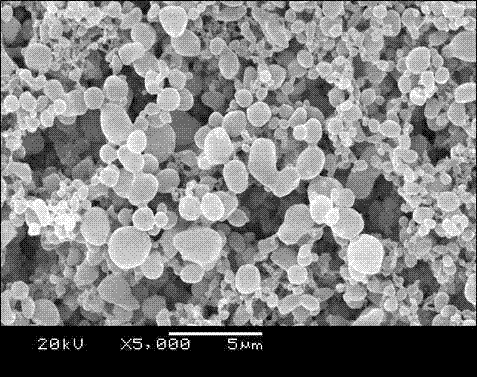
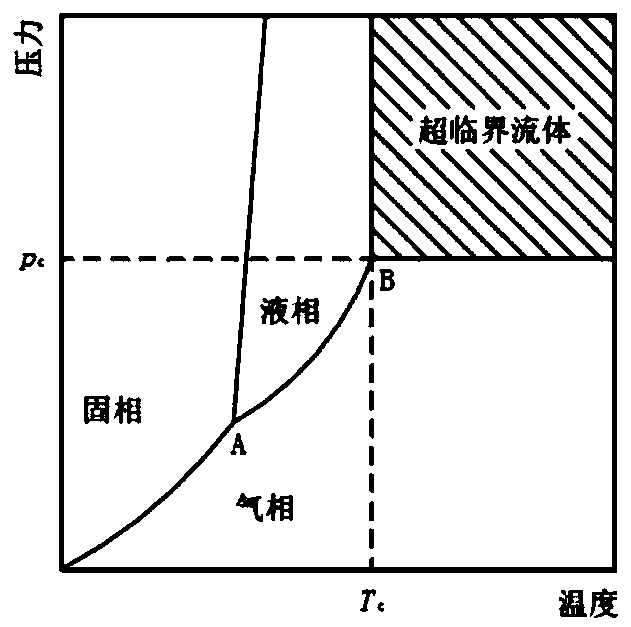
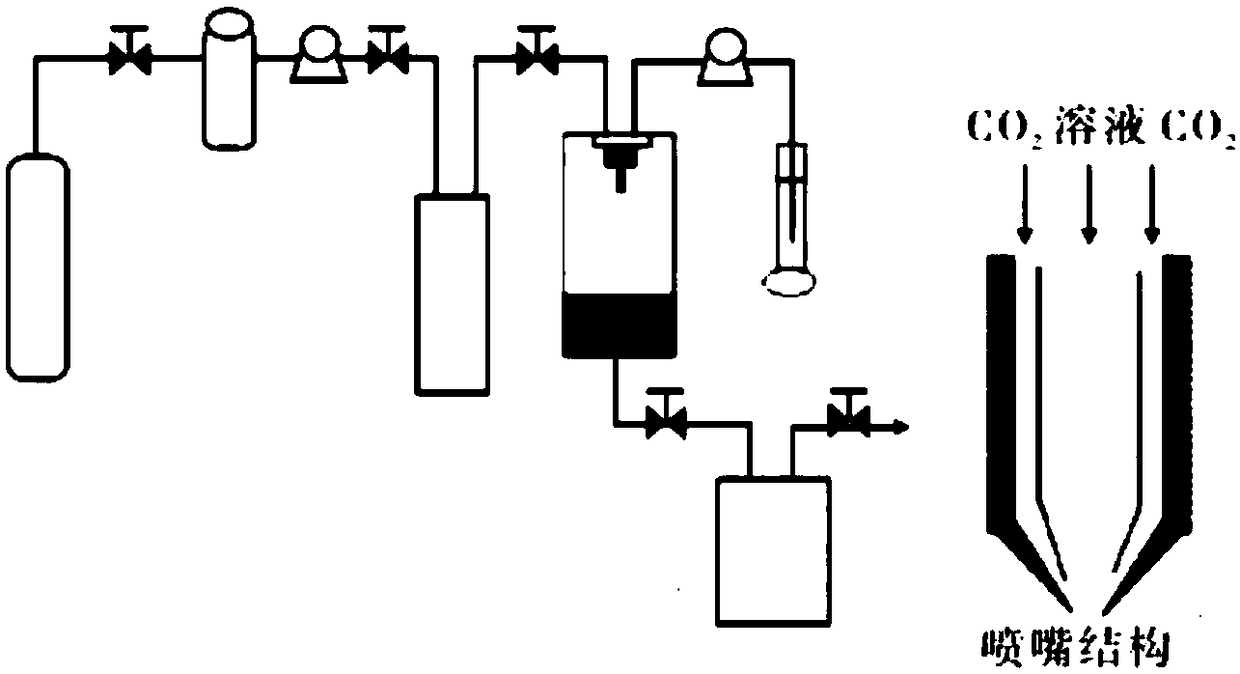
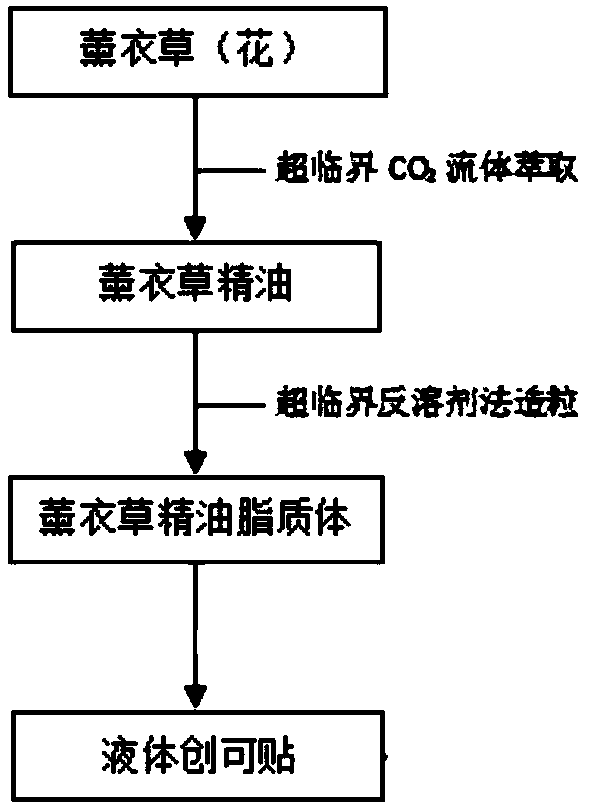
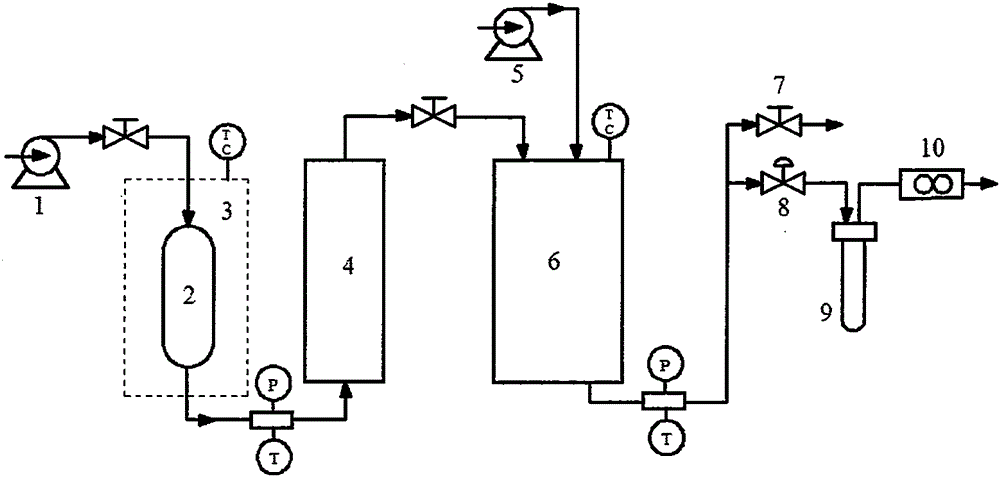
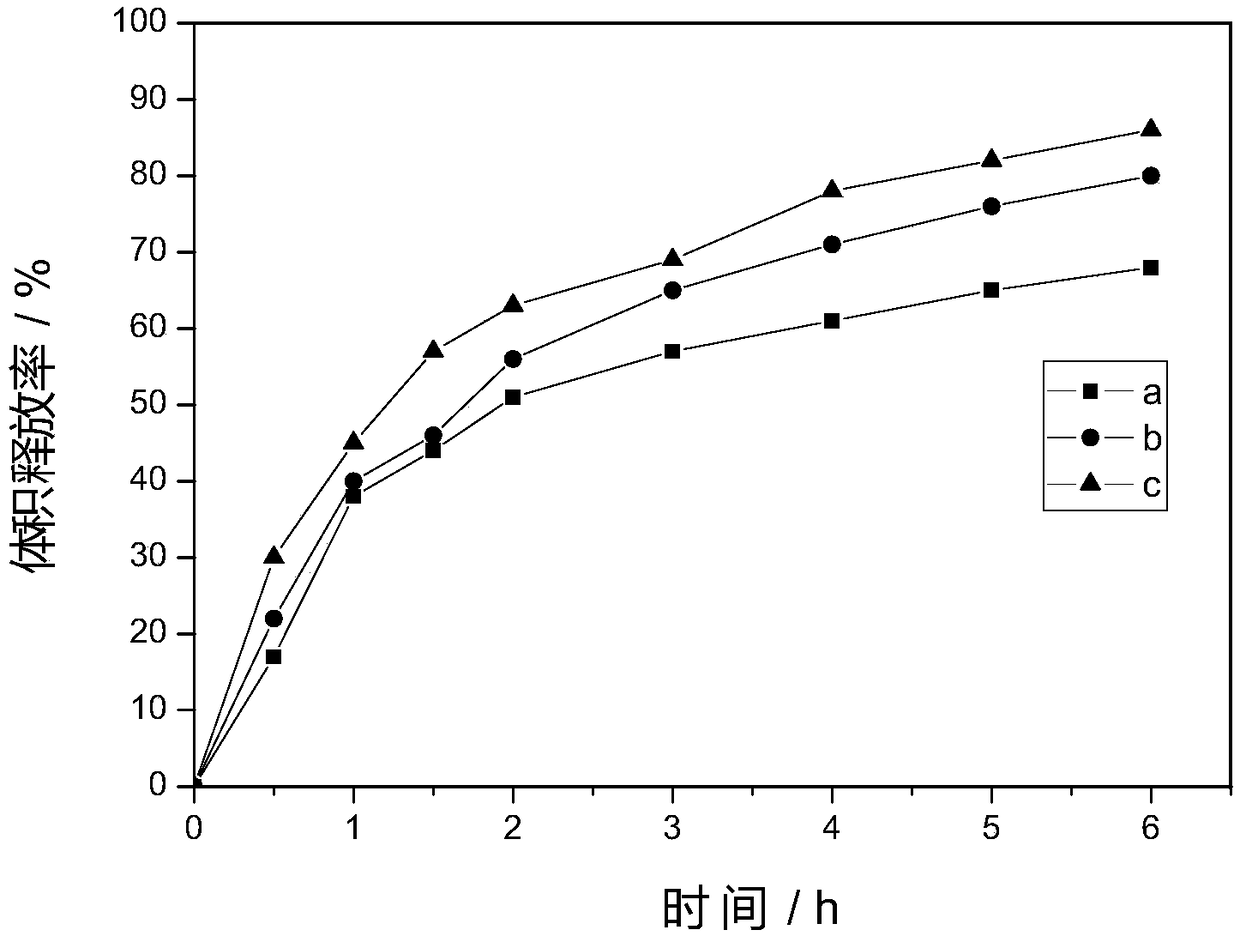
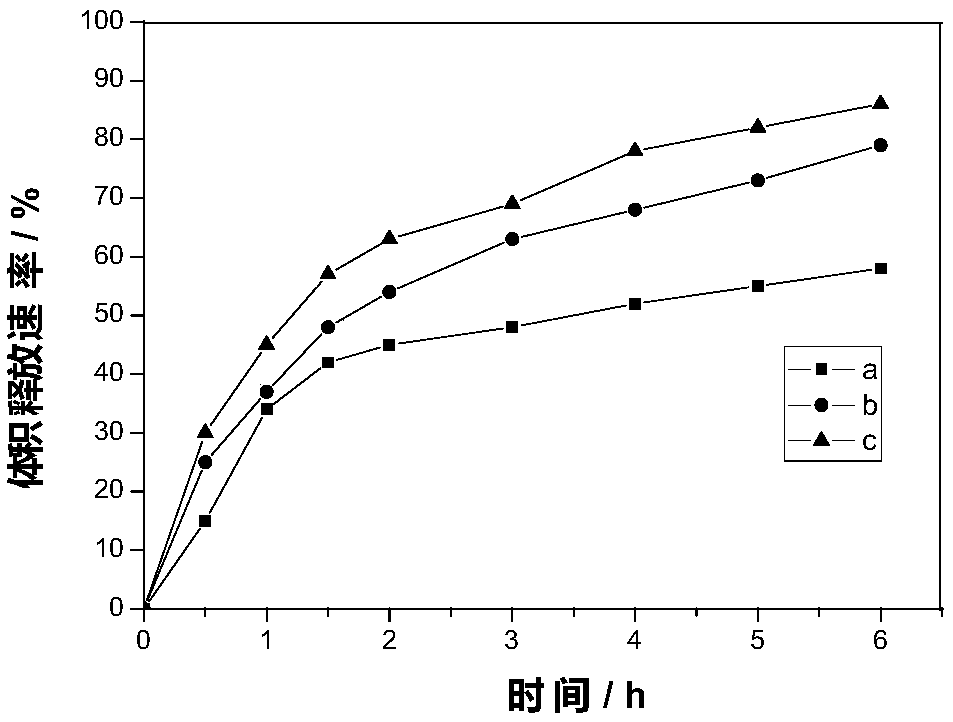
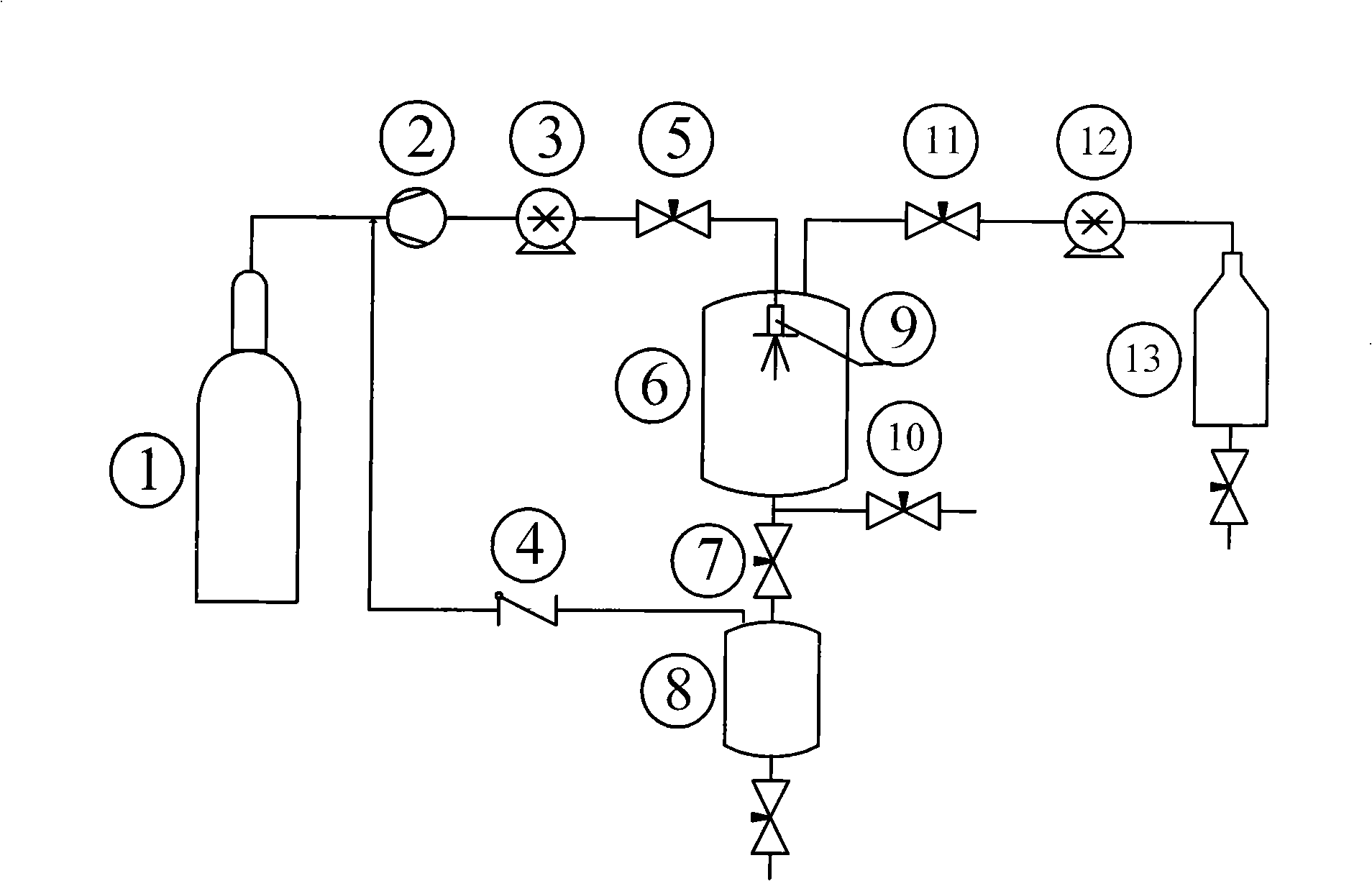
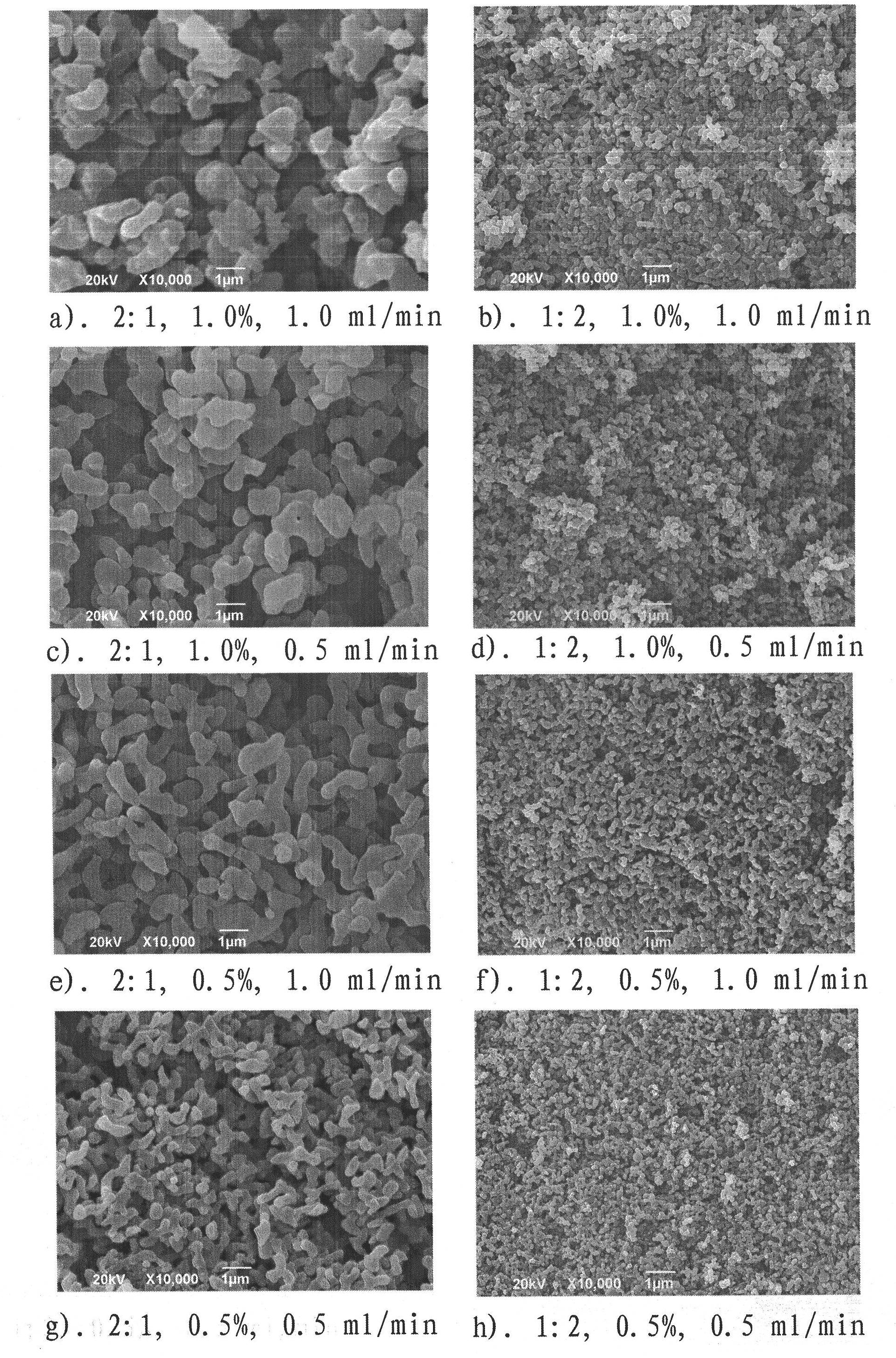
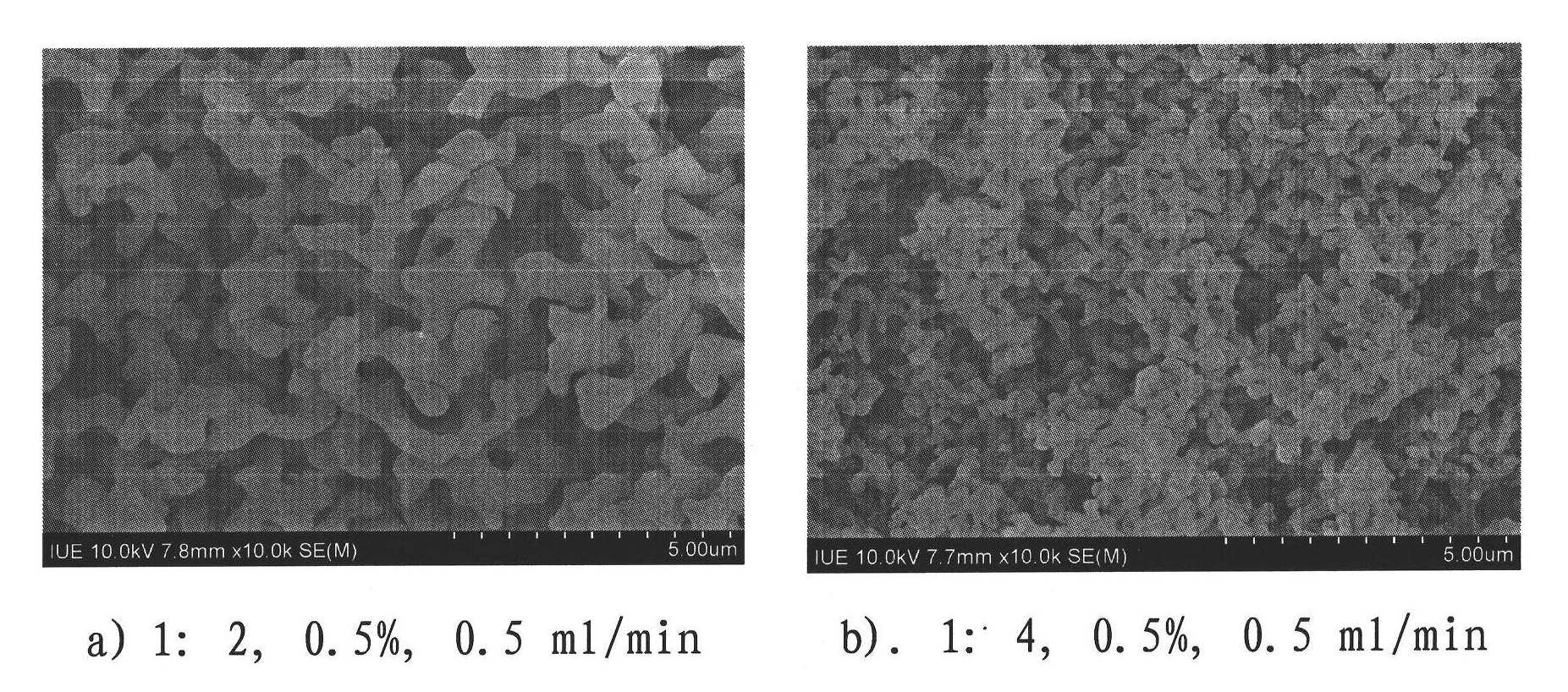
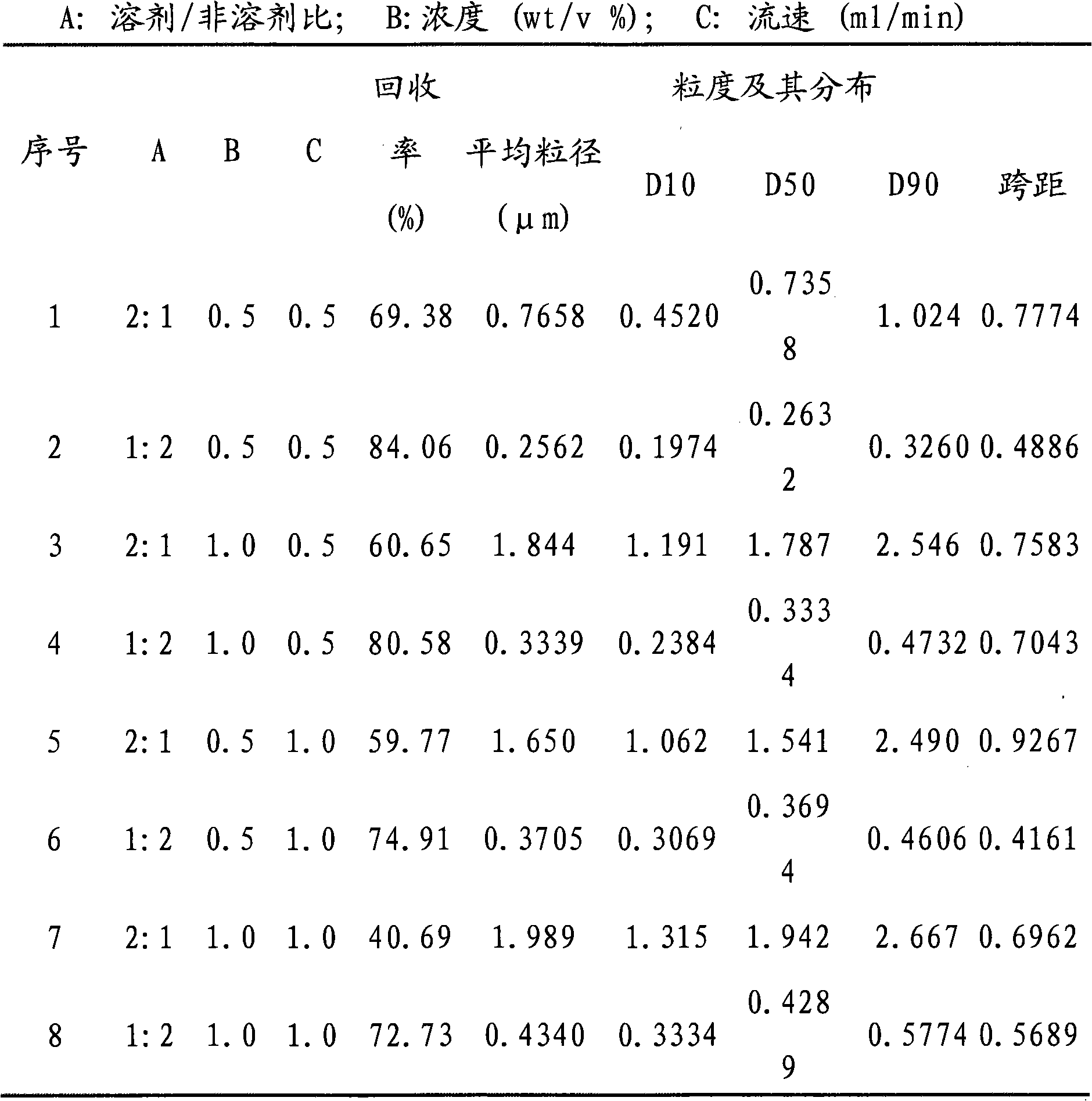
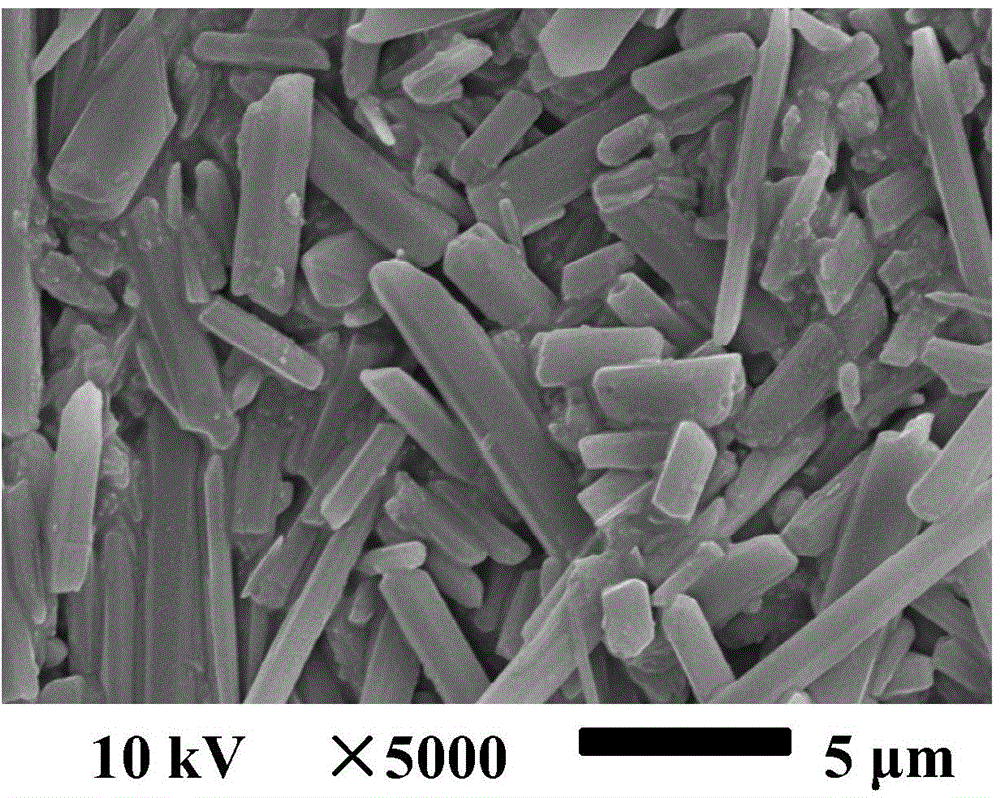
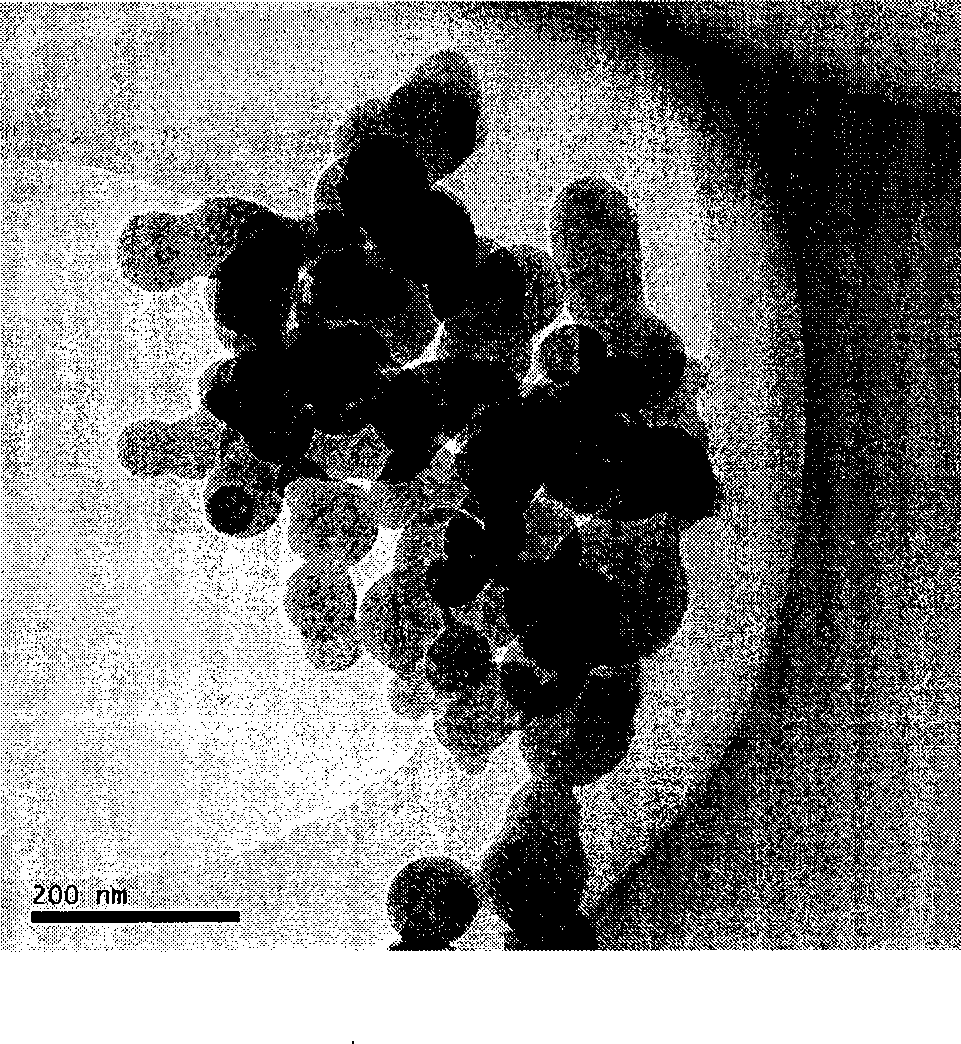Patents
Literature
57 results about "Supercritical anti solvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Equipment Assembly for and Method of Processing Particles
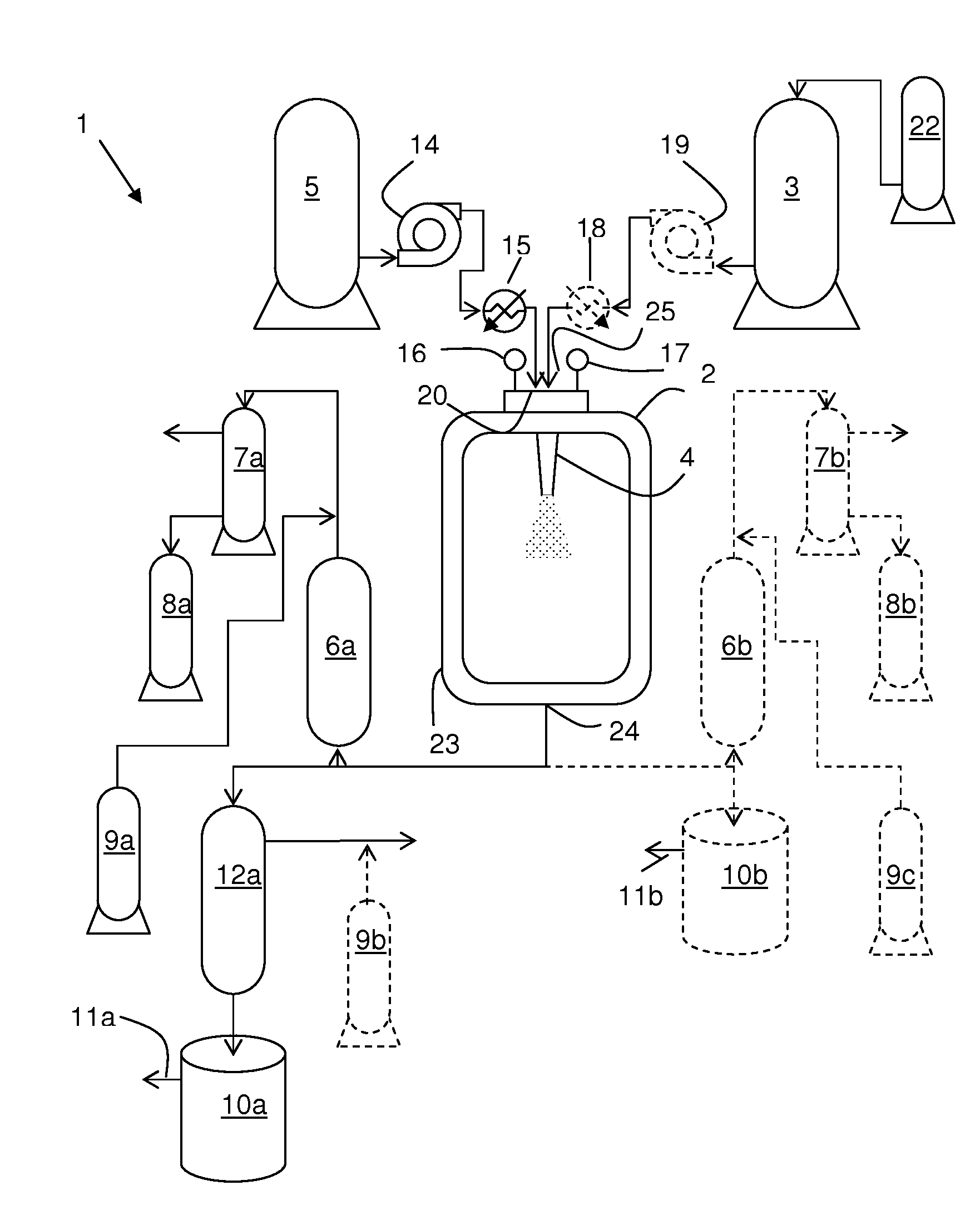
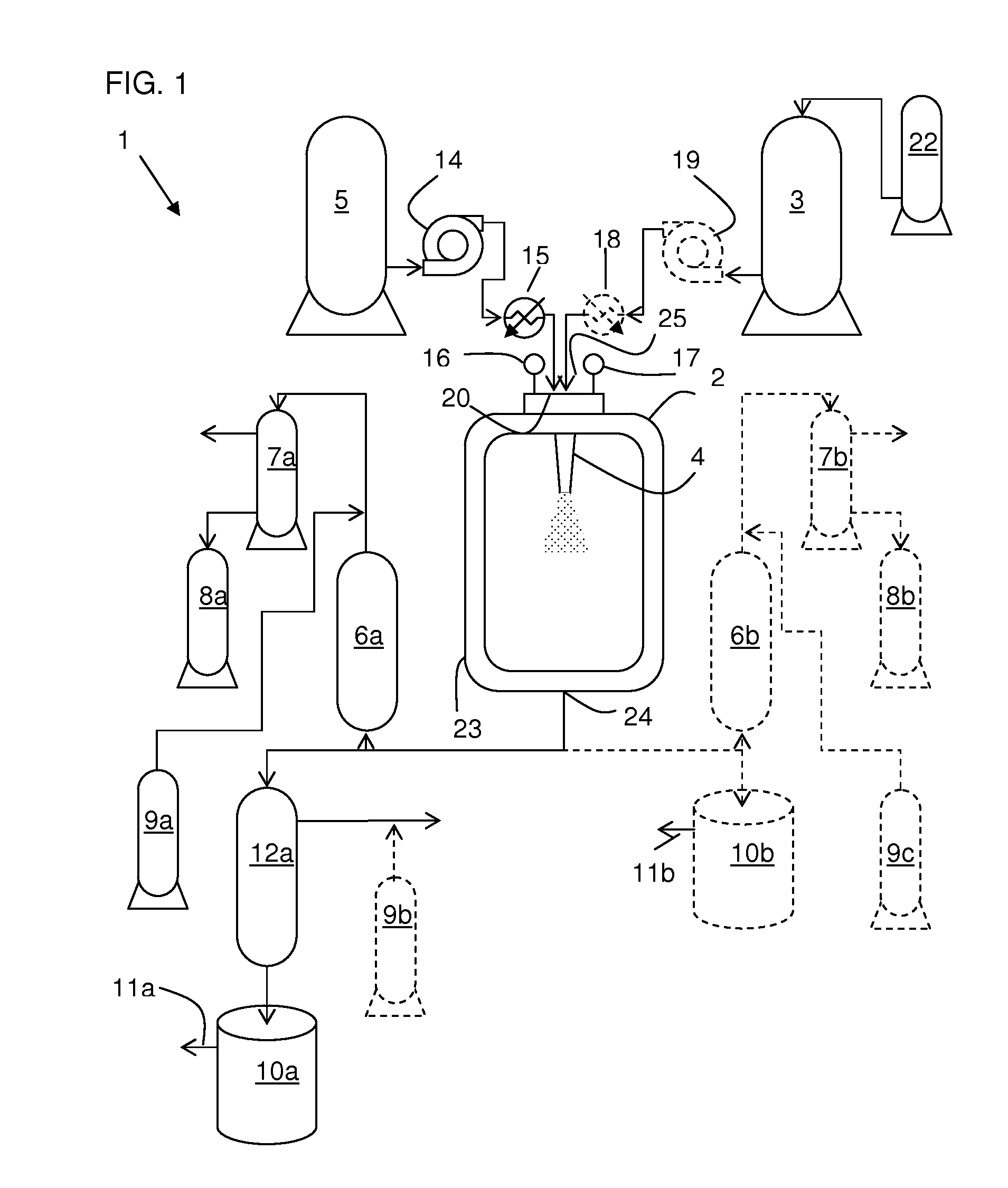
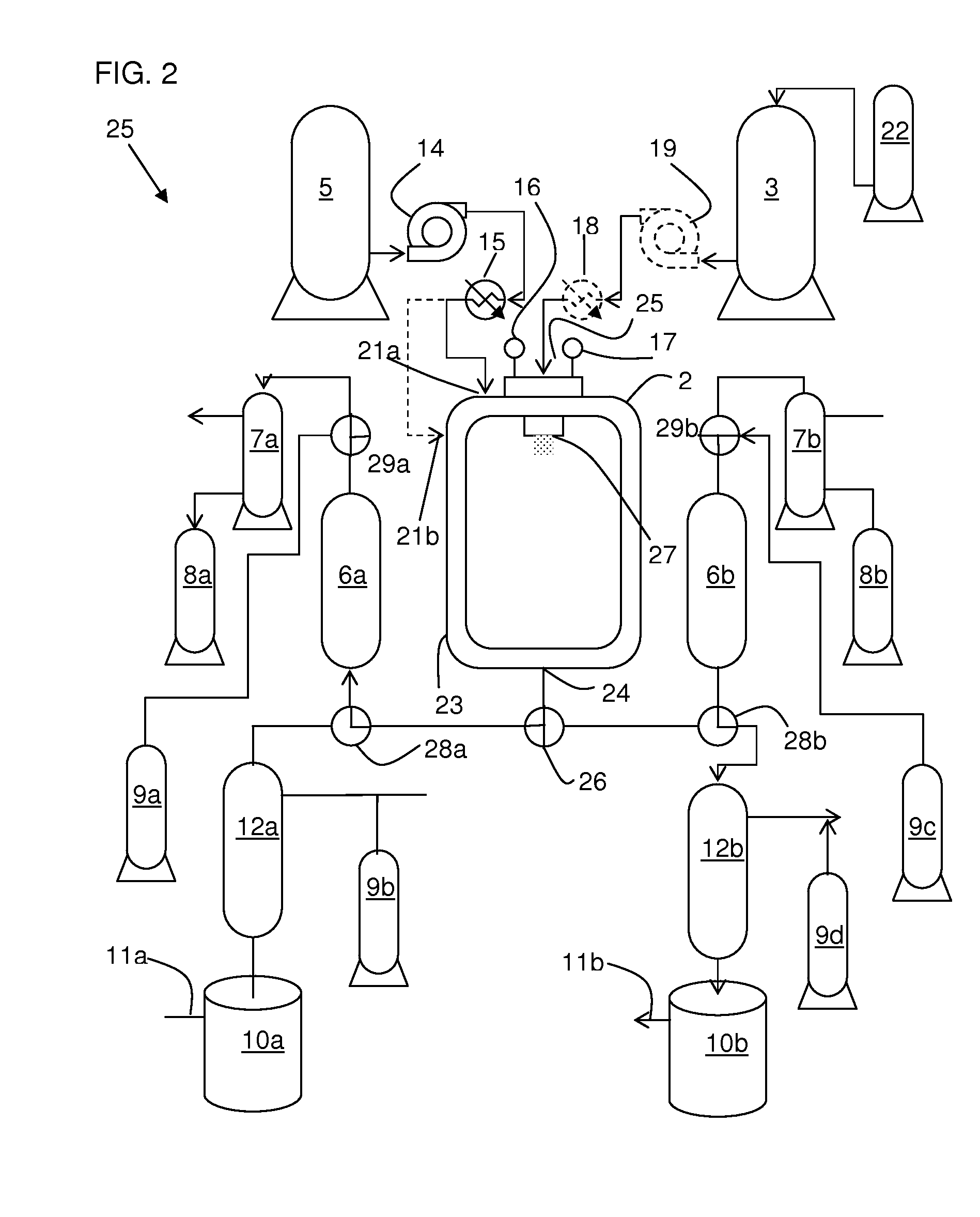
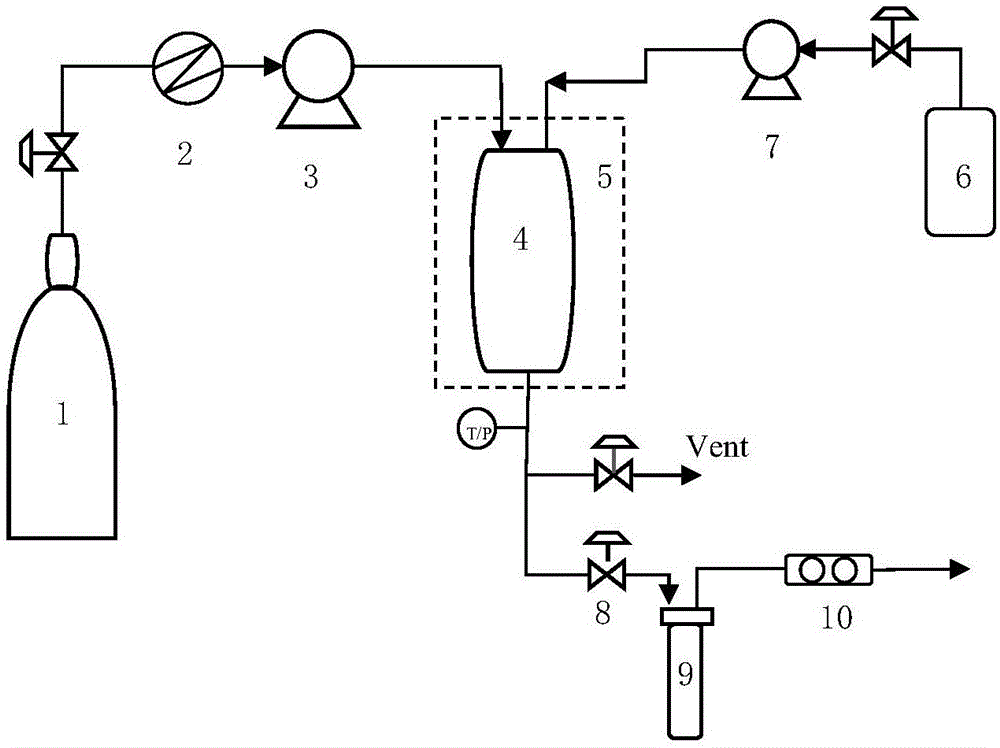
An equipment assembly for preparing, harvesting and collecting particles is disclosed. The assembly comprises a tandem filter system with one or more high pressure filters, one or more low pressure filters and one or more collection vessel. Particles can be prepared, harvested and collected continuously, semi-continuously or in a batch-type operation. A tandem filter system and its method of use are also disclosed. Particles made with the assembly and according the instant methods are also disclosed. The assembly provides improved particle harvesting and collection over other systems and permits continuous particle formation, in particular by dispersion of a solute-containing process fluid within a supercritical anti-solvent.
Owner:CRITITECH INC
Method for preparing naringenin/hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process
ActiveCN106265596AImprove solubilityHigh dissolution rateOrganic active ingredientsAntinoxious agentsSupercritical anti solventOrganic solvent
The invention discloses a method for preparing naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process, comprising the steps of S1, dissolving naringenin and hydroxypropyl-Beta-cyclodextrin in an organic solvent to obtain a sample solution; S2, introducing CO2 into a crystallizer, and adjusting temperature and pressure in the crystallizer; S3, continually introducing CO2 at a flow speed, maintaining the temperature and pressure in the crystallizer constant, and introducing the sample solution