Patents
Literature
1475results about "Spray delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. The invention also features compositions, and methods of use for the study, diagnosis, and treatment of traits, diseases and conditions that respond to the modulation of gene expression and / or activity in a subject or organism. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver biologically active molecules, such as antibodies (e.g., monoclonal, chimeric, humanized etc.), cholesterol, hormones, antivirals, peptides, proteins, chemotherapeutics, small molecules, vitamins, co-factors, nucleosides, nucleotides, oligonucleotides, enzymatic nucleic acids, antisense nucleic acids, triplex forming oligonucleotides, 2,5-A chimeras, dsRNA, allozymes, aptamers, decoys and analogs thereof, and small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules, to relevant cells and / or tissues, such as in a subject or organism. Such novel cationic lipids, microparticles, nanoparticles and transfection agents are useful, for example, in providing compositions to prevent, inhibit, or treat diseases, conditions, or traits in a cell, subject or organism. The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
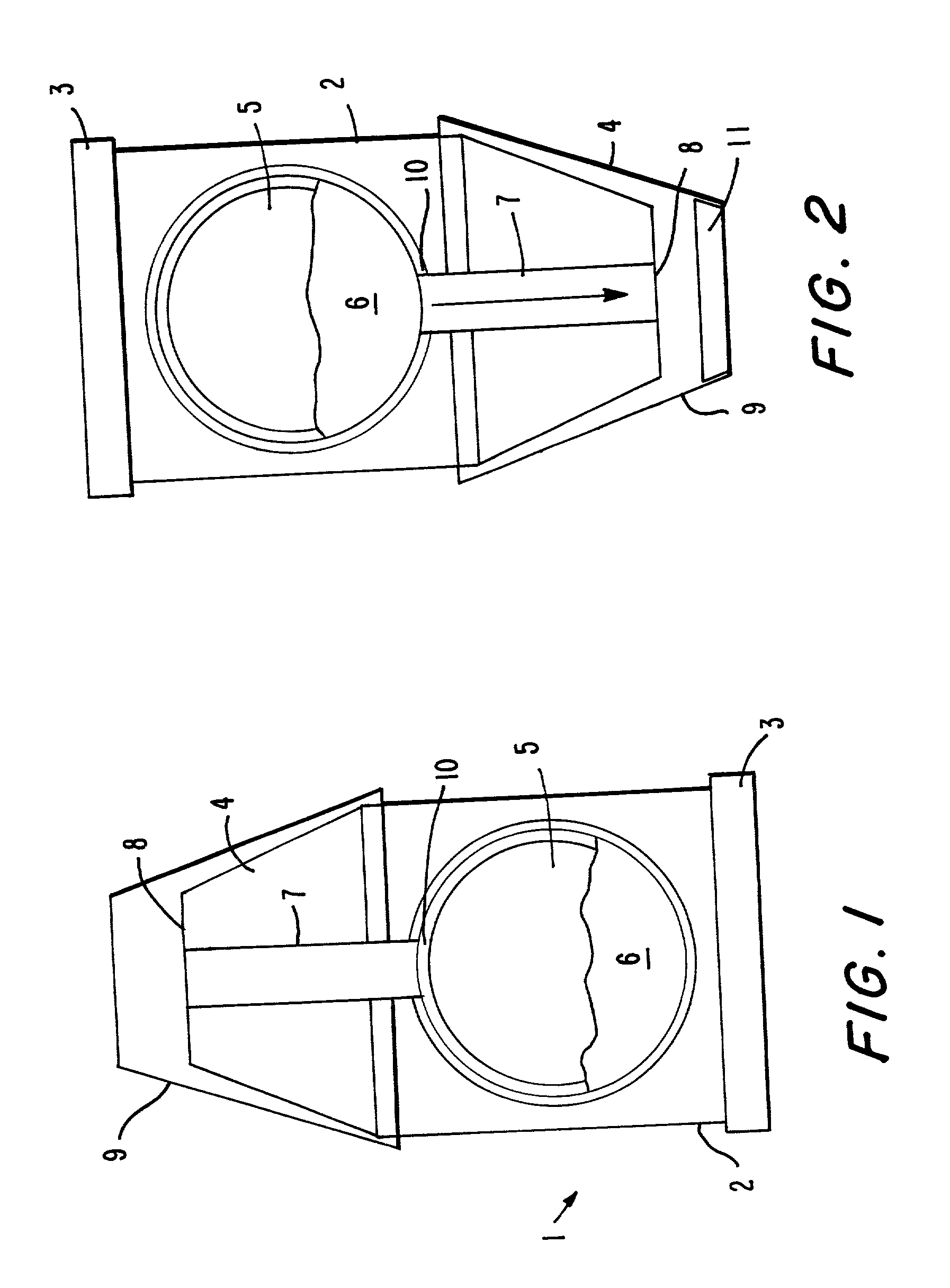
Substrates for drug delivery device and methods of preparing and use
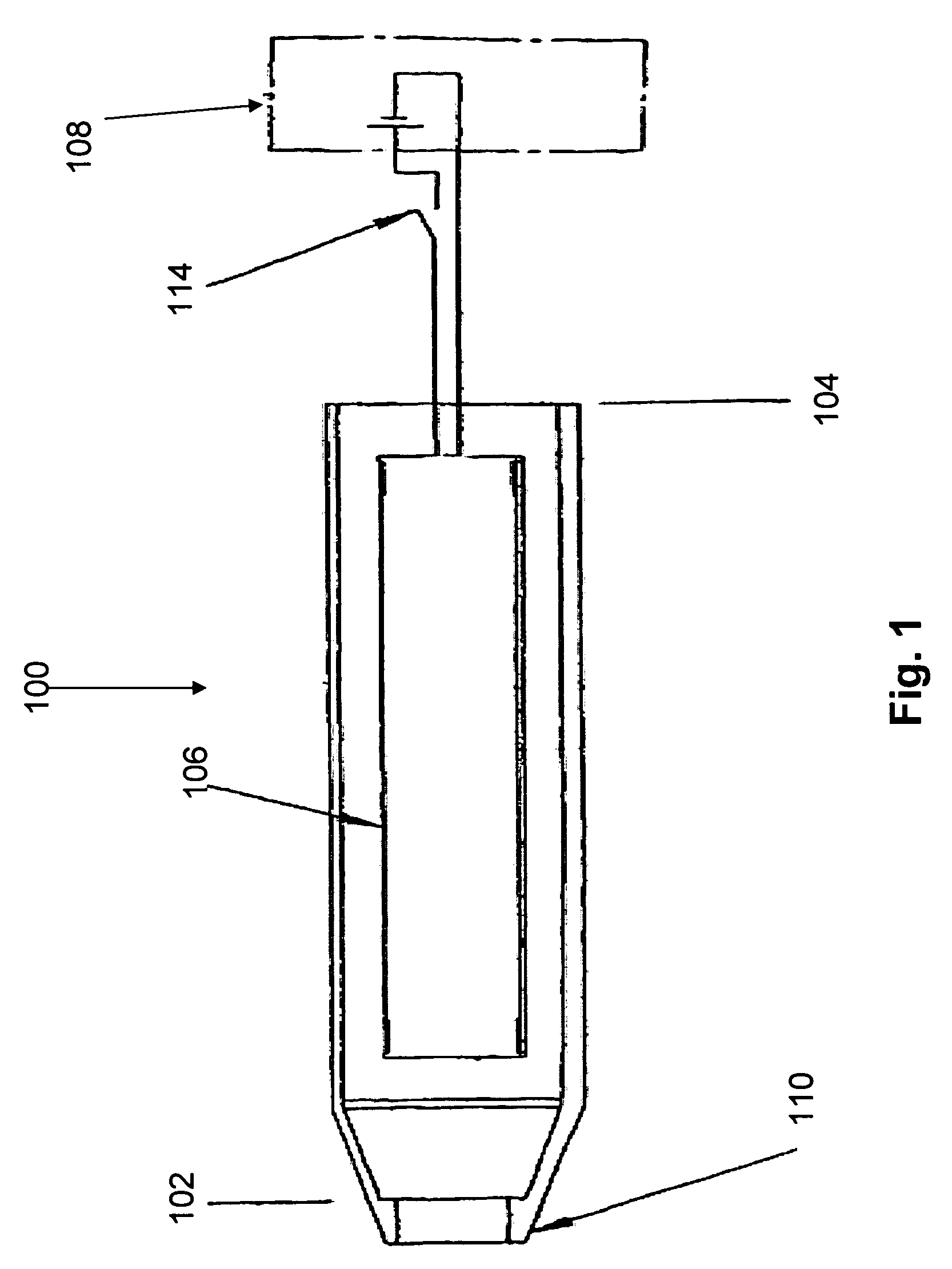
An assembly and method for producing a condensation aerosol are disclosed. The assembly includes a heat-conductive metal substrate with an oxidation resistant exterior surface and a drug composition film on the exterior surface and is for use in an aerosol device. The thickness of the film and the surface of the substrate is such that the aerosol formed by vaporizing and condensing the drug composition the aerosol contain 10% by weight or less drug-degradation products and at least 50% of the total amount of the drug composition in the film. The methods for treating the exterior surface include heat and chemical treatment and formation of a protective overcoat.
