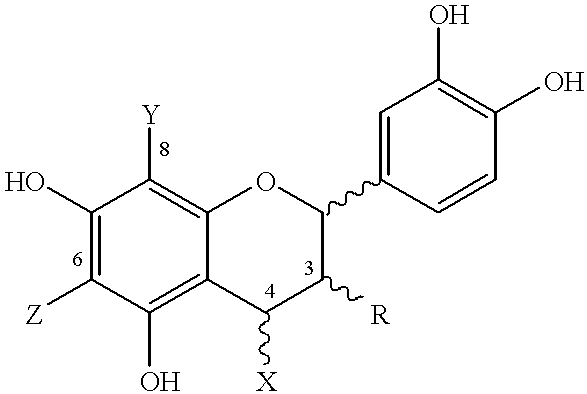
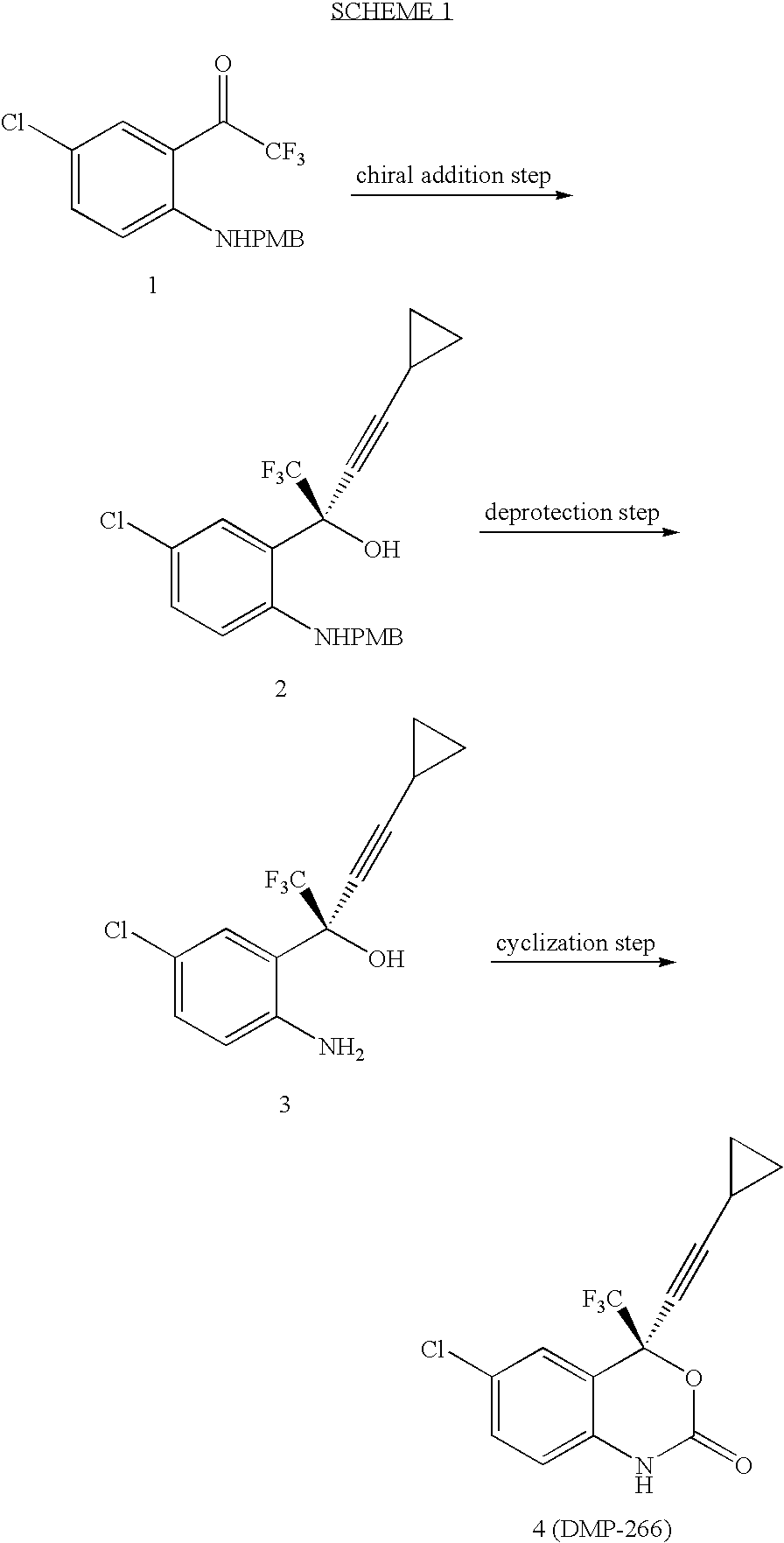
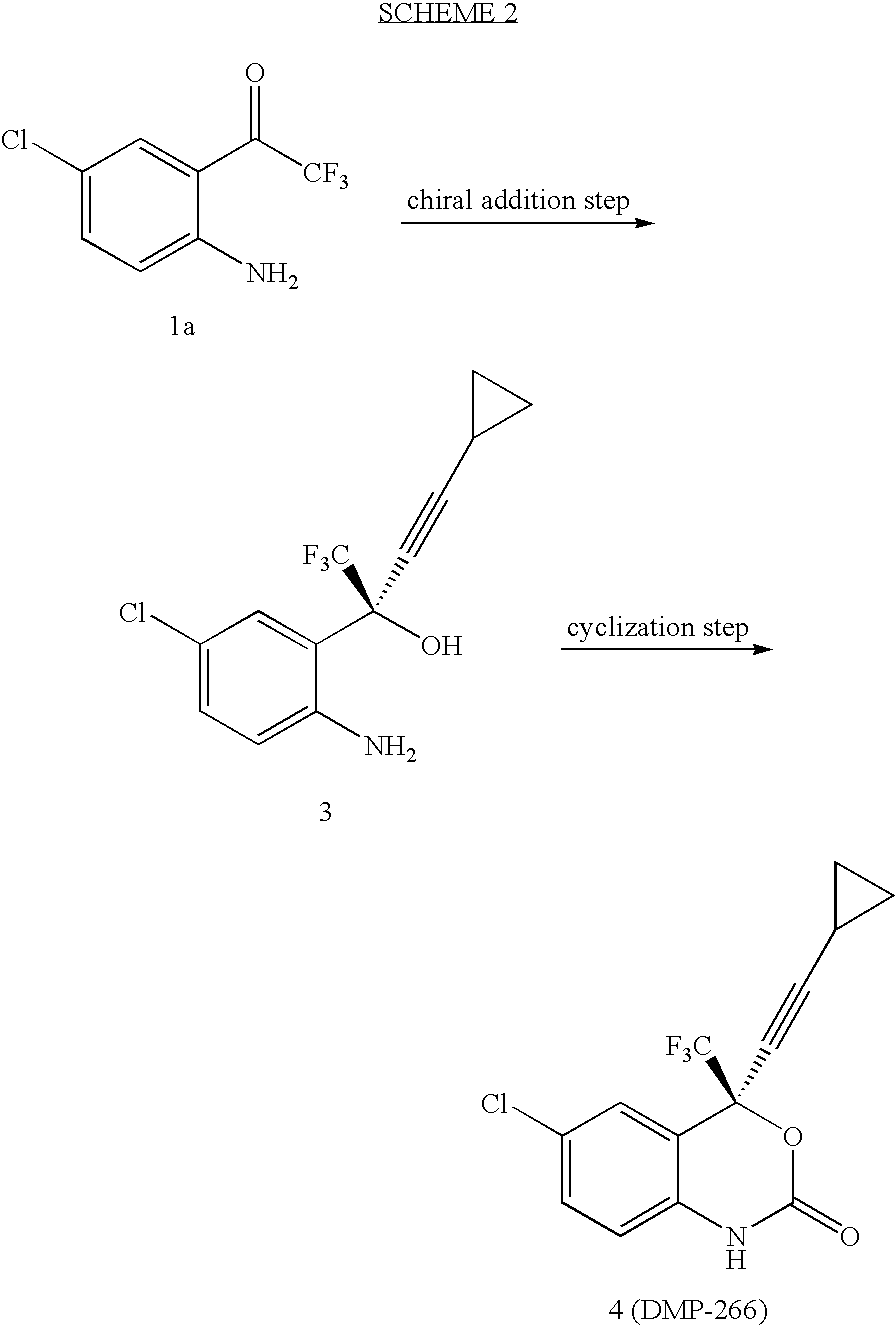
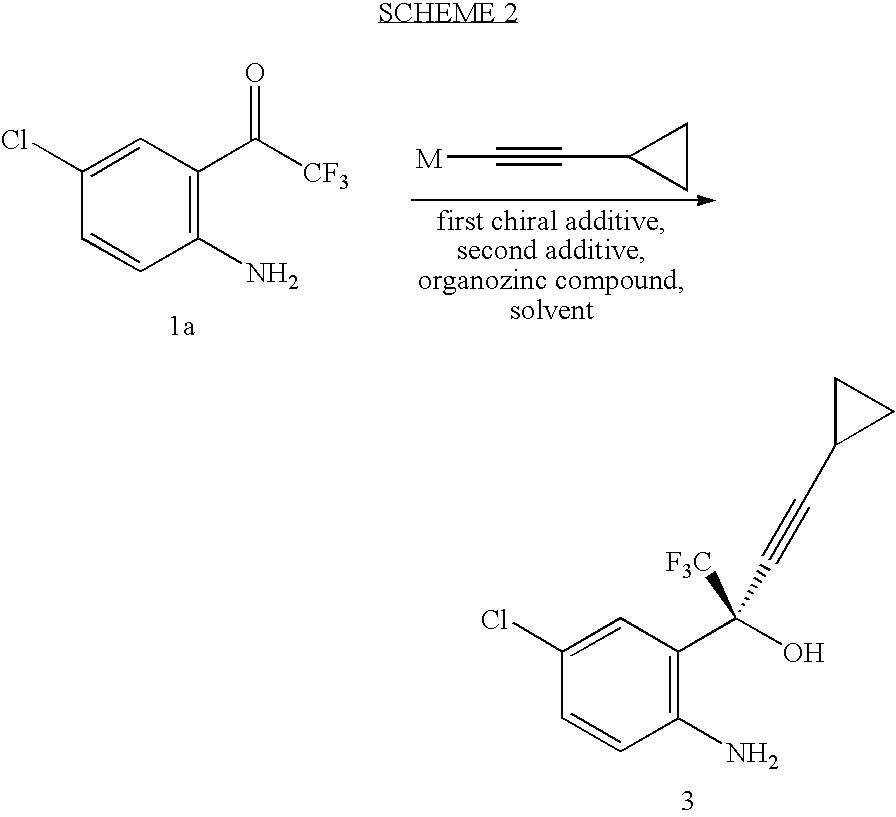
Patents
Literature
4221 results about "Tableting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tableting is a method of pressing medicine or candy into tablets. Confectionery manufacture shares many similarities with pharmaceutical production. A powder or granule mixture is prepared, a dye mold is filled, and then the mixture is compressed and ejected. While drug tablets are constrained to shapes that can be swallowed easily, candy tablets are chewable.
Direct compression metformin hydrochloride tablets
InactiveUS6117451AGood compressibilityImproved flowabilityPowder deliveryBiocideMetformin HydrochlorideHigh doses
Metformin Hydrochloride (herein referred to as metformin HCl) that may be 98.5%-100% pure is a high dose drug capable of being directly compressed with specific excipients into tablets having desired, hardness, disintegrating ability, and acceptable dissolution characteristics. Metformin HCl is not inherently compressible and thus presents formulation problems. Excipients used in the formulation enhance the flow and compaction properties of the drug and tableting mix. Optimal flow contributes to uniform die fill and weight control. The binder used ensures sufficient cohesive properties that allow metformin HCl to be compressed using the direct compression method. The tablets produced provide an acceptable in-vitro dissolution profile.
