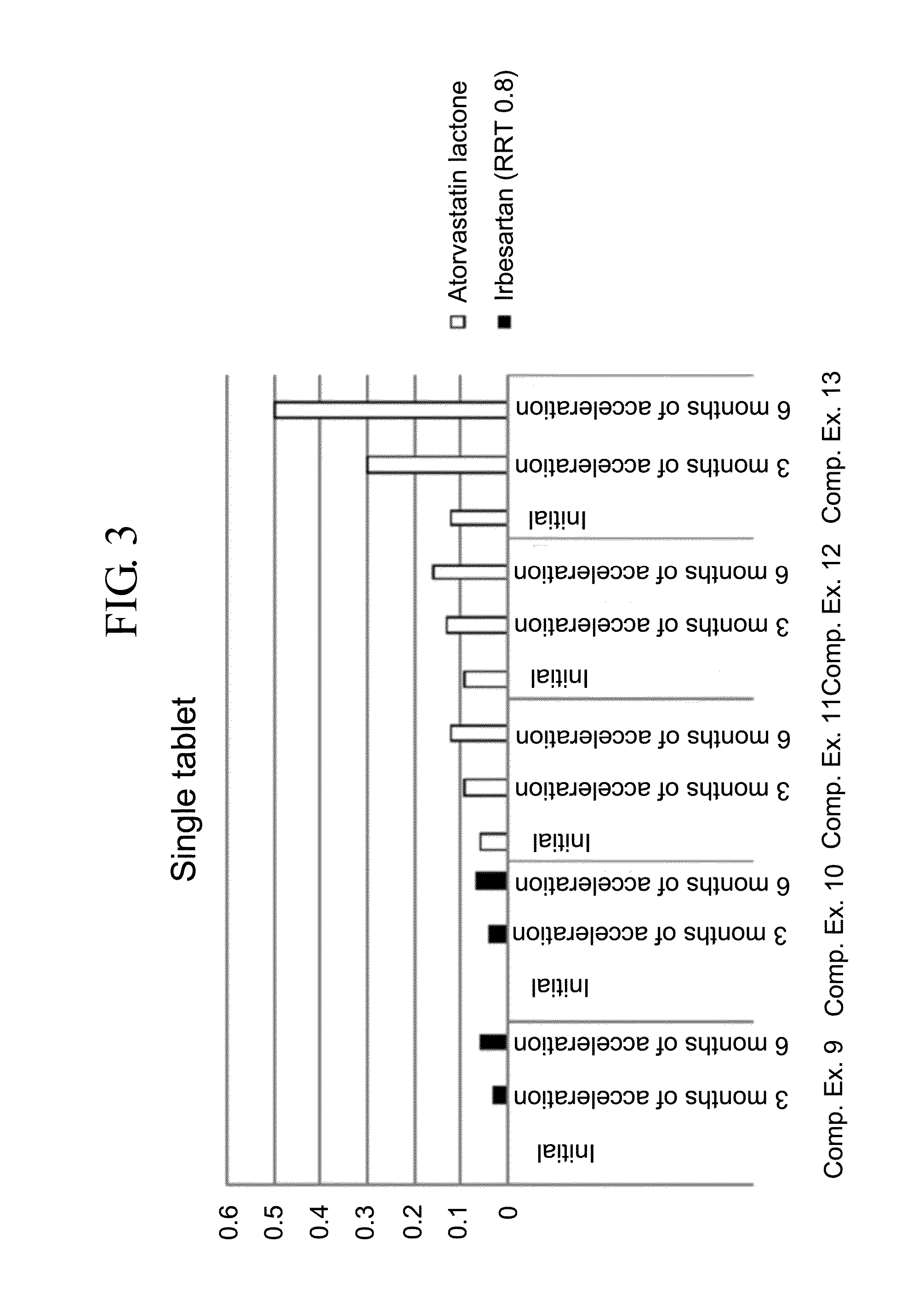
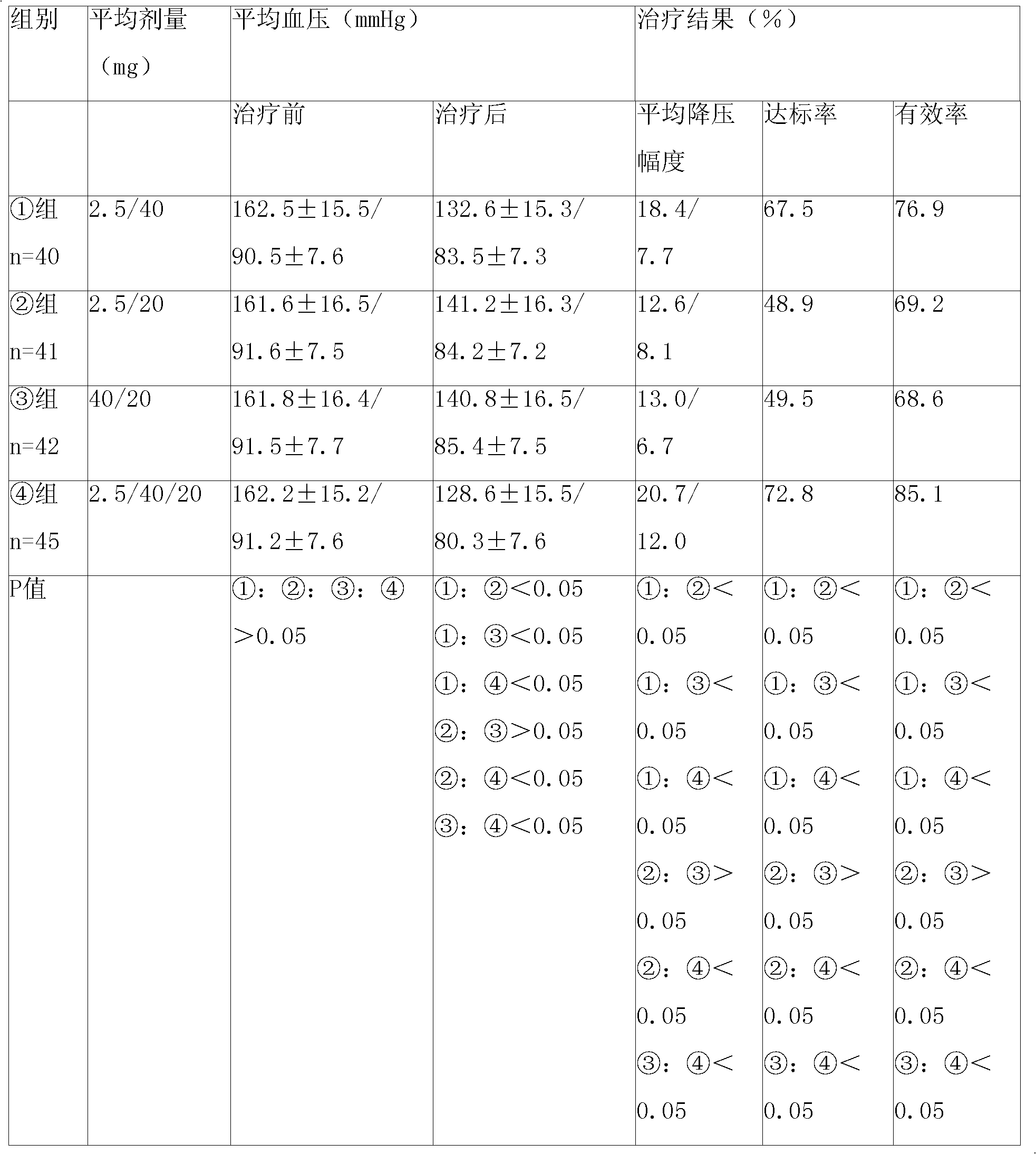
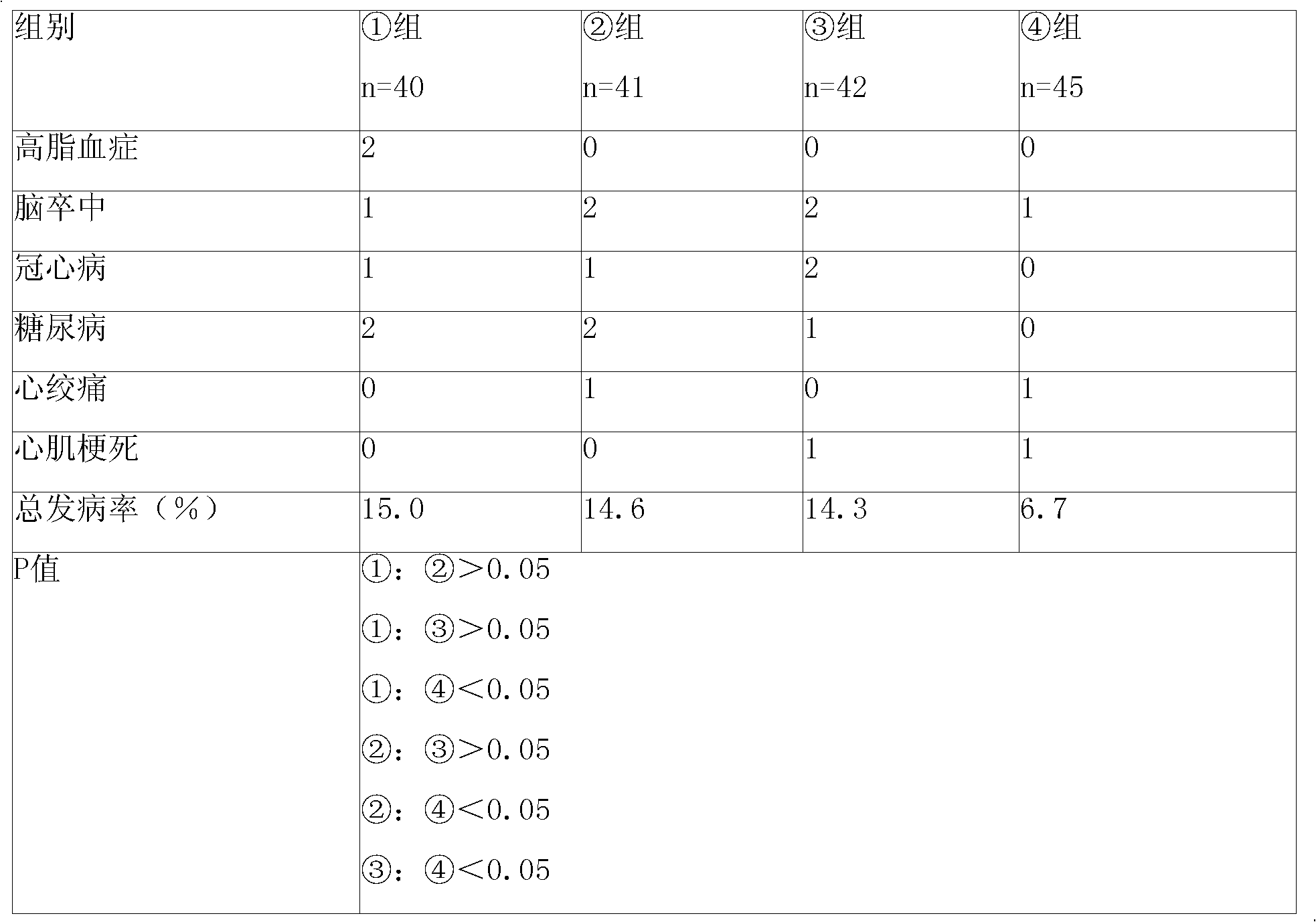
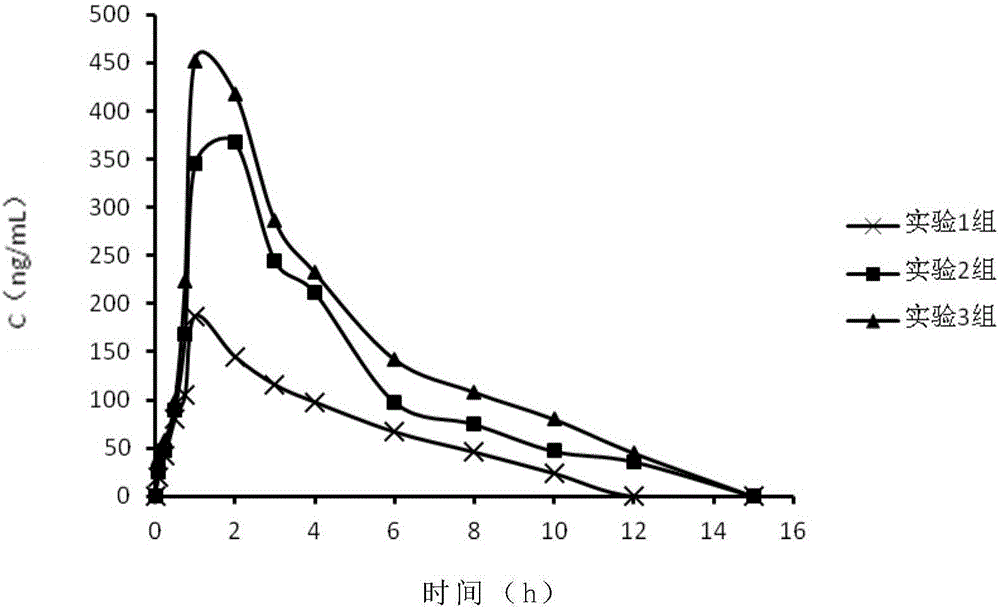
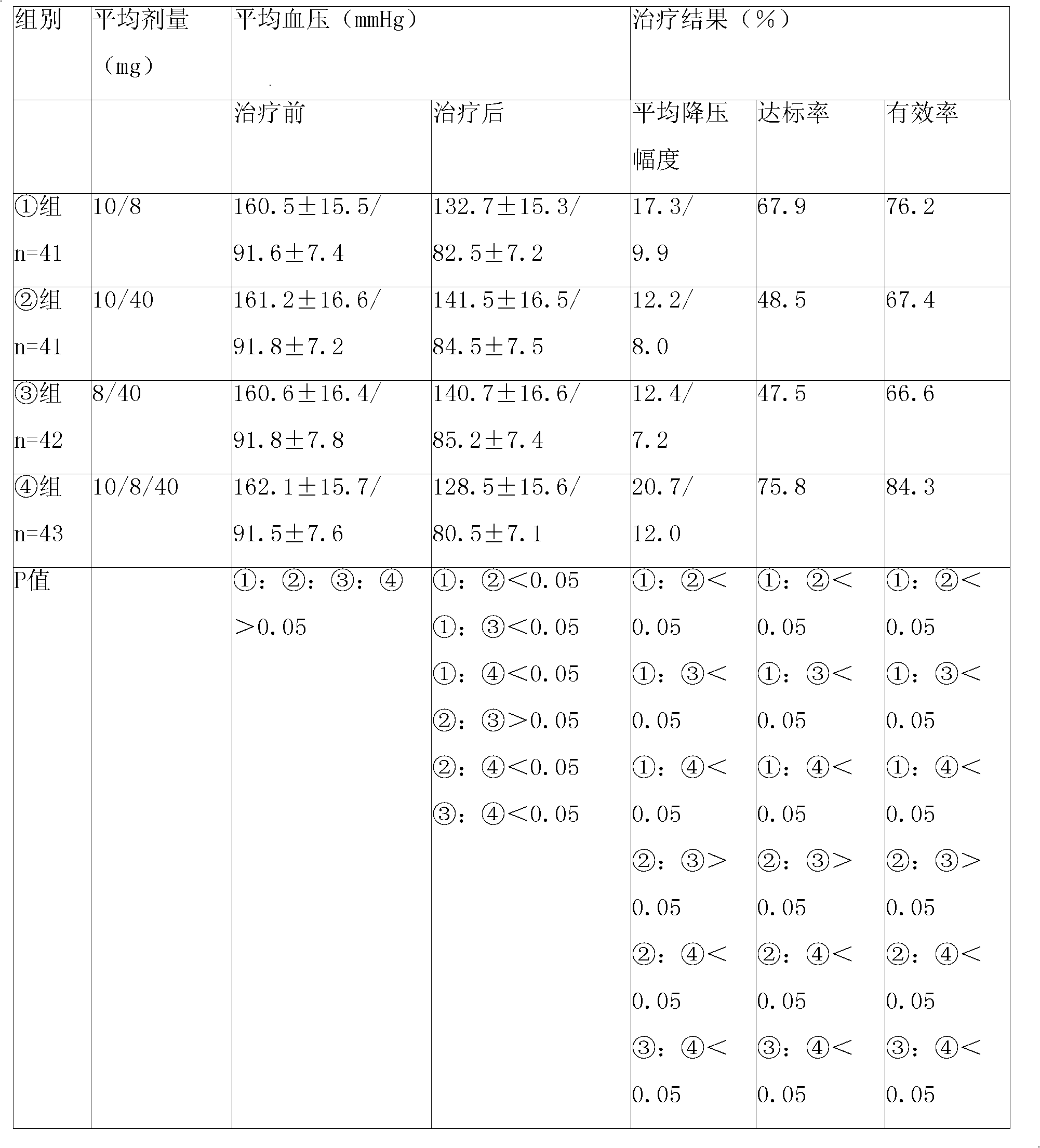Patents
Literature
154 results about "Irbesartan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
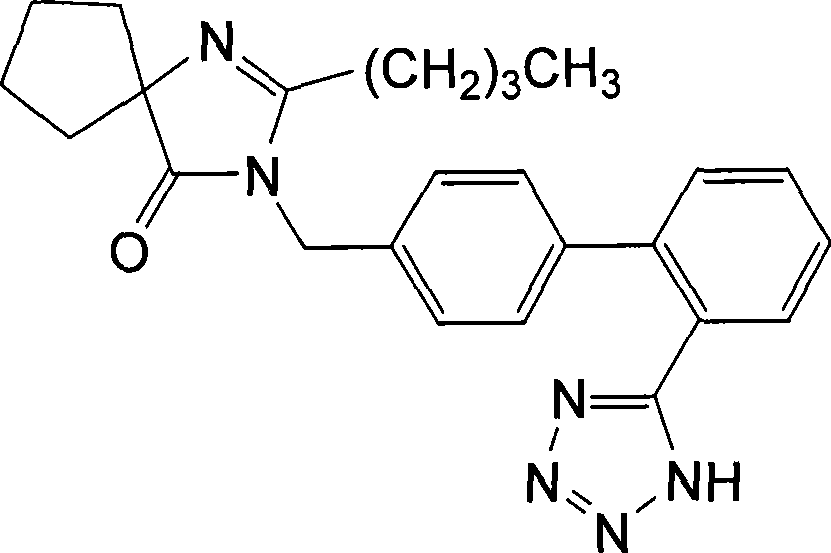
Irbesartan is used to treat high blood pressure (hypertension) and to help protect the kidneys from damage due to diabetes.
Pharmaceutical composition containing irbesartan
InactiveUS20050271720A1Reduce the amount of solutionHigh concentrationBiocideMetabolism disorderIrbesartanDissolution
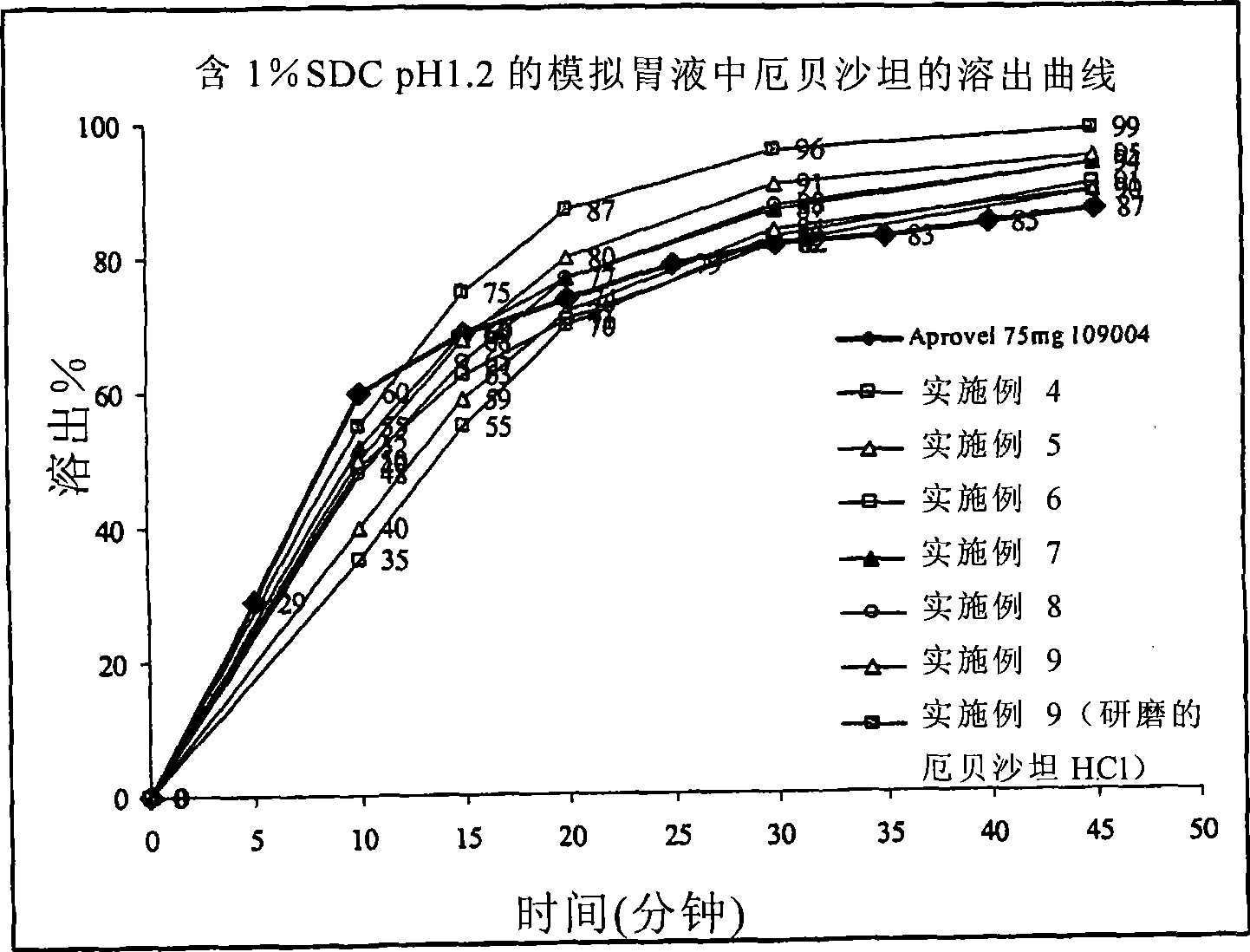
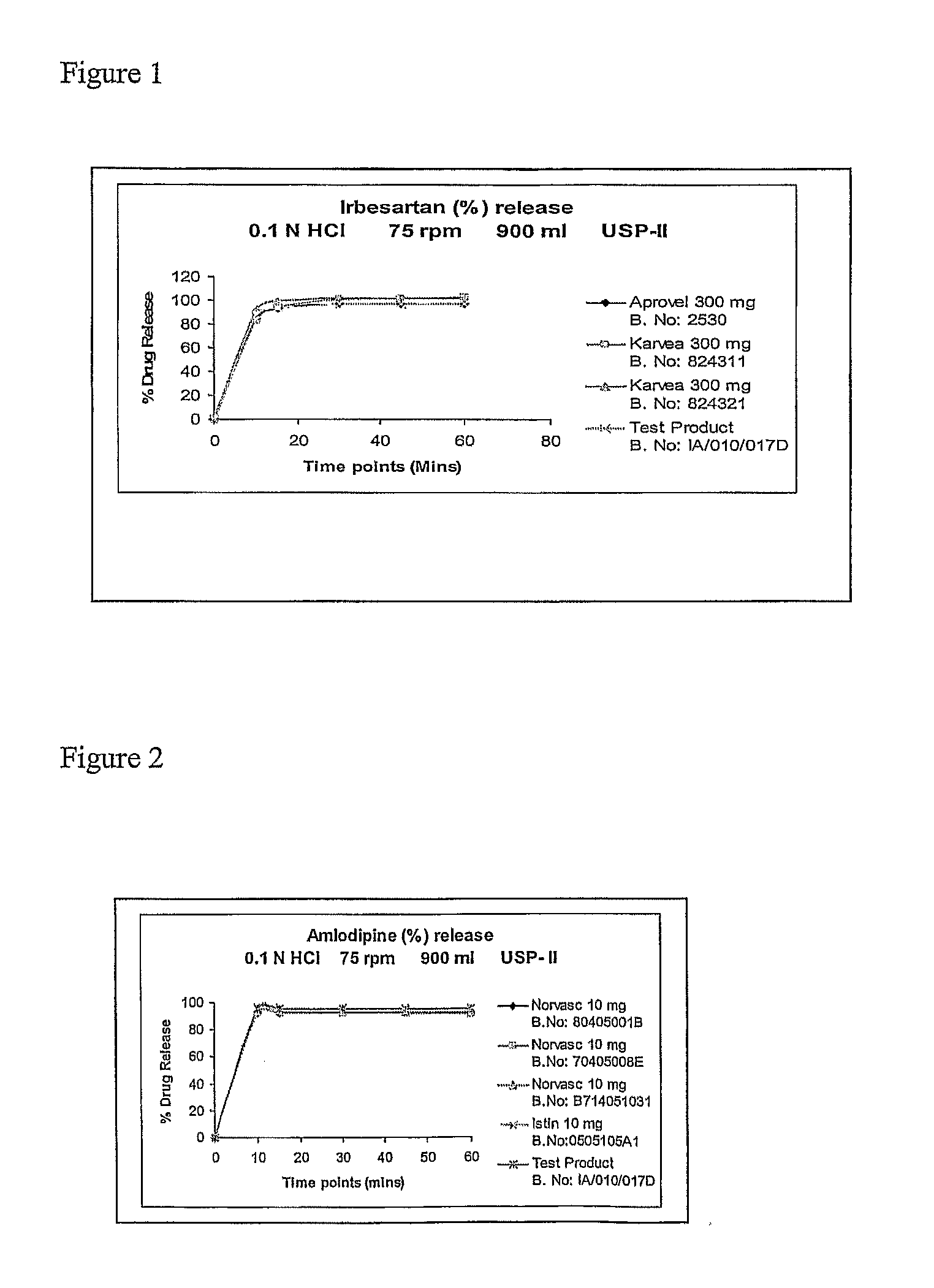
This invention relates to pharmaceutical compositions containing irbesartan, providing oral formulations with a high relative amount or concentration of irbesartan. In one embodiment, the present invention provides an oral formulation of irbesartan containing greater than 70% w / w irbesartan. In another embodiment, the invention provides an oral formulation of irbesartan which exhibits a dissolution profile according to which greater than about 85% of the Irbesartan is dissolved within about 30 minutes using USP apparatus 2, placing the tablet in 1000 mL of 0.1N hydrochloric acid at 37° C. with paddle speed of 50 rpm. The formulation can optionally contain at least one additional active ingredient.