Owner:ALEXZA PHARMA INC
Particles incorporating surfactants for pulmonary drug delivery
Improved aerodynamically light particles for drug delivery to the pulmonary system, and methods for their synthesis and administration are provided. In a preferred embodiment, the aerodynamically light particles are made of a biodegradable material and have a tap density less than 0.4 g / cm3 and a mass mean diameter between 5 mum and 30 mum. The particles may be formed of biodegradable materials such as biodegradable polymers. For example, the particles may be formed of a functionalized polyester graft copolymer consisting of a linear alpha-hydroxy-acid polyester backbone having at least one amino acid group incorporated therein and at least one poly(amino acid) side chain extending from an amino acid group in the polyester backbone. In one embodiment, aerodynamically light particles having a large mean diameter, for example greater than 5 mum, can be used for enhanced delivery of a therapeutic agent to the alveolar region of the lung. The aerodynamically light particles incorporating a therapeutic agent may be effectively aerosolized for administration to the respiratory tract to permit systemic or local delivery of wide variety of therapeutic agents.
Owner:MASSACHUSETTS INST OF TECH
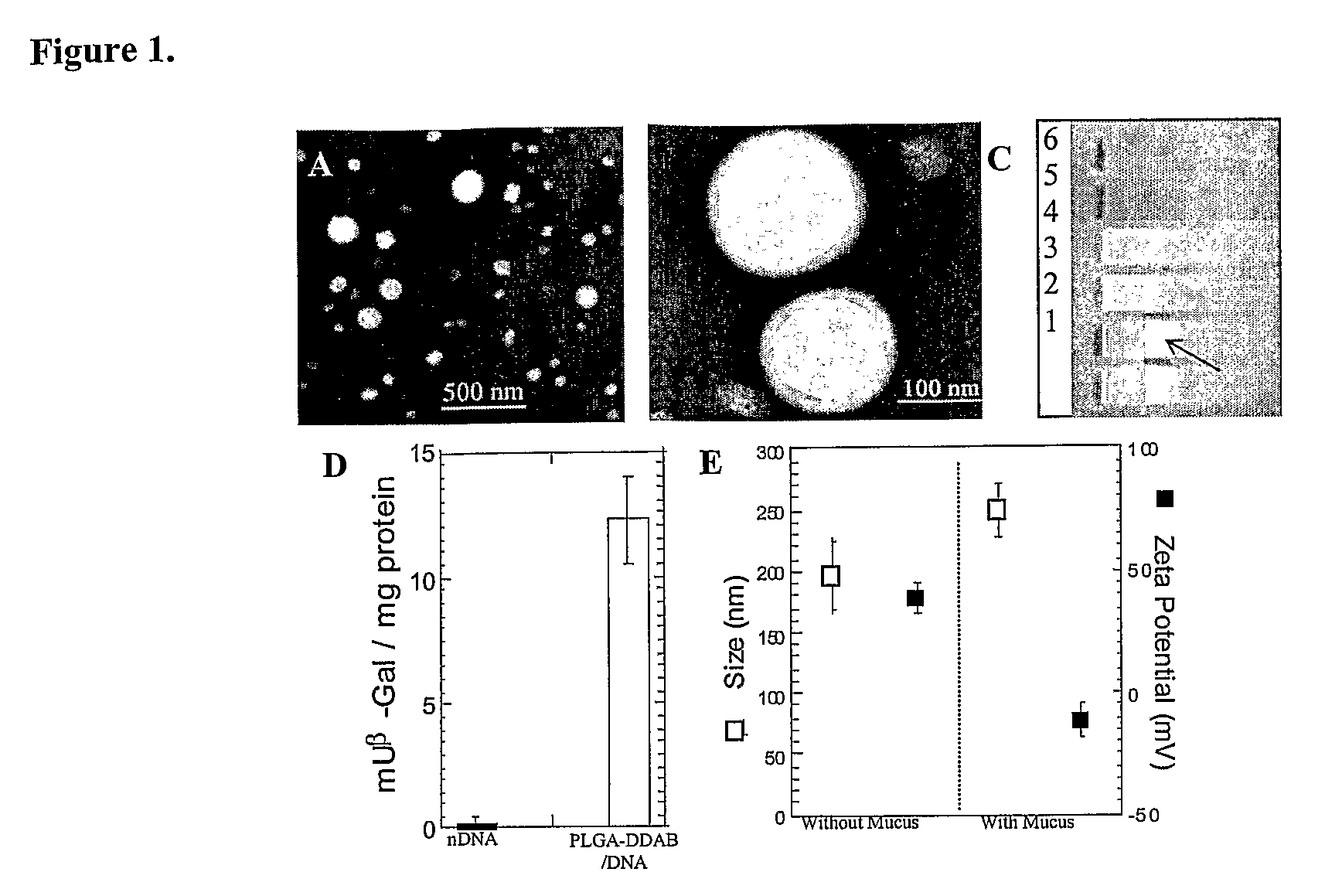
Drugs And Gene Carrier Particles That Rapidly Move Through Mucous Barriers
ActiveUS20080166414A1Easy adhesionPromote complexationPowder deliveryOrganic active ingredientsCrystallographyMucus
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
Compositions for inactivating pathogenic microorganisms, methods of making the compositions, and methods of use thereof
InactiveUS20060251684A1Reduce infectivityReduce morbiditySsRNA viruses negative-senseAntibacterial agentsPathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
Improved device and method for delivery of a medicament
ActiveUS20120255567A1Easy to useImprove the situationOrganic active ingredientsPowder deliveryDiseaseTherapeutic effect
The disclosure relates to an improved method of enhancing nicotine concentrations in a gaseous carrier. The methods are adaptable to the delivery of nicotine for therapeutic effect in various diseases, in particular nicotine for tobacco product use cessation, substitution and / or harm reduction. The disclosure further relates various devices and device design principles for practicing these methods.
Owner:PHILIP MORRIS PROD SA
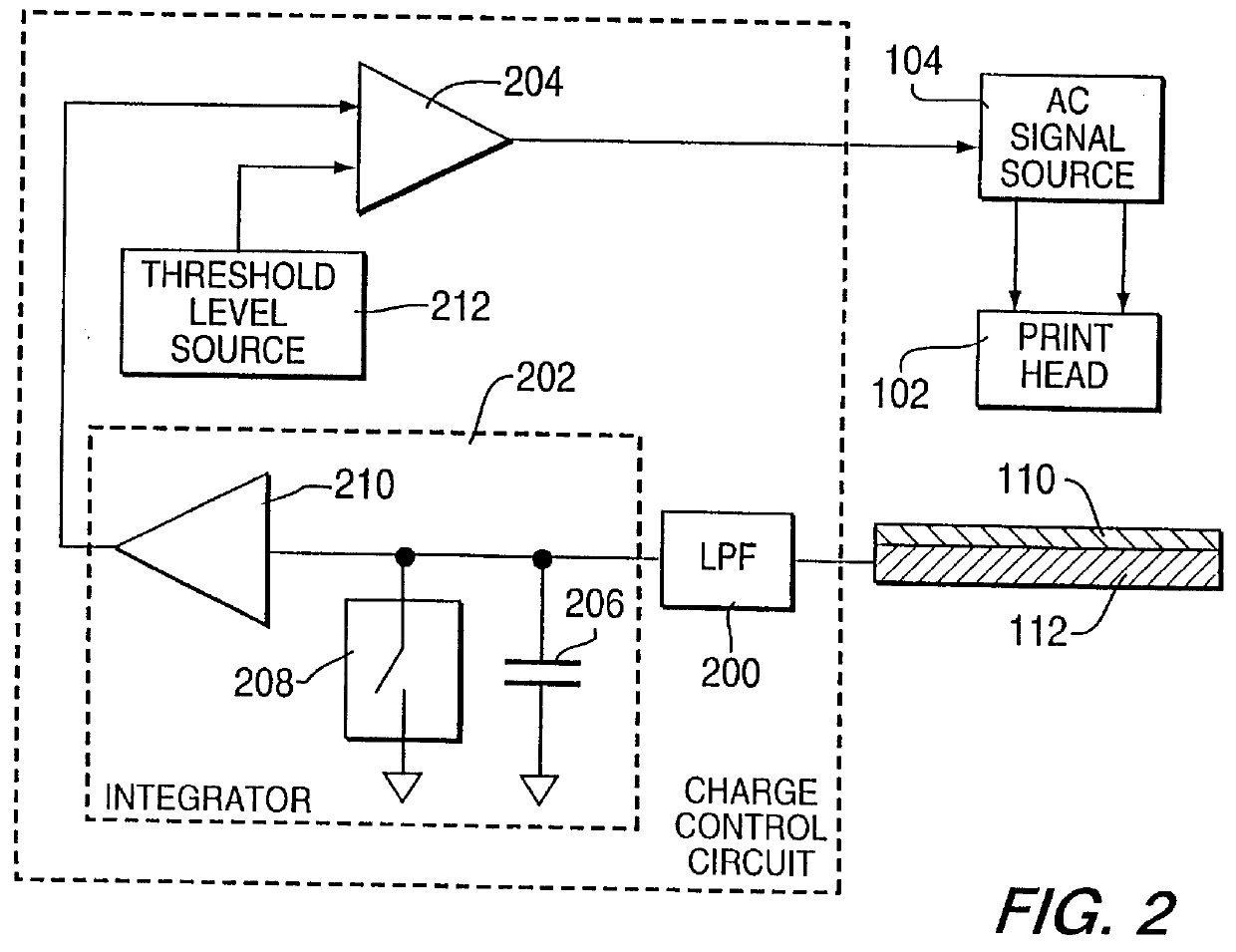
Method for electrostatically depositing a medicament powder upon predefined regions of a substrate
Method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, the apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
Method of Drug Formulation Based on Increasing the affinity of Crystalline Microparticle Surfaces for Active Agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
Method and apparatus for electrostatically depositing a medicament powder upon predefined regions of a substrate
A method for electrostatically depositing select doses of medicament powder at select locations on a substrate. Specifically, an apparatus contains a charged particle emitter for generating charged particles that charge a predefined region of a substrate and a charge accumulation control circuit for computing the amount of charge accumulated upon the substrate and deactivating the emitter when a selected quantity of charge has accumulated. Additionally, a triboelectric charging apparatus charges the medicament powder and forms a charged medicament cloud proximate the charged region of the substrate. The medicament particles within the medicament cloud electrostatically adhere to the charged region. The quantity of charge accumulated on the substrate at the predefined region and the charge-to-mass ratio of the medicament powder in the cloud control the amount (dose) of medicament deposited and retained by the substrate. Consequently, this apparatus accurately controls both medicament dosage and deposition location. Furthermore, since the substrate can be of any dielectric material that retains an electrostatic charge, the apparatus can be used to deposit medicament on substrates that are presently used in oral medicament consumption, e.g., substrates that are used to fabricate suppositories, inhalants, tablets, capsules and the like.
