Patents
Literature
7980results about "Pharmaceutical product form change" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
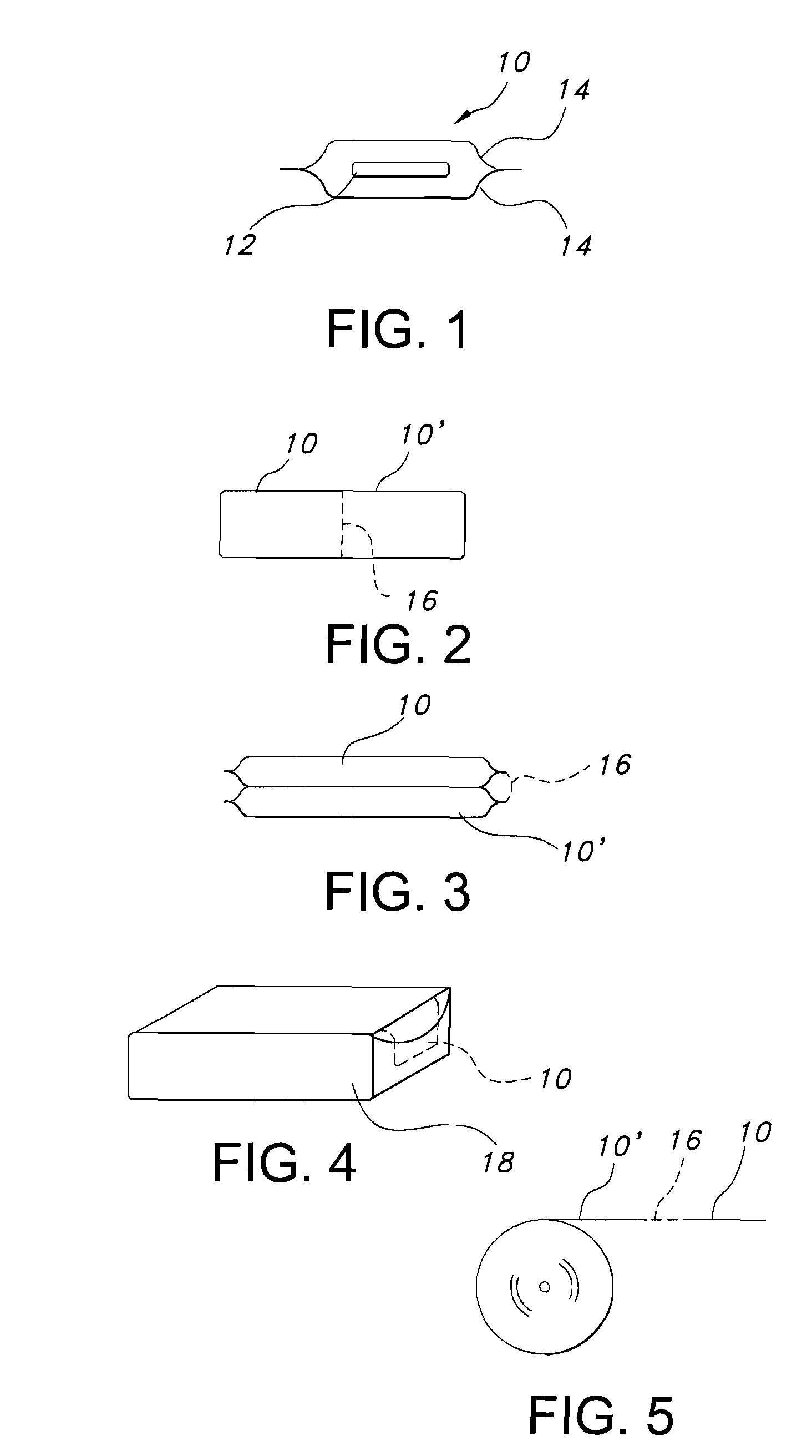
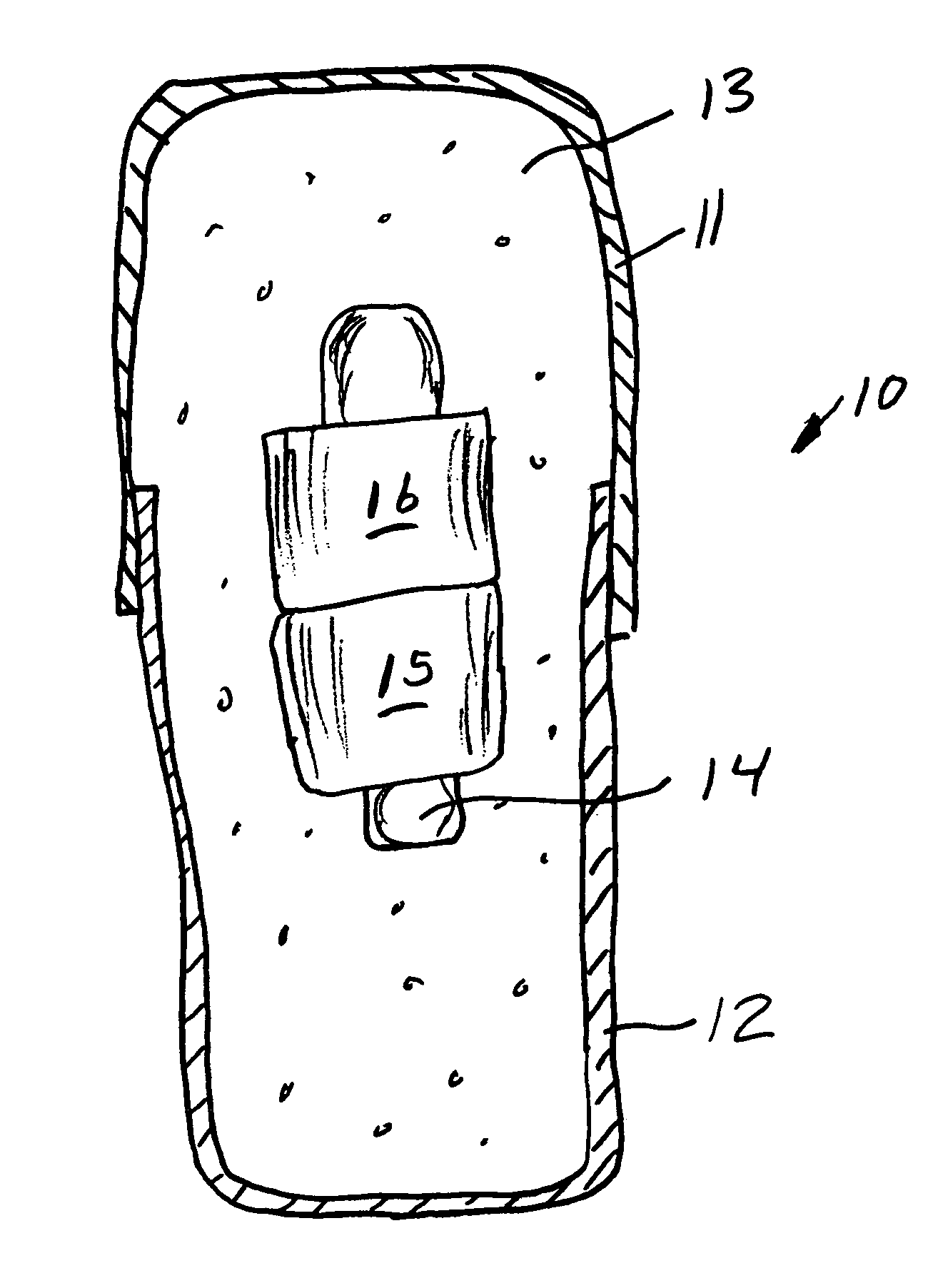
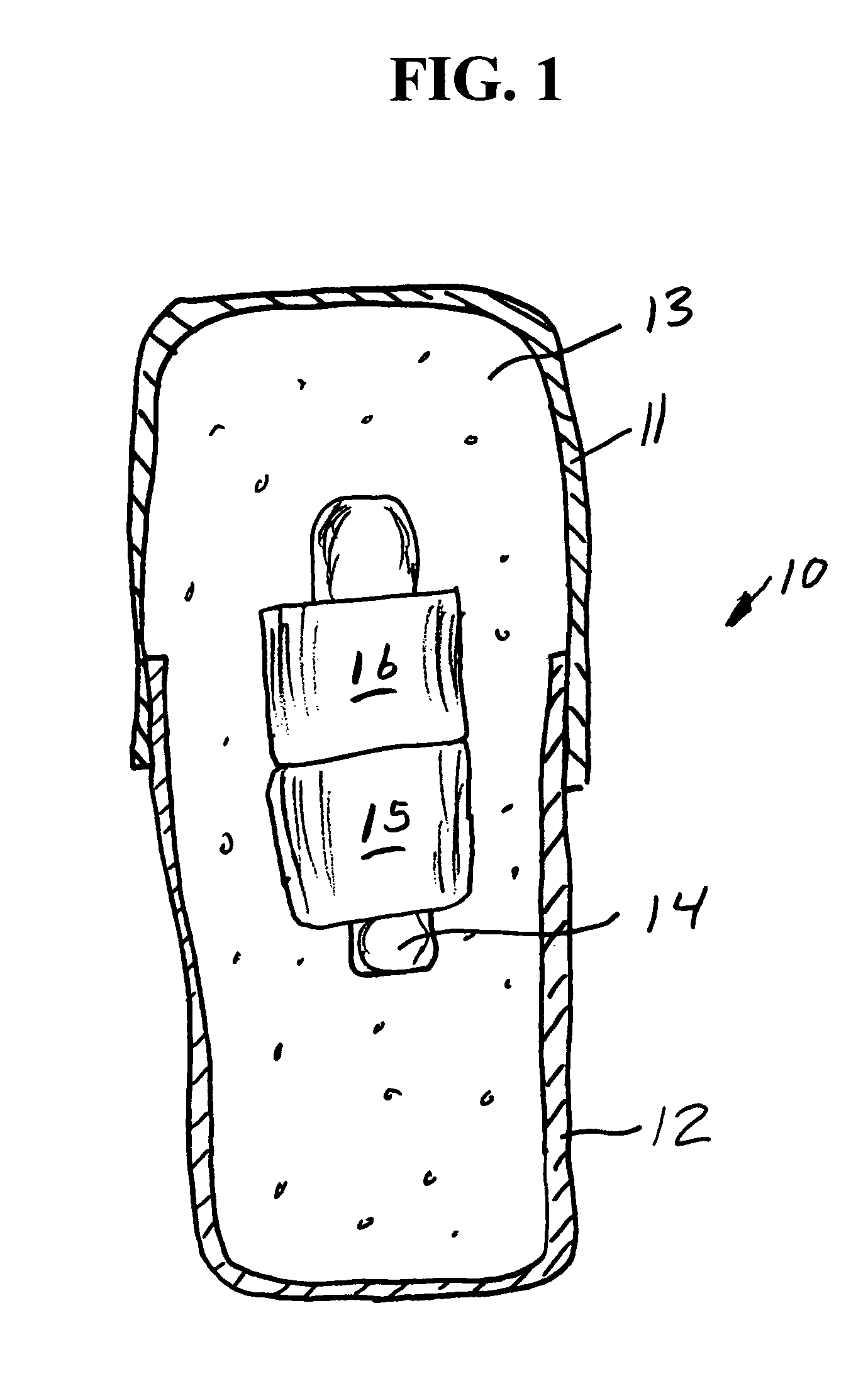
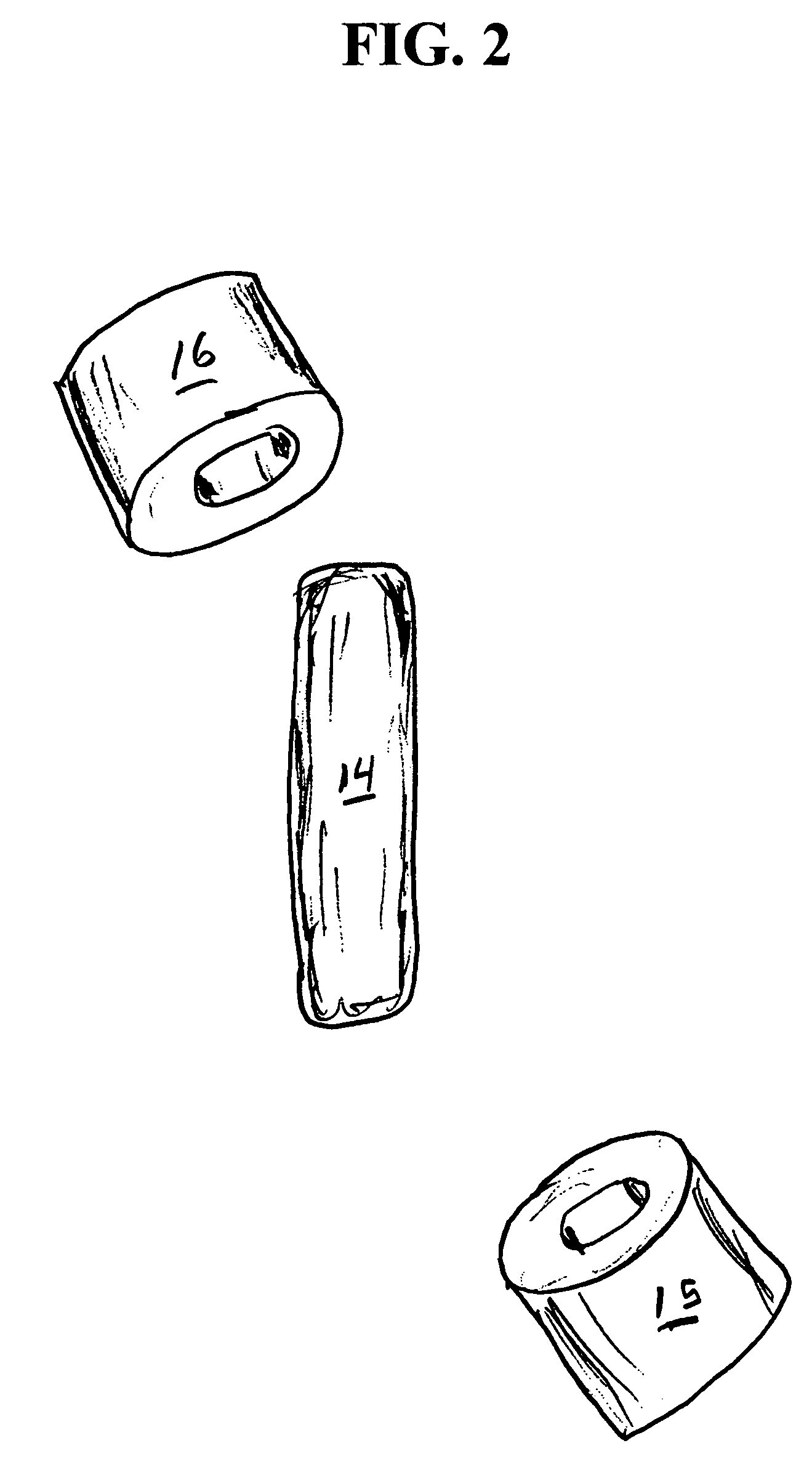
Methods for producing droplets for use in capsule-based electrophoretic displays
Methods are provided for forming a dispersion of substantially uniform droplets. An internal phase that includes a plurality of particles suspended in a first fluid is provided and an external phase including a second fluid is provided. The internal phase is vibrated and the internal phase is applied to the external phase. Either the internal phase or a combination of the internal and external phases form a series of droplets or complex droplets of substantially uniform size.
Owner:E INK CORPORATION
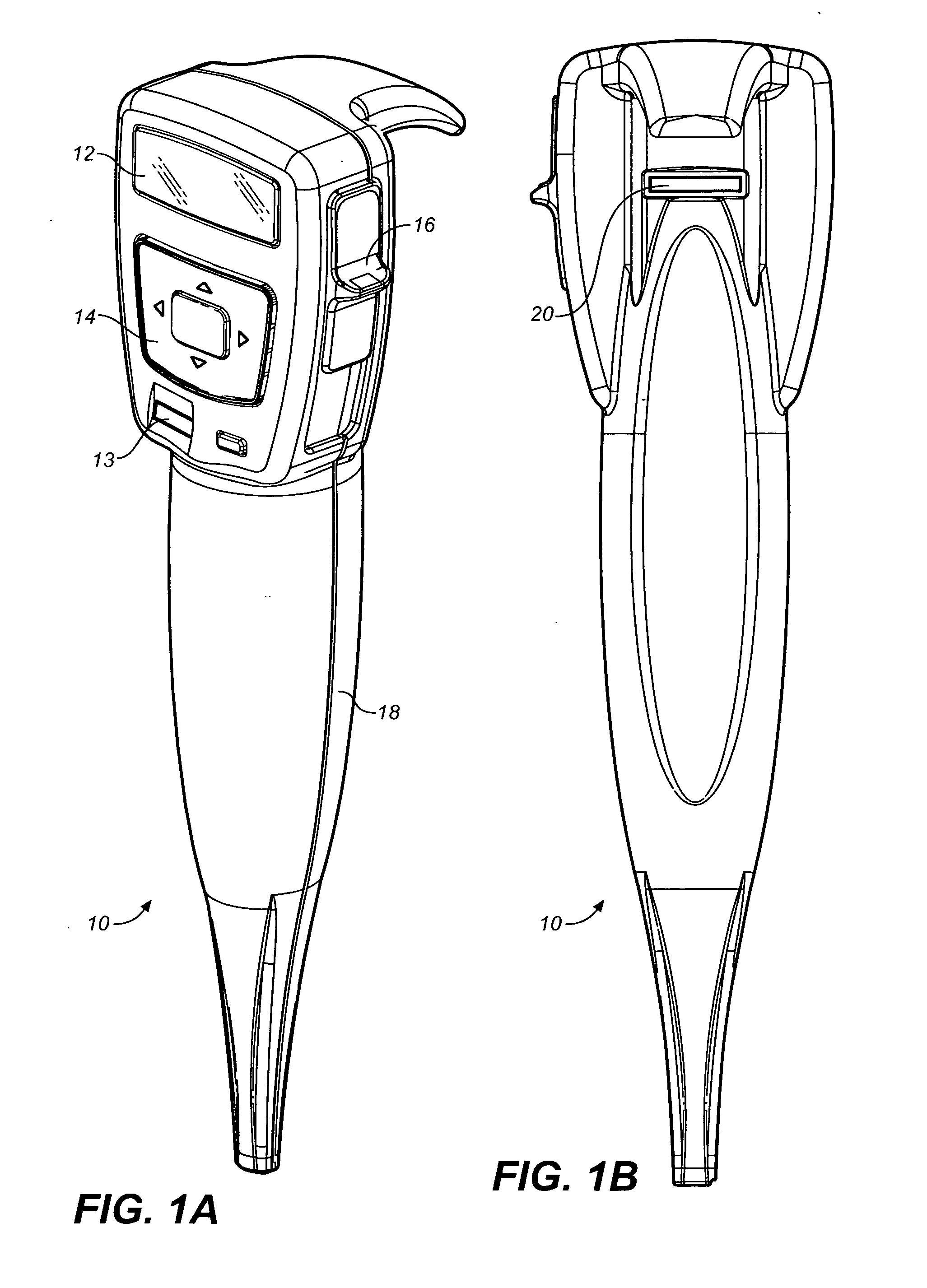
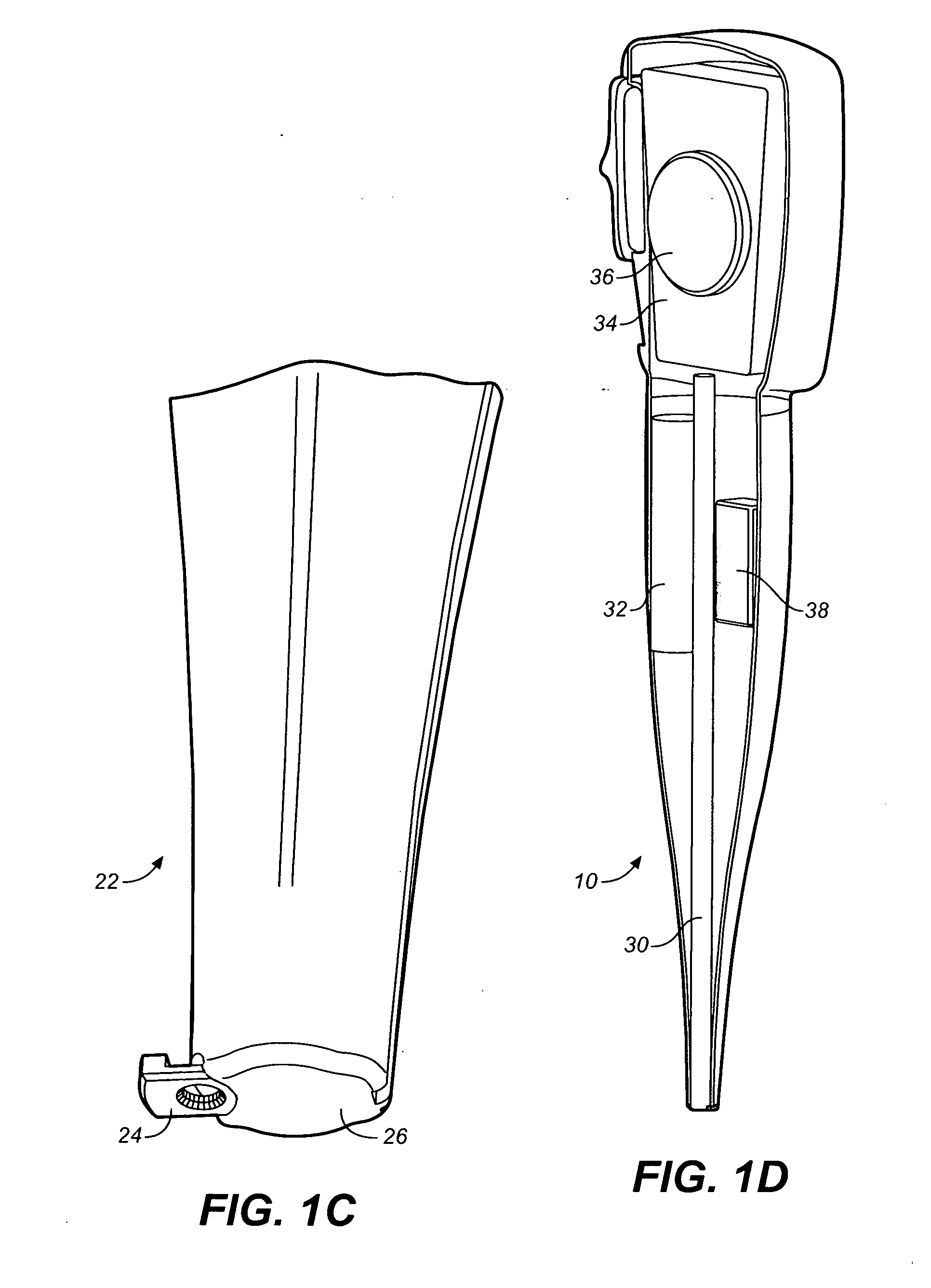
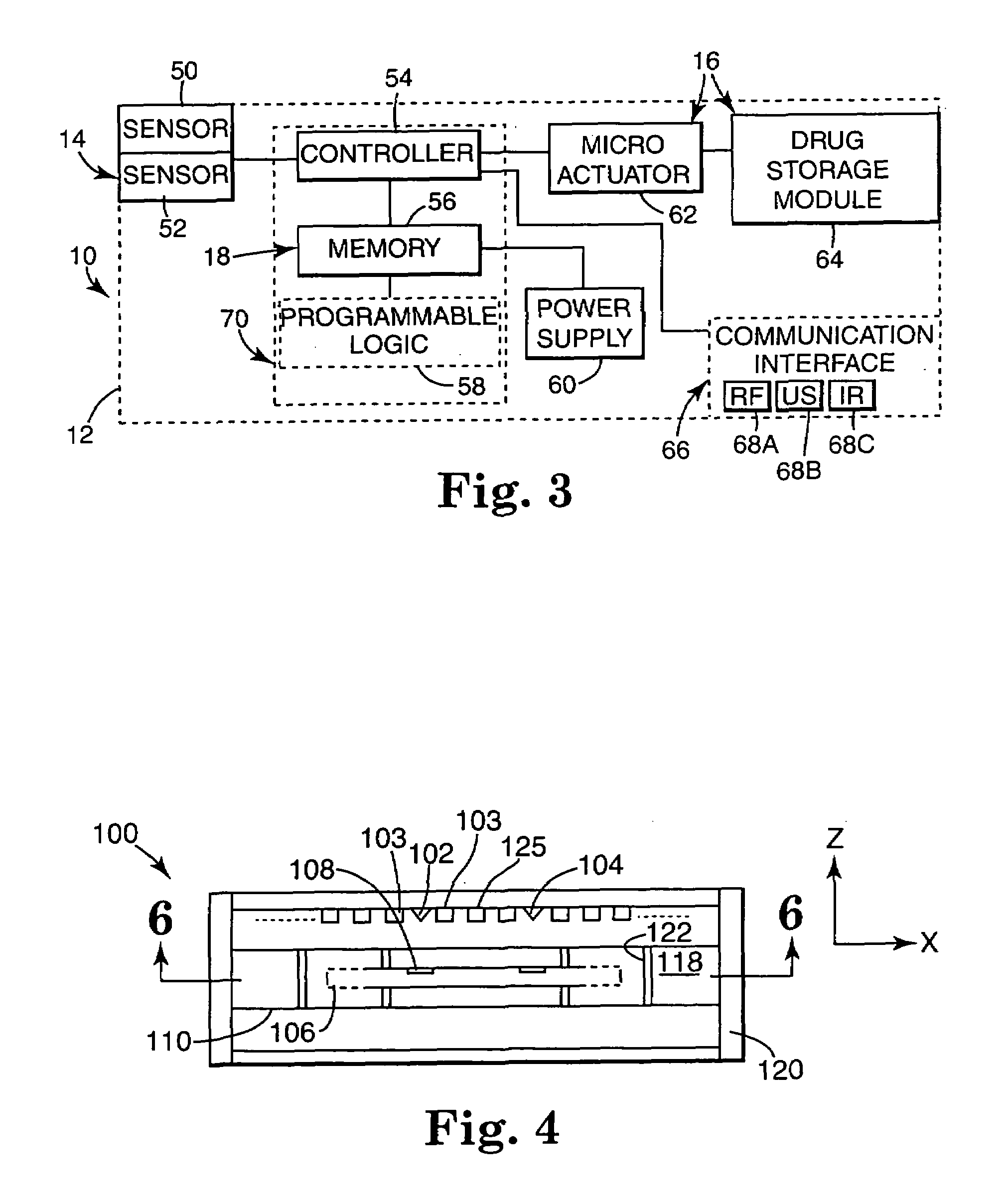
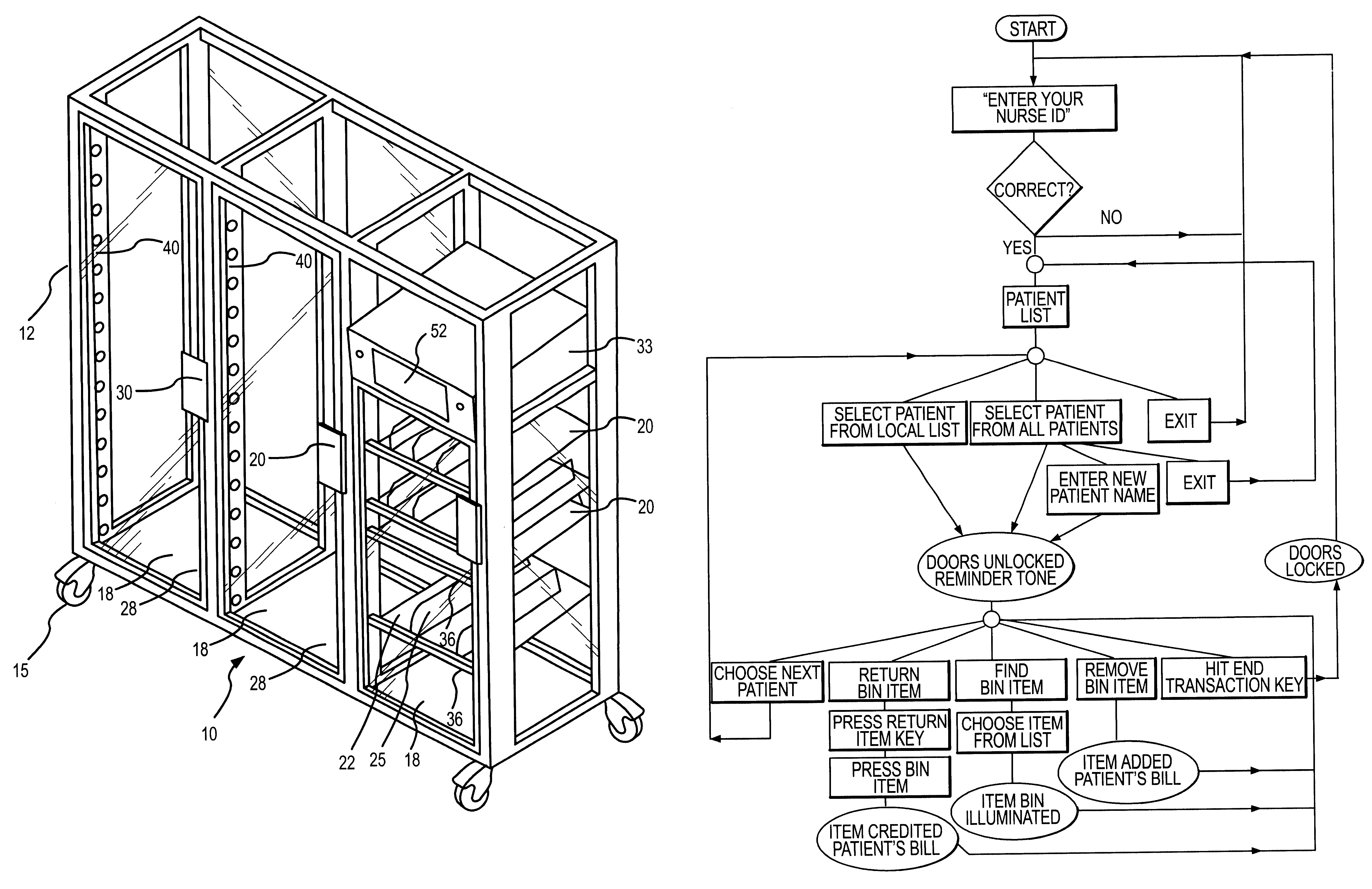