of step S1 into the crystallizer; S4, after introduction of the sample solution, continuously introducing CO2, maintaining the temperature and pressure in the crystallizer constant, and relieving the pressure over a period of time; when the pressure in the crystallizer drops to atmospheric pressure, opening the crystallizer to collect the naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules. The method provided herein wraps naringenin in HP-Beta-CD by using supercritical anti-solvent process, dissolving property of the naringenin in an aqueous solution is greatly improved, dissolvability is significantly improved, and bioavailability of the naringenin can be improved.
Owner:CHINA PHARM UNIV
Method for producing cerium-zirconium nanocomposite oxide fine particle with supercritical anti-solvent technology
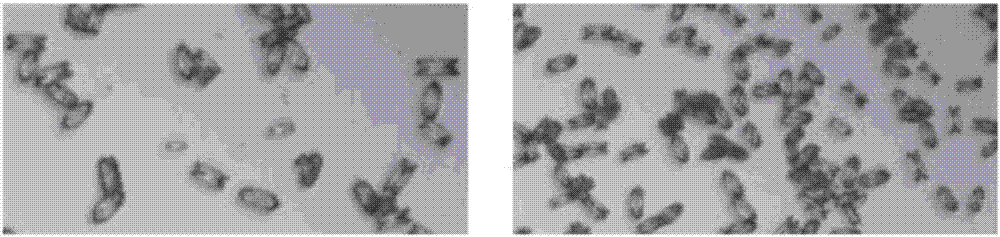
The invention relates to a method for preparing nanoscale composite Ce-Zr oxide particles by supercritical antisolvent (SAS) technique. The method comprises the following steps: dissolving a Ce salt and a Zr salt in a solution; separating out the soluble salts of the Ce and Zr in a manner of uniformly-dispersed nanoparticles through the antisolvent effect of supercritical fluids to obtain the nanoscale precursor particles of the supercritical antisolvent; and baking the precursor to obtain the nanoscale composite Ce-Zr oxide particles. The SAS technique is a pure physical process so as to obviate the complex steps, such as washing and drying, of the conventional coprecipitation method, and has the advantages of mild operation condition, easily controlled process, small particle size, uniform size distribution, uniform distribution of each composite component, repeated use of CO2 and solvent, environmental protection, etc.
Owner:TIANJIN UNIV
Novel crystal form of gefitinib and preparation method thereof based on supercritical anti-solvent technology
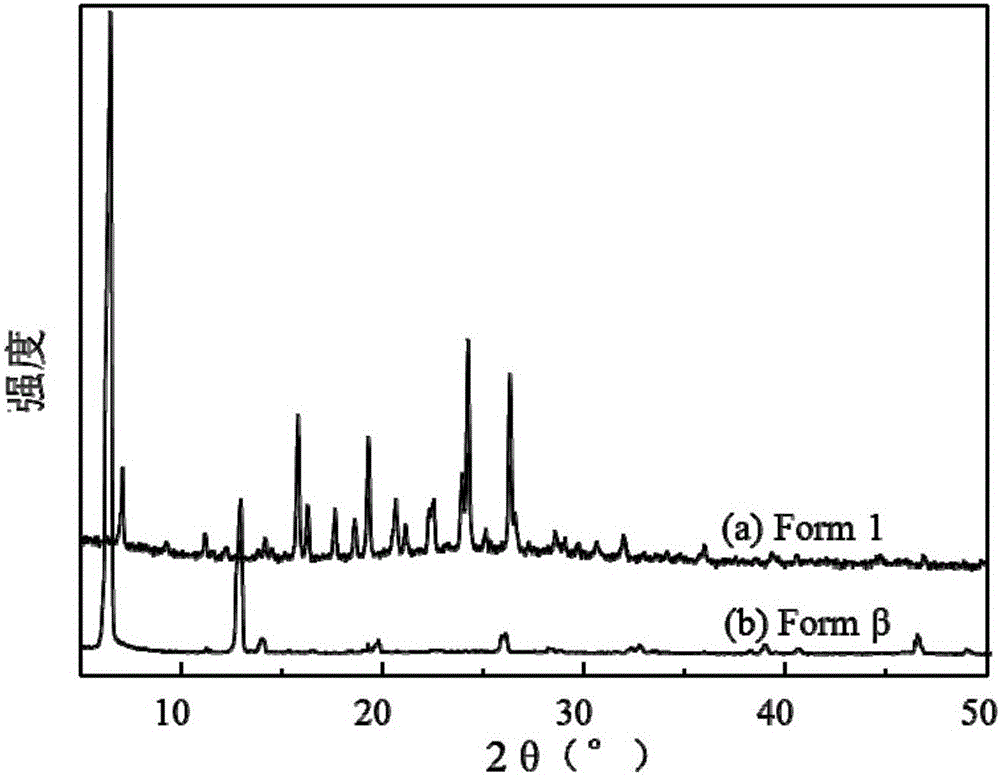
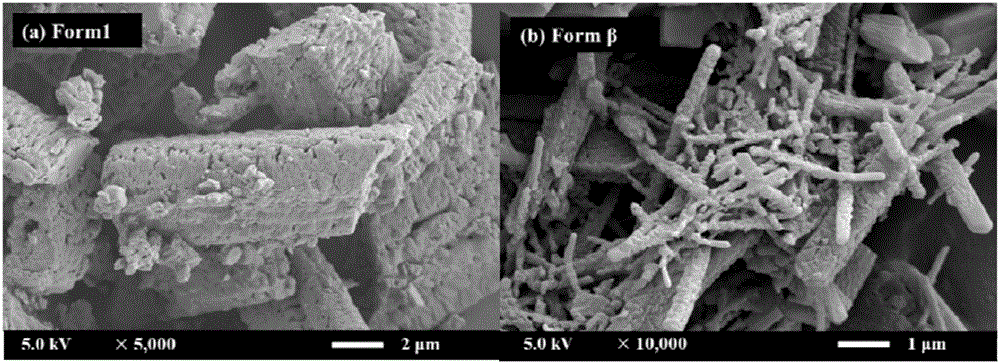
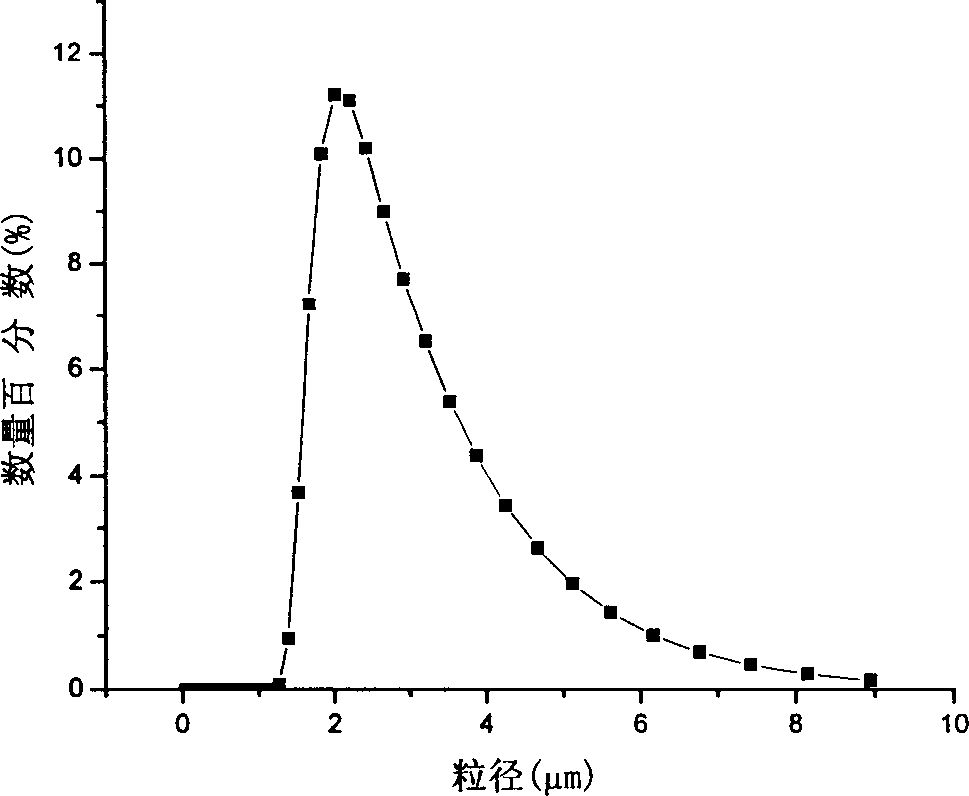
ActiveCN106083739ASmall particle sizeEasy to prepareOrganic chemistry methodsBulk chemical productionSolubilitySupercritical anti solvent
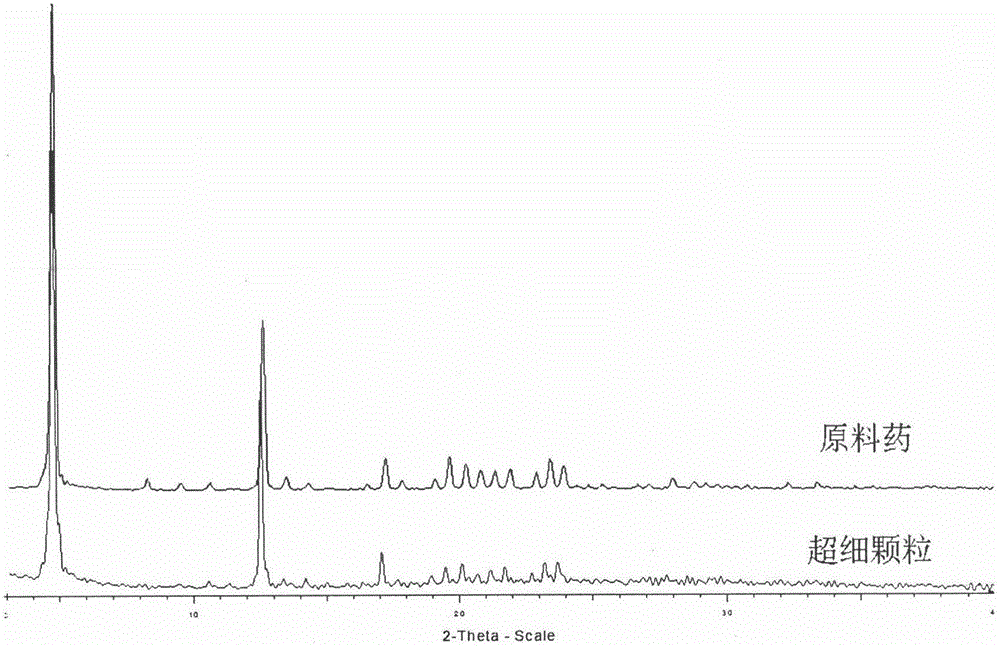