Owner:TEVA PHARM USA INC
Dispersible tablet for treating hypertension
The invention relates to a medicinal oral preparation containing Irbesartan and Hydrochlorothiazide, more specifically the dispersible tablets for enhancing the beneficial effects of Irbesartan and Hydrochlorothiazide.
Owner:江苏万高药业股份有限公司
Composition for lowering blood pressure and application thereof
InactiveCN101890165AImprove compliancePrevent or delay damageOrganic active ingredientsMetabolism disorderTasosartanValsartan
The invention provides a pharmaceutical composition which comprises calcium channel blockers of a medicinal dose, angiotensin II receptor antagonists of a medicinal dose, one or more of B vitamins of a medicinal dose and pharmaceutically acceptable carriers, wherein the calcium channel blockers are selected from amlodipine, felodipine, israbipine, nicardipine, nifedipine, nisoldipine, nitrendipine, lacidipine, diltiazem or verapamil; the angiotensin II receptor antagonists are selected from candesartan, telmisartan, losartan, valsartan, irbesartan, eprosartan, tasosartan or olmesartan; and the B vitamins are selected from one or more of vitamin B6, vitamin B12, folic acid and calcium leucovorin. The pharmaceutical composition of the invention can improve the curative effect of the hypotensor, enhance the target organ protecting action of the hypotensor, and reduce the morbidity of complications of angina, myocardial infarction and the like.
Owner:北京奥萨医药研究中心有限公司 +1
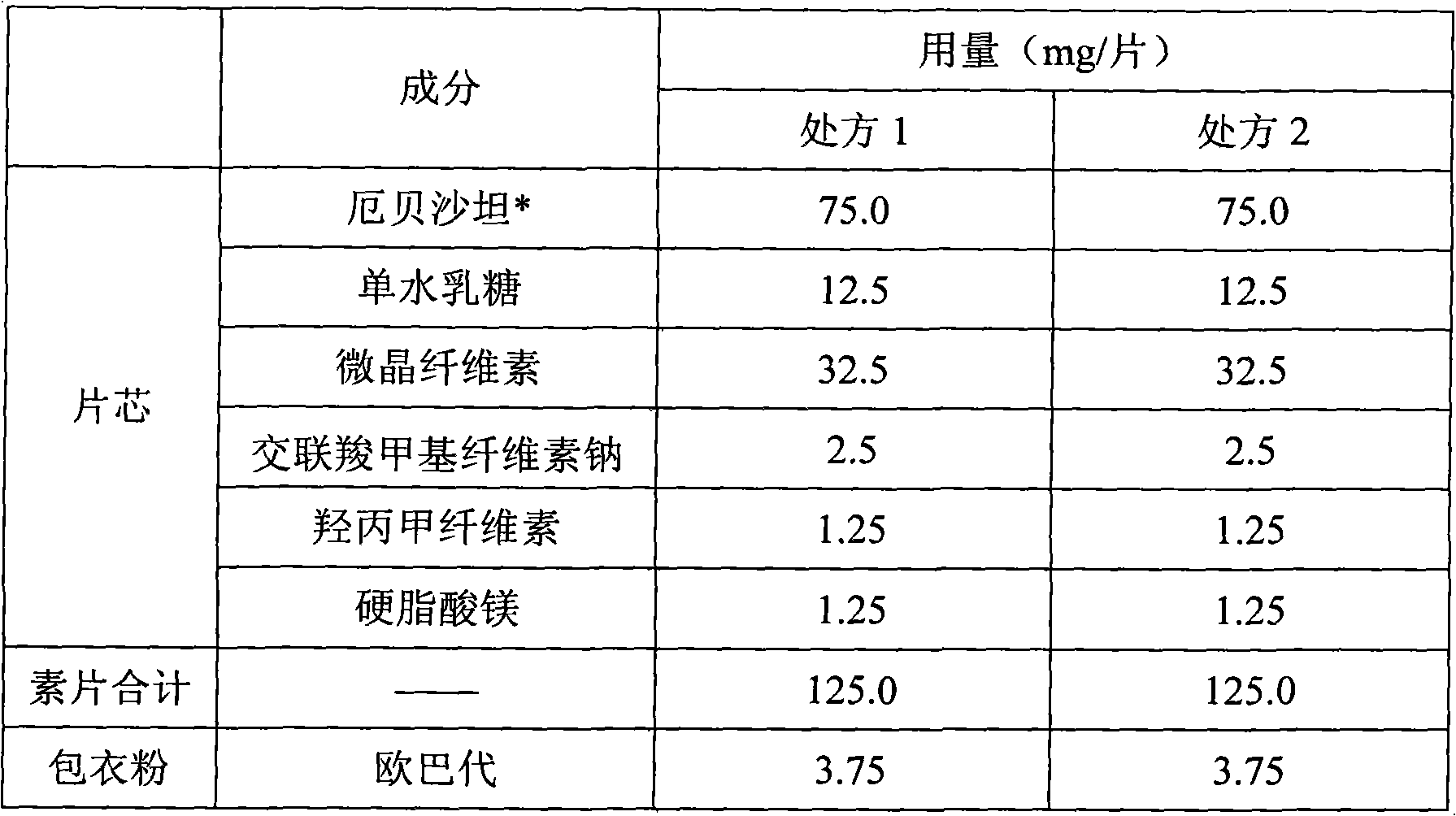
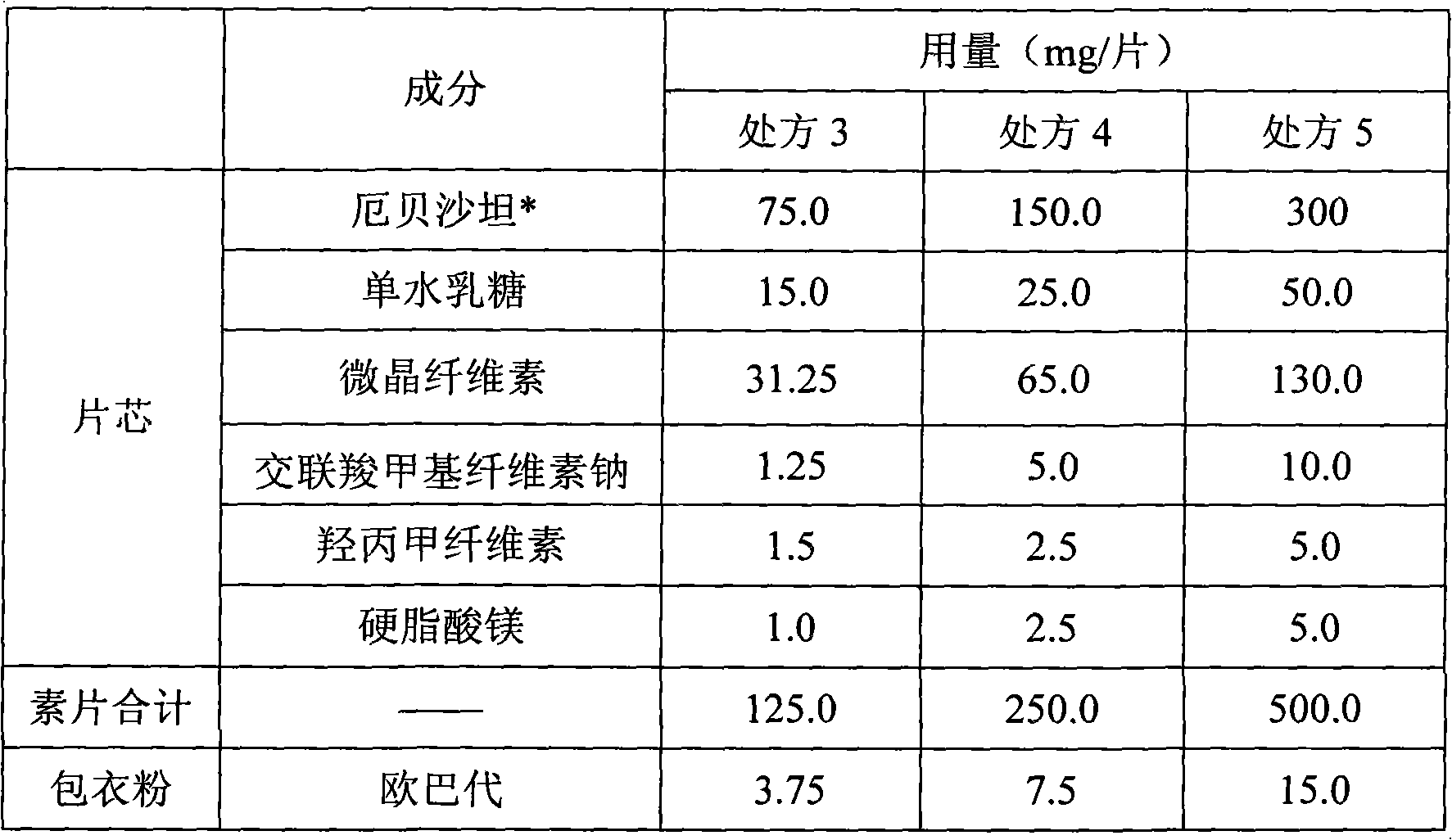
Pharmaceutical tablet compositions containing irbesartan
InactiveUS20090030052A1Rapid and complete drug releaseMaintain good propertiesBiocidePill deliveryIrbesartanLactose
A pharmaceutical tablet composition comprising irbesartan and lactose, said composition being essentially free of surfactant.