Owner:DELSYS PHARMA
Engineered particles and methods of use
InactiveUS7306787B2Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
Processes for making particle-based pharmaceutical formulations for pulmonary or nasal administration
InactiveUS20070178166A1Improve stabilityStability storage conditionPowder deliverySpray deliveryPowder mixtureNanoparticle
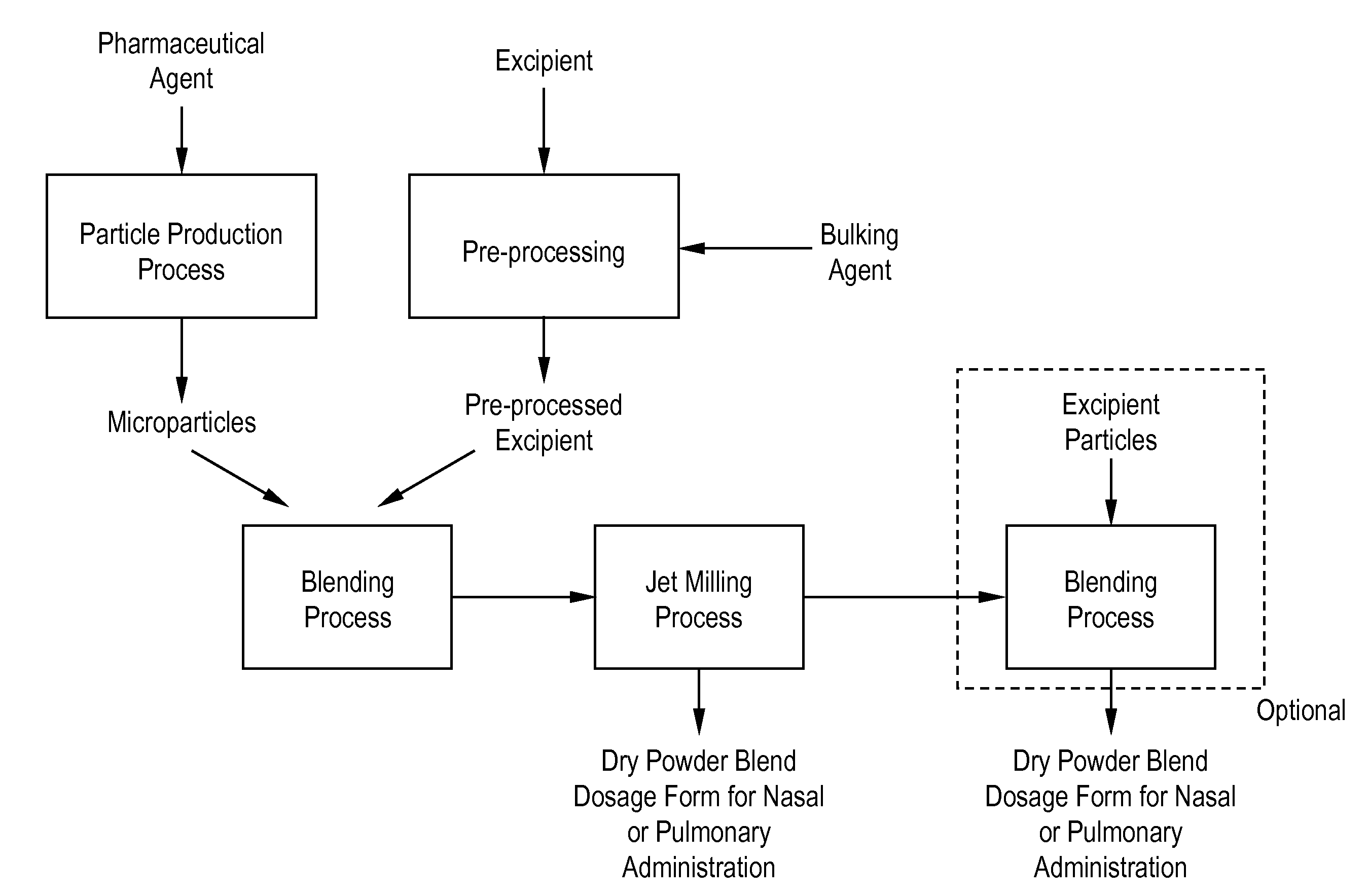
Dry powder pharmaceutical formulations for pulmonary or nasal administration are made to provide an improved respired dose. These formulations may be blends of milled blends and may include a phospholipid, alone or in combination with other excipient materials. In one case, the process includes the steps of (a) providing particles which comprise a pharmaceutical agent, (b) blending the particles with particles of at least one first excipient to form a first powder blend; (c) milling the first powder blend to form a milled blend which comprises microparticles or nanoparticles of the pharmaceutical agent; and (d) blending the milled blend with particles of a second excipient to form a blended dry powder blend pharmaceutical formulation suitable for pulmonary or nasal administration.
Owner:ACUSPHERE INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
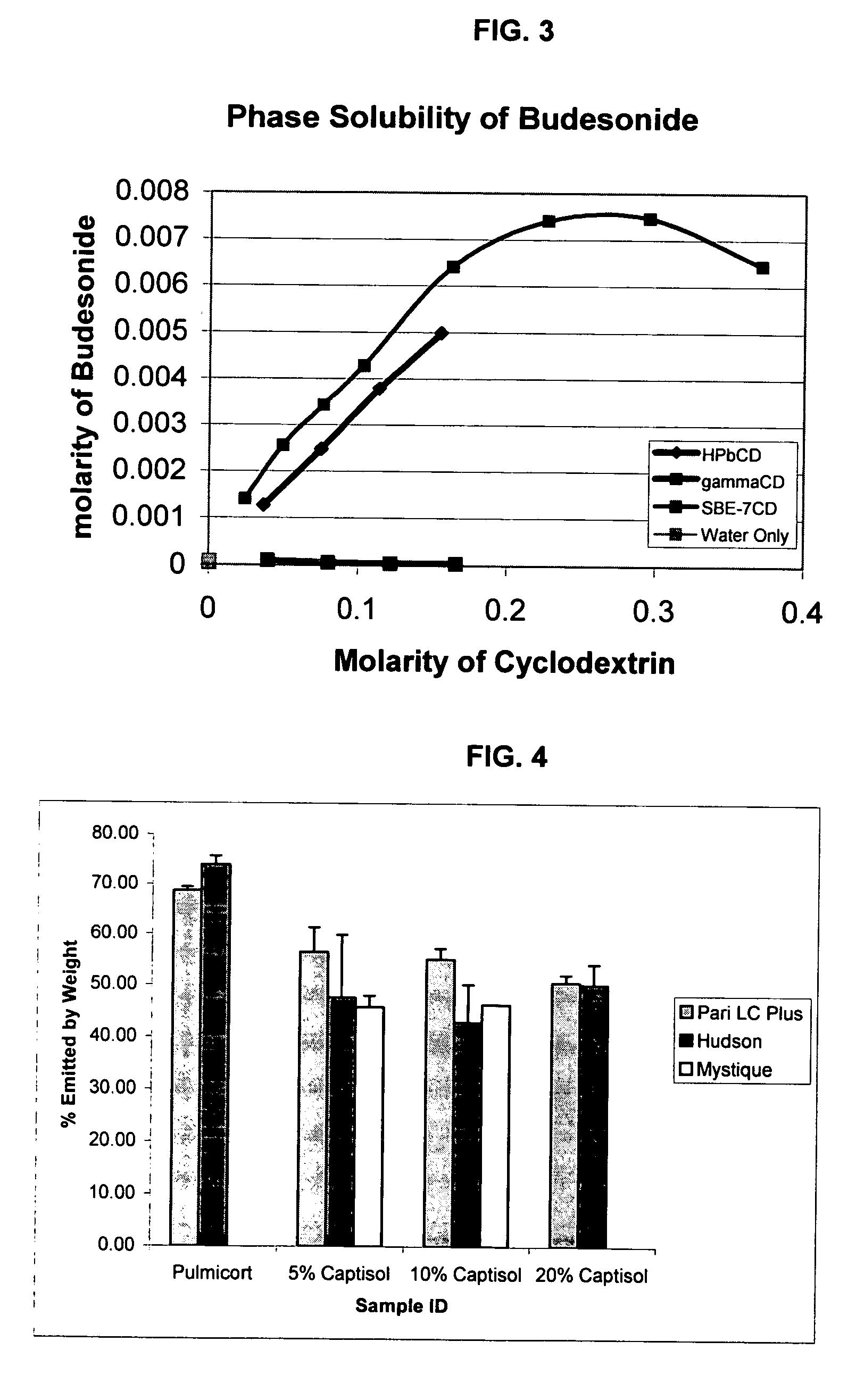
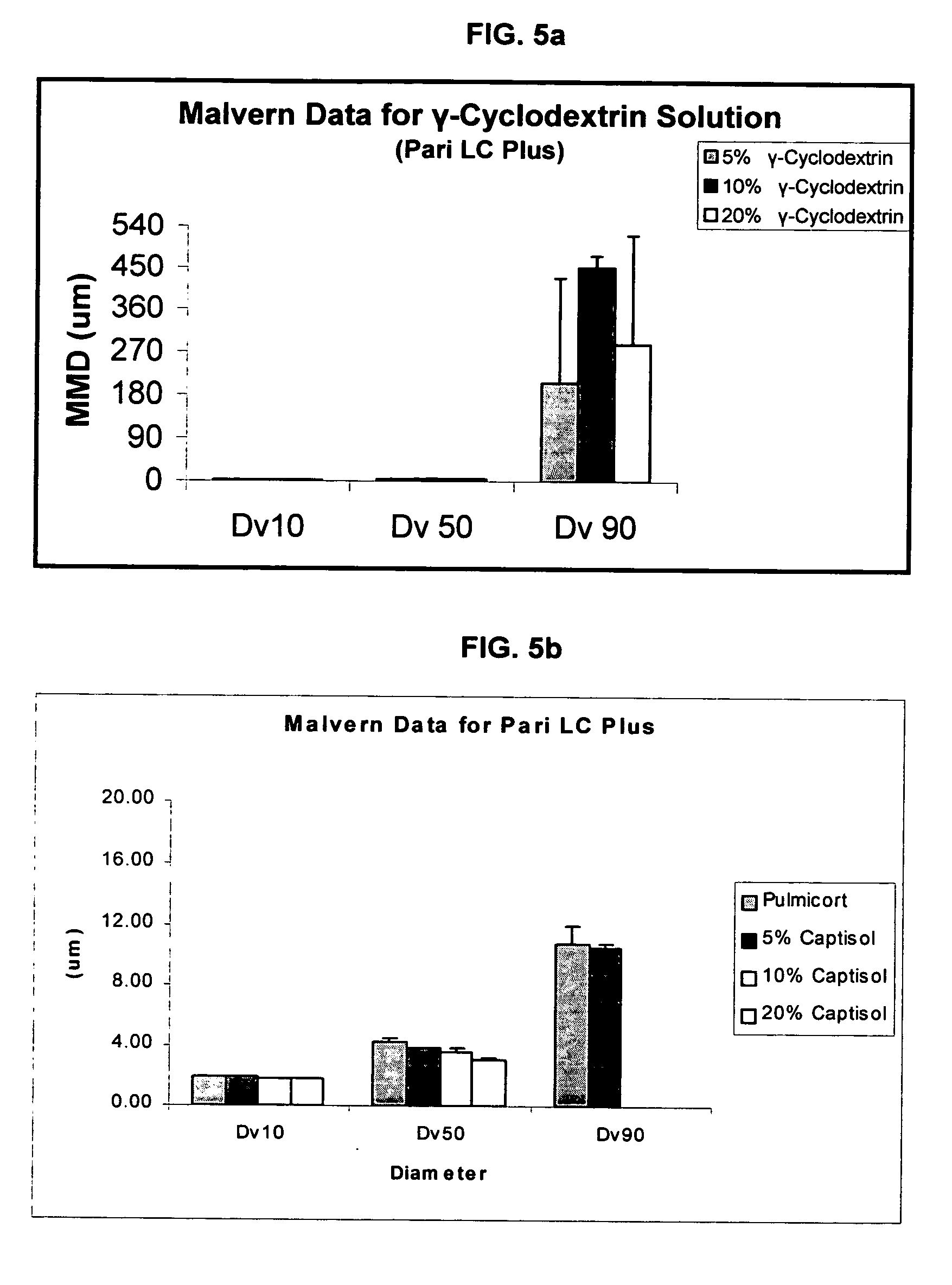
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Method of Drug Formulation Based on Increasing the Affinity of Active Agents for Crystalline Microparticle Surfaces
ActiveUS20070059374A1Reduce solubilityPromote associationPowder deliveryOrganic active ingredientsActive agentMicroparticle
Methods are provided for promoting the adsorption of an active agent to microparticles by modifying the structural properties of the active agent in order to facilitate favorable association to the microparticle.
Owner:MANNKIND CORP
