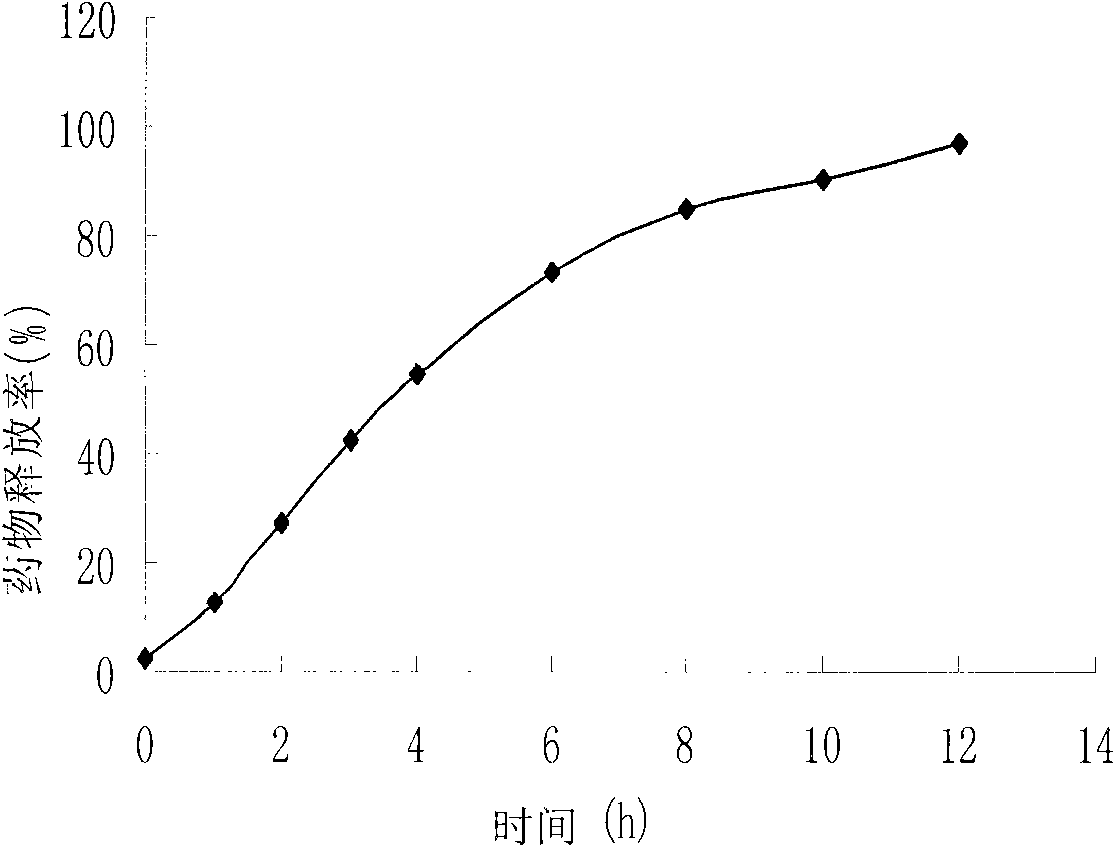
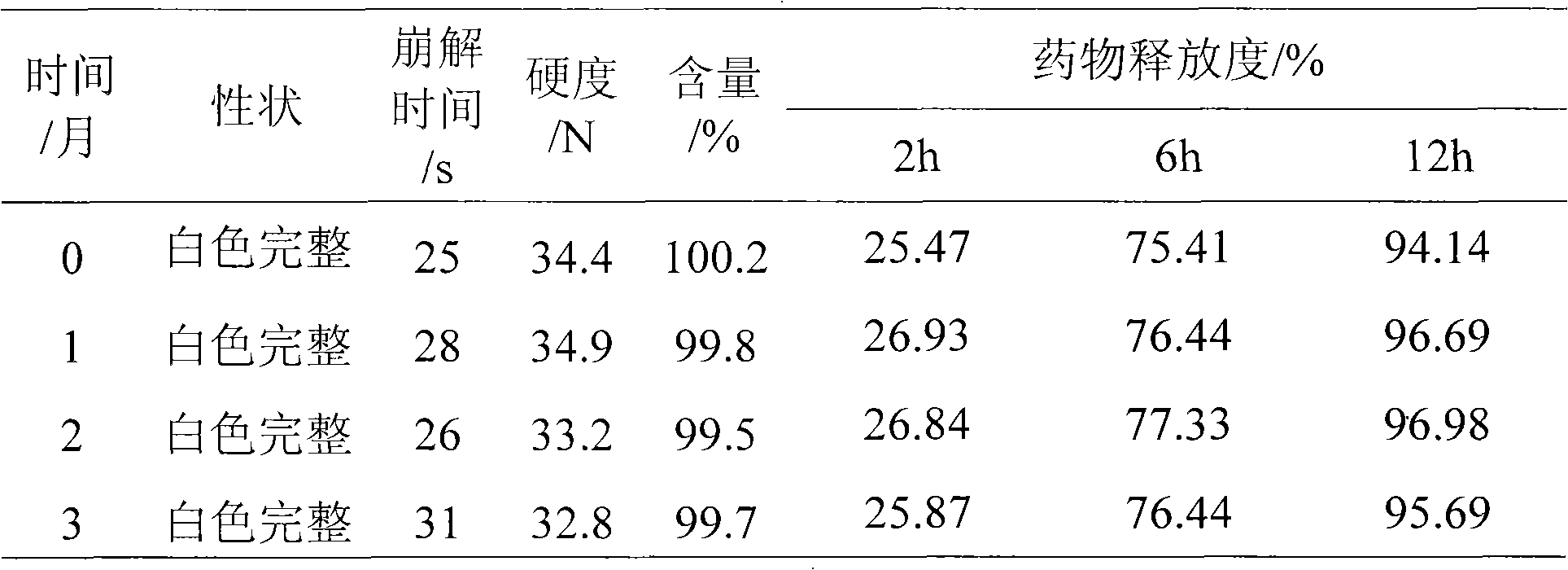
Water-insoluble medicine sustained-release pellet, sustained-release orally disintegrating tablet thereof and preparation method thereof
A technology of orally disintegrating tablets and sustained-release pellets, applied in the field of sustained-release pellets, can solve the problems of inability to achieve long-acting release, short slow release time, incomplete coating, etc., to maintain blood drug concentration, production steps Simple, selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 prepares acetaminophen sustained-release pellets
[0031] 1. pill heart medicine
[0032] Add 17g of polyvinylpyrrolidone PVP S630 and 100g of paracetamol to 560ml of 75% (v / v) ethanol to make a medicinal solution, wherein the concentration of paracetamol is 15g / 100ml, and the concentration of binder PVPS630 is 2.5% (wt) . Put the sucrose ball core with an average particle size of 0.15 mm in a fluidized bed for 10 minutes, and slowly spray the drug solution on the surface of the ball core. Weight gain after taking medicine: 45.0-50.0%. The operating conditions of the tangential jet fluidized bed are: the fan frequency is 20-23Hz; the material temperature is 33-35°C; the rotation frequency of the turntable is 100-150rpm; the atomization pressure of the atomizing spray gun is 0.10-0.15MPa; The liquid spray speed is 12~15g / min.
[0033] 2. Package isolation layer
[0034] Dissolve 15 g of hydroxypropyl methylcellulose (HPMC) in 300 ml of pure water, and s...
Embodiment 2
[0037] Example 2 Preparation of acetaminophen sustained-release pellets orally disintegrating tablets
[0038] Tablet preparation orally disintegrating tablet, one piece (mg)
[0039] Acetaminophen extended-release pellets 225
