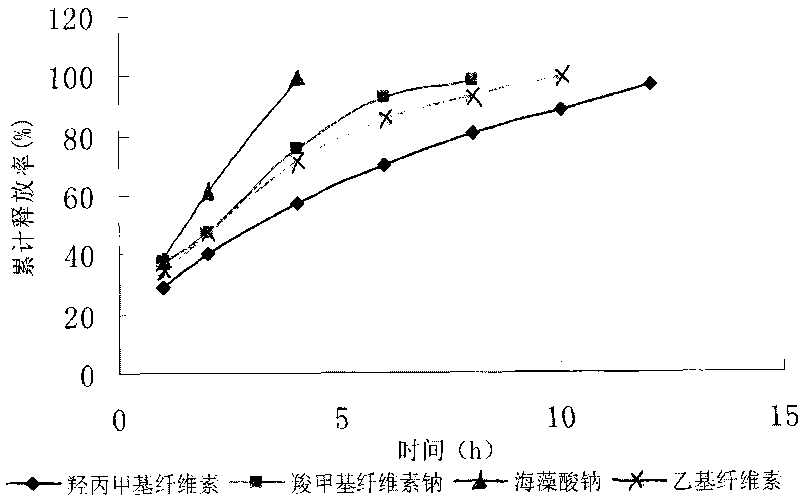
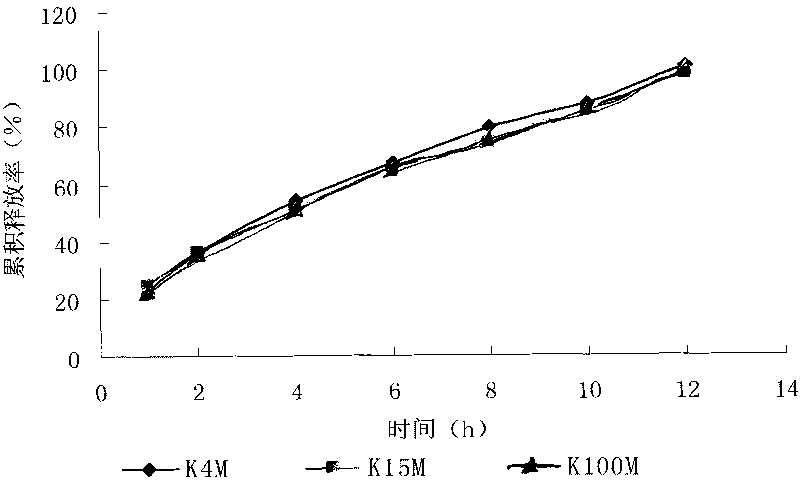
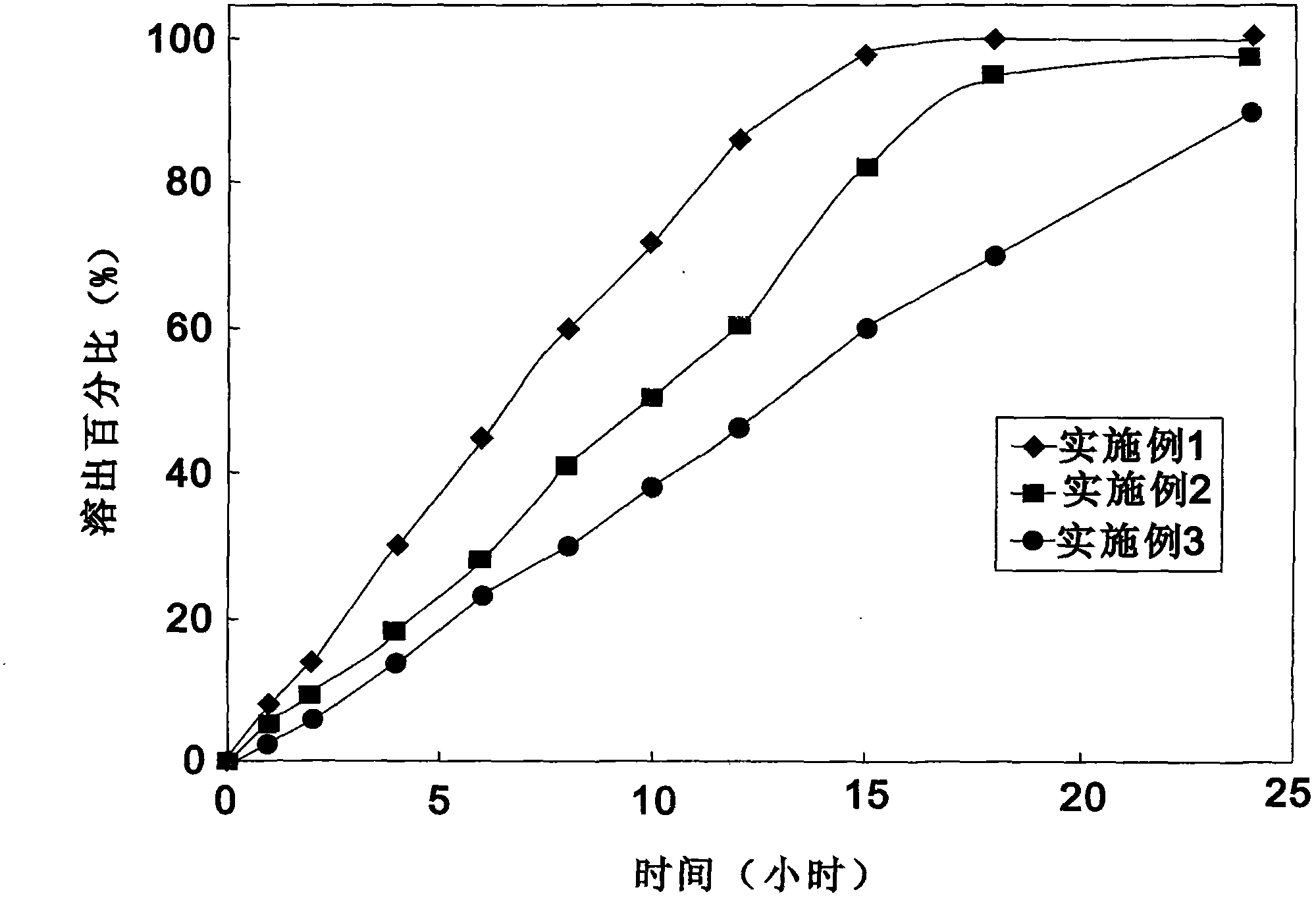
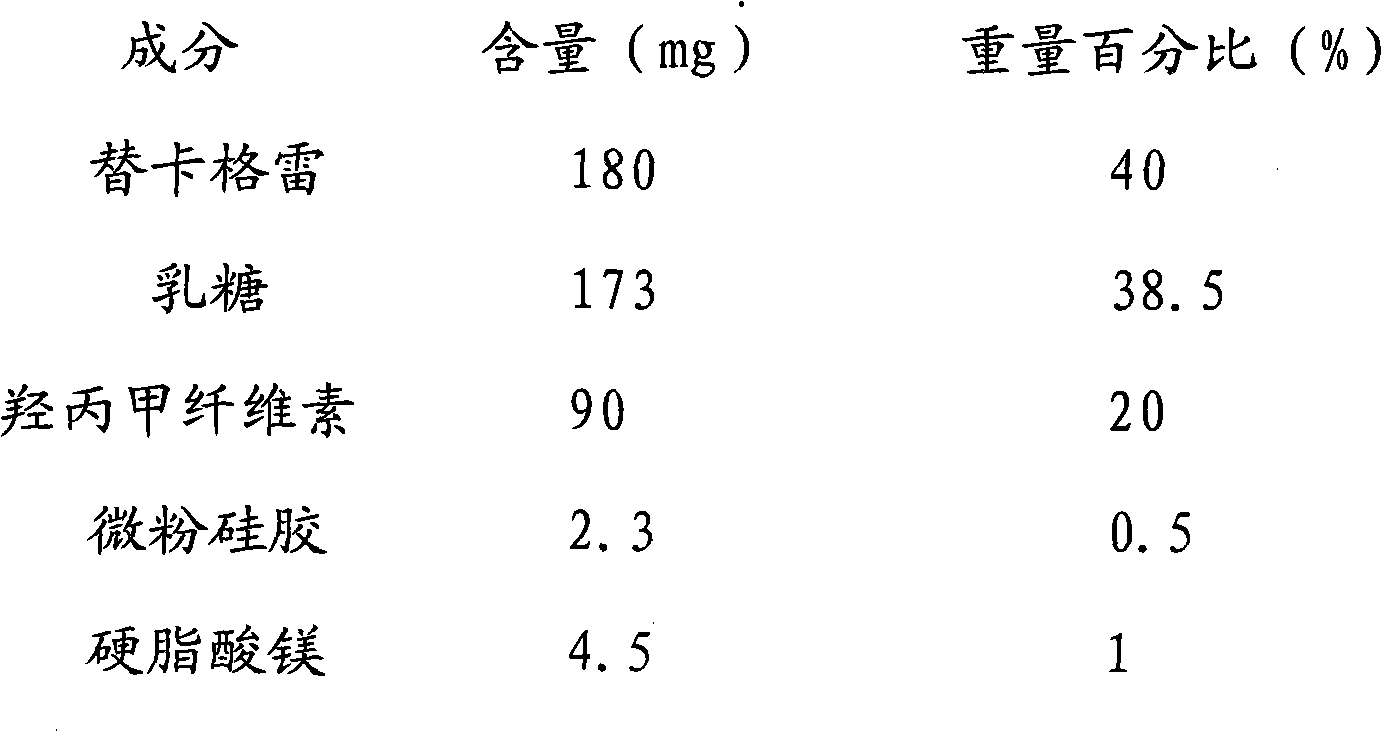
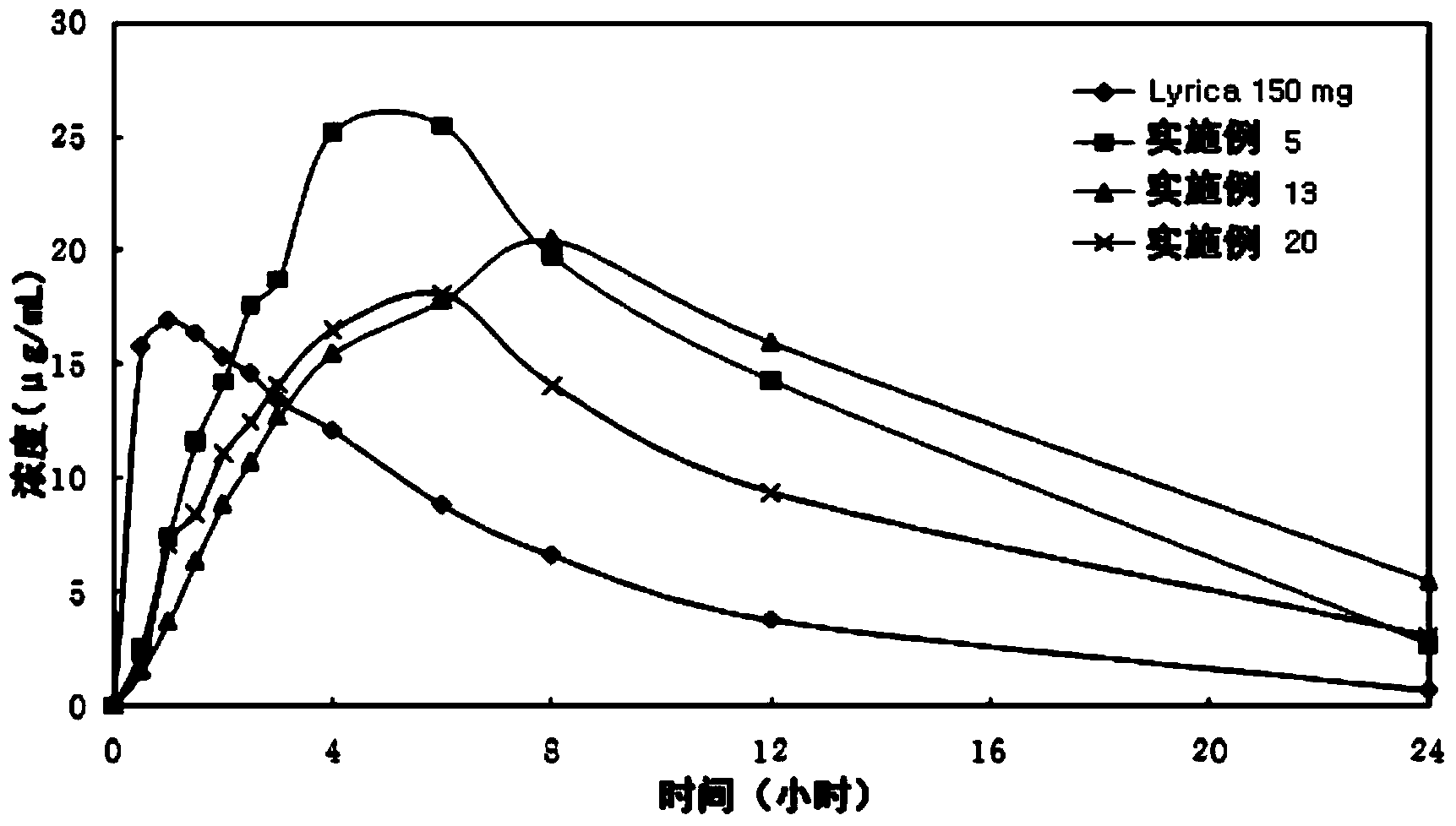
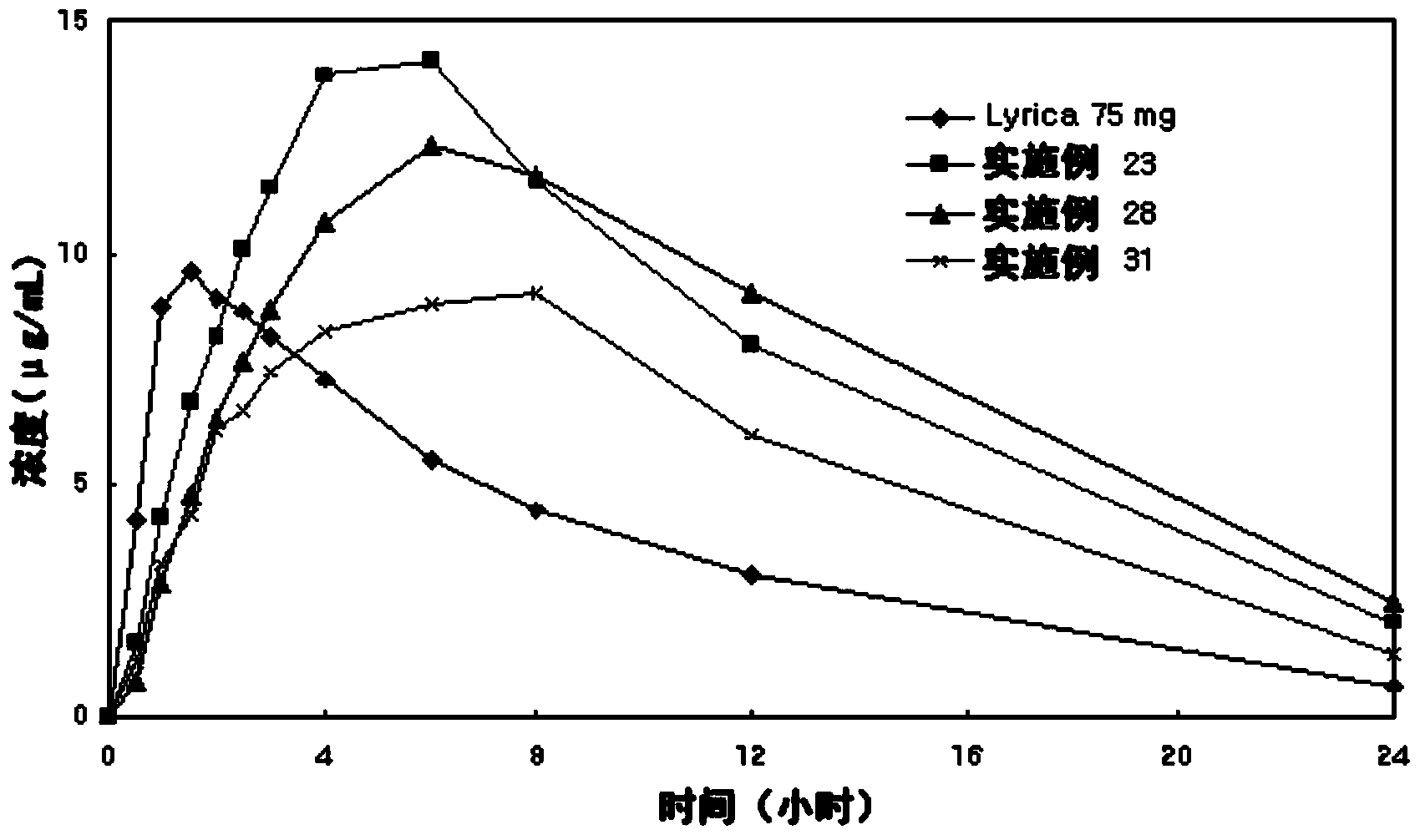
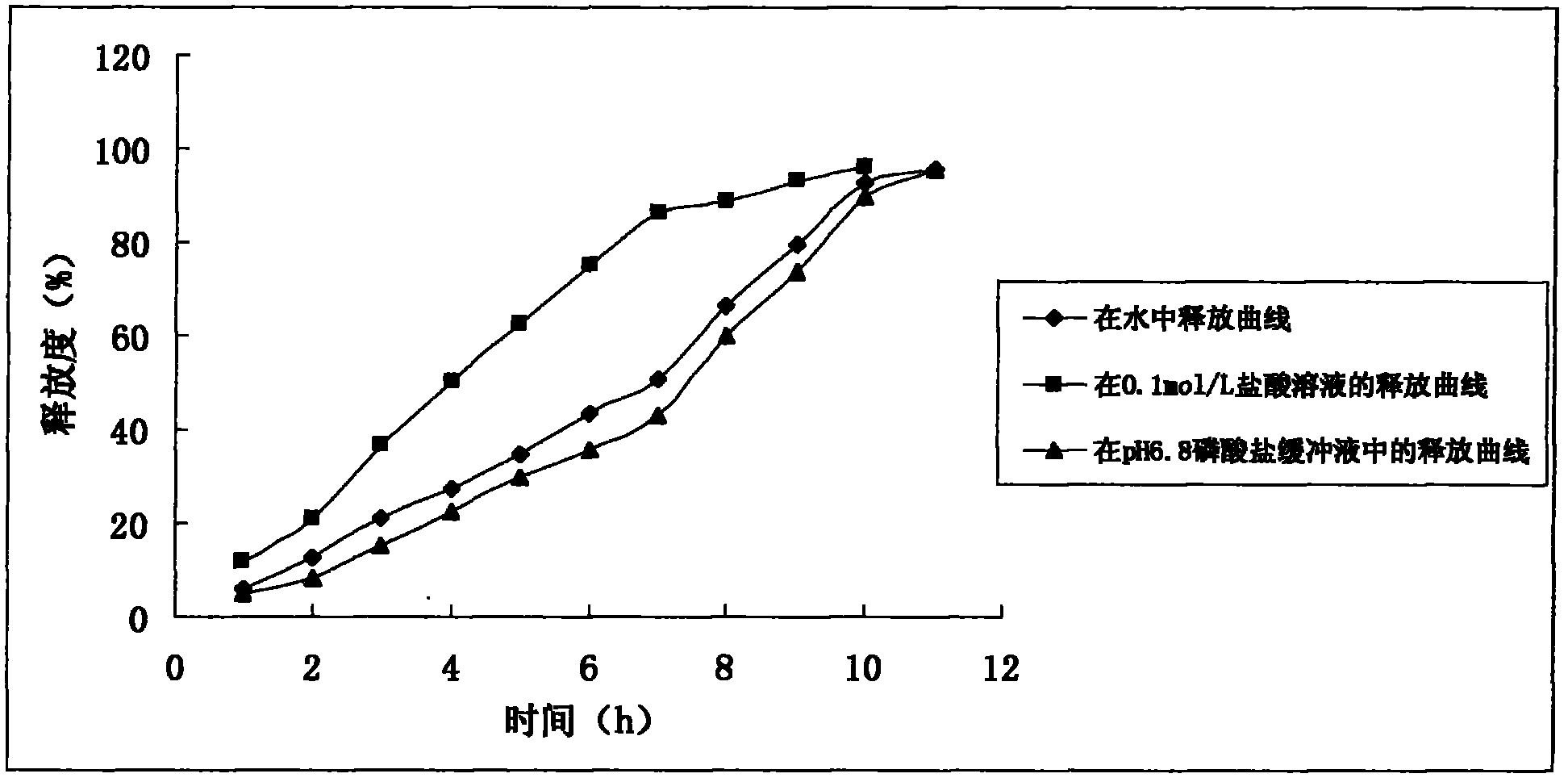
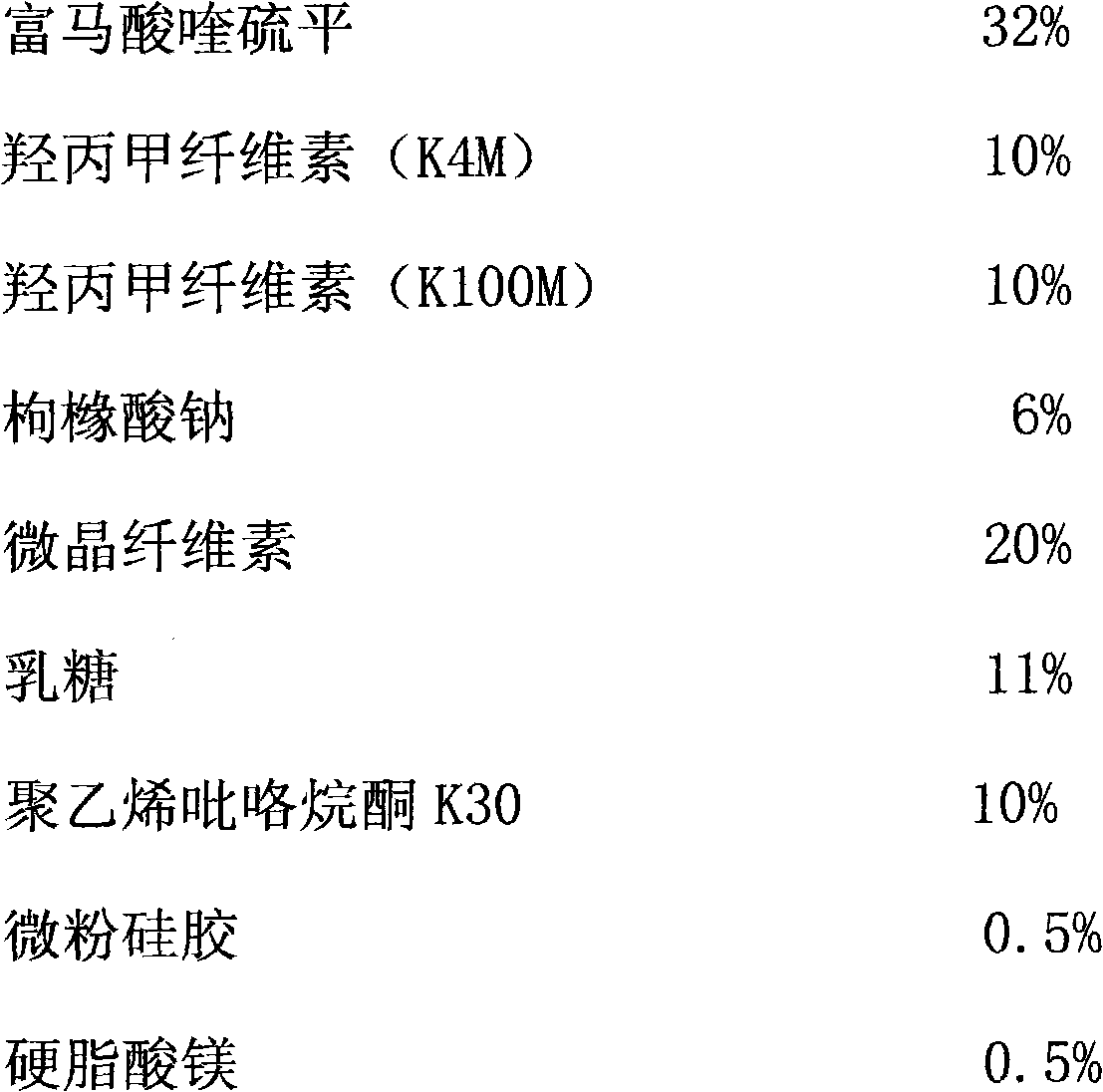
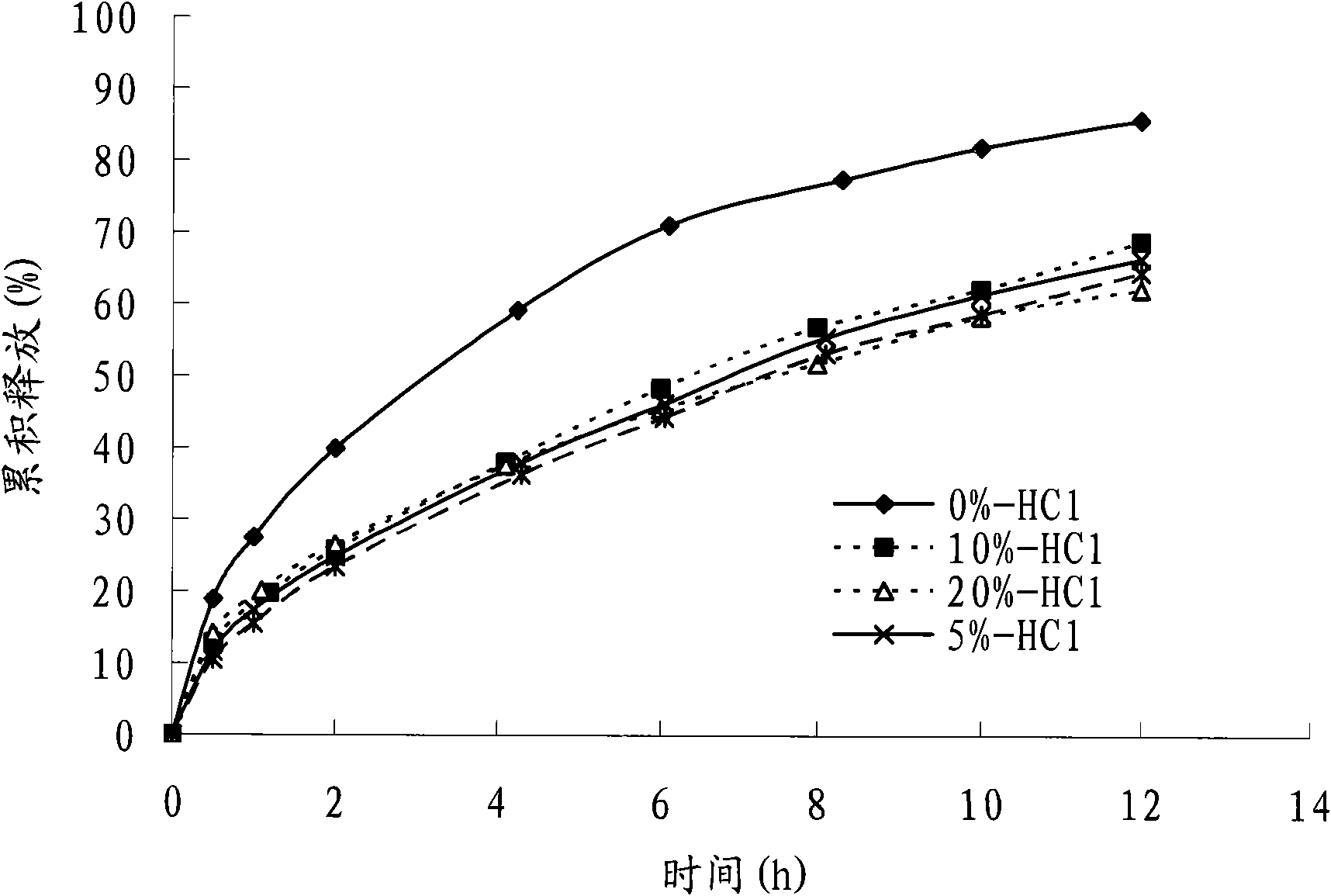
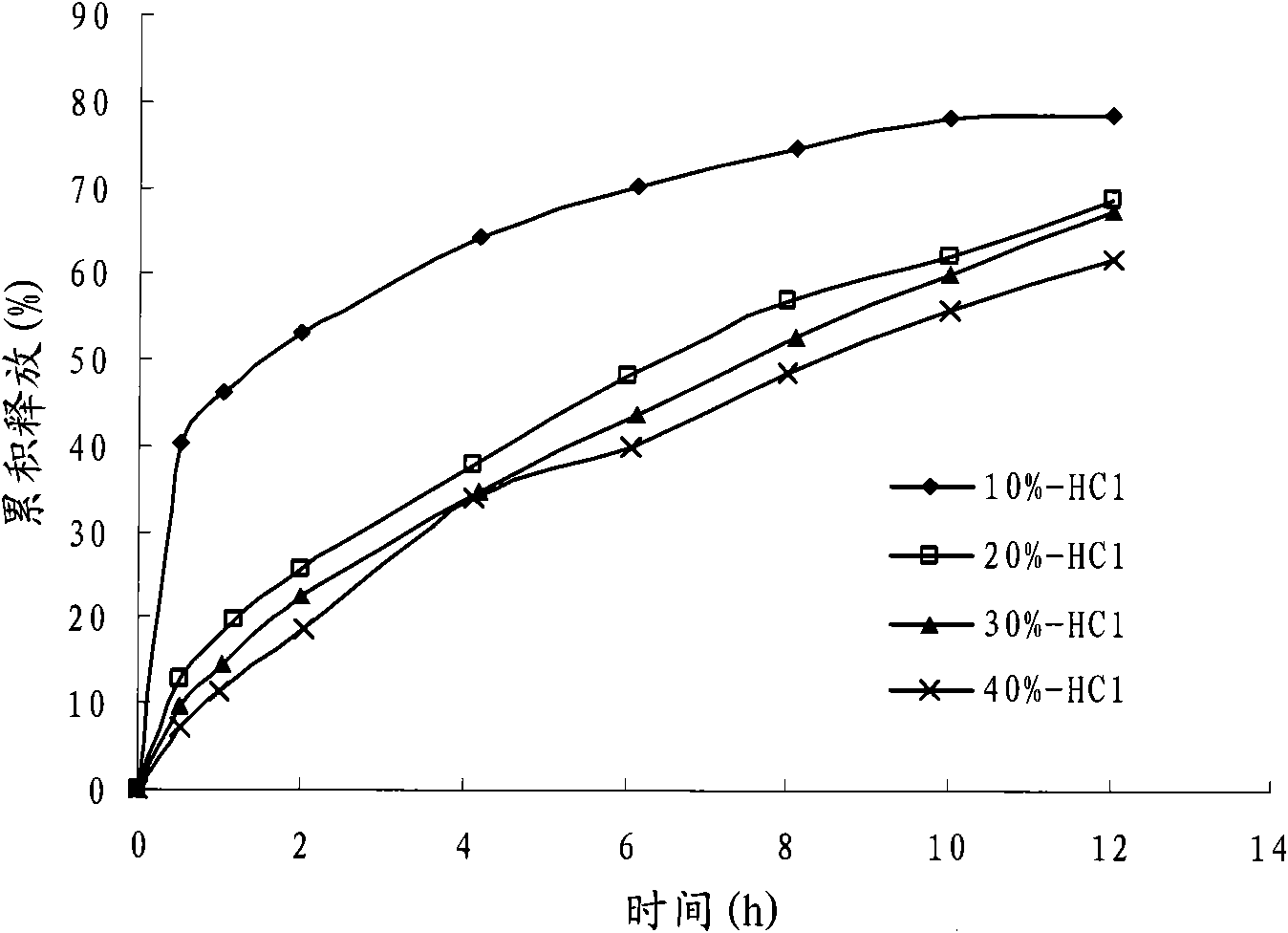
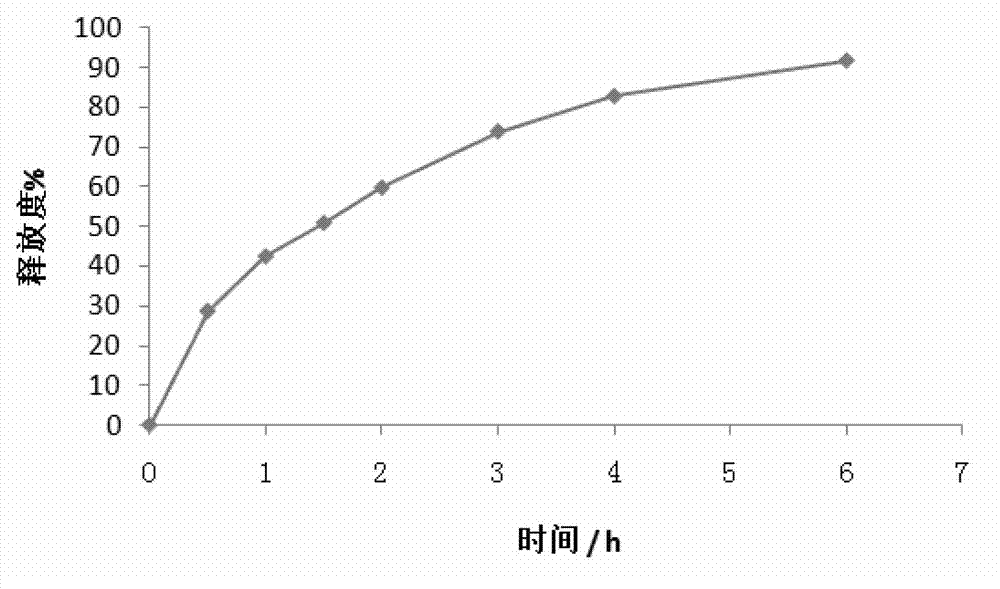
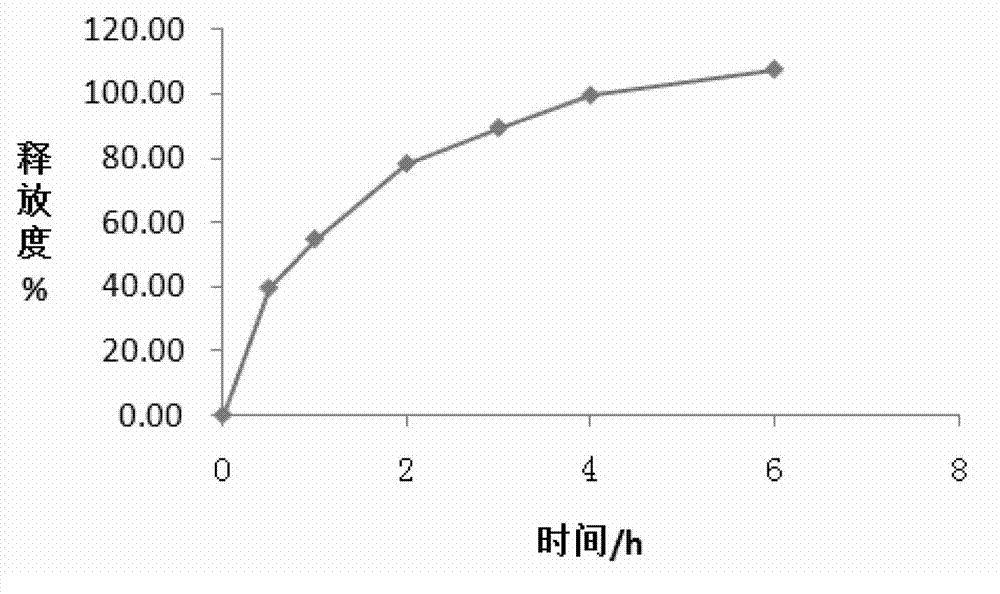
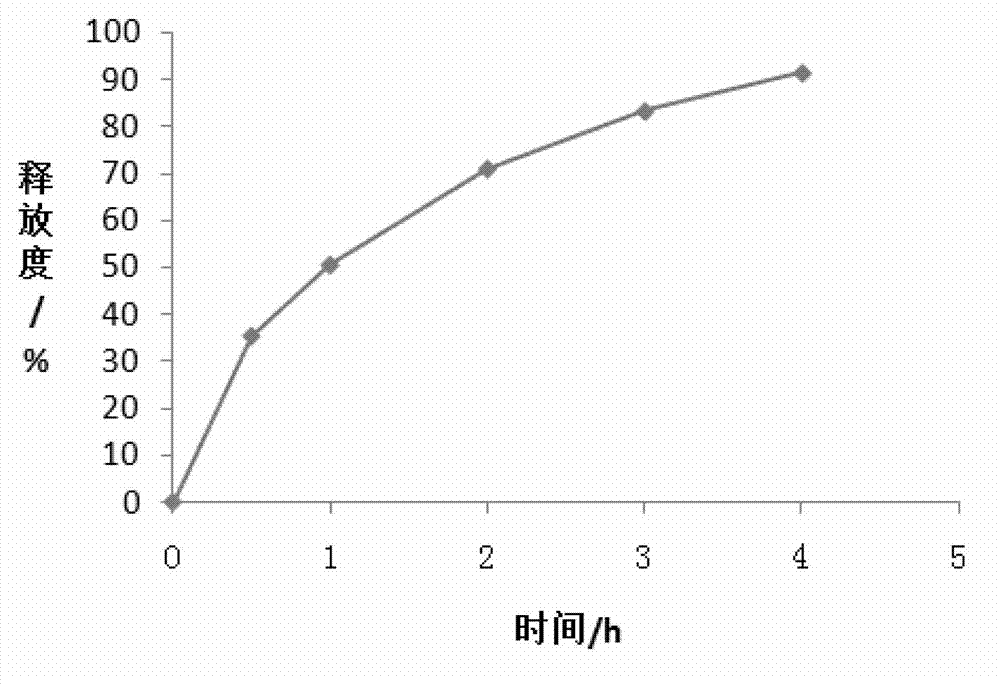
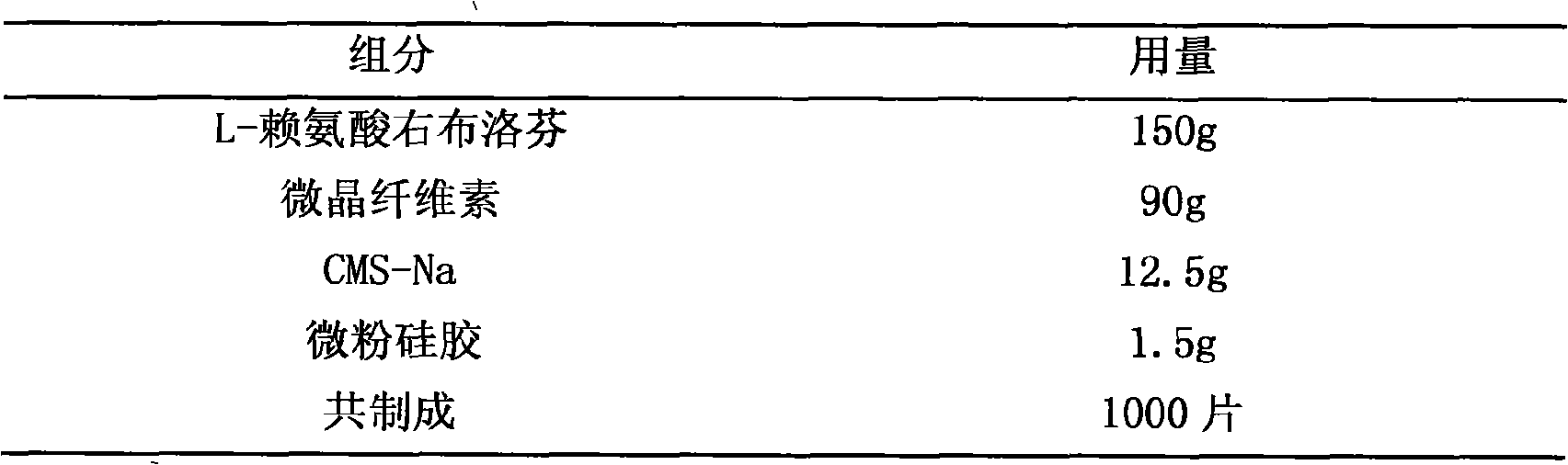