Nanoparticulate corticosteroid and antihistamine formulations
InactiveUS20060216353A1Less liver toxicityUseful in prophylaxis and chronic treatment of asthmaBiocidePowder deliveryPediatric patientMicroparticle
Compositions comprising a nanoparticulate corticosteroid and an antihistamine are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining an antihistamine with a nanoparticulate corticosteroid in a single formulation results in improved efficacy.
Owner:ALKERMES PHARMA IRELAND LTD
Compositions for inactivating pathogenic microorganisms, methods of making the compositons, and methods of use thereof
InactiveUS20050208083A1Reduce infectivityReduce morbidityAntibacterial agentsSsRNA viruses negative-sensePathogenic microorganismOrganic solvent
Nanoemulsion compositions with low toxicity that demonstrate broad spectrum inactivation of microorganisms or prevention of diseases are described. The nanoemulsions contain an aqueous phase, an oil phase comprising an oil and an organic solvent, and one or more surfactants. Methods of making nanoemulsions and inactivating pathogenic microorganisms are also provided.
Owner:NANOBIO CORP
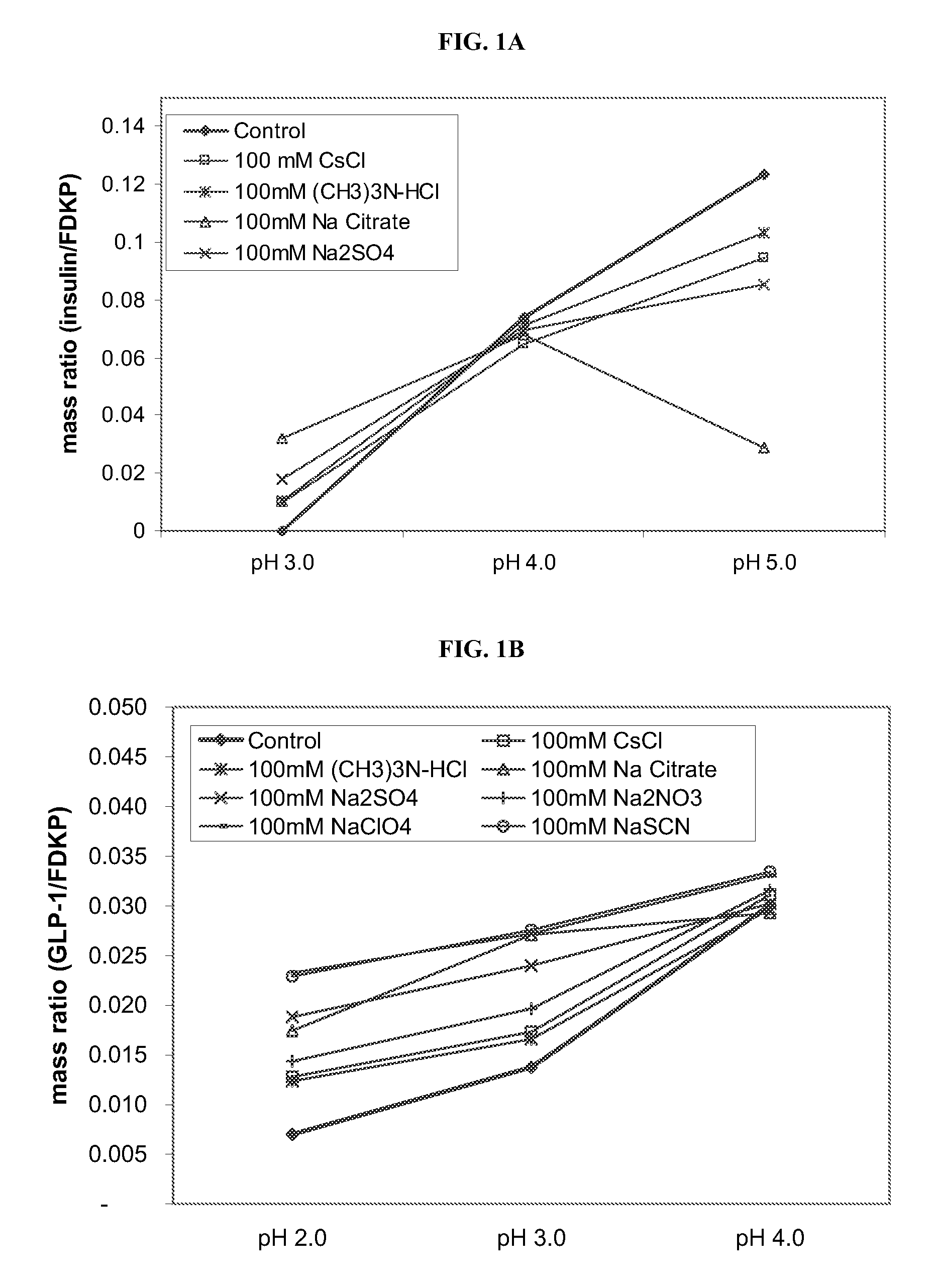
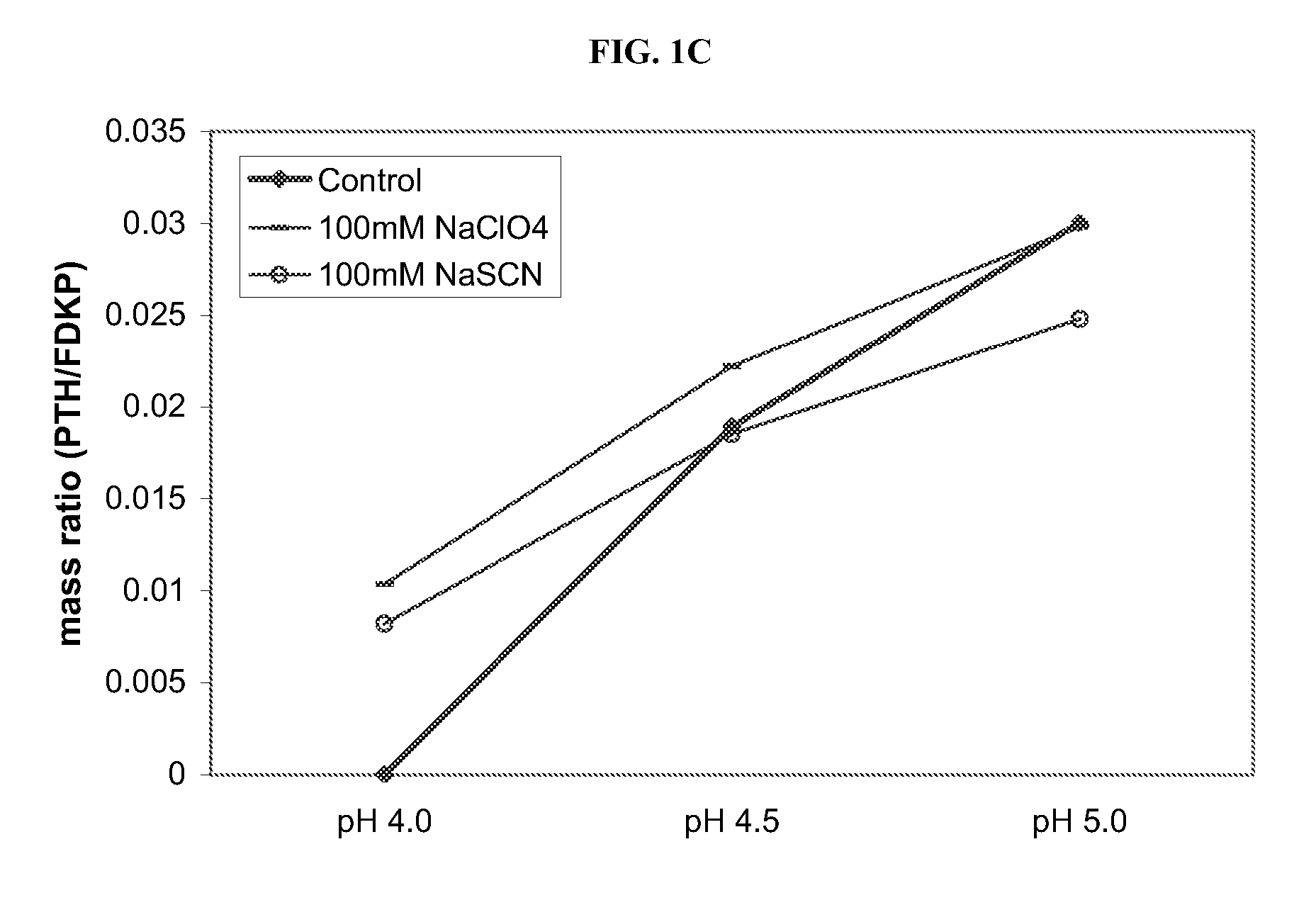
Compositions and methods for treating insulin resistance and diabetes mellitus
Provided are electrokinetically-altered fluids (gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating diabetes and diabetes-associated conditions or disorders (e.g., insulin resistance), or symptoms thereof. Provided are electrokinetically-altered ioinic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Semi-soft C-class immunostimulatory oligonucleotides
The invention relates to specific C-Class semi-soft CpG immunostimulatory oligonucleotides that are useful for stimulating an immune response. In particular the oligonucleotides are useful for treating allergy, such as allergic rhinitis and asthma, cancer and infectious disease, such as hepatitis B and hepatitis C.