Owner:ALEMBIC LTD
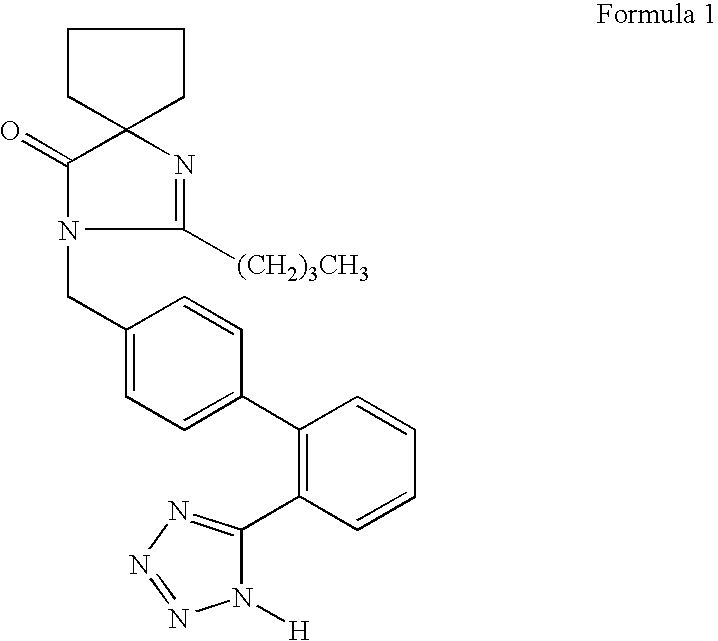
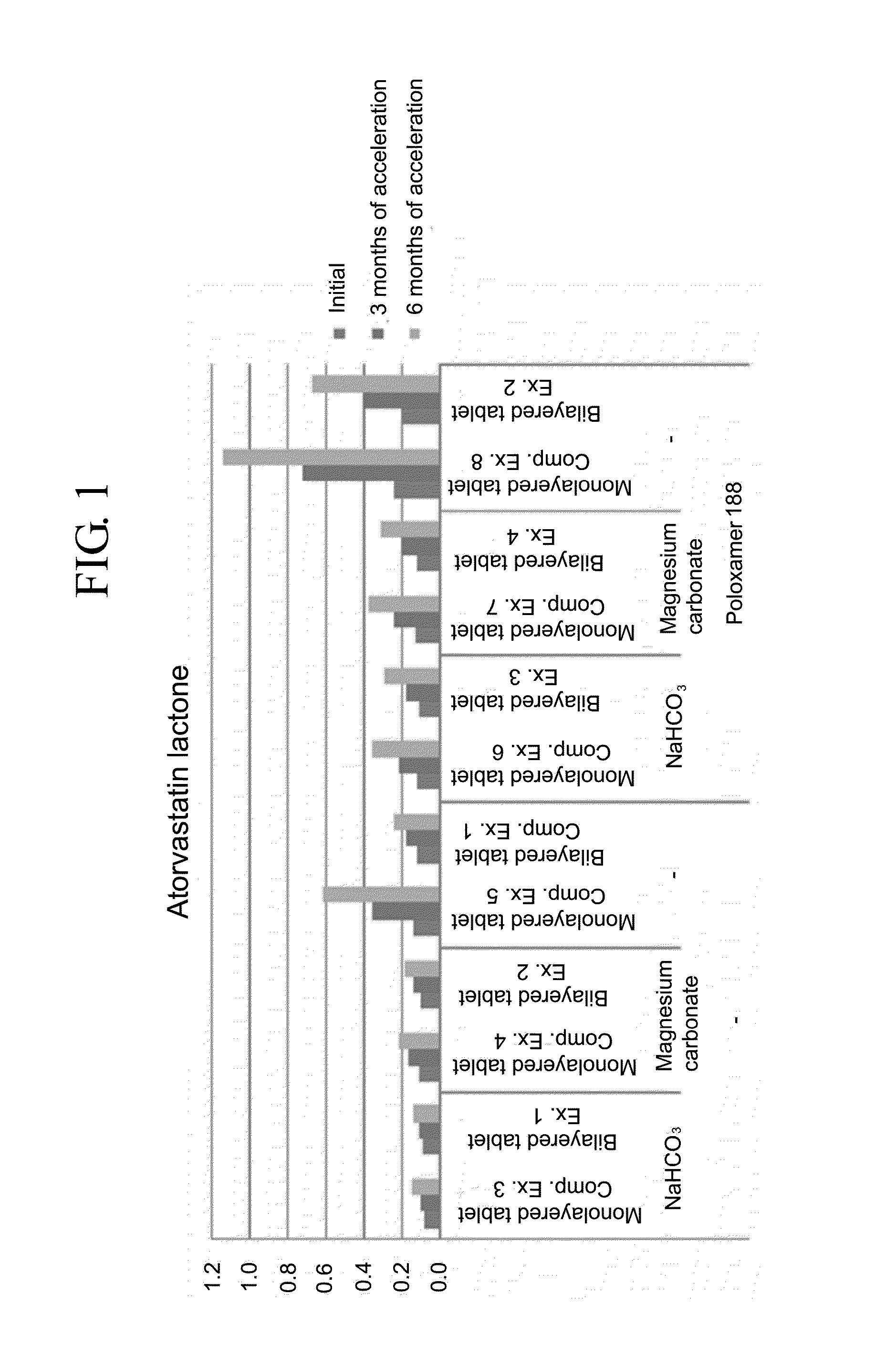
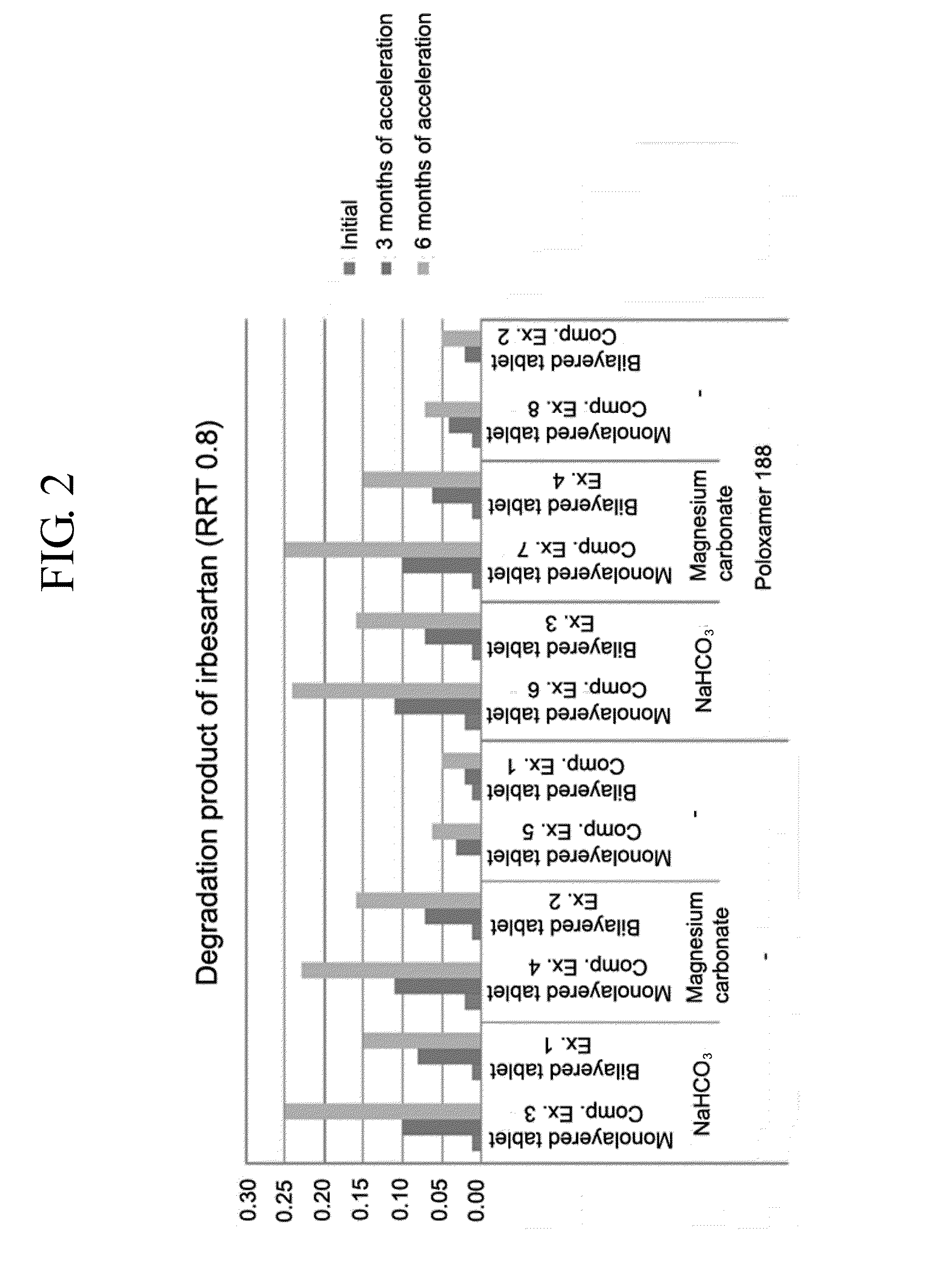
Pharmaceutical formulation in the form of bilayered tablets comprising hmg-coa reductase inhibitor and irbesartan
InactiveUS20130028974A1Increase ratingsImprove stabilityBiocideMetabolism disorderHMG-CoA reductaseIrbesartan
Provided is a pharmaceutical formulation in the form of a bilayered tablet consisting of a first layer containing irbesartan or a pharmaceutically acceptable salt thereof and a second layer containing an HMG-CoA reductase inhibitor and a basic additive, which can improve the dissolution rate and stability of irbesartan and an HMG-CoA reductase inhibitor to enhance the bioavailability of the drug compared to conventional complex formulations and to minimize the generation of the related compounds, thereby being effectively used as a stable and superior therapeutic agent for hypertension and hypercholesterolemia.
Owner:HANMI SCI CO LTD
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
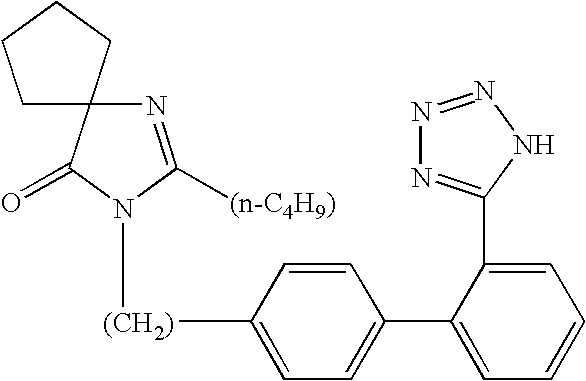
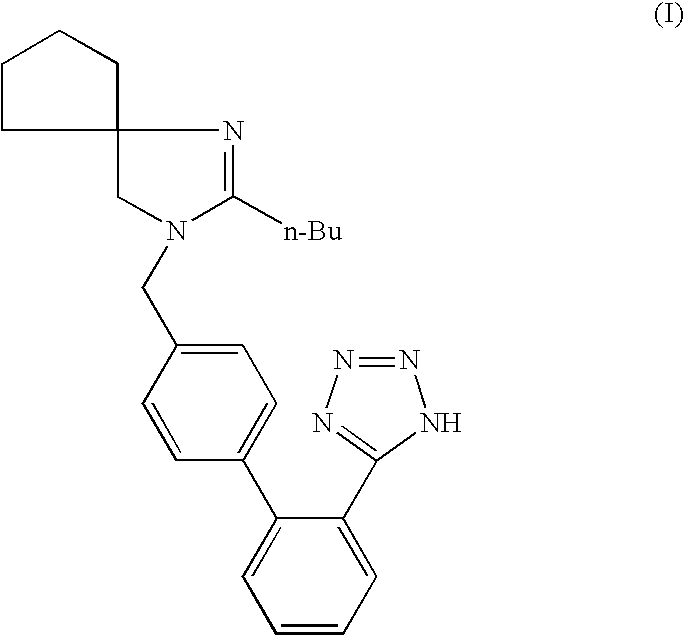
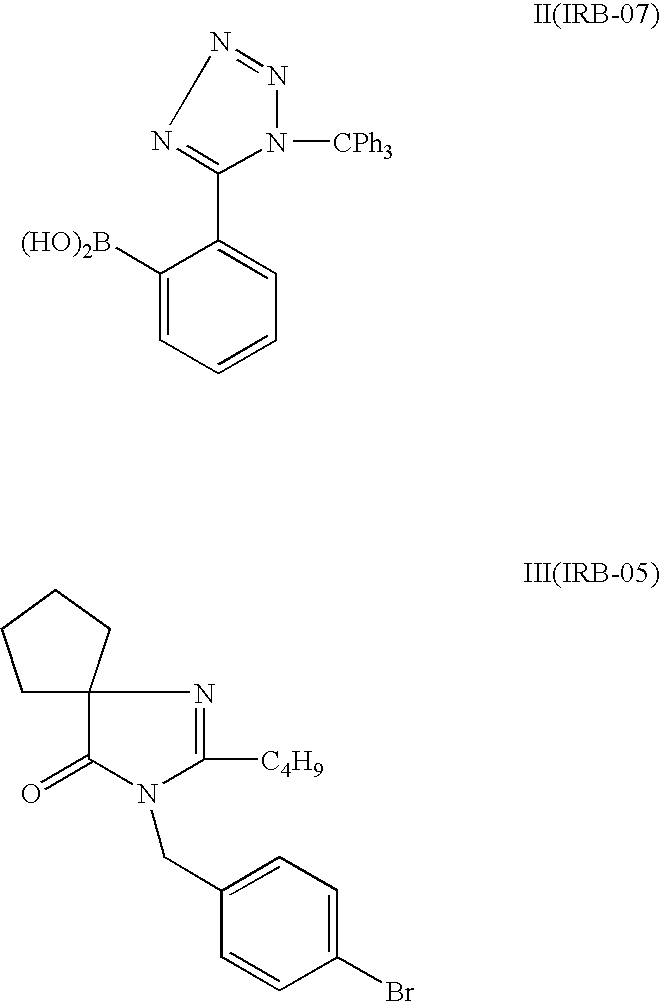
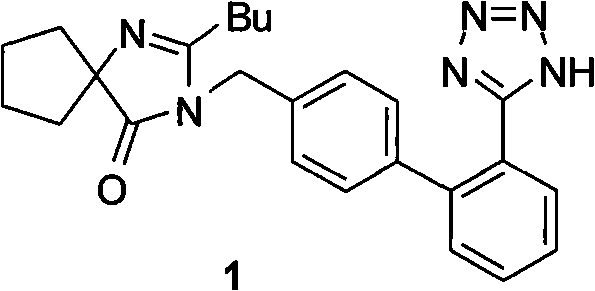
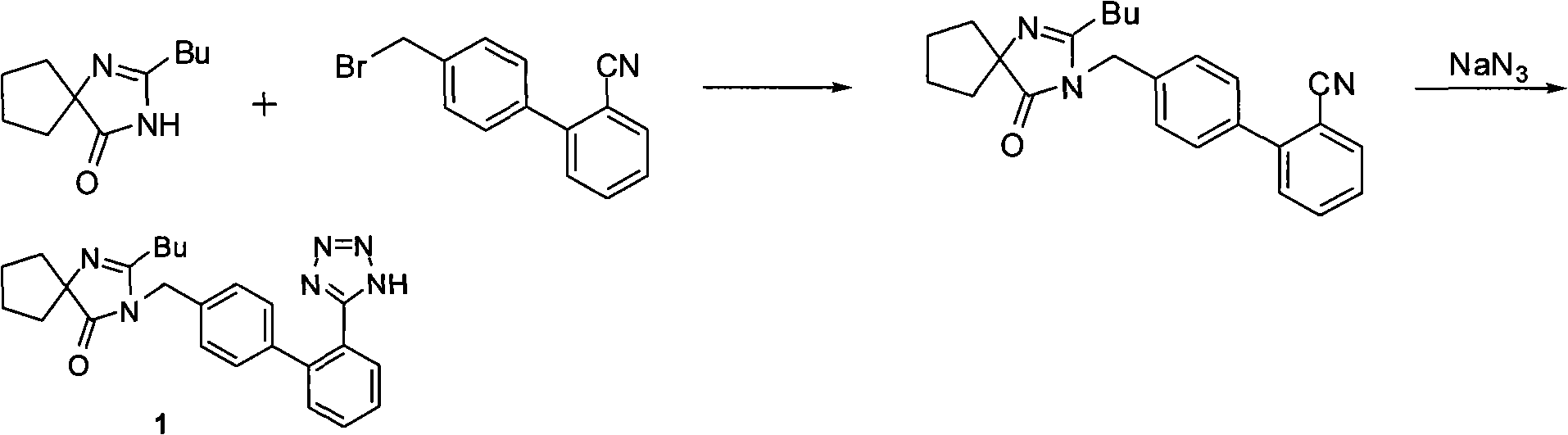
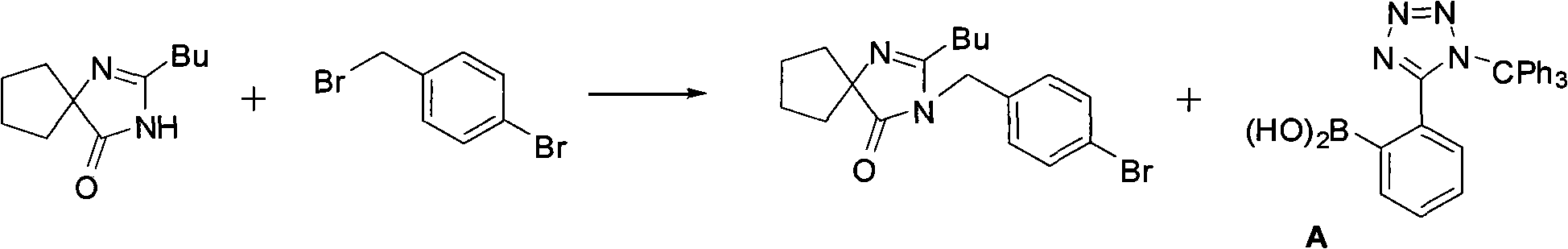
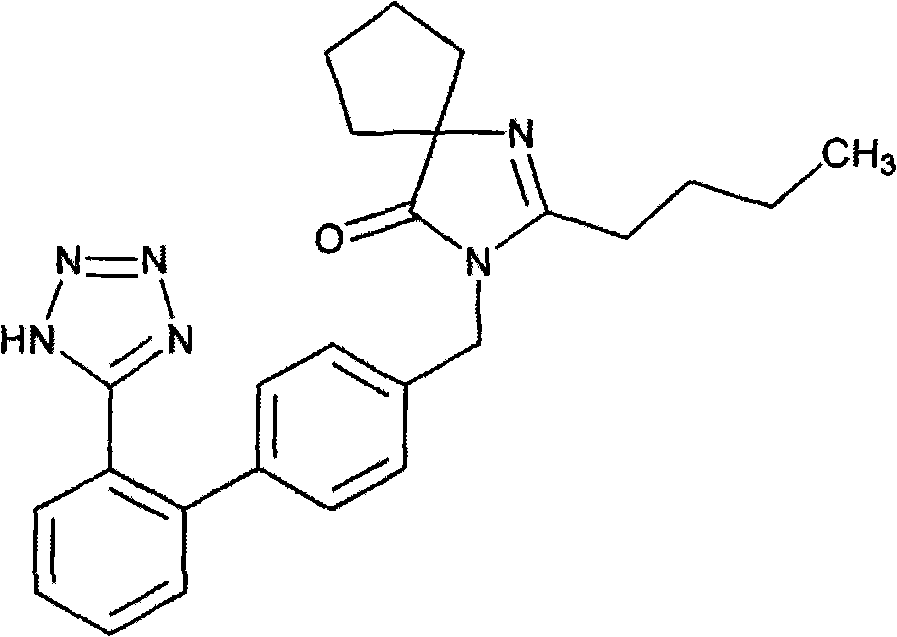
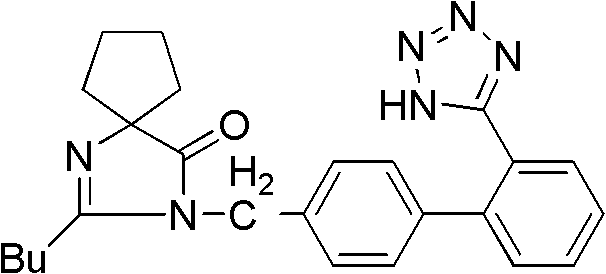
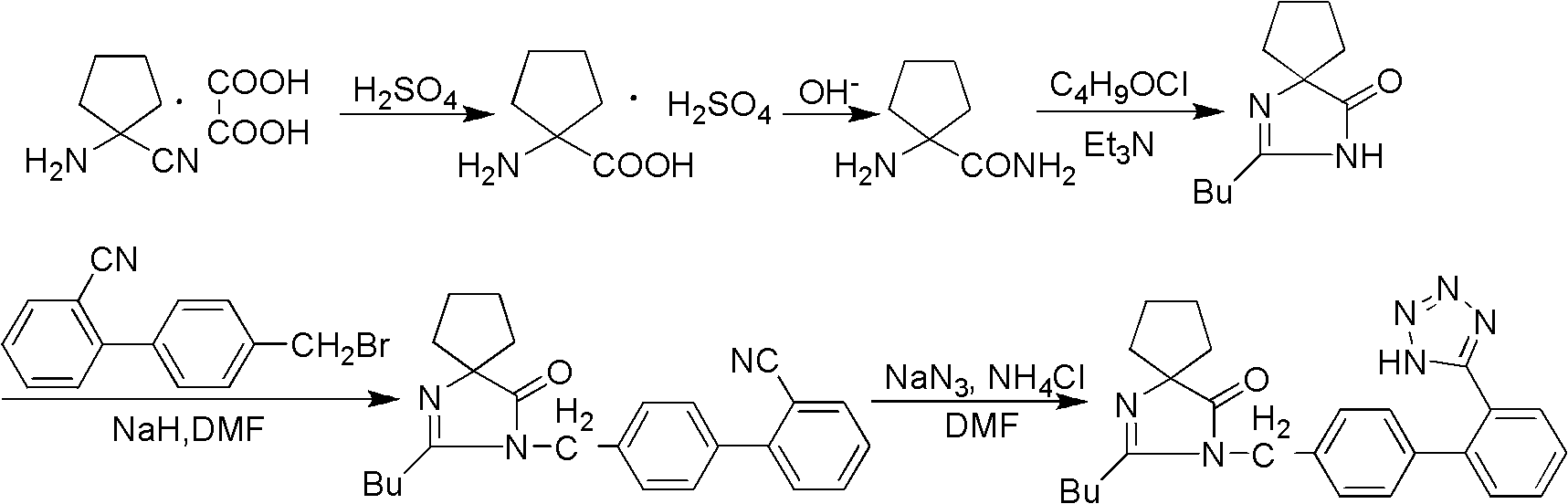
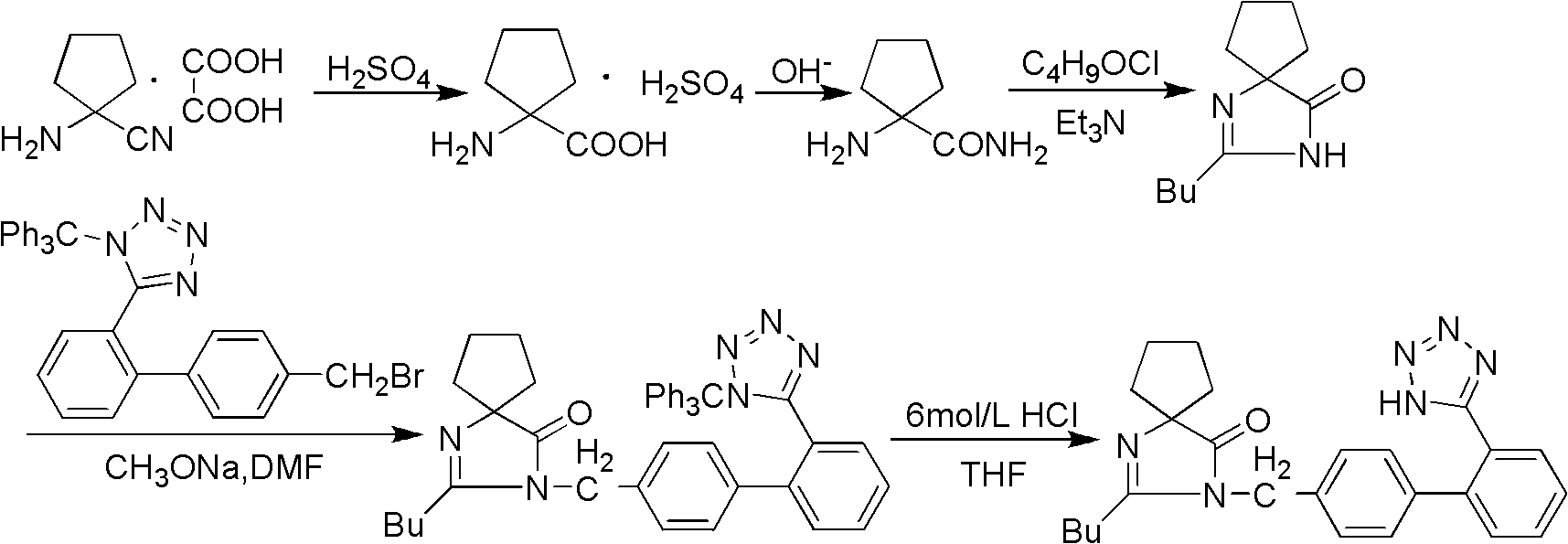
Synthesis of irbesartan
Provided are a method of making irbesartan via a Suzuki coupling reaction and a novel intermediate, 2-butyl-3-(4′-bromobenzyl)-1,3-diazaspiro[4.4]non-1-ene-4-one, for such process. The novel process includes the step of reacting such intermediate with a protected imidazolephenylboronic acid.
Owner:TEVA PHARM USA INC
Irbesartan sodium micro composite powder and tablets and preparation method thereof
ActiveCN102309456AHigh dissolution rateSmall particle sizeOrganic active ingredientsPowder deliveryIrbesartanMaterials science
