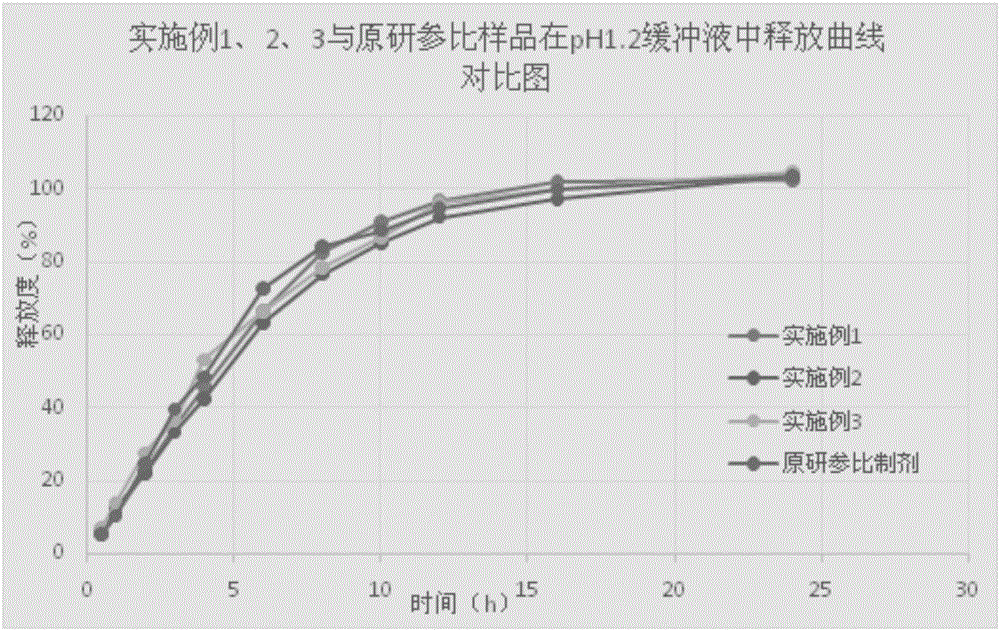
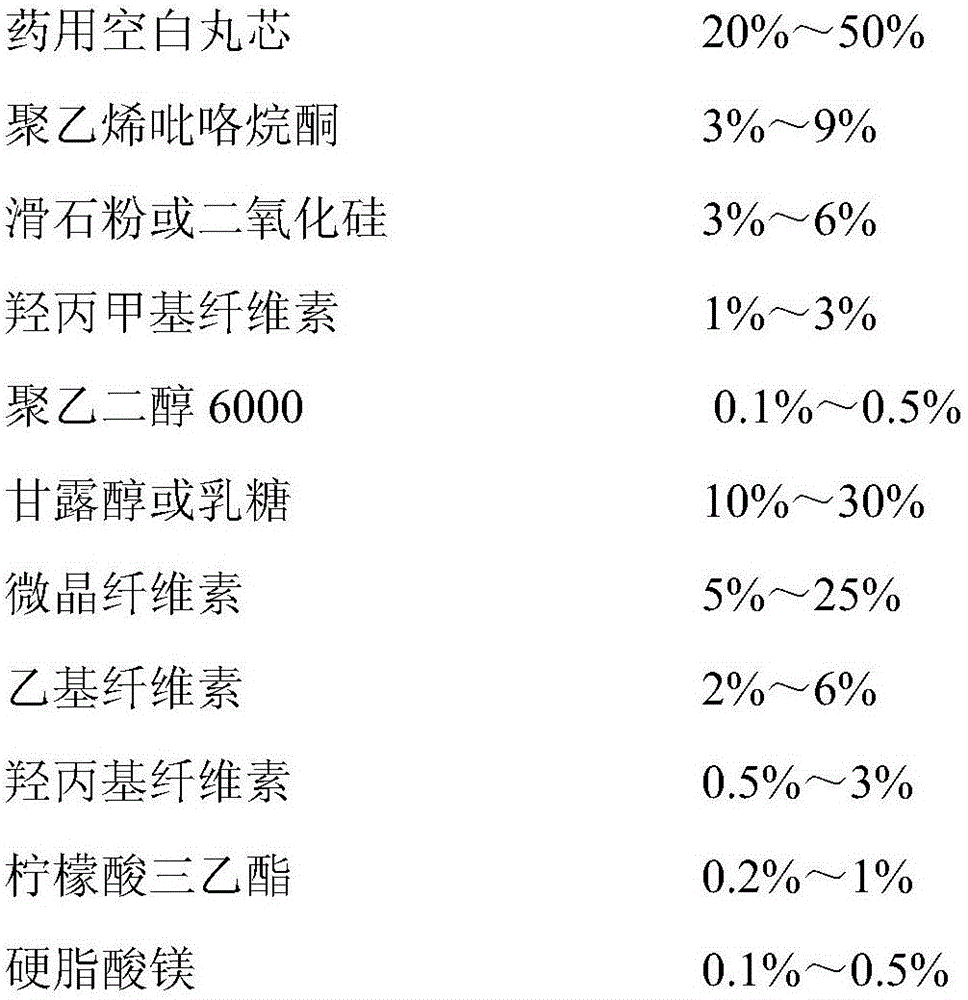
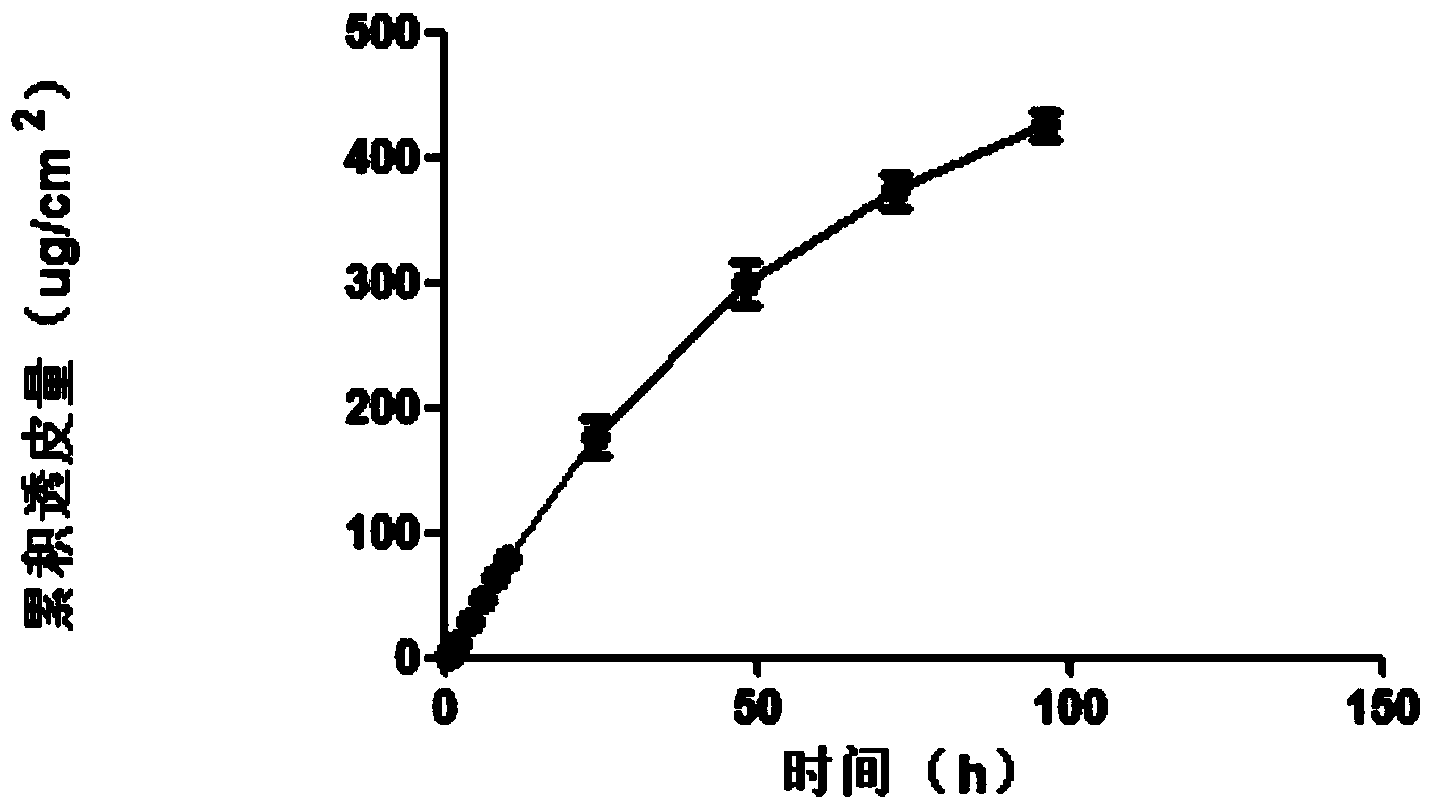
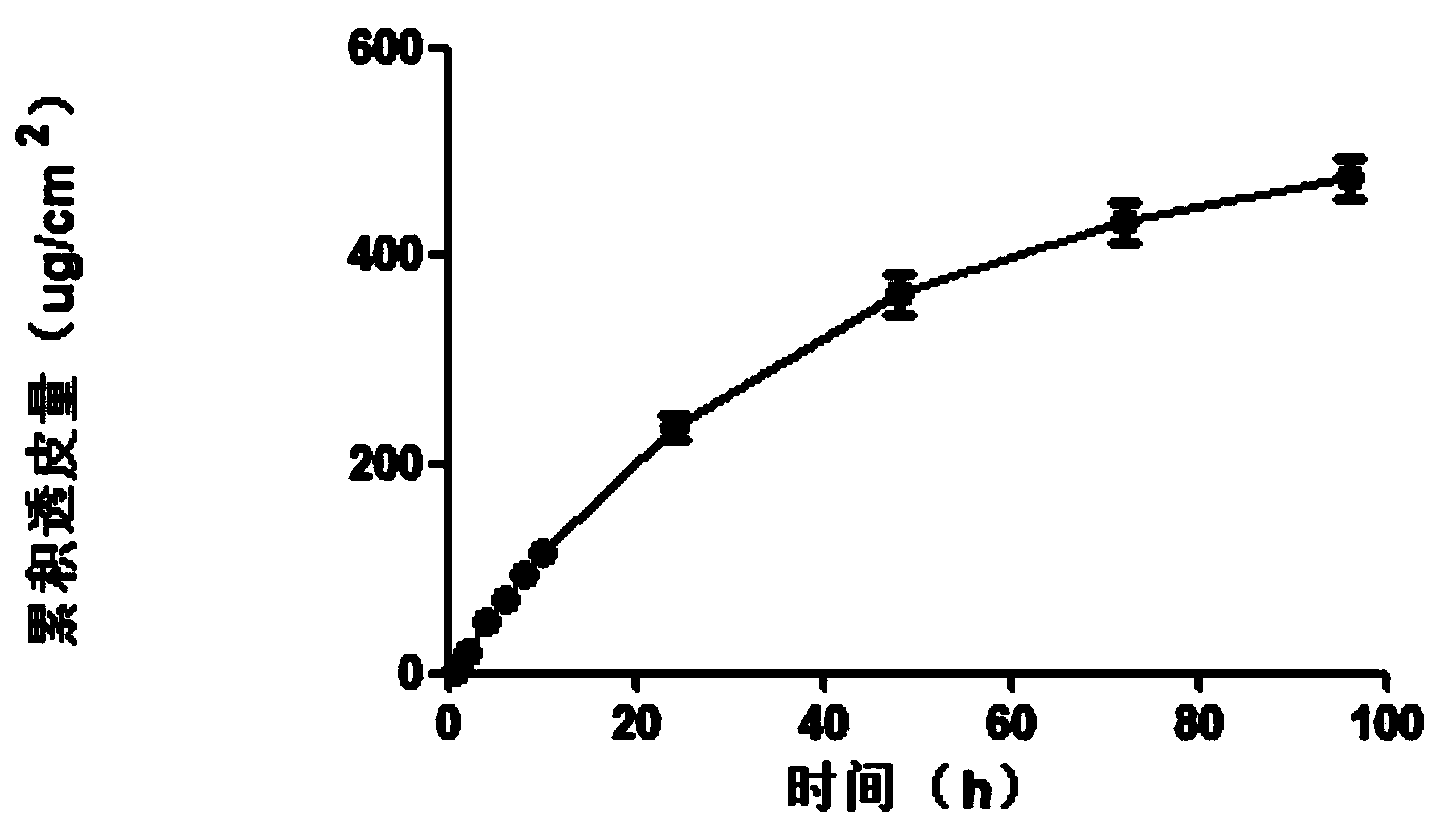
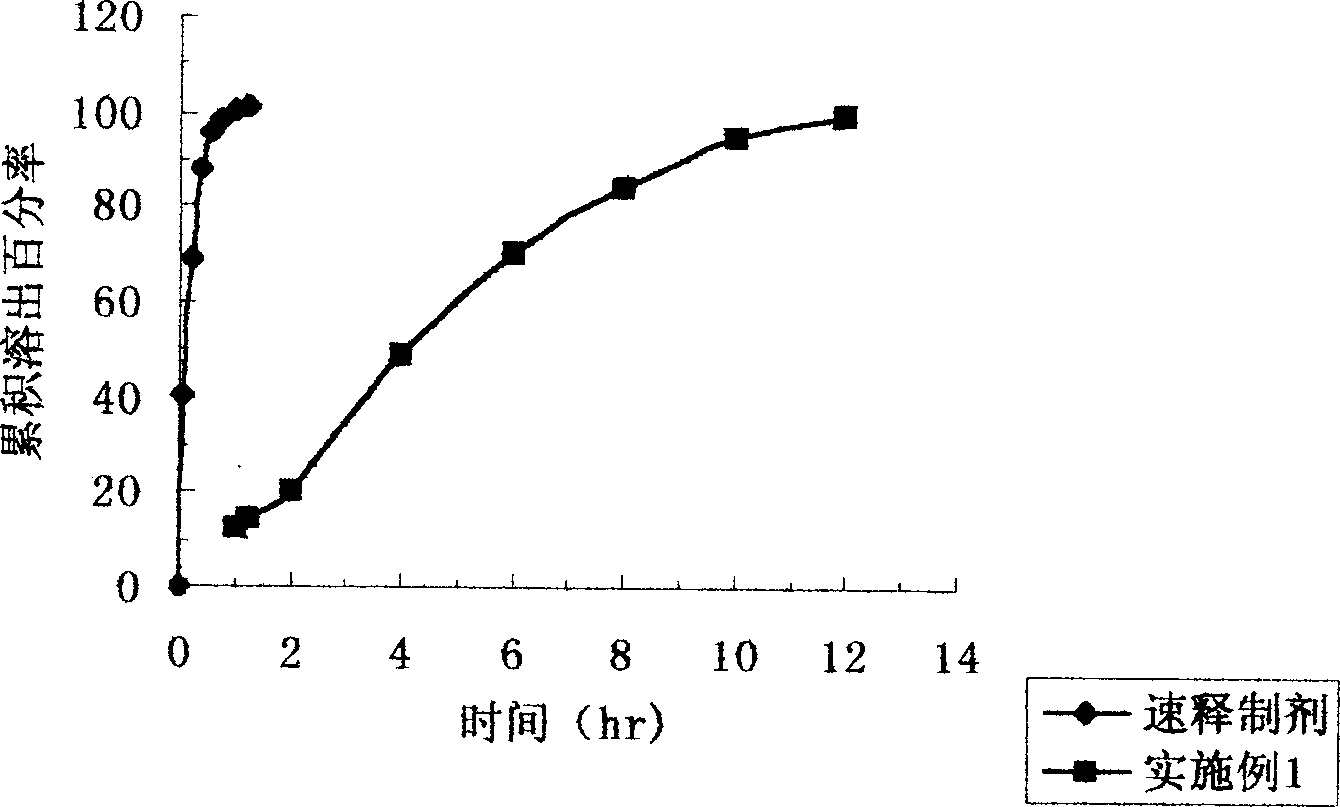
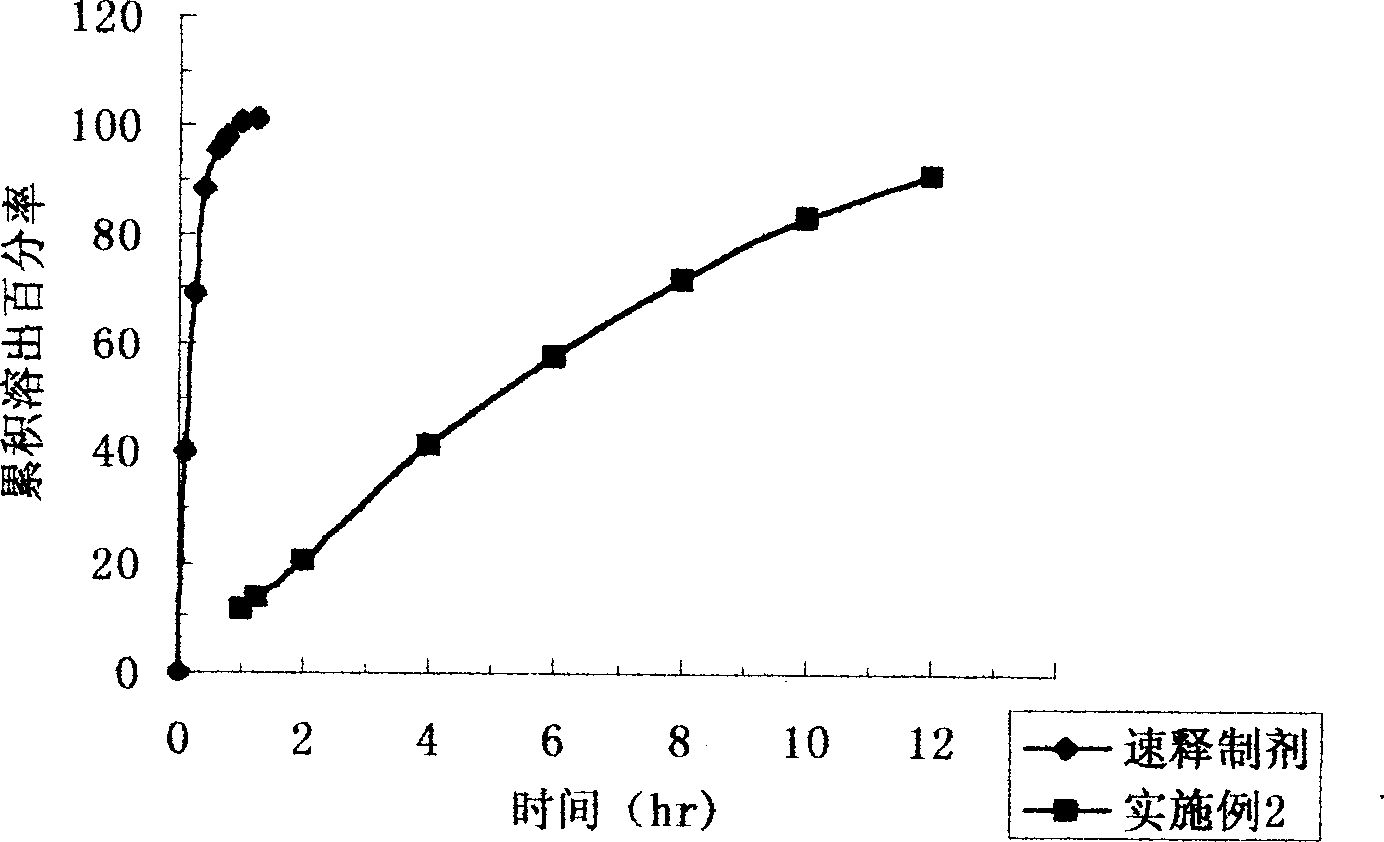
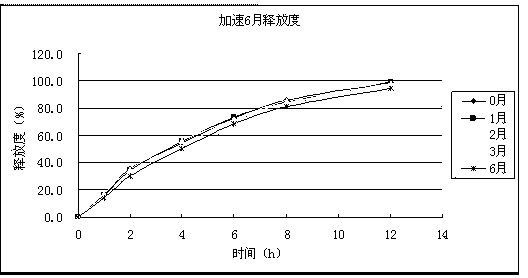
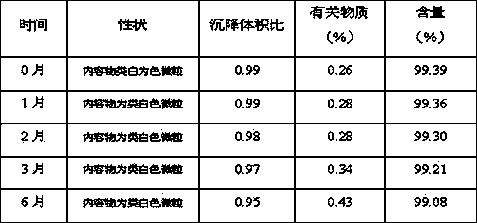
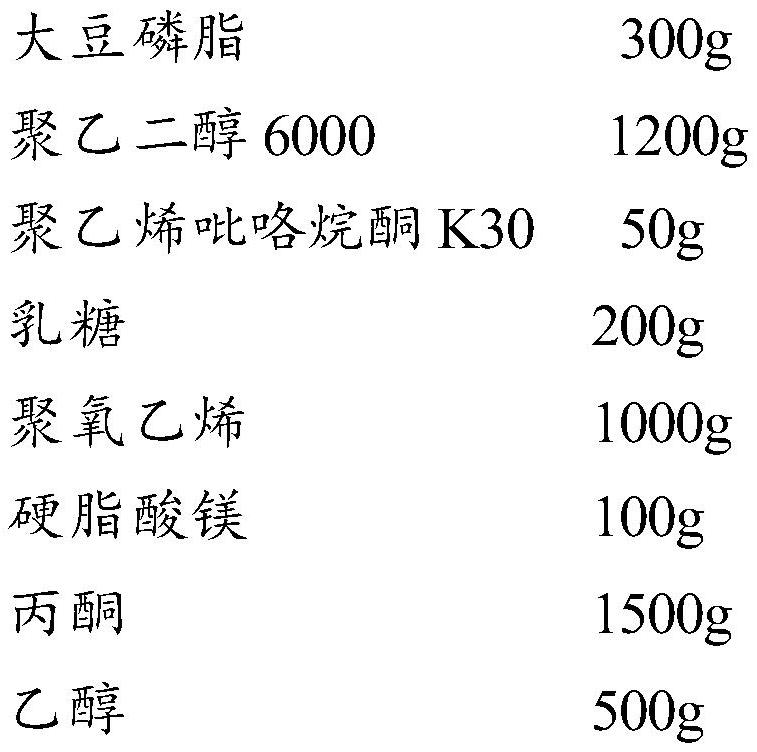
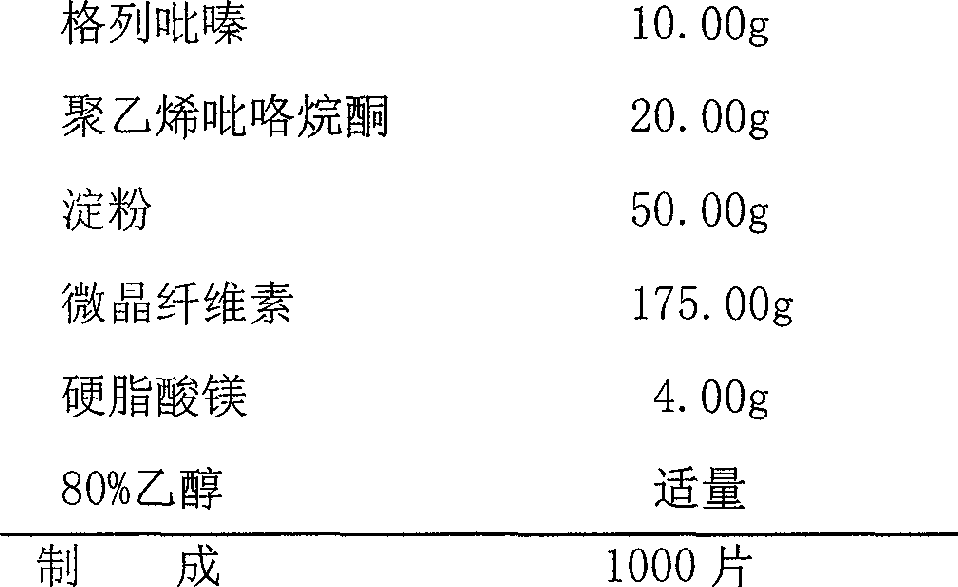
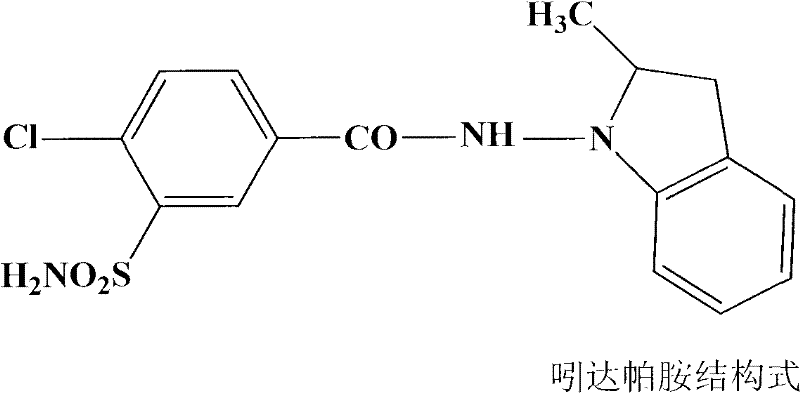
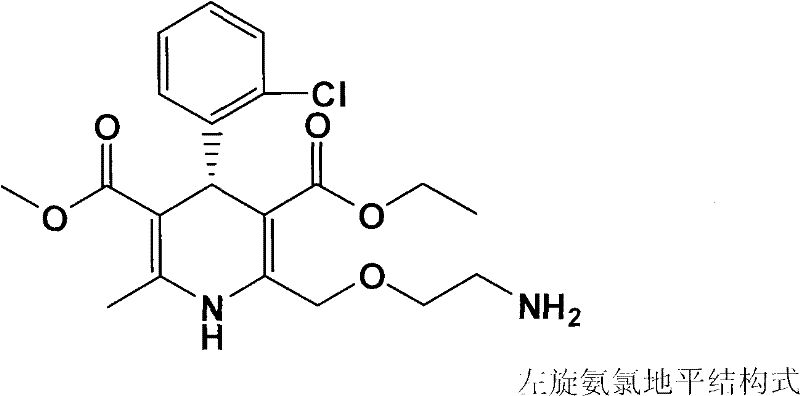
Patents
Literature
203results about How to "Reduce the frequency of taking" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
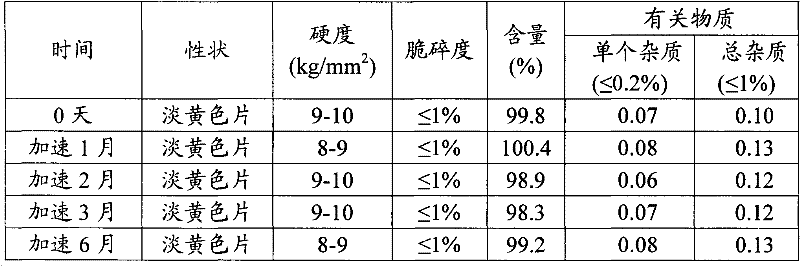
Metformin hydrochloride slowly released tablet and its preparation method
InactiveCN1415288APromote aerobic metabolismIncrease intakeOrganic active ingredientsMetabolism disorderExtended release tabletsAlcohol
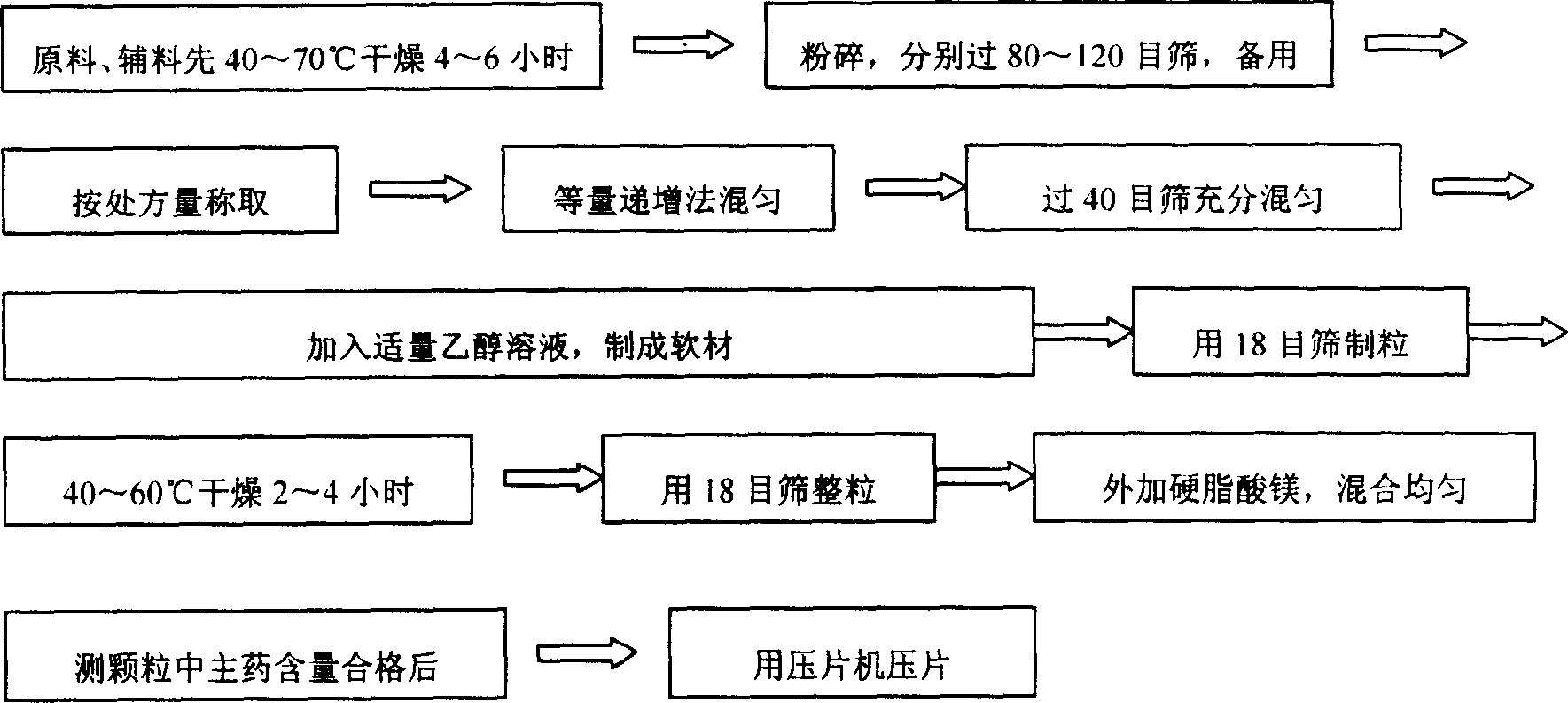
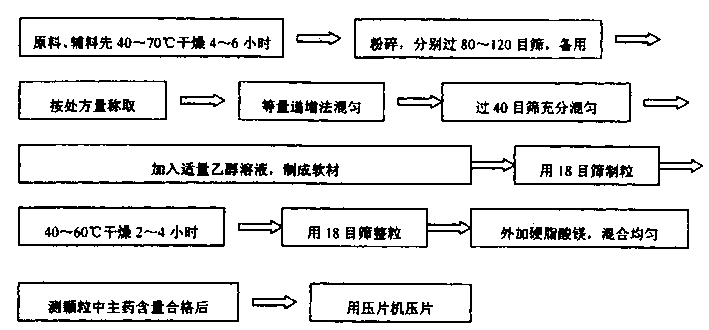
A slowly-releasing mellitin tablet is prepared from fluamine, excipient, adhesive and lubricant through drying at 40-70 deg.C for 4-6 hrs, pulverizing, sieving by 80-120 