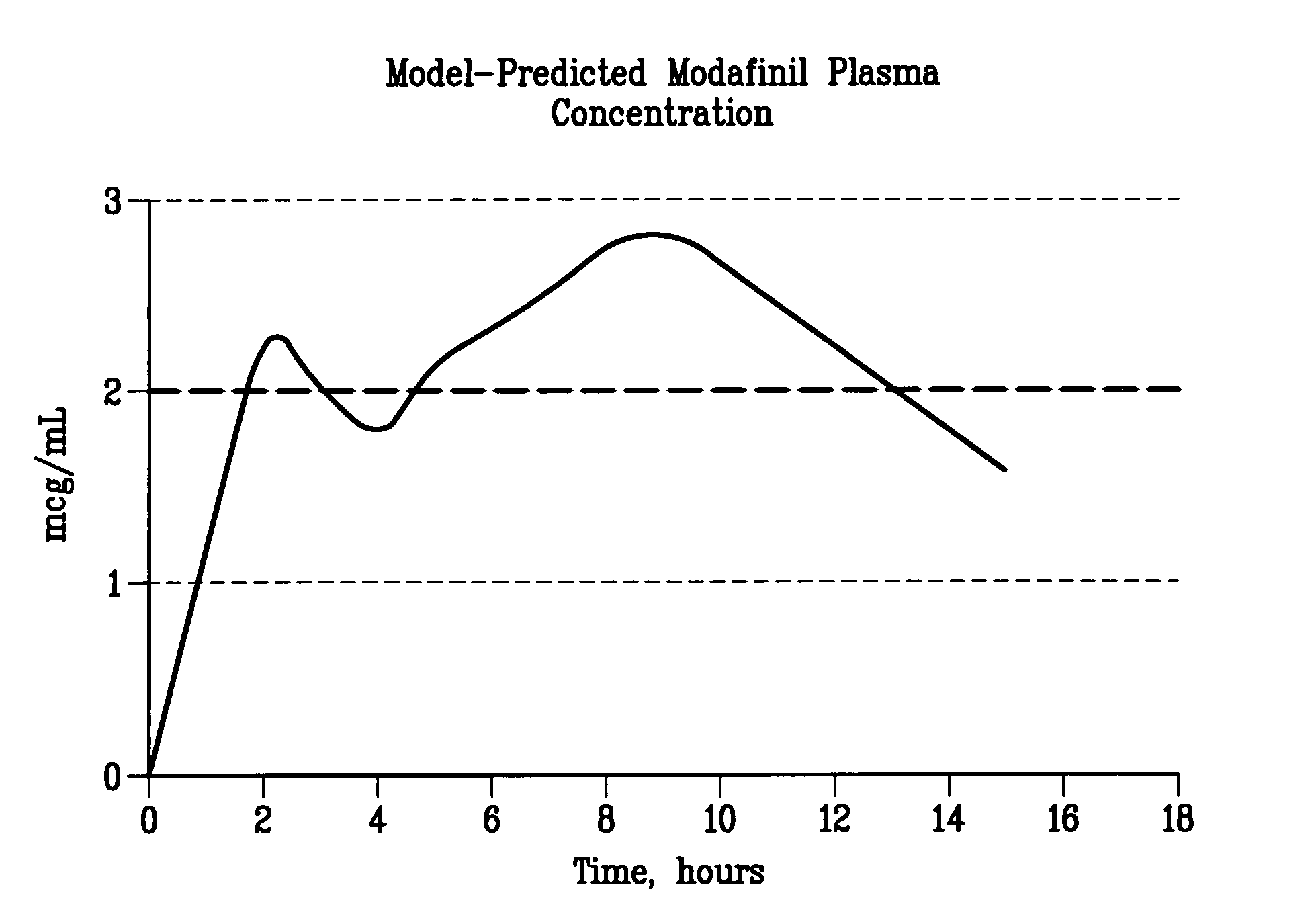
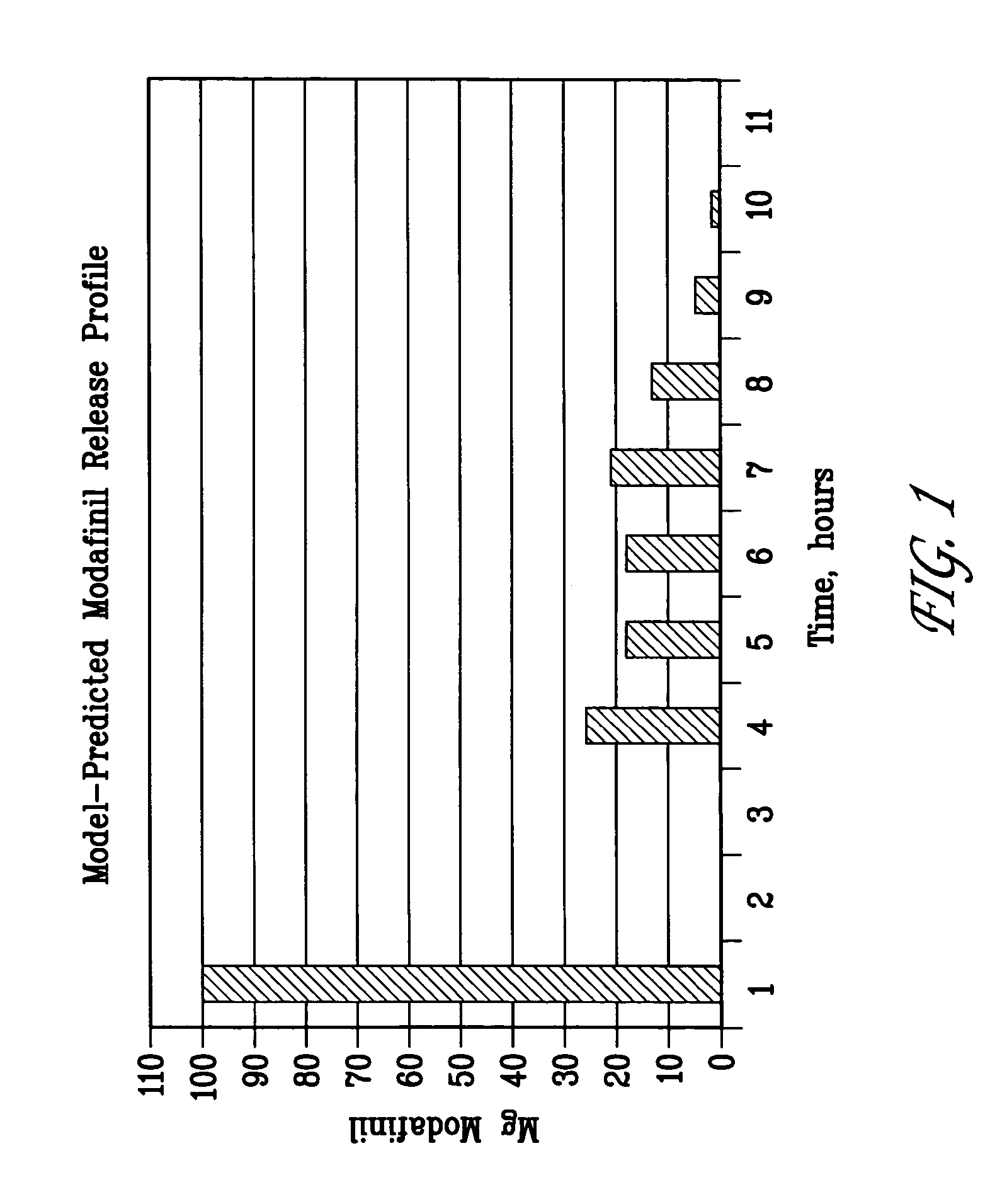
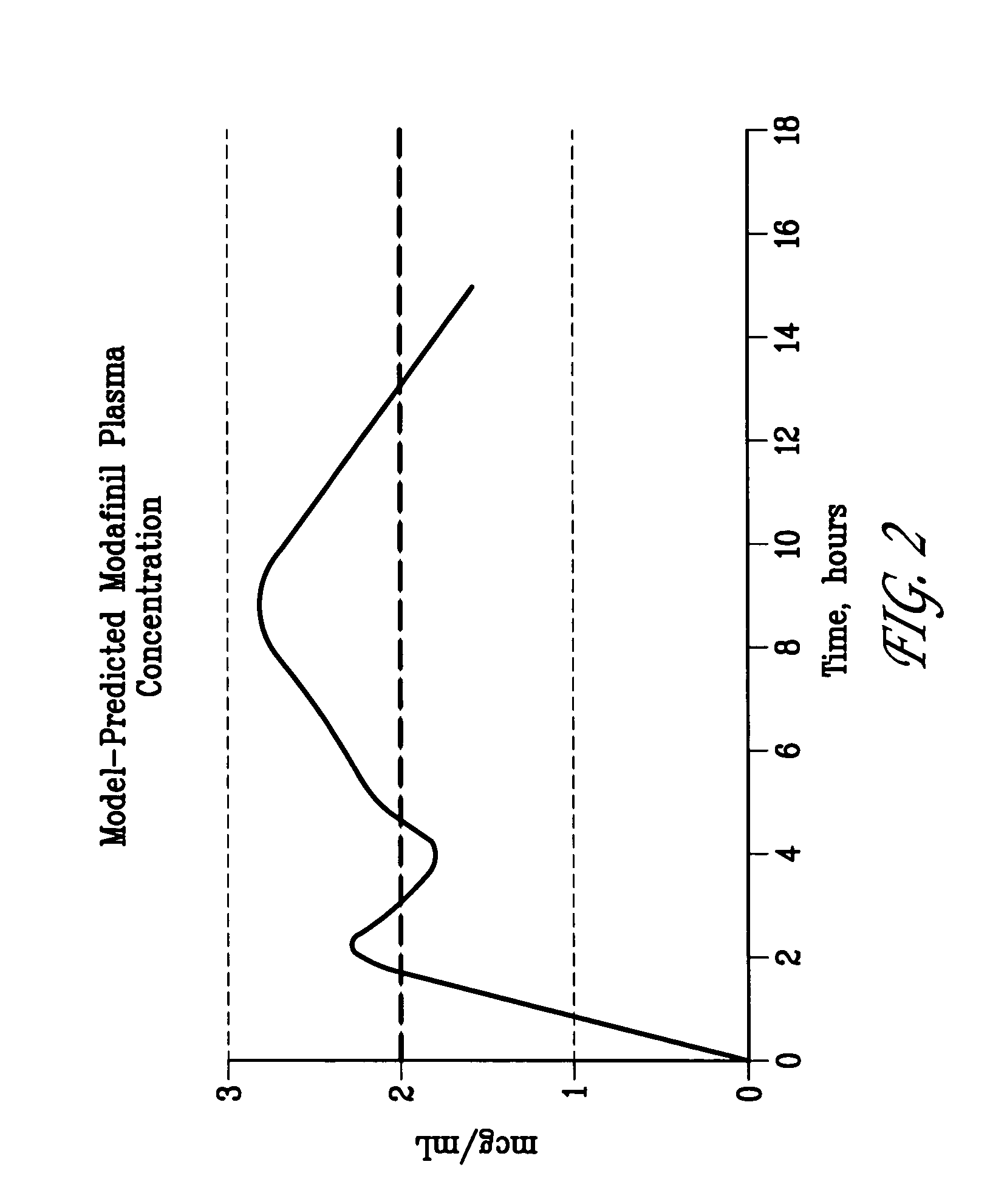
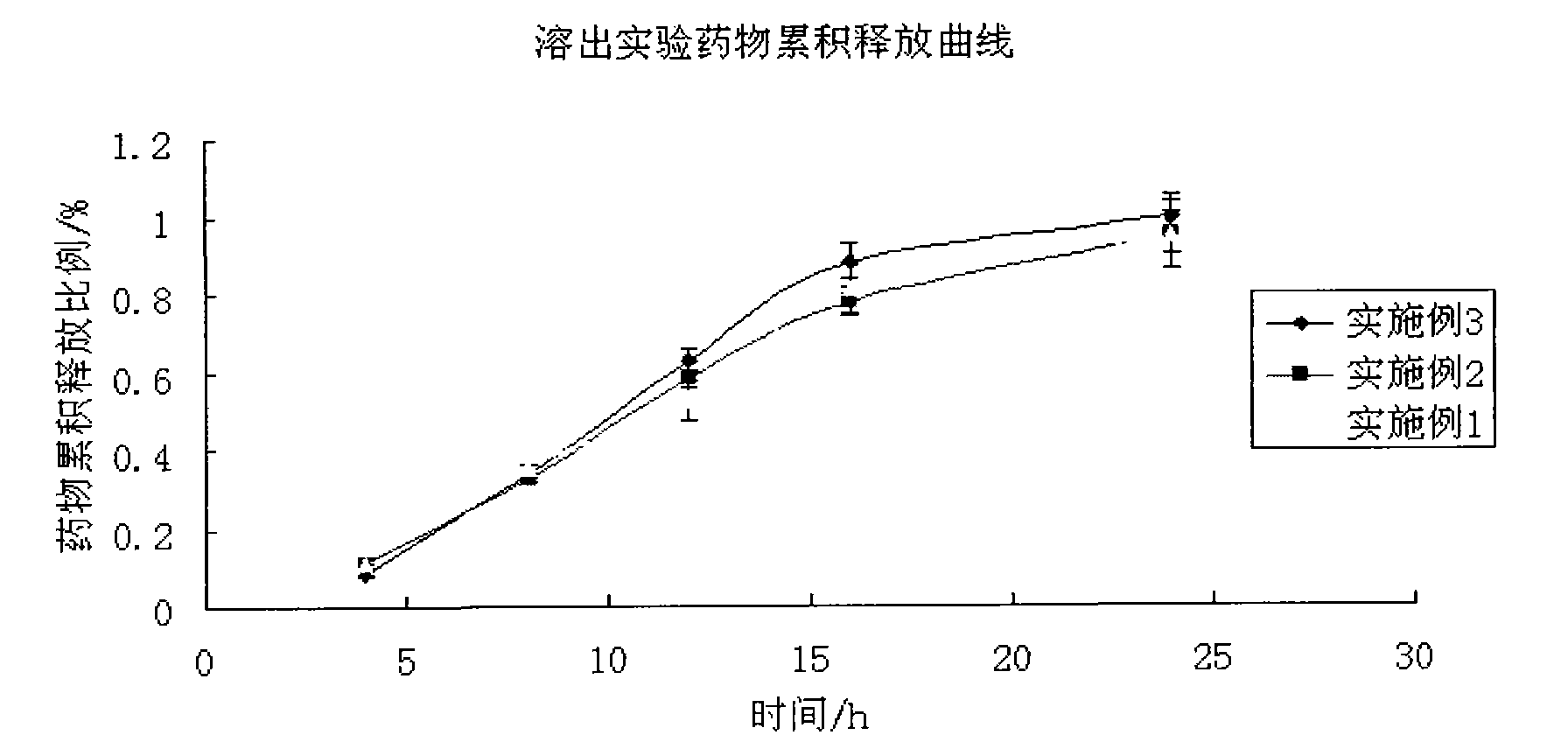
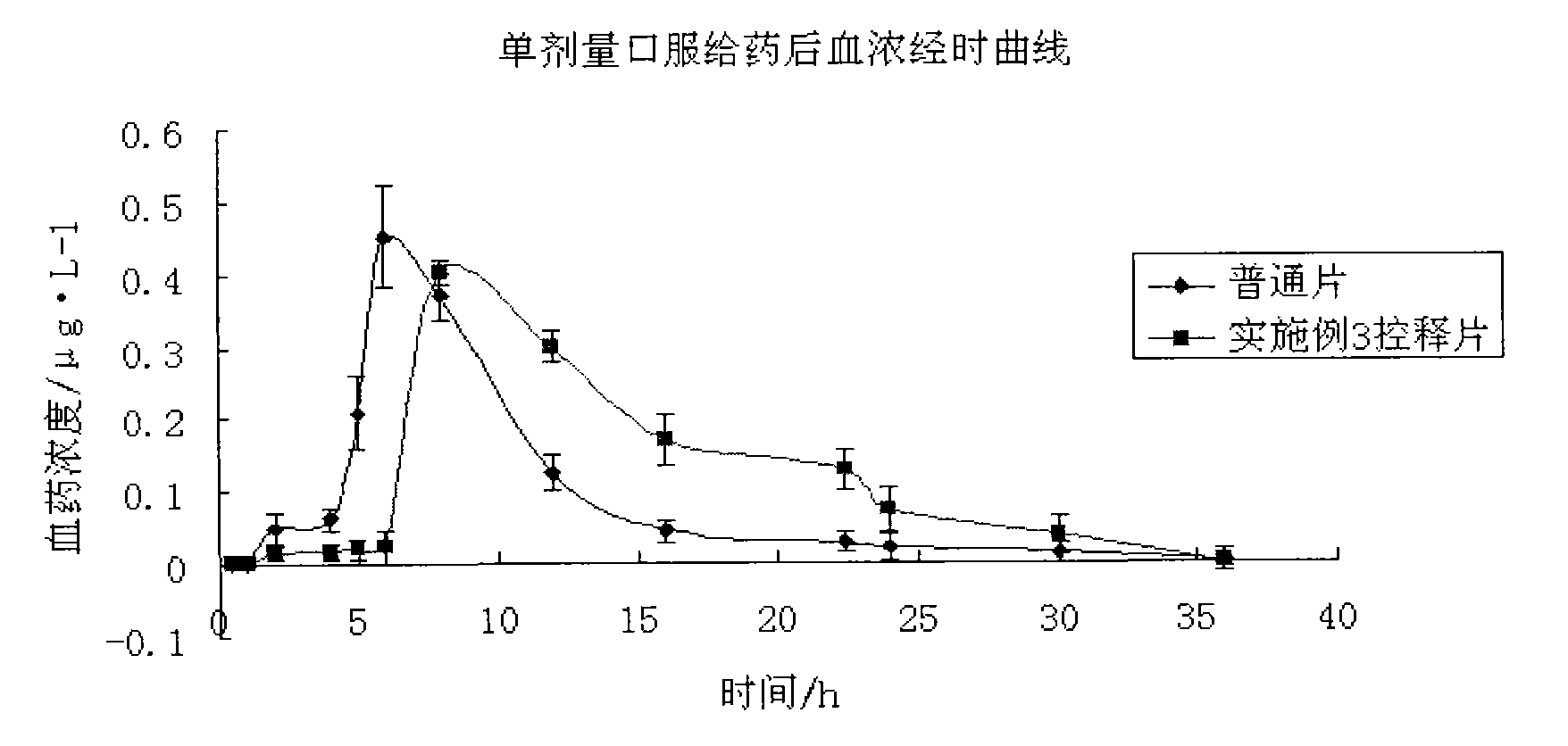
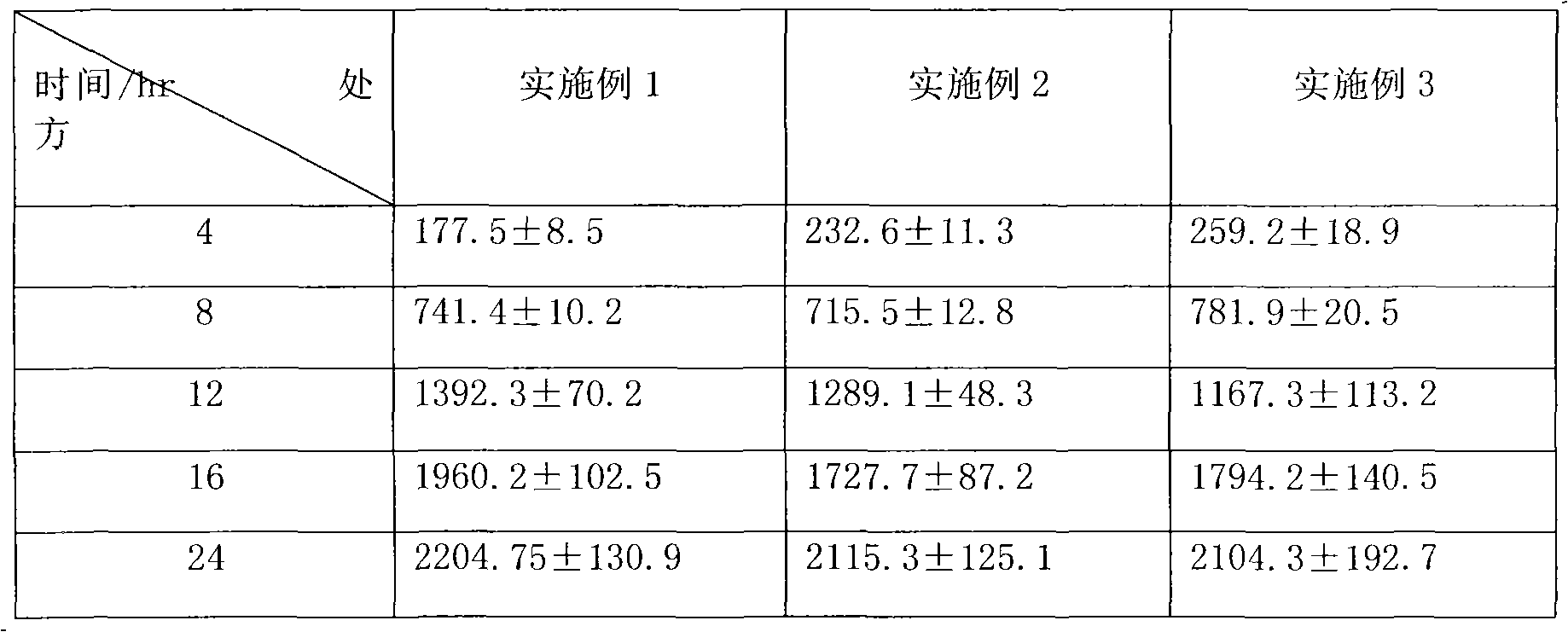
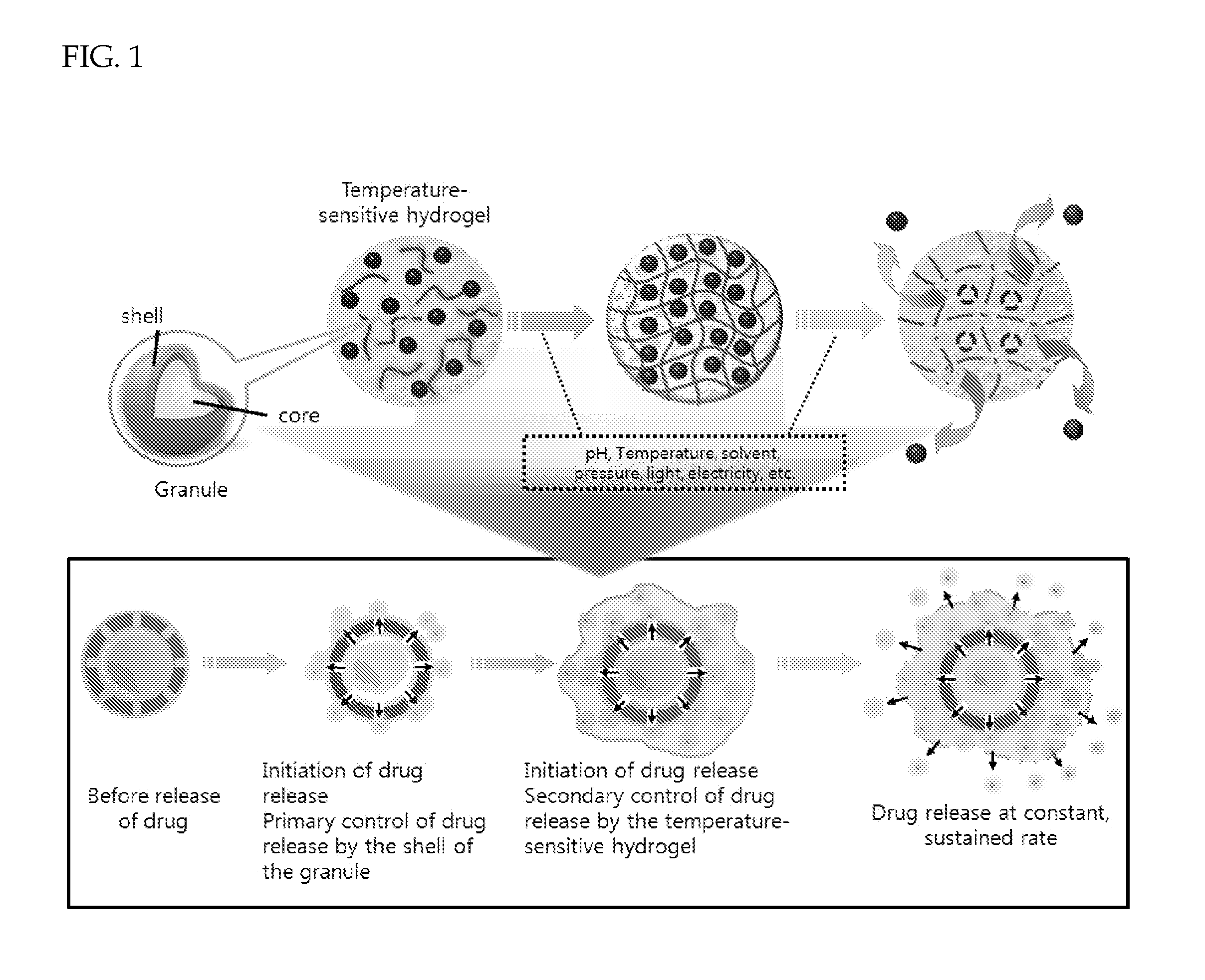
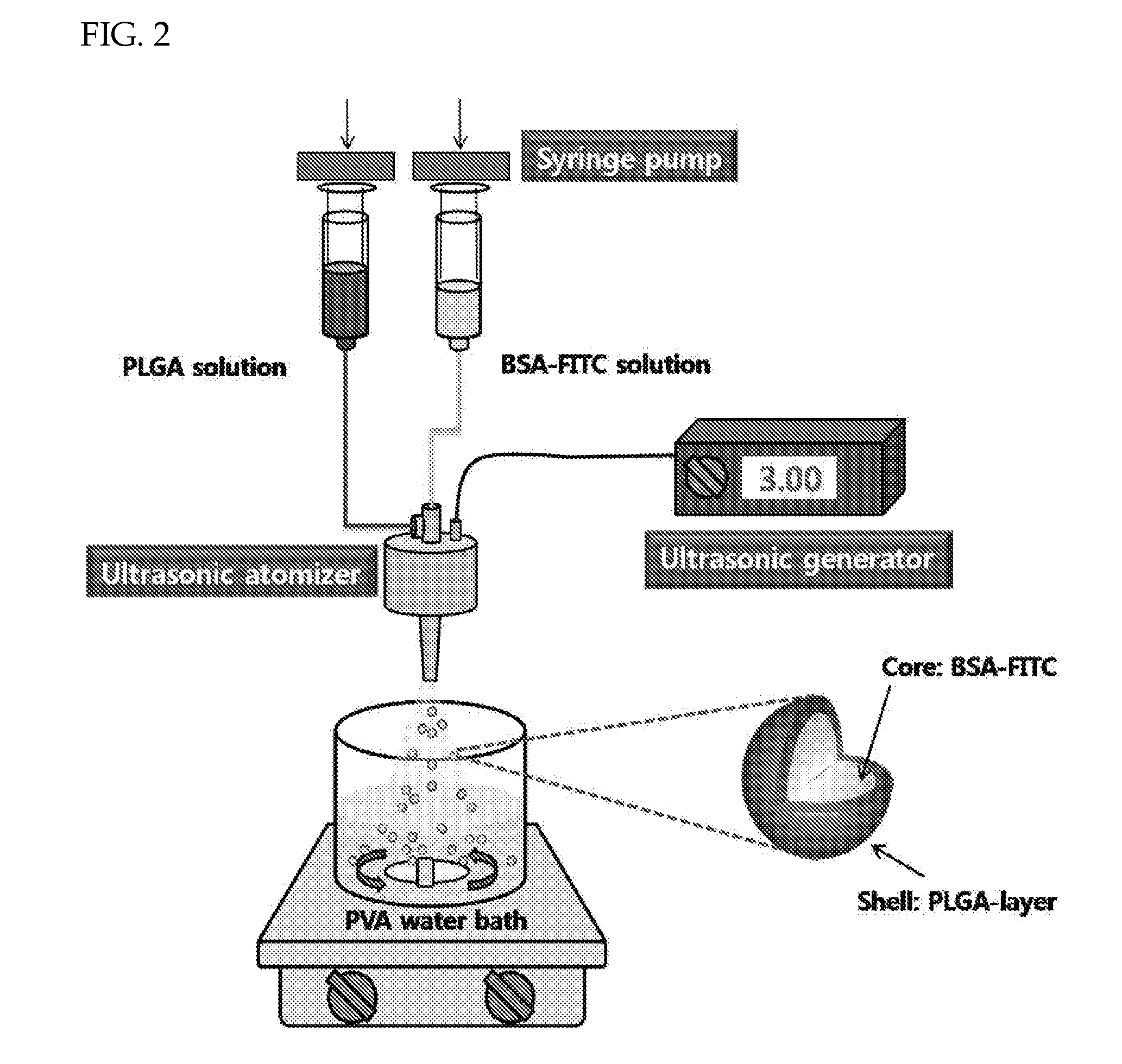
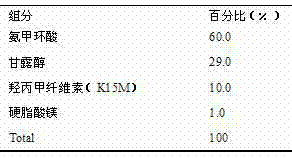
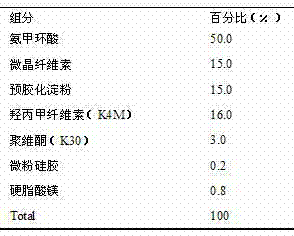
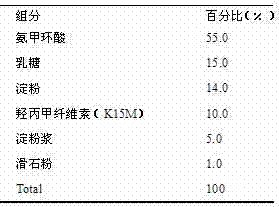
Patents
Literature
88results about How to "Maintain blood levels" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
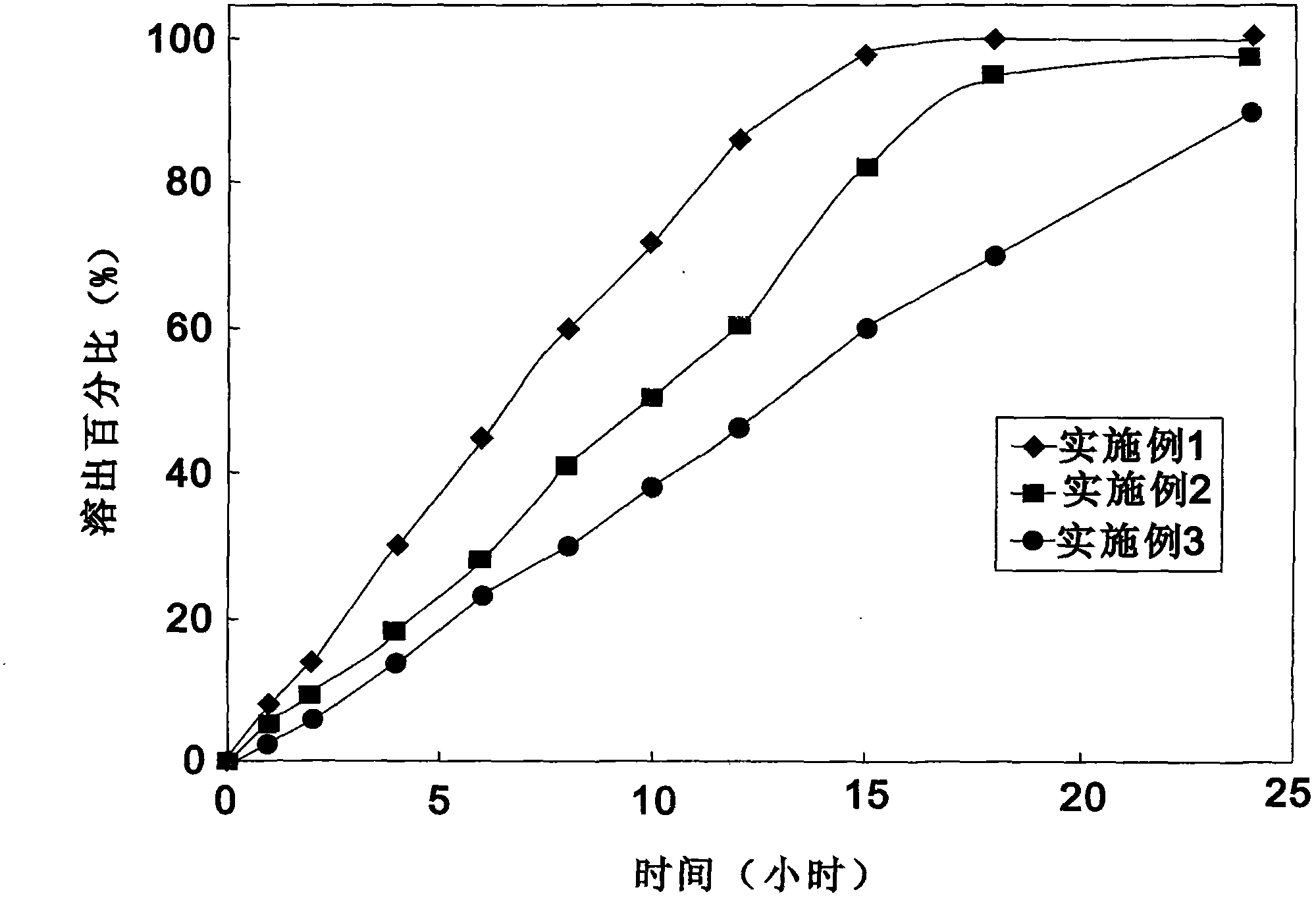
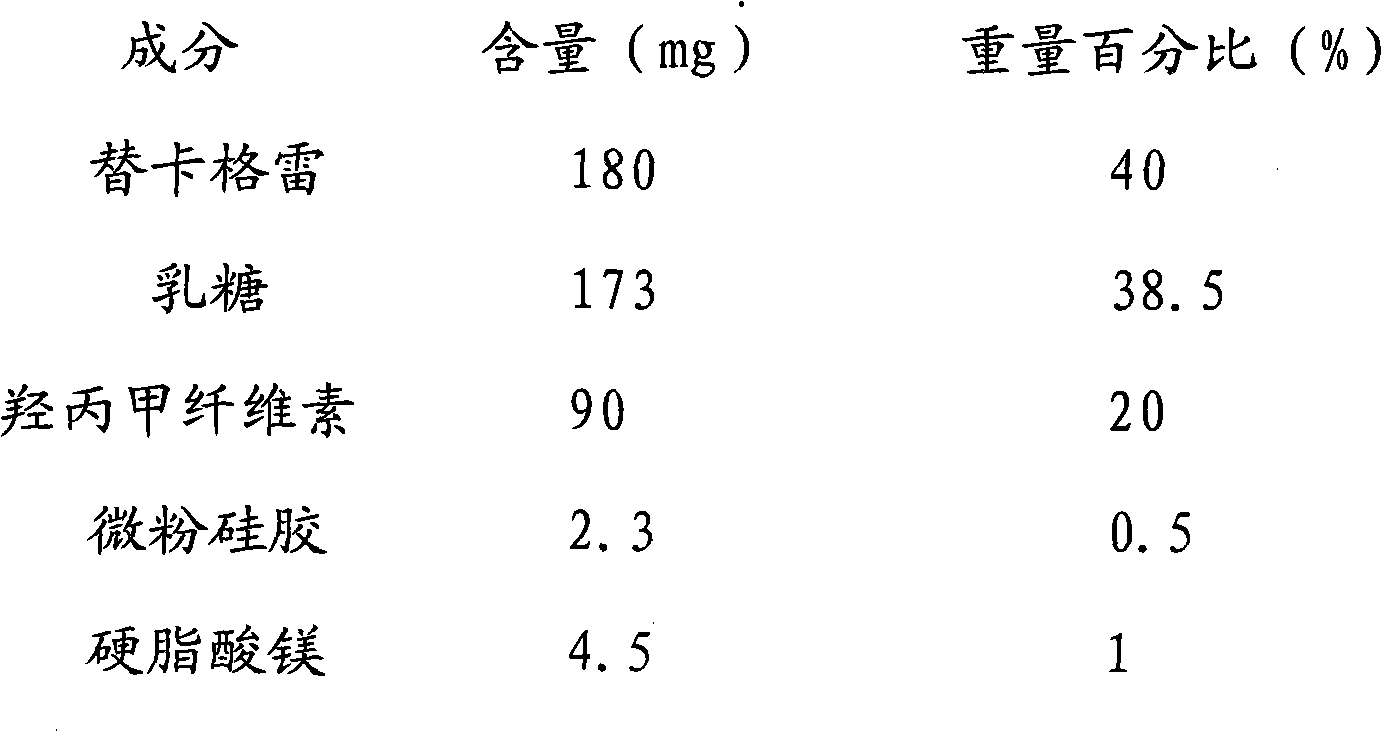
Ticagrelor sustained-release tablet system and preparation method thereof
InactiveCN102657629AMaintain blood levelsOrganic active ingredientsPharmaceutical delivery mechanismTicagrelorPediatrics
The invention provides a ticagrelor sustained-release tablet system and a preparation method of the ticagrelor sustained-release tablet system. The preparation method comprises the following steps of: firstly uniformly mixing 10-60% of ticagrelor, 5-60% of a filler and 5-60% of high molecular polymer in percentage by weight, adding a granulating solution to granulate; fully drying the obtained granules at a temperature of 50-60 DEG C, uniformly mixing the sieved granules with 0.25-10% of a lubricant and / or 0-10% of a flow aid, carrying out tabletting to obtain the ticagrelor matrix type sustained-release tablets, wherein the granulating solution is preferably water, an alcohol-water solution or absolute