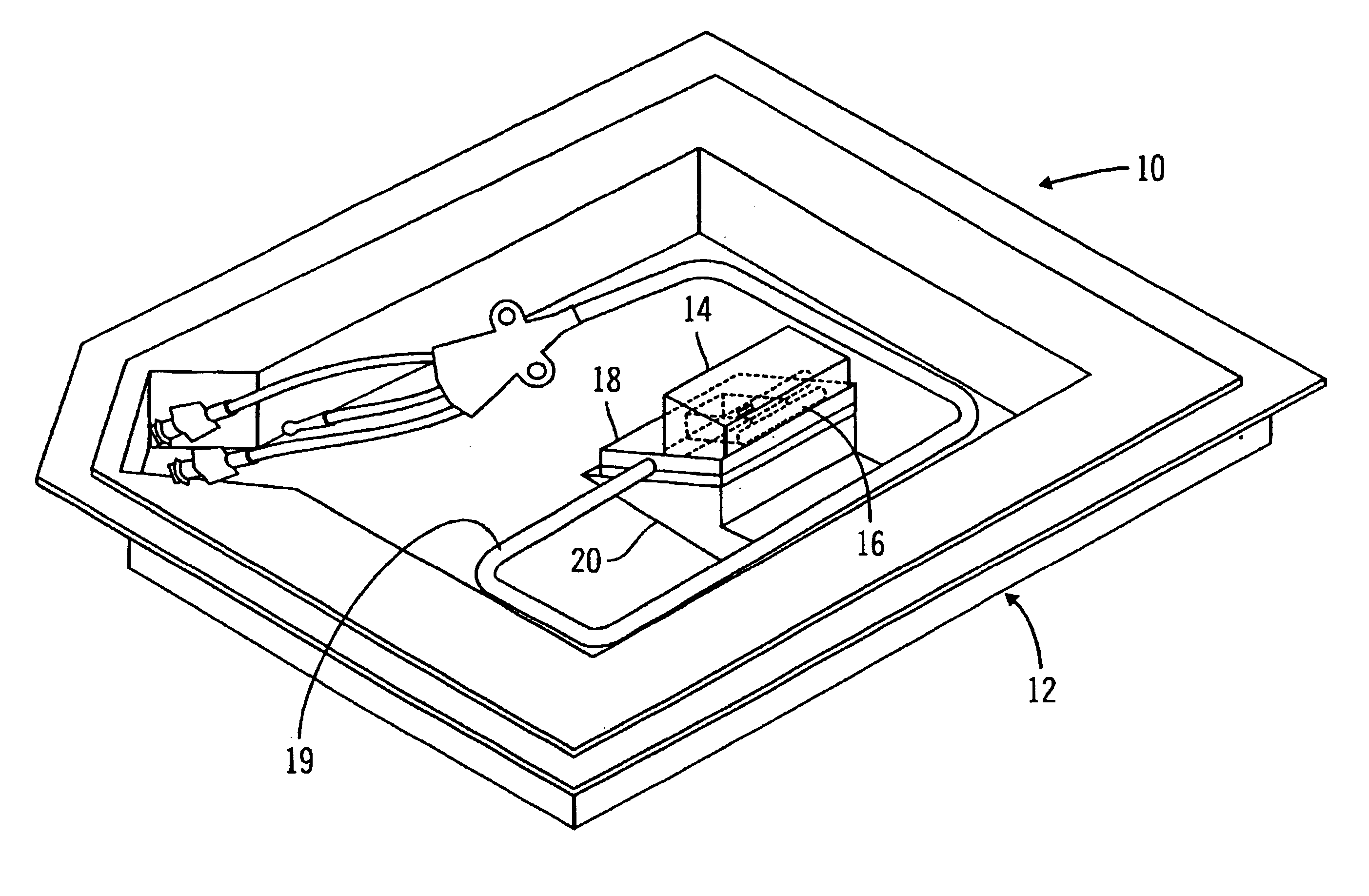
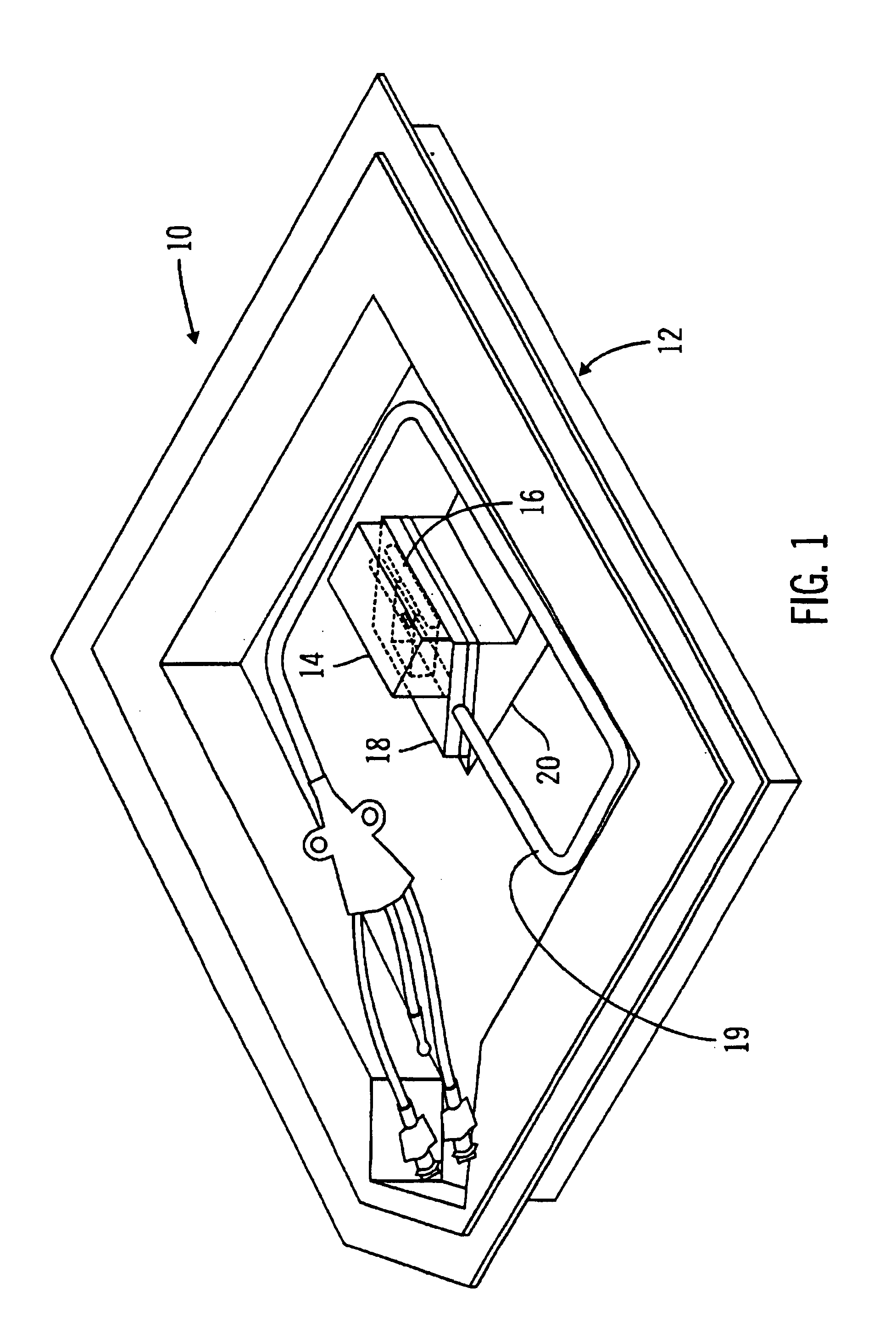
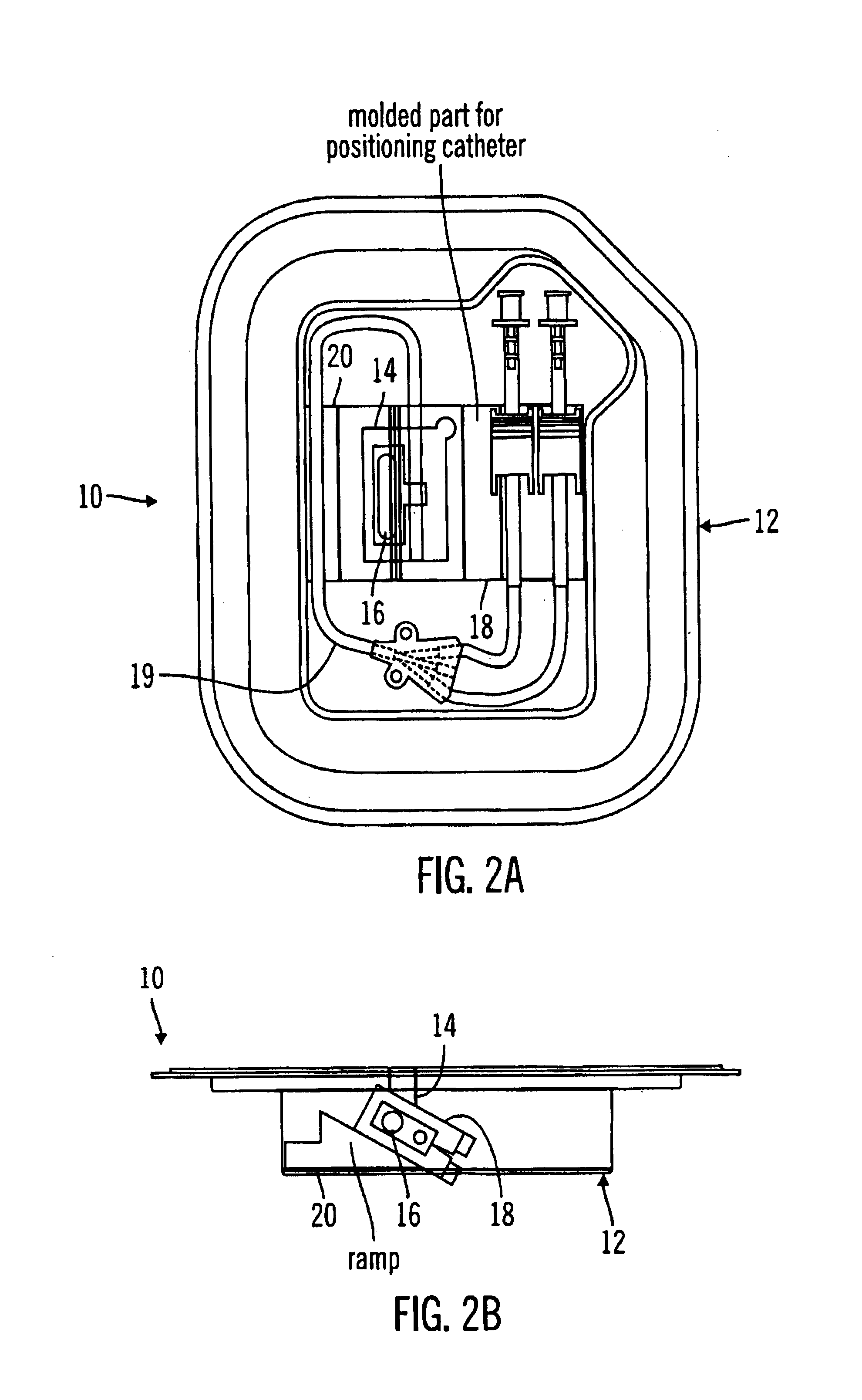
Glucose sensor package system
A glucose sensor package system that includes a glucose sensor and a protective package that indicates exposure to temperature changes to indicate proper temperature control. Also covered are methods of transporting and sterilizing the package. In addition, glucose sensors directed to various sizing and positioning of the electrodes on the glucose sensor are covered.
Owner:MEDTRONIC MIMIMED INC
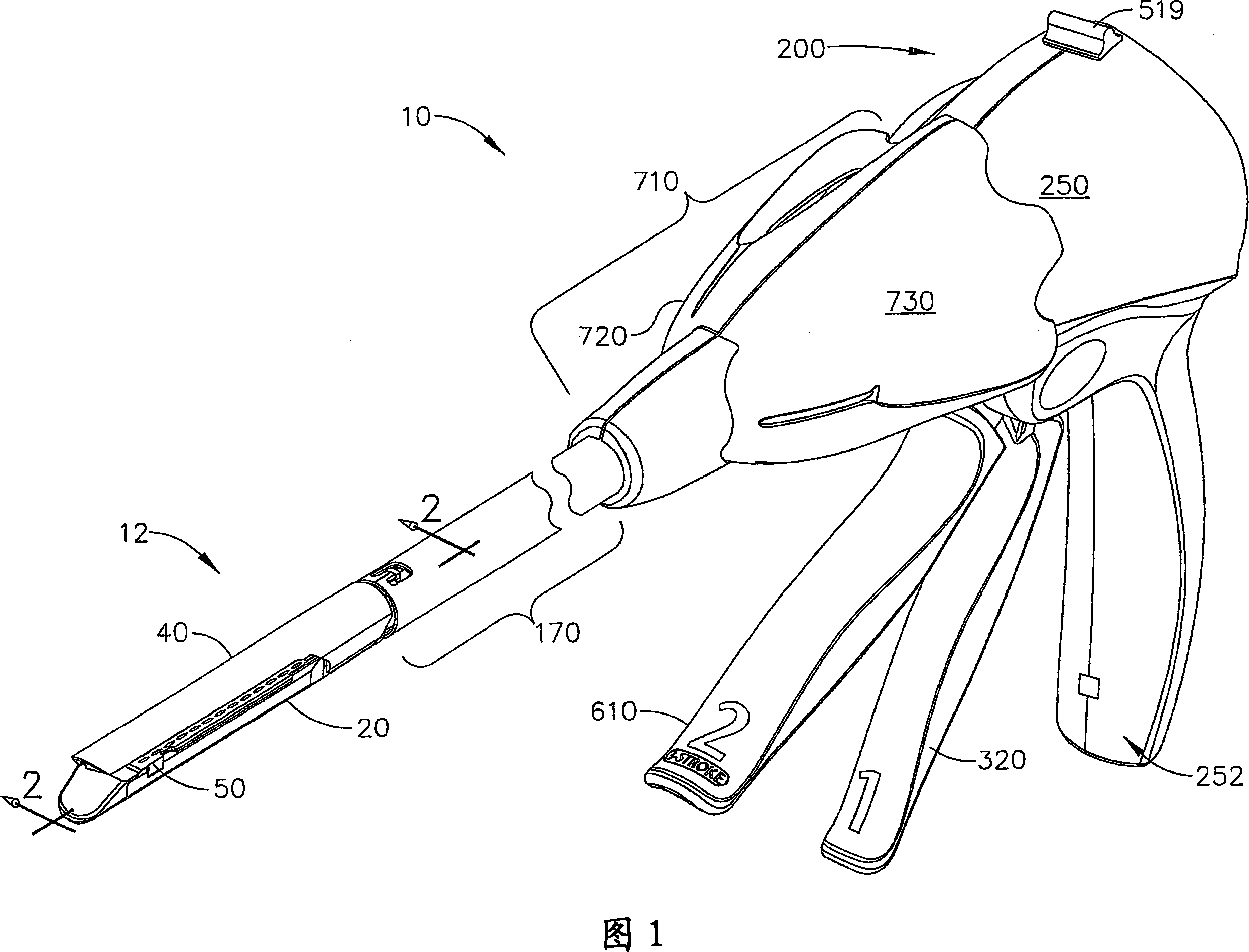
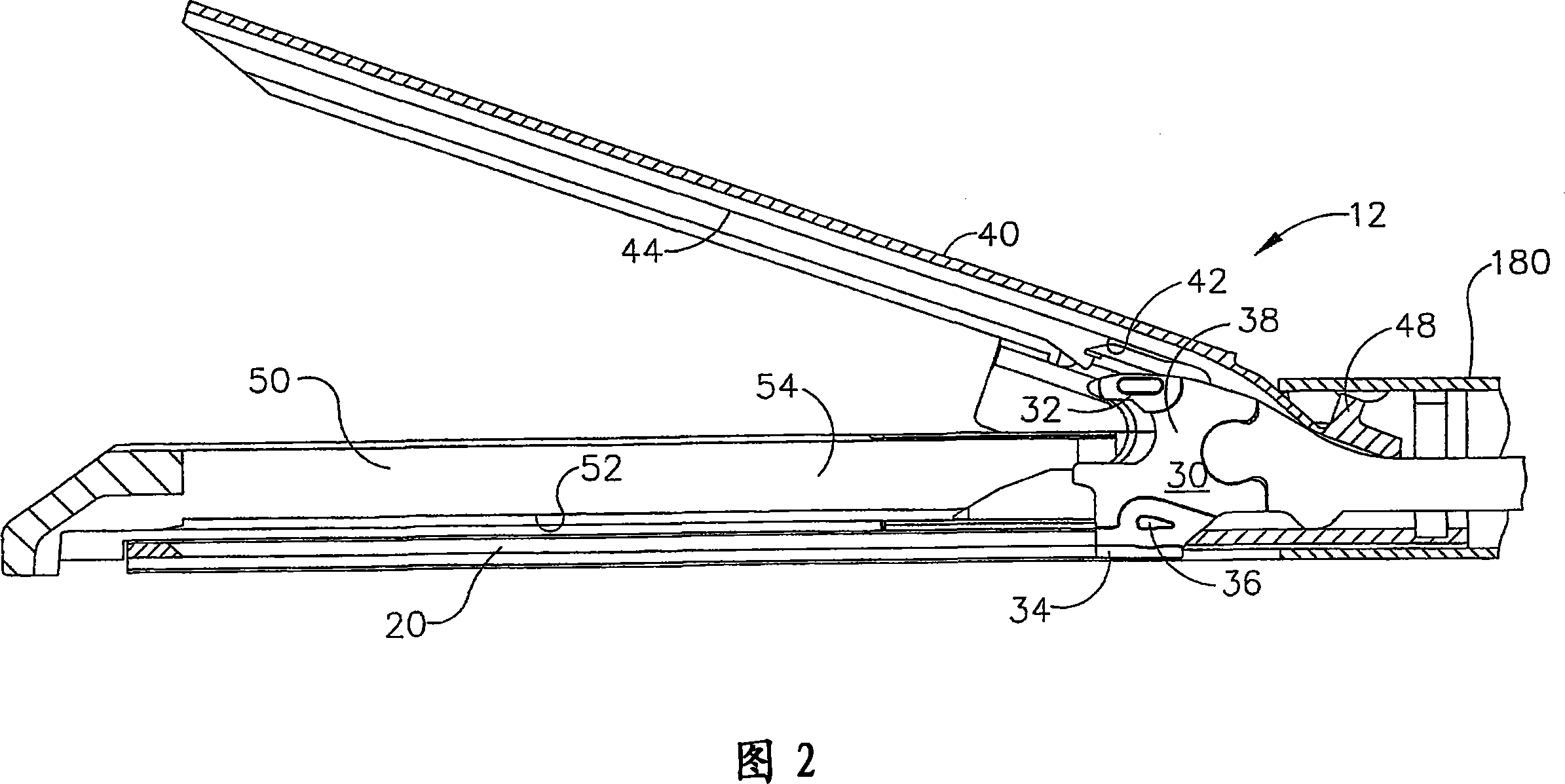
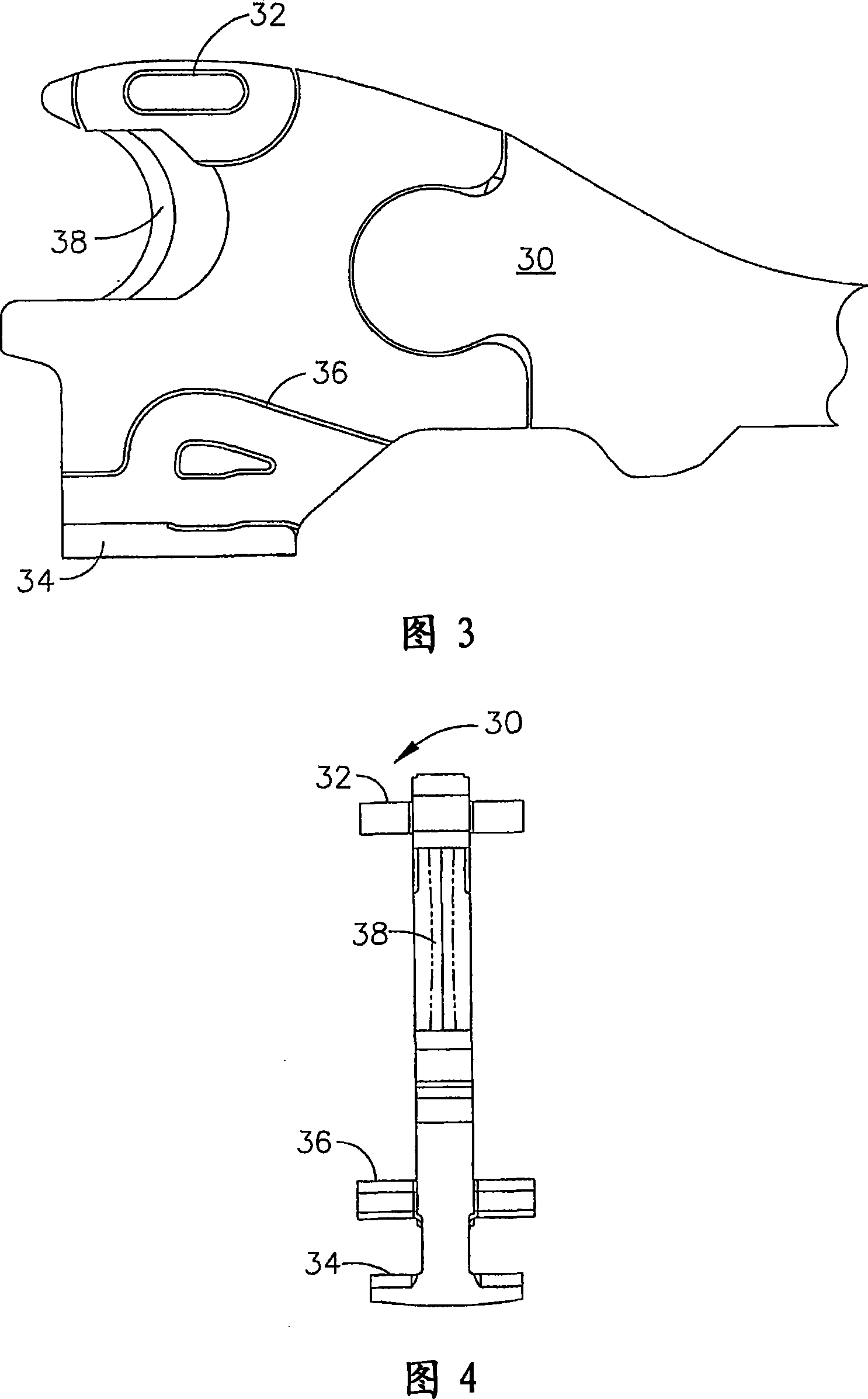
Manually driven surgical cutting and fastening instrument
A surgical cutting and fastening instrument that includes an elongate channel that is attached to a handle assembly by an elongate shaft assembly. The elongate channel is configured to receive a cartridge and has a pivotally translatable anvil attached thereto and a knife bar supported therein. The anvil may be selectively opened and closed by manipulating a closure trigger supported by the handle assembly. The knife bar may be distally advanced through the elongate channel by actuating a firing trigger that cooperates with a reversible rotary drive supported by the handle assembly. The knife bar may also be retracted to its starting position by actuating the firing trigger after the reversible rotary drive has been shifted to a retraction orientation.
