Patents
Literature
115 results about "Prednisone treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
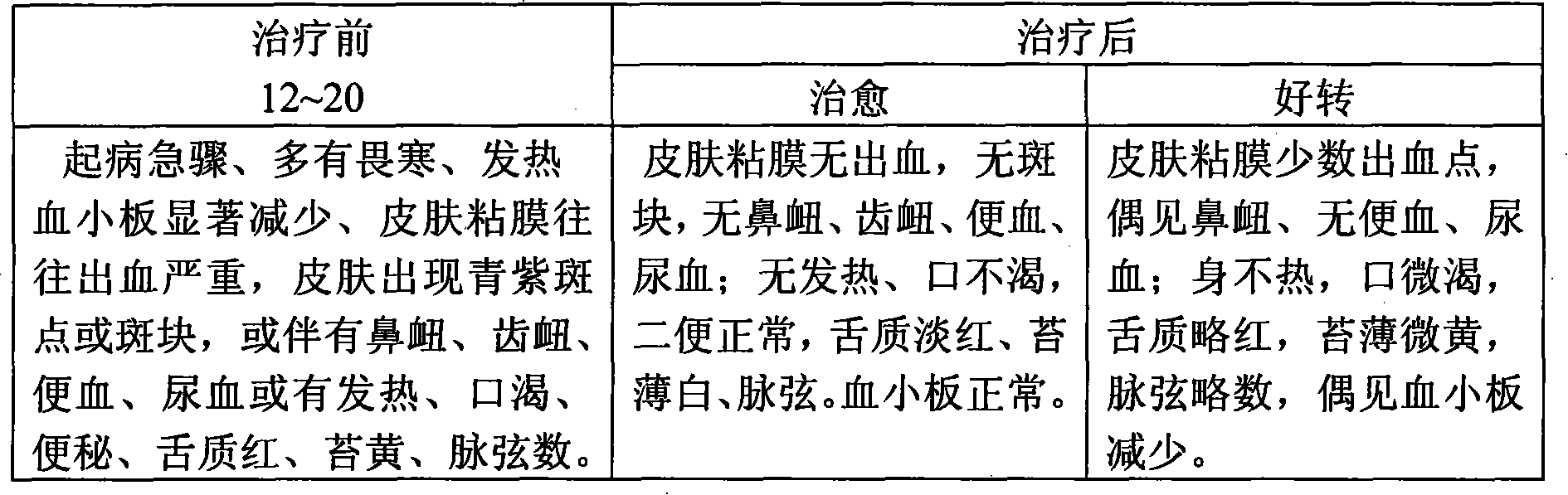
Prednisone can be used in the treatment of decompensated heart failure to increase renal responsiveness to diuretics, especially in heart failure patients with refractory diuretic resistance with large dose of loop diuretics.
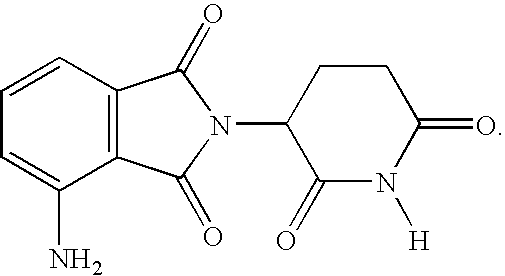
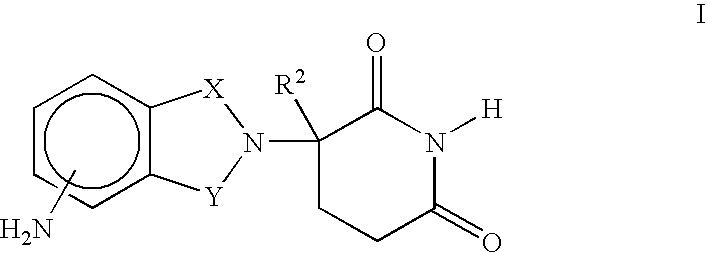
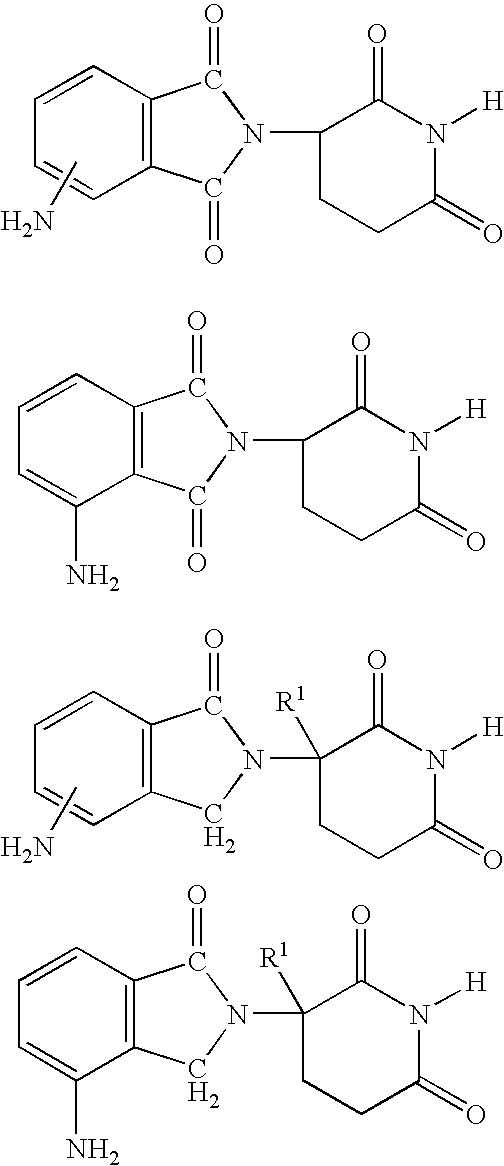
Methods for the treatment and management of myeloproliferative diseases using 4-(AMINO)-2-(2,6-dioxo(3-piperidyl)-isoindoline-1,3-dione in combination with other therapies
InactiveUS20090088410A1Reduce adverse effectsImprove toleranceBiocideAnimal repellantsJAK1 InhibitorMedicine
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of 4-(amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione, or a pharmaceutically acceptable salt, solvate or stereoisomer thereof, in combination with a second active agent. Particular second active agents are is prednisone, JAK1 inhibitor, JAK2 inhibitor, FLT3 inhibitor, BCL2 inhibitor, and HDAC inhibitor.
Owner:CELGENE CORP
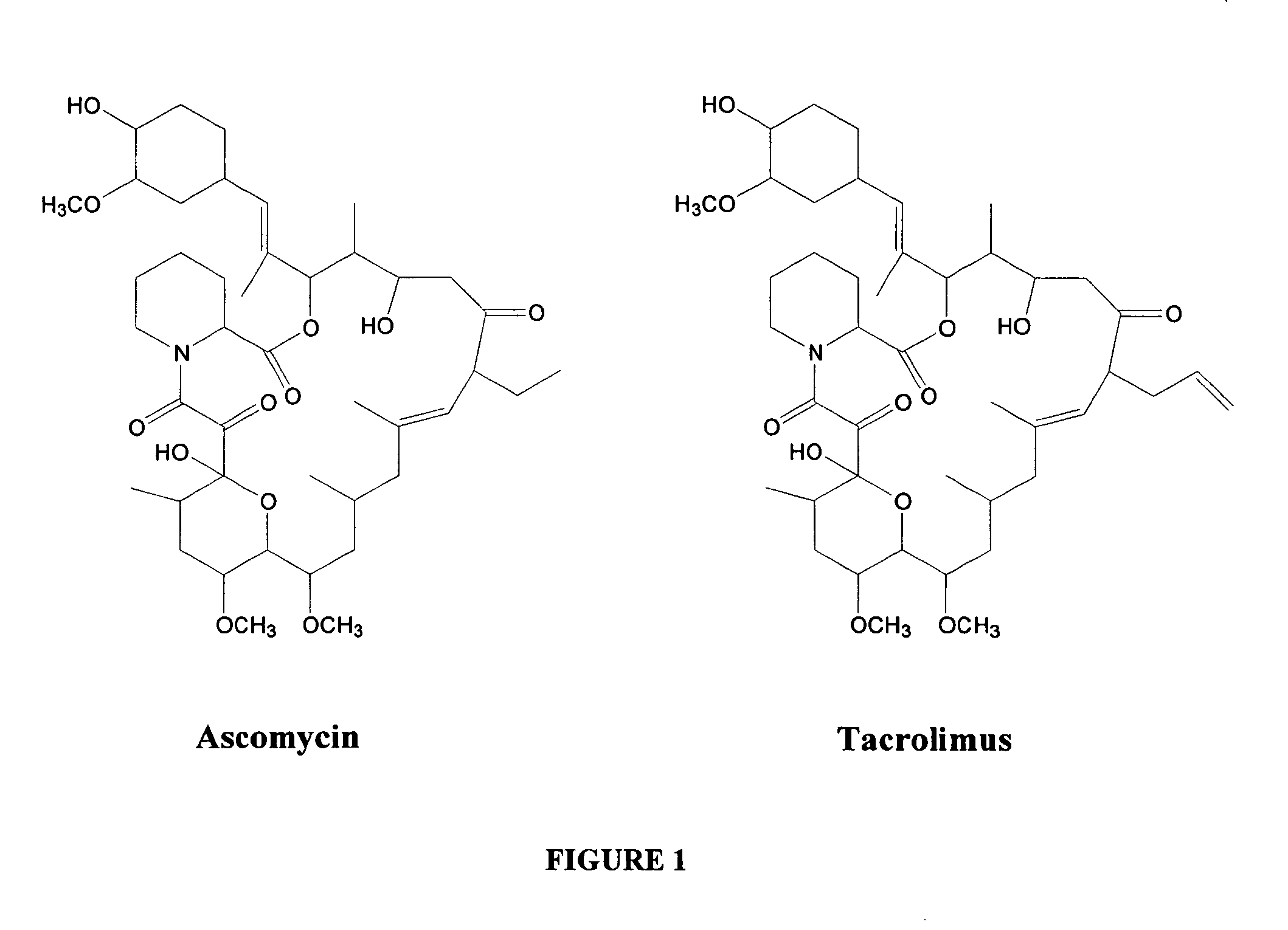
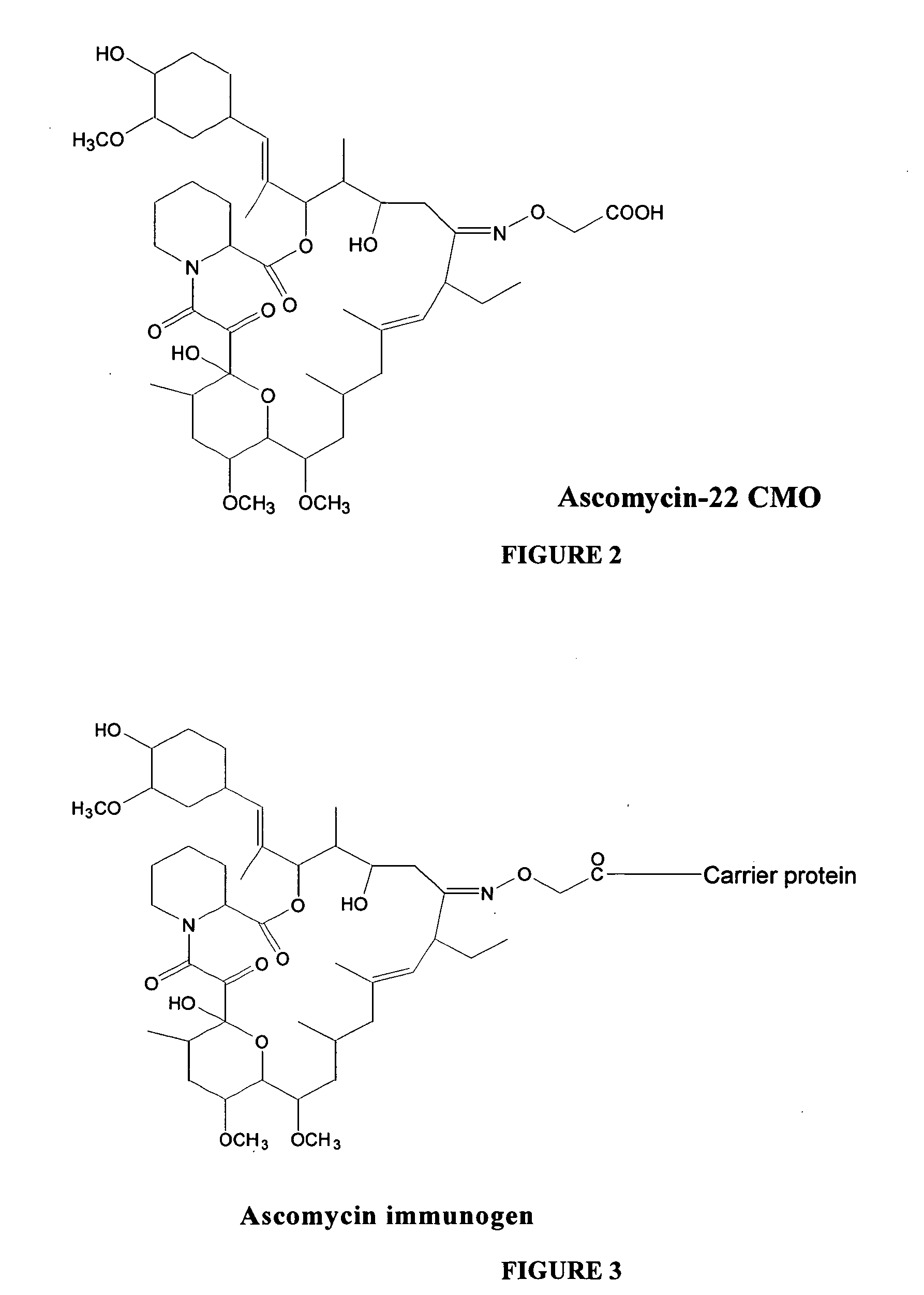
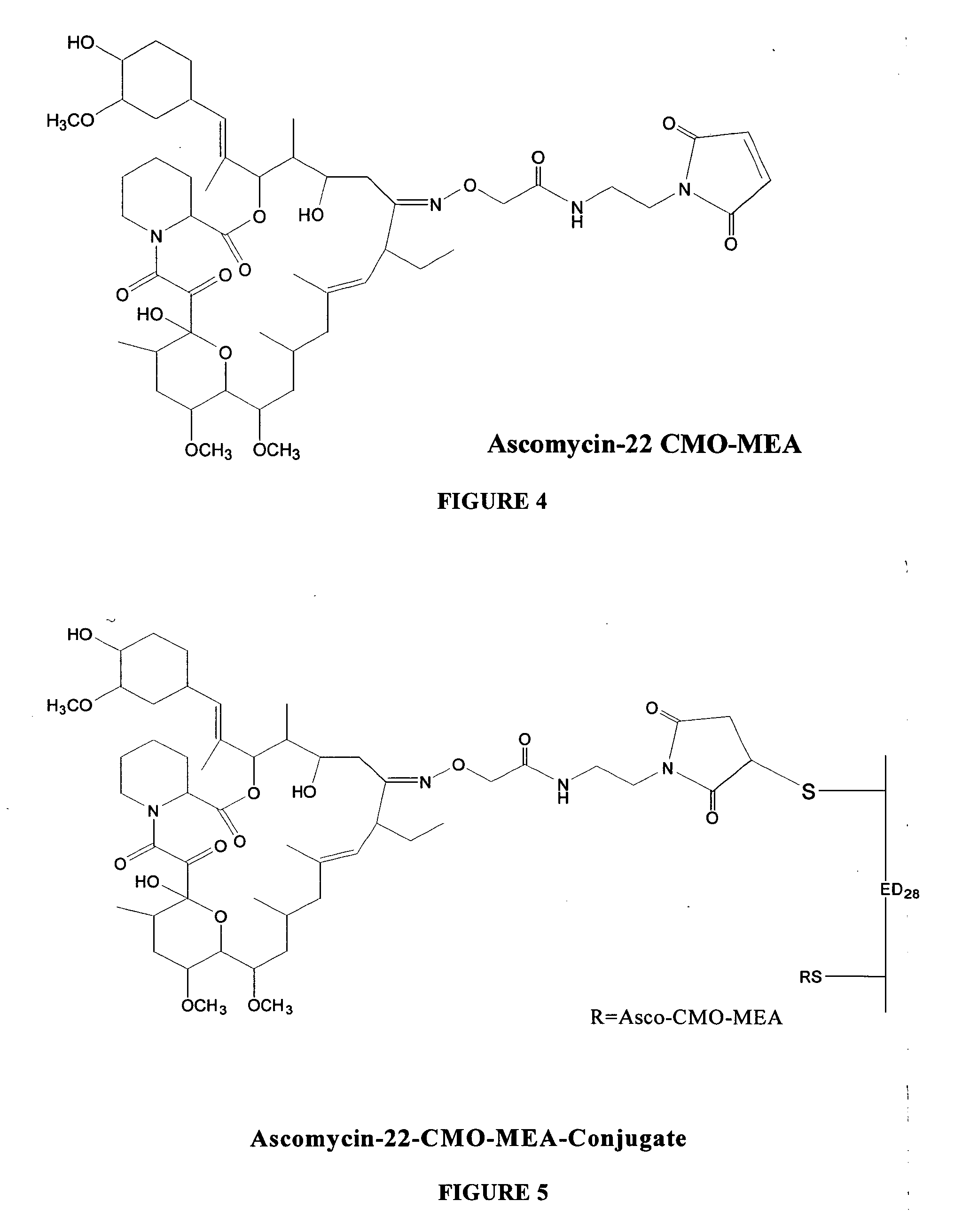
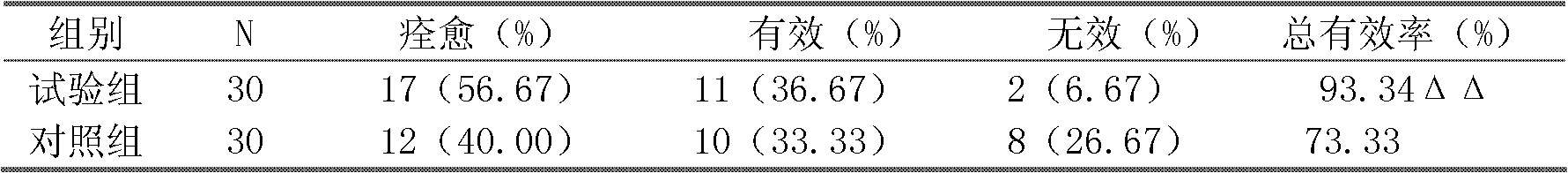
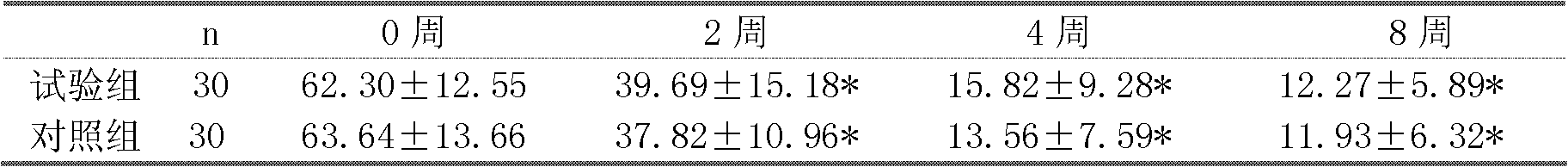
Hapten, immunogens and derivatives of ascomycin useful for preparation of antibodies and immunoassays
The invention teaches derivatives of ascomycin and methods of preparing immunogens and other conjugates useful in immunoassays for quantitatively measuring concentrations of tacrolimus in patient specimens. Antibodies produced from the disclosed immunogens capable of binding to tacrolimus with cross-reactivity of no more than 5% with each of 15-O-demethyl tacrolimus, 31-O-demethyl tacrolimus, and 13,31-O-didemethyl tacrolimus, less than 40% with 13-O-demethyl tacrolimus, and less than 1% with cyclosporin, rapamycin, mycophenolic acid, prednisone, hydrocortisol, and prednisolone are described. Further, immunoassays for measuring the concentration of tacrolimus using such antibodies are taught.
Owner:MICROGENICS CORP
Traditional Chinese medicine composition for treating subacute thyroiditis and preparation method thereof
ActiveCN103099902AImprove clinical symptoms and signsLow recurrence rateAntiviralsUnknown materialsDrug withdrawalClinical study
The invention discloses a traditional Chinese medicine composition for treating subacute thyroiditis. The composition is a preparation prepared from the following raw materials by weight: 15-30 parts of hedyotis diffusa, 9-15 parts of honeysuckle, 15-30 parts of dandelion, 15-30 parts of Chinese violet, 9-15 parts of root of common peony, 9-15 parts of radix scrophulariae, 9-15 parts of peach kernel, 9-15 parts of baked turtle shell and 15-30 parts of sweet wormwood. According to clinical study on the treatment of subacute thyroiditis, the traditional Chinese medicine composition provided by the invention has total effective rate of 93.34%, which is significantly better than 73.33% of a prednisone group, and can effectively reduce erythrocyte sedimentation rate and CRP of subacute thyroiditis patients, and effectively improve clinical symptoms of the