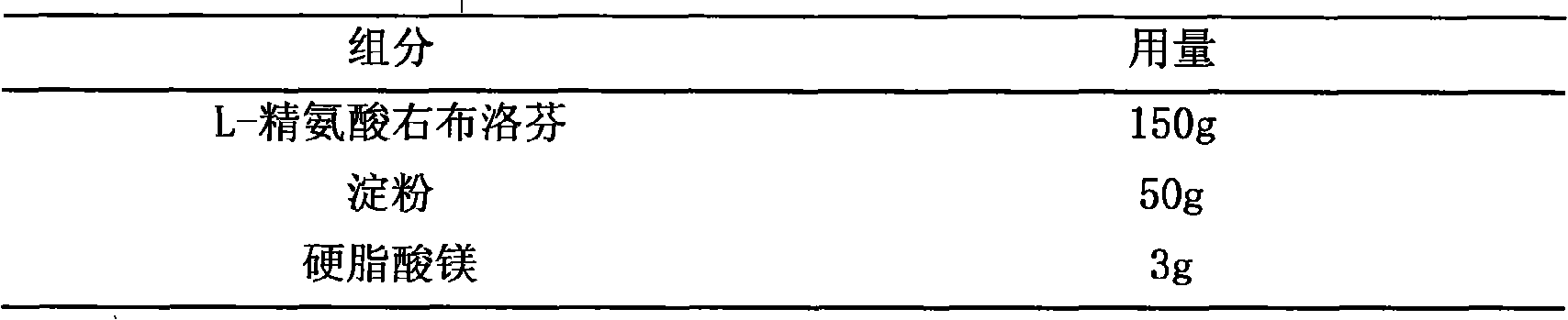
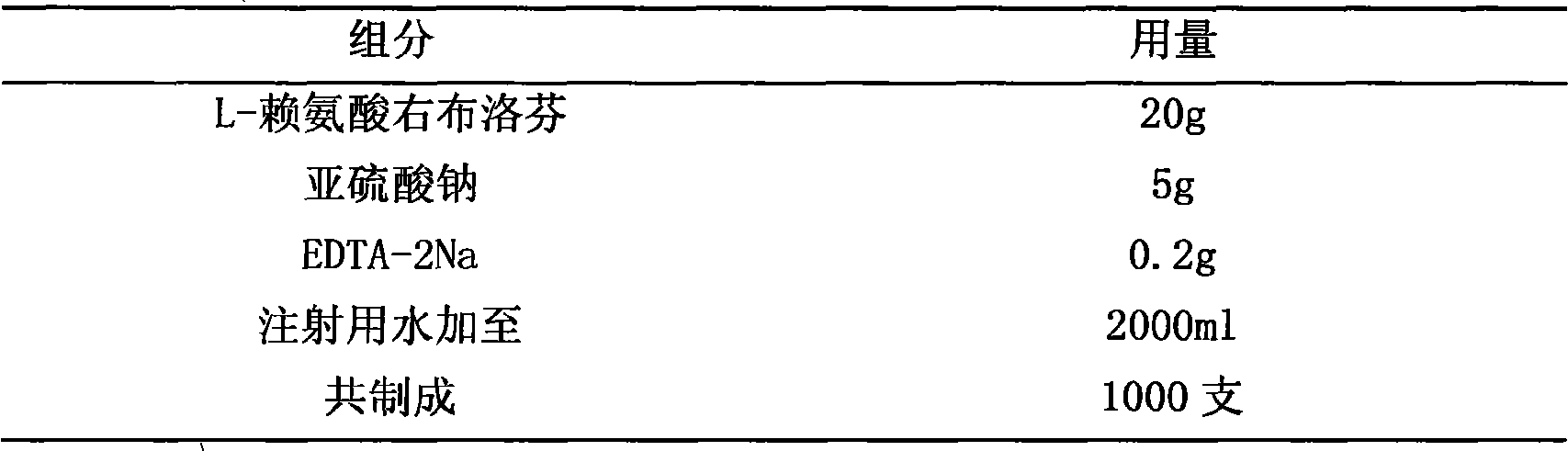
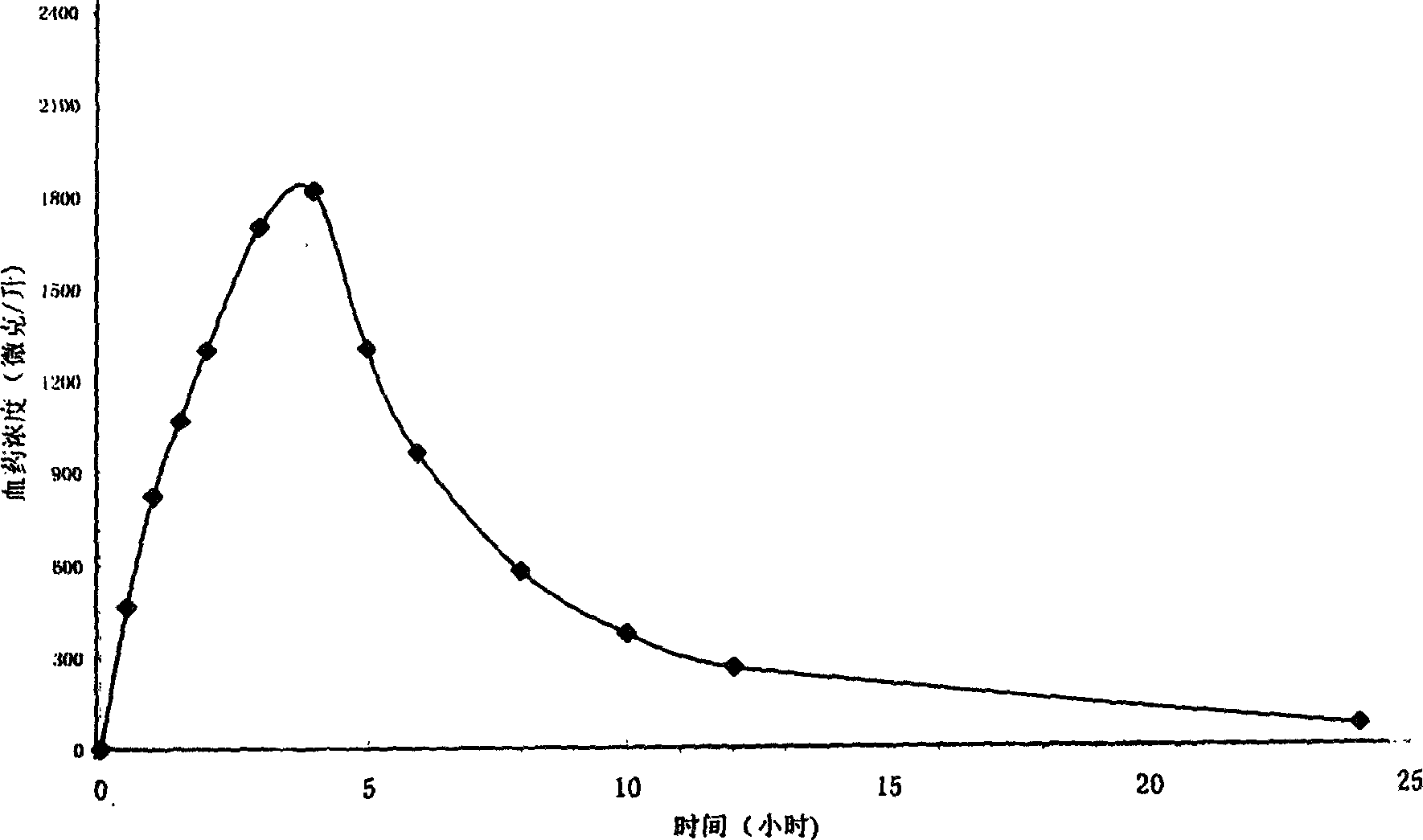
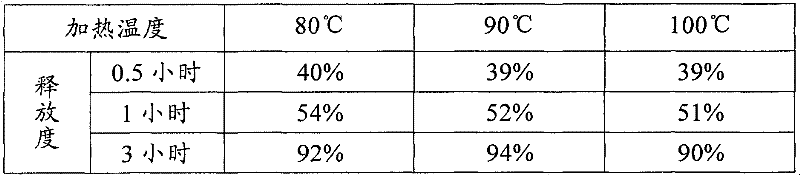
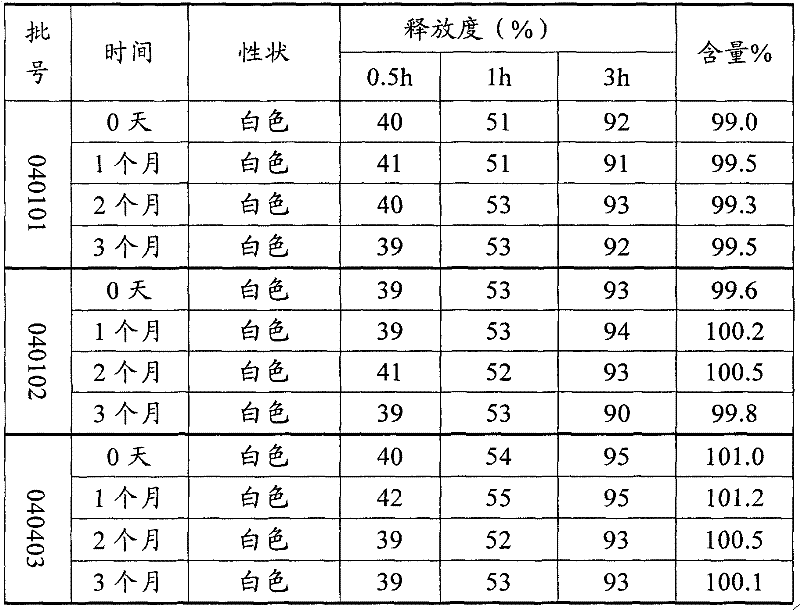
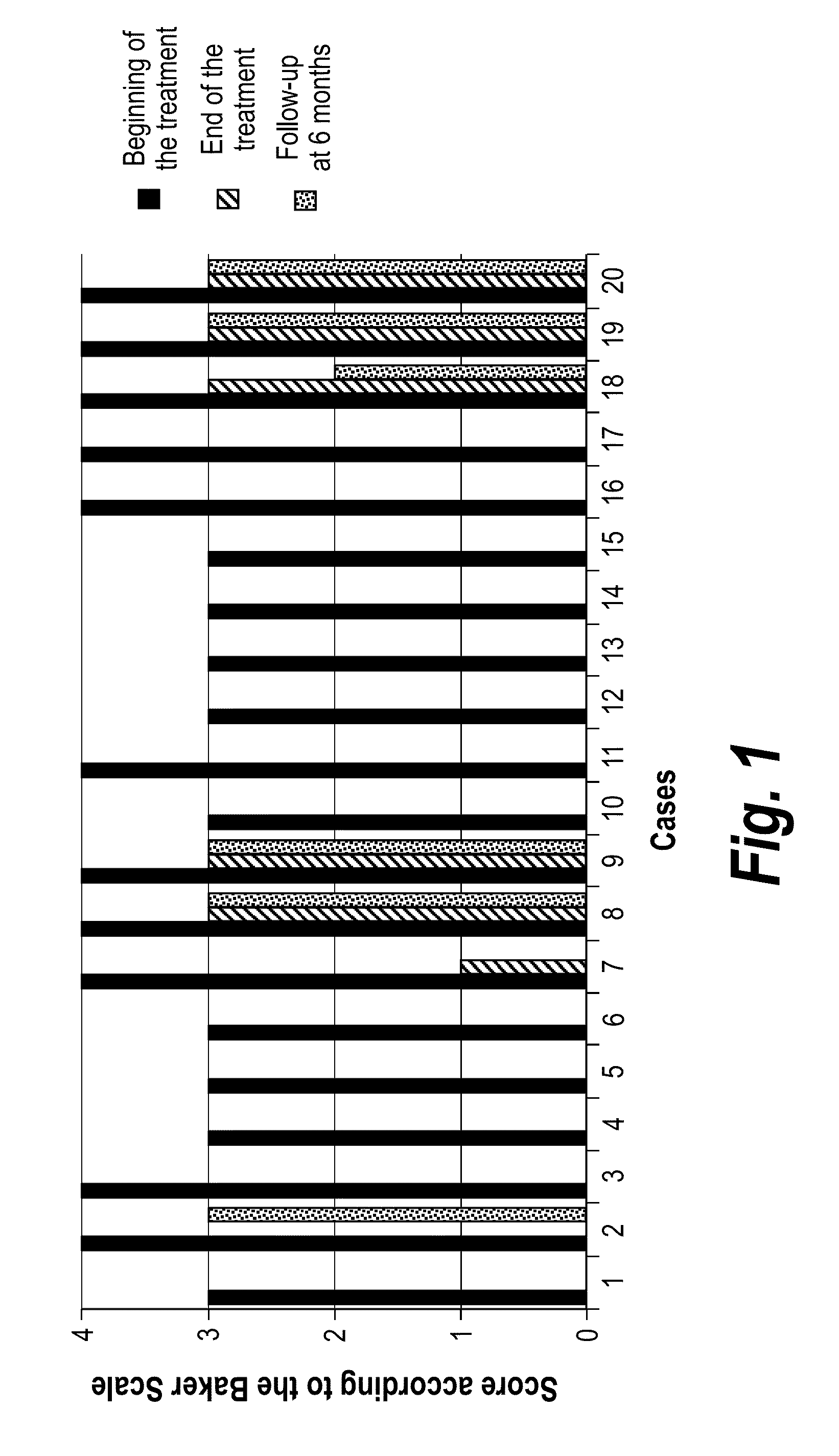
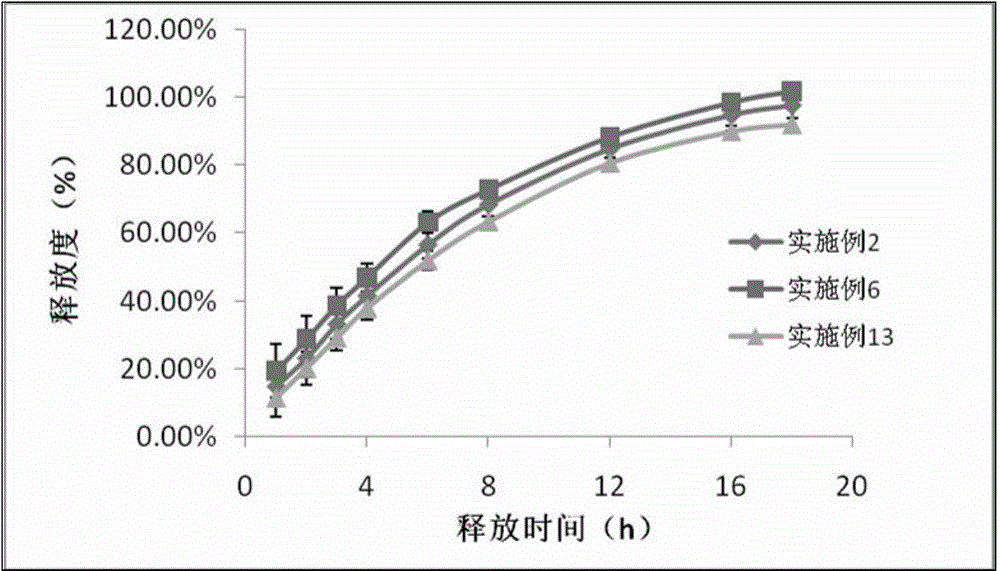
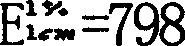Patents
Literature
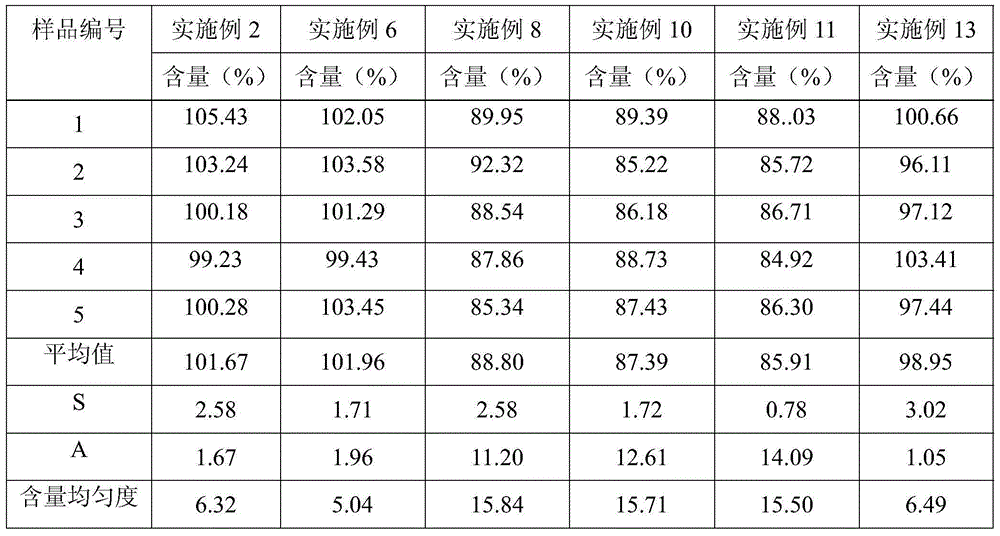
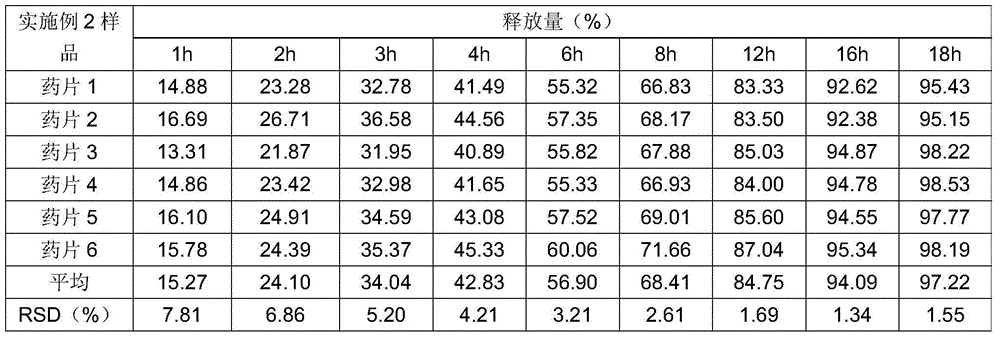
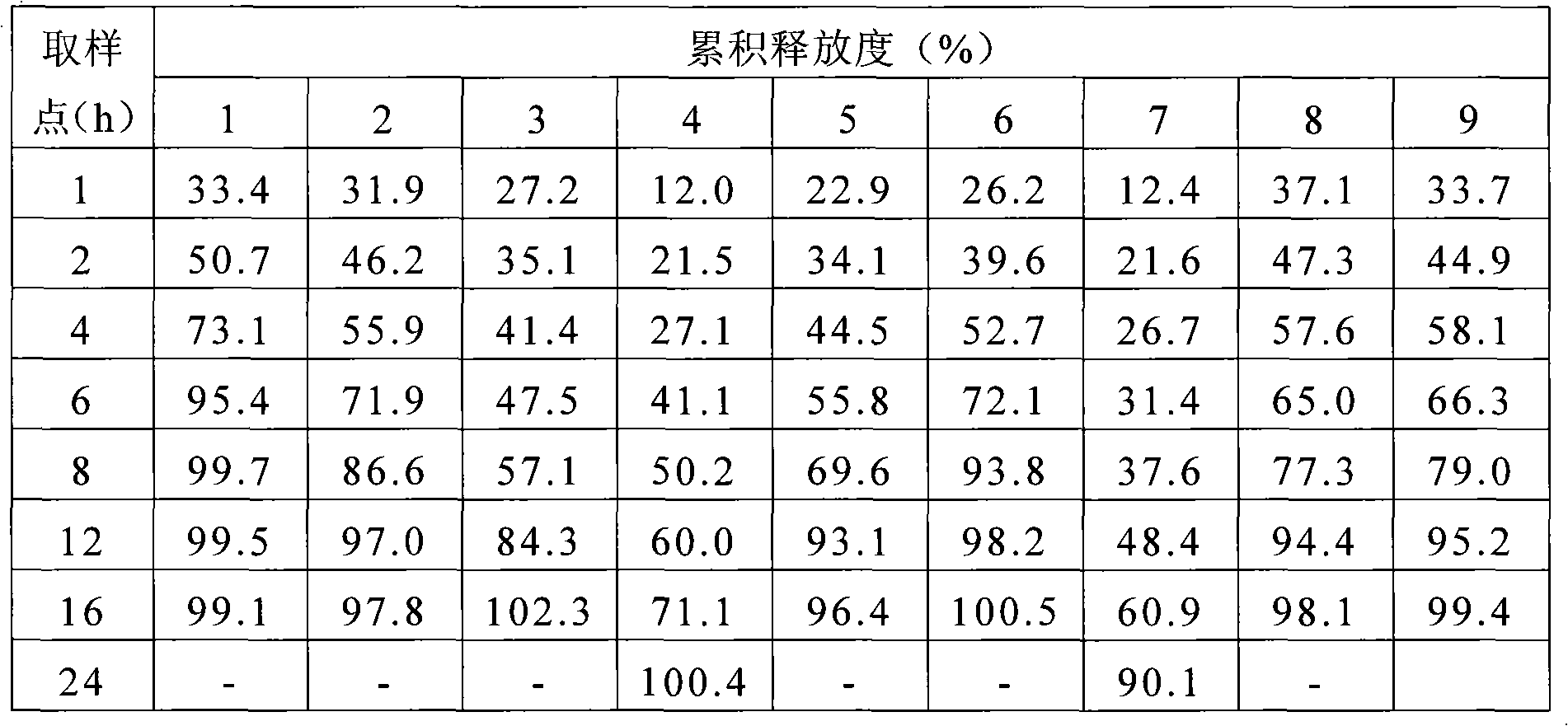
770 results about "Sustained Release Tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Xiral SR Sustained-Release Tablets are an