ethyl alcohol; the granule size of the ticagrelor is below 100 micrometers; and the content of the ticagrelor is 50-300mg in the preparation process. The ticagrelor matrix type sustained-release tablet system provided by the invention has the advantages that a patient can take the ticagrelor once a day to ensure that the drug dependence of the patient can be improved and the risk of myocardial infarction or apoplexy caused by acute thrombosis due to a dose of the ticagrelor missing of the patient is reduced.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE
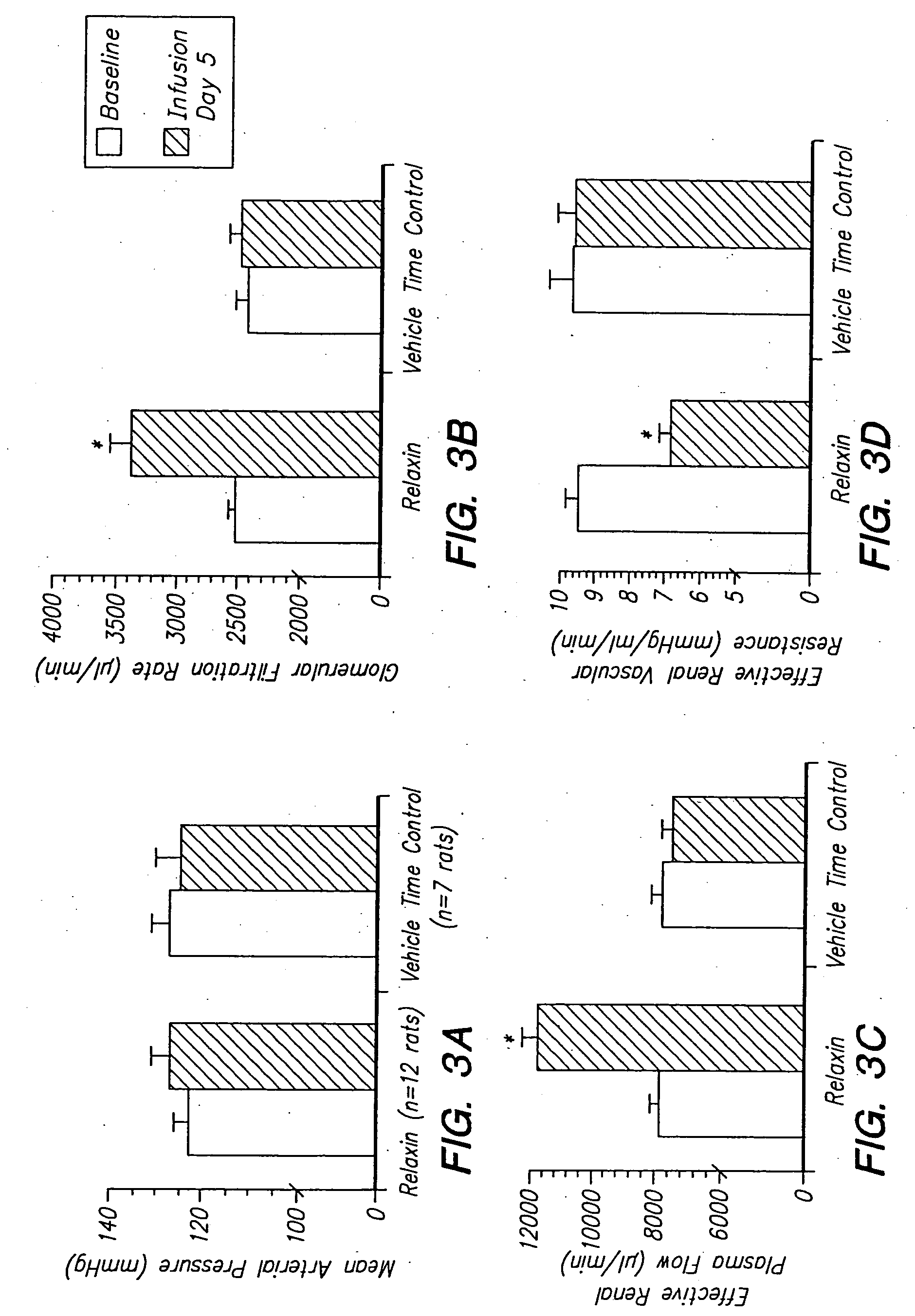
Use of relaxin to treat diseases related to vasoconstriction
InactiveUS20040266685A1Safety profileImprove securityPeptide/protein ingredientsRelaxinsArteriolar VasoconstrictionDisease
The invention relates to methods of treating diseases related to vasodilation, generally comprising administering to an individual an effective amount of a pharmaceutically active relaxin. Relaxin functions to increase both vasodilation and angiogenesis in males as well as females, and is therefore useful in treating a wide variety of diseases relating to vasoconstriction.
Owner:THE UNIVERSITY OF MEDICINE & DENTISTRY OF NEW JERSEY ROBERT WOOD JOHNSON MEDICAL SCHOOL +2
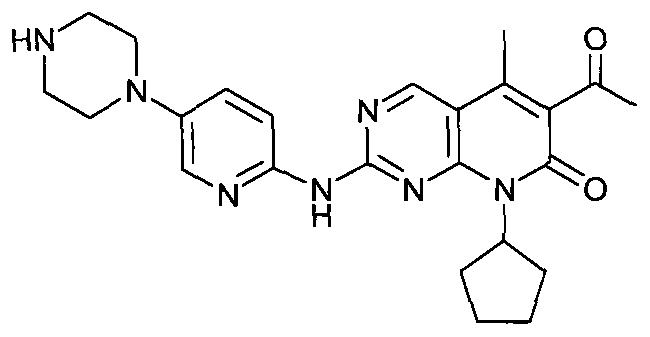
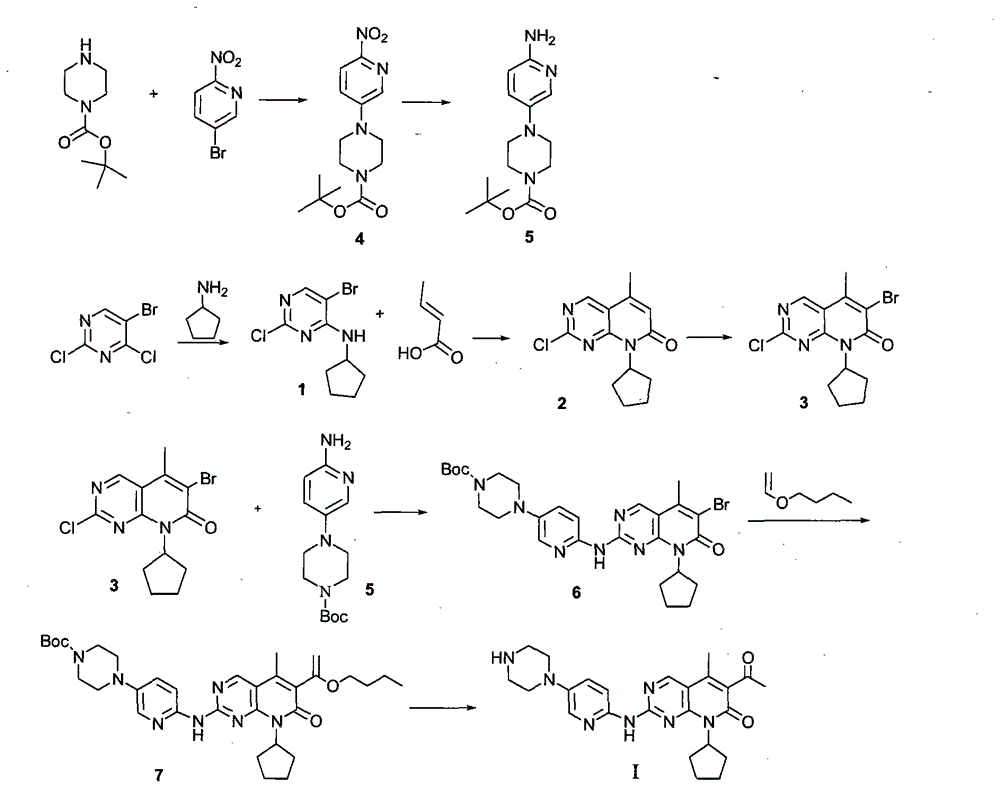
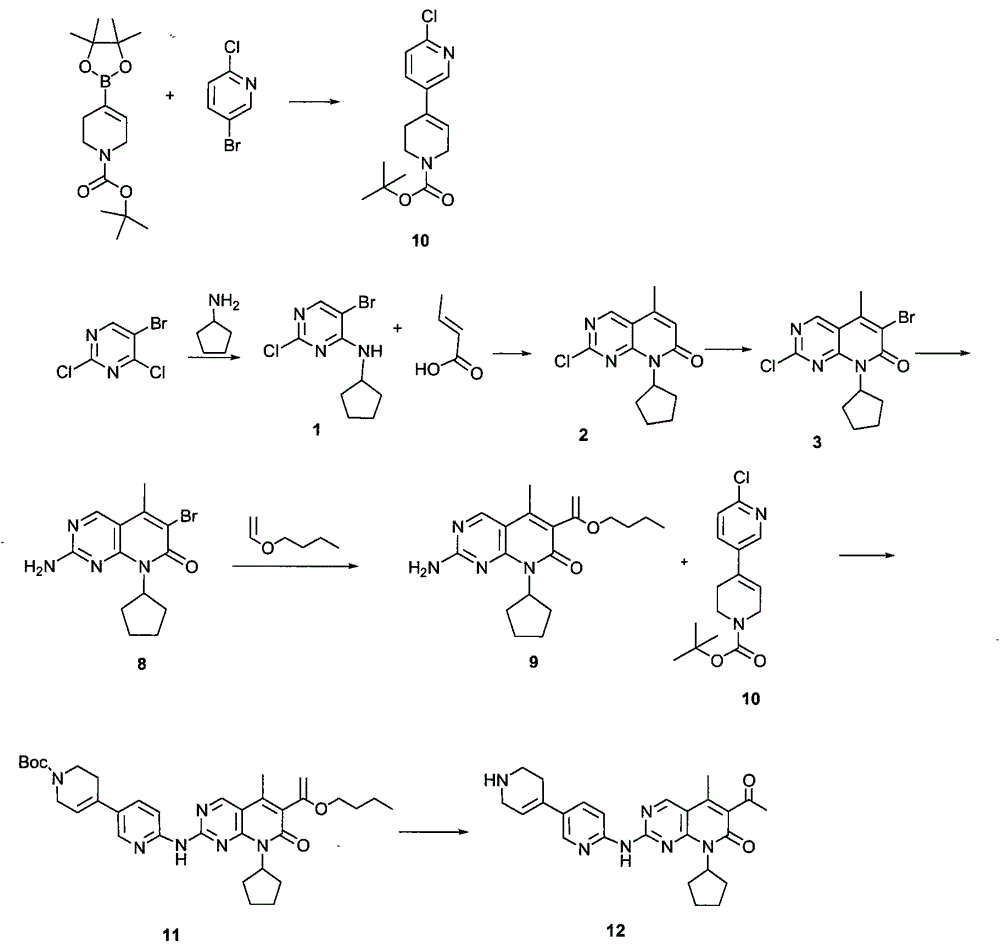
Novel synthesis method of CDK4 (cyclin-dependent kinase 4) inhibitor
ActiveCN104892604AMaintain blood levelsOrganic active ingredientsOrganic chemistrySynthesis methodsPyridine
The invention relates to a novel synthesis method of a CDK4 (cyclin-dependent kinase 4) inhibitor. A reaction of 2-chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one with acetylchloride is taken as an initial reaction, consequent reactions are performed, and the CDK4 inhibitor is prepared; the preparation method is simple and easy to implement, the yield is high, the quality is high, and industrial production is facilitated.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Nifedipine osmotic pump controlled release tablet and preparation method thereof
InactiveCN102138912AMaintain blood levelsGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineControlled Release Tablet
The invention provides a nifedipine osmotic pump controlled release tablet comprising a drug-containing layer tablet core, a booster layer tablet core, a coating membrane and a single drug-release pore on the surface of the controlled release tablet at one side of the drug-containing layer tablet core. The nifedipine osmotic pump controlled release tablet provided by the invention has stable medicament release velocity, basically realizes zero drug release within 0-20h and basically fully releases drug; therefore, the dosing number of a patient is reduced, and more stable blood drug concentration can be realized after a patient takes the drug. The nifedipine osmotic pump controlled release tablet provided by the invention is a safe, effective, stable, controllable and conveniently-applied medicament new preparation for clinically treating hypertension.