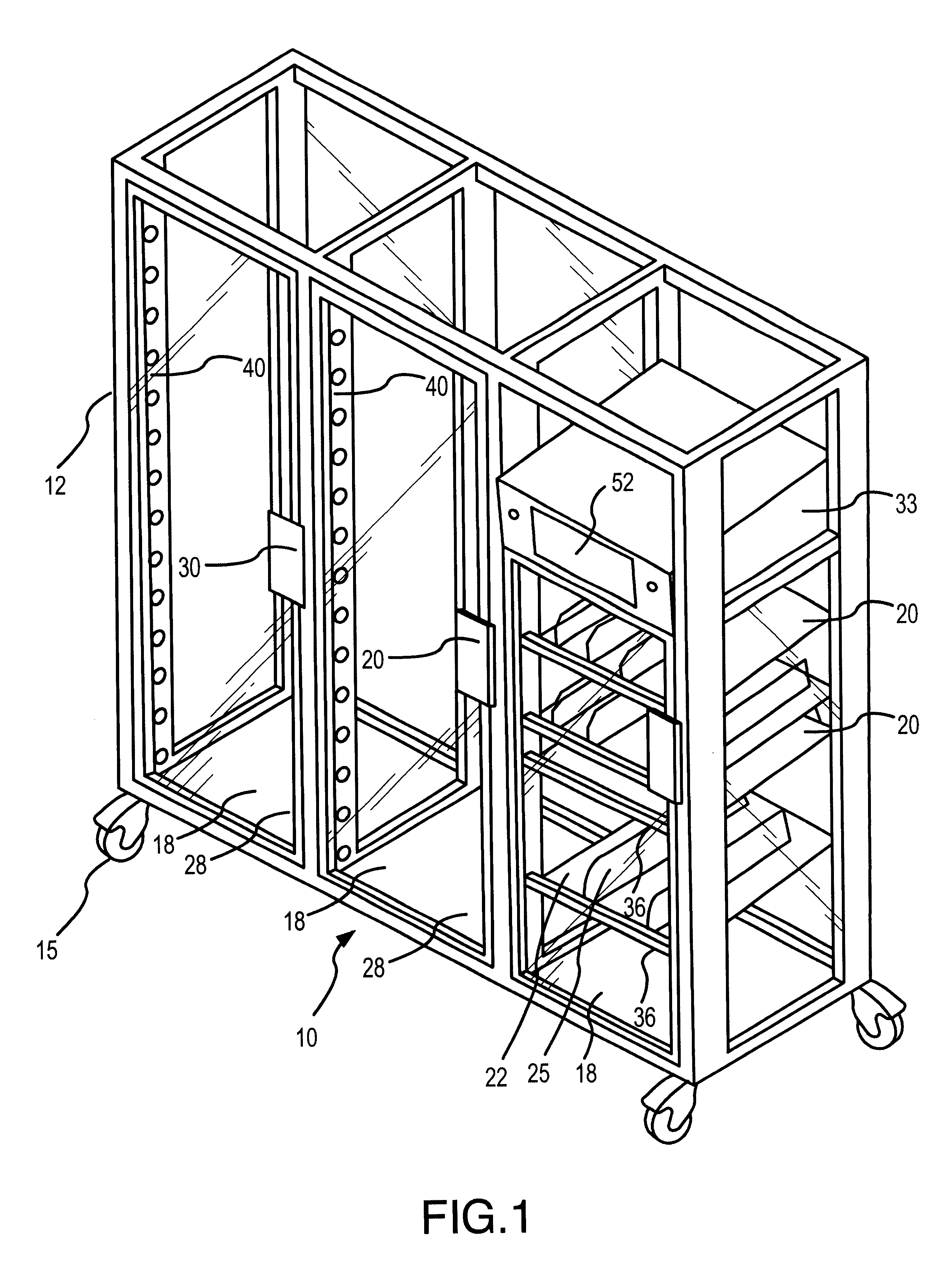
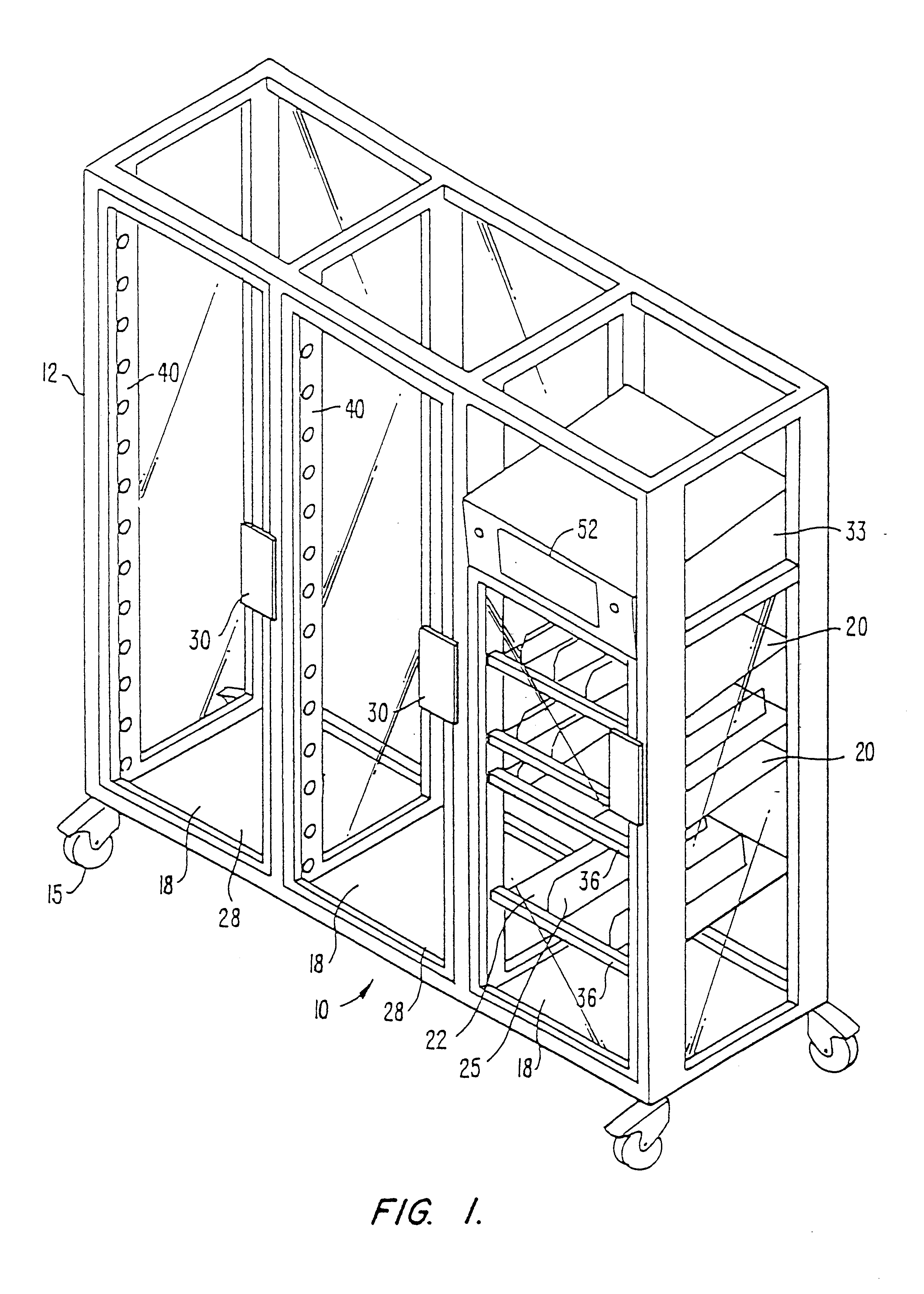
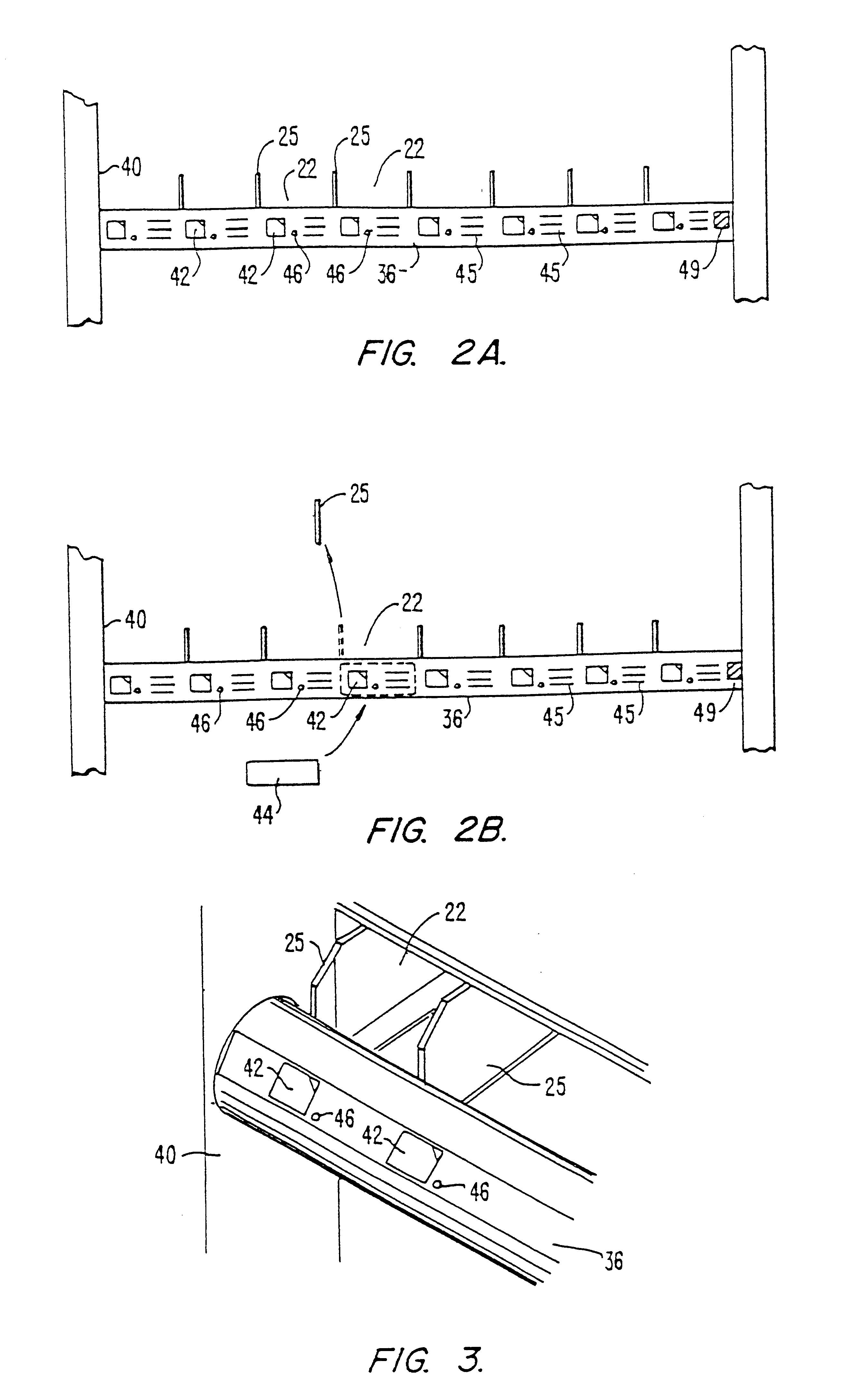
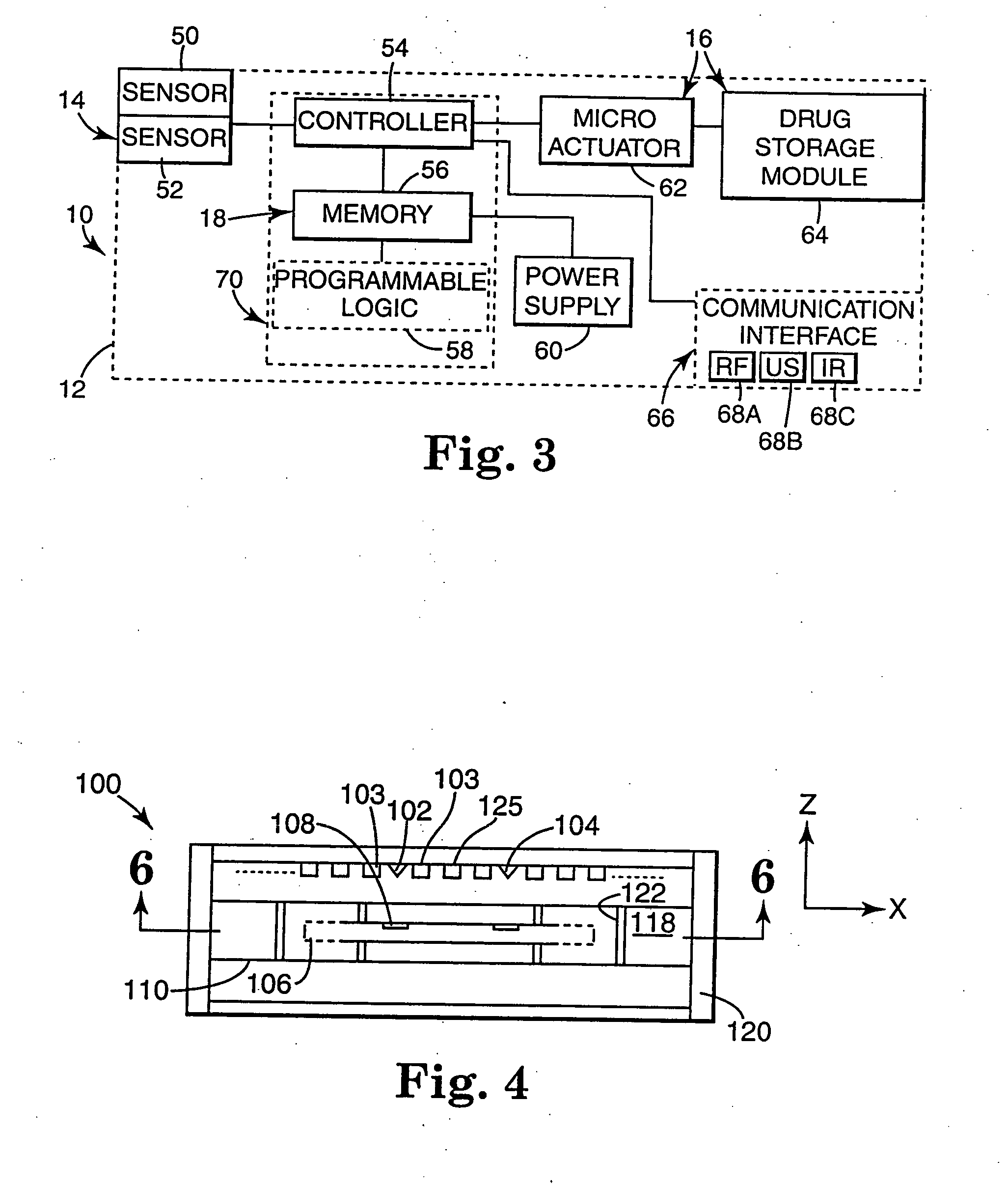
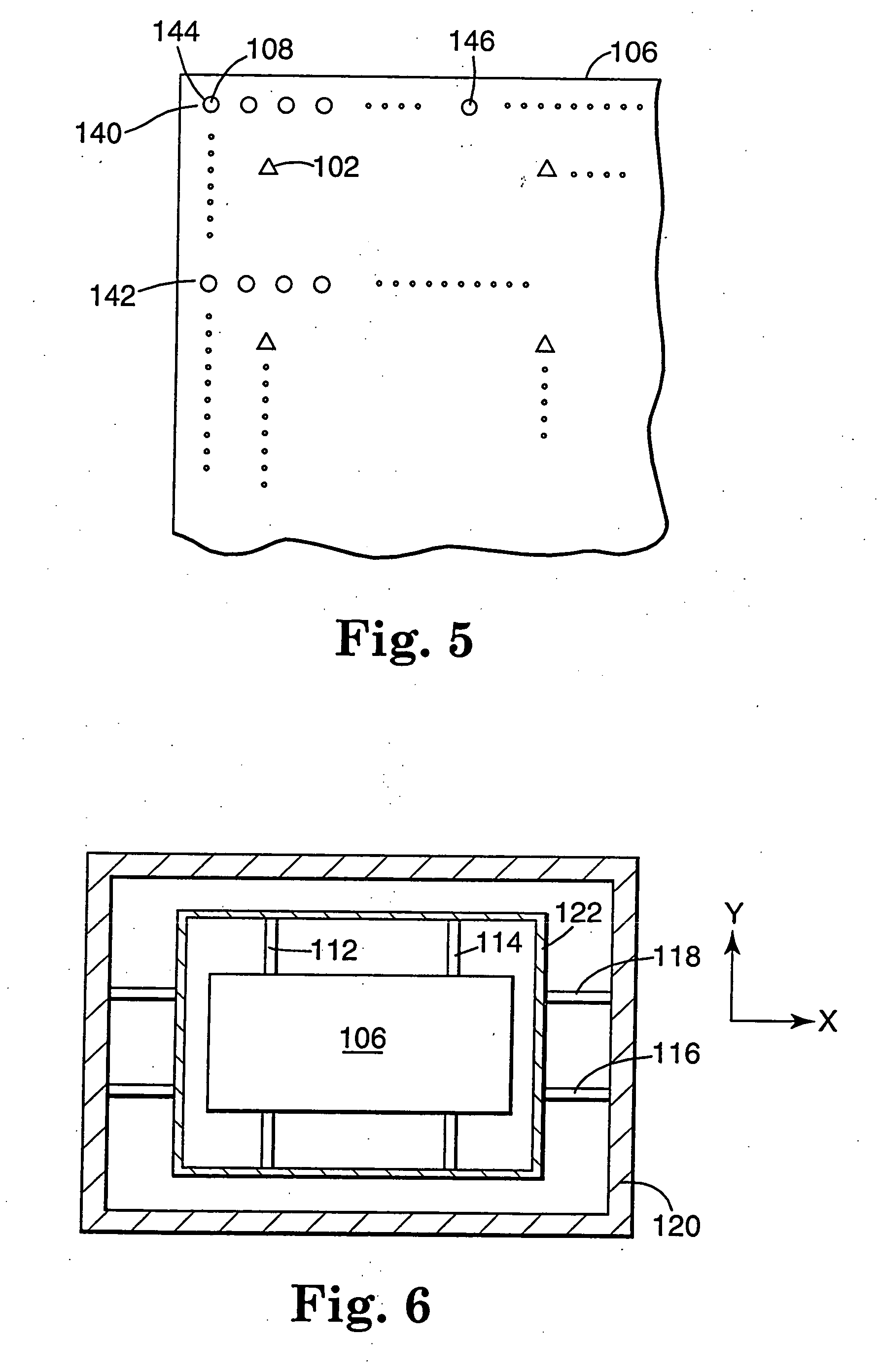
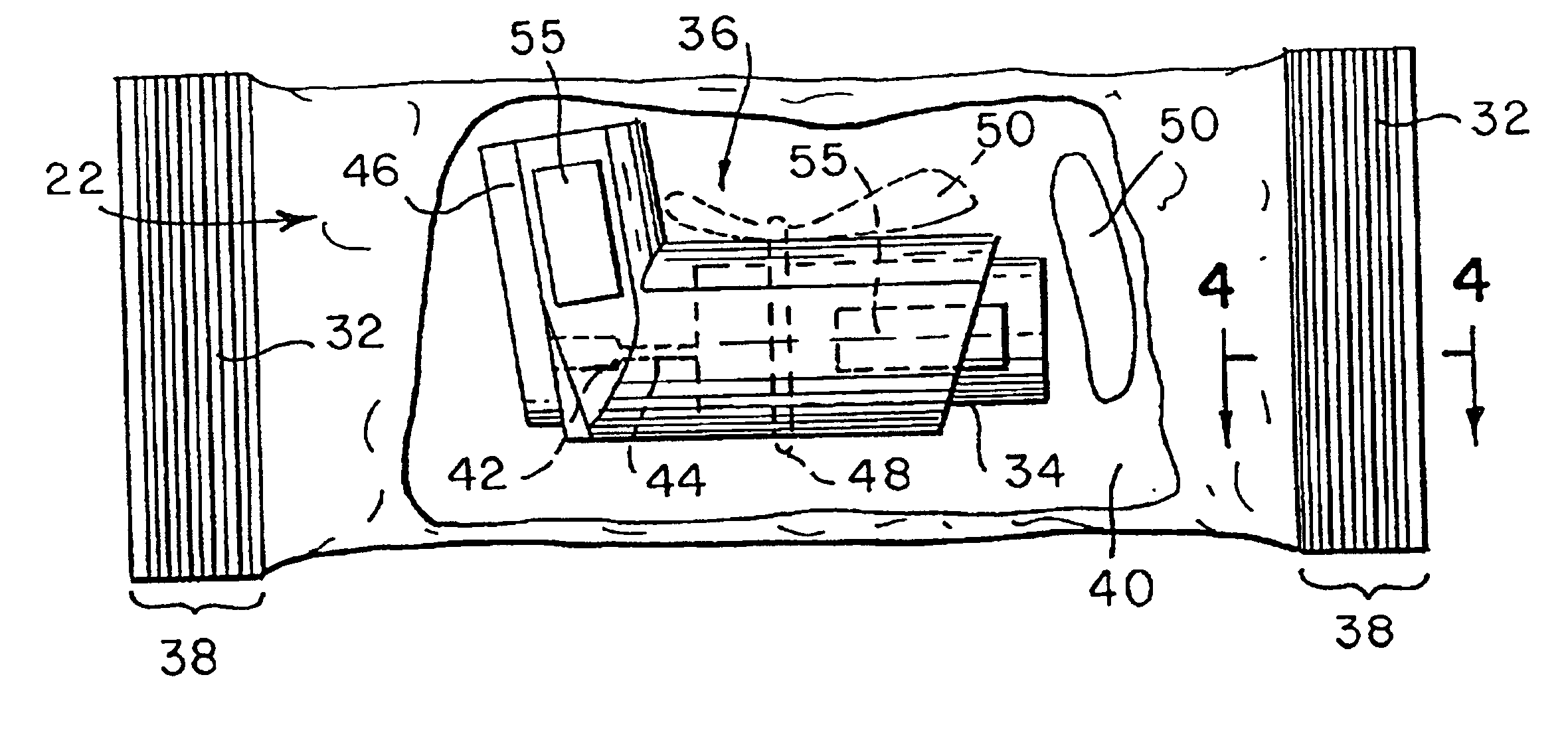
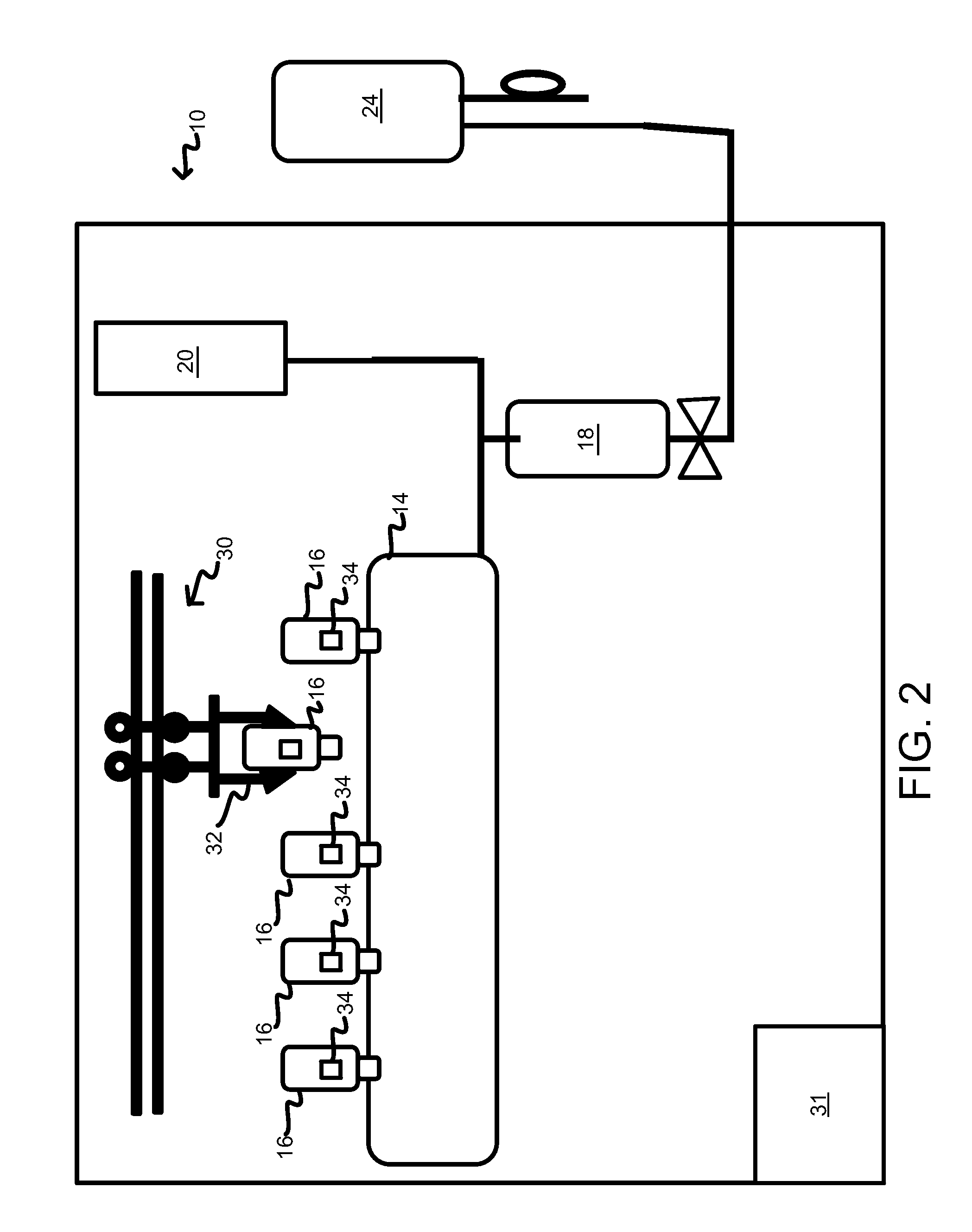
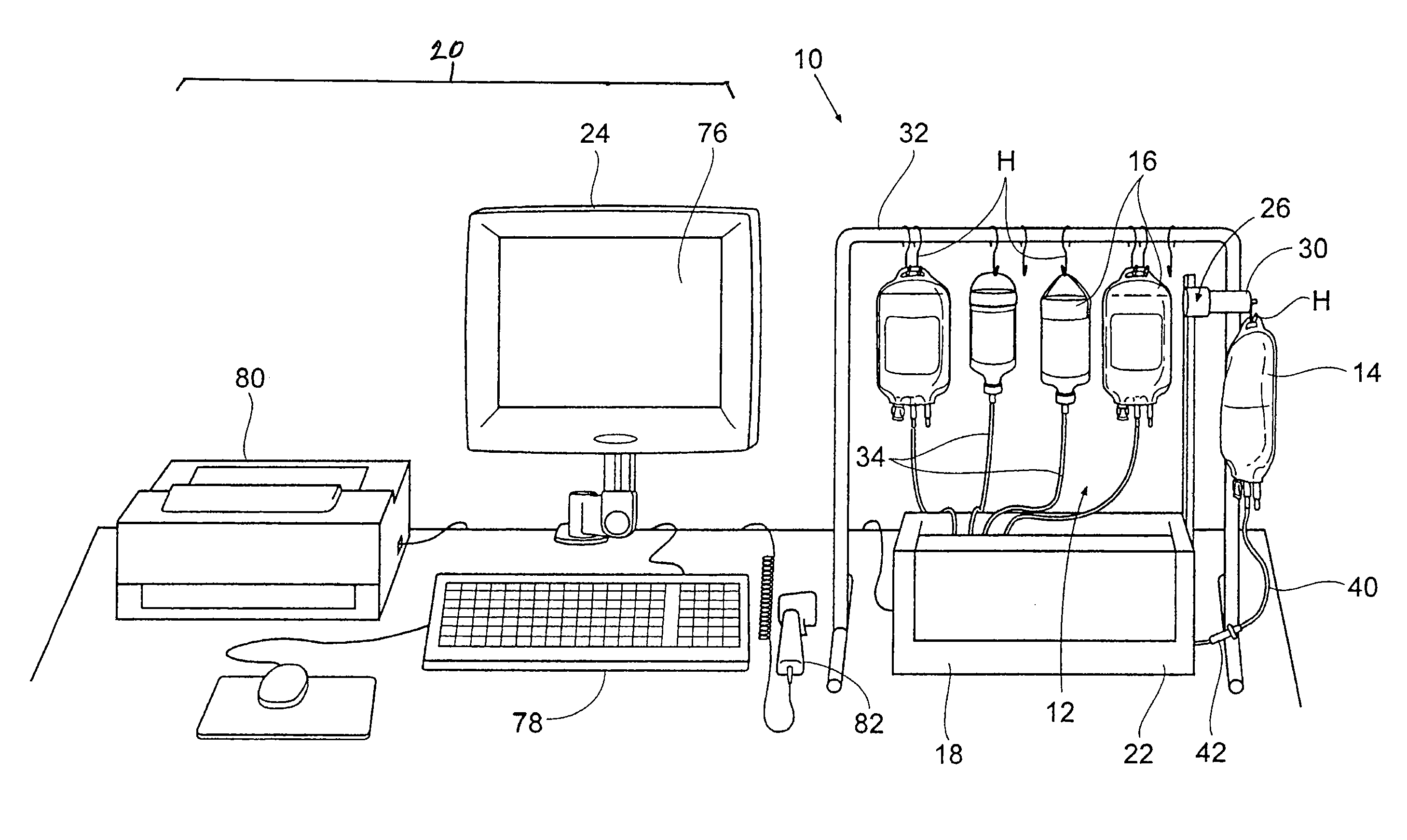
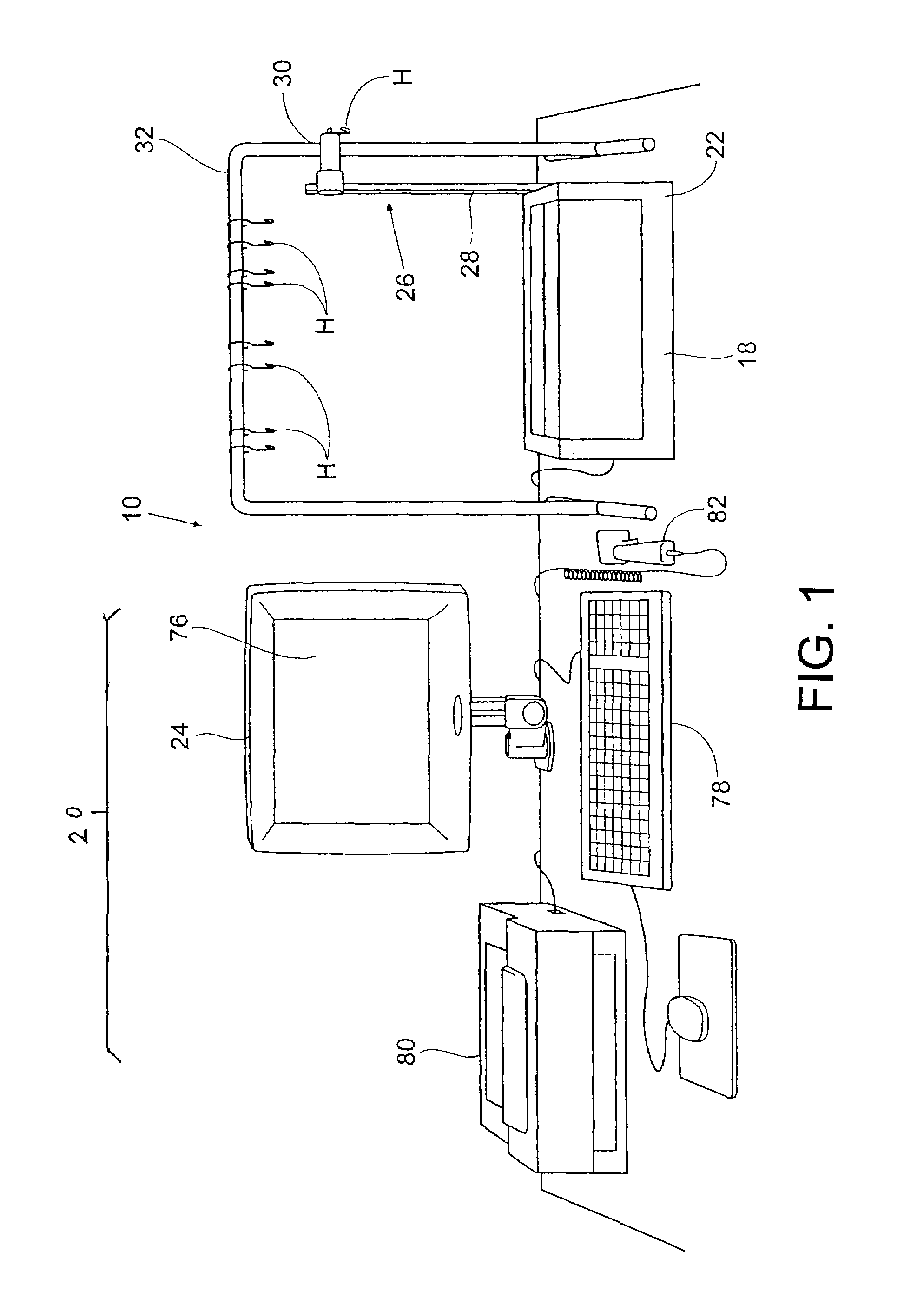
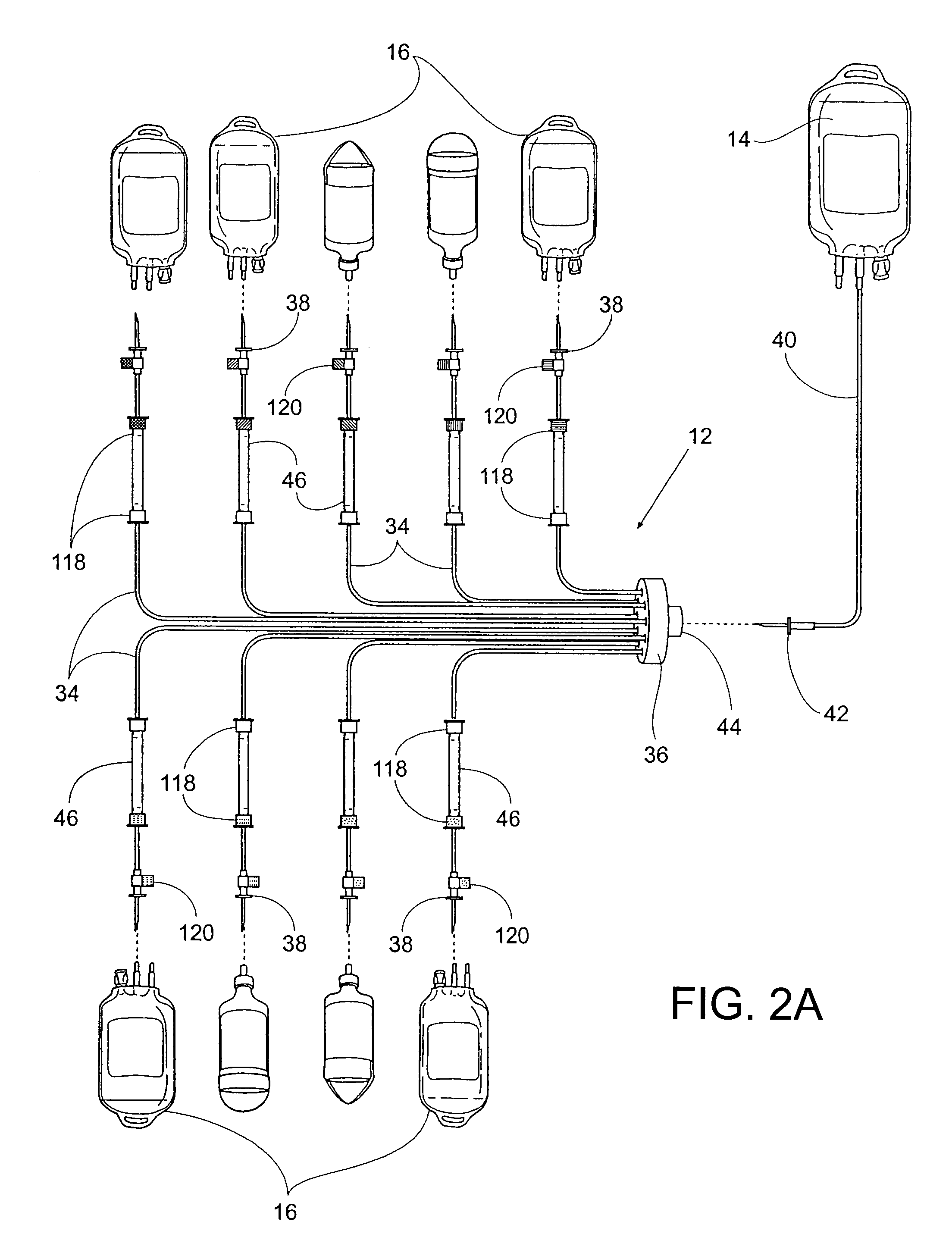
Drug storage and dispensing devices and systems comprising the same
ActiveUS20070186923A1Minimizing saliva influxPowdered material dispensingDrug and medicationsDrug StorageBiomedical engineering
Drug storage and dispensing devices for dispensing a drug dosage form to a patient are disclosed. The dispensing device has a programmable lock-out feature for locking the dispensing device and is capable of detecting the identity of a user. The invention further provides a method for the treatment of subject, by administering to the subject a drug dosage form using a dispensing device of the invention.