Owner:ETHICON ENDO SURGERY INC
Two-part package for medical implant
ActiveUS7712606B2Low costProcedure be minimizedDispensing apparatusHeart valvesBiomedical engineeringMedical treatment
Owner:BOSTON SCI SCIMED INC
Two-Part Package For Medical Implant
ActiveUS20070061008A1Low costMinimize preparation timeDispensing apparatusHeart valvesBiomedical engineeringMedical treatment
The invention provides a two-part package and method of use for a pre-attached medical implant and delivery tool system. The package includes a wet compartment and a dry compartment and allows a pre-attached implant and delivery tool system to be at least partially stored immersed in a fluid in the wet compartment and at least partially stored in the dry compartment. In one embodiment the implant comprises a replacement heart valve, and the heart valve is stored inside the wet compartment while the heart valve delivery tool remains dry in the dry compartment.
Owner:BOSTON SCI SCIMED INC
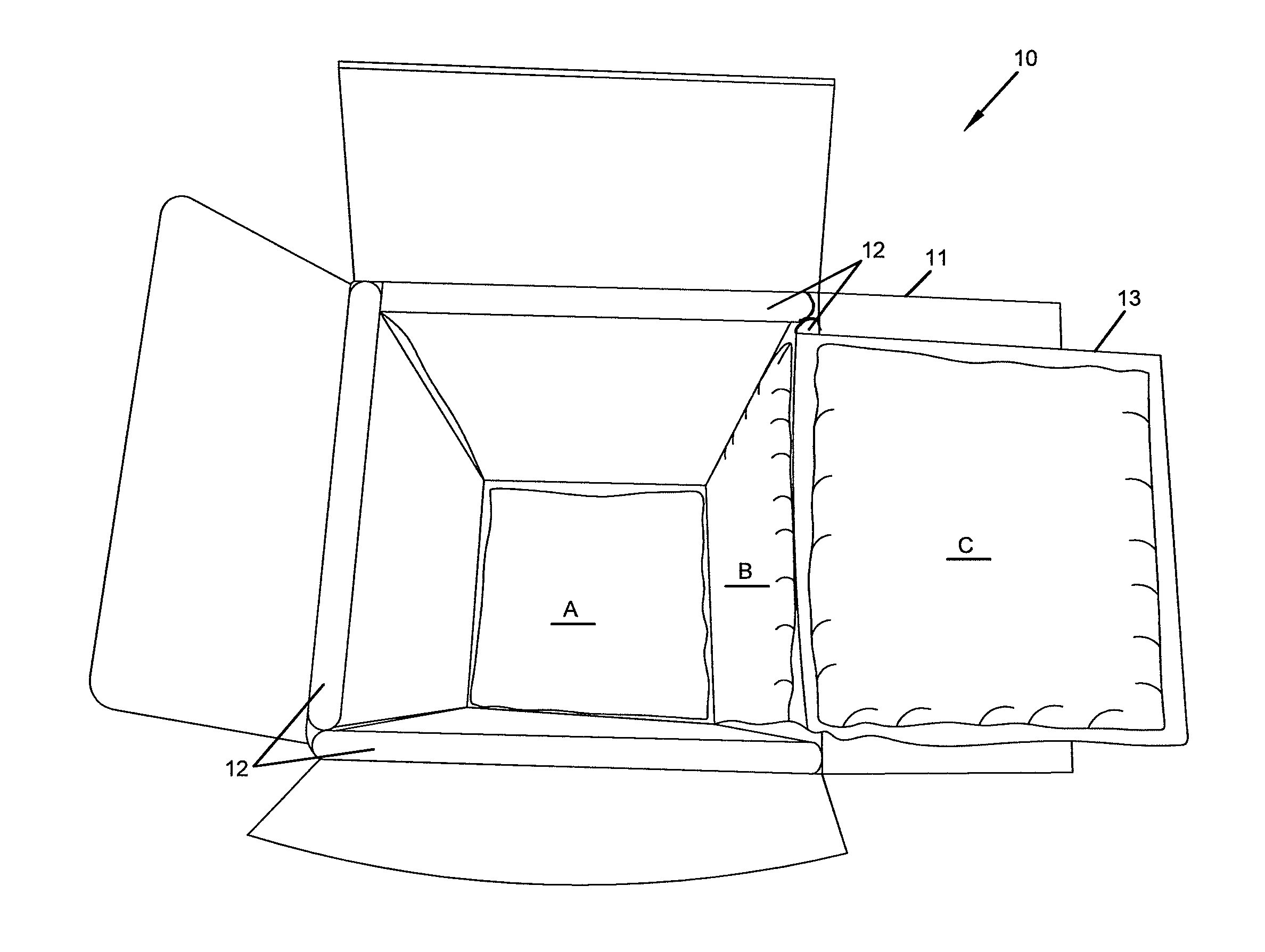
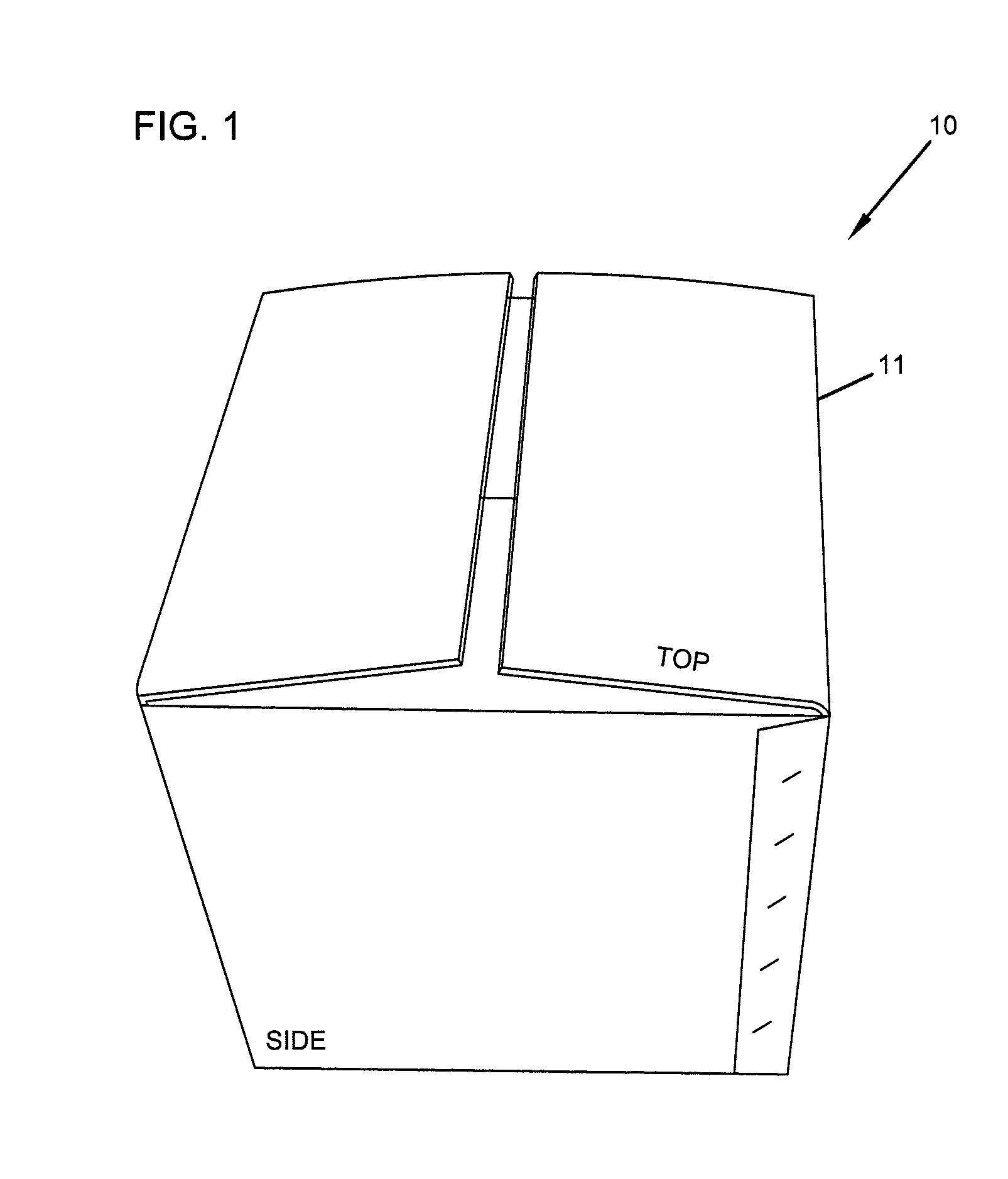
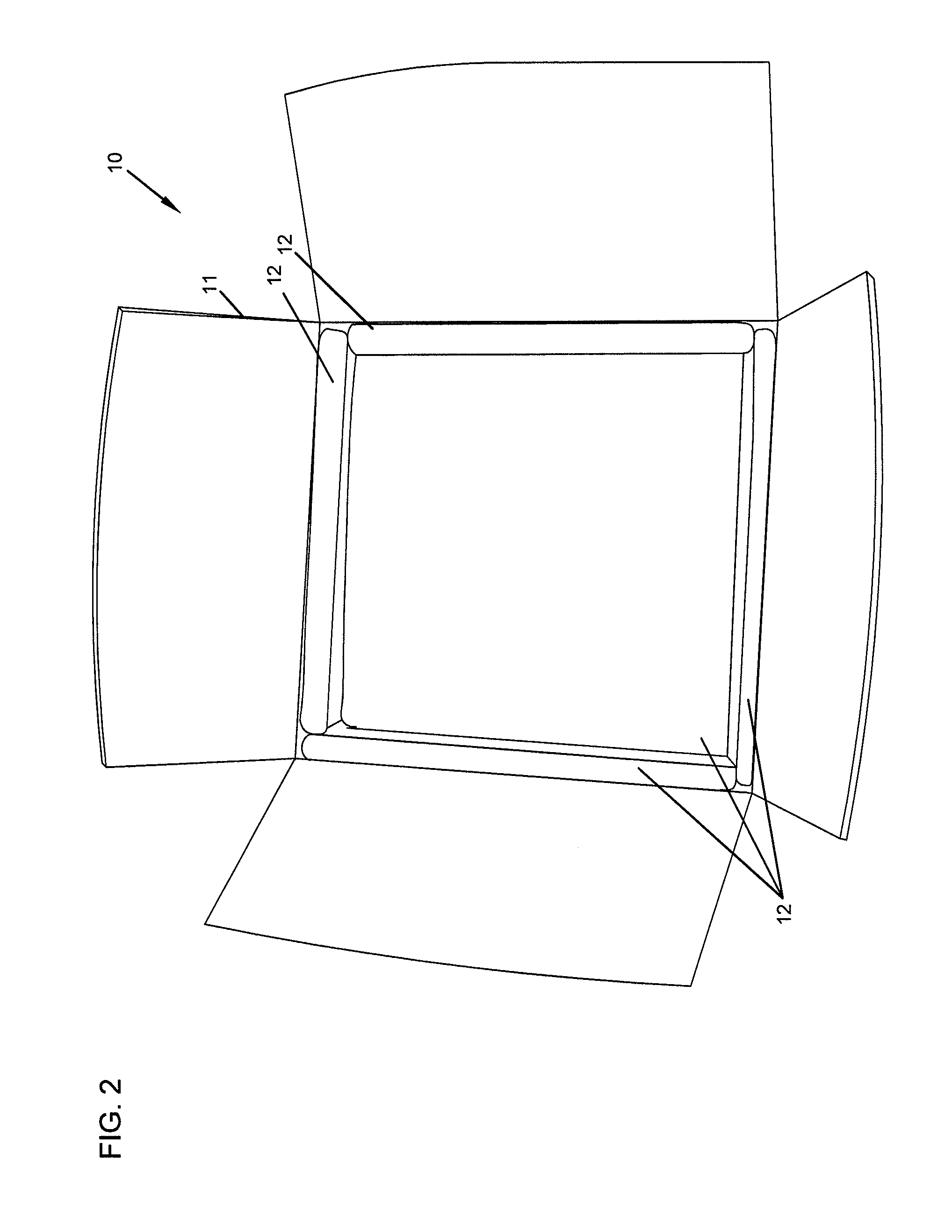
Repositionable base structure for a container
A base of a container including a bearing surface, a hinge, a first wall, and a second wall. The first wall sloping in a first direction from the bearing surface to the hinge, and the second wall sloping in a second direction away from the hinge. The second wall is adapted to be repositioned about the hinge with substantially no movement of the first wall.