antihistamine, decongestant, and anticholinergic combination. It works by blocking histamine, a substance in the body that causes sneezing, runny nose, and watery eyes.
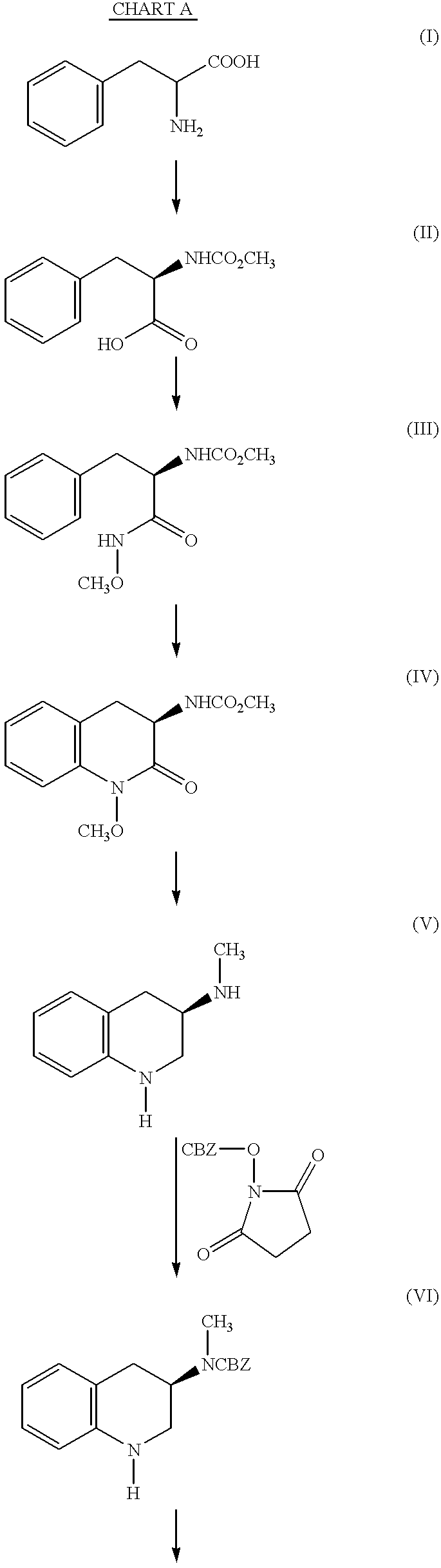
Sustained release tablet formulation to treat Parkinson's disease
The sustained release tablet of (R)-5,6-dihydro-5-(methylamino)-4H-imidazo[4,5-ij]-quinolin-2(1H)-one (Z)-2-butenedioate (1:1) which is disclosed permits twice daily administration of (R)-5,6-dihydro-5-(methylamino)-4H-imidazo[4,5-ij]-quinolin-2(1H)-one (Z)-2-butenedioate (1:1) to treat humans with Parkinson's disease.