Owner:PHARMALOGIX
Abuse resistant drug formulation
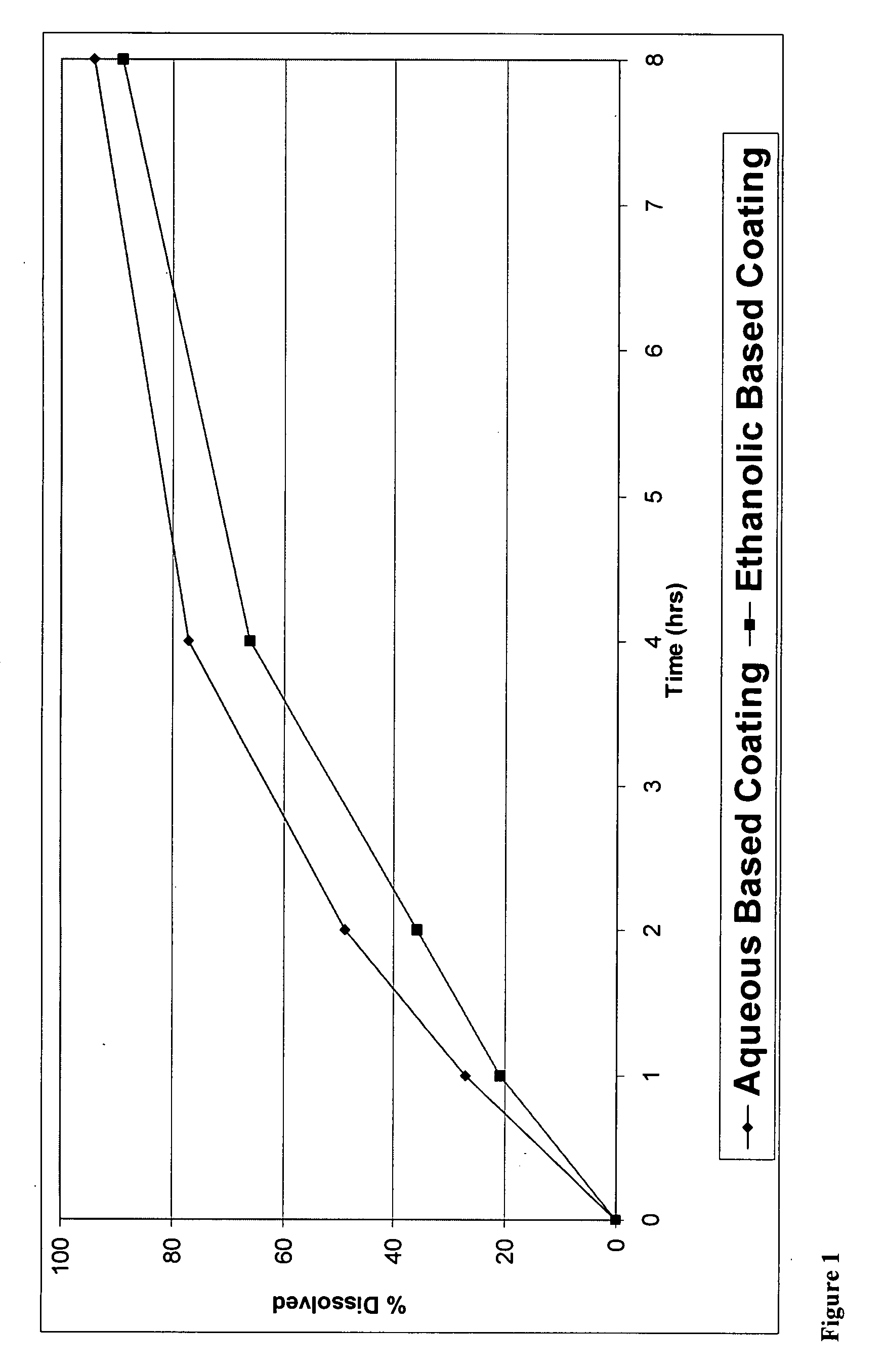
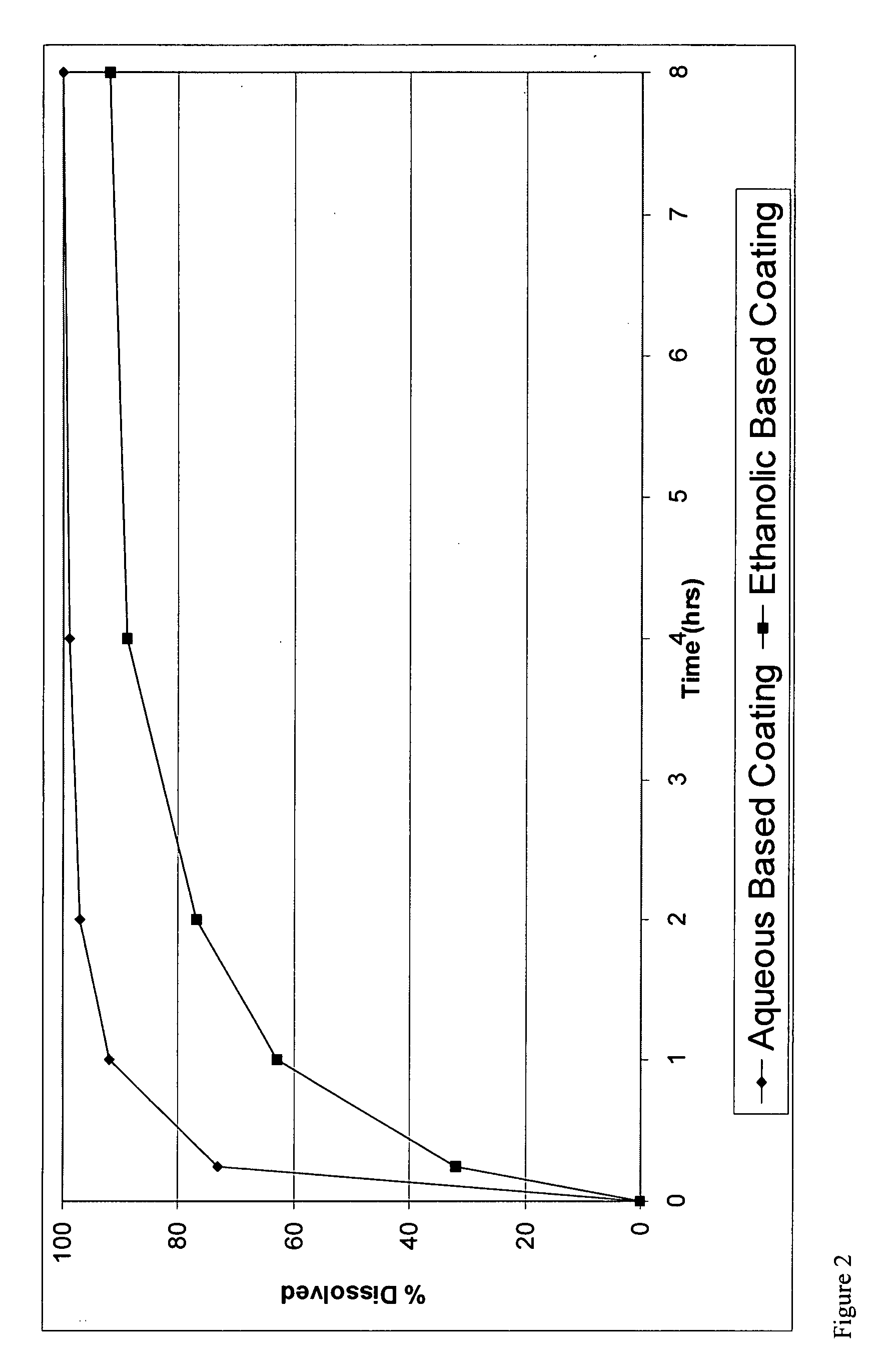
A pharmaceutical composition may include a granulate which may include at least one active pharmaceutical ingredient susceptible to abuse by an individual mixed with at least two materials, a first material that is substantially water insoluble and at least partially alcohol soluble and a second material that is substantially alcohol insoluble and at least partially water soluble, wherein the active pharmaceutical ingredient and the two materials are granulated in the presence of water and alcohol. The composition may also include a coating on the granulate exhibiting crush resistance which may have a material that is deposited on the granulate using an alcohol based solvent. The composition further comprises a second particle comprising a fat / wax. The present invention also includes a coated granulate, various dosage forms of the composition, as well as methods of production and tableting.
Owner:CIMA LABS +1
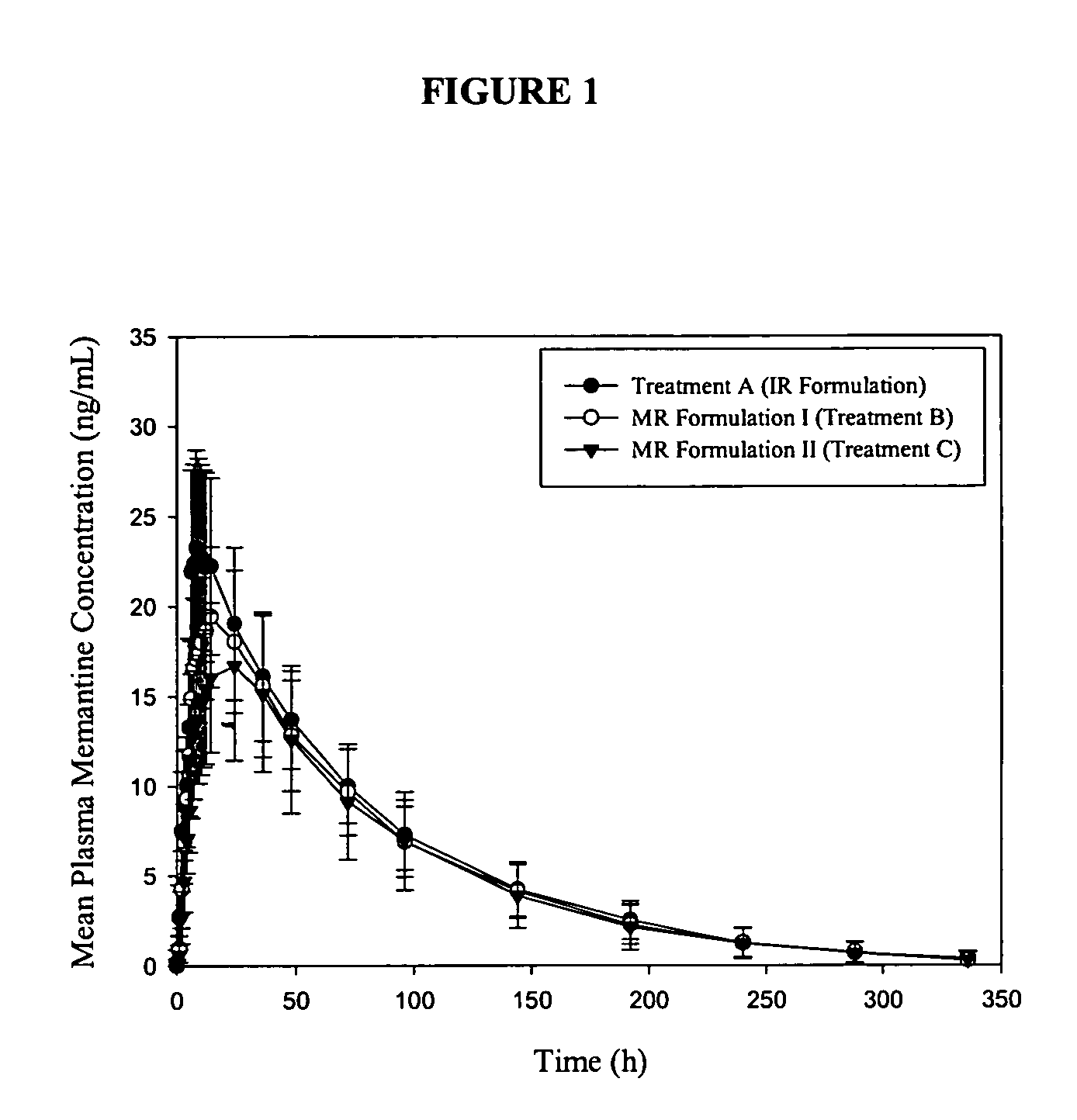
Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane
InactiveUS20060002999A1Improve bioavailabilityAdvantageous stability profileBiocideSenses disorderImmediate releasePharmaceutical medicine
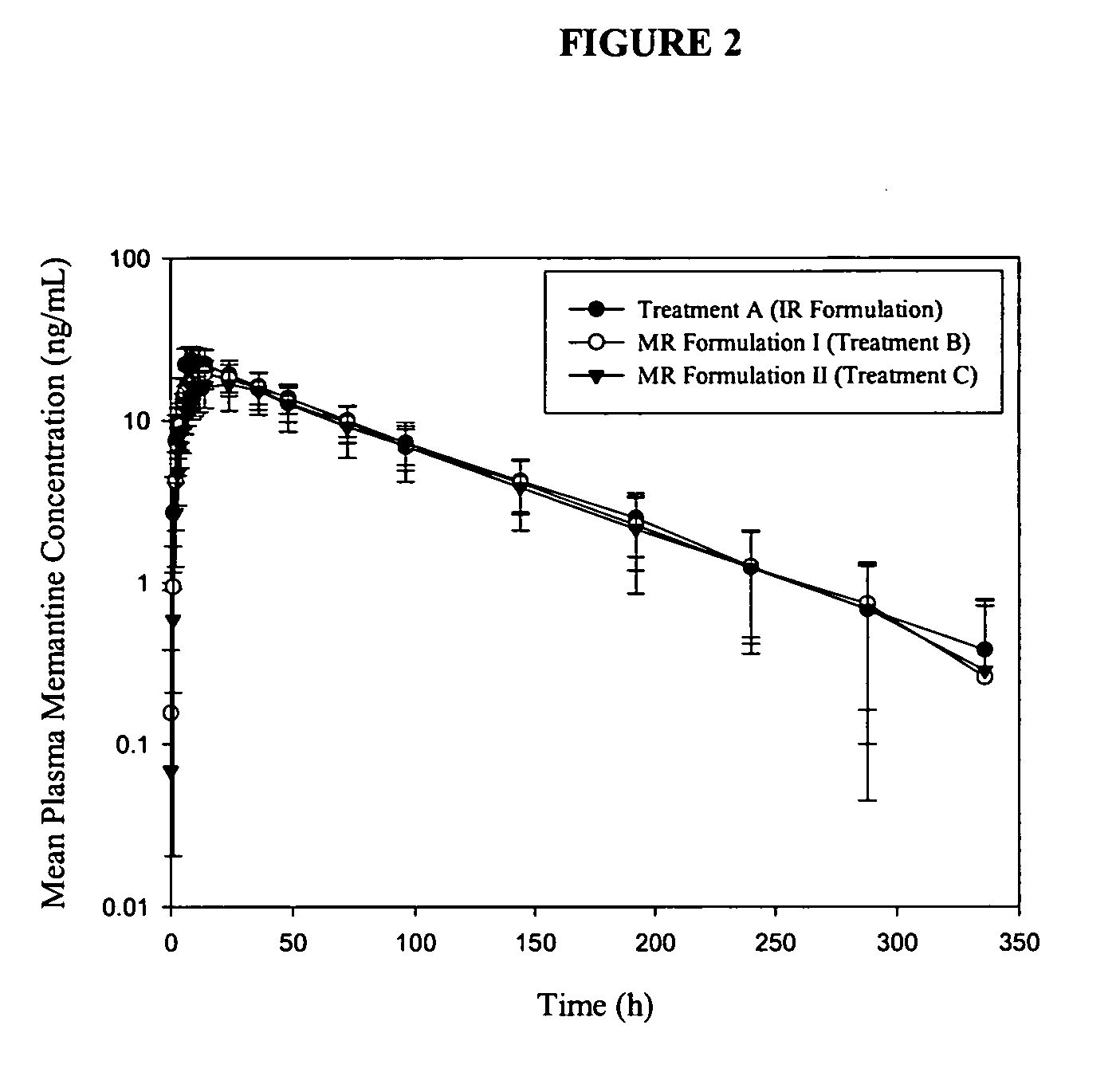
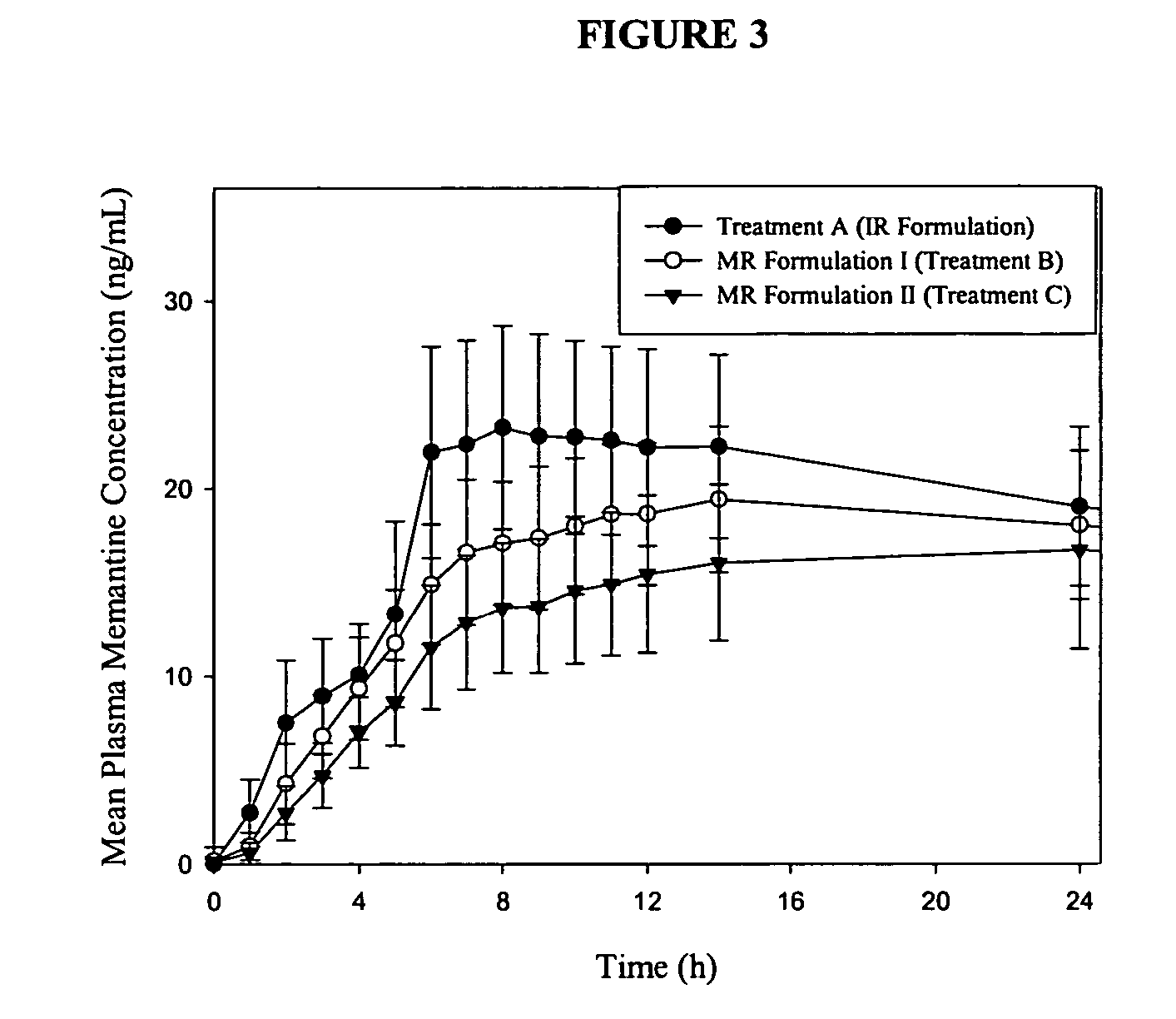
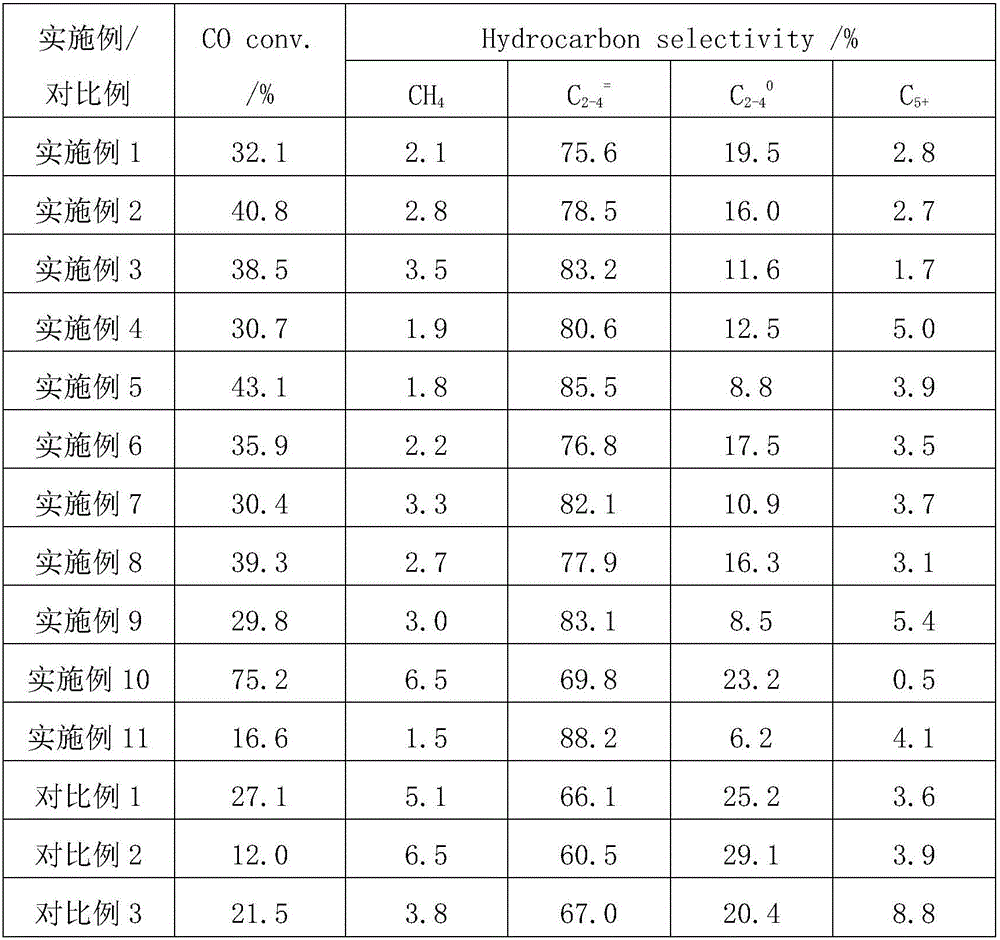
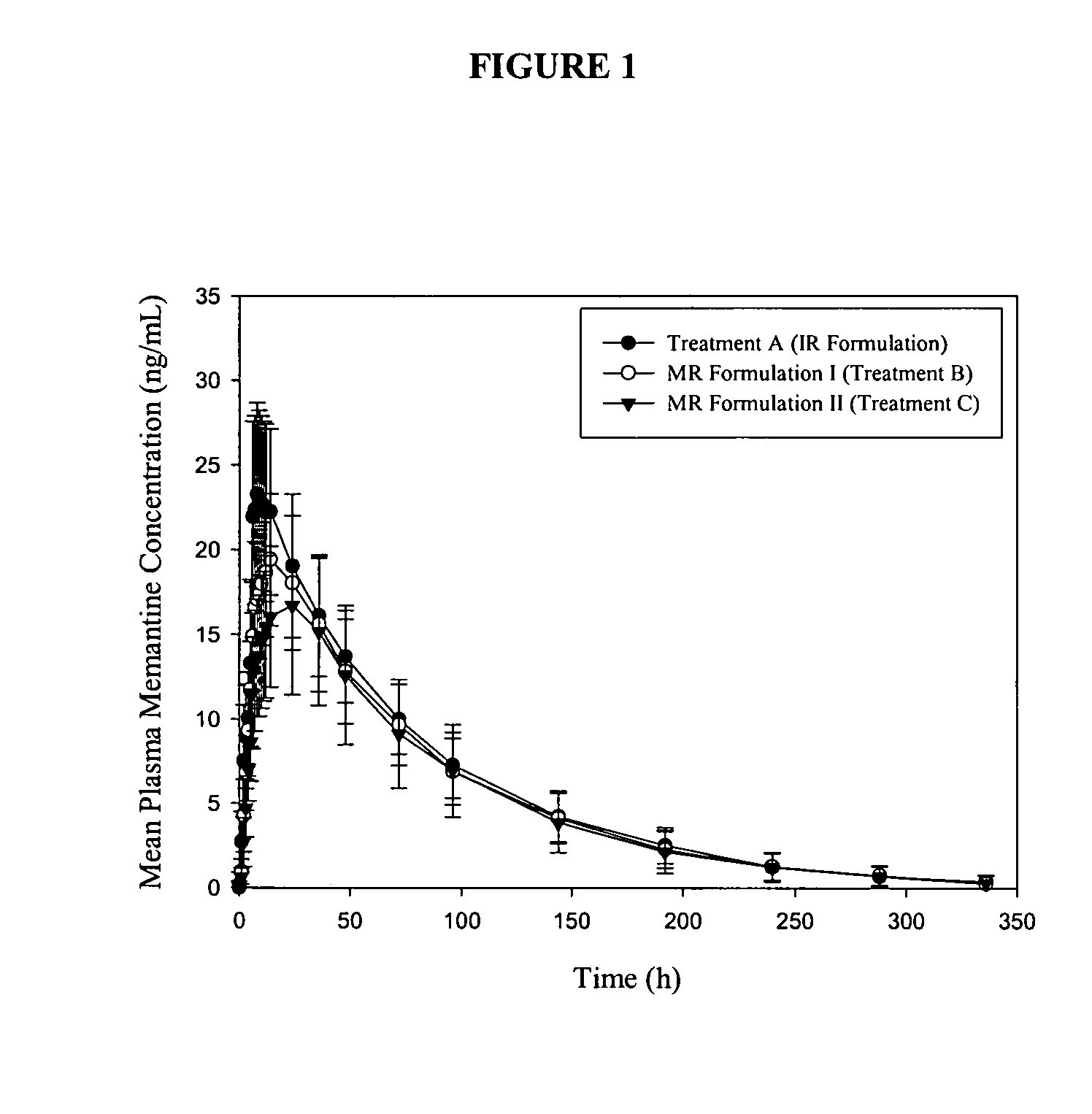
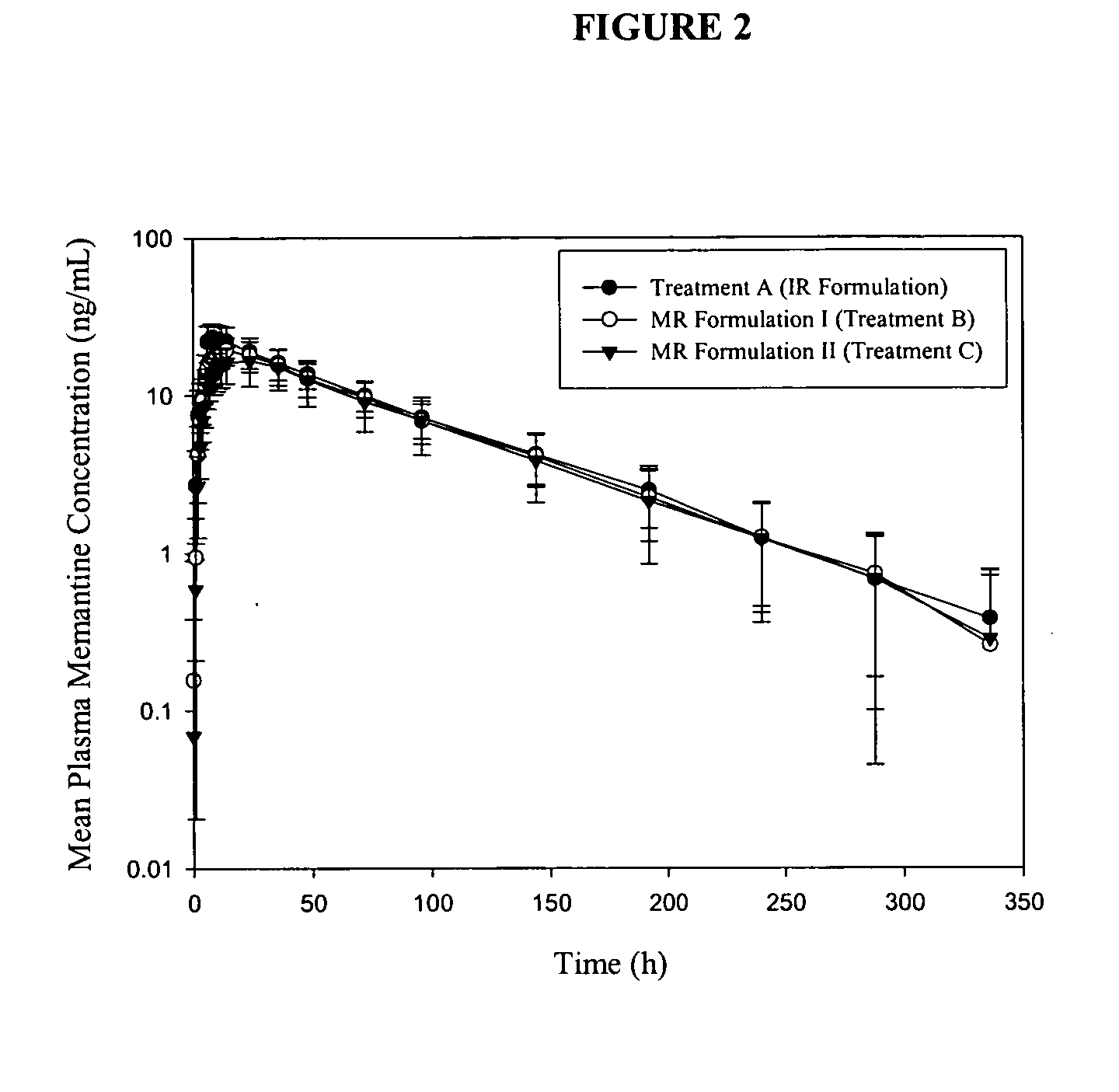
The present invention relates to an immediate release solid oral dosage form containing 1-aminocyclohexanes, preferably memantine or neramexane, and optionally a pharmaceutically acceptable coating, wherein the active ingredient exhibits dose proportionality and is released at a dissolution rate of more than about 80% within about the first 60 minutes following entry of said form into a use environment. The dosage form is direct compressed and has a hardness within the range of between about 3 and about 40 Kp, exhibits an average Tmax within the range of about 2 to about 8 hours with an active ingredient load within the range of about 2.5 to about 150 mg. The formulation allows for dose-proportional compositions for once daily or b.i.d. dosing, while maintaining a steady average range of Tmax.