meshes, proportionally mixing, mixing with alcohol solution, granularating by 18-mesh sieving, drying at 40-60 deg.C for 2-4 hrs, mixing with lubricant, and tabletting. Its advantages are long half-life (0.9-2.6 hr), low dosage and low by-effect.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Dimethyl biguanide sustained release capsule and preparation method thereof
InactiveCN1568950AReduce the frequency of takingStable blood concentrationOrganic active ingredientsMetabolism disorderDrugBiguanide
The invention discloses a dimethylbiguanide slow release capsule which includes capsule outer cover and slow release particles, wherein the slow release particles comprises dimethylbiguanide and hollow ball cores, retarding agent, slow release film forming material, bulking agent, plasticizing agent, adhesives and hole-forming agent.
Owner:范敏华
Dabrafenib mesylate sustained-release tablet and preparation method thereof
InactiveCN106539777AImprove securityReduce the frequency of takingOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletBlood concentration
The invention relates to a dabrafenib mesylate sustained-release tablet, which is prepared from the following raw materials and auxiliary materials: 1 part of dabrafenib mesylate, 0.3 to 0.9 part of sustained-release framework materials, 0.05 to 0.11 parts of glidants, 1.1 to 3.1 parts of filling agents and 1 to 6 parts of binding agents. The dabrafenib mesylate sustained release tablet prepared according to the invention has the advantages that the main medicine of dabrafenib mesylate is slowly released; the release period reaches up to 12h, so that the taking times of a product of the dabrafenib mesylate sustained-release tablet can be fewer than the taking times of a conventional capsule; the main medicine of the product of the dabrafenib mesylate is slowly released, so that the stable and durable effective blood concentration is provided; the blood concentration peak valley phenomenon is avoided or reduced; the use safety of the medicine is favorably improved; meanwhile, the stability of a product is high; the shelf life is as long as 24 months; and the preparation method is simple and feasible, and is worthy of being popularized and applied in markets.