Owner:COLEY PHARMA GRP INC +1
Delivery of drug esters through an inhalation route
The present invention relates to the delivery of drug esters through an inhalation route. Specifically, it relates to aerosols containing drug esters that are used in inhalation therapy. In a method aspect of the present invention, a drug ester is delivered to a patient through an inhalation route. The method comprises: a) heating a coating of a drug ester, on a solid support, to form a vapor; and, b) passing air through the heated vapor to produce aerosol particles having less than 5% drug ester degradation product. In a kit aspect of the present invention, a kit for delivering a drug ester through an inhalation route is provided which comprises: a) a thin coating of a drug ester composition and b) a device for dispensing said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
Delivery of drug esters through an inhalation route
The present invention relates to the delivery of drug esters through an inhalation route. Specifically, it relates to aerosols containing drug esters that are used in inhalation therapy. In a method aspect of the present invention, a drug ester is delivered to a patient through an inhalation route. The method comprises: a) heating a composition, wherein the composition comprises a drug ester, to form a vapor; and, b) allowing the vapor to cool, thereby forming a condensation aerosol comprising particles with less than 5% drug ester degradation product. In a kit aspect of the present invention, a kit for delivering a drug ester through an inhalation route is provided which comprises: a) a thin coating of a drug ester composition and b) a device for dispensing said thin coating as a condensation aerosol.
Owner:ALEXZA PHARMA INC
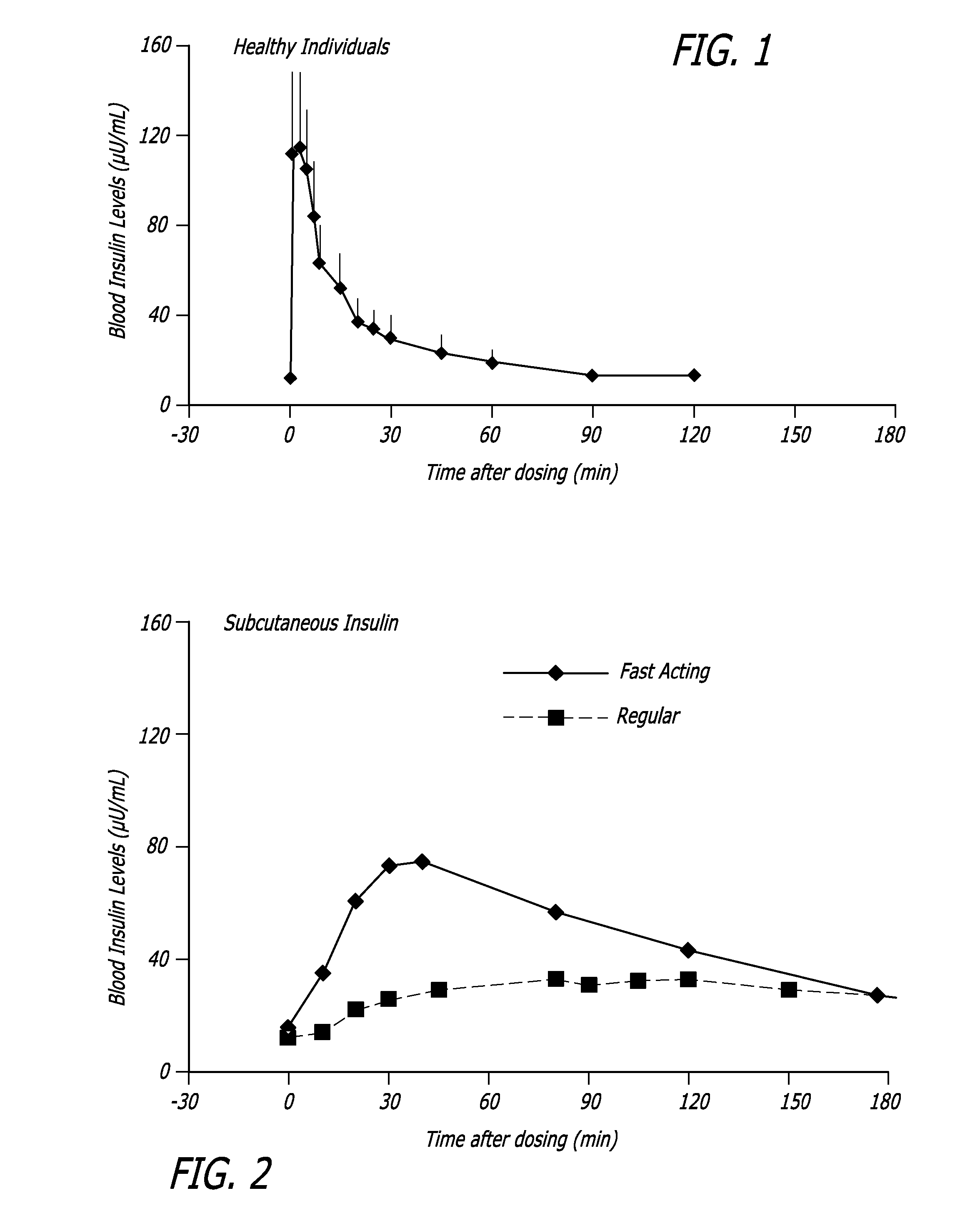