Owner:FOREST LAB HLDG LTD
Use of cocoa solids having high cocoa polyphenol content in tabletting compositions and capsule filling compositions
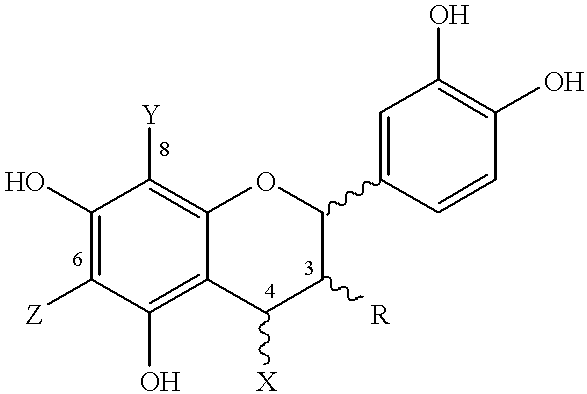
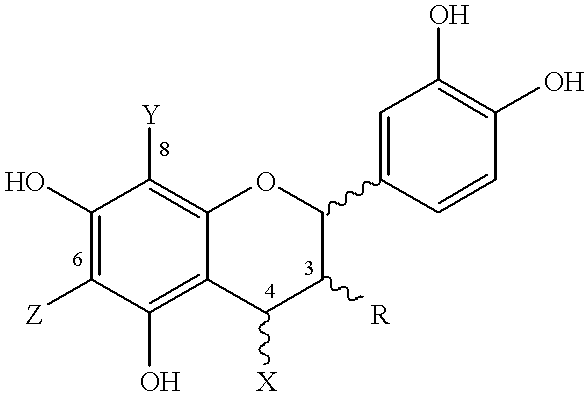
Disclosed and claimed are cocoa extracts, compounds, combinations thereof and compositions containing the same, such as polyphenols or procyanidins, methods for preparing such extracts, compounds and compositions, as well as uses for them, especially a polymeric compound of the formula An, wherein A is a monomer of the formula:wherein n is an integer from 2 to 18, such that there is at least one terminal monomeric unit A, and one or a plurality of additional monomeric units;R is 3-(alpha)-OH, 3-(beta)-OH, 3-(alpha)-O-sugar, or 3-(beta)-O-sugar;bonding between adjacent monomers takes place at positions 4, 6 or 8;a bond of an additional monomeric unit in position 4 has alpha or beta stereochemistry;X, Y and Z are selected from the group consisting of monomeric unit A, hydrogen, and a sugar, with the provisos that as to the at least one terminal monomeric unit, bonding of the additional monomeric unit thereto (the bonding of the additional monomeric unit adjacent to the terminal monomeric unit) is at position 4 and optionally Y=Z=hydrogen;the sugar is optionally substituted with a phenolic moiety, at any position on the sugar, for instance via an ester bond, andpharmaceutically acceptable salts or derivatives thereof (including oxidation products).
Owner:MARS INC
Compressed tablet formulation
This invention relates to a 50% drug loaded compressed tablet formulation for efavirenz. Efavirenz is a non-nucleoside reverse trancriptase inhibitor being studied clinically for use in the treatment of HIV infections and AIDS.
Owner:MERCK SHARP & DOHME CORP
Catalyst for preparing low-carbon olefins by one-step conversion of synthetic gas and preparation method thereof
ActiveCN106345514AImprove catalytic performanceImprove connectivityMolecular sieve catalystsSyngasMolecular sieve
A catalyst for preparing low-carbon olefins by one-step conversion of synthetic gas and a preparation method thereof relate to catalysts; the catalyst is made from 20-60% of a zirconium-based solid solution,30-70% of a double-micropore zeolite molecular sieve and 0.1-10% of metal oxides; the preparation method comprises: adding salt compounds of at least one element from IA, IIIA, VIIB, IB, IIB and the like into deionized water or alcohol to obtain solution A having a mass concentration of 0.1-15%; adding the zirconium-based solid solution into the solution A, heating, drying by distillation, and drying to obtain solid powder; adding the solid powder and the double-micropore zeolite molecular sieve into ethylene glycol, ultrasonically dispersing, and moving a filtered and washed sample to a vacuum drying box, drying at 50-100 DEG C for 2-24 h, calcining in a muffle furnace, and tableting obtained solid sample to obtain the catalyst.
Owner:XIAMEN UNIV
Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane
InactiveUS20060198884A1Improve stabilityBiocideSenses disorderImmediate releasePharmaceutical medicine
The present invention relates to an immediate release solid oral dosage form containing 1-aminocyclohexanes, preferably memantine or neramexane, and optionally a pharmaceutically acceptable coating, wherein the active ingredient exhibits dose proportionality and is released at a dissolution rate of more than about 80% within about the first 60 minutes following entry of said form into a use environment. The dosage form is direct compressed and has a hardness within the range of between about 3 and about 40 Kp, exhibits an average Tmax within the range of about 2 to about 8 hours with an active ingredient load within the range of about 2.5 to about 150 mg. The formulation allows for dose-proportional compositions for once daily or b.i.d. dosing, while maintaining a steady average range of Tmax.
Owner:FOREST LAB HLDG LTD
Over-coated chewing gum formulations including tableted center
Methods and products for delivering a medicament or agent to an individual are provided as well as methods for producing the product. The product includes a coating having a medicament or agent. The medicament or agent is present within the coating that surrounds a tableted gum center (the water soluble portion and a water insoluble base portion). By chewing the gum, the medicament or agent is released from the product. Continuing to chew the chewing gum creates a pressure within the buccal cavity forcing the agent or medicament directly into the systemic system of the individual through the oral mucosa contained in the buccal cavity. This greatly enhances the absorption of the drug into the systemic system as well as the bioavailability of the drug within the system.
Owner:WM WRIGLEY JR CO
Beta-lactam antibiotic-containing tablet and production thereof
InactiveUS6423341B1Antibacterial agentsOrganic active ingredientsLow-substituted hydroxypropylcelluloseEngineering
This invention provides beta-lactam antibiotic-containing tablets capable of being orally taken either as such owing to their being small-sized, hence still easily swallowable, or, in the case of administration to the aged encountering some difficulty in swallowing, in the form of dispersions resulting from easy self-disintegration upon being dropped into water in a glass as well as a method of producing the same. The tablets of this invention comprise, on the per-tablet basis, 60-85% by weight of a beta-lactam antibiotic, 1-10% by weight of low-substituted hydroxypropylcellulose and / or crosslinked polyvinylpyrrolidone as a disintegrator, and 0.5-2% by weight of a binder. Granules to be compressed for tableting are prepared using water or an aqueous solution of ethanol or the like.
Owner:ASTELLAS PHARMA INC
Liquid dosage forms having enteric properties of delayed and then sustained release
Owner:EASTMAN CHEM CO
High temperature resistant economical electrostatic powder coating for electric room heater and preparation method thereof
InactiveCN101565587AImprove heat resistanceAvoid harmFireproof paintsPowdery paintsMetallurgyHigh pressure
The invention discloses a high temperature resistant economical electrostatic powder coating for an electric room heater and a preparation method thereof. The formulation of the powder coating comprises the following compositions in percentage by weight: 53 to 57 percent of thermosetting resin, 3 to 7 percent of curing agent, 1 to 30 percent of pigment, 5 to 34 percent of filler and 2 to 5 percent of auxiliary agent. The powder coating is produced by adopting the method of premixing, melt extrusion and mixing, tabletting, cooling and crushing, fine grinding and classification, sieving and separation, and product, wherein the average particle size of the powder coating is between 25 and 30 mu m, and the thickness requirement between 50 and 60 mu m of an economic coating is met. The powder coating is suitable for coating by a high voltage electrostatic powder coating method and simultaneously is suitable for coating by a frictional electrostatic powder coating method.
Owner:杨彬
Tablet Quickly Disintegrating in Oral Cavity
ActiveUS20070275058A1Sufficient hardnessOral disintegration time is shortenedPowder deliveryBiocidePharmaceutical preservativesHardness
The present invention provides rapid disintegrating tablets in oral cavity having a shortened disintegration time in oral cavity as well as a sufficient hardness compared to rapid disintegrating tablets of the prior art. The above objective is solved by a composition in which the inorganic excipient and the disintegrating agent are dispersed in the complex particles consisting of mannitol and other saccharide(s) in a specific ratio, and rapid disintegrating tablets in oral cavity obtained by direct compression of the composition.
Owner:FUJI CHEM IND CO LTD
Stomach-nourishing spleen-strengthening chewing tablets and preparation method thereof
InactiveCN103356933AGood disintegrationLarge specific surface areaDigestive systemPill deliveryBiotechnologyNutrition
The invention relates to stomach-nourishing spleen-strengthening chewing tablets and a preparation method thereof, and belongs to the technical field of traditional Chinese medicines. The chewing tablets comprises the raw materials of, by weight: 6-9 parts of Mongolian milkvetch root, 5-8 parts of pilose asiabell root, 3-5 parts of ligusticum, 7-11 parts of common yam rhizome, 6-8 parts of dried longan, 8-12 parts of medlar, 7-9 parts of red date, 3-7 parts of dried tangerine peel, 8-12 parts of hawthorn fruit, 10-12 parts of stir-fried malt, 60-80 parts of microcrystalline cellulose, 10-20 parts of micro-powder silica gel, 2-10 parts of a hydroxymethyl cellulose, and 1-3 parts of a flavoring agent. The chewing tablets are prepared through three steps which are extraction, granulation, and tableting. The process provided by the invention is simple. The prepared chewing tablets have the advantages of rich nutrients, good taste, convenient application, and long effect. The tablets have the functions of stomach nourishing, spleen strengthening, qi invigorating, and digestion promoting.
