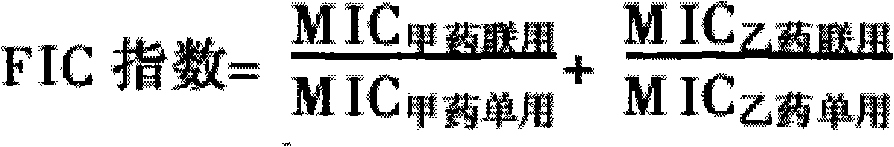Patents
Literature
147 results about "Prednisolone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
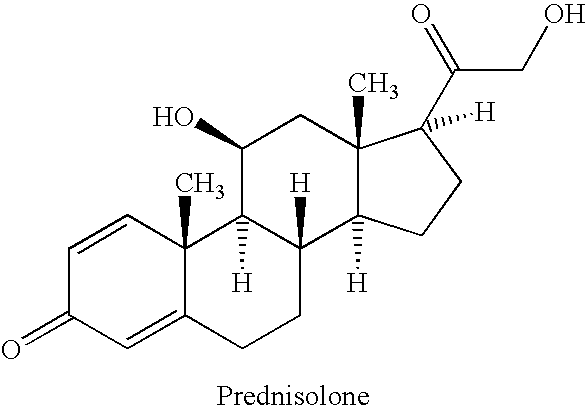
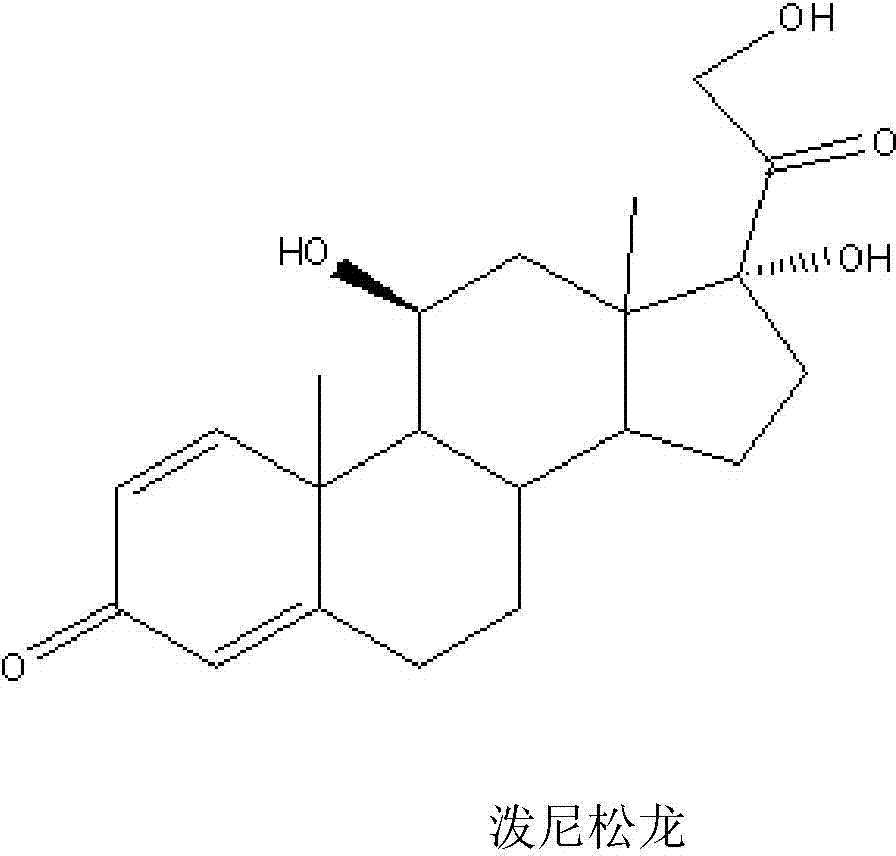
Prednisolone is a man-made form of a natural substance (corticosteroid hormone) made by the adrenal gland. It is used to treat conditions such as arthritis, blood problems, immune system disorders, skin and eye conditions, breathing problems, cancer, and severe allergies. It decreases your immune system's response to various diseases to reduce symptoms such as pain, swelling and allergic-type reactions..
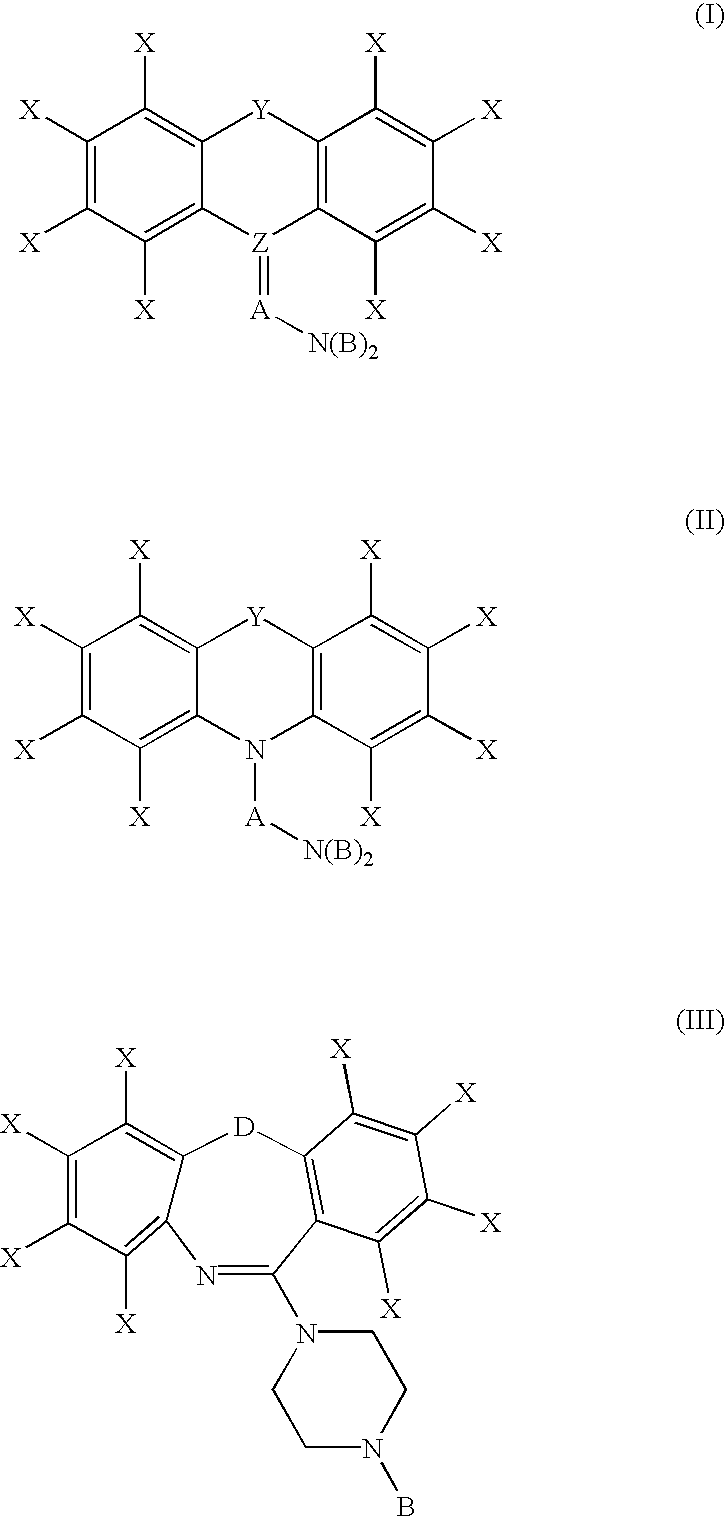
Combinations for the treatment of inflammatory disorders
InactiveUS6897206B2Suppression of TNFα levelBiocideCompound screeningTricyclic antidepressantTricyclic anti-depressants
The invention features a method for treating a patient having an inflammatory disorder, by administering to the patient (i) a tricyclic antidepressant (e.g., amoxapine); and (ii) a corticosteroid (e.g., prednisolone) simultaneously or within 14 days of each other in amounts sufficient to reduce or inhibit inflammation.
Owner:ZALICUS INC
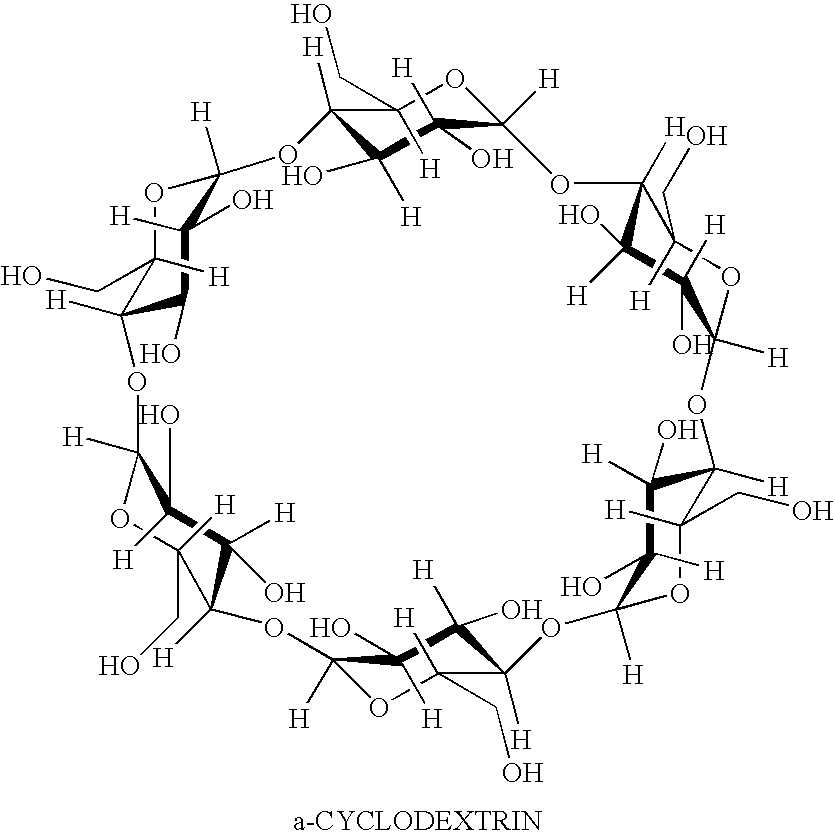
Prednisolone compositions