Owner:ACEIRX PHARM INC
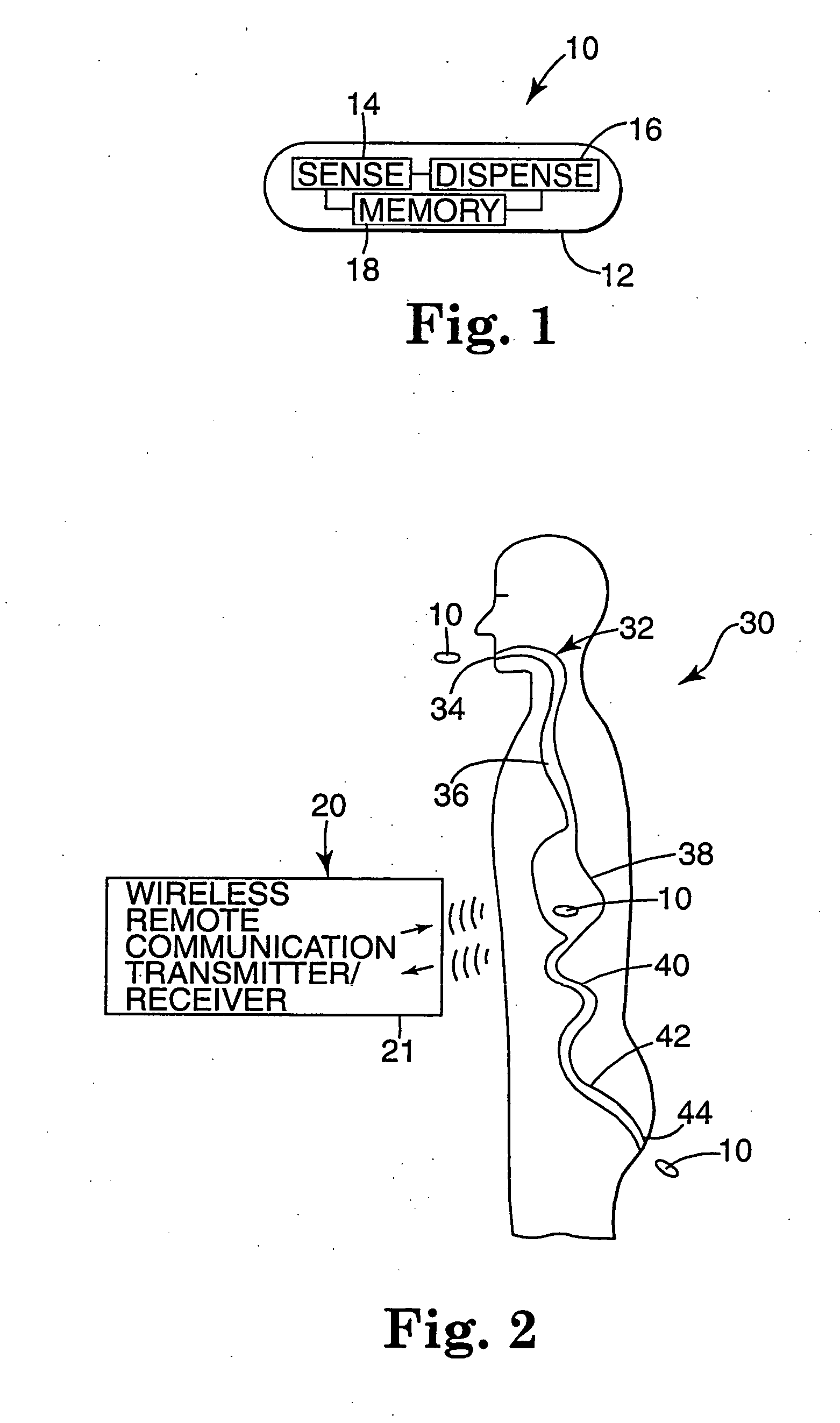
Internal drug dispenser capsule medical device
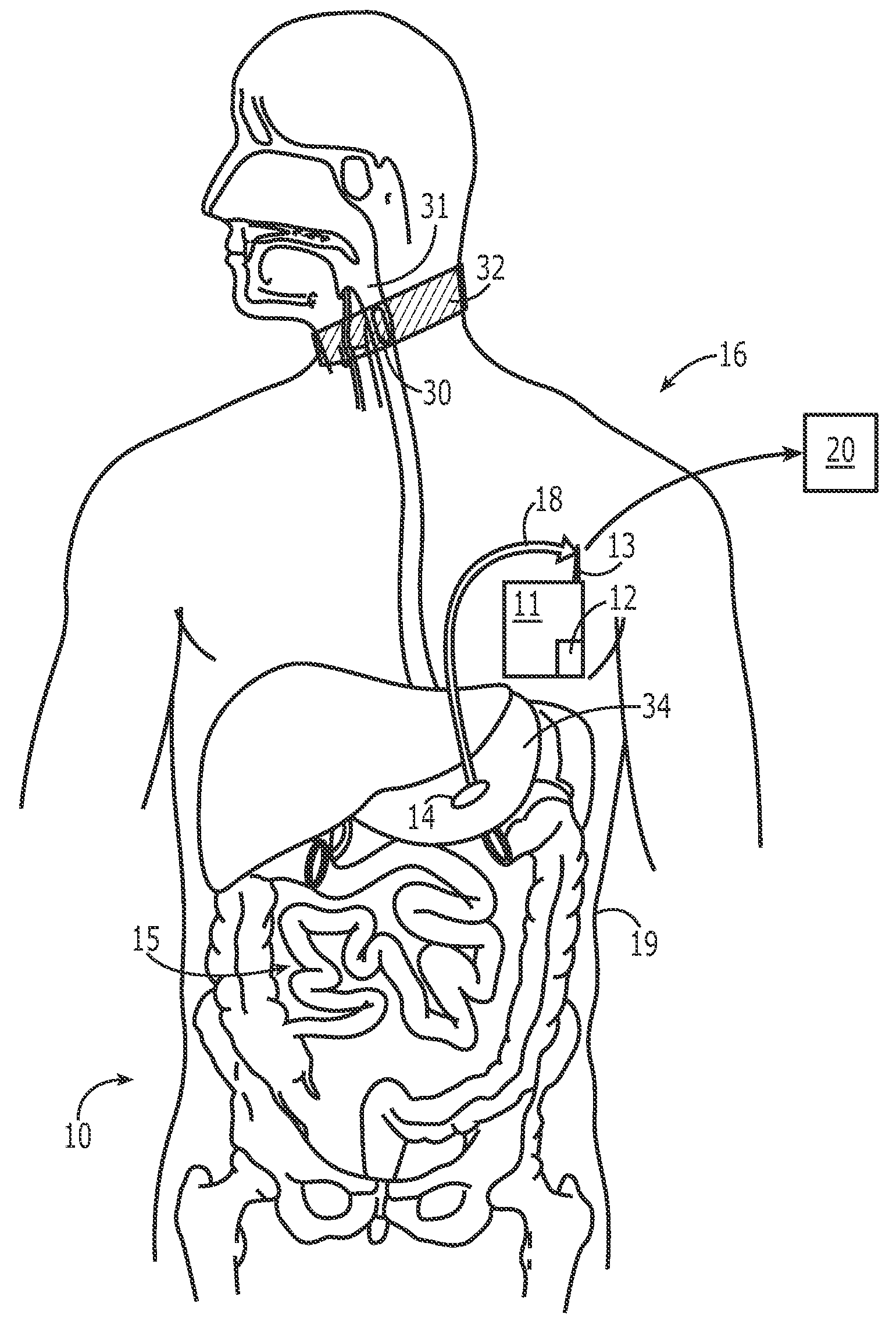
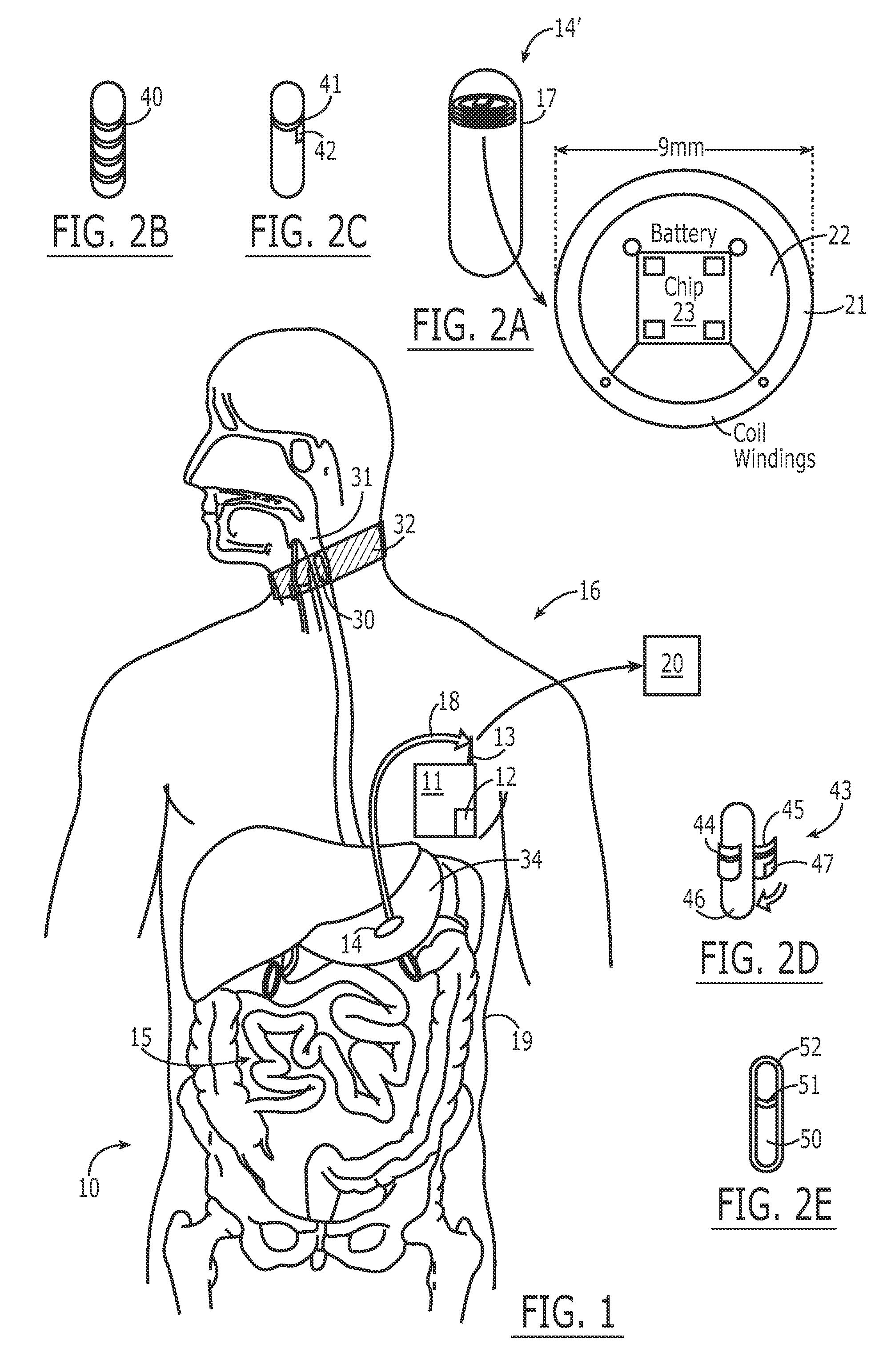
The present invention provides a swallowable internal drug medical device. The device includes a swallowable capsule. A sensing module is disposed in the capsule. A bioactive substance dispenser is disposed in the capsule. A memory and logic component is disposed in the capsule and in communication with the sensing module and the dispenser.
Owner:HEWLETT PACKARD DEV CO LP
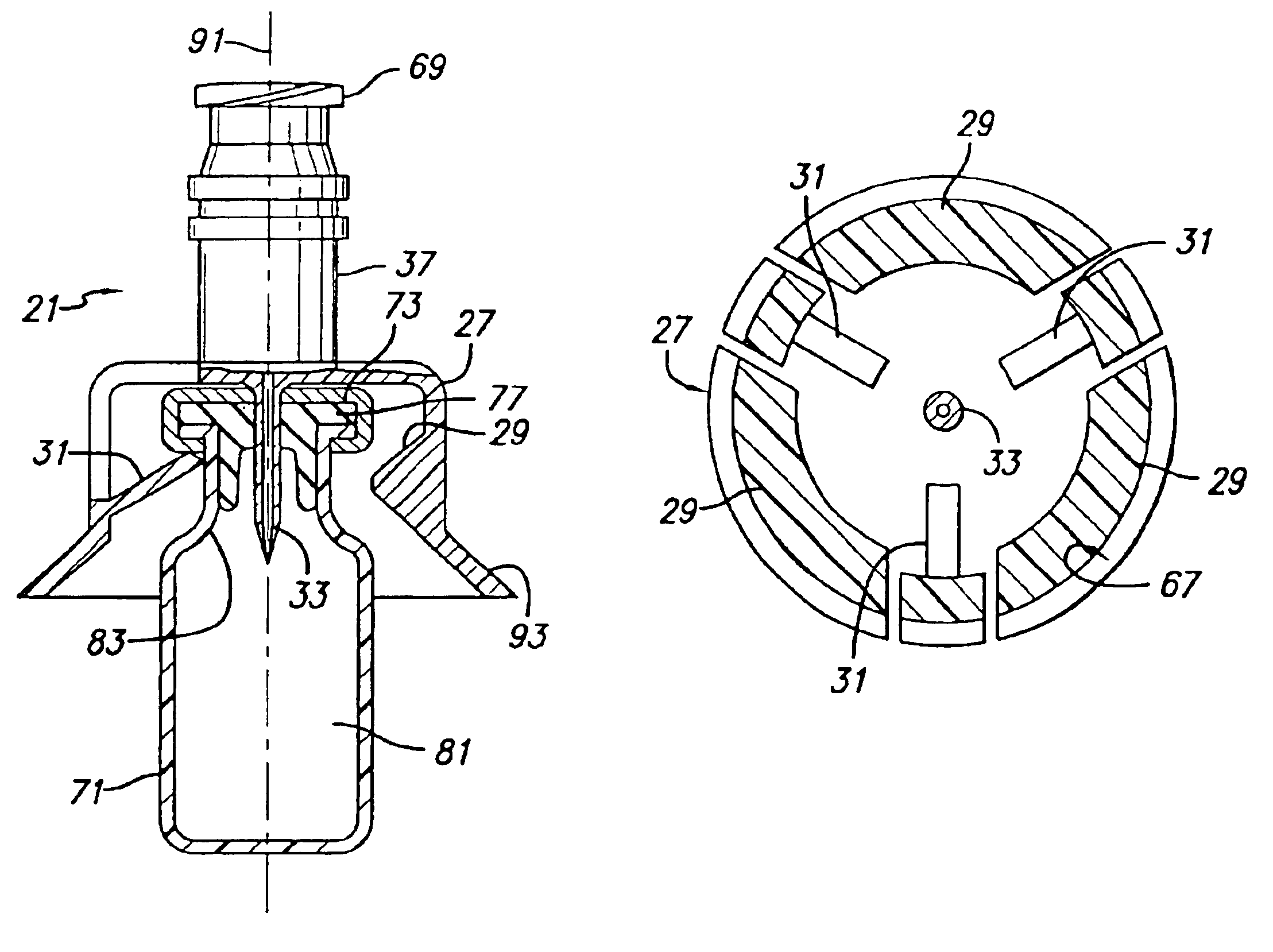
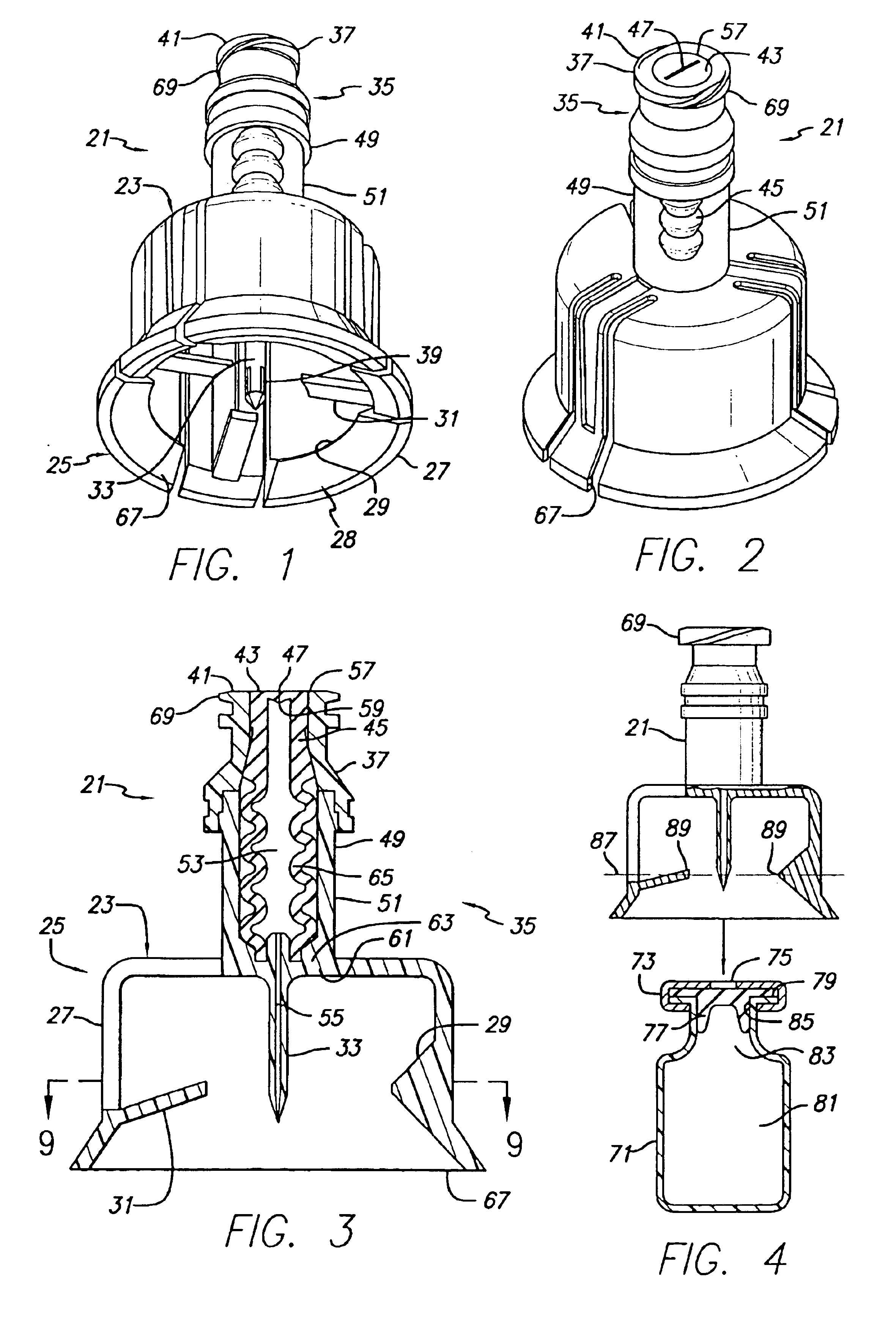
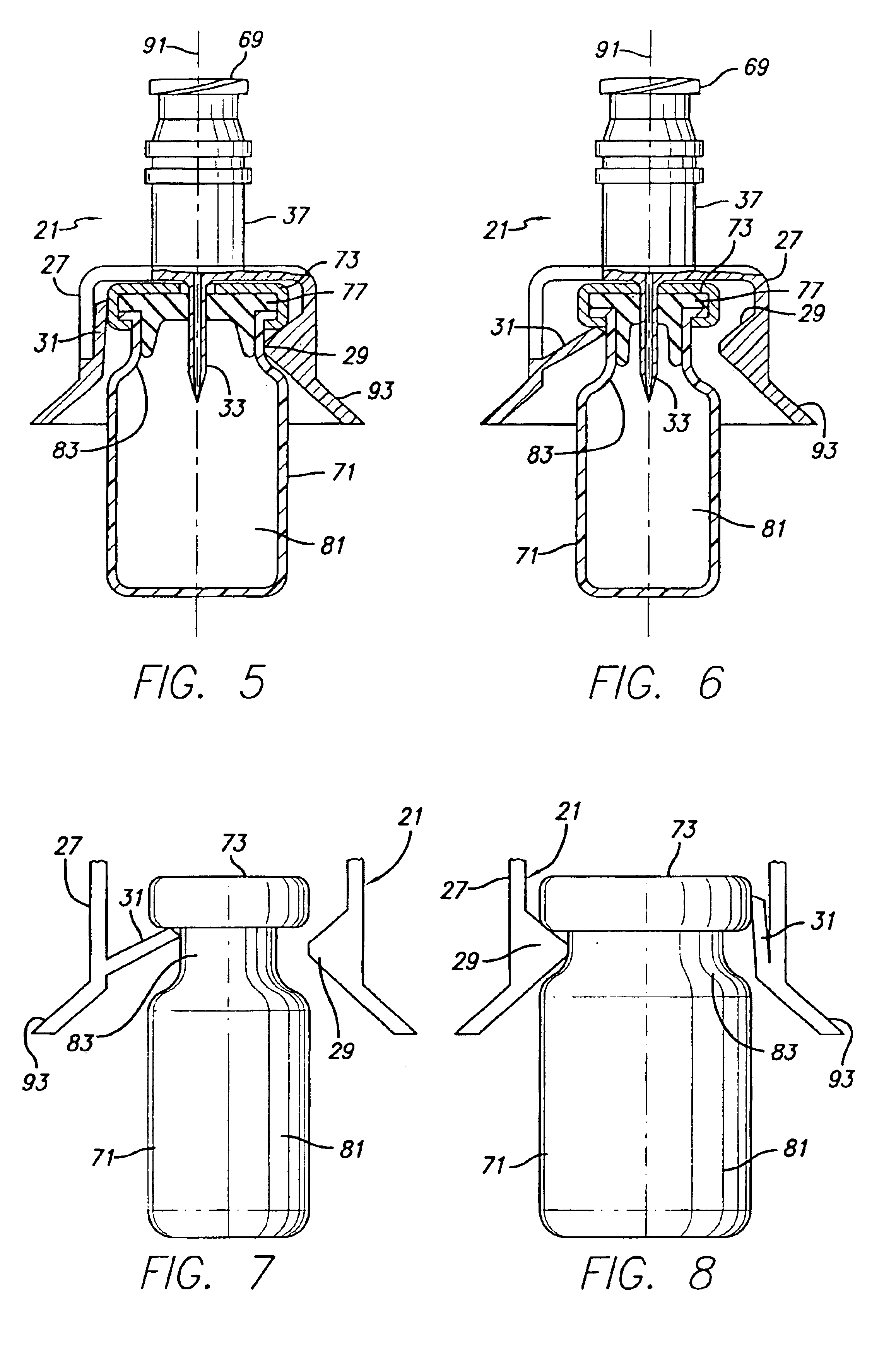
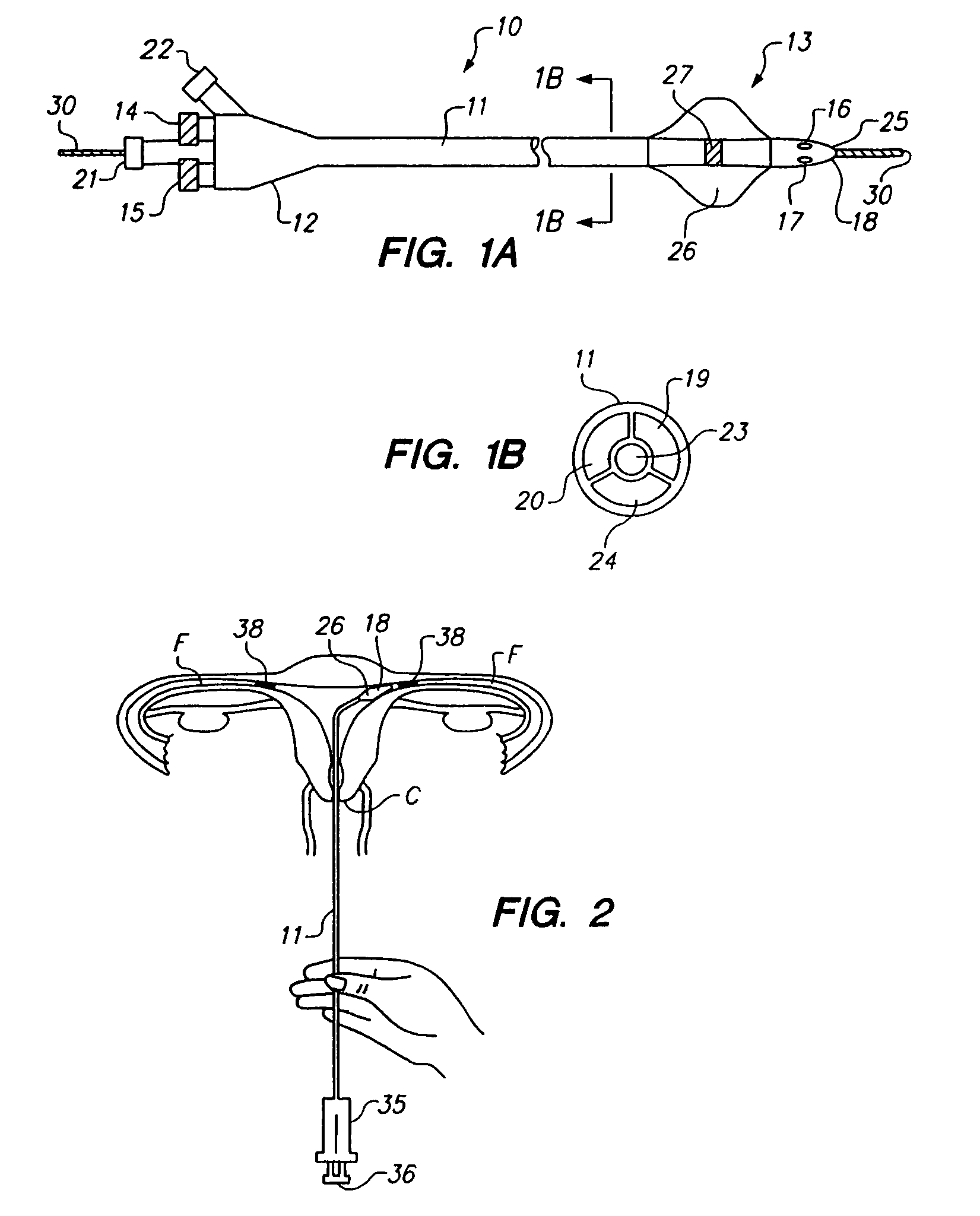
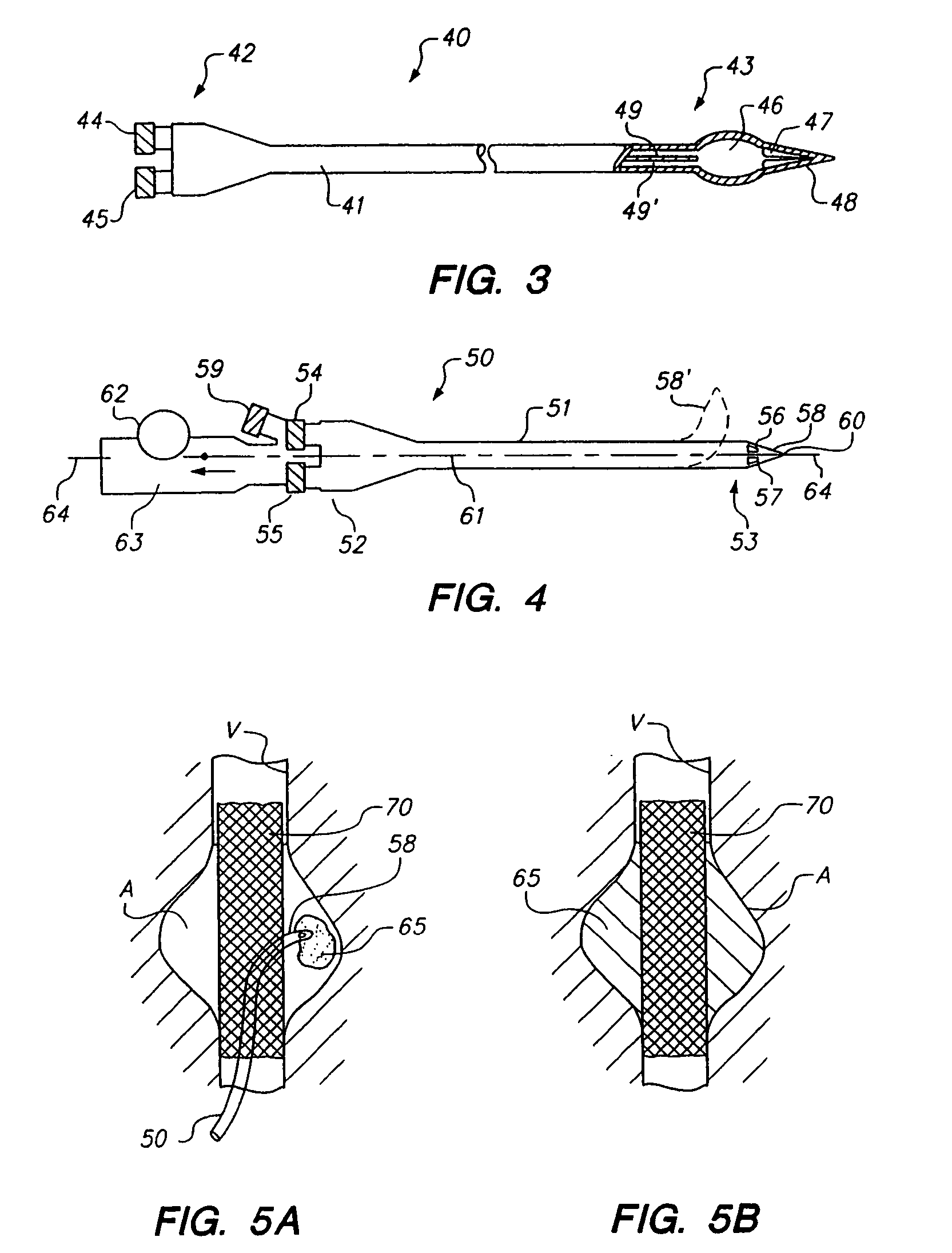
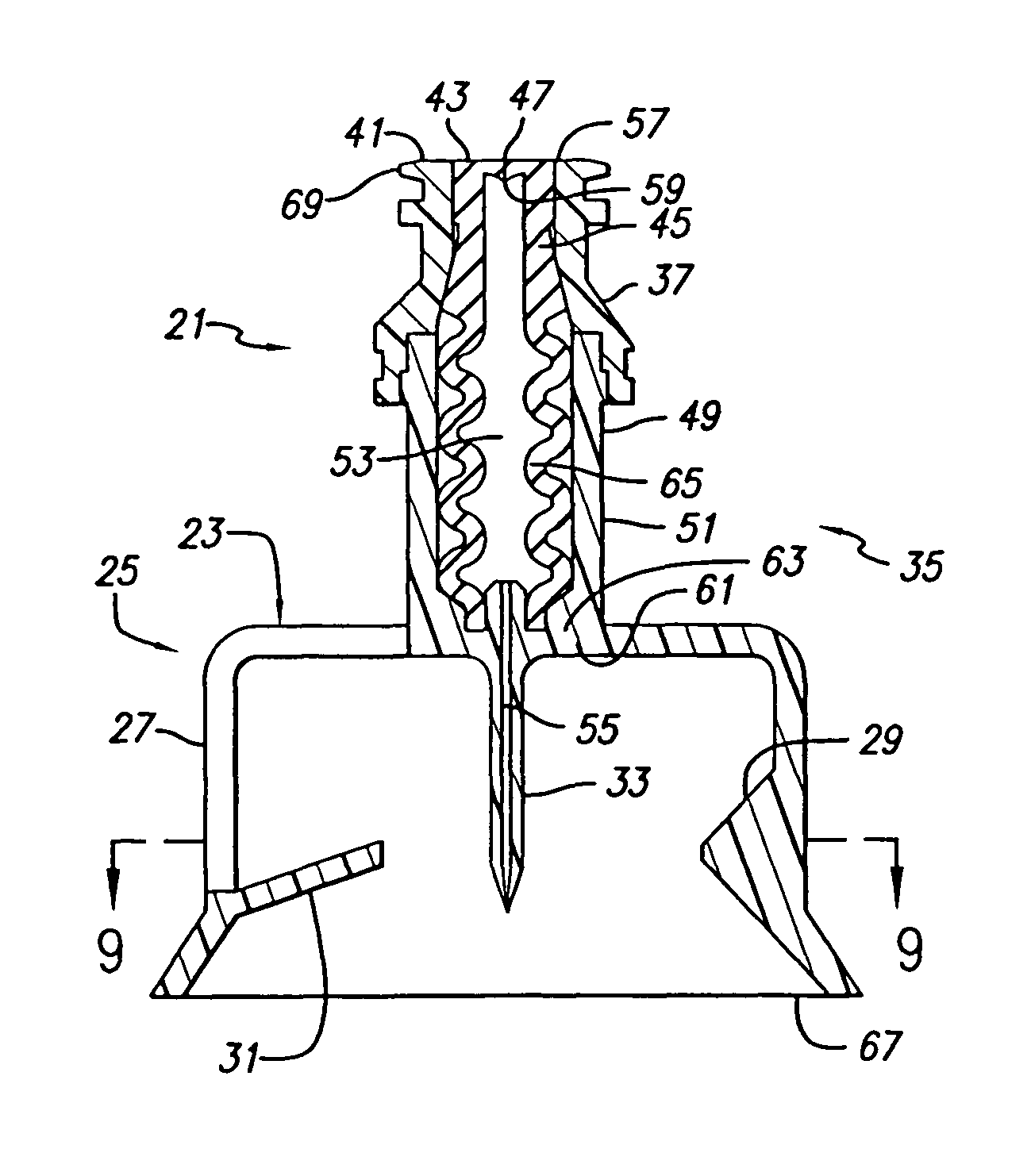
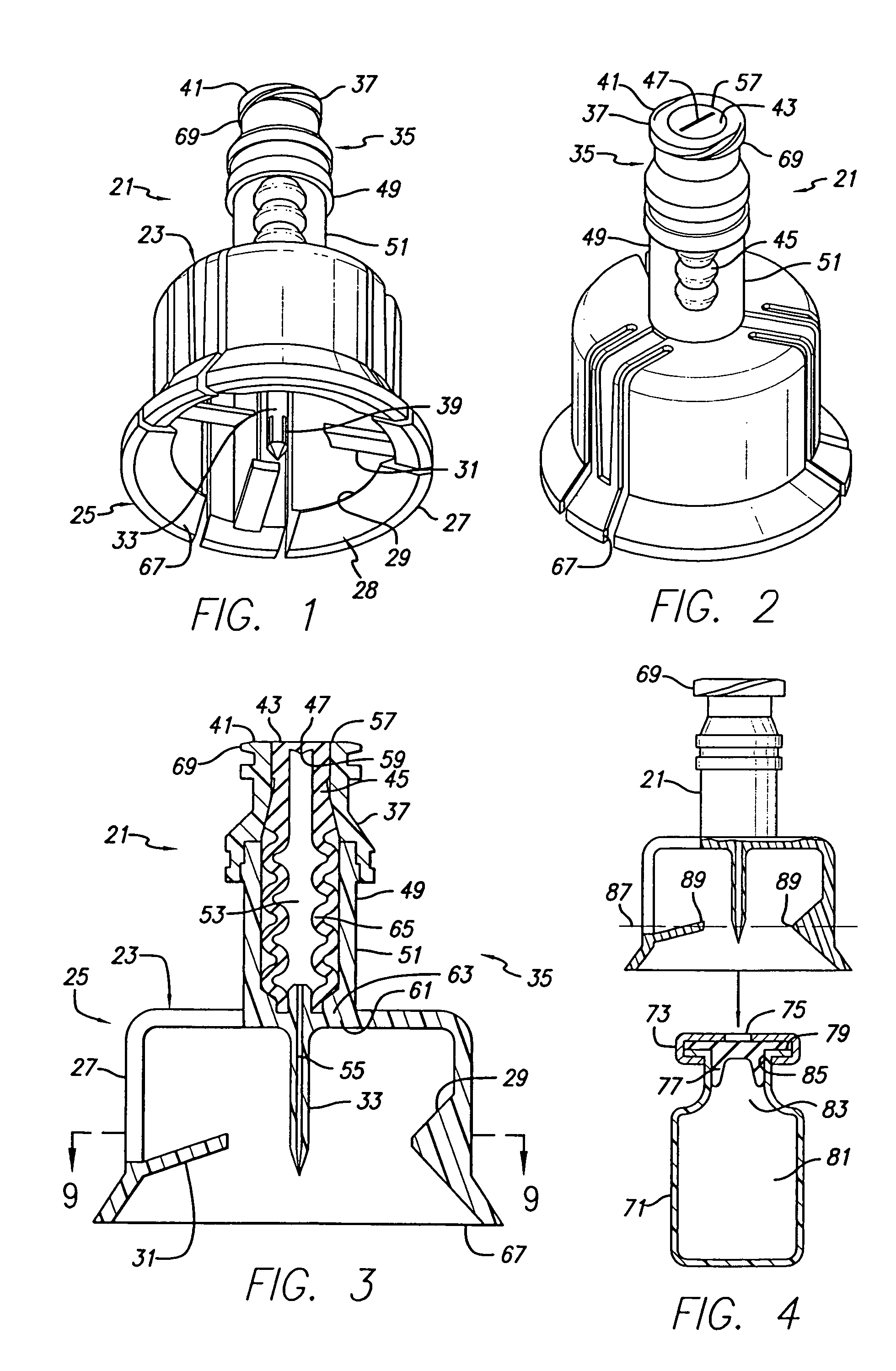
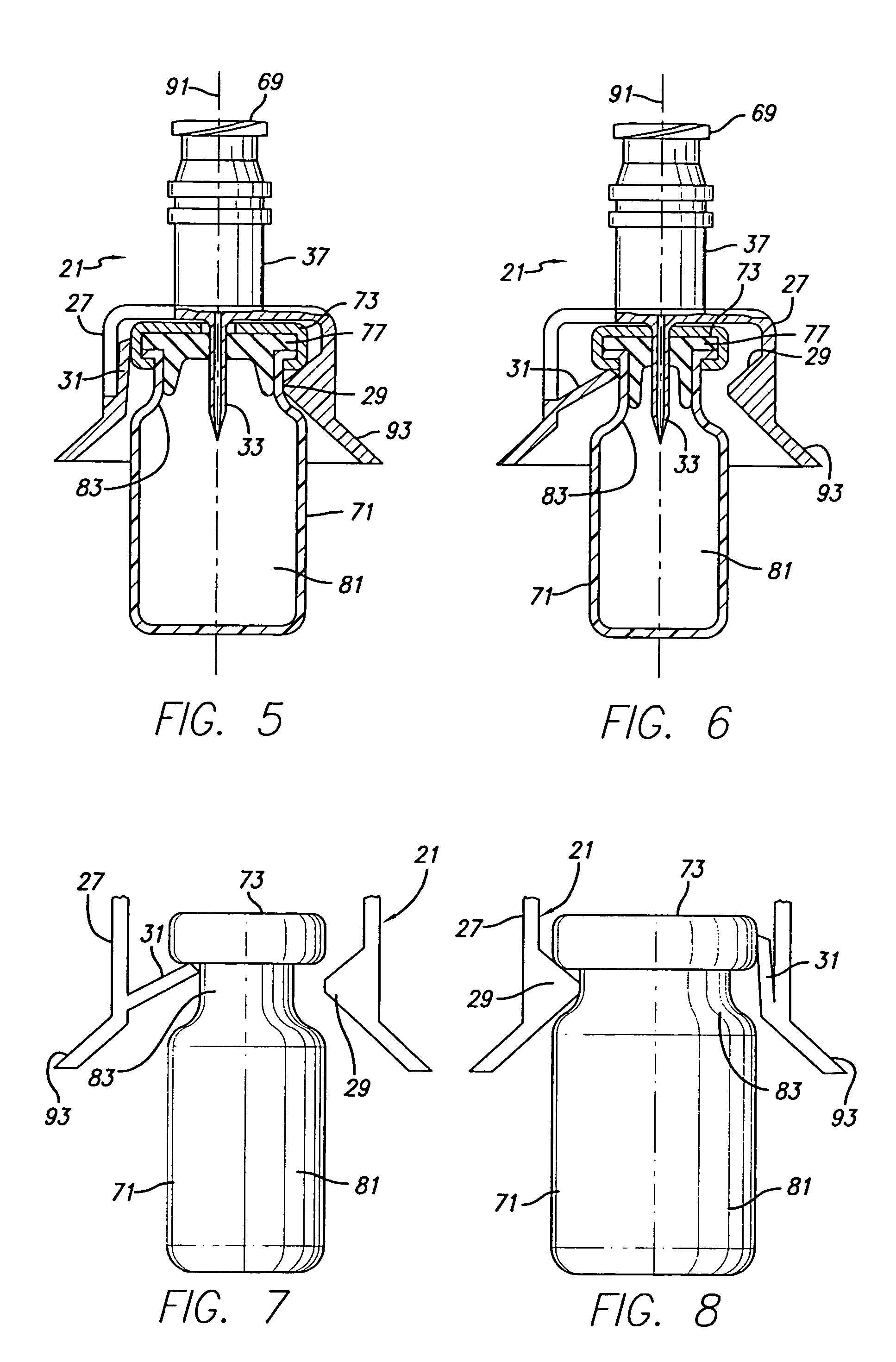
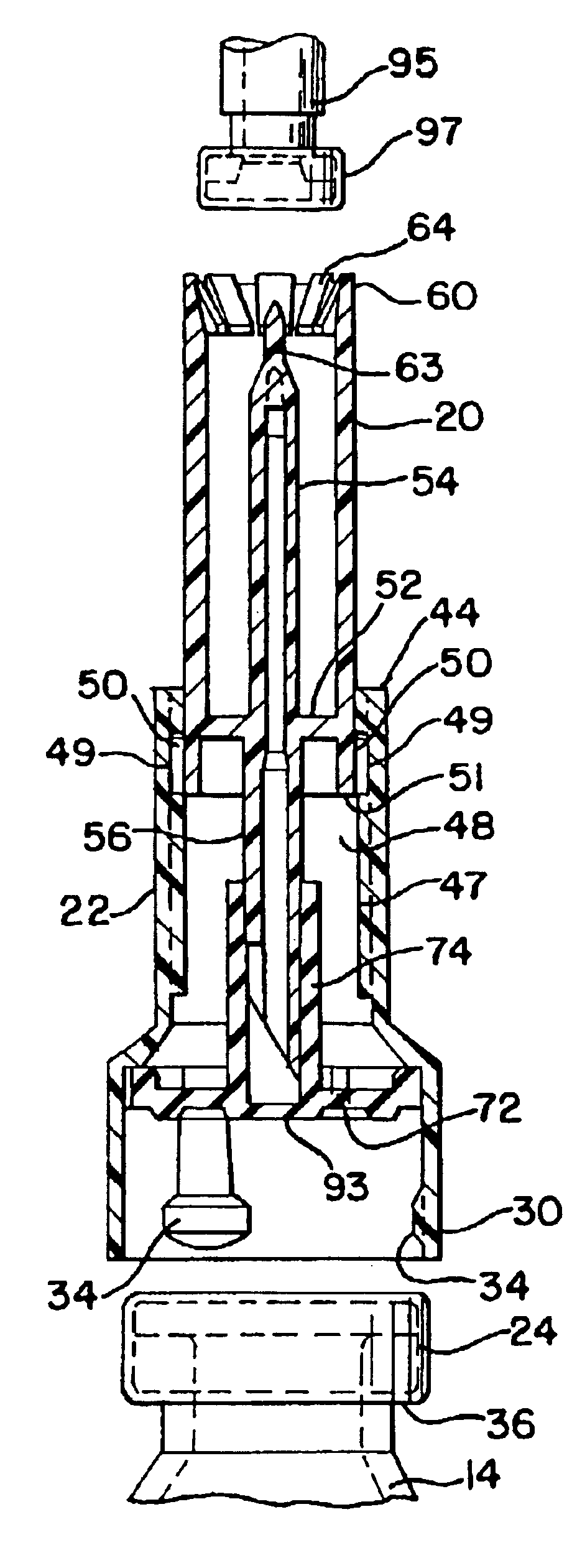
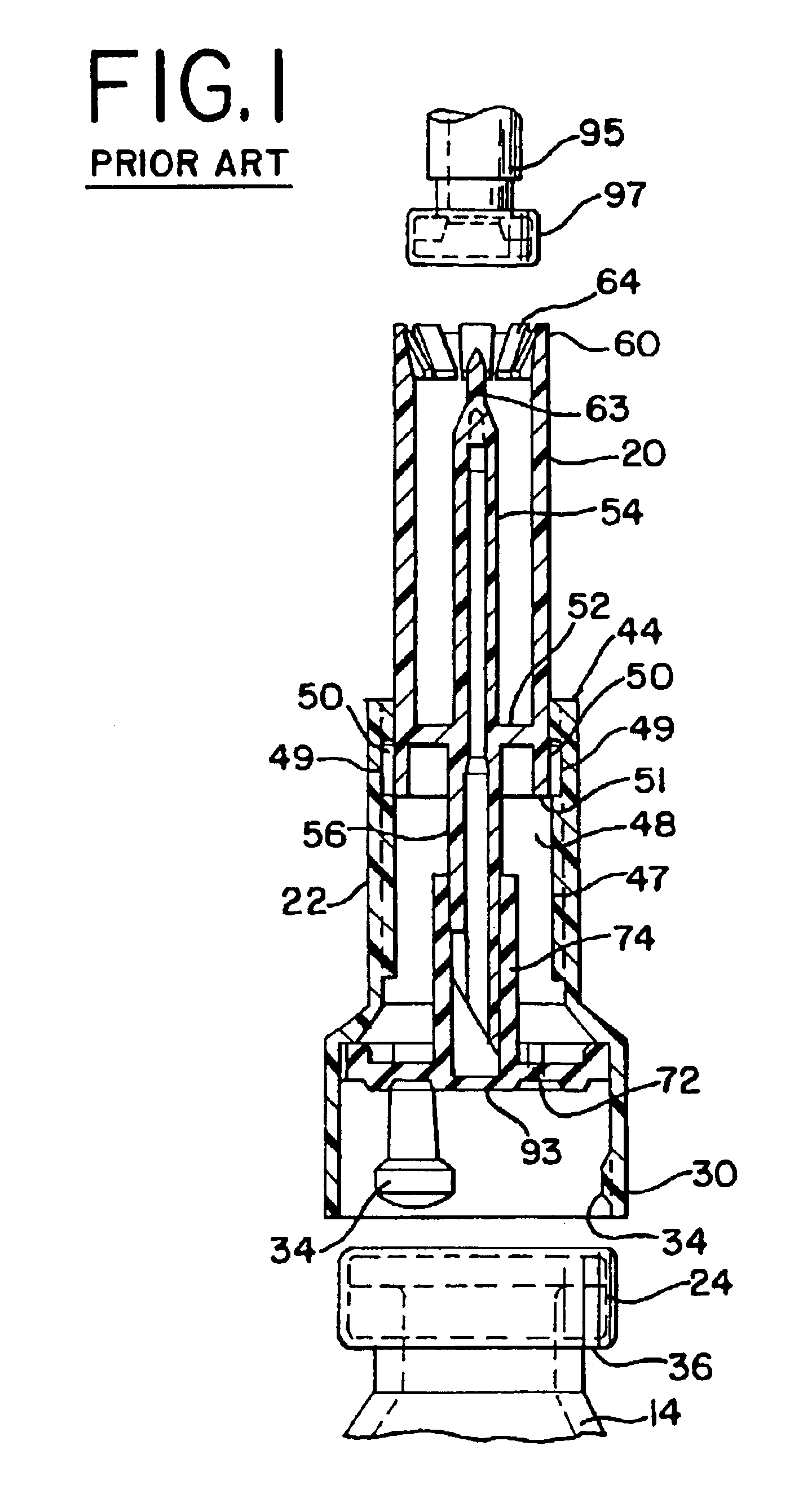
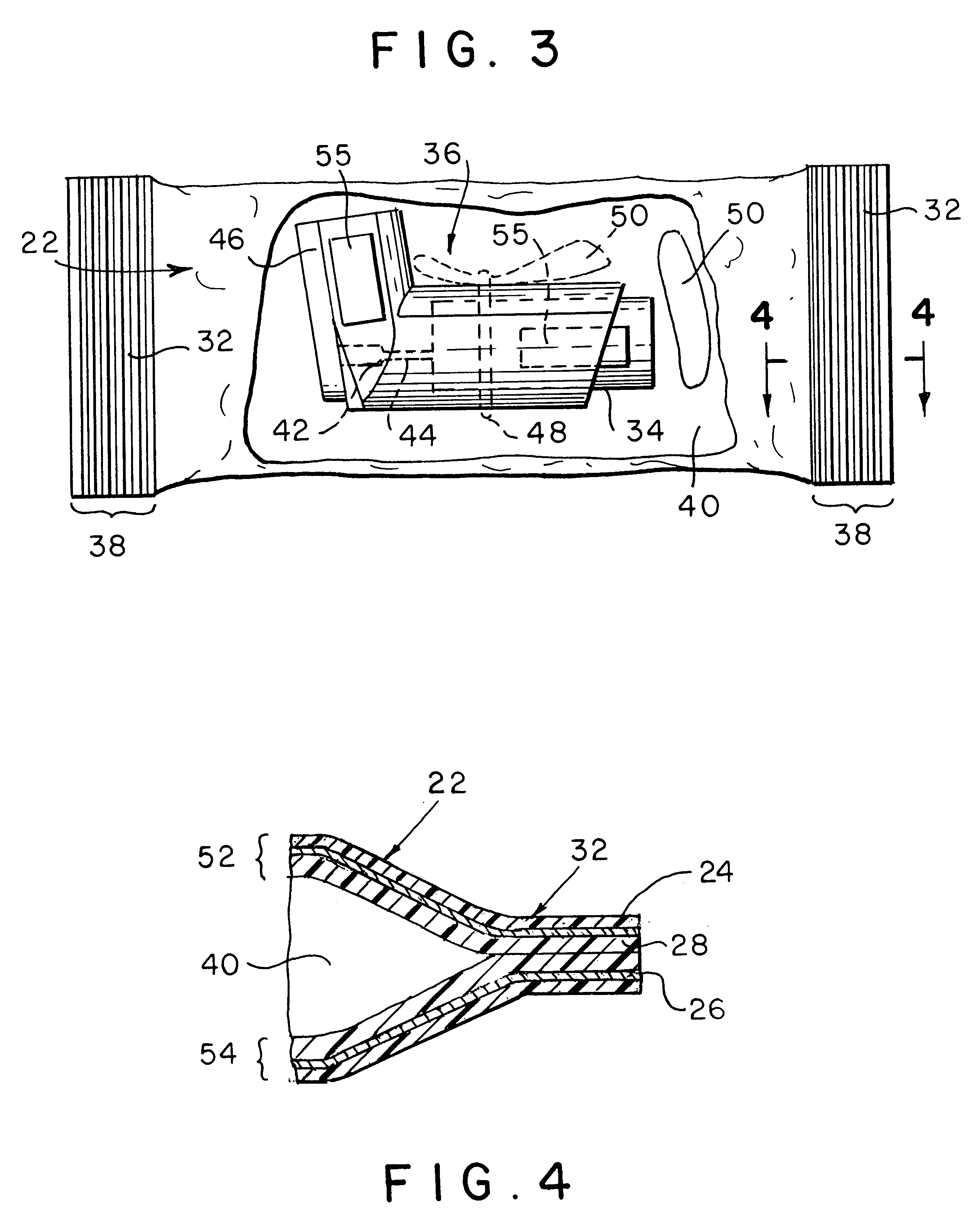
Vial adapter having a needle-free valve for use with vial closures of different sizes
A vial adapter having a needle-free valve, a sharpened cannula used to perforate a vial's rubber stopper, and a circular array of claws of different lengths to engage vial closures of different diameters. The array of claws includes a first set of claws each having a first length extending inwardly from the periphery of the housing of the adapter and a second set of claws alternating with the first set of claws and each having a longer length. The second set of claws are mounted so that they deflect and plastically deform out of the way in the case where the adapter is engaged with a vial that exceeds a predetermined size. The housing includes a shroud that is at least as long as the sharpened cannula to protect medical personnel who use the adapter from inadvertent punctures. The needle-free valve includes a resiliently deformable piston element with a naturally open bore. The interior of the piston provides a fluid flow path through the adapter. In one embodiment, the first set of claws of the adapter may be used with a vial closure of at approximately 20 mm in diameter and the second set of claws may be used with a vial closure of approximately 13 to 17 mm in diameter.
Owner:CAREFUSION 303 INC
Tamper resistant dosage forms
ActiveUS20090081290A1Reduces and prevents stickingBiocidePowder deliveryOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Process for making an ingestible film
