Patents
Literature
1089 results about "Orally disintegrating tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An orally disintegrating tablet or orally dissolving tablet (ODT) is a drug dosage form available for a limited range of over-the-counter (OTC) and prescription medications. ODTs differ from traditional tablets in that they are designed to be dissolved on the tongue rather than swallowed whole. The ODT serves as an alternative dosage form for patients who experience dysphagia (difficulty in swallowing) or for where compliance is a known issue and therefore an easier dosage form to take ensures that medication is taken. Common among all age groups, dysphagia is observed in about 35% of the general population, as well as up to 60% of the elderly institutionalized population and 18-22% of all patients in long-term care facilities ODTs may have a faster onset of effect than tablets or capsules, and have the convenience of a tablet that can be taken without water. During the last decade, ODTs have become available in a variety of therapeutic markets, both OTC and by prescription.
Orally disintegrable tablets
InactiveUS6328994B1Sufficient oral disintegrabilityHigh strengthOrganic active ingredientsPowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Orally disintegrating tablets and methods of manufacture
ActiveUS20050232988A1Effective taste-maskingOptimal size distributionPowder deliveryGranular deliveryOrally disintegrating tabletAlcohol sugars
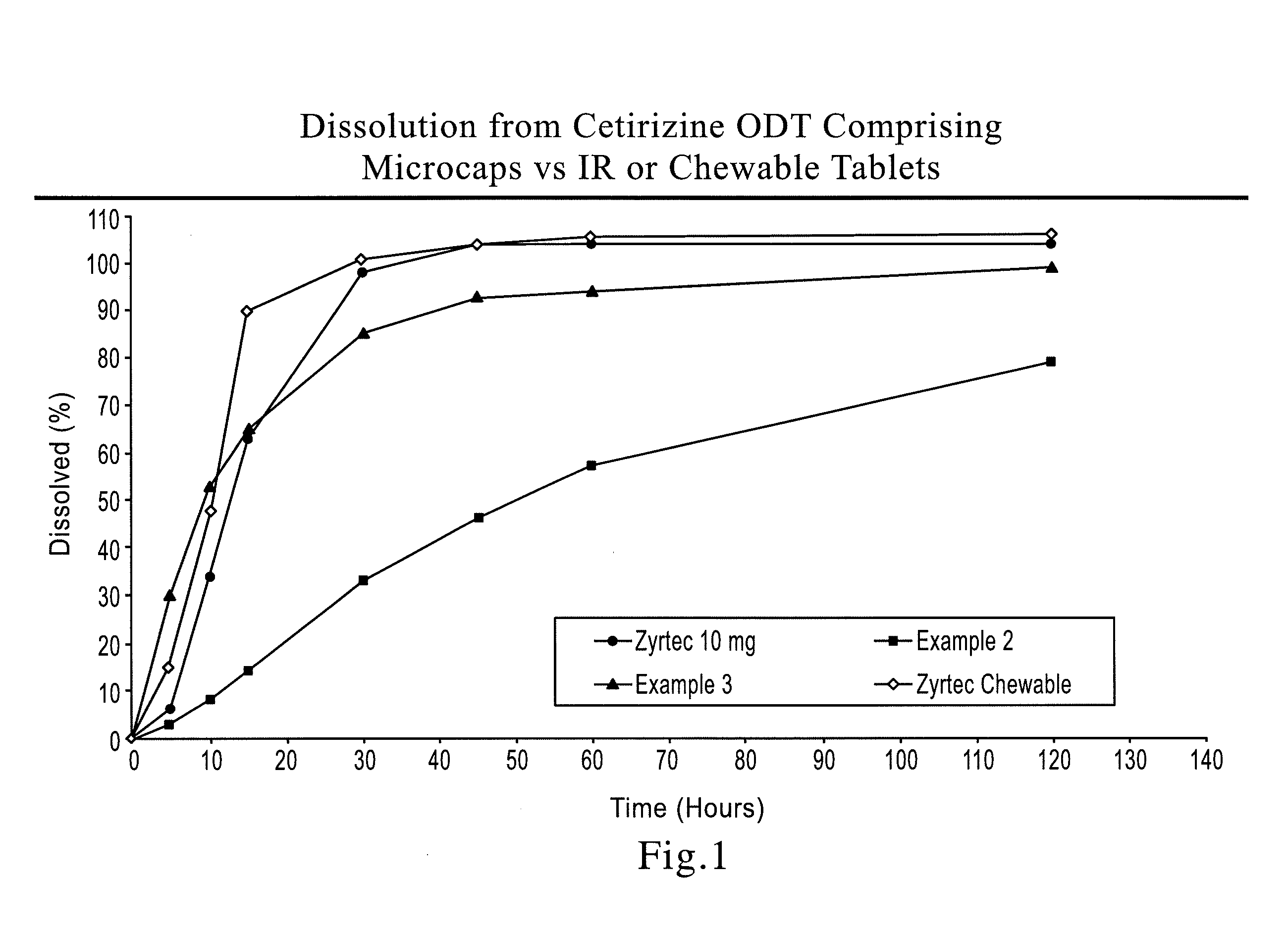
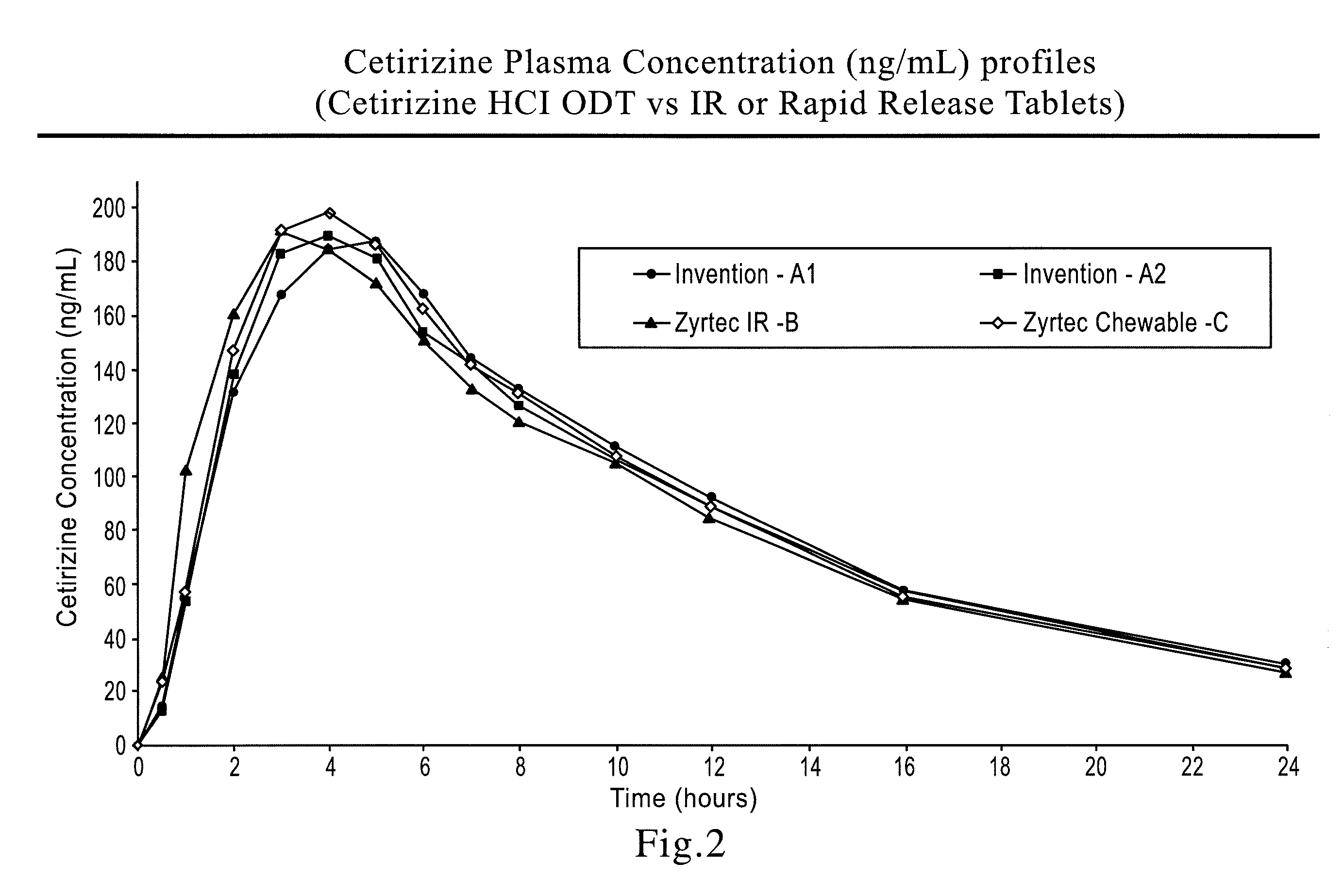
A tablet that rapidly disintegrates in the oral cavity comprising a compressed blend of rapidly dispersing microgranules prepared by granulating a sugar alcohol or a saccharide or a mixture thereof having an average particle size less than about 30 microns and a disintegrant, and a taste-masked microcapsule containing at least one drug, the microcapsule being prepared by granulating a pharmaceutically acceptable formulation comprising at least one drug in a therapeutically effective amount and at least one polymeric binder that improves resilience of the microgranules, wet milling the granulated mass, and microencapsulating the milled granules to provide microcapsules.
Owner:ADARE PHARM INC
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20060105038A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Orodispersible tablets
ActiveUS20100297031A1Short disintegration timeGood mechanical resistanceBiocideNervous disorderCalcium silicateOrally disintegrating tablet
This invention relates to a an orally disintegrating tablet obtainable by direct compression of a dry powdered mixture, said mixture comprising up to 15% by weight of calcium silicate, at least 50% of a diluent, a disintegrant agent and an active ingredient. It also relates to a process for preparing the tablets by homogeneous blending the specific excipients in powder form and subsequent direct compression of the mixture. Said tablets disintegrate quickly in the cavity of the mouth, in particular in less than 15 seconds.
Owner:LAB LESVI SL
Taste-masked pharmaceutical compositions with gastrosoluble pore-formers
InactiveUS20060105039A1Effective taste-maskingRapid/complete releaseOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active), coated with a taste-masking membrane comprising a water-insoluble polymer and one or more gastrosoluble inorganic or organic pore-formers (practically insoluble in water and saliva, but soluble in an acidic buffer), exhibit acceptable taste-masking when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:ADARE PHARM INC
Oral cavity disintegrating tablet and method of producing the same
ActiveUS20100278930A1Easy to produceDisintegrates quicklyBiocideOrganic active ingredientsSucroseOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing (a) one or more saccharides or sugar alcohols selected from the group consisting of mannitol, lactose, xylitol, sucrose, erythritol and glucose and (b) low substituted hydroxypropylcellulose and substantially free of a starch disintegrant, which tablet is produced by steps of granulating a composition containing the above-mentioned components (a) and (b) by an agitation granulation method, and compression-molding the obtained granulation product. The invention also provides a method of producing an orally disintegrating tablet substantially free of a starch disintegrant, including steps of granulating a composition containing the above-mentioned components by an agitation granulation method, and compression-molding the obtained granulation product.
