Method for preparing biological degradable polymer drug-carried fine particle by supercritical anti-solvent process
A technology of biodegradable and drug-loaded particles, which is applied in the fields of chemical and biomedical engineering, can solve the problems of low melting point and high solubility, and achieve the effects of high yield, high biological activity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Inject CO into the reactor 2 , adjust the pressure and temperature to a steady state (34°C, 14MPa). With 1g polylactic acid (L-PLA) and 0.1g norcantharidin (can be used for the treatment of primary liver cancer, the character is to be white crystalline powder; Aqueous solution shows acid reaction, easily soluble in acetone or hot water, in Slightly soluble in ethanol or cold water.) Dissolved in 50ml of dichloromethane to form a uniform and stable solution, wherein the concentration of polylactic acid is 20mg / ml, and the drug concentration of norcantharidin is 2mg / ml. Then the above solution was sprayed into the autoclave with a spray flow rate of 1ml / min. During the spraying process, the organic solvent swelled and the dissolving power was reduced, so that the original solute in the organic solvent was supersaturated to form a crystalline powder precipitate. After the solution was sprayed, CO was introduced into the reaction kettle. 2 , maintain the original pressure...
Embodiment 2
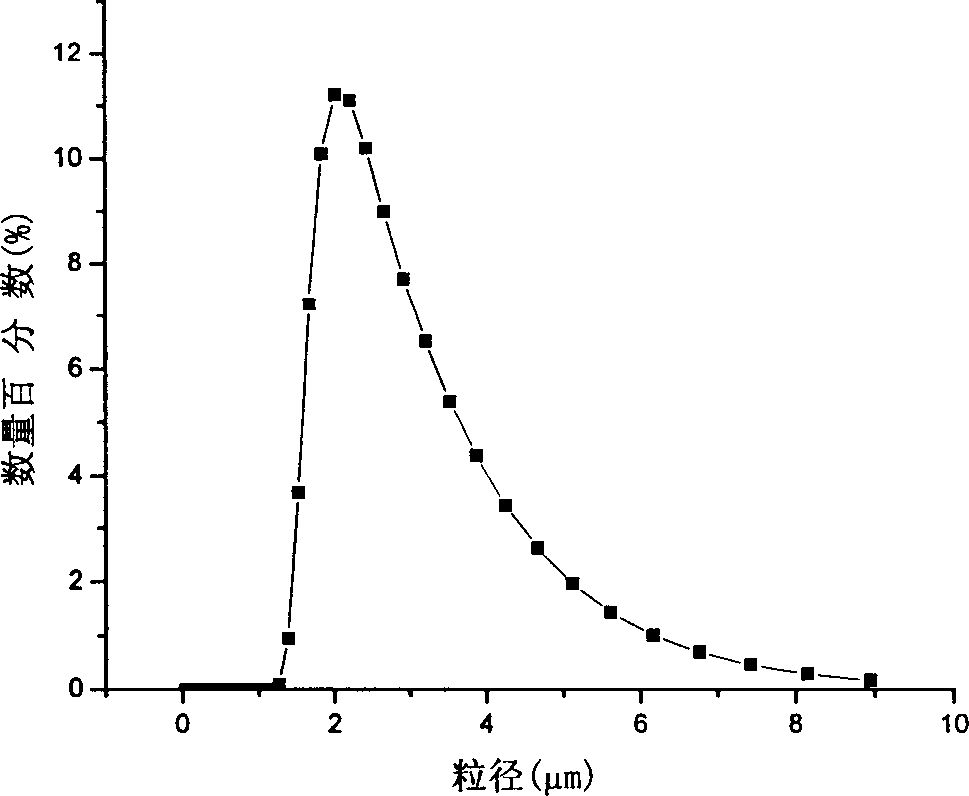
[0032] Inject CO into the reactor 2, adjust the pressure and temperature to a steady state (room temperature, 20MPa). 1g of polylactic acid (L-PLA) and 0.5g of norcantharidin were dissolved in 50ml of dichloromethane to form a uniform and stable solution, wherein the concentration of polylactic acid was 20mg / ml, and the concentration of norcantharidin was 10mg / ml. ml. Then the above solution was sprayed into the autoclave with a spray flow rate of 1ml / min. During the spraying process, the organic solvent swelled and the dissolving power was reduced, so that the original solute in the organic solvent was supersaturated to form a crystalline powder precipitate. After the solution was sprayed, CO was introduced into the reaction kettle. 2 , maintain the original pressure and temperature to wash the residual organic solvent, and the process lasts for 100 minutes. Finally, the pressure was slowly lowered for about 30 minutes, and the product was collected. The results of scannin...
Embodiment 3
[0034] The concentration of L-PLA was changed to 60 mg / ml, and other conditions were the same as in Example 1. The average particle size of the obtained microspheres was 3.562 μm, the standard deviation was 0.793 μm, and the drug encapsulation efficiency was 37.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com