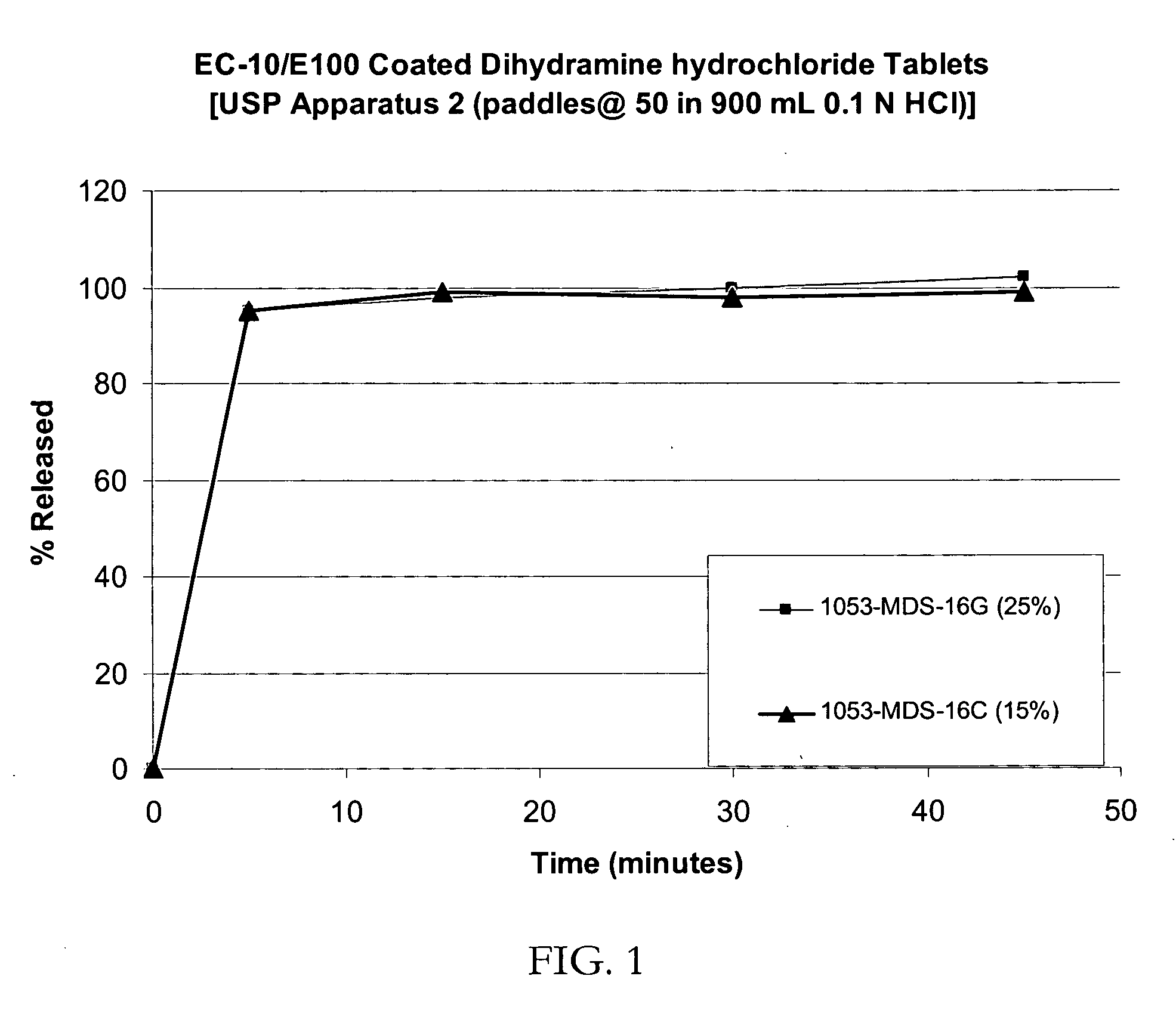
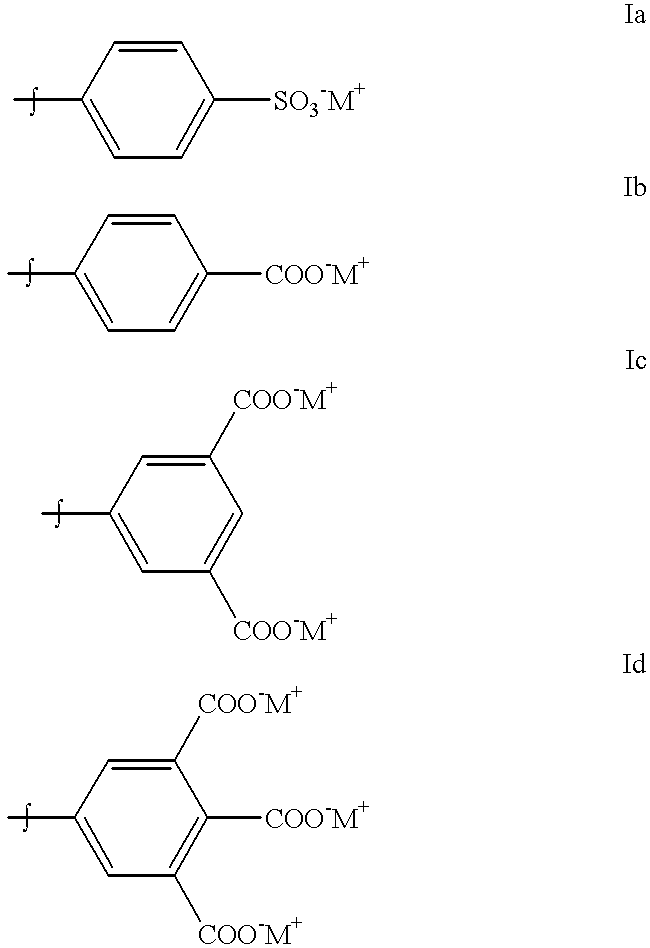
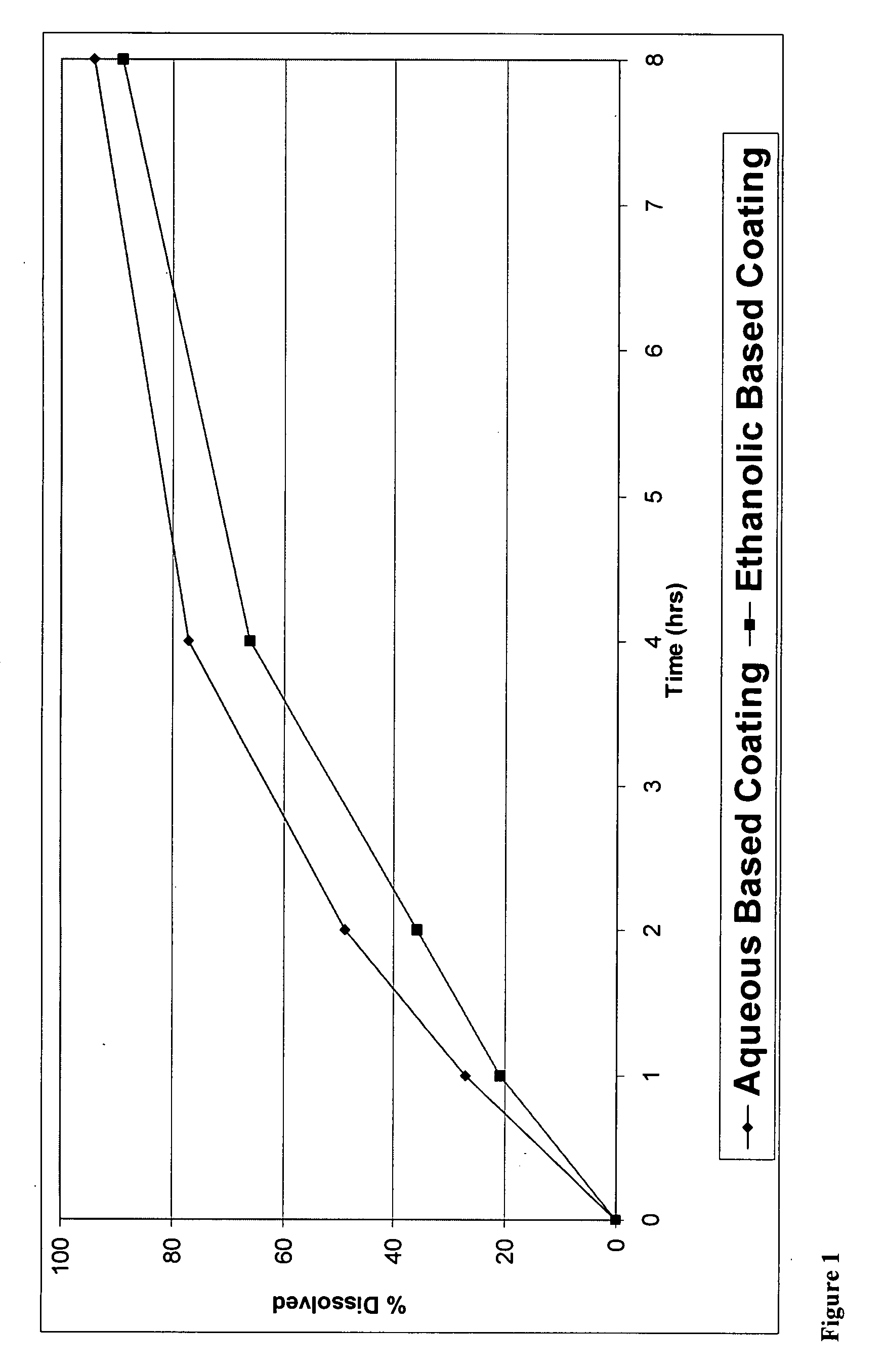
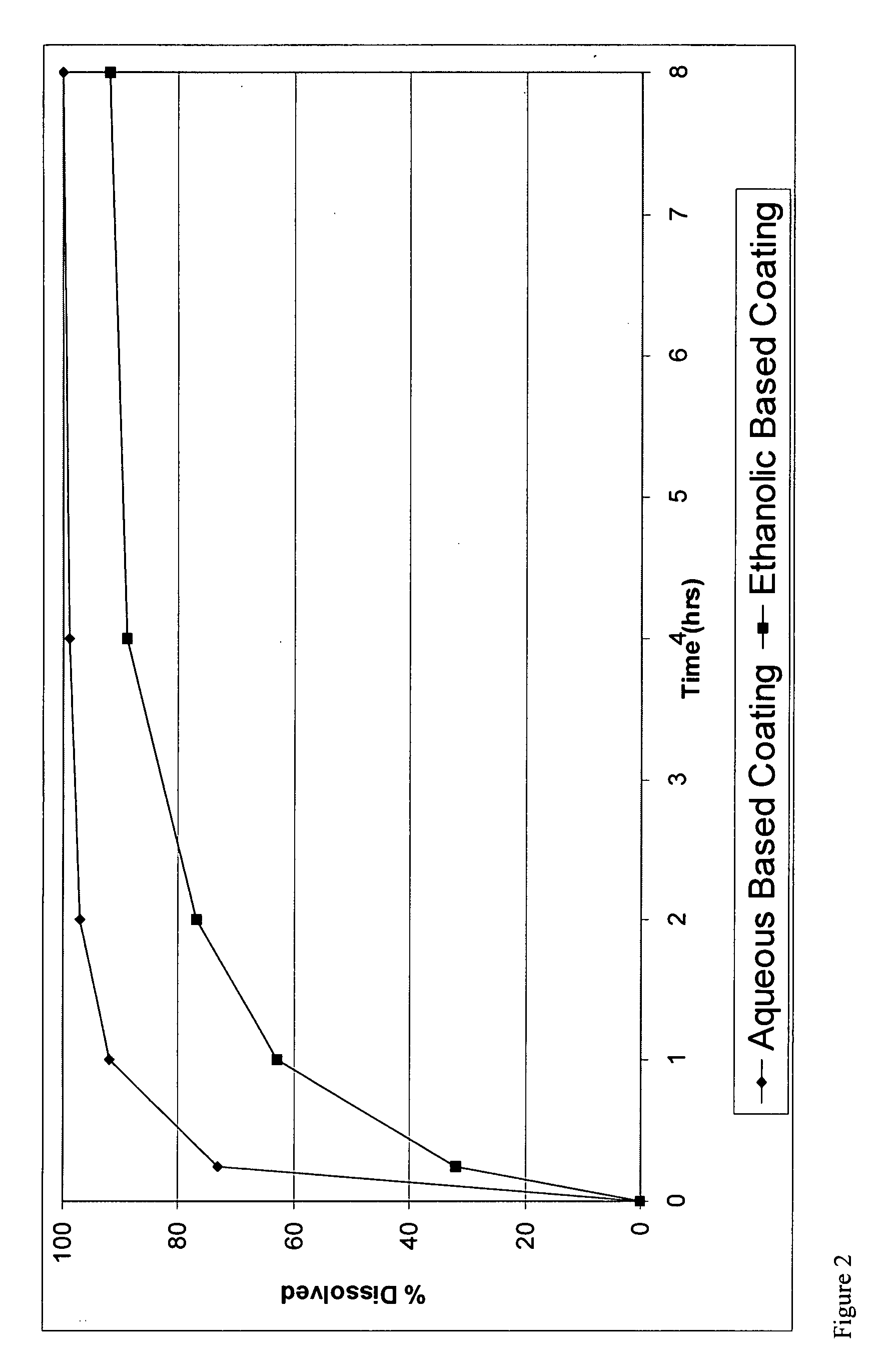
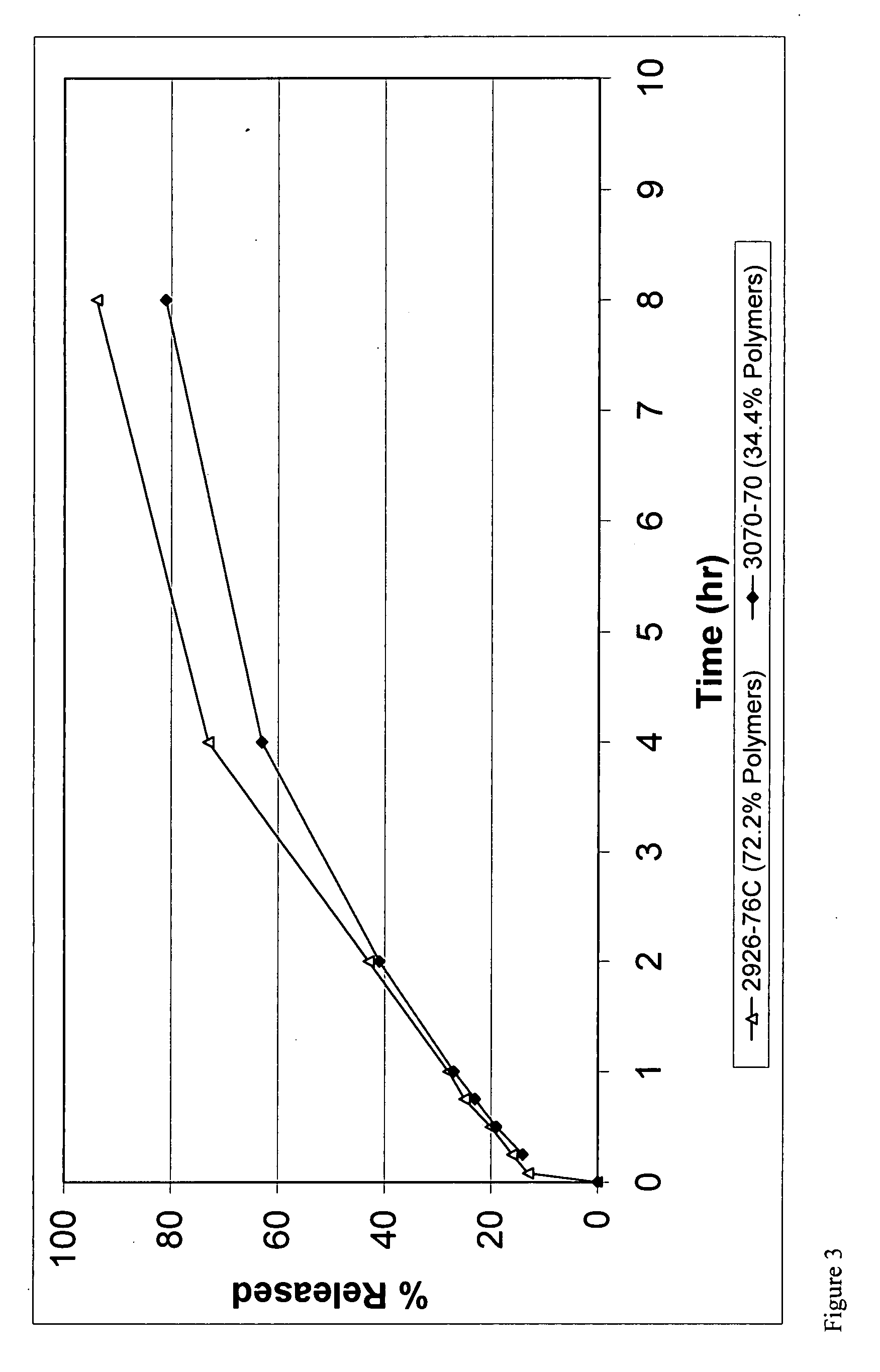
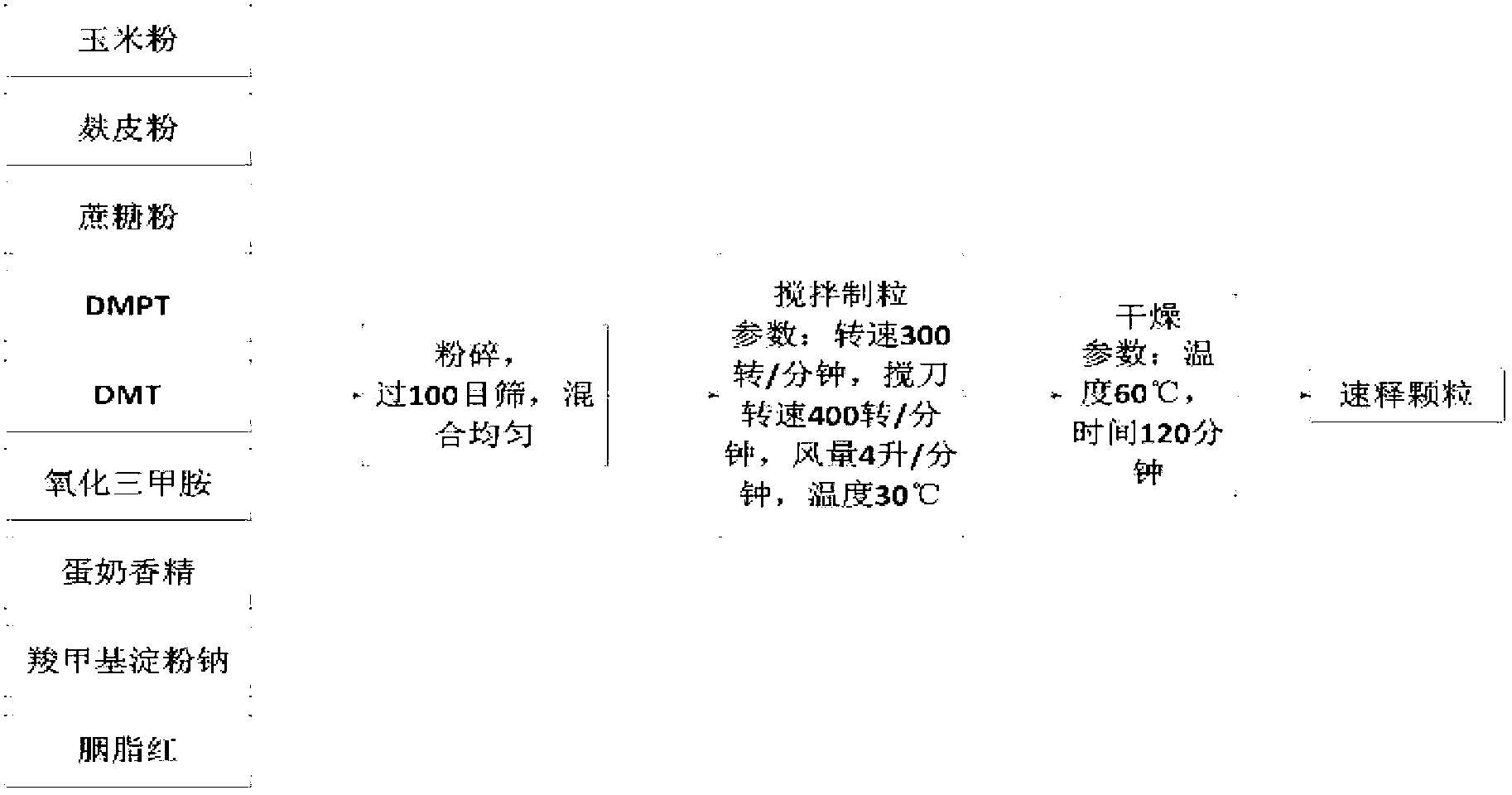Patents
Literature
4339 results about "Water insoluble" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insoluble comes from the Latin insolubilis meaning "that cannot be loosened.". When a substance is insoluble, it cannot be dissolved or loosened in water. Similarly, a situation that is insoluble has no hope of being solved.