The invention relates to film products containing desired levels of active components and methods of their preparation. Desirably, the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film. Desirably, the films may be exposed to temperatures above that at which the active components typically degrade without concern for loss of the desired activity.
Owner:AQUESTIVE THERAPEUTICS INC
Methods and apparatus for dispensing items
InactiveUS6609047B1Credit registering devices actuationDiagnosticsLight-emitting diodeComputer science
The invention provides methods and apparatus for dispensing items from a dispensing unit. According to the invention, the dispensing unit comprises a plurality of locations in which the items are held, a processor in which records corresponding to the items on the unit are stored, and a plurality of item switches corresponding to the locations in which the items are held. The item switches are connected to the processor so that a user of the dispensing unit can input records of items removed from the unit into the processor. The apparatus described is particularly suited for dispensing medical supplies although the apparatus will be usable for other types of items as well. Preferred embodiments will include a plurality of visual indicators, typically in the form of light emitting diodes, corresponding to the locations in which the items are held. Upon selection of a desired item from a list of items held by the unit, the visual indicator corresponding to the item is actuated so that the user can locate the desired item quickly and conveniently with the help of the visual indicator.
Owner:OMNICELL
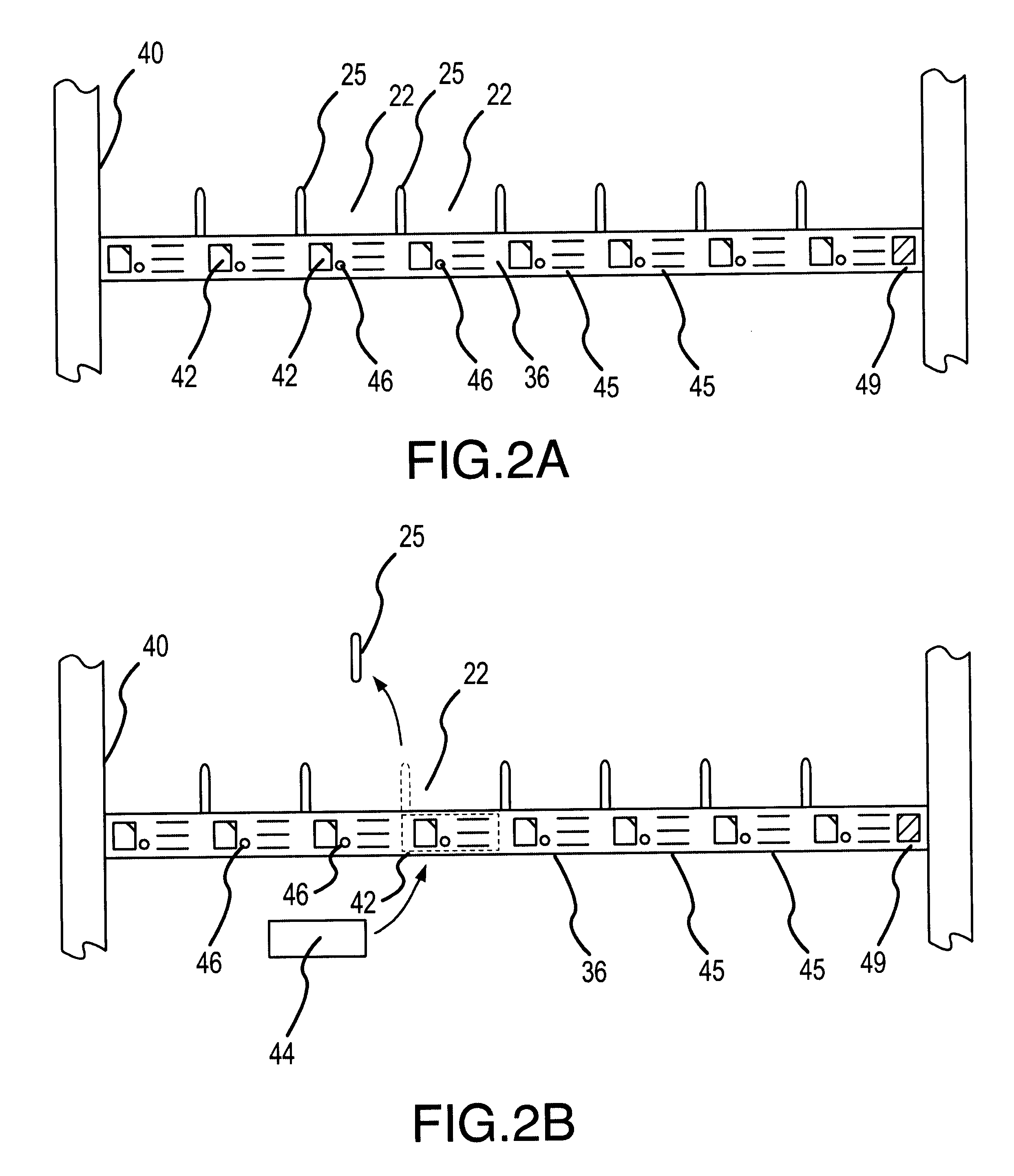
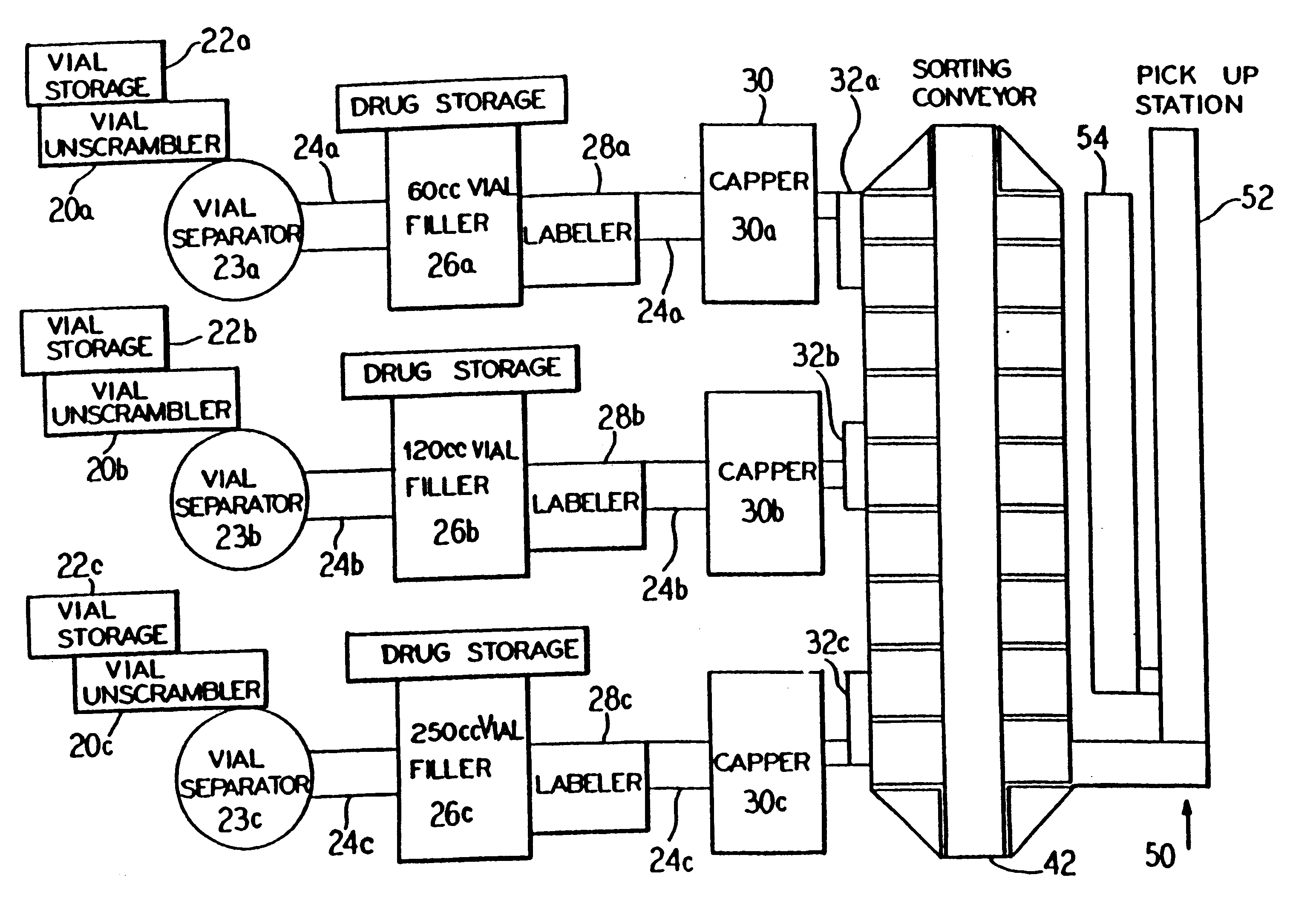
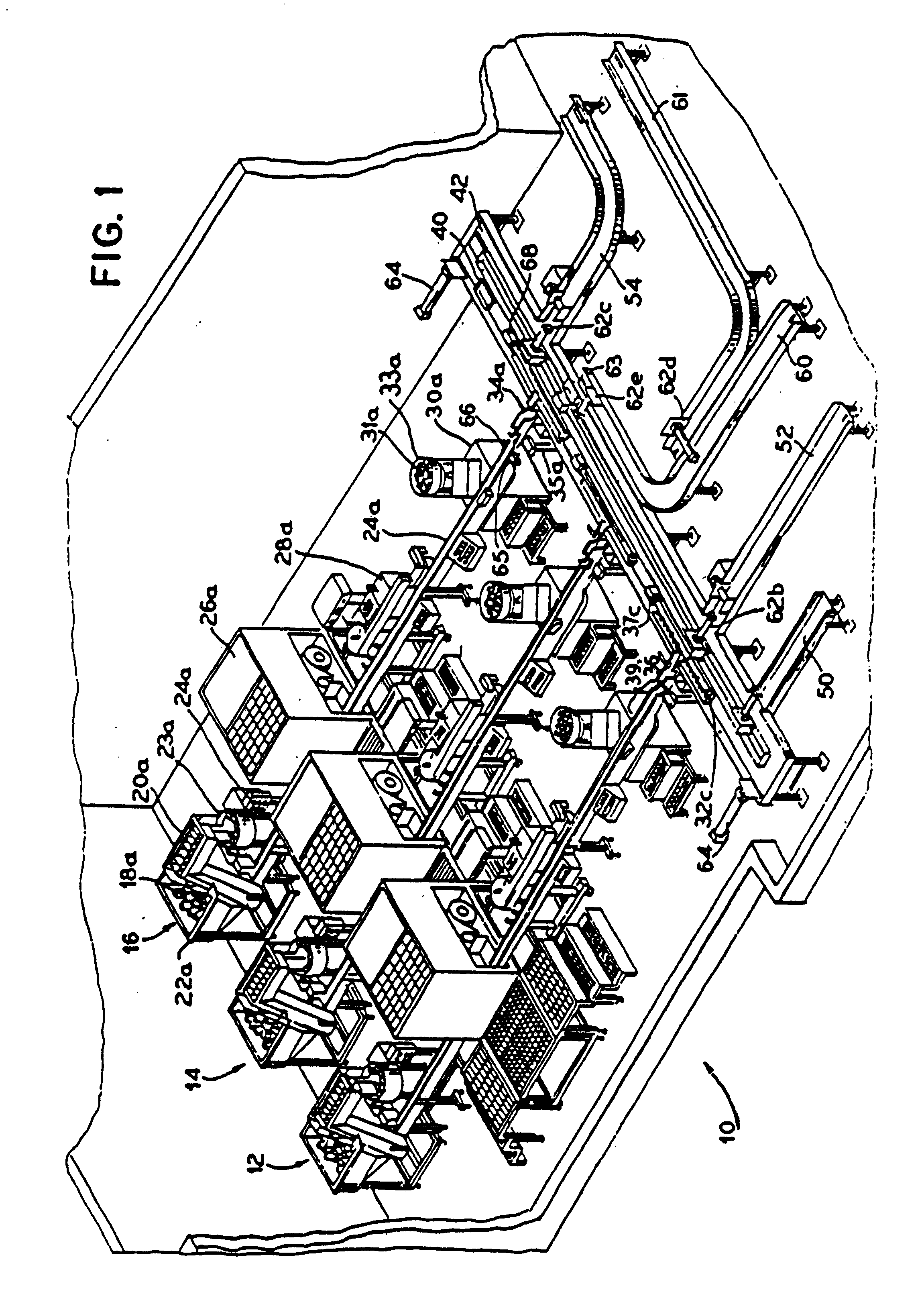
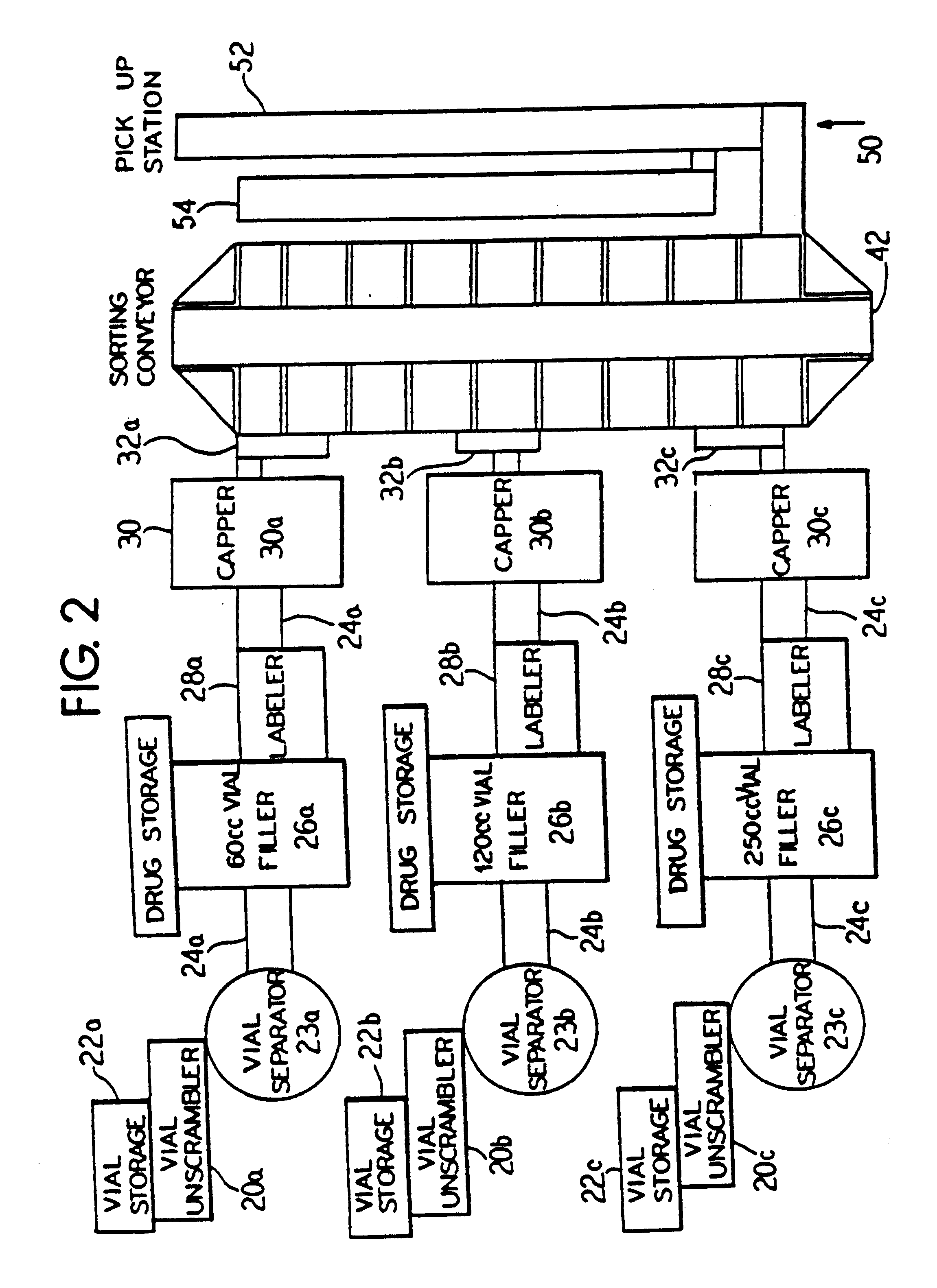
Automated prescription vial filling system
InactiveUSRE37829E1Reduction and inventoryLow costDrug and medicationsDigital data processing detailsMedical prescriptionBiomedical engineering
A method and apparatus for dispensing drugs, wherein a patient's order of one or more prescriptions is automatically filled. Various drugs are stored in three or more filler lines. A vial size is assigned to each line. When a prescription is filled, it is automatically assigned to a line in view of the vial size requirements and processed accordingly. Provisions are made for the inability to fill a prescription or order. Subsequently, all of the patient's prescriptions are collected and made available as a single order.