Owner:SAWAI PHARMA
Drug delivery systems comprising weakly basic drugs and organic acids
InactiveUS20070196491A1Increase probabilityAvoid eliminationPowder deliveryNervous disorderParticulatesRegimen
A pharmaceutical dosage form such as a capsule, a conventional or orally disintegrating tablet capable of delivering a nitrogen (N)-containing therapeutic agent having a pKa in the range of from about 5 to 14 into the body in a sustained-released fashion, in order to be suitable for a twice- or once-daily dosing regimen, comprises at least one organic acid, which solubilizes the therapeutic agent the drug prior to releasing it into the hostile intestinal environment wherein said weakly basic drug is practically insoluble. The unit dosage form is composed of a multitude of multicoated particulates (i.e., immediate-release beads, sustained-release beads and / or one or more timed, pulsatile-release bead populations) is designed in such a way that said weakly basic drug and said organic acid do not come into close contact during processing and / or storage for in-situ formation of acid addition compounds while ensuring that the acid is not depleted prior to completion of the drug release.
Owner:ADARE PHARM INC
Controlled-release compositions comprising a proton pump inhibitor
The present invention relates to pharmaceutical compositions, and methods of preparing such compositions, comprising one or more populations of controlled-release particles comprising one or more proton pump inhibitors. The present invention also relates to pharmaceutical dosage forms, including orally disintegrating tablets, tablets, capsules, and methods for their preparation.
Owner:APTALIS PHARMA +1
Orally disintegrable tablets
InactiveUS20020142034A1High strengthGood disintegrationBiocidePowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Taste masked topiramate composition and an orally disintegrating tablet comprising the same