patients; although the cooling time, improvement time of neck pain and regression time of neck tenderness are longer than those of the prednisone group, the goiter or nodule disappearance time shows no obvious difference from those of the prednisone group; relapse rate after drug withdrawal is 3.33%, which is significantly lower than 20% of the prednisone group; and the composition has little adverse reaction, which is significantly lower than that of the prednisone group. The invention also discloses a preparation method of the traditional Chinese medicine composition granules.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Preparation method of prednisone
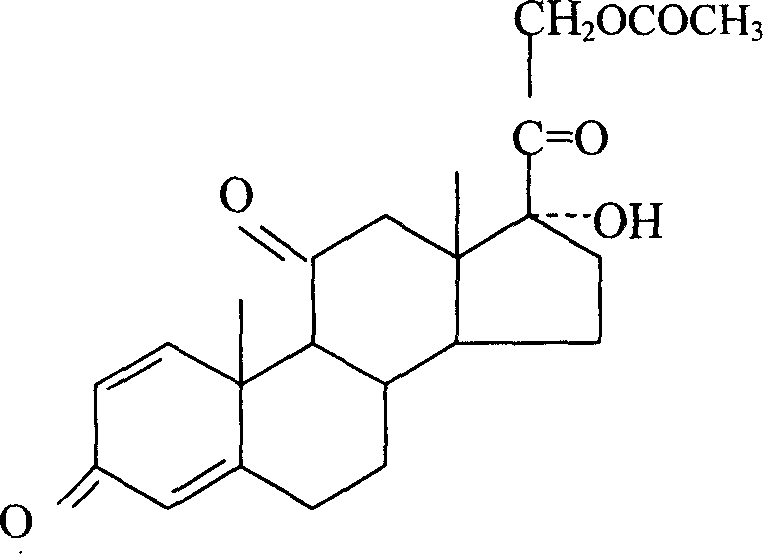
InactiveCN102617686AReduce recycling costsNo pollution in the processSteroidsPrednisone treatmentPollution
The invention discloses a preparation method of prednisone. The method comprises the following steps of: (a) preparing prednisone by taking a mold oxide (I) as a starting raw material, and undergoing a Platts oxidation reaction on the mold oxide to obtain a Platts substance (II); (b) undergoing a bromine addition reaction on the Platts substance (II) to obtain a bromine-added substance (III); (c) undergoing a bromine removing reaction on the bromine-added substance (III) to obtain a bromine-removed substance (IV); (d) undergoing a 21-bit iodine addition reaction on the bromine-removed substance (IV) to obtain prednisone (IV); and (k) undergoing a 21-bit replacement reaction on the substance (XI) obtained in the step (d) to obtain a replacement substance (IV). The preparation method has the advantages that: 1, after chromium waste water is treated, chromium can be used repeatedly, and the recovering cost of acetic acid can be reduced simultaneously; 2, totally-enclosed treatment is performed, waste water is drained after being concentrated, and all chromium is recovered, so that chromium pollution is avoided basically, and the concentration of acetic acid in water is reduced greatly simultaneously; and 3, a method for treating chromium waste water is simple, chromium is qualified, and adverse effects on a reaction are avoided.
Owner:浙江凯迪药业有限公司
Method for preparing sterides compound 17-alpha ester
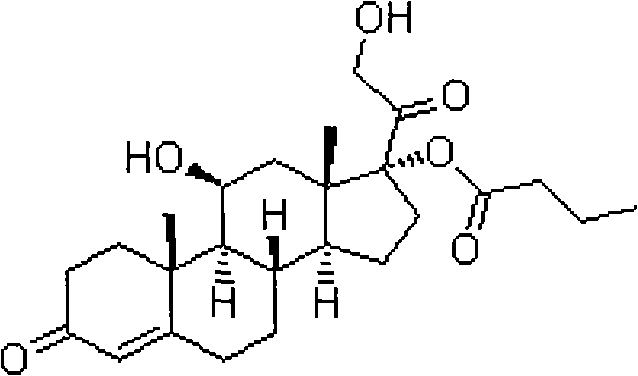
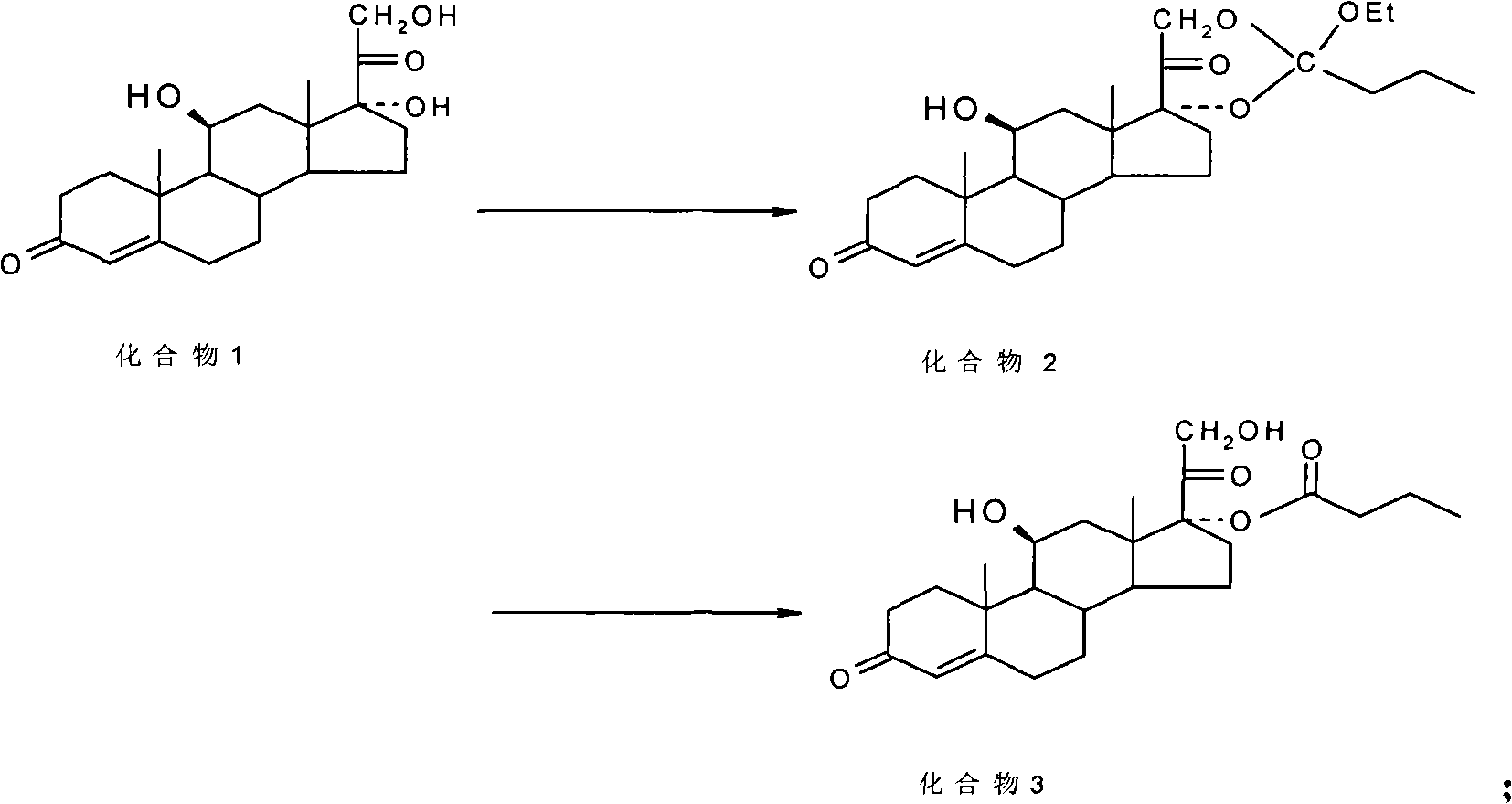
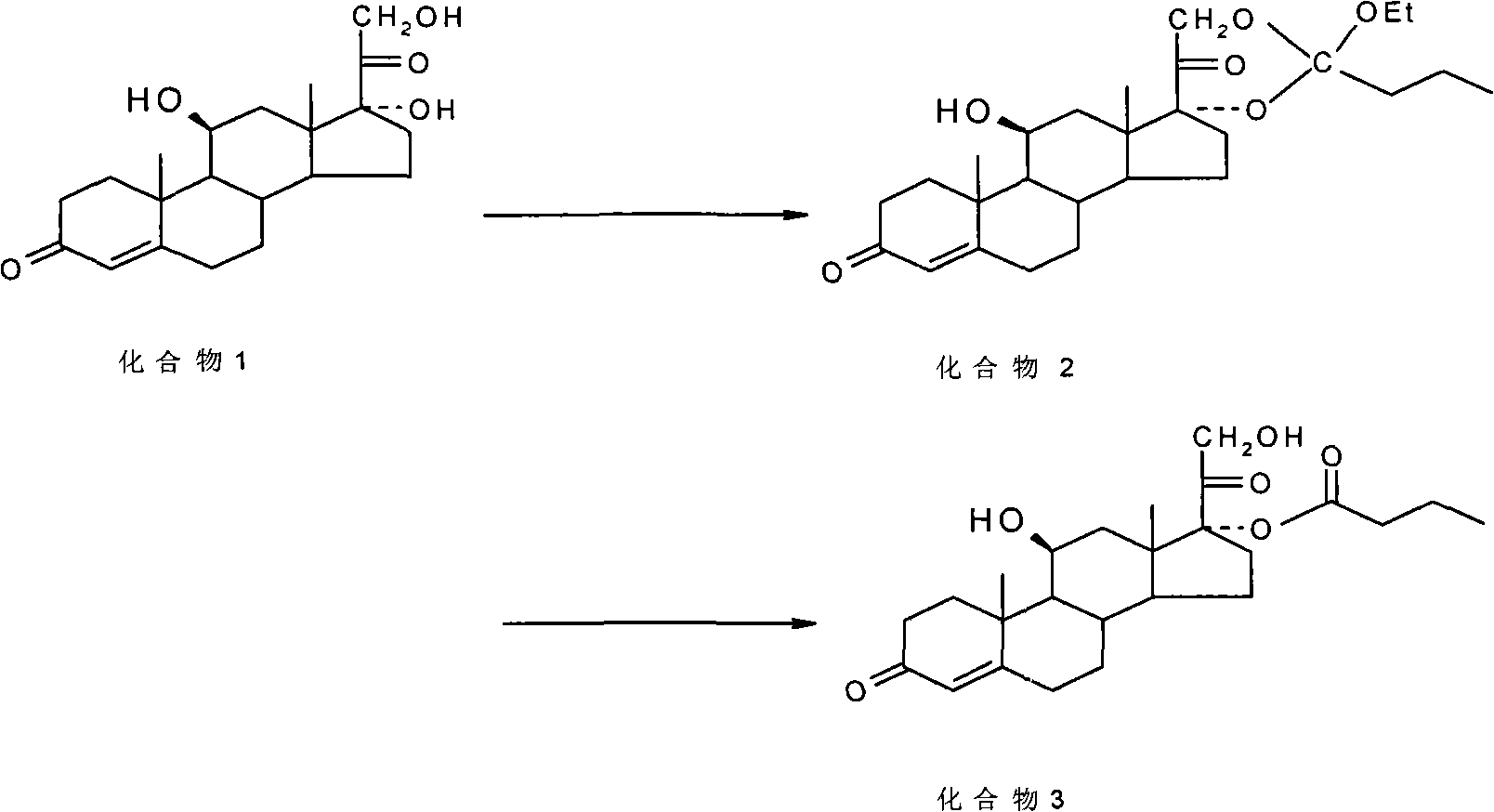
ActiveCN101891797AMake up for the impactPromote hydrolysisSteroids preparationPrednisoloneCompound 17
The invention discloses a method for preparing a sterides compound 17-alpha ester. The method comprises the following steps of: dissolving 17 alpha, 21-dihydroxyl sterides compound serving as a raw material in a cyclic ester solvent; adding an ester and a catalyst into the mixture and reacting the mixture to prepare a cyclic ester; and dissolving the prepared cyclic ester in a hydrolysis solvent, adding an orientation reagent and a hydrolysis reagent into the mixed solution and hydrolyzing the cyclic ester into the 17-alpha ester. The sterides compound is hydrocortisone, prednisolone, prednisone, hexadecadrol or betamethasone. The method has selective hydrolysis effect,, and can effectively enhance the conversion rate of a product, greatly increase product yield and greatly promote the preparation capability of a sterides medicament.