Owner:CHINA PHARM UNIV
Drug delivery formulation for controlling of initial burst and manufacturing method thereof
ActiveUS20120177740A1Maintain blood levelsMaximizing its therapeutic effectPowder deliveryPeptide/protein ingredientsBlood levelBiodegradable polymer
Provided is a drug delivery system for control of initial burst of a drug. More particularly, there are provided a drug delivery formulation including: a granule containing a biodegradable polymer and a drug; and a temperature-sensitive hydrogel, and a method for preparing the same. The presently disclosed drug delivery formulation can be prepared via a relatively simple process and allows a drug to be released slowly at a constant rate without initial burst and thus maintains a constant blood level of the drug for a long period of time. Consequently, it is capable of preventing the initial burst of the existing injection-type drug delivery formulations and slow-release granules and providing a desired release profile, including sustained release with time.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Tranexamic acid sustained-release solid composition and preparation method thereof
InactiveCN102525878ASolve the problem of too fast dissolution in waterMaintain blood levelsPeptide/protein ingredientsPharmaceutical non-active ingredientsBlood concentrationTranexamic acid
The invention discloses a tranexamic acid sustained-release solid composition and a preparation method thereof. The sustained-release solid composition is prepared by taking tranexamic acid as a raw material and adding a sustained-release framework material in a proper proportion. The prepared preparation is capable of achieving zero-order release within 3 h to keep blood concentration steady and is used for treating women suffering from menorrhagia clinically.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
Sustained release tablet containing donepezil hydrochloride active component as well as preparation method and application thereof
InactiveCN102309465AGood reproducibilityImprove consistencyNervous disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a sustained release tablet containing a donepezil hydrochloride serving as an active component. The sustained release tablet provided by the invention comprises the donepezil hydrochloride serving as an active component, a sustained-release material and other auxiliary materials, wherein the weight ratio of the donepezil hydrochloride serving as an active component to the sustained-release material is 1:(0.2-20), preferably 1:(0.5-10) and further preferably 1:(0.7-8). The sustained release tablet containing donepezil hydrochloride prepared by the invention is released steadily, which is favorable for the reduction of the fluctuation of the in-vivo medicament, thus the side effect is reduced, and the sustained release tablet is more suitable for treating patients suffering from Alzheimer-type dementia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Recombined human bFGF and PDGF-B duplicate adenovirus carrier and uses thereof
ActiveCN101319229AGood treatment effectReduce manufacturing costGenetic material ingredientsFermentationDiseaseCardiac muscle
The invention belongs to the gene therapy field. The technical problem to be solved is to provide a new gene therapy product capable of effectively treating diseases of a cardiovascular system. The invention particularly relates to an expression vector containing recombinant human bFGF and PDGF-B double genes, and a preparation method and an application thereof. The vector which contains the gene capable of encoding bFGF protein and the gene capable of encoding PDGF-B protein can simultaneously express the bFGF protein and the PDGF-BB protein in a eukaryotic cell. An optimal proposal is to make the gene capable of encoding the bFGF protein and the gene capable of encoding the PDGF-B protein be expressed respectively under the control of different promoters. Experiments show that: the recombinant vector has good application prospect for treating myocardial ischemia of coronary heart disease and provides a new choice for treating the diseases of the cardiovascular system.
Owner:GUANGXI WUZHOU PHARMA GRP
Double-controlled release gliclazide sustained-release capsules and preparation method thereof
ActiveCN102058563ALong-lasting effectMaintain blood levelsMetabolism disorderSulfonylurea active ingredientsSustained release pelletsSustained Release Capsule
The invention discloses double-controlled release gliclazide sustained-release capsules and a preparation method thereof. Contents filled into the sustained-release capsules are gliclazide sustained-release pellets of which surfaces are coated with quick release film coatings. Based on 1,000 capsules, the gliclazide sustained-release pellets are prepared from the following raw materials by weight: 20 to 30 grams of gliclazide, 30 to 50 grams of microcrystalline cellulose, 40 to 60 grams of lactose, 2 to 8 grams of hydroxypropyl methylcellulose 4000cp and 30 to 60 grams of water; and the quickrelease film coatings are prepared from the following raw materials by weight: 5 to 20 grams of gliclazide, 10 to 40 grams of EudragitRL100, 5 to 30 grams of talcpowder and 300 grams of ethanol at the volume concentration of 95 percent. Compared with the prior art, the capsules provided by the invention can develop the advantages of multi-unit preparations at the same time of meeting requirementson the quick response of medicaments and keeping a certain blood concentration; and the preparation method for the capsules is simple and easy to control.
Owner:桂林华信制药有限公司
Levofloxacin hydrochloride micropill capsule and preparation method thereof
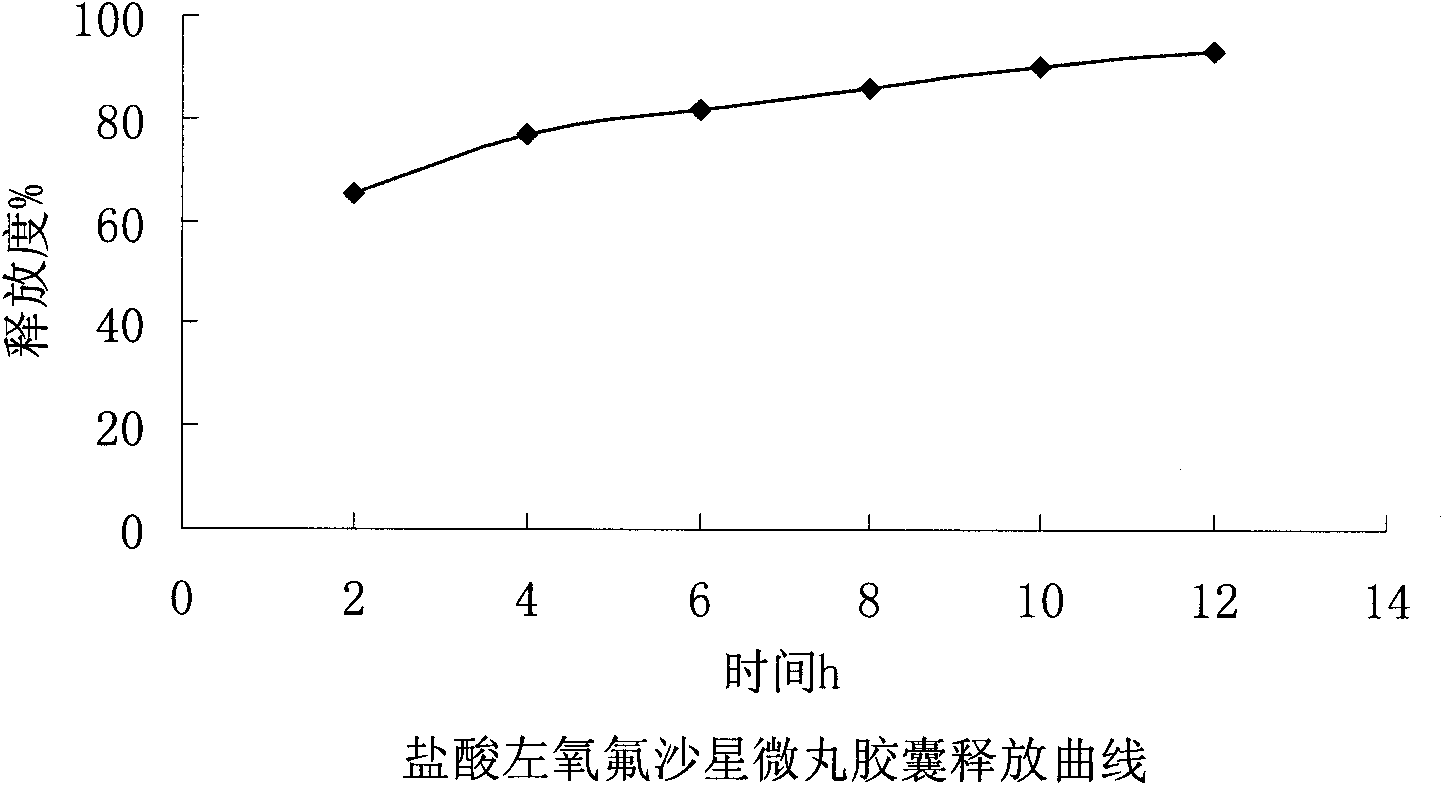
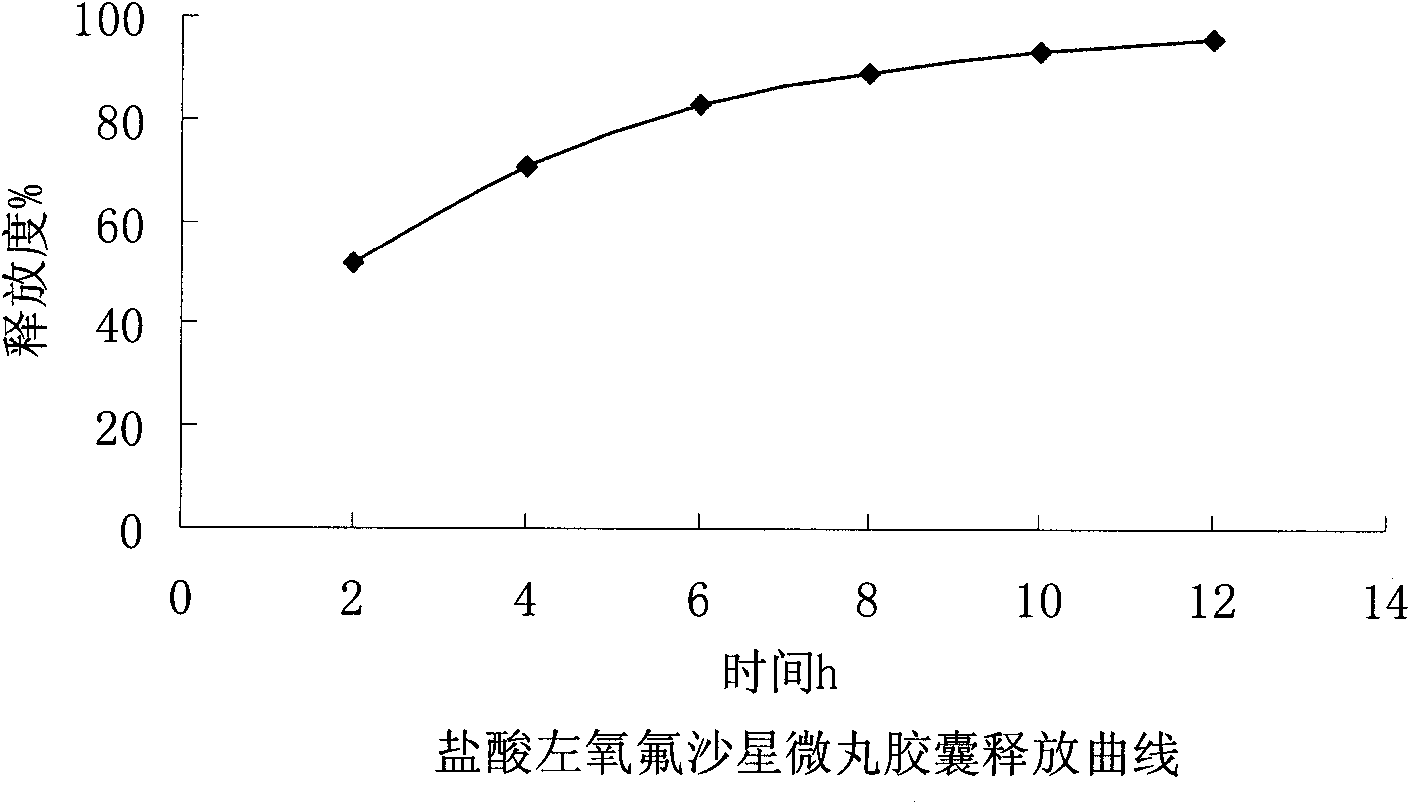
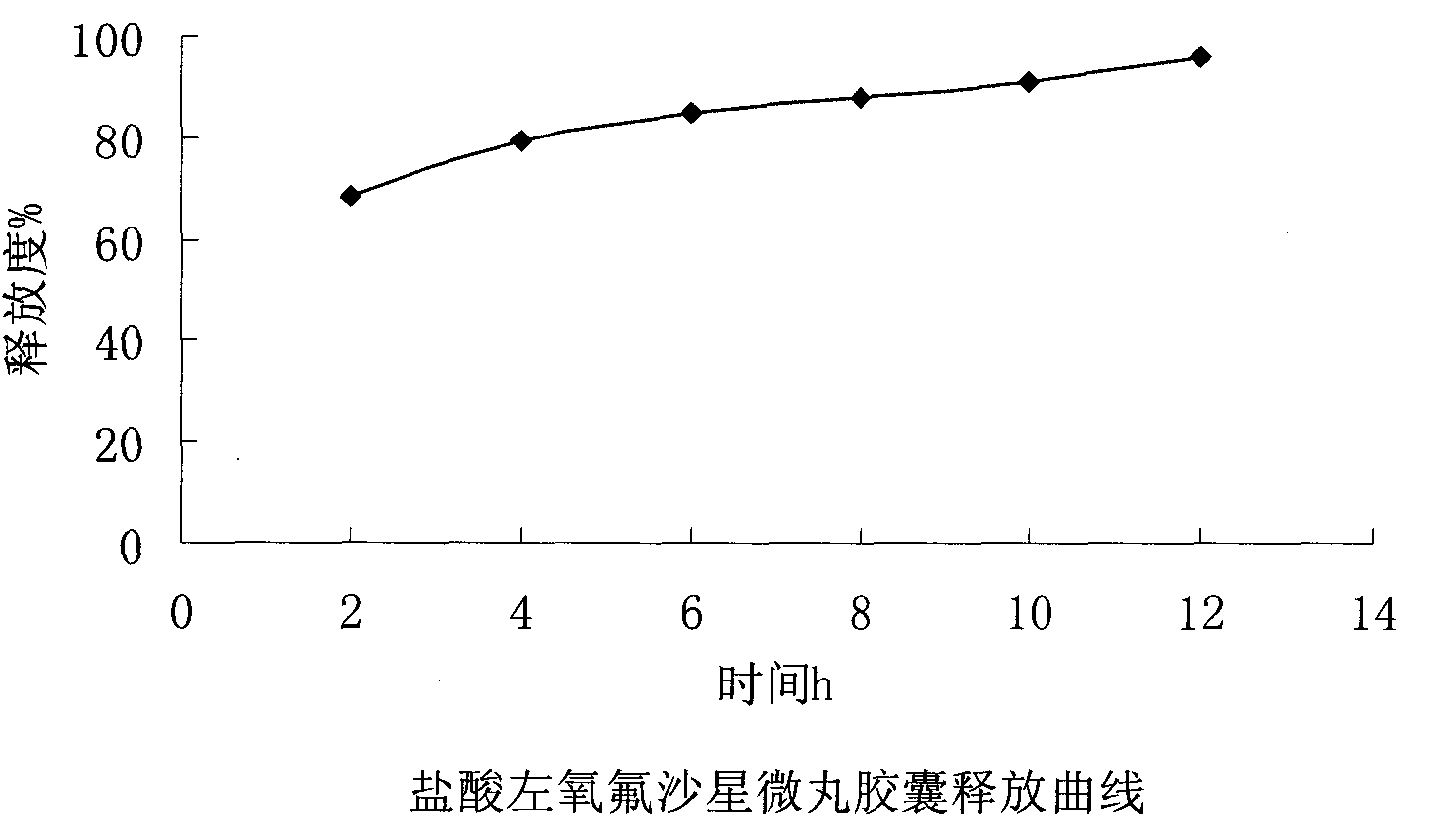
ActiveCN102106842AUniform sizeImprove liquidityAntibacterial agentsOrganic active ingredientsPlasticizerFluidized bed
The invention relates to a levofloxacin hydrochloride micropill capsule and a preparation method thereof. The levofloxacin hydrochloride micropill capsule comprises a pill core, a medicament-containing layer, a sustained-release coating layer and a quick-release layer, wherein levofloxacin hydrochloride is contained in the medicament-containing layer and the quick-release layer; and the sustained-release coating layer comprises the following materials in percentage by weight: 20 to 60 percent of levofloxacin hydrochloride, 30 to 55 percent of pill core, 10 to 25 percent of binding agent, 3 to 5 percent of sustained-release coating material, 0.3 to 3 percent of pore-forming agent and 0.1 to 1 percent of plasticizer. The levofloxacin hydrochloride micropill capsule has the advantages of high stability, small local stimulation of medicaments, high bioavailability and the like. Due to the adoption of a fluidized bed, the problems of large dust and low yield in a method of powder agglomerating in the background technology are solved.