Owner:CO2 PAC
Drug cartridge assembly and method of manufacture
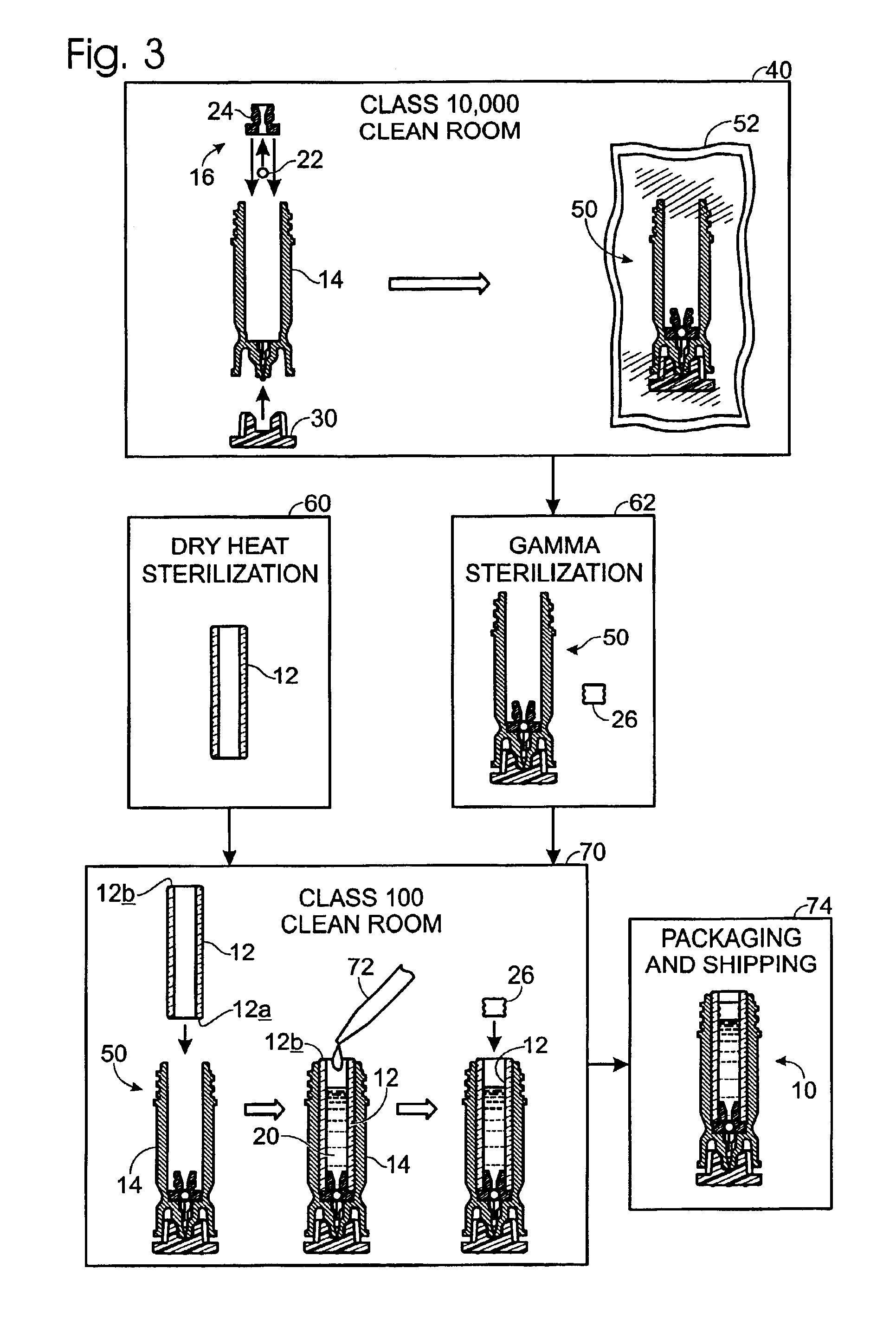
A method of manufacture for a drug cartridge assembly. The method includes providing a drug cartridge, providing a nozzle sub-assembly, and sterilizing the drug cartridge and nozzle sub-assembly. The method further includes assembling the drug cartridge and nozzle sub-assembly together in a configuration that enables ejection of liquid out of the drug cartridge through the nozzle sub-assembly. The method further includes filling the drug cartridge with a liquid, such as an injectable drug. The method may include separate sterilization of the drug cartridge and nozzle sub-assembly, using different sterilization processes. Portions of the method may be performed prior to sterilization within a first cleanroom, with subsequent steps being performed in a second cleanroom having a substantially lower particulate-per-volume rating than the first cleanroom.
Owner:BIOJECT
Packaging system
A packaging system for hydrating sterile devices without comprising the integrity of the sterilization. The packaging system may include an enclosure for enclosing a device requiring hydration, a container containing a hydrate, a base located within the interior of the enclosure and an activating member located within the interior of the enclosure. The container and the device may be located within a receptacle. The receptacle may rest on the base and the activating member may be affixed on top of the receptacle. A force may be exerted on an exterior portion of the enclosure such that the activating member pushes on the receptacle and crushes or ruptures the container. The hydrate located within the container is then released to the device, thereby hydrating the device without breaking the seal of the enclosure. The sterilized environment is therefore maintained and the device is hydrated.
Owner:MEDTRONIC MIMIMED INC
Package of sensitive articles
InactiveUS20050268573A1Convenient and effective and economicalSurgical furnitureDiagnosticsEngineeringOxygen
The invention is directed to the treating, e.g. sterilizing and packaging of medical devices and other articles, particularly such devices and articles which are reactive to oxygen or moisture containing atmospheres. The sterilized package has a container which includes at least one portion which is gas permeable and at least one portion which is gas impermeable and an interior in fluid communication with the gas permeable portion. The container may be sealed by applying an impermeable patch to the permeable portion thereof or by disposing the container in an enclosure formed of impermeable material and sealing the enclosure. After exposing the container to a sterilizing gas, the gas permeable portion may be removed or sealed off from the gas impermeable portion of the container in order to provide a package which is gas impermeable and can protect one or more articles within the interior of the container against environmental gases and moisture.
Owner:ALTAI MEDICAL TECH
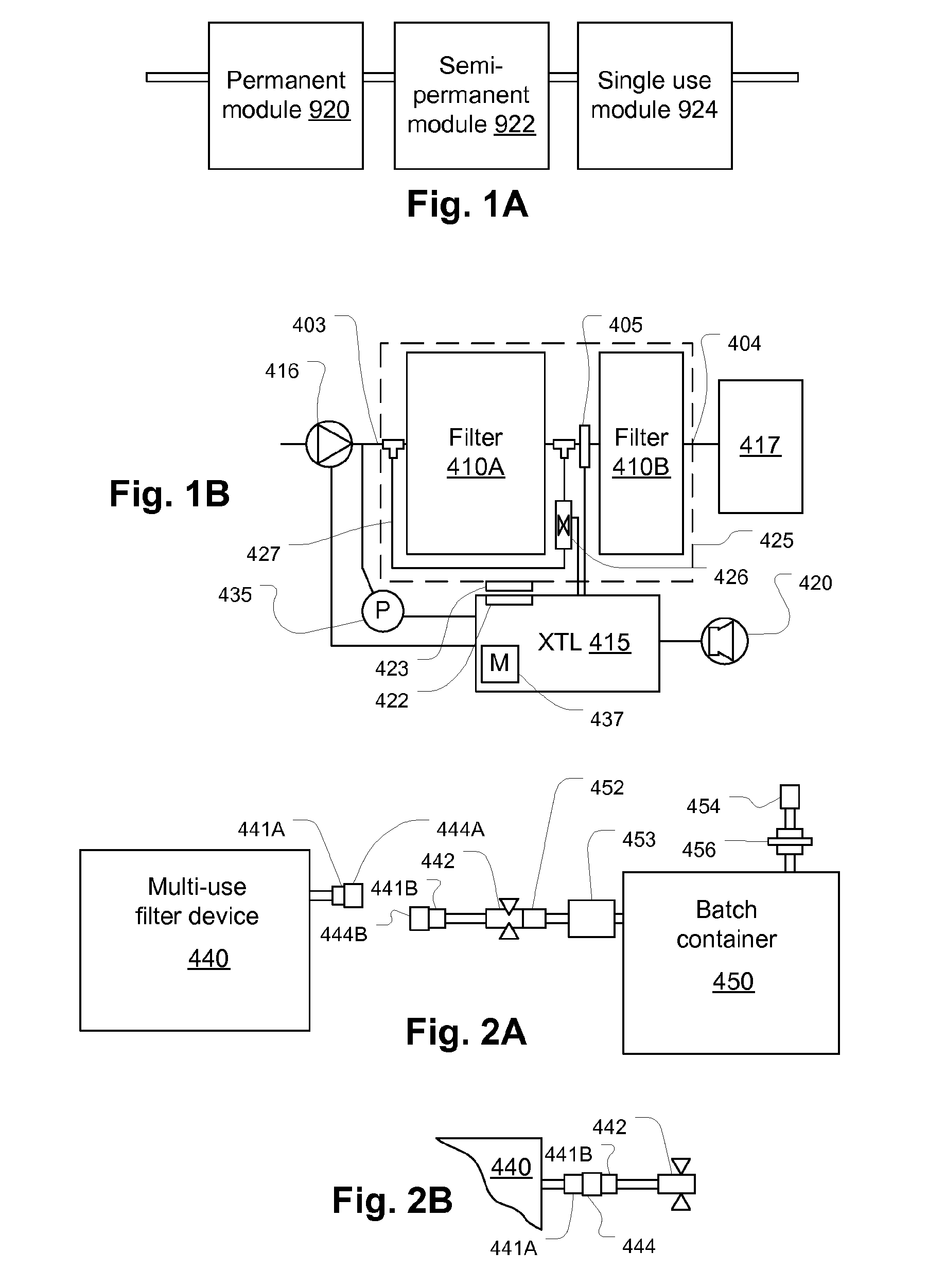
Filtration system for preparation of fluids for medical applications
ActiveUS20090182263A1Convenient and smoothIncrease the patency of the tubeEngine diaphragmsUltrafiltrationBlood treatmentsMedicine
Systems, methods, and devices for preparation of purified water and medicaments for various uses including blood treatment are described. Methods, devices, and systems for creating multiple-treatment batches are described.
Owner:NXSTAGE MEDICAL
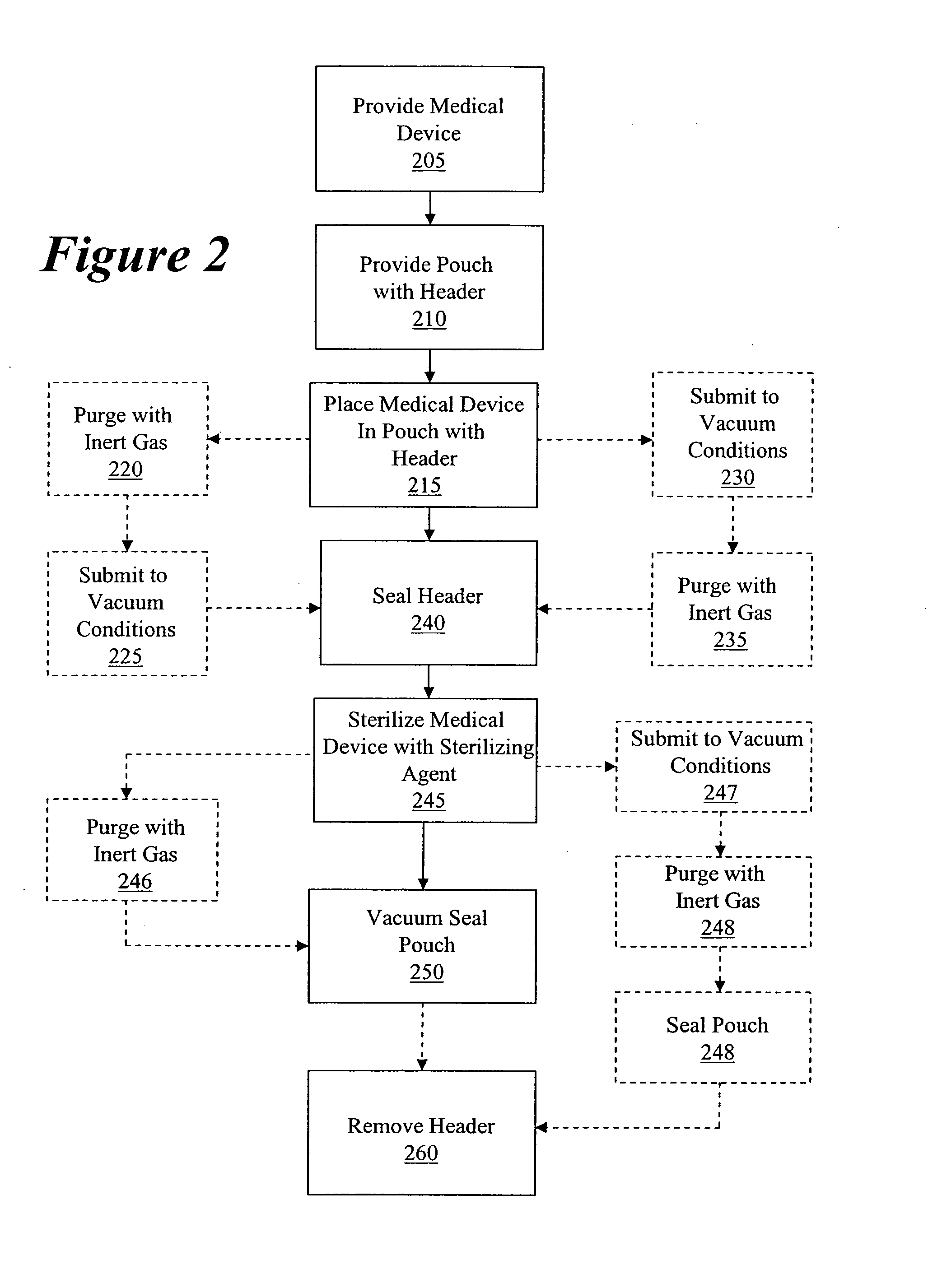
Packaging and sterilization of medical devices
InactiveUS20070084144A1Minimize timeExtended shelf lifeStentsSurgical furnitureCompound (substance)Fish oil
A method for the sterilization and packaging of a chemically sensitive medical device is provided. The chemically sensitive medical device has a coating derived from fish oil, a vitamin E compound or a combination thereof. The packaging pouch for the chemically sensitive medical device comprises a non-permeable chamber and a gas-permeable header. The sterilizing agent is administered to the packaged chemically sensitive medical device at a temperature of between about 20° C. and 40° C.