The invention discloses a novel crystal form of gefitinib and a preparation method thereof based on supercritical anti-solvent technology. The novel crystal form of gefitinib is a crystal form beta. The crystal form beta has a strongest characteristic peak in an XRPD pattern at a position where 2theta is equal to 6.7. The crystal form beta has main characteristic peaks in the XRPD pattern at positions where 2theta is about 6.7 and 13.0, and the relative intensity of the main characteristic peaks is greater than 20%. Characteristic peaks occur in the XRPD pattern when 2theta is 14.0, 19.7, 26.1, 32.8, 38.8, 40.5 and 46.5, and the relative intensity of the characteristic peaks is no more than 5%. The preparation method based on the supercritical anti-solvent technology is simple in process, mild in conditions and good in reproducibility; and the obtained crystal form beta of gefitinib is small in particle size, narrow in particle size distribution and high in solubility.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing biological degradable polymer drug-carried fine particle by supercritical anti-solvent process
InactiveCN1720902ANarrow particle size distributionImprove biological activityPeptide/protein ingredientsPharmaceutical product form changeParticulatesSupercritical anti solvent
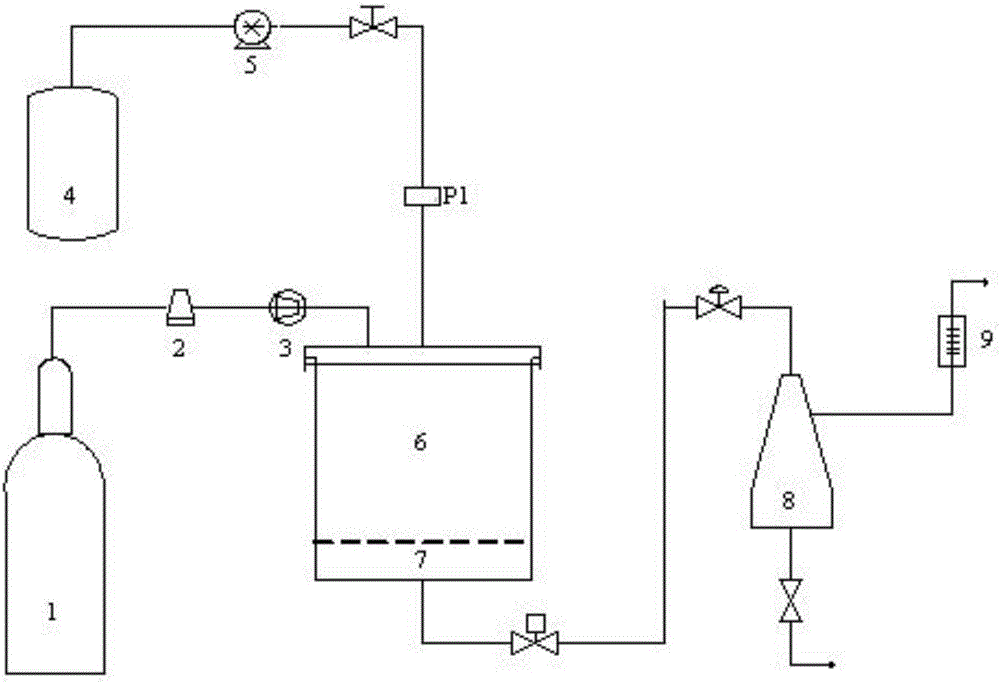
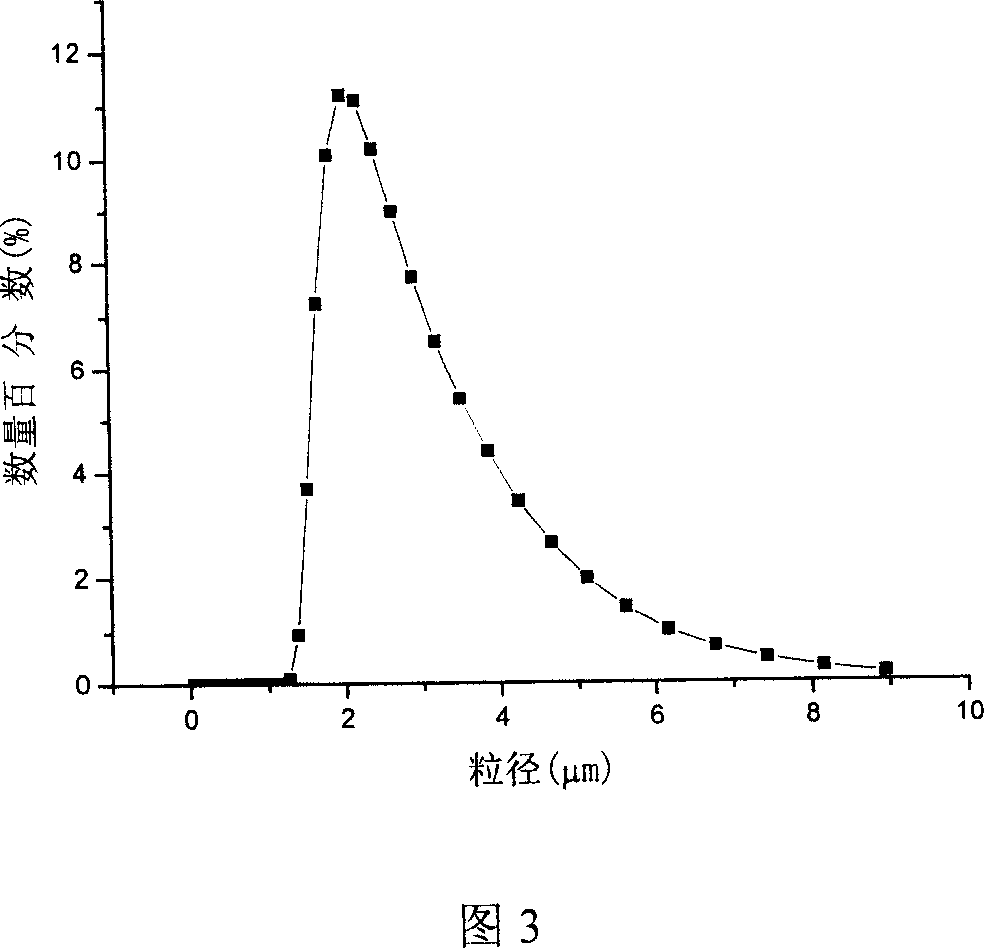
The invention relates to a process for preparing biodegradable polymeric medicine-carrying particles through supercritical fluid solvent-resisting procedure which mainly comprises the following steps, (1) letting CO2 into a crystallization reaction kettle and reaching a predetermined temperature and pressure, (2) spraying the polymer solution containing dissolved medicine into the reaction kettle through a purpose-made injector, depositing the particles, (3) collecting a certain amount of particles and stopping solution spraying, continuously letting in CO2 to purify the residual solvent, sustaining the procedure for 90-120 minutes, (4) finally evacuating under reduced pressure at operation temperature. The prepared submicron-sized particulate product has a grain size range of 0.5-5um.
Owner:TONGJI UNIV
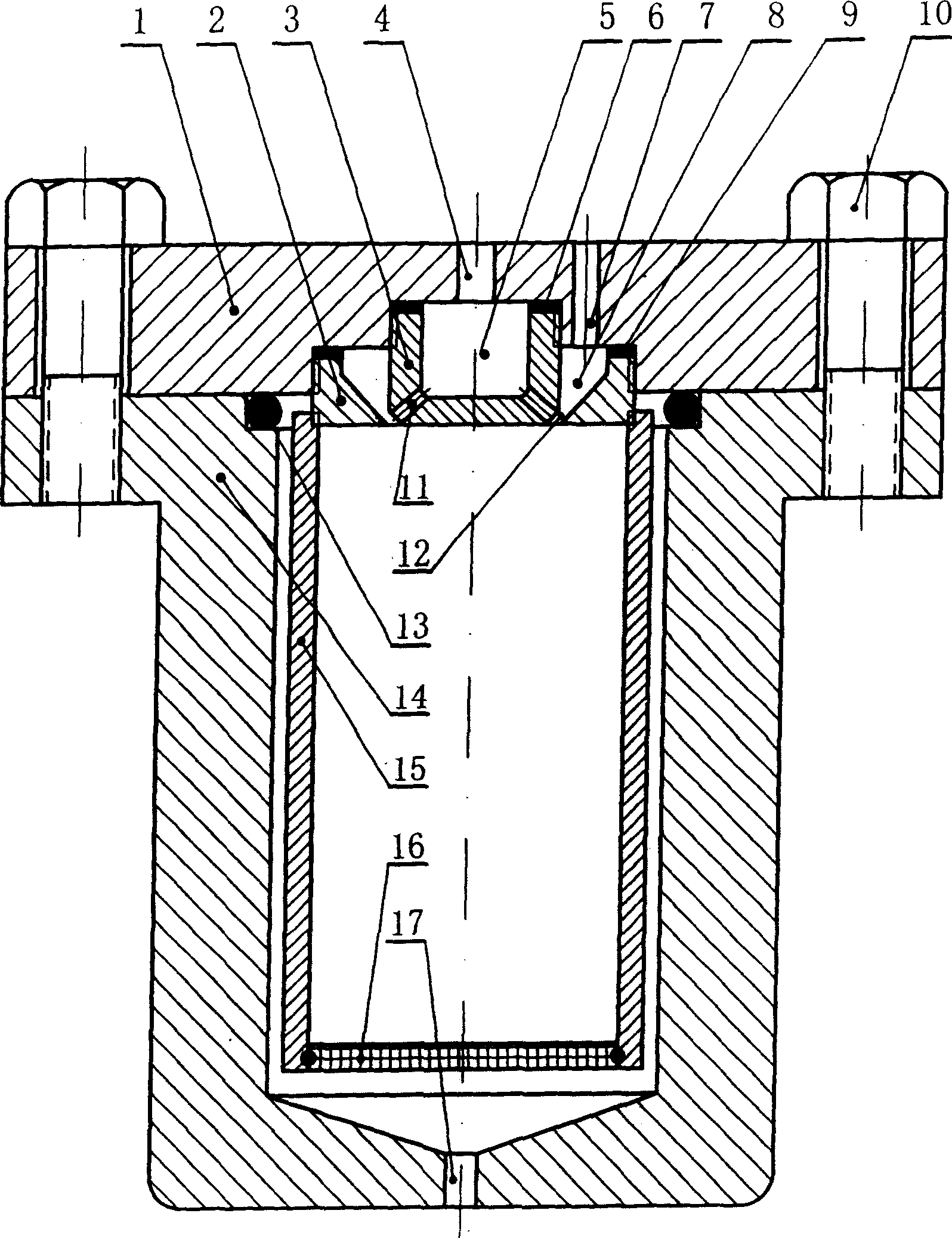
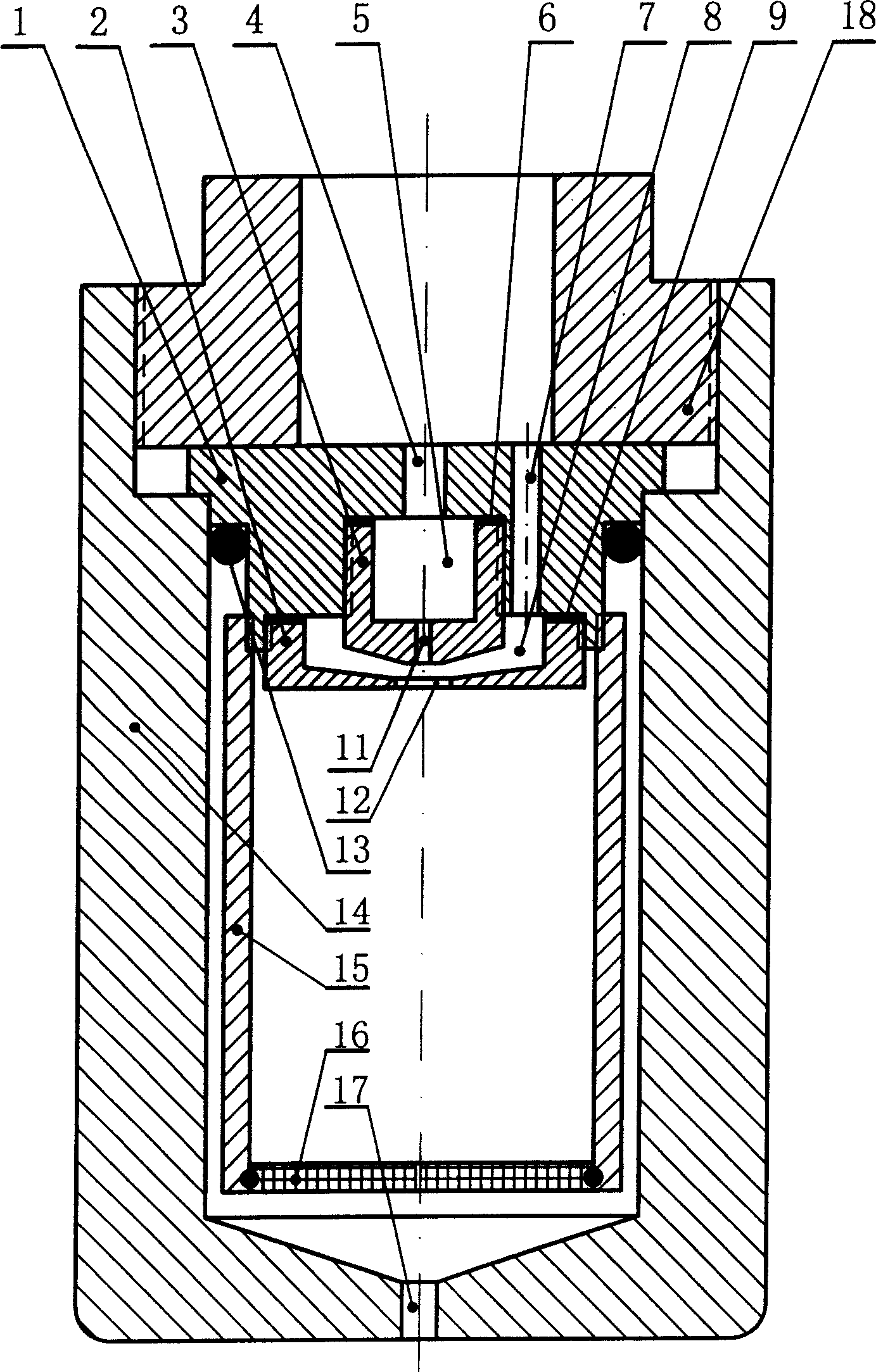
Crystallizing kettle for preparing superfine powder using supercritical anti-solvent process
InactiveCN1528505ASmall particle sizeEvenly distributedGranulation by liquid drop formationChemical/physical/physico-chemical nozzle-type rreactorsSolubilitySupercritical anti solvent