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
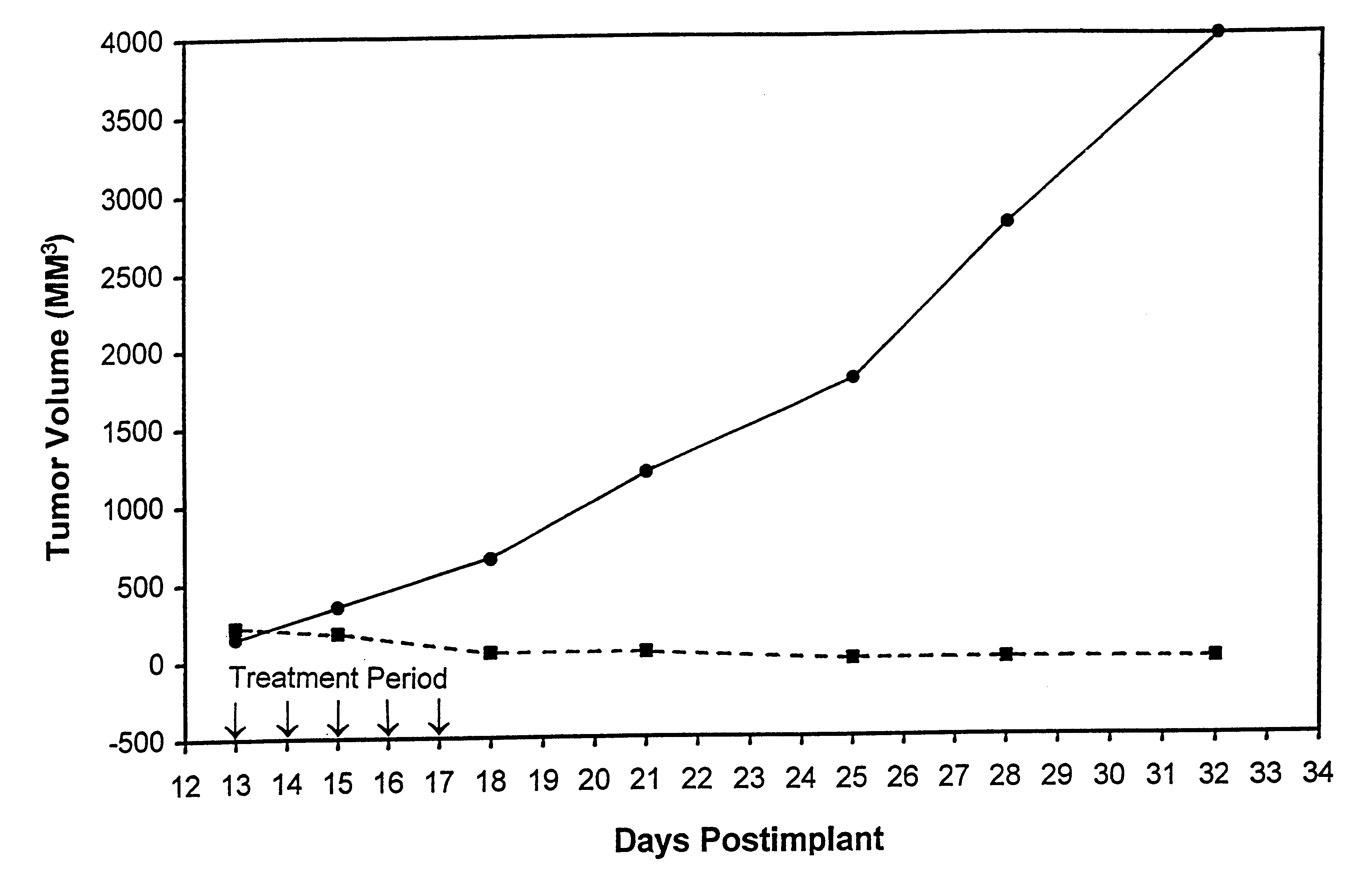
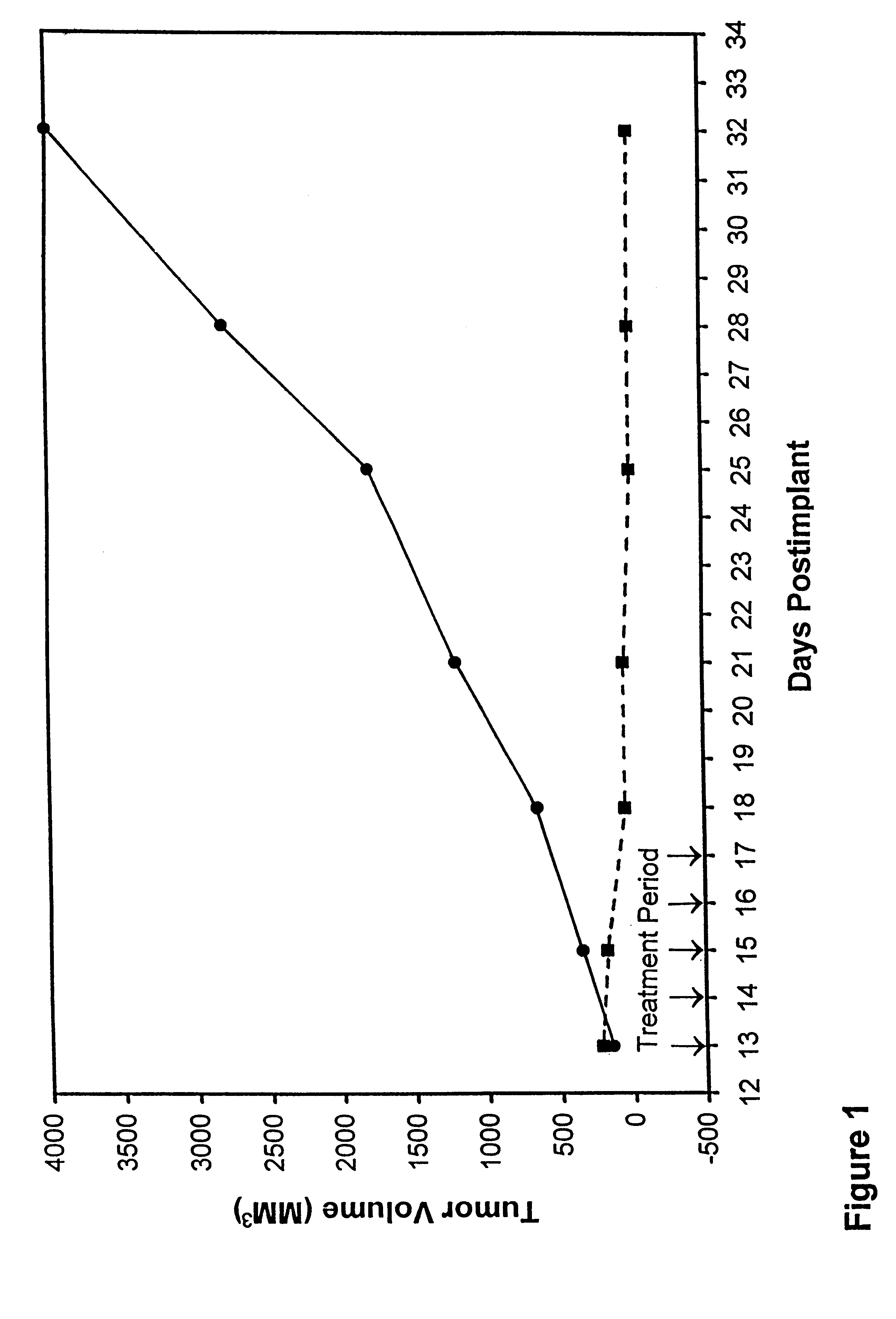
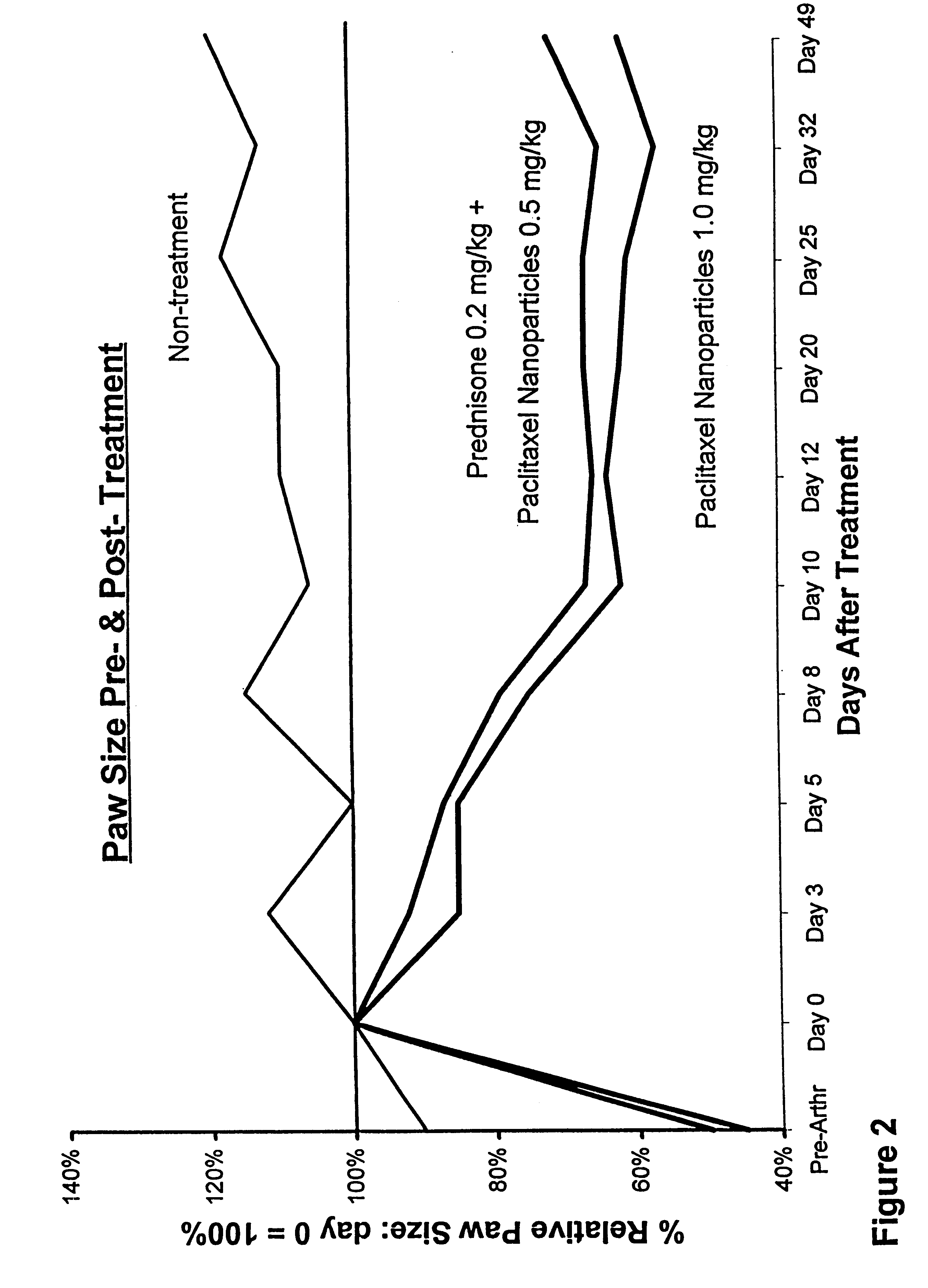
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Tobacco and/or tobacco substitute composition for use as a snuff in the oral cavity
InactiveUS20050061339A1Reduce releaseAvoid spreadingTobacco treatmentSide effectEnvironmental health
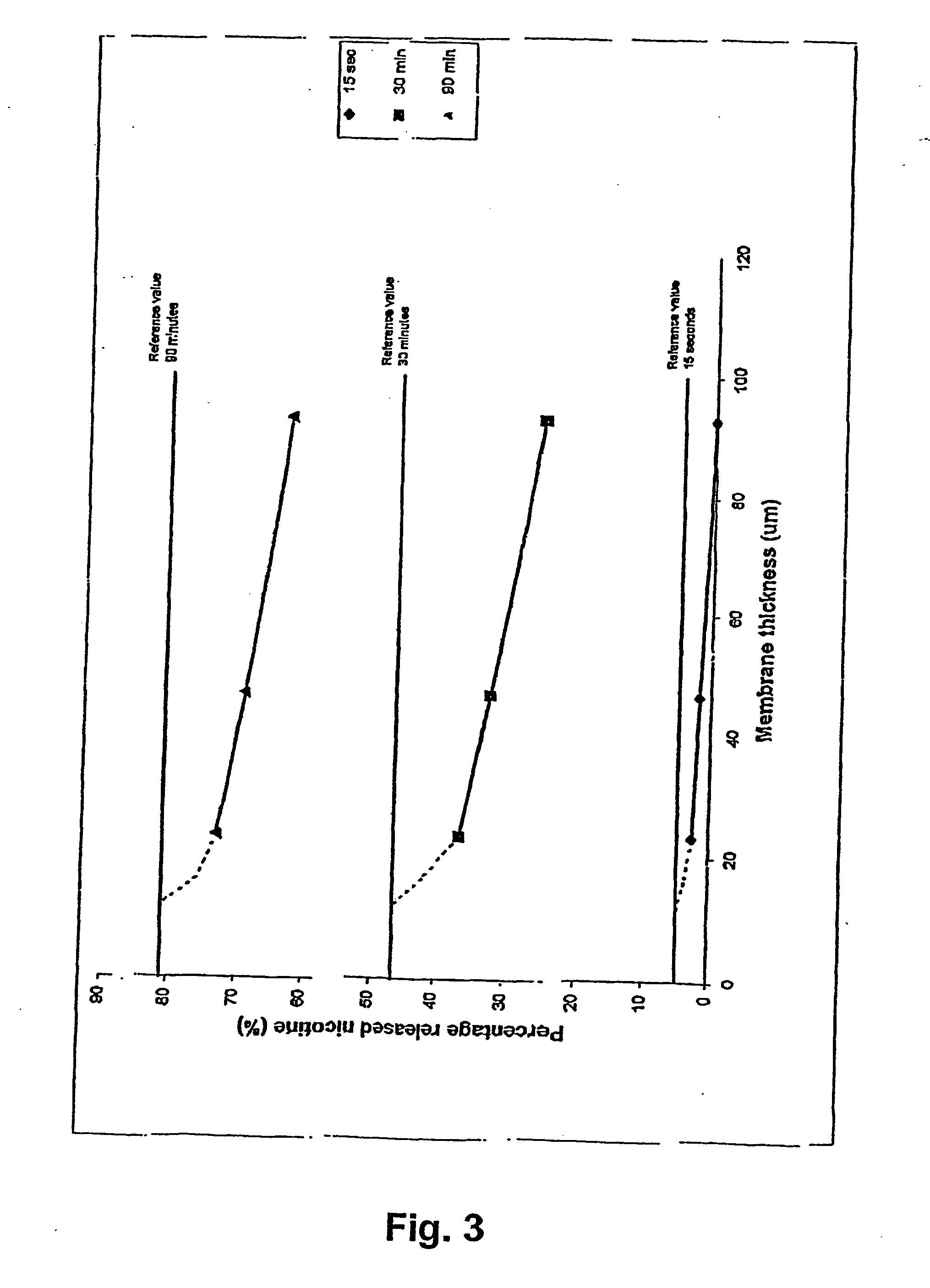
A novel composition for the use as snuff in the oral cavity, the composition comprising tobacco and / or a tobacco substitute encapsulated in a membrane material comprising one or more membranes at least one of which being water permeable and water-insoluble. A novel composition enables a selective release of e.g. nicotine while it at the same time reduces the release of substances, which normally lead to unwanted side effects. The novel compositions may be used as a healthier alternative to snuff and other tobacco products such as, e.g., cigarettes, cigars and pipe. Methods for giving up smoking, reducing nicotine craving, reducing side effects normally related to smoking and snuffing of tobacco as welt a method for the preparation of a composition according to the invention.