Owner:ZHEJIANG XIANJU PHARMA
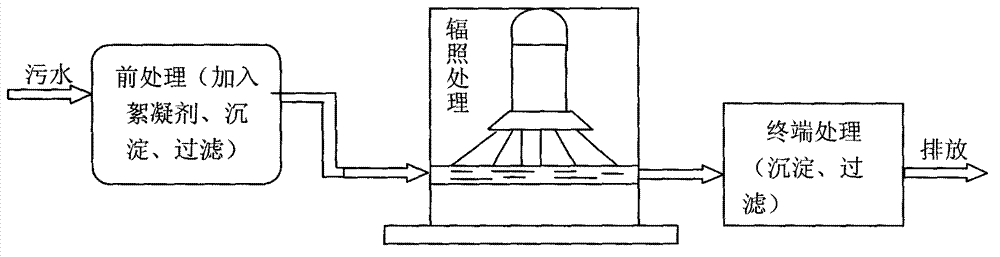
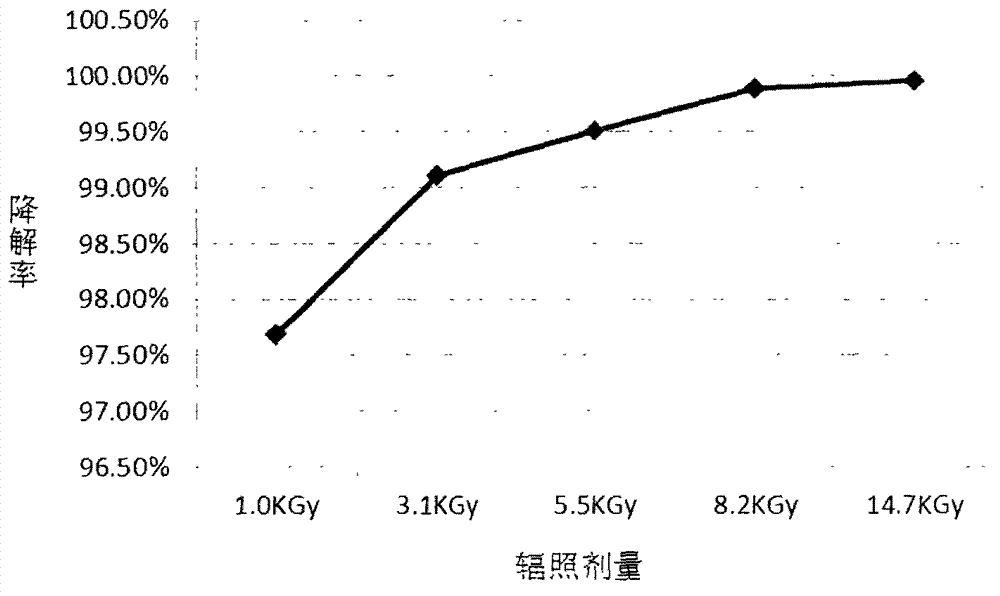
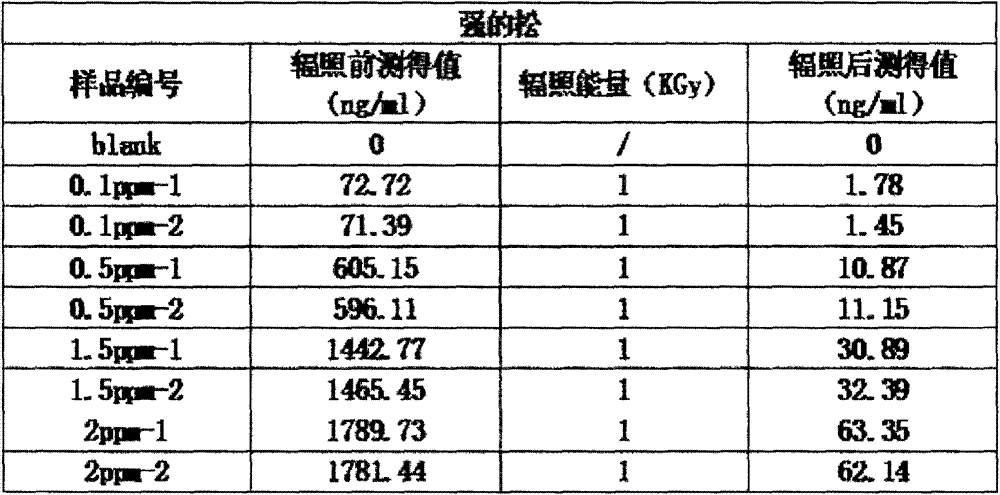
Method for degrading drug residue prednisone in waste water by utilizing irradiation
InactiveCN103172136AEfficient degradationEasy to operateWater/sewage treatment by irradiationWater contaminantsWastewaterEmission standard
The invention relates to a method for degrading drug residue prednisone in waste water by utilizing irradiation. The method provided by the invention comprises that irradiation treatment is carried out on a waste water solution containing prednisone pollutant by adopting a certain dosage electron beam accelerator irradiation technology, so that prednisone in the waste water solution is decomposed. By adopting the method provided by the invention, the prednisone in the waste water solution can be quickly and effectively degraded by virtue of low dosage irradiation, so that the concentration of prednisone in the waste water solution can meet safety and emission standards; and the operation is easy, no toxicity is added, and no residual harm is done.
Owner:INST OF HIGH ENERGY PHYSICS CHINESE ACAD OF SCI
Press-coated tablets of prednisone
The present invention provides for press-coated tablets of prednisone comprising a core comprising prednisone and a coating around the core. The present invention particularly discloses thickness of the coating applied to core having a convex shape for chronotherapeutic use. The present invention also provides for a process for preparing a press-coated tablet of prednisone and a method for treating conditions or pathology, the symptoms of which occur early in the morning.
Owner:CADILA HEALTHCARE LTD
Glucocorticoid synergist
InactiveCN101084910AImprove biological effectReduce dosageOrganic active ingredientsAntipyreticDiseaseDexamethasone
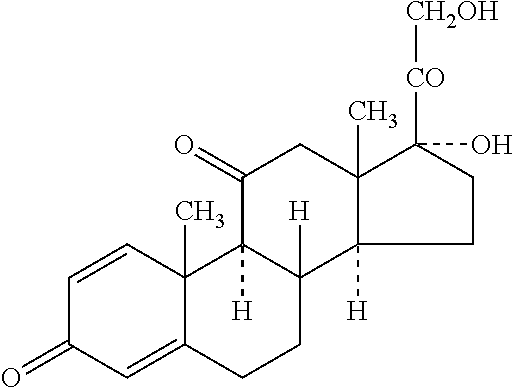
The invention relates to medical technology field, discloses a glucocorticoid synergist, and is characterized in adopting total ginsenoside as synergist. Its proportions are respectively as following: total ginsenoside 200mg-1200mg and dexamethasone 0.75-187.5mg; total ginsenoside 200mg-1200mg and betamethason 0.75-187.5mg; total ginsenoside 200-1200mg, and prednisone 5-1250mg, total ginsenoside 200-1200mg, and precortisyl 5-1250mg; total ginsenoside 200mg-1200mg, and urbason 4-1000mg; total ginsenoside 200-1200mg, and cortisone 25-6250mg; and total ginsenoside 200-1200mg, and hydrocortisone 20-5000mg.Total ginsenoside as glucocorticoid synergist can be used in the treatment of diseases requiring long term or a large amount of glucocorticoid such as systemic lupus erythematosus, nephrotic syndrome, polymyositis, and rheumatoid arthritis.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method of preparing Chinese medicine for treating idiopathic thromboeytopenic purpura idiopathic thromboeytopenic purpura
InactiveCN101244214ASmall side effectsAvoid destructionUnknown materialsBlood disorderSide effectThrombocytopenic purpura
The invention relates to a preparation method of a Chinese herbal medicine for treating idiopathic thrombocytopenic purpura, belonging to the technical field of the Chinese herbal medicine preparation method. At present, glucocorticoid such as prednisone is adopted to treat idiopathic thrombocytopenic purpura and is easy to bring side effect. The technical proposal is that: steep rhinoceros horn, root of rehmannia, root of herbaceous peony, herba cirsii, thistle, Chinese arborvitae twig, root of madder, palm, rhizoma imperatae, Chinese rhubarb, gardenia, gromwell, red peony root, pulsatilla chinensis, flos imperatae, loofah, spiranthes sinensis, herba ecliptae, cockscomb, Japanese dock root and tree peony bark in water and boil with slow fire to make into the Chinese herbal medicine for treating idiopathic thrombocytopenic purpura. The Chinese herbal medicine for treating idiopathic thrombocytopenic purpura has the advantages of having a stable curative effect after oral intake and avoiding anaphylactic reaction and gastrointestinal reaction caused by adopting western medicine as well as a plurality of restriction set by using glucocorticoid.