Owner:JIAMUSI UNIVERSITY
Composite containing pioglitazone HCL and repaglinide
InactiveCN101204394ASimplify useReduce the frequency of takingOrganic active ingredientsMetabolism disorderPIOGLITAZONE HCLRepaglinide
The invention relates to a composition which takes a pioglitazone hydrochloride and a repaglinide as an active ingredient and a preparation method thereof.The invention, taking the pioglitazone hydrochloride and the repaglinide as a medicinal active ingredient, is a medicinal composition mixedly formed with a carrier adoptive in pharmacy, and the invention can be prepared into oral preparations such as a troche, a capsule, a dispersible tablet, a chewable tablet, an orally disintegrating tablet, a buccal tablet, a liquid capsule, a soft capsule and a pill, etc. The composition can be used for curing type two diabetes.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Clonidine hydrochloride sustained-release tablet
ActiveCN105395506AGood effectReduce the frequency of takingOrganic active ingredientsNervous disorderClonidine HydrochloridePatient compliance
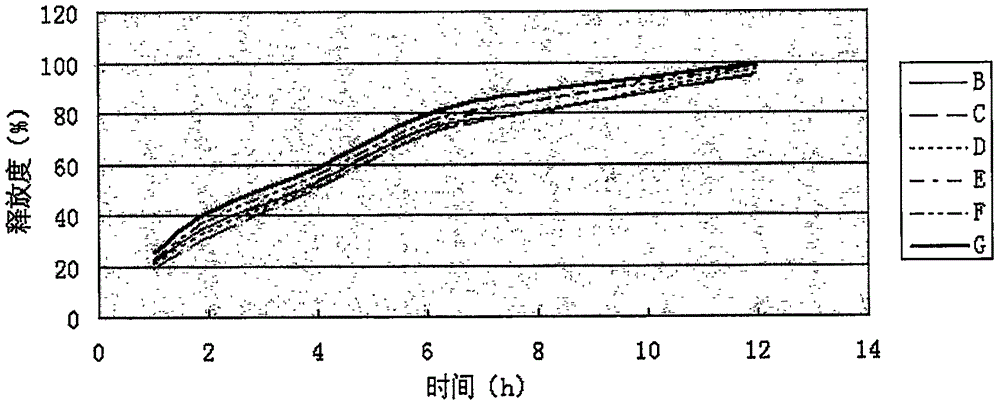
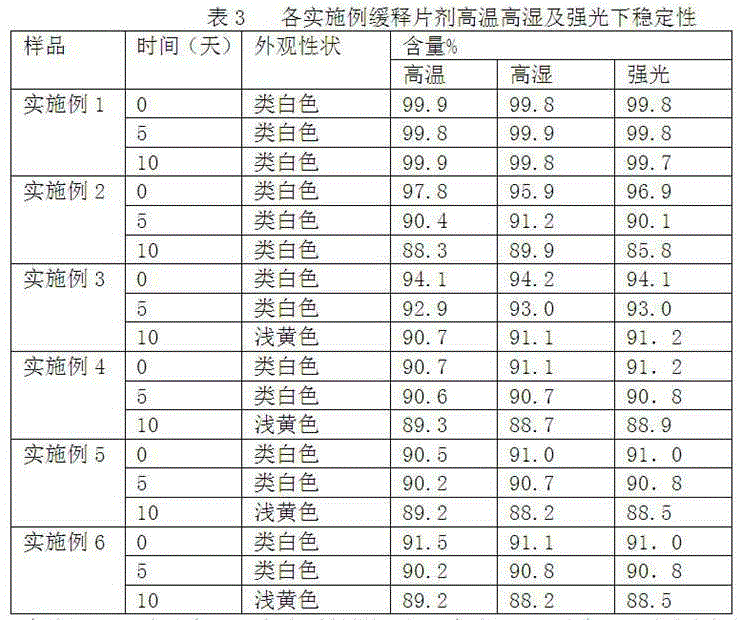
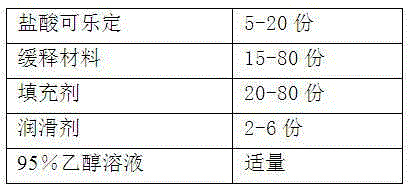
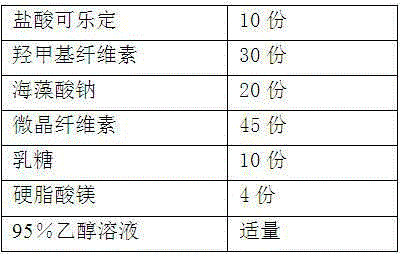
The invention provides a preparation method of a clonidine hydrochloride sustained-release tablet, and belongs to the technical field of a medicine. The clonidine hydrochloride sustained-release preparation disclosed by the invention consists of clonidine hydrochloride, a sustained-release material, a filling material, a lubricating agent and an ethanol solution with concentration being 95%. The clonidine hydrochloride, as a major ingredient, has an effect of lowering blood pressure and is effective on migraine, menopausal hectic fever and dysmenorrheal; the clonidine hydrochloride is applicable to the rapid drug treatment for addiction of opiates; and in 2010, the clonidine hydrochloride is approved to be used for treating attention deficit hyperactivity disorder in teenagers. The sustained-release preparation provided by the method is safe and effective, stable in quality, low in cost and low in administration efficiency, and the sustained-release preparation is capable of enhancing patient compliance and is capable of achieving the sustained release of drugs.
Owner:CP PHARMA QINGDAO CO LTD
Memantine hydrochloride slow release-donepezil quick release compound capsule
InactiveCN106727439AReduce the peak and valley phenomenon of blood drug concentrationReduce the frequency of takingNervous disorderAmine active ingredientsMemantine HydrochlorideFluidized bed
The invention provides a memantine hydrochloride slow release-donepezil quick release compound capsule. The compound capsule is prepared by jointly filling a capsule with memantine hydrochloride slow release pellets and donepezil quick release granules; the memantine hydrochloride slow release pellets are prepared by using medicinal empty pellet cores as original cores, dissolving memantine hydrochloride serving as main drug and proper excipients to form coating liquid, spraying the coating liquid to the bottom of a fluidized bed to form a drug-containing layer and sequentially coating the fluidized bed with an isolating layer and a slow release layer from inside to outside; the donepezil quick release granules are formed by subjecting donepezil hydrochloride serving as main drug and proper excipients to wet-process granulation, and the slow release pellets and quick release granules are filled into one same capsule according to a certain proportion of the main drug.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
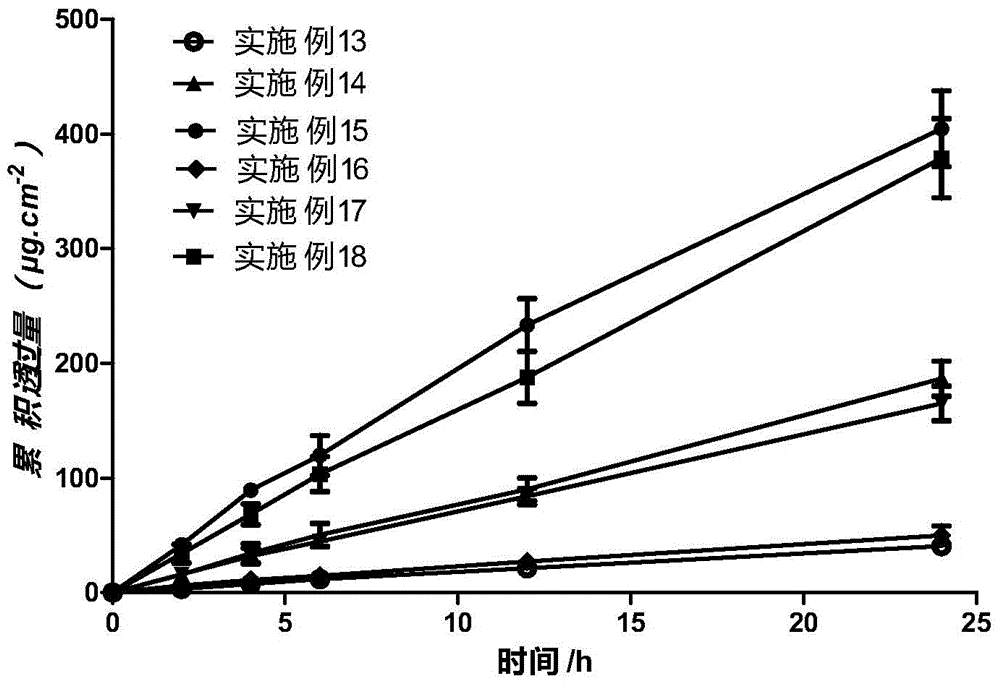
Transdermal delivery preparation in three-dimensional netty spatial configuration of agomelatine and preparation method thereof
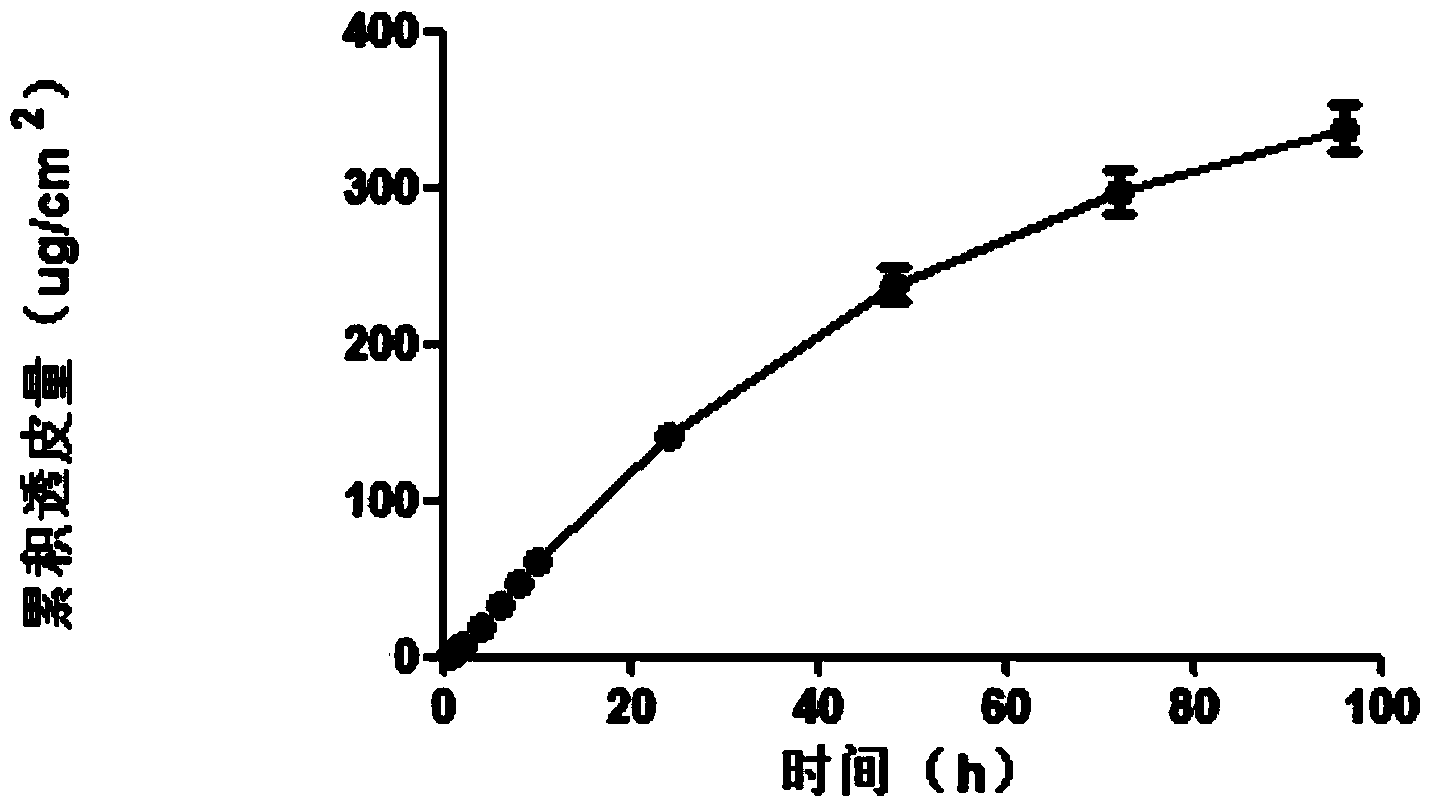
ActiveCN103830206AAvoid oral gastrointestinalAvoid liver first pass effectOrganic active ingredientsNervous disorderBlood concentrationSilicon dioxide
The invention discloses a transdermal delivery preparation in three-dimensional netty spatial configuration of agomelatine and a preparation method thereof. The transdermal delivery preparation consists of a backing layer, a medicine-loading system in a three-dimensional netty spatial configuration coated on the backing layer and an anti-sticking layer compounded on the system. The medicine-loading system in the three-dimensional netty spatial configuration comprises the following components in percentage by weight: 1-40% of agomelatine, 0-10% of nano-porous carbon dioxide, 40-90% of a pressure-sensitive adhesive, 1-30% of a transdermal penetration enhancer and 0-20% of a dispersant. The transdermal delivery preparation disclosed by the invention not only can continuously deliver the medicine in a transdermal manner for a longer time to maintain the constant blood concentration, but also is quick in transdermal absorption rate and high in transdermal absorptive amount, so that the preparation has the characteristics of stability and efficiency.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Simvastatin slow-release tablet and preparation method thereof
InactiveCN101590025AReduce the frequency of takingSmall side effectsOrganic active ingredientsMetabolism disorderAutomatic controlSide effect
The invention provides a simvastatin slow-release tablet and a preparation method thereof. The simvastatin slow-release tablet comprises a medicine-carrying tablet core and a coating layer coated outside the medicine-carrying tablet core, wherein the medicine-carrying tablet core comprises the following raw materials according to parts by weight: 7-13 parts of simvastatin, 120-140 parts of filler and 40-60 parts of adhesive; the existing short-acting simvastatin tablet is prepared into a coating type slow-release preparation form which controls the diffusion and dissolution of a medicine so as to delay the release of the medicine; after the medicine enters a human body, the coating layer slowly dissolves, so that the medicine is stably and durably released, the blood concentration is kept in a more stable effective state for a long time, the defect of large fluctuation of the effective blood concentration caused by the overlarge fluctuation of the blood concentration existing in the frequent administration of a conventional preparation form is avoided, and the toxic or side effect is reduced; and the simvastatin slow-release tablet is prepared in a closed one-step boiling granulator, thereby being hardly polluted, the quality of a finished product can be better guaranteed, and automatic control is convenient.