Owner:GALENICA
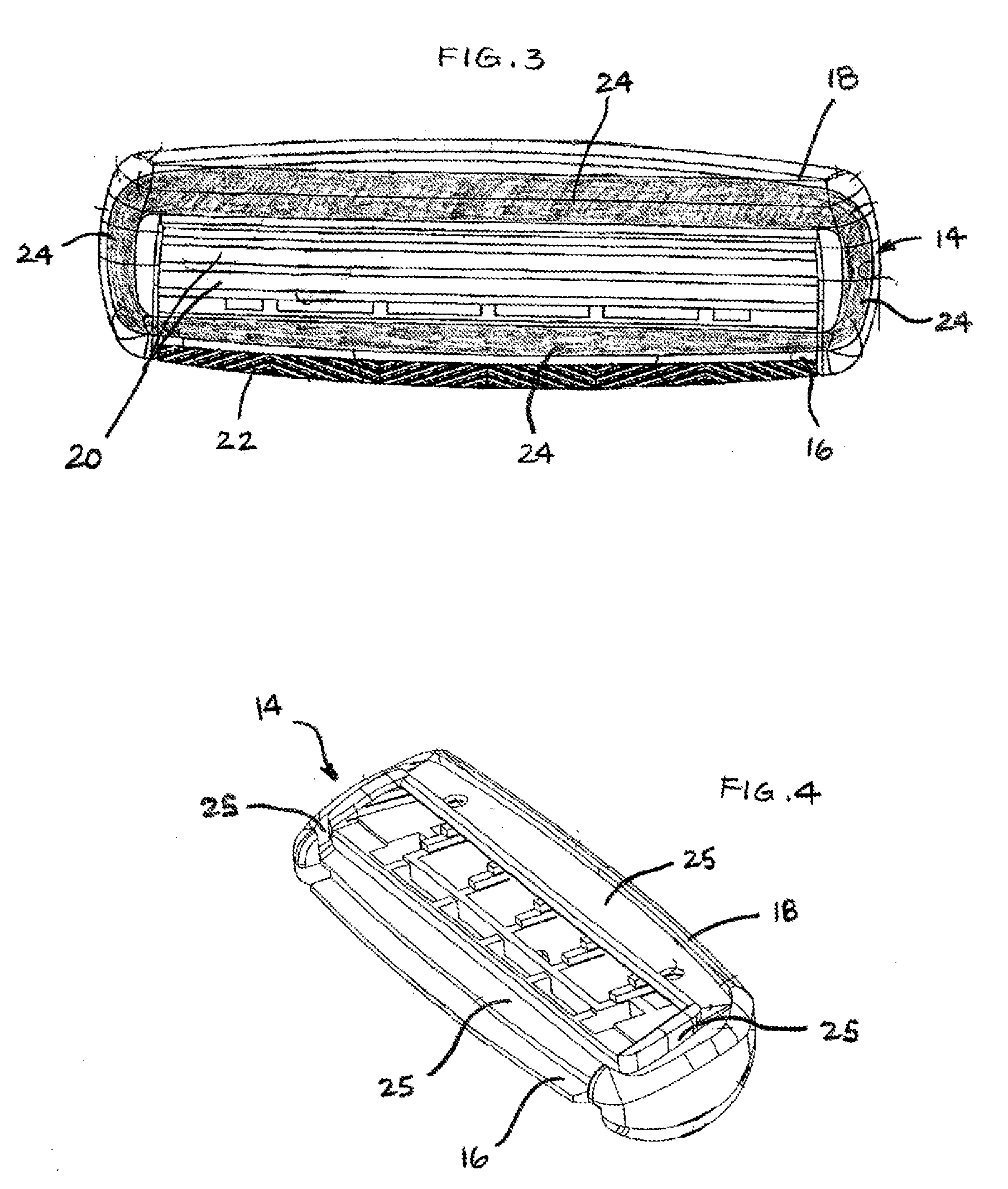
Shaving aid material
A shaving aid material is provided that includes a water-soluble lubricious shaving aid in combination with a water-insoluble erodable medium. In some embodiments, the shaving aid material further includes a water-soluble thermoplastic polymer, and may also include a plasticizer. Optional additional ingredients include emulsifiers, surfactants, skin conditioners, fragrances, depilatory agents, cleaning agents, medicinal agents, etc.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Implantable Tissue Compositions and Method
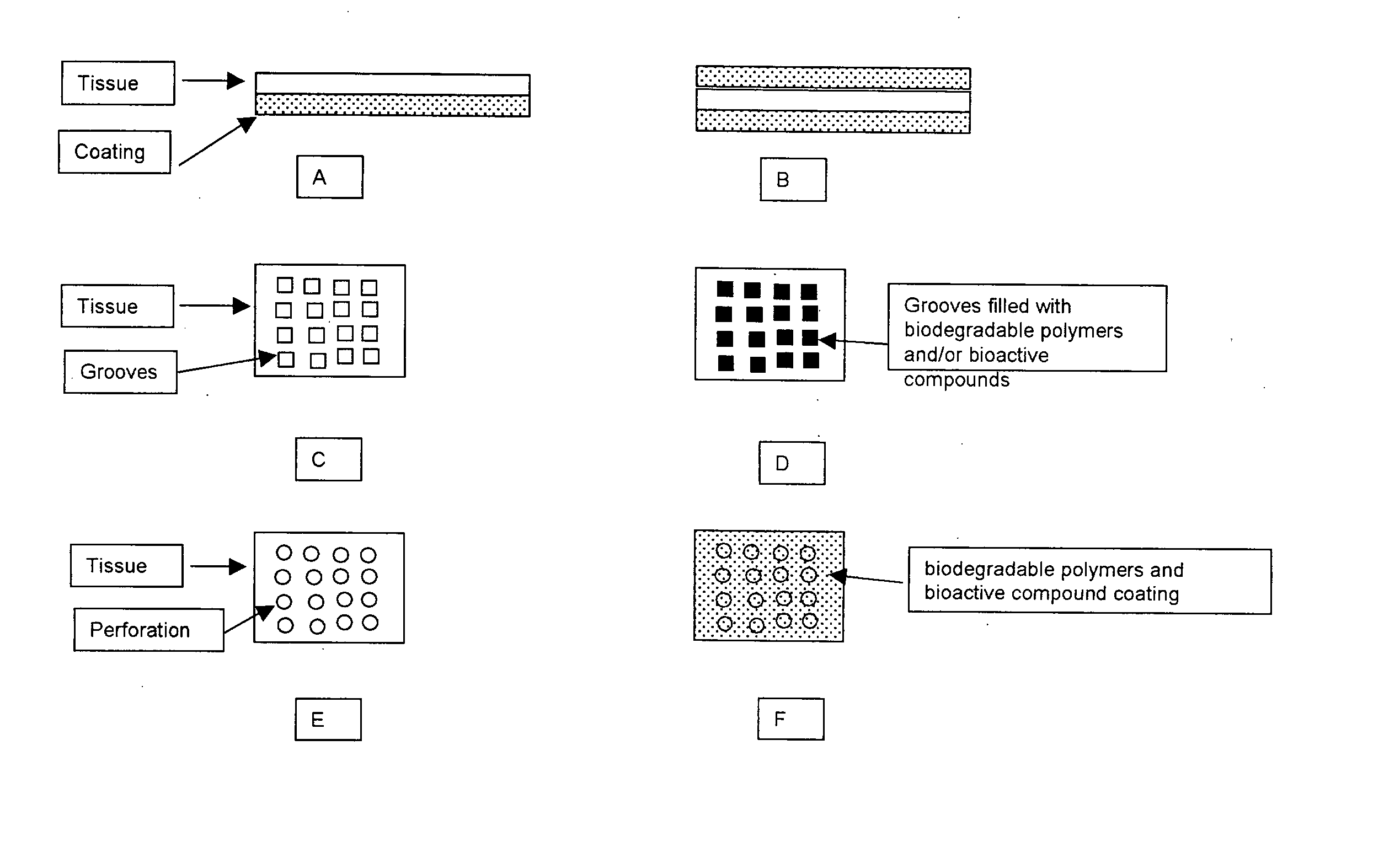
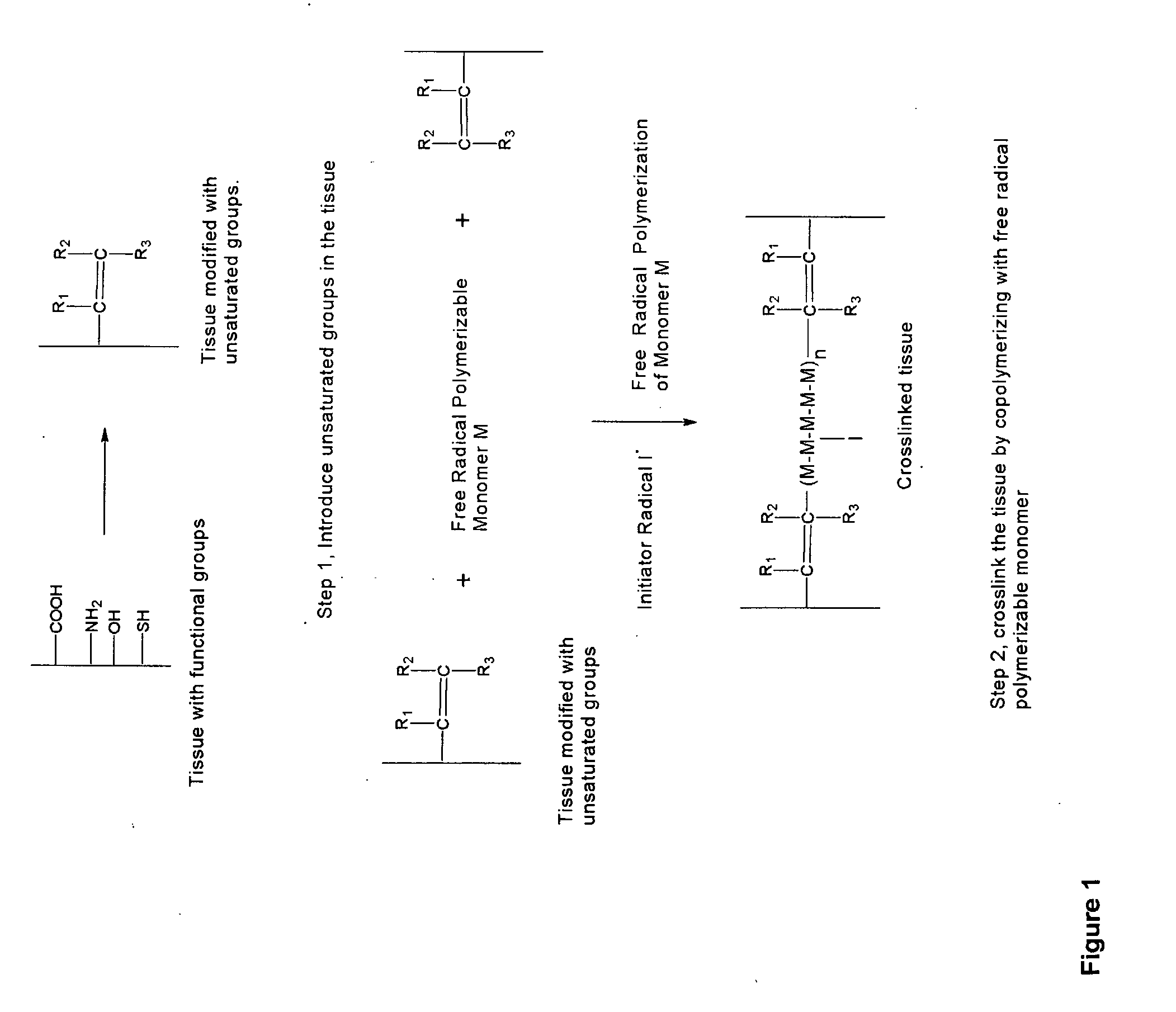
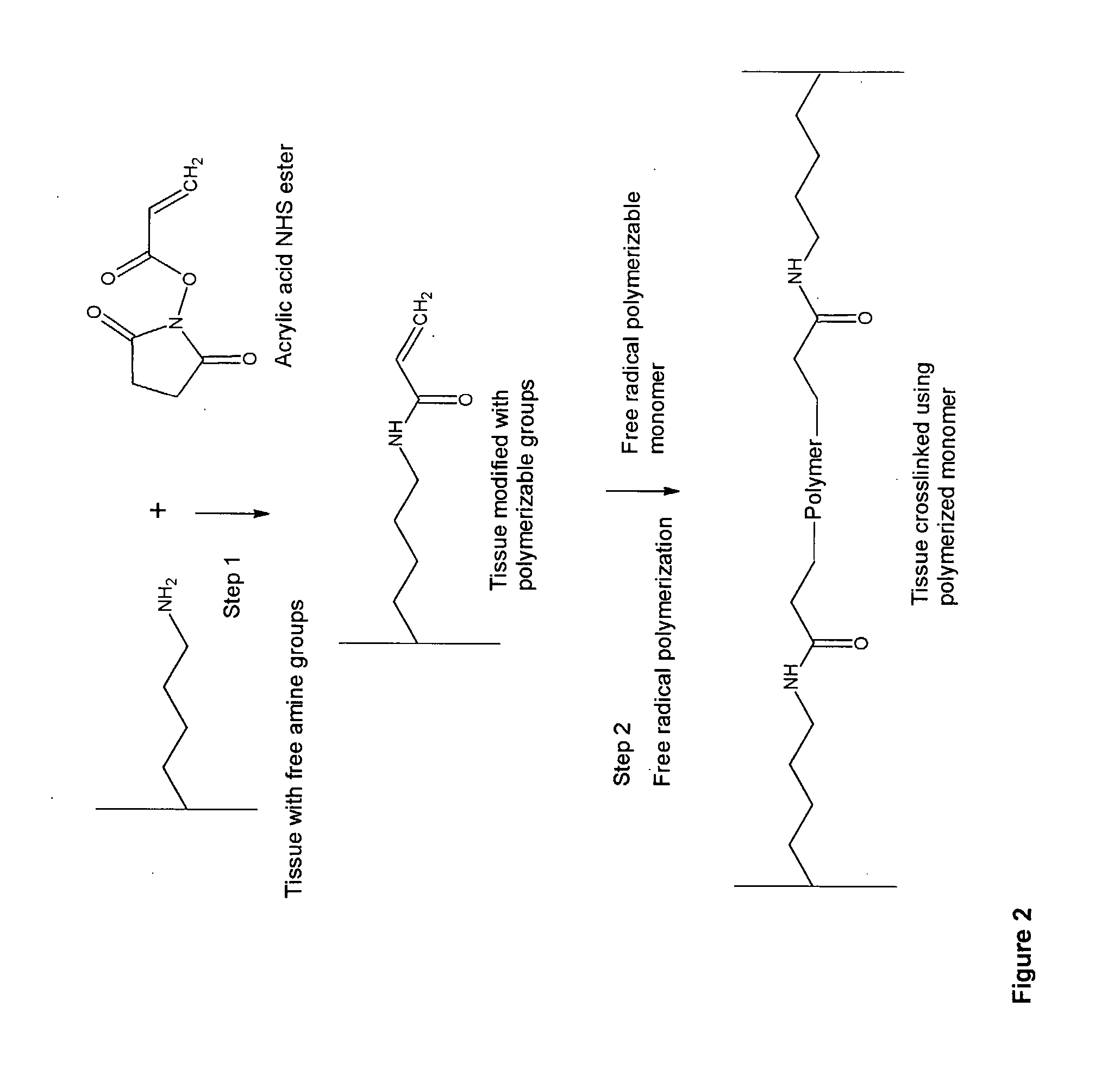
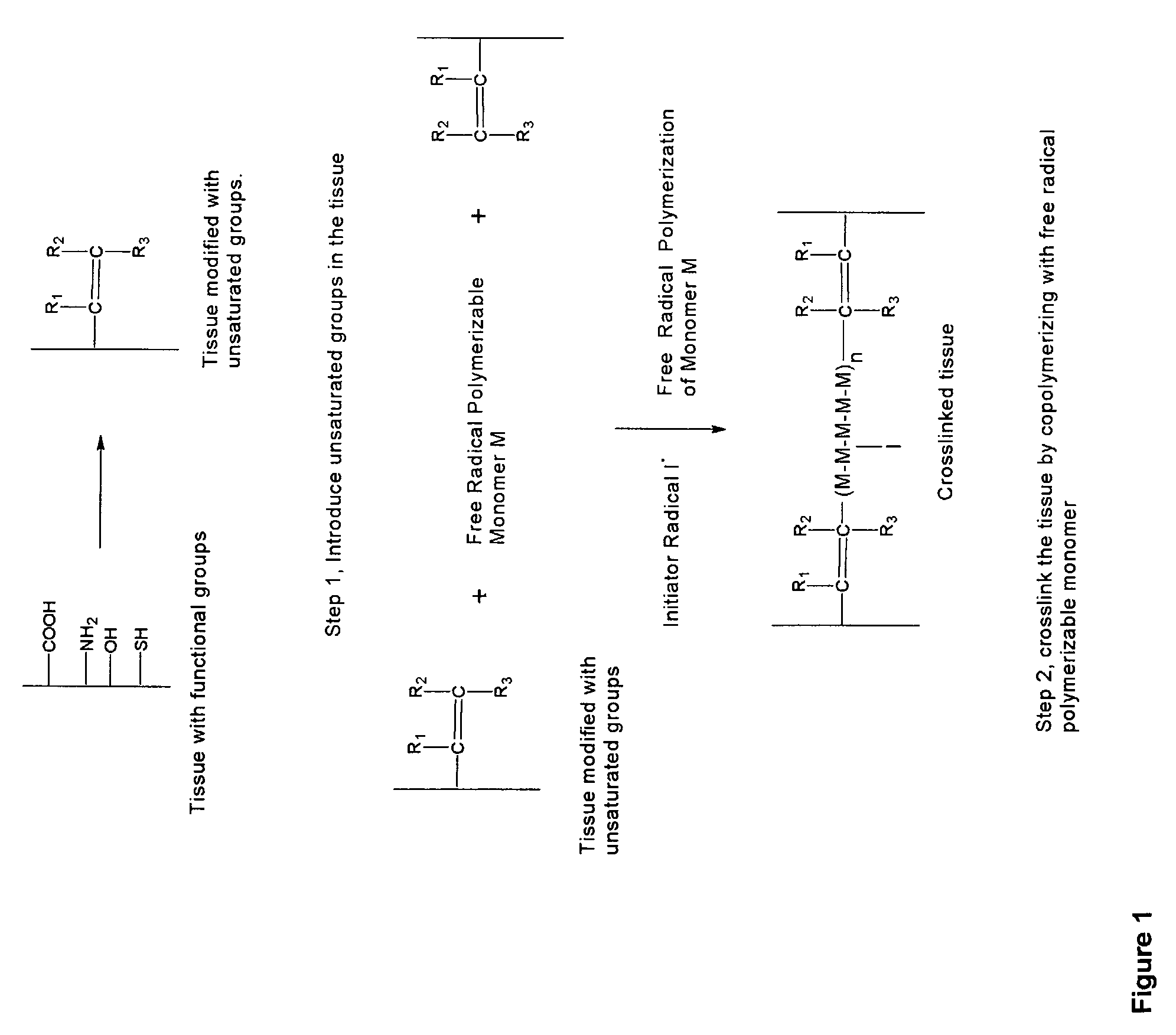
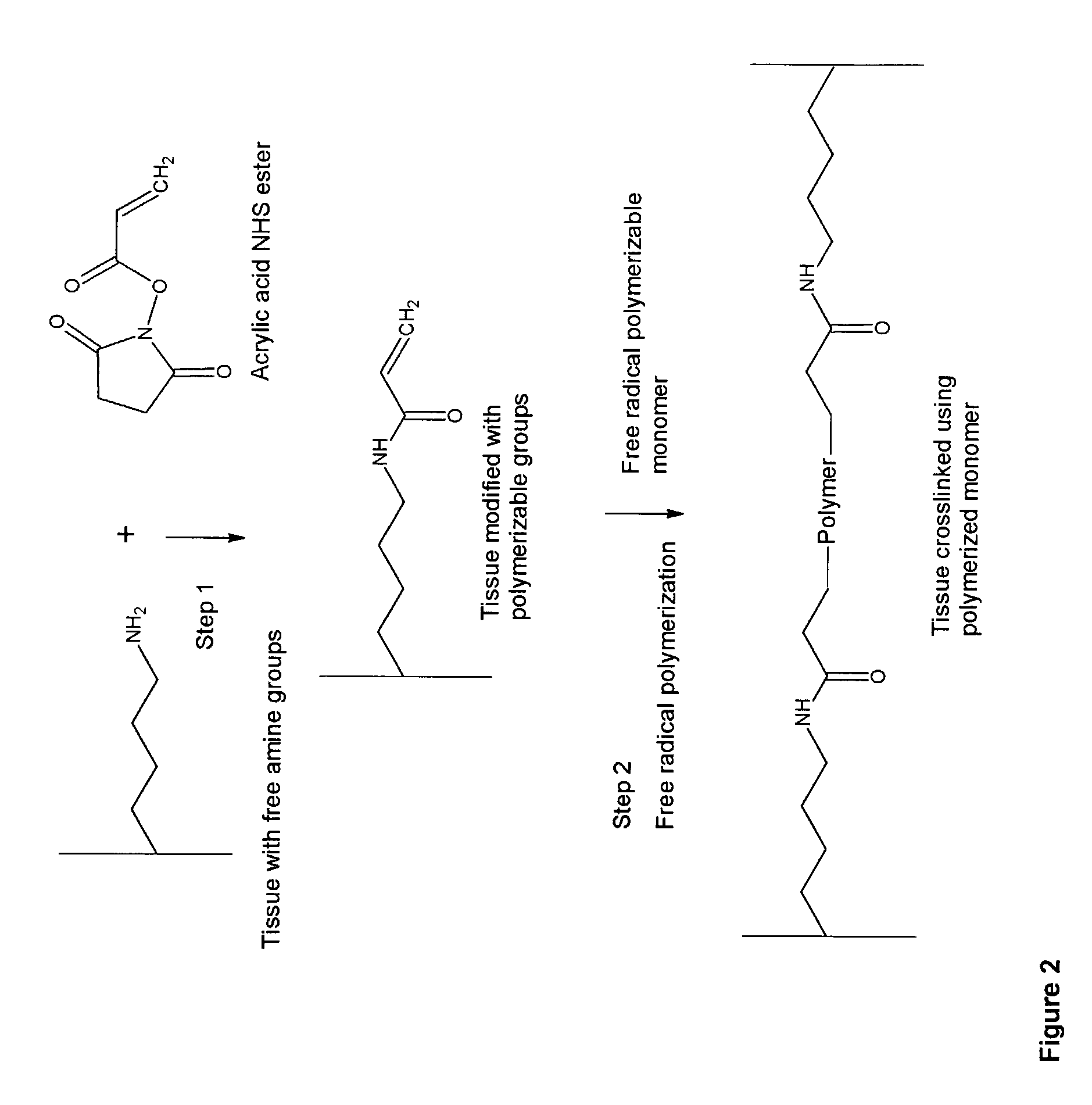
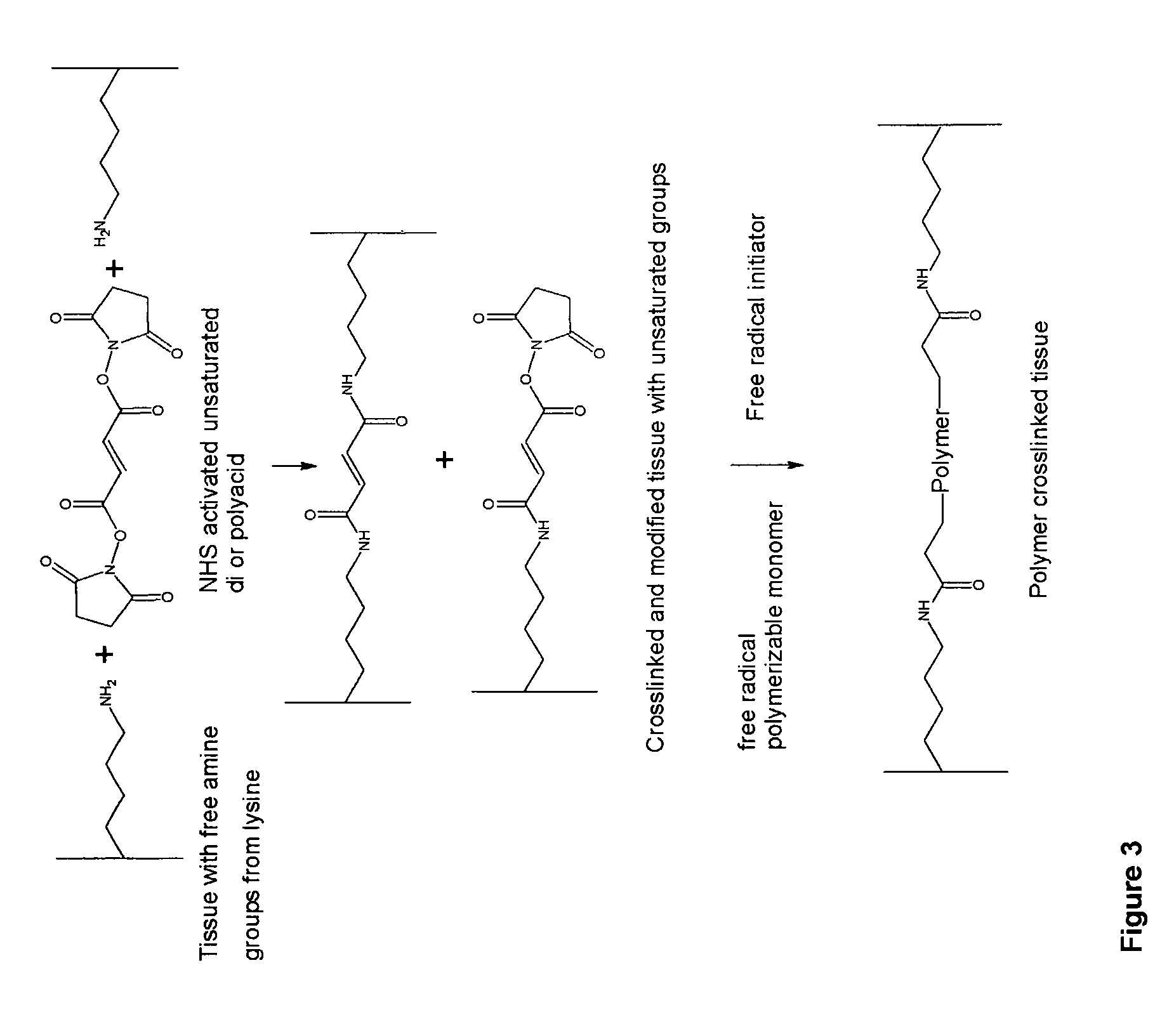
InactiveUS20070254005A1Promotes localized deliveryImprove overall lifespanNervous disorderAntipyreticWater insolubleFixation method
Novel implantable tissue fixation methods and compositions are disclosed. Methods and compositions of tissue, fixed using polymeric and / or variable length crosslinks, and di- or polymercapto compounds are described. Also described are the methods and compositions wherein the tissue is fixed using biodegradable crosslinkers. Methods and compositions for making radioopaque tissue are also described. Methods and compositions to obtain a degradable implantable tissue-synthetic biodegradable polymer composite are also described. Compositions and methods of incorporating