Owner:PHARMACIA & UPJOHN CO
Sustained-release tablet composition
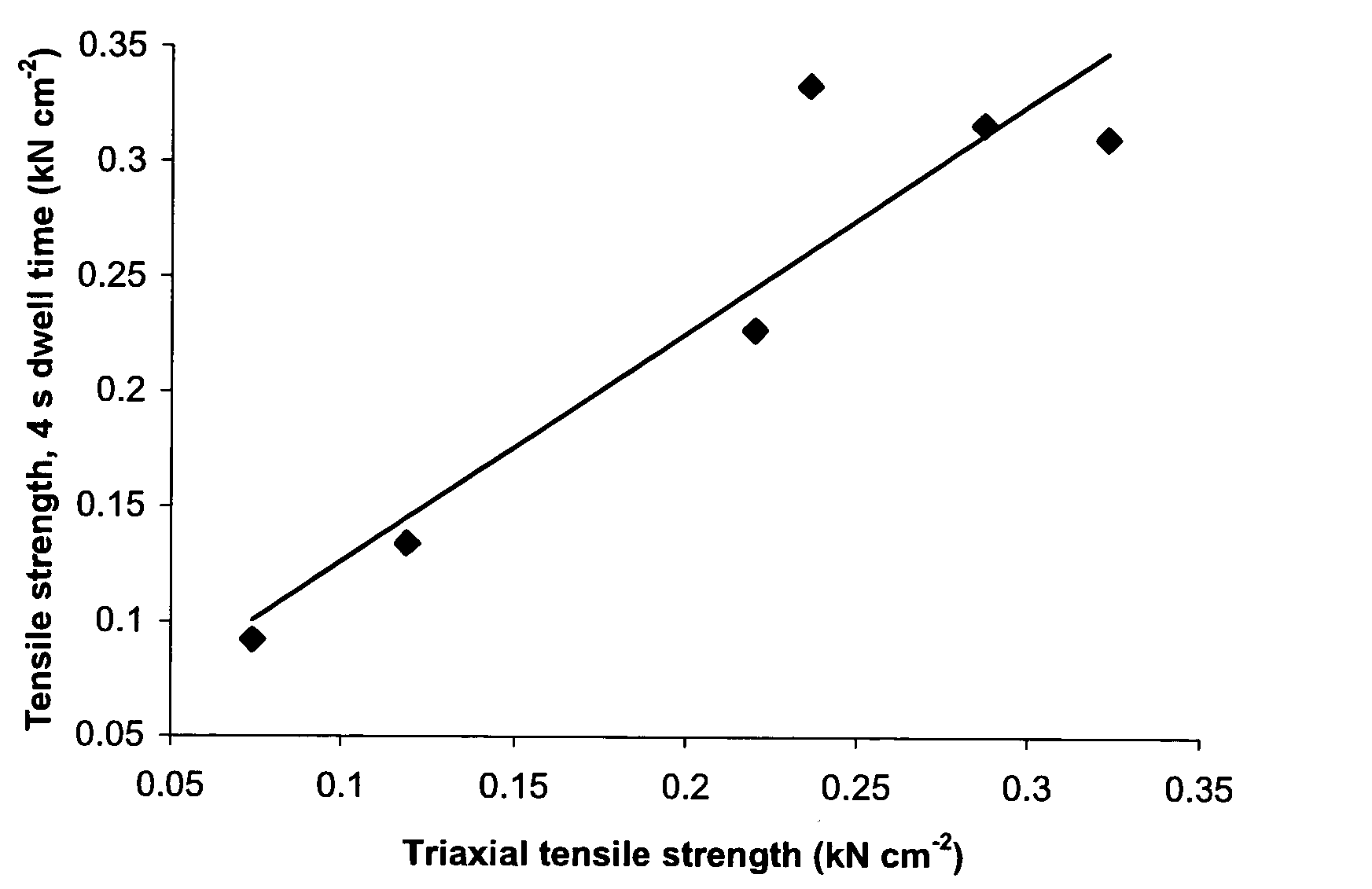
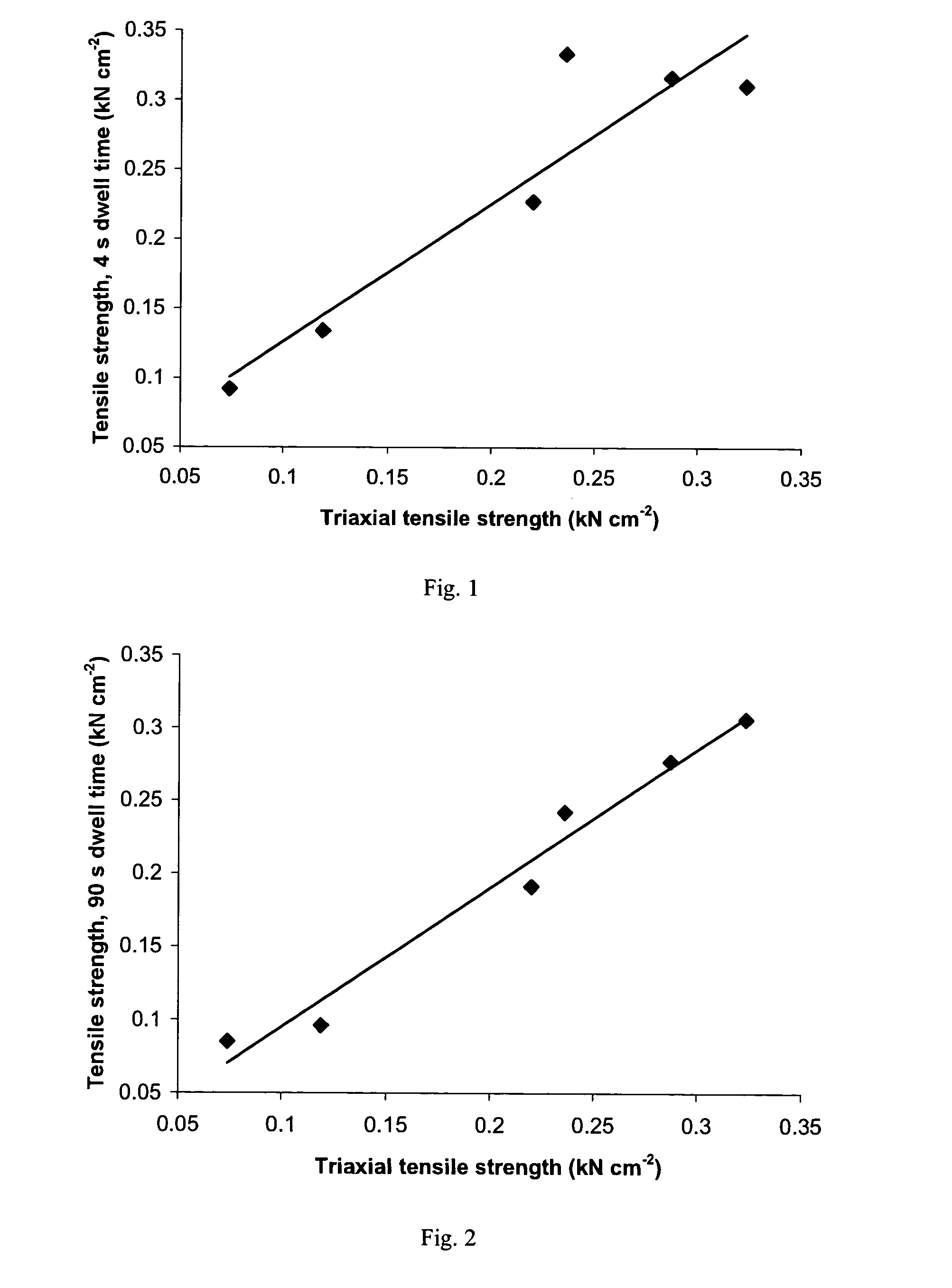
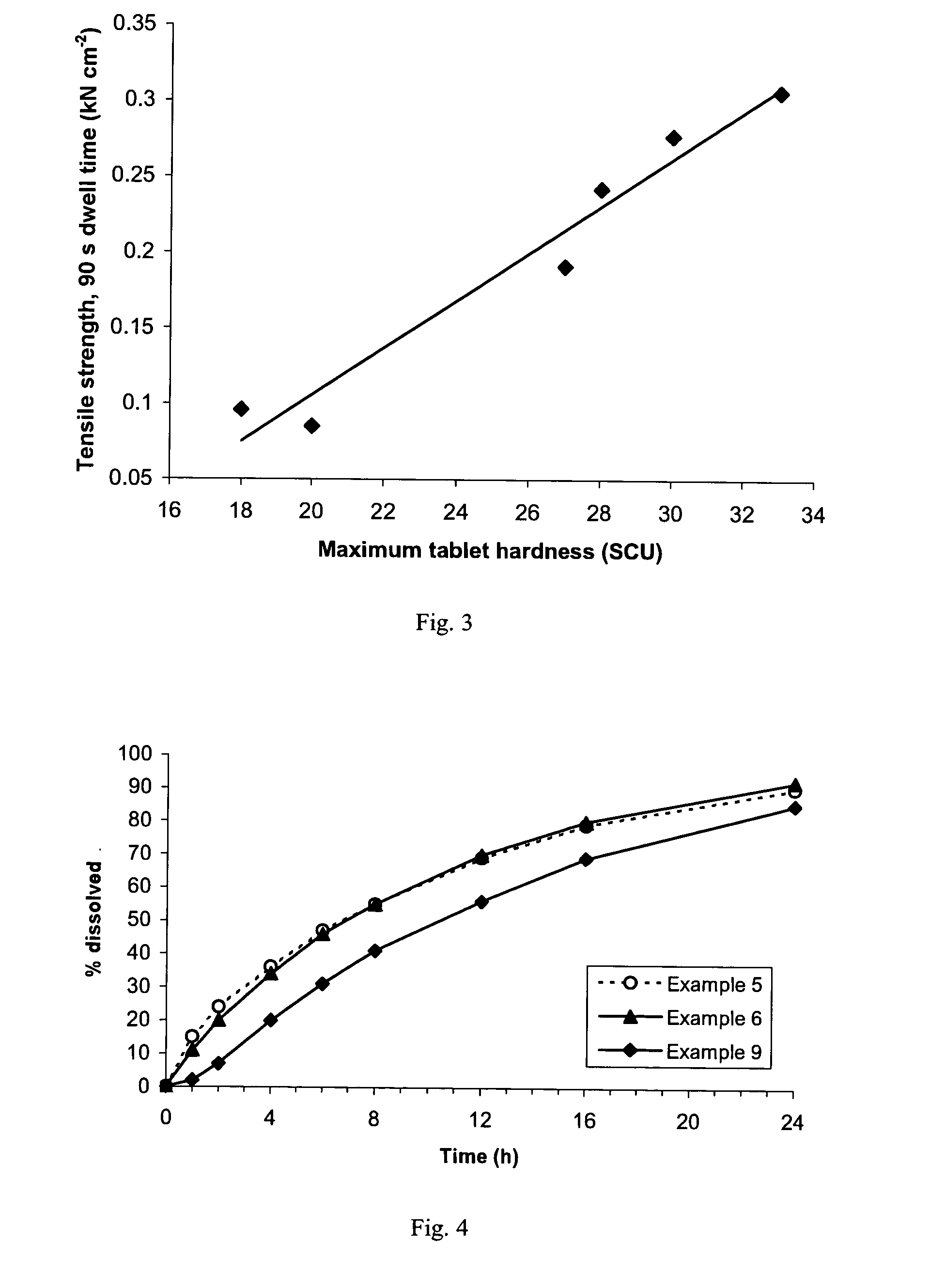
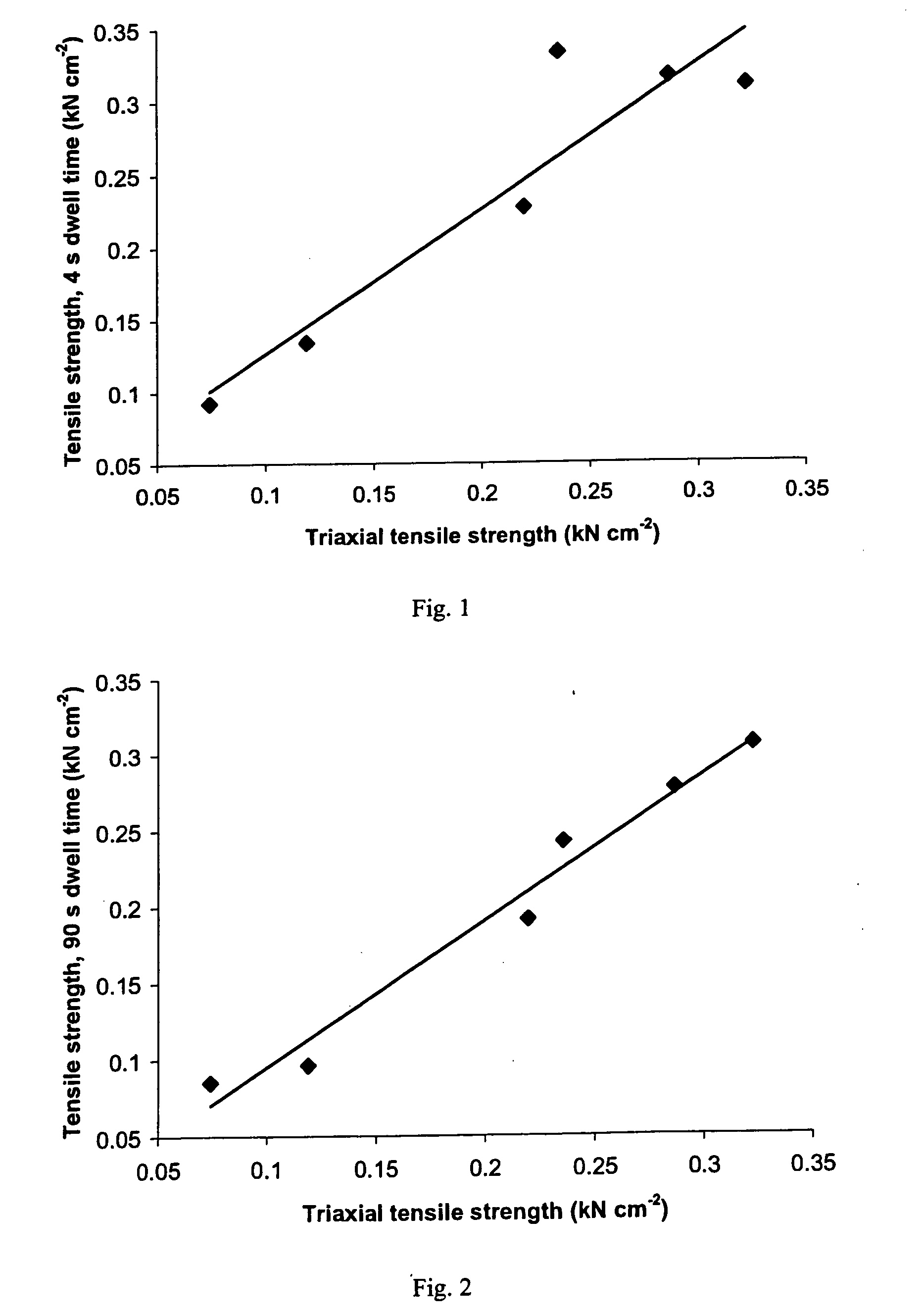
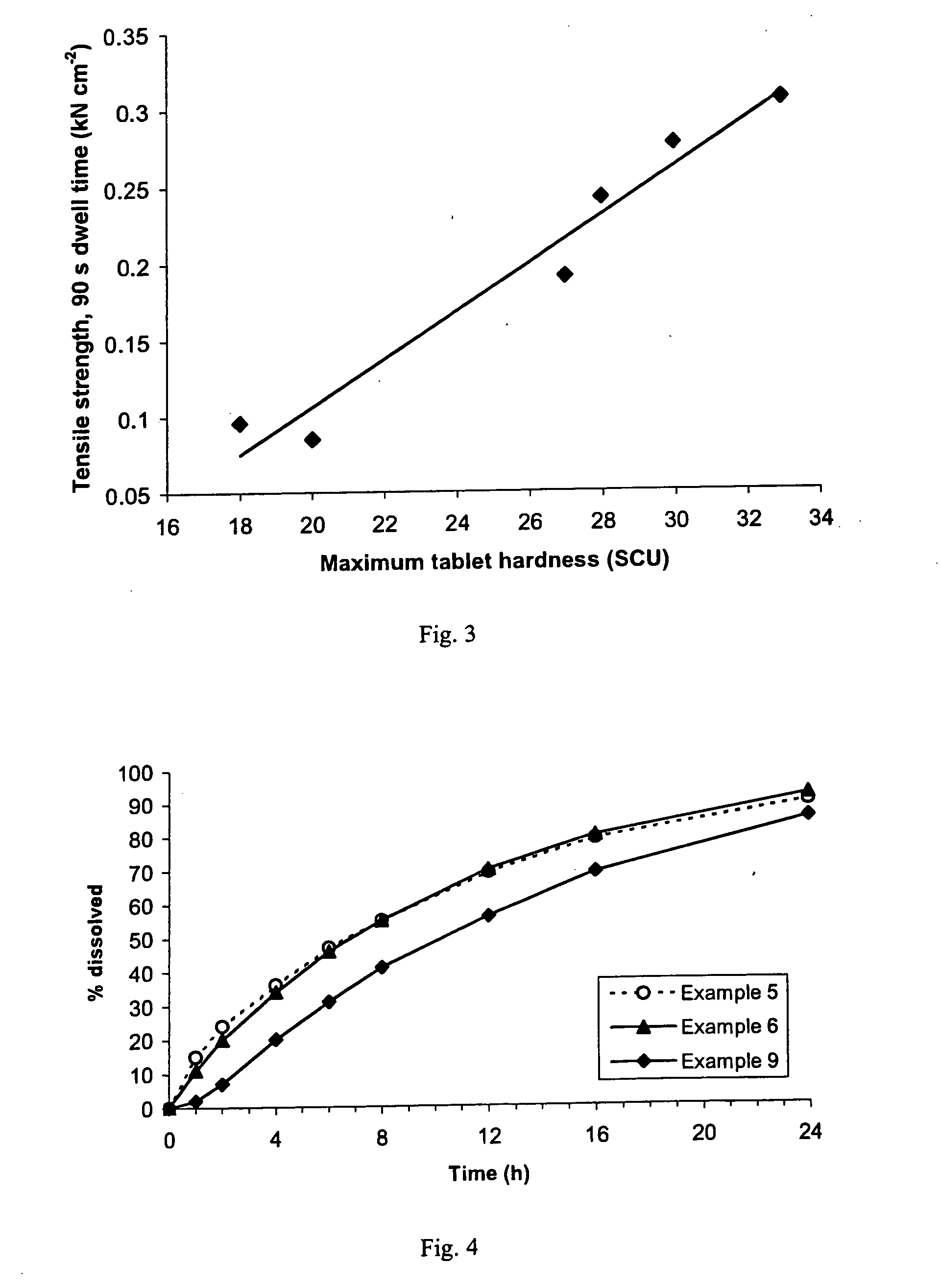
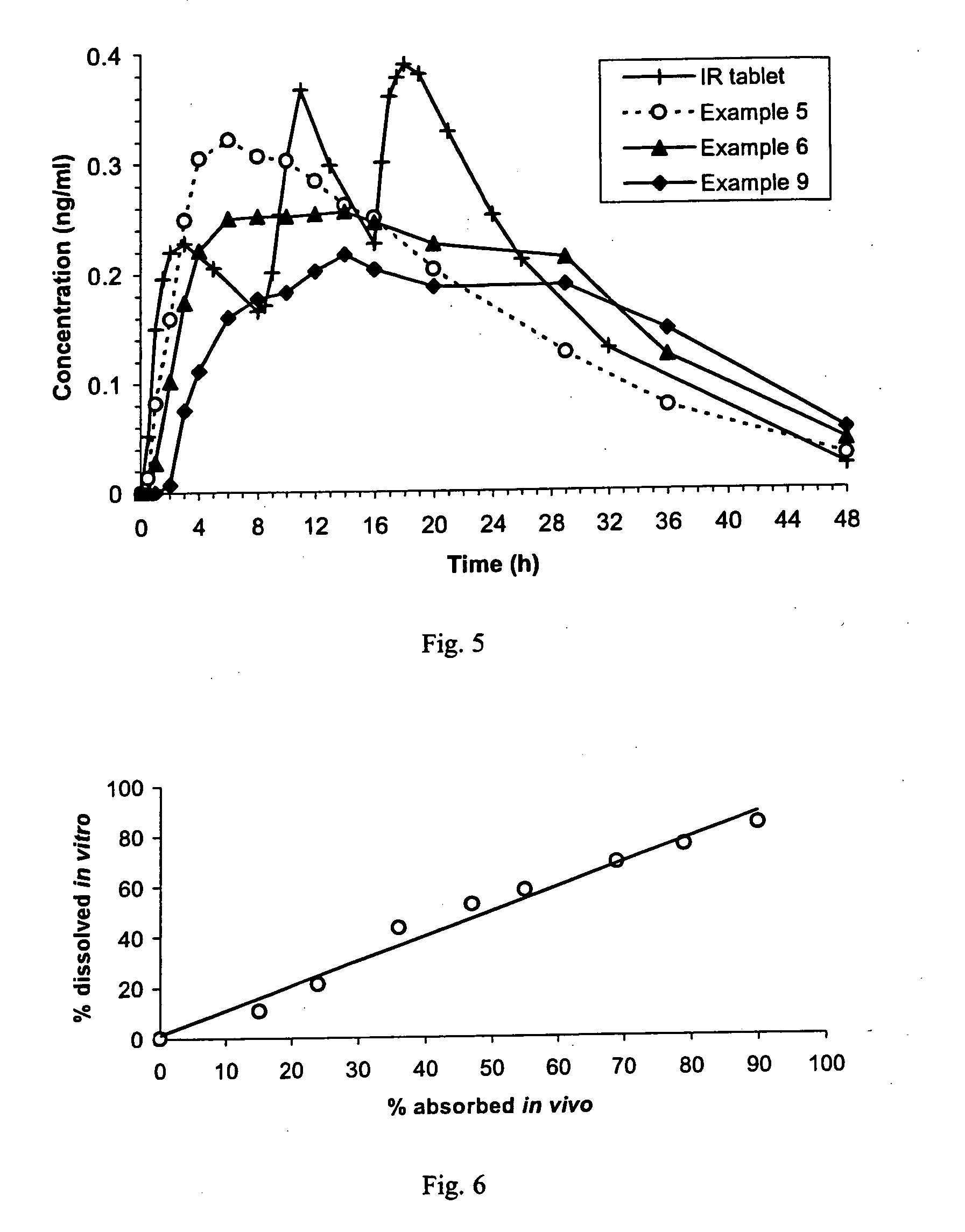
A sustained-release pharmaceutical composition in a form of an orally deliverable tablet comprises an active pharmaceutical agent having solubility not less than about 10 mg / ml, dispersed in a matrix comprising a hydrophilic polymer and a starch having a tensile strength of at least about 0.15 kN cm−2 at a solid fraction representative of the tablet.