The invention relates to a crystallizing kettle using the supercritical fluid desolvation course to prepare ultrafine powder, mainly composed of kettle body, material basket, inner and outer spray nozzles, etc. Its character: a penetrable straight spray hole is set on the cavity wall of the inner spray nozzle, and the outer surface of the inner spray nozzle and the inner surface of the outer spray nozzle form a supercritical fluid ringed channel, which gradually shrinks to become narrow to form a narrow and small spray hole. It can evenly disperse the solution into micro liquid drops in the supercritical fluid, so that the product has small grain size and uniformly distributed grains. It is applied to the field where the solvent in the solution can dissolve in the supercritical fluid but the solute in the solution does not dissolve in the supercritical fluid (or the solubility very small).
Owner:DALIAN UNIV OF TECH
Method for preparing irbesartan ultrafine particles by applying supercritical anti-solvent technology
ActiveCN105534923AHigh dissolution rateUniform particle size distributionOrganic active ingredientsMetabolism disorderSolubilitySupercritical anti solvent
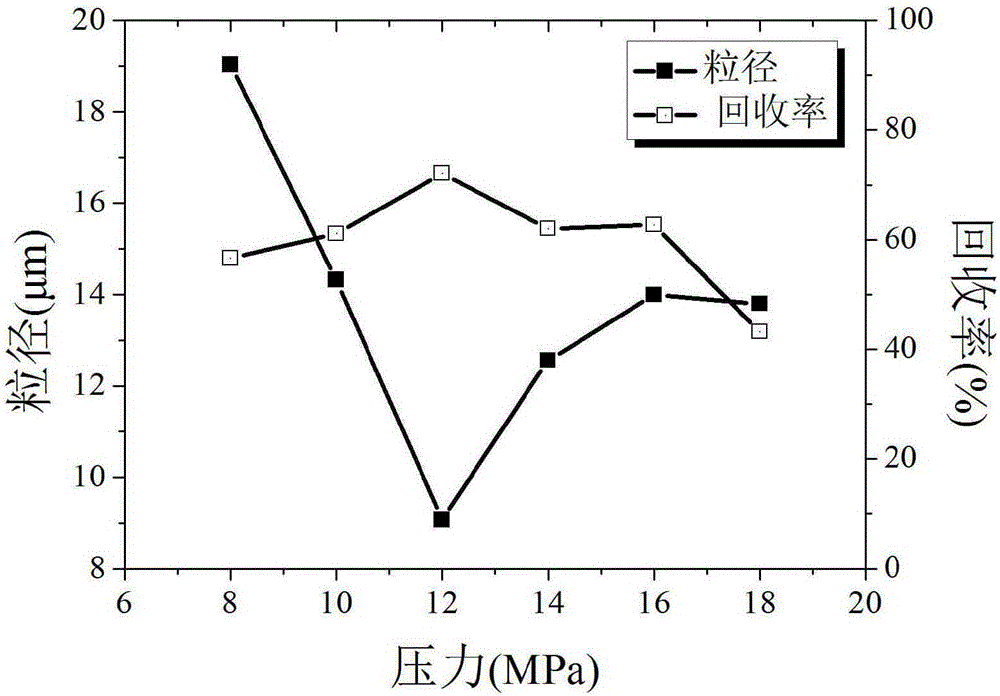
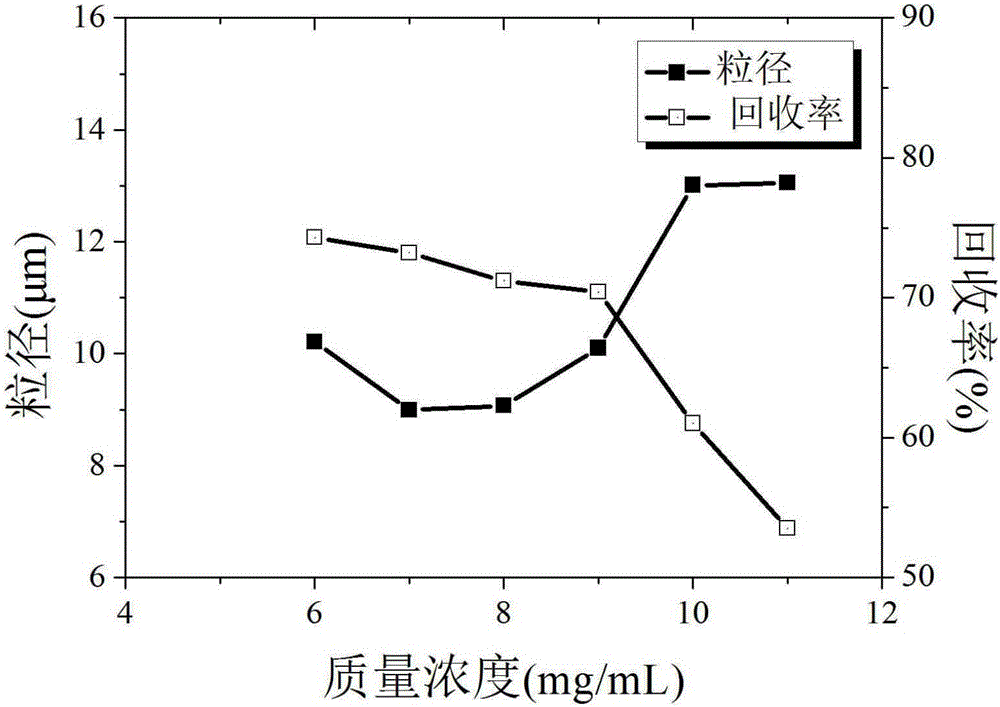
The invention discloses a process for preparing irbesartan ultrafine particles by applying a supercritical anti-solvent technology, and belongs to the technical field of medicinal dosage forms and supercritical technologies. The method is characterized in that an irbesartan solution is sprayed into a crystallization kettle through a supercritical fluid anti-solvent equipment system, and brick-shaped and amorphous-state irbesartan ultrafine particles are crystalized and precipitated in the crystallization kettle. According to the process, the shape and particle size of a medicament are controlled by changing parameters such as a solvent type, solution concentration, a solution sample introduction rate, a crystallization pressure and crystallization temperature. The irbesartan ultrafine particles prepared by the process have the advantages small particle sizes and narrow grain size distribution, and the dissolution rate and equilibrium solubility of the medicinal fine particles are improved remarkably.