Owner:SUZHOU TIANLING CHINESE TRADITIONAL MEDICINE SLICE
Confectionery product comprising agglomerated oil powder
InactiveUS20140141147A1Hard textureShape stableMilk preparationConfectioneryAdditive ingredientSugar food
The present invention relates to a solid confectionery product comprising pressure agglomerated powder ingredients, wherein said powder ingredients comprise an oil powder, the use of an oil powder for the preparation of a solid confectionery product, and processes for the preparation of a solid confectionery product compacting or shaping the ingredients including an oil powder by a pressure agglomeration process.
Owner:NESTEC SA
Fire-resistant powder coating material and its production process
InactiveCN1380370AOvercoming the flaws of explosionWith fire performanceFireproof paintsPowdery paintsEpoxyVitrification
The present invention relates to a decorative powder coating. It can be divided into two kinds of non-expansive fire-resisting powder coating and expansive fire-resisting powder coating. Its production process includes the following steps: heating proper quantity of polyester resin and / or epoxy resin to glass state temp., adding fire-resisting agent and / or carbon-forming agent, catalyst and foaming agent, stirring, cooling and pulverizing, mixing them with residual polyester resin and / or epoxy resin and other materials, stirring them, extruding, cooling, tabletting, pulverizing and sieving soas to obtain the invented products.
Owner:葛明槽
Tough sintering epoxy powder coating and preparation thereof
InactiveCN101353544AImprove toughnessImprove impact resistanceAnti-corrosive paintsPowdery paintsEpoxyCrusher
The invention relates to a tough fusion bonded epoxy powder coating and a preparation method thereof. The tough fusion bonded epoxy powder coating of the invention consists of the following components by mass percentage: linear phenolic epoxy resin, 30 percent to 50 percent; epoxy resin E-12, 0 percent to 20 percent; curing agent, 15 percent to 25 percent; leveling agent, 0 to 2 percent; and filling, 23 percent to 33 percent. The preparation method of the tough fusion bonded epoxy powder coating comprises the following steps: adding the components into a high-speed mixer for mixing according to mixture ratio and later into an extruder for melt extrusion, and then undertaking chip compression, cooling and crushing on a cold crawler type chip compression crusher, and later undertaking grinding, classification, cyclone separation and screening in a grinding machine, and finally obtaining the product. The tough fusion bonded epoxy powder coating of the invention is mainly used in the pipe anti-corrosion field, has the advantages of good toughness, high impact resistance performance, and simple preparation process, and is suitable for mass production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
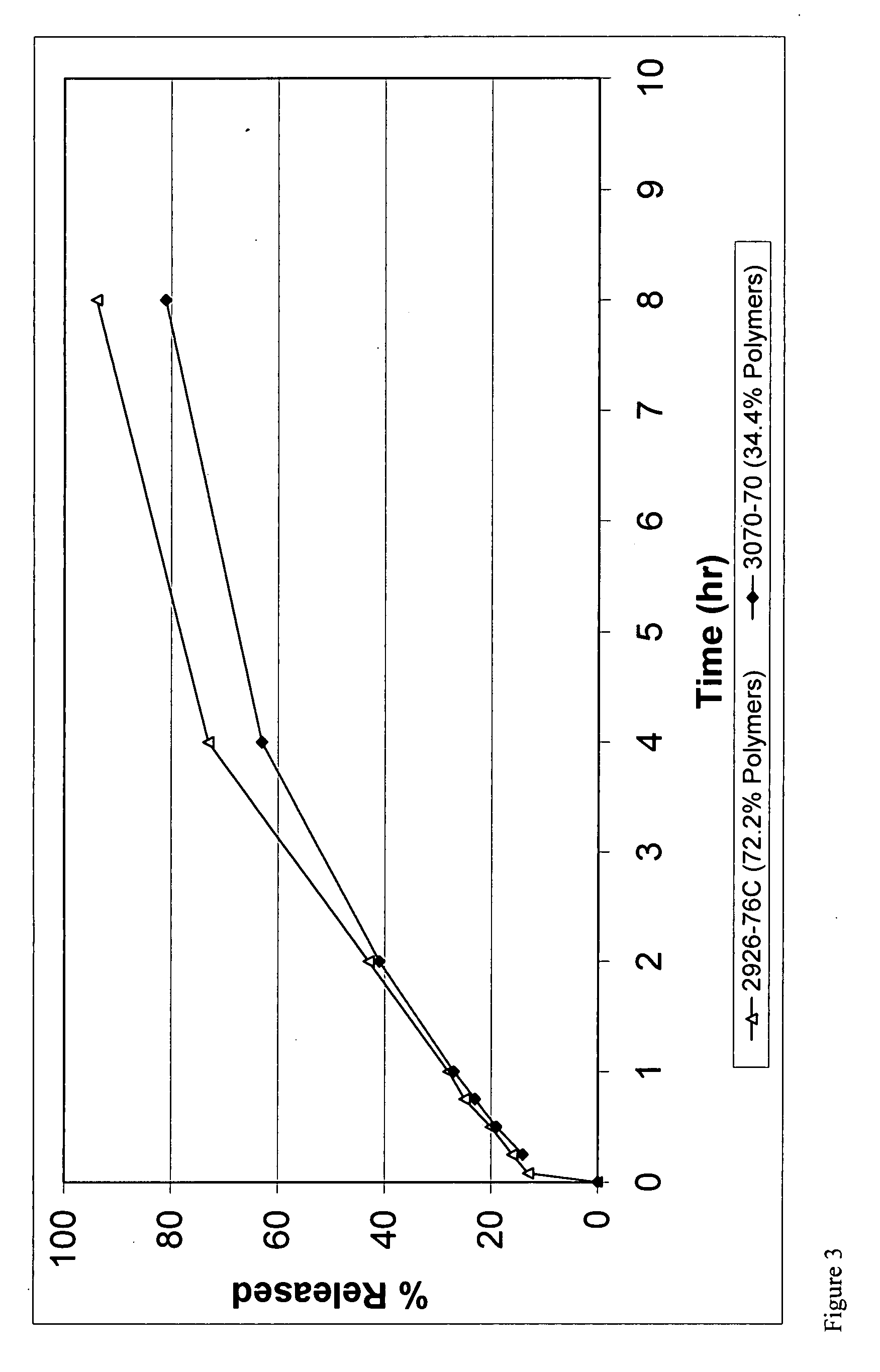
Compressible-Coated Pharmaceutical Compositions and Tablets and Methods of Manufacture
InactiveUS20110129530A1Improving tableting propertyImprove propertiesBiocideNervous disorderControlled releaseOrally disintegrating tablet
There is provided a method for preparing a pharmaceutical composition comprising compressible coated, taste-masked and / or controlled-release coated drug-containing particles, rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol, a saccharide, or a mixture thereof, and other optional, pharmaceutically acceptable excipients wherein the orally disintegrating tablet (ODT) or rapidly dispersing tablet (RDT) composition having acceptable tableting, organoleptic, and pharmacokinetic properties.
Owner:ADARE PHARM INC
Snack Cracker and Method for Making Same
InactiveUS20100215826A1Reduced level of saturated fatLower Level RequirementsDough treatmentLeguminous plant bakery productsChipped potatoesAdditive ingredient
The present invention discloses formulations for sheeted, baked fruit and vegetable crackers that have a light, crispy texture similar to a potato chip or cracker. Undehydrated ingredients are combined with dry ingredients and oil to make a dough, which is then sheeted and cut into pieces. The pieces are baked to produce vegetable and fruit snack crackers.
Owner:FRITO LAY TRADING CO GMBH
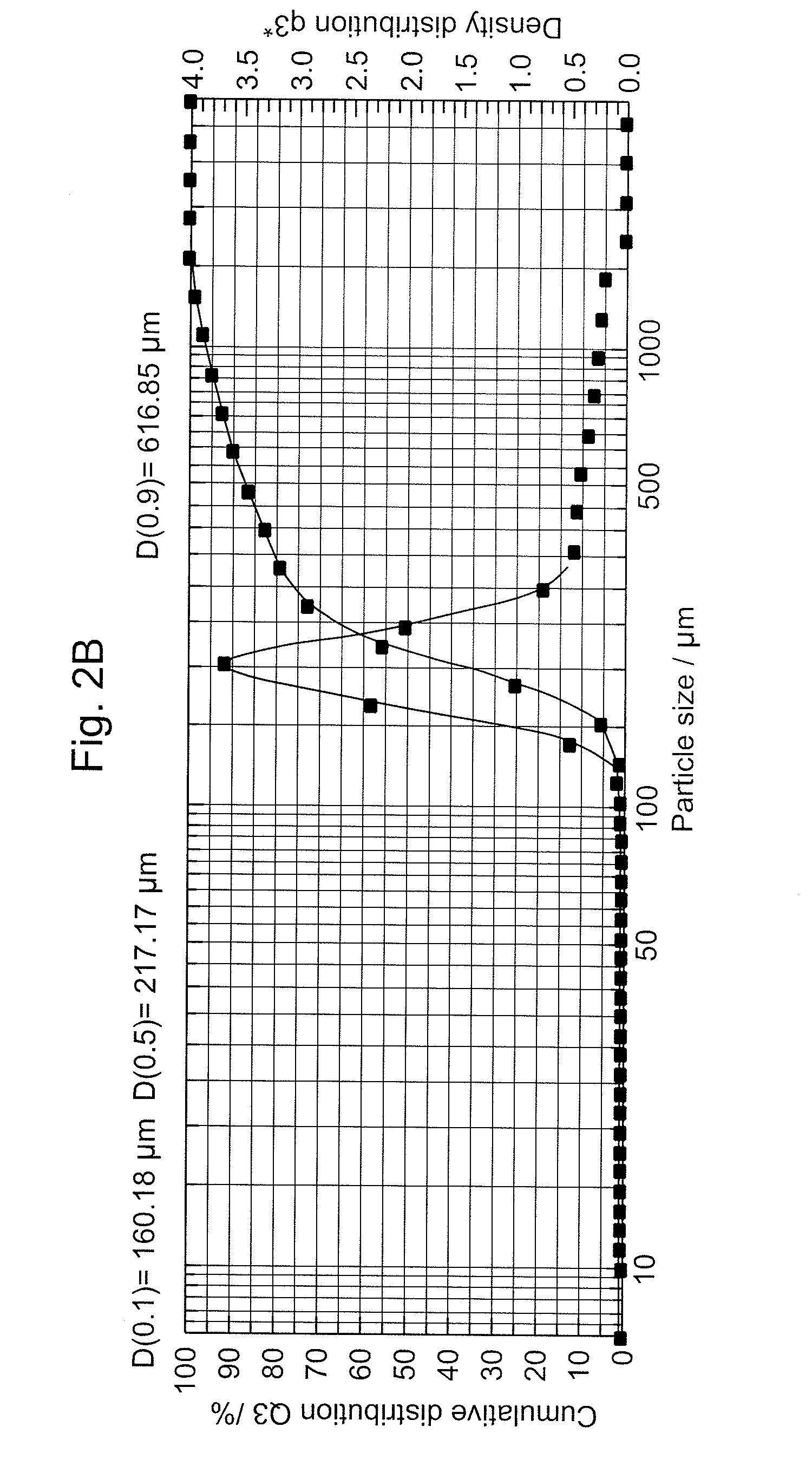
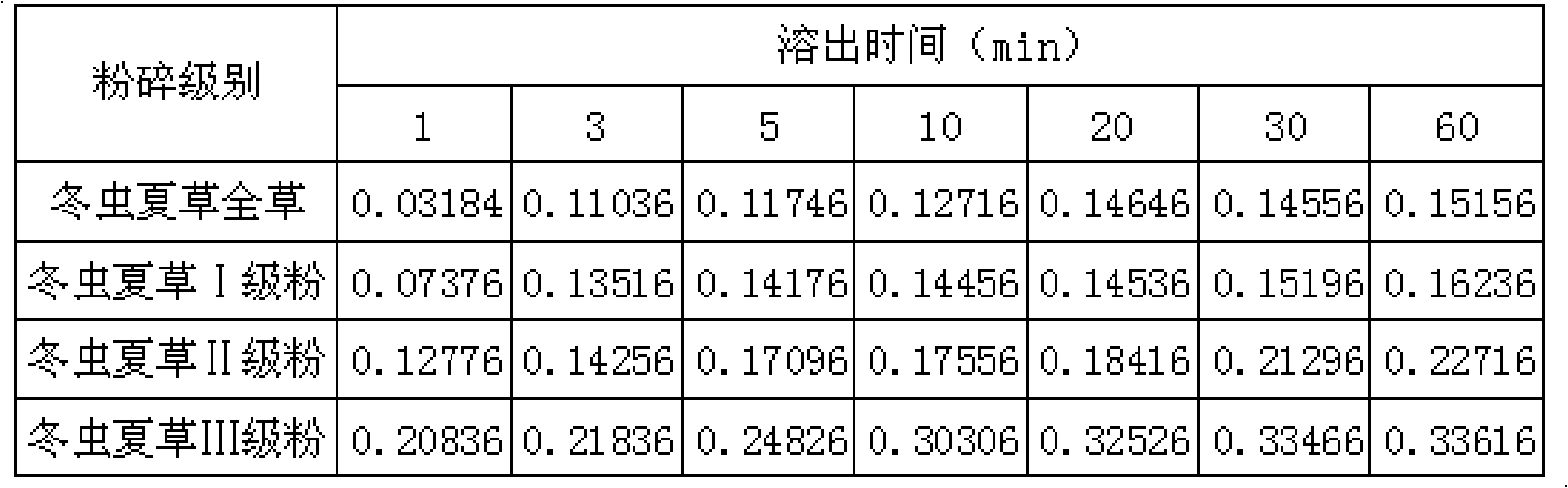
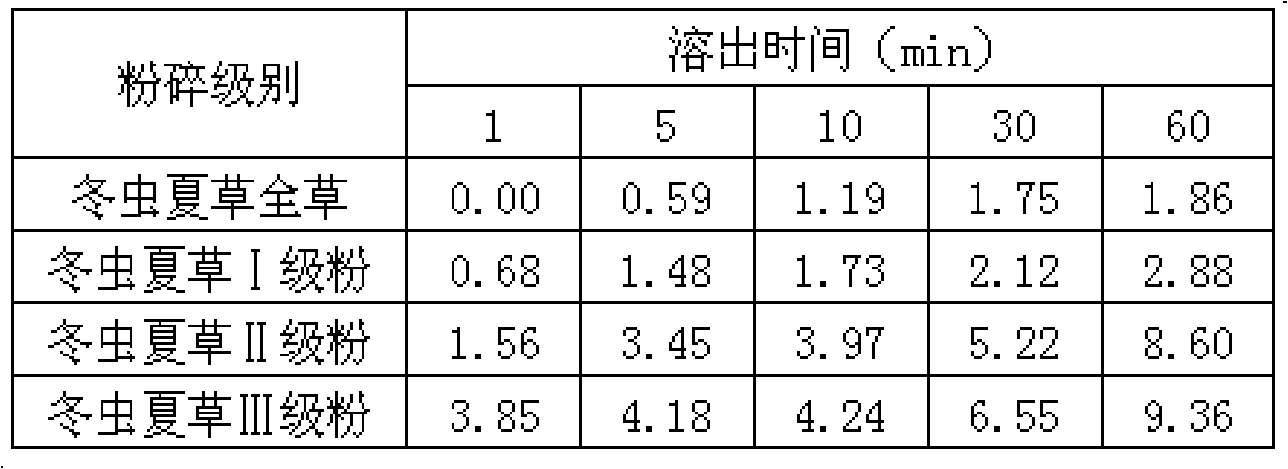
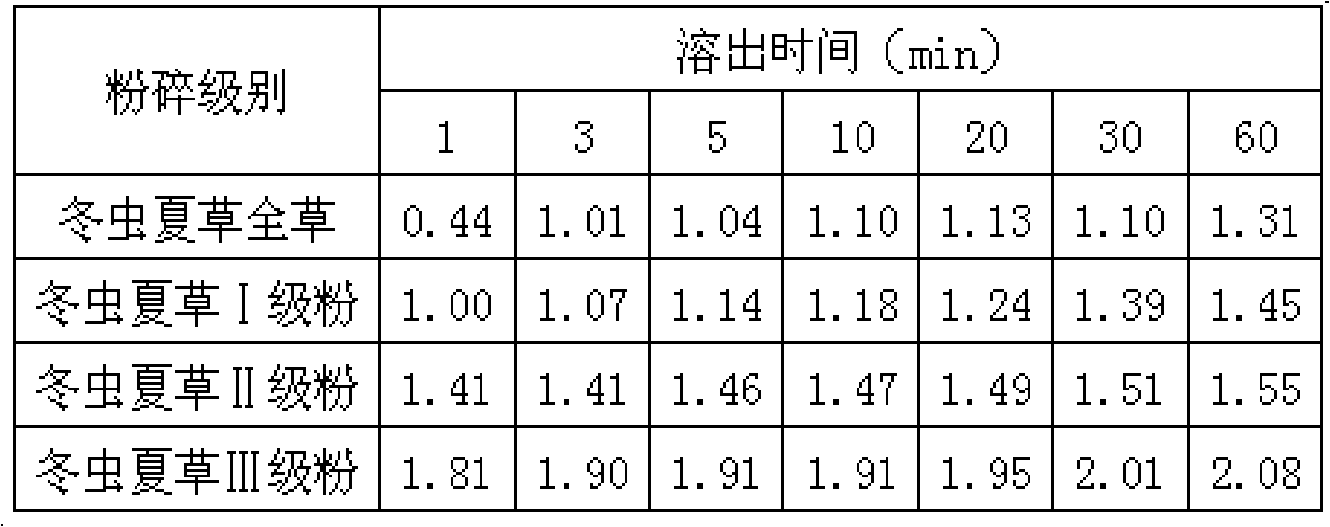
Aweto micropowder tablet and preparation method thereof
ActiveCN101332212AIncrease concentrationFully activePill deliveryImmunological disordersMedicineTableting
The present invention relates to a cordyceps sinensis powder tablet and a preparation method thereof, which pertains to the field of medicines and health products. The technical problem the present invention aims at providing a tablet containing cordyceps sinensis medicinal powder and no auxiliary material; the appearance, shape and harness of the tablet is consistent with the tablet quality standard. The cordyceps sinensis powder tablet only adopts cordyceps sinensis powder with the water content of 8 to 18 percent and the granule diameter of 1 to 150mum; no auxiliary material is added in the preparation process and the cordyceps sinensis powder is the only component. The aim of direct tablet forming can be achieved by controlling the water of the powder or by the process of second tabletting or dry granule tabletting. The process ensures that the tablet appearance is good; pockmark surface rate and fracture rate are low; tablet harness, disintegration rate and friability are consistent with the tablet quality requirement; the harness can also ensure that the tablet can not fracture in the preparation process when coating and film covering is carried out in the later stage.
