Patents
Literature
179 results about "Budesonide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain bowel conditions (such as Crohn's disease, ulcerative colitis).
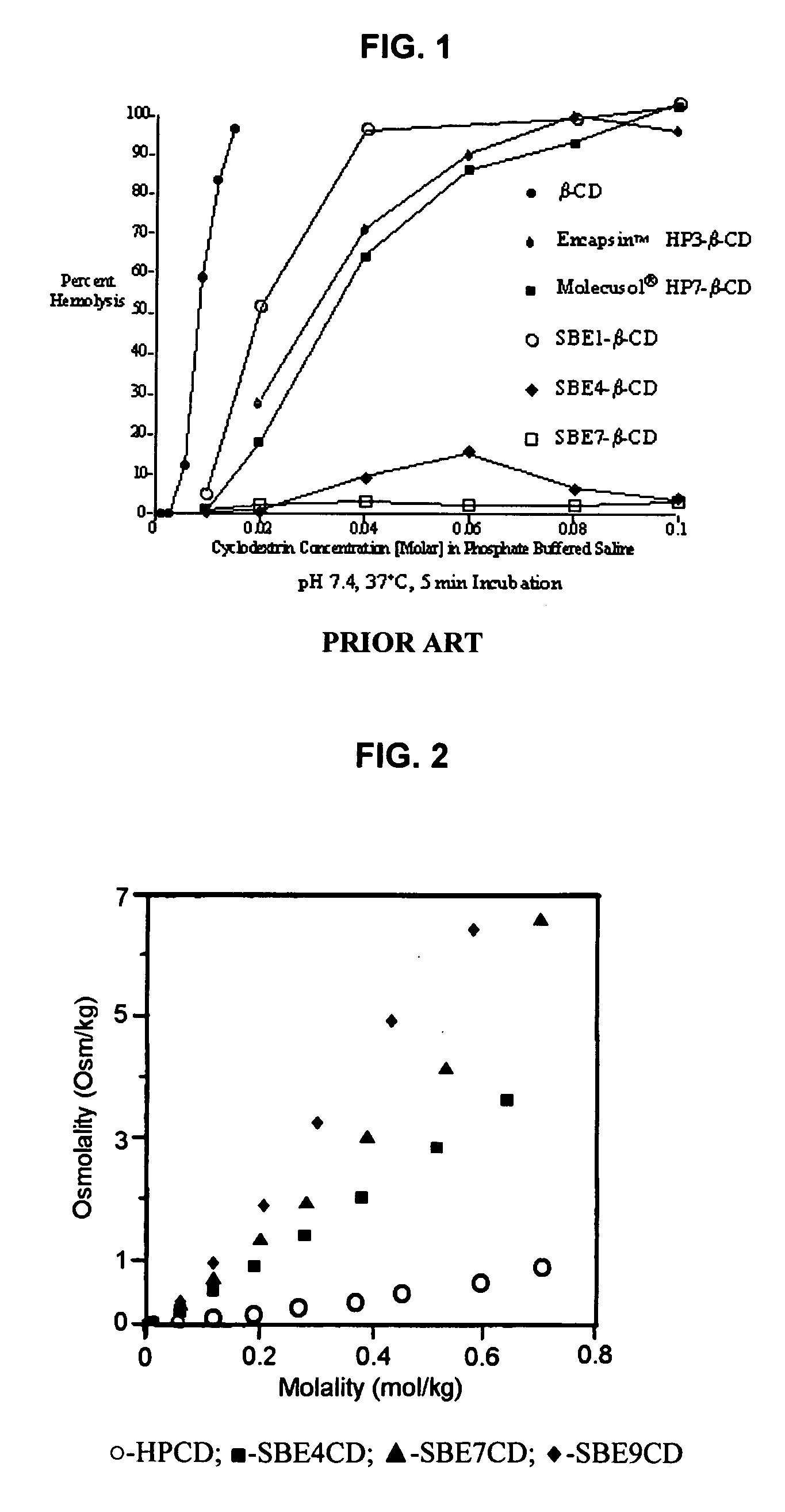
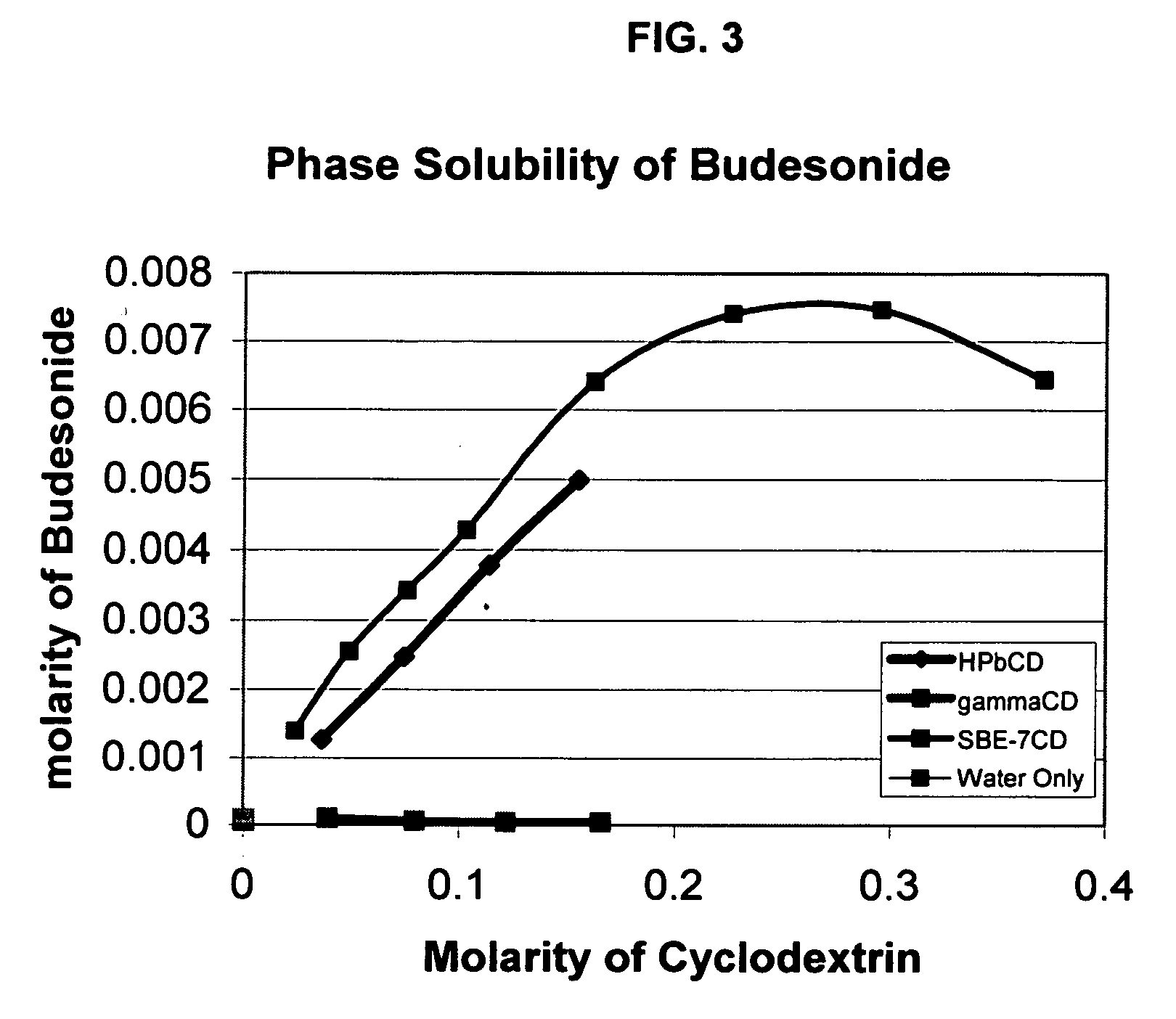
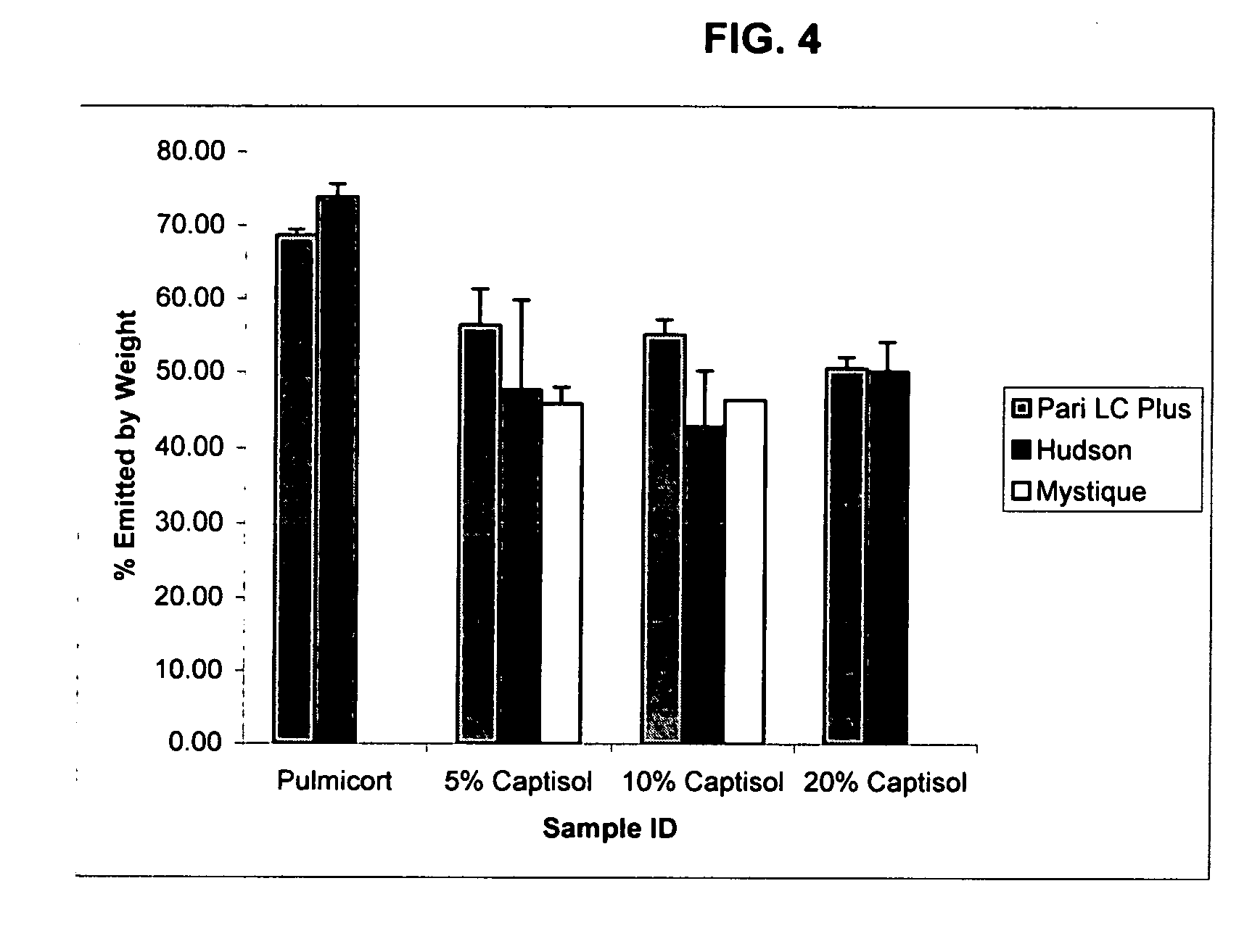
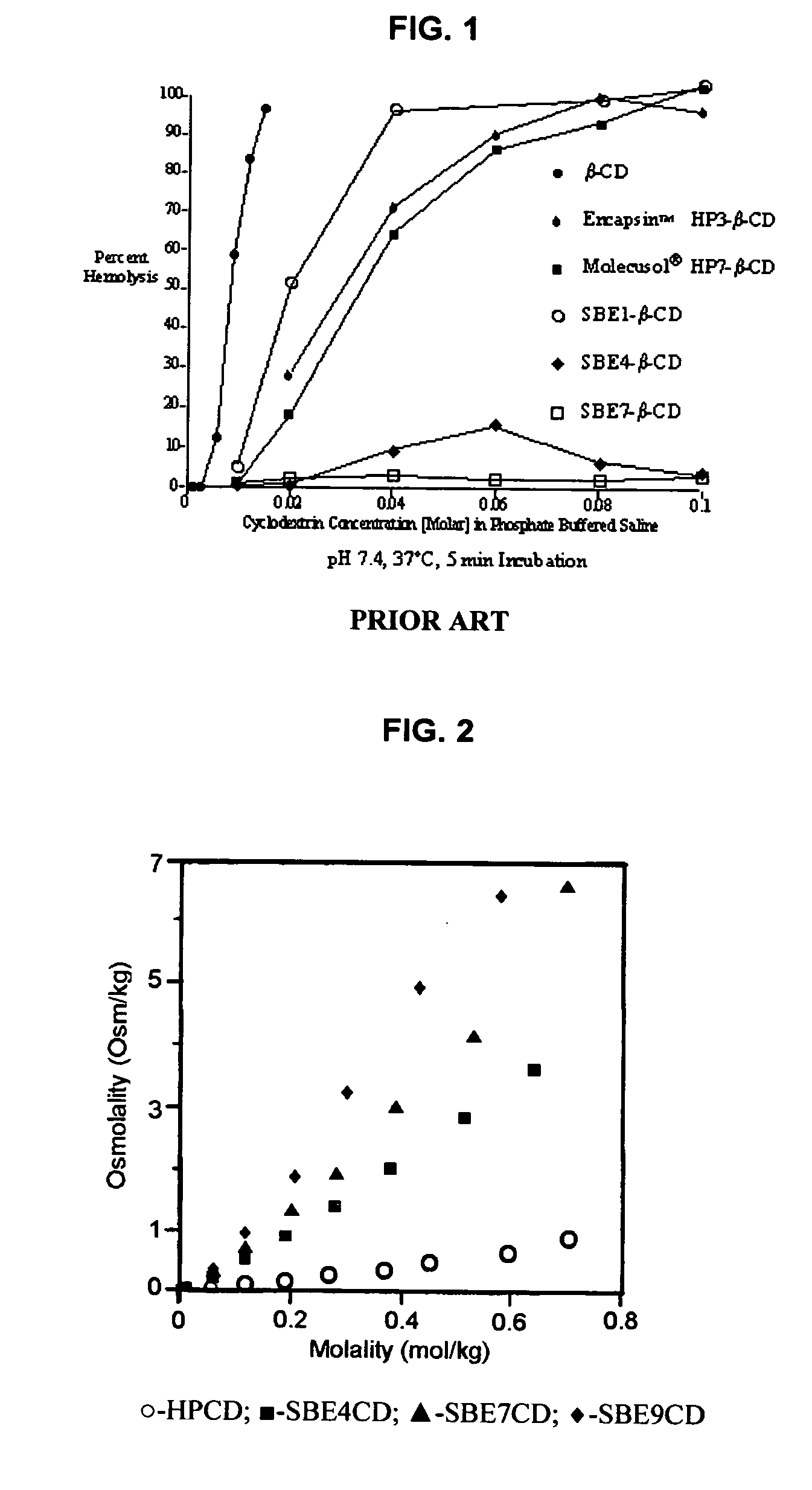
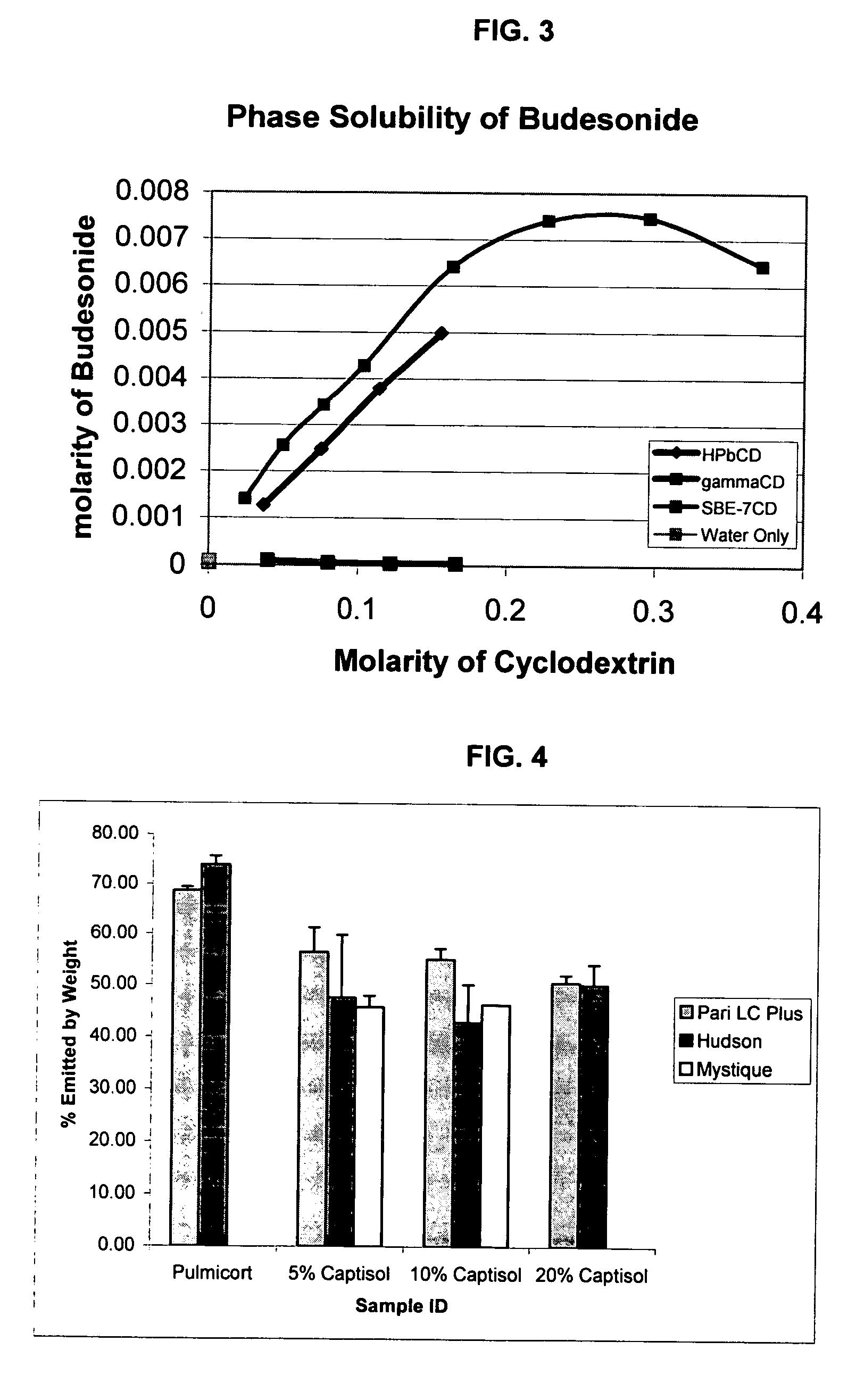
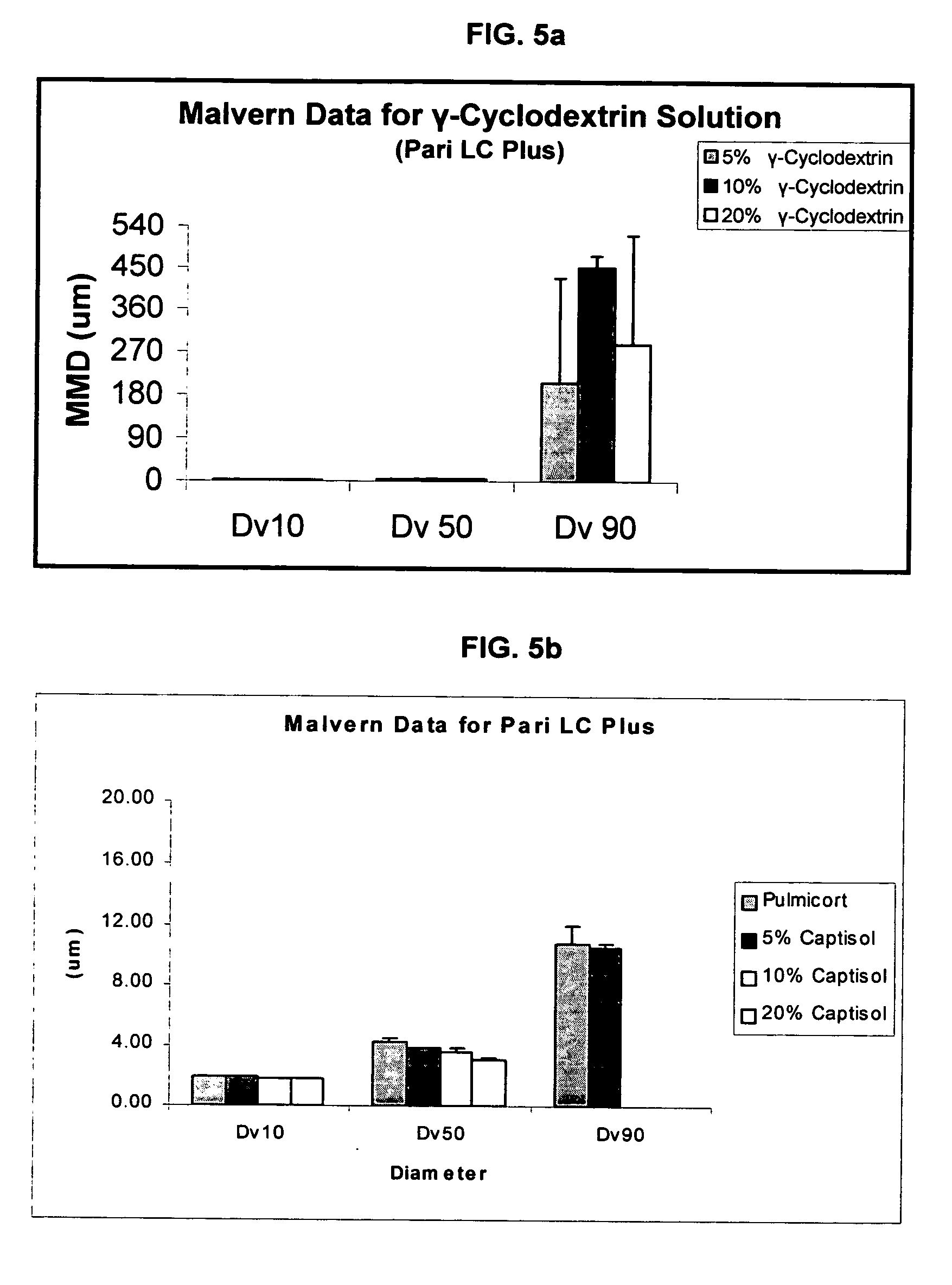
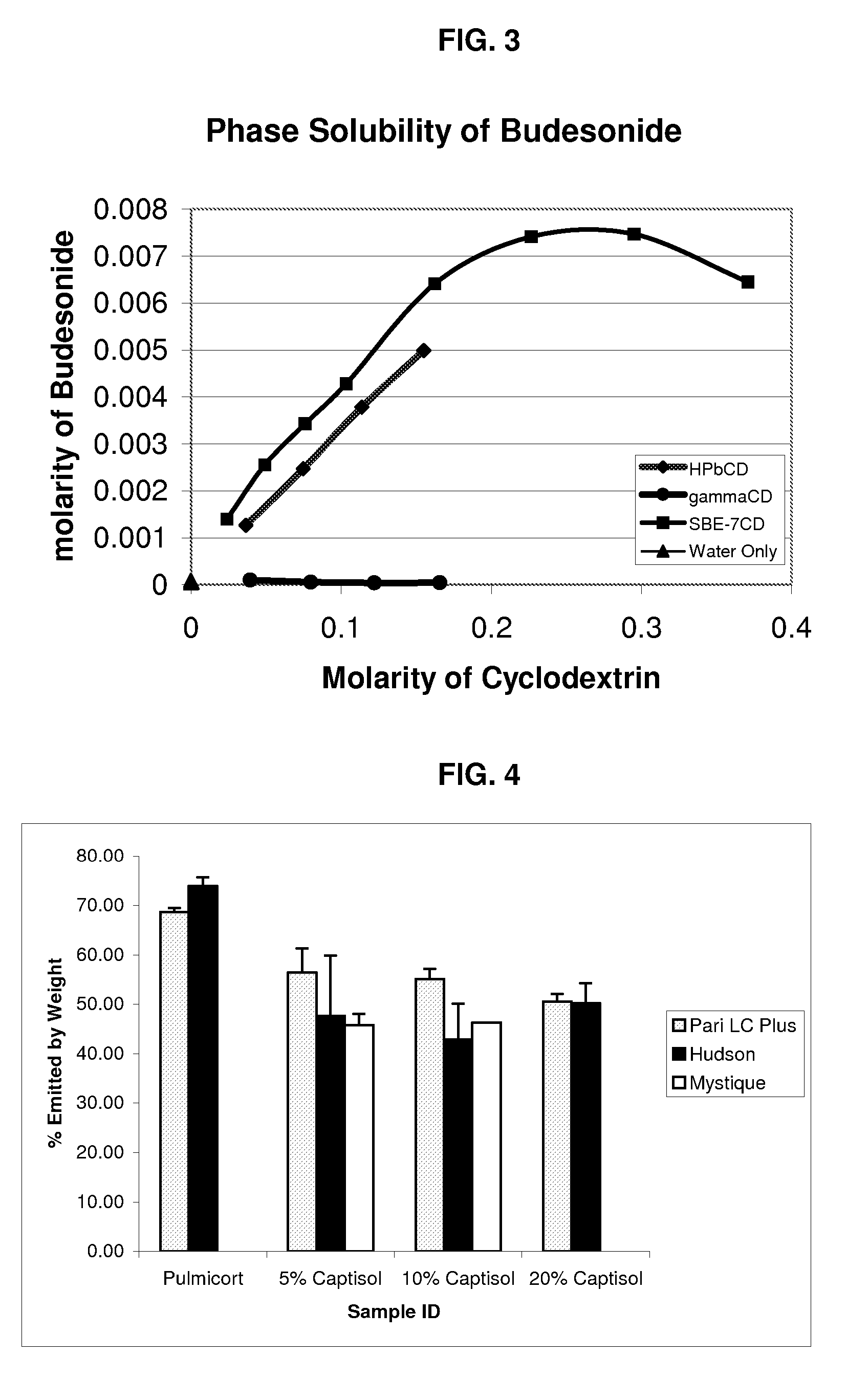
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid prepared from a unit dose suspension
InactiveUS20070020196A1Reduce the degradation rateIncrease productivityBiocideDispersion deliveryNebulizerCyclodextrin
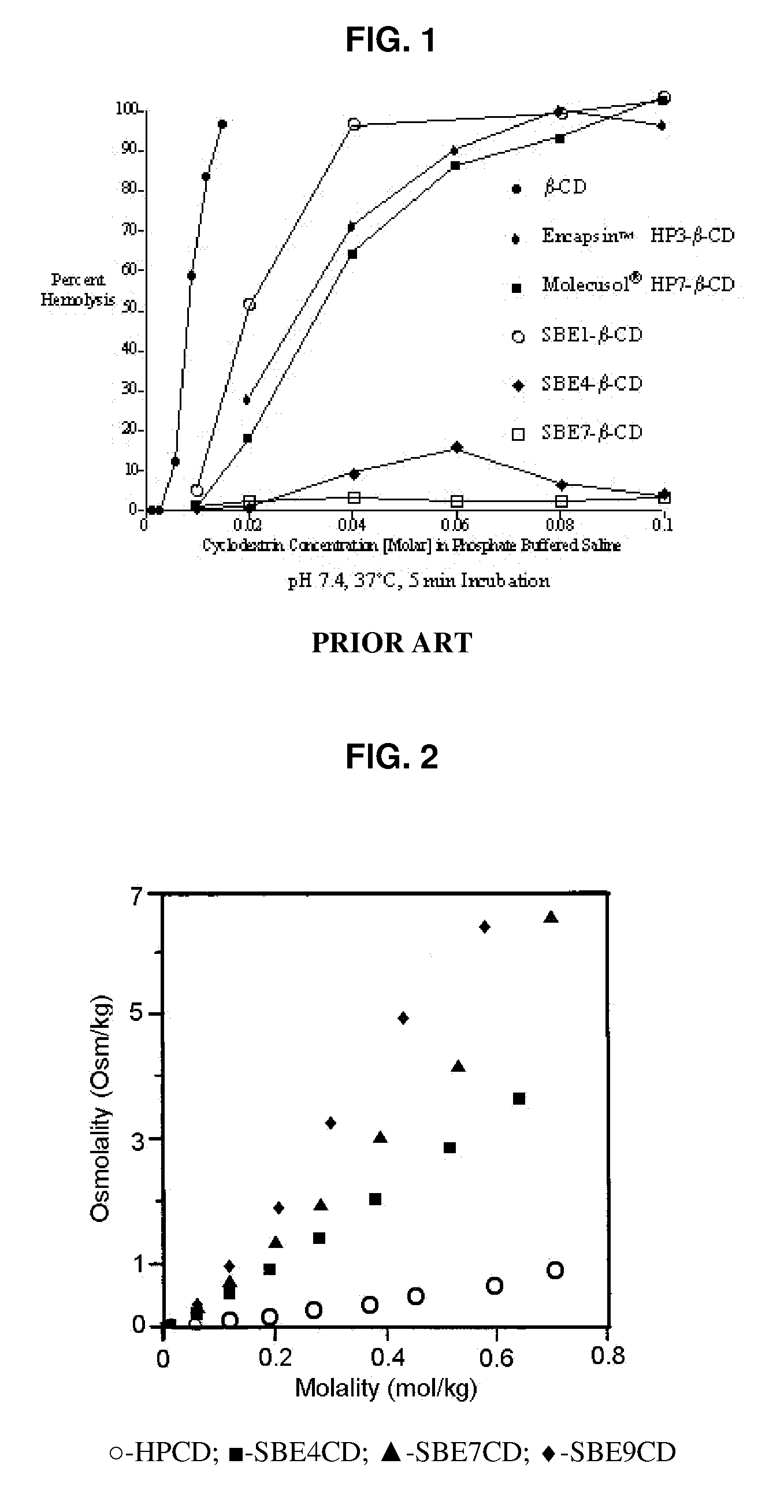
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Inhalant formulation containing sulfoalkyl ether cyclodextrin and corticosteroid
InactiveUS20070020299A1Reduce the degradation rateIncrease productivityBiocideOrganic active ingredientsNasal cavityNebulizer
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMACEUTICALS INC
Compositions and methods for treating cystic fibrosis
InactiveUS20100303917A1Increased mucus secretionReduce airflowBiocidePowder deliveryTobramycinCell membrane
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating cystic fibrosis or a symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered fluids optionally in combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). Particular embodiments comprise use and / or synergy with tobramycin for treating bacterial infection, and use and / or synergy with a bronchiodilator. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (like, membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions).