Owner:CHINA PHARM UNIV
Method for preparing estradiol ultrafine particles with supercritical anti-solvent technology
ActiveCN106432389ASmall particle sizeImprove solubilityEstrane derivativesBulk chemical productionSolubilitySupercritical anti solvent
The invention discloses a method for preparing estradiol ultrafine particles with a supercritical anti-solvent technology. The method comprises the steps as follows: step S1, estradiol is dissolved in an organic solvent, and an estradiol solution is prepared; step S2, CO2 is introduced into a crystallization kettle, and the temperature and pressure in the crystallization kettle are adjusted; step S3, CO2 is introduced continuously at a constant flow rate, and the estradiol solution prepared in step S1 is introduced into the crystallization kettle while the temperature and pressure in the crystallization kettle are kept unchanged; Step S4, CO2 is introduced continuously after the introduction of estradiol solution, the temperature and pressure in the crystallization kettle are kept unchanged, the pressure is relieved after a period of time, and after the pressure in the crystallization kettle is reduced to atmospheric pressure, the crystallization kettle is opened for collecting the estradiol ultrafine particles, wherein the sequence of the step S1 and the step S2 can be changed, and the organic solvent is a mixed solvent of acetone and ethanol. With the adoption of the method, estradiol with fine particle size can be prepared, the solubility of estradiol in water is improved, and accordingly, the bioavailability of estradiol is improved.
Owner:CHINA PHARM UNIV
Method for preparing carvedilol solid dispersions by virtue of supercritical anti-solvent technique
ActiveCN106309434AImproved dissolution propertiesCarvedilol solid dispersion with significantly improved dissolution propertiesPowder deliveryOrganic active ingredientsSupercritical anti solventOrganic solvent
The invention discloses a method for preparing carvedilol solid dispersions by virtue of a supercritical anti-solvent technique. The method comprises the following steps: (S1) dissolving carvedilol and a water-soluble carrier into an organic solvent, so as to obtain a carvedilol-carrier mixed solution; (S2) introducing CO2 into a crystallization kettle, and regulating the temperature and pressure in the crystallization kettle; (S3) continuing to introduce CO2 to keep the temperature and pressure in the crystallization kettle constant, and simultaneously introducing the mixed solution prepared in the step (S1) into the crystallization kettle; and (S4) after the carvedilol-carrier mixed solution is introduced, continuing to introduce CO2 to keep the temperature and pressure in the crystallization kettle constant, finally relieving the pressure, and after the pressure in the crystallization kettle is decreased to the atmospheric pressure, opening the crystallization kettle, and collecting the carvedilol solid dispersions. By virtue of the method, the carvedilol solid dispersions with obviously improved dissolution characteristics can be prepared, and furthermore, the bioavailability of indissolvable drugs can be improved.
Owner:CHINA PHARM UNIV
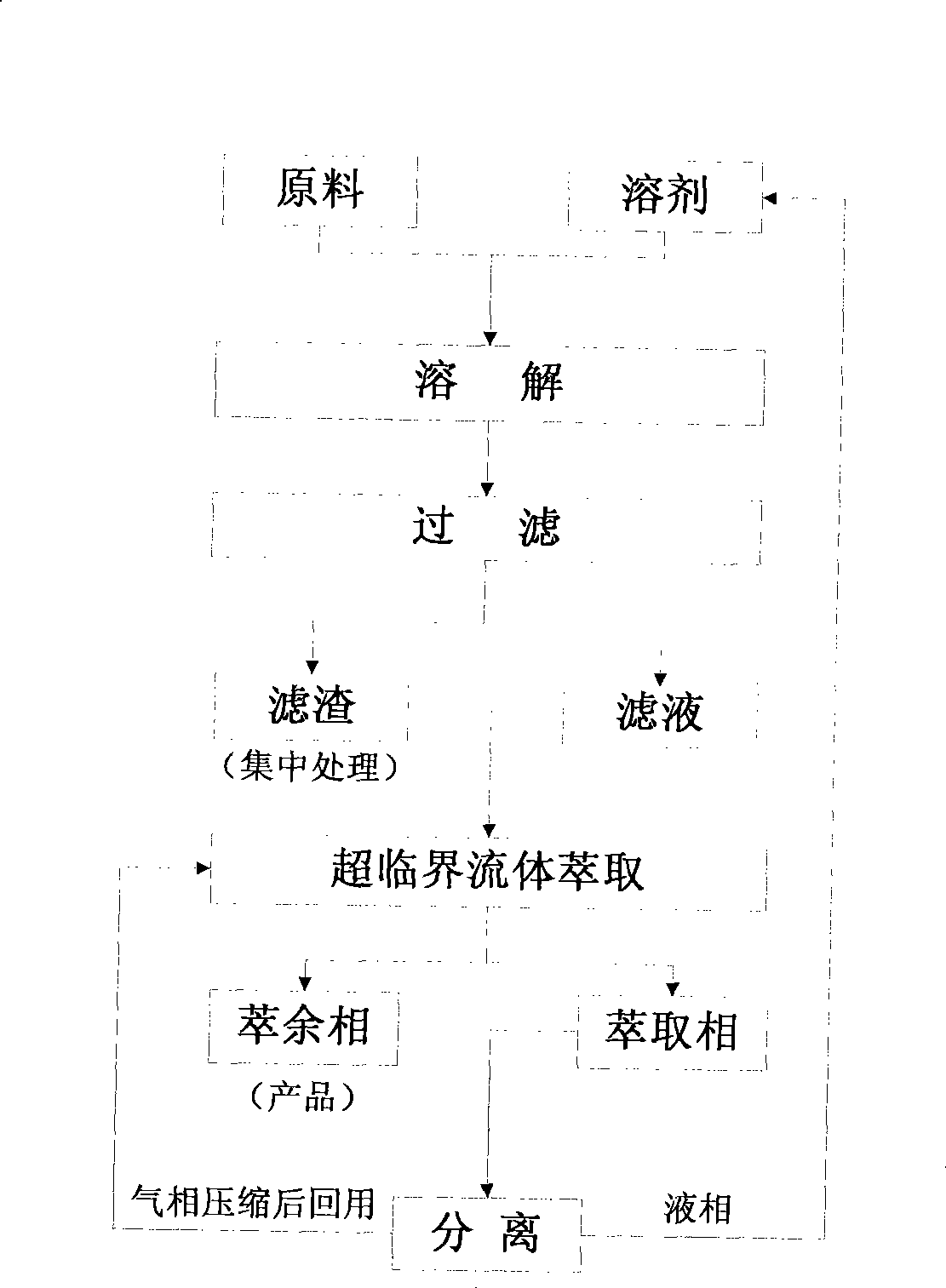
Method for recycling polyvinyl chloride or polyvinylidene chloride plastic
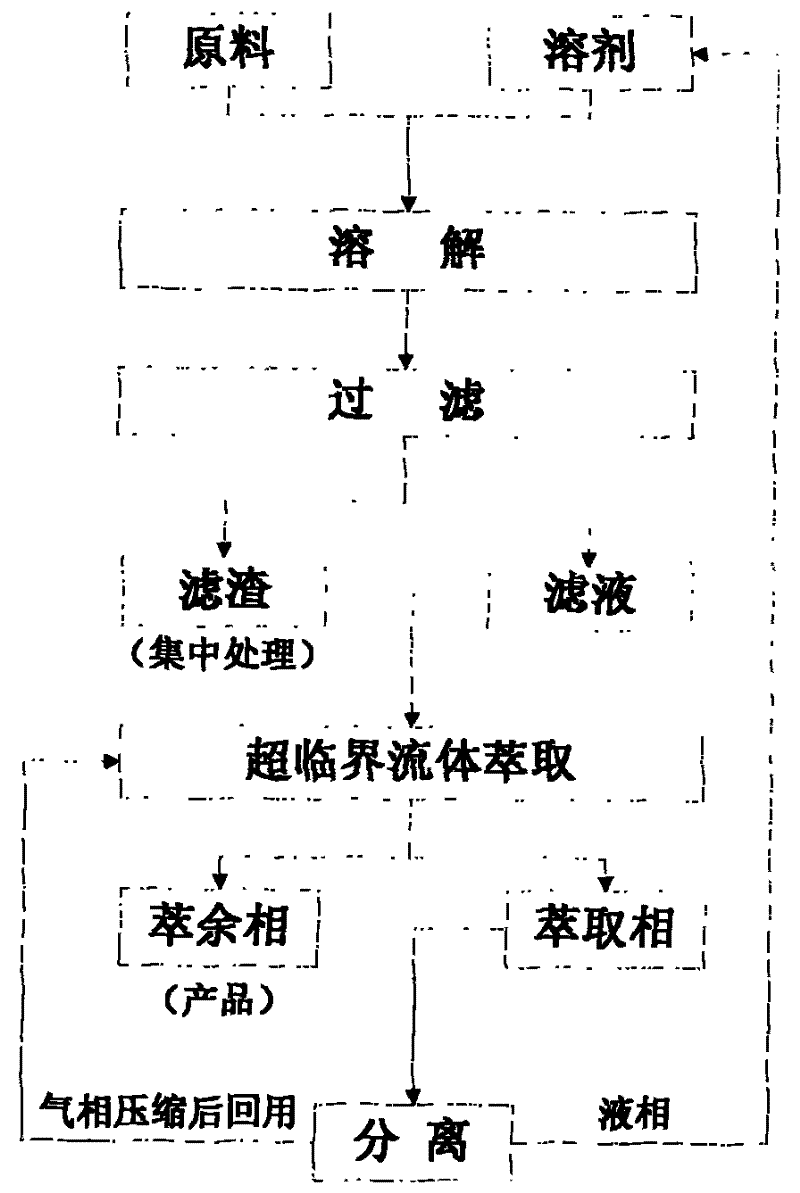
InactiveCN101367957BNo pollution in the processEasy to separatePlastic recyclingBulk chemical productionSupercritical anti solventPolyvinyl chloride
The invention discloses a method for reclaiming polyvinyl chloride (PVC) or PVDC plastics. The supercritical anti-solvent technology is used to reclaim PVC or PVDC plastic products; the shivers of the plastic products are dissolved in the solvent and the undissolved substances are filtered and removed; then supercritical fluid is made to contact with the filtrate, and the polymer precipitation inthe solution is dissolved; the solvent is extracted, and the supercritical fluid and solvent are reclaimed separately and recycled; and finally, the polymer particles are reclaimed. The solvent and the supercritical fluid achieved by the method can be recycled, and are pollution-free and environment friendly; the polymers can be precipitated and dried in a same reactor, and the process is very simple; the supercritical fluid and the solvent can be separated easily, and the energy consumption is low.
Owner:ZHEJIANG UNIV +1
Method for preparing biological degradable polymer drug-carried fine particle by supercritical anti-solvent process
InactiveCN1302766CNarrow particle size distributionImprove biological activityPeptide/protein ingredientsPharmaceutical product form changeParticulatesSupercritical anti solvent
The invention relates to a process for preparing biodegradable polymeric medicine-carrying particles through supercritical fluid solvent-resisting procedure which mainly comprises the following steps, (1) letting CO2 into a crystallization reaction kettle and reaching a predetermined temperature and pressure, (2) spraying the polymer solution containing dissolved medicine into the reaction kettle through a purpose-made injector, depositing the particles, (3) collecting a certain amount of particles and stopping solution spraying, continuously letting in CO2 to purify the residual solvent, sustaining the procedure for 90-120 minutes, (4) finally evacuating under reduced pressure at operation temperature. The prepared submicron-sized particulate product has a grain size range of 0.5-5um.
Owner:TONGJI UNIV
Method adopting supercritical CO2 fluid technology to produce water-soluble medicine controlled-release particles
InactiveCN102327186AAvoid contactHigh activity retentionPharmaceutical product form changeSupercritical anti solventMicrosphere
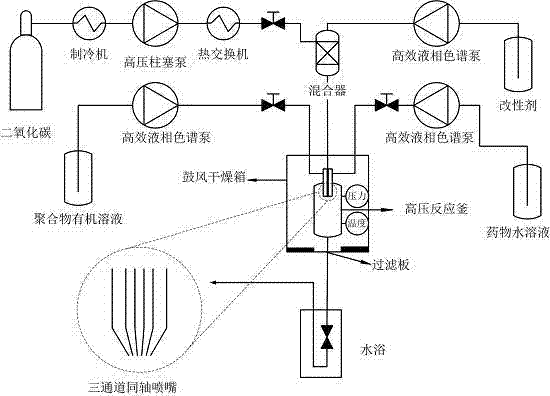
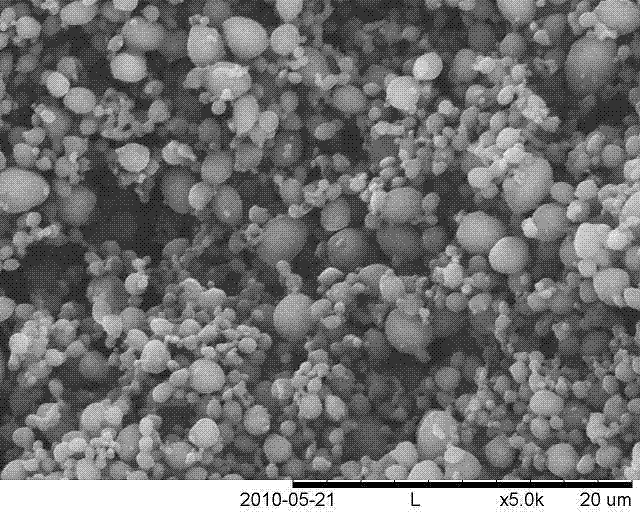
The invention relates to a method adopting a supercritical CO2 fluid technology to produce water-soluble medicine controlled-release particles, which belongs to the technical field of the novel preparation method and application of pharmaceutical dosage form variation. The traditional microsphere preparation method cannot keep the bioactivity of medicine, the supercritical anti-solvent technique is mild and safe, but is limited because the supercritical CO2 is difficult to be mixed with the polar solvent. In the invention, a mixer which is specially designed is adopted to mix the supercritical CO2 and ethanol, so the water blending performance is increased; and a triple-channel coaxial nozzle is adopted, the medicine aqueous solution, macromolecule carrier organic solution and ethanol are mixed with the supercritical CO2 to be simultaneously sprayed into a reactor through the inner layer of channel, the intermediate layer of channel and the outer layer of channel of the nozzle, extraction and separation are completed in one step, so medicine controlled-release particles are prepared. The method has mild conditions, the retentivity degree of the medicine activity is high, the residual amount of the organic solvent is low, and the operation is simple. The produced microspheres are smooth and spherical, have the particle size of 1-5mum, and the particle size distribution is narrow. The invention has application prospect in preparing water-soluble controlled-release microspheres.
Owner:SICHUAN UNIV
Supercritical anti-solvent breviscapine nanoparticle, preparation method of supercritical anti-solvent breviscapine nanoparticle, and breviscapine capsule and tablet
ActiveCN108159064AReduce distributionIncrease contact timePowder deliveryOrganic active ingredientsSupercritical anti solventNanoparticle
The invention relates to the field of pharmaceuticals, and particularly relates to a supercritical anti-solvent breviscapine nanoparticle, a preparation method of the supercritical anti-solvent breviscapine nanoparticle, a breviscapine capsule and a breviscapine tablet. The preparation method of the supercritical anti-solvent breviscapine nanoparticle comprises the following steps: enabling a breviscapine solution and a supercritical carbon dioxide fluid to contact, and precipitating to obtain the nanoparticle. The nanoparticle has the advantages of small particle size and even distribution ofparticles, the dissolving-out speed of the medicine is obviously improved, and the bioavailability of the medicine in vivo is improved.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Lavender essential oil lipidosome liquid band-aid and preparation method thereof
PendingCN109364290APromote regenerationPromote wound healingDermatological disorderLiposomal deliveryDiseaseSupercritical anti solvent
The invention discloses a preparation method of a lavender essential oil lipidosome liquid band-aid and belongs to the field of band-aids. The preparation method comprises the following steps that 1,a supercritical CO2 fluid extraction method is adopted to extract lavender essential oil from lavenders and / or lavender flowers; 2, refrigerated centrifugation, rectification, molecular distillation and / or adsorption techniques are adopted to remove impurities in the step 1; 3, a phase balance technique is adopted to optimize supercritical CO2 extraction of the essential oil in the step 2; 4, a supercritical anti-solvent method is adopted to prepare lavender essential oil lipidosome with the obtain lavender essential oil. Compared with the prior art, the preparation method has the advantages that cytothesis can be promoted, wound healing can be promoted and oily and pimple skin can astringe by utilizing lavenders, a treatment effect on scar fading is played, lavenders are prepared into thesprayable band-aid, and the lavender essential oil lipidosome liquid band-aid is quick and convenient to use and firm in fitting while the above effect is ensured. In addition, the symptoms of skin diseases such as psoriasis, dermatitis and eczema can also be relieved.