Owner:ATRIUM MEDICAL
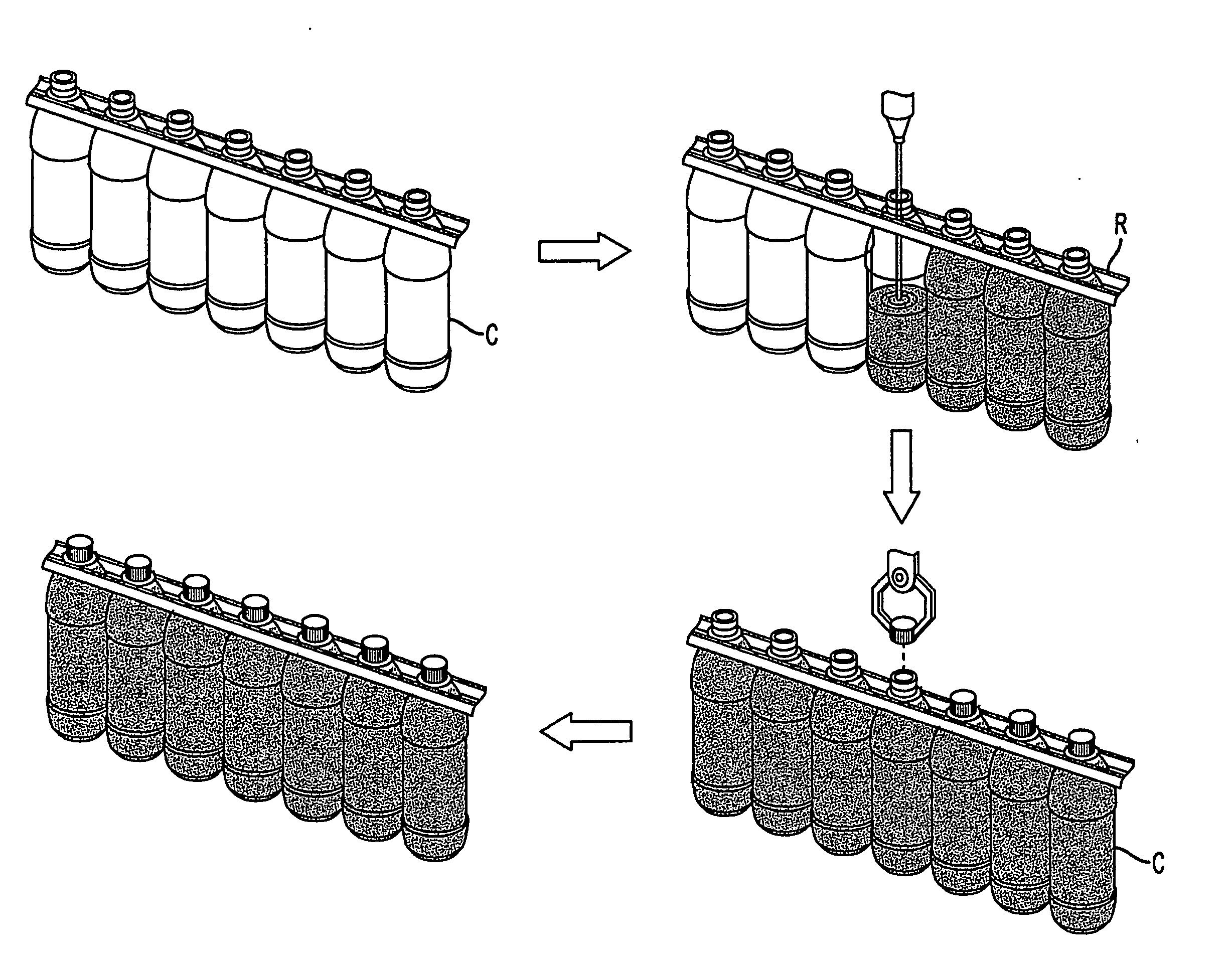
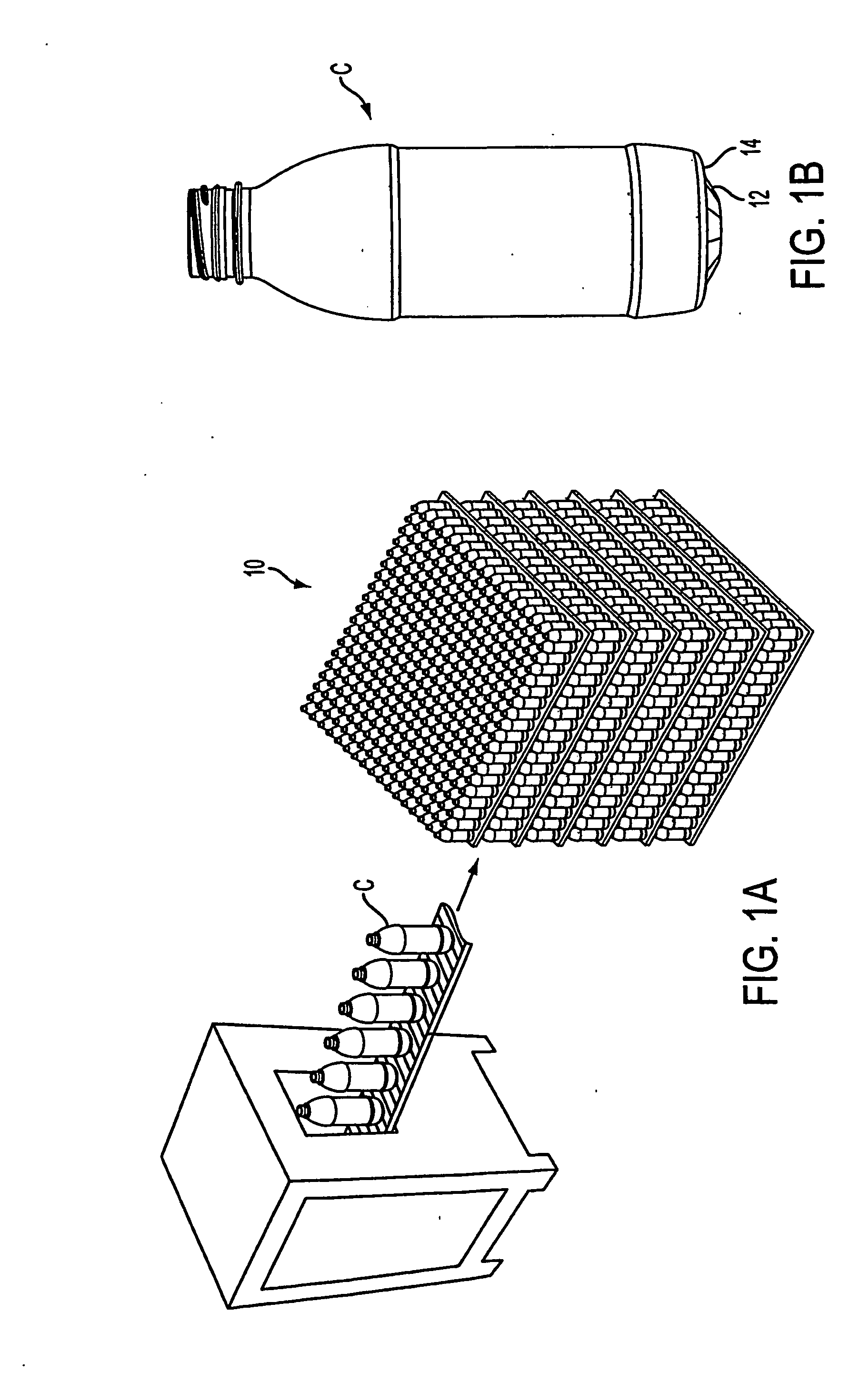
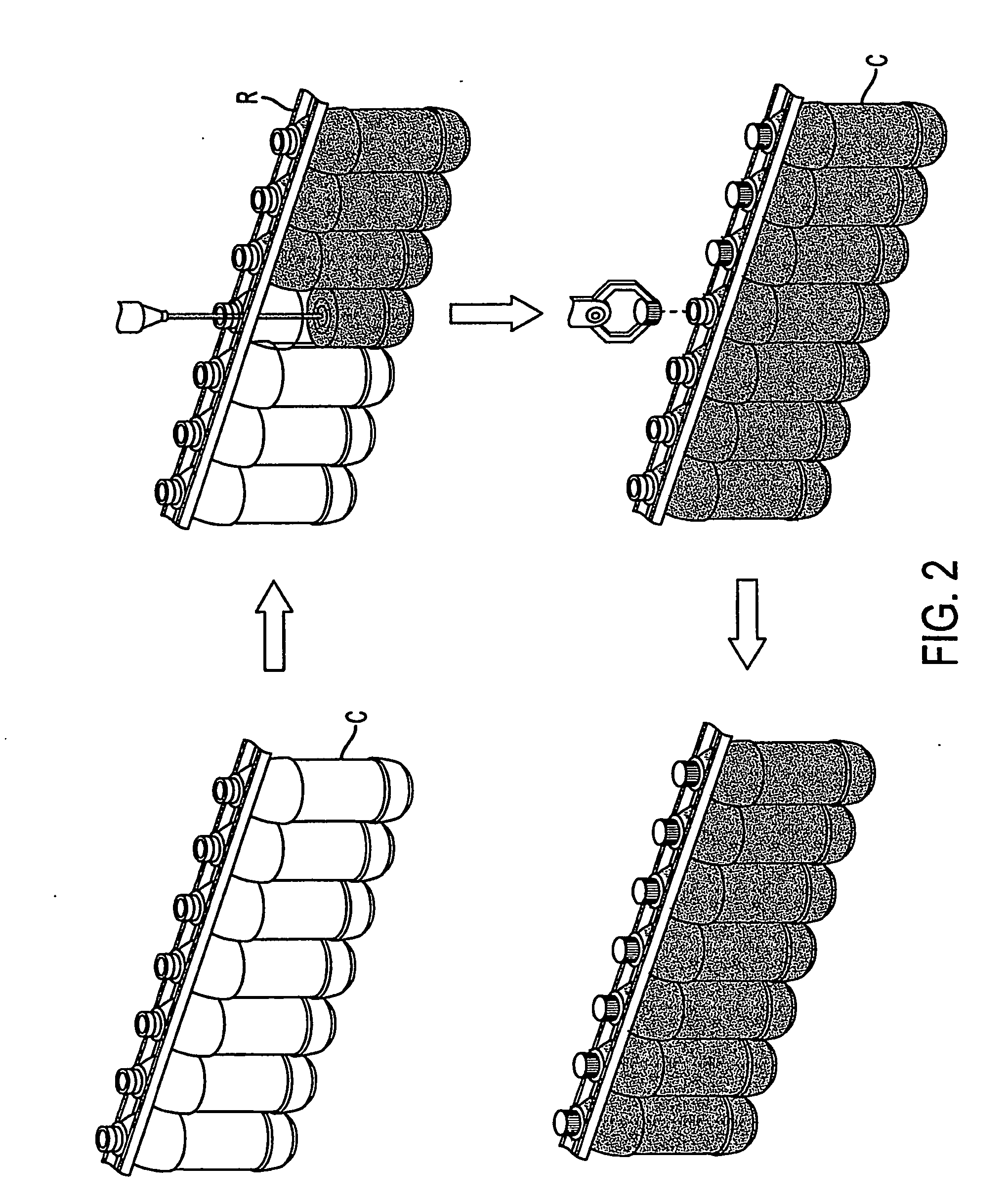
Container handling system
ActiveUS20070051073A1Smooth for label placementThorough removalCapsDecorative coversBlow moldingStructural geometry
A system for processing a simplified plastic container (C) that is to be filled with a hot product includes the step of blow-molding parison to form a container body, where the container body has a neck, a base, a side surface relatively free of structural geometry that surrounds an interior of the container body and, prior to being filled with the hot product, a projection (12) extending from the container body. After the container body is filled with a hot product in a production line, the neck of the filled container body is capped with a cap and then, the container body is cooled. During the cooling operation, the hot product is contracted so that the projection extending from the container can be pushed (P) into the container body like a traditional push-up so that the resultant, filled and cooled container body is relatively free of structural geometry.
Owner:CO2 PAC
Thermal Containment System Providing Temperature Maintaining Shipping Package with Segmented Flexible PCM Panels
InactiveUS20100314397A1Domestic cooling apparatusHeat storage plantsEngineeringPhase-change material
A packaging system having an outer container and a segment panel defining a plurality of phase change material (PCM) segments, with said segments being aligned with sides of the outer container interior upon wrapping around a payload and insertion into the outer container. The segmented panels may include edge tapers which facilitate the wrapping process with adjacent edge tapers being brought together during the wrapping process. A method of packaging using PCM-containing segments which are wrapped around a portion of a payload is also disclosed.
Owner:WILLIAMS PRESTON NOEL +1
Container closure with overlying needle penetrable and thermally resealable portion and underlying portion compatible with fat containing liquid product, and related method
InactiveUS20060231519A1Avoiding seal integrity problemGood product containmentCapsLiquid fillingLiquid productFormulary
A container and method are provided for storing fat containing liquid products, such as infant or baby formula, or other milk-based products. The container includes a body defining a storage chamber for receiving the aseptic fat containing liquid product, and a first aperture in fluid communication with the storage chamber. The body does not leach more than a predetermined amount of leachables into the fat containing liquid product and does not undesirably alter a taste profile of the fat containing liquid product. A container closure assembly includes a stopper receivable within the first aperture for hermetically sealing the storage chamber. The stopper includes a first material portion defining an internal surface in fluid communication with the storage chamber forming at least most of the surface area of the container closure that can contact any fat containing liquid product within the storage chamber and that does not leach more than a predetermined amount of leachables into the fat containing liquid product or undesirably alter a taste profile of the fat containing liquid product. A second material portion of the stopper either (i) overlies the first material portion and cannot contact any product within the storage chamber, or (ii) forms a substantially lesser surface area of the container closure that can contact any product within the storage chamber in comparison to the first material portion. The second material portion is needle penetrable for filling the storage chamber with product, and a resulting needle aperture formed in the second material portion is thermally resealable such as by the application of laser energy to seal the product within the storage chamber. A sealing portion of the container closure is engageable with the body prior to needle filling the storage chamber to thereby form a substantially dry hermetic seal between the container closure and body.
Owner:MEDINSTILL DEV
Tissue manipulation devices
Devices are provided for manipulating tissue during a surgical procedure. In certain embodiments, an end effector is operably coupled to the end of an elongate shaft. The end effector has at least one tissue support linkage movably coupled thereto such that upon application of a first actuation force thereto, the tissue support linkage moves laterally outward from within the end effector to enable the surgeon to manipulate / support adjacent tissue therewith. Upon application of another actuation force to the tissue support linkage, the tissue support linkage is caused to move substantially completely within the outer perimeter of the end effector to enable the end effector to be inserted through a lumen / opening or passageway. In various embodiments, the end effector may be selectively articulateable relative to the elongate shaft.
