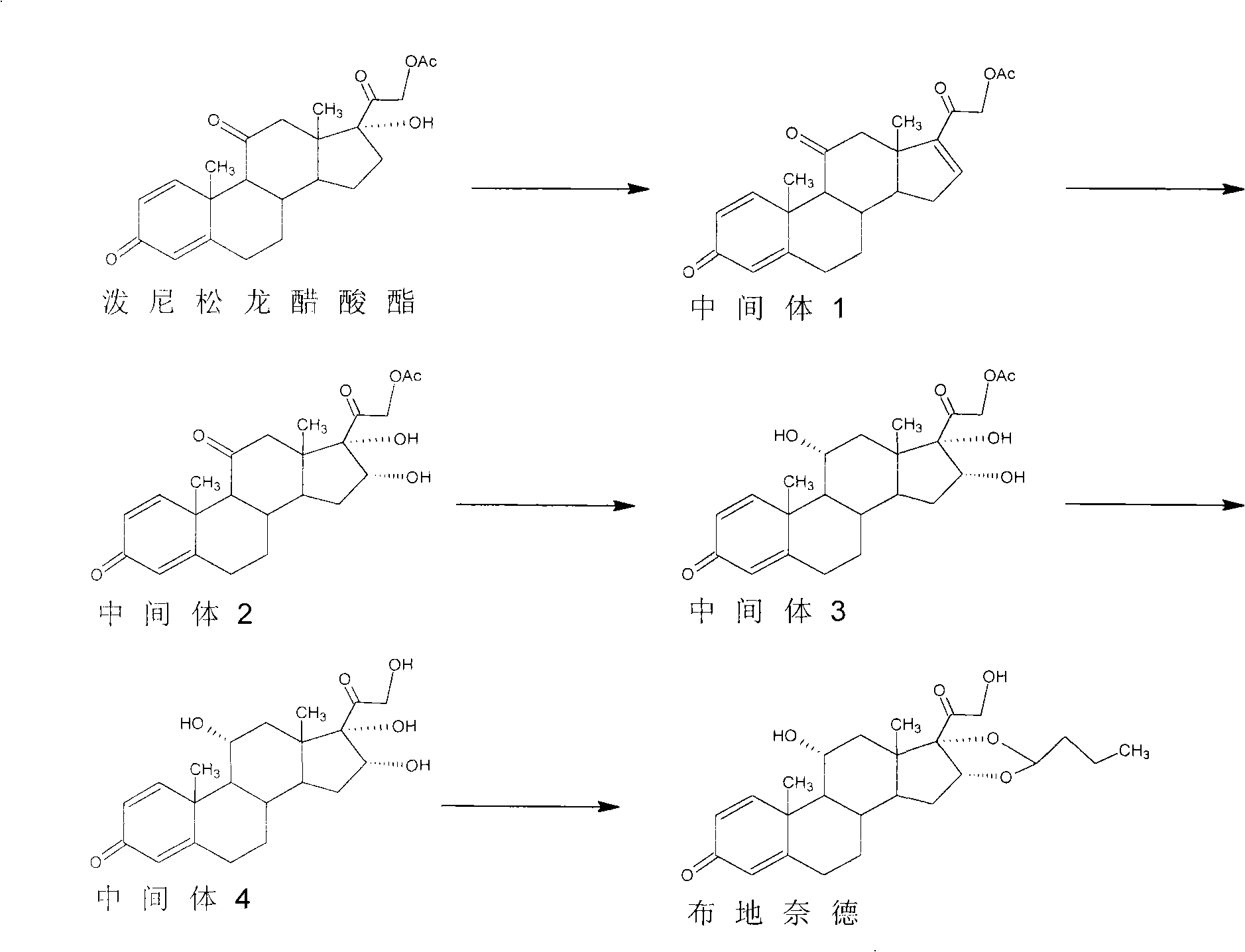
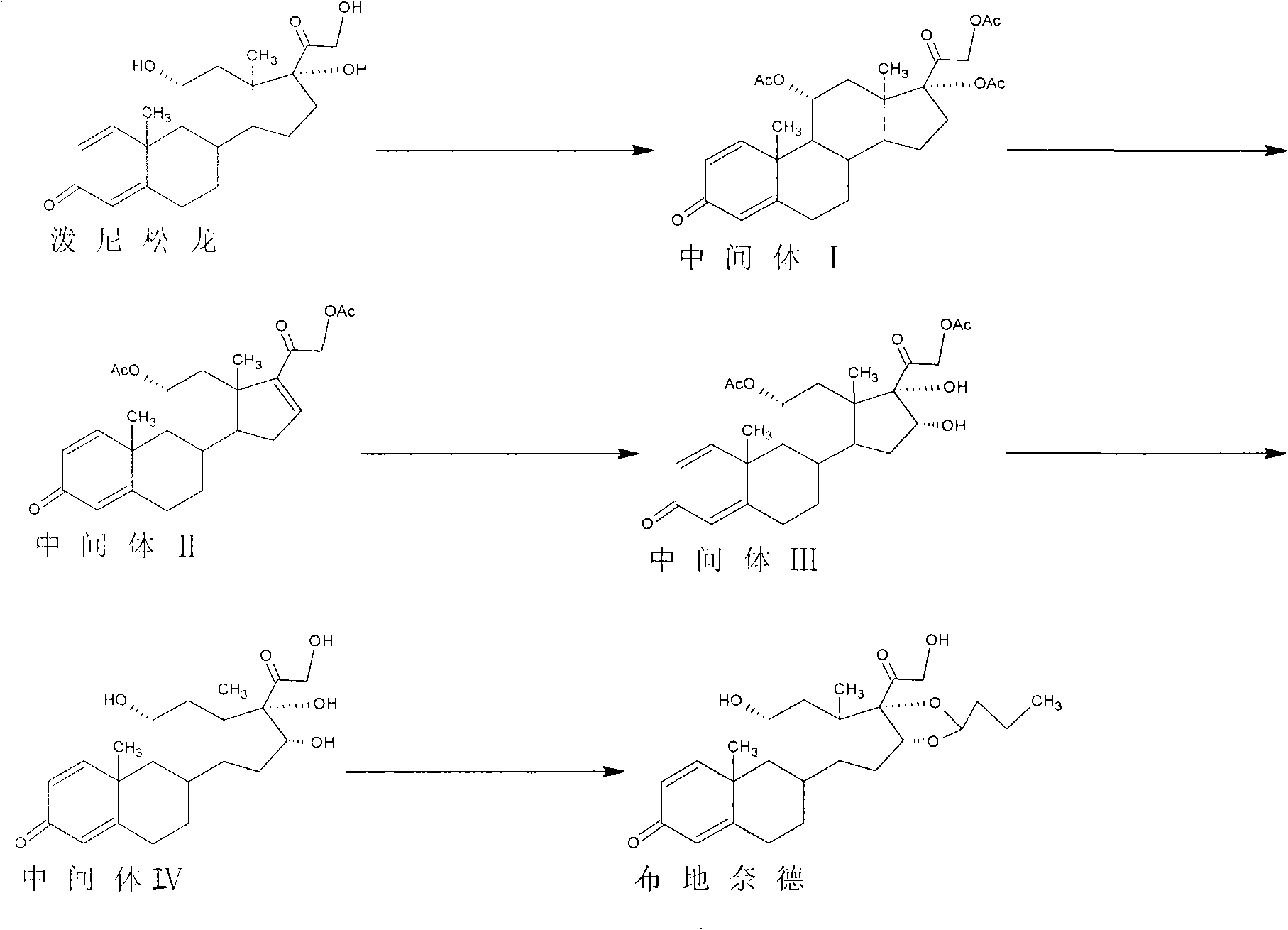
Preparation method of budesonide
An intermediate and diketone technology, which is applied in the field of preparation of budesonide, can solve the problems of slow reaction speed of intermediate preparation, unfavorable industrial production, and high industrial cost, so as to achieve easy control of reaction conditions, low production cost and guaranteed quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Add 100g of prednisolone (equivalent to 0.277mol), 400ml of dimethylformamide, 220ml of acetic anhydride, and 2g of 4-methylaminopyridine into the reactor, stir to raise the temperature and control the temperature at 80°C to react, TLC to track the reaction of raw materials It takes about 2.5 hours to complete, and then the reaction solution is cooled to room temperature. Pour the reaction solution into 6L of ice water, let it stand for 2 hours, filter with suction, wash with water, and dry to obtain shallow intermediate I (11,17,21-triacetoxy-1 , 4-pregnadiene-3,20-dione) yellow solid 124.8g (equivalent to 0.257mol). The molar yield of this step reaction is 92.8%.
[0029]2) Add 124.8g of intermediate I, 487ml of dimethylformamide, and 62.5g of potassium acetate into the reactor, stir and heat up to 100°C, keep warm for the reaction, TLC will take about 4 hours for the raw materials to react completely, and the reaction solution will drop to room temperature. The r...
Embodiment 2
[0036] 1) Add 80g of prednisolone (equivalent to 0.222mol), 300ml of dimethylformamide, 170ml of acetic anhydride, and 2g of 4-methylaminopyridine into the reactor, stir to raise the temperature, control the temperature at 100°C, and track the raw materials by TLC It takes about 