Owner:REVALESIO CORP
Compositions and methods for treating cystic fibrosis
Particular aspects provide electrokinetically-generated fluids (e.g., electrokinetically-generated gas-enriched fluids and solutions), and therapeutic compositions and methods comprising use thereof in treating at least one symptom of cystic fibrosis. In particular embodiments, at least one symptom of cystic fibrosis treated by the present invention include inhibition of Pseudomonas infection, synergy with tobramycin (including TOBI) for use against bacterial infection, and synergy with a bronchiodilator. In particular embodiments, the electrokinetically-generated fluids or therapeutic compositions and methods comprise combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). In certain aspects, the methods comprise regulating or modulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes).
Owner:REVALESIO CORP
Compositions and methods for treating cystic fibrosis
InactiveUS20100004189A1Increased mucus secretionReduce airflowBiocideCarbohydrate active ingredientsTobramycinCell membrane
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating cystic fibrosis or a symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered fluids optionally in combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). Particular embodiments comprise use and / or synergy with tobramycin for treating bacterial infection, and use and / or synergy with a bronchiodilator. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (like, membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions).
Owner:REVALESIO CORP
Inhalant formulation containing sulfoalkyl ether gamma-cyclodextrin and corticosteroid
InactiveUS20070020298A1Reduce the degradation rateIncrease productivityOrganic active ingredientsBiocideNasal cavityNebulizer
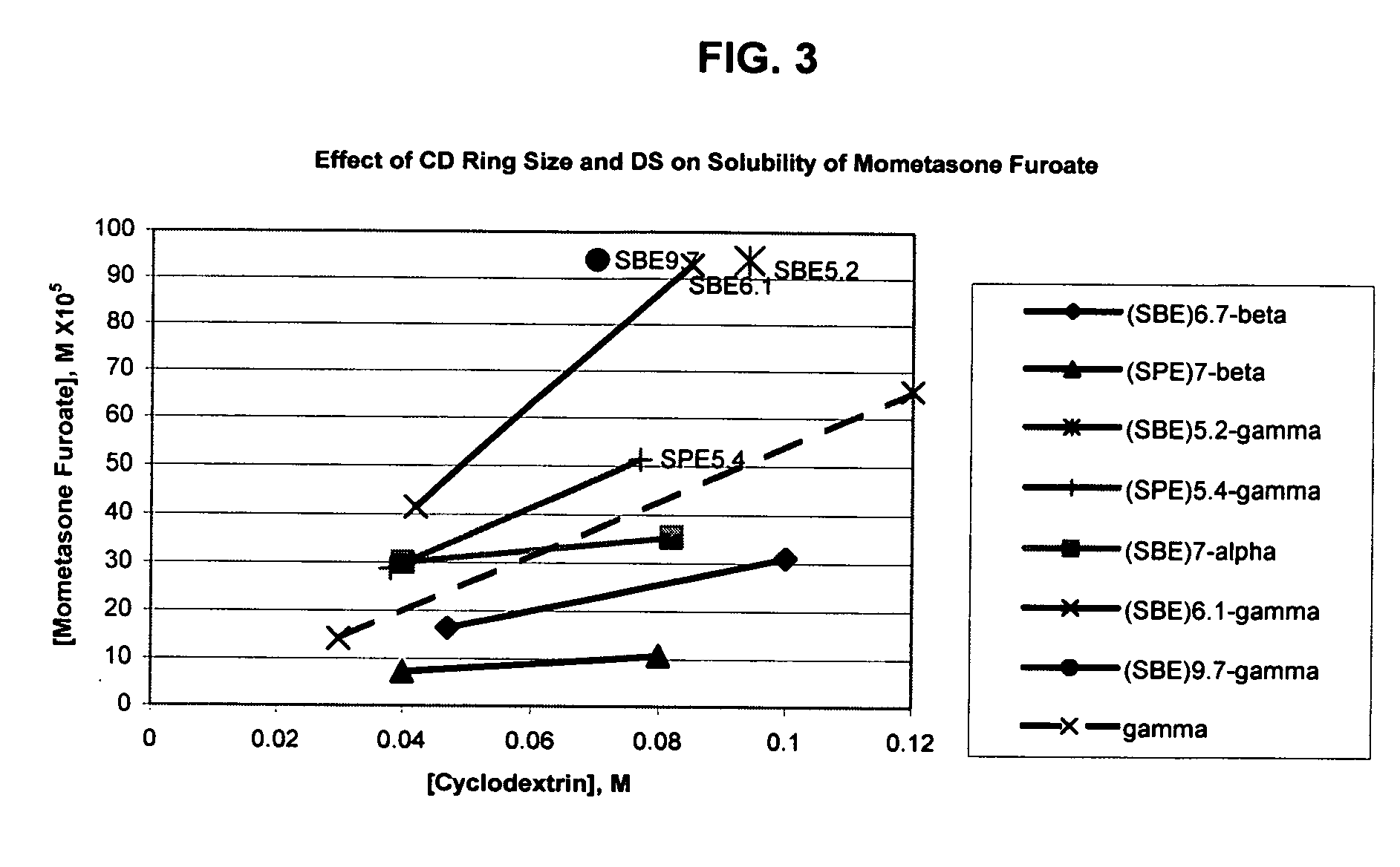
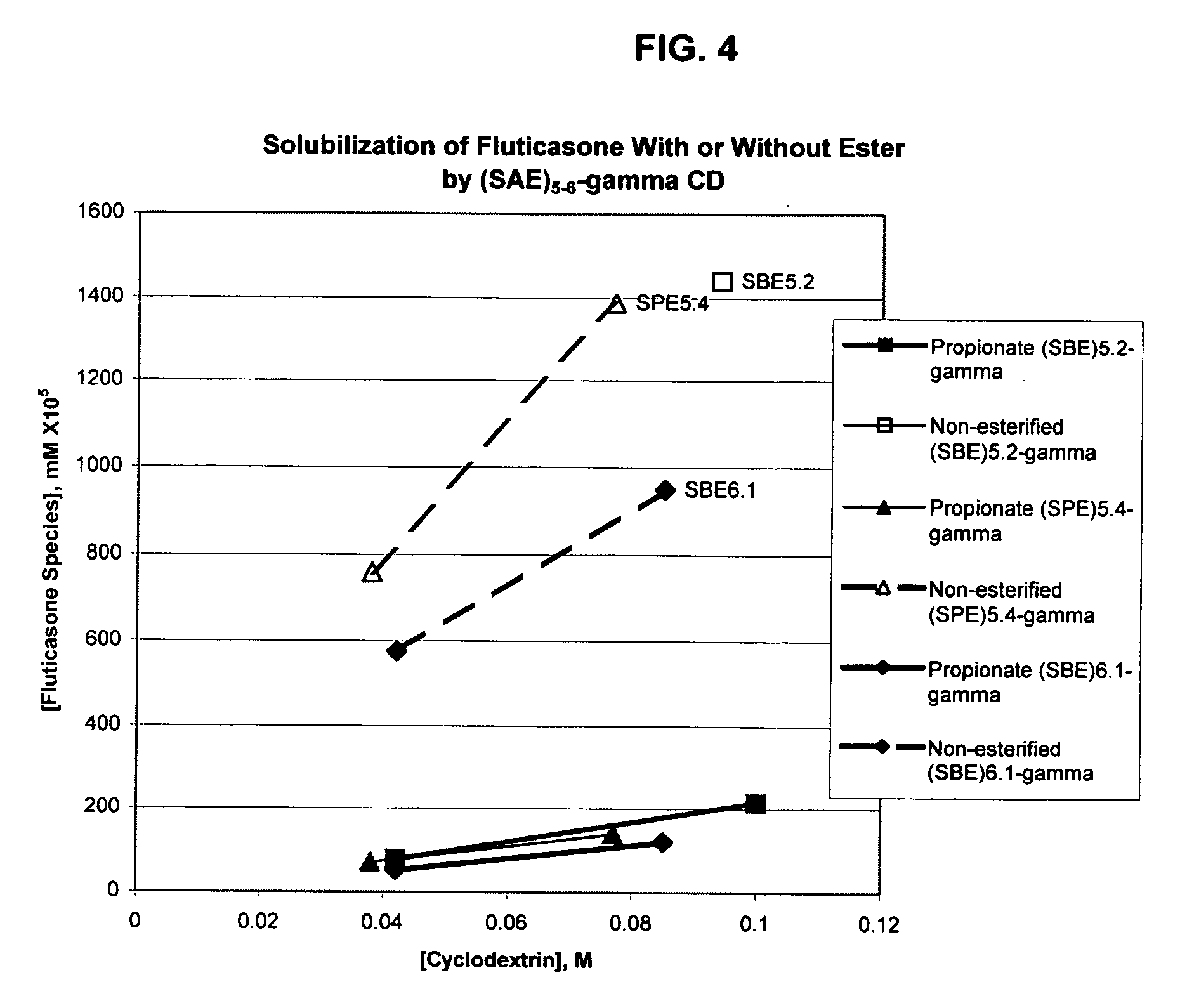
An inhalable formulation containing SAE-γ-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-γ-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation can include one or more additional therapeutic agents for use in combination with the corticosteroid. SAE-γ-CD is especially useful for solubilizing esterified corticosteroids.