Owner:PHARMACIA CORP
Sustained-release tablet composition of pramipexole
InactiveUS20050226926A1Organic active ingredientsNervous disorderSustained Release TabletHydrophilic polymers
A sustained-release pharmaceutical composition in a form of an orally deliverable tablet comprises a water-soluble salt of pramipexole, dispersed in a matrix comprising a hydrophilic polymer and a starch having a tensile strength of at least about 0.15 kN cm−2 at a solid fraction representative of the tablet.
Owner:BOEHRINGER INGELHEIM INT GMBH
Process for preparing sustained release tablets
The present invention is directed to the process of preparing a sustained release niacin tablet and the product prepared therefrom.
Owner:NOSTRUM PHARMA INC
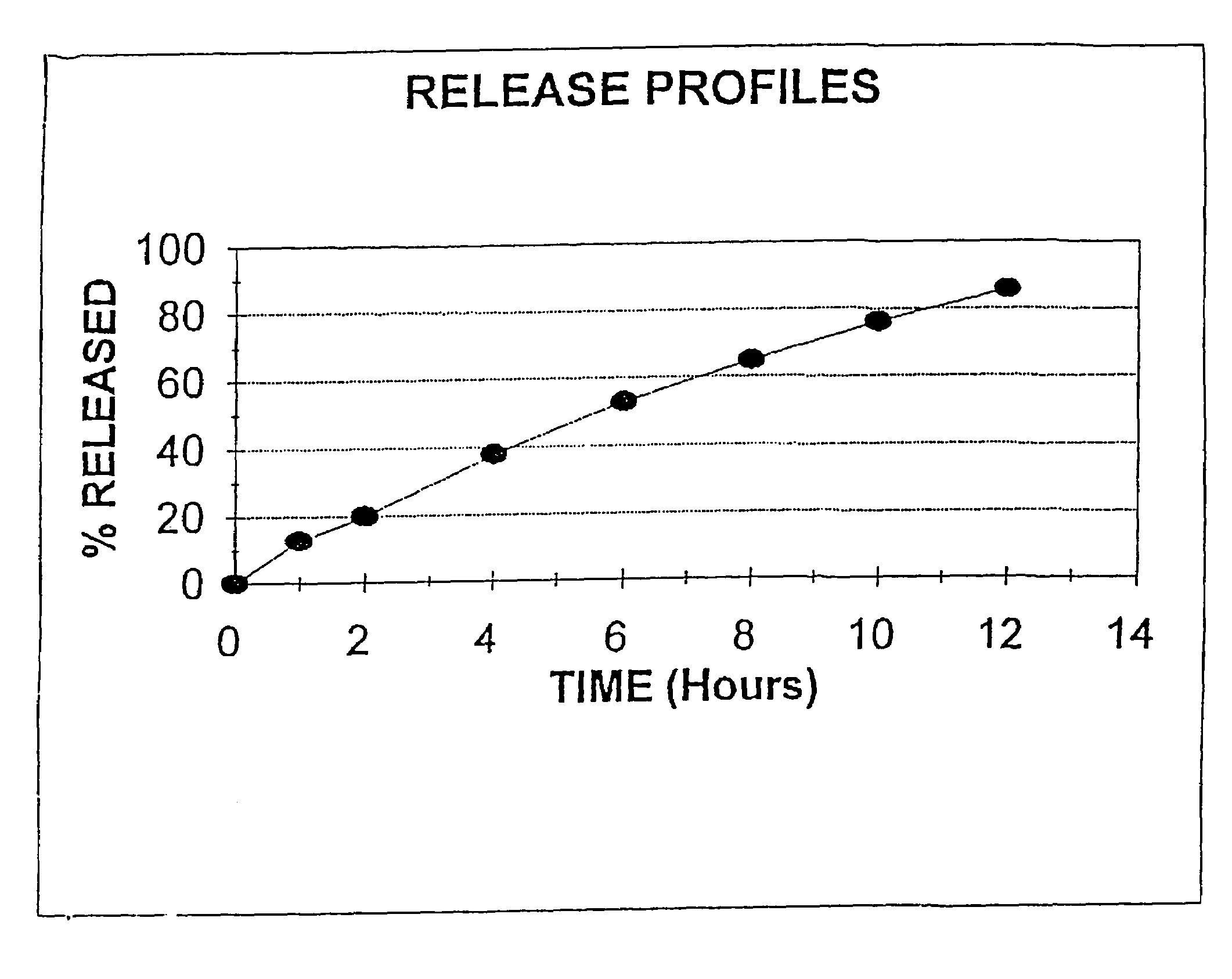
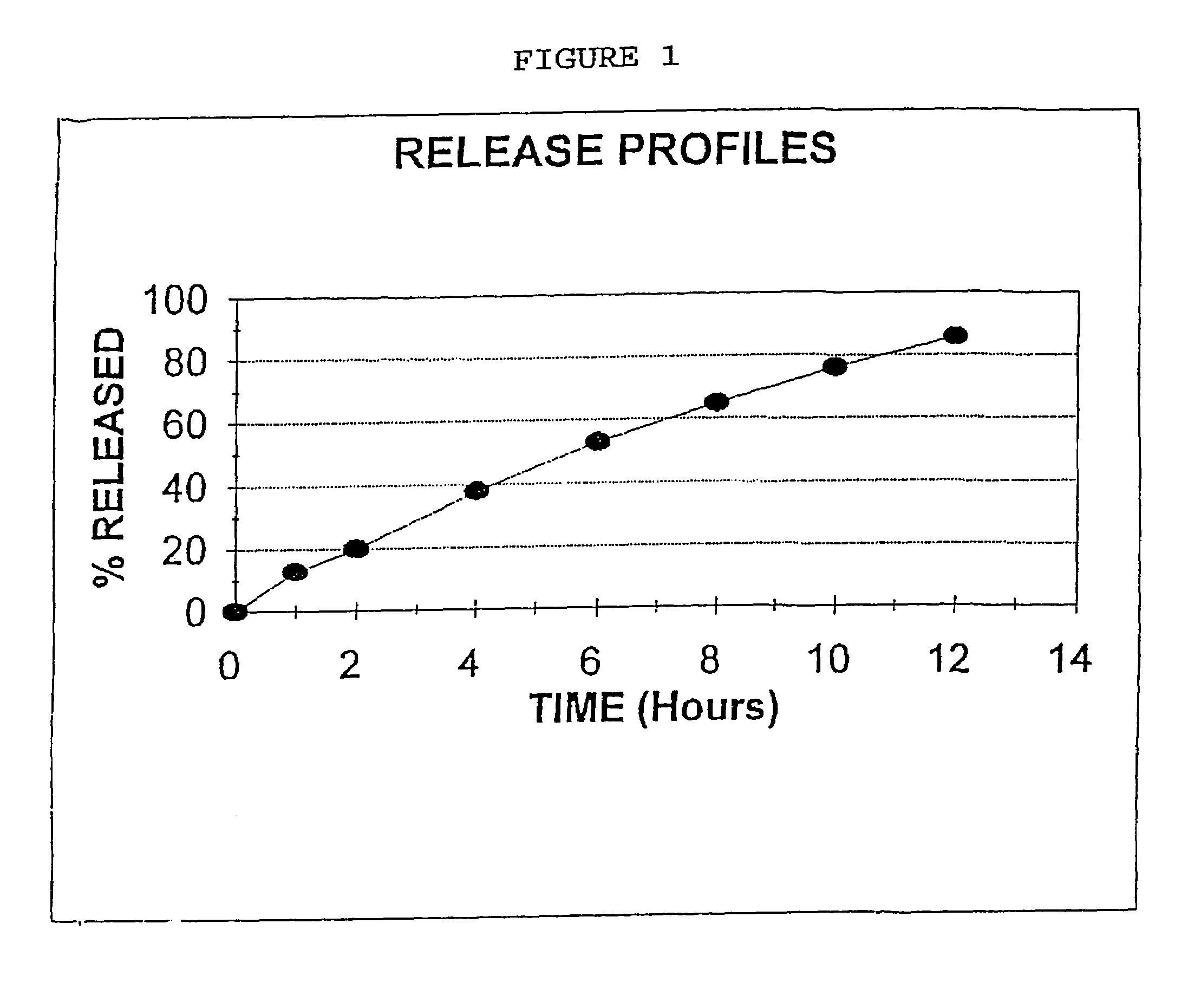
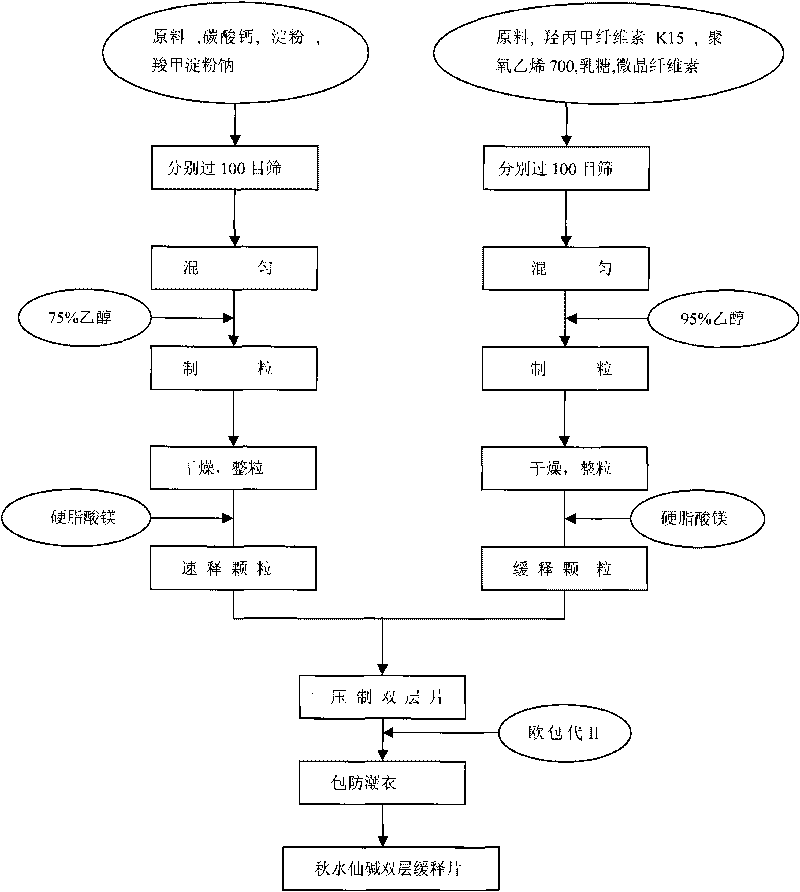
Colchicine bilayer sustained-release tablet and preparing method thereof
InactiveCN101732274AEffective plasma concentration stableStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a colchicine bilayer sustained-release tablet which comprises a quick release layer and a sustained-release layer, wherein the quick release layer mainly consists of colchicine, a disintegrant, a filler, a lubricant and an adhesive; the sustained-release tablet mainly consists of colchicine, a sustained-release matrix, a retarding agent, a filler and an adhesive; the weight ratio of the colchicine in the quick release layer to the colchicine in the sustained-release layer is (0.25-0.42) : 1; and the weight ratio of the quick release layer to the sustained-release layer is 1: 2 to 1: 4. The invention also discloses a method for preparing the colchicine bilayer sustained-release tablet. The colchicine bilayer sustained-release tablet releases the drugs through the quick release layer to quickly achieve the effective blood drug concentration, slowly releases the drugs through the sustained-release layer to exert and sustain the stable and uniform effective blood drug concentration, reduces the drug taking times and lightens the toxic or side function.
Owner:普尔药物科技开发(深圳)有限公司
Ticagrelor sustained-release tablet system and preparation method thereof
InactiveCN102657629AMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPediatrics
The invention provides a ticagrelor sustained-release tablet system and a preparation method of the ticagrelor sustained-release tablet system. The preparation method comprises the following steps of: firstly uniformly mixing 10-60% of ticagrelor, 5-60% of a filler and 5-60% of high molecular polymer in percentage by weight, adding a granulating solution to granulate; fully drying the obtained granules at a temperature of 50-60 DEG C, uniformly mixing the sieved granules with 0.25-10% of a lubricant and / or 0-10% of a flow aid, carrying out tabletting to obtain the ticagrelor matrix type sustained-release tablets, wherein the granulating solution is preferably water, an alcohol-water solution or absolute ethyl alcohol; the granule size of the ticagrelor is below 100 micrometers; and the content of the ticagrelor is 50-300mg in the preparation process. The ticagrelor matrix type sustained-release tablet system provided by the invention has the advantages that a patient can take the ticagrelor once a day to ensure that the drug dependence of the patient can be improved and the risk of myocardial infarction or apoplexy caused by acute thrombosis due to a dose of the ticagrelor missing of the patient is reduced.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE
Sustained release oral dosage forms of gabapentin
InactiveUS20050158380A1Extended gastric residence timeGood sustained releaseOrganic active ingredientsBiocideGabapentinSustained Release Tablet
The present invention relates to sustained release oral dosage forms of gabapentin and at least one rate controlling polymer, and a process for the preparation of the sustained release oral dosage forms, and a process for the preparation thereof. The sustained release tablet includes gabapentin or a pharmaceutically acceptable salt or hydrates thereof and at least one rate-controlling polymer such that the tablet provides therapeutically effective plasma levels of gabapentin for a period of up to about 12 hours.
Owner:RANBAXY LAB LTD
Sustained release tablets for treatment of aqueous environment and methods for making the same
InactiveUS20060165745A1BiocideDead animal preservationSustained Release TabletBULK ACTIVE INGREDIENT
The present invention provides tablets for treatment of aqueous environment that release at least one active ingredient in a sustained manner so as to provide the treated aqueous environment with the at least one active ingredient for a long period with a single dose. The present invention also provides methods for making the sustained release tablets.