Owner:AUTOMED TECH
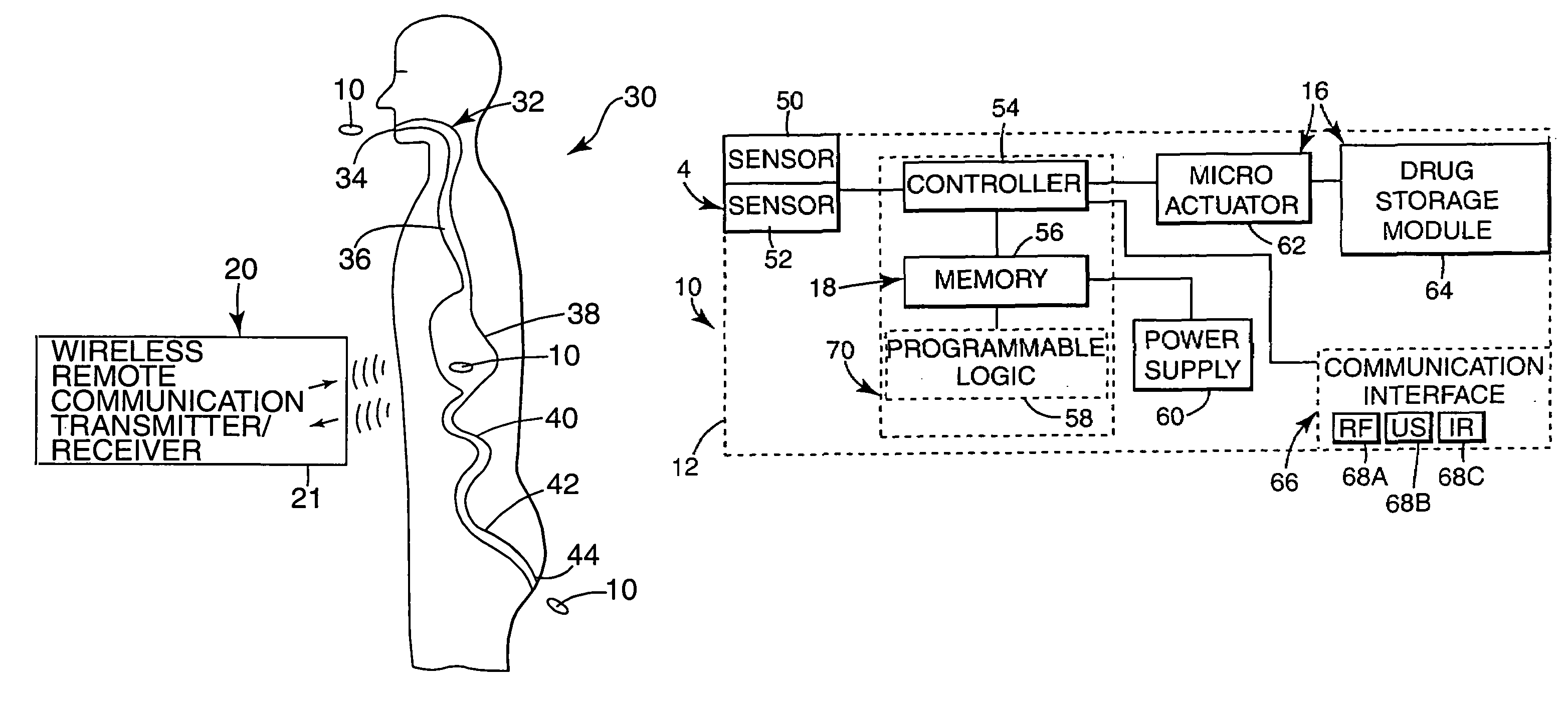
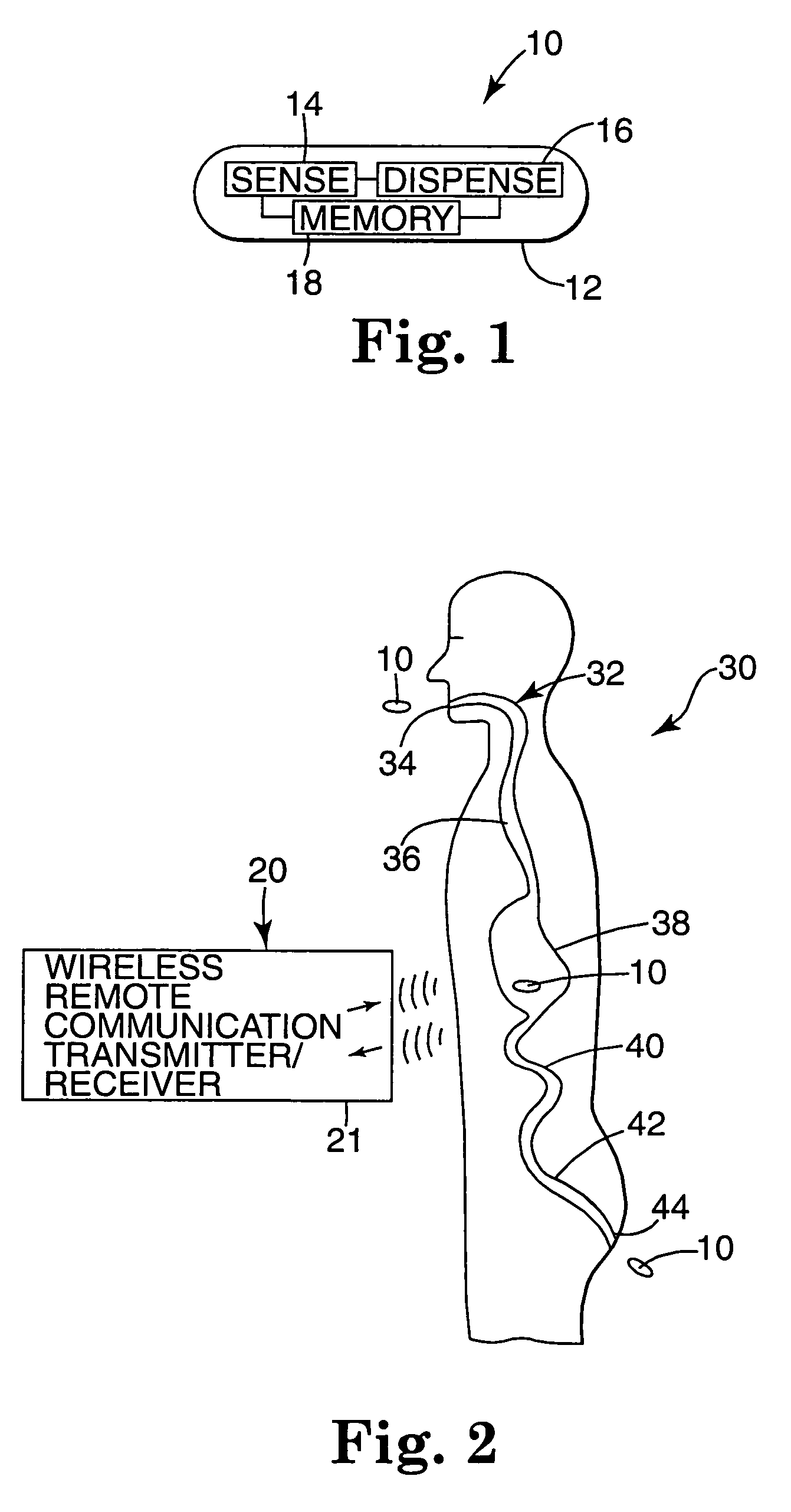
Medication compliance system and associated methods
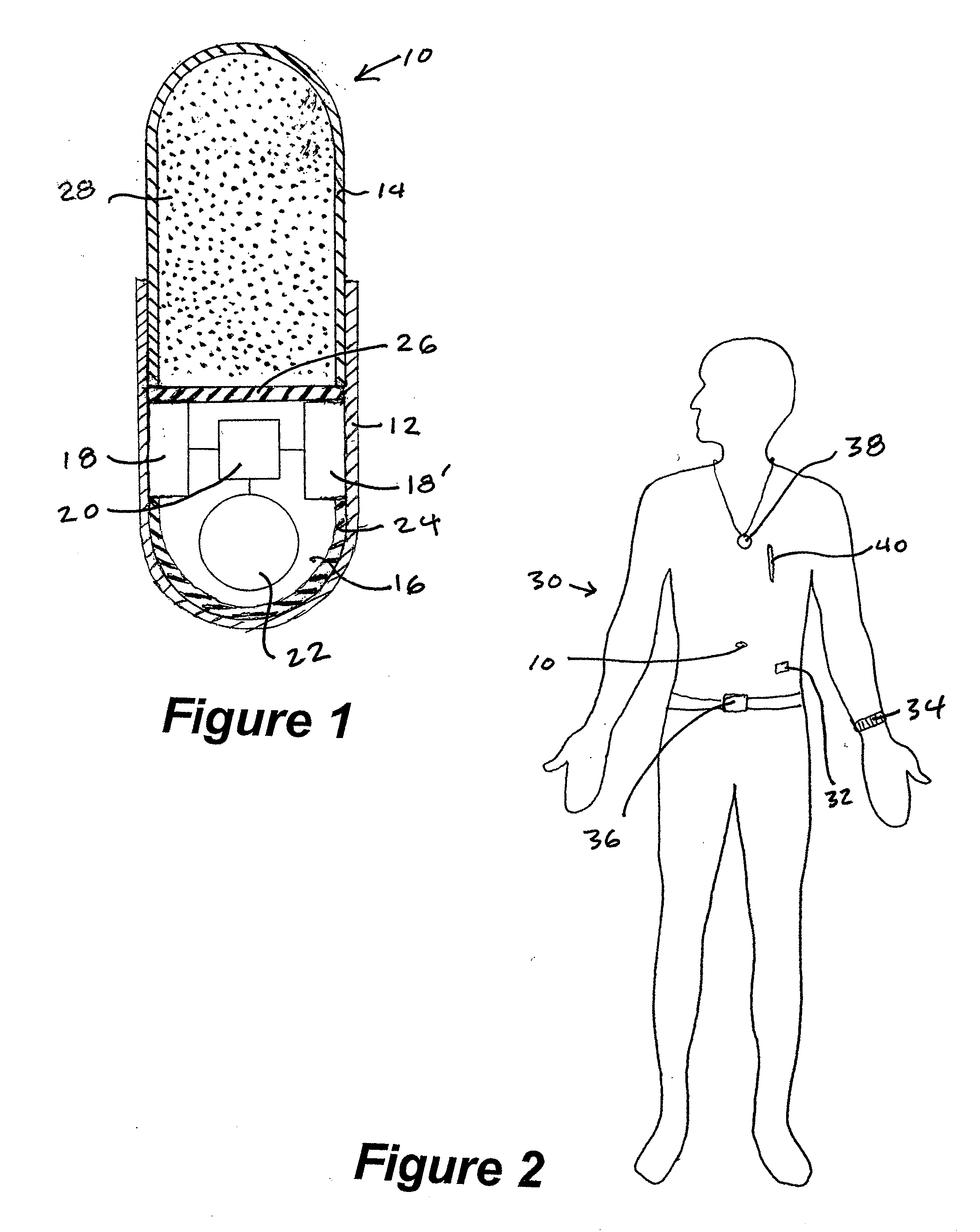
ActiveUS20070123772A1Reduce areaLessDrug and medicationsMedical devicesGastrointestinal tractDrug delivery
A system for monitoring medication compliance in a patient includes an electronic pill that includes a drug-transporting device and an antenna positioned on a surface of the drug-transporting device. A detector is positionable external a gastrointestinal tract of a patient for detecting a presence of the antenna in the patient gastrointestinal tract.
Owner:ETECTRX INC
Methods and apparatus for dispensing items
InactiveUS6272394B1Credit registering devices actuationDiagnosticsLight-emitting diodeMedical treatment
The invention provides methods and apparatus for dispensing items from a dispensing unit. According to the invention, the dispensing unit comprises a plurality of locations in which the items are held, a processor in which records corresponding to the items on the unit are stored, and a plurality of item switches corresponding to the locations in which the items are held. The item switches are connected to the processor so that a user of the dispensing unit can input records of items removed from the unit into the processor. The apparatus described is particularly suited for dispensing medical supplies although the apparatus will be usable for other types of items as well. Preferred embodiments will include a plurality of visual indicators, typically in the form of light emitting diodes, corresponding to the locations in which the items are held. Upon selection of a desired item from a list of items held by the unit, the visual indicator corresponding to the item is actuated so that the user can locate the desired item quickly and conveniently with the help of the visual indicator.
Owner:COMDISCO
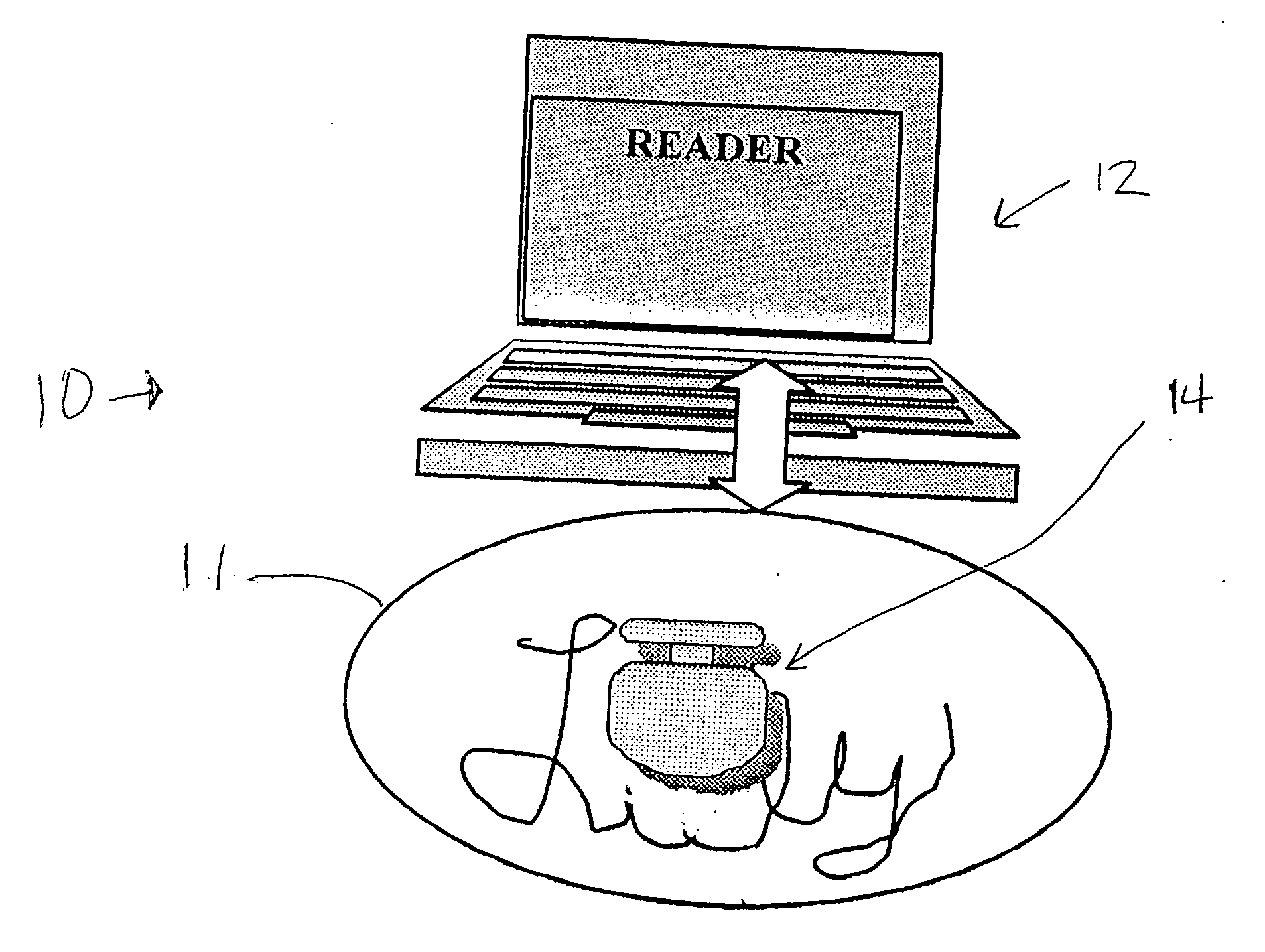
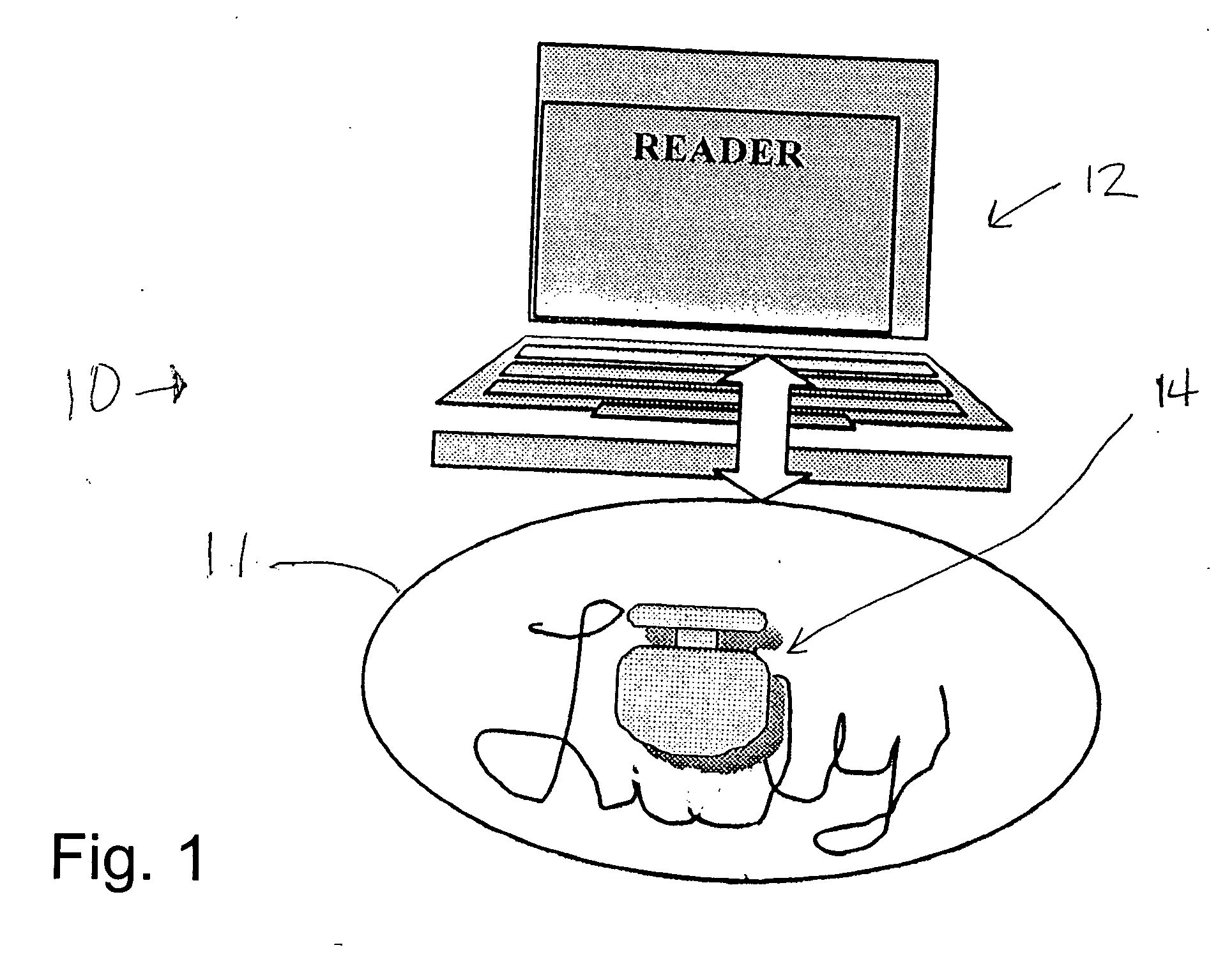
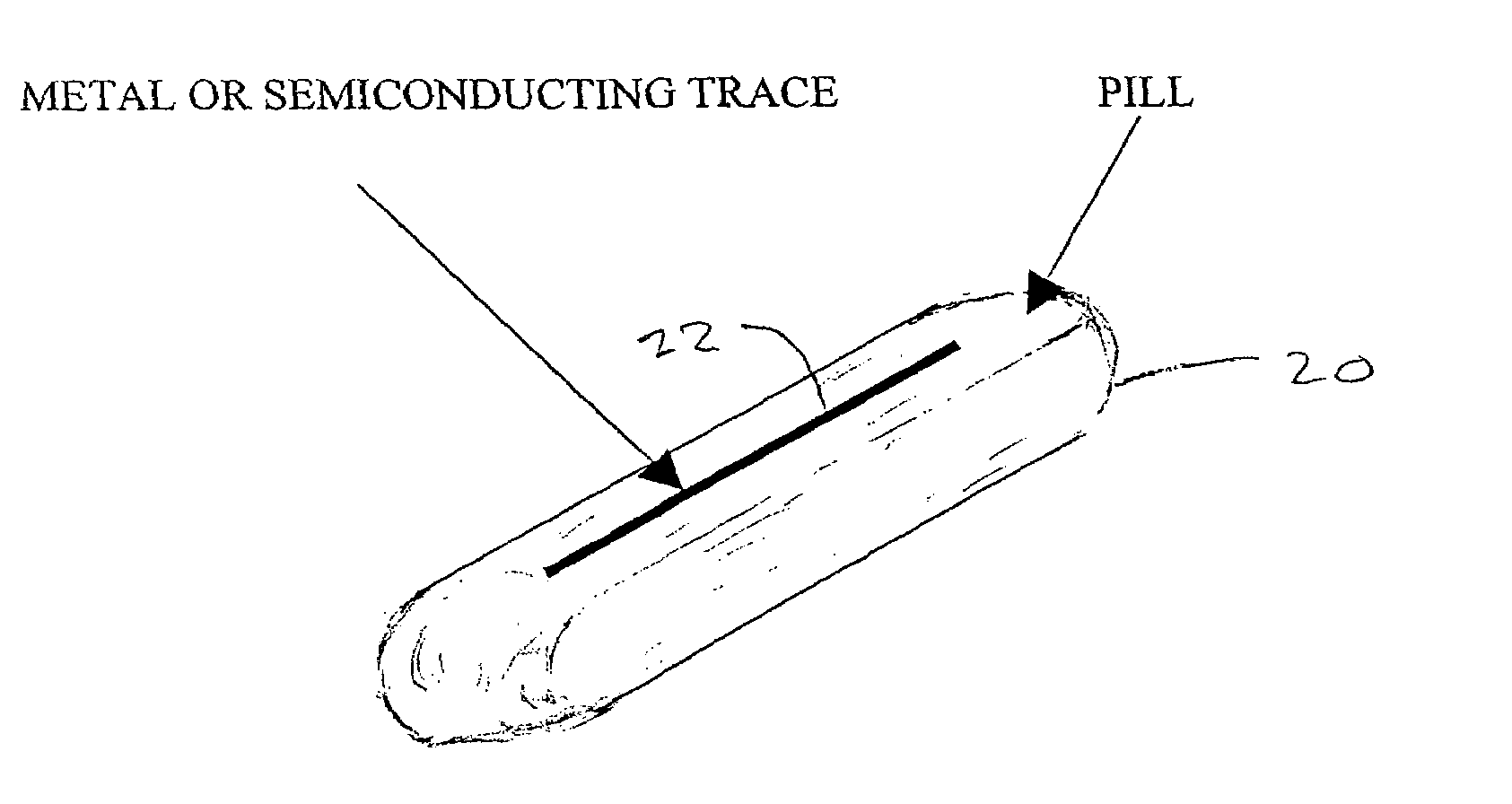
Trackable pills with electronic ID tags
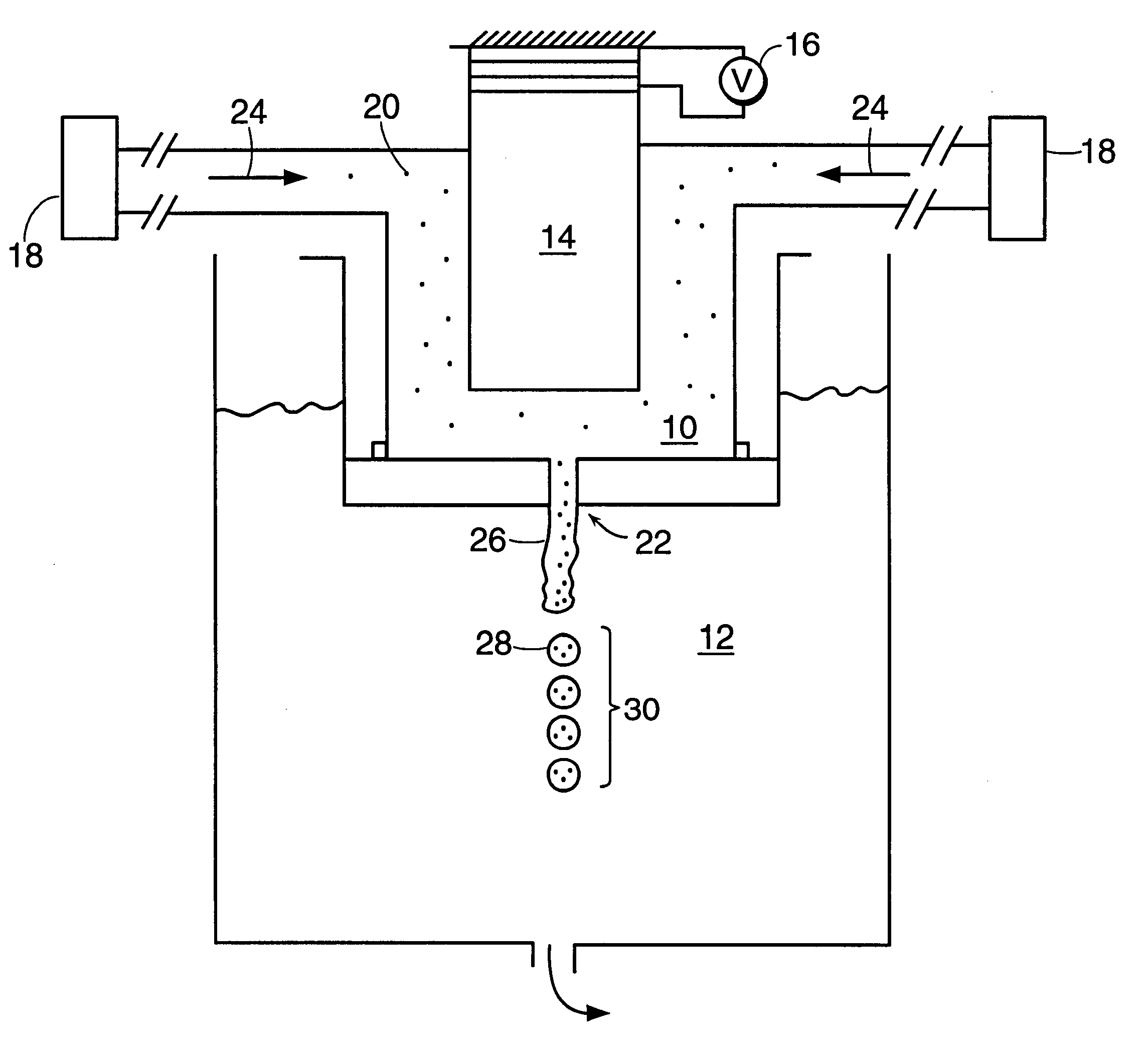
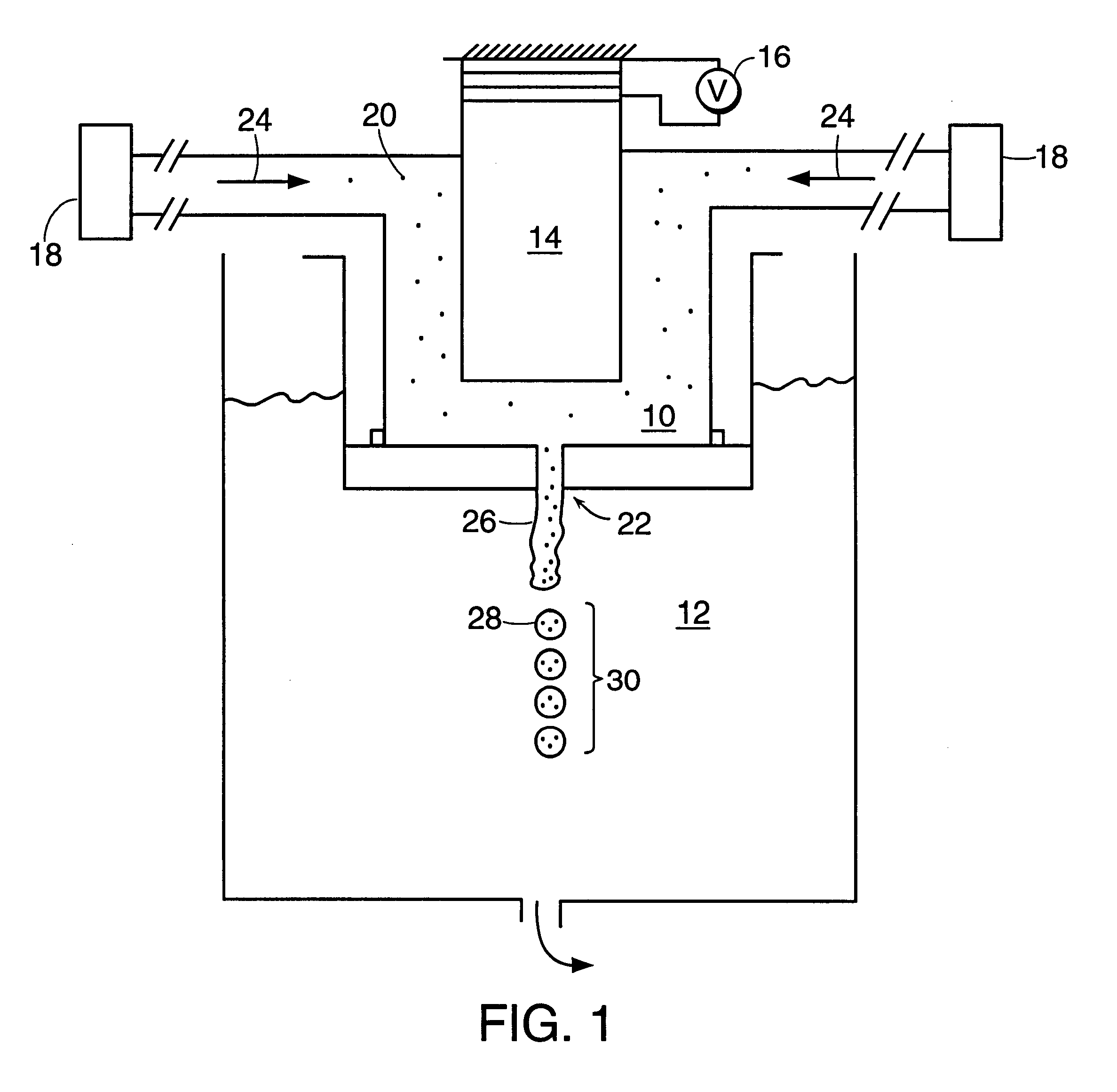
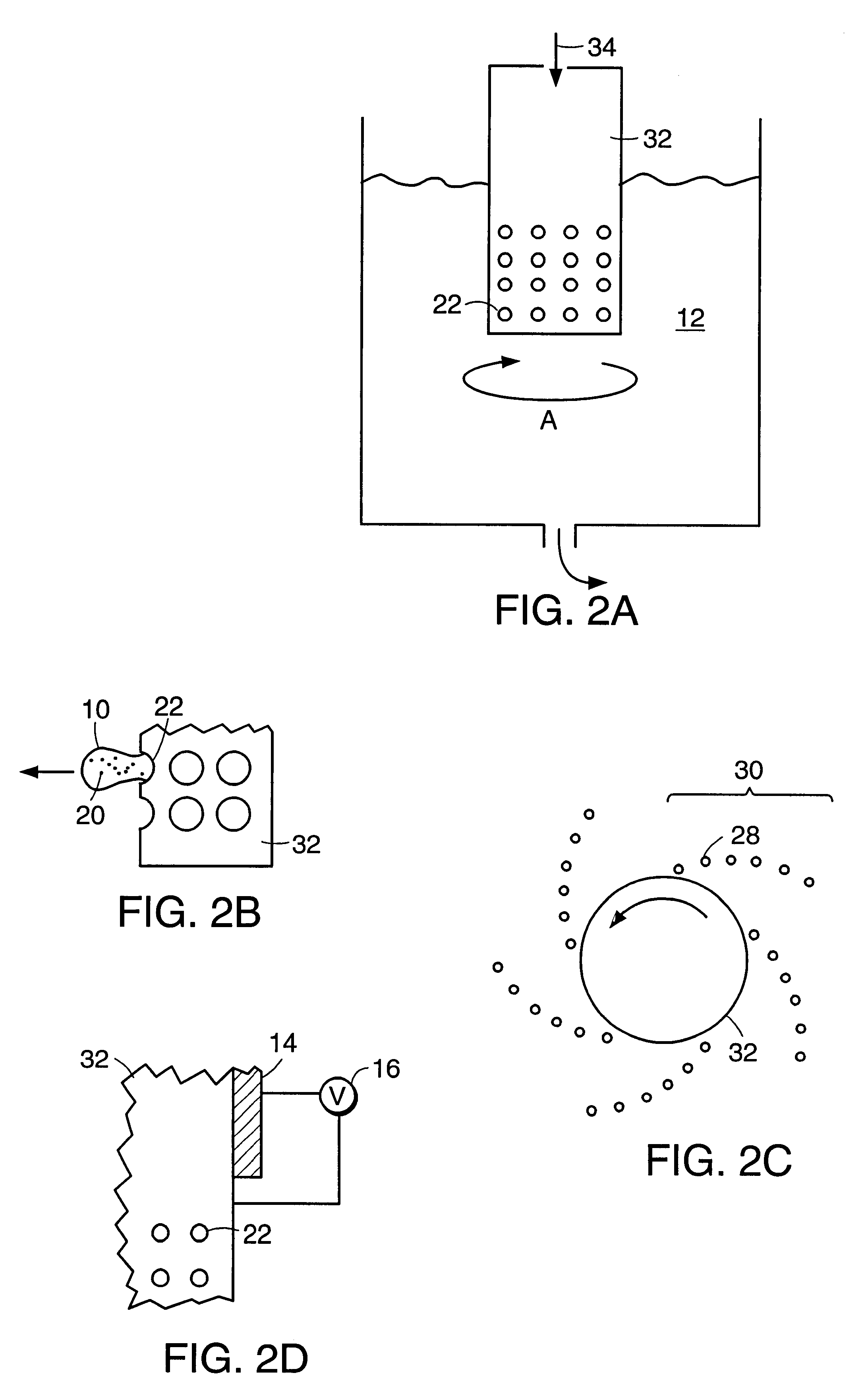
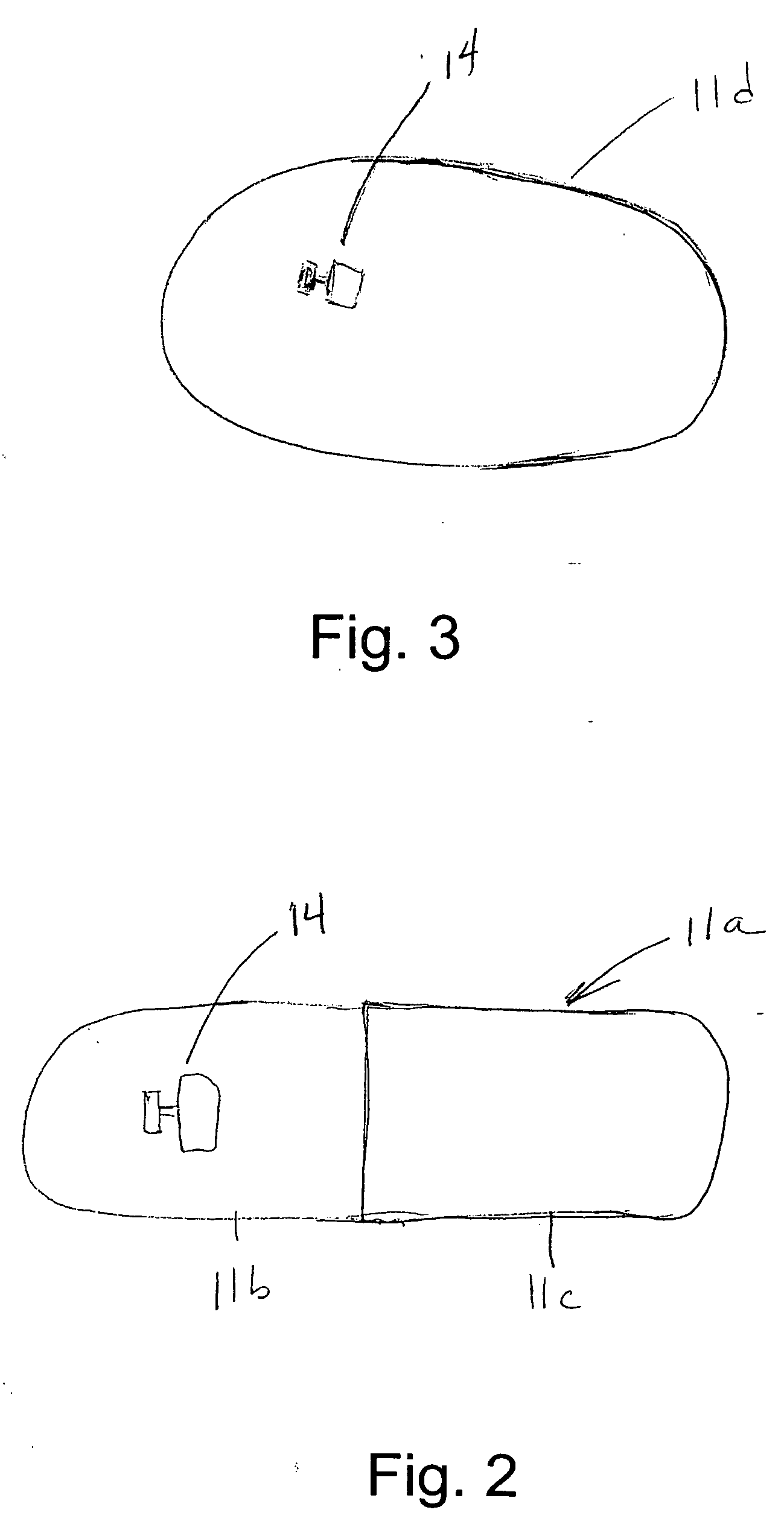
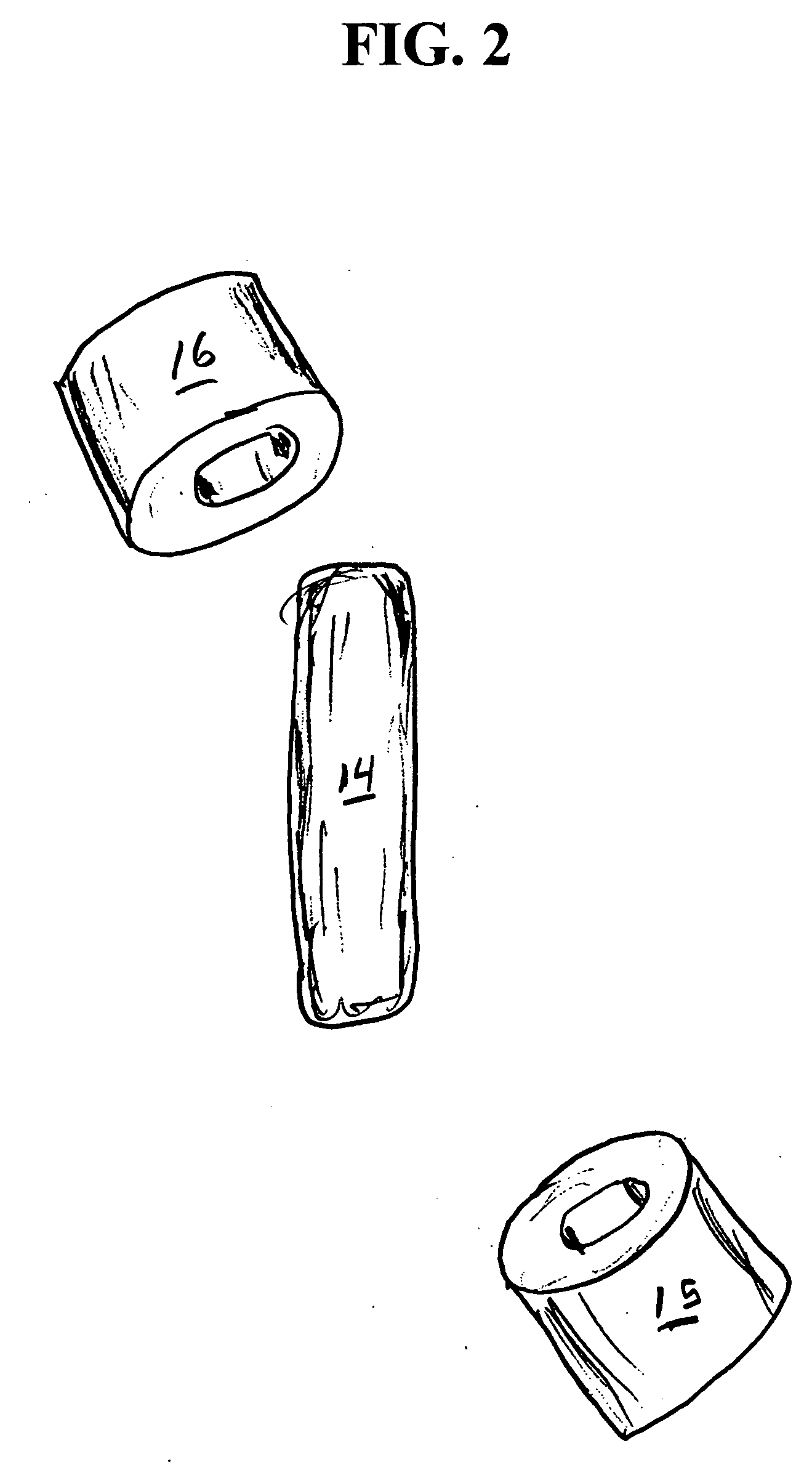
InactiveUS20060061472A1Prevent the tablet from dissolvingIncrease in sizeMemory record carrier reading problemsPharmaceutical product form changeConstant powerConductive materials
A medical pill intended for human or animal consumption includes an RF ID tag in or on the pill. The tag will respond to a nearby reader, the tag itself being without a battery or other constant power supply, capturing power from the reader's transmitted signal and storing a portion of that power in a power supply. An antenna for the RF ID tag may be integral with the tag or it may be transferred to the pill using conductive materials in the pill's coating, filler or binding agents, embedded within the pill, or printed onto the pill. If separate from the tag the antenna is electromagnetically coupled to the tag which has a small onboard antenna. The RF ID tag of each pill has data that are transmitted when the tag is interrogated by a signal from a reader. Incorporation of an ingestable ID tag is possible because of the tag's very small size compatible with ingestion and because the tag can contain an antenna within the pill that allows the tag to be read at a substantial distance. Several different methods for deactivating the RF ID tag after ingestion or use of the pill are disclosed. Medicaments other than oral pills can also have the ID tags.
Owner:TAGENT
Method and system for monitoring and analyzing compliance with internal dosing regimen
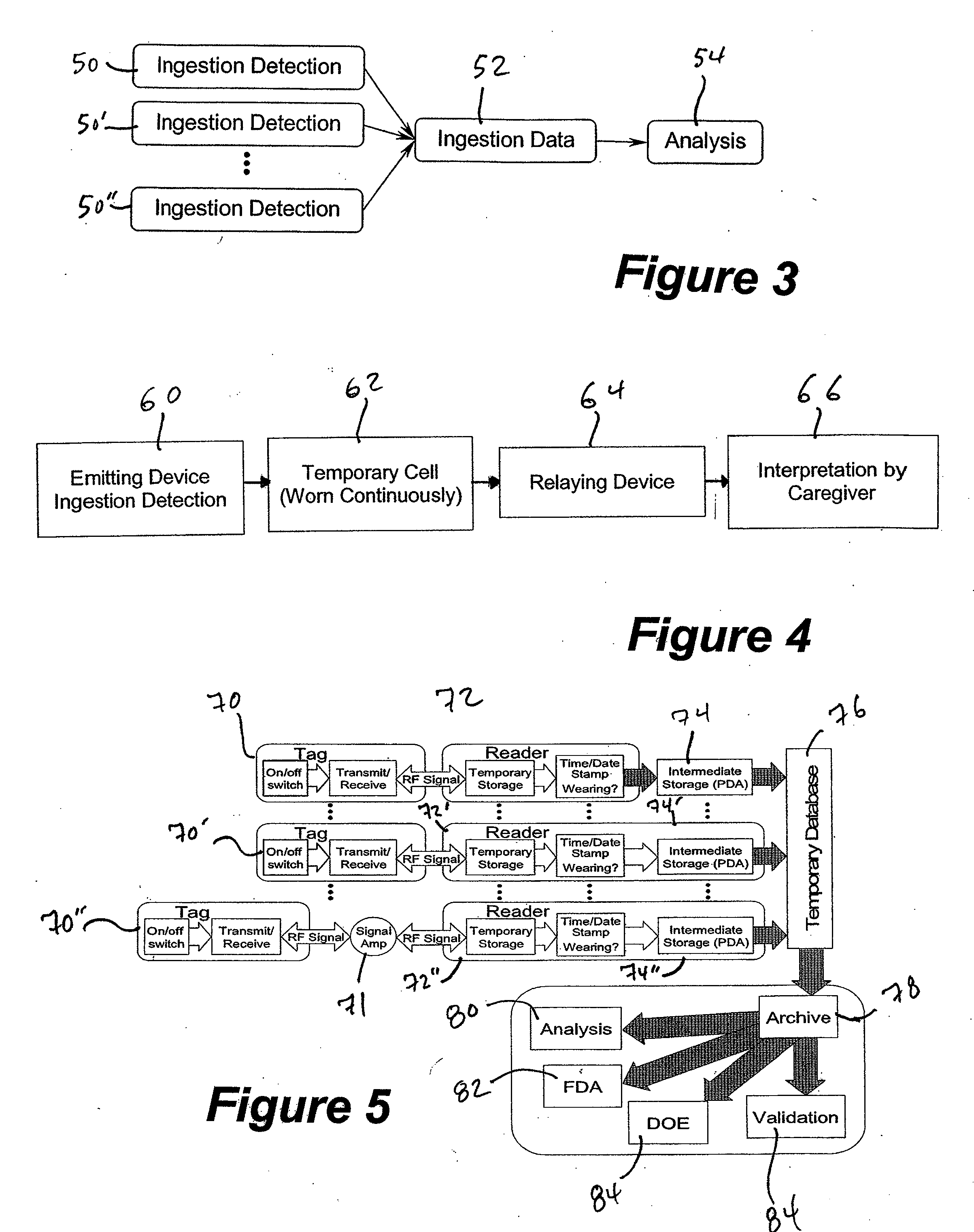
InactiveUS20070237719A1Ultrasonic/sonic/infrasonic diagnosticsCompounds screening/testingDosing regimenMedicine