Owner:HAINAN PULIN PHARMA +1
Decoction-free extraction process for precious Chinese herbal medicines
InactiveCN105232584AAvoid pollutionReduce lossesHydroxy compound active ingredientsLeech/worm material medical ingredientsDrug efficiencyChinese herbology
The invention discloses a decoction-free extraction process for precious Chinese herbal medicines. The process includes the steps: 1) cleaning, drying and grinding the Chinese herbal medicines into 20-30-mesh particles; 2) adopting a supercritical CO2 extraction process for removing heavy metals from the Chinese herbal medicine particles obtained at the step 1); 3) roasting the Chinese herbal medicine particles with the heavy metals removed at the step 2) for 50-60min at the temperature of 45-50 DEG C until the water content is 0.3-0.5wt%; 4) subjecting the Chinese herbal medicine particles roasted at the step 3) to ultrafine pulverization until the particle size is 0.35-0.75micrometer; 5) performing wall-breaking treatment, namely adding auxiliary materials into the Chinese herbal medicine particles obtained at the step 4), and extracting active ingredients by application of a low-temperature micro-nano wall-breaking technique. By effective rejection of heavy metal pollution, pharmacological functions of the Chinese herbal medicines can be improved, and side effects are reduced; by application of the cell wall breaking technique for powder processing, biological activity and bioavailability of the Chinese herbal medicines can be improved.
Owner:HENAN XINGZHI PATENT SERVICE CO LTD
Erigeron breviscapus slow release capsule and its preparation method
InactiveCN1205940CObvious sustained release in vitro characteristicsGood curative effectOrganic active ingredientsUnknown materialsSustained Release CapsuleAngina
The breviscapine sustained-release capsule and a preparation method thereof relate to a medicinal dosage form and a preparation process of a traditional Chinese medicine breviscapine sustained-release capsule. Capsules include the active ingredient scutellarin, blank ball cores and adhesives for adhering scutellarin powder to the blank cores and a coating layer for surface sealing. The adhesive and coating layers are made of polyvinylpyrrolidone Composed of ethyl cellulose, each capsule contains 60mg of breviscapine. During the preparation, the blank ball core is put into a coating granulator, scutellarin powder is added, and an adhesive is sprayed at the same time to make medicine-containing pellets, which are then sealed on the surface to make slow-release capsules. The present invention overcomes the disadvantages of poor dissolution rate and short drug action time of the original tablet form, can reach the required effective blood drug concentration in a short period of time, and can maintain the required blood drug concentration in a long period of time. High, reducing the daily dosage and frequency, only need to take twice a day, one capsule each time, it is a good maintenance drug for patients with coronary heart disease, angina pectoris, myocardial infarction and so on.
Owner:TSINGHUA UNIV
Carvedilol push-pull osmotic pump type controlled release preparation and preparation method thereof
InactiveCN102670545AMaintain blood levelsGood curative effectOrganic active ingredientsPill deliveryPush pullCurative effect
The invention relates to a carvedilol push-pull osmotic pump type controlled release preparation. The carvedilol push-pull osmotic pump type controlled release preparation comprises a medicine-containing layer tablet core, a boosting layer tablet core, a coating film and a single medicine releasing pore on the surface of a controlled release tablet on one side of the medicine-containing layer tablet core. According to the carvedilol push-pull osmotic pump type controlled release tablet disclosed by the invention, the medicine release accords with the zero-order release process and is basically complete, the administration frequencies of patients can be reduced and the peak-to-valley phenomenon which occurs after a general preparation is administrated is avoided; and plasma drug stability and durable curative effect in the release process of medicaments are obtained, and thus the safety and the effectiveness are improved.
Owner:CHINA PHARM UNIV
Cholesterol hydrophobic modification pullulan-donepezil-polysorbate 80 nano particle as well as preparation and application
InactiveCN108379241AHigh drug loadingHigh encapsulation efficiencyNervous disorderPharmaceutical non-active ingredientsDonepezilPullulan
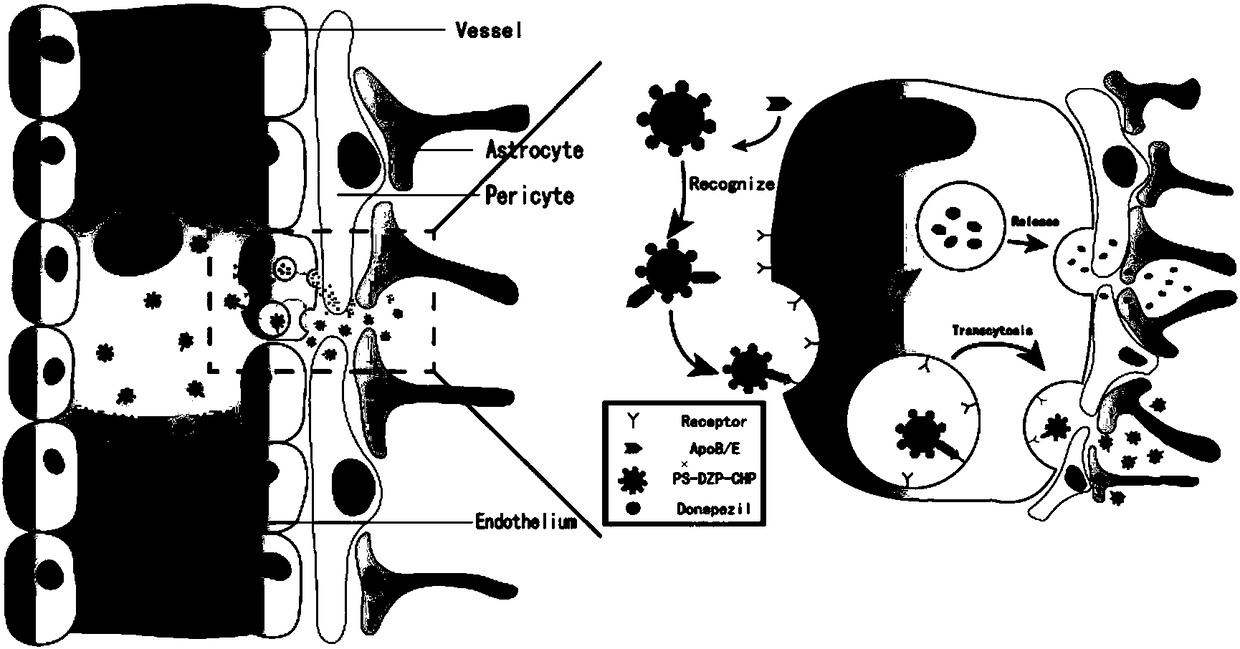
The invention discloses a cholesterol hydrophobic modification pullulan-donepezil-polysorbate 80 nano particle. The nano particle comprises a cholesterol hydrophobic modification pullulan nano particle, donepezil loaded in the cholesterol hydrophobic modification pullulan nano particle and polysorbate 80 adsorbed on the surface of the cholesterol hydrophobic modification pullulan nano particle. The cholesterol hydrophobic modification pullulan-donepezil-polysorbate 80 nano particle has good drug loading capacity, encapsulation efficiency and slow-release effect on the donepezil, and can be used for maintaining blood concentration within a longer time, prolonging medicine taking intervals and improving patient compliance. In addition, as the stability is improved, the dosage, which enters the whole body to circulate, is reduced, more nano particles stride a blood brain barrier before releasing medicine, so that the medicine is enriched at the brain, the release after location is realized, not only is the dosage reduced, but also the toxicity to a peripheral nervous system is reduced.