The invention discloses irbesartan sodium micro composite powder and tablets and a preparation method thereof, in particular to amorphous and crystallization irbesartan sodium micro composite powder and tablets and a preparation method thereof, and belongs to the field of nanometer medicinal preparations. In the method, the amorphous and crystalline irbesartan sodium micro composite powder and corresponding tablets are prepared by combining a liquid-phase precipitation method with a spray drying technology. In the amorphous irbesartan sodium micro composite powder and the tablets thereof, theaverage grain diameter of medicinal granules is between 200 and 900 nanometers, more than 90 percent of amorphous sodium micro composite powder can be dissolved out within 10 minutes, and more than 90 percent of amorphous sodium micro tablets can be dissolved out within 5 minutes. In the crystalline irbesartan sodium micro composite powder and the tablets thereof, the average grain diameter of the medicinal granules is between 500 and 2,000 nanometers, more than 90 percent of crystalline sodium micro composite powder can be dissolved out within 15 minutes, and more than 95 percent of crystalline sodium micro tablets can be dissolved out within 15 minutes.
Owner:BEIJING UNIV OF CHEM TECH
Celecoxib and irbesartan coamorphous substance
The invention relates to a celecoxib and irbesartan coamorphous substance. Solubility of the celecoxib and irbesartan coamorphous substance in various buffer solutions is remarkably increased. The celecoxib and irbesartan coamorphous substance is an amorphous state totally different from celecoxib and irbesartan crystals and is different from the celecoxib and irbesartan crystals in melting point, x-ray powder diffraction spectrogram, DSC (differential scanning calorimetry) diagram and infrared spectrum. Under Cu-Kalpha radiation, the x-ray powder diffraction spectrogram shown by degree 2theta has no sharp diffraction peak. The glass-transition temperature of the celecoxib and irbesartan coamorphous substance is about 74.5 DEG C.
Owner:CHINA PHARM UNIV
Technique for synthesizing Irbesartan
A process for synthesizing Ebeishatan features that the intermediate 2-n-butyl-3-[(2'-cyanobiphenyl-4- yl)methyl]-1,3-diazaspiro [4,4]-nonyl-1-ene-4-one can be directly used in next step without purification, and in the reaction where cyano is converted to tetrazazole, the mixture of tributyl tin chloride and sodium azide is used to replace tributyl tin azide. Its advantage is high output rate up to 75%.