A method and system for monitoring and analyzing compliance with an internal dosing regimen prescribed to be taken in multiple dose forms includes the steps of detecting internalization of a first dose form to generate a first data point, detecting internalization of a second dose form to generate a second data point, and analyzing the first data point and the second data point. The step of analyzing the first and second data points generates a metric of a variety of possible metric types. The first and second dose forms may be two of any plural number of sequentially-internalized dose forms which generate a like number of sequential data points. Subsequent internalizations of dose forms result in at least a like number of data points being generated. To effect the disclosed method a system is provided which includes at least two dose forms, a time stamp identifier operatively associated with each dose form, a receiving device for receiving the time stamp identifier data, and an analyzer for analyzing the received data,
Owner:DOW GLOBAL TECH LLC
Controlled release formulations coated with aqueous dispersions of acrylic polymers
InactiveUS6143353ADissolution stabilityIncrease weight gainPretreated surfacesMedical devicesWater insolubleActive agent
A stable solid controlled release formulation having a coating derived from an aqueous dispersion of a hydrophobic acrylic polymer includes a substrate including an active agent selected from the group consisting of a systemically active therapeutic agent, a locally active therapeutic agent, a disinfecting and sanitizing agent, a cleansing agent, a fragrance agent and a fertilizing agent, overcoated with an aqueous dispersion of the plasticized water-insoluble acrylic polymer. The formulation provides a stable dissolution of the active agent which is unchanged after exposure to accelerated storage conditions.
Owner:PURDUE PHARMA LP
Internal drug dispenser capsule medical device
The present invention provides a swallowable internal drug medical device. The device includes a swallowable capsule. A sensing module is disposed in the capsule. A bioactive substance dispenser is disposed in the capsule. A memory and logic component is disposed in the capsule and in communication with the sensing module and the dispenser.
Owner:HEWLETT PACKARD DEV CO LP
Method and package for storing a pressurized container containing a drug
Owner:GLAXO SMITHKLINE LLC
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device. In another embodiment, the instant invention is a device useful for oral drug delivery, consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) a first non-anti-collision RFID tag positioned in the capsule; (c) a second non-anti-collision RFID tag positioned in the capsule, so that if the RFID tags are interrogated by an RFID reader before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tags collide and so that after the dispersible material of the capsule has dispersed in the gastrointestinal system thereby allowing the first and second non-anti-collision tags to separate from each other, then the response of the RFID tags is sufficiently different from each other to determine that the capsule has dispersed in the gastrointestinal system
Owner:DOW GLOBAL TECH LLC
Prescription filling apparatus implementing a pick and place method
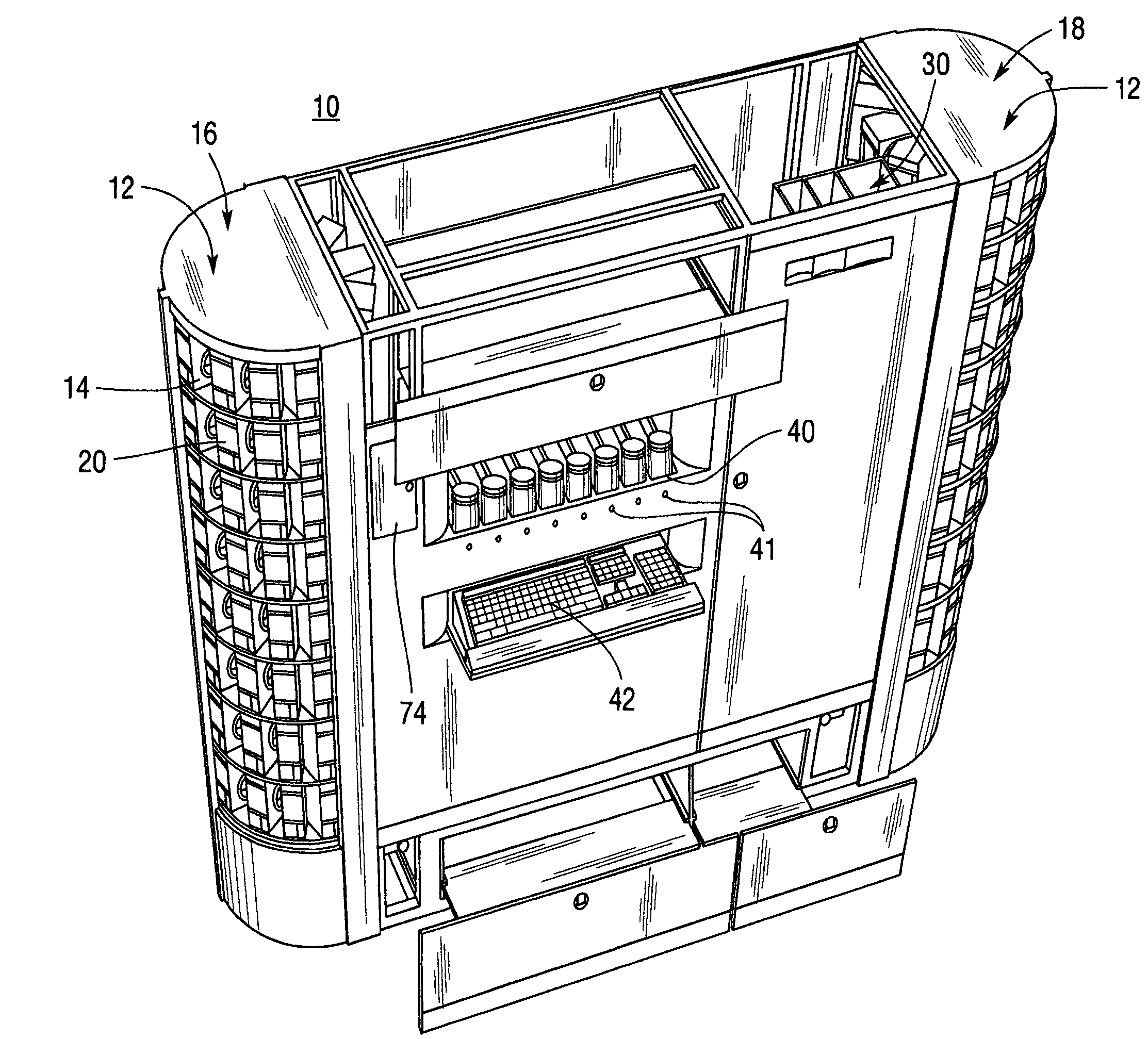
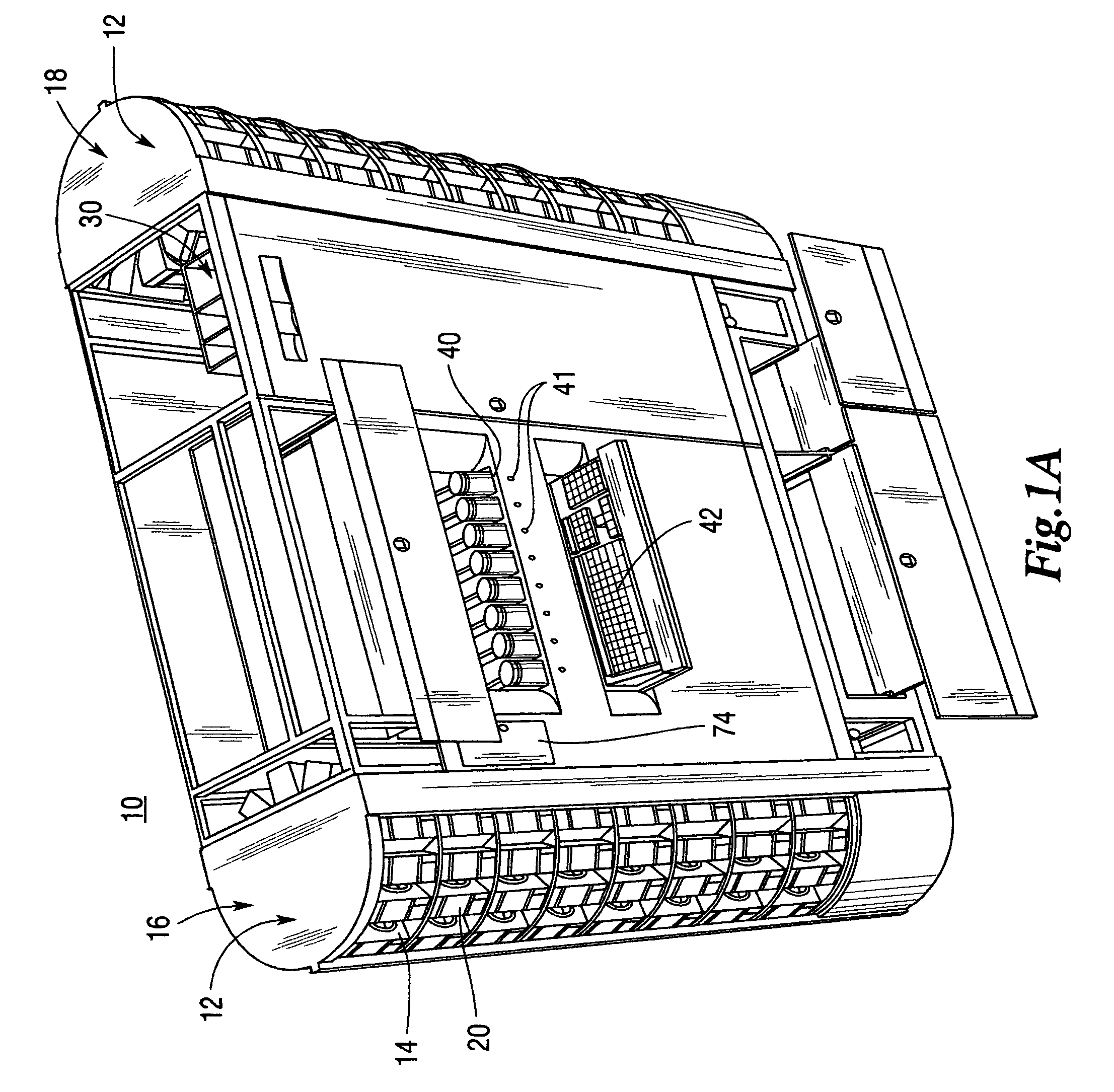
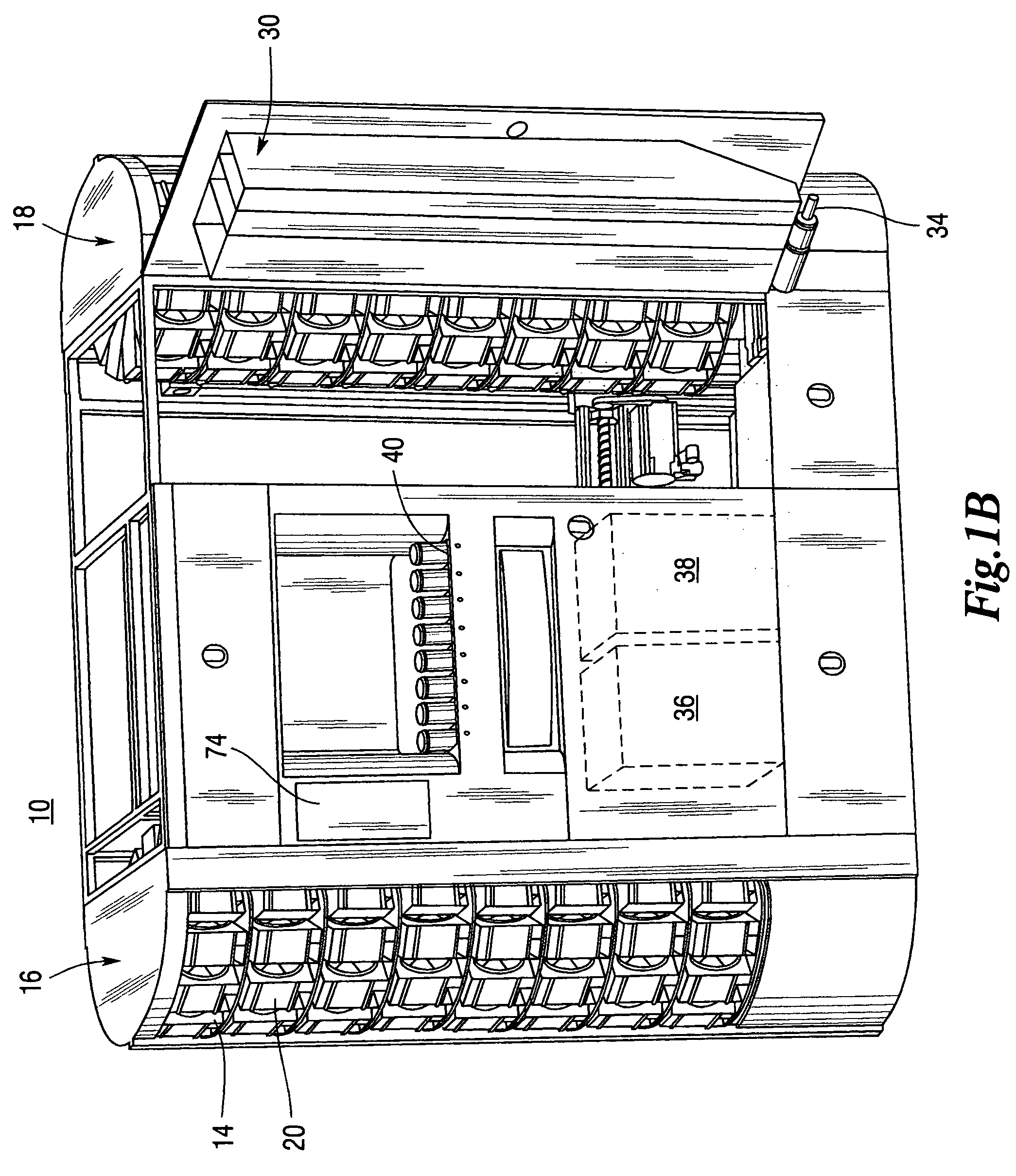
ActiveUS7228198B2Easy to scaleSmall footprintDigital data processing detailsSolid materialComputer control systemOutput device
An apparatus for filling vials comprises a shelving unit defining an array of storage locations. The shelving unit may be an array in an XY plane or one or more carousels. A plurality of storage containers are provided, each removably carried by one of the storage locations. A counting and dispensing unit, a source of vials, a label printer and application unit or units, and an output device are also provided. The output device may take a variety of forms such as an output chute, which is preferably used when a capping unit is provided, an output conveyor, a plurality of output lanes, and an output carousel, which may be a dedicated carousel or a portion of the carousel providing the plurality of storage locations. A computer controlled engagement device provides motion in a Z direction. The engagement device may be comprised of a first stage for engaging the storage containers and a second stage for engaging the vials. A computer controlled system carries the engagement device and moves the engagement device in XY directions among the plurality of storage locations, counting and dispensing unit, source of vials, label printer and application unit, and output device. Methods of operating and refilling the vial filling apparatus are also disclosed.