Owner:ETHICON ENDO SURGERY INC
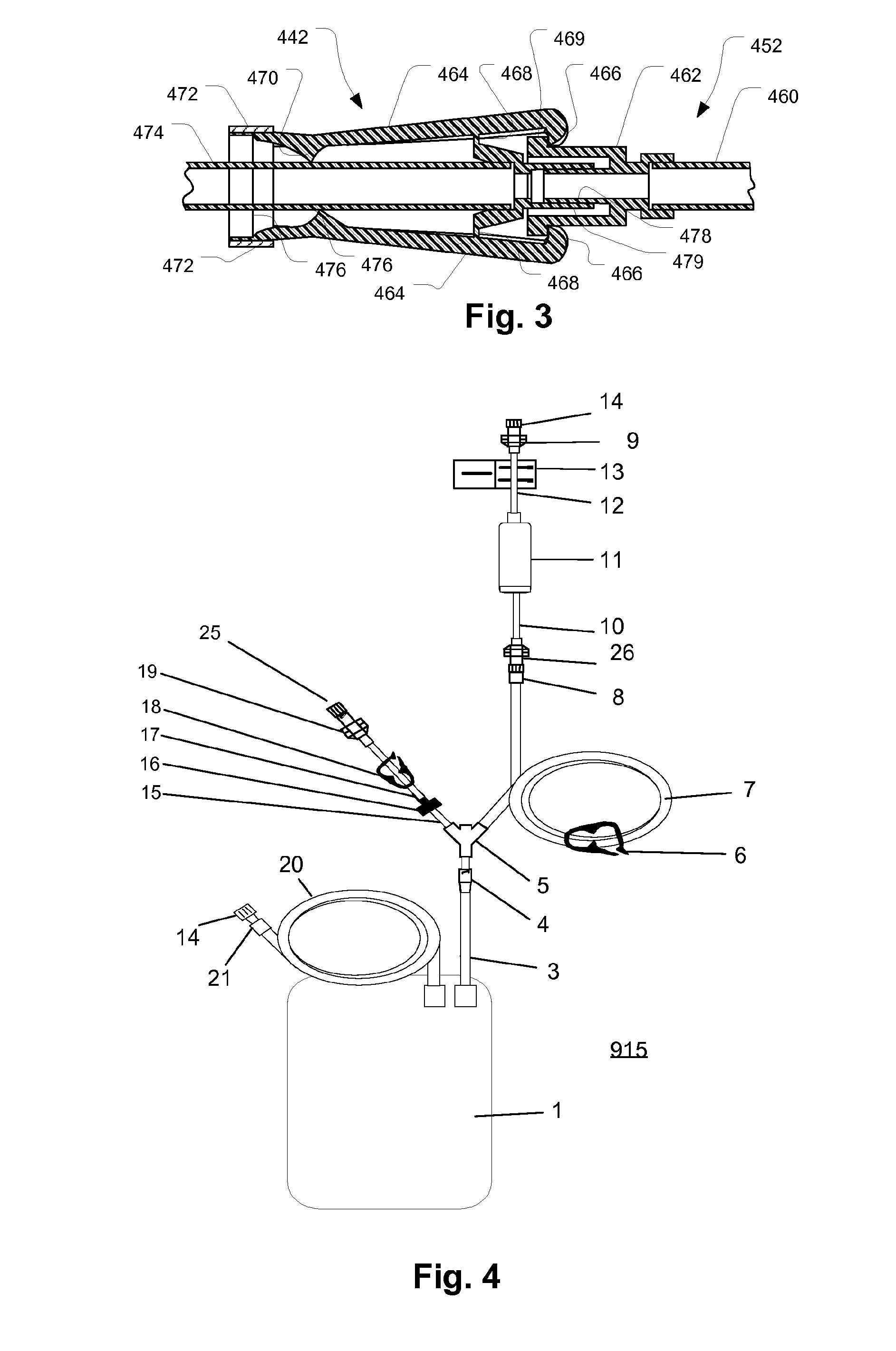
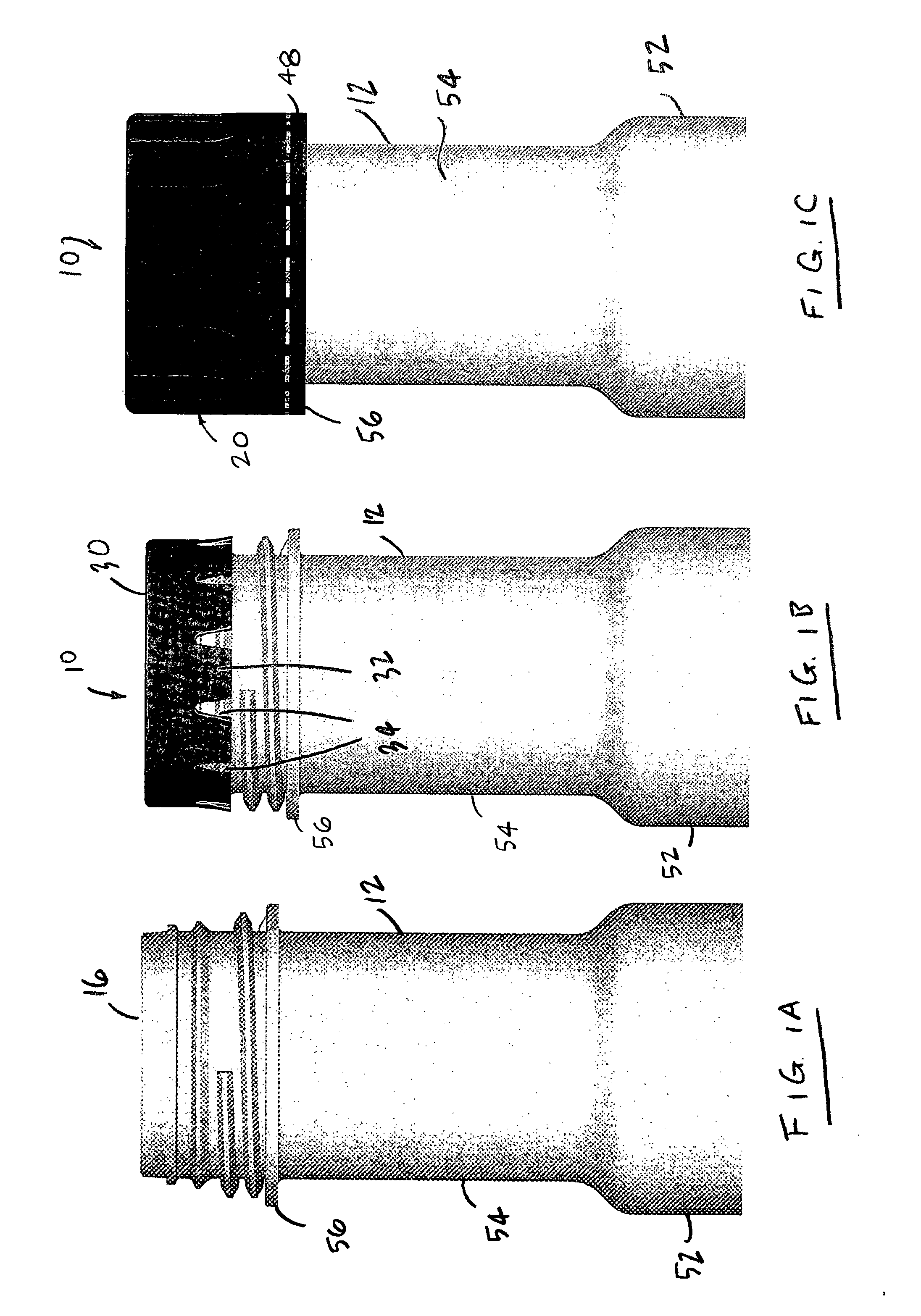
Sealed containers and methods of making and filling same
Disclosed is a uniquely configured medicament vial assembly which includes a storage vial, a stopper member and a securing ring. The vial assembly is configured to improve healthcare worker safety by providing a shielded gripping location to aid in the reduction of accidental needle sticks. The storage vial has a body portion which defines an interior chamber for storing a predetermined medicament and a neck portion through which medicament is received into and withdrawn from the interior chamber. The stopper member is inserted into the mouth of the vial and establishes a first seal. The securing ring is engaged with the mouth of the vial and adapted and configured for retaining the stopper member within the vial mouth and effectuating a second seal. The securing ring is formed from a thermoplastic and / or elastic material. Preferably, the securing ring is formed by molding the thermoplastic and / or elastic material over a portion of the storage vial and stopper member when engaged within the vial mouth.
Owner:MEDINSTILL DEV
Heat and hydrogen peroxide gas sterilization of container
The present invention discloses a method and apparatus for sterilizing containers with gas-phase hydrogen peroxide and heat on a linear form, fill and seal packaging machine. A partially formed container is subjected to multiple applications of gaseous hydrogen peroxide and hot air within a sterilization tunnel. The sterilization tunnel is maintained at a temperature greater than the condensation temperature of hydrogen peroxide. The present invention sterilizes the container allowing for filling of the container with a high acid product such as orange juice for ambient distribution. The container may be any number of possibilities such as TETRA REX TM gable top cartons, plastic bottles, and the like. The invention allows for the efficacious use of hydrogen peroxide gas having a concentration of up to 53%.
Owner:TETRA LAVAL HLDG & FINANCE SA
Sealed containers and methods of making and filling same
Owner:MEDINSTILL DEV
Method and device for pressurizing containers
Owner:INOFLATE
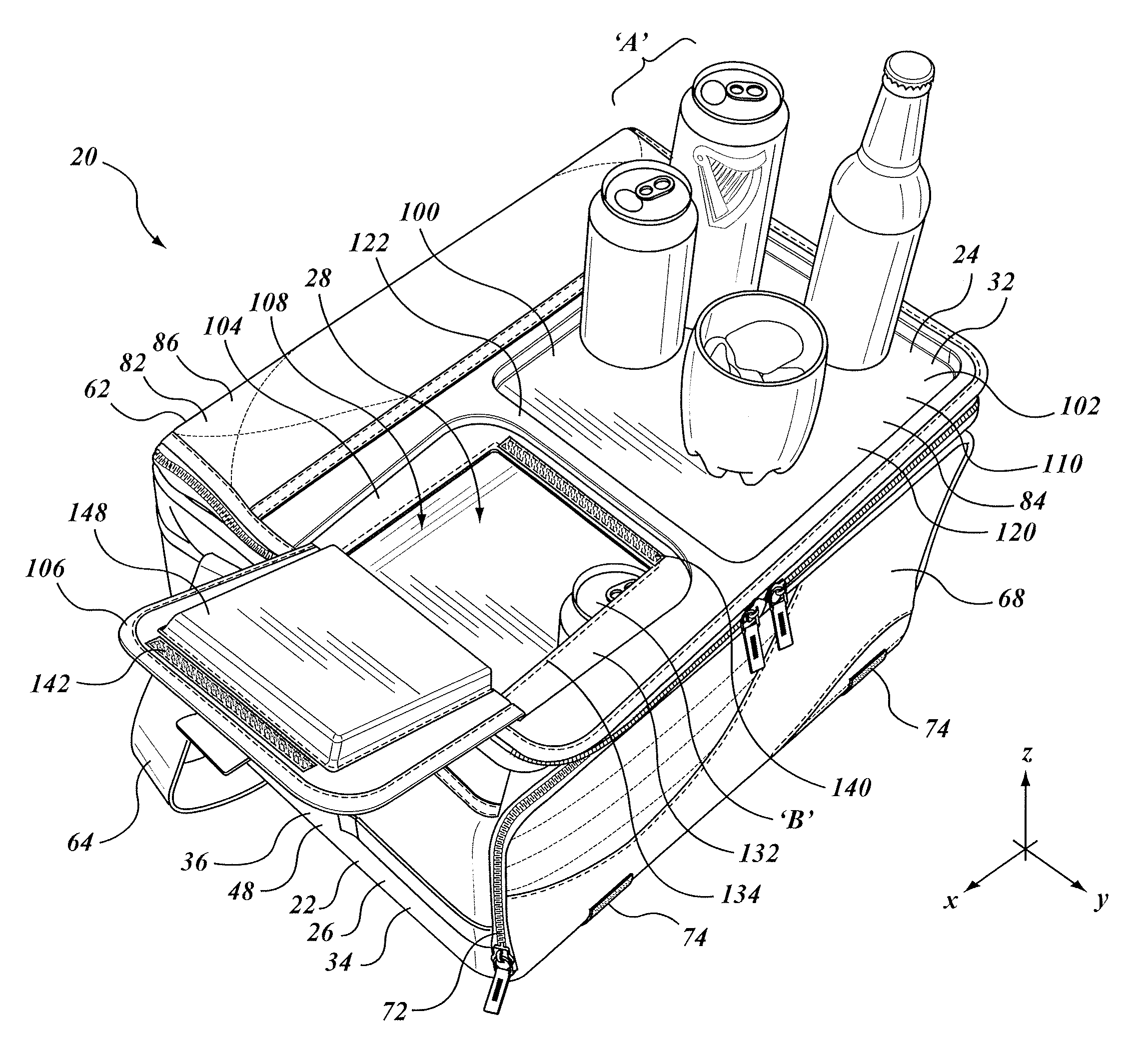
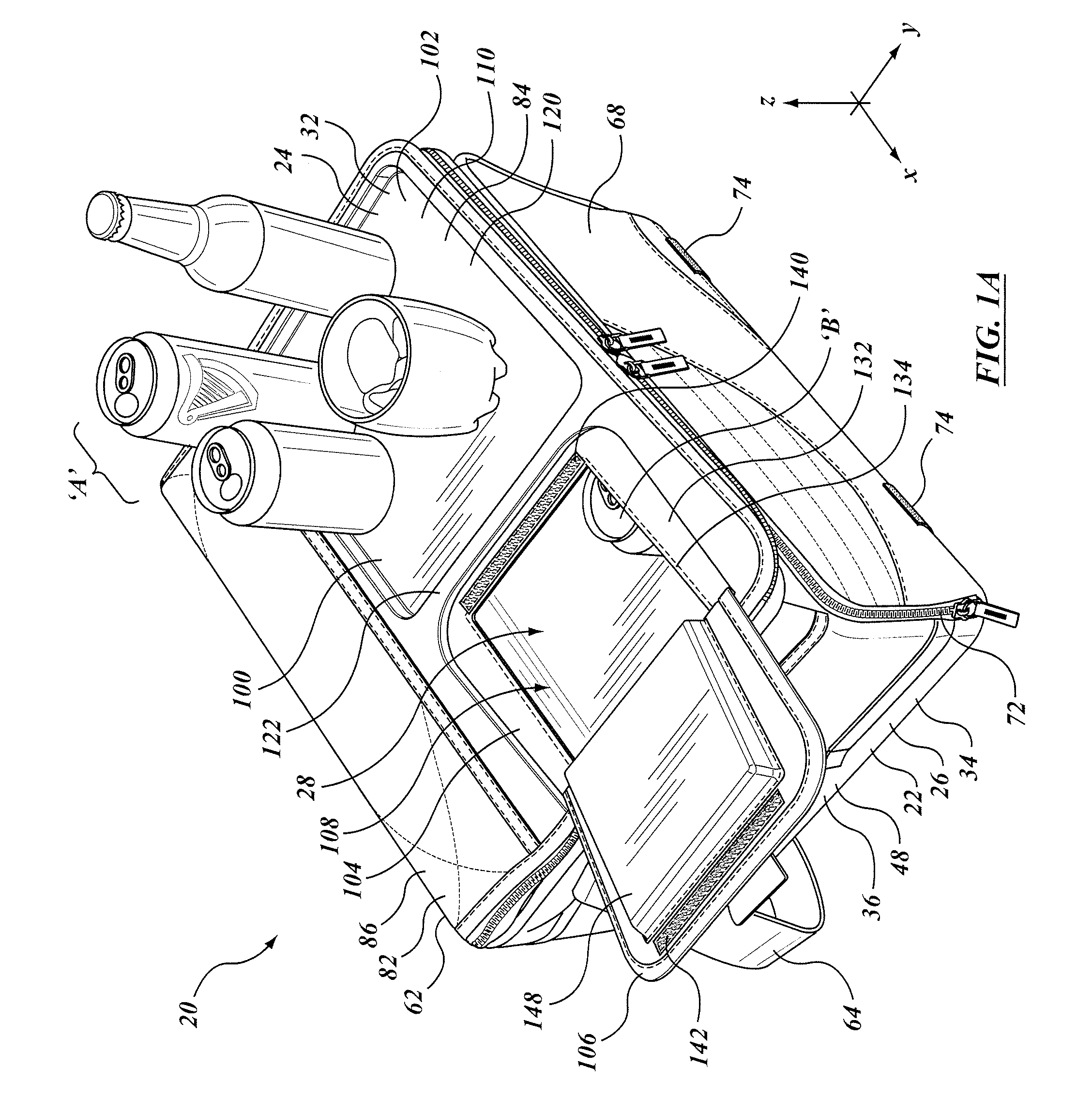
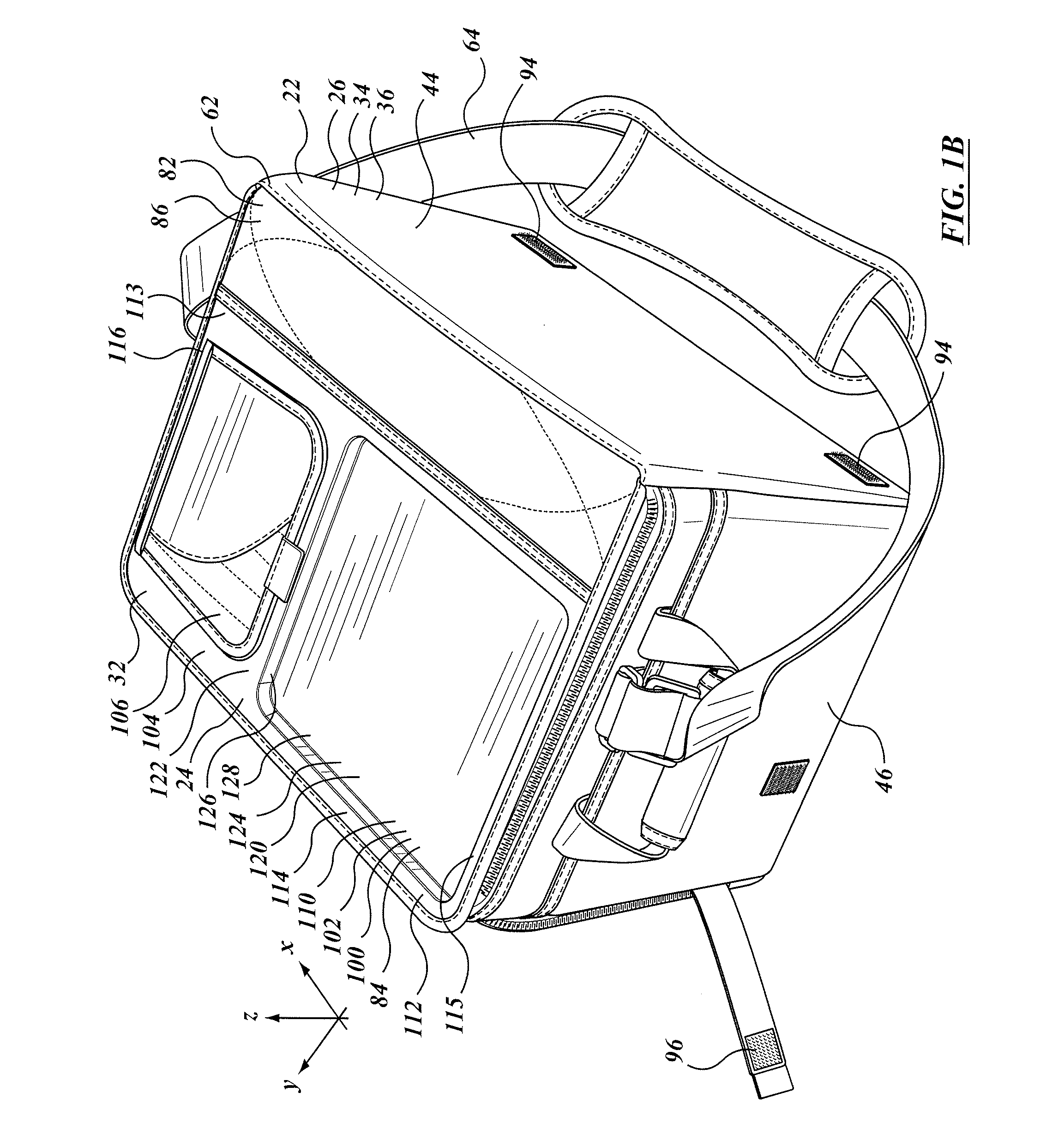
Insulated container with work surface
A soft sided insulated container assembly includes a first portion having an insulated, soft sided external wall structure, and a second portion that defined a lid co-operable with the first portion. The lid includes an auxiliary access opening and closure member that are smaller than the main opening, and that can be operated one-handed. A rigid work surface is provided, typically immediately adjacent to the secondary closure member, the work surface providing a place for holding or mixing a drink or for preparing a snack. In some embodiments the work surface may be movable relative to the container, whether slidable in a co-planar or parallel planar manner in translation, or folding as in a foldable shelf. The entire container assembly may be foldable or collapsible to a storage condition, and the rigid member, whether fixed or movable, does not impede that folding or collapsing.