Disclosed herein are compositions comprising cyclodextrin derivatives and prednisolone and prodrugs thereof, and methods related thereto. The use of soluble polyanionic polymers such as hydroxypropylmethylcellulose and others in relation to these compositions is also disclosed. Delivery of these prednisolone-related compounds to the back of the eye via topical ophthalmic administration is also disclosed.
Owner:ALLERGAN INC
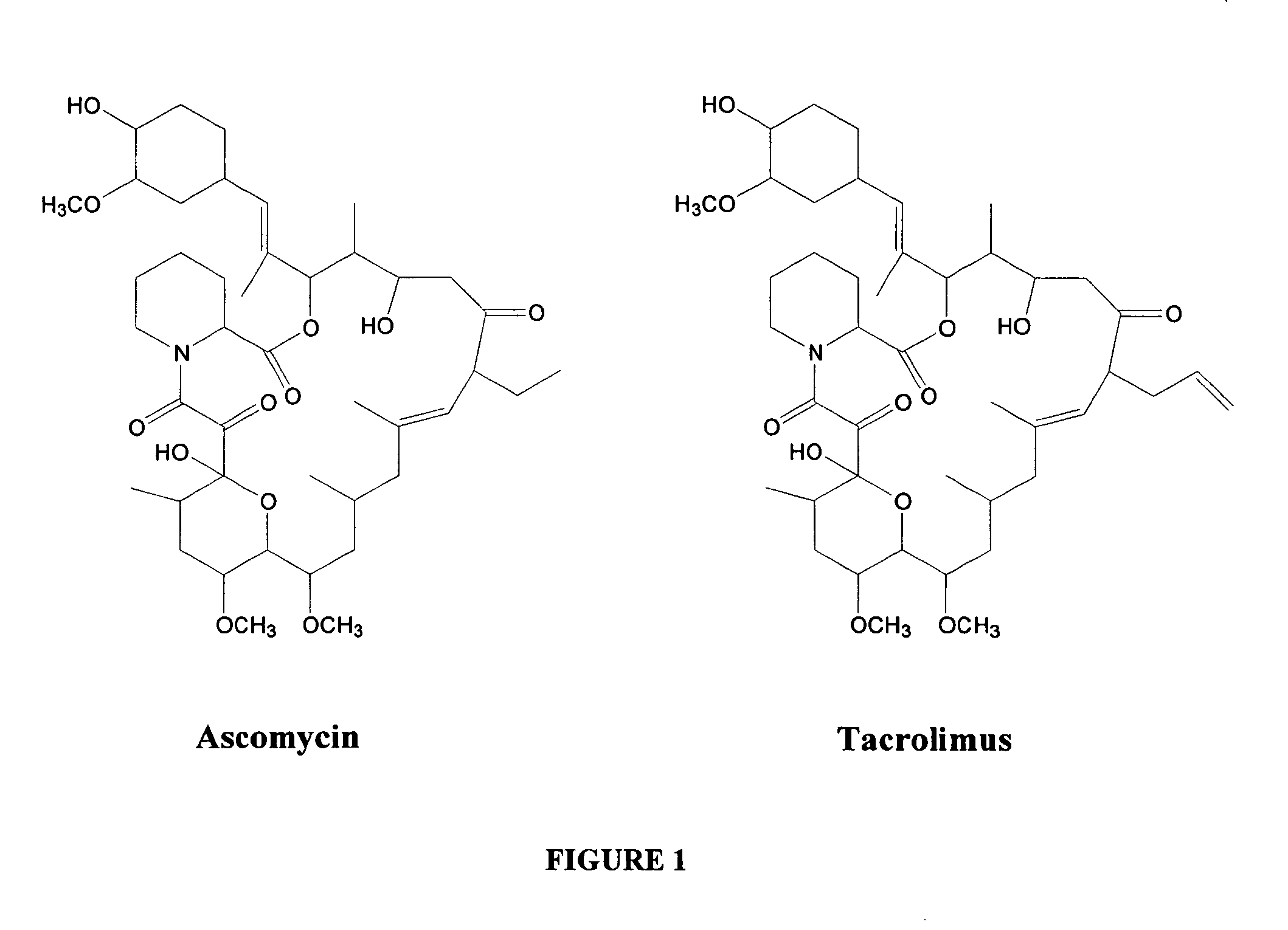
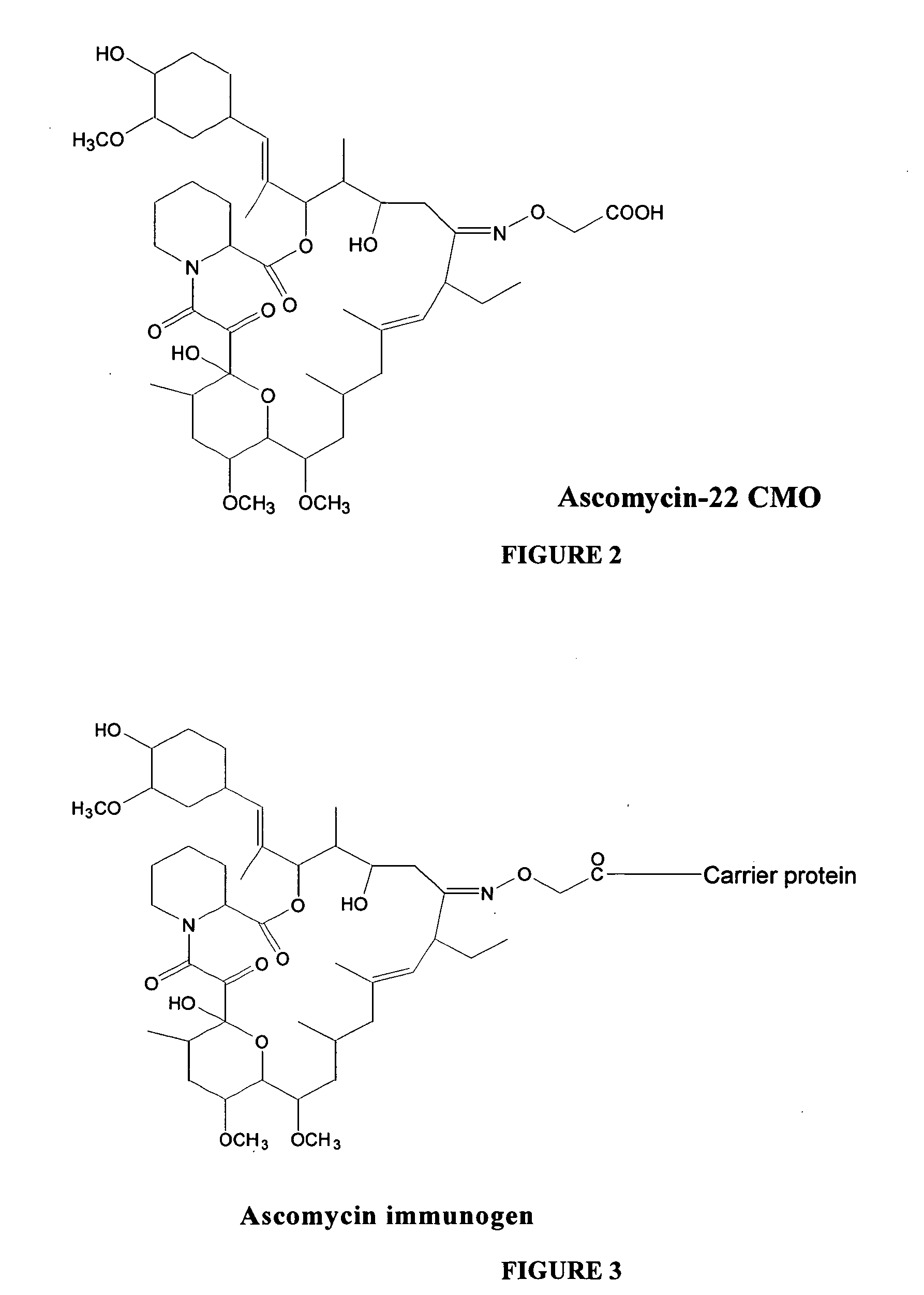
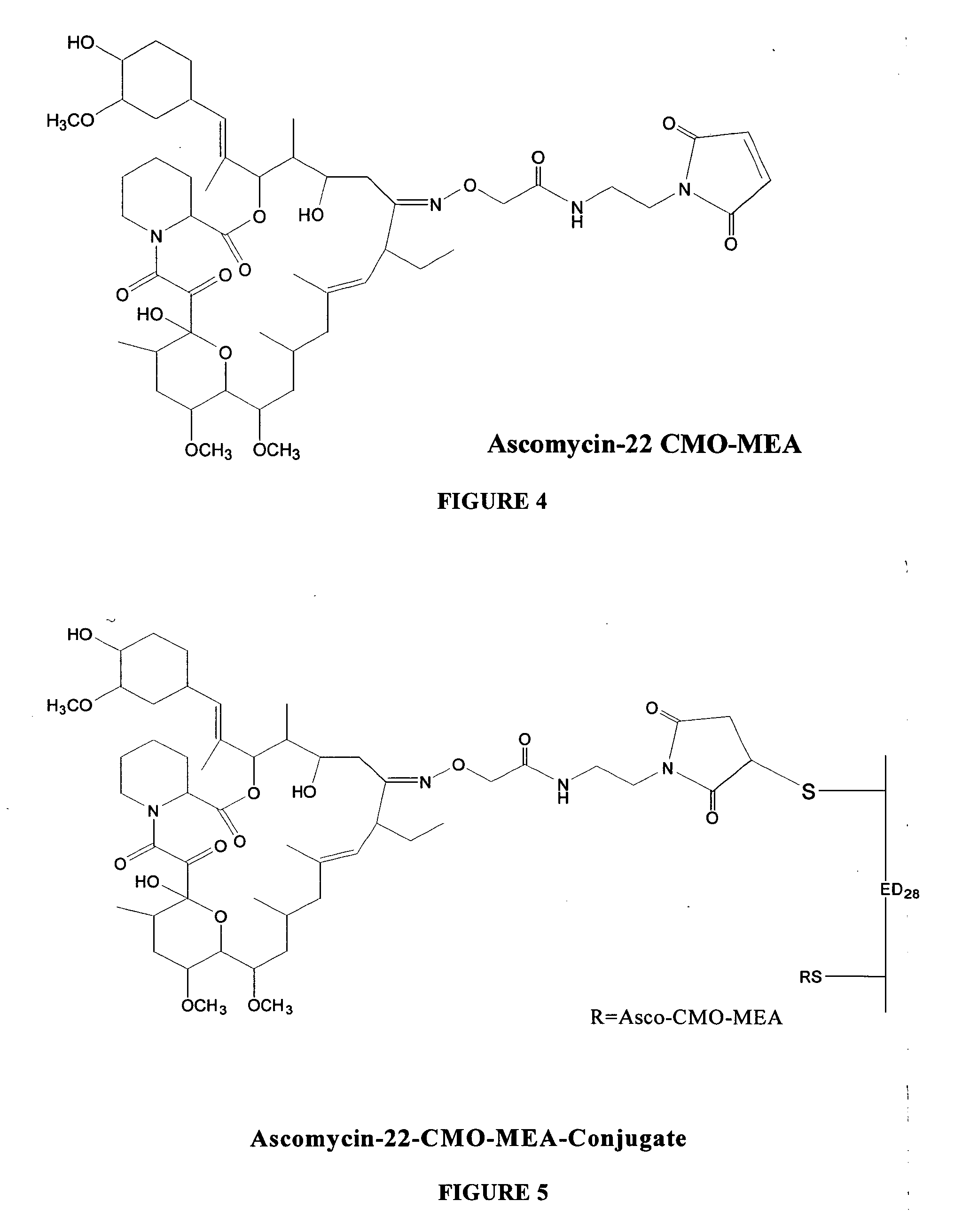
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
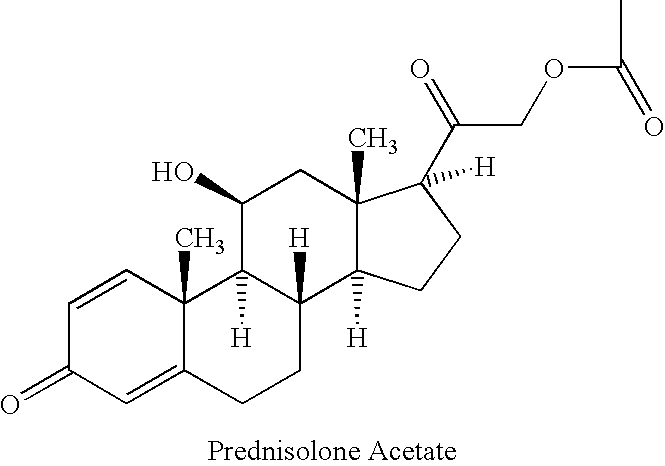
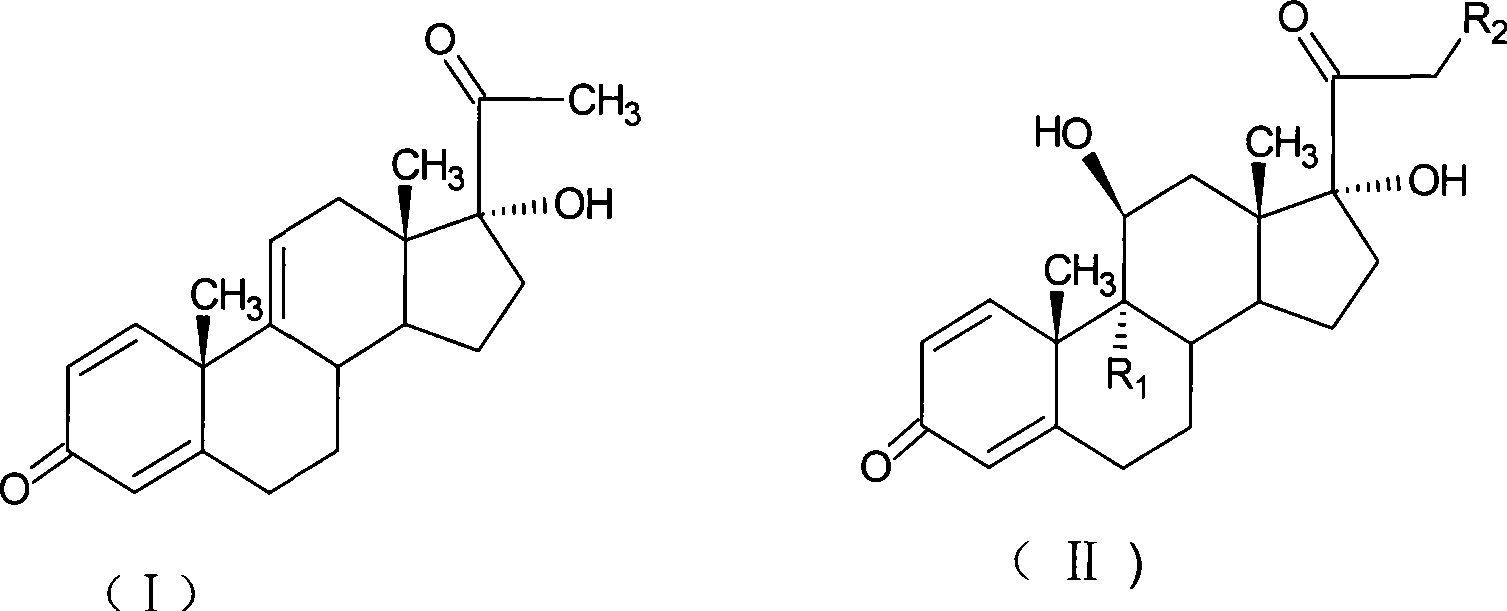
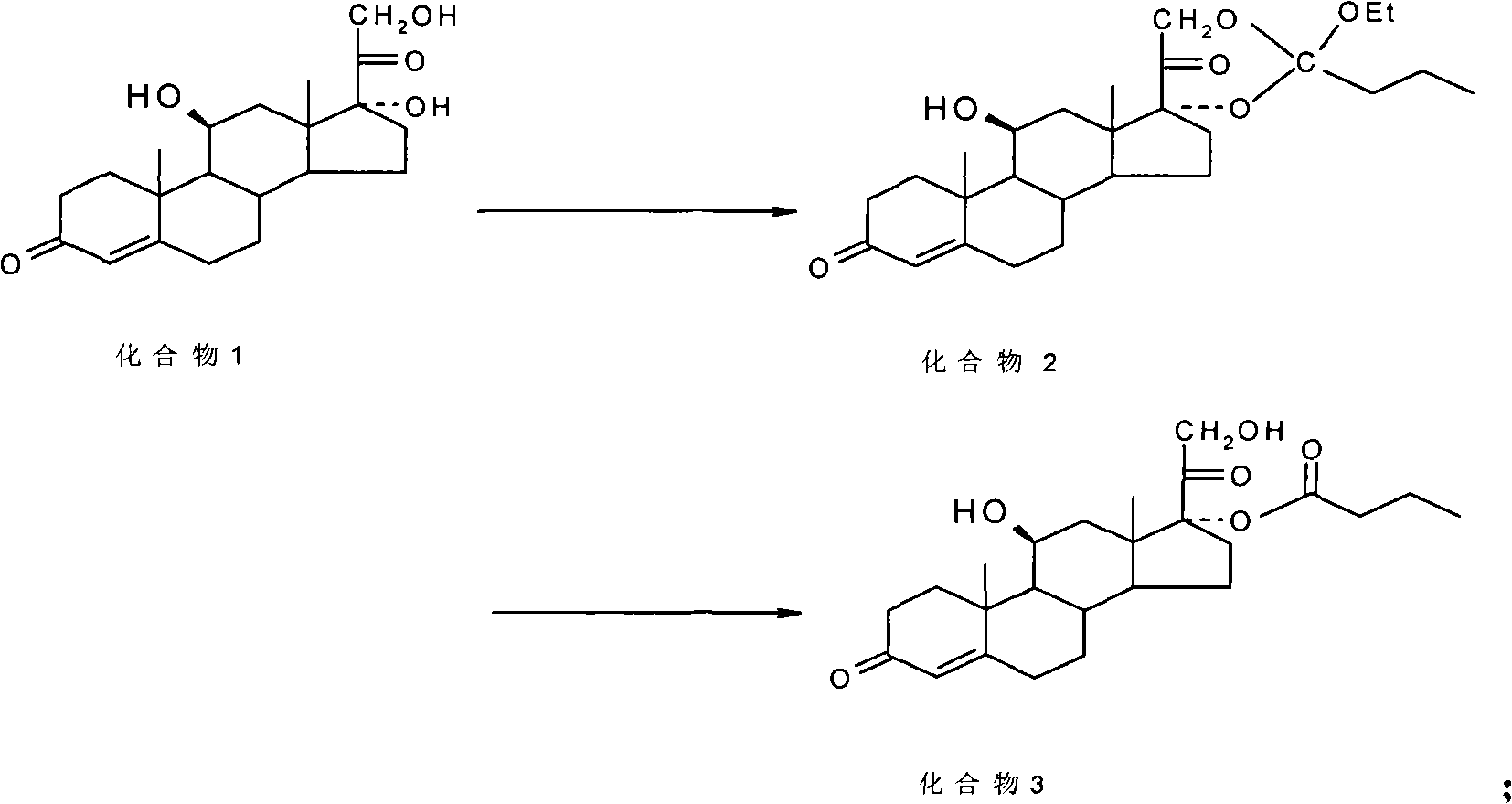
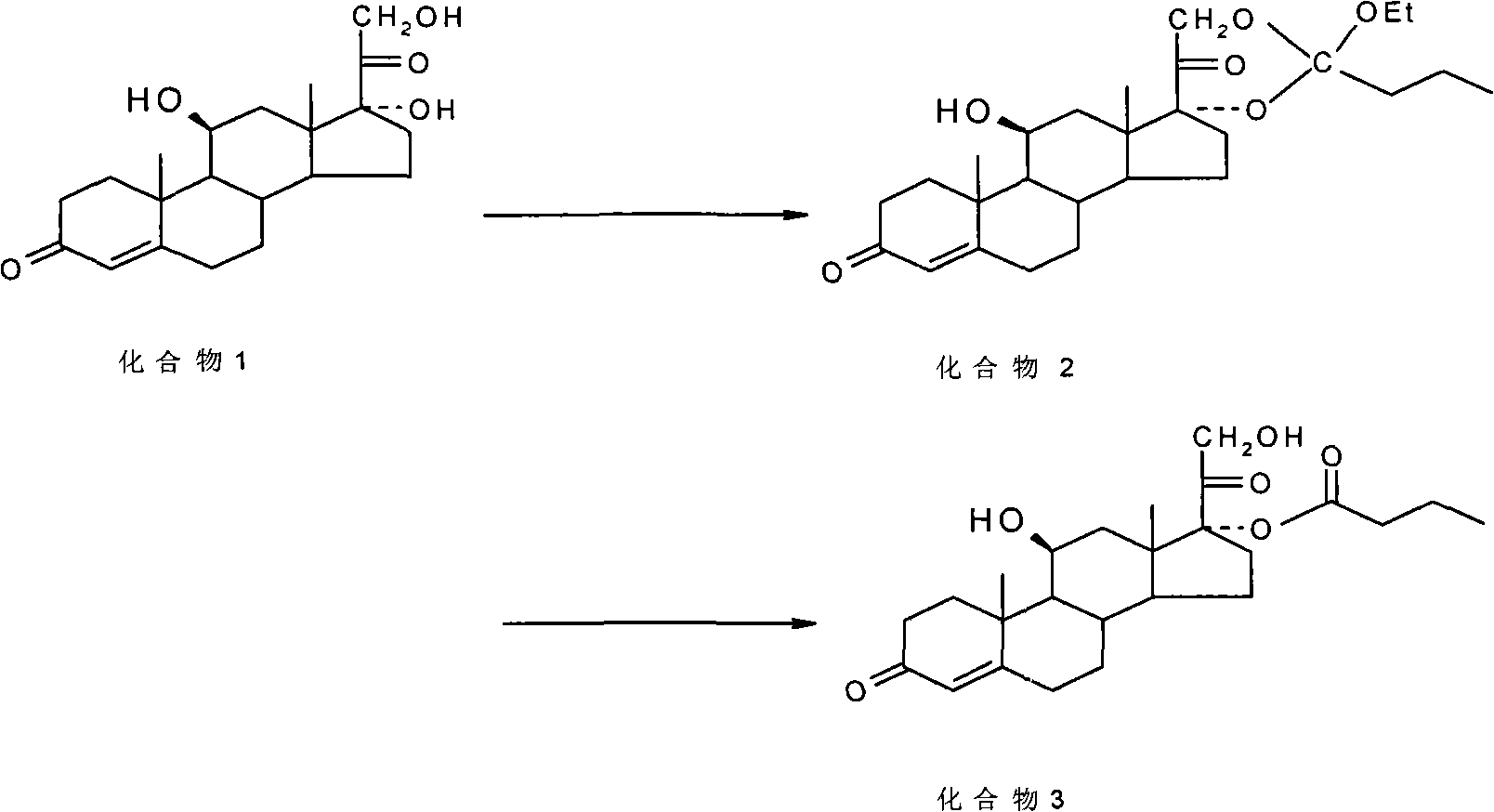
Preparation of metacortandralone and derivatives thereof
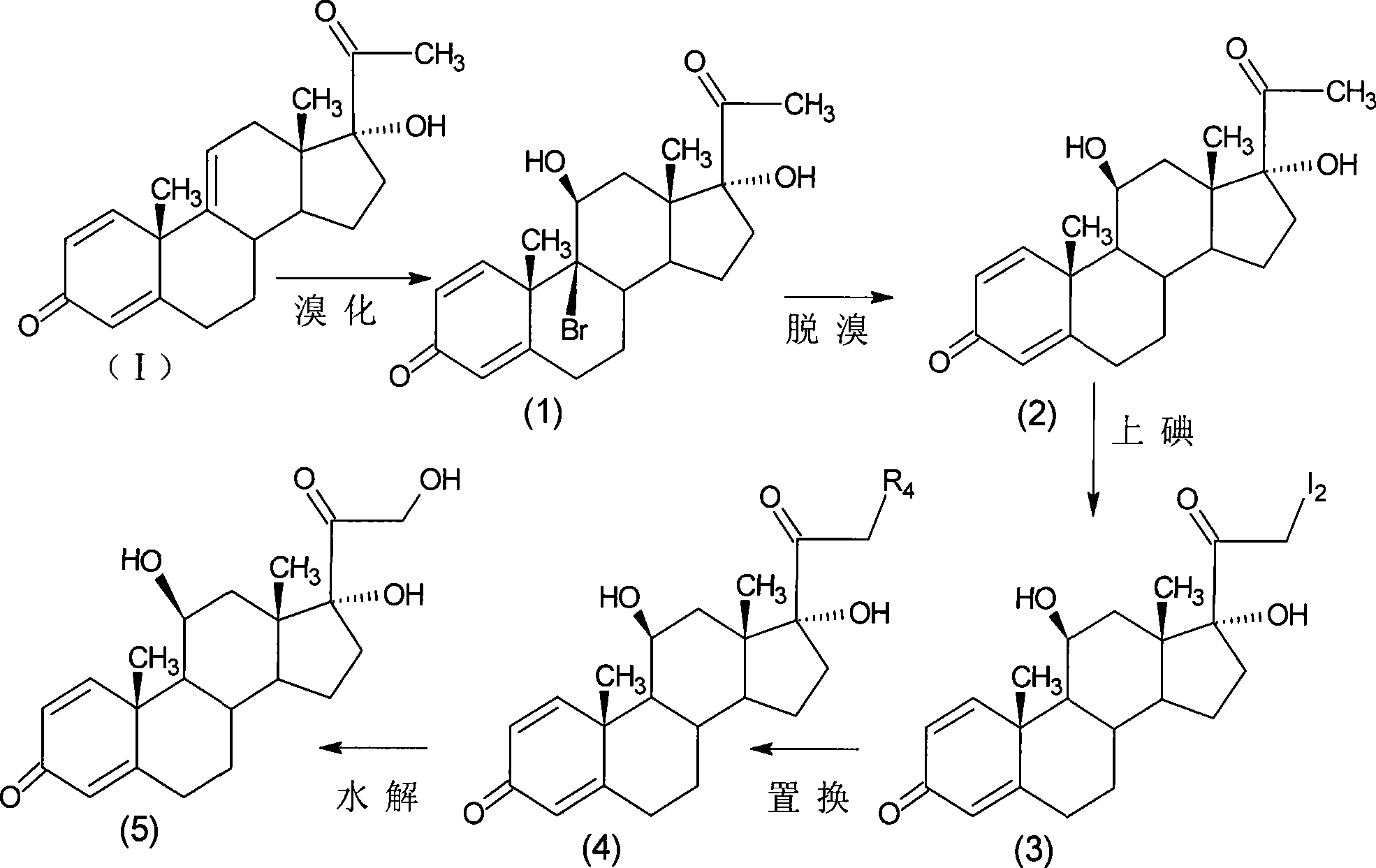
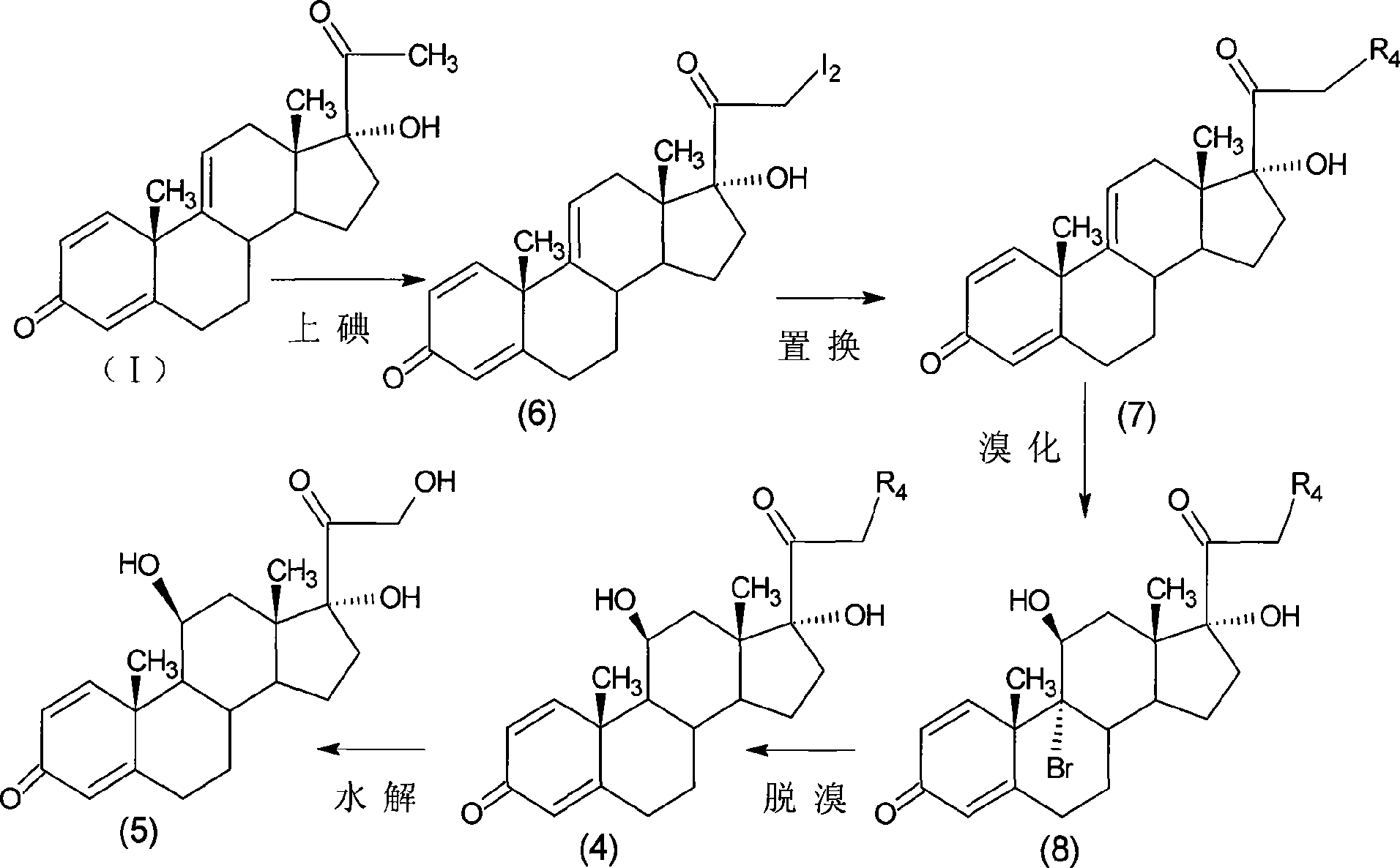
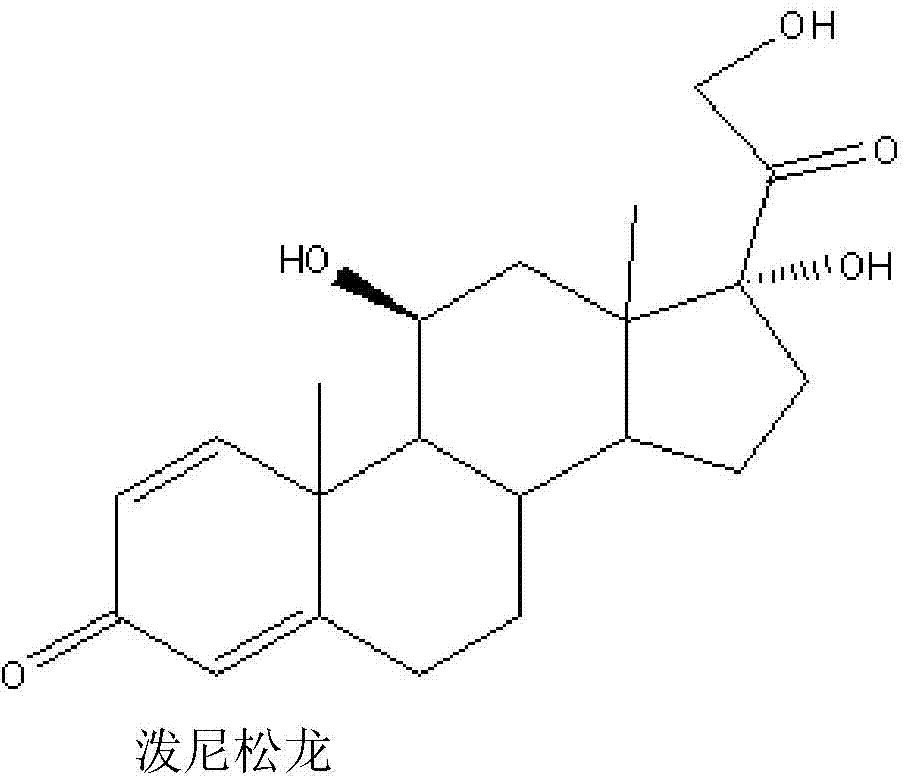
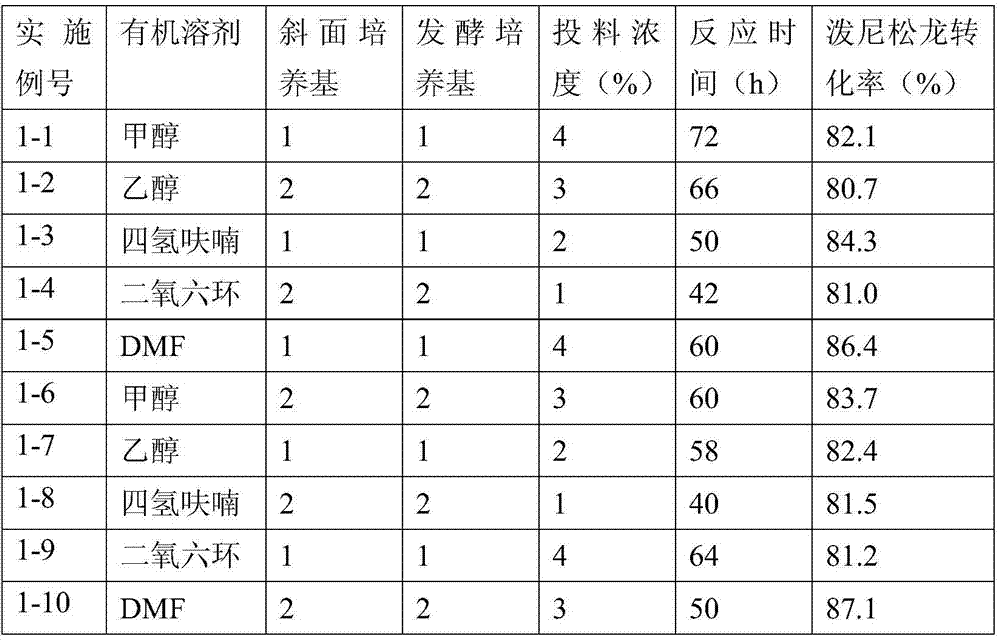
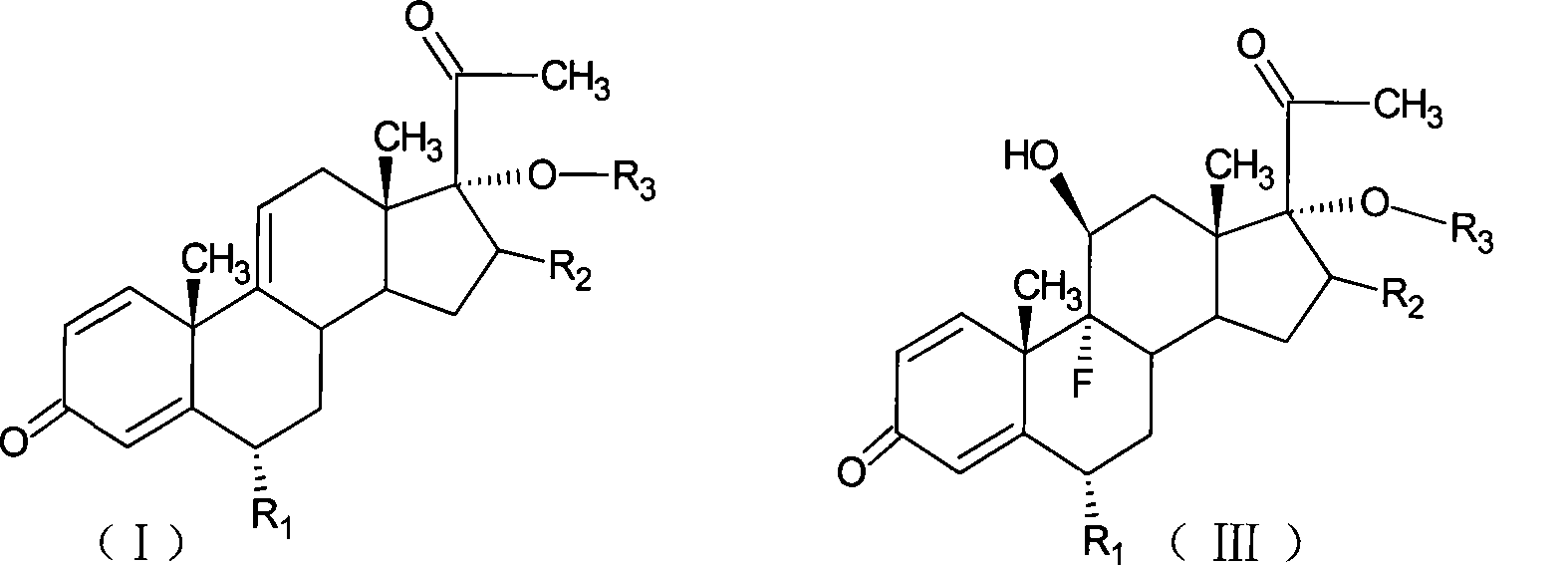
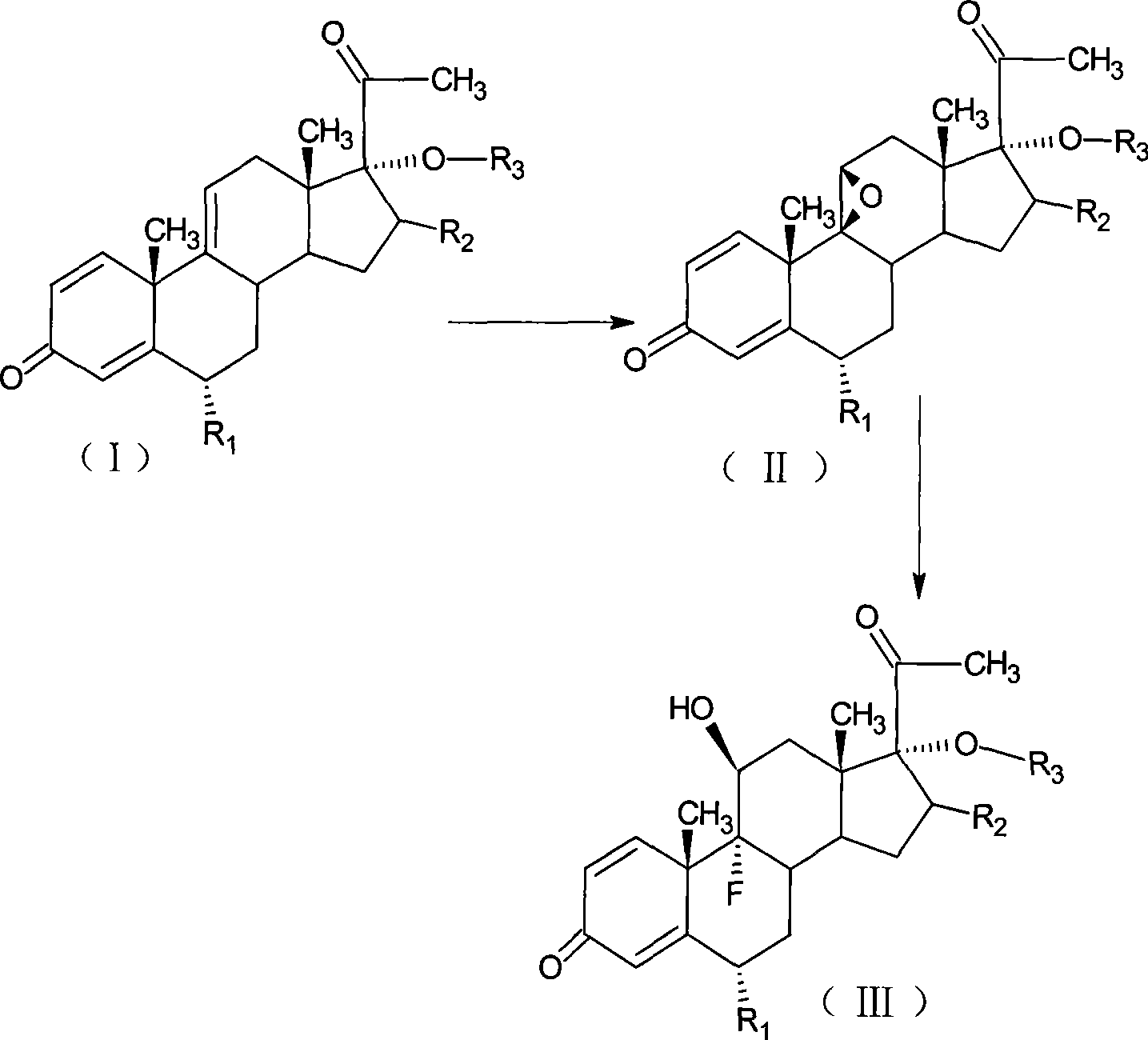
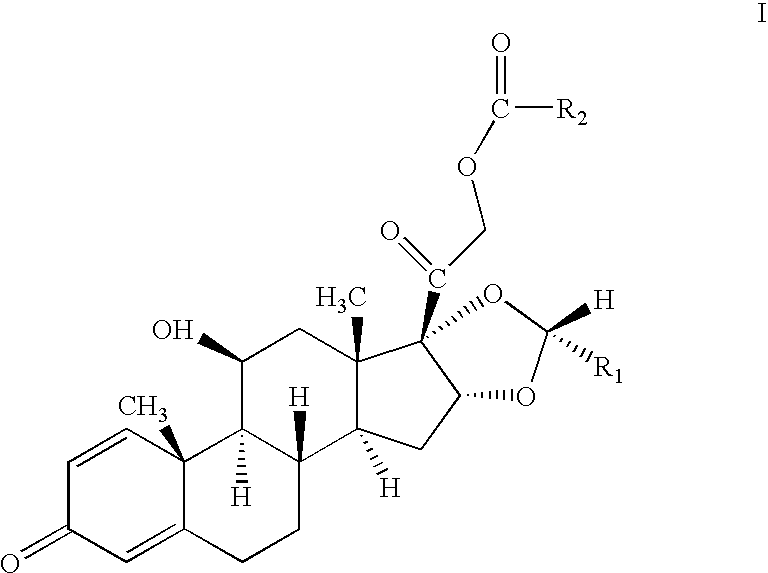
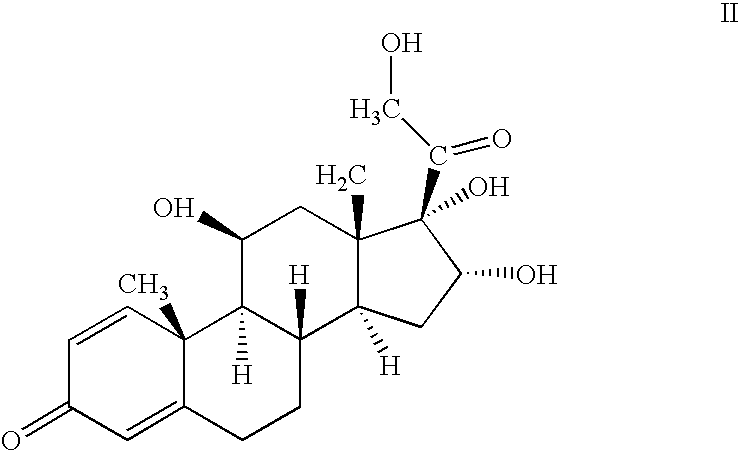
The invention relates to a preparation method of a steroid compound, in particular to the preparation for prednisolone and the derivative thereof, which takes 17-hydroxyl-1, 4, 9-triene- pregna-3, 20-diketone as the initiator and is improved by 9, 11th and 21st to obtain the prednisolone and the derivative thereof, such as prednisolone acetic ester, isoflupredone, and