Owner:CHEW YIWEN +1
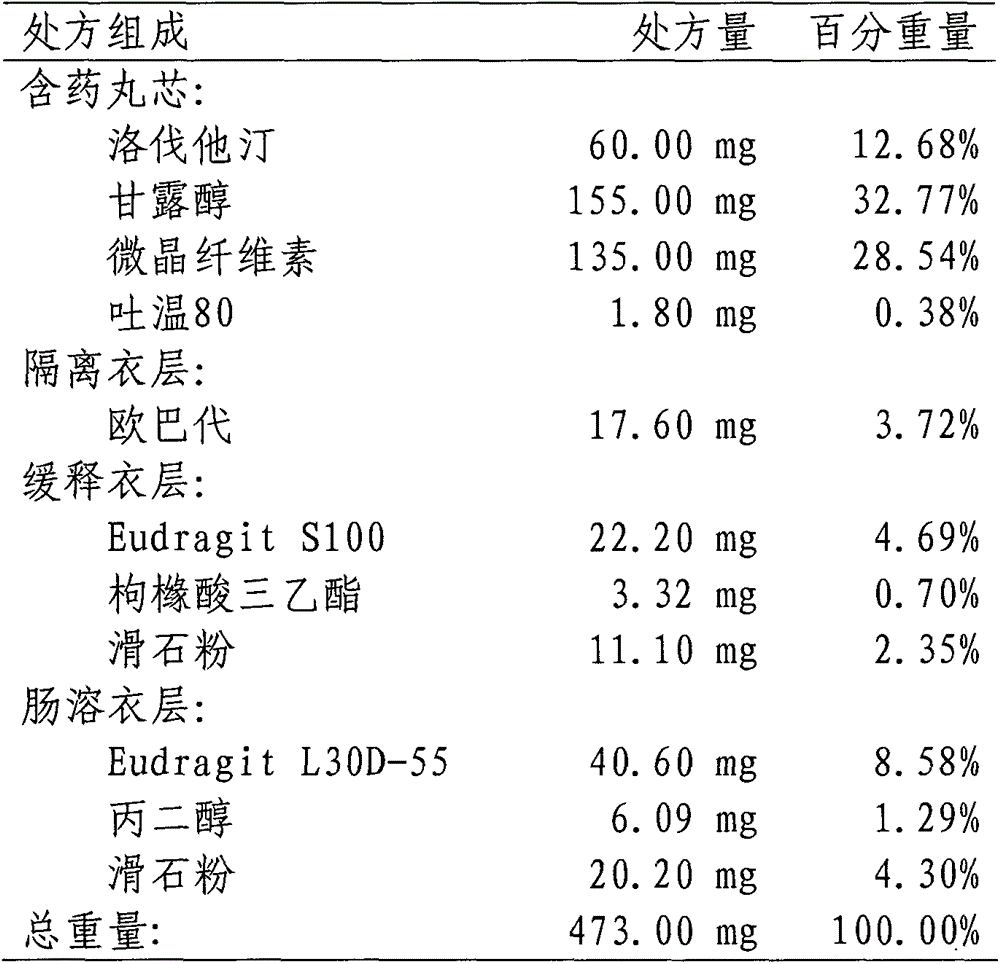
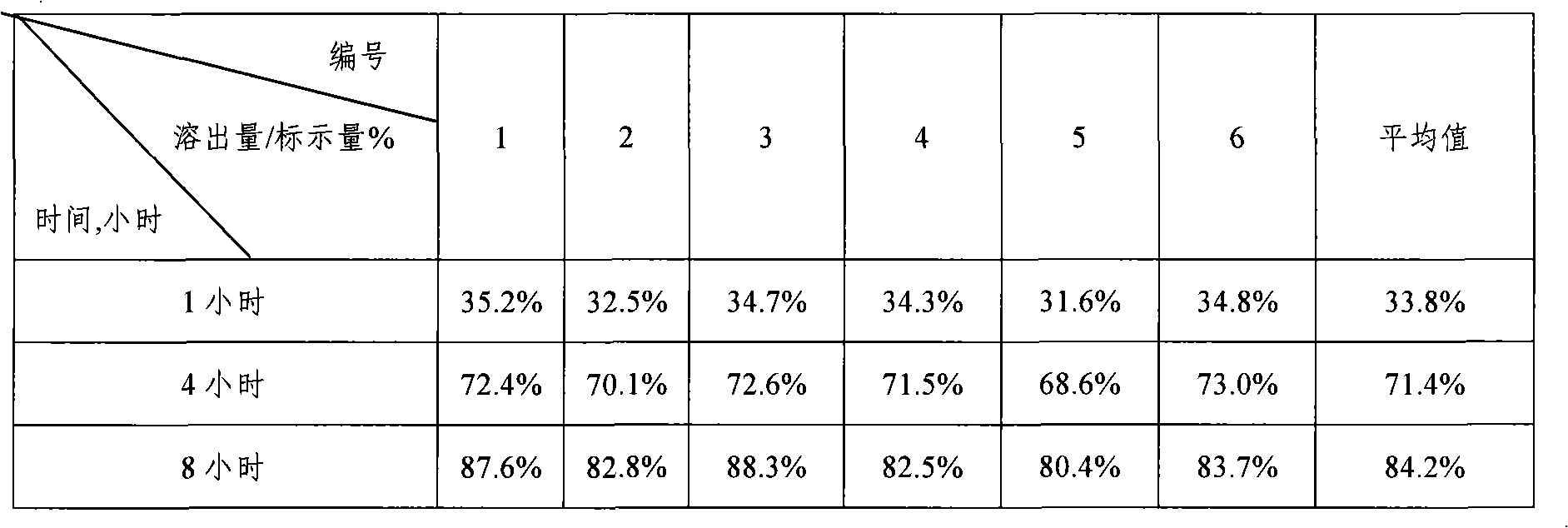
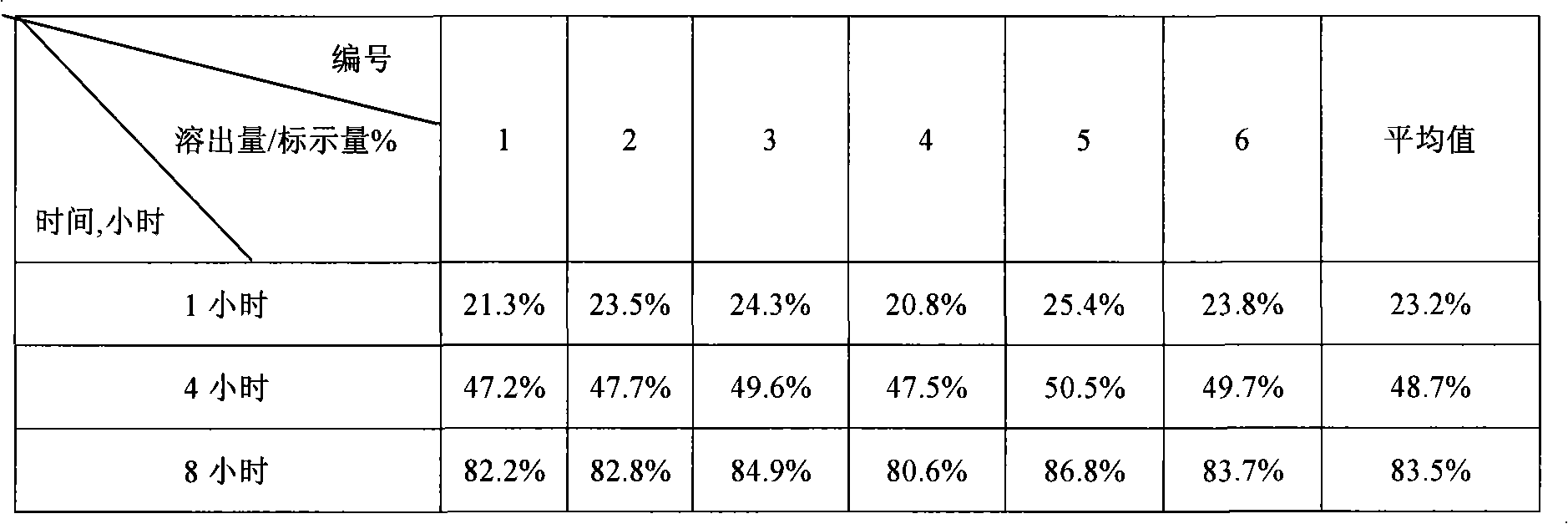
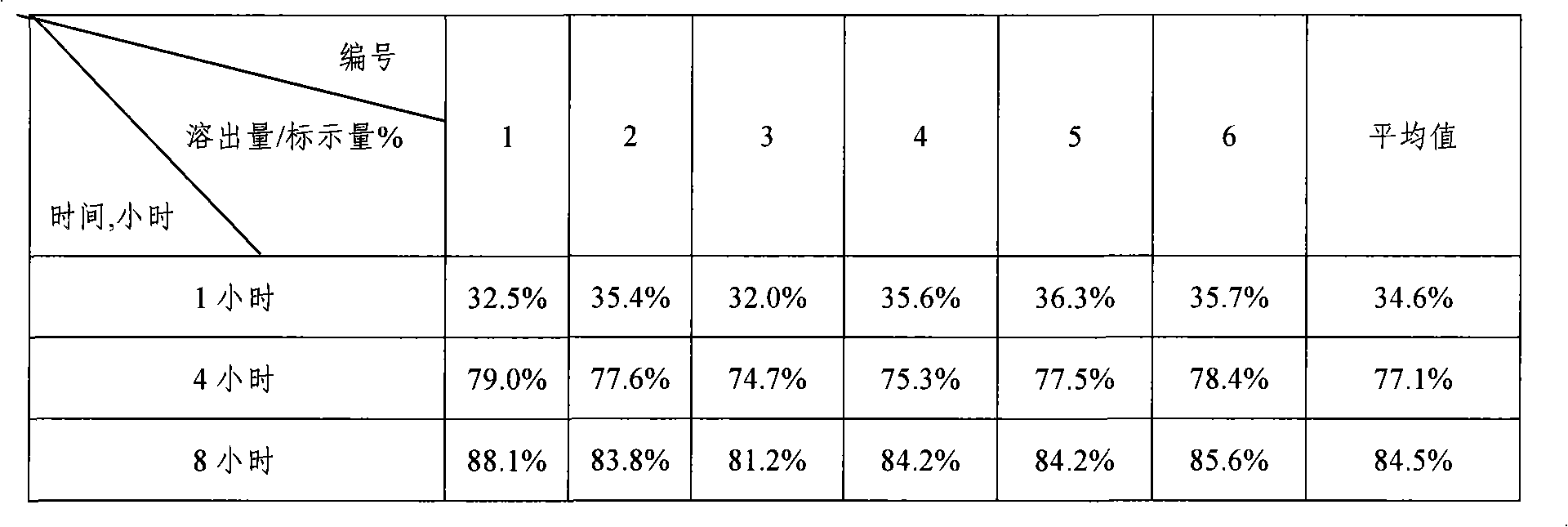
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
Double-layer sustained release tablets for compensating iron and preparation thereof
ActiveCN101322778AEasy to prepareImprove nutritional statusHeavy metal active ingredientsOrganic active ingredientsSustained Release TabletNutritional status
The invention relates to a double-layer sustained release tablet for supplementing iron, the double-layer sustained release tablet consists of a sustained release layer and a quick release layer laminating with each other at the weight ratio of 1:0.25-1:4; the sustained release layer consists of an iron-containing compound, a sustained release framework, an adhesive, a lubricant and a filler; the quick release layer consists of a traditional Chinese medicine for enriching the blood and / or invigorating vital energy, a filler, a disintegrating agent, an adhesive and a lubricant; the quick release layer can also be added with vitamins. In the double-layer sustained release tablet, by arranging the iron-containing compound in the sustained release layer and the traditional Chinese medicine in the quick release layer, the iron element is released slowly and uniformly, which increases the absorption rate, avoids gastrointestinal reaction caused by high-content iron; meanwhile the double-layer sustained release tablet supplements iron element and the traditional Chinese medicine which has the functions of enriching the blood and invigorating vital energy, thus fully improving the nutritional status caused by iron deficiency anemia and improving iron supplementing effect.
Owner:BEIJING COMPETITOR SPORTS SCI & TECH
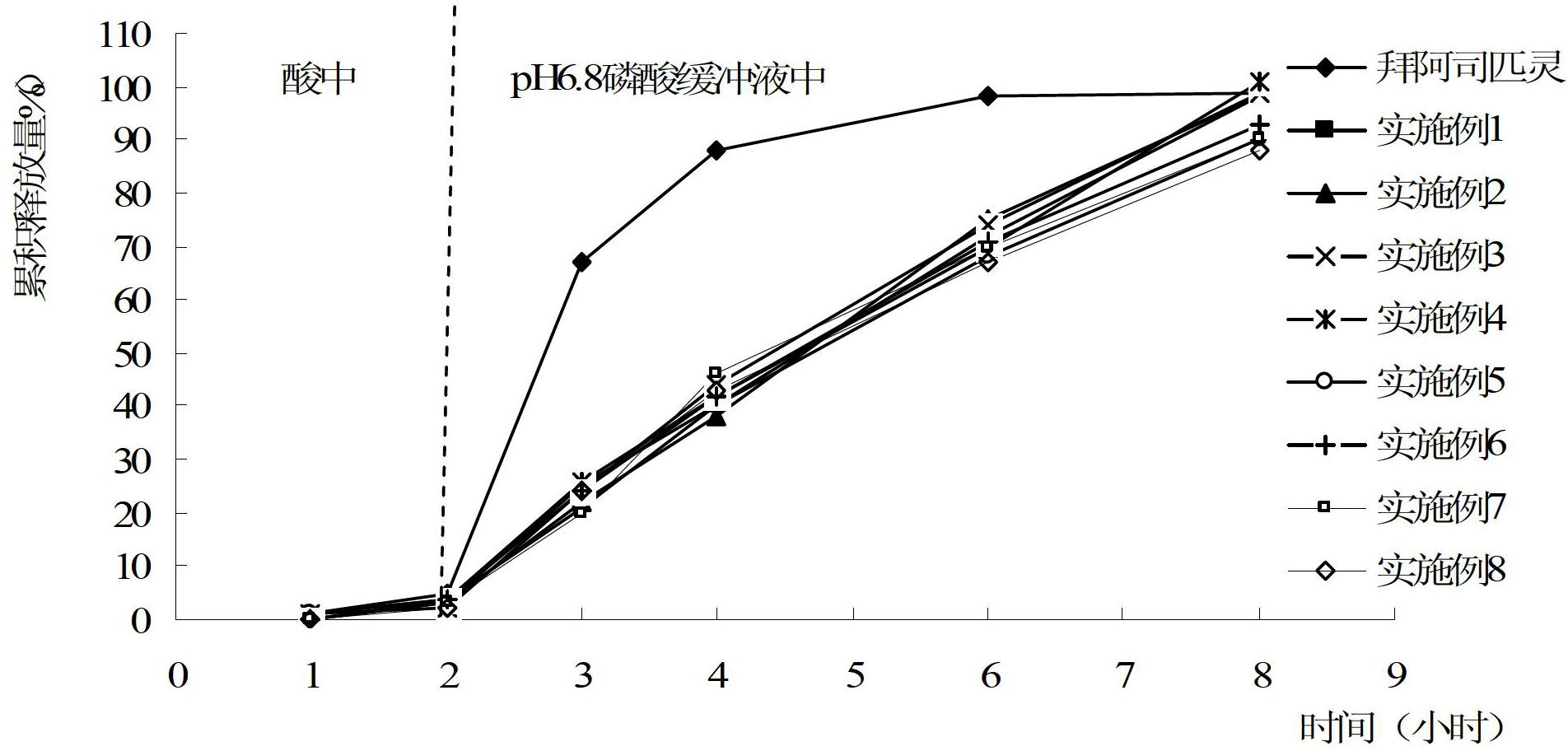
Preparation method of aspirin enteric-coated sustained-release preparation
ActiveCN102641254AAvoid degradation reactionsReduce the number of dosesOrganic active ingredientsAntipyreticSustained Release TabletRelease time
The invention relates to a preparation method of an enteric-coated sustained-release preparation, in particular to a preparation method of an aspirin enteric-coated sustained-release preparation. According to the preparation method, a prepared aspirin sustained-release tablet is used as a core tablet, and an enteric coating is packaged on the outer layer of the core tablet. In order to prevent aspirin in the core tablet from interacting with an enteric-coated material, an isolating layer can be packaged between the core tablet and the enteric coating. After the aspirin is prepared into the enteric-coated sustained-release preparation provided by the invention, the aspirin is not released or a little aspirin is released in a stomach, the release time in intestines is greatly extended, the phenomena of violent disintegration and dissolution do not occur in the intestines, the aspirin is released more slowly, the bioavailability is greatly increased, the degradation reaction of the aspirin in water is avoided, the frequency of administration is lower, and the compliance of a patient is improved.
Owner:SHANDONG XINHUA PHARMA CO LTD
Sustained release tablet comprising pregabalin through two-phase release-controlling system
ActiveCN103702664AExtended release profileIncrease buoyancyOrganic active ingredientsNervous disorderSustained Release TabletPolyethylene oxide
The invention provides a sustained release tablet having two-phase release-controlling system, which consists of a first release-controlling phase comprising pregabalin or its salt and hydroxypropyl methylcellulose; and a second release-controlling phase comprising polyethylene oxide as a swelling polymer, the first release-controlling phase being homogeneously dispersed in the second release-controlling phase.
Owner:YUHAN
Mustard essential oil fresh preserver and application thereof in refreshing fruits and vegetables
InactiveCN101711533AEasy to operateEasy to handleFruit and vegetables preservationAdditive ingredientCyclodextrin
The invention discloses a mustard essential oil fresh preserver and an application thereof in refreshing fruits and vegetables. The main active component of the mustard essential oil fresh preserver is mustard essential oil containing over 99% of allylisothiocyanate, and the mustard essential oil fresh preserver is mainly applied to a solid sustained release tablet and packaging bags made of plastic aluminum film paper. The solid sustained release tablet consists of 5%-10% of the mustard essential oil, 80%-85% of beta- cyclodextrin and 5%-10% of stearic acid. The preparation method comprises the following steps of: firstly, fully mixing and stirring the mustard essential oil and the alpha (beta)-cyclodextrin according to the proportion in a seal container, adding the stearic acid, mixing and stirring, directly packaging in the paper bags, sealing the opening in time, filling in the plastic bags, sealing by vacuumizing, or after using a tablet machine to make tablets, filling in the plastic film bags and sealing the opening, finally storing at dark places. The fresh preserver can slowly release the mustard essential oil, can be used combining with fruit and vegetable plastic film package, and is suitable for storing, conveying and refreshing various fruits and vegetables of apples, pears, grapes, jujubes, oranges, mangos, cherries, bananas, potatoes, onions, garlic and the like, thereby effectively restraining the growth of pathogenic organisms after picking up the fruits and the vegetables in the process of storing and refreshing and prolonging the refreshing time and the distance of the market circulation.
Owner:NAT ENG AN TECH RES CENT FOR PRESERVATION OF AGRI PROD TIANJIN
Sustained release tablet of quetiapine fumarate composition and preparation method of sustained release tablet
InactiveCN102218042AReduce weightIncrease toleranceOrganic active ingredientsNervous disorderSustained Release TabletOrganic acid
The invention discloses a sustained release tablet of a quetiapine fumarate composition, comprising the following components in percentage by weight: 25 to 40% of quetiapine fumarate, 2 to 8% of organic acid salt, 5 to 30% of a sustained release material and the balance of other pharmaceutical adjuvants, wherein the sustained release material is K type hydroxypropyl methylcellulose. The sustained release material is the K type hydroxypropyl methylcellulose and the organic acid salt is added, therefore, a stable sustained release skeleton can be formed by few sustained release materials, the raw material can be saved, the weight of unit preparation can be reduced, and the problem of difficulty in swallowing caused by large size of the preparation can be released and / or solved; the hydroxypropyl methylcellulose with different viscosities are used as the material of the skeleton so that the preparation can reach formulated sustained release effects and has the characteristics of strong controllability and stability in storage. By use of the preparation method capable of granulating in one step, the operation is simplified and the production efficiency can be improved.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Process for producing diabecron sustained release tablet
InactiveCN101428007AImprove bioavailabilityOrganic active ingredientsMetabolism disorderCelluloseMetformin Hydrochloride
The invention relates to a preparation method for metformin hydrochloride sustained-release tablets. The preparation method comprises the following steps: firstly, dissolving ethyl cellulose into ethanol solution, blending the ethyl cellulose with metformin hydrochloride to produce granules, and drying and sieving the granules at 45 to 55 DEG C; secondly, dissolving hydroxypropyl methylcellulose into ethanol solution to produce bonding agent, blending the granules prepared in step one, hydroxypropyl methylcellulose, ethyl cellulose and pore forming agent, adding the bonding agent, performing sieving and granulation, adding hydroxypropyl methylcellulose and lubricant, evenly blending, detecting the content, determining the tablet weight, and pressing into plain tablets; thirdly, dissolving film forming agent, plasticizer, masking agent and glidant for hydroxypropyl methylcellulose and ethyl cellulose into ethanol solution, completely stirring for more than 1 hour to produce sustained-release preparation coating solution, and finally obtaining the metformin hydrochloride sustained-release tablets by performing film coating to the plain tablets. The sustained-release preparation not only embodies the main characteristics of the sustained-release preparation for continuous sustained release of drug, but also presents high bioavailability.
Owner:上海天赐福生物工程有限公司
Medicinal composition containing ibuprofen sodium salt
InactiveCN102389423AOrganic active ingredientsNervous disorderSustained Release TabletBULK ACTIVE INGREDIENT
The invention relates to a medicinal composition containing ibuprofen sodium salt, which is prepared by taking ibuprofen sodium salt and other one or a plurality of medicinal components as active ingredients, and combing the active ingredients with pharmaceutically appropriate auxiliary materials. The medicinal composition can be made into oral preparations, including conventional tablets, dispersible tablets, sustained release tablets, capsules, sustained release capsules, soft capsules, particles, dry suspension, suspension and the like, and used for symptomatic treatment of being antipyretic, antiphlogistic, analgesic and anti-allergic, and improving sleeping.
Owner:FUKANGREN BIO PHARMA
Nifedipine sustained-release tablet
InactiveCN102125531AOrganic active ingredientsPharmaceutical delivery mechanismNifedipineSustained Release Tablet
The invention provides a nifedipine sustained-release tablet which contains a physiological effective dose of nifedipine and release blocker and a pharmaceutically acceptable excipient, wherein the nifedipine is dispersed in the release blocker; the release blocker is selected from macromolecular substances which can be swelled in water, such as hydroxypropyl methyl cellulose, sodium alginate, carboxymethyl cellulose, methyl cellulose and xanthan gum, and the amount of the used release blocker is 5-30 percent of the weight of the sustained-release tablet; and the nifedipine sustained-release tablet contains 15-30mg of nifedipine and also contains several of filler, a disintegrating agent, a surfactant, an adhesive, a lubricant and a glidant as the excipient. The nifedipine sustained-release tablet can be used for treating hypertension and angina pectoris.