substantially water-insoluble bioactive compounds in the implantable tissue are also disclosed. The use of membrane-like implantable tissue to make an implantable drug delivery patch are also disclosed. Also described are the compositions and methods to obtain a coated implantable tissue. Medical applications implantable tissue such as heart valve bioprosthesis, vascular grafts, meniscus implant, drug delivery patch are also disclosed.
Owner:PATHAK HLDG
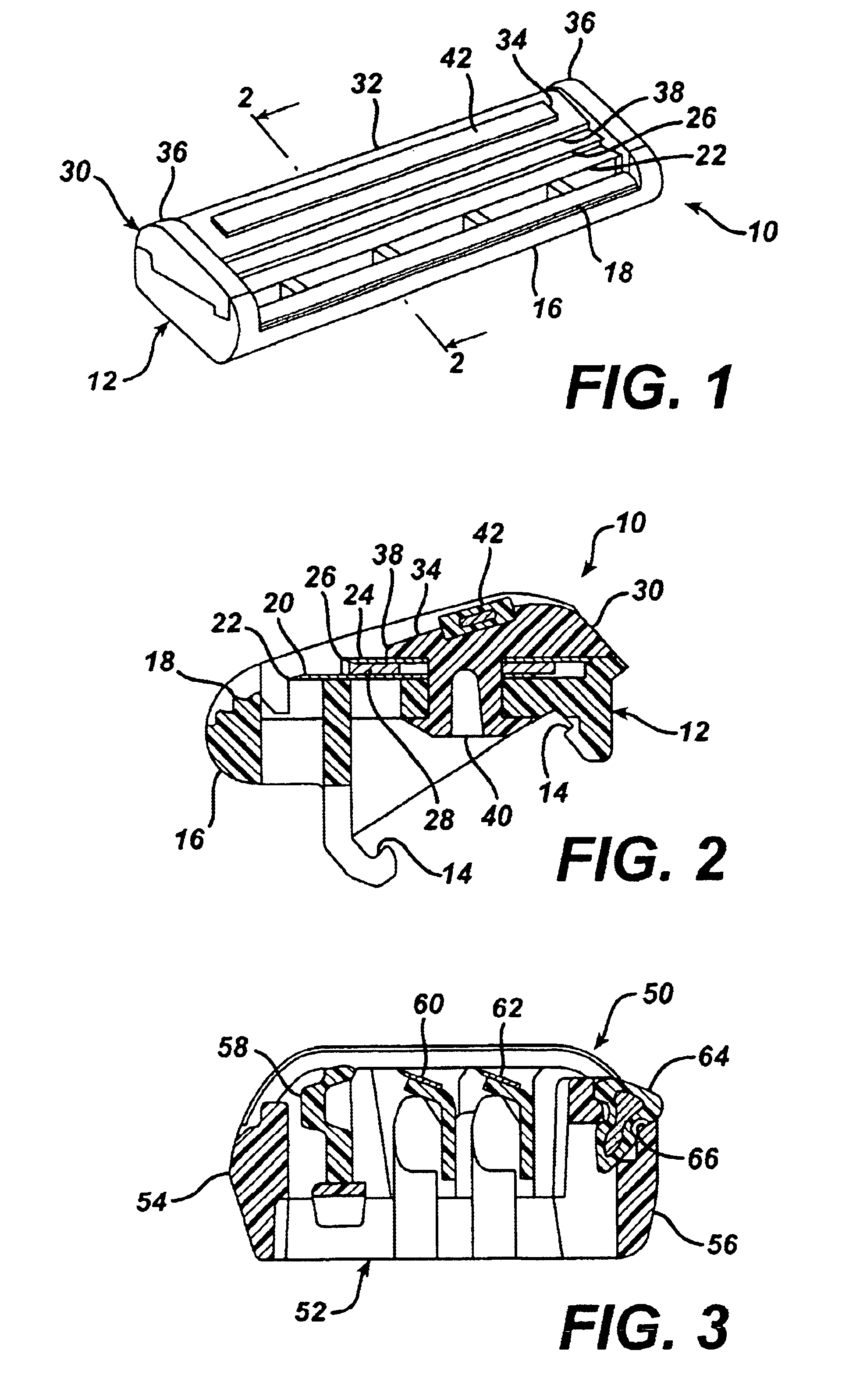
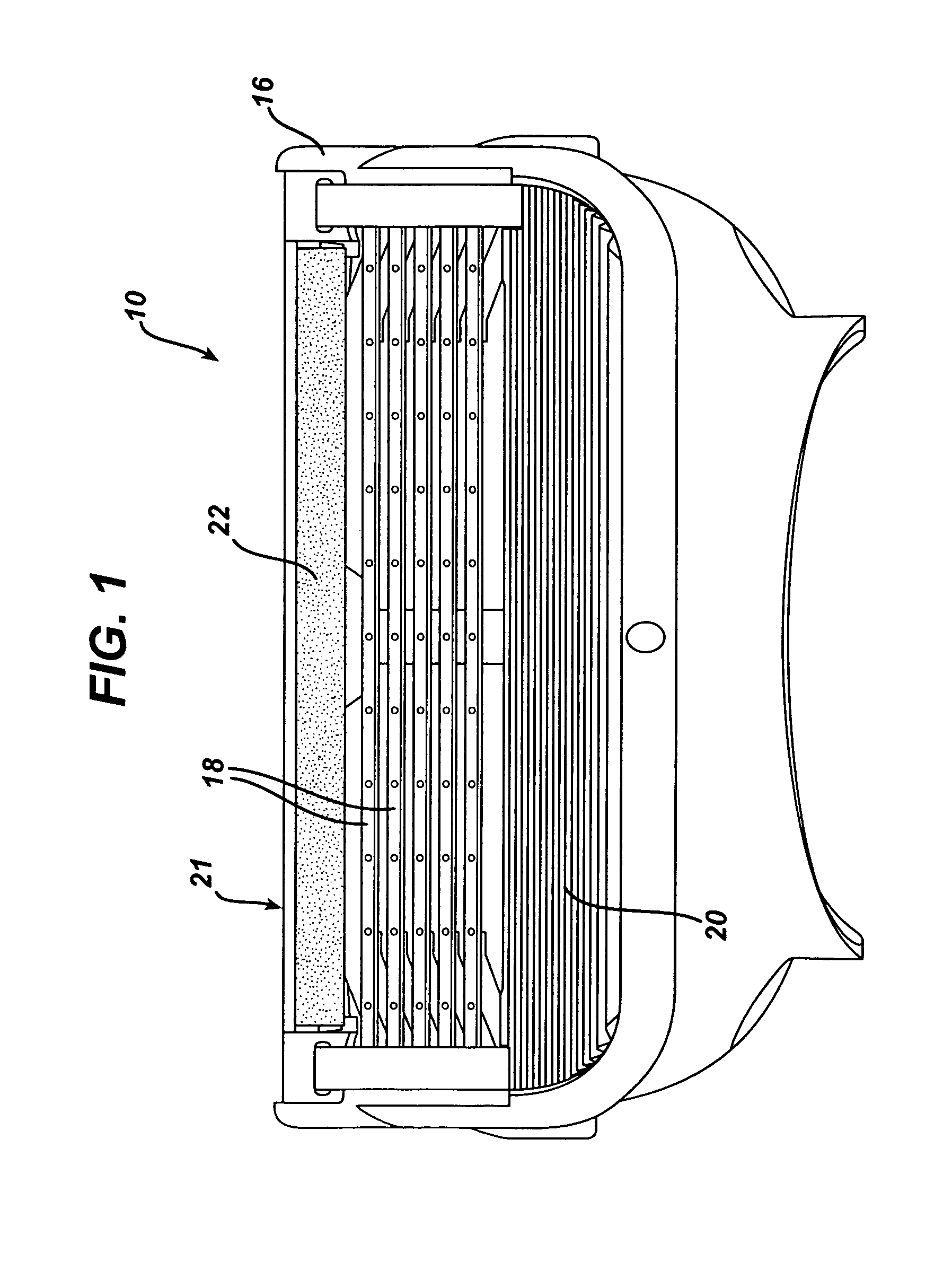
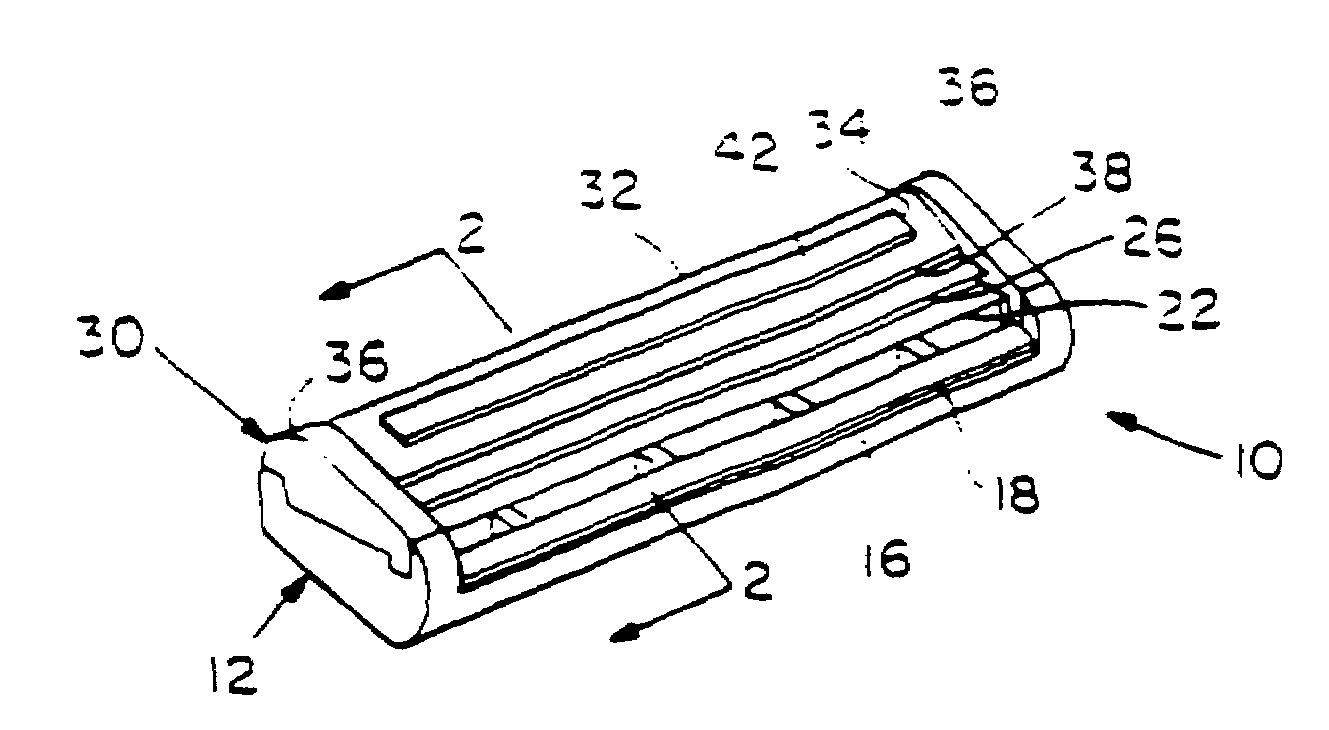
Shaving system
InactiveUS6944952B1High mechanical strengthImproved shaving aid release characteristicMeasurement devicesPhysics instrumentsWater insolubleRigid core
A shaving unit that comprises at least one blade and a skin engaging member that has a surface for engaging the user's skin adjacent the blade edge. The shaving unit may be of a disposable cartridge type adapted for coupling to and uncoupling from a razor handle or may be integral with a handle so that the complete razor is discarded as a unit when the blade or blades become dulled. The blade edge (or edges) cooperate with skin engaging surfaces to define a shaving geometry. The skin engaging member is comprised on an elongated sheath (or skin engaging layer) made of a mixture of water insoluble matrix and an effective amount of shaving aid and a rigid core (or non-skin engaging layer) extending axially through out said sheath. The axial position of the core need not be through the central axis.