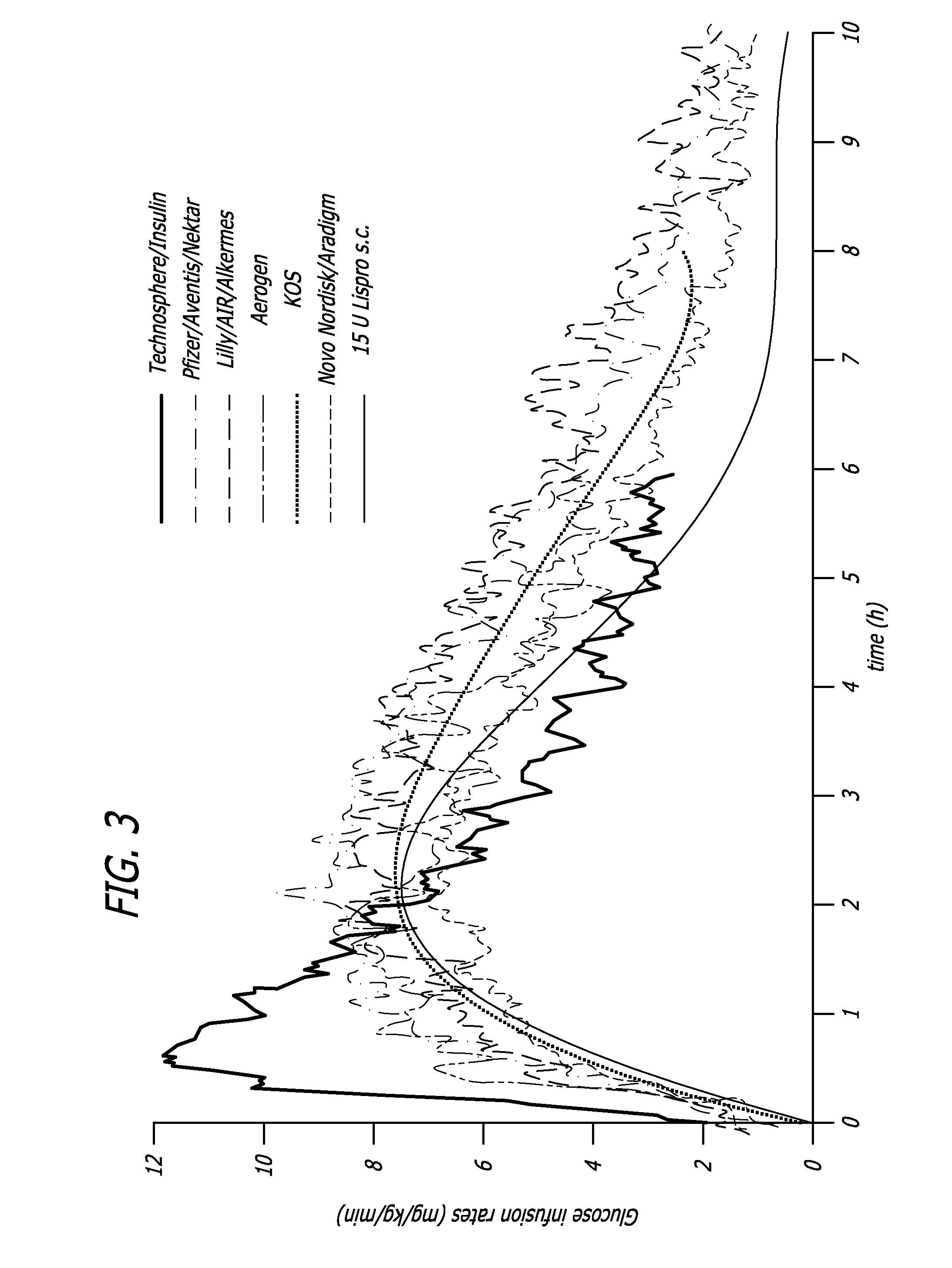
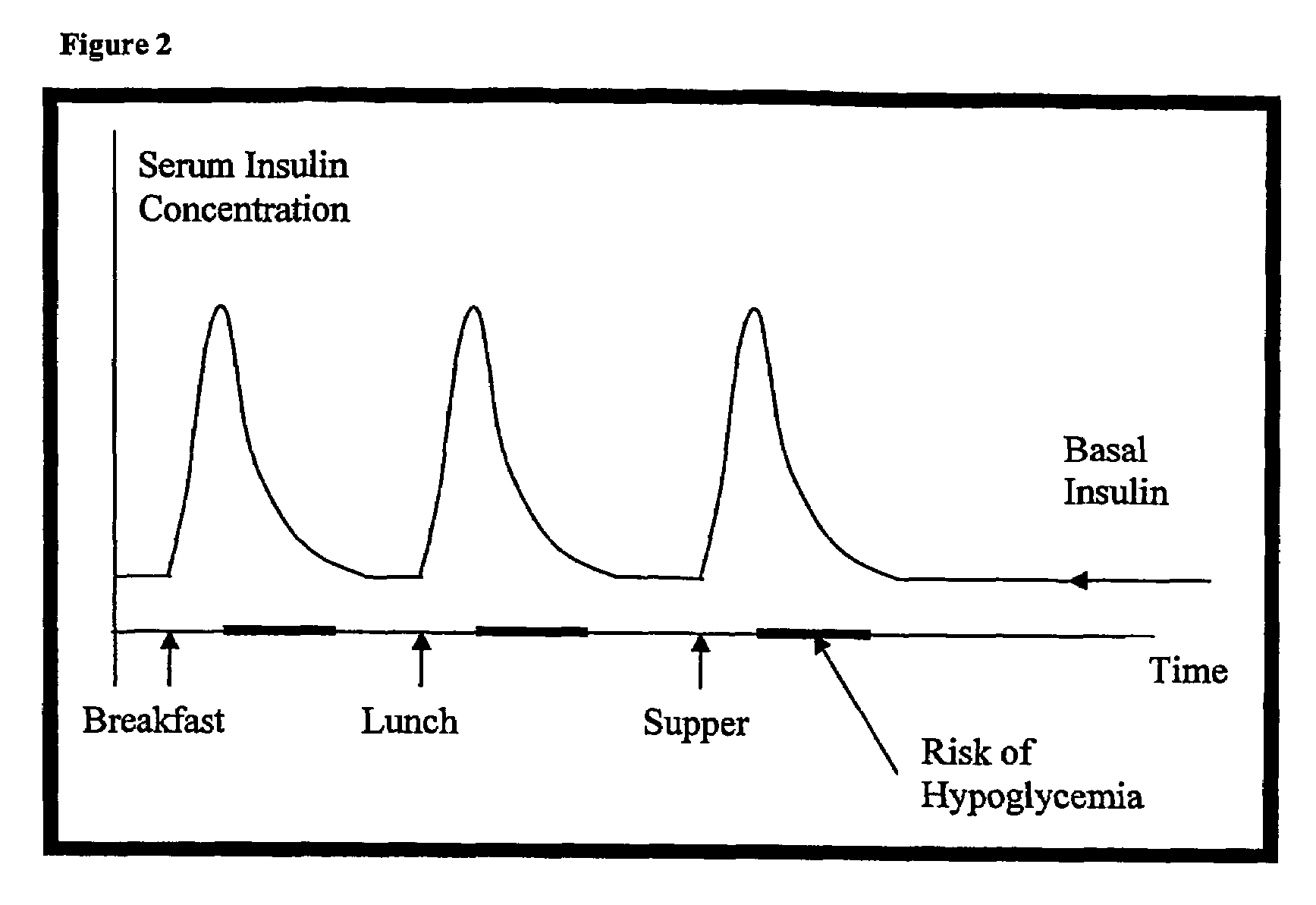
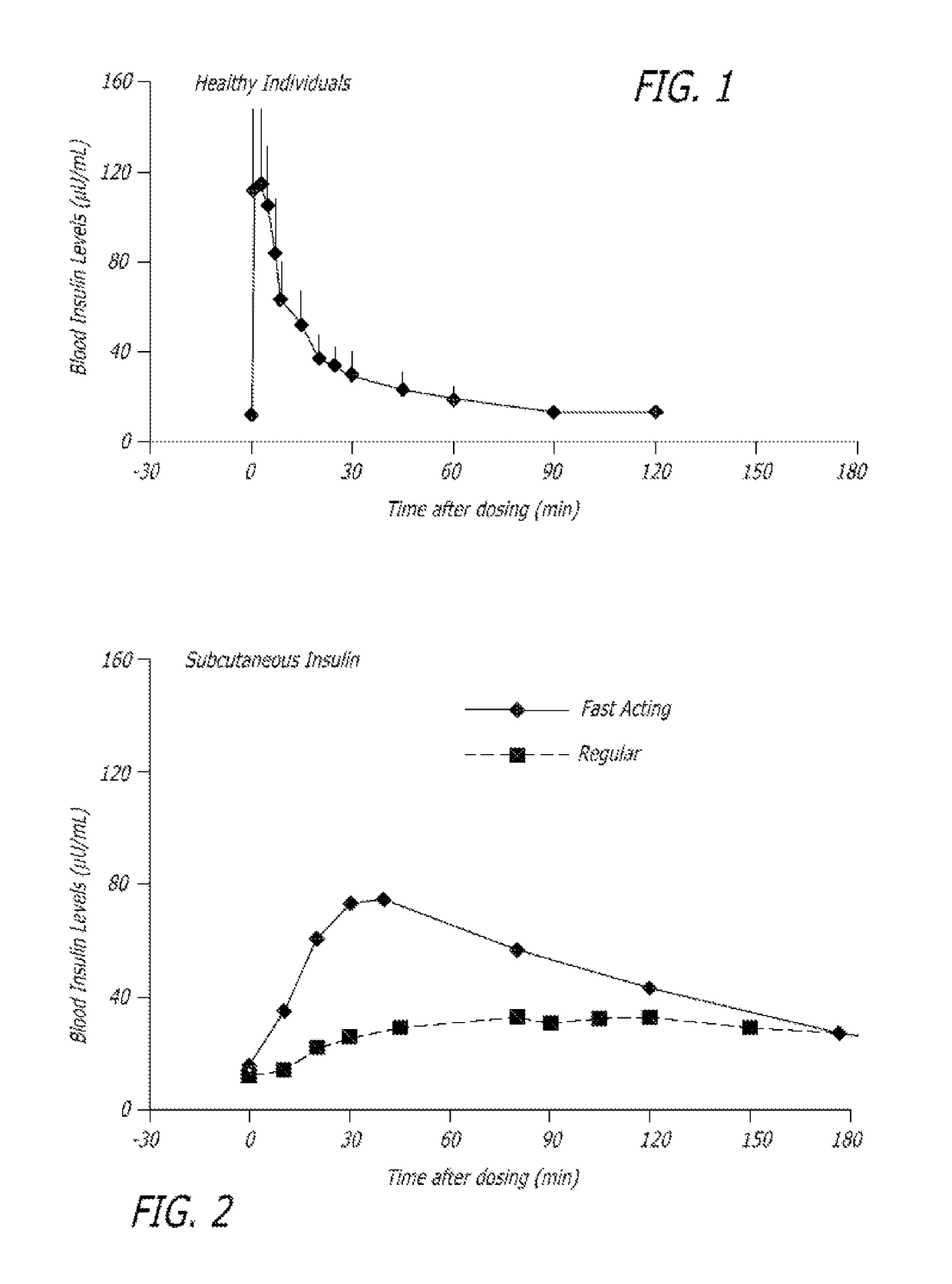
Superior control of blood glucose in diabetes treatment
InactiveUS20060239934A1Easy to controlReduce riskOrganic active ingredientsPowder deliveryPostprandial HypoglycemiaGlucose fluctuations
Methods related to the treatment of diabetes and improving the control of blood glucose levels are provided. In particular, methods are provided for effectively reducing postprandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration. Additionally, methods for effectively reducing post-prandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration along with a long-acting basal insulin.
Owner:MANNKIND CORP
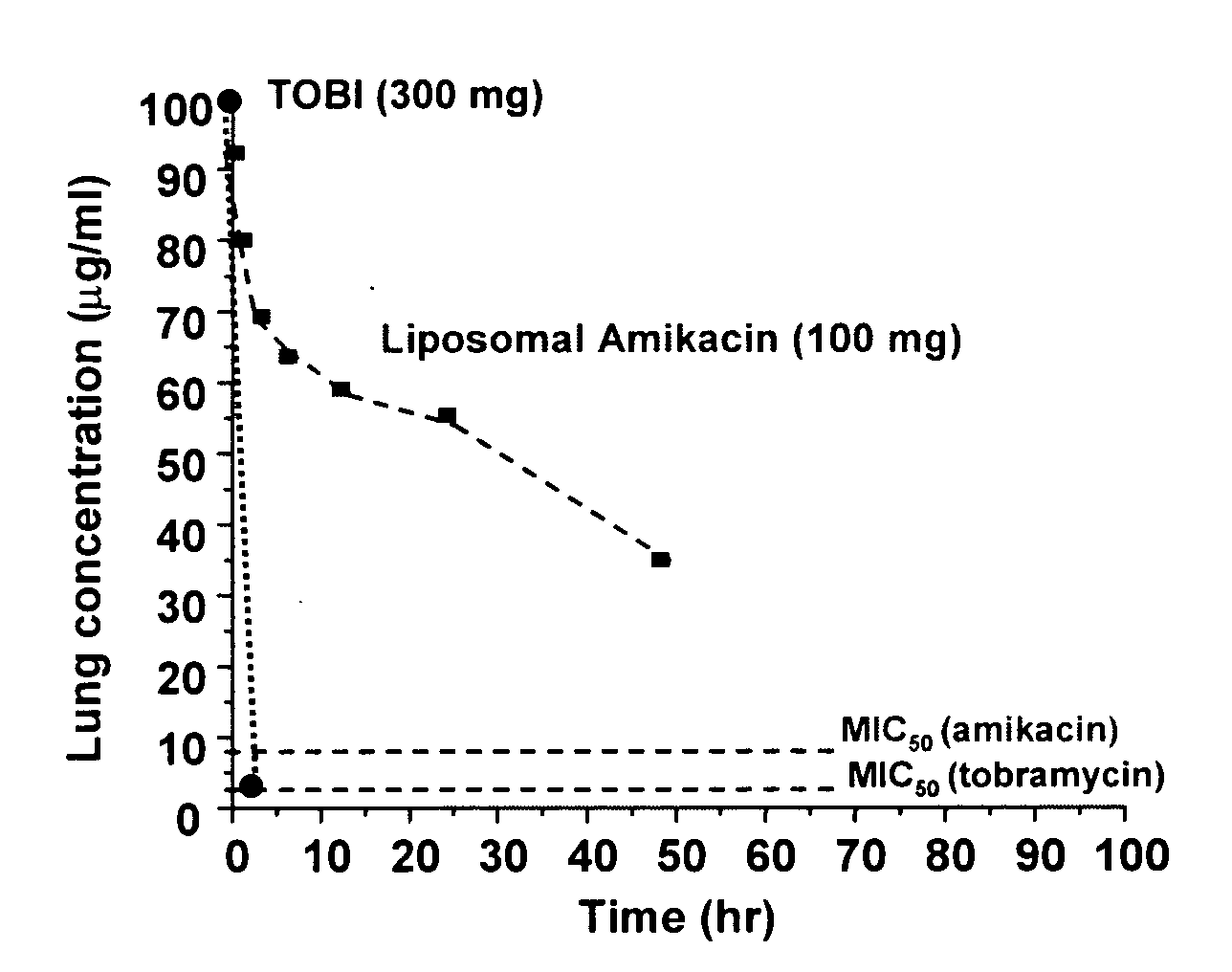
Lipid-based compositions of antiinfectives for treating pulmonary infections and methods of use thereof
ActiveUS20070196461A1Good treatment effectConvenient treatmentAntibacterial agentsPowder deliveryLipid formationPulmonary infection
A system for treating or providing prophylaxus against a pulmonary infection is disclosed comprising: a) a pharmaceutical formulation comprising a mixture of free antiinfective and antiinfective encapsulated in a lipid-based composition, and b) an inhalation delivery device. A method for providing prophylaxis against a pulmonary infection in a patient and a method of reducing the loss of antiinfective encapsulated in a lipid-based composition upon nebulization comprising administering an aerosolized pharmaceutical formulation comprising a mixture of free antiinfective and antiinfective encapsulated in a lipid-based composition is also disclosed.
Owner:INSMED INC
Aerosol drug formulations containing hydrofluoroalkanes and alkyl saccharides
InactiveUS6932962B1Function increaseGood dispersionPowder deliveryDispersion deliveryAerosol drugsActive agent