Owner:MCKESSON AUTOMATION SYST
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device.
Owner:DOW GLOBAL TECH LLC
Methods and apparatus for intraluminal deposition of hydrogels
Owner:INCEPT LLC
Compounder Apparatus
A containment assembly for enclosing a medication vial may comprise a first housing portion or interface portion having a proximal end and a distal end. The interface portion may include a housing wall which defines a channel spanning from the proximal end to the distal end. The channel may be open at the proximal and distal end. The containment assembly may further comprise at least one pierceable septum disposed at least at one of: on the proximal end of the channel and within the channel forming a barrier between the proximal end of the channel and distal end of the channel of the interface portion. The containment assembly may further comprise a variable-volume housing portion having a variable volume chamber. The variable-volume portion chamber of the variable-volume housing portion may be in fluid communication with the distal end of the channel.
Owner:DEKA PROD LLP
Apparatus and method for transferring data to a pharmaceutical compounding system
A system and method for use with a data entry system for providing input data to a pharmaceutical compounding device having an associated plurality of source solutions is provided. A first label comprising first indicia defining a desired pharmaceutical compound is generated. The first indicia is then provided to the pharmaceutical compounding device as an input. The pharmaceutical compounding device then prepares a pharmaceutical compound based on the first indicia and generates a second label comprising second indicia indicative of at least the contents of pharmaceutical compound. The first indicia and second indicia are provided to a comparison device where the contents of the actual pharmaceutical compound as indicated by the second indicia are compared to the desired pharmaceutical compound as indicated by the first indicia.
Owner:B BRAUN MEDICAL
Vial adapter having a needle-free valve for use with vial closures of different sizes
Owner:CAREFUSION 303 INC
Sliding reconstitution device with seal
Owner:BAXTER INT INC
Method and package for storing a pressurized container containing a drug
InactiveUS6179118B1Reduce manufacturing complexityReduce manufacturing costWrappersDiagnosticsWater vaporWaste management
Owner:GLAXO SMITHKLINE LLC
Trackable pills with electronic ID tags
InactiveUS7253716B2Prevent counterfeitingAccurate trackingMemory record carrier reading problemsPharmaceutical product form changeConstant powerEngineering
A medical pill intended for human or animal consumption includes an RF ID tag in or on the pill. The tag will respond to a nearby reader, the tag itself being without a battery or other constant power supply, capturing power from the reader's transmitted signal and storing a portion of that power in a power supply. An antenna for the RF ID tag may be integral with the tag or it may be transferred to the pill using conductive materials in the pill's coating, filler or binding agents, embedded within the pill, or printed onto the pill. If separate from the tag the antenna is electromagnetically coupled to the tag which has a small onboard antenna. The RF ID tag of each pill has data that are transmitted when the tag is interrogated by a signal from a reader. Incorporation of an ingestable ID tag is possible because of the tag's very small size compatible with ingestion and because the tag can contain an antenna within the pill that allows the tag to be read at a substantial distance. Several different methods for deactivating the RF ID tag after ingestion or use of the pill are disclosed. Medicaments other than oral pills can also have the ID tags.
Owner:TAGENT
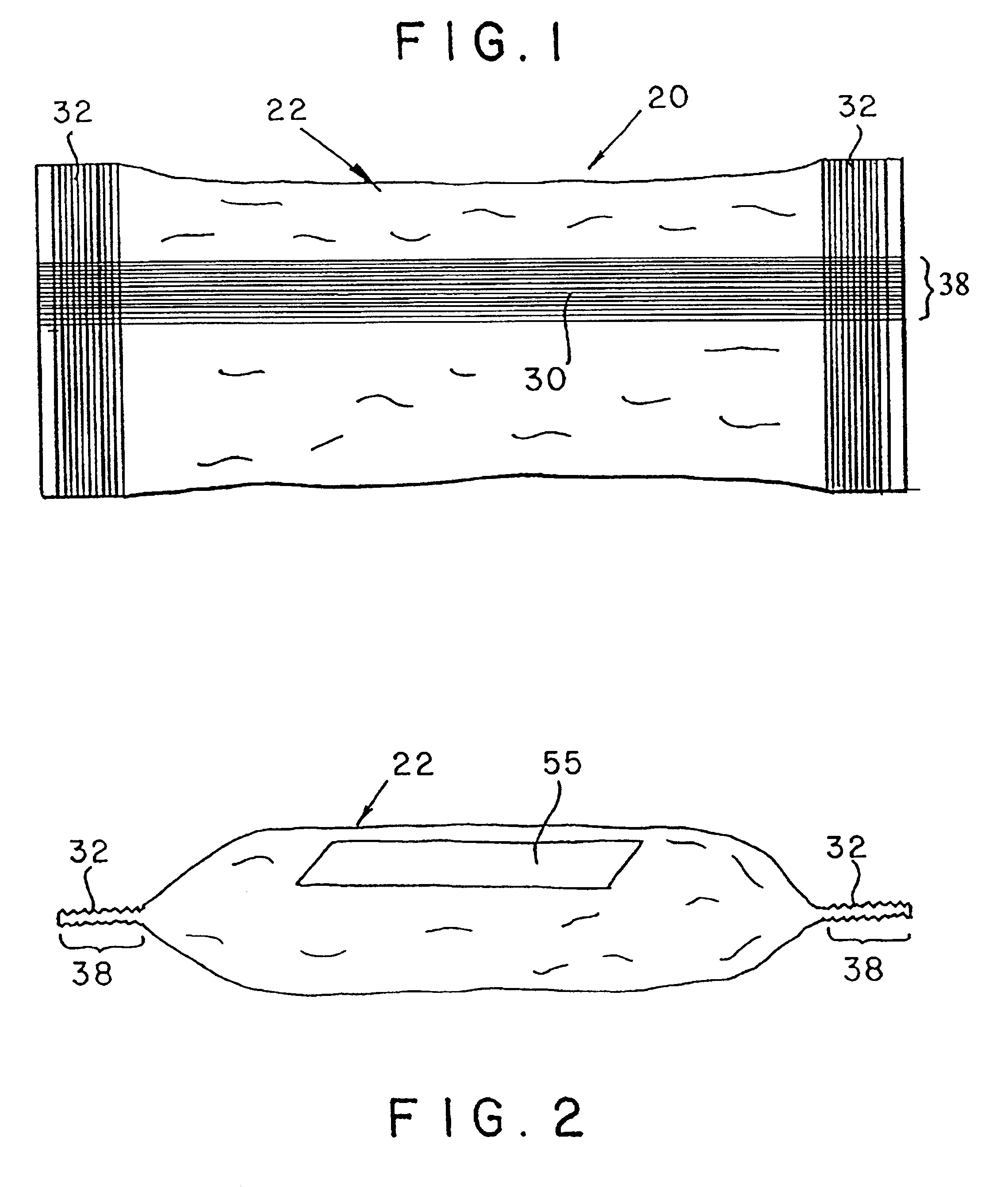
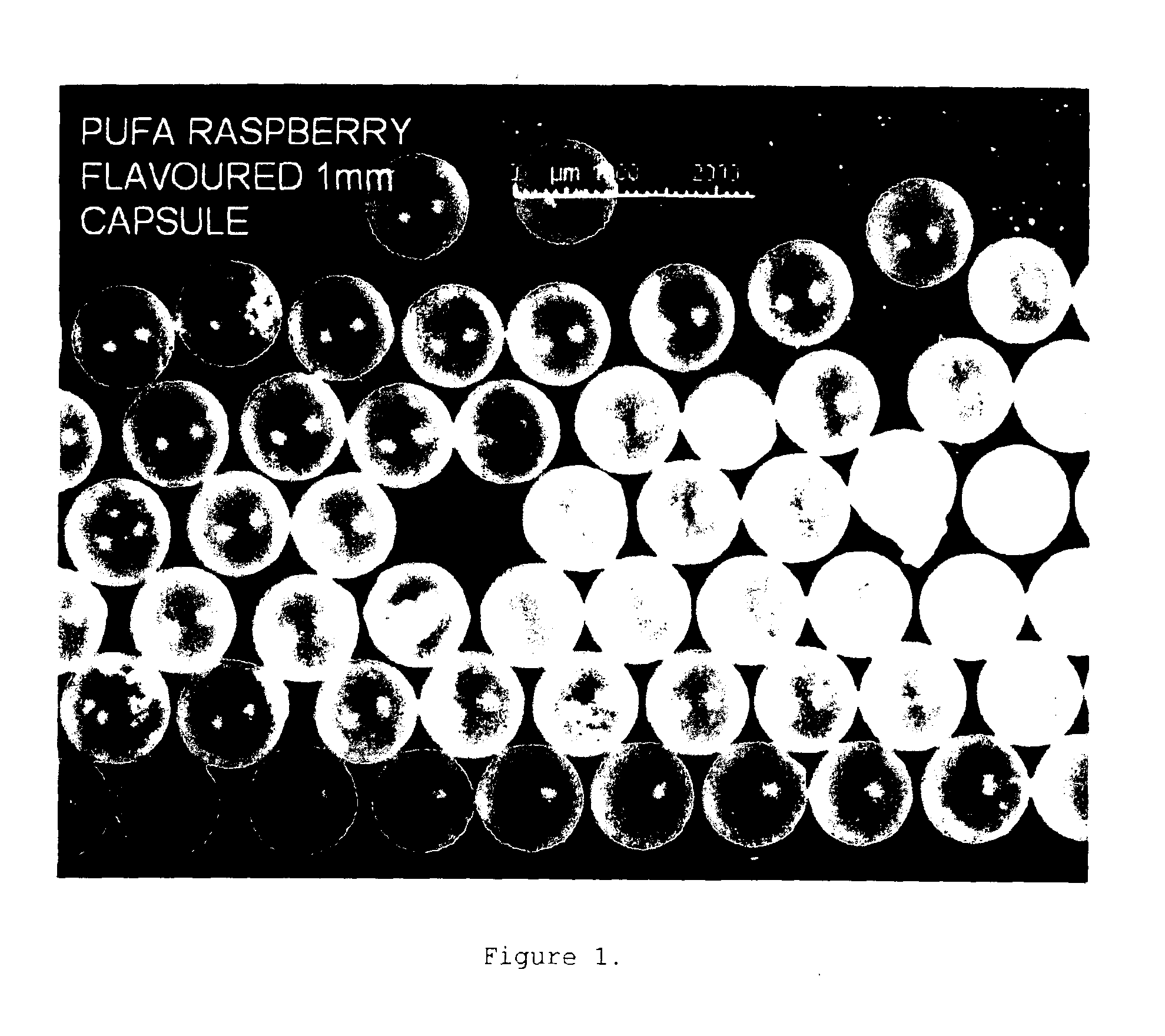
Seamless capsules containing high amounts of polyunsaturated fatty acids and a flavouring component
A seamless capsule includes a core and a shell, wherein the core includes at least one polyunsaturated fatty acid, and at least one flavouring component, the process for manufacturing the capsule and products containing the capsule are also disclosed.
Owner:V MANE FILS
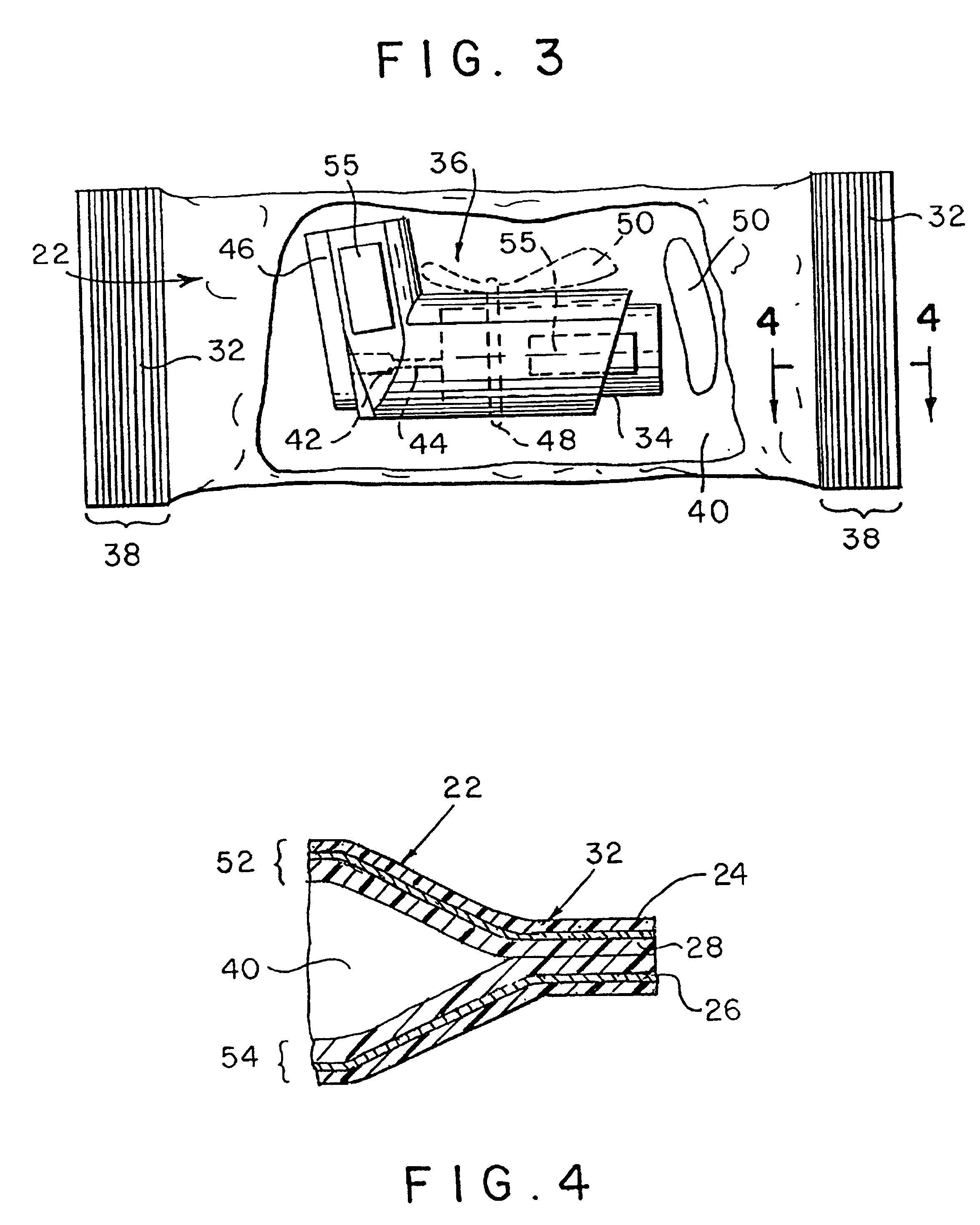
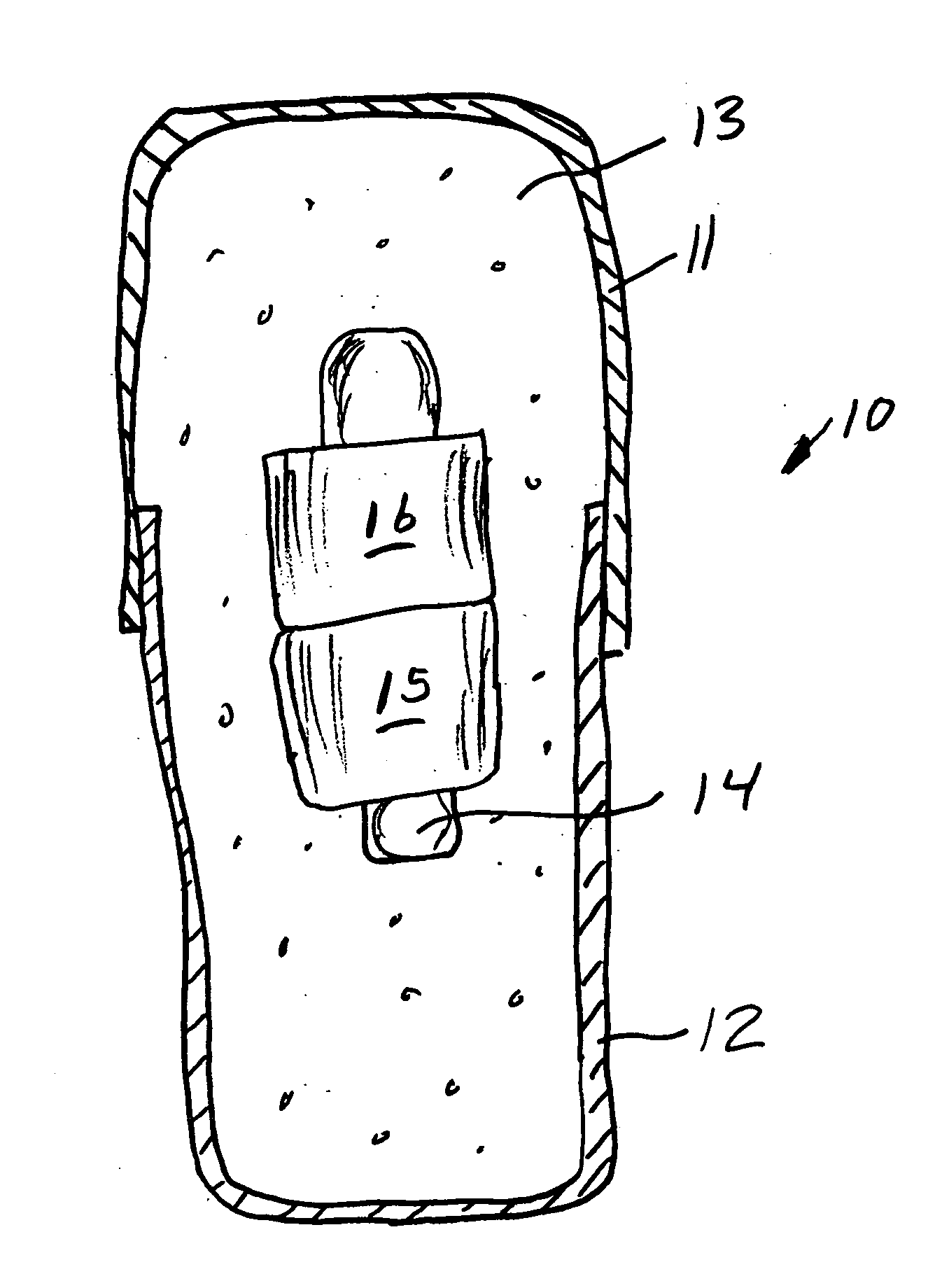
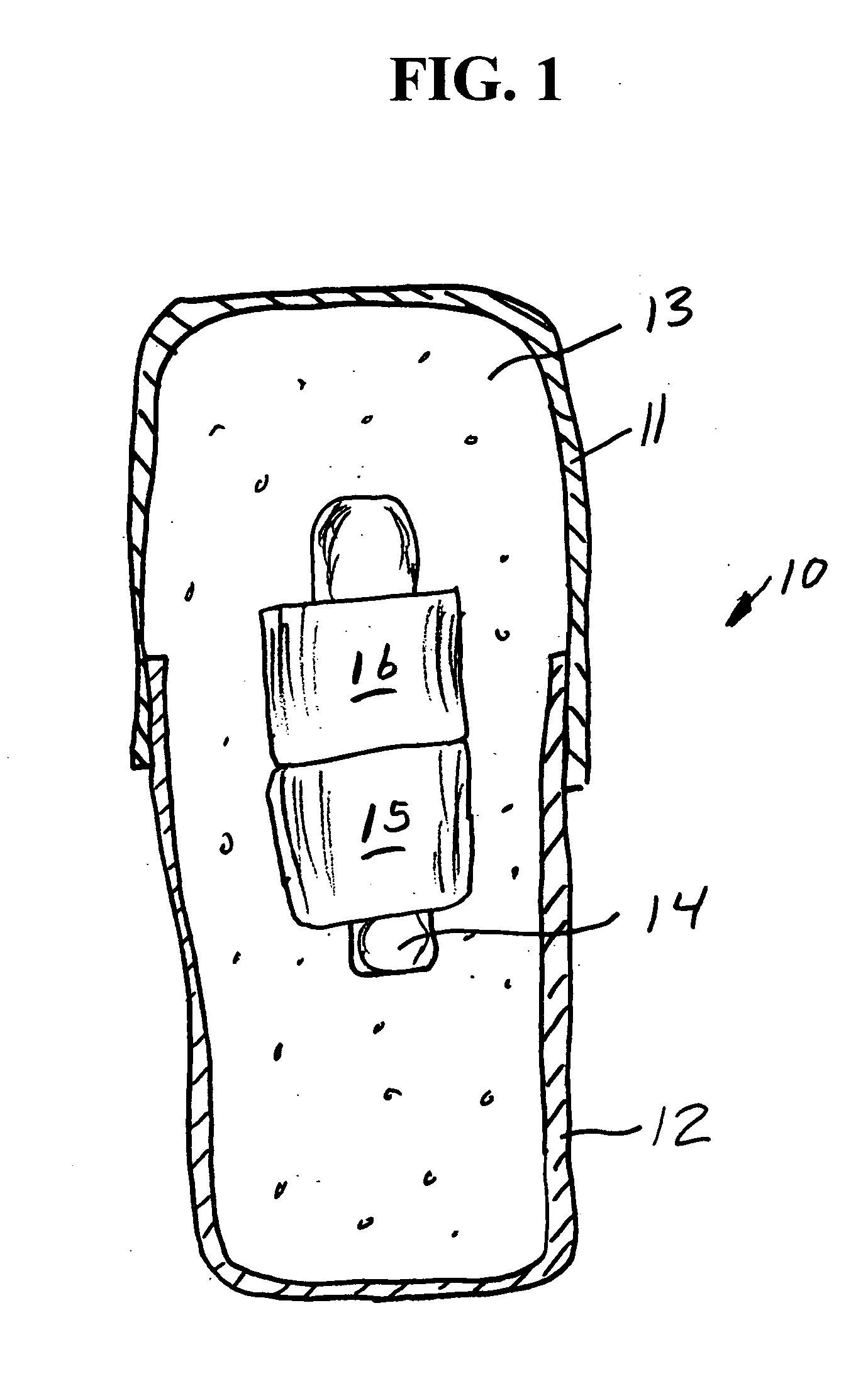