Owner:QINGHAI SPRING MEDICINAL RESOURCES TECHNOLOGY CO LTD
Method for extracting xanthoxylin and sanshoamides from pricklyash peel
The invention relates to a process for extracting xanthoxylin and sanshoamides through supercritical carbon dioxide. Firstly, the pricklyash peels are crushed and squashed into pieces, the squashed pricklyash peels are loaded in a supercritical extraction reactor for extraction, the extraction solvent is food-grade carbon dioxide, the extraction liquid sequentially enters a separation reactor I and a separation reactor II for separation, the pressure of the separation reactor I is 6-7 MPa, the temperature thereof is 25-32 DEG C and the time is 20-40 min; the pressure of the separation reactor II is 5-6 MPa, the temperature thereof is 22-26 DEG C and the time is 30-50 min; the products of the separation reactor I and the separation reactor II are respectively collected, the product of the separation reactor I is dehydrated and refined to prepare the sanshoamides, and the product of the separation reactor II is xanthoxylin; the sanshoamides, xanthoxylin and food oil are blended according to weight ratio of 1:1:30-100 to obtain supercritical pepper oil which can be used as flavoring oil for high-grade dinning industry.
Owner:CHENGUANG BIOTECH GRP CO LTD
Method of manufacturing tablet
InactiveUS20040131675A1Avoid breakingCoated granuleDigestive systemPharmaceutical product form changeRoom temperatureBiologically active substances
A method of manufacturing a tablet containing coated granules comprising compressing the coated granules containing biologically active substance and having a temperature exceeding a room temperature, whereby the tablet can be prevented from rupture of a part of a coating film of the granules at the time of tablet compression.
Owner:TAKEDA PHARMA CO LTD
Blueberry composite tablet candy capable of relieving visual fatigue and preparation method thereof
InactiveCN104397311ARelieve eye fatigueImprove eyesightConfectionerySweetmeatsBiotechnologyBlueberry extract
The invention discloses a blueberry composite tablet candy capable of relieving visual fatigue and a preparation method thereof. The blueberry composite tablet candy capable of relieving the visual fatigue comprises the following raw materials in percentage by weight: 13.25 percent of a blueberry extract, 0.5 percent of lutein esters, 0.3 percent of beta-carotene, 1.0 percent of citric acid, 64.45 percent of sorbitol, 2 percent of xylooligosaccharide, 3 percent of dextrin and 15 percent of microcrystalline cellulose, wherein the blueberry extract is extracted from a mixture of blackberry pomace dry matter and acid ethanol; the acid ethanol comprises ethanol and water with the volume percentage of 60 percent; the pH value of the acid ethanol is 1.5 to 3.0; the blackberry pomace dry matter and the acid ethanol are in the mass volume ratio of 1:50-1:70; the preparation method of the blueberry composite tablet candy capable of relieving the visual fatigue comprises the following main steps: 1, preparing the blueberry extract; 2, crushing; 3, mixing; 4, granulating; 5, tabletting; 6 packaging. The blueberry composite tablet candy capable of relieving the visual fatigue can effectively relieve the visual fatigue, can improve eyesight after long-term eating, and is convenient to take; a new using way is provided for utilizing fresh blueberry and fully playing intentional physiological and pharmacological efficacies.
Owner:HARBIN MO MEDICAL BIOLOGICAL TECH CO LTD
Calcium carbonate granulation
ActiveUS20050025811A1Increase shearHigh densityCalcium/strontium/barium carbonatesBiocideMedicineTableting
Highly compactable granulations and methods for preparing highly compactable granulations are disclosed. More particularly, highly compactable calcium carbonate granulations are disclosed. The granulations comprise powdered materials such as calcium carbonate that have small median particle sizes. The disclosed granulations are useful in pharmaceutical and nutraceutical tableting and provide smaller tablet sizes upon compression than previously available.
Owner:IVD LLC
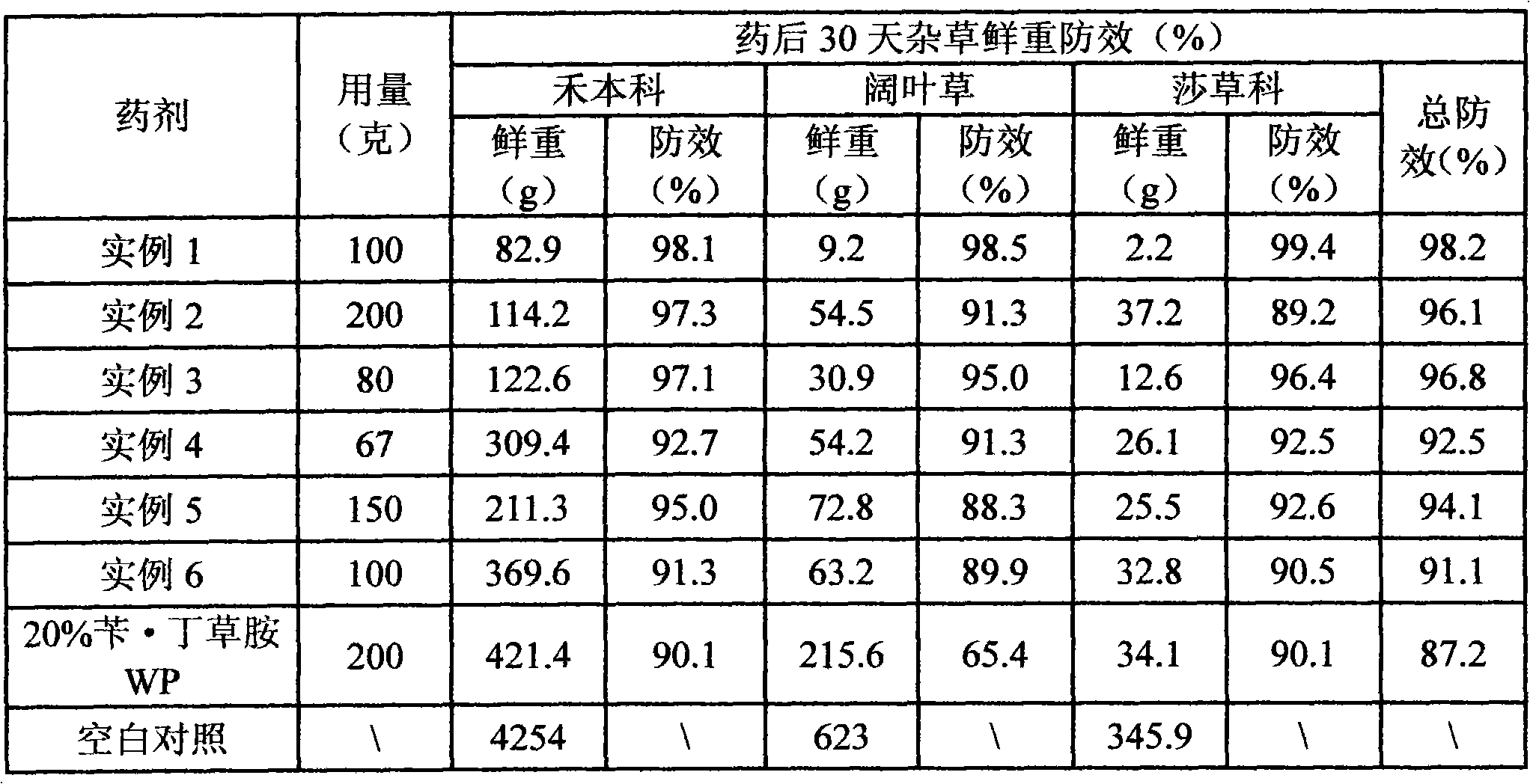
Herbicide effervescent tablets and preparation method thereof
The invention discloses herbicide effervescent tablets containing halosulfuronmethyl and pretilachlor and a preparation method thereof. The active ingredients of the herbicide effervescent tablets comprise 0.5 to 5 weight percent of halosulfuronmethyl, 10 to 25 percent of pretilachlor and the balance of an assistant. The effervescent tablets can be prepared by a direct dry-process tabletting method or a method of wet-process granulating first and then tabletting; the direct dry-process tabletting method comprises mechanically grinding the assistant, uniformly mixing with the assistant and directly tabletting the two raw material medicines; and the method of wet-process granulating first and then tabletting comprises drying the two raw material medicines and the ground assistant, mixing, adhering, granulating to prepare an acid soft material and an alkaline soft material and tabletting. The prepared effervescent tablets are new herbicide preparation products which are environment-friendly and convenient to use. The herbicide effervescent tablets can effectively prevent and control various weeds such as weed from broad leave family, grass family, cyperaceae family and the like in rice shooting fields and transplanting fields.