Owner:NANJING CHANGAO PHARM CO LTD
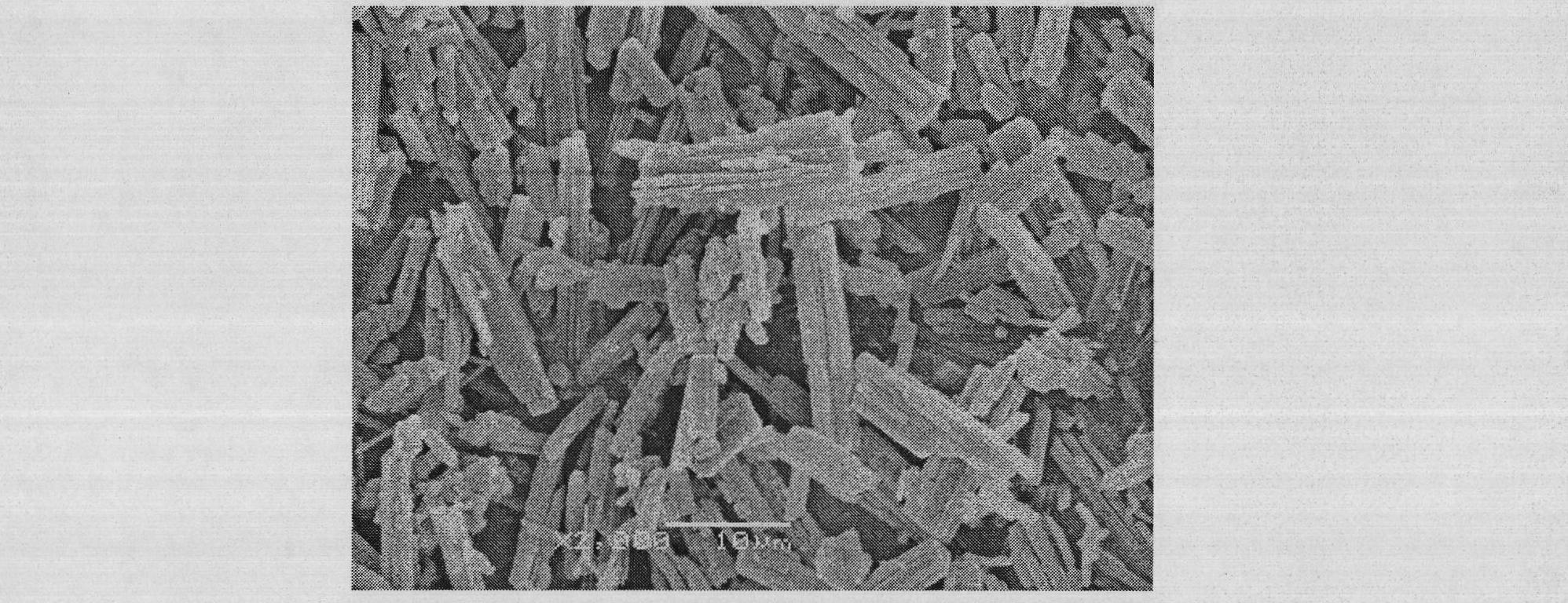
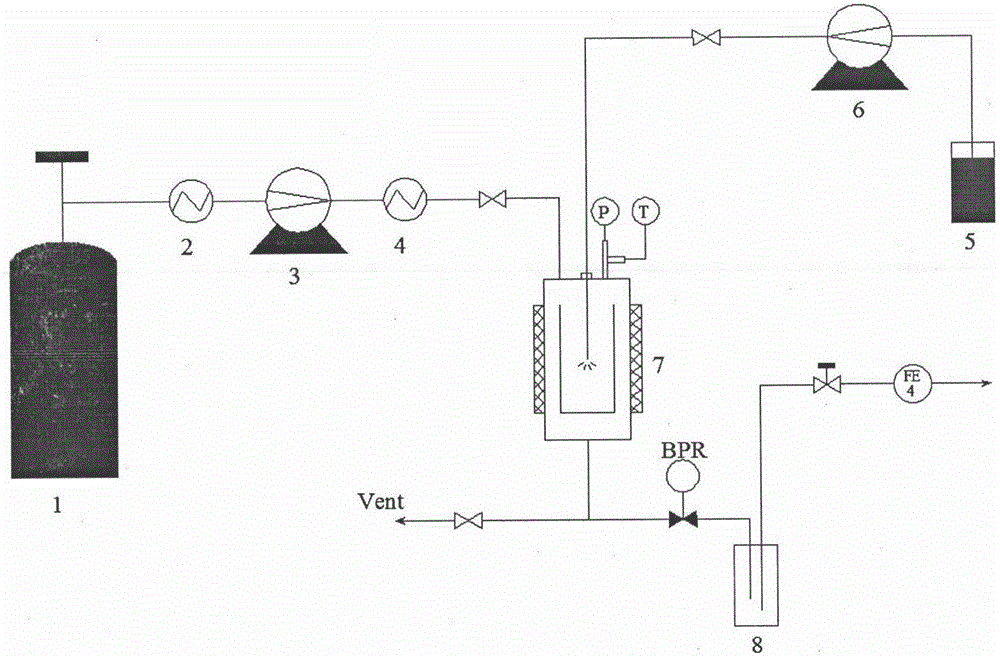
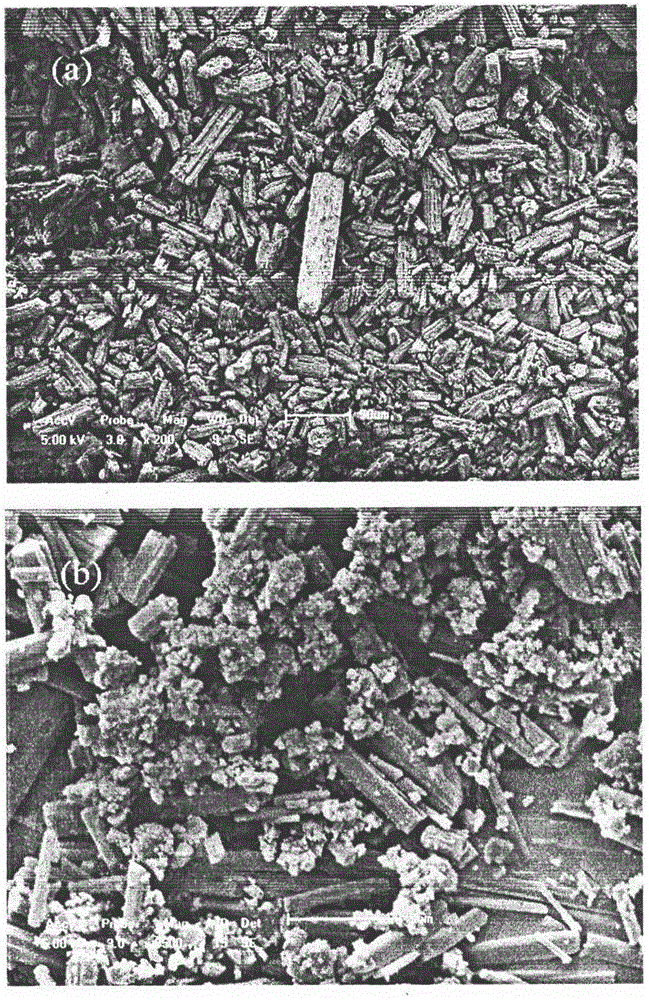
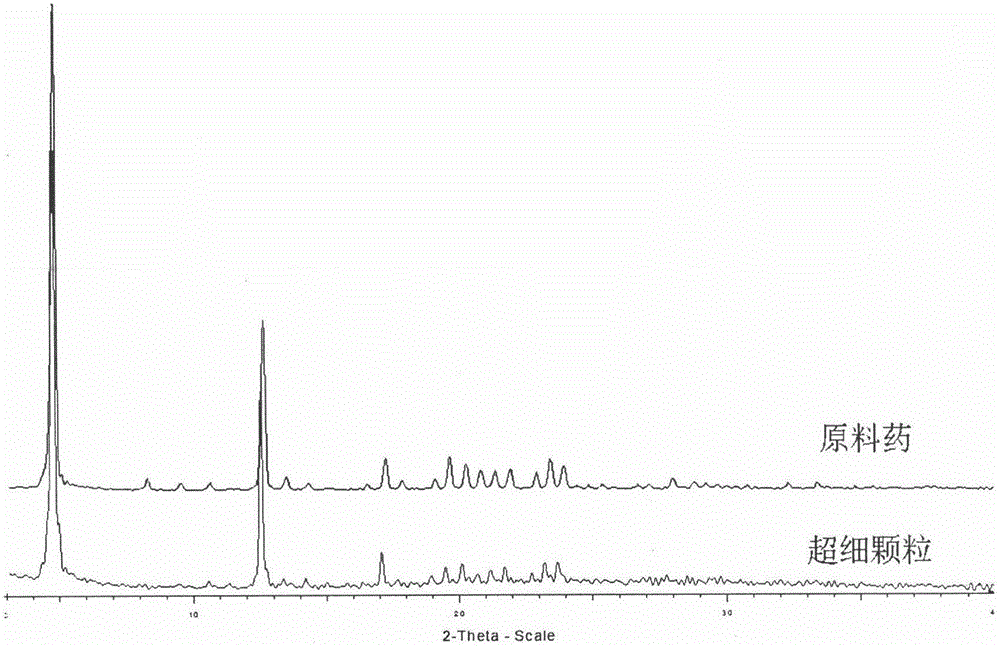
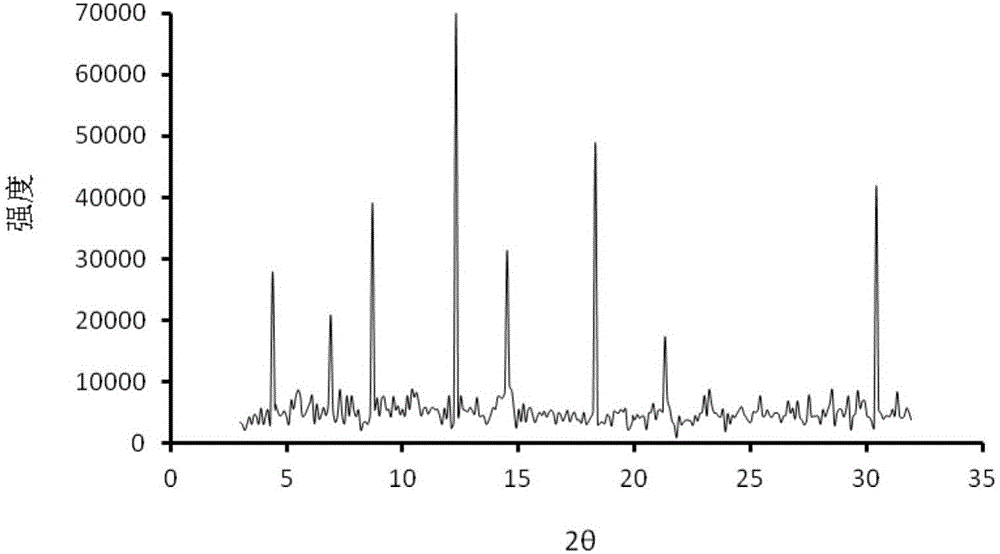
Method for preparing irbesartan ultrafine particles by applying supercritical anti-solvent technology
ActiveCN105534923AHigh dissolution rateUniform particle size distributionOrganic active ingredientsMetabolism disorderSolubilitySupercritical anti solvent
The invention discloses a process for preparing irbesartan ultrafine particles by applying a supercritical anti-solvent technology, and belongs to the technical field of medicinal dosage forms and supercritical technologies. The method is characterized in that an irbesartan solution is sprayed into a crystallization kettle through a supercritical fluid anti-solvent equipment system, and brick-shaped and amorphous-state irbesartan ultrafine particles are crystalized and precipitated in the crystallization kettle. According to the process, the shape and particle size of a medicament are controlled by changing parameters such as a solvent type, solution concentration, a solution sample introduction rate, a crystallization pressure and crystallization temperature. The irbesartan ultrafine particles prepared by the process have the advantages small particle sizes and narrow grain size distribution, and the dissolution rate and equilibrium solubility of the medicinal fine particles are improved remarkably.
Owner:CHINA PHARM UNIV
Angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof
ActiveCN106474479AGood treatment effectStable in natureMetabolism disorderGroup 5/15 element organic compoundsTasosartanValsartan
The present invention provides a an angiotensin receptor antagonist and creatine phosphate sodium complex and uses thereof, wherein the complex comprises an angiotensin receptor antagonist and creatine phosphate sodium, a molar ratio of the angiotensin receptor antagonist to the creatine phosphate sodium is 1:1-2, and the angiotensin receptor antagonist is selected from valsartan, losartan, irbesartan, telmisartan, eprosartan, candesartan, olmesartan, saprisartan, tasosartan, and elisartan. According to the present invention, the complex is formed by compounding the angiotensin receptor antagonist and the creatine phosphate sodium, and provides the unexpected double effect and the synergistic effect for treatment of heart failure and high blood pressure, the cocrystallization salt hydrate formed by linking the hydrogen bond has the stable characteristic, the pharmacokinetic property is significantly provided, and the positive application prospects are provided in the fields of anti-high blood pressure treatment and anti-heart failure treatment.