Owner:THE GILLETTE CO
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Abuse-resistant pharmaceutical compositions
An abuse-resistant controlled release pharmaceutical composition comprising a pharmaceutically effective amount of discrete particles of an active capable of abuse, wherein surfaces of said particles are wetted with a water insoluble coating material, and preferably wherein said composition comprises a matrix, in which said particles are distributed, and which renders the abuse-capable compound within the matrix difficult to separate from the matrix; and a method for the preparation of a controlled release pharmaceutical composition having a reduced potential for abuse, comprising applying a pressure force to a mixture comprising a water insoluble material, and particles of a pharmaceutically active compound capable of inducing in a subject a reaction that is physiologically or psychologically detrimental if administered in an immediate release dosage form, thereby resulting in surface coated particles, and incorporating said surface coated particles into a pharmaceutical composition
Owner:ELAN PHRMA INT LTD
Antigen delivery system and method of production
The present invention concerns polymer particle vaccine delivery systems in which a water insoluble protein antigen, e.g. a lipidated HpaA protein, is incorporated with particles comprising a polymer matrix. The present invention also concerns a method for incorporating such a water insoluble protein antigen with a polymer matrix in order to produce a polymer particle vaccine delivery system. In addition, the invention also provides a vaccine composition comprising the polymer particle delivery system. The vaccine can be used to treat and / or reduce the risk of for example Helicobacter infection.
Owner:ASTRAZENECA AB
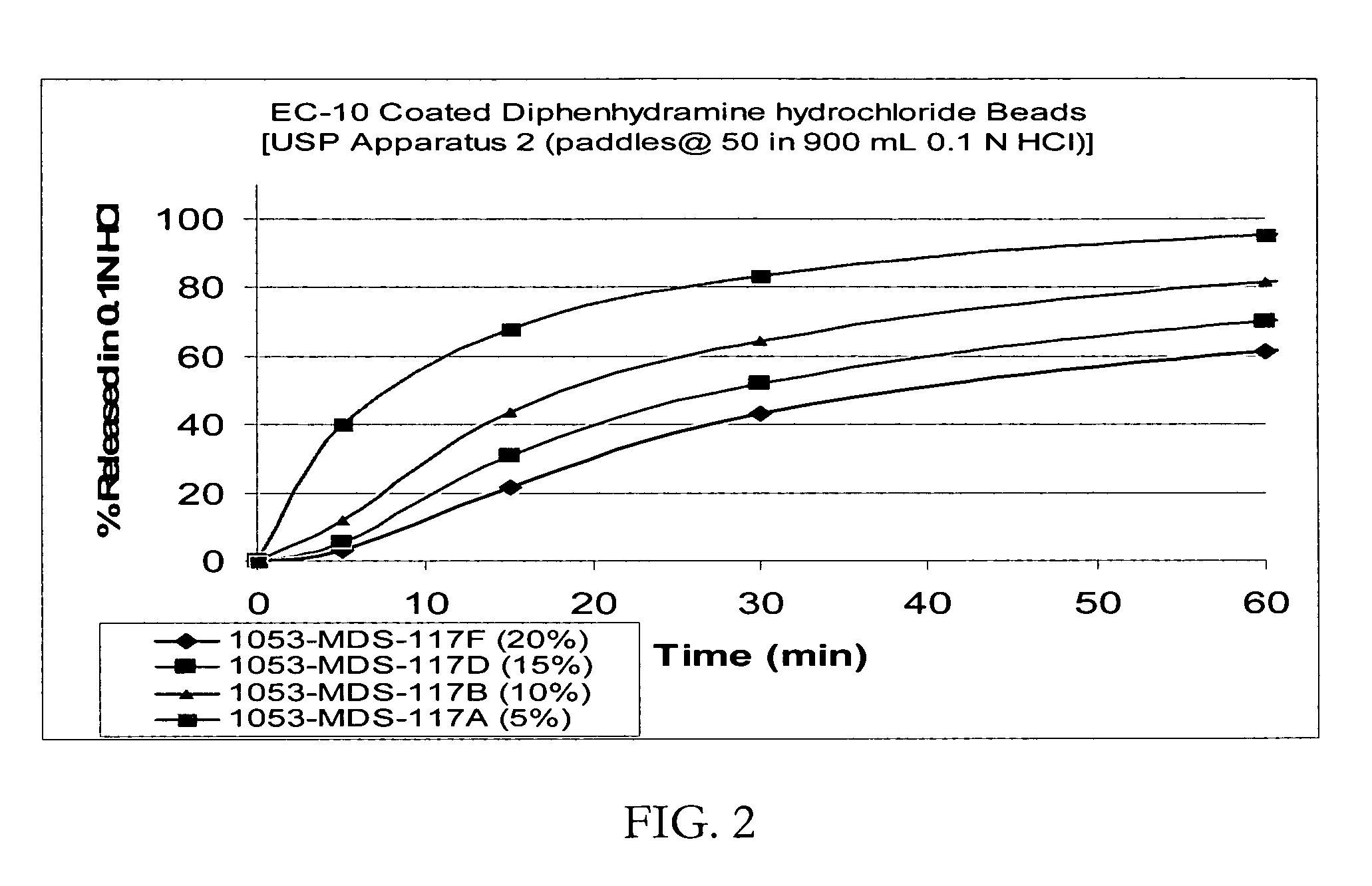
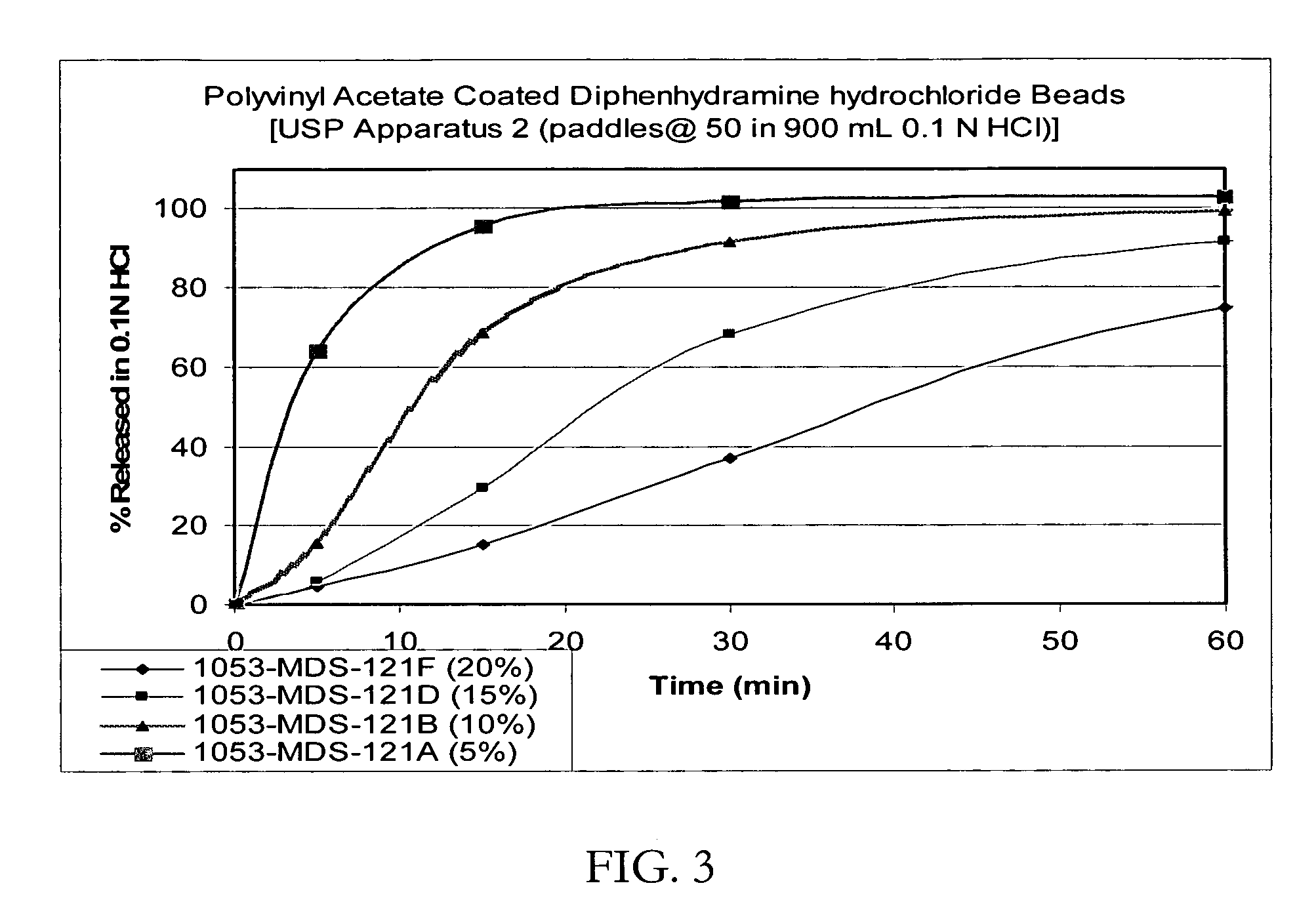
Controlled release formulations coated with aqueous dispersions of acrylic polymers
InactiveUS6143353ADissolution stabilityIncrease weight gainPretreated surfacesMedical devicesWater insolubleActive agent
A stable solid controlled release formulation having a coating derived from an aqueous dispersion of a hydrophobic acrylic polymer includes a substrate including an active agent selected from the group consisting of a systemically active therapeutic agent, a locally active therapeutic agent, a disinfecting and sanitizing agent, a cleansing agent, a fragrance agent and a fertilizing agent, overcoated with an aqueous dispersion of the plasticized water-insoluble acrylic polymer. The formulation provides a stable dissolution of the active agent which is unchanged after exposure to accelerated storage conditions.
Owner:PURDUE PHARMA LP
Wet shaving system including a mineral oil coated shaving aid
InactiveUS20080060201A1Easy to glideInhibit swellingMetal working apparatusWater insolubleWater soluble
A wet shaving system is disclosed including a blade member and a skin-engaging portion in proximity to said blade member, the skin-engaging portion comprising a solid polymeric shaving aid composite including an exposed portion containing a water-soluble shaving aid dispersed in a water-insoluble polymeric matrix, the water soluble shaving aid being coated with mineral oil.
Owner:THE GILLETTE CO
Processes to generate submicron particles of water-insoluble compounds
InactiveUS6177103B1Rapid of surface stabilizedRapid attainmentOrganic active ingredientsPowder deliveryWater insolubleChemical compound
Submicron particles of water-insoluble compounds, particularly drugs, are prepared by simultaneously stabilizing microparticulate suspensions of same with surface modifier molecules by rapid expansion into an aqueous medium from a compressed solution of the compound and surface modifiers in a liquefied gas and optionally homogenizing the aqueous suspension thus formed with a high pressure homogenizer.
Owner:JAGOTEC AG +1
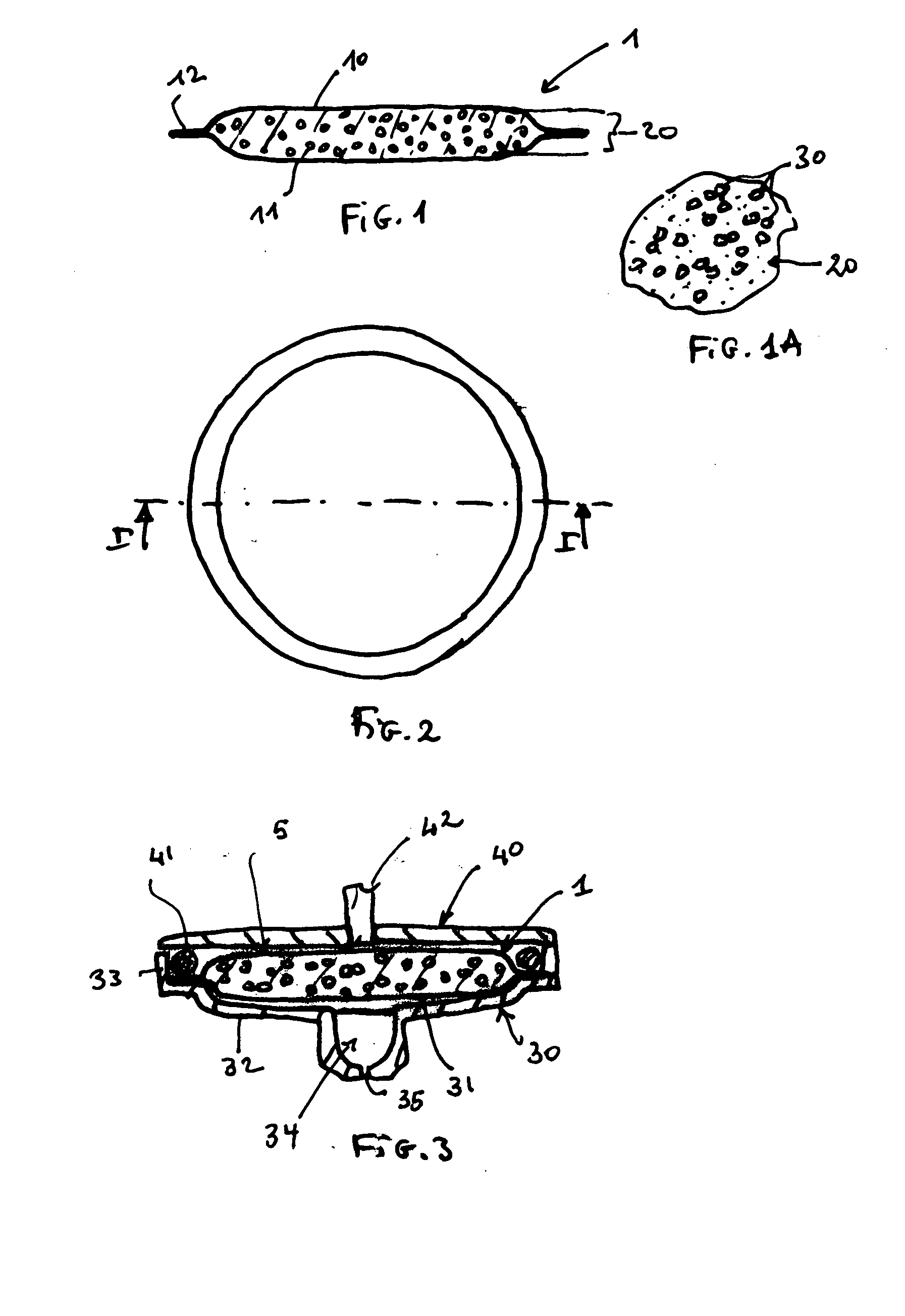
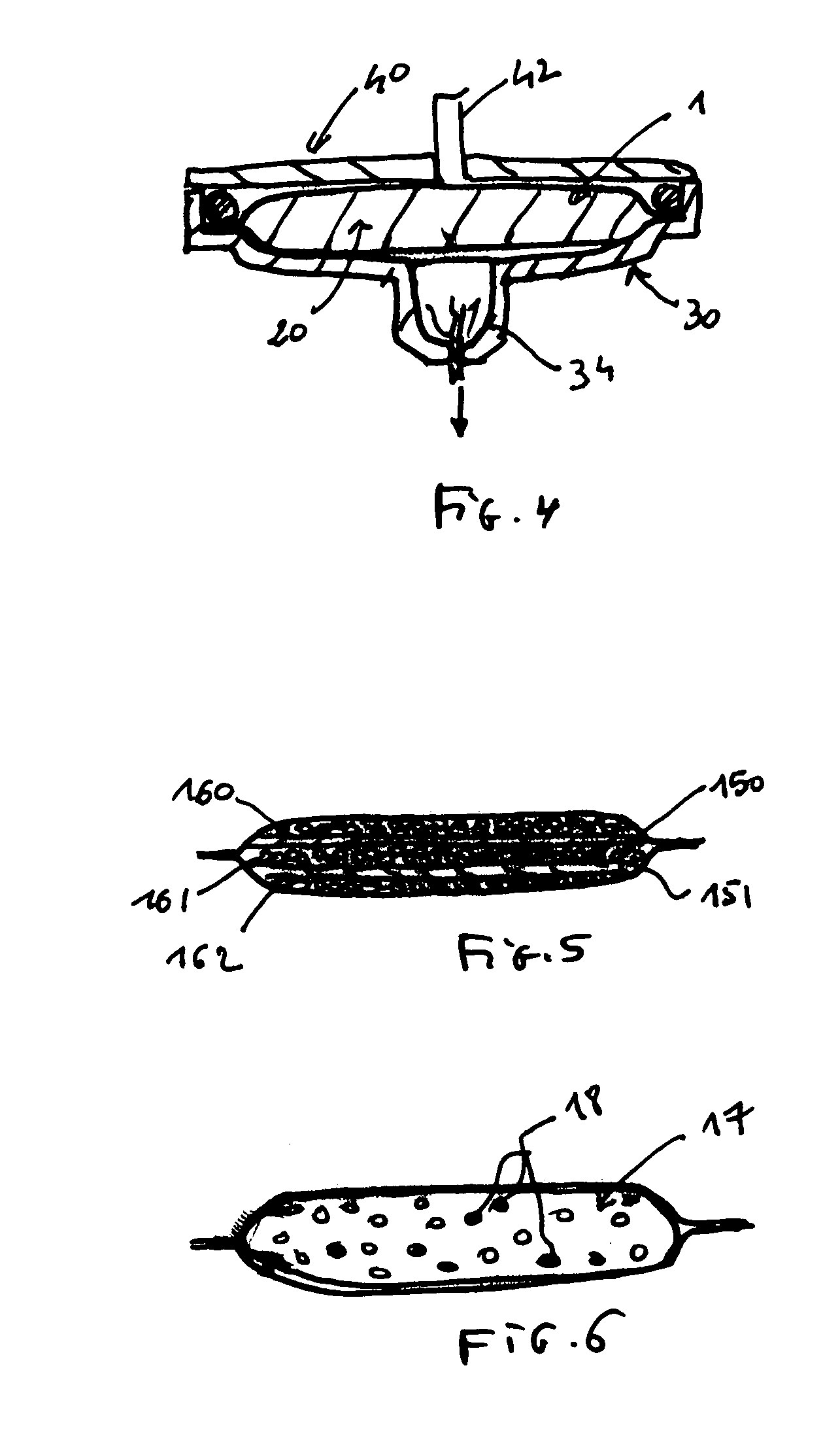
Slow-release matrix pellets and the production thereof
InactiveUS6290990B1Simple and low-cost productionHigh content of active substancesPharmaceutical non-active ingredientsGranular deliveryMaximum diameterPlasticizer
Slow-release matrix pellets with a spherical or lenticular shape and uniform maximum diameters in the range from 0.5 to 4 mm, composed ofa) 0.1-87% by weight of at least one biologically active compound,b) 5-50% by weight of at least one water-insoluble polymer,c) 5-45% by weight of at least one lipophilic component as plasticizer for polymer b),d) 3-40% by weight of a natural or semisynthetic gel former,e) 0-50% by weight of one or more conventional formulation aids.