the like. The invention further provides the application of a compound (I) in the preparation of a compound (II). As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the prednisolone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the triamcinolone products and the prednisolone products, thus greatly reducing the production cost and industrial conditions. R1 is equal to H, F, Cl and Br; R2 is equal to H, OH and OCOR3, wherein, R3 is equal to the alkyl with less than 11carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
Preparation method of R-budesonide
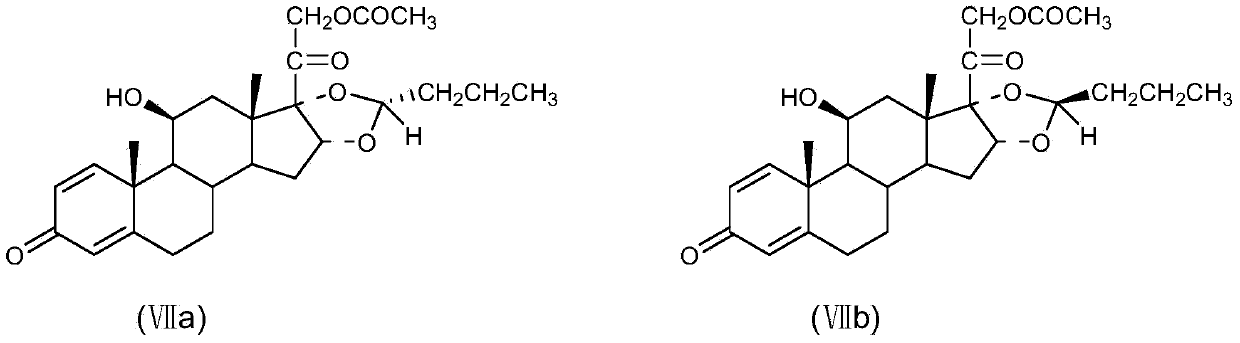
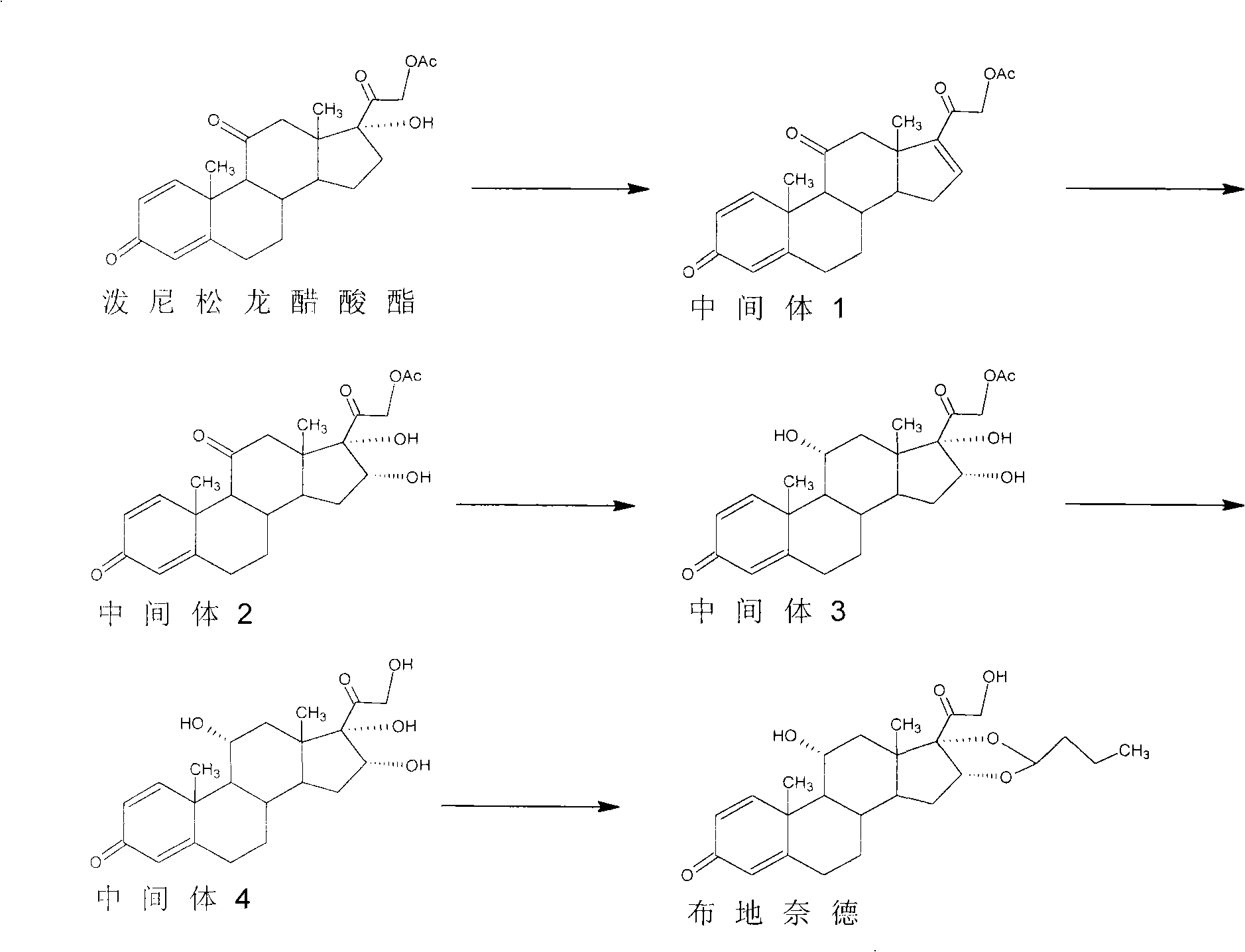
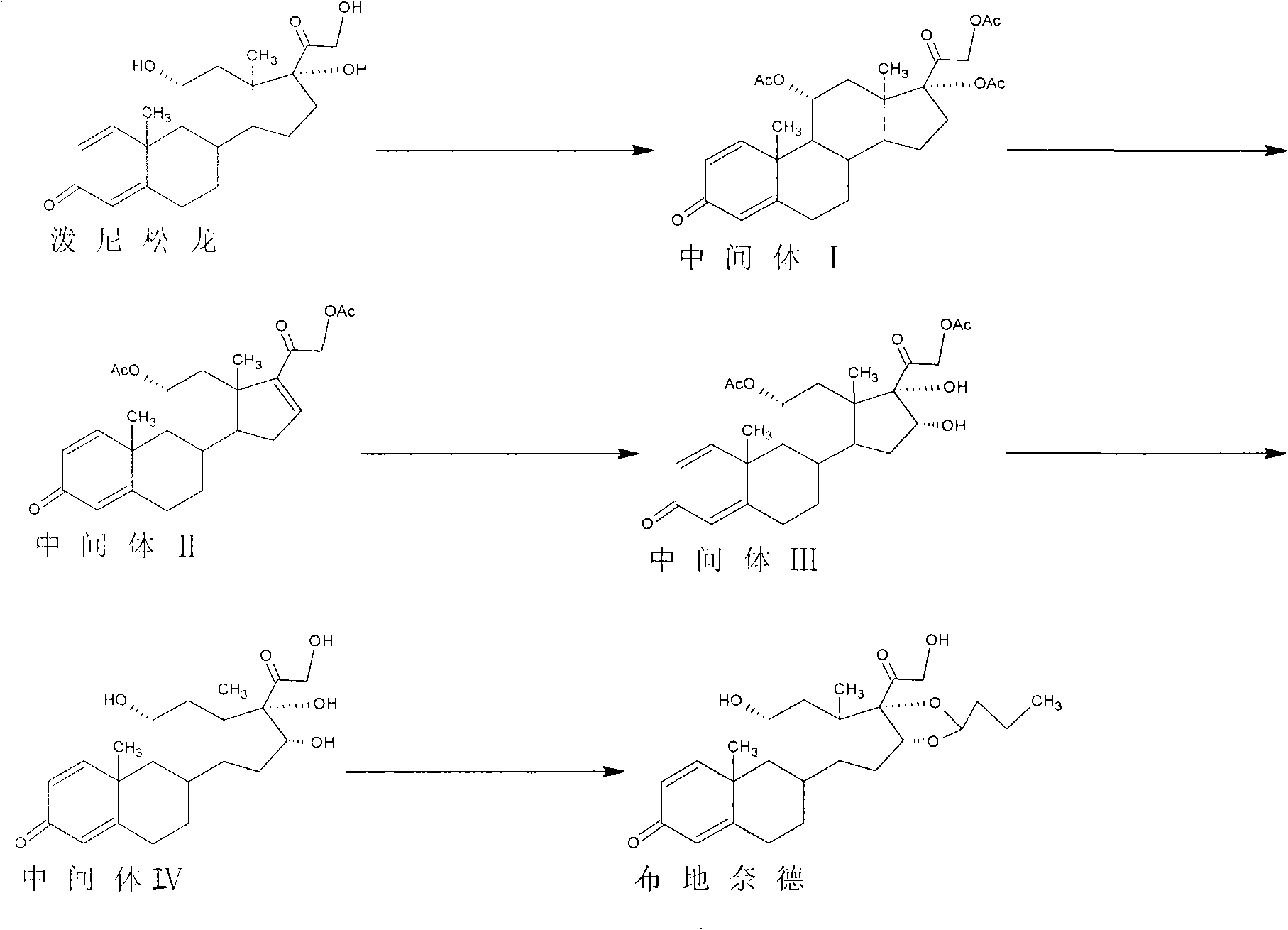
The invention discloses a preparation method of an adrenal cortex hormone medicament and particularly relates to a preparation method of a high-purity R-budesonide isomer. According to the method disclosed by the invention, prednisolone is used as an original raw material and the budesonide acetic ester is prepared by cyclization, ring opening, esterification, elimination, oxidation, cyclization and hydrolysis. The method disclosed by the invention has a simple process and high yield and is suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and preparation method thereof
InactiveCN101953784AExpanded antimicrobial spectrumHigh antibacterial efficacyAntibacterial agentsSolution deliveryPrednisoloneColistin Sulfate