Owner:王萍
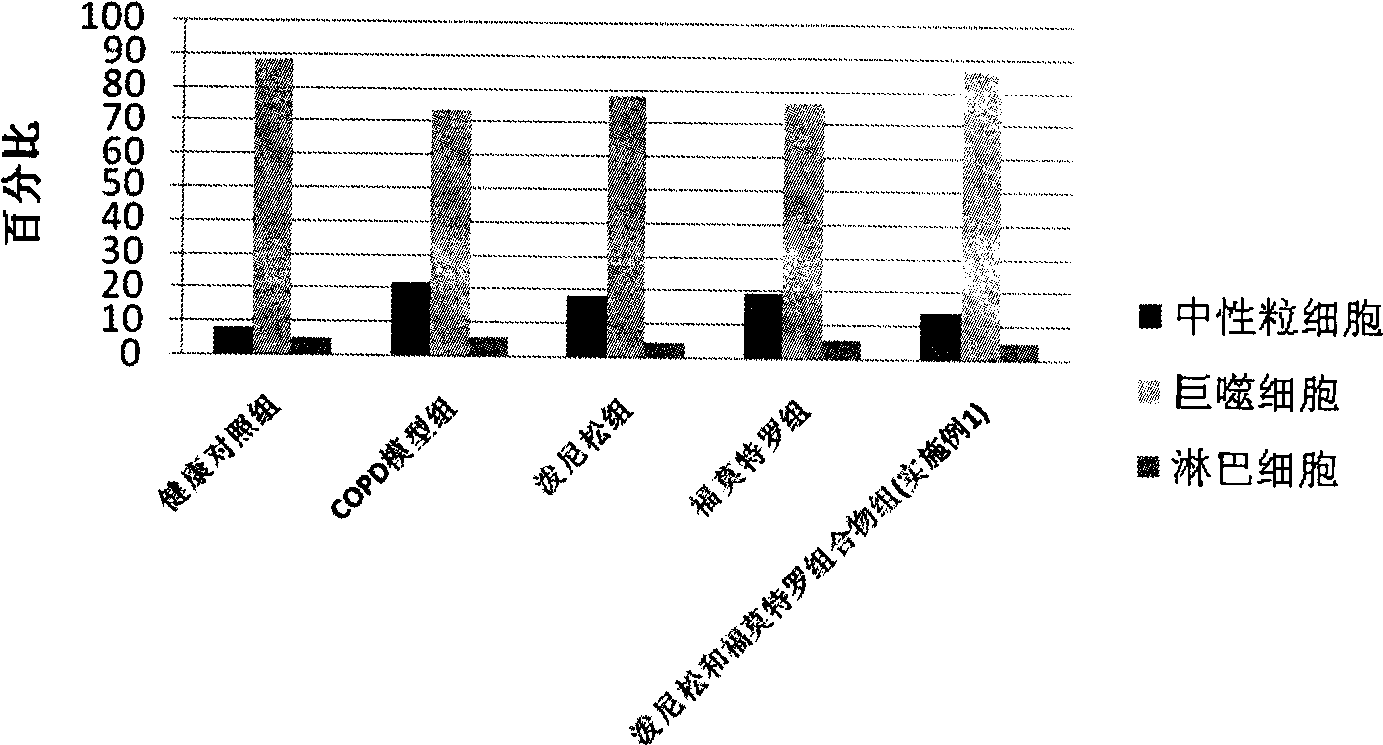
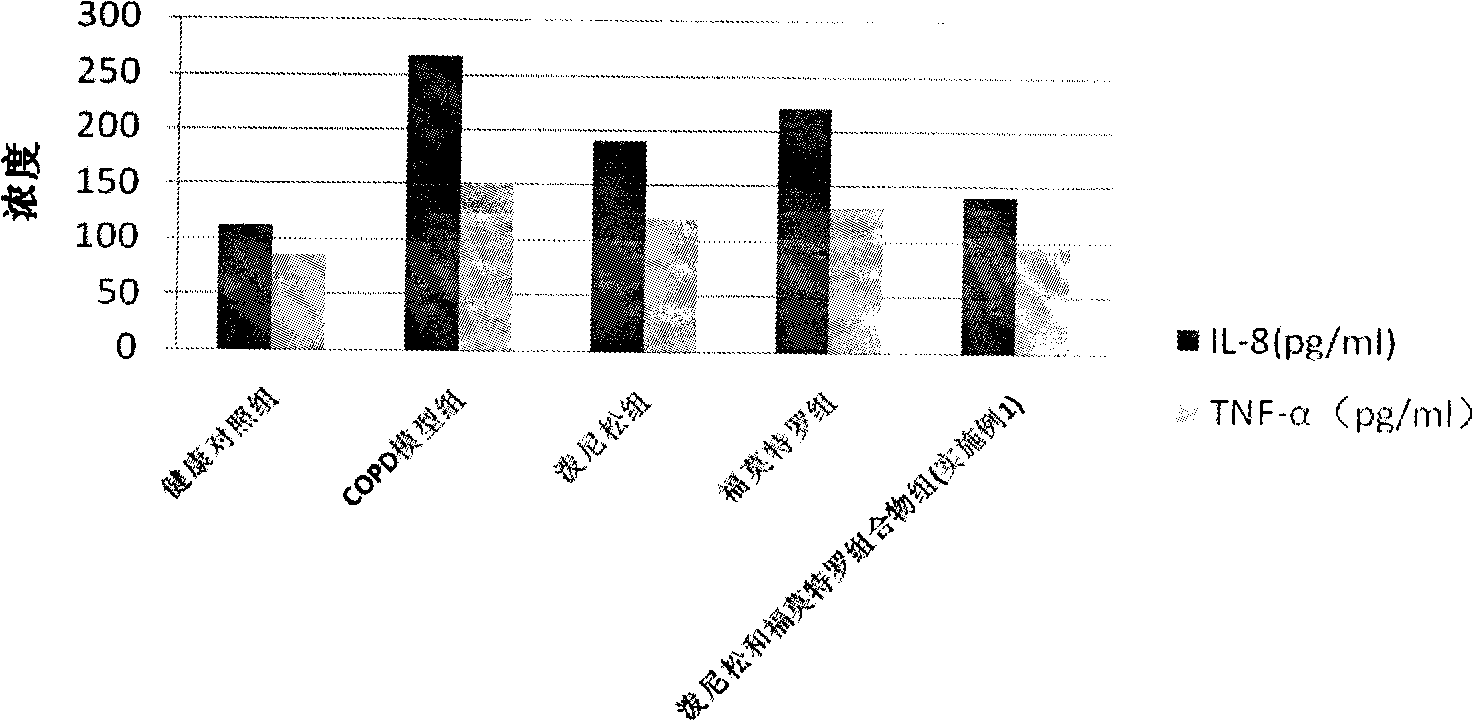
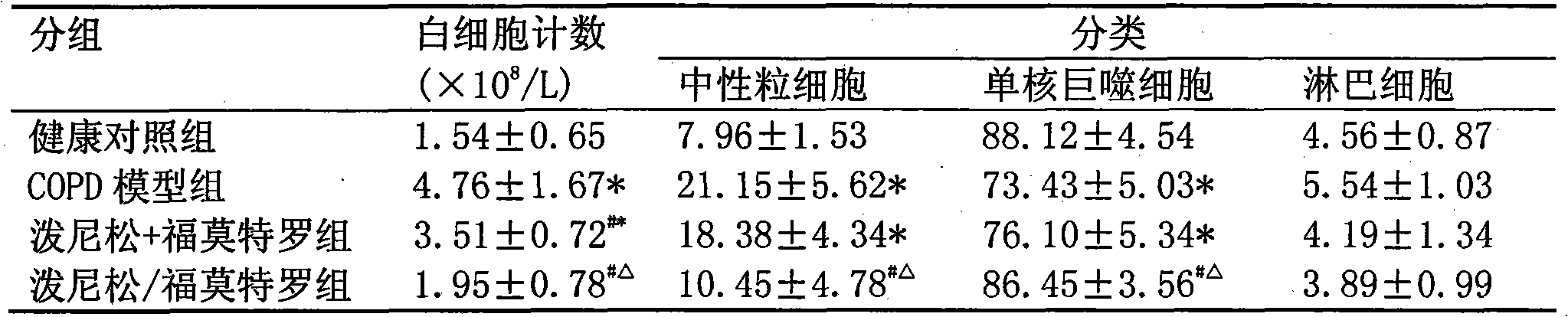
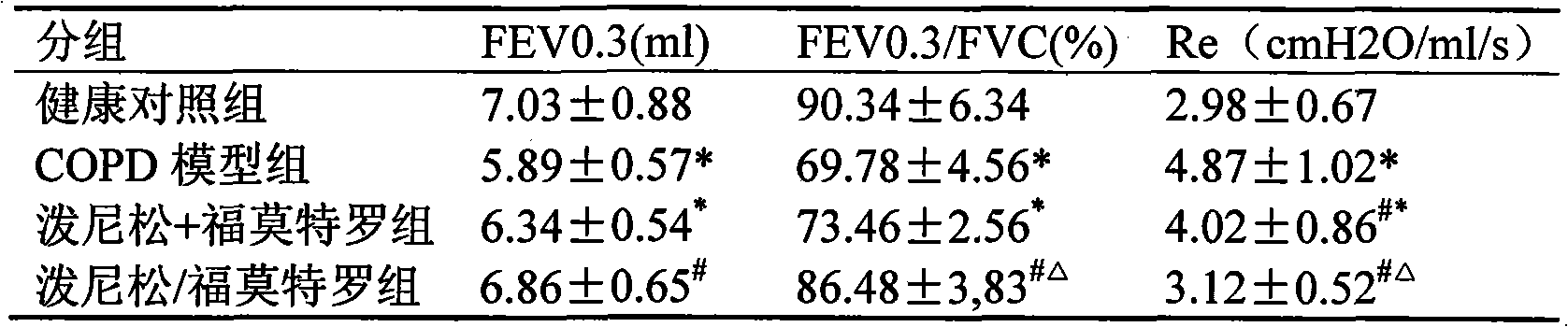
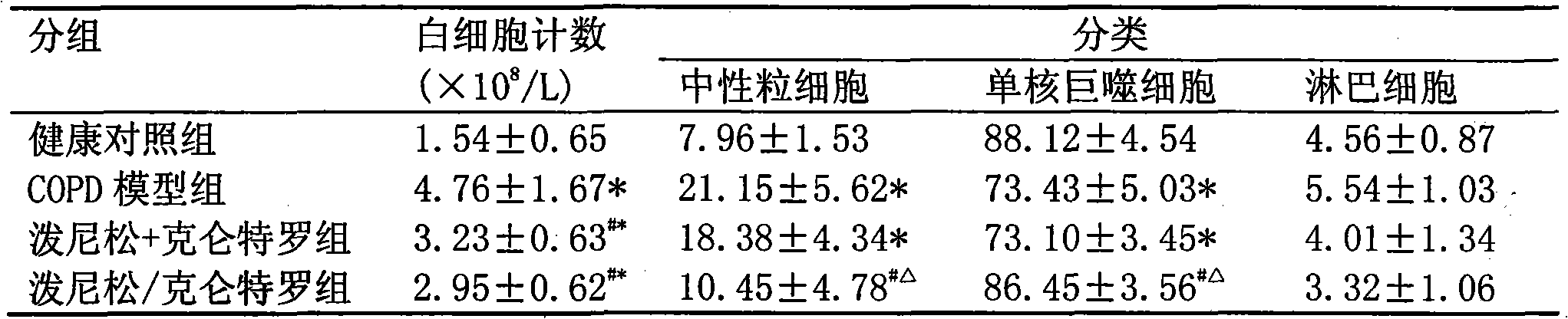
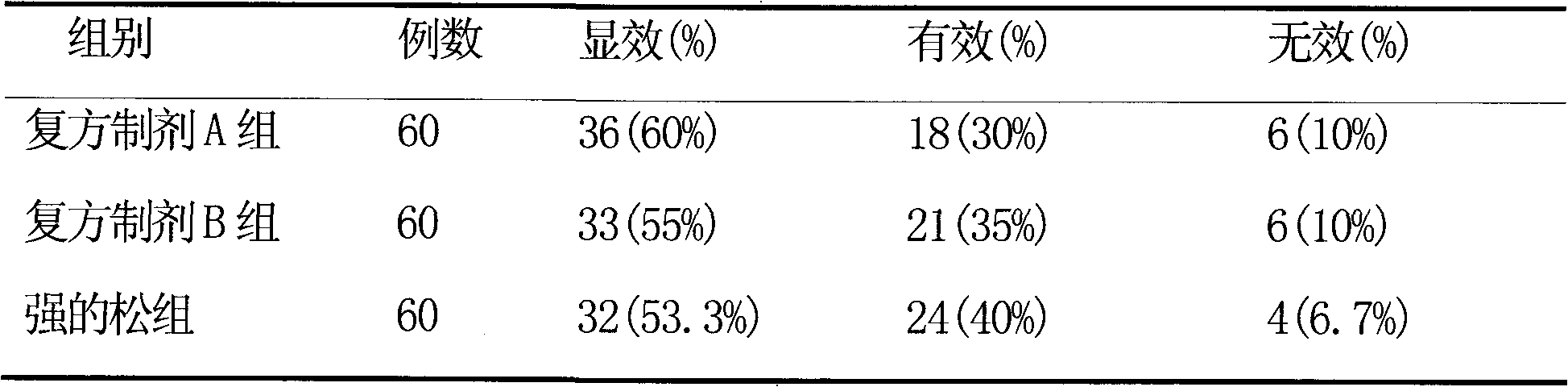
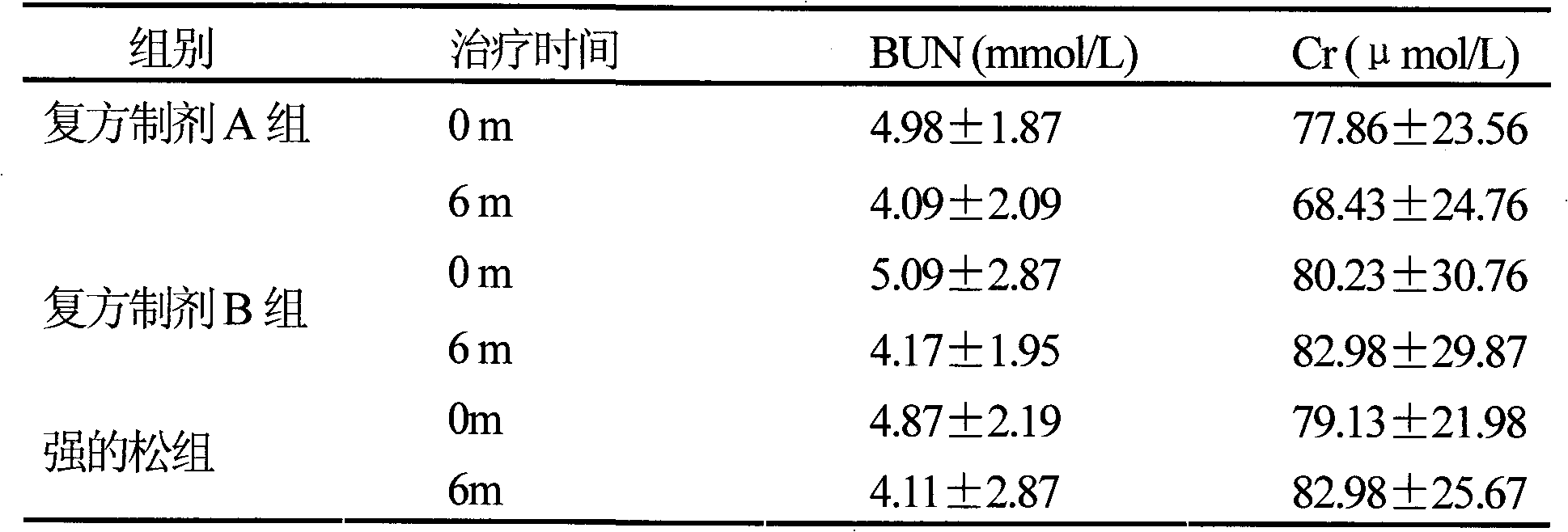
Pharmaceutical composition for the treatment of chronic obstructive pulmonary disease and bronchial asthma
InactiveCN101530618ALower doseReduce or avoid side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
The invention discloses a pharmaceutical composition for the treatment of chronic obstructive pulmonary disease (COPD) and bronchial asthma, which is composed of a glucocorticoid, a bronchodilator and a pharmaceutically acceptable auxiliary material or carrier; the composition is a preparation for oral use. The glucocorticoid in the inventive pharmaceutical composition is selected from prednisone, prednisolone, methylprednisolone, betamethasone, decamethasone or hydrocortisone; the bronchodilator is selected from formoterol, clenbuterol, procaterol or theophylline. The inventive composition has better therapeutic effect on the COPD and the bronchial asthma than independent administration of two ingredients, and has synergistic effect. The composition has easily-available raw materials, inexpensive price and increased medicine taking compliance as well as plays a significant role in preventing and treating the COPD and the bronchial asthma of patients in vast rural areas and patients in low-income class of the city in China.