Owner:BASF AG
Shaving system
InactiveUS7069658B2High mechanical strengthImproved shaving aid release characteristicMeasurement devicesPhysics instrumentsWater insolubleRigid core
A shaving unit that comprises at least one blade and a skin engaging member that has a surface for engaging the user's skin adjacent the blade edge is described herein. The shaving unit may be of a disposable cartridge type adapted for coupling to and uncoupling from a razor handle or may be integral with a handle so that the complete razor is discarded as a unit when the blade or blades become dulled. The blade edge (or edges) cooperate with skin engaging surfaces to define a shaving geometry. The skin engaging member is comprised of an elongated sheath (or skin engaging layer) made of a mixture of water insoluble matrix and an effective amount of shaving aid and a rigid core (or non-skin engaging layer) extending axially throughout said sheath. The axial position of the core need not be through the central axis.
Owner:THE GILLETTE CO
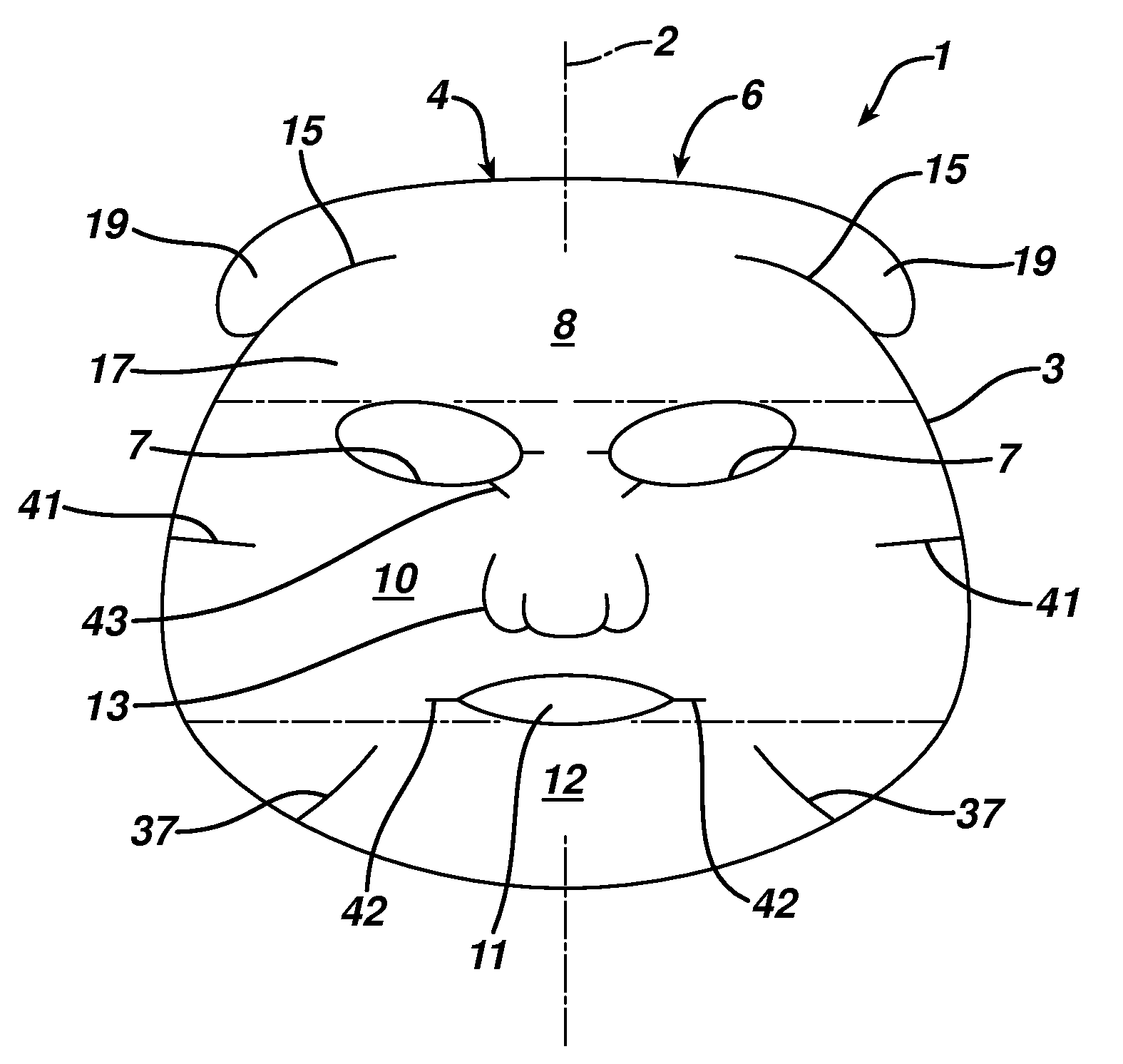
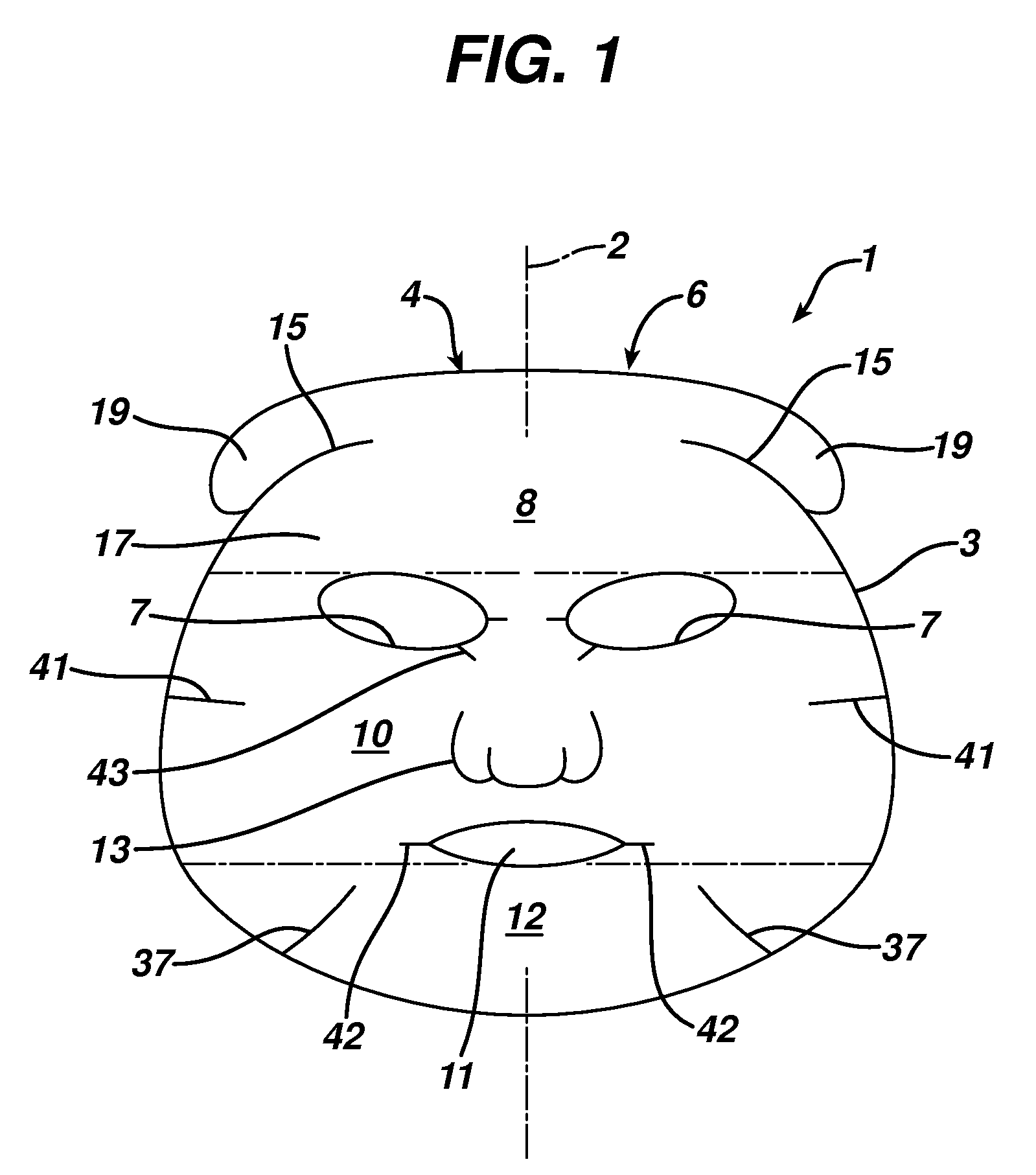
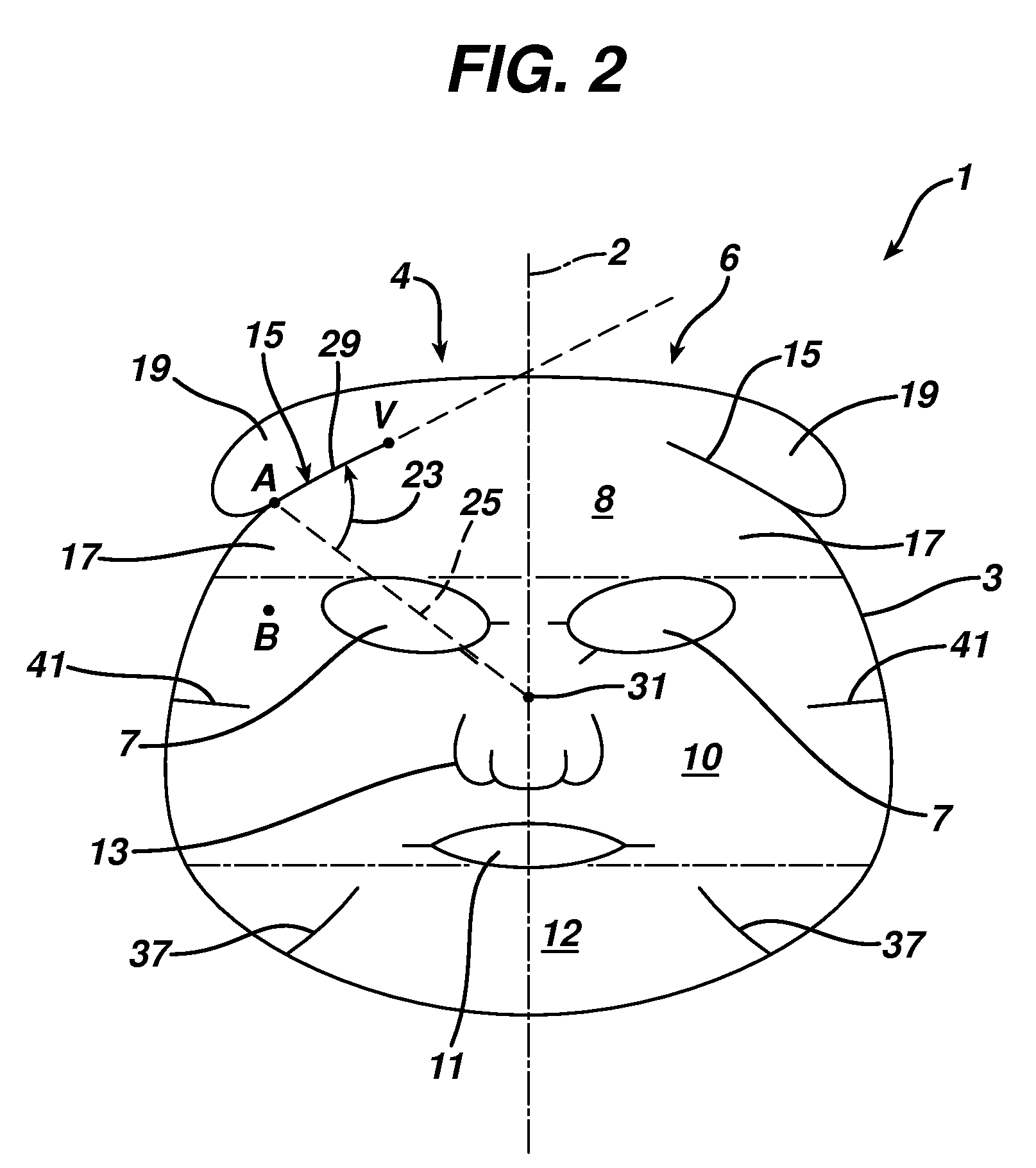
Facial mask
The present invention features a facial mask comprising a water-insoluble substrate sized and shaped to lie against and substantially coincident with a face of a human user. The water-insoluble substrate includes separation features that form laterally-extending tabs capable of overlapping the facial mask, improving its adaptability and fit. Methods of treating the skin using facial masks are also provided.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
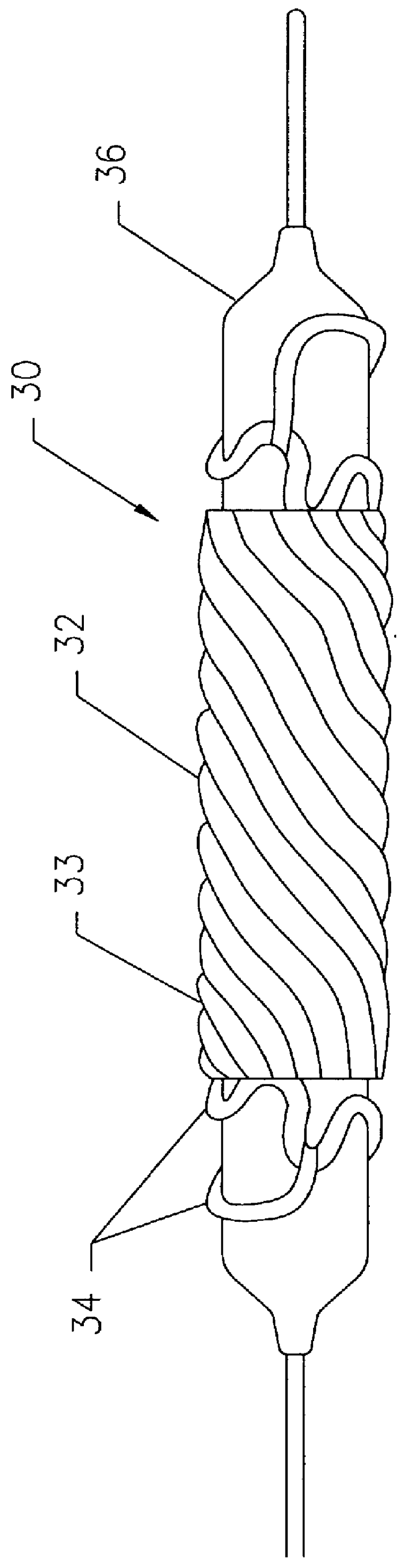
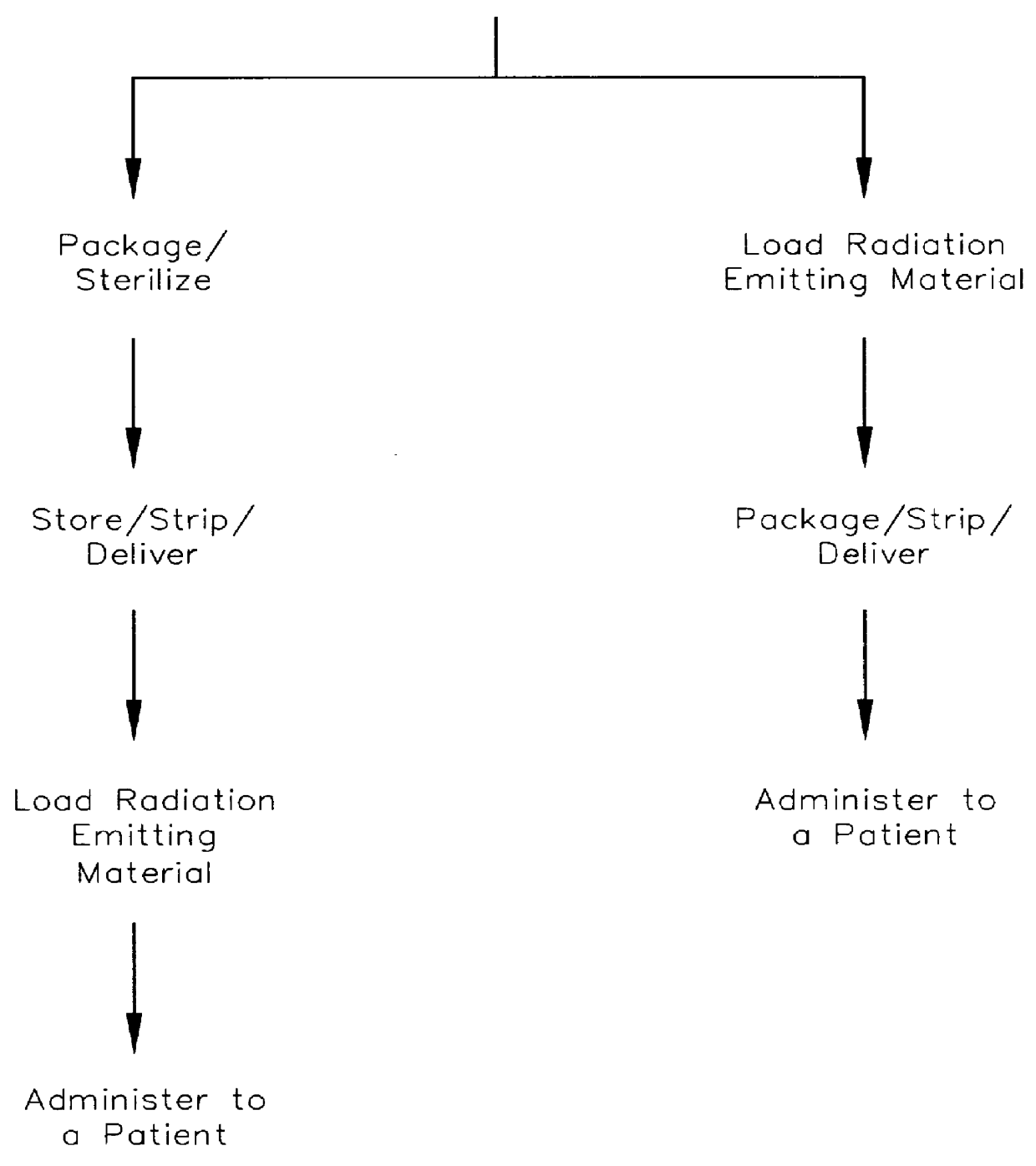
Medical device for delivering localized radiation
InactiveUS6106454AShort half-lifeUniform deliverySurgeryDiagnostic recording/measuringWater insolubleMedicine
A medical device useful for localized delivery of radiation in vivo is provided. The medical device includes a structure including a porous material; and a plurality of discrete particles including a water-insoluble radioactive salt dispersed throughout a substantial portion of the porous material. The water-insoluble radioactive salt is formed by contacting an aqueous radioactive salt solution with a heavy metal water-soluble salt dispersed throughout a substantial portion of the porous material. The heavy metal water-soluble salt can be dispersed in the porous material so that the device can be sterilized and the radioactive material can be loaded in the device in situ, for example, just prior to implantation.