2 hours for the reaction to complete, and the reaction solution is cooled to room temperature. The reaction solution is poured into 5L of ice water, allowed to stand for 1.6 hours, filtered with suction, washed with water, and dried to obtain shallow intermediate I (11,17,21-triacetoxy-1 , 4-pregnadiene-3,20-dione) yellow solid 99.7g (equivalent to 0.205mol), the molar yield yield of this step reaction is 92.3%.
[0037] 2) Add 99.7g of intermediate I, 400ml of dimethylformamide, and 45g of sodium acetate into the reactor, stir and raise the temperature to 120°C, keep warm for the reaction, TLC will take about 3 hours for the raw materials to react completely, and the reaction solution will drop to room...
Embodiment 3
[0044] 1) Add 150g of prednisolone (equivalent to 0.416mol), 600ml of dimethylformamide, 350ml of acetic anhydride, and 3g of 4-methylaminopyridine into the reactor, stir and raise the temperature, control the temperature at 100°C, and track the raw materials by TLC It takes about 3 hours for the reaction to complete, and the reaction solution is cooled to room temperature. The reaction solution is poured into 8L of ice water, allowed to stand for 2 hours, filtered with suction, washed with water, and dried to obtain shallow intermediate I (11,17,21-triacetoxy-1, 4-pregnadiene-3,20-dione) yellow solid 187.2g (equivalent to 0.385mol), the molar yield of this step reaction is 92.5%.
[0045] 2) Add 187.2g of intermediate I, 700ml of dimethylformamide, and 95g of potassium hydroxide into the reactor, stir and heat up to 110°C, keep warm for the reaction, TLC will take about 5 hours for the raw materials to react completely, and the reaction solution will drop to room temperature. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com