Owner:莫始平
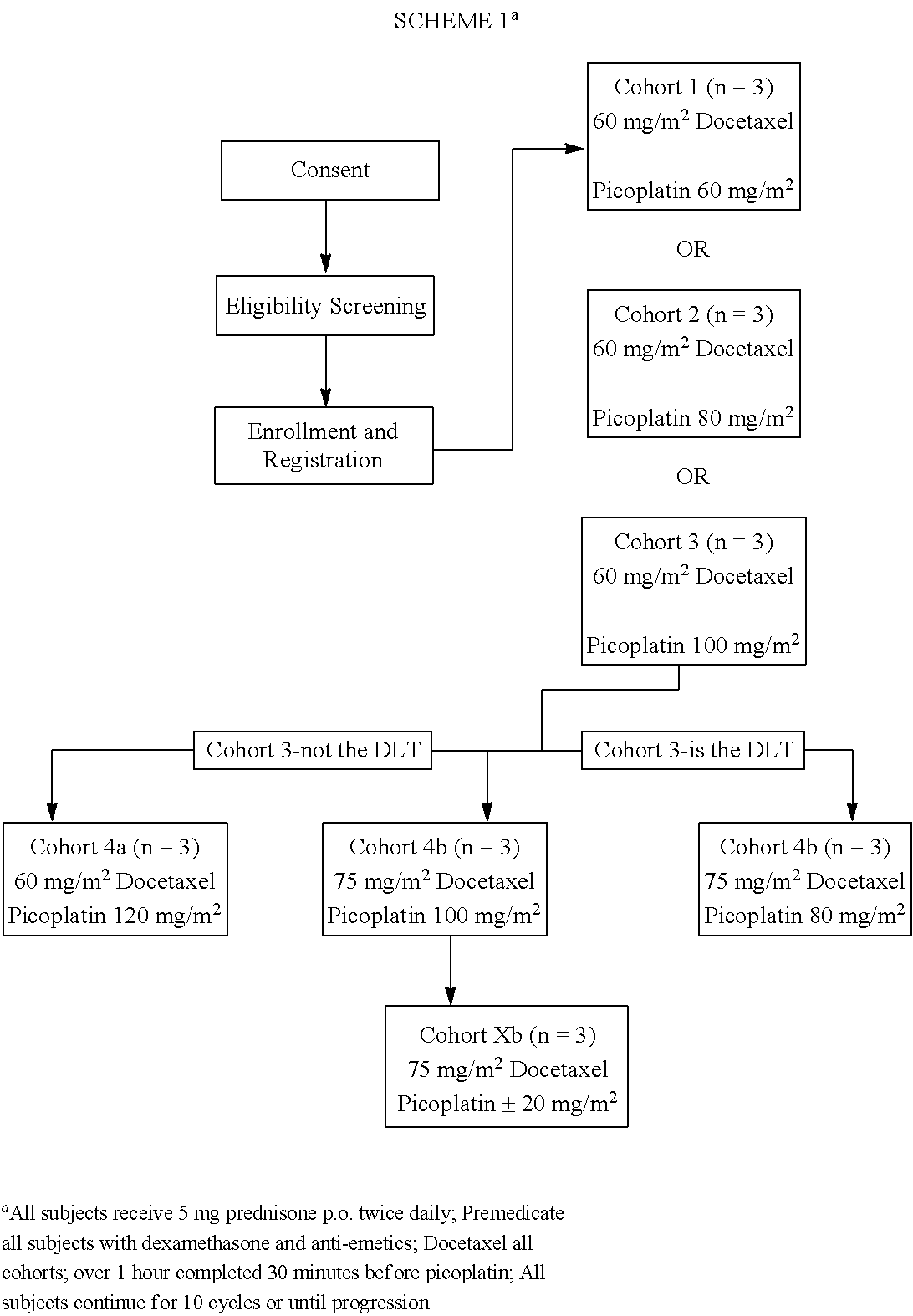
Use of picoplatin to treat prostate cancer
InactiveUS20120122825A1Improve the quality of lifeLower Level RequirementsBiocideOrganic active ingredientsRegimenDocetaxel-PNP
The invention provides a method of treatment of metastatic hormone-refractory prostate cancer involving substantially as concurrent administration of picoplatin and docetaxel. Prednisone may also be administered. Dosages and dosing regimens are provided.
Owner:SCHWEGMAN LUNDBERG& WOESSNER P A
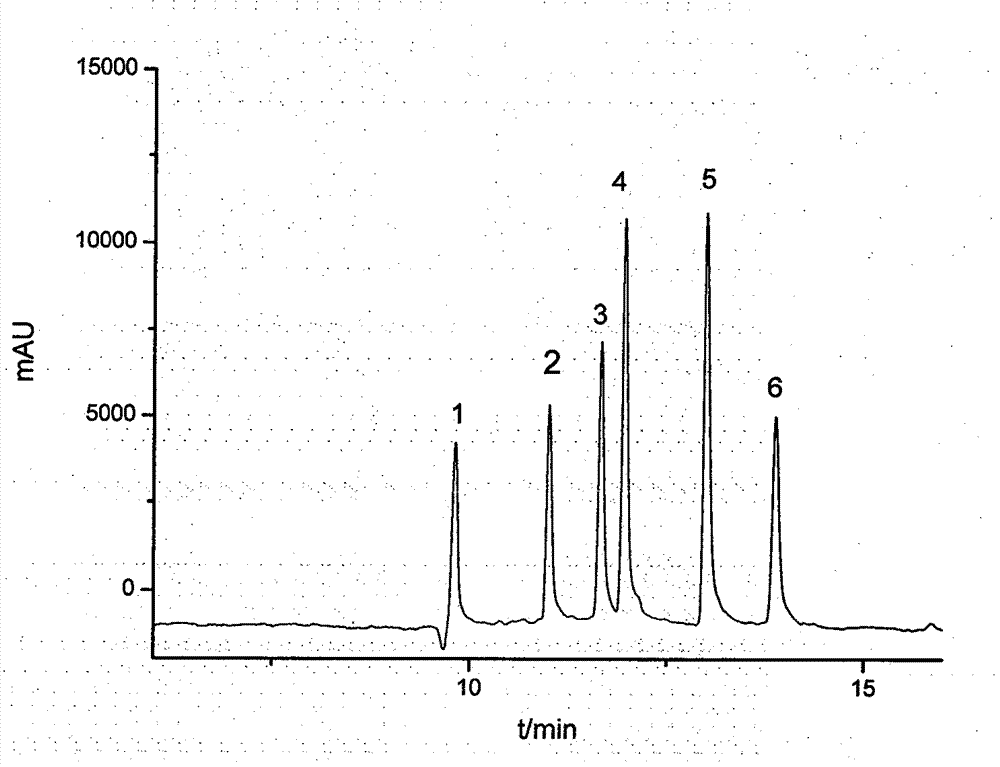
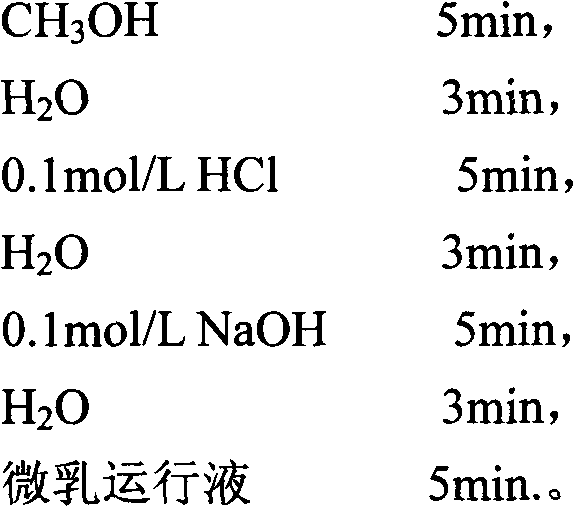
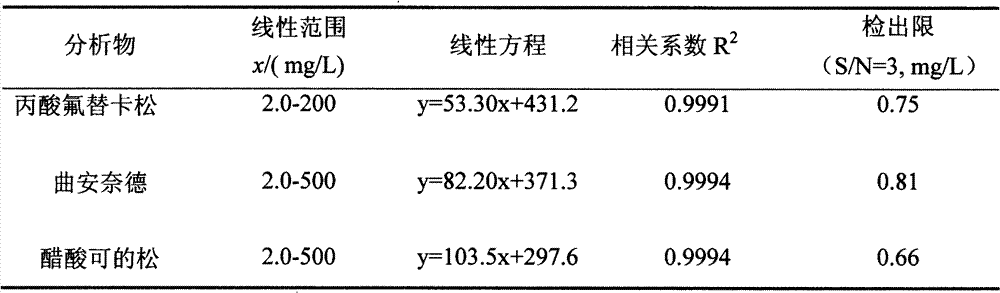
Method for separating and detecting six cortical hormones in skin-care cosmetic
The invention provides a method for separating and detecting six cortical hormones in a skin-care cosmetic. The six cortical hormones, i.e. fluticasone propionate, triamcinolone acetonide, cortisone acetate, dexamethasone, hydrocortisone and prednisone, in the cosmetic are separated and detected by a reverse micro emulsion electrokinetic chromatography (MEEKC) with ionic liquid and beta-cyclodextrin as additives. Compared with a conventional MEEKC separation and detection method, the method has higher separation degree. An experiment result shows that the method is simple in sample treatment, low in using amount, economic, high in speed, and high in separation degree and sensitivity. Capillary electrophoretic detection on the cortical hormones in the cosmetic is realized by the method.
Owner:JIANGNAN UNIV
Chinese and western compounded drug for treating alveolysis and its preparing method
InactiveCN1813941ANot poisonousExcellent treatment for dental diseasesOrganic active ingredientsDigestive systemCompounding drugsMedicine
The present invention discloses a medicine composition for effectively curing odontopathy and its preparation process. It is made up by using metronidazole, erythromycin, prednisone, crude gypsum, cimicifuga root and asarum as raw material through a certain preparation process. It can be made into capsule preparation, and its effective rate is above 95%.
Owner:房丽华
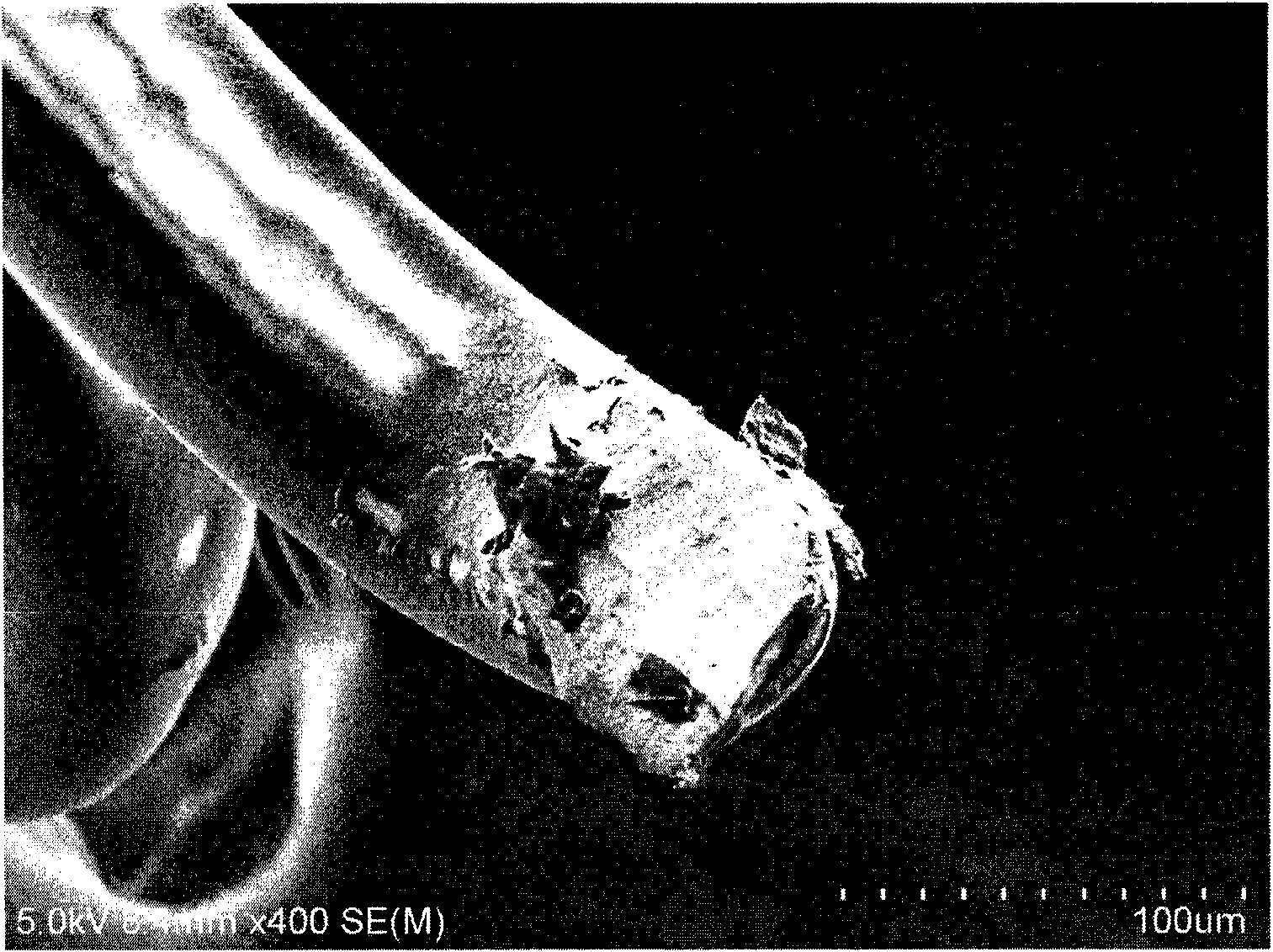
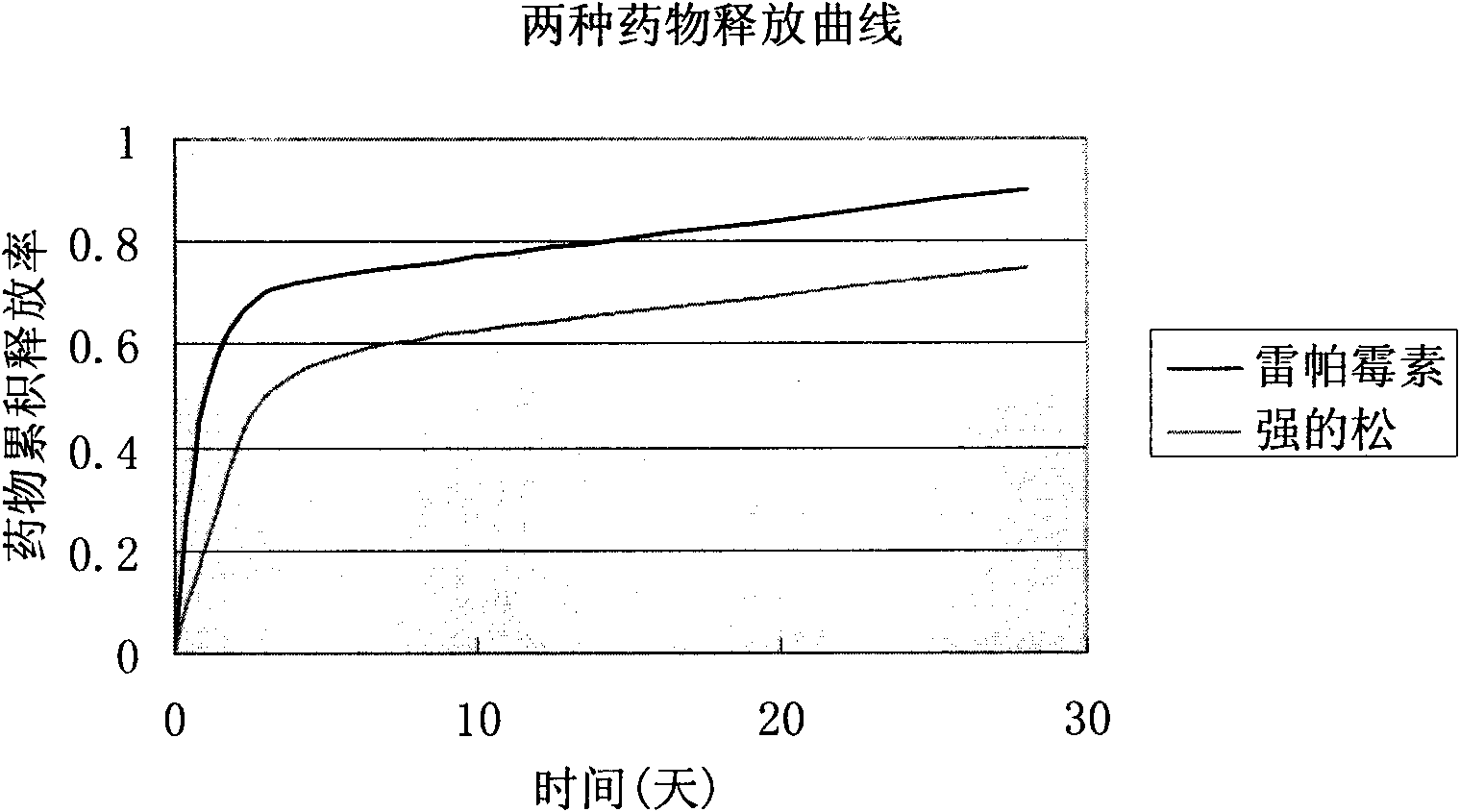
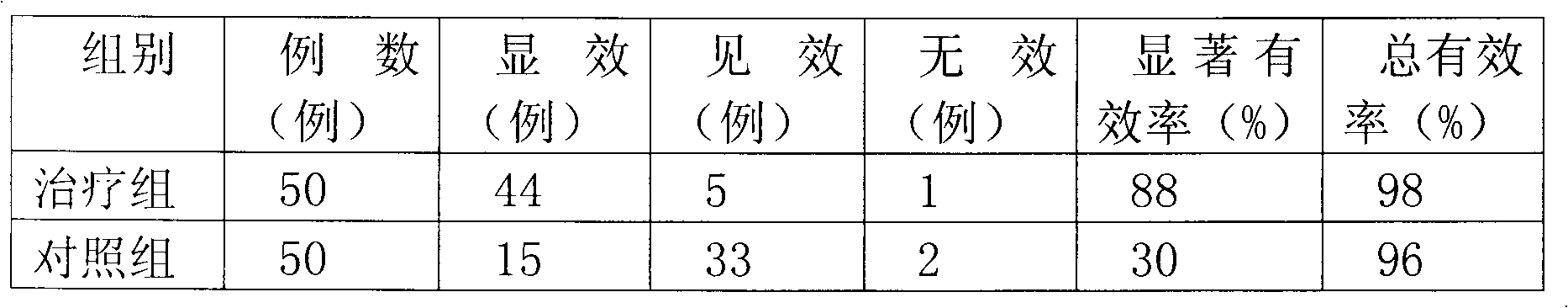
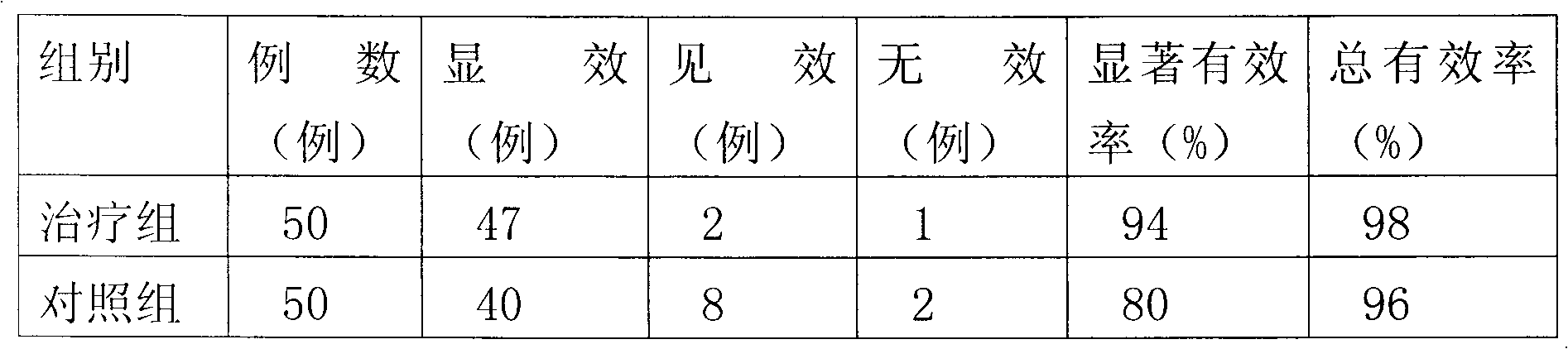
Biodegradable rapamycin-prednisone composite medicinal coat metal stent
InactiveCN101836910APrevent proliferationPromotes re-endothelializationStentsCoatingsThrombusPercent Diameter Stenosis
The invention provides a biodegradable rapamycin-prednisone composite medicinal coat metal stent, which comprises a metal stent, and active ingredients and a slow-release carrier wrapping the surface of the metal stent, wherein the active ingredients comprise rapamycin and prednisone, and the slow-release carrier is an in vivo degradable aliphatic polymer. The coat metal stent keeps the advantage of restenosis prevention of a medicinal coat metal stent, and solves the problem of late stent thrombosis along with full release of a slow-release coat; and the prednisone in the coat has the effect of inhibiting local inflammatory response caused by the carrier and the medicament. The coat metal stent of the invention is mainly used for interventional therapy of coronary heart disease, prevention of restenosis and reduction of late thrombosis formation after the stent is implanted.
Owner:XIAN BONUO ELECTRONICS TECH
Medicament for treating aphthosis and preparation method thereof
InactiveCN101961411AAvoid damageFast and high curative effectOrganic active ingredientsAnthropod material medical ingredientsSide effectLiver and kidney
The invention discloses a medicament for treating aphthosis and a preparation method thereof. The medicament is prepared from the following medicinal raw materials in part by weight: 10 to 30 parts of alum, 10 to 30 parts of non-toxic spider, 10 to 30 parts of evodia fruit, 1 to 3 parts of vitamin B2 and 5 to 15 parts of prednisone. The medicament has no toxic or side effect, is not metabolized by liver and kidney, is absorbed by local mucous membrane, and has the advantages of quick and high curative effect, remarkable treatment effect, short treatment course and combination of Chinese and western medicaments. The medicament does not need oral administration so as to reduce the injury of the oral medicaments to the liver and the kidney and fulfill the purpose of treating the aphthosis. Besides good antiphlogistic and analgesic functions, the medicament has obvious curative effect on impetigo.
Owner:屈广花
Prednisone pulse release tablet
InactiveCN104546785AEasy to take medicineChrono-rhythmicOrganic active ingredientsAntipyreticWeight gainingTime lag
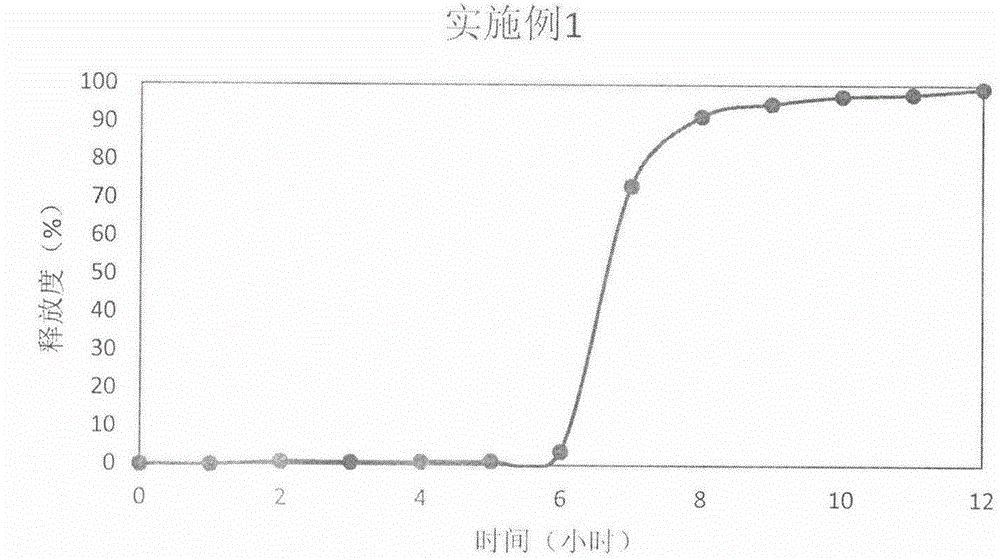
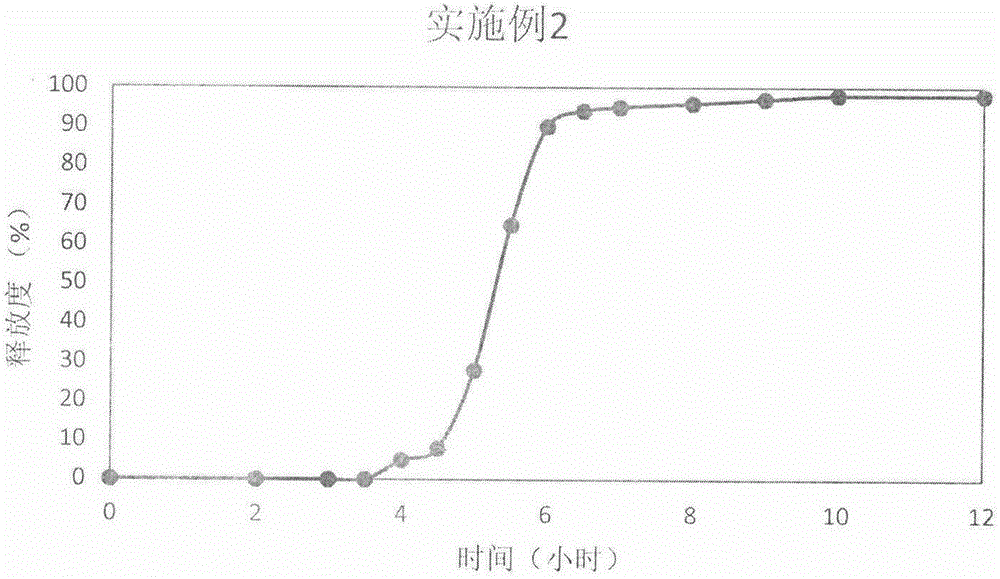