Owner:JIAOZUO XIANDA TRADE
Amoxicillin sustained-release preparation composition and preparation method thereof
InactiveCN101890006ASlow release rateReduce the frequency of takingAntibacterial agentsGranular deliveryMedicineDrug product
The invention discloses an amoxicillin sustained-release preparation composition and a preparation method thereof, and the amoxicillin sustained-release preparation composition is mainly prepared by amoxicillin bulk drug, sustained-release material and other proper auxiliary materials. The provided amoxicillin sustained-release preparation can slow down the release rate of main drug, reduces the medicine taking times and improves the compliance of patients. The invention provides the amoxicillin sustained-release preparation composition, and the preparation technique of the composition has good quality controllability and stability.
Owner:北京瑞伊人科技发展有限公司 +1
Novel composing prescription sustained-release preparation for treating high blood pressure and preparation method thereof
InactiveCN101185624AQuick-acting and long-actingReduce the frequency of takingPharmaceutical delivery mechanismHeterocyclic compound active ingredientsDissolutionDrug release
The invention relates to a sustained release preparation of a novel prescription for treating hypertension and the preparing method thereof. The novel prescription comprises a heart selective Beta1 receptor blocker metoprolol and an angiotensin II receptor antagonist (sartan drugs). The sustained release preparation consists of delayed release part and rapid release part, wherein the heart selective Beta1 receptor blocker metoprolol is the delayed release part, with first hour releasing 25-45%, fourth hour releasing 40-75% and eighth hour releasing over 75%; the angiotensin II receptor antagonist (sartan drugs) is the rapid release part, with 45 minutes dissolution over 75%. The composition has both rapid and prolonged action. The invention discloses in vitro drug release characteristics and preparation method thereof.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Transdermal drug delivery preparation with three-dimensional mesh stereoscopic configuration and preparation method of transdermal drug delivery preparation
InactiveCN104546804AImprove complianceFast absorption ratePharmaceutical non-active ingredientsSheet deliveryBlood concentrationMedicine
The invention discloses a transdermal drug delivery preparation with three-dimensional mesh stereoscopic configuration and a preparation method of the transdermal drug delivery preparation. The transdermal drug delivery preparation with the three-dimensional mesh stereoscopic configuration is composed of a transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration, a backing layer and an anti-sticking layer, wherein the backing layer is compounded on one side of the transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration; and the anti-sticking layer is compounded on the other side of the transdermal drug delivery system with the drug-loaded three-dimensional mesh stereoscopic configuration. With nanoporous silica as a carrier, the defects that a preparation is low in drug loading capacity, medicines are easily separated out and crystallized to affect the transdermal absorption efficiency, the adhesion performance of a pressure-sensitive adhesive system is not ideal enough, the pressure-sensitive adhesive system is easy to age, and the medicine stability is poor are solved; the long-term lasting transdermal penetration of the medicine can be effectively achieved; the stable blood concentration can be maintained; and the preparation has the characteristics of high transdermal absorption rate, high transdermal absorption amount, stability and high efficiency.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Curcumin carrier with temperature response
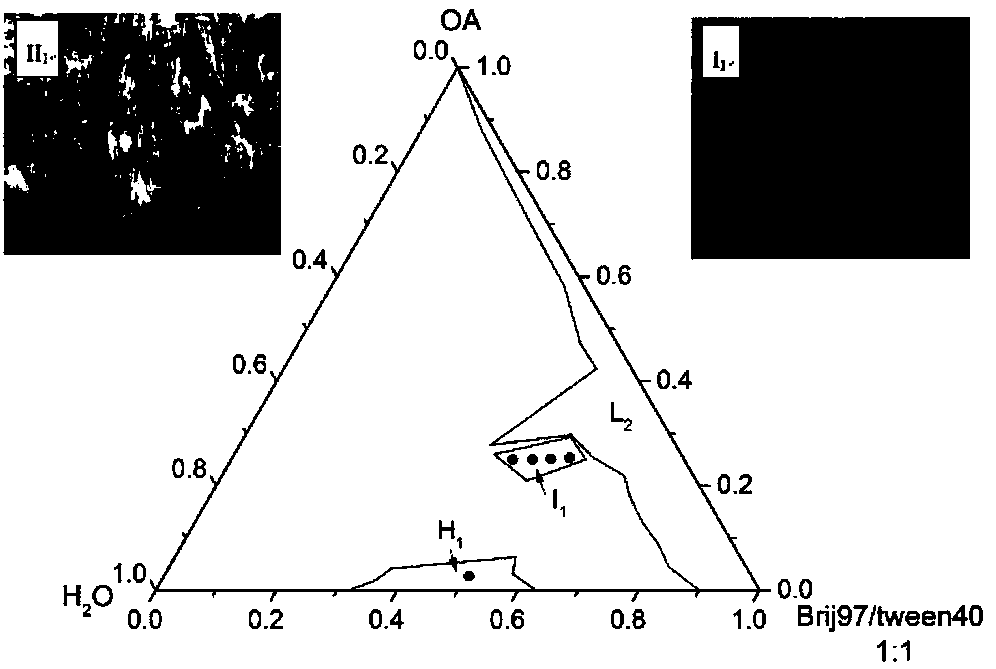
ActiveCN108451894AFast release rateQuick releasePharmaceutical delivery mechanismKetone active ingredientsDiseaseTemperature response