Owner:GUANGXI RES INST OF CHEM IND CO LTD +1
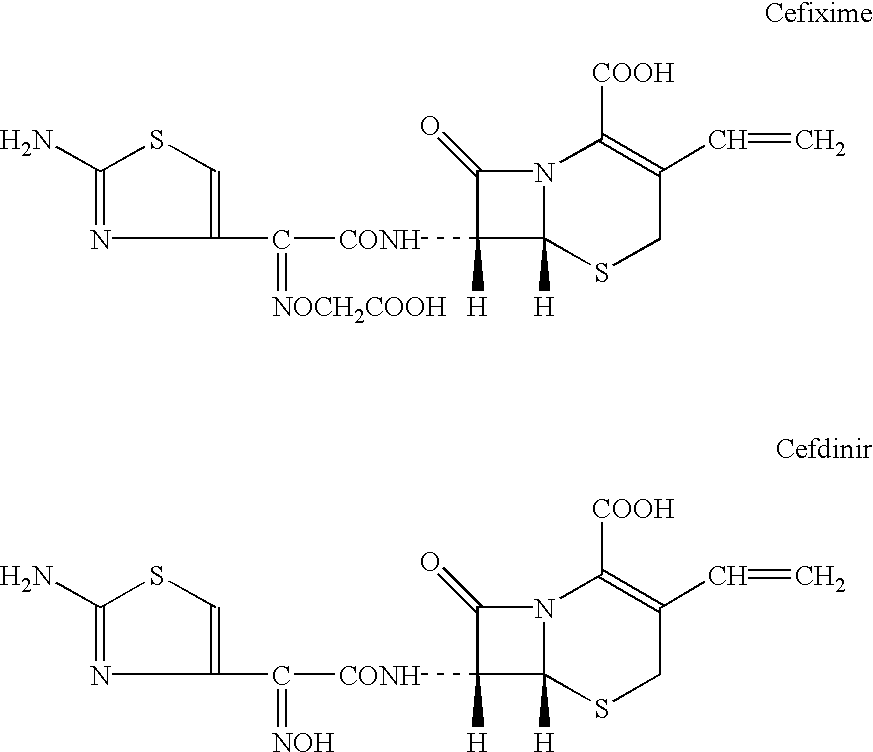
Cefixime dispersing tablet and preparation methods thereof
ActiveCN101606913AIncrease contentEasy to takeAntibacterial agentsOrganic active ingredientsMagnesium stearatePharmaceutical preservatives
The invention discloses a cefixime dispersing tablet and preparation methods thereof, belonging to the technical field of pharmaceutical preparation. The cefixime dispersing tablet comprises 40-420 mg of cefixime, 0-100 mg of starch, 0-250 mg of amylum pregelatinisatum, 10-80 mg of mannite, 0-150 mg of microcrystalline cellulose, 10-60 mg of carboxyrnethyl starch sodium, 2-20 mg of polyvidone K30, 0.4-10 mg of magnesium stearate, 0-10 mg of Steviosin and 0-10 mg of orange compound perfume. The preparation method comprises the following steps: evenly mixing basic remedies and excipients; adding adhesive to prepare granulates; drying; sorting and then adding with other excipients; and tabletting. The other preparation method is described as follows: the basic remedies are added after granulating. The invention aims to solve the technical problem that cefixime preparation has shorter disintegration time, better leachability, higher content of the basic remedies, production cost reduction, quality detection time reduction and production environment pollution reduction.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
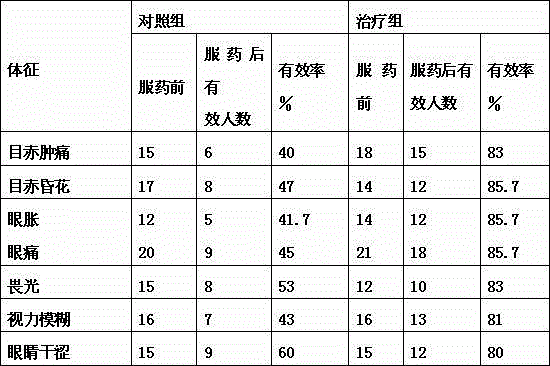
Eyesight-improving and nerves-soothing composition and preparation method thereof
An eyesight-improving and nerves-soothing composition comprises mulberries, downy rosemyrtle roots, boxthorn fruits, chrysanthemum, fleece-flower roots, cassia seeds, gorgon fruits and honey. Preparation steps for the eyesight-improving and nerves-soothing composition are as follows: the mulberries, the downy rosemyrtle roots and the chrysanthemum are respectively ground into powder and then mixed with honey to form an extractive; the boxthorn fruits, the fleece-flower roots, the cassia seeds and the gorgon fruits are ground into particles to be uniformly mixed, then water is added for decocting, the water is added in the proportion of 1 to 20 to 30, decocting is performed for 3 to 5 hours, and after decocting, a decoction solution is filtered for standby; the prepared extractive and the prepared decoction solution are mixed uniformly for standby; the prepared compound is sent to prepare compound powder through spray drying; the prepared compound powder is sent into a tablet machine for tabletting, so as to obtain solid particles with fixed shapes; the solid particles are packed into a sealed aluminium foil bag for boxing, so as to obtain the eyesight-improving and nerves-soothing composition.
Owner:陶玲云
Process for preparing brassica vegetable seedling powder chewable tablets and brassica vegetable seedling powder chewable tablet product
InactiveCN102511753ASmooth appearanceEmerald green colorFood shapingFood preparationBrassica creticaBroccoli raab
The invention relates to a process for preparing brassica vegetable seedling powder chewable tablets and a brassica vegetable seedling powder chewable tablet product, belonging to the technical field of deep processing of agricultural products. The process is characterized by comprising the following steps of: with brassica vegetable seedling powder, such as broccoli and the like as a main basic stock, compounding, preparing a soft material, granulating, drying, tabletting, sterilizing and packing to prepare the brassica vegetable seedling powder chewable tablet with the advantages of clean appearance, green color and crispness. The process for preparing the brassica vegetable seedling powder chewable tablet, disclosed by the invention, is simple, has high industrial degree and can realize complete utilization on the brassica vegetable seedling, such as broccoli and the like, thereby increasing the additional value of the brassica vegetable seedling. The brassica vegetable seedling powder chewable tablets, including the broccoli and the like, prepared by the process disclosed by the invention have functions of resisting oxidation, slowing aging and resisting inflammation and cancer and the like of human bodies, and is a kind of casual healthcare food. According to the product of the process for preparing the brassica vegetable seedling powder chewable tablets, disclosed by the invention, the content of 4-methylsulfinylglucosinolate is up to 185.0-413.0mg / 100g, and the content of sulforaphane provided by each chewable tablet is equal to that provided by 30-75g of fresh brassica vegetable, such as broccoli and the like.