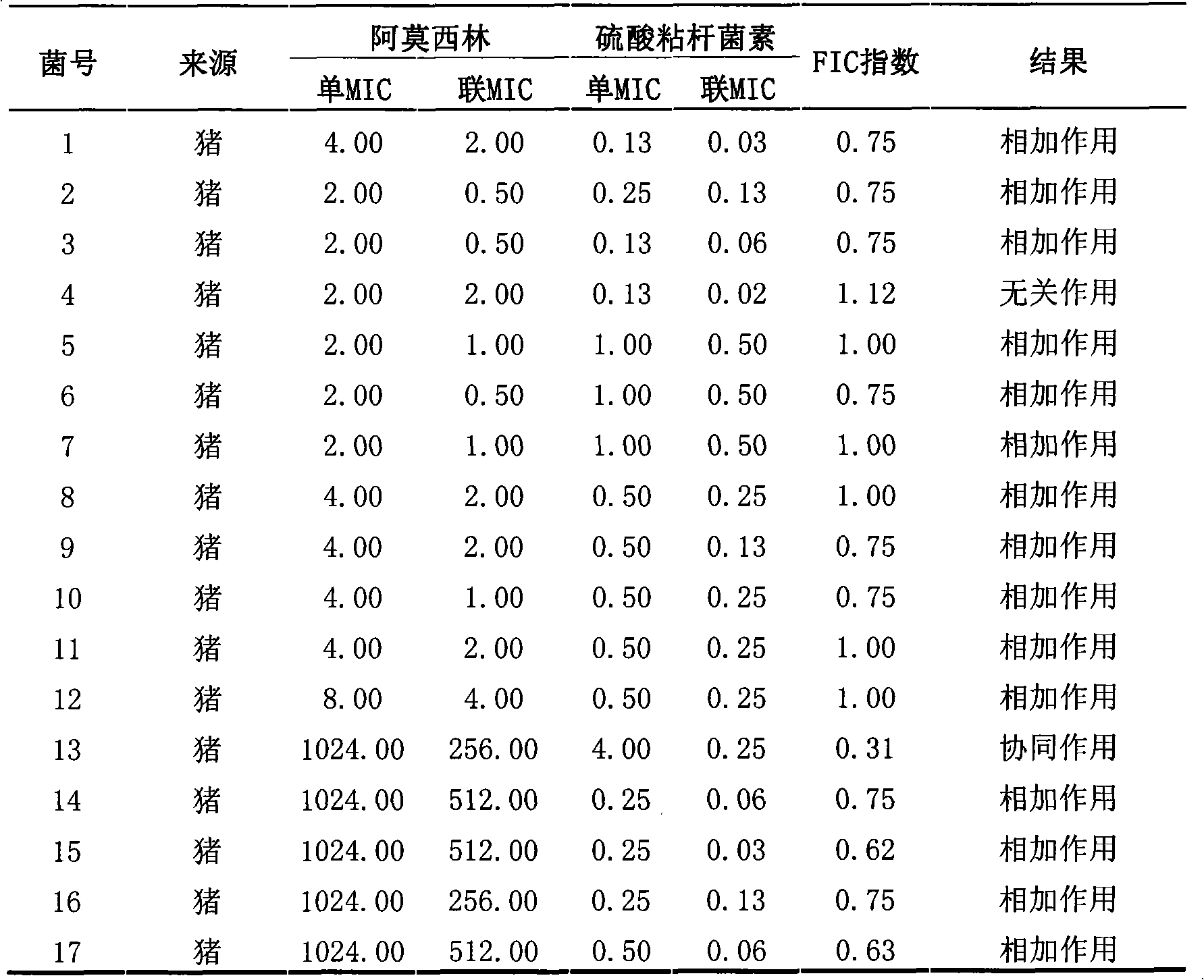
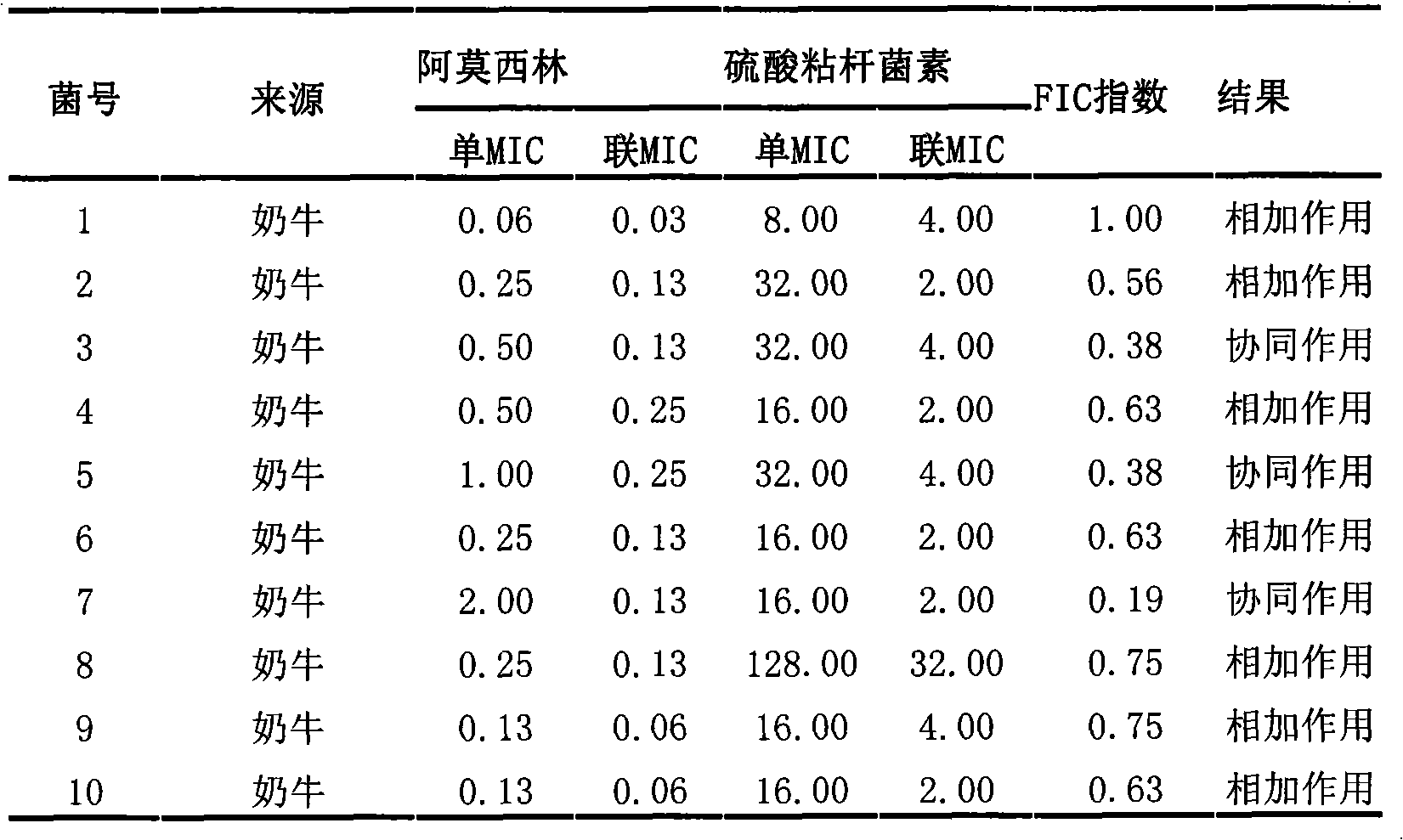
The invention discloses a veterinary suspension containing amoxicillin, colistin sulfate and prednisolone and a preparation method thereof. The veterinary suspension adopts the amoxicillin, the colistin sulfate and the prednisolone as active ingredients. The preparation method of the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone comprises the following steps of: (1) dissolving or dispersing a suspending agent, an antioxidant and a preservative in a hot dispersion medium to obtain a solution (A); (2) adding 40-80 percent of dispersion medium in a formula ratio in a colloid mill, starting the colloid mill, then slowly adding the solution (A) and adding a wetting agent while stirring after the solution (A) is fully added; (3) sequentially adding the amoxicillin, the colistin sulfate and the prednisolone after fully adding all the accessories, and grinding by adopting two alternate modes, i.e. an endless grinding mode and a non-endless grinding mode; and (4) detecting the grain fineness, stopping grinding when the grain fineness accords with the requirement, adding the dispersion medium to the formula ratio, mixing, canning, sealing and sterilizing to obtain the veterinary suspension containing the amoxicillin, the colistin sulfate and the prednisolone.