Owner:MEDTRONIC INC
Controlled release bait material and preparation method thereof
InactiveCN103004717AQuick and long-lasting releaseFast and long-lasting effectOther angling devicesWater insolubleMicrosphere
The invention relates to a controlled release bait material and a preparation method thereof. The controlled release bait material refers to small pills, micro-pills, microcapsules, microspheres, particles or sticks prepared from raw materials including a basic material, a feed attractant, a bonding agent, a hydrophilic high-polymer material, a water-insoluble high-polymer material, a quick-release material, a filler, a colorant, a flavoring agent and the like through a preparation means; and the controlled release bait material can be coated for improving the controlled release effect. The invention further discloses a preparation method of the bait material. The controlled release material has rapid and lasting attracting force on fishes, the fish attracting time can be adjusted according to the habit of a fisherman and the habits of fishes, the food ration and frequency of fishes are increased, the fishing success rate can be increased, the food intake of fishes in cultivation of fishes can be increased, the corrosion ratios of remnant feeds and feeds in water bodies are reduced, the utilization ratios of feeds is increased, and the water body environmental pollution is lowered. The preparation method provided by the invention is suitable for industrial production.
Owner:李群益 +2
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20060105038A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
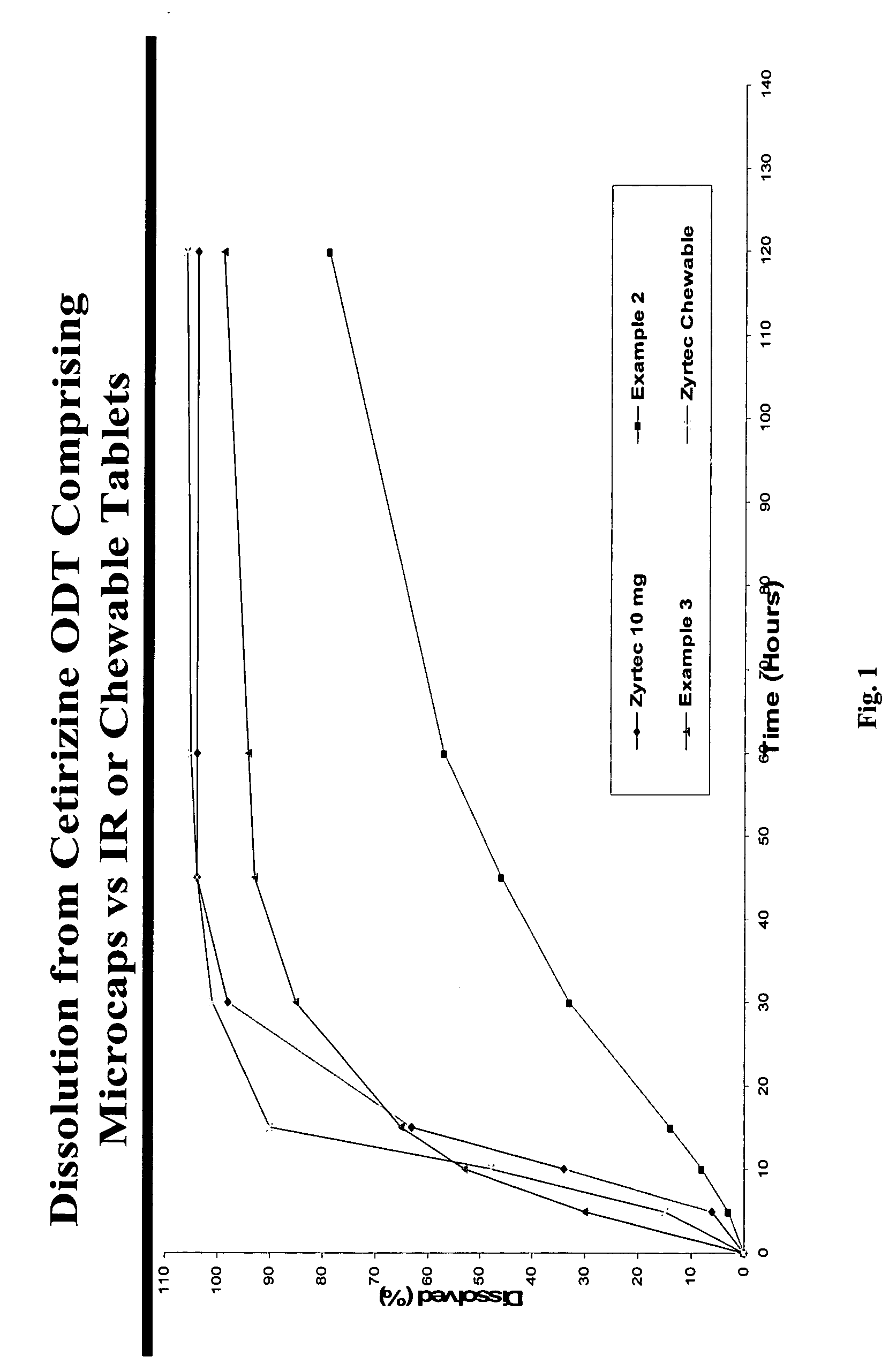
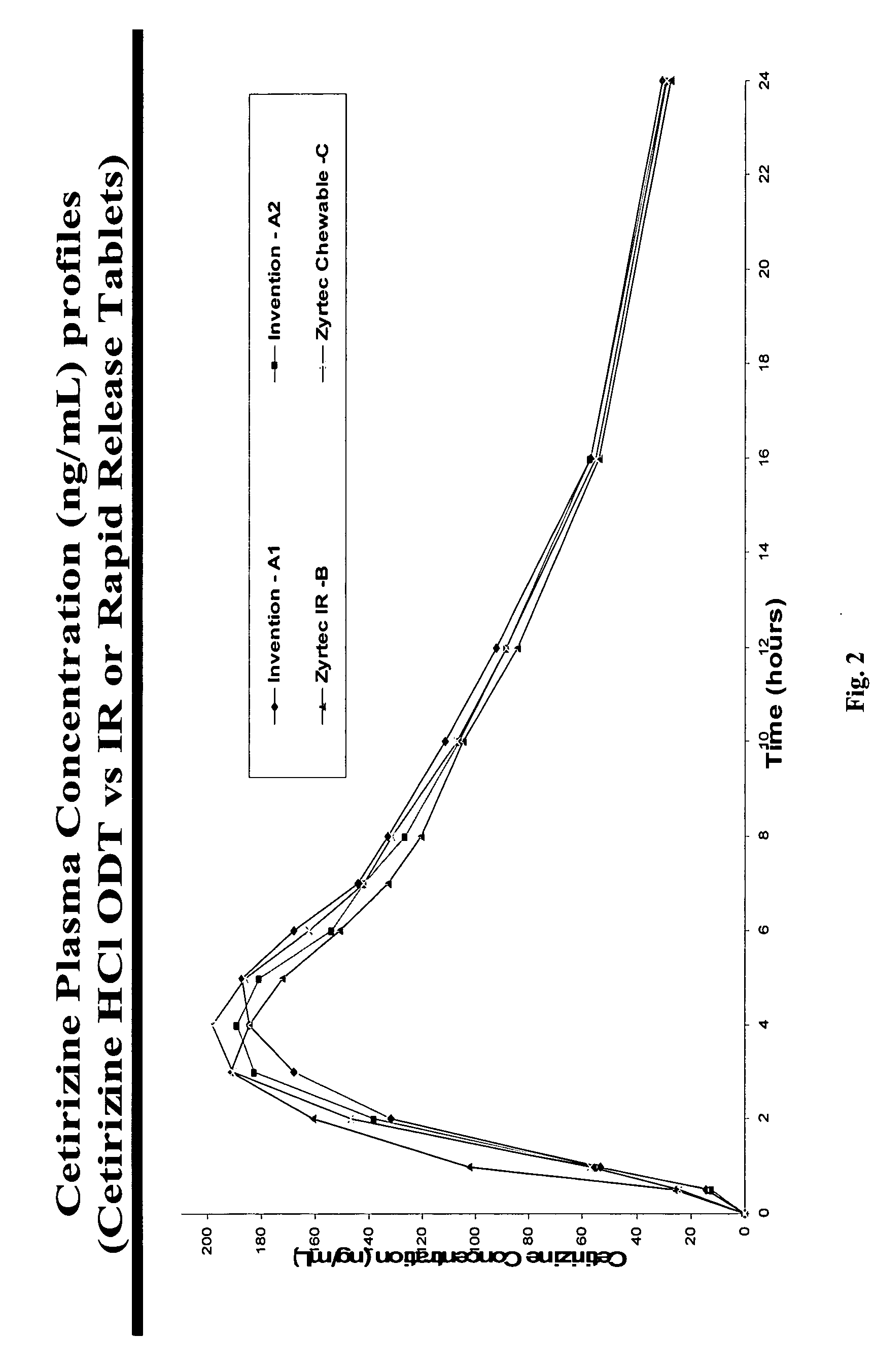
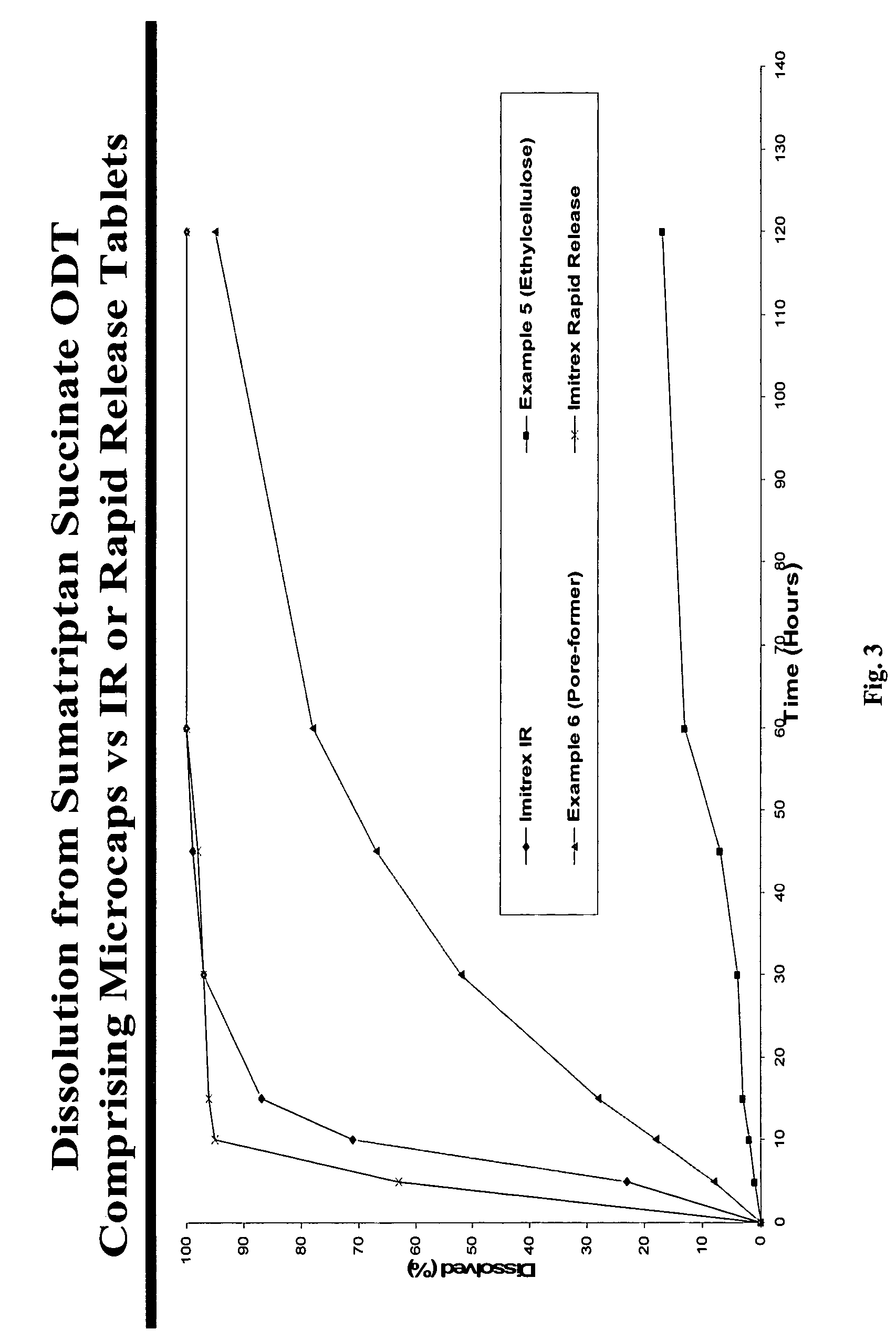
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
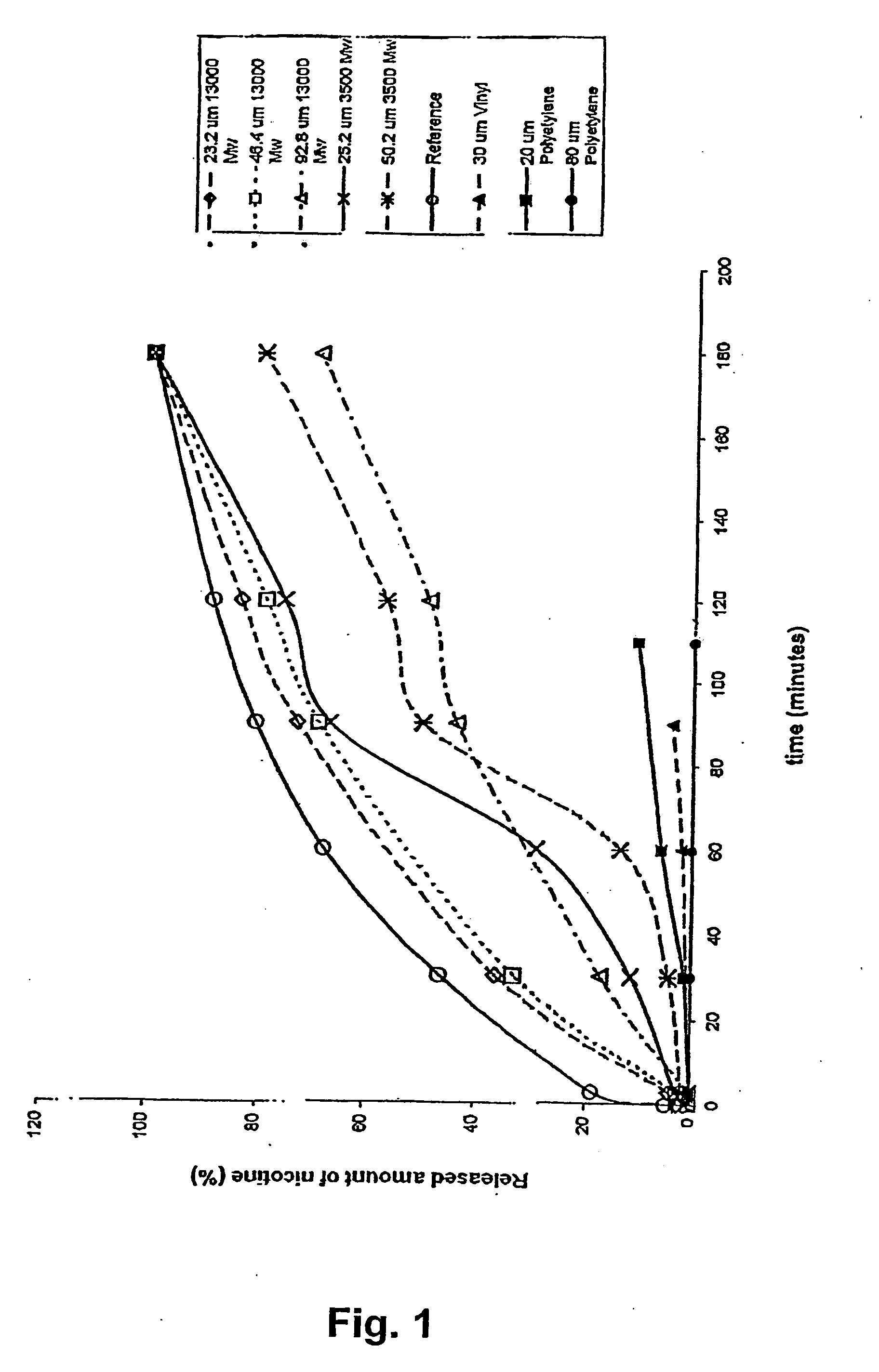
Loading and release of water-insoluble drugs
Owner:BOSTON SCI SCIMED INC
Binder compositions and associated methods
Disclosed are formaldehyde-free, thermally-curable, alkaline, aqueous binder compositions, curable to formaldehyde-free, water-insoluble thermoset polyester resins, and uses thereof as binders for non-woven fibers and fiber materials.
Owner:KNAUF INSULATION LLC
Disintegrant assisted controlled release technology
InactiveUS20060024361A1Controlled release rateEasy to controlOrganic active ingredientsBiocideControlled releaseWater insoluble
A disintegrant assisted controlled release device is disclosed. The device is a combination of a swelling disintegrant or super-disintegrant and water insoluble polymer or water soluble polymer, or both, and one or more water soluble or water insoluble active pharmaceutical ingredient(s). The said device is stabilized by a humectant or trehalose.
Owner:INTELLIPHARMACEUTICS
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Beverage portioned package for preparing a foamy beverage from soluble powder
InactiveUS20050158426A1Sufficient pressureSufficient amountPackaging foodstuffsWater insolubleWadding
A beverage portioned package for preparing a beverage in an extraction device of which the package is held or clamped between a water supplying part and a receiver of the device. The package has a first surface for the water to be forced to flow there through under pressure when the package is held or clamped in the device; a second surface for the flow of beverage to be forced to flow there through so to be collected in the receiver, wherein the package contains a portion of water-soluble beverage material and a portion of a filler. The filler comprising water insoluble material adapted to maintain the extraction pressure above the pressure created by the sole resistance of the first and second surfaces when the package is emptied of said soluble material.
Owner:NESTEC SA
Compositions and methods
Non-aerosol spray-on skin patch compositions as described comprising at least one substantially water insoluble film forming agent, at least one film plasticizer agent, at least one water soluble compound, and at least one organic solvent, the composition forming a flexible, porous and physiologically compatible skin patch when sprayed on to skin and allowed to dry. Also described are methods of improving wound healing by administering a physiologically active ingredient to a patient in need of such treatment.
Owner:KO THOMAS SAI YING
Antifungal nail lacquer and method using same
InactiveUS6224887B1Effective preventionEffective treatmentBiocideCosmetic preparationsAntifungalLacquer
A nail lacquer effective for the treatment or prevention of fungal infections, such as, onychomycosis, includes fungicidally effective amount of ciclopirox, econazole, or other antifungal agent in a clear, stable, film-forming lacquer vehicle which includes a water-insoluble film-forming polymer; 2-n-nonyl-1,3-dioxolane or similar penetration enhancer; and volatile solvent. A plasticizer for the film-forming polymer which is also compatible with the other components may be included although the preferred penetration enhancers may also function as plasticizer. The composition, when applied to the nails provides a hard, clear, water-resistant film containing the antifungal agent. The film is resistant to multiple washings and is effective in the treatment of onychomycosis.