In various embodiments, the present invention is directed to a taste masked pharmaceutical composition comprising a therapeutically effective amount of taste masked sulfamate-substituted monosaccharide particles comprising a sulfamate-substituted monosaccharide or a pharmaceutically acceptable salt or derivative thereof that are coated with one or more taste-masking layers, and optionally one or more of taste-masked neltrexone, 5-HT3 receptor antagonist, phentermine, and vitamin B-12. The present invention relates to methods of making the taste masked and ODT compositions, and methods of using the compositions for treating a patient subject to an epileptic condition, migraines, dysphagia, achieving / maintaining weight loss, or alcoholism or drug addiction.
Owner:APTALIS PHARMATECH
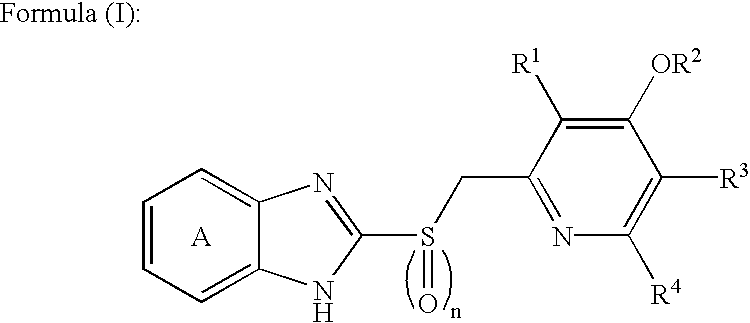
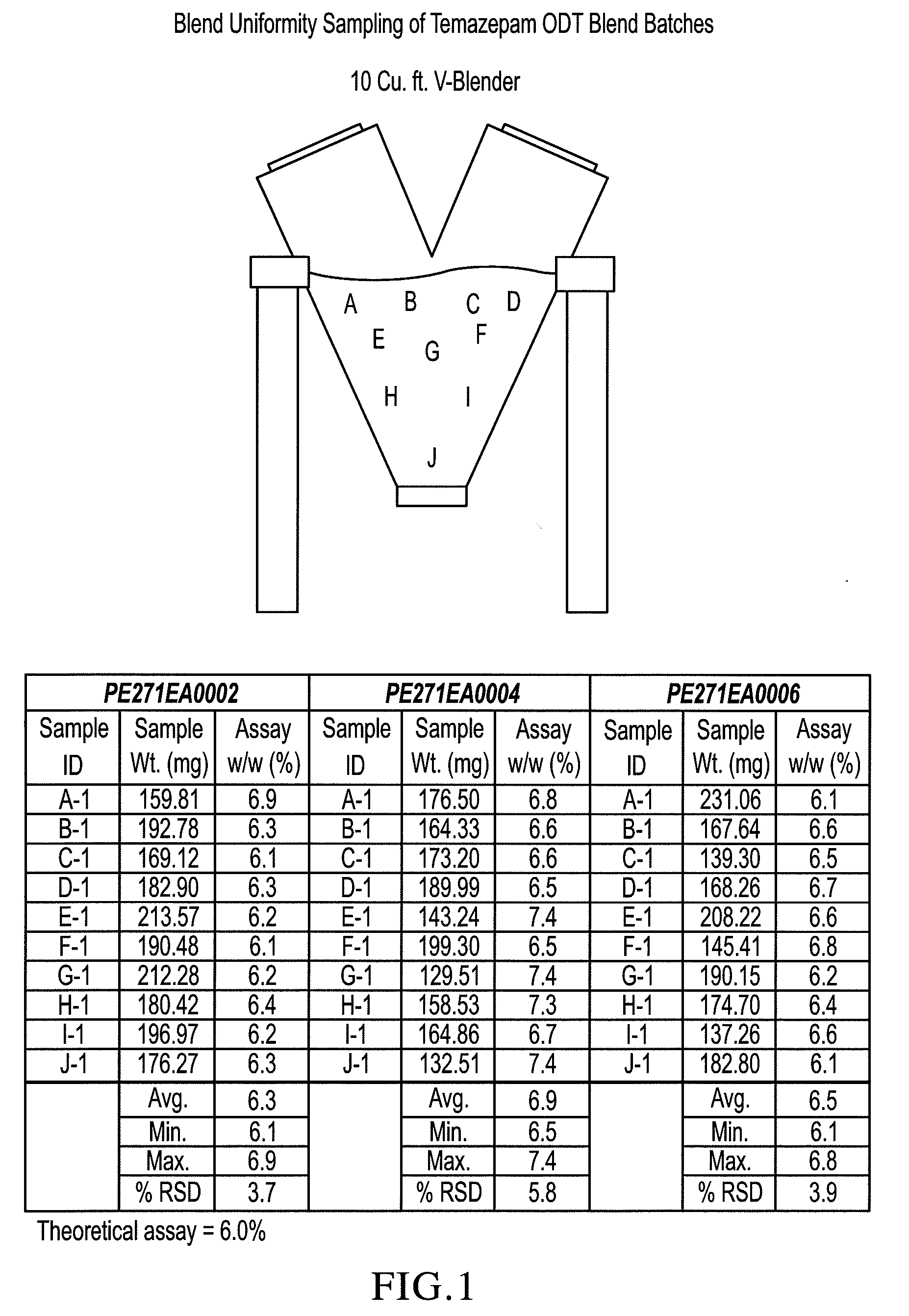
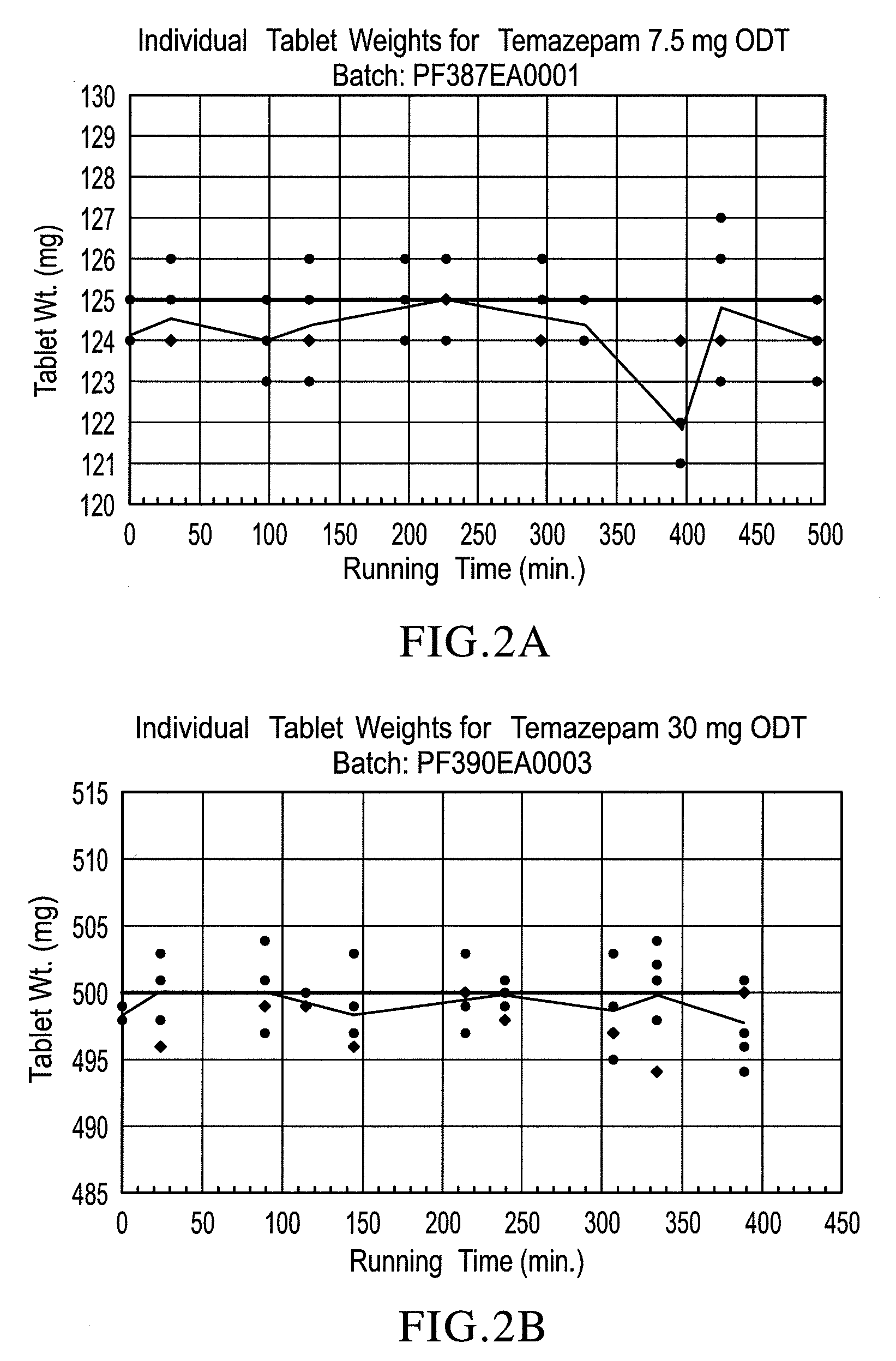
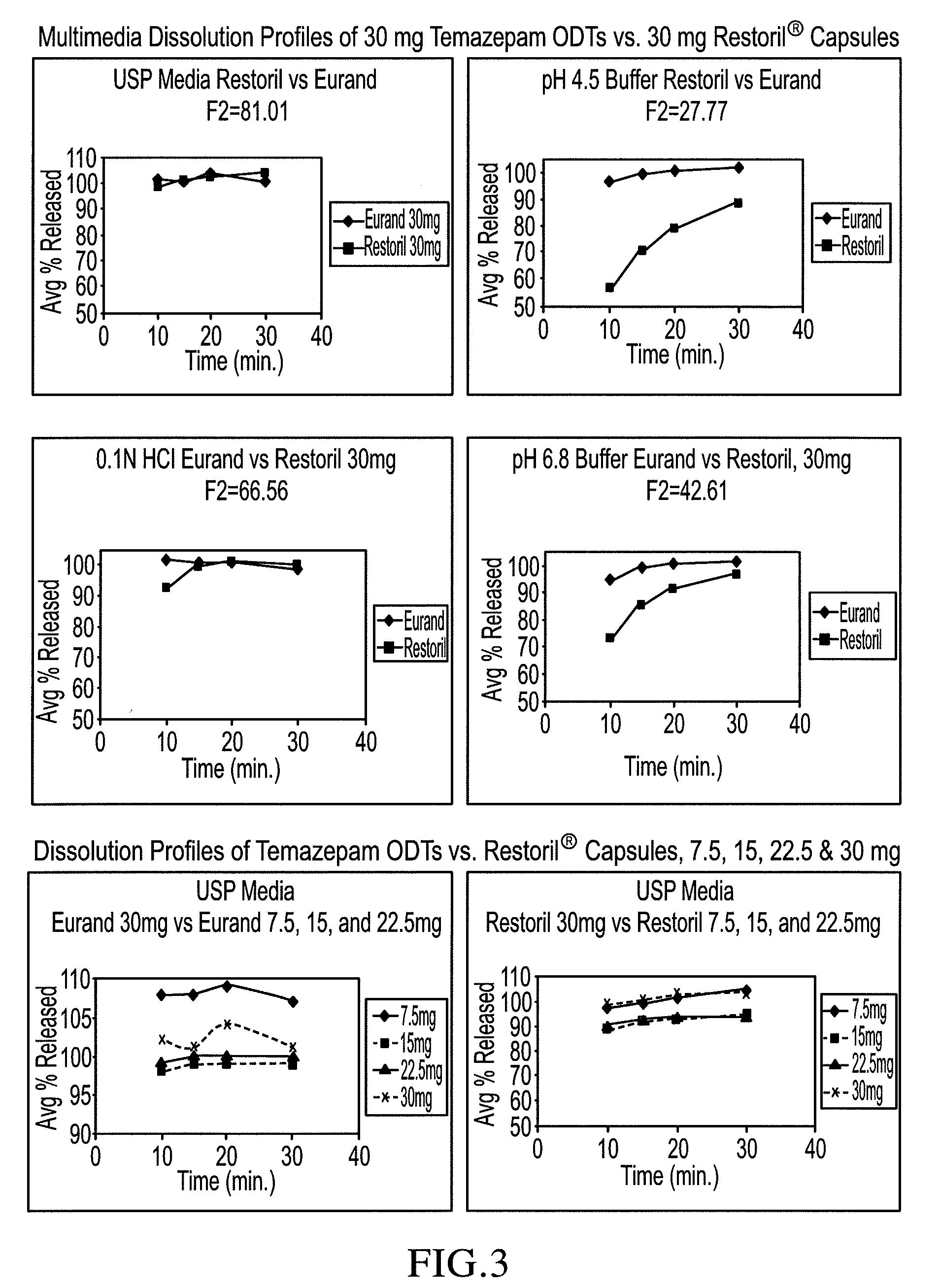
Orally disintegrating tablet compositions of temazepam
InactiveUS20090169620A1Patient compliance is goodBiocideNervous disorderTemazepamOrally disintegrating tablet
The compositions of the present invention are orally disintegrating tablet compositions comprising a therapeutically effective amount of at least one drug such as temazepam, 0.5-3% of an ODT binder polymer, a sugar alcohol and / or saccharide, and a disintegrant.
Owner:ADARE PHARM INC
Orally disintegrable tablets
InactiveUS7431942B2High strengthGood disintegrationPowder deliveryOrganic active ingredientsOrally disintegrating tabletEngineering
An orally disintegrable tablet of the present invention, which comprises (i) fine granules having an average particle diameter of 400 μm or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
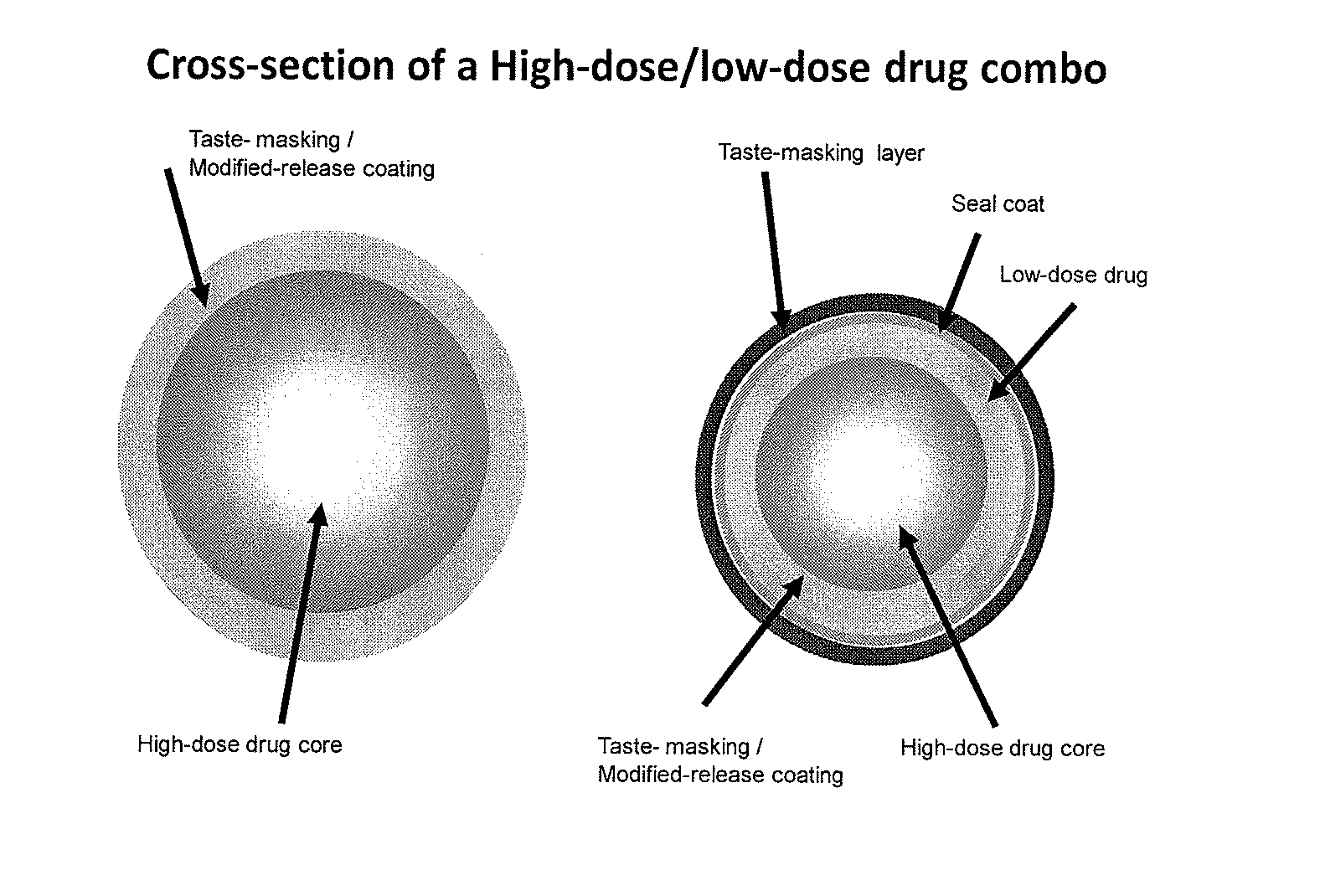
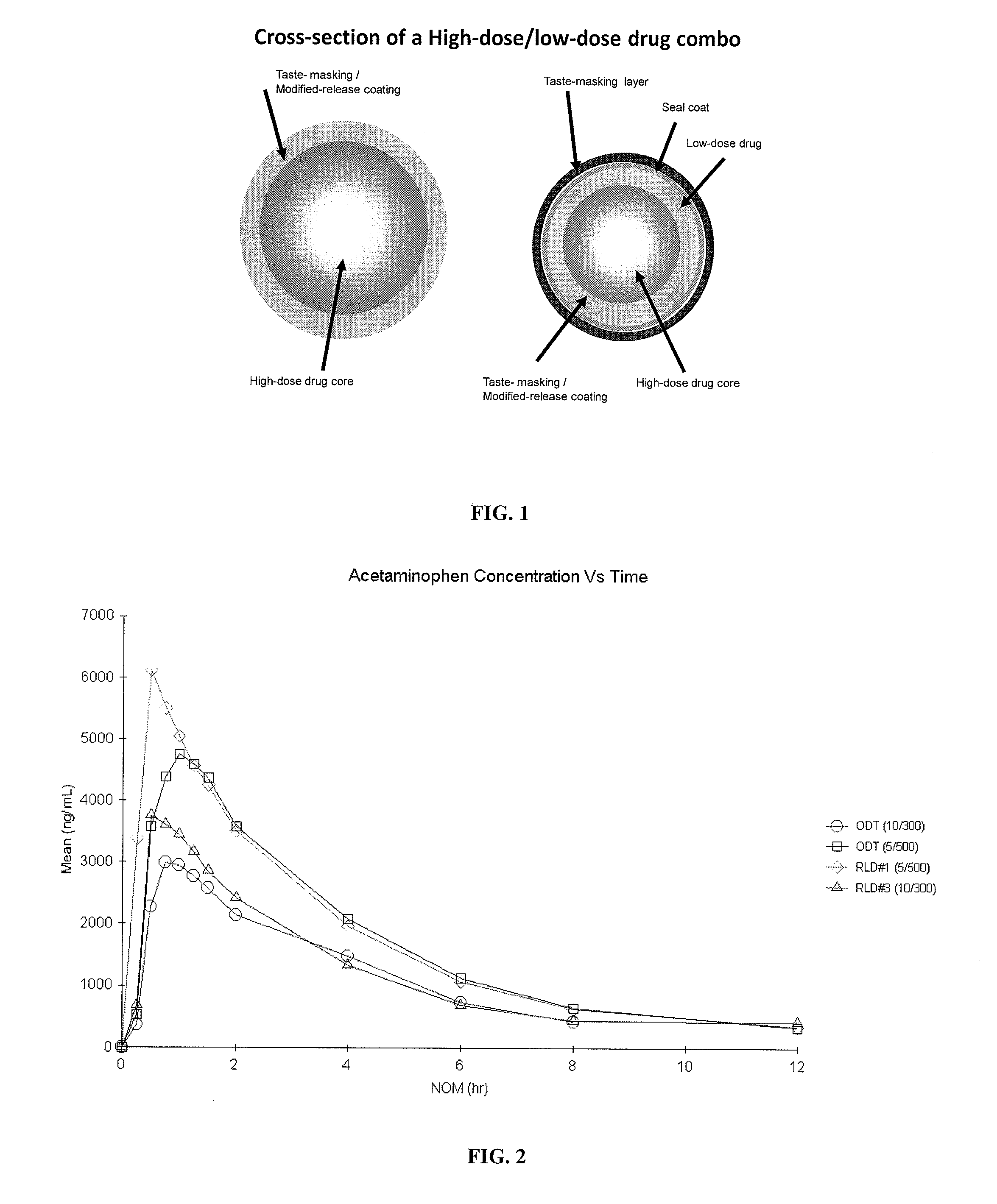
Orally Disintegrating Tablet Compositions Comprising Combinations of High and Low-Dose Drugs
The present invention is directed to pharmaceutical compositions comprising a plurality of taste-masked high-dose / low-dose drug-containing microparticles, dosage forms comprising such pharmaceutical compositions (such as an orally disintegrating tablet), and methods of making the pharmaceutical compositions and dosage forms of the present invention. Dosage forms comprising the pharmaceutical compositions of the present invention are improved homogeneous blends of high-dose and low-dose drugs which provide for more convenient and palatable administration of drug combinations, for example for treating pain, hyperglycemia, cardiovascular disease, and allergies.