Owner:上海中邦斯瑞生物药业技术有限公司
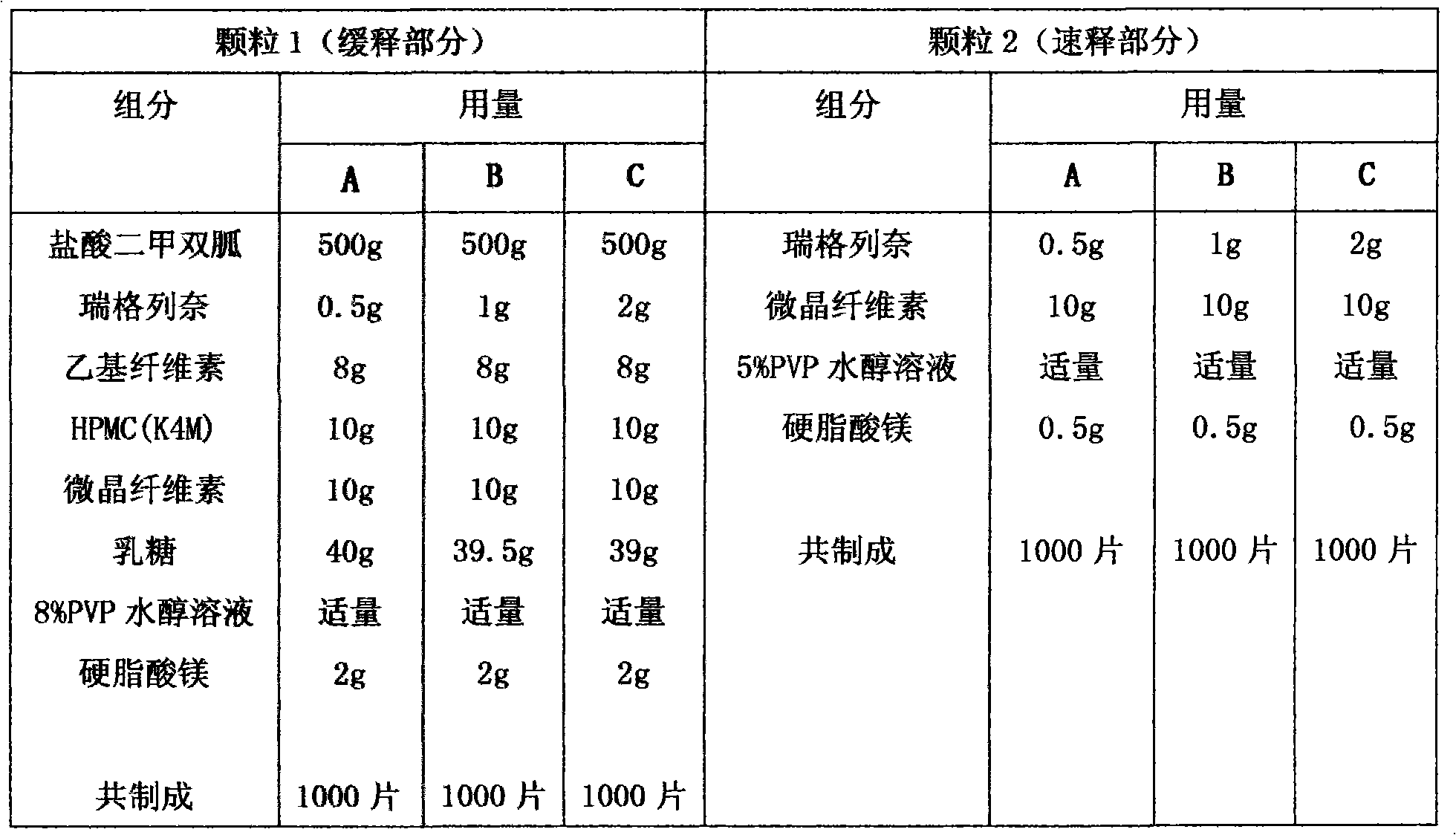
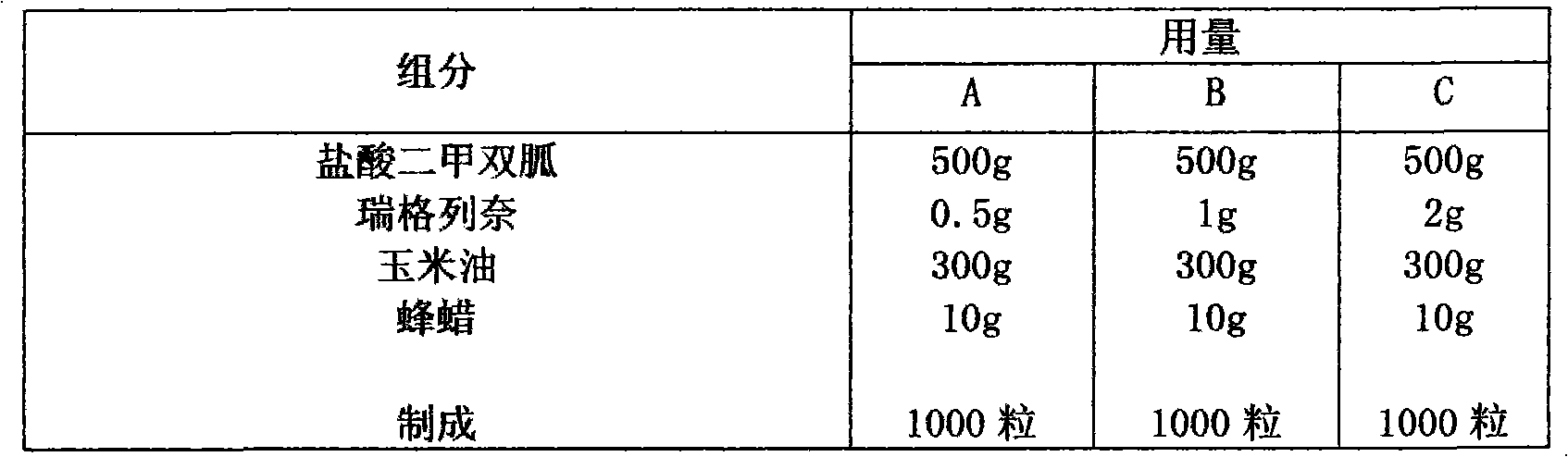
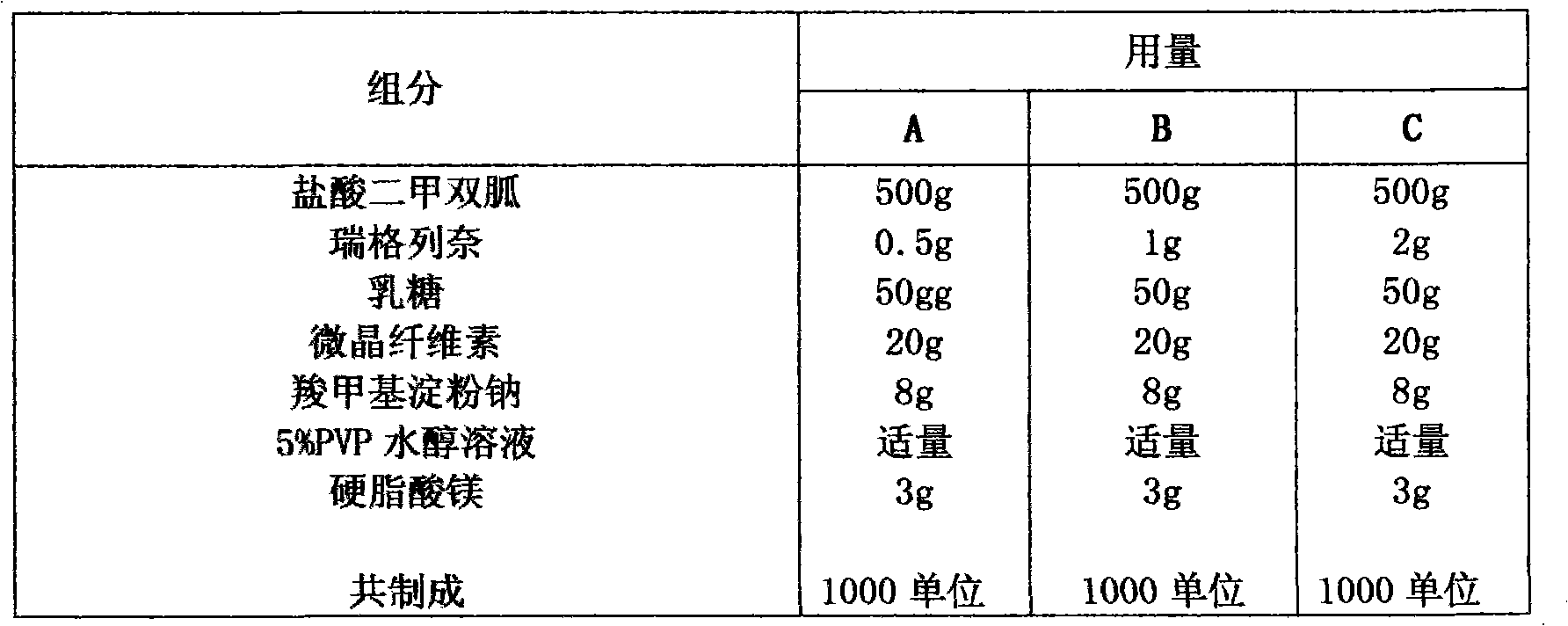
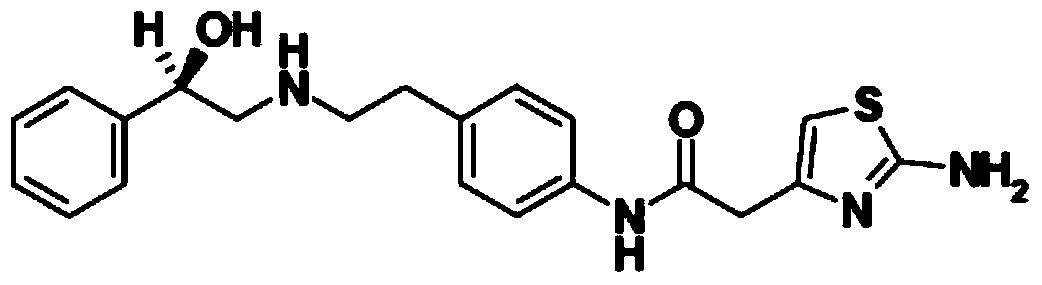
Compound with metformin and repaglinide, preparation method thereof and application thereof
InactiveCN101822672ALower glucose toleranceLower natural responsesOrganic active ingredientsMetabolism disorderInsulin dependent diabetesOrally disintegrating tablet
The invention relates to a composite composition with metformin hydrochloride and repaglinide as active ingredients, a preparation method thereof and application thereof and belongs to the technical field of medicaments. The composite composition is a medicinal composition which is mixed by using the metformin hydrochloride and repaglinide as the active ingredients and by using a carrier and can be prepared into sustained-release tablets, sustained-release granules, sustained-release capsules, common troches and capsules, granules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, liquid capsules, soft capsules, drop pills and other oral preparations. The composite composition is used for treating patients with I-type diabetes or II-type diabetes (non-insulin-dependent diabetes) and has synergistic effect on controlling blood sugar.
Owner:深圳南方盈信制药有限公司 +1
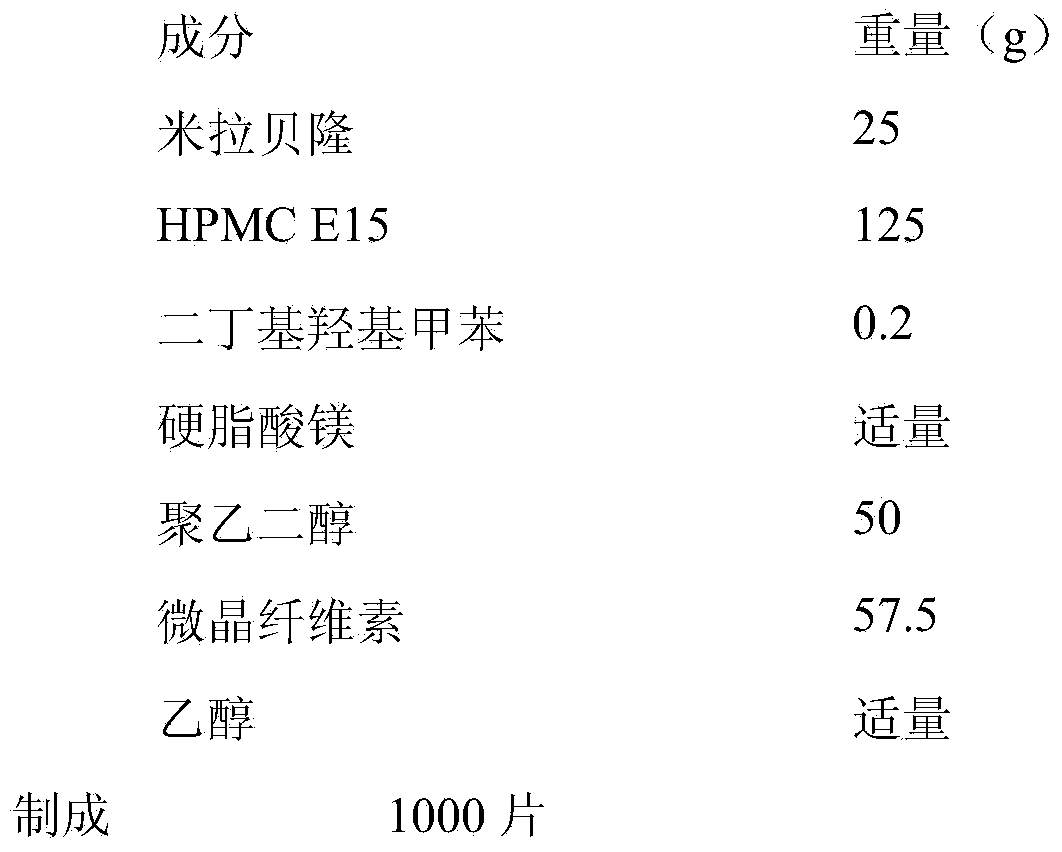
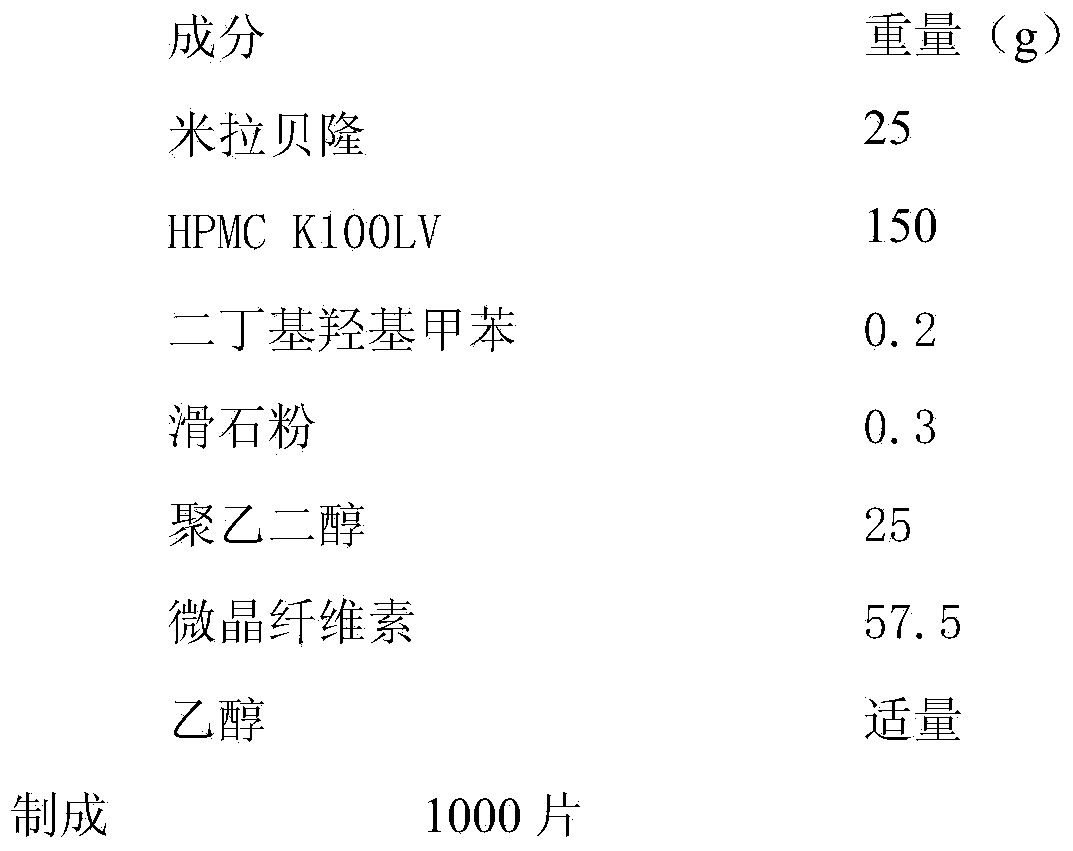
Merariveron sustained-release tablet and preparation method thereof
InactiveCN103655503AGood curative effectImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletAdhesive
The invention discloses a merariveron sustained-release tablet and a preparation method thereof. The merariveron sustained-release tablet is prepared from the following raw materials in percentage by weight: 5- 20% of merariveron, 10-70% of a skeleton material, 1- 5% of an antioxidant, 0.1-5% of a lubricant, 0-70% of a filler and a proper amount of an adhesive. The merariveron sustained-release tablet adopts a film coating, and thus the stability of the merariveron sustained-release tablet is improved. The sustained-release materials in the merariveron sustained-release tablet reduce influences, on release, of different PH valves. The influences, on the absorption of the merariveron sustained-release tablet, of food, are reduced, long-time stable effective drug concentration is kept, and thus the curative effect of the merariveron sustained-release tablet is improved. A patient only needs to take the merariveron sustained-release tablet once a day; the compliance of the patient is improved.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Combinations of HMG-COA reductase inhibitors and nicotinic acid and methods for treating hyperlipidemia once a day at night
InactiveUS20050255158A1Alter serum lipid levelReduce hyperlipidemiaSalicyclic acid active ingredientsMetabolism disorderLipid formationHMG-CoA reductase
The present invention relates to solid pharmaceutical combinations for oral administration comprising nicotinic acid or a nicotinic acid compound or mixtures thereof in an extended release form and an HMG-CoA reductase inhibitor, which are useful for altering lipid levels in subjects suffering from, for example, hyperlipidemia and atherosclerosis, without causing drug-induced hepatotoxicity, myopathy or rhabdomyolysis. The present invention also relates to methods of altering serum lipids in subjects to treat, for example, hyperlipidemia in hyperlipidemics, lipidemia in normolipidemics diagnosed with or predisposed to cardiovascular disease, and atherosclerosis, by administering such oral solid pharmaceutical combinations once per day as a single dose during the evening hours, without causing drug-induced hepatotoxicity, myopathy or rhabdomyolysis, or without causing in at least an appreciable number of individuals drug-induced hepatotoxicity, myopathy or rhabdomyolysis to such a level that discontinuation of such therapy would be required. More particularly, the present invention concerns oral solid pharmaceutical combinations comprised of, for example, (1) an HMG-CoA reductase inhibitor for immediate or extended release, (2) nicotinic acid, a nicotinic acid compound or mixtures thereof, and (3) a swelling agent to form a sustained release composition for extended release of the nicotinic acid or nicotinic acid compound or mixtures thereof for nocturnal or evening dosing for reducing serum lipids and increasing HDL-cholesterol. In accordance with the present invention, and by way of example, a composition for oral administration during the evening hours to alter serum lipids comprised of nicotinic acid and hydroxypropyl methylcellulose in the form of an extended or sustained release tablet or caplet coated with a coating comprising an HMG-CoA reductase inhibitor in immediate release form is disclosed. Also in accordance with the present invention, the pharmaceutical combinations may include a nonsteroidal anti-inflammatory agent for reducing the capacity of nicotinic acid or nicotinic acid compounds to provoke flushing reactions in individuals.
Owner:KOS LIFE SCI
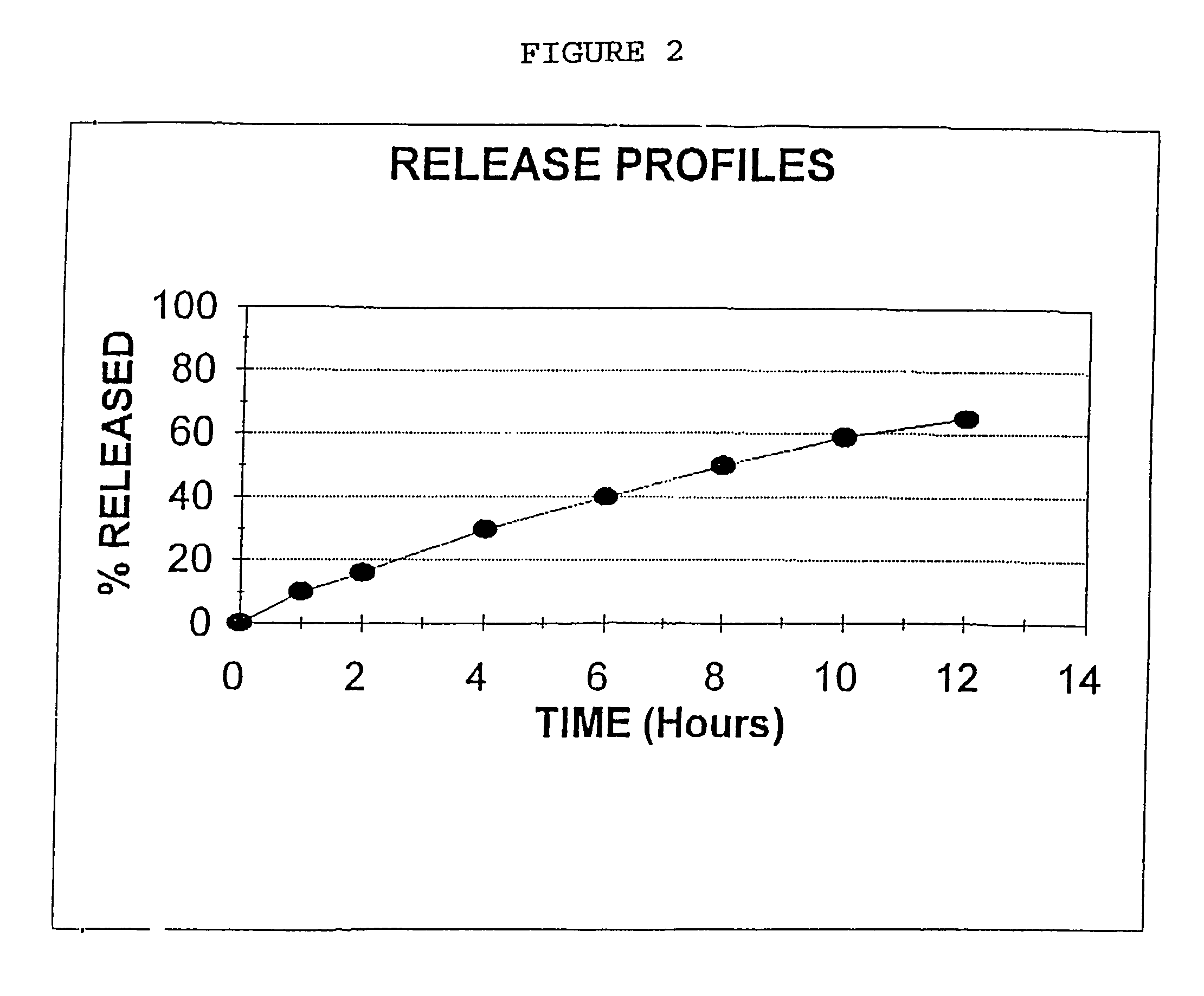
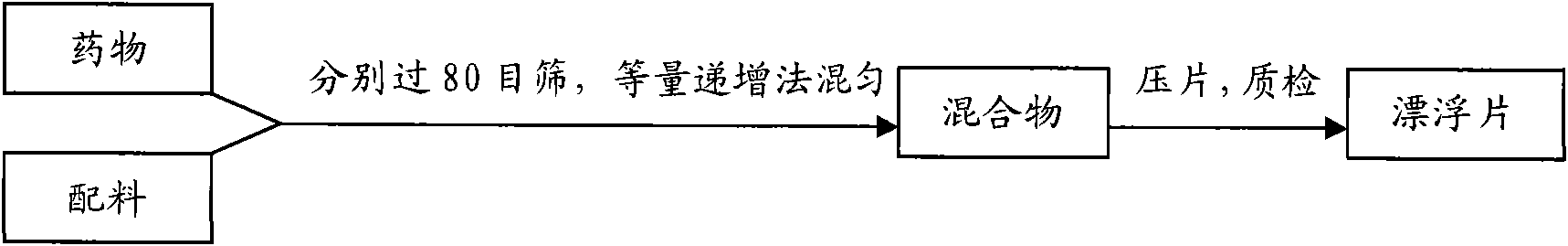
Colchicines gastric floating sustained-release tablet and method for preparing same
InactiveCN101536990ASchematic diagram of the preparation processOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a colchicines gastric floating sustained-release tablet, which comprises active components of colchicines and pharmaceutic adjuvant according to the weight ratio of 1:24-1,999, wherein the pharmaceutic adjuvant comprises a hydrophilic gel framework material, a effervescing agent, a floating assistant material, a filler, a pH value regulator and a lubricant. The colchicines gastric floating sustained-release tablet can swell quickly in gastric juice or a similar gastric juice medium and can float on the gastric juice for at least 4 hours. The invention also relates to a method for preparing the colchicines gastric floating sustained-release tablet. The colchicines gastric floating sustained-release tablet can reach the effective blood-drug concentration quickly after being taken and then release drugs slowly, and can maintain the balanced blood-drug concentration so as to reduce the dose times, relieve the toxic side effect and improve the bioavailability.