The invention discloses a prednisone pulse release tablet and a preparation method thereof. The prednisone pulse release tablet consists of a fast-release tablet core and a controlled-release coating layer, wherein the optimized coating weight gain rate of the controlled-release coating layer, relative to that of the fast-release tablet core, is 350-390%; the fast-release tablet core consists of drugs and an inner layer auxiliary material, wherein the drugs are prednisone and acetate thereof, the dose specification of the drugs is 1-5mg per tablet on the basis of prednisone; and the controlled-release coating layer consists of a coating framework material and a pore forming agent, wherein the coating framework material is a composition of hypromellose and ethyl cellulose, and the pore forming agent is mannitol. The prednisone pulse release tablet disclosed by the invention can ensure that the drug release time lag is adjusted between 2-9 hours by changing a prescription process, and the drug release time lag is preferably 4-8 hours according to specific needs of time pathology treatment performed clinically. The prescription of the prednisone pulse release tablet disclosed by the invention is not complex in composition, the preparation process adopts a simple and feasible powder direct tabletting method and a coating pressing method, and is suitable for large-scale industrial production.
Owner:CHINA PHARM UNIV
Treatment of diffuse large-cell lymphoma with Anti-cd20 antibody
InactiveUS20140030263A1Effective treatmentOrganic active ingredientsIn-vivo radioactive preparationsRegimenPrednisone treatment
The present invention concerns methods for the treatment of diffuse large cell lymphoma by administration of an anti-CD20 antibody and chemotherapy. Particular embodiments include the administration of anti-CD20 antibody in combination with chemotherapy comprising CHOP (cyclophosphamide, hydroxydaunorubicin / doxorubicin, vincristine, and prednisone / prednisolone) and / or in combination with a transplantation regimen.
Owner:BIOGEN INC
Application of chlorogenic acid in preparation of medicines for treating osteopetrosis
ActiveCN104606179AGood treatment effectLittle side effectsOrganic active ingredientsSkeletal disorderChlorogenic acidSide effect
The invention provides an application of chlorogenic acid in preparation of medicines for treating osteopetrosis. The chlorogenic acid can be used for effectively treating the osteopetrosis, the treatment effect is better than that of a combined drug of positive drugs such as prednisone and channels-soothing and blood-activating tablets, and the chlorogenic acid is proved to be a safe drug, has small side effects and is good in clinical application prospects.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Production method of prednisone hydrolysate
The present invention relates to a production method of prednisone hydrolysate. Said production method includes the following steps: using intermediate prednisone acetate as raw material, using chloroform and methyl alcohol mixed solution as solvent, using potassium hydroxide solution as catalyst, making them be reacted at low temperature, neutralizing, concentrating to make crystallization, cooling, filtering and drying so as to obtain the prednisone hydrolysate. Said invention can raise its yield and quality.
Owner:TIANJIN TIANYAO PHARM CO LTD
Drug-release type gel for treating fundus macular degeneration and preparation method thereof
InactiveCN109966243AAchieve sustained releaseLittle side effectsSenses disorderAerosol deliveryPeroxydisulfateNon invasive
The invention discloses drug-release type gel for treating fundus macular degeneration and a preparation method thereof. The drug-release type gel comprises an anti-angiogenesis drug, an inducing compound and gel and is characterized in that the inducing compound is used for increasing the permeation of the drug, and the gel attaches to the cornea during use and serves as the carrier of the drug;the anti-angiogenesis drug is selected from Lucentis or Compaq; the inducing compound is selected from optional one of dexamethasone, prednisone and cortisone or the combination of two of dexamethasone, prednisone and cortisone; the gel serving as the drug carrier is prepared by preparing a solution containing polydopamine and methacrylate gelatin, adding crosslinking agent N,N-methylene bisacrylamide and initiator potassium peroxydisulfate into the solution, and performing fixed-die forming to obtain the gel which does not contain the drug. The drug-release type gel has the advantages that non-invasive drug delivery is adopted, sustained drug release is achieved after the gel attaches to the cornea, and the anti-VEGF drug is allowed to act on the vitreous body by utilizing the permeability of the inducing compound.
Owner:SHANGHAI TONGREN HOSPITAL
Biological markers for evaluating therapeutic treatment of inflammatory and autoimmune disorders
InactiveUS20050002862A1ActionGood anti-inflammatory effectDisease diagnosisBiological testingAntigenDisease
Novel biological markers indicative of the action of an anti-inflammatory or immunosuppressive drug can be used to evaluate drug efficacy and compare local and systemic drug effects. They can also aid in comparison of different drugs, doses, and delivery routes. The biological markers include cell populations, cell surface antigen expression levels, and soluble factor concentrations. Measurement values of the novel biomarkers were shown to change significantly in allergic, atopic asthmatic, and healthy subjects after administration of prednisone.