Owner:CHINA AGRI UNIV +1
Preparation method of budesonide
The invention discloses a preparation method of budesonide. The budesonide is prepared sequentially through the following steps of: carrying out an esterification reaction on a starting material and acetic anhydride, wherein the starting material is prednisolone; carrying out a degreasing reaction in the presence of a catalyst which can be alkali or an alkali metal salt ; carrying out an oxidizing reaction with potassium permanganate in acid environment; carrying out an ester exchange reaction with alcohol in alkaline environment; carrying out a condensation reaction with n-butanal, and the like. In the preparation method, appropriate catalysts are added in the reactions, and reaction conditions are appropriately controlled so as to achieve the purposes of increasing the reaction rates inall the steps and improving the yield of intermediate products, and the product quality conforms to the standard of the European Pharmacopoeia. Meanwhile, all the steps have moderate reaction condition and easy control, low energy consumption, high product yield, small pollution, easy reaction raw material obtaining and low production cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
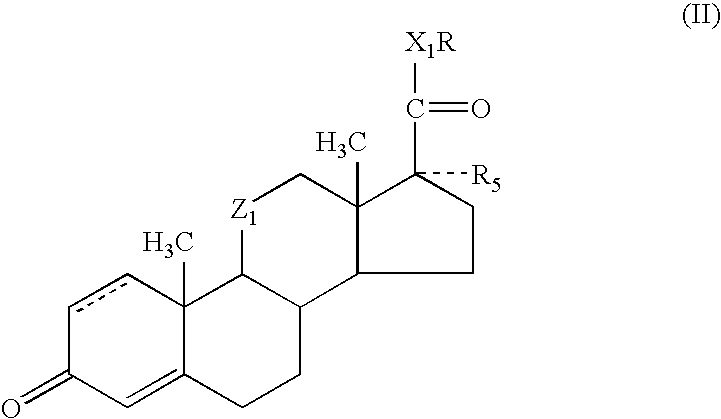
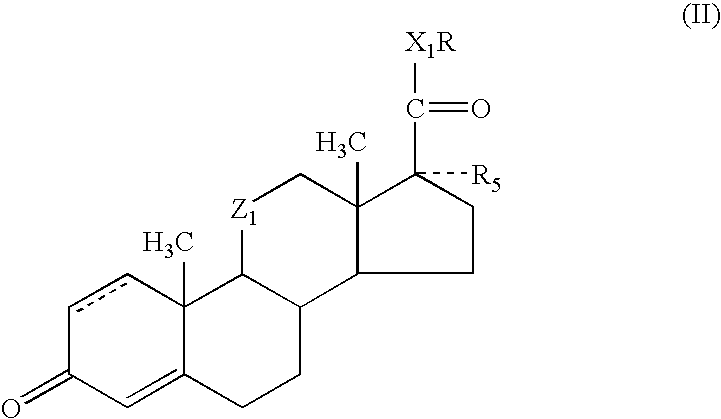
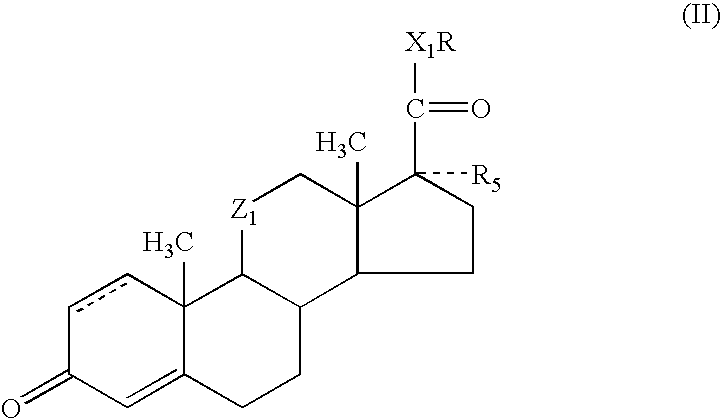
Enhancement of activity and/or duration of action of selected anti-inflammatory steroids for topical or other local application
ActiveUS20050020551A1High activityExtended durationOrganic active ingredientsAntipyreticPrednisoloneAlkoxy group
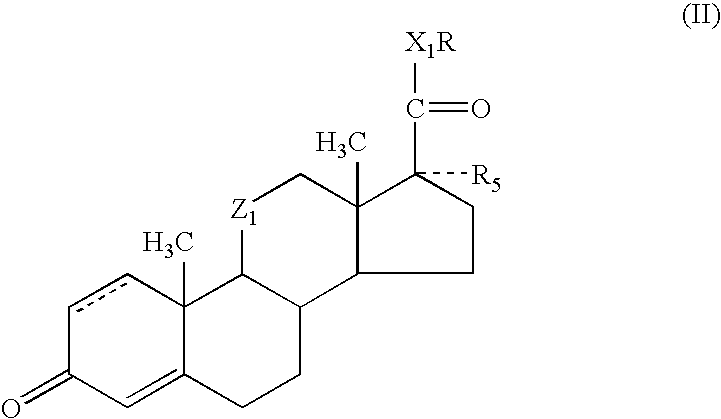
Methods and compositions for enhancing the activity and / or duration of action of soft anti-inflammatory steroids of the haloalkyl 17α-alkoxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylate type and the corresponding Δ1,4 compounds and of anti-inflammatory steroids of the hydrocortisone and prednisolone type are described. The enhancing agents have the formula: wherein R is H or C1-C4 alkyl; Z1 is carbonyl or β-hydroxymethylene; X1 is —O— or —S—; R5 is —OH, —OR6, —OCOOR6 or —OCOR7 wherein R6 is C1-C4 alkyl and R7 is C1-C4 alkyl, fluoromethyl or chloromethyl; and the dotted line in ring A indicates that the 1,2-linkage is saturated or unsaturated; with the proviso that when R is C1-C4 alkyl, then R5 is —OH.
Owner:BODOR LAB
Breast perfusion preparation for treating mammitis of milk cow in lactation period and preparation method thereof
InactiveCN101579347AGood therapeutic effectImprove stabilityAntibacterial agentsPharmaceutical delivery mechanismAntioxidantMilk cow's
Owner:CHINA AGRI UNIV
Pharmaceutical compositions for intraocular administration and methods for fabricating thereof
InactiveUS20160175323A1Removing issuePositive patient outcomeBiocideOrganic active ingredientsPrednisoloneMoxifloxacin
Pharmaceutical ophthalmic compositions are described, the compositions consisting essentially of a therapeutically effective quantity of an anti-bacterial agent (such as moxifloxacin), a therapeutically effective quantity of an anti-inflammatory agent (such as prednisolone), at least one pharmaceutically acceptable excipient and a pharmaceutically acceptable carrier. Methods for fabricating the compositions and using them are also described.
Owner:HARROW IP LLC
Perfusion medicament for treating milk cow mastitis and preparation method thereof
ActiveCN102488694ATreat both symptoms and root causesSolve the problem of easily inducing endotoxemia in dairy cowsAntibacterial agentsSexual disorderFLUNIXIN MEGLUMINEThird generation
The invention discloses a perfusion medicament for treating milk cow mastitis and a preparation method thereof. The perfusion medicament is prepared from the following raw materials by weight: 0.9-4.3g of cefquinome sulphate, 0.15-0.25g of prednisolone, 0.5-1.3g of flunixin meglumine, 1-1.5g of glycerin monostearate, 3-8g of Vaseline, 0.05g of ethylparaben and 65-70g of whiteruss. The preparationmethod comprises the following specific steps of: (1) putting one tenth of the liquid paraffin into a burdening tank in a certain weight ratio; (2) adding one third of the liquid paraffin into a heating tank; and (3) putting the rest of the liquid paraffin left after the steps (1) and (2) and auxiliary materials in the step (2) into an emulsifying tank, adding 0.9-4.3g of cefquinome sulfate in a certain weight ratio, and stirring till uniform distribution to obtain the perfusion medicament for treating milk cow mastitis. The perfusion medicament has the advantages of no induction of endotoxemia in a milk cow mastitis treating process, remarkable curative effect on mastitis and freeness from medicament tolerance.
Owner:QILU ANIMAL HEALTH PROD
Synthesis method of 16alpha-hydroxy prednisolone
ActiveCN102850423AEasy to prepareReduce pollutionSteroidsChemical recyclingDiphenylmethanePtru catalyst
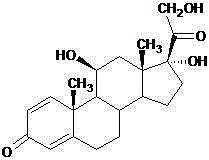
The invention provides a synthesis method of 16alpha-hydroxy prednisolone, belonging to the technical field of fine chemical synthesis method. The synthesis method comprises the following steps: using prednisolone with a structure as shown in formula (I) as a raw material, performing dehydration reaction to generate a double-bond compound under the action of gemini surfactant as shown in formula (II), then adding aqueous hydrogen peroxide solution for further reaction, and adding water to hydrolyze after finishing reaction so as to obtain the target product 16alpha-hydroxy prednisolone, wherein the gemini surfactant is diphenylmethane gemini surfactant. According to the synthesis method, gemini surfactant is simultaneously used as a reaction medium and a catalyst, and can be recovered and reused after reaction finishes, the process is simple, the after-treatment is convenient, the obtained products have the characteristics of high productivity, good efficiency, and less waste, the yield is greater than 85%, and the gemini surfactant can be recycled, and therefore the synthesis method is an economical, practical and environment-friendly technology and is suitable for popularization and application.
Owner:浙江东晖药业有限公司
Preparation of superfine prednisolone powder
InactiveCN101015558AHigh yieldTo overcome the solubility difference is not large enoughOrganic active ingredientsPowder deliveryPrednisoloneInstability
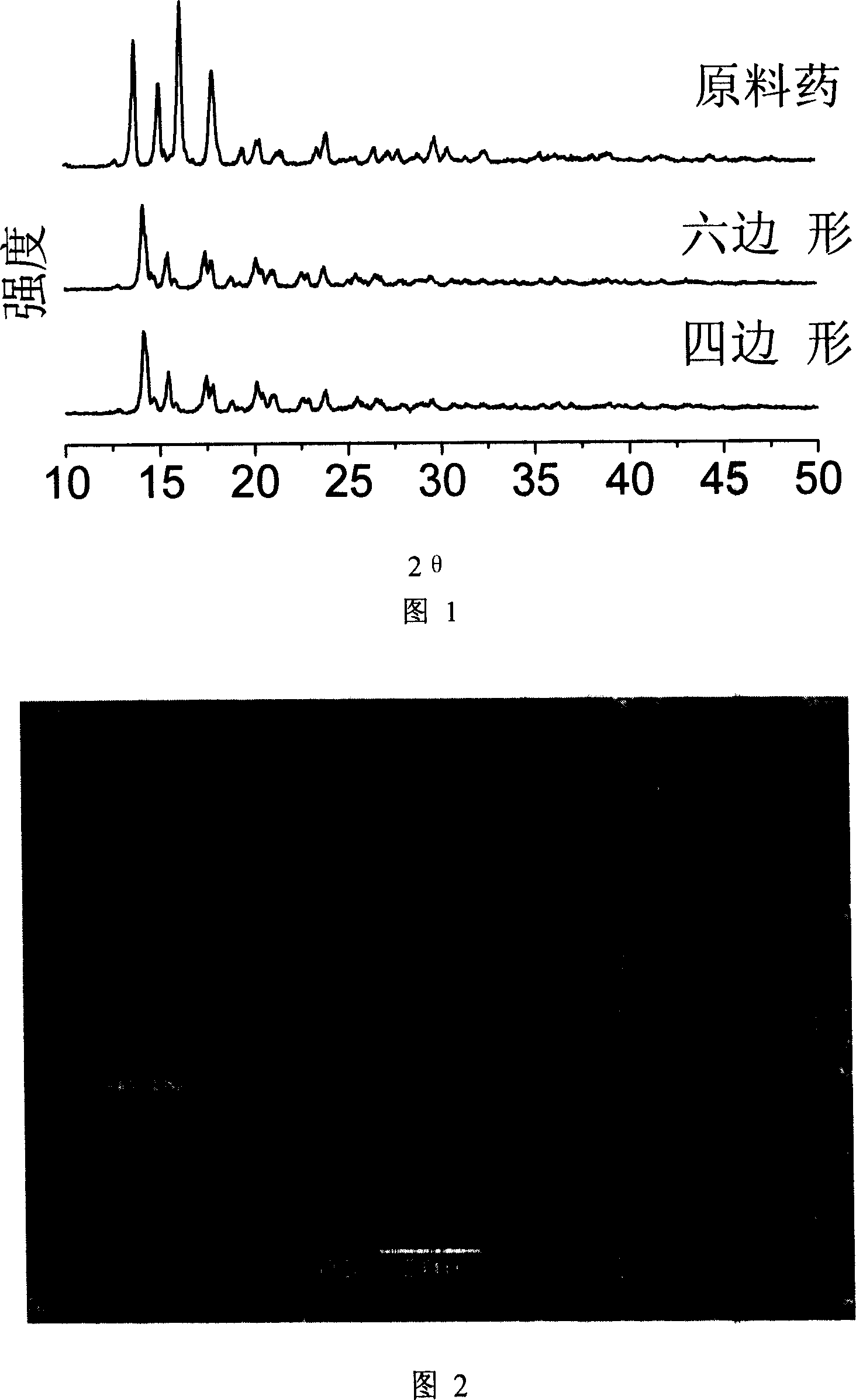
This invention relates to a method for preparing superfine prednisolone powder, and belongs to the field of micronization. Open question at present: particle diameter of prednisolone is small but can not obtain dry powder, or the dry powder has wide size distribution, bad redispersibility, and instability in water. The invention is to realize the preparation of prednisolone granule with controllable particle diameter, narrow particle size distribution, and superfine or nano-graded quadrilate and hexagon through inverse solvent recrystallization.the prednisolone of the invention has the advantages of controllable particle diameter, narrow particle size distribution, good redispersibility, stable micropowder, and good dissolving effect; and has prodigious dominance in the development of drug effect and new medicament form.
Owner:BEIJING UNIV OF CHEM TECH +1
Method for preparing sterides compound 17-alpha ester
ActiveCN101891797AMake up for the impactPromote hydrolysisSteroids preparationPrednisoloneCompound 17
The invention discloses a method for preparing a sterides compound 17-alpha ester. The method comprises the following steps of: dissolving 17 alpha, 21-dihydroxyl sterides compound serving as a raw material in a cyclic ester solvent; adding an ester and a catalyst into the mixture and reacting the mixture to prepare a cyclic ester; and dissolving the prepared cyclic ester in a hydrolysis solvent, adding an orientation reagent and a hydrolysis reagent into the mixed solution and hydrolyzing the cyclic ester into the 17-alpha ester. The sterides compound is hydrocortisone, prednisolone, prednisone, hexadecadrol or betamethasone. The method has selective hydrolysis effect,, and can effectively enhance the conversion rate of a product, greatly increase product yield and greatly promote the preparation capability of a sterides medicament.
Owner:ZHEJIANG XIANJU PHARMA
Method for preparing 6 beta-methylprednisolone
InactiveCN107602652AHigh purityImprove pharmacological activitySteroidsDehydrogenationMethylprednisolone
The invention discloses a method for preparing 6 beta-methylprednisolone. The method comprises the following steps: taking 6-methyl epoxides as raw materials, after Grignard reaction, hydrolysis, elimination, generating 6 beta-methyl elimination, and then after dehydrogenation by fermentation, iodine putting, replacement, hydrolysis, preparation of liquid phase purification, obtaining 6 beta-methylprednisolone. A reaction formula is as follows: the formula is shown in the description, the 6 beta-methylprednisolone of the invention is a major synthetic impurity in methylprednisolone, separation and purification of the 6 beta-methylprednisolone have great significance on impurity analysis.
Owner:ZHEJIANG XIANJU PHARMA
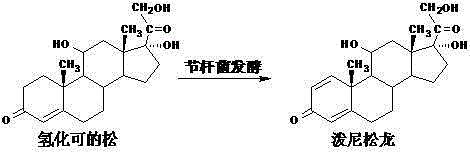
Method for preparing prednisolone through bio-fermentation in one step
The invention relates to a method for directly generating prednisolone through bio-fermentation in one step by using cortisone acetate as a raw material and Arthrobacter Simple ATCC 21032.
Owner:TIANJIN JINYAO GRP
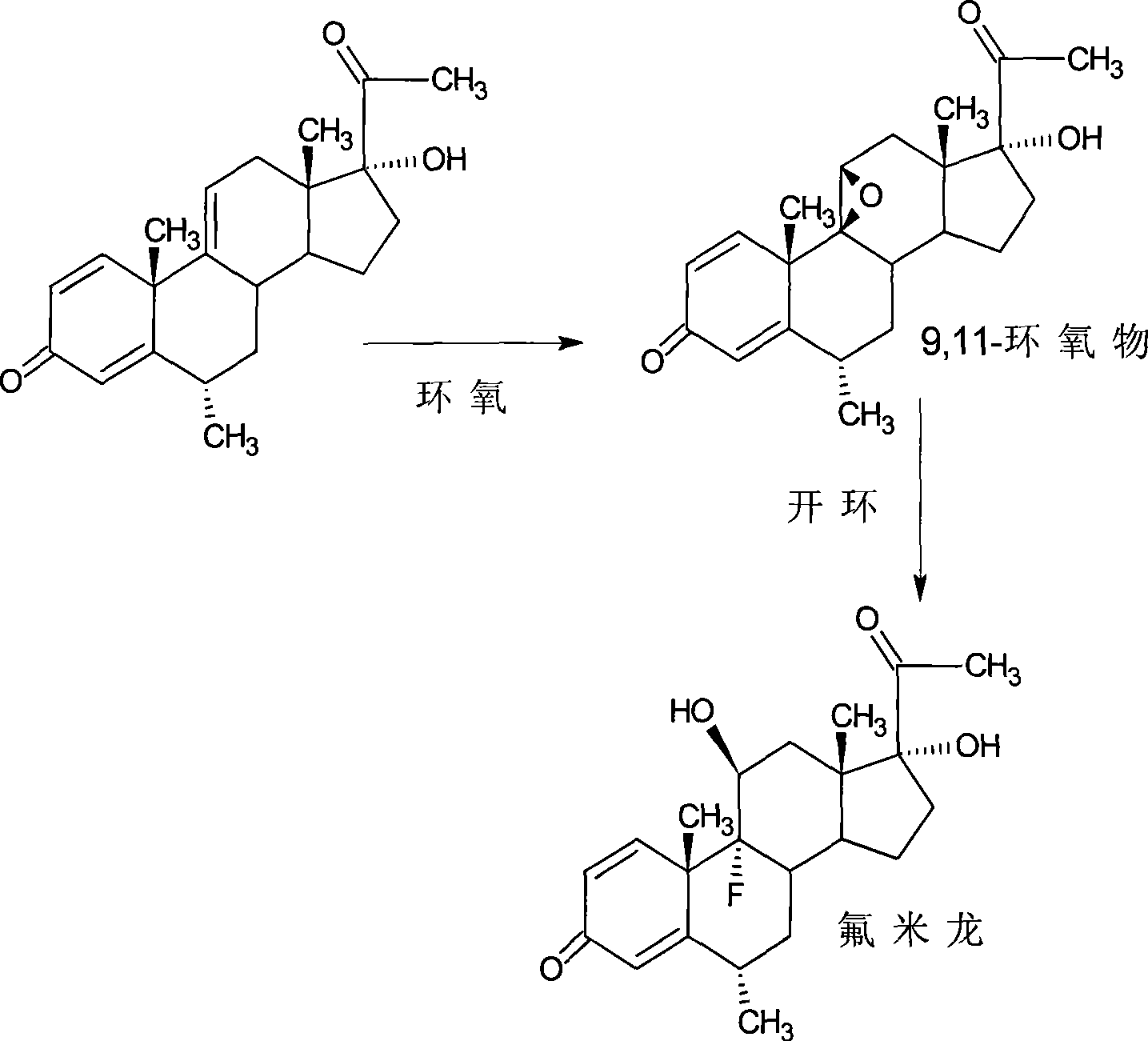
Preparation of fluorometholone and derivatives thereof
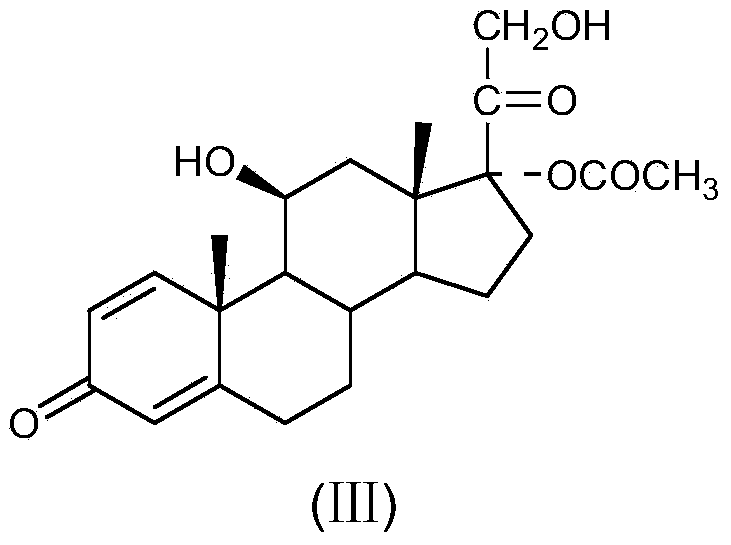
The invention relates to a preparation method of a steroid compound, in particular to the preparation of fluorometholone and the derivative thereof, which takes 6-methyl-17-hydroxyl-1, 4, 9-triene-pregna-3, 20-diketone as the initiator to design a brand new process line for synthesizing the fluorometholone and the derivative (III) thereof and further provides the application of a compound (I) in the preparation of the fluorometholone and the derivative (III) thereof. As the production process adopts the existing intermediate of the company as the initiator, the line is concise, the material is easy to obtain, expensive auxiliary materials are saved, and the yield and the cost are obviously superior to the historical synthetic method of the prednisolone and the derivative thereof; in addition, the adoption of the existing intermediate realizes the doubling production of the hexamethylprednisolone products and the fluorometholone products, thus greatly reducing the production cost and industrial conditions. Meanwhile, in the line for preparing a fluorometholone esterified ester, the disadvantages of easy esterification of 11th and too much side products are avoided. R1 is equal to the alkyl with less than 5 carbon atoms; R2 is equal to H, OH, the alkyl with less than 5 carbon atoms; R3 is equal to H, COR4, wherein, R4 is equal to the alkyl with less than 11 carbon atoms.