Owner:CYDEX PHARMACEUTICALS INC
Method for treating a respiratory disease
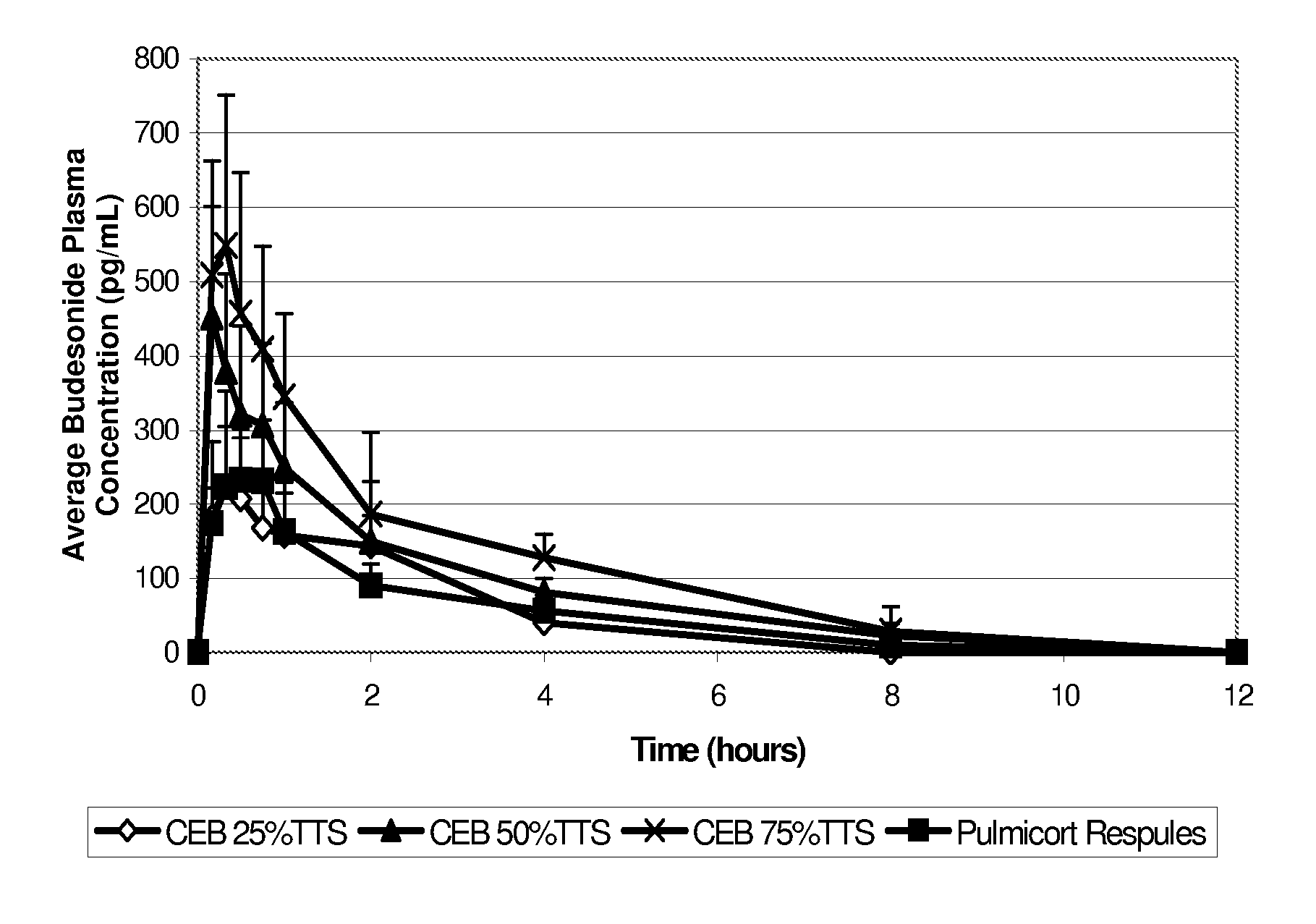
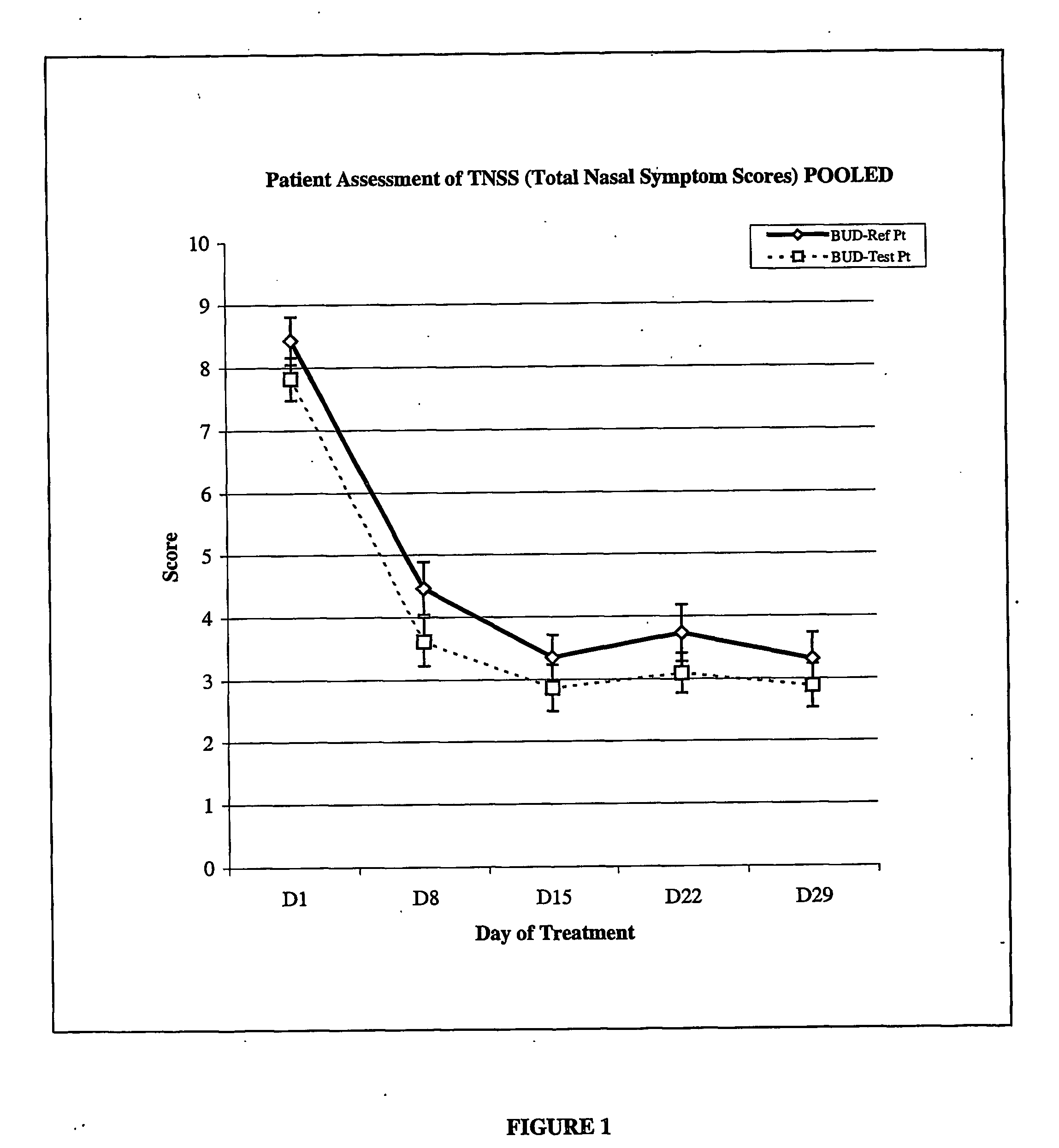
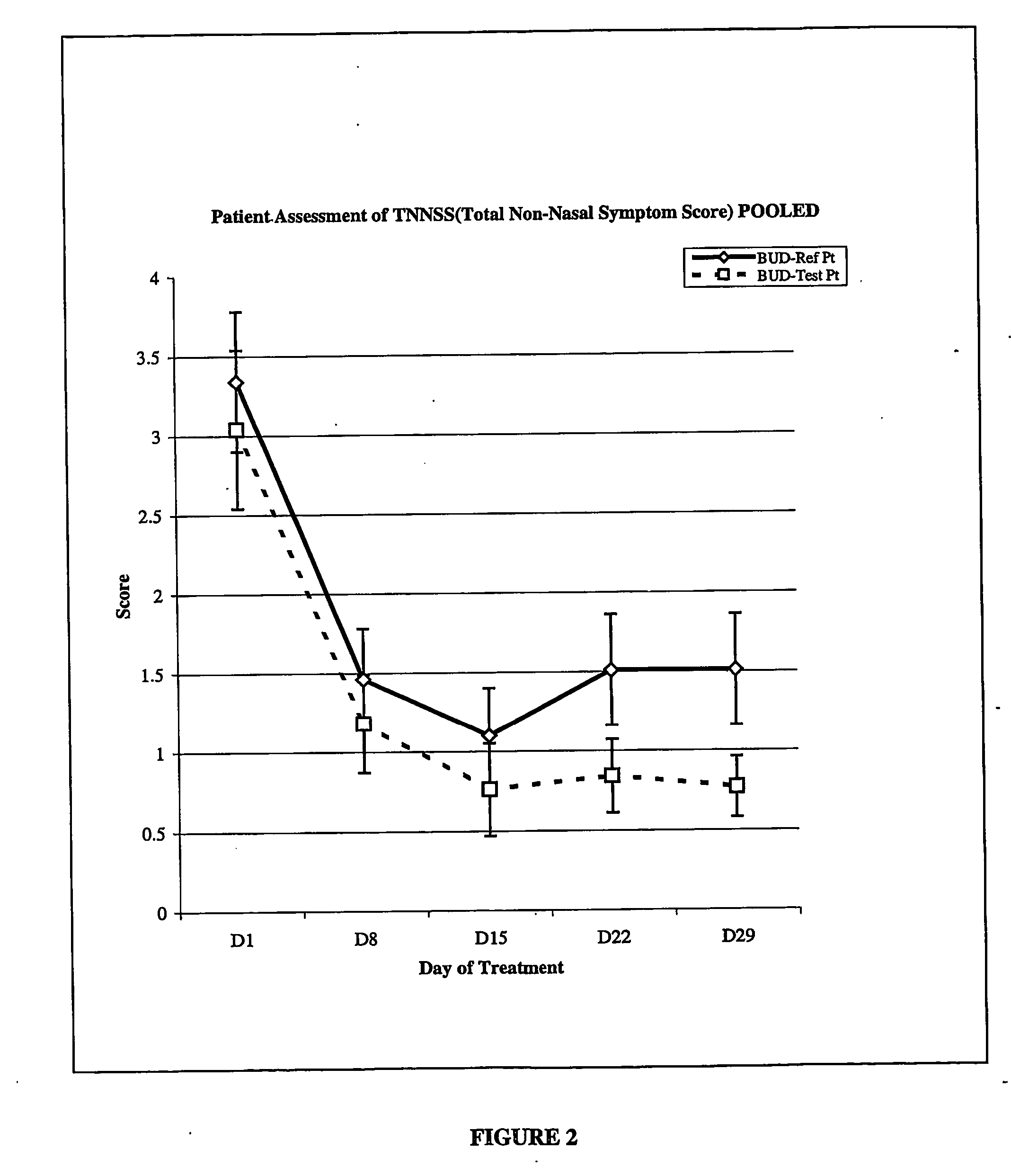
The invention provides a novel method of treating respiratory diseases, e.g., pediatric asthma, in a continuing regimen with not more than one daily dose of the drug budesonide using a nebulizer.