Owner:ADARE PHARM INC
Oral disintegrating dosage forms
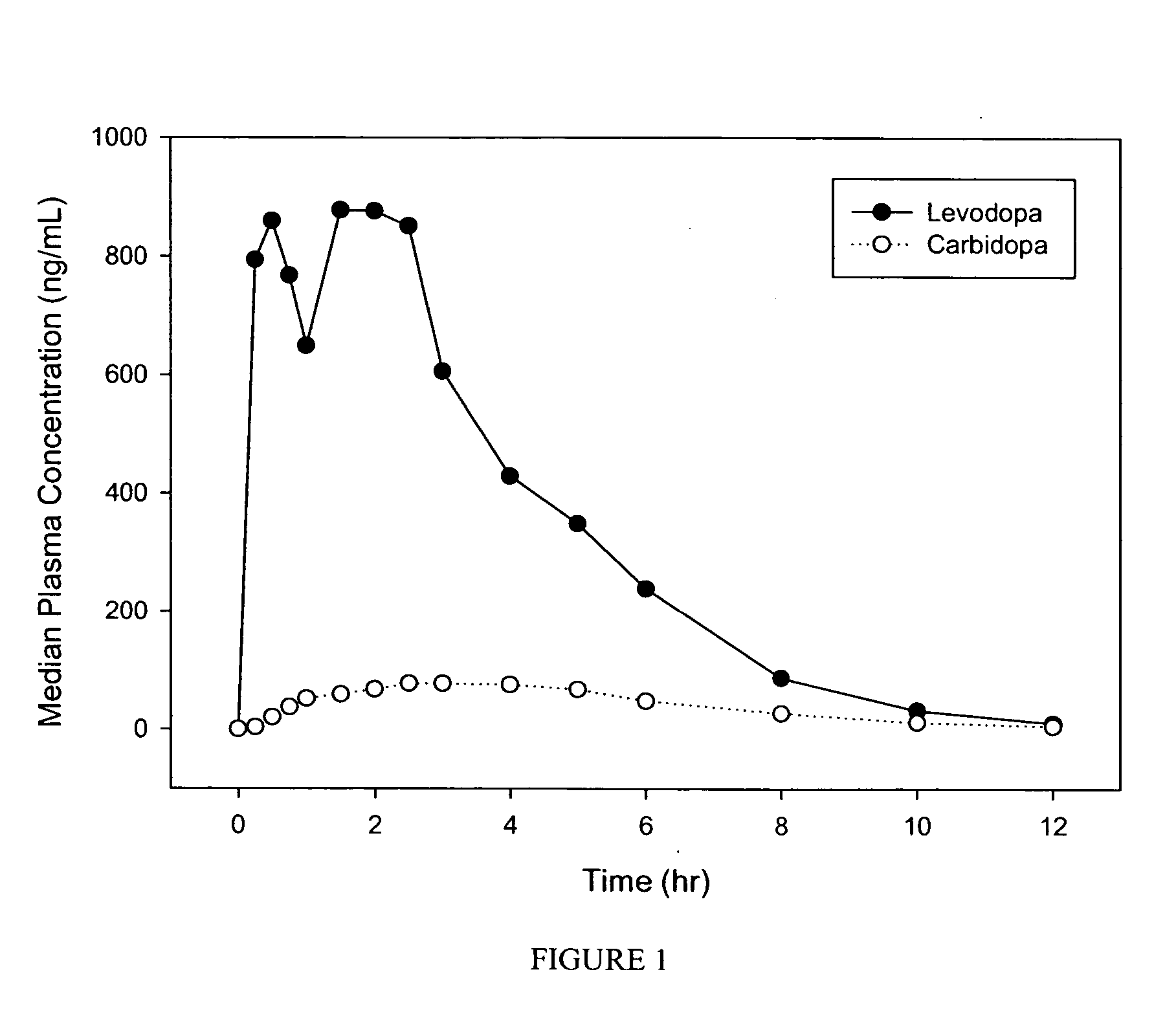
InactiveUS20050147670A1Good compressibilityImprove rapid dispersion characteristicOrganic active ingredientsBiocideImmediate releaseOrally disintegrating tablet
The invention is directed to pharmaceutical dosage forms having immediate release via rapid oral disintegration, specifically, orally disintegrating tablets containing levodopa and carbidopa. The invention further provides formulations containing relatively increased amounts of carbidopa than previously available, including, for example, formulations containing carbidopa-levodopa ratios of about 1:1 to about 1:3.
Owner:IMPAX LAB INC
Orally disintegrating tablet compositions of lamotrigine
The compositions of the present invention composition comprise a therapeutically effective amount of particles comprising lamotrigine, in combination with granules comprising a disintegrant, and a sugar alcohol and / or a saccharide. These compositions are useful in treating epilepsy and bipolar disorder, particularly for patients with dysphagia, and to improve compliance with bipolar patients.
Owner:ADARE PHARM INC
Memantine hydrochloride orally disintegrating tablet and its preparing method
InactiveCN1709229AExpand the application range of dosage formsImprove bioavailabilityNervous disorderPill deliveryMemantine HydrochlorideOrally disintegrating tablet
The present invention relates to a memantine hydrochloride oral disintegrant tablet and its preparation method. Its composition includes (by wt%) 2-20% of memantine hydrochloride as active component, 20-92% of filling agent, 5-30% of disintegrant agent and 1-30% of corrective agent.
Owner:FUKANGREN BIO PHARMA
Rapidly dispersing granules, orally disintegrating tablets and methods
InactiveUS20120282335A1High mechanical strengthDisintegrates quicklyAntibacterial agentsBiocideControlled releaseOrally disintegrating tablet
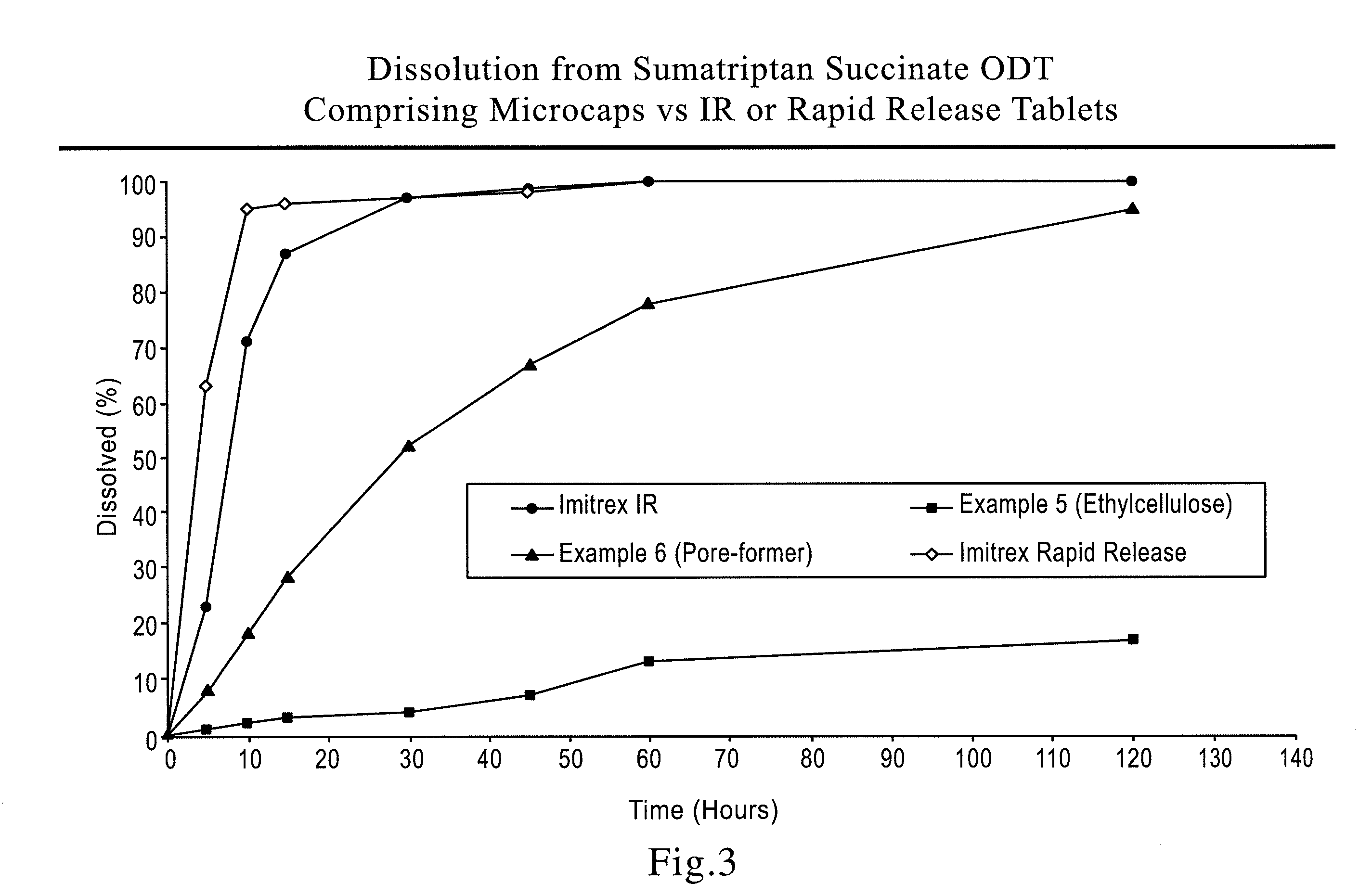
This invention relates to rapidly dispersing microgranules comprising at least one sugar alcohol or saccharide, at least one super disintegrant, and a pharmaceutically acceptable additive with multi-functionality (e.g., starch acting as a binder, disintegrant, diluent / filler, glidant, etc) at a low level, which can be formed by not only eliminating a wet milling step but also avoiding an extensive dry milling step. Furthermore, such rapidly dispersing microgranules could also comprise a pharmaceutically active agent thereby providing for a pharmaceutical composition, or the rapidly dispersing microgranules thus produced are suitable for blending with a pharmaceutically active agent that is optionally taste-masked and / or controlled release coated microparticles to also provide for a pharmaceutical composition and the invention is also directed to a method for manufacturing such rapidly dispersing microgranules in a high useable yield, as well as orally disintegrating tablets comprising such rapidly dispersing microgranules. The rapidly dispersing microgranules are also free flowing.