Owner:TIANJIN PHARMA GROUP CORP
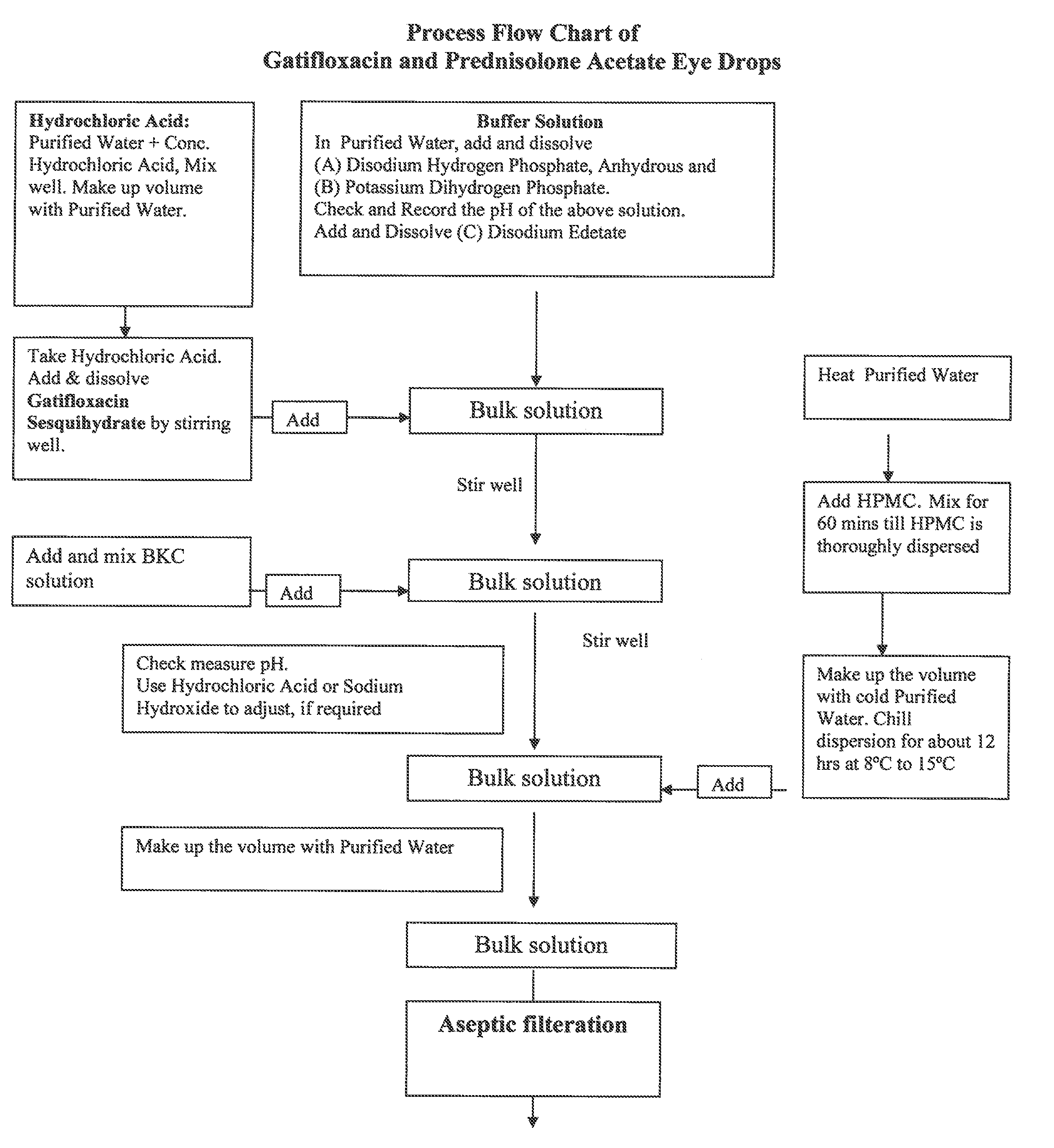
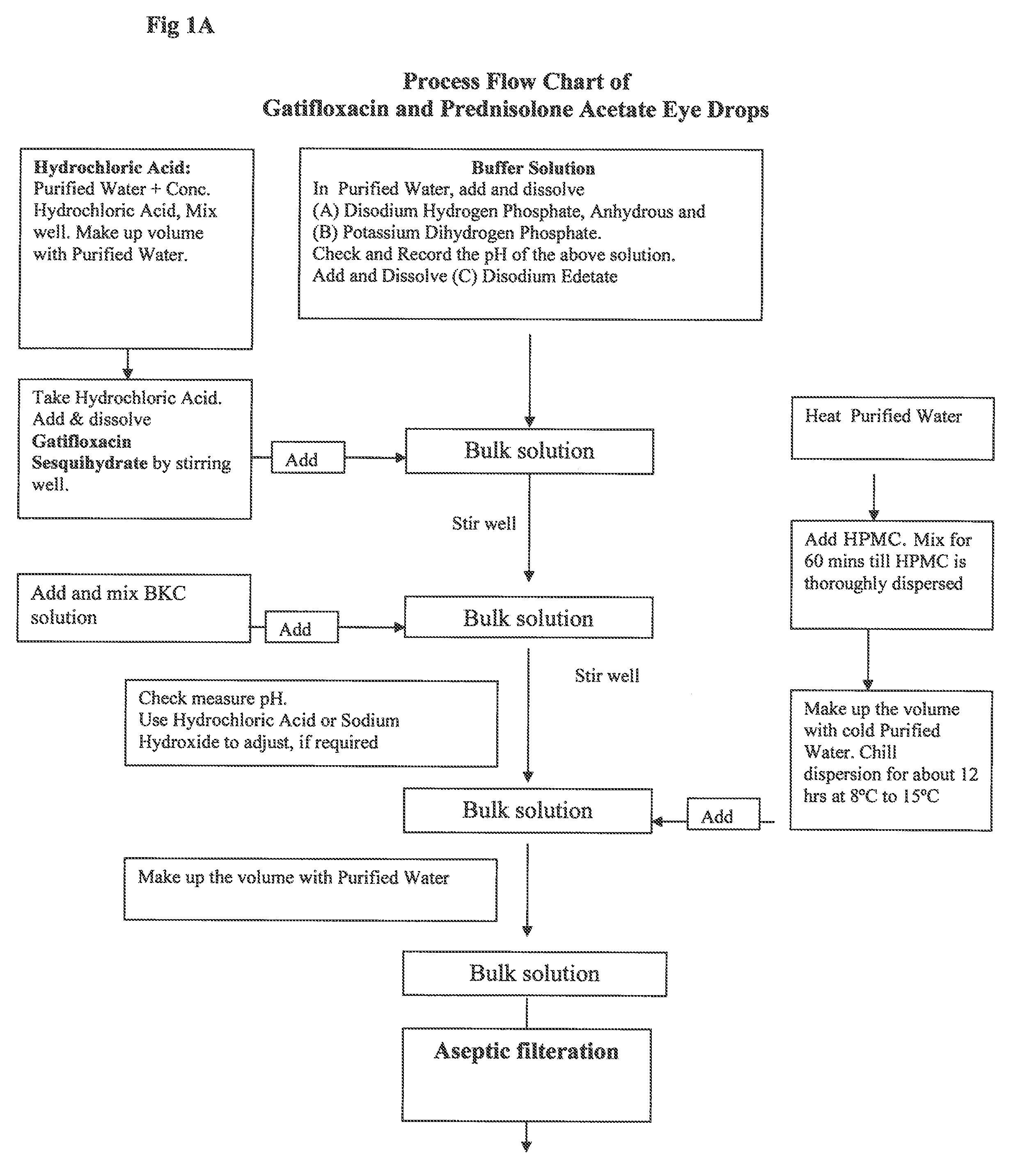
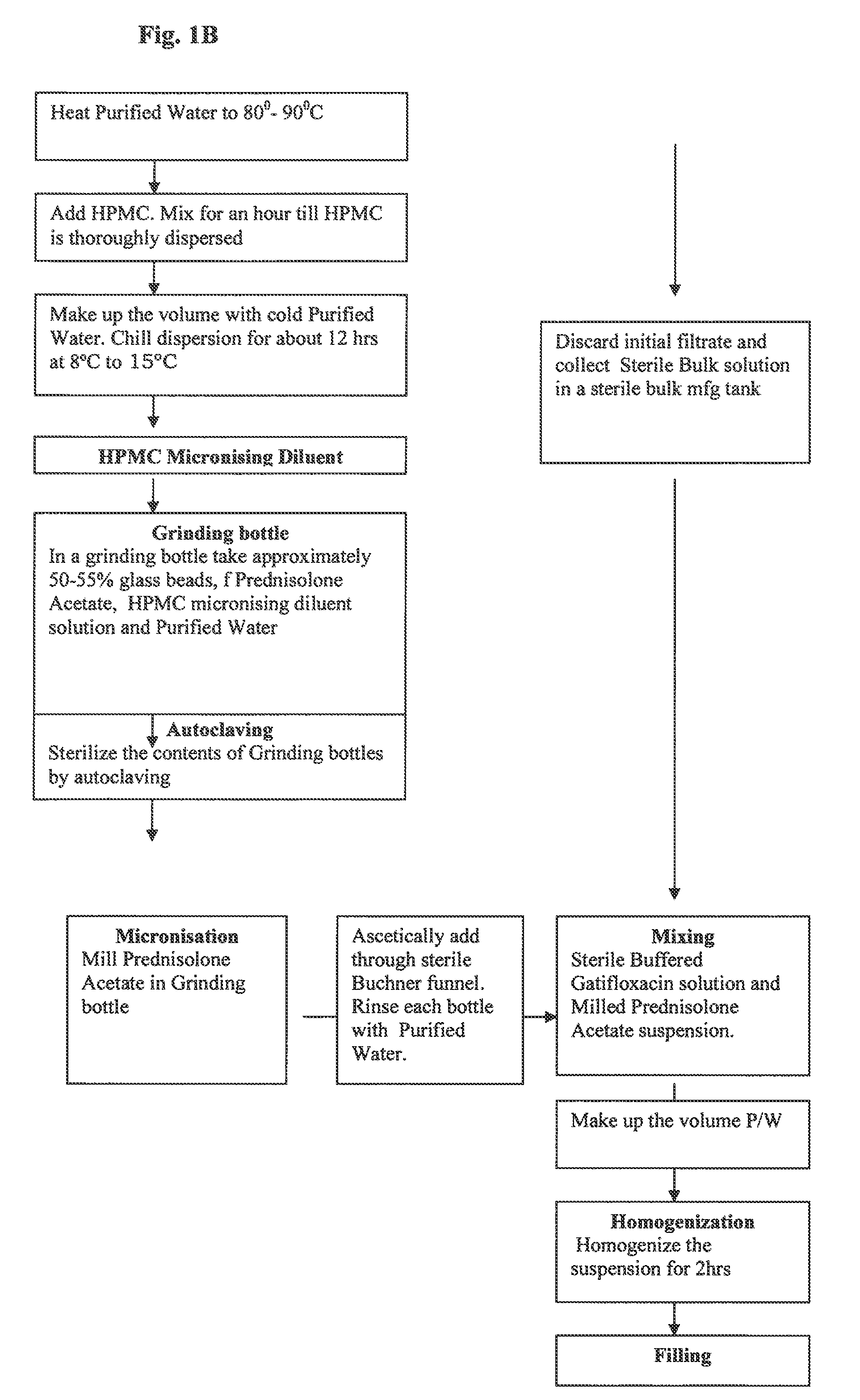
Ophthalmic Suspension for Ocular Use
InactiveUS20100063017A1More acceptabilityMore patient complianceAntibacterial agentsBiocidePrednisoloneGatifloxacin
A gatifloxacin and prednisolone topical ophthalmic pharmaceutical compositions for prevention and treatment of ophthalmic bacterial infections and inflammatory conditions associated with pre-surgical and / or post surgical ocular surgeries.