Owner:CALIFORNIA INNOVATIONS
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20130118127A1Increased durabilitySpectales/gogglesPackage sterilisationHydrophilic coatingPolymer science
The invention is related to a cost-effective method for making a silicone hydrogel contact lens having a crosslinked hydrophilic coating thereon. A method of the invention involves autoclaving, in a sealed lens package, a silicone hydrogel contact lens having a base coating of polyacrylic acid thereon in an aqueous solution in the presence of a water-soluble, crosslinkable hydrophilic polymeric material having epoxide groups, for a period of time sufficient to covalently attach the crosslinkable hydrophilic polymeric material onto the surface of the silicone hydrogel contact lens through covalent linkages each formed between one epoxide group and one of the carboxyl groups on and / or near the surface of the silicone hydrogel contact lens.
Owner:ALCON INC
Methods of providing antioxidants to a drug containing product
Owner:ABBOTT CARDIOVASCULAR
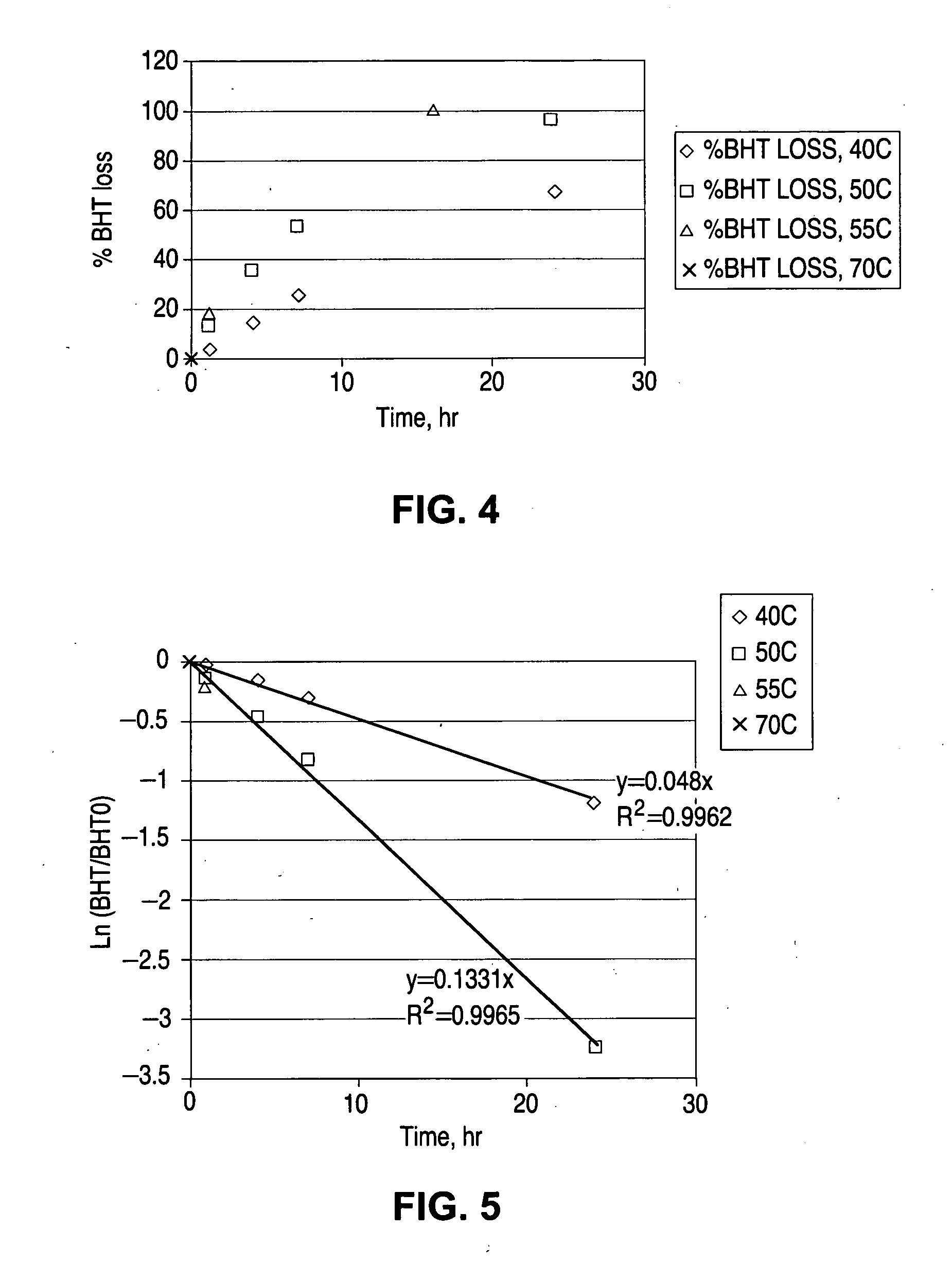
Sterilization system
InactiveUS6592816B1Accurate measurementMaterial analysis using wave/particle radiationElectric discharge tubesElectronRadiation
A sterilization system comprising: radiation source; optical and / or electrical sensors; and timing means; wherein the measurement of radiation by the optical sensor is substantially synchronized based on the timing means to the start and end of each pulse of radiation from the radiation source or to the start and end of the exposure of a product to the radiation source.
Owner:JOHNSON & JOHNSON VISION CARE INC
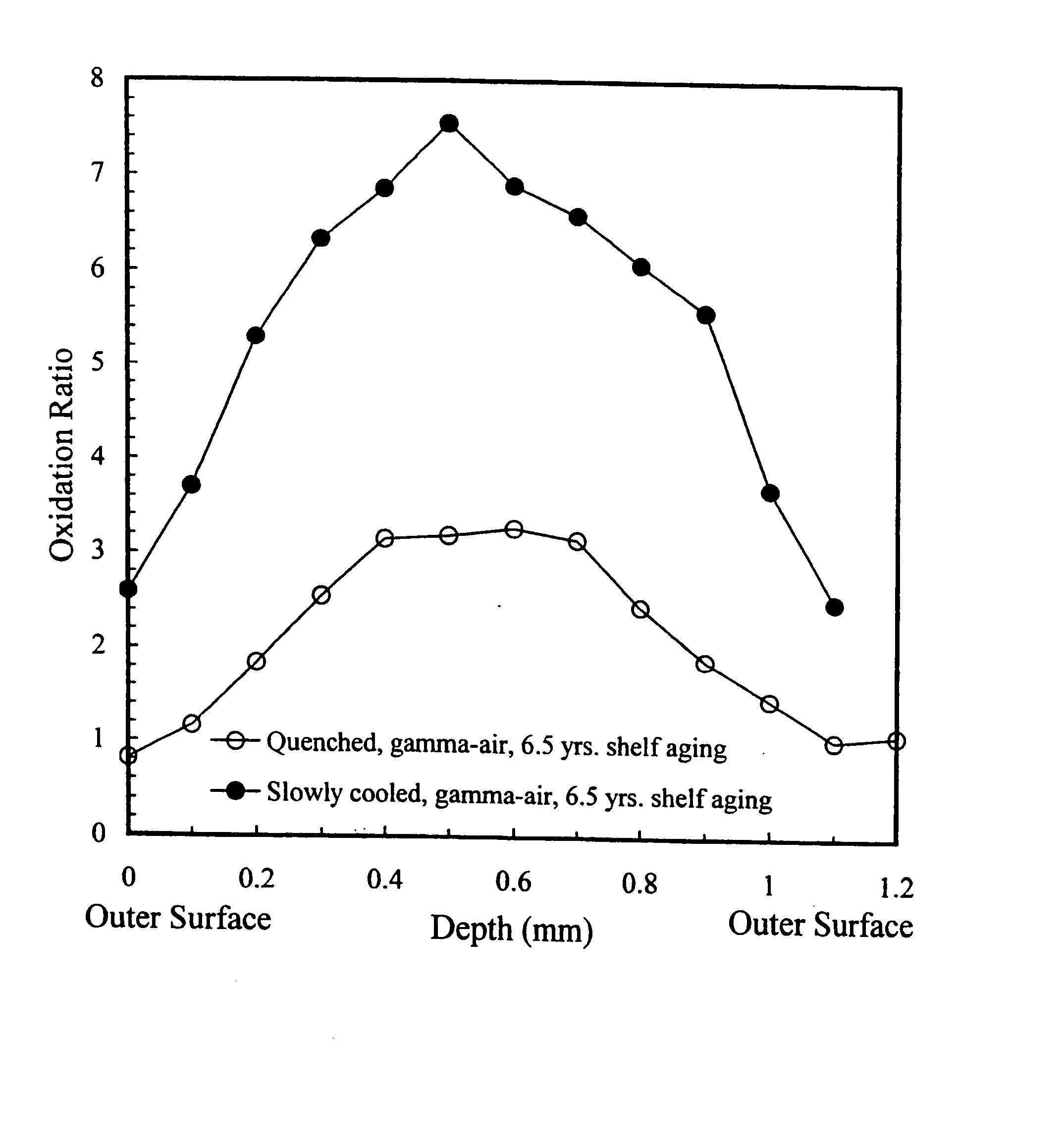
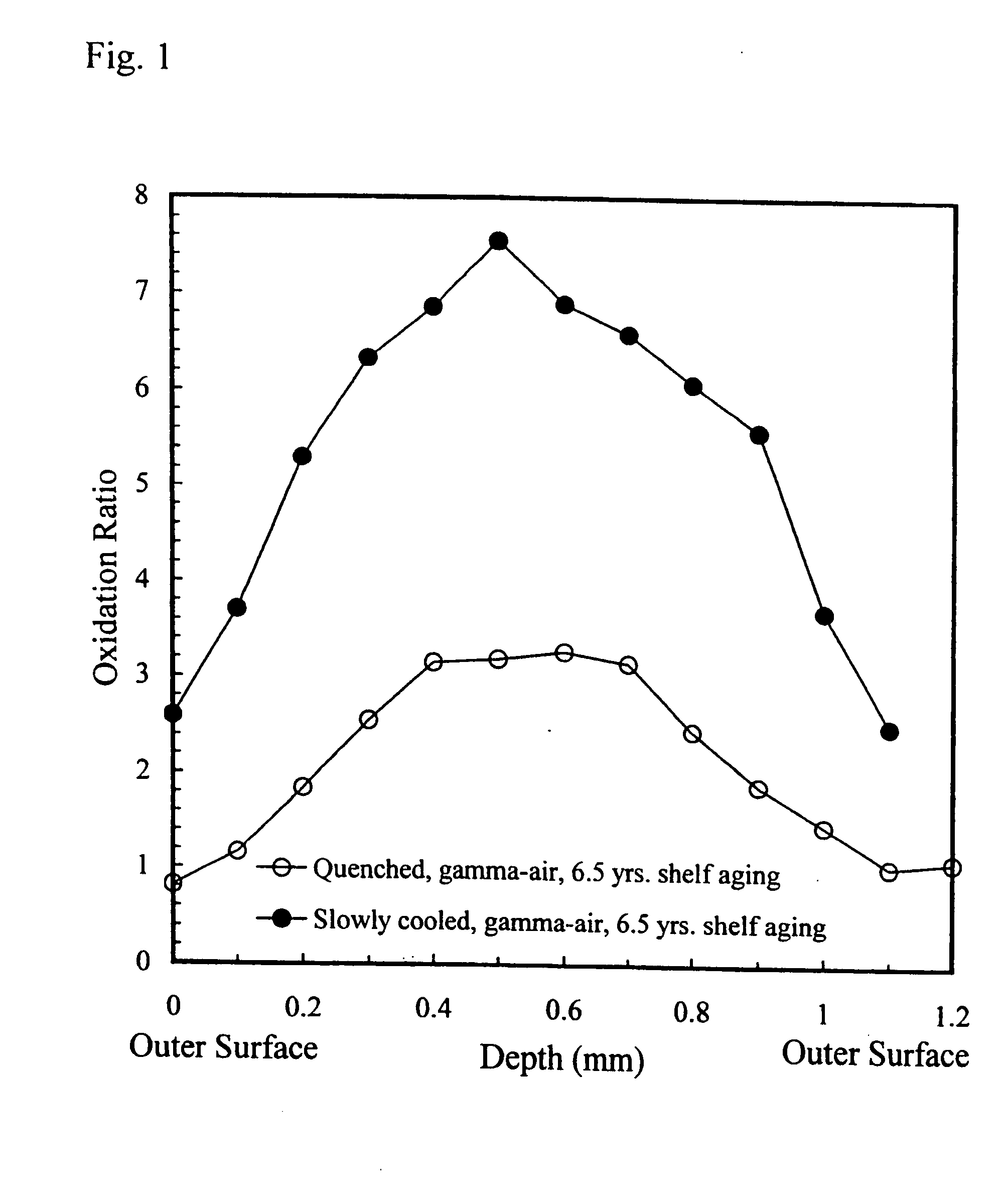
Oxidation-resistant and wear-resistant polyethylenes for human joint replacements and methods for making them
InactiveUS20070293647A1Improve wear resistanceImprove the immunitySurgeryPackage sterilisationPresent methodWear resistant
The present invention presents methods for making oxidation-resistant and wear-resistant polyethylenes and medical implants made therefrom. Preferably, the implants are components of prosthetic joints, e.g., a bearing component of an artificial hip or knee joint. The resulting oxidation-resistant and wear-resistant polyethylenes and implants are also disclosed.