The invention provides a curcumin carrier with temperature response. A lyotropic liquid crystal (hexagonal phase and three-dimensional phase) is built in a water medium with non-ionic surfactants (Brij97 and Tween40) and oleic acid to serve as a drug carrier of curcumin. When the temperature of the drug carrier provided by the application rises, the releasing rate of a sample increases, and the highest releasing rate is reached when the temperature of the drug carrier is approximate to the temperature of human body. The drug carrier can be released rapidly after being taken, and facilitates rapid treatment of diseases with drug. Moreover, the carrier has a better slow-releasing effect on the curcumin, and can reduce the taking times of the curcumin.
Owner:SHANDONG NORMAL UNIV
Cyclobenzaprine hydrochloride sustained-release capsules
ActiveCN104523655AGood effectReduce the frequency of takingOrganic active ingredientsMuscular disorderPatient complianceSustained-Release Preparations
The invention provides a method for preparing cyclobenzaprine hydrochloride sustained-release capsules and belongs to the technical field of medicines. The cyclobenzaprine hydrochloride sustained-release capsules disclosed by the invention consist of cyclobenzaprine hydrochloride, a sustained-release material, a diluent, an adhesive, a disintegrating agent and a lubricating agent, wherein the main component cyclobenzaprine hydrochloride has the effects of relieving muscle spasm and accompanying severe pain in skeletal muscles, and the sustained-release preparation can stably achieve the effects of relieving pain and spasm. The cyclobenzaprine hydrochloride sustained-release capsules are safe, effective, stable in quality, low in cost and low in administration frequency, and the patient compliance is enhanced.
Owner:CP PHARMA QINGDAO CO LTD
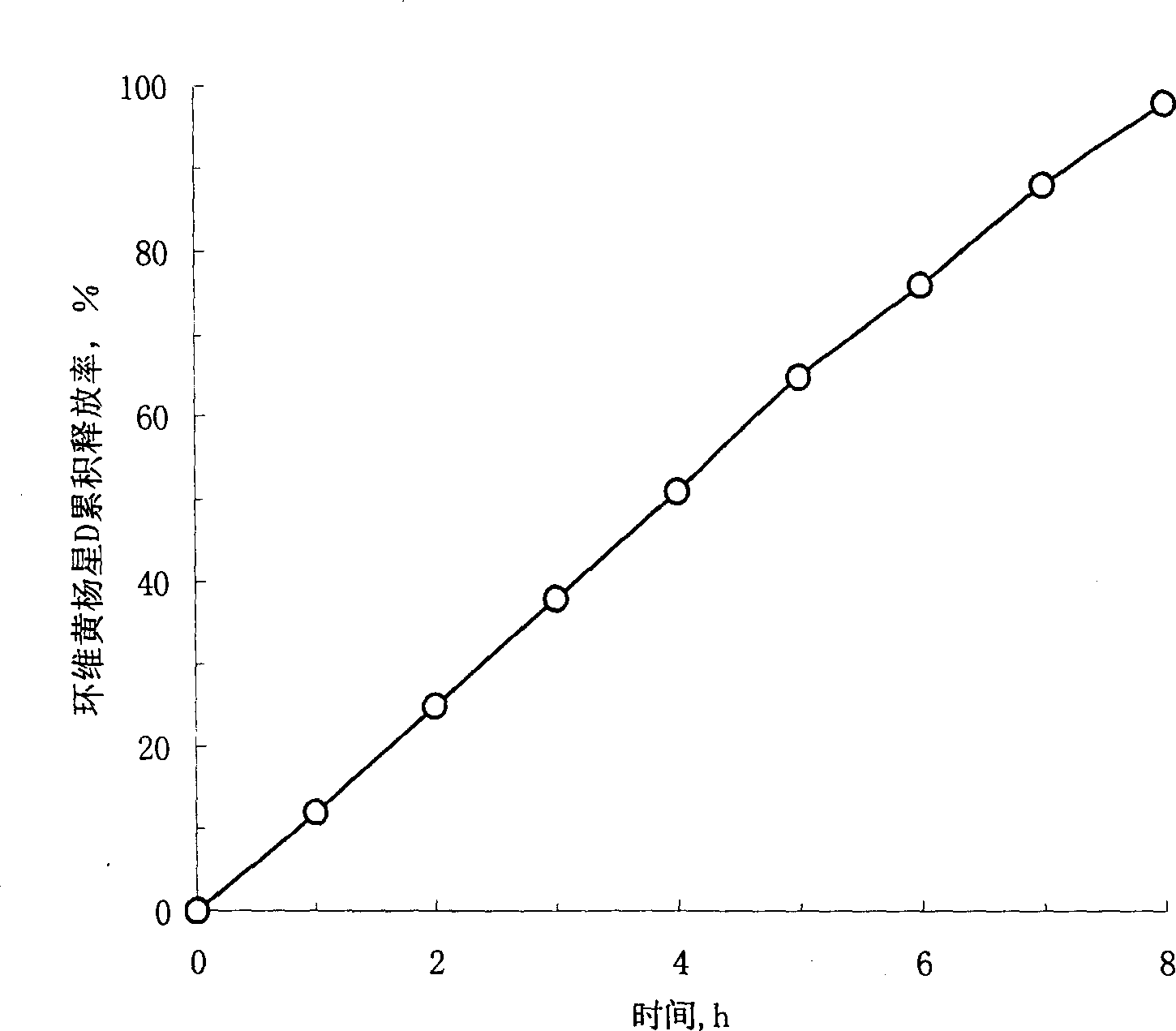
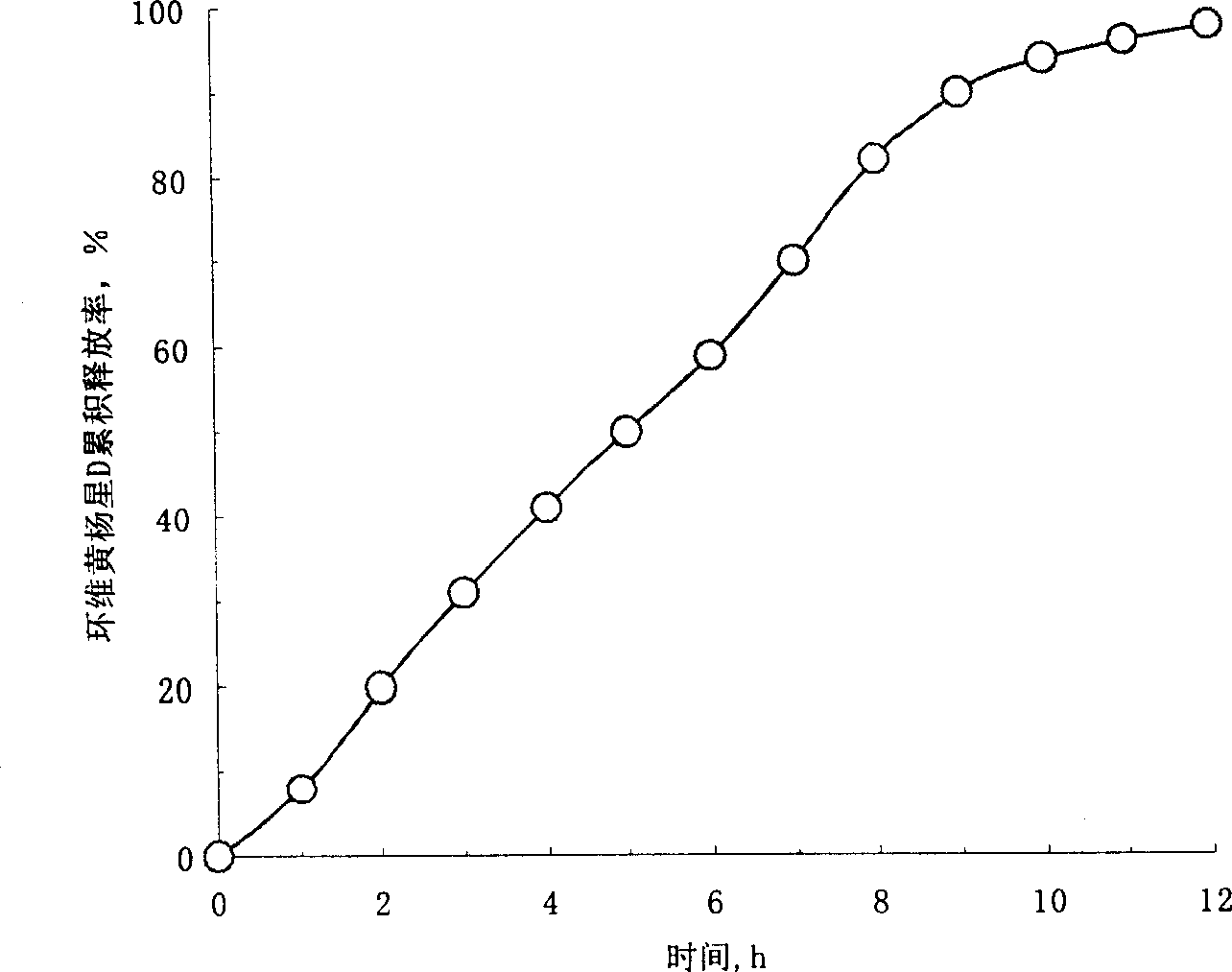
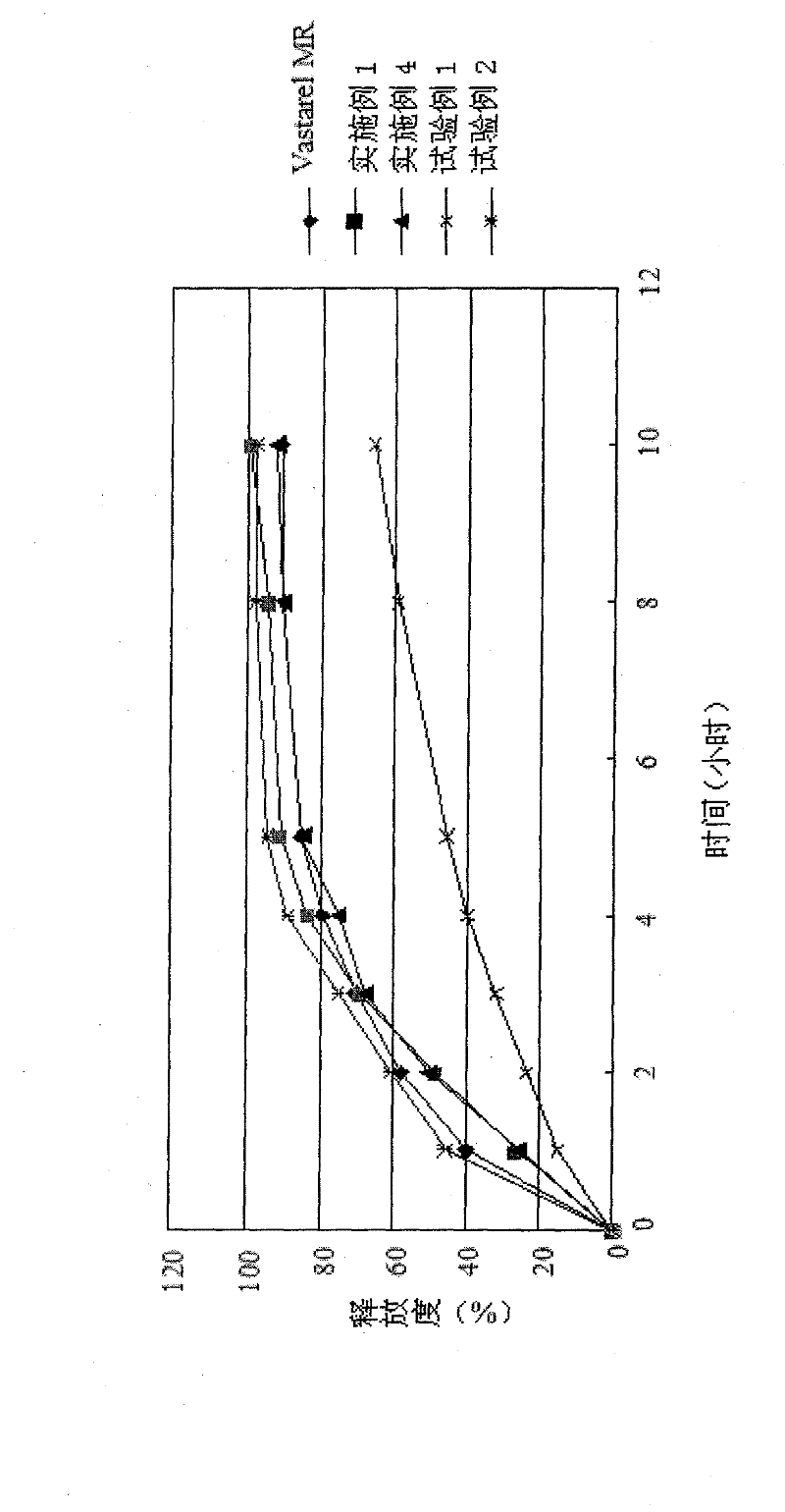
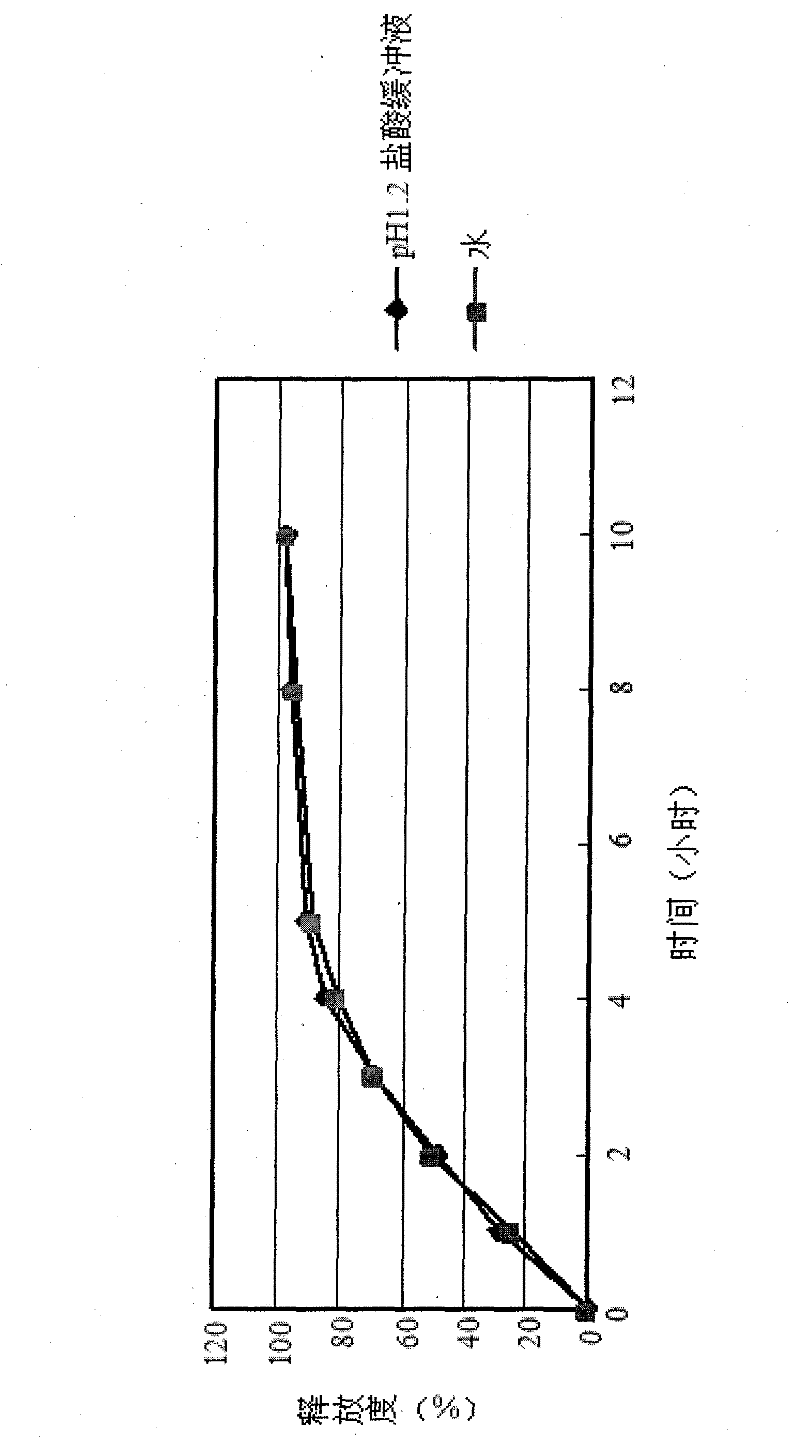
Osmosis pump controlled release preparation contg. Chinese medicine of cyclovirobuxine D, and preparing method thereof
InactiveCN1435178AImprove securityEasy to useOrganic active ingredientsPharmaceutical delivery mechanismControl releaseSemipermeable membrane
A controlled release medicine containing the Chinese-medicinal component cyclovirobuxine D is composed of the core tablet consisting of cyclovirobuxine D, penetrating active substance, acidic substance and high-molecular compound, and the coating film prepared from high-molecular compound. Its advantage is constant release speed.
Owner:BEIJING ZHONGHUI PHARM CO LTD
Trimetazidine dihydrochloride sustained release tablet and preparation method thereof
ActiveCN102670537AImprove complianceSimple stepsOrganic active ingredientsSenses disorderTrimetazidine DihydrochlorideFiller Excipient
The invention belongs to the field of medicinal preparations and particularly relates to a trimetazidine dihydrochloride sustained release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained release tablet takes polyvinyl acetate and mixture of povidone mixture and ethyl cellulose as sustained-release matrix materials and also contains a filling agent, a lubricating agent and other accessories. The release degree of the trimetazidine dihydrochloride sustained release tablet is not influenced by the granularity of raw material; the mixed sustained-release matrix material can be used for controlling the initial burst release of the trimetazidine dihydrochloride at earlier stage; the in vitro release of the sustained release tablet is not influenced by a pH environment; and meanwhile, the administration times of the medicament is reduced to two times from three times of general tablets, so that the administration adaptability of patients is improved. According to the preparation method of the trimetazidine dihydrochloride sustained release tablet, direct tabletting or granulation tabletting through a water-soluble solvent wet method can be adopted; and the preparation process is simple, and thus the trimetazidine dihydrochloride sustained release tablet can be continuously and stably produced in batch.