Owner:HUNAN NORMAL UNIVERSITY
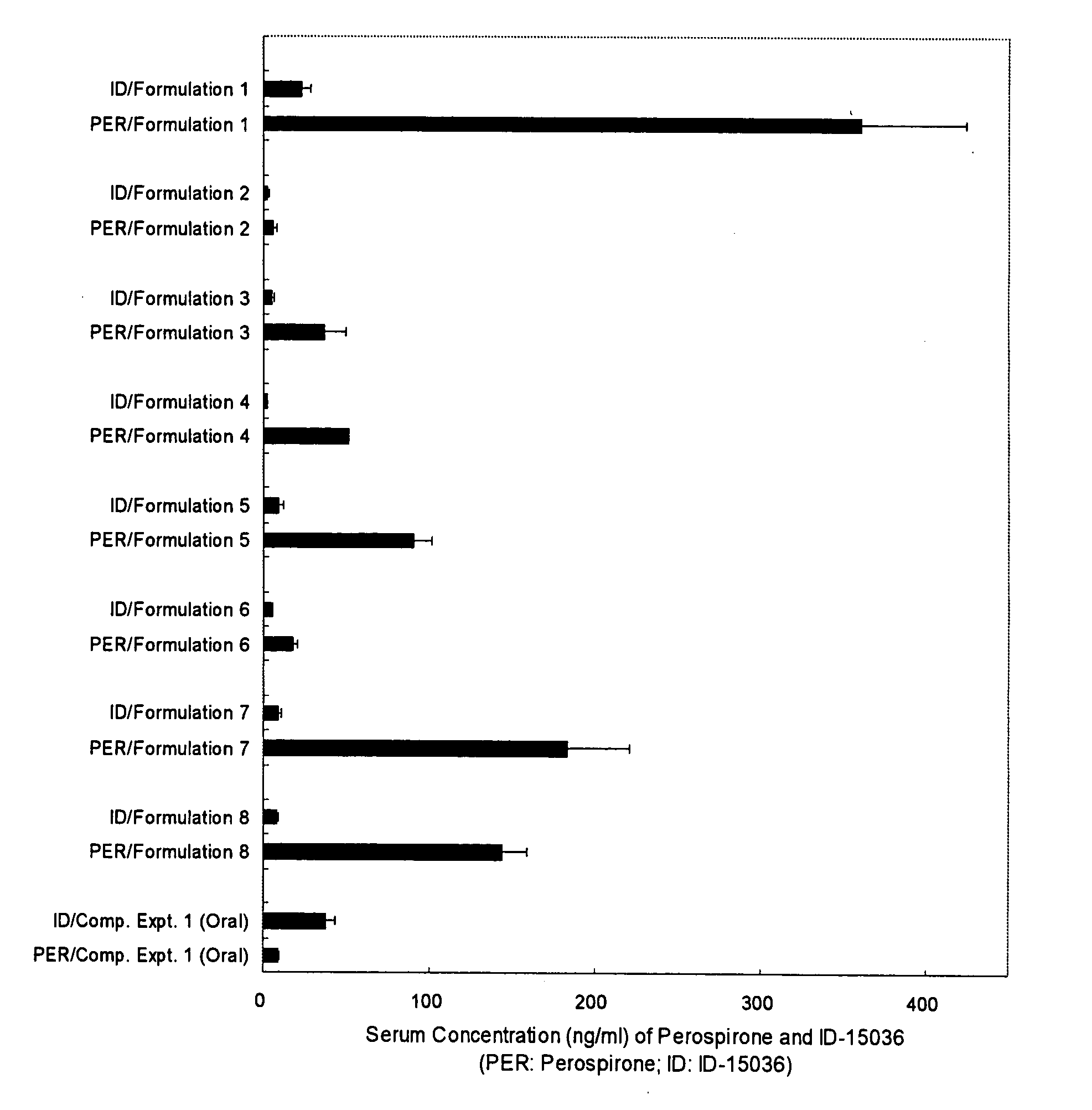
Pharmaceutical Composition for Transdermal Administration of Perospirone
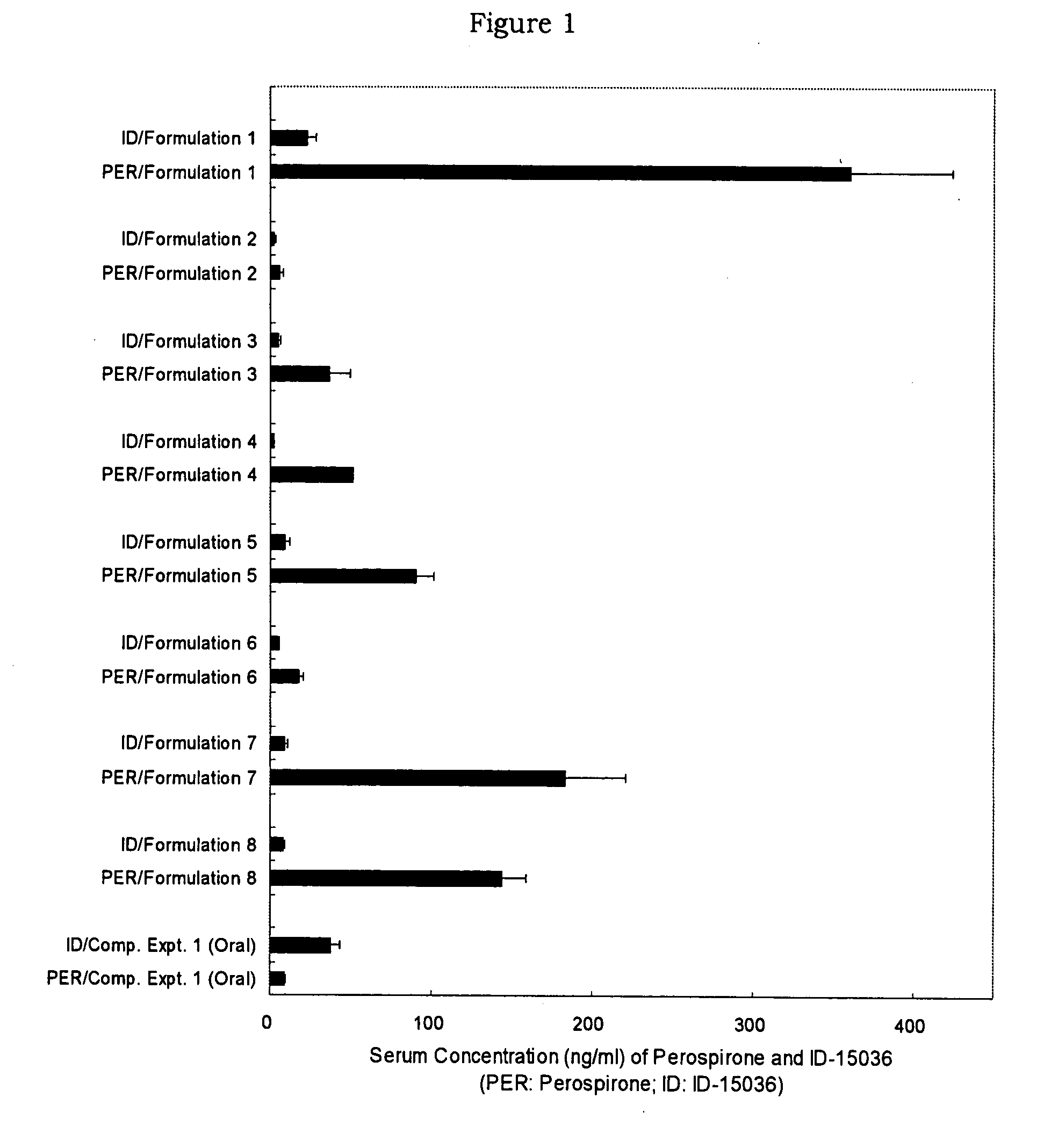
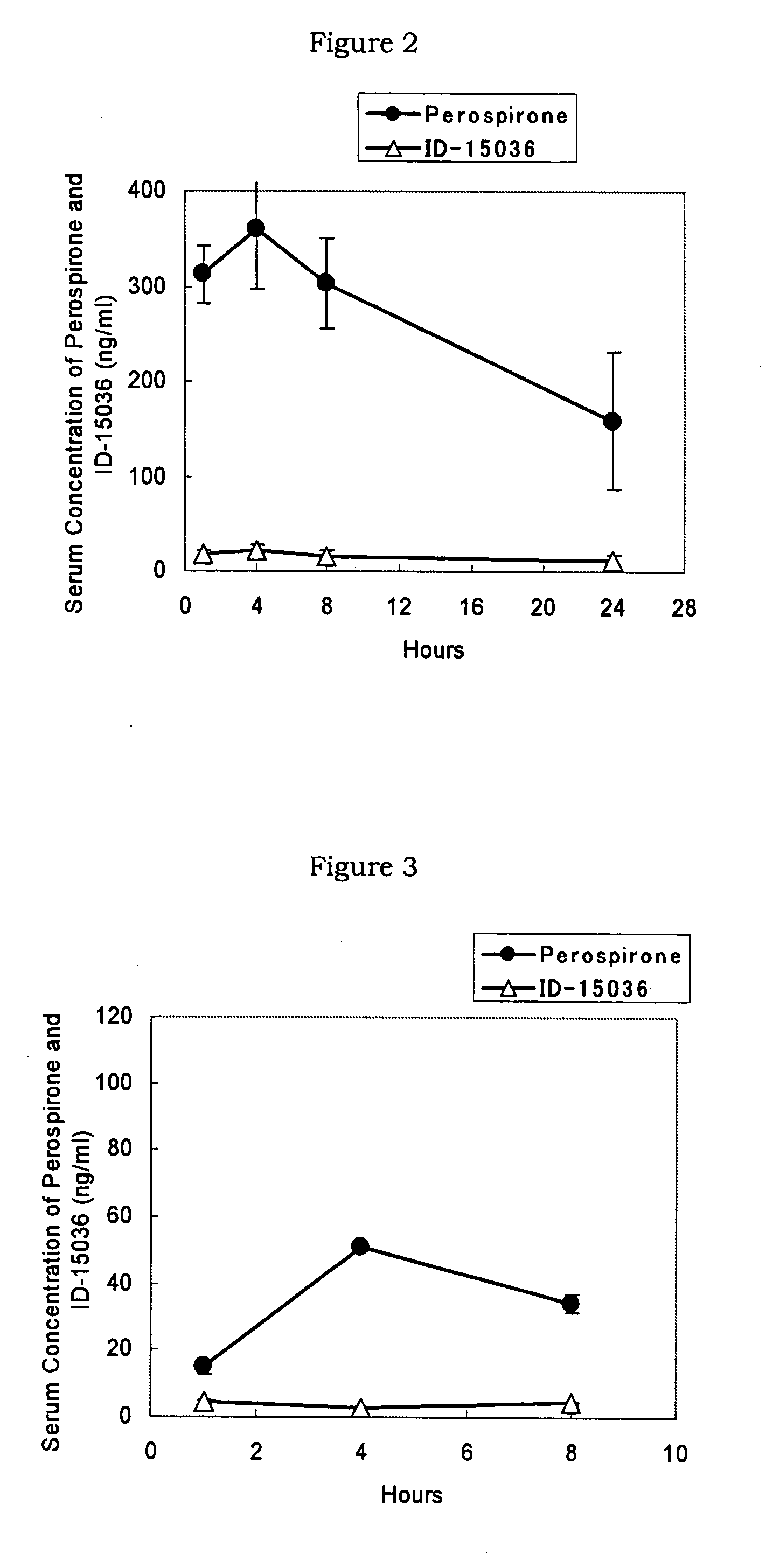
InactiveUS20070254887A1Reduce generationMaintain blood levelsBiocideNervous disorderBlood levelMetabolite
A pharmaceutical composition for transdermal administration comprising perospirone of the formula (1): or a pharmaceutically acceptable acid addition salt thereof, which can inhibit the generation of metabolites and continuously maintain the blood level of perospirone.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Compound sustained-release preparation and preparation method thereof
InactiveCN101780054AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsUrinary disorderBlood concentrationActive component
The invention relates to a compound sustained-release preparation and a preparation method thereof, and the compound sustained-release preparation comprises Epristeride, terazosin hydrochloride and pharmaceutically acceptable auxiliary materials, wherein the Epristeride and the terazosin hydrochloride are used as the active components, the Epristeride is prepared into a sustained-release part, and the terazosin hydrochloride is prepared into an ordinary-release part, so that the two medicines are kept in an effective blood concentration range in vivo, the adverse effect of the medicines and the administration frequency are reduced, and the safety and effectiveness of pharmacy and the compliance of patients are improved.
Owner:JIANGSU LIANHUAN PHARMA
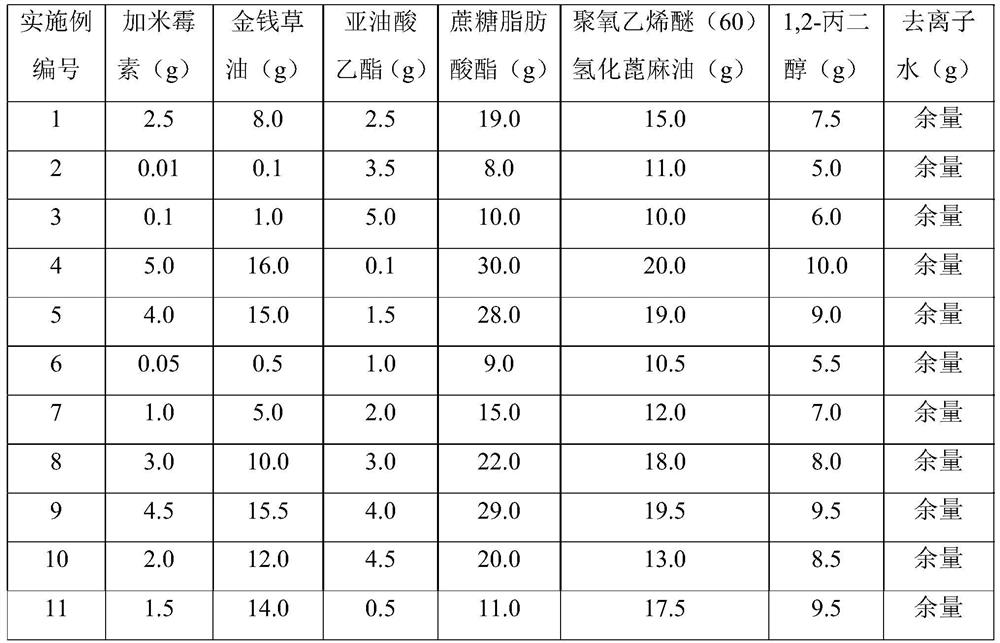
Gamithromycin emulsion, preparation method thereof and application of gamithromycin emulsion in prevention and treatment of porcine ileitis
ActiveCN112972379APrevention and treatment of ileitisConducive to clinical promotion and applicationAntibacterial agentsOrganic active ingredientsSucroseToxicology
The invention relates to a gamithromycin emulsion. Each 100g of the gamithromycin emulsion comprises the following components by weight of 0.01 to 5.0 g of gamithromycin, 0.1 to 16.0 g of lysimachia christinae hance oil, 0.1 to 5.0 g of ethyl linoleate, 8.0 to 30.0 g of sucrose fatty acid ester, 10.0 to 20.0 g of polyoxyethylene ether (60) hydrogenated castor oil, 5.0 to 10.0 g of 1, 2-propylene glycol and the balance of deionized water. Meanwhile, the invention further discloses a preparation method, the process operability is high, and production conversion is facilitated. The emulsion disclosed by the invention is in an oil-water mutual wrapping type, the prepared product can be infinitely diluted with water and can also be infinitely diluted with oil, after being diluted with water, the emulsion can meet the drinking water administration requirement and is convenient to use, and after being diluted with oil, the emulsion can be prepared into a slow-release emulsion, so that a long-acting antibacterial effect is achieved, and the administration frequency is reduced. The emulsion disclosed by the invention can be used for preventing and treating ileitis diseases caused by pig Lawsonia intracellularis infection, has an effect obviously better than that of the prior art, and has a wide market application prospect.
Owner:项朝荣
Pentoxifylline tablet controlled relasease tablet
InactiveCN1444943AMaintain blood levelsMaintain effective plasma concentrationOrganic active ingredientsNervous disorderDiseaseCellulose acetate
A slow-releasing torental table for treating peripheral obliteration vasculitis, sequelae of arteriosclerosis, and local ischemia is prepared from torental (20-70 wt.%), slow releasing agent (cellulose acetate and / or ethyl cellulose) (30-80 wt.%), and other assistants. Its advantages are high curative effect, durable action and low by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Oral quetiapine sustained-release tablet
InactiveCN102309466AExtended half-lifeImprove complianceOrganic active ingredientsNervous disorderDiseaseSide effect
The technical problem solved by the invention is to provide an oral sustained-release tablet of quetiapine and its salts which can effectively and continuously release for more than 12 hours, is slow in medical effect release, can stably maintain the effective plasma concentration for 24 hours, is long in release period, less in toxic or side effect, and convenient for administration; the main components are quetiapine with active components, a skeletal material with sustained-release effect, and an excipient; the invention is characterized in that on a basis of the total weight of the tablet, the tablet comprises 5%-50% of quetiapine with active components, 5%-50% of skeletal material with sustained-release effect, and 20%-50% of excipients. Polyoxyethylene with one or several different models is selected as the skeletal material. The excipient comprises a proper amount of sodium citrate dihydrate compounds. Polyoxyethylene is selected which can prolong the sustained-release period; the release period is greatly increased; the medicinal effect is released stably and slowly; the plasma concentration is guaranteed; the disease condition is controlled stably; patients are provided with 24-hour effective treatment; and the invention is widely applicable to single-dosage quetiapine treatment.
Owner:TIANJIN DEXUAN MEDICAL TECH
Irinotecan hydrochloride lipidosome composition and preparation method thereof
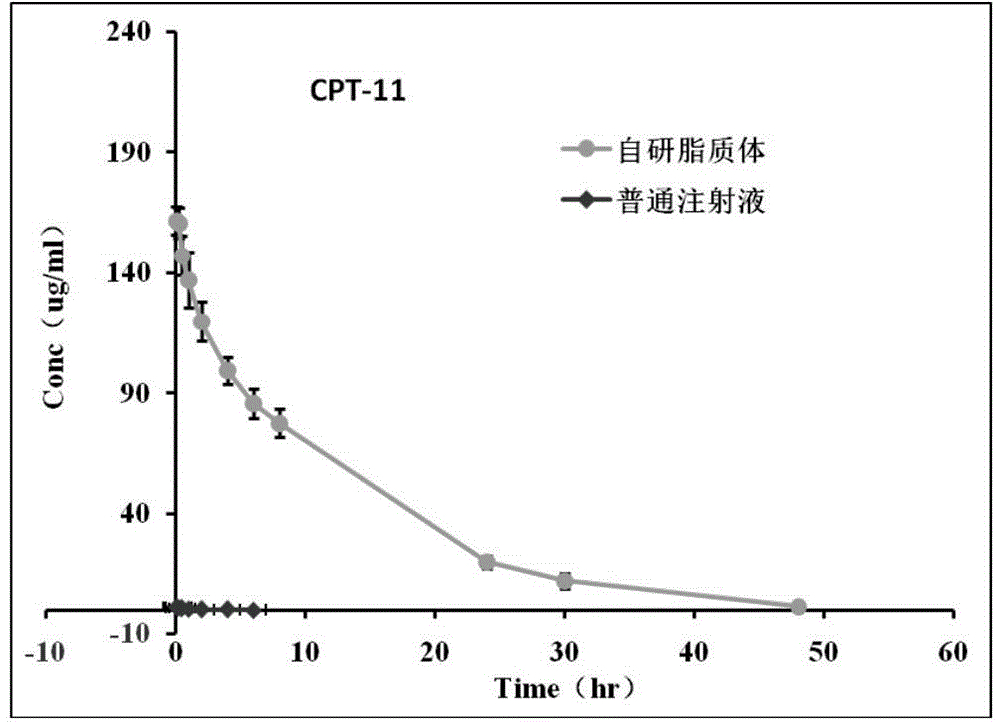
ActiveCN105982857ADoes not affect encapsulation rateNo additional high temperature processing timeOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolAdditive ingredient
The invention provides an irinotecan hydrochloride lipidosome composition, which consists of the following ingredients in parts by weight: 1 part of irinotecan hydrochloride, 2-6 parts of hydrogenated soybean phosphatide, 1.0-2.25 -parts of cholesterol and 0.04-0.20 parts of distearoyl phosphoethanolamine-polyethylene glycol 2000. The invention also provides a preparation method of the lipidosome composition. The lipidosome disclosed by the invention significantly reduces cost, and the lipidosome is good in stability,low in content of impurities, high in safety and easy for industrial mass production.