Owner:THE ORTHOPAEDIC HOSPITAL
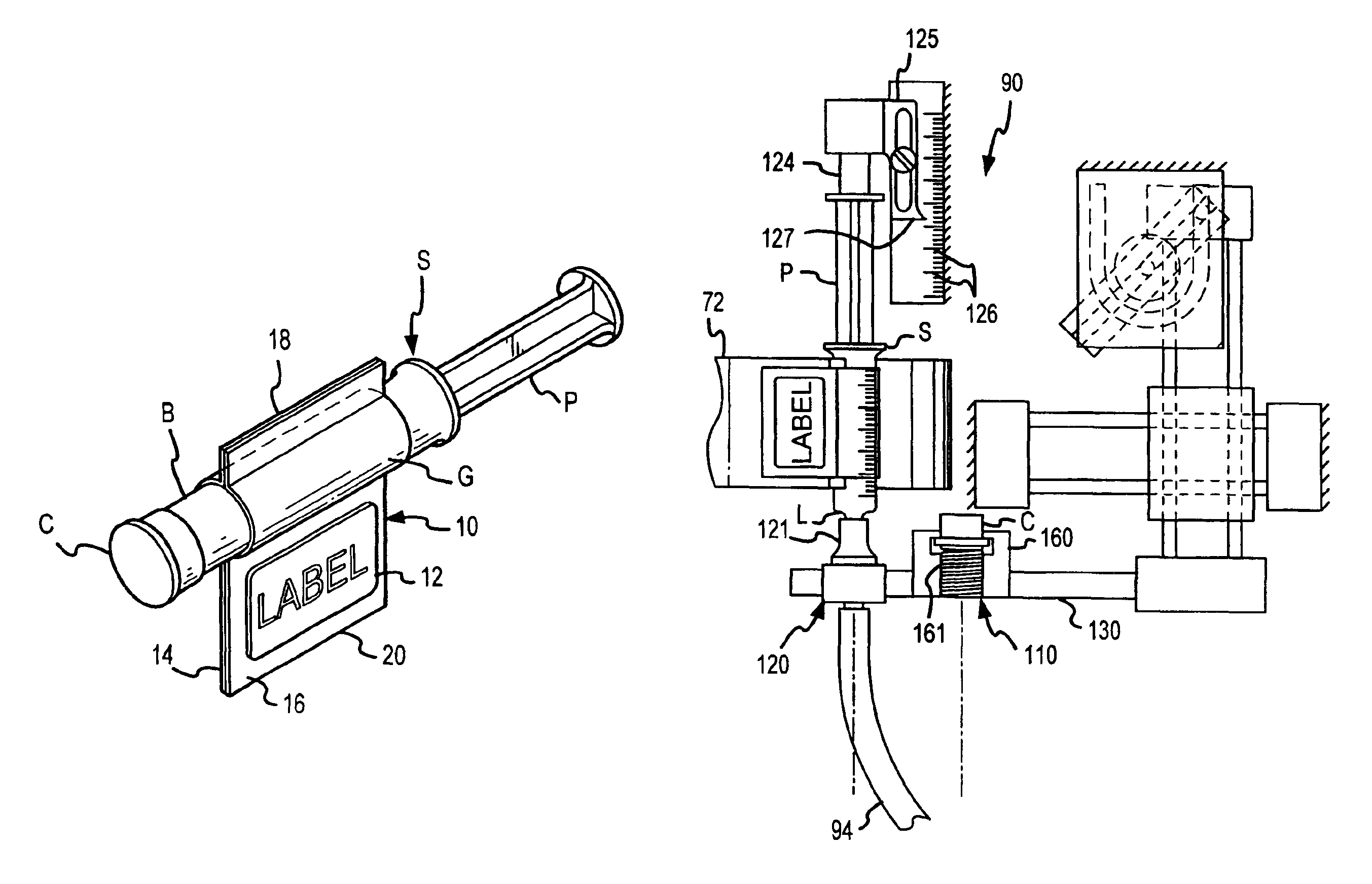
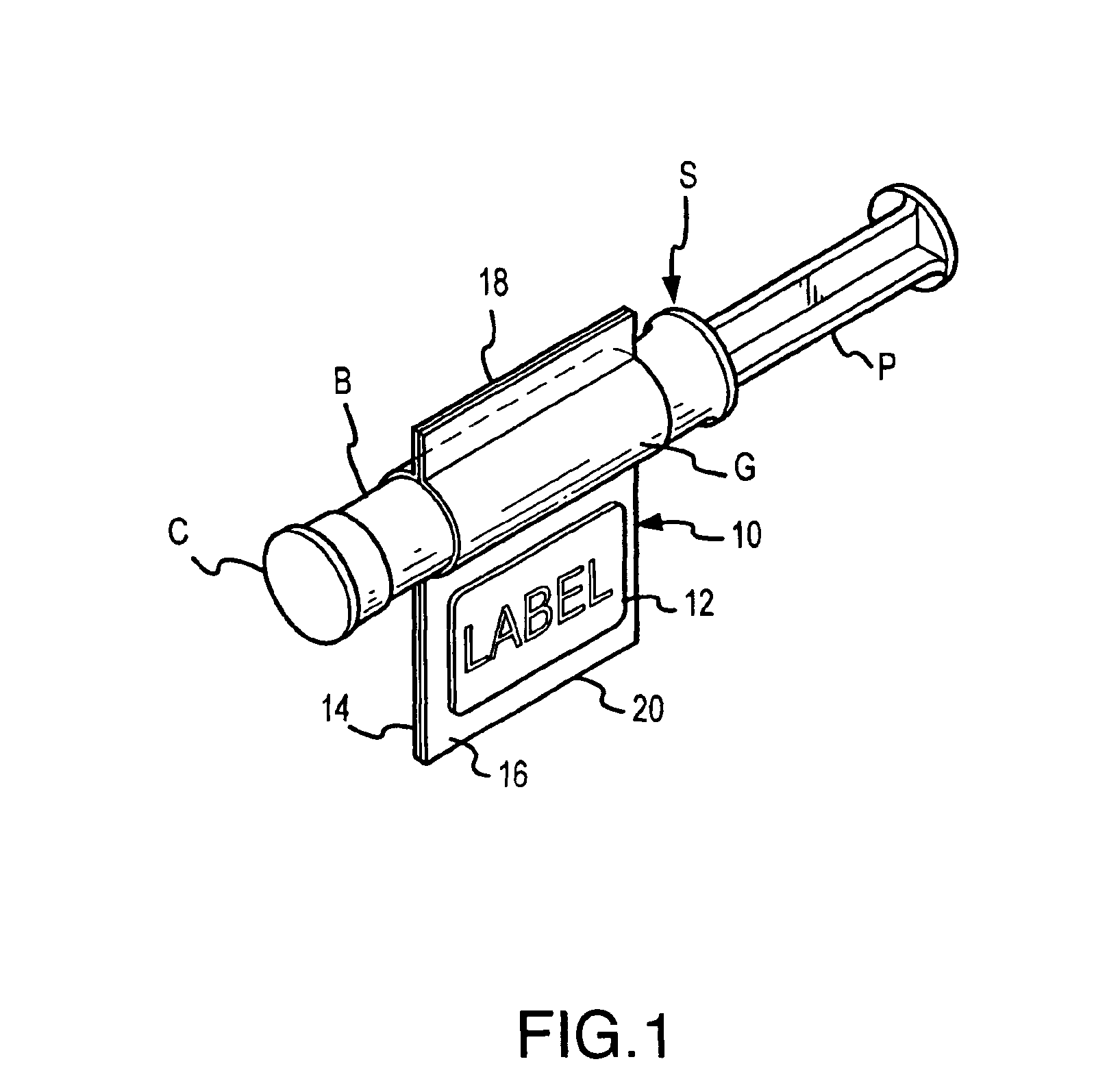
Method for filling and capping syringes
InactiveUS6976349B2Facilitates maintenance of sterilityImproved syringe fillingCapsAmpoule syringesManual handlingBiomedical engineering
An inventive method, system and apparatus are provided for syringe handling, and more particularly, for syringe labeling, filling and capping operations. To facilitate syringe handling, an inventive apparatus includes a plurality of syringe bodies interconnected in a predetermined orientation by a belt. Such belt may be of pliable construction and may define a predetermined spacing in between adjacent ones of the syringe bodies, such predetermined spacing corresponding with a distance between holders provided in a handling apparatus. The syringe handling apparatus may provide for the placement of contents-related information on belt segments between adjacent syringe bodies and for separating the belt segments, wherein a flap is left interconnected to each syringe body. The syringe handling apparatus may alternatively or also provide for automated filling of the syringe bodies wherein cap removal, filling and cap replacement operations are completed free from manual handling.
Owner:BAXTER ENGLEWOOD
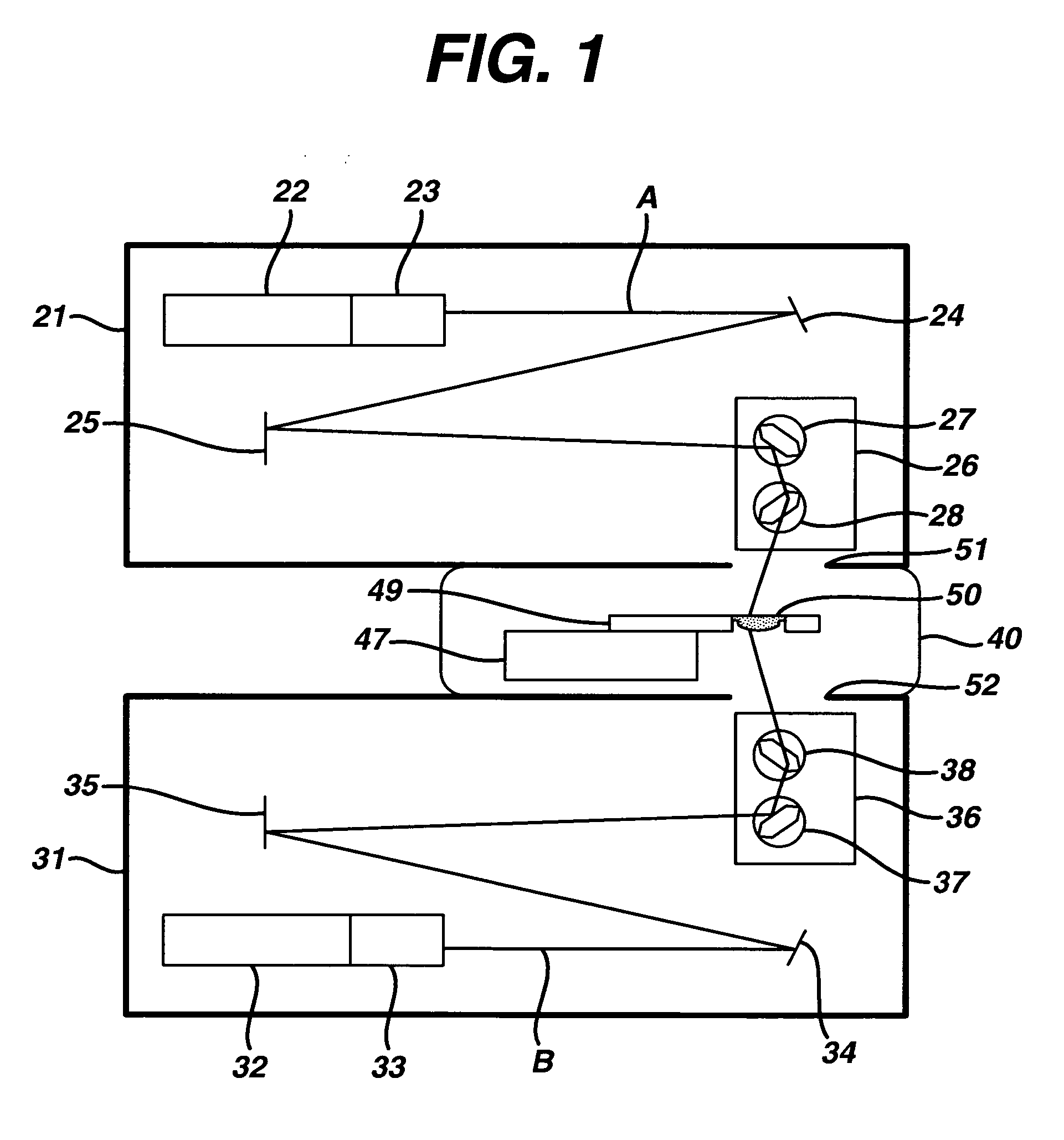
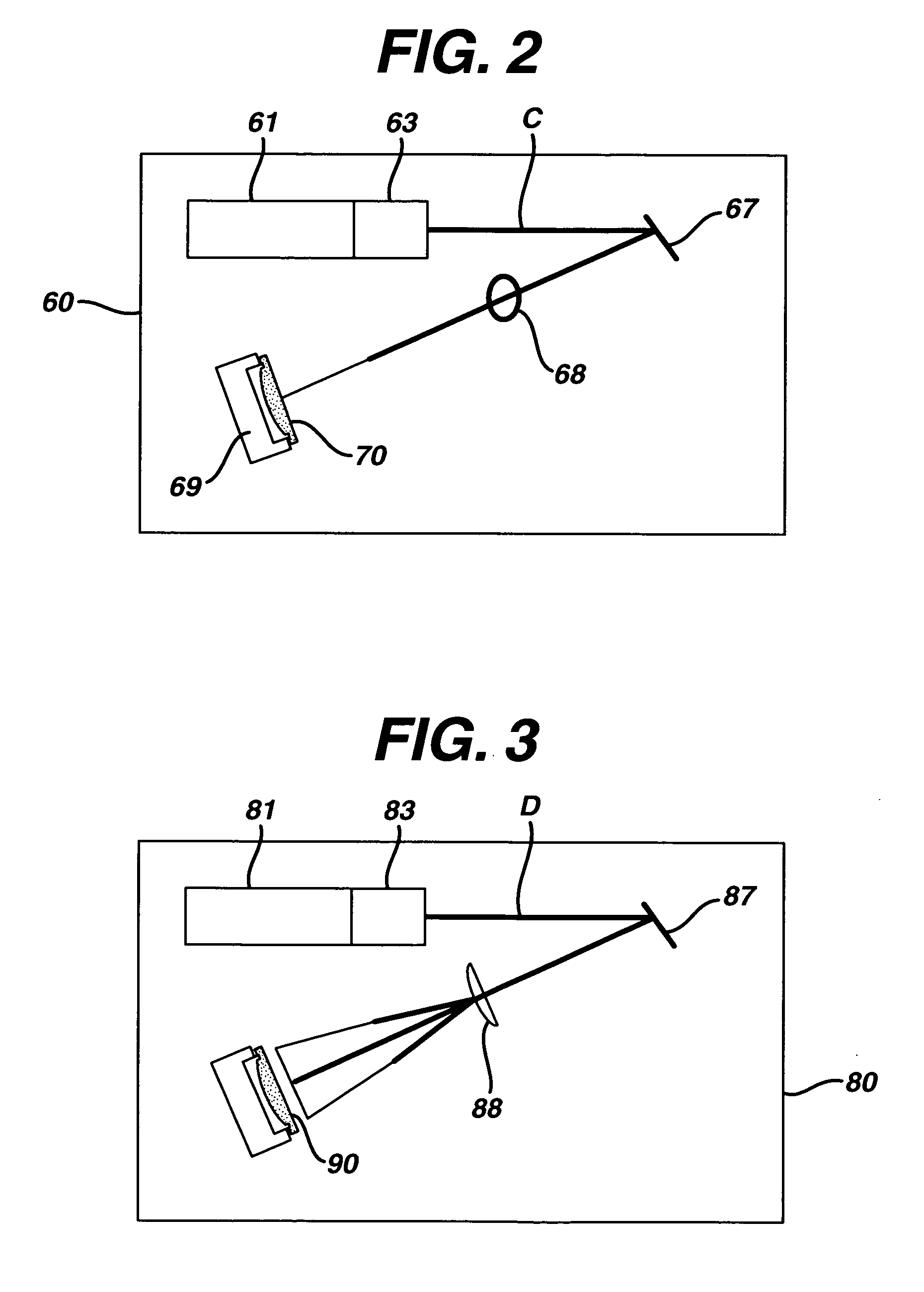
Method and apparatus of sterilization using monochromatic UV radiation source
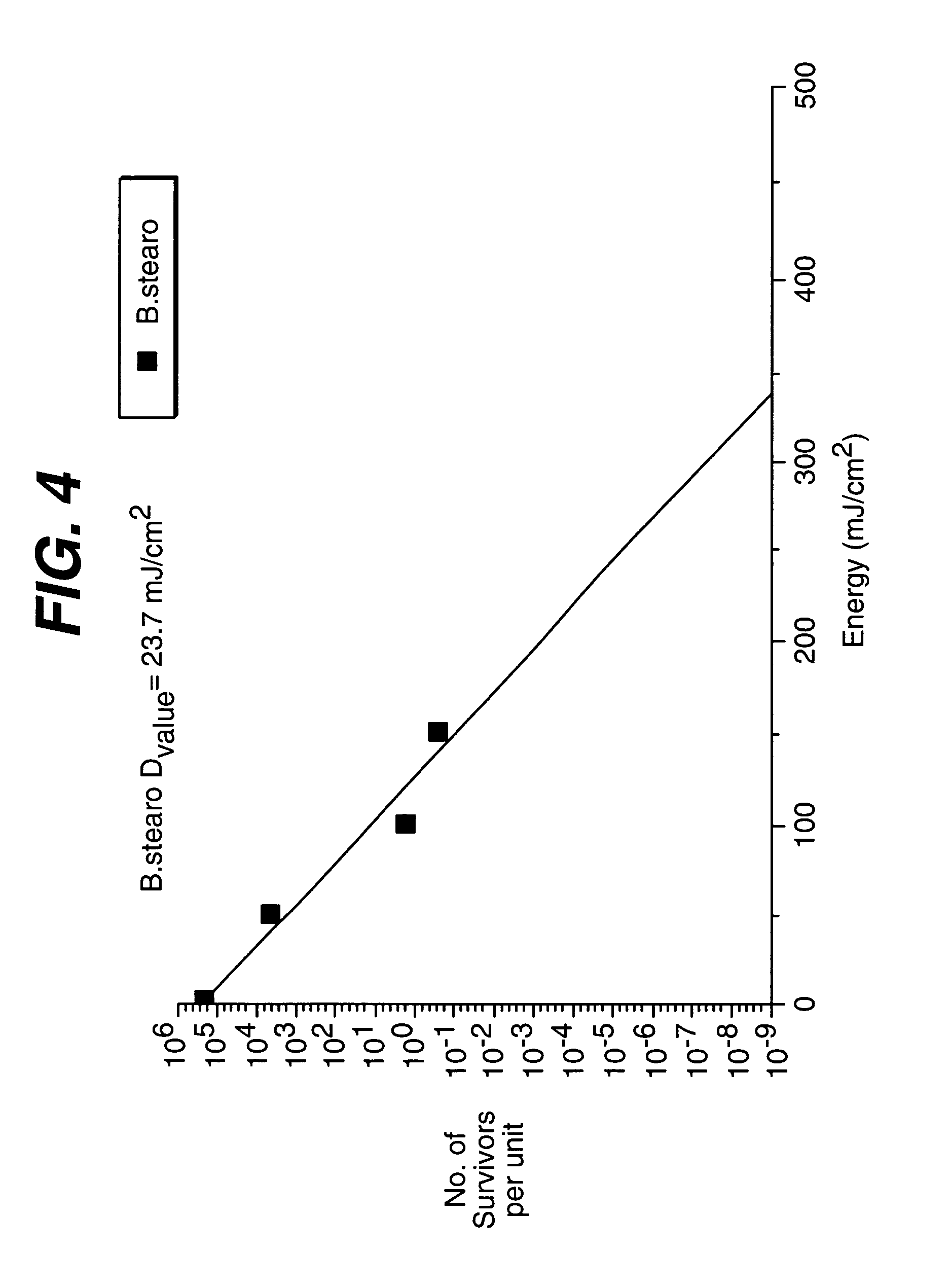
This invention provides a process of sterilizing a medical device, and preferably the contents of a sealed container which comprises said medical device, comprising the step of exposing said medical device to monochromatic ultraviolet radiation whereby the Dvalue of Bacillus stearothermophilus (ATCC 7953) is at least 23.7 mJ / cm2 monochromatic ultraviolet radiation at 257 nm to the spore. Further, this invention provides a process of sterilizing a medical device comprising the step of subjecting said medical device to monochromatic ultraviolet radiation wherein the minimum total energy density of said monochromatic ultraviolet radiation at 257 nm which reaches the microorganisms present on said medical device is at least 284 mJ / cm2. This invention further provides an apparatus for delivering UV radiation to a medical device for sterilization comprising a laser and a scanner for the laser such that at least 284 mJ / cm2 at 257 nm is applied to a treatment area for said medical device. This invention provides a process and apparatus in which sterilization can be achieved in less than 20 seconds, preferably less than 15 seconds, more preferably in less than 5 seconds. The process and apparatus are efficient and continuous.