Owner:ADARE PHARM INC
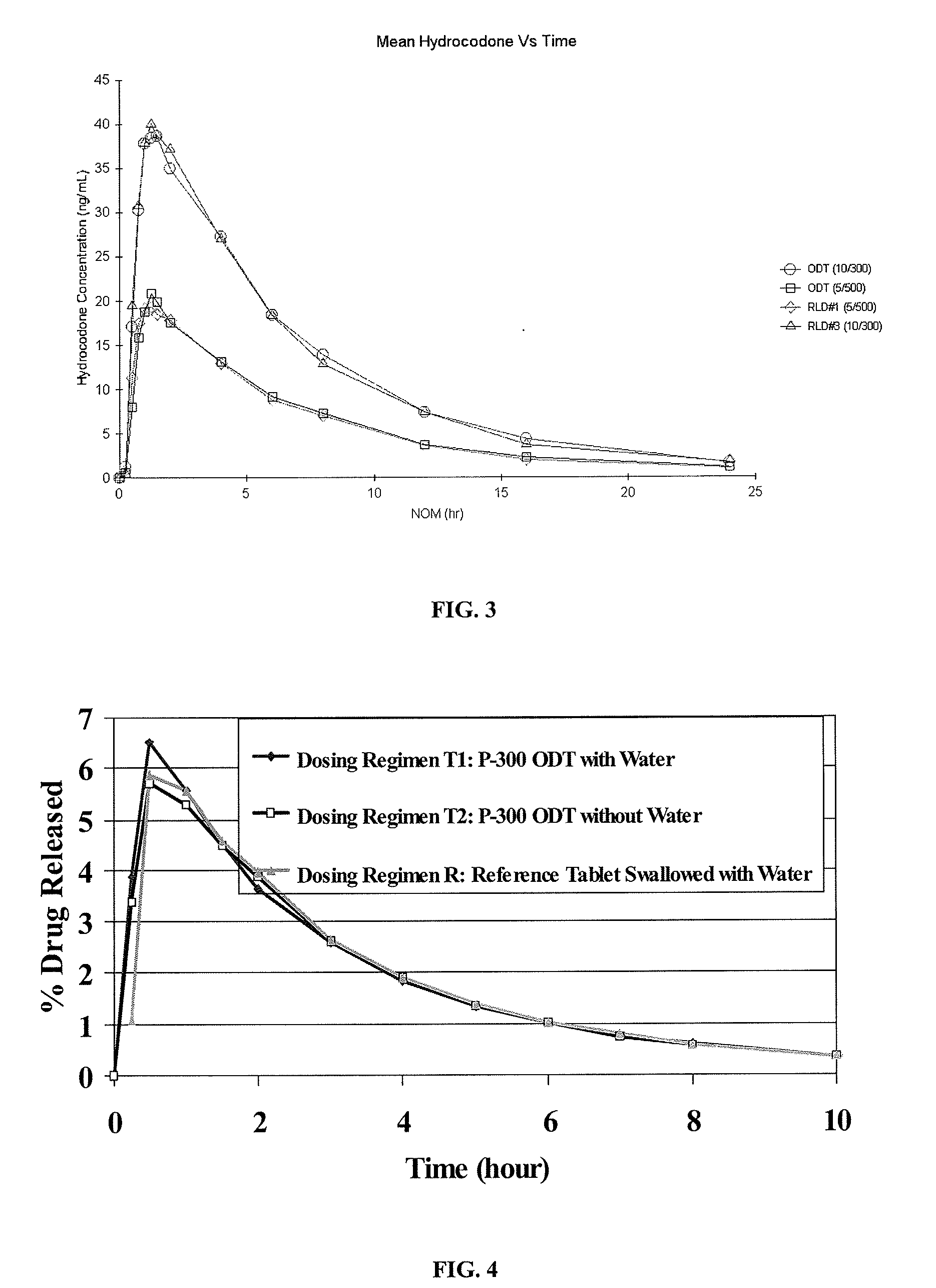
Granule and orally disintegrating tablet comprising oxycodone
The present invention relates to granules comprising oxycodone, as well as to orally disintegrating tablets including same and optionally acetaminophen.
Owner:ETHYPHARM SA
Taste-masked oral formulations of influenza antivirals
The present invention relates to taste-masked oral formulations of influenza antivirals. The taste-masked pharmaceutical formulations for oral administration comprise one or more influenza antivirals, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. Further, the taste-masked influenza antiviral formulations of the present invention are provided in the form of dispersible tablets, effervescent tablets, orally disintegrating tablets, chewable tablets, bite-dispersion tablets or the like, wherein the bitter taste of influenza antivirals is masked thereby providing palatable formulations.
Owner:RUBICON RES PTY LTD
Compressible-Coated Pharmaceutical Compositions and Tablets and Methods of Manufacture
InactiveUS20110129530A1Improving tableting propertyImprove propertiesBiocideNervous disorderControlled releaseOrally disintegrating tablet
There is provided a method for preparing a pharmaceutical composition comprising compressible coated, taste-masked and / or controlled-release coated drug-containing particles, rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol, a saccharide, or a mixture thereof, and other optional, pharmaceutically acceptable excipients wherein the orally disintegrating tablet (ODT) or rapidly dispersing tablet (RDT) composition having acceptable tableting, organoleptic, and pharmacokinetic properties.
Owner:ADARE PHARM INC
Disintegration piece taken through oral cavity containing Gimeracil and Oteracil Potassium with fluorine being added
InactiveCN1660105AOvercome the disadvantage of strong bitter tasteImprove complianceOrganic active ingredientsPill deliveryOTERACIL POTASSIUMOrally disintegrating tablet
Owner:LUNAN PHARMA GROUP CORPORATION
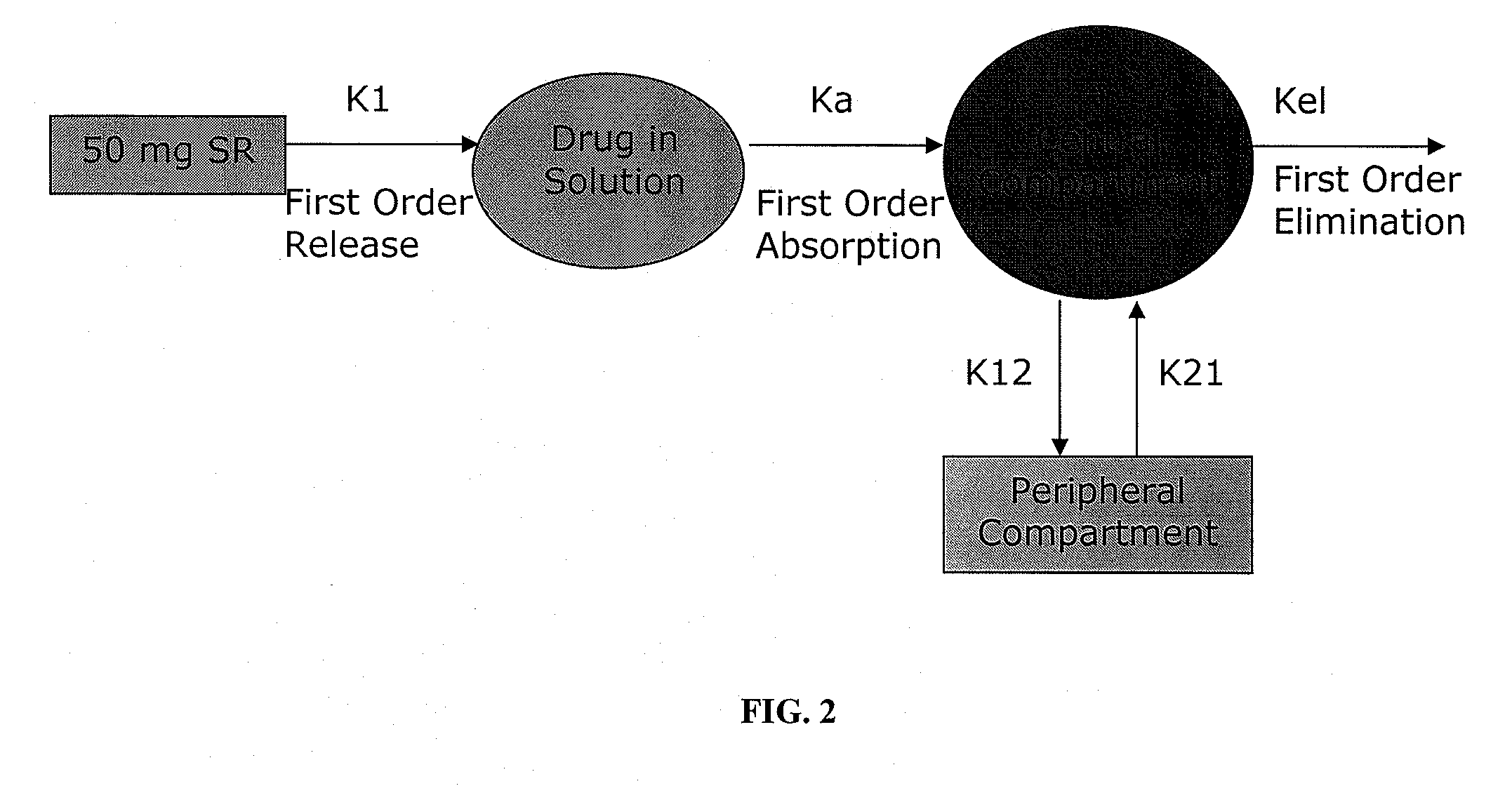
Compositions comprising weakly basic drugs and controlled-release dosage forms
InactiveUS20090258066A1Antibacterial agentsNervous disorderOrally disintegrating tabletMicroparticle