[0040] Mannitol 178
[0041] Microcrystalline Cellulose 87
[0042] Cross-linked polyvinylpyrrolidone 22
[0043] Hypromellose 11
[0044] aspartame 2
[0046] Microsilica 1
[0047] Talc 15
[0048] The preparation method is as follows:
[0049] Pass the acetaminophen sustained-release pellets obtained in Example 1 through a 60-mesh sieve, and the auxiliary materials that can be directly compressed: mannitol, microcrystalline cellulose, cross-linked polyvinylpyrrolidone, hydroxypropyl cellulose, magnesium stearate , talcum powder, micro-silica gel and aspartame are directly compressed with a tablet machine, and the hardness of the tablet is 3kg.
[0050] Get this sustained-release pellet orally disintegrating ta...
Embodiment 3
[0051] Embodiment 3 prepares diclofenac sodium sustained-release pellets
[0052] 1. Pills and medicines
[0053] Add 17g of polyvinylpyrrolidone PVP VA64 and 100g of diclofenac sodium to 560ml of 75% (v / v) ethanol, wherein the concentration of diclofenac sodium: 15g / 100ml, the concentration of binder: 2.5% (wt). Put the sucrose ball core with an average particle size of 0.10 mm in a fluidized bed for 10 minutes, and slowly spray the above-mentioned medicinal solution on the surface of the core. Weight gain after taking medicine: 45.0-50.0%. The operating conditions of the tangential jet fluidized bed are: the fan frequency is 20-23Hz; the material temperature is 33-35°C; the rotation frequency of the turntable is 100-150rpm; the atomization pressure of the atomizing spray gun is 0.10-0.15MPa; The liquid spray speed is 12~15g / min.
[0054] 2. Package isolation layer
[0055] Dissolve 13 g of hydroxypropyl methylcellulose in 300 ml of pure water, and slowly spray it on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com