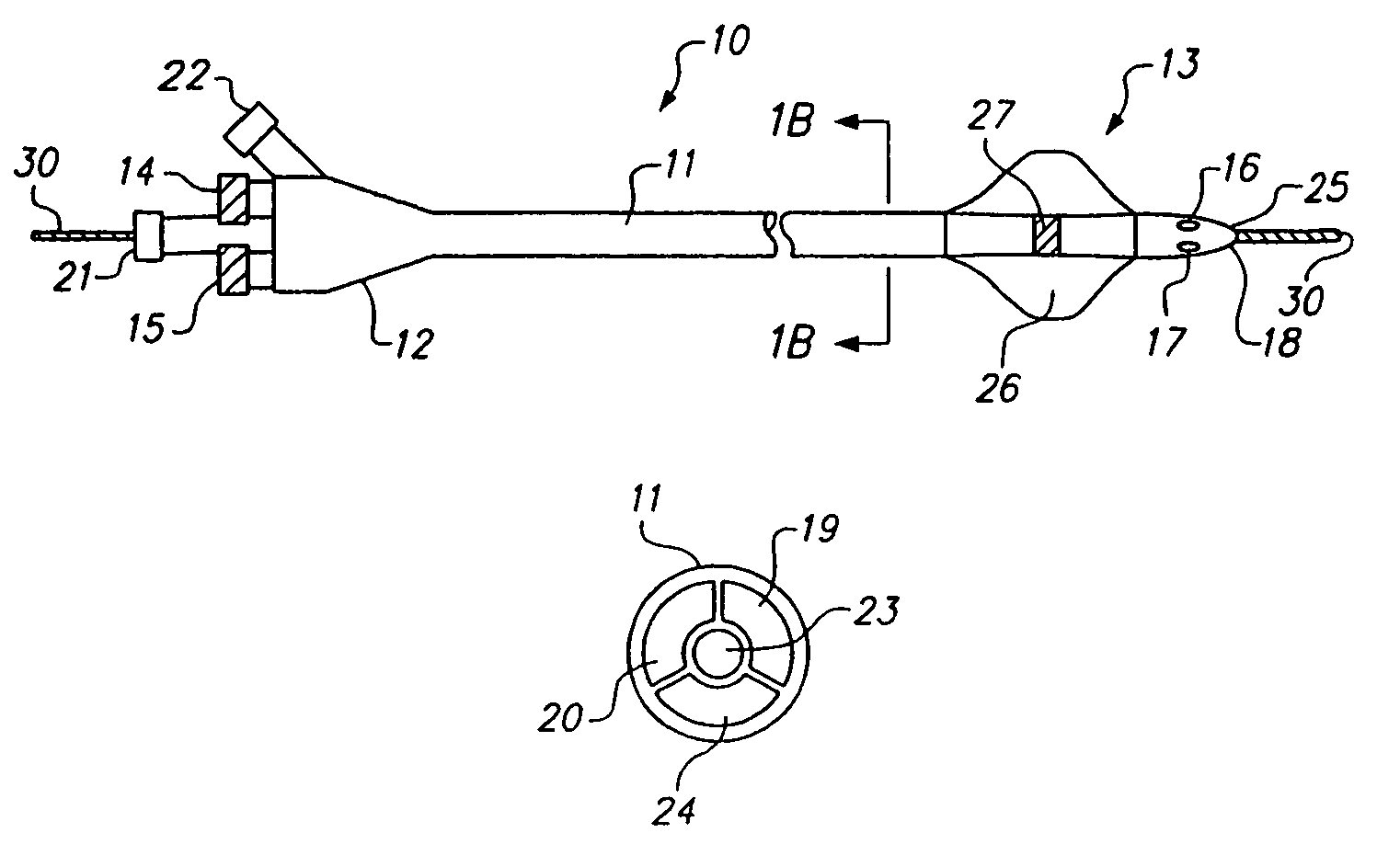
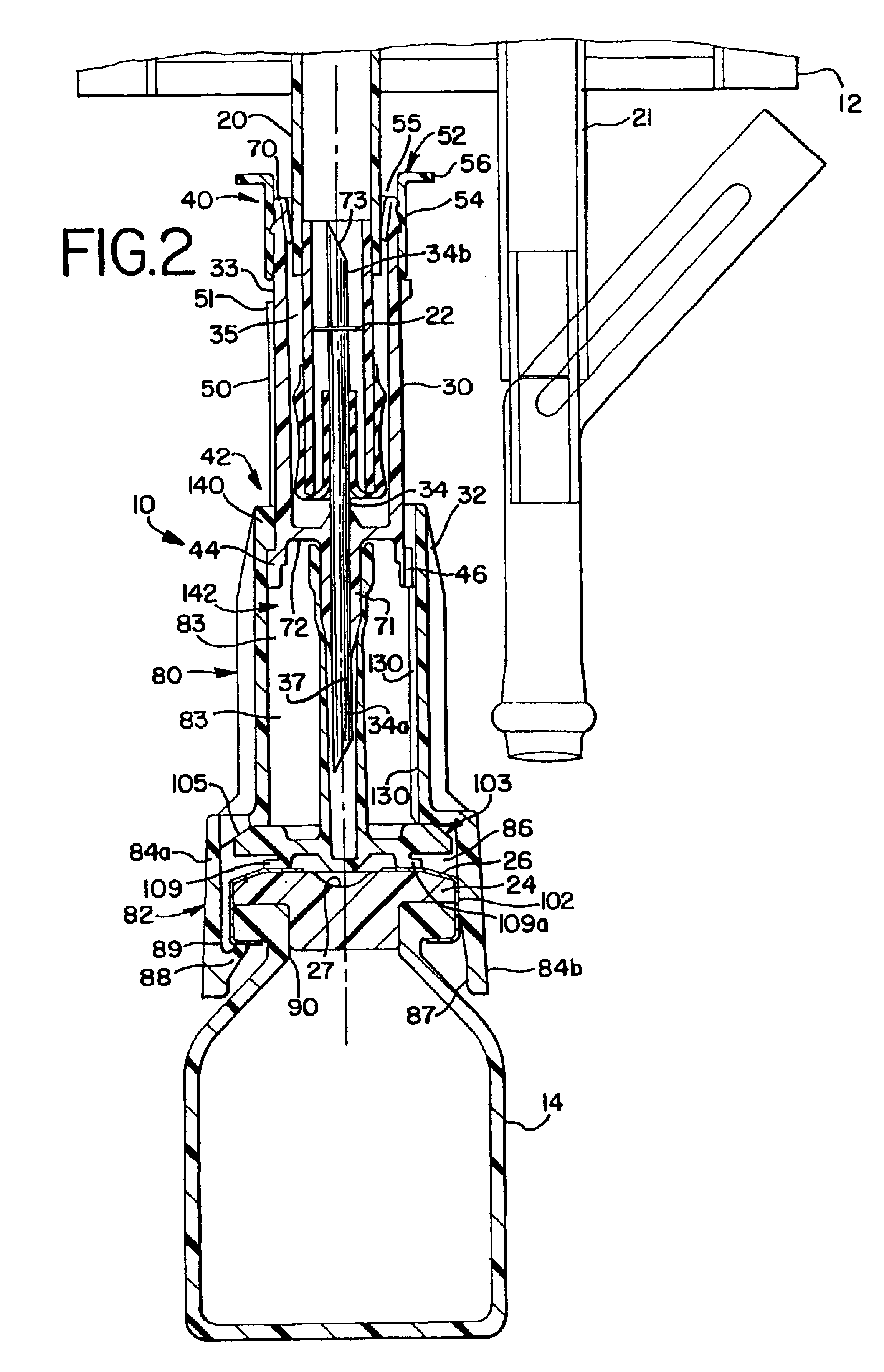
Sliding reconstitution device for a diluent container
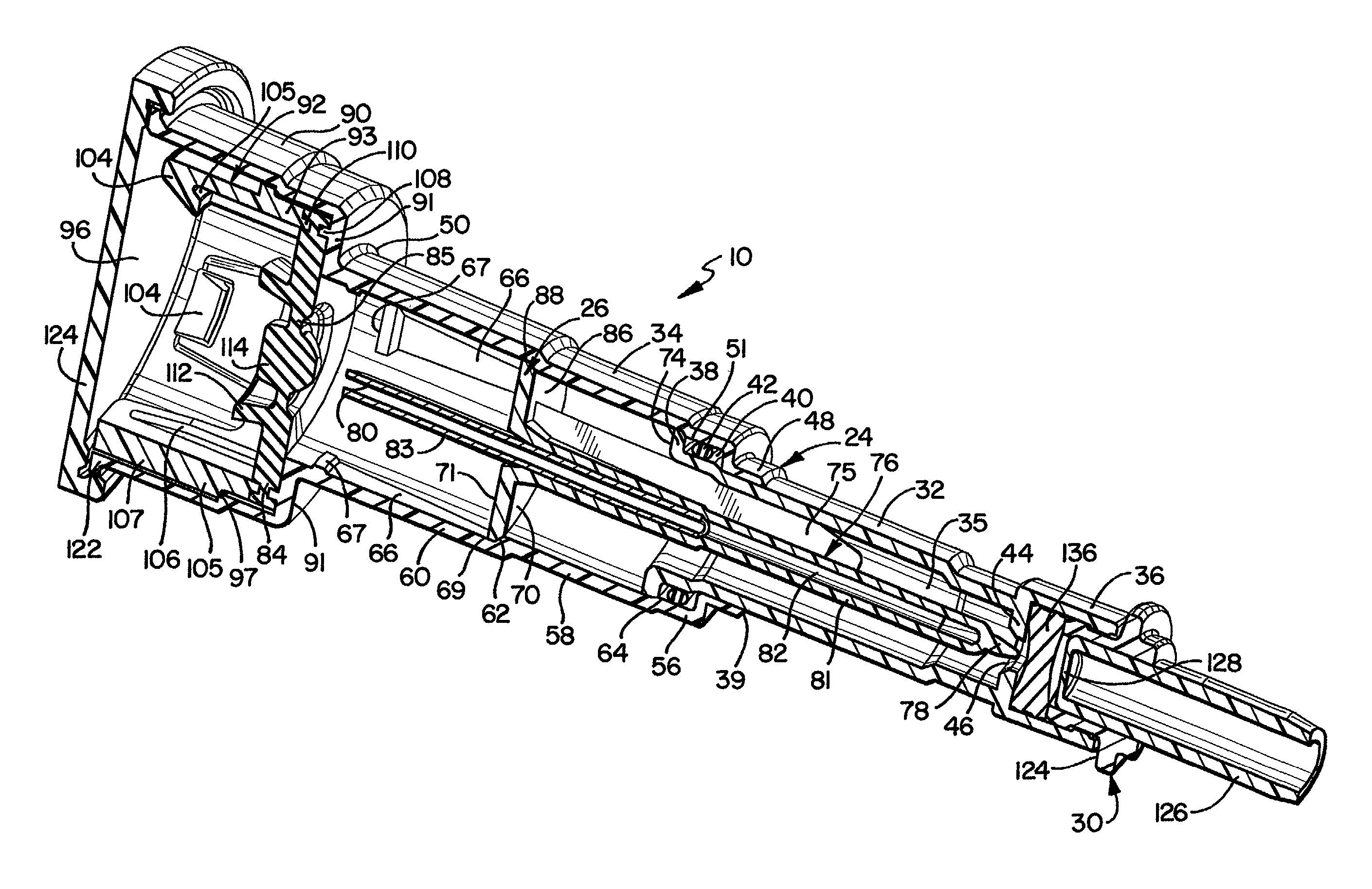
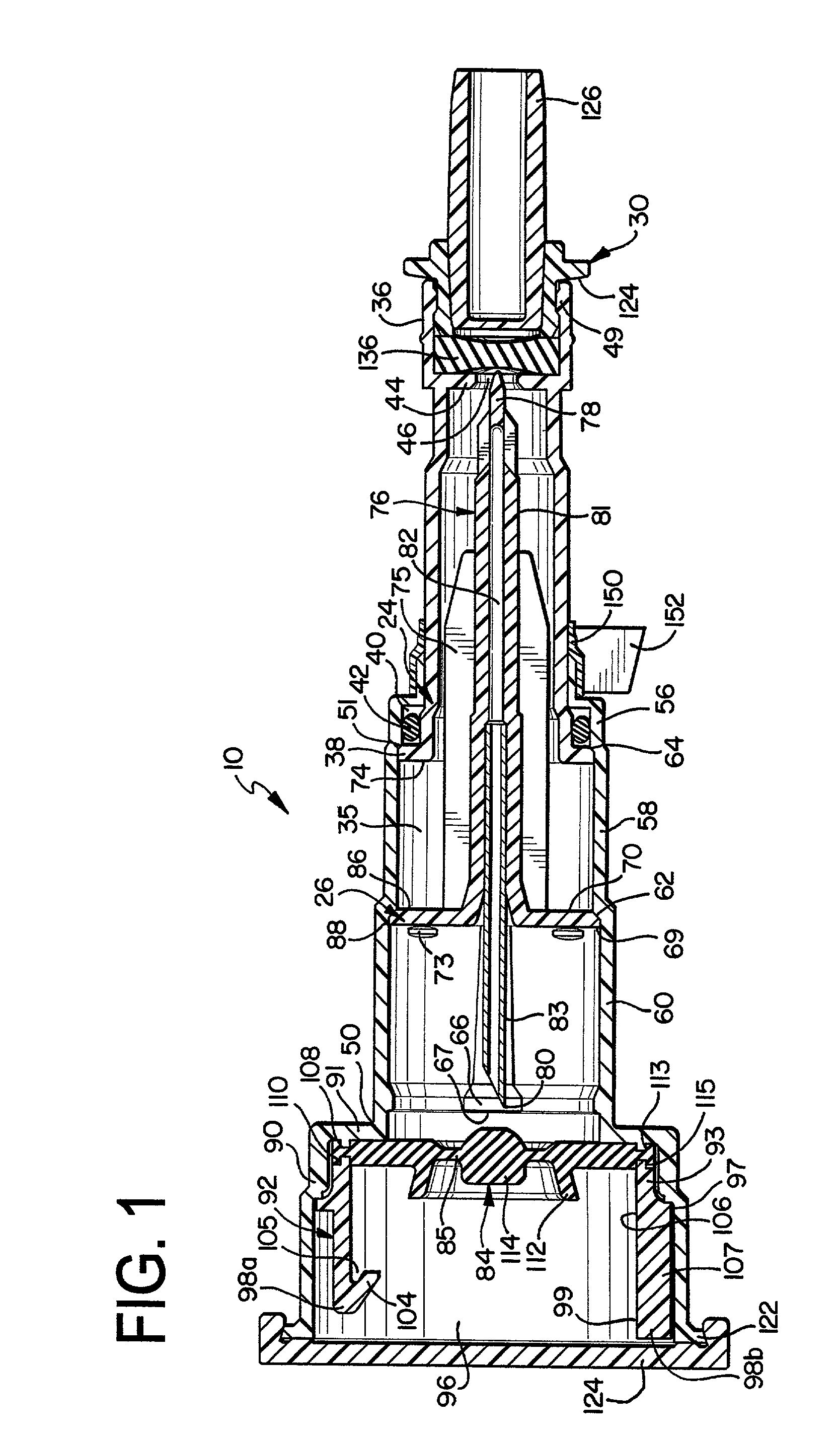
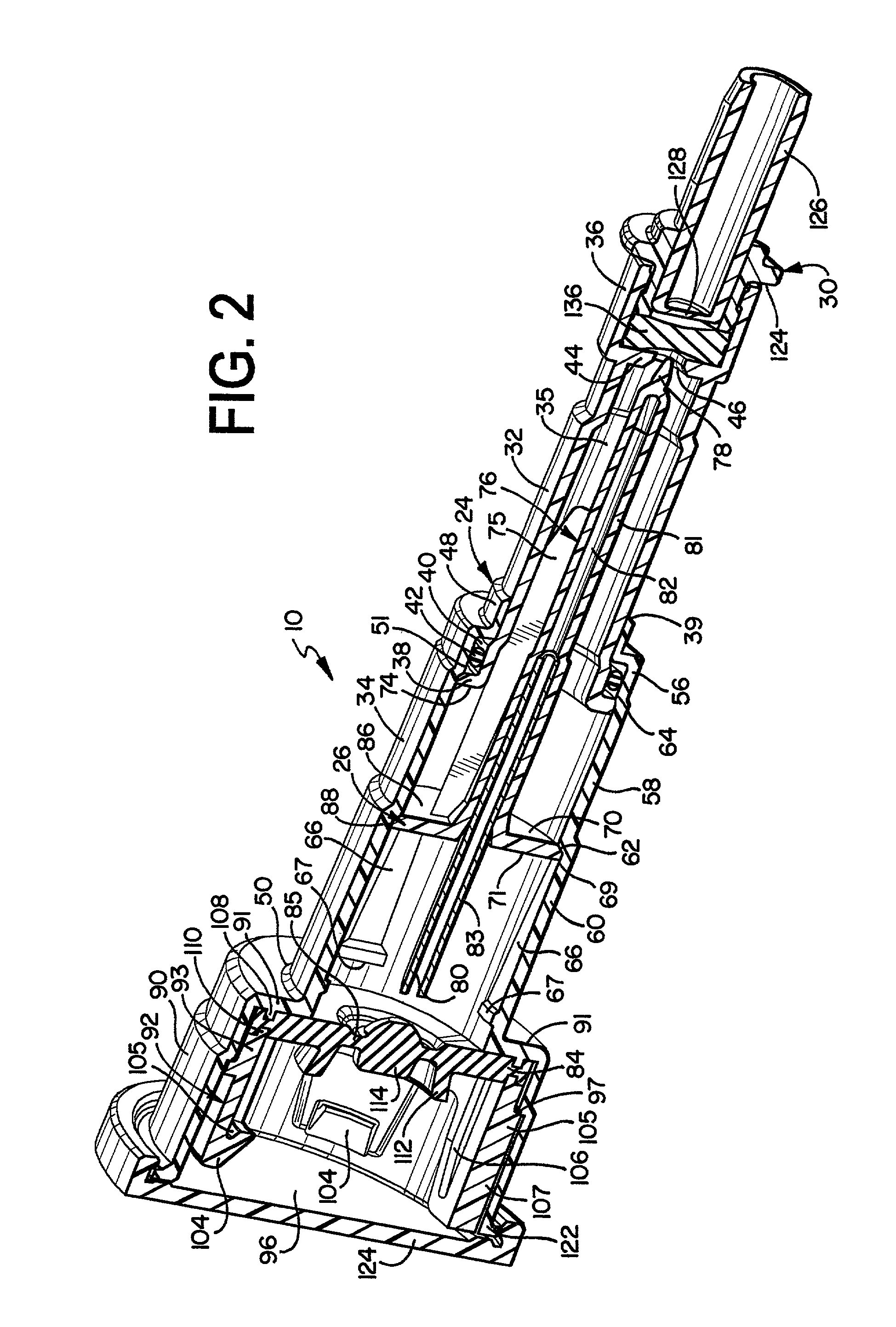
The present invention provides a reconstitution device (10) for placing a first container (12), such as a liquid container (e.g. flexible container or syringe), in fluid communication with a second container (14), such as a drug vial. To this end, there is provided a connector device (10) for establishing fluid communication between the diluent container (12) and the drug vial (14). The connector device (10) has a first sleeve member (32) having a first end (36) and a second end (38). The first sleeve member (32) has at the first end (36), a first attaching member (30) adapted to attach to the liquid container (12). The connector device (10) also has a second sleeve member (34) having a first end (48) and a second end (50). The second sleeve member (34) is movable axially with respect to the first sleeve member (32) from an inactivated position to an activated position. The second sleeve member (34) has a protuberance (66) on an inner surface of the second sleeve member (34). A second attaching member (28) is attached on the second end (50) of the second sleeve member (34) and is adapted to attach to the second container (14). The second attaching member (28) has a sealing member (84). A piercing member (76) is positioned in the chamber and projects within the sleeve members (32,34) for providing a fluid flow path from the first container (12) to the second container (14). The piercing member (76) is moveable from a first position in the inactivated position to a second position in the activated position wherein the piercing member (76) moves past the protuberance (66), the protuberance (66) preventing movement of the piercing member (76) back to the first position. The device (10) is movable from the inactivated position to the activated position by a force generally applied to the device outside the liquid container (12).
Owner:BAXTER INT INC
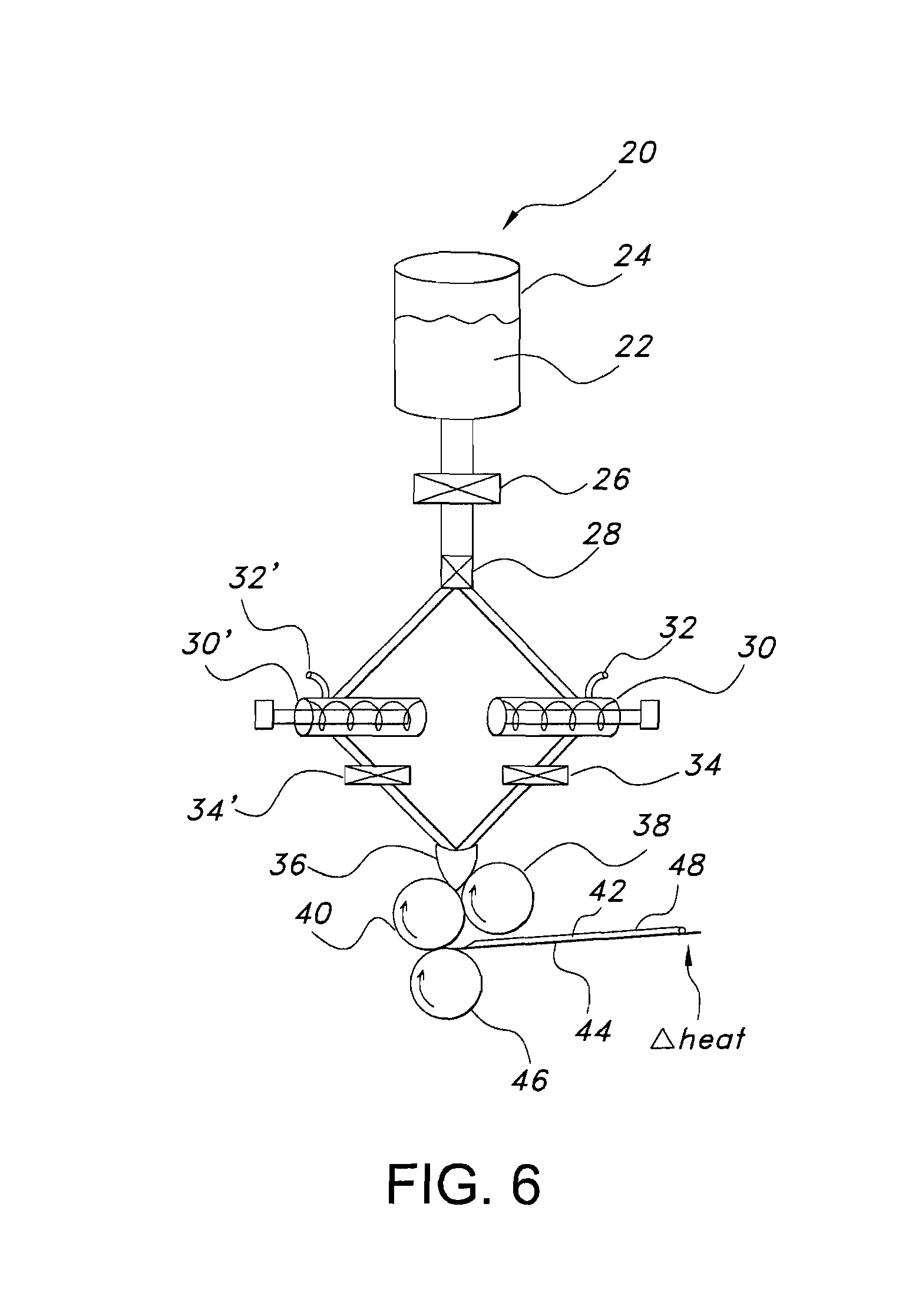
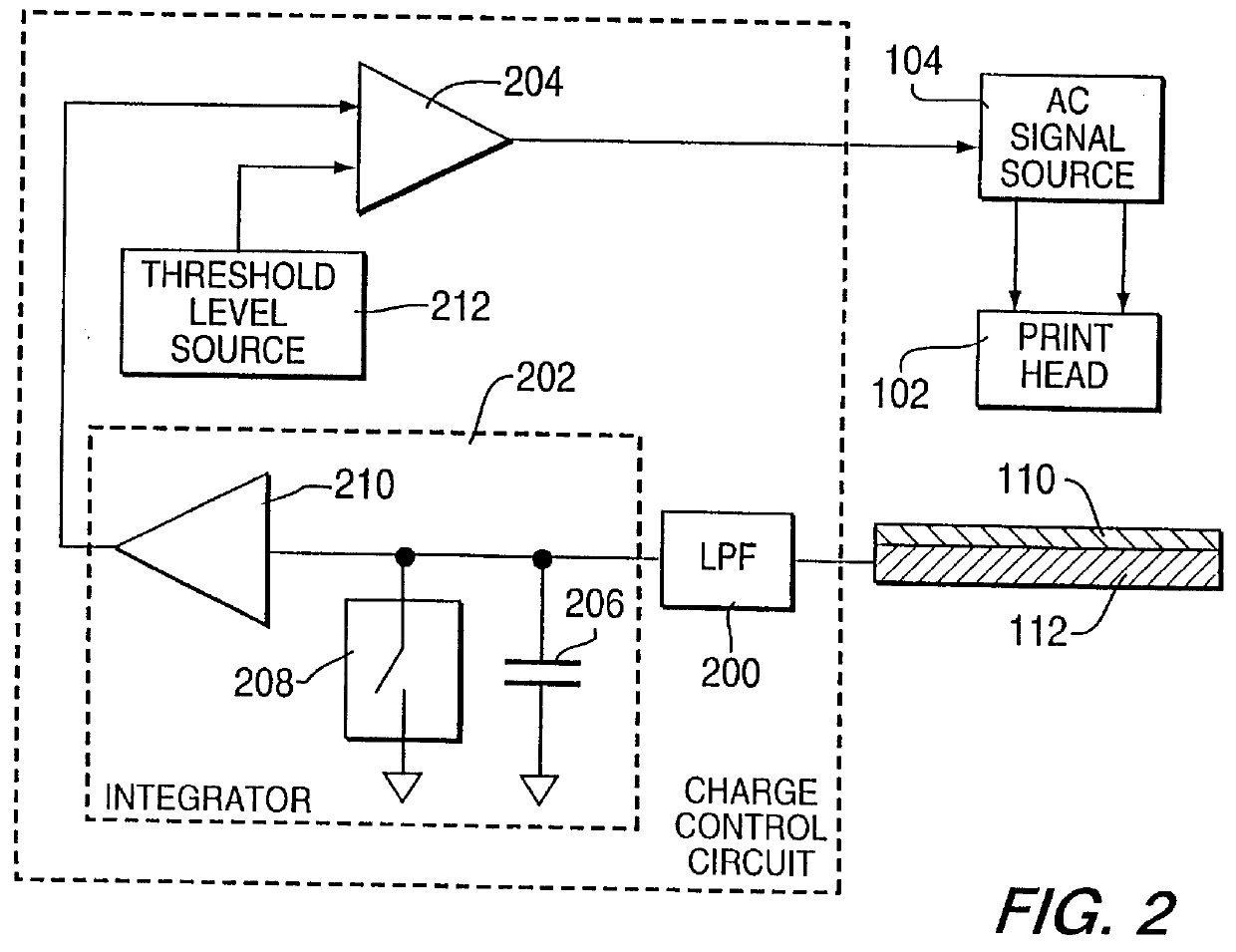
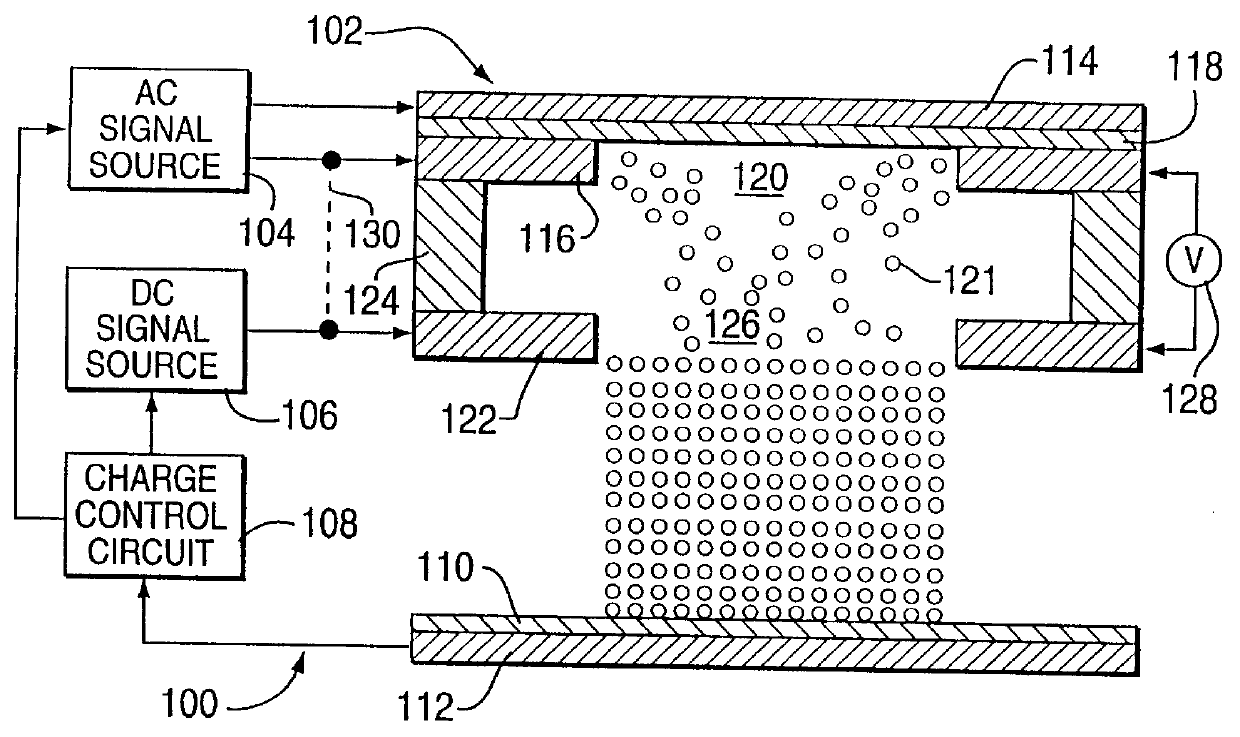
Method for electrostatically depositing a medicament powder upon predefined regions of a substrate
Method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, the apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
Ingestible event marker systems
ActiveUS20100185055A1Rapid and simple notationPrimary cell manufactureSurgeryComputer scienceDigestive tract
Ingestible event marker systems that include an ingestible event marker (i.e., an IEM) and a personal signal receiver are provided. Embodiments of the IEM include an identifier, which may or may not be present in a physiologically acceptable carrier. The identifier is characterized by being activated upon contact with a target internal physiological site of a body, such as digestive tract internal target site. The personal signal receiver is configured to be associated with a physiological location, e.g., inside of or on the body, and to receive a signal the IEM. During use, the IEM broadcasts a signal which is received by the personal signal receiver.
Owner:PROTEUS DIGITAL HEALTH INC
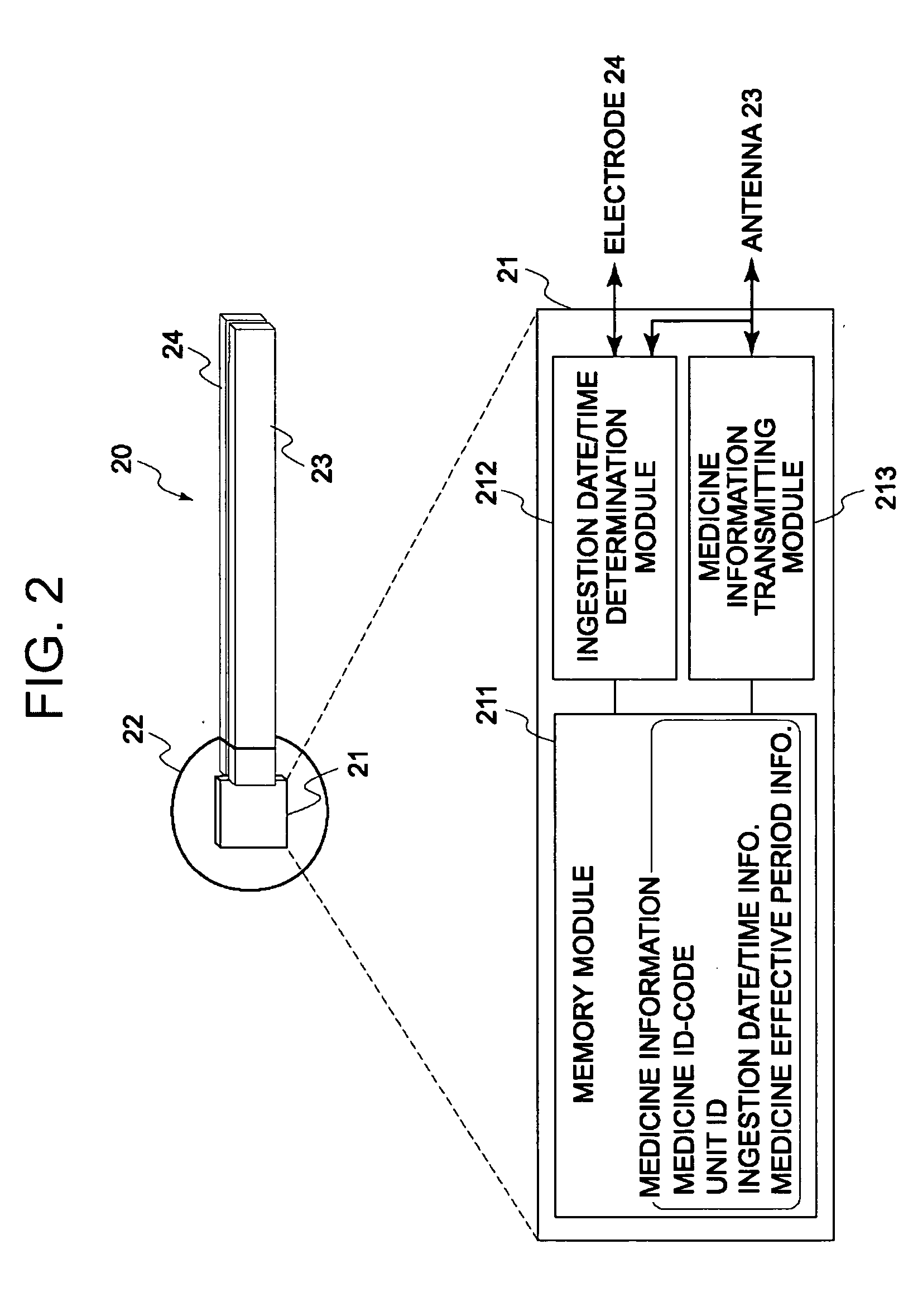
Medicine ingestion state management method, medicine and medicine ingestion state management device
ActiveUS20060145876A1Avoid overdosePrevent an excessive dosage of the medicine to the patientDrug and medicationsSurgeryMedication informationState management
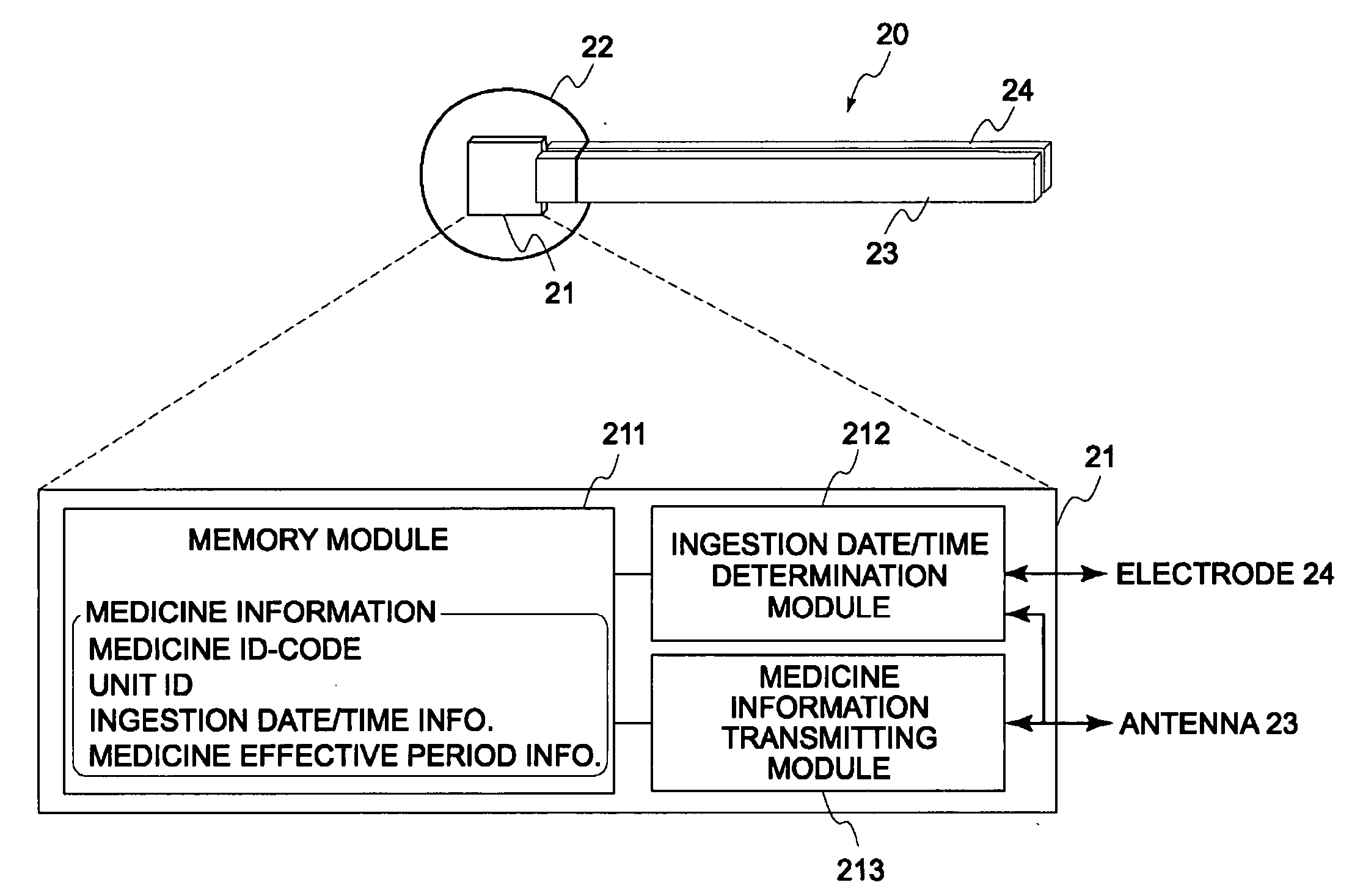
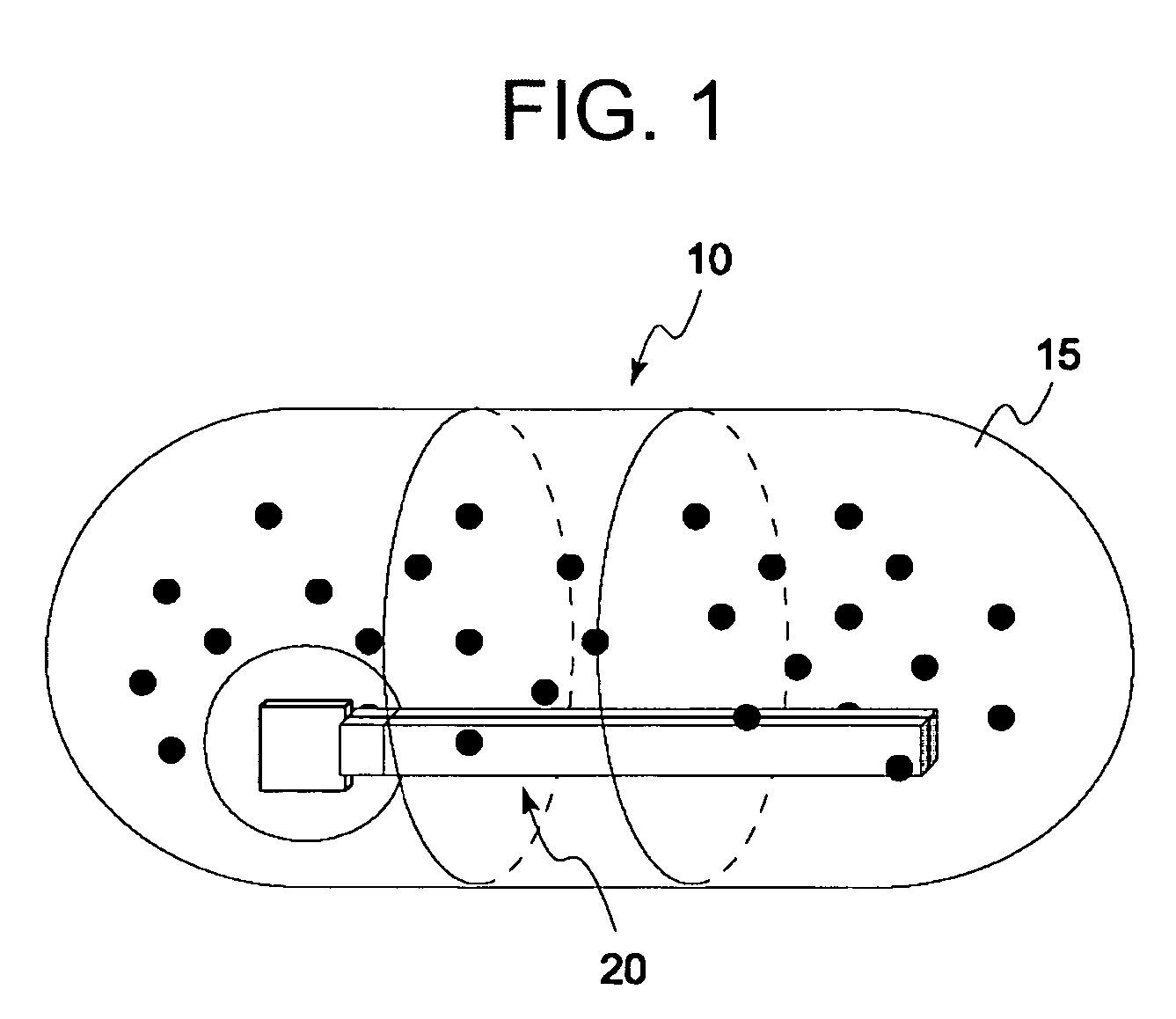
Disclosed is a medicine ingestion state management method capable of objectively managing medicine ingestion states of patients. The medicine ingestion state management method involves an operation of prescribing medicine (10) encapsulating, together with a medicament, a medicine information transmitting unit (20) having a function of transmitting medicine information capable of specifying a type and a quantity of the medicament to each individual patient, and an operation of grasping the medicine ingestion state of each patient by collecting the medicine information from each medicine information transmitting unit (20) in each patient.
Owner:FUJITSU LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com