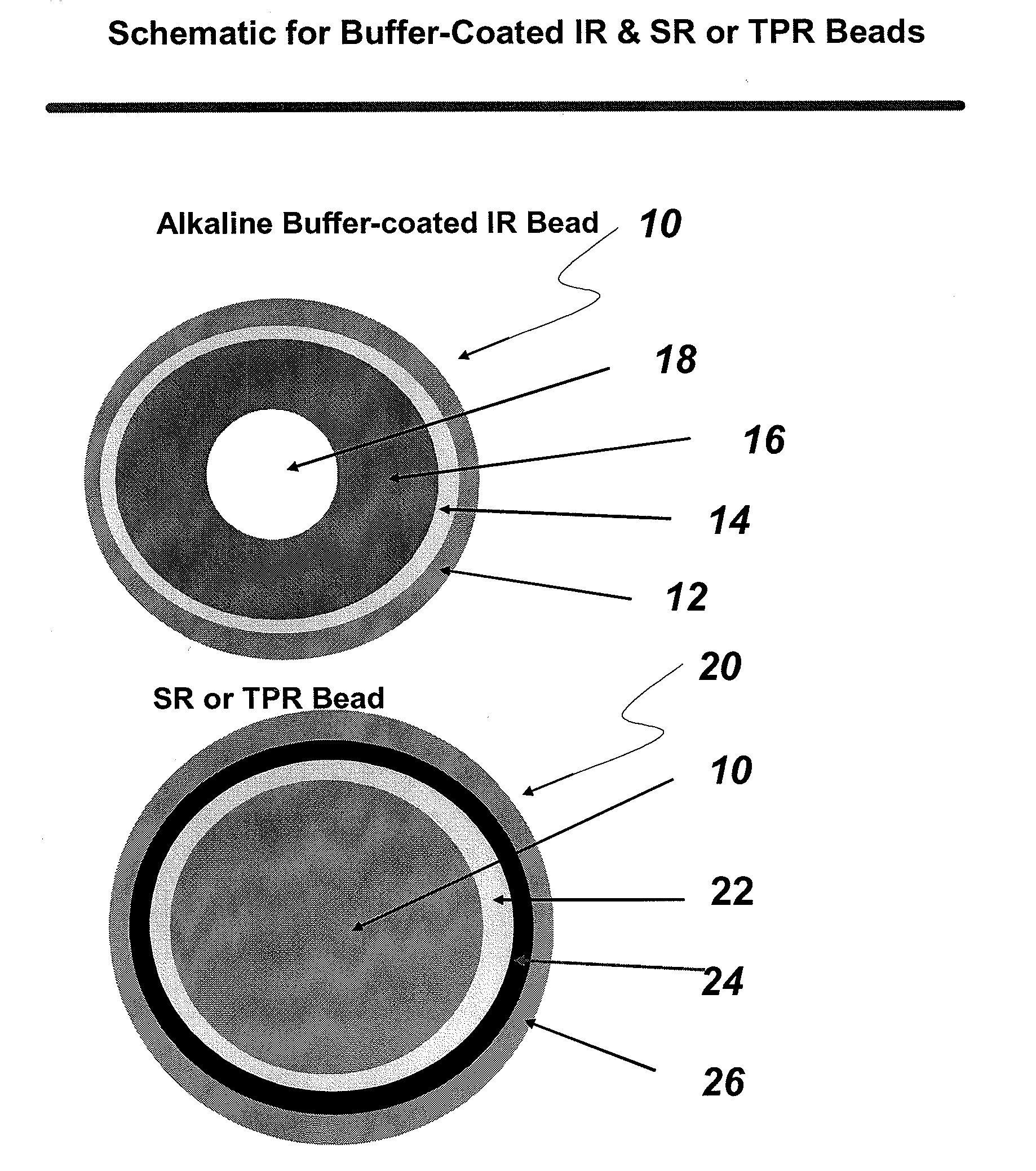
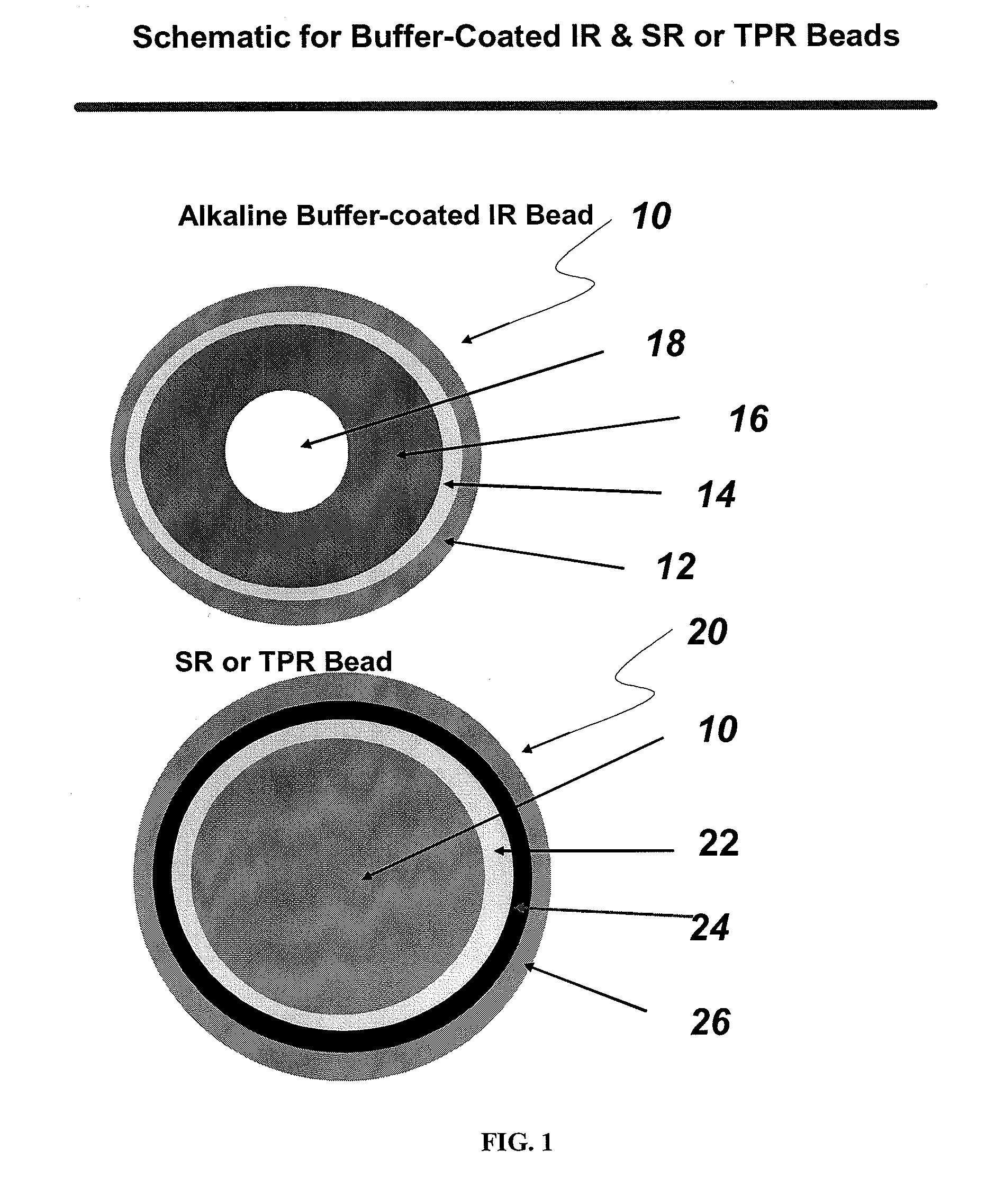
The present invention is directed to pharmaceutical compositions, and methods of making such compositions, comprising microparticles containing a weakly basic drug core, a layer of alkaline buffer, and a controlled-release coating. The present invention is also directed to pharmaceutical dosage forms, including orally disintegrating tablets, conventional tablets, and capsules, and methods for their preparation.
Owner:ADARE PHARM INC
Water-insoluble medicine sustained-release pellet, sustained-release orally disintegrating tablet thereof and preparation method thereof
InactiveCN101862297ASmall particle sizeHigh strengthOrganic active ingredientsAntipyreticSustained release pelletsSide effect
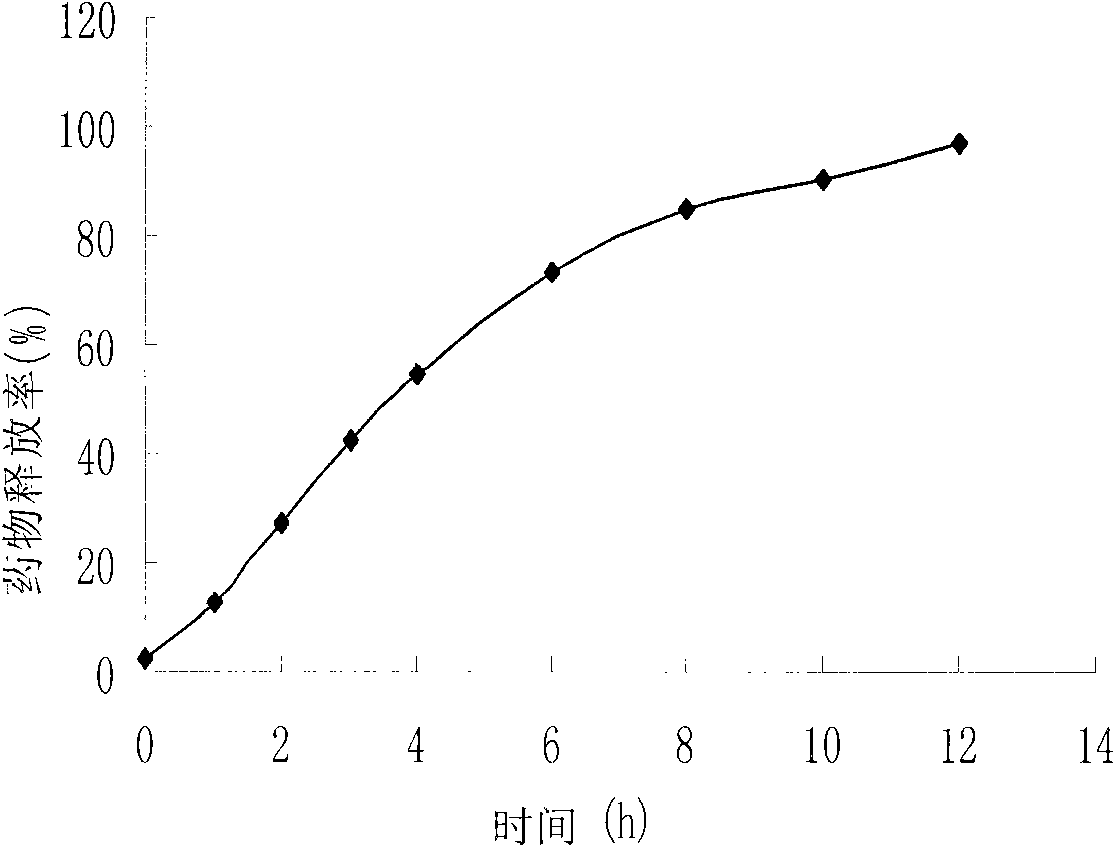
The invention discloses a water-insoluble medicine sustained-release pellet, a sustained-release orally disintegrating tablet thereof and a preparation method thereof. The water-insoluble medicine sustained-release pellet comprises a hollow pellet core, a medicine layer, an insulation layer and a sustained-release layer, wherein the sustained-release layer comprises the following ingredients: 54 to 88 percent of sustained-release materials, 2 to 30 percent of antitackiness agents and 1 to 30 percent of pore-foaming agents, wherein the percentage is the mass percentage in the sustained-release layer, wherein the sustained-release materials are one kind or several kinds of materials selected from ethyl acrylate and methyl methacrylate copolymers, polyvinyl acetate and ethyl cellulose. Through regulating the coating combinations and filling auxiliary materials to be pressed into orally disintegrating tablets, the medicine can be slowly released for more than 8 to 13 hours, so the stable blood medicine concentration can be maintained, the side effect is reduced, the medicine taking times can be reduced, and the medicine taking is convenient. The invention conforms to zero-grade release, has the advantages of high final accumulated medicine release amount, high efficacy, strong selectivity, good mouth feeling, simple production steps and high efficiency, and can be applied to large-scale production.