Owner:ASTRAZENECA AB
Pharmaceutical formulation for the active ingredient budesonide
InactiveUS20050089571A1Reduce riskImprove solubilityOrganic active ingredientsDigestive systemDissolutionBULK ACTIVE INGREDIENT
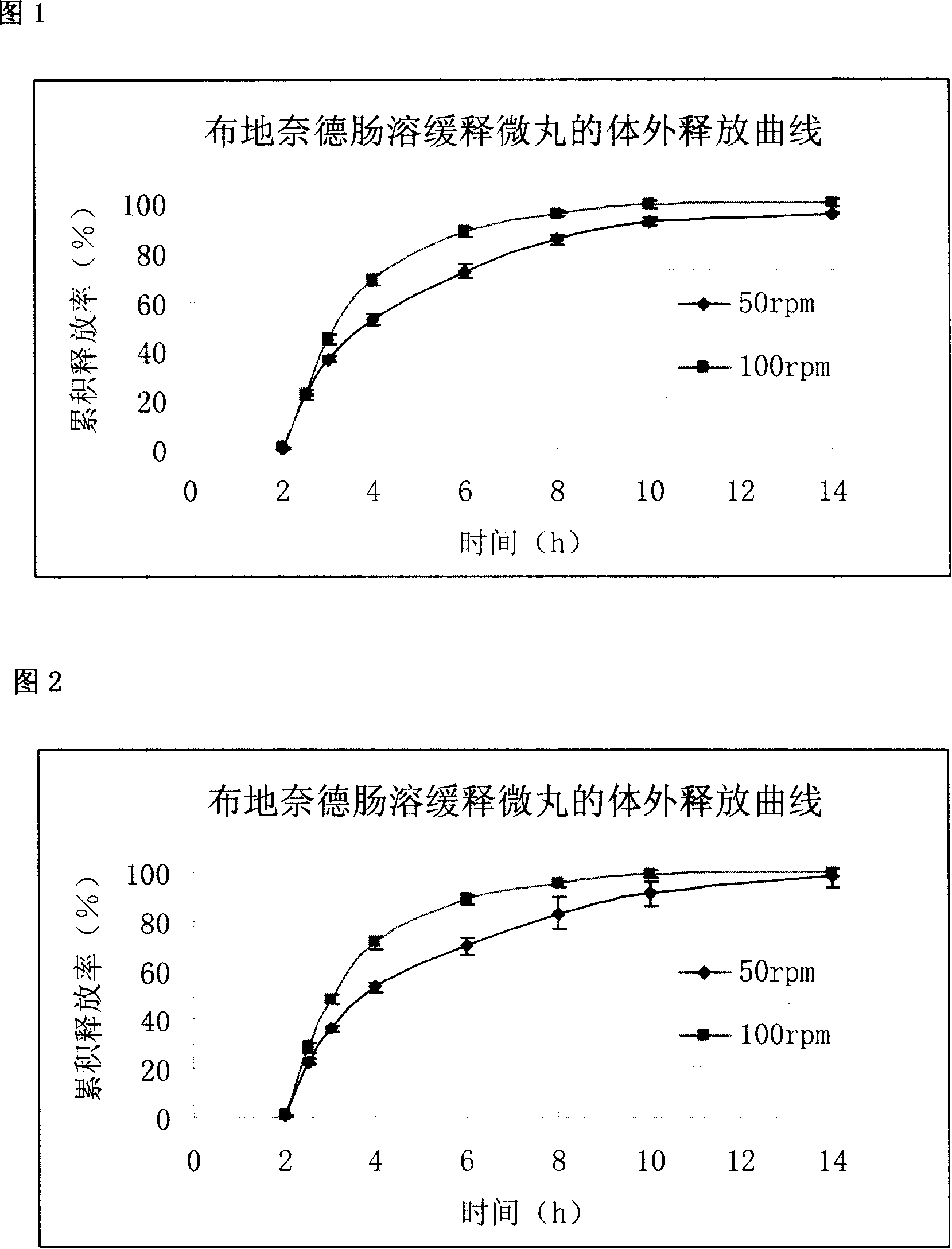
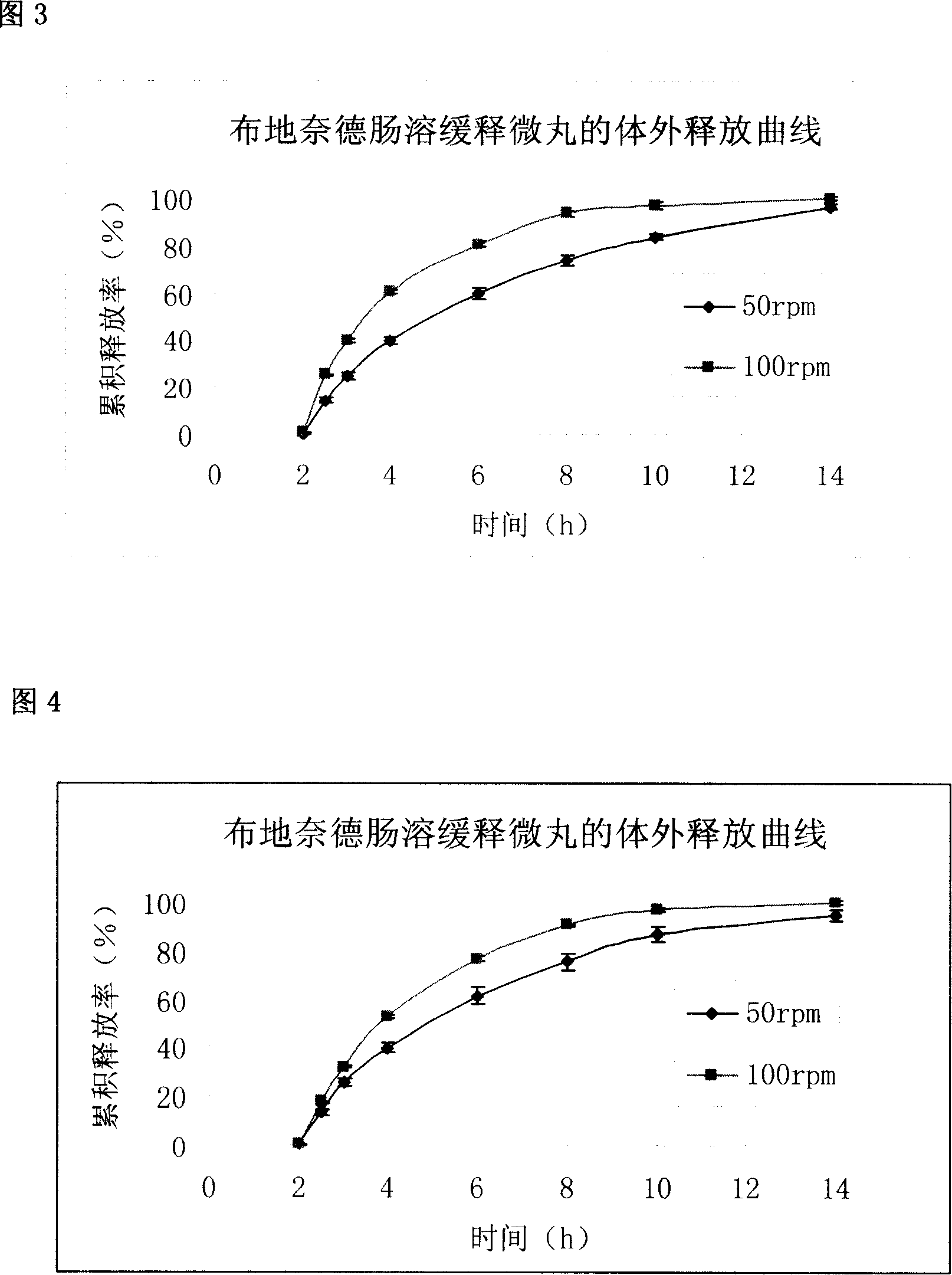
The invention relates to a pharmaceutical formulation containing essentially a) an inner layer which can optionally be applied to a core, with the active substance budesonide, bound with a binding agent; b) a middle layer with a polymer covering agent which is soluble in intestinal juice or retardant; and c) an outer envelope or outer layer which is resistant to stomach juice, said layers being able to contain in a manner known per se other pharmaceutically usual adjutants. The inventive formulation is characterised in that the binding agent is a polymer or a copolymer with acid groups and the formulation of the inner layer without the middle and outer layer releases the bound active ingredient in a release test according to USP XXIII monography <711> dissolution with apparatus 2 (addle) at a rotational speed of 100 / min in a phosphate buffer pH 7.5 after 30 min to a value of more than 80%.
Owner:EVONIK ROEHM GMBH
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid
InactiveUS20070202054A1Improve solubilityImprove stabilityBiocidePowder deliveryNebulizerCyclodextrin
An inhalable formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution, however, it can be stored as a dry powder, ready-to-use solution, or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus.
Owner:CYDEX PHARMA INC
Low dose corticosteroid composition
InactiveUS20060193783A1Improve toleranceReduce local irritationOrganic active ingredientsBiocideRecurrent sinusitisChronic sinusitis
The present invention relates to a low dose composition of budesonide suitable for administration of budesonide to mucosal membranes for the management of nasal symptoms associated with seasonal allergic rhinitis, perennial allergic rhinitis, perennial non-allergic rhinitis, nasal polyps, as well as prevention of post surgical polyps, chronic sinusitis and recurrent sinusitis comprising budesonide at a therapeutically effective dose of less than 16 mcg and a pharmaceutically acceptable liquid carrier.
Owner:SUN PHARMA INDS
Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract
ActiveUS20090191275A1Preventing and alleviating esophageal inflammationIncrease viscosity of compositionBiocideOrganic active ingredientsBudesonideDisease cause
Provided herein are methods for preventing or alleviating the symptoms of and inflammation associated with inflammatory diseases and conditions of the gastrointestinal tract, for example, those involving the esophagus. Also provided herein are pharmaceutical compositions useful for the methods of the present invention.
Owner:RGT UNIV OF CALIFORNIA
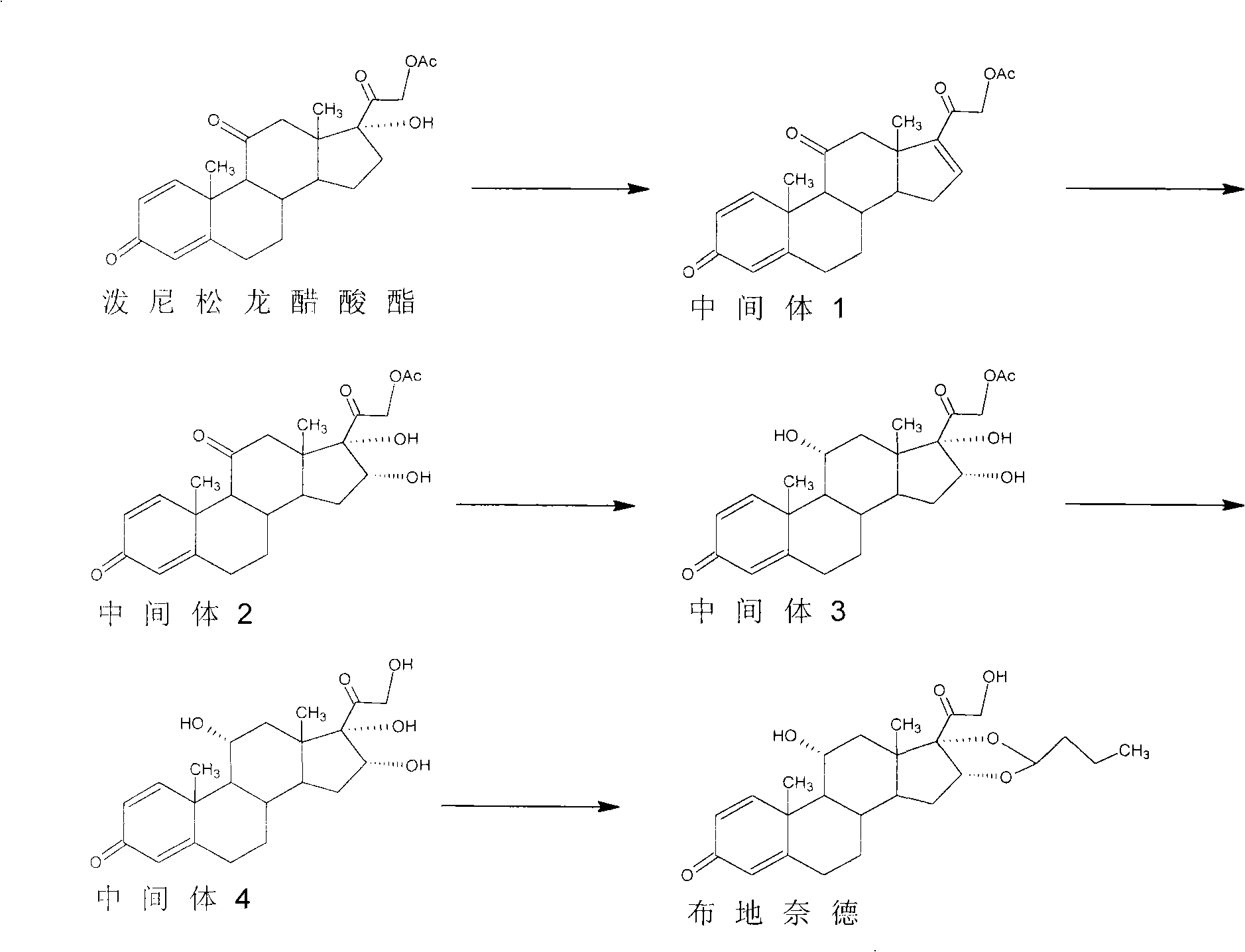
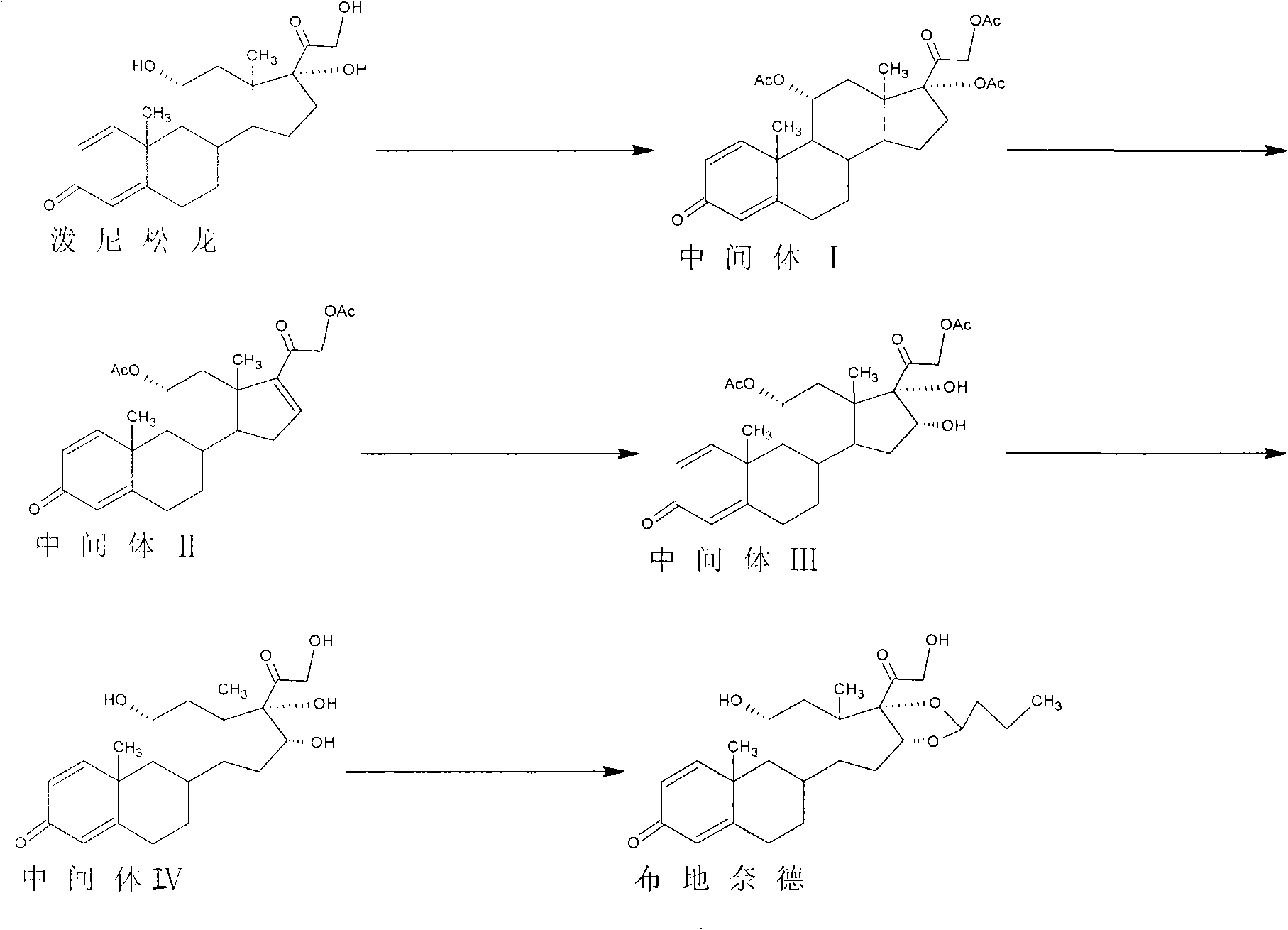
Preparation method of R-budesonide
The invention discloses a preparation method of an adrenal cortex hormone medicament and particularly relates to a preparation method of a high-purity R-budesonide isomer. According to the method disclosed by the invention, prednisolone is used as an original raw material and the budesonide acetic ester is prepared by cyclization, ring opening, esterification, elimination, oxidation, cyclization and hydrolysis. The method disclosed by the invention has a simple process and high yield and is suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
InactiveUS20070293460A1Adequate levelShortness of breathRespiratorsBiocideObstructive Pulmonary DiseasesCombination therapy
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and an aqueous solution comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium. A pharmaceutical composition is also described for the treatment of respiratory conditions and diseases comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic, and administering the solution to the patient using the nebulizer.