Owner:珠海赛隆药业股份有限公司(长沙)医药研发中心
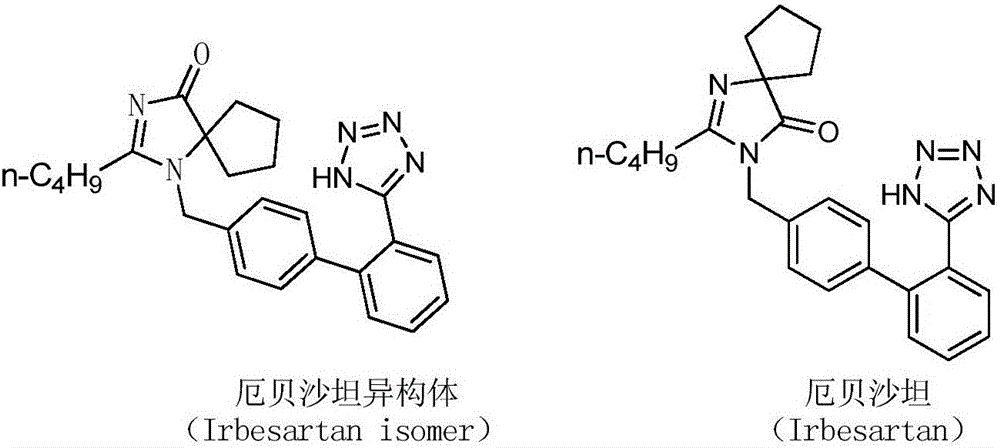
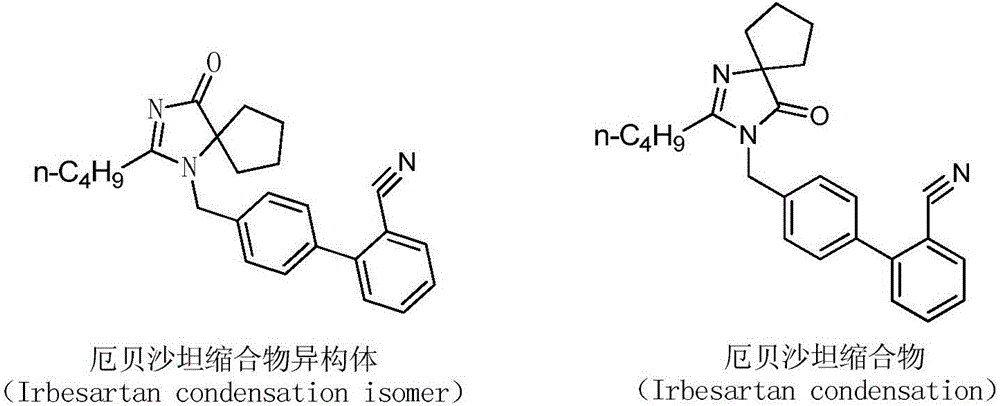
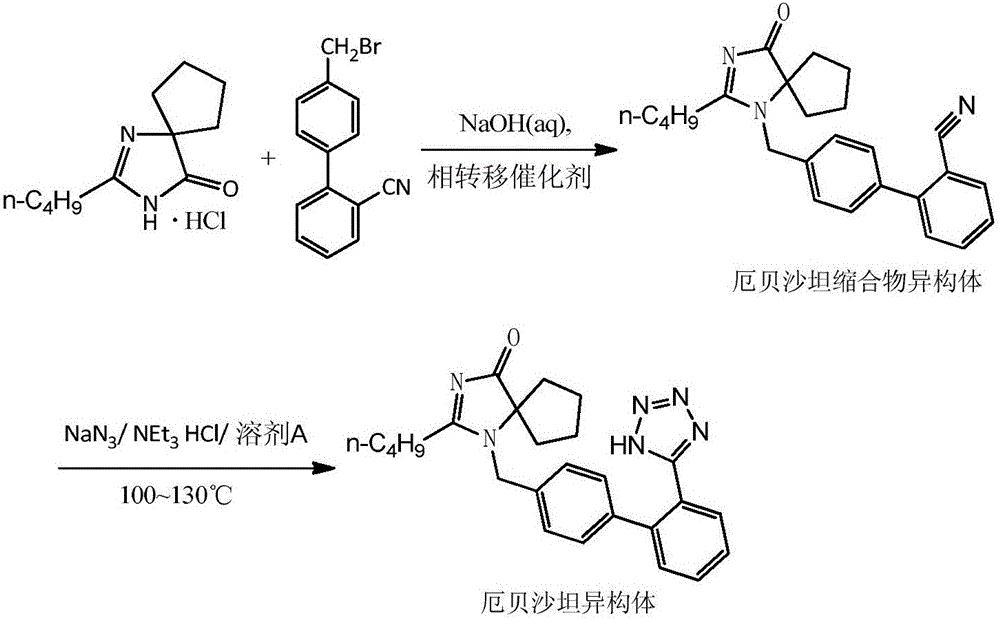
Preparation method of irbesartan isomer and irbesartan intermediate
InactiveCN106083826AHigh purityReduce energy consumptionOrganic chemistryChromatographic separationOrganic layer
The invention relates to a preparation method of an irbesartan isomer and an irbesartan condensation compound isomer as an irbesartan intermediate. The method comprises the following steps: 1) a phase transfer catalyst and an organic solvent are added to an irbesartan ring compound hydrochloride and biphenyl bromide, a sodium hydroxide solution is added, a reaction liquid is filtered after sufficient reaction of the raw materials, a filter cake is subjected to column chromatographic separation, and the irbesartan condensation compound isomer is obtained; 2) triethylamine hydrochloride, sodium azide and an organic solvent A are added to the irbesartan condensation compound isomer, the materials are heated to 100-130 DEG C and reacted, the sodium hydroxide solution and an organic solvent B are added, the mixture is stirred, left to stand and subjected to organic layer removal, hydrochloric acid is added to an aqueous-layer solution to regulate pH to 3.5-6.0, the solution is stirred and filtered, a filter cake is subjected to recrystallization with an organic solvent C or subjected to column chromatographic separation, and the irbesartan isomer is obtained. The irbesartan isomer impurity and the irbesartan intermediate prepared with the method have higher purity and lower energy consumption and cost. Meanwhile, the preparation method is simple to operate and environment-friendly and has important value in irbesartan production and research.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Irbesartan medicinal composition, as well preparation method and application thereof
The invention relates to an irbesartan medicinal composition, as well as a preparation method and application thereof. The preparation method comprises the following steps: preparing irbesartan solid dispersion which is prepared from irbesartan and a carrier material by adopting hot-melt granulation of a hot-melt extrusion granulator or a spray type solid dispersing agent preparation machine; mixing the irbesartan solid dispersion with a proper amount of diluting agent, disintegrating agent, surfactant, adhesive, flow aid and lubriant into tablets. According to the preparation method, irbessartan is prepared into a solid dispersing agent body and further prepared into a tablet, so that the dissolution rate is improved, and good absorption and biological utilization are achieved.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Method for synthesizing irbesartan and intermediate thereof
ActiveCN102432558APracticalSuitable for industrial productionOrganic chemistryIrbesartanMethyl group
The invention discloses a method for synthesizing irbesartan and an intermediate thereof, and belongs to the technical field of preparation methods for medicines. The method comprises the following steps of: protecting 5-(4'-methylbiphenyl-2-yl)-2-(1-methyl-1-phenylethyl)tetrazole by using alpha-methyl styrene under the catalysis of organic acid through the novel convenient reaction process, brominating a benzyl group, condensing a solid phase and a liquid phase under phase transfer to obtain irbesartan protected by 1-methyl-1-phenylethyl, performing acid hydrolysis on the protection to obtain the irbesartan and 2-phenyl-2-propanol, and recovering the 2-phenyl-2-propanol to obtain the alpha-methyl styrene. The invention has the advantages that: the synthetic process is suitable for industrial production, economic value can be produced, the synthetic process is safe, the cost for raw materials is saved, the subsequent product can be easily treated, the reaction raw material is single, and the synthetic method is convenient.
Owner:ZHEJIANG TIANYU PHARMA
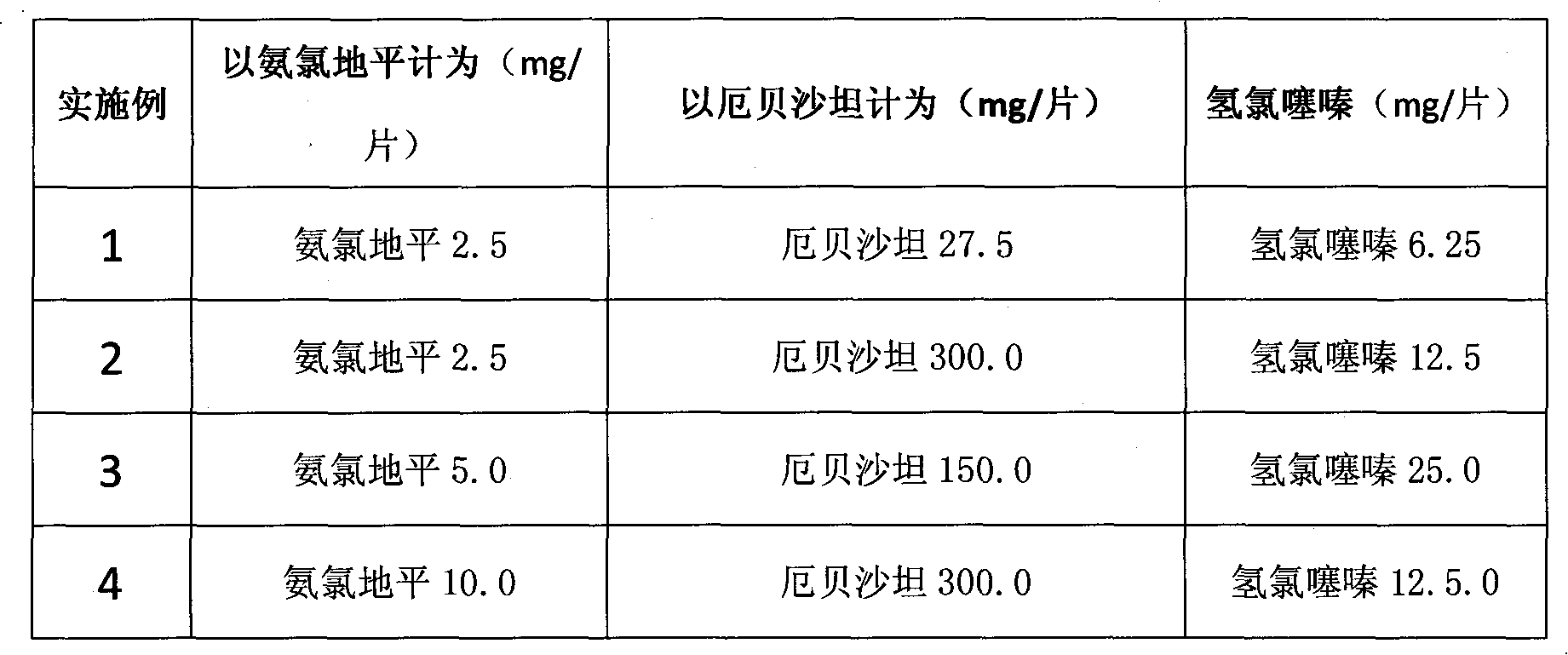
Amlodipine- and erbesartan-containing compound preparation for treating hypertension
InactiveCN101966187AQuick resultsHigh blood pressureOrganic active ingredientsCardiovascular disorderHydrochlorothiazideSide effect
The invention relates to an amlodipine- and erbesartan-containing compound preparation for treating hypertension, which comprises the following components: amlodipine or pharmaceutically acceptable salts of amlodipine, hydrochlorothiazide, and erbesartan or pharmaceutically acceptable salts of erbesartan which serve as main components, and a medicinal carrier, wherein in each preparation unit, the amlodipine or the pharmaceutically acceptable salts of amlodipine is 2.5 to 10.0mg based on the amlodipine, the erbesartan or pharmaceutically acceptable salts of erbesartan is 27.5 to 300.0mg based on the erbesartan, and the hydrochlorothiazide is 6.25 to 25.0mg. The compound preparation has the advantages that: through medicament combination to give full play to complementary action mechanisms of medicaments, the treatment effect is enhanced, the blood pressure quickly meets the standard, the qualification rate of the blood pressure is 80 percent, untoward effect related to dose increase of a certain medicament is reduced, and the longer action time is kept. The compound preparation has the characteristics of quick response, high qualification rate of blood pressure, small side effect and low cost.
Owner:邬林祥 +1
Medicinal composite containing irbesartan
InactiveCN101912390AReasonable dosageReduce joinOrganic active ingredientsPill deliveryPrillMedicine
The invention relates to a medicinal composite containing irbesartan, comprising irbesartan accounting for 30-70 wt% of preparation and pharmaceutical usable salt thereof, wherein the particle diameter of irbesartan D (V, 0.9), which is measured by a laser particle method is less than or equal to 200 mu m, and the composite is substantially micro powder free silica gel. The method of the invention can obtain a product with good dissolution and stability. The invention has the advantages that the invention chooses the irbesartan with certain range of particle diameter and auxiliary material to pelletize so as to obtain the particle with good flowability; meanwhile, the sticking problem generated by the electrostatic interaction of irbesartan can be solved; silica gel powder does not need to be added in a prescription, which omits the micro powder silica gel screening step, simplifies production technology, lowers production cost, improves production efficiency, lowers the production potential safety hazard brought by the micro powder silica gel and is more suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Irbesartan-containing pharmaceutical composition and preparing process thereof
ActiveCN104706640AAdvantages of preparation processQuick smashOrganic active ingredientsPharmaceutical delivery mechanismHydrochlorothiazideMedicine
The invention relates to an irbesartan-containing pharmaceutical composition and a preparing process thereof; the pharmaceutical composition comprises the following components: irbesartan, hydrochlorothiazide, lactose, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl methyl cellulose, silicon dioxide, magnesium stearate and a coating. Because a solid dispersoid technology is applied in the production manufacturing process, the materials are only subjected to conventional crushing treatment, and great change of the material physical properties are not caused; therefore, selection of a flow aid has no special requirements, and a high-quality and competitive-price precipitated silicon dioxide is selected in the premise of meeting the requirements of the production process.