Owner:SHANGHAI INST OF PHARMA IND +1
Synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and composition and formulation thereof
ActiveCN101537020APromote growth and reproductionStrong antagonistic effectOrganic active ingredientsBacteria material medical ingredientsBacillus licheniformisSynbiotics
The invention relates to a synbiotics of bacillus licheniformis and oligosaccharide class prebiotics and a composite and a formulation thereof, being characterized in that the synbiotics comprises bacillus licheniformis medical live bacterial powder and the oligosaccharide class prebiotics, wherein the bacillus licheniformis medical live bacterial powder contains live bacteria about 30 billion / gram; the weight ratio of the bacillus licheniformis medical live bacterial powder to the oligosaccharide class prebiotics is 1:1 to 76; the weight ratio of the medical live bacterial powder of the composite, the oligosaccharide class prebiotics and auxiliary materials is as follows: 1.25-5 percent of the bacillus licheniformis medical live bacterial powder, 5-95 percent of the oligosaccharide class prebiotics, 20-50 percent of preferable oligosaccharide class prebiotics, 30-65 percent of diluting agents, 1-20 percent of bonding agents, 1-15 percent of disintegrating agents, 0.1-3 percent of lubricating agents, 1-10 percent of coating agents, 0.01-0.1 percent of flavoring agents, 0.0001-0.001 percent of coloring agents, and 0.1-15 percent of suspending agents. The synbiotics composite is prepared into oral common tablets, oral cavity disintegration tablets, dispersing tablets, enteric-coated tablets, granular formulation, enteric-coated granular formulation, capsules, enteric-coated capsules, dry supensoid agents and external tablets according to a conventional process; and an in vitro test shows that each formulation can promote the growth and the reproduction of the synbiotics of bacillus licheniformis, restrain the growth of harmful bacteria, enhance the immunizing power of parasitifers, reduce diarrhea and enhance the health care.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
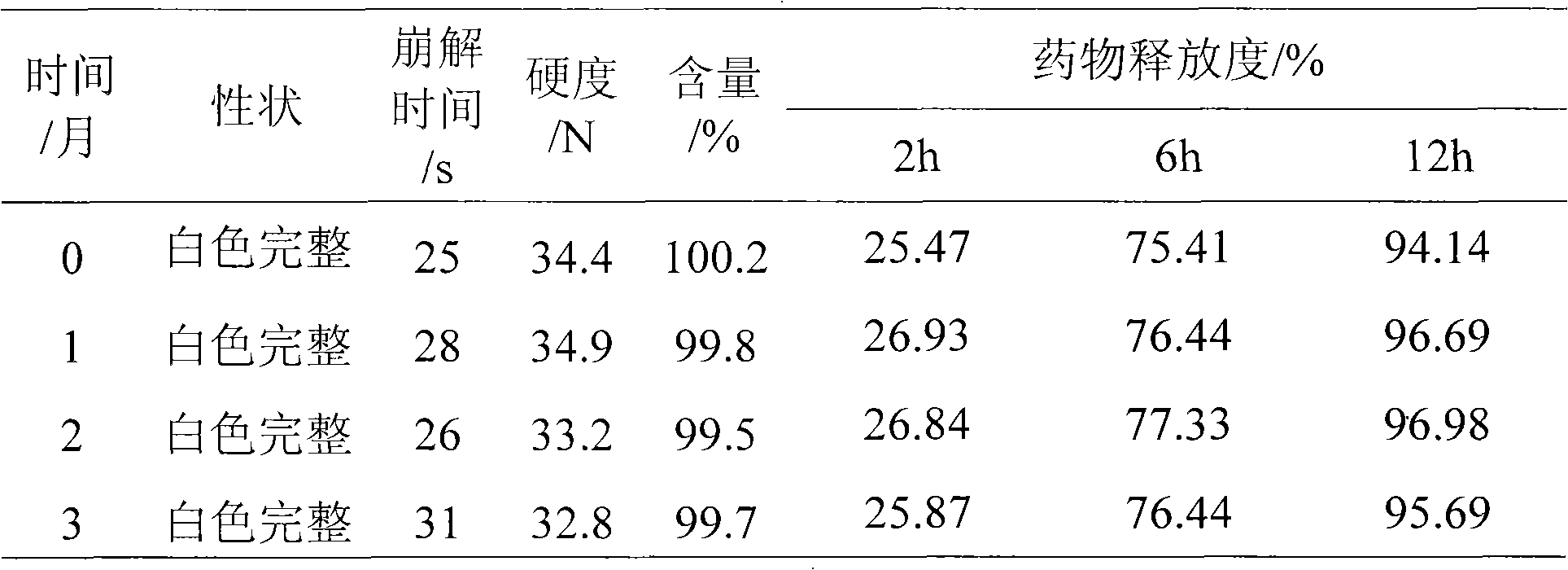
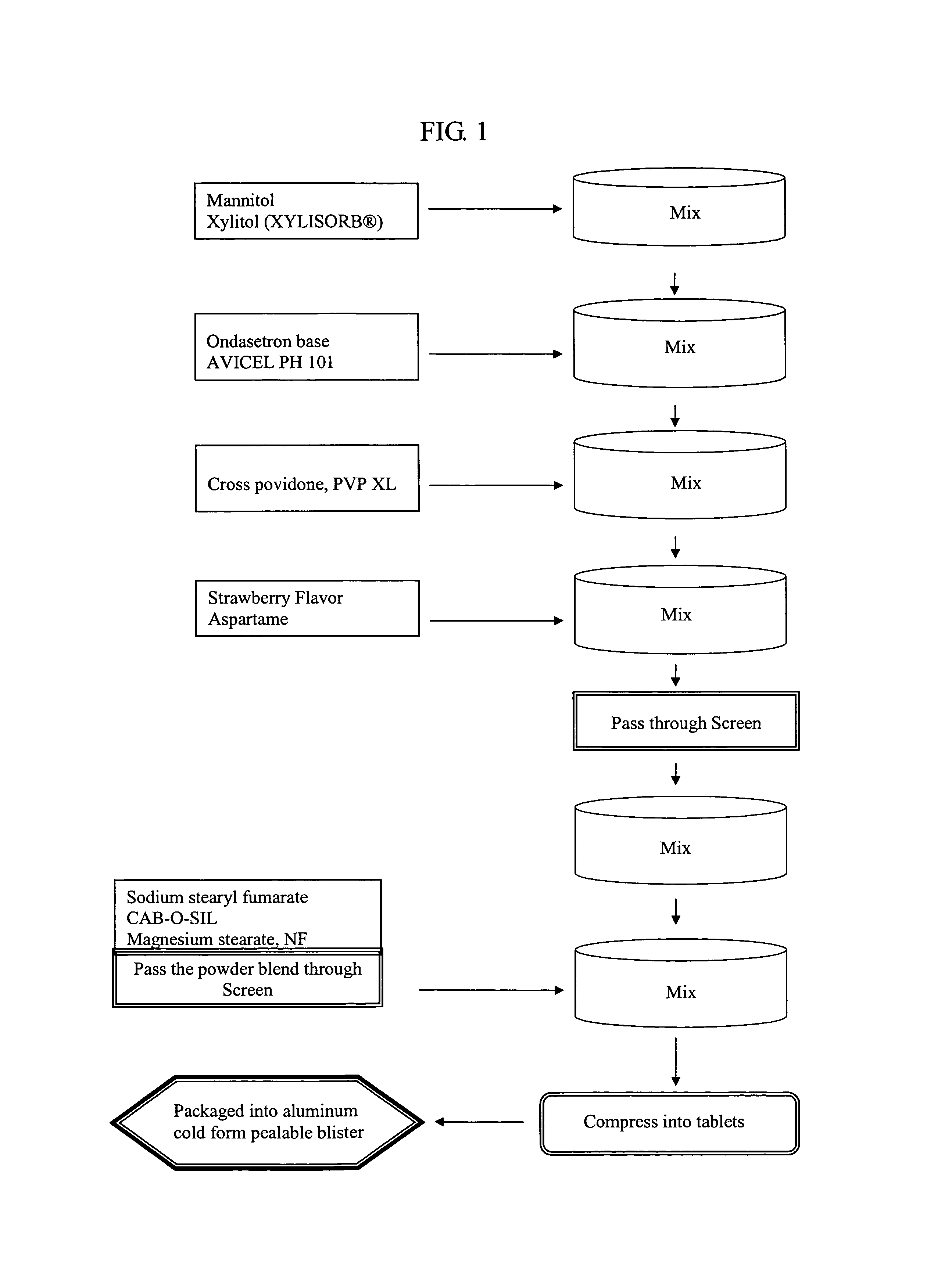
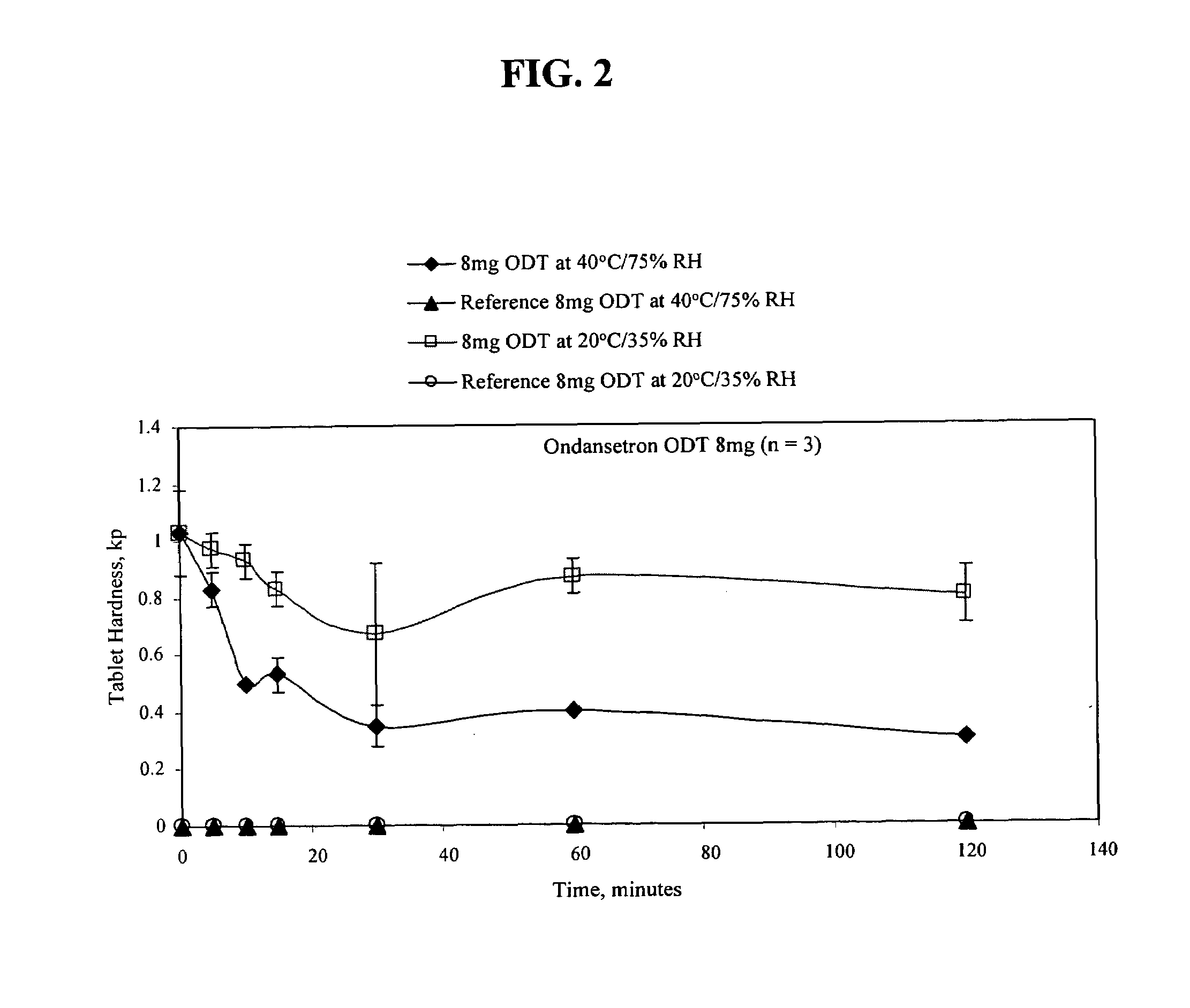
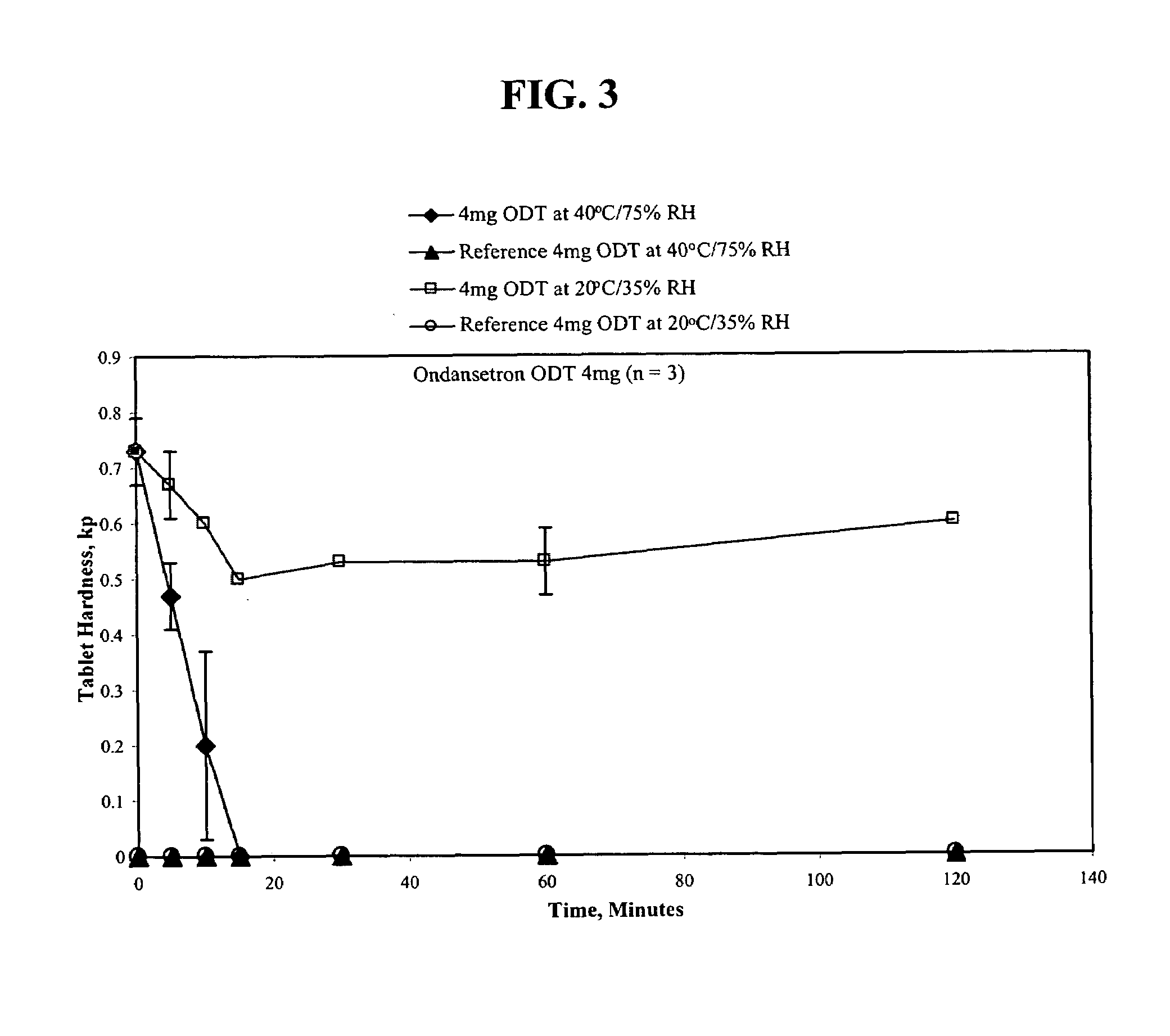
Ondansetron orally disintegrating tablets
InactiveUS7390503B1Safe and effective absorptionImprove bioavailabilityPowder deliveryPill deliveryWater dispersibleOrally disintegrating tablet
An ondansetron solid orally disintegrating dosage form for oral administration having at least one first water-dispersible component or water-insoluble cellulose derivative, a component having a —CHOH functional group, a disintegrating agent and at least one lubricant is provided. The dosage form can comprise ondansetron, a hydrophilic polymer such as microcrystalline cellulose, a component having a —CHOH functional group such as mannitol or