Owner:CMPD LICENSING
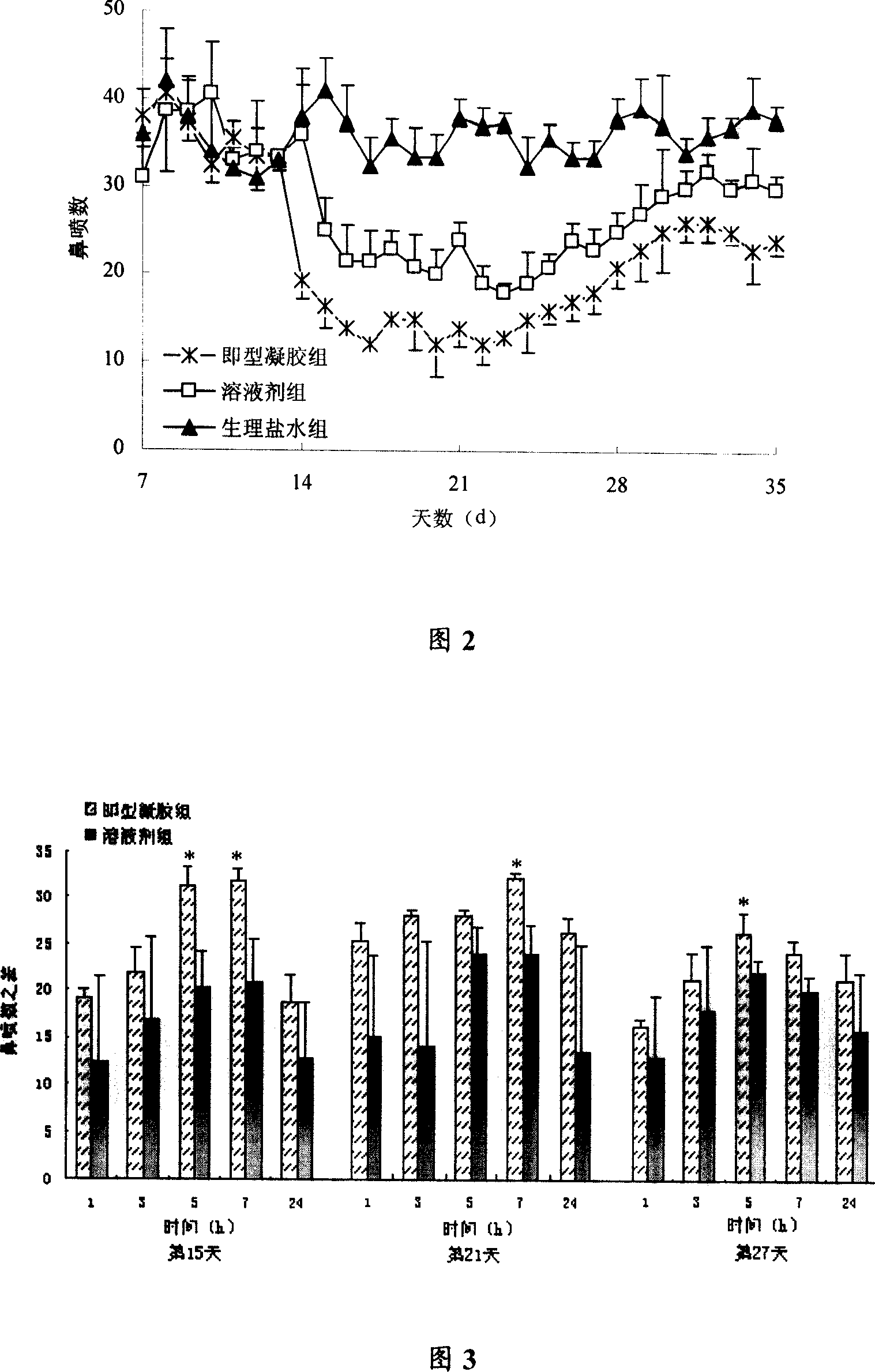
Instant gelling agent for treating allergic rhinitis
InactiveCN101015559AExtended stayOrganic active ingredientsPharmaceutical delivery mechanismNasal cavityGel preparation
The invention belongs to the field of western medicinal preparation, and relates to a preparation with suitable phase variation, detention time prolonging effects and topically administered in nasal cavity, and its preparation method. The method comprises dispersing momestasone furoate or budesonide in hydrophilic gel substrate prepared from acetyl-removed gellan gum and sodium alginate in micro powder form. the gel preparation is prepared and stored in solution from, and administered in solution spraying form; generates phase variation due to the change of environmental ionic strength, pH, or temperature after contacting with tunica mucosa of nasal cavity, and forms gel. The invention has the advantages of simple preparation and accurate administered dose; can prolong the contact time of medicine and schneiderian membrane; and improves therapeutic effect of the medicine to anaphylactic rhinitis.
Owner:FUDAN UNIV
Method for treating a respiratory disease
InactiveUS20050222111A1Readily and effectively usedImprove complianceAerosol deliveryRespiratory disorderDiseaseRegimen
The invention provides a novel method of treating respiratory diseases, e.g., pediatric asthma, in a continuing regimen with not more than one daily dose of the drug budesonide using a nebulizer.
Owner:ASTRAZENECA AB
Nebulizer Formulation
A sterile nebulizer formulation contains formoterol and budesonide in about 2 ml or less of saline and is for treatment of COPD and asthma and other airways diseases and disorders.
Owner:BREATH
Preparation method of budesonide
The invention discloses a preparation method of budesonide. The budesonide is prepared sequentially through the following steps of: carrying out an esterification reaction on a starting material and acetic anhydride, wherein the starting material is prednisolone; carrying out a degreasing reaction in the presence of a catalyst which can be alkali or an alkali metal salt ; carrying out an oxidizing reaction with potassium permanganate in acid environment; carrying out an ester exchange reaction with alcohol in alkaline environment; carrying out a condensation reaction with n-butanal, and the like. In the preparation method, appropriate catalysts are added in the reactions, and reaction conditions are appropriately controlled so as to achieve the purposes of increasing the reaction rates inall the steps and improving the yield of intermediate products, and the product quality conforms to the standard of the European Pharmacopoeia. Meanwhile, all the steps have moderate reaction condition and easy control, low energy consumption, high product yield, small pollution, easy reaction raw material obtaining and low production cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Delivery of a combination therapy for asthma and chronic obstructive pulmonary disease
A method of delivery of a combination therapy to the pulmonary system that includes providing a nebulizer and a fluid comprising a long-acting corticosteroid, a long-acting beta-agonist, and a long-acting anticholinergic in a pharmaceutically acceptable vehicle, and administering the solution to the patient using the nebulizer. The corticosteroid is budesonide, the beta-agonist is formoterol and the anticholinergic is tiotropium in a an aqueous solution, suspension or emulsion suitable for administration with the nebulizer.
Owner:RICHIES PHARMACY & MEDICAL SUPPLY
Budesonide intestines sustained release dextromethorphan pellets and method of manufacturing the same
InactiveCN101108171ARegulated release rateGood film formingOrganic active ingredientsAntipyreticSustained release pelletsMedicine
The invention belongs to the technical field of medicinal preparation and relates to an enteric preparation and sustained or controlled release preparation, in particular to an enteric controlled release micro-granule with budesonide and its preparation method. The invention, which comprises a quick release celphere, a controlled release layer and an enteric layer, takes budesonide as active ingredient of the drug to coordinate with medicinal carrier supplementary materials. After the release level testing, it is proved that the enteric controlled release micro-granule, which adopts different enteric materials, controlled release material and different coating conditions, does not release in stomach but began to release slowly after entering the small intestine with even release level. Therefore, the enteric controlled release micro-granule in the invention can effectively prevent the release in stomach, ensure the slow release in small intestine and the release of drug in the affected section of small incestine, ileocecus and colon.
Owner:FUDAN UNIV
Pharmaceutical formulation for treating the upper digestive tract
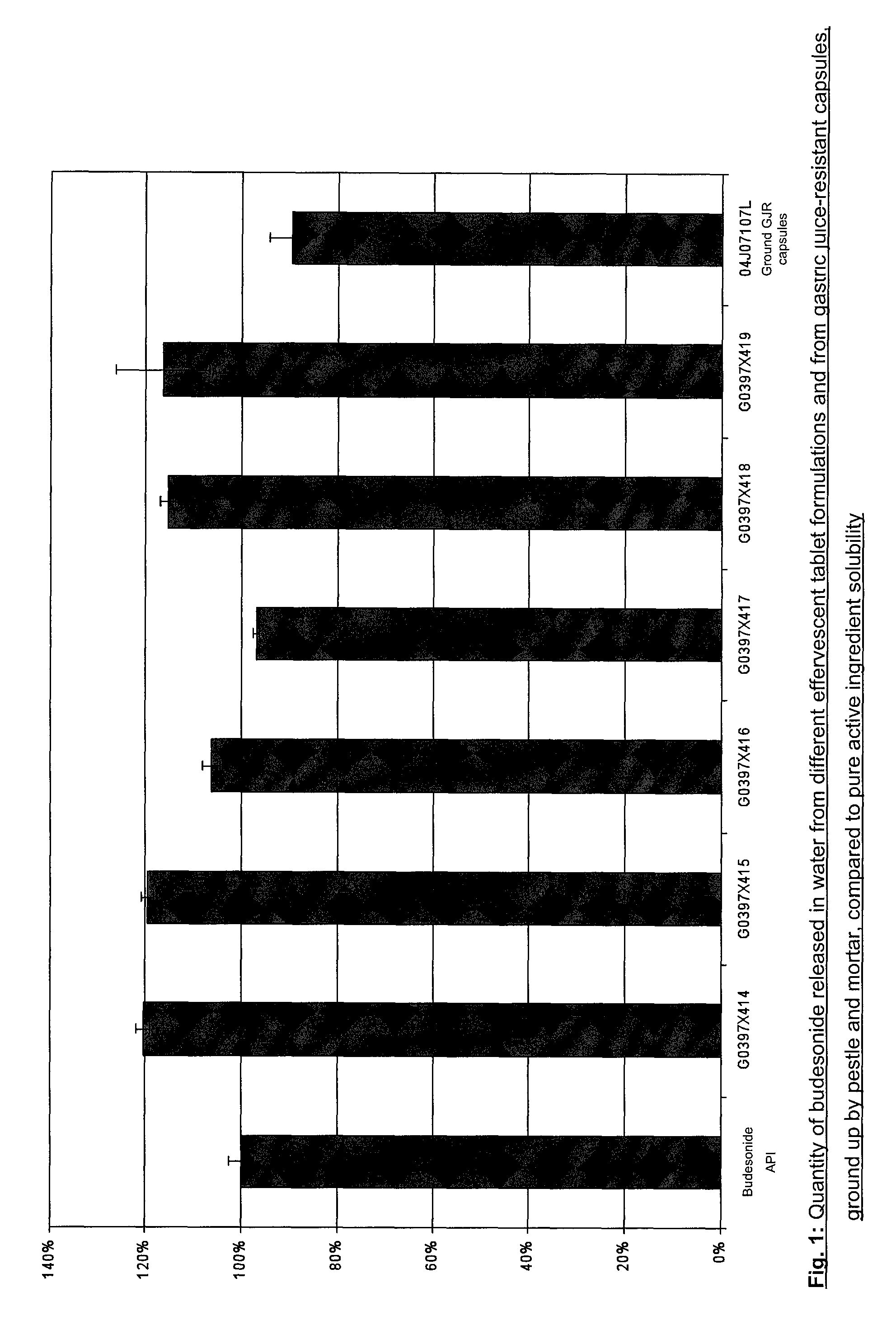
InactiveUS8580300B2Simple and accurate dosageLong-term stabilityOrganic active ingredientsBiocideEffervescent tabletOral medication
The invention relates to an effervescent tablet for preparing a mouth rinsing solution, wherein the effervescent tablet exhibits a high release rate of budesonide. A high availability of the active ingredient during use as a mouth rinsing solution on the inflamed mucosa of the upper digestive tract is thereby achieved. The advantage of the formulation according to the invention lies in the bioavailability comparable to oral forms of administration, which allows the formulation to be used safely over an extended period of time.