Owner:HUNAN KELUN PHARM RES CO LTD +1
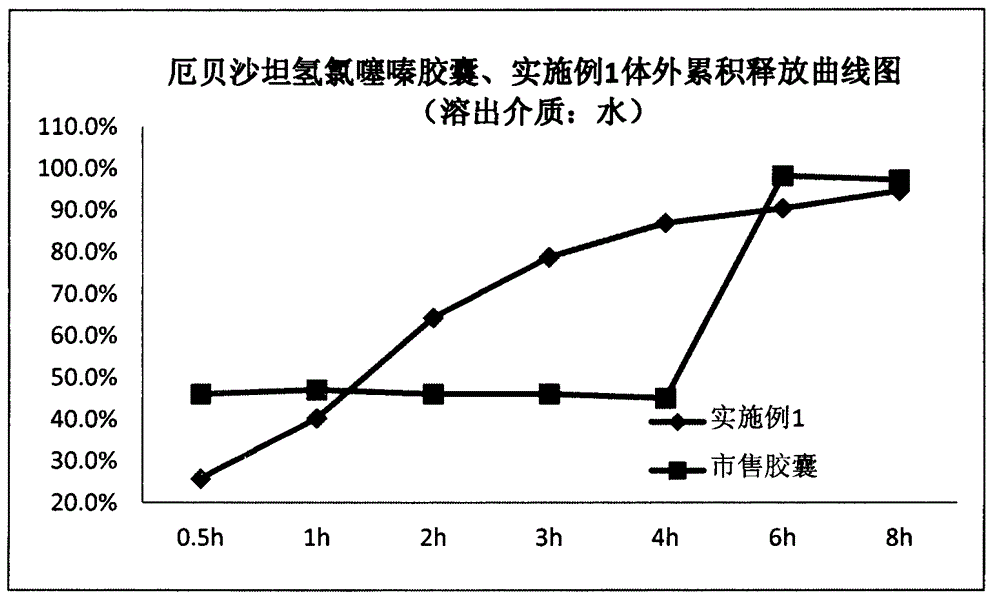
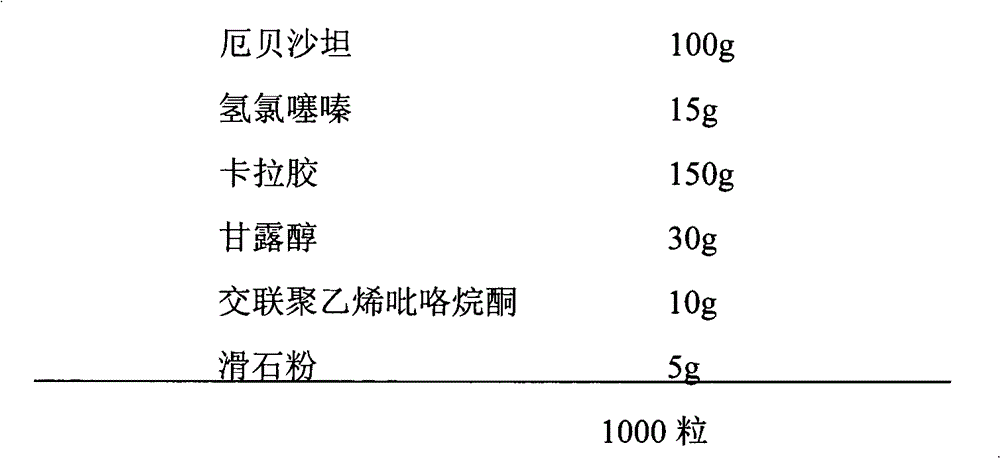
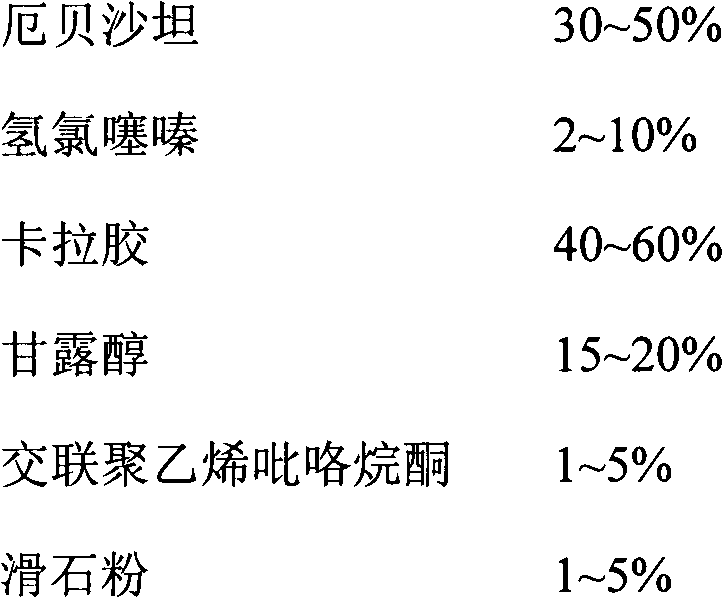
Irbesartan hydrochlorothiazide capsule
InactiveCN102743361AGood consistencyNot easy to releaseOrganic active ingredientsPharmaceutical delivery mechanismDrugCross-linked polyethylene
The invention discloses an irbesartan hydrochlorothiazide capsule. The capsule includes the components of irbesartan, hydrochlorothiazide, carrageenan, mannitol, crosslinked polyvinylpyrrolidone and talcum powder. The irbesartan hydrochlorothiazide capsule enables medicines to slowly release step by step, the phenomenon of sudden releasing cannot occur, blood concentration is stable, medicine effect can maintain more than 8 hours, adverse reaction caused by medicine sudden releasing is avoided, and pharmacy safety is added. The irbesartan hydrochlorothiazide capsule can be used for treating primary hypertension.
Owner:NANJING ZENKOM PHARMA
Acemetacin sustained-release preparation and preparation method
ActiveCN100563636CControl releaseRelease effective controlPowder deliveryOrganic active ingredientsDrug release rateControl release
The invention discloses an acemetacin slow-release preparation and a preparation method thereof, which belong to the technical field of medicine and are used to solve the problems of acemetacin's short plasma half-life and short duration of therapeutic action. It consists of an acemetacin drug core and a controlled-release layer wrapping the drug core. The controlled-release layer is composed of a xanthan gum-chitosan mixture, wherein the content of xanthan gum accounts for the total weight of xanthan gum-chitosan The percentage is 20% to 80%. In the present invention, the acemetacin drug core is coated with multiple layers of controlled-release layers, and the release speed of the drug is slowed down by increasing the number of layers of the controlled-release layer or the wrapping rate. Tests have shown that compared with conventional dosage forms, the preparation can well control the release of the drug, and can maintain the blood drug concentration of the therapeutic amount within 24 hours in a once-a-day administration mode. It overcomes the deficiencies of the prior art and provides an oral dosage form that can control the stable release of drugs for a long time, prolong the curative effect of drugs, reduce adverse reactions, and basically avoid incomplete drug release.
Owner:CSPC OUYI PHARM CO LTD
VEGF antibody-carrying ophthalmic thermosensitive hydrogel implant and preparation method thereof
ActiveCN107233570AHarm reductionPrecise positioningMetabolism disorderAerosol deliveryLiquid stateAnti vegf
The invention provides a VEGF antibody-carrying ophthalmic thermosensitive hydrogel implant and a preparation method thereof, and relates to a medical preparation and a preparation method thereof. The implant is a thermosensitive hydrogel for ophthalmic injection, and the phase change temperature of the thermosensitive hydrogel is 34DEG C-35DEG C; the thermosensitive hydrogel is selected from a thermosensitive NIPAAm hydrogel and carries a VEGF antibody; and the mass concentration of a thermosensitive hydrogel solution is 10%-20%. According to the VEGF antibody-carrying ophthalmic thermosensitive hydrogel implant, an anti-VEGF monoclonal antibody drug is slowly released, a relatively noninvasive method is used, the anti-VEGF monoclonal antibody drug and a gel are uniformly mixed to prepare a gel system which presents a liquid state in vitro and quickly changes into a semisolid state when injected into eyeballs, so the drug arrives the posterior segment through the injection mode to achieve the effect of locally slow release.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
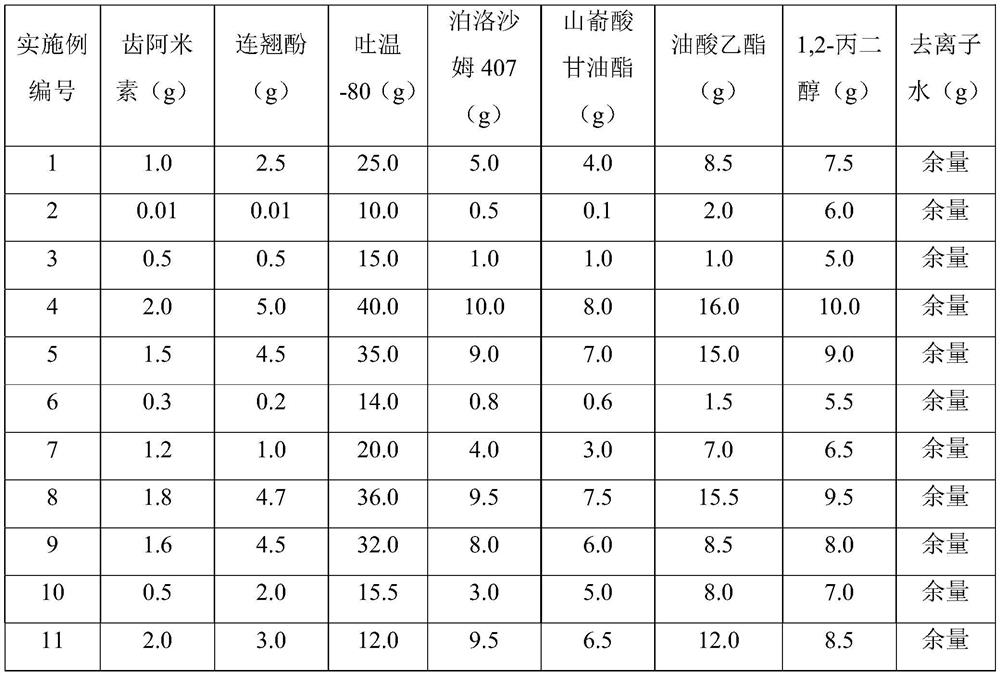
Novel traditional Chinese medicine sustained-release injection, preparation method and application to treatment of mycoplasma synovialis of poultry
ActiveCN111789883AEasy to useNo food safety issuesAntibacterial agentsOrganic active ingredientsBiotechnologyMethyl oleate
The invention relates to a novel traditional Chinese medicine sustained-release injection, a preparation method and an application to treatment of mycoplasma synovialis of poultry. Each 100 g of the injection comprises 0.01-2.0 g of visnagin, 0.01-5.0 g of forsythol, 10.0-40.0 g of tween-80, 0.5-10.0 g of poloxamer 407, 0.1-8.0 g of glyceryl behenate, 1.0-16.0 g of ethyl oleate, 5.0-10.0 g of 2-propanediol and the balance of deionized water. The invention further discloses a preparation method of the novel traditional Chinese medicine sustained-release injection. The method has high process operability and is beneficial to production transformation. The sustained-release injection is bicontinuous phase emulsion, the main component is a traditional Chinese medicine extract, and the problemof food safety due to drug residues can be solved; after injection, drugs can be slowly released locally in tissue, a long-acting effect is achieved, the injection administration frequency reduced, stress of poultry is reduced, and the market prospect is wide.
Owner:信阳市动物疫病预防控制中心
Novel hydrochloric acid tramadol sustained-release tablet
ActiveCN101095666AMaintain blood levelsImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismSide effectFilm coating
The invention discloses a new type sustained release tablets of tramadol hydrochloride and the preparation method. The sustained release tablets comprise tablet core and membrane clothing sheet. The tablet core contains sustained release capsule of tramadol hydrochloride with its weight being 80-90% of total weight of tablet core, and the sustained release capsule is prepared with tramadol hydrochloride and sustained release material with ratio being 1: 1.2-1.5. The sustained release capsule also contains 10% monoglyceride. The sustained release tablet is characterized by stable effective blood concentration, increased biological utilization rate, reduced side effect, good taste and small intake amount of one time per day.
Owner:CSPC OUYI PHARM CO LTD
'Huilaxitan' injection, and its prepn. method
InactiveCN1723893AMaintain blood levelsGuaranteed therapeutic effectOrganic active ingredientsNervous disorderAlcoholSolvent
An injection of aniracetam for intravenous injection is prepared from aniracetam, dissolved by propylene glycol solvent, the mixture of alcohol and water for injection, anti-oxidizing agent and pH regulator.
Owner:CHANGYUAN BIOLOGICAL PHARMA TECH WUXI
Radix ophiopogonis oligosaccharide slow-release tablet and preparation method thereof
InactiveCN106727384AReduce releaseEffective and stable releaseOrganic active ingredientsMetabolism disorderSide effectFiller Excipient
The invention discloses a radix ophiopogonis oligosaccharide slow-release tablet and a preparation method thereof. The radix ophiopogonis oligosaccharide slow-release tablet is prepared from the following raw materials in parts by mass: 1 part of active ingredient of radix ophiopogonis, 0.5 to 1.5 parts of slow-release framework material, 0.3 to 0.6 part of filling agent, and 0.01 to 0.02 part of lubricant, wherein the slow-release framework material preferably selects hydroxypropyl methyl cellulose, sodium carboxymethylcellulose or carboxyethyl cellulose; the filling agent comprises microcrystalline cellulose, lactose or starch; the lubricant comprises magnesium stearate, superfine silica powder or talcum powder. Compared with the prior art, the radix ophiopogonis oligosaccharide slow-release tablet has the advantages that after taking, the radix ophiopogonis oligosaccharide can be effectively and stably released in a human body, so that the effective plasma concentration and time can be maintained, and the peak and valley of plasma concentration can be avoided; under the premises of ensuring therapy effect, the side effect caused by overhigh plasma concentration can be avoided; the preparation technology is simple, and the radix ophiopogonis oligosaccharide slow-release tablet is suitable for industrial production.