Owner:普尔药物科技开发(深圳)有限公司
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Amino acid salt of (S)-ibuprofen and medicinal composition thereof
InactiveCN101265178AOrganic active ingredientsOrganic chemistryCoated tabletsSustained Release Tablet
The invention provides a preparation and synthesis method of basic amino acid medicine salt of a dextral optical isomer in ibuprofen that is the existing anti-inflammation antipyretic and analgesic, and a medicinal combination thereof. After being mixed with necessary auxiliary material, the medicine salt can be prepared into oral solid preparations such as oral liquid, granules, tablets, dispersible tablets, capsules, coated tablets, sustained release tablets, collocystis, etc. through necessary pharmaceutics operations, and can also be prepared into the preparations for intravenous injection such as hydro-acupuncture, freeze-dried powders, aseptic powders, high volume transfusions, etc.
Owner:FUKANGREN BIO PHARMA
Mosapride citrate sustained-release tablet
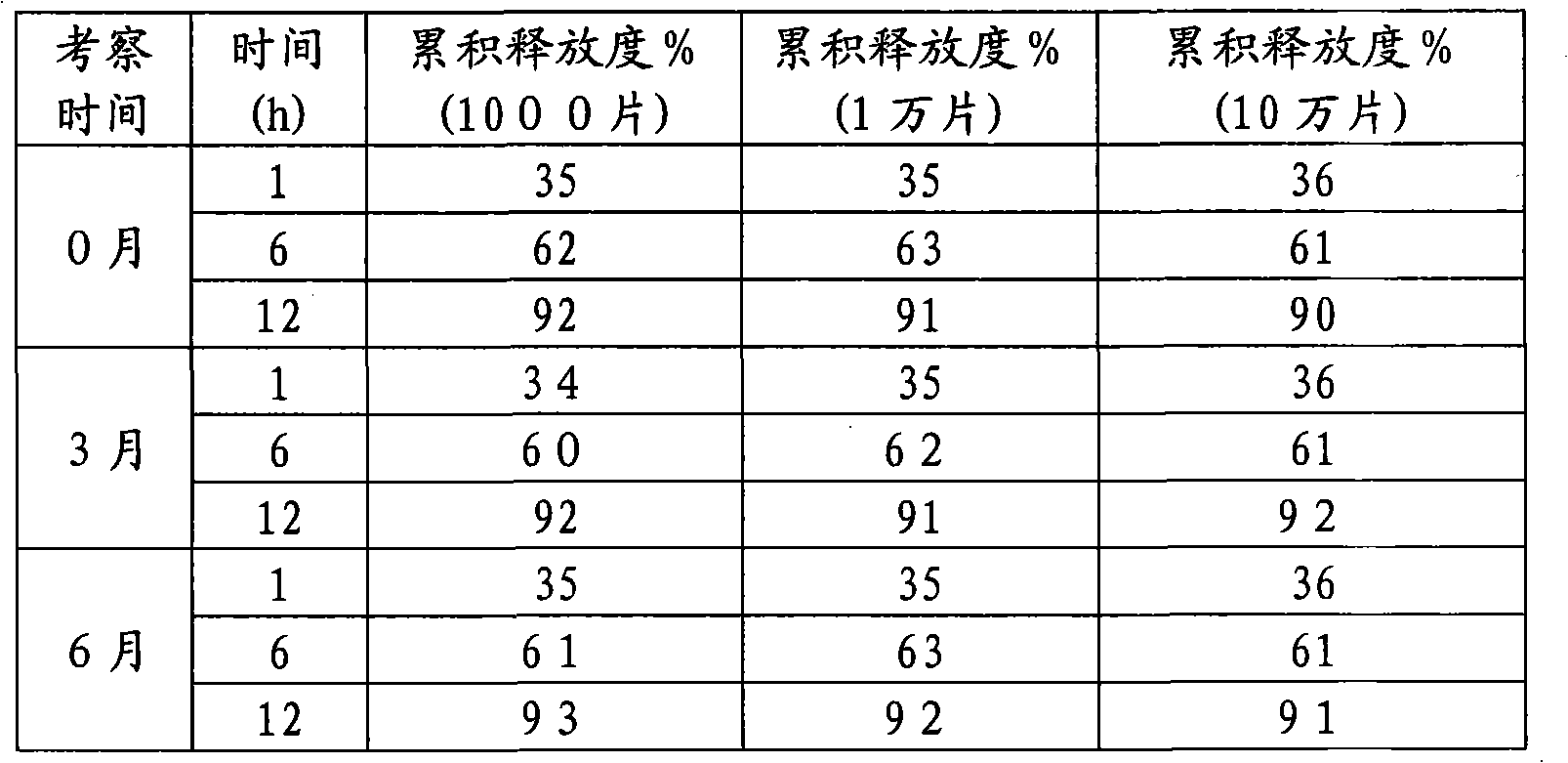
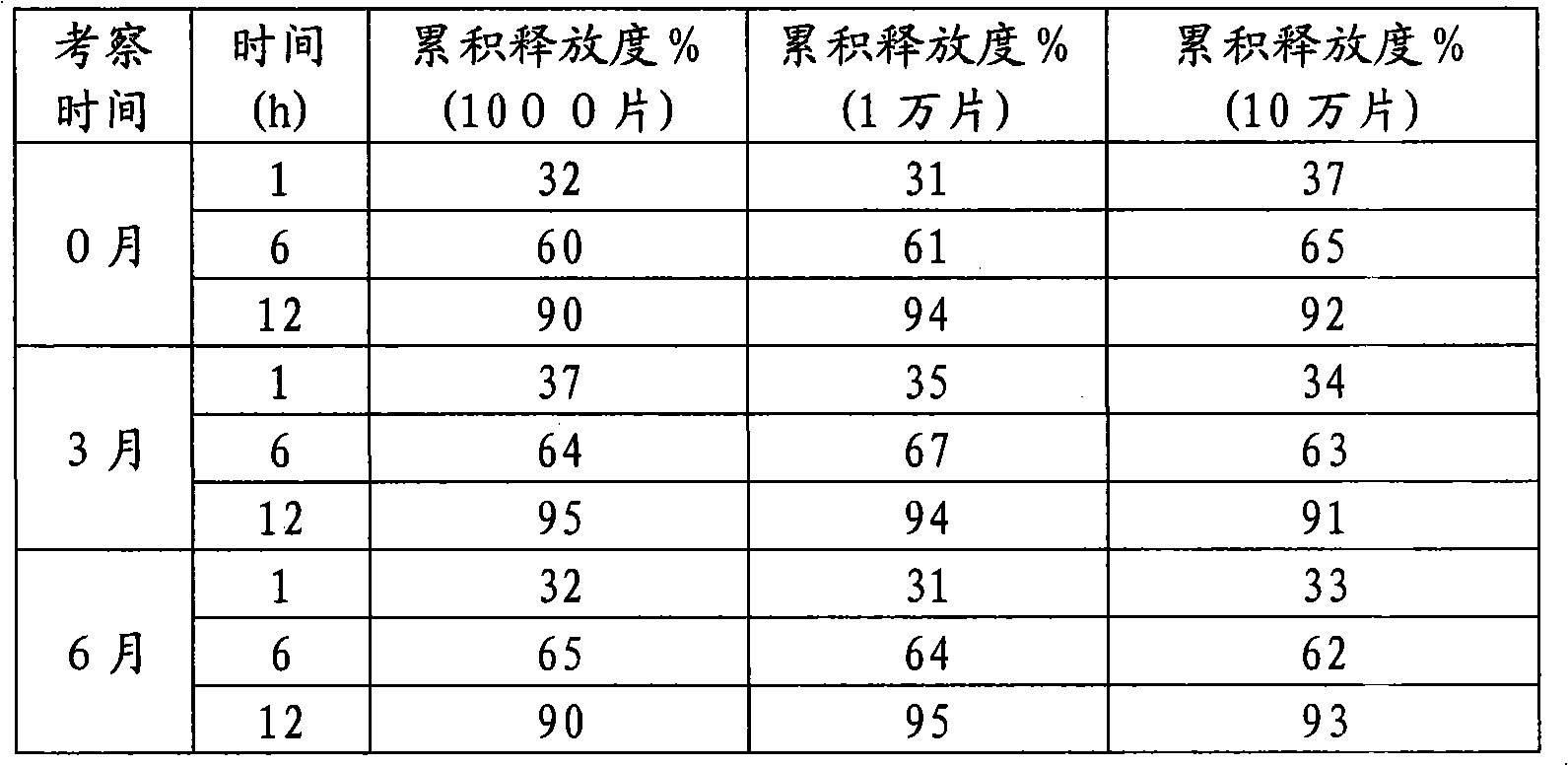
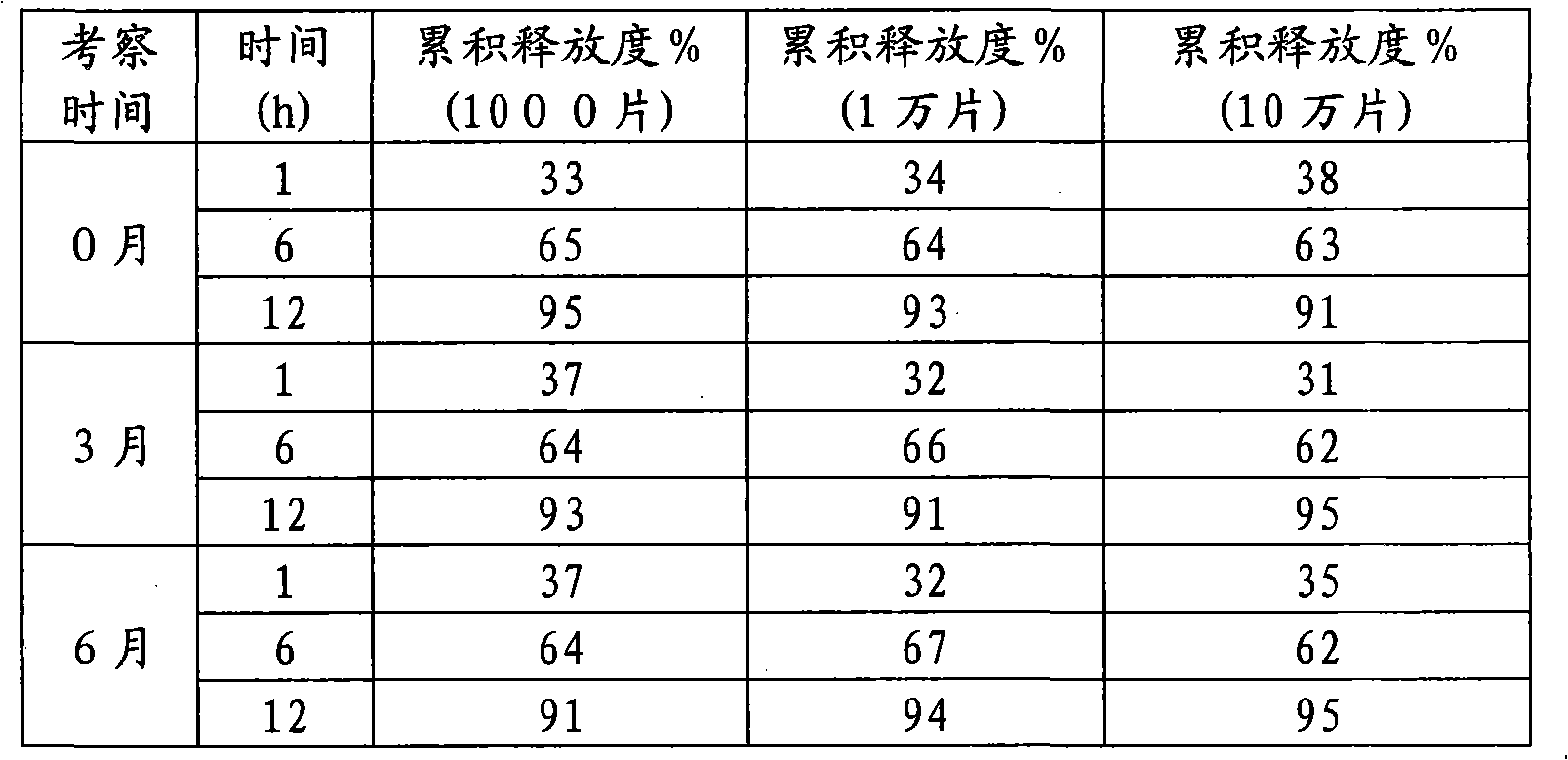
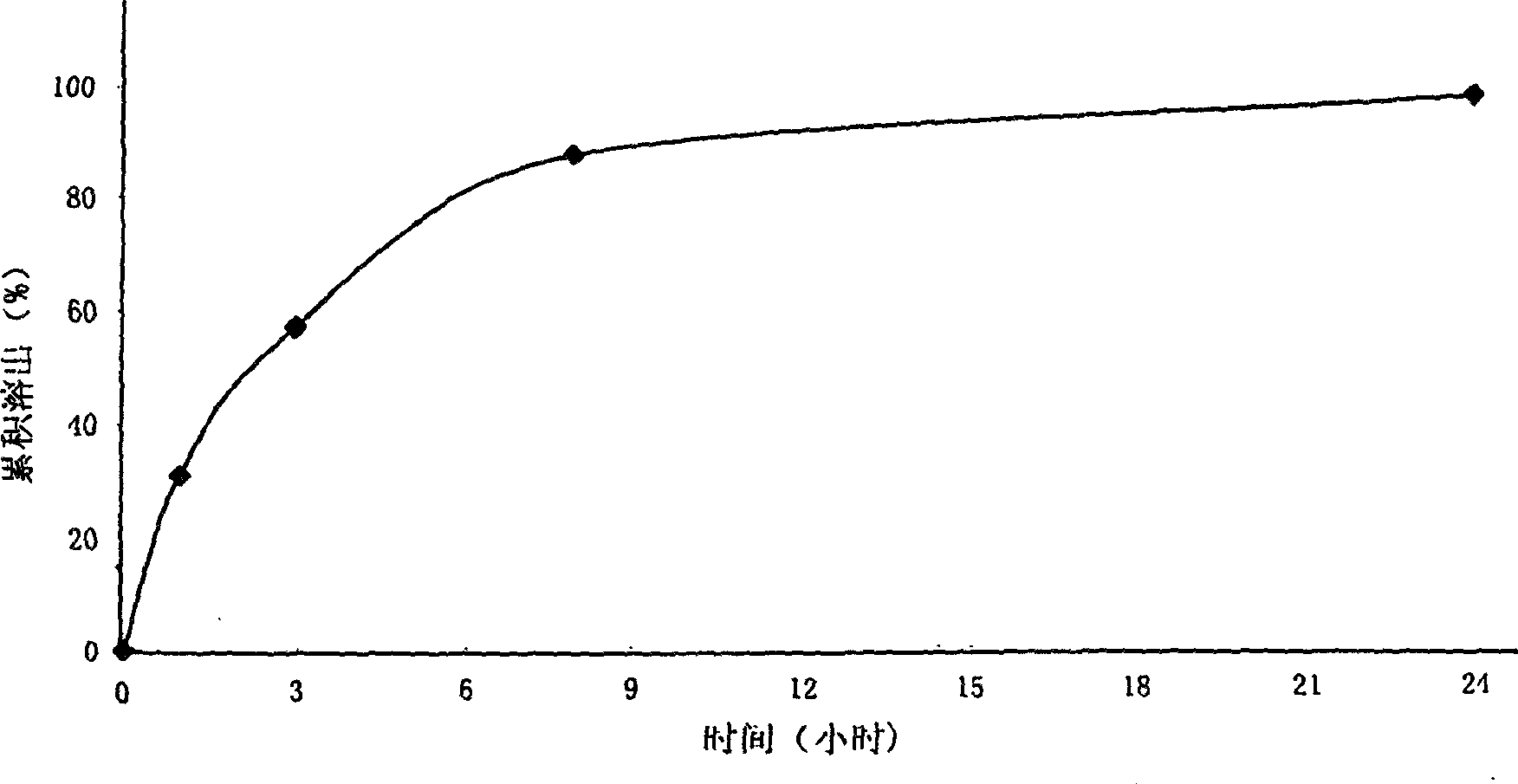
The invention discloses a mosapride citrate sustained-release tablet. The mosapride citrate sustained-release tablet comprises mosapride citrate, slow-release materials, a diluent, an adhesive, a lubricant and a coating material. A release degree of the mosapride citrate sustained-release tablet has good repeatability, regardless of being detected in an experiment or in mass production. After being subjected to stability monitoring for 6 months, the mosapride citrate sustained-release tablet still has an almost changeless release degree.
Owner:重庆健能医药开发有限公司
Metformin hydrochloride sustained-release tablet and method for preparing the same
ActiveCN1543937AConvenient amountReduce dosageOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
The invention provides a Metformin Hydrochloride slow release tablet and process for preparation, wherein the slow release tablet comprises metformin hydrochloride 46.5-70%, hydroxypropylcellulose 13.5-33.0%, micronization ethyl cellulose 10.0-14.0%, filling agent 1.3%-9.4%, and lubricating agent 1.2-1,5%. The preparation process comprises processing the prescribed raw material of metformin hydrochloride, cellulose glycollic ether, and the filling agent through the conventional tablet production process of granulation, drying, granulating, charging micronization ethyl cellulose, mixing homogenously with lubricant and tabletting.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Potassium citrate sustained release tablet and preparation method thereof
ActiveCN102240272A3 hours of increased releaseQuality improvementOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention provides a potassium citrate sustained release tablet and a preparation method thereof. A weight ratio of potassium citrate to a waxy sustained release matrix is 10:0.7-1.1, preferably 10:0.83-1.0, and most preferably 10:1.0. The sustained release tablet allows the concentration of citric acid radical in urine to be effectively increased, the fluctuation of the concentration of citrate in the urine to be reduced, and a stable and high level of the citrate concentration to be kept. More importantly, the three-hour release rate, the tabletting performance and the moisture resistance of the sustained release tablet are further improved.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Pharmaceutical composition containing pirfenidone in sustained-release tablet form
ActiveUS9408836B2Effective in regressionDeleterious effectNervous disorderAntipyreticHepatic fibrosisAnti fibrotic
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Sustained-Release Tablet and Process for Preparing the Same
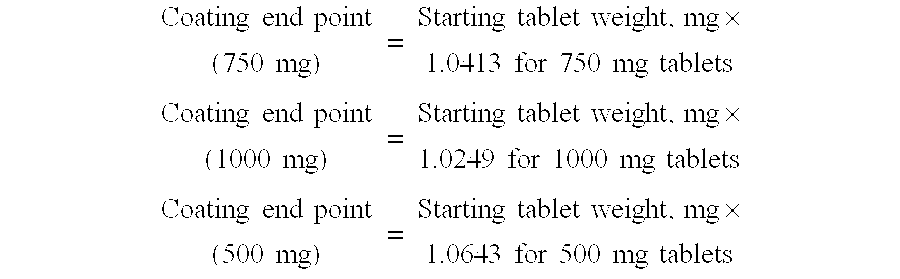
A method for producing a sustained-release tablet having improved stability and content uniformity is provided. The method involves first preparing a core tablet by granulating, drying, milling, blending, and compressing a mixture of active and inactive ingredients. Four coating layers are applied to the core tablet: an inner layer, an enteric coating layer, an active layer, and an outer layer. The active ingredient may be a tetracycline, such as doxycycline. A sustained-release tablet prepared according to the method is also described.
Owner:GALDERMA SA
Preparation method of clonidine hydrochloride sustained-release tablet
InactiveCN104138362AWell mixedIncrease in sizeOrganic active ingredientsNervous disorderSustained Release TabletFiller Excipient
The invention relates to a preparation method of a clonidine hydrochloride sustained-release tablet. The preparation method comprises the steps of dissolving a clonidine hydrochloride raw material into a proper amount of wetting agent by using a solvent dispersion method to prepare a solution containing 10-28.57mg / ml clonidine; adding the solution into a granulation pan filled with a filling agent and a binder at constant speed of 5-40ml / min to granulate; drying the prepared granules, screening by using a 50-100-mesh sieve, mixing the granules, a framework material, a flow aid and a lubricating agent, and tabletting to obtain the clonidine hydrochloride sustained-release tablet. By using the preparation method, the problem of non-uniform content generated in a preparation process of the clonidine hydrochloride sustained-release tablet can be effectively solved, the prepared clonidine hydrochloride sustained-release tablet is uniform in content, and the release behavior is in accord with the sustained-release characteristic. All process parameters in the preparation method can be correspondingly amplified according to the industrial production scale, so that the requirement for large-scale industrial production can be met.
Owner:LP PHARM (XIAMEN) CO LTD
Quetiapine sustained release tablets and method of preparing the same
ActiveCN101347413AGood slow releaseSimple processOrganic active ingredientsNervous disorderSustained Release TabletControl release
The invention belongs to the field of pharmaceutical preparations, more specifically relates to a seroquel sustained release tablet and a preparation method thereof. The sustained release tablet of the invention comprises 5%-50% of sustained release framework material with pH-dependent solubility according to weight percentage, and preferably comprises 15%-35% of the sustained release framework material with the pH-dependent solubility. The seroquel sustained release tablet prepared by the invention runs in the gastrointestinal tract by imitating sustained release and controlled release agents, the in vitro release rate thereof is determined, which proves that the sustained seroquel release tablet can continuously release for 6-24 hours. The seroquel sustained release tablet of the invention reduces dosage of sustained release material based on good sustained release performance and facilitates the administration of patients. The seroquel sustained release tablet of the invention has simple technology and is good for large-scale industrialized production at the same time.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com