Owner:新疆维吾尔自治区分析测试研究院 +1
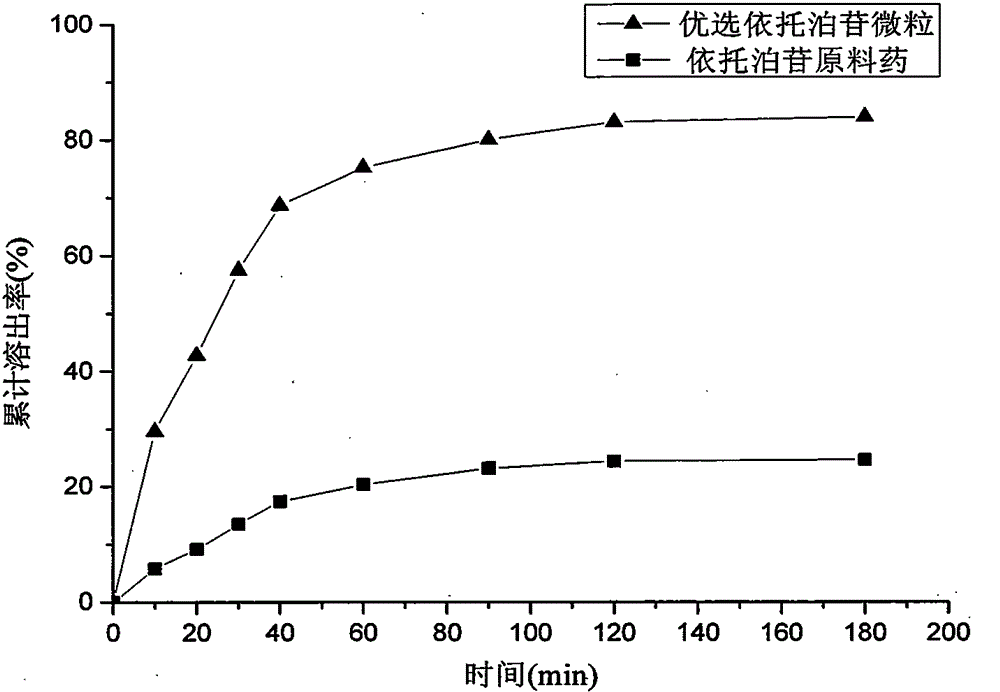
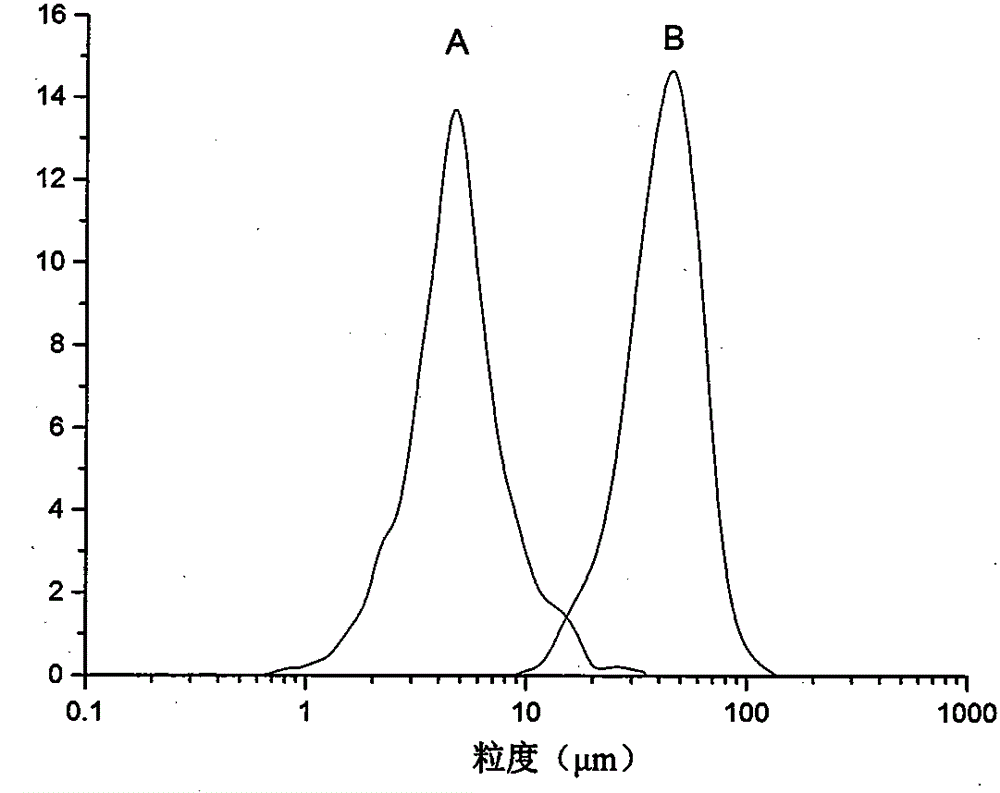
Method for preparing etoposide ultrafine particles by supercutical fluid technology
ActiveCN105030683AImprove solubilityOrganic active ingredientsPowder deliverySupercritical anti solventAnti solvent
The invention relates to a method for preparing etoposide ultrafine particles by a supercutical anti-solvent crystallization technology. The method can prepare the etoposide ultrafine particles with improved solubility. Through change of drug solvent types, a solution concentration, crystallization pressure, a crystallization temperature and a solution sample introduction flow velocity, drug particle sizes are effectively controlled in a range of 1-10 microns. The prepared etoposide ultrafine particles have a high dissolution rate.
Owner:CHINA PHARM UNIV
Method for recycling polyvinyl chloride or polyvinylidene chloride plastic
InactiveCN101367957ANo pollution in the processEasy to separatePlastic recyclingBulk chemical productionSupercritical anti solventPolyvinyl chloride
The invention discloses a method for reclaiming polyvinyl chloride (PVC) or PVDC plastics. The supercritical anti-solvent technology is used to reclaim PVC or PVDC plastic products; the shivers of the plastic products are dissolved in the solvent and the undissolved substances are filtered and removed; then supercritical fluid is made to contact with the filtrate, and the polymer precipitation in the solution is dissolved; the solvent is extracted, and the supercritical fluid and solvent are reclaimed separately and recycled; and finally, the polymer particles are reclaimed. The solvent and the supercritical fluid achieved by the method can be recycled, and are pollution-free and environment friendly; the polymers can be precipitated and dried in a same reactor, and the process is very simple; the supercritical fluid and the solvent can be separated easily, and the energy consumption is low.
Owner:ZHEJIANG UNIV +1
Composite nanometer particle obtained by coating curcumin eutectic crystal/piperine with polymers, and preparation of composite nanometer particle and application thereof to slow release pharmaceutical preparation
ActiveCN109432055AImprove physical and chemical propertiesImprove bioavailabilityAntibacterial agentsAntipyreticSupercritical anti solventAnti solvent
The invention belongs to the field of medicines, and particularly relates to a composite nanometer particle obtained by coating curcumin eutectic crystal / piperine with polymers. The composite nanometer particle comprises a core and a shell coating the core, wherein the core is a mixture of the curcumin eutectic crystal and the piperine; the shell is a hydrophilic polymer. The invention further discloses a preparation method and application of the composite nanometer particle. According to the preparation method provided by the invention, the composite nanometer particle with an in-situ coatingstructure is successfully prepared by creatively utilizing a curcumin eutectic crystal compound through a supercritical fluid anti-solvent technology (SAS). The invention provides the composite nanometer particle with the advantages of better dissolution performance, stability and bioavailability.
Owner:MEDONCARE PHARMA CO LTD
Composite nanometer particle obtained by coating curcumin eutectic crystal with polymers, preparation of composite nanometer particle and application thereof to pharmacy
ActiveCN109432056AImprove solubilityImprove stabilityAntibacterial agentsAntipyreticSupercritical anti solventAnti solvent
The invention belongs to the field of medicines, and particularly relates to a composite nanometer particle obtained by coating curcumin eutectic crystal with polymers. The composite nanometer particle comprises a core and a shell coating the surface of the core, wherein the core is the curcumin eutectic crystal and the shell is a hydrophilic polymer. The invention also discloses a preparation method and application of the composite nanometer particle. According to the preparation method provided by the invention, the composite nanometer particle with an in-situ coating structure is successfully prepared by creatively utilizing the curcumin eutectic crystal through a supercritical fluid anti-solvent method (SAS). The invention provides the composite nanometer particle with the advantages of better dissolution performance, stability and bioavailability.
Owner:MEDONCARE PHARMA CO LTD
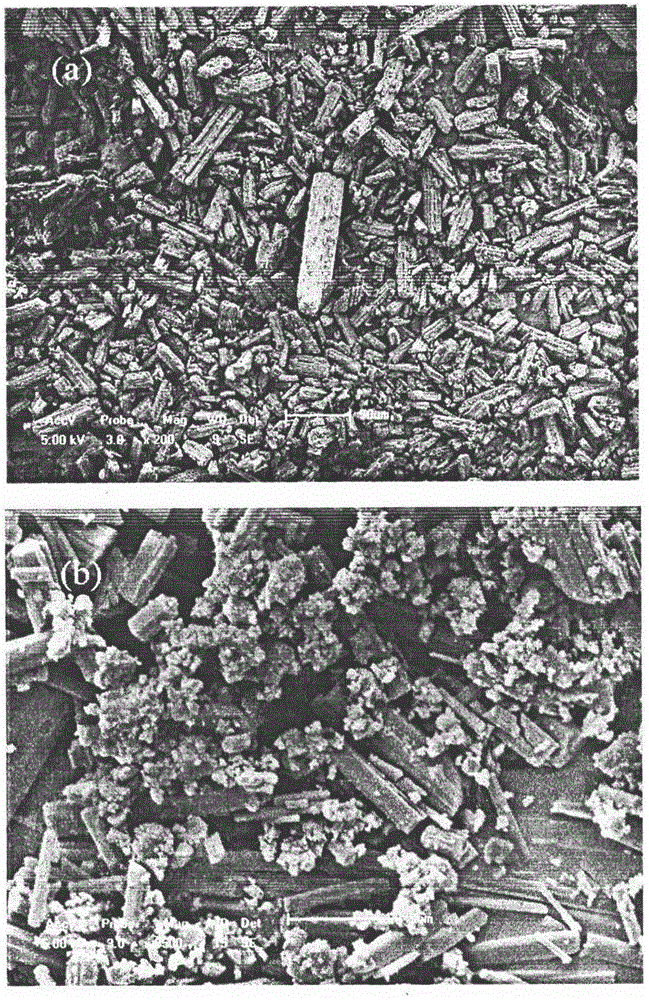
Super-critical anti-solvent preparation for water-soluble nano camptothecin powder
InactiveCN101264061ASimple processSimple and fast operationOrganic active ingredientsPowder deliverySolubilitySupercritical anti solvent
The invention relates to a supercritical anti-solvent preparation method of water-soluble nanoparticlization camptothecin powder, which is characterized in that, starting CO2 high-pressure pump, injecting CO2 in a high-pressure crystallizing reactor at a flow speed of 5L / h to 20L / h, stabilizing the temperature of the high-pressure crystallizing reactor between 32 DGR C and 75 DGR C, stabilizing the pressure between 7.5MPa and 25MPa, reaching a supercriticality; injecting DMSO or DMF solution with a camptothecin concentration of 1mg / ml to 5mg / ml in the high-pressure crystallizing reactor at a flow speed of 1ml / min to 30ml / min through a nozzle with a pore diameter of 20um to 1000um, dissolving out camptothecin powder with an average grain diameter of 100nm to 800nm, making CO2 circulate in the high-pressure crystallizing reactor for at least half an hour to dry the formed nanoparticlization camptothecin powder; separating DMSO or DMF solution from CO2 in a separation reaction in a pressure of 5MPa to 6.5MPa at temperature of 25 DGR C to 50 DGR C, recycling and reusing DMSO or DMF, making CO2 circulate directly for use. The nanoparticlization camptothecin powder has the advantages of smooth surface, uniform particle size distribution, good water solubility, no solvent remnant, non-pollution manufacturing technique, low cost, high yield, and easy industrialization.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
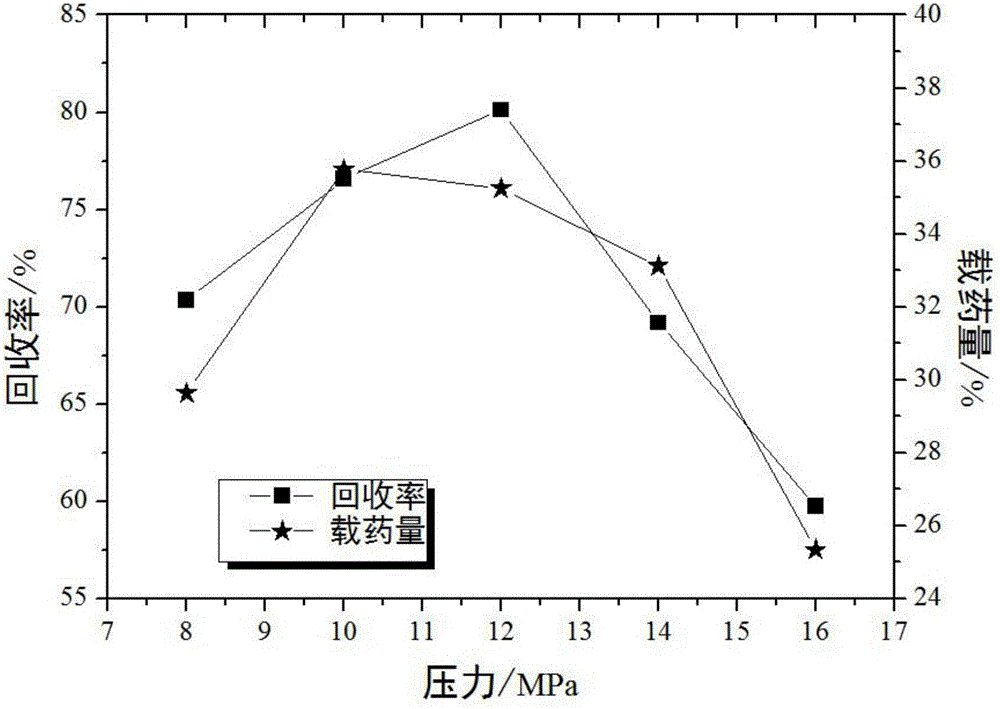
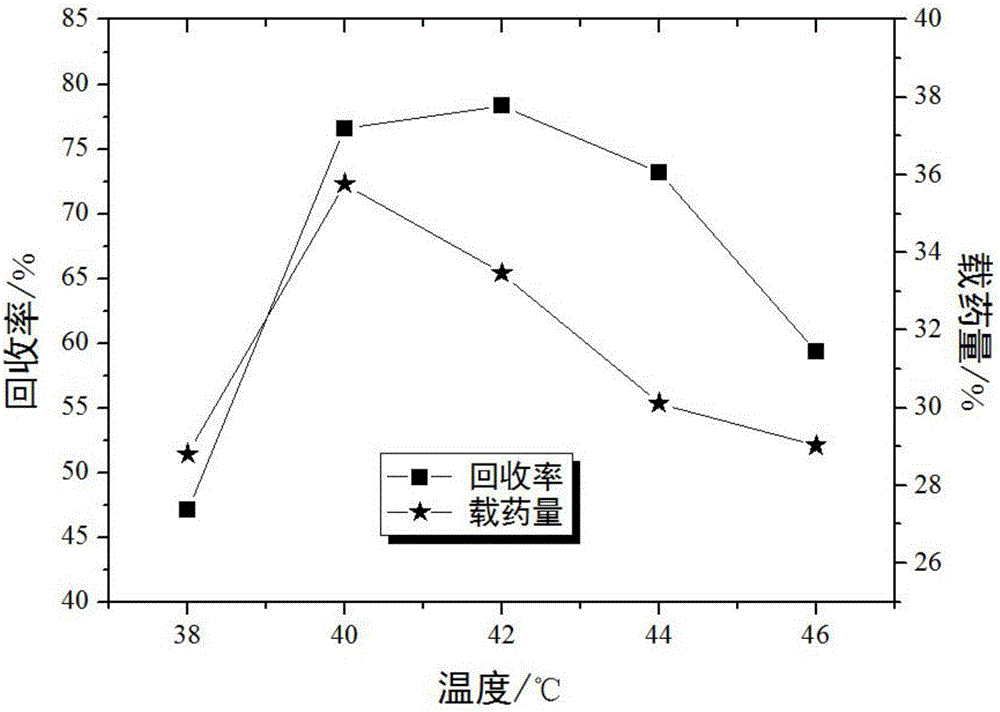
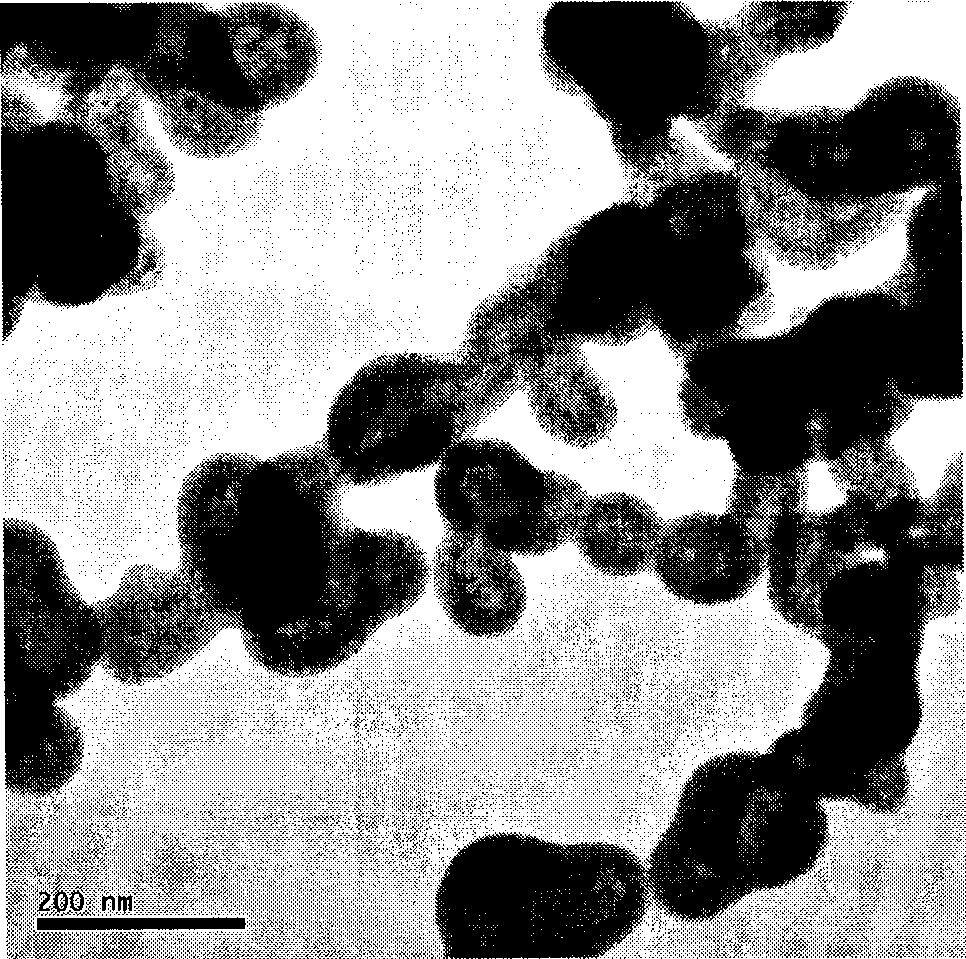
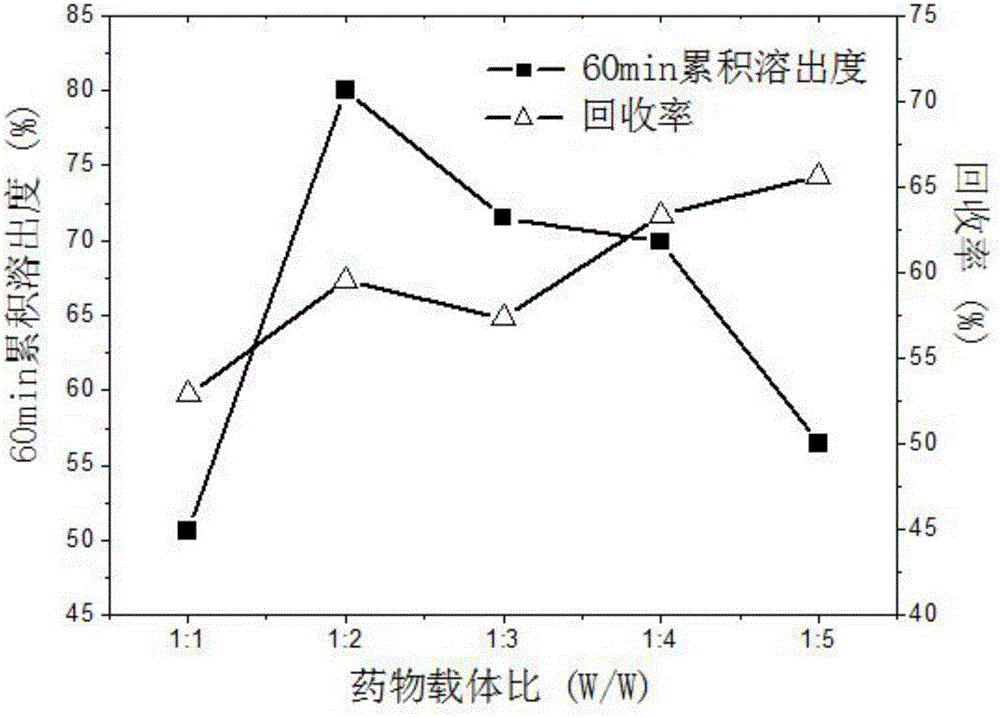
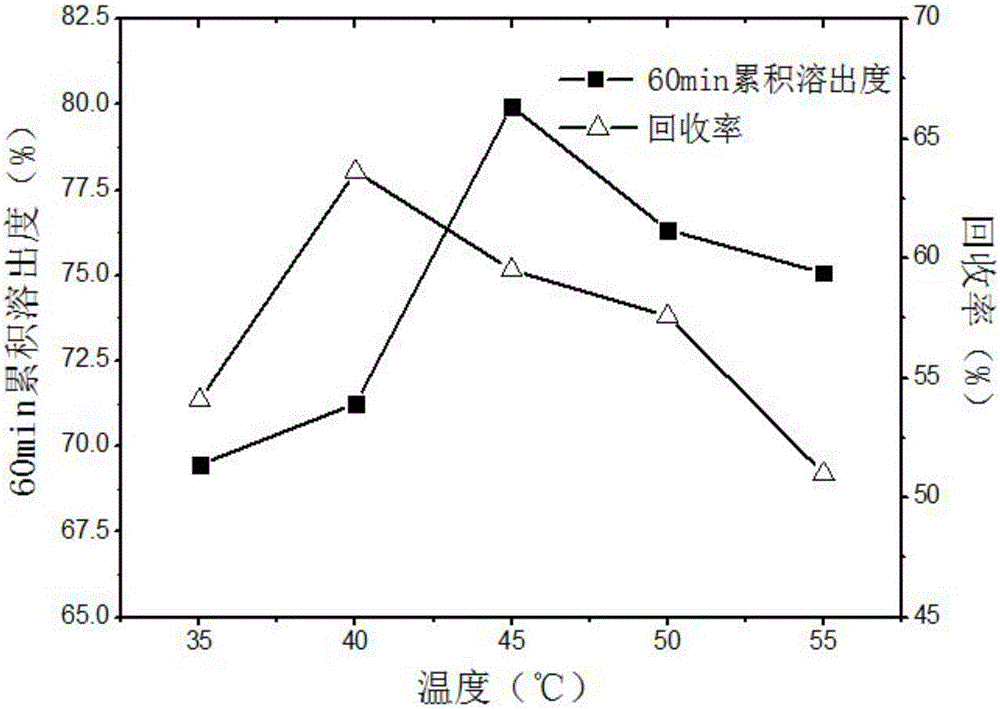
Method for preparing ultrafine drug particles in process of improving supercritical anti-solvent by nonsolvent method
InactiveCN102058996AHigh recovery rateReduce concentrationSolution crystallizationDrugs solutionNon solvent
The invention discloses a method for preparing ultrafine drug particles in process of improving supercritical anti-solvent by a nonsolvent method. The method comprises the following steps: dissolving a material in the mixed solution of an organic solvent and a non solvent to obtain an initial material solution with low concentration and high degree of saturation, wherein the organic solvent is a solvent which can be miscible with the supercritical fluid and the non solvent is another solvent which can be miscible with the organic solvent and can not dissolve the material; and then performing supercritical fluid anti-solvent treatment to obtain the ultrafine particles of the material. Experimental result shows that by adding the non solvent in the drug solution, the concentration can be reduced and the degree of saturation can be increased; then the supercritical carbon dioxide fluid anti-solvent process is performed so as to obviously reduce the particle size of the product and increase the recovery rate; and before the solution is supersaturated, the higher non solvent content is, the smaller drug particles are and the higher recovery rate is.