Owner:DR FALK PHARMA GMBH
Inhalant Formulation Containing Sulfoalkyl Ether Cyclodextrin and Corticosteroid Prepared from a Unit Dose Suspension
InactiveUS20110008325A1Improve solubilityImprove stabilityBiocideDispersion deliveryNebulizerCyclodextrin
An inhalable unit dose liquid formulation containing SAE-CD and corticosteroid is provided. The formulation is adapted for administration to a subject by nebulization with any known nebulizer. The formulation can be included in a kit. The formulation is administered as an aqueous solution or concentrated composition. The formulation is employed in an improved nebulization system for administering corticosteroid by inhalation. SAE-CD present in the formulation significantly enhances the chemical stability of corticosteroid, such as budesonide. A method of administering the formulation by inhalation is provided. The formulation can also be administered by conventional nasal delivery apparatus. The formulation is prepared by mixing SAE-CD, in solid or liquid (dissolved) form, with an inhalable suspension-based unit dose formulation.
Owner:CYDEX PHARMACEUTICALS INC
Use of composition comprising Formoterol and Budesonide for prevention or treatment of acute condition of asthma
InactiveCN1305380AOffset deteriorationIncrease inflammationPowder deliveryHydroxy compound active ingredientsChronic asthmaMedicine
Owner:ASTRAZENECA AB
Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract
ActiveUS8497258B2Inflammation can be prevented and alleviatedHigh viscosityBiocideOrganic active ingredientsInflammatory bowel diseaseBudesonide
Owner:RGT UNIV OF CALIFORNIA
Methods of treating ulcerative colitis
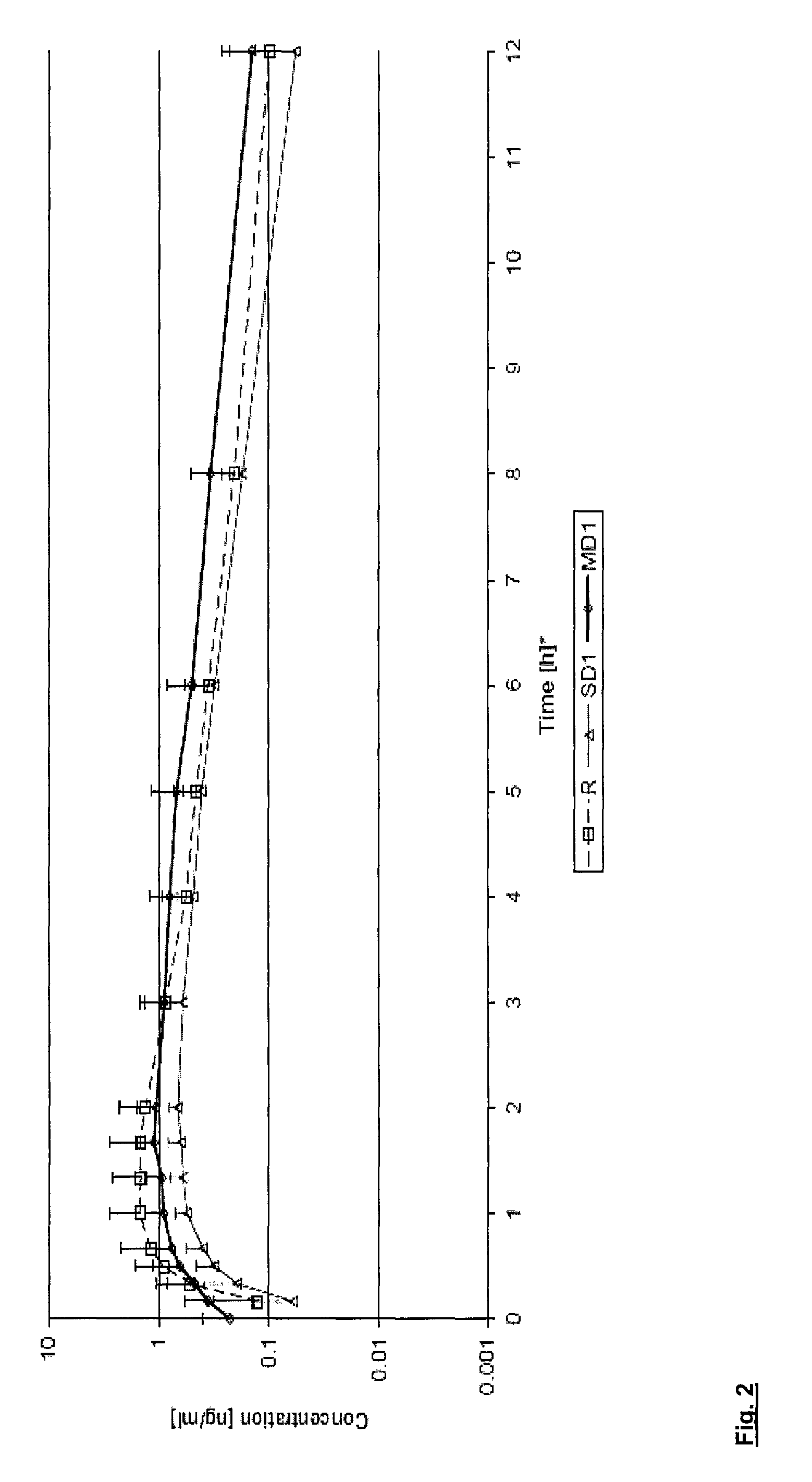
InactiveUS20140349982A1Decrease in systemic elimination rateDecrease in elimination rate constantOrganic active ingredientsDigestive systemMedicineUlcerative proctitis
Provided herein are methods of treating and inducing ulcerative colitis in a subject. Also provided are methods of treating subjects with mild to moderate active ulcerative colitis, including ulcerative proctitis and proctosigmoiditis. Also provided are methods of administering budesonide to a subject to treat ulcerative colitis, including ulcerative proctitis and proctosigmoiditis.
Owner:DR FALK PHARMA GMBH
Optimized pharmaceutical formulation for the treatment of inflammatory conditions of the esophagus
ActiveUS9867780B2Long retention timeWidely distributedOrganic active ingredientsAntipyreticEffervescent tabletHigh rate
Disclosed is an optimized pharmaceutical formulation for the treatment of inflammatory conditions of the esophagus. A pharmaceutical formulation in the form of an orodispersible effervescent tablet is stable, easy to produce, and can be used without dissolving same in a liquid. It is not necessary to drink anything with the tablet as this would reduce the time that the budesonide solution remains in the affected regions of the esophagus. The effervescent tablet of the invention surprisingly resulted in an unexpectedly high rate of histological remission in patients with active eosinophilic esophagitis.
Owner:DR FALK PHARMA GMBH
Budesonide nano crystallizing preparation and preparation method thereof
InactiveCN101961320AImprove stabilityPreparation process is easy to scale upOrganic active ingredientsPowder deliverySolubilityOral medication
The invention relates to application of a new medicament delivery system in budesonide delivery, in particular to a preparation method for a budesonide nano crystallizing mixed suspension and application thereof. Budesonide has poor water solubility and low bioavailability by oral administration, seriously influences the application in clinic and increases the economical burden of patients. Accordingly, the invention provides the preparation method of the budesonide nano crystallizing mixed suspension for more favorably solving the problem of poor water solubility. The budesonide nano crystallizing mixed suspension can rapidly dissolve effective components, shorten the action time, reduce adverse reaction and is safe and convenient for use. The invention provides the budesonide nano crystallizing mixed suspension which overcomes the defects in current clinic practice and has a plurality of other advantages.
Owner:SHANDONG XINBO PHARMA R&D
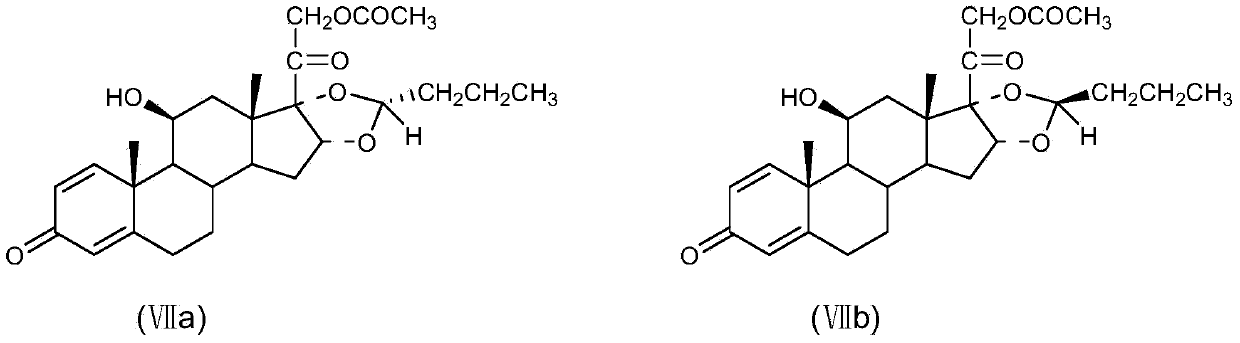
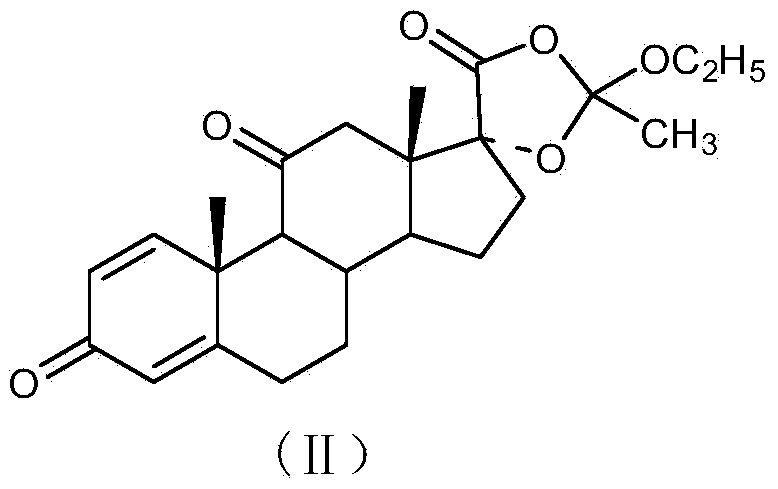
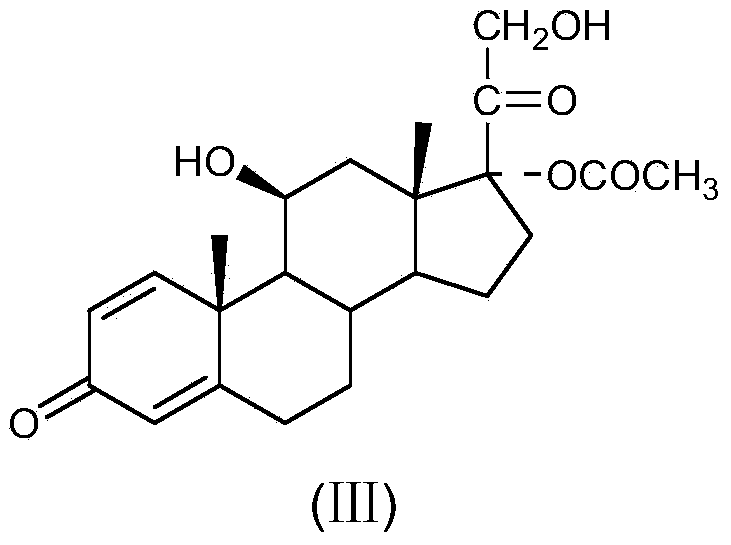
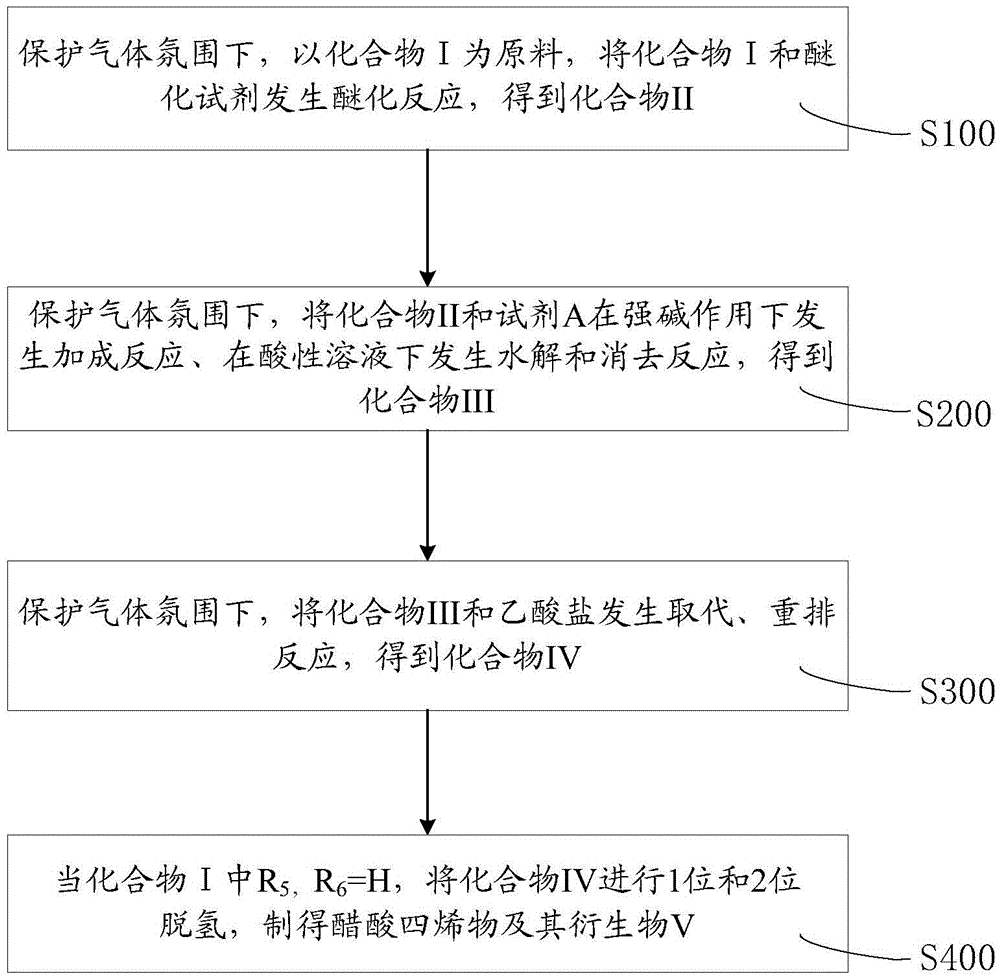
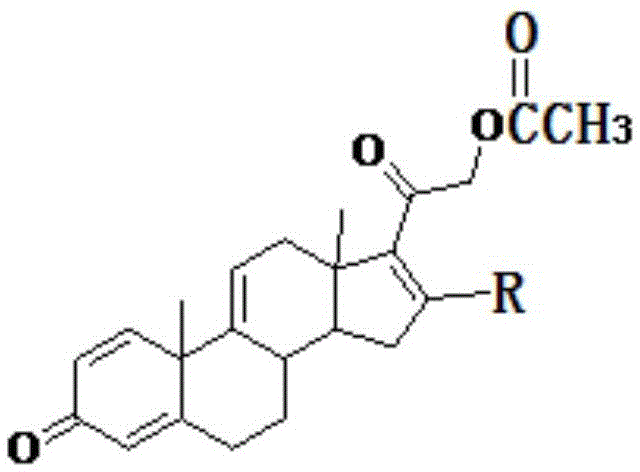
Method for preparing tetraene acetate and derivatives thereof
The invention relates to a method for preparing tetraene acetate and derivatives thereof. The method comprises the steps of obtaining a compound II through etherification reaction of a compound I and an etherification agent in the atmosphere of protective gases, obtaining a compound III through addition reaction of the compound II and a reagent A under the action of strong base and hydrolysis and elimination reaction of the compound II and the reagent A in the presence of an acid solution, obtaining a compound IV through substitution reaction and rearrangement reaction of the compound III and acetate, and conducting 1 position dehydrogenation and 2 position dehydrogenation on the compound IV to obtain the tetraene acetate and derivatives thereof. According to the method, the compound I is taken as the raw material and subjected to carbonyl etherification, addition, hydrolysis, elimination, rearrangement and dehydrogenation reaction to obtain the product, the raw material compound I is easy to obtain, cost is low, no precious metal is needed during preparation, reaction conditions are easy to control, operation is convenient, the method is suitable for large-scale industrial production, and the obtained tetraene acetate is an important intermediate for synthesis of dexamethasone, budesonide, betamethasone and other steroidal drugs.