Aerosol formulations suitable for use in pressurised metered dose inhalers comprise a hydrofluoroalkane propellant, an medicament for inhalation and a surfactant which is a a C8–C16 fatty acid or salt thereof, a bile salt, a phospholipid, or an alkyl saccharide.
Owner:ASTRAZENECA AB
Compositions and methods for treating asthma and other lung disorders
InactiveUS20100008997A1Powder deliveryOrganic active ingredientsObstructive Pulmonary DiseasesOxygen
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination treatment methods comprising administration of electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Method of drug formulation based on increasing the affinity of crystalline microparticle surfaces for active agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
Methods of determining film thicknesses for an aerosol delivery article
InactiveUS20050037506A1Efficient and reliable and accurateQuick fixPowder deliveryOrganic active ingredientsMedicineVaporization
Methods for determining the film thickness of a compound composition needed to provide a selected purity and yield of a condensation aerosol via vaporization of the compound composition have applications in aerosol delivery technology, in pulmonary drug delivery, and in other therapeutic treatment regimes. The methods for determining such film thickness, for use in a device having a film of drug composition to be aerosolized, include generating purities and yields of a drug composition by vaporizing films of the drug composition from substrates at two or more temperatures in the range of 150° C. to 500° C. and two or more film thickness in the range of 0.05 to 50 microns, determining from these yields and purities if a thickness and temperature exist where the aerosol has at least 90% purity and at least 50% yield, and repeated such measurements until the selected purity and yield requirement are met.