xylitol and a disintegrating agent such as crospovidone. The lubricant may be a mixture of magnesium stearate, sodium stearyl fumarate and colloidal silicon dioxide. The present invention provides a non-effervescent tablet comprising the ondansetron dosage form. Another aspect of the invention is the treatment of emesis such as nausea and vomiting caused by cancer chemotherapy and radiation by the administration of the ondansetron formulation of the present composition. Finally, a process of forming an ondansetron disintegrating tablet using the ondansetron dosage form is disclosed.
Owner:BARR LAB
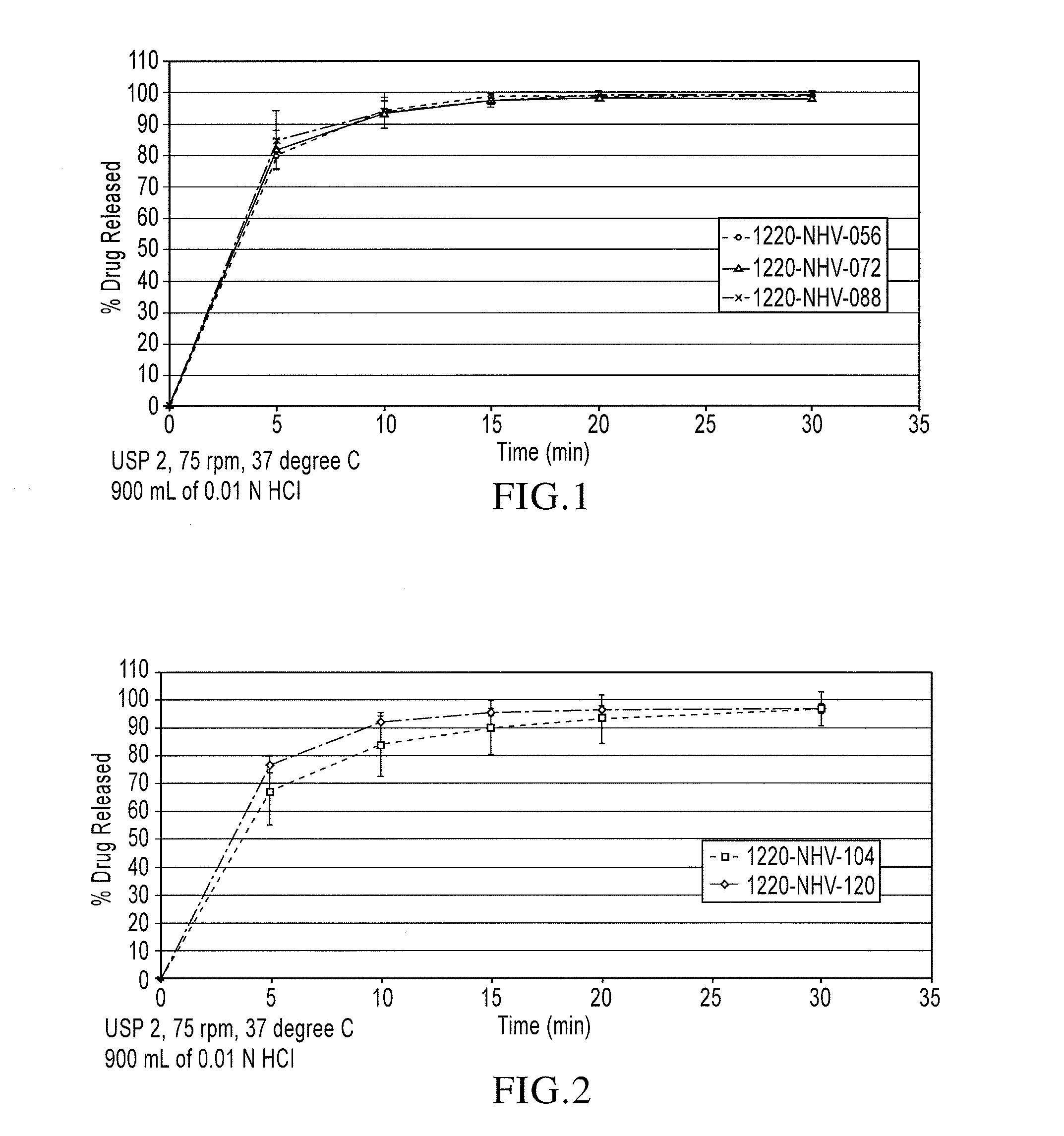
Orally disintegrating tablets and process for obtaining them
InactiveUS20060165781A1High dissolution rateImprove compression performancePill deliveryPharmaceutical non-active ingredientsMANNITOL/SORBITOLOrally disintegrating tablet
The tablets comprise: at least 59.5% spray-dried mannitol; active ingredient below or equal to 10%, where at least 90% in weight of the active ingredient has a particle size below 100 μm; microcrystalline cellulose 10-18%, with an average particle size of 50 μm and where at least 99% in weight of microcrystalline cellulose has a particle size below 250 μm; sodium croscarmellose 14%; and a lubricant agent 0.5-2%; where, unless specified otherwise, the percentages are expressed in weight of the total weight of the tablet. And also a process comprising: sieving and mixing of components except for the lubricant agent; mixing of all components; and direct compression of the final mixture. The tablets of the invention give lower disintegration times as well as good perception on the tongue after disintegration, and overcome the problem of insufficient mechanical resistance for packaging and transport operations.
Owner:WARNER CHILCOTT IBERIA
Lansoprazole orally disintegrating tablets
InactiveUS20070141151A1Dissolve fastPrevent degradationBiocidePill deliveryLansoprazoleOrally disintegrating tablet
The invention provides orally disintegrating tablets that readily disintegrates in the mouth, releasing enteric coated drug sub-tablets.
Owner:TEVA PHARM USA INC
Orally Disintegrating Tablets
InactiveUS20090208576A1High mechanical strengthNervous disorderAntipyreticCalcium silicateOrally disintegrating tablet
The present invention describes a directly compressible composite prepared by co-processing a water-soluble excipient and calcium silicate. The present invention further describes the incorporation of the co-processed composite into a tablet formulation. The orally disintegrating tablets are of optimal mechanical strength and disintegrate within 60 seconds in the oral cavity.
Owner:RUBICON RES PTY LTD
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20090263480A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryWater insolubleAdditive ingredient
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com