Owner:湖南成大生物科技有限公司
Materials and methods for treatment and diagnosis of disorders associated with oxidative stress
The subject invention pertains to materials and methods for the prevention and treatment of disease conditions associated with oxidative stress or a compromised reducing environment, including inflammatory bowel diseases such as Crohn's disease and ulcerative colitis. Another aspect of the subject invention concerns compositions formulated for administration as an enema. In one embodiment, a composition suitable for administration as an enema comprises an effective amount of 5-ASA and a steroid such as budesonide or hydrocortisone. The subject invention also concerns compositions formulated for oral administration. In one embodiment, a composition comprises alpha-lipoic acid, and / or N-acetyl-L-cysteine (N-A-C), and / or L-glutamine. The alpha-lipoic acid can be racemic alpha-lipoic acid, R-lipoic acid, or R-dihydro-lipoic acid. Methods of the invention include administration of compounds or compositions of the invention. In one embodiment, compounds or compositions of the invention are rectally instilled in a patient. In another embodiment, compounds or compositions are orally administered. The subject invention also concerns methods for screening for, assessing risk of developing, and / or diagnosing conditions associated with oxidative stress, such as ulcerative colitis and other inflammatory bowel disorders.
Owner:THERAPEUTIC RES
Use for budesonide and formoterol
The invention provides the use of formoterol and budesonide in the treatment of chronic obstructive pulmonary disease.
Owner:ASTRAZENECA AB
Particulate materials
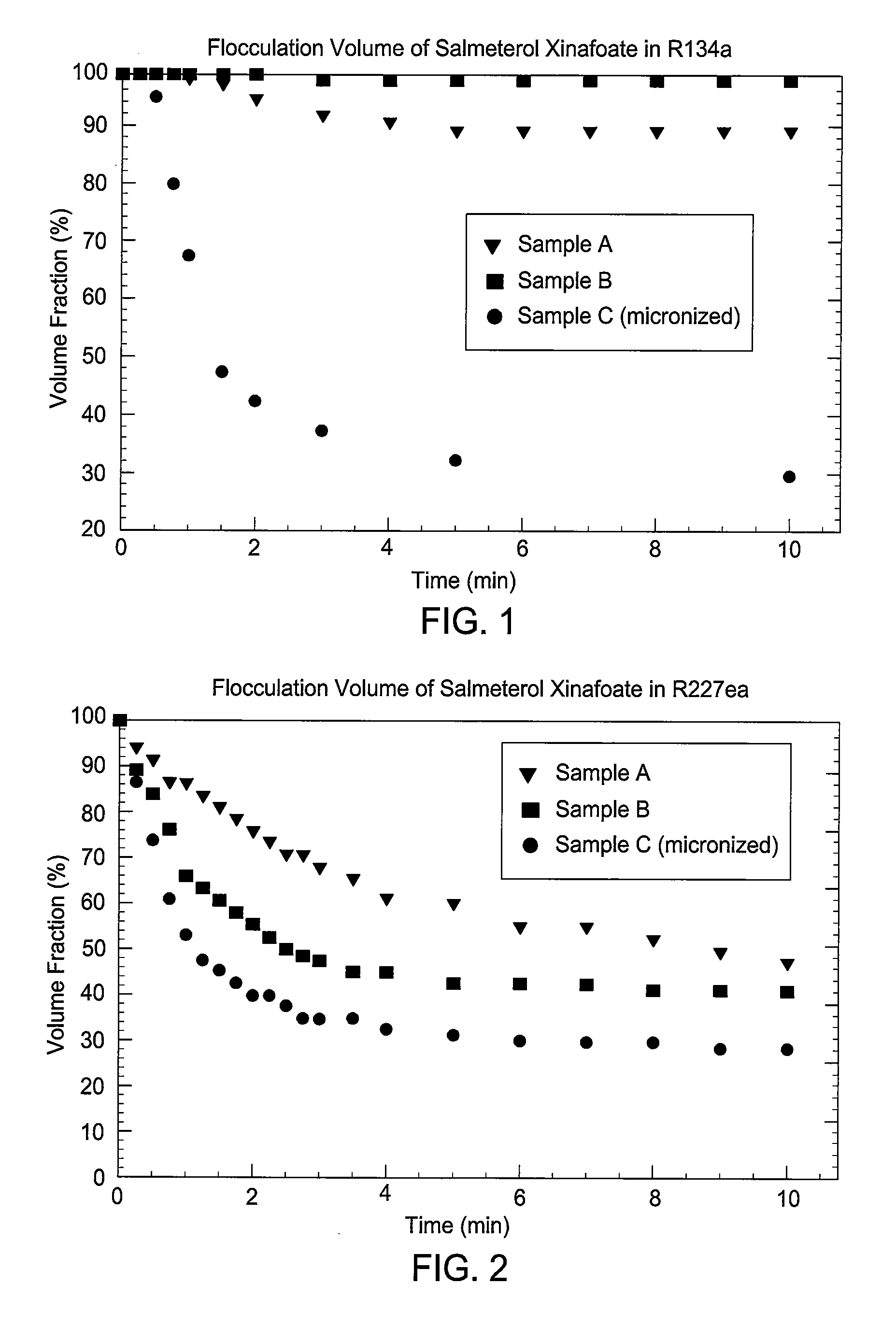
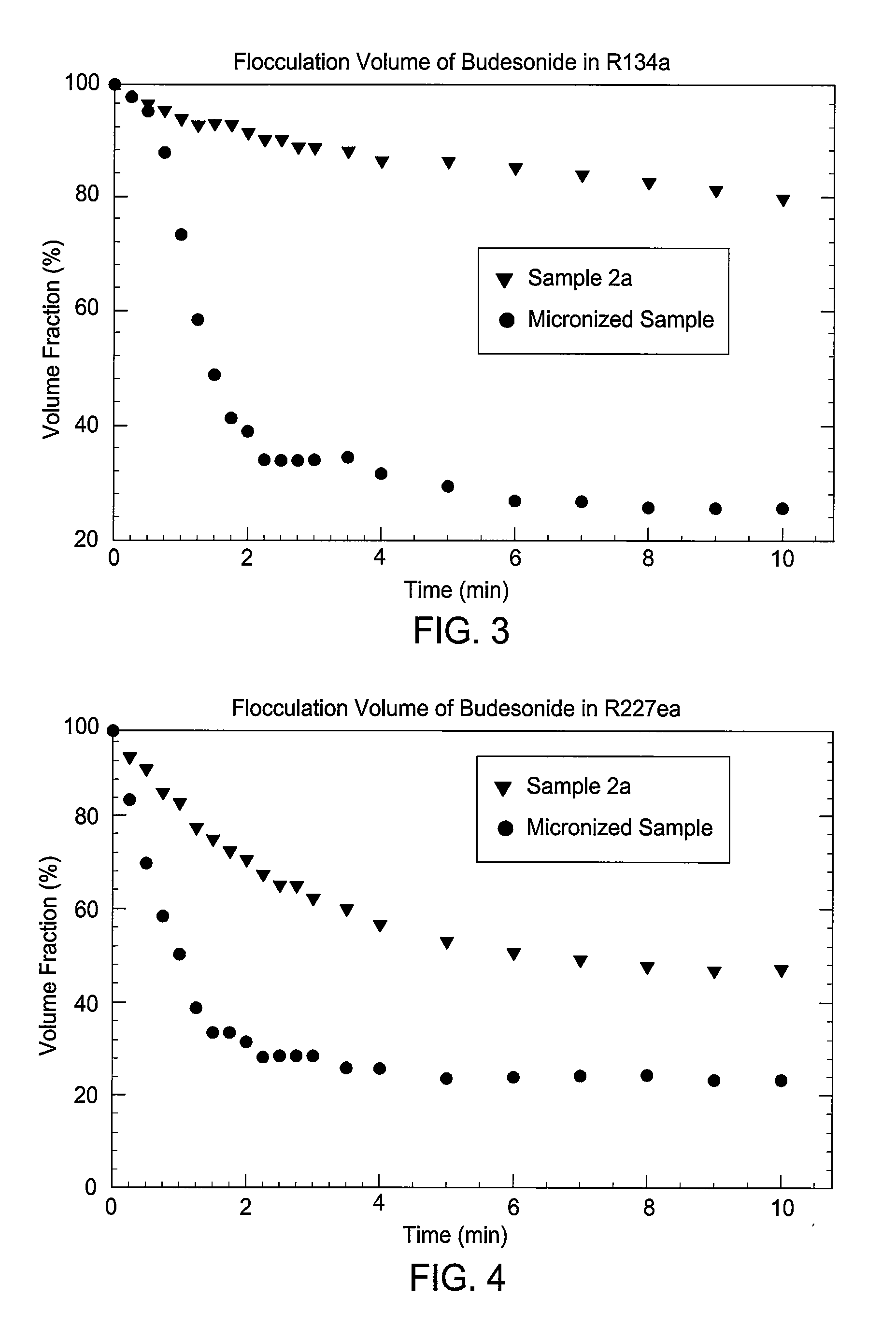
Embodiments of the invention relate to particles of active substances, methods for preparing the particles, formulations containing the particles, and metered dose inhalers containing the particles or formulations. In one embodiment, an inhaler contains an aerosol formulation containing a particulate active substance of non-micronized, solid particles having a mass median aerodynamic diameter of less than 10 μm. The particles may be suspended in a nonsolvent hydrofluorocarbon fluid vehicle (e.g., HFA 134a or 227ea) at a concentration within a range from about 0.2% w / v to about 5% w / v. The formulation exhibits a flocculation volume of about 85% or greater about 1 minute after mixing the particulate active substance and the vehicle. The particulate active substance may contain salmeterol xinafoate, budesonide, salbutamol sulfate, dihydroergotamine mesylate, risperidone-(9-hydroxy)-palmitate, bromocriptine mesylate, or derivatives thereof. In some examples, the active substance is dihydroergotamine mesylate.
Owner:NEKTAR THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com