Owner:HARBIN ZHENBAO PHARMA
Pharmaceutical formulation in the form of bilayered tablets comprising HMG-CoA reductase inhibitor and irbesartan
ActiveCN103002883AHigh dissolution rateImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsHMG-CoA reductaseDissolution
Owner:HANMI SCI CO LTD
Preparation method of irbesartan
InactiveCN102807564AEmission reductionSimple and fast operationOrganic chemistryAlkyl transferIrbesartan
The invention discloses a preparation method of irbesartan, which comprises the following steps of: 1) using irbesartan imidazole hydrochloride and 2-cyano-4'-bromomethylbiphenyl as raw materials, conducting alkylation reaction in a water-aprotic solvent non-homogeneous system under the effect of inorganic base and phase transfer catalyst to prepare irbesartan hydrocarbon; and 2) taking the irbesartan hydrocarbon prepared in the step 1 and azimino compounds and conducting cyclization reaction in polar solvent under the effect of cyclization catalyst to prepare irbesartan. The preparation method of irbesartan provided by the invention has the advantages of simplicity and convenience in operation, easiness in obtaining raw materials, easiness in realization of reaction conditions, high yield, low cost, environmental friendly and the like, and is suitable for industrial production.
Owner:珠海润都制药股份有限公司
Solid pharmaceutical composition comprising irbesartan
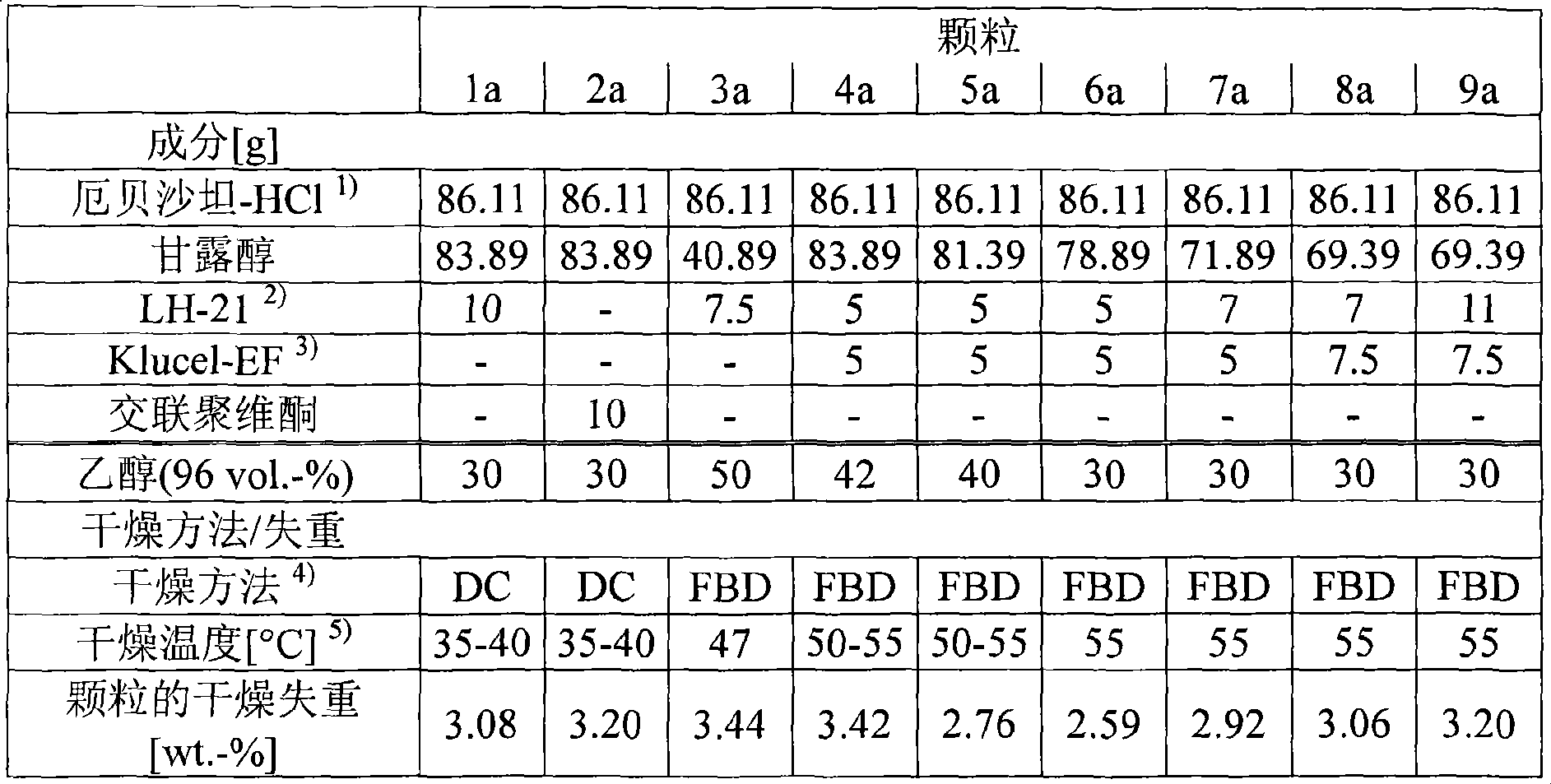
The present invention concerns preferably surfactant-free solid pharmaceutical formulations comprising, as an active ingredient, at least one of irbesartan and pharmaceutically acceptable salts thereof, and at least one disintegrant. Preferably, the active ingredient comprises irbesartan hydrochloride. Also, the present invention is directed to a process for the manufacture of such formulations, including a wet granulation process (A) and a direct granulation process (B).
Owner:KRKA D D NOVO MESTO
Irbesartan capsules and preparation method thereof
ActiveCN102697754AUniform particle sizeImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsPhysical chemistryIrbesartan
The invention discloses irbesartan capsules and a preparation method thereof. The irbesartan capsule mainly comprises irbesartan, pregelatinized starch, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl methylcellulose and magnesium stearate. A boiling one-step granulation method is adopted, which means that mixing, granulation and drying steps are completed in one step in a closed container of a boiling one-step granulator. The prepared particles have uniform granularity, good fluidity, small difference of capsule capacity, good solubility, less steps than the conventional wet-process granulation process, few production equipment, low production cost, high efficiency and high yield.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Pharmaceutical composition containing irbesartan and amlodipine benzenesulfonate and preparation method thereof
ActiveCN103860511AHigh dissolution rateImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBenzenesulfonates
The invention belongs to the technical field of medicine, and particularly relates to a pharmaceutical composition containing irbesartan and amlodipine benzenesulfonate and a preparation method thereof. The formula of the pharmaceutical composition comprises 10 parts by weight of irbesartan, 5 parts by weight of amlodipine benzenesulfonate (calculated according to amlodipine), 40 to 70 parts by weight of microcrystalline cellulose, 6 to 12 parts by weight of croscarmellose sodium, 3 to 9 parts by weight of hydroxypropyl methylcellulose, 2 to 8 parts by weight of colloidal silicon dioxide, 2 to 6 parts by weight of magnesium stearate and 2 to 6 parts by weight of a coating material. The pharmaceutical composition is prepared by a dry granulation tabletting process, and the shortcomings of a wet granulation process are overcome; and the pharmaceutical composition has the advantages of fast dissolution, uniform content, stable properties, simple production process and high production efficiency, and is suitable for industrial mass production.
Owner:HAINAN ZHONGJI MEDICAL TECH
"One-pot" synthesis of irbesartan intermediate
InactiveCN102285923ASimple process routeEasy to operateOrganic chemistryCyanide compoundHypertension medications
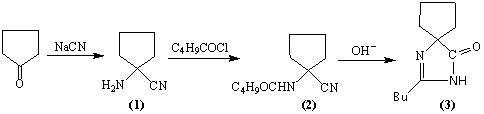
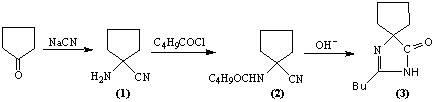
The invention relates to an improved synthesis process of an antihypertensive drug irbesartan intermediate 2-butyl-1,3-diazaspiro[4.4]nonane-1-en-4-one. Cyclopentanone undergoes an addition reaction to obtain 1-aminocyclopentyl cyanide. The cyanide is first acylated, then hydrolyzed and cyclized under alkaline conditions to obtain the product. The positive effect of the present invention is that the first hydrolysis, then acylation and cyclization reported in the prior art is improved to first acylation, then hydrolysis and cyclization, each step does not go through the separation process, and the "one pot method" is realized Production. The operation process is simplified, the product yield is improved, the product quality is guaranteed, the production cost is reduced, and it is more conducive to large-scale industrial production.
Owner:河南华商药业有限公司
Solid pharmaceutical fixed dose compositions comprising irbesartan and amlodipine, their preparation and their therapeutic application
ActiveUS20120171288A1Reduced and controlled impurityPowder deliveryBiocideMedicineAmlodipine besilate
The present invention is directed to solid stable pharmaceutical fixed dose compositions comprising irbesartan, amlodipine besilate and pharmaceutically acceptable excipients, to their preparation and to their therapeutic application.
Owner:SANOFI SA
Irbesartan gastric retention sustained-release pharmaceutical composition
InactiveCN101011393AOrganic active ingredientsPharmaceutical non-active ingredientsPharmacyIrbesartan
The invention relates to a method for preparing erbesatan stomach-held slow-release drug compound, which comprises the erbestan and acceptable medical shaping agents, to control the stop time in the stomach, to confirm the stable blood drug density of patient, improve safety and effect.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Drug composition containing irbesartan and hydrochlorothiazide
ActiveCN103040841APrevention of biological activityElevated C-reactive protein levelsOrganic active ingredientsCardiovascular disorderMortality rateIrbesartan
The invention discloses a drug composition containing irbesartan and hydrochlorothiazide. The drug composition is prepared into a tablet or a capsule by wet granulation of an active ingredient and a pharmaceutic adjuvant; the active ingredient comprises irbesartan and hydrochlorothiazide at a weight ratio of 1:(1.3-10); and the pharmaceutic adjuvant comprises a filler, a disintegrating agent, adhesive and a lubricant. With the adoption of the drug composition, impaired glucose tolerance is alleviated, the biological activity of insulin resistance is prevented, rising of a C-reactive protein level of a patient with hypertension can be prevented, the morbidity and mortality of and caused by various cardiovascular diseases in the future are reduced, and the drug composition has a very good improvement effect on the prognoses of the patient with the hypertension.
Owner:NANJING CHIA TAI TIANQING PHARMA
Pharmaceutical formulations containing irbesartan
Owner:LAB LESVI SL
Medicinal compound composition of irbesartan and hydrochlorothiazide and preparation method thereof
InactiveCN103655579ADissolution unchangedImprove stabilityOrganic active ingredientsCardiovascular disorderUse medicationCoated tablets
The invention discloses a compound composition of irbesartan and hydrochlorothiazide. The composition can be made into granules, capsules, tablets and coated tablets. By screening and adopting a specific adhesive, the dissolution rate of the active ingredient irbesartan can exceed 94%, and the dissolution rate of hydrochlorothiazide can exceed 96%; after the composition is placed for 10 days at a high temperature of 60 DEG C, the dissolution rates of the two active ingredients are basically kept unchanged; and therefore, the composition has good stability and can meet the needs of clinical medication.
Owner:石药集团中诺药业(石家庄)有限公司
Synthetic method for irbesartan
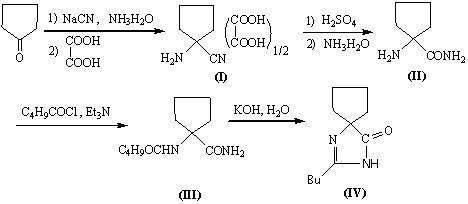
The invention discloses a synthetic method method for irbesartan. The method comprises the following steps of: hydrolyzing amino cyclopentyl cyanogen serving as a raw material in an alkaline solution to obtain amino cyclopentyl formamide; undergoing a cyclization reaction on the obtained amino cyclopentyl formamide and valeryl chloride to obtain 2-butyl-1,3-diazaspiro[4,4]nonane-1-alkene-4-ketone serving as an intermediate (I); undergoing a C-N coupling reaction on the intermediate (I) and 4-bromomethyl-2'-cyanobiphenyl to obtain 2-butyl-3-[(2'-cyanobiphenyl-4-radical)methyl]-1, 3-diazaspiro[4,4]nona-1-alkene-4-ketone serving as an intermediate (II); undergoing a sodium nitride cyclization reaction to obtain irbesartan serving as a target product. Due to the adoption of the synthetic method, the total yield of the irbesartan product is increased from below 45 percent to over 75 percent, the tonnage consumption the product is greatly lowered, and cost is greatly lowered. An operation process is simplified, so that the yield is increased, three-waste pollution is lowered, treatment is easy, and a novel process with high competitiveness is realized.
Owner:浙江弘盛药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com