Owner:ALLERGAN INC
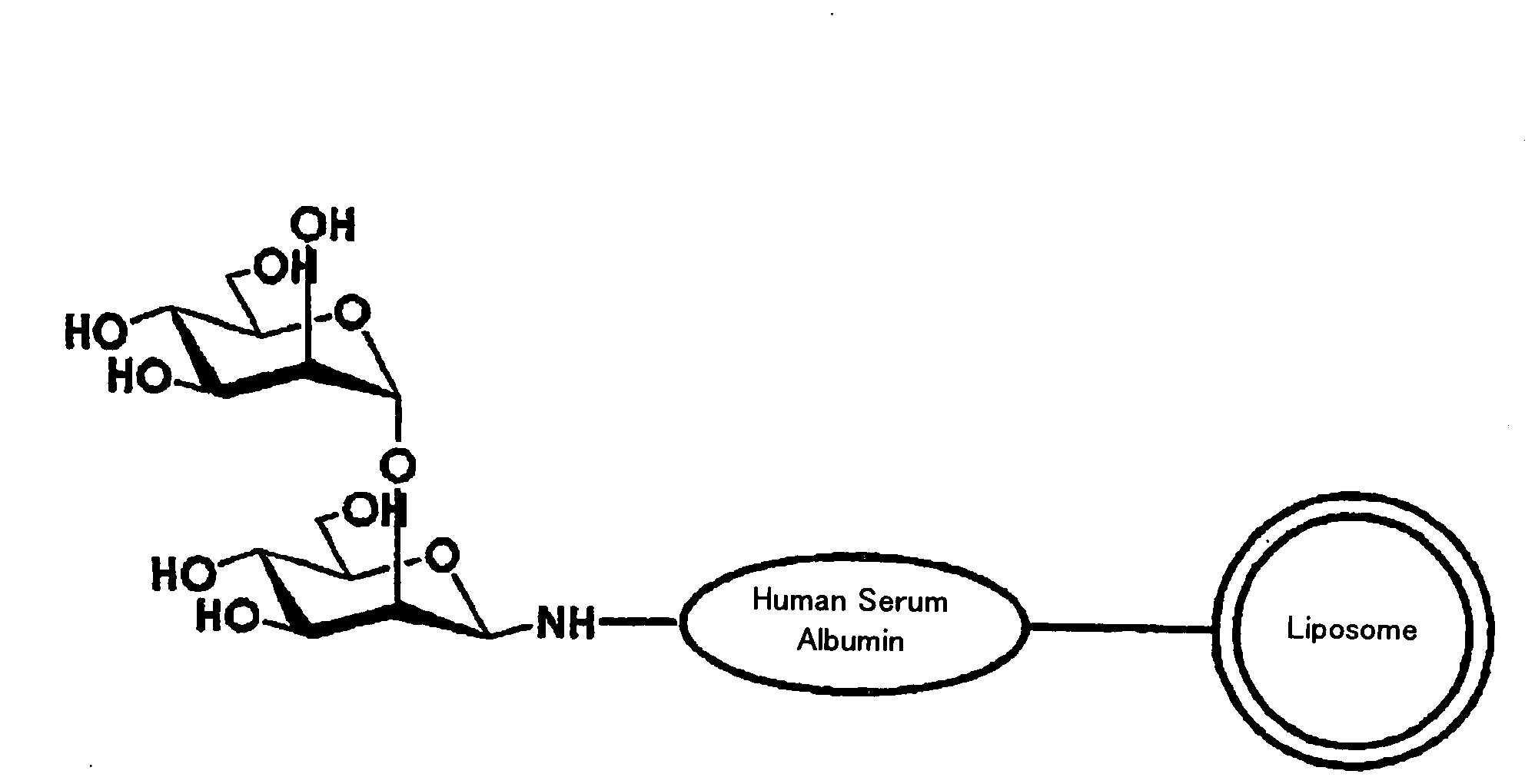
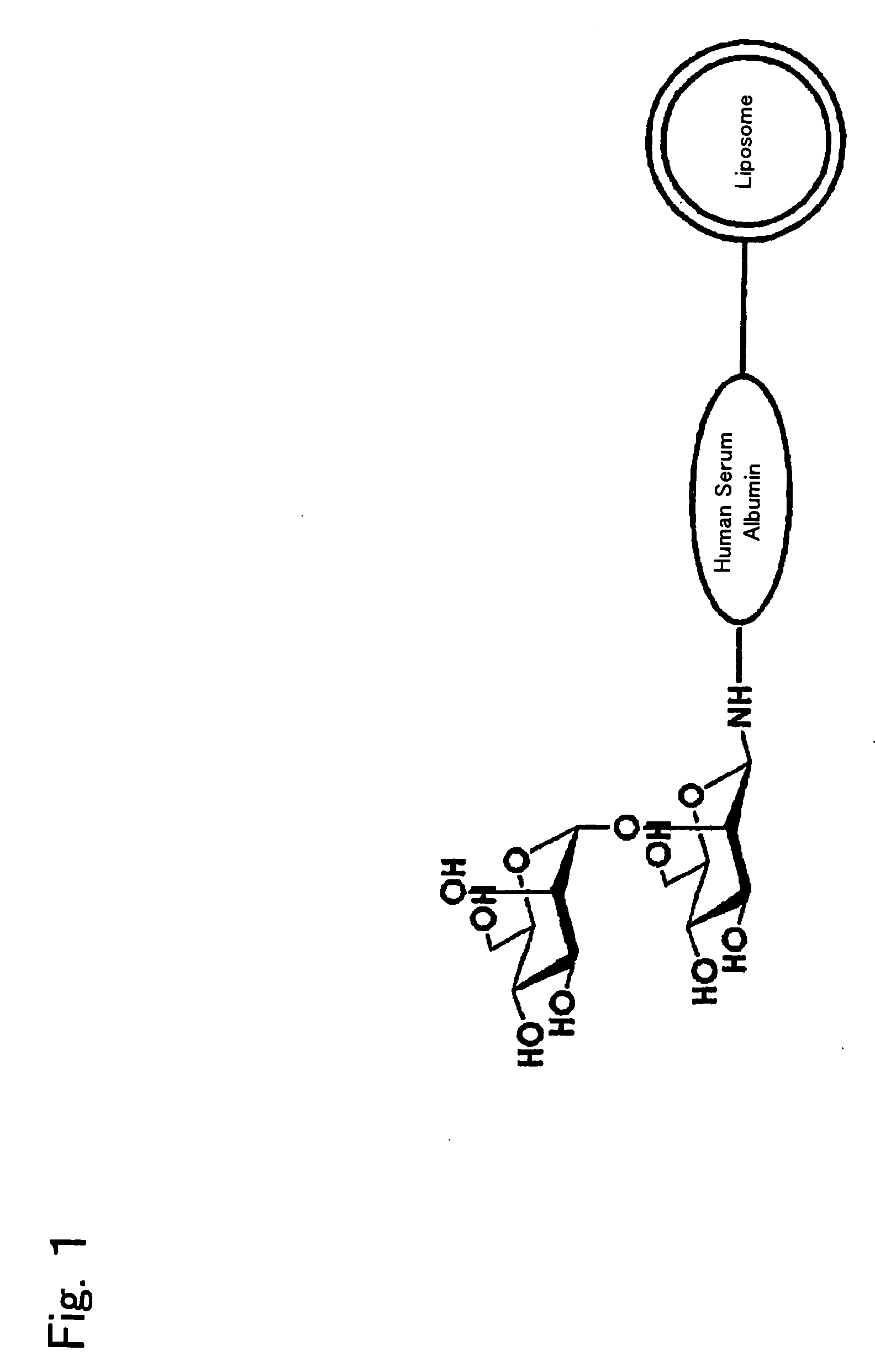
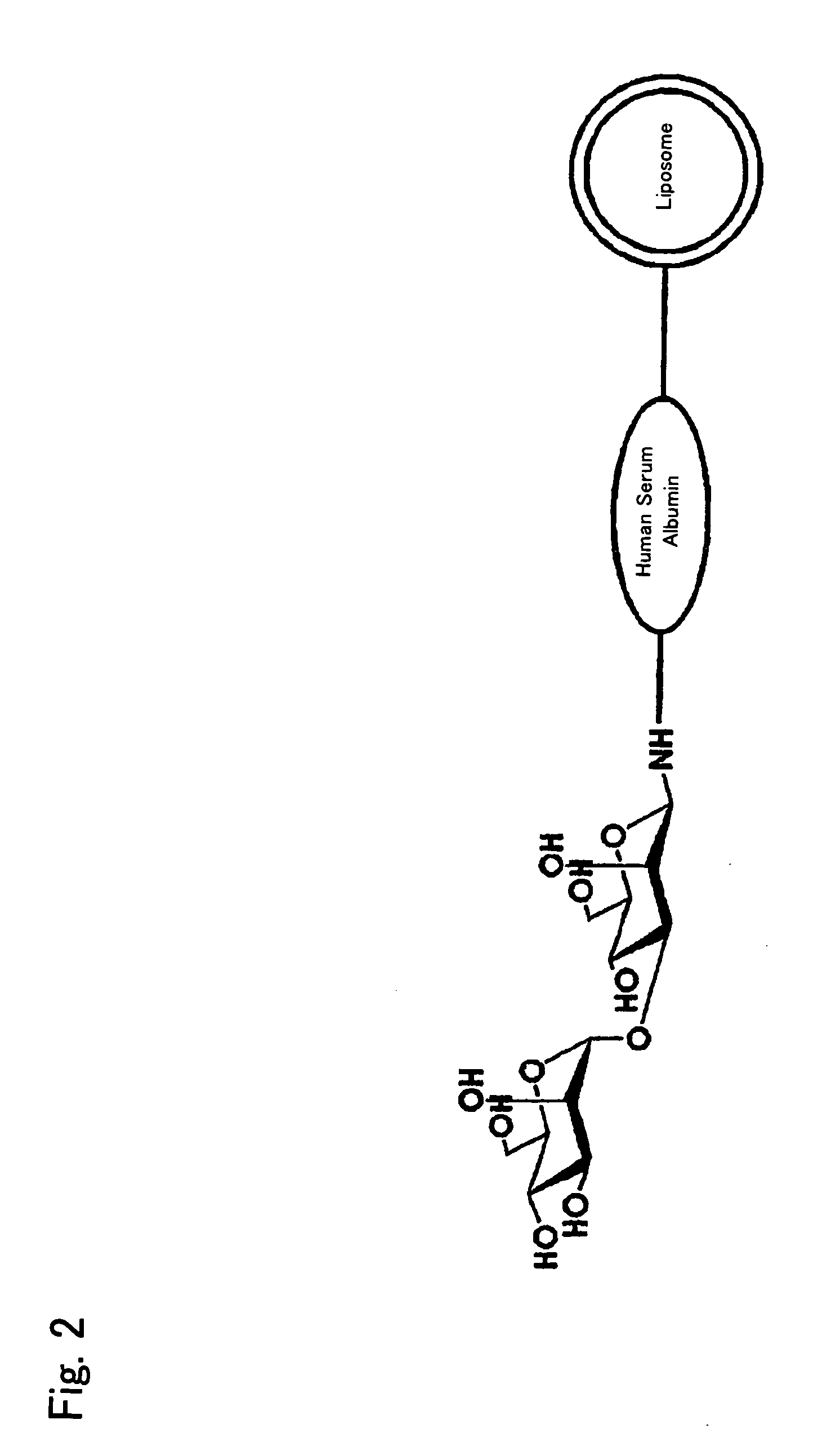
Liposome Preparation
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
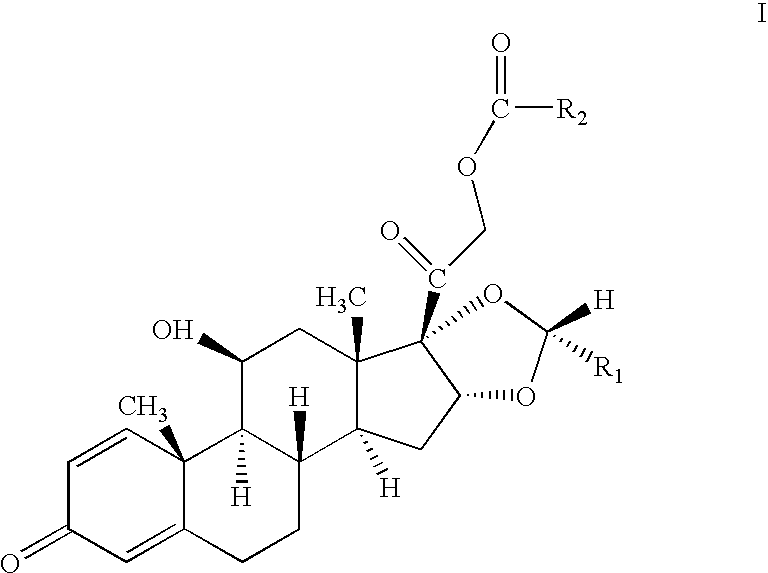
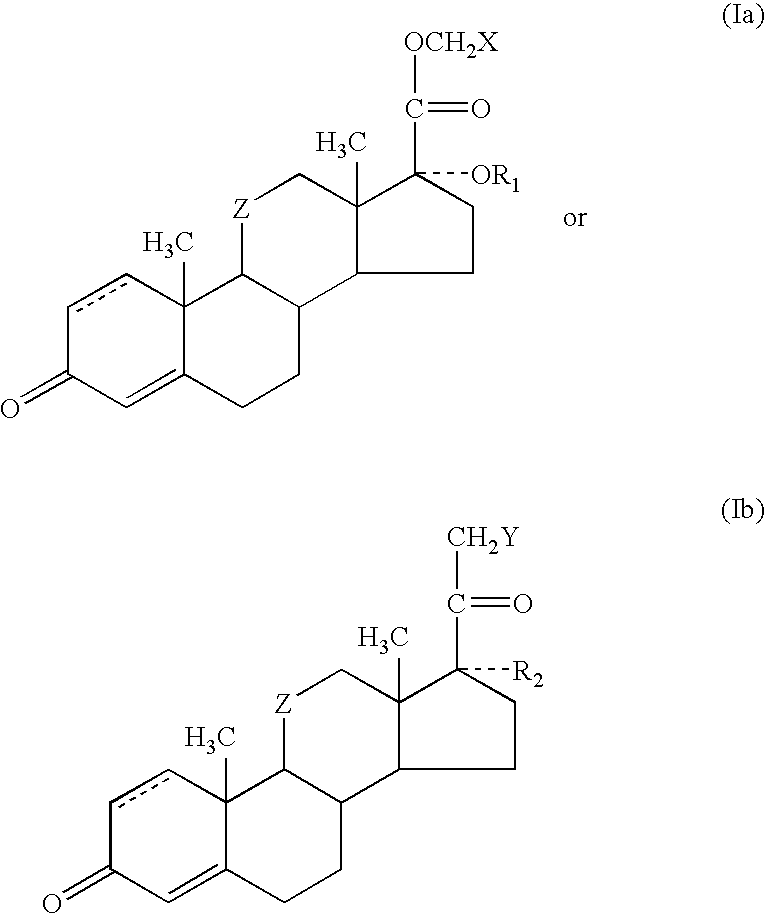
One-pot processes for preparing prednisolone derivatives
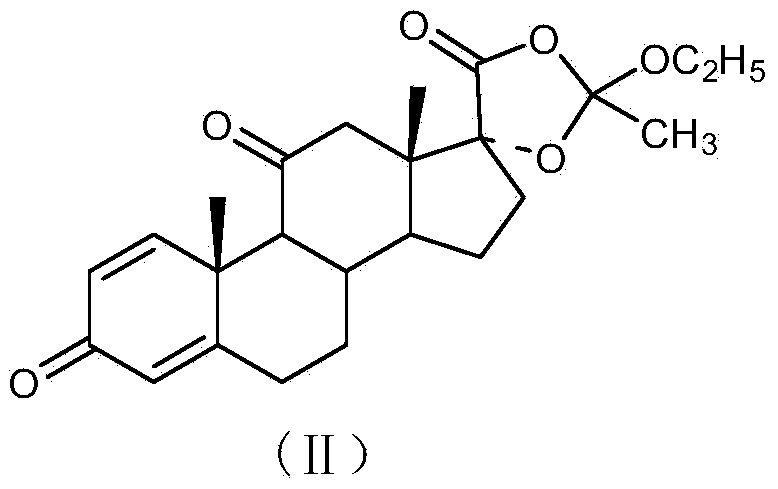
Disclosed is a one-pot process for the preparation of a prednisolone derivative of formula I, comprising reacting the compound of formula II with the compound of formula III and the compound of formula IV The process does not need to separate and purify the intermediate formed, and therefore the process reduces the reaction time and increases the yield of the product compared to the prior art.
Owner:ZHEJIANG XIANJU PHARMA
Preparation method of prednisolone
The invention relates to a preparation method of prednisolone. The preparation method comprises the steps of sequentially carrying out 3, 20-keto protective reaction, 11-keto reduction reaction, 21-hydroxyl esterification reaction, 3, 20-keto deprotection reaction and 21-acetic ester hydrolysis reaction by taking prednisone acetate as a raw material to obtain prednisolone. The invention provides a novel synthesis route sequentially comprising the steps of esterifying and deprotecting; nitrosification quenching reaction and resin hydrolysis reaction are omitted in the deprotection process; and ester hydrolysis reaction is finished under the protection of a mixed solvent and inert gases, so that the condition that byproducts are produced by hydrolysis reaction is avoided. The preparation method is novel in process route, simple and rapid in operation process, low in production cost and suitable for large-scale industrial production.
Owner:HUAZHONG PHARMA
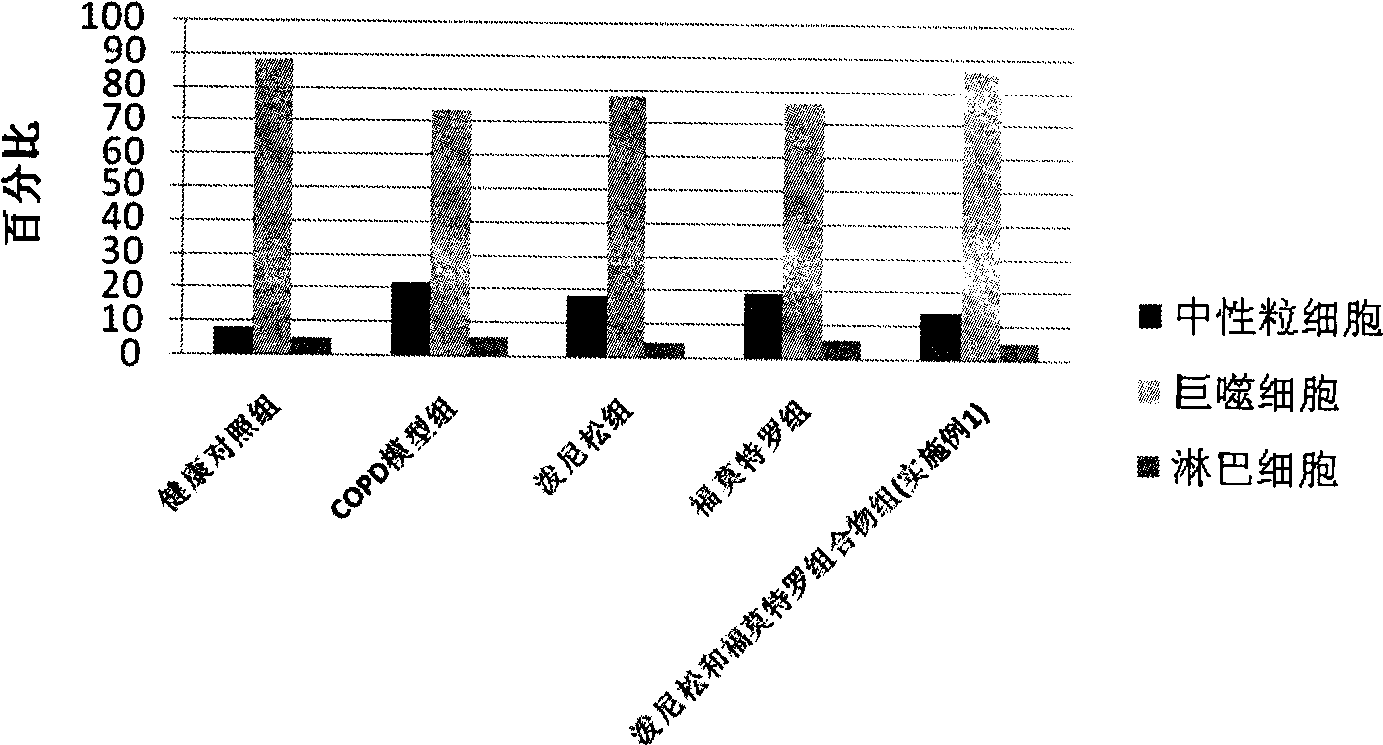
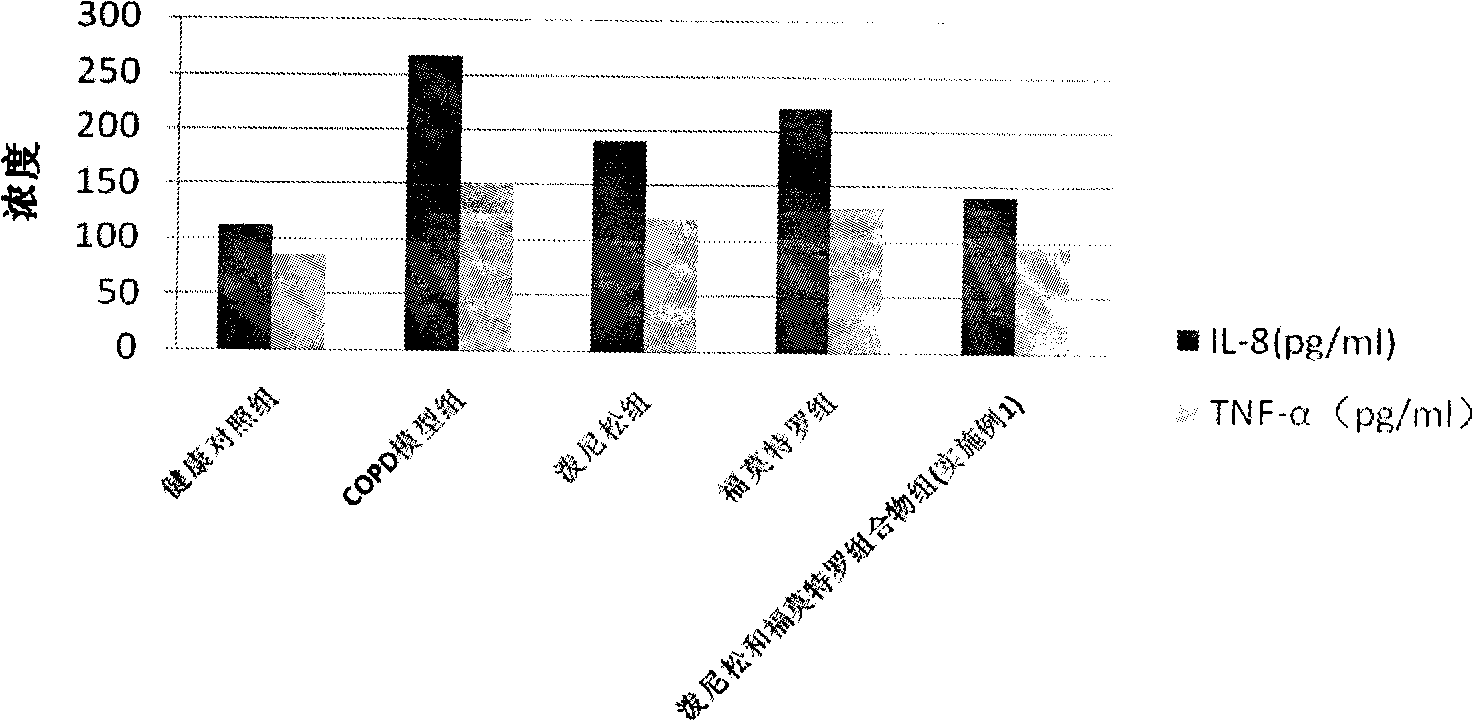
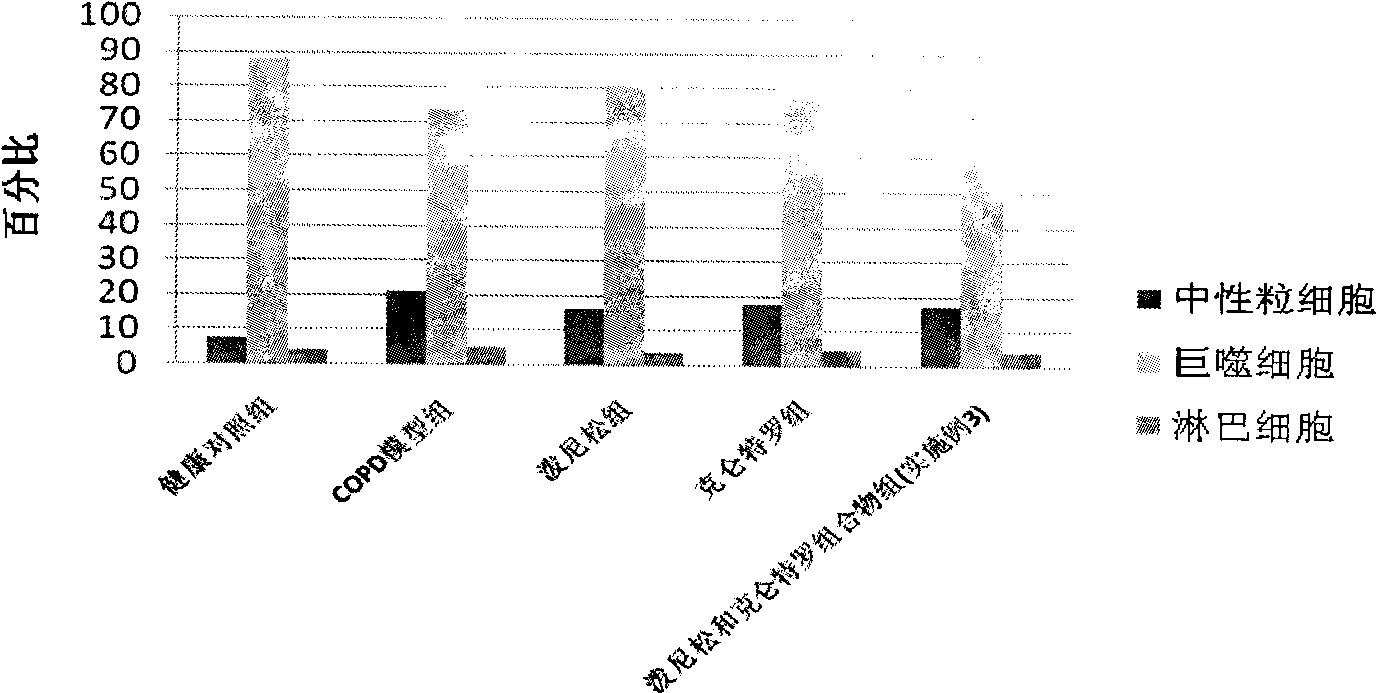
Pharmaceutical composition for the treatment of chronic obstructive pulmonary disease and bronchial asthma
InactiveCN101530618ALower doseReduce or avoid side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
The invention discloses a pharmaceutical composition for the treatment of chronic obstructive pulmonary disease (COPD) and bronchial asthma, which is composed of a glucocorticoid, a bronchodilator and a pharmaceutically acceptable auxiliary material or carrier; the composition is a preparation for oral use. The glucocorticoid in the inventive pharmaceutical composition is selected from prednisone, prednisolone, methylprednisolone, betamethasone, decamethasone or hydrocortisone; the bronchodilator is selected from formoterol, clenbuterol, procaterol or theophylline. The inventive composition has better therapeutic effect on the COPD and the bronchial asthma than independent administration of two ingredients, and has synergistic effect. The composition has easily-available raw materials, inexpensive price and increased medicine taking compliance as well as plays a significant role in preventing and treating the COPD and the bronchial asthma of patients in vast rural areas and patients in low-income class of the city in China.