Owner:HUAQIAO UNIVERSITY
Two novel crystal forms of 9-nitrocamptothecin and preparation methods thereof
ActiveCN104628737AImprove thermal stabilitySmall particle sizeOrganic chemistry methodsBulk chemical productionSolubilitySupercritical anti solvent
The invention discloses two novel crystal forms of 9-nitrocamptothecin and preparation methods thereof. In the X-ray powder diffraction pattern with copper target as a radiation source, the crystal form I has characteristic diffraction peaks at the diffraction angle 2theta of 5.8+ / -0.1 degrees, 7.4+ / -0.1 degrees, 8.8+ / -0.1 degrees, 10.5+ / -0.1 degrees, 11.3+ / -0.2 degrees, 11.7+ / -0 degrees, 15.7+ / -0.2 degrees, 17.8+ / -0.1 degrees, 18.7+ / -0.1 degrees, 21.1+ / -0.1 degrees, 24.1+ / -0.1 degrees and 26.2+ / -0.1 degrees and the crystal form II has the characteristic diffraction peaks at the diffraction angle 2theta is 6.1+ / -0.2 degrees, 6.7+ / -0.1 degrees, 7.5+ / -0.2 degrees, 9.4+ / -0.1 degrees, 11.4+ / -0.2 degrees, 12.4+ / -0.1 degrees, 14.1+ / -0.1 degrees, 15.1+ / -0.1 degrees, 17.6+ / -0.1 degrees, 19.7+ / -0.1 degrees, 22.6+ / -0.1 degrees, 23.8+ / -0.1 degrees, 25.2+ / -0.1 degrees, 27.3+ / -0.1 degrees and 29.2+ / -0.2 degrees. The preparation methods of the two novel crystal forms of 9-nitrocamptothecin are based on a supercritical anti-solvent process. The solubility of the prepared novel crystal forms I and II of 9-nitrocamptothecin is higher than that of the existing crystal forms of 9-nitrocamptothecin, and the thermal stability of the prepared novel crystal forms I and II of 9-nitrocamptothecin is more excellent than that of the existing crystal forms of 9-nitrocamptothecin.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing rabeprazole sodium by virtue of supercritical anti-solvent technique
InactiveCN106957302AAvoid easy to precipitate oilAvoid devitrificationOrganic chemistry methodsBulk chemical productionSolubilitySupercritical anti solvent
The invention discloses a method for preparing rabeprazole sodium by virtue of a supercritical anti-solvent technique. The final product rabeprazole sodium is prepared by virtue of the method. The method has the beneficial effects that a preparation manner is different from a traditional preparation process, rabeprazole sodium is prepared by virtue of the novel supercritical anti-solvent technique, the process is simple, conditions are mild, the reproducibility is good, the obtained product is relatively small in crystal form particle size, relatively narrow in size distribution and relatively high in solubility, the traditional solvent-out crystallization crystallization defects (disadvantages of difficulty in crystallization, easiness in formation of oily substances and the like) are avoided, the prepared product is high in purity and has no solvent residue, and any pollution is avoided.
Owner:SHANDONG YUXIN PHARMA CO LTD
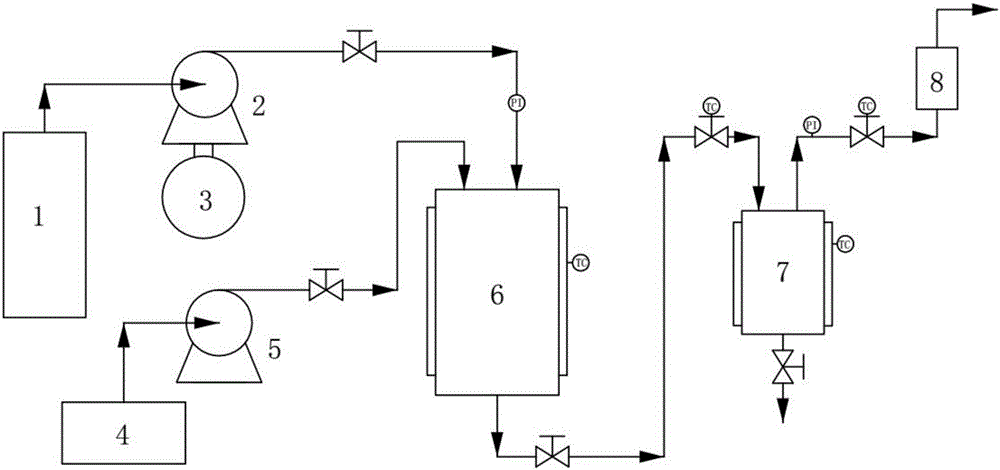
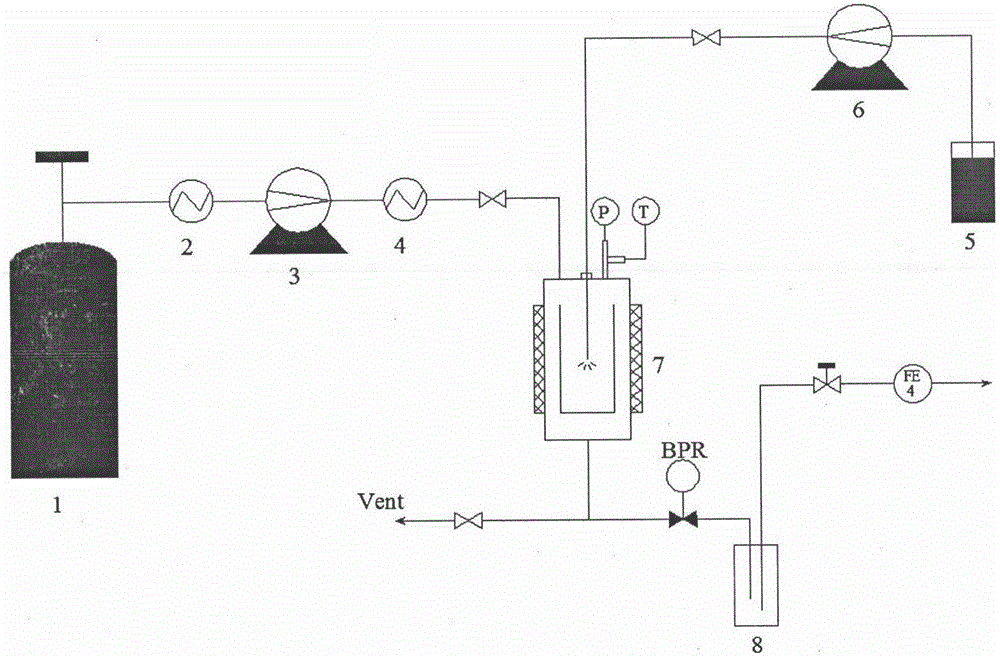
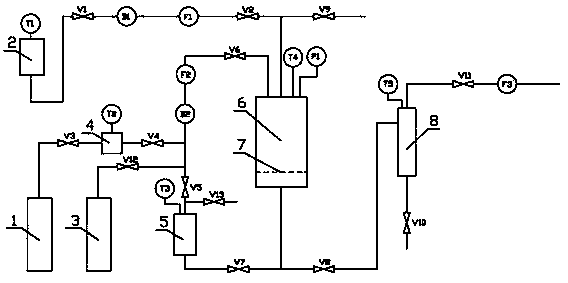
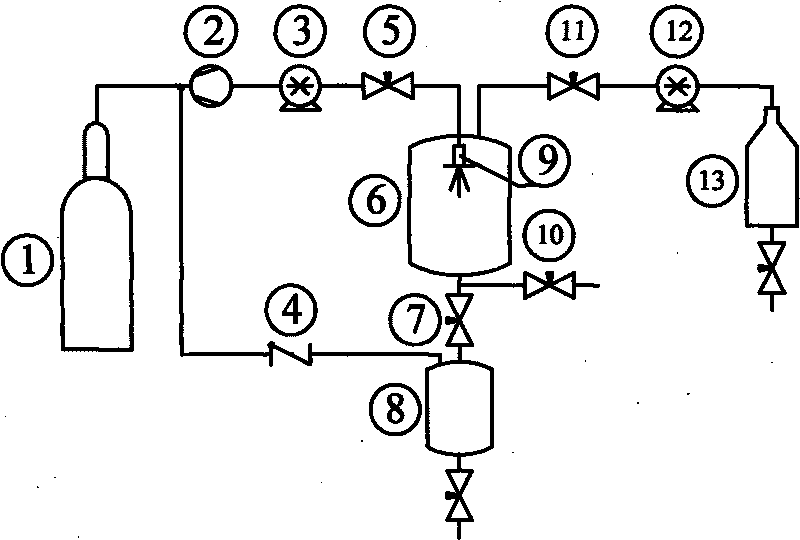
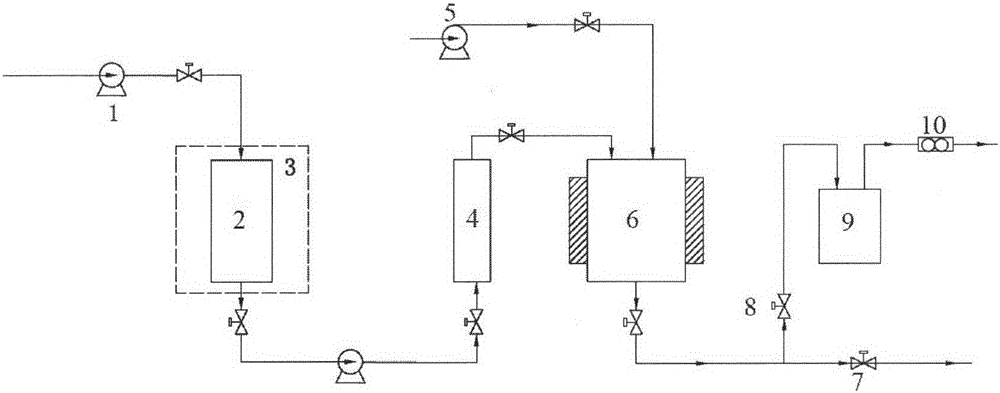
Improved device for preparing polymer drug-loaded particles by supercritical antisolvent method
ActiveCN108905884AImprove application efficiencySolve the problem of high drug loadingGranule coatingSolubilitySupercritical anti solvent
The invention discloses an improved device for preparing polymer drug-loaded particles by a supercritical antisolvent method, wherein the device includes a supercritical fluid supply branch, a solution supply branch, a supercritical fluid impregnation branch, a pressure control system and a granulation system. According to the device, for drugs insoluble in supercritical fluid, a solution containing a polymer and a drug and the supercritical fluid are used for preparing polymer drug-loaded particles in a granulating tank by the supercritical antisolvent method; for drugs soluble in the supercritical fluid, the polymer particles are prepared by an antisolvent method, and the polymer drug-loaded particles are prepared by a supercritical impregnation technology. The device realizes the coupling of a supercritical solution impregnation system and a supercritical anti-solvent system, and enables drugs with various solubility properties in the supercritical fluid to achieve high-efficiency loading in the polymer particles.
Owner:SOUTHEAST UNIV
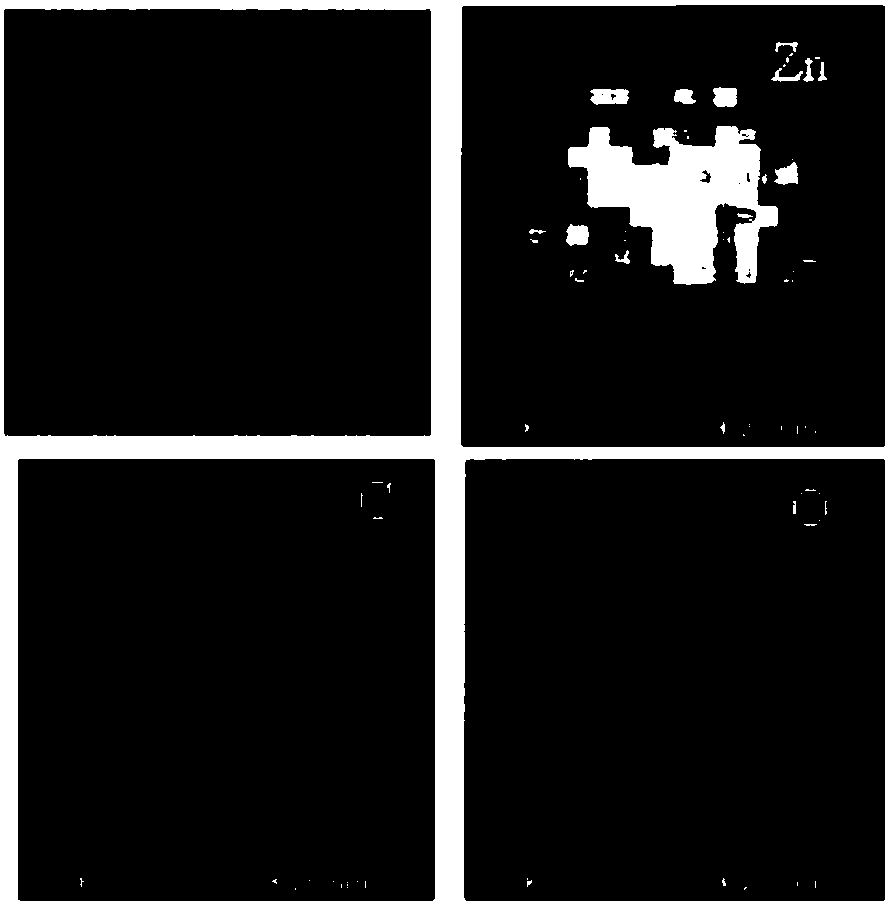
Method of preparing carbon-doped metal oxide nanoparticles
ActiveCN108117104AHigh puritySmall particle sizeZinc oxides/hydroxidesNano-carbonSupercritical anti solventMetal oxide nanoparticles
The invention provides a method of preparing carbon-doped metal oxide nanoparticles by means of the supercritical anti-solvent technology and high-temperature calcination. The method comprises the following steps: dissolving metal nitrate and a methyl vinyl ether-maleic anhydride copolymer in a solvent to form a mixed solution; then atomizing the mixed solution placed in a columnar piston injectorat a certain flow rate and injecting the mixed solution to a supercritical fluid; preparing polymer nanospheres of inorganic salt embedded with inorganic salt under the anti-solvent action of the supercritical fluid at a temperature of 308-330K and at a pressure of 10-12MPa; and calcining the nanospheres embedded with the metal nitrate and the methyl vinyl ether-maleic anhydride copolymer at a high temperature to finally obtain the carbon-doped metal oxide nanoparticles, the grain sizes of which are 10-100nm. The synthetic method is simple and easy to control operating condition, and the function and application of the metal oxide nanoparticles are improved greatly.