Owner:PPD BIOMARKER DISCOVERY SCI
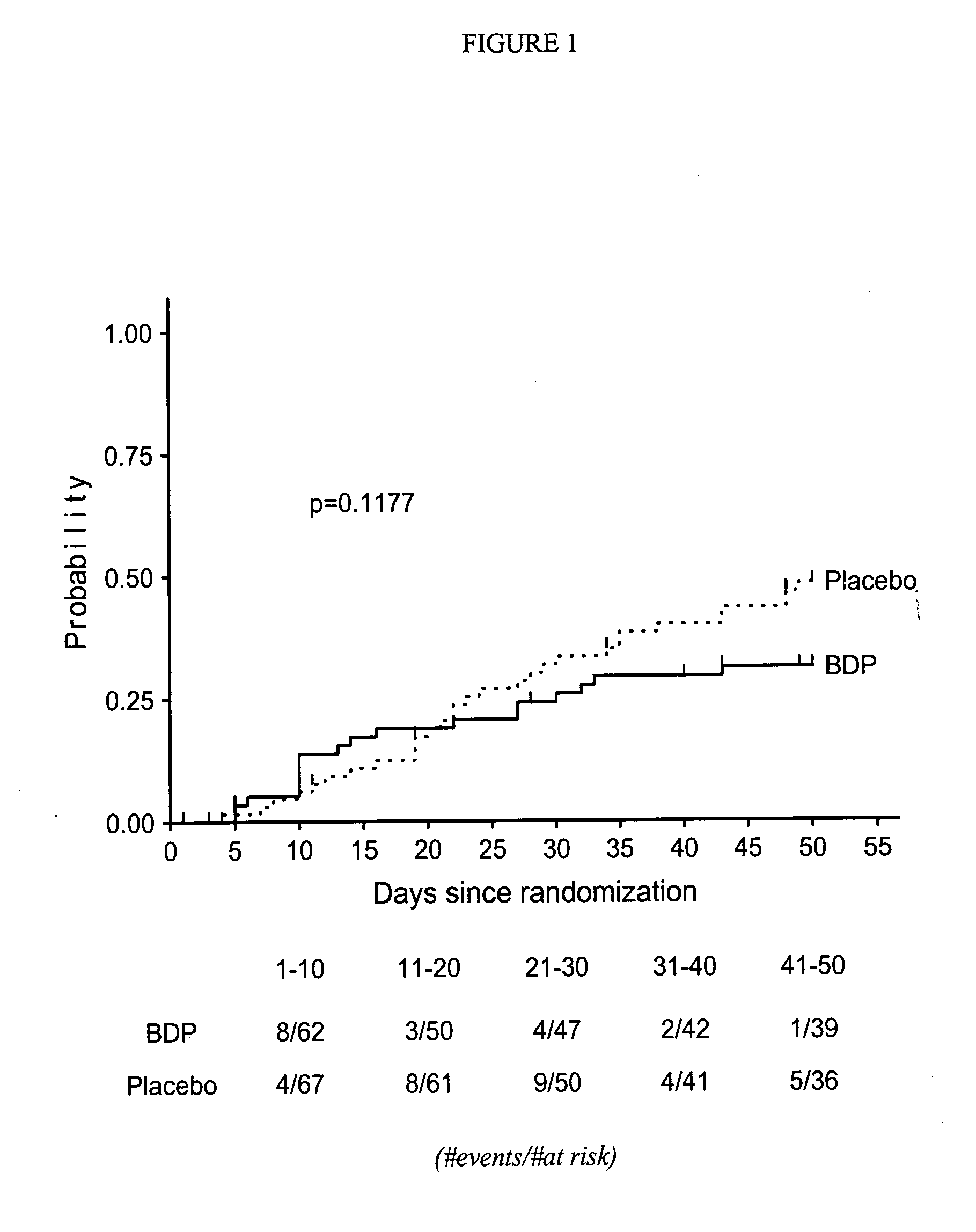
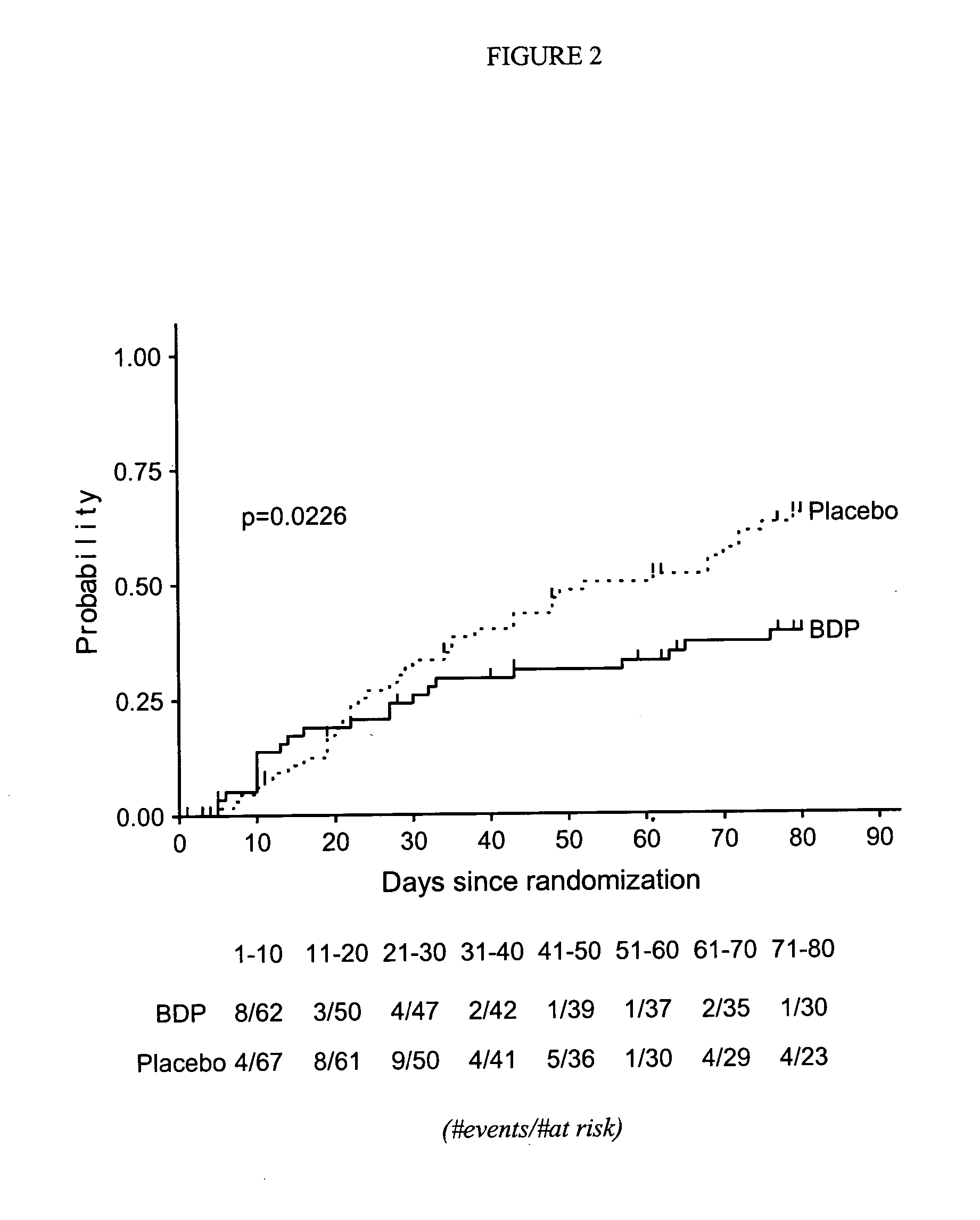
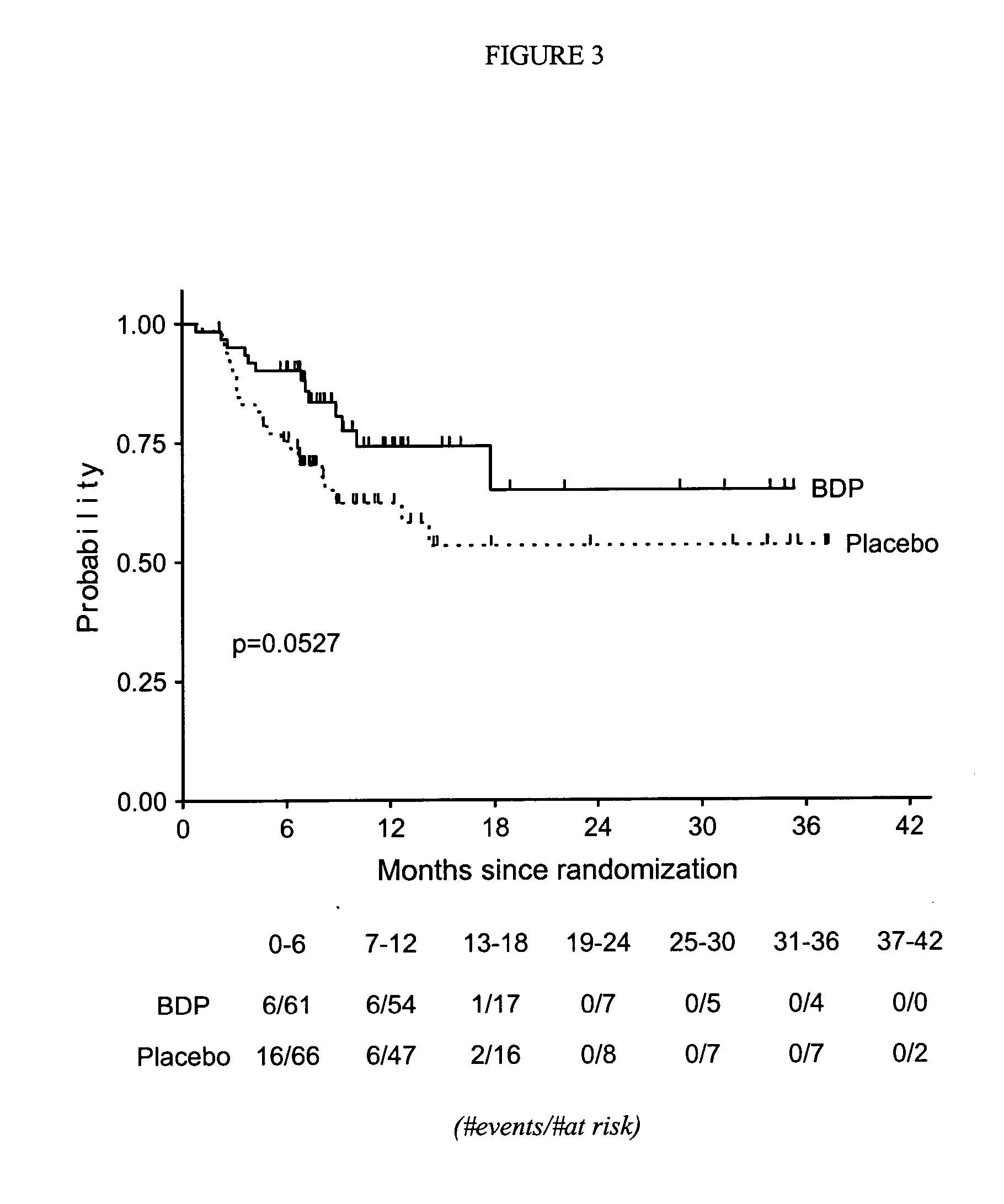
Treatment of graft-versus-host disease and leukemia with beclomethasone dipropionate and prednisone
InactiveUS20060252735A1Reduce mortalityOrganic active ingredientsBiocideHematopoietic cellCo administration
A method for reducing mortality associated with GVHD by treating the patent with an oral BDP regimen that involves co-administration of: 1) a high dose of prednisone (about 1-2 mg / kg / day) for about 10 days, which is then tapered rapidly over the following 7 days to a physiological replacement dose of about 0.0625 mg / kg / day for the remainder of the treatment, and 2) about 4-12 mg oral BDP q.i.d. for about 50 days, where the BDP is administered in both immediate release and enteric coated preparations. Another method is for treating leukemia by performing hematopoietic cell transplantation followed by said regimen. A significant reduction in patient mortality is observed 200 days after the start of these treatments
Owner:SOLIGENIX INC
External-use ointment for treatment of dermatopathy
InactiveCN106668864AWide range of treatmentHigh cure rateHydroxy compound active ingredientsAerosol deliveryDiseaseTherapeutic effect
The invention relates to an external-use ointment for treatment of dermatopathy. The ointment is prepared by mixing and blending the following medicines (by weight): 6-9 parts of sulfamethoxazole, 5-8 parts of chloramphenicol, 5-8 parts of prednisone, 3-5 parts of borneol, 0-8 parts of sulfur flour, 5-8 parts of vitamin E, 4-8 parts of vitamin complex B, 6-10 parts of VC, 5-7 parts of B1, 0.01-0.04 part of triamcinolone acetonide, 10 parts of urea ointment, 30 parts of a Chinese herbal antibacterial paste, 8-10 parts of chlorpheniramine, 4-8 parts of chloramphenicol raw powder, 6-10 parts of metronidazole, 10 parts of B6 ointment, 0-15 parts of terbinafine hydrochloride emulsifiable paste and 0-10 parts of ketoconazole emulsifiable paste. The ointment of the invention is used for treatment of skin diseases such as dermatitis, eczema, acne, yellow fluid ulcers, manus and tinea pedis, psoriasis, etc., can be used for realizing antibiosis, anti-inflammation, itching-relieving and antiallergic functions and the function of boosting body immunity, reduces exudation and inflammatory response and promotes inflammatory resorption of wounds and growth of granulation tissues, and has a good comprehensive treatment effect.