Owner:莫始平
Glucocorticosteroid and chemotherapy medicament carried by anticancer sustained-release agent
InactiveCN101502484AInhibition formationImprove permeabilitySolution deliveryPharmaceutical non-active ingredientsGlucocorticoidPolyethylene glycol
The invention provides an anti-cancer sustained-release agent co-carrying glucocorticoid and chemotherapeutic drugs and belongs to sustained-release injections. The anti-cancer sustained-release agent comprises sustained-release microspheres and a solvent, wherein, the sustained-release microspheres comprise anti-cancer active components and sustained-release auxiliary materials; and the solvent is a particular solvent containing a suspending agent. The glucocorticoid is selected from prednisolone, methylprednisolone, dexamethasone, betemethasone, triamcinolone acetonide or triamcinolone acetonide; the chemotherapeutic drugs are selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogues and the like; the sustained-release auxiliary materials are biocompatible high-polymers, such as polylactic acid and the copolymers thereof, polyethylene glycol, carboxyl-terminated polylactic acid copolymers, polyfatty acid dimer-sebacic acid copolymers, poly(erucic acid dimer-sebacic acid), poly(fumaric-co-sebacic acid), polifeprosan and the like; and the suspending agent with the viscosity being 100cp to 3,000cp (at the temperature of 20 to 30 DEG C) is selected from sodium carboxymethyl cellulose and the like. The anti-cancer active components and the sustained-release microspheres can further be prepared into sustained-release implants which can effectively inhibit the growth of tumors, alleviate edema and improve the curative effects of radiotherapy and chemotherapy by intra-tumor or peri-tumor injection or placement.
Owner:SHANDONG LANJIN PHARMA
Elemental nanoparticles of substantially water insoluble materials
InactiveUS20050129777A1Improve efficacyMinimize multiple dosingPowder deliveryOrganic active ingredientsWater insolubleProgesterones
This invention relates to a novel process of manufacture of nanoparticles of substantially water insoluble materials from emulsions. The emulsions have the ability to form a single liquid phase upon dilution of the external phase, instantly producing dispersible solid nanoparticles. The formed nanoparticles have average diameter of about 10 to 200 nm and are suitable for drug delivery and targeting of water insoluble therapeutic or diagnostic agents. Examples of such agents are methotrexate, progesterone, testosterone, prednisolone, and ibuprofen. Such agents can be used in a wide range of therapeutic and diagnostic treatments including treatment for cancer, hormonal therapy, and pain management.
Owner:HASSAN EMADELDIN M
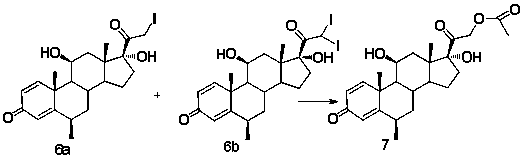
Method for preparing prednisolone
The invention discloses a method for preparing prednisolone. The method comprises the steps as follows: a dihydroxyprogesterone dehydrogenated substance is adopted as a raw material, an alpha hydroxyl in the 11th position is firstly transformed into beta hydroxyl, then reactions including iodination, replacement and the like are performed, and prednisolone and a derivative of prednisolone are obtained; the equation is shown in the specification. According to the method, the dihydroxyprogesterone dehydrogenated substance is adopted as the raw material for the first time for preparing prednisolone and the derivative of prednisolone, the total yield is 80% or higher, the production cost is reduced, and the method is applicable to large-scale industrial production.
Owner:ZHEJIANG XIANJU PHARMA
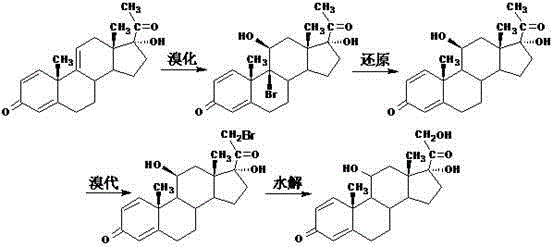
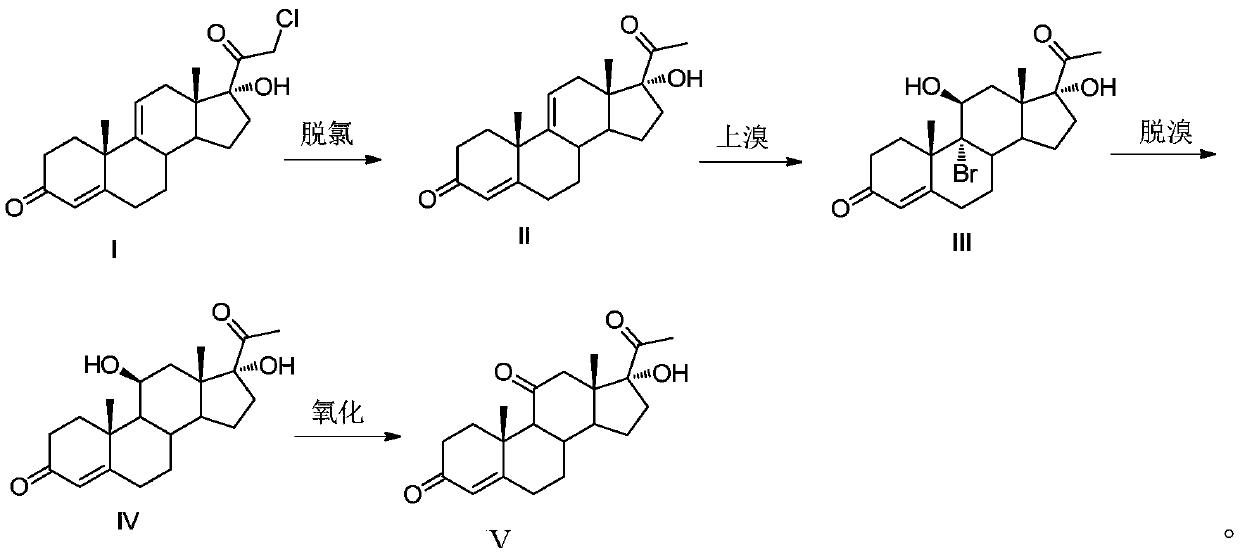
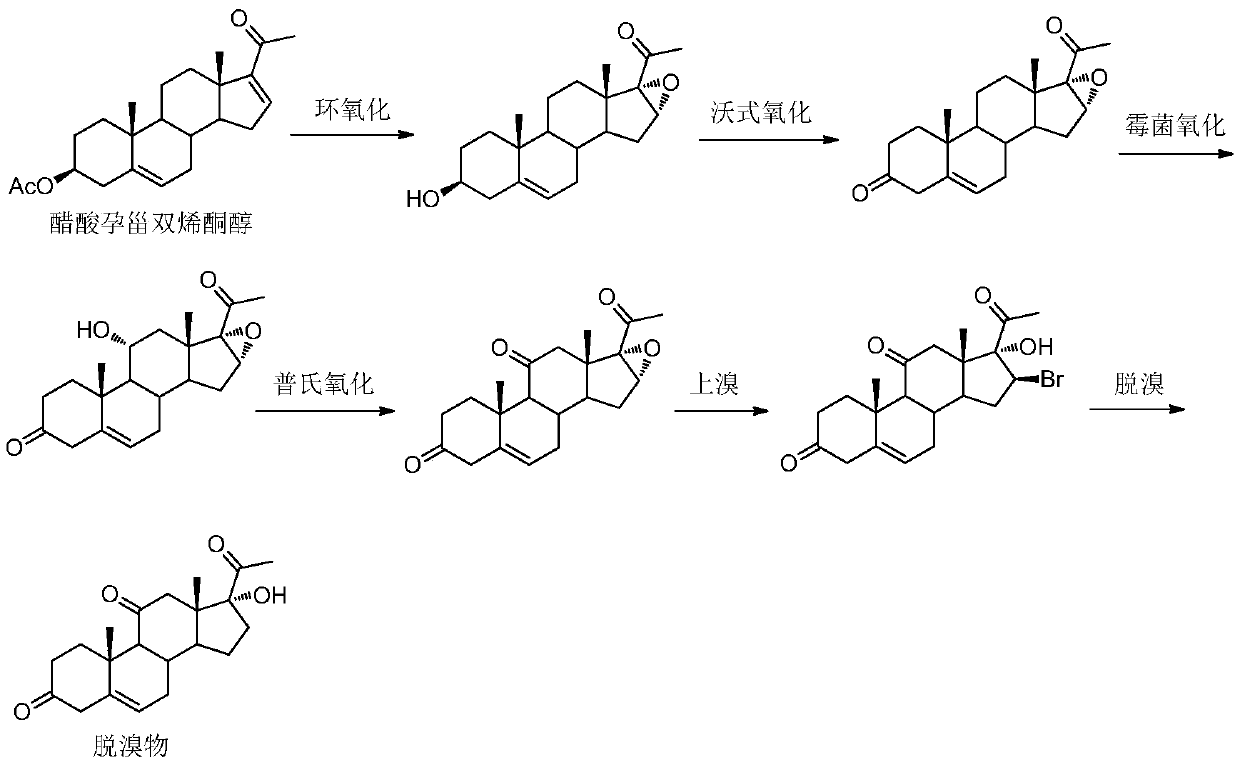
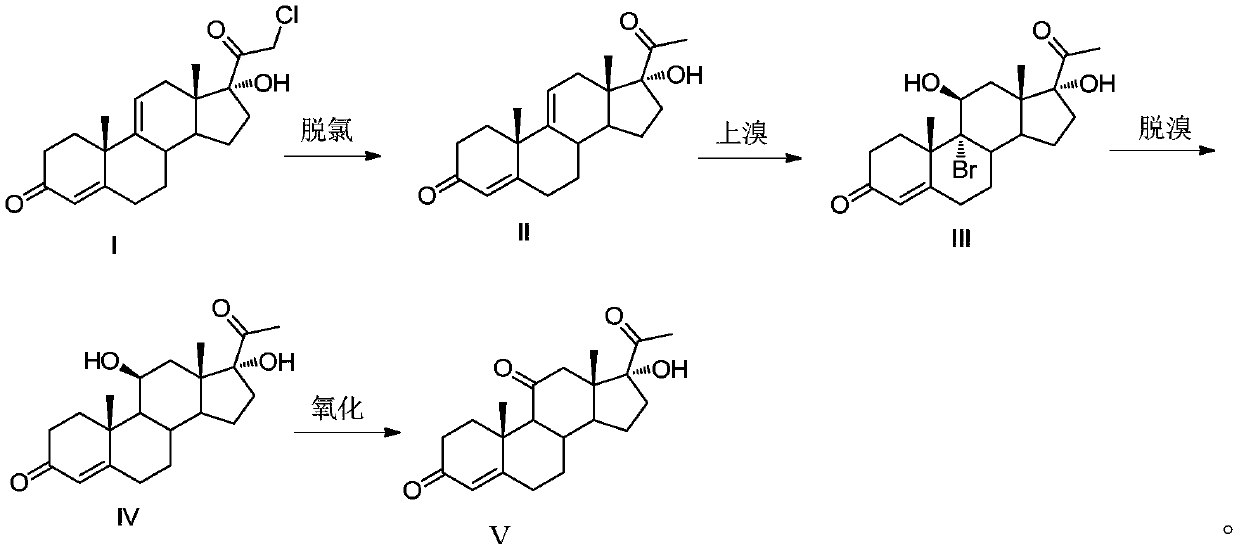
Methylprednisolone intermediate debrominated product and preparation method thereof
ActiveCN110698528AShort synthetic routeHigh yieldSteroidsBulk chemical productionPrednisoloneBiochemical engineering
The invention discloses a methylprednisolone intermediate debrominated product and a preparation method thereof. According to the preparation method, a compound shown as a formula I is used as a raw material, and a dechlorination reaction, a bromination reaction, a debromination reaction and an oxidation reaction are sequentially performed to prepare a compound (debrominated substance) shown as aformula V. The preparation method has the advantages of short synthetic route, high yield, low cost and easily available raw materials, is suitable for industrial production, and has very high industrial value.
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
Enhancement of activity and/or duration of action of selected anti-inflammatory steroids for topical or other local application
ActiveUS7419971B2Enhance topical and other local activity and duration of actionHigh activityOrganic active ingredientsPowder deliveryPrednisoloneAlkoxy group
Methods and compositions for enhancing the activity and / or duration of action of soft anti-inflammatory steroids of the haloalkyl 17α-alkoxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylate type and the corresponding Δ1,4 compounds and of anti-inflammatory steroids of the hydrocortisone and prednisolone type are described. The enhancing agents have the formula:wherein R is H or C1-C4 alkyl; Z1 is carbonyl or β-hydroxymethylene; X1 is —O— or —S—; R5 is —OH, —OR6, —OCOOR6 or —OCOR7 wherein R6 is C1-C4 alkyl and R7 is C1-C4 alkyl, fluoromethyl or chloromethyl; and the dotted line in ring A indicates that the 1,2-linkage is saturated or unsaturated; with the proviso that when R is C1-C4 alkyl, then R5 is —OH.
Owner:BODOR LAB
Treatment of diffuse large-cell lymphoma with Anti-cd20 antibody
InactiveUS20140030263A1Effective treatmentOrganic active ingredientsIn-vivo radioactive preparationsRegimenPrednisone treatment
The present invention concerns methods for the treatment of diffuse large cell lymphoma by administration of an anti-CD20 antibody and chemotherapy. Particular embodiments include the administration of anti-CD20 antibody in combination with chemotherapy comprising CHOP (cyclophosphamide, hydroxydaunorubicin / doxorubicin, vincristine, and prednisone / prednisolone) and / or in combination with a transplantation regimen.
Owner:BIOGEN INC
Ganoderma lucidum spores for treatment of autoimmune diseases
InactiveUS6893641B2Therapeutical activityRelieving/reducing symptomBiocideNervous disorderImmunologic disordersDisease
The present invention provides a method for treating a mammal with immunological disorders, particularly autoimmune disease, and most preferably systemic lupus erythematosus (SLE). The method includes oral administration of germination activated Ganoderma lucidum spores (“GLSs”) to the mammal. Additionally, a corticosteroid, such as prednisolone, can be co-administered with the GLSs to the mammal to achieve synergistic effect of treatment.
Owner:CHUNG CHEE KEUNG +1
Preparation method of R budesonide
The invention relates to the field of medicinal chemistry, in particular to a preparation method of new R budesonide, which is characterized in that a budesonide product is synthesized by carrying out microwave catalytic reaction on the 16-alpha hydroxyl prednisolone and n-butanal in the presence of a mixed catalyst, wherein the content of the R budesonide is more than 95 percent.
Owner:LUNAN BETTER PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com