Owner:HUAQIAO UNIVERSITY
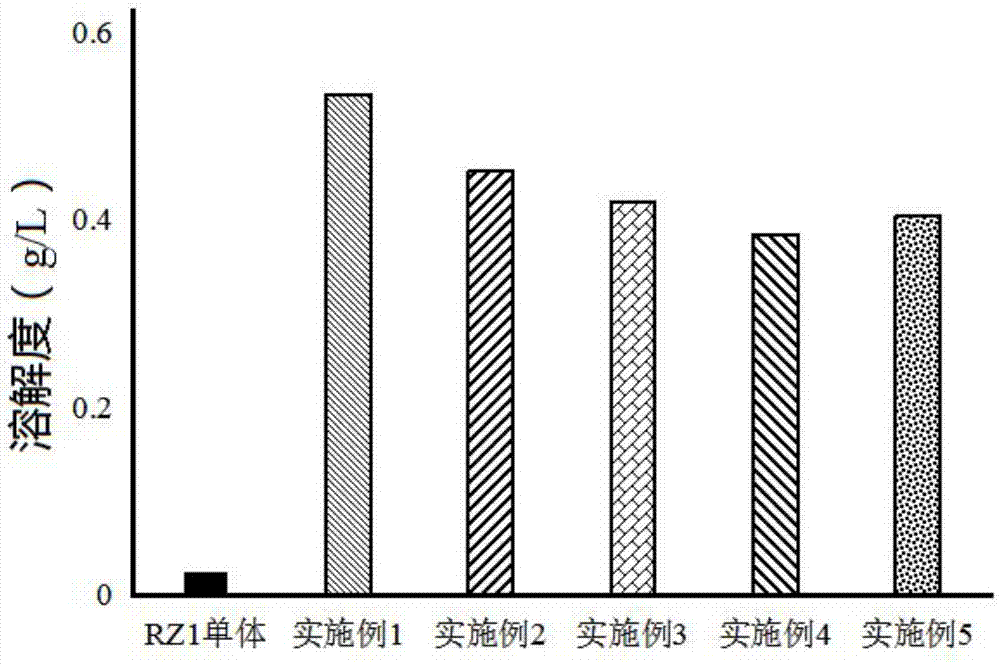
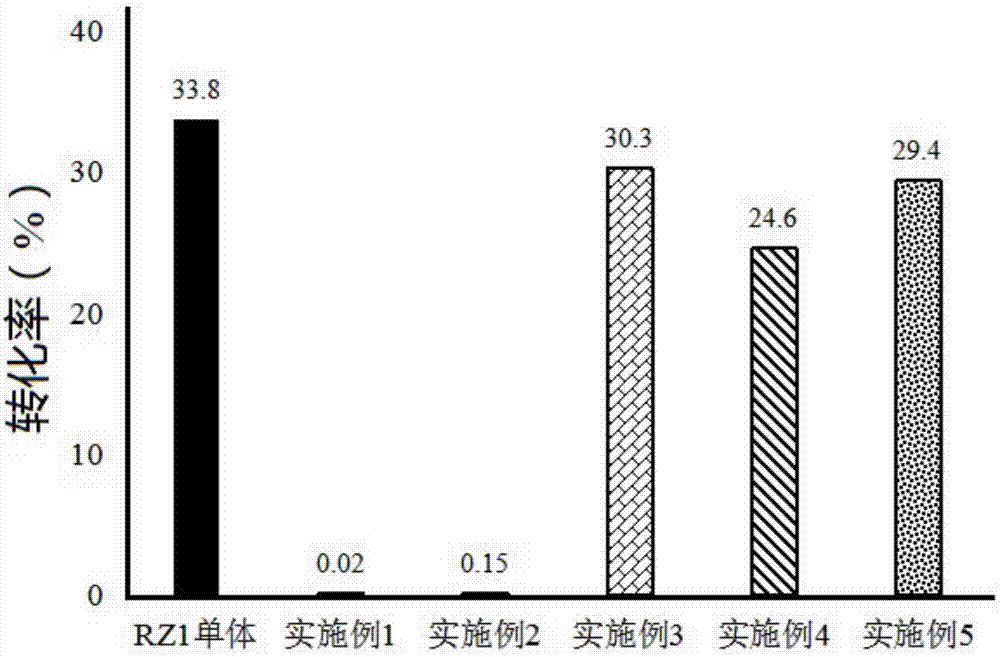
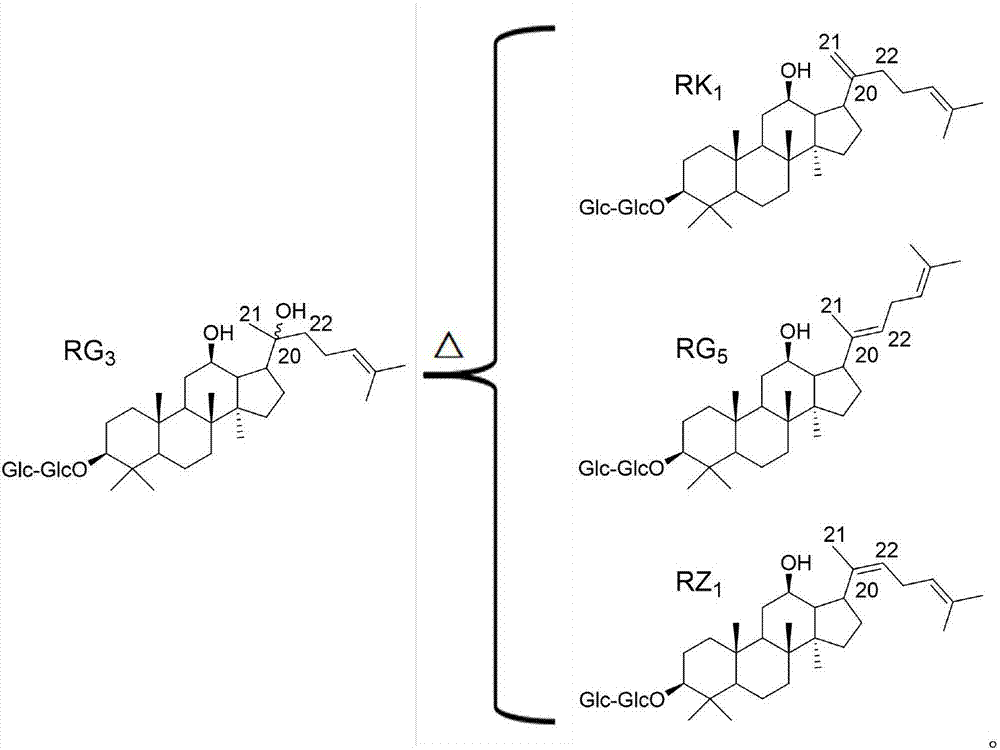
Ginsenoside RZ1 solid dispersion and preparation method thereof
ActiveCN107375215AInhibit transformationImprove solubilityPowder deliveryOrganic active ingredientsSolubilitySupercritical anti solvent
The invention relates to a ginsenoside RZ1 solid dispersion and a preparation method thereof. A solid carrier is povidone K-30. A supercritical anti-sovent method comprises the following preparation steps: step A, starting a device, introducing CO2 from the top of a kettle into a crystallization kettle to a preset pressure when the temperature of the crystallization kettle reaches a preset value, and adjusting the flow of a CO2 outlet valve to a set value; step B, pumping ginsenoside RZ1 and a povidone K-30 solution at a set volume flow into the crystallization kettle, wherein the solvent-containing CO2 enters a separation kettle, and the solvent is recovered in the separation kettle, CO2 is discharged, and then eluting the solution for 20-30min after the solution is fed; and step C, closing the CO2 inlet valve, emptying gas in the kettle, and opening the crystallization kettle to take out microparticles. The solvent method comprises the following preparation steps: stirring the ginsenoside RZ1 and the povidone K-30 solution at normal temperature; evaporating the mixture at a reduced pressure to remove the solvent; and drying and crushing the mixture. The povidone K-30 not only can improve the solubility of RZ1 in water, but also can inhibit isomerization of the ginsenoside RZ1.
Owner:YANTAI NEW ERA HEALTH IND
Preparation method for supercritical anti-solvent of water-soluble nano glycyrrhizic acid powder
InactiveCN101756905ASimple processSimple and fast operationOrganic active ingredientsPowder deliverySolubilitySupercritical anti solvent
The invention relates to a preparation method for the supercritical anti-solvent of water-soluble nano glycyrrhizic acid powder, and is characterized in that: a CO2 high-pressure pump is started up to inject CO2 into a high-pressure crystallizing kettle by flow rate of 5-20L / h, the temperature of the high-pressure crystallizing kettle is kept at 32-75 DEG C and the pressure is kept at 7.5-25MPa to reach a supercritical state; absolute ethyl alcohol with glycyrrhizic acid content of 1-5mg / ml is sprayed into the high-pressure crystallizing kettle through a spray nozzle with aperture of 20-1000um by flow rate of 1-30ml / min, glycyrrhizic acid powder with average diameter no more than 300nm is precipitated, and CO2 is continuously supplied into the high-pressure crystallizing kettle for at least half an hour to dry the formed nano glycyrrhizic acid powder; and the solvent, i.e. the absolute ethyl alcohol, is separated from CO2 in a separation kettle with pressure of 5-6.5MPa and temperature of 25-50 DEG C, the absolute ethyl alcohol is reused after the absolute ethyl alcohol is recovered, and CO2 gas is directly recycled. The invention has the advantages that the surface of the obtained nano glycyrrhizic acid powder is smooth, the size distribution is even, the water solubility is good, no residual solvent exists, the production technology causes no pollution, the cost is low, the yield is high and the industrialization is easy.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Technical study for preparing Nim-PLLA (nimesulide-poly-l-lactic acid) composite microsphere with supercritical anti-solvent method
InactiveCN105687141AHigh drug loadingUniform shapeAntipyreticAnalgesicsSolubilitySupercritical anti solvent
The invention relates to a method for preparing a Nim-PLLA (nimesulide-poly-l-lactic acid) composite microsphere with a supercritical anti-solvent crystallization technology. The nimesulide sustained-release microsphere with a sustained-release effect is prepared. The grain size of the drug composite microsphere can be effectively controlled through change of the drug solvent variety, the solution concentration, the crystallization pressure, the crystallization temperature, the flow velocity of the introduction of a solution sample and the like, and the grain size of the prepared microsphere is in a range from 1 mu m to 10 mu m. According to the drug sustained-release microsphere prepared with the method, the drug solubility and the drug sustained-release property are effectively improved.
Owner:CHINA PHARM UNIV
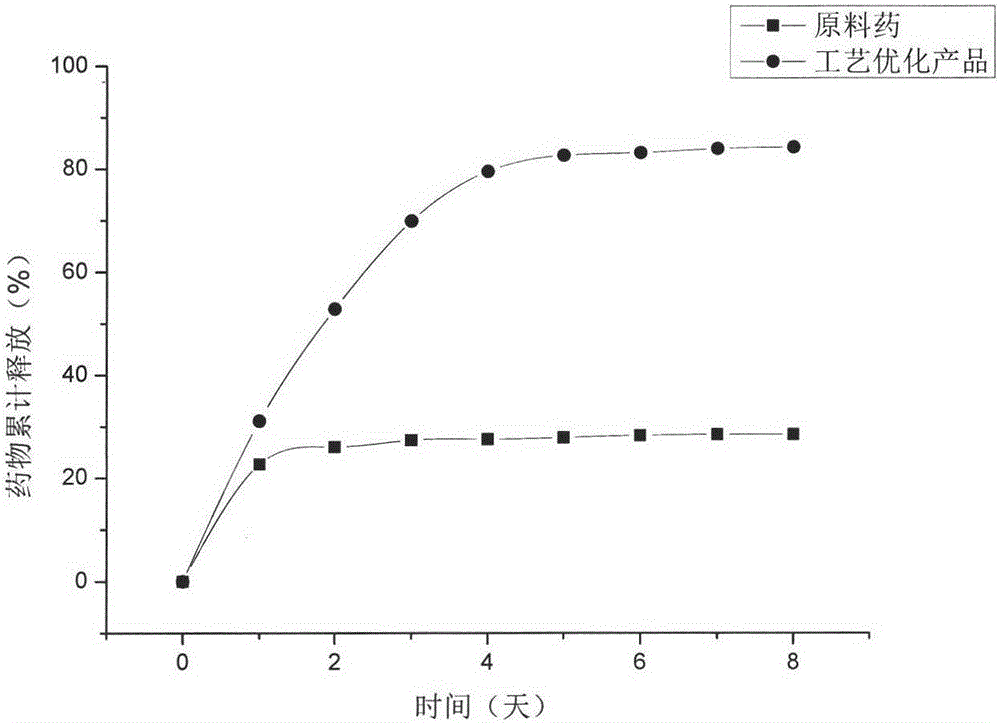
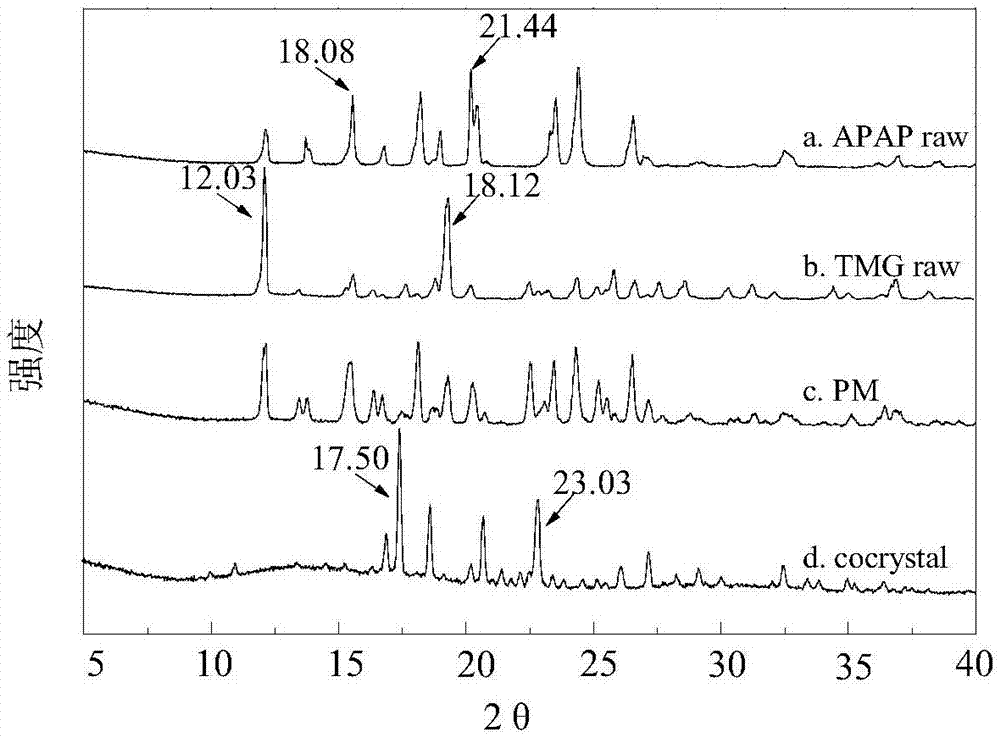
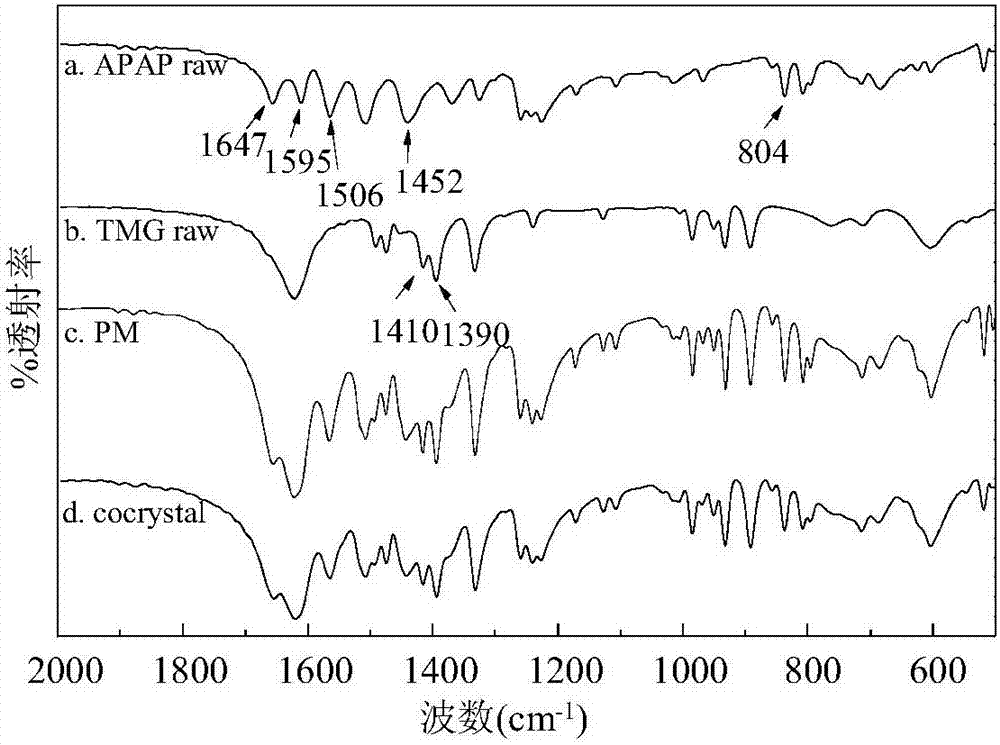
Method for preparing paracetamol-betaine cocrystal by supercritical anti-solvent particle production technique
ActiveCN107226784APromote growthSmall particle sizeProductsReagentsSolubilitySupercritical anti solvent
The invention discloses a method for preparing a paracetamol-betaine cocrystal by a supercritical anti-solvent particle production technique. The method comprises the following steps: adding paracetamol and betaine into an organic solvent respectively in a mole ratio of 1:1 or 1:2 to prepare a mixed solution; by using supercritical carbon dioxide as an anti-solvent, continuously introducing the anti-solvent into a settling kettle at a constant flow velocity by using a high-pressure pump; and when the temperature in the settling kettle is 35-50 DEG C and the pressure is 80-120bar, spraying the mixed solution into the settling kettle by using another high-pressure pump to precipitate the cocrystal drug particles. Compared with the grinding process in the past, the paracetamol-betaine cocrystal prepared by the method disclosed by the invention has the advantages of small particle size, uniform particle size distribution, high stability, higher solubility and higher dissolution rate. Different crystal forms can be obtained by changing the experimental conditions, thereby enhancing the bioavailability and drug effect of the paracetamol drug, and satisfying the medicinal requirements. The preparation method is simple to operate, has favorable repeatability, and can easily implement industrialized production.
Owner:SOUTH CHINA UNIV OF TECH
Composite nanoparticles of polymer coated curcumin eutectic/synergistic components, and preparation and application thereof in pharmacy
ActiveCN109589320AImprove physical and chemical propertiesImprove bioavailabilityAntibacterial agentsAntimycoticsSolubilityPharmacy
The invention belongs to the field of medicines, and particularly relates to composite nanoparticles of polymer coated curcumin eutectic / synergistic components. The composite nanoparticles comprise acore and a shell coating the surface of the core, the core is a mixture of curcumin eutectic and synergistic components, and the shell is a hydrophilic polymer. The invention also discloses a preparation method and application of the composite nanoparticles. In the preparation method, the curcumin eutectic is innovatively utilized, and the composite nanoparticles with in-situ coated structures aresuccessfully prepared by supercritical antisolvent crystallization (SAS). The composite nanoparticles have superior solubility, stability and bioavailability.
Owner:MEDONCARE PHARMA CO LTD
Improvements Relating To Inhalable Particles
InactiveUS20170056325A1Easy to inhaleReadily inhalable shapePowder deliveryOrganic active ingredientsInhalable particlesSupercritical anti solvent
A powder formulation, or pharmaceutical composition comprising or consisting of particles of an antimuscarinic agent, said particles being obtainable by supercritical anti-solvent (SAS) precipitation and having a D50 of 4 μm or less and a D90 of 10 μm or less. Methods of forming the formulation and composition are also disclosed, as are uses of the composition.
Owner:CRYSTEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com