Owner:毕明华
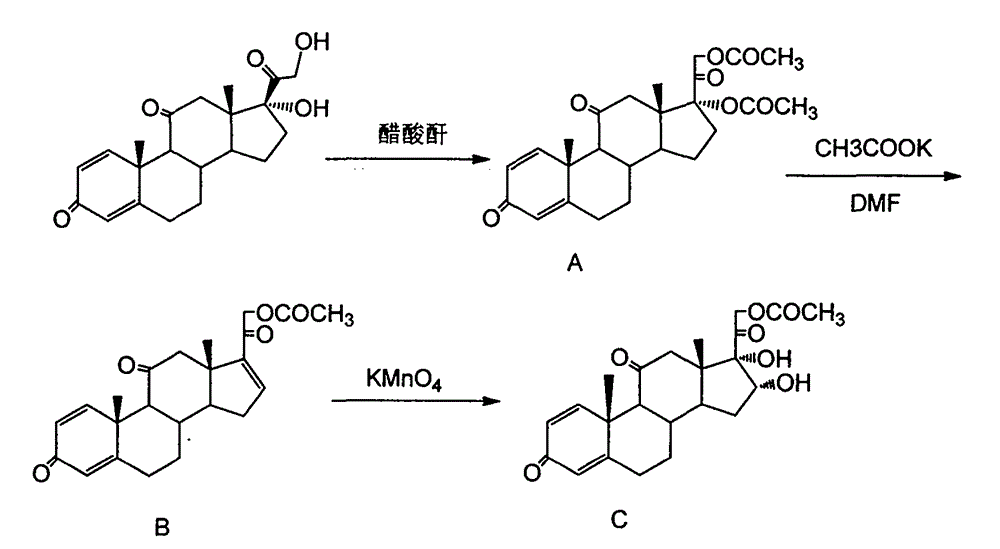
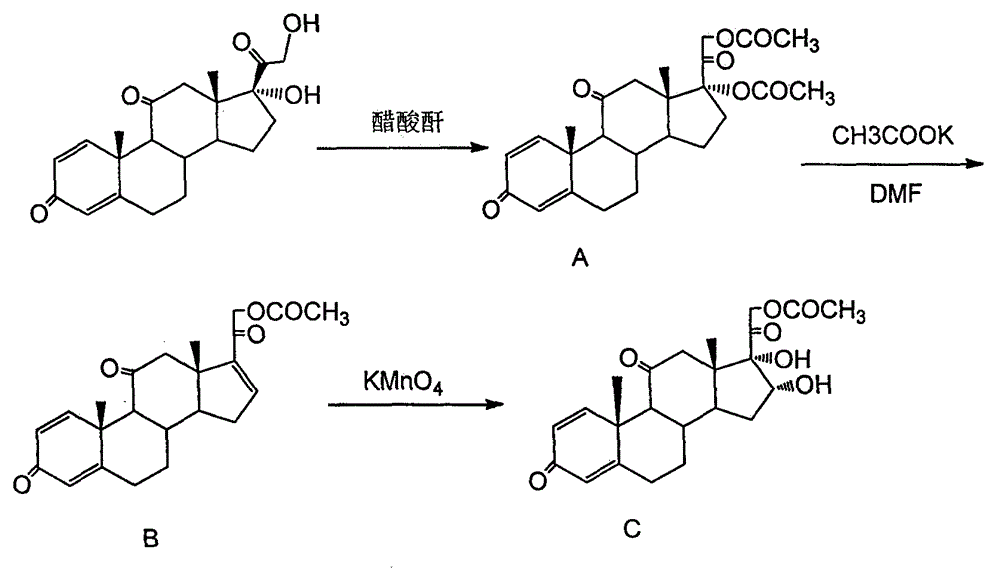
Preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone
The invention provides a preparation method of 16a,17a-dyhydroxyl-21-acetoxyl-1,4-pregnene diene-3,11,20-triketone. The preparation method comprises the following steps: step 1, by taking prednisone as a raw material, allowing the prednisone to have an esterification reaction with acetic oxide; step 2, performing a degreasing reaction by use of alkali; and step 3, oxidizing by use of potassium permanganate under an acidic condition. The method has the advantages of adopting raw materials which are low in cost and easy to obtain, simplifying the production process route and being simple to operate and suitable for large-scale industrial production.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
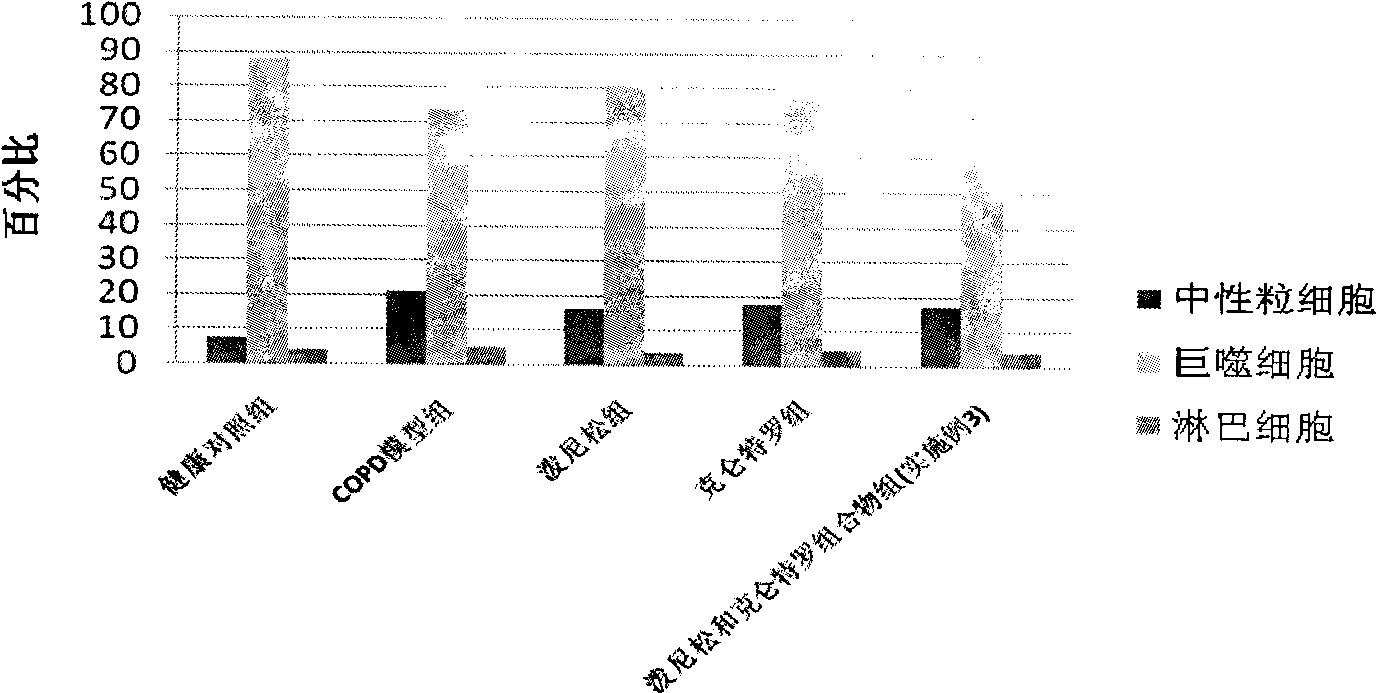
Medicine composition containing oral glucocorticoid and oral bronchodilator
InactiveCN101797258AReduce dosageEliminate side effectsRespiratory disorderHeterocyclic compound active ingredientsDiseaseAdditive ingredient
The invention discloses a medicine composition mainly containing oral glucocorticoid and an oral bronchodilator, as well as application thereof in preparing medicines for treating chronic obstructive pulmonary diseases (COPD) and bronchial asthma, which belongs to the technical field of medicine preparations. The oral glucocorticoid contained in the medicine composition is prednisone, prednisolone or methylprednisolone, and the oral bronchodilator is formoterol, clenbuterol or euphylline. The curative effect of the composition for treating the COPD or the bronchial asthma is better than that of separately using the two composition ingredients one after another, and the composition has the characteristics of synergistic action and low cost. Thus, the combination preparation of the oral glucocorticoid and the oral bronchodilator is of great significance to the treatment and the prevention of the COPD and the bronchial asthma of patients of lower income groups in wide rural and urban areas in China.
Owner:莫始平
Compound preparation for treating nephrotic syndromes
InactiveCN101856377AImprove biological effectLittle side effectsOrganic active ingredientsUrinary disorderSide effectAutoimmune disease
The invention relates to a compound preparation for treating nephrotic syndromes. A capsule of the compound preparation is prepared by combining 400 milligrams of ginsenoside and 10 to 20 milligrams of prednisone or 8 to 16 milligrams of meprednisone. The compound preparation not only enhances the biological effect of hormones, but also reduces the side effect of the hormones, so that the progress of the state of illness can be controlled more effectively. The compound preparation utilizes the effects of stress resistance, inflammatory resistance and the like of the ginsenoside used as main active ingredients of panax ginseng fully, has the two-way adjusting effect on the immunologic function and particularly has the synergistic effect on the prednisone, and is widely suitable for the treatment of autoimmune diseases.
Owner:中国人民解放军济南军区第四0一医院
Liquid medicine for curing burn and preparation method thereof
InactiveCN101371846ADermatological disorderHeterocyclic compound active ingredientsBurned skinAdditive ingredient
The invention relates to a liquid medicine for treating burn; the liquid medicine is prepared by the following ingredients in terms of part by weight: 90-110 portions of prednisone tablets, 70-110 portions of maleate chlor-trimeton tablets, 180-220 portions of vitamin B1 tablets, 13-17 portions of dexamethasone tablets, 5-8 portions of chloramphenicol eyedrop and 90-110 portions of distilled water. In preparation, all ingredients are taken in proportion; prednisone tablets, maleate chlor-trimeton tables, vitamin B1 tablets and dexamethasone tablets are dissolved in distilled water or smashed before being dissolved in distilled water, added with chloramphenicol eyedrop and evenly mixed. The antibiotic, anti-flammatory and anti-allergic functions of the liquid medicine can ensure the burnt skin does not be invaded by external bacteria and keep the cleanness, so that the burnt skin can be healed in a short time; the liquid medicine can be used for treating burn, scald, cauterized wound, and on the like, has the advantages of low treatment cost, short healing time, and no scar, and can also be used for treating tympanitis with good curative effect.
Owner:王文丰
Stem cell medicine for treating gonarthritis and using autologous adipose-derived stem cells for preparation
PendingCN108904783AIncrease the number ofQuality improvementOrganic active ingredientsPeptide/protein ingredientsSalidrosideMicrosphere
The invention discloses a stem cell medicine for treating gonarthritis and using autologous adipose-derived stem cells for preparation. The autologous adipose-derived stem cells are used for being induced and differentiated into chondrocytes, and the chondrocytes is combined with magnetic nano microspheres, salidroside, gynostemma pentaphyllum extract, prednisone, eucommia bark extract, atractylodes lancea extract, rehmannia glutinosa libosch root extract, erythrina indica lam extract, rabdosia rubescens extract, mixed stents and the like in a certain proportion to form the autologous adipose-derived stem cell medicine, and then the autologous adipose-derived stem cell medicine is injected into a gonarthrosis focus in the form of injection. In short, the autologous adipose-derived stem cell medicien has a good drug effect and a high curative effect, and can effectively repair articular cartilage and restore articular structures and functions.
Owner:广州杜德生物科技有限公司
Novel antitumoral use of cabazitaxel
The invention relates to a compound of Formula (I): which may be in base form or in the form of a hydrate or a solvate, in combination with prednisone or prednisolone, for its use as a medicament in the treatment of prostate cancer, particularly metastatic prostate cancer, especially for patients who are not catered for by a taxane-based treatment.
Owner:AVENTIS PHARMA INC
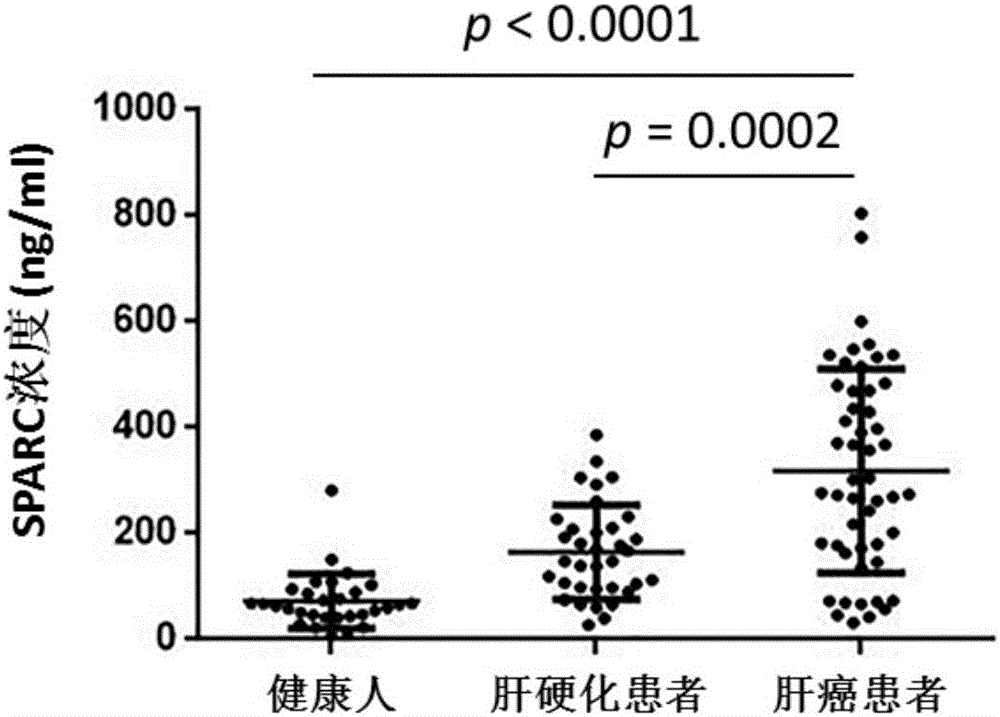
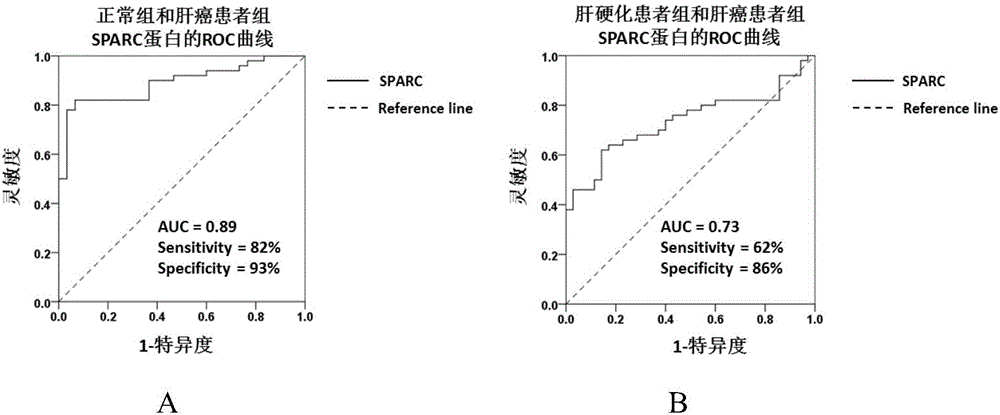
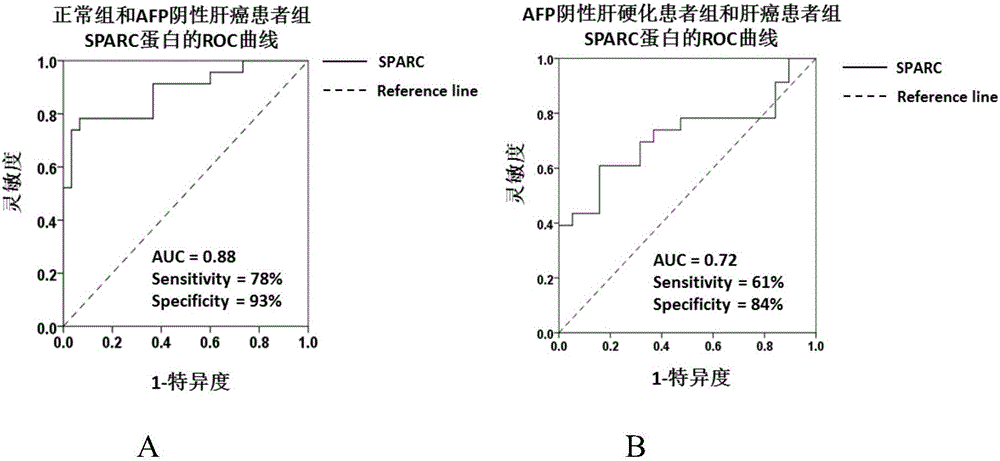
Application of substance for detecting SPARC (Satraplatin and Prednisone Against Refractory Cancer) protein in blood serum to preparation of kit for screening hepatocellular carcinoma
The invention discloses new application of a substance for detecting the concentration of an SPARC (Satraplatin and Prednisone Against Refractory Cancer) protein. The new application provided by the invention is application of a substance for detecting SPARC protein concentration to preparation of a product for screening or helping to screen the hepatocellular carcinoma. An experiment proves that the SPARC protein can be used as a tumor marker of the hepatocellular carcinoma; screening is carried out under the condition of taking normal persons as screening targets, the sensitivity of judging HCC patients is 82%, the specificity is 93% and the AUC (Area Under roc Curve) is 0.89; screening is carried out by taking LC (Liver Cirrhosis) patients as screening targets, the sensitivity of judging the HCC patients is 62%, the specificity is 86% and the AUC is 0.73; the SPARC protein can be used for diagnosing AFP (Alpha-fetoprotein) negative hepatocellular carcinoma; and the SPARC protein can also be combined with AFP to be used for screening or helping to diagnose the HCC from the normal persons or the LC patients.
Owner:BEIJING PROTEOME RES CENT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com