Owner:MACROCHEM CORP
Implantable tissue compositions and method
InactiveUS7919112B2Improve overall lifespanPromotes localized deliveryNervous disorderAntipyreticWater insolubleFixation method
Novel implantable tissue fixation methods and compositions are disclosed. Methods and compositions of tissue, fixed using polymeric and / or variable length crosslinks, and di- or polymercapto compounds are described. Also described are the methods and compositions wherein the tissue is fixed using biodegradable crosslinkers. Methods and compositions for making radio-opaque tissue are also described. Methods and compositions to obtain a degradable implantable tissue-synthetic biodegradable polymer composite are also described. Compositions and methods of incorporating substantially water-insoluble bioactive compounds in the implantable tissue are also disclosed. The use of membrane-like implantable tissue to make an implantable drug delivery patch are also disclosed. Also described are the compositions and methods to obtain a coated implantable tissue. Medical applications implantable tissue such as heart valve bioprosthesis, vascular grafts, meniscus implant, drug delivery patch are also disclosed.
Owner:PATHAK HLDG
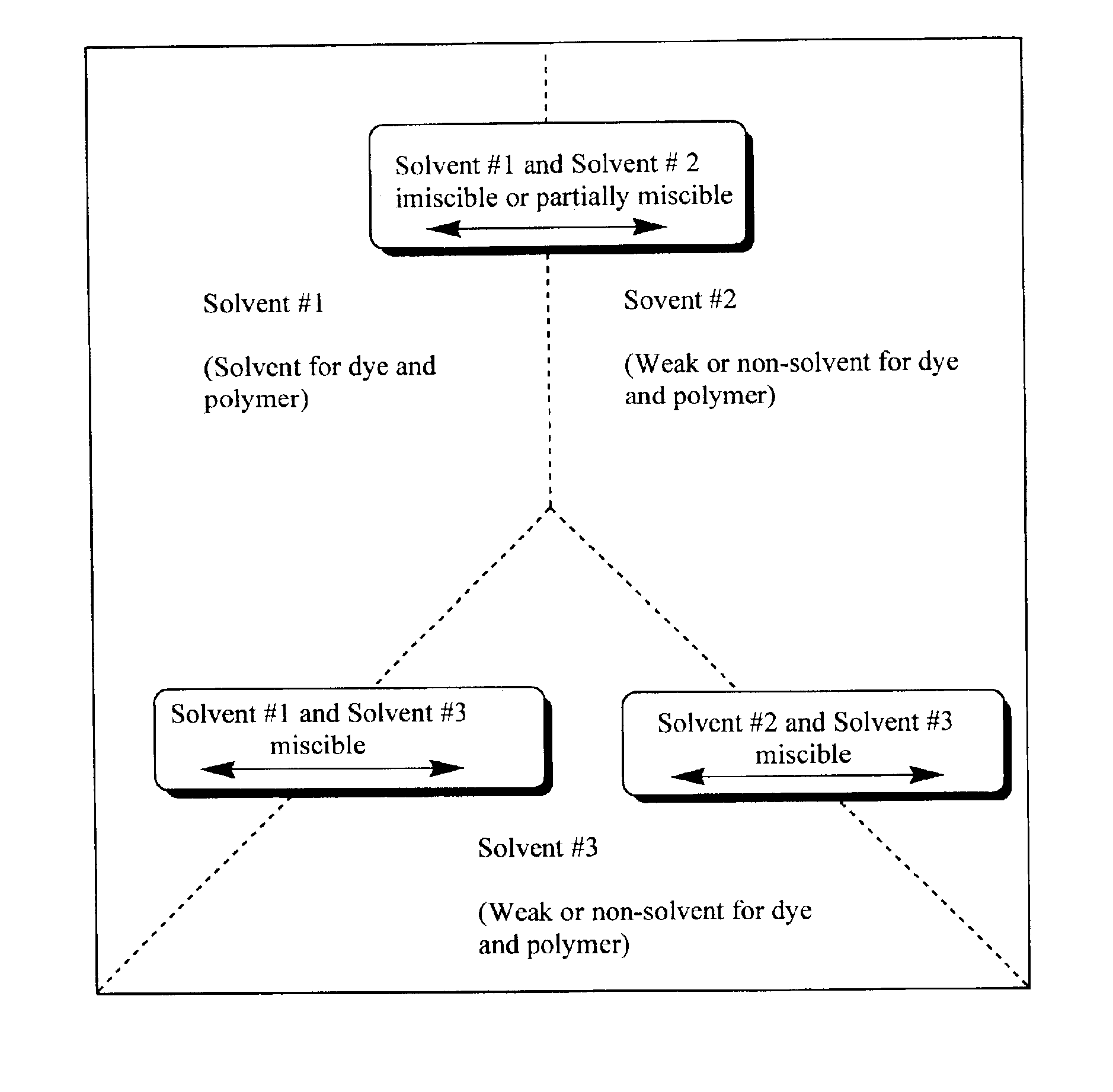
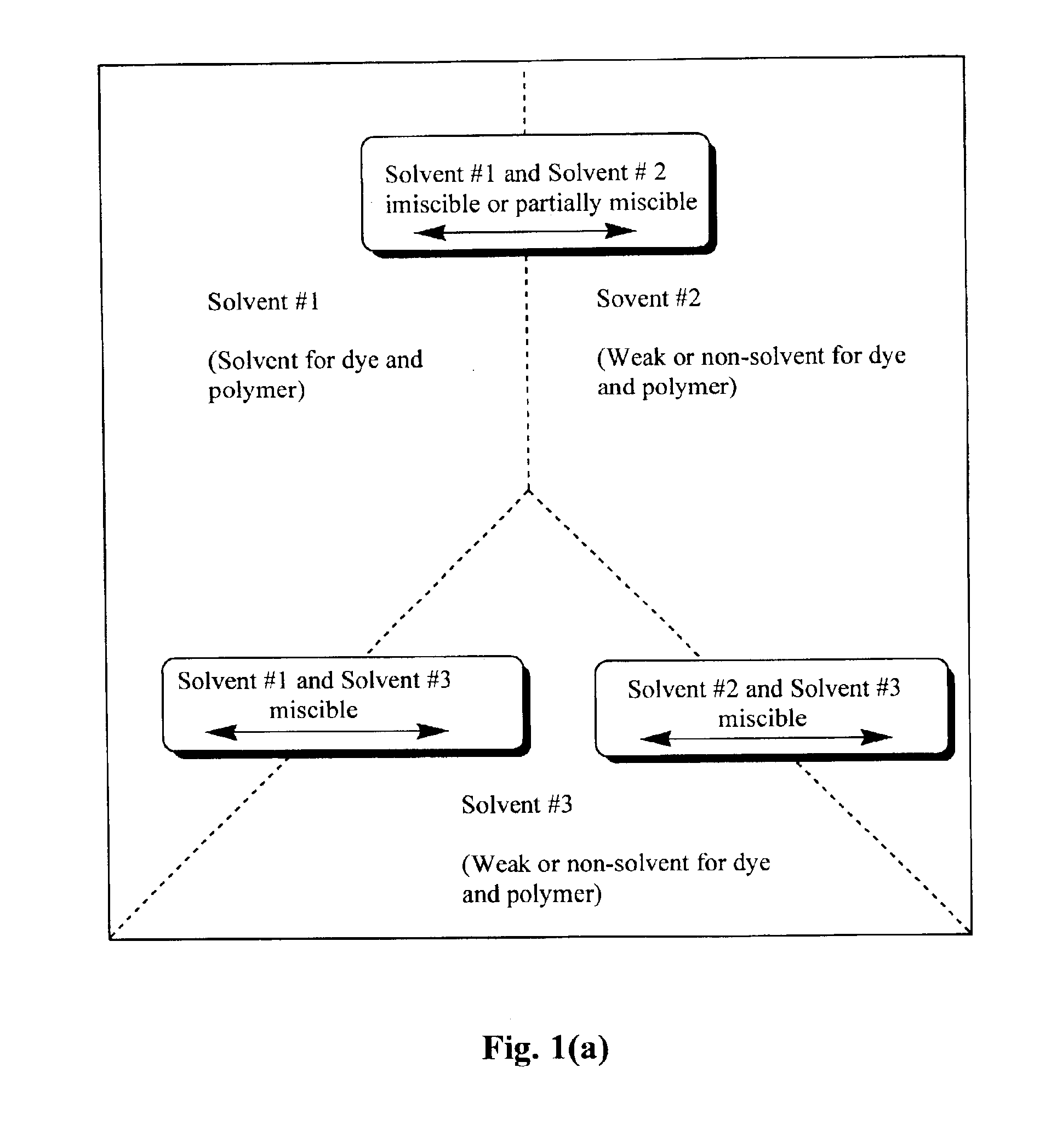
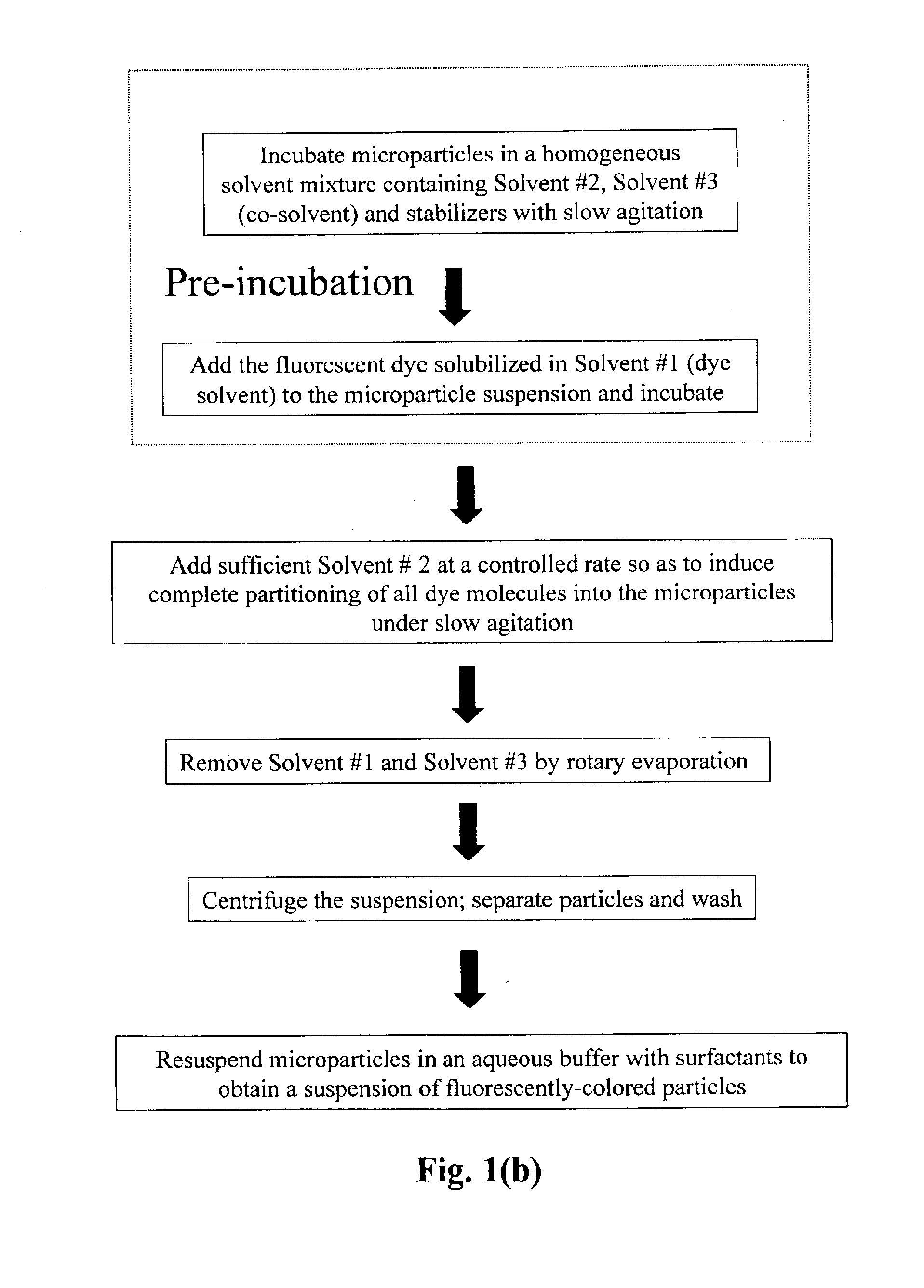
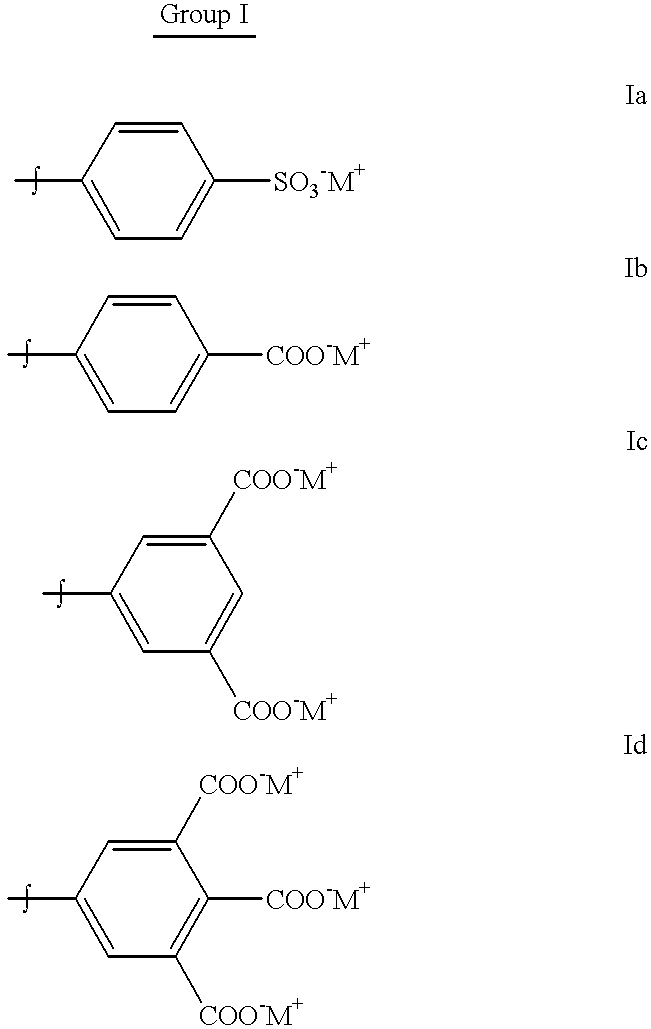
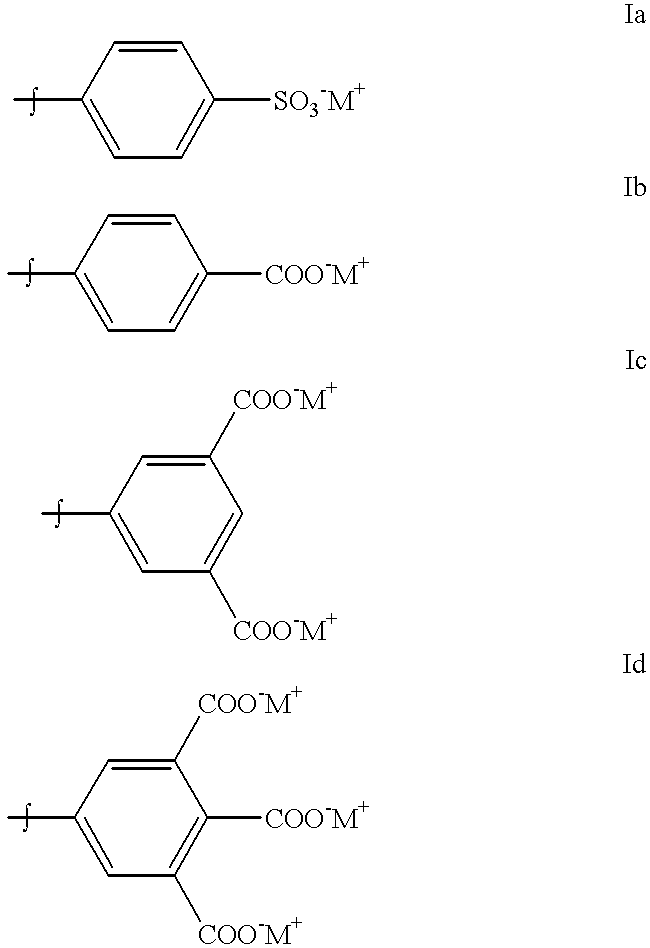
Production of dyed polymer microparticles
A dye, such as a fluorescent dye, is incorporated into polymer microparticles using a solvent system composed of a first solvent in which the dye and the microparticle polymer are soluble, a second solvent in which the dye and the microparticle polymer are not or only weakly soluble, and a third solvent in which the dye and the microparticle polymer are not or only weakly soluble. The first and second solvents are immiscible with each other, or at most partially miscible. The third solvent is miscible with the first and second solvents. The formulation provides substantially complete partitioning of the dye to the microparticles. The method may be used to obtain dyed polymer microparticle formed of cross-linked or non-cross-linked polymers. Libraries are provided comprising two or more sets of microparticles of different dye loadings. Fluorescent core-shell microparticles are produced from a mixture of microparticle cores incorporating one or more fluorescent dyes, a polymerization mixture comprising at least one polymerizable shell monomer, at least one free radical polymerization initiator comprising a water-insoluble oxidizing agent, and at least one water-soluble reducing agent.
Owner:BIOARRAY SOLUTIONS
Superior waterfastness and bleed control with specifically treated pigments for ink-jet printing
InactiveUS6221142B1Improve printing effectGood colorMaterial nanotechnologyDuplicating/marking methodsWater insolubleWater soluble
The ink of the invention comprises a vehicle and a colorant. The colorant is a water-insoluble pigment that has been chemically modified to be water dispersable by addition of functional groups to the surface of the pigment resulting in water soluble colorant particles. The performance of these pigments is improved by the addition of specific functional groups which provide improved black to color bleed control and high waterfastness.
Owner:HEWLETT PACKARD DEV CO LP
Cosmetic compositions having improved wear and beauty benefits
InactiveUS6503495B1Maintain integrityInhibit growthCosmetic preparationsImpression capsWater insolubleWater soluble
Owner:NOXELL CORP
Abuse resistant drug formulation
A pharmaceutical composition may include a granulate which may include at least one active pharmaceutical ingredient susceptible to abuse by an individual mixed with at least two materials, a first material that is substantially water insoluble and at least partially alcohol soluble and a second material that is substantially alcohol insoluble and at least partially water soluble, wherein the active pharmaceutical ingredient and the two materials are granulated in the presence of water and alcohol. The composition may also include a coating on the granulate exhibiting crush resistance which may have a material that is deposited on the granulate using an alcohol based solvent. The composition further comprises a second particle comprising a fat / wax. The present invention also includes a coated granulate, various dosage forms of the composition, as well as methods of production and tableting.
Owner:CIMA LABS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com