Owner:NANJING AGRICULTURAL UNIVERSITY
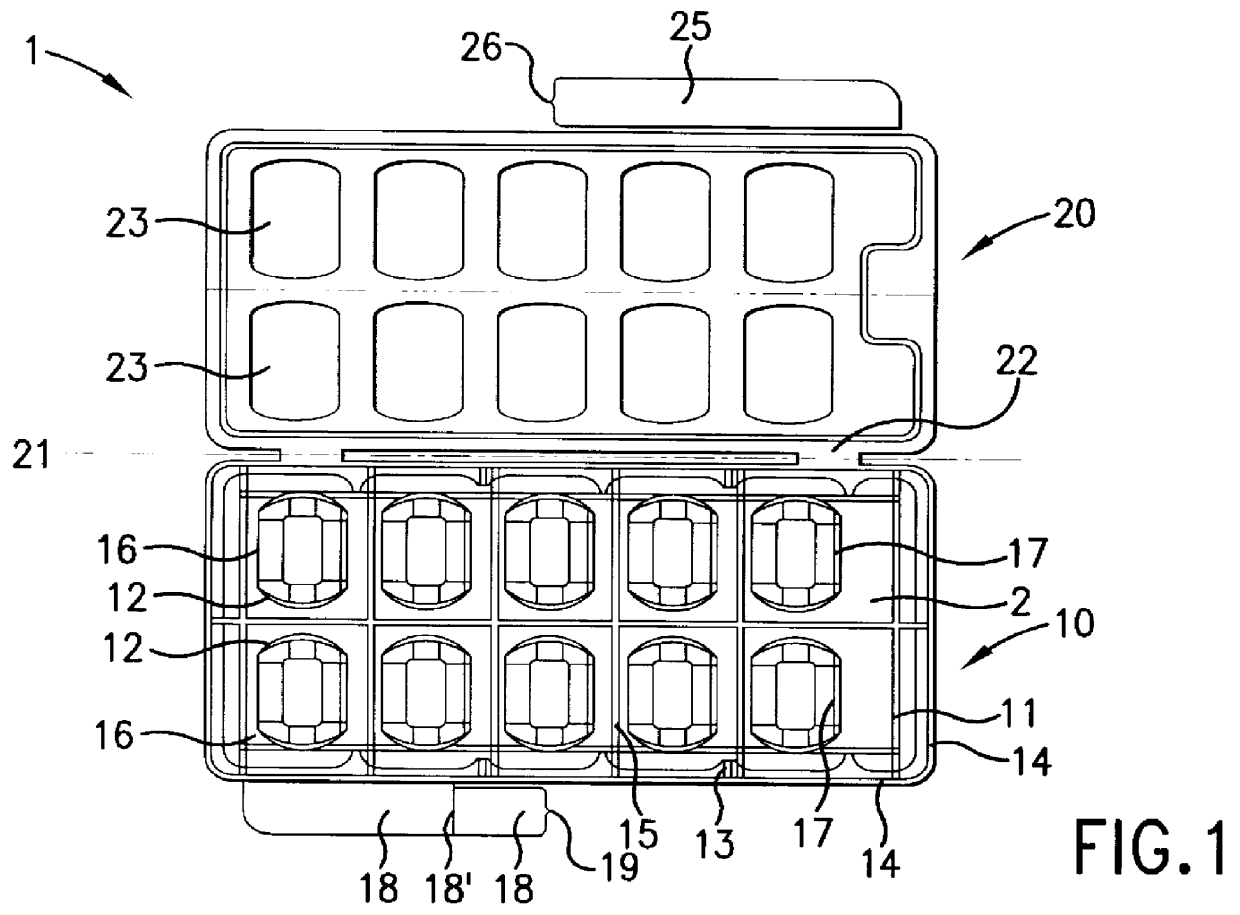
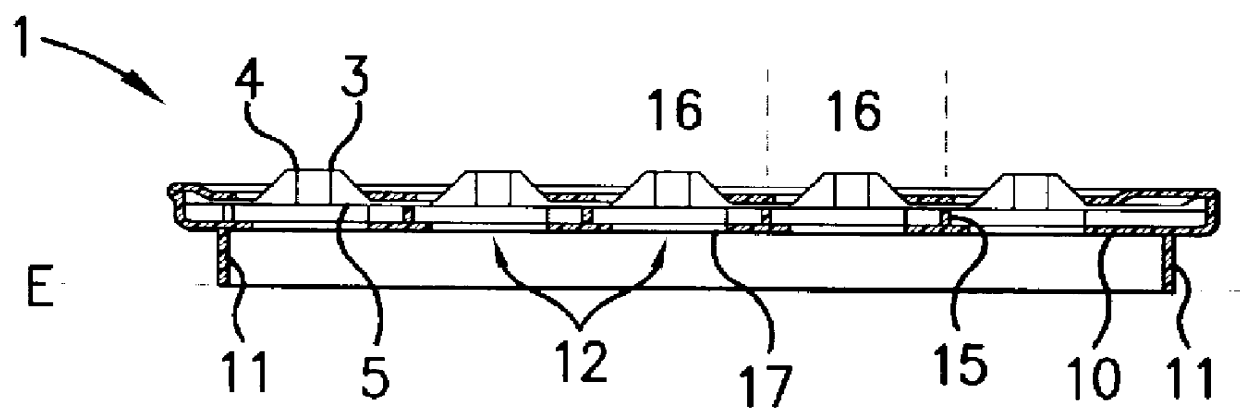
Device for pressing of tablets from a blister pack
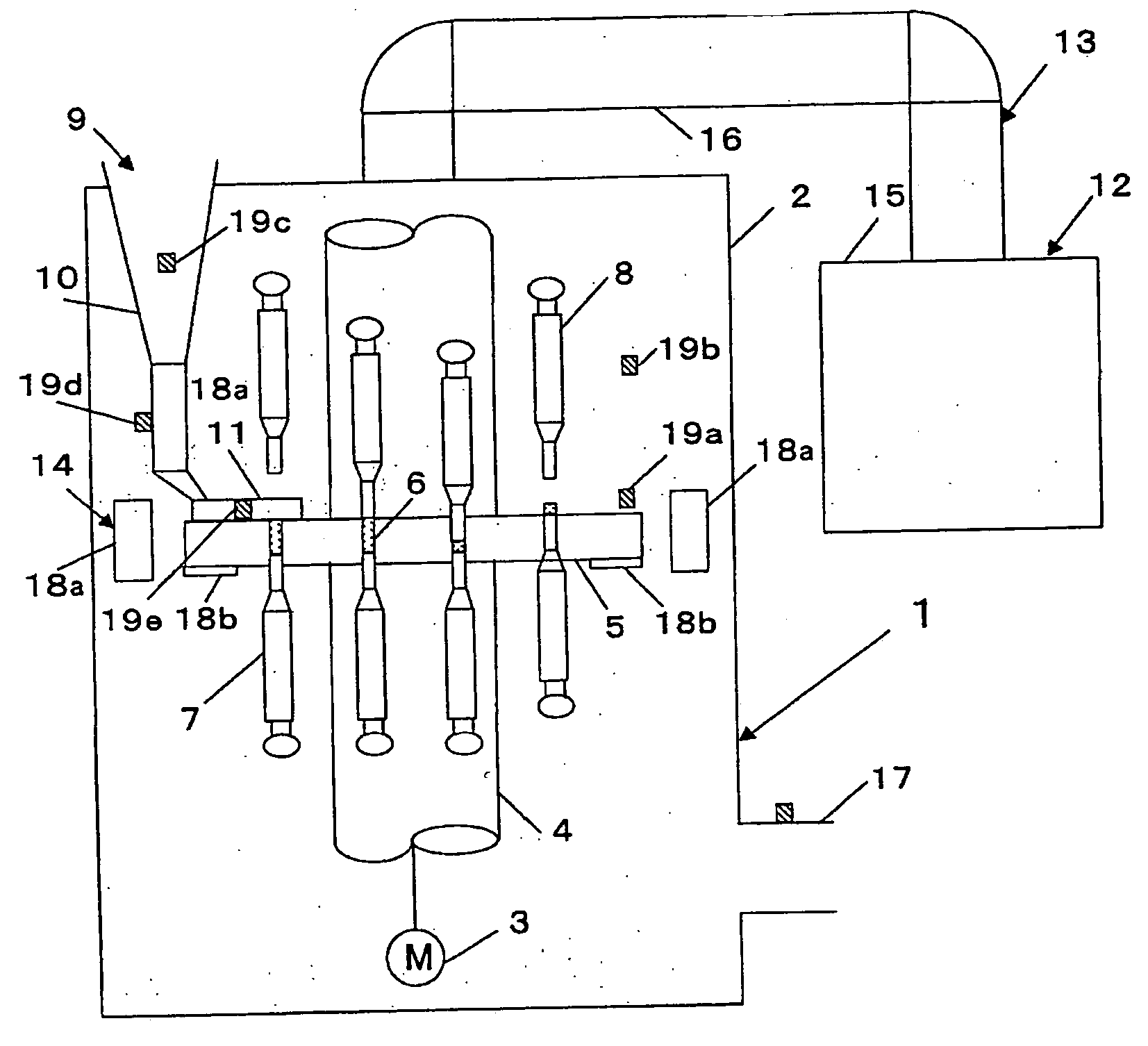
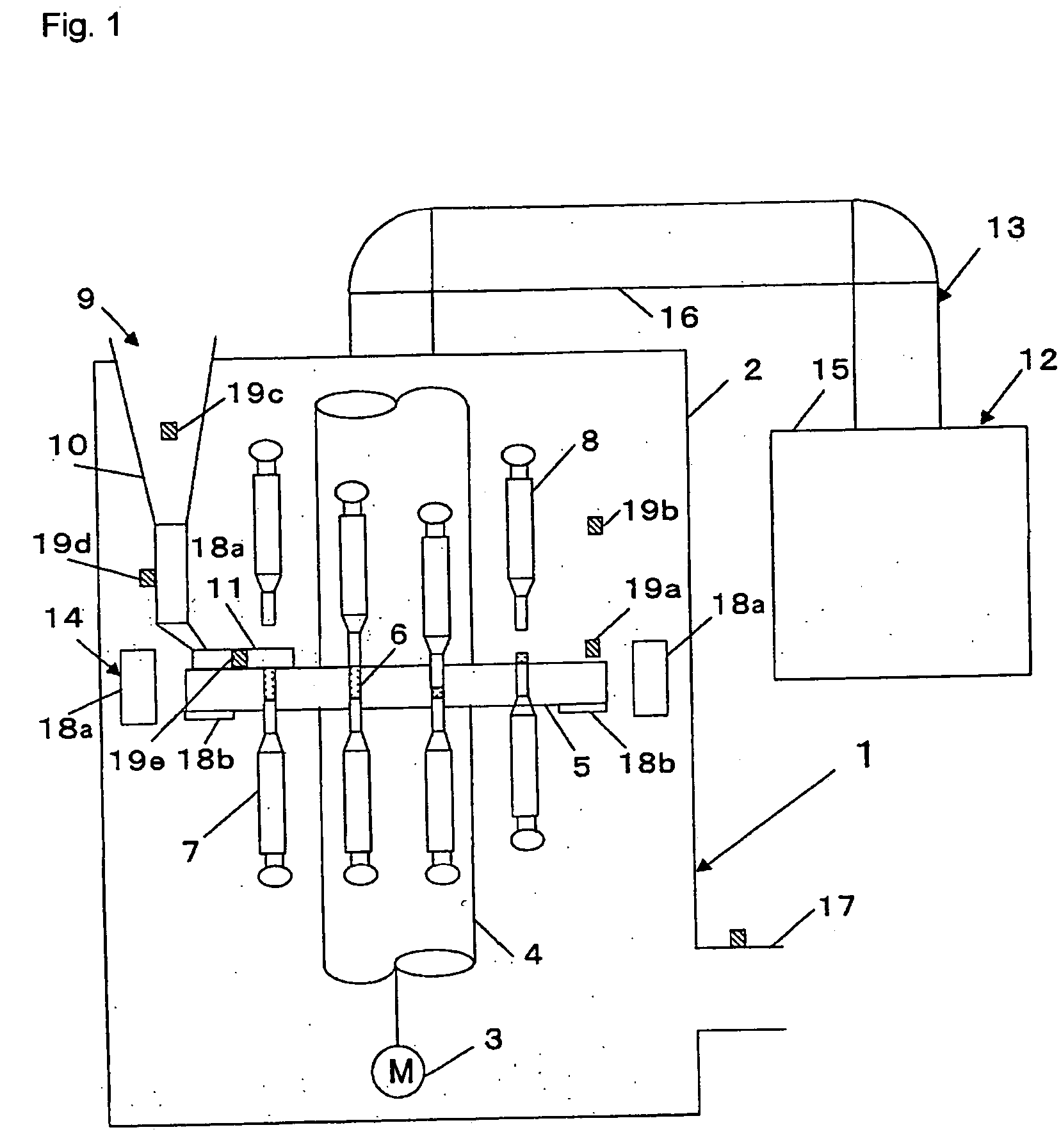
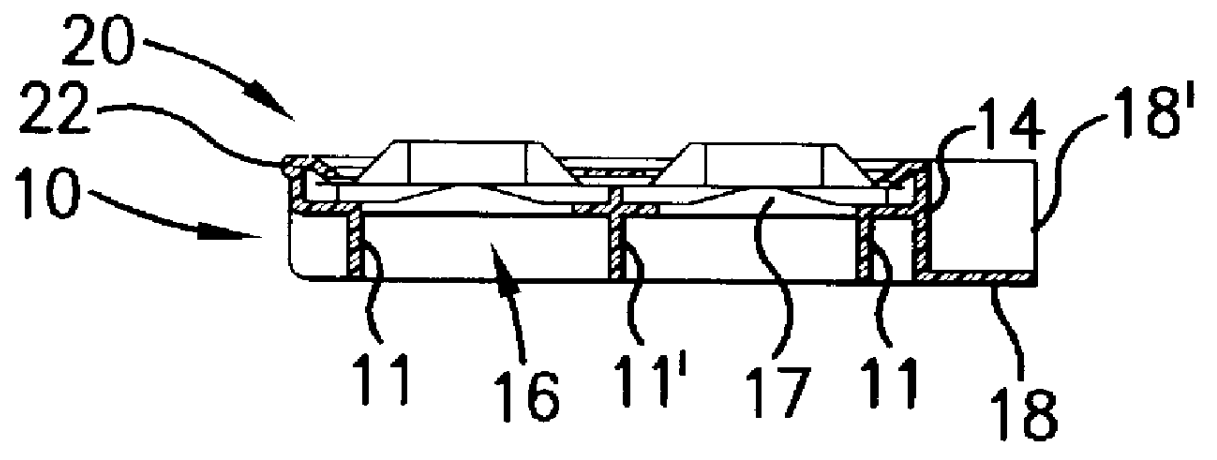
InactiveUS6155424APrecise arrangementSmall article dispensingOral administration devicePunchingEngineering
PCT No. PCT / CH97 / 00203 Sec. 371 Date Dec. 28, 1998 Sec. 102(e) Date Dec. 28, 1998 PCT Filed May 23, 1997 PCT Pub. No. WO98 / 00353 PCT Pub. Date Jan. 8, 1998A device for pressing of tablets from a blister pack. The device has a base plate and a cover plate. When closed, the two plates connected to each other by a hinge are superimposed in such a manner that openings in the cover plate are arranged substantially over openings in the base plate. The base plate is arranged using fins at a distance from a datum plane. Supporting bumps and embossed edges form a support plane on which the blister pack rests with the cover film thereof, for facilitating punching through the cover film when pressure is exerted on bumps of the deep-drawn plastic film of the blister pack. Tabs with corresponding locking noses lock the device. The device also allows disabled patients to remove the tablets required from the blister pack without further assistance.
Owner:CREATECHNIC
Tablet forming candy with refreshing, pharynx-clearing and throat-smoothing action
The invention relates to a compound extraction of refreshing and throat beneficiary effect, comprising essentially 1 to 5 portion of tea extraction, 0.5 to 5 portion of guanala extraction, 0.2 to 5 portion of balloonflower root extraction, 0.2 to 5 portion of Sterculiae Scaphigera extractuibm extraction, 10 to 60 portion of xylitol, 0.2 portion of grosvenor momordica extraction, o.1 to 1 portion of ginger oil, and 0.1 to 1 portion of menthol. The invention still relates to a sugar-free refreshing and throat beneficiary tabletting candy, comprising the compound extraction as the principal agent and medically acceptable accessories.
Owner:湖南新中意食品有限公司
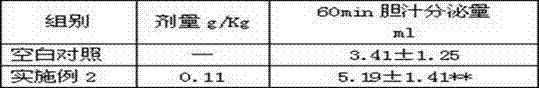
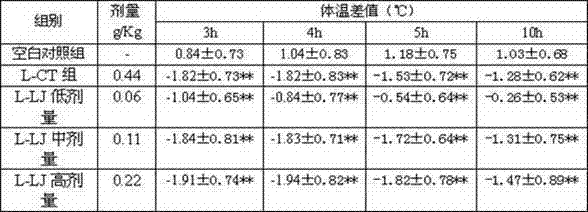
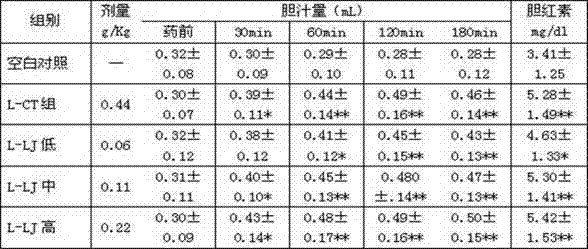
Preparation method of cholagogic tablet
InactiveCN102397451ADigestive systemSulfur/selenium/tellurium inorganic active ingredientsMedicinal herbsDesmodium
The invention relates to a preparation method of a cholagogic tablet, which comprises the following steps that: rhubarb is taken and extracted by microwave and is purified by macroporous resin columns to obtain the rhubarb extract; desmodium and honeysuckle are taken and extracted by microwave and are purified by macroporous adsorption resin and ion exchange resin to obtain desmodium-honeysuckle extract; costus root, rhizoma anemarrhenae, dyers woad leaves, radix bupleuri, root of herbaceous peony, radix scutellariae and Herba Artemisiae Capillaris are taken, pulverized and are extracted under a carbon dioxide (CO2) supercritical condition to obtain the supercritical extract; glauber salt is taken, heated and dissolved in the water to be filtered, the filtered liquid is mixed with the rhubarb extract, the desmodium-honeysuckle extract and the supercritical extract as well as dextrine and 70% ethanol to be made into granules, the granules are dried, carried out tabletting, and coated with a membrane. The cholagogic tablet which is prepared through the above method is convenient to take.
Owner:JIANGSU KANGHENG CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com