Owner:SUN YAT SEN UNIV
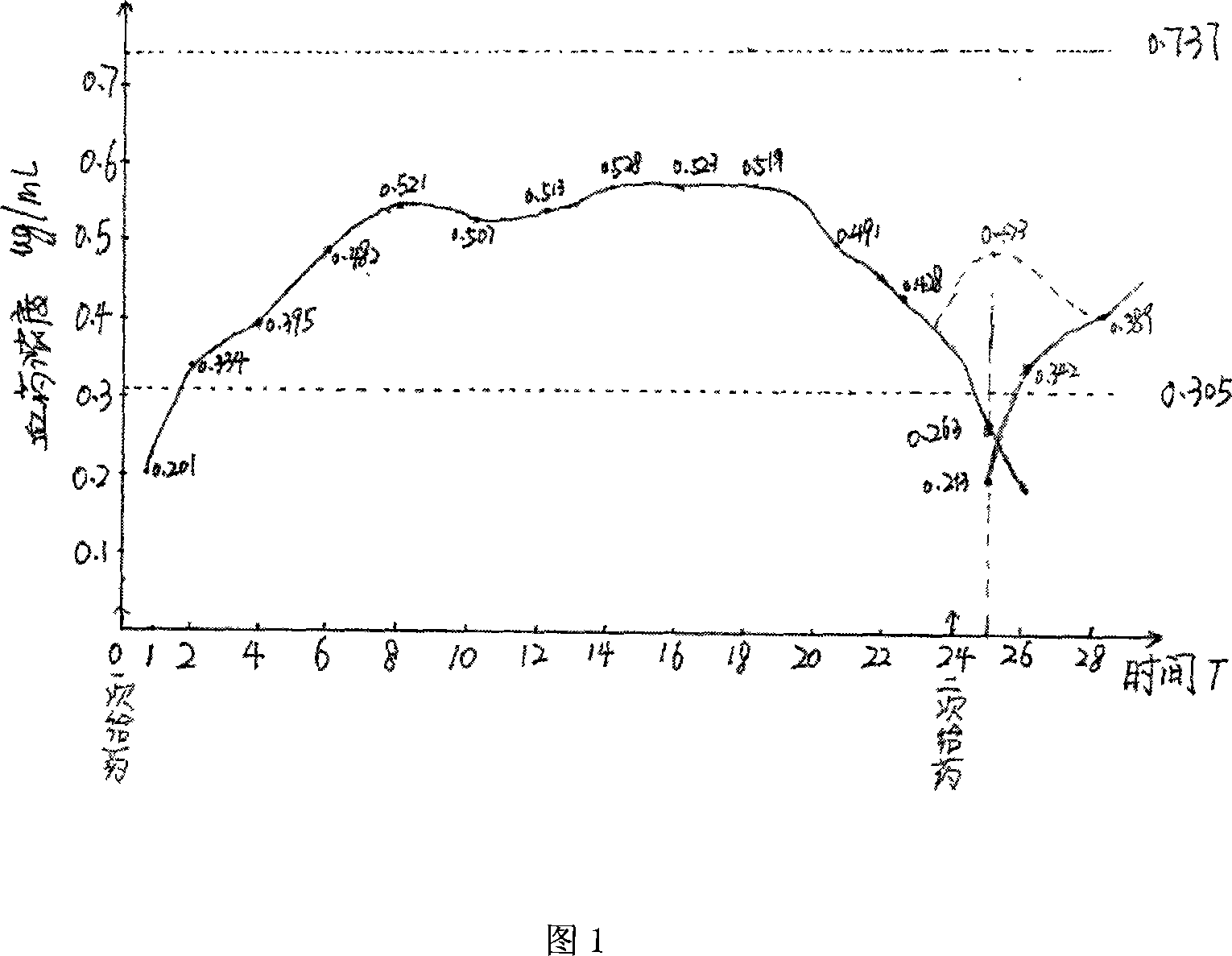
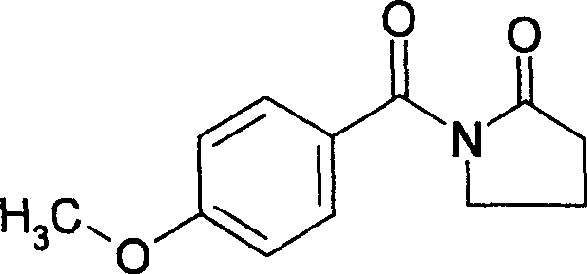
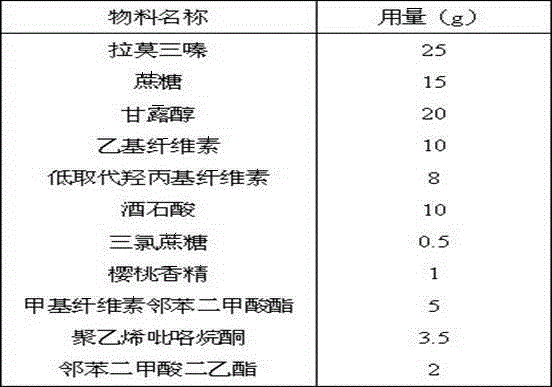
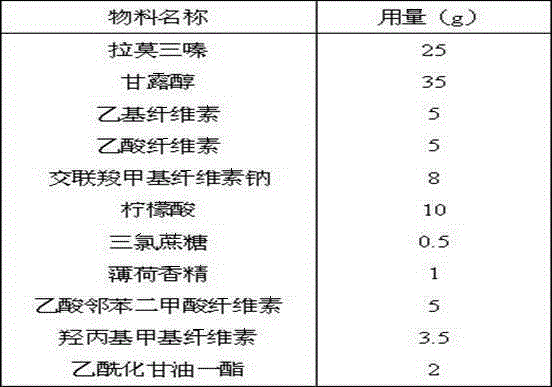
Lamotrigine orally disintegrating sustained release tablets
InactiveCN104473895AImprove Medication AdherenceControl blood drug concentrationNervous disorderPharmaceutical delivery mechanismOrally disintegrating tabletBULK ACTIVE INGREDIENT
The invention relates to a method for treating CNS diseases and provides lamotrigine orally disintegrating sustained release tablets which can be rapidly disintegrated in the oral cavity and can reflect very similar release rate unrelated to the pH value of the environment in the whole gastrointestinal tract. The preparation comprises lamotrigine, organic acids, a disintegrating agent, a polymer controlling release and an enteric polymer. The compositions can be used for treating epilepsy and bipolar disorder, particularly patients suffering from dysphagia and improving the compliance of patients suffering from the bipolar disorder. The orally disintegrating tablets are prepared according to the method disclosed by the invention, the release rate of active ingredients can be controlled, and the bioequivalence and clinical safety and effectiveness of the product are guaranteed.
Owner:万全万特制药(厦门)有限公司
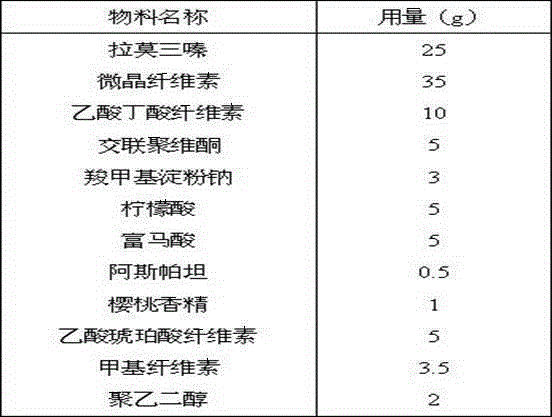
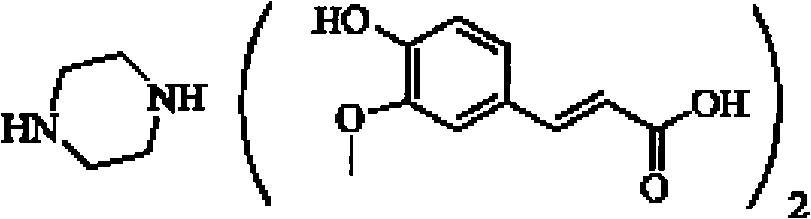
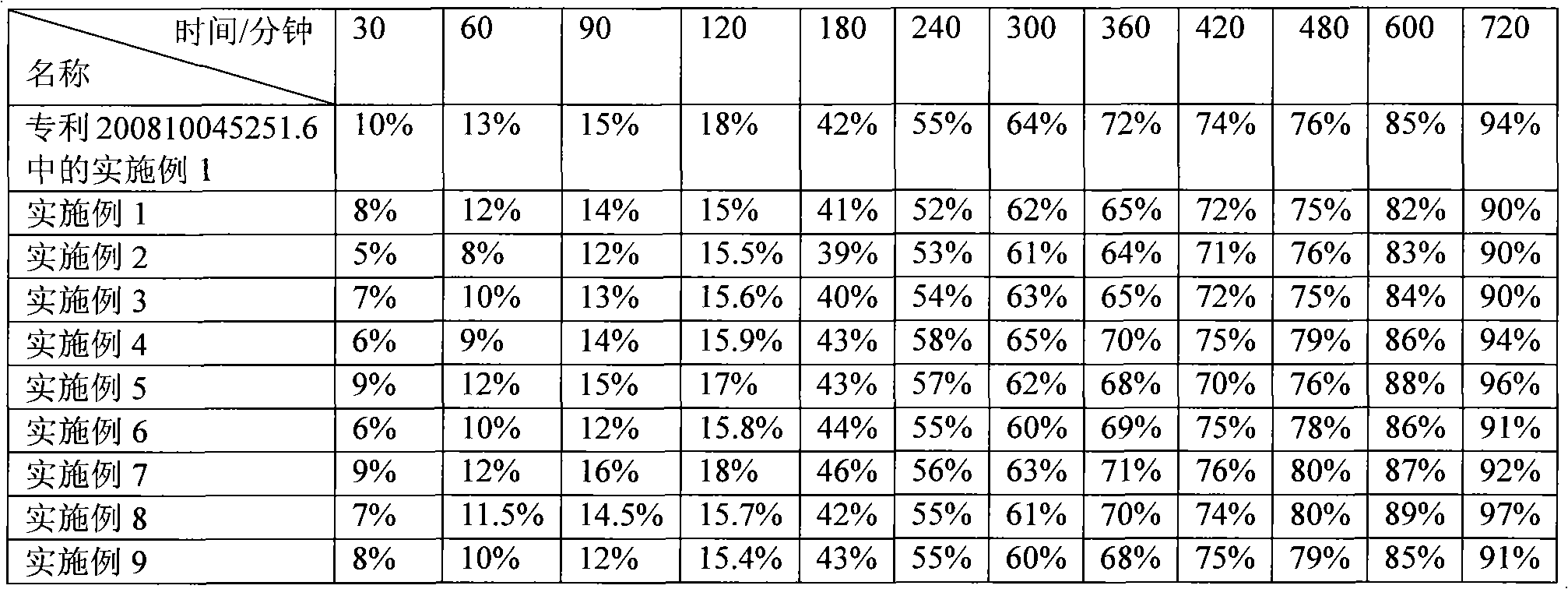
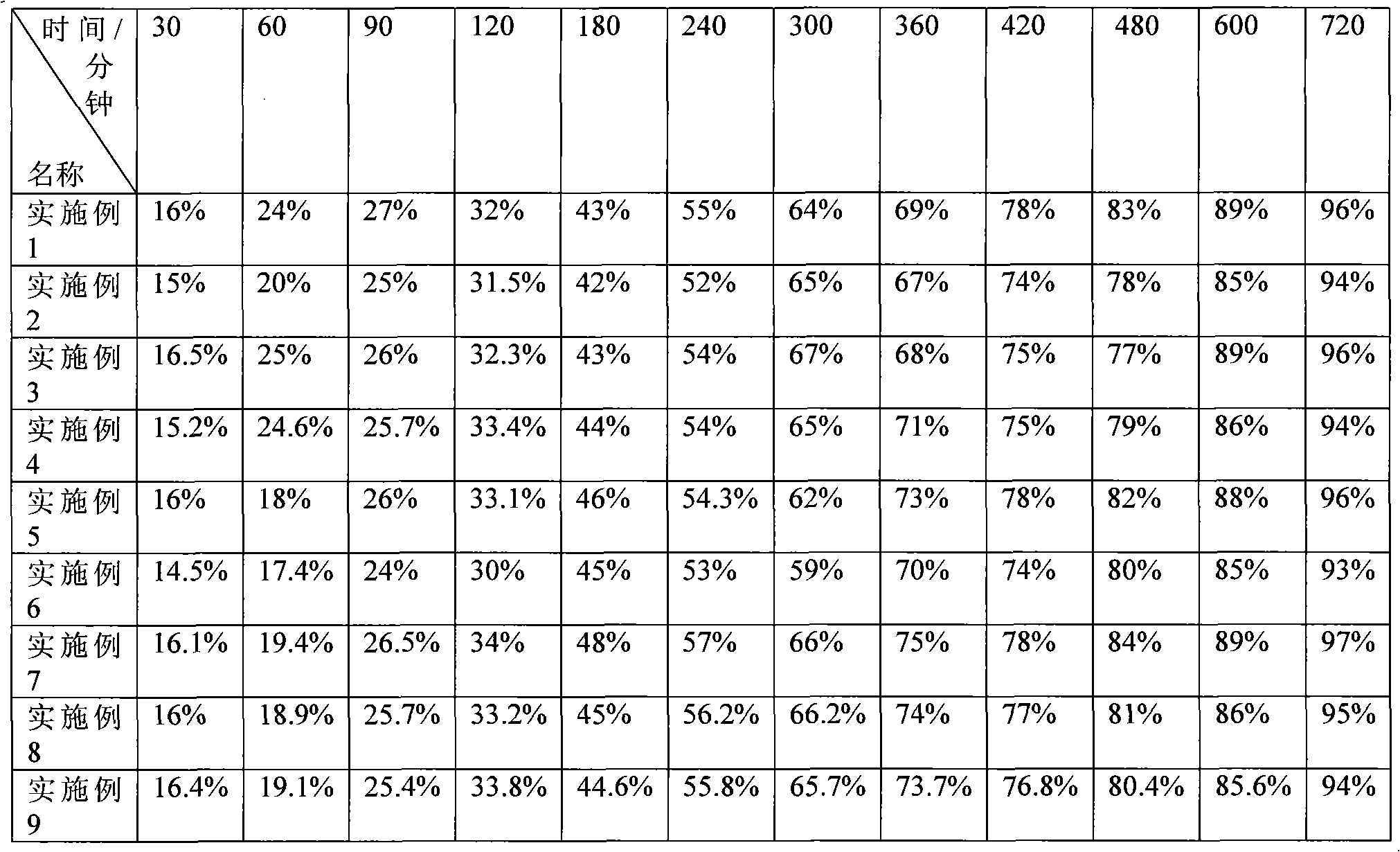
Piperazine ferulate sustained-release tablet and its preparation method
ActiveCN102335153AImprove release resultsImprove liquidityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveDissolution
The invention describes a piperazine ferulate sustained-release tablet and its preparation method. The piperazine ferulate sustained-release tablet consists of piperazine ferulate, a framework material, a diluent, a lubricant, an adhesive, and optionally a coating material. In the invention, starch1500 (shanda in Chinese) is added into the diluent to improve the release of the sustained-release preparations. Meanwhile, a strict preparation process is employed so as to make the tablet of the invention reach a release result approximate to human in vivo release. With simple analysis of dissolution and release, the tablet and its preparation method are suitable for large scale production. According to a rotating basket method for in vitro drug release of sustained-release preparations in the 2010 edition of Chinese pharmacopoeia, in 900ml of water, the release rate of the tablet in the invention can be 25-35% accumulatively in 2h, 45-60% in 4h, 60-75% in 6h, and over 90% in 12h. A detailed study of the in vitro release result of the tablet indicates that the piperazine ferulate sustained-release tablet reaches good sustained-release effects and can have delayed release within 12h, so that the medicine taking time can be reduced.
Owner:浙江四维医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com