Owner:ALEXZA PHARMA INC
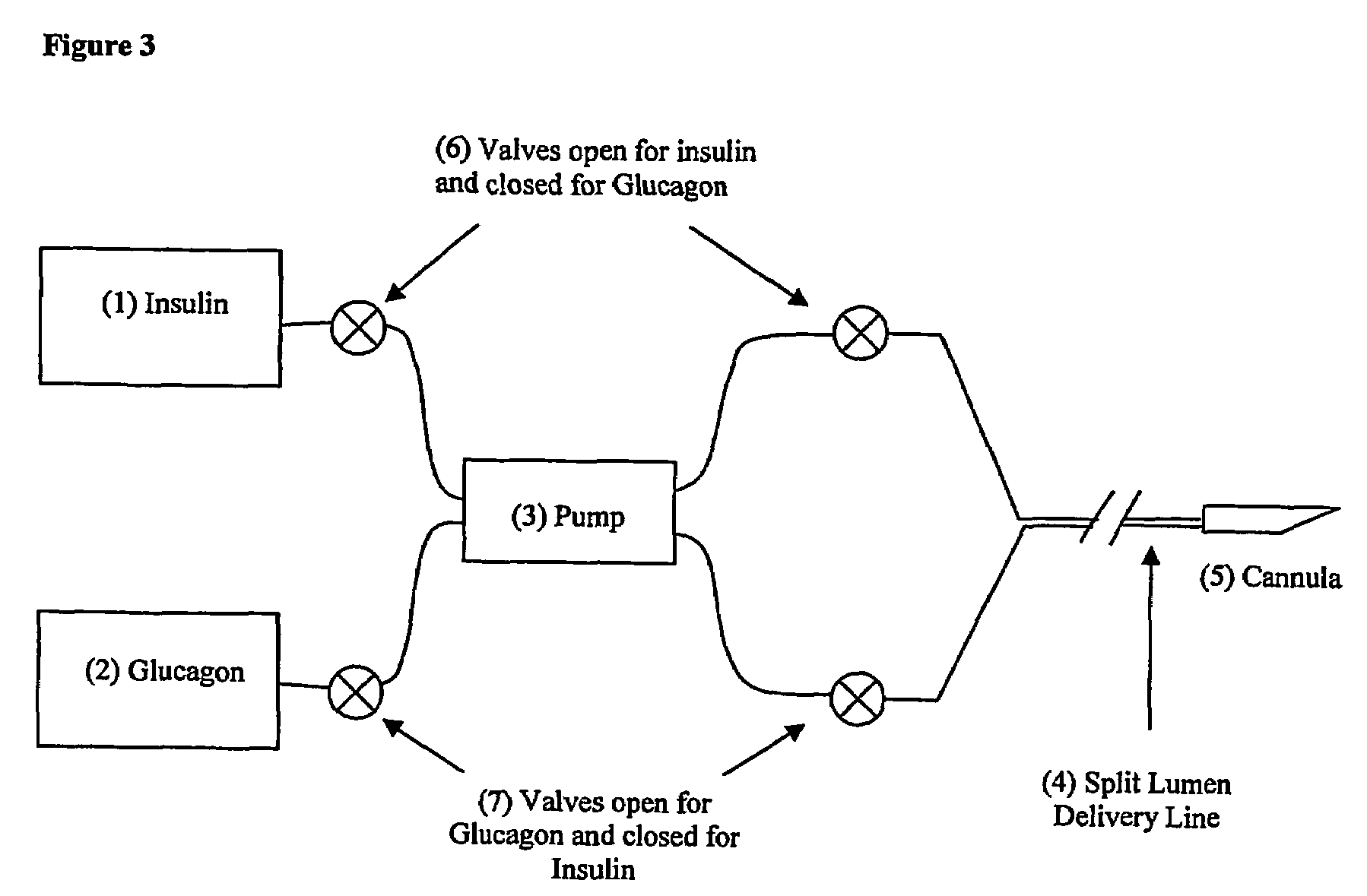
Compositions and methods for the prevention and control of insulin-induced hypoglycemia
InactiveUS7314859B2Extended half-lifePowder deliveryDispersion deliveryInsulin induced hypoglycemiaCvd risk
Pharmaceutical composition comprising both insulin and glucagon can be administered to control and treat diabetes while reducing or eliminating the risk of insulin-induced hypoglycemia.
Owner:DIOBEX INC
Delivery of diazepam through an inhalation route
The present invention relates to the delivery of compounds for the treatment of anxiety disorders and symptoms of such disorders through an inhalation route. Specifically, it relates to aerosols containing that are used in inhalation therapy. In a method aspect of the present invention, diazepam is administered to a patient through an inhalation route. The method comprises: a) heating a composition, comprising diazepam to form a vapor; and, b) allowing the vapor to cool, thereby forming a condensation aerosol with less than 5% of the drug degradation products. In a kit aspect of the present invention, a kit for delivering diazepam through an inhalation route is provided which comprises: a) a thin coating of a diazepam composition; and, b) a device for dispending said thin coating as a condensation aerosol
Owner:ALEXZA PHARMA INC
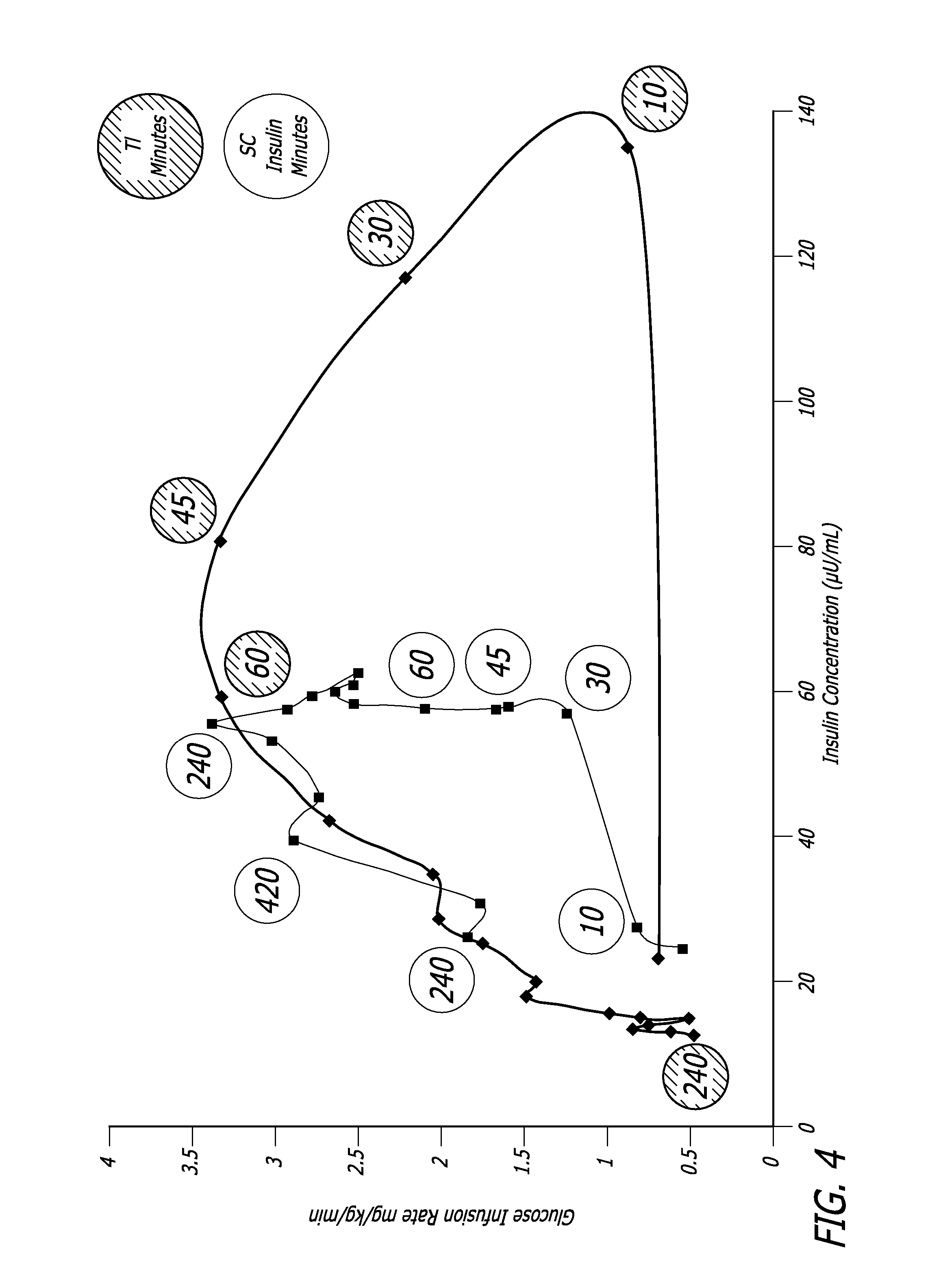
Method of treating diabetes type 2 by metformin and an ultrarapid acting insulin
Disclosed herein are improved methods of treating hyperglycemia with a combination of an ultrarapid acting insulin and insulin glargine comprising prandial administration of the ultrarapid insulin, and administration of a first dose of insulin glargine within 6 hours of waking for a day.
Owner:MANNKIND CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com