Owner:QILU PHARMA
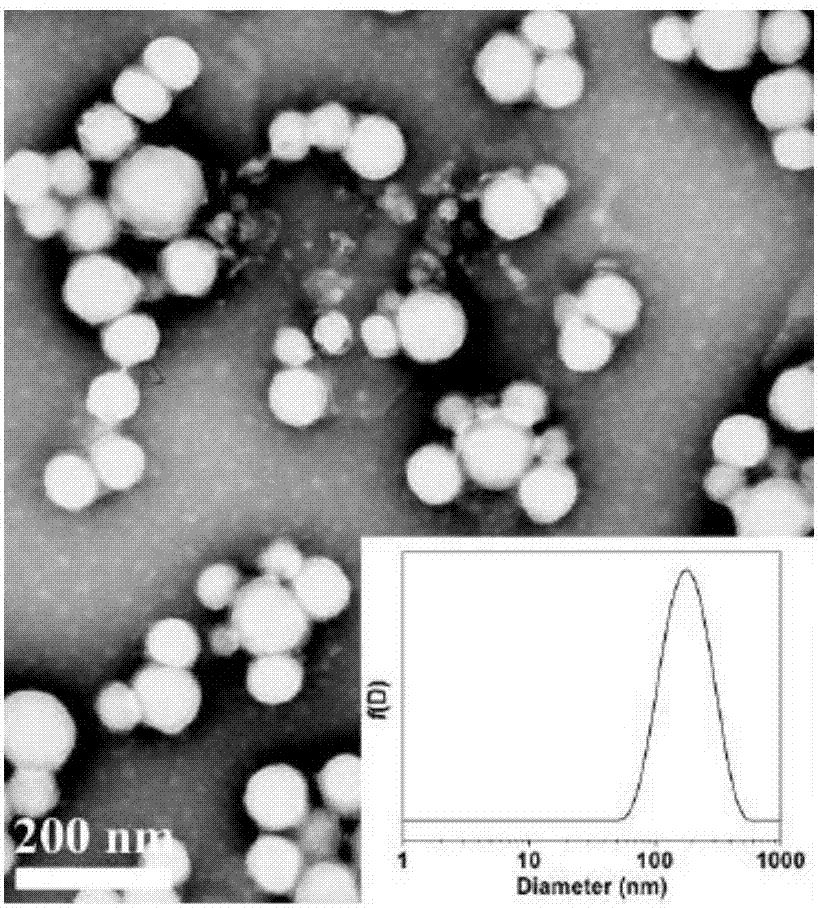
Composite nanocapsule-injectable hydrogel double-drug-loading sustained release system and preparation method thereof
InactiveCN107496382AProlong the action timePlay a fixedNervous disorderAerosol deliveryMedicineNerve repair
The invention discloses a composite nanocapsule-injectable hydrogel double-drug-loading sustained release system, comprising a nanocapsule used for encapsulating a drug, and injectable hydrogel, wherein the average particle size of the nanocapsule is 100nm-1000nm, and the weight volume ratio of the composite nanocapsule to the injectable hydrogel is (1 to 1) to (1 to 10); the injectable hydrogel is prepared from a high molecular polymer and a crosslinking agent; the nanocapsule is prepared from a high molecular base material; a local anesthetic, a nerve repair drug or a contrast medium can be encapsulated in the nanocapsule, so that the effect of slowly releasing the drug is achieved; injectable hydrogel matrix in the double-drug-loading sustained release system can be used for locating a nanocapsule carrier in a human body, so that the whole drug sustained release system is fixed at the site of lesion, and the long-term slow release treatment for the site of lesion is facilitated. The composite nanocapsule-injectable hydrogel double-drug-loading sustained release system has good application prospects in the fields such as analgesia, tumor therapy, local anaesthesia and nerve repair which need local long-term drug slow release.
Owner:SHANGHAI JIAO TONG UNIV +1
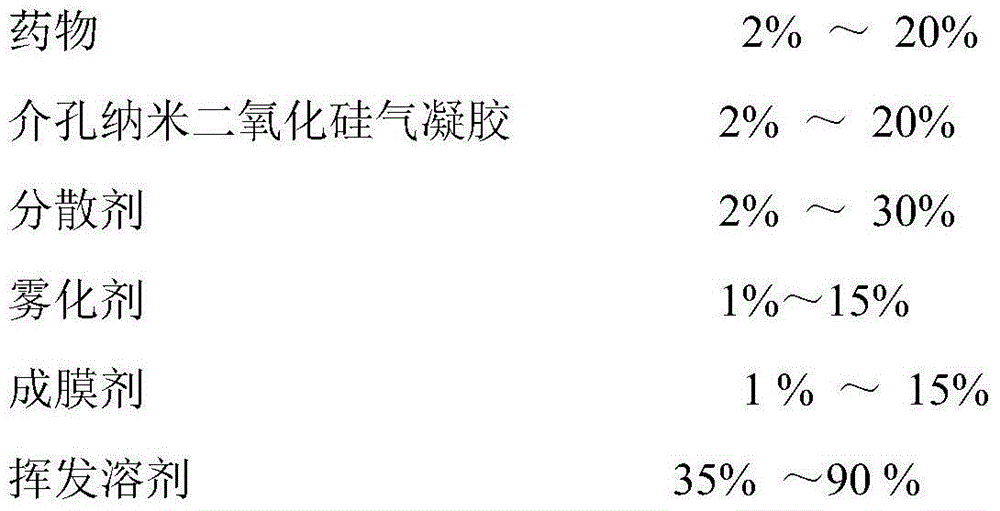
Transdermal spray preparation for plastic mist membranization and preparation method of transdermal spray preparation
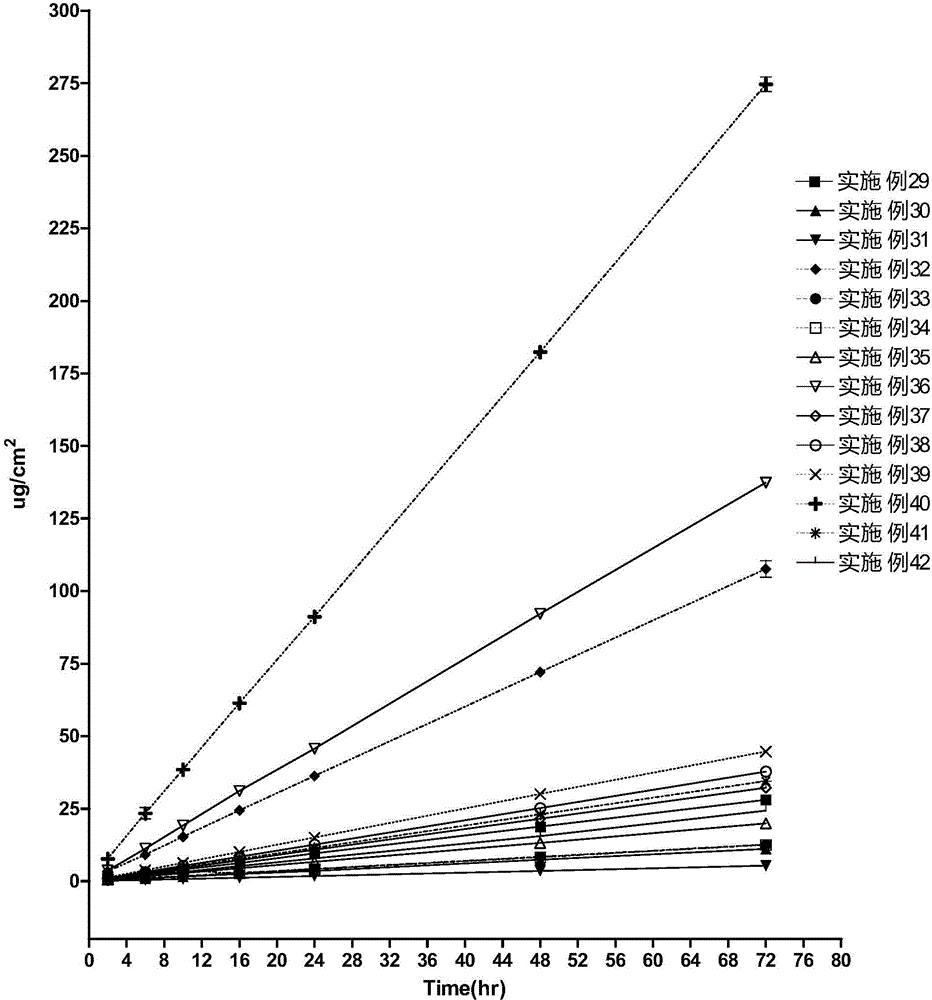
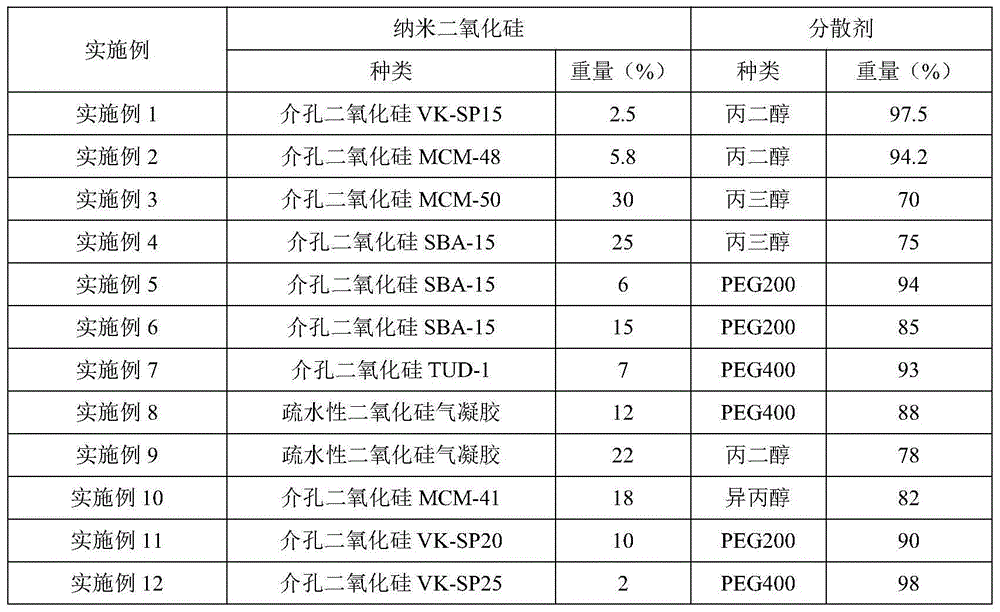
ActiveCN104414975APromote absorptionImprove complianceOrganic active ingredientsAntipyreticPorosityControlled release
The invention discloses a transdermal spray preparation for plastic mist membranization and a preparation method of the transdermal spray preparation. The transdermal spray preparation for plastic mist membranization comprises the following components in percentage by mass: 2%-20% of medicine, 2%-20% of mesoporous nano silica aerogel, 2%-30% of a dispersing agent, 1%-15% of atomizing agent, 1%-15% of a film-forming agent and 35-90% of a volatile solvent. The mesoporous nano silica aerogel is a controlled-release mesoporous nanometer carrier; the porosity of the mesoporous nano silica aerogel is 90%-99.8%; the aperture of the mesoporous nano silica aerogel is 20-100nm; the three-dimensional nano particle size is 2-70nm; the specific surface area is 100-1,000m<2> / g; the density is 0.003-30g / cm<3>; and the heat-conducting coefficient is 0.01-0.018w / m.k. According to the transdermal spray preparation, long-time continuous transdermal penetration of the medicine can be effectively achieved; the constant blood concentration is maintained; and the preparation has the characteristics of high transdermal absorption rate, high transdermal absorption amount and is stable and efficient.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Sustained release preparation of roxithromycin
InactiveCN1415305AGood curative effectGreat tasteAntibacterial agentsOrganic active ingredientsRoxithromycinAdhesive
A showly-releasing roxithromycin contains roxithromycin (30-80 wt.%), slow-releasing assistant (hydroxypropylmethyl cellulose, etc) (10-40 wt.%), and others (hole-forming agent, adhesive, lubricant, etc). Its advantages are long active period (24 hrs.), and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Memantine hydrochloride slow-release dry suspension and preparation method thereof
ActiveCN103417483ASimple preparation processEasy to operatePowder deliveryPharmaceutical product form changeDrug release rateMemantine Hydrochloride
The invention relates to a memantine hydrochloride slow-release dry suspension and a preparation method thereof. The memantine hydrochloride slow-release dry suspension comprises the following materials, by weight: 5-20% of memantine hydrochloride, 10-40% of ion exchange resin, 0.5-10% of a hydrophilic or water-soluble accessory, 5-20% of a dressing material and 10-50% of other accessories. The preparation technology of the memantine hydrochloride slow-release dry suspension is simple, easy for operation and control, and suitable for industrialized large production. The obtained memantine hydrochloride slow-release dry suspension has stable drug release rate and high accumulation drug release rate; after administration, patients can obtain stable plasma concentration level, so as to effectively reduce administration frequency and toxic and side effects; meanwhile, the memantine hydrochloride slow-release dry suspension solves the problems of medication validity, security and compliance for the elderly.