Owner:JOHNSON & JOHNSON VISION CARE INC
Silicone hydrogel lens with a grafted hydrophilic coating
ActiveUS8409599B2Easy to implementMinimal orDental implantsPackage sterilisationHydrophilic coatingRoom temperature
The invention provides a cost-effective method for applying a hydrophilic coating onto a silicone hydrogel contact lens based on Fenton chemistry. The hydrophilic coating is covalently attached onto the contact lens at room temperature without UV irradiation. The invention also provides silicone hydrogel contact lenses having a hydrophilic coating obtained according to the method of the invention.
Owner:ALCON INC
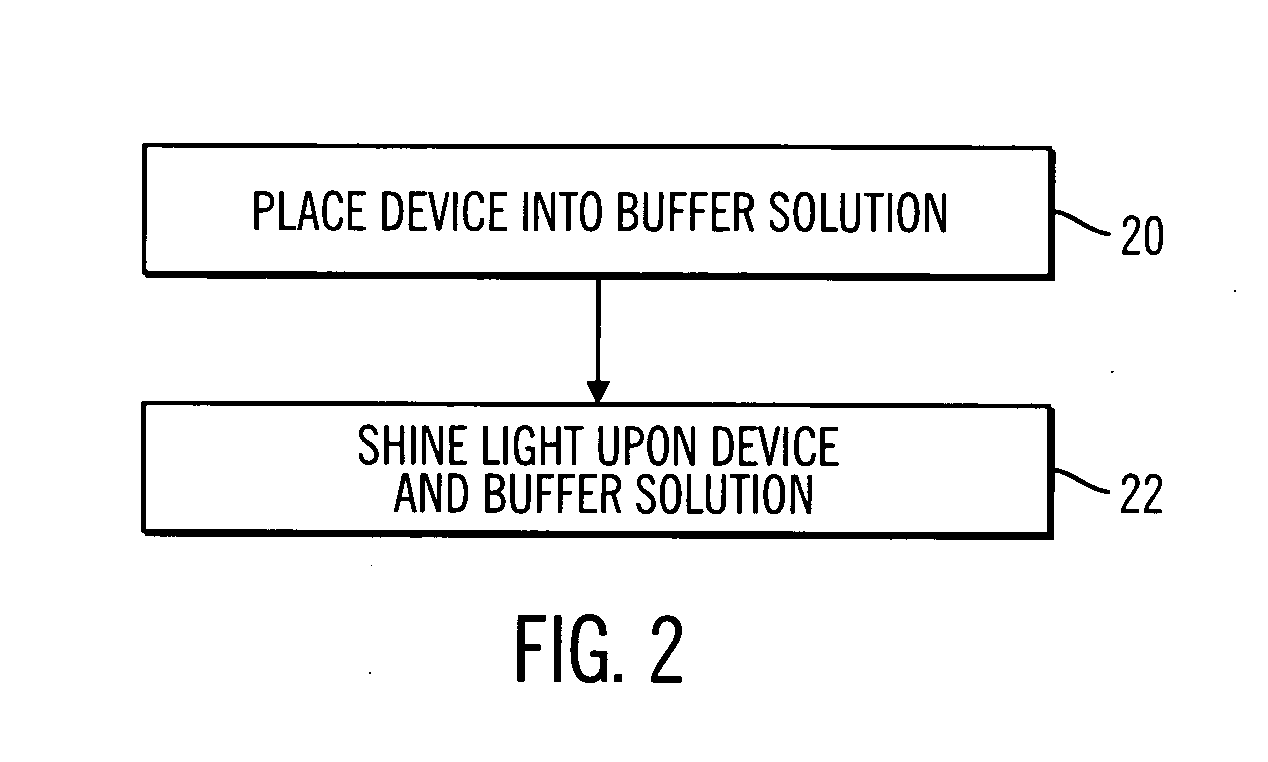
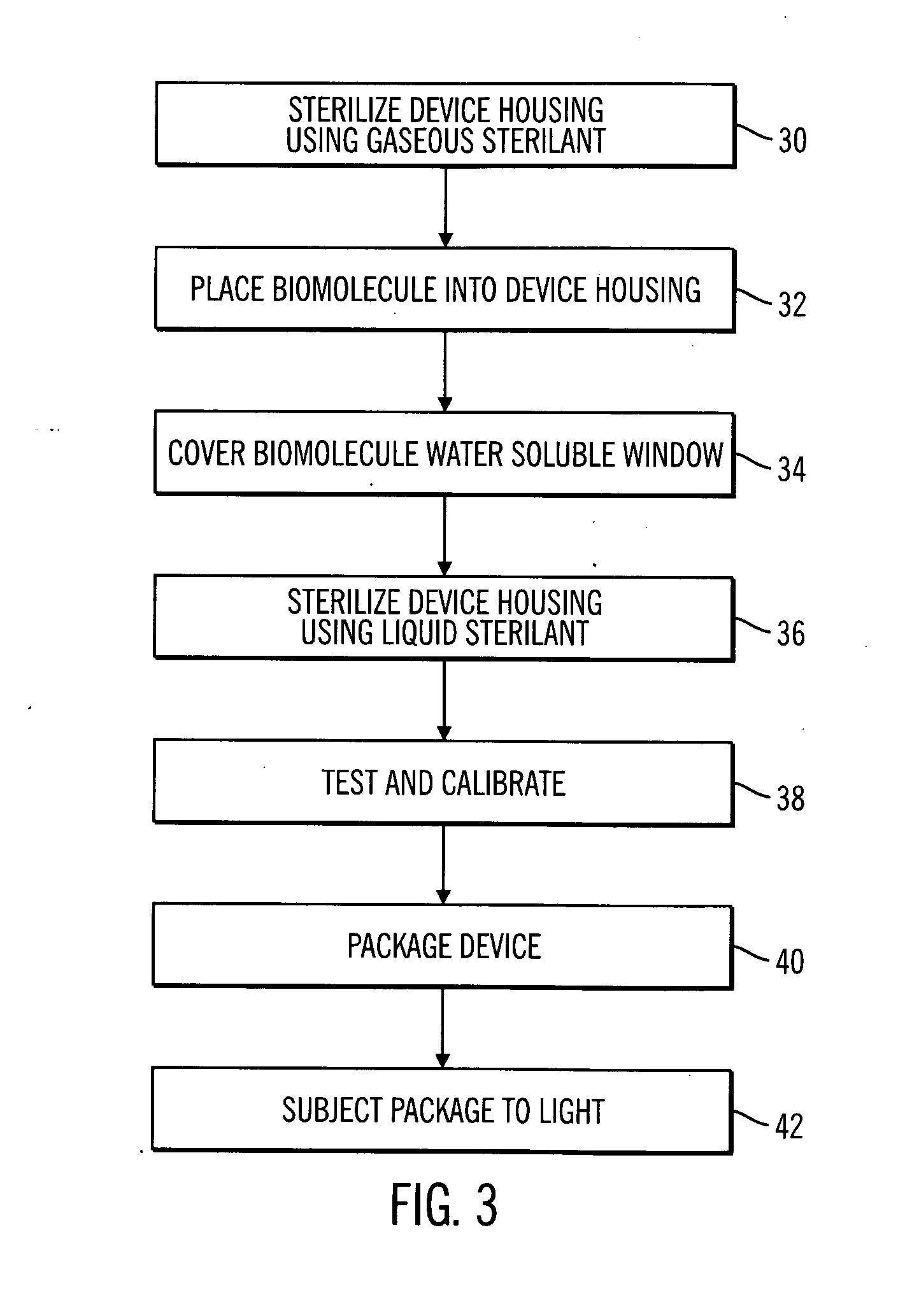
Sterile device and method for producing same
A sterile device immersed in a sterile buffer and a method for providing same. The sterile device may be a medical device such as a biosensor having a biomolecule as a sensing element such as, for example, a glucose oxidase enzyme. The buffer may be a bicarbonate solution. Both the device and the buffer may be packaged and stored over long term while maintaining sterilization. The sterilization method may comprise a combination of gaseous, liquid and light sterilization.
Owner:MEDTRONIC MIMIMED INC
Wound dressing and apparatus for forming same
A multi-layered wound dressing includes a moisture-retaining portion for enhancing the healing of a wound. The wound dressing includes an intermediate layer that has both water soluble and water insoluble fibers. An apparatus that includes a cutting tool and a reservoir of liquid to pre-moisten a portion of the dressing may be used to manufacture the dressings.
Owner:POLYREMEDY
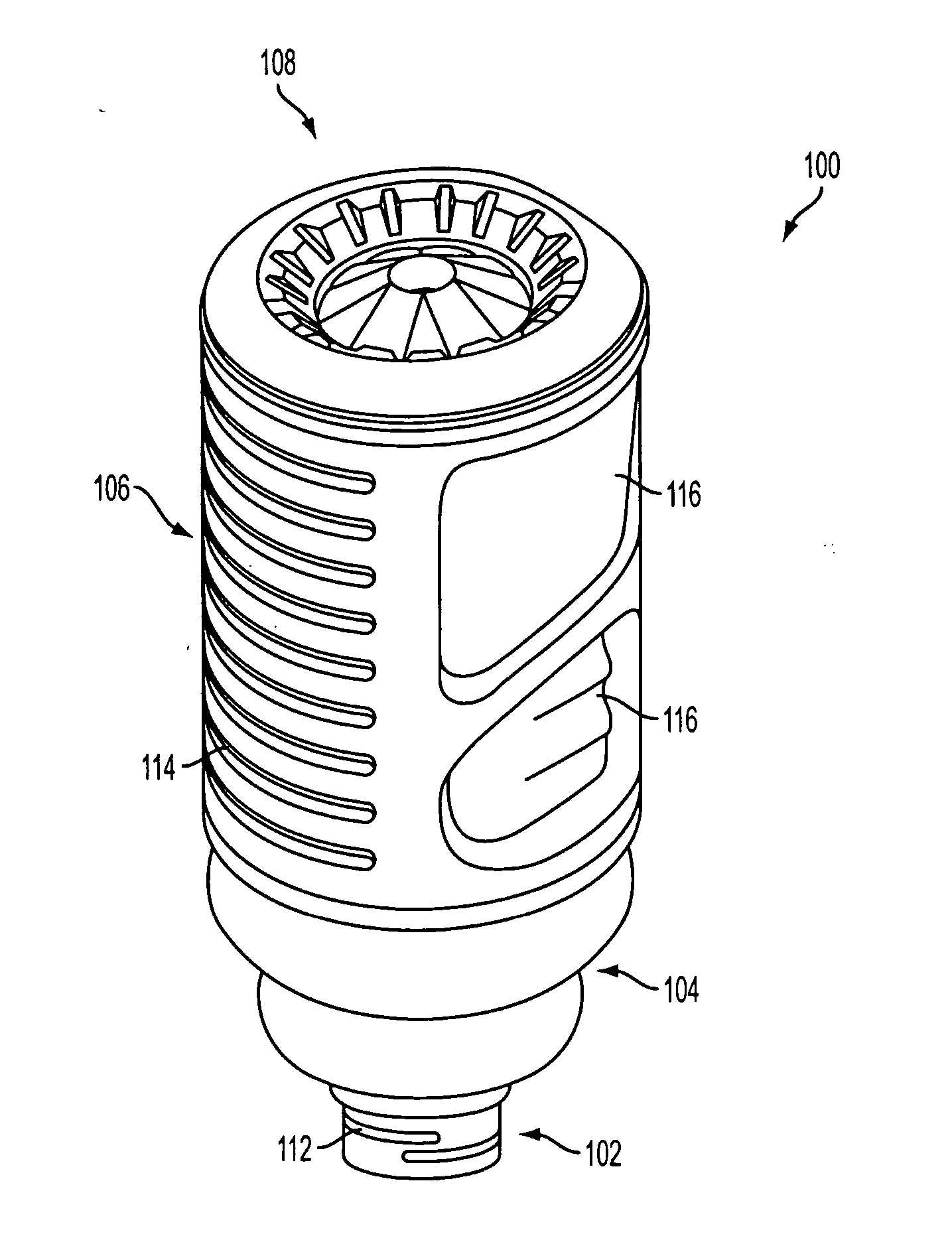
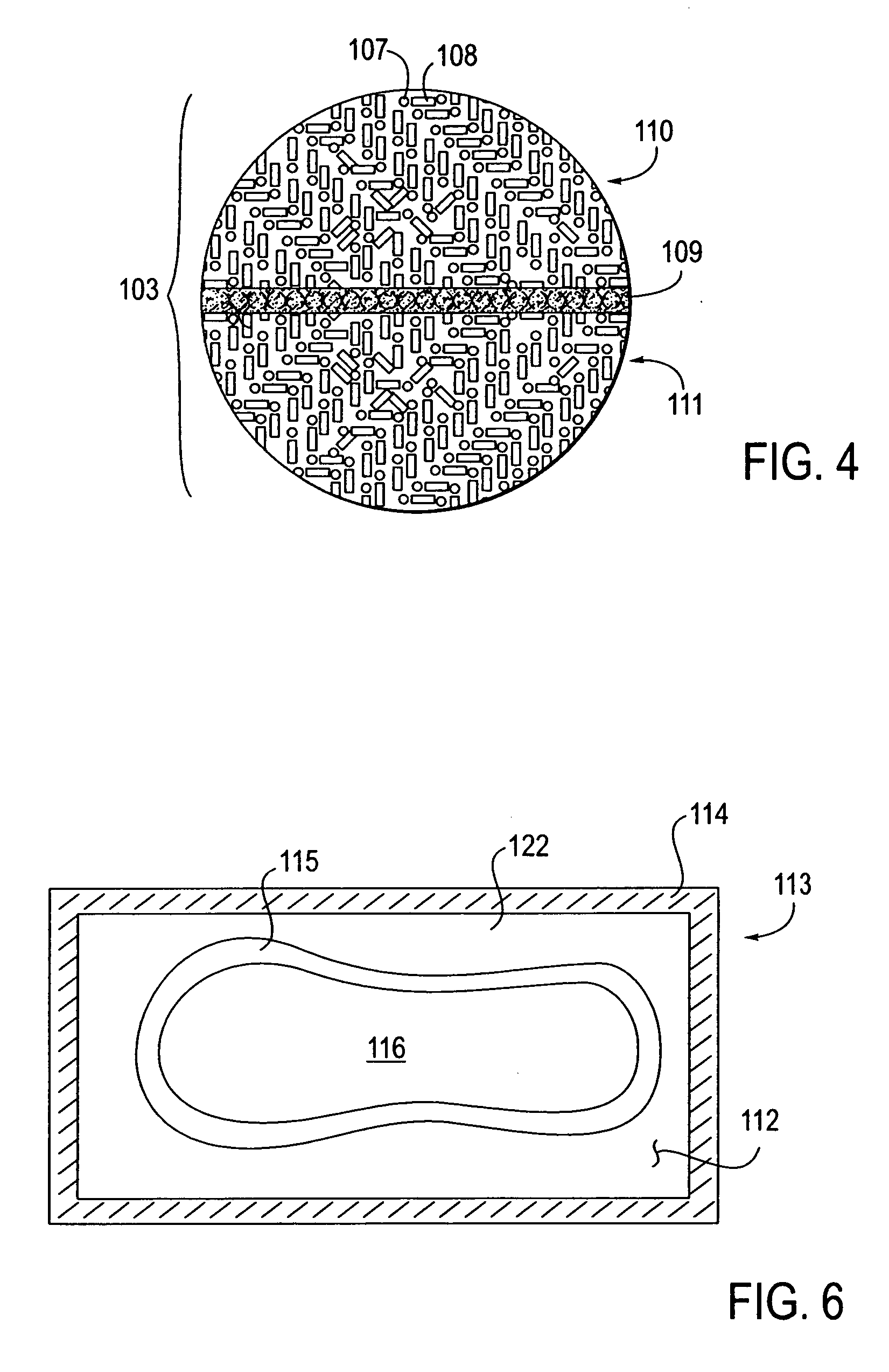
Ethanol-free gel formulation cartridge for e-vaping device
A cartridge for an e-vaping device includes an ethanol-free gel formulation. The ethanol-free gel formulation includes a vapor former, water, and a biopolymer. The biopolymer may be included in an amount ranging from about 0.01% by weight based on the weight of the ethanol-free gel formulation to about 2.0% by weight based on the weight of the ethanol-free gel formulation. The biopolymer may be one or more of agar, kappa carrageenan, gelatin, sodium alginate, gellan gum, pectin, and combinations thereof. The cartridge also includes a heater configured to heat liquid from the gel formulation to a temperature sufficient to release a liquid / semi-liquid component from the gel, which component thereupon forms a vapor.
Owner:AKRIA CLIENT SERVICES LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com