Owner:HAINAN PULIN PHARMA +1
Sustained-release preparation of compound metformin hydrochloride rosiglitazone and preparation method thereof
InactiveCN101683340AImprove complianceReduce the frequency of takingOrganic active ingredientsMetabolism disorderMalaiseMedicine
The invention relates to a sustained-release preparation of compound metformin hydrochloride rosiglitazone and a preparation method thereof; wherein, rosiglitazone and metformin hydrochloride have slow releasing characteristic in an in-vitro dissolution test, the rosiglitazone is released by 10-30 percent for a first hour, and is released by 60-80 percent for a fourth hour, and is released by morethan 80 percent for an eighth hour; the metformin hydrochloride is released by 15-40 percent for a first hour, is released by 50-70 percent for a fourth hour, and is released by more than 75 percentfor an eighth hour. The formula is mild and has prolonged action of glucose reduction, so as to avoid adverse reaction such as hypoglycemia and gastrointestine malaise, reduce taking times (one or twotimes each day), and improve compliance of a patient. The invention discloses the in-vitro drug-releasing characteristic of the sustained-release preparation and the preparation method thereof.
Owner:北京利乐生制药科技有限公司
Transdermal patch containing proton pump inhibitor drug and preparation method of transdermal patch
InactiveCN107929268AAvoid oral gastrointestinalReduce the influence of gastrointestinal environmentOrganic active ingredientsDigestive systemTransdermal patchBlood concentration
The invention relates to a transdermal patch containing a proton pump inhibitor drug. The transdermal patch is characterized by mainly comprising a back lining layer, a drug library layer and an anti-sticking layer, wherein the drug library layer contains the following components in percentage by weight: 0.1%-40% of active components, 20%-90% of a drug library matrix, 1%-5% of a stabilizer, 1%-30%of a transdermal absorption enhancer and 0-20% of a dispersing agent. According to the transdermal patch, long-time continuous transdermal penetration of the drug can be effectively realized, a constant blood concentration can be maintained, and a preparation is high in transdermal absorption speed and transdermal absorbing capacity; and the transdermal patch has the characteristics of stability,high efficiency, relatively high pharmaceutical safety and the like.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
Policosanol-containing solid dispersion, controlled release tablet and preparation method thereof
ActiveCN114053241ASmall particle sizeControlled release rateHydroxy compound active ingredientsMetabolism disorderActive agentPolicosanol
The invention relates to the technical field of policosanol preparations, and provides a policosanol-containing solid dispersion, the policosanol-containing solid dispersion comprises policosanol, a surfactant and a carrier, and the surfactant is a nonionic surfactant used for auxiliary dispersion; the particle size of the solid dispersion is not greater than 5 microns. The invention provides a controlled-release tablet containing policosanol and a preparation method of the controlled-release tablet. The tablet comprises a double-layer tablet core and a controlled-release film layer wrapping the double-layer tablet core; the double-layer tablet core is composed of a drug-containing layer and a boosting layer; the drug-containing layer comprises policosanol, a surfactant, a filler, a carrier, an adhesive and a lubricant; the content of policosanol in each controlled-release tablet is more than or equal to 5mg. The particle size of policosanol in the drug can be greatly reduced, the dispersity and solubility of policosanol can be improved, the release rate of policosanol can be controlled, the release time and the medicine taking interval can be prolonged, and then the bioavailability of policosanol in a human body and the medicine taking compliance of policosanol can be improved.
Owner:湖北中古生物制药有限公司
Ibuprofen slow-release suspension and preparation method thereof
InactiveCN101810571AMask the spicinessImprove complianceOrganic active ingredientsAntipyreticCurative effectSuspending Agents
The invention relates to an ibuprofen slow-release suspension and a preparation method thereof, relating to medicines, in particular to the technical field of the pharmacy of chemical medicines. The preparation method of the ibuprofen slow-release suspension comprises the following steps of: firstly mixing a flavoring agent, an antiseptic and pure water, and heating to be prepared into simple syrup; preparing ibuprofen and glutin into an ibuprofen microcapsule; then mixing the simple syrup, the ibuprofen microcapsule and a suspending agent, adding edible pigments and the flavoring agent, and uniformly stirring. The invention has the advantages of simple process, easy production and operation, good curative effect of the medicines and convenient carrying and preservation.
Owner:祝瑞章
Sustained release composns preparation of Fudosteine and clarithromycin
InactiveCN1833652AEasy to useGood treatment effectPowder deliveryOrganic active ingredientsFUDOSTEINEDisease
A slowly-releasing composite medicine for treating the diseases in respiratory system is prepared from Fuduositan (15-30 Wt %), clarithromycin (10-40), and auxiliary (rest) including paralyser.
Owner:GUANGZHOU YOUYI PHARMA SCI & TECH DEV +1
Glipizide enteric sustained-release preparation composition and method for preparing the same
InactiveCN101502517AReduce the frequency of takingSlow release rateOrganic active ingredientsMetabolism disorderPatient complianceIrritation
The invention discloses a glipizide enteric sustained-release preparation combination and a preparation method thereof. The glipizide enteric sustained-release preparation combination is mainly prepared from glipizide bulk drugs, sustained-release materials, enteric materials and other appropriate auxiliary materials. The glipizide enteric sustained-release preparation provided by the invention can not only prevent glipizide from disintegrating in the stomach and causing irritation to gastric mucosa and avoid the adverse reactions caused by the administration, such as nausea, abdominal pain, diarrhea and the like, but also deaccelerate the release rate of drugs, reduce the frequency of administration and improve the patient compliance. The invention provides a novel form of drug featuring higher safety and better patient compliance and having the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Zinc gluconate pellets and preparation method thereof
InactiveCN101703478AGood dispersionQuick effectOrganic active ingredientsMetabolism disorderGluconic acidDissolution
The invention provides a zinc gluconate pellet preparation, which is prepared from zinc gluconate and pharmaceutic adjuvant, and is characterized in that: the pharmaceutic adjuvant is an excipient and an adhesive, wherein in the pellet preparation, the weight percent of the zinc gluconate is 10 to 80 percent, the weight percent of the excipient is 12 to 89 percent and the weight percent of the adhesive is 1 to 8 percent. The pellet preparation can be prepared into a slow release preparation or an enteric-coated preparation. The zinc gluconate pellet preparation has high dissolution rate, and high bioavailability; and the method is simple, convenient, and easy to operate.
Owner:JF PHARMALAND TECH DEV
Microsphere for injuecting 'Shuang Hualian' for treating viral disease of bird and preparation thereof
InactiveCN101347523AImprove complianceEfficient killingAntiviralsPharmaceutical non-active ingredientsDiseaseSide effect
The invention relates to a microsphere used for injecting Shuanghuanglian which is used for treating poultry viral diseases and a preparation method thereof; the microsphere consists of 2-60% of Chinese medicinal material extracts which are taken as active ingredients and 39-95% of biodegradable medicinal high-molecular excipients which have microsphere weight and have molecular weight ranging from 4,000 to 9,000,000 by weight proportion; wherein, the weight percentage of medicinal materials is as follows: 13-40% of Chinese goldthread, 15-45% of honeysuckle, 25-61% of forsythia and 11-40% of milk veteh. The medicines are prepared into microspheres by emulsifying dispersion method. The beneficial effects of the invention is that: (1) developing a new medicine preparation for preventing and treating poultry viral diseases and increasing disease resistance of the body; (2) reasonable prescription and strong antiviral ability; (3) advanced technique, stable preparation and long storage time; (4) broad medicine sources and no toxicity and side effect, little drug resistance generated and meeting the international sanitary inspection requirements. Especially, the microsphere has significant effect on the treatment of poultry infectious bursal disease, untypical Newcastle disease, infective bronchitis, etc.
Owner:TIANJIN SHENGJI GRP CO LTD
Compound medicinal composition for reducing blood pressure, and compound tablet for reducing blood pressure
ActiveCN102327263AProlong biological half-lifeReduce the frequency of takingOrganic active ingredientsPill deliveryHypotensive actionCompounded preparations
The invention discloses a compound medicinal composition for reducing blood pressure and a compound tablet for reducing blood pressure. The compound medicinal composition for reducing blood pressure comprises levamlodipine or pharmaceutically acceptable salt thereof and indapamide, wherein the weight ratio of indapamide to levamlodipine is 1:(1.5-12). The novel compound preparation for reducing blood pressure improves the effect of reducing blood pressure by using synergism among medicines, has a stable effect of reducing blood pressure and excellent cost performance, reduces a side effect, and can be widely popularized and applied to crowds. The compound tablet for reducing blood pressure has high stability and can effectively prolong a validity period.
Owner:KANGYA OF NINGXIA PHARMA +1
Preparation method of puerarin sustained-release dropping pill
InactiveCN104622828AImprove Medication AdherenceReduce releaseOrganic active ingredientsSenses disorderWater bathsSide effect
The invention relates to a preparation method of a puerarin sustained-release dropping pill. The method comprises the following steps: (1) weighing hydrophobic matrix and hydrophilic matrix, fully melting and mixing evenly under a water bath heating condition, adding puerarin powder and mixing evenly; (2) starting the dropping pill, and preheating for 30 minutes; (3) starting a pill dropping machine stirring system and stirring for 10 minutes; (4) turning on a condensate liquid level adjusting knob and adjusting the dropping distance; (5) mounting a dripping head in a pill dropping machine; (6) dropping a liquid medicine into condensate; (7) collecting sustained-release dropping pills; and (8) sucking up condensate on the surfaces of the sustained-release dropping pills with filter paper and medical gauze, so as to obtain the puerarin sustained-release dropping pills. An optimal preparation technology of the novel puerarin sustained-release dropping pill is optimized; the targets of delaying drug release and reducing toxic and side effects can be reached; meanwhile, the medication frequency can be reduced; the medication compliance of patients is improved; the method has relatively large application value; and important reference is provided for pharmaceutical companies and clinical research and development.
Owner:TAIYUAN INST OF TECH
Salidroside sustained-release tablet and preparation method thereof
InactiveCN102258493AReduce the frequency of takingReduce the fluctuation of blood drug concentration in the bodyOrganic active ingredientsPharmaceutical delivery mechanismOral medicationSizing
The invention provides a salidroside sustained-release tablet and a preparation method thereof. This slow-release tablet includes 2.0-60.00 parts by weight of salidroside, and 10.00-95.00 parts by weight of slow-release blocking material, wherein the slow-release blocking material includes: hydroxypropylmethylcellulose or hydroxypropylmethylcellulose Mixture with other cellulose. Its preparation method is to mix salidroside, slow-release retarding materials and fillers in the prescription amount of raw materials, add binders to make soft materials, granulate, dry, granulate, and granulate according to the conventional process of tablets. The dry granules after granulation are added with a lubricant, mixed evenly, and then compressed into tablets. The salidroside sustained-release tablet of the present invention can maintain a longer therapeutic effect after oral administration.
Owner:NANJING JIZHONG MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com